Translate this page into:
A novel study on synthesis of egg shell based activated carbon for degradation of methylene blue via photocatalysis
⁎Corresponding author. awaisahmed@gcuf.edu.pk (Awais Ahmad)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract

Abstract
Egg shell-based activated carbon was successfully synthesized by the simple chemical activation process. Orthophosphoric acid and sodium hydroxide used as an activation agent. XRD pattern reveals the hexagonal structure of activated carbon. The functional group presents in activated carbon was identified using FT-IR spectroscopy. SEM images show irregular shapes of carbon. The photocatalytic performance of investigated activated carbon by illuminating methylene blue dye under UV–Visible irradiations. Photocatalytic activity of activated carbon results maximum degradation efficiency of 83%. Adsorption efficiency have been increased with respect of time for degradation of dye. Free radicals and superoxide’s play a significant role is decolourization of methylene blue. Photocatalytic activity of activated carbon synthesized by Orthophosphoric acid results shows the high degradation efficiency when compared to NaOH.
Keywords
Activated carbon
Photocatalytic activity
Methylene blue
Hexagonal
1 Introduction
In recent years, nanotechnology is a tool which has increasingly become an important topics for researchers to develop new devices using the nanoparticles to design, characterization of nanosized materials such as metal/metal oxide and from preparation aspects. These nanosized materials could potentially exposure in many different fields, such as drug delivery systems, biomedical-medicine, catalysis, and imaging/sensing etc. Among them, nanomaterials are appealing materials for applications as catalysis, due to their tremendous surface areas coupled with superior optical/electronic properties compared to larger-sized bulk materials (Erdoğan, 2020). Significant contributors to water pollution are textile industries that affect the biological oxygen demand level of water and pose a severe threat to aquatic fauna and flora. There are plenty of dyes which are present in water as pollutants and are troublesome to treat (Singh et al., 2017). Activated carbon have proven to be a tremendous substance by adsorbing the dye through chemical or physical bond over the surface to degrade dye and result in dye removal. The quality of Adsorption over the surface is owed to various physico chemical activities, including Pore volume, surface area, pore diameter(DP) and pore size distribution. Slight alteration in these properties greatly enhance the adsorption power of the activated charcoal (Kalantary et al., 2016; Zhao et al., 2020). Biomass such as Onion leaves (Hernández-Barreto et al., 2020) Sugar cane leaves (Li et al., 2016) Tobacco stalk (Bolbol et al., 2019), Cabbage (Zhang et al., 2019), Date palm (Alagha et al., 2020), Rice husk (He et al., 2019) Sewage sludge (Alagha, 2020), Tamarind seed (Andas and Satar, 2018), Teak saw dust (Armynah et al., 2014); Mosambi peels (Singh et al., 2019), Pea shells (Geçgel et al., 2013); Rubber wood sawdust (Kumar et al., 2005), Jute fiber (Senthilkumaar et al., 2005) and Mango seed kernel (Vasanth and Kumaran, 2005) have been utilized for the preparation of activated carbon. The chemical activation method which involves chemical activators such as H3PO4, KOH and ZnCl2 and then gets impregnated with resulting char. Execution of chemical activation can be achieved in a single step, whereas activation and carbonization are done simultaneously. A uni-step chemical activation method can scale down operational time, cost and energy consumption (Khanday et al., 2017). Porous carbonaceous absorbent, AC has better absorption ability than other absorbents (Marrakchi et al., 2016). Photocatalysis is time taking processes, due to the generation of electrons and holes pair via light that can easily recombine (Marrakchi et al., 2016). Various treatment techniques and strategies have been reported previously to eradicate the dyes from dye-bearing effluents. Among them, adsorption is a favourable method for eliminating coloured contaminants. This process is also significant for being a non-destructive process because it just involves the relocation of pollutants from one phase to another, rather than being eliminated. Therefore, the adsorbent regeneration is required prior to its reuse (Khanday et al., 2017).
In this present study, activated carbon was successfully synthesized by the chemical activation process with various activation agents. As the present investigation results, the comparison of Orthophosphoric acid and NaOH activation agent. Structural, morphological and optical properties of synthesized activated carbon were characterized using XRD, SEM and FT-IR spectroscopy. Methylene blue removal of activated carbon was experimentally visualized using a UV–Visible irradiation technique.
2 Materials and method
Egg shell was collected from the local market. Orthophosphoric acid and NaOH were purchased from Madras scientific chemicals, Tirunelveli, India. Methylene blue was obtained from Merck.
2.1 Preparation of activated carbon
Egg shells were washed and dried at sunlight for a day. Crush the powder and grounded it into a very fine powder. The powdered egg shell was well mixed with a 4:1 ratio of an activating agent (NaOH and H3PO4) and egg shell. Stirrer the solution for an hour. Place the solution 24 h for the activation process. Filter the solution and dried for 550 °C for 3 h. Thus activated carbon is obtained. Obtained carbon was stored for further characterization.
2.2 Characterization of activated carbon (AC)
The structural and phase of investigated AC was investigated using XRD. Crystallographic patterns were recorded using PAN analytical XPERT PRO Diffractometer. FT-IR spectra were recorded using a Perkin Elmer spectrometer with KBr pellets. FT-IR spectra were recorded from 4000 to 600 cm−1. Nano Metrix visualized the particle size of investigated activated carbon. The surface morphology of activated carbon was visualized using Joel JSM 6390 Scanning Electron microscope.
2.3 Photocatalytic activity
The dye degradation efficiency of activated carbon was recorded using UV–Visible irradiation. 50 ml/L of methylene blue is mixed with 0.50 g of active material. The mixture of activated carbon and methylene blue were well mixed using stirrer and centrifuged. Degradation of methylene blue removal was experimentally carried out using UV–Visible irradiations. The dye degradation efficiency of activated carbon was calculated using
η- removal efficiency
C0- Initial concentration (mg/L) of methylene blue
Ct- Concentration of methylene blue at equilibrium (mg/L) (Zhang et al., 2019).
3 Results and discussion
3.1 XRD analysis
Crystal structure, micro strain, density dislocation and average crystalline size were calculated from the XRD spectrum. XRD pattern of activated carbon was shown in Fig. 1. X-ray diffraction peaks observed at 29.5°, 36.1°, 39.5°, 43.4°, 47.7°, 48.6°, and 57.7° corresponds to Braggs reflection planes of (2 0 1), (1 0 5), (1 0 7), (2 0 7), (2 0 6), (3 0 4), and (3 1 4) respectively. Obtained diffraction peaks show the hexagonal crystal structure of carbon and well-matched with JCPDS: 721,616 (Amarasinghe and Wanniarachdhi, 2019). In addition to that peak at 23.1° corresponds to (0 1 2) reflection plane corresponds to the rhombohedral structure of CaCO3. From XRD pattern shows a mixed crystal phase with a hexagonal structure of carbon. Variations in average crystalline, change in peak intensity peak shift due to the influence of activating agent and egg shell components. For H3PO4 activated egg shell results in high crystalline peaks when compared to NaOH. The addition of NaOH activation agent results indicates the removal of silica and ash content. There is a slight variation in the XRD pattern of activated carbon due to the activation agent. Phosphoric acid increases the porous structure of carbon.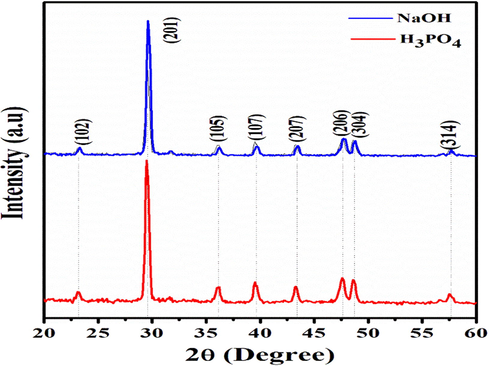
XRD of Egg shell based activated carbon (XRD: X-ray diffraction).
3.2 Fourier transform infrared Spectroscopy.
Functional group and chemical components present in egg shell based activated carbon were recorded using FT-IR spectroscopy. FT-IR spectra of AC is depicted in Fig. 2. The hydroxyl group presents at 3369 and 3359 cm−1. The observed peak at 2916 and 2878 cm−1 corresponds to C—H interaction with the surface of the carbon. A climax at 1430 cm−1 attributed to the carbonate group vibration (Balasubramanian et al., 2019). The band observed at 1064 cm−1 corresponds to the vibration of the phosphate group. 948 corresponds to the vibration of calcium carbonate. The band observed at 878 and 800 cm−1 due to the vibration of carbonate. The peak observed at 699 cm−1 due to C—H vibration. A spike at 512 cm−1 represents the vibration of PO43− (Tangboriboon et al., 2012). Change in peak intensity is due to Protoporphyrin IX pigment present in the egg shell. When phosphoric acid interacted with phenolic and carbonyl groups of carbon results P-Carbon (C-O-P). Formation of C—O—P bond results development of microspores on the surface of carbon (Shalma et al., 2008). The presence of nitrogen, oxygen, and hydrogen quickly bond with other metals on the surface. This result in decreasing activation energy for methylene blue degradation and more surface area to the activated sites on the AC (Luo, 2009).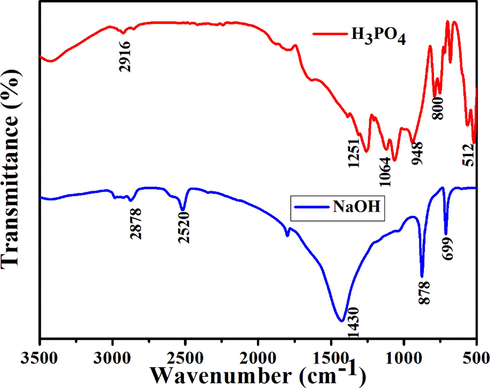
FT-IR spectra of Egg shell based activated carbon (Red) H3PO4 (b) NaOH. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.3 Scanning electron microscope
The structural morphology of AC was visualized by implementing SEM technique. SEM images of activated carbon were shown in Fig. 3(a,b). The morphological view of activated carbon from the egg shell reveals irregular shape. Investigated activated carbon result ununiformed structure of carbon. The acid acts as a dehydration agent that inhibits tar formation. The calcination process enhances the surface area. Thermal effect or temperature is one of the most influencing factors for activated carbons. This process on the surface area forms the active sites. The active sites are known to be the material absorption process (Pezoti et al., 2016), which results in micropores present in activated carbon. Activated carbon prepared by NaOH the activation agent results from the smooth surface of carbon with less amount of microspores (Islam et al., 2015).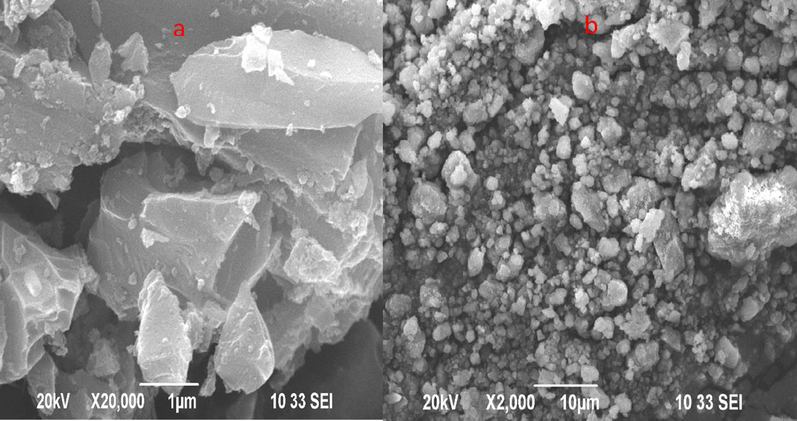
FESEM images Egg shell based activated carbon (a’), NaOH (b), H3PO4.
3.4 Particle size analyzer
The Average particle size value of investigated activated carbon shows 89 nm for NaOH activation and 88 nm for Orthophosphoric acid activation. DLS spectra of activated carbon were shown in Fig. 4. Investigated activated carbon results poly dispersive index of 0.765 and 0.57 respectively.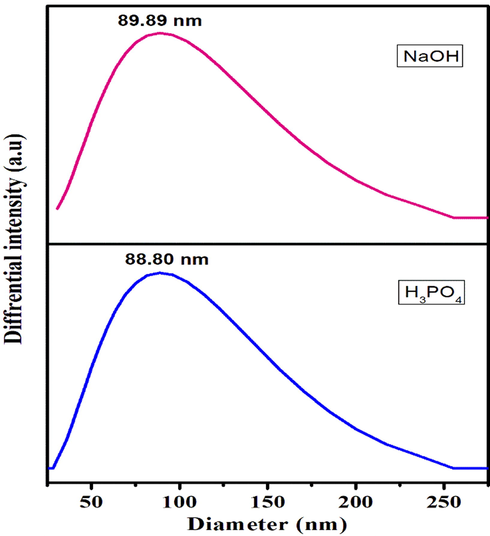
DLS spectra of Egg shell based activated carbon.
3.5 Photocatalytic activity
Photodegradation of methylene blue results in the absorption efficiency of activated carbon. Photocatalytic activity of activated carbon was experimentally studied using UV–Visible irradiation technique. Activated carbon used as an active source to degrade dye molecules. Photodegradation of activated carbon was recorded at every 30 min’ interval of time. Fig. 5.a and b. shows, photodegradation of methylene blue (see Fig. 6).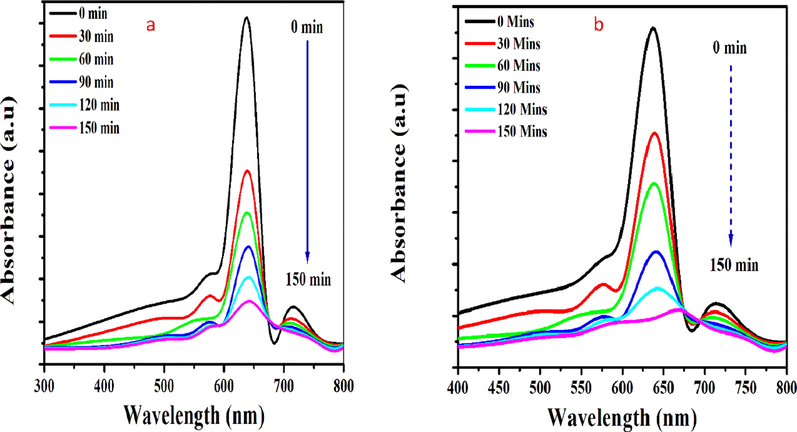
shows Photocatalytic activity of Egg shell based activated carbon (a) With H3PO4 84% (b) with NaOH 74%
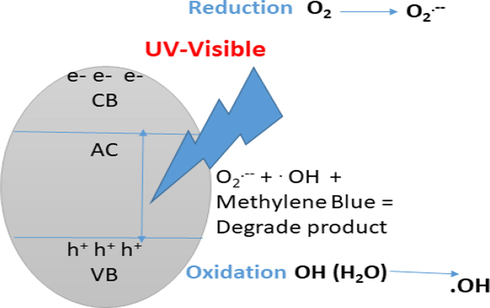
Schematic representation about photocatalysis and its impact on degradation of organic pollutant i.e. Methylene Blue.
The UV–Visible absorption band of MB monomer in water molecules corresponds to 665 nm region which shows n-π* transition of MB (Ilomuanya and Igwilo, 2017). Excitation of electrons from the CB to VB generates a hole behind. Which causes photo to generate electron and spots on the surfaces of the carbon OH group present in activated carbon interact with holes to form OHO free radical. Oxygen molecules interact with the carbon surface to form superoxide’s (O2….). Organic pollutant (MB) can neutralize to H2O and CO2 and mineral acids present in activated carbon are responsible for dye removal (Jayakumar et al., 2017). Decolourization of methylene blue is due to the destruction of Azo bonds (—N⚌N—). During the degradation process, dye molecules were converted to leuco Methylene blue, which results informs in change the colour formation. Carbon + hѵ → h+ + e− h+ + O-H → OH---- e−+ O2 → O2---- MB + ROS → CO2 + H2O
Activated carbon results show the maximum degradation efficiency of about 74% and 83% for NaOH activation and H3PO4 activated carbon. % R of methylene blue dye. % R at 0, 30, 60, 90, 120and 150 min were 29.90, 45.17, 49.59, 76.09, and 83.96, respectively for H3PO4 and 32, 43, 56, 66, 68, 74 %R for NaOH (Ahmad et al., 2020). The absorption spectra reveal the degradation of MB from aqueous medium increases as the reaction time increases (Bagheri et al., 2015). The surface of AC is enriched mainly carboxyl, carbonyl, lactone, phenolic hydroxyl groups, and many oxygen-containing functional groups together, which determine its adsorption performance. There are three main modes of action which increase the photodegradation of MB, the first is to give electrons-to be affected by electrons, the second is electrostatic action, and the third is coordination reaction (Muzarpar et al., 2020). The photocatalytic activity results tell the fact of increases in methylene blue removal from 0 to 30 min. The degradation rate slows after 30 to 120 min for both samples. MB decomposes to leuco methylene blue after 120 min. The photocatalytic activity of AC prepared using (H3PO4) was higher when compared to NaOH. By adding phosphoric acid as an activation agent which gives the carbon results to C-O-P formation due to the carbon phosphorous formation to the surface. The results reassure the microspores distribution on the surface of AC. Active microspores present in the activated carbon results give high degradation efficiency when compared to NaOH. Degradation efficiency increases with high porosity, spectacular adsorption capacity, and enhanced active sites for the reacting species during chemical reaction (Hameed, 2020; Ramli et al., 2014; Huang et al., 2011; Hoseinzadeh Hesas et al., 2013; Foo and Hameed, 2012; Deng et al., 2010).
4 Conclusion
In this work, activated carbon was successfully synthesized by the chemical activation method. The rich amount of carbon having high absorbent due to the surface area and porous nature of the material. XRD pattern reveals a mixed phase of activated carbon with a hexagonal crystal structure of carbon. FT-IR identified the functional group present in activated carbon. Particle size increases for NaOH activation. SEM images reveal the irregular form of activated carbon with active microspores. Photodegradation of activated carbon results maximum degradation efficiency of 83% at 120 min. Degradation efficiency rate increases from 0 to 30 min. From the photocatalytic activity of activated carbon results, the investigated activated carbon is highly applicable for wastewater treatment.
Acknowledgements
This work was funded by the Researchers Supporting Project Number (RSP-2020/243) King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Biosynthesis, chararectrisation and photocatalytic degradation of methylene blue removal using silver nanoparticles. Mater. Today Proc. 2020
- [CrossRef] [Google Scholar]
- Comparative adsorptive removal of phosphate and nitrate from wastewater using Biochar-MgAl LDH nanocomposites: coexisting anions effect and mechanistic studies. Nanomaterials. 2020;2020(10):336.
- [CrossRef] [Google Scholar]
- Magnetic Mg-Fe/LDH intercalated activated carbon composites for nitrate and phosphate removal from wastewater: insight into behavior and mechanisms. Nanomaterials. 2020;10:1361.
- [CrossRef] [Google Scholar]
- Eco-friendly photocatalyst derived from Eggshell waste for dye degradation. J. Chem. 2019:8184732.
- [Google Scholar]
- Synthesis and characterization of tamarind seed activated carbon using different types of activating agents: a comparison study. Mater. Today: Proc.. 2018;5:17611-17617.
- [Google Scholar]
- Annealing effect on the particle size and chemical composition of activated carbon obtained from vacuum furnace of teak sawdust. AIP Conf. Proc.. 2014;1617:19.
- [CrossRef] [Google Scholar]
- Functionised activated carbon derived from biomass for photocatalysis applications perspective. Int. J. Photoenergy 2015
- [CrossRef] [Google Scholar]
- Electrochemical performances of activated carbon prepared using eggshell waste, SN. Appl. Sci. 2019
- [Google Scholar]
- Layered double hydroxide–loaded biochar as a sorbent for the removal of aquatic phosphorus: behavior and mechanism insights. Arab. J. Geosci.. 2019;12:503.
- [CrossRef] [Google Scholar]
- Optimization of preparation of activated carbon from cotton stalk by microwave assisted phosphoric acid-chemical activation. J. Hazard. Mater.. 2010;182:2.
- [Google Scholar]
- Preparation and stabilization of Ag nanoparticles with N-vinyl-2-pyrrolidone grafted-poly (vinyl alcohol) in an organic medium and investigation of their usability in the catalytic dye decolorization. Colloid Interface Sci. Commun.. 2020;34
- [CrossRef] [Google Scholar]
- Porous structure and adsorptive properties of pineapple peel based activated carbons prepared via microwave assisted KOH and K2CO3 activation. Microporous Mesoporous Mater.. 2012;148:191-195.
- [Google Scholar]
- Geçgel, Ünal, Özcan, Gülce, Gürpınar, Gizem Çağla, 2013. Removal of methylene blue from aqueous solution by activated carbon prepared from Pea Shells (Pisum sativum). J. Chem. 2013, Article ID 614083, 9p. http://dx.doi.org/10.1155/2013/614083.
- Synthesis of Si/Cu amorphous adsorbent for efficient removal of methylene blue dye from aqueous media. J. Inorg. Organomet. Polym. Mater. 2020
- [CrossRef] [Google Scholar]
- Efficient phosphate removal from wastewater by MgAl-LDHs modified hydrochar derived from tobacco stalk. Bioresour. Technol. Rep.. 2019;8:100348
- [CrossRef] [Google Scholar]
- Adsorption and photocatalytic study of phenol using composites of activated carbon prepared from onion leaves (Allium fistulosum) and metallic oxides (ZnO and TiO2) Catalysts. 2020;10:574.
- [CrossRef] [Google Scholar]
- The effects of a microwave heating method on the production of activated carbon from agricultural waste: a review. J. Anal. Appl. Pyrolysis. 2013;100:1-11.
- [Google Scholar]
- Comparative study on characterization of activated carbons prepared by microwave and conventional heating methods and application in removal of oxytetracycline (OTC) Chem. Eng. J.. 2011;171:1446-1453.
- [Google Scholar]
- Effect of pore size and morphology of activated charcoal prepared from midribs of Elaeis Guineensis on adsorption of poisons using meteronidazole and Escherichia coli O157:H7 as a case study. J. Microsc. Ultrastruct.. 2017;4(1):32-38.
- [Google Scholar]
- Methylene blue adsorption on factory-rejected tea activated carbon prepared by conjunction of hydrothermal carbonization and sodium hydroxide activation processes. J. Taiwan Inst. Chem. Eng.. 2015;52:57-64.
- [Google Scholar]
- Photocatalytic degradation of methylene blue by nickel oxide nanoparticles. Mater. Today Proc.. 2017;4:11690-11695.
- [Google Scholar]
- The survey of Malathion removal using magnetic graphene oxide nanocomposite as a novel adsorbent: thermodynamics, isotherms, and kinetic study. Desalin. Water Treat.. 2016;57(58):28460-28473.
- [Google Scholar]
- Cross-linked beads of activated oil palm ash zeolite/chitosan composite as a bio-adsorbent for the removal of methylene blue and acid blue 29 dyes. Int. J. Biol. Macromol.. 2017;95:895-902.
- [Google Scholar]
- Mesoporous zeolite-activated carbon composite from oil palm ash as an effective adsorbent for methylene blue. J. Taiwan Inst. Chem. Eng.. 2017;70:32-41.
- [Google Scholar]
- Adsorption of bismark brown dye on activated carbons prepared from rubberwood sawdust (Hevea brasiliensis) using different activation methods. J. Hazard. Mater.. 2005;126(1–3):63-70.
- [Google Scholar]
- Enhancing phosphate adsorption by Mg/Al layered double hydroxide functionalized biochar with different Mg/Al ratios. Sci. Total Environ.. 2016;559:121-129.
- [CrossRef] [Google Scholar]
- The performance of phosphoric acid in the preparation of activated carbon-containing phosphorous species from rice husk residue. J. Mater. Life Sci.. 2009;54:5008-5021.
- [Google Scholar]
- Mesoporous-activated carbon prepared from chitosan flakes via single-step sodium hydroxide activation for the adsorption of methylene blue. Int. J. Biol. Macromol.. 2016;98:233-239.
- [Google Scholar]
- Cross-linked chitosan/sepiolite composite for the adsorption of methylene blue and reactive orange 16. Int. J. Biol. Macromol.. 2016;93:1231-1239.
- [Google Scholar]
- Exploration sustainable base material for activated carbon production using agriculture waste as raw materials: a review. IOP Conf. Series: Mater. Sci. Eng.. 2020;864
- [CrossRef] [Google Scholar]
- NaOH-activated carbon of high surface area produced from guava seeds as a high-efficiency adsorbent for amoxicillin removal: Kinetic, isotherm and thermodynamic studies. Chem. Eng. J.. 2016;288:778-788.
- [Google Scholar]
- Ramli, Zatil Amali Che, Asim, Nilofar, Isahak, Wan N.R.W., Emdadi, Zeynab, Ahmad-Ludin, Norasikin, Ambar Yarmo, M., Sopian, K., 2014. Photocatalytic degradation of methylene blue under UV light irradiations on prepared carbonaceous TiO2, Sci. World J. https://doi.org/10.1155/2014/415136.
- Adsorption of methylene blue onto jute fiber carbon: kinetics and equilibrium studies. J. Colloid Interface Sci.. 2005;284(1):78-82.
- [Google Scholar]
- Fourier transform infrared spectra of technologically modified calcium phosphate. RTU Res. Inf. Syst. 2008:68-71.
- [Google Scholar]
- Removal of methylene blue dye using activated carbon prepared from biowaste precursor. Indian Chem. Eng. 2017
- [CrossRef] [Google Scholar]
- Removal of methylene blue dye using activated carbon prepared from biowaste precursor. Indian Chem. Eng. 2019 ISSN: 0019-4506 (Print) 0975-007X
- [Google Scholar]
- Preparation of calcium oxide from egg shell via calcination. Mater. Sci. 2012:31-322.
- [Google Scholar]
- Removal of methylene blue by mango seed kernel powder. Biochem. Eng. J.. 2005;27(1):83-93.
- [Google Scholar]
- Adsorption of phosphate from aqueous solution by vegetable biochar/layered double oxides: fast removal and mechanistic studies. Bioresour. Technol.. 2019;284:65-71.
- [CrossRef] [Google Scholar]
- Metal-based nanocatalysts via a universal design on cellular structure. Adv. Sci.. 2020;7(3):1902051.
- [Google Scholar]







