Translate this page into:
Oxidation of sulfide mineral and metal extraction analysis in the microwave-assisted roasting pretreatment of refractory gold ore
⁎Corresponding author. szulle@chem-eng.its.ac.id (Siti Zullaikah)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The recovery of gold from refractory ore could guarantee sustainable development in the gold industry. However, there are many challenges in the treatment of ore, such as environmental pollution and low recovery. In this regard, the current research focuses on the pretreatment of refractory gold ore and investigates the effect of microwave power, the role of additives, and water on the oxidation and decomposition of sulfide minerals. Pretreatment using microwave-assisted roasting with NaClO3 as an additive to the oxidizing agent is a new method of processing refractory gold ore. Oxidation was studied through sulfur and chlorine mass balance analysis and the degree of metal extraction from sulfide minerals. SEM analysis was carried out to complete the study of the phase change of compounds in the oxidation of mineral sulfides. Roasting of refractory gold ore with a composition of 180 kg NaClO3 and 180 kg water/ton ore reached a temperature of 470 °C at a microwave power of 400 watts for 30 minutes. Total oxidized sulfur reached 90.6%, while only 10.4 % of the sulfur was released, and chlorine release was 49.5%. In addition, water leaching was conducted to investigate the extraction rate of metals after roasting. The results showed that the water leaching extraction of Cu, Zn, Pb, and Fe reached 84.3%, 97.6%, 22.8%, and 8.4%, respectively. Applying microwaves with appropriate concentrations of additives and water resulted in high oxidation of sulfide minerals (>90%) and low sulfur emissions in roasting refractory gold ore with relatively low power, temperature, and time. Moreover, leaching this roasted gold ore resulted in gold extraction of 92.5%; however, leaching of refractory gold without pretreatment resulted in gold extraction of only 47.5%.
Keywords
Refractory Gold Ore
Sulfide Mineral
Microwave-Assisted Roasting
Sulfide Mineral Oxidation
Water Leaching
Gold Extraction
1 Introduction
The world's demand for gold has been increasing recently due to its many applications, including jewelry and electronics, and is foreseen as an essential metal in the digital economy era (Calvo et al., 2022). Statista reported the world's gold demand in Q4 2022, jewelry 602.5, technology 72, investment 245.7, and central banks and other institutions 417.1 metric tons (Statista, 2023). The primary source for producing gold metal is gold ores. Metallurgically, gold ore is grouped into free-milling and refractory ores (Amaya et al., 2013; Sousa et al., 2022). There are also other classifications, namely free-milling, complex gold ores, and refractory gold ores (Ahtiainen et al., 2021). Free milling gold ore generally has a high gold content and can be processed by direct cyanidation with an extraction rate of more than 95 %. Refractory ores consist of sulfide minerals such as pyrite (FeS2), chalcopyrite (CuFeS2), galena (PbS), sphalerite (ZnS), arsenopyrite (FeAsS), and other minerals. Pyrite and arsenopyrite are generally the gold-carrying sulfide minerals in refractory gold ores (Ahtiainen et al., 2021; Corrans & Angove, 1991). Refractory gold ore is classified based on its recovery into mildly (80–95 %), moderately (50–80 %), and highly (less than 50 %) refractory (Asamoah et al., 2021; Mabwe, 2020).
The world's gold reserves originating from non-refractory ore are 906 million troy ounces, while refractory ore reserves are 288 million troy ounces, with production growth in the 2015–2019 period of 1.5 % and 3.3 %, respectively (Motta et al., 2021). Gold production from refractory gold ore has increased due to the depletion of free-milled gold ore (Msumange et al., 2020). The depletion of high-grade gold ore reserves also causes high energy consumption and environmental impacts in the mining sector (Jose-Luis et al., 2019). The reserves of high-grade gold ore are getting smaller, which has encouraged the gold processing industry to utilize other sources of raw materials, such as tailings left over from primary processing (Askarova et al., 2021). The gold mining industry has also begun processing refractory gold ore (Larrabure & Rodríguez-Reyes, 2021; Peng et al., 2021). Refractory gold ore resources are abundant, and effective processing can bring high economic benefits (P. Zhu et al., 2012). Refractory gold ore is a vital gold resource (Gui et al., 2022). The processing of gold from refractory ore has become an essential guarantee for the sustainability of the gold mining industry but with challenges such as low gold recovery and significant environmental pollution (Farjana et al., 2019). The chemicals used in leaching gold ore can harm human health and seriously pollute the environment (Peng et al., 2021).
Processing refractory gold ore with conventional methods results in low recovery of gold with high environmental impact (Qin, Guo, Tian, Yu, et al., 2021). Leaching gold from refractory ore faces obstacles, including kinetic or thermodynamic barriers to cyanide diffusion, re-adsorption of preg-robbing gold or silver, and high cyanide consumption (Larrabure & Rodríguez-Reyes, 2021). One of the main problems in extracting gold from refractory ores is that most particles are smaller than 10 μm (Amdur et al., 2022). The mobility of gold and silver metals from refractory ores is deficient because they are encapsulated in sulfide minerals, arsenic sulfide, carbon, or clay, making it challenging to extract them (Canıeren & Karagüzel, 2021).
Refractory gold ores require pretreatment to liberate the gold from encapsulated sulfide minerals by decomposing the sulfide minerals into oxides or other chemical compounds easily soluble in water or other solvents in the leaching process. Pretreatment and processing of refractory gold ore are becoming increasingly important and an inevitable trend for gold development due to the depletion of high-grade ores (Mabwe, 2020). Extraction of gold from refractory gold ore without any side reactions is a highly desirable process in the future (Qin, Guo, Tian, & Zhang, 2021). Due to a large amount of mineral waste, the pretreatment of refractory gold ore is a crucial problem (Piervandi, 2023). One of the significant environmental challenges is arsenic-containing minerals processing (Ng et al., 2023). Arsenic is a common pollutant and impurity in complex gold ores (Liu et al., 2022). Arsenic (As) has been well-documented as a toxic and carcinogenic element since 1970 (Samouhos et al., 2021). Gold ores that are increasingly complex with low gold content have encouraged the development of innovations in their processing (Ahtiainen et al., 2021).
In the conventional process, to overcome the presence of sulfide minerals, pretreatment is carried out by roasting at a temperature of around 700 °C (Amaya et al., 2013). Other methods include ultra fine grinding (UFG) (Bıyıklı et al., 2022; Celep et al., 2015, 2019; Celep & Yazici, 2013; Corrans & Angove, 1991; González-Anaya et al., 2011), pressure oxidation (Ahtiainen et al., 2021; Canıeren & Karagüzel, 2021; Dyson et al., 2022; Faraz et al., 2014; Koslides & Ciminelli, 1992; S. Lee et al., 2022; Liu et al., 2022; L. Zhang, Guo, Tian, Zhong, & Qin, 2022; L. Zhang, Guo, Tian, Zhong, Li, et al., 2022), bio-oxidation (Berkinbayeva et al., 2023; K. Y. Cheng et al., 2021; J. Lee et al., 2021; Q. Li et al., 2020; McNeice et al., 2021; Yin et al., 2020), ozonation (Krylova, 2022; Piervandi, 2023), ultrasound pretreatment (Fu et al., 2017; Gui et al., 2021, 2022, 2023; Guo et al., 2019; Hu et al., 2020; G. Zhang et al., 2016) and a combination of these pretreatment methods.
The grinding process is one of the energy-intensive comminution stages in mineral processing (Adewuyi et al., 2020; Somani et al., 2017). Comminution is a process stage with low energy efficiency (Napier-Munn, 2015; Tromans, 2008). The pressure oxidation method requires a corrosion-resistant autoclave and is carried out at high pressure. The bio-oxidation process generally requires a relatively long time, which hinders achieving production capacity (Gökelma et al., 2016). Ultrasound pretreatment requires an external heat energy source and a reagent as an oxidizing agent.
Microwave-assisted extraction (MAE) can be an alternative for treating sulfide minerals in refractory gold ore processing. During pretreatment, microwave applications can be used at the comminution stage (Batar, 2004; Sudarsono et al., 1998) or the roasting as pretreatment stage (Amankwah & Ofori-Sarpong, 2011; Amaya et al., 2013; Cho et al., 2020; Choi et al., 2017; F. Lin et al., 2021; Lovas et al., 2011; Su et al., 2011). This method can provide rapid heating radiation (Nanthakumar et al., 2007), cause micro-cracks (Adewuyi et al., 2020), and trigger decomposition reactions of sulfide minerals (Cho et al., 2020). Microwave technology can also be applied to mineral extraction or leaching stages with significant advantages, including shorter processing time and lower energy consumption (Cho et al., 2020).
The microwave-assisted roasting method for processing refractory gold has been studied by several researchers previously. However, there is no literature addressing the role of NaClO3 additive as an oxidizing agent during microwave-assisted roasting. Therefore, in this study, the effect of microwave power and the role of additives on the oxidation of sulfide minerals in microwave-assisted roasting of refractory gold ore were investigated. The decomposition of sulfide minerals during roasting has been investigated by analyzing the extraction of metals such as Cu, Zn, Pb, and Fe, which are soluble in water. The release of sulfur into the atmosphere and the decomposition of sulfide minerals are indicative of the oxidation of sulfide minerals during microwave-assisted roasting. Oxidation can break the encapsulation of sulfide minerals in gold particles, thereby increasing the leaching yield of pretreated refractory gold ore. In the final section of this study, the gold extraction rate of the roasted gold ore was investigated and compared with the gold extraction rate without roasting.
2 Materials and methods
2.1 Materials
The refractory gold ore sample, mined in the Garut District, West Java – Indonesia, was kindly provided by PT ANTAM. The gold ore samples were lump-sized between 10 and 20 cm. The additive sodium chlorate (NaClO3) > 98 %, from Merck, was used as an oxidizing agent. Sodium chlorate has been used as an oxidizing agent in extracting copper and zinc sulfide minerals (Kariuki et al., 2009). Hydrochloric acid 37 %, from Merck was used as a solvent in extracting gold from refractory ores with the oxidizer NaClO3 5 % (w/v) (Y. Cheng et al., 2013). Whatman Grade 5 filter paper was used to separate residue from the leaching solution.
2.2 Refractory gold ore characterization and preparation
In the early stages, mineralogical analysis was carried out on selected samples to determine the types of minerals contained in the refractory gold ore. Mineralogical analysis was conducted using an Olympus CX-31P optical microscope, USA, and the X-ray diffraction method using the Benchtop Powder X-Ray Diffraction (XRD) Rigaku Miniflex 600. The morphology of the mineral grains in the sample was analyzed using the scanning electron microscope (SEM) with energy dispersive X-ray spectroscopy (EDS) Phenom ProX G5 by Thermo Scientific.
Bulk samples were prepared through crushing, drying, and grinding to obtain gold ore with a grain size > 75 μm (Amankwah & Pickles, 2009; Cho et al., 2020; Nanthakumar et al., 2007; F. Zhu et al., 2018). The crushing was carried out using a laboratory jaw crusher and grinding using a BICO Mill BM-1, Braun International, USA. Sampling and sieving were performed using a rotary sample divider and sieve shaker Retsch AS200Tap, Germany. The elemental gold content of the prepared bulk sample was determined by the fire-assay method (Amankwah & Pickles, 2009; Cho et al., 2020; Nanthakumar et al., 2007; F. Zhu et al., 2018) and measured by Atomic Absorption Spectrophotometer (AAS) Agilent FS 240. Elements other than gold in the sample were analyzed by the X-ray fluorescence (XRF) method (H. Li et al., 2018; Nheta et al., 2020; Wang et al., 2019). In this study, the Rigaku NEXCG XRF was used for elements analysis.
A prepared refractory gold ore of 50 g was added and mixed with 180 kg/ton of NaClO3 in the gold ore. Distilled water of 180 kg/ton of gold ore was added and remixed until it achieved homogeneity. The mixture was then granulated.
2.3 Microwave-assisted roasting
Prepared gold ore samples were placed into a fire clay crucible and then heated in a microwave oven for 30 min (F. Zhu et al., 2018), with a microwave power between 300 and 800 W (Amankwah & Ofori-Sarpong, 2020). The roasting process used a household microwave, Sharp R-753GX, China, with a frequency of 2.45 GHz and power up to 1000 W with 100-watt intervals. The roasting process flow and equipment scheme were modified from previous research (Amankwah & Ofori-Sarpong, 2020; Choi et al., 2017; Su et al., 2011).
Furthermore, the temperature measurement was carried out shortly after roasting was completed. The temperature generated from microwaves was measured with an infrared thermometer KIMO Kiray 300, Francis. The roasted samples were cooled and weighed to determine their weight after roasting. Four grams of roasted samples were leached with distilled water at 50 °C for 60 min. Samples from roasting and residue from water leaching were subjected to chemical and mineralogy analysis. Water leaching aimed to determine the level of decomposition of sulfide minerals through the analysis of metal extracted in water.
Changes in mineral surface morphology before and after pretreatment were investigated by scanning electron method using the SEM Desktop Scanning Electron Microscope (Phenom ProX G5 by Thermo Scientific). The thermodynamic analysis of the roasting reaction of sulfide minerals was carried out with the help of the HSC Chemistry 8 software from Outotec.
During roasting, sulfur dioxide, chlorine, and arsenic compounds were released into the atmosphere. During water leaching, elements such as Fe, Cu, Zn, S, and Cl dissolved into a solution. The weight loss of roasted and leached ore (residue) was compared to their initial weight. The weight loss on the roasting and water-soluble part during water leaching was determined by the formula:
: Weight of mixed ore and additive before roasting.
: Weight of roasted ore
: Weight of residue after water leaching.
The fraction by weight of an element released into the atmosphere during roasting was calculated by the formula:
: Elemental weight of initial sample (before roasting).
: Elemental weight of the sample after roasting.
: Elemental weight released into the atmosphere during roasting.
Elemental weight fraction in residue, calculated by the formula:
: Elemental weight in residue after leaching.
The oxidation and decomposition of sulfide minerals were studied by analyzing the weight loss during roasting and extraction from roasted minerals during water leaching. Element extraction was calculated using the formula:
The mass balance of the elements in roasting and water leaching was calculated by the formula:
2.4 Leaching experiment
Leaching aims to compare gold extraction rates from roasted and unroasted gold ores. Leaching was carried out using a 1000-ml three-neck flask with 100 mL 3 M HCl as solvent and 5 % (w/v) NaClO3 oxidizer at a temperature of 40 °C. NaClO3 5 % (w/v) was added at a feed rate of 0.25 mL/minute. After the NaClO3 feeding was complete, leaching continued for 5 min. After that, the residue is immediately separated from the pregnant leach solution (PLS). The Au content in the solution was analyzed using AAS.
Gold extraction rate is calculated using a formula:
: Weight of Au in PLS.
: Weight of Au in gold ore (roasted gold ore).
3 Results and discussion
3.1 Refractory gold ore characteristics
Mineralogical characteristics and surface morphology of the refractory gold ore sample are shown in Fig. 1 - Fig. 3. The optical mineralogical analysis is presented in Fig. 1. The sample contains chalcopyrite (Ch), covellite (Cv), galena (Ga), hematite (He), pyrite (Py), and sphalerite (Sp). The presence of these sulfide minerals was confirmed in previous studies (Arif et al., 2020; Purwanto et al., 2020; Yuningsih et al., 2012). Fe2O3 and PbO were confirmed from the scanning results with SEM (Fig. 2). Pyrite is the dominant mineral with a relatively large particle size. Chalcopyrite, covellite, galena, hematite, and sphalerite are relatively small, and some are encapsulated in pyrite grains.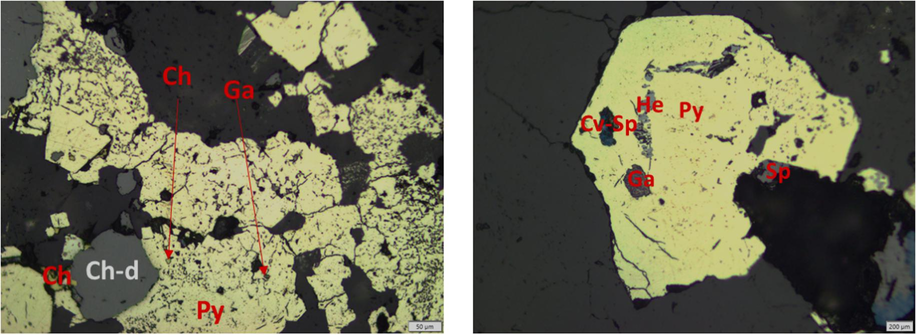
Optical images of refractory gold ore, Ch (chalcopyrite - CuFeS2), Ch-d (chalcopyrite disease), Cv (covellite - CuS), Ga (galena - PbS), He (hematite – Fe2O3), Py (pyrite – FeS2), Sp (sphalerite - ZnS).
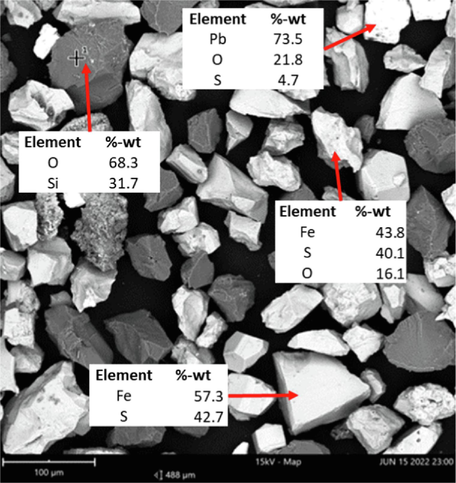
SEM image and EDS mapping of refractory gold ore.
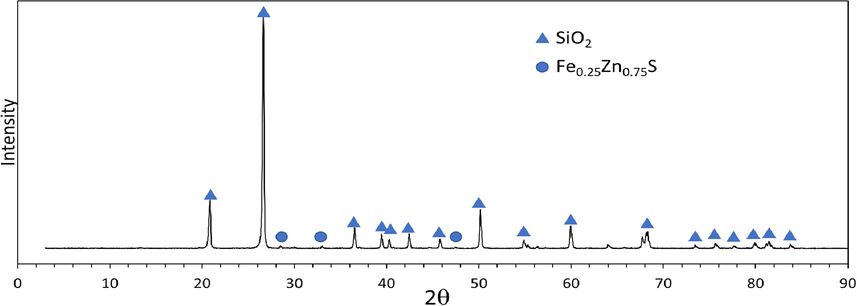
XRD pattern of refractory gold ore.
SEM analysis also showed that iron (Fe) and lead (Pb) in refractory gold ore formed sulfide minerals and some in the form of oxide minerals. XRD analysis (Fig. 3) identified two main mineral compounds: silicone dioxide (SiO2) and the mineral sphalerite associated with iron, forming the Fe0.25Zn0.75S complex.
The chemical composition of the gold ore samples is presented in Table 1. The Au content is relatively low at 2.13 ppm, and Ag is 35.05 ppm. Previous studies have shown that the gold content in the ore varies between 0.04 and 2.18 ppm (Purwanto et al., 2020). Fe content is the highest metal element, with 22.75 ppm (2.28 %) and a total sulfur content of 13.95 ppm (1.40 %). Based on the S content in the gold ore, it can be concluded that it belongs to the low-refractory gold ore (Arif et al., 2020; Purwanto et al., 2020).
Elements
Fe
Cu
Al
Pb
Zn
S
Au
Ag
Content [ppm]
22,750
1,065
14,650
2,995
3,925
13,950
2.13
35.05
3.2 Effect of microwave power on temperature and weigh loss of roasted ore
Temperature strongly influenced the oxidation of sulfide minerals in roasting. In general, the higher temperature generated a faster roasting process. The study of the effect of microwave power on temperature is presented in Fig. 4a. The higher the microwave power resulted in the higher roasting temperature. This study showed a significant difference in temperature rise due to the presence of additives and water during the roasting process. The roasting of wet pellets with water 180 kg/ton ore and NaClO3 180 kg/ton ore resulted in a higher temperature rise than the others. Roasting wet pellets without NaClO3 also showed a reasonably good response. The addition of NaClO3 as an oxidizing agent also responded well to microwaves, resulting in higher temperatures.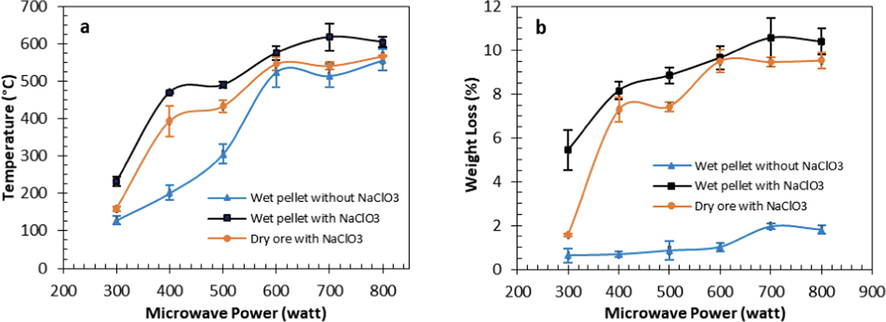
Effect of microwave power on (a) temperature and (b) weight loss during roasting; wet pellet (water 180 kg/ton ore), NaClO3 180 kg/ton ore and roasting time 30 menit.
The temperature generated during microwave heating is significantly influenced by the mineral types of responses to microwaves. The refractory gold ore used in this study primarily consists of pyrite, with smaller quantities of chalcopyrite, sphalerite, and galena. Previous research reported that pyrite and chalcopyrite minerals generated temperatures of 670 °C and 780 °C when heated at 900 W for 1 min (Lovas et al., 2011). During the roasting process, transforming sulfide minerals into oxide minerals altered their response to microwaves. Hematite gave a temperature response of 118 °C when heated with a power of 900 W for 1 min (Lovas et al., 2011), while heating with a power of 500 W for 4 min generated a temperature of > 980 (Haque, 1999). In the current work, it was observed that heating at 800 W generated lower temperatures than at 700 W. The generated temperature was related to the decomposition of sulfide minerals into oxides, especially the change of pyrite into hematite, where hematite responded less well to very high microwave power (Lovas et al., 2011).
The increased temperature affected weight loss during roasting, as presented in Fig. 4b. This loss indicated a decomposition reaction of sulfide minerals. Weight loss occurs due to the release of gases formed during roasting. Roasting with NaClO3 (wet and dry) resulted in a higher weight loss than roasting without additives. The generated temperature were 129 °C for wet pellets without NaClO3, 232 °C for wet pellets with NaClO3, and 159 °C for dry ore with NaClO3 at 300 W. The resulting weight loss is 0.62 %, 5.4 % and 1.6 % respectively. At a power temperature of 700 W, weight loss reached 10.6 % because the roasting temperature achieved the highest, 618 °C.
Weight loss in roasting without NaClO3 was due to the release of sulfur during oxidation. The reactions include releasing SO2 gas through the reaction Eq. (9) – (14). The roasting process without aeration resulted in a low quantity of O2 in the sample; consequently, the emission of SO2 was relatively low. The low release of SO2 causes the weight loss in roasting without additives to be relatively low. The release of SO2 and Cl2 during roasting with NaClO3 caused high weight loss. The release of SO2 and Cl2 must be kept as low as possible to reduce the environmental impact.
Weight loss in both wet pellets (wet roasting) and dry ore (dry roasting) with NaClO3 may have occurred due to the reaction of released Cl2 and SO2, referring to Eq. (9) - (14), Eq.(22) and Eq. (23). The decomposition of NaClO3 at temperatures higher than 265 °C produced O2 gas, Eq. (15) which reacted with sulfide minerals as shown in Eq. (9) – (14).
3.3 Role of additive on metal extraction
The role of additives and the effect of microwave power on the decomposition of sulfide minerals in gold ore can be observed from the extraction rate of soluble metals during the water leaching of roasted ore. The results of this study can be seen in Fig. 5. Roasting without NaClO3 with limited O2 content resulted in relatively low metal extraction. Meanwhile, roasting with NaClO3 using dry and wet pellets resulted in higher metal extraction.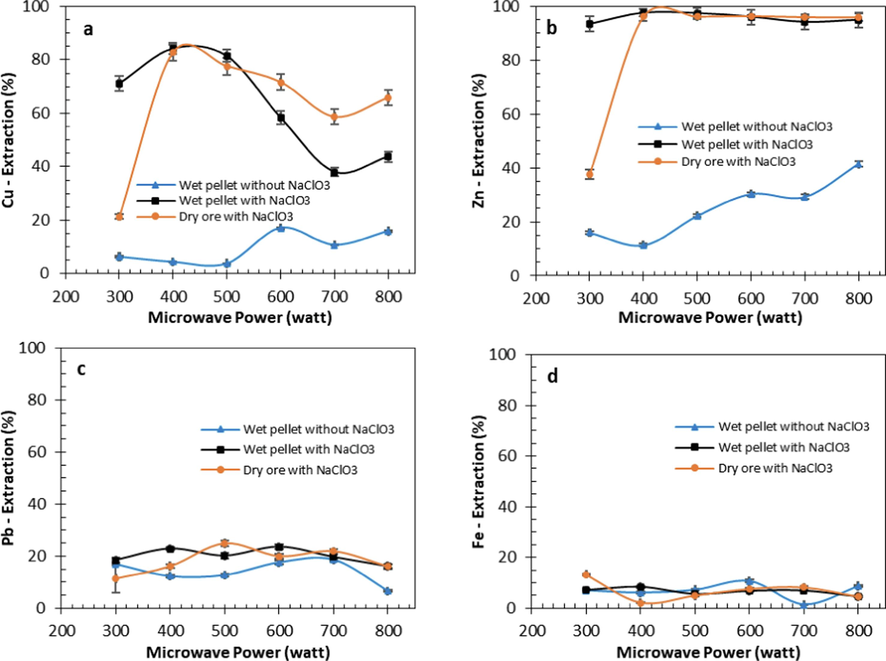
Effect of microwave roasting power on metal extraction during water leaching at 50 °C, 250 rpm and leaching time 60 min; wet pellet (water 180 kg/ton ore), NaClO3 180 kg/ton ore (a) copper, (b) zinc, (c) lead, (d) iron.
In roasting without NaClO3, the extraction of Cu and Zn was relatively low. The minimum amount of oxidizer in roasting without NaClO3 and aeration caused the oxidation reaction of CuFeS2 and ZnS to be less than optimal. In addition, the oxidation reaction without additives also produced oxide compounds (ZnO, CuS, and Fe2O3), which were difficult to dissolve in water leaching. The oxidation reactions without NaClO3 are indicated in equations Eq. (9) and Eq. (10).
Extraction of Cu and Zn reached 84.3 % and 97.6 %, respectively, when roasting with NaClO3, both wet pellets and dry gold ore, at a power of 400 W or more for 30 min. Copper and zinc dissolved in water leaching increased significantly with microwave power from 300 to 400 W (Fig. 5a and Fig. 5b). Decomposition reaction of copper sulfide minerals is shown in Eq. (16) and Eq. (17). In comparison, oxidation of zinc sulfide corresponds to Eq. (18) and Eq. (19). At microwave power higher than 400 W, copper extraction rate decreased (Kamariah et al., 2022). High temperatures at a power of more than 400 W cause the decomposition of CuSO4 compounds into oxide compounds at 540 – 730 °C, which were difficult to dissolve in water (Tanaka & Koga, 1990).
In the water leaching of roasted gold ore at 800 W (Fig. 5a), Cu extraction was slightly higher than at 700 W. This difference can be attributed to the lower temperature generated at 800 W compared to 700 W. CuSO4 undergoes decomposition at high temperatures ranging from 540 °C to 730 °C, resulting in the formation of copper oxide. At 800 W with a temperature of 605 °C, the decomposition of CuSO4 into copper oxide was less extensive compared to 700 W with a temperature of 618 °C, resulting in higher Cu extraction at 800 W than at 700 W.
At a power higher than 400 W, zinc extraction was as high as the extraction at a power lower than 400 W (Kamariah et al., 2022). The thermal decomposition of ZnSO4 was higher than 800 °C (Ingraham & Marier, 1967; Narayan et al., 1988).
The extraction of Pb and Fe was relatively low compared to the Cu and Zn extraction for all conditions. The increase in microwave power from 300 to 800 W did not affect Pb and Fe extraction. The highest Pb extraction was 24.8 % and 22.8 % for roasting with NaClO3 using dry and wet roasting methods. The highest Fe extraction reached 13 % in dry roasting with NaClO3. Previous research also showed that the extraction of Pb and Fe was relatively low compared to the extraction of Cu and Zn (Kamariah et al., 2022). Oxidation of iron and lead from sulfide minerals formed PbO Eq. (11) and Eq. (20), PbSO4 Eq. (21), and Fe2O3 Eq. (12) or Fe3O4 Eq. (14). These compounds had low solubility during water leaching. Low iron extraction was also due to some of the iron minerals in refractory gold ore samples as oxide minerals such as hematite, magnetite, and in the form of limonite (Arif et al., 2020; Purwanto et al., 2020).
The formation of FeCl3 Eq. (22) led to an increase in Fe extraction during water leaching. However, FeCl3 decomposed at a relatively low temperature of 400 °C, and formed Fe2O3 (Kanungo & Mishra, 1996). In contrast, the formation of FeSO4 Eq. (22) and Fe2(SO4)3 Eq. (23) and Eq. (24) remained relatively stable at high-temperature roasting, as the decomposition temperatures for FeSO4 Eq. (23) were in the range of 525 – 650 °C and for Fe2(SO4)3, they were in the range of 625 – 710 °C (Masset et al., 2006).
In the current study, extraction of Cu, Zn, Pb, and Fe respectively reached 84.3 %, 97.6 %, 22.8 %, and 8.4 % in roasting with wet pellets of refractory gold ore, water 180 kg/ton ore, NaClO3 180 kg/ton ore, 400 W (470 °C) for 30 min. Previous research showed that the extraction of Cu, Zn, Pb, and Fe was ±80 %, ±65 %, ±15 %, and ±25 %, respectively, at roasting temperatures of 400 – 550 °C for 60 min (Kamariah et al., 2022). Current research showed a higher extraction rate of metals such as Cu, Zn, and Pb at shorter roasting times.
3.4 SEM analysis of roasted ore and residue
SEM analysis was carried out to study the effects of roasting and water leaching on the mineral surface morphology and determine the mineral decomposition during the pretreatment process. Sulfur and oxygen contents in the sulfide mineral before roasting were 4.7 – 42.7 % and 16.1 – 21.8 %, respectively. The sulfur content in roasted ore particles was identified to be between 1.6 – 19.8 %, while the oxygen content was 23.8 – 46.3 %. Sulfur content decreased, and oxygen increased due to the oxidation of sulfide minerals and phase changes from sulfides to oxides (Fig. 6). SEM analysis confirmed cracks formation in pyrite and sphalerite-pyrite minerals. SEM-EDS mapping analysis of sphalerite mineral grains and a small portion of covellite showed that the sulfur content was still relatively high and had not undergone decomposition.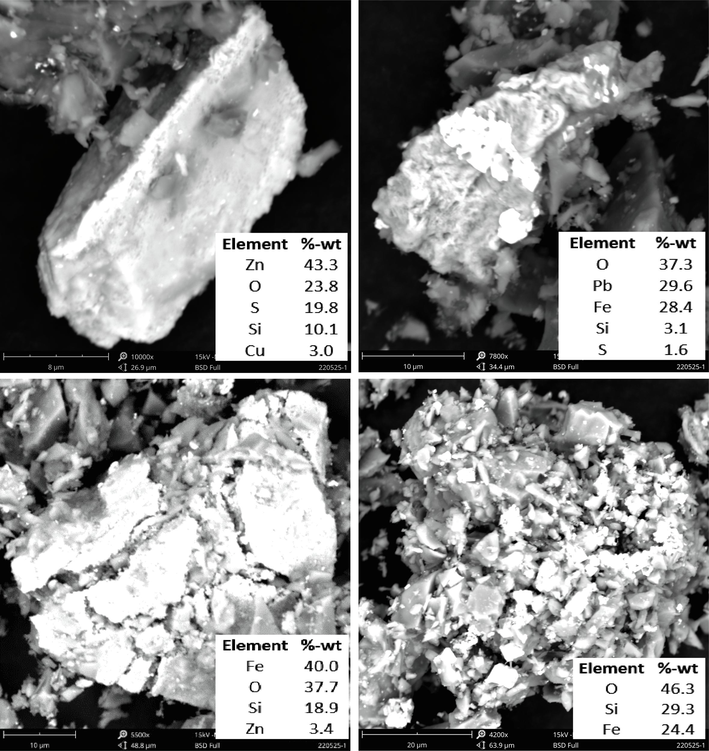
Morphology and EDS of roasted gold ore (wet pellet, water 180 kg/ton ore, without NaClO3, microwave power 700 W and roasting time 30 min).
SEM analysis of roasted ore showed that the sulfur content in sulfide mineral decreased significantly to 1.7 % – 4.2 %, while oxygen increased to 16.3 % − 41.2 % at 700 W with NaClO3 for 30 min roasting time, as shown in Fig. 7. Elements of Na and Cl derived from additives were still identified in mineral grains even though their levels have decreased relatively compared to the initial content of Na and Cl. The grain surface morphology, with no visible micro-cracks, looks different compared to roasting without additives. The identification results with EDS mapping of roasted grain with NaClO3 at 700 W identified the presence of Fe2O3, Fe2(SO4)3, NaCl and Na2SO4 compounds.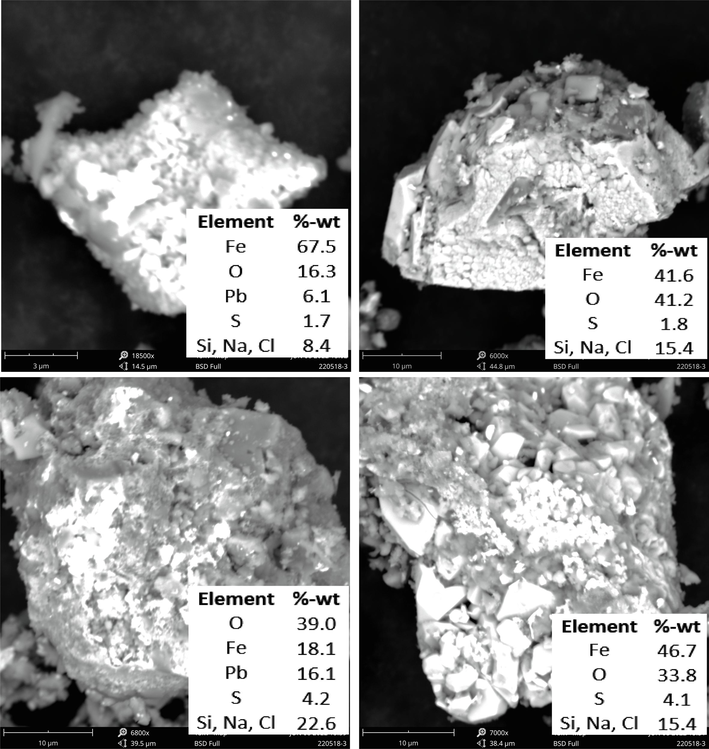
Morphology and EDS of roasted ore (wet pellet, water 180 kg/ton ore, NaClO3 180 kg/ton ore, microwave power 700 W and roasting time 30 min).
The water leaching test has been studied and presented in Fig. 8. SEM analysis of water leaching samples showed that sulphur was not identified as mineral grains. The decreasing sulphur content was due to the dissolving of sulphate compounds during the water leaching process. SEM analysis of residue also showed that the remaining sulphur in roasted ore with additives was a sulphate compound. The sulphate compounds were easily extracted during water leaching. EDS mapping also showed that the compounds identified in the grains were Fe2O3.
Morgphology and EDS of residue after water leaching (wet pellet with water 180 kg/ton ore, NaClO3 180 kg/ton ore, microwave power 700 W, and roasting time 30 min), leaching temperatur 50 °C for 60 min.
3.5 Mass balance analysis of sulfur and chlorine in roasted ore and residue
The released sulfur gases such as SO2 due to the oxidation of sulfide minerals is a critical problem in the roasting of refractory gold ores. The sulfur release was calculated by mass balance analysis of sulfur in microwave-assisted roasting. The sulfur release was strongly influenced by temperature and oxygen concentration in the roasting. This research also studied the release of chlorine gas due to using NaClO3 as an oxidizing agent. The mass balance analysis of sulfur and chlorine is shown in Fig. 9 and Fig. 10.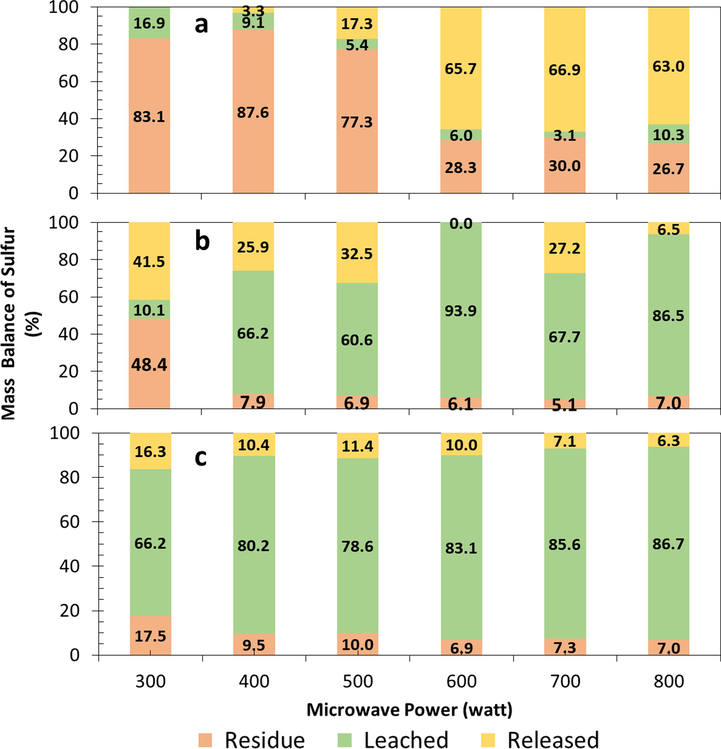
Sulfur mass balance in the roasting and water leaching process: (a) wet pellet with water 180 kg/ton ore, and without additive, (b) dry ore with NaClO3 180 kg/ton ore, (c) wet pellet with water 180 kg/ton ore and NaClO3180 kg/ton ore.
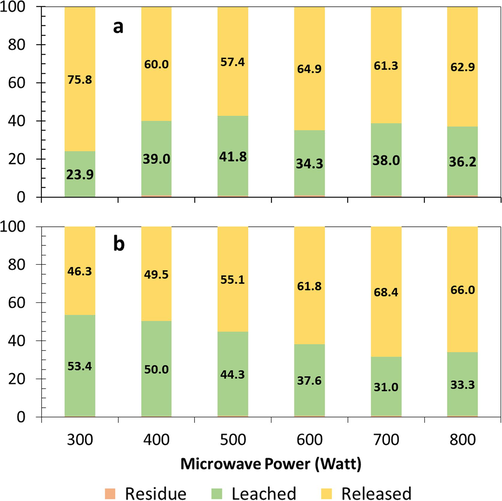
Chlorine mass balance during the roasting and water leaching process dry ore with NaClO3 180 kg/ton ore, (b) wet pellet with water 180 kg/ton ore and NaClO3180 kg/ton ore.
The mass balance analysis of sulfur on roasting without NaClO3 (Fig. 9a) indicated that the higher microwave power resulted in the higher sulfur release. The sulfur release increased in correlation with the roasting temperature (Fig. 4). The sulfur release was measured in roasting at 400 W or higher. The highest sulfur release was achieved at 700 W, reaching 66.9 %. A minimal sulfur dissolved, with majority remaining in the residue and not dissolving during the water leaching. Sulfur in residue reached more than 77 % in roasting without NaClO3 at 300 – 500 W for 30 min. Roasting without NaClO3 corresponds to Eq. (9) - Eq. (14). Water had no role in the wet roasting process because the water evaporated before the decomposition of sulfide minerals at temperatures above 100 °C.
The mass balance analysis of sulfur on roasting refractory gold ore with 180 kg/ton NaClO3 and dry method is presented in Fig. 9b. Sulfur release occurred at a low power of 300 W, reaching 41.5 % and then decreased with increasing microwave power. Sulfur release in dry roasting with NaClO3 was lower than in wet roasting without NaClO3. Sulfur release in dry roasting with NaClO3 is shown in reactions Eq. (16), Eq. (18), Eq. (20), Eq. (22), and Eq. (23). Sulfur dissolved when water leaching reached above 60 % in roasting with a power of more than 400 W. In contrast, roasting without additives only achieved an average lower than 10 %. Water-soluble sulfur was very high at the roasting power of 400 W, resulting in deficient sulfur remaining in the residue, ≤7.9 %. This dissolved sulfur indicates that there has been a decomposition of sulfide minerals into compounds that are soluble in water (Amankwah & Ofori-Sarpong, 2020; Ma et al., 2016; Qin, Guo, Tian, Yu, et al., 2021).
The mass balance analysis of sulfur on roasting refractory gold ore with 180 kg/ton NaClO3 additive and wet method is presented in Fig. 9c. The highest sulfur release was only 16.3 % and tended to decrease with increasing microwave power. Wet roasting with additives is shown in reactions Eq. (17), Eq. (19), Eq. (21), and Eq. (24). Sulfur release in wet roasting was lower than in dry roasting. The addition of water played a role in reducing the release of sulfur into the atmosphere during roasting. In addition, water played a role in increasing sulfur dissolution during water leaching. This phenomenon indicated the higher formation of sulfate compounds in wet roasting compared to dry roasting.
Wet roasting of refractory gold ore with NaClO3 reduces the sulfur release by only 10.4 %, and the sulfur that dissolves during water leaching reaches 80.2 % at 400 W of power for 30 min. The temperatures generated in the 400-watt on wet and dry roasting were 470 and 394 °C, respectively. The total decomposition of sulfur reached 90.6 %. Whereas in dry conditions, the total decomposition of sulfur reached 93.1 %, and the release of sulfur was 25.9 %. Microwave roasting results were higher than conventional processes, carried out at 550 °C with low aeration, and only reduced the sulfur content by 50 % (De Michelis et al., 2013) or lower than 50 % (Dosmukhamedov et al., 2022). Some previous studies mentioned that the optimum roasting temperature of refractory gold ore was between 500 – 700 °C (González et al., 2021; F. Lin et al., 2021; Y. Lin et al., 2022; Qin, Guo, Tian, Yu, et al., 2021; Sousa et al., 2022). Additives played a role in increasing the decomposition of mineral sulfides (H. Li et al., 2018; F. Zhu et al., 2018). In the current research, NaClO3 additives and water also reduced the release of sulfur into the atmosphere.
Oxidation of sulfur from sulfide minerals significantly influences the degree of extraction in gold ore leaching. High gold extraction is achieved at high degrees of sulfur oxidation. Sulfur oxidation correlated linearly with gold extraction during leaching (Ahtiainen et al., 2021; Faraz et al., 2014). The degree of sulfur oxidation in the current study was more than 90 %. The results of this oxidation are expected to increase the degree of gold extraction by more than 90 % during the leaching process.
Chlorine release was one of the focuses of attention in this study due to the use of NaClO3 as an oxidizing agent. Mass balance analysis of chlorine is shown in Fig. 10. Dry roasting of gold ore (Fig. 10a) produced high chlorine release, between 57.4 % − 75.8 %. Roasting of wet pellets of gold ore released chlorine between 46.3 % − 68.4 %, as shown in Fig. 10b. Wet roasting released less chlorine than dry roasting. In wet roasting, the increase in power caused a relatively high increase in chlorine release. Water contained in wet roasting significantly reduced the level of chlorine released into the atmosphere.
The decomposition of sulfide minerals with the addition of NaClO3 in wet roasting involved chemical reactions that release chlorine (Eq.22 and Eq.23) or those that do not release chlorine Eq. (16) - Eq. (21), Eq. (24). Sulfur and chlorine mass balance analysis showed that wet roasting produced low sulfur and chlorine releases with a high sulfur decomposition, 90.6 % at 400 W of power for 30 min. Previous studies mentioned that the oxidation of sulfide mineral by more than 90 % in pretreatment could increase gold extraction by more than 90 % in the leaching of refractory gold ore (Ahtiainen et al., 2021; Faraz et al., 2014).
3.6 Leaching of roasted gold ore
The leaching tests showed that the oxidation degree of sulfide minerals greatly influenced the extraction of gold in leaching, as presented in Fig. 11. Direct leaching of refractory gold without pretreatment resulted in 47.5 % gold extraction. Previous researchers reported that gold extraction was relatively low when leaching was carried out directly on refractory gold without pretreatment (Celep et al., 2019; Qin, Guo, Tian, Yu, et al., 2021; Salazar-Campoy et al., 2020). Non-cyanide leaching research conducted by L. Zhang in 2022 on weakening refractory gold without pretreatment only resulted in 7.6 % gold extraction (L. Zhang, Guo, Tian, Zhong, Li, et al., 2022).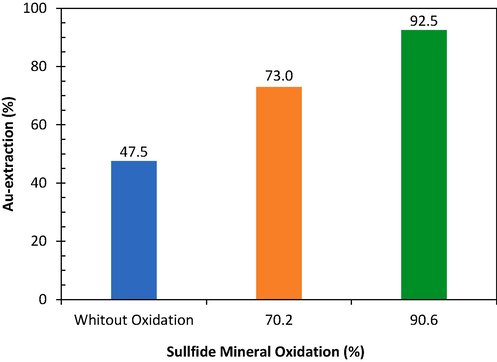
Effect of sulfide mineral oxidation on Au extraction in acid leaching with 100 mL HCl 3 M, 14 mL NaClO3 5 % (w/v), 0.25 mL/min NaClO3 feeding rate, 250 rpm, liquid–solid ratio 10 at leaching temperature 40 °C.
The leaching of roasted gold ore with 70.2 % and 90.6 % sulfide mineral oxidation resulted in gold extraction of 73.0 % and 92.5 %, respectively. Previous research reported that the oxidation of sulfide minerals greatly determined the extraction of gold from refractory extraction. Sulfide oxidation of 48.4 % and 95.0 % resulted in gold extraction of 71.1 % and 95 % (Faraz et al., 2014). Other researchers reported that 97 % oxidation of sulfide minerals achieved 99 % gold extraction (Ahtiainen et al., 2021).
The results of the leaching test on roasted gold ore showed that pretreatment is an essential stage in the processing of refractory gold ore. Gold extraction can be increased significantly from 47.5 % without pretreatment to 92.5 % after pretreatment with 90.6 % sulfide mineral oxidation.
4 Conclusions
Wet roasting with water 180 kg/ton ore and without NaClO3 at 300 – 800 W for 30 min increased the temperature from 129 to 557 °C. Total oxidized sulfur reached 12.4 % and 73.3 % at 400 and 800 W for 30 min, respectively. The sulfur release reached 63.0 % on roasting with 800 W for 30 min.
Wet roasting with water 180 kg/ton and NaClO3 180 kg/ton ore at 300 – 800 W for 30 min increased the temperature from 189 to 620 °C. Total oxidized sulfur reached 90.6 % and 93.0 % at 400 and 800 W for 30 min, respectively. The sulfur release reached 10.4 % and 6.3 %, and chlorine release reached 49.5 % and 66.0 % at 400 and 800 W for 30 min, respectively.
Dry roasting with NaClO3 180 kg/ton ore at 300 – 800 W for 30 min increased the temperature from 159 to 567 °C. Total oxidized sulfur reached 92.1 % and 93.0 % at 400 and 800 W for 30 min, respectively. The sulfur release was at 25.9 % and 6.5 %, and chlorine at 60 % and 62.9 %, at 400 and 800 W for 30 min, respectively.
In the current study, extraction of Cu, Zn, Pb, and F reached 84.3 %, 97.6 %, 22.8 %, and 8.4 %, respectively, for wet roasting of refractory gold ore, with 180 kg/ton ore, NaClO3 180 kg/ton ore, 400 W for 30 min.
Direct leaching of refractory gold without pretreatment resulted in gold extraction of 47.5 %. In contrast, leaching roasted gold ore with 90.6 % oxidation of sulfide minerals results in gold extraction of 92.5 %.
Pretreatment of refractory gold ore with microwave-assisted roasting methods requires optimization to reduce the release of sulfur and chlorine into the atmosphere, reducing roasting time. The combination of microwave power and time is critical to increasing the efficiency of the roasting process.
Acknowledgement
The authors are grateful to PT ANTAM for providing refractory gold ore samples for this study and to the Ministry of Education, Culture, Research, and Technology for financial support (Penelitian Disertasi Doktor 2022).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Methods of ore pretreatment for comminution energy reduction. Minerals. 2020;10(5)
- [CrossRef] [Google Scholar]
- Simultaneous sulfide oxidation and gold dissolution by cyanide-free leaching from refractory and double refractory gold concentrates. Miner. Eng.. 2021;170
- [CrossRef] [Google Scholar]
- Microwave heating of gold ores for enhanced grindability and cyanide amenability. Miner. Eng.. 2011;24(6):541-544.
- [CrossRef] [Google Scholar]
- Microwave roasting of flash flotation concentrate containing pyrite, arsenopyrite and carbonaceous matter. Miner. Eng.. 2020;151
- [CrossRef] [Google Scholar]
- Microwave roasting of a carbonaceous sulphidic gold concentrate. Miner. Eng.. 2009;22(13):1095-1101.
- [CrossRef] [Google Scholar]
- Amdur, A. M., Fedorov, S. A., & Matushkina, A. N. (2022). Extraction of gold from definitely processing ores and technogenic waste by their high-temperature treatment and subsequent centrifugal separation. Mining Informational and Analytical Bulletin, 11–1, 95–106. 10.25018/0236_1493_2022_111_0_95.
- Karakteristik Mineralisasi Endapan Epitermal Pada Prospek Arinem. Geomine. 2020;8(3):193-202.
- [Google Scholar]
- Refractory gold ores and concentrates part 1: mineralogical and physico-chemical characteristics. Miner. Process. Extract. Metall.: Trans. Inst. Mining Metall.. 2021;130(3):240-252.
- [CrossRef] [Google Scholar]
- Optimization of the combined beneficiation scheme and increase in the performance of highly efficient refractory gold-bearing ores under development. IOP Conf. Ser.: Mater. Sci. Eng.. 2021;1047(1)
- [CrossRef] [Google Scholar]
- Theory and applications of microwave energy in communition. Key Eng. Mater.. 2004;264–268(II):1399-1402.
- [CrossRef] [Google Scholar]
- The novel use of microalgae in the greening of the metallurgical industry. J. Ecol. Eng.. 2023;24(1):299-306.
- [CrossRef] [Google Scholar]
- Bıyıklı, B., Sevgül, S., & Dündar, H. (2022). The Effect of Fine Grinding on Cyanide Leaching of Gold Mine Tailings. Proceedings of the 27th International Mining Congress and Exhibition of Turkey, IMCET 2022, 895–903.
- The influence of ore grade decline on energy consumption and GhG emissions: The case of gold. Environ. Develop.. 2022;41(November 2021):1-11.
- [CrossRef] [Google Scholar]
- Silver metal extraction from refractory silver ore using chloride-hypochlorite and hydrochloric acid media under high pressure. Mining Metall. Explor.. 2021;38(2):1215-1223.
- [CrossRef] [Google Scholar]
- Improvement of silver extraction by ultrafine grinding prior to cyanide leaching of the plant tailings of a refractory silver ore. Miner. Process. Extr. Metall. Rev.. 2015;36(4):227-236.
- [CrossRef] [Google Scholar]
- Ultra fine grinding of silver plant tailings of refractory ore using vertical stirred media mill. Trans. Nonferrous Metals Soc. China (English Ed.). 2013;23(11):3412-3420.
- [CrossRef] [Google Scholar]
- Effect of ultra-fine grinding on extraction of gold and silver from a refractory flotation tailings by cyanide leaching. Int. Mining Congr. Exhibit. Turkey 2019:826-833.
- [Google Scholar]
- Effect of initial cell concentration on bio-oxidation of pyrite before gold cyanidation. Minerals. 2021;11(8)
- [CrossRef] [Google Scholar]
- Fast and effective gold leaching from a desulfurized gold ore using acidic sodium chlorate solution at low temperature. Ind. Eng. Chem. Res.. 2013;52(47):16622-16629.
- [CrossRef] [Google Scholar]
- Recovery of gold from the refractory gold concentrate using microwave assisted leaching. Metals. 2020;10(5)
- [CrossRef] [Google Scholar]
- Microwave pretreatment for thiourea leaching for gold concentrate. Metals. 2017;7(10)
- [CrossRef] [Google Scholar]
- Ultra fine milling for the recovery of refractory gold. Miner. Eng.. 1991;4(7–11):763-776.
- [CrossRef] [Google Scholar]
- Roasting and chlorine leaching of gold-bearing refractory concentrate: Experimental and process analysis. Int. J. Min. Sci. Technol.. 2013;23(5):709-715.
- [CrossRef] [Google Scholar]
- Chlorination treatment for gold extraction from refractory gold-copper-arsenic-bearing concentrates. Sustainability (Switzerland). 2022;14(17):1-14.
- [CrossRef] [Google Scholar]
- Near-technical limit gold recovery from a double refractory carlin-type ore after pre-treatment by high-temperature pressure oxidation. Mining Metall. Explor.. 2022;39(4):1563-1570.
- [CrossRef] [Google Scholar]
- Improved recovery of a low-grade refractory gold ore using flotation-preoxidation-cyanidation methods. Int. J. Min. Sci. Technol.. 2014;24(4):537-542.
- [CrossRef] [Google Scholar]
- Life cycle analysis of copper-gold-lead-silver-zinc beneficiation process. Sci. Total Environ.. 2019;659:41-52.
- [CrossRef] [Google Scholar]
- Synergistic extraction of gold from the refractory gold ore via ultrasound and chlorination-oxidation. Ultrason. Sonochem.. 2017;37:471-477.
- [CrossRef] [Google Scholar]
- A review on alternative gold recovery reagents to cyanide. J. Mater. Sci. Chem. Eng.. 2016;04(08):8-17.
- [CrossRef] [Google Scholar]
- González, J. A. V., Rodríguez, H. R. P., Norberto Damián Ñique Gutierrez, Silva, L. A., & Zavaleta, N. E. (2021). Treatment of a carbonaceous gold ore by oxidative leaching-roasting and direct roasting. Proceedings of the LACCEI International Multi-Conference for Engineering, Education and Technology. 10.18687/LEIRD2021.1.1.39.
- Gold recovery optimization of a refractory concentrate by ultrafine grinding - A laboratory study. Miner. Metall. Process. 2011;28(2):95-101.
- [CrossRef] [Google Scholar]
- Gui, Q., Fu, L., Hu, Y., Di, H., Liang, M., Wang, S., & Zhang, L. (2023). Gold extraction using alternatives to cyanide: Ultrasonic reinforcement and its leaching kinetics. Minerals Engineering, 191(May 2022), 107939. 10.1016/j.mineng.2022.107939.
- The mechanism of ultrasound oxidation effect on the pyrite for refractory gold ore pretreatment. Arab. J. Chem.. 2021;14(4)
- [CrossRef] [Google Scholar]
- Mechanism of synergistic pretreatment with ultrasound and ozone to improve gold and silver leaching percentage. Appl. Surf. Sci.. 2022;576
- [CrossRef] [Google Scholar]
- Selective removal of antimony from refractory gold ores by ultrasound. Hydrometall.. 2019;190
- [CrossRef] [Google Scholar]
- Microwave energy for mineral treatment processes - A brief review. Int. J. Miner. Process.. 1999;57(1):1-24.
- [CrossRef] [Google Scholar]
- Leaching kinetics of antimony from refractory gold ore in alkaline sodium sulfide under ultrasound. Chem. Eng. Res. Des.. 2020;164:219-229.
- [CrossRef] [Google Scholar]
- Kinetics of the thermal decomposition of znso4 and zno·2znso4. Can. Metall. Q.. 1967;6(3):249-261.
- [CrossRef] [Google Scholar]
- The energy needed to concentrate minerals from common rocks: The case of copper ore. Energy. 2019;181:494-503.
- [CrossRef] [Google Scholar]
- Conventional versus microwave-assisted roasting of sulfidic tailings: Mineralogical transformation and metal leaching behavior. Miner. Eng.. 2022;183(May):107587
- [CrossRef] [Google Scholar]
- Thermal dehydration and decomposition of FeCl3·xH2O. J. Therm. Anal.. 1996;46(5):1487-1500.
- [CrossRef] [Google Scholar]
- Chlorate-based oxidative hydrometallurgical extraction of copper and zinc from copper concentrate sulfide ores using mild acidic conditions. Hydrometall.. 2009;96(1–2):72-76.
- [CrossRef] [Google Scholar]
- Pressure oxidation of arsenopyrite and pyrite in alkaline solutions. Hydrometall.. 1992;30:87-106.
- [Google Scholar]
- Efficiency of ozone application for extraction of metals from mineral raw materials. Russ. J. Non-Ferrous Metals. 2022;63(3):247-255.
- [CrossRef] [Google Scholar]
- A review on the negative impact of different elements during cyanidation of gold and silver from refractory ores and strategies to optimize the leaching process. Miner. Eng.. 2021;173
- [CrossRef] [Google Scholar]
- Toward sustainable solution for biooxidation of waste and refractory materials using neutrophilic and alkaliphilic microorganisms - A review. ACS Appl. Bio Mater.. 2021;4(3):2274-2292.
- [CrossRef] [Google Scholar]
- Enhanced gold recovery from alkaline pressure oxidized refractory gold ore after its mechanical activation followed by thiosulfate leaching. J. Sustain. Metall.. 2022;8(1):186-196.
- [CrossRef] [Google Scholar]
- Effect of Acidithiobacillus ferrooxidans and Leptospirillum ferrooxidans on preg-robbing of gold by graphite from thiourea leaching solution. J. Clean. Prod.. 2020;261
- [CrossRef] [Google Scholar]
- Extraction of gold and silver in the selective chlorination roasting process of cyanidation tailing. Separ. Sci. Technol. (Philadelphia). 2018;53(3):458-466.
- [CrossRef] [Google Scholar]
- Lin, F., Feng, X. T., Lu, G. M., Su, X. X., Li, S. P., & Zhang, J. yu. (2021). Study on Microwave Heating Order and Electromagnetic Characteristics of Copper and Gold Ores. Rock Mechanics and Rock Engineering, 54(5), 2129–2143. 10.1007/s00603-021-02376-4.
- Rapid gold cyanidation from a sulfur-high and arsenic-high micro-fine concentrate via facile two-stage roasting pre-treatment. Miner. Eng.. 2022;190
- [CrossRef] [Google Scholar]
- Optimization of arsenic fixation in the pressure oxidation of arsenopyrite using response surface methodology. Miner. Process. Extr. Metall. Rev. 2022
- [CrossRef] [Google Scholar]
- The application of microwave energy in mineral processing-A review. Acta Montanistica Slovaca Ročník. 2011;16(December):137-148.
- [Google Scholar]
- Pretreatment of arsenic-bearing gold ore with microwave-assisted alkaline leaching. Green Process. Synth,. 2016;5(3):269-273.
- [CrossRef] [Google Scholar]
- Potential pre-treatment and processing routes for recovery of gold from complex (Refractory) gold ores. a review. Proc. Int. Conf. Indus. Eng. Oper. Manage.. 2020;59:1708-1715.
- [Google Scholar]
- Gold extraction from refractory sulfide gold concentrates: A comparison of bio-oxidation and neutral atmospheric pre-treatment and economic implications. J. Sustain. Metall.. 2021;7(3):1354-1367.
- [CrossRef] [Google Scholar]
- Motta, G., Polcyn, M., & Saragosa, E. (2021). Refractory gold ores: Challenges and opportunities for a key source of growth (Issue March). https://www.mckinsey.com/industries/metals-and-mining/our-insights/refractory-gold-ores-challenges-and-opportunities-for-a-key-source-of-growth.
- Investigation of the amenability of a copper-rich refractory gold ore to cyanide leaching. Sci. Min. J.. 2020;59(4):225-234.
- [CrossRef] [Google Scholar]
- Microwave pretreatment of a double refractory gold ore. Miner. Eng.. 2007;20(11):1109-1119.
- [CrossRef] [Google Scholar]
- Is progress in energy-efficient comminution doomed? Miner. Eng.. 2015;73:1-6.
- [CrossRef] [Google Scholar]
- A study of zinc sulfate decomposition at low heating rates. Ind. Eng. Chem. Res.. 1988;27(6):1050-1058.
- [CrossRef] [Google Scholar]
- The fate of the arsenic species in the pressure oxidation of refractory gold ores: Practical and modelling aspects. Miner. Process. Extr. Metall. Rev.. 2023;44(2):155-187.
- [CrossRef] [Google Scholar]
- Nheta, W., Nkwinika, M., & Mailula, M. (2020). Optimisation of gold recovery from small scale custom mills. Proceedings of the World Congress on Mechanical, Chemical, and Material Engineering, 1–8. 10.11159/mmme20.134.
- Peng, K. B., Gao, L. K., Rao, B., Gong, Z. H., Shen, H. R., Gao, G. Y., He, F., & Zhang, M. (2021). Research progress of non-cyanide gold leaching in gold mines. Gongcheng Kexue Xuebao/Chinese Journal of Engineering, 43(7), 871–882. 10.13374/j.issn2095-9389.2020.11.15.001.
- Pretreatment of refractory gold minerals by ozonation before the cyanidation process: A review. J. Environ. Chem. Eng.. 2023;11(1)
- [CrossRef] [Google Scholar]
- Gold mineralization and deposit type in Arinem Cisewu and its surrounding, Garut Regency, West Java, Indonesia. AIP Conf. Proc.. 2020;2245:3-8.
- [CrossRef] [Google Scholar]
- Qin, H., Guo, X. yi, Tian, Q. hua, & Zhang, L. (2021). Recovery of gold from refractory gold ores: Effect of pyrite on the stability of the thiourea leaching system. International Journal of Minerals, Metallurgy and Materials, 28(6), 956–964. 10.1007/s12613-020-2142-9.
- Recovery of gold from sulfide refractory gold ore: Oxidation roasting pretreatment and gold extraction. Miner. Eng.. 2021;164
- [CrossRef] [Google Scholar]
- Comparative study of gold extraction from refractory pyritic ores through conventional leaching and simultaneous pressure leaching/oxidation. Mining Metall. Explor.. 2020;37(4):1279-1284.
- [CrossRef] [Google Scholar]
- Arsenic release through refractory gold ore processing. In: Immobilization and Decontamination Approaches. Current Opinion in Environmental Science and Health. 2021. p. :20.
- [CrossRef] [Google Scholar]
- Pre-treatment of rocks prior to comminution – A critical review of present practices. Int. J. Min. Sci. Technol.. 2017;27(2):339-348.
- [CrossRef] [Google Scholar]
- Sousa, R., Regufe, M. J., Fiúza, A., Leite, M. M., & Futuro, A. (2022). A systematic review of sustainable gold extraction from raw ores using alternative leaching reagents. In Extractive Industries and Society (Vol. 9, pp. 1–15). 10.1016/j.exis.2021.101018.
- Statista. (2023). Demand for gold worldwide from 1st quarter of 2016 to 4th quarter of 2022, by purpose. https://www.statista.com/statistics/274684/global-demand-for-gold-by-purpose-quarterly-figures/.
- Experimental study on microwave pretreatment with some refractory flotation gold concentrate. Adv. Mat. Res.. 2011;158:71-75.
- [CrossRef] [Google Scholar]
- Sudarsono, A., Sudaryanto, & Pramusanto. (1998). Perubahan struktur kristal dan indeks kerja bijih emas Cimanggu, Jawa Barat akibat pemanasan gelombang mikro. Proc. ITB, 30(3), 25–32.
- The thermal decomposition of basic copper(II) sulfate. J. Chem. Edu.. 1990;67(7):612-614.
- [Google Scholar]
- Mineral comminution: Energy efficiency considerations. Miner. Eng.. 2008;21(8):613-620.
- [CrossRef] [Google Scholar]
- Yin, L., Yang, H. ying, Lu, L. shan, Sand, W., Tong, L. lin, Chen, G. bao, & Zhao, M. miao. (2020). Interfacial alteration of pyrite caused by bioleaching. Hydrometallurgy, 195. 10.1016/j.hydromet.2020.105356.
- The Arinem Te-bearing gold-silver-base metal deposit, West Java, Indonesia. Resour. Geol.. 2012;62(2):140-158.
- [CrossRef] [Google Scholar]
- Zhang, L., Guo, X. yi, Tian, Q. hua, Zhong, S. ping, Li, D., Qin, H., Xu, X. yang, & Makuza, B. (2022). Non-cyanide Extraction of Gold from Carlin-Type Gold Concentrate by Pressure Oxidation Pretreatment–Jarosite Decomposition–Fine Grinding: An Eco-Friendly Process and Its Mechanism. Journal of Sustainable Metallurgy, 8(3), 1299–1317. 10.1007/s40831-022-00567-z.
- Zhang, L., Guo, X. yi, Tian, Q. hua, Zhong, S. ping, & Qin, H. (2022). Extraction of gold from typical Carlin gold concentrate by pressure oxidation pretreatment - Sodium jarosite decomposition and polysulfide leaching. Hydrometallurgy, 208, 105743. 10.1016/j.hydromet.2021.105743.
- Ultrasound-intensified leaching of gold from a refractory ore. ISIJ Int.. 2016;56(4):714-718.
- [CrossRef] [Google Scholar]
- Kinetics of leaching refractory gold ores by ultrasonic-assisted electro-chlorination. Int. J. Miner. Metall. Mater.. 2012;19(6):473-477.
- [CrossRef] [Google Scholar]
- Gold extraction from cyanidation tailing using microwave chlorination roasting method. Metals. 2018;8(12):1-11.
- [CrossRef] [Google Scholar]







