Translate this page into:
A combined computational & electrochemical exploration of the Ammi visnaga L. extract as a green corrosion inhibitor for carbon steel in HCl solution
⁎Corresponding authors. ercleehs@hanyang.ac.kr (Han-Seung Lee), hlgaz@hanyang.ac.kr (Hassane Lgaz)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Natural-based corrosion inhibitors have gained great research interest thanks to their low cost and higher performance. In this work, the chemical composition of the methanolic extract of Ammi visnaga umbels (AVU) was evaluated by gas chromatography (GC) coupled with mass spectrometry (MS) and applied for corrosion inhibition of carbon steel (CS) in 1.0 mol/L HCl using chemical and electrochemical techniques along with scanning electron microscope (SEM) and theoretical calculations. A total of 46 compounds were identified, representing 89.89% of the overall chemical composition of AVU extract, including Edulisin III (72.88%), Binapacryl (4.32%), Khellin (1.97%), and Visnagin (1.65%). Chemical (Weight loss) and electrochemical (potentiodynamic polarization curves (PPC), and electrochemical impedance spectroscopy (EIS)) techniques revealed that investigated extract can be used as an effective corrosion inhibitor for carbon steel in 1.0 mol/L HCl solution. At a low dose of 700 ppm, the inhibitory action of AVU extract reached an inhibition efficiency of 84 percent. According to polarization tests, the investigated extract worked as a mixed inhibitor, protecting cathodic and anodic corrosion reactions. The EIS test showed that upon the addition of AVU extract to HCl solution, the polarization resistance increased while the double layer decreased. SEM images showed a protected CS surface in the inhibited solution. Quantum chemical calculations by Density Functional Theory (DFT) for the main components confirmed the major role of heteroatoms and aromatic rings as adsorption sites. Molecular dynamics (MD) simulation was used to study the adsorption configuration of the main components on the Fe(1 1 0) surface. Outcomes from this study further confirmed the significant advantage of using plant-based corrosion inhibitors for protecting metals and alloys.
Keywords
Corrosion inhibition
Green inhibitor
Carbon steel
Ammi visnaga
DFT
Molecular dynamics
1 Introduction
Corrosion is a thermodynamically spontaneous process that involves the chemical or electrochemical interaction of metals or alloys with the surrounding constituents, resulting in the change of metals or alloys to a more stable state (Jafari et al., 2019; Laamari et al., 2011). The negative consequences of corrosion significantly impact metallic structures, maintenance costs, and public welfare. The approximated global cost of corrosion is $2.5 trillion (USD), which is around 3.4% of industrialized nations' Gross Domestic Product (GDP). Some 15% ($ US 375 billion) − 35% ($ US 875 billion) range of this cost can be neglected by accomplishing felicitous corrosion prevention tactics (Koch et al., 2016). Carbon steel is one of the preferred engineering materials in the industrial arena, including oil and gas transportation pipelines, downhole casing, reinforced concrete, vehicles, refining, and chemical processing, despite its non-corrosion resistance, particularly in acidic circumstances (Mobin et al., 2017). Carbon steel is a significant building component because of its exceptional mechanical properties and low cost. On the other hand, pickling, acid cleaning of boilers, descaling, oil well acidizing, and other industrial applications of acidic solutions are routinely used to remove deposited rust, iron oxide scale for enhancing oil production (Khalaf et al., 2020). The most often used corrosive acids during such processes are hydrochloric acid and sulphuric acid (Aourabi et al., 2020; Hemapriya et al., 2020; Lgaz et al., 2020a).
The addition of corrosion inhibitors to acidic solutions is the first choice to reduce their aggressiveness and thus the corrosion rate. Inhibitors with electronegative elements including O, S, N, P, conjugation, double or triple bonds, and aromatic rings provide greater corrosion mitigation (Verma et al., 2021). These compounds work by adsorbing onto the surface of a metal substrate and producing a protective layer, making it impervious to the harsh external environment. The degree of corrosion protection provided by an inhibitor is, in most cases, proportional to its concentration and depends on its physicochemical properties (Verma et al., 2018c). It has been reported in many studies that increasing the inhibitor concentration increases fractional surface coverage by an extra number of inhibitor molecules, which may be limited to a fixed optimal concentration due to surface saturation (Mobin et al., 2020).
The use of harmful synthetic inhibitors, especially inorganic ones, is no longer permitted due to increased environmental awareness. As a result, current research focuses on non-hazardous, biodegradable, less expensive, and ecologically friendly corrosion inhibitors. Plant extracts represent a plenteous category of corrosion inhibitors as most of these materials are easily obtainable and non-poisonous (Saxena et al., 2018). Many studies examining plant extracts have revealed that various extracts of leaf, root, bark, stem, pulp, and fruit are being investigated as corrosion inhibitors for various metals and alloys (Verma et al., 2018a). Plant extracts such as Eucalyptus leaves (Dehghani et al., 2019a), Primula vulgaris flower extract (Majd et al., 2019) Aloe vera (Abiola and James, 2010), ginger (Gadow and Motawea, 2017; Liu et al., 2019), turmeric rhizomes (Al-Fakih et al., 2015), olives leaf (Harb et al., 2020), olive roots, stems, and leaves (Bouknana et al., 2015), Mentha rotundifolia leaves (Khadraoui et al., 2014), Jathropa leaf (Ikpeseni et al., 2021), Cashew nut sheel (Furtado et al., 2019), Hibiscus leaf extract (Hoai et al., 2019), Coriandrum Sativum L. Seeds Extract (Kadiri et al., 2018) Henna Extract (Fouda et al., 2019), Amaranthus (Srivastava, 2020), Myrmecodia pendans Extract (Pradityana et al., 2016), Castor oil (Farhadian et al., 2020), binary mixture of sesame and castor oil (Oyekunle et al., 2019), Azadirachta indica (Sharma et al., 2015), neem (Sharma et al., 2009), Halopitys Incurvus Extract (Benabbouha et al., 2018), potato (Shekhar et al., 2021), Lagerstroemia speciosa leaf extract (Mobin et al., 2019b) and pineapple stem extract (Mobin et al., 2019a) among others have been investigated as good corrosion inhibitors. Experiments were carried out in acidic, alkaline, and neutral environments to test their corrosion inhibition performance for various ferrous and non-ferrous alloys. Polyphenolic secondary metabolites, such as flavonoids, tannins, resveratrol tannin, and coumarin, gallic acid, sterols, alkaloid berberine pyrrolidine, ascorbic acids, amino acids, and carotene, are among the main constituents found in these plants. The corrosion inhibition effect is due mainly to synergistic adsorption of different components or those with a higher percentage.
Ammi visnaga (Khella) belongs to the Apiaceae/Umbelliferae family. It is an annual herbaceous folk plant that grows wild in the Mediterranean area, particularly in Egypt, Morocco, and the Islamic Republic of Iran, with bi- or tripinatisect linear segmented leaves and huge compound umbels of white flowers. It is referred to as “Bechnikha” in the local Moroccan dialect. This is an important part of the local floristic history (Chraka et al., 2020). The literature review reveals that Ammi visnaga has been used to treat psoriasis, asthma, vitiligo recovery, hypoglycemia, and toxic bites (Bhagavathula et al., 2015). Many common drugs have been developed from khella, including amiodarone, nifedipine, and cromolyn. The ability of Ammi visnaga seeds to reduce carbon steel corrosion in the acidic medium (Zaher et al., 2020) and the essential oil of the plant to prevent brass corrosion in NaCl media (Chraka et al., 2020) has been documented.
In light of these facts, herein, the methanolic extract of Ammi visnaga dry umbels was chosen to study its anti-corrosive performance for carbon steel in 1 mol/L HCl medium by employing weight loss measurements, electrochemical impedance spectroscopy, potentiodynamic polarization curves coupled with surface characterization by scanning electron microscope (SEM). The principal constituents prevailing in AVU extract were identified utilizing GC/MS. It should be noted here that for the well acidization process, which is a maintenance technique to remove mineral deposits from wells, a high HCl concentration ranging from 15 to 20 wt% is used (Reynolds, 2020). To limit the corrosion effect on equipment, a corrosion inhibitor formulation (CIF) composed of inhibitor molecules, intensifiers, surfactants, metal chelating agents is added to HCl solution (Brezinski, 1999; Reynolds, 2020). Bearing this in mind, the present work is a contribution to investigate the corrosion inhibiting properties of AVU extract, and therefore its potential incorporation into a CIF. The 1.0 mol/L HCl is selected as a reference for laboratory tests.
Furthermore, considering the presence of several compounds, theoretical investigation of the major components can provide useful insights into their electronic properties. Therefore, the active sites are responsible for interacting with the metal surface (Bahlakeh et al., 2019a; Dehghani et al., 2019b, 2020b, 2020c). These insights can be achieved by implementing DFT calculations of selected components. In addition, molecular dynamics simulation is an interesting choice for studying the adsorption of major components on the metal surface. Taking these into consideration, these theoretical tools are used in the present work to investigate the adsorption behavior of AVU extract’s components on the steel surface.
2 Material and methods
2.1 Collection and preparation of methanolic extract
The plant was collected in October 2015 in the Sidi Slimane region of Morocco, a rural section of the Rabat-Salé-Kenitra area. Ammi visnaga umbels were crushed and ground into a fine powder. Soxhlet extraction with methanol was performed (Jensen, 2007). To this end, 15 g of the powder was put in a cartridge, and 300 mL of solvent (99.8% methanol) was used to extract it. The extraction process was continued until the solvent retrieved was colorless (6 h). The solvent rich in extracted compounds was collected in a flask, then allowed to evaporate completely in a rotary evaporator at mild temperature (40 °C) to remove the solvent, after which it was weighed. The extraction yield was 18.2%. The resulting extract was kept at 4 °C.
2.2 Chromatographic characterization
The chromatographic analysis of the methanolic extract of Ammi visnaga umbels was carried out using a Bruker 456 GC Triple Quadrupole EVOQ gas chromatography-mass spectrometer equipped with an 8400 series auto-injector (Bruker, Germany). The system was equipped with an RXI-5SIL MS column (30 m × 0.25 mm × 0.25 μm film thickness, Bruker, Germany). The temperature was set from 35 °C to 300 °C at 5 °C min−1 and then held at 300 °C for 10 min. Helium gas was used as a carrier gas with a constant flow rate of 1.5 mL min−1. A 1.0 μl of samples were automatically injected into the non-split mode. The temperature of the mass spectrometry interface was 280 °C. For CG mass detection, an electron ionization system with ionization energy of 70 eV was used and the scanning range was 10–600 amu. The identification and percentage composition of the compounds were performed using the mass spectrometry library and the NIST 2014, 11th Edition and WILEY 5th Edition spectrometer data bank.
2.3 Corrosion tests
2.3.1 Carbon steel specimens and aggressive solution
Carbon steel was employed as the working electrode in this investigation, and its chemical and mass composition are: 0.370% C, 0.230% Si, 0.680% Mn, 0.016% S, 0.077% Cr, 0.011% Ti, 0.059% Ni, 0.009% Co, 0.160% Cu, and balance Fe. A clean surface condition is required for optimum measurement reliability. The samples' surfaces were mechanically polished using abrasive papers (carbon, silica) with increasing particle sizes ranging from 80 to 1200 mm, then rinsed with distilled water, acetone and dried. The corrosive solution (1.0 mol/L) was made with bidistilled water from a concentrated 37% HCl solution. Pre-trial tests were carried out to select the appropriate concentration range, which is fixed between 150 and 700 ppm of AVU extract.
2.3.2 Gravimetric measurements
Gravimetric experiments were performed to determine the concentration impact of AVU extract on carbon steel corrosion at different temperatures (303 to 333 K). The carbon steel specimens were immersed in 1.0 mol/L HCl for 12 h, according to the ASTM G1-90 designation (Metals, 2017). The experiments were conducted in a double-walled cell with a condenser and a thermometer. The electrolyte was kept at the desired temperature by a circulating water thermostat. The specimens were immersed in 250 mL beaker containing the 250 mL electrolyte. After the immersion, the carbon steel specimens were completely cleaned with demineralized water and gently scrubbed with a bristle brush to eliminate the corrosion products. They were then cleaned with acetone and twice distilled water. Finally, the test specimens were dried to check that their weight remained consistent. Each gravimetric test value is the average of three tests. The corrosion rate and inhibition efficiency (ηw%) can be calculated by using Eq. (1) and Eq. (2), respectively:
2.3.3 Electrochemical studies
PGZ 301 Potentiostat/Galvanostat controlled by Volatamaster 4 software package was used to perform all electrochemical measurements. A conventional three-electrodes cell was used consisting of the CS serves as the working electrode, saturated calomel electrode (SCE) as the reference electrode, and platinum wire acts as the counter electrode. Prior to each experiment, the working electrode was dipped in HCl solution without and with different concentrations of the extract for 30 min to reach the steady-state open circuit potential (OCP). After that, electrochemical impedance spectroscopy (EIS) experiment was performed by applying a frequency range from 100 kHz to 0.01 Hz at an AC voltage amplitude of 10 mV. Nyquist and Bode plots were used to represent the EIS data, and Zview software was used to obtain the appropriate equivalent circuit model. All tests were performed in an aerated and non-stirred condition. Then, the potentiodynamic polarization experiment was achieved in the potential range of −800 to −200 mV/SCE applying a sweep rate of 0.5 mV/s. The corrosion current density (icorr), anodic Tafel slope (βa), cathodic Tafel slope (βc), and corrosion potential (Ecorr) were determined using Nova 2.1 program. The following relationship was used to quantify inhibition efficiency (ƞPPC%) using the calculated values of icorr.
Applying polarization resistance (Rp) obtained from the impedance data of the Nyquist plots, the ƞEIS% was measured.
2.3.4 SEM analysis
A scanning electron microscope (SEM) was used for surface characterization of the carbon steel. Steel samples were treated as described above before immersion in inhibited solution for 12 h before tests. A Hitachi TM-1000 SEM at an accelerating voltage of 15 kV was used for SEM analysis of steel surface.
2.4 Theoretical calculations
The geometry optimization and frontier molecular orbitals of the main components of AVU extract were determined using the Dmol3 module implemented in Material Studio software [27] using Generalized gradient approximation (GGA) with double numerical basis sets plus polarization (DNP) [28] and COSMO solvation model [29]. All other parameters correspond to “fine” quality in the Dmol3 code.
MD simulation by Forcite module of Materials Studio software was used to investigate the interaction of the four main components of AVU extract with Fe(1 1 0) surface. The pure iron crystal was imported from Materials Studio database and cleaved along (1 1 0) plane. Then, the Fe (1 1 0) plane of four layers was enlarged to 6 × 6 supercell, and a vacuum slab with a zero thickness was created. Fe (1 1 0) plane was chosen as the first layer as it is the most stable iron plan. The second layer that contains one AVU extract’s components, 491 water molecules, 9H3O+ and 9Cl- was created by Amorphous cell module of Materials Studio. Then, both layers were combined by Build layers tool of Materials Studio, and the resulted simulation box had a dimension of 12.39 × 12.39 × 35.97 Å3. Although the simulation system is a simplistic model of the corrosion inhibition process, it is still useful in making qualitative and comparative analyses, which can boost the understanding of the inhibitor's adsorption behavior. Other parameters were COMPASS force field, NVT ensemble, 303 K, 5000 ps simulation time, and 1 fs time step. The energy of interaction between molecules and Fe(1 1 0) surface was determined according to the following equation:
3 Results and discussion
3.1 Identification of AVU extract’s components
Fourty six chemicals accounting for 89.89% of the overall chemical composition of Ammi visnaga umbels' methanolic extract were found (Table 1). The Edulisin III is the most abundant component, accounting for 72.88% of the total composition, followed by Binapacryl (4.32%), then Khellin (1.97%), and Visnagin (1.65%) with modest percentages.
N°
Compound
Retention time
%
1
Dimethylethylamine
5.478
0.623
2
4-Ethyl-2,3-dihydrofuran
5.823
0.034
3
3-Oxobutyramide
7.597
0.434
4
Linalol
24.346
0.176
5
cis-3-Hexenyl iso-butyrate
30.962
0.310
6
2,6-Dimethyl-1,7-octadiene-3,6-diol
36.554
0.175
7
(Z)-2-nonadecene
44.287
0.069
8
Neryl (S)-2-methylbutanoate
50.973
0.043
9
2,4-Bis-(tert.-butyl-)-phenol
51.187
0.070
10
Geranyl vinyl ether
61.777
0.055
11
Dodecyl isobutyrate
64.374
0.180
12
Estragole
65.080
0.063
13
Farnesol
67.237
0.761
14
Visnagin
77.561
1.658
15
2-(2-vinylcyclohexyl)-3-butyn-2-ol
79.329
0.194
16
cis,trans-.alpha.-Farnesene
84.694
1.127
17
Khellin
86.049
1.977
18
Isopimpinellin
86.164
0.199
19
Butyl 4,7,10,13,16,19-docosahexaenoate
86.239
0.125
20
Doconexent
87.821
0.746
21
Caryophyllene oxide
89.076
0.133
22
Glyceryl linolenate
92.251
0.076
23
5.alpha.-Estran-3-on-17.beta.-ol
92.306
0.042
24
2-Methylenecholestan-3-ol
92.965
0.083
25
Isolongifolene
96.145
0.126
26
Decursin
98.188
0.464
27
Cnidimine
100.341
1.949
28
Edulisin III
104.128
72.886
29
Binapacryl
107.090
4.325
30
Docosane
112.464
0.379
31
Andrographolide
121.942
0.409
Total
89.891
The molecular structures and organic class of major constituents of the methanolic extract of Ammi visnaga umbels are listed in Table 2. According to data from the literature, GC–MS analysis of the methanolic extract of Ammi visnaga umbels has not been studied to date; however, the two main furanochromones (khellin and visagin) have already been isolated in several studies (Franchi et al., 1985) using different methods such as the capillary electrophoresis method (Günaydın and Erim, 2002). Moreover, with a new supercritical fluid extraction technique, these active principles were studied and then analyzed by reverse-phase HPLC [41] and separated in different stages of development of the fruit of Ammi visnaga (Bishr et al., 2018). In contrast, binapacryl and Edulisin III were never identified in extracts of Ammi visnaga. However, Edulisin III was previously isolated in (Mizuno et al., 1994) from the fruit of Angelica edulis Miyabe (Umbelliferous).
Compounds
Chemical group
Chemical formula
(%)
Khellin
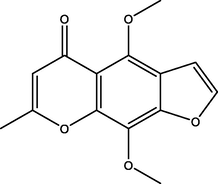
Furanochromons
C14H12O5
1.97
Visnagin
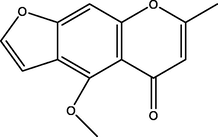
C13H10O4
1.65
Edulisin III
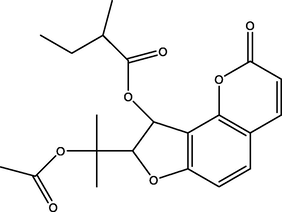
Coumarins
C21H24O7
72.88
Binapacryl
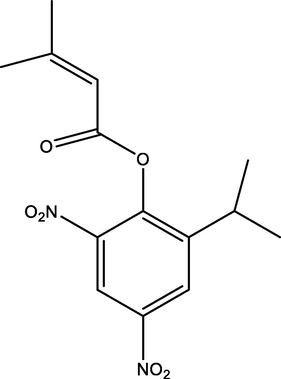
Dinitrophenols
C15H18N2O6
4.32
3.2 Gravimetric measurements
3.2.1 Effect of AVU extract concentration
The gravimetric experiment was carried out to determine AVU extract's protective capability at successively increasing concentrations. Table 3 lists the derived parameters such as corrosion rate (mg cm-2h−1), surface coverage, and inhibition efficiency (percentage). According to the findings, AVU extract may effectively resist carbon steel corrosion under tested acidic medium. Furthermore, when the concentration of the inhibitor increases, the corrosion rate reduces, thus the corrosion-preventive activity of AVU extract improves with concentration, with the greatest concentration being 700 ppm. The ability of AVU extract's natural chemical constituents to adsorb onto the metal surface is mainly the reason for its corrosion resistance ability. The synergistic adsorption of different AVU extract’s components may create a protective barrier on the steel surface, thus protecting it against the extrinsic attack of acidic solution's aggressive ions (Aslam et al., 2020). The proportion of surface covered with adsorbed AVU extract molecules determines the amount of corrosion protection. The adsorbed molecules of AVU extract grow on the metal surface as its concentration increases. The θ value, is a critical variable since it reflects the percentage of metal substrate surface covered by AVU extract molecules.
[AVU] (ppm)
CR (mg cm−2h−1)
ϴ
ηw (%)
1.0 M HCl
1.135 ± 0.0072
–
–
150
0.5108 ± 0.0098
0.55
55
250
0.3746 ± 0.0076
0.67
67
450
0.2384 ± 0.0047
0.79
79
700
0.1817 ± 0.0088
0.84
84
3.2.2 Effect of temperature and activation parameters
Considering the well-known effect of temperature on most of chemical reactions, a better understanding of the corrosion inhibition process can be achieved by studying the effect of temperature on the inhibition performance of tested inhibitor. To this end, the effect of temperature on the corrosion rate is investigated using weight loss experiments at four different temperatures in the range of 303–333 K with 10 K gap. Results are represented in Table 4. It can be seen that the increase of temperature accelerates the metal dissolution rate in both uninhibited and inhibited mediums. However, the addition of the optimum concentration of AVU extract to the 1.0 mol/L HCl results in a significant decrease of corrosion rate compared to uninhibited solutions. The maximum inhibition efficiency is obtained at 303 K; however, it shows only a small decrease by 5% at 333 K, signifying that the presence of AVU extract in the blank solution has an excellent corrosion inhibition effect.
Medium
303 K
313 K
323 K
333 K
CR (mg cm−2h−1)
ηw (%)
CR (mg cm−2h−1)
ηw (%)
CR (mg cm−2h−1)
ηw (%)
CR (mg cm−2h−1)
ηw (%)
Blank
1.135
–
2.466
–
5.032
–
10.029
–
Blank + 700 ppm AVU extract
0.181
84
0.443
82
0.956
81
2.107
79
The reaction kinetics of corrosion follow Arrhenius and transition state equations as follow:
Plots of ln CR vs. 1000/T and ln CR/T vs. 1000/T are shown in Fig. 1(a) and 1(b), representing Arrhenius and transition state equations, respectively. Activation parameters calculated from slopes and intercept of plots are listed in Table 5. Results show a higher activation energy in the presence of AVU extract compared to that of blank solution. It suggests the increase of the energy barrier for the corrosion reaction, resulting in reduced rate of corrosion process (Verma et al., 2016). The positive ΔHa values refer to the endothermic nature of the dissolution process (Azzaoui et al., 2017). Besides, the increased ΔSa value signifies an increase in the degree of disorder compared with the blank (Azzaoui et al., 2017).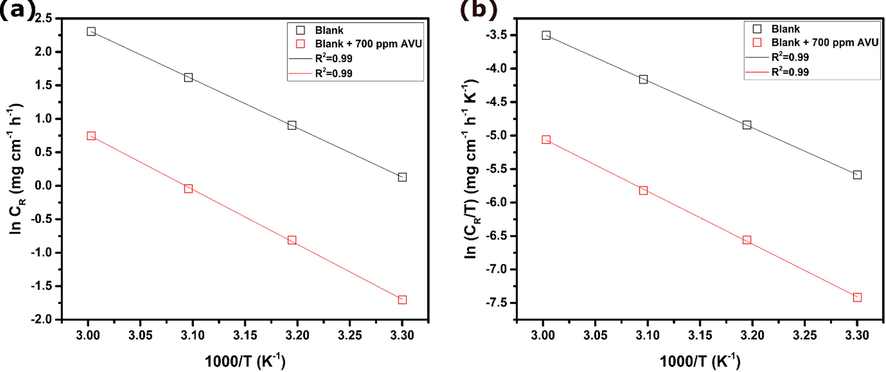
Arrhenius (a) and transition state (b) plots for carbon steel in 1.0 mol/L HCl without and with 700 ppm of AVU extract.
Medium
Ea (kJ/mol)
(kJ/mol)
(J/mol K)
Blank
60.79
58.15
−51.86
AVU extract
68.09
65.46
−42.92
3.3 Electrochemical impedance spectroscopy
The EIS is a non-destructive and very effective instrumental approach for determining the corrosion processes occurring at the metal-corrosive electrolyte interface. Fig. 2(a) shows Nyquist curves for carbon steel in a 1.0 mol/L HCl solution with and without different AVU extract concentrations. The diameter of capacitive loops is directly related to inhibitor concentration, implying that greater concentrations of AVU extract might more effectively protect against the attack of aggressive acidic species. The single semicircle can be ascribed to the charge transfer process at the solution/electrode interface (Aslam et al., 2019). The charge transfer process controls the corrosion reaction of steel, and the inhibitor presence does not change the steel dissolution mechanism (Znini et al., 2012). It is also clear that these semicircles are imperfect due to the frequency dispersion (Larabi et al., 2004).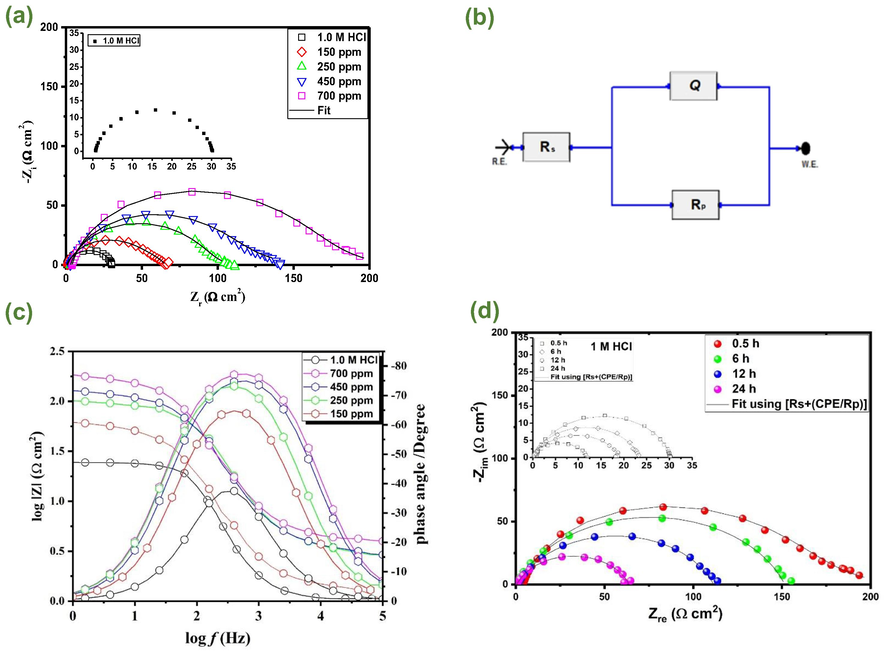
Impedance results of carbon steel in 1.0 mol/L HCl without and with different concentrations of AVU extract at 303 K; (a) Nyquist plots, (b) Equivalent electrical circuit, (c) Bode plots, and (d) Nyquist plots of carbon steel in 1.0 mol/L HCl and with 700 ppm of AVU extract at different immersion times.
The circuit Rs(RpCPE) shown in Fig. 2(b) is used to model the impedance output explicitly. Rs, Rp, and CPE represent solution resistance, polarization resistance, and the constant phase element, respectively. To address the fluctuations in capacitance behavior, CPE is employed instead of capacitance (Bahlakeh et al., 2019b; Dehghani et al., 2020a). The following relationship may be used to calculate the CPE impedance (ZCPE) (Orazem and Tribollet, 2008).
here, Y0 represents proportionality constant, n is the CPE exponent, which ranges from −1 to + 1 and j is an imaginary number (j= (-1)1/2. ω is the angular frequency in rad−1. Cdl is estimated by the following equation (Saha et al., 2016a):
Cdl may also be represented as follows:
Table 6 shows the electrochemical parameters acquired from the EIS results using the equivalent circuit. It can be shown in Table 6 that when AVU extract is present in the acid solution, Rp values increase, and Cdl values decrease in contrast to the untreated media. Furthermore, as the AVU extract concentration is raised between 150 and 700 ppm, the Rp values rise. In contrast, the Cdl values fall, indicating a significant double-layer behavior change due to AVU extract adsorption onto the steel substrate. It can be explained by an increased thickness and/or decreased local dielectric constant, as Helmholtz model suggested (Fouda et al., 2013). This adsorption occurred simultaneously with the displacement of H2O molecules or other ions from the steel substrate. As a consequence of the successful adsorption of AVU extract molecules on the steel surface, a drop in the rate of corrosion and an increase in inhibitory efficiency are observed.
[AVU extract] (ppm)
Rp (Ω cm2)
n
Y0*10-4 (sn Ω−1 cm−2)
Cdl (μF cm−2)
ηEIS (%)
1.0 mol/L HCl
29.35
0.89
1.7610
92
0.33
–
150
68.46
0.86
1.6601
80.09
6.08
57
250
97.84
0.84
1.4612
65.06
3.87
70
450
122.89
0.84
1.1632
51.79
4.65
74
700
172.66
0.85
0.7870
36.86
5.23
83
The impedance response of AVU extract’s addition to HCl solution can also be obtained in Bode modulus and Bode phase graphs, as illustrated in Fig. 2(c). Compared to the corrosive electrolytic solution, the phase angle increases to greater values with larger concentrations of AVU extract in the middle frequency domain, implying the formation of a protective barrier that provides a superior capacitance response in the presence of AVU extract. Furthermore, relative to the corrosive electrolyte reference, the magnitudes of absolute impedance in the lower frequency zone have been moved towards greater values. This confirms the Nyquist representation's conclusion, and both show the effective adsorption of AVU extract’s molecules onto the steel surface, preventing it, therefore, from corrosion. As shown in Table 2, AVU extract composition contains many molecules with several heteroatoms, aromatic rings, and double bonds. These would work as adsorption sites when AVU extract is add to the acidic solution, interacting with the steel surface via physical and chemical interactions. These reactive sites will be investigated in more detail in the theoretical sections.
3.4 Effect of different immersion times
The effect of immersion time on the protective layer developed on the surface can be determined by observing the evolution of the polarization resistance of carbon steel at various immersion times in an aggressive environment without and with the inclusion of the inhibitor. Carbon steel samples were subjected for 0.5–24 h to the solutions containing a higher concentration of AVU extract, i.e., 700 ppm. Fig. 2(d) illustrates the recorded electrochemical impedance diagrams in the form of Nyquist plots. Table 7 shows the electrochemical parameter values obtained using the equivalent circuit model after curve processing. The results show that on increasing immersion time from 0.5 to 12 hr, the Rp values decrease in the uninhibited and inhibited medium. However, the values of Rp remain significantly higher compared with blank solution. When the immersion time is increased from 12 to 24 hrs., some of the adsorbed AVU extract molecules are mainly desorbed from the carbon steel surface. As a result, the surface area covered decreased. However, since the performance is directly related to the blank solution, it can be noticed that the AVU extract is highly effective when the steel dissolution is very high (24 h, Rp = 12 Ohm cm2 compared to 63 Ohm cm2 in the presence of AVU extract). On the basis of the experimental results, it can be said that the AVU extract can act as an excellent acid corrosion inhibitor under present experimental conditions.
Inhibitor and Time (h)
n
Blank
0.5
29
0.89
1.7610
92
0.33
–
6
23
0.84
2.5114
94
2.45
–
12
18
0.83
2.9866
102
3.24
–
24
12
0.88
3.0891
144
1.14
–
AVU extract
0.5
172
0.85
0.7870
36
5.23
83
6
150
0.85
0.8181
37
7.36
84
12
110
0.83
1.0032
39
5.47
83
24
63
0.86
1.0527
46
3.27
81
3.5 Potentiodynamic polarization curves
Open circuit potential plots and potentiodynamic polarization curves for carbon steel samples excluding and including varying concentrations of AVU extract in 1.0 mol/L HCl medium are represented in Fig. 3(a)-(b). Derived parameters and inhibition efficacies (ƞPPC%) are listed in Table 8. From OCP plots (Fig. 3(a)), it can be noticed that a steady state potential is attained in the absence and in the presence of different concentrations of AVU extract. The steady state potential values of carbon steel in the presence of inhibitor’s concentrations are all around that of blank solution, without significant potential shifting, suggesting a mixed inhibition effect. A careful analysis of Tafel curves expresses that cathodic as well as anodic arms got altered when AVU extract is added into acidic medium, and the curves moved towards lower corrosion current densities on adding accretive concentrations of AVU extract in acidic solution. It affirms that AVU extract limited both steel oxidation and hydrogen ions' reduction at carbon steel surface. As reported in the literature (Palaniappan et al., 2019), a compound can be labeled as anodic/cathodic type when the shift in Ecorr is at least 85 mV with respect to blank solution. By inspecting the data in Table 8, it can be detected that the AVU extract in the considered system functioned as a mixed-type inhibitor. These results substantiated that the investigated AVU extract significantly lowered the anodic dissolution reaction and moderately cathodic reaction to some extent. The values of the cathodic slopes βc vary delicately with the addition of the green inhibitor, unlike the anode slopes βa, which fluctuate a little more, which declares the methanolic extract of the umbels as a mixed inhibitor.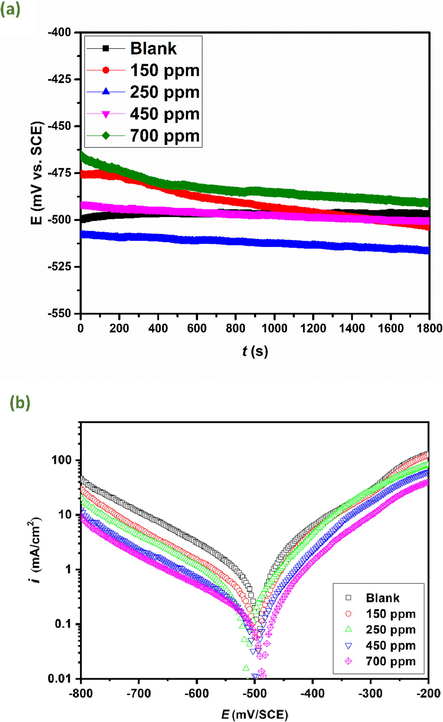
Open circuit potential plots (a) and polarization curves (b) of carbon steel in 1 mol/L HCl with and without addition of different concentrations of AVU extract at 303 K.
[AVU extract] (ppm)
-Ecorr (mV/SCE)
-βc (mV dec-1)
βa (mV dec-1)
icorr (μA cm−2)
ηTafel (%)
1.0 M HCl
496.0
87.5
139.7
576.0
–
150
503.2
78.5
132.6
276.36
55
250
517.7
76.1
137.1
180.48
67
450
500.4
71.7
133.1
129.72
79
700
491.3
76.3
147.1
107.16
84
3.6 Surface characterization by SEM
SEM is a reliable tool to analyze the surface and morphologies of a wide range of materials. SEM can monitor the surface change during corrosion inhibition. The morphology of the carbon steel surface in the absence and in the presence of 700 ppm g/L of AVU extract in 1.0 mol/L HCl solution is represented in Fig. 4. By inspecting this Figure, differences can be easily distinguished between protected and unprotected steel surface. The unprotected surface (Fig. 4(a)) is seriously corroded, and there is the formation of corrosion products. According to our recent XPS analysis, those corrosion products are mainly iron oxides such as FeO/Fe3O4, and Fe2O3 due to the iron oxidation and chloride attack (Lgaz et al., 2020b). In contrast, no severe corrosion attack is observed on the steel surface after adding 700 ppm of AVU extract to HCl solution. It signifies that the AVU extract can inhibit the acid solution and limit its aggressiveness during the immersion. As evidenced by previous results, this can be explained by the effective adsorption of different AVU extract's components, forming a robust protective barrier against corrosion.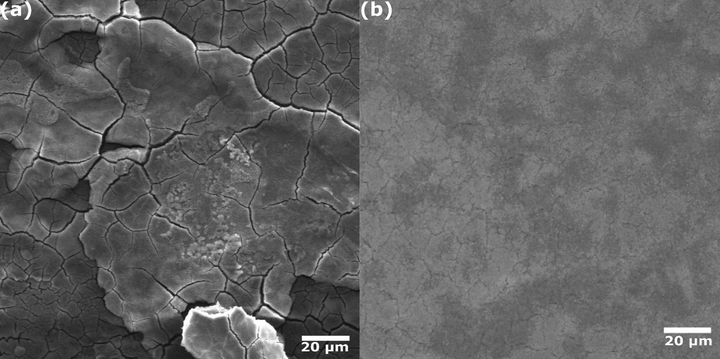
SEM images of carbon steel in (a) 1.0 mol/L HCl and (b) in presence of 700 ppm of AVU extract after 12 h immersion.
3.7 DFT calculations
Quantum chemical calculations are recognized as one of the most efficient tools to get information about the electronic and reactivity of chemical compounds (Boulhaoua et al., 2021; Guo et al., 2020). These calculations find broad applications in corrosion inhibition studies (Bahlakeh et al., 2019a; Olasunkanmi et al., 2020). Global reactivity descriptors like Highest Occupied Molecular Orbitals (HOMO), Lowest Unoccupied Molecular Orbitals (LUMO), and energy gap (
, etc. can be obtained from DFT calculations. These parameters are related to a single molecule; therefore, they have two advantages and one limitation. They allow the identification of potential adsorption sites and reactivity of the investigated molecule, and further the comparison of this reactivity with other molecules, which gives initial insights on why a molecule is more reactive than others (Fan et al., 2019; Liu et al., 2021b, 2021a). However, the main limitation of this approach is that it focusses only on the single molecule without considering its interaction with the surface of the metal (Kumar et al., 2020; Poberžnik et al., 2020). In this work, the AVU extract is regarded as one inhibitor even though it contains many components. Therefore, efforts will be limited to investigating frontier molecular orbitals iso-surfaces of the four molecules with higher percentages and the local reactivity of the Edulisin III, which is believed to be the main responsible for the corrosion inhibition effect of the AVU extract. Fig. 5 represents the iso-surfaces of the HOMO and LUMO of Edulisin III, Binapacryl, Khellin, and Visnagin. The HOMO density is related to the ability of a molecule or a part of it to donate electrons (Dutta et al., 2017; Meng et al., 2021). The inspection of results in Fig. 5 reveals that the HOMO density is distributed over the chromone moiety of the Edulisin III, suggesting that this part may be responsible for donating electrons to vacant d-orbitals of iron upon its adsorption.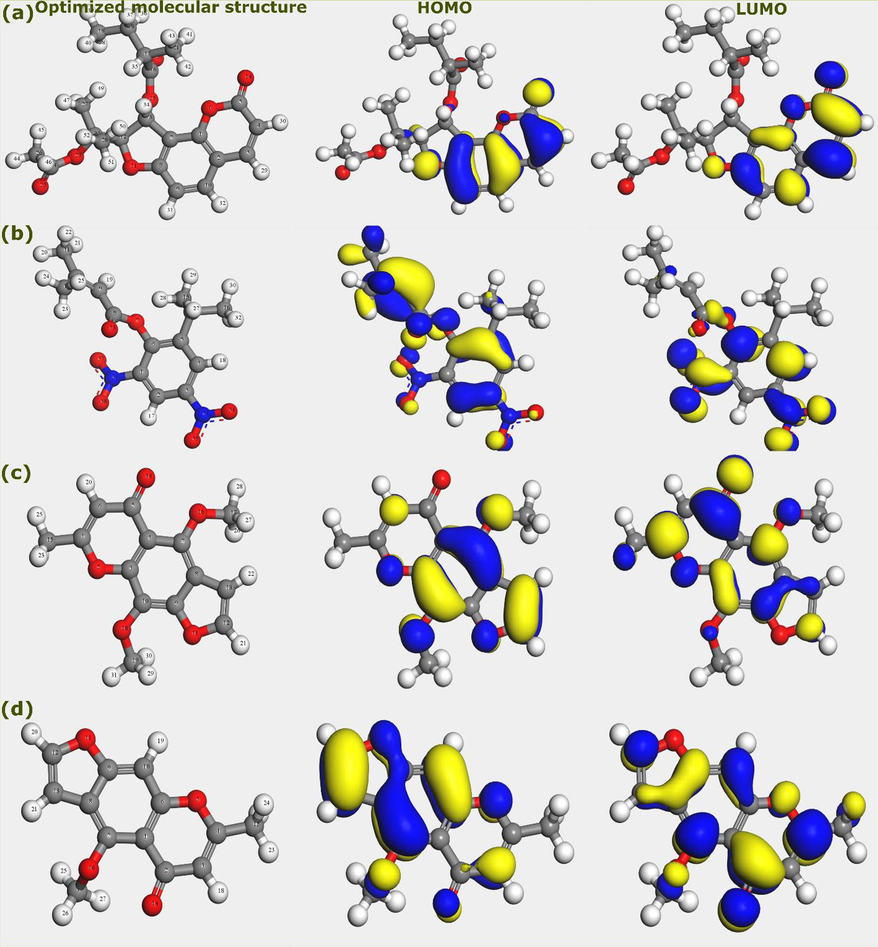
Optimized molecular structures, HOMO and LUMO iso-surfaces of AVU extract’s components; (a) Edulisin III, (b) Binapacryl, (c) Khellin, and (d) Visnagin.
In the other three molecules, i.e., Binapacryl, Khellin, and Visnagin, the HOMO density is observed on the entire molecular structure. It signifies that the whole molecule can be involved in interactions with the steel surface. Moving to the LUMO iso-surfaces, which indicate the tendency of a molecule to accept electrons from the metal surface. This is usually described as a back-donation process (Saha et al., 2016b). In the case of Edulisin III, the LUMO distribution is like that of HOMO, i.e., over the chromone moiety, suggesting that it is the main reactive part and responsible for Donor-Acceptor (D-A) interactions with iron atoms. Interestingly, the same goes for other molecules with intensities a bit lower than HOMO iso-surfaces. These remarks can be explained by the presence of several heteroatoms, especially the oxygen atom and aromatic rings. The oxygen atom can donate its electron pairs when interacting with vacant d-orbitals of iron. On the other hand, the back-donation of electrons to π* orbitals is an important step in strengthening bonds and coordination with the metal surface.
Condensed Fukui functions iso-surfaces of the Edulisin III molecule are shown in Fig. 6. A large iso-surface represents a high Fukui function value and indicates that the atom or part of the molecule is chemically softer than other parts (Damej et al., 2021; El Aoufir et al., 2020). Fig. 6(a) shows parts of Edulisin III susceptible to be attacked by nucleophiles, while Fig. 6(b) shows those atoms or parts susceptible to be attacked by electrophiles. Results in Fig. 6 strengthen the conclusion that the chromone moiety is the most reactive part in the Edulisin III molecule. It is responsible for almost all D-A interactions of these components with steel surface, thus making a vital contribution to the corrosion inhibition performance of the investigated extract.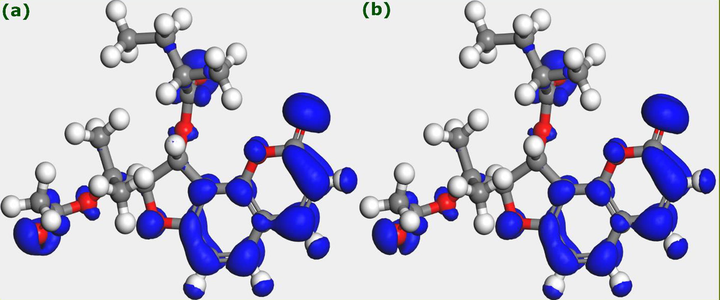
Fukui function iso-surfaces of Edulisin III, (a)
, and (b)
.
3.8 Molecular dynamics simulation
The corrosion inhibition of carbon steel by organic molecules is an adsorption process. The reactive sites of the molecules interact with the steel surface through physical and or chemical interactions. Therefore, a reliable simulation of the corrosion inhibition should involve both interactive species, i.e., molecule and the metal. To this end, researchers have turned their attention towards molecular dynamics simulation for getting useful insights into the adsorption process (Verma et al., 2018b). In this work, MD simulation was performed for the four main components of AVU extract. Fig. 7 shows the top and side views of the most stable adsorption geometries. The adsorption systems include one molecular structure of the components along with water molecules, chlorine, and hydronium ions in interaction with Fe(1 1 0) surface. It can be seen that after the simulation equilibrium, all components tend to move close to the iron surface.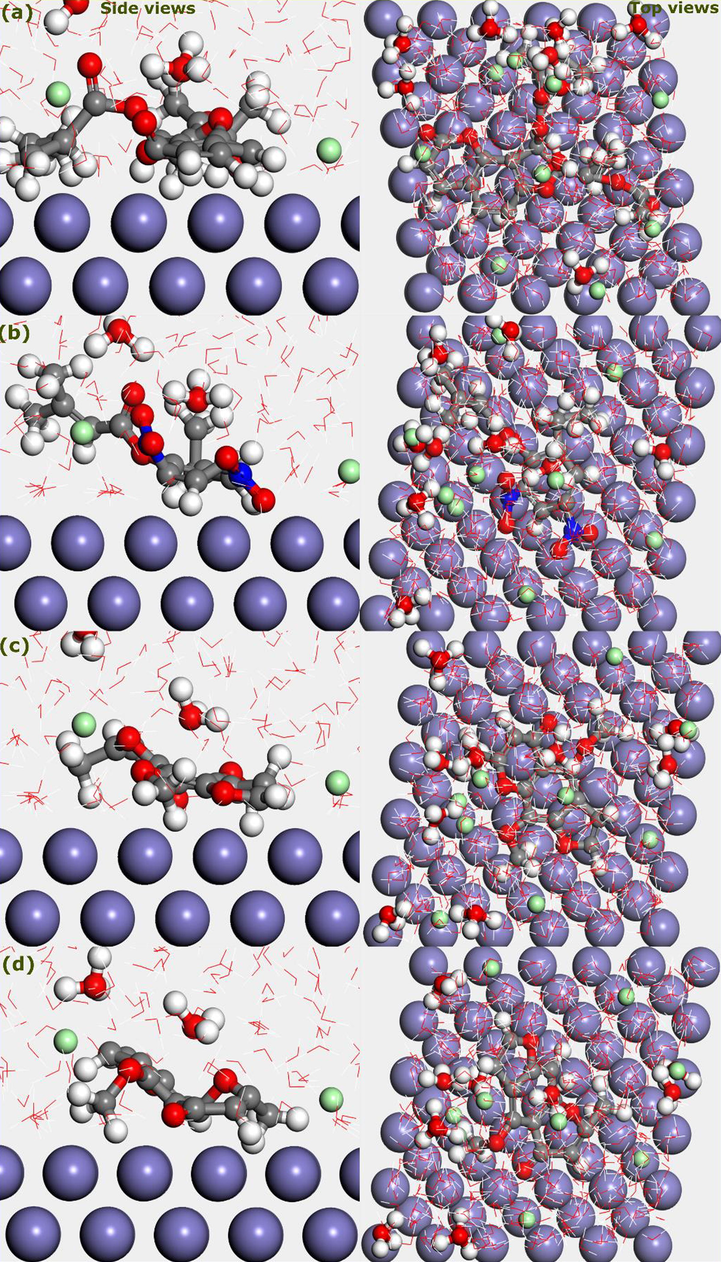
Top and side views of the most stable geometries of AVU extract’s components; (a) Edulisin III, (b) Binapacryl, (c) Khellin, and (d) Visnagin.
Interestingly, all aromatic rings adopt a parallel configuration over the Fe(1 1 0). This remark strengthens the DFT results, which showed that aromatic rings are highly reactive parts. The parallel adsorption mode gives a chance to strong D-A interactions and thus high coverture of the surface. The calculated interaction energies of the Edulisin III, Binapacryl, Khellin, and Visnagin over Fe(1 1 0) surface are – 324.5, −241.7, −306.5, and −297.4 kJ/mol, respectively. It indicates that all components strongly interact with Fe(1 1 0) surface, with Edulisin outperforms other molecules.
3.9 Comparison with similar materials
Recently, there has been a great effort towards developing green and eco-friendly alternatives to traditional, especially toxic corrosion inhibitors (Afia et al., 2013; Bammou et al., 2014; Oguzie et al., 2012). Besides, the development of conventional organic inhibitors comes with other unwanted issues related to expensive solvents, reagents, and catalysts and environmental problems due to the discharge of chemicals into the surrounding environments. These factors and others have turned the researchers' attention to more safe alternatives such as plant-based materials. Since then, many new environmentally friendly corrosion inhibitors have appeared and applied for protecting different metals and alloys. In the literature, several review papers report the application of plant-based materials in different experimental conditions (Alrefaee et al., 2021), and references therein. Herein, for a comparative purpose, a few plant-based corrosion inhibitors are listed in Table 9 with some experimental conditions. All reported inhibitors have been investigated in experimental conditions similar to the present work. Generally speaking, a plant-based corrosion inhibitor with an inhibition efficiency over 80% in a highly acidic solution has a great industrial application potential given its non-toxic nature and, therefore, the possibility for application in low corrosive mediums. However, reported results show that most inhibitors have high corrosion inhibition performance. The extract under investigation shows an inhibition efficiency of 84% at a very low concentration of 700 ppm. Compared with some reported results, and considering concentration/efficiency relationship, the present extract has excellent corrosion inhibiting properties, which outperforms most reported ones. Together, these promising results once again demonstrates the importance of this class of materials for protecting metals in highly aggressive solutions.
Inhibitor
Optimum conc./Material/Solution
Highest inhibition efficiency (%)
Reference
Tunbergia fragrans
500 ppm/Mild steel/1.0 M HCl
81.0
(Muthukumarasamy et al., 2020)
Magnolia grandiflora
500 ppm/Q235 steel/1.0 M HCl
85.0
(Chen et al., 2020)
Pterocarpus santalinoides leaves extract
0.7 g/L/Carbon steel/1.0 M HCl
85.2
(Dehghani et al., 2020b)
Opuntia elatior fruit
500 mg/L/Mild steel/1.0 M HCl
79.7
(Loganayagi et al., 2014)
Rosa canina fruit extract
800 ppm/Mild steel/1.0 M HCl
86
(Sanaei et al., 2019)
Taxus baccata extract
600 ppm/Mild steel/1.0 M HCl
83
(Hanini et al., 2019)
Haematostaphis barteri Leaves Extract
40 g/L/Mild steel/1.0 M HCl
73
(Ishak et al., 2019)
Elaeoselinum thapsioides (BEET) butanolic extract
900 ppm/Carbon steel/1.0 M HCl
82
(Benahmed et al., 2020)
AVU
700 ppm/Mild steel/1.0 M HCl
84
This work
3.10 Corrosion inhibition mechanism
Experimental results showed that the addition of tested inhibitor to the 1.0 mol/L HCl solution decreased the double layer capacitance compared to that obtained in the blank solution. Moreover, a substantial decrease is observed in both anodic and cathodic corrosion reactions after the addition of AVU extract to 1.0 mol/L solution. These conclusions suggest that AVU extract’s components act mainly by adsorption on the steel surface, increasing the double layer thickness, and therefore decreasing its capacitance. Unsurprisingly, most organic corrosion inhibitors have similar inhibition mechanism; that is the adsorption on steel surface by replacing pre-adsorbed water molecules (Fan et al., 2021; Ma et al., 2020). This adsorption is usually a two-step process.
-
-
The steel surface is found to have a positive charge under the same experimental conditions, i.e., 1.0 mol/L (Solmaz, 2014a). Similarly, inhibitor molecules having heteroatoms will be protonated in this acidic medium (Solmaz, 2014b). In this situation, the adsorption of molecules seems impossible due to electrostatic repulsions. However, chlorine ions, which are first pre-adsorb on the positively charged surface, make it possible by playing an intermediate role between protonated molecules and positively charged steel surface (Fan et al., 2020). This process is called physisorption, and it is paramount for the next step.
-
-
The electrostatic attraction between protonated molecules and pre-adsorbed chlorine ions would facilitate the chemical adsorption of AVU extract’s components. After approaching the steel surface, molecules having reactive adsorption sites like heteroatoms and π-electrons would participate in chemical interactions with empty d-orbitals of iron (Fan et al., 2020). It can be noticed that molecules with high percentage are all having several oxygen atoms. These atoms will act as adsorption sites and share surplus electrons with vacant d-orbitals of iron (Haque et al., 2018). Furthermore, it has been shown from MD simulations that all molecules adopt a nearly parallel disposition over the iron surface. This adsorption mode could facilitate the interactions between π-electrons of aromatic rings and the iron atoms. Together, heteroatoms and π-electrons play a significant role in this step, known as a chemisorption process.
-
-
The adsorption of AVU extract’s components on steel surface by sharing electrons with vacant d-orbitals of iron can lead to the accumulation of electrons on its surface making it more negative. Consequently, to relieve this extra negative charge, the iron surface can transfer electrons to a vacant π* (antibonding) orbital of the inhibitor molecule via retro-donation (Mobin and Rizvi, 2017). This would strengthen the adsorption of molecules and thus leading to an enhanced corrosion protection.
The corrosion inhibition by AVU extract may involve all its components; however, Edulisin III seems to have a higher contribution due to its incomparable percentage with other components. Given this fact, a proposed adsorption mechanism is represented in Fig. 8 taking the Edulisin III as a representative example.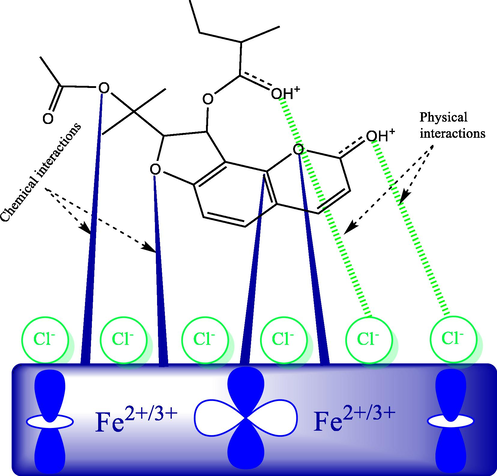
A graphical representation of the potential corrosion inhibition mechanism; case of Edulisin III.
4 Conclusion
In the present work, the corrosion inhibition performance of Ammi visnaga extract was investigated for carbon steel in 1.0 mol/L medium employing chemical, electrochemical, surface characterization techniques along with DFT and MD simulation. The characterization of AVU extract by GC/MS revealed the presence of Edulisin III (72.88%), Binapacryl (4.32%), Khellin (1.97%), and Visnagin (1.65%) as major constituents. The AVU's methanolic extract was shown to be a potent corrosion inhibitor for carbon steel in 1.0 mol/L HCl medium, with an inhibition efficacy of 84% at 700 ppm. The extract was found to have a mixed inhibition effect, blocking both anodic and cathodic corrosion reactions. Further, the addition of AVU extract to HCl solution led to a high polarization resistance and a small double-layer capacitance, as evidenced from EIS tests. DFT calculations showed that the chromone moiety of the Edulisin III is the most reactive part, acting simultaneously as electron donating and accepting region. Results from MD revealed that the four components adopted a nearly parallel configuration over the Fe(1 1 0) surface. Insights from the present work further highlight the significant advantage of natural-based corrosion inhibitors in protecting metals and alloys.
Acknowledgments
The authors would like to thank the Deanship of Scientific Research at Umm Al- Qura University, Saudi Arabia, for supporting this work by Grant Code. 20-SCI-1-01-0002. This research was supported by basic science research program through the National Research Foun-dation (NRF) of Korea funded by the Ministry of Science, ICT and Future Planning (No. 2015R1A5A1037548).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- The effects of Aloe vera extract on corrosion and kinetics of corrosion process of zinc in HCl solution. Corros. Sci.. 2010;52:661-664.
- [Google Scholar]
- Comparative study of corrosion inhibition on mild steel in HCl medium by three green compounds: Argania spinosa press cake, kernels and hulls extracts. Trans. Indian Inst. Met.. 2013;66:43-49.
- [Google Scholar]
- Turmeric and ginger as green inhibitors of mild steel corrosion in acidic medium. J. Mater. Environ. Sci.. 2015;6:1480-1487.
- [Google Scholar]
- Challenges and advantages of using plant extract as inhibitors in modern corrosion inhibition systems: Recent advancements. J. Mol. Liq.. 2021;321:114666
- [CrossRef] [Google Scholar]
- Valorization of Zea mays hairs waste extracts for antioxidant and anticorrosive activity of mild steel in 1 M HCl environment. Arabian J. Chem.. 2020;13:7183-7198.
- [CrossRef] [Google Scholar]
- Gravimetric, electrochemical, and morphological studies of an isoxazole derivative as corrosion inhibitor for mild steel in 1M HCl. Arabian J. Chem.. 2020;13:7744-7758.
- [Google Scholar]
- Inhibitory effect of sodium carboxymethylcellulose and synergistic biodegradable gemini surfactants as effective inhibitors for MS corrosion in 1 M HCl. J. Mater. Res. Technol.. 2019;8:4521-4533.
- [CrossRef] [Google Scholar]
- Eco friendly green inhibitor Gum Arabic (GA) for the corrosion control of mild steel in hydrochloric acid medium. Corros. Sci.. 2017;129:70-81.
- [Google Scholar]
- Novel cost-effective and high-performance green inhibitor based on aqueous Peganum harmala seed extract for mild steel corrosion in HCl solution: Detailed experimental and electronic/atomic level computational explorations. J. Mol. Liq.. 2019;283:174-195.
- [CrossRef] [Google Scholar]
- Corrosion inhibition of steel in sulfuric acidic solution by the chenopodium ambrosioides extracts. J. Assoc. Arab Universities Basic Appl. Sci.. 2014;16:83-90.
- [CrossRef] [Google Scholar]
- Red Algae Halopitys Incurvus extract as a green corrosion inhibitor of carbon steel in hydrochloric acid. J. Bio-and Tribo-Corrosion. 2018;4:1-9.
- [Google Scholar]
- Adsorption and corrosion inhibition properties of butanolic extract of Elaeoselinum thapsioides and its synergistic effect with Reutera lutea (Desf.) Maires (Apiaceae) on A283 carbon steel in hydrochloric acid solution. Chem. Africa. 2020;3:251-261.
- [CrossRef] [Google Scholar]
- Ammi Visnaga in treatment of urolithiasis and hypertriglyceridemia. Pharmacognosy Res.. 2015;7:397.
- [Google Scholar]
- Supercritical fluid extraction of γ-Pyrones from Ammi visnaga L. fruits. Future J. Pharm. Sci.. 2018;4:57-62.
- [Google Scholar]
- Aqueous extracts of olive roots, stems, and leaves as eco-friendly corrosion inhibitor for steel in 1 MHCl medium. Int. J. Ind. Chem.. 2015;6:233-245.
- [Google Scholar]
- Synthesis, structural analysis and corrosion inhibition application of a new indazole derivative on mild steel surface in acidic media complemented with DFT and MD studies. Colloids Surf. A. 2021;617:126373
- [CrossRef] [Google Scholar]
- New Environmental Options for Corrosion Inhibitor Intensifiers. Presented at the SPE/EPA Exploration and Production Environmental Conference, OnePetro. 1999
- [CrossRef] [Google Scholar]
- Magnolia grandiflora leaves extract as a novel environmentally friendly inhibitor for Q235 steel corrosion in 1 M HCl: Combining experimental and theoretical researches. J. Mol. Liq.. 2020;311:113312
- [Google Scholar]
- Aging time effect of Ammi visnaga (L.) lam essential oil on the chemical composition and corrosion inhibition of brass in 3% NaCl medium. Experimental and theoretical studies. Mater. Today:. Proc.. 2020;22:83-88.
- [Google Scholar]
- Experimental and theoretical explorations of S-alkylated mercaptobenzimidazole derivatives for use as corrosion inhibitors for carbon steel in HCl. J. Mol. Liq.. 2021;331:115708
- [CrossRef] [Google Scholar]
- Designing a novel targeted-release nano-container based on the silanized graphene oxide decorated with cerium acetylacetonate loaded beta-cyclodextrin (β-CD-CeA-MGO) for epoxy anti-corrosion coating. Chem. Eng. J.. 2020;400:125860
- [CrossRef] [Google Scholar]
- Green Eucalyptus leaf extract: a potent source of bio-active corrosion inhibitors for mild steel. Bioelectrochemistry. 2019;130:107339
- [Google Scholar]
- Experimental complemented with microscopic (electronic/atomic)-level modeling explorations of Laurus nobilis extract as green inhibitor for carbon steel in acidic solution. J. Ind. Eng. Chem.. 2020;84:52-71.
- [CrossRef] [Google Scholar]
- Integrated modeling and electrochemical study of Myrobalan extract for mild steel corrosion retardation in acidizing media. J. Mol. Liq.. 2020;298:112046
- [CrossRef] [Google Scholar]
- A combined experimental and theoretical study of green corrosion inhibition of mild steel in HCl solution by aqueous Citrullus lanatus fruit (CLF) extract. J. Mol. Liq.. 2019;279:603-624.
- [CrossRef] [Google Scholar]
- Effect of substitution on corrosion inhibition properties of 2-(substituted phenyl) benzimidazole derivatives on mild steel in 1M HCl solution: A combined experimental and theoretical approach. Corros. Sci.. 2017;123:256-266.
- [CrossRef] [Google Scholar]
- The effect of the alkyl chain length on corrosion inhibition performances of 1,2,4-triazole-based compounds for mild steel in 1.0 M HCl: Insights from experimental and theoretical studies. J. Mol. Liq.. 2020;303:112631
- [CrossRef] [Google Scholar]
- Fabrication, characterization and efficient surface protection mechanism of poly (trans-cinnamaldehyde) electropolymerized coatings for EH36 steel in simulated seawater. Colloids Surf. A. 2021;629:127434
- [Google Scholar]
- Revealing the assembly mechanism of an octadecylamine based supramolecular complex on mild steel in condensate water: correlative experimental and theoretical studies. J. Mol. Liq.. 2019;292:111446
- [Google Scholar]
- Trazodone as an efficient corrosion inhibitor for carbon steel in acidic and neutral chloride-containing media: Facile synthesis, experimental and theoretical evaluations. J. Mol. Liq.. 2020;311:113302
- [Google Scholar]
- A theoretical and experimental study of castor oil-based inhibitor for corrosion inhibition of mild steel in acidic medium at elevated temperatures. Corros. Sci.. 2020;175:108871
- [Google Scholar]
- Henna extract as green corrosion inhibitor for carbon steel in hydrochloric acid solution. Int. J. Electrochem. Sci.. 2019;14:4668-4682.
- [Google Scholar]
- Ginger extract as green corrosion inhibitor for steel in sulfide polluted salt water. J. Korean Chem. Soc.. 2013;57:272-278.
- [Google Scholar]
- High performance liquid chromatography analysis of the furanochromones khellin and visnagin in various organs of Ammi visnaga (L.) Lam. at different developmental stages. J. Ethnopharmacol.. 1985;14:203-212.
- [Google Scholar]
- Eco-friendly corrosion inhibitors based on Cashew nut shell liquid (CNSL) for acidizing fluids. J. Mol. Liq.. 2019;284:393-404.
- [Google Scholar]
- Investigation of the corrosion inhibition of carbon steel in hydrochloric acid solution by using ginger roots extract. RSC Adv.. 2017;7:24576-24588.
- [Google Scholar]
- Determination of khellin and visnagin in Ammi visnaga fruits by capillary electrophoresis. J. Chromatogr. A. 2002;954:291-294.
- [Google Scholar]
- Newly synthesized triazolopyrimidine derivative as an inhibitor for mild steel corrosion in HCl medium: an experimental and in silico study. J. Mater. Res. Technol.. 2020;9:6568-6578.
- [CrossRef] [Google Scholar]
- Influence of different polyphenol extracts of Taxus baccata on the corrosion process and their effect as additives in electrodeposition. Sustainable Chem. Pharm.. 2019;14:100189
- [CrossRef] [Google Scholar]
- Microwave-induced synthesis of chitosan schiff bases and their application as novel and green corrosion inhibitors: experimental and theoretical approach. ACS Omega. 2018;3:5654-5668.
- [CrossRef] [Google Scholar]
- Olive leaf extract as a green corrosion inhibitor of reinforced concrete contaminated with seawater. Arabian J. Chem.. 2020;13:4846-4856.
- [Google Scholar]
- Utilization of biowaste as an eco-friendly biodegradable corrosion inhibitor for mild steel in 1 mol/L HCl solution. Arabian J. Chem.. 2020;13:8684-8696.
- [CrossRef] [Google Scholar]
- An improved corrosion resistance of steel in hydrochloric acid solution using Hibiscus sabdariffa leaf extract. Chem. Pap.. 2019;73:909-925.
- [Google Scholar]
- Thermodynamic parameters and adsorption mechanism of corrosion inhibition in mild steel using jatropha leaf extract in hydrochloric acid. Arabian Journal for Science and Engineering 2021:1-11.
- [Google Scholar]
- Ishak, A., Adams, F.V., Madu, J.O., Joseph, I.V., Olubambi, P.A., 2019. Corrosion Inhibition of Mild Steel in 1M Hydrochloric Acid using Haematostaphis barteri Leaves Extract. Procedia Manufacturing, The 2nd International Conference on Sustainable Materials Processing and Manufacturing, SMPM 2019, 8-10 March 2019, Sun City, South Africa 35, 1279–1285. 10.1016/j.promfg.2019.06.088
- Corrosion inhibition of carbon steel immersed in a 1M HCl solution using benzothiazole derivatives. Arabian J. Chem.. 2019;12:1387-1394.
- [CrossRef] [Google Scholar]
- Coriandrum Sativum. L seeds extract as a novel green corrosion inhibitor for mild steel in 1.0 M hydrochloric and 0.5 M sulfuric solutions. Anal. Bioanal. Chem.. 2018;10:249-268.
- [Google Scholar]
- Inhibitive effect by extract of Mentha rotundifolia leaves on the corrosion of steel in 1 M HCl solution. Res. Chem. Intermed.. 2014;40:961-972.
- [Google Scholar]
- Cationic gemini-surfactants based on waste cooking oil as new ‘green’inhibitors for N80-steel corrosion in sulphuric acid: a combined empirical and theoretical approaches. J. Mol. Struct.. 2020;1203:127442
- [Google Scholar]
- Koch, G., Varney, J., Thompson, N., Moghissi, O., Gould, M., Payer, J., 2016. International measures of prevention, application, and economics of corrosion technologies study. NACE international 216.
- Amino acids as copper corrosion inhibitors: A density functional theory approach. Appl. Surf. Sci.. 2020;514:145905
- [CrossRef] [Google Scholar]
- Corrosion inhibition of carbon steel in hydrochloric acid 0.5M by hexa methylene diamine tetramethyl-phosphonic acid. Arabian J. Chem.. 2011;4:271-277.
- [CrossRef] [Google Scholar]
- Synergistic influence of poly(4-vinylpyridine) and potassium iodide on inhibition of corrosion of mild steel in 1M HCl. J. Appl. Electrochem.. 2004;34:833-839.
- [CrossRef] [Google Scholar]
- Improved corrosion resistance of mild steel in acidic solution by hydrazone derivatives: An experimental and computational study. Arabian J. Chem.. 2020;13:2934-2954.
- [CrossRef] [Google Scholar]
- Lgaz, H., Saha, S.Kr., Chaouiki, A., Bhat, K.S., Salghi, R., Shubhalaxmi, Banerjee, P., Ali, I.H., Khan, M.I., Chung, I.-M., 2020b. Exploring the potential role of pyrazoline derivatives in corrosion inhibition of mild steel in hydrochloric acid solution: Insights from experimental and computational studies. Construction and Building Materials 233, 117320. 10.1016/j.conbuildmat.2019.117320
- Anti-corrosive mechanism of poly (N-ethylaniline)/sodium silicate electrochemical composites for copper: Correlated experimental and in-silico studies. J. Mater. Sci. Technol.. 2021;72:202-216.
- [Google Scholar]
- Long-term protective mechanism of poly (N-methylaniline)/phosphate one-step electropolymerized coatings for copper in 3.5% NaCl solution. J. Alloy. Compd.. 2021;872:159752
- [Google Scholar]
- Effect of ginger extract as green inhibitor on chloride-induced corrosion of carbon steel in simulated concrete pore solutions. J. Cleaner Prod.. 2019;214:298-307.
- [Google Scholar]
- Opuntiol: An active principle of Opuntia elatior as an eco-friendly inhibitor of corrosion of mild steel in acid medium. ACS Sustainable Chem. Eng.. 2014;2:606-613.
- [Google Scholar]
- Understanding the anticorrosive mechanism of a cross-linked supramolecular polymer for mild steel in the condensate water: comprehensive experimental, molecular docking, and molecular dynamics investigations. J. Mol. Model.. 2020;26:1-17.
- [Google Scholar]
- Green method of carbon steel effective corrosion mitigation in 1 M HCl medium protected by Primula vulgaris flower aqueous extract via experimental, atomic-level MC/MD simulation and electronic-level DFT theoretical elucidation. J. Mol. Liq.. 2019;284:658-674.
- [Google Scholar]
- Efficient corrosion inhibition by sugarcane purple rind extract for carbon steel in HCl solution: mechanism analyses by experimental and in silico insights. RSC Adv.. 2021;11:31693-31711.
- [Google Scholar]
- Metals, A.C.G.-1 on C. of, 2017. Standard practice for preparing, cleaning, and evaluating corrosion test specimens. ASTM international.
- Structures of New Coumarins and Antitumor-Promoting Activity of Coumarins from Angelica edulis1. Planta Med.. 1994;60:333-336.
- [Google Scholar]
- Experimental and theoretical assessment of almond gum as an economically and environmentally viable corrosion inhibitor for mild steel in 1 M HCl. Sustainable Chem. Pharm.. 2020;18
- [CrossRef] [Google Scholar]
- Bio-/environment-friendly cationic gemini surfactant as novel corrosion inhibitor for mild steel in 1 M HCl solution. J. Surfactants Deterg.. 2017;20:57-74.
- [Google Scholar]
- Pineapple stem extract (Bromelain) as an environmental friendly novel corrosion inhibitor for low carbon steel in 1 M HCl. Measurement. 2019;134:595-605.
- [Google Scholar]
- Corrosion mitigation of mild steel in acidic medium using Lagerstroemia speciosa leaf extract: A combined experimental and theoretical approach. J. Mol. Liq.. 2019;286:110890
- [Google Scholar]
- Adsorption and corrosion inhibition behavior of hydroxyethyl cellulose and synergistic surfactants additives for carbon steel in 1M HCl. Carbohydr. Polym.. 2017;156:202-214.
- [CrossRef] [Google Scholar]
- Adsorption and corrosion inhibition performance of Tunbergia fragrans extract on mild steel in acid medium. Materials Today: Proceedings, International Conference on Nanotechnology: Ideas, Innovation and Industries. 2020;33:4054-4058.
- [CrossRef] [Google Scholar]
- Natural products for materials protection: mechanism of corrosion inhibition of mild steel by acid extracts of Piper guineense. The Journal of Physical Chemistry C. 2012;116:13603-13615.
- [Google Scholar]
- Adsorption and Corrosion Inhibition Potentials of Salicylaldehyde-based Schiff Bases of Semicarbazide and p-Toluidine on Mild Steel in Acidic Medium: Experimental and Computational Studies. Surf. Interfaces. 2020;21:100782
- [CrossRef] [Google Scholar]
- Corrosion inhibition of mild steel using binary mixture of sesame and castor oil in brine solution. Mater. Today Commun.. 2019;21:100691
- [Google Scholar]
- Rapid investigation expiry drug green corrosion inhibitor on mild steel in NaCl medium. Mater. Sci. Eng., B. 2019;249:114423
- [Google Scholar]
- DFT study of n-alkyl carboxylic acids on oxidized aluminum surfaces: From standalone molecules to self-assembled-monolayers. Appl. Surf. Sci.. 2020;525:146156
- [CrossRef] [Google Scholar]
- Pradityana, A., Shahab, A., Noerochim, L., Susanti, D., 2016. Inhibition of corrosion of carbon steel in 3.5% NaCl solution by Myrmecodia Pendans extract. Int. J. Corrosion 2016.
- A technical playbook for chemicals and additives used in the hydraulic fracturing of shales. Energy Fuels. 2020;34:15106-15125.
- [Google Scholar]
- Novel Schiff-base molecules as efficient corrosion inhibitors for mild steel surface in 1 M HCl medium: experimental and theoretical approach. PCCP. 2016;18:17898-17911.
- [CrossRef] [Google Scholar]
- Evaluating electronic structure of quinazolinone and pyrimidinone molecules for its corrosion inhibition effectiveness on target specific mild steel in the acidic medium: a combined DFT and MD simulation study. J. Mol. Liq.. 2016;224:629-638.
- [Google Scholar]
- Use of Rosa canina fruit extract as a green corrosion inhibitor for mild steel in 1M HCl solution: A complementary experimental, molecular dynamics and quantum mechanics investigation. J. Ind. Eng. Chem.. 2019;69:18-31.
- [CrossRef] [Google Scholar]
- Use of Sida cordifolia extract as green corrosion inhibitor for mild steel in 0.5 M H2SO4. J. Environ. Chem. Eng.. 2018;6:694-700.
- [CrossRef] [Google Scholar]
- Corrosion inhibition of Neem (Azadirachta indica) leaves extract as a green corrosion inhibitor for Zinc in H2SO4. Green Chem. Lett. Rev.. 2009;2:47-51.
- [Google Scholar]
- Potential of Azadirachta indica as a green corrosion inhibitor against mild steel, aluminum, and tin: a review. J. Anal. Sci. Technol.. 2015;6:1-16.
- [Google Scholar]
- Ethanol extract of waste potato peels for corrosion inhibition of low carbon steel in chloride medium. Mater. Today:. Proc.. 2021;44:2267-2272.
- [Google Scholar]
- Investigation of corrosion inhibition mechanism and stability of Vitamin B1 on mild steel in 0.5 M HCl solution. Corros. Sci.. 2014;81:75-84.
- [CrossRef] [Google Scholar]
- Investigation of adsorption and corrosion inhibition of mild steel in hydrochloric acid solution by 5-(4-Dimethylaminobenzylidene)rhodanine. Corros. Sci.. 2014;79:169-176.
- [CrossRef] [Google Scholar]
- Mild Steel Corrosion Inhibition, in 4 N Sulphuric Acid, by a Green Inhibitor. Portugaliae Electrochimica Acta. 2020;38:99-106.
- [Google Scholar]
- Thiol (-SH) substituent as functional motif for effective corrosion protection: A review on current advancements and future directions. J. Mol. Liq.. 2021;324:115111
- [CrossRef] [Google Scholar]
- An overview on plant extracts as environmental sustainable and green corrosion inhibitors for metals and alloys in aggressive corrosive media. J. Mol. Liq.. 2018;266:577-590.
- [CrossRef] [Google Scholar]
- Molecular dynamics and Monte Carlo simulations as powerful tools for study of interfacial adsorption behavior of corrosion inhibitors in aqueous phase: A review. J. Mol. Liq.. 2018;260:99-120.
- [CrossRef] [Google Scholar]
- Substituents effect on corrosion inhibition performance of organic compounds in aggressive ionic solutions: A review. J. Mol. Liq.. 2018;251:100-118.
- [CrossRef] [Google Scholar]
- Adsorption behavior of glucosamine-based, pyrimidine-fused heterocycles as green corrosion inhibitors for mild steel: experimental and theoretical studies. J. Phys. Chem. C. 2016;120:11598-11611.
- [Google Scholar]
- Zaher, A., Chaouiki, A., Salghi, R., Boukhraz, A., Bourkhiss, B., Ouhssine, M., 2020. Inhibition of mild steel corrosion in 1M hydrochloric medium by the methanolic extract of Ammi Visnaga L. lam seeds. Int. J. Corros. 2020.
- Essential oil of Salvia aucheri mesatlantica as a green inhibitor for the corrosion of steel in 0.5M H 2SO 4. Arabian J. Chem.. 2012;5:467-474.
- [CrossRef] [Google Scholar]







