Translate this page into:
Magnetic covalent organic framework with ionic tags as an efficient catalyst in the preparation of 2,3-disubstituted thiazolidine-4-ones and N‑amino-2-pyridones
⁎Corresponding authors. myarie.5266@gmail.com (Meysam Yarie), m.yarie92@basu.ac.ir (Meysam Yarie), zolfi@basu.ac.ir (Mohammad Ali Zolfigol) mzolfigol@yahoo.com (Mohammad Ali Zolfigol)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
General route for the catalytic preparation of thiazolidine-4-ones and N–amino-2-pyridones.
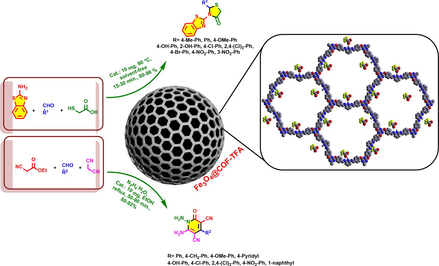
Abstract
A new pyridine-based magnetic COF decorated with trifluoroacetate as ionic sections (Fe3O4@COF-TFA) was designed and constructed. In a comprehensive study, Fe3O4@COF-TFA was investigated using FT-IR, EDS, elemental mapping, SEM, TEM, VSM and TGA/DTG analysis. Fe3O4@COF-TFA was successfully used as a heterogeneous catalyst for construction of 2,3-disubstituted thiazolidine-4-ones and N–amino-2-pyridones via a multi-component synthetic strategy.
Abstract
The functionalization of magnetic nanoparticles with covalent organic frameworks (COFs) will endow them inspiring characteristics. Herein, a new magnetic covalent organic framework (MCOF) decorated with trifluoroacetate as ionic sections (Fe3O4@COF-TFA) was designed and constructed. To confirm the successful synthesis of this hybrid material, several techniques including Fourier-transform infrared spectroscopy (FT-IR), thermogravimetric analysis (TGA), derivative thermogravimetry (DTG), field-emission scanning electron microscopy (FE-SEM), energy-dispersive X-ray spectroscopy (EDS), elemental mapping, transmission electron microscopy (TEM), vibrating-sample magnetometer (VSM) and X-ray diffraction analysis (XRD) were applied. TG/DTG analyses show that Fe3O4@COF-TFA has an excellent thermal stability up to 450 ℃. The FE-SEM analysis revealed that catalyst has the island-like morphology and according to TEM analysis, it has spherical geometry with nanometer scale particle size. Also, surface area of Fe3O4@COF and Fe3O4@COF-TFA are 74 and 12 m2.g−1, respectively. Fe3O4@COF-TFA was applied as a recoverable heterogeneous catalyst for construction of 2,3-disubstituted thiazolidine-4-ones and N‑amino-2-pyridones via a multi-component synthetic strategy. It is worth mentioning that synthesized derivatives have a good yield and short reaction times.
Keywords
Covalent organic framework
Heterogeneous catalyst
N‑amino-2-pyridones Thiazolidine-4-one
1 Introduction
Since the emergence of COFs as strong covalently linked two- and three-dimensional organic structures, these versatile materials have attracted considerable interest (Waller et al., 2015; Kandambeth et al., 2018). These reticular, sustainable as well as environmentally gentle materials possess unique properties such as high thermal and chemical stability, regulatable physicochemical characteristics, metal-free and flexible scaffolds, extremely low density, high surface area, tolerable charge-carrier mobility, and large pore sizes (Bayatani et al., 2023; Tan et al., 2023; Geng et al., 2020). COFs have been profoundly deliberated in electronics (Yang and Börjesson, 2022), energy storage (Cao et al., 2019), solar cells (Wu et al., 2018), gas capture and separation (Abdoli, 2023), water treatment (Wang et al., 2020), self-healing materials (Zhang et al., 2022), catalysis (Yusran et al., 2020) and drug delivery (Bai et al., 2016). In recent years, COFs have been enormously applied in many catalytic transformations (Guo and Jiang, 2020; Yarie, 2021; Torabi, 2021). These materials due to intrinsic catalytic activities, well-defined active sites, designability and excellent stability have a robust partnership in catalytic and photocatalytic processes (Yang et al., 2020), CO2 fixation utility (Yang et al., 2020), multi-component reactions (Yao et al., 2021), electrocatalytic processes (Chen et al., 2022) and coupling reactions (Qiu et al., 2020). Due to the easy post-modification of COFs by active metallic groups, acidic or basic moieties and ionic sections, it is possible to design robust catalytic systems in a targeted approach (Gao et al., 2019; Segura et al., 2019). Also, to address the limitations of COFs and improve their catalytic activity, these compounds can be designed as hybrid composites by incorporation of magnetic nanoparticles, graphene oxides, carbon nanotubes, MXenes and MOFs (Zhuang and Wang, 2021; Zhao et al., 2021; Wang et al., 2022; Cai et al., 2019).
In the last years, the development, and applications of magnetic nanoparticles (MNPs) have been the subject of intensive research by many industrial and academic researchers. They have motivated them to focus on synthetic routes and extraordinary as well as vast applications of MNPs (Ma et al., 2021; Mokhtary, 2016). The lucrative utilities of MNPs in medicine, biosensing, agriculture, drug delivery and catalytic processes is undeniable (Zandipak and Sobhanardakani, 2018; Sobhanardakani et al., 2018). MNPs due to their high thermal and chemical stability, ease of separation and reuse, high specific surface area, cheap and accessible as well as environmentally friendly nature are constantly gaining much attention (Gloag et al., 2019). Due to the excellent performance of MNPs, these compounds have found their position as an integral part of new sciences and appear as hybrid systems with other useful chemical compounds such as MOFs, COFs, DESs, etc. (Zhao et al., 2019; Torabi et al., 2022; Torabi et al., 2020; Zhuang et al., 2020; Govan and Gun’ko, 2014). Hence, these materials have represented good catalytic applications in many organic reactions such as multi-component (Torabi et al., 2021), oxidation/reduction (Payra et al., 2017), coupling (Shaikh and Zahir, 2022), asymmetric synthesis (Primitivo et al., 2021) and protection processes (Khaef et al., 2020).
Recently, the incorporation of MNPs within the COFs to achieve unique heterogeneous catalytic systems has received the attention of chemists and their emphasis. (Sharma et al., 2020). On the other hand, modification of heterogeneous catalysts by ionic liquids can enhance their catalytic utilities (Ghasemi et al., 2020). Due to the fruitful performance of ionic liquids in catalytic transformations, there are several reports regarding MNPs, or COFs decorated by ionic liquid. Therefore, the functionalization of MNPs-COF hybrids by ionic sections deserves more attention because it can open a new window to the world of catalysis (Anizadeh et al., 2022; Chen et al., 2021; Alishahi et al., 2023).
According to the literatures, 2-pyridone containing heterocycles have a brilliant background in many areas such as functional materials, organic dyes, agricultural compounds, versatile synthons, biological compounds, and pharmaceutical molecules (Zarei et al., 2023; Tavassoli et al., 2023; Fujii et al., 2013). Also, 2-pyridones are widely found in natural products such as amrinone, milrinone, and ciclopirox (Hernández et al., 2013; Fossa et al., 2003). In addition, N‑amino-2-pyridones as one of the most important families of 2-pyridone, have been widely studied by chemists. Fabulous medicinal properties such as antimicrobial (Ahadi et al., 2021), anticancer (Amer et al., 2021), anticoagulant (Babaee et al., 2020), anti-HIV (Zhang et al., 2022) antimalarial (Sangwan et al., 2022), anticonvulsant (Keshk et al., 2021), antimalarial (Hurtado-Rodríguez et al., 2022), and antihypertensive (Hernández, xxxx) have been reported for N‑amino-2-pyridone bearing molecules.
Due to their fundamental and superior applications in vast domains of chemistry such as natural products, agricultural chemistry, pharmaceuticals, and functional materials, thiazolidine-4-one scaffolds have gained significant importance (Tawfeek et al., 2022). However, the most important uses of thiazolidine-4-ones is their exceptional and innumerable pharmaceutical and medicinal utilities such as anticancer (Ansari et al., 2020), antioxidant (Jaiswal et al., 2019), anti-infammatory (Jaiswal et al., 2019), cardiovascular (Jaiswal et al., 2019), antimicrobial (Jaiswal et al., 2019) and anti-HIV (Jaiswal et al., 2019). Furthermore, these compounds are well-known in hybrid drugs which are as good as marketed drugs (Khan et al., 2022).
In continuous of our research on MCOFs (Alishahi et al., 2023), we designed and synthesized a new pyridine-based MCOF decorated with trifluoroacetate as ionic sections namely Fe3O4@COF-TFA. After the characterization of Fe3O4@COF-TFA, its catalytic behavior was tested for the preparation of 2,3-disubstituted thiazolidine-4-ones and N‑amino-2-pyridones via a multi-component strategy (Baharfar et al., 2019) (Scheme 1; 2).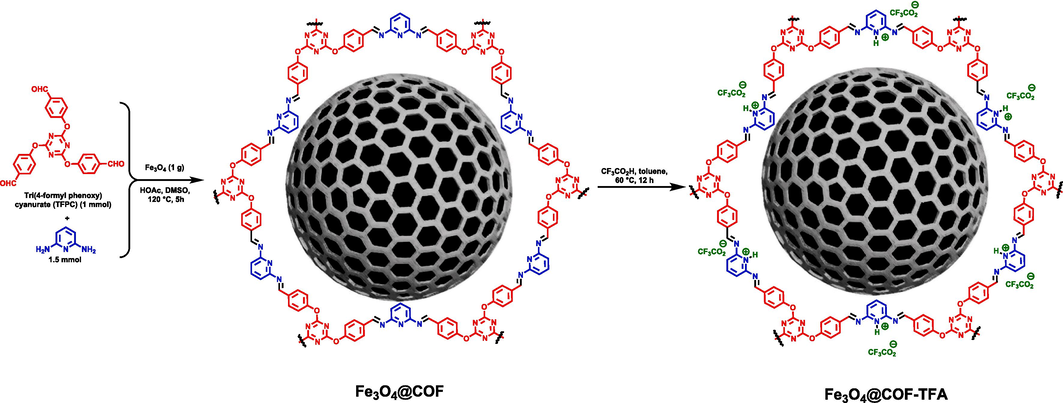
Synthesis of Fe3O4@COF and Fe3O4@COF-TFA.
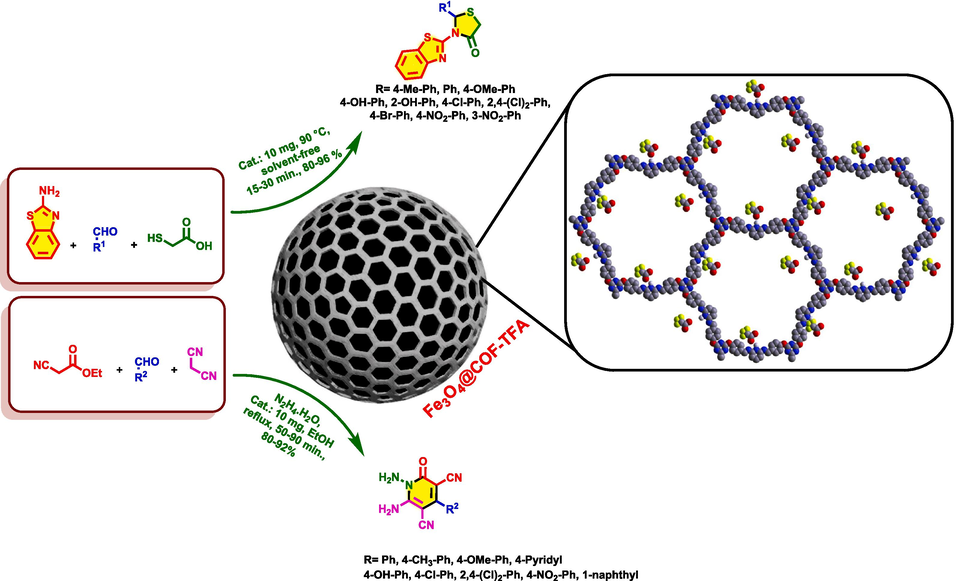
General route for the catalytic preparation of thiazolidine-4-ones and N‑amino-2-pyridones.
2 Results and discussion
Magnetic catalysts and catalytic systems are currently attracting great attention from researchers and industries. In continuous of our previous research on MCOFs (Alishahi et al., 2023), Fe3O4@COF-TFA was designed, constructed and applied as a catalyst for the multi-component synthesis of thiazolidine-4-ones and N‑amino-2-pyridones. At first, various characterization techniques were used to approve the structure, morphology, and physical properties of Fe3O4@COF-TFA. In continued its application in the synthesis of thiazolidine-4-ones and N‑amino-2-pyridones was explored.
2.1 Characterization
Several characteristic techniques supported the proposed chemical structure of Fe3O4@COF-TFA. The FT-IR spectrum of tri(4-formyl phenoxy) cyanurate (TFPC) illustrates stretching vibrations characteristic of C=O at 1701 cm−1 and the breathing mode of the triazine ring is observed at 808 cm−1 (See ESI). Also, the FT-IR spectrum of 2,6-diaminopyridine shows the typical peaks of NH2 about at 3372 and 3392 cm−1 and C=N bond (pyridine ring) at 1627 cm−1 (Fig. 1a). The disappearance of C=O in FT-IR of TFPC and NH2 signals of 2,6-diaminopyridine as well as the appearance of a new signal about 1631 cm−1 (imine functional group) verified the successful synthesis of Fe3O4@COF (Fig. 1b). Also, according to the FT-IR spectrum of Fe3O4@COF-TFA (Fig. 1c), the existence of broader peaks compared to the previous step confirmed the successful addition of TFA to Fe3O4@COF. Of note, the new peak at 1643 cm−1 can be related to the carbonyl functional group of TFA.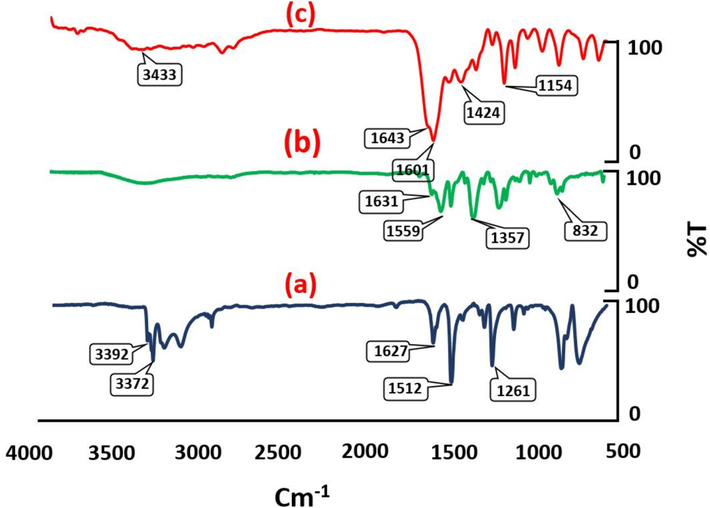
(a) FT-IR spectrum of 2,6-diaminopyridine, (b) FT-IR spectrum of Fe3O4@COF, (c) FT-IR spectrum of Fe3O4@COF-TFA.
EDS spectra of Fe3O4@COF and Fe3O4@COF-TFA samples were illustrated in Fig. 2a-b, which is well in accordance with our expectations. It is worth mentioning that C, N, Fe, and O signals for Fe3O4@COF and C, N, Fe, O and F signals for Fe3O4@COF-TFA can be seen in EDS results which appearance of F signal, in turn, confirms the chemical structure of Fe3O4@COF-TFA. In addition, the uniform distribution of elements in elemental mapping analysis confirmed the EDS results (Figs. 3, 4).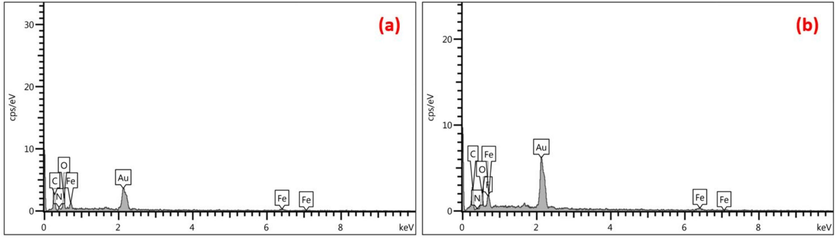
EDS spectra of (a) Fe3O4@COF and (b) Fe3O4@COF-TFA.
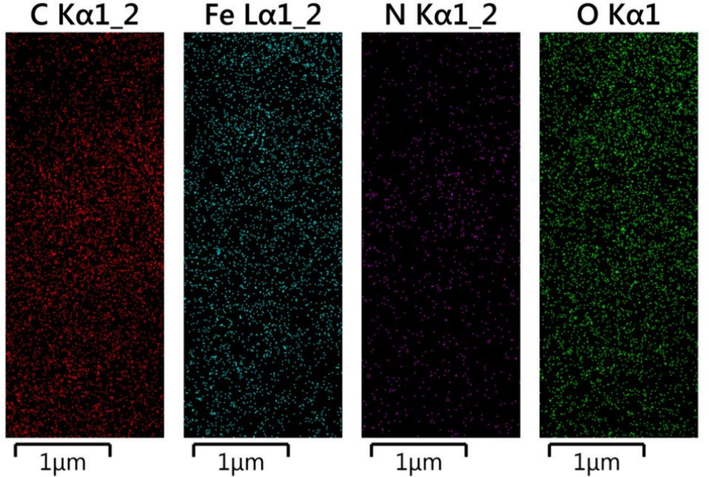
Elemental distribution of Fe3O4@COF.
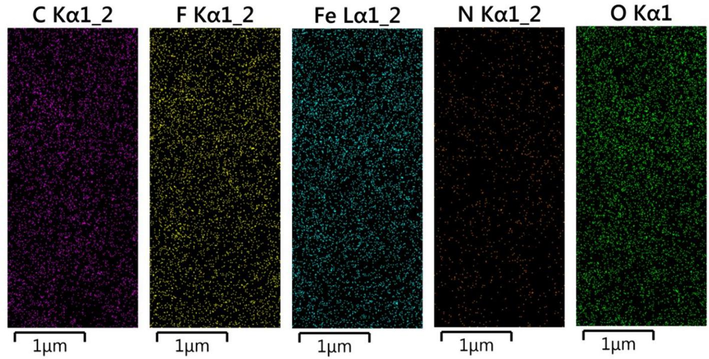
Elemental distribution of Fe3O4@COF-TFA.
FE-SEM and TEM analysis were used to investigate the morphology of the prepared MCOFs. According to FE-SEM images, the island-like morphologies were illustrated for both Fe3O4@COF (Fig. 5a-b) and Fe3O4@COF-TFA (Fig. 5c-d). Some parts have filamentous morphologies which confirmed the construction of polymeric scaffolds at the surface of Fe3O4. Nevertheless, the relatively porous structure for Fe3O4@COF and Fe3O4@COF-TFA was revealed by these images. TEM analysis was applied as proof of FE-SEM images. Accordingly, TEM images show the spherical geometry for the structure of Fe3O4@COF (Fig. 5e-f) and Fe3O4@COF-TFA (Fig. 5g-h) and the organic layers are well represented at the surface of Fe3O4. In addition, TEM images revealed that the size of the magnetic nanoparticles in both Fe3O4@COF and Fe3O4@COF-TFA are in nanometer scale (Fig. 5e-h).
FESEM images of Fe3O4@COF (a, b), Fe3O4@COF-TFA (c, d) and TEM images of Fe3O4@COF (e, f) and Fe3O4@COF-TFA (g, h).
Nitrogen adsorption/desorption isotherms of Fe3O4@COF and Fe3O4@COF-TFA are depicted in Fig. 6. According to these diagrams, the mesoporous structure of the prepared compounds is approved. In addition, the BET surface area of Fe3O4@COF and Fe3O4@COF-TFA are 74 and 12 m2.g−1, respectively. This reduction in the amount of surface area can be attributed to the post-synthetic modification of Fe3O4@COF by trifluoroacetic acid. In addition, The BJH analysis was used to determine the pore size distribution and the results show the pore size distribution for Fe3O4@COF and Fe3O4@COF-TFA mainly lies between 2 to 20 nm and 2 to 60 nm respectively (Fig. 7).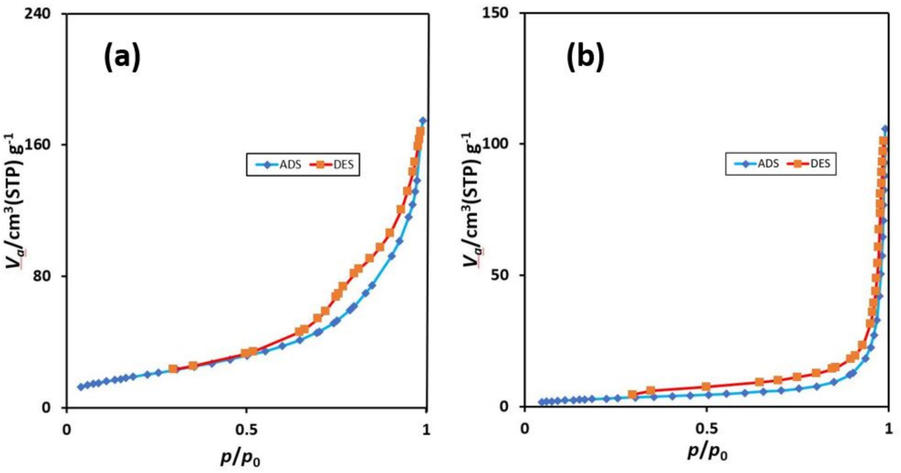
Nitrogen adsorption/desorption isotherms of (a) Fe3O4@COF and (b) Fe3O4@COF-TFA.
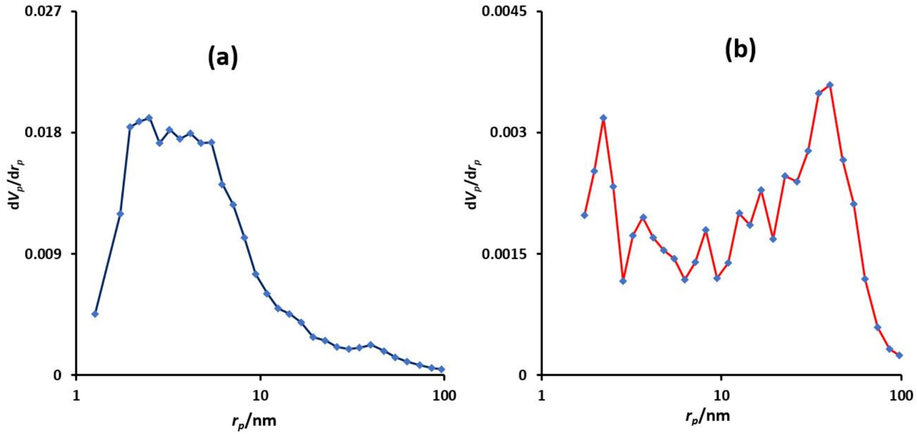
Pore size distribution curves of Fe3O4@COF (a) and Fe3O4@COF-TFA (b).
In another study, the magnetic properties of the prepared MCOFs were measured by using VSM analysis. Both Fe3O4@COF and Fe3O4@COF-TFA have a remarkable magnetization value which are about 50 and 40 emu/g, respectively which guarantees their recovering feasibility by using a suitable external magnet. Nevertheless, the magnetic value of Fe3O4 is about 70 emu/g (Alishahi et al., 2023) which shows the reduction in the magnetic value of Fe3O4@COF in comparison to Fe3O4 confirmed the successful addition of organic layers to Fe3O4 nanoparticles. Also, the diminishing of the magnetic value for Fe3O4@COF-TFA is due to the addition of TFA to the supported organic layers on Fe3O4@COF (Fig. 8).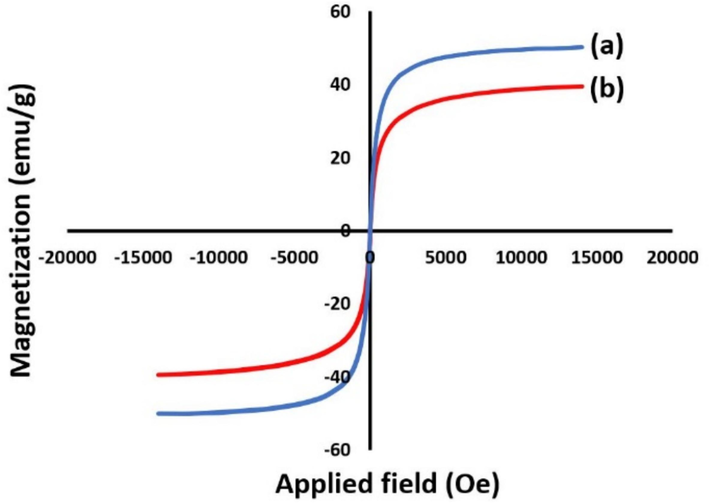
VSM curves of (a) Fe3O4@COF and (b) Fe3O4@COF-TFA.
The study of the thermal stability of MCOFs is imperative. Bearing this idea in mind, TG/DTG analysis was used for the evaluation of the thermal stability of Fe3O4@COF-TFA. The analysis was performed from an ambient temperature up to 600 ℃ under an inert atmosphere. According to TG/DTG curves (Fig. 9), Fe3O4@COF-TFA has excellent thermal stability up to about 450 ℃ and gradual weight loss up to 165 ℃ is because of the evaporation of trapped solvents from the pores and surface of Fe3O4@COF-TFA. The weight loss up to about 40 % before 600 ℃ confirmed the presence of acceptable amounts of organic layers on the surface of Fe3O4. According to XRD analysis, although the both Fe3O4@COF and Fe3O4@COF-TFA has an amorphous structure, but the diagnostic signals related to Fe3O4 by cubic lattice are at 30◦, 35◦, 43◦, 53◦,57◦ and 62◦ confirmed the preservation of the crystalline structure of Fe3O4 (Fig. 10).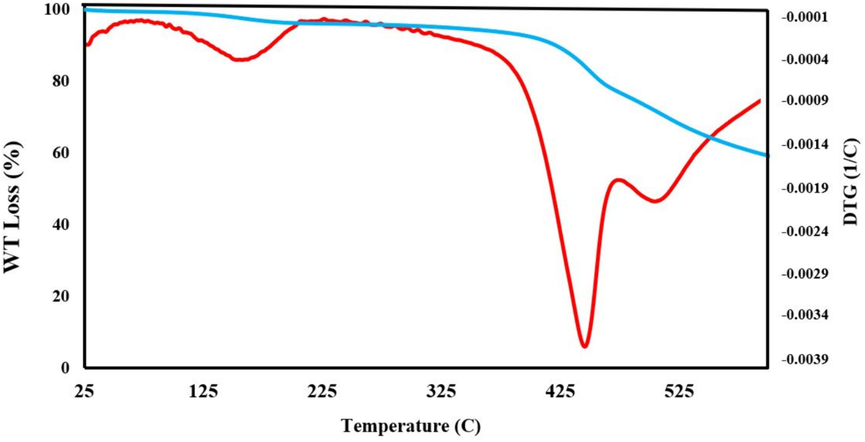
TGA/DTG curves of Fe3O4@COF-TFA.
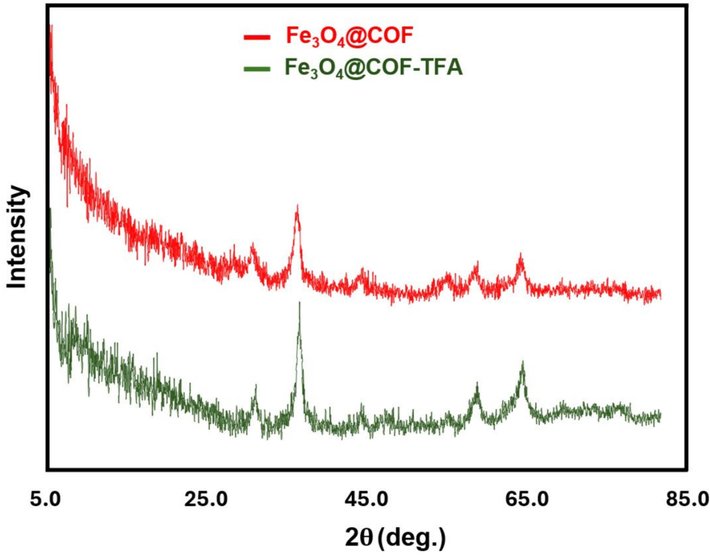
XRD pattern of Fe3O4@COF and Fe3O4@COF-TFA.
The predictable catalytic potential of Fe3O4@COF-TFA, encouraged us to use it as a heterogeneous catalyst for the construction of the 2,3-disubstituted thiazolidine-4-ones and N‑amino-2-pyridones via multi-component synthetic route. Hence, we focused on finding the optimal reaction conditions. For this goal, the synthesis of molecule 1f was used as a model reaction among 2,3-disubstituted thiazolidine-4-ones and several influential parameters such as temperature, solvent and the amounts of the catalyst were explored. The model reaction does not have good progress in the absence of catalyst and only a negligible amount of product was obtained after 60 min. In the following, three amounts of catalyst including 5, 10 and 20 mg were used and according to the results, 10 mg Fe3O4@COF-TFA was selected as the best amount of catalyst. About optimization of temperature, the model reaction has admirable results at 90 ℃ compared to lower temperatures. In addition, the role of solvent was markedly studied, and the model reaction was carried out in different polar (protic and aprotic) and nonpolar solvents including H2O, MeOH, EtOH, CH2Cl2, EtOAc, CHCl3, and n-hexane. Nevertheless, the presence of the solvent did not impressive effect on the progress of reaction and solvent-free conditions were selected as the best conditions. More details can be seen in Table 1. Regarding the synthesis of N‑amino-2-pyridones, molecule 2f was selected as a model compound. Unlike the previous reaction, performing the model reaction in the presence of protic solvents such as EtOH and MeOH is more efficient than in solvent-free conditions. Anyway, considering the yield of desired product and green chemistry principles, the best results were obtained by using 10 mg Fe3O4@COF-TFA as a catalyst in refluxing EtOH (Table 2).

Entry
Solvent
Catalyst loading (mg)
Temperature (oC)
Time (min.)
Yieldb (%)
TON
TOF (min−1)
1
–
–
90
30
–
–
–
2
–
–
90
60
15
–
–
3
–
5
90
30
45
9000
300
4
–
10
90
30
93
9300
310
5
–
20
90
30
92
4600
153.3
6
–
10
110
30
95
9500
316.6
7
–
10
75
30
80
8000
266.6
8
–
10
50
40
60
4000
100
9
–
10
25
90
–
0
0
10
H2O
10
Reflux
30
_
0
0
11
CH3OH
10
Reflux
30
45
4500
150
12
C2H5OH
10
Reflux
30
30
3000
100
13
CH2Cl2
10
Reflux
30
40
4000
133.3
14
EtOAc
10
Reflux
30
52
5200
173.3
15
n-hexane
10
Reflux
30
–
−
−
16
CHCl3
10
Reflux
30
–
−
−
Entry
Solvent
Temperature (°C)
Catalyst loading (mg)
Time (min.)
Yield (%) b
TON
TOF (min−1)
1
−
70
10
60
50
5000
83.3
2
−
80
10
60
58
5800
96.6
3
−
90
10
60
62
6200
103.3
4
−
100
10
60
54
5400
90
5
EtOH
70
10
60
70
7000
116.6
6
EtOH
Reflux
10
60
90
9000
150
7
EtOH
Reflux
−
60
50
−
−
8
EtOH
Reflux
20
60
92
4600
76.6
9
EtOH
Reflux
15
60
75
5000
83.3
10
MeOH
Reflux
10
60
65
6500
108.3
11
n‐Hexane
Reflux
10
60
30
3000
50
12
EtOAc
Reflux
10
60
40
4000
66.6
13
H2O
Reflux
10
60
−
0
0
14
C2H5OH
Reflux
10
30
trace
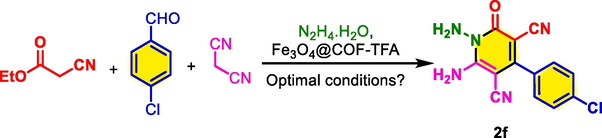
To account for the optimum reaction conditions for the synthesis of 2,3-disubstituted thiazolidine-4-ones and N‑amino-2-pyridones, the scope and limitations of the reactions were investigated upon using different substituted aryl-aldehydes. Therefore, an assortment of aromatic aldehydes with electron-poor and electron-rich aryl groups and, benzaldehyde was used for the synthesis of the desired molecules. It is worth mentioning that all the achieved molecules have good yields and short reaction times (Table 3, 4). Also, the melting points of the previously reported molecules are embedded in related tables (Kalhor and Banibairami, 2020; Mahapartra and Patanik, 1984; Dolganov et al., 2022).

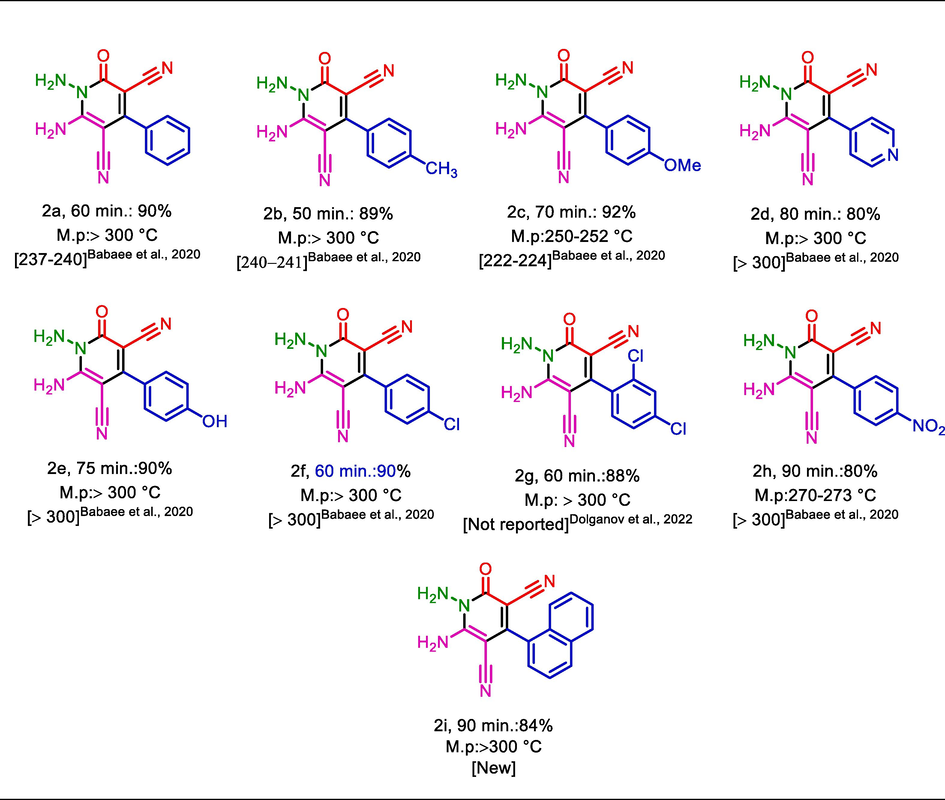
Also, in a comparative study, we discussed the catalytic activity and efficiency of Fe3O4@COF-TFA with some of the previously reported procedures for the preparation of 1b and 2a compounds. We found that the Fe3O4@COF-TFA shows a good and acceptable catalytic performance under mild reaction conditions with short reaction time and high yield of product. The recycling and reusing ability of Fe3O4@COF-TFA is another benefit of this system which this point is not observed in most of the previously reported catalysts (Table 5, 6).
Entry
Catalyst
Time
Yield (%)
1
Fe3O4@COF-TFA (10 mg), solvent-free, 90 ℃ [this work]
30 min.
80
2
1,4‑diethyl‑1,4‑diazabicyclo[2,2,2]octane‑1,4‑diium perchlorate (2 mol %), H2O, reflux (Pinate and Makone, 2023)
70 min.
88
3
zinc(II) chloride, benzene, reflux (Srivastava et al., 2008)
15 h
72
4
Fe3O4@SiO2@(CH2)3–urea–benzoic acid, solvent-free, 80 ℃ (Fazl et al., 2022)
15 min.
85
5
HClO4 immobilized on silica, toluene, 100 ℃ (Kumar et al., 2013)
6 h
70
Entry
Catalyst
Time
Yield (%)
1
Fe3O4@COF-TFA (10 mg), EtOH, reflux [this work]
60 min.
90
2
Piperidine (2 mol%), H2O, 20 ℃ (Hosseini and Bayat, 2018)
12 h
90
3
Potassium fluoride impregnated over alumina EOH/H2O, 20 ℃ (Kshiar et al., 2018)
20 min
87
4
Cobalt sulfide (0.4 mol%), EtOH, reflux (Safaei-Ghomi et al., 2019)
35 min.
88
5
Zinc(II) oxide (8 mol%), EtOH, reflux (Safaei-Ghomi et al., 2014)
40 min.
91
6
Piperidine, EtOH, 80 ℃ (Ranjbar-Karimi et al., 2018)
50 min.
50
To investigate the key role of the components of this hybrid catalytic system, the 1f molecule was prepared in the presence of Fe3O4, Fe3O4@COF, and TFA compared to Fe3O4@COF-TFA. The results showed that each of these catalysts can have a catalytic role to some extent. The free hydroxy groups of Fe3O4 have inherent catalytic activity and high specific surface of COF increased the number of effective interactions. Therefore, their synergistic effects with TFA can improve the catalytic potential of Fe3O4@COF-TFA (Table 7).
Entry
Catalyst
Load of catalyst
Yield (%)
1
Fe3O4@COF-TFA
10 mg
93
2
Fe3O4
10 mg
25
3
Fe3O4@COF
10 mg
44
4
TFA
10 mol%
50
Also, we suggested a plausible mechanistic route for the synthesis of 2,3-disubstituted thiazolidine-4-ones by using Fe3O4@COF-TFA as a heterogeneous catalyst (Scheme 3). In this mechanism, firstly, the carbonyl group of aryl aldehyde is activated by the catalyst and subjected to a nucleophilic attack reacted from 2-aminobenzothiazole to achieve the intermediate a. Then, the reaction of intermediate a with thioglycolic acid leads to the formation of intermediate b. In the next step, via a nucleophilic intermolecular cyclization as well as the removal of H2O, the target product is synthesized.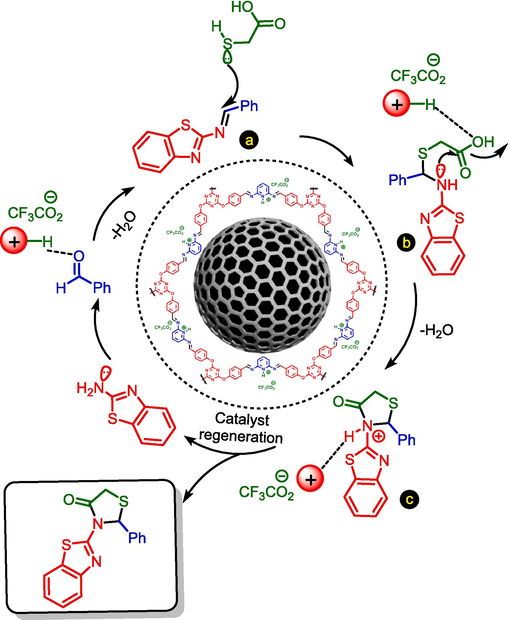
The suggested mechanistic route for the synthesis of 2,3-disubstituted thiazolidine-4-ones by using Fe3O4@COF-TFA as a catalyst.
Moreover, we suggested a plausible mechanism for synthesized N-amino-2-pyridones. At first, aryl aldehyde is activated by an acidic section of the catalyst and reacts with activated malononitrile which leads to Knoevenagel adduct a. Also, hydrazine hydrate reacts by ethyl cyanoacetate, and intermediate b is produced. In the next step, due to the reaction of intermediate b by intermediate a, intermediate c is performed. Continuously, intermediate f is performed via intermolecular cyclization reaction as well as two tautomerization processes. Finally, the target product is achieved via a cooperative vinylogous anomeric-based oxidation (Alabugin et al., 2021) (Scheme 4).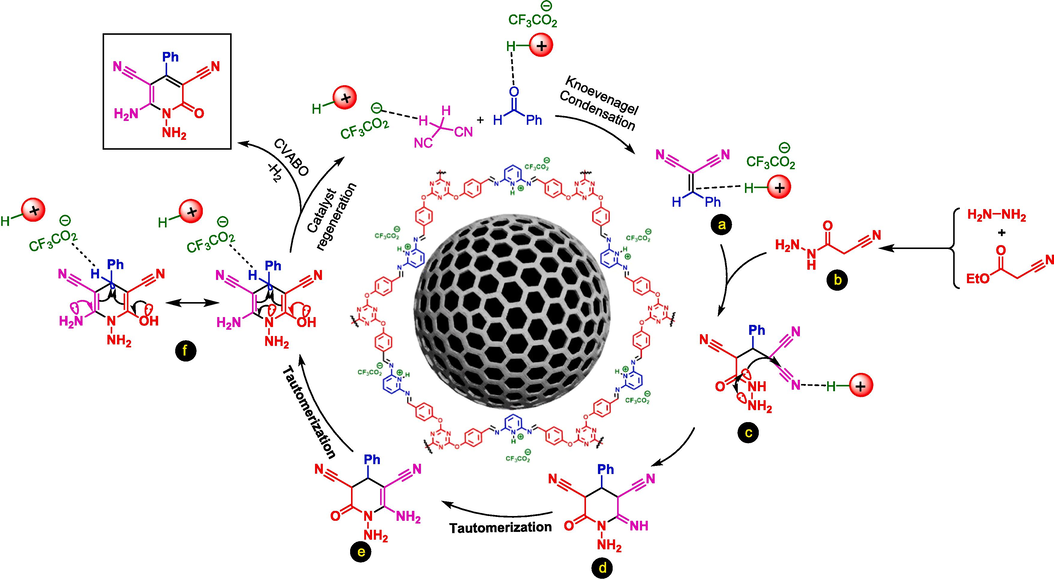
The suggested plausible mechanism for the preparation of N–amino-2-pyridones by using Fe3O4@COF-TFA as a catalyst.
With this in mind that recovering and reusing ionic catalysts is convenient, one of our goals for the design of Fe3O4@COF-TFA is its easy recovering and reusing capability. Hence, we delve to the recovery test of Fe3O4@COF-TFA towards the preparation of desired molecule 1f under optimal reaction conditions. After completing each run of the model reaction, Fe3O4@COF-TFA was separated from the mixture of the reaction by using an external magnet and washed with EtOH three times, desiccated, and used in the next run. The results prove that this catalyst can be recovered and reused up to 5 times (Fig. 11). In addition, FESEM and FT-IR analyses were carried out from the recovered catalyst which exhibits that the catalyst has good stability, and its morphology has not changed after running the reaction (ESI).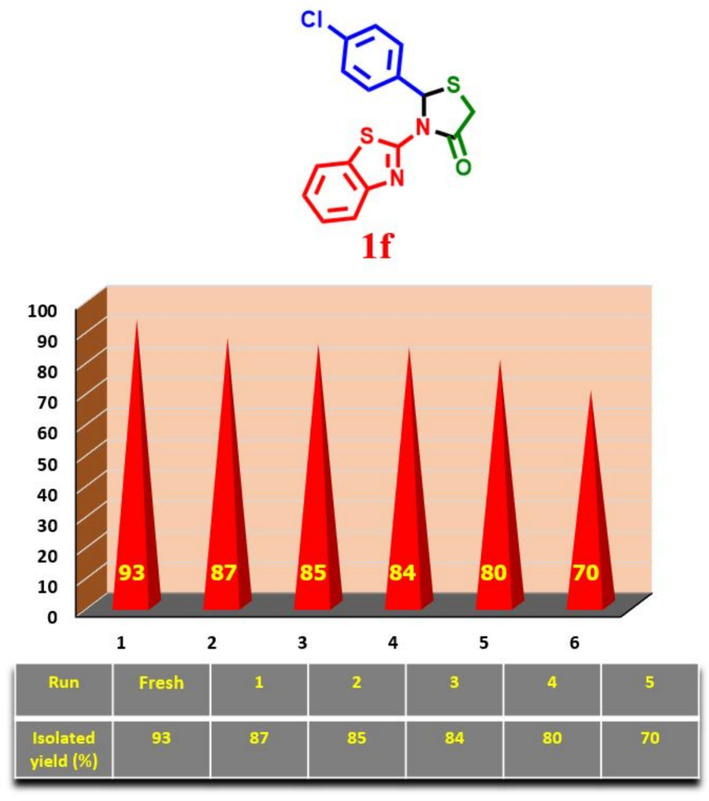
Recovering and reusing test of Fe3O4@COF-TFA in the synthesis of 1f under optimum conditions.
3 Experimental section
3.1 Synthesis of Fe3O4@COF-TFA
At first, required chemical compounds including Fe3O4 (Torabi et al., 2021) and TFPC (Torabi et al., 2023) were synthesized based on the previously reported methods. The desired products were synthesized according to our recently reported educational synthetic organic method (Zolfigol et al., 2024). In the next step, TFPC (1 mmol, 0.441 g), 2,6-diaminopyridine (1.5 mmol, 0.163 g), Fe3O4 (1 g) and dimethyl sulfoxide (50 mL) were added into a 100 mL flask and sonicated at room temperature for 30 min. After that, 2 mL of acetic acid as a catalyst was added to the flask and heated at 120 ℃ for 5 h. Then, bulky precipitate was separated by using an external magnet and was washed several times with THF, DCM and MeOH. Then, the precipitate was dried at 100 ℃ for 12 h. In the next step, Fe3O4@COF (1 g) was treated with TFA (0.2 mL) in toluene at 60 ℃ for 12 h and finally washed by EtOH (3 20 mL) and dried at 80 ℃.
3.2 Preparation of 2,3-disubstituted thiazolidine-4-one derivatives by using Fe3O4@COF-TFA as a catalyst
Aryl aldehyde derivatives (1 mmol), 2-aminobenzothiazole (1 mmol, 0.150 g), thioglycolic acid (1 mmol, 0.092 g) and Fe3O4@COF@TFA (0.01 g) were added to the round bottom flask under solvent-free conditions and heated at 90 ℃. The progress of the reaction was monitored by using TLC technique. By completing of reaction, the organic compounds were dissolved in DCM (20 mL) while the catalyst is insoluble and was separated by using an external magnet. After that, the DCM was removed and the remained solid was washed by EtOH to yield pure products. Finally, the products were desiccated at 80 ℃.
3.3 Preparation of N-amino-2-pyridones derivatives by using Fe3O4@COF-TFA as a catalyst
In a 10 mL round-bottomed flask, aryl aldehyde (1 mmol), ethyl cyanoacetate (1 mmol, 0.113 g), malononitrile (1 mmol, 0.066 g) and hydrazine monohydrate (1 mmol, 0.050 g), Fe3O4@COF-TFA (0.01 g) and EtOH (10 mL) were added, and the mixture of reaction was vigorously stirred under reflux conditions. The progress of reactions was checked out by TLC techniques. After completing of reaction, the catalyst was separated from the mixture of the reaction. Then, the solvent was removed and the remained solid was washed by cold EtOH to yield pure products. Finally, the products were desiccated at 80 ℃.
3.4 Spectral data
3.4.1 3-(Benzo[d]thiazol-2-yl)-2-phenylthiazolidine-4-one (1b)
M.p. = 168–170 °C, 1H NMR (301 MHz, DMSOd6) δppm 8.06 (d, J=6 Hz, 1H, Aromatic), 7.76 (d, J=3 Hz, 1H, Aromatic), 7.67 (d, J=9 Hz, 1H, Aromatic), 7.46 – 7.34 (m, 5H, Aromatic), 7.21 (d, J=9 Hz, 1H, Aromatic), 6.95 (s, 1H, CH), 4.25 (d, J=15 Hz, 1H, CH), 4.10 (d, J=15 Hz, 1H, CH). 13C NMR (76 MHz, DMSOd6) δppm 172.2, 156.4, 148.0, 137.2, 133.8, 132.5, 130.1, 128.4, 126.9, 125.0, 122.5, 121.9, 60.4, 32.2.
3.4.2 3-(Benzo[d]thiazol-2-yl)-2-(4-methoxyphenyl)thiazolidine-4-one (1c)
M.p. = 159–161 °C, FT-IR (KBr, υ, cm−1): 3063, 1697, 1505, 1441, 1375, 758. 1H NMR (301 MHz, DMSOd6) δppm 8.04 (d, J=9 Hz, 1H, Aromatic), 7.70 (d, J=9 Hz, 1H, Aromatic), 7.46–7.33 (m, 4H, Aromatic), 6.91 – 6.88 (m, 3H, Aromatic and CH), 4.33 (d, J=15 Hz, 1H, CH), 4.05 (d, J=15 Hz, 1H, CH), 3.72 (s, 3H, CH3). 13C NMR (76 MHz, DMSOd6) δppm 172.2, 159.4, 156.4, 148.2, 133.5, 131.8, 127.5, 126.8, 124.9, 122.4, 121.8, 63.1, 55.6, 32.4.
3.4.3 3-(Benzo[d]thiazol-2-yl)-2-(2-hydroxyphenyl)thiazolidine-4-one (1e)
M.p. = 246–249 °C, 1H NMR (301 MHz, DMSOd6) δppm 10.17 (s, 1H, OH), 8.04 (s, 1H, Aromatic), 7.68 (s, 1H, Aromatic), 7.38 – 7.35 (m, 2H, Aromatic), 7.12 (s, 1H, Aromatic), 6.95–6.88 (m, 3H, Aromatic), 6.71 (s, 1H, CH), 4.15 (d, J=15 Hz, 1H, CH), 4.3 (d, J=15 Hz, 1H, CH). 13C NMR (76 MHz, DMSOd6) δppm 172.2, 156.4, 148.0, 137.2, 133.8, 132.5, 131.8, 130.1, 128.4, 127.4, 126.9, 125.0, 122.5, 121.9, 60.4, 32.2.
3.4.4 3-(Benzo[d]thiazol-2-yl)-2-(2,4-dichlorophenyl)thiazolidine-4-one (1 g)
M.p. = 220–222 °C, FT-IR (KBr, υ, cm−1): 2986, 1702, 1508, 1470, 1369, 751. 1H NMR (301 MHz, DMSOd6) δppm 8.03 (d, J=9 Hz, 1H, Aromatic), 7.68 (d, J=9 Hz, 1H, Aromatic), 7.42 – 7.24 (m, 5H, Aromatic), 6.94 (s, 1H, CH), 4.31 (d, J=15 Hz, 1H, CH), 4.05 (d, J=15 Hz, 1H, CH). 13C NMR (76 MHz, DMSOd6) δppm 172.2, 156.5, 148.1, 141.6, 131.8, 129.2, 128.5, 126.9, 125.9, 124.9, 122.4, 121.8, 63.2, 32.4.
3.4.5 3-(Benzo[d]thiazol-2-yl)-2-(4-bromophenyl)thiazolidine-4-one (1 h)
M.p. = 197–200 °C, FT-IR (KBr, υ, cm−1): 2970, 1703, 1508, 1444, 1374, 1444, 761. 1H NMR (301 MHz, DMSOd6) δppm 8.05 (d, J=9 Hz, 1H, Aromatic), 7.68 (d, J=9 Hz, 1H, Aromatic), 7.56–7.33 (m, 6H, Aromatic), 6.94 (s, 1H, CH), 4.33 (d, J=15 Hz, 1H, CH), 4.06 (d, J=15 Hz, 1H). 13C NMR (76 MHz, DMSOd6) δppm 172.2, 156.5, 148.1, 141.6, 131.8, 129.2, 128.5, 126.8, 125.9, 124.9, 122.4, 121.8, 63.2, 32.4.
3.4.6 1,6-Diamino-2-oxo-4-phenyl-1,2-dihydropyridine-3,5-dicarbonitrile (2a)
M.p. = 300 °C, FT-IR (KBr, υ, cm−1): 3455, 3402, 3307, 3253, 2222, 2208, 1643, 1611, 1464. 1H NMR (301 MHz, DMSOd6) δppm 8.51 (s, 2H, NH2), 7.86–7.42 (m, 5H, Aromatic), 5.70 (s, 2H, NH2). 13C NMR (76 MHz, DMSOd6) δppm 160.1, 159.8, 157.2, 135.1, 130.7, 129.1, 128.5, 116.9, 116.0, 86.9, 74.9, 40.8, 40.5, 40.2, 39.9, 39.7, 39.4, 39.1.
3.4.7 1,6-Diamino-2-oxo-4-(p-tolyl)-1,2-dihydropyridine-3,5-dicarbonitrile (2b)
M.p. = 300 °C, FT-IR (KBr, υ, cm−1): 3446, 3399, 3307, 3245, 2219, 2206, 1642, 1618, 1599, 1525. 1H NMR (301 MHz, DMSOd6) δppm 8.48 (s, 2H, NH2), 7.51–7.18 (m, 4H, Aromatic), 5.68 (s, 2H, NH2), 2.41 (s, 3H, CH3). 13C NMR (76 MHz, DMSOd6) δppm 160.1, 159.8, 157.2, 140.6, 132.1, 129.6, 128.5, 117.0, 116.1, 86.8, 74.8, 40.8, 40.5, 40.3, 40.0, 39.7, 39.4, 39.1, 21.5.
3.4.8 1,6-diamino-4-(2,4-dichlorophenyl)-2-oxo-1,2-dihydropyridine-3,5-dicarbonitrile (2 g)
M.p. = 300 °C, FT-IR (KBr, υ, cm−1): 3412, 3291, 31943081, 2224, 1671, 1674, 1608, 1568. 1H NMR (301 MHz, DMSOd6) δppm 8.67 (s, 2H, NH2), 7.93 (d, J=2.0 Hz, 1H, Aromatic), 7.69–7.66 (m, 1H, Aromatic), 7.59–7.57 (m, 1H, Aromatic), 5.72 (s, 2H, NH2). 13C NMR (76 MHz, DMSOd6) δppm 159.4, 157.0, 156.8, 136.1, 133.2, 132.4, 131.7, 130.0, 128.6, 116.0, 115.1, 87.5, 75.3, 40.8, 40.5, 40.3, 40.0, 39.7, 39.4, 39.2.
3.4.9 1,6-diamino-4-(naphthalen-1-yl)-2-oxo-1,2-dihydropyridine-3,5-dicarbonitrile (2i)
M.p. = 300 °C, FT-IR (KBr, υ, cm−1): 3400, 3325, 3274, 3217, 2217, 1638, 1589, 1523, 1474, 779. 1H NMR (301 MHz, DMSOd6) δppm 8.56 (s, 2H, NH2), 8.13–8.07 (m, 2H, Aromatic), 7.75–7.55 (m, 5H, Aromatic), 5.72 (s, 2H, NH2). 13C NMR (76 MHz, DMSOd6) δppm 159.7, 159.4, 157.1, 133.5, 133.0, 130.4, 129.9, 129.0, 127.8, 127.1, 126.4, 125.9, 124.9, 116.5, 115.6, 88.5, 76.4, 40.8, 40.6, 40.3, 40.0, 39.7, 39.4, 39.2.
4 Conclusion
In summary, an ionic containing magnetic COF was successfully synthesized by constructing imine-linked COF on the surface of Fe3O4 and post-synthetic acidification approach by using TFA. The obtained Fe3O4@COF-TFA was precisely characterized by FT-IR, TGA/DTG, FESEM, EDS, elemental mapping, TEM, VSM and XRD analysis. Fe3O4@COF-TFA due to its porosity and catalytic active sites, exhibit excellent catalytic performance in the multi-component synthesis of 2,3-disubstituted thiazolidine-4-one and N–amino-2-pyridone derivatives. The objected multi-component reactions were performed under mild conditions with selectivity and high yield of products. N–amino-2-pyridones derivatives were synthesized in EtOH as a green solvent. On the other hand, the recovery and reusability of catalyst as a critical point of ionic catalytic systems were investigated. Accordingly, Fe3O4@COF-TFA was recovered and reused up to 5 times in the synthesis of 1f. Therefore, this work demonstrates a new approach to the development of COF-based catalysts for many catalytic transformations.
CRediT authorship contribution statement
Erfan Abdoli: Investigation, Methodology. Morteza Torabi: Conceptualization, Investigation, Methodology, Writing – original draft. Meysam Yarie: Conceptualization, Investigation, Methodology, Validation, Writing – original draft. Mohammad Ali Zolfigol: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Writing – review & editing.
Acknowledgments
We thank the Bu-Ali Sina University for financial support to our research group.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Catalytic applications of porous organic polymers in CO2 fixation. Iran. J. Catal.. 2023;13:379.
- [Google Scholar]
- Synthesis and decarboxylation of functionalized 2-pyridone-3-carboxylic acids and evaluation of their antimicrobial activity and molecular docking. Iran. J. Pharm. Res.. 2021;20:456.
- [Google Scholar]
- (a). Alabugin, I. V., Kuhn, L., Medvedev, M. G., Krivoshchapov, N. V., Vil, V. A., Yaremenko, I. A., Mehaffy, P., Yarie, M., Terent’ev, A. O., Zolfigol, M. A., 2021. Stereoelectronic power of oxygen in control of chemical reactivity: the anomeric effect is not alone. Chem. Soc. Rev. 50, 10253. (b) Alabugin, I.V., Kuhn, L., Krivoshchapov, N.V., Mehaffy, P., Medvedev, M. G. 2021. Anomeric effect, hyperconjugation and electrostatics: Lessons from complexity in a classic stereoelectronic phenomenon. Chem. Soc. Rev. 50, 10212. (c) Tavassoli, A., Yarie, M., Torabi, M., Zolfigol, M. A., 2023. Application of novel magnetic H-bonding catalyst for synthesis of hybrid pyridine-triazole derivatives bearing indole or sulfonamide segments. J. Phys. Chem. Solids, 186, 111786. (d) Zarei, N., Zolfigol, M. A., Torabi, M., Yarie, M., 2023. Synthesis of new hybrid pyridines catalyzed by Fe3O4@ SiO2@ urea-riched ligand/Ch-Cl. Sci. Rep. 13, 9486.
- Nanoarchitectonics of magnetic covalent organic framework with sulfonic acid tags for catalytic preparation of triazolo quinazolinones and 4H-pyrimidobenzothiazoles. J. Solid State Chem.. 2023;324:124119
- [Google Scholar]
- Recent advances in chemistry and pharmacological aspects of 2-pyridone scaffolds. J. Saudi Chem. Soc.. 2021;25:101259
- [Google Scholar]
- Urea-dithiocarbamic acid functionalized magnetic nanoparticles modified with Ch-Cl: Catalytic application for the synthesis of novel hybrid pyridones via cooperative geminal-vinylogous anomeric-based oxidation. J. Mol. Liq.. 2022;364:120016
- [Google Scholar]
- Rhodium (II)-catalyzed annulative coupling of β-ketothioamides with α-diazo compounds: access to highly functionalized thiazolidin-4-ones and thiazolines. J. Org. Chem.. 2020;85:8320.
- [Google Scholar]
- Synthesis of metal–organic frameworks MIL-101 (Cr)-NH2 containing phosphorous acid functional groups: Application for the synthesis of N-amino-2-pyridone and pyrano [2, 3-c] pyrazole derivatives via a cooperative vinylogous anomeric-based oxidation. ACS Omega. 2020;5:6240.
- [Google Scholar]
- Synthesis and characterization of MgO nanoparticles supported on ionic liquid-based periodic mesoporous organosilica (MgO@PMO-IL) as a highly efficient and reusable nanocatalyst for the synthesis of novel spirooxindole-furan derivatives. Appl. Organomet. Chem.. 2019;33:e4805.
- [Google Scholar]
- Nanoscale covalent organic frameworks as smart carriers for drug delivery. Chemcomm. 2016;52:4128.
- [Google Scholar]
- Fabrication of an imidazolium-based magnetic ionic porous organic polymer for efficient heterogeneous catalysis of Betti reaction. J. Mol. Liq.. 2023;390:122863
- [Google Scholar]
- One-step construction of hydrophobic MOFs@ COFs core–shell composites for heterogeneous selective catalysis. Adv. Sci.. 2019;6:1802365.
- [Google Scholar]
- (a). Cao, S., Li, B., Zhu, R., Pang, H., 2019. Design and synthesis of covalent organic frameworks towards energy and environment fields. Chem. Eng. J. 355, 602, (b) Yu, F., Li, C., Li, W., Yu, Z., Xu, Z., Liu, Y., Wang, B., Na, B., Qiu, J., 2024. Π‐Skeleton tailoring of olefin‐linked covalent organic frameworks achieving low exciton binding energy for photo‐enhanced uranium extraction from seawater. Adv. Func. Mater. 34, 2307230, (c) Wei, T., Sun, C., Guo, X., Zhou, Y., Wang, M., Qiu, X., Wang, Q., Tang, Y., 2024. Petaloid bimetallic metal-organic frameworks derived ZnCo2O4/ZnO nanosheets enabled intermittent lithiophilic model for dendrite-free lithium metal anode. J. Colloid Interface Sci. 664, 596.
- Construction of pyridine-based chiral ionic covalent organic frameworks as a heterogeneous catalyst for promoting asymmetric Henry reactions. Org. Lett.. 2021;23:1748.
- [Google Scholar]
- Methods to make conductive covalent organic frameworks for electrocatalytic applications. Chinese J. Struc. Chem.. 2022;41:2212107.
- [Google Scholar]
- 7-Aryl-3-(hydroxymethyl)-5-oxo-1, 2, 3, 5-tetrahydro [1, 2, 4] triazolo [1, 5-a] pyridine-6, 8-dicarbonitriles: Synthesis and predicted biological activity. Russ. J. Gen. Chem.. 2022;92:185.
- [Google Scholar]
- Synthesis and application of novel urea–benzoic acid functionalized magnetic nanoparticles for the synthesis of 2, 3-disubstituted thiazolidin-4-ones and hexahydroquinolines. RSC Adv.. 2022;12:16342.
- [Google Scholar]
- Synthesis and pharmacological characterization of functionalized 2-pyridones structurally related to the cardiotonic agent milrinone. Bioorg. Med. Chem.. 2003;11:4749.
- [Google Scholar]
- Post-synthetic modification of phenylboronic acid-functionalized magnetic covalent organic frameworks for specific enrichment of N-linked glycopeptides. ACS. Sustain. Chem. Eng.. 2019;7:18926.
- [Google Scholar]
- Covalent organic frameworks: design, synthesis, and functions. Chem. Rev.. 2020;120:8814.
- [Google Scholar]
- Ionically tagged magnetic nanoparticles with urea linkers: Application for preparation of 2-aryl-quinoline-4-carboxylic acids via an anomeric-based oxidation mechanism. ACS Omega. 2020;5:3207.
- [Google Scholar]
- Advances in the application of magnetic nanoparticles for sensing. Adv. Mater.. 2019;31:1904385.
- [Google Scholar]
- Recent advances in the application of magnetic nanoparticles as a support for homogeneous catalysts. Nanomater. 2014;4:222.
- [Google Scholar]
- Covalent organic frameworks for heterogeneous catalysis: principle, current status, and challenges. ACS. Cen. Sci.. 2020;6:869.
- [Google Scholar]
- Synthesis, ex vivo and in silico studies of 3-cyano-2-pyridone derivatives with vasorelaxant activity. Eur. J. Med. Chem.. 2013;70:669.
- [Google Scholar]
- Hernández, F., Sánchez, A., Rendón-Vallejo, P., Millán-Pacheco, C., Alcaraz, Y., Delgado, F., Estrada-Soto, S., Synthesis, ex vivo and in silico studies of 3-cyano-2-pyridone derivatives with vasorelaxant activity. Eur. J. Med. Chem. 70, 669.
- An efficient and ecofriendly synthesis of highly functionalized pyridones via a one-pot three-component reaction. RSC Adv.. 2018;8:27131.
- [Google Scholar]
- Bioactive 2-pyridone-containing heterocycle syntheses using multicomponent reactions. RSC Adv.. 2022;12:35158.
- [Google Scholar]
- [3+ 2]-annulation of azaoxyallyl cations and thiocarbonyls for the assembly of thiazolidin-4-ones. Org. Lett.. 2019;21:5848.
- [Google Scholar]
- Design of a new multi-functional catalytic system Ni/SO3H@zeolite-Y for three-component synthesis of N-benzo-imidazo-or-thiazole-1,3-thiazolidinones. RSC Adv.. 2020;10:41410.
- [Google Scholar]
- Covalent organic frameworks: chemistry beyond the structure. J. Am. Chem. Soc.. 2018;141:1807.
- [Google Scholar]
- Synthesis, physicochemical and vibrational spectral properties of 2–pyridone and 2–aminopyridine derivatives: An experimental and theoretical study. J. Mol. Struct.. 2021;1225:129136
- [Google Scholar]
- Catalytic application of sulfamic acid-functionalized magnetic Fe3O4 nanoparticles (SA-MNPs) for protection of aromatic carbonyl compounds and alcohols: Experimental and theoretical studies. RSC Adv.. 2020;10:44946.
- [Google Scholar]
- Mercaptobenzimidazole-Based 1, 3-thaizolidin-4-ones as antidiabetic agents: Synthesis, in vitro α-glucosidase inhibition activity, and molecular docking studies. ACS Omega. 2022;7:28041.
- [Google Scholar]
- A three component one-pot synthesis of N-amino-2-pyridone derivatives catalyzed by KF-Al2O3. Synth. Commun.. 2018;48:1816.
- [Google Scholar]
- Supported protic acid-catalyzed synthesis of 2, 3-disubstituted thiazolidin-4-ones: enhancement of the catalytic potential of protic acid by adsorption on solid supports. Green Chem.. 2013;15:2872.
- [Google Scholar]
- (a). Ma, Z., Mohapatra, J., Wei, K., Liu, J. P., Sun, S., 2021. Magnetic nanoparticles: Synthesis, anisotropy, and applications. Chem. Rev. 123, 3904, (b) Wu, L., Mendoza-Garcia, A., Li, Q., Sun, S., 2016. Organic phase syntheses of magnetic nanoparticles and their applications. Chem. Rev. 116, 10473.
- Mahapartra, B. D., Patanik, R. C., 1984. Fungicidal activities and mass spectral studies of some Schiff bases derived from p. hydroxy benzaldehyde and their derivatives. J. Ind. Chem. Soc. 1061.
- Recent advances in catalysts immobilized magnetic nanoparticles. J. Iran. Chem. Soc.. 2016;13:1827.
- [Google Scholar]
- Recent advances on Fe-based magnetic nanoparticles in organic transformations. J. Nanosci. Nanotechnol.. 2017;17:4432.
- [Google Scholar]
- Novel DABCO based acidic ionic liquid as a green protocol for the synthesis of thiazolidin-4-one derivatives and cytotoxic activity evaluation on human breast cancer cell line. J. Sulphur Chem.. 2023;44:20.
- [Google Scholar]
- Preparation and asymmetric induction evaluation of the first ephedrine-based ligands immobilized on magnetic nanoparticles. ACS Omega. 2021;6:35641.
- [Google Scholar]
- Hierarchically porous covalent organic frameworks assembled in ionic liquids for highly effective catalysis of C-C coupling reactions. Green. Chem.. 2020;22:2605.
- [Google Scholar]
- Dipyrido [1, 2-b: 3′, 4′-e][1, 2, 4] triazine scaffolds from pentafluoropyridine. J. Heterocycl. Chem.. 2018;55:2516.
- [Google Scholar]
- An efficient method for the synthesis of N-amino-2-pyridones using reusable catalyst ZnO nanoparticles. J. Chem. Res.. 2014;38:583.
- [Google Scholar]
- Nano-Co3S4 as a retrievable and robust catalyst for the synthesis of 2-oxo-pyridines and 5-oxo-[1, 2, 4] triazolo [2, 3-a] pyridines. Org. Prep. Proced. Int.. 2019;51:388.
- [Google Scholar]
- A score years’ update in the synthesis and biological evaluation of medicinally important 2-pyridones. Eur. J. Med. Chem.. 2022;232:114199
- [Google Scholar]
- Post-synthetic modification of covalent organic frameworks. Chem. Soc. Rev.. 2019;48:3903.
- [Google Scholar]
- Pd complex of ferrocenylphosphine supported on magnetic nanoparticles: A highly reusable catalyst for transfer hydrogenation and coupling reactions. J. Organomet. Chem.. 2022;973:122395
- [Google Scholar]
- Recent development of covalent organic frameworks (COFs): synthesis and catalytic (organic-electro-photo) applications. Mater. Horiz.. 2020;7:411-454.
- [Google Scholar]
- Removal of heavy metal (Hg (II) and Cr (VI)) ions from aqueous solutions using Fe2O3@ SiO2 thin films as a novel adsorbent. Process. Saf. Evironment. Protect.. 2018;120:348.
- [Google Scholar]
- Laparoscopic pyeloplasty with concomitant pyelolithotomy–is it an effective mode of treatment? Urol. Int.. 2008;80:306.
- [Google Scholar]
- Application of new multi-H-bond catalyst for the preparation of substituted pyridines via a cooperative vinylogous anomeric-based oxidation. Res. Chem. Intermed.. 2023;49:679.
- [Google Scholar]
- Design, synthesis, crystal structures and biological evaluation of some 1, 3-thiazolidin-4-ones as dual CDK2/EGFR potent inhibitors with potential apoptotic antiproliferative effects. Arab. J. Chem.. 2022;15:104280
- [Google Scholar]
- Magnetic phosphonium ionic liquid: Application as a novel dual role acidic catalyst for synthesis of 2′-aminobenzothiazolomethylnaphthols and amidoalkyl naphthols. Res. Chem. Intermed.. 2020;46:891.
- [Google Scholar]
- Synthesis of triarylpyridines with sulfonate and sulfonamide moieties via a cooperative vinylogous anomeric-based oxidation. Sci. Rep.. 2021;11:16846.
- [Google Scholar]
- A magnetic porous organic polymer: catalytic application in the synthesis of hybrid pyridines with indole, triazole and sulfonamide moieties. RSC Adv.. 2022;12:8804.
- [Google Scholar]
- Construction of a new 2D coral-like covalent organic framework as CuI nanoparticles carrier for the preparation of diverse triazoles. Arab. J. Chem.. 2023;16:105090
- [Google Scholar]
- In situ synthesis of 2D/2D MXene-COF heterostructure anchored with Ag nanoparticles for enhancing Schottky photocatalytic antibacterial efficiency under visible light. J. Colloid. Interface. Sci.. 2022;608:735.
- [Google Scholar]
- Covalent organic frameworks for separation applications. Chem. Soc. Rev.. 2020;49:708.
- [Google Scholar]
- Highly conjugated three-dimensional covalent organic frameworks based on spirobifluorene for perovskite solar cell enhancement. J. Am. Chem. Soc.. 2018;140:10016.
- [Google Scholar]
- Electroactive covalent organic frameworks: a new choice for organic electronics. Trends. Chem.. 2022;4:60.
- [Google Scholar]
- Docking site modulation of isostructural covalent organic frameworks for CO2 fixation. Chem. Eur. J.. 2020;26:4510.
- [Google Scholar]
- Covalent organic frameworks for photocatalytic applications. Appl. Catal. B. 2020;276:119174
- [Google Scholar]
- Sulfonic acid and ionic liquid functionalized covalent organic framework for efficient catalysis of the Biginelli reaction. J. Org. Chem.. 2021;86:3024.
- [Google Scholar]
- Novel mesoporous Fe3O4/SiO2/CTAB–SiO2 as an effective adsorbent for the removal of amoxicillin and tetracycline from water. Clean Technol. Environ. Policy. 2018;20:871.
- [Google Scholar]
- Novel urea-functionalized magnetic nanoparticles as a heterogeneous hydrogen bonding catalyst for the synthesis of new 2-hydroxy pyridines. Pahs.. 2023;43:3072.
- [Google Scholar]
- Effect of covalent organic framework modified graphene oxide on anticorrosion and self-healing properties of epoxy resin coatings. J. Colloid. Interface. Sci.. 2022;608:1025.
- [Google Scholar]
- Design, synthesis, and mechanistic study of 2-pyridone-bearing phenylalanine derivatives as novel HIV capsid modulators. Molecules. 2022;27:7640.
- [Google Scholar]
- Ruthenium nanoparticles confined in covalent organic framework/reduced graphene oxide as electrocatalyst toward hydrogen evolution reaction in alkaline media. Ind. Eng. Chem. Res.. 2021;60:11070.
- [Google Scholar]
- Magnetic nanoparticles@ metal-organic framework composites as sustainable environment adsorbents. J. Nanomater.. 2019;2019:1454358.
- [Google Scholar]
- Magnetic COFs as catalyst for Fenton-like degradation of sulfamethazine. Chemosphere. 2021;264:128561
- [Google Scholar]
- Magnetic COFs for the adsorptive removal of diclofenac and sulfamethazine from aqueous solution: Adsorption kinetics, isotherms study and DFT calculation. J. Hazard. Mater.. 2020;385:121596
- [Google Scholar]
- The importance of non-stoichiometric ratio of reactants in organic synthesis. J. Chem. Edu.. 2024;101:877.
- [Google Scholar]







