Translate this page into:
Targeting T2Rs, a feasible approach for natural bitter agents from traditional Chinese medicine modulate ABC transporters to treat respiratory diseases
⁎Corresponding authors at: State Key Laboratory of Southwestern Chinese Medicine Resources, School of Pharmacy, Chengdu University of Traditional Chinese Medicine, Chengdu 611137, China. wuchunjie@cdutcm.edu.cn (Chun-Jie Wu), pengwei@cdutcm.edu.cn (Wei Peng)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background
It’s known that respiratory diseases are the top of the list of systemic diseases, and accumulating evidence suggests that one of the important reasons for the high incidence of respiratory diseases is the difficulty in delivering drugs effectively to the respiratory system.
Purpose
In this review, we summarized the potential roles of targeting T2Rs in combination with bitter compounds for the treatment of respiratory diseases, and also discussed the potential of ABC transmembrane transporter proteins to deliver bitter compounds to cells to combat drug resistance, providing a reference for future studies on bitter receptor therapy related to respiratory diseases.
Results
The airway epithelium cells serve as a lung barrier against the invasion of various harmful substances in the respiratory system, and many receptors have been found to exist in the airway epithelium cells. Interestingly, it’s reported that lots of bitter compounds (quercetin, resveratrol, etc.) can reduce oxidative stress and other responses in respiratory diseases via bitter taste receptors (T2Rs).
Conclusion
Collectively T2Rs, seem as feasible drug targets and alternative treatment option for for natural bitter agents from traditional Chinese medicine to respiratory diseases in the future.
Keywords
Bitter taste receptor
Respiratory diseases
Bitter compounds
ABC transporters
- COVID-19
-
Corona Virus Disease 2019
- COPD
-
chronic obstructive pulmonary disease
- CRS
-
chronic rhinosinusitis
- CF
-
cystic fibrosis
- T2Rs
-
bitter taste receptors
- ABC transporters
-
ATP-binding cassette transporters
- GPCR
-
G-protein coupled receptor
- c NMP
-
Cyclic nucleotide
- c AMP
-
Cyclic adenosine monophosphate
- IP3
-
Inositol (1,4,5) triphosphate
- DAG
-
Diacylglycerol
- T1Rs
-
taste 1 receptors
- GLP-1
-
glucagon-like peptide 1
- PD
-
Parkinson's disease
- PTC
-
phenylthiocarbamide
- IL-4/5/13/33
-
Interleukin 4/5/13/33
- 6-PTU
-
6-n-propyl 2-thiouracil
- GPT
-
generative pre-trained transformer
- P-g p
-
P-glycoprotein
- MRP1
-
Multi-drug resistance associated protein
- RCT
-
reverse cholesterol transport
- LXRs
-
liver X receptors
- BLM
-
bleomycin
- EMT
-
epithelial mesenchymal transformation
- TNF-α
-
Tumor necrosis factor-α
- HASM
-
human airway smooth muscle
- SNPs
-
single nucleotide polymorphisms
- Ach E
-
Acetylcholinesterase
- VEC
-
Vascular endothelial cells
- CHOP
-
CCAAT/enhancer binding proteins homologous protein
- AHR
-
Airway hyperresponsiveness
- BALF
-
Bronchoalveolar lavage fluid
Abbreviations
1 Introduction
Respiratory diseases are reported to be one of the leading causes of death worldwide (Greene et al., 2011; Troeger et al., 2018). In particular, with the sudden outbreak of the Corona Virus Disease 2019 (COVID-19), our focus has intensified towards optimizing therapeutic approaches for respiratory ailments. The main lesions of respiratory diseases are in the trachea, bronchi, lungs and chest, and the common respiratory diseases include asthma, chronic obstructive pulmonary disease (COPD), chronic rhinosinusitis (CRS) and cystic fibrosis (CF) (McMahon et al., 2022). Clinical symptoms of respiratory diseases mostly manifest as coughing, wheezing, chest tightness, chest pain, even respiratory distress and hypoxia in severe cases, eventually leading to death from respiratory failure (Khattak et al., 2021). Most of the above symptoms are associated with smoking, indoor air pollution from solid fuels, ambient particles, and lysozyme or other bacteriocins (Soriano et al., 2020). Currently, most of available therapeutical methods for respiratory diseases are bronchodilators, inhaled glucocorticoids and antibacterial drugs (Celli and Wedzicha, 2019). In addition, monoclonal antibody therapies (biologics) are introduced to target specific cytokines and their functions (Nayak et al., 2019). Although these methods can alleviate acute exacerbations of respiratory diseases, the toxic side effects such as drug resistance, high cost limit the extensive and long-term use of the current available drugs. Therefore, finding more feasible drugs with fewer side effects and better efficacy is still a challenge for respiratory diseases.
It's reported that bitter taste receptors (T2Rs) play important roles in the development of oral diseases. Besides, T2Rs are also expressed in extraoral tissues and exhibit in various thereby garnering escalating attention from researchers as novel therapeutic targets against diverse diseases. Previous studies have shown that T2R10 can promote the chemosensitivity of pancreatic cancer cells (Stern et al., 2018), thereby increasing the drug resistance in these cells. Addiyionally, some bitter compounds such as flavonoids have shown therapeutic effects on respiratory diseases (Hui et al., 2013; Mocanu et al., 2015b). These lipophilic (Duarte et al., 2022b) and trans-biofilm agents possess the ability to stimulate T2Rs, facilitating their entry into the cell membrane, for example, the antimalarial drug artesunate stimulates T2R14 to improve bronchodilation. There are also many bitter compounds that can improve bioavailability by modulating ATP-binding cassette transporters (ABC transporters). For example, quercetin could downregulate the expression of ABCA3, thereby reducing oxidative stress and improving its own bioavailability. ABC transporters are specific proteins located on cell membranes, and abnormal expression of the protein family is an important contributor to the development of drug resistance in many cells. These proteins utilize the energy generated by ATP hydrolysis to transport many chemotherapeutic drugs into and out of respiratory cells. Therefore, this review aims to summarize recent advancements in T2Rs, explore how bitter compounds targeting T2Rs can effectively treat respiratory diseases, elucidate the regulatory effects of bitter compounds on ABC transmembrane and shed light on the collective involvement of all three components in respiratory diseases. The ultimate goal is to provide valuable insights for future studies investigating the diverse roles played by T2Rs.
2 T2Rs and bitter compounds in traditional Chinese medicines
The T2Rs are a subset of G-protein coupled receptors (GPCRs), which play a crucial role in cancer by regulating tumorigenesis, proliferation, migration, and invasion of cancer cells. GPCRs and their ligands serve as significant therapeutic targets for cancer treatment. After binding to T2Rs, bitter compounds can be transduced through the following three pathways: (1) Bitter receptors are stimulated to activate the α-taste conductor-PDE-cyclic nucleotide (c NMP) pathway, resulting in a decrease in cytoplasmic cyclic adenosine monophosphate (c AMP) and an increase in calcium ion (Ca2+) levels, finally calcium ions depolarize the membrane. (2) Activation of β, γ-isosin- PLC-1,4,5-triphosphate inositol (IP3), Phosphatidylcholine β activation produces IP3 and diacylglycerol (DAG), resulting in the release of intracellular calcium ions (Ca2+) (Bufe et al., 2002; Chandrashekar et al., 2006). (3) Pathways independent of GPCR/G protein mechanism (Fig. 1).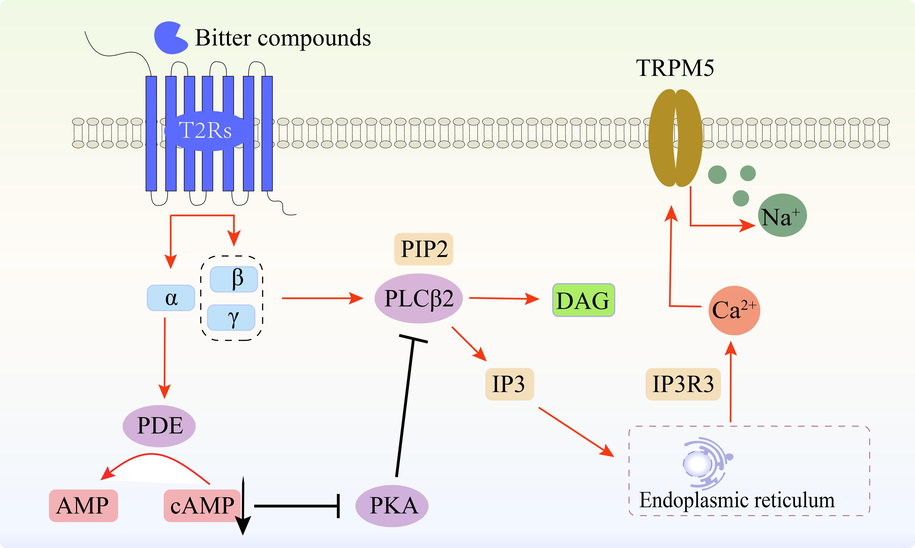
Signal transduction pathway of bitter compounds.
2.1 Taste receptors
Taste is an important perceptual response for people, which is known as the body’s “nutritional gatekeeper”, maintaining the balance of nutrients in the body (Liu et al., 2017b). Taste is detected by taste cells in the upper epidermis (Yang, 2004) of the tongue, which transmit taste signals to the brain. There are five basic taste responses: sweet, sour, bitter, salty and umami (L-glutamic acid taste). Sweetness indicates the presence of carbohydrates; sourness indicates the presence of food spoilage and unripe fruit; bitterness can prevent mammalian poisoning by avoiding the intake.
of some toxic substances; saltiness controls the intake of sodium ions and maintains the body's water balance; Umami is thought to be associated with protein-rich foods. Taste cells were divided into four types: I, Ⅱ, Ⅲ and Ⅳ, bitter, sweet and umami tastes were sensed by type Ⅱ cells (Lajtha et al., 2007).
Taste receptors are involved in a variety of important physiological processes in living organisms and are also important drug targets, and can be divided into the first family of taste 1 receptors (T1Rs) and the second family of T2Rs, and the first family of taste can be divided into three conformations, T1R1, T1R2 and T1R3. Umami receptor is a heterodimer composed of T1R1 and T1R3, which is mainly co-expressed in type Ⅱ cells of taste buds in the anterior part of the tongue. Sweet taste receptors are composed of T1R2 and share T1R3 with umami taste receptors (Nelson et al., 2002; Nelson et al., 2001). Bitter taste receptor cells were mainly distributed in the vallate papillae, foliate papillae and fungiform papillae (Rozengurt and Sternini, 2007), and each cell contained a variety of T2Rs (Gabriel, 2015); The Ca2+ channel was activated by activating TRPV1. The nerve fibers that transmit salty taste signals are mainly distributed in the dorsal and ventral parts of the tongue (Caterina et al., 1997). Sour taste is sensed primarily by taste receptor cells on the tongue and palate epithelium (Liman et al., 2014).
Outside the oral cavity, only umami, sweet, and bitter taste belong to GPCRs, and Wauson et al. (2012) conducted a comprehensive investigation on the distribution of T1R1/T1R3 receptors and demonstrated their presence in various tissues, organs, and cells. There is already evidence suggesting that sweet taste receptors may play a role in immune regulation and response. Moreover, these receptors are expressed in gastrointestinal enteroendocrine L-cells and K-cells, which exert physiological effects through the secretion of glucagon-like peptide) and glucose-dependent insulinotropic peptide (Kojima and Nakagawa, 2011). Additionally, Deshpande and Liggett were the first to report the expression of T2Rs in airway smooth muscle cells as well as their involvement in T2Rs-mediated bronchodilation induced by bitter taste agonists. The potential use of T2Rs as screening targets for asthma treatment has garnered significant attention (Deshpande et al., 2010).
2.2 Structure and function of T2Rs
Bitter taste receptor, a member of GPCRs (Behrens and Meyerhof, 2011), is encoded by a gene family consisting of 30 genes. Its structure consists of a protein with an extracelluar N-terminus, 7 transmembrane α-helices, and intracellular C-terminus. It contains three corresponding intracellular loops and three extracellular loops (Chandrashekar et al., 2000), which can convert extracellular stimuli into intracellular stimuli. The three loops are highly conserved within cells and function as the region responsible for G-protein coupling, while the extracellular N-terminus is short and polymorphic. The diversity observed in bitter taste receptors enables them to bind to various bitter substances. T2Rs encompass 25-1, -3, -4, -5, -7, -8, -9, -10, -13, -14, -16, -38, -39, -40, -41, -42, -43, -44, -45, -46, -47, -48, 49, 50, 60), accounting for approximately 4% of human GPCRs (Pydi et al., 2012) (Fig. 2).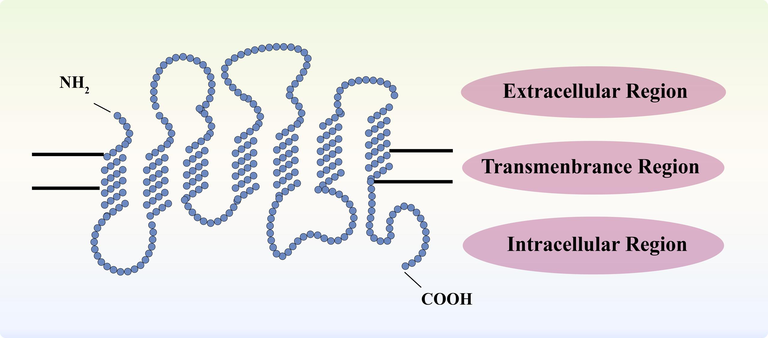
Structural diagram of T2Rs.
The expression and distribution of T2Rs have developed rapidly from early gastrointestinal endocrine cells to respiratory epithelial cells (Finger et al., 2003), pancreas, thymus, heart (Foster et al., 2013), brain, testis and other tissues. The study showed by Singh et al. (2011). used reverse transcriptase polymerase chain reaction to reveal the presence of T2R4, T2R107 and T2R38 transcripts in the brain stem of mice, the results showed that endogenous of functional T2R4 occurs in these cells. Wu et al. (2005). confirmed by reverse transcriptase polymerase chain reaction that gene expression for T2Rs was detected in the gastrointestinal mucosa of mice. Similarly, immunostaining and galactoside staining demonstrated T2R5 expression in the testis (Fehr et al., 2007).
In addition, various bitter compounds can activate a bitter taste receptor (Meyerhof et al., 2010), and different bitter taste receptors can also be activated by a single bitter compound (Reichling et al., 2008), which may be different from the concentration threshold of receptor activation induced by bitter compounds. As mentioned above, T2R14 can be activated by compounds such as the active ingredient of absinthine, woodruff bitter toxin, sodium benzoate, and 1,8-naphthalaldehydic acid (Behrens et al., 2004), in contrast, aristolochic acid can activate T2R44 and T2R46(Pronin et al., 2004). Many bitter compounds such as alkaloids, flavonoids and polyphenols have significant effects in the treatment of respiratory diseases. After the interaction between bitter compounds and T2Rs, bitter signal transduction makes the brain feel bitter taste. Therefore, the combination of bitter compounds with T2Rs has become a new avenue to treat diseases.
2.3 Bitter compounds in traditional Chinese medicines
Bitter compounds are mainly derived from plants (such as phenyl thiourea, blue sheen, aloin, etc.), animals, or result from aging and deterioration during food processing food produced by microorganisms (e.g., acyl homoserine lactone produced by gram-negative bacteria Pseudomonas aeruginosa, erythromycin sugar derived from actinomycetes, postpartum fungus, etc.) (Drewnowski and Gomez-Carneros, 2000; Kingsbury, 1964; Murata and Sata, 2000).). Although there is no clear correlation between bitterness and toxicity (Glendinning, 1994), its unpleasant taste can prevent the ingestion of some toxic substances.
There are many naturally occurring bitter compounds in traditional Chinese medicines. Denatonium, commonly known as “bitter essence”, is currently recognized as the most bitter compound in the world and is mainly found in lotus (Nelumbo nucifera Gaertn)(Civantos et al., 2021); Citrulline belongs to terpenoids, which are generally enriched in citrus fruits (Citrus reticulata Blanco), especially in the highest concentration within the seeds (Ji et al., 2014). Limonin has been reported to activate T2Rs and play an anti-inflammatory role in the airways; Quercetin, naringin and kaempferol are flavonoids with a bitter taste that mainly found in Alpinia officinarum Hance, panax ginseng, ginkgo biloba L. and other plants; Aristolochic acid is another bitter compound found in Asarum plants, its derivatives aristolochic acid I and II are toxic and can bind to T2R43 and T2R44, activating them. Furthermore, familiar traditional Chinese medicines such as Aloe vera, Artemisia annua and Andrographis paniculata are rich in aloin, chloroquine and andrographolide (Shimizu et al., 2021), which are ligands for T2Rs and have been validated in vivo experiments. Oregano has shown beneficial effects on improving symptoms of CRS as well as treating asthma (Erenler et al., 2016; Hoang et al., 2023).
There are various kinds of bitter compounds, which can be roughly divided into flavonoids, alkaloids, salts, terpenoids, hydroxyl fatty acids, amino acids, amides, N-heterocyclic compounds, thiourea, urea, esters, carbonyl compounds, phenols, crown ethers, steroids, halogenated or acetylated sugars, and metal ions (Dubois et al., 2008; Hans-Dieter and Herbert, 1985). Among them, flavonoids are natural plant secondary metabolites with a phenyl benzopyrans structure. They possess the characteristics of few side effects and low toxicity and are preferred in the drug molecules for cancer prevention (Hui et al., 2013; Mocanu et al., 2015a). Some naturally occurring bitter compounds such as bitter gourd extract have been shown to inhibit breast genes and promote apoptosis (Ray et al., 2010). Alkaline compounds consisting of pyridine, tetrahydropyrrole, quinoline, and isoquinoline. Nearly all of them possess a bitter taste, with the intensity of bitterness increasing in proportion to the strength of the alkaloid.
2.4 Application of T2Rs-bitter compounds in human diseases
Recently, the expression of T2Rs has been increasingly found in extra-oral tissues, including the brain (Santos et al., 2019), gastrointestinal tract (Lee et al., 2019), and urogenital system (Kumar and Cheng, 2021; Li, 2013; Xu et al., 2013) (Fig. 3).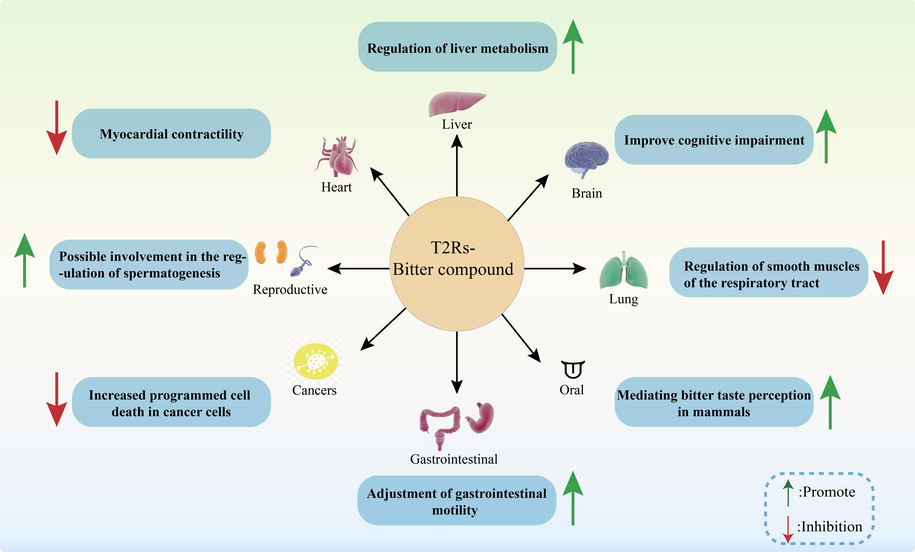
Application of T2Rs-bitter compounds in human diseases.
In brain, bitter compounds have shown to ameliorate cognitive impairment and improve behavioral performance in mouse (Wei et al., 2019) and disease (Corpas et al., 2019; Pierzynowska et al., 2019; Sabogal-Guáqueta et al., 2015; Yang et al., 2017). These compounds mediate reduced apoptosis by regulating expression and activity (Du et al., 2018; Ghofrani et al., 2015; Wang et al., 2017a; Zhang et al., 2018); Moreover, T2Rs may play a relevant role in Parkinson's disease (PD) by their bitter compounds such as naringenin (Garcia-Esparcia et al., 2013), Singh et al. (2011) used RT-PCR and immunohistochemistry to analyze the expression of bitter taste receptors in brain cells, finding that T2R4 binds to the bitter compounds like denatonium benzoate and quinine, leading to increased intracellular calcium levels. This suggests that T2Rs are expressed in multiple regions that the of T2R14 by resveratrol facilitates ABCG2 transport (Duarte et al., 2020), thereby modulating the neural activity of resveratrol. Various bitter receptors found in the gastrointestinal tract also play a crucial role in regulating gastrointestinal motility and gastric acid secretion (Depoortere, 2014), particularly within the large intestine and colon. Kaji et al. (2009) studied the expression of T2Rs in the human intestine through various molecular biotechnology techniques, discovering that T2Rs mediate colonic epithelial anion secretion induced by a bitter compound called6-PTU in rats while promoting rapid increases in Ca2+ concentration through other bitter compounds such as Cycloheximideand Denatonium (Wu et al., 2002). In addition, phenylthiocarbamide (PTC) activated T2R38 in colorectal adenocarcinoma model Caco-2 cells, increasing the expression and activity of ABCB1 in intestinal cells and mouse gut (Jeon et al., 2011).
Most T2Rs have been detected in the respiratory system (Shaik et al., 2016), indicating their respiratory diseases. In Hariri's study (Hariri et al., 2017b), T2Rs were found to be crucial regulators of sinus immune responses and potential therapeutic targets, with T2R14 influencing cytokine release in primary and cultured airway cells, thereby triggering the anti-inflammatory effects of flavonoids.
It is worth noting, however, that T2Rs may not necessarily exert their function solely within the oral cavity. Recently, Lu et al. (2021) utilized the CRISPR/Cas9 gene-editing technique to delete three bitter taste receptors - T2R143/T2R135/T2R126. The findings demonstrated that bronchiectasis does not require T2Rs, which aligns with Liu et al.'s study (Liu et al., 2017a) where T2R143/T2R135/T2R126 were found to be expressed in the airways of mice and other non-taste epithelial tissues instead of their canonical locations. Collectively, further to elucidate the role of T2Rs in the airway and other tissues. Subsequently, we will discuss the potential of combining T2Rs with bitter compounds for treating respiratory diseases.
3 Application of T2Rs and bitter compounds in respiratory diseases
The common characteristics of respiratory diseases encompass heightened airway obstruction, inflammation within the airways, and stress. Alleviating or modifying these features constitutes the primary approach in contemporary solutions.
3.1 Application of bitter compounds in respiratory diseases
3.1.1 Application of bitter compounds in traditional Chinese medicines
Several bitter compounds have been found to inhibit the stimulation of inflammation by primary and cultured airway cells (Hariri et al., 2017a; Hariri et al., 2017b), thus showing potential as therapeutic agents for the treatment of acute respiratory viral infections (Ren et al., 2020) (Table 1). Quinine, denatonium, naringin (Ni et al., 2021), quercetin and kaempferol have all been identified as having the ability to reduce interleukin 4 (IL-4) levels in vitro and in vivo models of asthma. Among these compounds, naringin and quercetin have demonstrated significant efficacy.(Guihua et al., 2016; Park et al., 2009; Ren et al., 2021; Shi et al., 2009). Amentoflavone and quercetin have been reported to exhibit efficacy in improving bronchial inflammation (Cai et al., 2019; Nanua et al., 2006; Rogerio et al., 2010). In addition, limonin, denatonium and 6-n-Propyl-2-thiouracil (6-PTU) have demonstrated the ability to enhance smooth muscle contraction by inducing cellular apoptosis (Doggrell, 2011; Sakai et al., 2016; Wen et al., 2015a). Quinine activates T2Rs by increasing capillary frequency and stimulating NO production, which enhances respiratory innate immune defense (Workman et al., 2018) The receptors for denatonium (T2R4, T2R10) and chloroquine (T2R3, T2R10) were expressed in generative pre-trained transformer (GPT) (Pulkkinen et al., 2012), and The downstream signaling pathway of GPCR can be regulated by a regulator of G protein signal 21, which directly influences bitter-mediated c AMP formation and intracellular calcium flow in airway epithelial cells (Cohen et al., 2012). Denatonium has been demonstrated to induce apoptosis in airway epithelial cells by damaging mitochondria (Wen et al., 2015b). Moreover, it should be noted that bitter taste receptors exhibit species-specificity, making it uncertain whether a natural compound acting as an agonist in a mouse TAS receptor would elicit the same response in humans. In the upper respiratory tract, hTAS2R46 and hTAS2R38 are present, and when stimulated with phenylthiouride, these receptors display low levels of calcium response. This leads to a high intracellular production of NO, resulting in bacterial infection and bronchiectasis, ultimately contributing to asthma development (Carey et al., 2017).
Compound
Disease
Structure
Biological activity
Cells/Animals
Ref
Naringin
Normal respiratory
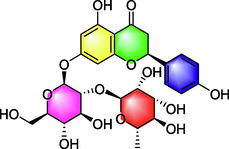
Improving memory;
Inhibiting TNF-α, AChE;Wistar rats
(Kaur and Prakash, 2020)
Inhibiting Amyloid deposits;
Increasing VEC proliferation;
Inhibiting endothelial cell apoptosis
Inhibiting GRP78, CHOP, caspase-12 and Cyt c proteins;Culture of Rat VEC in Vitro
(Shangguan et al., 2017)
Asthma
Inhibiting OVA-induced eosinophil counts;
Decreasing IL-4 and INF-γ;
Inhibiting Th2 cells and enhancing Th1 cells;
Inhibiting GABA3 and increasing T-bet.Ovalbumin was used to establish asthma model mice
(Guihua et al., 2016)
Inhibiting cough and AHR;
Inhibiting Leukocytes, IL-4, IL-5 and IL-13 in BALF.Guinea
(Ren et al., 2021)
Promoting the proliferation of the 16HBE14o-, BEAS-2B, and A549 cells,
Nontoxicity to the airway epithelial structure and function;
Promoting ACEs proliferation16HBE14o-, BEAS-2B, and A549 cells
(Ni et al., 2021)
Decreasing traction gradually
Decreaseing bronchial airway resistance in miceASMCs cultured in vitro or bronchial airways of Balb/c mice in vivo
(Wang et al., 2016)
Decreasing levels of IL4, IL13, CCL5, CCL11 and iNOS
inhibiteing pulmonary IkappaBalpha degradation and NF-kappaB DNA-binding activityMice were sensitized and challenged with ovalbumin
(Shi et al., 2009)
COPD
Decreasing neutrophil/lymphocyte/platelet counts and MDA content in blood;
Upregulating AQP1 in the lung tissuesLPS/CS-induced mice
(Zhang et al., 2022)
CF
Attenuating DPM-induced injury
Increasing DPM-induced CFTR, AQP1, and AQP5 mRNA and protein expressionDPM-induced mice and cell
(Shi et al., 2019)
Chronic bronchitis
Decreasing IL-8, LTB4 and TNF-α;
Increasing the content of lipoxin A4 (LXA4) in BALFGuinea pig model
(Luo et al., 2012)
Quercetin
Normal respiratory
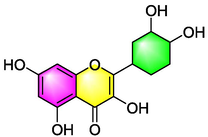
Inhibiting ET and NO synthesis;
Decreasing NOX2, TNF-α, IL-1 and IL-6;Lung epithelial A549 cells
(Sul and Ra, 2021)
Asthma
Decreasing eosinophil counts in BALF, blood, and lung parenchyma;
Decreasing neutrophils in blood and IL-5 in lung homogenates.BALB/c mice
(Gong et al., 2014)
Increasing levels of IL-4;
Increasing IFN-γ.OVA-induced asthma model mice
(Park et al., 2009)
CRS
Increasing transepithelial Cl- transport and CBF in MNSE and HSNE cultures;
Primary HSNE and MNSE cells
(Zhang et al., 2011)
CF
Activating CFTR-mediated anion transport in respiratory epithelial cells;
Inhibiting Cl- conductance at high concentration.Fisher rat thyroid and CFBE41o- cells
(Pyle et al., 2010)
Acute respiratory distress syndrome
Decreasingd MMP-9 activity;
Suppressing LPS-induced lung inflammation, and that an HO-1-dependent pathway mediated these cytoprotective effectsMouse model of ALI were established by challenging intratracheally LPS
(Takashima et al., 2014)
Inhibiting TNF-alpha-induced PI 3-kinase activity, Akt, NF-kB and IL-8
Human airway epithelial cells
(Nanua et al., 2006)
Airway hyperinflammation
Inhibiting levels of IL-5, IL-4, NF-kappaB, P-selectin expression and the mucus production in the lung
Mice
(Rogerio et al., 2010)
COPD
Corticosteroid sensitivity was restored
human monocytic U937 cells
(Mitani et al., 2017)
Amentoflavone
Asthma
Attenuating airway hyperresponsiveness and goblet cell hyperplasia;
Increasing IL-4, IL-5 and IL-13, IgE, IFNγ;
Reducing eotaxin, NFAT1 and c-Myc protein in lung tissue of OVA-challenged rats.Ova-induced rats
(Ferrera et al., 2007)
Pleurisy
Decreasing SOD and GSH depletion and MDA and MPO generation; IL-1β and TNF-α
Increasing HO-1, NQO1, and γ-GCL levels, IL-10Wild-type (WT) and Nrf2-deficient (Nrf2) mice
(Hou et al., 2022)
Inflammation
Ihibiting of C3 and negative regulation of the B cell receptor /NF-κB signaling pathways andHMGB1
Lung inflammation from cold exposure
(Cai et al., 2019)
Acute lung injury
Increasing Nrf2-GCLc signaling, enhancement of GSH antioxidant defense, reduction of oxidative stress and final amelioration of inflammation and histological injury of lung
CLP-induced septic rats
(Zong and Zhang, 2017)
Kaempferol
Asthma
Inhibiting TNF-α induced adhesion of molecule-1 and eosinophil integrin β2 in epithelial cells;
Inhibiting eosinophils in airway and lung tissue;Human airway epithelial BEAS-2B cells and eosinophils
(Gong et al., 2012)
Inhibiting total white blood cell and eosinophil count in BALF;
Inhibiting expression of CD4+, B220+, MHC class II and CD40 in BAL cells;
Inhibiting Th2 cytokines (IL-5 and IL-13);BALB/c mice
(Gong et al., 2012)
Modulating PAR1 activation attenuates fibrotic airway remodeling through bronchial EMT;
Decreasing collagen deposition, epithelial secretions and goblet hyperplasia;BEAS-2B cells and BALB/c mice
(Medeiros et al., 2009)
CF
Immunofluorescence demonstrated a favorable change in the intracellular distribution of CFTR
IB3-1cells
(Lim et al., 2004)
Chloroquine
Asthma
Inhibiting immunoglobulin E, IL-4/-13 and TGF-β1 in BALF to attenuate AHR, inflammation and remodeling;
Inhibiting ASM cell proliferation (PCNA), hypertrophy (α-SMA) and accessory secretion (MMP-9 and MMP-13).HDM sensitized mice and ASM cells
(Rogerio et al., 2007)
Resveratrol
Asthma
Inhibiting IL-4,IL-5;
Suppressing airway hyperresponsiveness, eosinophilia, and mucus hypersecretionOVA-induced allergic mouse
(Lee et al., 2009)
COPD
Decreasing IL-1 and IL-8;
Increasing IFN-α and miR-34Rats
Dendritic cells(Wang et al., 2015; Wang et al., 2017b)
Isorhamnetin
Asthma
Decreasing IL-1β, IL-6, IL-8 and CXCL-10.
Human bronchial epithelial cell line BEAS-2B cells
(Ren et al., 2022)
COPD
Decreasing IL-6
Mice
(Xu et al., 2022)
Apigenin
CRS
Inhibiting α-SMA, fibronectin and type I collagen;
Inhibiting MAPK (p-38, JNK) and NF-κB activation induced by TGF-β1;
Reducing migration and collagen contractile activity to inhibit fibroblast functional activity.Nasal fibroblasts and isolated subnasal turbinate tissue
(Yang et al., 2018)
COPD
Inhibiting CD38 and H2O2–-induced senescence
hydrogen peroxide (H2O[Formula: see text]- or doxorubicin (DOXO)-induced senescence model
(Li et al., 2021)
Chlorogenic acid
CRS
Promoting Cl- transport mediated by CFTR..
Mouse and human sinus epithelium
(Illing et al., 2015)
Quinine
Increasing ciliary beat frequency and NO production.
CRS patients
(Workman et al., 2018)
Baicalin
Asthma
Reversing OVA-induced oxidative stress and inflammation
Upregulating miR-103OVA-induced asthmatic mouse
(Ma et al., 2014; Zhai and Wang, 2022)
Decreasing OVA-IgE, IL-6, TNF-α and NF-kB.
(Liu et al., 2016a)
CRS
Decreasing IL-33-dependent expression of trypsin-like in mast cells.
Human airway epithelial cells and human mast cells
(Yoshida et al., 2021)
COPD
Improving lung function and HPA axis function
36-week side stream cigarette smoke induced rat model
(Wang et al., 2018)
Inhibiting apoptosis and TNF-α, IL-6 and IL-8 levels in MLE-12 cells;
Increasing HSP72 expressionMLE-12 cells
(Hao et al., 2021)
Increasing IL-10, SOD, GSH and level of p-IκBa/IκB;
Decreasing the protein levels of MYD88, p-NF-κBp65/NF-κBp65, TLR2, and TLR4Rats
(Ju et al., 2022)
Inhibiting TNF-α, IL-β and NF-kB;
Increasing HDAC2Cigarette smoke (extract) (CS/CSE)-induced airway inflammation
(Li et al., 2012; Zhang et al., 2021)
Genistein
Asthma
Decreasing OVA-induced airway inflammation, Th2-type cytokines
Increasing Th1-type cytokinesOVA-induced mice
(Gao et al., 2012)
CF
Chloride ion outflow.
CF bronchial epithelium is CFBE1o-
(Andersson and Roomans, 2000)
COPD
Inhibiting NF-κB,TNF-α and MMP
Lymphocytes
(Liu et al., 2016b)
Limonin
CF
Reducing inflammatory factors;
Increasing T2Rs in lung.Rats
(Doggrell, 2011)
6-n-Propyl-2-thiouracil
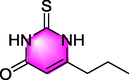
Inhibiting smooth muscle contraction.
Rat and mouse
(Sakai et al., 2016)
Denatonium
Asthma
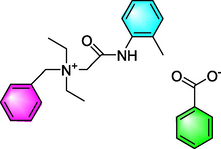
TAS2R4, TAS2R10 were expressed in GPT
Inhiting contractions induced by carbachol.Guinea pig
(Pulkkinen et al., 2012)
CF
Damaging mitochondria;
Inducing apoptosis in airway epithelial cells.Airway epithelial cells
(Wen et al., 2015a)
Thymol
Asthma
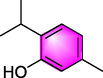
Improving the most prominent inflammation characteristics of asthma
Balb/c mice
(Mohammadi et al., 2018)
COPD
Reducing lung emphysema and inflammation in mice.
Mices
(Games et al., 2016)
Carvacrol
Asthma
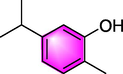
Reducing the values of AEC, IgE, IL-4, IL-5, IL-13, TNF-α, IFN-γ, iNOS and MDA.
Rats
(Ezz-Eldin et al., 2020)
COPD
Indicating a preventive effect on tracheal responsiveness and pathological changes of the lung.
Guinea pigs
(Gholami Mahtaj et al., 2015)
3.1.2 Application of ABC transporters and bitter compounds
The integrity of the respiratory epithelial barrier serves as the primary defense mechanism for preserving lung health. Bitter compounds exhibit distinctphobic ones permeate phosphol through diffusion within the organism, while hydrophilic ones access target cells via specific membrane transport proteins (Duarte et al., 2022a). Numerous bitter compounds possess hydrophobic properties, resulting in limited bioavailability due to restricted absorption and stability.
ABC transporters are membrane proteins found in respiratory epithelium that facilitate the transport of various substrates across biofilms using energy. They play a crucial role in protecting cells from exposure to diverse substances (van der Deen et al., 2005). Additionally, ABC transporters are essential for maintaining the bioavailability of bitter compounds within the respiratory system. Some well-studied examples of ABC transporters include P-glycoprotein (P-g p), multi-drug resistance associated protein (MRP1), ABCA1, ABCG1(Chai et al., 2017) and ABCA3(Peca et al., 2015). ABCA1 and ABCG1 play a role in reverse cholesterol transport (RCT) by facilitating the efflux of cellular cholesterol and phospholipids, and their transcriptional regulation is mediated by liver X receptors (LXRs) (Zhao and Dahlman-Wright, 2010). ABCA3 functions as a phospholipid exporter specifically involved in the synthesis of pulmonary surfactants. Studies conducted on knockout mice have confirmed that the absence of ABC transporters disrupts lipid homeostasis, reduces surfactant production, impairs respiratory physiology, and increases the expression of inflammatory cytokines in lung cells (Bates et al., 2005; McCarthy et al., 2022; Rindler et al., 2017).
Flavonoids possess the potential for treating respiratory diseases; however, their limited bioavailability in the lung hampers their advantageous health effects. Consequently, it is comprehended that flavonoids modulate the expression and activity of ABC transporters in vivo/in vitro. In vitro models, RLE/ABCA3 cells utilized to investigate the inhibitory effect of quercetin on ABCA3 expression, thereby suppressing bleomycin (BLM)-induced epithelial mesenchymal transformation (EMT) (Takano et al., 2020) in alveolar type II cells and reducing ntracellular ROS levels, consequently mitigating oxidative stress and alleviating inflammation. The presence and activity of these ABC transporters may also influence the delivery of pulmonary drugs to their target sites. Mice lacking ABCG1, ABCA1, and ABCA3 of inflammatory factors IL-1β (Hamilton et al., 2002) and tumor necrosis factor-α (TNF-α) in their lungs (Baldán et al., 2008). These inflammatory factors can cause inflammation,oxidative damage, airway fibrosis and mucogenesis, which affect the treatment of respiratory diseases (Mukhopadhyay et al., 2006). Furthermore, Mercier et al. (2018) established a pharmacological model using RPMI2650 cells to study nasal mucosa and observed functional activity of and MRP1 transporters at the cellular level. Apigenin directly interacts with ABC transmembrane transporter CFTR (Ferrera et al., 2007), expressed in the parietal membrane of epithelial cells. The synergistic combination of apigenin-quercetin downregulates ABC transporter expression and inhibit membrane transporter protein (P-g p) activity and phase II enzyme function, thereby reducing compound metabolism and increasing the potential bioavailability of apigenin (Ravisankar et al., 2019). This highlights the relationship between quercetin and ABC transporter expression.
Both in vitro and animal models have shown that bitter compounds can regulate the expression of ABC transporters, which should be further studied. Overall, novel therapeutic approaches hold promise for enhancing drug resistance and augmenting drug bioavailability.
3.2 Exploration of T2Rs as an intermediate in the treatment of respiratory diseases
The activation of T2Rs by bitter compounds leads to changes in the levels of Ca2+, NO, cAMP (Upadhyaya et al., 2014), thereby reducing oxidative stress in the respiratory tract and resulting in relaxation or constriction of the airways. However, this alteration in airway tone often exacerbates airway inflammation response observed in most respiratory diseases. Studies have shown that activation of extra-oral T2Rs (T2R4, T2R14, T2R38) can prevent inflammation (Carey et al., 2017). Therefore, it is suggested that the activation of T2Rs may be associated with inflammatory responses in respiratory diseases and these responses serve as indicators for evaluating the anti-inflammatory and antioxidant effects induced compound-mediated activation (Fig. 4).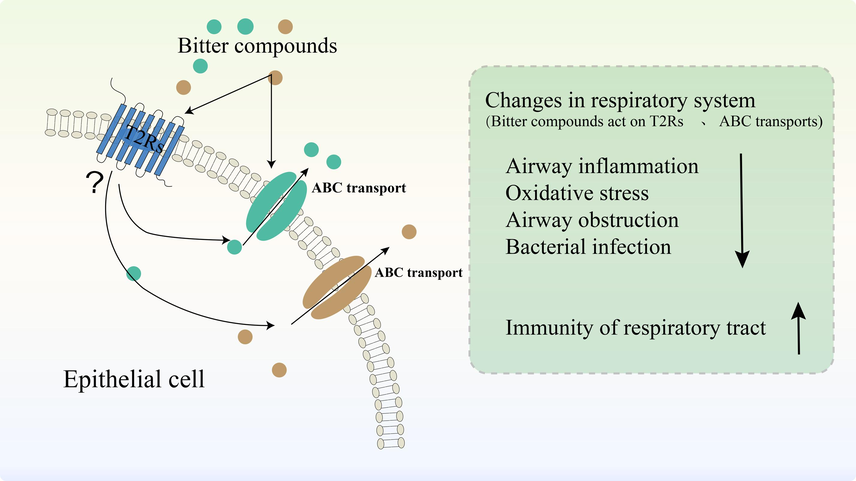
T2Rs act as intermediates for the transport of bitter compounds. T2Rs act as intermediates that can bind directly to bitter compounds and may regulate ABC transporters activity at the epithelial cell membrane. In addition, bitter compounds can directly affect ABC transporters activity. It is also indicated that bitter compounds and T2Rs caused some changes in the respiratory system: airway obstruction, and bacterial infections↓; Immunity of respiratory tract↑.
3.2.1 Asthma and COPD
Asthma is a chronic inflammatory disease of the airway, characterized by the contraction of human airway smooth muscle (HASM) due to an increase in local bronchoconstrictor substances. This leads to airway inflammation and obstruction of airflow (Mims, 2015; Vogelmeier et al., 2017). Deshpande et al. (2010) initially reported the expression of T2Rs in human airway smooth muscle cells. Both quinine and denatonium, which are T2R agonists and bitter compounds, have been shown to improve bronchiectasis by reducing Ca2+ oscillation frequency (Tan and Sanderson, 2014) and Ca2+ sensitivity. Additionally, it has been found that artesunate, an antimalarial drug, may enhance Ca2+ flow through binding with T2R14(Wang et al., 2019). Flavonoids such as chloroquine, naringenin and kaempferol inhibits the expression activities of inflammatory cytokines TNF-α, IL-4, IL-5, and IL-13, reducing airway resistance in inflammatory rats and asthmatic mice (Devillier et al., 2015; Gong et al., 2012; Ren et al., 2021; Shi et al., 2009), ultimately improving cough. Cuiyuncao exerts anti-inflammatory function through the T2R10/IP3R1/NFAT1 dependent signaling pathway (Desv (Yu et al., 2017)). The above findings suggests that targeting T2Rs may be a potential therapeutic approach for asthma treatment.
3.2.2 CRS
CRS is an inflammation of sinuses and nasal mucosa, thus anti-inflammatory effects of flavonoids may play a protective role against CRS (Liu et al., 2022). The role of T2Rs in sinusitis and their contribution to chronic rhinosinusitis have been extensively studied (Adappa et al., 2016; Adappa et al., 2013; Adappa et al., 2014; Carey et al., 2016; Dżaman et al., 2016; Gallo et al., 2016; Mfuna Endam et al., 2014; Rom et al., 2017). Apigenin inhibits functional activity of nasal fibroblasts by reducing the migration and collagen contractile activities (Yang et al., 2018). Numerous studies indicate that T2R38 plays a predominant role (Lee and Cohen, 2014; Lee and Cohen, 2015a; Lee and Cohen, 2015b), particularly in individuals with T2R38AVI/AVI (Hariri et al., 2017b), single nucleotide polymorphisms (SNPs) in the T2R38 gene may affect susceptibility to CRS and reduce oxidative stress. T2Rs and related signaling pathways could potentially serve as targets for treating chronic rhinosinusitis (Dżaman et al., 2016). Expression of T2R38 has been found enhance upper respiratory innate immunity (Douglas and Cohen, 2017). Baicalin has been shown to stimulate T2R14, leading to the inhibition of IL-33 expression (Yoshida et al., 2021).
3.2.3 CF
CF is characterized by the presence of chronic bacterial infections, bronchiectasis, excessive production of airway mucus, and elevated levels of intracellular (d'Angelo et al., 2014). Quercetin and genistein can reduce the conductivity of chloride ions in rats or cells (Andersson and Roomans, 2000; Pyle et al., 2010; Jaggupilli et al., 2017). Treatment with quinine in CuFi-1 cells resulted in a dose-dependent intracellular Ca2+ levels and bronchoconstriction, indicating functional expression of T2Rs in these cells. In addition, studies have shown that the bitter agonist denatonium can activate primary nasal epithelial cells HSP90 and reduce NO production stimulated by T2Rs, which plays an important role in airway antimicrobial. These fingdings suggest that targeting T2Rs may hold promise for the treatment of cystic fibrosis. Numerous studies have described the effects of bitter compounds against CF.
4 Summary and discussion
This review discusses that bitter compounds from plants targeting T2Rs may be a viable treatment for respiratory diseases and are considered as potential novel bronchodilators. T2Rs are important intermediates that regulate the action of bitter compounds on tissues or cells, as reflected by the reduction of inflammatory factors, and bitter compounds reduce oxidative stress and alleviate the inflammatory response by stimulating T2Rs. Notably, the low bioavailability of bitter compounds and their ability to cross epithelial cell membranes greatly limit their effective treatment of disease, whereas binding to ABC transporters not only reduces inflammation levels, but also effectively reduces compound metabolism and increases bioavailability. In terms of taste, most of the active pharmaceutical ingredients with poor taste are bitter and are an important cause of poor compliance and adherence to medication in many patients, especially in children, which severely limits the development and clinical application of many formulations. Innovative taste correction and taste masking techniques can be used, such as the addition of sweeteners such as aspartame and steviol, bitter taste receptor inhibitors such as propoxur, enterodiol and γ-aminobutyric acid or direct coating of bitter compounds with acrylic resins and β-cyclodextrins, etc. Sweeteners can activate sweet taste receptors to produce sweet taste, thus balancing the bitter taste of traditional Chinese medicine substances. Bitter taste blockers can competitively bind to bitter taste receptors, thereby reducing the frequency of bitter taste signal transduction. Inclusion complexes can directly block the bitter taste from touching the mouth. The above methods effectively enable these bitter ingredients to retain their activity and improve their taste, making it easier for patients to accept the drug in the mouth to treat their diseases. In conclusion, the natural bitter compounds present in plants can continue to be studied in depth in the future to explore their potential ability to combine with T2Rs to treat diseases.
In summary, targeting T2Rs in combination with bitter compounds from plants is a promising therapeutic approach, while how to improve the bioavailability of bitter compounds in lung respiratory epithelial cell membranes remains to be addressed. When both T2Rs and ABC transporters are present in the cell membrane, it is possible to use bitter blockers (Trp-Trp (Ojiro et al., 2021) etc.) and ABC transporters inhibitors (Propofol (Greene et al., 2011)) to block the perception of bitter compounds simultaneously and separately, and analyze the degree of uptake of bitter compounds in the lung epithelial cell membrane by some indicators.
such as changes in Ca2+ content and changes in the level of inflammatory factors produced by the activation of T2Rs by bitter compounds. These experimental hypotheses will clarify the mechanism and bioavailability of bitter compounds through T2Rs and ABC transporters in the treatment of respiratory diseases, and are of great importance for the future treatment of respiratory diseases.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Genetics of the taste receptor T2R38 correlates with chronic rhinosinusitis necessitating surgical intervention. Int. Forum Allergy Rhinol.. 2013;3:184-187.
- [CrossRef] [Google Scholar]
- The bitter taste receptor T2R38 is an independent risk factor for chronic rhinosinusitis requiring sinus surgery. Int. Forum Allergy Rhinol.. 2014;4:3-7.
- [CrossRef] [Google Scholar]
- TAS2R38 genotype predicts surgical outcome in nonpolypoid chronic rhinosinusitis. Int. Forum Allergy Rhinol.. 2016;6:25-33.
- [CrossRef] [Google Scholar]
- Activation of deltaF508 CFTR in a cystic fibrosis respiratory epithelial cell line by 4-phenylbutyrate, genistein and CPX. Eur. Respir. J.. 2000;15:937-941.
- [CrossRef] [Google Scholar]
- Loss of ABCG1 results in chronic pulmonary inflammation. J. Immunol. (Baltimore, Md. : 1950). 2008;180:3560-3568.
- [CrossRef] [Google Scholar]
- Pulmonary abnormalities due to ABCA1 deficiency in mice. Am. J. Physiol. Lung Cell. Mol. Physiol.. 2005;289:L980-L989.
- [CrossRef] [Google Scholar]
- The human taste receptor hTAS2R14 responds to a variety of different bitter compounds. Biochem. Biophys. Res. Commun.. 2004;319:479-485.
- [CrossRef] [Google Scholar]
- Gustatory and extragustatory functions of mammalian taste receptors. Physiol. Behav.. 2011;105:4-13.
- [CrossRef] [Google Scholar]
- The human TAS2R16 receptor mediates bitter taste in response to β-glucopyranosides. Nat. Genet. 2002
- [CrossRef] [Google Scholar]
- Amentoflavone ameliorates cold stress-induced inflammation in lung by suppression of C3/BCR/NF-κB pathways. BMC Immunol.. 2019;20:49.
- [CrossRef] [Google Scholar]
- Taste Receptors in Upper Airway Immunity. Adv Otorhinolaryngol.. 2016;79:91-102.
- [CrossRef] [Google Scholar]
- Sinonasal T2R-mediated nitric oxide production in response to Bacillus cereus. Am. J. Rhinol. Allergy. 2017;31:211-215.
- [CrossRef] [Google Scholar]
- The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816-824.
- [CrossRef] [Google Scholar]
- Update on clinical aspects of chronic obstructive pulmonary disease. N. Engl. J. Med.. 2019;381:1257-1266.
- [CrossRef] [Google Scholar]
- Examining the role of ABC lipid transporters in pulmonary lipid homeostasis and inflammation. Respir. Res.. 2017;18:41.
- [CrossRef] [Google Scholar]
- Denatonium benzoate bitter taste perception in chronic rhinosinusitis subgroups. Int. Forum Allergy Rhinol.. 2021;11:967-975.
- [CrossRef] [Google Scholar]
- Regulator of G-protein signaling-21 (RGS21) is an inhibitor of bitter gustatory signaling found in lingual and airway epithelia. J. Biol. Chem.. 2012;287:41706-41719.
- [CrossRef] [Google Scholar]
- Resveratrol induces brain resilience against alzheimer neurodegeneration through proteostasis enhancement. Mol. Neurobiol.. 2019;56:1502-1516.
- [CrossRef] [Google Scholar]
- Improving the efficacy of inhaled drugs in cystic fibrosis: challenges and emerging drug delivery strategies. Adv. Drug Deliv. Rev.. 2014;75:92-111.
- [CrossRef] [Google Scholar]
- Taste receptors of the gut: emerging roles in health and disease. Gut. 2014;63:179-190.
- [CrossRef] [Google Scholar]
- Bitter taste receptors on airway smooth muscle bronchodilate by localized calcium signaling and reverse obstruction. Nat. Med.. 2010;16:1299-1304.
- [CrossRef] [Google Scholar]
- The pharmacology of bitter taste receptors and their role in human airways. Pharmacol. Ther.. 2015;155:11-21.
- [CrossRef] [Google Scholar]
- Bitter taste receptors as a target for bronchodilation. Expert Opin. Therapeut. Targets 15. 2011;10(1517/14728222)
- [Google Scholar]
- Taste receptors mediate sinonasal immunity and respiratory disease. Int. J. Mol. Sci.. 2017;18
- [CrossRef] [Google Scholar]
- Bitter taste, phytonutrients, and the consumer: a review. Am. J. Clin. Nutr.. 2000;72:1424-1435.
- [CrossRef] [Google Scholar]
- Epigallocatechin gallate reduces amyloid beta-induced neurotoxicity via inhibiting endoplasmic reticulum stress-mediated apoptosis. Mol. Nutr. Food Res.. 2018;62:e1700890.
- [CrossRef] [Google Scholar]
- The bitter taste receptor TAS2R14 regulates resveratrol transport across the human blood-cerebrospinal fluid barrier. Biochem. Pharmacol.. 2020;177:113953
- [CrossRef] [Google Scholar]
- The druggability of bitter taste receptors for the treatment of neurodegenerative disorders. Biochem. Pharmacol.. 2022;197:114915
- [CrossRef] [Google Scholar]
- The druggability of bitter taste receptors for the treatment of neurodegenerative disorders. Biochem. Pharmacol.. 2022;197:114915
- [CrossRef] [Google Scholar]
- The correlation of TAS2R38 gene variants with higher risk for chronic rhinosinusitis in Polish patients. Otolaryngologia polska = Polish Otolaryngol.. 2016;70
- [Google Scholar]
- Isolation and identification of chemical constituents from Origanum majorana and investigation of antiproliferative and antioxidant activities. J. Sci. Food Agric.. 2016;96:822-836.
- [CrossRef] [Google Scholar]
- Potential anti-inflammatory and immunomodulatory effects of carvacrol against ovalbumin-induced asthma in rats. Life Sci.. 2020;242:117222
- [CrossRef] [Google Scholar]
- Expression of the G-protein alpha-subunit gustducin in mammalian spermatozoa. J. Compara. Physiol. A Neuroethol. Sens. Neural Behav. Physiol.. 2007;193:21-34.
- [CrossRef] [Google Scholar]
- Characterization of a 7,8-benzoflavone double effect on CFTR Cl(-) channel activity. J. Membr. Biol.. 2007;220:1-9.
- [CrossRef] [Google Scholar]
- Solitary chemoreceptor cells in the nasal cavity serve as sentinels of respiration. PNAS. 2003;100:8981-8986.
- [CrossRef] [Google Scholar]
- Expression, regulation and putative nutrient-sensing function of taste GPCRs in the heart. PLoS One. 2013;8:e64579.
- [Google Scholar]
- TAS2R38 taste receptor gene and chronic rhinosinusitis: new data from an Italian population. BMC Med. Genet.. 2016;17:54.
- [CrossRef] [Google Scholar]
- Structurally related monoterpenes p-cymene, carvacrol and thymol isolated from essential oil from leaves of lippia sidoides Cham. (verbenaceae) protect mice against elastase-induced emphysema. Molecules. 2016;21
- [CrossRef] [Google Scholar]
- Genistein attenuated allergic airway inflammation by modulating the transcription factors T-bet, GATA-3 and STAT-6 in a murine model of asthma. Pharmacology. 2012;89:229-236.
- [CrossRef] [Google Scholar]
- Functional genomics reveals dysregulation of cortical olfactory receptors in Parkinson disease: novel putative chemoreceptors in the human brain. J. Neuropathol. Exp. Neurol.. 2013;72:524-539.
- [CrossRef] [Google Scholar]
- Naringenin improves learning and memory in an Alzheimer's disease rat model: Insights into the underlying mechanisms. Eur. J. Pharmacol.. 2015;764:195-201.
- [CrossRef] [Google Scholar]
- The effect of zataria multiflora and its constituent, carvacrol, on tracheal responsiveness and lung pathology in Guinea Pig model of COPD. Phytother. Res.: PTR. 2015;29:730-736.
- [CrossRef] [Google Scholar]
- Is the bitter rejection response always adaptive? Physiol. Behav.. 1994;56:1217-1227.
- [CrossRef] [Google Scholar]
- Kaempferol suppresses eosionphil infiltration and airway inflammation in airway epithelial cells and in mice with allergic asthma. J. Nutr.. 2012;142:47-56.
- [CrossRef] [Google Scholar]
- Inhibition of airway epithelial-to-mesenchymal transition and fibrosis by kaempferol in endotoxin-induced epithelial cells and ovalbumin-sensitized mice. Lab. Invest.. 2014;94:297-308.
- [CrossRef] [Google Scholar]
- Probenecid inhibits the human bitter taste receptor TAS2R16 and suppresses bitter perception of salicin. PLoS One. 2011;6:e20123.
- [CrossRef] [Google Scholar]
- Naringin protects ovalbumin-induced airway inflammation in a mouse model of asthma. Inflammation. 2016;39:891-899.
- [CrossRef] [Google Scholar]
- Contribution of efflux pump activity to the delivery of pulmonary therapeutics. Curr. Drug Metab.. 2002;3:1-12.
- [CrossRef] [Google Scholar]
- Bitter compounds: Occurrence and structure-activity relationships. Food Rev. Intl. 1985
- [CrossRef] [Google Scholar]
- Baicalin alleviates chronic obstructive pulmonary disease through regulation of HSP72-mediated JNK pathway. Mol. Med. (Cambridge, Mass.). 2021;27:53.
- [CrossRef] [Google Scholar]
- Plant flavones enhance antimicrobial activity of respiratory epithelial cell secretions against Pseudomonas aeruginosa. PLoS One. 2017;12:e0185203.
- [Google Scholar]
- Flavones modulate respiratory epithelial innate immunity: Anti-inflammatory effects and activation of the T2R14 receptor. J. Biol. Chem.. 2017;292:8484-8497.
- [CrossRef] [Google Scholar]
- Herbal medicines for rhinosinusitis: A systematic review and network meta-analysis. Curr. Allergy Asthma Rep.. 2023;23:93-109.
- [CrossRef] [Google Scholar]
- Amentoflavone ameliorates carrageenan-induced pleurisy and lung injury by inhibiting the NF-κB/STAT3 pathways via Nrf2 activation. Front. Pharmacol.. 2022;13:763608
- [CrossRef] [Google Scholar]
- Flavonoids, flavonoid subclasses and breast cancer risk: a meta-analysis of epidemiologic studies. PLoS One. 2013;8:e54318.
- [CrossRef] [Google Scholar]
- Chlorogenic acid activates CFTR-mediated Cl-secretion in mice and humans: Therapeutic implications for chronic rhinosinusitis. Otolaryngol. Head Neck Surg.. 2015;153:291-297.
- [CrossRef] [Google Scholar]
- Analysis of the expression of human bitter taste receptors in extraoral tissues. Mol. Cell. Biochem.. 2017;426:137-147.
- [CrossRef] [Google Scholar]
- Gut bitter taste receptor signalling induces ABCB1 through a mechanism involving CCK. Biochem. J. 2011;438:33-37.
- [CrossRef] [Google Scholar]
- Identification of novel compounds for human bitter taste receptors. Chem. Biol. Drug Des.. 2014;84:63-74.
- [CrossRef] [Google Scholar]
- Baicalin inhibits inflammation in rats with chronic obstructive pulmonary disease by the TLR2/MYD88/NF-κBp65 signaling pathway. Evid. Based Complement. Alternat. Med.. 2022;2022:7273387.
- [CrossRef] [Google Scholar]
- Secretory effects of a luminal bitter tastant and expressions of bitter taste receptors, T2Rs, in the human and rat large intestine. Am. J. Physiol. Gastrointest. Liver Physiol.. 2009;296:G971-G981.
- [CrossRef] [Google Scholar]
- Involvement of the nitric oxide signaling in modulation of naringin against intranasal manganese and intracerbroventricular β-amyloid induced neurotoxicity in rats. J. Nutr. Biochem.. 2020;76:108255
- [CrossRef] [Google Scholar]
- The role of hydrogen sulfide in respiratory diseases. Biomolecules. 2021;11
- [CrossRef] [Google Scholar]
- Botany (Book reviews: Poisonous plants of the United States and Canada) Science. 1964;145
- [CrossRef] [Google Scholar]
- The role of the sweet taste receptor in enteroendocrine cells and pancreatic β-cells. Diabetes Metab. J.. 2011;35:451-457.
- [CrossRef] [Google Scholar]
- A hypothesis: Bitter taste receptors as a therapeutic target for the clinical symptoms of SARS-CoV-2. Pharmazie. 2021;76:43-54.
- [CrossRef] [Google Scholar]
- Lajtha, A., Oja, S.S., Schousboe, A., Saransaari, P., 2007. Handbook of Neurochemistry and Molecular Neurobiology. Springer US, https://doi.org/10.1007/978-0-387-30373-4.
- Sinonasal solitary chemosensory cells “taste” the upper respiratory environment to regulate innate immunity. Am. J. Rhinol. Allergy. 2014;28:366-373.
- [CrossRef] [Google Scholar]
- Role of the bitter taste receptor T2R38 in upper respiratory infection and chronic rhinosinusitis. Curr. Opin. Allergy Clin. Immunol.. 2015;15:14-20.
- [CrossRef] [Google Scholar]
- Taste receptors in innate immunity. Cell. Mol. Life Sci.. 2015;72:217-236.
- [CrossRef] [Google Scholar]
- Therapeutic potential of ectopic olfactory and taste receptors. Nat. Rev. Drug Discov.. 2019;18:116-138.
- [CrossRef] [Google Scholar]
- Anti-inflammatory and anti-asthmatic effects of resveratrol, a polyphenolic stilbene, in a mouse model of allergic asthma. Int. Immunopharmacol.. 2009;9:418-424.
- [CrossRef] [Google Scholar]
- Taste perception: from the tongue to the testis. Mol. Hum. Reprod.. 2013;19:349-360.
- [CrossRef] [Google Scholar]
- Baicalin is anti-inflammatory in cigarette smoke-induced inflammatory models in vivo and in vitro: A possible role for HDAC2 activity. Int. Immunopharmacol.. 2012;13:15-22.
- [CrossRef] [Google Scholar]
- Apigenin alleviates oxidative stress-induced cellular senescence via modulation of the SIRT1-NAD[Formula: see text]-CD38 axis. Am. J. Chin. Med.. 2021;49:1235-1250.
- [CrossRef] [Google Scholar]
- Modulation of deltaF508 cystic fibrosis transmembrane regulator trafficking and function with 4-phenylbutyrate and flavonoids. Am. J. Respir. Cell Mol. Biol.. 2004;31:351-357.
- [CrossRef] [Google Scholar]
- Effects of resveratrol and genistein on nuclear factor-κB, tumor necrosis factor-α and matrix metalloproteinase-9 in patients with chronic obstructive pulmonary disease. Mol. Med. Rep.. 2016;13:4266-4272.
- [CrossRef] [Google Scholar]
- [Pharmacoeconomic evaluation of Xiangju Capsules in treatment of chronic rhinosinusitis] Zhongguo Zhong yao za zhi = Zhongguo zhongyao zazhi = China J. Chinese Mater. Med.. 2022;47
- [Google Scholar]
- Members of bitter taste receptor cluster Tas2r143/Tas2r135/Tas2r126 are expressed in the epithelium of murine airways and other non-gustatory tissues. Front. Physiol.. 2017;8:849.
- [CrossRef] [Google Scholar]
- Advances in research on food umami flavor. J. Chinese Instit. Food Sci. Technol.. 2017;17
- [Google Scholar]
- Baicalin attenuates inflammation in mice with OVA-induced asthma by inhibiting NF-κB and suppressing CCR7/CCL19/CCL21. Int. J. Mol. Med.. 2016;38:1541-1548.
- [CrossRef] [Google Scholar]
- Genetic deletion of the Tas2r143/Tas2r135/Tas2r126 cluster reveals that TAS2Rs may not mediate bitter tastant-induced bronchodilation. J. Cell. Physiol.. 2021;236:6407-6423.
- [CrossRef] [Google Scholar]
- Naringin attenuates enhanced cough, airway hyperresponsiveness and airway inflammation in a guinea pig model of chronic bronchitis induced by cigarette smoke. Int. Immunopharmacol.. 2012;13:301-307.
- [CrossRef] [Google Scholar]
- Anti-asthmatic effects of baicalin in a mouse model of allergic asthma. Phytother. Res.: PTR. 2014;28:231-237.
- [CrossRef] [Google Scholar]
- Autoimmune pulmonary alveolar proteinosis. Am. J. Respir. Crit. Care Med.. 2022;205:1016-1035.
- [CrossRef] [Google Scholar]
- The bitter end: T2R bitter receptor agonists elevate nuclear calcium and induce apoptosis in non-ciliated airway epithelial cells. Cell Calcium. 2022;101:102499
- [CrossRef] [Google Scholar]
- Preventive and curative glycoside kaempferol treatments attenuate the TH2-driven allergic airway disease. Int. Immunopharmacol.. 2009;9:1540-1548.
- [CrossRef] [Google Scholar]
- Pharmacological characterization of the RPMI 2650 model as a relevant tool for assessing the permeability of intranasal drugs. Mol. Pharm.. 2018;15:2246-2256.
- [CrossRef] [Google Scholar]
- The molecular receptive ranges of human TAS2R bitter taste receptors. Chem. Senses. 2010;35:157-170.
- [CrossRef] [Google Scholar]
- Genetic variations in taste receptors are associated with chronic rhinosinusitis: a replication study. Int. Forum Allergy Rhinol.. 2014;4:200-206.
- [CrossRef] [Google Scholar]
- Asthma: definitions and pathophysiology. Int. Forum Allergy Rhinol.. 2015;5(Suppl 1):S2-S6.
- [CrossRef] [Google Scholar]
- Quercetin restores corticosteroid sensitivity in cells from patients with chronic obstructive pulmonary disease. Exp. Lung Res.. 2017;43:417-425.
- [CrossRef] [Google Scholar]
- Chemoprevention of breast cancer by dietary polyphenols. Molecules. 2015;20:22578-22620.
- [CrossRef] [Google Scholar]
- Chemoprevention of breast cancer by dietary polyphenols. Molecules. 2015;20:22578-22620.
- [CrossRef] [Google Scholar]
- Immunomodulatory effects of Thymol through modulation of redox status and trace element content in experimental model of asthma. Biomed. Pharmacother. = Biomedecine Pharmacotherapie. 2018;105
- [Google Scholar]
- Role of TNFalpha in pulmonary pathophysiology. Respir. Res.. 2006;7:125.
- [CrossRef] [Google Scholar]
- Isolation and structure of pulcherrimine, a novel bitter-tasting amino acid, from the sea urchin (Hemicentrotus pulcherrimus) ovaries. J. Agric. Food Chem.. 2000;48:5557-5560.
- [CrossRef] [Google Scholar]
- Quercetin blocks airway epithelial cell chemokine expression. Am. J. Respir. Cell Mol. Biol.. 2006;35:602-610.
- [CrossRef] [Google Scholar]
- Bitter taste receptors for asthma therapeutics. Front. Physiol.. 2019;10:884.
- [CrossRef] [Google Scholar]
- Naringin as a plant-derived bitter tastant promotes proliferation of cultured human airway epithelial cells via activation of TAS2R signaling. Phytomedicine. 2021;84:153491
- [CrossRef] [Google Scholar]
- Trp-Trp acts as a multifunctional blocker for human bitter taste receptors, hTAS2R14, hTAS2R16, hTAS2R43, and hTAS2R46. Biosci. Biotech. Bioch.. 2021;85:1526-1529.
- [CrossRef] [Google Scholar]
- Quercetin regulates Th1/Th2 balance in a murine model of asthma. Int. Immunopharmacol.. 2009;9:261-267.
- [CrossRef] [Google Scholar]
- ABCA3, a key player in neonatal respiratory transition and genetic disorders of the surfactant system. Biochem. Soc. Trans.. 2015;43:913-919.
- [CrossRef] [Google Scholar]
- Autophagy-dependent mechanism of genistein-mediated elimination of behavioral and biochemical defects in the rat model of sporadic Alzheimer's disease. Neuropharmacology. 2019;148:332-346.
- [CrossRef] [Google Scholar]
- Identification of ligands for two human bitter T2R receptors. Chem. Senses. 2004;29:583-593.
- [CrossRef] [Google Scholar]
- The bitter taste receptor (TAS2R) agonists denatonium and chloroquine display distinct patterns of relaxation of the guinea pig trachea. Am. J. Physiol. Lung Cell. Mol. Physiol.. 2012;303:L956-L966.
- [CrossRef] [Google Scholar]
- Recent advances in structure and function studies on human bitter taste receptors. Curr. Protein Pept. Sci.. 2012;13:501-508.
- [CrossRef] [Google Scholar]
- Activation of the cystic fibrosis transmembrane conductance regulator by the flavonoid quercetin: potential use as a biomarker of ΔF508 cystic fibrosis transmembrane conductance regulator rescue. Am. J. Respir. Cell Mol. Biol.. 2010;43:607-616.
- [CrossRef] [Google Scholar]
- Combined cereal and pulse flavonoids show enhanced bioavailability by downregulating phase II metabolism and ABC membrane transporter function in Caco-2 model. Food Chem.. 2019;279:88-97.
- [CrossRef] [Google Scholar]
- Bitter melon (Momordica charantia) extract inhibits breast cancer cell proliferation by modulating cell cycle regulatory genes and promotes apoptosis. Cancer Res.. 2010;70:1925-1931.
- [CrossRef] [Google Scholar]
- Functions of human bitter taste receptors depend on N-glycosylation. J. Neurochem.. 2008;106:1138-1148.
- [CrossRef] [Google Scholar]
- Isorhamnetin attenuates TNF-α-induced inflammation, proliferation, and migration in human bronchial epithelial cells via MAPK and NF-κB pathways. Anatomical Rec. (Hoboken, N.J.: 2007). 2021;304:901-913.
- [CrossRef] [Google Scholar]
- Traditional Chinese medicine for COVID-19 treatment. Pharmacol. Res.. 2020;155:104743
- [CrossRef] [Google Scholar]
- Chloroquine attenuates asthma development by restoring airway smooth muscle cell phenotype via the ROS-AKT pathway. Front. Pharmacol.. 2022;13:916508
- [CrossRef] [Google Scholar]
- Alveolar injury and regeneration following deletion of ABCA3. JCI Insight. 2017;2
- [CrossRef] [Google Scholar]
- Anti-inflammatory activity of quercetin and isoquercitrin in experimental murine allergic asthma. Inflamm. Res.. 2007;56:402-408.
- [CrossRef] [Google Scholar]
- Anti-inflammatory effect of quercetin-loaded microemulsion in the airways allergic inflammatory model in mice. Pharmacol. Res.. 2010;61:288-297.
- [CrossRef] [Google Scholar]
- The impact of bitter taste receptor genetics on culturable bacteria in chronic rhinosinusitis. Rhinology. 2017;55:90-94.
- [CrossRef] [Google Scholar]
- Taste receptor signaling in the mammalian gut. Curr. Opin. Pharmacol.. 2007;7:557-562.
- [CrossRef] [Google Scholar]
- The flavonoid quercetin ameliorates Alzheimer's disease pathology and protects cognitive and emotional function in aged triple transgenic Alzheimer's disease model mice. Neuropharmacology. 2015;93:134-145.
- [CrossRef] [Google Scholar]
- Denatonium and 6-n-propyl-2-thiouracil, agonists of bitter taste receptor, inhibit contraction of various types of smooth muscles in the rat and mouse. Biol. Pharm. Bull.. 2016;39:33-41.
- [CrossRef] [Google Scholar]
- Bitter taste receptors: Extraoral roles in pathophysiology. Int. J. Biochem. Cell Biol.. 2016;77:197-204.
- [CrossRef] [Google Scholar]
- Naringin inhibits vascular endothelial cell apoptosis via endoplasmic reticulum stress- and mitochondrial-mediated pathways and promotes intraosseous angiogenesis in ovariectomized rats. Int. J. Mol. Med.. 2017;40:1741-1749.
- [CrossRef] [Google Scholar]
- Naringenin inhibits allergen-induced airway inflammation and airway responsiveness and inhibits NF-kappaB activity in a murine model of asthma. Can. J. Physiol. Pharmacol.. 2009;87:729-735.
- [CrossRef] [Google Scholar]
- Regulation effects of naringin on diesel particulate matter-induced abnormal airway surface liquid secretion. Phytomedicine. 2019;63:153004
- [CrossRef] [Google Scholar]
- Expression profiles and functional characterization of common carp (Cyprinus carpio) T2Rs. Biochem. Biophys. Rep.. 2021;28:101123
- [CrossRef] [Google Scholar]
- Functional bitter taste receptors are expressed in brain cells. Biochem. Biophys. Res. Commun.. 2011;406:146-151.
- [CrossRef] [Google Scholar]
- Prevalence and attributable health burden of chronic respiratory diseases, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Respir. Med.. 2020;8:585-596.
- [CrossRef] [Google Scholar]
- Overcoming chemoresistance in pancreatic cancer cells: role of the bitter taste receptor T2R10. J. Cancer. 2018;9:711-725.
- [CrossRef] [Google Scholar]
- Quercetin prevents LPS-induced oxidative stress and inflammation by modulating NOX2/ROS/NF-kB in lung epithelial cells. Molecules. 2021;26
- [CrossRef] [Google Scholar]
- Suppressive effect of quercetin against bleomycin-induced epithelial-mesenchymal transition in alveolar epithelial cells. Drug Metab. Pharmacokinet.. 2020;35:522-526.
- [CrossRef] [Google Scholar]
- Protective effects of intratracheally administered quercetin on lipopolysaccharide-induced acute lung injury. Respir. Res.. 2014;15:150.
- [CrossRef] [Google Scholar]
- Bitter tasting compounds dilate airways by inhibiting airway smooth muscle calcium oscillations and calcium sensitivity. Br. J. Pharmacol.. 2014;171:646-662.
- [CrossRef] [Google Scholar]
- Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect. Dis.. 2018;18:1191-1210.
- [CrossRef] [Google Scholar]
- Dextromethorphan mediated bitter taste receptor activation in the pulmonary circuit causes vasoconstriction. PLoS One. 2014;9:e110373.
- [Google Scholar]
- ATP-binding cassette (ABC) transporters in normal and pathological lung. Respir. Res.. 2005;6:59.
- [CrossRef] [Google Scholar]
- Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summary. Am. J. Respir. Crit. Care Med.. 2017;195:557-582.
- [CrossRef] [Google Scholar]
- Naringenin reduces oxidative stress and improves mitochondrial dysfunction via activation of the Nrf2/ARE signaling pathway in neurons. Int. J. Mol. Med.. 2017;40:1582-1590.
- [CrossRef] [Google Scholar]
- The effects of resveratrol on inflammation and oxidative stress in a rat model of chronic obstructive pulmonary disease. Molecules. 2017;22
- [CrossRef] [Google Scholar]
- Evaluation of pharmacological relaxation effect of the natural product naringin on in vitro cultured airway smooth muscle cells and in vivo ovalbumin-induced asthma Balb/c mice. Biomed. Rep.. 2016;5:715-722.
- [CrossRef] [Google Scholar]
- Baicalin exerts anti-airway inflammation and anti-remodelling effects in severe stage rat model of chronic obstructive pulmonary disease. Evid. Based Complement. Alternat. Med.. 2018;2018:7591348.
- [CrossRef] [Google Scholar]
- Artesunate attenuates airway resistance in vivo and relaxes airway smooth muscle cells in vitro via bitter taste receptor-dependent calcium signalling. Exp. Physiol.. 2019;104:231-243.
- [CrossRef] [Google Scholar]
- Resveratrol inhibits dysfunction of dendritic cells from chronic obstructive pulmonary disease patients through promoting miR-34. Int. J. Clin. Exp. Path.. 2015;8:5145-5152.
- [Google Scholar]
- The G protein-coupled taste receptor T1R1/T1R3 regulates mTORC1 and autophagy. Mol. Cell. 2012;47:851-862.
- [CrossRef] [Google Scholar]
- Increased BBB permeability contributes to EGCG-caused cognitive function improvement in natural aging rats: pharmacokinetic and distribution analyses. Acta Pharmacol. Sin.. 2019;40:1490-1500.
- [CrossRef] [Google Scholar]
- Denatonium inhibits growth and induces apoptosis of airway epithelial cells through mitochondrial signaling pathways. Respir. Res.. 2015;16:13.
- [CrossRef] [Google Scholar]
- The role of quinine-responsive taste receptor family 2 in airway immune defense and chronic rhinosinusitis. Front. Immunol.. 2018;9:624.
- [CrossRef] [Google Scholar]
- Expression of bitter taste receptors of the T2R family in the gastrointestinal tract and enteroendocrine STC-1 cells. PNAS. 2002;99:2392-2397.
- [CrossRef] [Google Scholar]
- Genomic organization, expression, and function of bitter taste receptors (T2R) in mouse and rat. Physiol. Genom.. 2005;22:139-149.
- [CrossRef] [Google Scholar]
- Functional characterization of bitter-taste receptors expressed in mammalian testis. Mol. Hum. Reprod.. 2013;19:17-28.
- [CrossRef] [Google Scholar]
- Isorhamnetin alleviates airway inflammation by regulating the Nrf2/Keap1 pathway in a mouse model of COPD. Front. Pharmacol.. 2022;13:860362
- [CrossRef] [Google Scholar]
- Apigenin alleviates TGF-β1-induced nasal mucosa remodeling by inhibiting MAPK / NF-kB signaling pathways in chronic rhinosinusitis. PLoS One. 2018;13:e0201595.
- [Google Scholar]
- A systematic strategy for discovering a therapeutic drug for Alzheimer's disease and its target molecule. Front. Pharmacol.. 2017;8:340.
- [CrossRef] [Google Scholar]
- Baicalin suppresses type 2 immunity through breaking off the interplay between mast cell and airway epithelial cell. J. Ethnopharmacol.. 2021;267:113492
- [CrossRef] [Google Scholar]
- Selaginella uncinata flavonoids ameliorated ovalbumin-induced airway inflammation in a rat model of asthma. J. Ethnopharmacol.. 2017;195:71-80.
- [CrossRef] [Google Scholar]
- Baicalin regulates the development of pediatric asthma via upregulating microRNA-103 and mediating the TLR4/NF-κB pathway. J. Receptor Signal Transduct. Res.. 2022;42:230-240.
- [CrossRef] [Google Scholar]
- Protective role of naringenin against Abeta25-35-caused damage via ER and PI3K/Akt-mediated pathways. Cell. Mol. Neurobiol.. 2018;38:549-557.
- [CrossRef] [Google Scholar]
- Baicalin ameliorates cigarette smoke-induced airway inflammation in rats by modulating HDAC2/NF-κB/PAI-1 signalling. Pulm. Pharmacol. Ther.. 2021;70:102061
- [CrossRef] [Google Scholar]
- Quercetin increases cystic fibrosis transmembrane conductance regulator-mediated chloride transport and ciliary beat frequency: therapeutic implications for chronic rhinosinusitis. Am. J. Rhinol. Allergy. 2011;25:307-312.
- [CrossRef] [Google Scholar]
- Naringin suppressed airway inflammation and ameliorated pulmonary endothelial hyperpermeability by upregulating Aquaporin1 in lipopolysaccharide/cigarette smoke-induced mice. Biomed. Pharmacother. = Biomedecine & Pharmacotherapie. 2022;150
- [Google Scholar]
- Liver X receptor in cholesterol metabolism. J. Endocrinol.. 2010;204:233-240.
- [CrossRef] [Google Scholar]
- Amentoflavone prevents sepsis-associated acute lung injury through Nrf2-GCLc-mediated upregulation of glutathione. Acta Biochim. Pol.. 2017;64:93-98.
- [CrossRef] [Google Scholar]







