A critical review on quercetin bioflavonoid and its derivatives: Scope, synthesis, and biological applications with future prospects
⁎Corresponding author. dsangeetha@vit.ac.in (Sangeetha Dhanaraj)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract

Abstract
Lifestyle is an important factor in health, and researchers are trying to see the adverse effect on human health of a change in lifestyle. Many people follow unhealthy lifestyles, which affect human physical and mental health. Millions of people suffer from various problems like cardiovascular disease, overweight, malnutrition, hypertension, stress, violence, etc. There are many medicines that can cure these diseases, but they have side effects. When a man consumes green leaves and vegetables in his diet, his chances of reducing the risk of any disease increase. Every plant has primary and secondary metabolites. Flavonoid is a secondary metabolite found in various plants that plays an important role in the survival of the plant by performing physiological activities and being able to withstand adverse effects from the environment. Quercetin is one such bioflavonoid found in many plants, like onions, citrus fruits, parsley, etc. It comes under the category of flavone, with five hydroxy groups and a chromen-4-one skeletal structure. It has various applications, which include anti-cancer, anti-bacterial, anti-viral, anti-inflammatory, anti-diabetic, etc. However, the use of quercetin is limited due to its poor bioavailability, poor aqueous solubility, lower stability in the gastrointestinal tract, and first-pass effect. To enhance the application of quercetin, various derivatives of quercetin and its metal complex are required that are water soluble, which helps them reach the target site without any first-pass effect and provides them with stability. This review is divided into three main sections: The first section explains about flavonoids and their types; the second section explains about quercetin, its various methods of synthesis, its derivatives, and its metal complex; and the last section represents the complete applications of quercetin, with the drawbacks and how to overcome or deal with the drawbacks.
Keywords
Flavonoids
Quercetin
Anti-cancer
Anti-inflammatory
First pass effect
Poor aqueous solubility
1 Introduction
As individuals, we might have wondered how plants survive. But the answer is simple: plants survive by producing primary and secondary metabolites with other external sources like light, water, quality of soil, etc., which involve complex mechanisms that aid the survival of plants through photosynthesis and metabolic cycles. Many plants have a unique pigment called flavonoid, which cannot be produced in the human body since it is a phytochemical. Flavonoids are polyphenolic compounds, which are bioactive secondary metabolites (Mosunova et al., 2021). The main difference between primary and secondary metabolites is that secondary metabolites are not required for plant growth or reproduction; these functions are carried out by primary metabolites, mainly proteins, lipids, carbohydrates, etc. But secondary metabolites play a significant role in the survival of the plant (Mathesius, 2018). Some of the secondary metabolites are terpenoids, alkaloids, phenolic compounds, flavonoids, steroids, etc (Kessler and Kalske, 2018). Almost 50,000 secondary metabolites have been discovered in the plant kingdom (Pang et al., 2021) and medicinal plants, which mainly depend on secondary metabolites for their action (Teoh, 2016). Flavonoids are made of an aromatic ring with at least one hydroxyl group (Panche et al., 2016). They have variable phenolic structures and are found in parts of the plant like the bark, stem, flower, fruits, etc (Havsteen, 2002). Flavonoids are classified based on their molecular structure; the four main classes of flavonoids are flavanone, flavone, catechin, and anthocyanin, whose general structures are depicted in (Fig. 1) (Rice-Evans et al., 1996).
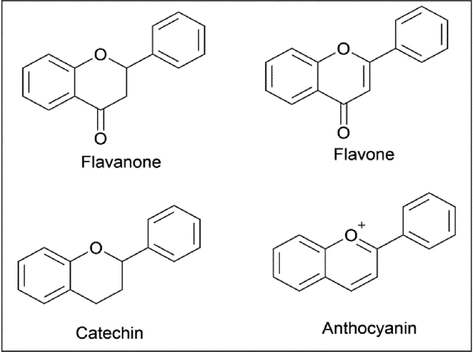
- General structure of four main class of flavonoids.
It was found that the human diet includes a higher number of flavonoids, the most abundant flavonoid is flavonol. Flavonoids give flowers their flavour, colour, and aroma; they also protect enzymes and prevent fat oxidation (Tomas-Barberan and Clifford, 2000). Since flavonoids play an important role in the aroma of flowers, this attracts pollinators, and ultimately pollination occurs in plants; they also help in fruit dispersion, which helps in seed germination and finally leads to the growth and development of the seedling (Dudek et al., 2016). Stress is one of the major issues in plants during drought conditions. Flavonoids act as a unique UV filter that help plants cope with biotic and abiotic stresses (Takahashi and Ohnishi, 2004). In addition to being drought resistant, flavonoids are also helpful in increasing freezing tolerance; sometimes, due to mutation, there might be a positive or negative effect depending upon the situation; the flavonoid character can be enhanced or diminished, but if it is diminished, it becomes hard for the plant to survive and to perform the function (Jorgensen, 1995; Pandey and Rizvi, 2009). Cotton is one of the major crops of India; however, researchers work on cotton to improve its resistance and defence against pathogens, herbivores, etc. Bacillus thuringiensis (Bt) cotton is widespread for its resistant ability, but further growing research uses strategies utilising flavonoids in pest management and developing the plant with desired characters. In addition to Bacillus thuringiensis (Bt) cotton flavonoids are important to improve the pest resistance of bananas (Hölscher et al., 2016; Nix et al., 2017; Zafar et al., 2020). Flavonoids play an important role in plant-bacteria interactions, since they are structure-specific and control the functions of nodulation, gene expression, and infection of nitrogen-fixing rhizobia (Liu and Murray, 2016; Peters et al., 1986; Spaink et al., 1989). Apart from inducing flavonoids to increase resistance, there are natural sources of flavonoids that are available. Different classes of flavonoids in various food sources are listed below in (Table 1) (Kumar and Pandey, 2013a; Nijveldt et al., 2001).
| Class | Compounds | Dietary source |
|---|---|---|
| Flavanones | Hesperidin Naringenin Naringin Taxifolin |
Oranges, Grapefruits, Lemon and other citrus fruits |
| Flavone | Quercetin Rutin Chrysin Apigenin Luteolin Kaempferol Myricetin Sibelin |
Onions, parsley, lettuce, olives Cranberries, Buckwheat Berries, Fruit skins Apple skin, red wine Celery Broccoli Fruit peels Grapes |
| Catechins | Epicatechin Catechin Epigallocatechin |
Tea Red wine Tea |
| Anthocyanins | Apigenidin Cyanidin Peonidin Pelargonidin Petunidin |
Cherry Berries Red graphs Raspberries Red wine |
Flavonoids having functional hydroxyl groups mediate antioxidant effects by scavenging free radicals or by chelating metal ions (Kumar et al., 2013b; Kumar and Pandey, 2013b). Prevention of radical generation might be crucial during the chelation of metals, which can damage target biomolecules (Kumar et al., 2013a; Leopoldini et al., 2006). Flavonoids have good health promoting properties since they have high antioxidant properties both in vitro and in vivo and can induce a protective system in humans (Cook, 1996; Rice-evans et al., 1995). They can regulate growth factors in plants, such as auxin. However, in the case of bacteria and fungi, there is the presence of biosynthetic genes that can produce flavonoids (Agati et al., 2012; Fangchuan et al., 2011). Apart from flavanones, flavones, and chalcones, flavonoids are also in the form of isoflavones and flavanols, which can be produced by bacteria with antioxidant properties (Song et al., 2014; Sülsen et al., 2017).
Flavonoids are biosynthesized from derivatives of acetic acid or phenylalanine using a specific pathway known as the shikimic acid pathway. Flavonols and flavones have many compounds called 2-benzo-γ-pyrone (narrow-sense flavonoids) (Wang et al., 2018c).
1.1 Flavanones
Flavanones are a type of flavonoid that are generally found in citrus fruits such as lemon, orange, etc (Table 1). They are responsible for the bitter taste of the fruit and peel in citrus fruits. Hesperitin and naringenin come under this category (Agrawal, 2011; Terahara, 2015). The main structural difference between flavanones and flavones is that flavones have a double bond present between the 2 and 3 positions, which is absent in flavanones (Fig. 1) (Iwashina, 2013). Isomerization of 2′ hydroxychalcones gives 2-arylchroman-4-ones by a ring closure mechanism with a stereogenic centre. When comparing natural flavanones, they are optically active and have an S configuration (Brahmachari, 2008). According to studies, sweet oranges contain 19.6 mg of aglycones per 100 g edible fruit, and the striking fact is that flavanones are 95% when measured. In the case of tangerines, flavanones were found to be 96% (Peterson et al., 2006). The concentration of flavanones will differ when measured in different fruits and vegetables. Natural flavanones are linked with sugars, in the form of 7-O-glycosides (Dias et al., 2021). Flavanones have free radical-scavenging properties, which show good health benefits like (YAO et al., 2004) anti-inflammatory action, anti-tumour activity, antioxidant activity, control of cholesterol levels, etc (Lambert et al., 2005; McPhail et al., 2003; Woo, 2013; Zhang et al., 2005).
1.2 Flavone
Flavones are mainly found in onions, parsley, lettuce, olives, cranberries, buckwheat, etc (Table 1). Chamomile and parsley have the highest flavone content, with quercetin, rutin, chrysin, etc. as some examples of flavones. The main source of flavone is from plants; mainly, they are conjugated as 7-O-glycosides with acetyl moieties (Gebhardt et al., 2005). They can be hydrolyzed with enzymes or acids even before analysis (Engelhardt et al., 1993; Hostetler et al., 2017). They have a double bond between the 2 and 3 positions and are oxidised at the C4 position (Martens and Mithöfer, 2005). They act as primary pigments in white or cream-colored flowering plants and co-pigments in blue-coloured flowering plants (Harborne and Williams, 2000). They have anti-inflammatory and antioxidant activity (Soto-Blanco, 2022). A considerable amount of flavone helps in cardiovascular and neuropathological diseases (Arredondo et al., 2015). Flavones are a signal molecule for initiating the colonisation of roots by nitrogen-fixing bacteria and fungi (mycorrhiza) (Rolfe, 1988; Siqueira et al., 1991). They protect plants by acting as natural pesticides against insects, bacteria, and fungi (McNally et al., 2003).
1.3 Catechins
Catechins are polyphenols found in tea, red wine, etc (Table 1). They account for 75% of the polyphenol compounds in tea leaves, with many chemical features, for example the hydroxy group (Singh et al., 2011b). Epicatechin, catechin, and epigallocatechin are a few types of catechin (Jin et al., 2006). They help with anti-allergenic activities. Since allergy is an abnormal immune cell response, by controlling the amount of histamine binding to histamine receptors, we can prevent allergy. Studies are conducted with catechin, which was extracted from tea and analysed; it was confirmed that it prevents the binding of histamine to receptors (OHMORI et al., 1995). Also help in anti-inflammatory, anti-microbial, antioxidant, and anti-arthritic activities (Aoshima et al., 2009; Bae et al., 2020; Foyet et al., 2015; Thring et al., 2009). Catechins play an important role in protecting the human body against UV radiation, promoting cell activity (Kim et al., 2015b; Zhang et al., 2017). Catechins are extensively used in medical, pharmaceutical, cosmetic, etc (Thornfeldt, 2005).
1.4 Anthocyanins
Anthocyanins is also a type of polyphenolic compound found in cherries, berries, red grapes, raspberries, etc (Table 1). They are glycosylated polyphenolic compounds with different colours ranging from red, orange, blue, and even purple (Tanaka and Ohmiya, 2008). Since they are water-soluble, they are stored in cell vacuoles. There are about 600 anthocyanins identified, among which apigenidin, cyanidin, peonidin, pelargonidin, and petunidin are prominent ones (Kong et al., 2003; Liu et al., 2018; Smeriglio et al., 2016). Anthocyanins help plants withstand adverse conditions for both biotic and abiotic stress (Ahmed et al., 2014). Due to their attractive colour, humans have various benefits like anti-obesity, anti-inflammatory properties, antioxidants, cardio-vascular activities, etc (Bors et al., 1990; He et al., 2011; Khoo et al., 2017; Rechner and Kroner, 2005; Tsuda et al., 2003).
1.5 Others
Isoflavonoids, neoflavonoids, chalcones, etc. are subgroups of flavonoids. Isoflavonoids are mainly found in soyabeans, beans, and lentils (legume seeds) (Gacek, 2014). They have anti-carcinogenic potential and antioxidant activities (Bolca, 2014; Popa and Rusu, 2017; Spagnuolo et al., 2015). Neoflavonoids have a 4-phenylchromen backbone and are mainly found in the bark of Mesua thwaitesii; they are mainly anti-inflammatory (Iinuma et al., 1987; Nishimura et al., 2000; Wu et al., 2011). Chalcones are also referred to as open-chain flavonoids because of the absence of one ring from the basic flavonoid structure. They are found in berries like strawberries, bearberries, etc (Rozmer and Perjési, 2016; Rudrapal et al., 2021). Arbutin, chalconaringenin, phloridzin, etc., are some of the examples of chalcone (Gomes et al., 2017). They help with neurological disorders and cardiovascular diseases (Cheng et al., 2014; García-Calderón et al., 2020).
Flavonoids have a unique structure and function that allows them to interact with specific enzymes, resulting in an effective polyparamacological behaviors. This way, flavonoids show good activity against many diseases and protect us from foods we consume that have rich flavonoid content (Balasuriya and Rupasinghe, 2012; Falcone Ferreyra et al., 2012; Gao et al., 2015; Giuliani et al., 2014; Krych and Gebicka, 2013; Rakers et al., 2014).
In general, if flavonoids are present in the daily diet, which helps in the prevention of cardiovascular disease (Egert and Rimbach, 2011), one of the most important functions of flavonoids is that they have anti-cancer activity (Chandra Tiwari and Husain, 2017; Lin et al., 2005; Ozcan et al., 2014; Saxena et al., 2012). Several epidemiological studies provide evidence for the consumption of fresh fruits and vegetables that have high concentrations of flavonoids that show good activity against stroke (Pietta, 2000). When we see the structure–activity relationship of bioactive flavonoids, which have good anti-bacterial and anti-viral activity, they show therapeutic activities against Escherichia coli, influenza virus, hepatitis C virus, and canine distemper virus (Carvalho et al., 2013; Mierziak et al., 2014; Nguyen et al., 2013; Shibata et al., 2014) by attributing chemical structure by hydroxylation, methoxylation, etc (Kim et al., 2011). Flavonoids, in addition to their anti-cancer, anti-bacterial, and anti-virus properties, play an important role in the treatment of diabetics, as the disease is now seen in both children and adults. Diabetes is a serious issue because it is a chronic disease and has adverse effects on the human body, mainly kidney failure, stress, slow wound healing, eye problems, high blood glucose levels, being overweight or obese, etc. Flavonoids, help in maintaining diabetics and act as a barrier from their adverse effects on the human body (Ha et al., 2018; Moller, 2001; Nazir et al., 2018; Vu et al., 2020), also showed that people consuming approximately 5–80 g per day of fruits and vegetables containing flavonoids had a reduced risk of myocardial infection. Studies have also shown that people consuming 600 g of fruits and vegetables containing flavonoids have a decreased risk of coronary heart disease (Borges et al., 2010; Lock et al., 2005). One of the greatest challenges faced by neuropsychologists is that today a lot of people suffer from Alzheimer’s disease and atherosclerosis. Alzheimer’s disease is a progressive neurologic disorder that causes the brain to shrink, and it is usually associated with dementia; whereas, atherosclerosis is a condition that can narrow or harden the arteries, mainly due to cholesterol lining called atheromatous plaque deposition over time. Once the arteries are blocked, blood flow is reduced, which can lead to heart attacks, strokes, etc. In such cases, flavonoids are used to treat this complex puzzle called Alzheimer’s disease and atherosclerosis to a certain extent, but a complete cure is not possible (Bondi et al., 2017; Castañeda-Ovando et al., 2009; Lee et al., 2016, 2009; Rafieian-Kopaei et al., 2014). In this review, quercetin’s structural aspects, method of synthesis, and main applications, which are required in the medical field, are discussed.
2 Quercetin
Quercetin (3,4,5,7-tetrahydroxyflavonol) belongs to the class of flavonoids, which are naturally occurring polyphenols. The name quercetin is derived from Quercetum (oak forest), named after Quercus in 1857. Quercetin is a yellow solid with a bitter taste; it is sparingly soluble in alcohol and soluble in glacial acetic acid but not soluble in water since it is hydrophobic in nature (Baghel et al., 2012; Scholz and Williamson, 2007). Quercetin is composed of two benzene rings linked by a heterocyclic pyrone ring, as shown in (Fig. 2). The IUPAC nomenclature of quercetin is 2-(3′,4′-dihydroxyphenyl)-3,5,7-trihydrochromen-4-one. They are mainly present in onions, apples, berries, red wine, green tea, etc (Almeida et al., 2018; Anand David et al., 2016b; Ding et al., 2021; Sakai et al., 2022; Shi et al., 2021; Tu et al., 2022). They are also found in medicinal plants like Hypericum perforatum, Ginkgo biloba, etc. The quercetin content of certain selected foods is listed below in (Table 2 and Table 3) (Häkkinen et al., 1999; Wiczkowski et al., 2008; Williamson and Manach, 2005). Quercetin has a keto-carbonyl group, and salt can be generated with strong acid since the oxygen atom at the first carbon is basic (Yang et al., 2020). Quercetin has a lot of applications, like being anti-inflammatory, treating cardiovascular disease, being anti-cancer, having anti-angiogenic activity, being anti-depressive, etc (Babaei et al., 2018; Chirumbolo, 2010; Dajas, 2012; Horowitz and Zunszain, 2015; Li et al., 2020; Salehi et al., 2020; Suganthy et al., 2016; Vauzour, 2014; Wang et al., 2019; Yang et al., 2018). Quercetin also has antioxidant properties with free radical scavenging, xanthine oxidase inhibitors, modulation of gene expression, reduction of leukocyte immobilization, NOS (Nitric Oxide Synthase) inhibitors, etc (Arai et al., 2000; Chang et al., 1993; Chondrogianni et al., 2010; Costantino et al., 1999; IIO et al., 1986; Mandel et al., 2004; Mohd Sairazi and Sirajudeen, 2020; Rahman et al., 1990; Shutenko et al., 1999). Recently, quercetin was also granted GRAS (Generally Recognized as Safe) status by the United States Food and Drug Organization (de Barros et al., 2022). The dose of quercetin is usually 400–600 mg of a coated tablet, taken one–three times between meals. In one experiment, 500–1000 mg of quercetin were administered for an upper respiratory tract infection (Heinz et al., 2010a, 2010b; Henson et al., 2008; Li et al., 2016; NIEMAN et al., 2009). As said earlier, quercetin is not soluble in water, so in certain cases, a protein-digesting enzyme is extracted from pineapple, which increases the absorption. Most of the time, quercetin is blended with one or more additives (Horwitz, 2018; Rodríguez De Luna et al., 2020).
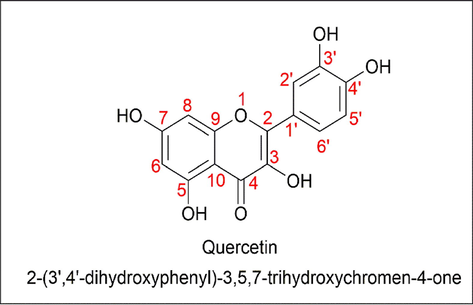
- Structure of Quercetin.
| Food Source | Quercetin Content (mg/100 g) |
|---|---|
| Green tea leaves, dry | 255.55 |
| Black tea leaves, dry | 204.66 |
| onions | 13.27 |
| Spinach | 4.28 |
| Apple with skin | 4.42 |
| Broccoli | 3.21 |
| Red Wine | 0.84 |
| Family | Pharmacological Activity | Examples |
|---|---|---|
| Apiaceae | Lowers blood pressure | Apium graveolens |
| Amaryllidaceae | Cardioprotective, antioxidant | Allium cepa |
| Moringaceae | Antibacterial, antihypertensive | Moringa oleifera |
| Apiaceae | Wound healing | Centella asiatica |
| Hypericaceae | Neurological effects | Hypericum perforatum |
| Brassicaceae | Reduce risk of stroke, neuropathy, prevents cancer | Brassica oleracea |
| Brassicaceae | Reduce the risk of cancer | Nasturtium officinale |
| Rosaceae | Decrease the risk of cardiovascular disease | Malus domestica |
| Ericaceae | Urinary tract infections | Vaccinium oxycoccus |
| Theaceae | Antiviral, antidiabetic | Camellia sinensis |
3 Synthesis
3.1 Synthesis of quercetin
Elena Vladimirovna Vetrova et al. (Vetrova et al., 2017) used the conventional hydrolysis method of rutin. This procedure involves dissolving 0.10 g of rutin in 1.5 ml of methanol and adding 0.25 ml of concentrated hydrochloric acid. The reaction was kept at 100 ℃ for 3 h. Once the hydrolysis is complete, the precipitate is filtered and dried at 80 ℃ for 3 h (Scheme 1).
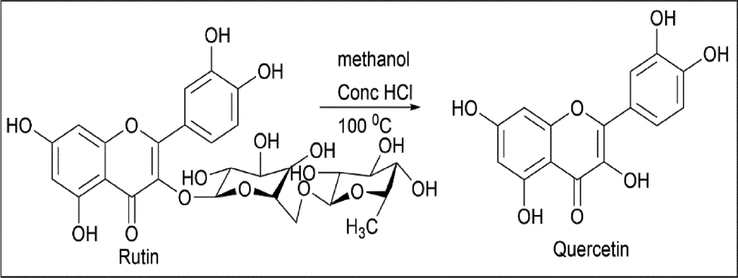
- Synthesis of Quercetin by hydrolysis of Rutin.
The biosynthesis of quercetin is done by using cinnamoyl-CoA, as shown by Ajay Sharma et al. (Sharma et al., 2018; Singh et al., 2021) cinnamoyl-CoA and malonyl-CoA used as starting material, and in the presence of chalcone synthase, which acts as a catalytic enzyme, results in the formation of naringenin-chalcone. Naringenin-chalcone is treated with chalcone isomerase for the isomerisation reaction, which leads to the formation of naringenin. Naringenin is treated with flavanone 3-hydroxylase and flavonoid 3′-hydroxylase, which give dihydrokaempferol and eriodictyol, respectively, by hydroxylation reactions at the C3 and C3′ positions. The resultant compound, dihydrokaempferol, was treated with flavonoid 3′-hydroxylase, and eriodictyol was treated with flavanone 3-hydroxylase so that hydroxylation takes place at the C3′ and C3 positions of dihydrokaempferol and eriodictyol, which results in the formation of dihydroquercetin. It is further reacted with flavonol synthase, resulting in the formation of quercetin by forming a double bond (Scheme 2).
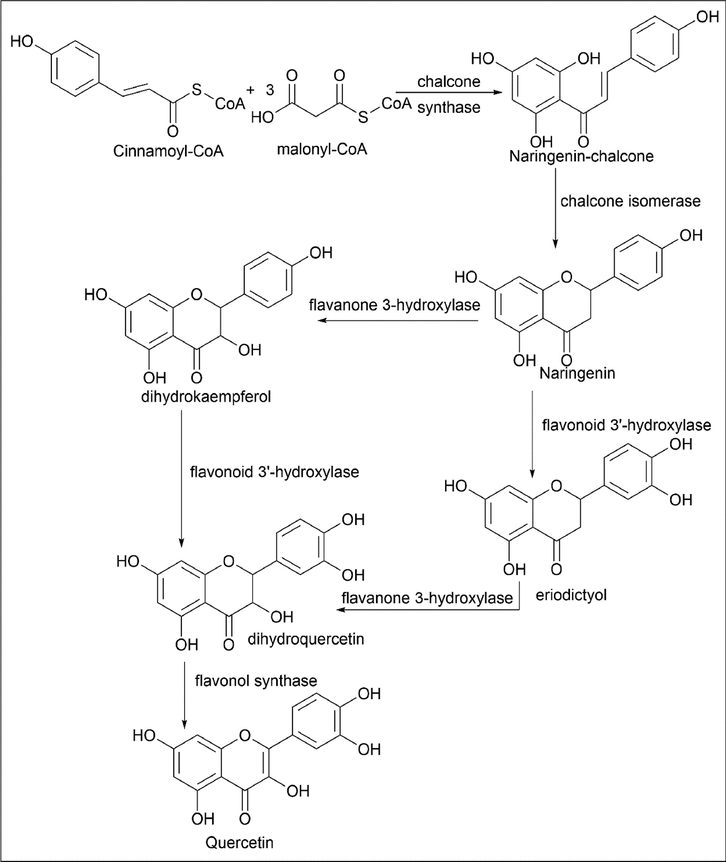
- Biosynthesis of Quercetin using Cinnamoyl-CoA and Malonyl-CoA.
3.2 Derivatives of quercetin
Duan et al. (Duan et al., 2017) synthesised an acylated quercetin analogue using rutin as the starting material. 40 mmol of rutin and 133 mmol of K2CO3 were added to 160 ml of DMF under nitrogen conditions at room temperature for about one hour. For the above solution, 133 mmol of benzyl bromide was added dropwise and stirred for 3 h at 40 ℃ under nitrogen condition, then the pH was adjusted to 6.0 using acetic acid at 10% (v/v) in an ice bath. About 300 ml of deionized water was added and filtered. For 600 ml of 95% (v/v) of ethanol, the filtered residue was added at 70 ℃ HCl was added, and hydrolysis continued at 70 ℃ for 2 h. The suspension was filtered and washed with ice water until its pH became neutral to yield hydrolysate. In 250 ml of DCM, the hydrolyzate was dissolved, followed by the addition of equimolar chlorides (butyryl chloride, propionyl chloride, etc) and TEA. After the reaction was completed, it was extracted with 1 mol/L HCl. A solution of saturated aqueous solution of NaHCO3 and deionized water was used to wash the organic layer and dry it. In 5000 ml of ethanol/dioxane (1:1), the acylated compound was dissolved, Pd/C was added, and the mixture was stirred at room temperature under hydrogen at atmospheric pressure for about 2 h. The Pd/C was removed by filtering, and an acylated quercetin crude product was obtained (Scheme 3).
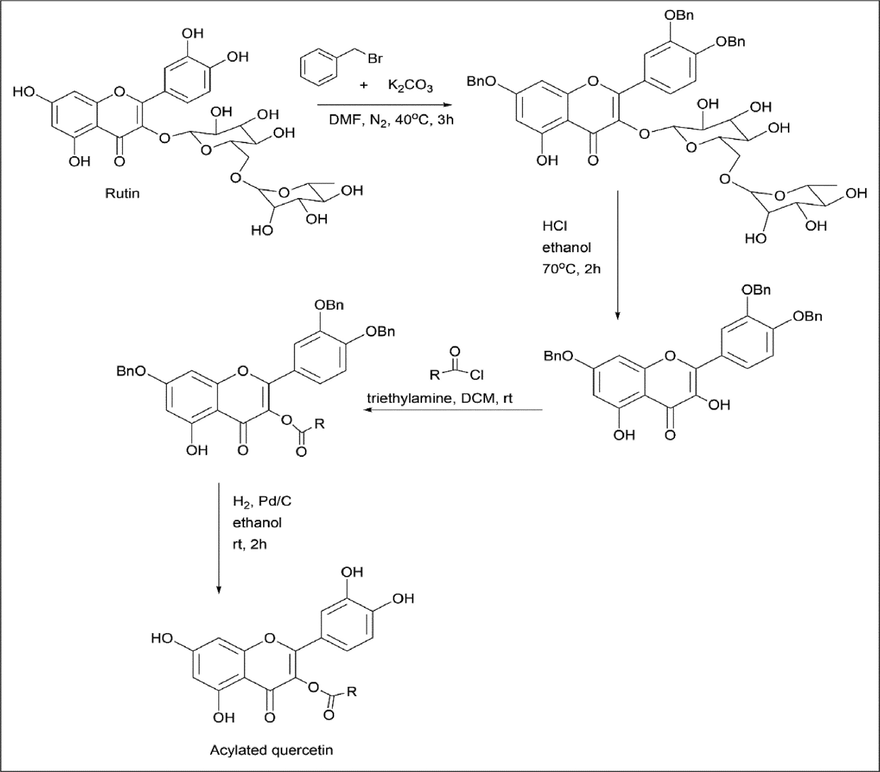
- Synthesis of Acylated Quercetin.
Osonga et al. (Osonga et al., 2017) used quercetin as the starting material, to which dibenzyl phosphate was added, which gets substituted on the OH group of quercetin in the presence of methyl cyanide and carbon tetrachloride. Once the reaction is over, dry DCM is added, H2 is added, 5% Pd/C is added so that the benzyl group is converted to an OH group to form quercetin pentaphosphate (Scheme 4).
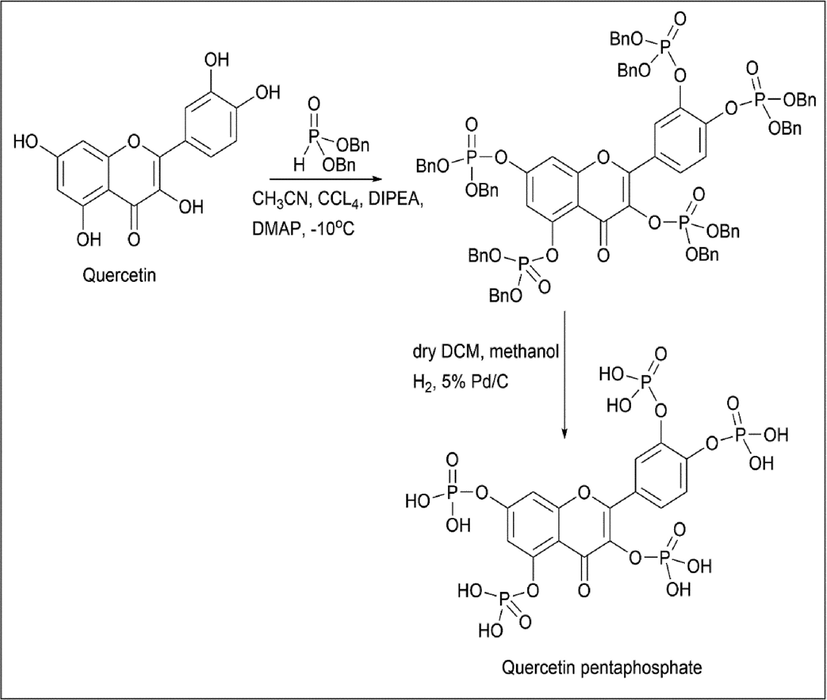
- Synthesis of Quercetin Pentaphosphate.
Mihyang Kim et al. (Kim et al., 2015a) alkylated quercetin to form pentamethoxyquercetin. 3 mmol of quercetin was dissolved in 120 ml of DMF, 90.1 mmol of K2CO3 and methyl iodide were added to the reaction mixture and stirred for 3 h at room temperature. Ethyl acetate was used for extraction and washed with brine, dried over anhydrous MgSO4, and then filtered and concentrated under reduced pressure. Purified using vacuum liquid chromatography using hexane and ethyl acetate to yield pentamethoxyquercetin (Scheme 5).
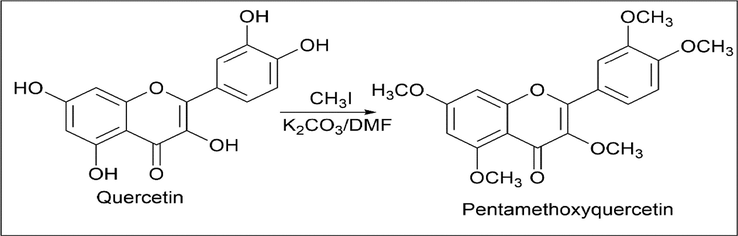
- Synthesis of Pentamethoxyquercetin.
Martina Danihelova et al. (Danihelová et al., 2012) used a similar method for alkylation of quercetin using 1-chloropropan-2-ol. Quercetin was dissolved in DMF; K2CO3 and 1-chloropropane was added. Once the reaction is over, the compound is filtered and dried (Scheme 6).
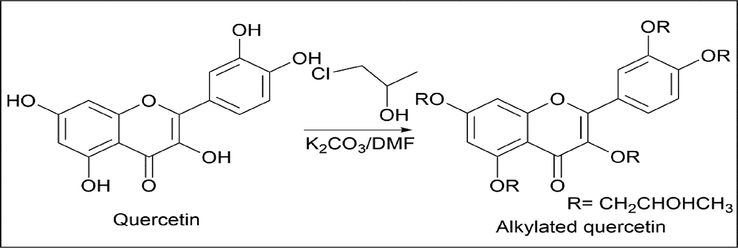
- Synthesis of Alkylated Quercetin.
Darsandhari et al. (Darsandhari et al., 2019) used enzymatic synthesis of quercetin derivatives. Here, three derivatives of quercetin were synthesised, namely quercetin 3-O-β-D-glucoside, quercetin 3-O-β-D-lactoside, 3′ sialyllactosyl quercetin and 6′ sialyllactosyl quercetin.
The synthesis of quercetin 3-O-β-D-glucoside is done by one pot synthesis using 100 mM tris buffer, 20 mM MgCl2, 150 mM acetyl phosphate, 50 mM glucose 1-phosphate, 2 mM UMP, 8 mM quercetin, and 1 mM ATP. The enzymes used is ACK, GalU, and UGT78K1, incubated at 37 ℃ (Scheme 7).
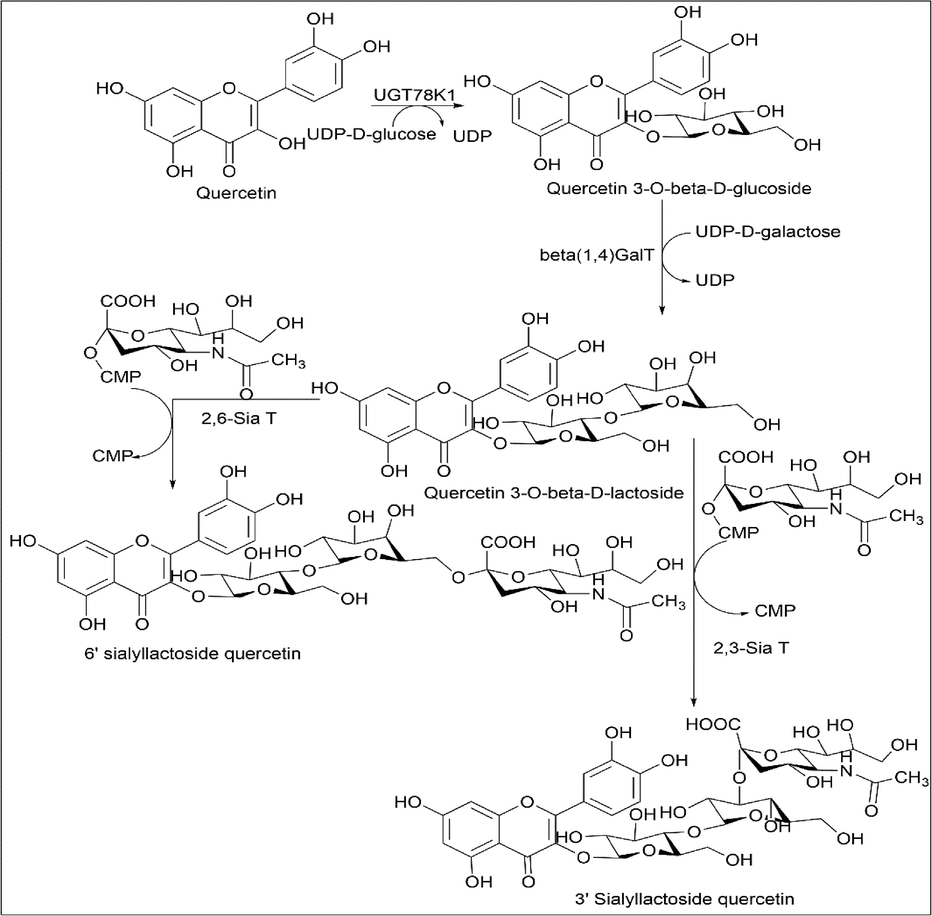
- Synthesis of Quercetin 3-O-β-D-glucoside, Quercetin 3-O-β-D-lactoside, 3′ sialyllactosyl quercetin and 6′ sialyllactosyl quercetin.
The synthesis of quercetin 3-O-β-D-lactoside was also prepared using enzyme, 50 mM of tris-Cl buffer (pH 7), 10 mM of UDP-α-d-lactoside, MnCl2, and 20% crude β (1,4) GalT. To this, 2 mM quercetin 3-O-β-D-glucoside was added and incubated for 2 h at 37 ℃ (Scheme 7).
The synthesis of 3′ sialyllactosyl quercetin and 6′ sialyllactosyl quercetin involves 25 mM tris-HCl buffer (pH 7.5), 20 mM MgCl2·6H2O, and 5 mM quercetin3-O-β-D-lactoside dissolved in DMSO. CMP-NeuNAc was used as a donor along with the sailyltransferase enzymes, 10 mM of CMP-NeuNAc and 50 µg/ml of sailyltransferase were added. The reaction mixture was incubated at 37 ℃, unreacted protein was removed by centrifugation (Scheme 7).
Rubin Thapa Magar et al.(Magar and Sohng, 2020) used a different strategy of synthesising a derivative of quercetin using gram-negative bacteria called Escherichia coli by metabolic engineering. E. coli is designed or engineered in such a way that it expresses only the desired biocatalysts, which are isolated from various sources while suppressing other genes in E. coli. This engineered E. coli is used for the biotransformation of quercetin. Engineered E. coli produce nucleoside diphosphate sugar (NDP-sugar) and glycosyltransferases (GTs). For the methylation of quercetin O-methyl transferase (OMTs) use S-adenosylmethionine, releasing S-adenosylhomocysteine, and glycosyltransferases use NDP-sugar for the formation of quercetin glycosides (Scheme 8).
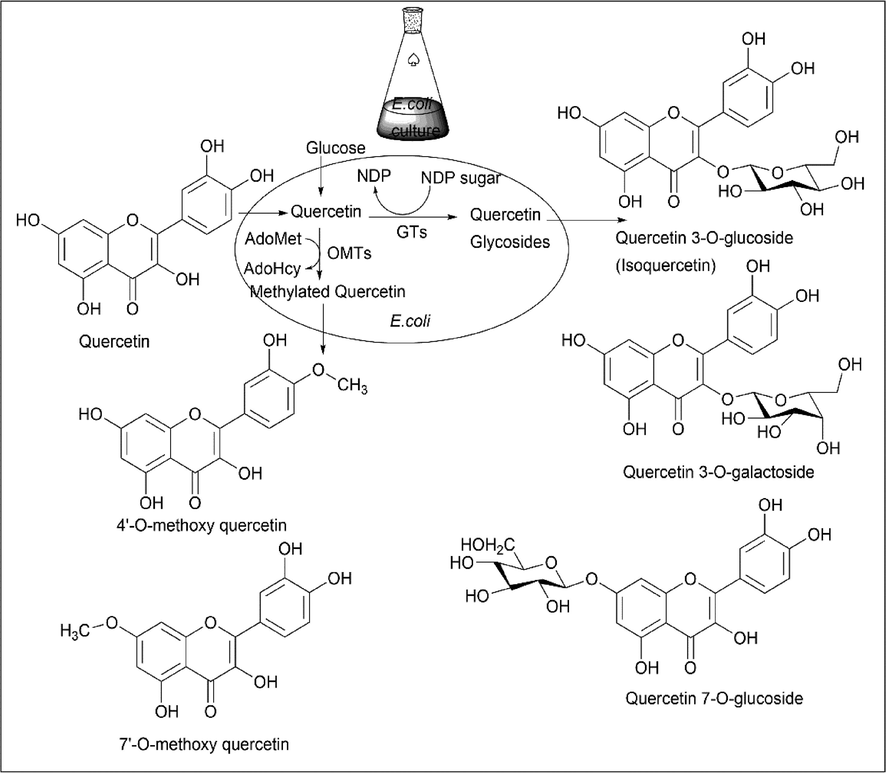
- Synthesis of derivative of Quercetin using E. coli.
Moon-Hee Choi et al. (Choi et al., 2021) used quercetin to synthesise a novel derivative called 3,7-dioleylquercetin by a modified method of Kato et al. Quercetin and oleyl bromide were used as starting materials, and generally the reaction takes place by SN2 reaction. Simultaneously, reactions occur on the 3,7, and 4′ positions of quercetin OH groups. The synthesis was performed by adjusting the equivalent ratio so that the oleyl moiety attaches to the 3, 7 OH groups. Once the reaction is complete, oleyl moieties are obtained (Scheme 9).
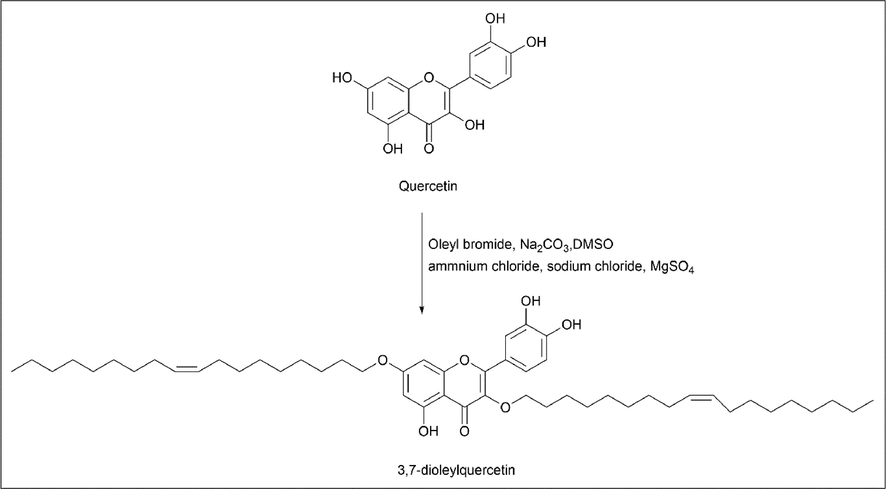
- Synthesis of 3.7-dioleyquercetin.
Mohammed Kajjout et al. (Kajjout and Rolando, 2011) protected and synthesised a derivative of quercetin. Quercetin was treated with dichlorodiphenylmethane at 180 ℃ for its protection (Scheme 10). The synthesis of 3-O-β-D-glucoside and 3-O-β-D-glucuronide is done by using protected quercetin as starting material and treating it with 2-(acetoxymethyl)-6-bromotetrahydro-2H-pyran-3,4,5-triyl triacetate in the presence of K2CO3/DMF, which forms a complex structure by replacing the Br group of 2-(acetoxymethyl)-6-bromotetrahydro-2H-pyran-3,4,5-triyl triacetate, which is treated with benzyl bromide, which acts as a protection for the OH group of the quercetin complex. The quercetin complex is treated with sodium methoxide and methanol to convert all O-acetyl groups to OH groups, which further react with H2/Pd/C and NaOCl for 3-O-β-D-glucoside and 3-O-β-D-glucuronide, respectively (Scheme 11).
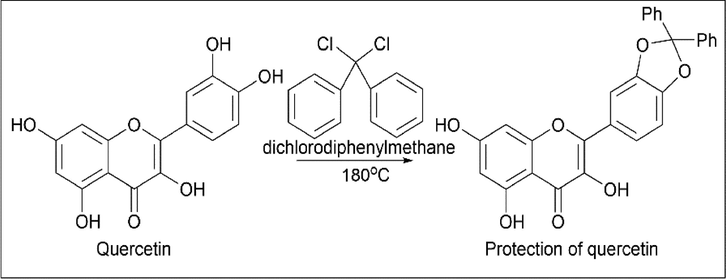
- Protection of Quercetin.
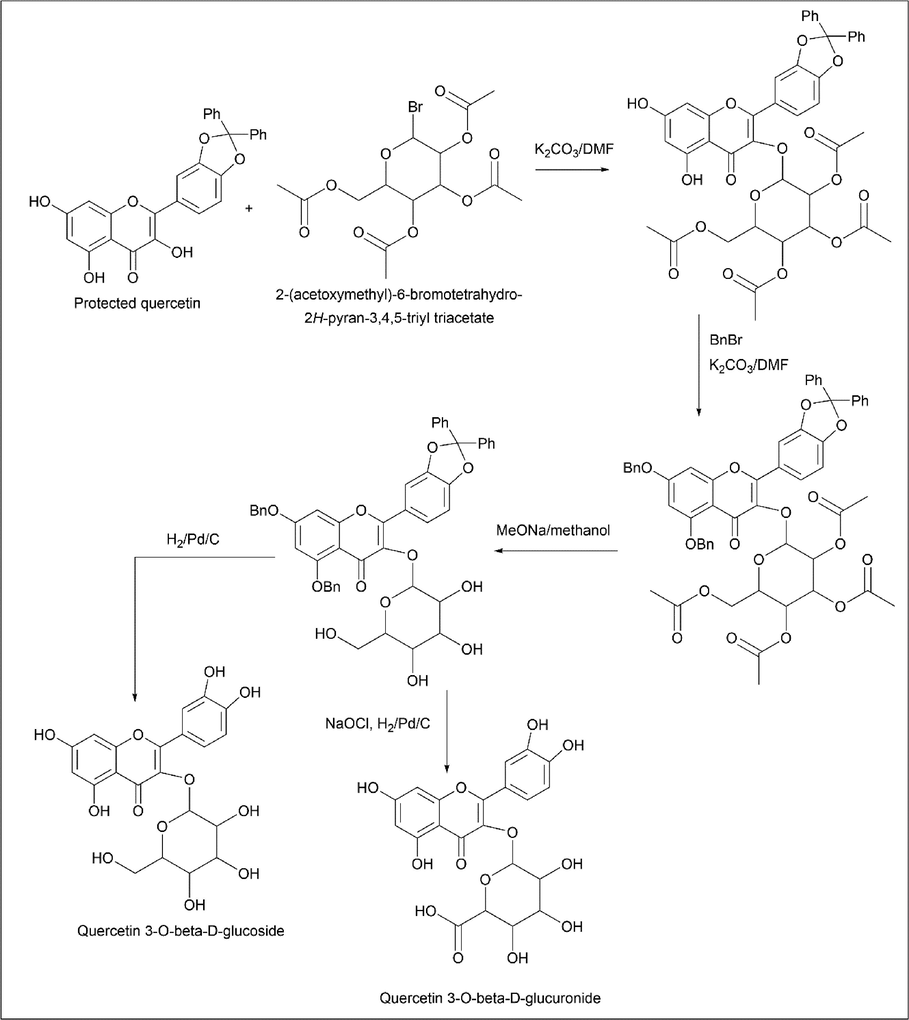
- Synthesis of Quercetin 3-O-β-D-glucoside and Quercetin 3-O-β-D-glucuronide.
Ketan V. Hirpara et al. (Hirpara et al., 2009) synthesised a fullerene derivative of quercetin. Quercetin was reacted with methyl iodide and K2CO3 to give tetra-methoxy quercetin and penta-methoxy quercetin, respectively. Tetra-methoxy quercetin was reacted with p-TsCl to give a tosylated product called tetramethylated monotosylated quercetin. Penta-methoxy quercetin was reacted with aluminium bromide so that one methoxy group was converted to an OH group by the demethylation process (Scheme 12).
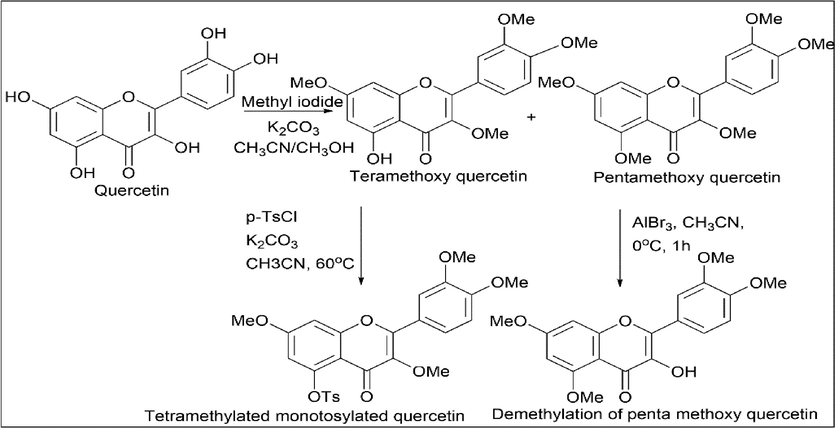
- Tosylation and demethylation of Quercetin.
Tetra-methoxy quercetin was treated with 3-iodo-1-propanol to give a propanol derivative of tetra-methoxy quercetin, further reacting with methyl malonyl chloride to give corresponding malonate esters, which on reacting with C60 by the Bingel reaction (cyclopropanation reaction) yield a fullerene derivative of quercetin (Scheme 13).
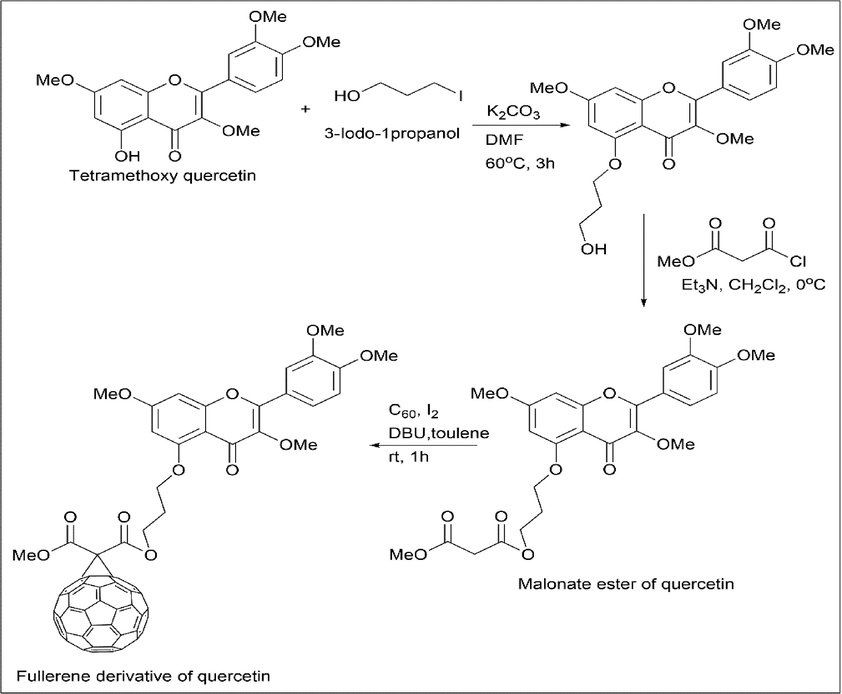
- Synthesis of fullerene derivative of Quercetin.
Komei Kato et al. (Kato et al., 2016) synthesised isorhamnetin (3′-O-methylquercetin) and azaleatin (5-O-methylquercetin) using quercetin. Quercetin was treated with 3 equivalents of benzyl bromide and 3 equivalents of K2CO3 to give the protected product 3,4′,7-tri-O-benzylquercetin, which further reacted with methyl iodide, hydrogen, and palladium to give isorhamnetin (3′-O-methylquercetin) (Scheme 14).
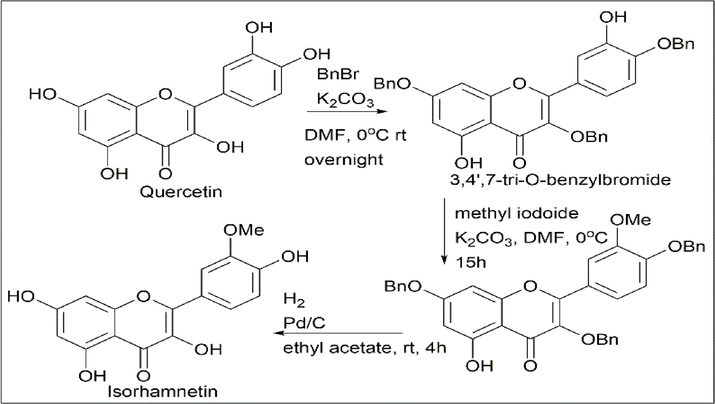
- Synthesis of Isorhamnetin.
Quercetin was treated with 4 equivalents of benzyl bromide and 4 equivalents of K2CO3 to give 3,3′,4′,7-tetra-O-benzylquercetin, further reacted with methyl iodide, hydrogen, and palladium to give Azaleatin (5-O-methylquercetin) (Scheme 15).
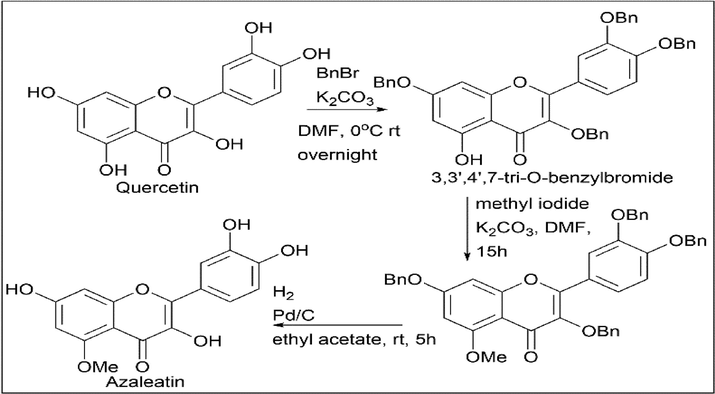
- Synthesis of Azaleatin.
Evgeny V. Buravlev et al. (Buravlev et al., 2018) synthesised the C-8 monoaminomethyl derivative of quercetin. Quercetin was reacted with amine in 1,4-dioxane or ethanol, aqueous formaldehyde was treated with hydrochloric acid, and the removal of solvated organic solvents was done. Heterocyclic secondary amine was used as an amine source in the reaction (Scheme 16).
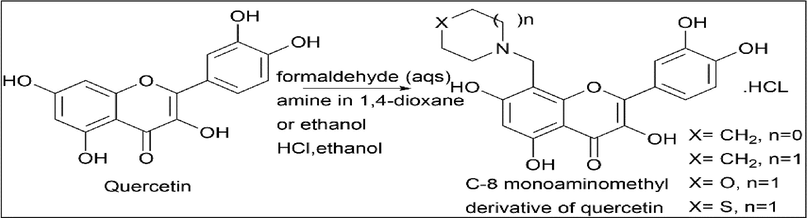
- Synthesis of C-8 monoaminomethyl derivative of Quercetin.
Tomas C. Tempesti et al. (Tempesti et al., 2012) synthesised quercetin-CF3 using rutin as the starting material. 0.08 mmol of rutin and 0.04 mmol of 2-chloro-5-(trifluoromethyl)-1,3-dinitrobenzene was dissolved in 5 ml of DMF and stirred with 100 mg of K2CO3 at 80 ℃ for about 15 h. The solution was extracted using ethyl acetate and water. Following separation of the organic and aqueous phases, the aqueous phase was washed three times with ethyl acetate. The organic phase was evaporated and a solid was obtained (Scheme 17).
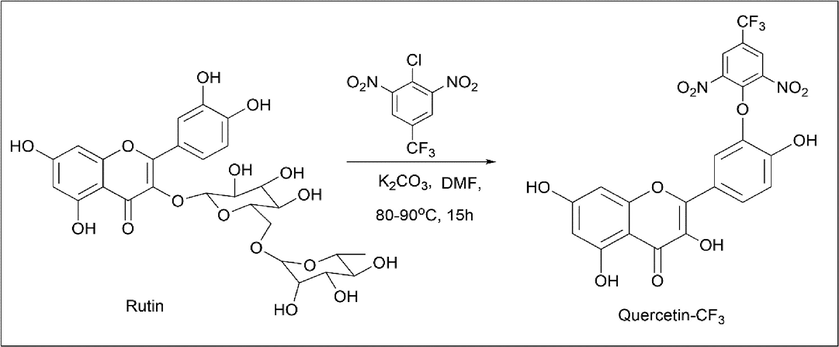
- Synthesis of Quercetin-CF3.
Chatzikonstantinou et al. (Chatzikonstantinou et al., 2020) synthesised peracetylated quercetin (PQ). 200 mg of quercetin, 1.25 ml of acetic anhydride, and 15 ml of pyridine were taken and refluxed in a nitrogen atmosphere. Once the reaction was complete, it was quenched with ice water and washed with ethyl acetate and diethyl ether. Yellow solid was obtained (Scheme 18).
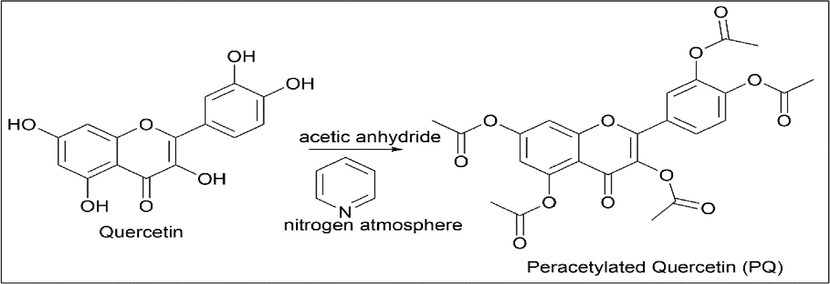
- Synthesis of Peracetylated Quercetin.
Mishurov et al. (Mishurov et al., 2016) synthesised tetraglycidyl quercetin. 2 g of quercetin, 1.0 ml of epichlorohydrin, and 0.50 g of N-pentadecyl-N-benzyl-N, N-dimethyl ammonium chloride were added to DMSO and heated for 30 min at 80 ℃ in an argon atmosphere. A solution of aqueous NaOH was added and heated for 4 h at 80 ℃. Once a concentrated precipitate was obtained, it was called diglycidyl quercetin since quercetin and epichlorohydrin were taken in a 1:2 ratio.
To synthesise triglycidyl quercetin, quercetin, and epichlorohydrin, they were taken in a 1:3 ratio.
To synthesise tetraglycidyl quercetin, quercetin and epichlorohydrin were taken in a 1:4 ratio (Scheme 19).
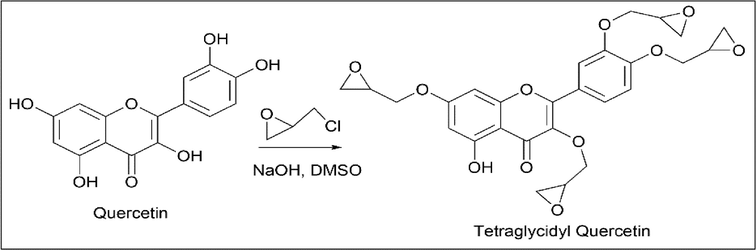
- Synthesis of Tetraglycidyl Quercetin.
Ayan Mukherjee et al. (Mukherjee et al., 2019) studied the regioselectivity of quercetin. Quercetin was reacted with benzyl bromide and K2CO3 to give a protected form of quercetin, namely tribenzyl quercetin (60%) and tetrabenzyl quercetin (33%). Tribenzyl quercetin was reacted with methyl bromoacetate; K2CO3 results in a monomethyl ester substituted product at the C3′ position; it was further reacted with Pd(OH)2/C, THF for deprotection or debenzylation of quercetin by replacing the benzyl group with an OH group. 2 N NaOH and acetone are used to convert the methoxy group to the OH group.
Tribenzyl quercetin was treated with methyl bromo ester to give a dimethyl ester substituted product at the C3′ and C5 positions, further reacting with Pd(OH)2/C, THF for the deprotection or debenzylation of quercetin by replacing the benzyl group with an OH group. 2 N NaOH and acetone are used to convert the methoxy group to the OH group.
Similarly, tetrabenzyl quercetin was reacted with methyl bromoacetate, K2CO3 results in a monomethyl ester substituted product at the C5 position, and further reacted with Pd(OH)2/C, THF for the deprotection or debenzylation of quercetin by replacing the benzyl group with an OH group. 2 N NaOH and acetone to convert the methoxy group to the OH group (Scheme 20).
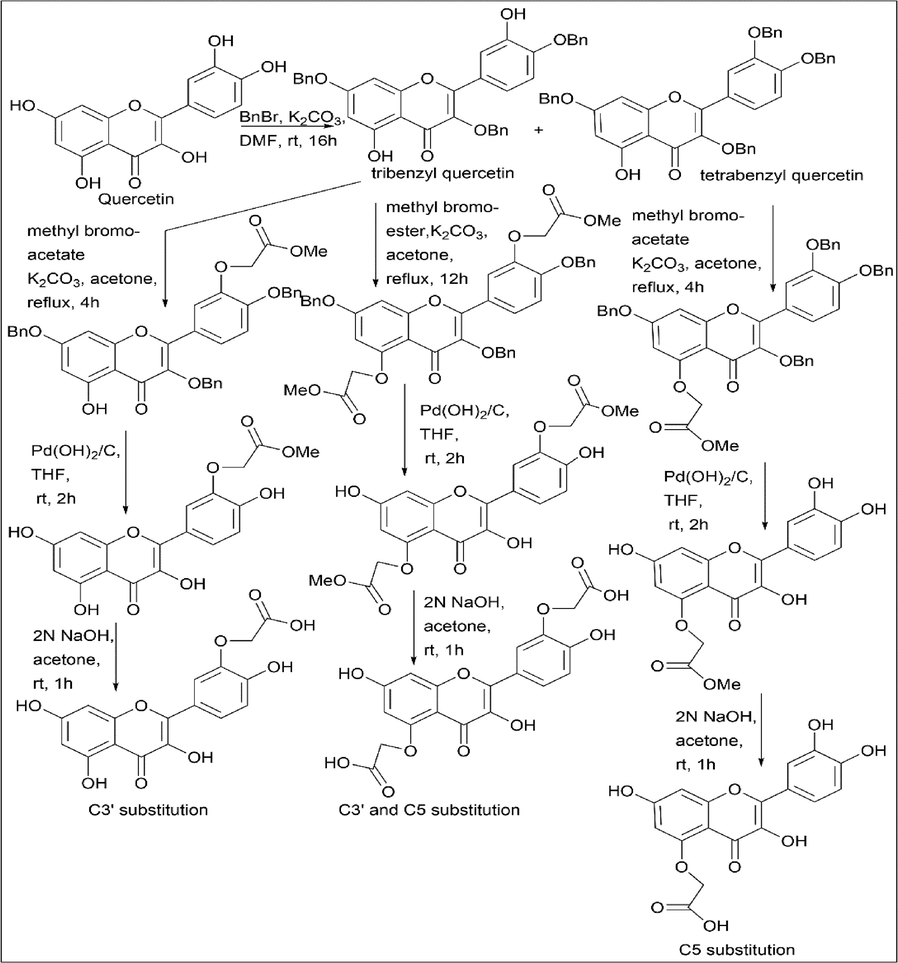
- Regioselectivity of Quercetin derivative.
Isika et al. (Isika et al., 2020) synthesised quercetin derivative called 2,2′,2″-((2-(3,4-bis(2-amino-2-oxoethoxy)phenyl)-4-oxo-4H-chromene-3,5,7-triyl)tris(oxy))triacetamide. 2 mmol of quercetin were dissolved in 10 ml of DMF which forms a brown solution. 15 mmol of anhydrous K2CO3 was added and stirred at room temperature for about an hour. To this, 14 mmol of ethyl chloroacetate was added slowly and stirred at room temperature under nitrogen gas. A solid product was obtained; to this above-solid 1 mmol of THF/H2O (1:2) was added so that the solid will dissolve. Once dissolved, 10 mmol of LiOH·H2O were added and stirred at room temperature to obtain the solid product. This above mentioned solid of about 0.7 mmol was mixed with thionyl chloride and refluxed to form an acyl chloride intermediate. This product was transferred into ice-cold ammonium hydroxide (aqueous) and stirred for two hours at cold condition and stirred at room temperature for about 4 h to obtain 2,2′,2″-((2-(3,4-bis(2-amino-2-oxoethoxy)phenyl)-4-oxo-4H-chromene-3,5,7-triyl)tris(oxy))triacetamide (Scheme 21).
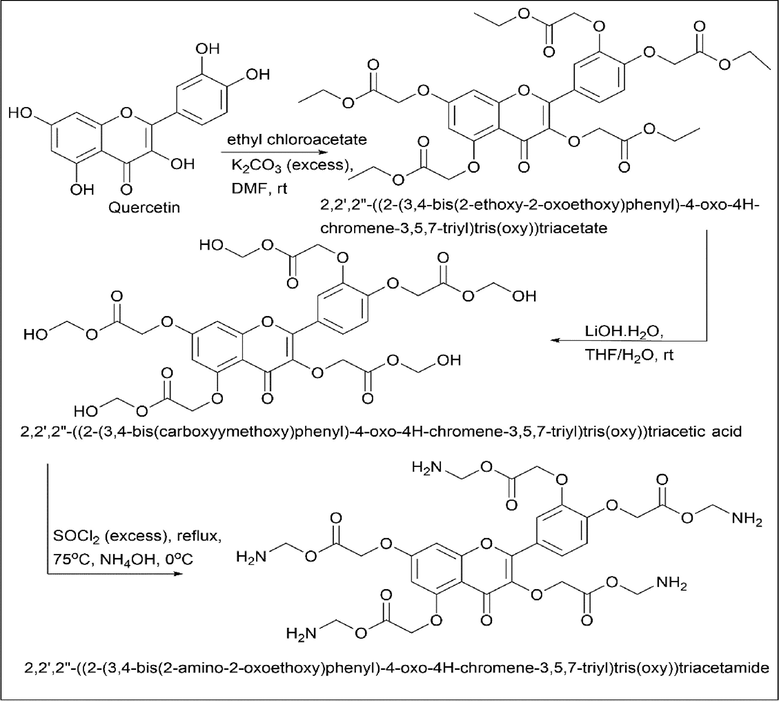
- Synthesis of 2,2′,2″-((2-(3,4-bis(2-amino-2-oxoethoxy)phenyl)-4-oxo-4H-chromene-3,5,7-triyl)tris(oxy))triacetamide.
Zhong et al. (Zhong et al., 2015) synthesised 7-O-substituted quercetin derivative. Peracetylation of quercetin using Ac2O and pyridine further reacted with thiophenol and imidazole for regioselective deacetylation of the 7-O-Ac group at 0 ℃, which gives 7-O-monodeprotected flavonol. Using substituted benzyl bromides, the 7th position was alkylated. Deprotection (deacetylation) is done using methanolic ammonia, which results in 7-O-arylmethylquercetin (Scheme 22).
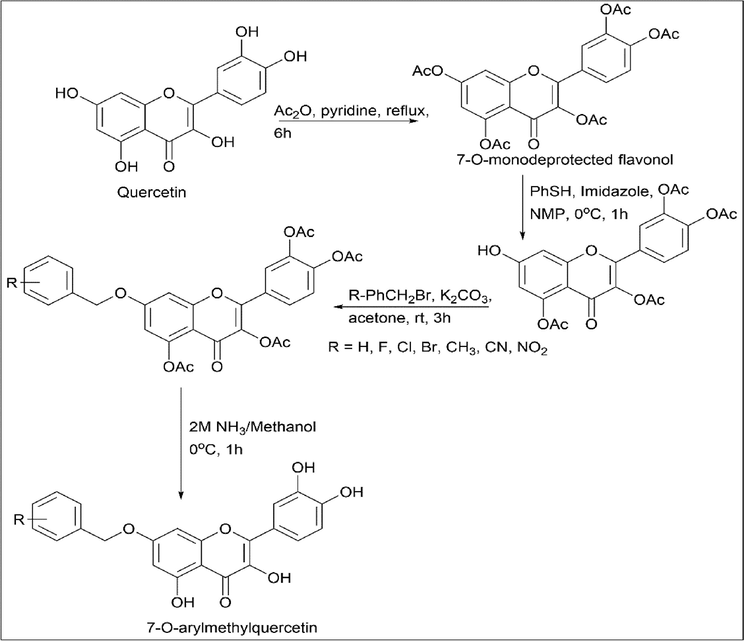
- Synthesis of 7-O-substituted Quercetin derivative.
E. Woznicka et al. (Woźnicka et al., 2015) synthesised quercetin-8-sulfonic acid and its sodium salt, quercetin-8-sulfonic acid. 2 g of quercetin was taken in a round bottom flask and 8 cm3 of concentrated sulfuric acid was added. The reaction mixture was stirred at 18–20 ℃ for 2 h. Once the reaction is complete, ice-cold water is added to the reaction mixture, and an orange precipitate is obtained, filtered, recrystallized, and dried at room temperature.
The sodium salt of quercetin-8-sulfonic acid was obtained by neutralising the post sulfonic mixture with 20% NaOH (pH 4). The yellow precipitate was obtained, filtered, recrystallized, and dried at room temperature (Scheme 23).
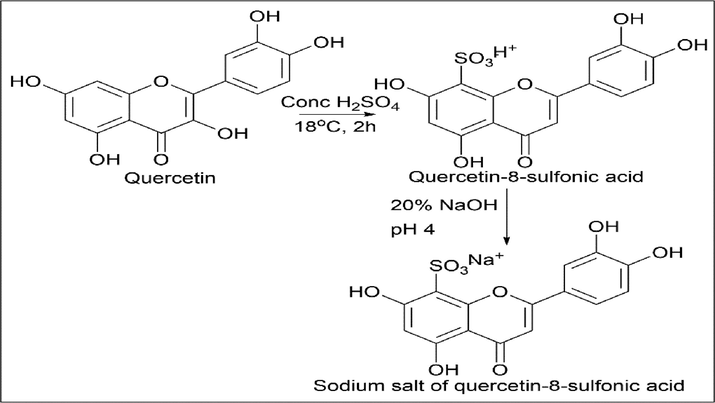
- Synthesis of Quercetin-8-sulfonic acid and its Sodium salt of quercetin-8-sulfonic acid.
Mahendra Thapa et al. (Thapa et al., 2012) synthesised 3-aminopropyl quercetin. Quercetin was reacted with sodium carbonate and benzyl chloride in DMF to form tribenzyl quercetin. Tribenzyl quercetin was treated with sodium hydride and N-(3-iodopropyl) phthalimide and further reacted with hydrazine, followed by hydrogen and palladium, to give 3-aminopropyl quercetin (Scheme 24).
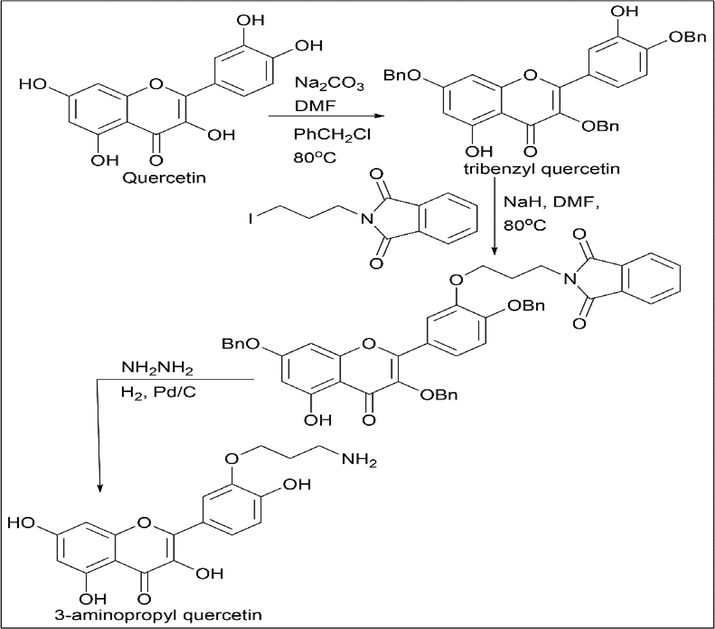
- Synthesis of 3-aminopropyl quercetin.
3.3 Synthesis of quercetin metal complex
Zahra Moodi et al. (Moodi et al., 2021) synthesised a metal complex of quercetin. Here, copper (Ⅱ) has been used as a metal for complex synthesis. In this method, first a Schiff base was synthesised with quercetin and ethanolamine and the corresponding metal complex was synthesised.
The synthesis of Schiff base involves an equimolar amount of quercetin and ethanolamine. After dissolving quercetin in 7 ml of ethanol, a few drops of glacial acetic acid were added dropwise to this ethanol amine after 30 min and refluxed at 60 °C for 8 h with stirring. Orange crystalline precipitate was obtained (Scheme 25).
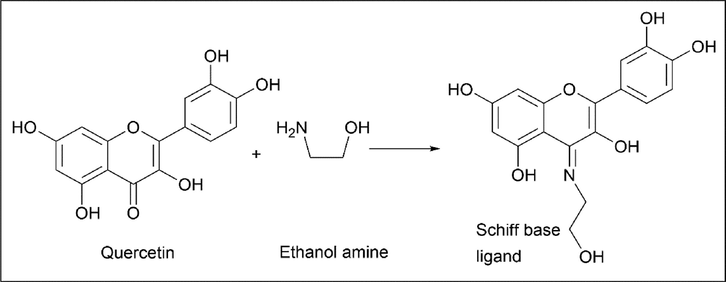
- Synthesis of Schiff base ligand from Quercetin.
The synthesis of a metal complex with copper as the metal was achieved. 0.5 mmol of ligand was dissolved in 1 ml of 10% NaOH and in 20 ml of deionized water and stirred for 10 min at room temperature. Once an orange solution is obtained, 0.5 mmol of Cu(OAc)2 dissolved in 10 ml of deionized water is added dropwise under ultrasonic irradiation at room temperature and sonicated for 30 min. centrifuged and dried in vacuum at 80 ℃ for 6 h (Scheme 26).
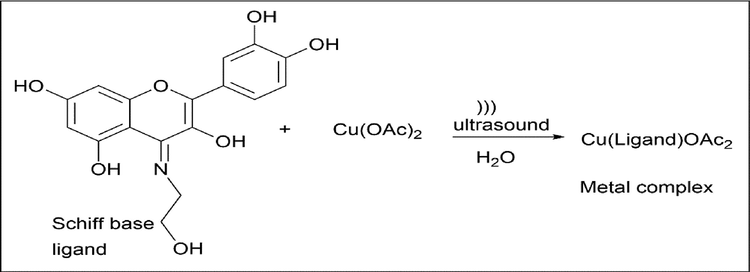
- Synthesis of metal complex of Quercetin by Schiff base ligand.
Wildson Max Barbosa da Silva et al. (da Silva et al., 2020) worked on a metal complex of quercetin with FeSO4·7H2O, Cu (CH3COO)2, Zn (CH2COO)2, NiCl2, and CoCl2 with quercetin as a ligand. Quercetin was dissolved in 10 ml of methanol and metal salt in 10 ml of distilled water with the stoichiometric ratio of 2:1 and stirred at room temperature for 20 min. Three drops of triethylamine were added to the reaction mixture, causing an immediate colour change, and the mixture was stirred for three hours. The reaction mixture was left at a low temperature for about two days so that precipitation was complete, filtered, and stored under vacuum in a desiccator (Scheme 27).
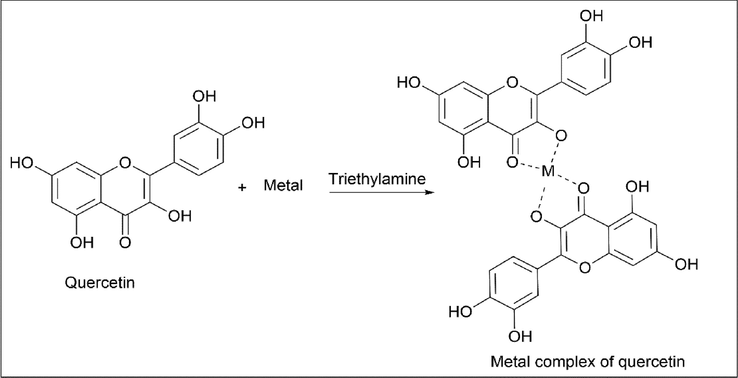
- Synthesis of Quercetin metal complex with Triethylamine.
Alina Bravo et al. (Bravo and Anacona, 2001; Kalinowska et al., 2021) also followed a similar method for synthesising quercetin metal complex using 2 mmol quercetin and 1 mmol metal like CoCl2·6H2O, MnCl2·4H2O, CdCl2, or HgCl2 in 40 ml ethanol, which was refluxed for about 10 h. It was left overnight, once a solid is formed, it was dried (Scheme 28).
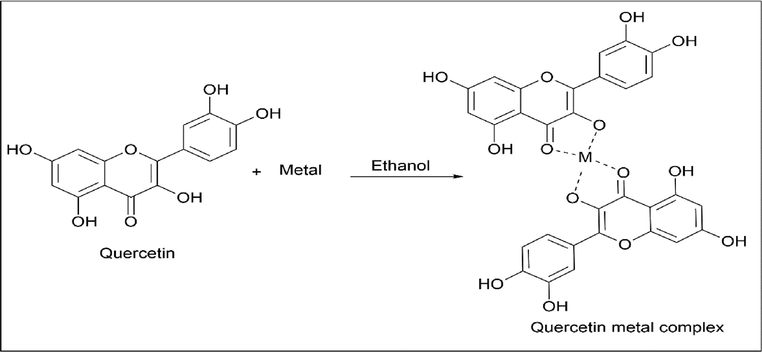
- Synthesis of Quercetin metal complex with ethanol as solvent.
Kirankumar Shastrala et al. (Shastrala et al., 2021) synthesised in a similar manner the complexes of Zn(Ⅱ), Ni(Ⅱ), Co(Ⅱ), Cu(Ⅱ), etc prepared using respective metal chlorides. However, Mo(Ⅳ) prepared using ammonium salt, and VO(Ⅳ) was prepared using Vanadyl sulphate. Quercetin and metal were taken in a (2:1) ratio. A methanolic solution of quercetin and a methanolic or aqueous solution of metal salt were used for the reaction. The pH was adjusted to 10 by adding NaOH solution (Scheme 29).
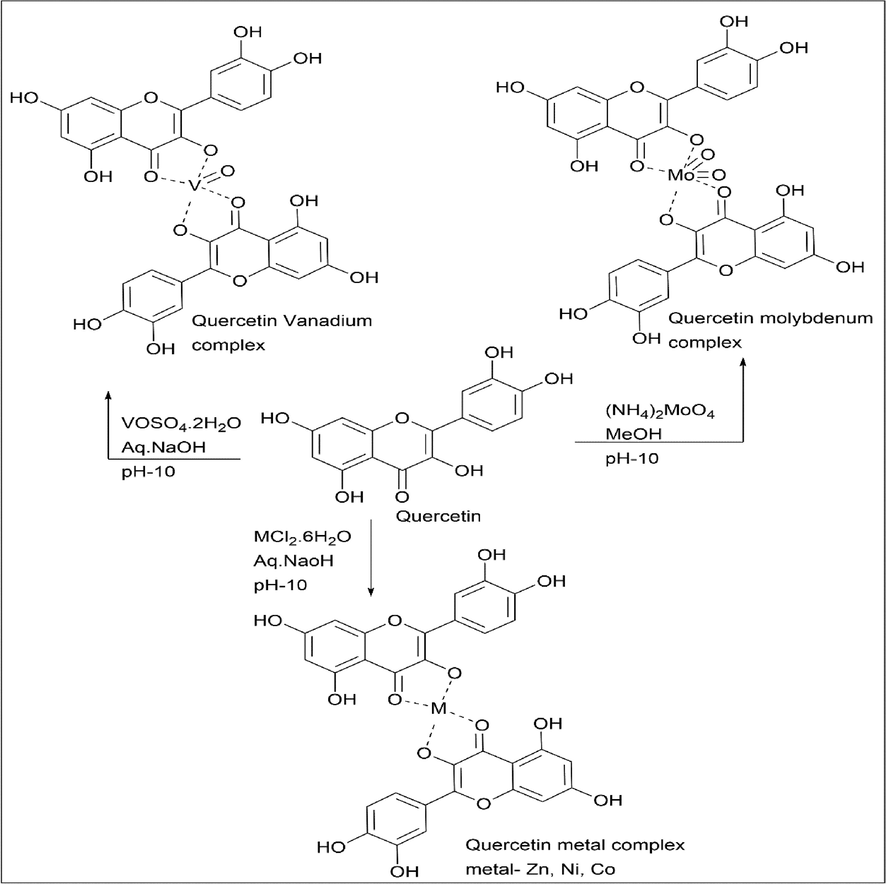
- Synthesis of Quercetin vanadium, molybdenum, and other metal complex.
Phakorn Papan et al. (Papan et al., 2020) worked on the iron(III)-quercetin complex. The synthesis method involves using quercetin hydrate, of which 0.0050 mol was added to 500 ml of methanol and allowed to stir until it dissolved. The colour of the solution is yellow. The pH of the solution was adjusted to 12 using 50% NaOH to convert it into deprotonated form (Scheme 30). Iron(III) chloride, 0.0025 mol in 500 ml ultrapure water, and added to the quercetin solution. The reaction was incubated for 2 h at 60 ℃. The dark yellow product was collected and stored for future analysis (Scheme 31).
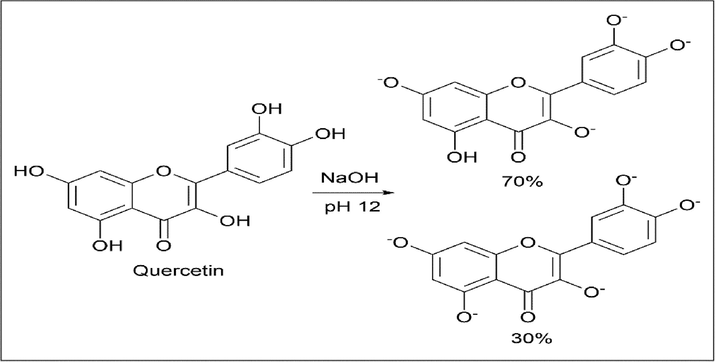
- Synthesis of Deprotonated form of Quercetin.
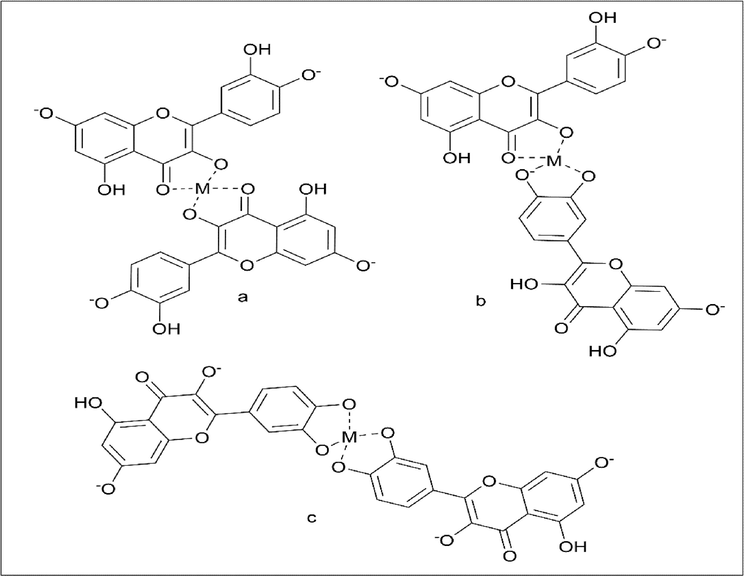
- Synthesis of Iron quercetin metal complex with different attachment.
Birjess Bukhari et al. (Bukhari et al., 2009) took a different approach by using a 1:2 ratio of quercetin and a metal like copper to form Cu-quercetin. Synthesis involves using a two-necked, round-bottom flask. 0.01 mol of quercetin was added to 20 ml of methanol, and once it had been dissolved, solid 0.02 mol of CuSO4·.5H2O was added and stirred for 15 min at room temperature. A colour change was noticed from yellow to brownish yellow. Once the reaction is complete, the product is washed with t-butanol and dried. The yield of Cu-quercetin complex was about 77% (Scheme 32).
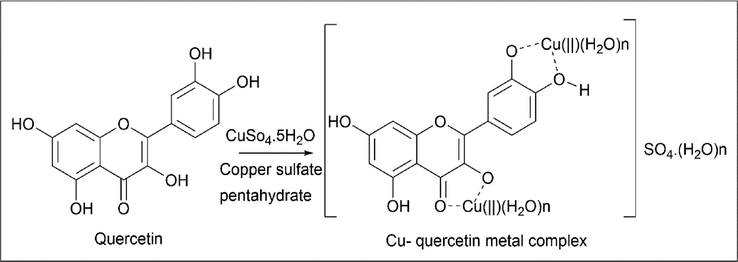
- Synthesis of Cu-quercetin metal complex.
Sartaj Tabassum et al. (Tabassum et al., 2013) worked on heterobimetallic complexes. Synthesis of monometallic complexes [Cu (Que)2(H2O)2] and [Zn (Que)2(H2O)2] and further heterobimetallic complexes were formed using Sn2Cl4.
3.3.1 Synthesis of monometallic complexes [Cu (Que)2(H2O)2] and [Zn (Que)2(H2O)2]
1 mmol of Cu (NO3)2·6H2O / Zn (NO3)2 was used as a metal source, and 2 mmol quercetin was refluxed to give a monometallic complex.
3.3.2 Synthesis of a heterobimetallic complex [Cu (Que)2(H2O)2-Sn2Cl4]
1 mmol of Cu (NO3)2·6H2O was dissolved in methanol, and to this 2 mmol of Sn2Cl4 was added dropwise and refluxed for about 6 h. The resultant brown color precipitate was obtained by adding ether to the concentrated solution. The compound was washed with ether and dried (Scheme 33). A similar procedure was carried out for [Zn (Que)2(H2O)2].
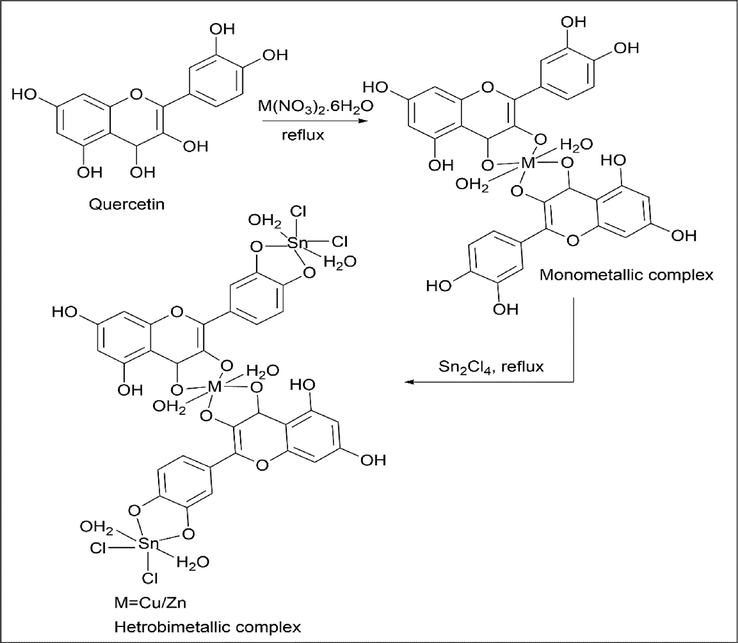
- Synthesis of Heterobimetallic complex.
Ravichandran et al.(Ravichandran et al., 2014) synthesized a quercetin-cadmium metal complex. 0.01 mol/L of quercetin was dissolved in 20 ml of ethanol in a 50 ml round-bottom flask. To this yellow-colored solution, 0.02 mol/L of cadmium nitrate was added quickly. After about 2 h of stirring at room temperature, the yellow colour changes to dark green, indicating the formation of a metal complex. The reaction mixture was filtered and evaporated, and a solid product was obtained. Unreacted material was washed with water (Scheme 34).
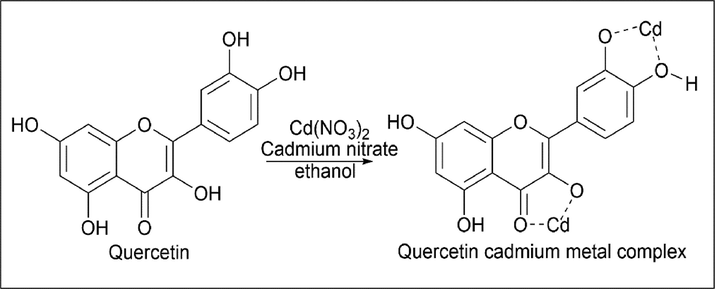
- Synthesis of Quercetin cadmium metal complex.
4 Applications
4.1 Anti-bacterial, anti-fungal, and anti-viral
Quercetin has anti-bacterial activity against a wide range of gram-positive and gram-negative bacteria (Anand David et al., 2016a). Some gram-negative bacteria are more resistant to quercetin than gram-positive bacteria, but certain derivatives of quercetin have potent antibacterial agents against both gram-positive and negative strains of bacteria (Nguyen and Bhattacharya, 2022; S. Wang et al., 2018a). Quercetin reacts with the bacterial cell membrane, and the reaction is determined by quercetin’s hydroxyl groups (Roy et al., 2022). The activity of the drug against bacteria is achieved by growing them in an agar medium with quercetin 25 µmol/ml dissolved in DMSO and diluted to 6*10-3 µmol/ml using the double dilution method. Culture media were spread by bacterial suspension (100 µl) and 100 µl of drug in drug discs. The zone of inhibition is measured after 24 h of incubation at 37 ℃ (Wang et al., 2018b). Minimum inhibitory concentration (MIC) will vary for different bacterial strains; for example, Streptococcus sanguis, Streptococcus mutans, Streptococcus sobrinus, etc., have MIC values ranging from 1 to 8 mg/mL (Yi Shu, 2011) Staphylococcus aureus has a MIC of 20 µg/mL (Jaisinghani, 2017). Depending on the strain of bacteria, the MIC value will change accordingly. Some bacteria are responsible for food spoiling, but when bacteria were treated with quercetin, they showed MIC, which results in killing bacteria (Montone et al., 2021).
The proposed mechanism of quercetin inhibiting bacterial activities involves three main mechanisms.
-
The perforation of quercetin damages the bacterial cytoplasmic membrane
-
Blocking the synthesis of nucleic acids and inhibiting energy metabolism
-
Targeting gyrase in bacteria (Ahmad et al., 2015; Osonga et al., 2019; Plaper et al., 2003).
Quercetin is well known for its anti-bacterial and anti-viral activities, but its anti-fungal activities are not well documented. However, quercetin has anti-fungal activity against Aspergillus niger, Aspergillus fumigatus, etc (Batiha et al., 2020; Yin et al., 2021). Quercetin shows anti-fungal activity to manage clinical Candida albicans biofilms and sensitise fluconazole-resistant Candida albicans isolated to fluconazole (Gao et al., 2016; Shahzad et al., 2014; Singh et al., 2015).
Quercetin was studied for anti-viral activities since it has a promising effect in inhibiting protease, reverse transcriptase, and polymerase and binding to viral capsid proteins (Agrawal et al., 2020; Bachmetov et al., 2012; Shinozuka et al., 1988; Spedding et al., 1989). A certain derivative of quercetin, namely quercetin 7-rhamnoside, acts against the porcine epidemic diarrhoea virus (Choi et al., 2009). Porcine epidemic diarrhoea virus causes acute diarrhoea, dehydration, vomiting, etc (Jung et al., 2020). Quercetin inhibits the activity of the Flaviviridae family, which consists of enveloped positive-strand RNA viruses. Pestivirus, Hepacivirus, etc genera come under this category, Hepatitis C belongs to the Hepacivirus genus (di Petrillo et al., 2022; Roudot-Thoraval, 2021). There is much literature supporting the antiviral properties of quercetin, both in vitro and in vivo. Quercetin has dose-dependent antiviral activity against herpes viruses (HSV-1 and HSV-2) in cell culture (Lee et al., 2017b; Lyu et al., 2005). They can inhibit respiratory viruses that are present in cultured cells, which show a protective effect on the lung (de Palma et al., 2008; Kumar et al., 2003). They also show therapeutic effects at an early stage of COVID (Kalil et al., 2021; Khan et al., 2022).
4.2 Anti-inflammatory
Quercetin was reported to have anti-inflammatory activities (Morikawa et al., 2003; Oršolić et al., 2004). Researchers have worked both in vitro and in vivo to find how efficiently quercetin works on inflammation (Kempuraj et al., 2005). Quercetin possesses both gastrointestinal cytoprotective and mast cell stabilising activities (B. Penissi et al., 2003). It has immunosuppressive activity on dendritic cells (Huang et al., 2010). They mainly inhibit AA metabolism and the production of eicosanoids (potent inflammatory mediators). Further, the effect of quercetin was examined on the COX-2 AA pathway, but no specific data were available for its potential to inhibit the COX-1 AA pathway (Lee et al., 2017c; Lesjak et al., 2018).
Marijia et al. (Azeem et al., 2022) worked on derivatives of quercetin; they are quercetin-3-O-glucuronide, isorhamnetin, isorhamnetin-3-O-glucoside, tamarexitin, etc. In the inflammatory response on COX-1, results showed that anti-inflammatory activity was seen more by tamarexitin. In recent times, COVID-19 has created a great impact in every country considering that SARS-CoV-2 is a beta coronavirus that causes severe inflammatory pneumonia. As is known, inflammation causes damage to our bodies and is even responsible for the deaths of a few people. Quercetin is a carbohydrate-free flavonoid, and this gives it a great ability to reduce inflammation (Saeedi-Boroujeni and Mahmoudian-Sani, 2021; Zhou et al., 2020).
Despite the evidence that quercetin shows anti-inflammation activities, there is no proper mechanism that can explain the effect of quercetin’s activity against inflammation (Chen et al., 2016).
Studies have revealed that polyphenolic compounds like quercetin, curcumin, etc, can enhance the phosphorylation of AMP-activated protein kinase (AMPK) (Wang et al., 2014). Quercetin suppresses inflammation by modulating AMP-activated protein kinase (Hung et al., 2015; Qiu et al., 2018; Sato and Mukai, 2020). To study the effect of inflammation in vivo, studies were done in mice expressing the human CRP gene. The term effect of quercetin had a positive effect on inflammation, but in the case of in vitro short-term quercetin is enough to act on inflammation (Bischoff, 2008; Boots et al., 2008b; Ghosh, 1999; Kleemann et al., 2011; Rogerio et al., 2010; Zadelaar et al., 2007). Recent studies revealed that quercetin suppressed proinflammatory cytokines through attenuation of NF-kappa B and p38 expression in human mast cells (Comalada et al., 2005; Karuppagounder et al., 2016; Min et al., 2007; Nair et al., 2006). In addition to quercetin, iso-quercetin is also an effective inflammation suppressor, suggesting a potential for treating allergies (Rogerio et al., 2007). In the case of quercetin derived from plants, it can affect inflammatory mediators, and they produce secondary messengers that lead to pro-inflammatory molecule expression (Calixto et al., 2004, 2003; Cho et al., 2003; Rather and Bhagat, 2020).
4.3 Anti-diabetic
Diabetes mellitus has become a major health issue all over the world. Diabetic disease leads to an increase in blood glucose levels because the body is unable to metabolize the sugar. It is a metabolic disease characterized by chronic hyperglycemia, which results in defects in insulin secretion (Craig et al., 2009; Galtier, 2010; Kharroubi, 2015). They are caused by genetics, changes in lifestyle, etc. They are further classified as type Ⅰ and type Ⅱ diabetics (Canivell and Gomis, 2014; M.D., 2020; Wilkin, 2009). In type Ⅰ, T cells destroy β cells of Islets of Langerhans; this leads to imbalance and disturbs equilibrium, as they are insulin-dependent (Daneman, 2006; Devendra et al., 2004; Ferrannini et al., 2010; Kilic et al., 2014). Blood glucose increases since the insulin level is low. A person suffers from polyurea, polydipsia, polyphagia, etc (Dabelea et al., 2014; DiMeglio et al., 2018).
In the case of type Ⅱ they are insulin independent (Kahn, 1994); they have loss of insulin receptor function, that is, the pancreas has insulin production but becomes insensitive once it binds to the insulin receptor (Galicia-Garcia et al., 2020; Roden and Shulman, 2019; Weyer et al., 1999). As a result of the increased cell number and size, more insulin is produced. When β cell number is increasing, it is referred to as hyperplasia, and when it increases in size, it is called hypertrophy (Muir et al., 2016; Schuster, 2010). It produces the protein Amylin, which uses all the energy and nutrients. Eventually, a reverse takes place from hypertrophy to hypotrophy, that is, size decreases, β cells start dying, and insulin production decreases (Druet et al., 2006; Robertson, 1995). This can be caused by genetics as well as acquired factors.
In recent times, researchers have found that quercetin has anti-diabetic activity. When quercetin was administered over a longer duration (14–28 days and ≥ 28 days) of intervention, it had a significant effect on serum glucose levels with a shorter duration (Bule et al., 2019). When quercetin was tested on plasma glucose concentration, control rats, i.e., non-diabetic rats, had no effect, but there was a significant reduction in the blood glucose level of diabetic rats after 8–10 days at the two doses used (Table 4) (Haddad and Eid, 2017; Vessal et al., 2003). To improve quercetin activity, scientists tried using polymer-based carriers like chitosan, liposomes, PLA, PLGA, polyphosphoesters, etc. They are non-toxic, easily synthesized, and have other advantages that help quercetin reach the target and perform the specific function (Mukhopadhyay et al., 2012; Mukhopadhyay and Prajapati, 2015; Pinto, 2010; Xu et al., 2007a; Xu et al., 2007b). Extensive preclinical and clinical studies should be carried out to make a prominent anti-diabetic drug, and it should be approved by the FDA (Yao et al., 2019; Yi et al., 2021).
| Flavonoid | Metabolites produced from flavonoids | Function | Mechanism of action | Model |
|---|---|---|---|---|
| Quercetin |
|
Antihyperglycemic effect and hypolipemic effect | Inhibit insulin dependent activation of P13K, inhibit GLUT2 which reduces the absorption of glucose in small intestine, block the activity of tyrosine kinase. Improv the recovery of cell proliferation, improve glucose absorption, reduces lipid peroxidation |
In-vitro studies involve skeletal muscle cells hepatocyte RINm5F β cells. In-vivo studies involves rats streptozotocin (STZ)-induced diabetic rats. |
4.4 Cardiovascular disease prevention
Flavonoids can deliver anticipated cardioprotective action in cardiovascular diseases and ageing (Egert et al., 2009; Peluso, 2006). Recent studies showed that quercetin plays an important role in cardiovascular protection (Deng et al., 2020). In 1993, an investigation that was performed on humans showed a positive correlation between dietary intake of flavonoids, including quercetin, and a lower risk of cardiovascular disease in both men and women (Hertog et al., 1993). Traditional Chinese medicine is widely used in Southeast Asia for cardiovascular disease. An extract from Ginkgo biloba leaves had the flavonoid quercetin, which showed a significant effect on cardiovascular activities. When analyzed, quercetin had pharmacokinetic action on 47 cardiovascular disease-related targets and 12 KEGG (Kyoto Encyclopedia of Genes and Genomes) signaling pathways (Wu et al., 2019b). From experiments, mice, rats, rabbits, and other animals that have the same cardiac physiology also exhibit the same functional capacity for quercetin (Chirumbolo, 2012; Patel et al., 2018; Zahedi et al., 2013). In addition to cardiovascular activity, quercetin also reduces blood pressure; certain cardio favored actions, etc (Islam et al., 2014; Lakhanpal and Rai, 2008; Larson et al., 2010; Perez-Vizcaino and Duarte, 2010).
Researchers found that quercetin has been speculated to inhibit the protein kinase involved in the Ca2+ sensitizing mechanism, which helps in smooth muscle contraction, which helps reduce hypertension and ease the flow of blood in blood vessels (Larson et al., 2012). They have a direct effect on vascular smooth muscle tone, where quercetin-related flavonoids exert a vasodilator effect in isolated arteries (Duarte et al., 1993b, 1993a; Fitzpatrick et al., 1993). Quercetin and its metabolites are more potent in coronary arteries when compared to conductance vessels since they are resistant (Dagher et al., 2021; Pérez-Vizcaı́no et al., 2002).
G1-phase of the cell cycle is blocked by quercetin, which subsequently down-regulated CDKs and cyclins and up-regulated the CDK inhibitor p21 expression in vascular smooth muscle cells (Perez-Vizcaino et al., 2006; Wang et al., 2022a). Since quercetin is abundant and researchers have worked on it in vitro and in vivo, clinical studies showed that quercetin is cardioprotective by also reducing blood pressure and having antioxidant potential, which makes way for smooth blood flow (Chen et al., 2021b; Duarte et al., 2001; Kachur et al., 2017; Manna and Jain, 2015; Mirsafaei et al., 2020). Hypertension, dyslipidemia, diabetes, and obesity are also causes of cardiovascular disease. So, quercetin has been proposed for cardiovascular disease protection since it has anti-thrombotic, anti-dyslipidemia, and anti-hypertension properties (Chen et al., 2021a; Feng et al., 2022; Kadoglou et al., 2021; Li et al., 2021b; Tabasco et al., 2011).
An investigation confirmed the effect of quercetin (120 mg per day) for two months on patients had a positive effect on chronic systemic inflammation who had coronary artery disease (Brunetti et al., 2014; Chekalina et al., 2018; Ishizawa et al., 2011; Knekt et al., 1996; Papakyriakopoulou et al., 2022). Also, quercetin is associated with endothelial dysfunction, which helps against cardiovascular disease by vasodilation (Bondonno et al., 2015; Costa et al., 2022; Edwards et al., 2007; Knekt et al., 2000; Kozłowska and Szostak-Węgierek, 2022). But further research must confirm the complete function and confirm the hypothesis.
4.5 Neurological disorder prevention
Quercetin has a good effect on neurodegenerative disorders, and for neurodegenerative disease, inflammation plays a key role (Alam et al., 2016; Islam et al., 2021; Sharifi-Rad et al., 2020). Early brain injury triggers neuroinflammation, which is a secondary reaction that leads to significant neuronal damage, ultimately creating different neuropathologies that act as a driving force (Glass et al., 2010; Sharifi-Rad et al., 2021; Smith et al., 2012; Tsoukalas et al., 2021). Glial cells, including microglia and astrocytes, in the central nervous system (CNS) act as an immune system that maintains the normal structure of neurons by defending against pathogens (Badshah et al., 2016; Khan et al., 2018; Williams et al., 2008). There is an issue with the potential use of quercetin, where researchers are not sure whether it passes the blood–brain barrier (BBB) in in vivo, but in vitro studies indicated that with the BBB model, quercetin enters the brain (Amanzadeh et al., 2019; Faria et al., 2010; Schaffer and Halliwell, 2012; Youdim et al., 2004). When quercetin 30 mg/kg was administered to rats, studies revealed that rats had increased memory function, also found in brain tissue, and had a positive effect on neurological studies (de Boer et al., 2005; Haleagrahara et al., 2009; Huebbe et al., 2010; PARK et al., 2018; Yang et al., 2014). Quercetin is known for its role in neurodegenerative diseases since they have the capacity to modulate microRNA (miRNA) expression (Benameur et al., 2021). As mentioned, quercetin plays a key role in Alzheimer’s disease and Parkinson’s disease and is a recent derivative of natural food sources such as vitamins and phytochemicals, which are safe, functional foods, etc. Since vitamin C is water soluble, a high intake of this vitamin C decreased the risk of Alzheimer’s disease, and people with higher plasma concentrations of vitamin C had better memory performance (Gupta et al., 2017; Heo and Lee, 2004; Masaki et al., 2000; Perrig et al., 1997; Samini, 2019).
Neuroinflammation is also a serious issue that may be caused by some primary detrimental disorders, be induced by β-amyloid aggregates, or be due to neoplasms (hyperproliferated cells). In addition to neurons, even gliosis is involved in pathological changes related to neurodegeneration and CNC disorder (Heneka et al., 2015; Hooten et al., 2015; Lyman et al., 2014; Testa et al., 2014; Troubat et al., 2021; Wróbel-Biedrawa et al., 2022). Analysis of quercetin in the brain showed that when quercetin was administered orally in conjugated form, it was accumulated in the brain as (quercetin-3-O-glucoronide and isorhamnetin-3-O-glucuronide), but methylquercetin-3-O-glucoronide was not quantifiable (Bitan et al., 2003; Dajas et al., 2015; Ho et al., 2013; Ishisaka et al., 2011; Muñoz-Reyes et al., 2021). Autophagy is a cellular destructive mechanism involving the recycling and removal of damaged and aggregated proteins by a lysosomal degradative process. This is an integral component of the central nervous system (Siokas et al., 2021). Genes related to autophagic processes lead to neurodegeneration by mutation when quercetin is administered (Ciechanover and Kwon, 2015; Qu et al., 2014; Regitz et al., 2014).
4.6 Antioxidant
A molecule that can produce free radicals by oxidation reaction can be inhibited by antioxidants. Antioxidants can be natural or synthetic; there are many dietary antioxidants like vitamin C, vitamin E, etc (Blokhina, 2003; Geethangili and Ding, 2018). Many flavonoids have antioxidant properties; one of them is quercetin, which is known for its antioxidant properties (Flora and Pachauri, 2010; Lakhanpal and Rai, 2007). The property of quercetin to act against various diseases is due to its antioxidant properties, because of which the study of quercetin’s antioxidant property has become a research hotspot (Boots et al., 2008a; Chen, 2006; Liao et al., 2018).
Quercetin maintains oxidative balance, exhibiting strong antioxidant activity. In vivo studies of quercetin showed antioxidant activity through its effect on glutathione enzymatic activity, toxicology factors, reactive oxygen species from the environment, signal transduction, etc (Ademosun et al., 2016; Jin et al., 2016; Kinaci et al., 2012; Kobori et al., 2015; Qi et al., 2022; Song et al., 2013). Quercetin’s chemical structure plays an important role as an antioxidant; it has a hydroxyl group (OH) mainly in the B and C rings, which contribute more than the A ring (Zheng et al., 2017b). Studies also showed that quercetin can act as an antioxidant agent and reduce cellular levels of mixed disulfide proteins (Ashfaq et al., 2006; Ishige et al., 2001; Michala and Pritsa, 2022). SPLET (sequential proton loss electron transfer) mechanisms are important antioxidant mechanisms in which antioxidants trap free radicals. Phenolic antioxidants have confirmed the important role of SPLET in polar solvents (Leopoldini et al., 2010; Vagánek et al., 2014; Xue et al., 2014; Zheng et al., 2017a). Recently, chemical studies revealed that quercetin metal complexes have better antioxidant activity than quercetin alone (Chen et al., 2005; Xu et al., 2019). Comparing quercetin and curcumin, quercetin has a higher reduction potential and total antioxidant capacity; it reduces lipopolysaccharide-induced reactive oxygen species to a normal level and ultimately reduces lipopolysaccharide-induced No production (Zhang et al., 2011).
In certain cases, there can be DNA damage by means of free radicals or chemicals, which cause various types of damage to our body by causing diseases such as diabetes, cancer, nerve disease, etc (Selvaraju et al., 2012; Song et al., 2020; Stone et al., 2011). So, to protect DNA from these adverse effects, natural and synthetic antioxidants are required, which can protect DNA against damage. Researchers found quercetin has antioxidant ability, which can protect DNA from these external effects, but the mechanism is still unknown (Janjua et al., 2009; Kanakis et al., 2007, 2005; Oliveira-Brett and Diculescu, 2004; Silva et al., 2008; Srivastava et al., 2016). In recent time, the effect of chalcogens on the scavenging power of quercetin was studied using functional theory; quercetin has a high peroxyl radical scavenging property at pH 7–10, which is attributed to its antioxidant abilities and chelating agent (Castañeda-Arriaga et al., 2020; de Souza and De Giovani, 2004; Galano et al., 2016; Simunkova et al., 2021; Ullah et al., 2020). Quercetin can prevent the oxidation of low-density lipoproteins by scavenging free radicals (Murota and Terao, 2003). There is further research required to enhance the bioavailability of flavonoids to exert antioxidant activity (Hollman and Katan, 1997).
4.7 Anti-cancer
Cancer is abnormal uncontrolled cell growth which spreads all over the body (metastasis) and cause damage to various organs (Chaffer and Weinberg, 2011). Cancer is caused by various reason like genetics, UV rays, CFC’S, tobacco, PVC, mutation, etc (Blackadar, 2016). Cure for cancer is chemotherapy, radiotherapy, surgery but all these methods have side effects (Baskar et al., 2012; Nurgali et al., 2018). Some studies have indicated that phytochemicals like polyphenol, flavones, flavonoids have anti-cancer activity (Kopustinskiene et al., 2020). One such example is quercetin which has wide range of activity on lung, colon, ovarian, liver, breast, pancreatic, prostate, bladder, gastric, bone cancer, etc (Fig. 3, Table 5) (Asgharian et al., 2022b; Baruah et al., 2016; Rauf et al., 2018).
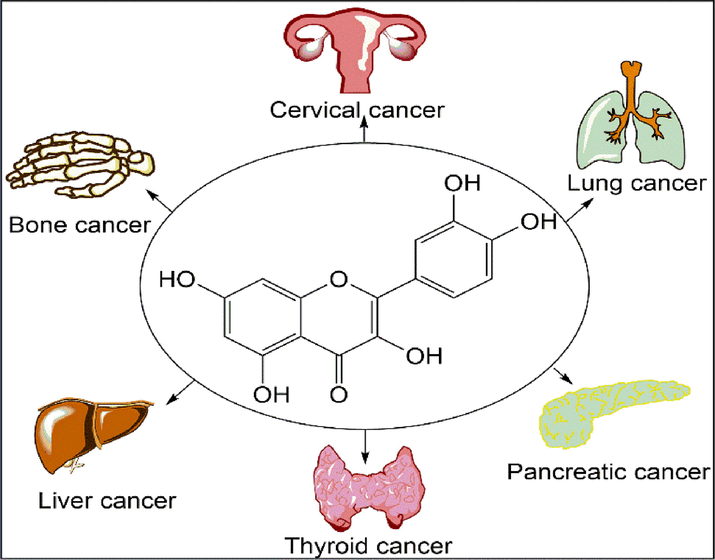
- Quercetin acting on various types of cancer.
| Quercetin and types | Type of cancer | Mechanism | Dose(s) | Model |
|---|---|---|---|---|
| Quercetin | Gastric cancer | Suppresses the growth of gastric cancer in human by provoking mitochondrial-dependent apaptosis by repression of PI3K/Akt signaling | 20 µM | In vitro |
| Quercetin targeted via phenyl boronic acid and zinc oxide nanopatrticles (PBA-ZnO-Q) | Breast cancer | This can induce apoptotic cell death in breast cancer with combination of oxidative stress and mitochondrial damage | 8 µg/ml | In vitro and in vivo |
| Quercetin | Colorectal cancer | Promote 5-fluorouracil-induced apoptosis in MSI CRC cells via p53 regulation | 1 µM, 100 µM | In vitro |
| Quercetin | Oral cancer | Enhanced apoptosis in human oral cancer SAS cells by mitochondria and endoplasmic reticulum-mediated signaling pathways | 40 µM | In vitro |
| Quercetin | Thyroid cancer | Decreases cell proliferation and promoted apoptosis through caspase activation and down regulation of Hsp90 expression | 10 µM | In vitro |
| Quercetin | Lung cancer | Promotes apoptosis in lung cancer via modulation of p53 posttranslational modifications and antiproliferative and antimetastatic effect on A549 non-small cell lung cancer via impact on the cytoskeleton | 100 mg/kg and 74 µM | In vivo and in vitro |
| Quercetin | Pancreatic cancer | Overexpression of microRNA let-7c and suppress pancreatic cancer progression | 50 µM | In vivo and in vitro |
| Gold-quercetin into poly (DL-lactide-co-glycolide) nanoparticles | Liver cancer | Suppress liver cancer progression by AP-2β/hTERT, impeding caspase/cyto-cpathway, inactivating NF-kB/COX-2 | 30–50 µg/ml | In vivo and in vitro |
| Quercetin | Prostate cancer | Converts the doxorubicin resistance of prostate cancer cells via targeting the c-met/P13K/AKT pathway | 10 µM | in vitro |
While acceptable dosages of quercetin are used to treat cancer, there are no detrimental side effects on normal cells (Tang et al., 2020). In vitro and in vivo tests of quercetin's anti-cancer efficacy revealed that it inhibits metastasis and angiogenesis and encourages apoptosis (programmed cell death) (Ingham and Schwartz, 2017).
Due to changes in lifestyle, breast cancer has recently become a more severe issue for women. The active flavonoid quercetin, which inhibits breast cancer by inhibiting signal transduction, also prevents free radicals from impairing low-density lipoproteins, so quercetin, which acts as a lipid peroxidation inhibitor, acts against cancer cells (Ahmed et al., 2021; Ezzati et al., 2020; Wang et al., 2018a). Glioblastoma multiforme (GBM) is a connective tissue malignant tumor, considered the most aggressive type of brain cancer (Afshari et al., 2019; Ostrom et al., 2015). It is important to develop potential treatments for glioblastoma. Quercetin was able to inhibit cell migration (Liu et al., 2017; Tavana et al., 2020). Collectively, gliomas are malignant brain tumors that go untreated despite the availability of multiple therapeutic options. Researchers also found that quercetin inhibits cell viability and migration of gliomas (Pan et al., 2015; Zhang et al., 2015). Lung cancer is the leading cause of death worldwide. The main reason for lung cancer is using tobacco products, smoking, air pollution, radon gas, etc (Lemjabbar-Alaoui et al., 2015). Lung cancer is divided into non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC) (dela Cruz et al., 2011). Quercetin inhibits NSCLC by quercetin-induced mitotic catastrophe, which involves perturbation of mitotic microtubules, which leads to monopolar spindle formation and failure of cytokinesis, which is caused by depletion of actin filaments by quercetin (Klimaszewska-Wiśniewska et al., 2017). Ovarian cancer is a malignant tumor that affects women’s reproductive tract, and it is ranked as the seventh most prevalent female cancer across the world (Vafadar et al., 2020). Since quercetin has two benzene rings joined by a pyrone ring, it acts as two antioxidant pharmacophores and helps in removing free radicals (Dhalaria et al., 2020; Shafabakhsh and Asemi, 2019; Thomasset et al., 2007). Quercetin has a cytotoxic effect on cancer cells through several mechanisms (Vargas and Burd, 2010). Cell cycle arrest is achieved by depolymerization of cellular microtubules when quercetin directly binds to tubulin (Gupta and Panda, 2002). Just like women suffer from ovarian cancer, men also suffer from prostate cancer. Prostate cancer is a common male malignant tumor, and the incident rate of new cases is 27% (Siegel et al., 2014; YANG et al., 2015). It is the second leading malignant tumor, followed by lung cancer; it is uncontrolled growth in the prostate gland (Hussain et al., 2021). Researchers found that the flavonoid quercetin decreases the function of androgen receptors by suppressing their expression (Ghafouri-Fard et al., 2021). Morris et al.(Kim et al., 2019; MORRIS et al., 2006) studied the action of quercetin by isolating prostate cancer cells from affected tissue and treating them with quercetin. Quercetin was able to diminish the primary prostate epithelial cells by reducing DNA synthesis or micro-RNA, where cancer cells are sensitive to chemotherapy. Pancreatic cancer is a common cancer of the gastrointestinal tract (GIT). It is characterized as exocrine pancreas and ductal adenocarcinoma tumors (Asgharian et al., 2021; Scarpa et al., 2018). The possibility of developing pancreatic cancer is 1.5% in both genders but is generally seen in older people between the ages of 70 and 80 (Rahib et al., 2014; Sanabria Mateos and Conlon, 2016). Quercetin inhibits cell proliferation in vitro and in vivo in the MIA PaCa-2 cell line of pancreatic cells (Angst et al., 2013). Pancreatic ductal adenocarcinoma is a highly aggressive type of tumor that has developed resistance to gemcitabine and other cytotoxic drugs, so researchers are finding new ways to develop a potent drug against pancreatic cancer, where quercetin has led them to positive results in pancreatic cancer (Fan et al., 2016; Hasegawa et al., 2014). Considering liver cancer, hepatocellular carcinoma (HCC) is the most frequent cancer (Bréchot et al., 1998). Patients also have a history of liver cirrhosis, which is caused by the Hepatitis B or C virus (Shiratori, 1995; Wu et al., 2019a).There are very few reports on how quercetin proliferates tumors, but when studied with cell lines like KIM-1, KYN-2, KYN-3, HAK-1B, HAK-2, HAK-5, HAK-6, KMCH-1, KMCH-2, they showed a synergic effect when treated with quercetin (HISAKA et al., 2020). Skin cancer is also common now due to changes in lifestyle and the use of harmful chemicals on the skin. Melanoma is a type of skin cancer that develops from neural crest-derived melanocytes (Harris et al., 2016). Melanocytes have a special feature in that they express the oxidative enzyme tyrosinase, which oxidizes quercetin into reactive adducts (Kubo et al., 2007; Metodiewa et al., 1999). Keratinocyte malignancy is generally seen over the face and arms as it is exposed to ultraviolet rays (Narayanan et al., 2010). Non-melanomatous skin cancer (NMSC) is more common than melanomas, but it is less deadly and easier to treat. Treatment with flavonoids can proliferate cancer (Pop and Diaconeasa, 2021). But researchers are working hard to find the effectiveness of quercetin on many types of cancer.
4.8 Anti-allergy
With a sudden change in environment and lifestyle, the human immune system is not able to tolerate even a simple allergen like pollen grains. Different changes in the environment will contribute to this problem, like indoor and outdoor allergens, air pollution, food, water, etc (Kawai et al., 2007; Takano and Inoue, 2017). The immune system reacts quickly when it encounters some allergens, which ultimately leads to the secretion of allergy-related mediators that generate allergic symptoms (Singh et al., 2011a). Decarboxylation of histidine produces histamine by releasing carbon dioxide; once histamine is released, it exerts its function by binding to various types of histamine receptors found in various cells throughout the body, which causes allergy (Maintz and Novak, 2007; Parsons and Ganellin, 2006; Thangam et al., 2018). Quercetin has a potential effect on allergic diseases by inhibiting histamine production. Current therapies such as β2-agonists and corticosteroids are limited due to their side effects, so the effect of quercetin on allergy studies such as asthma and atopic dermatitis has been extensively studied (Djukanovic, 2002; Nakamura et al., 2011; Traub and Murray, 2020; Ward, 2002).
Fewtrell and Gomperts examined the effect of quercetin on secretion from antigen sensitized mast cells and found histamine had an inhibitory effect on histamine secretion mediated by antigen, but when considering ionophores, they had very little effect (Fewtrell and Gomperts, 1977). Quercetin has an inhibitory action on mast cells because of which it causes a regression in the release of tryptase, monocyte chemoattractant protein 1 (MCP-1), and IL-6 (Mlcek et al., 2016; Shaik et al., 2006). Allergic asthma is a chronic inflammatory lung disease. Asthma has symptoms like wheezing, airway hyperresponsiveness, and airway obstruction (He et al., 2020). When analyzed, quercetin had a positive effect when tested in vitro and in vivo by the immediate phase response and the late phase response of an allergic reaction. In the experimental studies, mice were injected intraperitoneally with 8 or 16 mg/kg/day in 200 µl of quercetin every day, and quercetin showed a strong indication of a decrease in allergic airway inflammation and hyperresponsiveness by an anti-allergic effect in the Th1/Th2 immune response (Kumazawa et al., 2014; Lee et al., 2007; Park et al., 2009; Nutakki, 2020). In one study, quercetin was able to inhibit liver fibrosis using a murine model of experimental infection with Schistosoma japonicum; quercetin modulates airway remodeling in asthmatic patients and acts as a bronchodilator (Fortunato et al., 2012; Jafarinia et al., 2020; Townsend and Emala, 2013; Xu et al., 2006). Quercetin exhibits a potent effect on cellular and humoral immune functions in pre-clinical investigation with resveratrol, genistein, and epigallocatchol-3-gallate, which can modulate allergic sensitization and have a direct effect on mast cells, inhibiting mediator release (Jantan et al., 2015; Kimata et al., 2000; Kobori et al., 2016; Marzocchella et al., 2011; Yang et al., 2013). Sometimes quercetin alone may not be able to act against allergies in certain cases, but a combination of drugs can work effectively. Quercetin along with luteolin can suppress β-hexosaminidase activity as an index of anti-allergy activity (Hashimoto et al., 2006; Mizuno et al., 2017; Ueda et al., 2002; Yamashita et al., 2016). In 2017, Luo et al. (Cruz et al., 2012; Luo et al., 2018) worked on various herbs and investigated that an herb with quercetin had a special feature of bronchodilators, or relaxing the airways, in the case of asthma and certain chronic obstructive pulmonary diseases. Similar tests were mast cell-dependent; in vivo studies showed that mice displayed increased airway response. Patients with asthma have mast cells but not T cells or eosinophils that can localize within the bronchial smooth muscle bundles. Several mast cell mediators have unique functions in airway smooth muscle function, where mast cells involved in asthmatic airway smooth muscle involve CXCL10 and CXCR3 (Bradding, 2003; Bradding et al., 2006; Brightling et al., 2005; Christensen et al., 2006; Komolafe and Pacurari, 2022; Reuter et al., 2008; Satarkar and Patra, 2022).
4.9 Anti-depressant
Today's population is significantly impacted by depression, which is a critical global health issue. Depression is a mental disorder that relates to emotion and has neurovegetative symptoms that do not allow a person to focus on anything while damaging the quality of life (Joo, 2017; Lee et al., 2017a; Malhi and Mann, 2018). Recently, the World Health Organization (WHO) reported that worldwide, almost 280 million people will suffer from depression in 2021, and depression can lead to suicide. As every day reports, the suicide rate is increasing due to depression and is mostly seen in teenagers (Goyal et al., 2009; Grover et al., 2010; World Health Organization, 2021). Biologically, depression can be caused by nerve damage, aplastic disorder, inflammation, stress, genetics, and other psychological factors, etc (Delva and Stanwood, 2021; Felger, 2018; Mahar et al., 2014; Ménard et al., 2016; Yohn et al., 2017).
The cure for depression is antidepressants; one such antidepressant is quercetin. The main effect of quercetin was studied in comparison with that of classic antidepressants like fluoxetine and imipramine. Since depression is associated with diabetics, studies revealed that quercetin has the potential to be employed as a therapy for depression associated with diabetes (Anjaneyulu et al., 2003; Erenmemisoglu et al., 2010; Holzmann et al., 2015; Plumb et al., 1999). A Chinese herb like Chaihu Shugansan was able to improve depression, which was much better than fluoxetine, duloxetine, etc. Quercetin with other components can improve depression synergistically, and clinical studies revealed that quercetin monotherapy is required for treating depression (Li et al., 2014; Qin et al., 2013; Wang et al., 2022b; Wang et al., 2012; Wei et al., 2021). When diabetic rats were treated with quercetin, it showed that 50 mg/kg an effective anti-depressant, but when a high dose of 100 mg/kg was used, it was ineffective. This result was obtained when a forced swim test was done on rats after administration of quercetin for 21 days and analyzed by measuring plasma adrenocorticotropic hormone (ACTH) and corticosterone (CORT) levels (Asgharian et al., 2022a; Demir et al., 2016; Kawabata et al., 2010; Said Mohammadi et al., 2014; Vahl et al., 2005). To investigate the protective effect of quercetin on the cell viability of cultured HepG2 (Human Hepatoma) cells that were subjected to stress induced by tert-butyl hydroperoxide, all doses from 0.1 µM to 10 µM of quercetin were given to mice, and it was found that pretreatment with 10 µM worked effectively on stress (Alía et al., 2005; Alía et al., 2006a; Alía et al., 2006b; Choi et al., 2010; Kim et al., 2013). Brain-derived neurotrophic factor (BDNF) is also associated with depression and cardiovascular disease. Estrogen receptor α (ER α) is a receptor that acts against depression and cardiovascular disease. In the tail suspension test (TST) and forced swimming test (FST), it was proven that quercetin exhibits similar behavior to ER α, which acts against depression and cardiovascular disease by reducing immobility (Chen et al., 2008; Hwang et al., 2020; Malacara et al., 2004; Wang et al., 2021; Zhu et al., 2018). Since quercetin is naturally available in many plants, researchers worked on Coccinia indica (Cucurbitaceae), which was already in use in Ayurveda for depression, but claims are still not validated. So, researchers worked by extracting quercetin using methanol, and the extract significantly reduced the duration of immobility at an FST dose of 400 mg/kg which did not affect locomotor activity, which confirmed its antidepressant activity (Randhawa et al., 2015). Already there are a lot of other synthetic drugs in the market for depression like clomipramine, tranylcypromine, and fluoxetine, but all these drugs have some side effects like constipation, dry mouth, restlessness, headaches, etc., because of which scientists are working from a plant source that acts as an anti-depressant and has no side effects (Dziukas and Vohra, 1991; Ghaemi et al., 2001; Majeroni and Hess, 1998; Rahmatullah et al., 2012).
4.10 Anti-arthritic and interstitial cystitis prevention
Arthritis means “disease of the joints.” It is a chronic inflammation that often co-exists with pain and structural damage (Golds et al., 1983; Senthelal et al., 2022). More than 100 different types of arthritis have been reported, of which osteoarthritis is the most common, followed by non-inflammatory arthritis. Sometimes our immune system acts against our body through an autoimmune response like ankylosing spondylitis, rheumatoid arthritis, psoriatic arthritis, etc (Bullock et al., 2018; Walecki, 2013; Zhu et al., 2019). Sometimes arthritis can be caused by infection, like Lyme’s arthritis (Quintero et al., 2021; Tamási and Szekanecz, 2007; Wolfe and Ang, 2017). Quercetin has anti-inflammatory and anti-nociceptive effects. When researchers worked on women with rheumatoid arthritis, quercetin had a positive effect. In clinical studies, 50 women with rheumatoid arthritis were administered quercetin 500 mg/day for about 8 weeks, which reduced early morning stiffness, and joint count because of quercetin (Jackson et al., 2006; Javadi et al., 2017).
As discussed earlier, rheumatoid arthritis is an autoimmune disorder and a chronic inflammatory joint disease with the main pathological conditions of synovial membrane inflammation, an autoimmune response, oxidative stress, bone damage, etc (Aletaha and Smolen, 2018; Choudhary et al., 2018; Gegout et al., 1994; Quiñonez-Flores et al., 2016; Smolen et al., 2016). Studies revealed that quercetin decreased synovial inflammation by reducing joint clinical parameters and inflammatory cytokines and decreasing oxidative stress (Tang et al., 2022). In comparison with quercetin and hesperidin, quercetin had the ability to reduce edema when administered (Emim et al., 2011; Formica and Regelson, 1995; Guardia et al., 2001; Pelzer et al., 1998). Curcumin and quercetin are both antioxidant molecules with a lot of advantages, like being anti-tumor, anti-proliferative, anti-inflammatory, etc. When the inhibitory effects of both were studied on inflammation associated with arthritis using assays like neutrophil activation, synoviocyte function, and angiogenesis, both curcumin and quercetin were able to inhibit neutrophil activation, which thus confirmed that both can be suitable candidates for preclinical evaluation of anti-inflammatory agents (Arora et al., 2015; Banerjee et al., 2003; Bengmark, 2006; Hahm et al., 2004; Hui et al., 1997; Tudan et al., 2003).
Interstitial cystitis is a painful bladder syndrome, a chronic condition with suprapubic pain and chronic inflammation, and patients feel pain mainly in the suprapubic region with a strong sense of urgency to urinate (Gupta et al., 2015; Marcu et al., 2018; Sholan, 2020; Theoharides et al., 2008; Tyagi et al., 2018). Frequent urination may occur at night or during the day, with pain when passing urine (dysuria) and pain during sexual intercourse (dyspareunia) (Cvach and Rosamilia, 2015). Recently, it was proven that the oral bioflavonoid form of quercetin is clinically effective in men with chronic pelvic pain syndrome disorders like interstitial cystitis. Similar studies were done on interstitial cystitis in 22 patients and documented that the Cysta-Q complex (equivalent to 500 mg quercetin) for four weeks caused improvement in the patients (FORREST and SCHMIDT, 2004; Katske et al., 2001; Nickel, 2004; Winter et al., 2015). Acute cystitis is treated with antibiotic agents, but only a small amount reaches the bladder, so to reach antibiotics in the bladder, intravesical administration is required, avoiding the first-pass effect (Alidjanov et al., 2018; di Vico et al., 2020). Quercetin, when encapsulated in a micelle, which is a biodegradable nanoparticle, is a safe and efficient intravenous drug delivery system. Quercetin was able to reduce inflammatory cell infiltration of bladders with acute cystitis (Gou and Wang, 2012; Lee and Cima, 2011).
5 Drawbacks of quercetin
Despite having so many applications, mainly anti-tumor, anti-diabetic, anti-inflammatory, etc., due to the drawbacks of quercetin, the therapeutic index is limited in pharmacological applications. Since quercetin is hydrophobic in nature, it is difficult to administer it orally (Guo and Bruno, 2015; Li et al., 2009). Oral bioavailability has been illustrated to be less than 17% in animals, and in humans it is less than 2% (Gao et al., 2011; Sak, 2014). However, in natural food sources where quercetin is attached to glycoside, for example, in the case of onions, where quercetin is attached to glucose to form quercetin-3-glucoside, quercetin glucoside is more bioavailable than quercetin aglycone because of its water-soluble nature based on the octanol–water partition coefficient; however, if given to humans, a specific dosage is required (Guo et al., 2014; Lee and Mitchell, 2012; Rothwell et al., 2013; Wolffram et al., 2002). Quercetin undergoes the first-pass effect, i.e., quercetin is metabolized in the liver once it is entered into our body due to its poor absorption in the gastrointestinal tract since it is surrounded by a mucus layer with 90% water content, and it undergoes the first-pass effect (gastrointestinal instability) (Date et al., 2011; Li et al., 2021a; Macierzanka et al., 2011; NIELSEN et al., 1998). Quercetin is also associated with some drawbacks like headaches, stomachaches, and a high dosage of quercetin can lead to kidney related problems (Hu et al., 2010; Mulholland et al., 2001). Scientists are still working on its bioavailability, water solubility, first-pass effect, stability, and no side effects on humans.
5.1 How to overcome the drawbacks of quercetin
Quercetin has poor permeability and instability; numerous approaches have been undertaken that help in drug delivery (Cai et al., 2013; Ersoz et al., 2020). To ensure complete safety, polymeric nanoparticles can act as a carrier of quercetin, and nanoparticles should have the following properties: being non-toxic, protecting the drug in the gastrointestinal tract, not altering the properties of the drug, increasing bioavailability, reaching the specific target, having a prolonged sustained release profile from the nanoparticle, controlled release, and being biodegradable (Bilia et al., 2014; Chiu et al., 2012; Khosravi Zanjani et al., 2014; Petri, 2015; Sezgin et al., 2007; Zhang et al., 2008). Fungi called Penicillium brevicompactum produce a secondary metabolite called mycophenolic acid, which, along with liposomes and quercetin, helps target breast cancer (Patel et al., 2020, 2016; Puel et al., 2005). To enhance its poor aqueous solubility and stability, quercetin was encapsulated in polymeric micelles. Polymeric micelles are an excellent candidate for drug delivery in cancer (Dian et al., 2014; Hamaguchi et al., 2005). In certain other cases, cancer-associated fibroblasts (CAFs) are stromal cells in the tumor environment that create a physical barrier for drugs to penetrate the tumor, making drug delivery difficult. In such cases, the liposome system of quercetin helps in the sequential delivery of drugs to the target (Duan et al., 2022; Lv et al., 2018a, 2018b; Munot et al., 2022; Tran et al., 2014; Truffi et al., 2019). Synthesizing various derivatives of quercetin, which is soluble in aqueous medium, helps in drug delivery (Kashyap et al., 2016). The metal complex of quercetin acts as a more potent anti-cancer agent than quercetin itself (Massi et al., 2017; Uivarosi and Munteanu, 2017). It was possible to increase the aqueous solubility of quercetin with rebaudioside or rubusoside treatment without altering its properties. As Ru concentration increases, the solubility of quercetin in aqueous medium also increases (Kumari et al., 2011; Nguyen et al., 2015). Nanotechnology and nanoparticle carriers have a unique property in drug delivery that helps quercetin reach the target and perform its function (Fig. 4) (Couvreur and Vauthier, 2006; Curcio et al., 2012).
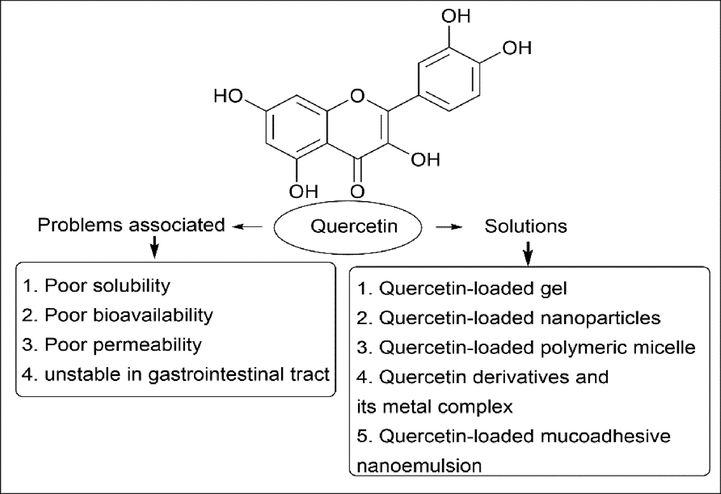
- Problems associated with quercetin and solutions.
6 Conclusion
Quercetin is a flavonoid with many applications, but it is still not recognized in the field of pharmacology due to the drawbacks associated with it. Researchers are working to improve its bioavailability, aqueous solubility, avoiding first pass effect, etc. The synthesis of derivatives that are soluble in water, the synthesis of metal complex and checking their therapeutic action on various diseases are being done. Researchers have found polymeric or metal nanoparticle conjugation of quercetin is best for various conditions, including cancer treatment. In the future, quercetin can be conjugated or encapsulated with some biopolymer, or the synthesis of quercetin derivatives that are hydrophilic, stable in the gastrointestinal tract, etc., can be used in the application of drug delivery that can overcome the drawbacks of quercetin and act as a potent drug.
Acknowledgments
Special thanks to Vellore Institute of Technology for the support. We would also thank the anonymous reviewers for their constructive comments.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Antioxidative Properties and Effect of Quercetin and Its Glycosylated Form (Rutin) on Acetylcholinesterase and Butyrylcholinesterase Activities. J. Evid Based Complementary Altern. Med.. 2016;21:NP11-NP17.
- [CrossRef] [Google Scholar]
- Auraptene-induced cytotoxicity mechanisms in human malignant glioblastoma (U87) cells: role of reactive oxygen species (ROS) Excli J.. 2019;18:576-590.
- [CrossRef] [Google Scholar]
- Flavonoids as antioxidants in plants: Location and functional significance. Plant Sci.. 2012;196:67-76.
- [CrossRef] [Google Scholar]
- Pharmacological activities of flavonoids: a review. Int. J. Pharm. Sci. Nanotechnol.. 2011;4:1394-1398.
- [CrossRef] [Google Scholar]
- Quercetin: antiviral significance and possible COVID-19 integrative considerations. Nat. Prod. Commun.. 2020;15 1934578X2097629
- [CrossRef] [Google Scholar]
- Therapeutic potential of flavonoids and their mechanism of action against microbial and viral infections—A review. Food Res. Int.. 2015;77:221-235.
- [CrossRef] [Google Scholar]
- Quercetin offers chemopreventive potential against breast cancer by targeting a network of signalling pathways. Res. J. Pharm. Technol. 2021:2829-2839.
- [CrossRef] [Google Scholar]
- Characterization of dihydroflavonol 4-reductase (DFR) genes and their association with cold and freezing stress in Brassica rapa. Gene. 2014;550:46-55.
- [CrossRef] [Google Scholar]
- Inflammatory process in Alzheimer’s and Parkinson’s diseases: central role of cytokines. Curr. Pharm. Des.. 2016;22:541-548.
- [CrossRef] [Google Scholar]
- Diagnosis and Management of Rheumatoid Arthritis. J. Am. Med. Assoc.. 2018;320:1360.
- [CrossRef] [Google Scholar]
- Response of the antioxidant defense system to tert -butyl hydroperoxide and hydrogen peroxide in a human hepatoma cell line (HepG2) J. Biochem. Mol. Toxicol.. 2005;19:119-128.
- [CrossRef] [Google Scholar]
- Influence of quercetin and rutin on growth and antioxidant defense system of a human hepatoma cell line (HepG2) Eur. J. Nutr.. 2006;45:19-28.
- [CrossRef] [Google Scholar]
- Quercetin protects human hepatoma HepG2 against oxidative stress induced by tert-butyl hydroperoxide. Toxicol. Appl. Pharmacol.. 2006;212:110-118.
- [CrossRef] [Google Scholar]
- Reevaluation of the acute cystitis symptom score, a self-reporting questionnaire. Part I. Dev. Diagnosis Differential Diagnosis. Antibiotics. 2018;7:6.
- [CrossRef] [Google Scholar]
- Bioavailability of quercetin in humans with a focus on interindividual variation. Compr. Rev. Food Sci. Food Saf.. 2018;17:714-731.
- [CrossRef] [Google Scholar]
- Application of quercetin in neurological disorders: from nutrition to nanomedicine. Rev. Neurosci.. 2019;30:555-572.
- [CrossRef] [Google Scholar]
- Overviews of biological importance of quercetin: a bioactive flavonoid. Pharmacogn. Rev.. 2016;10:84.
- [CrossRef] [Google Scholar]
- Overviews of biological importance of quercetin: a bioactive flavonoid. Pharmacogn. Rev. 2016
- [CrossRef] [Google Scholar]
- The flavonoid quercetin inhibits pancreatic cancer growth in vitro and in vivo. Pancreas. 2013;42:223-229.
- [CrossRef] [Google Scholar]
- Antidepressant activity of quercetin, a bioflavonoid, in streptozotocin-induced diabetic mice. J. Med. Food. 2003;6:391-395.
- [CrossRef] [Google Scholar]
- Antimicrobial activity of fullerenes and their hydroxylated derivatives. Biocontrol Sci.. 2009;14:69-72.
- [CrossRef] [Google Scholar]
- Dietary intakes of flavonols, flavones and isoflavones by japanese women and the inverse correlation between quercetin intake and plasma LDL cholesterol concentration. J. Nutr.. 2000;130:2243-2250.
- [CrossRef] [Google Scholar]
- Curcumin loaded solid lipid nanoparticles ameliorate adjuvant-induced arthritis in rats. Eur. J. Pain. 2015;19:940-952.
- [CrossRef] [Google Scholar]
- Arredondo, F., Echeverry, C., Blasina, F., Vaamonde, L., Díaz, M., Rivera, F., Martínez, M., Abin-Carriquiry, J.A., Dajas, F., 2015. Flavones and flavonols in brain and disease, in: bioactive nutraceuticals and dietary supplements in neurological and brain disease. Elsevier, pp. 229–236. https://doi.org/10.1016/B978-0-12-411462-3.00025-4.
- Quercetin impact in pancreatic cancer: an overview on its therapeutic effects. Oxid. Med. Cell. Longev.. 2021;2021:1-13.
- [CrossRef] [Google Scholar]
- Pharmacological effects and therapeutic potential of natural compounds in neuropsychiatric disorders: an update. Front. Pharmacol.. 2022;13
- [CrossRef] [Google Scholar]
- Potential mechanisms of quercetin in cancer prevention: focus on cellular and molecular targets. Cancer Cell Int.. 2022;22:257.
- [CrossRef] [Google Scholar]
- The relationship between plasma levels of oxidized and reduced thiols and early atherosclerosis in healthy adults. J. Am. Coll. Cardiol.. 2006;47:1005-1011.
- [CrossRef] [Google Scholar]
- An insight into anticancer, antioxidant, antimicrobial, antidiabetic, and anti-inflammatory effects of quercetin: a review. Polym. Bull. 2022
- [CrossRef] [Google Scholar]
- Quercetin in food: possible mechanisms of its effect on memory. J. Food Sci.. 2018;83:2280-2287.
- [CrossRef] [Google Scholar]
- Suppression of hepatitis C virus by the flavonoid quercetin is mediated by inhibition of NS3 protease activity. J. Viral Hepat.. 2012;19:e81-e88.
- [CrossRef] [Google Scholar]
- Protective effect of lupeol against lipopolysaccharide-induced neuroinflammation via the p38/c-Jun N-terminal kinase pathway in the adult mouse brain. J. Neuroimmune Pharmacol.. 2016;11:48-60.
- [CrossRef] [Google Scholar]
- Activity of catechins and their applications. Biomed. Dermatol.. 2020;4:8.
- [CrossRef] [Google Scholar]
- I S S N 2278–4357 a review of quercetin: antioxidant and anticancer properties. World J. Pharm. Pharm. Sci.. 2012;1
- [Google Scholar]
- Antihypertensive properties of flavonoid-rich apple peel extract. Food Chem.. 2012;135:2320-2325.
- [CrossRef] [Google Scholar]
- Modulation of inflammatory mediators by ibuprofen and curcumin treatment during chronic inflammation in rat. Immunopharmacol. Immunotoxicol.. 2003;25:213-224.
- [CrossRef] [Google Scholar]
- Quercetin modulates Wnt signaling components in prostate cancer cell line by inhibiting cell viability, migration, and metastases. Tumor Biol.. 2016;37:14025-14034.
- [CrossRef] [Google Scholar]
- Cancer and radiation therapy: current advances and future directions. Int. J. Med. Sci.. 2012;9:193-199.
- [CrossRef] [Google Scholar]
- The pharmacological activity, biochemical properties, and pharmacokinetics of the major natural polyphenolic flavonoid: quercetin. Foods. 2020;9:374.
- [CrossRef] [Google Scholar]
- The potential neuroprotective role of free and encapsulated quercetin mediated by mirna against neurological diseases. Nutrients. 2021;13:1318.
- [CrossRef] [Google Scholar]
- Curcumin, an atoxic antioxidant and natural NFκB, Cyclooxygenase-2, Lipooxygenase, and inducible nitric oxide synthase inhibitor: a shield against acute and chronic diseases. J. Parenter. Enteral Nutr.. 2006;30:45-51.
- [CrossRef] [Google Scholar]
- Flavonoids loaded in nanocarriers: an opportunity to increase oral bioavailability and bioefficacy. Food Nutr. Sci.. 2014;05:1212-1327.
- [CrossRef] [Google Scholar]
- Quercetin: potentials in the prevention and therapy of disease. Curr. Opin. Clin. Nutr. Metab. Care. 2008;11:733-740.
- [CrossRef] [Google Scholar]
- Amyloid β-protein (Aβ) assembly: Aβ40 and Aβ42 oligomerize through distinct pathways. Proc. Natl. Acad. Sci.. 2003;100:330-335.
- [CrossRef] [Google Scholar]
- Historical review of the causes of cancer. World J Clin Oncol. 2016;7:54.
- [CrossRef] [Google Scholar]
- Antioxidants, oxidative damage and oxygen deprivation stress: a review. Ann. Bot.. 2003;91:179-194.
- [CrossRef] [Google Scholar]
- Bolca, S., 2014. Bioavailability of Soy-Derived Isoflavones and Human Breast Cancer, in: Polyphenols in Human Health and Disease. Elsevier, pp. 1241–1256. https://doi.org/10.1016/B978-0-12-398456-2.00094-3.
- Alzheimer’s disease: past, present, and future. J. Int. Neuropsychol. Soc.. 2017;23:818-831.
- [CrossRef] [Google Scholar]
- The efficacy of quercetin in cardiovascular health. Curr. Nutr. Rep.. 2015;4:290-303.
- [CrossRef] [Google Scholar]
- Health effects of quercetin: from antioxidant to nutraceutical. Eur. J. Pharmacol.. 2008;585:325-337.
- [CrossRef] [Google Scholar]
- In vitro and ex vivo anti-inflammatory activity of quercetin in healthy volunteers. Nutrition. 2008;24:703-710.
- [CrossRef] [Google Scholar]
- Identification of flavonoid and phenolic antioxidants in black currants, blueberries, raspberries, red currants, and cranberries. J. Agric. Food Chem.. 2010;58:3901-3909.
- [CrossRef] [Google Scholar]
- Bors, W., Heller, W., Michel, C., Saran, M., 1990. [36] Flavonoids as antioxidants: Determination of radical-scavenging efficiencies. pp. 343–355. https://doi.org/10.1016/0076-6879(90)86128-I.
- The role of the mast cell in asthma: a reassessment. Curr. Opin. Allergy Clin. Immunol.. 2003;3:45-50.
- [CrossRef] [Google Scholar]
- The role of the mast cell in the pathophysiology of asthma. J. Allergy Clin. Immunol.. 2006;117:1277-1284.
- [CrossRef] [Google Scholar]
- Naturally occurring flavanones: an overview. Nat. Prod. Commun.. 2008;3 1934578X0800300
- [CrossRef] [Google Scholar]
- Metal complexes of the flavonoid quercetin: antibacterial properties. Transit. Met. Chem.. 2001;26
- [CrossRef] [Google Scholar]
- Impact of HBV, HCV and GBV-C/HGV on hepatocellular carcinomas in Europe: results of a European concerted action. J. Hepatol.. 1998;29:173-183.
- [CrossRef] [Google Scholar]
- The CXCL10/CXCR3 Axis mediates human lung mast cell migration to asthmatic airway smooth muscle. Am. J. Respir. Crit. Care Med.. 2005;171:1103-1108.
- [CrossRef] [Google Scholar]
- Early inflammatory cytokine response: a direct comparison between spontaneous coronary plaque destabilization vs angioplasty induced. Atherosclerosis. 2014;236:456-460.
- [CrossRef] [Google Scholar]
- Synthesis, characterization and antioxidant activity copper–quercetin complex. Spectrochim. Acta A Mol. Biomol. Spectrosc.. 2009;71:1901-1906.
- [CrossRef] [Google Scholar]
- Antidiabetic effect of quercetin: a systematic review and meta-analysis of animal studies. Food Chem. Toxicol.. 2019;125:494-502.
- [CrossRef] [Google Scholar]
- Rheumatoid arthritis: a brief overview of the treatment. Med. Princ. Pract.. 2018;27:501-507.
- [CrossRef] [Google Scholar]
- Synthesis and membrane-protective properties of aminomethyl derivatives of quercetin at the C-8 position. Chem. Papers. 2018;72:201-208.
- [CrossRef] [Google Scholar]
- Bioavailability of quercetin: problems and promises. Curr. Med. Chem.. 2013;20:2572-2582.
- [CrossRef] [Google Scholar]
- Anti-Inflammatory compounds of plant origin. Part I. Action on arachidonic acid pathway, nitric oxide and nuclear factor κ B (NF-κB) Planta Med. 2003
- [CrossRef] [Google Scholar]
- Anti-inflammatory compounds of plant origin. Part II. Modulation of pro-inflammatory cytokines, chemokines and adhesion molecules. Planta Med. 2004
- [CrossRef] [Google Scholar]
- Diagnosis and classification of autoimmune diabetes mellitus. Autoimmun. Rev.. 2014;13:403-407.
- [CrossRef] [Google Scholar]
- In vitro inhibition of canine distemper virus by flavonoids and phenolic acids: Implications of structural differences for antiviral design. Res. Vet. Sci.. 2013;95:717-724.
- [CrossRef] [Google Scholar]
- Chalcogen effects on the primary antioxidant activity of chrysin and quercetin. New J. Chem.. 2020;44:9073-9082.
- [CrossRef] [Google Scholar]
- Chemical studies of anthocyanins: a review. Food Chem.. 2009;113:859-871.
- [CrossRef] [Google Scholar]
- A perspective on cancer cell metastasis. Science. 2011;1979(331):1559-1564.
- [CrossRef] [Google Scholar]
- Biological activities and role of flavonoids in human health - a review. Indian J. Sci. Res.. 2017;12
- [Google Scholar]
- Inhibitory effects of flavonoids on xanthine oxidase. Anticancer Res.. 1993;13:2165-2170.
- [Google Scholar]
- Lipase immobilized on magnetic hierarchically porous carbon materials as a versatile tool for the synthesis of bioactive quercetin derivatives. Bioresour. Technol. Rep.. 2020;9
- [CrossRef] [Google Scholar]
- Quercetin reduces the transcriptional activity of NF-kB in stable coronary artery disease. Indian Heart J.. 2018;70:593-597.
- [CrossRef] [Google Scholar]
- CiteSpace II: Detecting and visualizing emerging trends and transient patterns in scientific literature. J. Am. Soc. Inf. Sci. Technol.. 2006;57:359-377.
- [CrossRef] [Google Scholar]
- Quercetin attenuates cardiac hypertrophy by inhibiting mitochondrial dysfunction through SIRT3/PARP-1 pathway. Front. Pharmacol.. 2021;12
- [CrossRef] [Google Scholar]
- Therapeutic Effects of Quercetin on Inflammation, Obesity, and Type 2 Diabetes. Mediators Inflamm.. 2016;2016:1-5.
- [CrossRef] [Google Scholar]
- Pharmacokinetics and modeling of quercetin and metabolites. Pharm. Res.. 2005;22:892-901.
- [CrossRef] [Google Scholar]
- New horizons in the roles and associations of COX-2 and novel natural inhibitors in cardiovascular diseases. Mol. Med.. 2021;27:123.
- [CrossRef] [Google Scholar]
- The Function and Catalysis of 2-Oxoglutarate-Dependent Oxygenases Involved in Plant Flavonoid Biosynthesis. Int. J. Mol. Sci.. 2014;15:1080-1095.
- [CrossRef] [Google Scholar]
- The role of quercetin, flavonols and flavones in modulating inflammatory cell function. Inflamm. Allergy Drug Targets. 2010;9:263-285.
- [CrossRef] [Google Scholar]
- Role of quercetin in vascular physiology. Can. J. Physiol. Pharmacol.. 2012;90:1652-1657.
- [CrossRef] [Google Scholar]
- Perorally active nanomicellar formulation of quercetin in the treatment of lung cancer. Int. J. Nanomed.. 2012;651
- [CrossRef] [Google Scholar]
- Quercetin suppresses proinflammatory cytokines production through MAP kinases and NF-κB pathway in lipopolysaccharide-stimulated macrophage. Mol. Cell. Biochem.. 2003;243:153-160.
- [CrossRef] [Google Scholar]
- Antiviral activity of quercetin 7-rhamnoside against porcine epidemic diarrhea virus. Antiviral Res.. 2009;81:77-81.
- [CrossRef] [Google Scholar]
- A Tocotrienol-Rich fraction from grape seeds inhibits oxidative stress induced by tert -Butyl hydroperoxide in HepG2 cells. J. Med. Food. 2010;13:1240-1246.
- [CrossRef] [Google Scholar]
- Novel quercetin derivative of 3,7-Dioleylquercetin shows less toxicity and highly potent tyrosinase inhibition activity. Int. J. Mol. Sci.. 2021;22:4264.
- [CrossRef] [Google Scholar]
- Anti-ageing and rejuvenating effects of quercetin. Exp. Gerontol.. 2010;45:763-771.
- [CrossRef] [Google Scholar]
- Experimental animal models for rheumatoid arthritis. Immunopharmacol. Immunotoxicol.. 2018;40:193-200.
- [CrossRef] [Google Scholar]
- CXCL10 Is the Key Ligand for CXCR3 on CD8 + Effector T Cells Involved in Immune Surveillance of the Lymphocytic Choriomeningitis Virus-Infected Central Nervous System. J. Immunol.. 2006;176:4235-4243.
- [CrossRef] [Google Scholar]
- Degradation of misfolded proteins in neurodegenerative diseases: therapeutic targets and strategies. Exp. Mol. Med.. 2015;47:e147-e.
- [CrossRef] [Google Scholar]
- In vivo quercitrin anti-inflammatory effect involves release of quercetin, which inhibits inflammation through down-regulation of the NF-κB pathway. Eur. J. Immunol.. 2005;35:584-592.
- [CrossRef] [Google Scholar]
- Flavonoids-Chemistry, metabolism, cardioprotective effects, and dietary sources. J. Nutr. Biochem.. 1996;7:66-76.
- [CrossRef] [Google Scholar]
- 1-Benzopyran-4-one Antioxidants as Aldose Reductase Inhibitors. J. Med. Chem.. 1999;42:1881-1893.
- [CrossRef] [Google Scholar]
- Nanotechnology: intelligent design to treat complex disease. Pharm. Res.. 2006;23:1417-1450.
- [CrossRef] [Google Scholar]
- Definition, epidemiology, and classification of diabetes in children and adolescents. Pediatr. Diabetes. 2009;10:3-12.
- [CrossRef] [Google Scholar]
- Kalanchoe pinnata inhibits mast cell activation and prevents allergic airway disease. Phytomedicine. 2012;19:115-121.
- [CrossRef] [Google Scholar]
- Quercetin-Imprinted nanospheres as novel drug delivery devices. J Funct Biomater. 2012;3:269-282.
- [CrossRef] [Google Scholar]
- Review of intravesical therapies for bladder pain syndrome/interstitial cystitis. Transl. Androl. Urol.. 2015;4:629-637.
- [CrossRef] [Google Scholar]
- Evidence for Quercetin as a Dietary Supplement for the Treatment of Cardio-Metabolic Diseases in Pregnancy: A Review in Rodent Models. Foods. 2022;11:2772.
- [CrossRef] [Google Scholar]
- Synthesis of quercetin-metal complexes, in vitro and in silico anticholinesterase and antioxidant evaluation, and in vivo toxicological and anxiolitic activities. Neurotox. Res.. 2020;37:893-903.
- [CrossRef] [Google Scholar]
- Trends in the prevalence of ketoacidosis at diabetes diagnosis: the SEARCH for diabetes in youth study. Pediatrics. 2014;133:e938-e945.
- [CrossRef] [Google Scholar]
- Therapeutic potential of quercetin to alleviate endothelial dysfunction in age-related cardiovascular diseases. Front. Cardiovasc. Med.. 2021;8
- [CrossRef] [Google Scholar]
- Life or death: Neuroprotective and anticancer effects of quercetin. J. Ethnopharmacol.. 2012;143:383-396.
- [CrossRef] [Google Scholar]
- Quercetin in brain diseases: Potential and limits. Neurochem. Int.. 2015;89:140-148.
- [CrossRef] [Google Scholar]
- Lipophilization of flavonoids for their food, therapeutic and cosmetic applications. Acta Chimica Slovaca. 2012;5:59-69.
- [CrossRef] [Google Scholar]
- Enzymatic synthesis of novel quercetin sialyllactoside derivatives. Nat. Prod. Res.. 2019;33:1944-1952.
- [CrossRef] [Google Scholar]
- Lecithin-based novel cationic nanocarriers (Leciplex) II: improving therapeutic efficacy of quercetin on oral administration. Mol. Pharm.. 2011;8:716-726.
- [CrossRef] [Google Scholar]
- Design of quercetin-loaded natural oil-based nanostructured lipid carriers for the treatment of bacterial skin infections. Molecules. 2022;27:8818.
- [CrossRef] [Google Scholar]
- Tissue distribution of quercetin in rats and pigs. J. Nutr.. 2005;135:1718-1725.
- [CrossRef] [Google Scholar]
- Selective inhibitors of picornavirus replication. Med. Res. Rev.. 2008;28:823-884.
- [CrossRef] [Google Scholar]
- Antioxidant properties of complexes of flavonoids with metal ions. Redox Rep.. 2004;9:97-104.
- [CrossRef] [Google Scholar]
- Lung cancer: epidemiology, etiology, and prevention. Clin. Chest Med.. 2011;32:605-644.
- [CrossRef] [Google Scholar]
- Dysregulation of brain dopamine systems in major depressive disorder. Exp. Biol. Med.. 2021;246:1084-1093.
- [CrossRef] [Google Scholar]
- Antidepressant-like effects of quercetin in diabetic rats are independent of hypothalamic–pituitary–adrenal axis. Acta Neuropsychiatr. 2016;28:23-30.
- [CrossRef] [Google Scholar]
- Therapeutic potential of quercetin as an antiatherosclerotic agent in atherosclerotic cardiovascular disease: a review. Evid. Based Complement. Alternat. Med.. 2020;2020:1-12.
- [CrossRef] [Google Scholar]
- Bioactive compounds of edible fruits with their anti-aging properties: a comprehensive review to prolong human life. Antioxidants. 2020;9:1123.
- [CrossRef] [Google Scholar]
- Quercetin and its derivates as antiviral potentials: a comprehensive review. Phytother. Res.. 2022;36:266-278.
- [CrossRef] [Google Scholar]
- Acute Cystitis Symptom Score (ACSS): clinical validation of the Italian version. Antibiotics. 2020;9:104.
- [CrossRef] [Google Scholar]
- Enhancing oral bioavailability of quercetin using novel soluplus polymeric micelles. Nanoscale Res. Lett.. 2014;9:684.
- [CrossRef] [Google Scholar]
- Plant flavonoids: chemical characteristics and biological activity. Molecules. 2021;26:5377.
- [CrossRef] [Google Scholar]
- Mechanisms of kidney cell pyroptosis in chronic kidney disease and the effects of traditional chinese medicine. Evid. Based Complement. Alternat. Med.. 2021;2021:1-10.
- [CrossRef] [Google Scholar]
- Airway inflammation in asthma and its consequences: Implications for treatment in children and adults. J. Allergy Clin. Immunol.. 2002;109:S539-S548.
- [CrossRef] [Google Scholar]
- Characterization of insulin secretion and resistance in Type 2 diabetes of adolescents. J. Clin. Endocrinol. Metab.. 2006;91:401-404.
- [CrossRef] [Google Scholar]
- Sequential delivery of quercetin and paclitaxel for the fibrotic tumor microenvironment remodeling and chemotherapy potentiation via a dual-targeting hybrid micelle-in-liposome system. ACS Appl. Mater. Interfaces. 2022;14:10102-10116.
- [CrossRef] [Google Scholar]
- Synthesis of regioselectively acylated quercetin analogues with improved antiplatelet activity. Mol. Med. Rep.. 2017;16:9735-9740.
- [CrossRef] [Google Scholar]
- Vasodilator effects of quercetin in isolated rat vascular smooth muscle. Eur. J. Pharmacol.. 1993;239:1-7.
- [CrossRef] [Google Scholar]
- Vasodilatory effects of flavonoids in rat aortic smooth muscle. Structure-activity relationships. Gen. Pharmacol. Vasc. S.. 1993;24:857-862.
- [CrossRef] [Google Scholar]
- Antihypertensive effects of the flavonoid quercetin in spontaneously hypertensive rats. Br. J. Pharmacol.. 2001;133:117-124.
- [CrossRef] [Google Scholar]
- The occurrence of flavonoids and related compounds in flower sections of papaver nudicaule. Plants. 2016;5:28.
- [CrossRef] [Google Scholar]
- Quercetin reduces blood pressure in hypertensive subjects1. J. Nutr.. 2007;137:2405-2411.
- [CrossRef] [Google Scholar]
- Quercetin reduces systolic blood pressure and plasma oxidised low-density lipoprotein concentrations in overweight subjects with a high-cardiovascular disease risk phenotype: a double-blinded, placebo-controlled cross-over study. Br. J. Nutr.. 2009;102:1065-1074.
- [CrossRef] [Google Scholar]
- Which sources of flavonoids: complex diets or dietary supplements? Adv. Nutr.. 2011;2:8-14.
- [CrossRef] [Google Scholar]
- Pharmacological evaluation of the anti-inflammatory activity of a citrus bioflavonoid, hesperidin, and the isoflavonoids, duartin and claussequinone, in rats and mice. J. Pharm. Pharmacol.. 2011;46:118-122.
- [CrossRef] [Google Scholar]
- Determination of flavone C-glycosides in tea. Zeitschrift fur Lebensmittel-Untersuchung und -Forschung. 1993;197:239-244.
- [CrossRef] [Google Scholar]
- Effect of some antidepressants on glycaemia and insulin levels of normoglycaemic and alloxan-induced hyperglycaemic mice. J. Pharm. Pharmacol.. 2010;51:741-743.
- [CrossRef] [Google Scholar]
- Quercetin-loaded nanoparticles enhance cytotoxicity and antioxidant activity on C6 glioma cells. Pharm. Dev. Technol.. 2020;25:757-766.
- [CrossRef] [Google Scholar]
- A review on anti-cancer properties of Quercetin in breast cancer. Life Sci.. 2020;248:117463
- [CrossRef] [Google Scholar]
- Flavonoids: biosynthesis, biological functions, and biotechnological applications. Front. Plant Sci.. 2012;3
- [CrossRef] [Google Scholar]
- Continuous exposure of pancreatic cancer cells to dietary bioactive agents does not induce drug resistance unlike chemotherapy. Cell Death Dis.. 2016;7:e2246-e.
- [CrossRef] [Google Scholar]
- Advances in microbial heterologous production of flavonoids. Afr. J. Microbiol. Res.. 2011;5:2566-2574.
- [CrossRef] [Google Scholar]
- Flavonoid transport across RBE4 cells: A blood-brain barrier model. Cell. Mol. Biol. Lett.. 2010;15
- [CrossRef] [Google Scholar]
- Felger, J.C., 2018. Role of Inflammation in Depression and Treatment Implications. pp. 255–286. https://doi.org/10.1007/164_2018_166.
- 3-(3-Hydroxyphenyl)propionic acid, a microbial metabolite of quercetin, inhibits monocyte binding to endothelial cells via modulating E-selectin expression. Fitoterapia. 2022;156:105071
- [CrossRef] [Google Scholar]
- Progression to Diabetes in Relatives of Type 1 Diabetic Patients: Mechanisms and Mode of Onset. Diabetes. 2010;59:679-685.
- [CrossRef] [Google Scholar]
- Quercetin: A novel inhibitors of Ca2+ influx and exocytosis in rat peritoneal mast cells. Biochimica et Biophysica Acta (BBA) -. Biomembranes. 1977;469:52-60.
- [CrossRef] [Google Scholar]
- Endothelium-dependent vasorelaxing activity of wine and other grape products. Am. J. Physiol.-Heart Circulatory Physiol.. 1993;265:H774-H778.
- [CrossRef] [Google Scholar]
- Chelation in metal intoxication. Int. J. Environ. Res. Public Health. 2010;7:2745-2788.
- [CrossRef] [Google Scholar]
- Review of the biology of quercetin and related bioflavonoids. Food Chem. Toxicol.. 1995;33:1061-1080.
- [CrossRef] [Google Scholar]
- Interstitial cystitis, chronic nonbacterial prostatitis and chronic pelvic pain syndrome in men: a common and frequently identical clinical entity. J. Urol.. 2004;172:2561-2562.
- [CrossRef] [Google Scholar]
- Quercetin: a flavonoid with the potential to treat asthma. Brazilian J. Pharm. Sci.. 2012;48:589-599.
- [CrossRef] [Google Scholar]
- Anti-inflammatory and anti-arthritic activity of a methanol extract from Vitellaria paradoxa stem bark. Pharmacognosy Res.. 2015;7:367.
- [CrossRef] [Google Scholar]
- Soy and legume seeds as sources of isoflavones: selected individual determinants of their consumption in a group of perimenopausal women. Menopausal Rev.. 2014;1:27-31.
- [CrossRef] [Google Scholar]
- Food antioxidants: chemical insights at the molecular level. Annu. Rev. Food Sci. Technol.. 2016;7:335-352.
- [CrossRef] [Google Scholar]
- Pathophysiology of Type 2 Diabetes Mellitus. Int. J. Mol. Sci.. 2020;21:6275.
- [CrossRef] [Google Scholar]
- Definition, epidemiology, risk factors. Diabetes Metab.. 2010;36:628-651.
- [CrossRef] [Google Scholar]
- Preparation of a chemically stable quercetin formulation using nanosuspension technology. Int. J. Pharm.. 2011;404:231-237.
- [CrossRef] [Google Scholar]
- Ameliorative effects of baicalein in MPTP-induced mouse model of Parkinson’s disease: A microarray study. Pharmacol. Biochem. Behav. 2015;133:155-163.
- [CrossRef] [Google Scholar]
- Quercetin assists fluconazole to inhibit biofilm formations of fluconazole-resistant candida albicans in in vitro and in vivo antifungal managements of vulvovaginal candidiasis. Cell. Physiol. Biochem.. 2016;40:727-742.
- [CrossRef] [Google Scholar]
- Flavonoids and Isoflavonoids Biosynthesis in the Model Legume Lotus japonicus; Connections to Nitrogen Metabolism and Photorespiration. Plants. 2020;9:774.
- [CrossRef] [Google Scholar]
- Molecular evolution of flavonoid dioxygenases in the family Apiaceae. Phytochemistry. 2005;66:1273-1284.
- [CrossRef] [Google Scholar]
- A review of the phytochemistry and pharmacology of Phyllanthus urinaria L. Front. Pharmacol.. 2018;9
- [CrossRef] [Google Scholar]
- Characterization of zymosan-induced arthritis in the rat: Effects on joint inflammation and cartilage metabolism. Life Sci.. 1994;55:PL321-PL326.
- [CrossRef] [Google Scholar]
- Effectiveness and safety of long-term antidepressant treatment in bipolar disorder. J. Clin. Psychiatry. 2001;62:565-569.
- [CrossRef] [Google Scholar]
- Emerging impact of quercetin in the treatment of prostate cancer. Biomed. Pharmacother.. 2021;138:111548
- [CrossRef] [Google Scholar]
- Quercetin inhibits LPS-induced nitric oxide and tumor necrosis factor-α production in murine macrophages. Int. J. Immunopharmacol.. 1999;21:435-443.
- [CrossRef] [Google Scholar]
- The flavonoid quercetin inhibits thyroid-restricted genes expression and thyroid function. Food Chem. Toxicol.. 2014;66:23-29.
- [CrossRef] [Google Scholar]
- Mechanisms underlying inflammation in neurodegeneration. Cell. 2010;140:918-934.
- [CrossRef] [Google Scholar]
- Lymphocyte transformation to connective tissue antigens in adult and juvenile rheumatoid arthritis, osteoarthritis, ankylosing spondylitis, systemic lupus erythematosus, and a nonarthritic control population. Cell. Immunol.. 1983;82:196-209.
- [CrossRef] [Google Scholar]
- Chalcone derivatives: promising starting points for drug design. Molecules. 2017;22:1210.
- [CrossRef] [Google Scholar]
- Treating acute cystitis with biodegradable micelle-encapsulated quercetin. Int. J. Nanomed.. 2012;2239
- [CrossRef] [Google Scholar]
- Study of prevalence of depression in adolescent students of a public school. Ind. Psychiatry J.. 2009;18:43.
- [CrossRef] [Google Scholar]
- An overview of Indian research in depression. Indian J. Psychiatry.. 2010;52:178.
- [CrossRef] [Google Scholar]
- Anti-inflammatory properties of plant flavonoids. Effects of rutin, quercetin and hesperidin on adjuvant arthritis in rat. Il Farmaco. 2001;56:683-687.
- [CrossRef] [Google Scholar]
- Endogenous and exogenous mediators of quercetin bioavailability. J. Nutr. Biochem.. 2015;26:201-210.
- [CrossRef] [Google Scholar]
- Quercetin bioavailability is associated with inadequate plasma vitamin C status and greater plasma endotoxin in adults. Nutrition. 2014;30:1279-1286.
- [CrossRef] [Google Scholar]
- Quercetin treatment reduces the ERK mediated release of proinflammatory cytokines in cadmium mediated cholinergic dysfunction in rats: a process of memory impairments. J. Neurol. Sci.. 2017;381:197.
- [CrossRef] [Google Scholar]
- A multidisciplinary approach to the evaluation and management of interstitial cystitis/bladder pain syndrome: an ideal model of care. Transl. Androl. Urol.. 2015;4:611-619.
- [CrossRef] [Google Scholar]
- Perturbation of microtubule polymerization by quercetin through tubulin binding: a novel mechanism of its antiproliferative activity. Biochemistry. 2002;41:13029-13038.
- [CrossRef] [Google Scholar]
- Chalcone derivatives from the root bark of Morus alba L. act as inhibitors of PTP1B and α-glucosidase. Phytochemistry. 2018;155:114-125.
- [CrossRef] [Google Scholar]
- The antidiabetic potential of quercetin: underlying mechanisms. Curr. Med. Chem.. 2017;24:355-364.
- [CrossRef] [Google Scholar]
- Synthetic curcumin analogs inhibit activator protein-1 transcription and tumor-induced angiogenesis. Biochem. Biophys. Res. Commun.. 2004;321:337-344.
- [CrossRef] [Google Scholar]
- Content of the flavonols quercetin, myricetin, and kaempferol in 25 edible berries. J. Agric. Food Chem.. 1999;47
- [CrossRef] [Google Scholar]
- Flavonoid quercetin protects against swimming stress-induced changes in oxidative biomarkers in the hypothalamus of rats. Eur. J. Pharmacol.. 2009;621:46-52.
- [CrossRef] [Google Scholar]
- NK105, a paclitaxel-incorporating micellar nanoparticle formulation, can extend in vivo antitumour activity and reduce the neurotoxicity of paclitaxel. Br. J. Cancer. 2005;92:1240-1246.
- [CrossRef] [Google Scholar]
- Advances in flavonoid research since 1992. Phytochemistry. 2000;55:481-504.
- [CrossRef] [Google Scholar]
- Quercetin as an emerging anti-melanoma agent: a four-focus area therapeutic development strategy. Front. Nutr.. 2016;3
- [CrossRef] [Google Scholar]
- MicroRNA-1246 expression associated with CCNG2-mediated chemoresistance and stemness in pancreatic cancer. Br. J. Cancer. 2014;111:1572-1580.
- [CrossRef] [Google Scholar]
- Effects of combined administration of quercetin, rutin, and extract of white radish sprout rich in kaempferol glycosides on the metabolism in rats. Biosci. Biotech. Bioch.. 2006;70:279-281.
- [CrossRef] [Google Scholar]
- The biochemistry and medical significance of the flavonoids. Pharmacol. Ther.. 2002;96:67-202.
- [CrossRef] [Google Scholar]
- Frequency of signs and symptoms in persons with asthma. Respir. Care. 2020;65:252-264.
- [CrossRef] [Google Scholar]
- Evaluation of antidiabetic potential of selected traditional Chinese medicines in STZ-induced diabetic mice. J. Ethnopharmacol.. 2011;137:1135-1142.
- [CrossRef] [Google Scholar]
- Quercetin supplementation and upper respiratory tract infection: a randomized community clinical trial. Pharmacol. Res.. 2010;62:237-242.
- [CrossRef] [Google Scholar]
- A 12-week supplementation with quercetin does not affect natural killer cell activity, granulocyte oxidative burst activity or granulocyte phagocytosis in female human subjects. Br. J. Nutr.. 2010;104:849-857.
- [CrossRef] [Google Scholar]
- Neuroinflammation in Alzheimer’s disease. Lancet Neurol.. 2015;14:388-405.
- [CrossRef] [Google Scholar]
- Post-160-km race illness rates and decreases in granulocyte respiratory burst and salivary IgA output are not countered by quercetin ingestion. Int. J. Sports Med.. 2008;29:856-863.
- [CrossRef] [Google Scholar]
- Protective effects of quercetin and Vitamin C against oxidative stress-induced neurodegeneration. J. Agric. Food Chem.. 2004;52:7514-7517.
- [CrossRef] [Google Scholar]
- Dietary antioxidant flavonoids and risk of coronary heart disease: the Zutphen Elderly Study. Lancet. 1993;342:1007-1011.
- [CrossRef] [Google Scholar]
- Quercetin and its derivatives: synthesis, pharmacological uses with special emphasis on anti-tumor properties and prodrug with enhanced bio-availability. Anticancer Agents Med. Chem.. 2009;9:138-161.
- [CrossRef] [Google Scholar]
- Quercetin Suppresses Proliferation of Liver Cancer Cell Lines In Vitro. Anticancer Res. 2020;40:4695-4700.
- [CrossRef] [Google Scholar]
- Identification of brain-targeted bioactive dietary quercetin-3- O -glucuronide as a novel intervention for Alzheimer’s disease. FASEB J.. 2013;27:769-781.
- [CrossRef] [Google Scholar]
- Absorption, metabolism, and health effects of dietary flavonoids in man. Biomed. Pharmacother.. 1997;51:305-310.
- [CrossRef] [Google Scholar]
- Phenylphenalenones accumulate in plant tissues of two banana cultivars in response to herbivory by the banana weevil and banana stem weevil. Plants. 2016;5:34.
- [CrossRef] [Google Scholar]
- Antidepressant-like effect of quercetin in bulbectomized mice and involvement of the antioxidant defenses, and the glutamatergic and oxidonitrergic pathways. Pharmacol. Biochem. Behav. 2015;136:55-63.
- [CrossRef] [Google Scholar]
- Protective and Toxic Neuroinflammation in Amyotrophic Lateral Sclerosis. Neurotherapeutics. 2015;12:364-375.
- [CrossRef] [Google Scholar]
- Neuroimmune and neuroendocrine abnormalities in depression: two sides of the same coin. Ann. N. Y. Acad. Sci.. 2015;1351:68-79.
- [CrossRef] [Google Scholar]
- Horwitz, R.J., 2018. The Allergic Patient, in: Integrative Medicine. Elsevier, pp. 300-309.e2. https://doi.org/10.1016/B978-0-323-35868-2.00030-X
- Flavones: food sources, bioavailability, metabolism, and bioactivity. Adv. Nutr.: An Int. Rev. J.. 2017;8:423-435.
- [CrossRef] [Google Scholar]
- Preparation and enhanced oral bioavailability of cryptotanshinone-loaded solid lipid nanoparticles. AAPS PharmSciTech. 2010;11:582-587.
- [CrossRef] [Google Scholar]
- Immunosuppressive effect of quercetin on dendritic cell activation and function. J. Immunol.. 2010;184:6815-6821.
- [CrossRef] [Google Scholar]
- Effect of dietary quercetin on brain quercetin levels and the expression of antioxidant and Alzheimer’s disease relevant genes in mice. Pharmacol. Res.. 2010;61:242-246.
- [CrossRef] [Google Scholar]
- Paclitaxel selectively induces mitotic arrest and apoptosis in proliferating bovine synoviocytes. Arthritis Rheum.. 1997;40:1073-1084.
- [CrossRef] [Google Scholar]
- Quercetin is a potent anti-atherosclerotic compound by activation of SIRT1 signaling under oxLDL stimulation. Mol. Nutr. Food Res.. 2015;59:1905-1917.
- [CrossRef] [Google Scholar]
- Quercetin and its nano-scale delivery systems in prostate cancer therapy: paving the way for cancer elimination and reversing chemoresistance. Cancers (Basel). 2021;13:1602.
- [CrossRef] [Google Scholar]
- The role of estrogen receptors and their signaling across psychiatric disorders. Int. J. Mol. Sci.. 2020;22:373.
- [CrossRef] [Google Scholar]
- Revised structure of neoflavone in Coutarea hexandra. Phytochemistry. 1987;26:3096-3097.
- [CrossRef] [Google Scholar]
- Effects of flavonoids on xanthine oxidation as well as on cytochrome c reduction by milk xanthine oxidase. J. Nutr. Sci. Vitaminol. (Tokyo). 1986;32:635-642.
- [CrossRef] [Google Scholar]
- Cell-Cycle therapeutics come of age. J. Clin. Oncol.. 2017;35:2949-2959.
- [CrossRef] [Google Scholar]
- Flavonoids protect neuronal cells from oxidative stress by three distinct mechanisms. Free Radic. Biol. Med.. 2001;30:433-446.
- [CrossRef] [Google Scholar]
- Accumulation of orally administered quercetin in brain tissue and its antioxidative effects in rats. Free Radic. Biol. Med.. 2011;51:1329-1336.
- [CrossRef] [Google Scholar]
- Pharmacology in health food: metabolism of quercetin in vivo and its protective effect against arteriosclerosis. J. Pharmacol. Sci.. 2011;115:466-470.
- [CrossRef] [Google Scholar]
- Novel quercetin and apigenin-acetamide derivatives: design, synthesis, characterization, biological evaluation and molecular docking studies. RSC Adv.. 2020;10:25046-25058.
- [CrossRef] [Google Scholar]
- Neuropharmacological effects of quercetin: a literature-based review. Front. Pharmacol.. 2021;12
- [CrossRef] [Google Scholar]
- An update on the cardiovascular effects of quercetin, a plant flavonoid. Curr. Nutr. Food Sci.. 2014;10:36-48.
- [CrossRef] [Google Scholar]
- Flavonoid properties of five families newly incorporated into the order caryophyllales (Review) Bull Natl Mus Nat Sci. 2013;39
- [Google Scholar]
- The antioxidants curcumin and quercetin inhibit inflammatory processes associated with arthritis. Inflamm. Res.. 2006;55:168-175.
- [CrossRef] [Google Scholar]
- Quercetin with the potential effect on allergic diseases. Allergy, Asthma Clin. Immunol.. 2020;16:36.
- [CrossRef] [Google Scholar]
- Spectrophotometric analysis of flavonoid–DNA binding interactions at physiological conditions. Spectrochim Acta A Mol. Biomol. Spectrosc.. 2009;74:1135-1137.
- [CrossRef] [Google Scholar]
- Plant-derived immunomodulators: an insight on their preclinical evaluation and clinical trials. Front. Plant Sci.. 2015;6
- [CrossRef] [Google Scholar]
- The effect of quercetin on inflammatory factors and clinical symptoms in women with rheumatoid arthritis: a double-blind, randomized controlled trial. J. Am. Coll. Nutr.. 2017;36:9-15.
- [CrossRef] [Google Scholar]
- Separation of catechin compounds from different teas. Biotechnol. J.. 2006;1:209-213.
- [CrossRef] [Google Scholar]
- Amelioration of particulate matter-induced oxidative damage by vitamin c and quercetin in human bronchial epithelial cells. Chemosphere. 2016;144:459-466.
- [CrossRef] [Google Scholar]
- Cosuppression, flower color patterns, and metastable gene expression states. Science. 1995;1979(268):686-691.
- [CrossRef] [Google Scholar]
- Porcine epidemic diarrhea virus (PEDV): an update on etiology, transmission, pathogenesis, and prevention and control. Virus Res.. 2020;286:198045
- [CrossRef] [Google Scholar]
- The interplay between statins and adipokines. Is this another explanation of statins’ ‘pleiotropic’ effects? Cytokine. 2021;148:155698
- [CrossRef] [Google Scholar]
- Insulin action, diabetogenes, and the cause of Type II Diabetes. Diabetes. 1994;43:1066-1085.
- [CrossRef] [Google Scholar]
- Regiospecific synthesis of quercetin O-β-d-glucosylated and O-β-d-glucuronidated isomers. Tetrahedron. 2011;67:4731-4741.
- [CrossRef] [Google Scholar]
- Baricitinib plus Remdesivir for Hospitalized Adults with Covid-19. N. Engl. J. Med.. 2021;384:795-807.
- [CrossRef] [Google Scholar]
- Co(II) complex of quercetin-spectral, anti-/pro-oxidant and cytotoxic activity in hacat cell lines. Appl. Sci.. 2021;11:9244.
- [CrossRef] [Google Scholar]
- DNA interaction with naturally occurring antioxidant flavonoids quercetin, kaempferol, and delphinidin. J. Biomol. Struct. Dyn.. 2005;22:719-724.
- [CrossRef] [Google Scholar]
- An overview of DNA and RNA bindings to antioxidant flavonoids. Cell Biochem. Biophys.. 2007;49:29-36.
- [CrossRef] [Google Scholar]
- Molecular targets of quercetin with anti-inflammatory properties in atopic dermatitis. Drug Discov. Today. 2016;21:632-639.
- [CrossRef] [Google Scholar]
- Molecular mechanisms of action of quercetin in cancer: recent advances. Tumor Biol.. 2016;37:12927-12939.
- [CrossRef] [Google Scholar]
- Effects of Functional Groups and Sugar Composition of Quercetin Derivatives on Their Radical Scavenging Properties. J. Nat. Prod.. 2016;79
- [CrossRef] [Google Scholar]
- Treatment of interstitial cystitis with a quercetin supplement. Tech. Urol.. 2001;7:44-46.
- [Google Scholar]
- Suppressive effect of quercetin on acute stress-induced hypothalamic-pituitary-adrenal axis response in Wistar rats. J. Nutr. Biochem.. 2010;21:374-380.
- [CrossRef] [Google Scholar]
- Flavonoids and related compounds as anti-allergic substances. Allergol. Int.. 2007;56:113-123.
- [CrossRef] [Google Scholar]
- Flavonols inhibit proinflammatory mediator release, intracellular calcium ion levels and protein kinase C theta phosphorylation in human mast cells. Br. J. Pharmacol.. 2005;145:934-944.
- [CrossRef] [Google Scholar]
- Plant secondary metabolite diversity and species interactions. Annu. Rev. Ecol. Evol. Syst.. 2018;49:115-138.
- [CrossRef] [Google Scholar]
- Neuroprotective effect of quercetin against the detrimental effects of LPS in the adult mouse brain. Front. Pharmacol.. 2018;9
- [CrossRef] [Google Scholar]
- Oral Co-Supplementation of Curcumin, Quercetin, and Vitamin D3 as an Adjuvant Therapy for Mild to Moderate Symptoms of COVID-19—Results From a Pilot Open-Label. Randomized Controlled Trial. Front Pharmacol. 2022;13
- [CrossRef] [Google Scholar]
- Diabetes mellitus: The epidemic of the century. World J. Diabetes. 2015;6:850.
- [CrossRef] [Google Scholar]
- Anthocyanidins and anthocyanins: colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res.. 2017;61:1361779.
- [CrossRef] [Google Scholar]
- Microencapsulation of probiotics by calcium alginate-gelatinized starch with chitosan coating and evaluation of survival in simulated human gastro-intestinal condition. Iran J. Pharm. Res.. 2014;13:843-852.
- [Google Scholar]
- The islet estrogen receptor-α is induced by hyperglycemia and protects against oxidative stress-induced insulin-deficient diabetes. PLoS One. 2014;9:e87941.
- [Google Scholar]
- Protective effects of oligomeric and polymeric procyanidin fractions from defatted grape seeds on tert-butyl hydroperoxide-induced oxidative damage in HepG2 cells. Food Chem.. 2013;137:136-141.
- [CrossRef] [Google Scholar]
- Anti-melanogenic effects of black, green, and white tea extracts on immortalized melanocytes. J. Vet. Sci.. 2015;16:135.
- [CrossRef] [Google Scholar]
- MicroRNA targeting by quercetin in cancer treatment and chemoprotection. Pharmacol. Res.. 2019;147:104346
- [CrossRef] [Google Scholar]
- Synthesis of alkyl quercetin derivatives. J. Korean Soc. Appl. Biol. Chem.. 2015;58:343-348.
- [CrossRef] [Google Scholar]
- Flavanones and rotenoids from the roots of Amorpha fruticosa L. that inhibit bacterial neuraminidase. Food Chem. Toxicol.. 2011;49:1849-1856.
- [CrossRef] [Google Scholar]
- Effects of luteolin, quercetin and baicalein on immunoglobulin E-mediated mediator release from human cultured mast cells. Clin Exp Allergy. 2000;30:501-508.
- [CrossRef] [Google Scholar]
- Effects of quercetin on apoptosis, NF-κB and NOS gene expression in renal ischemia/reperfusion injury. Exp. Ther. Med.. 2012;3:249-254.
- [CrossRef] [Google Scholar]
- Anti-inflammatory, anti-proliferative and anti-atherosclerotic effects of quercetin in human in vitro and in vivo models. Atherosclerosis. 2011;218:44-52.
- [CrossRef] [Google Scholar]
- Antiproliferative and antimetastatic action of quercetin on A549 non-small cell lung cancer cells through its effect on the cytoskeleton. Acta Histochem.. 2017;119:99-112.
- [CrossRef] [Google Scholar]
- Flavonoid intake and coronary mortality in Finland: a cohort study. BMJ. 1996;312:478-481.
- [CrossRef] [Google Scholar]
- Quercetin intake and the incidence of cerebrovascular disease. Eur. J. Clin. Nutr.. 2000;54:415-417.
- [CrossRef] [Google Scholar]
- Chronic high intake of quercetin reduces oxidative stress and induces expression of the antioxidant enzymes in the liver and visceral adipose tissues in mice. J. Funct. Foods. 2015;15:551-560.
- [CrossRef] [Google Scholar]
- Quercetin suppresses immune cell accumulation and improves mitochondrial gene expression in adipose tissue of diet-induced obese mice. Mol. Nutr. Food Res.. 2016;60:300-312.
- [CrossRef] [Google Scholar]
- CXC Chemokines in the Pathogenesis of Pulmonary Disease and Pharmacological Relevance. Int J Inflam. 2022;2022:1-16.
- [CrossRef] [Google Scholar]
- Analysis and biological activities of anthocyanins. Phytochemistry. 2003;64:923-933.
- [CrossRef] [Google Scholar]
- Targeting Cardiovascular Diseases by Flavonols: An Update. Nutrients. 2022;14:1439.
- [CrossRef] [Google Scholar]
- Catalase is inhibited by flavonoids. Int. J. Biol. Macromol.. 2013;58:148-153.
- [CrossRef] [Google Scholar]
- Effects of Quercetin on Mushroom Tyrosinase and B16–F10 Melanoma Cells. Molecules. 2007;12:1045-1056.
- [CrossRef] [Google Scholar]
- Calotropis procera root extract has the capability to combat free radical mediated damage. ISRN Pharmacol.. 2013;2013:1-8.
- [CrossRef] [Google Scholar]
- Antioxidant mediated protective effect of Parthenium hysterophorus against oxidative damage using in vitro models. BMC Complement. Altern. Med.. 2013;13:120.
- [CrossRef] [Google Scholar]
- Chemistry and biological activities of flavonoids: an overview. Sci. World J.. 2013;2013:1-16.
- [CrossRef] [Google Scholar]
- Phenolic content, reducing power and membrane protective activities of Solanum xanthocarpum root extracts. Vegetos- An Int. J. Plant Res.. 2013;26:301.
- [CrossRef] [Google Scholar]
- Effect of Quercetin on lipid peroxidation and changes in lung morphology in experimental influenza virus infection. Int. J. Exp. Pathol.. 2003;84:127-134.
- [CrossRef] [Google Scholar]
- Nanoencapsulation and characterization of Albizia chinensis isolated antioxidant quercitrin on PLA nanoparticles. Colloids Surf. B Biointerfaces. 2011;82:224-232.
- [CrossRef] [Google Scholar]
- Potential use of dietary natural products, especially polyphenols, for improving Type-1 allergic symptoms. Curr. Pharm. Des.. 2014;20:857-863.
- [CrossRef] [Google Scholar]
- Quercetin: A Versatile Flavonoid. Internet J. Medical Update - EJOURNAL. 2007;2
- [CrossRef] [Google Scholar]
- Role of quercetin in cardiovascular diseases. Internet J. Medical Update - EJOURNAL. 2008;3
- [CrossRef] [Google Scholar]
- Inhibition of carcinogenesis by polyphenols: evidence from laboratory investigations. Am. J. Clin. Nutr.. 2005;81:284S-291S.
- [CrossRef] [Google Scholar]
- Quercetin: A Treatment for Hypertension?—A Review of Efficacy and Mechanisms. Pharmaceuticals. 2010;3:237-250.
- [CrossRef] [Google Scholar]
- Therapeutic potential of quercetin to decrease blood pressure: review of efficacy and mechanisms. Adv. Nutr.. 2012;3:39-46.
- [CrossRef] [Google Scholar]
- Cost of high prevalence mental disorders: findings from the 2007 Australian national survey of mental health and wellbeing. Aust. New Zealand J. Psychiatry. 2017;51:1198-1211.
- [CrossRef] [Google Scholar]
- Contribution of flavonoids to the antioxidant properties of common and tartary buckwheat. J. Cereal Sci.. 2016;68:181-186.
- [CrossRef] [Google Scholar]
- An intravesical device for the sustained delivery of lidocaine to the bladder. J. Control. Release. 2011;149:133-139.
- [CrossRef] [Google Scholar]
- d -pinitol regulates Th1/Th2 balance via suppressing Th2 immune response in ovalbumin-induced asthma. FEBS Lett.. 2007;581:57-64.
- [CrossRef] [Google Scholar]
- The anti-HSV-1 effect of quercetin is dependent on the suppression of TLR-3 in Raw 264.7 cells. Arch. Pharm. Res.. 2017;40:623-630.
- [CrossRef] [Google Scholar]
- Pharmacokinetics of quercetin absorption from apples and onions in healthy humans. J. Agric. Food Chem.. 2012;60:3874-3881.
- [CrossRef] [Google Scholar]
- Anti–inflammatory effect of quercetin and galangin in LPS–stimulated RAW264.7 macrophages and DNCB–induced atopic dermatitis animal models. Int. J. Mol. Med. 2017
- [CrossRef] [Google Scholar]
- (−)-Epigallocatechin-3-gallate prevents lipopolysaccharide-induced elevation of beta-amyloid generation and memory deficiency. Brain Res.. 2009;1250:164-174.
- [CrossRef] [Google Scholar]
- Lemjabbar-Alaoui, H., Hassan, O.U., Yang, Y.-W., Buchanan, P., 2015. Lung cancer: Biology and treatment options. Biochimica et Biophysica Acta (BBA) - Reviews on Cancer 1856, 189–210. https://doi.org/10.1016/j.bbcan.2015.08.002
- Iron chelation by the powerful antioxidant flavonoid quercetin. J. Agric. Food Chem.. 2006;54:6343-6351.
- [CrossRef] [Google Scholar]
- Pyranoanthocyanins: a theoretical investigation on their antioxidant activity. J. Agric. Food Chem.. 2010;58:8862-8871.
- [CrossRef] [Google Scholar]
- Antioxidant and anti-inflammatory activities of quercetin and its derivatives. J. Funct. Foods. 2018;40:68-75.
- [CrossRef] [Google Scholar]
- Gut microbiota: a novel regulator of cardiovascular disease and key factor in the therapeutic effects of flavonoids. Front. Pharmacol.. 2021;12
- [CrossRef] [Google Scholar]
- Traditional chinese medicine in depression treatment: from molecules to systems. Front. Pharmacol.. 2020;11
- [CrossRef] [Google Scholar]
- Enhancement of gastrointestinal absorption of quercetin by solid lipid nanoparticles. J. Control. Release. 2009;133:238-244.
- [CrossRef] [Google Scholar]
- Enhancement of oral bioavailability of quercetin by metabolic inhibitory nanosuspensions compared to conventional nanosuspensions. Drug Deliv.. 2021;28:1226-1236.
- [CrossRef] [Google Scholar]
- Antidepressant-like effects of Chaihu-Shugan-San via SAPK/JNK signal transduction in rat models of depression. Pharmacogn. Mag.. 2014;10:271.
- [CrossRef] [Google Scholar]
- A bibliometric analysis and visualization of medical big data research. Sustainability. 2018;10:166.
- [CrossRef] [Google Scholar]
- Lin, G., Chan, S.S.-K., Chung, H.-S., Li, S.-L., 2005. Chemistry and biological activities of naturally occurring phthalides. pp. 611–669. https://doi.org/10.1016/S1572-5995(05)80065-1
- The role of flavonoids in nodulation host-range specificity: an update. Plants. 2016;5:33.
- [CrossRef] [Google Scholar]
- Low concentration of quercetin antagonizes the invasion and angiogenesis of human glioblastoma U251 cells. Onco Targets Ther.. 2017;10:4023-4028.
- [CrossRef] [Google Scholar]
- Anthocyanin biosynthesis and degradation mechanisms in solanaceous vegetables: a review. Front. Chem.. 2018;6
- [CrossRef] [Google Scholar]
- The global burden of disease attributable to low consumption of fruit and vegetables: implications for the global strategy on diet. Bull World Health Organ. 2005;83 https://doi.org//S0042-96862005000200010
- [Google Scholar]
- Polygonum aviculare L. extract and quercetin attenuate contraction in airway smooth muscle. Sci. Rep.. 2018;8:3114.
- [CrossRef] [Google Scholar]
- Nanoplatform Assembled from a CD44-Targeted Prodrug and Smart Liposomes for Dual Targeting of Tumor Microenvironment and Cancer Cells. ACS Nano. 2018;12:1519-1536.
- [CrossRef] [Google Scholar]
- Targeting intracellular MMPs efficiently inhibits tumor metastasis and angiogenesis. Theranostics. 2018;8:2830-2845.
- [CrossRef] [Google Scholar]
- Neuroinflammation: The role and consequences. Neurosci. Res.. 2014;79:1-12.
- [CrossRef] [Google Scholar]
- Antiherpetic activities of flavonoids against herpes simplex virus type 1 (HSV-1) and type 2 (HSV-2) in vitro. Arch. Pharm. Res.. 2005;28:1293-1301.
- [CrossRef] [Google Scholar]
- Diabetes mellitus diabetes mellitus. Ferri’s Clinical Advisor. 2020;2020(512):432-441.
- [CrossRef] [Google Scholar]
- Adsorption of bile salts to particles allows penetration of intestinal mucus. Soft Matter. 2011;7:8077.
- [CrossRef] [Google Scholar]
- A Review on Structure, Modifications and Structure-Activity Relation of Quercetin and Its Derivatives. J. Microbiol. Biotechnol.. 2020;30:11-20.
- [CrossRef] [Google Scholar]
- Stress, serotonin, and hippocampal neurogenesis in relation to depression and antidepressant effects. Neurosci. Biobehav. Rev.. 2014;38:173-192.
- [CrossRef] [Google Scholar]
- Histamine and histamine intolerance. Am. J. Clin. Nutr.. 2007;85:1185-1196.
- [CrossRef] [Google Scholar]
- The pharmacologic treatment of depression. J. Am. Board Family Med.. 1998;11:127-139.
- [CrossRef] [Google Scholar]
- The relationship of estrogen receptor-α polymorphism with symptoms and other characteristics in post-menopausal women. Maturitas. 2004;49:163-169.
- [CrossRef] [Google Scholar]
- Cell signaling pathways in the neuroprotective actions of the green tea polyphenol (-)-epigallocatechin-3-gallate: implications for neurodegenerative diseases. J. Neurochem.. 2004;88:1555-1569.
- [CrossRef] [Google Scholar]
- Obesity, oxidative stress, adipose tissue dysfunction, and the associated health risks: causes and therapeutic strategies. Metab. Syndr. Relat. Disord.. 2015;13:423-444.
- [CrossRef] [Google Scholar]
- Interstitial cystitis/bladder pain syndrome. Semin. Reprod. Med.. 2018;36:123-135.
- [CrossRef] [Google Scholar]
- Dietary flavonoids: molecular mechanisms of action as anti- inflammatory agents. Recent Pat. Inflamm. Allergy Drug Discov.. 2011;5:200-220.
- [CrossRef] [Google Scholar]
- Association of vitamin E and C supplement use with cognitive function and dementia in elderly men. Neurology. 2000;54:1265-1272.
- [CrossRef] [Google Scholar]
- Research progress in the modification of quercetin leading to anticancer agents. Molecules. 2017;22:1270.
- [CrossRef] [Google Scholar]
- Flavonoid functions in plants and their interactions with other organisms. Plants. 2018;7:30.
- [CrossRef] [Google Scholar]
- Complex C -Glycosyl flavonoid phytoalexins from Cucumis s ativus. J. Nat. Prod.. 2003;66:1280-1283.
- [CrossRef] [Google Scholar]
- Kinetic and stoichiometric assessment of the antioxidant activity of flavonoids by electron spin resonance spectroscopy. J. Agric. Food Chem.. 2003;51:1684-1690.
- [CrossRef] [Google Scholar]
- Pathogenesis of depression: Insights from human and rodent studies. Neuroscience. 2016;321:138-162.
- [CrossRef] [Google Scholar]
- Quercetin may act as a cytotoxic prooxidant after its metabolic activation to semiquinone and quinoidal product. Free Radic. Biol. Med.. 1999;26:107-116.
- [CrossRef] [Google Scholar]
- Quercetin: a molecule of great biochemical and clinical value and its beneficial effect on diabetes and cancer. Diseases. 2022;10:37.
- [CrossRef] [Google Scholar]
- Flavonoids as important molecules of plant interactions with the environment. Molecules. 2014;19:16240-16265.
- [CrossRef] [Google Scholar]
- Quercetin inhibits expression of inflammatory cytokines through attenuation of NF-κB and p38 MAPK in HMC-1 human mast cell line. Inflamm. Res.. 2007;56:210-215.
- [CrossRef] [Google Scholar]
- Molecular and biological functions of quercetin as a natural solution for cardiovascular disease prevention and treatment. Plant Foods Hum. Nutr.. 2020;75:307-315.
- [CrossRef] [Google Scholar]
- Synthesis, molecular structure and optical properties of glycidyl derivatives of quercetin. Struct. Chem.. 2016;27:285-294.
- [CrossRef] [Google Scholar]
- Enhancement of anti-inflammatory and anti-allergic activities with combination of luteolin and quercetin in in vitro co-culture system. Food Sci. Technol. Res.. 2017;23:811-818.
- [CrossRef] [Google Scholar]
- Quercetin and its anti-allergic immune response. Molecules. 2016;21:623.
- [CrossRef] [Google Scholar]
- Natural products and their bioactive compounds: neuroprotective potentials against neurodegenerative diseases. Evid. Based Complement. Alternat. Med.. 2020;2020:1-30.
- [CrossRef] [Google Scholar]
- New drug targets for type 2 diabetes and the metabolic syndrome. Nature. 2001;414:821-827.
- [CrossRef] [Google Scholar]
- Lactoferrin, quercetin, and hydroxyapatite act synergistically against pseudomonas fluorescens. Int. J. Mol. Sci.. 2021;22:9247.
- [CrossRef] [Google Scholar]
- Quercetin as a precursor for the synthesis of novel nanoscale Cu (II) complex as a catalyst for alcohol oxidation with high antibacterial activity. Bioinorg. Chem. Appl.. 2021;2021:1-9.
- [CrossRef] [Google Scholar]
- Inhibitory effect of quercetin on carrageenan-induced inflammation in rats. Life Sci.. 2003;74:709-721.
- [CrossRef] [Google Scholar]
- Selenium- or quercetin-induced retardation of DNA synthesis in primary prostate cells occurs in the presence of a concomitant reduction in androgen-receptor activity. Cancer Lett.. 2006;239:111-122.
- [CrossRef] [Google Scholar]
- Mosunova, O., Navarro-Muñoz, J.C., Collemare, J., 2021. The Biosynthesis of Fungal Secondary Metabolites: From Fundamentals to Biotechnological Applications. In: Encyclopedia of Mycology. Elsevier, pp. 458–476. https://doi.org/10.1016/B978-0-12-809633-8.21072-8.
- Adipose tissue fibrosis, hypertrophy, and hyperplasia: correlations with diabetes in human obesity. Obesity. 2016;24:597-605.
- [CrossRef] [Google Scholar]
- Semisynthetic quercetin derivatives with potent antitumor activity in colon carcinoma. ACS Omega. 2019;4:7285-7298.
- [CrossRef] [Google Scholar]
- Quercetin in anti-diabetic research and strategies for improved quercetin bioavailability using polymer-based carriers – a review. RSC Adv.. 2015;5:97547-97562.
- [CrossRef] [Google Scholar]
- Strategies for effective oral insulin delivery with modified chitosan nanoparticles: a review. Prog. Polym. Sci.. 2012;37:1457-1475.
- [CrossRef] [Google Scholar]
- Pre-clinical and clinical study of QC12, a water-soluble, pro-drug of quercetin. Ann. Oncol.. 2001;12:245-248.
- [CrossRef] [Google Scholar]
- A comparative study of quercetin-loaded nanocochleates and liposomes: formulation, characterization, assessment of degradation, and in vitro anticancer potential. Pharmaceutics. 2022;14:1601.
- [CrossRef] [Google Scholar]
- Transit and metabolic pathways of quercetin in tubular cells: involvement of its antioxidant properties in the kidney. Antioxidants. 2021;10:909.
- [CrossRef] [Google Scholar]
- Antioxidative flavonoid quercetin: implication of its intestinal absorption and metabolism. Arch. Biochem. Biophys.. 2003;417:12-17.
- [CrossRef] [Google Scholar]
- The Flavonoid Quercetin Inhibits Proinflammatory Cytokine (Tumor Necrosis Factor Alpha) Gene Expression in Normal Peripheral Blood Mononuclear Cells via Modulation of the NF-κβ System. Clin. Vaccine Immunol.. 2006;13:319-328.
- [CrossRef] [Google Scholar]
- Attenuation of Transforming Growth Factor–β–Stimulated Collagen Production in Fibroblasts by Quercetin-Induced Heme Oxygenase–1. Am. J. Respir. Cell Mol. Biol.. 2011;44:614-620.
- [CrossRef] [Google Scholar]
- Review: Ultraviolet radiation and skin cancer. Int. J. Dermatol.. 2010;49:978-986.
- [CrossRef] [Google Scholar]
- The burden of Diabetes, Its Oral Complications and Their Prevention and Management. Open Access Maced J Med Sci. 2018;6:1545-1553.
- [CrossRef] [Google Scholar]
- Antimicrobial activity of quercetin: an approach to its mechanistic principle. Molecules. 2022;27:2494.
- [CrossRef] [Google Scholar]
- The influence of flavonoid compounds on the in vitro inhibition study of a human fibroblast collagenase catalytic domain expressed in E. coli. Enzyme Microb. Technol.. 2013;52:26-31.
- [CrossRef] [Google Scholar]
- Enhancement of quercetin water solubility with steviol glucosides and the studies of biological properties. Functional Foods in Health and Disease. 2015;5:437.
- [CrossRef] [Google Scholar]
- In vitro biotransformation of flavonoids by rat liver microsomes. Xenobiotica. 1998;28:389-401.
- [CrossRef] [Google Scholar]
- Effects of Quercetin and EGCG on Mitochondrial Biogenesis and Immunity. Med. Sci. Sports Exerc.. 2009;41:1467-1475.
- [CrossRef] [Google Scholar]
- Flavonoids: a review of probable mechanisms of action and potential applications. Am. J. Clin. Nutr.. 2001;74:418-425.
- [CrossRef] [Google Scholar]
- Structures of 4-aryl-coumarin (neoflavone) dimers isolated from Pistacia chinensis BUNGE and their estrogen-like activity. Chem Pharm Bull (Tokyo). 2000;48:505-508.
- [CrossRef] [Google Scholar]
- Flavonoid profile of the cotton plant Gossypium hirsutum: a review. Plants. 2017;6:43.
- [CrossRef] [Google Scholar]
- Editorial: adverse effects of cancer chemotherapy: anything new to improve tolerance and reduce sequelae? Front. Pharmacol.. 2018;9
- [CrossRef] [Google Scholar]
- Immunomodulatory effect of Quercetin on dysregulated Th1/Th2 cytokine balance in mice with both type 1 diabetes and allergic asthma. J. Appl. Pharm. Sci.. 2020;10:80-87.
- [CrossRef] [Google Scholar]
- Antiallergic constituents from oolong tea stem. Biol. Pharm. Bull.. 1995;18:683-686.
- [CrossRef] [Google Scholar]
- Electrochemical study of quercetin–DNA interactions: Part I Analysis in incubated solutions. Bioelectrochemistry. 2004;64:133-141.
- [CrossRef] [Google Scholar]
- Immunomodulatory and antimetastatic action of propolis and related polyphenolic compounds. J. Ethnopharmacol.. 2004;94:307-315.
- [CrossRef] [Google Scholar]
- Synthesis and characterization of novel flavonoid derivatives via sequential phosphorylation of quercetin. Tetrahedron Lett.. 2017;58:1474-1479.
- [CrossRef] [Google Scholar]
- Antimicrobial Activity of a New Class of Phosphorylated and Modified Flavonoids. ACS Omega. 2019;4:12865-12871.
- [CrossRef] [Google Scholar]
- CBTRUS Statistical Report: primary brain and central nervous system tumors diagnosed in the United States in 2008–2012. Neuro Oncol.. 2015;17:iv1-iv62.
- [CrossRef] [Google Scholar]
- Phenolics in human health. Int. J. Chem. Eng. Applications. 2014;5:393-396.
- [CrossRef] [Google Scholar]
- Quercetin promotes cell apoptosis and inhibits the expression of MMP-9 and fibronectin via the AKT and ERK signalling pathways in human glioma cells. Neurochem. Int.. 2015;80:60-71.
- [CrossRef] [Google Scholar]
- Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev.. 2009;2:270-278.
- [CrossRef] [Google Scholar]
- Linking plant secondary metabolites and plant microbiomes: a review. Front. Plant Sci.. 2021;12
- [CrossRef] [Google Scholar]
- Potential pharmaceutical applications of quercetin in cardiovascular diseases. Pharmaceuticals. 2022;15:1019.
- [CrossRef] [Google Scholar]
- Iron (III)-Quercetin complex: synthesis, physicochemical characterization, and MRI cell tracking toward potential applications in regenerative medicine. Contrast Media Mol. Imaging. 2020;2020:1-22.
- [CrossRef] [Google Scholar]
- Quercetin regulates Th1/Th2 balance in a murine model of asthma. Int. Immunopharmacol.. 2009;9:261-267.
- [CrossRef] [Google Scholar]
- Quercetin attenuates neuronal cells damage in a middle cerebral artery occlusion animal model. J. Vet. Med. Sci.. 2018;80:676-683.
- [CrossRef] [Google Scholar]
- Parsons, M.E., Ganellin, C.R., 2006. Histamine and its receptors. Br J Pharmacol 147, S127–S135. https://doi.org/10.1038/sj.bjp.0706440
- Therapeutic potential of quercetin as a cardiovascular agent. Eur. J. Med. Chem.. 2018;155:889-904.
- [CrossRef] [Google Scholar]
- Production of mycophenolic acid by Penicillium brevicompactum—A comparison of two methods of optimization. Biotechnol. Rep,. 2016;11:77-85.
- [CrossRef] [Google Scholar]
- Liposomal delivery of mycophenolic acid with quercetin for improved breast cancer therapy in SD rats. Front. Bioeng. Biotechnol.. 2020;8
- [CrossRef] [Google Scholar]
- Flavonoids attenuate cardiovascular disease, inhibit phosphodiesterase, and modulate lipid homeostasis in adipose tissue and liver. Exp. Biol. Med.. 2006;231:1287-1299.
- [CrossRef] [Google Scholar]
- Acute and chronic antiinflammatory effects of plant flavonoids. Il Farmaco. 1998;53:421-424.
- [CrossRef] [Google Scholar]
- Review: Role of mast cells in gastrointestinal mucosal defense. Biocell 2003:27.
- [CrossRef] [Google Scholar]
- Endothelial function and cardiovascular disease: Effects of quercetin and wine polyphenols. Free Radic. Res.. 2006;40:1054-1065.
- [CrossRef] [Google Scholar]
- Flavonols and cardiovascular disease. Mol. Aspects Med.. 2010;31:478-494.
- [CrossRef] [Google Scholar]
- Endothelium-Independent vasodilator effects of the flavonoid quercetin and its methylated metabolites in rat conductance and resistance arteries. J. Pharmacol. Experimental Therapeutics. 2002;302:66-72.
- [CrossRef] [Google Scholar]
- The relation between antioxidants and memory performance in the old and very old. J. Am. Geriatr. Soc.. 1997;45:718-724.
- [CrossRef] [Google Scholar]
- A plant flavone, luteolin, induces expression of Rhizobium meliloti nodulation genes. Science. 1986;1979(233):977-980.
- [CrossRef] [Google Scholar]
- Flavanones in oranges, tangerines (mandarins), tangors, and tangelos: a compilation and review of the data from the analytical literature. J. Food Compos. Anal.. 2006;19:S66-S73.
- [CrossRef] [Google Scholar]
- Xanthan gum: A versatile biopolymer for biomedical and technological applications. J. Appl. Polym. Sci.. 2015;132:n/a-n/a.
- [CrossRef] [Google Scholar]
- Site-specific drug delivery systems within the gastro-intestinal tract: From the mouth to the colon. Int. J. Pharm.. 2010;395:44-52.
- [CrossRef] [Google Scholar]
- Characterization of quercetin binding site on DNA gyrase. Biochem. Biophys. Res. Commun.. 2003;306:530-536.
- [CrossRef] [Google Scholar]
- Antioxidant properties of flavonol glycosides from green beans. Redox Rep.. 1999;4:123-127.
- [CrossRef] [Google Scholar]
- Recent advances in phenolic metabolites and skin cancer. Int. J. Mol. Sci.. 2021;22:9707.
- [CrossRef] [Google Scholar]
- Popa, D.-S., Rusu, M.E., 2017. Isoflavones: Vegetable Sources, Biological Activity, and Analytical Methods for Their Assessment. In: Superfood and Functional Food - The Development of Superfoods and Their Roles as Medicine. InTech. https://doi.org/10.5772/66531.
- Byssochlamys nivea as a source of mycophenolic acid. Appl. Environ. Microbiol.. 2005;71:550-553.
- [CrossRef] [Google Scholar]
- Quercetin: its antioxidant mechanism, antibacterial properties and potential application in prevention and control of toxipathy. Molecules. 2022;27:6545.
- [CrossRef] [Google Scholar]
- Chaihu-Shugan-San, an oriental herbal preparation, for the treatment of chronic gastritis: a meta-analysis of randomized controlled trials. J. Ethnopharmacol.. 2013;146:433-439.
- [CrossRef] [Google Scholar]
- Quercetin attenuates mitochondrial dysfunction and biogenesis via upregulated AMPK/SIRT1 signaling pathway in OA rats. Biomed. Pharmacother.. 2018;103:1585-1591.
- [CrossRef] [Google Scholar]
- Quercetin alleviates high glucose-induced Schwann cell damage by autophagy. Neural Regen. Res.. 2014;9:1195.
- [CrossRef] [Google Scholar]
- Oxidative stress relevance in the pathogenesis of the rheumatoid arthritis: a systematic review. Biomed Res. Int.. 2016;2016:1-14.
- [CrossRef] [Google Scholar]
- Arthritis and diagnostics in lyme disease. Trop. Med. Infect Dis.. 2021;6:18.
- [CrossRef] [Google Scholar]
- Atherosclerosis: process, indicators, risk factors and new hopes. Int. J. Prev. Med.. 2014;5:927-946.
- [Google Scholar]
- Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res.. 2014;74:2913-2921.
- [CrossRef] [Google Scholar]
- Complexes involving quercetin, DNA and Cu(II) Carcinogenesis. 1990;11:2001-2003.
- [CrossRef] [Google Scholar]
- Medicinal plants and formulations used by the Soren clan of the Santal Tribe in Rajshahi district, Bangladesh for treatment of various ailments. Afr. J. Tradit. Complement. Altern. Med.. 2012;9
- [CrossRef] [Google Scholar]
- Inhibitory potency of flavonoid derivatives on influenza virus neuraminidase. Bioorg. Med. Chem. Lett.. 2014;24:4312-4317.
- [CrossRef] [Google Scholar]
- Screening of antidepressant activity and estimation of quercetin from Coccinia indica using TLC densitometry. Pharm. Biol.. 2015;53:1867-1874.
- [CrossRef] [Google Scholar]
- Quercetin as an innovative therapeutic tool for cancer chemoprevention: molecular mechanisms and implications in human health. Cancer Med.. 2020;9:9181-9192.
- [CrossRef] [Google Scholar]
- Anticancer potential of quercetin: a comprehensive review. Phytotherapy Res.. 2018;32:2109-2130.
- [CrossRef] [Google Scholar]
- Antioxidant study of quercetin and their metal complex and determination of stability constant by spectrophotometry method. Food Chem.. 2014;146:472-478.
- [CrossRef] [Google Scholar]
- Anthocyanins and colonic metabolites of dietary polyphenols inhibit platelet function. Thromb. Res.. 2005;116:327-334.
- [CrossRef] [Google Scholar]
- Amyloid-beta (Aβ 1–42)-induced paralysis in Caenorhabditis elegans is inhibited by the polyphenol quercetin through activation of protein degradation pathways. Mol. Nutr. Food Res.. 2014;58:1931-1940.
- [CrossRef] [Google Scholar]
- Mast cell-derived tumour necrosis factor is essential for allergic airway disease. Eur. Respir. J.. 2008;31:773-782.
- [CrossRef] [Google Scholar]
- The relative antioxidant activities of plant-derived polyphenolic flavonoids. Free Radic. Res.. 1995;22:375-383.
- [CrossRef] [Google Scholar]
- Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med.. 1996;20:933-956.
- [CrossRef] [Google Scholar]
- Antagonist: diabetes and insulin resistance–philosophy, science, and the multiplier hypothesis. J. Lab. Clin. Med. 1995
- [Google Scholar]
- Environmentally friendly methods for flavonoid extraction from plant material: impact of their operating conditions on yield and antioxidant properties. Sci. World J.. 2020;2020:1-38.
- [CrossRef] [Google Scholar]
- Anti-inflammatory activity of quercetin and isoquercitrin in experimental murine allergic asthma. Inflamm. Res.. 2007;56:402-408.
- [CrossRef] [Google Scholar]
- Anti-inflammatory effect of quercetin-loaded microemulsion in the airways allergic inflammatory model in mice. Pharmacol. Res.. 2010;61:288-297.
- [CrossRef] [Google Scholar]
- Flavones and isoflavones as inducing substances of legume nodulation. Biofactors. 1988;1:3-10.
- [Google Scholar]
- Rothwell, J.A., Perez-Jimenez, J., Neveu, V., Medina-Remon, A., M’Hiri, N., Garcia-Lobato, P., Manach, C., Knox, C., Eisner, R., Wishart, D.S., Scalbert, A., 2013. Phenol-Explorer 3.0: a major update of the Phenol-Explorer database to incorporate data on the effects of food processing on polyphenol content. Database 2013, bat070–bat070. https://doi.org/10.1093/database/bat070.
- Epidemiology of hepatitis C virus infection. Clin. Res. Hepatol. Gastroenterol.. 2021;45:101596
- [CrossRef] [Google Scholar]
- Antimicrobial efficacy of quercetin against vibrio parahaemolyticus biofilm on food surfaces and downregulation of virulence genes. Polymers (Basel). 2022;14:3847.
- [CrossRef] [Google Scholar]
- Naturally occurring chalcones and their biological activities. Phytochem. Rev.. 2016;15:87-120.
- [CrossRef] [Google Scholar]
- Chalcone scaffolds, bioprecursors of flavonoids: chemistry, bioactivities, and pharmacokinetics. Molecules. 2021;26
- [CrossRef] [Google Scholar]
- Anti-inflammatory potential of Quercetin in COVID-19 treatment. J. Inflamm.. 2021;18:3.
- [CrossRef] [Google Scholar]
- Chronic administration of quercetin prevent spatial learning and memory deficits provoked by chronic stress in rats. Behav. Brain Res.. 2014;270:196-205.
- [CrossRef] [Google Scholar]
- Site-Specific anticancer effects of dietary flavonoid quercetin. Nutr. Cancer. 2014;66:177-193.
- [CrossRef] [Google Scholar]
- Sakai, E., Farhana, F., Yamaguchi, Y., Tsukuba, T., 2022. Potentials of natural antioxidants from plants as antiosteoporotic agents. pp. 1–28. https://doi.org/10.1016/B978-0-12-823944-5.00002-8.
- Therapeutic potential of quercetin: new insights and perspectives for human health. ACS Omega. 2020;5:11849-11872.
- [CrossRef] [Google Scholar]
- The neuro-protective effects of quercetin. Res. J. Pharm. Technol.. 2019;12:561.
- [CrossRef] [Google Scholar]
- Evolution, expression and functional analysis of CXCR3 in neuronal and cardiovascular diseases: a narrative review. Front. Cell Dev. Biol.. 2022;10
- [CrossRef] [Google Scholar]
- Modulation of chronic inflammation by quercetin: the beneficial effects on obesity. J. Inflamm. Res.. 2020;13:421-431.
- [CrossRef] [Google Scholar]
- Flavonoids and phenolic acids as antioxidants in plants and human health. Int. J. Pharm. Sci. Rev. Res. 2012
- [Google Scholar]
- Genetic unrelatedness of co-occurring pancreatic adenocarcinomas and IPMNs challenges current views of clinical management. Gut. 2018;67:1561-1563.
- [CrossRef] [Google Scholar]
- Do polyphenols enter the brain and does it matter? Some theoretical and practical considerations. Genes Nutr.. 2012;7:99-109.
- [CrossRef] [Google Scholar]
- Interactions Affecting the Bioavailability of Dietary Polyphenols in Vivo. Int. J. Vitam. Nutr. Res.. 2007;77:224-235.
- [CrossRef] [Google Scholar]
- Obesity and the development of type 2 diabetes: the effects of fatty tissue inflammation. Diabetes Metab Syndr Obes. 2010;253
- [CrossRef] [Google Scholar]
- Diabetes, oxidative stress, molecular mechanism, and cardiovascular disease – an overview. Toxicol. Mech. Methods. 2012;22:330-335.
- [CrossRef] [Google Scholar]
- Senthelal, S., Li, J., Ardeshirzadeh, S., Thomas, M.A., 2022. Arthritis. PMID-30085534
- Investigation of pluronic and PEG-PE micelles as carriers of meso-tetraphenyl porphine for oral administration. Int. J. Pharm.. 2007;332:161-167.
- [CrossRef] [Google Scholar]
- Quercetin: a natural compound for ovarian cancer treatment. J. Ovarian Res.. 2019;12:55.
- [CrossRef] [Google Scholar]
- Utilising polyphenols for the clinical management of Candida albicans biofilms. Int. J. Antimicrob. Agents. 2014;44:269-273.
- [CrossRef] [Google Scholar]
- Role of quercetin (a natural herbal compound) in allergy and inflammation. J. Biol. Regul. Homeost. Agents. 2006;20:47-52.
- [Google Scholar]
- Diet, lifestyle and cardiovascular diseases: linking pathophysiology to cardioprotective effects of natural bioactive compounds. Int. J. Environ. Res. Public Health. 2020;17:2326.
- [CrossRef] [Google Scholar]
- Cinnamomum species: bridging phytochemistry knowledge, pharmacological properties and toxicological safety for health benefits. Front. Pharmacol.. 2021;12
- [CrossRef] [Google Scholar]
- Therapeutic charm of quercetin and its derivatives: a review of research and patents. Pharm. Pat. Anal.. 2018;7
- [CrossRef] [Google Scholar]
- Synthesis, characterization, and pharmacological evaluation of some metal complexes of quercetin as P-gp inhibitors. Futur J. Pharm. Sci.. 2021;7:99.
- [CrossRef] [Google Scholar]
- The effects of quercetin combined with nucleopolyhedrovirus on the growth and immune response in the silkworm (Bombyx mori) Arch. Insect Biochem. Physiol.. 2021;108
- [CrossRef] [Google Scholar]
- The flavonoid apigenin inhibits hepatitis C virus replication by decreasing mature microRNA122 levels. Virology. 2014;462–463:42-48.
- [CrossRef] [Google Scholar]
- Inhibitory effect of flavonoids on DNA-dependent DNA and RNA polymerases. Experientia. 1988;44:882-885.
- [CrossRef] [Google Scholar]
- Characteristic difference of hepatocellular carcinoma between hepatitis B- and C- viral infection in Japan. Hepatology. 1995;22:1027-1033.
- [CrossRef] [Google Scholar]
- Sholan, R., 2020. Clinical manifestations and results of cystoscopy in women with interstitial cystitis/bladder pain syndrome. North Clin Istanb. https://doi.org/10.14744/nci.2020.23245.
- Antibacterial activity of quercetin on oral infectious pathogens. Afr. J. Microbiol. Res.. 2011;5
- [CrossRef] [Google Scholar]
- Influence of the antioxidant quercetin in vivo on the level of nitric oxide determined by electron paramagnetic resonance in rat brain during global ischemia and reperfusion. Biochem. Pharmacol.. 1999;57:199-208.
- [CrossRef] [Google Scholar]
- Oxidative DNA damage protection and repair by polyphenolic compounds in PC12 cells. Eur. J. Pharmacol.. 2008;601:50-60.
- [CrossRef] [Google Scholar]
- Antioxidant vs. Prooxidant properties of the flavonoid, kaempferol, in the presence of Cu(II) Ions: A ROS-Scavenging activity, fenton reaction and DNA damage study. Int. J. Mol. Sci.. 2021;22:1619.
- [CrossRef] [Google Scholar]
- The role of quercetin in plants. Plant Physiol. Biochem.. 2021;166:10-19.
- [CrossRef] [Google Scholar]
- Dietary polyphenols in the prevention and treatment of allergic diseases. Clin Exp Allergy. 2011;41:1346-1359.
- [CrossRef] [Google Scholar]
- Green tea catechin, epigallocatechin-3-gallate (EGCG): mechanisms, perspectives, and clinical applications. Biochem. Pharmacol.. 2011;82:1807-1821.
- [CrossRef] [Google Scholar]
- Quercetin sensitizes fluconazole-resistant candida albicans to induce apoptotic cell death by modulating quorum sensing. Antimicrob. Agents Chemother.. 2015;59:2153-2168.
- [CrossRef] [Google Scholar]
- ADORA2A rs5760423 and CYP1A2 rs762551 Polymorphisms as Risk Factors for Parkinson’s Disease. J. Clin. Med.. 2021;10:381.
- [CrossRef] [Google Scholar]
- Stimulation of vesicular-arbuscular mycorrhiza formation and growth of white clover by flavonoid compounds. New Phytol.. 1991;118:87-93.
- [CrossRef] [Google Scholar]
- Chemistry, Pharmacology and Health Benefits of Anthocyanins. Phytother. Res.. 2016;30:1265-1286.
- [CrossRef] [Google Scholar]
- Role of pro-inflammatory cytokines released from microglia in neurodegenerative diseases. Brain Res. Bull.. 2012;87:10-20.
- [CrossRef] [Google Scholar]
- Microbial biosynthesis of medicinally important plant secondary metabolites. Nat. Prod. Rep.. 2014;31:1497-1509.
- [CrossRef] [Google Scholar]
- Antioxidant effect of quercetin against acute spinal cord injury in rats and its correlation with the p38MAPK/iNOS signaling pathway. Life Sci.. 2013;92:1215-1221.
- [CrossRef] [Google Scholar]
- Mechanism of antioxidant properties of quercetin and quercetin-DNA complex. J. Mol. Model.. 2020;26:133.
- [CrossRef] [Google Scholar]
- Soto-Blanco, B., 2022. Herbal glycosides in healthcare, in: Herbal Biomolecules in Healthcare Applications. Elsevier, pp. 239–282. https://doi.org/10.1016/B978-0-323-85852-6.00021-4.
- Genistein and cancer: current status, challenges, and future directions. Adv. Nutr.. 2015;6:408-419.
- [CrossRef] [Google Scholar]
- Symbiotic properties of rhizobia containing a flavonoid-independent hybrid nodD product. J. Bacteriol.. 1989;171:4045-4053.
- [CrossRef] [Google Scholar]
- Inhibition of reverse transcriptases by flavonoids. Antiviral Res.. 1989;12:99-110.
- [CrossRef] [Google Scholar]
- Quercetin, a natural flavonoid interacts with DNA, arrests cell cycle and causes tumor regression by activating mitochondrial pathway of apoptosis. Sci. Rep.. 2016;6:24049.
- [CrossRef] [Google Scholar]
- Chemistry and structural biology of DNA damage and biological consequences. Chem. Biodivers.. 2011;8:1571-1615.
- [CrossRef] [Google Scholar]
- Bioactive effects of quercetin in the central nervous system: Focusing on the mechanisms of actions. Biomed. Pharmacother.. 2016;84:892-908.
- [CrossRef] [Google Scholar]
- Potential of terpenoids and flavonoids from asteraceae as anti-inflammatory, antitumor, and antiparasitic agents. Evid. Based Complement. Alternat. Med.. 2017;2017:1-2.
- [CrossRef] [Google Scholar]
- Effect of grape polyphenols on lactic acid bacteria and bifidobacteria growth: Resistance and metabolism. Food Microbiol.. 2011;28:1345-1352.
- [CrossRef] [Google Scholar]
- New modulated design and synthesis of quercetin–CuII/ZnII–Sn2IV scaffold as anticancer agents: in vitro DNA binding profile, DNA cleavage pathway and Topo-I activity. Dalton Trans.. 2013;42:10029.
- [CrossRef] [Google Scholar]
- The significance of the study about the biological effects of solar ultraviolet radiation using the exposed facility on the international space station. Biol. Sci. Space. 2004;18:255-260.
- [CrossRef] [Google Scholar]
- Environmental pollution and allergies. J. Toxicol. Pathol.. 2017;30:193-199.
- [CrossRef] [Google Scholar]
- Biological therapy of arthritis and systemic autoimmune diseases. Orv. Hetil.. 2007;148:63-70.
- [CrossRef] [Google Scholar]
- Seeing is believing: engineering anthocyanin and carotenoid biosynthetic pathways. Curr. Opinion Biotechnol.. 2008;19:190-197.
- [CrossRef] [Google Scholar]
- Pharmacological basis and new insights of quercetin action in respect to its anti-cancer effects. Biomed. Pharmacother.. 2020;121:109604
- [CrossRef] [Google Scholar]
- Pharmacological aspects of natural quercetin in rheumatoid arthritis. Drug Des. Devel. Ther.. 2022;16:2043-2053.
- [CrossRef] [Google Scholar]
- Quercetin: a promising phytochemical for the treatment of glioblastoma multiforme. Biofactors. 2020;46:356-366.
- [CrossRef] [Google Scholar]
- Antifungal activity of a novel quercetin derivative bearing a trifluoromethyl group on Candida albicans. Med. Chem. Res.. 2012;21:2217-2222.
- [CrossRef] [Google Scholar]
- Teoh, E.S., 2016. Secondary Metabolites of Plants, in: Medicinal Orchids of Asia. Springer International Publishing, Cham, pp. 59–73. https://doi.org/10.1007/978-3-319-24274-3_5.
- Flavonoids in foods: a review. Nat. Prod. Commun.. 2015;10 1934578X1501000
- [CrossRef] [Google Scholar]
- Loading into Nanoparticles Improves Quercetin’s Efficacy in Preventing Neuroinflammation Induced by Oxysterols. PLoS One. 2014;9:e96795.
- [CrossRef] [Google Scholar]
- The role of histamine and histamine receptors in mast cell-mediated allergy and inflammation: the hunt for new therapeutic targets. Front. Immunol.. 2018;9
- [CrossRef] [Google Scholar]
- Synthesis and antiviral activity of substituted quercetins. Bioorg. Med. Chem. Lett.. 2012;22:353-356.
- [CrossRef] [Google Scholar]
- Interstitial cystitis: bladder pain and beyond. Expert Opin. Pharmacother.. 2008;9:2979-2994.
- [CrossRef] [Google Scholar]
- Dietary polyphenolic phytochemicals—promising cancer chemopreventive agents in humans? A review of their clinical properties. Int. J. Cancer. 2007;120:451-458.
- [CrossRef] [Google Scholar]
- Cosmeceuticals containing herbs: fact, fiction, and future. Dermatol. Surg.. 2005;31:873-880. discussion 880
- [CrossRef] [Google Scholar]
- Anti-collagenase, anti-elastase, and anti-oxidant activities of extracts from 21 plants. BMC Complement. Altern. Med.. 2009;9:27.
- [CrossRef] [Google Scholar]
- Flavanones, chalcones and dihydrochalcones - nature, occurrence, and dietary burden. J. Sci. Food Agric.. 2000;80:1073-1080.
- [CrossRef] [Google Scholar]
- Quercetin acutely relaxes airway smooth muscle and potentiates β-agonist-induced relaxation via dual phosphodiesterase inhibition of PLCβ and PDE4. Am. J. Physiol.-Lung Cellular Mol. Physiol.. 2013;305:L396-L403.
- [CrossRef] [Google Scholar]
- Quercetin-containing self-nanoemulsifying drug delivery system for improving oral bioavailability. J. Pharm. Sci.. 2014;103:840-852.
- [CrossRef] [Google Scholar]
- Traub, M., Murray, M.T., 2020. Aphthous Stomatitis. In: Textbook of Natural Medicine. Elsevier, pp. 1114-1117.e1. https://doi.org/10.1016/B978-0-323-43044-9.00147-3.
- Neuroinflammation and depression: a review. Eur. J. Neurosci.. 2021;53:151-171.
- [CrossRef] [Google Scholar]
- Nano-Strategies to target breast cancer-associated fibroblasts: rearranging the tumor microenvironment to achieve antitumor efficacy. Int. J. Mol. Sci.. 2019;20:1263.
- [CrossRef] [Google Scholar]
- A novel nutraceutical formulation can improve motor activity and decrease the stress level in a murine model of middle-age animals. J. Clin. Med.. 2021;10:624.
- [CrossRef] [Google Scholar]
- Dietary cyanidin 3-O-β-D-Glucoside-Rich purple corn color prevents obesity and ameliorates hyperglycemia in mice. J. Nutr.. 2003;133:2125-2130.
- [CrossRef] [Google Scholar]
- Effect of kaempferol on hedgehog signaling pathway in rats with –chronic atrophic gastritis – Based on network pharmacological screening and experimental verification. Biomed. Pharmacother.. 2022;145:112451
- [CrossRef] [Google Scholar]
- The effect of inhibiting topoisomerase I and II on the antiapoptotic response associated with pro-inflammatory crystals of calcium pyrophosphate dihydrate in human neutrophils. Inflamm. Res.. 2003;52:8-17.
- [CrossRef] [Google Scholar]
- Recent advances in imaging and understanding interstitial cystitis. F1000Res. 2018;7:1771.
- [CrossRef] [Google Scholar]
- Luteolin as an anti-inflammatory and anti-allergic constituent of perilla frutescens. Biol. Pharm. Bull.. 2002;25:1197-1202.
- [CrossRef] [Google Scholar]
- Uivarosi, V., Munteanu, A., 2017. Flavonoid Complexes as Promising Anticancer Metallodrugs. In: Flavonoids - From Biosynthesis to Human Health. InTech. https://doi.org/10.5772/67879.
- Important flavonoids and their role as a therapeutic agent. Molecules. 2020;25:5243.
- [CrossRef] [Google Scholar]
- Quercetin and cancer: new insights into its therapeutic effects on ovarian cancer cells. Cell Biosci.. 2020;10:32.
- [CrossRef] [Google Scholar]
- Reaction enthalpies of OH bonds splitting-off in flavonoids: the role of non-polar and polar solvent. Comput. Theor. Chem.. 2014;1050:31-38.
- [CrossRef] [Google Scholar]
- Comparative analysis of ACTH and corticosterone sampling methods in rats. Am. J. Physiol.-Endocrinol. Metab.. 2005;289:E823-E828.
- [CrossRef] [Google Scholar]
- Hormesis and synergy: pathways and mechanisms of quercetin in cancer prevention and management. Nutr. Rev.. 2010;68:418-428.
- [CrossRef] [Google Scholar]
- Effect of flavonoids on learning, memory and neurocognitive performance: relevance and potential implications for Alzheimer’s disease pathophysiology. J. Sci. Food Agric.. 2014;94:1042-1056.
- [CrossRef] [Google Scholar]
- Antidiabetic effects of quercetin in streptozocin-induced diabetic rats. Comparative Biochem. Physiol. Part C: Toxicol. Pharmacol.. 2003;135:357-364.
- [CrossRef] [Google Scholar]
- A simple way for the preparation of natural antioxidant quercetin from rutin by subcritical water. J. Nat. Sci. Biol. Med.. 2017;8:213.
- [CrossRef] [Google Scholar]
- Antioxidant and antidiabetic activities of flavonoid derivatives from the outer skins of Allium cepa L. J. Agric. Food Chem.. 2020;68:8797-8811.
- [CrossRef] [Google Scholar]
- Quercetin inhibits angiotensin II-induced vascular smooth muscle cell proliferation and activation of JAK2/STAT3 pathway: a target based networking pharmacology approach. Front. Pharmacol.. 2022;13
- [CrossRef] [Google Scholar]
- Meta-analysis of the clinical effectiveness of traditional Chinese medicine formula Chaihu-Shugan-San in depression. J. Ethnopharmacol.. 2012;141:571-577.
- [CrossRef] [Google Scholar]
- Canonical Chinese medicine formula Danzhi-Xiaoyao-San for treating depression: a systematic review and meta-analysis. J. Ethnopharmacol.. 2022;287:114960
- [CrossRef] [Google Scholar]
- Bioactive flavonoids in medicinal plants: Structure, activity and biological fate. Asian J. Pharm. Sci.. 2018;13:12-23.
- [CrossRef] [Google Scholar]
- Quercetin exerts antidepressant and cardioprotective effects in estrogen receptor α-deficient female mice via BDNF-AKT/ERK1/2 signaling. J. Steroid Biochem. Mol. Biol.. 2021;206:105795
- [CrossRef] [Google Scholar]
- Novel insights of dietary polyphenols and obesity. J. Nutr. Biochem.. 2014;25:1-18.
- [CrossRef] [Google Scholar]
- Antidepressant active ingredients from herbs and nutraceuticals used in TCM: pharmacological mechanisms and prospects for drug discovery. Pharmacol. Res.. 2019;150:104520
- [CrossRef] [Google Scholar]
- Quercetin Inhibits Breast Cancer Stem Cells via Downregulation of Aldehyde Dehydrogenase 1A1 (ALDH1A1), Chemokine Receptor Type 4 (CXCR4), Mucin 1 (MUC1), and Epithelial Cell Adhesion Molecule (EpCAM) Med. Sci. Monit.. 2018;24:412-420.
- [CrossRef] [Google Scholar]
- Bacteriostatic effect of quercetin as an antibiotic alternative in vivo and its antibacterial mechanism in vitro. J. Food Prot.. 2018;81:68-78.
- [CrossRef] [Google Scholar]
- Airway inflammation, basement membrane thickening and bronchial hyperresponsiveness in asthma. Thorax. 2002;57:309-316.
- [CrossRef] [Google Scholar]
- Mechanism of Xiaoyao San in treatment of depression, breast hyperplasia, and functional dyspepsia based on network pharmacology. Zhongguo Zhong Yao Za Zhi. 2021;46:4230-4237.
- [CrossRef] [Google Scholar]
- The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J. Clin. Investig.. 1999;104:787-794.
- [CrossRef] [Google Scholar]
- Quercetin from shallots (Allium cepa L. var. aggregatum) is more bioavailable than its glucosides. J. Nutr.. 2008;138
- [CrossRef] [Google Scholar]
- The accelerator hypothesis: a review of the evidence for insulin resistance as the basis for type I as well as type II diabetes. Int J Obes. 2009;33:716-726.
- [CrossRef] [Google Scholar]
- Hippocampal Poly(ADP-Ribose) Polymerase 1 and Caspase 3 Activation in Neonatal Bornavirus Infection. J. Virol.. 2008;82:1748-1758.
- [CrossRef] [Google Scholar]
- Bioavailability and bioefficacy of polyphenols in humans. II. Review of 93 intervention studies. Am. J. Clin. Nutr. 2005
- [CrossRef] [Google Scholar]
- A case control study reveals that polyomaviruria is significantly associated with interstitial cystitis and vesical ulceration. PLoS One. 2015;10:e0137310.
- [Google Scholar]
- Biologic therapies for autoimmune and connective tissue diseases. Immunol. Allergy Clin. North Am.. 2017;37:283-299.
- [CrossRef] [Google Scholar]
- Quercetin-3-Glucoside is transported by the glucose carrier SGLT1 across the brush border membrane of rat small intestine. J. Nutr.. 2002;132:630-635.
- [CrossRef] [Google Scholar]
- Dietary flavonoid intake and risk of stomach and colorectal cancer. World J. Gastroenterol.. 2013;19:1011.
- [CrossRef] [Google Scholar]
- World Health Organization, 2021. World Health Organization - News room [WWW Document]. Depression. https://www.who.int/news-room/fact-sheets/detail/depression.
- New sulfonic derivatives of quercetin as complexing reagents: synthesis, spectral, and thermal characterization. J. Therm. Anal. Calorim.. 2015;120:351-361.
- [CrossRef] [Google Scholar]
- A flavonoid on the brain: quercetin as a potential therapeutic agent in central nervous system disorders. Life. 2022;12:591.
- [CrossRef] [Google Scholar]
- Anti-inflammatory and Cytotoxic Neoflavonoids and Benzofurans from Pterocarpus santalinus. J. Nat. Prod.. 2011;74:989-996.
- [CrossRef] [Google Scholar]
- Quercetin shows anti-tumor effect in hepatocellular carcinoma LM3 cells by abrogating JAK2/STAT3 signaling pathway. Cancer Med.. 2019;8:4806-4820.
- [CrossRef] [Google Scholar]
- Systematic investigation of quercetin for treating cardiovascular disease based on network pharmacology. Comb. Chem. High Throughput Screen.. 2019;22:411-420.
- [CrossRef] [Google Scholar]
- Inhibition of quercetin on liver fibrosis due to Schistosoma japonicum infection and on the expression of immediate early gene and metalloproteinase 1 inhibitor in liver tissue of mice. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi. 2006;24:148-149.
- [Google Scholar]
- Antioxidant activities of quercetin and its complexes for medicinal application. Molecules. 2019;24:1123.
- [CrossRef] [Google Scholar]
- Encapsulation and release of a hydrophobic drug from hydroxyapatite coated liposomes. Biomaterials. 2007;28:2687-2694.
- [CrossRef] [Google Scholar]
- Preparation of dual crosslinked alginate–chitosan blend gel beads and in vitro controlled release in oral site-specific drug delivery system. Int. J. Pharm.. 2007;336:329-337.
- [CrossRef] [Google Scholar]
- Density functional theory study of the structure–antioxidant activity of polyphenolic deoxybenzoins. Food Chem.. 2014;151:198-206.
- [CrossRef] [Google Scholar]
- A novel in vitro co-culture model comprised of Caco-2/RBL-2H3 cells to evaluate anti-allergic effects of food factors through the intestine. J. Immunol. Methods. 2016;435:1-6.
- [CrossRef] [Google Scholar]
- A systematic review of acupuncture and Chinese herbal medicine for postpartum depression. Complement. Ther. Clin. Pract.. 2018;33:85-92.
- [CrossRef] [Google Scholar]
- Anti-apoptotic and anti-oxidative roles of quercetin after traumatic brain injury. Cell. Mol. Neurobiol.. 2014;34:797-804.
- [CrossRef] [Google Scholar]
- Polyphenols differentially inhibit degranulation of distinct subsets of vesicles in mast cells by specific interaction with granule-type-dependent SNARE complexes. Biochem. J. 2013;450:537-546.
- [CrossRef] [Google Scholar]
- Quercetin in prostate cancer: chemotherapeutic and chemopreventive effects, mechanisms, and clinical application potential. Oncol. Rep.. 2015;33:2659-2668.
- [CrossRef] [Google Scholar]
- Quercetin: its main pharmacological activity and potential application in clinical medicine. Oxid. Med. Cell. Longev.. 2020;2020:1-13.
- [CrossRef] [Google Scholar]
- Estimated daily quercetin intake and association with the prevalence of type 2 diabetes mellitus in Chinese adults. Eur. J. Nutr.. 2019;58:819-830.
- [CrossRef] [Google Scholar]
- Flavonoids in food and their health benefits. Plant Foods Hum. Nutr.. 2004;59:113-122.
- [CrossRef] [Google Scholar]
- The therapeutic effects and mechanisms of quercetin on metabolic diseases: pharmacological data and clinical evidence. Oxid. Med. Cell. Longev.. 2021;2021:1-16.
- [CrossRef] [Google Scholar]
- Quercetin amelioratesAspergillus fumigatuskeratitis by inhibiting fungal growth, toll-like receptors, and inflammatory cytokines. Int. Immunopharmacol.. 2021;93:107435
- [CrossRef] [Google Scholar]
- Flavonoid permeability across an in situ model of the blood–brain barrier. Free Radic. Biol. Med.. 2004;36:592-604.
- [CrossRef] [Google Scholar]
- Mouse models for atherosclerosis and pharmaceutical modifiers. Arterioscler. Thromb. Vasc. Biol.. 2007;27:1706-1721.
- [CrossRef] [Google Scholar]
- Insect resistance management in Bacillus thuringiensis cotton by MGPS (multiple genes pyramiding and silencing) J. Cotton Res.. 2020;3:33.
- [CrossRef] [Google Scholar]
- Does quercetin improve cardiovascular risk factors and inflammatory biomarkers in women with Type 2 Diabetes: a double-blind randomized controlled clinical trial. Int. J. Prev. Med.. 2013;4:777-785.
- [Google Scholar]
- Zhang, M., Swarts, S.G., Yin, L., Liu, C., Tian, Y., Cao, Y., Swarts, M., Yang, S., Zhang, S.B., Zhang, K., Ju, S., Olek, D.J., Schwartz, L., Keng, P.C., Howell, R., Zhang, L., Okunieff, P., 2011. Antioxidant Properties of Quercetin. pp. 283–289. https://doi.org/10.1007/978-1-4419-7756-4_38.
- Shikonin inhibits the migration and invasion of human glioblastoma cells by targeting phosphorylated β-Catenin and phosphorylated PI3K/Akt: a potential mechanism for the anti-glioma efficacy of a traditional chinese herbal medicine. Int. J. Mol. Sci.. 2015;16:23823-23848.
- [CrossRef] [Google Scholar]
- Structure activity relationships and quantitative structure activity relationships for the flavonoid-mediated inhibition of breast cancer resistance protein. Biochem. Pharmacol.. 2005;70:627-639.
- [CrossRef] [Google Scholar]
- Physicochemical characterization and antioxidant activity of quercetin-loaded chitosan nanoparticles. J. Appl. Polym. Sci.. 2008;107:891-897.
- [CrossRef] [Google Scholar]
- Sucrose esters improve the colloidal stability of nanoethosomal suspensions of (−)-epigallocatechin gallate for enhancing the effectiveness against UVB-induced skin damage. J. Biomed. Mater. Res. B Appl. Biomater.. 2017;105:2416-2425.
- [CrossRef] [Google Scholar]
- Antioxidant activity of quercetin and its glucosides from propolis: a theoretical study. Sci. Rep.. 2017;7:7543.
- [CrossRef] [Google Scholar]
- Solvent effects on the intramolecular hydrogen-bond and anti-oxidative properties of apigenin: a DFT approach. Dyes Pigm.. 2017;141:179-187.
- [CrossRef] [Google Scholar]
- Discovery of metal ions chelator quercetin derivatives with potent anti-HCV activities. Molecules. 2015;20:6978-6999.
- [CrossRef] [Google Scholar]
- A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270-273.
- [CrossRef] [Google Scholar]
- Ankylosing spondylitis: etiology, pathogenesis, and treatments. Bone Res.. 2019;7:22.
- [CrossRef] [Google Scholar]
- Associations between ERα/β gene polymorphisms and osteoporosis susceptibility and bone mineral density in postmenopausal women: a systematic review and meta-analysis. BMC Endocr. Disord.. 2018;18:11.
- [CrossRef] [Google Scholar]







