Translate this page into:
Chemical composition and antibacterial activity of essential oil of Pelargonium graveolens and its fractions
⁎Corresponding author. a.amechrouq@umi.ac.ma (Ali Amechrouq)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University. Production and hosting by Elsevier.
Abstract
This work aims to study the chemical composition and evaluate the antibacterial activity of Pelargonium graveolens essential oil and its fractions collected in the Er-Rachidia region of Morocco. GC-MS analysis of Pelargonium graveolens essential oil and its fractions yielded majority compounds such as; epi-γ-Eudesmol (16.67 %), Geraniol (12.54 %), β-Citronellol (12, 34 %), Citronellyl formate (7.70 %) and Geranyl tiglate (5.21 %), for the crude essential oil of Pelargonium graveolons, while the fractions that is obtained by preparative plate chromatography gave the following compounds, fraction 1 consists mainly of β-Citronellol (35. 83 %) Geraniol (38.78 %), fraction 2 is dominated by epi-γ-Eudesmol (55. 10 %) and α-agorofuran (8.41 %)), as well as fraction 3 is revealed the presence of Geranylgeraniol (23.70 %) and epi-γ-Eudesmol (17.53 %) and Phenylethyl tiglate (12.01 %), fraction 4 consists mainly of Phenylethyl tiglate (58.19 %) and α-agorofuran (8.49 %), then the fraction 5 is represented by the compound majorities such as Geranyl tiglate (30.75 %) and Geranyl butanoate (10.94 %), while the fraction 6 is characterized mainly by 1-Isopropyl-4,7-dimethyl-1,2,3,5,6,8a-hexahydronaphthalene (12.08 %). Concerning the antibacterial activity of the essential oil and its fractions 1, 2, 3, and 4 showed bactericidal power against all tested bacteria: Listeria monocytogenes, Escherichia coli, Staphylococcus aureus, and Salmonella typhimurium, also fractions 5 and 6 have bactericidal power against Escherichia coli, Salmonella typhimurium and no power against Staphylococcus aureus and Listeria monocytogenes.
Keywords
Pelargonium graveolens
Essential oil
Fractions
GC-MS
Antibacterial activity
1 Introduction
Currently, scientific researchers have made enormous progress to substitute synthetic antioxidants with potent natural compounds with fewer side effects (Oussaid et al., 2020). The maps contain biologically active chemical elements in the form of secondary metabolites. These are the main components that give the plant its medicinal properties. Indeed, essential oils extracted from aromatic and medicinal plants are composed of complex mixtures of secondary metabolites, and can be used in different applications such as aromatherapy, perfumes, pharmaceuticals, detergents, and cosmetics (Khan et al., 2018). As a result, it has long been used as a natural preservative for foods and beverages due to the presence of antimicrobial compounds (Nychas et al., 2003).
The Moroccan flora presents considerable biodiversity. It has many aromatic and medicinal plants rich in secondary metabolites with important therapeutic and pharmacological properties. We are interested in studying geranium plants from the perspective of valorizing our region's natural resources.
Scented geranium (Pelargonium graveolens) is a member of the medicinal geranium family (Mainardi et al., 2009). It is extensively used by local communities as a fresh or dried culinary herb. It is also known for its pharmacological properties in the treatment of fever, diarrhea, bronchitis, gastroenteritis, and other respiratory diseases (Tahan and Yaman, 2013; Tajkarimi et al., 2010). Also, their essential oils are used in the synthesis of perfumes and cosmetics and the food industry. And various pharmacological properties, such as antibacterial, immunostimulant, antioxidant, hypoglycemic, anti-inflammatory, and inflammatory, have been demonstrated (Boukhris et al., 2012; Boukhatem et al., 2013a; Zhuang et al., 2009; Moyo and Van Staden, 2014).
This study aims to determine, on the one hand, the chemical composition of the essential oil of Pelargonium graveolens leaves as well as these fractions obtained by preparative thin layer chromatography by GC-MS and, on the other hand, to evaluate their antimicrobial activities against four bacterial strains: Salmonella typhimurium, Escherichia coli, Staphylococcus aureus, and Listeria monocytogenes.
2 Material and methods
2.1 Plant material
The aerial part (stems, leaves, and flowers) of Pelargonium graveolens was collected in May 2020 in the region of Er-Rachidia (Morocco) (31° 55′ 38.05″ N 4° 25′ 42.593″ W).
The plant was identified by the botanist Professor Ibn Tattou Mohammed at the Scientific Institute of Rabat (Morocco). The aerial part of Pelargonium graveolens were dried under open-air conditions, shielded from light, and kept at room temperature. Subsequently, they were carefully stored in the laboratory until they were ready for use in the study.
2.2 Extraction of essential oil (EO)
The extraction is done by hydrodistillation in a Clevenger-type apparatus. A mixture of 100 g of plant material and 1000 mL of water was boiled for 3 h to obtain the EO. The yield of essential oils is expressed as the quantity of oil obtained per 100 g of dry plant material. The essence obtained is stored in glass bottles at a temperature (4 °C) and protected from light. The yields are expressed on the dry matter (in mL/100 g of raw material).
2.3 Preparative chromatography
This technique consists of using 20 × 20 cm plates of DC Kieselguhr 60 F254. The thickness of the layers is 25 mm and the solvents of migration used are hexane and ether (10 % Eth/Hex). The migration distance of the solvent is 15 cm.
The mixture to be separated is deposited as a narrow band by the juxtaposition of spots along the starting line. After separating the constituents of the mixture into parallel bands, each band is successfully detached with a spatula and eluted with methanol.
2.4 Chromatographic analysis
Essential oils were analyzed using a Perkin Elmer auto system XL chromatograph equipped with an autosampler and a non-polar column (Rtx-1) coupled to a Perkin Elmer Turbo Mass detector. The carrier gas was helium (1 mL/min) with a pressure of 25 psi applied to the top of the column. The injector temperature was 250 °C, and the detector temperature was 280 °C. The heating program consisted of a ramp up from 60 °C to 230 °C at a rate of 2 °C/min followed by a 45 min plateau at 230 °C. The injection was performed in fractionated mode with a fractionation ratio of 1/50. The volume of the sample injected was 0.2 µL. Detection was performed with a four-pole filter analyzer. Molecules are typically bombarded with a 70-eV electron beam. The unit is connected to the computer system that manages the NIST mass spectra library.
2.5 Antibacterial activity
2.5.1 Bacterial strains and growth conditions
The bacterial strains used in this study (Listeria monocytogenes, Salmonella typhimurium, Staphylococcus aureus, and Escherichia coli) were obtained from the Laboratory of Microbiology and Health, Faculty of Sciences, Moulay Ismail University, Morocco. Bacterial strains from frozen stocks (−80 °C) were spread on Mueller Hinton agar and incubated at 37 °C for 24 h. Then prepare, a bacterial suspension in sterile distilled water and adjust to 0.5 McFarland equivalent (108 CFU/mL).
2.5.2 Disc-diffusion assay
The disk diffusion method tested the antibacterial activity of the essential oils and their fractions. Petri dishes containing Mueller Hinton agar were spread with 100 µL of the bacterial suspension. Next, spread 10 µL of essential oil and amoxicillin on a 6 mm diameter sterile paper disk. Then incubate the dish at 37 °C for 24 h and measure the diameter of the inhibition zone (including the disk) in millimeters.
2.5.3 Broth microdilution method
The minimum inhibitory concentration and minimum bactericidal concentration of essential oils against four strains were determined using the broth microdilution method described by Bouymajane et al (Bouymajane et al., 2022). Briefly, a 50 µL volume of Mueller Hinton broth supplemented with DMSO was added to sterile, flat-bottomed 96-well microplates. Next, a 50 µL volume of essential oil and its fractions (prepared in 25 % (v/v) DMSO) was added to the first microplate and mixed to determine the serial dilution. Next, add 50 µL of bacterial suspension and 50 µL of MHB-DMSO to each well. Wells containing bacterial suspensions of MHB-DMSO and wells containing essential oils and MHB-DMSO were used as positive and negative controls, respectively.
All microplates were incubated at 37 °C for 24 h. Next, a 50 µL volume of 2, 3, 5-triphenyl tetrazolium chloride (TTC) was added to each microplate well and reincubated at 37 °C for 30 min. MIC was determined as the lowest concentration of essential oil that showed no visible bacterial growth. BMC was determined as the lowest concentration of essential oil or fractions that produced no bacterial colonies. Microplate wells showing no visible bacterial growth were spread on Petri dishes containing MHA and incubated at 37 °C for 24 h. All experiments were performed in triplicate.
3 Results and discussion
3.1 Chromatographic analysis
Before performing the bactericidal activity, a gas chromatographic analysis coupled with mass spectrometry of the essential oil was performed (Fig. 1). The chemical composition of the essential oil and its fractions were determined and represented in Table 1.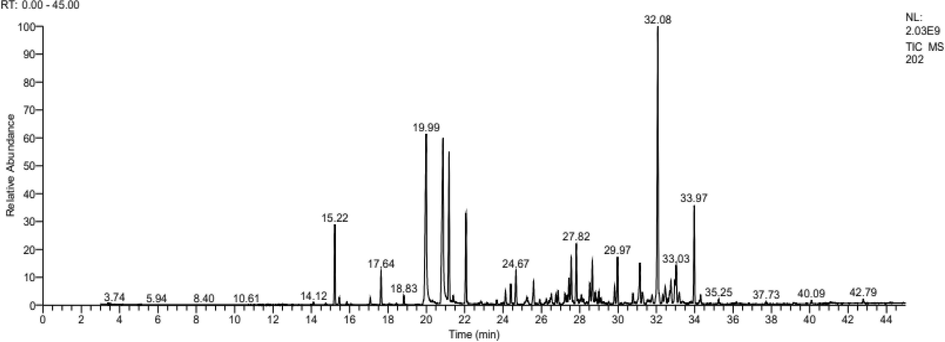
Chromatogram of the analysis of essential oil of Pelargonium graveolens collected in Er- Rachidia.
Chemical Compounds
Formulas
EO of P. graveolens
F1
F2
F3
F4
F5
F6
Linalool
C10H18O
4.06
0.67
–
–
–
–
–
β-Citronellol
C10H20O
12.34
35.83
3.19
–
–
–
–
Geraniol
C10H18O
12.54
38.78
0.40
–
–
–
–
Citronellyl formate
C11H20O2
7.70
–
–
–
–
7.61
–
Geranyl formate
C11H18O2
0.00
–
–
–
–
6.83
–
Geranyl acetate
C12H20O2
1.90
−
−
−
−
−
−
Germacrene D
C15H24
2.53
–
–
–
–
–
4.81
Viridflorene
C15H24
3.39
–
–
–
–
–
7.66
Geranyl butanoate
C14H24O2
2.51
–
–
–
–
10.94
–
Phenylethyl tiglate
C13H16O2
2.70
–
–
12.01
58.19
–
–
epi-γ-Eudesmol
C15H26O
16.67
0.10
55.10
17.53
1.08
–
–
Geranyl tiglate
C15H24O2
5.21
–
–
–
–
30.75
–
β-caryophellene
C15H24
1.40
–
–
–
–
–
–
1-Isopropyl-4,7-dimethyl-1,2,3,5,6,8a-hexahydronaphthalene
C15H24
2.40
–
–
–
–
–
12.08
Geranyl geraniol
C20H34O
–
–
–
23.70
–
–
–
Citronellyl butanoate
C14H26O2
0.62
–
–
–
–
2.87
–
γ-cadinene
C15H24
1.18
–
–
–
–
–
3.09
α-agorofuran
C15H24O
0.98
–
8.41
–
8.49
–
–
Neryl hexanoate
C16H28O2
0.50
–
–
–
–
1.13
–
Total (%)
78.63
75.38
67.01
53.24
67.76
60.13
27.64
Oxygenated monoterpenes
28.94
75.28
3.59
–
–
–
0.00
Hydrocarbon sesquiterpenes
10.09
0.00
0.0
0.0
0.0
0.0
27.64
Oxygenated sesquiterpenes
24.04
0.10
63.51
17.53
9.57
30.75
0.0
Other
21.14
0.00
0.00
12.01
58.19
60.13
0.00
GC/MS analyses of Pelargonium graveolens essential oil and its collected fractions showed the presence of five major constituents (Fig. 1 and Table1): epi-γ-Eudesmol (16.67 %), Geraniol (12.54 %), β-Citronellol (12, 34 %), Citronellyl formate (7.70 %) and Geranyl tiglate (5.21 %). This result is similar to that reported by Boukhris et al (Boukhris et al., 2013), Moutaouafiq et al (Moutaouafiq et al., 2019), Rana et al (Rana et al., 2002), Boukhatem et al (Boukhatem et al., 2013a), and Wei et al (Wei et al., 2022).
In effect, the fractions F1, F2, F3, F4, F5 and F6 represent respectively 28.8 % (0.576 g), 18.7 % (0.374 g), 1.7 % (0.034 g), 3 % (0.06 g),12.5 % (0.25 g) and 12 % (0.24 g) of the total essential oil. The F1 fraction consists mainly of β-Citronellol (35.83 %) and Geraniol (38.78 %), the F2 fraction is dominated by the presence of oxygenated sesquiterpenes (Epi-γ-Eudesmol (55.10 %) and α-agorofuran (8.41 %)), fraction F3 revealed the presence of Geranyl geraniol (23.70 %), epi-γ-Eudesmol (17.53 %) and Phenylethyl tiglate (12.01 %), fraction F4 consists mainly of Phenylethyl tiglate (58.19 %) and α-agorofuran (8. 49 %), fraction F5 represented majority compounds such as Geranyl tiglate (30.75 %) and Geranyl butanoate (10.94 %), Citronellyl formate (7.61 %) and Geranyl formate (6. 83 %), so fraction F6 is characterized by the presence of sesquiterpene hydrocarbons predominantly (27.64 %), such as 1-Isopropyl-4,7-dimethyl-1,2,3,5,6,8a-hexahydronaphthalene (12.08 %) and Viridfloreen (7.66 %), Germacren D (4.81 %) and γ-cadinene (3.09 %) (Table 1).
3.2 Antibacterial activity
The essential oil of Pelargonium graveolens and its fractions were tested against four bacterial layers (Escherichia coli, Salmonella typhimirium, Staphylococcus aureus, and Listeria monocytogenes). The results obtained are presented in Table 4.
The results of the essential oil disk diffusion method (Fig. 2 and Table 2) indicated that Salmonella typhimirium is more sensitive due to the appearance of a higher zone of inhibition (15 ± 0. 22 mm). This value is higher than that obtained for the antibiotic amoxicillin (14.5 ± 0.15 mm), followed by Listeria monocytogenes (13 ± 0.13 mm), Staphylococcus aureus (12 ± 0.12 mm) and Escherichia coli (9 ± 0.11 mm). AMX: Amoxicillin (antibiotic).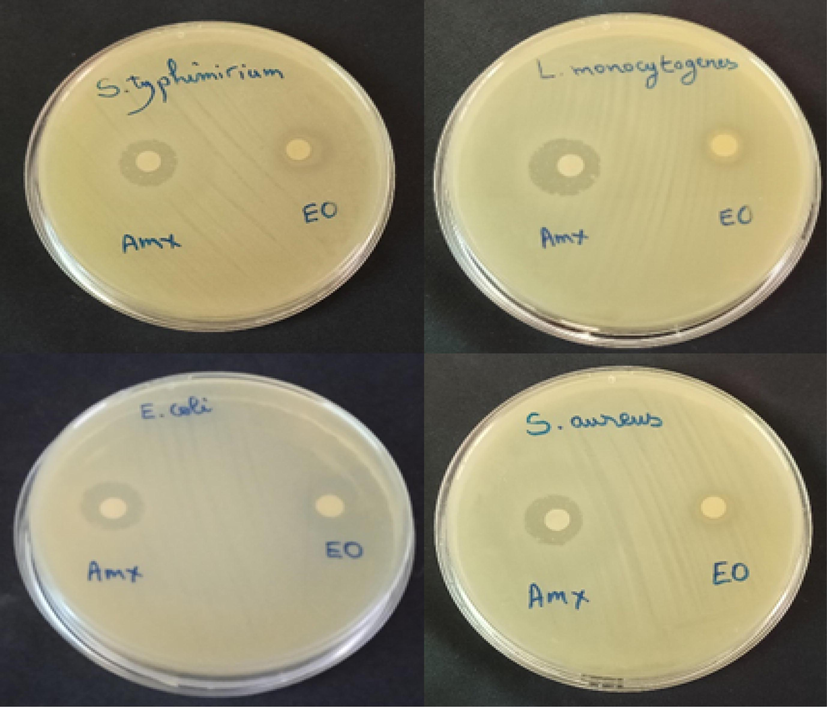
Method of diffusion on disc of essential oil of Pelargonium graveolens.
Bacteria
Diameter of the inhibition zone (mm)
EO
AMX
Escherichia coli
9.00 ± 0.11
16.00 ± 0.21
Salmonella typhimirium
15.00 ± 0.22
14.50 ± 0.15
Staphyloccocus aureus
12.00 ± 0.12
14.00 ± 0.18
Listeria monocytogenes
13.00 ± 0.13
18.00 ± 0.11
The results of the disk diffusion method of the essential oil fractions of Pelargonium graveolens (Fig. 3 and Table 3) showed that the F1 fraction has a very high area (19 mm) against the Salmonella typhimurium strain. It is due to the presence of β-Citronellol (35.83 %) and (38.78 %) in the F1 fraction. Previous studies have been reported on the antibacterial activity of essential oil rich in citronellol, which showed very potent antimicrobial activity (Prashar et al., 2003; Si et al., 2006; S. Hassane et al., 2012). Kim et al. proved that carvacrol, citral, and geraniol showed potent antibacterial activity against Salmonella typhimurium (Kim et al., 1995). Fraction F2 shows a diameter of the inhibition zone of 12 mm against Listeria monocytogenes, and fraction F3 shows high and similar diameters for Salmonella Typhimurium and Staphylococcus aureus strains (14 mm). This antibacterial activity may be due to the majority of compounds Geranyl geraniol (23.70 %), epi-γ-Eudesmol (17.53 %), and Phenylethyl tiglate (12.01 %), which is in agreement with the literature (Ngom et al., 2014). For fractions F4, F5 and F6, they revealed a low activity.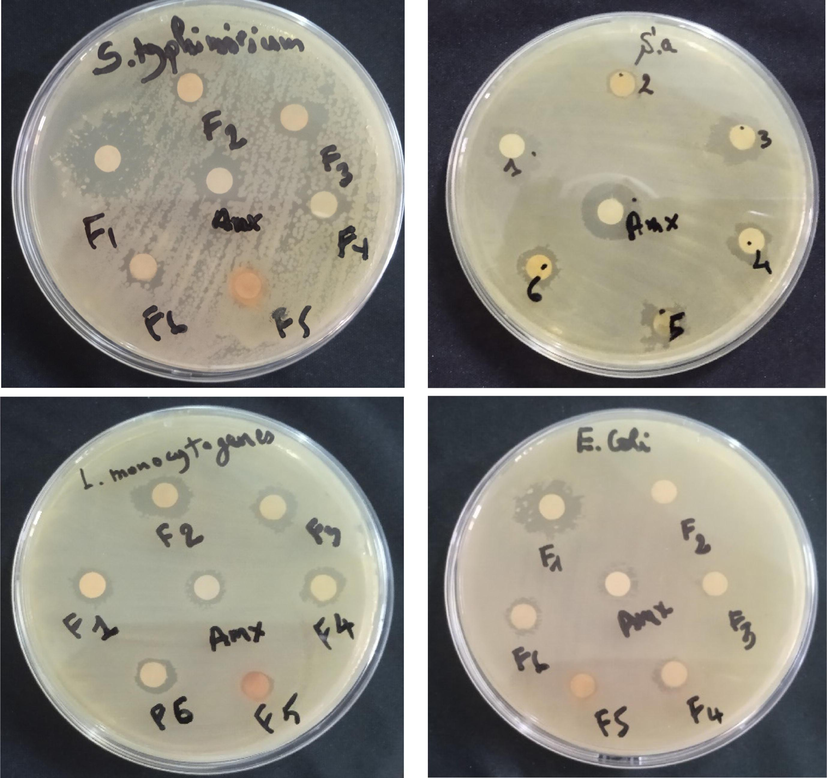
Disc diffusion method of Pelargonium graveolens essential oil fractions.
Diameter of the inhibition zone (mm)
Fractions
Bacteria
F1
F2
F3
F4
F5
F6
AMX
Escherichia coli
14
9
6
10
5
6
10
Salmonellatyphimirium
19
9
14
9
9
9
11
Staphyloccocus aureus
13
8
14
11
9
10
15
Listeria monocytogenes
9
12
10
11
7
10
9
Bacterial strains
Escherichia coli
Salmonella typhimirium
Staphylococcus aureus
Listeria monocytogenes
(%)
MIC
MBC
MBC/MIC
MIC
MBC
MBC/MIC
MIC
MBC
MBC/MIC
MIC
MBC
MBC/MIC
EO
1.04
2.08
2
1.04
2.08
2
2.08
2.08
1
1.04
2.08
2
F1
2.08
4.16
2
1.04
4.16
4
1.04
4.16
4
1.04
4.16
4
F2
1.04
4.16
4
2.08
4.16
2
2.08
4.16
2
4.16
8.33
2
F3
2.08
8.33
4
2.08
4.16
2
2.08
8.33
4
2.08
–
–
F4
4.16
8.33
2
4.16
8.33
2
8.33
8.33
1
8.33
8.33
1
F5
4.16
8.33
2
8.33
8.33
1
4.16
–
–
4.16
–
–
F6
4.16
–
–
4.16
8.33
2
4.16
–
–
4.16
–
–
The results of the Minimum Inhibition Concentration (MIC) (Fig. 4) indicated that the essential oil of Pelargonium graveolens presented a bactericidal effect against all bacteria, Escherichia coli, Staphyloccocus aureus, Salmonella Typhimurium and Listeria monocytogenes, with the MIC value ranging between 1.04 % and 2.08 %. These results are similar to those obtained by Atailia et al (Atailia and Djahoudi, 2015). Who found an interesting antibacterial activity with a MIC value of 1 % and 1.5 % for Gram-positive and Gram-negative bacteria, respectively (Table 4).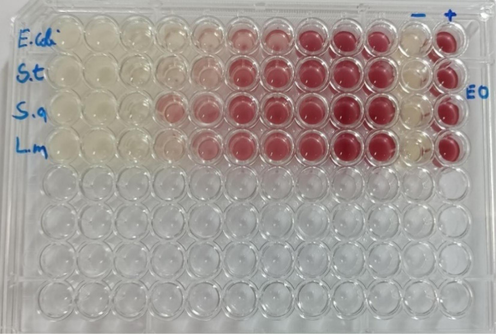
Microdilution method of Pelargonium graveolens essential oil (E.Coli: Escherichia coli;S.T: Salmonella typhimirium;S.a: Staphyloccocus aureus;L.m: Listeria monocytogenes).
The potent antibacterial activity of Pelargonium graveolens essential oil is mainly due to its chemical composition, which is rich in alcohols and terpene phenols (β-Citronellol, Geraniol, Linalool, epi-γ-Eudesmol). Many authors have studied the antimicrobial activity of the main compounds of essential oils, classifying them in the following order: phenols, alcohols, aldehydes, ketones, ethers, and hydrocarbons (Cox et al., 2001; Bourkhiss et al., 2010; Farag et al., 1989).
The results of the fractions of the essential oil of Pelargonium graveolens (Fig. 5) showed that the values of MIC vary between 1.04 % and 8.33 %. Fraction 1 showed a bactericidal effect for the Escherichia coli bacteria and a bacteriostatic effect for the other bacteria tested with a ratio of CMB/CMI equal to 4. Fraction 2 revealed a bactericidal effect for Salmonella typhimurium, Staphylococcus aureus, and Listeria monocytogenes bacteria with a ratio of 2 and a bacteriostatic effect for Escherichia coli. Fraction 3 showed a bactericidal effect for Salmonella typhimurium and a bacteriostatic effect for Escherichia coli and Staphylococcus aureus. Fraction 4 showed a bactericidal effect for all tested bacteria with BMC/MIC ratios between 1 and 2. While fractions 5 and 6 showed a bactericidal effect for Salmonella typhimurium (Table 4).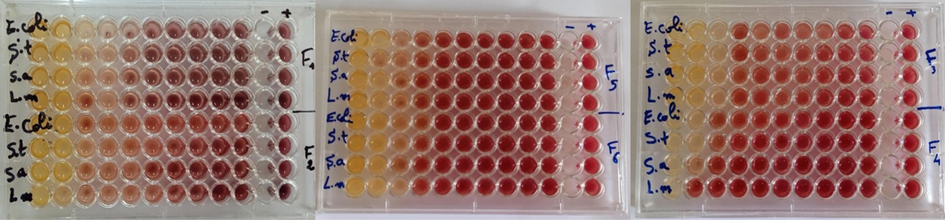
Microdilution method of six fractions of essential oil of Pelargonium graveolens.
4 Conclusion
GC-MS analysis of the essential oil of Pelargonium graveolens and its fractions showed that essential oil is rich in epi-γ-Eudesmol, Geraniol, β-Citronellol, Citronellyl formate, and Geranyl tiglate, fraction 1 consists mainly of β-Citronellol and Geraniol, fraction two is dominated by epi-γ-Eudesmol and α-agorofuran, as well as fraction three revealed the presence of Geranylgeraniol and epi-γ-Eudesmol and Phenylethyl tiglate, fraction 4 consists mainly of Phenylethyl tiglate and α-agorofuran, fraction five is represented by such majorities as Geranyl tiglate and Geranyl butanoate, while fraction six is characterized principally by 1-Isopropyl-4,7-dimethyl-1,2,3,5,6,8a-hexahydronaphthalene, in addition. The essential oil of Pelargonium graveolens and its fractions showed a bactericidal effect on all tested bacteria, in which fractions 5 and 6 possessed bactericidal effects on Escherichia coli, Salmonella typhimirium, and no effect on Staphyloccocus aureus and Listeria monocytogenes.
Indeed, these preliminary results highlighted that Pelargonium graveolens essential oil can be considered a suitable alternative for use in the food industry as a natural antimicrobial agent.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Composition chimique et activité antibactérienne de l’huile essentielle de géranium rosat (Pelargonium graveolens L’Hér.) cultivé en Algérie. Phytothérapie. 2015;13:156-162.
- [CrossRef] [Google Scholar]
- Rose geranium essential oil as a source of new and safe anti-inflammatory drugs. Libyan J. Med.. 2013;8:22520.
- [CrossRef] [Google Scholar]
- Essential oil of Algerian rose-scented geranium (Pelargonium graveolens): Chemical composition and antimicrobial activity against food spoilage pathogens. Food Control. 2013;34:208-213.
- [CrossRef] [Google Scholar]
- Hypoglycemic and antioxidant effects of leaf essential oil of Pelargonium graveolens L’Hér. in alloxan induced diabetic rats. Lipids Health Dis.. 2012;11:81.
- [CrossRef] [Google Scholar]
- Chemical composition and biological activities of polar extracts and essential oil of rose-scented geranium, Pelargonium Graveolens. Phytother. Res. PTR. 2013;27:1206-1213.
- [CrossRef] [Google Scholar]
- Production et caractérisation de l’huile essentielle de la sciure de bois de tetraclinis articulata (vahl) masters. Bull. Société R. Sci. Liège 2010
- [Google Scholar]
- Phenolic Compounds, Antioxidant and Antibacterial Activities of Extracts from Aerial Parts of Thymus zygis subsp. gracilis, Mentha suaveolens and Sideritis incana from Morocco. Chem. Biodivers.. 2022;19:e202101018.
- [Google Scholar]
- Interactions between components of the essential oil of Melaleuca alternifolia. J. Appl. Microbiol.. 2001;91:492-497.
- [CrossRef] [Google Scholar]
- Antimicrobial activity of some Egyptian spice essential oils. J. Food Prot.. 1989;52:665-667.
- [CrossRef] [Google Scholar]
- S. Hassane, S.O., Ghanmi, M., Satrani, B., Farah, A., Mansouri, N., Chaouch, A., 2012. Activité antifongique contre la pourriture du bois de l’huile essentielle de Pelargonium x asperum Erthrt. Ex Willd des îles Comores. Bull. Société R. Sci. Liège.
- The composition of the essential oil and aqueous distillate of Origanum vulgare L. growing in Saudi Arabia and evaluation of their antibacterial activity. Arab. J. Chem.. 2018;11:1189-1200.
- [CrossRef] [Google Scholar]
- Antibacterial activity of carvacrol, citral, and geraniol against salmonella typhimurium in culture medium and on fish cubes. J. Food Sci.. 1995;60:1364-1368.
- [CrossRef] [Google Scholar]
- Complementary and alternative medicine: herbs, phytochemicals and vitamins and their immunologic effects. J. Allergy Clin. Immunol.. 2009;123:283-294. quiz 295–296
- [CrossRef] [Google Scholar]
- Antifungal activity of pelargonium graveolens essential oil and its fractions against wood decay fungi. J. Essent. Oil Bear. Plants. 2019;22:1104-1114.
- [CrossRef] [Google Scholar]
- Medicinal properties and conservation of Pelargonium sidoides DC. J. Ethnopharmacol.. 2014;152:243-255.
- [CrossRef] [Google Scholar]
- Ngom, S., Diop, M., Mbengue, M., Faye, F., Kornprobst, J.M., 2014. Composition chimique et propriétés antibactériennes des huiles essentielles d’Ocimum basilicum et d’Hyptis suaveolens (L.) Poit récoltés dans la région de Dakar au Sénégal 9.
- Nychas, G.-J., Skandamis, P., Tassou, C., 2003. Antimicrobials from herbs and spices. In: Natural Antimicrobials For the Minimal Processing of Foods. pp. 176–200. https://doi.org/10.1533/9781855737037.176.
- Assessment of the Chemical/biological activities of extracts and essential oil of Rosmarinus officinalis L. from the Oriental region of Morocco. Moroc. J. Chem.. 2020;8:8-744.
- [CrossRef] [Google Scholar]
- Antimicrobial action of palmarosa oil (Cymbopogon martinii) on Saccharomyces cerevisiae. Phytochemistry. 2003;63:569-575.
- [CrossRef] [Google Scholar]
- Chemical constituents of essential oil of Pelargonium graveolens leaves. Int. J. Aromather.. 2002;12:216-218.
- [CrossRef] [Google Scholar]
- Antimicrobial activity of essential oils and structurally related synthetic food additives towards selected pathogenic and beneficial gut bacteria. J. Appl. Microbiol.. 2006;100:296-305.
- [CrossRef] [Google Scholar]
- Can the Pelargonium sidoides root extract EPs® 7630 prevent asthma attacks during viral infections of the upper respiratory tract in children? Phytomedicine. 2013;20:148-150.
- [CrossRef] [Google Scholar]
- Antimicrobial herb and spice compounds in food. Food Control. 2010;21:1199-1218.
- [CrossRef] [Google Scholar]
- Comparison of chemical composition and activities of essential oils from fresh leaves of Pelargonium graveolens L′Herit. extracted by hydrodistillation and enzymatic pretreatment combined with a solvent-free microwave extraction method. Ind. Crop. Prod.. 2022;186:115204
- [CrossRef] [Google Scholar]
- Effect of citronellol and the Chinese medical herb complex on cellular immunity of cancer patients receiving chemotherapy/radiotherapy. Phytother. Res.. 2009;23:785-790.
- [CrossRef] [Google Scholar]







