Translate this page into:
New sesquineolignan glycoside isomers from the aerial parts of Leonurus japonicus and their absolute configurations
⁎Corresponding authors. tianhui1009@126.com (Hui Tian), xiling@cdutcm.edu.cn (Liang Xiong)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
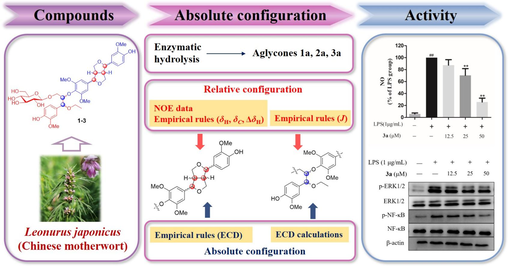
The acid water-soluble fraction of the 95% EtOH extract of Leonurus japonicus exhibited significant anti-inflammatory activity by suppressing the p-ERK/ERK ratio and iNOS expression. A further phytochemical investigation of this acid water-soluble fraction led to the isolation of three previously undescribed sesquineolignan glycosides, leolignosides A–C (1–3), and their planar structures were elucidated using HR-ESI-MS and 1D and 2D NMR. To determine the complicated absolute configuration of sesquineolignan glycosides, coupling constants, NOESY data, empirical rules, enzymatic hydrolysis, and ECD data were comprehensively used. These isolated compounds were also screened for anti-inflammatory activity. Encouragingly, leolignosides A–C all suppressed LPS-induced NO overproduction in RAW 264.7 macrophages. The aglycone (3a) of leolignoside C was chosen for further investigation for its activity and the available amount. Similar to the acid water-soluble fraction, compound 3a inhibited NO overproduction and decreased the p-ERK/ERK and p-NF-κB/NF-κB ratios, indicating that 3a may exert anti-inflammatory effects through the ERK/NF-κB signaling pathway.
Keywords
Leonurus japonicus
Sesquineolignan glycosides
Absolute configuration
Anti-inflammation
1 Introduction
The aerial parts of Leonurus japonicus Houtt. (Chinese motherwort), known as an “excellent medicine for obstetrical and gynecological diseases”, have been used as an important medicine for treating postpartum hemorrhage and regulating menstruation in China for >1800 years (Miao et al., 2019). Given its role in clinical practice, our previous studies focusing on its traditional efficacy achieved several results. For example, alkaloids are the main material basis of motherwort for uterine contraction and hemostasis (Liu et al., 2018). Meanwhile, alkaloids also play a key role in treating postpartum uterine subinvolution due to their pro-angiogenic effects (Zhou et al., 2020). Motherwort terpenoids and steroids have significant vasodilatory and anti-platelet aggregation activities (Peng et al., 2013; Ye et al., 2014; Xiong et al., 2015a; Xiong et al., 2015b; Zhou et al., 2015; Zhou et al., 2019). In addition, flavonoids (Liu et al., 2018) and other compounds (Zhou et al., 2013) were also studied. Although some lignans have been reported from the fruits of L. japonicus (Tian et al., 2021; Peng et al, 2021), a survey of the literature revealed that no more than 20 lignans have been reported from L. japonicus, and these do not include sesquineolignans.
Chirality is an essential attribute of most natural products and is closely related to their pharmacological activities. Multiple chiral centers are usually present in lignans, which makes it difficult to determine their absolute configurations. This is especially true for sesquineolignans containing multiple isolated chiral centers, as well as rotatable branched chains. Although great efforts have been made to determine the configurations of lignans, many studies only reported planar structures or relative configurations of lignans, especially sesquineolignans (Macías et al., 2004; Yin et al., 2016; Zhuang et al., 2018; Zhao et al., 2020), reflecting the notion that the determination of the absolute configurations of sesquineolignans is difficult. As part of an effort focused on bioactive secondary metabolites from motherwort, three new sesquineolignan glycosides (1–3) with the same planar structure and different absolute configurations were discovered (Fig. 1). To determine their absolute configurations, coupling constants, NOESY data analysis, enzymatic hydrolysis, empirical rules, and electronic circular dichroism (ECD) calculations were used in this study.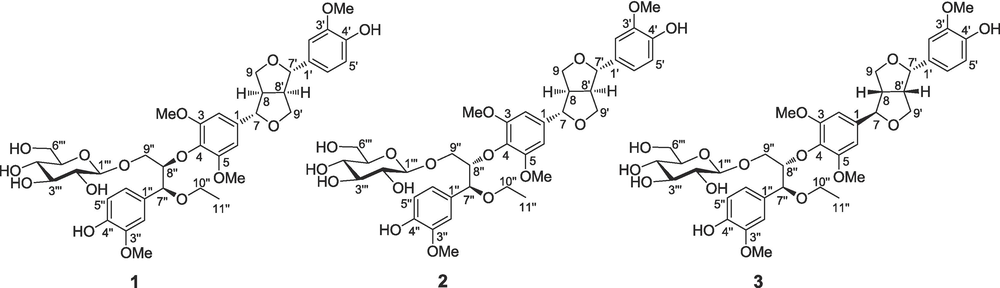
Structures of sesquineolignan glycosides 1–3.
In addition, inspired by the traditional efficacy of motherwort and its clinical application in inflammatory gynecological diseases, the anti-inflammatory activities of the extract and the sesquineolignans were evaluated. NF-κB is a key regulator of inflammatory responses and commonly forms a complex with IκB in the cytoplasm. When the cells are stimulated by inducers, IκB is expeditiously phosphorylated, ubiquitylated, and degraded. Then, NF-κB translocates to the nucleus and binds to cognate DNA binding sites, triggering the transcription of many genes, including cytokines, chemokines, and enzymes. Inducible nitric-oxide synthase (iNOS) plays an important role in NO synthesis and secretion, which initiate and sustain the inflammatory process. What’s more, a lot of natural products could exert anti-inflammatory activity through the NF-κB/iNOS pathway (Li et al., 2019; Sun et al., 2017; Zuo et al., 2024). Therefore, the expressions of related proteins were also investigated in this study.
2 Materials and methods
2.1 General experimental procedures
A Rudolph Autopol-I automatic polarimeter (Rudolph Research Analytical, USA) was applied to measure optical rotations. IR spectra were recorded using a Cary 600 FT-IR microscope (Agilent Technologies Inc., CA, USA). ECD spectra were detected on a Chirascan-plus Circular Dichroism spectrometer (Applied Photophysics Ltd., Leatherhead, England). A Bruker TIMS-TOF-MS instrument (Bruker Corporation, Billerica, MA, USA) was applied to acquire HR-ESI-MS data. NMR data were obtained using a Bruker-AVIIIHD-600 spectrometer (Bruker Corporation, Billerica, MA, USA) with the solvent peaks as the references. Column chromatography was performed using silica gel (200–300 mesh, Yantai Institute of Chemical Technology, Yantai, China), MCI gel CHP 20P (40–60 μm, Mitsubishi Chemical, Co., Japan), and Toyopearl HW-40F (TOSOH, Tokyo, Japan). TLC was performed using GF254 silica gel plates (Anhui Liangchen Silicon Source Material Co. Ltd., Anhui, China). HPLC separation was carried out on an Agilent 1100 instrument equipped with a Zorbax SB-C18 (9.4 × 250 mm2, 5 μm). The hydrolysis reactions were carried out with snailase (Beijing BioDee Biotechnology Co., Ltd., Beijing, China). Curcumin was used as a positive control and purchased from Sichuan Weikeqi Biological Technology Co., Ltd. (Sichuan, China). NO levels were detected by nitric oxide assay kit from Beyotime Institute of Biotechnology (Shanghai, China). Rabbit anti-phospho-ERK1/2 (Thr202/Tyr204) and anti-ERK1/2 antibodies were purchased from Cell Signaling Technology (CST; Danvers, Massachusetts, US). Rabbit anti-phospho-NF-κB (Ser536) and anti-NF-κB antibodies were obtained from Chengdu Zen Bioscience Co., Ltd. (Chengdu, China). Rabbit anti-iNOS was bought from Beyotime Institute of Biotechnology (Shanghai, China). Rabbit anti-β-actin antibody was gained from GeneTex Inc. (Irvine, California, USA). Peroxidase-conjugated AffiniPure goat anti-rabbit immunoglobulin G (IgG; [H+L]) was purchased from ZSGB-BIO Commerce Store (Beijing, China). Shanghai EpiZyme Biotechnology Co., Ltd. (Shanghai, China) supplied Omni-ECL TMFemtoLiqht chemiluminescence Kit.
2.2 Plant material
The aerial parts of L. japonicus were purchased from Sichuan Neautus Traditional Chinese Medicine Co., Ltd. in 2019 and identified by Prof. Jihai Gao at Chengdu University of Traditional Chinese Medicine. A voucher specimen (LJ-20190517) has been deposited at the school of pharmacy, Chengdu university of Traditional Chinese Medicine, China.
2.3 Extraction and isolation
The aerial parts of L. japonicus (100 kg) were refluxed three times with 95 % EtOH (3 h each time) to afford an EtOH extract, which was evaporated to give a residue (5.5 kg). The residue was suspended in water containing 1 % hydrochloric acid and partitioned with EtOAc. The acid water-soluble fraction (AWSF, 660 g) was subjected to a silica gel column using a gradient elution of ethyl acetate–methanol–ammonia (100:7:5–0:7:5) to afford 11 fractions (F1–F11). Eluting with a step gradient of 10–100 % MeOH in H2O, F5 was separated using flash chromatography over MCI gel to obtain six subfractions (F5-1–F5-6). F5-5 was fractioned via silica gel column chromatography over ethyl acetate–methanol–ammonia (100:5:5–0:5:5) to afford nine subfractions F5-5-1–F5-5-9. F5-5-4 was further purified using Toyopearl HW-40F column chromatography (50 % MeOH in H2O), followed by reserved-phase semi-preparative HPLC (50 % MeOH in H2O) to afford 1 (2.3 mg, tmin = 102.4 min), 2 (1.3 mg, tmin = 90.2 min), and 3 (1.9 mg, tmin = 112.8 min).
Leolignoside A (1): white powder;
−4.8 (c 0.04, MeOH); UV (MeCN) λmax (log ε) 193 (4.4), 281 (3.0) nm; IR (ATR) νmax 3296, 2938, 2856, 1634, 1567, 1514, 1463, 1025, 771, 722 cm−1; (+)-HR-ESI-MS m/z 797.2995 [M+Na]+ (calcd. for C39H50O16Na, 797.2997); 1H (CDCl3, 600 MHz) and 13C NMR (CDCl3, 150 MHz) data, see Table 1.
No.
1
2
3
δH
δC
δH
δC
δH
δC
1
−
137.1
−
136.9
−
137.0
2
6.56 br.s
103.4
6.48 br.s
103.5
6.51 br.s
103.7
3
−
153.2
−
153.2
−
153.2
4
−
135.5
−
135.3
−
135.4
5
−
153.2
−
153.2
−
153.2
6
6.56 br.s
103.4
6.48 br.s
103.5
6.51 br.s
103.7
7
4.71 d (5.4)
86.1
4.68 d (5.4)
85.9
4.38 d (7.2)
87.8
8
3.06 m
54.6
3.02 m
54.6
2.85 m
54.8
9
4.25 dd (9.6, 7.2)
3.90 a
71.7
4.26 a
3.88 a
71.7
4.12 br.d (9.6)
3.85 a
71.1
1′
−
132.9
−
132.9
−
130.4
2′
6.90 d (1.8)
108.7
6.89 d (1.8)
108.7
6.94 d (1.8)
108.4
3′
−
146.9
−
146.9
−
146.6
4′
−
145.4
−
145.4
−
144.8
5′
6.90 d (8.4)
114.4
6.90 d (8.4)
114.4
6.89 d (8.4)
114.4
6′
6.82 dd (8.4, 1.8)
119.1
6.82 dd (8.4, 1.8)
119.1
6.78 dd (8.4, 1.8)
118.5
7′
4.76 d (4.8)
85.9
4.74 d (5.4)
85.9
4.86 d (5.4)
82.2
8′
3.12 m
54.2
3.09 m
54.1
3.32 m
50.2
9′
4.29 dd (9.0, 7.2)
3.90 a
72.2
4.26 a
3.88 a
72.1
3.83 a
3.31 a
70.0
1″
−
131.0
−
131.4
−
131.5
2″
6.76 d (1.8)
109.2
6.87 d (1.8)
109.8
6.88 d (1.2)
109.9
3″
−
146.6
−
146.4
−
146.4
4″
−
145.1
−
145.2
−
145.2
5″
6.81 d (8.4)
114.2
6.81 a
113.8
6.82 d (8.4)
113.8
6″
6.66 dd (8.4, 1.8)
120.2
6.80 a
121.0
6.81 dd (8.4, 1.2)
120.9
7″
4.53 d (3.6)
81.2
4.59 d (6.6)
80.7
4.60 d (6.0)
80.8
8″
4.36 ddd (7.8, 3.6, 1.8)
85.7
4.44 ddd (6.6, 3.6, 3.0)
83.3
4.42 ddd (6.0, 3.6, 3.0)
83.4
9″
4.19 dd (11.4, 1.8)4.06 dd
(11.4, 7.8)69.5
4.24 a
3.85 a
68.4
4.24 dd (11.4, 3.6)
3.85 a
68.3
10″
3.45 q (7.2)
65.3
3.43 q (7.2)
65.0
3.44 q (7.2)
65.0
11″
1.25 t (7.2)
15.6
1.19 t (7.2)
15.4
1.19 t (7.2)
15.4
1′′′
4.43 d (7.8)
104.1
4.36 d (7.8)
103.1
4.35 d (7.8)
103.0
2′′′
3.24 dd (8.4, 7.8)
74.0
3.35 t (8.4)
73.9
3.34 a
73.8
3′′′
3.50 dd (9.0, 8.4)
76.6
3.52 t (9.0)
76.8
3.53 t (9.0)
76.8
4′′′
3.52 dd (9.0, 7.2)
70.5
3.56 t (9.0)
70.8
3.56 t (9.0)
70.8
5′′′
3.36 m
75.3
3.33 m
75.4
3.34 a
75.4
6′′′
3.86 a3.77 dd
(12.0, 4.8)62.6
3.87 a
3.78 dd (12.0, 5.4)62.8
3.88 dd (12.0, 3.6)
3.78 dd (12.0, 4.8)62.8
OMe-3, 5
3.83 s
56.4
3.75 s
56.5
3.76 s
56.5
OMe-3′
3.91 s
56.1
3.91 s
56.1
3.92 s
56.1
OMe-3″
3.84 s
56.1
3.84 s
56.0
3.86 s
56.1
OH-4′
5.62 s
−
5.58 s
OH-4″
5.56 s
−
5.56 s
Leolignoside B (2): white powder; −9.5 (c 0.04, MeOH); UV (MeCN) λmax (log ε) 202 (3.9), 280 (2.7) nm; IR (ATR) νmax 3355, 2925, 2854, 1666, 1595, 1517, 1457, 1075, 820, 742, cm−1; (+)-HR-ESI-MS m/z 797.2995 [M+Na]+ (calcd. for C39H50O16Na, 797.2997); 1H (CDCl3, 600 MHz) and 13C NMR (CDCl3, 150 MHz) data, see Table 1.
Leolignoside C (3): white powder; −4.8 (c 0.04, MeOH); UV (MeCN) λmax (log ε) 199 (4.3), 281 (3.0) nm; IR (ATR) νmax 3297, 2919, 2852, 1637, 1557, 1516, 1461, 1074, 763, 720 cm−1; (+)-HR-ESI-MS m/z 797.2995 [M+Na]+ (calcd. for C39H50O16Na, 797.2997); 1H (CDCl3, 600 MHz) and 13C NMR (CDCl3, 150 MHz) data, see Table 1.
2.4 Enzymatic hydrolysis
Compounds 1 (1.9 mg), 2 (1.0 mg), and 3 (1.5 mg) in H2O (4 mL) were hydrolyzed with snailase (15 mg) at 37 °C for 48 h, respectively. After that, EtOAc was applied to extract aglycones three times (3 × 16 mL). Then, the aglycones 1a (1.1 mg), 2a (0.6 mg), and 3a (0.8 mg) were purified using HPLC (50 % MeCN in H2O), and their structures were determined using 1H NMR (Table 2) and HR-ESI-MS. Compound 1a:
−16.0 (c 0.03, MeOH); CD (MeCN) 209 (Δε + 4.50), 239 (Δε + 0.41), 279 (Δε + 0.41) nm; (+)-HR-ESI-MS m/z 635.2461 [M+Na]+ (calcd. for C33H40O11Na, 635.2468). 2a:
− 3.6 (c 0.03, MeOH); CD (MeCN) 217 (Δε + 0.14), 233 (Δε − 0.48), 282 (Δε + 0.10) nm; (+)-HR-ESI-MS m/z 635.2463 [M+Na]+ (calcd. for C33H40O11Na, 635.2468). 3a:
+11.5 (c 0.03, MeOH); CD (MeCN) 216 (Δε + 0.23), 232 (Δε − 0.22), 289 (Δε + 0.09) nm; (+)-HR-ESI-MS m/z 635.2464 [M+Na]+ (calcd. for C33H40O11Na, 635.2468).
No.
1a
2a
3a
1
−
−
−
2
6.51 br.s
6.51 br.s
6.53 br.s
3
−
−
−
4
−
−
−
5
−
−
−
6
6.51 br.s
6.51 br.s
6.53 br.s
7
4.70 d (5.4)
4.70 a
4.39 d (7.2)
8
3.04 m
3.04 m
2.85 m
9
4.24 dd (9.0, 7.2)
3.87 a
4.25 dd (9.0, 7.8)
3.90 a
4.12 br.d (9.6)
3.85 dd (9.6, 6.0)
1′
−
−
−
2′
6.94 d (1.8)
6.96 br.s
6.96 br.s
3′
−
−
−
4′
−
−
−
5′
6.86–6.92 a
6.94 d (8.4)
6.89 d (7.8)
6′
6.86–6.92 a
6.89 a
6.87 a
7′
4.73 d (5.4)
4.73 d (4.8)
4.85 d (5.4)
8′
3.09 m
3.08 m
3.31 m
9′
4.25 dd (9.0, 7.2)
3.89 a
4.24 dd (9.0, 7.2)
3.90 a
3.83 m
3.31 m
1″
−
−
−
2″
6.86–6.92 a
6.88 br.s
6.88 br.s
3″
−
−
−
4″
−
−
−
5″
6.86–6.92 a
6.88 a
6.88 a
6″
6.81 dd (8.4, 1.8)
6.81 br.d (7.8)
6.78 br.d (7.8)
7″
4.69 d (3.6)
4.70 d (6.0)
4.71 d (6.6)
8″
4.08 m
4.08 m
4.08 m
9″
3.96 dd (12.0, 3.0)
3.63 dd (12.0, 3.0)3.95 m
3.70 dd (11.4, 3.0)3.97 m
3.70 m
10″
3.48 q (7.2)
3.48 q (7.2)
3.48 q (7.2)
11″
1.21 t (7.2)
1.21 t (7.2)
1.21 t (7.2)
OMe-3, 5
3.74 s
3.74 s
3.74 s
OMe-3′
3.90 s
3.90 s
3.92 s
OMe-3″
3.89 s
3.89 s
3.90 s
OH-4′
5.61 s
5.60 s
5.58 s
OH-4″
5.57 s
5.56 s
5.56 s
2.5 Cell culture
RAW 264.7 macrophages were purchased from the Shanghai Cell Bank of the Chinese Academy of Sciences and cultured in DMEM containing 10% FBS, 100 U/mL penicillin, and 100 µg/mL streptomycin at 37 °C in a humidified atmosphere with 5 % CO2.
2.6 Anti-inflammatory activity assay
The doses of the tested compounds were chosen according to a cell viability assay in normal RAW 264.7 cells using CCK-8 kits. Then, in the anti-inflammatory assay, RAW 264.7 cells were seeded in a 96-well plate (5 × 104 cells per well) and incubated for 24 h. Subsequently, the cells were treated with 1 μg/mL LPS for 24 h with or without various concentrations of tested compounds (curcumin was used as a positive control) (Huang et al., 2022, Li et al., 2021). Then, the supernatants were collected and assayed for NO production with an NO kit using Griess reagent. Absorbance was measured using a microplate reader at a wavelength of 570 nm. NO levels in samples were determined based on a standard curve.
2.7 Western blot analysis
RAW 264.7 cells were pretreated with compound 3a (12.5, 25, and 50 μM) for 1 h and then stimulated with 1 μg/mL LPS. To obtain total proteins, the cells were lysed in RIPA buffer containing proteinase inhibitors and crushed with an ultrasonic cell pulverizer. Equal quantities of protein (30 μg per lane) were separated using SDS-PAGE and transferred to PVDF membranes, which were blocked in 5% non-fat milk for 2 h at room temperature and incubated with primary antibodies against ERK, p-ERK, NF-κB, p-NF-κB, iNOS, and β-actin overnight at 4 °C. After three washes with TBST, membranes were incubated with HRP-conjugated goat anti-rabbit secondary antibodies for 1 h at room temperature and washed again. Protein bands were visualized by enhanced chemiluminescence. Western blot images were captured using a Tanon 5200 chemiluminescence imaging system.
2.8 Statistical analysis
Data are presented as mean ± standard deviation (SD). All experiments were conducted at least three times. One-way analysis of variance was conducted for comparisons among multiple groups, and the least significant difference test was used for comparisons between two groups. Figures were prepared using GraphPad Prism version 5.0 (GraphPad Software, Inc., San Diego, CA, US). In all comparisons, P<0.05 was considered statistically significant.
3 Results
3.1 Inhibitory effect of AWSF on NO production in RAW 264.7 cells
To exclude the possibility that the cytotoxicity of AWSF may contribute to its anti-inflammatory effect, the viability of RAW 264.7 cells treated with various AWSF concentrations was evaluated using the CCK-8 assay. As shown in Fig. 2A, AWSF exhibited no cytotoxic effect at concentrations of 6.25, 12.5, 25, 50, and 100 μg/mL. Therefore, it is rational to apply these concentrations in the subsequent experiments.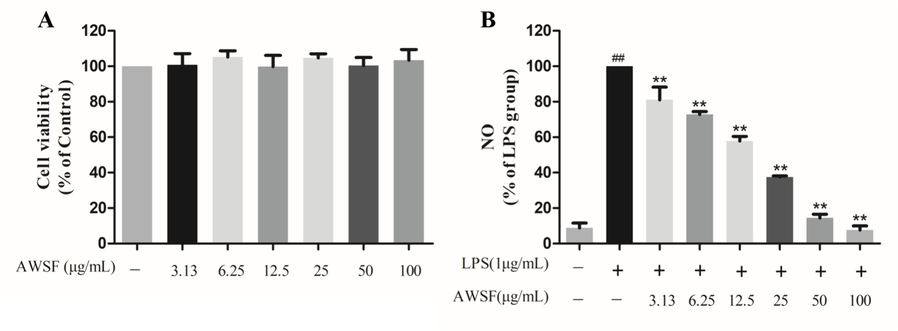
Inhibitory effect of AWSF on LPS-induced NO production in RAW 264.7 cells. (A) Effect of AWSF on the survival rate of normal macrophages. (B) Effect of AWSF on NO release from macrophages. Data are shown as mean ± SD of three independent experiments. ##P < 0.01 vs. untreated control; **P < 0.01 vs. model group.
Because overproduction of NO is a typical marker for inflammation in activated macrophages, Griess reagent kits were applied to assess extracellular NO concentrations. As presented in Fig. 2B, NO production in RAW 264.7 macrophages was markedly increased by stimulation with LPS, and AWSF could significantly decrease the NO content in a dose-dependent manner with an IC50 value of 14.59 μg/mL.
3.2 AWSF decreased the protein expression of p-ERK and iNOS in LPS-activated RAW 264.7 cells
NO, a pro-inflammatory mediator, is synthesized excessively by iNOS when macrophages are stimulated by LPS (Feng et al., 2021, Xiang et al., 2018). Thus, we speculated that iNOS might be involved in the effect of AWSF on NO release. In addition, numerous studies have reported that ERK, a member of the MAPK family, is critical for activating NF-κB, which could subsequently activate iNOS expression (Peng et al., 2015, Zou et al., 2018). Considering that AWSF is a mixture, it is acceptable to preliminarily explore whether AWSF decreases NO overproduction by suppressing ERK and iNOS protein expression. As shown in Fig. 3, AWSF suppressed LPS-activated protein expression of p-ERK and iNOS in a dose-dependent manner, indicating that AWSF may exert anti-inflammatory activity by inhibiting p-ERK and iNOS expression.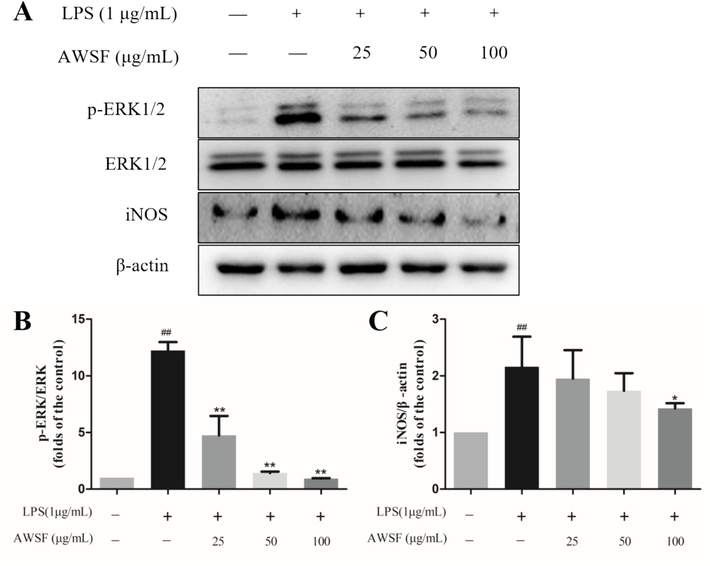
Effect of AWSF on the expression of inflammatory-related proteins. (A) Expression levels of p-ERK1/2 (Thr202/Tyr204) and iNOS. (B) p-ERK/ERK ratio. (C) iNOS/β-actin ratio. Data are shown as mean ± SD. ##P < 0.01 vs. untreated control; *P < 0.05 and **P < 0.01 vs. model group (n = 3).
3.3 Structure elucidation of the compounds isolated from AWSF
Compound 1 was obtained as a white powder. HR-ESI-MS analysis of 1 gave an [M+Na]+ at m/z 797.2995 revealing a molecular formula of C39H50O16 (calcd. for C39H50O16Na, 797.2997). The IR spectrum of 1 displayed strong signals at 1634, 1567, 1514, and 3296 cm−1, supporting the presence of aromatic rings and hydroxy groups. To provide more detailed structural information, the 1H NMR spectrum (Table 1) displayed typical signals that corresponded to two 1,3,4-trisubstituted aromatic rings [δH 6.90 (1H, d, J=1.8 Hz), 6.90 (1H, d, J=8.4 Hz), 6.82 (1H, dd, J=8.4, 1.8 Hz), 6.81 (1H, d, J=8.4 Hz), 6.76 (1H, d, J=1.8 Hz), and 6.66 (1H, dd, J=8.4, 1.8 Hz)], a 1,3,4,5-tetrasubstituted aromatic ring [δH 6.56 (2H, br.s)], two exchangeable aromatic hydroxy protons (δH 5.62 and 5.56), four aromatic methoxy groups (δH 3.91, 3.84, and 3.83), multiple oxygenated methylene and methine groups, two non-oxygenated methine groups, and a methyl group. The 13C NMR data (Table 1) in combination with DEPT and HSQC spectra exhibited carbon signals corresponding to the above protons, including 19 methine signals [eight aromatic (δC 120.2, 119.1, 114.4, 114.2, 109.2, 108.7, 103.4, 103.4), nine oxygenated (δC 104.1, 86.1, 85.9, 85.7, 81.2, 76.6, 75.3, 74.0, 70.5), and two non-oxygenated (δC 54.6, 54.2)], five oxygenated methylene signals (δC 72.2, 71.7, 69.5, 65.3, 62.6), and five methyl signals [a methyl group (δC 15.6) and four methoxy groups (δC 56.4, 56.4, 56.1, 56.1)]. In addition, the 13C NMR and DEPT data indicated the presence of 10 aromatic quaternary carbons attributed to three phenyl units. From the above data, it can be seen that 1 contains three C6-C3 moieties substituted with four aromatic methoxy groups, two phenolic hydroxy groups, and a glycosyl group. Moreover, according to the coupling constant of the anomeric proton (J=7.8 Hz) and the 13C chemical shifts of the sugar moiety, it is inferred that there is a β-glucopyranosyl (Xiong et al., 2013). Therefore, compound 1 is a sesquineolignan β-glucopyranoside.
A rigorous survey of the literature revealed that 1 and (−)-(7R,7′R,7″R,8S,8′S,8″S)-4′,4″-dihydroxy-3,3′,3″,5-tetramethoxy-7,9′:7′,9-diepoxy-4,8″-oxy-8,8′-sesquineolignan-7″,9″-diol (Xiong et al., 2011) have a very similar sesquineolignan skeleton, both of which are linked by medioresinol and guaiacylglycerol. The main difference is that 1 has one more β-glucopyranosyl unit and an ethoxy group than the compound reported in the literature. Detailed comparison of the 13C NMR data between the two compounds showed that C-7″ and C-9″ resonances in 1 were shifted by ΔδC+8.7 and +8.8 ppm, respectively, indicating that the β-glucopyranosyl and ethoxy groups are attached at the C-7″ and C-9″ positions. The inference of the planar structure of 1 was confirmed by 2D NMR including 1H–1H COSY, HSQC, and HMBC (Fig. 4). In the HMBC spectrum, H-7 shared correlations with C-9, C-8′, and C-9′, while H-7′ displayed correlations with C-8, C-9, and C-9′, in combination with the COSY correlations of H-7/H-8/H2-9, H-7′/H-8′/H2-9′, and H-8/H-8′, confirming the existence of the tetrahydrofuran ring in 1. Two symmetric methoxy groups are connected to the C-3 and C-5 positions based on the HMBC correlations of H-2/6 with C-1, C-3/5, C-4, and C-7, as well as OMe-3/5 with C-3/5. A methoxy group and a hydroxy group were determined to be located at the C-3′ and C-4′ positions, respectively, relying on the correlations from H-5′ to C-1′, C-3′, and C-6′; from H-6′ to C-2′ and C-4′; from OMe-3′ to C-3′; from OH-4′ to C-3′, C-4′, and C-5′; and from H-8′ to C-1′, C-2′, and C-3′. Similarly, it can be determined that a methoxy group and a hydroxy group were attached to C-3″ and C-4″, respectively. Furthermore, according to the HMBC correlations of H-7″ with C-1″, C-2″, C-6″, C-8″, and C-9″, together with the COSY correlations of H-7″/H-8″/H2-9″, it is inferred that 1 contains a guaiacylglycerol fragment. The ethoxyl group at C-7″ was established by the HMBC correlation of H-7″ with C-10″, while the β-glucopyranosyl group was connected to C-9″ based on the HMBC correlation of H-1′″ with C-9″. Although no HMBC signal for the ether bond between H-8″ and C-4 was observed, the connection of 8″-O-4 can be inferred from the molecular formula and the chemical shifts of H-8″ (δH 4.36), C-8″ (δC 85.7), and C-4 (δC 135.5). Therefore, the planar structure of 1 was determined as 4′,4″-dihydroxy-3,3′,3″,5-tetramethoxy-7″-ethoxy-7,9′:7′,9-diepoxy-4,8″-oxy-8,8′-sesquineolignan-9″-O-β-glucopyranoside.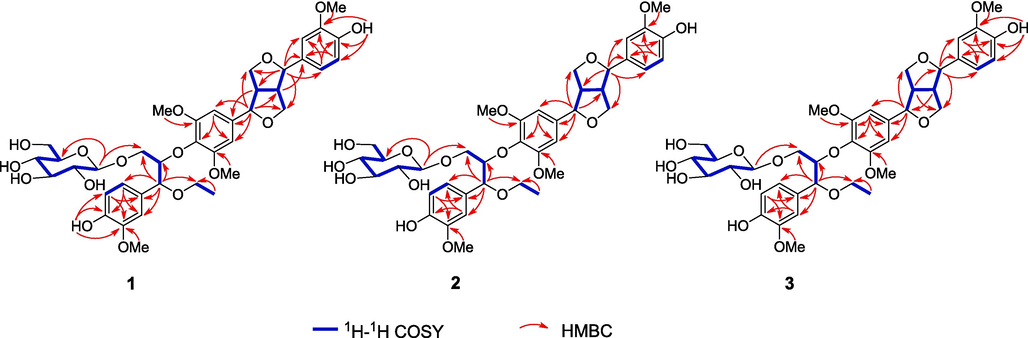
Key HMBC and 1H–1H COSY correlations of compounds 1–3.
The relative configuration of 1 was determined through a comprehensive analysis of coupling constants, chemical shifts, and NOESY data. First, the 1H NMR and 13C NMR shifts for the bis-tetrahydrofuran ring in 1 were analyzed, revealing that both H-7 and H-7′ were at 4.6–5.1 ppm, H-8 and H-8′ at 3.0–3.2 ppm, H-9a and H-9′a at 4.2–4.4 ppm, H-9b and H-9′b at 3.8–4.0 ppm, C-7 and C-7′ at 82–87 ppm, C-8 and C-8′ at 54–56 ppm, and C-9 and C-9′ at 71–74 ppm. According to empirical rules (Xiong et al., 2011, Shi, 2009, Greger and Hofer, 1980), it can be deduced that the bis-tetrahydrofuran lignan fragment of 1 is of the eq/eq type, and H-7/H-8 and H-7′/H-8′ are in trans orientation. These relative configurations can further be confirmed by ΔδH–9a, 9b (0.35 ppm) and ΔδH–9′a, 9′b (0.39 ppm) (Shao et al., 2018), both of which were in the range of 0.3–0.4 ppm. The above inference is also consistent with the NOESY data of 1, in which H-2/6 showed correlations to H-8, while H-2′/H-6′ shared correlations with H-8′. Regarding the guaiacylglycerol fragment, the relative configuration was determined by the coupling constant of H-7″ and H-8″ (J7″,8″). According to the empirical rules for 8,4′-oxolignans, J7″,8″ can be used for the determination of their relative configurations and is influenced by the tested solvent (Xiong et al., 2011, Gan et al., 2008). Therefore, the guaiacylglycerol unit of 1 was inferred to be an erythro configuration based on J7″,8″ = 3.6 Hz (CDCl3).
To further determine the absolute configuration, compound 1 was enzymatically hydrolyzed to obtain aglycone 1a (Table 2) and a glucose. The glucose was identified as D-glucose using TLC and
compared with a D-glucose reference standard (Xiong et al., 2013, Husdon and Dale, 1916). Because the cotton peaks in ECD spectra are widely used to determine the absolute configurations of lignans, the ECD data of 1 were measured. Numerous studies have shown that in 8,4′-oxyneolignans, the Cotton effect at 230–240 nm can be used to determine the configuration of C-8 in the aryl glycerol units (positive for 8S and negative for 8R) (Xiong et al., 2011, Gan et al., 2008, Huo et al., 2008, Greca et al., 1994), while in the eq/eq-type 7,9′:7′,9-diepoxylignans, the Cotton effect at around 280 nm can be validated for the configuration assignment of the bis-tetrahydrofuran ring (positive for 7S,7′S,8R,8′R and negative for 7R,7′R,8S,8′S) (Shi, 2009, Hofer and Schölm, 1981, Hosokawa et al., 2004). However, in the sesquineolignans with a bis-tetrahydrofuran ring and an aryl glycerol unit, these empirical rules could not be applied to determine the absolute configuration of the aryl glycerol unit but could be used only for configuration determination of the bis-tetrahydrofuran lignan fragment. This is because the bis-tetrahydrofuran lignan fragment could also generate an obvious Cotton effect at 230–245 nm in addition to the Cotton effect at around 280 nm (Hofer and Schölm, 1981; Hosokawa et al., 2004; Muhit et al., 2016; Li et al., 2008,2014), which means that the Cotton effect at 230–245 nm of such sesquineolignans is caused by a comprehensive effect of the aryl glycerol unit and the bis-tetrahydrofuran lignan fragment. In this study, to begin with, a positive Cotton effect at 279 nm (Δε + 0.41) indicated that the eq/eq-type bis-tetrahydrofuran lignan fragment in 1a had a 7S,7′S,8R,8′R configuration. Then, the absolute configuration of the guaiacyl glycerol unit in 1a was determined through ECD calculations, using the time-dependent density functional theory method. Based on the erythro relative configuration of the guaiacyl glycerol unit, the absolute configuration of C-7″ and C-8″ might be 7″S,8″R or 7″R,8″S. Thus, compound 1a could be inferred as (7S,7′S,7″S,8R,8′R,8″R)-1a or (7S,7′S,7″R,8R,8′R,8″S)-1a. ECD calculations showed that the experimental ECD spectrum of 1a matched the calculated ECD spectrum of (7S,7′S,7″S,8R,8′R,8″R)-1a (Fig. 5). Consequently, the structure of 1 was determined as (–)-(7S,7′S,7″S,8R,8′R,8″R)-4′,4″-dihydroxy-3,3′,3″,5-tetramethoxy-7″-ethoxy-7,9′:7′,9-diepoxy-4,8″-oxy-8,8′-sesquineolignan-9″-O-β-D-glucopyranoside (named leolignoside A).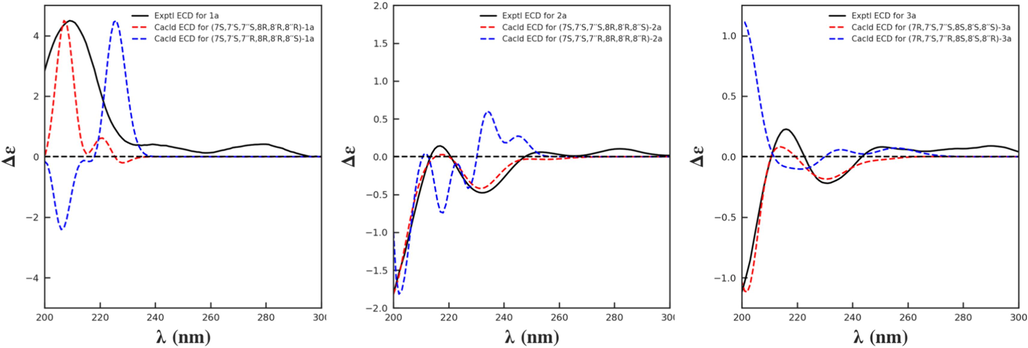
Experimental and calculated ECD spectra of compounds 1a–3a.
Compound 2 showed very similar spectroscopic data to those of 1, indicating that 2 is also a sesquineolignan derivative with a similar structure. HR-ESI-MS displayed that 2 had the same molecular formula as 1 (C39H50O16), thus 2 was inferred to be an isomer of 1. Detailed comparison of the 1H and 13C NMR data between 2 and 1 (Table 1) revealed that the most prominent difference is that C-8″ resonance in 2 was shifted by ΔδC−2.4 ppm, while the coupling constant of H-7″ and H-8″ (J7″,8″) in 2 was increased to 6.0 Hz. Thus, it can be assumed that 2 is the C-8″ epimer of 1. 2D NMR data analysis further verified that 2 had the same planar structure as 1 (Fig. 1). Moreover, based on 1H and 13C NMR data of the bis-tetrahydrofuran ring, it was clear that the bis-tetrahydrofuran lignan fragment of 2 was also of the eq/eq type. In particular, ΔδH–9a, 9b and ΔδH–9′a, 9′b (0.3–0.4 ppm) (Shao et al., 2018), together with the NOESY correlations of H-2/H-6 with H-8 and H-2′/H-6′ with H-8′, confirmed it was of the eq/eq type. The relative configuration of the guaiacyl glycerol fragment was determined as threo according to the coupling constant of J7″,8″ = 6.6 Hz (Xiong et al., 2011, Gan et al., 2008).
Using the same methods as described for 1, compound 2 was enzymatically hydrolyzed to obtain its aglycone 2a (Table 2) and a D-glucose. The ECD spectrum of 2a showed a positive Cotton effect at 282 nm, similar to that of 1a, indicating that the eq/eq-type bis-tetrahydrofuran lignan fragment of 2a was also a 7S,7′S,8R,8′R configuration. Then, compound 2a was assumed as (7S,7′S,7″S,8R,8′R,8″S)-2a or (7S,7′S,7″R,8R,8′R,8″R)-2a for ECD calculation according to the above threo configuration of the guaiacyl glycerol unit. As shown in Fig. 5, the calculated ECD spectrum of (7S,7′S,7″S,8R,8′R,8″S)-2a agreed with the experimental ECD spectrum of 2a. Therefore, compound 2 was determined to be (–)-(7S,7′S,7″S,8R,8′R,8″S)-4′,4″-dihydroxy-3,3′,3″,5-tetramethoxy-7″-ethoxy-7,9′:7′,9-diepoxy-4,8″-oxy-8,8′-sesquineolignan-9″-O-β-D-glucopyranoside (leolignoside B).
Compound 3 showed similar spectroscopic data and the same molecular formula as 1 and 2, inferring that 3 may be a sesquineolignan glycoside analog with the same planar structure. This deduction was demonstrated through 2D NMR experiments. Further comparison of 1H and 13C NMR data of 3 and 2 uncovered that the resonance signals of the guaiacyl glycerol β-D-glucopyranoside fragment in 3 and 2 were almost the same, including the chemical shifts and coupling constants. Thus, the relative configuration of the guaiacyl glycerol unit in 3 was also threo. Applying empirical rules (Shi, 2009, Greger and Hofer, 1980), the 1H and 13C NMR data of the bis-tetrahydrofuran ring in 3 indicated that the bis-tetrahydrofuran lignan unit is not of the eq/eq type but of the eq/ax type. The relative configuration was further verified by ΔδH–9a, 9b (0.27 ppm, in the range of 0.205–0.36 ppm) and ΔδH–9′a, 9′b (0.52 ppm > 0.50 ppm) (Shao et al., 2018). After enzyme hydrolysis of 3, the aglycone 3a (Table 2) and a D-glucose were obtained. In the ECD spectrum of 3a, a positive Cotton effect at 289 nm suggested that the eq/ax-type bis-tetrahydrofuran lignan fragment of 3a has a 7R,7′S,8S,8′S configuration (Muhit et al., 2016, Li et al., 2008, Li et al., 2014). Then, two possible isomers, (7R,7′S,7″R,8S,8′S,8″R)-3a and (7R,7′S,7″S,8S,8′S,8″S)-3a, were applied to ECD calculations. The results showed that 7R,7′S,7″S,8S,8′S,8″S was the most probable configuration for 3a (Fig. 5). Accordingly, compound 3 was determined to be (–)-(7R,7′S,7″S,8S,8′S,8″S)-4′,4″-dihydroxy-3,3′,3″,5-tetramethoxy-7″-ethoxy-7,9′:7′,9-diepoxy-4,8″-oxy-8,8′-sesquineolignan-9″-O-β-D-glucopyranoside (leolignoside C).
3.4 Inhibitory effects of compounds 1a–3a on NO production in RAW 264.7 cells
Similar to AWSF, compounds 1a–3a were first evaluated for their cytotoxic effects in RAW 264.7 cells. As presented in Fig. 6A, these compounds all showed no cytotoxicity at concentrations of 12.5, 25, and 50 μM, which provided evidence for further anti-inflammatory assessments.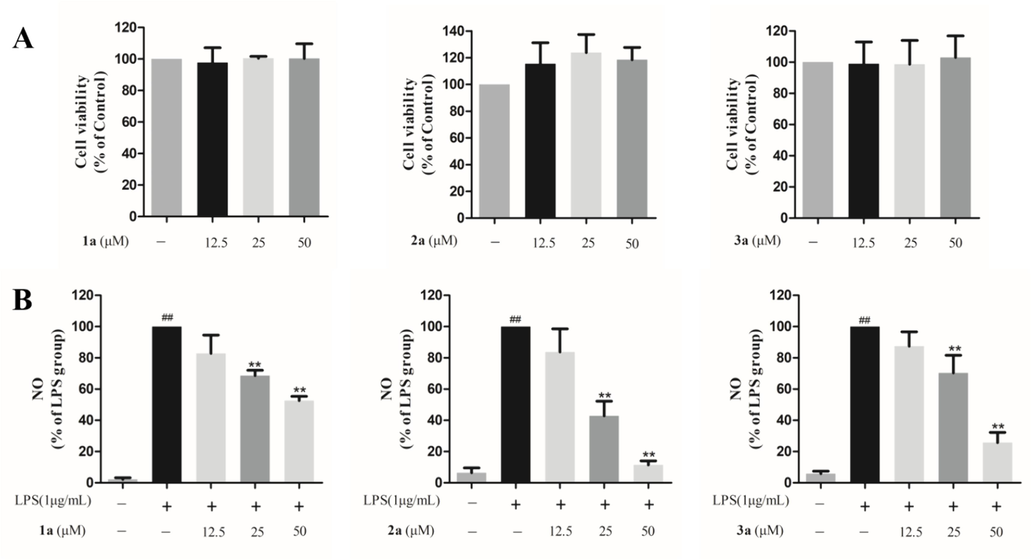
Effects of compounds 1a–3a on NO release from macrophages. (A) Effects of compounds 1a–3a on the survival rate of normal macrophages. (B) Effects of compounds 1a–3a on NO release from macrophages. Data are shown as mean ± SD of three independent experiments. ##P < 0.01 vs. untreated control; **P < 0.01 vs. model group.
The results shown in Fig. 6B reveal that sesquineolignans 1a, 2a, and 3a exerted inhibitory effects on NO release in LPS-injured RAW 264.7 cells. The IC50 values of 2a and 3a was 22.67 and 33.58 μM, respectively. Curcumin was used as a positive control (IC50 = 27.90 μM). Although compound 2a showed the most potent effect, its inhibitory effect on inflammatory-related protein expression was not investigated because the amount of sample available was low. Compound 3a was selected for the subsequent experiments.
3.5 Inhibitory effect of compound 3a on p-ERK and p-NF-κB expression in LPS-injured RAW 264.7 cells
Based on the preliminary exploration of AWSF, the inhibitory effect of compound 3a on protein expression was also examined. As shown in Fig. 7, the LPS-induced increase in p-ERK and p-NF-κB was reversed by 3a. However, unfortunately, iNOS expression was not investigated due to the limited amount of 3a. In summary, compound 3a exerted anti-inflammatory activity through the ERK/NF-κB signaling pathway.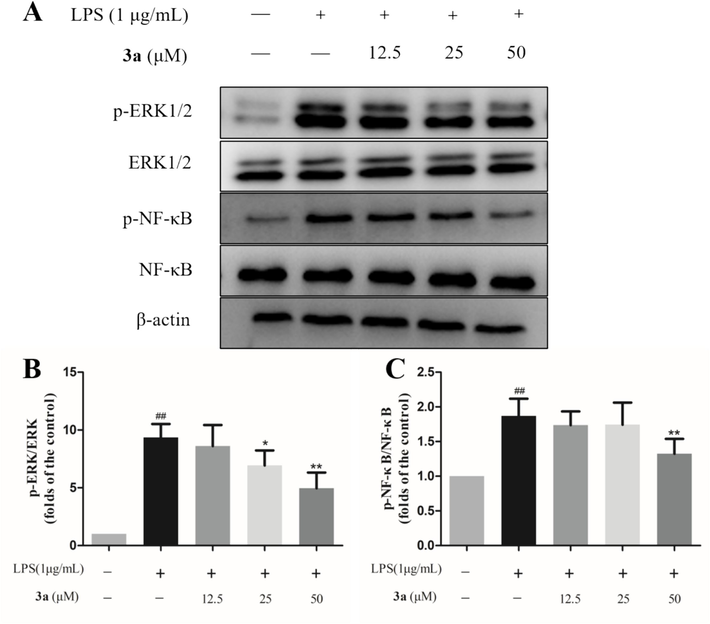
Effect of compound 3a on the expression of inflammation-related proteins. (A) Expression levels of p-ERK1/2 (Thr202/Tyr204) and p-NF-κB (Ser536). (B) p-ERK/ERK ratio. (C) p-NF-κB/NF-κB ratio. Data are shown as mean ± SD. ##P < 0.01 vs. untreated control; *P < 0.05 and **P < 0.01 vs. model group (n = 3).
4 Discussion
It is difficult to determine the configurations of lignans, especially for sesquineolignans, due to their multiple chiral centers on the rings and the chains. Over the years, researchers have attempted to solve this problem, and some empirical rules have been summarized. In the 7,9′:7′,9-diepoxylignans, the relative configuration of the bis-tetrahydrofuran ring could be determined through NOE data analysis coupled with empirical rules of the 1H and 13C NMR data (Shi, 2009; Greger and Hofer, 1980; Shao et al., 2018). Particularly, ΔδH–9a, 9b and ΔδH–9′a, 9′b are available for the determination of the relative configuration (eq/eq type: ΔδH–9a, 9b and ΔδH–9′a, 9′b in the range of 0.3–0.4 ppm; eq/ax type: ΔδH–9a, 9b in the range of 0.205–0.36 ppm, ΔδH–9′a, 9′b > 0.50 ppm) (Shao et al., 2018). For determination of the bis-tetrahydrofuran moiety, the Cotton effect at around 280 nm was often applied (eq/eq type: positive for 7S,7′S,8R,8′R and negative for 7R,7′R,8S,8′S; eq/ax type: positive for 7R,7′S,8S,8′S and negative for 7S,7′R,8R,8′R) (Shi, 2009; Hofer and Schölm, 1981; Hosokawa et al., 2004; Muhit et al., 2016; Li et al., 2008; Li et al., 2014). Regarding 8,4′-oxyneolignans with an aryl glycerol unit, the coupling constant of H-7 and H-8 (J7,8) is widely used to determine the relative configuration (erythro type: J7,8 ≤ 4.0 Hz; threo type: J7,8 ≥ 6.0 Hz) (Xiong et al., 2011; Gan et al., 2008). When it comes to the absolute configuration of the aryl glycerol unit, the Cotton effect at 230–240 nm in the ECD spectrum is usually applied (positive for 8S and negative for 8R) (Xiong et al., 2011; Gan et al., 2008; Huo et al., 2008; Greca et al., 1994). However, for the sesquineolignans with a bis-tetrahydrofuran lignan fragment and an aryl glycerol unit, the Cotton effect at 230–240 nm is contributed by both the aryl glycerol and 7,9′:7′,9-diepoxy lignan chromophores in the molecules. Thus, the above empirical rules are not suitable for determining the absolute configuration of aryl glycerol units in sesquineolignans. In the present study, to determine the complicated absolute configuration of sesquineolignan glycosides, coupling constants, NOESY data, empirical rules, enzymatic hydrolysis, and ECD data were comprehensively used, which made the conclusion more reliable.
5 Conclusions
In the present study, we discovered that the AWSF of the 95 % EtOH extract of L. japonicus had significant anti-inflammatory activity by suppressing NO overproduction and iNOS and p-ERK expression in LPS-activated macrophages. Then, the chemical composition of AWSF was investigated. Three new sesquineolignan glycosides were isolated, and their complicated absolute configurations were determined based on coupling constants, NOESY data, empirical rules, enzymatic hydrolysis, and ECD data. All sesquineolignan aglycones showed anti-inflammatory activities, and 3a was chosen for further study. The results suggest that 3a may decrease NO production through the ERK/NF-κB signaling pathway.
CRediT authorship contribution statement
Lan Bu: Investigation, Writing – original draft. Qin-Mei Zhou: Investigation, Writing – original draft. Cheng Peng: Methodology, Project administration. Hong-Zhen Shu: Investigation. Fei Zhou: Data curation, Methodology. Guang-Xu Wu: Investigation. Fei Liu: Data curation, Methodology. Hui Tian: Supervision, Writing – review & editing. Liang Xiong: Funding acquisition, Supervision, Writing – review & editing.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant Nos. 82022072 and 81872991), the Innovation Team Program of Jinan City (Grant No. 202228038), and “Xinglin Scholar” Plan of Chengdu University of Traditional Chinese Medicine (Grant No. XKTD2022006).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Polyisoprenylated benzophenone derivatives from Garcinia cambogia and their anti-inflammatory activities. Food Funct.. 2021;12:6432-6441.
- [CrossRef] [Google Scholar]
- Glycosides from the root of Iodes cirrhosa. J. Nat. Prod.. 2008;71:647-654.
- [CrossRef] [Google Scholar]
- New unsymmetrically substituted tetrahydrofurofuran lignans from Artemisia absinthium. Tetrahedron. 1980;36:3551-3558.
- [CrossRef] [Google Scholar]
- Steteochemistry of tetrahydrofurofuran derivatives—circular dichroism and absolute configuration. Tetrahedron.. 1981;37:1181-1186.
- [CrossRef] [Google Scholar]
- A new lignan from Balanophora abbreviata and inhibition of lipopolysaccharide (LPS)-induced inducible nitric oxide synthase (iNOS) expression. Chem. Pharm. Bull.. 2004;52:1265-1267.
- [CrossRef] [Google Scholar]
- Six pairs of enantiomeric phthalide dimers from the rhizomes of Ligusticum chuanxiong and their absolute configurations and anti-inflammatory activities. Bioorg. Chem.. 2022;127:105970
- [CrossRef] [Google Scholar]
- Neolignan glycosides from Symplocos caudata. Phytochemistry. 2008;69:788-795.
- [CrossRef] [Google Scholar]
- Studies on the forms of D-glucose and their mutarotation. J. Am. Chem. Soc.. 1916;889:320-328.
- [CrossRef] [Google Scholar]
- Minor furofurano lignans from the Tibetan herb, Lancea Tibetica. Planta Med.. 2008;74:1391-1396.
- [CrossRef] [Google Scholar]
- Polyacetylene glucosides from the florets of Carthamus tinctorius and their anti-inflammatory activity. Phytochemistry. 2021;187:112770
- [CrossRef] [Google Scholar]
- Rutaecarpine ameliorated sepsis-induced peritoneal resident macrophages apoptosis and inflammation responses. Life Sci.. 2019;228:11-20.
- [CrossRef] [Google Scholar]
- Isolation and identification of phase Ⅰ metabolites of phillyrin in rats. Fitoterapia. 2014;97:92-97.
- [CrossRef] [Google Scholar]
- Alkaloids and flavonoid glycosides from the aerial parts of Leonurus japonicus and their opposite effects on uterine smooth muscle. Phytochemistry. 2018;145:128-136.
- [CrossRef] [Google Scholar]
- Bioactive lignans from a cultivar of Helianthus annuus. J. Agric. Food Chem.. 2004;52:6443-6447.
- [CrossRef] [Google Scholar]
- Leonurus japonicus (Chinese motherwort), an excellent traditional medicine for obstetrical and gynecological diseases: a comprehensive overview. Biomed. Pharmacother.. 2019;117:109060
- [CrossRef] [Google Scholar]
- Furofuran lignan glucosides with estrogen-inhibitory properties from the Bangladeshi medicinal plant Terminalia citrina. J. Nat. Prod.. 2016;79:1298-1307.
- [CrossRef] [Google Scholar]
- A bicyclic diterpenoid with a new 15,16-dinorlabdane carbon skeleton from Leonurus japonicus and its coagulant bioactivity. Molecules. 2013;18:13904-13909.
- [CrossRef] [Google Scholar]
- 8-O-4' Neolignans from the fruits of Leonurus japonicus. Nat. Prod. Res. Dev.. 2021;2021(33):1320-1325.
- [CrossRef] [Google Scholar]
- Panax notoginseng flower saponins (PNFS) inhibit LPS-stimulated NO overproduction and iNOS gene overexpression via the suppression of TLR4-mediated MAPK/NF-kappa B signaling pathways in RAW264.7 macrophages. Chin. Med. (United Kingdom). 2015;10:1-11.
- [CrossRef] [Google Scholar]
- An efficient method for determining the relative configuration of furofuran lignans by 1H NMR spectroscopy. J. Nat. Prod.. 2018;81:1023-1028.
- [CrossRef] [Google Scholar]
- Vaccaria hypaphorine alleviates lipopolysaccharide-induced inflammation via inactivation of NFκB and ERK pathways in Raw 264.7 cells. BMC Compl. Altern. Med.. 2017;1:120.
- [CrossRef] [Google Scholar]
- New lignans from the fruits of Leonurus japonicus and their hepatoprotective activities. Bioorg. Chem.. 2021;115:105252
- [CrossRef] [Google Scholar]
- Semi-mechanism-based pharmacodynamic model for the anti-inflammatory effect of baicalein in LPS-stimulated RAW264.7 macrophages. Front. Pharmacol.. 2018;9:1-9.
- [CrossRef] [Google Scholar]
- Lignans and neolignans from Sinocalamus affinis and their absolute configurations. J. Nat. Prod.. 2011;74:1188-1200.
- [CrossRef] [Google Scholar]
- Phenolic glucosides from dendrobium aurantiacum var. denneanum and their bioactivities. Molecules. 2013;18:6153-6160.
- [CrossRef] [Google Scholar]
- Bis-spirolabdane diterpenoids from Leonurus japonicus and their anti-platelet aggregative activity. Fitoterapia. 2015;100:1-6.
- [CrossRef] [Google Scholar]
- Leonuketal, a spiroketal diterpenoid from Leonurus japonicus. Org. Lett.. 2015;17:6238-6241.
- [CrossRef] [Google Scholar]
- Leonurusoleanolides E-J, minor spirocyclic triterpenoids from Leonurus japonicus fruits. J. Nat. Prod.. 2014;77:178-182.
- [CrossRef] [Google Scholar]
- Anti-complementary components of Helicteres angustifolia. Molecules. 2016;21:1-9.
- [CrossRef] [Google Scholar]
- A new lignan glycoside from Astragalus yunnanensis. J. Asian Nat. Prod. Res.. 2020;22:594-600.
- [CrossRef] [Google Scholar]
- Stachydrine promotes angiogenesis by regulating the VEGFR2/MEK/ERK and mitochondrial-mediated apoptosis signaling pathways in human umbilical vein endothelial cells. Biomed. Pharmacother.. 2020;131:110724
- [CrossRef] [Google Scholar]
- Aromatic compounds from Leonurus japonicus Houtt. Biochem. Syst. Ecol.. 2013;51:101-103.
- [CrossRef] [Google Scholar]
- Steroids from the aerial parts of Leonurus japonicus. Phytochem. Lett.. 2015;12:287-290.
- [CrossRef] [Google Scholar]
- New triterpenoids from Leonurus japonicus (Lamiaceae) Biochem. Syst. Ecol.. 2019;82:27-30.
- [CrossRef] [Google Scholar]
- Neolignan and phenylpropanoid compounds from the fruits of Illicium simonsii Maxim. Nat. Prod. Res.. 2018;32:2468-2475.
- [CrossRef] [Google Scholar]
- Anti-inflammatory sesquiterpenoids from the Traditional Chinese Medicine Salvia plebeia: regulates pro-inflammatory mediators through inhibition of NF-κB and Erk1/2 signaling pathways in LPS-induced Raw264.7 cells. J. Ethnopharmacol.. 2018;210:95-106.
- [CrossRef] [Google Scholar]
- Essential oil from Ligusticum chuanxiong Hort. alleviates lipopolysaccharide-induced neuroinflammation: Integrating network pharmacology and molecular mechanism evaluation. J. Ethnopharmacol.. 2024;319:117337
- [CrossRef] [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2024.105932.
Appendix A
Supplementary data
The following are the Supplementary data to this article:Supplementary Data 1
Supplementary Data 1







