Translate this page into:
Applying green analytical chemistry (GAC) for development of stability indicating HPLC method for determining clonazepam and its related substances in pharmaceutical formulations and calculating uncertainty
⁎Corresponding author. Tel.: +20 1223729152. a.badr@sigma-pharm.com (Ahmed Badr Eldin),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Clonazepam contains one benzodiazepine ring in its chemical structure which makes it vulnerable to degradation. In this study, green analytical chemistry approach was applied in attempts for the development of validated stability indicating RP-HPLC method for determining clonazepam and its related substances in pharmaceutical formulation. Validation has been performed according to ICH guidelines. Assay was capable of simultaneous monitoring of the intact drug in the presence of its related substances within the same run. HPLC assay involved an ODS column and a mobile phase composed of 2% sodium dodecyl sulfate, 0.05 M sodium acetate buffer pH 3.5 and isopropanol in ratio (25:55:20) at a flow rate of 1.5 mL/min and detection was carried out at 254 nm. HPLC method allowed good resolution between the peaks that corresponded to the active pharmaceutical ingredients and its degradation products with good linearity, precision, accuracy, specificity, LOD and LOQ. The expanded uncertainty (0.33%) of the method was also estimated from method validation data. This analytical technique is not only ecofriendly but also faster than the conventional liquid chromatographic system official in the USP-36.
Keywords
Green analytical chemistry
Clonazepam
Related substances
Uncertainty
HPLC
Stability indicating method
1 Introduction
Due to scientific and public concern about the environment pollution, environmentally friendly practices have been introduced in different areas of society and research. In green analytical chemistry, sample preparation and LC analysis need special attention because hazardous solvents are often used. European medicine agency (EMA) mentioned that solvents like methanol and tetrahydrofuran are ranked by as hazardous solvents (ICH Topic Q3C (R4), 2009) and because of their inherent toxicity, safe detoxification of the waste solvents is essential, which may lead to high to very high disposal costs. Possibilities toward green LC include reducing solvent use, switching to more benign solvents and/or eliminating organic solvents (Armenta et al., 2008; Kerton and Marriott, 2013; Taylor, 2010; Clark and Tavener, 2006). Clonazepam [5-(2-Chlorophenyl)-7-nitro-3H-1,4-benzodiazepin-2(1H)-one] is mainly used as anticonvulsant, muscle relaxant and anxiolytic agent. Clonazepam is slightly soluble in acetone, chloroform, acetic anhydride, hardly soluble in methanol, isopropanol, ether, almost insoluble in water. In all of the aforementioned LC techniques, including the USP-36 relevant monograph, tetrahydrofuran and methanol have been used as a part of mobile phase and extraction procedure. The present investigation describes a rapid, accurate and precise RP-HPLC method for the determination of Clonazepam and its related compound A & B (Fig. 1) in pharmaceutical dosage forms within quality control laboratories without the need to use of hazardous, toxic solvents since this drug is being marketed in domestic and international market.
Structures of clonazepam and its related compounds (a) clonazepam (b) clonazepam related A and (c) clonazepam related B.
2 Experimental
2.1 Materials and chemicals
USP – reference standard materials of clonazepam (99.88%), related compound A and related compound B in addition to excipient used for preparing the placebo were kindly provided by Sigma pharmaceutical industries (Egypt). All solvents and reagents were of HPLC grade, and were purchased from Sigma-Aldrich. HPLC grade water was obtained through a Milli-Q system (Millipore, Milford, USA) and was used to prepare all solutions.
Commercial samples of clonazepam tablets, manufactured by Sigma pharmaceutical industries – Egypt, were used in the applications.
2.2 Apparatus
Shimadzu HPLC system (Shimadzu, Kyoto, Japan), set to recycle the mobile phase and was equipped with a System Controller CBM-20A, Solvent Delivery Unit LC-20A, On-line Degassing Unit DGU-20A, and Photo-diode Array detector SPD-M20A. The peak areas were integrated automatically by computer using a Shimadzu LC solution V1.24 SP1 software program. A 20 μL volume of sample was introduced into Auto-Sampler SIL-20A injector. Photo cabinet “Atlas Suntest CPS Plus” – Germany has been used for photodegradation.
2.3 Analytical procedures
2.3.1 Reference method
According to the relevant monograph in the USP-36 (USP, 2013) the elution was carried out on a BDS C8 Hypersil column (150 mm × 4.6 mm, 5 μm particle size) from Thermo scientific (Massachusetts, USA). All analyses were performed at ambient temperature under isocratic conditions with a mobile phase of ammonium phosphate buffer solution pH 8.0: methanol: tetrahydrofuran at a flow rate of 1.5 mL/min in ration (60:52:13, v/v), using UV detection at 254 nm. Diluent solution prepared by mixing water, methanol, and tetrahydrofuran in ration 60:52:13 was used for standard and sample preparation.
2.3.2 Proposed liquid chromatography method
2.3.2.1 Preparation of stock solutions and standards
Stock standard solution of clonazepam, related compound A and related compound B was prepared in mobile phase at a concentration of 0.2 mg/ml. Series of dilutions at concentration levels of 4–140 μg/mL were obtained from stock solution by the appropriate dilution in mobile phase.
2.3.2.2 Sample preparation
Samples, of randomly selected clonazepam tablets, were dissolved and diluted to the appropriate volumes to get a concentration of 0.04 mg/ml using mobile phase as solvent. All samples were filtered through nylon sample filter (Whatman, 0.22 μm).
2.3.2.3 Method development and chromatographic conditions
A variety of mobile phases were investigated in the development of a stability-indicating HPLC method for the analysis of clonazepam and its related compound in pharmaceutical preparations. The suitability of mobile phase was decided on the basis of green analytical chemistry principles, selectivity, sensitivity of the assay, stability studies, and separation of clonazepam from the known related substances (A&B) beside the impurities formed during forced degradation studies. The elution was carried out on a BDS C8 Hypersil column (250 mm × 4.6 mm, 5 μm particle size) from Thermo scientific (Massachusetts, USA). All analyses were performed at ambient temperature under isocratic conditions with a mobile phase of isopropanol: 2% sodium dodecyl sulfate (SDS): 0.05 M sodium acetate buffer (pH 3.5 ± 0.05) in ratio (20:25:55, v/v) at a flow rate of 1.5 mL/min, using DAD detector at 254 nm.
2.3.2.4 Stability indicating capabilities of the proposed method
The stability-indicating capability of the method was determined by its ability to separate the reference degradation substances (A&B) from the drug of interest in addition to subjecting reference solution of clonazepam (40 μg/mL) to forced degradation conditions by acidic, basic, oxidative, and photolytic conditions to evaluate the interferences in the quantitation of clonazepam. Standard solutions in 1 M hydrochloric acid and 1 M sodium hydroxide were used for the acidic and basic hydrolysis, respectively. Both solutions were kept at ambient temperature for 12 h. For oxidative degradation, solutions were prepared in hydrogen peroxide (3%) solution and kept at ambient temperature for 4 h. Photodegradation was induced by irradiating the neutral solution at 1.2 million lux hours for 24 h. The distance between the light source and the sample was maintained at 25 cm. These solutions were diluted with mobile phase to final concentration of 40 μg/ml and were injected into chromatographic system.
2.3.2.5 Linearity
Linearity was evaluated by determining the response of a series of dilutions of thirteen standard solutions from clonazepam and nine standard solutions of its related compounds (A&B) at a concentration range of 4–140 μg/mL and 4–64 μg/mL, respectively. Each dilution was injected in triplicate to plot the calibration curve. Slope, intercept, and regression coefficient (R2) of the calibration curves were calculated to ascertain linearity of the method.
2.3.2.6 Precision
For method repeatability, assay of authentic samples solutions was repeatedly performed six times on the same day (intra-day). For reproducibility, freshly prepared solutions at aforementioned concentration level were analyzed at different days (inter-day), and results were statistically evaluated in terms of % RSD. The authentic samples were prepared by addition of the suitable amount of standard and the related compounds (A&B) to the placebo. These samples were handled as descried under assay preparation to give the desired concentration.
2.3.2.7 Accuracy
Accuracy was calculated as the deviation of the mean from nominal concentration. To assess accuracy, freshly prepared placebo of the clonazepam pharmaceutical formulations was spiked with various amounts of clonazepam and its related compounds (A&B) to obtain the concentration levels of 30, 40 and 50 μg/ml. Each solution was injected in triplicate.
2.3.2.8 Limit of detection (LOD) and limit of quantitation (LOQ)
The LOD is taken as the lowest concentration of an analyte in a sample that can be detected, but not necessarily quantified, under the stated conditions of the test while the LOQ is the lowest concentration of an analyte in a sample that can be determined with acceptable precision and accuracy under the stated conditions of test. They can be calculated from the linear calibration curve where LOD is the 3 times of residual standard deviation of the regression line (at the lower concentration) divided by the slope, while LOD is ten times the division product (Shrivastava and Gupta, 2011).
2.3.2.9 Robustness
In order to check the robustness, the effect of small but deliberate variations in the chromatographic conditions was evaluated. The conditions studied were flow rate (altered by ±0.01 mL/min), and pH of buffer solution (altered by ±0.1). These chromatographic variations were evaluated for retention time, peak area, number of theoretical plate and asymmetry factor for each clonazepam and its related compound.
2.3.2.10 Selectivity and system suitability
Selectivity of this method was indicated by the absence of any excipient interference at the retention times of the peaks of clonazepam and its related compounds with an acceptable resolution in the system suitability solution. The absence of interfering peak was evaluated by injecting a blank sample consisting of diluent and placebo. The test is only valid if the resolution between clonazepam related A and clonazepam related B is more than 2 as stated in the relevant monograph in the USP 36 (USP, 2013).
2.3.2.11 Uncertainty of the method
Among the different sources of uncertainty, the uncertainty associated with calibration appears to be the most important source in the overall uncertainty (ICH guideline Q2B, 2005).
The quantification of clonazepam by was assess through the calibration curve equation (Y = aX + b), where Y is the response, a is the slope, X is the concentration of clonazepam and b is the intercept. Based on this information, the uncertainty of HPLC’s result was estimated by the following equation: where UA% is the expanded uncertainty, t1-α/n-2 is the t-student for confidence level of 1-α and n-2 degrees of freedom (n correspond to the numbers of standard employed to obtain calibration curve), A% is the result of sample (in percentage), σY is the standard deviation of the areas obtained in chromatograms of sample, Y is the average of the areas obtained in chromatograms of sample, σb is the error for the intercept and σa is the error for the slope.
3 Results and discussions
3.1 Optimization of the chromatographic conditions
The HPLC procedure was optimized with a view to develop an ecofriendly stability-indicating method. No internal standard was used because no extraction or separation step was involved. Considering the green chemistry fundamentals (Clark et al., 2006), various solvent systems as mobile phases were tried for the development of a green and environmentally benign HPLC method. Of several solvents and solvent mixtures investigated, the mobile phase of isopropanol: 2% SDS: 0.05 M sodium acetate buffer (pH 3.5 ± 0.05) in ratio (20:25:55, v/v) was found to furnish sharp, well defined peaks with very good symmetry (around 1) and appropriate separation (NLT 2) between the peak of interest and the related compounds as a system suitability requirement in the relevant monograph in the USP-36 (Fig. 2).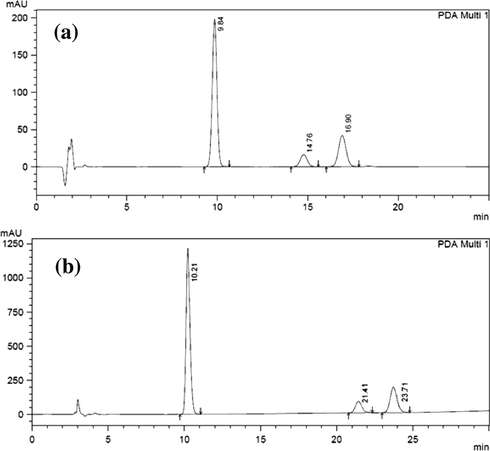
Separation of system suitability solution using (a) the proposed green method and (b) the USP-36 method (Clonazepam at 9.84, related compound A at 14.86 and related compound B at 16.90) and (b) the USP-36 method (Clonazepam at 10.21, related compound A at 21.41 and related compound B at 23.71).
Other organic modifiers and surfactants were tried such as Brij-L23, tween 20 and tween 80 did not resulted in chromatographic system as good as proposed method.
3.2 Method validation
3.2.1 Linearity
The calibration curves of clonazepam, clonazepam related A and clonazepam related B were evaluated by the linear least square analysis. The calibration curve plotted between concentration of drug and peak area and the regression equations were calculated and the correlation coefficients of the calibration curves were found to be R2 > 0.999 in all samples types. Linearity was established in concentration range of 4–140 μg/ml for clonazepam and 4–64 μg/ml for clonazepam related compounds A & B as reported in Table 1. The linear regression data for the calibration plot were indicative of a good linear relationship between peak area and concentration over a wide range. The correlation coefficient was indicative of high significance. The intercept of the ordinate showed the calibration plot did not deviate from linearity.
Clonazepam
Clonazepam related compound A
Clonazepam related compound B
Concentration (μg/ml)
Peak area
Concentration (μg/ml)
Peak area
Concentration (μg/ml)
Peak area
4
354,436
4
43,266
4
116,673
8
714,285
8
87,160
8
240,323
16
1,438,491
16
176,453
16
493,393
24
2,153,360
24
273,040
24
739,427
32
2,880,450
32
367,352
32
978,949
40
3,594,755
40
451,953
40
1,232,962
48
4,323,766
48
545,139
48
1,475,217
56
5,042,151
56
638,419
56
1,715,512
64
5,775,803
64
734,168
64
1,926,860
80
7,304,113
R2
0.9998
R2
0.9997
100
9,080,737
Slope
11496.851
Slope
30429.064
120
10,822,764
Intercept
4458.937
Intercept
3781.023
140
12,556,717
R2
0.9999
Slope
90217
Intercept
231.86
3.2.2 Accuracy
Accuracy was determined by the standard addition method. Three sets of placebo preparations were spiked with 32, 40 and 48 μg/ml of clonazepam and related substances reference standards and the mixtures were analyzed by the proposed method. The experiment was performed in triplicate. Good recoveries (101.33–99.40%) were obtained at each concentration level with a very low % RSD (0.97–0.11%) which indicated the accuracy of the proposed method (Table 2).
Amount taken (μg)
Amount found (μg)
Percent recovery
Mean percentage recovery
RSD%
Clonazepam
32
31.87
99.59%
99.57%
0.11
40
39.78
99.45%
48
47.84
99.67%
Clonazepam related compound A
32
32.28
100.88%
99.77%
0.97
40
39.62
99.05%
48
47.71
99.40%
Clonazepam related compound B
32
32.16
100.50%
100.97%
0.42
40
40.53
101.33%
48
48.52
101.08%
3.2.3 Precision
3.2.3.1 Analysis repeatability
It was evaluated by carrying out the analysis of the six homogenous solutions of same test sample and content of related substances. The determinations were carried out one after the other under conditions as similar as possible. The relative standard deviation was calculated from the results of the obtained observations (Table 3).
Exp. No.
Repeatability on day 1
Repeatability on day two
Clonazepam
Related A
Related B
Clonazepam
Related A
Related B
1
99.90%
100.06%
100.92%
100.00%
100.16%
101.02%
2
99.93%
100.33%
101.16%
100.23%
100.63%
101.46%
3
99.96%
100.30%
100.99%
100.16%
100.50%
101.19%
4
99.97%
100.30%
101.76%
100.37%
100.70%
102.17%
5
99.79%
100.27%
101.91%
99.89%
100.37%
102.01%
6
99.93%
100.94%
101.65%
100.13%
101.14%
101.86%
Mean
99.91
100.37
101.40
100.13
100.58
101.62
SD
0.064
0.298
0.427
0.168
0.334
0.464
RSD (%)
0.064
0.297
0.421
0.168
0.332
0.456
3.2.3.2 Intermediate precision
The intermediate precision of the method was checked by determining precision on a different instrument, analysis being performed in different laboratory on a different day. The relative standard deviation was calculated from the results of the obtained observations.
In all cases the RSD was lower than 2 (Table 3).
3.2.4 Specificity
The specificity of the method was evaluated through its ability to discriminate between the peak of the parent drug and those due to its related compounds A & B (Fig. 2).
In the same regard a forced degradation study was established, clonazepam stock solution was exposed to various stress conditions. On treating with 5 N NaOH extensive degradation has been occurred, when treated with 1 N NaOH for one hour, the height of peak was reduced and four new peaks of degradation products were observed (Fig. 3). Almost the same has been happened on treating with 1 N HCl for one hour (Fig. 4). Clonazepam was found to degrade more rapidly in oxidative conditions. Upon reflux with 30% hydrogen peroxide for 1 h, peak height was reduced, with the appearance of two new degradation peaks (Fig. 5). One degradation peak has been separated with dramatic change in the peak of the parent drug has been happened on exposing to photo-irradiation conditions in photo cabinet (Fig. 6).The above results show the method is stability indicating.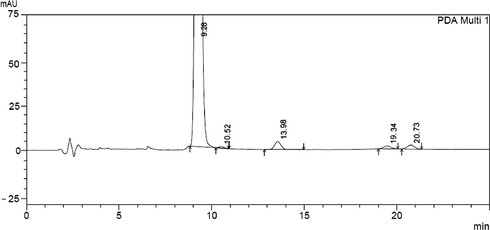
Clonazepam solutions under basic degradation conditions (Clonazepam at 9.28, basic degradation products at 10.52, 13.98, 19.34 and 20.73).
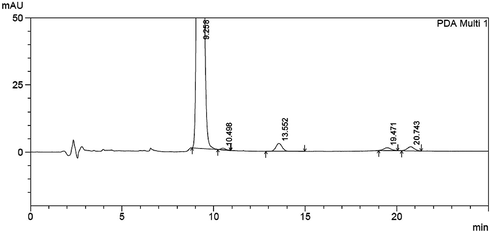
Clonazepam solution under acidic degradation conditions (Clonazepam at 9.26, acidic degradation products at 10.50, 13.55, 19.47 and 20.74).
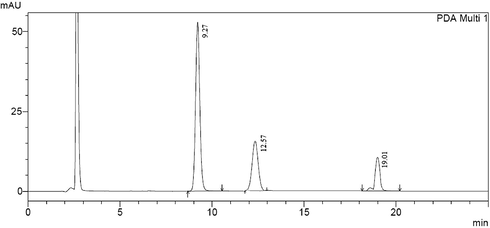
Clonazepam solution under oxidative degradation conditions (Clonazepam at 9.27, oxidative degradation products at 12.75 and 19.01).
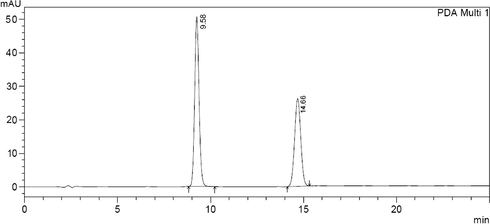
Clonazepam solution under photo-irradiation conditions (Clonazepam at 9.58, photo degradation product at 14.66).
3.2.5 Detection (LOD), quantification (LOQ) limits and system suitability
Limit of detection (LOD) and the limit of quantitation (LOQ) were calculated as 3 S.D. and 10 S.D. of the blank, respectively, according to the treatment by Miller and Miller (Sadeghi et al., 2013). Before injecting solutions, the column was equilibrated for at least 20 min with the mobile phase. Their values prove the sensitivity of the proposed method and that it can be used for detection and quantification of trace amounts of clonazepam and its related compounds. The obtained system suitability parameters were satisfactory according to the relevant USP-36 monograph (Table 4).
Parameters
Clonazepam
Clonazepam related compound A
Clonazepam related compound B
LOD (μg/ml)
0.024
0.386
0.357
LOQ (μg/ml)
0.0799
1.2875
1.1893
Theoretical plates (h)
18625.64
8854.82
9570.59
Tailing factor (T)
1.104
0.988
1.032
Resolution
–
6.865
2.815
3.2.6 Robustness
The robustness of an analytical procedure, as defined by the ICH refers to its capability to remain unaffected by small and deliberate variations in method parameters (Płotka et al., 2013). Robustness was determined by recording the effect of slight change on the separation conditions of buffer and pH. Table 5 shows that the theoretical plates, tailing factor and resolution of clonazepam and its related substances are not changed significantly which indicates the proper robustness of the method.
Drug name
Variations
Chromatographic parameters
Retention time
Area
Theoretical plates
Asymmetry
Clonazepam
Change in buffer pH
9.83
3594745
18620.52
1.103
Flow rate at 1.51 ml/min
9.75
3594760
18624.83
1.102
Clonazepam related compound A
Change in buffer pH
14.66
451943
8840.77
0.998
Flow rate at 1.51 ml/min
14.56
451961
8857.94
1.009
Clonazepam related compound B
Change in buffer pH
16.85
1232974
9511.48
1.026
Flow rate at 1.51 ml/min
16.75
1232950
9514.59
1.031
3.2.7 Uncertainty estimation
The expanded uncertainty amount (0.33% at confidence level of 95%), was estimated based on the error of the slope and the intercept. Considering HPLC method, a six standard calibration curve may decrease the uncertainty estimated, as it decrease the t-student multiplier. Increasing the number of injections per standard level may also contribute in the reduction of final uncertainty (De Melo Abreu et al., 2005; Lourenço, 2012). Other sources of uncertainties, e.g. uncertainties of volumetric glassware, were not considered due to its non significant contribution into the total budget of the uncertainty.
3.3 Application
Samples of clonazepam 0.5 mg tablets (n = 6) were analyzed for the parent drug and its related substances by this method and the results showed a percent recovery of 100.1% and a RSD of 0.60% for 6 replicates while the related substances were within the accepted limits. The method was used for analyzing samples of the stability program of the drug product also for analysis of raw materials samples which give results between 99.6% and 100.2%.
4 Conclusion
The proposed RP-HPLC method is simple, selective, rapid, accurate, precise, reproducible, robust, sensitive and stability indicating. Therefore, the method was applied to the assay of clonazepam in tablets dosage form. The method is also simple in terms of sensitivity, use of an environmentally benign mobile phase, simple extraction procedures, relatively lower retention time and no internal standard is required. These advantages make this method superior for the routine analysis of clonazepam and its related substances in commercially available formulations. The method could also be applied for the prediction of shelf life in the stability studies of the mentioned formulations because it is having stability-indicating properties. The replacement of widely-used hazard solvents and chemicals with new, innocuous, and less toxic ones provides environmentally benign alternatives to the more hazardous chemicals and processes in the field of drug/pharmaceutical analysis. Finally, estimation of the uncertainty is important fulfill one of the ISO 17025 requirements aiming to guarantees the quality and reliability of the obtained results.
Authors’ contribution
Abdallah Shalaby and Ahmed Badr Eldin participated in the work conception, design, and coordination, helped to draft the paper and revised it critically for important intellectual content. Mahmoud Abdallah, Moataz Shaldam and Mohamed Abdallah participated in the design and carried out the HPLC analysis and wrote the paper. All authors read and approved the final paper.
Acknowledgement
The authors are grateful to Sigma pharmaceutical industries – Egypt for providing the facilities to carry out this study.
References
- Screening of grapes and wine for azoxystrobin, kresoxim-methyl and trifloxystrobin fungicides by HPLC with diode array detection. Food Addit. Contam.. 2005;22:549-556.
- [Google Scholar]
- ICH Topic Q3C (R4), 2009. Impurities: guideline for residual solvents (CPMP/ICH/283/95) accessed February 15, 2014.
- ICH guideline Q2B, 2005. ICH Guidelines: validation of analytical procedures. Text and Methodology Q2 (B), 2005.
- Alternative Solvents for Green Chemistry (second ed.). Cambridge, UK: Royal Society of Chemistry; 2013. 149–174
- Simple estimation of uncertainty in the quantification of cefazolin by HPLC and bioassay. J. Chromatogr. Sep. Tech.. 2012;3:1-3.
- [Google Scholar]
- Validation and uncertainty estimation of an ecofriendly and stability-indicating HPLC method for determination of diltiazem in pharmaceutical preparations. J. Anal. Methods Chem.. 2013;353814:1-10.
- [Google Scholar]
- Methods for the determination of limit of detection and limit of quantitation of the analytical methods. Chron. Young Sci.. 2011;2:21-25.
- [Google Scholar]
- Principles into practice: setting the bar for green chemistry. Environ. Health Perspect.. 2010;118:A254-A257.
- [Google Scholar]
- United States Pharmacopoeia 36/National Formulary 31. Clonazepam Monogr. 2013:3050-3052.
- [Google Scholar]







