Translate this page into:
A potential antifungal agent: Insight into the antifungal mechanism against Phomopsis sp
⁎Corresponding author. wxue@gzu.edu.cn (Wei Xue)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
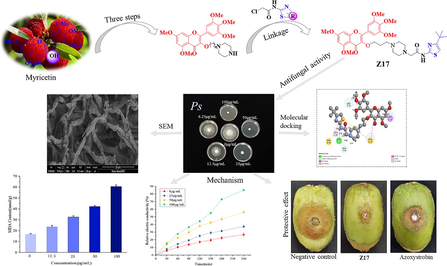
19 myricetin derivatives containing thiazole were designed and synthesized. Their fungicidal activities in vitro against ten species of plant pathogenic fungi were evaluated. Bioassay results indicated that some of compounds exhibited remarkable antifungal activities. Among them, Z17 showed the strongest antifungal activity against Phomopsis sp, with in vitro EC50 of 12.3 µg/mL, which was superior to those of the control drug azoxystrobin (32.2 µg/mL) and fluopyram (77.7 µg/mL). In addition, Z18 also had the inhibitory activity against the Alternaria brassicae, with an EC50 value of 32.5 µg/mL, which was much higher than azoxystrobin (49.3 µg/mL). Z17 exhibited good protective activity (62.5 %) against Phomopsis sp on kiwifruit in vivo at 200 µg/mL. The results of in vivo experiments revealed that Z18 could effectively defend against the infestation of cabbage by Alternaria brassicae, and improve the protection ability of the crop. Scanning electron microscopy (SEM) and fluorescence microscopy (FM) demonstrated that Z17 could destroy the integrity of cell membrane of pathogen Phomopsis sp, thus affecting the normal growth of mycelia. The results of the mechanism research further confirmed that the action of Z17 changed the mycelial morphology of Phomopsis sp, affected the permeability of cells, increased the leakage of cytoplasm and MDA contents. Molecular docking simulation results revealed that compound Z17 could readily bind with the active site of SDH and could be a potential SDH inhibitor. In summary, this study provides new ideas for effectively controlling plant fungal diseases and developing new green chemical pesticide products.
Keywords
Myricetin
Thiazole
Antifungal activity
Mechanism of action research
1 Introduction
Plant pathogenic fungi seriously influence the flourishing development of world agriculture, posing a serious threat to global food security. At the same time, they also can endanger the normal development and growth of crops, resulting in reducing production and significant losses of fruits and vegetables. Some fungal toxins produced by plant pathogenic fungi can even endanger the health of humans and animals (Gao et al., 2023; Zhang et al., 2023a, 2023b). Kiwifruit is a popular fruit that contains many nutrients such as vitamins, polyphenols, and pectin (Figiel-Kroczyńska, 2021). Unfortunately, it is easy to be infected by pathogenic fungi such as Phomopsis sp after picking, resulting in fruit rot, leaf peel necrosis and other plant diseases, which leads to a decline in fruit quality and yield (Zhou et al., 2023). Sclerotinia sclerotiorum (S. sclerotiorum) and Rhizoctonia solani (R. solani) are extremely harmful to rape and rice, and the economic losses caused by these two pathogenic fungi are as high as 80 and 50 % each year (Dey et al., 2016; Liu et al., 2022). Cruciferous vegetables are rich in nutrients and good for human health. Eating more cruciferous vegetables can prevent the occurrence of cancer (Zeng et al., 2023). Alternaria brassicae (A. brassicae) is one of the most common and destructive fungal diseases in cruciferous family, and spreads in different places mainly through seed transmission and harms the leaves and the whole plant, which leads to a serious decline in the quality and quantity of cabbage (Belmas et al., 2018). At present, traditional chemical fungicides, including benzimidazoles, dithiocarbamates, SDH inhibitors, and azoles, are still the most commonly used methods for controlling various plant pathogens due to their high efficiency, broad spectrum, economy, and ease of access. However, with the extensive and long-term application of fungicides, an increasing number of pathogens have accelerated the emergence and development of drug resistance, causing serious environmental problems. This greatly reduces the effectiveness of fungicides and requires an increase in dosage during application process, leading to a vicious cycle (Gao et al., 2023). Therefore, it is urgent to discover and create efficient and low toxic green antifungal drugs by using natural products as the framework for structural modification.
At present, the application of natural products for drug molecular design in agriculture has become one of the hotspots. Myricetin is a natural polyhydroxy flavonoid compound that can be extracted from many plants, fruits, vegetables, and beverages, such as bayberry bark, tomatoes, onions, red wine and so on (Ong and Khoo, 1997). In the early research work of our group, it was found that myricetin and its derivatives had a wide range of biological activities, such as antiviral (Tang et al., 2020), antibacterial (Rashed et al., 2014; Liu et al., 2021), and anticancer (Xue et al., 2015). For example, Peng et al. designed and synthesized a series of myricetin derivatives containing 1,3,4-oxadiazole sulfide, which showed good antiviral activity. Compound A (Fig. 1) showed obvious curative and protective effects against tobacco mosaic virus, with EC50 values of 195.2 ± 4.4, 189.9 ± 3.5 μg/mL, which was superior to that of ningnanmycin (296.4 ± 3.8, 307.6 ± 4.4 μg/mL) (Peng et al., 2021). Jiang et al. introduced the active groups piperidine and dithiocarbamate into myricetin. B (Fig. 1) exhibited the excellent antibacterial activity against Xac (Xanthomonas axonopodis pv. citri), with EC50 value of 0.1 μg/mL, which was better than that of thiazoin (60.0 μg/mL) (Jiang et al., 2020). Cao et al. constructed a series of myricetin derivatives containing isoxazole, among which C (Fig. 1) demonstrated good activity against S. sclerotiorum, with EC50 value of 13.2 μg/mL, which was higher than that of the control agent azoxystrobin 23.0 μg/mL (Cao et al., 2023). Liu et al. introduced a myricetin derivative containing pyrazol piperazine amide, and D (Fig. 1) had an EC50 of 16.9 μg/mL against Phomopsis sp, which was better than that of azoxystrobin 50.7 μg/mL (Liu et al., 2023). Therefore, myricetin was expected to be used as the lead compound in this study to obtain some novel compounds with higher antifungal activity.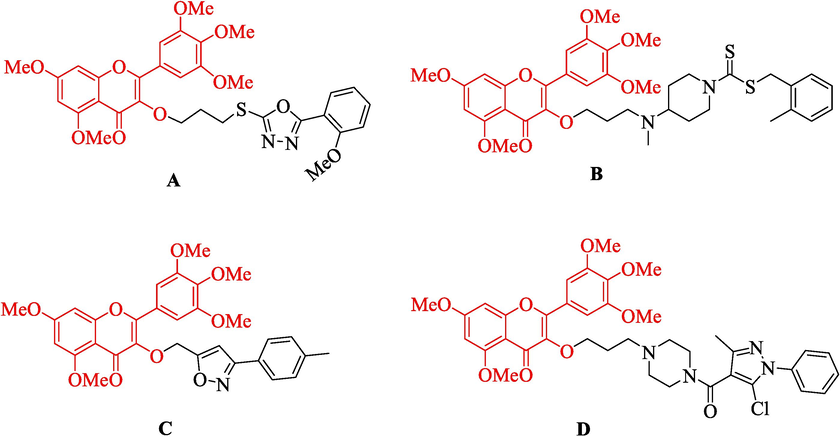
Structures of myricetin derivatives previously reported by our group.
Thiazole rings containing two heteroatoms N and S are a good pharmacodynamic molecule and are widely found in natural products (Rouf and Tanyeli, 2015). It has been widely used due to its antibacterial (Zhu et al., 2021), anticancer (Cai et al., 2016), insecticidal (Borcea et al., 2021), anti-inflammatory (Kamat et al., 2020), antiviral properties (KanagasAbapathy et al., 2023), and high bioavailability and good biocompatibility (Meng et al., 2022). For example, commercially available fungicide isothiazolinone (Silva et al., 2020), antifungal agent abafungin (Borelli et al., 2008), insecticide thiamethoxam (Ferreira-Junior et al., 2018) and the anti-cancer drug dasatinib (Bolf et al., 2023) all contain thiazole ring structures (Fig. 2). Therefore, the innovation of thiazole structure and its extensive biological activity have attracted widespread attention from pharmaceutical chemists.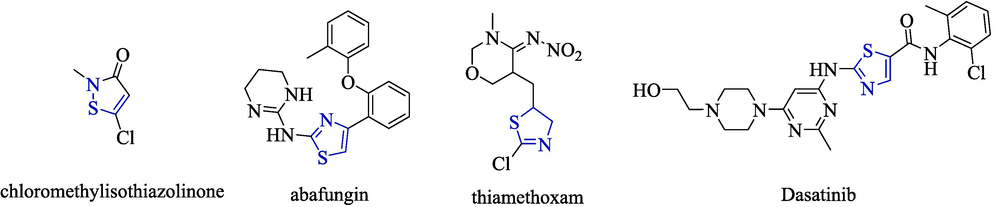
Thiazole active group fragment.
On the basis of these considerations, we assumed to introduce thiazole functional groups into the structure of myricetin through active splicing, and a series of myricetin derivatives containing thiazole structures were designed and synthesized (Fig. 3). Subsequently, the antifungal activity of the target compounds were evaluated and screened. Through antifungal activity screening and studying the mechanism of action, we found out the compound Z17 with broad-spectrum antifungal activity, which provided sufficient support for further development of myricetin derivatives.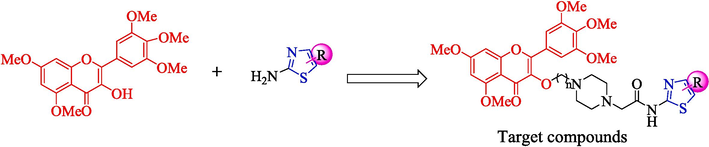
The design ideas of target compounds.
2 Materials and methods
2.1 Instruments and chemicals
The measurement of melting points was carried out using an XT-4 binocular microscope without calibration (Beijing, China). The relevant information for NMR was obtained by using a 500 MHz nuclear magnetic resonance instrument (JEOL-ECX500, Japan Electronics Co., Tokyo, Japan). High-resolution mass spectrometry (HRMS) used the Thermo Scientific Q Active mass spectrometer (Missouri, USA). SEM data obtained from FEI Nova Nano 450 (Oregon, USA). Observing the staining of cell hyphae using Olympus BX53 fluorescence microscope (Tokyo, Japan). The permeability of cell membranes was measured using a conductivity meter Leici DDSJ-3O8F (Shanghai, China). The content of MDA was determined by the Multiskan FC microplate reader assay (Shanghai, China). The release of cell contents was recorded by an N-5000 ultraviolet spectrophotometer (Shanghai, China). All reagents and solvents used in the experiment were purchased from Shanghai Titan Chemical Co., Ltd. (Shanghai, China) and Beijing Solarbio Technology Co., Ltd. (Beijing, China). All reagents are analytically pure and can be used directly.
2.2 Synthesis
2.2.1 Synthesis of intermediates 1–5
Intermediates 1 and 2 were synthesized by referring to the literature (Chen et al., 2019). The intermediates 3 to 5 were prepared according to the methods reported in the literature and slightly modified (Das et al., 2006; Zhou et al., 2022; Zhang et al., 2023a, 2023b). Firstly, 5.73 mmol 4-tert-butoxypiperazine, 5.73 mmol K2CO3, and 9.98 mL acetonitrile solvent were added to the round-bottom flask, heated at 80 ℃ for 0.5 h, and then adding intermediate 2. The reaction was monitored by TLC (dichloromethane:methanol = 30:1). After the reaction was completed, the reaction system was poured into ice water and white solid was precipitated to obtain intermediate 3. With methanol as the solvent, intermediate 3 (1.59 mmol) was added into a 100 mL round-bottomed flask, stirring at 80 ℃ for 10 min, then slowly adding 0.59 mL of HCl to the reaction vessel using a pipette, and continue refluxing for 2 h to gain intermediate 4. The different substitutions of 2-aminothiazole (8.76 mmol), 0.92 mL pyridine and 9.15 mL acetonitrile were successively added into the round-bottom flask and stirred at room temperature for 0.5 h, after adding 0.84 mL chloroacetyl chloride under the condition of ice bath. At the end of the reaction, the intermediate 5 was obtained by vacuum concentration and filtration.
2.2.2 Synthesis of target compounds Z1-Z19
The synthetic steps of the target compounds Z1-Z19 referred to the methods reported in the literature (He et al., 2020; Peng et al., 2023). 6.75 mL DMF was used as solvent, 3.54 mmol K2CO3 as acid binder, 0.88 mmol intermediate 4 was added to a round-bottom flask, stirred at room temperature for 45 min until solid dissolution, and then 1.77 mmol intermediate 5 was added to the reaction system and heated at 80 ℃ for 12 h. After the reaction was complete, the system was poured into ice water. The oil was extracted by dichloromethane and dried with anhydrous Na2SO4, then concentrated under reduced pressure. The obtained oil was further purified by column chromatography (ethyl acetate: methanol = 30:1 v/v).
2.3 Antifungal activity assay in vitro
The inhibitory effect of Z1-Z19 on fungi in vitro was tested with mycelium growth rate method (Fang et al., 2022). In addition, compounds with better activity were selected for further determination of the half effective concentration (EC50). The commercial agents azoxystrobin and fluopyram were used as positive controls. Each experiment was repeated three times.
2.4 Antifungal bioassay in vivo
According to the methods reported in the literature (Zhou et al., 2023), the inhibitory activity of Z17 against Phomopsis sp on kiwifruit was evaluated at 200 and 100 μg/mL with azoxystrobin as the positive control. The effect of Z18 against A. brassicae on cabbage was evaluated by using the azoxystrobin as positive control, and the disease degree was graded as follows. There is grade 0, there is no disease. At grade 1, the lesion area accounted for less than 5 % of the leaf area. At grade 2, the lesion area accounted for 6–10 % of the leaf area. At grade 3, the lesion area accounted for 11–25 % of the leaf area. At grade 4, the lesion area accounted for 26–50 % of the leaf area. At grade 5, the lesion area accounted for more than 50 % of the leaf area, with azoxystrobin as the positive control (He et al., 2021; Zhang et al., 2022). Each experiment was repeated three times.
2.5 Scanning electron microscope and fluorescence microscope
With reference to the previous literature (Fang et al., 2022; Xie et al., 2022), Z17 was tested by scanning electron microscope and fluorescence microscope to observe the morphological changes of mycelia, with the aim of studying the inhibitory effect of Z17 on Phomopsis sp further.
2.6 Formation of sclerotia
The experiment of Z17 on sclerotia formation was performed by previously reported in the literature (Zhang et al., 2018).
2.7 Permeability of cell membranes
According to previous reports, the permeability of cell membranes was analyzed by measuring the relative conductivity of mycelium (Wang et al., 2020).
2.8 Release of cellular contents
Ultraviolet spectrophotometer was used to measure the absorbance of the supernatant at 260 and 280 nm, respectively, thereby to judge the degree of cytoplasmic release (Cao et al., 2023).
2.9 Determination of malondialdehyde content
The content of malondialdehyde (MDA) reflects the degree of lipid peroxidation of animal and plant cell membranes. The higher the content of MDA is, the higher the degree of lipid peroxidation of cell membranes is, and the more serious the damage to cell membranes is. According to the methods reported in the literature, the content of MDA in Z17 was studied (Chen et al., 2023; Hu et al., 2023).
2.10 Molecular docking
To further investigate whether SDH is a potential target for action, we conducted molecular docking of Z17 and fluopyram (Succinate dehydrogenase inhibitor) with SDH (Liu, et al., 2023). Discovery studio 2019 tools and PyMOL Win software were used to simulate and verify the binding ability of Z17 to SDH active proteins (PDB: 2FBW).
3 Results and discussion
3.1 Chemistry
The Z1-Z19 were synthesized according to the designed route of Scheme 1. All the compounds were characterized by NMR and HRMS, the detailed data are in the Supporting material.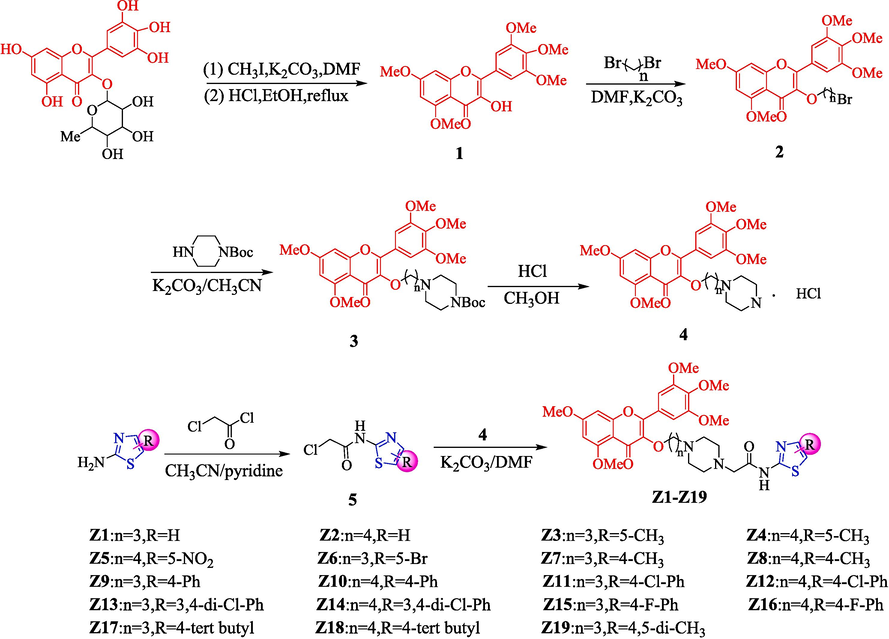
Synthetic route of compounds Z1-Z19.
3.2 In vitro antifungal activity
Z1-Z19 were tested for in vitro inhibitory activities against 10 plant fungi at 100 μg/mL. The test results were shown in Table 1 that Z17 had a certain inhibitory activity on all the test strains. Z17 possessed the best inhibitory effect on Phomopsis sp, with an inhibition rate of 94.3 %, which was significantly higher than those of the azoxystrobin (65.2 %) and fluopyram (51.9 %). Besides, Z17 also exhibited good inhibitory effects on R. solani, S. sclerotiorum, Fusarium graminearum, Botrytis cinerea, and A. brassicae, with inhibition rates of 80.3, 83.3, 69.5, 75.8, and 69.0 %, respectively, which were superior to those of azoxystrobin 74.5, 62.4, 60.9, 68.8, and 59.5 %. At the same time, Z6 also had good inhibitory effects on S. sclerotiorum and Phomopsis sp, with inhibition rates of 80.8 and 91.7 %, which were better than the those of azoxystrobin 62.4 and 65.2 %. The inhibition rates of Z4 on Phomopsis sp and Phytophthora capsici were 70.4 and 80.2 %, which were better than the those of azoxystrobin 65.2 and 71.2 %. The inhibitory rate of Z18 on A. brassicae was 72.0 %, which was higher than the control drug azoxystrobin 59.5 %.
Compounds
Inhibition rates (%) a
Ps
Ss
Bd
Rs
Fg
Pc
Bc
Ab
Cg
Fo
Z1
47.4 ± 0.9
41.6 ± 2.9
22.7 ± 2.2
58.6 ± 1.9
34.8 ± 1.9
63.9 ± 1.4
59.2 ± 2.7
36.2 ± 2.4
22.7 ± 2.2
18.7 ± 3.4
Z2
66.5 ± 0.9
59.6 ± 2.3
29.3 ± 2.3
58.2 ± 2.3
30.9 ± 2.7
59.7 ± 1.2
61.3 ± 1.2
33.2 ± 2.3
29.3 ± 2.3
21.8 ± 1.6
Z3
49.6 ± 2.4
60.8 ± 2.4
24.8 ± 1.8
54.0 ± 3.0
33.9 ± 1.9
73.8 ± 1.7
60.0 ± 2.0
33.6 ± 4.3
24.8 ± 1.8
19.8 ± 7.2
Z4
70.4 ± 1.2
66.5 ± 1.8
33.1 ± 2.6
57.7 ± 1.7
36.5 ± 3.7
80.2 ± 2.4
65.8 ± 2.3
41.4 ± 1.2
33.1 ± 2.6
19.8 ± 4.8
Z5
28.3 ± 3.5
5.3 ± 3.8
13.2 ± 1.4
54.8 ± 2.0
16.7 ± 4.2
30.5 ± 2.9
50.8 ± 1.1
22.4 ± 2.0
13.2 ± 1.4
7.1 ± 5.0
Z6
91.7 ± 1.7
80.8 ± 1.6
26.4 ± 2.3
63.2 ± 1.8
43.8 ± 1.7
57.5 ± 1.2
65.8 ± 1.1
62.1 ± 3.1
26.4 ± 2.3
30.6 ± 6.5
Z7
65.7 ± 0.9
60.0 ± 3.3
19.8 ± 2.7
49.8 ± 1.4
37.3 ± 1.9
58.4 ± 0.9
51.7 ± 5.0
37.1 ± 1.9
19.8 ± 2.7
26.6 ± 1.6
Z8
73.5 ± 0.9
62.0 ± 2.3
26.4 ± 2.3
45.2 ± 2.2
37.8 ± 1.7
60.9 ± 3.4
52.1 ± 2.2
37.5 ± 1.7
26.4 ± 2.3
27.0 ± 1.7
Z9
53.5 ± 2.7
60.8 ± 2.0
24.8 ± 1.1
39.7 ± 2.0
27.5 ± 3.7
37.8 ± 3.4
62.1 ± 0.9
37.1 ± 2.8
24.8 ± 1.1
26.2 ± 3.3
Z10
69.1 ± 3.7
62.4 ± 1.1
23.6 ± 0.9
45.2 ± 0.9
32.2 ± 1.9
38.6 ± 0.9
65.0 ± 2.8
49.1 ± 1.2
23.6 ± 0.9
16.3 ± 5.9
Z11
53.9 ± 3.5
64.9 ± 2.6
18.6 ± 1.7
51.5 ± 4.4
38.6 ± 1.7
56.2 ± 1.4
66.7 ± 2.3
35.3 ± 1.4
18.6 ± 1.7
24.6 ± 5.5
Z12
34.3 ± 1.7
45.7 ± 1.6
24.8 ± 1.1
49.8 ± 1.4
38.2 ± 2.9
30.0 ± 3.7
66.3 ± 2.7
34.1 ± 4.7
24.8 ± 1.1
13.9 ± 5.9
Z13
50.0 ± 2.7
43.3 ± 1.6
12.8 ± 0.9
58.6 ± 3.7
36.5 ± 3.1
37.3 ± 2.8
55.0 ± 2.4
30.6 ± 3.9
12.8 ± 0.9
10.7 ± 3.2
Z14
20.4 ± 1.3
32.7 ± 1.2
7.9 ± 3.8
57.7 ± 1.7
29.2 ± 3.5
54.1 ± 3.4
58.8 ± 3.1
29.3 ± 1.2
7.9 ± 3.8
1.2 ± 2.2
Z15
40.4 ± 3.7
53.9 ± 1.6
16.1 ± 3.2
51.5 ± 1.8
39.5 ± 2.4
45.1 ± 1.2
63.8 ± 4.2
43.1 ± 2.0
16.1 ± 3.2
19.8 ± 3.7
Z16
30.9 ± 2.8
50.6 ± 4.2
14.0 ± 1.1
47.3 ± 1.4
35.6 ± 3.2
28.8 ± 2.4
62.1 ± 3.3
45.3 ± 0.9
14.0 ± 1.1
21.0 ± 4.7
Z17
94.3 ± 0.9
83.3 ± 2.1
58.7 ± 2.3
80.3 ± 1.7
69.5 ± 3.7
66.5 ± 1.4
75.8 ± 2.3
69.0 ± 0.0
56.2 ± 3.0
53.6 ± 2.2
Z18
19.1 ± 1.5
16.7 ± 1.4
61.2 ± 3.3
53.6 ± 3.9
37.3 ± 4.0
36.5 ± 7.7
43.8 ± 3.1
72.0 ± 1.7
61.2 ± 3.3
7.1 ± 3.6
Z19
78.7 ± 1.7
61.2 ± 4.7
25.2 ± 3.2
54.0 ± 3.4
47.6 ± 4.0
54.9 ± 1.2
58.8 ± 1.9
44.0 ± 1.9
25.2 ± 3.2
27.0 ± 2.6
YMr.
40.4 ± 0.1
29.0 ± 2.1
24.4 ± 1.8
26.2 ± 1.9
33.0 ± 3.2
29.2 ± 1.9
43.8 ± 1.2
7.4 ± 2.4
33.9 ± 1.8
17.1 ± 5.9
Azb
65.2 ± 2.4
62.4 ± 1.1
66.1 ± 1.1
74.5 ± 1.7
60.9 ± 2.2
71.2 ± 0.9
68.8 ± 2.7
59.5 ± 1.2
66.1 ± 1.1
51.2 ± 1.8
Fl b
51.9 ± 1.6
–
–
–
–
–
–
–
–
–
As shown in Table 1, we could see that the introduction of a thiazole moiety with antifungal activity into myricetin can significantly enhance its inhibitory effect. Therefore, we studied the control effects of different substituents on plant pathogens by changing the structure of thiazole and analyzed their structure–activity relationships. When the R = 4-C(CH3)3 and R = 4-Ph substituents, their in vitro antifungal activities were better than that of R = 5-Br and R = 4-F-Ph, such as Z17 (n = 3, R = 4-C(CH3)3) > Z6 (n = 3, R = 5-Br), Z9 (n = 3, R = 4-Ph) > Z16 (n = 4, R = 4-F-Ph). This indicates that when the R group was an electron donating group, myricetin derivatives have better antifungal activities. In addition, we have found that brominated alkanes with a carbon atom number of 3 have better antifungal activity than those with a carbon atom number of 4, such as (Z17, n = 3, R = 4-C(CH3)3 > (Z18, n = 4, R = 4-C(CH3)3), (Z11, n = 3, R = 4-Cl-Ph) > (Z12, n = 4, R = 4-Cl-Ph), which indicated that shortening the carbon chain can make the binding between compounds closer and have better antifungal effects.
In summary, when the number of C atoms in brominated alkanes was 3 and the R group on the thiazole group was an electron donating group, the antifungal activities increase significantly. In order to further verify the antifungal efficacy, EC50 test was conducted on some compounds. As shown Fig. 4 and Table 2, Z17 had the optimal EC50 value for Phomopsis sp (12.3 µg/mL), which was superior to those of azoxystrobin (32.2 µg/mL) and fluopyram (77.7 µg/mL). The EC50 value of Z18 against A. brassicae was 32.5 µg/mL, which was better than the that of azoxystrobin (49.3 µg/mL).
In vitro antifungal effects of Z17 against Phomopsis sp at different concentrations.
Compounds
Phytopathogen
Regression equation
r
EC50(μg/mL)a
Z6
Ss
y = 2.6288x + 0.9667
0.9569
34.2
Z17
Ss
y = 1.4578x + 3.0769
0.9732
20.9
Az
Ss
y = 0.8128x + 3.7499
0.9812
34.5
Z6
Ps
y = 1.5401x + 3.3079
0.9960
12.6
Z8
Ps
y = 1.6356x + 2.4724
0.9836
35.1
Z17
Ps
y = 1.8964x + 2.9318
0.9857
12.3
Z19
Ps
y = 1.0323x + 3.5524
0.9347
25.3
Az
Fl
Ps
Ps
y = 0.6440x + 4.0290
y = 1.5210x + 2.12420.9903
0.980832.2
77.7
Z4
Pc
y = 1.1789x + 3.2549
0.9923
30.2
Az
Pc
y = 0.7478x + 3.8068
0.9809
39.4
Z17
Rs
y = 2.1291x + 1.6775
0.9853
36.4
Az
Rs
y = 0.5320x + 4.7451
0.9936
3.0
Z17
Fg
y = 1.2113x + 3.0195
0.9918
43.2
Az
Fg
y = 0.5534x + 4.4612
0.9986
9.4
Z17
Bc
y = 1.6081x + 2.2809
0.9708
49.1
Az
Bc
y = 1.2816x + 2.7232
0.9987
59.8
Z17
Ab
y = 1.9509x + 1.7919
0.9833
44.1
Z18
Ab
y = 1.7018x + 2.4278
0.9829
32.5
Az
Ab
y = 0.3965x + 4.3289
0.9853
49.3
3.3 Z17 in vivo fungicidal activities resist Phomopsis sp
The results of in vivo experiments showed that Z17 had good protective and curative effects (Fig. 5, Table 3) on kiwifruit infected with Phomopsis sp at 200 µg/mL. The protective effect of Z17 was 62.5 % on kiwifruit, superior to that of the azoxystrobin 40.7 %. The curative effect of Z17 (31.4 %) was close to that of azoxystrobin (31.1 %). Therefore, we can draw the following conclusion that Z17 could reduce the diameter of the lesion significantly, thereby inhibiting Phomopsis sp growth and exhibiting good in vivo antifungal activity. As shown in Fig. 5, Z17 showed no obvious toxicity to kiwifruit at a high concentration.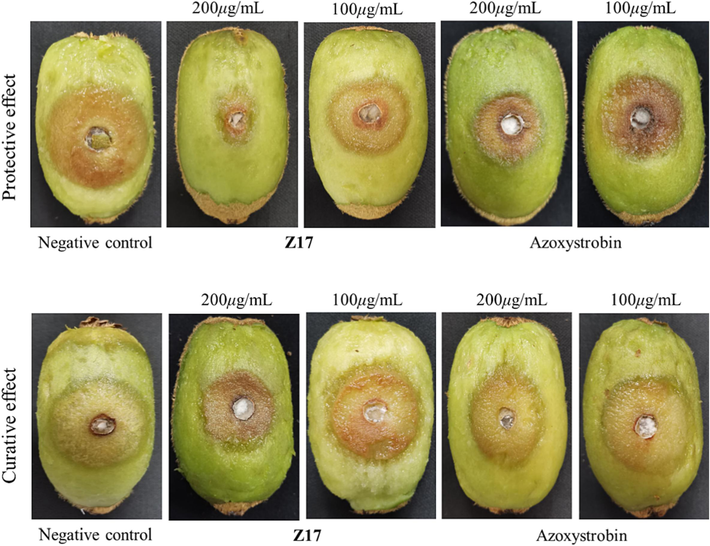
The protective and curative effect of Z17 and azoxystrobin against Phomopsis sp on kiwifruit.
Compound
Concentrations
(μg/mL)Curative effect
Protective effect
Lesion length
Control
Lesion length
Control
(mm ± SD)
effeicacy(%)a
(mm ± SD)
effeicacy(%)a
Z17
200
28.6 ± 0.5
31.4
16.2 ± 0.7
62.5
100
35.5 ± 0.9
14.8
28.2 ± 0.8
35.7
Az
200
28.7 ± 0.4
31.1
25.6 ± 1.0
40.7
100
36.0 ± 0.0
13.6
35.3 ± 0.5
18.3
Control
0
41.7 ± 0.8
–
43.2 ± 0.9
–
3.4 Z18 in vivo fungicidal activities resist A. brassicae
According to Fig. 6 and Table 4, it was observed that the negative control produced a large number of spot lesions and leaf necrosis and wilt, while the leaves treated with Z18 only formed a small number of scattered spot lesions regardless of curative or protective, and the number of spots was less than that of the control drug azoxystrobin. At 200 μg/mL, the protective and curative effects of Z18 against A. brassicae on cabbage were 84.4, 81.9 %, which were superior to that of azoxystrobin 57.9, 43.5 %. In conclusion, Z18 exhibits excellent protective and curative activities against A. brassicae.
The protective and curative effect of Z18 and azoxystrobin against A. brassicae on cabbage.
Compound
Concentrations
(μg/mL)Curative effect
Protective effect
Lesion length
Control
Lesion length
Control
(mm ± SD)
effeicacy(%)a
(mm ± SD)
effeicacy(%)a
Z18
200
1.8 ± 0.5
81.9
1.7 ± 0.0
84.4
100
6.3 ± 0.4
36.9
4.7 ± 0.4
56.6
Az
200
5.6 ± 0.5
43.5
4.6 ± 0.3
57.9
100
7.3 ± 0.1
26.5
7.1 ± 0.1
35.3
Control
0
10.0 ± 0.5
–
11.0 ± 0.4
–
3.5 Observations on the morphology of Phomopsis sp
3.5.1 Scanning electron microscope (SEM) experiment of Z17
The mechanism of Z17 on Phomopsis sp was further studied by using SEM, which is consistent with the previous experimental conclusions. The mycelia morphology of Phomopsis sp treated with Z17 changed significantly. As shown in Fig. 7, the mycelium of group A without drug treatment was smooth and full in morphology, good in extension and uniform in thickness. When Z17 was 50 μg/mL (group B), the mycelium began to flatten, the surface was depressed, and the growth was obviously inhibited. When the concentration of the compound increased to 100 μg/mL (group C), the surface damage of the mycelium was aggravated, severe atrophy occurred, and the morphological folds were shriveled. In conclusion, with the increase of compound concentration, the degree of damage to mycelia intensified, which indicated that Z17 could destroy the mycelia morphology of Phomopsis sp and inhibit its normal growth.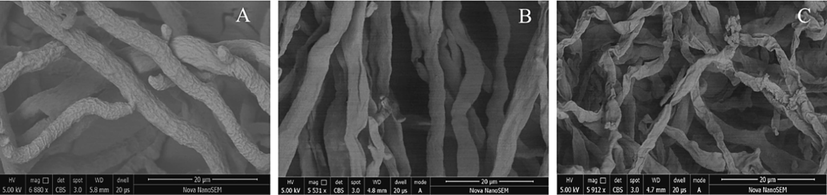
SEM images of the hyphae of Phomopsis sp after treatment with Z17. (A) 0 μg/mL, (B) 50 μg/mL, (C) 100 μg/mL. Scale bar for (A-C) are 20 μm.
3.5.2 Fluorescence microscope (FM) imaging experiment of Z17
The Fig. 8 showed the situation of Z17 treated with Phomopsis sp at 0, 50, 100 μg/mL and stained with PI. As can be seen from Fig. 8 - A and A1, untreated (0 μg/mL) mycelium was thin and could not be stained by PI. When Z17 was 50 and 100 μg/mL (Fig. 8 - B and B1, C and C1), the mycelia had a strong shape, uneven surface, emitted strong red fluorescence obviously, and the fluorescence became stronger and stronger with the increase of drug concentration. In short, Z17 could damage the integrity of mycelial cell membranes and have a strong impact on mycelial morphology.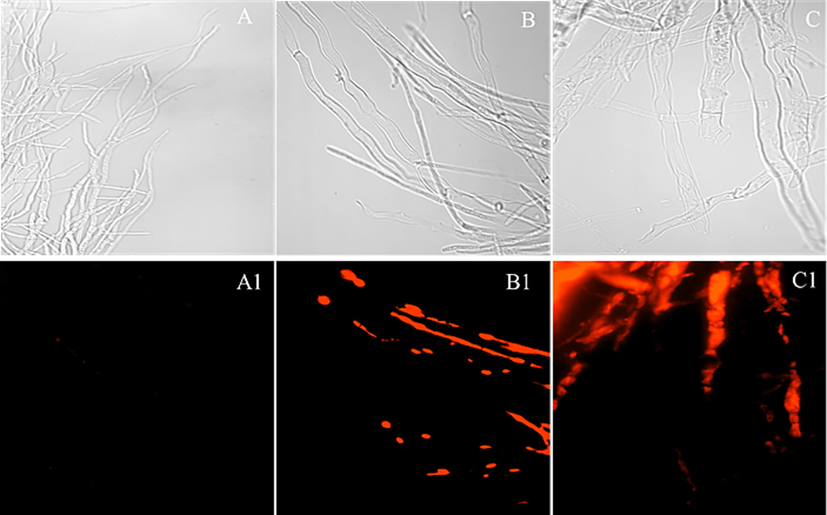
Morphological observation by FM of Phomopsis sp treated with Z17. (A-A1) 0 μg/mL, (B-B1) 50 μg/mL, (C-C1) 100 μg/mL.
3.6 Inhibition effect of Z17 on the formation of sclerotia of Phomopsis sp
As shown in Fig. 9 and Table 5, Z17 had a certain inhibitory effect on the formation of Phomopsis sp sclerotia. At a concentration of 100 μg/mL, the inhibition rate of Z17 reached 98.0 %, which was better than that of azoxystrobin (96.5 %). It can be seen that the number of sclerotia was almost completely inhibited. And when the concentration was 50 μg/mL, a small amount of sclerotium was produced. With the reduction of the concentration of the drug, the number of sclerotia increased, but both were better than the commercial drug azoxystrobin. This indicated that Z17 had a good effect on inhibiting the formation of Phomopsis sp sclerotia.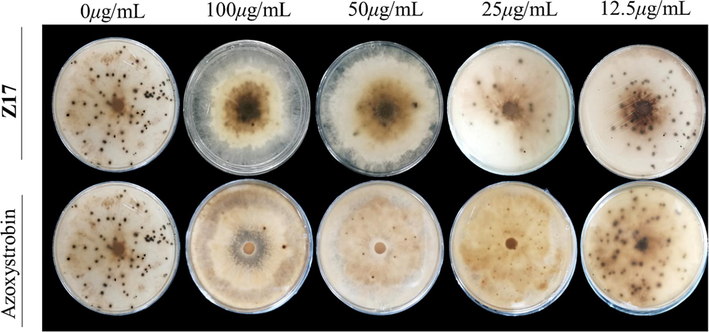
The inhibitory effect of Z17 on the formation of sclerotia of Phomopsis sp.
Compound
Concentrations (μg/mL)
Number of sclerotia
Inhibition ration(%)a
Z17
12.5
57.3 ± 1.3
14.4
25.0
20.0 ± 0.7
70.1
50.0
8.3 ± 0.5
87.6
100.0
1.3 ± 0.5
98.0
Az
12.5
67.0 ± 2.1
–
25.0
49.0 ± 0.8
26.9
50.0
10.0 ± 0.6
85.1
100.0
2.3 ± 0.6
96.5
Control
0.0
67.0 ± 0.8
–
3.7 Effect of Z17 on the cell membrane permeability of Phomopsis sp
Cell membranes are important for maintaining the integrity of cell structures and normal life activities. As shown in Fig. 10, compared with the blank control group, the relative conductivity of the mycelium increased with time after being treated with Z17. And when the concentration of Z17 was at 25, 50, 100 μg/mL, the relative conductivity also increased gradually. In particular, when Z17 was 50 and 100 μg/mL, the rising trend was more evident and increased in a concentration gradient. In conclusion, Z17 could change permeability of cell membrane and promote cell death of Phomopsis sp.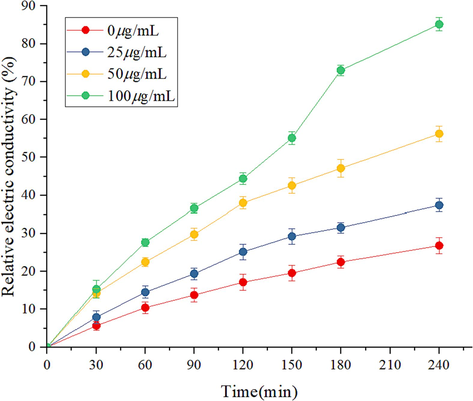
Changes in cell membrane permeability of Z17 against Phomopsis sp.
3.8 Effect of Z17 on the cytoplasmic leakage of Phomopsis sp
As can be seen from the Fig. 11, the absorbance value of the Z17 treatment group was significantly higher than that of the blank control. Especially, when the concentration was 50 μg/mL, the permeability of cytoplasmic contents increased significantly. Therefore, we could speculate that Z17 could destroy cell membrane structure of the mycelia, thereby promote the release of intracellular contents. It was consistent with the results of SEM.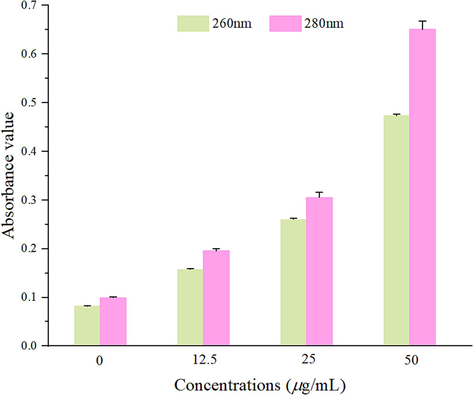
Release of cellular contents from Phomopsis sp after treatment with Z17.
3.9 Determination of MDA content by Z17
The results of Z17 on the content of MDA was shown in Fig. 12. Compared with the control group (0 μg/mL), the content of MDA in the mycelia treated with different concentrations of Z17 (12.5, 25, 50, 100 μg/mL) increased significantly with the increase of concentration, which suggested that Z17 can seriously damage the cell membrane of Phomopsis sp, and the damage degree was proportional to the concentration of the compound.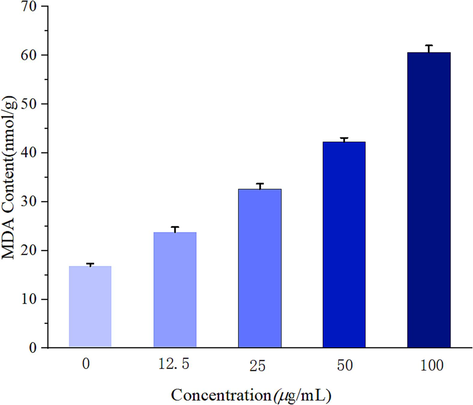
MDA content of Phomopsis sp after treatment with Z17.
3.10 Molecular docking of Z17 with SDH
The molecular docking simulation results indicated that Z17 and fluopyram (SDHI) dock into the active protein pocket of SDH in a similar mode of action. As shown in Fig. 13 A-a, Z17 and SDH active protein had a strong hydrogen bond interaction between the amide bond and the key amino acid residue LEU 63, and the distance between the amide bond and LEU 63 was 1.45 Å. In addition, amino acid residues PRO 64 (2.54 Å) and LEU 62 (2.67 Å) interacted with the piperazine ring and methoxy group of Z17 through conventional hydrogen bonding. LEU 61 (2.2 Å) forms Pi-Sigma bonds with aromatic rings. PHE 86 (5.86 Å) and TYR 85 (5.44 Å, 5.82 Å) bind with S element and benzene ring through Pi-Sulfur and Pi-Pi T-shaped bonds. ALA 60 (4.12, 4.41 Å) formed Alkyl interactions and Pi-Alkyl hydrophobic with thiazole ring and tert-butyl groups. In Fig. 13 B-b, fluopyram was connected to amino acid residues VAL 53 (2.55 Å) and GLY 52 (2.70 Å) by a conventional hydrocarbon bond. And PHE 56(3.66 Å) formed the Pi-Pi Stacked interaction. LEU 83(5.42 Å), ILE 93(4.75 Å), LEU 91(4.83 Å), LEU 55(5.21 Å) form Alkyl interactions and Pi-Alkyl hydrophobics. In summary, Z17 formed multiple interactions with the amino acid residues of SDH, making it tightly bound to SDH. Meanwhile, the binding energy of Z17 was −89.3 kcal mol−1, which was slightly higher than that of flupyram (-−51.9 kcal mol−1). It was further proved that SDH may be one of the action sites of Z17. These results suggested that Z17 may destroy the mitochondrial tricarboxylic acid cycle, thus leading to the death of pathogens.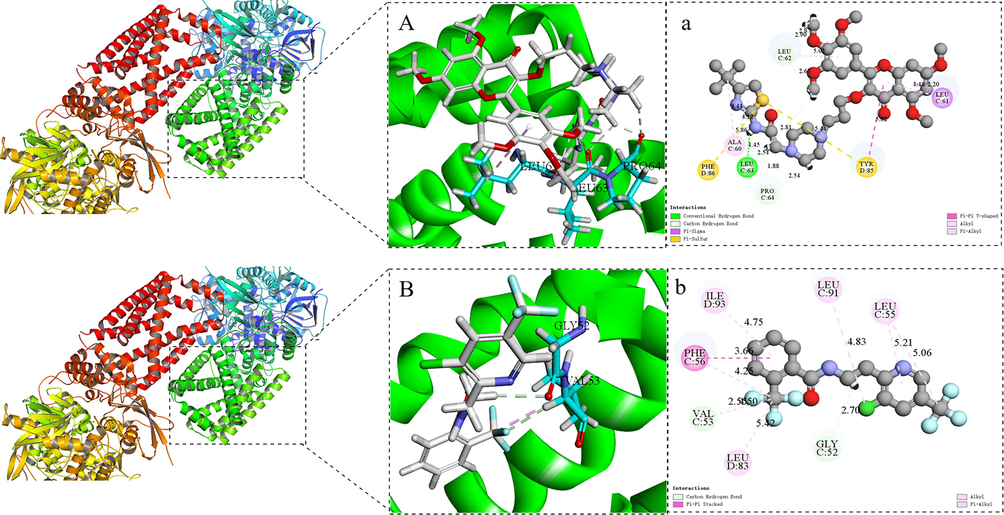
Molecular docking modes of Z17 (A-a) and fluopyram (B-b) with SDH.
4 Conclusions
19 myricetin derivatives containing thiazole were designed and synthesized. The antifungal activity test results indicated that Z17 and Z18 had good inhibitory activities. The EC50 value of Z17 against Phomopsis sp was 12.3 µg/mL, which was superior to those of the control drug azoxystrobin (32.2 µg/mL) and fluopyram (77.7 µg/mL). The in vivo experiment results indicated that Z17 had the good protective activity against Phomopsis sp (62.5 %) on kiwifruit, which was better than that of azoxystrobin (40.7 %) at 200 µg/mL. Z18 possessed an excellent protective and curative activity against A. brassicae (84.4, 81.9 %) on cabbage, which was superior to that of azoxystrobin (57.9, 43.5 %) at 200 µg/mL. The mechanism studies, such as molecular docking, SEM, FM, sclerotium formation, cell membrane permeability, cell content release, and MDA content were all consistent with the preliminary screening results, which further indicated that Z17 possessed the excellent antifungal activity. In view of this, we could affirm that Z17 exhibited excellent antifungal activity, which also confirmed that the myricetin derivatives containing thiazole can be further developed as potential fungicides. As a result, the current work provides a new research direction and some theoretical basis for the creation of green pesticides.
Acknowledgements
This research was completed with the support of the National Natural Science Foundation of China (No. 21867003), the Science Foundation of Guizhou Province (No. 20192452).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Genome sequence of the necrotrophic plant pathogen alternaria brassicicola abra43. Genome Announc.. 2018;6 e01559-17
- [Google Scholar]
- Dasatinib and trametinib promote anti-tumor metabolic activity. Cells. 2023;12:1374-1383.
- [Google Scholar]
- An overview of the synthesis and antimicrobial, antiprotozoal, and antitumor activity of thiazole and bisthiazole derivatives. Molecules. 2021;26:624-649.
- [Google Scholar]
- Modes of action of the new arylguanidine abafungin beyond interference with ergosterol biosynthesis and in vitro activity against medically important fungi. Chemotherapy. 2008;54:245-259.
- [Google Scholar]
- Synthesis and anticancer activity of novel thiazole-5-carboxamide derivatives. Appl. Sci.. 2016;6:8-17.
- [Google Scholar]
- Design, synthesis and bioactivity of myricetin derivatives for control of fungal disease and tobacco mosaic virus disease. RSC Adv.. 2023;13:6459-6465.
- [Google Scholar]
- Synthesis and antibacterial and antiviral activities of myricetin derivatives containing a 1,2,4-triazole schiff base. RSC Adv.. 2019;9:23045-23052.
- [Google Scholar]
- Design, synthesis and biological activity of chalcone derivatives containing pyridazine. Arab. J. Chem.. 2023;16:1-11.
- [Google Scholar]
- 2-aminothiazole as a novel kinase inhibitor template. structure-activity relationship studies toward the discovery of N-(2-chloro-6-methylphenyl)-2-[[6-[4-(2-hydroxyethyl)-1-piperazinyl)]-2-methyl-4-pyrimidinyl] amino)]-1,3-thiazole-5-carboxamide (Dasatinib, BMS-354825) as a potent pan-src kinase inhibitor. J. Med. Chem.. 2006;49:6819-6832.
- [Google Scholar]
- Identification and agro-morphological characterization of rice genotypes resistant to sheath blight. Australas Plant Pathol.. 2016;45:145-153.
- [Google Scholar]
- Discovery of arylox, arylthio, and arylamino containing acethydrazides as fungicidal agents. J. Agric. Food Chem.. 2022;71:920-933.
- [Google Scholar]
- Effects of a thiamethoxam-based insecticide on the life history of chironomus xanthus. Water, Air, Soil Pollut.. 2018;229:376-384.
- [Google Scholar]
- Actinidia (mini kiwi) fruit quality in relation to summer cutting. Agronomy. 2021;11:964-978.
- [Google Scholar]
- Synthesis and antifungal activity of curcumol derivatives. Chem. Biodiversity.. 2023;20:e202300442
- [Google Scholar]
- Synthesis and antibacterial activity of novel myricetin derivatives containing sulfonylpiperazine. Chem. Pap.. 2020;75:1021-1027.
- [Google Scholar]
- Discovery of zeylenone from uvaria grandiflora as a potential botanical fungicide. Pest Manage. Sci.. 2021;77:5407-5417.
- [Google Scholar]
- Venturicidin A is a potential fungicide for controlling fusarium head blight by affecting deoxynivalenol biosynthesis, toxisome formation, and mitochondrial structure. J. Agric. Food Chem.. 2023;71:12440-12451.
- [Google Scholar]
- Antibacterial activities of novel dithiocarbamate-containing 4H-chromen-4-one derivatives. J. Agric. Food Chem.. 2020;68:5641-5647.
- [Google Scholar]
- Pyridine and thiazole-based hydrazides with promising anti-inflammatory and antimicrobial activities along with their in silico studies. ACS Omega.. 2020;5:25228-25239.
- [Google Scholar]
- Synthesis, characterization and molecular docking studies of highly functionalized and biologically active derivatives of 2-aminothiazole. J. Mol. Struct.. 2023;1275:134593
- [Google Scholar]
- Design, synthesis, antibacterial activity, antiviral activity, and mechanism of myricetin derivatives containing a quinazolinone moiety. ACS Omega.. 2021;6:30826-30833.
- [Google Scholar]
- Analysis of tissue-specific defense responses to sclerotinia sclerotiorum in brassica napus. Plants. 2022;11:2001-2015.
- [Google Scholar]
- Synthesis and biological activity of myricetin derivatives containing pyrazole piperazine amide. Int. J. Mol. Sci.. 2023;24:10442-10457.
- [Google Scholar]
- Design, synthesis, and antifungal activity of flavonoid derivatives containing thiazole moiety. Chem. Pap.. 2022;77:877-885.
- [Google Scholar]
- Antibacterial and antiviral activities of 1,3,4-oxadiazole thioether 4H-chromen-4-one derivatives. J. Agric. Food Chem.. 2021;69:11085-11094.
- [Google Scholar]
- Design, synthesis, and fungicidal activities of novel piperazine thiazole derivatives containing oxime ether or oxime ester moieties. Pest Manage. Sci.. 2023;79:1977-1986.
- [Google Scholar]
- Antibacterial and antifungal activities of methanol extract and phenolic compounds from diospyros virginiana L. Ind. Crops Prod.. 2014;59:210-215.
- [Google Scholar]
- Bioactive thiazole and benzothiazole derivatives. Eur. J. Med. Chem.. 2015;97:911-927.
- [Google Scholar]
- Isothiazolinone biocides: chemistry, biological, and toxicity profiles. Molecules. 2020;25:991-1012.
- [Google Scholar]
- Synthesis and antiviral activity of novel myricetin derivatives containing ferulic acid amide scaffolds. New J. Chem.. 2020;44:2374-2379.
- [Google Scholar]
- Bioactivity-guided synthesis accelerates the discovery of 3-(Iso)quinolinyl-4-chromenones as potent fungicide candidates. J. Agric. Food Chem.. 2020;69:491-500.
- [Google Scholar]
- Synthesis and bioactivity evaluation of 5-trifluoromethyl-1H-pyrazole-4-carboxamide derivatives as potential anticancer and antifungal agents. J. Heterocycl. Chem.. 2022;59:1759-1767.
- [Google Scholar]
- Novel myricetin derivatives: design, synthesis and anticancer activity. Eur. J. Med. Chem.. 2015;97:155-163.
- [Google Scholar]
- Bioactive compounds in cruciferous sprouts and microgreens and the effects of sulfur nutrition. J. Sci. Food Agric.. 2023;103:7323-7332.
- [Google Scholar]
- Discovery of β-carboline oxadiazole derivatives as fungicidal agents against rice sheath blight. J. Agric. Food Chem.. 2018;66:9598-9607.
- [Google Scholar]
- Controlling black spot of postharvest broccoli by meyerozyma guilliermondii and its regulation on ROS metabolism of broccoli. Biol. Control.. 2022;170:104938
- [Google Scholar]
- Synthesis, antibacterial and antifungal activity of myricetin derivatives containing piperidine and amide fragments. Pest Manage. Sci.. 2023;79:4795-4808.
- [Google Scholar]
- Discovery of N-phenylpropiolamide as a novel succinate dehydrogenase inhibitor scaffold with broad-spectrum antifungal activity on phytopathogenic fungi. J. Agric. Food Chem.. 2023;71:3681-3693.
- [Google Scholar]
- Design, synthesis, and antifungal activity of novel chalcone derivatives containing a piperazine fragment. J. Agric. Food Chem.. 2022;70:1029-1036.
- [Google Scholar]
- Design, synthesis and antifungal activity of novel 1,4-pentadiene-3-one containing quinazolinone. Int. J. Mol. Sci.. 2023;24:2599-2612.
- [Google Scholar]
- Synthesis of novel thiazolyl hydrazine derivatives and their antifungal activity. J. Chem.. 2021;2021:1-8.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2023.105480.
Appendix A
Supplementary data
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1
The Supporting Information includes the characterization data, HNMR and HRMS spectrogram for the target compounds.







