Translate this page into:
Remediation of heavy metals contaminated soil by enhanced electrokinetic technology: A review
⁎Corresponding author. jxlgcm@163.com (Ming Chen)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Electrokinetic (EK) remediation is based on EK concepts that can successfully remove heavy metals (HMs) from soil. Traditional EK remediation is limited by focusing effect, electrode polarization, soil acidification/alkalization, and other variables. Thus, many enhanced technologies have been studied to improve EK remediation. This review systematically summarizes the progress of research on enhanced technologies for EK remediation by optimizing EK system key components (ion exchange membranes, electrodes, electrolytes, and power supply), and the advantages and disadvantages of each technology were also compared. Specifically, appropriate electrode materials with high conductivity and EK activity positively influence HMs removal during the EK process. The arrangement of electrode matrix, electrode exchange, and electrode approaching significantly impact HMs removal efficiency and energy consumption cost. Additionally, selecting suitable electrolytes can enhance the dissolution of soil HMs to improve the remediation rate. AC is not commonly used for HMs removal but can effectively combine with phytoremediation techniques. Furthermore, we summarize the application of EK remediation on a field scale and the effects of EK remediation on soil resilience. Finally, we propose the following research prospects: (1) the development of enhanced technology should be based on practical considerations for application on a field scale, (2) soil resilience should be an important indicator for the evaluation of EK remediation, and (3) variation of HMs fraction before and after EK remediation need to be concerned.
Keywords
Electrokinetic remediation
Heavy metals
Soil
Enhanced technology
1 Introduction
Approximately 20 % of China's arable land is polluted by HMs, which leads to the annual production of 12 million tons of contaminated crops (Zheng et al., 2020). The China Soil Pollution Survey Bulletin indicates an overall excess rate of 16.1 % for total soil HMs, with excess rates of 7.0 %, 1.6 %, 2.7 %, 1.5 %, and 1.1 % for Cd, Hg, As, Pb, and Cr, respectively (Yang et al., 2018). Overall, soil HMs pollution severely threatens the environment and human health, necessitating the urgent implementation of effective remediation measures (Manikanta et al., 2023b, 2023a; Mounesh et al., 2024, 2023). Compared with other technologies (phytoremediation, chemical fixation, etc.), EK remediation exhibits better behavior in HMs removal and low secondary pollution. Much research has elaborated on the principles and key components of EK remediation (Verma et al., 2023).
However, only HMs in dissolved ionic form and adsorbed by colloids can be removed through EK remediation, which limits its remediation efficiency. Electrolytic reactions near the anode and cathode induce soil acidification and alkalization, adversely affecting soil microorganisms (Li et al., 2020). Non-uniform soil pH distribution causes a “focusing effect” that hinders ion migration, severely affecting remediation effectiveness (Zhao and Xiao, 2022). Polarization on the electrode surface and the formation of precipitates from alkali metals reduce the EK performance, thus lowering the remediation efficiency (Hu et al., 2023). Therefore, traditional EK remediation is inadequate for current needs, requiring enhancement through optimizing components within the EK remediation apparatus (Vocciante et al., 2016).
Table 1 summarizes the methods for enhancing EK remediation. Briefly, EK remediation can be enhanced by changing the components such as membranes, electrodes, electrolytes, and power supply in the EK system. We will review these technologies further and summarize the recent research progress in the latter sections. Besides, Zhang et al. (2023) reported an interesting method to enhanced EK remediation. Briefly, biochar is mixed into Cr-contaminated soil for EK remediation. And the biochar-based bipolar electrochemistry construction promotes the transformation between Cr (III) and Cr (VI), it means a way to reduce the toxicity of variable valence HMs (such as Cr and As) (Sangrulkar et al., 2023). While this method does not remove HMs directly, it is not within our scope of review.
Methods
Advantage
Disadvantage
Components
Specifically
Membranes
Cation exchange membrane
Block OH– in cathode entering soil slurry and inhibit focusing effect.
The use of membranes will increase cost expenditures.
Electrolytes
Facilitating HMs dissolution
Accelerate EK to reduce energy consumption and promote HMs dissolution to improve removal efficiency.
Electrolytes with good solubilization effects, such as strong acids, are harmful to soil.
pH adjustment
Eliminate soil acidification/alkalization and inhibit the focusing effect.
—
Electrodes
Approaching electrodes
Compress focusing effect area into a smaller range and reduce energy consumption.
Electrode movement is inconvenient, especially on a pilot scale.
Polarity exchange
Eliminate soil acidification/alkalization,
It may reduce the removal rate of HMs because HMs are constantly changing the moving direction.
Electrode dimensional matrices
Improve HMs removal rate.
The mechanism of
electrode matrices effect on the remediation rate is unclear, and the effect on energy consumption is uncertain.
Power supply
AC
No soil acidification/alkalization.
It cannot directly remove HMs and is often combined with phytoremediation.
Asymmetric AC
Less nutrient loss.
—
This study comprehensively reviews the EK remediation for HMs removal from soil, and systematically summarizes the recent explorations of the primary components (membranes, electrolytes, electrodes, power supply) of the EK remediation system. Furthermore, the review focuses more on the actual usability of technology, including (1) the field application of EK remediation and (2) the impact of EK remediation on soil resilience.
2 EK remediation conception
The EK remediation process involves preparing a soil suspension by mixing soil with an electrolyte solution and applying direct current (DC) to the suspension. Under the influence of the electric field, HMs ions in the soil move toward the cathode/anode. This process significantly reduces the concentration of HMs in the soil within the sample chamber. EK remediation experimental setups typically consist of four main components: the soil sample chamber, electrolyte chamber (anode and cathode chambers), electrodes, and power source. The electrolyte chamber and the sample chamber are separated by a filtration membrane (Fig. 1).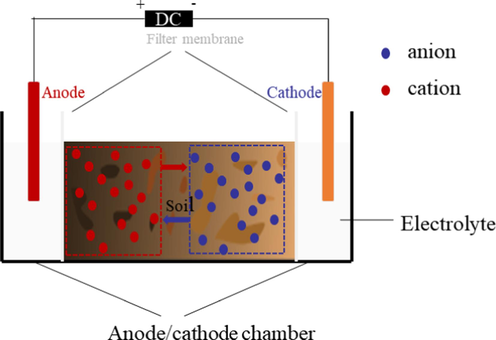
Schematic of EK remediation equipment.
During the EK remediation process, water electrolysis reactions occur in the vicinity of the electrodes, as represented by eqs. (1) and (2): Anode: 2H2O − 4e- → 4H+ + O2(1) Cathode: 2H2O + 2e- → H2 + 2OH–(2)
In the EK remediation, the anode undergoes oxidation, producing H+ ions and causing soil acidification near the electrode. On the other hand, the cathode undergoes reduction, generating OH– ions and causing soil alkalization in the vicinity. Under the influence of the electric field, HMs ions exhibit directional movement through three main mechanisms: electromigration, electrofiltration, and electrophoresis (Kong et al., 2023). Due to EK effects, cationic HMs migrate toward the cathode, while anionic HMs move toward the anode.
In most cases, only HMs in the free ion state and those bound to colloids can migrate under the influence of the electric field during EK remediation. Research indicates that EK remediation primarily targets exchangeable, carbonate, and reducible forms of HMs in the soil rather than organic-bound and residual forms (Zhou et al., 2020). According to the above EK remediation reaction equations, the anode and cathode undergo acidification and alkalization, respectively, influenced by the H+ and OH– produced. H+ and OH– generated at both ends of the electrode also move towards the opposite electrode under the electric field. When they meet and neutralize each other, the pH value at the meeting point changes significantly, leading to the precipitation of migrated HMs ions. This phenomenon has a significant inhibitory effect on HMs removal, known as the “focusing effect” (Ng et al., 2016).
3 Enhanced technologies for EK remediation
Traditional electrical remediation commonly employs DC, utilizing remediation devices and mechanisms as described above. In electrical remediation, enhancing the migration of HMs and overcoming the “focusing effect” are crucial aspects for improving the efficiency of EK remediation, which can be achieved by optimizing the key components of the electrical remediation apparatus, such as electrolytes, ion-exchange membranes, approaching electrodes, electrode matrices, and new electrode materials, among others.
3.1 Application of ion exchange membranes
Ion exchange membranes (EM) can eliminate the “focusing effect” and enhance the efficiency of EK remediation. EMs are polymer membranes containing ion groups that exhibit selective permeability to ions. Based on their selectivity to ions, they can be categorized into anion exchange membranes (AEM) and cation exchange membranes (CEM). Placing CEM between the soil and the cathode chamber allows only HMs cations to enter the cathode chamber while preventing OH– from entering the soil, thus avoiding forming focused areas. The movement of OH-generated at the cathode towards the anode is a major hindrance to removing HMs (cations) from the soil. Therefore, the application of CEM can increase the removal efficiency of HMs.Similarly, using AEM between the soil and the anode chamber can prevent H+ ions from entering the soil, preventing soil acidification. The use of EM effectively controls the pH of the soil, thereby improving the efficiency of EK remediation. At the same time, the application of EM on the EK process is also called Electrodialytic remediation (ED), a membrane-based technique Liu et al., 2020.
In the case of CEM application, metal ions (Cu2+, Cd2+, etc.) will form precipitates with OH– at the cathode after passing through CEM, which may reduce membrane flux or lead to membrane fouling and increase costs (Song et al., 2019). Kim et al. (2005) proposed a solution to membrane fouling by adding an auxiliary reservoir system between CEM and soil samples. This method employs a solution circulation system to transfer OH– into the auxiliary reservoir, where HMs precipitation occurs, instead of allowing HMs to pass through CEM and form sediments on the membrane surface. However, strict control of the rate of OH– transfer in the circulation system is required to apply this method. Song et al. (2020) studied the EK remediation of HMs-contaminated soil from a discarded electroplating factory using a double-layer CEM. The results showed that under the conditions of EDTA as the electrolyte, the EK remediation efficiency of the double-layer CEM for Cu and Ni was 3 to 10 times higher than that of traditional EK remediation. However, the removal efficiency of Cr did not significantly increase, possibly due to the different forms of existence compared to Cu and Ni. In soil environments, Cr exists as oxygen-containing anions, and CEM prevents its entry into the anode chamber. Therefore, the selection and arrangement of EM should be based on the types and forms of existence of the HMs present.
Traditional ion EM is made from ion exchange resin, and to pursue better membrane performance, some researchers have reported using ion exchange fibers to prepare membranes(EFM) (Swanckaert et al., 2022). Ion exchange fibers are a class of fiber materials with diameters ranging from 50 to 500 nm and a length of several km with excellent chemical and mechanical properties, showing potential as alternatives to EM (Roddecha et al., 2012). EFM, prepared by ion exchange fibers, has distinct properties such as high molecular orientation, high surface to volume ratio, high porosity and interconnected three-dimensional structure, which are beneficial for ion exchange performance (Swanckaert et al., 2022). Attribute to the large pore hydrophilic structure of EFM, ions can freely migrate within them like in an aqueous solution (Basta et al., 1998). Researchers have confirmed that EFM application has a higher removal rate of HMs in soil than EM (Souilah et al., 2012).
3.2 Electrolytes
The regulation of electrolytes involves two aspects: (1) selecting suitable electrolytes to increase the solubility of HMs and (2) adjusting the pH of the electrolyte solution to suppress the “focusing effect,” especially neutralizing OH– in the anode chamber. Soil solution is a crucial medium for the migration of soluble species, and EK remediation can be viewed as the transfer of pollutants through electrical migration and electroosmosis in liquids. Therefore, electrolytes play a significant role in EK remediation (Li et al., 2019). Table 2 summarizes the progress of the research in enhanced EK remediation through electrolyte adjustment.
Electrolyte
HMs/content (mg/kg)
Remediation effects
literature
Citric acid
Cr /2097.4)
Citric acid removed 23.6 % of total Cr.
Wu et al., 2016
Citric acid, aspartic acid
Cr(VI) /8.25
Cr /11.2Citric acid can promote the removal of Cr and Cr (VI), but aspartic acid can only promote the removal of Cr (VI)
Fu et al., 2017
Citric acid
Pb/5491.9
When citric acid is used as the cathode electrolyte, the maximum removal rate of Pb reaches 87.1 %, and it can slowly and effectively control the pH rise of the cathode.
Zhang et al., 2017
EDTA
Pb/3529
The removal rate of Pb is 92.7 %, which is significantly higher than using nitric acid as the electrolyte.
Lee and Kim, 2010
Acetic acid
Cu/300 ∼ 1200
Zn/250 ∼ 1000Compared with the control group, the removal rates of Cu and Zn increased by more than 15 %, and the current intensity increased from 33 to 70 mA to 71–123 mA.
An et al., 2017
Anaerobic microbial agents
As/4023
The species of As changed, and it promotes mobility but has no significant impact on the final removal rate of As. Energy consumption can be saved by shortening the EK remediation time.
Lee et al., 2009
CaCl2- humic acid
Cd/1.72
The total amount of Cd decreased from 1.72 mg/kg to 1.20 mg/kg, and the effective state content of Cd decreased from 0.44 to 0.27 mg/kg.
Hu et al., 2021
GLDA, rhamnolipids
Cu/1352.1
Zn/731.6
Cr/202.5
Pb/52.5
Ni/175.1
Mn/1033.3The combination of GLDA and rhamnolipids leads to higher HMs removal rates. The removal rates of Cu, Zn, Cr, Pb, Ni, and Mn by mixed electrolytes are 70.6 %, 82.2 %, 89.%, 60.0 %, 88.4 %, and 70.0 %, respectively.
Tang et al., 2017
Citric acid, polyaspartic acid
Pb/1000
Both citric acid and polyaspartic acid can enhance the removal of Pb, but the enhancement effect of polyaspartic acid is not significant.
Wen et al., 2017
Tween 80、Brij 35、EDTA
Pb/17 ∼ 23
Zn/11 ∼ 18
Ni/10 ∼ 12All electrolytes improved the repair efficiency due to their solubilizing effect on HMs. The order of HMs' desorption/removal ability is Zn > Ni > Pb.
Saberi et al., 2018
Ascorbic acid, oxalic acid (adjust anode pH)
As193.5
The removal rate of As increased from 18.7 % to 25.3 %.
Ryu et al., 2017
Citric acid (control cathode pH)
Cu/672
Ni/94.4
Cr/188Cu, Ni, and Cr removal rates were 68.8 %, 53.3 %, and 52.9 %, respectively. After controlling the cathode pH, the removal rate of Cr increased to 93.3 %.
Fan et al., 2015
Sodium sulfate (control for anion/anode)
As/145.7
Cu /278.1
Zn/246.0
Pb/516.8Cu, Zn, and Pb removal rates are 54.7 %, 44.4 %, 3.9 %, and 68.9 %, respectively.
Yang et al., 2014
Control cathode pH
Cd/250
The highest removal rate of Cd reaches 93 %.
Almeira et al., 2012
Control cathode pH
Cd/1.19
Compared with polarity exchange (65.3 %), using acid-base neutralization to regulate electrolyte pH results in a lower Cd removal rate (58.3 %).
Hu et al., 2017
3.2.1 Hms solubilization by electrolyte
The physicochemical properties/parameters of soil suspensions (pH, Zeta potential, conductivity, HMs solubility, etc.) change with the addition of electrolytes, and these factors significantly impact the efficiency of EK remediation (Wang et al., 2021). Therefore, selecting appropriate electrolytes to enhance the migration of HMs can strengthen EK remediation. According to the theory of soil washing technology, some washing agents can enhance the solubility of HMs, including inorganic washing agents (acid/base/salt solutions), surfactants, and chelating agents (Zheng et al., 2024). In summary, the study of soil washing agents provides insights into the selection of electrolytes, aiming to improve the solubility of HMs and enhance the efficiency of EK remediation. More details about washing agents can be found in previous review (Zheng et al., 2022). pH is a crucial factor influencing the leaching ability of HMs in solid waste. Generally, low pH favors the dissolution of cationic HMs, while high pH favors the dissolution of anionic HMs (Król et al., 2020). The precipitation process between metals and electrolytes should be considered for electrolyte selection. For example, and can form precipitates with cationic HMs, reducing the migration of HMs. Additionally, using HCl as an electrolyte can lead to the generation of chlorine gas if inappropriate voltage is applied (Wang et al., 2023). Therefore, is commonly used as an electrolyte because it has minimal side reactions (such as gas or precipitate formation) during the EK process. Compared to inorganic strong acids, some weak organic acids as electrolytes have better prospects. Pre-adding small organic molecules (acetic acid, lactic acid, humic acid, citric acid, ascorbic acid, polyaspartic acid, etc.) to the soil has a solubilizing effect on HMs, leading to an increase in the removal rate of HMs by 15 %-59 % (Ryu et al., 2017; Wu et al., 2016). Moreover, these weak acids are environmentally friendly and biodegradable, causing less harm to the soil than inorganic strong acids. Specific microorganisms, especially Cr and As, can also alter the mobility of HMs in soil due to microbial physiological processes (Verma et al., 2023).
EDTA can form stable soluble complexes with HMs, promoting the desorption of HMs from the soil surface and enhancing their mobility. Cameselle et al. (2021) used EDTA and citric acid as electrolytes and compared them with deionized water. The results showed that 0.5 M citric acid as an electrolyte could effectively avoid the precipitation of HMs as hydroxides in the cathode region. Citrate ions formed positively charged complexes with HMs, allowing smooth migration to the cathode region and removal. Using EDTA/NaOH as anode/cathode also prevents soil acidification, and the negatively charged complexes formed between EDTA and metal ions promote their movement toward the electrode in the electric field, effectively increasing the removal efficiency of HMs. However, EDTA is poorly biodegradable and can persist in the soil, leading to soil degradation (Jelusic et al., 2014; Jez and Lestan, 2016). Some readily biodegradable electrolytes, such as ethylenediaminetetraacetic acid (EDDS) and ethylene glycol tetraacetic acid (EGTA), have gradually been studied (Beiyuan et al., 2018). When selecting electrolytes, consideration should be given to both removal efficiency and the long-term impact on the soil environment. Furthermore, electrode corrosion is a common issue during the EK remediation process. Therefore, adding electrolyte solutions with anti-corrosion functions is beneficial for EK remediation. Wen et al. (2017) reported that polyaspartic acid, used as an electrolyte, could effectively alleviate the corrosion of stainless steel electrodes.
3.2.2 pH adjustment of electrolyte
It is important to note that the pH of soil suspension should be adjusted before the start of EK remediation (Torabi et al., 2021). Here, the emphasis is on adjusting the pH of the electrolyte solution in the electrode chamber during EK remediation. Due to the influence of H+ and OH– generated in the anode and cathode chambers, the pH of the electrolyte solution undergoes significant changes. Moreover, excess H+ and OH– ions may cause the reversal of the electroosmotic flow direction during the migration process, which can hinder the enrichment of HMs cations to the cathode and reduce the remediation efficiency. Therefore, controlling the pH of the electrolyte solution in the electrode chamber is crucial during EK remediation. Adding alkaline and acidic solutions to the anode and cathode chambers to neutralize the generated H+ and OH– ions achieve electrode depolarization. The electrolyte was acidified in the cathode chamber with nitric acid to remove 98 % of Cd from kaolin (Almeira et al., 2012). Some studies continuously renewed the electrolyte in the cathode chamber with deionized water to remove the produced OH– ions (Hicks and Tondorf, 1994). However, continuously introducing an external solution into the EK device increases the electrolyte volume, leading to increased subsequent processing costs, contradicting the principles of clean production and sustainable development. Lee developed an electrolyte circulation system based on the principle of acid-base neutralization, regulating the pH of the electrode by pumping the electrolyte from the anode chamber to the cathode chamber (Lee and Yang, 2000). This method pumps the electrolyte from the anode chamber to the cathode chamber, reducing the pH of the cathode electrolyte by 4 units (from 12 to 8).
Conversely, pumping the electrolyte from the cathode chamber to the anode chamber to neutralize H+ ions is theoretically feasible (Zhou et al., 2005). This method was improved by controlling the volume of electrolyte solution pumped into the anode by real-time monitoring of the pH in the cathode chamber, thereby regulating the pH in the cathode chamber. Adjusting the pH of the electrode can promote electrode depolarization, ensuring a higher level of EK remediation efficiency and making it an indispensable enhanced EK remediation technique (Mao et al., 2019). Additionally, some weak acids have a certain buffering capacity. Therefore, in addition to using acid-base legal control of electrode pH, specific electrolytes with pH control functions have prompted the combined use of electrolyte enhancement and pH control in some studies (Table 1).
3.3 Electrodes
3.3.1 Electrode materials
The foundation of EK remediation lies in promoting the migration of HMs through the soil solution by electric current. Therefore, suitable electrode materials ensure adequate current intensity, improving remediation efficiency. Appropriate materials can be used as standalone electrodes or coatings on electrode surfaces. Electrode materials for EK remediation are typically chosen to be chemically inert but possess high activity at the interface (Telepanich et al., 2021). Common electrode materials for EK remediation include titanium, stainless steel, and graphite (Mu’azu and Essa, 2020).
The oxide films, a passive layer, will be generated on the surface of electrodes such as Ti, Pt, Ag, Au, during the EK remediation. The above passive layer will reduce electrode reaction activity and affect EK remediation's efficiency. Roughening and coating the electrode surface with specific metal oxide coatings can enhance electrode reaction activity and improve remediation efficiency. Metal oxides such as SnO2, Sb2O5, IrO2, Ta2O5, IrO2, and RuO2 are commonly used on electrode surfaces, and elemental metals like Pt and Sn are also used as electrode coatings. According to Muazu et al., a Ti electrode coated with iridium showed a high removal efficiency for Pb (Mu’azu and Essa, 2020b). A Ti electrode coated with ruthenium-iridium achieved high removal efficiency for Pb while reducing energy consumption (Li et al., 2021). To enhance electrode performance, many synthesized polymer materials, such as polypyrrole, polyaniline, etc., are used as electrode materials for EK remediation. These materials, known as conductive polymers, are applied as electrode coatings on flexible electrodes, providing a larger contact area and more contact points for reactions with HMs. Wang et al., 2019 reported using a polyaniline electrode for the EK remediation of Cr-contaminated soil, demonstrating that the polyaniline electrode promoted the removal of Cr through adsorption. Additionally, electrode materials with good conductivity and high EK activity are preferred (Gidudu and Chirwa, 2022; Kim et al., 2021; Qu et al., 2023; Wang et al., 2019). The generation of H+ and OH– during the electrolysis process can inhibit the migration of HMs; therefore, the higher overpotential caused by the evolution of oxygen and hydrogen during the EK process should be considered. Table 3 summarizes studies comparing the EK remediation efficiency of different electrode materials. Overall, synthetic conductive polymers as electrode materials show higher EK remediation efficiency, followed by graphite electrodes and some metal electrodes such as stainless steel and titanium. In general, electrode materials should exhibit good EK performance, a large specific surface area, and chemical stability to ensure improved energy utilization and remediation efficiency. Hamdi et al. (2025) summarized the advantages and disadvantages of several electrode materials (Pt/Ti, IrO2/Ti, Graphite, Iron), and more details can be found in the literature.
Electrode materials
HMs/content (mg/kg)
Remediation effect
Literature
Stainless steel, graphite plate, titanium plate, titanium
Cu/—
Cd/—The removal efficiency of Cu and Cd by graphite electrodes is relatively high, with 40.3 % and 95.2 %, respectively, and the current during the repair process of graphite electrodes is significantly higher than that of other electrodes.
Fang et al., 2014
Graphite, stainless steel, titanium
Cd/—
The removal rate of Cd by graphite electrode is 73 %, which is higher than that of stainless steel electrode (44 %) and titanium electrode (40 %).
Zhang et al., 2017
Graphite, stainless steel, titanium
U/—
The removal rate of U is 34.1 % higher than that of stainless steel electrodes (15.88 %) and titanium electrodes (18.8 %).
Li et al., 2021
Graphite, stainless steel, titanium
Pb/462.7
The removal efficiency of graphite, stainless steel, and titanium electrodes for Pb is 77 %, 64 %, and 54 %, respectively.
Cai et al., 2016
Graphite, Platinum Plated Titanium, PET-CNT
Cd/67.32
Cu/347.5
Ni/302.3
Pb /353.2
Zn/353.2PET-CNT as a cathode can significantly increase the current and electroosmotic flow and improve the removal efficiency of HMs. The removal rates of Cd, Cu, Ni, Pb, and Zn by PET-CNT were 89.7 %, 63.6 %, 90.7 %, 19.2 %, and 88.7 %, respectively. Compared with graphite and platinum-plated titanium electrodes, the removal rates of Cd, Ni, and Zn have been improved by at least 30 %.
Yuan et al., 2016
Polypyrrole, graphite
Cr/500
The removal rates of Cr by polypyrrole and graphite electrodes are 92 % and 81 %, respectively.
Wang et al., 2019a
Polyaniline, graphite
Cr/500
The removal rates of Cr by polyaniline and graphite electrodes are 60 % and 41 %, respectively.
Wang et al., 2019b
3.3.2 Optimization by approaching electrodes
Soil near the anode typically has a lower pH, resulting in higher mobility and remediation efficiency of HMs. To alleviate the “focusing effect” that restricts HMs migration, the electrode approaching is employed to enhance EK remediation. Specific electrode approaches involve fixing one end of the electrode and continuously moving the other toward the fixed end. As the distance between the electrodes decreases, the area affected by the focusing effect diminishes. Essentially, the electrode approaching includes both anode and cathode approaching, depending on the type and nature of the target HMs (Zhang et al., 2014).
Research on anode approaching is more extensive than cathode approaching, and its schematic is illustrated in Fig. 2. The anode moves toward the fixed cathode, and the H+ generated during the movement is maintained in the appropriate position, causing the influence area of the anode to expand during the electrode movement. Therefore, soils treated with the electrode approaching method usually undergo more pronounced soil acidification (Han et al., 2021). The advantages of approaching the anode as an enhanced EK remediation method include 1) reducing the metal precipitation area to improve remediation efficiency and 2) decreasing the ion migration distance to save energy. Cai et al. achieved a copper removal efficiency of 62.0 % using the electrode approaching technique, significantly higher than a fixed anode (38.9 %) Cai et al., 2016. Furthermore, anode approaching significantly reduces energy consumption during the EK remediation by shortening the distance between electrodes (Gidudu and Chirwa, 2020; Zan et al., 2020). Some studies have reported the application of cathode approaching techniques. For example, Yao et al. (2020)investigated the impact of cathode approaching on As removal, indicating that compared to traditional EK remediation, cathode approaching increased As removal rate from 4.2 % to 20.3 %, albeit with higher energy consumption. Ng et al. (2016) reported the EK remediation of soil contaminated with Pb-Cr compounds, alkalizing the soil during cathode approaching. Although energy consumption decreased by 22.5 %, no significant enhancement was observed in Pb or Cr ions migrating. The reasons for the varying impacts of the cathode approach on energy consumption in these two studies need further investigation. Li et al. (2012) pointed out that current density initially increases in the early stages of cathode approaching but gradually decreases afterward. It is due to the weakening of ion intensity in the pore fluid and the electrode's polarization effect during the cathode approach (Oumellal et al., 2011; Wang et al., 2020). Thus, using appropriate electrode materials for depolarization can alleviate the high energy consumption caused by cathode approaching techniques. Additionally, Zeng et al. (2023) studied the effects of anode approaching, cathode approaching, and bipolar approaching on the EK remediation of soil As. The results showed that all three methods could enhance the migration of As, with a migration rate of 31.5 % achieved with bipolar approaching. This study provides a new perspective for electrode approaching methods. Table 4 summarizes research on enhancing EK remediation using electrode approaching. In general, anode approaching is more suitable for removing cationic HMs. Moreover, energy consumption during the EK process in electrode approaching studies is also a research focus.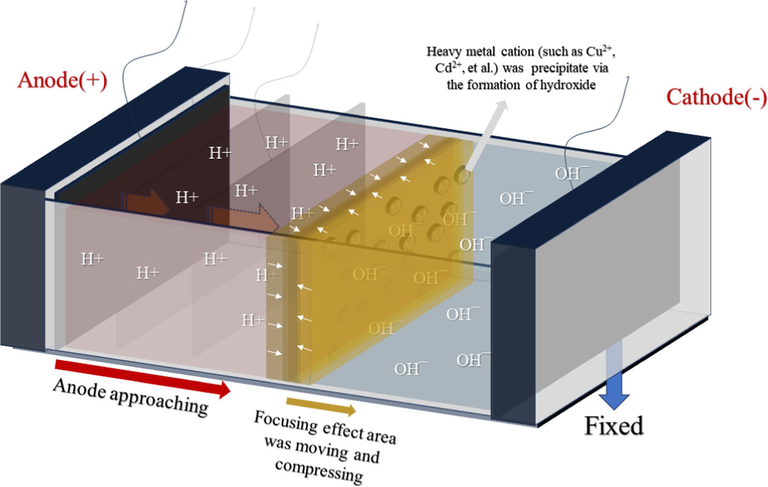
Schematic of approaching anode for EK remediation.
Methods
HMs/content (mg/kg)
Remediation effect
Literature
Cathode approaching
Cr/797.9
Pb/402.2There was no significant increase in Pb and Cr removal rates, but the energy consumption decreased by 22.5 %.
Ng et al., 2016
Cathode approaching
As/185
The removal rate of As has increased from 4.2 % to 20.3 %, increasing energy consumption.
Yao et al., 2020
Anode approaching
Cd/—
The removal rate of Cd by the fixed anode is 68, and that of Cd by the anode approaching method is 95 %. The energy consumption of the anode approaching is reduced by 65 %.
Wan et al., 2019
Anode approaching
Cu/584
Zn/1267
Cr/136
Pb/81
Ni/130
Mn/654The removal rates of Cu, Zn, Cr, Pb, Ni, and Mn reached 52 %, 57 %, 60 %, 47 %, 53 %, and 54 %, respectively.
Tang et al., 2021
Anode approaching
Pb/1000
Anode approaching increases the removal rate of Pb from 72 % to 84 %, and reduces repair time and energy consumption.
Zhang et al., 2014
Anode approaching
Pb/1685
Zn/6342
Cu/668
Cd/121The HMs removal rate was increased, and energy consumption was reduced 40 %.
Li et al., 2021
3.3.3 Optimization by polarity exchange
Polarity exchange refers to the intermittent change in electrode polarity during the EK remediation process. This alternation causes one side of the electrode to periodically generate H+ and OH– ions, maintaining the soil pH in the neutral range (Zhao et al., 2022). This prevents the excessive accumulation of OH– ions at the cathode, which could lead to HMs precipitation and impact remediation efficiency. Polarity exchange can alleviate the negative effects caused by the focusing effect and enhance treatment efficiency (Sun et al., 2021a).
Zhou et al. (2018) reported that exchange electrodes have a higher efficiency in removing HMs than fixed electrodes. The total removal rates for total Cr, Cr(VI), and Cr(III) were 43.7 %, 91.9 %, and 19.3 %, respectively. Hu et al. (2017) compared the effects of two methods, controlling electrode pH and polarity exchange, on the removal efficiency of Cd in farmland soil, and the study indicated that polarity exchange could increase the remediation efficiency from 40 % to 65 %, surpassing the efficiency of controlling electrode pH (58 %). Additionally, some studies suggest that when the frequency of polarity exchange is set at every 96 h/cycle, the removal rates for Cr and Cd are 70 % and 82 %, respectively, exceeding the 57 % and 49 % of traditional EK remediation. With an increase in polarity exchange frequency every 48 h/cycle, the removal rates for Cr and Cd increased to 88 % and 94 % (Lu et al., 2012). The studies mentioned above found that increasing the frequency of polarity exchange promotes the removal of HMs. During the polarity exchange process, the direction of HMs migration continuously changes. In practical field applications, excessive polarity exchange due to large soil pollution areas may hinder HMs from reaching the electrodes, reducing remediation efficiency. Some studies have also observed decreased HM removal rates with increased polarity exchange. Sun et al., 2021b investigated the impact of polarity exchange frequency on the EK remediation of Cr-contaminated soil. The results showed that as the polarity exchange frequency increased from 84 h/cycle to 24 h/cycle, the Cr removal rate decreased from 96 % to 82 %. However, due to the alternating production of H+ and OH– ions and acid-base neutralization during the polarity exchange process, the method has minimal impact on soil pH, making it an environmentally friendly approach.
3.3.4 Electrode dimensional matrices
In the EK process, the strength and effective area of the electric field are controlled by the number and spatial arrangement of electrodes (Cuevas et al., 2016). Therefore, the efficiency of EK remediation is influenced by the arrangement of electrodes. Horizontal electric fields are widely used in traditional EK remediation, where electrodes are generally vertically arranged column electrodes. Electrode matrices are divided into one-dimensional and two-dimensional matrices based on the ratio of anodes to cathodes (Krcmar et al., 2018). The former represents traditional matrix arrangements (anode: cathode ratio of 1:1), but this does not mean that the number of anodes and cathodes is both 1; it could also be a parallel column of anodes and cathodes. Fig. 3a shows a common one-dimensional electrode matrix. In recent years, the application of two-dimensional electrode matrices, where electrodes of opposite polarity are placed within a geometric area surrounded by multiple electrodes, has become more widespread. Two-dimensional electrode matrices include multiple-anode and multiple-cathode matrix forms (Fig. 3b, c). Two-dimensional electrode matrices with a single cathode and multiple anodes have configurations that include triangles, squares, hexagons, and octagons.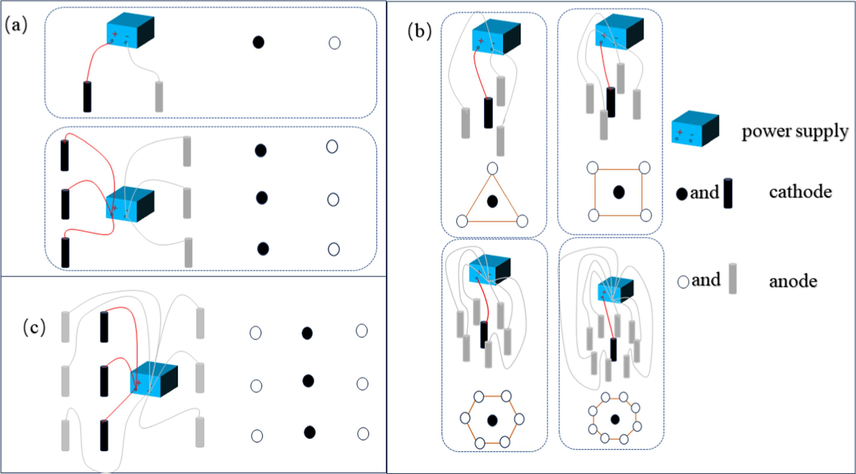
The schematic of one-dimensional and two-dimensional electrode matrices.
Kim et al. (2021) reported using one-dimensional and two-dimensional electrode matrices for the EK remediation of multi-metal-contaminated soil. The removal rates for Cd, Cu, Ni, Pb, and Zn using the one-dimensional electrode matrix method (4 anodes/4 cathodes) were 69.1 %, 69.2 %, 74.7 %, 28.3 %, and 71.3 %, respectively. The removal rates for the two-dimensional electrode matrix method (6 anodes/1 cathode) were 79.8 %, 82.6 %, 83.7 %, 34.3 %, and 81.1 %, respectively. The two-dimensional electrode matrix achieved higher remediation efficiency. Still, energy consumption increased by 41.0 %, attributed to the dense electrode matrix generating high-density current and causing soil heating, leading to energy wastage. Genc's study showed that the remediation efficiency of the two-dimensional electrode matrix (8 anodes/1 cathode) was lower than the traditional electrode arrangement (1 anode/1 cathode) (Turer and Genc, 2005). In conclusion, the mechanism by which electrode matrices affect the EK remediation rate is unclear. However, using the appropriate electrode matrix can improve remediation efficiency or save energy consumption in specific application scenarios.
4 AC EK remediation and enhanced technology
4.1 Application of conventional AC
In some studies, polarity exchange can alleviate soil pH changes near the electrodes. To some extent, AC can be considered as DC with high-frequency polarity exchange. Therefore, AC is also applied in the EK remediation of HMs in soil. Luo et al. (2018) conducted field-scale experiments comparing the EK remediation efficiency of DC and AC. The results indicated that DC was more effective than AC in promoting the accumulation of HMs on the soil surface and reducing the leaching risk of HMs in the soil. Unlike DC, the spatial distribution of HMs in the soil does not change with AC; instead, AC only alters the form distribution of HMs in the soil. Studies have shown that AC can enhance the migration force of HMs ions and strengthen plant uptake by promoting plant growth Liu et al., 2020. Therefore, alternating electric fields are often combined with plant remediation methods (Wei et al., 2019).
Acosta-Santoyo et al., (2017) compared the effects of different electric fields on ryegrass in polluted soil. Compared to the control group, three types of electric fields (DC, AC, and polarity exchange DC) increased ryegrass biomass by 44 %, 125 %, and 45 %, respectively. Greater biomass implies that plants can extract more pollutants from the soil, resulting in higher remediation efficiency. The mechanism by which AC promotes plant growth may be attributed to bidirectional electro-osmosis, which promotes water movement in the soil, potentially carrying soluble nutrient ions to the plant roots. Root membranes may undergo periodic hyperpolarization and depolarization under the influence of the electric field, which can enhance plant absorption of trace elements and promote plant growth. In addition, due to the presence of the alternating electric field, HMs in the soil have a more remarkable migration ability and are more easily absorbed by plants. Because the process of plant absorption of HMs is slow, the enhanced migration ability of HMs by the electric field may pose environmental risks, such as adverse effects on soil fauna and flora, microorganisms, and groundwater, among others. HMs activity and plant growth positively correlate with voltage, but excessively high metal activity can inhibit plant growth. Cang et al. (2011) reported that excessively high voltage can also inhibit plant growth. Suitable voltage intensity should be selected before implementing EK remediation. Activated HMs in the soil may migrate through groundwater, expanding the scope of pollution. In the practical application of this method, the following points should be noted: (1)Sites with continuous and stable waterproof layers below the contaminated soil and deep groundwater burial conditions have better implementation conditions; (2) Sites with obvious groundwater flow directions, pollution control can be combined with groundwater-permeable reaction walls; (3) Plant species should be selected that are fast-growing and have large biomass; (4) Rainy seasons should be avoided appropriately to prevent precipitation from causing the expansion of the contaminated area.
4.2 Asymmetric AC EK remediation
Xu et al. (2019) introduced a novel EK remediation technique based on asymmetric AC electrochemistry. Asymmetric AC EK remediation involves incorporating electrochemistry into a soil washing system to achieve the circulation and regeneration of soil washing solutions. The schematic diagram of the remediation device is shown in Fig. 4, where the electrolyte circulates in the soil through a pump, undergoing multiple washing cycles. Electrodes are placed on one side of the soil body, and the electrolyte flows sequentially through the soil-electrode-pump and circulates again from the other end of the soil. Asymmetric AC is applied to the electrodes, meaning the voltage changes are uneven. The operating voltage alternates between 5 V and −10 V, with durations of 0.5 ms and 2.0 ms, respectively (Fig. 4). Under the influence of the electric field, HMs present in the soil are reduced to metallic elements on the electrode during the EK washing process.Moreover, conventional nutrients such as Ca, Na, K, and Mg do not undergo reduction reactions during the EK process. They remain in a chemical equilibrium state between the circulating electrolyte and the soil sample, avoiding excessive nutrient loss due to repeated washing cycles. Long-term experiments and plant pot experiments conducted by researchers strongly support this conclusion.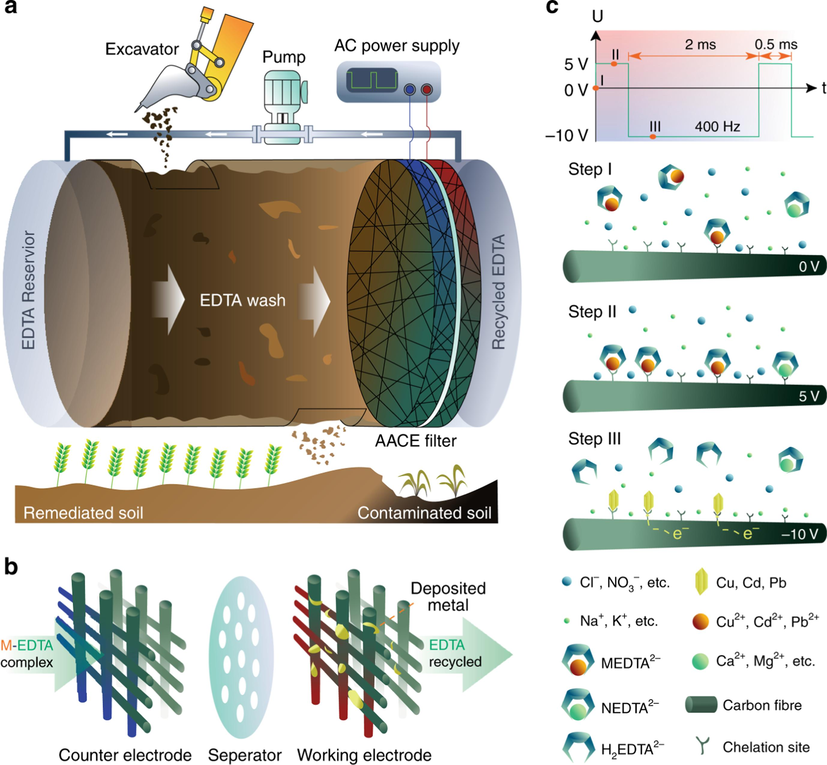
Schematic of asymmetric AC application for EK remediation (Xu et al., 2019).
Han et al. (2023) reported removing 97 % Cd and 93 % Pb from sludge using asymmetric AC EK remediation. Several parameters in the process can be adjusted to optimize the remediation efficiency. For example, increasing the positive bias voltage (negative bias voltage being −10 V) from 2 V to 3 V can improve the removal efficiency of Cr from 87 % to 99 % (Xie et al., 2022). Soil with a Cr concentration of 1200 mg/kg was remediated using asymmetric AC for 24 h, reducing Cr to a safe level of 30 mg/kg. The effectiveness of asymmetric AC EK remediation also depends on the distribution of HMs forms in the soil. Lu et al. reported the removal efficiency of Cd, Pb, Cu, and Zn in soil using asymmetric AC with positive and negative bias voltages of 3 V/-8V (Lu et al., 2023). The results showed a significant removal effect for Cd and Pb but a poorer removal effect for Cu and Zn, attributed to the predominant presence of Cu and Zn in the tested soil in the form of Fe/Mn oxides, which are difficult to dissolve and enter the EK washing system.
4.3 Enhanced technology for AC EK remediation
AC can be considered a form of DC with high-frequency polarity changes, an enhancement technique in traditional EK remediation. Because the impact of AC on contaminated soil significantly differs from DC, enhancement techniques applicable to AC fields are also valuable for research. Most enhancement techniques used in DC EK remediation are equally appropriate to AC. Selecting suitable electrolytes to enhance HMs solubilization positively affects AC EK remediation. Some electrolytes with anti-corrosive properties can effectively protect the electrodes (Wen et al., 2017). Choosing appropriate electrode materials to prevent corrosion and ensure current intensity strengthens AC EK remediation. Compared to a DC field, AC does not lead to focusing effects. Therefore, measures such as ion exchange membranes, controlling electrode pH, and electrode approach, which aim to avoid focusing effects, may not apply to enhancing AC EK remediation.
Recent studies indicate that AC may affect the plant remediation process by stimulating the activity of specific microorganisms. Ding et al. (2022) reported that AC promotes the growth of Acidobacteri and increases the bioavailability of uranium (U) while reducing its stress on plants, resulting in better U enrichment absorption by plants. Therefore, enhancing AC electrokinetic remediation could be achieved by inoculating specific microbial agents (Yin et al., 2020). Additionally, asymmetric AC, as a particular form of AC, can be enhanced by controlling parameters such as AC frequency, duty cycle, electrolyte flow rate, and electrode distance in the remediation device and power source.
5 Application of EK remediation at filed and pilot scale
Researchers have conducted reliable field applications of EK remediation. Liu et al. (2016) implemented field-scale EK remediation for Cu-contaminated soil at a former electroplating site. The project involved an 8 × 2 × 1 m remediation area with cylindrical titanium electrodes. Citric acid and lactic acid were used as cathodic electrolytes with treatment durations of 24 and 17 days, respectively. The soil, classified as loamy soil, had an average Cu content of 884 mg/kg. The primary process included civil construction, installation of electrodes/power supply equipment, electrification, wastewater recovery, and treatment. The isolated soil in the remediation area was separated from the surrounding region by PVC panels. The added electrolyte, consisting of biodegradable small-molecule organic acids, had minimal long-term impact on the soil and mitigated cathode alkalization during the EK process. The Cu removal rates from the anode and cathode after treatment with lactic acid were 52.6 % and 35.7 %, respectively, while citric acid resulted in 27.2 % and 17.5 % removal rates. The total energy consumption for lactic acid treatment was 15.7 kWh/m3, lower than many existing studies. Using numerical simulations to guide field applications of EK remediation can optimize operational parameters and reduce trial-and-error costs. Mao et al. (2019) studied a model simulation-based approach to guide field experiments for EK remediation. This research first optimized experimental parameters through simulation, determining that the polarity exchange cycle should be 48 h and the remediation time should be 226 h. Predictions for Pb removal rates were made based on these parameters. Subsequently, field remediation experiments were conducted according to the optimized parameters, and the obtained Pb removal rate closely matched the predicted results (with an error of 5–10 %).
While there have been many theoretical and experimental studies on EK remediation and its enhancement techniques, reports on on-site remediation are limited. Table 4 summarizes some instances of field-scale testing of EK remediation techniques. Although some application cases demonstrate the feasibility of EK remediation, many of the technologies studied in the laboratory are challenging to implement on a field scale. Numerous enhancement techniques have been developed to address issues with traditional EK remediation. However, most of these techniques exhibit excellent performance in laboratory-scale studies but encounter limitations in field-scale remediation processes. For example, conductive polymer electrodes and graphite electrodes offer superior remediation effects, but the cases summarized in Table 4 mainly involve metal electrodes. This is because field-scale EK remediation requires larger electrode sizes, and the manufacturing cost of conductive polymers is high.
In contrast, the application of large-sized graphite electrodes is limited by their mechanical properties (Wang et al., 2021; Hou et al., 2020). Therefore, metal electrodes have more potential applications in field-scale remediation. It is feasible to enhance the application of EK remediation on a field scale by increasing the dissolution capacity of HMs through electrolyte enhancement (Liu et al., 2016). Additionally, the construction cost of engineering facilities during the EK remediation process and the energy consumption are factors limiting the application of this technology. In the cases summarized in Table 5, there is significant variation in energy consumption levels (0.6–271 kWh/m3), but in most studies, energy consumption levels are relatively high. Consequently, in some reports, the feasibility of microbial fuel cells and solar power generation technology in applying EK remediation is also crucial to facilitate the transition of this technology from the laboratory to field-scale use (Abbas and Rafatullah, 2021; Hassan et al., 2015).
Soil
Scale
(length × width × height)
(m × m × m)HMs/content (mg/kg)
Remove rate
Time
(day)Energy consumption
(kWh/m3)literature
Paddy soil
4 × 1 × 0.5,
20 × 10 × 0.5Cd/3.68
87 %, 74 %
14
2, 0.6
Cai et al., 2021
Surface soil of electronic waste dismantling site
2 × 1 × 1
Cd/0.82
Pb/93.1
Cu/178.6—
90
—
Luo et al., 2018
Paddy soil
5 × 3.5 × 1.5
As/12.8
Cu/13.1
Pb/44.143.2 %
17.7 %
71.2 %28
34
Kim et al., 2013
Electroplating plant topsoil
8 × 2 × 1
Cu/ 186.7 ∼ 1578
35.7 %∼52.6 %
17
15.7
Liu et al., 2016
Paddy soil
4 × 2 × 1.5
As/0.54 ∼ 183.9
Cu/13.3 ∼ 220.3
Pb/11.5 ∼ 504.6−3.4 ∼ 20.7 %
−48.8 ∼ 12.1 %
−31.2 ∼ 23.9 %104
51 ∼ 210
Kim et al., 2012
Paddy soil
3.5 × 5 × 1.5
As/10.1 ∼ 12.8
Cu/13.1 ∼ 27
Pb/39.2 ∼ 78.35.9 ∼ 50.6 %
−21.1 ∼ 33.7 %
50.6 ∼ 81.2 %28
34 ∼ 181
Kim et al., 2011
River sediment
12 × 10 × 4
Pb/345.3
90 %
9.4
0.86
Mao et al., 2019
Saline alkali soil clay
—
Zn/—
Pb/—
Cd/—31.9 %
38.1 %
36.0 %35
271
Mu’azu et al, 2019
Paddy soil
4 × 2 × 1.5
As/0.54 ∼ 184
Cu/13.3 ∼ 220
Pb/11.5 ∼ 5053.4 ∼ 20.7 %
−48.8 ∼ 12.1 %
−31.2 ∼ 23.9 %102
51 ∼ 210
Kim et al., 2012
Sediment
1 × 1 × 0.54
Zn/2313 ∼ 3123
1.1 %
100
537
Li et al., 2009
Red soil
1 × 0.8 × 0.7
Cu/829
75.7 %
70
244
Zhou et al., 2006
Sand clay soil
0.9 × 0.8 × 0.9
Pb/1187 ∼ 3041
70 ∼ 85 %
270
1620
Alshawabkeh et al., 2005
Sand soil
9.14 × 4.57 × 3
Cd/5 ∼ 20
Cr/80 ∼ 100070 %
78 %200
208
GENT, 2004
6 The effects of EK on resilience of soil
The resilience after EK remediation is of paramount importance for soil sustainability (Wang et al., 2021). EK remediation may have several negative impacts on the soil as follows: (1) acidification or alkalization caused by H+ and OH–, (2) the effects of electrolytes on soil, and (3) the loss of nutrient elements. Many studies reported the response of soil physicochemical properties and enzyme activities to EK remediation (Iannelli et al., 2015; Masi et al., 2016). Xu et al. (2021) reported EK remediation for Cd-contained soil by adding HNO3, acetic acid, and EDTA. The results revealed that HNO3 addition caused a high Cd removal rate but significantly reduced microorganism diversity. Besides, HNO3-EK remediation reduced the activity of soil enzymes. Xu et al. (2022) revealed that EK remediated soil with HNO3 as electrolytes was at risk of acidification (low pH), salinization (high ), barren (low DOC), and micro-ecology imbalance (phyto- pathogenic bacteria). And they suggested neutralization, fertilization, and bactericides was necessary management for soil. Electrolytes with poor biodegradability (EDTA, DTPA, etc.), will have negative effects on soil resilience (Zhong et al., 2014). Yang et al., (2020) reported the feasibility of using environmentally friendly electrolytes (GLDA) to enhance EK remediation. It is worth pointing out that the effects of EK remediation on soil resilience are not all negative. Fan et al., (2022) reported that microbial taxa were changed by electric field, and it is led to variations in the functional gene components. Especially, the expression of the metabolisms was promoted, which could promote the degradation of pollutants. Overall, microbial communities are influenced by electric fields and also determine the soil's resilience. The effects of EK remediation on soil resilience and soil microbiology should be taken seriously, ignoring the resilience might overestimate the impact of EK remediation. Thus, soil resilience will be an important evaluation index for evaluating the effect of EK remediation in future research.
7 Conclusion
EK remediation is an emerging method with advantages such as simplicity, in-situ application, environmental friendliness, and minimal secondary pollution. The conventional components of the EK system are the power supply, electrodes, electrolytes, and ion exchange membranes. EK remediation can be strengthened by optimizing these factors. Specifically, appropriate electrode materials with high conductivity and EK activity positively influence HMs removal during the EK process. The arrangement of electrode matrix, electrode exchange, and electrode approaching significantly impact HMs removal efficiency and energy consumption cost. Additionally, selecting suitable electrolytes can enhance the dissolution of soil HMs to improve the remediation rate. AC is not commonly used for HMs removal but can effectively combine with phytoremediation techniques. The use of asymmetric AC provides a new approach to EK remediation.
8 Perspectives
Various enhanced technologies have been applied on a laboratory scale and have posed good results. After applying it on a field scale, the device installation and operation process becomes more complex, limiting the application of enhanced technologies. This is an urgent consideration when developing enhanced technologies. Besides, resilience is crucial for the soil after remediation. Thus, soil resilience should be an important indicator for evaluating EK remediation. The fraction of HMs has a significant effect on EK remediation efficiency, and it also determines the environmental risks. More studies should be concerned with the variation of HMs fraction before and after EK remediation, which was ignored in most existing studies.
Funding.
This work was funded by the National Key R&D Program of China [No. 2019YFC1805100], the National Natural Science Foundation of China [No.51664025], the Jiangxi Provincial Natural Science Foundation [No. 20232ACB203026], the Science and Technology Project of Ganzhou City [No. 2023PNS27982], the Science and Technology Project of Jiangxi Education Department [No. GJJ214407].
Ethics approval.
Not applicable, research does not report on or involve the use of any animal or human data or tissue.
Consent to participate.
Not applicable.
Consent for publication
The authors consent for publication of the manuscript in Environmental Science and Pollution Research.
CRediT authorship contribution statement
Xiaojun Zheng: Project administration, Writing – original draft, Writing – review & editing. Qi Li: Data curation, Funding acquisition, Methodology. Zongli Wang: Writing – review & editing. Ming Chen: Funding acquisition, Supervision.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Recent advances in soil microbial fuel cells for soil contaminants remediation. Chemosphere. 2021;272:129691
- [CrossRef] [Google Scholar]
- Electrokinetic – enhanced ryegrass cultures in soils polluted with organic and inorganic compounds. Environ. Res.. 2017;158:118-125.
- [CrossRef] [Google Scholar]
- Simultaneous removal of cadmium from kaolin and catholyte during soil electrokinetic remediation. Desalination. 2012;300:1-11.
- [CrossRef] [Google Scholar]
- Pilot-scale electrokinetic cleanup of lead-contaminated soils. J. Geotech. Geoenviron. Eng.. 2005;131:283-291.
- [CrossRef] [Google Scholar]
- Effect of electrokinetic remediation on heavy metal contaminated soil by adding acetic acid. Chinese Journal of Environmental Engineering. 2017;11:5283-5290.
- [Google Scholar]
- Electroextraction of Pb2+ ions from diluted solutions by a process combining ion-exchange textiles and membranes. Desalination. 1998;120:175-184.
- [CrossRef] [Google Scholar]
- Combined application of EDDS and EDTA for removal of potentially toxic elements under multiple soil washing schemes. Chemosphere. 2018;205:178-187.
- [CrossRef] [Google Scholar]
- Enhanced electrokinetic remediation of copper-contaminated soils near a mine tailing using the approaching-anode technique. J. Environ. Eng.. 2016;142:04015079.
- [CrossRef] [Google Scholar]
- Effects of electrode material on electrode remediation of Lead – contaminated soil Near a mine tailing. Environmental Science and Management. 2016;41:108-111.
- [Google Scholar]
- In situ electrokinetic (EK) remediation of the total and plant available cadmium (Cd) in paddy agricultural soil using low voltage gradients at pilot and full scales. Sci. Total Environ.. 2021;785:147277
- [CrossRef] [Google Scholar]
- Enhanced electrokinetic remediation for the removal of heavy metals from contaminated soils. Appl. Sci.. 2021;11:1799.
- [CrossRef] [Google Scholar]
- Effects of electrokinetic-assisted phytoremediation of a multiple-metal contaminated soil on soil metal bioavailability and uptake by indian mustard. Sep. Purif. Technol.. 2011;79:246-253.
- [CrossRef] [Google Scholar]
- Assessment of IrO 2 -Ta 2 O 5 |Ti electrodes for the electrokinetic treatment of hydrocarbon-contaminated soil using different electrode arrays. Electrochim. Acta. 2016;208:282-287.
- [CrossRef] [Google Scholar]
- Enhancement effects of the coupling between AC electric field and Fusarium sp. A-2 on the remediation of uranium contaminated soil by Macleaya cordata. China Environ. Sci.. 2022;42:258-266.
- [CrossRef] [Google Scholar]
- Mechanism of bio-electrokinetic remediation of pyrene contaminated soil: effects of an electric field on the degradation pathway and microbial metabolic processes. J. Hazard. Mater.. 2022;422:126959
- [CrossRef] [Google Scholar]
- Electrokinetic remediation of an electroplating contaminated soil with different enhancing electrolytes. Zhongguo Huanjing Kexue/china Environmental Science. 2015;35:1458-1465.
- [Google Scholar]
- Research on electrokinetic remediation of copper and cadmium contaminated soil with different electrodes. Guangdong Chemical Industry. 2014;41
- [Google Scholar]
- Electrokinetic remediation of chromium (Cr)-contaminated soil with citric acid (CA) and polyaspartic acid (PASP) as electrolytes. Chem. Eng. J.. 2017;316:601-608.
- [CrossRef] [Google Scholar]
- Bench- and field-scale evaluation of chromium and cadmium extraction by electrokinetics. J. Hazard. Mater.. 2004;110:53-62.
- [CrossRef] [Google Scholar]
- The combined application of a high voltage, low electrode spacing, and biosurfactants enhances the bio-electrokinetic remediation of petroleum contaminated soil. J. Clean. Prod.. 2020;276:122745
- [CrossRef] [Google Scholar]
- The role of pH, electrodes, surfactants, and electrolytes in electrokinetic remediation of contaminated soil. Molecules. 2022;27:7381.
- [CrossRef] [Google Scholar]
- Hybrid and enhanced electrokinetic system for soil remediation from heavy metals and organic matter. J. Environ. Sci.. 2025;147:424-450.
- [CrossRef] [Google Scholar]
- Free nitrous acid-assisted asymmetrical alternating current electrochemistry (FNA-AACE) for multi-heavy metals decontamination in waste activated sludge. Water Res.. 2023;242:120259
- [CrossRef] [Google Scholar]
- Critical review of electro-kinetic remediation of contaminated soils and sediments: mechanisms, performances and technologies. Water Air Soil Pollut.. 2021;232:335.
- [CrossRef] [Google Scholar]
- Solar powered electrokinetic remediation of Cu polluted soil using a novel anode configuration. Electrochim. Acta. 2015;181:58-67.
- [CrossRef] [Google Scholar]
- Electrorestoration of metal contaminated soils. Environ. Sci. Tech.. 1994;28:2203-2210.
- [CrossRef] [Google Scholar]
- Research progress of porous electrode materials based onsupercapacitors. Journal of Functional Materials/gongneng Cailiao. 2020;51
- [Google Scholar]
- Applying a nanocomposite hydrogel electrode to mitigate electrochemical polarization and focusing effect in electrokinetic remediation of a Cu- and Pb-contaminated loess. Environ. Pollut.. 2023;333:122039
- [CrossRef] [Google Scholar]
- Effect of pH regulation method on cadmium removal in Paddy soils by electrokinetic leaching. Journal of Changjiang River Scientific Research Institute. 2017;34:19-23.
- [Google Scholar]
- Performance of electrokinetic removal of cadmium from contaminated soil treated with CaCl2 -humic acid. Journal of Changjiang River Scientific Research Institute. 2021;38:60-65.
- [Google Scholar]
- Electrokinetic remediation of metal-polluted marine sediments: experimental investigation for plant design. Electrochim. Acta. 2015;181:146-159.
- [CrossRef] [Google Scholar]
- Revitalization of EDTA-remediated soil by fertilization and soil amendments. Ecol. Eng.. 2014;73:429-438.
- [CrossRef] [Google Scholar]
- EDTA retention and emissions from remediated soil. Chemosphere. 2016;151:202-209.
- [CrossRef] [Google Scholar]
- Research and field experiences on electrokinetic remediation in South Korea. Sep. Purif. Technol.. 2011;79:116-123.
- [CrossRef] [Google Scholar]
- Electrokinetic remediation of heavy metal-contaminated soils: performance comparison between one- and two-dimensional electrode configurations. J. Soil. Sediment.. 2021;21:2755-2769.
- [CrossRef] [Google Scholar]
- Enhanced electrokinetic extraction of heavy metals from soils assisted by ion exchange membranes. J. Hazard. Mater.. 2005;118:93-102.
- [CrossRef] [Google Scholar]
- In situ field scale electrokinetic remediation of multi-metals contaminated paddy soil: influence of electrode configuration. Electrochim. Acta. 2012;86:89-95.
- [CrossRef] [Google Scholar]
- Field application of in situ electrokinetic remediation for As-, Cu-, and Pb-contaminated paddy soil. Water Air Soil Pollut.. 2013;224:1698.
- [CrossRef] [Google Scholar]
- Inorganic electrified membrane: from basic science to performance translation. ACS ES&T Engineering. 2023;3:2123-2146.
- [CrossRef] [Google Scholar]
- Application of hexagonal two dimensional electrokinetic system on the nickel contaminated sediment and modelling the transport behavior of nickel during electrokinetic treatment. Sep. Purif. Technol.. 2018;192:253-261.
- [CrossRef] [Google Scholar]
- An assessment of pH-dependent release and mobility of heavy metals from metallurgical slag. J. Hazard. Mater.. 2020;384:121502
- [CrossRef] [Google Scholar]
- Heavy metal removal from shooting range soil by hybrid electrokinetics with bacteria and enhancing agents. Environ. Sci. Tech.. 2010;44:9482-9487.
- [CrossRef] [Google Scholar]
- A new method to control electrolytes pH by circulation system in electrokinetic soil remediation. J. Hazard. Mater.. 2000;77:227-240.
- [CrossRef] [Google Scholar]
- A novel combination of anaerobic bioleaching and electrokinetics for arsenic removal from mine tailing soil. Environ. Sci. Tech.. 2009;43:9354-9360.
- [CrossRef] [Google Scholar]
- Coupling electrokinetics and phytoremediation to remove uranium from contaminated soil: a laboratory pilot-scale study. ACS Earth Space Chem.. 2021;5:3448-3457.
- [CrossRef] [Google Scholar]
- Comparison of approaching and fixed anodes for avoiding the ‘focusing’ effect during electrokinetic remediation of chromium-contaminated soil. Chem. Eng. J.. 2012;203:231-238.
- [CrossRef] [Google Scholar]
- Electrochemical combined remediation of chromium contaminated oil based on strengthening by anode consumption. Environ. Eng. 2020
- [Google Scholar]
- Pilot-scale electrokinetic movement of HCB and Zn in real contaminated sediments enhanced with hydroxypropyl-β-cyclodextrin. Chemosphere. 2009;76:1226-1232.
- [CrossRef] [Google Scholar]
- Electrokinetic remediation of antibiotic-polluted soil with different concentrations of tetracyclines. Environ. Sci. Pollut. Res.. 2019;26:8212-8225.
- [CrossRef] [Google Scholar]
- Efficient electrokinetic remediation of heavy metals from MSWI fly ash using approaching anode integrated with permeable reactive barrier. Environ. Sci. Pollut. Res.. 2021;28:22156-22169.
- [CrossRef] [Google Scholar]
- Field-scale electrokinetic remediation of heavy metal contaminated sites. Chinese Journal of Environmental Engineering. 2016;10:3877-3883.
- [Google Scholar]
- Simultaneous removal of trivalent chromium and hexavalent chromium from soil using a modified Bipolar membrane electrodialysis system. Environ. Sci. Tech.. 2020;54:13304-13313.
- [CrossRef] [Google Scholar]
- Characteristics and opportunities of electrokinetic-assisted phytoremediation of heavy metal contaminated soil. Chemical Indutry and Engineering Progress. 2020;39:5252-5265.
- [CrossRef] [Google Scholar]
- Electrokinetic remediation of chromium- and cadmium-contaminated soil from abandoned industrial site. Sep. Purif. Technol.. 2012;98:216-220.
- [CrossRef] [Google Scholar]
- Sustainable decontamination of heavy metal in wastewater and soil with novel rectangular wave asymmetrical alternative current electrochemistry. J. Hazard. Mater.. 2023;442:130021
- [CrossRef] [Google Scholar]
- A real scale phytoremediation of multi-metal contaminated e-waste recycling site with Eucalyptus globulus assisted by electrical fields. Chemosphere. 2018;201:262-268.
- [CrossRef] [Google Scholar]
- P. Manikanta N. Mounesh R.R., Mohanty, J., Balakrishna, R.G., Sandeep, S., Nagaraja, B.M., CdO decorated with polypyrrole nanotube heterostructure: potent electrocatalyst for the detection of antihistamine drug promethazine hydrochloride in environmental samples Langmuir 39 2023 11099 11107 10.1021/acs.langmuir.3c01445.
- P. Manikanta N. Mounesh R.R., Sandeep, S., Nagaraja, B.M., Development of novel microsphere structured – calcium tungstate as efficacious electrocatalyst for the detection of antibiotic drug nitrofurantoin Journal of Materials Chemistry B 11 2023 11600 11611 10.1039/D3TB02087H.
- Pilot-scale electro-kinetic remediation of lead polluted field sediments: model designation, numerical simulation, and feasibility evaluation. Environ. Sci. Eur.. 2019;31:25.
- [CrossRef] [Google Scholar]
- Ligand-enhanced electrokinetic remediation of metal-contaminated marine sediments with high acid buffering capacity. Environ. Sci. Pollut. Res.. 2016;23:10566-10576.
- [CrossRef] [Google Scholar]
- R. Mounesh K.R.V., Pandith, A., Eldesoky, G.E., Nagaraja, B.M., Novel nitrogen-rich anchored nickel (II) phthalocyanine with composite of multiwalled carbon nanotubes on modified glassy carbon electrode: sensitive and selective electrocatalytic activity of nitrite Applied Organom Chemis 38 2024 10.1002/aoc.7302 e7302.
- Electrochemical, ultrasensitive, and selective detection of nitrite and H2O2: novel macrostructured phthalocyanine with composite MWCNTs on a modified GCE. Langmuir. 2023;39:1665-1676.
- [CrossRef] [Google Scholar]
- Comparative performance evaluation of anodic materials for electro-kinetic removal of Lead (II) from contaminated clay soil. Soil Sediment Contam. Int. J.. 2020;29:69-95.
- [CrossRef] [Google Scholar]
- Scale-up of hybrid electrokinetic–adsorption technique for removal of heavy metals from contaminated saline-sodic clay soil. Journal of King Saud University - Engineering Sciences. 2019;31:122-130.
- [CrossRef] [Google Scholar]
- Remediation of Pb/Cr co-contaminated soil using electrokinetic process and approaching electrode technique. Environ. Sci. Pollut. Res.. 2016;23:546-555.
- [CrossRef] [Google Scholar]
- The failure mechanism of nano-sized Si-based negative electrodes for lithium ion batteries. J. Mater. Chem.. 2011;21:6201.
- [CrossRef] [Google Scholar]
- Application of novel polypyrrole/melamine foam auxiliary electrode in promoting electrokinetic remediation of Cr(VI)-contaminated soil. Sci. Total Environ.. 2023;876:162840
- [CrossRef] [Google Scholar]
- Mechanical properties and ionic conductivity of electrospun quaternary ammonium ionomers. J. Membr. Sci.. 2012;389:478-485.
- [CrossRef] [Google Scholar]
- A combination of reducing and chelating agents for electrolyte conditioning in electrokinetic remediation of As-contaminated soil. J. Taiwan Inst. Chem. Eng.. 2017;70:252-259.
- [CrossRef] [Google Scholar]
- Simultaneous removal of polycyclic aromatic hydrocarbon and heavy metals from an artificial clayey soil by enhanced electrokinetic method. J. Environ. Manage.. 2018;217:897-905.
- [CrossRef] [Google Scholar]
- Advancements in biochar-based electrodes for improved performance of microbial fuel cells. Bioresource Technology Reports. 2023;24:101684
- [CrossRef] [Google Scholar]
- Migration and decomplexation of metal-chelate complexes causing metal accumulation phenomenon after chelate-enhanced electrokinetic remediation. J. Hazard. Mater.. 2019;377:106-112.
- [CrossRef] [Google Scholar]
- EDTA-enhanced electrokinetic remediation of aged electroplating contaminated soil assisted by combining dual cation-exchange membranes and circulation methods. Chemosphere. 2020;243:125439
- [CrossRef] [Google Scholar]
- Electroremediation of contaminated soil by heavy metals using ion exchange fibers. Electrochim. Acta. 2012;86:138-141.
- [CrossRef] [Google Scholar]
- Electrokinetic remediation of Cd-contaminated soil using low voltage gradients coupled with array adsorption zone and polarity exchange. Process Saf. Environ. Prot.. 2021;153:117-129.
- [CrossRef] [Google Scholar]
- Influence of polarity exchange frequency on electrokinetic remediation of Cr-contaminated soil using DC and solar energy. Process Saf. Environ. Prot.. 2021;153:117-129.
- [CrossRef] [Google Scholar]
- A review on ion-exchange nanofiber membranes: properties, structure and application in electrochemical (waste)water treatment. Sep. Purif. Technol.. 2022;287:120529
- [CrossRef] [Google Scholar]
- Removal of heavy metal from sludge by the combined application of a biodegradable biosurfactant and complexing agent in enhanced electrokinetic treatment. Chemosphere. 2017;189:599-608.
- [CrossRef] [Google Scholar]
- Coupled with EDDS and approaching anode technique enhanced electrokinetic remediation removal heavy metal from sludge. Environ. Pollut.. 2021;272:115975
- [CrossRef] [Google Scholar]
- Graphene-alginate fluids as unconventional electrodes for the electrokinetic remediation of Cr(VI) Water Air Soil Pollut.. 2021;232:334.
- [CrossRef] [Google Scholar]
- Electrokinetic removal of Cd and cu from mine tailing: EDTA enhancement and voltage intensity effects. Journal of Hazardous, Toxic, and Radioactive Waste. 2021;25:05020007.
- [CrossRef] [Google Scholar]
- Assessing effect of electrode configuration on the efficiency of electrokinetic remediation by sequential extraction analysis. J. Hazard. Mater.. 2005;119:167-174.
- [CrossRef] [Google Scholar]
- Microbial technologies for heavy metal remediation: effect of process conditions and current practices. Clean Techn. Environ. Policy. 2023;25:1485-1507.
- [CrossRef] [Google Scholar]
- Enhancements in ElectroKinetic remediation technology: environmental assessment in comparison with other configurations and consolidated solutions. Chem. Eng. J.. 2016;289:123-134.
- [CrossRef] [Google Scholar]
- Wan, Y., Wang, A., Shen, M., 2019. Restoration of Cadmium Contaminated Soil Using Approaching Anode Method of Polygonal Electrode. Ekoloji Dergisi.
- Application of a multi-electrode system with polyaniline auxiliary electrodes for electrokinetic remediation of chromium-contaminated soil. Sep. Purif. Technol.. 2019;224:106-112.
- [CrossRef] [Google Scholar]
- Application of polypyrrole flexible electrode for electrokinetic remediation of Cr(VI)-contaminated soil in a main-auxiliary electrode system. Chem. Eng. J.. 2019;373:131-139.
- [CrossRef] [Google Scholar]
- Research progress of positive electrode material recycling technology for retired lithium batteries. Research Progress of Positive Electrode Material Recycling Technology for Retired Lithium Batteries. 2023;42:2702-2716.
- [Google Scholar]
- Remediation of heavy metal-contaminated soils by electrokinetic technology: mechanisms and applicability. Chemosphere. 2021;265:129071
- [CrossRef] [Google Scholar]
- A review of green remediation strategies for heavy metal contaminated soil. Soil Use Manag.. 2021;37:936-963.
- [CrossRef] [Google Scholar]
- Electrode material–ionic liquid coupling for electrochemical energy storage. Nat. Rev. Mater.. 2020;5:787-808.
- [CrossRef] [Google Scholar]
- Review on combined electrokinetic and phytoremediation technology for soil contaminated by heavy metal. Journal of Nanjing Forestry University (Natural Sciences Edition). 2019;43:154-160.
- [Google Scholar]
- Enhanced electrokinetic remediation of heavy metals contaminated soils by stainless steel electrodes as well as the phenomenon and mechanism of electrode corrosion and crystallization. Environmental Science. 2017;38:1209-1217.
- [CrossRef] [Google Scholar]
- Focus on factors affecting pH, flow of Cr and transformation between Cr(VI) and Cr(III) in the soil with different electrolytes. Electrochim. Acta. 2016;211:652-662.
- [CrossRef] [Google Scholar]
- Asymmetrical alternating current electrochemically-mediated washing method for sustainable remediation of Cr(VI)-contaminated soil. J. Hazard. Mater.. 2022;437:129088
- [CrossRef] [Google Scholar]
- Remediation of heavy metal contaminated soil by asymmetrical alternating current electrochemistry. Nat. Commun.. 2019;10:1-8.
- [Google Scholar]
- Enhanced electrokinetic remediation for Cd-contaminated clay soil by addition of nitric acid, acetic acid, and EDTA: effects on soil micro-ecology. Sci. Total Environ.. 2021;772:145029
- [CrossRef] [Google Scholar]
- Resilience of Cd contaminated clay soil after nitric acid and acetic acid enhanced electrokinetic remediation. J. Environ. Chem. Eng.. 2022;10:108071
- [CrossRef] [Google Scholar]
- Yang, X., Zhou, M., Cang, L., Ji, Q., Xie, J., 2020. Enhanced Electrokinetic Remediation of Heavy-Metals Contaminated Soil in presence tetrasodium N, N-bis(carboxymethyl) glutamic acid (GLDA) as chelator. International Journal of Electrochemical Science 15, 696–709. 10.20964/2020.01.15.
- The transport behavior of As, Cu, Pb, and Zn during electrokinetic remediation of a contaminated soil using electrolyte conditioning. Chemosphere. 2014;117:79-86.
- [CrossRef] [Google Scholar]
- A review of soil heavy metal pollution from industrial and agricultural regions in China: pollution and risk assessment. Sci. Total Environ.. 2018;642:690-700.
- [CrossRef] [Google Scholar]
- Electrokinetic-enhanced remediation of actual arsenic-contaminated soils with approaching cathode and Fe0 permeable reactive barrier. J. Soil. Sediment.. 2020;20:1526-1533.
- [CrossRef] [Google Scholar]
- Study on the remediation of cadmium contaminated soil by cadmium-tolerant strains combined with electrokinetic remediation technology. Acta Sci. Circumst.. 2020;40:2212-2219.
- [Google Scholar]
- Electrokinetic remediation of heavy metals contaminated kaolin by a CNT-covered polyethylene terephthalate yarn cathode. Electrochim. Acta. 2016;213:140-147.
- [CrossRef] [Google Scholar]
- An approaching-theoretical-capacity anode material for aqueous battery: hollow hexagonal prism Bi2O3 assembled by nanoparticles. Energy Storage Mater.. 2020;28:82-90.
- [CrossRef] [Google Scholar]
- Effect of the approaching electrode on the soil arsenic migration and speciation transformation during electrokinetic remediation. Chinese Journal of Environmental Engineering. 2023;17:1294-1302.
- [Google Scholar]
- Zhang, H., Xu, H., Zi, H., Tang, Y., Zheng, X., Wang, P., Huang, J., Wu, H., Song, P., Wu, linxin, Liu, Z., Mao, W., Song, X., Jia, M., 2023. Investigation of the redox behavior of biochar-based bipolar electrochemistry in porous media. Chemical Engineering Journal 470, 144384. 10.1016/j.cej.2023.144384.
- Effects of electrode material on the electrokinetic remediation of cadmium-contaminated soil. Guangdong Chemical Industry. 2017;44:205-207.
- [Google Scholar]
- Electrokinetic remediation of Pb-contaminated tailing soil with enhancing reagents ascorbic acid and citric acid. Research of Environmental Sciences. 2017;30:1138-1145.
- [CrossRef] [Google Scholar]
- Enhanced electrokinetic remediation of lead-contaminated soil by complexing agents and approaching anodes. Environ. Sci. Pollut. Res.. 2014;21:3126-3133.
- [CrossRef] [Google Scholar]
- Electrokinetic remediation of Cd-contaminated soil using low voltage gradients coupled with array adsorption zone and polarity exchange. Process Saf. Environ. Prot.. 2022;157:81-91.
- [CrossRef] [Google Scholar]
- A review of heavy metal removal from soil by electrokinetic remediation. Earth and Environment. 2022
- [Google Scholar]
- Assessment of zeolite, biochar, and their combination for stabilization of multimetal-contaminated soil. ACS Omega. 2020;5:27374-27382.
- [CrossRef] [Google Scholar]
- Remediation of heavy metal-contaminated soils with soil washing: a review. Sustainability. 2022;14:13058.
- [CrossRef] [Google Scholar]
- Leaching of heavy metals from tungsten mining tailings: a case study based on static and kinetic leaching tests. Environ. Pollut.. 2024;342:123055
- [CrossRef] [Google Scholar]
- Presynaptic nicotinic acetylcholine receptors and the modulation of circuit excitability. Nicotinic Receptors. 2014;137–167
- [CrossRef] [Google Scholar]
- Electrokinetic remediation of a Cu–Zn contaminated red soil by controlling the voltage and conditioning catholyte pH. Chemosphere. 2005;61:519-527.
- [CrossRef] [Google Scholar]
- Pilot-scale electrokinetic treatment of a Cu contaminated red soil. Chemosphere. 2006;63:964-971.
- [CrossRef] [Google Scholar]
- Exchange electrode-electrokinetic remediation of Cr-contaminated soil using solar energy. Sep. Purif. Technol.. 2018;190:297-306.
- [CrossRef] [Google Scholar]
- Removal of cadmium in contaminated kaolin by new-style electrokinetic remediation using array electrodes coupled with permeable reactive barrier. Sep. Purif. Technol.. 2020;239:116544
- [CrossRef] [Google Scholar]







