Translate this page into:
Design, synthesis and antifungal activity of indole derivatives containing 1,3,4-oxadiazole
⁎Corresponding authors. zcwang@gzu.edu.cn (Zhenchao Wang), wxue@gzu.edu.cn (Wei Xue)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
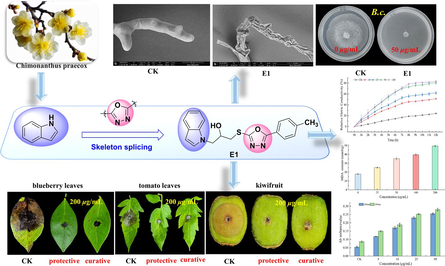
In this study, 21 indole derivatives containing 1,3,4-oxadiazole were designed and synthesized, the results of the biological activity test showed that the target compounds had certain antifungal activity against 12 plant pathogenic fungi in vitro, among which. E1 showed excellent bioactivity against Botrytis cinerea (B.c.), Tomato Botrytis cinerea (F.M.) and Phomopsis sp (P.s.), with median effective concentration (EC50) values of 2.8, 5.1 and 5.2 µg/mL, which are higher than those of the control drug azoxystrobin (Az) at 15.2, 31.2 and 15.2 µg/mL. In vivo tests on blueberry leaves, tomato leaves and kiwifruit showed that E1 at 200 µg/mL offered stronger protective effect against B.c. (91.9 %) than Az (83.8 %) in blueberry leaves, F.M. (83.3 %) than Az (72.9 %) in tomato leaves and P.s. (89.3 %) than Az (86.9 %) in kiwifruit. Scanning electron microscope (SEM) experiments showed that B.c. hyphae treated with E1 had abnormal shrinkage and obvious morphological changes. The results of the mechanism study showed that E1 could change the integrity of the cell wall and cell membrane of pathogen B.c., which led to the increase in malondialdehyde (MDA), cell leakage and permeability and the rupture of the cell membrane. Because of their strong antifungal effects on plant fungus, indole derivatives containing 1,3,4-oxadiazole were predicted to develop into novel fungicides.
Keywords
Indole
1,3,4-oxadiazole
Antifungal activity
Botrytis cinerea
Tomato Botrytis cinerea
Phomopsis sp
1 Introduction
Plant fungal diseases were the main problem facing global agricultural production (Cui et al., 2020), which resulted in 10 % of post-harvest losses and a 20 % annual reduction in agricultural output. Furthermore, the mycotoxins produced by certain plant pathogenic fungi were dangerous to both human and animal health (Bräse et al., 2009). Plant pathogenic fungi such as B.c., F.M. and P.s. had a substantial impact on agriculture (Gonzalez-Fernandez et al., 2011). reducing crop output and quality significantly. B.c. and F.M., for example, can infect over 200 plants, including vegetables and minor fruit harvests (Ren et al., 2020). Plant pathogenic fungi are primarily accountable for fruit rot, leaf necrosis, and other diseases, collectively resulting in billions of dollars of annual losses globally (Ma et al., 2019; Tang et al., 2020). Fungicides were therefore crucial for maintaining production stability as well as increasing crop yield and quality (Liu et al., 2021). Furthermore, persistent fungicide usage has led to a number of drawbacks, including residual toxicity, drug resistance and environmental issues (Ren et al., 2020). Thus, in order to preserve plants, new environmental protection fungicides must be developed (Tleuova et al., 2020).
The design and development of molecules based on the structure of natural products is now one of the most active areas of drug research. Indole (Fig. 1) was an important natural substance that was a nitrogen-containing benzopyrrole alkaloid. Chimonanthus praecox's main skeleton was indole (Duan et al., 2023; Nunes, 2011). and its derivatives exhibit significant biological activity such as antifungal (Zheng et al., 2023), antibacterial (Silva et al., 2019; Tleuova et al., 2020), anticancer (Ribas e Ribas et al., 2016; Wang et al., 2022), antiviral (Seiber, 2011; Qian et al., 2010), excellent candidates for metal ion sensing (Alharthi, 2023) and so on. Indole derivatives were becoming a source of natural goods for health and environmental protection (Wu et al., 2007), which had become a hot issue in life science and chemistry as market demand grew.
The structures of indole and 1,3,4-oxadiazole.
One of the key five-membered nitrogen-containing heterocyclic compounds is 1,3,4-oxadiazole (Fig. 1), which has been studied extensively in both medicine (Nowakowska, 2007) and organic synthesis (Chen et al., 2010). The 1,3,4-oxadiazole skeleton has been extensively studied and developed in the process of physiologically active recombination due to its incorporation of diverse functional building blocks (Tao et al., 2019; Fu et al., 2020). Fig. 2 depicts a commercial medicament that containing 1,3,4-oxadiazole. Furthermore, 1,3,4-oxadiazole compounds had anticancer (Du et al., 2013), antibacterial (Ouyang et al., 2021), antiviral (Jiang et al., 2020), antifungal (Zhu et al., 2016), insecticidal (Wang et al., 2019) and other biological properties.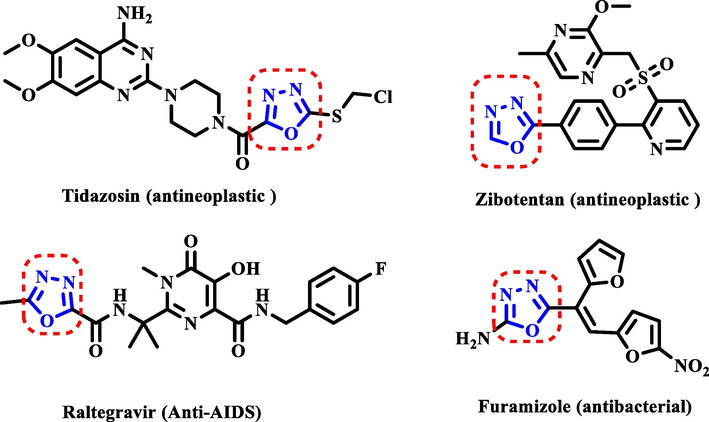
Some commercial drugs contain 1,3,4-oxadiazole moiety.
To discover novel pesticide compounds exhibiting superior antifungal activities, we synthesized 21 indole derivatives incorporating the 1,3,4-oxadiazole moiety, leveraging the principle of active splicing in drug synthesis (Fig. 3 and Scheme 1). The in vitro antifungal activity of all the target compounds was tested against 12 plant pathogenic fungi using the mycelial growth rate method. The results of antifungal tests demonstrated that E1 exhibited a pronounced inhibitory effect against B.c., F.M. and P.s. Furthermore, in vivo experiments were conducted on blueberry leaves, tomato leaves and kiwifruit, utilizing compound E1 at concentrations of 200 and 100 µg/mL. In order to further validate the antifungal activity of the studied series of compounds, SEM experiments and light microscope observation experiments showed that E1 could alter the integrity of the cell wall and cell membrane of the pathogenic bacterium B.c., leading to abnormal cell crumpling and leakage of contents, which led to an increase in the MDA, cellular leakage and cell permeability. This study identified indole derivatives exhibiting antifungal activities, establishing a theoretical foundation for the research, development and design of novel pesticides.
Design of target compounds.
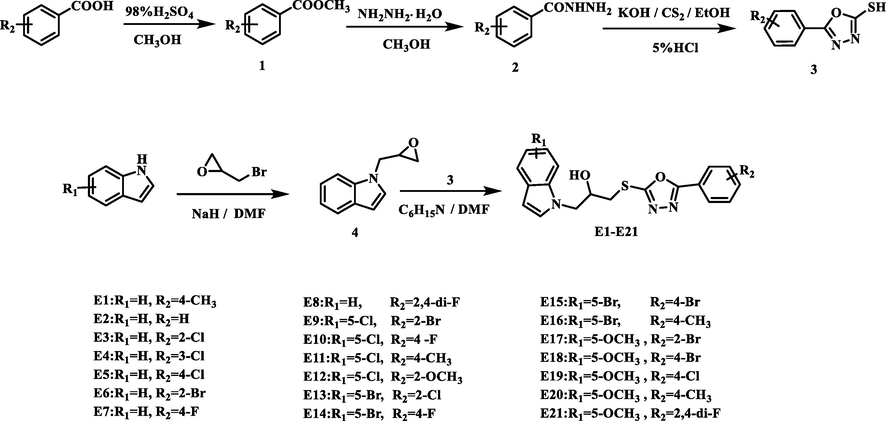
Synthetic route of compounds E1-E21.
2 Materials and methods
2.1 Instruments and chemicals
2.1.1 Instruments
Experimental equipment utilized in this study is referenced from previously reported literature, providing a basis for equipment selection and experimental design (Zhou et al., 2023; Peng et al., 2022; Liu et al., 2023; Hu et al., 2023). Melting point measurements were performed by using the X-4B melting point instrument (Shanghai INESA Co., Ltd., Shanghai, China) without any modifications. The spectrum data were acquired by using a 500 NMR spectrometer (Bruker, Karlsruhe, Germany) and an ASCEND400 NMR spectrometer (Bruker, Germany). HRMS data were collected by using a hybrid quadrupole mass spectrometer from Thermo Scientific (Waltham, MA, USA). Cell permeability was measured by using the Leici DDSJ-3O8F conductivity meter (Shanghai Instrument & Electric Science Instrument Co., Ltd., China). Data for SEM were collected on an FEI Nova Nano 450 (Hillsboro, OR, USA). Cell leakage was detected by using the N-5000 UV spectrophotometer (Shanghai Yoke Instrument Co., Ltd., China). The microstructure was examined by using an optical microscope, specifically the Olympus CX21 model (Olympus, Japan).
2.1.2 Chemicals
Chemicals can be referenced based on previously reported literature (Ibrahim et al., 2017; Ibrahim et al., 2017; Li et al., 2017; Zhou et al., 2018; Meşeli et al., 2021; Peng et al., 2022). Reaction materials and chemicals, including carbon disulfide, epibromohydrin, substituted indole, various substituted benzoic acids, hydrazine hydrate and azoxystrobin (all A.R. grade) were obtained from Shanghai Titan Technology Co., Ltd., all analytical-grade reagents and solvents, including anhydrous ethanol, anhydrous methanol and N, N-dimethylformamide (DMF) were commercially obtained. Additional chemical materials were sourced from Bositai Technology Co., Ltd. (Chongqing, China).
2.2 Synthesis
2.2.1 Synthesis of intermediates 1–4
Intermediates 1–4 were synthesized using slightly modified procedures reported in the literature (Peng et al., 2021; Ibrahim et al., 2017; Li et al., 2017; Zhou et al., 2018; Meşeli et al., 2021). (Using E2 as an illustrative example, intermediates 1–4 are methyl benzoate, benzohydrazide, 5-phenyl-1,3,4-oxadiazole-2-thiol and 1-(oxiran-2-ylmethyl)-1H-indole, respectively). Different substitutes for benzoic acid were used in acidic conditions, such as methanol as a solvent in an esterification reaction that took 7 h to produce intermediate 1, methanol as a solvent in a reaction that took 7 h to produce intermediate 2, ethanol as a solvent in an alkaline condition that took 8 h to produce carbon disulfide (CS2), and finally 5 % HCl acidification to produce intermediate 3. In the replacement of indole as a raw material in the strong alkaline conditions of NaH and bromopropylene oxide at 45 °C at a temperature of heating and reflux for 6 h to produce intermediate 4.
2.2.2 Synthesis of target compounds E1-E21
Dissopropylamine (8.66 mmol) and 20 mL of DMF were added to a round-bottom flask along with Intermediate 4 (2.89 mmol). Intermediate 3 (3.18 mmol) was added to the reaction mixture after 30 min. TLC was used to track the reaction's course for around 10 h at 77 °C. TLC was used to carry out the reaction. The reaction was heated, then combined with 90 mL of distilled water and extracted with ethyl acetate. The upper liquid phase was recovered, dried, filtered and the ethyl acetate solvent was removed after three washes with saturated brine. Finally, E1-E21 were purified by column chromatography (petroleum ether: ethyl acetate = 10:1, v/v) (Ibrahim et al., 2017). The synthetic route of E1-E21 was shown in Scheme 1. The Supporting Information contains detailed characterization data for E1-E21.
2.3 Antifungal bioassays
2.3.1 In vitro antifungal activity assay
According to the mycelium growth rate approach mentioned in references (Jang et al., 2012; Wan et al., 2015; Ibrahim et al., 2017). Dimethylsulfoxide (DMSO) was utilized as a blank control, whereas Az served as the positive control. At a concentration of 100 µg/mL, the antifungal activity of E1-E21 against 12 types of B.c., Rhizoctonia solani (R.s.), Phytophthora capsica (P.c.), Sclerotinia sclerotiorum (S.s.), F.M., P.s., Neoscytalidium dimidiatum (N.d.), Colletotrichum gloeosporioides (C.g.), pepper anthracnose (P.a.), Botryosphaeria dothidea (B.d.), Fusarium graminearum (F.g.) and Fusarium asiaticum (F.a.) were determined in vitro. Concurrently, the EC50 values for compounds exhibiting strong antifungal properties were determined, with the experimental protocol outlined in the Supporting Information.
2.3.2 In vivo antifungal activity assay
The experiment was carried out in accordance with the procedure described in the literature (Zhou et al., 2015; Wang et al., 2023; Zhou et al., 2023). By using DMSO as a blank control and Az as positive control, the curative and protective activities of E1 with the greatest antifungal activity on blueberry leaves, tomato leaves and kiwifruit in vivo were evaluated. The experimental method was provided in the Supporting Information.
2.3.3 Scanning electron microscopy (SEM) characterization
The approach described in the literature was used to conduct the SEM experiment (Su et al., 2021; Zhang et al., 2018). B.c. mycelium was treated with various doses of E1. The experimental approach was described in the Supporting Information.
2.3.4 Cytoplasmic leakage volume assay
To investigate other possible action modes of E1 on B.c. mycelium, the absorbance of the supernatant of B.c. mycelium treated with E1 was measured by using an ultraviolet spectrophotometer at 260 and 280 nm, as described in the literature (Liu et al., 2023; Ma et al., 2019). Secondly, the MDA content of E1 was measured to indirectly reflect the degree of tissue peroxidation damage to the mycelium membrane (Chen et al., 2023; Zhou et al., 2023). In this investigation, two distinct approaches were utilized to investigate the degree of E1 damage to B.c. mycelium, which resulted in cytoplasmic leakage. Experimental methods were provided in the Supporting Information.
2.3.5 Optical microscope characterization
By using an optical microscope, the effect of the most active E1 on B.c. mycelium was observed according to the method reported in the literature (Liu et al., 2023).
2.3.6 Characteristics of cell membrane permeability
The effect of E1 on cell permeability was assessed with the method described in the literature (Efenberger-Szmechtyk et al., 2021; Shang et al., 2019; Zhou et al., 2023), and the relative conductivity of mycelium suspensions containing varying doses of E1 was determined at various times. The experimental approach was described in the Supporting Information.
3 Results and discussion
3.1 Chemistry
Following the synthetic route outlined in Scheme 1, a series of indole derivatives (E1-E21) incorporating the 1,3,4-oxadiazole moiety were synthesized. The structure of the target compounds was preliminary characterized by NMR and HRMS, and detailed data were provided in the Supporting Information.
3.2 In vitro antifungal activity analysis
The inhibitory activity of the target compounds against 12 distinct plant pathogenic fungi was assessed at a concentration of 100 µg/mL using mycelium growth inhibition assay, with Az serving as the control agent for comparison. Table 1 shows all 21 indole derivatives with the 1,3,4-oxadiazole moiety inhibited B.c. by 74.7–100.0 %, outperforming the commercial Az (72.4 %). E1, E6 and E9 exhibited 100 % inhibition against B.c., demonstrating strong anti-B.c. activity. E2 (78.0 %) and E3 (76.8 %) showed better inhibition of S.s. than Az (75.6 %), with minimal impact overall. Simultaneously, it exerts an inhibitory effect on F.M. Specifically, the inhibitory range of E1, E2, E3, E7, E10, E11, E14, E15, E16, E17, E18, E19 and E20 was 68.1–80.5 %, exceeding the inhibitory effect of the control drug Az, which was 66.1 %. All 21 compounds synthesized concurrently demonstrated good inhibitory activity against P.s., with an inhibitory range of 67.9–89.6 %, outperforming the control drug Az (66.7 %). Among these compounds, E5 (80.9 %), E7 (83.0 %), E9 (77.3 %), E13 (86.1 %), E16 (86.1 %) and E17 (74.2 %) demonstrated superior inhibitory effects against N.d. compared to the control medication Az (72.4 %). They exhibit certain inhibitory effects against C.g., with E1 (72.7 %), E2 (67.4 %) and E3 (69.4 %) demonstrating stronger inhibitory actions compared to Az (62.4 %). E1 (71.6 %) and E3 (69.0 %) demonstrated stronger inhibitory effects on P.a. compared to Az (68.5 %). Similarly, E6 (61.8 %) displayed higher inhibitory activity against F.g. than Az (57.9 %). To verify the antifungal activity of these compounds, we determined the EC50 values of the compounds exhibiting stronger inhibitory activity than the control agent at a concentration of 100 μg/mL. The results are presented in Table 2 and Fig. 4. The EC50 values of E1, E2, E3, E6, E7, E9, E16 and E21 against B.c. were 2.8, 3.4, 8.1, 8.0, 5.6, 4.3, 6.2 and 8.4 μg/mL, respectively, these values were superior to that of Az (15.2 μg/mL), and with E1 performing best with an EC50 of 2.8 μg/mL. In addition, E1, E2, E3, E14 and E16 exhibited EC50 values of 5.1, 10.5, 5.2, 14.1 and 14.8 μg/mL against F.M., outperforming Az (31.2 μg/mL). Meanwhile, E1, E2, E3, E5, E6, E8, E16, E18, E20 and E21 showed EC50 values of 5.2, 6.3, 7.5, 9.0, 5.9, 5.8, 9.4, 5.5, 7.9 and 7.1 μg/mL against P.s., which was superior to that of Az (15.2 μg/mL). And lastly E1, E2 and E3 showed EC50 values of 13.9, 14.7 and 8.9 μg/mL against S.s., which was better than that of Az (18.9 μg/mL). Based on these results, E1 demonstrates potent broad-spectrum antifungal activity. In summary, the incorporation of 1,3,4-oxadiazole-containing indole derivatives into the structure enhances antifungal activity.
Compd.
Inhibition rate (%)
B.c.b
R.s.
P.c.
S.s.
F.M.
P.s.
N.d.
C.g.
P.a.
B.d.
F.g.
F.a.
E1
100
41.4 ± 1.5
36.5 ± 1.1
53.7 ± 1.4
80.5 ± 1.6
89.6 ± 1.7
35.8 ± 0.9
72.7 ± 0.0
71.6 ± 0.0
45.1 ± 1.5
51.0 ± 1.6
49.4 ± 0.9
E2
99.6 ± 0.9
53.7 ± 1.6
49.2 ± 1.1
78.0 ± 1.4
69.3 ± 0.9
85.4 ± 1.7
46.4 ± 1.4
67.4 ± 2.6
64.7 ± 2.4
47.7 ± 1.3
43.6 ± 1.7
50.2 ± 0.9
E3
94.6 ± 1.7
55.6 ± 1.5
48.4 ± 1.1
76.8 ± 1.2
77.3 ± 1.8
87.1 ± 0.9
54.2 ± 0.7
69.4 ± 1.1
69.0 ± 1.4
35.2 ± 1.7
56.0 ± 1.9
56.9 ± 0.9
E4
84.0 ± 0.9
70.9 ± 1.8
38.1 ± 0.0
60.6 ± 1.6
64.1 ± 1.4
74.2 ± 1.1
38.8 ± 0.9
55.0 ± 0.9
52.6 ± 2.4
15.2 ± 1.1
34.7 ± 1.6
39.3 ± 1.7
E5
77.4 ± 1.7
67.5 ± 1.1
37.3 ± 1.1
58.9 ± 0.9
64.9 ± 1.1
82.1 ± 1.7
80.9 ± 1.7
35.1 ± 0.9
59.5 ± 1.9
50.8 ± 2.8
51.7 ± 1.6
51.9 ± 1.7
E6
100
73.9 ± 1.0
36.9 ± 1.2
62.6 ± 1.8
56.6 ± 0.9
87.5 ± 1.4
62.4 ± 0.9
32.6 ± 0.9
62.5 ± 1.3
43.2 ± 1.3
61.8 ± 1.7
50.6 ± 1.2
E7
90.7 ± 0.0
71.3 ± 0.8
33.7 ± 1.6
62.6 ± 1.8
75.3 ± 1.7
67.9 ± 1.7
83.0 ± 0.9
45.0 ± 0.9
61.2 ± 1.4
26.1 ± 1.7
43.6 ± 1.7
48.5 ± 2.3
E8
75.5 ± 1.1
65.7 ± 1.0
42.9 ± 2.3
59.8 ± 1.8
61.8 ± 1.4
78.8 ± 1.2
55.5 ± 1.4
38.4 ± 0.9
54.3 ± 1.2
24.6 ± 0.8
47.5 ± 1.1
42.7 ± 0.9
E9
100
66.4 ± 0.0
38.1 ± 0.0
46.7 ± 0.9
65.3 ± 1.8
74.6 ± 1.7
77.3 ± 0.9
34.7 ± 1.1
55.2 ± 2.4
18.9 ± 1.1
52.1 ± 1.1
45.2 ± 0.9
E10
94.2 ± 1.7
75.7 ± 1.5
47.6 ± 1.3
61.8 ± 1.1
68.1 ± 1.1
74.2 ± 1.8
64.8 ± 0.9
34.3 ± 1.2
57.8 ± 1.2
43.2 ± 1.3
52.1 ± 1.1
45.2 ± 0.9
E11
94.9 ± 0.9
73.1 ± 1.3
49.2 ± 1.1
57.3 ± 1.2
75.7 ± 0.9
69.2 ± 1.1
61.8 ± 1.1
43.4 ± 0.9
53.4 ± 1.4
48.5 ± 1.1
47.9 ± 1.1
49.0 ± 1.2
E12
96.9 ± 2.2
71.3 ± 0.8
44.8 ± 0.9
54.9 ± 1.2
55.4 ± 1.1
72.9 ± 0.9
58.2 ± 1.1
31.4 ± 1.1
51.3 ± 0.9
43.9 ± 1.1
57.5 ± 1.1
41.4 ± 1.2
E13
97.3 ± 1.6
67.5 ± 1.1
39.7 ± 1.1
46.7 ± 1.6
64.9 ± 1.7
70.4 ± 0.9
86.1 ± 0.9
45.9 ± 0.9
52.6 ± 1.2
52.3 ± 1.3
45.6 ± 1.1
36.0 ± 1.2
E14
98.8 ± 1.1
78.0 ± 0.8
33.7 ± 0.9
70.3 ± 0.9
74.5 ± 1.7
75.4 ± 0.9
66.7 ± 0.9
35.5 ± 1.4
52.2 ± 1.3
68.9 ± 1.7
57.5 ± 1.1
51.5 ± 1.2
E15
98.8 ± 1.1
67.5 ± 1.1
46.0 ± 1.1
61.0 ± 1.4
75.3 ± 1.7
74.2 ± 1.8
60.6 ± 0.9
37.6 ± 0.9
49.1 ± 1.2
37.5 ± 1.1
48.6 ± 0.8
46.4 ± 1.2
E16
98.8 ± 1.1
78.4 ± 1.0
48.4 ± 1.1
70.3 ± 1.6
75.3 ± 1.1
84.6 ± 0.9
86.1 ± 0.9
40.9 ± 0.9
68.1 ± 1.2
45.1 ± 1.5
47.1 ± 0.8
46.9 ± 1.7
E17
75.5 ± 1.1
64.9 ± 1.6
31.3 ± 0.9
54.1 ± 0.9
73.7 ± 1.4
72.1 ± 2.2
74.2 ± 1.3
44.2 ± 1.2
57.8 ± 2.4
20.8 ± 0.8
37.1 ± 1.6
40.2 ± 0.9
E18
90.3 ± 0.9
71.3 ± 0.8
39.7 ± 1.1
54.1 ± 0.9
72.5 ± 2.9
82.9 ± 0.9
53.9 ± 0.9
27.7 ± 1.7
62.5 ± 1.9
38.6 ± 1.3
59.5 ± 1.1
39.3 ± 2.2
E19
74.7 ± 0.9
70.1 ± 1.0
31.7 ± 1.1
46.7 ± 0.9
74.1 ± 2.1
73.3 ± 1.1
51.8 ± 0.9
33.5 ± 0.9
52.6 ± 1.2
33.7 ± 1.5
53.7 ± 1.3
42.7 ± 0.9
E20
63.4 ± 1.1
64.6 ± 0.8
36.5 ± 1.1
47.6 ± 1.2
71.7 ± 0.9
79.6 ± 0.9
66.4 ± 0.9
47.1 ± 1.1
53.0 ± 0.9
19.3 ± 1.7
58.3 ± 1.3
40.2 ± 0.9
E21
91.8 ± 1.1
59.0 ± 1.0
38.1 ± 1.3
46.3 ± 1.4
62.9 ± 1.8
78.8 ± 1.9
67.0 ± 0.7
31.8 ± 1.2
61.2 ± 0.0
10.2 ± 1.1
46.3 ± 1.6
33.5 ± 1.2
Azc
72.4 ± 0.9
86.6 ± 1.3
61.1 ± 1.7
75.6 ± 0.0
66.1 ± 0.9
66.7 ± 1.1
72.4 ± 0.7
62.4 ± 1.7
68.5 ± 1.7
72.0 ± 2.1
57.9 ± 0.8
66.5 ± 1.8
Comp.
Pathogens
Regression equation
Correlation coefficient (r)
EC50 (µg/mL)
E1
B.c.
y = 1.2820x + 4.4289
0.9805
2.8
E2
B.c.
y = 1.3824x + 4.2676
0.9865
3.4
E3
B.c.
y = 1.4319x + 3.6964
0.9834
8.1
E6
B.c.
y = 1.1322x + 3.9748
0.9966
8.0
E7
B.c.
y = 1.2106x + 4.0952
0.9736
5.6
E9
B.c.
y = 1.0571x + 4.3265
0.9390
4.3
E16
B.c.
y = 1.3392x + 3.9345
0.9874
6.2
E21
B.c.
y = 0.9753x + 4.0997
0.9792
8.4
Azb
B.c.
y = 1.2756x + 3.4941
0.9719
15.2
E1
F.M.
y = 0.7209x + 4.4875
0.9914
5.1
E2
F.M.
y = 0.8248x + 4.1566
0.9947
10.5
E3
F.M.
y = 0.7323x + 4.4745
0.9546
5.2
E14
F.M.
y = 0.8012x + 4.0792
0.9882
14.1
E16
F.M.
y = 1.0992x + 3.7151
0.9879
14.8
Azb
F.M.
y = 0.8368x + 3.7501
0.9882
31.2
E1
P.s.
y = 0.9305x + 4.3315
0.9701
5.2
E2
P.s.
y = 1.1486x + 4.0831
0.9671
6.3
E3
P.s.
y = 1.2638x + 3.8911
0.9878
7.5
E5
P.s.
y = 1.1266x + 3.9234
0.9859
9.0
E6
P.s.
y = 0.9664x + 4.2582
0.9985
5.9
E8
P.s.
y = 0.7920x + 4.3938
0.9252
5.8
E16
P.s.
y = 1.1072x + 3.9203
0.9845
9.4
E18
P.s.
y = 0.8859x + 4.3441
0.9792
5.5
E20
P.s.
y = 0.9939x + 4.1062
0.9805
7.9
E21
P.s.
y = 1.0582x + 4.0994
0.9770
7.1
Azb
P.s.
y = 1.2756x + 3.4941
0.9719
15.2
E1
S.s.
y = 0.6179x + 4.2931
0.9924
13.9
E2
S.s.
y = 1.0074x + 3.8229
0.9973
14.7
E3
S.s.
y = 0.8141x + 4.2253
0.9735
8.9
Azb
S.s.
y = 0.8912x + 3.8616
0.9860
18.9
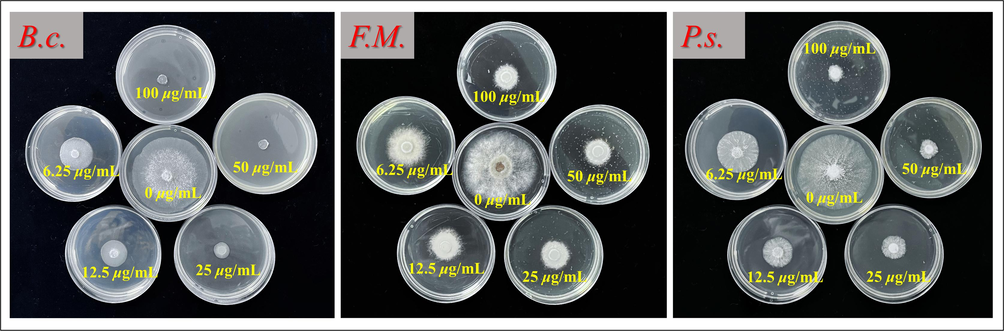
The EC50 figure of antifungal activities of E1 against Botrytis cinerea (B.c.), Tomato Botrytis cinerea (F.M.) and Phomopsis sp (P.s.).
3.3 Structure-activity relationship of antifungal activity
Utilizing the data presented in Table 1 along with the structure of the target compounds, a detailed structure–activity relationship analysis was conducted, revealing that the introduction of 1,3,4-oxadiazole into the indole moiety significantly enhances antifungal activity. This series of compounds demonstrated remarkable antifungal activity against B.c. Introducing either electron-withdrawing or electron-donating groups further enhances their antifungal activity. Specifically, the presence of the 1,3,4-oxadiazole moiety effectively boosts antifungal activity. In relation to other examined fungi, compounds with R1 = H exhibited stronger inhibitory effects than those with R1= (Br, Cl). This was evident in the anti-S.s. activity of E2 and E3, the anti-F.M. activity of E1, E2, E3 and E7, as well as the inhibitory activities against P.s. (E1, E2, E3, E5 E6), C.g. (E1, E2, E3) and P.a. (E1, E2). Furthermore, when R2 = 4-CH3-Ph, H, 2-Cl-Ph, 3-Cl-Ph, 4-Cl-Ph, 2-Br-Ph and 4-F-Ph, it inhibits several fungi. Moreover, the compound's antifungal activity test suggests that the indole ring R1 can exhibit strong antifungal activity in the presence of electron-donating substituents. Regardless of whether R2 features electron-donating or electron-withdrawing substituents, the antifungal activity of this series of compounds can be enhanced. Specifically, E1 (R1 = H, R2 = CH3) exhibited remarkable antifungal activity against multiple fungal species. As a result of the aforementioned analysis, the highly bioactive representative E1 was selected for subsequent in vivo and mechanistic investigations.
3.4 Bioactivity test results of E1 against B.c., f.m. And p.s. In vivo
The in vitro and EC50 experimental results demonstrated that E1 exhibited excellent antifungal activity against three plant pathogens: B.c., F.M. and P.s. To further investigate the inhibitory effect of E1 in a real-world setting, fresh, uniformly sized and undamaged blueberry leaves, tomato leaves and kiwifruit fruits were chosen for experimental evaluation, using agricultural fungicide Az as a control. E1 showed superior fungicidal activity against B.c. compared to the control. Its in vivo performance on blueberry leaves with EC50 = 2.8 μg/mL was tested and results are in Table 3 and Fig. 5. Firstly, E1 exhibited protective activities of 91.9 % and 75.8 % at 200 and 100 µg/mL, outperforming Az with 83.8 % and 56.6 %, respectively. Its curative efficacies were 86.3 % and 51.6 % at the same concentrations, surpassing Az's 55.8 % and 24.2 %.
Compound
Concentration
(μg/mL)Curative effect
Protective effect
Lesion length a
(mm ± SD)controlling
efficacy (%)Lesion length a
(mm ± SD)Controlling
efficacy (%)
E1
200
7.2 ± 0.4
86.3 ± 1.9
6.3 ± 0.7
91.9 ± 3.8
100
12.7 ± 0.5
51.6 ± 2.4
9.0 ± 0.6
75.8 ± 2.9
Az b
200
12.0 ± 0.6
55.8 ± 2.9
7.7 ± 0.5
83.8 ± 2.4
100
17.0 ± 0.8
24.2 ± 4.2
12.2 ± 0.9
56.6 ± 4.6
control
–
20.8 ± 0.7
–
21.5 ± 0.5
–
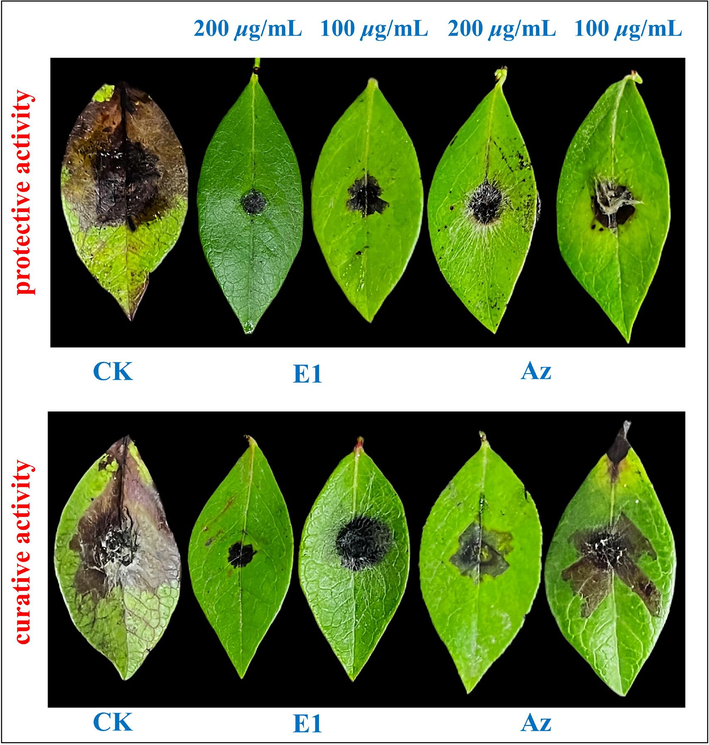
In vivo control effects of E1 against Botrytis cinerea (B.c.) on blueberry leaves.
Secondly, E1 with excellent anti-F.M. activity (EC50 = 5.1 μg/mL) in tomato leaves was studied in vivo, as shown in Table 4 and Fig. 6. The protective activity of E1 was 83.3 and 71.9 % at 200 and 100 µg/mL, respectively, which was superior to that of Az (72.9 and 55.2 %, respectively). In terms of curative efficacy, E1 demonstrated an equivalent performance of 79.2 % at 200 μg/mL compared to Az's 80.2 %. Notably, E1′s effective rate of 62.3 % at 100 μg/mL surpassed Az's rate of 37.7 %. At the same time, kiwifruit was a favorite fruit for people, but fungal infection will greatly reduce the yield and death of fruit trees, so it was more significant to study drugs with resistance to P.s. Therefore, the current study aimed to further investigate E1 excellent anti-P.s. activity (EC50 = 5.2 μg/mL) through in vivo experiments. As presented in Table 5 and Fig. 7. The protective activity of E1 was 89.3 % and 79.4 % at concentrations of 200 and 100 µg/mL, respectively, exceeding the corresponding values for Az, which were 86.9 % and 69.6 %. Similarly, the curative efficacy of E1 at the same concentrations was 85.7 % and 68.8 %, respectively, outperforming Az's curative efficacy of 77.7 % and 66.5 %. Thus, we can conclude that E1 had better curative and protective activity than Az in vivo, which was consistent with the crude screening and EC50 results. It was further confirmed that the inhibition was better when R2 was an electron-giving group such as -CH3. These intriguing findings imply that the integration of 1,3,4-oxadiazole into indoles holds promise as a potential agricultural fungicide for the management of fungal diseases.
Compound
Concentration
(μg/mL)Curative effect
Protective effect
Lesion length a
(mm ± SD)controlling
efficacy (%)Lesion length a
(mm ± SD)controlling
efficacy (%)
E1
200
8.7 ± 0.5
79.2 ± 2.5
7.7 ± 0.5
83.3 ± 2.5
100
11.7 ± 0.7
62.3 ± 3.9
9.5 ± 0.8
71.9 ± 4.0
Az b
200
8.5 ± 0.8
80.2 ± 4.0
9.3 ± 0.9
72.9 ± 4.9
100
16.0 ± 0.6
37.7 ± 3.0
12.2 ± 0.4
55.2 ± 1.9
control
–
22.7 ± 0.5
–
21.0 ± 0.8
–
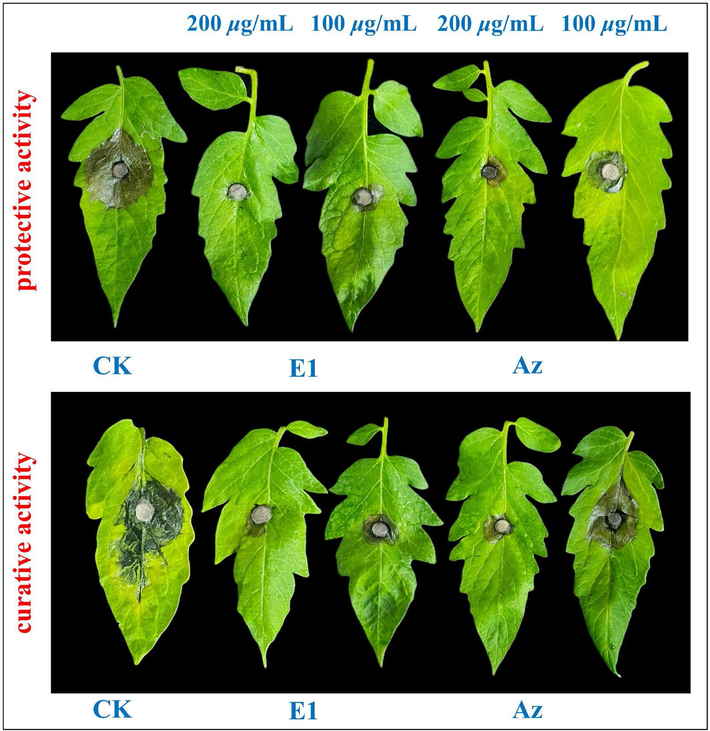
In vivo control effects of E1 against Tomato Botrytis cinerea (F.M.) on tomato leaves.
Compound
Concentration (μg/mL)
Curative effect
Protective effect
Lesion length a(mm ± SD)
Controlling fficacy (%)
Lesion length a (mm ± SD)
Controlling efficacy (%)
E1
200
10.3 ± 0.7
85.7 ± 2.0
8.8 ± 0.7
89.3 ± 1.9
100
16.7 ± 0.5
68.8 ± 1.3
12.3 ± 0.5
79.4 ± 1.3
Az b
200
13.3 ± 0.9
77.7 ± 2.5
9.7 ± 0.7
86.9 ± 2.0
100
17.5 ± 0.8
66.5 ± 2.1
15.8 ± 0.4
69.6 ± 1.0
control
–
42.3 ± 0.5
–
40.7 ± 0.9
–

In vivo control effects of E1 against Phomopsis sp (P.s.) on kiwifruit.
3.5 Observation results of mycelium morphology by E1 scanning electron microscope (SEM)
The mycelium of B.c. was further analyzed by using SEM, with the findings presented in Fig. 8. Notably, the morphology of the mycelium treated with E1 exhibited substantial alterations. In contrast, the untreated group exhibited an intact and robust mycelium. However, when the concentration of E1 was 50 μg/mL, the mycelia appeared curled and wrinkled and the surface was uneven. When the drug concentration increased to 100 μg/mL, the mycelium wrinkled more obviously, shrunk, the mycelium content leaked and the mycelium ruptured. Therefore, it was concluded that the inhibitory effect of E1 on B.c. was achieved by altering the integrity of the cell wall and cell membrane of B.c., disrupting its mycelial morphology and affecting mycelial growth. The reason for the 100 % inhibition of B.c. by E1 in the in vitro inhibition test results at a concentration of 100 μg/mL was further verified.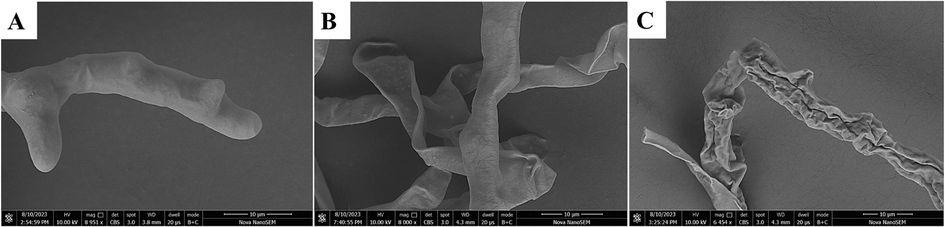
SEM images of the hyphae of Botrytis cinerea (B.c.) after treatment with E1. (A) 0 µg/mL, (B) 50 µg/mL and (C) 100 µg/mL. Scale bar for (A-C) are 20 µm.
3.6 The implications of E1 on the cytoplasmic leakage of b.c.
In order to investigate other potential mechanisms of action of E1, the current study employed two different methods to assess the degree of mycelial damage subsequent to the compound's activity, which results in the leakage of cytoplasmic contents. The results were displayed in Fig. 9. In particular, the absorbance of the mycelial supernatant of blueberry gray mold treated with E1 at 260 and 280 nm was measured using a UV spectrophotometer. Furthermore, during the course of 10 h, the absorbance of the mycelial supernatant of the mycelium treated with E1 at 260 and 280 nm was proportionate to the concentration. Second, the concentration of MDA can serve as an indirect indicator of the extent of tissue peroxidative damage to the mycelial membrane, the higher the content, the more severe the damage to the cell membrane. When the concentration of E1 (0, 25, 50, 100 and 200 µg/mL) was applied to B.c. mycelium, the MDA content grew progressively. These concentrations were all better than what the control group had. The results showed that E1 causes damage to cell membranes, with the degree of damage increasing with concentration. The degree of cytoplasmic content leakage rose, which shows that E1 may also destroy the structure of the cell membrane by destroying the permeability of the cell membrane, so that drugs can penetrate bacteria and combine with some enzymes, affecting the activity of enzymes and thus achieving an antifungal effect.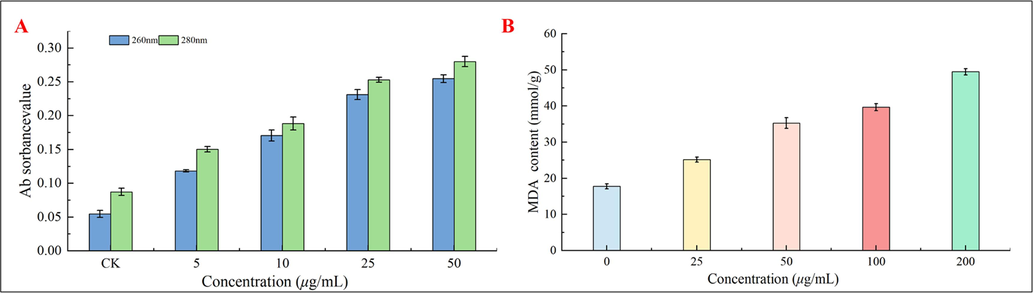
Leakage of cytoplasmic contents after E1 action on Botrytis cinerea (B.c.) hyphae was determined by UV spectrophotometry (A) and MDA (B).
3.7 E1 observation through the light microscope on the hyphae morphology
After treatment with E1 (25 μg/mL), the mycelium of B.c. was observed under an optical microscope (A, C) (10/1.25) and (B, D) (40/0.65), as shown in Fig. 10. Specifically, in the blank control group (A, B) (0.5 % DMSO) without drug treatment, the mycelium surface was smooth and had few branches. However, after E1 (C, D) treatment, it was observed that the mycelium branches increased, the mycelium was disorderly and staggered and the mycelium thickness distribution was uneven. It can be proven that the compound had a good inhibitory effect on the growth of fungi. This further explains why E1 was able to inhibit mycelial growth with excellent inhibitory activity against B.c.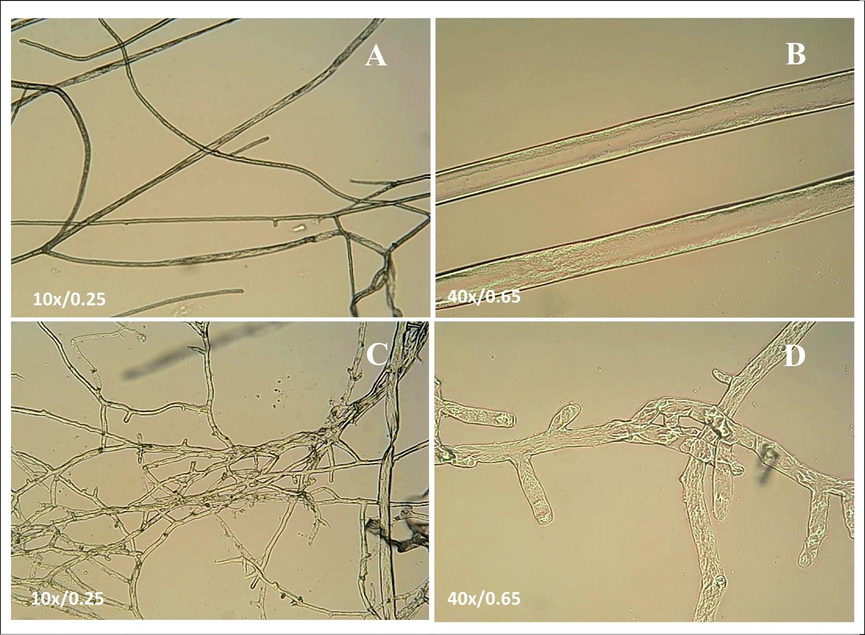
The optical microscope images of Botrytis cinerea (B.c.) treated with E1. (A, B) 0 μg/mL and (C, D) 25 μg/mL. Scale bar for (A, C) are 10x/0.25 and Scale bar for (B, D) are 40x/0.65.
3.8 The impact of E1 on b.c. Cell membrane permeability
Cell membranes play a crucial role in maintaining the structural integrity of cells and ensuring normal cellular life activities. The relative conductivity serves as a metric to assess the permeability of these membranes. As depicted in Fig. 11, the relative conductivity of the experimental group treated with different concentrations of E1 (0, 10, 25, 50 and 100 μg/mL) increased significantly after 12 h. When compared to the blank control group, the relative conductivity of the mycelium treated with E1 exhibited a time-dependent increase. Furthermore, as the drug concentration increased, the conductivity of the mycelium also rose, exhibiting a more pronounced upward trend compared to that of the control group. The findings revealed that E1 had a considerable influence on the permeability of the cell membrane of B.c. mycelium, potentially damaging the cell membrane and causing mycelium mortality. The longer the period and the greater the concentration, the more severe the cell membrane rupture, resulting in the loss of the contents, and therefore the higher the conductivity. It increases its permeability to achieve the inhibitory action.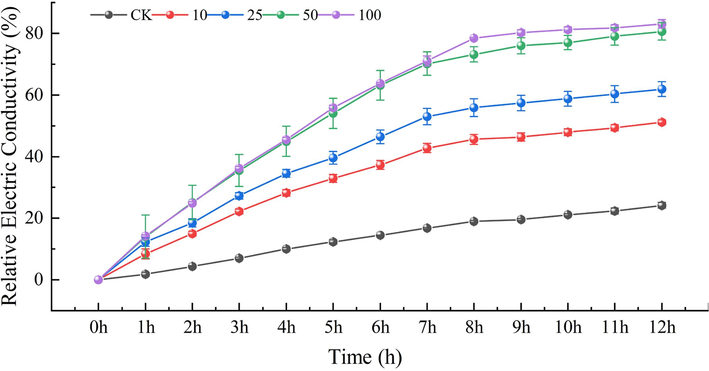
Changes in cell membrane permeability of E1 against Botrytis cinerea (B.c.).
4 Conclusion
In this study, 21 indole derivatives containing the 1,3,4-oxadiazole moiety were designed and synthesized. These target compounds were tested for antifungal activity against 12 plant pathogenic fungi. The antifungal activity test results demonstrated that the target compounds exhibited antifungal activity against the tested plant pathogenic fungi. Among them, E1 showed excellent antifungal activity against B.c., F.M., P.s. and S.s. its EC50 values of 2.8, 5.1, 5.2 and 13.9 μg/mL were superior to those of Az, which were 15.2, 31.2, 15.2 and 18.9 μg/mL respectively. In vivo activity tests showed that the protective and curative effects of E1 on blueberry leaves (91.9 % and 86.3 %) were better than Az (83.8 and 55.8 %) at the concentration of 200 μg/mL. SEM results further confirmed that E1 could cause mycelium curling and folding, mycelium surface unevenness, and mycelium structure disruption. In addition, E1 could change the integrity of the cell wall and cell membrane of pathogenic bacteria B.c., and cause the cell membrane to rupture and the contents to leak. This resulted in enhanced malondialdehyde levels and cell membrane permeability. In conclusion, the derivatives of the 1,3,4-oxadiazole structure introduced in the indole structure had good broad-spectrum antifungal activity. The present study provides a research basis for the development of new antifungal drugs.
CRediT authorship contribution statement
Bangcan He: Performed the experiments, Analyzed the data, Writing – original draft, Writing – review & editing, Visualization. Yuzhi Hu: Investigation, Verify and evaluate the antifungal activity of the target compounds. Piao Mao: Investigation, Verify and evaluate the antifungal activity of the target compounds. Tianyu Deng: Investigation, Verify and evaluate the antifungal activity of the target compounds. Yuhong Wang: Provided the material for evaluating the antifungal activities. Xingping Luo: Provided the material for evaluating the antifungal activities. Hongqian Zou: Provided the material for evaluating the antifungal activities. Zhenchao Wang: Conceptualization, Design of experiments, Funding acquisition, Data supervision, Provision of experimental mapping software, Methodology. Wei Xue: Conceptualization, Design of experiments, Funding acquisition, Data supervision, Provision of experimental mapping software, Methodology. All authors have given approval to the final version of the manuscript.
Acknowledgements
The authors gratefully acknowledge the National Nature Science Foundation of China (22007022), the Science Foundation of Guizhou Province (No. 20192452).
References
- A review on indole-based colorimetric and fluorescence sensors for the detection of heavy metal ions. Chem. Pap.. 2023;77:7379-7394.
- [Google Scholar]
- Chemistry and biology of mycotoxins and related fungal metabolites. Chem. Rev.. 2009;109:3903-3990.
- [Google Scholar]
- Design, synthesis and biological activity of chalcone derivatives containing pyridazine. Arab. J. Chem.. 2023;16:104852
- [Google Scholar]
- Synthesis of new chalcone derivatives containing a rhodanine-3-acetic acid moiety with potential anti-bacterial activity. Eur. J. Med Chem.. 2010;45:5739-5743.
- [Google Scholar]
- Simple analogues of quaternary benzo[c]phenanthridine alkaloids: discovery of a novel antifungal 2-phenylphthalazin-2-ium scaffold with excellent potency against phytopathogenic fungi. J. Agric. Food Chem.. 2020;68:15418-15427.
- [Google Scholar]
- Novel 1,3,4-oxadiazole thioether derivatives targeting thymidylate synthase as dual anticancer/antimicrobial agents. Bioorg. Med. Chem.. 2013;21:2286-2297.
- [Google Scholar]
- Research status of indole-modified natural products. RSC Med. Chem.. 2023;14:2535-2563.
- [Google Scholar]
- Antibacterial mechanisms of aronia melanocarpa (michx.), chaenomeles superba lindl. and cornus mas L. leaf extracts. Food Chem.. 2021;350:129218
- [Google Scholar]
- New chalcone derivatives: synthesis, antiviral activity and mechanism of action. RSC Adv.. 2020;10:24483-24490.
- [Google Scholar]
- Contribution of proteomics to the study of plant pathogenic fungi. J. Proteome Res.. 2011;11:3-16.
- [Google Scholar]
- Synthesis and biological activities of novel chalcone derivatives containing pyrazole oxime ethers. Fitoterapia.. 2023;166:105458
- [Google Scholar]
- Design, synthesis, molecular modeling and anti-hyperglycemic evaluation of novel quinoxaline derivatives as potential PPARγ and SUR agonists. Bioorg. Med. Chem.. 2017;25:1496-1513.
- [Google Scholar]
- 5-HT2C receptor selectivity and structure–activity relationship of N-methyl-N-(1-methylpiperidin-4-yl)benzenesulfonamide analogs. Bioorg. Med. Chem. Lett.. 2012;22:347-352.
- [Google Scholar]
- Antibacterial activities of novel dithiocarbamate-containing 4H-chromen-4-one derivatives. J. Agric. Food Chem.. 2020;68:5641-5647.
- [Google Scholar]
- Design, synthesis, biological evaluation, and molecular docking of chalcone derivatives as anti-inflammatory agents. Bioorg. Med. Chem. Lett.. 2017;27:602-606.
- [Google Scholar]
- Synthesis and biological activity of myricetin derivatives containing pyrazole piperazine amide. Int. J. Mol. Sci.. 2023;24:10442.
- [Google Scholar]
- Design, synthesis, antibacterial activity, antiviral activity, and mechanism of myricetin derivatives containing a quinazolinone moiety. ACS Omega.. 2021;6:30826-30833.
- [Google Scholar]
- Efficacy of rapamycin in modulating autophagic activity of Botrytis cinerea for controlling gray mold. Postharvest Biol. Tec.. 2019;150:158-165.
- [Google Scholar]
- Design, synthesis, antibacterial activity evaluation and molecular modeling studies of new sulfonamides containing a sulfathiazole moiety. New J. Chem.. 2021;45:8166-8177.
- [Google Scholar]
- A review of anti-infective and anti-inflammatory chalcones. Eur. J. Med. Chem.. 2007;42:125-137.
- [Google Scholar]
- Biological control of postharvest diseases of fruit. Eur. J. Plant Pathol.. 2011;133:181-196.
- [Google Scholar]
- Antibacterial and antiviral activities of 1,3,4-oxadiazole thioether 4H-chromen-4-one derivatives. J. Agric. Food Chem.. 2021;69:11085-11094.
- [Google Scholar]
- Novel 1,3,4-oxadiazole sulfonate/carboxylate flavonoid derivatives: synthesis and biological activity. Pest Manage. Sci.. 2022;79:274-283.
- [Google Scholar]
- China: Forward to the green pesticides via a basic research program†. J. Agric. Food Chem.. 2010;58:2613-2623.
- [Google Scholar]
- Design, synthesis, antiviral bioactivity, and mechanism of the ferulic acid ester-containing sulfonamide moiety. ACS Omega.. 2020;5:19721-19726.
- [Google Scholar]
- Ribas e Ribas, A.D., Spolti, P., Del Ponte, E.M., Donato, K.Z., Schrekker, H., Fuentefria, A.M., 2016. Is the emergence of fungal resistance to medical triazoles related to their use in the agroecosystems? A mini review. Braz. J. Microbiol. 47, 793-799.
- Sustainability and agricultural and food chemistry. J. Agric. Food Chem.. 2011;59:1-21.
- [Google Scholar]
- Insecticidal and antifungal activities of Rheum palmatum L. anthraquinones and structurally related compounds. Ind. Crop Prod.. 2019;137:508-520.
- [Google Scholar]
- Trichoderma/pathogen/plant interaction in pre-harvest food security. Fungal Biol-UK. 2019;123:565-583.
- [Google Scholar]
- Design, synthesis, and antibacterial activity of novel myricetin derivatives containing sulfonate. Monatsh Chem.. 2021;152:345-356.
- [Google Scholar]
- Synthesis and antiviral activity of novel myricetin derivatives containing ferulic acid amide scaffolds. New J. Chem.. 2020;44:2374-2379.
- [Google Scholar]
- Synthesis and in vitro and in vivo biological activity evaluation and quantitative proteome profiling of oxadiazoles bearing flexible heterocyclic patterns. J. Agric. Food Chem.. 2019;67:7626-7639.
- [Google Scholar]
- Recent advances and remaining barriers to producing novel formulations of fungicides for safe and sustainable agriculture. J. Control Release.. 2020;326:468-481.
- [Google Scholar]
- Synthesis, antiviral bioactivity of novel 4-thioquinazoline derivatives containing chalcone moiety. Molecules.. 2015;20:11861-11874.
- [Google Scholar]
- Crucial role of the Ca2+/CN signaling pathway in the antifungal activity of seselin against Botrytis cinerea. J. Agric. Food Chem.. 2023;71:9772-9781.
- [Google Scholar]
- Flavonoids from sedum aizoon L. inhibit Botrytis cinerea by negatively affecting cell membrane lipid metabolism. Appl. Microbiol Biotechnol.. 2022;106:7139-7151.
- [Google Scholar]
- Synthesis and insecticidal evaluation of novel N-pyridylpyrazole derivatives containing diacylhydrazine/1,3,4-oxadiazole moieties. J. Heterocyclic Chem.. 2019;56:1330-1336.
- [Google Scholar]
- Anti-tobacco mosaic virus (TMV) triterpenoid saponins from the leaves of Ilex oblonga. J. Agric. Food Chem.. 2007;55:1712-1717.
- [Google Scholar]
- Discovery of β-carboline oxadiazole derivatives as fungicidal agents against rice sheath blight. J. Agric. Food Chem.. 2018;66:9598-9607.
- [Google Scholar]
- Synthesis and antifungal activity of indole derivatives. Chem. Nat Compd.. 2023;59:111-118.
- [Google Scholar]
- Identification of botryosphaeriaceae species causing kiwifruit rot in sichuan province. China. Plant Dis.. 2015;99:699-708.
- [Google Scholar]
- Antiviral properties and interaction of novel chalcone derivatives containing a purine and benzenesulfonamide moiety. Bioorg Med Chem. Lett.. 2018;28:2091-2097.
- [Google Scholar]
- Design, synthesis and antifungal activity of novel 1,4-pentadiene-3-one containing quinazolinone. Int. J. Mol. Sci.. 2023;24:2599.
- [Google Scholar]
- Synthesis and optimization of novel allylated mono-carbonyl analogs of curcumin (MACs) act as potent anti-inflammatory agents against LPS-induced acute lung injury (ALI) in rats. Eur. J. Med. Chem.. 2016;121:181-193.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2024.105758.
Appendix A
Supplementary data
The following are the Supplementary data to this article:Supplementary Data 1
Supplementary Data 1







