Translate this page into:
Design, synthesis and antibacterial activity of coumarin-3-carboxylic acid derivatives containing acylhydrazone moiety
⁎Corresponding authors. gxh200719@163.com (Xiuhai Gan), feng8513@sina.com (Gang Feng)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
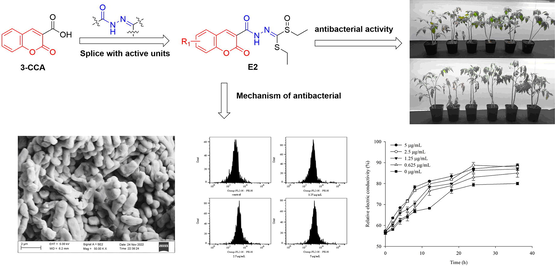
Plant diseases caused by bacteria lead to enormous yield and economic losses in agricultural production. To develop innovative bactericides for controlling plant disease, a series of coumarin-3-carboxylic acid (3-CCA) derivatives containing acylhydrazone thioether/sulfoxide were designed and synthesized by utilizing the principles of bioisosterism and active splicing. E2 exhibited the best antibacterial activities against Xoo, Rs and Ac. Meanwhile, the protective efficacies of E2 were comparable to a commercial bactericide kasugamycin against Ac and Rs in vivo. E2 was remarkable for producing potent antimicrobial activity by disrupting the integrity of the bacterial membrane. The study suggested 3-CCA derivatives containing the acylhydrazone single-sulfoxide possessed great potential for development of novel bactericides.
Abstract
Plant diseases caused by bacteria lead to enormous yield and economic losses in agricultural production. To develop innovative bactericides for controlling plant disease, a series of coumarin-3-carboxylic acid derivatives containing acylhydrazone thioether/sulfoxide were designed and synthesized by utilizing the principles of bioisosterism and active splicing. The bactericidal activities of these derivatives were subsequently evaluated. The result indicated that E2 exhibited the highest antibacterial activities in vitro against Xanthomonas oryzae pv. oryzae, Ralstonia solanacearum, and Acidovorax citrulli, with EC50 values of 2.97, 1.17, and 1.23 µg/mL, respectively. Moreover, in vivo experiments showed that E2 exhibited robust protective properties against Ralstonia solanacearum, and Acidovorax citrulli, with efficacies of 72.52 % and 63.90 %, respectively, at a concentration of 100 µg/mL, which was comparable to the positive control kasugamycin at the same concentration. Scanning electron microscopy analysis revealed that E2 induced changes in the cellular morphology of Acidovorax citrulli, such as shrinkage and collapse. Subsequent investigation revealed that E2 had the capacity to compromise the structural stability of the bacterial membrane of Ralstonia solanacearum. This study represents the first report on the antibacterial activities of this series of coumarin-3-carboxylic acid derivatives containing the acylhydrazone thioether/single-sulfoxide moiety, and the results suggest that coumarin-3-carboxylic acid derivatives containing the acylhydrazone single-sulfoxide may hold potential as effective antibacterial agents. And coumarin-3-carboxylic acid derivatives exhibited promise as plant bacterial agent to prevent rice bacterial leaf blight, tomato bacterial wilt, and melon bacterial fruit blotch.
Keywords
Acylhydrazone
Antibacterial activity
Coumarin-3-carboxylic acid
Ralstonia solanacearum
Single-sulfoxide
- (1H NMR)
-
1H nuclear magnetic resonance
- (13C NMR)
-
13C nuclear magnetic resonance
- (HRMS)
-
High-resolution mass spectrometry
- (EC50)
-
Median effective concentration
- (3-CCA)
-
Coumarin-3-carboxylic acid
- (SEM)
-
Scanning electron microscopy
- (DMSO)
-
Dimethylsulfoxide
- (Xoo)
-
Xanthomonas oryzae pv.oryzae
- (Rs)
-
Ralstonia solanacearum
- (Ac)
-
Acidovorax citrulli
Abbreviations
1 Introduction
Plant bacterial diseases, including rice bacterial leaf blight, tomato bacterial wilt, and melon bacterial fruit blotch, caused by Xanthomonas oryzae pv. oryzae (Xoo), Ralstonia solanacearum (Rs) and Acidovorax citrulli (Ac), respectively, have the potential to cause a substantial reduction in crop yield and quality, thereby significantly impacting food production and security (Huang et al., 1997). For example, bacterial fruit blotch caused by Ac has severely cut production in Cucumis (Zhu et al., 2023). To date, the use of copper compounds and antibiotics remains a crucial method for controlling plant bacterial diseases. However, the overuse of conventional bactericides may result in the emergence of resistance to targeted pathogens, environmental contamination, and health complications (Zhang et al., 2019; Carpane et al., 2020; Chita et al., 2020). Thus, there is an immediate need to explore innovative bactericides that possess novel mechanisms of action and superior efficacy in managing bacterial infections in plants.
Due to their diverse structural characteristics, unique mode of actions, and potential environmental benefits, natural products serve as a significant source of lead compounds for the development of new pesticides (Gao et al., 2007; Lin et al., 2012; Zhang et al., 2012; G et al., 2013; Gerwick and Sparks, 2014; Xiao et al., 2014; Zhang et al., 2015). Among these compounds, coumarin-3-carboxylic acid (3-CCA) has garnered considerable attention due to its multifaceted biological activities, including antitumor (Chimenti et al., 2004; Zhang et al., 2018), anticancer (Ji et al., 2020), and antimicrobial properties (Lin et al., 2012). Notably, the splicing of pyrazole amide and hydrazide at the 3-carboxyl site of 3-CCA has yielded lead compounds with enhanced antibacterial, antifungal, and anti-cancer activities, as evidenced by previous studies (Wei et al., 2017; Liu et al., 2018; Yu et al., 2018; Esfahani et al., 2021). Despite the absence of any documented reports on the antibacterial activity of 3-CCA against phytopathogenic bacteria, our previous study has revealed its remarkable broad-spectrum antibacterial properties against a diverse range of plant pathogenic bacteria and could break cell membranes (Zhu et al., 2023). Additionally, the simple structure of 3-CCA presents a promising avenue for further modification aimed at enhancing its antibacterial efficacy.
Acylhydrazone is a compound of the Schiff base variety that arises from the condensation of acylhydrazine with aldehydes or ketones. The presence of acylhydrazone in compounds confers a diverse range of biological activities, including herbicidal (Zeng et al., 2022), insecticidal (Sun et al., 2015; Ren et al., 2021), bactericidal (Abdelrahman et al., 2017; Zhang et al., 2020; Zhou et al., 2021), and antiviral properties (Chen et al., 2016; Xie et al., 2020), which are crucial in the development of novel pesticides and medicines. Notable commercial drugs that contain the acylhydrazone structure include benquinox, furazolidone, and nitrofurazone (Fig. 1). Consequently, the incorporation of acylhydrazone fragments in the development of coumarin-3-carboxylic acid derivatives with potent bactericidal activity is a viable approach.
The commercial antibacterial drugs containing acylhydrazone moiety.
Unfortunately, there is a lack of reported information regarding the antibacterial properties of 3-CCA derivatives that contain the acylhydrazone skeleton. As a result, this study aimed to address this gap by designing and synthesizing a range of 3-CCA derivatives that incorporated the acylhydrazone moiety (as depicted in Fig. 2 and Fig. 3) through an active splicing method. The synthesized compounds were then evaluated for their bactericidal activities, with the ultimate goal of identifying potential lead compounds for the development of innovative bactericides that can control plant diseases.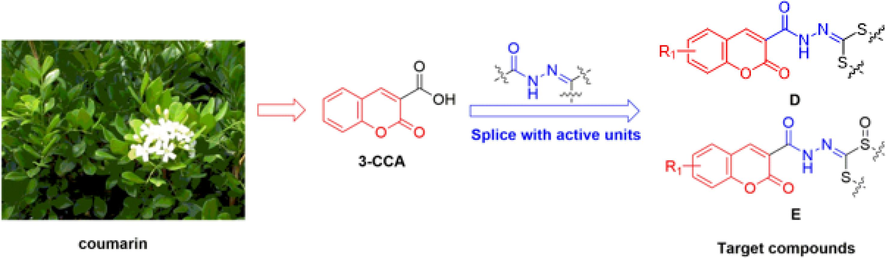
Design of the target compounds.
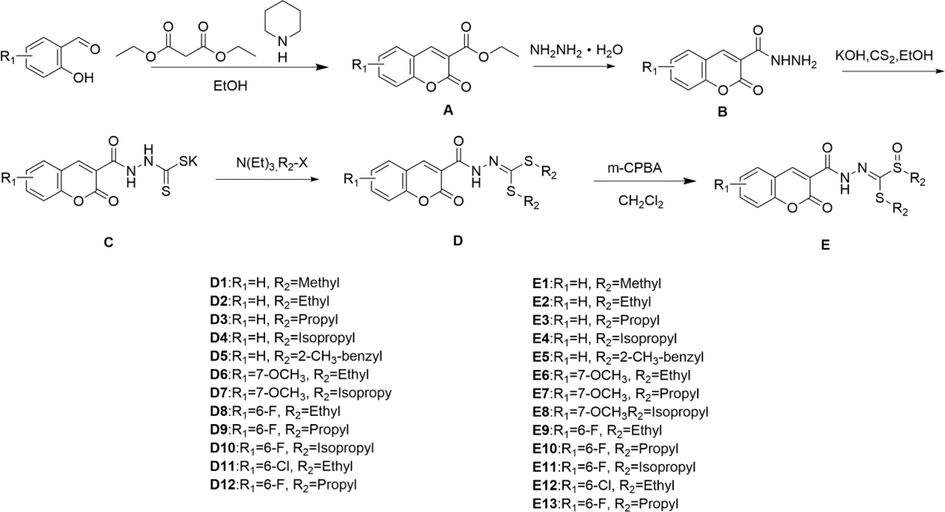
Synthesis route of the target compounds.
2 Materials and methods
2.1 Instruments
All chemical reagents utilized in this study were commercially available and were not subjected to further purification. The chemical reaction processes were monitored through thin-layer chromatography (TLC) under an ultraviolet lamp. The melting points of the compounds were determined using a melting point apparatus (Shanghai INESA Optical Instrument Co., Ltd., China) without temperature calibration. The 1H and 13C NMR spectra were obtained using a Bruker DKX500 NMR spectrometer (Bruker; Karlsruhe, Germany) with CDCl3 or DMSO‑d6 as the solvent and TMS as the internal standard. The Thermo Scientific Q Exactive (Thermo Scientific, Missouri, MO) was utilized to obtain high-resolution mass spectrometry (HRMS) data of the compounds(Fig. 4).
Images of the devices used in this study.
2.2 Chemicals
The present study procured various reaction materials from Shanghai Titan Technology Co., Ltd (Shanghai, China) and other chemical materials from Bositai Technology Co., Ltd (Chongqing, China). Coumarin-3-carboxylic acid (3-CCA, 95 %) was obtained from Bide Pharmatech Ltd. (Shanghai, China), while kasugamycin (65 %) and a water solution (2 %) were sourced from Shenzhen Novoxin Agrochemical Co., Ltd (Shenzhen, China). All chemical reagents and solvents utilized in the study were of analytical purity.
2.3 Bacteria
In this study, three phytopathogenic bacterial strains, namely Xoo (bacterial leaf blight of rice), Ac (bacterial fruit blotch), and Rs (tomato bacterial wilt), were subjected to in vitro antibacterial screening. These strains were preserved for long-term use by storing them in 20 % glycerol at −80 °C. The strains were cultured either on Luria-Bertani agar (LA) plates, which contained 10 g of tryptone, 5 g of yeast extract, 10 g of NaCl, 16 g of agar, and 1 L of distilled water, or in LB broth (without agar) at 28 °C in the dark.
2.4 Synthesis
2.4.1 Synthesis of intermediates A-C
As shown in Fig. 3, to the solution of substituted salicylaldehyde (10 mmol) in EtOH was added, diethyl malonate (12 mmol) and piperidine (2 mmol) under an argon atmosphere. After addition, the reaction mixture was heated to reflux for 12 h to produce intermediates A. Then using dichloromethane as the solvent, intermediates A (10 mmol) and hydrazine hydrate (15 mmol) were stirred at −5°C for 10–30 mins. Intermediates B were saturated with sodium chloride and extracted with dichloromethane. Subsequently, the intermediates B, KOH (12 mmol), and carbon disulphide (15 mmol) were mixed and refluxed in ethanol. After the completion of the reaction as monitored by TLC, the solvent was removed in vacuum. The raw product was washed with ethanol to obtain intermediates C.
2.4.2 Synthesis of target compounds D1-D12
To the solution of intermediates C (10 mmol) in DMF (25 mL) was added different halogenated hydrocarbons (24 mmol) and potassium carbonate (15 mmol). The reaction mixture was stirred for 5–8 h at room temperature until TLC detection showed the reaction was finished. The saturated aqueous ammonium chloride solution was poured into the reaction mixture to obtain a crude product, which was purified by recrystallization or column chromatography to get target compounds D1-D12.
2.4.3 Synthesis of target compounds E1-E13
Based on the target compounds D, the target compounds E (10 mmol) were oxidized by m-CPBA (12 mmol) with CH2Cl2 as the solvent at room temperature, then target compounds E1-E13 were obtained by extraction and purification. The structures of target compounds were confirmed with 1H NMR, 13C NMR, and HRMS, and the data are described in the Supporting Information.
The representative data for compound E2 are presented in the following: ethyl (E)-(ethylsulfinyl)-N-(2-oxo-2H-chromene-3-carbonyl)methanehydrazonothioate (E2): Yield: 27.93 %, yellow solid, m.p. 142.4–143.7 ℃. 1H NMR (400 MHz, CDCl3) δ 12.58 (s, 1H), 9.08 (s, 1H), 7.79–7.74 (m, 2H), 7.50–7.42 (m, 2H), 3.39–3.30 (m, 1H), 3.23–3.01 (m, 3H), 1.42 (t, J = 7.4 Hz, 3H), 1.35 (t, J = 7.5 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 161.19, 158.40, 157.63, 154.71, 150.81, 135.21, 130.27, 125.77, 118.41, 117.17, 116.90, 47.70, 28.99, 16.05, 6.89. HRMS (ESI) m/z for C15H16O4N2NaS2 [M + H]+ calcd: 375.04437, found: 375.04309.
2.4.4 X-ray diffraction
X-ray single-crystal diffraction experiments were conducted at a temperature of 170 K, using Cu Kα rays as the incident light. The data collection angles ranged from 8.192° to 144.272°, and the independent diffraction points were 2613 (Rint = 0.0284, Rsigma = 0.0307). Compound E2 was recrystallized from a mixture of ethanol and dichloromethane to obtain a suitable single crystal, with dimensions of 0.14 mm × 0.12 mm × 0.1 mm for diffraction analysis.
2.5 Antibacterial activity test in vitro
In vitro, antimicrobial activities of all target compounds against Xoo, Rs and Ac were evaluated using a turbidity assay (Li et al., 2014), with kasugamycin and 3-CCA as the positive control and dimethyl sulfoxide (DMSO) as the blank control. The concentrations of compounds used for screening were 50 and 25 µg/mL respectively.
2.6 Antibacterial activities of E2 against Ac and Rs in vivo
In vivo, antibacterial activities of target compound E2 against Ac and Rs were evaluated by greenhouse pot experiments (Li et al., 2013; Li et al., 2015; Wang et al., 2016; Seong et al., 2019) respectively. Kasugamycin water solution (2 %) was used as the positive control. The disease symptoms were evaluated each day after inoculation using a disease index (DI) calculated from a modified 0 to 9 disease severity scale: 0, no symptoms; 1, 3, 5 and 7, necrotic lesions on approximately 25 %, 50 %, 75 % and 100 % of the cotyledons, respectively; and 9, total death of the seedling. The disease index and control effect were calculated each day based on the following formula:
2.7 Observation of microstructure
The morphologies and microstructures of compound E2 against Ac were observed by scanning electron microscopy (SEM) (Liu et al., 2021).
2.8 Effect of E2 on the membrane integrity of Rs
The present study assessed the effect of E2 on the membrane permeability of Ac, utilizing the methodology outlined by (Ernst et al. 2000). A suspension of Rs bacteria was prepared and subjected to varying concentrations of E2, employing the same procedures as the previous membrane permeability analysis. Following a 4-hour treatment, the bacteria were collected via centrifugation at 12000 × g for 5 min and subsequently incubated with propidium iodide (PI, 30 μM) for 20 min in the dark at room temperature. Subsequently, the unbound dye underwent a comprehensive washing process with the PBS buffer, and the PI fluorescence of both treated samples and controls was assessed via flow cytometry. The experimentation was conducted thrice, with each treatment executed in triplicate.
2.9 Statistical analyses
The SPSS software (version 20.0, IBM Corp., Armonk, NY, USA) was utilized for variance analysis during data analysis. Statistical significance was determined as P < 0.05 through the application of Duncan’s Multiple Range Test, and the outcomes were depicted in lowercase alphabets in both figures and tables. Sigma Plot (version 12.5, Systat Software Inc., San Jose, CA, USA) was employed to generate graphs.
3 Results and discussion
3.1 Chemistry
The synthesis of target compounds is illustrated in Fig. 3, and synthetic procedures and methods are described in the Materials and Methods section. The structures of the target compounds were confirmed through the analysis of 1H NMR, 13C NMR and HRMS data, which were listed in the Supporting Information. Furthermore, the structure of compound E2 was authenticated via X-ray diffraction analysis, as depicted in Fig. 5.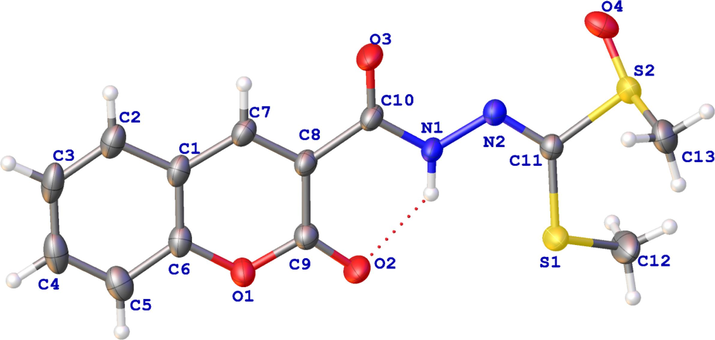
X-ray crystal structure of compound E2.
3.2 Antibacterial activity of 3-CCA derivatives against Xoo, Rs and Ac in vivo
The in vitro antibacterial activity of twenty-five 3-CCA derivatives containing acylhydrazone thioether and single-sulfoxide moieties were determined against Xoo, Rs and Ac. The results were tabulated in Table 1. Notably, the acylzone-sulfoxide compounds E1 and E2 exhibited significant efficacy in controlling plant pathogenic bacteria at a concentration of 50 μg/mL, with inhibitions of 96.21 %, 95.94 % and 99.75 % against Xoo, Rs and Ac, respectively. These values were higher than those of the lead compound 3-CCA and were comparable to the positive control kasugamycin. Compound E3, an acylzone-sulfoxide, exhibited significant antibacterial activity against Xoo and Rs, with inhibitory rates of 93.83 % and 99.99 % at a concentration of 50 μg/mL, respectively. These rates were higher than those of the lead compounds and comparable to the positive control. Conversely, the bactericidal activity of acylhydrazone thioethers was observed to be lower, ranging from 4 % to 47 %.
Sample
Inhibition (%)
Xoo
Rs
Ac
50 μg/mL
25 μg/mL
50 μg/mL
25 μg/mL
50 μg/mL
25 μg/mL
D1
32.46
22.13
3.98
4.71
15.07
11.77
D2
27.15
21.54
5.41
2.74
6.41
5.75
D3
50.42
47.2
1.78
0.31
9.69
6.98
D4
19.49
9.64
15.69
13.32
14.69
8.61
D5
18.75
6.53
8.40
5.65
16.37
6.34
D6
46.93
44.57
1.58
0.25
41.82
17.42
D7
37.19
31.47
2.99
11.01
10.57
0.45
D8
31.88
24.7
0.46
0.26
14.68
8.60
D9
59.03
48.46
7.54
0.68
10.15
5.45
D10
45.11
2.21
1.16
0.26
11.58
11.50
D11
43.73
0.46
21.52
19.24
18.16
3.97
D12
4.06
4.33
13.88
6.71
32.61
26.75
E1
92.93
89.76
99.06
96.64
99.97
98.16
E2
96.21
93.17
95.94
94.54
99.75
94.16
E3
93.83
87.09
99.99
99.91
74.86
54.40
E4
41.38
38.84
13.55
7.61
12.33
5.84
E5
46.47
39.40
14.23
11.88
7.91
2.23
E6
82.91
63.11
88.15
60.10
68.25
47.49
E7
95.87
93.69
99.23
79.06
97.80
98.23
E8
88.05
84.69
97.17
88.75
98.55
97.44
E9
71.82
62.10
74.97
58.95
70.61
42.46
E10
7.45
0.33
19.13
12.38
30.72
28.77
E11
86.48
69.87
97.84
39.19
25.76
5.72
E12
92.69
80.81
56.34
5.87
55.28
28.06
E13
80.61
32.56
6.84
5.48
70.79
57.72
3-CCA
47.68
38.18
61.40
47.52
57.23
38.95
Kasugamycin
91.92
90.83
98.94
86.55
98.59
32.36
The antibacterial activity of compounds E6-E8, which were modified with 7-methoxyl, was found to be higher than that of acylhydrazone thioether but lower than that of E1-E3. At a concentration of 50 μg/mL, these compounds were effective against Xoo, Rs, and Ac, with inhibition rates ranging from 60.10 % to 98.55 %. Additionally, compounds E9, E11, E12, and E13, which were derived from 6-chloro and 6-fluoro, exhibited strong selective antibacterial activity against Xoo, with inhibition rates of 71.82 %, 86.48 %, 92.69 %, and 80.61 % at 50 μg/mL, respectively.
The aforementioned results suggested that the 3-carboxyl moiety might serve as the active site of 3-CCA, thereby presenting a promising avenue for further modifications aimed at developing more potent antibacterial agents. To this end, a total of twenty-five 3-CCA derivatives were synthesized in this investigation through the incorporation of acylhydrazone thioether and single-sulfoxide groups at the 3-carboxyl position of the parent compound. The results obtained indicate that the antibacterial activities of 3-CCA derivatives containing acylhydrazone single-sulfoxide outperformed those containing acylhydrazone thioether. Furthermore, the bacterial activity was significantly impacted by the substituent R2. The structure–activity relationship (SAR) analysis of R2 substitutions revealed that the substitution of R2 with benzyl or bulky groups resulted in reduced antibacterial activity compared to methyl or ethyl groups, which was consistent with the findings of (Zhang et al. 2022). Nevertheless, the structural modifications had an overall detrimental effect on the activity, and no beneficial substitution for the benzene ring of coumarin was observed. The introduction of F and Cl as electron withdrawing groups resulted in a significant reduction in antibacterial activity compared to the absence of substituents. Similarly, the introduction of a methoxy group as an electron donor group resulted in inferior antibacterial activity. Consequently, the sulfoxide moiety may serve as an antibacterial fragment, and sulfoxide compounds may have the potential as antibacterial agents against plant diseases.
3.3 Bacterial virulence assays of high activity compounds
Based on the results of the initial screening, high-activity compounds, namely compounds E1, E2, E3, E6, E7 and E8, were selected for further investigation of their antibacterial virulence. As presented in Table 2, E2 exhibited the most potent antibacterial activity against Xoo, Rs and Ac, with EC50 values of 2.97 μg/mL, 1.17 μg/mL and 1.23 μg/mL, respectively, surpassing those of the lead compound 3-CCA and the positive control kasugamycin. Similarly, E1 also demonstrated remarkable bactericidal activity against Xoo, Rs and Ac, with EC50 values of 3.43 μg/mL, 1.94 μg/mL and 2.46 μg/mL, respectively. However, other modification sites of coumarins also showed moderate antibacterial activity which EC50 values ranged from 2.78 to 9.92 μg/mL on Xoo, Rs and Ac respectively.
Bacteria
Compounds
LC-P
r
EC50 (μg/mL)
95 %FL (μg/mL)
Xoo
E1
Y = 3.9752 + 1.9151x
0.9653
3.43
2.35–5.00
E2
Y = 4.3844 + 1.3012x
0.9743
2.97
1.95–4.52
E3
Y = 4.0204 + 1.5017x
0.9839
4.49
3.11–6.48
E6
Y = 3.7316 + 1.3528x
0.9983
8.66
5.96–12.58
E7
Y = 4.2631 + 1.5348x
0.9929
3.02
2.00–4.56
E8
Y = -3.8780 + 1.9498x
0.9980
3.76
2.77–5.12
3-CCA
Y = 2.6739 + 1.5292x
0.9952
33.20
22.97–47.99
Kasugamycin
Y = 4.1624 + 1.1718x
0.9464
5.19
3.30–8.15
Rs
E1
Y = 4.4807 + 1.8019x
0.9813
1.94
1.43–2.64
E2
Y = 4.9010 + 1.4810x
0.9507
1.17
0.77–1.78
E3
Y = 4.0498 + 1.4119x
0.9815
4.71
3.21–6.92
E6
Y = 4.0023 + 1.0729x
0.9968
8.51
5.05–14.34
E7
Y = 4.3511 + 1.4173x
0.9610
2.87
1.95–4.23
E8
Y = 4.3441 + 1.4788x
0.9690
2.78
1.91–4.03
3-CCA
Y = 3.3851 + 1.1059x
0.9960
28.86
18.16–45.85
Kasugamycin
Y = 3.9657 + 1.3179x
0.9940
6.09
4.04–9.19
Ac
E1
Y = 4.4881 + 1.3112x
0.9895
2.46
1.63–3.69
E2
Y = 4.8650 + 1.5056x
0.9965
1.23
0.82–1.85
E3
Y = 4.0763 + 1.0702x
0.9831
7.30
4.44–12.00
E6
Y = 4.0282 + 0.9750x
0.9998
9.92
5.53–17.80
E7
Y = 4.1132 + 1.7174x
0.9941
3.28
2.29–4.71
E8
Y = 4.0509 + 1.5035x
0.9691
4.28
2.97–6.17
3-CCA
Y = 3.3787 + 1.1849x
0.9932
23.35
15.24–35.79
Kasugamycin
Y = 3.9056 + 1.1019x
0.9852
9.85
5.81–16.67
3.4 Bacterial activity of E2 against Ac and Rs in vivo
Pot experiments were undertaken to investigate the protective and curative properties of E2 against Ac (Table 3 and Fig. 6). The results indicated that E2 demonstrated a remarkable protective effect against Ac, with an efficacy of 72.52 % at a concentration of 100 μg/mL. This efficacy was comparable to that of the positive control, kasugamycin (71.84 %), and significantly superior to other concentrations. Furthermore, E2 exhibited significant curative effects against Ac, with a curative efficacy of 62.07 % at the same concentration. No significant difference was observed when compared to the control agent, kasugamycin (63.40 %). Note: Columns represent the mean ± SE. Different letters in the column represent significant difference at P0.05 level, the same as following.
Sample
Concentration(µg/mL)
Protective effect
Curative effect
Disease index
efficacy (%)
Disease index
efficacy (%)
E2
25
38.68 ± 3.56b
35.24c
53.91 ± 1.89b
25.90c
50
23.46 ± 2.14c
60.70b
35.80 ± 1.23c
50.83b
100
16.46 ± 2.57d
72.52a
27.16 ± 2.14d
62.63a
Kasugamycin
100
16.87 ± 2.85d
71.84a
26.60 ± 2.76d
63.40a
control
/
59.67 ± 3.77a
/
72.84 ± 2.47a
/

The protective and curative effect of E2 against melon bacterial fruit blotch in vivo.
The results of our study indicated that E2 exhibited remarkable protective and curative effects against Rs in vivo, as evidenced by the data presented in Table 4 and Fig. 7. Specifically, the efficacy of E2 against Rs was determined to be 63.90 % at a concentration of 100 μg/mL, which was not significantly different from that of Kasugamycin (64.34 %). Additionally, E2 demonstrated a moderate curative effect against Rs, with an efficacy of 55.55 % at 100 μg/mL, which was lower than that of kasugamycin (60.34 %).
Sample
Concentration(µg/mL)
Protective effect
Curative effect
Disease index
efficacy (%)
Disease index
efficacy (%)
E2
25
30.04 ± 1.43b
44.45c
43.89 ± 6.01b
33.08d
50
23.87 ± 2.85c
56.11b
34.57 ± 3.27c
47.15c
100
19.75 ± 4.28d
63.90a
29.01 ± 1.85d
55.55b
Kasugamycin
100
19.34 ± 1.43d
64.34a
25.93 ± 2.14d
60.34a
control
/
54.32 ± 5.38a
/
65.43 ± 6.42a
/

The protective and curative effect of E2 against tomato bacterial wilt in vivo.
3.5 Effect of E2 on the hyphae morphology of Rs
The present study utilized scanning electron microscopy to investigate the effect of E2 on the ultrastructure of Rs. The results, depicted in Fig. 8, indicate that the control treatment (7-A) yielded bacterial cells that were characterized by a mellow, full, elongated, and rod-shaped morphology. However, exposure to 1.25 μg/mL of E2 resulted in some cells displaying depression (7-B), and as the concentration of E2 increased, the cells exhibited more severe shrinkage (7-C). Notably, cells treated with 5 μg/mL of E2 displayed significant shrinkage and distortion (7-D).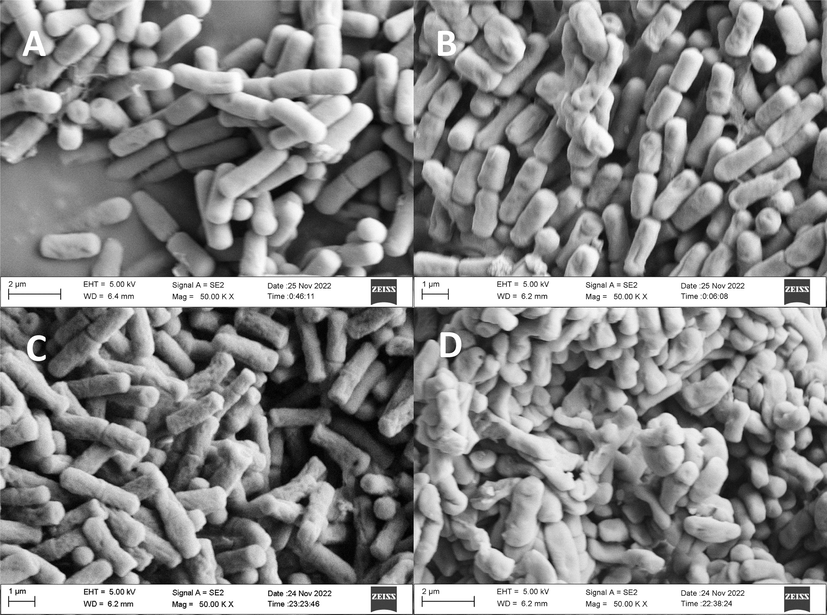
SEM observations of Rs treated with E2 (A) CK; (B) E2 at 1.25 µg/ mL; (C) E2 at 2.5 µg/ mL and (D) E2 at 5 µg/ mL.
3.6 Effects of E2 on cell membrane integrity of Rs
This study employed a conductivity meter and flow cytometer to assess the impact of E2 on the integrity of the Rs cell membrane. The findings indicated that the relative conductivity of the solution increased when the bacterial cell membrane was compromised releasing of intracellular electrolytes. By measuring the relative electrical conductivity, the effect of E2 on cell membrane permeability could be determined. The findings, illustrated in Fig. 9, indicated a direct correlation between the concentrations of E2 and the conductivity values. Notably, a marked escalation in relative conductivity was observed at the 4-hour mark post-treatment, with the conductivity values of E2 at 1.25, 2.5 and 5 μg/mL significantly surpassing those of the control. The conductivity values for all treatments reached their zenith at the 24-hour mark post-treatment.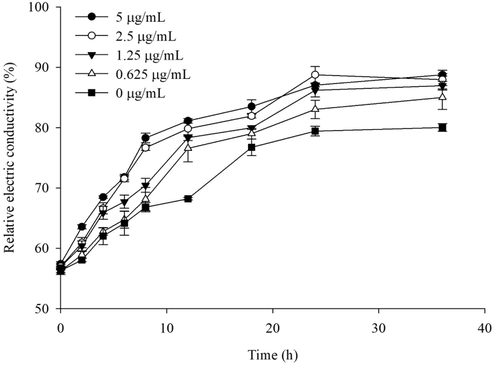
Effect of compound E2 on relative conductivity of Rs.
The bacterial cell membrane can be penetrated by PI, which subsequently binds to nucleic acid and elicits a fluorescence response that can be detected via flow cytometry. This fluorescence response is frequently employed to evaluate the integrity of the bacterial cell membrane. As illustrated in Fig. 10, the fluorescence intensity was primarily distributed between 0 and 105 in the absence of E2 treatment. However, following E2 treatment, the fluorescence intensity of Rs increased. Furthermore, the fluorescence intensity increased progressively with increasing E2 concentrations, peaking at a concentration of 5 μg/mL.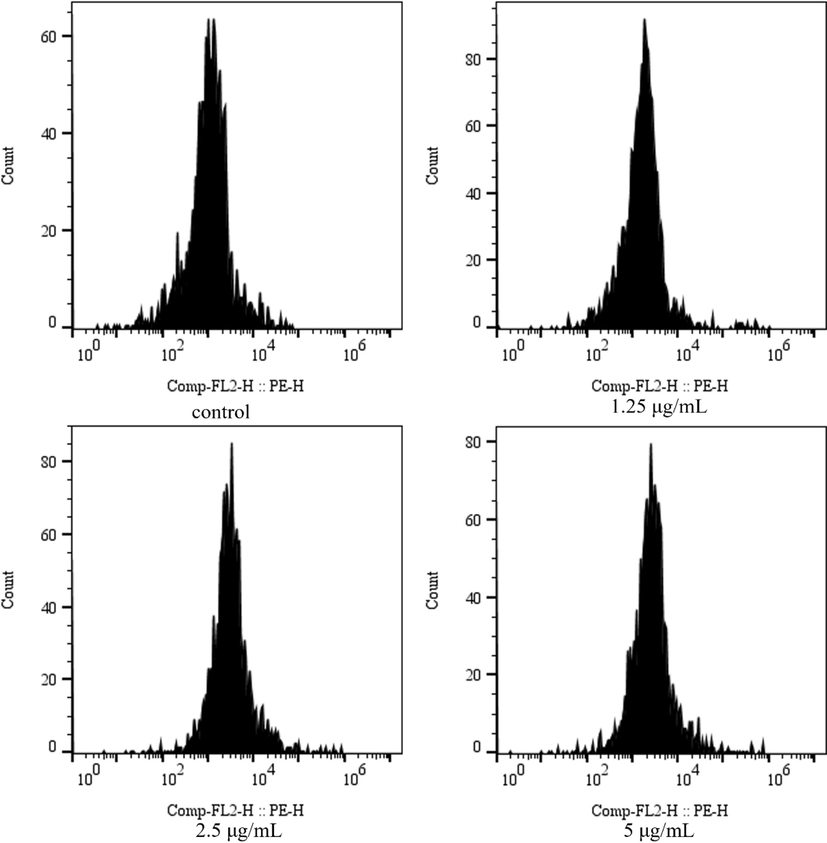
Effect of compound E2 on cell membrane integrity of Rs.
The cell membrane is a critical component in maintaining the structural integrity of cells and facilitating normal cellular functions, rendering it a crucial target for antibacterial interventions (Hendrich et al., 2003). Several natural antibacterial agents, including thymol (Harnvoravongchai et al., 2018), protocatechualdehyde (Li et al., 2016), and berberine (Peng et al., 2015), can exert antibacterial effects by modifying the permeability and integrity of bacterial cell membranes (Ismail et al., 2020; Langeveld et al., 2014). The findings of this study demonstrated that treatment of Rs cells with E2 resulted in a characteristic wrinkled and withered surface, increased conductivity, and elevated fluorescence intensity of PI bound to the nucleus. These results indicated E2 could significantly undermine the integrity of the Rs cell membrane.
4 Conclusions
In summary, a total of twenty-five novel 3-CCA derivatives containing the acylhydrazone were synthesized, and several compounds demonstrated noteworthy antibacterial properties. Notably, compound E2 exhibited the most potent antibacterial activities against Rs and Ac both in vitro and in vivo, and disrupted bacterial membrane integrity, resulting in significant antimicrobial activity. The findings from the analysis of structural-activity relationship indicated that 3-CCA derivatives containing an acylhydrazone single-sulfoxide moiety with short linear alkane substituents (methyl/ethyl) exhibited a significantly higher antibacterial activity compared to those substituted with benzyl or other large groups. This study represents the first report on the antibacterial activities of this particular series of 3-CCA derivatives containing the acylhydrazone thioether/single-sulfoxide moiety, and the results suggest that 3-CCA derivatives containing the acylhydrazone single-sulfoxide may hold potential as effective antibacterial agents. And coumarin-3-carboxylic acid derivatives exhibited promise as plant bacterial agent to prevent rice bacterial leaf blight, tomato bacterial wilt, and melon bacterial fruit blotch.
Acknowledgement
This work was supported financially by the High-level Talents Foundation of Basic and Applied Basic Research Plan (Natural Science Field) of Hainan Province(320RC697), Key Laboratory of Low-carbon Green Agriculture in Northeastern China, Ministry of Agriculture and Rural Affairs P. R. China (Grant No.LCGANE08), the National Natural Science Foundation of China (NSFC No. 32372598), the Fundamental Research Fund of Academy of Tropical Agricultural Science (1630042022009) and Chinese Academy of Tropical Agricultural Sciences for Science and Technology Innovation Team of National Tropical Agricultural Science Center (CATASCXTD202305).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Design, synthesis and 2D QSAR study of novel pyridine and quinolone hydrazone derivatives as potential antimicrobial and antitubercular agents. Eur. J. Med. Chem.. 2017;138:698-714.
- [Google Scholar]
- Management of northern corn leaf blight using nativo (trifloxistrobin + tebuconazole) fungicide applications. Crop Prot.. 2020;127:104982
- [Google Scholar]
- Design, synthesis, and biological activities of spirooxindoles containing acylhydrazone fragment derivatives based on the biosynthesis of alkaloids derived from tryptophan. J. Agric. Food Chem.. 2016;64:6508-6516.
- [Google Scholar]
- Inhibition of monoamine oxidases by coumarin-3-acyl derivatives: biological activity and computational study. Bioorg. Med. Chem. Lett.. 2004;14:3697-3703.
- [Google Scholar]
- Granulysin, a T cell product, kills bacteria by altering membrane permeability. J. Immunol.. 2000;165:7102-7108.
- [Google Scholar]
- Synthesis of some novel coumarin isoxazol sulfonamide hybrid compounds, 3D-QSAR studies, and antibacterial evaluation. Sci. Rep.. 2021;11:20088.
- [Google Scholar]
- Natural products for pest control: An analysis of their role, value and future. Pest Manage. Sci.. 2014;70:1169-1185.
- [Google Scholar]
- Antimicrobial effect of asiatic acid against Clostridium difficile is associated with disruption of membrane permeability. Front. Microbiol.. 2018;9:2125.
- [Google Scholar]
- Lipids as a target for drugs modulating multidrug resistance of cancer cells. Curr. Drug Targets. 2003;4:23-30.
- [Google Scholar]
- Pyramiding of bacterial blight resistance genes in rice: Markerassisted selection using RFLP and PCR. Theor. Appl. Genet.. 1997;95:313-320.
- [Google Scholar]
- Angiotensin-converting enzyme and renin inhibition activities, antioxidant properties, phenolic and flavonoid contents of Cuphea ignea A. DC. J. Reports Pharm. Sci.. 2020;9:92-96.
- [Google Scholar]
- Synthesis and anticancer activity of new coumarin-3-carboxylic acid derivatives as potential lactate transport inhibitors. Bioorg. Med. Chem.. 2020;29:115870
- [Google Scholar]
- Synergy between essential oil components and antibiotics: A review. Crit. Rev. Microbiol.. 2014;40:76-94.
- [Google Scholar]
- Effect of subinhibitory concentrations of chlorogenic acid on reducing the virulence factor production by Staphylococcus aureus. Foodborne Pathog. Dis.. 2014;11:677-683.
- [Google Scholar]
- Effect of chitosan solution on the inhibition of Acidovorax citrulli causing bacterial fruit blotch of watermelon. J. Sci. Food Agric.. 2013;93:1010-1015.
- [Google Scholar]
- Antibacterial activities against rice bacterial leaf blight and tomato bacterial wilt of 2-mercapto-5-substituted-1,3,4-oxadiazole/thiadiazole derivatives. Bioorg. Med. Chem. Lett.. 2015;25:481-484.
- [Google Scholar]
- Evaluation of the antibacterial effects and mechanism of action of protocatechualdehyde against Ralstonia solanacearum. Molecules. 2016;21:754.
- [Google Scholar]
- Synthesis and antifungal activity of novel sclerotiorin analogues. J. Agric. Food Chem.. 2012;60:4480-4491.
- [Google Scholar]
- Synthesis and antibacterial activities of novel 4-hydroxy-7-hydroxy- and 3-carboxycoumarin derivatives. Molecules. 2012;17:10846-10863.
- [Google Scholar]
- Novel coumarin-pyrazole carboxamide derivatives as potential topoisomerase Ⅱ inhibitors: Design, synthesis, and antibacterial activity. Eur. J. Med. Chem.. 2018;157:81-87.
- [Google Scholar]
- Transcriptome analysis on the mechanism of ethylicin inhibiting Pseudomonas syringae pv. actinidiae on kiwifruit. Microorganisms. 2021;9:724.
- [Google Scholar]
- Antibacterial activity and mechanism of berberine against Streptococcus agalactiae. Int. J. Clin. Exp. Path.. 2015;8:5217-5223.
- [Google Scholar]
- Regioselective hemisynthesis and insecticidal activity of C8-hydrazones/acylhydrazones/sulfonylhydrazones coumarin-type derivatives of osthole. Bioorg. Med. Chem. Lett.. 2021;40:127962
- [Google Scholar]
- Design, synthesis, and insecticidal activity of some novel diacylhydrazine and acylhydrazone derivatives. Molecules. 2015;20:5625-5637.
- [Google Scholar]
- Quorum-sensing contributes to virulence, twitching motility, seed attachment and biofilm formation in the wild type strain Aac-5 of Acidovorax citrulli. Microb. Pathog.. 2016;100:133-140.
- [Google Scholar]
- Discovery of 2H-chromen-2-one derivatives as G Protein-Coupled Receptor-35 agonists. J. Med. Chem.. 2017;60:362-372.
- [Google Scholar]
- Secondary metabolites from the endophytic Botryosphaeria dothidea of Melia azedarach and their antifungal, antibacterial, antioxidant, and cytotoxic activities. J. Agric. Food Chem.. 2014;62:3584-3590.
- [Google Scholar]
- Synthesis and antiviral/fungicidal/insecticidal activities study of novel chiral indole diketopiperazine derivatives containing acylhydrazone moiety. J. Agric. Food Chem.. 2020;68:5555-5571.
- [Google Scholar]
- Design, synthesis and antifungal activity evaluation of coumarin-3-carboxamide derivatives. Fitoterapia. 2018;127:387-395.
- [Google Scholar]
- Novel pyrazole amides as potential 4-hydroxyphenylpyruvate dioxygenase inhibitors. J. Agric. Food Chem.. 2022;70:7400-7411.
- [Google Scholar]
- Design, synthesis and biological evaluation of novel furoxan-based coumarin derivatives as antitumor agents. Med. Chem. Res.. 2018;27:1198-1205.
- [Google Scholar]
- Synthesis and fungicidal activity of novel pimprinine analogues. Eur. J. Med. Chem.. 2012;53:283-291.
- [Google Scholar]
- Synthesis and antifungal activity of novel streptochlorin analogues. Eur. J. Med. Chem.. 2015;92:776-783.
- [Google Scholar]
- Synthesis and antibacterial activities of 2-oxo-N-phenylacetamide derivatives containing a dissulfone moiety target on Clp. J. Agric. Food Chem.. 2022;70:9356-9366.
- [Google Scholar]
- Discovery of novel bis-sulfoxide derivatives bearing acylhydrazone and benzothiazole moieties as potential antibacterial agents. Pestic. Biochem. Phys.. 2020;167:104605
- [Google Scholar]
- Response of soil microbes after direct contact with pyraclostrobin in fluvo-aquic soil. Environ. Pollut.. 2019;255:113164
- [Google Scholar]
- Discovery of efficient inhibitors against pyruvate dehydrogenase complex component E1 with bactericidal activity using computer aided design. Pestic. Biochem. Phys.. 2021;177:104894
- [Google Scholar]
- Antibacterial activities of coumarin-3-carboxylic acid against Acidovorax citrulli. Front. Microbiol.. 2023;14:1207125.
- [Google Scholar]
Appendix A
Supplementary material
Characterization data, 1H and 13C NMR spectra and HRMS of compounds D1-D12, and E1-E13 are described in the Supporting Information. Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2023.105389.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary Data 1
Supplementary Data 1







