Translate this page into:
Adsorption of various inorganic and organic pollutants by natural and synthetic zeolites: A critical review
⁎Corresponding authors. dehmaniy@gmail.com (Younes Dehmani), t.lamhasni@gmail.com (Taibi Lamhasni), francodison@gmail.com (Dison S.P. Franco)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Zeolites are microporous crystalline aluminosilicates with high surface area and uniform pore size. Natural and synthetic zeolites have been used to adsorb organic and inorganic compounds in aqueous media due to thier particular physicochemical properties and the low cost of the process. The flexibility of zeolites to remove pollutants from water, such as dyes, heavy metal ions, and phenolic compounds, is discussed in this document in the context of contemporary research. This review briefly consolidates the currently available literature to comprehend the structure of zeolites and their synthesized by hydrothermal method. Later, this manuscript is present different parameters to study the adsorption mechanisms of organic and inorganic contaminants using the zeolites. The main adsorption processes using zeolites as adsorbents include chelation, surface adsorption, natural processes, diffusion, electrostatic interaction and complexation. In addition, the research demonstrates that the dominant models in the isothermal and kinetic study of adsorption are the Langmuir and the pseudo-second-order models. We can assess the beneficial applicability of zeolite materials for real wastewater treatment in the future by comparing their adsorption capacities for removing harmful substances from water to those of other adsorbents and crude zeolites.
Keywords
Adsorption
Synthetized zeolites
Organic and inorganic pollutants

1 Introduction
The question of water treatment, conservation, and quality could be a universal issue. Pollutants discharged into springs, and aquatic systems have evolved considerably in quantity and quality(Bhaskar et al., 2020). More complex products enrich simple domestic discharges, and also the sewerage networks have very diverse characteristics since they now collect industrial, commercial and artisanal discharges (Akani and Nwankwo, 2018; Bhaskar et al., 2020; Ma et al., 2016a). Pollution, a fancy and taxing phenomenon of world development, could be a degradation of a natural environment by chemical substances and industrial wastes affecting, to varying degrees, all urban, industrial, and rural areas and, therefore, the aquatic environment. It impacts the soil, water, and air and may reach ditches, rivers, canals, marshes, lakes, the ocean, and groundwater (Rong et al., 2015). Among all the harmful and priority chemical pollutants, aromatic compounds, including substituted phenolic compounds (nitrophenols, chloroprenes…), are detected in leachates at alarming concentrations. Organic and inorganic compounds that cause unpleasant tastes and odors are detected in surface water, groundwater and wastewater. These are the highest ten micropollutants on the list of organic and inorganic compounds classified by the U.S. (Akani and Nwankwo, 2018; Ismagilov et al., 2007; Luis, 2006; Ma et al., 2016a; Sun et al., 2015). The main techniques include biological degradation, membrane filtration (El-Kordy et al., 2022b), advanced oxidation, photocatalytic degradation, electrochemical oxidation, and particularly those supported adsorption phenomena (El-Kordy et al., 2022a). Of all the techniques listed, solid-state adsorption processes are highly effective in removing organic compounds from wastewater. Adsorption processes provide many advantages over other techniques, like low capital and operating costs, easy use, the potential use of naturally available solid supports, high efficiency, and simple apparatus in eliminating pollutants, even at trace levels(Abdellaoui et al., 2020; Abou Oualid et al., 2020; Afiqah et al., 2019; Ahmad et al., 2021; Eltaweil et al., 2020; Hosseini et al., 2021; Moosavi et al., 2020). Adsorption methods are widely used to separate organic and inorganic pollutants from domestic and industrial wastewater. Therefore, very important research efforts are devoted to developing varied alternative adsorbents with high sorption capacities to uptake pollutants from wastewater(Alshabib and Onaizi, 2019; Erkonak et al., 2008; Lin et al., 2020; Mandal and Das, 2019; Mirmohamadsadeghi et al., 2012; Petrie et al., 2015; Sun et al., 2015; Vuppala et al., 2019). Many types of adsorbents exist, the most common being zeolite. It is a superior adsorbent because of its large specific area, various surface functions, and porosity. Nevertheless, the disadvantage of zeolite is its high preparation and regeneration cost and, thus, the limit of its adsorption capacity. Nevertheless, recent data from the scientific literature show that zeolites have proven to be very effective in the field of liquid effluent treatment.
Nevertheless, the bibliographic data on the microstructure and the rendering of removal in environmental reactions remains insufficient. Therefore, this paper aims to provide preliminary knowledge on the technical feasibility of the zeolite adsorption process by evaluating the results of published studies. Several studies and reviews of zeolite adsorption have investigated the elimination of organic pollutants from aqueous solutions (Chaouati et al., 2013; Cheng et al., 2016; Kragović et al., 2019; Lin et al., 2020; Merrikhpour and Jalali, 2013; Na chat et al., 2022; Razavi et al., 2021), while others have focused on removing inorganic pollutants (Elhakim et al., 2021; Hailu et al., 2017; Ma et al., 2016b; Marszałek et al., 2022; Mehraban et al., 2007; Yang et al., 2019a). Accordingly, The present literature review focuses simultaneously on the adsorption of organic and inorganic pollutants on natural and synthesised zeolites.
The essay examines the essential structural characteristics of zeolites and their industrial uses. Isotherms, kinetics, mathematical models, and adsorption mechanisms typically used to describe processes are all covered in-depth. Studies on equilibrium, thermodynamics, and characterization are also covered. There is also a summary of how these models have been used in zeolite adsorption studies already performed. One of the strengths of this review is its thorough synthesis of recent research results on adsorption of various organic and inorganic pollutants using natural and synthetic zeolites. This makes it easier to identify the crucial variables that would improve adsorption efficiency. The effect of the best-performing parameters such as pH, initial concentration, zeolite properties, nature of the organic pollutants.
The first zeolite was identified and named by Axel F. Cronstedt in 1756. When he noticed that this particular sort of rock could hold onto water within its structure and release it when heated, he came up with the term “zeolite” from the Greek words “zeo” to boil and “lithos” to stone,which had previosly been used in literature.(de Aquino et al., 2020; Kim et al., 2022a; Madhu et al., 2022; Ramezani Shabolaghi and Irani, 2022; Wang and Chen, 2021). Zeolites are members of the mineral family and are more precisely categorized as alumino-silicates having a distinct crystalline structure. Al, Si, and O are the three primary components of them. The arrangement of these components in tetrahedral SiO4 and AlO4 configurations creates various zeolite kinds. Four oxygen atoms are covalently linked to the center Al or Si atom. The formula TO4 is frequently used to denote the core atom along with the oxygen atoms. The central atom is also known as the [T] atom(Alborzi et al., 2022; Feng et al., 2022; Li et al., 2022b; Wang and Chen, 2021).
The Structure Commission of the International Zeolite Association(SC-IZA) is responsible for assigning codes for the proper nomenclature of zeolite frameworks; IUPAC accepts these codes (Mccusker and Baerlocher, 2019). The three capital Roman letters that make up the nomenclature code define the topology of the atomic lattice T, regardless of its chemical makeup or symmetry. Take LTA, MOR, or FAU as examples. In addition, a hyphen, such as -CLO, -LIT, or -PAR, is inserted before the code in the scenario where there is a broken framework, one whose periodicity at a certain time is not constant. (Li et al., 2022b; Wang et al., 2021a).
The location where the material was found or discovered, a researcher's name, a company's name, or whether the material has a particular property are all frequent factors that affect the letters that are utilized(El Alouani et al., 2021), and so forth 218 zeolite frames had been given codes by the IZA-SC before July 2014(He et al., 2021; Ji and Zhang, 2022).
Zeolites have desirable characteristics that are governed by their structural makeup. The basic tetrahedral structure known as the Primary Building Unit (PBU) serves as the foundation for the zeolite framework. Due to the T atom's position at the PBU's center and its function as a connector between PBUs, the oxygen atoms are positioned at the corners of each unique tetrahedron. The frame type explains the connections and distribution of the T atoms throughout the three-dimensional network.
Each zeolite has a specific kind of structure, which also establishes characteristics like topology and pore size(He et al., 2021; Khan et al., 2021). Regardless of the zeolite's chemical makeup, the framework establishes its identity. However, there is a limitation when the [TO4] tetrahedra are bound to one another. Due to the negative charge contained in each PBU, which causes unfavorable interactions during the creation of zeolites, Lowenstein's rule (Rong et al., 2015)states that there cannot be Al—O-—i bonds, i.e., two nearby AlO4tetrahedra. For this reason, in any zeolite framework, the minimum value of Si/Al is always 1(Chaibi et al., 2021; Razavi et al., 2021). Linking multiple PBUs leads to more complex secondary building unit (SBUs) structures. Rings are the most basic SBU types and can be divided into several categories based on how many T atoms they contain. For instance, a ring is a 4-ring if it contains four T atoms, and so on (Lv et al., 2022; Mauer et al., 2022). The pores of zeolites surrounded by several rings can be classified as very large (consisting of 14 rings), large (12 rings), medium (10 rings), and small (8 rings)(Lee et al., 2019). When the ring defines an oriented face of the three-dimensional frame, the word “window” is used. Zeolites can have frameworks with anything from four to twenty rings. (dos Santos et al., 2021; Kiwaan et al., 2021; Wang et al., 2021b)).
A more intricate SBU cage construction is created when these rings are combined. Meier first provided eight kinds of cages (Lepreux, 2005), but as characterization techniques advanced, it became possible to identify other types of cages. Cavities are categorized according to the type and quantity of rings (Zhang et al., 2019b). For instance, a cage [4662] has 8 windows: 6 are created by the 4 rings, and the 6 rings create 2. Fig. 1 shows examples of construction units and pore/cage sizes of three zeolite structures such as zeolite A (LTA), sodalite (SOD) and faujazite (FAU). Each type of zeolite is classified according to the number and type of rings. The Formation of the LTA, SOD (sodalite) and FAU (faujasite) structures from 4R and 6R PerBUs, with rings of 4 and 6 tetrahedra, respectively. In the structural models, the T elements in the framework are located at the intersection of edges, with oxygen atoms in the middle of the edges.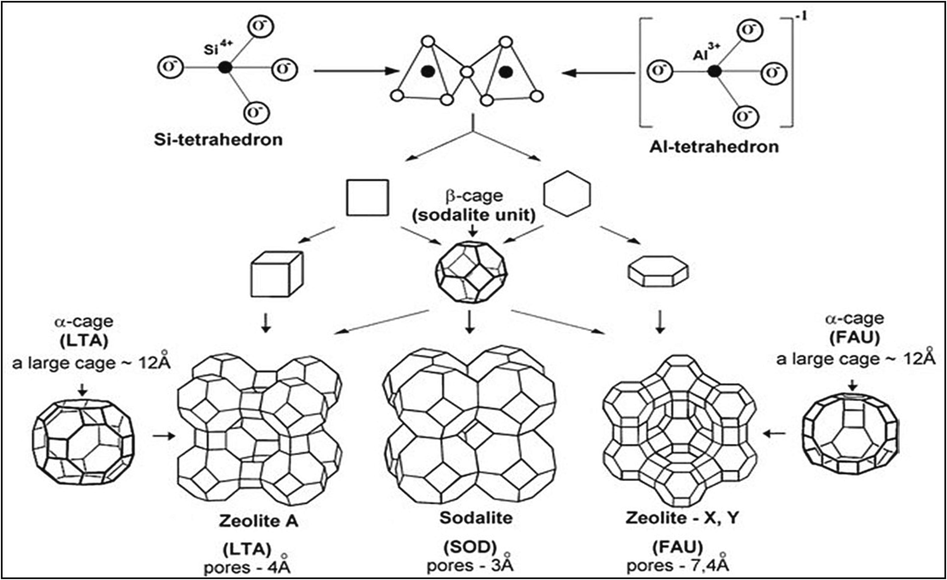
Structural unit of zeolite, exemples zeolite A (LTA), sodalite (SOD), and faujasite (FAU) (Scientific, 2017).
The oxygen atoms, which act as linking atoms between the T atoms, are absent from the graphical representation of the cages, as can be seen,to grasp the overall structure better. Greek letters are also used to identify some Zeolite structures. The latter is well-known for its inclusion in the popular A, X, and Y zeolites (Kim et al., 2021). In reality, X and Y zeolites have different chemical compositions but share the same structural element, FAU. Finally, cages and annular connections can form channels and pores in one, two, or three dimensions,with the junction of the channels resulting in the formation of the complete zeolite structure that we showed previously in the Fig. 1 (Ouyang et al., 2021).
2 Synthesized zeolites
Scientists have created synthetic methods in response to the first production of natural zeolites. The procedures used to create the synthesized zeolites have been repeated and altered to produce the same or different materials, occasionally with considerably different properties from the final output. Regardless of their origins, zeolitic minerals often develop under hydrothermal settings in nature (Kim et al., 2021). The goal of the earliest synthetic methods was to faithfully mimic the conditions that led to the production of natural zeolites, according to Fig. 2.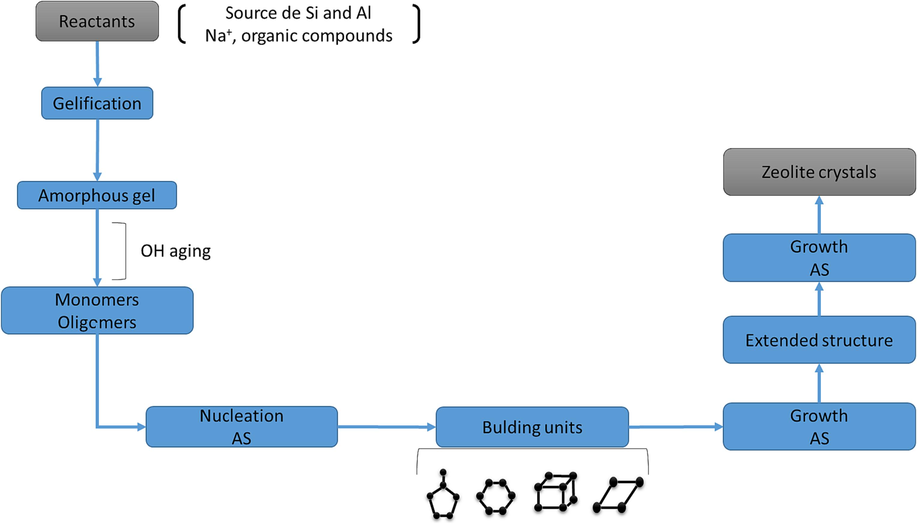
Diagram of the hedrothermalsynthesis of zeolites.
According to its definition, hydrothermal refers to heterogeneous reactions that dissolve and recrystallize materials that are typically rather intractable (Sadki and Saidi, 2014). As the name implies, hydrothermal techniques typically involve the use of water at relatively high pressure (between 1 and 100 MPa), typically autogenous, and temperatures (ranging from 100 to 1000 °C) (Mthombeni et al., 2015). At the same time, a more current definition specifies that a hydrothermal process is one that is performed above ambient temperature and one bar (Lin et al., 2016). In this process, the pressure in the reactor increases as a function of temperature, but it is not common to know the pressure in the reactor. Therefore, the vapour pressure at this temperature is used as a reference.
The process is occasionally referred to as solvothermal when an alternative solvent is used in place of water. However, some authors have gone one step further and given the process a name based on the solvent it uses, such as glycothermal and ammonothermic(Liu et al., 2022).
A hydrothermal environment needs the presence of alkaline media in the form of basic solutions (Li et al., 2020). As a result, an alkaline media, a solvent, a supply of silica (SiO2), and an alumina (Al2O3) are required to create zeolites. They are finally put together to react at high temperatures to achieve crystallization after aging. According to Cundy(Pei et al., 2022), the hydrothermal approach can be used to produce zeolite minerals in the following manner:
-
Silica and alumina from amorphous sources are dissolved and packed in extremely alkaline solutions with Na + or K + cations (NaOH or KOH).
-
The already-formed mixture is heated to increase pressure inside the autoclave and speed up the reaction product.
-
Before the reaction temperature of the amorphous gel. After that, it experiences what is known as induction time (the precursors remain amorphous, and no crystallographic phase is detected). When the first crystals form, this phase concludes.
-
The crystallization period, which follows the induction period, is when nearly all of the material progressively transforms into crystals
-
Following crystallization, recovery is accomplished through filtration, washing, and drying. In the event that use has taken place, the biological skeleton is eliminated using a high-temperature calcination procedure.
All scientific and technological advancements in the zeolites field have been made possible by manipulating and researching some important variables. Composition, time, temperature, agitation, and reaction kinetics are the primary determinants of zeolite synthesis (mechanisms).
3 Adsorption of various pollutants
Many scientific works on adsorption in zeolite systems have been carried out in different laboratories from different backgrounds. This is how several types of adsorbents of natural (zeolites, natural clays, etc.) or synthetic (activated carbons, bridged clays, silica gels, resins, etc.) origins have been prepared and tested in the adsorption of several organic (phenols, humic acids, dyes, pesticides, etc.) and inorganic micropollutants, in particular heavy metals.
On the other hand, the scientific literature offers us very few critical works on the adsorption of organic and inorganic compounds(Ahmed and Hameed, 2020; El Alouani et al., 2021; Elhakim et al., 2021; Jia et al., 2022; Marszałek et al., 2022; Sharma et al., 2022). Generally, the various works relating to adsorption (mainly organic and inorganic compounds) have emphasized the influence of certain parameters such as solubility, pH, temperature, the adsorbate nature, and the hydrophobic and organophilic properties of the different adsorbents used(Dehmani et al., 2020a, 2020b; Feng et al., 2020; Lütke et al., 2019; Nakhjiri et al., 2021; Wang et al., 2020; Yao et al., 2020; Zhang et al., 2021). Furthermore, in comparison with conventional adsorbents and to enhancenatural materials, several scientific kinds of research have focused on using zeolites to improve their surface adsorption properties.
These modifications, which are of physicochemical types based essentially on ion exchange, generally lead, depending on the nature of the modification, to obtaining hydrophobic adsorbents and heterogeneous catalysts. In the field of adsorption and despite their thermal instabilities, hydrophobic and organophilic, have been widely used in the depollution of water contaminated by certain organic micropollutants, in particular phenols, pesticides, dyes, etc.(Ba Mohammed et al., 2021, 2020; Beauchet et al., 2010; Sellaoui et al., 2021).
Since the 1990s, many studies have been performed on the adsorption of organic and inorganic pollutants on natural and modified zeolites(Armaroli et al., 1999; Clark and Snurr, 1999; Du et al., 1999; Hernández-Huesca et al., 1999; Huber and Knözinger, 1999; Ivanov et al., 1999; Khelifa et al., 1999; Mello and Eić, 1999; Rouquerol et al., 1999; Tatlier and Erdem-Şenatalar, 1999; Timonen and Pakkanen, 1999). The Table 1 presentsmost of the published works on using zeolites and zeolite-based materials in adsorption of organic and inorganic pollutants. The adsorbed quantity of pollutants on zeolite materials is very important in toxic and harmful amount of about 40.7 mg/g in the work of Wen PingCheng(Ping et al., 2016). A rate exceeds 98 % with an adsorbed amount exceeding 70 mg/g in the work of Bochera and his colleagues (Ba Mohammed et al., 2019a).
Qmax (mg/g)
R (%)
Kinetic mode
Isotherm model
Ref
Oxytetracyclinehydrochloride
83.3
PSO
Langmuir
(Başkan et al., 2022)
CO2
212.9
Langmuir
(Jedli et al., 2022)
CO2
167.2
(June Choi et al., 2022)
CO2
27.2
(Kwon et al., 2022)
Ammonium
17.8
PSO and Elovich
Sips
(Nguyen et al., 2022)
NH4+ and NO3–
98
Rhodamine 6G
71
Thomas et Adams-Bohart
(Ameh et al., 2022)
Metal ions Pb2+
487.8
PSO
Langmuir
(Ameh et al., 2022)
Metal ions Cd2+
194.0
PSO
Langmuir
[92]
Methyleneblue
48.7
91
PSO
Freundlich
(Cheng et al., 2021)
Ammonium
11.8
(Muscarella et al., 2021)
CO2
132.0
(June Choi et al., 2022)
CO2
44
(Kim et al., 2022b)
Nickel
97
Avramikinetic
Redlich-Peterson
[43]
Ammonia and
88
(Ma et al., 2022)
Phosphate
99
[96]
Propionicacid
516.0
PSO
(Na chat et al., 2022)
VOCs
3 % CO2 and 97 % CH4
(Sun et al., 2022)
Co(II)
10.8
PSO
Langmuir
(Gupta et al., 2011)
Cu(II)
38.1
PSO
F and L
(Zhan et al., 2013)
As(V)
38.2
PSO
Freundlich
(Suazo-Hernández et al., 2019)
Cd(II)
78.1
PSO
Langmuir
(Ahali Abadeh and Irannajad, 2017)
Ni(II)
76.3
PSO
Freundlich
(Ahali Abadeh and Irannajad, 2017)
Pb(II)
123.0
PSO
Freundlich
(Nah et al., 2006)
Zn(II)
30.0
PSO
Langmuir
(Fungaro and Graciano, 2007)
Ga(III)
86.9
PSO
Langmuir
(Zhao et al., 2015)
In(III)
94.7
PSO
Langmuir
Cs(II)
77.5
Freundlich
(Eljamal et al., 2019)
Cs(II)
81.4
–
Tόth
(Rahman et al., 2021)
Cr(VI)
48.3
PSO
Langmuir
(Angaru et al., 2021)
Cd(II)
196.0
Langmuir
(Elwakeel et al., 2017)
Cu(II)
118.1
PSO
Langmuir
(Li et al., 2020)
Cu(II)
77.8
PSO
Langmuir
(Kouznetsova et al., 2020)
Th(IV)
9.2
PSO
Freundlich
(Kaygun and Akyil, 2007)
Sr(II)
0.9
PSO
Temkin
(Yusan and Erenturk, 2011)
Cu(II)
719.3
PSO
Langmuir
(Dinu and Dragan, 2010)
(Humelnicu et al., 2011)
Co(II)
467.9
PSO
Langmuir
UiO2(II)
536.3
PSO
Sips
Th(IV)
438.5
PSO
Sips
(Humelnicu et al., 2011)
V(V)
277.7
Elovich
Freundlich
(Salehi et al., 2020)
Cr(VI)
344.8
PSO
Langmuir
(Mthombeni et al., 2015)
V(V)
74.9
PSO
Langmuir and Sips
(Mthombeni et al., 2016)
Pb(II)
138.8
–
Langmuir
(Nakamoto et al., 2017)
Cd(II)
56.2
–
Langmuir
(Nakamoto et al., 2017)
ReactiveOrange5
239.3
PSO
Freundlich
(Piri et al., 2019)
Reactive orange16
90.0
PSO
Freundlich
Congored
40.6
PSO
Freundlich
Methyleneblue
222.7
PSO
Langmuir
(Lin et al., 2016)
Methyleneblue
28.4
PSO
Langmuir
(Majid et al., 2019)
Methylene blue
49.0
PSO
l
Congored
161.3
PSO
Langmuir
(Madan et al., 2019)
Methyleneblue
53.2
PSO
Langmuir
(Zhu et al., 2014)
Malachite green
48.5
Langmuir
RhodamineB
64.4
PSO
Langmuir
(Cheng et al., 2017)
Methyleneblue
94.4
PSO
Freundlich
(Huang et al., 2019)
RhodamineB
32.7
Langmuir
(Li et al., 2020)
Methyleneblue
555.5
PSO
Langmuir
(Shui et al., 2020)
ReactiveRed 120
19.6
PSO
HillandSips
(Dehghani et al., 2017)
ReactiveRed 196
39.0
Jovanovic
Brilliantgreen
90.0
PSO
Langmuir
(Shamsudin and Shahadat, 2019)
Methylene Orange
8.3
PSO
Langmuir
(Yang et al., 2019b)
Methyleneblue
151.5
PSO
Freundlich
(Khanday et al., 2017)
Acidblue29
212.7
PSO
Freundlich
Methylene Orange
287.0
PSO
Freundlich
(Radoor et al., 2021)
Congored
19.0
PSO
Freundlich
(Radoor et al., 2020)
Malachite green
29.5
PSO
Langmuir
(Radoor et al., 2021)
Brilliantgreen
1461.3
PSO
Langmuir
(Mittal et al., 2020b)
Methyleneblue
661.9
PSO
Langmuir
(Mittal et al., 2020a)
Basicyellow 28
180.0
PSO
Freundlich
(Panic and Velickovic, 2014)
Basicyellow 28
117.0
PSO
Langmuir
(Panic and Velickovic, 2014)
Methyleneblue
81.9
Freundlich
(Sabarish and Unnikrishnan, 2018a)
MethylOrange
76.4
Freundlich
(Sabarish and Unnikrishnan, 2018b)
Methylene Orange
153.0
Freundlich
(Habiba et al., 2017)
AcidBlue74
16.6
Freundlich
(Ghanavati et al., 2021)
Hexavalentchromium
25.6
PSO
l
(Liu et al., 2022)
Phenol
PSO
l
(Ba Mohammed et al., 2019a)
Zn(II)
80.4
PSO
Langmuir
(Zhang et al., 2020)
Co(II)
9.1
(Lin et al., 2020)
Mn(II)
8.6
(Lin et al., 2020)
Cd (II)
92.4
(Zhang et al., 2019a)
Phenol
PSO
Freundlich
(Yousef et al., 2011)
Phenol
40.7
PSO
Redlich-Peterson
(Cheng et al., 2016)
RushdiI. Yousefutilized a natural zeolite in the removal of phenol, the results in squash utilized the zeolite in adsorption with a removal rate greater than 90 % and a Qmax = 356.5 mg/g (Yousef et al., 2011). Zeolites are good adsorbents for dyes elimination, analysis of literature data shows that the adsorption capacity of Reactive Orange 5 is about 239 mg/g, Reactive orange 16 is about 90 mg/g and Congo red at a Q = 40 mg/g in the work of F. Piri(Piri et al., 2019)under normal conditions. An adsorbed quantity of methylene blue on a zeolite-based material in the work of Z. Shui(Shui et al., 2020)exceeds 555.5 mg/g. Finally, all published works show that zeolitic materials present a good and efficient choice for wastewater treatment.
pollutants for the environment. For example, a rate exceeds 99 % for cadmium in the works of D. Li (Li et al., 2022a), Z. AhaliAbadeh(Ahali Abadeh and Irannajad, 2017), K. Z. Elwakeel(Elwakeel et al., 2017), and K. Nakamoto(Nakamoto et al., 2017), very important results for a pollutant more dangerous for human health and the environment, phenol with low concentrations poses serious environmental questions. For this reason, the scientific research carried out in the last years on eliminating this pollutant, especially the adsorption, keeps a great place in the treatment methods used to eliminate phenols and phenolic compounds. This study exhibits the effectiveness of zeolite and zeolite-based materials in the adsorption of phenol. An adsorbed amount of about 40.7 mg/g in the work of Wen PingCheng(Ping et al., 2016). A rate exceeds 98 % with an adsorbed amount exceeding 70 mg/g in the work of Bochera and his colleagues (Ba Mohammed et al., 2019a).
RushdiI.Yousef utilized a natural zeolite in the removal of phenol; the results in squash utilized the zeolite in adsorption with a removal rate greater than 90 % and a Qmax = 356.5 mg/g (Yousef et al., 2011). Zeolites are good adsorbents for dyes elimination; analysis of literature data shows that the adsorption capacity of Reactive Orange 5 is about 239 mg/g, Reactive orange 16 is about 90 mg/g and Congo red at a Q = 40 mg/g in the work of F. Piri(Piri et al., 2019)under normal conditions. An adsorbed quantity of methylene blue on a zeolite-based material in the work of Z. Shui(Shui et al., 2020)exceeds 555.5 mg/g. Finally, all published works show that zeolitic materials present a good and efficient choice for wastewater treatment.
Prior to being released into aquatic systems, wastewater must be cleaned of organic and inorganic pollutants. A crucial topic of scientific inquiry for a long time has been the creation of efficient methods for this goal. Adsorption, precipitation, advanced oxidation, ozonation, coagulation-flocculation, ion exchange, solvent extraction, membrane filtration, and reverse osmosis have been tested to treat water that contains organic pollutants. However, the solid-state adsorption method has shown to be the most successful at removing organic and inorganic contaminants from wastewater of all the technologies discussed.
Compared to the other methods outlined, the adsorption process has several benefits, including low capital and operational costs and the potential use of a variety of naturally occurring solid adsorbent media. In order toeliminate organic and inorganic contaminants from home and commercial wastewater, adsorption technology has been extensively used. Many studies have focused on finding low-cost adsorbents with high adsorption capacities for typical contaminants and increasing their effectiveness for uptaking. Adsorbents have historically been used to treat wastewater, including activated carbon, clay minerals, agricultural wastes, and zeolites (Sharma and Feng, 2017). As efficient adsorbents for inorganic and organic contaminants in wastewater, zeolites have drawn much interest. The pore size and cages that result in size selectivity make up the empty space in zeolites. Zeolite utilized as an adsorbent is sometimes referred to as a molecular sieve because, from a molecular perspective, the zeolite lattice behaves to solid particles like an ordinary sieve.
For a given structure, the pore diameter and the diameter of the cages connected by the windows are fixed, but the size and number of cations present can still modify them. For example, the window diameters of zeolite A exchanged with K+, Na+ or Ca2+ are 0.3, 0.4, and 0.5 nm, respectively.
Much research was carried out on the organic and inorganic pollutants uptaking by using inexpensive adsorbents, including charcoal,zeolites, clay, and rock minerals (Fig. 3). The results in Fig. 3 show the increase in the number of works interested in the use of the adsorption process in the treatment of organic and inorganic pollutants. The strong point of this study is that it tells about the evolution of works based on zeolites and materials based on zeolite in adsorption. Moreover, a great percentage of these materials are compared with other solids.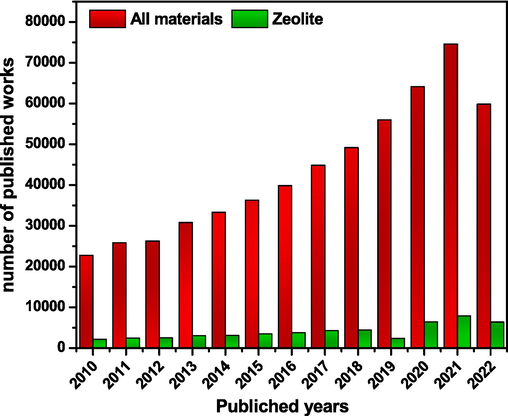
Evolution of the number of works published in the journal on the adsorption of organic and inorganic pollutants.
Zeolites have been used in several research chapters and review papers to absorb both inorganic and organic contaminants (Abbasi, 2019; Aksu and Yener, 1999; Ioannou and Simitzis, 2009; Li et al., 2017, 2019; Liu et al., 2019; Mrunal et al., 2019; Sheela and Nayaka, 2012; Vikrant et al., 2019; Zhang et al., 2017). Work detailing the interactions between zeolites and organic and inorganic contaminants has been published (Guan et al., 2021; Mthombeni et al., 2016, 2015; Nakamoto et al., 2017; Piri et al., 2019). Some review articles target very specific organic substances, like phenols (Shakir et al., 2008) and alcohols (Elhakim et al., 2021). While some zeolite adsorption papers focused on the adsorption of heavy metals (Dehmani et al., 2020a), others have examined the removal of the dye from aqueous solutions (Abbas-Ghaleb et al., 2019; Choi et al., 2018; Huang et al., 2021; Jodeh et al., 2015). The subject of this criticism is the adsorption of organic and inorganic contaminants onto natural and modified zeolites. The adsorption capacity prediction of a system is not easily envisaged since it is strongly dependent on many factors, such as the properties of the adsorbent (particle size, porosity, surface area) and the adsorbate (structure, ionic charge, functional groups, water solubility, pKa, polarity, functionality). The adsorbent molecular weight and size, solution circumstances (such as the solvent, temperature, solute concentration, pH, ionic strength, and solute competition),the solid–liquid interface interactions, and the experimental apparatus type are some of these factors.
4 Effects of adsorption parameters
The pH is a significant factor in any adsorption study because it affects the adsorbent structure and the adsorption mechanism. In addition, this factor is important in the electrostatic mechanism. This factor depends on the origin of the water to be treated and its treatment process (coagulation, flocculation, oxidation). It is, therefore, useful to know the adsorption efficiency at different pH. We have represented some pH favorable to the adsorption of some organic and inorganic pollutants on different zeolites(Alkaim et al., 2014; Frost and Griffin, 1977; Griffin et al., 1977; Johnson, 1990; Miller et al., 1983; MULJADI et al., 1966).
For example, the variations of the quantity adsorbed as a function of pH from acidic to basic medium, from an initial concentration of phenol 60 mg/L and zeolite in the case of the adsorption of phenol go from 75 to 11 mg/g in the work of Bouchra Ba Mohammed. Three regions of the pH effect on the adsorption of the lord metals (Cd, Ni, Cu, and Pb) were noticed by HajarMerrikhpou(Yousef and El-Eswed, 2009);zeolite adsorption capacity increased as the initial pH of the solution increased from 2 to 4 (region 1). However, the zeolite adsorption performance remained almost unchanged in region 2 (pH between 4 and 7). These results were justified by the effect of pH on the structural properties of zeolite, an effect dependent on the dominant charge on the solid surface, whichis dependent on zero charge pH. It may be noted that the phenol adsorption capacity remains almost unchanged at pH between 3.3 and 8.6;however, it strongly decreases when the pH becomes higher than 8.6. Thus, at pH higher than phenol pKa (9.89), phenol dissociates and appears as phenolate ions (C6H5O−); these arguments are given by Wen Ping Cheng (Cheng et al., 2016)when studied the pH effect on the phenol adsorption on the composite zeolite X/activated carbon.
In his research, Rushdi I. Yousef (Yousef and El-Eswed, 2009) investigated the adsorption of phenol and chlorophenols on Jordanian zeolite tuff in buffered solutions with pH values of 4.0, 6.0, 10.5, and the pKa of phenols. The pH range was selected so that two values (4.0 and 6.0) were below the pKa of phenols, one was at the pKa of phenols, and one was at a pH higher than the pKa (10.5). The impact of phenol dissociation on adsorption has been thus investigated. The pH of the Jordanian zeolite tuff (6.9, Section 3.1), where the zeolite surface charge is positive and negative, respectively, was lower and higher than these pH values.
The amount adsorbed is proportional to the adsorbent surface area that is accessible to the adsorbate, which is frequently different from the material's total specific surface area. As a result, it depends on the material's accessible surface area, which might be challenging to determine. The porosity is influenced by how the pores' sizes are distributed. It reflects the microporous adsorbents' internal structure. (Haffane et al., 2016; Jeguirim et al., 2018). The porosity of adsorbent material is crucial when it comes to the adsorption of aromatic organic compounds.
Macroporous carbon has a better adsorption capacity than mesoporous carbon in the case of macromolecules. Indeed, the smaller size involvesa larger contact surface. This will then increase its retention power. Moreover, this surface increases when the support is porous. Industrial adsorbents (mainly zeolites) develop huge specific surfaces (600 to about 1200 m2/g). Several works have shown the relationship between the adsorbed quantity and the textural parameters (Aazza et al., 2017; de Aquino et al., 2020). As an example, Nan Jiang shows an important difference in the quantity adsorbed of phenol of zeolite modefié one passes from 837 to 1304 mg/g for a zeolite faujasite of textural parameters (727 m2/g and 0. 5136 cm3/g) to another of parameters (789 m2/g 0.5233 cm3/g)(Jiang et al., 2020). The same result to the work of NourrdineChaouati;Briefly, the specific surface, the diameters of pores, and the volume of the pores have an important role in the adsorption for that in the synthesis of this material it is necessary to take into consideration these parameters(Chaouati et al., 2013).
The purpose of post-synthesis modifications of zeolites is to adjust the physical and chemical properties to the use for which the zeolite is intended. Synthetic zeolites whose compensating cation is an alkali metal (Na or K) have practically zero acidity. Therefore, ion exchange is the essential method for adjusting the acid-base properties of zeolites. The alkaline cations can be partially or exchanged either directly by protons in an acid medium (if the stability of the structure allows it) or indirectly by ammonium ions subsequently decomposed by calcination into volatile NH3 and protons. In addition, they can be exchanged by multivalent cations (Ca2+, La3+, Ce°, etc.), which makes zeolites able to catalyze acid reactions. The nature of the exchanged cation and the degree of exchange determine the acidity and activity of the zeolite. The exchange of the zeolite by multivalent cations modifies its acidity and its selectivity and improves its thermal stability.
The parameters determining the speed and degree of the exchange are:
-
–
The structural characteristics and the Si/Al ratio of the zeolite.
-
–
The nature, the charge, and the size of the cationic species.
-
–
The concentration of cationic species in the exchange solution.
-
–
Temperature.
-
–
The formation of acid sites using hydrolysis.
The chemical composition of a zeolite can be modified by reducing the number of aluminum atoms present in the network, which is done by dealumination. These operations can be carried out by various treatments, particularly acid treatment, hydrothermal treatment, and isomorphic substitution.
The number of aluminum in the crystal lattice of the zeolites, which defines both the number and the strength of the acid sites, is adjusted by dealumination; this is the main way to modify the acidity and the Si/Al ratio of a zeolite. The Si/Al ratio, therefore, conditions the strength of the acid sites.
From the results of Table 3, it is concluded that the adsorbed amount of organic and inorganic pollutants was affected by the effect of modéfication undergone by the zeolitic material. It is noticed that in the case of methylene blue, under conditions close to the level of temperature initial concentration and pH, it is found that the adsorbed amount increases from the order of 28 to 660 mg/g for a modified zeolite. In several authors' works on cadmium adsorption on zeolitic materials, it is noticed that the adsorbed quantity exceeds 190 mg/g compared with a non-modified zeolite 50 mg/g.
Zeolite name
Surface area (m2 g−1)
Pore volume (cm3 g−1)
Si/Al ratio from XRF analysis
QLF
(µmol/g)
FAU250
727
0.5136
409
1304
FAU50
698
0.5160
43
1271
FAU40
606
0.4819
32
825
FAU30
789
0.5233
31
837
BEA250
516
0.3022
286
414
BEA150
524
0.3243
107
420
BEA75
563
0.3720
78
530
MOR120
431
0.2687
113
529
HFAU (5)
690
0.357
5
13.610
HFAU (5) modified
670
0.360
4.239
HFAU (60)
906
0.485
60
24.512
HFAU (60) modified
821
0.473
21.958
Adsorbedquantity mg/g
Cd(II)
MB
MG
78.25
48.7
48.59
196
222.78
29.58
52.21
28.41
92.47
49.02
94.481
555.56
151.51
661.91
5 Adsorption isotherms
The adsorption isotherms are experimental curves representing at constant temperature the adsorbed quantity per mass of adsorbent (qe in mg/g) at the concentration remaining in the fluid phase after the adsorption equilibrium (Ce in g/L). They are expressed in mathematical equations, these isotherms are intended to describe the adsorption process. Their appearance may represent certain phenomena involved: monolayer or multilayer adsorption and lateral interaction between molecules.
An in-depth search of the bibliographical data shows the modeling of the exemplary results of the adsorption of pollutants on zeolitic materials by the mathematical modules: Langmuir, Freundlich, Temkin, and Sips. The Fig. 4 shows that the Langmuir model describes 52 % of the works published, 34 % of the works are described by the Freundlich model, and 7 % by the Sips model. The rest of the work is described by other models by Tempkin, Redlich-Peterson, and others. An important remark is that the Freundlich or Langmuir model can describe the adsorption isotherm for the same adsorbate and in the same temperature range. As an example the case of the adsorption of phenol in the work of R. I. Yousef we find the model of Freundlich, and in the work of B. Ba Mohammed we find Langmuir.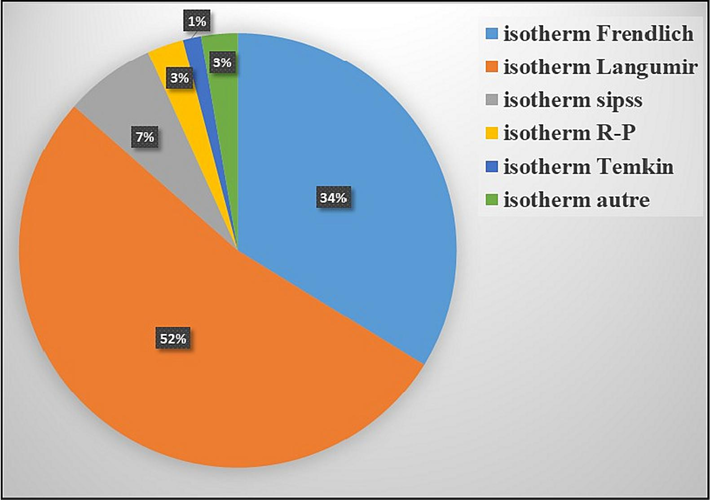
Modeling statistics of the adsorption isotherm on zeolite.
6 Adsorption kinetics
The kinetics of adsorption describes the decrease in the concentration of the adsorbate in the solution as a function of contact time (Dehbi et al., 2019). Knowledge of the kinetics of adsorption is of considerable practical interest in the optimal use of an adsorbent and in the knowledge of the factors to be optimized to optimize to manufacture or improve an adsorbent leading to the fastest kinetics possible (Hassan et al., n.d.). The adsorption rate of a solute from a solution depends on many factors, including the adsorbent nature, the adsorbate, and the stirring rate of the medium. Therefore, it is very important to know the speed and the mechanism by which the materials eliminate the pollutant. For that, three kinetic models have been applied in order to evaluate the adsorption parameters. The models applied are:
-
-
The pseudo-first-order model.
-
-
The pseudo-second-order model.
-
-
The model of intra-particle diffusion.
As shown in Fig. 5 the majority of the adsorption works of organic and inorganic pollutants follow a pseudo-second-order model (95 %); this is something that is in agreement with the nature of the zeolitic solid surface and the role played by the functional groups of zeolite and the ionic exchanges of this material.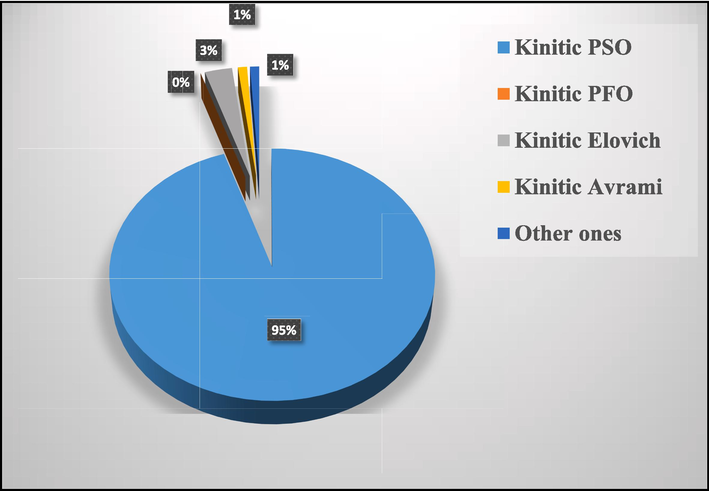
Modeling statistics of adsorption kinetics on Zeolite.
7 Adsorption thermodynamics
Two types of adsorption exist, chemical adsorption and the other physical. Chemical adsorption forms a covalent bond between the adsorbent's surface and the adsorbate species. The energies of attraction put in this type of adsorption are very high, and the values of these energies are above 200 kJ/mol. In addition, physical adsorption is a process with weak interaction forces between the adsorbent and the adsorbent put in this type are of electrostatic nature (<80 kJ mol−1), hydrogen bond (<60 kJ/mol), and type VanderWaals(Sabbatini et al., 2009) (<30 kJ/mol). When we put a solid (zeolite) in contact with a solution, each molecule of the latter manifests a capacity to adsorb on the surface of the solid; several conditions affect this phenomenon as the temperature, concentration, pH, the adsorbent nature, and the structural properties and textures of the adsorbent and their nature(Rajendran et al., 2022).
The temperature: Adsorption is an endothermic or exothermic phenomenon, depending on the adsorbent nature and molecules adsorbed, so the temperature has a very important role depending on the nature of the adsorption process (exothermic or endothermic. In the adsorption of organic and inorganic pollutants process, it is observed in this investigation that both processes exist even in the case of the same adsorbate. The chosen solid is noticed in several works indexed in the first table and the following table (Tables 4 and 2) that the temperature effect depends on the type of zeolite used. In work as an example, we conclude that the adsorption of phenol on natural zeolite is isothermal, a rare case in the literature. This justifies the importance of using natural zeolite in treating organic pollutants in aquatic systems. As for the inorganic pollutants, we notice in Y. Pei's work that the variation of the adsorbate does not affect the effect of temperature on the adsorption. Increasing the temperature leads to an improvement in the adsorption capacity of either nickel or cadmium ions. It is concluded in this part that the adsorption of zeolitic materials is a spontaneous process, and the other thermodynamic parameters are dependent on the type of zeolite used.
Temperature (K)
Thermodynamic parameter
Type of interaction
Ref
ΔH/kJ·mol−1
ΔS/J·mol−1·K−1
ΔG/kJ·mol−1
Ni+(zeolite X)
298
318
31.039
118.581
−6.670
endothermic
(Pei et al., 2022)
Cd+(zeolite X)
298
318
13.329
45.532
−1.150
endothermic
(Pei et al., 2022)
Phenol (faujasitezeolite)
298
313
18.69
14.62
−4.33
endothermic
(Ba Mohammed et al., 2019b)
Phenol (naturalzeolites)
298
318
−10.16
−0.0053
−8.58
exothermic
(Yousef et al., 2011)
8 Conclusions and future outlooks
Aquatic systems containing organic and inorganic contaminants have been managed by using natural and engineered zeolites as adsorbent materials. The technical feasibility of zeolite adsorption techniques is in the function of the flexibility to remove organic and inorganic impurities present in these water sources, even though zeolites are acknowledged as a widely accessible and affordable alternative adsorbent. Additionally, some types of natural or altered zeolites could be appropriate for some applications but not others. So that existing information in the area can regularly be habituated to understanding each zeolite's technical performance as an adsorbent for the intended application, this review paper seeks to highlight the adsorption performance of various zeolites for diverse pollutants.
However, compared to carbon-based materials and graphene/zeolite, several investigations have found that the adsorption capabilities of 2,4-dichlorophenol and bisphenol are 10 % and 40 % higher, respectively. Zeolite-based adsorption methods also have advantages over other biological processes in cost (approximately 20 times less expensive than activated carbon) and byproduct release into the environment. Furthermore, organic and inorganic substances will not be eliminated by chemical pollutants. Although zeolites' adsorption efficiency is considerably improved by using surfactants in their preparation, further research should investigate and analyze the potential of thermally and surface-modified zeolites. Examining the possibility of organic/inorganic composites for pollutant removal is another way to enhance zeolite technology's adsorption performance potentially. In reality, the zeolite properties (nature, type,size), structure of the target pollutants, and operating conditions strongly affect the adsorption capabilities of both primitive and modified zeolites.
In order to maximize the use of particular zeolites against particular contaminants, a comprehensive approach must be used. Although the use of zeolite as an organic compound adsorbent was investigated about 70 years ago, only a few industrial uses, like oil spills, are in use nowadays. Therefore, the potential of zeolite-based adsorbents in comparison to other conventional techniques has been highlighted in this review. Nevertheless, most of the research reported in the literature is carried out on a small scale in the lab. Therefore, applying the performance statistics to large-scale facilities is impossible.
Additionally, an absence of reproducibility of performance and a variety of contradictory results are reported within the literature. In published studies, the regeneration of used clays is not examined. These are considered significant obstacles to using zeolite-based adsorbents in practical settings because organic and inorganic contaminants are invariably mixed in wastewater. Therefore, additional research is required to address these issues utilizing wastewater generated under industrial operating circumstances.
Acknowledgements
The corresponding author thanks all his co-workers who have contributed to this research.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Ortho-Nitro-Phenol adsorption onto alumina and surfactant modified alumina: kinetic, isotherm and mechanism. J. Environ. Chem. Eng.. 2017;5:3418-3428.
- [CrossRef] [Google Scholar]
- Isopropyl Alcohol Total Oxidation Over Platinum Supported on Alumina and Mullite. Chem. Afr.. 2019;2:335-341.
- [CrossRef] [Google Scholar]
- Adsorption of phenol, hydrazine and thiophene on stanene monolayers : A computational investigation. Synth. Met.. 2019;247:26-36.
- [CrossRef] [Google Scholar]
- Synthesis of zirconium-modified merlinoite from fly ash for enhanced removal of phosphate in aqueous medium : Experimental studies supported by Monte Carlo/SA Simulations. Chem. Eng. J.. 2020;404:126600
- [CrossRef] [Google Scholar]
- Eco-Efficient Green Seaweed Codium decorticatum Biosorbent for Textile Dyes : Characterization, Mechanism, Recyclability, and RSM Optimization. ACS Omega 2020
- [CrossRef] [Google Scholar]
- Environmental Technology & Innovation Enhancing adsorption of malachite green dye using base-modified Artocarpus odoratissimus leaves as adsorbents. Environ. Technol. Innov.. 2019;13:211-223.
- [CrossRef] [Google Scholar]
- Removal of Ni and Cd ions from aqueous solution using iron dust-zeolite composite: Analysis by thermodynamic, kinetic and isotherm studies. Chem. Res. Chin. Univer.. 2017;33:318-326.
- [CrossRef] [Google Scholar]
- Adsorption of malachite green by activated carbon derived from gasified Hevea brasiliensis root. Arab. J. Chem.. 2021;14:103104
- [CrossRef] [Google Scholar]
- Insight into the co-pyrolysis of different blended feedstocks to biochar for the adsorption of organic and inorganic pollutants: A review. J. Clean. Prod.. 2020;265:121762
- [CrossRef] [Google Scholar]
- Monitoring the Microbial Load at Chosen Critical Control Points in the Production of Kunun-zaki. IOSR J. Environ. Sci.. 2018;12:41-46.
- [CrossRef] [Google Scholar]
- The usage of dried activated sludge and fly ash wastes in phenol biosorption/adsorption: Comparison with granular activated carbon. J. Environ. Sci. Health - Part A Toxic/hazardous Subst. Environmen. Eng.. 1999;34:1777-1796.
- [CrossRef] [Google Scholar]
- A theoretical investigation into the comparative adsorption between dissolved oxygen and oxygenate species on zeolite 3.7 Å during aviation fuel treatment for thermal stability improvement. Fuel. 2022;317:123451
- [CrossRef] [Google Scholar]
- Effect of pH on adsorption and photocatalytic degradation efficiency of different catalysts on removal of methylene blue. Asian J. Chem.. 2014;26:8445-8448.
- [Google Scholar]
- A review on phenolic wastewater remediation using homogeneous and heterogeneous enzymatic processes : Current status and potential challenges. Sep. Purif. Technol.. 2019;219:186-207.
- [CrossRef] [Google Scholar]
- Column adsorption of Rhodamine 6G over Na–P/SOD zeolite synthesised from aluminosilicate secondary waste. J. Clean. Prod.. 2022;338:130571
- [CrossRef] [Google Scholar]
- Encapsulated zerovalent iron/nickel-fly ash zeolite foam for treating industrial wastewater contaminated by heavy metals. Mater. Today Chem.. 2021;22:100577
- [CrossRef] [Google Scholar]
- A FT-IR study of the adsorption of C5 olefinic compounds on NaX zeolite. Vib. Spectrosc. 1999;20:85-94.
- [CrossRef] [Google Scholar]
- Adsorptive removal of phenol using faujasite-type Y zeolite: Adsorption isotherms, kinetics and grand canonical Monte Carlo simulation studies. J. Mol. Liq.. 2019;296:111997
- [CrossRef] [Google Scholar]
- Adsorptive removal of phenol using faujasite-type Y zeolite: Adsorption isotherms, kinetics and grand canonical Monte Carlo simulation studies. J. Mol. Liq.. 2019;296
- [CrossRef] [Google Scholar]
- Fe-ZSM-5 zeolite for efficient removal of basic Fuchsin dye from aqueous solutions: Synthesis, characterization and adsorption process optimization using BBD-RSM modeling. J. Environ. Chem. Eng.. 2020;8:104419
- [CrossRef] [Google Scholar]
- Enhanced removal efficiency of NaY zeolite toward phenol from aqueous solution by modification with nickel (Ni-NaY) J. Saudi Chem. Soc.. 2021;25:101224
- [CrossRef] [Google Scholar]
- Investigation of adsorption properties of oxytetracycline hydrochloride on magnetic zeolite/Fe3O4 particles. Adv. Powder Technol.. 2022;33
- [CrossRef] [Google Scholar]
- Catalytic oxidation of VOCs on NaX zeolite: Mixture effect with isopropanol and o-xylene. Appl. Catal. B. 2010;100:91-96.
- [CrossRef] [Google Scholar]
- Evaluation on the Activity of Surfactant Immobilized and Metal Cation Exchanged Impregnated Montmorillonite Nanoclays on Oxidation of Benzyl Alcohol. Chem. Afr.. 2020;3:351-361.
- [CrossRef] [Google Scholar]
- TGA investigation of water and ethanol adsorption over LTA zeolites. Micropor. Mesopor. Mater.. 2021;324:111285
- [CrossRef] [Google Scholar]
- Adsorption of phenol from aqueous solution onto zeolites y modified by silylation. C. R. Chim.. 2013;16:222-228.
- [CrossRef] [Google Scholar]
- Phenol adsorption equilibrium and kinetics on zeolite X/activated carbon composite. J. Taiwan Inst. Chem. Eng.. 2016;62:192-198.
- [CrossRef] [Google Scholar]
- Novel adsorption materials based on graphene oxide/Beta zeolite composite materials and their adsorption performance for rhodamine B. J. Alloy. Compd.. 2017;708:255-263.
- [CrossRef] [Google Scholar]
- NaP1 zeolite synthesized via effective extraction of Si and Al from red mud for methylene blue adsorption. Adv. Powder Technol.. 2021;32:3904-3914.
- [CrossRef] [Google Scholar]
- A seasonal observation on the distribution of engineered nanoparticles in municipal wastewater treatment systems exemplified by TiO 2 and ZnO. Sci. Total Environ.. 2018;625:1321-1329.
- [CrossRef] [Google Scholar]
- Adsorption isotherm sensitivity to small changes in zeolite structure. Chem. Phys. Lett.. 1999;308:155-159.
- [CrossRef] [Google Scholar]
- CO2 adsorption capacity of zeolites synthesized from coal fly ashes. Fuel. 2020;276:118143
- [CrossRef] [Google Scholar]
- Hematite Iron Oxide Nanoparticles (α-Fe2O3): Synthesis and Modelling Adsorption of Malachite Green. J. Environ. Chem. Eng.. 2019;103394
- [CrossRef] [Google Scholar]
- Removing Reactive Red 120 and 196 using chitosan/zeolite composite from aqueous solutions: Kinetics, isotherms, and process optimization. J. Ind. Eng. Chem.. 2017;51:185-195.
- [CrossRef] [Google Scholar]
- Removal of phenol from aqueous solution by adsorption onto hematite (α-Fe2O3): Mechanism exploration from both experimental and theoretical studies. Arab. J. Chem.. 2020;13:5474-5486.
- [CrossRef] [Google Scholar]
- Kinetic, thermodynamic and mechanism study of the adsorption of phenol on Moroccan clay. J. Mol. Liq.. 2020;312:113383
- [CrossRef] [Google Scholar]
- Evaluation of Cu2+, Co2+ and Ni2+ ions removal from aqueous solution using a novel chitosan/clinoptilolite composite: Kinetics and isotherms. Chem. Eng. J.. 2010;160:157-163.
- [CrossRef] [Google Scholar]
- CO2 adsorption in a zeolite-based bench scale moving bed prototype: Experimental and theoretical investigation. Chem. Eng. Res. Des.. 2021;171:225-236.
- [CrossRef] [Google Scholar]
- Comparative study of mean-field theory and Monte Carlo simulation of supercritical methane adsorption in zeolites. Chem. Phys. Lett.. 1999;307:413-418.
- [CrossRef] [Google Scholar]
- Application of geopolymers for treatment of water contaminated with organic and inorganic pollutants: State-of-the-art review. J. Environ. Chem. Eng.. 2021;9:105095
- [CrossRef] [Google Scholar]
- Direct Z-scheme of WO3/GO decorated with silver nanoparticles for synergetic adsorption and photocatalytic activity for organic and inorganic water pollutants removal. Appl. Surf. Sci.. 2021;564:150410
- [CrossRef] [Google Scholar]
- Iron based nanoparticles-zeolite composites for the removal of cesium from aqueous solutions. J. Mol. Liq.. 2019;277:613-623.
- [CrossRef] [Google Scholar]
- Experimental study of phenol removal from aqueous solution by adsorption onto synthesized Faujasite-type Y zeolite. Desalin. Water Treat.. 2022;277:144-154.
- [CrossRef] [Google Scholar]
- Preparation of sodalite and faujasite clay composite membranes and their utilization in the decontamination of dye effluents. Membranes. 2022;12:1-18.
- [CrossRef] [Google Scholar]
- Mesoporous magnetic biochar composite for enhanced adsorption of malachite green dye : Characterization, adsorption kinetics, thermodynamics and isotherms. Adv. Powder Technol.. 2020;31:1253-1263.
- [CrossRef] [Google Scholar]
- Retention of copper, cadmium and lead from water by Na-Y-Zeolite confined in methyl methacrylate shell. J. Environ. Chem. Eng.. 2017;5:3698-3710.
- [CrossRef] [Google Scholar]
- Treatment of olive mill wastewater by supercritical water oxidation. J. Supercrit. Fluids. 2008;46:142-148.
- [CrossRef] [Google Scholar]
- Functionalized construction of biochar with hierarchical pore structures and surface O-/N-containing groups for phenol adsorption. Chem. Eng. J.. 2020;410:127707
- [CrossRef] [Google Scholar]
- Synthesis and VOCs adsorption performance of surfactant-templated USY zeolites with controllable mesopores. Chem. Phys. Lett.. 2022;798
- [CrossRef] [Google Scholar]
- Effect of pH on Adsorption of Arsenic and Selenium from Landfill Leachate by Clay Minerals. Soil Sci. Soc. Am. J.. 1977;41:53-57.
- [CrossRef] [Google Scholar]
- Adsorption of zinc ions from water using zeolite/iron oxide composites. Adsorpt. Sci. Technol.. 2007;25:729-740.
- [CrossRef] [Google Scholar]
- Application of electrospun Polyamide-6/Modified zeolite nanofibrous composite to remove Acid Blue 74 dye from textile dyeing wastewater. J. Text. Inst.. 2021;112:1730-1742.
- [CrossRef] [Google Scholar]
- The Capture Threshold Of Male Pink Bollworm1/ Moth With Gossyplure, And Its Effect On Boll Infestation And Frequency Of Insecticidal Treatment. J. Environ. Sci. Health Part A: Environ. Sci. Eng.. 1977;12:431-449.
- [CrossRef] [Google Scholar]
- Catalytic combustion of volatile organic compounds (VOCs) over structured Co3O4 nano-flowers on silicalite-1/SiC foam catalysts. Micropor. Mesopor. Mater.. 2021;323:111173
- [CrossRef] [Google Scholar]
- Adsorption of cobalt(II) from aqueous solution onto hydroxyapatite/zeolite composite. Adv. Mater. Lett.. 2011;2:309-312.
- [CrossRef] [Google Scholar]
- Effect of deacetylation on property of electrospun chitosan/PVA nanofibrous membrane and removal of methyl orange, Fe(III) and Cr(VI) ions. Carbohydrate Polymers. 2017;177:32-39.
- [CrossRef] [Google Scholar]
- Etude de l ’ effet de purification et de modification d ’ une argile locale sur les propriétés structurales et texturales (Investigation of the effect of purification and modification of a local clay on its structural and textural properties) J. Mater. Environ. Science. 2016;7:525-530.
- [Google Scholar]
- Preparation and characterization of cationic surfactant modified zeolite adsorbent material for adsorption of organic and inorganic industrial pollutants. J. Environ. Chem. Eng.. 2017;5:3319-3329.
- [CrossRef] [Google Scholar]
- Hassan, O., Youne, D., Lahcen, M., Mohamed, A., n.d. The phenol adsorption in an aqueous environment by Moroccan clay Summary : 1–22.
- Hydrothermal transformation of geopolymers to bulk zeolite structures for efficient hazardous elements adsorption. Sci. Total Environ.. 2021;767:144973
- [CrossRef] [Google Scholar]
- Adsorption equilibria and kinetics of CO2, CH4 and N2 in natural zeolites. Sep. Purif. Technol.. 1999;15:163-173.
- [CrossRef] [Google Scholar]
- Heterogeneous photoelectro-Fenton using ZnO and TiO2 thin film as photocatalyst for photocatalytic degradation Malachite Green. Appl. Surface Sci. Adv.. 2021;6:100126
- [CrossRef] [Google Scholar]
- Efficient removal of methylene blue from aqueous solutions using magnetic graphene oxide modified zeolite. J. Colloid Interface Sci.. 2019;543:43-51.
- [CrossRef] [Google Scholar]
- Experimental and theoretical investigation of reconstruction and active phases on honeycombed Ni3N-Co3N/C in water splitting. Appl Catal B. 2021;297:120461
- [CrossRef] [Google Scholar]
- Adsorption of CO on sodium containing X- and Y-zeolites and determination of the aluminum distribution. Appl. Catal. A. 1999;181:239-244.
- [CrossRef] [Google Scholar]
- Adsorption characteristics of UO22+ and Th4+ ions from simulated radioactive solutions onto chitosan/clinoptilolite sorbents. J. Hazard. Mater.. 2011;185:447-455.
- [CrossRef] [Google Scholar]
- Adsorption kinetics of phenol and 3-nitrophenol from aqueous solutions on conventional and novel carbons. J. Hazard. Mater.. 2009;171:954-964.
- [CrossRef] [Google Scholar]
- Oxidation of organic compounds in a microstructured catalytic reactor. Chem. Eng. J.. 2007;135
- [CrossRef] [Google Scholar]
- Adsorption of hydrocarbons by ZSM-5 zeolites with different SiO2/Al2O3 ratios: A combined FTIR and gravimetric study. Appl. Catal. B. 1999;21:243-258.
- [CrossRef] [Google Scholar]
- Adsorption of CO2 onto zeolite ZSM-5: Kinetic, equilibrium and thermodynamic studies. Fuel. 2022;321:124097
- [CrossRef] [Google Scholar]
- Adsorption/reduction of nitrogen dioxide on activated carbons: Textural properties versus surface chemistry – A review. Chem. Eng. J.. 2018;347:493-504.
- [CrossRef] [Google Scholar]
- Adsorption of cerium (III) by zeolites synthesized from kaolinite after rare earth elements (REEs) recovery. Chemosphere. 2022;303:134941
- [CrossRef] [Google Scholar]
- Water-Insoluble Cyclodextrin-based nanocubes for highly efficient adsorption toward diverse organic and inorganic pollutants. Sep. Purif. Technol.. 2022;291:120970
- [CrossRef] [Google Scholar]
- Adsorption of triclosan, trichlorophenol and phenol by high-silica zeolites: Adsorption efficiencies and mechanisms. Sep. Purif. Technol.. 2020;235:1-9.
- [CrossRef] [Google Scholar]
- Removal of Methylene Blue from Industrial Wastewater in Palestine Using Polysiloxane Surface Modified with Bipyrazolic Tripodal Receptor. Moroccan J. Chem.. 2015;1:140-156.
- [Google Scholar]
- Effect of pH, Temperature, and Concentration on the Adsorption of Cadmium on Goethite. Environ. Sci. Tech.. 1990;24:112-118.
- [CrossRef] [Google Scholar]
- Effect of framework Si/Al ratio on the adsorption mechanism of CO2 on small-pore zeolites: II. Merlinoite. Chem. Eng. J.. 2022;446:137100
- [CrossRef] [Google Scholar]
- Study of the behaviour of thorium adsorption on PAN/zeolite composite adsorbent. J. Hazard. Mater.. 2007;147:357-362.
- [CrossRef] [Google Scholar]
- Revealing and elucidating chemical speciation mechanisms for lead and nickel adsorption on zeolite in aqueous solutions. Colloids Surf. A Physicochem. Eng. Asp.. 2021;623:126711
- [CrossRef] [Google Scholar]
- Cross-linked beads of activated oil palm ash zeolite/chitosan composite as a bio-adsorbent for the removal of methylene blue and acid blue 29 dyes. Int. J. Biol. Macromol.. 2017;95:895-902.
- [CrossRef] [Google Scholar]
- Adsorption of propene on NaX zeolite exchanged with Zn2+ and Cu2+. Appl. Catal. A. 1999;178:61-68.
- [CrossRef] [Google Scholar]
- Comprehensive evaluation of 3A, 4A, 5A, and 13X zeolites for selective 1-octene adsorption over n-octane. J. Ind. Eng. Chem.. 2022;110:274-285.
- [CrossRef] [Google Scholar]
- CO2 adsorption on zeolite 13X modified with hydrophobic octadecyltrimethoxysilane for indoor application. J. Clean. Prod.. 2022;337:130597
- [CrossRef] [Google Scholar]
- Dynamic adsorption/desorption of p-xylene on nanomorphic MFI zeolites: Effect of zeolite crystal thickness and mesopore architecture. J. Hazard. Mater.. 2021;403
- [CrossRef] [Google Scholar]
- Experimental and electrical studies of Na-X zeolite for the adsorption of different dyes. J. Mol. Liq.. 2021;332:115877
- [CrossRef] [Google Scholar]
- Sorption and mechanism studies of Cu2þ, Sr2þ and Pb2þ ions on mesoporous aluminosilicates/zeolite composite sorbents. Water Sci. Technol.. 2020;82:984-997.
- [CrossRef] [Google Scholar]
- Influence of Alginate Encapsulation on Point of Zero Charge (pH pzc) and Thermodynamic Properties of the Natural and Fe(III)-Modified Zeolite. Procedia Manuf.. 2019;32:286-293.
- [CrossRef] [Google Scholar]
- Tailoring the CO2 selective adsorption properties of MOR zeolites by post functionalization. J. CO2 Util.. 2022;62:102064
- [CrossRef] [Google Scholar]
- Treatment technologies of palm oil mill effluent (POME)and olive mill wastewater (OMW): A brief review. Environ. Technol. Innov.. 2019;15:100377
- [CrossRef] [Google Scholar]
- Lepreux, S., 2005. Approche de d ́ eveloppement centr ́ e d ́ ecideur et a l ’ aide de patron de Syst ‘ emes Interactifs d ’ Aide ‘ a la D ́ ecision Application a 5–6.
- Adsorption of indium (III) from aqueous solution on raw, ultrasound- and supercritical-modified chitin: Experimental and theoretical analysis. Chem. Eng. J.. 2019;373:1247-1253.
- [CrossRef] [Google Scholar]
- Li, H., Zheng, F., Wang, J., Zhou, J., Huang, X., Chen, L., Hu, P., Gao, J. ming, Zhen, Q., Bashir, S., Liu, J.L., 2020. Facile preparation of zeolite-activated carbon composite from coal gangue with enhanced adsorption performance. Chemical Engineering Journal 390, 124513. https://doi.org/10.1016/j.cej.2020.124513.
- Adsorption behavior of phenol by reversible surfactant-modified montmorillonite: Mechanism, thermodynamics, and regeneration. Chem. Eng. J. 2017
- [CrossRef] [Google Scholar]
- Adsorption of CdII by synthetic zeolite under multi-factor using response surface methodology. Colloids Surf. A Physicochem. Eng. Asp.. 2022;647:129165
- [CrossRef] [Google Scholar]
- Atomic layer deposition precisely modified zeolite 13X: Physicochemical synergistic adsorption of space molecular contaminants. Appl. Surface Sci.. 2022;590:153084
- [CrossRef] [Google Scholar]
- Synthesis of zeolite/hydrous metal oxide composites from coal fly ash as efficient adsorbents for removal of methylene blue from water. Int. J. Miner. Process.. 2016;148:32-40.
- [CrossRef] [Google Scholar]
- Selective adsorption of Co(II)/Mn(II) by zeolites from purified terephthalic acid wastewater containing dissolved aromatic organic compounds and metal ions. Sci. Total Environ.. 2020;698:134287
- [CrossRef] [Google Scholar]
- Poisoning and competitive adsorption effects during phenol hydrogenation on platinum in water-alcohol mixtures. Appl. Catal. A: General. 2019;585:117199
- [CrossRef] [Google Scholar]
- Green method to synthesize magnetic zeolite/chitosan composites and adsorption of hexavalent chromium from aqueous solutions. Int. J. Biol. Macromol.. 2022;194:746-754.
- [CrossRef] [Google Scholar]
- Isopropanol adsorption-oxidation over V2O5 - A mass spectrometry study. J. Mol. Catal. A Chem.. 2006;247:31-35.
- [CrossRef] [Google Scholar]
- Preparation of activated carbon from black wattle bark waste and its application for phenol adsorption. J. Environ. Chem. Eng.. 2019;7:103396
- [CrossRef] [Google Scholar]
- Adsorption behavior and mechanism of mixed heavy metal ions by zeolite adsorbent prepared from lithium leach residue. Microporous Mesoporous Mater.. 2022;329:111553
- [CrossRef] [Google Scholar]
- Adsorption of phenol and Cu(II) onto cationic and zwitterionic surfactant modified montmorillonite in single and binary systems. Chem. Eng. J.. 2016;283:880-888.
- [CrossRef] [Google Scholar]
- Simultaneous adsorption of ammonia and phosphate using ferric sulfate modified carbon/zeolite composite from coal gasification slag. J. Environ. Manage.. 2022;305:114404
- [CrossRef] [Google Scholar]
- Adsorption of phenol, phosphate and Cd(II) by inorganic-organic montmorillonites: A comparative study of single and multiple solute. Colloids Surf. A Physicochem. Eng. Asp.. 2016;497:63-71.
- [CrossRef] [Google Scholar]
- Adsorption dynamics of Congo red dye removal using ZnO functionalized high silica zeolitic particles. Appl. Surf. Sci.. 2019;487:907-917.
- [CrossRef] [Google Scholar]
- Rubik’s cube shaped organic template free hydrothermal synthesis and characterization of zeolite NaA for CO2 adsorption. Fuel. 2022;317:123492
- [CrossRef] [Google Scholar]
- Modification of Zeolite by Magnetic Nanoparticles for Organic Dye Removal. Arab. J. Sci. Eng.. 2019;44:5457-5474.
- [CrossRef] [Google Scholar]
- Phenol adsorption from wastewater using clarified sludge from basic oxygen furnace. J. Environ. Chem. Eng.. 2019;7:103259
- [CrossRef] [Google Scholar]
- Simultaneous adsorption of organic and inorganic micropollutants from rainwater by bentonite and bentonite-carbon nanotubes composites. J. Water Process Eng.. 2022;46
- [CrossRef] [Google Scholar]
- Combination of X-ray powder diffraction and adsorption calorimetry for the characterization of calcium exchanged LTA zeolites. Microporous Mesoporous Mater.. 2022;337
- [CrossRef] [Google Scholar]
- Mccusker, L.B., Baerlocher, C., 2019. 4.6. Zeolites 452–464.
- Mehraban, Z., Farzaneh, F., Shafiekhani, A., 2007. Synthesis and characterization of a new organic – inorganic hybrid NiO – chlorophyll- a as optical material 29, 927–931. https://doi.org/10.1016/j.optmat.2006.02.007.
- Breakthrough study of so2 and H2O adsorption on zeolites from ternary mixtures. Stud. Surf. Sci. Catal.. 1999;125:657-666.
- [CrossRef] [Google Scholar]
- Comparative and competitive adsorption of cadmium, copper, nickel, and lead ions by Iranian natural zeolite. Clean Techn. Environ. Policy. 2013;15:303-316.
- [CrossRef] [Google Scholar]
- Effect of pressure on the sorption of Yb by montmorillonite. Clay Clay Miner.. 1983;31:17-21.
- [CrossRef] [Google Scholar]
- An efficient method for clay modification and its application for phenol removal from wastewater. Appl. Clay Sci.. 2012;59–60:8-12.
- [CrossRef] [Google Scholar]
- High efficiency removal of methylene blue dye using κ-carrageenan-poly(acrylamide-co-methacrylic acid)/AQSOA-Z05 zeolite hydrogel composites. Cellul.. 2020;27:8269-8285.
- [CrossRef] [Google Scholar]
- Zeolite-Y incorporated karaya gum hydrogel composites for highly effective removal of cationic dyes. Colloids Surf A Physicochem Eng Asp. 2020;586:124161
- [CrossRef] [Google Scholar]
- Chinese Journal of Chemical Engineering Extraordinary adsorption of acidic fuchsine and malachite green onto cheap nano-adsorbent derived from eggshell. Chin. J. Chem. Eng.. 2020;28:1591-1602.
- [CrossRef] [Google Scholar]
- Cu2O nanoparticles for adsorption and photocatalytic degradation of methylene blue dye from aqueous medium. Environ. Nanotechnol. Monit. Manage.. 2019;12
- [CrossRef] [Google Scholar]
- Adsorption of hexavalent chromium onto magnetic natural zeolite-polymer composite. J. Taiwan Inst. Chem. Eng.. 2015;50:242-251.
- [CrossRef] [Google Scholar]
- Vanadium (V) adsorption isotherms and kinetics using polypyrrole coated magnetized natural zeolite. J. Taiwan Inst. Chem. Eng.. 2016;66:172-180.
- [CrossRef] [Google Scholar]
- THE MECHANISM OF PHOSPHATE ADSORPTION BY KAOLINITE, GIBBSITE, AND PSEUDOBOEHMITE: PART I. THE ISOTHERMS AND THE EFFECT OF pH ON ADSORPTION. J. Soil Sci.. 1966;17:212-228.
- [CrossRef] [Google Scholar]
- Ammonium adsorption, desorption and recovery by acid and alkaline treated zeolite. Bioresour. Technol.. 2021;341:125812
- [CrossRef] [Google Scholar]
- Na chat, N., Sangsuradet, S., Tobarameekul, P., Worathanakul, P., 2022. Modified hierarchical zeolite X derived from riceberry rice husk for propionic acid adsorption. Materials Chemistry and Physics 282, 125933. https://doi.org/10.1016/j.matchemphys.2022.125933.
- Removal of Pb ion from water by magnetically modified zeolite. Miner. Eng.. 2006;19:1452-1455.
- [CrossRef] [Google Scholar]
- Mordenite zeolite - Polyethersulfone composite fibers developed for decontamination of heavy metal ions. J. Environ. Chem. Eng.. 2017;5:513-525.
- [CrossRef] [Google Scholar]
- Preparation of magnetic double network nanocomposite hydrogel for adsorption of phenol and p-nitrophenol from aqueous solution. J. Environ. Chem. Eng.. 2021;9:105039
- [CrossRef] [Google Scholar]
- Adsorption removal of ammonium from aqueous solution using Mg/Al layered double hydroxides-zeolite composite. Environ. Technol. Innov.. 2022;25
- [CrossRef] [Google Scholar]
- Dynamic ammonia adsorption by FAU zeolites to below 0.1 ppm for hydrogen energy applications. Int. J. Hydrogen Energy. 2021;46:32559-32569.
- [CrossRef] [Google Scholar]
- Removal of model cationic dye by adsorption onto poly(methacrylic acid)/zeolite hydrogel composites: Kinetics, equilibrium study and image analysis. Sep. Purif. Technol.. 2014;122:384-394.
- [CrossRef] [Google Scholar]
- Article Stellerite-seeded facile synthesis of zeolite X with excellent aqueous Cd 2 + and Ni 2 + adsorption performance Article Stellerite-seeded facile synthesis of zeolite X with excellent aqueous Cd 2 + and Ni 2 + adsorption performance. Chin. J. Chem. Eng. 2022
- [CrossRef] [Google Scholar]
- A review on emerging contaminants in wastewaters and the environment: Current knowledge, understudied areas and recommendations for future monitoring. Water Res.. 2015;72:3-27.
- [CrossRef] [Google Scholar]
- Ping, W., Gao, W., Cui, X., Hong, J., Feng, R., 2016. Phenol adsorption equilibrium and kinetics on zeolite X / activated 0, 1–7. https://doi.org/10.1016/j.jtice.2016.02.004.
- Enhanced adsorption of dyes on microwave-assisted synthesized magnetic zeolite-hydroxyapatite nanocomposite. J. Environ. Chem. Eng.. 2019;7:103338
- [CrossRef] [Google Scholar]
- Removal of anionic dye Congo red from aqueous environment using polyvinyl alcohol/sodium alginate/ZSM-5 zeolite membrane. Sci. Rep.. 2020;10:1-15.
- [CrossRef] [Google Scholar]
- An efficient removal of malachite green dye from aqueous environment using ZSM-5 zeolite/polyvinyl alcohol/carboxymethyl cellulose/sodium alginate bio composite. J. Polym. Environ.. 2021;29:2126-2139.
- [CrossRef] [Google Scholar]
- Statistical techniques for the optimization of cesium removal from aqueous solutions onto iron-based nanoparticle-zeolite composites. Environ. Sci. Pollut. Res.. 2021;28:12918-12931.
- [CrossRef] [Google Scholar]
- A critical and recent developments on adsorption technique for removal of heavy metals from wastewater-A review. Chemosphere. 2022;303:135146
- [CrossRef] [Google Scholar]
- Ethanol adsorption in cation-exchanged linde type L zeolite, studied by molecular simulations. Comput. Theor. Chem.. 2022;1207:113498
- [CrossRef] [Google Scholar]
- Adsorption and photocatalytic removal of SO2 using natural and synthetic zeolites-supported TiO2 in a solar parabolic trough collector. J. Clean. Prod.. 2021;310:127376
- [CrossRef] [Google Scholar]
- Adsorption-photodegradation synergetic removal of methylene blue from aqueous solution by NiO/graphene oxide nanocomposite. Powder Technol.. 2015;275:322-328.
- [CrossRef] [Google Scholar]
- Rouquerol, F., Rouquerol, J., Sing, K., 1999. Adsorption by Clays, Pillared Layer Structures and Zeolites. Adsorption by Powders and Porous Solids 355–399. https://doi.org/10.1016/b978-012598920-6/50012-9.
- Polyvinyl alcohol/carboxymethyl cellulose/ZSM-5 zeolite biocomposite membranes for dye adsorption applications. Carbohydr. Polym.. 2018;199:129-140.
- [CrossRef] [Google Scholar]
- PVA/PDADMAC/ZSM-5 zeolite hybrid matrix membranes for dye adsorption: Fabrication, characterization, adsorption, kinetics and antimicrobial properties. J. Environ. Chem. Eng.. 2018;6:3860-3873.
- [CrossRef] [Google Scholar]
- Iron oxide adsorbers for arsenic removal: A low cost treatment for rural areas and mobile applications. Desalination. 2009;248:184-192.
- [CrossRef] [Google Scholar]
- Adsorption d ’ un colorant cationique d ’ un milieu aqueux sur une argile locale activée (adsorption of dyes on activated local clay in aqueous solution) Mater. Environ. Sci.. 2014;5:2060-2065.
- [Google Scholar]
- Enhanced adsorption properties of zirconium modified chitosan-zeolite nanocomposites for vanadium ion removal. Int. J. Biol. Macromol.. 2020;164:105-120.
- [CrossRef] [Google Scholar]
- Bulgarian Academy of Sciences. Space Research and Technology Institute. Aerospace Research in Bulgaria. 29, 2017. Sofia. 2017;2015:10-29.
- [Google Scholar]
- Trapping of Ag+, Cu2+, and Co2+ by faujasite zeolite Y: New interpretations of the adsorption mechanism via DFT and statistical modeling investigation. Chem. Eng. J.. 2021;420:127712
- [CrossRef] [Google Scholar]
- Removal of catechol from aqueous solutions by adsorption onto organophilic-bentonite. J. Hazard. Mater.. 2008;150:765-773.
- [CrossRef] [Google Scholar]
- Cellulose/bentonite-zeolite composite adsorbent material coating for treatment of N-based antiseptic cationic dye from water. J. Water Process Eng.. 2019;29:100764
- [CrossRef] [Google Scholar]
- Water depollution using metal-organic frameworks-catalyzed advanced oxidation processes: A review. J. Hazard. Mater. 2017
- [CrossRef] [Google Scholar]
- Carbon nano-structures and functionalized associates: Adsorptive detoxification of organic and inorganic water pollutants. Inorg. Chem. Commun.. 2022;141:109579
- [CrossRef] [Google Scholar]
- Kinetics and thermodynamics of cadmium and lead ions adsorption on NiO nanoparticles. Chem. Eng. J.. 2012;191:123-131.
- [CrossRef] [Google Scholar]
- Synthesis of a novel zeolite-activated carbon composite using lithium-silicon-powder waste for ammonia-nitrogen and methylene blue removal. Ind. Eng. Chem. Res.. 2020;59:14616-14624.
- [CrossRef] [Google Scholar]
- Synthesis and characterization of zeolite-based composites functionalized with nanoscale zero-valent iron for removing arsenic in the presence of selenium from water. J. Hazard. Mater.. 2019;373:810-819.
- [CrossRef] [Google Scholar]
- Adsorption, separation and regeneration of cation-exchanged X zeolites for LNG purification: Li+, K+, Mg2+ and Ca2+. Microporous Mesoporous Mater.. 2022;340:112032
- [CrossRef] [Google Scholar]
- Treatment of phenolic wastewater by combined UF and NF/RO processes. Desalination. 2015;355:68-74.
- [CrossRef] [Google Scholar]
- Effects of thermal gradients in a solar adsorption heat pump utilizing the zeolite-water pair. Appl. Therm. Eng.. 1999;19:1157-1172.
- [CrossRef] [Google Scholar]
- A qualitative 1H NMR study of CHCl3 adsorption on conjugated acid-base pairs in cation exchanged Y-zeolites. Microporous Mesoporous Mater.. 1999;30:327-333.
- [CrossRef] [Google Scholar]
- Adsorption performance of standard biochar materials against volatile organic compounds in air: A case study using benzene and methyl ethyl ketone. Chem. Eng. J.. 2019;123943
- [CrossRef] [Google Scholar]
- Olive mill wastewater integrated purification through pre-treatments using coagulants and biological methods: Experimental, modelling and scale-up. J. Clean. Prod.. 2019;236:117622
- [CrossRef] [Google Scholar]
- Adsorption of phenol and bisphenol A on river sediments: Effects of particle size, humic acid, pH and temperature. Ecotoxicol. Environ. Saf.. 2020;204:111093
- [CrossRef] [Google Scholar]
- A periodic density functional theory study on methanol adsorption in HSAPO-34 zeolites. Chem. Phys. Lett.. 2021;771:138532
- [CrossRef] [Google Scholar]
- Unraveling the adsorption and diffusion properties of hexamethyldisiloxane on zeolites by static gravimetric analysis. Water Res.. 2021;197
- [CrossRef] [Google Scholar]
- Simultaneous adsorption of phosphate and zinc by lanthanum modified zeolite. Environ. Technol. Innov.. 2021;24:101906
- [CrossRef] [Google Scholar]
- Treatment of paper mill wastewater using a composite inorganic coagulant prepared from steel mill waste pickling liquor. Sep. Purif. Technol.. 2019;209:238-245.
- [CrossRef] [Google Scholar]
- Preparation and Characterization of Cationic Water-Soluble Pillar[5]arene-Modified Zeolite for Adsorption of Methyl Orange. ACS Omega. 2019;4:17741-17751.
- [CrossRef] [Google Scholar]
- Promotion mechanism of natural clay colloids in the adsorption of arsenite on iron oxide particles in water. Chem. Eng. J.. 2020;392:123637
- [CrossRef] [Google Scholar]
- The effect of pH on the adsorption of phenol and chlorophenols onto natural zeolite. Colloids Surf. A Physicochem. Eng. Asp.. 2009;334:92-99.
- [CrossRef] [Google Scholar]
- Adsorption characteristics of natural zeolites as solid adsorbents for phenol removal from aqueous solutions: Kinetics, mechanism, and thermodynamics studies. Chem. Eng. J.. 2011;171:1143-1149.
- [CrossRef] [Google Scholar]
- Adsorption Characterization of Strontium on PAN/Zeolite Composite Adsorbent. World J. Nuclear Sci. Technol.. 2011;01:6-12.
- [CrossRef] [Google Scholar]
- Preparation and characterization of surfactant-modified hydroxyapatite/zeolite composite and its adsorption behavior toward humic acid and copper(II) Environ. Sci. Pollut. Res.. 2013;20:2512-2526.
- [CrossRef] [Google Scholar]
- Facile synthesis of hollow p-Cu2O/n-ZnO microspheres with enhanced photocatalytic H2 production. Chem. Phys. Lett.. 2019;734:136748
- [CrossRef] [Google Scholar]
- Excellent adsorption of Zn(II) using NaP zeolite adsorbent synthesized from coal fly ash via stage treatment. J. Clean. Prod.. 2020;258:120736
- [CrossRef] [Google Scholar]
- Modification of synthetic zeolite X by thiourea and its adsorption for Cd (II) Mater. Lett.. 2019;236:233-235.
- [CrossRef] [Google Scholar]
- Synthesis of reduced graphene oxide/NiO nanonanocomposites for the removal of Cr(VI) from aqueous water by adsorption. Microporous Mesoporous Mater.. 2017;255:7-14.
- [CrossRef] [Google Scholar]
- Utilization of bark waste of Acacia mangium: The preparation of activated carbon and adsorption of phenolic wastewater. Ind. Crop. Prod.. 2021;160:113157
- [CrossRef] [Google Scholar]
- Synthesis of CoFe2O4-zeolite materials and application to the adsorption of gallium and indium. J. Chem. Eng. Data. 2015;60:1338-1344.
- [CrossRef] [Google Scholar]
- Facile one-pot synthesis of novel spherical zeolite-reduced graphene oxide composites for cationic dye adsorption. Ind. Eng. Chem. Res. 2014
- [CrossRef] [Google Scholar]






