Supercritical carbon dioxide chelation extraction of heavy metal ions from drilling fluid waste: Experiment and simulation
⁎Corresponding authors. mabo@brpetro.com (Bo Ma), wangrh@upc.edu.cn (Ruihe Wang)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
This study evaluates the application of supercritical carbon dioxide chelation extraction technology for treating heavy metal ions (Zn2+ and Cr3+) in drilling fluid waste. Through a combination of experimental and molecular dynamics simulation methods, the influence of extraction parameters (temperature, pressure, duration, and chelating agents) on the extraction efficiency are investigated. Findings show that increased duration and pressure significantly improve extraction efficiency, while temperature has a complex effect, initially increasing efficiency but plateauing and slightly decreasing at higher temperatures. The optimum extraction condition with pressure of 220 bar, temperature 348.15 K and an extraction duration of 70 min using ethylene diamine tetraacetic acid (EDTA) as the chelating agent has been determined. Importantly, molecular simulation analysis revealed that citric acid outperforms EDTA in terms of Zn2+ and Cr3+ aggregation by forming larger aggregates with greater numbers of molecules while reducing overall aggregate count. Furthermore, optimization of extraction pressure in the EDTA system shows potential benefits. These results suggest that supercritical carbon dioxide chelation extraction technology has strong potential for environmentally friendly and reliable waste management in the drilling industry. This study represents a significant step forward in developing sustainable solutions for heavy metal removal from drilling fluid waste.
Keywords
Supercritical carbon dioxide
Chelation extraction
Heavy metal ions
Drilling fluid waste
Environmental remediation
Molecular simulation
1 Introduction
In the oil and gas drilling process, drilling fluid waste containing a significant amount of mineral oil, organic compounds, and various heavy metal substances is classified as hazardous waste according to industry regulations (Ali et al., 2013). Oil-based drilling fluid waste exhibits high oil content and chemical oxygen demand values, while water-based drilling fluid waste presents severe issues with excessive heavy metal levels. Once released into the environment, heavy metal ions are challenging to degrade or migrate, not only posing a threat to normal biological activities but also tending to accumulate in the food chain at elevated concentrations, ultimately posing health risks to humans (Awual and Hasan, 2015). Consequently, these pollutants are strictly prohibited by environmental regulations both domestically and internationally (Ma et al., 2019).
Conventional treatment methods involve transforming dissolved heavy metals into insoluble compounds or concentrating and separating them without altering their chemical form. Current approaches for extracting heavy metals from drilling fluid waste often face several limitations that hinder their effectiveness and scalability (Chanmiya Sheikh et al., 2023; Eren et al., 2024). Traditional methods, such as chemical precipitation, adsorption, and ion exchange, can be costly, generate secondary waste streams, and may not achieve high removal efficiencies, particularly for complex matrices like drilling fluid waste (Karimi-Maleh et al., 2021). Chemical precipitation, for instance, requires the addition of chemicals that can increase the overall treatment cost and potentially introduce new contaminants. Adsorption methods, while effective in some cases, may suffer from issues like low adsorption capacity, selectivity issues, and challenges associated with regeneration and disposal of the adsorbents. Ion exchange resins, though efficient, are prone to fouling and require frequent regeneration, which can be both costly and energy intensive. Moreover, many of these methods are not suitable for large-scale applications due to their complexity, high operating costs, and the potential for re-emission of metals during waste disposal. Biological treatment methods, such as bioremediation, are environmentally friendly but are often slow, highly dependent on environmental conditions, and may not be effective for all types of heavy metals and contaminants present in drilling fluid waste (Hossain et al., 2024; Ghoreishi et al., 2012).
Therefore, from an environmental protection perspective coupled with economic considerations it becomes imperative to seek an efficient method for eliminating heavy metal contamination from drilling mud waste that is environmentally friendly without causing additional pollution. Supercritical Fluid Extraction (SFE) has garnered widespread attention within scientific circles as a novel separation technology widely applied across various fundamental fields including food processing, chemical engineering biotechnology among others. Carbon dioxide stands out as a preferred extraction solvent due to its low critical point temperature; low viscosity; non-toxicity; non-flammability; excellent chemical stability; cost-effectiveness absence of by-products among other advantages (Cheng et al., 2020). In recent years, SFE has garnered significant attention for its potential applications in environmental remediation due to its high extraction efficiency, minimal solvent usage, and reduced environmental impact. Studies have demonstrated the effectiveness of SFE using supercritical carbon dioxide (SCCO2), the most used supercritical solvent, in extracting pollutants such as polycyclic aromatic hydrocarbons (PAHs), polychlorinated biphenyls (PCBs), and heavy metals from various waste streams and contaminated soils (Korzenski et al., 2022).
However, despite its numerous benefits carbon dioxide's non-polar nature results in low efficiency when extracting polar substances particularly metal ions (Skpska, 2021). Additionally due to their positive charge nuclei supercritical carbon dioxide cannot directly extract electroneutral substances thus making direct extraction of metal ions challenging. Nevertheless, recent advancements in Supercritical Fluid Chelating Extraction (SFCE) technology offer an effective solution addressing these limitations through coordination bonds between chelating agents and charged metal ions generating electrically neutral stable easily soluble complexes which can be separated from the original matrix via mass transfer into supercritical fluid phase (Kang et al., 2018; Gopalan et al., 2003). Supercritical CO2 extraction harnesses the unique properties of CO2 in its supercritical state to effectively dissolve and extract heavy metals from diverse matrices. The solubilizing capacity of Supercritical CO2, bolstered by the utilization of chelating agents, facilitates the formation of stable complexes with heavy metals, enabling their mass transfer and diffusion from the matrix into the supercritical fluid. Following extraction, techniques such as depressurization, absorption, adsorption, or phase separation are typically employed to separate heavy metals from the supercritical CO2 solvent, allowing for their recovery and facilitating reuse of the supercritical fluid. Given its high extraction efficiency fast speed good selectivity lack of solvent-related secondary pollution characteristics supercritical carbon dioxide offers new prospects for removing heavy metals effectively (Ramazanov and Shakhbanov, 2019).
Studies have demonstrated that supercritical carbon dioxide chelation extraction technology can achieve highly efficient extractions exceeding 99 % for toxic heavy metals such as mercury copper silver cadmium chromium present in wastewater using specific chelating agents neutralizing metallic ion charges forming neutral chelates combined with another polar carrier agent facilitating effective extraction by supercritical carbon dioxide (Clifford et al., 2001; Liu et al., 2019) The ligands employed for extraction must exhibit good solubility in supercritical CO2 and possess the ability to stably complex with the target metal ions, allowing for effective migration of the resulting chelate complexes in supercritical CO2. The unique nature of coordination reactions, including geometric specificity, contributes to the excellent selectivity of chelate extraction. For instance, crown ether ligands can form highly stable chelate complexes with metal ions of specific ionic radii, enabling high selectivity in the extraction of metal ions (Iso et al., 2006; Chao et al., 2010).
While this technique has been successfully employed for extracting toxic heavy metals from synthetic solid waste matrices there is currently no reported research on applying this approach specifically for treating heavy metals present in drilling fluid wastes either domestically or internationally. This study aims at leveraging the physical–chemical advantages offered by supercritical carbon dioxide utilizing an improved experimental setup designed for chelating-heavy-metal-extraction experiments employing different parameters (temperature pressure time). The objective is to provide practical and theoretical guidance on extraction condition, like pressure, temperature, duration and chelating agent, towards field application process research involving supercritical CO2 treatment of drilling fluid wastes thereby achieving harmless disposal practices contributing both theoretical practical value towards developing applicable technologies for managing industrial wastes efficiently.
2 Lab experiment
2.1 Experimental equipment
Fig. 1 below illustrates the simulation of the supercritical carbon dioxide chelation extraction system, which consists of the following: (1) Four Mixing Extraction Reactors (including liquid storage tank and reaction tank). It is composed of kettle body, kettle cover, sealing ring, etc. The kettle body and kettle cover are made of 316 L with a capacity of 1000 ml and an inner diameter of 72 mm. The maximum working pressure is 400 bar and the highest working temperature is 423.15 K ℃. The sealing is achieved by fluor rubber ring. There are air inlet and exhaust ports on the upper end of the kettle body. There are liquid discharge port, pressure measurement port, and temperature measurement port at the bottom of the kettle. (2) Magnetically coupled stirrer. The stirring force is transmitted by the coupling of internal and external magnetic steel. It is a magnetically coupled stirrer without dynamic sealing and leakage. The operating pressure is 400 bar and the highest working temperature is 423.15 K. The magnetic steel adopts high-temperature resistant cobalt-containing magnetic steel, which maintains magnetism at 423.15 K. (3) Stirring motor and speed control. It consists of a speed-regulating motor and a speed controller. The stirring speed is adjustable from 60 to 1000 r/min. The speed is adjusted by the debugger on the control box, and the rotational speed and torque values are displayed. It can also be controlled by a computer. (4) Pressure measurement system (manufactured by Huaan Scientific, China). The pressure is measured by a pressure transmitter. The pressure transmitter has a range of 500 bar, is made of 316 L, and has a measurement accuracy of 0.1 bar. The pressure value can be displayed on the digital display meter on the control box. (5) Temperature control system (manufactured by Huaan Scientific, China). The temperature control system consists of a temperature controller, industrial heating jacket, PT100 temperature probe, control circuitry, etc. The highest working temperature is 423.15 K with a temperature control accuracy of ±1 K. The temperature value can be displayed on the digital display meter on the control box or collected by computer control. (6) Valves and fittings. The kettle body is equipped with a CO2 gas injection control valve and a gas vent valve. A discharge valve is installed at the bottom of the kettle body. All pipelines and valves in contact with fluids are made of 316 L. The burst pressure of the explosion-proof valve is 425 bar. (7) Electrical control box. The control box panel is equipped with a temperature controller, pressure digital display meter, speed meter, pressure and temperature alarm switches, power switch, and heating switch. The control circuitry is installed inside the box.
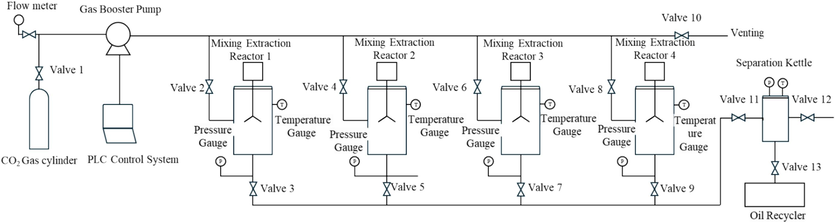
- Schematic diagram of the experiment equipment.
2.2 Experimental materials
The experimental materials include CO2 gas (supplied by Huanghui Gas, China, 99.95 % purity). Drilling fluid waste containing heavy metals which was obtained from oilfield in South China sea. The drilling fluid waste not only contains a variety of chemical additive waste and drilling cuttings waste, oil, including a variety of toxic compounds, such as benzene, phenols, etc., but also contains various heavy metals, such as zinc, chromium, copper, lead. Chelation agent: ethylene diamine tetraacetic acid (EDTA) (supplied by Minghao Chemical, China, 99 % purity) and citric acid (CA) (supplied by Anhui Ruikesi, China, 99 % purity). Cosolvents: Water. Fig. 2 shows the drilling fluid waste before experiment and after experiment. The drilling fluid sample is oil-based mud with the characteristics of deep black in color and a pungent odor.

- Drilling fluid waste before experiment and after experiment.
2.3 Experimental procedure
2.3.1 Preparation of drilling fluid waste samples
For the sample collection process, an all-metal sealed bucket is used for collection, and nitrogen displacement protection is used to prevent the oxidation of heavy metal ions or volatilization of organic components. The collection process is strictly controlled without pollution source contact and quickly packaged and refrigerated to maintain the original condition of the sample. Each sampling point was sampled three times to ensure the stability and reproducibility of the results. Immediately after collection, the sample is placed in a dry, light-free environment for temporary storage and then transferred to a cryopreservation device to prevent possible contamination changes caused by biological activity. The samples were removed from the freezer the night before determination and allowed to thaw naturally at room temperature.
2.3.2 Supercritical carbon dioxide chelation extraction process
Place the experimental medium inside, screw the kettle cover clockwise, and connect the motor control line. 10 g of drilling fluid waste was selected for each experiment, and thorough mixing was conducted before each experiment to ensure the homogeneity of the sample composition. Filter paper was utilized as the substrate during the experiment. The required entrainment agent (Water) and chelating agent (Citric Acid and EDTA) were added and thoroughly blended.
Turn on the HAPUMP-200C CO2 constant speed and pressure pump, as well as the gas cylinder, and close the venting valve 10. Open the main gas inlet switch and the corresponding kettle body inlet switch counterclockwise. Depending on the number of extraction kettles in use, selectively open valves 2, 4, 6, 8, and close valves 3, 5, 7, 9.
Let in the gas and pressurize. Use the laboratory's PLC control system to control the gas booster pump. Observe the pressure gauge of the extraction kettle. After pressurization is complete, screw down and close the inlet switch valve 1 clockwise. Set and control the heating temperature using the temperature controller of the stirring extraction kettle. After pressurization is complete, screw down and close the inlet switch clockwise.
After the experiment, first turn off the temperature control button to cool down, turn off the stirrer, and close inlet valves 2, 4, 6, 8. Open the corresponding kettle body drain valves 3, 5, 7, 9 at the bottom counterclockwise, and open valves 11 and 13 to separate the discharged liquid.
2.3.3 Heavy mental and extraction efficiency analysis
The detection of heavy metal ions involves the use of primary X-ray photons or other microscopic ions to excite the atoms in the sample, inducing them to emit fluorescence (secondary X-rays). This method is utilized for material composition analysis and chemical state research. The Zetium XRF instrument (manufactured by Panalytical, Netherlands) is employed for testing, primarily to determine the content of Zn2+ and Cr3+in drilling fluid waste before (
3 Experiment results and discussion
3.1 The impact of extraction duration on extraction efficiency
The experiments (see Table 1) were carried out under controlled conditions of 140 bar pressure and 328.15 K temperature, using EDTA as the chelating agent, to investigate the specific impact of extraction time on the efficiency of extracting Zn2+ and Cr3+ from drilling fluid waste. The findings from these experiments are depicted in Fig. 3, illustrating the correlation between extraction time and efficiency.
| No. | Duration/min | Temperature/K | Pressure/bar | Carrier | Chelating agent | Ratio of sample to Carrier | Ratio of sample to chelating agents | Cr3+ Extraction Efficiency | Zn2+ Extraction Efficiency |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 70 | 328.15 | 140 | Water | EDTA | 4:1 | 4:1 | 24.32% | 47.67% |
| 2 | 70 | 308.15 | 140 | Water | EDTA | 4:1 | 4:1 | 0.12% | 0.02% |
| 3 | 70 | 348.15 | 140 | Water | EDTA | 4:1 | 4:1 | 31.43% | 56.96% |
| 4 | 70 | 328.15 | 180 | Water | EDTA | 4:1 | 4:1 | 44.20% | 59.20% |
| 5 | 70 | 328.15 | 220 | Water | EDTA | 4:1 | 4:1 | 51.07% | 66.79% |
| 6 | 10 | 328.15 | 140 | Water | EDTA | 4:1 | 4:1 | 14.73% | 12.77% |
| 7 | 130 | 328.15 | 140 | Water | EDTA | 4:1 | 4:1 | 44.20% | 51.07% |
| 8 | 190 | 328.15 | 140 | Water | EDTA | 4:1 | 4:1 | 48.12% | 54.02% |
| 10 | 70 | 328.15 | 80 | Water | EDTA | 4:1 | 4:1 | 2.49% | 4.24% |
| 12 | 70 | 348.15 | 80 | Water | EDTA | 4:1 | 4:1 | 4.26% | 8.49% |
| 13 | 70 | 348.15 | 100 | Water | EDTA | 4:1 | 4:1 | 18.32% | 20.86% |
| 14 | 70 | 348.15 | 180 | Water | EDTA | 4:1 | 4:1 | 53.04% | 66.79% |
| 15 | 70 | 348.15 | 220 | Water | EDTA | 4:1 | 4:1 | 59.34% | 73.66% |
| 16 | 70 | 328.15 | 140 | Water | Citric Acid | 4:1 | 4:1 | 32.68% | 54.86% |
| 17 | 70 | 368.15 | 100 | Water | EDTA | 4:1 | 4:1 | 24.14% | 17.68% |
| 18 | 70 | 368.15 | 140 | Water | EDTA | 4:1 | 4:1 | 38.83% | 51.07% |
| 19 | 70 | 368.15 | 180 | Water | EDTA | 4:1 | 4:1 | 56.96% | 61.87% |
| 20 | 70 | 368.15 | 220 | Water | EDTA | 4:1 | 4:1 | 63.84% | 70.71% |
| 21 | 70 | 368.15 | 80 | Water | EDTA | 4:1 | 4:1 | 5.14% | 6.73% |
| 22 | 70 | 328.15 | 100 | Water | EDTA | 4:1 | 4:1 | 12.43% | 14.02% |
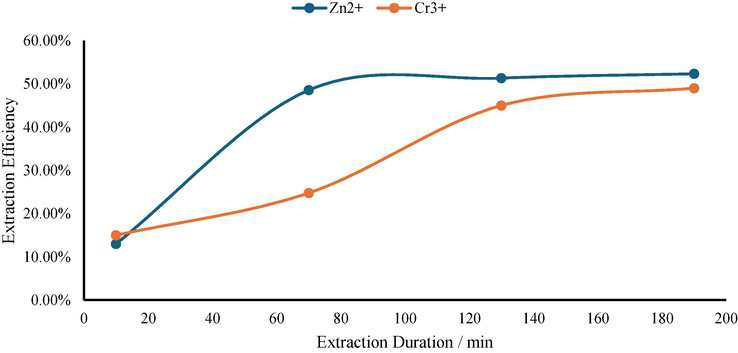
- The influence of extraction duration to extraction efficiency (Zn2+ and Cr3+).
As illustrated in the figure, there is a notable increase in extraction efficiency with an extension of extraction time from 10 min to 70 min. This enhancement can be attributed to improved contact between supercritical carbon dioxide and drilling fluid waste, facilitating increased dissolution of Zn2+ and Cr3+ in supercritical carbon dioxide. With prolonged extraction time, a greater amount of these ions can diffuse into supercritical carbon dioxide, resulting in an overall improvement in extraction efficiency.
However, it is worth noting that the rise in extraction efficiency becomes marginal when extending the extraction time beyond 70 min up to 190 min. This phenomenon can be explained by considering that under given temperature and pressure conditions, the solubility of Zn2+ and Cr3+ in drilling fluid waste within supercritical carbon dioxide reaches a fixed value. Once equilibrium is reached, further extension of extraction time does not significantly enhance extraction efficiency.
Based on these experimental results, an optimal extraction time of 70 min is determined for achieving maximum efficiency while also taking economic factors into account. This duration allows for sufficient removal of Zn2+ and Cr3+ from drilling fluid waste while minimizing overall cost and processing time.
3.2 The impact of extraction temperature on extraction efficiency
Fig. 4 illustrates the variation in extraction efficiency with temperature under specific conditions: a pressure of 140 bar, an extraction time of 70 min, and the use of EDTA as the chelating agent. The results reveal that initially, the extraction efficiency increases and then gradually decreases as temperature rises. Notably, at a temperature of 348.15 K, the extraction efficiency reaches a maximum of 78.1 %. In the process of extracting metal ions, temperature exerts a critical influence on extraction efficiency, which is more intricate than other factors. To gain deeper insights, let's explore three potential reasons for the impact of temperature on extraction efficiency.
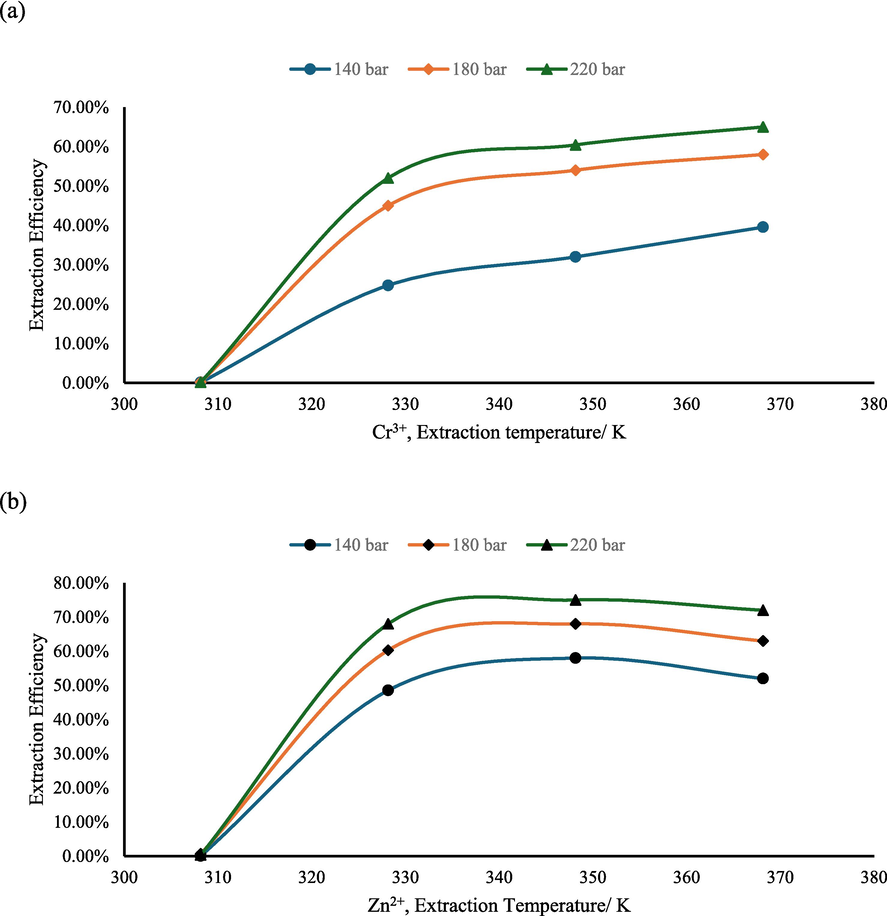
- The influence of extraction temperature to extraction efficiency (Zn2+ and Cr3+).
Firstly, as temperature rises, molecular thermal motion intensifies, accelerating desorption rates of metal ions from filter paper and favoring efficient extraction. In simpler terms, heat promotes more effective release of metal ions from filter paper.
Secondly, higher temperatures lead to increased saturated vapor pressure for substances; this enhances volatility for complexing agents and metal complexes leading to improved solubility in supercritical carbon dioxide. Consequently, accelerating complexation reactions ultimately enhances overall extraction efficiency by facilitating easier mixing between complexing agents/metal complexes and supercritical carbon dioxide resulting in more effective extractions.
Lastly but importantly to note is that increasing temperatures result in decreased density in supercritical carbon dioxide reducing solubility for both complexing agents and metal complexes – an unfavorable factor impacting overall extraction efficiency. Essentially higher temperatures have both beneficial effects while also reducing critical solubility levels necessary for successful extractions.
Hence it becomes evident that varying temperatures delicately balance these three factors' impact on overall extractive efficiencies; understanding this balance allows potential optimization towards enhanced efficiencies within processes involving metal ion extractions.
3.3 The impact of extraction pressure on extraction efficiency
To delve deeper into the influence of extraction pressure on extraction efficiency, experiments were conducted using EDTA as the chelating agent, an extraction temperature of 328.15 K, and an extraction time of 70 min. The results of these experiments are presented in Fig. 5. As the figure illustrates, the extraction efficiency exhibits a trend of initial increase followed by a relatively stable as the extraction pressure rises. Specifically, the highest extraction efficiency of 70 % is achieved under a pressure of 220 bar.
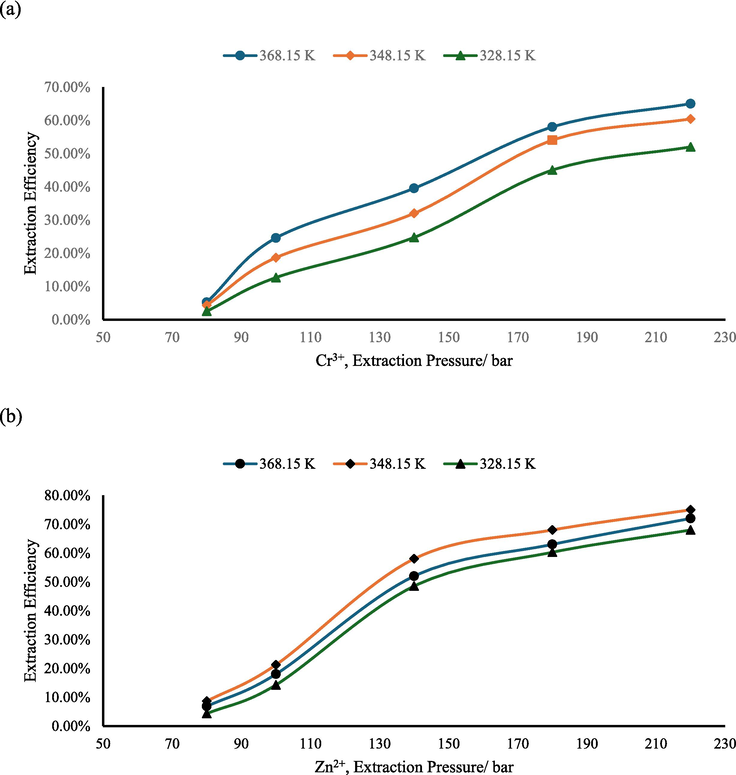
- The influence of extraction pressure to extraction efficiency (Zn2+ and Cr3+).
The pressure of supercritical carbon dioxide is a crucial factor that significantly impacts the efficiency of metal extraction. This is primarily due to the direct influence of extraction pressure on the density of supercritical carbon dioxide. As the pressure increases, the density of carbon dioxide also increases, which leads to an increase in its solubility for metal chelates. This, in turn, enhances the efficiency of metal ion extraction. In simpler terms, by adjusting the pressure, the density of supercritical carbon dioxide can be controlled, which directly affects its ability to dissolve metal chelates and, ultimately, the efficiency of metal ion extraction. At the same time, high-pressure conditions have high requirements for the pressure resistance and safety of the equipment, and the extraction efficiency has not been significantly improved, so the choice of extraction pressure in the experimental study is not the greater the better. Therefore, precise control over the extraction pressure is essential for achieving optimal metal extraction efficiency.
3.4 The impact of chelating agents on extraction efficiency
Experiments were carried out under pressure of 140 bar and temperature of 328.15 K to study the influence of different chelating agents on extraction efficiency. When employing EDTA and citric acid as chelating agents, the extraction efficiencies for Cr3+ were 24.32 % and 32.68 %, respectively, while those for Zn2+ were 47.67 % and 54.86 %. The extraction efficiency is different to remove Cr3+ and Zn2+ by using Citric Acid and EDTA. And the extraction efficiency of Citric Acid is relatively higher than of EDTA when extracting Cr3+ and Zn2+.
4 Molecular simulation study on the extraction of heavy metal ions by supercritical carbon dioxide chelation
4.1 Modeling and simulation details
4.1.1 Model construction
In this study, a simulation system was constructed comprising different chelating agents (Citric Acid (CA) and EDTA) and metal ions (Zn2+ and Cr3+) (Fig. 6). The simulation system consisted of 2000 carbon dioxide molecules, 200 water molecules, 6 EDTA molecules, and the corresponding number of Zn2+ and Cr3+ ions with their respective charge (Feng and Mather, 2002). After equilibration at the designated target temperature and pressure, the initial dimensions of the simulation system were approximately 8.0 × 8.0 × 8.0 nm3.
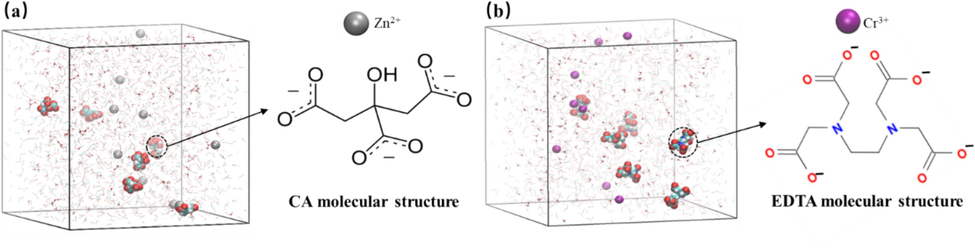
- Initial model of EDTA with metal ions (Zn2+ and Cr3+).
4.1.2 Simulation settings
The molecular dynamics simulations were performed using the GROMACS software package (version 5.1.5) (Feng and Mather, 2002). The Simple Point Charge Extended (SPC/E) parameters were employed for modeling water molecules, while the Trappe force field parameters were used to describe CO2 molecules (Zhu et al., 2019; Xiong et al., 2019). The structural files and force field parameters for EDTA molecules were obtained from the ATB website. Interactions between different atoms were calculated using Lorentz–Berthelot combination rules (Sohrevardi et al., 2017; Nobre et al., 2006). A Leap-frog algorithm with a time step of 1 fs was utilized to integrate the equations of motion. For long-range electrostatic interactions, Particle-Mesh-Ewald with a cutoff radius of 1.0 nm was applied. Van der Waals interactions between atoms were described by Lennard-Jones potential with a cutoff radius of 1.0 nm. The Parrinello-Rahman barostat and Nose-Hoover thermostat were employed to control pressure and temperature with time constants set at 1.0 ps and 0.5 ps, respectively. Semi-isotropic pressure coupling was selected to allow independent fluctuations in the xy and z dimensions of the system, considering periodic boundary conditions in three-dimensional space. The initial model underwent energy minimization followed by NVT simulation for 100 ps before conducting NPT simulations for an additional 200 ps under various target temperature–pressure conditions (140 bar–328 K; 140 bar–368 K; 100 bar–328 K; 220 bar–328 K). Finally, eight sets of dynamic simulations lasting 50 ns each were carried out under different target temperature–pressure conditions.
4.2 The impact of different chelating agents on extraction efficiency
The precise comprehension of the molecular process of chelating agent-mediated extraction of metal ions, such as citric acid and EDTA, serves as a fundamental guide for applications and further exploration of their microscopic properties. The molecular process of Zn2+ and Cr3+ extraction by citric acid and EDTA to form aggregates is depicted in Fig. 7. Initially, several preliminary aggregates composed of hydrated chelating agents and metal ions are formed (Fig. 7a–d). Subsequently, these small aggregates diffuse within the continuous supercritical CO2 phase, coalescing with water molecules to gradually assemble into larger aggregates exhibiting characteristics akin to those of microemulsion droplets. By 8 ns, a stable reverse micelle aggregate containing most chelating agents and water molecules has been established. This morphology persists as a stable reverse micelle aggregate over time.
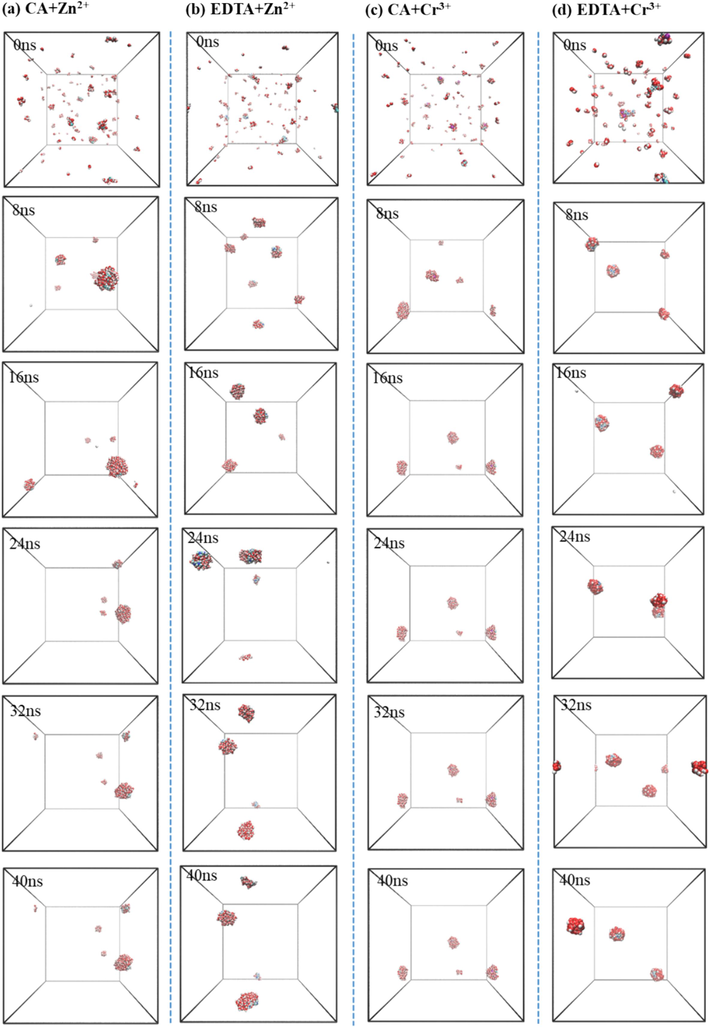
- Self-assembly of different chelating agents extracting Zn2+ (a, b) and Cr3+ (c, d) in supercritical CO2 system.
The evolution over time in terms of maximum aggregate size and the number of aggregates within the system is illustrated in Fig. 8a–b. It can be observed that compared to the EDTA system, the citric acid system exhibits a higher number of molecules in the largest Zn2+ and Cr3+ aggregate while maintaining fewer total aggregates at any given time point.
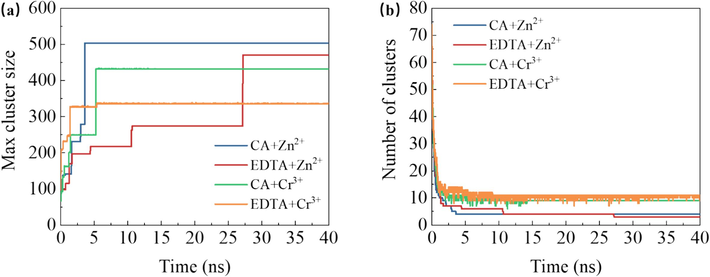
- The maximum cluster atomic number (a) and the number of clusters (b) in the system with citric acid and EDTA over time.
When the same chelating agent is used to extract different metal ions, the variation of the chelating agent's gyration radius over time can provide insights into the kinetic characteristics of the complexes formed between the chelating agent and various metal ions (Fig. 9a). The gyration radius dynamics reflect the rate, stability, and dissociation kinetics of these complexes. Significant fluctuations in gyration radius over time for complexes formed by different metal ions with a common chelating agent indicate lower stability and potential for dissociation reactions. Conversely, smaller fluctuations suggest greater stability.
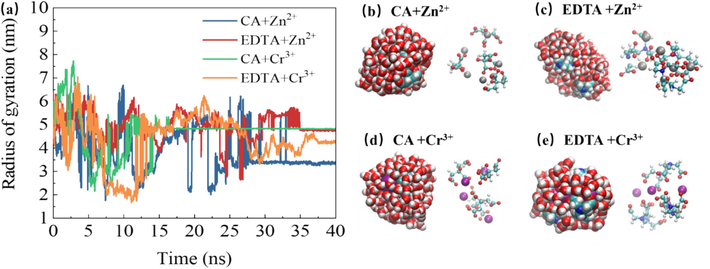
- Evolution of gyration radius over time in different supercritical carbon dioxide systems (a) and molecular structure of the largest clusters at 40 ns (b–e).
During the initial stage (0–25 ns), systems containing citric acid exhibit relatively smaller rotational radius fluctuations compared to EDTA systems. This suggests that Zn2+ and Cr3+ form more stable complexes with citric acid at a faster rate than with EDTA. Additionally, larger rotational radii may increase contact surface area with water molecules affecting interactions between chelating agents and metals.
At 40 ns termination point, systems containing citric acid display relatively smaller rotational radii compared to those containing EDTA indicating differences in spatial conformation influenced by metal ion type and their interaction with chelating agents as depicted in Fig. 9b–e.
The solvent-accessible surface area (SASA) can be utilized to analyze the interaction between chelating agents and water molecules (Li et al., 2015). In this study, the calculation of the contact surface area between the chelating agent and water molecules (Fig. 10) was employed to assess solvation characteristics. A larger SASA value indicates a greater contact area between the chelating agent and water molecules.
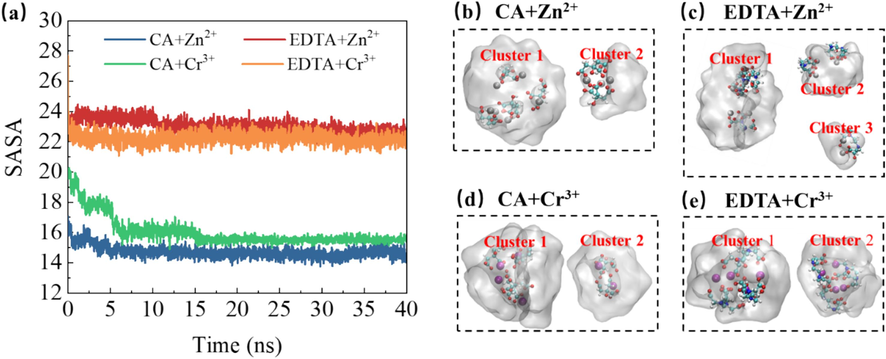
- The solvation accessible surface area (SASA) of citrate ligands in different metal ion systems evolves over time.
In comparison with citric acid, the EDTA system exhibits a relatively larger SASA value due to its possession of multiple coordination sites and a more substantial molecular structure. This property facilitates the formation of stable complexes with metal ions in aqueous solution, corroborating findings from rotational radius data.
It is evident that there are relatively fewer aggregated groups in the citric acid system, indicating a tighter complexation morphology between metal ions and chelating agents. The resulting complex can effectively interact with water molecules to form stable aggregates, thereby enhancing stability and solubility in aqueous solutions while facilitating sustained presence within them.
4.3 Molecular simulation of chelating agent extraction of metal ions under different temperature and pressure conditions
The precise comprehension of the molecular process involved in chelating agent extraction of metal ions is crucial for guiding practical applications and further exploration of its microscopic properties. The molecular simulation depicts the process of EDTA extracting zinc ions to form aggregates under identical pressure conditions (140 bar) but at different temperatures (328 K and 368 K), as illustrated in Fig. 2. Initially, several preliminary aggregates composed of hydrated EDTA and ions are formed, as shown in Fig. 11a and b. Subsequently, these small aggregates diffuse within the continuous supercritical CO2 phase, interacting with water molecules, gradually assembling into larger aggregates exhibiting characteristics resembling those of microemulsion droplets. By 8 ns, a stable aggregate containing a majority of EDTA chelating agents and most water molecules has been formed. This stable reverse-phase microemulsion aggregate morphology is maintained thereafter. The evolution over time of the maximum aggregate size and number is depicted in Fig. 11(c) and (d). It can be observed that at 15 ns, the maximum number of molecules in the system's largest aggregate has been reached while the number of aggregates has decreased to a minimum. Comparatively, under conditions at 368 K, there are more molecules in the system's largest aggregate but fewer total aggregates compared to conditions at 328 K.
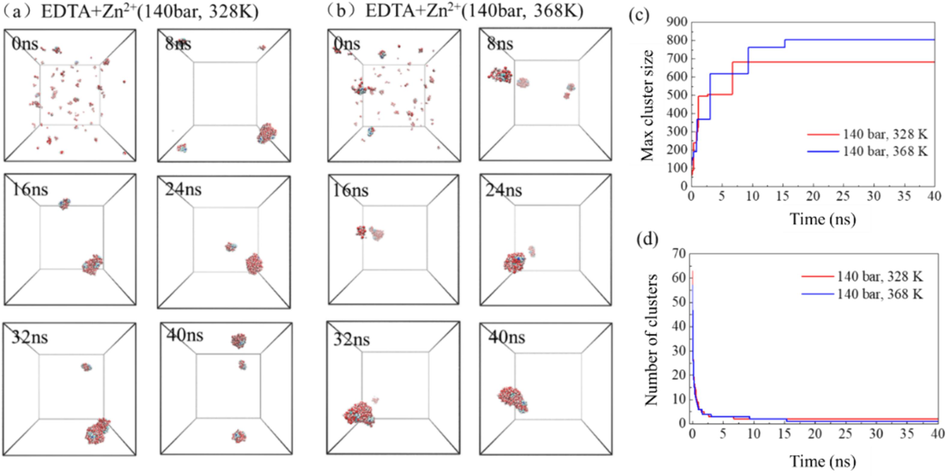
- The extraction of Zn2+ by EDTA chelating agent in supercritical carbon dioxide system at different temperatures (a–b), the evolution of the maximum cluster atomic number (c) and the number of clusters (d) in the system over time.
The molecular process of EDTA extracting Zn2+ and forming aggregates under different pressures is shown in Fig. 12. Similar to the aggregate process of EDTA in the system under different temperatures, it finally assembled into a large inverse micelle. However, at 8 ns, a stable aggregate containing most of the EDTA chelating agent and most of the water molecules has been formed in the system. After that, the aggregate form remains stable. The evolution of the maximum aggregate and the number of aggregates in the system with time is shown in Fig. 12 (c and d). It can be seen that compared with the system under 100 bar, the maximum aggregate of EDTA in the system under 220 bar pressure has more molecules and less number of aggregates formed finally.
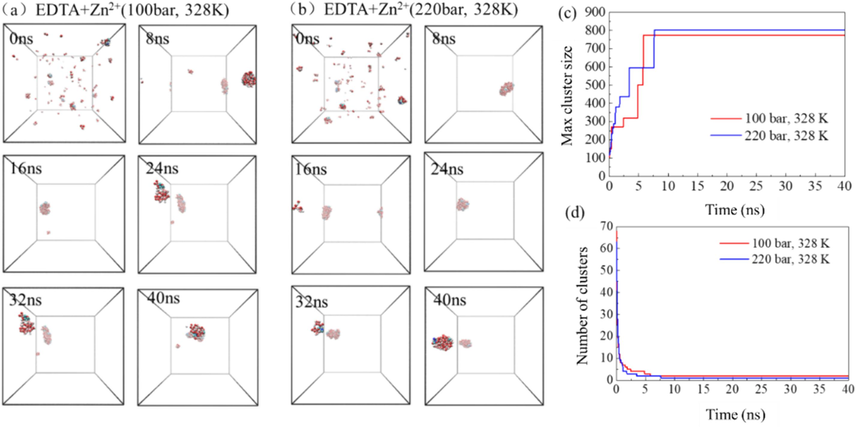
- The extraction of Zn2+ by EDTA chelating agent in supercritical carbon dioxide system under different pressures (a–b), the evolution of the maximum cluster atomic number (c) and the number of clusters (d) in the system over time.
Based on the simulation results of the Zn2+ system, under the same pressure conditions, higher temperature can increase the number of molecules in the maximum aggregate and reduce the number of aggregates formed. The effect of higher pressure is similar when the temperature is controlled.
The molecular process of forming aggregates by EDTA extraction of Cr3+ at different temperatures and pressures is shown in Fig. 13. The aggregation process is similar to that of the Zn2+-containing system, starting with the formation of several preliminary aggregates composed of hydrated EDTA and ions (Fig. 13a and b). Then, small aggregates diffuse and aggregate with water molecules in the continuous supercritical CO2 phase, and gradually assemble into a larger inverse micelle. But at higher temperatures, it takes longer for the maximum number of aggregate molecules to reach the maximum (23 ns after equilibrium). Compared with 328 K, the maximum aggregate molecules formed at 368 K have more numbers of molecules, but the number of aggregates is less.
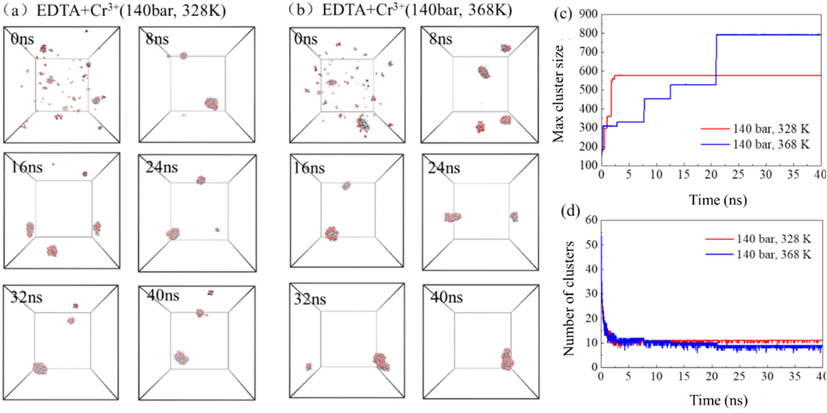
- The self-assembly process of EDTA chelating agent extracting Cr3+ in supercritical carbon dioxide system at different temperatures (a–b), the maximum cluster atomic number (c) and the number of clusters (d) in the system over time.
The molecular process of forming aggregates by EDTA extraction of Cr3+ at different temperatures and pressures is shown in Fig. 14. The aggregation process of EDTA is similar to that of the system at different temperatures, and finally assembles into a larger inverse micelle. But at 11 ns, a stable aggregate containing most of the EDTA chelator and most of the water molecules has been formed in the system, and has maintained a stable aggregate form since then, but the number of clusters in the system is relatively large (Fig. 14c and d). Compared with 100 bar, the maximum aggregate molecules of EDTA in the 220 bar pressure system are more, and the number of aggregates formed is similar.
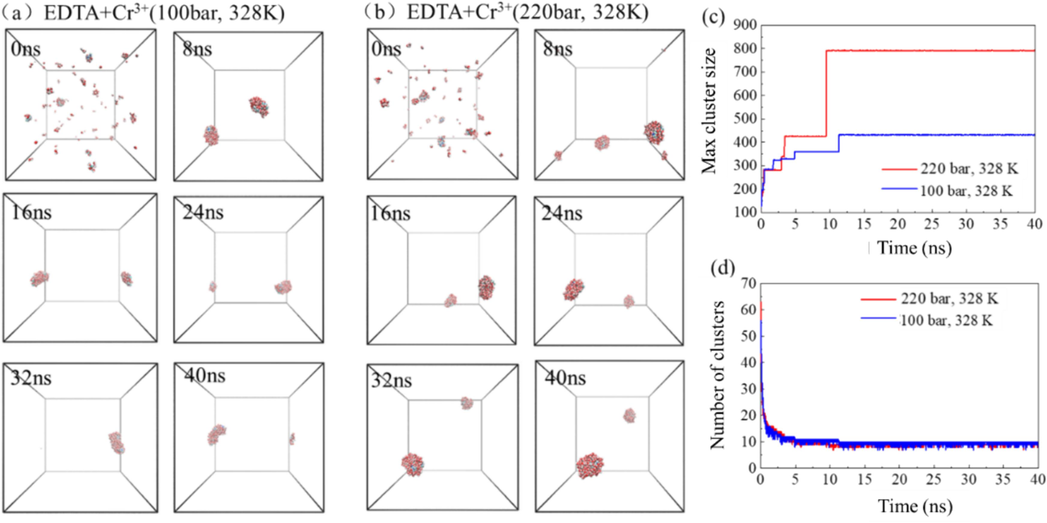
- The self-assembly process of EDTA chelating agent extracting Cr3+ in supercritical carbon dioxide system under different pressures (a-b), the maximum cluster atomic number (c) and the number of clusters (d) in the system evolve with time.
Solvent accessible surface area (SASA) can be used to analyze the interaction between chelating agents and water molecules. In this study, information about the solvation characteristics of the chelating agent and water can be obtained by calculating the surface area of the chelating agent in contact with water molecules (Fig. 15). A larger SASA value indicates a larger contact area between the chelating agent and water molecules. In the Zn2+-containing system, under the same conditions, the SASA value is relatively lower at higher temperatures and pressures, indicating that the contact area between EDTA and water molecules is smaller, which also means that the aggregates formed by EDTA and metal ions are closer. In the Cr3+-containing system, the SASA value of EDTA is relatively higher at 140 bar and 368 K than at other temperature and pressure conditions.。.
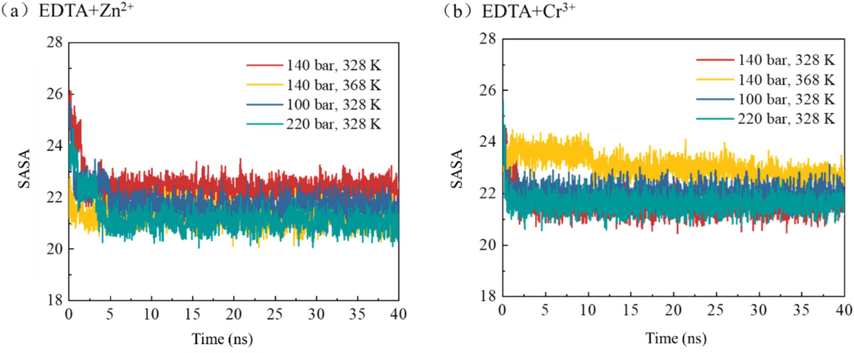
- The solvent-accessible surface area (SASA) of EDTA chelating agents under different temperature and pressure conditions evolves over time.
5 Conclusions
This study demonstrates the efficacy of supercritical carbon dioxide chelation extraction in efficiently removing Zn2+ and Cr3+ from drilling fluid waste, surpassing industry standards for heavy metal removal. The technique exhibits a strong potential for environmentally friendly and reliable waste management in the drilling industry.
Key findings indicate that extraction efficiency improves with increased duration and pressure, highlighting the importance of optimizing these parameters. Interestingly, while efficiency initially increases with temperature, it plateaus and slightly decreases at higher temperatures due to competing effects on density and saturated vapor pressure. These insights provide valuable guidance for process optimization. The optimum extraction condition is extraction pressure 220 bar, temperature 348.15 K and extraction duration of 70 min with the chelating agents of EDTA.
Molecular simulation analysis reveals that the citric acid system outperforms the EDTA system in terms of Zn2+ and Cr3+ aggregation, with a higher number of molecules in the largest aggregates and fewer total aggregates overall. Notably, under higher pressures (220 bar), the EDTA system shows an increase in the number of molecules in the largest aggregate, indicating potential benefits of pressure optimization.
The practical implications of this research underscore the need for exploring alternative chelating agents to further enhance extraction efficiency and cost-effectiveness. Additionally, future work should focus on optimizing extraction conditions to maximize performance, as well as conducting pilot-scale studies to validate the scalability and feasibility of this technology for industrial applications.
CRediT authorship contribution statement
Bo Ma: Writing – original draft, Project administration, Data curation. Ruihe Wang: Supervision, Conceptualization. Hongjian Ni: Methodology. Jaifang Xu: Simulation guide. Caiyun Xiao: Experiment support. Jie Chen: Simulation support.
Acknowledgement
The financial is supported by “the Fundamental Research Funds for the Central Universities (24CX02013A). We sincerely thank our colleagues from China University of Petroleum (East China) for helping with the theory research.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Ali, I.A., Dahche, A., Karrani, S., 2013. ADCO's Drilling Waste Management System Towards Zero Waste Disposals[C]// SPE/IADC Middle East Drilling Technology Conference and Exhibition.
- Fine-tuning mesoporous adsorbent for simultaneous ultra-trace palladium(ii) detection, separation and recovery. J. Ind. Eng. Chem.. 2015;21:507-515.
- [Google Scholar]
- Toxic cadmium (II) monitoring and removal from aqueous solution using ligand-based facial composite adsorbent. J. Mol. Liq. 2023
- [Google Scholar]
- Research progress of chelating agents in supercritical CO2 chelating extraction of heavy metal ions[J] J. Anal. Sci. 2010
- [Google Scholar]
- Effects of supercritical CO2 treatment temperatures on mineral composition, pore structure and functional groups of shale: implications for CO2 sequestration. Sustainability.. 2020;12(9):3927.
- [Google Scholar]
- Modelling of the extraction of uranium with supercritical carbon dioxide[J] J. Nucl. Sci. Technol. 2001
- [Google Scholar]
- Synthesis of zeolite from industrial wastes: a review on characterization and heavy metal and dye removal. Environ. Sci. Pollut. Res.. 2024;31(29):41791-41823.
- [Google Scholar]
- Solubility of hydrogen sulfide in n-eicosane at elevated pressure[J] J. Chem. Eng. Data. 2002;37(4):412-413.
- [Google Scholar]
- Supercritical extraction of toxic heavy metals from aqueous waste via cyanex 301 as chelating agent. J. Supercrit. Fluids. 2012;72:288-297.
- [Google Scholar]
- Gopalan, Aravamudan S., Wai, Chien M., Jacobs, Hollie K., 2003. [ACS Symposium Series] Supercritical Carbon Dioxide Volume 860 (Separations and Processes) || Supercritical Fluid Extraction of Actinides and Heavy Metals for Environmental Cleanup: A Process Development Perspective[J].
- Benign separation, adsorption, and recovery of rare-earth yb(iii) ions with specific ligand-based composite adsorbent. Process Saf. Environ. Prot.. 2024;185:367-374.
- [Google Scholar]
- Extraction of uranium (VI) from nitric acid solution into supercritical carbon dioxide containing tri-n-butylphosphate[J] Chem. Lett.. 2006;1995(5):365-366.
- [Google Scholar]
- The extraction of metal contaminants using supercritical carbon dioxide[J] Int. J. Chemoinform. Chem. Eng. 2018
- [Google Scholar]
- Recent advances in removal techniques of cr(vi) toxic ion from aqueous solution: a comprehensive review. J. Mol. Liq. 2021:329.
- [Google Scholar]
- Korzenski, M., Xu, C., Baum, T., 2022. Supercritical carbon dioxide: the next generation solvent for semiconductor wafer cleaning technology. advanced materials technology.
- Parameterization of highly charged metal ions using the 12-6-4 lj-type nonbonded model in explicit water. J. Phys. Chem. B. 2015;119(3):883-895.
- [Google Scholar]
- A new theoretical model of steady-state characteristics of supercritical carbon dioxide natural circulation[J] Energy 2019
- [Google Scholar]
- Experimental study on harmless disposal of waste oil-based mud using supercritical carbon dioxide extraction. Fuel. 2019;252:722-729.
- [Google Scholar]
- Supercritical carbon dioxide extraction of pigments from Bixa orellana seeds (experiments and modeling) [J] Braz. J. Chem. Eng. 2006
- [Google Scholar]
- Supercritical fluid extraction of oils and waxes from grape seeds with carbon dioxide[J] Russ. J. Phys. Chem. B 2019
- [Google Scholar]
- Extraction of galactolipids from waste by-products: the feasibility of green chemistry methods[D] Appl. Sci. 2021
- [Google Scholar]
- Transport properties of mixtures composed of acetone, water, and supercritical carbon dioxide by molecular dynamics simulation. J. Supercrit. Fluids. 2017;130:321-326.
- [Google Scholar]
- Molecular dynamics simulation of supercritical co2 microemulsion with ionic liquid domains: structures and properties. Chin. J. Chem. Eng.. 2019;27(11)
- [Google Scholar]







