Translate this page into:
Piceatannol, a comprehensive review of health perspectives and pharmacological aspects
⁎Corresponding authors. hala.aljaber@bau.edu.jo (Hala I. Al-Jaber), ak_shakya@ammanu.edu.jo (Ashok K. Shakya), mmubarak@ju.edu.jo (Mohammad S. Mubarak)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
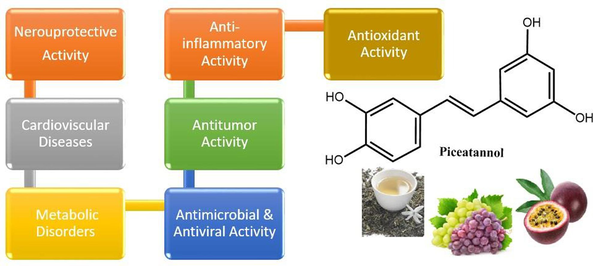
Piceatannol, a natural polyphenolic stilbenoid found in numerous fruits and vegetables such as grapes, passionate fruit, blueberries, and white tea, is well recognized for its diverse pharmacological effects. Numerous previous research studies demonstrated the intriguing bioactivity of piceatannol, including its antioxidant, anti-inflammatory, and cancer preventive and neuroprotective properties. It has also shown potential benefits in managing hypercholesterolemia, atherosclerosis, angiogenesis, and cardiovascular diseases. Accordingly, this comprehensive review aims to provide an updated overview and covers the recent literature dealing with the chemistry of piceatannol, its bioavailability, pharmacological activities, and potential health benefits. In addition, the review will focus on the medicinal and traditional significance of piceatannol in combating various diseases and ailments.
Keywords
Piceatannol
Bioavailability
Antioxidant activity
Anti-inflammatory
Antiproliferative activity
Antimicrobial activity
Cardiovascular diseases
Metabolic disorders
Neuroprotective activity
- 5-LOX
-
5-lipoxygenase
- A549 cells
-
lung cancer cell lines
- ABTS
-
2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid))
- ACC
-
acetyl-CoA carboxylase
- AChE
-
acetylcholinesterase
- AGEPs
-
advanced glycation end-products
- Akt/mTOR
-
protein kinase B (AKT)/mammalian target of rapamycin
- Akt
-
protein kinase B (AKT)
- Anp
-
albumin nanoparticles
- AP-1
-
activator protein-1
- ARPE
-
human retinal pigment epithelium cell
- ATGL
-
adipose triglyceride lipase
- Aβ25-35
-
amyloid beta-peptide (25-35)
- B16F10
-
malignant B16F10 melanoma cells
- BACE1
-
β-site amyloid precursor protein cleaving enzyme 1
- Bax
-
Bcl-2-associated X protein
- Bcl-2
-
B-cell lymphoma 2
- Bcl-xL
-
B-cell lymphoma-extra large (Bcl-xL)
- bFGF
-
basic fibroblast growth factor
- bmDCs
-
bone marrow-derived dendritic cells
- BPH
-
benign prostatic hyperplasia
- beBSA
-
bovine serum albumin
- Cal51
-
human Cal51 (breast cancer) cells
- CAT
-
catalase
- CC-3
-
cleaved caspase-3
- CCK8
-
cell counting kit-8 assay method
- CD36
-
cluster of differentiation 36
- cdk4
-
cyclin-dependent kinase 4
- CGI-58
-
comparative gene identification-58
- CIRI
-
cerebral ischemia/reperfusion injury
- CK
-
creatine kinase
- COMT
-
catechol-O-methyl transferase
- COX-1
-
cyclooxygenase-1
- COX-2
-
cyclooxygenase-2
- CPT1α
-
Carnitine palmitoyltransferase 1α
- CS/Pla-PicNps
-
chitosan/poly-lactic acid coated piceatannol nanoparticles
- DPPH
-
2,2-diphenyl-1-picrylhydrazyl
- DSS
-
dextran sulfate sodium
- EB-EH
-
estradiol benzoate induced-endometrial hyperplasia
- EGFR
-
epidermal growth factor receptor
- EJ cells
-
bladder cancer cell lines EJ cells
- eNOS
-
endothelial nitric oxide synthase
- ER
-
endoplasmic reticulum
- Erk
-
eExtracellular signal-regulated kinase
- EtOAc
-
ethyl acetate
- EtOH
-
ethanol
- FHF
-
fulminant hepatic failure
- FoxO1
-
forkhead box protein O1
- FRAP
-
ferric reducing antioxidant power assay
- FXR
-
farnesoid X Receptor
- Glo-I
-
glyoxalase I
- GLU
-
glucuronidase
- GSH
-
glutathione peroxidase
- GST
-
glutathione-S-transferase
- HaCaT
-
normal human keratinocyte cell line
- HAT
-
histone acetyltransferase
- hCMV
-
human cytomegalovirus
- HCT116
-
colorectal cancer cells
- HepG2
-
hepatoblastoma cancer cells
- HF-fed
-
high fat-fed
- HI
-
hypoxic-ischemic
- HIF-1α
-
hypoxia-inducible factor-1α
- HIV-1
-
human immunodeficiency virus-1
- HL-60
-
MOLT-4, THP-1, U937, and K562, different types of leukemia cancer cell lines
- hMSC
-
human mesenchymal stem cells
- Ho-1
-
heme oxygenase-1
- HSC cells
-
hepatic stellate cells
- HT1080
-
human fibrosarcoma cell line
- HT-29 cells
-
human colorectal adenocarcinoma
- HUVECs
-
human umbilical vein endothelial cells
- ICAM
-
intercellular adhesion molecule-1
- IKK
-
inhibitor of nuclear factor-κB (IκB) kinase
- IL-10
-
interleukin-10
- IL-12
-
interleukin-12
- IL-1β
-
interleukin-1β
- IL-6
-
interleukin-6
- IL-8
-
interleukin-8
- iNOS
-
inducible nitric oxide synthase
- IV injection
-
intravenous injection
- J82 cells
-
bladder cancer cell lines
- JAK-STAT3
-
janus kinase pathway/signal transducer and activator of transcription 3
- LLC
-
Lewis lung carcinoma
- LPS
-
lipopolysaccharide
- MAPK
-
p38-mitogen-activated protein kinase
- MCF-7
-
Michigan cancer foundation-7
- MDA
-
malondialdehyde
- MGO
-
methylglyoxal
- miRNAs/miRs
-
microRNAs
- MMP3/9
-
metaloroteinase enzyme 3/9
- MMP-9
-
matrix metalloproteinase-9
- MRC-5 cells
-
normal lung fibroblast MRC-5 cells
- MRI
-
magnetic resonance imaging
- MRP1
-
multidrug resistance-associated protein 1
- mTOR
-
mammalian target of rapamycin
- NAC
-
N-acetyl-L-cysteine
- NAFLD
-
non-alcoholic fatty liver disease
- NCI-H522 cells
-
NCI-H522 human lung cancer cells
- NEFAs
-
non-esterified fatty acids
- NF-κB
-
nuclear factor kappa B
- Nps
-
nanoparticles
- Nrf2
-
nuclear factor erythroid 2–related factor 2
- ORAC
-
oxygen radical antioxidant capacity
- OS
-
osteosarcoma
- OSCC
-
oral squamous cell carcinoma
- P. acne
-
Propionibacterium acnes
- P. edulis
-
Passiflora edulis
- PANC-1
-
PaCa-2 cells, pancreatic cancer cell lines
- PARP
-
poly-ADP ribose polymerase
- PC-3
-
22Rv1, LNCaP, and VCaP, prostate cancer cells
- PCSK9
-
proprotein convertase subtilisin/kexin type 9
- PDGF
-
platelet-derived growth factor
- PD-L1
-
programmed cell death ligand 1
- PGE2
-
prostaglandin E2
- PI3K
-
phosphatidylinositol 3‑kinase
- PLIN1
-
perilipin1
- PMs
-
peritoneal macrophages
- PPARα
-
peroxisome proliferator-activated receptor α
- PTEN
-
phosphatase and TENsin homolog deleted on chromosome 10
- PTIȮ
-
2-phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl 3-oxide radical
- PTP-1B
-
protein tyrosine phosphatase-1B
- ROS
-
reactive oxygen species
- RPE-19
-
retinal pigment epithelium cells
- SAR
-
structure activity relationships
- SGC7901
-
BGC823, MKN28, MGC803, HGC27, and AGS cells, human gastric cancer cell lines
- SAM
-
S-adenosyl-l-methionine
- Sirt1
-
sirtuin 1
- SK-MES-1
-
human lung squamous cell carcinoma
- SMAD4
-
mothers against decapentaplegic homolog 4
- SNEDDS
-
self-nano-emulsifying drug delivery system
- SOD
-
super-oxide dismutase
- StS
-
sulfatase
- STS
-
stilbene synthase
- STAT
-
signal transducers and activators of transcription
- T3SS
-
type III secretion system
- TAC
-
total antioxidant capacity
- TEU
-
trolox equivalent unit
- TG
-
thapsigargin
- TNBS
-
2,4,6-trinitrobenzene sulfonic acid
- TNF-α
-
tumor necrosis factor alpha
- UGT
-
uridine 5′-diphospho-glucuronosyltransferase
- UV
-
ultraviolet radiation
- UV-C
-
ultraviolet-C
- VCAM
-
vascular cell adhesion molecule-1
- VEGF
-
vascular endothelial growth factor
- w/w %
-
weight by weight percent
- Wnt
-
wingless/integrated signaling
- XIAP
-
X-linked inhibitor of apoptosis protein
- XO
-
xanthine oxidase
- γH2AX
-
phosphorylated histone variant H2AX
Abbreviations
1 Introduction
Natural products have always played a crucial role in drug development throughout the history of humankind. This was obvious from the ancient prescriptions of herbal remedies and magical spills in Egyptian medical papyri or clay tablets in cuneiform from Mesopotamia, all dating back in time even before 2600 BCE. These prescriptions were successfully used for the treatment of many ailments, including those related to gastrointestinal, urinary tract, gynecological, and dental problems (Elagbar et al., 2020; Metwaly et al., 2021). The accumulation of knowledge in the field of traditional medicine has led to grouping, organizing, and categorizing these herbal remedies according to their effects on diseases, and thus biomedicine and chemistry came to shed light not only on the active ingredients of medicinal plants but also to investigate their mechanisms of action (Al-Jaber, 2007). Moreover, consequent efforts led to the isolation and characterization of many compounds that were consolidated into main groups and subgroups of well-known secondary metabolites based on their structural features. Accordingly, different classes of terpenoids, flavonoids, phenolic acids, stilbenoids, alkaloids, and many others were identified and characterized with a wide range spectrum of bioactivities.
Stilbenoids, such as piceatannol and resveratrol, are among the most important classes of secondary metabolites, classified as phenolic acid derivatives. Compounds belonging to this class of secondary metabolites are characterized by C6-C2-C6 carbon skeleton (cis and trans-forms, Fig. 1), linking them, in biochemical terms, to phenylpropanoids and sharing the main biosynthetic pathway of chalcones. Resveratrol and its natural analog piceatannol (Scheme 1) are the most recognized natural stilbenoids in plants (Rivière et al., 2012). These two compounds are well known for their interesting pharmacological effects, which are directly linked to their structure. Along with many other stilbenoids, resveratrol and piceatannol are known to exert a wide spectrum of bioactivities, including antioxidant, antimicrobial, anti-diabetic, anti-inflammatory, antiproliferative, cardioprotective, and neuroprotective effects (Akinwumi et al., 2018).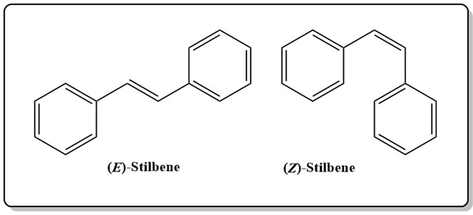
Main skeleton of stilbenes.
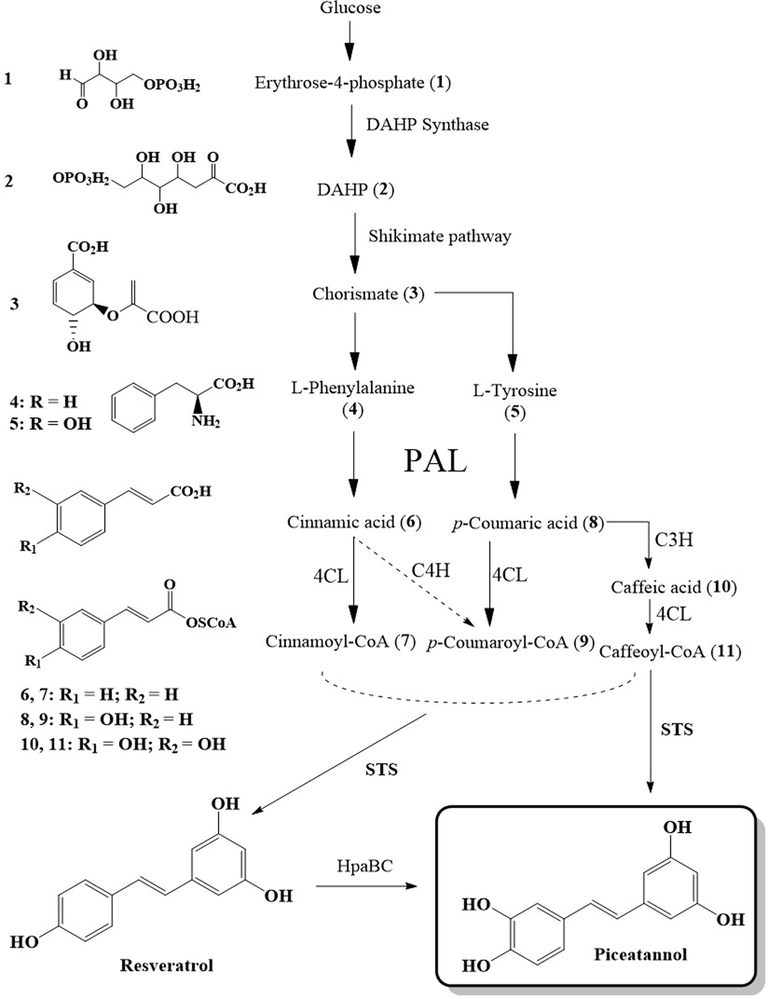
Phenylpropanoid biosynthetic pathway of stilbenes, resveratrol, and piceatannol.
Piceatannol, (known also as 3,3′,4,5′-tetrahydroxy-trans-stilbene, (E)-4-(3,5-dihydroxystyryl)benzene-1,2-diol, Fig. 2) occurs as a pale yellow powder (C14H12O4, 244.24 g/mol). Despite its poor solubility in water due to its relatively nonpolar character, it is soluble in organic solvents like ethanol and dimethyl sulfoxide (DMSO). The melting point of this compound is 223–226 °C (Seyed et al., 2016). Although this compound occurs in the trans and cis forms, the trans-isomer is the more abundant (Piotrowska et al., 2012). The IR (KBr) spectrum of this compound shows absorption bands due to the OH groups (3511, 3346 cm−1) and C=C bonds (1600, 1633 cm−1) (Osamudiamen et al., 2020). The 1H NMR (400 MHz, acetone‑d6 at 25 °C) spectrum of this compound is characterized by the CH=CH protons at δH 6.82 and 6.95, each resonating as a doublet with a high 3J coupling constant (≈16 Hz) indicating trans-configuration of the olefinic unit (Ferré-Filmon et al., 2005). Piceatannol was isolated for the first time from the heartwood of Vouacapoua americana (Shrestha et al., 2019; Banik et al., 2020). It is particularly present in numerous edible fruits such as blueberries, pomegranates, passion fruit seeds, white and red grapes, peanuts, Japanese knotweed, and white tea among others (Shrestha et al., 2019; Banik et al., 2020) in addition to Asian legumes. Different techniques were described for the extraction of this stilbene. These included simple solvent extraction (maceration at room temperature), with and without ultrasonication or microwave, employing supercritical fluid extraction or high-pressure techniques. Still, in most cases, piceatannol and many other stilbenes were recovered from plant material with conventional solid–liquid extraction, utilizing mainly alcohol or hydroalcoholic solvent (de Santana et al., 2017; Rotta et al., 2020). In this context, Viganóet al. (2016) reported the use of a high-pressure liquid extraction technique for extracting piceatannol from passion fruit seeds, the best recovery conditions were attained at 70 °C and using aqueous ethanol mixture (75 %) conditions. Ultrasound extraction of passion fruit seeds in ethanol or acetone provided a high recovery yield of piceatannol when compared to Soxhlet extraction using the same solvents (Krambeck et al., 2020). Piceatannol and other stilbenes are classified as phytoalexins synthesized by plants in response to external stress like UV radiation, fungal stress, or heavy metal contamination of the soil. For this compound, these factors were essential for its synthesis or enhancement of its content (Kershaw et al., 2022; Piotrowska et al., 2012).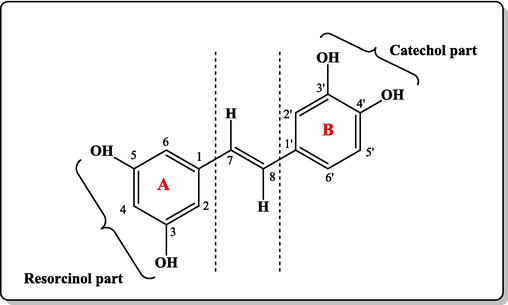
Structure of piceatannol.
Recently, piceatannol has drawn significant interest as evidenced by the increasing number of research works on this compound, its analogs, and derivatives. This interest was mainly attributed to its diverse pharmacological properties, which hold promise for the prevention and treatment of various human diseases. In this regard, research work revealed that piceatannol exhibits interesting anti-proliferative properties against different types of cancer. It has also shown a promise in addressing hypercholesterolemia and atherosclerosis and is well recognized for its antioxidant, anti-inflammatory, antidiabetic, and photoprotective effects in addition to showing preventive and curative effects in cardiovascular and cognitive diseases.
Based on the preceding discussion, the present work focuses on the pharmacological and health benefits of piceatannol and its related analogs/derivatives. Thus, relevant literature describing the biosynthesis, bioavailability, antioxidant, anti-inflammation, antitumor, cardiovascular, and metabolic effects of piceatannol its analogs and derivatives were obtained from different sources, including PubMed, Google Scholar, Scopus, and Science Direct. We strongly believe that this review will significantly add to the field and provide great help to researchers. However, further research efforts are needed to explore the potentials of this compound, its analogs, and derivatives.
2 Biosynthesis of piceatannol in plants
The phenylpropanoid pathway is responsible for the biosynthesis of stilbenes and their oxygenated derivatives in plants as shown in Scheme 1. Biosynthesis involves the production of the amino acids, phenylalanine (L-Phe, 4) or tyrosine (L-Tyr, 5) from glucose by the shikimate (Scheme 1) or arogenate pathway. These two amino acids are synthesized using 3-deoxy-D-arabino-heptulsonate-7-phsophate (DAHP) synthase through condensation of phosphoenolpyruvate (PEP) and erythrose-4-phosphate (1) to produce DAHP (2). DAHP (2) is then enzymatically transformed to chorismate (CHO, 3), the main precursor for the amino acids (L-Phe or L-Tyr) required for the biosynthesis of phenylpropanoids. The biosynthesis of simple stilbenes starts with the production of cinnamic acid (6), p-coumaric acid (8), or caffeic acid (10) from either L-Phe (4) or L-Tyr (5) by an enzymatic deamination reaction utilizing phenylalanine ammonia lyase (PAL). The acids are then converted to their cinnamoyl-CoA (7) and p-coumaroyl-CoA (9) or caffeoyl-CoA derivatives through the action of 4-coumaroyl-CoA ligase (4CL) and coenzyme A (CoA). Then, utilizing stilbene synthase (STS), simple monomeric stilbenes like resveratrol, piceatannol, and others are formed from the reaction of the corresponding CoA-ester derivative (cinnamoyl-CoA (7), p-coumaroyl-CoA (9) or caffeoyl-CoA (11)) and three malonyl-CoA in a single reaction. Piceatannol can also be obtained by the metabolic hydroxylation of resveratrol (Thapa et al., 2019; Dubrovina and Kiselev, 2017). The stilbenes obtained can then be methylated, prenylated, or glycosylated with specific enzymes.
3 Bioavailability and metabolism
In addition to its natural sources, piceatannol is also produced by CYP450 metabolism of resveratrol. However, in vivo metabolism of this compound results in glucuronidated, methylated, and sulfated derivatives. Examples of identified metabolites include rhapontigenin, isorhapontigenin, mono and di-glucuronide piceatannol in addition to O-methyl piceatannolmonoglucuronide, and O-methyl piceatannolmonosulfate (Dai et al., 2020). Due to its better bioavailability profile and therapeutic effects, piceatannol is more interesting than resveratrol. However, limited work has been conducted to investigate the bioavailability of piceatannol.
As with resveratrol, piceatannol has been reported to suffer from extensive phase II metabolism, which results in the formation of sulfonation and glucuronidation products. Piceatannol is more stable in the hepatic microsome, and phase I enzymes has no significant effects on its metabolism. The ortho-substitution pattern of the hydroxyl groups in piceatannol had a great contribution to this stability, as many stilbenoids with meta-hydroxyl groups were associated with poor bioavailability and metabolic instability.
The main metabolic pathway of piceatannol is shown in Scheme 2A, which emphasizes phase II metabolism including sulfation, glucuronidation, and O-methylation. This process involves several enzymatic pathways including sulfation enzymes (sulfatase, sulfotransferase), glucuronidation enzymes: (glucuronidase (GLU), uridine 5′-diphospho-glucuronosyltransferase (UGT)), and O-methylation enzymes and related cofactors (catechol-O-methyltransferase (COMT) and S-adenosyl-l-methionine (SAM)) in addition to CYP450 enzyme responsible for the metabolism of resveratrol to piceatannol (Dai et al., 2020). Due to the lack of clinical studies related to phase I metabolism of piceatannol, Rajanet al. (2024) conducted an in silico investigation to predict the metabolites related to the interaction of piceatannol with CYP450 enzyme in phase I metabolism. Epoxidation, methylation, and aromatic hydroxylation (Scheme 2B) were the main biotransformation products of piceatannol catalyzed by CP450 isoforms including CYP3A4, CYP2D6 & CYP2C9.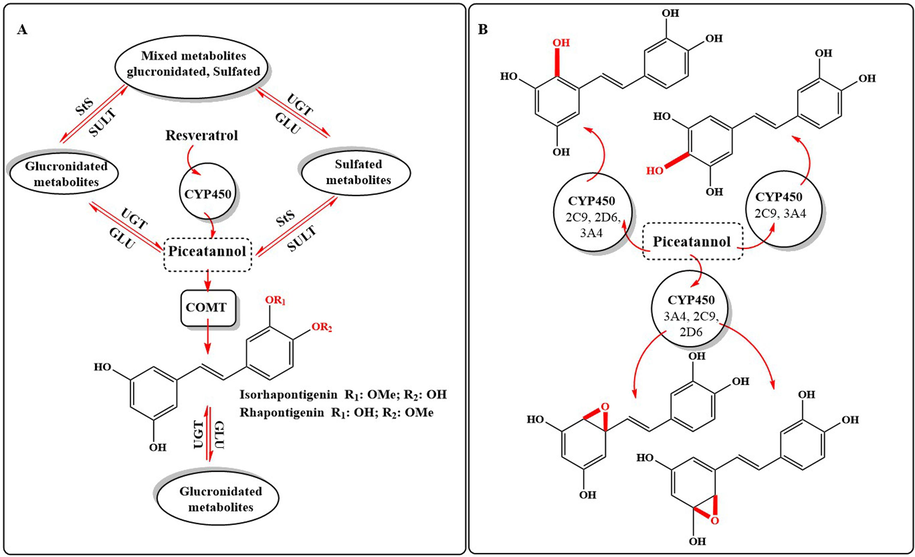
Metabolic pathways of piceatannol in A, phase II metabolism, B, in silico predicted phase I biotransformation metabolites.
4 Antioxidant activity
Oxidative stress is a condition associated with the imbalance between natural antioxidant defence systems and the production of reactive oxygen species (ROS) (Rauf et al., 2024); this imbalance is responsible for many serious pathological conditions. High generation of ROS induces oxidative stress, perturbs redox homeostasis, and damages the functionality of biomolecules like proteins, lipids, and DNA, resulting in various serious diseases like cancer, diabetes, cardiovascular, and cognitive disorders (Rauf et al., 2024). ROS are categorized into two groups including free radical species like the hydroxyl radical (•OH) and superoxide anion (O2• −) while the other is related to non-radical species like hydrogen peroxide (H2O2). These species are produced as byproducts of cellular metabolism and are important due to their prominent effect on cellular damage. However, other important reactive species produced by metabolic reactions are nitric oxide radical (NO•) and the non-radical peroxynitrite anion (ONOO–) (Fernandes et al., 2018). It is the main role of antioxidants, whether natural or synthetic, to relieve the oxidative stress of these highly reactive molecules. Medicinal plants, especially those rich with phenolic compounds and flavonoids, exhibit strong antioxidant activity and are considered as a valuable source of functional and nutraceutical formulations (Salehi et al., 2020).
Stilbenoids, due to their structural features, are recognized for their strong antioxidant activity. In this regard, methylation or glycosylation of the hydroxyl groups or even replacement with other substituents is responsible for the profound effect on their antioxidant power. Molecular coupling studies revealed that the antioxidant and neuroprotective attributes of stilbenoids are closely related to the number and position of OH groups in their bibenzyl skeleton. Skeletons with ortho-OH groups, as in piceatannol, exhibit stronger antioxidant and neuroprotective activities (Wen et al., 2018a). Similarly, the presence of two 3′,4′-dihydroxy groups (catechol part − ring B) in piceatannol plays a critical role in its ability to scavenge free radicals and capture the unpaired electrons (Rajan et al., 2024) which results in the formation of the highly stabilized piceatannol-semiquinone radical.
The antioxidant activity of piceatannol and its derivatives was extensively investigated, both in vitro and in vivo. Within the in vitro context, piceatannol obtained from Maackia amurensis showed strong ABTS•+ scavenging activity (IC50: 6.73 μM) that was higher than trolox (IC50: 16.83 μM), resveratrol (IC50: 11.15 μM), trans-ferulic acid (IC50: 13.51 μM), and chlorogenic acid (IC50: 27.23 μM) (Kim et al., 2017). Piceatannol also displayed interesting antioxidant activity at concentration levels ranging between 10 and 25 μM, which was 2-fold higher than trolox, in addition to inhibiting eicosanoid synthesis and Caco-2 growth (41.1 ± 2.3 %, p ≤ 0.05) (Storniolo and Moreno 2019). Tarbeeva et al. (2023) reported the antioxidant activity of piceatannol isolated from M. amurensis using the DPPH• and FRAP assays. Piceatannol exhibited stronger DPPH• radical scavenging activity (1.05 ± 0.12 µg/mL) as compared to ascorbic acid and quercetin (5.83 ± 0.49 and 2.81 ± 0.12 µg/mL, respectively). It also showed moderate FRAP activity (15.71 ± 1.43 µM Fe2+/µM piceatannol) when compared to ascorbic acid (3.58 ± 0.29 Fe2+/µM ascorbic acid) and quercetin (5.53 ± 0.55 µM Fe2+/µM quercetin) (Tarbeeva et al., 2023). It additionally demonstrated higher scavenging power against hypochlorous acid (HOCl) (IC50: 1.2 ± 0.5 µg/mL) and O2•− radical anion (IC50: 7.3 ± 0.02 µg/mL) (dos Santos et al., 2022) as compared to quercetin (IC50: 1.9 ± 0.3; 8.8 ± 0.3 µg/mL.; respectively). However, the DPPH• radical scavenging power of piceatannol was slightly lower than quercetin (6.3 ± 1.3 µg/mL; 4.8 ± 1.0 µg/mL., respectively). Piceatannol was the main component responsible for the antioxidant activity observed in the extract obtained from passion fruit seeds (20.4 ± 2.1 µg/mL) (dos Santos et al., 2022).
Hao et al. (2019) indicated that piceatannol exhibits interesting protective effects against H2O2-induced damage in retinal pigment epithelium cells (RPE-19). Furthermore, it reduced H2O2-induced cell death in RPE cells by 64.4 % and significantly decreased the production of ROS by 75.0 % (Hao et al., 2019). Similarly, Tang et al. (2019) reported that piceatannol and its derivative rhaponiticin (12, Fig. 3) exhibit interesting xanthine oxidase (XO) inhibition capacity (IC50: 6.44 µM; 5.97 µM., respectively) that was higher than allopurinol (IC50: 52.0 µM). Piceatannol also displayed interesting oxygen radical antioxidant capacity (ORAC IC50: 30.8 ± 3.9 mmol trolox equivalent unit (TEU)/g) (Biais et al., 2017) and exerted significant radical scavenging power against six active oxygen radicals (HO•, O2• ‾, RO•, t-BuOO•, Me•) and singlet oxygen 1O2• (IC50: 2.78, 0.11, 0.36, 7.70, 1.86, 7.0 TEU, respectively) that was higher than resveratrol and trolox (Sueishi et al., 2017). The observed activity was mainly attributed to the o-hydroxylation pattern in piceatannol’s skeleton. On the other hand, 3,3′,4′,5-tetramethoxypiceatannol (13, Fig. 3), displayed lower radical scavenging power against each of HO•, O2• ‾, RO•, t-BuOO•, Me• and 1O2• as compared to piceatannol (Sueishi et al., 2017).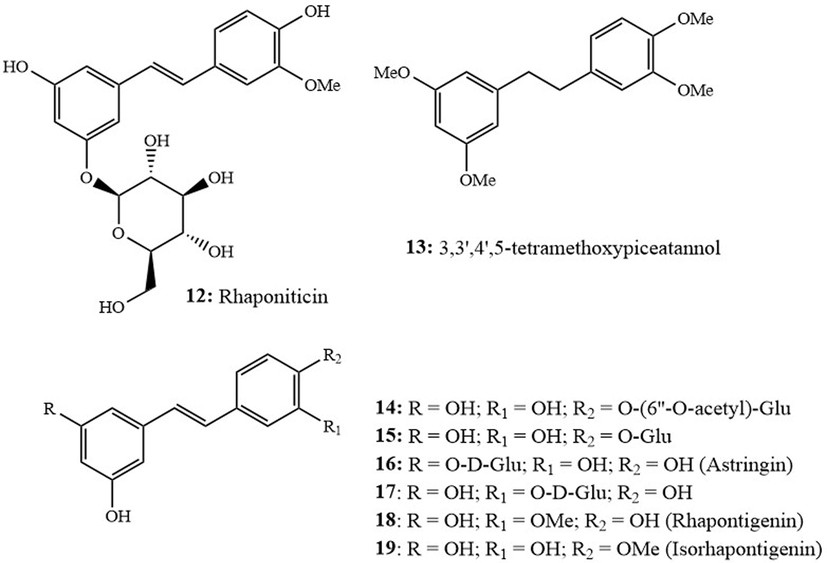
Structures of rhaponiticin (12) and 3,3′,4′,5′-tetramethoxypiceatannol (13), piceatannol-4′-O-β-D-(6′'-O-acetyl)-glucoside (14), piceatannol-4′-O-β-D-glucoside (15), astringin (16), piceatannol-3′-O-β-glucopyranoside (17), rhapontigenin (18) and isorhapontigenin (19).
Several piceatannol derivatives were investigated for their in vitro antioxidant potential. Fei et al. (2017) studied the DPPH• scavenging power of piceatannol-4′-O-β-D-(6′'-O-acetyl)-glucoside (14, Fig. 3) and piceatannol-4′-O-β-D-glucoside (15, Fig. 3). Both compounds demonstrated moderate activity, as evidenced by their IC50 values (137.32; 144.94 μM., respectively) when compared to ascorbic acid (25.40 μM). Astringin (16, Fig. 3), another derivative of piceatannol (known also as piceatannol-3-β-D-glucopyranoside), exerted strong scavenging activity against O2• ‾ radical anion and effectively inhibited ferroptosis. Both astringin (16) and piceatannol exhibited notable levels of scavenging activity against phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl 3-oxide radicals (PTIO•), which was associated with the ability of these two stilbenes to donate hydrogen atoms (Chen et al., 2021). In terms of in vivo studies, Moustafa et al. (2021), employed stressed animal models, exposed to γ-radiation of reserpine administration to study the effect of piceatannol on the mitochondrial biogenesis pathway and its role in controlling stress-caused disturbances. Results indicated that piceatannol modulated the imbalance between oxidants and antioxidants and thus enhanced both mitochondrial biogenesis and function in addition to its ability to regulate inflammatory and apoptotic responses. These findings suggest the possible development of piceatannol-adjunctive therapy for patients subjected to radiotherapy treatment (Moustafa et al., 2021).
Several published reports dealt with the effect of piceatannol on improving neurological functions. In this regard, Wang et al. (2020a) investigated the in vivo effect of this compound using a cerebral ischemia/reperfusion injured (CIRI) mouse model (C57BL/6 mice) where several important parameters were collected and studied including neurological dysfunction (neurological score and rotarod test), cognitive condition (Morris water maze test), histological studies (hematoxylin and eosin staining method), expression levels of the genes, sirtuin 1 (Sirt1), FoxO1, cleaved caspase-3 (CC-3), Bax, and B-cell lymphoma 2 (Bcl-2). In addition, the in vitro and in vivo apoptotic cells (TUNEL assay) were used to monitor the production of ROS and the antioxidant enzymes such as catalase (CAT), glutathione (GSH) peroxidase and superoxide dismutase (SOD). Results revealed that piceatannol exhibited an interesting effect on enhancing neurological functions and mitigating hippocampal neuronal damage after CIRI. Furthermore, it concomitantly enhanced the CC-3 levels, Bax expressions, and intracellular ROS while reducing the levels of non-enzymatic enzyme antioxidants in the CIRI-mouse model. Both low and high doses of piceatannol notably reduced ROS generation and the expressions of proteins linked to apoptosis, while elevating the levels of antioxidant enzymes. Additionally, it remarkably triggered the Sirt1/FoxO1 pathway. Upon suppression of the Sirt1/FoxO1 pathway, there was an increase in TUNEL positive cells and CC-3 expression, with an increase in CC-3 within neurons. It is worth mentioning that the high dose of piceatannol did not induce an elevation in non-enzymatic enzyme levels. It was concluded that piceatannol potentially exerts a neuroprotective impact on hippocampal neurons by involving the Sirt1/FoxO1 pathway (Wang et al., 2020a).
The antioxidant and neuroprotective properties of piceatannol and other related stilbenoids against Aβ25-35-induced neurotoxicity (Aβ25-35-indneuroTox) in primary cortex neurons of rats were also investigated (Wen et al., 2018a). According to this study, piceatannol showed strong capacity to eliminate •OH and DPPH• radicals as compared to resveratrol and trans-4-hydroxystilbene (26.82 %, 13.07 %, 6.25 %, respectively). It also effectively inhibited the intracellular accumulation of ROS in neurons (417 % for piceatannol at 20 µM). The neuroprotective effects of piceatannol against Aβ25-35-indneuroTox proceeded by modulating the PI3K/Akt signaling pathway and downstream mitochondria-mediated and caspase-dependent signaling pathways. In an in vitro study, Treml et al. (2019) used THP-1 macrophage-like cells and pyocyanin-induced oxidative stress to investigate the antioxidant or pro-oxidant properties of piceatannol, piceatannol-3′-O-β-glucopyranoside (17, Fig. 3), isorhapontigenin (19, Fig. 3) along with sixteen other stilbenoids. Moreover, the impact of these selected compounds on the expression of CAT, GSH, and heme oxygenase-1 (Ho-1) enzymes was examined in addition to investigating their effect on the activation of the nuclear factor erythroid 2-related factor 2 (Nrf2). Results revealed that piceatannol and piceatannol-3′-O-β-glucopyranoside (17) exhibited a significant reduction in ROS levels by 53.8 % and 41.4 %, respectively. These two compounds showed strong antioxidant effects after short- (1 h) and long-term (24 h) incubation with pyocyanin-stimulated cells. Piceatannol affected molecular targets associated with antioxidant defense, such as AP-1, Nrf2, Ho-1, COX, iNOS, NF-κB, and IKK. Moreover, piceatannol showed more potent direct scavenging activity and greater inhibition of COX-2, NF-κB, and the production of the pro-inflammatory cytokines TNF-α and IL-1β. Furthermore, incubation of cells with piceatannol at concentrations of 10–20 µM increased Ho-1 expression (Treml et al., 2019).
The antioxidant effect of several monomeric stilbenoids on the bone marrow-derived dendritic cells (bmDCs) was investigated by Johnsenet al. (2023) by measuring the effect of tested compounds on the microbially-induced intracellular ROS formation, as determined by oxidation of 6-carboxy-2′,7′-dichlorodihydrofluorescein diacetate. Results indicated that lipopolysaccharide (LPS)-induced ROS formation diminished for cells treated with the stilbenoid monomers resveratrol and piceatannol as compared to resveratrol methylated derivatives or other dimeric stilbenoids. This investigation also reported that piceatannol and resveratrol showed the highest total antioxidant capacity (TAC) rates in ABTS•+ and DPPH• assays (Johnsen et al., 2023). Piceatannol was also tested for its possible efficacy in the prevention and/or treatment of nonalcoholic fatty liver disease (NAFLD) in HepG2 hepatocytes (Yang et al., 2020). Results showed that piceatannol significantly lowers fat accumulation in HepG2 cells by suppressing lipogenesis reduced fatty acids (cluster of differentiation 36 (CD36)) and promoting oxidation (fulminant hepatic failure (FHF), peroxisome proliferator-activated receptor α (PPARα), and carnitine palmitoyltransferase 1α (CPT1α)). Results also revealed that the pathways related to the antioxidative effects could contribute to piceatannol's impact on improving steatosis. The results of this study emphasized the importance of using plant-derived phytochemicals as therapeutic agents for future NAFLD treatments (Yang et al., 2020). Piceatannol also has a beneficial effect as a supplement in preventing age-related reduction in lipid metabolism by partially improving antioxidant capacity. This indicates the possible application of this stilbene in antioxidant supplementation as a strategy to counteract age-related muscle function loss and improve whole-body metabolism (Tsukamoto-Sen et al., 2021). Summarized in Table 1 are the main results for the in vitro antioxidant activity of piceatannol and its derivatives (see Table 1).
Compound
Assay
IC50 value
References
Piceatannol
DPPH•
1.05 ± 0.12 μg/mL
(Tarbeeva et al., 2023)
6.3 ± 1.3 μg/mL
(dos Santos et al., 2022)
18 μM for 50% inhibition of DPPH
(Hosoda et al., 2021)
FRAP
15.71 ± 1.43 μM Fe2+/μM piceatannol
(Tarbeeva et al., 2023)
ABTS
6.73 ± 0.04 μM
(Kim et al., 2017)
HOCl
1.2 ± 0.5 μg/mL
(dos Santos et al., 2022)
O2•−
7.3 ± 0.02 μg/mL
(dos Santos et al., 2022)
30.7 ± 0.4 μM
(Chen et al., 2021)
PTIO•
312.5 ± 12.0 μM (pH 4.5); 219.8 ± 7.0 μM (pH 7.4)
(Chen et al., 2021)
ORAC
30.8 ± 3.9 mmol trolox Equivalent/g
(Biais et al., 2017)
Fe3+
68.2 ± 5.3 μM
(Chen et al., 2021)
Piceatannol-4'-O-β-D-(6''-O-acetyl)-glucoside (14)
DPPH•
137.32 μM
(Fei et al., 2017)
Piceatannol-4′-O-β-D-glucoside (15)
Fe3+
178.9 ± 2.8 μM
(Chen et al., 2021)
Astringin (Piceatannol-3-β-D-glucopyranoside) (16)
Fe3+
178.9 ± 2.8 μM
(Chen et al., 2021)
O2•−
37.8 ± 0.3 μM
(Chen et al., 2021)
PTIO•
411.1 ± 25.6 μM (pH4.5); 368.8 ± 12.9 μM (pH 7.4)
(Chen et al., 2021)
5 Anti-inflammatory activity
Inflammation is a defensive process of the immune system in response to harmful agents like infection or injuries (Rakib et al., 2023). Based on the time scale of illness, inflammation can be defined as either acute or chronic. Chronic inflammations, which last for a long period, have been directly or indirectly associated with chronic disorders like atherosclerosis, cancer, diabetes, and mitochondrial infections. Inflammatory response results from the production of several pro-inflammatory mediators that work as part of a complex regularity system, involving inducers, sensors, mediators, and effectors. The inflammation response is initiated by signals produced from inducers; mediators then modify the functional state of the injured tissue/organ to adjust the set of conditions causing the inflammation response (Prottay et al., 2024).
Stilbenoids are well recognized for their anti-inflammatory effects through their ability to target cyclooxygenases (COX-1, COX-2), iNOS, leukotrienes, NF-κB, tumor necrosis factor α, interleukins, and many others (Dvorakova and Landa 2017). In this respect, piceatannol was identified as a selective inhibitor of spleen tyrosine Syk kinase (Piotrowska et al., 2012), in addition to its in vitro effects in targeting kinases (serine/threonine kinases, phosphinositide-3-kinase) and other enzymes like COX-1, COX-2, ATPase, MMP-2, and MMP-9 (Dvorakova and Landa, 2017).
The anti-inflammatory properties of some natural and synthetic stilbenoids were examined with particular emphasis on their effect on inflammatory gene expression and activation of the P13K/Akt pathway (Eräsalo et al., 2018). This pathway is involved in pivotal cellular functions including the regulation of survival, motility, differentiation, and proliferation of leukocytes in addition to NF-κB-mediated transcription. Studies involved testing each of pinosylvin (20), monomethylpinosylvin (21), pterostilbene (22) (Fig. 4), resveratrol, piceatannol, rhaponiticin (12), astringin (16), and rhapontigenin (18) (Fig. 3). Of all tested compounds, piceatannol significantly inhibited Akt phosphorylation, in a dose-dependent manner, while the glycosylated derivatives did not show any interesting effect. It also down-regulated the production of the pro-inflammatory mediators NO (at 2.6 µM), IL6 (at 13.0 µM), and MCP1 (at 4.0 µM) in J774 activated macrophages. However, glycosylated piceatannol derivatives (12) and (16) were inactive. Moreover, using an in vivo carrageenan-induced paw edema mouse model of inflammation, piceatannol (at 30 mg/kg dose) showed an interesting anti-inflammatory response that was comparable to the two drugs dexamethasone (2 mg/kg) and LY294002 (15 mg/kg).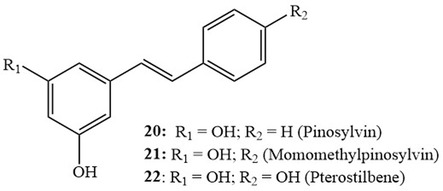
Structures of pinosylvin (20), monomethylpinosylvin (21), and pterostilbene (22).
Piceatannol and other related stilbenoids exerted their anti-inflammatory effect by inhibiting the PI4K/Akt pathway (Eräsalo et al., 2018). In another study, piceatannol, along with twenty-five other stilbenoids were screened in vitro for their ability to inhibit COX-1, COX-2, and 5-LOX enzymes. Piceatannol showed anti-inflammatory activity comparable to the positive control zileuton and had the highest inhibitory effect on the NF-κB/AP-1 pathway as compared to all tested 25 stilbenoids (Leláková et al., 2019). Piceatannol also inhibited LPS-induced oxidative stress and the resulting inflammatory reaction in brain endothelial cancer cell lines by suppressing the NF-κB and MAPK signaling pathways coupled with down-regulation of adhesion molecules (ICAM-1 and VCAM-1) which in total contributed to the reduction of ROS generation. The positive impacts of piceatannol on brain endothelial cells indicated the importance of extending more research efforts to validate the potential in vivo therapeutic effects of this compound in neurological disorders (Zhou et al., 2022). Other published reports showed that piceatannol is responsible for the anti-inflammatory effect observed for both passion fruit passage and the extract obtained from C. garettiana heartwood (Baseggio et al., 2021; Panthong et al., 2020). Within this context, Baseggio et al. (2021) revealed that piceatannol-rich extract of passion fruit bagasse exhibits anti-inflammatory effects on the liver during prostate cancer progression in TRAMP mice, in addition to significantly inhibiting TNF-α and IL-6 production at a concentration of 10-30 μM (Panthong et al., 2020). In contrast, Zhu et al. (2020) analyzed the function and mechanism of action of piceatannol in keratinocytes stimulated by Propionibacterium acnes. Results revealed that piceatannol exerts no harmful effects on the normal human keratinocyte cell line HaCaT but effectively inhibited P. acnes-induced proliferation. Moreover, piceatannol promoted translocation and targeted gene transcription of Nrf2, resulting in reduced levels of ROS. Piceatannol also impeded the movement of p65 (a component of NF-κB) into the nucleus and the release of inflammatory cytokines like interleukin-6 (IL-6), TNF-α, and interleukin-8 (IL-8), which indicated the ability of piceatannol to mitigate proliferation and migration of P. acnes-induced HaCaT cells. This was mainly achieved by leveraging the antioxidant and anti-inflammatory properties of piceatannol. Such a result highlighted the potential use of this stilbene for the treatment of acne vulgaris (Zhu et al., 2020).
A comparative study was conducted by Johnsen et al. (2023) to assess the immune-modulating effect of some selected monomeric and dimeric stilbenoids on the production of bacterially induced cytokines (IL-12, IL-10, and TNF-α) in murine bone marrow-derived dendritic cells (bmDCs) in addition to their possible effects on the LPS-induced production of ROS. Results showed that stimulation with increasing multiplicity infection of E. coli (strain, Nissle 1917) causes a dose-dependent increase of IL-10, a slight decrease in IL-12, and no effect on TNF-α production. However, the administration of piceatannol as well as resveratrol significantly inhibited E. coli-induced production of IL-12 in a dose-dependent manner. At high concentration levels (30 and 40 µM), piceatannol was the most potent inhibitor of IL-12 production (≈82 % at 40 µM) and displayed, with resveratrol, a significant inhibition of IL-10 production (Johnsen et al., 2023). This inhibition effect was mainly attributed to their ability to mitigate ROS generation.
There is substantial evidence that correlates the significant increase in activated T cells with inflammation, indicating their key role in fostering an inflammatory reaction through the release of numerous pro-inflammatory cytokines. Immune cells, like T cells and macrophages, are known for their role in controlling autoimmune diseases and regulating inflammation. Activated T cells increase with inflammation and are responsible for the production of several pro-inflammatory cytokines. Rakib et al. (2023) demonstrated that piceatannol effectively reduces the activation of CD4 and CD8 T cells, with a stronger effect on CD8 T cells, mainly through induction of regulatory T cells (Tregs) in a dose-dependent manner. Piceatannol decreased the expression of several pro-inflammatory mediators in immune cells and adipocytes and regulated adipocyte shape, size, and lipid deposition. In addition to its antiadipogenic and apoptotic activities, findings from this investigation confirmed the beneficial anti-inflammatory properties of piceatannol and its potential health benefits for the treatment of various inflammatory diseases (Rakib et al., 2023).
Piceatannol is known for its limited bioavailability resulting from poor solubility in aqueous systems (0.5 g/L H2O). Attempts to enhance the bioavailability of piceatannol were achieved using different drug delivery systems. A self-nanoemulsifying drug delivery system (SNEDDS) was formulated to enhance the solubility of piceatannol (Bachmaier et al., 2022). This formulation exerted interesting preventive effects against estradiol benzoate-induced endometrial hyperplasia (EB-EH), which was attributed to the antioxidant, anti-inflammatory, and proapoptotic activities of piceatannol in addition to modulating NF-κB and Nrf2/Ho-1 signaling pathways (Binmahfouz et al., 2022). In another study, uniformly sized albumin nanoparticles (Anp ≈120 nm in diameter) with and without piceatannol were utilized to in vivo characterize mouse neutrophils (Bachmaier et al., 2022). Two distinct neutrophil subsets coexisted simultaneously in various tissues including bone marrow, peripheral blood, spleen, and lungs in normal conditions, and following the exposure to inflammatory stimuli, one demonstrating high endocytosed Anp (Anp-high) while the other failed to uptake these nanoparticles (Anp-low). The Anp-high neutrophil subsets displayed elevated production of ROS, as well as chemokines and cytokines associated with inflammation. Piceatannol loaded-Anp formulation effectively targeted Anp-high subset and ameliorated the impact of poly-microbial sepsis by reducing tissue inflammation while preserving the essential neutrophilic host-defense capabilities (Bachmaier et al., 2022).
Several in vivo studies were conducted to investigate the anti-inflammatory effects of this compound. In this regard, the anti-inflammatory effect of piceatannol (at 10 mg/kg) was tested on 2,4,6-trinitrobenzene sulfonic acid TNBS-treated mice to investigate inflammation-driven immune dysfunction in rodent models of colitis. This Syk inhibitor significantly reduced colonic expression of pro-inflammatory genes TNFα, IL-1β, INFγ while markedly increasing the expression of the anti-inflammatory genes TGFβ and IL-10. Furthermore, piceatannol caused a significant reduction in cellular infiltrate, as evidenced by the decreased expression levels of the immune cell marker genes Cd11b, Cd8, Cd4, Dx5, Cd38, c-myc and FoxP3. Similar observations were made in a different colitis model involving dextran sulfate sodium (DSS), where piceatannol effectively lowered the expression of mRNAs associated with inflammation, including MCP-1, IL-1β, INFγ, and TNFα, which were induced by DSS in the colon (Biagioli et al., 2017). Piceatannol protected mice from inflammatory aging-induced hearing loss (ARHL) and also from defects in the inner hair cells (IHC) and spiral ganglion, which was attributed to the caspase-11 GSDMD pathway (Yang et al., 2023). These findings supported the promising implementation of piceatannol for treating hearing loss problems.
6 Anti-proliferative activity
Piceatannol is known for its significant preventive and therapeutic effects against several types of cancers, including breast, liver, spleen, kidney, prostate, and colon cancers. This is ascribed to its ability to modulate several cell-signaling pathways, affecting the expression of related proteins and genes, in addition to its ability to induce apoptosis related to the production of ROS and other mechanisms. Piceatannol is known for inhibiting important factors involved in cell proliferation and inflammation in cancer cells like COX-2. It also selectively mitigates Syk protein involved in the maintenance of vascular integrity in addition to regulating and modulating immune and inflammatory responses of hematopoietic cells. Also, it is known for inhibiting enzymes involved in cell growth and survival like ATPase in addition to downregulating the AKT/mTOR, thus affecting the proliferation of cancer cells. Several mechanisms are involved, including the ability of piceatannol to induce apoptosis, mitigate ROS, and regulate various cellular signaling pathways. In vitro and in vivo studies revealed its ability to induce tumor regression and apoptosis in addition to its effects on suppressing angiogenesis, invasion, migration, and metastasis (Banik et al., 2020), thus revealing its antitumor effects against several types of cancers such as lung cancer, lymphoma, and colorectal cancer (Huangfu et al., 2023; Cao et al., 2020; Nayyab et al., 2020).
Special interest on the chemistry and mechanism of action of piceatannol against various types of cancers was the main topic of the review by Baniket al. (2020). Piceatannol exerted its antitumor effects by regulating Akt/mTOR, NF-κB and JAK-STAT3 pathways in addition to its effect on the induction of mitochondrial dependent and independent pathways of apoptosis, through lowering the levels of anti-apoptotic proteins and enhancing the levels of pro-apoptotic proteins. The review also emphasized the antimetastatic potency of piceatannol in suppressing MMPs, Wnt/β-catenin signaling pathway, and PI3K/Akt/ mTOR, epithelial to mesenchymal transition (Banik et al., 2020). The anticancer activity of piceatannol was attributable to its ability to modulate the EGFR signaling pathway by either suppressing the EGFR phosphorylation or EGFR expression (Rakib et al., 2023). Furthermore, the review by Nayyab et al. (2020) highlighted the effect of piceatannol on modulating JAK/STAT, Wnt/β-catenin, and mTOR pathway in different cancers and shed light on its effect in the regulation of microRNAs in some cancers.
Hosoda et al. (2021) indicated that the anti-oxidative and anti-apoptotic effects of piceatannol were mediated by the Nrf2 and Ho-1. Piceatannol (at 50 mM) successfully induced mitochondrial depolarization and apoptosis in C2C12 myoblast cells. Moreover, piceatannol had superior antioxidant and anti-apoptotic effects when compared to resveratrol, mitigating apoptosis via SIRT1-dependent and independent mechanisms. Piceatannol could be considered a promising therapeutic agent for the treatment of diseases connected to ROS; however, more research work should be conducted to investigate the safety and pharmacokinetics of piceatannol in humans (Hosoda et al., 2021).
The anxiolytic effect of piceatannol and its potential impact on stress-induced growth of Lewis lung carcinoma (LLC) was investigated by Yoshizawa et al. (2020). The anxiolytic properties of piceatannol were assayed using the elevated plus maze test in addition to evaluating its pharmacological modulation effect on mice injected LLC. Results indicated that low concentration levels of piceatannol (3 mg/kg) exhibit interesting anxiolytic effects, due to its inhibitory effects on glyoxalase 1 (Glo-I). However, administration of piceatannol at high concentration levels (30 mg/kg) revealed significant antitumor effects as evidenced by its ability to suppress stress-induced tumor growth (Yoshizawa et al., 2020).
Several studies indicated that the antitumor effects of piceatannol are related to its ability to modulate microRNA expression (miRNAs/miRs), but the mechanism of action on osteosarcoma (OS) cells was not clear. miRNAs are a class of small noncoding RNAs (21–23 nucleotides), identified in mammals, and are known for their role in regulating gene expression involved in pivotal biological and pathological processes like proliferation, differentiation, and carcinogenesis. Piceatannol inhibited Saos‑2 and MG‑63 OS cell proliferation and induced apoptosis in a dose-dependent manner. The therapeutic effect of piceatannol was achieved by altering miRNAs, mainly downregulating the oncogene miR-21 responsible for OS proliferation, invasion, and apoptosis suppression. The study revealed that the effect of piceatannol on human OS cells was weakened by the overexpression of miR-21 by blocking the PTEN/AKT signaling pathway. Consequently, it was concluded that the mechanism observed in piceatannol-induced apoptosis may be regulated by the miR-21/PTEN/AKT axis in human OS cells (Zheng and Wu, 2020). Piceatannol also induced apoptosis in colon cancer cells by upregulating miR-129 (Zhang et al., 2014).
Piceatannol exerted strong antitumor activity against NCI-H522 human lung cancer cells with an IC50 value of 0.76 µM (Takasawa et al., 2017). The compound was also reported to exhibit significant antitumor activity against human lung squamous cell carcinoma (SK-MES-1) (IC50: 7.64 ± 0.5 µg/mL) that was stronger than resveratrol (>22.8 µg/mL) and its dimer (E)-ε-viniferin (IC50: 35.8 ± 1.4 µg/mL). Furthermore, piceatannol enhanced the cytotoxic and apoptotic effects of the drug, gemcitabine, against A549 lung cancer cell line (non-small cell type). This synergistic effect of the piceatannol-gemcitabine combination enhanced the expression of the Bcl-2 proapoptotic protein family (Sáez et al., 2018). In addition, piceatannol showed a better antiproliferative effect against bladder cancer cell lines J82 compared to both resveratrol and (E)-ε-viniferin (6.7 ± 0.3; 10.1 ± 0.6 and 25.7 ± 0.5 µg/mL., respectively). However, it showed no appreciable effect on normal lung fibroblast MRC-5 cells (Sáez et al., 2018).
The cytotoxicity of piceatannol against A549 cell lines was improved by utilizing an optimized formula of piceatannol-loaded bilosome zein (Biz) (Alhakamy et al.,2021). In vitro experiments using the optimized (Piceatannol-Biz) formulation significantly improved the cytotoxic potentials against A549 cell lines as evidenced by the reduction in the IC50, increased anti-proliferative activity, elevated levels of apoptosis and necrosis cell populations, and increased intracellular caspase-3 concentration levels (Alhakamy et al., 2021). Additionally, piceatannol efficiently inhibited the proliferation of several human gastric cancer cell lines, including SGC7901, BGC823, MKN28, MGC803, HGC27, and AGS (Huangfu et al., 2023). The most sensitive cells to piceatannol treatment were SGC7901, BGC823, and MKN28, showing IC50 values < 10 μmol/L after 48 h of incubation. The other cells (MGC803, HGC27, and AGS) were less sensitive to piceatannol (IC50 values ranging from 17.0–37.1 μmol/L). A detailed study on SGC7901 and BGC823 cells indicated that piceatannol causes a decrease in DNA replication and increased DNA breakage. Inhibition of tumor cells was mainly attributed to the effect of piceatannol on the phosphorylation level of Beclin-1, which affected its binding to Bcl-2 and UV- resistance-associated gene and consequently disturbed the balance between autophagy and apoptosis. It was also noticed that piceatannol induces a potent synergistic effect with the mTOR inhibitor drug “everolimus” and over a wide range of dose responses, concluding the possible application of combinatorial piceatannol/everolimus therapy in future clinical trials for gastric cancer patients (Huangfu et al., 2023).
Piceatannol exhibited the highest antiproliferative effect against Colon-26 cells (IC50: 5.87 mg/mL) as compared to other compounds detected in Itadori leaf extracts like neochlorogenic acid, rutin, and quercetin (Takemoto et al., 2023). It also showed higher anticancer activity against Caco-2 and human colorectal adenocarcinoma HT-29 (42.0 ± 9.8 µM; 69.5 ± 4.6 µM) cell lines (González-Sarrías et al., 2022) as compared to the results of oxyresveratrol (23, Fig. 5) (IC50: 55.4 ± 2.5; 83.4 ± 5.8 µM, respectively). This activity was strongly attributed to the ortho-dihydroxy substitution pattern in piceatannol in addition to its pKa1 value. Results also indicated that the lower pKa1 value of piceatannol contributed to increasing its activity against Caco-2 cells while its higher hydrophobicity contributed to improving its activity against Ht-29 cells compared to oxyresveratrol (Fig. 5) (González-Sarrías et al., 2022).
Oxyresveratrol (23).
Piceatannol displayed interesting antitumor effects, in a time- and dose-dependent manner, against two pancreatic cell lines, PANC-1 (IC50: 60 µM) and MIA PaCa-2 (IC50: 90 µM), in addition to significantly changing the expression of apoptosis-related proteins and genes, inhibiting colony formation abilities, invasion, and migration of cancerous cells, which indicated the possible consideration of piceatannol as a chemotherapeutic agent for the treatment of pancreatic cancer (Ayan et al., 2022). In this investigation, piceatannol triggered apoptosis through the mitochondrial pathway in both types of cancer cell lines and was characterized by a reduction in mitochondrial membrane potential, an increase in intercellular ROS, an increase in caspase-3–7-8–9 activity, and upregulation of proapoptotic genes like BAX, cytochrome C and reduction of anti-apoptotic proteins including Bcl-2 and TNFR1(Ayan et al., 2022). Moreover, piceatannol demonstrated an interesting protective rule in diminishing cancer-associated wasting as evidenced by its ability to reduce lipolysis by at least 50 % in both CCM-induced lipolysis cancer induced by cytokines and PANC-1-derived cancer conditioned media (CCM), in vitro. Furthermore, piceatannol modulated the stability of lipolytic proteins and protected tumor-bearing mice against weight loss in the early stages of cancer-associated cachexia (CAC) by preserving adipose tissue, with no effect on survival (Kershaw et al., 2022).
The effect of using resveratrol and piceatannol in the upregulation of programmed cell death ligand 1 (PD-L1) expression in breast and colorectal cancer cells was reported by Lucas et al. (2018). Resveratrol significantly increased the expression of PD-L1 in Cal51 breast cancer cell lines. Piceatannol, on the other hand, caused a marked increase in the level of PD-L1 in HCT116 colorectal cells. The synergistic effect of a combination of both stilbenoids was tested against several breast cancer (Cal51, BT549, BT474, and SKBR3) and colorectal (HCT116, SW480, HT29, and SW620) cell lines. The 100-combo combination prepared from resveratrol and piceatannol (50 μM each) demonstrated a synergistic upregulation of PDL-1 in Cal51 (≥4.5-fold) and SW620 cells (≥3.5-fold) than 50 μM of either compound tested alone. The upregulation of PD-L1 was mediated by the transcription NF-κB factor. Furthermore, treatment with the combo 100 formulation reduced tumor cell survival as evidenced by increased DNA damage (γH2AX), caspase-3 cleavage, decreased levels of survival markers (p38-MAPK/c-Myc), and G1-to-S cell cycle arrest (Lucas et al., 2018).
Liver fibrosis is considered a primary factor for hepatocarcinogenesis, in which Syk kinase is thought to be involved in malignant transformation in fibrotic liver. Accordingly, Syk inhibitors, like piceatannol, would play an important role in preventing hepatocarcinogenesis. The in vivo experiment conducted by the Torres-Hernandez et al. (2019) revealed that treatment of liver fibrosis with piceatannol led to an increase in the expression of p16 and p53 tumor suppressor genes while the expressions of Bcl-xL and SMAD4 decreased. The hepatic expression of genes involved in angiogenesis (Angpt2, Ccl2), apoptosis (Apaf1, Bcl2l11, Birc3, Casp7), cell cycle regulation (Mki67, Ccnd2), and cellular senescence (Map2k1, Serpinb2) were also altered by piceatannol treatment in addition to its ability to mitigate hepatic stellate cell cellular proliferation (Torres-Hernandez et al., 2019).
Several drug delivery systems were designed to enhance the bioactivity of piceatannol. Within this context, piceatannol nanoparticles coated with chitosan/poly-lactic acid (CS/Pla-PicNp) were synthesized and evaluated for their impact on human liver (HepG2), lung (A549), and breast (MCF7) cancer cell lines in terms of inhibiting proliferation and inducing apoptosis (Dhanapal and Ravindrran, 2018). These CS/Pla-PicNps significantly inhibited the proliferation of all tested cancer cell lines in a dose-dependent manner, revealing IC50 concentration values of 5, 10, and 10 µg/mL, respectively. This was attributed to the effect of these CS/Pla-PicNps on the expression of apoptotic proteins and their ability to trigger apoptosis through mitochondrial-dependent pathways in cancer cells (Dhanapal and Ravindrran, 2018). The antiproliferative, pro-apoptotic, and antioxidant properties of piceatannol against MCF-7 cancer cell lines also increased upon loading in Zein-nanoparticles. The designed nanoparticles demonstrated 24 times lower IC50 value as compared to piceatannol alone (0.71 ± 0.06 μg/mL; 17.4 μg/mL., respectively) (Algandaby and Al-Sawahli, 2021).
Hu et al. (2020) investigated the in vitro and in vivo inhibitory effect of piceatannol on vascular endothelial growth factor (VEGF)-mediated angiogenesis using human colorectal adenocarcinoma cells (HUVEC). Results revealed that piceatannol inhibits VEGF-mediated cell proliferation, cell migration, and tube formation in the HUVECs without affecting cell viability. It also inhibited the formation of intestinal veins in vivo in the embryo of zebrafish. These researchers were also able to identify the mechanism of piceatannol in inducing antiangiogenic activity, proposing that piceatannol was able to bind to VEGF, inhibiting activation of the VEGF receptors and preventing the downstream signaling and production of phosphorylated eNOS, Erk, and Akt. Based on these results, it was concluded that piceatannol can down-regulate the VEGF-mediated angiogenic activity with no cytotoxic effect by decreasing the amount of the VEGF binding to the receptors. Accordingly, it was suggested that piceatannol could be used as an efficient therapeutic agent to reduce the incidence of diseases related to angiogenesis (Hu et al., 2020).
The role of piceatannol in statin resistance/tolerance in association with its effect on the PCSK9 expression related to its p300 inhibitory (p300i) activity was also investigated (Kim et al., 2020). In this study, HepG2 cells were exposed to statin (rosuvastatin and simvastatin) with or without piceatannol in a delipidated serum medium. Piceatannol epigenetically was able to regulate the expression of statin-induced PCSK9 expression by decreasing the activity of p300 HAT (Kim et al., 2020). In another study, piceatannol was examined for its protective effects against cisplatin-induced kidney damage in rats (Wahdan et al., 2019). It was found that the administration of piceatannol at a dose of 10 mg/kg for seven days, starting two days before a single cisplatin injection (7 mg/kg), counteracted the rise in nephrotoxicity markers caused by cisplatin and restored normal kidney structure. Additionally, piceatannol reversed cisplatin-induced reductions in Nrf2 expression and the mRNA levels of antioxidant enzymes such as Ho-1, cysteine ligase catalytic and modifier subunits, superoxide dismutase, and glutathione-S-transferase (GST) activities. Furthermore, piceatannol could restrain cisplatin-induced inflammation in addition to suppressing NF-κB factor activation and lowering tissue levels of IL-1β, TNF-α, COX-2, and iNOS. Cisplatin-induced apoptosis was hindered by piceatannol through decreasing p53 and cytochrome-C expression in addition to lowering caspase-3 activity. These results indicated the nephroprotective effects of piceatannol against cisplatin-induced kidney damage with special emphasis on its role in modulating the Nrf2/Ho-1 signaling pathway while inhibiting inflammatory and apoptotic processes. Similarly, piceatannol was found to decrease the cisplatin-induced increase in serum neurotensin levels and platinum accumulation in the sciatic nerve and was responsible for mitigating the microscopic alterations observed in nerve axons and restoring normal myelin thickness (Wahdan et al., 2021).
Piceatannol was also reported to have a significant effect on the proliferation and apoptosis of EJ bladder cell lines (Li et al., 2020). EJ cell lines were incubated with different concentrations of piceatannol and then, CCK8 and western blot methods were used to evaluate its effect on the cell cycle, apoptosis, and its related signal pathways. Flow cytometric results revealed the dose- and time-dependent effect of piceatannol on EJ cell proliferation that was blocked at the G0/G1 phase as compared to the control group (p < 0.05). Apoptosis of these cell lines was also enhanced by this stilbene in a time- and dose-dependent manner. Western blotting results indicated that piceatannol upregulates the protein expression and downregulates Akt phosphorylation when compared to the control. These results suggest that piceatannol exerts its inhibiting effects of EJ cell proliferation and apoptosis by activating the PTEN/Akt signaling pathway (Li et al., 2020).
Results obtained by Lundqvist et al. (2017) focused on piceatannol’s capacity to suppress prostate cancer cell lines like LNCaP and RWPE cells that rely primarily on androgen simulation. Piceatannol inhibited dihydrotestosterone-induced activation of the androgen receptor and reduced andogenic signaling in LNCaP cells at concentrations ≥ 1 μM and concentration levels ≤ 5 μM in RWPE cells (Lundqvist et al., 2017). Similarly, Kido et al. (2020) investigated the inhibitory effect of piceatannol against PC-3, 22Rv1, LNCaP, and VCaP prostate cancer cells. This study involved exposing these cancer cell lines to different concentrations of piceatannol (10–40 μM), and then different parameters were analyzed including cell viability, Western blot, lactate measurement, and flow cytometric analysis. Results showed that piceatannol reduces the viability of all tested cell lines in a time- and concentration-dependent manner, causing cell cycle arrest at the G0/G1 phase and apoptosis in LNCaP and 22Rv1 cells. Administration of piceatannol also changed the levels of p53, p21, cyclin D1 proteins, and cyclin-dependent kinase 4 (cdk4) with no effect on glucose metabolism in these prostate cancer cells. The crucial steps for the anticancer activity of piceatannol against prostate cancer and those linked to androgen-dependent phenotype are cell cycle arrest and p53 modulation (Kido et al., 2020).
To overcome the poor pharmacokinetics of piceatannol caused by its poor water solubility, a self-nano emulsifying drug delivery system (SNEDDS) formulation was designed to evaluate the preventive effects of piceatannol against testosterone-induced benign prostatic hyperplasia (BPH) in rats (Eid and Abdel-Naim, 2020). In this in vivo study, seven groups of rat models received several treatments that included piceatannol-SNEDDS formulation, testosterone, and combinations of testosterone with varying doses of piceatannol or finasteride over a period of four weeks. The Piceatannol-SNEDDS formulation exhibited interesting antiproliferative effects by affecting specific protein and gene expressions associated with cell growth and apoptosis. This formulation reduced prostate enlargement, histopathological changes, and oxidative stress markers. Results of the immunohistochemical analysis confirmed the positive effect of piceatannol-SNEDDS in mitigating testosterone-induced inflammatory markers like TNF-α, IL-6, COX-2, iNOS, NF-κB, and preserving Nrf2 expression while increasing the expression of genes associated with antioxidant responses. Moreover, this formulation exhibited protective effects against experimentally induced BPH by modulating the Nrf2/HO-1/NFκB axis (Eid and Abdel-Naim, 2020).
Numerous studies reported the antitumor effects of piceatannol against blood cancer like leukemia and lymphoma. Previously, Siedlecka-Kroplewska et al. (2019) reported the effect of piceatannol in the process of inducing autophagy in the human T lymphoblast leukemia cell line (MOLT-4). Piceatannol increased LC3-II protein levels and reduced p62/SQSTM1 protein levels. It also triggered apoptosis in MOLT-4 cells, a process characterized by phosphatidylserine externalization, caspase-3 activation, and disruption of mitochondrial membrane potential, internucleosomal DNA fragmentation, PARP1 cleavage, chromatin condensation, and cell nucleus fragmentation. Despite these interesting effects, MOLT-4 cells developed resistance to piceatannol’s toxicity upon prolonged exposure, thus indicating the possible risk of multidrug resistance associated with piceatannol administration as a chemotherapeutic agent during leukemia treatment (Siedlecka-Kroplewska et al., 2019). Piceatannol also displayed interesting cytotoxic effects against the THP-1 human monocytic leukemia cell lines with an IC50 value of 6.7 ± 1.0 μM (Leláková et al., 2019). Piceatannol also exhibited interesting antitumor effects against HL-60, MOLT-4, THP-1, U937, and K562 leukemia cell lines. At a dose of 14 µM (corresponding to IC90), piceatannol promoted caspase-dependent apoptosis, marked experimentally by activation of caspase-3, fragmentation of internucleosomal DNA, and fragmentation of cell nuclei instead of triggering autophagy in HL-60 cell lines (Siedlecka-Kroplewska et al., 2021). Despite this interesting action, HL-60 developed resistance to piceatannol’s harmful effects through mechanisms linked to multidrug resistance-associated protein 1 (MRP1) activity. Such resistance developed by HL-60 cancer cell lines again indicated the possible risks of multidrug resistance associated with using piceatannol in chemotherapy, consequently underscoring the importance of its use in combination with other chemotherapeutic agents (Siedlecka-Kroplewska et al., 2021).
Other types of leukemia cancer cell lines, including THP-1, U937, and K562 cells (Jin et al., 2018) were also affected by piceatannol. The observed cytotoxic effect of this stilbene was associated with the induction of DNA damage and an increase in the proportion of cells in the sub-G1 phase of the cell cycle, in addition to inhibiting ROS generation. The presence of N-acetyl-L-cysteine (NAC), a known potent ROS scavenger, notably inhibited piceatannol-induced apoptosis. This effect strongly indicated that apoptosis induced by piceatannol did not result from its ability to inhibit ROS generation but was mainly attained by the downregulation of Bcl-2 and X-linked inhibitor of apoptosis protein (XIAP). Thus, the observed antitumor effects of piceatannol against HL-60, THP-1, U937, and K562 cancer cells were independent of its antioxidant activity (Jin et al., 2018). Additionally, research findings indicated that piceatannol exhibits cytotoxic effects against unstimulated RAW 264.7 cells, another macrophage-like cell line transformed by the Abelson leukemia virus derived from BALB/c mice, with an IC50 value of 20.1 μM (MTT assay method, after 24 h of treatment) that persisted at a similar level after 48 h of treatment. Data obtained from trypan blue dye exclusion assays indicated that piceatannol showed LC50 values of 8.9 μM and 9.8 μM after 24 and 48 h of incubation in RAW 264.7 macrophages, respectively (Achy-Brou and Billack, 2017). The observed cytotoxic activity of piceatannol against RAW 264.7 macrophages was higher than that observed for resveratrol (MTT IC50 (24/48 h), 20.1, 22.7 µM; trypan blue assay IC50 (24/48 h), 9.6, 10.2 µM). Additionally, the effect of piceatannol on the viability of peritoneal macrophages (PMs) isolated from Nrf2+/+ and Nrf2-/- mice was estimated. Results indicated that piceatannol induces concentration-dependent cell death in the primary cells with no significant difference between Nrf2+/+ (LC50 110 µM) and Nrf2-/- macrophages (LC50 100 ∝ M) (Achy-Brou and Billack, 2017).
In addition, piceatannol showed an inhibitory effect against malignant B16F10 melanoma cells (Yu et al., 2021). It decreased the viability of B16F10 cells in a concentration-dependent manner, which was accompanied by a reduction in invasion ability and an elongation of wound healing time. The observed cytotoxic effect against malignant B16F10 melanoma cells was attributed to Syk kinase inhibition (Yu et al., 2021). Piceatannol also exerted a robust inhibitory effect on the progression of oral squamous cell carcinoma (OSCC) tumors. During 36 days of an in vivo experiment conducted on a mice model, administration of piceatannol at 20 and 40 mg/kg/day doses significantly decreased tumor growth, in a dose-dependent pattern, from day 27 and 24 onward, respectively, as compared to the control group. The results revealed that piceatannol attenuated the expression of VEGF and matrix metalloproteinase-9 (MMP-9) in tumor tissues (Gao et al., 2017). Piceatannol (at 50 µM dose) successfully increased the percent apoptosis in stimulated T cells from 13.5 to 28.1 % as compared to the control (Rakib et al., 2023).
Other tumor cells were also affected by treatment with this stilbenoid. Piceatannol improved apoptosis of H2O2-retinal pigment epithelium cell ARPE-19, leading to a reduction in the levels of key proteins involved in apoptosis, including Bax/Bcl-2, cleaved CC-3, and cleaved PARP. Furthermore, piceatannol treatment activated the Nrf2 signaling pathway, suggesting the possible role of this stilbene in lowering the risk of age-related macular degeneration (Hao et al., 2019). Moreover, the inhibitory effect of piceatannol-3-O-β-D-glucopyranoside against human fibrosarcoma (HT1080) and human umbilical vein endothelial cells (HUVEC) was reported (Kim and Ma, 2019). This glucoside exhibited no effect on the proliferation of both cell lines at 100 μM dose, yet it suppressed the metastatic ability of HT1080 cells, reduced the production of proangiogenic factors in HT1080 cells under normoxic and hypoxic conditions, and suppressed hypoxia-induced activation of the hypoxia-inducible factor (HIF)-1α pathway. Treatment with piceatannol-3-O-β-D-glucopyranoside significantly reduced HUVEC angiogenic activity, including migration and tubular structure formation, and markedly inhibited spontaneous and VEGF-induced vessel formation (Kim and Ma, 2019). Table 2 summarizes the antitumor results for piceatannol and its related derivatives. Increased the time spent in the open arms of the EPMT compared to the control group at 3 mg/kg. Significantly suppressed stress-induced tumor growth enhancement at 30 mg/kg. Suppressed cell proliferation and induced cell apoptosis in a dose-dependent fashion. Caused overexpression of miR-21 by blocking the PTEN/AKT signaling pathway. Human lung squamous cell carcinoma SK-MES-1 Non-small cell type, A549 Normal lung fibroblast MRC-5 cells. Exhibited Strong antitumor activity against SK-MES-1. Enhanced the cytotoxic 2& apoptotic effects of gemcitabine against A549 due to increasing the expression of the Bcl-2 pro apoptotic protein family. Showed no appreciable effect against MRC-5 cells. NCI-H522 human lung cancer cells Demonstrated strong antitumor activity. J82 bladder cancer cell lines Exhibited antiproliferative effect. Human gastric cancer cell lines of SGC7901, BGC823, and MKN28 MGC803, HGC27, and AGS <10 μmol/L after 48 h of incubation Range: 17.0–37.1 μmol/L Most sensitive cells to piceatannol treatment were SGC7901, BGC823, and MKN28. Caused dysfunction of the pathogenic gene expression network in gastric cancer cells. Induced a potent synergistic effect with the mTOR inhibitor everolimus and over a wide range of dose responses. T cell Induced apoptosis in activated T cells. Piceatannol increased the frequency of apoptosis in these cells in a dose-dependent manner. Piceatannol at 50 μM dose increased the % apoptosis in stimulated T cells from 13.5% to 28.1% (compared to control). Mouse colon (Colon-26) cancer cells Strong anticancer Caco-2 Exhibited strong anticancer activity which was attributed to the ortho-dihydroxy substitution pattern in piceatannol in addition to its pKa1 values. Human colorectal adenocarcinoma HT-29 Lower pKa1 value of piceatannol enhanced its activity against Caco-2 cells. Higher hydrophobicity enhanced its activity against Ht-29 cells. PANC-1 MIA PaCa-2 60 μM 90 μM Caused antitumor effect in a time- and dose- dependent manner. Significantly changed the expression of apoptosis related proteins and genes. Inhibited colony formation abilities, invasion and migration of cancerous cells. Triggered apoptosis via the mitochondrial pathway in both cancer cell lines which was characterized by lowering mitochondrial membrane potential and causing an increase in Caspase-9 activity and upregulation of the proapoptotic genes. Caused a marked increase in PD-L1 level. Increased the expression of p16 and p53 tumor suppressor genes. Lowered the expression of Bcl-xL and SMAD4 Mitigated HSC cellular proliferation. Cell proliferation was affected in a time and dose dependent manner. Caused an inhibiting effects of EJ cells proliferation and apoptosis by activating the PTEN/Akt signaling pathway. Decreased the viability for all tested cell lines in a time and concentration dependent manner. Caused cell cycle arrest at G0/G1 phase & apoptosis in LNCaP and 22Rv1 cells. Caused change in levels of proteins p53, p21, cyclin D1 and cyclin-dependent kinase 4 (cdk4). Caused no change in glucose metabolism. In LNCaP cells, it notably reduced the androgenic signaling at concentrations ≥ 1 μM. In RWPE cells, caused a significant decrease in androgenic signaling at concentrations ≤ 5 μM. At 14 μM: it promoted caspase-dependent apoptosis, caused activation of caspase-3, fragmentation of internuclesomal DNA and fragmentation of cell nuclei. HL-60 developed resistance to piceatannol’s harmful effects through mechanisms linked to MRP1 activity. Increased the LC3-II protein levels and decreased the p62/SQSTM1 protein levels. Prolonged exposure to piceatannol decreased its toxic effects on MOLT-4 cells. THP-1 human monocytic leukemia cell line Showed high toxic activity. Leukemia cancer cell lines, including THP-1, U937, and K562 cells Cytotoxic effects against these cells were associated with the induction of DNA damage, increase in the proportion of cells in the sub-G1 phase of the cell cycle, in addition to inhibiting the generation of ROS. Cytotoxic effects. Decreased cell viability upon piceatannol administration in a concentration dependent manner. Reduced tumor growth in a dose dependent pattern. Attenuated VEGF and matrix metalloproteinase-9 (MMP-9) expression in tumor tissues. Caused a decrease in the levels of key proteins involved in apoptosis like Bax/Bcl-2, Cleaved Caspase-3, and Cleaved PARP. Caused an activation of the Nrf2 signaling pathway. Caused a decrease in the IC50, an increase in the anti-proliferative activity, an increase in the levels of apoptosis and necrosis cell populations, and an increase in intracellular caspase-3 concentration. Increased antiproliferative, pro-apoptotic and antioxidant properties against (IC50 of formulation is 24-times lower than that of piceatannol (17.4 μg/mL). HepG2 human liver MCF7 Breast cancer cell lines A549 Lung cancer cells 5 mg/mL 10 mg/mL 10 mg/mL Caused a significant inhibition of proliferation of HepG2, A549, and MCF7 cancer cell lines in a dose and time- dependent-manner. Mechanism involved influencing the expression of apoptotic proteins, triggering apoptosis through mitochondria-dependent pathways in the tested cancer cells. Decreased the prostate enlargement, histopathological changes, and oxidative stress markers. Antiproliferative effects by influencing specific protein and gene expressions associated with cell growth and apoptosis. Mitigated testosterone-induced inflammatory markers like TNF-α, IL-6, COX-2, iNOS, NF-κB, and preserved Nrf2 expression. Increased the expression of genes associated with antioxidant responses. Cal51, BT549, BT474 and SKBR3 Breast cancer cell lines HCT116, SW480, HT29 and SW620 Colorectal cell lines Caused a synergistic upregulation of PDL-1 in Cal51 (≥ 4.5-fold) and SW620 cells (≥ 3.5-fold). The 100 combo decreased the tumor cell survival, increased DNA damage (γH2AX), caspase 3 cleavage, decreased levels of survival markers (p38-MAPK/c-Myc), and G1-to-S cell cycle arrest. HT1080 (human fibrosarcoma) Cells not affected at 10 μM dose level. Suppressed the metastatic ability of HT1080 cells. Decreased the production of proangiogenic factors under normoxic and hypoxic conditions. Suppressed hypoxia-induced activation of HIF-1α pathway. HUVECs (human umbilical vein endothelial cells) Cells not affected at 10 μM dose level. Lowered the angiogenic activity, including migration and tubular structure formation, markedly inhibited spontaneous and VEGF-induced vessel formation.
Compound
Cell line
IC50 value
Notes
References
Piceatannol
7-week-old C57BL/6N mice//LLC
–
(Yoshizawa et al., 2020)
OS (Osteosarcoma) cells
–
(Zheng and Wu, 2020)
7.64 ± 0.5 μg/mL
(Sáez et al., 2018)
0.76 μM
(Takasawa et al., 2017)
6.7 ± 0.3 μg/mL
(Sáez et al., 2018)
(Huangfu et al., 2023)
–
(Rakib et al., 2023)
5.87 mg/mL
(Takemoto et al., 2023)
42.0 ± 9.8 μM
(González-Sarrías et al., 2022)
69.5 ± 4.6 μM
Pancreatic cell lines:
(Ayan et al., 2022)
HCT116 colorectal cells
–
(Lucas et al., 2018)
Hepatic stellate cell (HSC) cellular proliferation
–
Liver treatment with piceatannol resulted in:
(Torres-Hernandez et al., 2019)
Bladder cancer cell line EJ
–
(Li et al., 2020)
PC-3, 22Rv1, LNCaP, & VCaP prostate cancer cells
–
(Kido et al., 2020)
LNCaP and RWPE prostate cancer cells
–
(Lundqvist et al., 2017)
HL-60, MOLT-4, THP-1, U937 & K562 leukemia cell lines
–
(Siedlecka-Kroplewska et al., 2021)
Human T lymphoblast leukemia cell line (MOLT-4)
–
(Siedlecka-Kroplewska et al., 2019)
6.7 ± 1.0 μM
(Leláková et al., 2019)
–
(Jin et al., 2018)
RAW 264.7 leukemia virus-transformed cell line
20.1 μM
(Achy-Brou & Billack, 2017)
B16F10 Malignant melanoma cells
–
(Yu et al., 2021)
OSCC (Oral squamous cell carcinoma tumor progression)
–
(Gao et al., 2017)
ARPE-19 Retinal pigment epithelium cell lines
–
(Hao et al., 2019)
A549 Lung cancer cell lines
22.3 ± 3.4 μM
(Alhakamy et al., 2021)
Piceatannol loaded bilosome-zein
A549 Lung cancer cell lines
5.78 ± 2.3 μM
(Alhakamy et al., 2021)
Piceatannol-zein nanoparticles
MCF-7 Breast cancer cell lines
0.71 ± 0.06 μg/mL
(Algandaby and Al-Sawahli, 2021)
Chitosan/poly (lactic acid)-coated piceatannol nanoparticles (CS/Pla-PicNps)
(Dhanapal and Ravindrran, 2018)
Piceatannol-SNEDDS
Testosterone-induced benign prostatic hyperplasia (BPH)
–
(Bachmaier et al., 2022)
Resveratrol & piceatannol combination (The 100 combo; 50 μM each)
–
(Lucas et al., 2018)
Piceatannol-3-O-β-D-glucopyranoside
–
(Kim and Ma, 2019)
–
7 Antimicrobial activity
Microbial infectious diseases are one of the most significant causes of morbidity. The discovery of new antimicrobial agents is quite challenging due to the antibiotic resistance developed by bacteria in addition to the difficulties that accompany the tedious synthesis and screening of new drugs. Phenolic compounds from natural sources, especially stilbenoids, are promising candidates in this field. Stilbenoids can exert their antimicrobial effect through several mechanisms of interaction with microbes, by either inducing cell membrane damage, DNA degradation mediated by oxidative stress, or increasing cell membrane permeability that results in the leakage of intracellular proteins and nucleic acids (Li et al., 2024). Several reports described the antimicrobial activity of piceatannol and other related stilbenoids, most studies however, focused on the effect against different strains of S. aureus. In this regard, piceatannol was reported to exhibit anti-staphylococcal activity (MIC 64–256 µg/mL) against six standard S. aureus strains, including two clinical isolates, which was lower than the one observed for pterostilbene (22, MIC 32–128 µg/mL, Fig. 4) (Zakova et al., 2018). Structure-activity relationships (SAR) revealed that hydroxyl groups at the ortho-position (at 3′ and −4′ of ring B, catechol part of piceatannol skeleton) play a crucial role in the inhibitory effect of piceatannol. SAR studies also revealed that the presence of one methoxy group at position A-3 (ring A, resorcinol part of piceatannol skeleton) produces a stronger antibacterial effect against S. aureus compared to compounds with a methoxy group located at ring B of the stilbenoid skeleton (Zakova et al., 2018). Picetannol was also responsible for the dose- and time-dependent bactericidal effects observed for the dried ethanolic extract of Cassia garettiana heartwood, against S. aureus, methicillin-resistant (MR) S. aureus, S. epidermidis, E. coli, Salmonella typhi, Salmonella typhimurium, Klebsiella pneumoniae, and Shigella dysenteriae while showing no activity against Pseudomonas aeruginosa (Panthong et al., 2020).
Jusuf et al. (2020) investigated the inhibitory effect of the extract obtained from the seeds of the purple variant Passiflora edulis Sims var. edulis against Propionibacterium acnes. This piceatannol-rich extract exhibited significant antibacterial activity against P. acnes that was comparable to the activity of the positive controls, clindamycin, and erythromycin (Jusuf et al., 2020). Similarly, Sundin et al. (2020) showed that compounds with a benzofuran core structure like the synthesized resveratrol-piceatannol hybrid (24, Fig. 6) exerted significant to moderate activities against the Type III secretion system (T3SS) in the serotype of gram-negative pathogenic bacteria Yersinia pseudotuberculosis and P. aeruginosa (Sundin et al., 2020).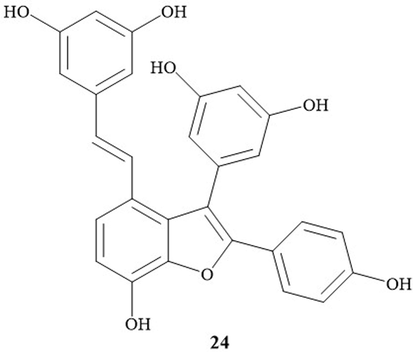
Synthesized resveratrol-piceatannol hybrid (24).
Shi et al. (2022) investigated the antibiotic activity of piceatannol, in addition to evaluating the synergistic effect and mechanism of action of piceatannol combined with some selected antibiotics, the activity was tested against six S. aureus strains (ATCC 29213, J-28, J-6, J-11, J-14 and J-9). Piceatannol showed an inhibitory effect against S. aureus strains (MIC, 64–128 µg/mL; MBC, 512–256 µg/mL). The combination of piceatannol with gentamicin, vancomycin, cefotaxime, and methicillin showed an additive effect against most of the tested S. aureus strains. In this respect, the combination of piceatannol and ciprofloxacin displayed significant synergistic effects against S. aureus strains. Piceatannol enhanced the sensitivity of S. aureus to ciprofloxacin by dissipating the bacterial proton motive force without increasing cell membrane permeability. Additionally, piceatannol inhibited bacterial ATP synthesis and exerted little effect on the haemolytic activity of mammalian erythrocytes, and displayed low levels of resistance development, indicating its safe administration in combination with other drugs as novel antibiotics (Shi et al., 2022). Parndaeng et al. (2023) studied the antimicrobial activity of five compounds isolated from Streblus taxoides wood including piceatannol and its reduced analog dihydropiceatannol (25, Fig. 7) against various bacterial strains including S. aureus, S. epidermidis, Cutibacterium acnes, and methicillin-resistant (MR) S. aureus. Both compounds showed moderate to low activity against all tested strains (S. aureus, 64, 128 µg/mL; S. epidermidis, 64, 128 µg/mL; C. acnes, > 256, > 256 µg/mL; (MR) S. aureus, 64, 128 µg/mL., respectively) as compared to the positive controls used (oxacillin, 128 µg/mL; vancomycin, 0.125 µg/mL).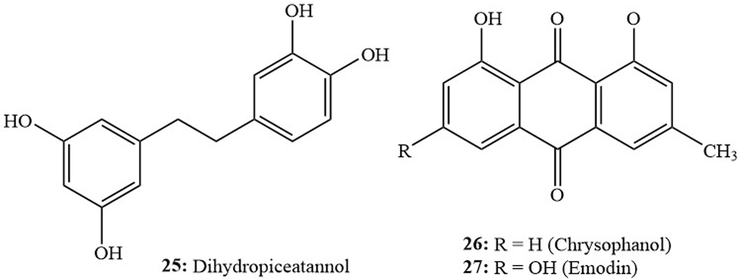
Dihydropiceatannol (25), chrysophanol (26) and emodin (27).
In addition, the antiviral potential of some stilbenoids was also evaluated, with most studies focusing only on resveratrol. However, the limited number of investigations reporting the antiviral potential of piceatannol, and its mechanism of action confirmed the effect of the hydroxylation pattern in piceatannol on the observed antiviral effects (Zlodeeva et al., 2023; Zheng et al., 2021; Wang et al., 2020b; Nakajima et al., 2020).
Herpes human cytomegalovirus (hCMV) gets activated once the immune system becomes weak. Piceatannol had a suppressing effect on hCMV. It decreased the expression of hCMV immediate-early and early proteins along with the replication of hCMV DNA in a dose dependent manner (Wang et al., 2020b). It additionally decreased ROS production and significantly suppressed the hCMV-induced cellular senescence, which was indicated by decreasing the activity of senescence galactosidase (SA-β-Gal). Piceatannol suppressed hCMV virus by inhibiting p16INK4a activation and the cellular senescence induced by this virus to reduce or hold the infection of hCMV. Piceatannol thus could be considered a potent effective drug in the treatment of chronic infection with hCMV (Wang et al., 2020b). Piceatannol also exhibited interesting anti-hepatitis B activity (HBV) with an IC50 value of 96.2 ± 4.6 µM (Nakajima et al., 2020). In a study involving the antiviral activity of various compounds isolated from the Cassia abbreviate (Yang et al., 2021), piceatannol exhibited significant activity against HIV-1 (IC50, 3.58 ± 0.27 µM) compared to the two positive controls tested enfuvirtide (IC50: 0.0096 ± 0.001 µM) and plerixafor (IC50: 0.075 ± 0.009 µM). Piceatannol also demonstrated inhibitory effects against HIV-1 infection within a dual-chamber assay simulating the female genital tract (Zheng et al., 2021) in addition to revealing microbicide effects against HSV infection. SAR studies showed that piceatannol pharmacophoric groups, characterized by the hydroxylation pattern of the two phenyl groups (resorcinol and catechol patterns), are strictly required to inhibit HIV-1 entry suggesting that further work is required on optimized synthetic derivatives of piceatannol to investigate potential therapeutic efficacy in humans (Zheng et al., 2021). Recently, Zlodeeva et al. (2023) reported the effect of piceatannol and other polyphenolic compounds as inhibitors of viral fusion through a lipid-mediated mechanism. These researchers found that piceatannol displayed a remarkable capacity in impeding the calcium (Ca)-induced fusion of negatively charged vesicles, and the extent to which this fusion was hindered depends on the hydroxylation pattern of the polyphenolic framework. Substantial enhancement of the inhibition process was observed when there were at least two OH groups in each of the phenolic rings, as exemplified by piceatannol (Zlodeeva et al., 2023). Table 3. Summarizes the most important results for the antimicrobial activity of piceatannol and some of its related derivatives. S. aureus S. epidermidis C. acnes methicillin resistant S. aureus 64 μg/mL 64 μg/mL >256 64 μg/mL SAR studies confirmed the effect of ortho-OH groups at 3' & 4' - ring B on the inhibitory effect of piceatannol. Presence of -OCH3 at 3'-of ring A increased the antibacterial effect against S. aureus. Inactive. S. aureus S. epidermidis C. acnes Methicillin Resistant S. aureus 128 μg/mL 128 μg/mL >256*128 μg/mL Y. pseudotuberculosis P. aeruginosa Significant to moderate activities. Showed microbicide effect against HSV infection. SAR studies confirmed the effect of the hydroxylation pattern in piceatannol and its inhibitory effect against HIV-1. Decreased the expression of hCMV immediate-early and early proteins- & required replication in a dose-dependant manner. Inhibited ROS production. Suppressed the hCMV induced cellular senescence. Lowered the activity of SA-β-Gal). Inhibited the activation of p16INK4a.
Compound
Species
IC50 value
Notes
References
Antimicrobial activity
Piceatannol
–
(Parndaeng et al., 2023)
S. aureus
250, 500 μg/mL
(Zakova et al., 2018)
MR. S. aureus
250, 500 μg/mL
S. epidermidis
250, 250 μg/mL
E. coli
500, 500 μg/mL
S. typhi
500, 500 μg/mL
S. typhimurium
500, 500 μg/mL
K. pneumonia
1000, 1000 μg/mL
S. dysenteriae
500, 500 μg/mL
P. aeruginosa
> 1000, >1000 μg/mL
Dihydropiceatannol (25)
–
(Parndaeng et al., 2023)
Resveratrol-piceatannol hybrid (24)
–
(Sundin et al., 2020)
Antiviral activity
Piceatannol
HIV-1
3.58 ± 0.27 μM
(Zheng et al., 2021)
Human cytomegalovirus (hCMV)
–
(Wang et al., 2020b)
8 Metabolic disorders
In line with its interesting antioxidant, anti-inflammatory, and antitumor activities, piceatannol has also shown activities against several metabolic disorders. In particular, the antidiabetic potentials of this compound have drawn special attention. Dos Santos et al., (2022) investigated the anti-glycation and antidiabetic potentials of the ethanolic P. edulis seed extract (EtOH-PESE) along with piceatannol. Piceatannol exerted high inhibitory activities against the formation of advanced glycation end-products (AGEPs) and high methylglyoxal (MGO) trapping capacity (65 %) that were higher than those observed for EtOH-PESE (21.9 %). The observed antidiabetic activity of piceatannol, reported in terms of α-glucosidase, was 12-fold higher than acarbose (IC50: 20.4 ± 7.6 l and 251.6 ± 4.5 (µg/mL, respectively). Piceatannol showed higher inhibitory potential towards α-glucosidase than α-amylase and dipeptidyl-peptidase 4 (DPP4) (dos Santos et al., 2022). Similarly, the inhibitory effect of piceatannol along with resveratrol and oxyresveratrol (Fig. 5) against the formation of AGEPs was also investigated using antiglycation assay in bovine serum albumin BSA-acrolein and BSA-methylglyoxal model (Wang et al., 2019b). Piceatannol displayed the most significant inhibitory effects with an IC50 value of 2.44 mM and 0.19 mM in the antiglycation assay within the BSA-acrolein and BSA-methylglyoxal models, respectively, that exceeded those observed for the positive control aminoguanidine (IC50: 11.11 mM and 1.21 mM, respectively), oxyresveratrol (16.58; 0.19 mM) and resveratrol (49.02; 5.71 mM) (Wang et al., 2019b).
Piceatannol was also reported to enhance the glyoxalase enzyme system's function and reduce MGO-induced damage by increasing Glo-I activity and GSH levels while alleviating oxidative damage and inflammation and improving mitochondrial biogenesis. Piceatannol lowered MGO-induced-ER (endoplasmic reticulum) stress and autophagy in addition to displaying interesting inhibitory effects against diabetic osteopathy (Suh et al., 2018), thus confirming the promising therapeutic effects of piceatannol for the treatment of diabetes and degenerative disorders related to carbonyl stress. Furthermore, based on an in vivo experiment, piceatannol showed inhibitory effects against mammalian-glucosidase activity, at a dose ranging from 14 to 84 µg/mL (Zhang et al., 2017). While the administration of piceatannol to high-fat fed C57Bl/6 mice at a dose of 14 mg/kg BW significantly inhibited the effect on postprandial blood glucose levels, resveratrol required a higher dose to achieve a similar effect (30 mg/kg BW). Since the observed effect was achieved upon the administration of each stilbene 60 min before consumption of sucrose or glucose, this strongly indicated that these two stilbenes exert their effect mainly by delaying the absorption of carbohydrates after sucrose or starch consumption which consequently resulted in a notable reduction in postprandial blood glucose concentrations (Zhang et al., 2017).
In Thai herbal traditional medicine, Senna siamea has been extensively used for the treatment of diabetes (Nuankaew et al., 2021). Compounds isolated from the different extracts of this plant showed interesting anti-insulin resistance effects in zebrafish larvae, including resveratrol, piceatannol, dihydropiceatannol (25), chrysophanol (26), and emodin (27) (Fig. 7). Off these compounds, piceatannol and dihydropiceatannol (25) showed the highest α-glucosidase inhibitory effect (IC50: 4.76 ± 1.81; 13.09 ± 0.17 µM., respectively) that exceeded the activity observed for the positive control acarbose (47.21 ± 1.41 µM) (Nuankaew et al., 2021). The efficacy of piceatannol in the early stage of diabetic nephropathy was proposed by the Borgohain research group (2017). Their research revealed that oral treatment with piceatannol (at a dose containing 30 and 50 mg/kg) for 14 days significantly restores blood sugar levels to normal. In addition, renal histopathology studies further validated the anti-diabetic kidney damage properties of piceatannol, the effects were more significant at 50 mg/kg dose level. This study highlighted the protective effects of piceatannol against diabetic renovascular damage indicating its possible clinical application in the early stage of diabetic nephropathy (Borgohain et al., 2017). Piceatannol was also reported to exhibit significant effects on adipocyte lipolysis by inhibiting nonesterified fatty acids (NEFAs) and glycerol release with concomitant reduction of ATGL, CGI- 58, and PLIN1 expression in adipocytes (Kwon et al., 2022). Degradation of these proteins was mediated by upregulation of the autophagy-lysosome pathway. Additionally, piceatannol administration lowered fasting-induced serum glycerol levels in healthy mice, lipolysis, central adiposity, and hyperinsulinemia in diet-induced obese mice. These findings underscore the importance of using foods in reducing lipolysis and its associated metabolic disorders (Kwon et al., 2022).
The potential gastroprotective properties of piceatannol on Indo-induced gastric injuries were investigated using an in vivo rat model (Shaik and Eid, 2022). Administration of piceatannol at 5 and 10 mg/kg doses effectively counteracted the increase in ulcer and lesion indices, acid production, and histological abnormalities induced by Indo-induced gastric ulcers. From a mechanistic point of view, piceatannol significantly reduced the levels of malondialdehyde (MDA), the by-product of lipid peroxidation, while simultaneously increasing the content of GSH and enhancing the activities of SOD and CAT. Furthermore, piceatannol exhibited pronounced suppression of inflammatory parameters including COX-2, IL-6, TNF-α and NF-κB while boosting levels of both mucin and prostaglandin E2 (PGE2). It also stimulated angiogenesis, as evidenced by the upregulation of proangiogenic factors including VEGF, basic fibroblast growth factor (bFGF), and platelet-derived growth factor (PDGF). The results of this study revealed the protective effect of piceatannol against Indo-induced gastric ulcers that were facilitated through its antioxidative, anti-inflammatory, and angiogenic mechanisms (Shaik and Eid, 2022).
Regarding liver diseases and liver failure, Wen et al. (2018b) examined the in vivo impact of piceatannol on D-GalN/LPS-induced FHF, in addition to its in vitro effect on pro-inflammatory cytokine production and ROS release induced by the thapsigargin (TG)-induced ER stress. In vivo experimental results revealed that piceatannol markedly decreased mortality rates and lowered serum levels of alanine transaminase and aspartic aminotransferase. It also mitigated liver damage caused by D-GalN/LPS in mice. Furthermore, piceatannol decreased the expression of pro-inflammatory cytokines (TNF-α, IL-1β, and IL-6) as well as the expression of stress markers including CHOP and phosphorylated-IRE1α. It also attenuated oxidative stress in the livers of mice treated with D-GalN/LPS. The in vitro results were in total agreement with the in vivo observations, showing that piceatannol suppressed the release of pro-inflammatory cytokines, inflammasome activation, and ROS production induced by TG, both with and without LPS priming in J774A.1 macrophage. These findings strongly suggest the use of piceatannol as a therapeutic agent to prevent acute liver failure (Wen et al., 2018b).
Similarly, Jiang et al. (2020) examined the potential inhibitory effect of piceatannol on UGT enzymes and assessed the risk of food-drug interactions by inhibiting UGTs. Furthermore, a systematic evaluation of piceatannol's inhibitory effects on UGTs using high-performance liquid chromatography, which involved measuring the rates of formation for 4-methyl umbelliferone glucuronide and imipramine N-glucuronide was conducted. The findings revealed that piceatannol exhibits a wide spectrum of inhibitory effects against human UGTs. Quantitative risk prediction analysis indicated that combining piceatannol with medications mainly metabolized by UGT1A6, UGT1A7, UGT1A8, and UGT1A9 could lead to food-drug interactions. Therefore, precautions are recommended when piceatannol-rich food is administered alongside drugs metabolized by UGT enzymes (Jiang et al., 2020).
9 Obesity and fat accumulation
The effect of piceatannol on postmenopausal obesity and its mechanism of action was recently reported by Arisawa et al. (2023). In vivo investigation was particularly related to the effect of piceatannol on body and tissue weight in high-fat-fed (HF-fed) ovariectomized C57BL/6L mice. Piceatannol significantly decreased fat volume and suppressed elevated serum total cholesterol in the HF-fed ovariectomized mice group compared to the control group, with no effect on blood glucose/insulin levels or lipogenesis in either ovariectomized or control-operated mice. Additionally, piceatannol activated the phosphorylation of hormone-sensitive lipase in the ovariectomized mice group but did not affect the expression of adipose triglyceride lipase. This effect was partially attributed to lipolysis associated with the activation of phospho-HSL in white adipose tissue and the modulation of the SIRT1/PGC1α/UCP1 pathway in brown adipose tissue (Arisawa et al., 2023).
The potential antiadipogenic and delipidating effects of piceatannol in human adipocytes derived from stroma cells were also reported (Carpéné et al., 2018). Human mesenchymal stem cells (hMSC) were obtained from adipose tissues of lean and obese individuals and then differentiated into mature adipocytes to assess the effect of piceatannol on their functions. Results showed that piceatannol, during all stages of adipocyte differentiation, significantly reduces lipid synthesis and accumulation in murine and hMSC-derived adipocytes. Moreover, it alleviated glucose transportation into adipocytes and mitigated the expressions of adipogenic regulators like PPAR-γ, FAS, and glucose transporter GLUT4. Such results indicated the promising effects of piceatannol for treating metabolic disorders associated with obesity (Carpéné et al., 2018). Similarly, the antiadipogenic properties of piceatannol and other compounds in 3 T3-L1 pre-adipocytes were reported (Mosqueda-Solís et al., 2017). The cells were subjected to varying concentrations of piceatannol, specifically 25, 10, or 1 μM, for a duration of 8 days. There was a significant reduction in lipid accumulation when the cells were exposed to 25 μM of piceatannol, resulting in a notable decrease in triacylglycerol content of approximately 31.6 ± 8.6 % (Mosqueda-Solís et al., 2017). The effect of piceatannol on fat accumulation was assessed using Caenorhabditis elegans as an animal model. Treatment of C. elegans nematodes with piceatannol at concentrations of 50 and 100 μM effectively decreased fat accumulation under normal and high-glucose conditions. The reduction in fat accumulation occurred due to the compound’s ability to suppress the genes associated with lipid synthesis and potentially promote lipolysis (Shen et al., 2017a).
10 Cardiovascular diseases
Several studies have reported promising and curative effects of piceatannol and its related derivatives on cardiovascular disease, which could be linked to its antioxidant and anti-inflammatory activities. However, the JAK2/STAT3 signaling pathway is strongly involved in many biological processes including inflammation and processes linked to tumor proliferation, apoptosis, and metastasis, and is strongly related to organ damage, septic shock, and uncontrolled inflammatory response. The inhibition of this signaling pathway is of importance for preventing myocardial injury. Xie et al. (2021) investigated the actual role of piceatannol in ameliorating cardiac function by inhibiting this signaling pathway in a septic heart model. Molecular docking, molecular dynamic simulations, and surface plasma resonance imaging studies indicated stable binding of the Jak2-piceatannol complex with favorable binding energy (–8.279 kJ/mol) revealing a high binding affinity of piceatannol to the JAK2 gene. In vivo and in vitro studies revealed that in addition to its ability to enhance cardiac function, treatment with piceatannol decreased sepsis-induced myocardial loss and suppressed myocardial inflammatory responses. Piceatannol potentially helped restore compromised cardiac function by mitigating sepsis-induced apoptosis and inflammation in both septic mice and H9c2 cells by inhibiting the JAK2/SAT3 pathway (Xie et al., 2021). Piceatannol also exerted protective effects on H9c2 cardiomyocytes against H2O2-induced damage in a dose-dependent pattern (Wang et al., 2019a). At a 40 µM dose, piceatannol restored cell viability and mitochondrial viability to a level above 80 %.
The protection effect of H9c2 cells against the induced oxidative stress was achieved by upregulating SOD activity and decreasing the production of creatine kinase (CK) and lactate dehydrogenase in addition to inhibiting the H2O2-induced reduction of mitochondrial membrane potential. Furthermore, administration of piceatannol at 10, 20, and 40 mg/mL dose levels significantly decreased Ca2+ level in H9c2 cells, in addition to exerting a significant regulating effect on P13K-Akt signaling pathway in these cardiomyocytes which is related to the preservation of normal cardiac functions (Wang et al., 2019a). Oxidative stress induced by hypoxia and cardiomyocyte apoptosis is among the essential processes in the progression of heart failure. Piceatannol displayed a distinct effect against hypoxia-induced stress for H9c2 cardiomyocytes which was achieved by suppressing oxidative stress. Administration of piceatannol at 2 µM dose post 48 hypoxia-induced stress caused a significant reduction in NO release. Additionally, treatment with piceatannol decreased lipid peroxidation in a dose-dependent manner. Moreover, 18 h pre-treatment with 2 µM piceatannol increased the expression of MnSOD protein (Boccellino et al., 2019). Piceatannol and other synthetic derivatives including 5-(3,5-dimethoxystyryl)-2-methoxyphenol (28), 4-(3,5-dimethoxystyryl)-2-methoxyphenol (29), and 4-(3,5-dimethoxystyryl) benzene-1,2-diol (30) (Fig. 8) were assayed for their inhibitory effects against 5-lipoxygenase (5-LOX). Compounds 29 and 30 showed more potent inhibitory effects against 5-LOX as concluded from the results of the cell-based assay (29: 0.07 ± 0.01; 30: 0.05 ± 0.01 µM), and cell-free assay (29: 0.78 ± 0.22; 30 0.32 ± 0.03 µM) as compared to compound 28 (5.48 ± 1.99 µM; 0.78 ± 0.22 µM), piceatannol (0.24 ± 0.09 µM; 1.1 ± 0.27 µM), resveratrol (4.9 ± 0.6 µM; 63.4 ± 2.1 µM) and the positive control (Zileuton, 1.1 ± 0.40 µM; 0.56 ± 0.1 µM) (Boccellino et al., 2019).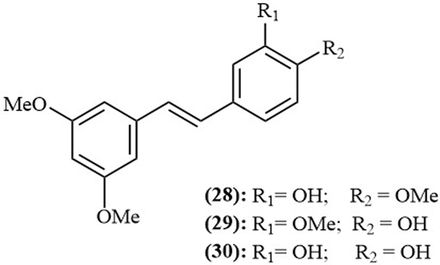
Synthesized methylated derivatives of piceatannol 28, 29 and 30.
The electrophysiological effects of piceatannol on the whole heart model experiment of atrial fibrillation were investigated (Frommeyer et al., 2019). Results indicated that simultaneous infusion of 10 μM piceatannol in rabbit heart increased the refractory atrial period and induced a significant slowing of atrial conduction and intrinsic heart rate, thus indicating the effect of the compound on stabilizing atrial repolarization (Frommeyer et al., 2019). Piceatannol also exerted a significant inhibitory effect on maximum INa in failing cardiomyocytes as compared to the control (Chang et al., 2018). Moreover, the inhibitory effect of piceatannol against signal transducer and activators of transcription (STAT) signaling pathway stimulated fetal lung growth, in a dose-dependent manner (Piairo et al., 2018). Administration of piceatannol at 10 ng/mL was recognized as the optimal concentration that had the most significant effect on growth stimulation when compared to lower doses (0.1 and 1 ng/mL) or the control (DMSO) (Piairo et al., 2018).
11 Neuroprotective activity
Several studies reported on the neuroprotective effects of piceatannol and some of its related derivatives, especially on cognitive diseases such as Alzheimer’s, epilepsy, and brain damage related to alcohol consumption during pregnancy. In phase II metabolism and mediated by COMT, a rapid clearance of piceatannol to rhapontigenin (18) and isorhapontigenin (19) was observed after intravenous (IV) injection in rats (4.02 % ± 0.61 % and 17.70 % ± 0.91 %, respectively). Accordingly, piceatannol was identified as one of the COMT inhibitors, with potential activity for the treatment of Alzheimer's disease (Dai et al., 2020). Recently, Sie et al. (2023) reported the effect of piceatannol, rhapontigenin (18), isorhapontigenin (19), gentol and resveratrol on Alzheimer’s diseases-associated factors, including inhibition of acetylcholinesterase (AChE) and amyloid-β peptide aggregation. Piceatannol showed the highest inhibitory activity against AChE (IC50: 281.47 µM) when compared to gentol (IC50: 275.53 µM). The AChE inhibitory effect of rhapontigenin (18) and isorhapontigenin (19) was almost comparable to resveratrol (IC50: 3322.3; 321.91 and 345.82 µM., respectively). Similarly, piceatannol exerted the highest inhibitory activity against amyloid-β peptide aggregations (IC50: 0.48 µM) which was higher than resveratrol (1.76 µM), gentol (IC50, 1.41 µM), isorhapontigenin (19) (2.93 µM), and rhapontigenin (18) (1.94 µM). The in vivo experiments revealed that pre-treatment with piceatannol (50 mg/kg, 1 × 7 days) ameliorates scopolamine-induced cognitive dysfunctions of amnesiac ICR mice (Sie et al., 2023). These findings were in total agreement with the results obtained by Biais et al. (2017), in which piceatannol exerted a notable neuroprotective effect against Alzheimer’s disease due to its ability to inhibit β-amyloid aggregation (Biais et al., 2017).
Other piceatannol derivatives isolated from the roots of Rheum lhasaense including piceatannol-3′-O-[2′'-(3,5-dihydroxy-4-methoxybenzoyl)]-β-D-glucopyranoside (31) and piceatannol-3′-O-(2′'-galloyl)-β-D-glucopyranoside (32) (Fig. 9), were screened for their AChE inhibition activity along with piceatannol-3′-O-glucoside (17), polydatin (33), resveratrol, and some other stilbenoids, phenolic acid derivatives, and flavonoids. Compounds 31 and 32 revealed relatively low AChE inhibition activity (IC50: 38.93 ± 1.66; 45.18 ± 8.83 µM., respectively) as compared to the positive control tacrine (0.76 ± 0.05 µM), but significantly stronger than the inhibition strength observed for piceatannol-3′-O-glucoside (17) (287.83 ± 69.32 µM) and resveratrol (IC50: 1709 µM) (Liu et al., 2021). However, piceatannol-3′-O-β-D-glucopyranoside, isolated from R. lhasaense, was investigated, in vivo, for its cognitive impairment along with the underlying mechanism of action. Results showed that administration of this compound (40 mg/kg and 160 mg/kg) mitigates scopolamine-induced cognitive dysfunction in mice by targeting BACE1-MMP3/9 pathway (Wang et al., 2023).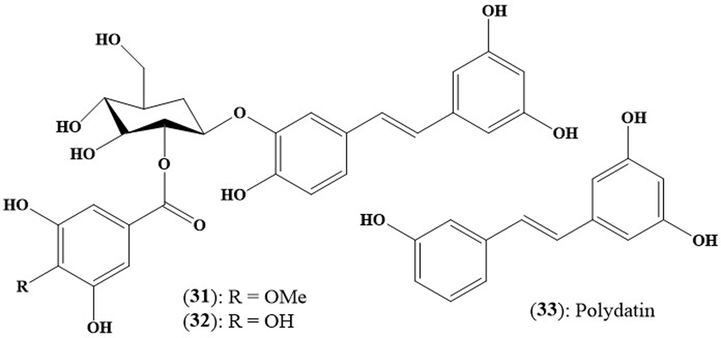
Structures of compounds 31, 32, and 33.
The possible use of resveratrol and piceatannol as natural alternatives for controlling epileptic seizures in pentylenetetrazole (PTZ)-induced seizures in adult zebrafish (Danio rerio) was investigated (Pedroso et al., 2022). Although the two compounds did not slow the PTZ-induced seizures or their development in adult zebrafish, possible activity could be observed with higher doses in models considering chronic administration (Pedroso et al., 2022).
Nutrition intake plays a vital role in neonatal neuroprotection and neurodevelopment, especially during pregnancy and breast-feeding. Alcohol exposure may have a deleterious effect on the development of the neonatal brain as it may increase the chance of the brain developing hypoxic-ischemic encephalopathy, which is the main cause of fetal mortality and neurological disability. Dumont et al. (2020) evaluated the consumption of trans-resveratrol and trans-piceatannol during pregnancy and breastfeeding in rats with hypoxic-ischemic (HI) neonate rat brain damage, sensorimotor, and cognitive impairments, concerning moderate alcohol consumption. In their experiment, trans-resveratrol and trans-piceatannol (each at 0.15 mg/kg/day) were administered in drinking water, with or without alcohol. Results revealed that consumption of moderate alcohol did not increase the brain lesions (measured by MRI) but it did increase brain impairment. Results also revealed that while administration of trans-resveratrol accompanied by alcohol consumption did not reverse the deleterious effect of HI coupled with maternal alcoholism, piceatannol supplementation interestingly caused the recovery of all the sensory and cognitive functions in a dose-dependent manner. The observed effect of piceatannol supplementation perfectly corresponded to the consumption of one passion fruit per day (Dumont et al., 2020).
12 Miscellaneous: Cosmetic, Anti-tyrosinase, and others
Piceatannol has been reported to have beneficial cosmetic effects on the skin including promotion of collagen production and inhibition of melanin synthesis. The beneficial use of piceatannol in the sun protection industry has been emphasized by Shi et al. (2020), who investigated the UV-photoprotective mechanism of piceatannol, and resveratrol extracted from grape’s skin. The accumulation of these two stilbenes on grapes’ skin increases with UV-C radiation. Moreover, UV radiation did not affect the stability of these two compounds, which indicated their ability to absorb UV radiation, thus suggesting the potential application of these two stilbenes in the sun protection industry, especially in the synthesis of new formulations like sunscreen products, sun protective clothing, or fabrics (Shi et al., 2020). Also, the effect of piceatannol-rich passion fruit seed extract on the skin of 32 healthy Japanese women aged 35–54 with dry skin was reported (Maruki-Uchida et al., 2018). In this study, two capsules of passion fruit seed extract (containing 5 mg of piceatannol) were consumed daily by the women for 8 weeks, while the placebo (control) group received dextrin. Data related to skin hydration and other parameters were collected at different time intervals during drug administration (0, 4, and 8 weeks). When compared to the skin properties before the study, piceatannol administration was associated with a significant increase in skin moisture, mainly after 4 and 8 weeks of treatment in addition to lowering the loss of trans-epidermal water decreased over time. Results showed a notable decrease in perspiration and fatigue in the piceatannol-treated group as compared to the placebo group (Maruki-Uchida et al., 2018). This suggests the beneficial use of piceatannol to promote healthy aging and combat age-related diseases in humans.
Hyperpigmentation usually follows inflammation triggered by bacterial infections. Tyrosinase is the main enzyme known to be involved in the hyperpigmentation process. However, the unique hydroxylation pattern of the bibenzyl skeleton of stilbenes is mainly responsible for their anti-tyrosinase activity. In a study by Parndaeng et al. (2023), piceatannol and dihydropiceatannol (25) extracted from Streblus taxoides wood, were investigated for their anti-tyrosinase activity. While piceatannol demonstrated moderate inhibition activity (IC50: 149.73 ± 0.86 µg/mL), dihydropiceatannol (25) showed greater tyrosinase inhibition power (IC50: 35.65 ± 0.98 µg/mL). The lower anti-tyrosinase activity observed for piceatannol was linked to the lack of oxygenation pattern found in the resorcinol skeleton (Likhitwitayawuid, 2008). It is noteworthy that both, piceatannol and dihydropiceatannol (25) showed no significant cytotoxic effects On B16-F1 melanoma cells, with cell viability remaining above 80 %. Docking studies revealed that the bibenzyl structure of both compounds was anchored at the active site of mushroom tyrosinase, indicating a mode of inhibition as a competitive inhibitor (Parndaeng et al., 2023).
The study by Ha et al. (2018) revealed that piceatannol exhibits a notable inhibition of protein tyrosine phosphatase-1B (PTP-1B), as evidenced by its IC50 value (4.81 ± 0.21 μM), when compared to the positive control, ursolic acid (IC50: 11.34 ± 0.01 μM). The observed inhibitory effect of piceatannol on PTP-1B was associated with the presence of two hydroxyl groups at positions 3′ and 4′ within the stilbene structure. It was reported that piceatannol 4′-methyl ether displayed lower inhibitory activity against PTP-1B, with an IC50 exceeding 100 μM, thus further confirming the effect of the substitution pattern on its effectiveness (Ha et al., 2018). On the other hand, several polyphenolic compounds were tested for their mammalian arginase inhibitory effect including resveratrol, piceatannol, and their condensation oligomers scirpusin A (34) and cyperusphenol A (35) (Fig. 10). The two oligomers showed an interesting arginase inhibitory effect (IC50: 17.6 ± 2.2 µM, 19.4 ± 1.3 µM) that were close to that observed for piceatannol (IC50: 12.6 ± 0.6 µM) despite being slightly less active than the positive control (Nω-hydroxy-nor-L-arginine, IC50: 1.7 ± 0.2 µM) (Arraki et al., 2021). The arginase inhibitory activity of piceatannol was also compared to resveratrol and other phenolic acids (chlorogenic acid, caffeic acid, quinic acid) and flavonoids ((−)-epicatechin, taxifolin, quercetin, fisetin, and kaempferol). Among all tested compounds, chlorogenic acid (IC50: 10.6 μM) and piceatannol (IC50: 12.1 μM) showed the highest arginase inhibitory activity (Bordage et al., 2017).
Structures of scirpusin A (34) and cyperusphenol A (35).
Piceatannol was investigated for its effect on the life span of Caenorhabditis elegans (Shen et al., 2017b). Results revealed that at 50 and 100 µM doses, piceatannol significantly extended the lifespan of C. elegans without altering growth rate, worm size, and progeny production. Piceatannol also delayed the age-related decrease in pumping rate and locomotive activity, in addition to protecting against heat and oxidative stress. Additionally, the results obtained are in favor of the positive impact of piceatannol in issues related to promoting healthy aging and combating age-related diseases in humans. Furthermore, piceatannol-3′-O-β-D-xylopyranoside (36, Fig. 11), isolated from Rheum undulatum showed an interesting potent binding affinity for estrogen receptors as compared to 17β-estrodiol (Park et al., 2018).
Structures of compounds 23.
13 Conclusions
This review has consolidated the latest important biological activity findings of piceatannol and its analogs/derivatives. In addition to being more metabolically stable than its precursor resveratrol, other factors contributed to its significant and wide spectrum of bioactivities. SAR studies confirmed the effect of the ortho-dihydroxyl groups in ring B (catechol moiety, Fig. 2) in the wide spectrum bioactivity of this molecule. Blocking of these hydroxyl groups, by methylation, reduced the observed antioxidant activity of piceatannol and mitigated its ability to inhibit enzymes responsible for the formation of ROS or nitrogen-reactive species (Treml et al., 2019). However, despite lowering its antioxidant activity, structural modification contributed to increasing the bioavailability of this molecule and consequently enhanced its activity in certain pathways. The wide range of pharmacological characteristics observed for piceatannol reveals an astonishing therapeutic promise for several medical disorders. This is attributed to the effect of piceatannol in mediating numerous signaling pathways and thus exerting its effect by affecting protein/gene expressions and enzyme activities (Fig. 12).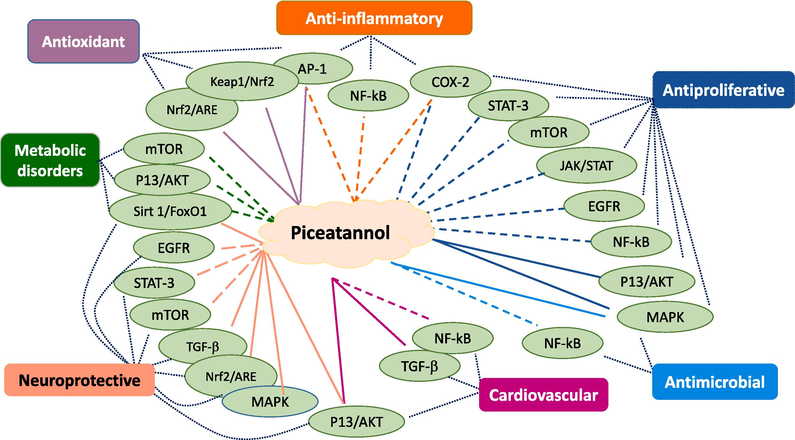
Effects of piceatannol on various common pathways connected to its bioactivities (dashed line: inhibition, solid line: enhancement of the indicated pathway).
Furthermore, piceatannol offers itself as a desirable molecule for future drug development and supplementary medicine because of its anti-inflammatory and antioxidant properties as well as its potential to treat cancer, cardiovascular diseases, neurological and cognitive disorders, metabolic disorders, and diseases connected with ROS (Rakib et al., 2023; Zhou et al., 2022, Suh et al., 2018; Borgohain et al., 2017). Piceatannol also displayed interesting properties associated with promoting healthy aging, combating age-related diseases in humans, and skin care improvement. Additionally, the combination of piceatannol with various drugs (antibiotics, chemotherapeutic agents) revealed interesting synergistic effects indicating the possible application of combinatorial drug therapy for the treatment of bacterial infections and cancer treatment (Huangfu et al., 2023). Nevertheless, to completely understand its therapeutic actions, ideal dosage, potential side effects, and the possibility of drug resistance, more research efforts, including clinical trials and mechanistic studies—are required. The therapeutic applications of piceatannol present promising opportunities for enhancing human health and tackling intricate medical issues in the years to come, notwithstanding these obstacles.
CRediT authorship contribution statement
Hala I. Al-Jaber: Writing – review & editing, Writing – original draft, Supervision, Resources, Investigation, Data curation, Conceptualization. Ashok K. Shakya: Writing – review & editing, Resources, Data curation, Conceptualization. Mahmoud A. Al-Qudah: Writing – review & editing, Resources, Data curation. Lina M. Barhoumi: Writing – review & editing, Resources, Data curation. Hana E. Abu-Sal: Writing – original draft, Resources, Data curation. Hazem S. Hasan: Writing – original draft, Investigation, Data curation. Nezar Al-Bataineh: Resources. Sultan Abu-Orabi: Writing – review & editing, Resources. Mohammad S. Mubarak: Writing – review & editing, Conceptualization.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- A comparative assessment of the cytotoxicity and nitric oxide reducing ability of resveratrol, pterostilbene and piceatannol in transformed and normal mouse macrophages. Drug Chem. Toxicol.. 2017;40(1):36-46.
- [CrossRef] [Google Scholar]
- Biological activities of stilbenoids. Int. J. Mol. Sci.. 2018;19(3):792.
- [CrossRef] [Google Scholar]
- Augmentation of anti-proliferative, pro-apoptotic and oxidant profiles induced by piceatannol in human breast carcinoma MCF-7 cells using zein nanostructures. Biomed. Pharmacother.. 2021;138:111409
- [CrossRef] [Google Scholar]
- Piceatannol-loaded bilosome-stabilized zein protein exhibits enhanced cytostatic and apoptotic activities in lung cancer cells. Pharmaceutics. 2021;13:638.
- [CrossRef] [Google Scholar]
- Chemical constituents of Flora of Jordan – Part (XXI). Chemical constituents of Salvia palaestina and Osyris alba. Amman, Jordan: The University of Jordan; 2007. PhD thesis,
- Piceatannol prevents obesity and fat accumulation caused by estrogen deficiency in female mice by promoting lipolysis. Nutrients. 2023;15:1374.
- [CrossRef] [Google Scholar]
- Mammalian arginase inhibitory activity of methanolic extracts and isolated compounds from Cyperus Species. Molecules. 2021;26(6):1694.
- [CrossRef] [Google Scholar]
- Piceatannol induces apoptotic cell death through activation of caspase-dependent pathway and upregulation of ROS-mediated mitochondrial dysfunction in pancreatic cancer cells. Mol. Biol. Rep.. 2022;49:11947-11957.
- [CrossRef] [Google Scholar]
- Albumin nanoparticle endocytosing subset of neutrophils for precision therapeutic targeting of inflammatory tissue Injury. ACS Nano. 2022;16(3):4084-4101.
- [CrossRef] [Google Scholar]
- Piceatannol: a natural stilbene for the prevention and treatment of cancer. Pharmacol. Res.. 2020;153:104635
- [CrossRef] [Google Scholar]
- Systemic antioxidant and anti-inflammatory effects of yellow passion fruit bagasse extract during prostate cancer progression. J. Food Biochem.. 2021;46(3):e13885
- [CrossRef] [Google Scholar]
- Genetic and pharmacological dissection of the role of spleen tyrosine kinase (Syk) in intestinal inflammation and immune dysfunction in inflammatory bowel diseases. Inflamm. Bowel Dis.. 2017;24(1):123-135.
- [CrossRef] [Google Scholar]
- Antioxidant and cytoprotective activities of grapevine stilbenes. J. Agric. Food Chem.. 2017;65(24):4952-4960.
- [CrossRef] [Google Scholar]
- Piceatannol SNEDDS attenuates estradiol-induced endometrial hyperplasia in rats by modulation of NF-kB and Nrf2/HO-1 Axes. Nutrients. 2022;14:1891.
- [CrossRef] [Google Scholar]
- Protective effect of piceatannol and bioactive stilbene derivatives against hypoxia-induced toxicity in H9c2 cardiomyocytes and structural elucidation as 5-LOX inhibitors. Eur. J. Med. Chem.. 2019;180:637-647.
- [CrossRef] [Google Scholar]
- Investigation of mammal arginase inhibitory properties of natural ubiquitous polyphenols by using an optimized colorimetric microplate assay. Planta Med.. 2017;83:647-653.
- [CrossRef] [Google Scholar]
- Small molecule inhibiting nuclear factor-kB ameliorates oxidative stress and suppresses renal inflammation in early stage of alloxan-induced diabetic nephropathy in Rat. Basic Clin. Pharmacol. Toxicol.. 2017;120(5):442-449.
- [CrossRef] [Google Scholar]
- Overview of cellular mechanisms and signaling pathways of piceatannol. Curr. Stem Cell Res. Ther.. 2020;15(1):4-10.
- [CrossRef] [Google Scholar]
- The dietary antioxidant piceatannol inhibits adipogenesis of human adipose mesenchymal stem cells and limits glucose transport and lipogenic activities in adipocytes. Int. J. Mol. Sci.. 2018;19(7):2081.
- [CrossRef] [Google Scholar]
- Inhomogeneous down-regulation of INa underlies piceatannol pro-arrhythmic mechanism in regional ischemia-reperfusion. Pacing Clin. Electrophysiol.. 2018;41(9):1116-1122.
- [CrossRef] [Google Scholar]
- Comparison of ferroptosis-inhibitory mechanisms between ferrostatin-1 and dietary stilbenes (piceatannol and astringin) Molecules. 2021;26(4):1092.
- [CrossRef] [Google Scholar]
- Biotransformation of piceatannol, a dietary resveratrol derivative: Promises to human health. Mol. Nutr. Food Res.. 2020;64(2):e1900905
- [CrossRef] [Google Scholar]
- Optimization of the antioxidant polyphenolic compounds extraction of yellow passion fruit seeds (Passiflora edulis Sims) by response surface methodology. J. Food Sci. Technol.. 2017;54(11):3552-3561.
- [CrossRef] [Google Scholar]
- Chitosan/poly (lactic acid)-coated piceatannol nanoparticles exert an in vitro apoptosis activity on liver, lung and breast cancer cell lines. Artif Cells Nanomed. Biotechnol.. 2018;46(sup1):274-282.
- [CrossRef] [Google Scholar]
- Antidiabetic, antiglycation, and antioxidant activities of ethanolic seed extract of Passiflora edulis and piceatannol in vitro. Molecules. 2022;27(13):4064.
- [CrossRef] [Google Scholar]
- Regulation of stilbene biosynthesis in plants. Planta. 2017;246(4):597-623.
- [CrossRef] [Google Scholar]
- Maternal alcoholism and neonatal hypoxia-ischemia: Neuroprotection by stilbenoid polyphenols. Brain Res.. 2020;738:146798
- [CrossRef] [Google Scholar]
- Anti-inflammatory activity of natural stilbenoids, A Review. Pharmacol. Res.. 2017;124:126-145.
- [CrossRef] [Google Scholar]
- Piceatannol attenuates testosterone-induced benign prostatic hyperplasia in rats by modulation of Nrf2/HO-1/NFκB Axis. Front. Pharmacol.. 2020;11:614897
- [CrossRef] [Google Scholar]
- Phytochemical diversity and pharmacological properties of Rhus coriaria. Chem. Biodiversity. 2020;17(4):e1900561
- [CrossRef] [Google Scholar]
- Natural stilbenoids have anti-inflammatory properties in Vivo and down-regulate the production of inflammatory mediators NO, IL6, and MCP1 Possibly in a PI3K/Akt-Dependent Manner. J. Nat. Prod.. 2018;81(5):1131-1142.
- [CrossRef] [Google Scholar]
- Phenolic constituents from Rheum nobile and their antioxidant activity. Nat. Prod. Res.. 2017;31(24):2842-2849.
- [CrossRef] [Google Scholar]
- Fernandes, R. P. P., Trindade, M. A., de Melo, 2018. Natural Antioxidants and Food Applications: Healthy Perspectives, In: Grumezescu, A., Holban, A-M (Eds.). In: Alternative and Replacement Foods, 1st edition 17. Elsevier Science, pp. 31–64. doi:10.1016/B978-0-12-811446-9.00002-2.
- Stereoselective synthesis of (E)-hydroxystilbenoids by Ruthenium-catalyzed cross-Metathesi. Eur. J. Org. Chem.. 2005;15:3319-3325.
- [CrossRef] [Google Scholar]
- Acute electrophysiologic effects of the polyphenols resveratrol and piceatannol in rabbit atria. Clin. Exp. Pharmacol. Physiol.. 2019;46(1):94-98.
- [CrossRef] [Google Scholar]
- Activated spleen tyrosine kinase promotes malignant progression of oral squamous cell carcinoma via mTOR/S6 signaling pathway in an ERK1/2-independent manner. Oncotarget. 2017;8(48):83900-83912.
- [CrossRef] [Google Scholar]
- Main determinants affecting the antiproliferative activity of stilbenes and their gut microbiota metabolites in colon cancer cells: A structure–activity relationship study. Int. J. Mol. Sci.. 2022;23:15102.
- [CrossRef] [Google Scholar]
- PTP1B inhibitory activity and molecular docking analysis of stilbene derivatives from the rhizomes of Rheum undulatum L. Fitoterapia. 2018;131:119-126.
- [CrossRef] [Google Scholar]
- Piceatannol protects human retinal pigment epithelial cells against hydrogen peroxide induced oxidative stress and apoptosis through modulating PI3K/Akt signaling pathway. Nutrients. 2019;11(7):1515.
- [CrossRef] [Google Scholar]
- Different antioxidative and antiapoptotic effects of piceatannol and resveratrol. J. Pharmacol. Exp. Ther.. 2021;376:385-396.
- [CrossRef] [Google Scholar]
- Piceatannol, a natural analog of resveratrol, exerts anti-angiogenic efficiencies by blockage of vascular endothelial growth factor binding to its receptor. Molecules. 2020;25(17)
- [CrossRef] [Google Scholar]
- Piceatannol enhances Beclin-1 activity to suppress tumor progression and its combination therapy strategy with everolimus in gastric cancer. Sci. China Life Sci.. 2023;66(2):298-312.
- [CrossRef] [Google Scholar]
- Piceatannol exhibits potential food-drug interactions through the inhibition of human UDP-glucuronosyltransferase (UGT) in Vitro. Toxicol. in Vitro. 2020;67:104890
- [CrossRef] [Google Scholar]
- Piceatannol-induced apoptosis is reversed by N-acetyl-L-cysteine through restoration of XIAP expression. Biol. Pharm. Bull.. 2018;41(9):1372-1378.
- [CrossRef] [Google Scholar]
- Investigation of the effects of monomeric and dimeric stilbenoids on bacteria-induced cytokines and LPS-induced ROS formation in bone marrow-derived dendritic cells. Int. J. Mol. Sci.. 2023;24:2731.
- [CrossRef] [Google Scholar]
- Antibacterial activity of passion fruit purple variant (Passiflora edulis Sims var. edulis) seeds extract against Propionibacterium acnes. Clin. Cosmet. Investig. Dermatol.. 2020;13:99-104.
- [CrossRef] [Google Scholar]
- Piceatannol, a dietary polyphenol, alleviates adipose tissue- loss in pre-clinical model of cancer-associated cachexia via lipolysis inhibition. Nutrients. 2022;14:2306.
- [CrossRef] [Google Scholar]
- Prevention of prostate cancer in transgenic adenocarcinoma of the mouse prostate mice by yellow passion fruit extract and antiproliferative effects of its bioactive compound piceatannol. J. Cancer Prev.. 2020;25(2):87-99.
- [CrossRef] [Google Scholar]
- Rapid identification and isolation of inhibitors of rat lens aldose reductase and antioxidant in Maackia amurensis. Biomed. Res. Int.. 2017;2017:4941825.
- [CrossRef] [Google Scholar]
- Piceatannol reduces resistance to statins in hypercholesterolemia by reducing PCSK9 expression through p300 acetyltransferase inhibition. Pharmacol Res.. 2020;161:105205
- [CrossRef] [Google Scholar]
- Piceatannol-3-O-β-D-glucopyranoside (PG) exhibits in vitro anti-metastatic and anti-angiogenic activities in HT1080 malignant fibrosarcoma cells. Phytomedicine. 2019;57:95-104.
- [CrossRef] [Google Scholar]
- Identification and quantificatin of stilbenes (piceatannol and resveratrol) in Passiflora edulis by-products. Pharmaceuticals (basel). 2020;13(4):73. PMID: 32326010; PMCID: PMC7243114
- [CrossRef] [Google Scholar]
- Piceatannol antagonizes lipolysis by promoting autophagy-lysosome-dependent degradation of lipolytic protein clusters in adipocytes. J. Nutr. Biochem.. 2022;105:108998.
- [CrossRef] [Google Scholar]
- Parallel in vitro and in silico investigations into anti-inflammatory effects of non-prenylated stilbenoids. Food Chem.. 2019;285:431-440.
- [CrossRef] [Google Scholar]
- Progress of antimicrobial mechanisms of stilbenoids. Pharmaceutics. 2024;16:663.
- [CrossRef] [Google Scholar]
- Piceatannol inhibits proliferation and induces apoptosis of bladder cancer cells through regulation of the PTEN/AKT signal pathway. Cell Mol. Biol. (noisy-Le-Grand). 2020;66(3):181-184.
- [CrossRef] [Google Scholar]
- Stilbenoids isolated from the roots of Rheum lhasaense under the guidance of the acetylcholinesterase inhibition activity. J. Nat. Med.. 2021;75:372-380.
- [CrossRef] [Google Scholar]
- Upregulation of PD-L1 expression by resveratrol and piceatannol in breast and colorectal cancer cells occurs via HDAC3/p300-mediated NF-κB signaling. Int. J Oncol.. 2018;53(4):1469-1480.
- [CrossRef] [Google Scholar]
- Resveratrol, piceatannol and analogs inhibit activation of both wild-type and T877A mutant androgen receptor. J. Steroid Biochem. Mol. Biol.. 2017;174:161-168.
- [CrossRef] [Google Scholar]
- Effect of passion fruit seed extract rich in piceatannol on the skin of women: A randomized, placebo-controlled, double-blind trial. J. Nutr. Sci. Vitaminol.. 2018;64(1):75-80.
- [CrossRef] [Google Scholar]
- Metwaly, A. M., Ghoneim, M. M., Eissa, I. H., Elsehemy, I. A., Mostafa, A. E., Hegazy, M. M., Afifi, W. M., Dou, D., 2021. Traditional ancient Egyptian medicine: A review. Saudi J. Biol. Sci. 2021, 28 (10), 5823–5832. doi: 10.1016/j.sjbs.2021.06.044.
- Screening of potential anti-adipogenic effects of phenolic compounds showing different chemical structure in 3T3-L1 preadipocytes. Food Funct.. 2017;8(10):3576-3586.
- [CrossRef] [Google Scholar]
- Piceatannol promotes hepatic and renal AMPK/SIRT1/PGC-1α mitochondrial pathway in rats exposed to reserpine or gamma-radiation. Int. J. Immunopathol. Pharmacol.. 2021;35(1–15) 20587384211016194
- [CrossRef] [Google Scholar]
- Non-nucleoside hepatitis B virus polymerase inhibitors identified by an in vitro polymerase elongation assay. J. Gastroenterol.. 2020;55(4):441-452.
- [CrossRef] [Google Scholar]
- Piceatannol mediated regulation of deregulated signaling pathways in different cancers: Tumbling of the ninepins of molecular oncology. Cell Mol. Biol. (noisy-Le-Grand). 2020;66(6):157-163.
- [CrossRef] [Google Scholar]
- Anti-insulin resistance effect of constituents from Senna siamea on zebrafish model, its molecular docking, and structure–activity relationships. J. Nat. Med.. 2021;75:520-531.
- [CrossRef] [Google Scholar]
- Trans-resveratrol, piceatannol and gallic acid, potent polyphenols isolated from Mezoneuron benthamianum effective as anticaries, antioxidant and cytotoxic agents. Sci. Afr.. 2020;7:e00244
- [CrossRef] [Google Scholar]
- Bactericidal effect and anti-Inflammatory activity of Cassia garettiana Heartwood extract. Sci. World J.. 2020;1653180
- [CrossRef] [Google Scholar]
- Estrogenic activity of constituents from the rhizomes of Rheum undulatum Linné. Bioorg. Med. Chem. Lett.. 2018;1528(4):552-557.
- [CrossRef] [Google Scholar]
- Chemical constituents from Streblus taxoides wood with their antibacterial and antityrosinase activities plus in silico study. Antibiotics. 2023;12:319.
- [CrossRef] [Google Scholar]
- Evaluation of resveratrol and piceatannol anticonvulsant potential in adult zebrafish (Danio rerio) Neurochem. Res.. 2022;47:3250-3260.
- [CrossRef] [Google Scholar]
- STATs in lung development: Distinct early and late expression, growth modulation and signaling dysregulation in congenital diaphragmatic hernia. Cell Physiol. Biochem.. 2018;45(1):1-14.
- [CrossRef] [Google Scholar]
- Biological activity of piceatannol: Leaving the shadow of resveratrol. Mutat. Res. Rev. Mutat. Res.. 2012;750(1):60-82.
- [CrossRef] [Google Scholar]
- Sclareol exerts an anti-inflammatory effect, possibly through COXs inhibition pathway, in vivo and in silico studies. Pharmaceut. Sci. Adv.. 2024;2:100029
- [CrossRef] [Google Scholar]
- Predicting phase-I metabolism of piceatannol, an in silico study. In Silico Pharmacol.. 2024;12:52.
- [CrossRef] [Google Scholar]
- Piceatannol induces regulatory T cells and modulates the inflammatory response and adipogenesis. Biomed. Pharmacother.. 2023;161:114514
- [CrossRef] [Google Scholar]
- Rauf, A., Khalil A. A., Awadallah, S., Khan, S. A., Abu-Izneid, T., Kamran, M., Hemeg, H. A., Mubarak, M. S., Khalid, A., Wilairatana, P., 2024. Reactive oxygen species in biological systems, pathways, associated diseases, and potential inhibitors —A review. Food Sci. Nutr. 12, 675–693. doi: 10.1002/fsn3.3784.
- Natural stilbenoids: distribution in the plant kingdom and chemotaxonomic interest in Vitaceae. Nat. Prod. Rep.. 2012;29:1317-1333.
- [CrossRef] [Google Scholar]
- Use of passion fruit seed extract (Passiflora edulis Sims) to prevent lipid oxidation in dairy beverages during storage and simulated digestion. LWT. 2020;123:10908.
- [CrossRef] [Google Scholar]
- Oligostilbenoids in Vitis vinifera L. Pinot Noir grape cane extract: Isolation, characterization, in vitro antioxidant capacity and anti-proliferative effect on cancer cells. Food Chem. 2018;1(265):101-110.
- [CrossRef] [Google Scholar]
- Achillea spp.: A comprehensive review on its ethnobotany, phytochemistry, phytopharmacology and industrial applications. Cell Mol Biol (noisy-Le-Grand). 2020;66(4):78-103.
- [Google Scholar]
- A comprehensive review on the chemotherapeutic potential of piceatannol for cancer treatment, with mechanistic insights. J. Agric. Food Chem.. 2016;64(4):725-737.
- [CrossRef] [Google Scholar]
- Piceatannol affects gastric ulcers induced by indomethacin: Association of antioxidant, anti-Inflammatory, and angiogenesis mechanisms in rats. Life. 2022;12:356.
- [CrossRef] [Google Scholar]
- Piceatannol reduces fat accumulation in Caenorhabditis elegans. J. Med. Food.. 2017;20(9):887-894.
- [CrossRef] [Google Scholar]
- Piceatannol extends the lifespan of Caenorhabditis elegans via DAF-16. Biofactors. 2017;43(3):379-387.
- [CrossRef] [Google Scholar]
- Mechanism of synergy between piceatannol and ciprofloxacin against Staphylococcus aureus. Int. J. Mol. Sci.. 2022;23:15341.
- [CrossRef] [Google Scholar]
- Ultrafast nonadiabatic photoisomerization dynamics mechanism for the UV photoprotection of stilbenoids in grape skin. Chem. Asian. J.. 2020;15(9):1478-1483.
- [CrossRef] [Google Scholar]
- Biosynthesis of resveratrol and piceatannol in engineered microbial strains: achievements and perspectives. Appl. Microbiol. Biotechnol.. 2019;103(7):2959-2972.
- [CrossRef] [Google Scholar]
- Inhibition of acetylcholinesterase and amyloid-β aggregation by piceatannol and analogs: Assessing in vitro and in vivo impact on a murine model of scopolamine-induced memory impairment. Antioxidants. 2023;12(7):1362.
- [CrossRef] [Google Scholar]
- Induction of autophagy, apoptosis and aquisition of resistance in response to piceatannol toxicity in MOLT-4 human leukemia cells. Toxicol. in Vitro. 2019;59:12-25.
- [CrossRef] [Google Scholar]
- Piceatannol, a structural analog of resveratrol, is an apoptosis inducer and a multidrug resistance modulator in HL-60 human acute myeloid leukemia cells. Int. J. Mol. Sci.. 2021;22:10597.
- [CrossRef] [Google Scholar]
- Resveratrol analogs with antioxidant activity inhibit intestinal epithelial cancer Caco-2 cell growth by modulating arachidonic acid Cascade. J. Agric. Food Chem.. 2019;67(3):819-828.
- [CrossRef] [Google Scholar]
- Resveratrol analogues like piceatannol are potent antioxidants as quantitatively demonstrated through the high scavenging ability against reactive oxygen species and methyl radical. Bioorg. Med. Chem. Lett.. 2017;27(23):5203-5206.
- [CrossRef] [Google Scholar]
- Protective effects of piceatannol on methylglyoxal-induced cytotoxicity in MC3T3-E1 osteoblastic cells. Free Radic. Res.. 2018;52(6):712-723.
- [CrossRef] [Google Scholar]
- Exploring resveratrol dimers as virulence blocking agents - Attenuation of type III secretion in Yersinia pseudotuberculosis and Pseudomonas aeruginosa. Sci. Rep.. 2020;10(1):2103.
- [CrossRef] [Google Scholar]
- Piceatannol, a natural trans-stilbene compound, inhibits human glyoxalase I. Bioorg. Med. Chem. Lett.. 2017;27(5):1169-1174.
- [CrossRef] [Google Scholar]
- Inhibition of colorectal cancer cell proliferation by treatment with Itadori leaf extract. J. Oleo Sci.. 2023;72(2):199-209.
- [CrossRef] [Google Scholar]
- Screening and evaluation of xanthine oxidase inhibitors from Gnetum parvifolium in China. Molecules. 2019;24(14):2671.
- [Google Scholar]
- Neuroprotective and antiherpetic properties of polyphenolic compounds from Maackia amurensis Heartwood. Molecules. 2023;28(6):2593.
- [CrossRef] [Google Scholar]
- Biotechnological advances in resveratrol production and its chemical diversity. Molecules. 2019;24(14):2571.
- [CrossRef] [Google Scholar]
- Targeting SYK signaling in myeloid cells protects against liver fibrosis and hepatocarcinogenesis. Oncogene. 2019;38(23):4512-4526.
- [CrossRef] [Google Scholar]
- Antioxidant activity of selected stilbenoid derivatives in a cellular model system. Biomol.. 2019;9(9)
- [CrossRef] [Google Scholar]
- Effect of antioxidant supplementation on skeletal muscle and metabolic profile in aging mice. Food Funct.. 2021;12:825-833.
- [CrossRef] [Google Scholar]
- Sequential high pressure extractions applied to recover piceatannol and scirpusin B from passion fruit bagasse. Food Res. Int.. 2016;85:51-58.
- [CrossRef] [Google Scholar]
- Piceatannol protects against cisplatin nephrotoxicity via activation of Nrf2/HO-1 pathway and hindering NF-κB inflammatory cascade. Naunyn–Schmiedeberg’s. Arch. Pharmacol.. 2019;392(11):1331-1345.
- [CrossRef] [Google Scholar]
- Piceatannol ameliorates behavioural, biochemical and histological aspects in cisplatin-induced peripheral neuropathy in rats. Basic Clin. Pharmacol. Toxicol.. 2021;129(6):486-495.
- [CrossRef] [Google Scholar]
- Investigating the chemical profile of Rheum lhasaense and its main ingredient of piceatannol-3′-O-β-D-glucopyranoside on ameliorating cognitive impairment. Biomed. Pharmacother.. 2023;160:114394
- [CrossRef] [Google Scholar]
- Inhibiting the formation of advanced glycation end-products by three stilbenes and the identification of their adducts. Food Chem.. 2019;295:10-15.
- [CrossRef] [Google Scholar]
- Piceatannol pretreatment alleviates acute cardiac injury via regulating PI3KAkt-eNOS signaling in H9c2 cells. Biomed. Pharmacother.. 2019;109:886-891.
- [CrossRef] [Google Scholar]
- Piceatannol protects against cerebral ischemia/reperfusion induced apoptosis and oxidative stress via the Sirt1/FoxO1 signaling pathway. Mol. Med. Rep.. 2020;22(6):5399-5411.
- [CrossRef] [Google Scholar]
- Inhibitory effects of piceatannol on human cytomegalovirus (hCMV) in vitro. J. Microbiol.. 2020;58(8):716-723.
- [CrossRef] [Google Scholar]
- Antioxidant activity and neuroprotective activity of stilbenoids in rat primary cortex neurons via the PI3K/Akt signalling Pathway. Molecules. 2018;23(9):2328.
- [CrossRef] [Google Scholar]
- Piceatannol attenuates D-GalN/LPS-induced hepatoxicity in mice: Involvement of ER stress, inflammation and oxidative stress. Int. Immunopharmacol.. 2018;64:131-139.
- [CrossRef] [Google Scholar]
- Piceatannol protects against sepsis-induced myocardial dysfunction via direct inhibition of JAK2. Int. Immunopharmacol.. 2021;96:107639
- [CrossRef] [Google Scholar]
- Chemical constituents of Cassia abbreviata and their anti-HIV-1 activity. Molecules. 2021;26(9):2455.
- [CrossRef] [Google Scholar]
- Piceatannol attenuates fat accumulation and oxidative stress in steatosis-induced HepG2 cells. Curr. Res. Food. Sci.. 2020;3:92-99.
- [CrossRef] [Google Scholar]
- Piceatannol protects against age-related hearing loss by inhibiting cellular pyroptosis and inflammation through regulated Caspase11-GSDMD pathway. Biomed. Pharmacother.. 2023;163:114704
- [CrossRef] [Google Scholar]
- The putative glyoxalase 1 inhibitor piceatannol exhibits both anxiolytic-like and antitumor effects in mice. Anticancer Res.. 2020;40(6):3271-3276.
- [CrossRef] [Google Scholar]
- Effect of piceatannol against malignant melanoma in vivo and in vitro. Hua Xi Kou Qiang Yi Xue Za Zhi. 2021;39(4):413-418.
- [CrossRef] [Google Scholar]
- Zakova, T., Rondevaldova, J., Bernardos, A., Landa, P., Kokoska, L., 2018. The relationshipbetween structure and in vitro antistaphylococcal effect of plant-derived stilbenes. Acta Microbiol. Immunol. Hung. 65 (4), 467–476. doi: 10.1556/030.65.2018.040.
- Piceatannol promotes apoptosis via up-regulation of microRNA- 129 expression in colorectal cancer cell lines. Biochem. Biophys. Res. Commun.. 2014;452(775–781):2014.
- [CrossRef] [Google Scholar]
- α-Glucosidase inhibitory effect of resveratrol and piceatannol. J. Nutr. Biochem.. 2017;47:86-93.
- [CrossRef] [Google Scholar]
- Piceatannol suppresses proliferation and induces apoptosis by regulation of the microRNA–21/phosphatase and tensin homolog/protein kinase B signaling pathway in osteosarcoma cells. Mol. Med. Rep.. 2020;22(5):3985-3993.
- [CrossRef] [Google Scholar]
- Active components from Cassia abbreviata prevent HIV-1 entry by distinct mechanisms of action. Int. J. Mol. Sci.. 2021;22:5052.
- [CrossRef] [Google Scholar]
- Piceatannol protects brain endothelial cell line (bEnd. 3) against lipopolysaccharide-Induced inflammation and oxidative stress. Molecules. 2022;27(4):1206.
- [CrossRef] [Google Scholar]
- Piceatannol inhibits P. acnes-induced keratinocyte proliferation and migration by downregulating oxidative stress and the inflammatory response. Inflammation. 2020;43(1):347-357.
- [CrossRef] [Google Scholar]
- The degree of hydroxylation of phenolic rings determines the ability of flavonoids and stilbenes to inhibit calcium-mediated membrane fusion. Nutrients. 2023;15:1121.
- [CrossRef] [Google Scholar]







