Translate this page into:
Optimization and Techno-Economic analysis of catalytic gasification of wheat straw biomass using ASPEN PLUS model
⁎Corresponding authors. mammar@gcuf.edu.pk (Muhammad Ammar), jdwcheju@jejunu.ac.kr (Dong-Won Jung)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Biomass gasification efficiently produces heat, electricity, and power. However, removing harmful contaminants from raw syngas is crucial. Tar production is a challenge due to blockage, plugging, and corrosion. Tar steam reforming (TSR) is the most promising technique, converting high molecular weight hydrocarbons into CO, CO2, H2, and CH4. In this study, a model of biomass gasification using wheat straw as biomass feedstock has been developed using Aspen Plus. The gasification flow sheet encompasses gasification, catalytic filter candle, gas cleaning, impurity removal reactor, separator, and subsequent sorbent reactors. Ni-based catalysts with Ni ratios (5%, 10%, and 15%) are used to simulate TSR reactions. Results show that the 15% Ni-Co-Al2O3 catalyst outperforms the 10% and 5% Ni-Co-Al2O3 catalysts. This study explores the impact of temperature, catalyst loadings, and steam-to-carbon (S/C) ratios on toluene conversion and hydrogen yield in catalytic steam reforming, along with temperature, steam-to-biomass ratios, and equivalence ratio on syngas fraction in gasification. It includes a techno-economic analysis of wheat straw gasification to improve syngas energy through efficient tar conversion.
Keywords
Biomass gasification
Tar removal
Catalytic steam reforming
Aspen plus
Techno-economic analysis
- GR
-
Gasifier Reactor
- CS
-
Catalyst Sorbent
- CFC
-
Ceramic Filter Candles
- BG
-
Biomass Gasification
- UBT
-
Uniform Bed Temperature
- HGC
-
Hot Gas Cleaning
- MC
-
Moisture Content
- VM
-
Volatile Matter
- PA
-
Proximate Analysis
- UA
-
Ultimate Analysis
- CG
-
Char Gasification
- FBR
-
Fluidize Bed Reactor
- CF
-
Ceramic Filter
- WGSR
-
Water Gas Shift Reaction
- WGR
-
Water Gas Reaction
- QRM
-
Quasi-Equilibrium Method
- FBG
-
Fluidize Bed Gasifier
Abbreviations
1 Introduction
Carbonaceous feedstock stands out among renewable energy sources as an optimal choice for heat and power generation. Its abundance and capacity to deliver substantial energy make it particularly promising. Moreover, compared to fossil fuels, it offers the advantage of significantly lower environmental impacts. With increasing demand for fossil fuels, concerns arise regarding their finite nature and environmental impact. Human activities, particularly the combustion of fossil fuels, have led to significant emissions of greenhouse gases, contributing to climate change. The rise in the global population further exacerbates the demand for energy resources (Xie et al., 2012). Consequently, the costs associated with fossil fuels have increased, and the effects of climate change have become more pronounced. To mitigate greenhouse gas emissions, these developments have prompted a shift towards sustainable energy sources, such as wind, hydropower, biomass, solar, and other renewable technologies (Lahijani et al., 2013; Patil et al., 2011). The combustion of fossil fuels releases greenhouse gases, exacerbating environmental issues. This has spurred interest in the utilization of renewable energy sources. While various renewable energy sources exist, biomass has emerged as a promising alternative to fossil fuels (Molino et al., 2016; Eriksson et al., 2012). Biomass has the potential to replace fossil fuels partially and can be converted into various states of matter, making it a versatile substitute (Nieminen et al., 2012). Bio-syngas, produced from solid biomass through gasification, hold promise for large-scale applications in industrial chemical processes. It can produce liquid biofuels, specialty chemicals, electricity, and heat generation (Larkum et al., 2012; Kaushal and Tyagi, 2017).
Several methods exist for generating energy from biomass and municipal solid waste, including thermal, chemical, biological, and physical techniques (Shahbaz et al., 2022; Dai et al., 2015). These methods can convert biomass and municipal solid waste into heat, power, gaseous, and liquid fuels (Shahbaz et al., 2019). Gaseous fuels such as syngas and hydrogen and liquid fuels like ethanol, methanol, and bio-oils can be produced through these processes (Chen et al., 2015; Vannice, 1975). However, bio-syngas may contain harmful impurities, including sulfur compounds, soot, tar, and chloride compounds, while primarily consisting of hydrogen (H2), carbon monoxide (CO), and methane (CH4) (Yu et al., 2015; Barisano et al., 2012; Shahbaz et al., 2020). Tar, a major obstacle in biomass gasification, impedes commercialization efforts (Shehzad et al., 2018;Jiang et al., 2016). It comprises a complex mixture of organic compounds that can condense at ambient temperatures, causing valve and pipeline obstructions (Lynd et al., 2011; Ahmed et al., 2014). Catalytic steam reforming is an efficient method for tar removal during biomass gasification, converting tar into hydrogen-rich gas, thereby increasing the heating value of syngas and regulating the H2/CO ratio crucial for liquid fuels synthesis via the Fischer-Tropsch process (Eriksson et al., 2012; Taupe et al., 2016). Various catalysts have been proposed for catalytic steam reforming, including dolomites, alkali metal catalysts, zeolites, novel metal catalysts, mayenite minerals, magnesites, olivine, and nickel-based catalysts (Muzyka et al., 2015; Kwon et al., 2012). However, despite their excellent activity, nickel-based catalysts face challenges such as rapid deactivation due to metal sintering and carbon deposition, limiting their effectiveness (Patil et al., 2011; Antonopoulos et al., 2012). Alumina, with its high surface area and thermal and mechanical stability, is an ideal support for nickel-based catalysts (Patil et al., 2011; Antonopoulos et al., 2012). Carbon deposition is a common issue in hydrogen production reactions, and various methods have been explored to mitigate it, including substrate modification, nickel alloying with transition metals (e.g., Fe, Cu, Co), alkaline earth metal addition, and synthesis route optimization (Devi et al., 2005; Allesina et al., 2013).
The simulation of wheat straw gasification presented in this study involves a comprehensive approach to address various impurities, including tar. It employs advanced gas cleaning and conditioning techniques using a combination of catalyst sorbents (CS) in the gasifier reactor (GR), catalytic agents in the freeboard for tar removal, and subsequent sorbent reactors for H2S and HCl removal. The simulation, conducted using Aspen Plus, encompasses the entire process involving three reactors: a sorbent reactor utilizing a chemical agent in the gasifier temperature range, a stoichiometric reactor activating ceramic filter candles (CFC) at gasifier temperature, and lower-temperature sorbent reactors. The primary objective of this simulation is to evaluate the overall performance of this advanced process configuration in removing pollutants such as hydrocarbons, H2S, HCl, and NH3 from syngas through catalytic methods, considering varying operating conditions such as temperature and steam-to-biomass ratio during the gasification process to optimize the system. Additionally, this study investigates the catalytic steam reforming of tar using novel nickel-based alumina-supported catalysts. Due to its prevalence, toluene is selected as a representative component of biomass tar. Modified nickel-based catalysts, Ni-Co/Al2O3 with 5%, 10%, and 15% compositions, are evaluated. The catalytic agents are systematically investigated regarding performance during toluene catalytic steam reforming and the effects of support modification and Ni incorporation on their properties. These findings may provide valuable insights for developing integrated and novel nickel-based catalysts for tar catalytic steam reforming in biomass gasification. Additionally, this study conducts a techno-economic analysis of wheat straw gasification using Aspen Plus.
2 Material and methods
2.1 Biomass characteristics
The wheat straw is utilized as a biomass feedstock in the study subjected to the gasification process. The proximate and ultimate analyses of wheat straw are readily available in Table 1.
Proximate analysis
Moisture Contents
9 %
Volatile Matter
62 %
Fixed Carbon
23 %
Ash
6 %
Ultimate analysis
Carbon
46 %
Hydrogen
6 %
Oxygen
40 %
Nitrogen
7 %
Sulfur
1 %
2.2 Catalytic gasification of biomass using Aspen plus
In the study, the simulation model is developed using Aspen Plus. The Gibbs free energy minimization equilibrium model is designed to model the catalytic gasification of wheat straw. The primary steps and components involved in the catalytic gasification of wheat straw are biomass decomposition, gasification, gas cleaning, catalytic filter candles, impurities removal reactor, and separator.
2.2.1 Model development
The assumptions for the development of the model of catalytic gasification of wheat straw are as follows:
-
The model is zero-dimensional prototypical.
-
The catalytic gasification process is performed under steady-state circumstances.
-
The process is isothermal, and there is an isothermal uniform bed temperature (UBT) within the reactor with no temperature and pressure gradient.
-
All gases behave ideally.
-
Drying and pyrolysis are prompted inside a bubbling fluidized bed (BFB); principally, it consists of CO, H2, CO2, H2O, and CH4.
-
The char produced from the pyrolysis zone is 100% carbon (graphite).
-
The catalytic agent is marked off as a conventional solid in the Aspen Plus databank by its physical and chemical properties.
-
The sulfur entirely reacts to make H2S, and just a tiny part of N transforms into NH3, and a major part of N makes nitrogen oxides (Molino et al., 2016; Andrew et al., 2016).
For the physical properties, methods such as Ideal & RK-SOAVE (Redlich-Kwong-Soave), RKS-BM (Redlich-Kwong-Soave equation with the Berthelot Mixing rules), and PR-BM (Peng-Robinson equation with the Berthelot Mixing rules) are deemed suitable for gasification occurring at high-temperature components with minor gas constituents. Aspen Plus automatically retrieves binary interaction parameters from its database. In configuring the non-conventional properties, biomass and ash contents are inputted as nonconventional solids during the simulation, alongside their ultimate and proximate analyses. To determine the enthalpy and density of the non-conventional components, the models “DCOALIGT” for density and “HCOALGEN” for enthalpy are chosen.
2.2.2 Flow sheet development
The Aspen Plus flow sheet of the developed model is shown in Fig. 1, while all the units are described in Table 2. In a single reactor, the gasification process encompasses three simultaneous stages: drying, pyrolysis, and gasification. However, these stages are distinctly emphasized in various blocks within the simulation. The power plant primarily features a gasifier that transforms wheat straw into syngas, supported by a hot gas cleaning (HGC) system. The “ WBIOMASS ” stream is initially established using wheat straw, with a constant 200 kg/h flow rate. Ambient air is introduced to the wet biomass to reduce moisture. “WBIOMASS” is designated as a non-conventional stream, with ultimate and proximate analyses provided, alongside mass flow rate and thermodynamic specifications. The wet biomass is supplied at 25°C and 1 bar pressure and is mixed with air in a mixer. Component attributes for biomass in the NCPSD substream are configured, with PROXANAL, ULTANAL, and SULFANAL measured as weight % on a dry basis, excluding moisture in PROXANAL. This mixture is then directed to the “RStoic Reactor” for drying. In the RStoic block, the moisture content is removed from the biomass. At 1 bar pressure and with a heat duty of 0 Btu/hr, the RStoic block is employed, although biomass drying is not typically treated as a chemical reaction. Aspen Plus software assigns all non-conventional constituents a molecular weight of 1.0 as a standard. The “Calculator Block” determines the dried biomass's moisture content (MC) and calculates the corresponding biomass conversion to water. Upon completing the drying process, the “MCINCPSD” stream class is utilized, as biomass decomposition converts to carbon, necessitating a stream class inclusive of conventional solids. Carbon is considered a solid in this process.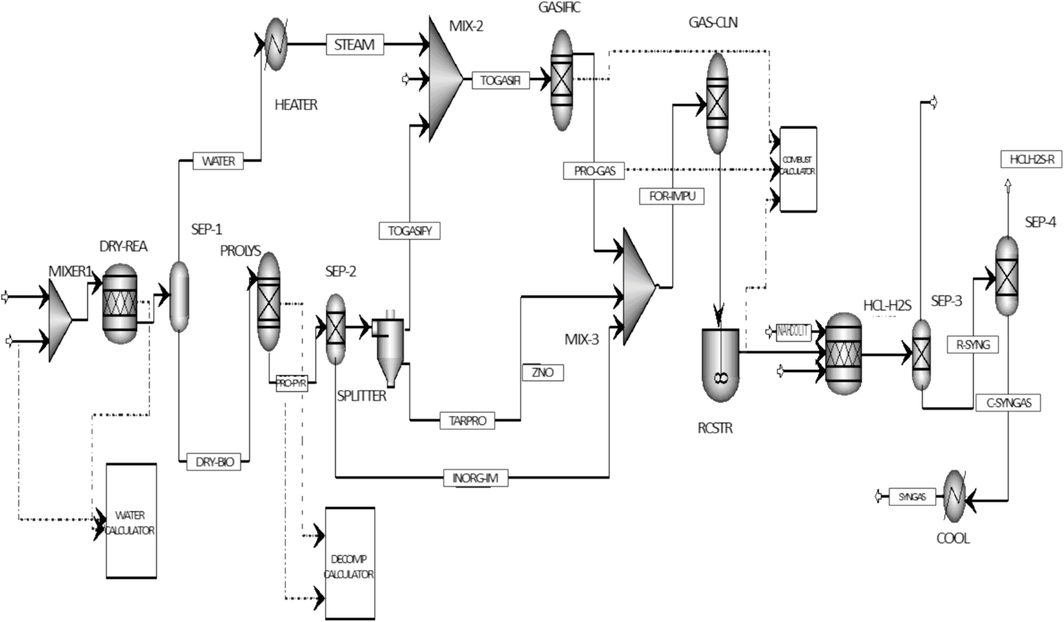
The flow sheet of a simulation model for gasification progress.
Aspen Plus model name
Block ID
Description
MIXER
MIXER
Mixer - mixes air with the wet biomass.
MIXER-2
Mixer - blends oxidizing fluid in the “TOGASIFY” stream.
MIXER-3
Mixer - blends the “PRO-GAS” stream beginning at gasified in “INORG-IM” and “TAR-PRO.”
RSTOIC
DRY-REA
RStoic reactor - Reduces the fuel moisture content.
NAHCOLIT
RStoic reactor - mockup the reaction amid nahcolite and HCl.
RYield
PROLYS
RYield reactor - alters the non-conventional stream “BIOMASS” to their conventional components.
RGIBBS
GASIFIC
Gibbs free energy reactor - mocks up the processes of drying, pyrolysis, partial oxidation, and gasification. It constricts the synthetic balance for the set-out reactions to allow the syngas composition with particular temperature accessibility instead of specific reactions.
GAS-CLN
Gibbs free energy reactor will help carry out a nickel reaction with tar at high temperatures.
SSPLIT
SPLITTER
Splitter - divides unreacted char from char to scorch.
RCSTR
RCSTR
RCSTR - catalyst filter reaction will be carried out by it.
HEATER
HEATER
Heat exchanger - Steam heat exchanger.
COOL
Heat exchanger - Syngas cooling.
SEPARATORS
SEP-1
Separator - separates the dry biomass and water
SEP-2
Separator - set apart the feed within two streams: “VOLATILE” with “INORG-IM.”
SEP-3
Separator - set apart the ash, char, H2S, and HCl.
SEP-4
Separator - remove water and other by-products.
2.2.3 Biomass decomposition
The process is intricate due to the myriad of chemical and physical transformations occurring rapidly and simultaneously. When dry biomass is heated within a temperature range of 200–500 °C, without oxygen or any other oxidizing agent, it decomposes into various products, including volatiles, known as tar or condensable hydrocarbons, gases, and solid char. The Aspen Plus reactor model, “RYIELD,” is utilized to simulate the breakdown of the supplied material. At this juncture, biomass converts into its constituent elements, comprising hydrogen, carbon, ash, sulfur, oxygen, and nitrogen, by specifying the yield supply based on the ultimate biomass analysis. Yield distributions are calculated in accordance with the ultimate analysis of the feedstock, aided by the calculator block and FORTRAN statements. Since the decomposing of biomass produces the elemental components S, Cl, and N; as a result, it produces H2S, HCl, and NH3 by succeeding reactions:
The products produced from the pyrolysis zone are separated using a SEP-2 in the flowsheet. N2, Cl2, and S value equal 1 for the fractional conversion. The inorganic impurities are supplied to the “MIXER-3”. The splitter is used to split the streams into different sub-streams. Here, one stream is mixed with oxidizing fluid and steam in the “MIXER-2” and then is transferred to “GASIFIC” for gasification. The second stream is split for the tar products, as shown in Fig. 1. The system is in equilibrium. Hence, the simulation of tar production in the gasifier “GASIFIC” is impossible. Thus, “TAR-PRO” is also supplied to “MIXER-3” (Table 2). The amount of tar, besides gases, must equal the volatile matter in proximate analysis. Furthermore, it is presumed that biomass's complete oxygen and hydrogen components are unconfined throughout the degasification procedure. Although unrestrained, volatile matter combines different products like CO2, CH4, CO, H2O, H2, and prime tar (Tar) (Eriksson et al., 2012).
2.2.4 Gasification
During the drying and devolatilization stage, char emerges as the primary product, often depicted solely as carbon. Devolatilization char gasification (CG) represents a highly intricate process involving heat, mass transfer, and chemical interactions. To simplify, CG is categorized into heterogeneous and homogeneous reactions. The gasifier plays a pivotal role in gasification, with the fluidized bed reactor (FBR) exhibiting superior performance due to its high reaction rates and conversion efficiency. A quasi-equilibrium temperature (QET) method is utilized to model the fluidized-bed biomass gasification process, ensuring a specific syngas composition. In the simulation, the RGibbs reactor, referred to as “GASIFIC” in the flow sheet, simulates the gasification process, with products from the pyrolysis zone directed to the gasifier. This reaction commences in the presence of the gasifying agent. The Gibbs free energy minimization chemical technique, implemented through RGibbs reactors, establishes equilibrium constants for each reaction based on gasifier temperature, ensuring a balanced syngas composition. Operating conditions for the gasification system are set at 800 °C and 1 bar pressure, with steam serving as the sole oxidizing agent; thus, water is supplied to the boiler to generate steam, which is then superheated to 200 °C and 1 bar pressure. At thermodynamic equilibrium, the total Gibbs free energy of the system is minimized, determined by a specific equation:
where n and μ are specified as moles number and chemical potential of species “i” correspondingly. Hence, the main reactions are in the “GASIFIC” section.
Boudouard
The products from the Gasification and tar products are transferred to the “MIXER-3,” where the impurities are delivered to “GAS-CLN’ for the cleaning process.
2.2.5 Gas cleaning
Prior to any significant catalytic action steps, such as shifting and reforming reactors, the raw petroleum gas within the gasifier undergoes cleaning, necessitating the removal of ammonia (NH3), particulates (fly ash), alkali metals, reduced sulfur compounds (H2S, COS, etc.), and halides (HCl, HBr, etc.). Moreover, since the hydrogen yield is sensitive to CO, the purified hydrogen gas yield must also remove CO to the required extent. The raw gasifier petroleum vapor is scrubbed of particulates along the flow channels to prevent blockage by passing it over a hot flue channel using CFC components designed for exceptionally high temperatures. The filter accumulates fly ash, occasionally removed by inert gas back-flushing to maintain adequate pressure drop across the filter. Reliable, cost-effective hot flue filters include ceramic filters (CF), commercially available through Siemens Westinghouse for various applications, including integrated gasification combined cycle (IGCC), pressurized fluidized bed combustion (PFBC), and biomass gasification. These CF types undergo extensive sub-industry testing, encompassing full-scale analysis across a wide range of operating conditions, including gas flow rate, temperature, pressure, and dust loading. An extraordinary temperature of 1037 °C is required for this process.
In fluidized bed gasifiers, chemically active bed materials can aid in cleaning biomass gasification gas by reducing tar load. Besides their catalytic tar decomposing effect, these bed materials influence the sintering properties of the bed. By selecting an appropriate bed material, higher temperatures or pressures can be used in the gasifier without issues. Common bed materials used in biomass gasification are low-cost natural minerals. Nickel catalysts are the most widely studied non-metallic catalysts for biomass gasification gas cleaning. Nickel catalysts have been researched as bed materials and catalysts for a separate reformer after the gasifier. Gas cleaning processes can enhance the performance of the biomass gasifier. For cleaning processes, the nickel catalyst is used in bed gas cleaning. The “CLEANING” RGibbs reactor is utilized for replication in Aspen Plus, operating at 800 °C and 1 bar. Under fixed conditions, the gas yield can be increased by reducing tar and organic contaminants within the gasifier bed with catalyst assistance.
2.2.6 Catalytic filter candles
An RCSTR reactor simulates the catalytic filter candles. The diverse elements measured in lieu of this model are naphthalene, phenol, and toluene. Subsequently, the products produced from the “GASIFIC” are delivered to the RCSTR reactor in which the tar steaming process occurred in the existence of nickel catalyst Ni-Co/Al2O3 with the ratio of 5%, 10%, and 15 %wt. So, the reactions arising inside the “RCSTR” block are signified via Eq. (10) to Eq. (13), respectively.
Steam reforming
2.2.7 Impurities removal reactor
Two types of sorbents, regenerable and non-regenerable, are employed in gas cleaning technology to remove chlorine and sulfur from petroleum vapors. Regenerable sorbents, like ZnTiO3, have undergone significant development, particularly in integrated gasification combined cycle (IGCC) systems for coal-based gasification, along with other types based on iron oxides (Fe2O3), manganese (Mn2O3), and copper (CuO). Non-regenerable sorbents, including sulfa treat iron oxide and conventional zinc oxide (ZnO), are economically available and widely used for desulfurization applications at temperatures up to 260 °C for iron oxide and 400 °C for zinc oxide. Chlorine removal, also commercially available, can be accomplished using non-regenerable resources like sodium-supported alumina or other sodium-containing sorbents.
In this simulation, the Rstoic reactor “HCl-H2S” eliminates contaminants. Since HCl, H2S, and NH3 are significant contributors to environmental and corrosion issues, gas cleaning is imperative to mitigate these impurities. Therefore, a multifunctional reactor is considered to simultaneously remove all these contaminants in hot cleaning gas, employing sorbents. HCl interferes with the adsorption of H2S, necessitating multiple phases to achieve the desired mg/L level for these inorganic impurities. Zinc-based sorbents are the primary contenders for H2S removal, typically conducted at a mid-temperature of 400 °C. Additionally, cerium or copper-based sorbents are preferred to reduce H2S below 1 mg/L. The cleaning ability of different oxides for H2S varies with temperature, with Ce and Cu > Zn > Co > Mo > Mn > Fe > Ni > Sn. Alkali-based sorbents, predominantly potassium and sodium combinations, are favored for HCl removal at mid- and high-temperatures. The most effective sorbent for HCl removal is nahcolite (NaHCO3), capable of reducing HCl impurities to concentrations below 1 mg/L in the 526–650 °C temperature range. The reduction of hydrogen sulfide using the sorbents listed in Table 3, employing the REQUIL reactor. Each sorbent is evaluated at its highest stability temperature. The values of other components remain unchanged. Table 3 illustrates the assessment of sorbent flow rates necessary to reduce the H2S concentration to below 1 mg/L, with reference to the chosen temperature range presented in the reactor with the different composition of syngas production. Consequently, the REQUIL block ensures all contaminants are maintained below regulatory limits.
Sorbent Type
Sorbent Flow rate (kg/h)
Sorbent Temperature (oC)
H2S level (mg/L)
Copper oxide
0.3
600
0.7
Zinc oxide
0.3
450
0.7
Cerium oxide
0.3
600
0.7
Iron oxide
0.3
600
0.7
2.2.8 Separator
The raw syngas produced from the gasification zone go through cleaning. Then, the separator “SEP-3” removes the impurities like ash, char, H2S, and HCl. Then, the “R-SYNG” is passed through the “SEP-4”. Choosing a heat exchanger is to cool the syngas to remove water.
2.3 Techno-economic analysis
Techno-economics analysis is an inquiry to evaluate the technical and economic growth of the catalytic gasification of wheat straw. According to this analysis, techno-economics analysis for wheat straw gasification is performed at optimized operating conditions. In the sustenance of techno-economic analysis, the costs involved and calculated standards have been described in Fig. 2. Certain parameters and values are deliberated in Table 4 for economic assessment.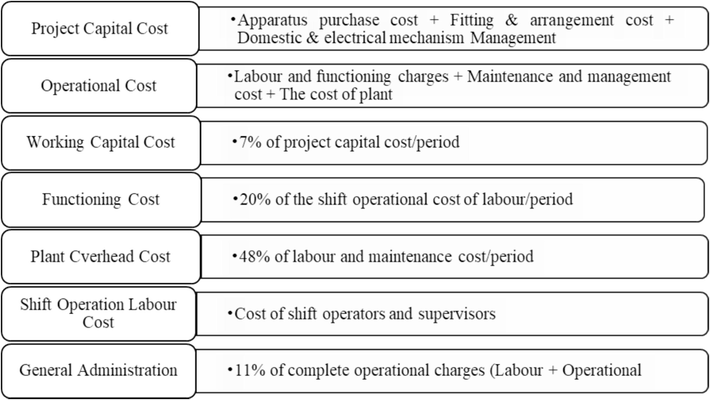
Elaboration of the costs and calculations involved in Aspen plus.
Specifications
Values
No. of periods analysis for 15 years
15
Weeks per day
300 ∼ 320 day
Number of workers/ shift
3
Hours per period
8766
EPC cost
33 weeks
Construction phase time
20 weeks
Start-up-time
20 weeks
Number of administrators/shift
1
Unit cost for the machinist
25/h
Unit cost for supervisor
40/h
Devaluation
Straight line
Interest rate 15 %/year
15 %/year
Salvage value
15 year
3 Results and discussion
3.1 Reforming behaviour
3.1.1 Effects of temperatures and catalyst loadings on toluene conversion
The reaction temperature has an intense effect on tar reforming. The reaction temperature is between 500 and 1000 °C with an increment of 100. Fig. 3(a) and 3(b) illustrate the remarkable toluene conversions and hydrogen yield at different temperatures. Fig. 3(a) shows that the toluene conversion extraordinarily increases over without catalyst and all %Ni loading 5%-15% Ni-Co/ Al2O3 as the temperature increases. In the case without a catalyst, toluene seems stable initially. The efficiency of toluene conversion is very low at 600 °C, i.e. 13%, and topsy-turvy increase with only 31% conversion at 700 °C. As the temperature increases, toluene starts to lose its stability. As a result, at 800–900 °C temperature, toluene conversion drastically increases, and 63% conversion occurs. However, toluene conversion is also increased by temperature rise in the case of a catalyst; nevertheless, the toluene conversion rate is significantly higher than without a catalyst. This shows a remarkable approach for tar conversion in the presence of a catalyst. In Fig. 3(a), the toluene conversion enhances from 29% to 95%, 25% to 90%, and 21% to 87% for 15% Ni-Co/Al2O3, 10% Ni-Co/Al2O3 and 5%Ni-Co/Al2O3 as the temperature increases from 500 °C to 1000 °C, respectively.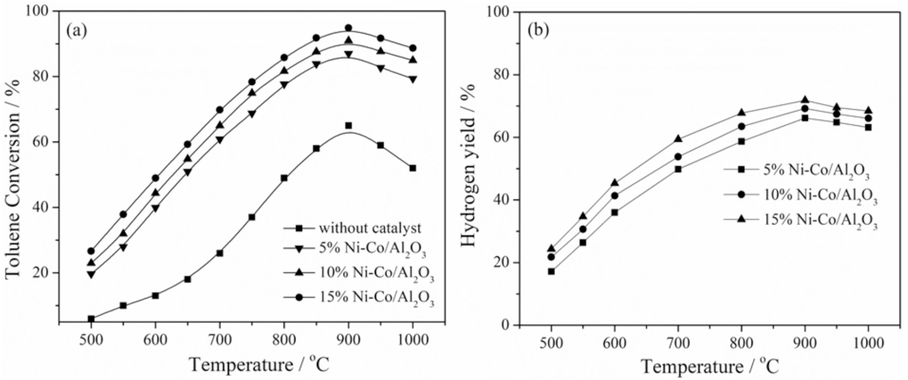
Effects of temperatures on toluene conversion (a) and hydrogen yield (b).
The data presented in Fig. 3(a) highlights the substantial conversion efficiencies of toluene across various %Ni loadings and temperatures. Notably, as temperatures increase, the breakdown of tars into smaller particles such as H2 and CO becomes more efficient. This phenomenon is integral to understanding steam reforming processes' catalytic activity and product distribution. The H2, CO, and CO2 concentrations are closely tied to the temperature-dependent dynamics of the water gas shift reaction (WGSR). This reaction plays a pivotal role in adjusting the gas composition and is a key factor in optimizing the efficiency of steam-reforming reactions using nickel catalysts. The enhanced steam-reforming activity observed at higher temperatures is attributed to the increased catalytic activity of nickel metal. However, it is crucial to note that a delicate balance must be maintained while steam reforming is thermodynamically favorable at elevated temperatures. High temperatures can lead to challenges such as reduced hydrogen creation due to the exothermic nature of certain reactions, as described by Le Chatelier's principle (Kaushal and Tyagi, 2017; Yan et al., 2016). At around 900 °C, a critical transition point is observed where further increases in temperature lead to a slight decrease in toluene conversion efficiency across all %Ni loadings. This decline can be attributed to catalyst deactivation caused by carbon deposition and sintering processes. These mechanisms result in the formation of coke on the catalyst surface, which inhibits active sites and diminishes the efficiency of toluene removal. The detrimental impact of coke formation underscores the importance of managing temperature ranges to mitigate catalyst deactivation. By optimizing temperature conditions within a suitable range, it becomes possible to maintain catalytic effectiveness and uphold efficient toluene conversion rates. Additionally, strategies to prevent or reduce coke formation, such as catalyst regeneration techniques, are crucial for sustaining long-term conversion efficiency and process stability.
Fig. 3(b) illustrates the %Ni loadings influence hydrogen yield. In Fig. 3(b), hydrogen yield increases with increasing temperature for all %Ni loadings. Fig. 3(b) explains that hydrogen yield at lower reaction temperatures is more significant than at higher temperatures. Hydrogen yield for 5% Ni-Co/Al2O3, 10% Ni-Co/Al2O3, and 15% Ni-Co/Al2O3 are 66%, 68% and 71% at 900 °C, respectively. However, the reverse reaction consumes more carbon dioxide and hydrogen with increasing temperature. Hence, it is found that the hydrogen percentage yield increment is getting lower with the rising temperature. In addition to the effects of steam reforming and WGS reactions on hydrogen yield, the partial CO2, CO, and H2 are formed. The methanation reactions can decrease the hydrogen yield. During this simulation, it is noted that the formation of CH4 is extraordinary at a lesser temperature in Ni-Co/Al2O3 catalyst presence; the reason for this higher value of CH4 is that Co is present in the catalyst can mainly stimulate the methanation reaction from H2 (Li et al., 2014). It has been noted that the concentration of CH4 is very high at a lower temperature of 600 °C, equated with a higher temperature, and H2 concentration is decreased in the presence of a catalyst (Yan et al., 2016; Šulc et al., 2012). Understanding and analyzing diminishing returns on conversion rates at higher temperatures or catalyst loadings provides critical insights into process optimization by guiding decisions on optimal operating conditions, catalyst efficiency, energy utilization, economic feasibility, sustainability, and process stability. These insights are instrumental in developing efficient and sustainable chemical processes.
3.1.2 Effect of S/C ratio on toluene conversion and hydrogen yield
The impacts of the S/C ratio on toluene conversion and hydrogen yield at 900 °C are studied at the S/C ratio varied from 0.5 to 2.0, as shown in Fig. 4(a) and 4(b). It illustrates that as the S/C ratio increases, the proficiency of toluene conversion extraordinarily improves, and Fig. 4(a) demonstrates the increment of toluene conversion. For the reforming reactions, it is noted that in Fig. 4(b), the partial steam pressure is higher for the higher S/C ratio. Strengthening the reforming reactions can intensify the partial steam pressure; as a result, the concentration of hydrogen production can increase. Le Chatelier’s principle also explains that WGS reactions to produce hydrogen and hydrogen yield can be promoted by escalating the values of S/C ratios (Vannice, 1975; He et al., 2010). Then again, good steam is used at a high S/C ratio to initiate tar reforming and cracking, prompting higher gas and hydrogen yields. Moreover, higher water pressure favors the balance of WGSR headed for hydrogen yield and can diminish coke formation on the impetus by advancing the gasification of carbonaceous intermediates. Incidentally, the inordinate steam may prompt some unfriendly impacts. The first is that exorbitant water may cause extra energy to separate steam from the dryness of delivered gas. The subsequent one is the diminishing tar decay brought about by the diminishing changing temperature due to the heat consumed by steam.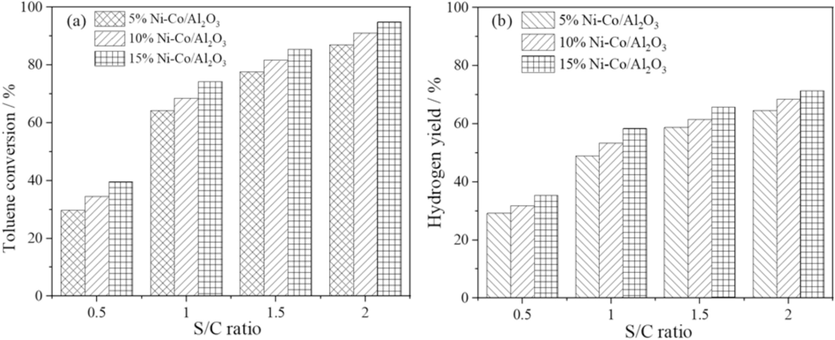
S/C ratio on toluene conversion (a) and hydrogen yield (b).
3.2 Gasification behaviour
3.2.1 Effect of gasifier temperature on syngas fraction
The gasifier temperature is altered between 600 °C and 1200 °C. The influence of gasifier temperature on syngas mole fraction is shown in Fig. 5. The carbon in the biomass at 600 °C temperature is not oxidized wholly, so this does not favor syngas production. However, with the rising temperature, the carbon further deteriorates, increasing the conversion rate. The syngas' incomplete scorch methane and carbon exist at very low temperatures. However, as the temperature rises, the Boudouard and methanation reactions contribute, and the unburned carbon begins to transform into carbon monoxide by the Boudouard reaction. The reverse methanation reaction forms H2 from methane. So, if the gasifier's temperature is increased, it can favor the carbon monoxide and hydrogen yield; thus, the heating value of gas can be enriched. According to the methanation reaction, with the temperature rise, the mole fraction of hydrogen can be increased, and it is noted that the methane mole fraction in syngas is dropped down. It is also seen that the yield of CO and H2 began to drop down at more significant temperatures because of the water gas reaction (WGR). Carbon dioxide and carbon monoxide mole fractions depend on the gasifier temperature via the Boudouard reaction (Park et al., 2010; Chan et al., 2019). Although the temperature upsurges, the CO2 mole fraction can be reduced, and the mole fraction of CO can escalate. Thus, the water gas reaction is more favorable for producing CO and H2.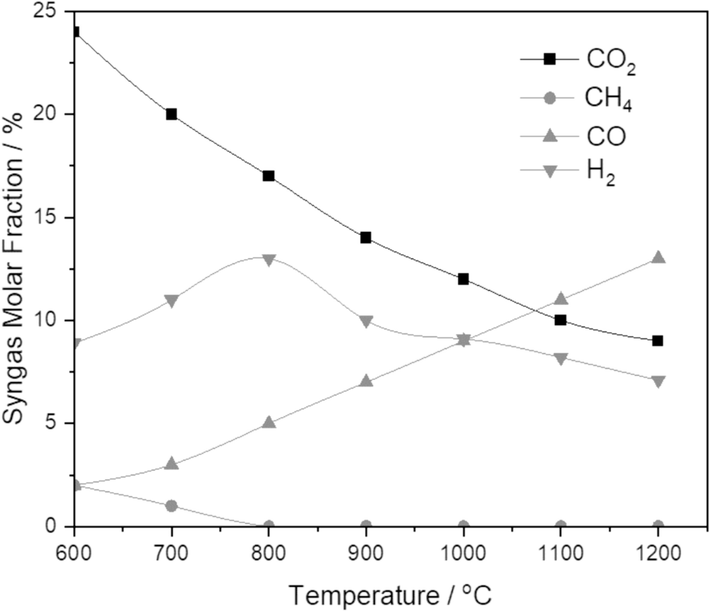
Effect of gasifier temperature on syngas fraction.
3.2.2 Effect of steam-to-biomass ratio on syngas fraction
The steam to biomass ratio (SBR) can be described as the mass flow rate of the steam supplied into the gasifier divided by the biomass feed rate on a dry basis, and this is one of the most significant process constraints strangled in steam gasification (Allesina et al., 2013; Li and Gong, 2014). Steam, as a gasifying agent, is considered an effective pro to enhance the molecular fraction of H2. The concentration of H2O is increased by injecting the steam into the gasification process. The steam to biomass has been from 0 to 2.0; the consequence is shown in Fig. 6. The influence of injecting steam on syngas production has been investigated. Here, the syngas molar fractions are considered to have a role in SBR·H2O partial pressure can be escalated to the inner side of the gasification reactor by using steam as a gasifying agent and promoting the WGS, steam reforming reaction, and WGR. The steam intensifies the mole fraction of CO2 and H2. According to the water gas reaction, the mole fraction of CO2 decreases by increasing the steam.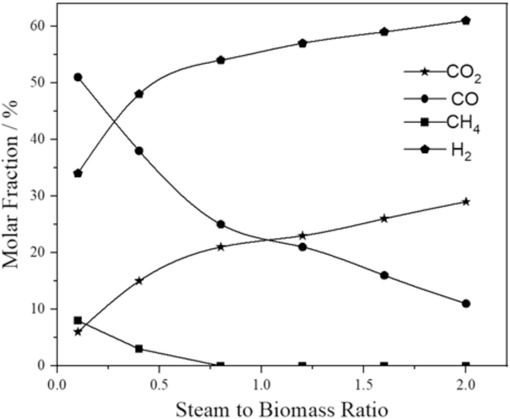
Effect of steam-to-biomass ratio on syngas fraction.
3.2.3 Effect of equivalence ratio on syngas fraction
Equivalence ratio (ER) is the ratio of the stoichiometric amount of air to the amount of air delivered to the gasifier. It can be directly evolved with the air (Villa-Gomez et al., 2011). This means that surging the equivalence ratio can increase the amount of oxygen delivered to the gasifier. Consequently, it can also increase the carbon conversion within the gasoline. The excessive quantity of O2 can cause the fuel to be oxidized wholly. So, syngas starts to drop down. The amount of H2 and CO is escalated initially because fuel conversion efficiency increases. However, the influence of the complete combustion of fuel can be seen, which lessens syngas production at a certain point of 0.3, which can be noticeably grasped in Fig. 7. The consequences of this complete combustion lead the CGE values to fall. The heating values of the syngas are to be reduced after this point. The Boudouard reaction is endothermic; char reacts with CO2 by increasing the temperature to form CO. The amount of Char at 0.3 ER value is enough for the Boudouard reaction, so CO increases and decreases for CO2. However, beyond this limit, with the increasing value of ER, the amount of char is inadequate; hence, it can increase CO2 and decrease CO. The water gas reaction is endothermic. Hydrogen and carbon monoxide production is escalated through the rising temperature and ER; thus, an extra amount of char is used. The methanation reaction is exothermic, increasing ER and temperature; it reduces CH4 formation. The methane reforming reaction is endothermic, meaning the amount of CH4 is less because, with the increasing temperature, it prefers a forward reaction.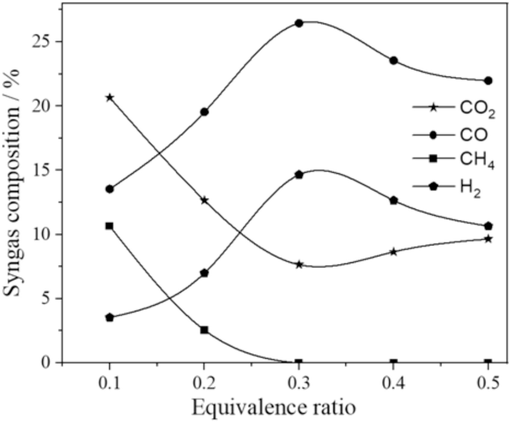
Effect of equivalence ratio on syngas fraction.
3.3 Techno-economic analysis
It is vital to conclude the lucrative economic sustainability for every single yield. The results of the techno-economic analysis of wheat straw gasification are presented in Table 5. Table 5 illustrates the total processing cost for wheat straw gasification. The total utility, operating, capital, purchased equipment, and installation costs are explained. The price of $2.8 M without raw material is a prerequisite for gasifying the wheat straw. However, $4.8 M is the total cost for wheat straw gasification comprising raw material costs. The net CO2 emanation is 5745.25 kg CO2-e/h. Furthermore, regarding rudimentary production, the revenue from wheat straw gasification is accessible in Table 6.
Items
Cost
Total capital cost (USD)
5,340,347
Total operating cost (USD/Year)
1,572,247
Total utility cost (USD/Year)
56,756
Purchased equipment cost (USD)
195,365
Total installation cost (USD)
1,066,576
Raw material cost (USD/Year)
1,204,145
Total cost (USD/Year)
2,865,354
Total cost (USD/Year) (with raw material cost)
4,779,567
Total Revenue (USD/Year)
2,518,256
Net Carbon dioxide emanations (kg CO2-e/h)
5745.25
Combustion energy (MW)
−1.926
Water removal (MW)
−0.90086
Product
Product flow (kg/h)
Product cost (USD)
Ash + Char
267.32
$5 M
Syngas (dry)
1583.54
$16.5 M
Total
1850.86
$21.7 M
4 Conclusion
This study focuses on the simulation of tar and particle removal during wheat straw gasification within a fluidized bed gasifier using Aspen Plus software. The simulation employs Gibbs free energy minimization to determine the equilibrium composition of the syngas. Temperature significantly influences syngas composition, with attention to the steam-to-biomass ratio. Unwanted byproducts such as toluene, HCl, H2S, and NH3 are considered and modelled for removal during gas cleaning. The gas cleaning process involves catalytic tar removal using nickel-based catalysts and adsorption-based removal of HCl and H2S using sorbents. Specifically, This paper systematically investigates the catalytic steam reforming of toluene over various Ni-Co/Al2O3 catalysts (5%, 10%, and 15% Ni-Co/Al2O3). The catalytic performance of different Ni compositions is evaluated to understand their effectiveness in promoting toluene steam reforming and subsequent conversion to syngas components. The findings revealed that the 15% Ni-Co/Al2O3 catalyst outperformed in terms of activity and resistance to carbon deposition during toluene catalytic conversion. In steam reforming, the toluene conversion is monitored at each concentration level under various operating conditions, including temperature, catalyst loading, and S/C ratio. The optimum conditions for maximum toluene conversion are determined for each catalyst composition. Higher Ni-Co/Al2O3 catalyst concentrations exhibit superior performance in promoting toluene steam reforming and enhancing syngas production from biomass feedstock. The results demonstrate the efficient conversion of tar into H2, CO2, CH4, and CO at elevated temperatures and S/C ratios. High-temperature operation and optimal S/C ratios show promising yields of H2. The study further explores the influence of gasifier temperature, steam-to-biomass ratio, and equivalence ratio on syngas composition in gasification. The removal of H2S using ZnO sorbent and HCl using Nacholite is also investigated. A techno-economic analysis of wheat straw gasification is also conducted, providing insights into the economic feasibility of the process.
CRediT authorship contribution statement
Muhammad Faheem Ul Hasnain: Conceptualization, Data curation, Writing – original draft. Hassan Osaf Ali: Data curation, Writing – original draft. Shahid Aziz: Formal analysis, Investigation. Patrick Mountapmbeme Kouotou: Software, Validation. Muhammad Waqas: Conceptualization, Supervision, Validation, Writing – review & editing. Syed Murawat Abbas Naqvi: Visualization. Muhammad Hassaan Athar: Software, Validation, Visualization. Muhammad Ammar: Conceptualization, Supervision, Validation, Writing – review & editing. Imran Shah: Validation, Writing – review & editing. Dong-won Jung: Funding acquisition, Writing – review & editing.
Acknowledgment
This project was supported by the 2024 scientific promotion program funded by Jeju National University.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Thermodynamics analysis of refinery sludge gasification in adiabatic updraft gasifier. Scientific World Journal. 2014;2014
- [Google Scholar]
- Modeling and investigation of the channeling phenomenon in downdraft stratified gasifiers. Bioresour. Technol.. 2013;146:704-712.
- [Google Scholar]
- Practical achievements on biomass steam gasification in a rotary tubular coiled-downdraft reactor. Waste Manag. Res.. 2016;34:1268-1274.
- [Google Scholar]
- Modelling of a downdraft gasifier fed by agricultural residues. Waste Manag.. 2012;32:710-718.
- [Google Scholar]
- Biomass gasification and in-bed contaminants removal: Performance of iron enriched Olivine and bauxite in a process of steam/O2 gasification. Bioresour. Technol.. 2012;118:187-194.
- [Google Scholar]
- A novel real-time monitoring and control system for waste-to-energy gasification process employing differential temperature profiling of a downdraft gasifier. J. Environ. Manage.. 2019;234:65-74.
- [Google Scholar]
- Biomass to hydrogen-rich syngas via catalytic steam gasification of bio-oil/biochar slurry. Bioresour. Technol.. 2015;198:108-114.
- [Google Scholar]
- Gasification of woody biomass. Annual Review of Chemical and Biomolecular Engineering. 2015;6:77-99.
- [Google Scholar]
- Pretreated olivine as tar removal catalyst for biomass gasifiers: investigation using naphthalene as model biomass tar. Fuel Process. Technol.. 2005;86:707-730.
- [Google Scholar]
- Characterization of Scots pine stump–root biomass as feed-stock for gasification. Bioresour. Technol.. 2012;104:729-736.
- [Google Scholar]
- Towards efficient hydrogen production from glycerol by sorption enhanced steam reforming. Energ. Environ. Sci.. 2010;3:1046-1056.
- [Google Scholar]
- Solid–gaseous phase transformation of elemental contaminants during the gasification of biomass. Sci. Total Environ.. 2016;563:724-730.
- [Google Scholar]
- Advanced simulation of biomass gasification in a fluidized bed reactor using ASPEN PLUS. Renew. Energy. 2017;101:629-636.
- [Google Scholar]
- New candidate for biofuel feedstock beyond terrestrial biomass for thermo-chemical process pyrolysis/gasification) enhanced by carbon dioxide (CO2) Bioresour. Technol.. 2012;123:673-677.
- [Google Scholar]
- Ash of palm empty fruit bunch as a natural catalyst for promoting the CO2 gasification reactivity of biomass char. Bioresour. Technol.. 2013;132:351-355.
- [Google Scholar]
- Selection, breeding and engineering of microalgae for bioenergy and biofuel production. Trends Biotechnol.. 2012;30:198-205.
- [Google Scholar]
- Strategies for improving the performance and stability of Ni-based catalysts for reforming reactions. Chem. Soc. Rev.. 2014;43:7245-7256.
- [Google Scholar]
- Integrated drying and incineration of wet sewage sludge in combined bubbling and circulating fluidized bed units. Waste Manag.. 2014;34:2561-2566.
- [Google Scholar]
- A global conversation about energy from biomass: the continental conventions of the global sustainable bioenergy project, Interface. Focus. 2011;1:271-279.
- [Google Scholar]
- Biomass gasification technology: The state of the art overview, Journal of Energy. Chemistry. 2016;25:10-25.
- [Google Scholar]
- Preliminary studies on the treatment of wastewater from biomass gasification. Waste Manag.. 2015;44:135-146.
- [Google Scholar]
- Towards optimizing wood development in bioenergy trees. New Phytol.. 2012;194:46-53.
- [Google Scholar]
- Steam reforming of biomass gasification tar using benzene as a model compound over various Ni supported metal oxide catalysts. Bioresour. Technol.. 2010;101:S101-S103.
- [Google Scholar]
- Biomass downdraft gasifier with internal cyclonic combustion chamber: Design, construction, and experimental results. Bioresour. Technol.. 2011;102:6286-6290.
- [Google Scholar]
- An insight into the anaerobic co-digestion of municipal solid waste and food waste: influence of co-substrate mixture ratio and substrate to inoculum ratio on biogas production. Appl. Biochem. Biotechnol.. 2019;187:1356-1370.
- [Google Scholar]
- Impact of C/N ratios and organic loading rates of paper, cardboard and tissue wastes in batch and CSTR anaerobic digestion with food waste on their biogas production and digester stability. SN Applied Sciences. 2020;2:1-13.
- [Google Scholar]
- Mesophilic batch co-digestion of municipal solid waste and food waste: improved digester performance and stability at optimum organic loading rates. J. Chem. Technol. Biotechnol.. 2022;97:633-642.
- [Google Scholar]
- Modeling and comparative assessment of bubbling fluidized bed gasification system for syngas production–a gateway for a cleaner future in Pakistan. Environ. Technol.. 2018;39:1841-1850.
- [Google Scholar]
- Biomass waste gasification–Can be the two stage process suitable for tar reduction and power generation? Waste Manag.. 2012;32:692-700.
- [Google Scholar]
- Updraft gasification of poultry litter at farm-scale–A case study. Waste Manag.. 2016;50:324-333.
- [Google Scholar]
- The catalytic synthesis of hydrocarbons from H2CO mixtures over the group VIII metals: III. Metal-Support Effects with Pt and Pd Catalysts, Journal of Catalysis. 1975;40:129-134.
- [Google Scholar]
- Effect of sulfide concentration on the location of the metal precipitates in inversed fluidized bed reactors. J. Hazard. Mater.. 2011;192:200-207.
- [Google Scholar]
- Syngas production by two-stage method of biomass catalytic pyrolysis and gasification. Bioresour. Technol.. 2012;110:603-609.
- [Google Scholar]
- Simulation of biomass-steam gasification in fluidized bed reactors: model setup, comparisons and preliminary predictions. Bioresour. Technol.. 2016;221:625-635.
- [Google Scholar]
- Co-gasification of biosolids with biomass: Thermogravimetric analysis and pilot scale study in a bubbling fluidized bed reactor. Bioresour. Technol.. 2015;175:51-58.
- [Google Scholar]







