Translate this page into:
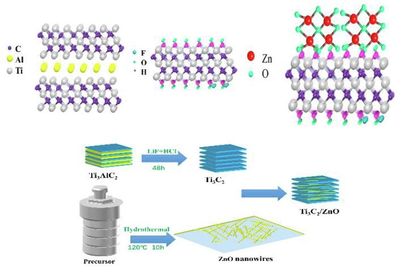
Synthesis of Ti3C2Tx nanosheets / ZnO nanowires composite material for NO2 gas sensing
⁎Corresponding authors. liu-chenhui@hotmail.com (Chenhui Liu), wfkm0202@126.com (Fang Wang)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
NO2 is a toxic gas that poses a significant threat to the environment and human health. In this work, one-dimensional ZnO nanowires and two-dimensional layered titanium carbide (Ti3C2Tx) have been prepared by hydrothermal and selective chemical etching methods, respectively. The Ti3C2Tx/ZnO composites are synthesized by electrostatic self-assembly method. The characterization results indicate that the ZnO nanowires are uniformly dispersed on the surface of layered Ti3C2Tx to form a heterostructure. Meanwhile, the gas sensitive performance of Ti3C2Tx/ZnO composite material is investigated and compared with that of pure Ti3C2Tx. The results reveal that the composite exhibits a high selectivity as well as sensitivity with a response value (S = Rg/Ra) of 18.66 to 100 ppm NO2 at 250 ℃, which is 4.3 times higher than that of pure Ti3C2Tx. This proves that the prepared composite material is an effective sensing material for detecting NO2 gas.
Keywords
ZnO nanowires
Ti3C2Tx/ZnO
NO2 gas sensing
Electrostatic self-assemble
1 Introduction
The rapid global industrialization has led to severe environmental degradation, especially air pollution, which poses a serious risk to human health (Bhowmick et al., 2022; Keerthana et al., 2022). NO2 is one of the most harmful gases originating from fuel combustion and automobile exhaust emissions, which not only produces acid rain but also causes serious atmospheric pollution. In particular, long-term exposure to ppmlevel NO2 can cause lung inflammation, asthma, tissue hypoxia, and other diseases in humans (Yuan et al., 2018; Liu et al., 2022; Fan et al., 2022). Therefore, it is crucial to develop a highly sensitive and selective NO2 gas sensor that can detect lowconcentration (ppb-level) NO2 (Shuvo et al., 2020). The resistive Metal-Oxide-Semiconductor (MOS)-based gas sensors based on the gas–solid reaction principle are widely used for detecting low-concentration NO2 gas because of their low cost,simple synthesis route, portability, and high stability (Teterycz et al., 2004). The commonly used metal oxides in the wide-band-gap MOS gas sensors are ZnO (Qiu et al., 2014)、SnO2 (Wei et al.,2004)、WO3 (Kanda et al., 2005)、TiO2 (Li et al., 2018)、Co3O4, W18O49 and CuO, However, the application of these sensors is limited by several problems, such as high operating temperature, long response time, poor selectivity, and broad spectral response to gases with similar redox characteristics (Chen et al., 2018; Zheng et al., 2021). To overcome these problems, it is essential to improve the semiconducting property of metal oxide by combining it with a twodimensional (2D) material, which can increase the effective contact area between the sensitive material and gas surface, accelerating the free charge transfer during the gassolid reaction process (Teterycz et al., 2004; Yang et al., 2021; Wang et al., 2021). This is a feasible method to improve the selectivity and reduce the operating temperature of the NO2 gas sensor.
MXenes are an emerging family of 2D transition metal carbides and/or nitrides, which are generally synthesized by chemical etching of the A element in the MAX phases. They have a graphene-like 2D layered structure, and their general chemical composition can be expressed as Mn+1XnTx (n = 1–3), where M is an early transition metal such as Ti, V, Nb, and Ta, X is C or N, and Tx represents the surface functional groups such as –OH, −O, and −F (Gogotsi et al., 2019; Naguib et al., 2012;Naguib et al., 2011). Compared to the other 2D materials, MXene with a large specific surface area and excellent electrical conductivity exhibits (Orangi et al., 2020). Furthermore, a large number of functional groups are present on the surface of MXenes, which can provide abundant active sites for gas adsorption and surface reaction. In addition, owing to their unique layered structure, MXenes are also widely used in photocatalysis, energy storage (Naguib et al., 2011; Fan et al., 2018; Orangi et al., 2020), electromagnetic shielding g (Li et al., 2021), etc. Until now, several kinds of MXenes have been successfully synthesized by etching and exfoliating various MAX phases, such as Ti3C2Tx, V2C, Nb2C, Nb4C3, Ti4N3, and Mo2C (Liu et al., 2019; Lee et al., 2019; Halim et al., 2016). However, pure 2D MXenes have some limitations as a gas sensing material, such as low sensitivity, poor selectivity, and slow response and recovery.
Notably, the gas-sensing performance of MXene such as 2D Ti3C2Tx can be enhanced by combining it with ZnO to form a composite gas sensitive material. This strategy can fully utilize the excellent gas-sensitive characteristics of ZnO as well as the large specific surface area and multiple efficient conductive channels of 2D Ti3C2Tx. In this work, the Ti3C2Tx/ZnO composites are synthesized by using uniform ZnO nanowires and Ti3C2Tx via the electrostatic self-assembly method. The results reveal that the Ti3C2Tx/ZnO composites exhibit excellent NO2 gas sensing properties, and the fabricated sensor based on the composites displays a good response of 18.66 to 100 ppm NO2 with outstanding selectivity. Additionally, the mechanism for enhancing the sensing response is explored. The enhanced sensing properties of the ZnO/MXene composite material with the two-dimensional conductive network indicate its immense potential in gas sensing applications.
2 Experimental
2.1 Raw materials and equipment
The chemicals used were Zn(NO3)·6H2O, NaOH, C6H12N4 (HMTA), NaF, CH3CH2OH, LiF (purity ≥ 99 %, Aladdin's reagent), HCl, Ti3AlC2 MAX powder, and polydiallyl dimethyl ammonium chloride (PDDA, Aladdin's reagent). Deionized water was used throughout the experiment.
The experimental equipment included JJ1023BC electronic scale, TG16-WS centrifugal machine, SK330BT supersonic cleaner, DHG-9145A drying oven, XinYi-10 freeze drier, OTF-1200X tube furnace, DF-101S magnetic stirrer, and SD101 fourchannel gas sensitive measuring instrument.
2.2 Sample Preparation
Synthesis of one-dimensional ZnO nanowires
The ZnO nanowires were fabricated by a hydrolysis approach. Firstly, solution A was obtained by dissolving 1.19 g Zn(NO3)·6H2O and 0.169 g NaOH in 40 ml distilled water under continuous stirring. Then, 0.28 g C6H12N4(HMTA) and 0.84 g NaF were dissolved in 40 ml distilled water to obtain solution B. Next, the solutions A and B were mixed for 0.5 h through magnetic stirring. Subsequently, the mixed solution was transferred to a polytetrafluoroethylene (PTFE)-lined autoclave, which was then heated at 120 ℃ in a constant-temperature furnace for 10 h. The white precipitate was Collected, then. The precipitate was separated by centrifugation and cleaned with deionized water for several times until the solution was neutral. Finally, the samples were dried in a drying oven at 80 °C for 8–10 h to obtain a white powder for further use (Wang et al., 2004). The one-dimensional (1D) ZnO nanowires were obtained by heating the dried sample to 400 ℃ at a rate of 5 ℃/ min in a tube furnace and maintaining it for 2 h.
Preparation of 2D Ti3C2Tx
Firstly, 1 g LiF powder was dissolved in 10 mL HCl with a concentration of 9 mol/L and stirred at room temperature for 30 min. In an ice bath, 1 g Ti3AlC2 MAX powder was slowly added to the above solution to avoid initial overheating of the solution due to exothermic reaction, and then the solution was transferred to an oil bath at 35 °C and continuously stirred for 24 h (Li et al., 2018; Kim et al., 2018; He et al., 2021; Pei et al., 2021). Then, the powder was dispersed for 1 h to obtain Ti3C2Tx. After centrifugation at 3500 rpm for 5 min, the supernatant was discarded, and the precipitate was washed with deionized water to a pH of 6. Finally, the sample was vacuum dried for 10 h to obtain the Ti3C2Tx powder.
Fabrication of Ti3C2Tx/ZnO composites by electrostatic self-assembly
Firstly, 1 g of the synthesized 1D ZnO nanowires was dispersed in 300 mL of 5 % PDDA, followed by ultrasonication for 3 h. After filtration, the PDDA-modified ZnOPDDA was washed with deionized water and dried for 12 h in a drying oven at 70 ℃. Subsequently, 1 g PDDA-modified ZnO powder was dissolved in 10.00 mL deionized water. Then, a certain amount of Ti3C2Tx was added, and the powder was vigorously stirred under the protection of argon gas for 10 h. Here, the composite material samples with the Ti3C2Tx mass fraction of 14 %, 25 %, and 40 % are denoted as M1, M2, and M3, and the pure Ti3C2Tx MXene is called M.
2.3 Characterization of Ti3C2TX/ZnO composites
The prepared Ti3C2Tx/ZnO composites were characterized by X-ray diffraction (XRD; A25X, Bruker, Germany), scanning electron microscopy (SEM; NOVA NANOSEM −450, FEI, USA), high-resolution transmission electron microscopy(HRTEM, JEM-2100Plus, JEOL Ltd., Japan), X-ray photoelectron spectroscopy (XPS; Thermo Fisher, Thermo Scientific, USA), and Brunauer-Emmett-Teller (BET) surface area analysis (BELSORP, Microtrac MRB, Japan).
2.4 Fabrication of gas sensing device
An appropriate amount of Ti3C2Tx/ZnO was placed in a beaker, and a small amount of pre-prepared organic solvent was added to form a thick liquid slurry. The slurry sample was printed on an alumina matrix by using screen printing to form a material film with a thickness of 5–10 μm. Then, these alumina matrixes were dried at 70℃ for 40 min and calcined at 350 ℃ for 2 h, followed by heat treatment at 550℃ for 2 h to enhance the mechanical bond of nanowires in the films (Hou et al., 2021). Finally, a thick porous film was formed for gas-sensing performance test.
2.5 Gas-sensing performance test
The gas-sensing performance of the samples was evaluated using a four-channel testing device (SD101, Wuhan Huachuang Ruike Tech. Co. Ltd., Wuhan, China). The test system included a computer, a gas flow controller, and a four-channel test chamber. During the test, the test chip was placed in a closed chamber filled with air. When the resistance showed a stable baseline, different working temperatures and different concentrations of target gas were set. At least three samples of each type were tested, and the average values were calculated. The gas response can be evaluated as follows: where Ra is the sample resistance in air, and Rg is the sample resistance in the presence of NO2 gas.
3 Results and discussion
3.1 Morphology and composition
The crystal structure of the prepared samples was examined by XRD, and the results are shown in Fig. 1. It can be seen that the ZnO sample has narrow and sharp characteristic peaks without any impurity peak. The diffraction peaks are located at 31.8°, 34.3°, 36.35°, 47.54°, 56.53°, 62.92°, 67.9°, and 69.1°, which correspond to the (100), (002), (101), (102), (103), (112), and (201) crystal planes, respectively (Keerthana et al., 2022; Gong et al., 2014; Srinivasan et al., 2022) in accordance with the Standard JCPDS Card No. 36–1451. The XRD results suggest that the prepared ZnO sample is a typical feldspathic zincite structure.. The diffraction peaks of Ti3AlC2 precursor are in good agreement with the Standard JCPDS Card No. 52–0875 (Li et al., 2019), and there are no impurity peaks, which proves that Ti3AlC2 is of high purity. The typical characteristic peaks at 9.65°and 39.13°correspond to the (002) and (104) crystal planes, respectively. However, these diffraction peaks of Ti3AlC2 precursor are not observed in the spectra of pure Ti3C2Tx and composite samples M1, M2 and M3, while the strong characteristic peak corresponding to (002) plane of Ti3C2Tx appears at 6.9°. This indicates that through selective etching of Ti3AlC2 precursor by LiF + HCl solution, Ti3C2Tx with high purity has been successfully prepared. Comparing the XRD patterns of composite materials M1, M2 and M3 with those of pristine ZnO and Ti3C2Tx, the presence of the independent characteristic diffraction peak of ZnO can be verified. Meanwhile, the characteristic diffraction peak of Ti3C2Tx appears at 6.9°, indicating that ZnO and Ti3C2Tx are successfully combined. Besides, the relative diffraction peak intensity of Ti3C2Tx decreases as the relative content of Ti3C2Tx decreases from 40 % (M3) to 25 % (M2) and then to 14 % (M1).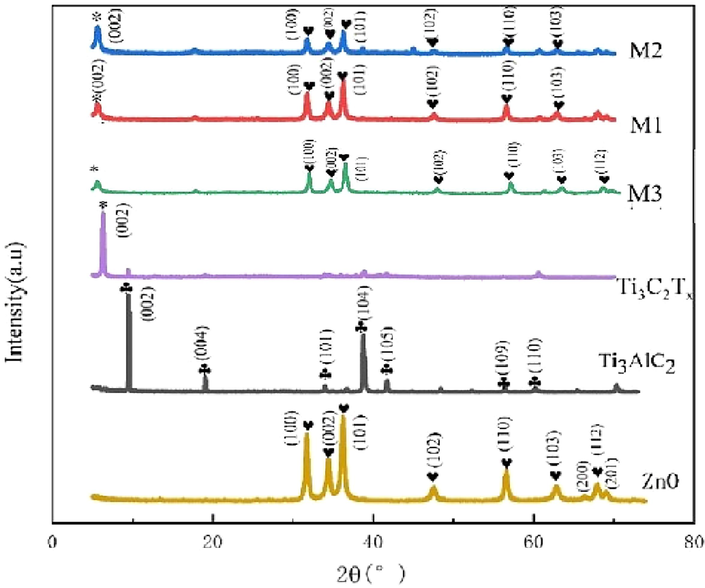
XRD patterns of Ti3AlC2, Ti3C2Tx MXene sheets, ZnO nanowires, and M1, M2 and M3 composite materials.
The micromorphology of the samples was investigated by SEM. Fig. 2(a-d) show the SEM images of Ti3AlC2 MAX phase, pure Ti3C2Tx, ZnO nanowires, and Ti3C2Tx/ZnO composite material, respectively. Fig. 2(e-f) show the SEM image of Ti3C2Tx/ZnO composite material at high magnification. It is clear from Fig. 2(a-b) that the Ti3AlC2 layers are tightly adhered together without stratification. After etching, the spacing between the layers becomes significantly larger, resembling a typical open stacked sheet structure (Han et al., 2020). This indicates that the Al atom layer in the Ti3AlC2 precursor structure can be selectively etched by LiF + HCl system, resulting in a typical 2D accordion morphology, with obvious microscopic pores between the layers. Besides, it can be seen in. Fig. 2(c) that the surface of ZnO nanowires is smooth and compact, with excellent crystallinity, and the dispersion is uniform. Fig. 2(e-g) show that the 1D ZnO nanowires are uniformly distributed on the surface and between the layers of 2D Ti3C2Tx. The composite samples have a 2D layered structure, where the ZnO nanowires are inserted in the 2D layered structure of Ti3C2Tx, indicating the successful synthesis of Ti3C2Tx/ZnO. EDS elemental mapping was employed to reveal the spatial distribution of elements in the as-formed Ti3C2Tx/ZnO, as shown in Fig. 2(h-l). It is shown that the C, O, Ti, and Zn elements are uniformly distributed throughout the heterostructures. It is showed that the surface of Ti3C2Tx sample formed through etching by LiF + HCl has −O and −F functional groups, while the nanowires on the surface of sheet structure are ZnO, which is consistent with the results of SEM and TEM.
SEM images of (a) Ti3AlC2 MAX phase; (b) pure Ti3C2Tx nanosheets; (c) ZnO nanowires; (d-g) Ti3C2Tx/ZnO composite materials. (h-l) EDS mapping analysis of C, O, F, Ti, and Zn.
The microstructure and internal crystal structure of the Ti3C2Tx/ZnO composite were examined by HRTEM. Fig. 3(a) shows that the ZnO nanowires are uniformly distributed on the surface and between the layers of Ti3C2Tx, which is consistent with the SEM results. Fig. 3(c) presents the HRTEM image of the composite, where the lattice fringe spacings of 0.26 and 0.307 nm are attributed to the (0 0 2) plane of ZnO and the (0 0 2) plane of Ti3C2Tx, respectively. These results are consistent with the previous characterizations.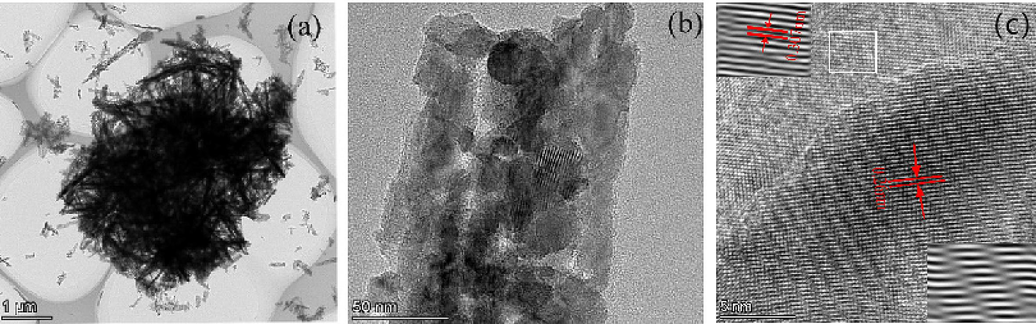
(a-b) TEM image of the synthesized Ti3C2Tx/ZnO composites from different perspectives; (c) HRTEM image showing the interlayer spacing of the synthesized Ti3C2Tx/ZnO composites.
The specific surface area and pore structure of Ti3C2Tx/ZnO and Ti3C2Tx samples were analyzed by N2 adsorption–desorption isotherm measurement. The results are shown in Fig. 4. The isotherm reveals a H3-type hysteresis loop at a relative pressure (P/P0) of 0.7–1.0, indicating that the samples have an obvious mesoporous structure. The BET specific surface area of M1, M2 and M3 are calculated to be 23.92,24.22 and 24.75 m2/g, respectively.The difference among M1, M2 and M3 is very small. This indicates that the effect of the difference in titanium carbide content in the composites on the specific surface area is minimal.The BET specific surface area of M is calculated to be 7.2923 m2/g. This implies that the specific surface area of the Ti3C2Tx/ZnO composite material is nearly three times higher than that of Ti3C2Tx, indicating the potential of the proposed structure in gas sensing application.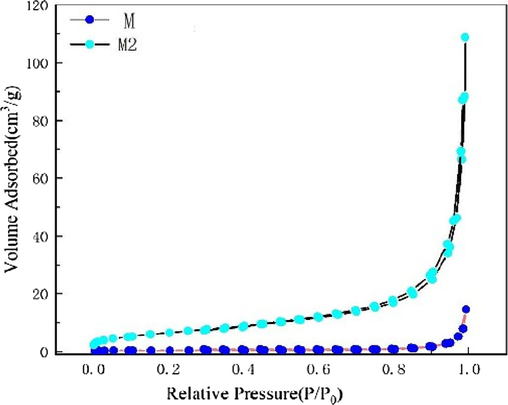
N2 adsorption–desorption isotherms of Ti3C2Tx (M)and Ti3C2Tx/ZnO composite.
XPS was used to examine the elemental composition and chemical state of different materials. Fig. 5(a) shows the full survey spectrum of ZnO, where only Zn, O, and C elements can be observed. This confirms a high purity of the ZnO nanowires. The highresolution XPS spectrum of Zn 2p in Fig. 5(b) shows two strong peaks at 1021.8 and 1044.9 eV, which are attributed Zn 2p3/2 and Zn 2p1/2, respectively, indicating the normal oxidation state of Zn2+ in ZnO nanowires (Zhao et al., 2021). Besides, the spacing between the two Zn 2p peaks is 23.1 eV, which is consistent with the energy splitting of ZnO. Fig. 5(c) shows the high-resolution XPS spectrum of O1s, where the peak at 529.53 eV can be assigned to O2– in ZnO nanowires, while the peak at 530.28 eV is ascribed to the surface hydroxyl group formed due to the reaction of chemisorbed oxygen with water in the atmosphere (Zhao et al., 2019).Fig. 5(d) shows the full survey spectrum of Ti3C2Tx. It is evident that the Ti3C2Tx sheets are composed of Ti, C, O, and F elements. The spectra of individual elements was verified by Gaussian fitting. In the fitted C 1 s spectrum, there are four peaks at the binding energies of 280.63, 283.58, 284.33, and 288.28 eV, which can be indexed to C-Ti, C-C, C-O, and CHx-C-O bonds (Halim et al., 2016; Wu et al., 2017), respectively, proving a typical carbon bonding in Ti3C2TxMXene. As shown in Fig. 5(e), the Ti 2p XPS spectra of Ti3C2Tx can be divided into six peaks at the binding energies of 453.9, 454.98, 458.23, 460.18, and 464.8 eV, which correspond to Ti-C(Ti2+), Ti-X(Ti3+), TiO2-X-Fx, C-Ti-Fx, and TiO2(Ti4+), respectively (Halim et al., 2014).Fig. 5(g) shows the full survey spectrum of Ti3C2Tx/ZnO composite. The characteristic peaks of Ti, O, C, Z, and F can be observed in the full spectrum, which is consistent with the EDS mapping results. The C 1 s spectra shows three peaks corresponding to different carbon bonds: C-O, C-C, and C-Ti-Tx. The fitted Ti 2p spectra shows four different peaks, which represent C-Ti-OxFx, Ti-C, C-Ti-Ox, and CTi-Fx, TiO2. However, compared to the Ti 2p spectrum of Ti3C2Tx, the area of C-Ti-Fx peak is significantly decreased because the attached ZnO has added the oxygen terminal to Ti3C2Tx.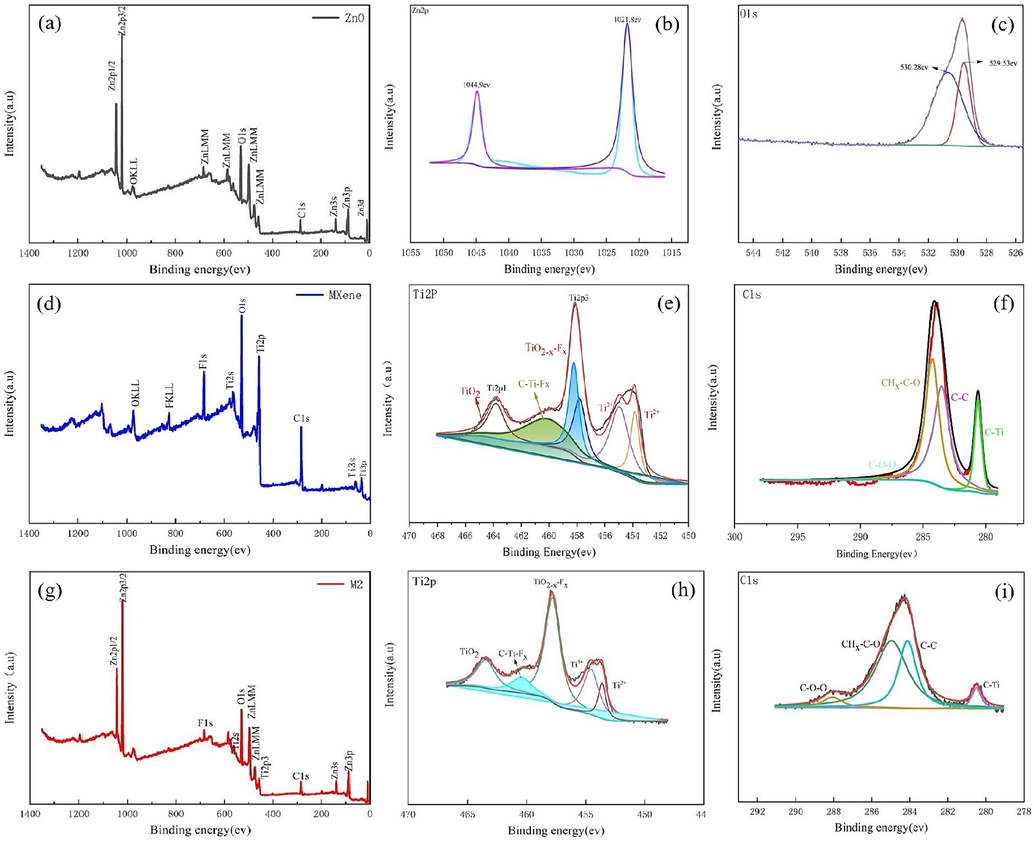
(a) XPS full survey spectrum of ZnO; XPS spectra of (b); Zn 2p and (c) C 1 s for ZnO; (d) XPS full survey spectrum of Ti3C2Tx; high-resolution XPS spectra of (e) Ti 2p and (f) C 1 s for Ti3C2Tx;(g) XPS full survey spectrum of Ti3C2Tx/ZnO; high-resolution XPS spectra of (h) Ti 2p and (i) C 1 s for Ti3C2Tx/ZnO composite.
3.2 Gas-sensing properties
To determine the optimal operating temperature of composites and Ti3C2Tx/ZnO pure Ti3C2Tx, the sensing response of the samples M, M1, M2, and M3 to 100 ppm NO2 at different operating temperatures was examined, and the results are shown in Fig. 6(a). As the operating temperature increases, the response values of M, M1, M2, and M3 gas sensors first increase and then decrease. The response of all the samples becomes maximum at 250 ℃. Specifically, the maximum response values of M, M1, M2, and M3 to 100 ppm NO2 are 4.3, 17.07, 18.66, and 8.5, respectively. Therefore, 250℃ is selected as the optimal operating temperature of all the sensors. Fig. 6(b) shows the dynamic response-recovery curves of different sensors at the optimal operating working temperature. It is evident that the response value of all the composite materials is higher than that of the pure Ti3C2Tx. The response of sample M2 reaches a maximum of 18.66. Specifically, compared to the pureTi3C2Tx, the sensitivity of Ti3C2Tx/ZnO composite is enhanced by almost 4.3 times. Further, within the range of 100 s, the sensitivity of the four different sensor materials rises rapidly.the M2 is determined to represent the best gas-sensing performances of Ti3C2Tx/ZnO composites in the future measurements. Moreover, all of the responses of these sensors based on Ti3C2Tx/ZnO composites are much higher than thatof their pristine components, indicating that the gas-sensing performances can be improved by the pure Ti3C2Tx. Fig. 6(c) shows that the sensor exhibits extremely high sensitivity for ppm-level NO2 gas at 250 ℃. In particular, the response of the Ti3C2Tx/ ZnO composite shows a good linear relationship with the NO2 concentration (R2 = 0.95344), which indicates that the Ti3C2Tx/ZnO composite-based sensor can be used for real-time detection of NO2 gas. Fig. 6(d) shows that good linear fits are also acquired.The limit of detection (LOD) of the sensor based on the Ti3C2Tx/ZnO composite is estimated to be 0.105 ppm based on the equation LOD = 3σD/σ (here, σD is the standard deviation of noise during the response and σ is the slope of the linear part).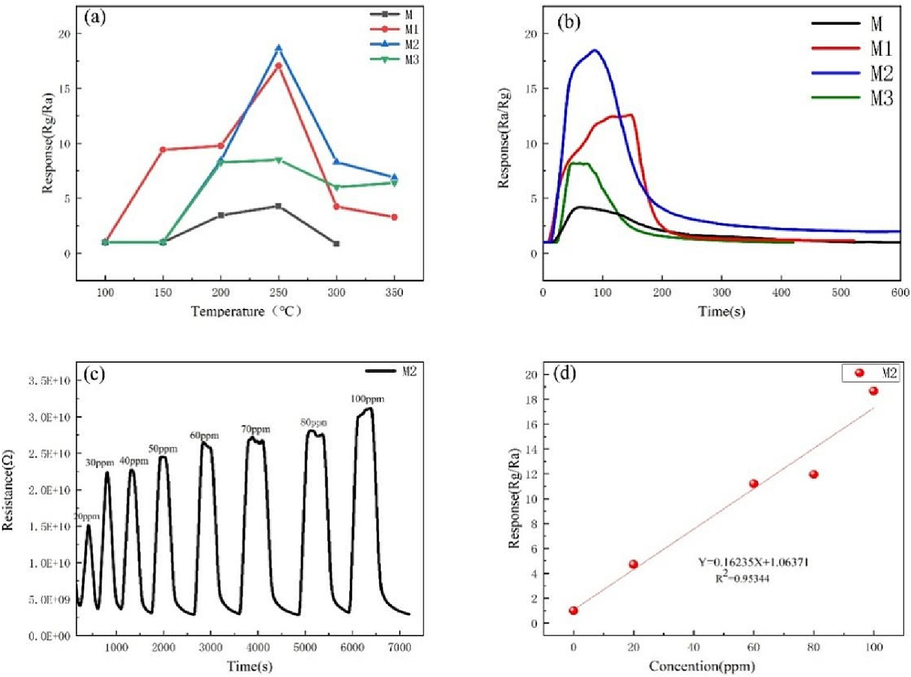
(a) Response of materials towards 100 ppm NO2 at different temperatures. (b)Response-recovery of different materials to 100 ppm NO2 at 250 ℃. (c) Dynamic response-recovery curves for different NO2 concentrations (20–100 ppm) at 250 ℃. (d) Linear fitting of the response curve of Ti3C2Tx/ZnO composite to 0–100 ppm NO2 at 250 ℃.
Repeatability and long-term stability are the two primary performance indicators for the practical application of a gas sensor. The repeatability of a gas sensor is measured by changing the gas concentration. Fig. 7(a) shows the dynamic resistance vs. time curve of the M2 composite sample for five cycles under exposure to 100 ppm NO2. The M2 composite shows an excellent repeatability without any significant attenuation in the response under alternate exposure to air and 100 ppm NO2. Furthermore, Fig. 7(b) shows the response of Ti3C2Tx/ZnO composite exposed to 100 ppm NO2 for a continuous 30 days. The test was performed once every 5 days for 6 consecutive times, and the results show a slight fluctuation or negligible reduction, indicating good long-term stability. At the optimal operating temperature, the response and recovery curves of samples M and M2 exposed to 100 ppm NO2 are shown in Fig. 7(c,d). After NO2 gas is injected, the response of samples M and M1 increases rapidly and becomes stable at the maximum value. The response time and recovery time of M2 are calculated to be 17 and 102 s, respectively, which are both lower than those of Ti3C2Tx gas sensor. The results demonstrate that the Ti3C2Tx/ZnO composite-based sensor exhibits a comparatively fast response/recovery to NO2 gas at 250℃. Besides, it can be seen that the sensor cannot fully recover to its initial resistance. This may be because the sensor measures the concentration of target gas at a high temperature, and a certain amount of residual NO2 gas molecules are adsorbed on the sample, occupying the effective adsorption sites of oxygen, which inevitably reduces the recovery resistance value. The improvement in the gas sensitivity of Ti3C2Tx can be ascribed to the formation of heterojunction between Ti3C2Tx and ZnO (Wang et al., 2022). The good conductive network provided by 2DTi3C2Tx can greatly enhance the mobility of free charge carriers in the gas–solid reaction process, improve the reaction rate at the gas–solid interface, facilitate the gas adsorption, and shorten the response/ recovery time.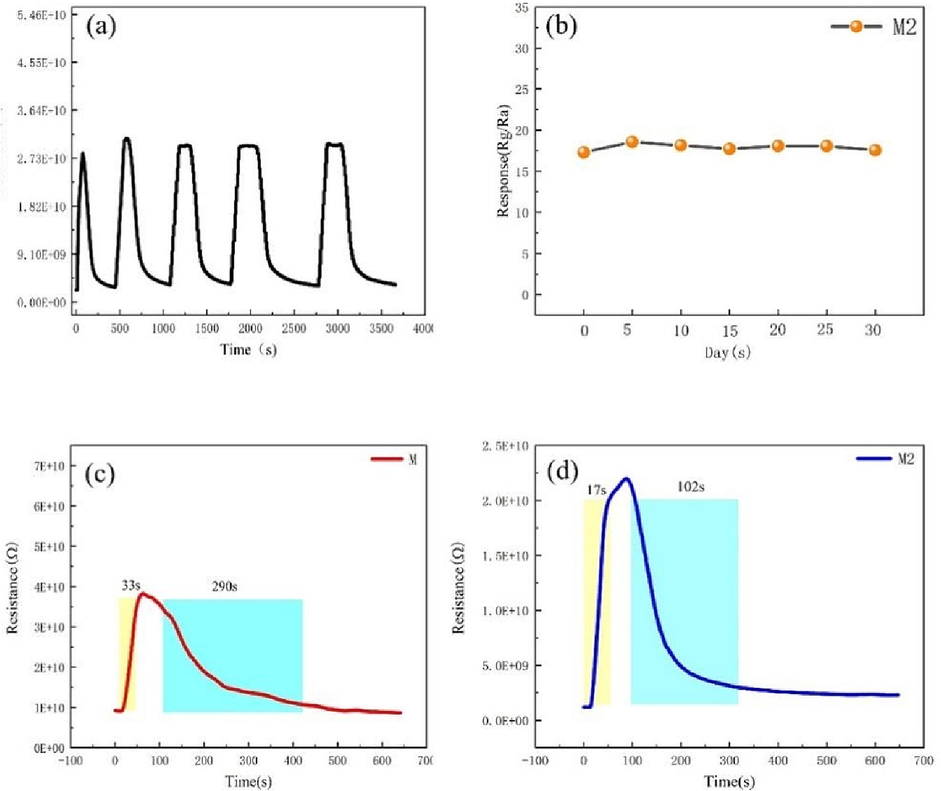
(a) Cyclic transient sensing response towards 100 ppm NO2; (b) long-term stability of Ti3C2Tx/ZnO composite; (c-d) response and recovery time of the sensors based on M and M2 composites to 100 ppm NO2 at 250 ℃.
Selectivity is an important characteristic of gas sensors. To evaluate the ability of the gas sensor to recognize NO2 and avoid the interference of background gases, the selectivity of Ti3C2Tx/ZnO composites was tested. Fig. 8 shows the response of Ti3C2Tx/ZnO composite-based sensor to six different gases (ethanol, acetone, acetaldehyde, methanol, NO2 and SO2) at the optimal operating temperature of 250 ℃. The gas response values of M2 sensor to 1000 ppm ethanol, acetone, acetaldehyde, and methanol are 0, 0, 1.94, and 0.86, respectively. The gas response values of M1 and M2 sensors to 100 ppm SO2 are 3.87 and 5.02. Thus, the response of Ti3C2Tx/ZnO composite-based sensor to NO2 is much higher than that to other gases, indicating its excellent selectivity to NO2. This suggests that the M1 and M2 gas sensitive elements show good response and selectively toward NO2 in the presence of several background gases.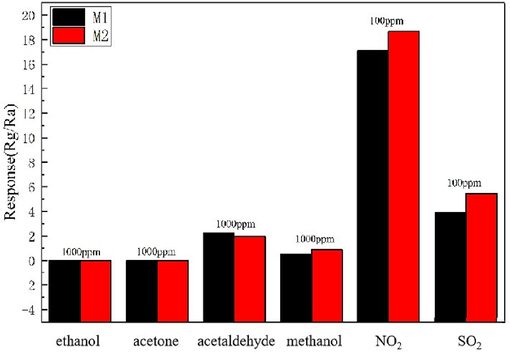
Selectivity of Ti3C2Tx/ZnO composite to various target gases.
3.3 Gas sensing mechanism
Herein, a possible mechanism for the gas sensing property of Ti3C2Tx/ZnO composite material is established based on the modulation of depletion layer. After the contact in vacuum, the charge carrier transfer process occurs at the Ti3C2Tx interface due to the difference in Fermi energy and then stops until the static charge equilibriumis reached when the Fermi level (Ef) flattens under the action of internal electric field, causing band bending, as shown in Fig. 9(c). When the Ti3C2Tx/ZnO gas sensor is exposed to air, O2 molecules can not only capture free electrons from the conduction band of ZnO and convert them into O2–, but also obtain electrons from Ti3C2Tx through surface functional groups (–OH and −O, −F) to produce O2–. The adsorbed O2– ions can further gain electrons to form O-ions, causing an increase in the Schottky barrier and the formation of electron depletion layer (Su et al., 2019), as shown in Fig. 9(d). When the Ti3C2Tx/ZnO gas sensor is exposed to NO2, which is a typical oxidizing gas with high electron affinity, NO2 can be adsorbed on surface of Ti3C2Tx/ZnO composite and capture electrons form NO2–. The depletion layer at the interface widens, and the resistance increases, as shown in Fig. 9(e). As NO2 further reacts with a large amount of O2-on the material surface to form NO2–, the ZnO conduction band and Ti3C2Tx continue to lose electrons. Besides, the depletion layer further widens and the resistance increases, and the adsorbed O-ions on the surface of ZnO spheres also play a major part in the chemical adsorption of NO2 (Yang et al., 2016; Al-hadeethi et al., 2017).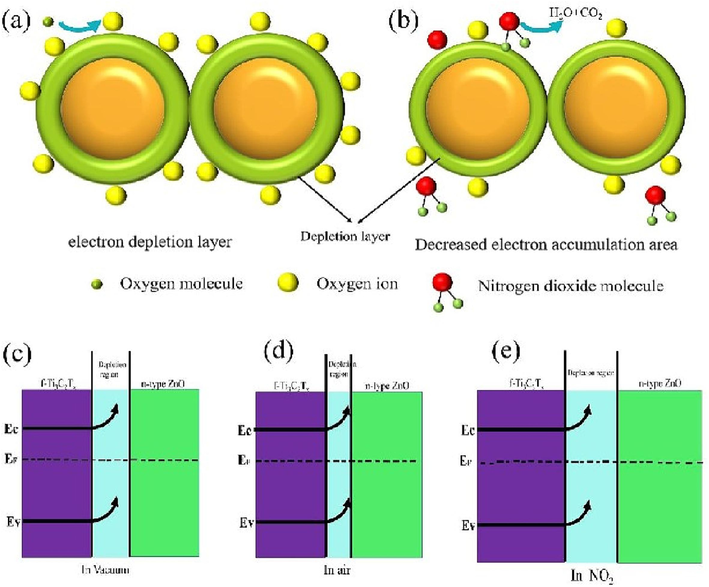
(a-b) Schematic illustration of the NO2 gas sensing mechanism of Ti3C2Tx/ZnO nanocomposite. Schottky barrier in (c) vacuum, (d) air, and (e) NO2.
4 Conclusions
The Ti3C2Tx/ZnO nanocomposites were successfully synthesized through the electrostatic self-assembly method. The microstructure of the composites with different contents of Ti3C2Tx were analyzed. Furthermore, the optimal operating temperature, concentration, stability, response and recovery time, and selectivity of the Ti3C2Tx/ZnO composite-based gas sensor to NO2 gas were investigated. The Ti3C2Tx/ZnO nanocomposites containing 25 % Ti3C2Tx exhibited the best NO2 sensing performance with a response value of 18.66, fast response and recovery (17/102 s), and outstanding selectivity compared to pure Ti3C2Tx. The response of Ti3C2Tx/ZnO nanocomposites toward 100 ppm NO2 gas was almost 4.3 times higher that of pure Ti3C2Tx, which could be ascribed to the preferred surface termination group, large specific surface area of Ti3C2Tx, and heterogeneous structure constructed by ZnO and Ti3C2Tx.
CRediT authorship contribution statement
Jiyun Gao: Data curation, Investigation, Methodology, Writing – original draft, Writing – review & editing. Youyou Yin: Data curation, Formal analysis. Yongjing Guo: Data curation, Formal analysis. Lijuan Jia: Data curation, Investigation. Futing Xia: Data curation, Formal analysis, Writing – review & editing. Chenhui Liu: Funding acquisition, Supervision. Ming Hou: Formal analysis, Investigation. Fang Wang: Supervision, Writing – original draft.
Acknowledgments
This research was funded by National Natural Science Foundation of China, No.52304400; Youth Fund Project of Yunnan Province, No.202201AU070156; Ten Thousand Talent Plans for Young Top-notch Talents of Yunnan Province (YNWR-QNBJ-2019-066).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Synthesis, characterization and acetone gas sensing applications of Ag-doped ZnO nanoneedles. Ceramics International.. 2017;43:6765-6770.
- [CrossRef] [Google Scholar]
- Sensitive and selective CO2 gas sensor based on CuO/ZnO bilayer thin-film architecture. Journal of Alloys and Compounds.. 2022;903:163871
- [CrossRef] [Google Scholar]
- In-situ growth of ZnO nanowire arrays on the sensing electrode via a facile hydrothermal route for high-performance NO2 sensor. APPLIED SURFACE SCIENCE.. 2018;435:1096-1104.
- [CrossRef] [Google Scholar]
- Fast and recoverable NO2 detection achieved by assembling ZnO on Ti3C2Tx MXene nanosheets under UV illumination at room temperature. Nanoscale.. 2022;14:3441-3451.
- [CrossRef] [Google Scholar]
- A nanoporous MXene film enables flexible supercapacitors with high energy storage. Nanoscale.. 2018;10:9642-9652.
- [CrossRef] [Google Scholar]
- Synthesis and characterization of flower-like ZnO nanostructures via flower-like ZnOHF intermediate. Materials Letters.. 2014;127:36-39.
- [CrossRef] [Google Scholar]
- Transparent conductive two-dimensional titanium carbide epitaxial thin films. Chemistry of Materials.. 2014;26:2374-2381.
- [CrossRef] [Google Scholar]
- X-ray photoelectron spectroscopy of select multi-layered transition metal carbides (MXenes) APPLIED SURFACE SCIENCE.. 2016;362:406-417.
- [CrossRef] [Google Scholar]
- Beyond Ti3C2Tx: MXenes for electromagnetic interference shielding. ACS Nano.. 2020;14:5008-5016.
- [CrossRef] [Google Scholar]
- MXene/SnO2 heterojunction based chemical gas sensors. Sensors and Actuators b: Chemical.. 2021;329:129275
- [CrossRef] [Google Scholar]
- Room temperature gas sensing under UV light irradiation for Ti3C2Tx MXene derived lamellar TiO2-C/g-C3N4 composites. APPLIED SURFACE SCIENCE.. 2021;535:147666
- [CrossRef] [Google Scholar]
- Development of a WO3 thick-film-based sensor for the detection of VOC. Sensors and Actuators b: Chemical.. 2005;108:97-101.
- [CrossRef] [Google Scholar]
- Hierarchical ZnO/CuO nanostructures for room temperature detection of carbon dioxide. Journal of Alloys and Compounds.. 2022;897:162988
- [CrossRef] [Google Scholar]
- Kim S. J., Koh H.-J., Ren C. E., Kwon O., Maleski K., Cho S.-Y., Anasori B., Kim C.-K., Choi Y.-K., Kim J., 2018. Metallic Ti3C2T x MXene gas sensors with ultrahigh signal-to-noise ratio. ACS nano. 12, 986-993.https://doi.org/10.1021/acsnano.7b07460.
- Two-dimensional vanadium carbide MXene for gas sensors with ultrahigh sensitivity toward nonpolar gases. ACS Sensors.. 2019;4:1603-1611.
- [CrossRef] [Google Scholar]
- Ti3C2 MXene-derived Ti3C2/TiO2 nanoflowers for noble-metal-free photocatalytic overall water splitting. Applied Materials Today.. 2018;13:217-227.
- [CrossRef] [Google Scholar]
- Li M., Lu J., Luo K., Li Y., Chang K., Chen K., Zhou J., Rosen J., Hultman L., Eklund P., 2019. Element replacement approach by reaction with Lewis acidic molten salts to synthesize nanolaminated MAX phases and MXenes. Journal of the American Chemical Society. 141, 4730-4737. https://doi.org/10.1021/jacs.9b00574.
- Advances in MXene films: synthesis, assembly, and applications. Transactions of Tianjin University.. 2021;27:217-247.
- [CrossRef] [Google Scholar]
- Pursuit of a high-capacity and long-life Mg-storage cathode by tailoring sandwich-structured MXene@carbon nanosphere composites. Journal of Materials Chemistry a.. 2019;7:16712-16719.
- [CrossRef] [Google Scholar]
- Enhanced room-temperature NO2 sensing performance of SnO2/Ti3C2 composite with double heterojunctions by controlling co-exposed and facets of SnO2. Sensors and Actuators b: Chemical.. 2022;365:131919
- [CrossRef] [Google Scholar]
- Two-dimensional nanocrystals produced by exfoliation of Ti3AlC2. ADVANCED MATERIALS.. 2011;23:4248-4253.
- [CrossRef] [Google Scholar]
- On the topotactic transformation of Ti2AlC into a Ti–C–O–F cubic phase by heating in molten lithium fluoride in air. Journal of the American Ceramic Society.. 2011;94:4556-4561.
- [CrossRef] [Google Scholar]
- Two-Dimensional Transition Metal Carbides. ACS Nano.. 2012;6:1322-1331.
- [CrossRef]
- A Review of the Effects of Electrode Fabrication and Assembly Processes on the Structure and Electrochemical Performance of 2D MXenes. Advanced Functional Materials.. 2020;30:20053051-200530522.
- [CrossRef] [Google Scholar]
- Ti3C2TX MXene for sensing applications: recent progress, design principles, and future perspectives. ACS Nano.. 2021;15:3996-4017.
- [CrossRef] [Google Scholar]
- Qiu M. Y., 2014. Investigation on the gas sensing properties of ZnO thin films. Applied Mechanics and Materials. 685, 144-148. https://doi.org/10.4028/www.scientific.net/AMM.685.144.
- Sulfur-doped titanium carbide MXenes for room-temperature gas sensing. ACS Sensors.. 2020;5:2915-2924.
- [CrossRef] [Google Scholar]
- Zinc oxide nanoparticles from Coriandrum sativum as sensor for detection of n-butanol and nitric oxide gas. Materials Today: Proceedings.. 2022;151:1760-1764.
- [CrossRef] [Google Scholar]
- Glucose-assisted synthesis of hierarchical flower-like Co3O4 nanostructures assembled by porous nanosheets for enhanced acetone sensing. Sensors and Actuators b: Chemical.. 2019;288:699-706.
- [CrossRef] [Google Scholar]
- Study on physico-chemical properties of tin dioxide based gas sensitive materials used in condensation reactions of n-butanol. Applied Catalysis a: General.. 2004;274:49-60.
- [CrossRef] [Google Scholar]
- Hydrothermal synthesis and photoluminescence properties of ZnO nanowires. Solid State Communications.. 2004;132:269-271.
- [CrossRef] [Google Scholar]
- SnO-SnO2 modified two-dimensional MXene Ti3C2Tx for acetone gas sensor working at room temperature. Journal of Materials Science & Technology.. 2021;73:128-138.
- [CrossRef] [Google Scholar]
- Mesoporous MXene/ZnO nanorod hybrids of high surface area for UV-activated NO2 gas sensing in ppb-level. Sensors and Actuators b: Chemical.. 2022;353:2022.
- [CrossRef] [Google Scholar]
- A novel SnO2 gas sensor doped with carbon nanotubes operating at room temperature. Sensors & Actuators B Chemical.. 2004;101:81-89.
- [CrossRef] [Google Scholar]
- Few-layer MXenes delaminated via high-energy mechanical milling for enhanced sodium-ion batteries performance. ACS APPLIED MATERIALS & INTERFACES.. 2017;9:39610-39617.
- [CrossRef] [Google Scholar]
- Flexible resistive NO2 gas sensor of three-dimensional crumpled MXene Ti3C2Tx/ZnO spheres for room temperature application. Sensors and Actuators b: Chemical.. 2021;326:128828
- [CrossRef] [Google Scholar]
- High sensitivity and good selectivity of ultralong MoO3 nanobelts for trimethylamine gas. Sensors and Actuators b: Chemical.. 2016;226:478-485.
- [CrossRef] [Google Scholar]
- A flexible VOCs sensor based on a 3D MXene framework with a high sensing performance. Journal of Materials Chemistry a.. 2018;6:18116-18124.
- [CrossRef] [Google Scholar]
- Room temperature formaldehyde sensing of hollow SnO2/ZnO heterojunctions under UV-LED activation. IEEE Sensors Journal.. 2019;19:7207-7214.
- [CrossRef] [Google Scholar]
- P-n junctions based on CuO-decorated ZnO nanowires for ethanol sensing application. APPLIED SURFACE SCIENCE.. 2021;538:148140
- [CrossRef] [Google Scholar]
- Engineering SnO2 nanorods/ethylenediamine-modified graphene heterojunctions with selective adsorption and electronic structure modulation for ultrasensitive room-temperature NO2 detection. Nanotechnology.. 2021;32:155505
- [CrossRef] [Google Scholar]







