Translate this page into:
A review of the historical records, chemistry, pharmacology, pharmacokinetics and edibility of Angelica dahurica
⁎Corresponding author at: School of Pharmacy, Binzhou Medical University, Yantai 264003, China. 2665275709@qq.com (Long Dai)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Background
Angelica dahurica (Hoffm.) Benth. & Hook. f. ex Franch. & Sav. (AD) is a tall perennial herb named Baizhi in Chinese Medical Works. Its medicinal use was first recorded in Shennong's Classic of Material Medical, the first herbal monograph in ancient China.
Aim of the review
This review systematically summarises and evaluates traditional applications, botany, chemistry, and pharmacology of AD. This review aims to support the researchers who would like to explore its further potential as a medical agent.
Materials and methods
The information was collected from Web of Science (https://www.webofscience.com), PubMed (https://pubmed.ncbi.nlm.nih.gov), CNKI (https://kns.cnki.net), Dissertations, Chinese Pharmacopoeia, and Government documents. The keyword used in the literature search was Angelica dahurica. The chemical structures of the compounds in AD were obtained from either research articles or PubChem. The historical origin and ethnopharmacology of AD were reviewed in detail. In addition, relevant information was obtained from regional and global unpublished sources. The plant's name and its classifications were confirmed using the Medicinal Plant Name Service (http://mpns.kew.org) and Plants of the World Online (https://powo.science.kew.org).
Results
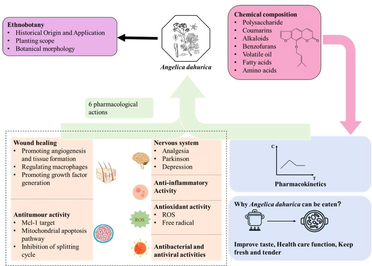
Over time, AD has been used extensively for its medicinal uses. The bioactive chemical compound present in it is imperatorin (IMP). It has anti-inflammatory, analgesic, and other therapeutic effects, and it is used as a marker for the quality evaluation of AD. The most studied part of the plant is the root. The root contains coumarins and volatile oil. Modern science has found that AD has the ability to treat skin wounds, analgesic, anti-inflammatory, anti-tumor, anti-depressant, and antioxidant effects, etc.
Conclusions
This paper presents a detailed comparative analysis of available resources, confirming the origin, traditional applications, and therapeutic uses of AD. Most importantly, it is widely welcomed as a therapeutic drug. The results of several studies showed that the chemical components of AD were more deeply involved in the central nervous system and histopathological mechanisms. Moreover, AD not only has medical effects but also can be used as food condiments and food accessories.
Keywords
Angelica dahurica
Medicinal use
Chemical composition
Pharmacological effects
Pharmacokinetics
Edibility
- AD
-
Angelica dahurica (Hoffm.) Benth. & Hook. f. ex Franch. & Sav.
- ADEE
-
AD ethanol extract
- ADP
-
Angelica dahurica polysaccharides
- AKT
-
protein kinase B
- ABTS
-
2,2-azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt
- Bcl-2
-
B-cell lymphoma-2
- Bax
-
Bcl-2-Associated X
- CGRP
-
Calcitonin gene related peptide
- COX-2
-
cycloxygenase-2
- CAMK Ⅱ
-
Ca2+/calmodulin-dependent kinase Ⅱ
- DL
-
duliang Soft Capsule
- DA
-
dopamine
- DPPH
-
2,2-diphenyl-1-picrylhydrazyl
- EGF
-
epidermal growth factor
- ERK
-
extracellular signal-regulated kinase
- ET
-
endothelin
- FIB
-
fibrinogen
- GPR119
-
G-protein-coupled receptor 119
- GLP-1
-
glucagon-like peptide-1
- HPLC
-
high performance liquid chromatography
- HUVEC
-
human umbilical vein endothelial cells
- HIF-1α
-
hypoxia-inducible factor-1α
- H1N1 and H9N2
-
influenza A viruses
- HL-60
-
human promyelocytic leukaemia cells
- hKv1.5
-
human Kv1.5 channel
- IMP
-
imperatorin
- IL-6
-
interleukin-6
- IL-12
-
interleukin 12
- IL-1β
-
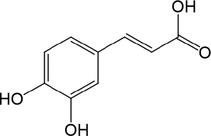
interleukin-1β
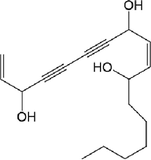
- IκB
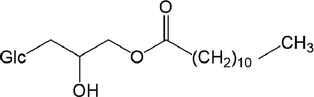
-
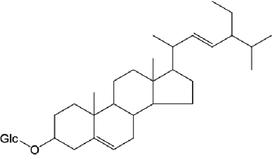
inhibitor of NF-κBα
- IFN-γ
-
interferon-gamma
- INOS
-
inducible nitric oxide synthase
- JNK
-
c-jun n-terminal kinase
- LDH
-
lactate dehydrogenase
- LAD2 cells
-
human laboratory of allergic disease 2
- LC-MS/MS
-
liquid chromatographic-tandem mass spectrometric method
- Mcl-1
-
myeloid cell leukaemia 1
- MAPK
-
mitogen-activated protein kinase
- MDC/CCL22
-
macrophage-derived chemokines
- MRGPRX2
-
mas-related g protein-coupled receptor x 2
- NE
-
norepinephrine
- NF-κB
-
nuclear factor kappa-B
- NADPH
-
nicotinamide adenine dinucleotide phosphate
- OGD-R
-
oxygen-glucose deprivated/reperfusion
- OXPHOS
-
oxidative phosphorylation
- PDGF-β
-
platelet-derived growth factor-β
- P53
-
tumour suppressor gene
- PD
-
parkinson's disease
- PSD
-
post synaptic density
- PGE2
-
prostaglandin E2
- PXR
-
pregnane X receptor
- ROS
-
reactive oxygen species
- ROCC
-
receptor-operated Ca2+ channel
- SH-SY5Y
-
human neuroblastoma cells
- SAZ
-
synaptic active zone
- TGF-β1
-
transforming growth factor-β1
- TNF-α
-
tumor necrosis factor-α
- TLR4
-
toll-like receptor 4
- TRPV1
-
proteins form calcium channels that transmit pain to the central nervous system
- TARC/CCL17
-
thymus and activation-regulated chemokines
- VEGF
-
vascular endothelial growth factor
- VDCC
-
voltage-dependent calcium channel
- WHO
-
World Health Organization
- YB
-
Yuanhu-Baizhi herbal pair
- 6-OHDA
-
6-hydroxydopamine
- 5-HT
-
5-hydroxytryptamine
Abbreviations
1 Introduction
Baizhi, also known as Xiangbaizhi, Latin name is Angelica dahurica (Hoffm.) Benth. & Hook. f. ex Franch. & Sav. It is mainly grown in China. Five different names exist for AD in the plant database (https://powo.science.kew.org, last accessed at 07/09/2022). AD is famous for its medicinal and edible uses. It is included in the Chinese government's “Traditional Food and Chinese Medicinal Materials Catalogue” (Zhou and Na, 2022). It is well known that the roots of AD have the effects of expelling wind, relieving pain, dehumidifying, and relieving itching (Zhu et al., 2022). It is often used to treat the symptoms of wind-cold migraine, rhinitis and, dermatocyst. The main active ingredients in AD imperatorin (IMP). It have shown efficacy in treating tumors and antioxidants. This review systematically investigated the uses of AD regarding its historical uses, medicinal preparations, chemical compounds present, pharmacological activities, mechanisms of action, pharmacokinetics, etc (Li et al., 2020).
Currently, only a very few review articles on AD are already published. Therefore, this article reviews the historical applications, chemical composition, pharmacological uses, mechanisms of action, and pharmacokinetics of AD. In addition, the research gaps between traditional uses and modern clinical research are identified to guide future research.
1.1 Historical records
Bai Zhi (白芷) first appeared in the poem Li Sao of Qu Yuan in China's Warring States Period. Many alternate names for AD were recorded in Qu Yuan's Chu ci (楚辞), such as “Bai Zhi (白芷)”, “Fang Zhi (芳芷)”, “ Zhi (茝)”, “Bi Zhi (辟芷)”, “Yao Zhi (药芷)” and “Fang Xiao (芳虈)”. During the Warring States Period, between nobles, it was often made into a sachet. Since then, AD, as a representative of noble quality, had become the object of poetry in all dynasties (Zhang, 1986). In the pre-Qin and Han dynasties, AD was also called li (蓠), Fu Li (苻蓠), Xiao (虈), and Wan (莞) (Xu, 2013). AD was mostly used in vanilla at this time, and its medical use was not recorded in detail.
The first clear record of the medicinal use of AD was recorded in the Shennong's Classic of Material Medical (神农本草经) of the Han Dynasty, which indicates that AD had the functions of dispelling wind, stopping bleeding, and beautifying, and it is still used today. In Wei and Jin dynasties, it was recorded in Ming Yi Bie Lu (名医别录) that AD could cure wind evil, vomiting, head dizziness, and itching. It could be used to make a plaster. This book added the functions and aliases of AD. At this time, AD was still growing in the wild, and there was no cultivation. Later, in the Southern and Northern Dynasties, Tao Hongjing's Collective Notes to the Canon of Materia medica (本草经集注) described that AD leaves could be used in bathing to add fragrance. Traditional Chinese religious Daoists used it to remove corpse insects and made incense (Tao, 1994). It was written in Master Lei's Discourse on Drug Processing (雷公炮炙论) that Sang gong teng (One Chinese herbal medicine) and Iris lacteal var. chinensis (Fisch.) Koidz. (Ma Li) were commonly used as counterfeits of AD. Two works of the Tang Dynasty, Yao Xing Lun (药性论) and Ri Hua Zi Ben Cao (日华子本草) recorded that AD could remove facial scars and could be used to beautify the face (Tang, 1957). Later, empress Dowager ci xi's famous beauty secret recipe “Yurong powder (玉荣散)”, AD was its main medicine. By the Song Dynasty, AD had been widely known. Song Su recorded the detailed characteristic morphology of AD in Notes on Ben Cao Tu Jing (本草图经) (Song, 1988). Jia You Ben Cao (嘉佑本草) recorded that the first artificial cultivation of AD appeared in Shandong, China (Niu et al., 2018). Therefore, AD was more widely distributed after the Song Dynasty and gradually began to be cultivated. According to plant morphology, AD at this time should be is A. dahurica (Fish. ex Hoffm.) Benth. et Hook. f. var. formosana (Boiss.) Shan et Yuan (Chinese vernacular names: Hang bai zhi). After the Jin and Yuan dynasties, the efficacy of AD was further detailed. AD tastes aromatic, and the role of treating cold. The ability of AD to open the orifices (通窍) was first described in the Yao Yong Fa Xing (药用法象). After the Ming Dynasty, there were many records about AD. Mi Lan recorded another source of AD, Heracleum scabridum Franch (Chinese vernacular name: Dian bai zhi) in his work on Materia Medica of Southern Yunnan (滇南本草) (Lan, 1959). Later, Wang et al. (2020) thought AD should belong to H. scabridum. The Compendium of Materia Medica (本草纲目) (Tao, 1994), known as the pharmaceutical treasure trove, summarised the previous records of AD in detail, including plant morphology, efficacy, and classical prescriptions. At this time, cultivated AD gradually replaced wild AD and became the mainstream of commodity. During the Ming and Qing dynasties, Hangbaizhi became the mainstream commoditiy. According to Wang et al. (2001) AD at this time was more likely to be the Angelica dahurica var. formosana (Boissieu) Yen (Chinese vernacular name: Tai wan bai zhi). Angelica dahurica (Fisch. ex Hoffm.) Benth. et Hook. f (Chinese vernacular names: Yu bai zhi, Qi bai zhi) was recorded in the Yao Wu Chu Chan Bian (药物出产辨). Wang et al. (2004) believed that Yu bai zhi should be a variety brought back to Changge County from other places due to commodity exchanges during the Qianlong period of the Qing dynasty. After modern research and investigation, Wang et al. (2020) speculated that Yu bai zhi should be a wild AD cultivated in North China after domestication. In modern times, from the Chinese Pharmacopoeia published in 1930 to the 2020 edition of Chinese Pharmacopoeia, there was a systematic and detailed description of AD's medicinal parts, properties, identification, content determination, taste, and function. With the deepening of research, AD, a traditional Chinese medicine, has gradually been known worldwide. Table 1 briefly lists the development process of AD in China according to the changes in dynasties.
Period
Author
Monographs
Warring States period
Yuan Qu
Li Sao, Chu Ci
Pre-qin and Han dynasties
Unknown
Shennong Materia Medica Classic
Wei and jin dynasty
Hongjing Tao
Ming Yi Bie Lu
Northern and Southern dynasties
Hongjing Tao
Collective Notes to the Canon of Materia medica
Xiao Lei
Master Lei's Discourse on Drug Processing
Tang dynasty
Unknown
Drug property theory
Five Dynasties and Ten kingdoms
Zihua Ri
Ri Hua Zi Ben Cao
Bei song dynasty
Song Su
Ben Cao Tu Jing
Yuxi Zhang
Jia You Ben Cao
Unknown
Unknown
Yao Yong Fa Xing
Ming dynasty
Shizhen Li
Compendium of Materia Medica
1930
Renshan Chen
Yao Wu Chu Chan Bian
Recent period
Chinese government
Chinese Pharmacopoeia
At present, the number of Chinese patent medicine prescriptions of AD was 557, and the number of traditional Chinese medicine prescriptions was 1841 (https://www.da.yaozh.com, last accessed at 07/06/2022). Among them, Bai Zhi Gao and Huo Xiang Zheng Qi He Ji were the most famous and widely used prescriptions. Modern clinical studies had shown that AD combined with other chinese herbal medicines could effectively treat headache, vitiligo, psoriasis, constipation, and other diseases (https://www.da.yaozh.com, last accessed at 07/06/2022). In traditional Chinese therapies, AD was often combined with other medicinal plants such as Ligusticum chuanxiong Hort., Notopterygium incisum Ting ex H.T. Chang and Panax ginseng (https://powo.science.kew.org, last accessed at 07/09/2022). However, the possible interactions, synergistic effects, and underlying action mechanisms between the bioactive compounds present in AD and other medicinal plants remain unknown and should be further investigated. In addition, AD could be used not only as medicine but also as healthy food. AD was approved by the Chinese Health and Family Planning Commission as an herbal medicine that can be used as a health food (Yang et al., 2021). In China, more than 700 kinds of health foods containing AD had been recorded, with purported curative powers ranging from functions of removing spots from beauty, anti-fatigue, increasing immunity, regulating blood lipids, and improving sleep (https://www.da.yaozh.com, last accessed at 07/06/2022). Such products include Rain Brand Beauty Oral Liquid of Forest (Beauty Oral Liquid), Panlong Yunhai Shiliwei Capsule (Sleep improvement capsule), and Zhiwangpai Yishen Capsule (Relieving fatigue capsule).
AD is present in at least 8 provinces in China. Such as Neimenggu, Hebei, Henan, Sichuan, Anhui, Zhejiang, Fujian, and Taiwan. AD is suitable for planting in sandy loam and loam with flat terrain, deep soil layer, loose and fertile soil, and good drainage, but not in sandy land, saline-alkali land, gravel land, and low-lying land. The general geographical distribution of AD in China is shown in Fig. 1.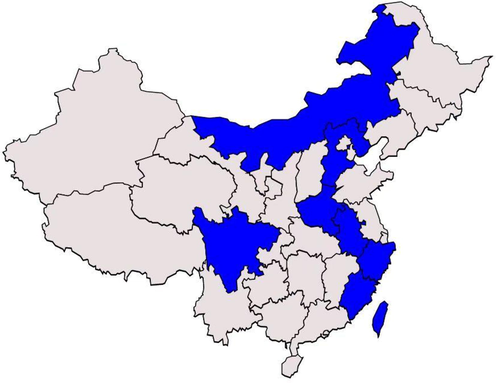
The general geographical distribution of AD in China.
1.2 Botanical morphology of AD
In China, AD mainly has three cultivars, namely A. dahurica (Fish. ex Hoffm.) Benth. et Hook. f. var. formosana (Boiss.) Shan et Yuan, Angelica dahurica (Fisch. ex Hoffm.) Benth. et Hook. f and Angelica dahurica var. formosana (Boissieu) Yen (https://powo.science.kew.org, last accessed at 07/09/2022). The botanical morphology is as follows (Wang et al., 2020; Lian et al., 2019). Plant morphology (A) (B) and decoction pieces morphology (C) of AD are shown in Fig. 2.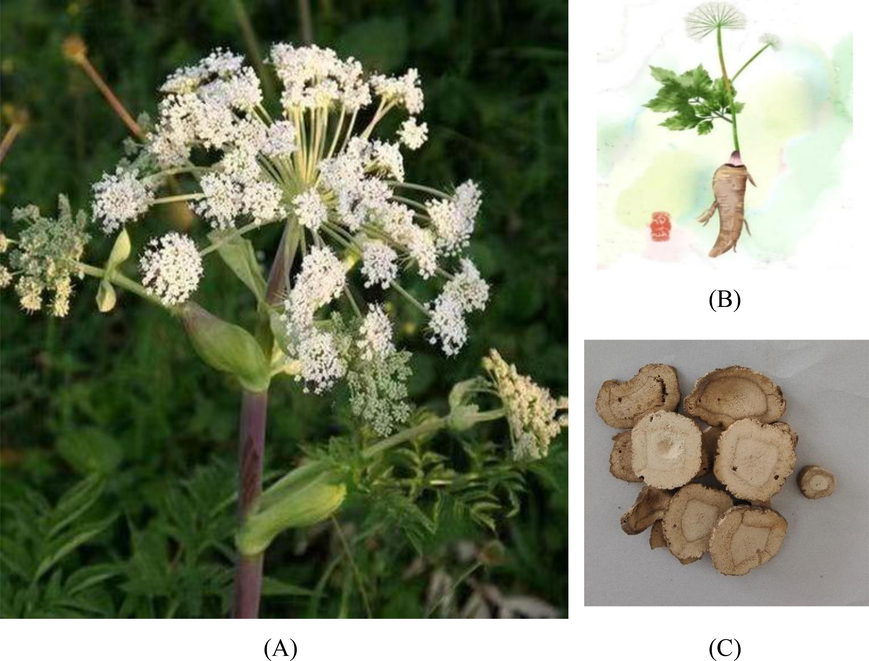
Plant morphology (A) (B) and decoction pieces morphology (C) of AD.
AD is a perennial tall herbaceous plant; the plant height generally grows to 1–2.5 m. The roots are cylindrical, branching, 3–5 cm in diam. The outer skin is yellowish-brown or brown, with a strong odor. Stem base 2–5 cm in diam, sometimes 7–8 cm, usually purplish, hollow, longitudinally furrowed.
Basal leaves have a pinnate split leaf, long petiole, and lower petiole with tabloid cauline margin membranous sheath. Upper stem leaves have 2 to 3 pinnate, leaves blade contour oblong or triangular, 15–30 cm long and 10–25 cm wide, petiole 15 cm long, lower membranous sheath cystic dilated, glabrous or rare, often purple. Terminal segments are oblong, ovate or linear-lanceolate, sessile, 2.5–7 cm long and 1–2.5 cm wide, margin with irregular white cartilaginous coarsely serrate, base on both sides are often unequal, Along the leaves axis down into a wing.
Leaves below inflorescence simplified into leafless, abaxially dilated cystic sheaths, glabrous outside. Inflorescences terminal or lateral, 10–30 cm in diam. Peduncles 5–20 cm long, Inflorescence peduncle, rachis, and pedicel have short brown hair. The spokes of the main umbrella range from 18 to 40, with the central main umbrella sometimes reaching as high as 70. The bract morphology is usually an ovate-expanded sheath. Small bracts composed of 5 to 10, linear-lanceolate, membranous, flower white, no calyx teeth, petals obovate, ovary glabrous or short-haired, styles 2 times longer than the base of the short conical style. Fruits oblong to oval, yellowish-brown, sometimes purplish, 4–7 mm long and 4–6 mm wide, glabrous, dorsal edges flattened, thick and obtuse, sub-spongy. The rib is wider; the lateral rib is winged, narrower than the fruit body. Tubing 1 in the groove, tubing 2 in the combined surface. The flowering period is July-August and the fruiting period is August-September. In Table 2, we analyzed the differences in plant morphology of three AD base sources.
The base source
Differences
A. dahurica (Fish. ex Hoffm.) Benth. et Hook. f. var. formosana (Boiss.) Shan et Yuan (Chinese vernacular names: Hang bai zhi)
Plants 1–1.5 m tall, stems and leaves sheaths are mostly yellowish green. The root is long conical, the upper part is nearly square, the surface is gray-brown, there are most of the larger hole-like lateral protuberances, slightly arranged into several vertical, hard and heavy, the section is white, Powder property sense is bigger.
Angelica dahurica (Fisch. ex Hoffm.) Benth. et Hook. f (Chinese vernacular names: Yubaizhi, Qi bai zhi)
Fruits without ridges, apex dented stem scar conical, scented, but not pungent.
Angelica dahurica var. formosana (Boissieu) Yen (Chinese vernacular names: Tai wan bai zhi)
Fruits and seeds have hairs, but the ovary and fruit of lower branches have no hairs or few hairs.
2 Chemical composition of AD
2.1 Polysaccharide
AD polysaccharides had an antioxidant effect. In the in vitro coagulation experiment, ADPS-1b, ADPS-2, ADPS-3a, and ADPS-3b were found to cause FIB (fibrinogen) coagulation to produce the anticoagulant effect. ADPS-1a had no anticoagulant effect (Wang et al., 2017). It had been reported that the content of polysaccharides in AD in Anguo of Hebei Province and Hangzhou of Zhejiang Province is relatively high (Zhou et al., 2015). In Table 3, the structural composition and related information of AD polysaccharides are in detail.
Number
Compound
Monosaccharide species and molar ratio
Relative molecular mass
References
1
ADP1
Arabinose: Mannose: Glucose: Galactose 10.20: 1.00: 63.71: 5.82
<1.0 × 104
Xu et al. (2011)
2
ADP2
Arabinose: Mannose: Glucose: Galactose 14.55: 1.00: 14.12: 14.91
1.39 × 106
Xu et al. (2011)
3
ADP3
Rhamnose: Arabinose: Glucose: Galactose 1.66: 7.21: 1.00: 21.82
<1.0 × 104
Xu et al. (2011)
4
ADP4
Rhamnose: Arabinose: Glucose: Galactose 1.13: 2.97: 1.00: 5.32
1.00 × 105
Xu et al. (2011)
5
ADPS-1a
Xylose: Mannose: Glucose: Galactose 0.31: 0.22: 26.1: 0.11
1.53,8 × 105
Wang et al. (2017)
6
ADPS-1b
Arabinose: Xylose: Mannose: Glucose: Galactose 0.10: 0.26: 0.07: 15.3: 1.37
8312
Wang et al. (2017)
7
ADPS-2
Rhamnose: Rrabinose: Xylose: Mannose: Glucose: Galactos 0.34: 1.79: 0.35: 0.40: 15.8: 5.59
1.117 × 105
Wang et al. (2017)
8
ADPS-3a
Rhamnose: Arabinose: Xylose: Mannose: Glucose: Galactose 1.06: 2.21: 0.13: 0.41: 1.68: 4.97
3766
Wang et al. (2017)
9
ADPS-3b
Rhamnose: Arabinose: Xylose: Mannose: Glucose: Galactose 0.18: 0.36: 0.25: 0.09: 13.5:1 0.59
96,680
Wang et al. (2017)
10
ADP
→3)-Manp-(1→, →4, 6)-Galp-(1→, →4)-Galp-(1→, →3)-Glcp-(1→, →5)-Araf-(1→, →2)-Galp-(1 → 0.32: 0.57: 0.29: 0.95: 0.71: 0.26
6.09 × 103
Dong et al. (2021)
2.2 Coumarins
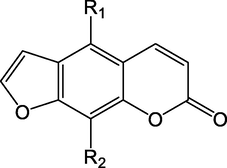
Coumarins in AD had many pharmacological activities, especially in the central nervous system, such as relief of pain (Pang et al., 2021). Studies had shown that 15, 18 coumarins had a strong stimulating effect on alkaline phosphatase (AP). Estrogen could promote the secretion of alkaline phosphatase in Ishikawa cells, which indicated that coumarins had a certain estrogenic effect (Piao et al., 2006). PPAR-γ was a receptor expressed in macrophages related to lipid and glucose metabolism. Compounds 52, 53, and 57 showed obvious ligand-binding activity of PPAR-γ (Matsuo et al., 2020). Some benzofuran compounds mentioned below also had this effect. The main coumarin components are shown in Table 4. Serial number: 11–63.
Number
Compound
Molecular formula
References
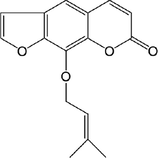
11
Imperatorin (IMP)
C16H14O4
Wei, and Ito, (2006)
12
Oxypeucedanin
C16H14O5
Wei, and Ito, (2006)
13
Iso-imperatorin
C16H14O4
Wei, and Ito, (2006)
14
Oxypeucedanin hydrate
C16H16O6
Piao et al. (2006)
15
9-hydroxy-4-methoxy-ooralen
C12H8O5
Piao et al. (2006)
16
Byakangelicin
C17H18O7
Piao et al. (2006)
17
Pabulenol
C16H14O5
Piao et al. (2006)
18
Alloisoim peratorin
C16H14O4
Piao et al. (2006)
19
Neobyakangelicol
C17H16O6
Piao et al. (2006)
20
Byakangelicol
C17H16O6
Piao et al. (2006)
21
Phellotorin
C17H16O5
Piao et al. (2006)
22
Xanthotoxol
C11H6O4
Zhang et al. (2009)
23
5-methoxy-8-hydroxypsoralen
C12H8O5
Zhang et al. (2009)
24
Xanthotoxin
C12H8O4
Zhang et al. (2009)
25
Isopimpinellin
C13H10O5
Zhang et al. (2009)
26
Bergapten
C12H8O4
Zhang et al. (2009)
27
Iso-oxypeucedanine
C16H14O5
Zhang et al. (2009)
28
Iso-byakangelicin
C17H18O7
Zhang et al. (2009)
29
Suberosin
C15H16O3
Zhang et al. (2009)
30
Apaensin
C17H16O6
Zhang et al. (2009)
31
8-geranoxypsoralen
C21H22O4
Zhang et al. (2009)
32
Iso-tert-O-methylbyakangelicin
C18H20O7
Zhang et al. (2009)
33
8-geranoxy-5-methoxypsoralen
C22H24O5
Zhang et al. (2009)
34
5-demethoxyisodahuribirin A
C32H28O9
Zhang et al. (2009)
35
Sodahuribirin A
C33H30O10
Zhang et al. (2009)
36
Scopoletin
C10H8O4
Zheng et al. (2010)
37
Osthole
C15H16O3
Zheng et al. (2010)
38
Psoralen
C11H6O3
Zheng et al. (2010)
39
Cnidilin
C17H16O5
Zheng et al. (2010)
40
Pimpinellin
C13H10O5
Xie et al. (2010)
41
Osthenol
C14H14O3
Xie et al. (2010)
42
Byakangelicin
C17H18O7
Xie et al. (2010)
43
Auraptenol
C15H16O4
Xie et al. (2010)
44
Angelicin
C11H6O3
Pfeifer et al. (2016)
45
Umbelliferone
C9H6O3
Pfeifer et al. (2016)
46
2′'R–neobyakangelico
C17H16O6
Zhang et al. (2019)
47
Oxypeucedanin ethanolate
C19H22O5
Zhang et al. (2019)
48
Iso-byakangelicol
C17H17O6
Zhang et al. (2019)
49
Columbianetin
C14H14O4
Zhang et al. (2019)
50
(-)-marmesin
C14H14O4
Zhang et al. (2019)
51
7-hydroxy-6-(2′,3′,4′-trihydroxy-isopentanyl)-coumarin
C14H16O6
(Matsuo et al. (2020)
52
1′-O-β-D-glucopyranosyl-3′-Hydroxynodakenetin
C20H24O11
Niu et al. (2002)
53
(3′S)-hydroxy-nodakenetin 4′-O-β-D-piofuranosyl-(1 → 6)-β-D-glucopyranoside.
C26H34O14
Matsuo et al. (2020)
54
columbianetin 2′-O-β-D-glucopyranoside
C20H24O10
Kim et al. (2010)
55
Olumbianetin2′-O-β-D-apiofuranosyl-(1 → 6)-β-D-glucopyranosid
C25H32O13
VanWagenen et al. (1988)
56
R-(+)-oxypeuce-Danin hydrate sucrose ether
C28H36O16
Matsuo et al. (2020)
57
2′′-sulfo-(±)-oxypeucedaninhydrate.
C13H10O7S
Matsuo et al. (2020)
58
Byakangelicin3′′-O-β-D-glucopyranosid
C23H28O13
Thastrup, Lemmich, (1983)
59
Yakangelicin3′′-O-β-D-apiofuranosyl-(1 → 6)-β-D-gluco-pyranosi
C28H36O16
Jia et al. (2008)
60
R-(+)-byakangelicin sucrose ether
C29H38O17
Matsuo et al. (2020)
61
2′′-sulfo-(±)-byakangelicin
C17H18O10S
Matsuo et al. (2020)
62
5,8-bis-(2,3-dihydroxy-3-me-thylbutyloxy)-psoral
C21H26O9
Kwon et al. (1997)
63
3′′-O-β-D-glucopyranosyl-5,8-bis (2,3-dihydroxy-3-me-thylbutyloxy)-psorale
C27H36O15
Matsuo et al. (2020)
64
Dahurines A
C17H20N2O6
Qi et al. (2019)
65
Dahurines B
C16H23NO4
Qi et al. (2019)
66
Dahurines C
C16H23NO4
Qi et al. (2019)
67
Dahurines D
C12H15NO3
Qi et al. (2019)
68
Dahurines E
C23H31NO4
Qi et al. (2019)
69
Dahurines F
C17H27NO4
Qi et al. (2019)
70
(8R,11S,12R)-funebral
C12H15NO4
Qi et al. (2019)
71
(8R,11S,12R)-3,4-dihydro-3-amino-4,5-dimethylfuran2[5H]-one-2-formyl pyrrole
C11H13NO3
Qi et al. (2019)
72
4″-butyl-2-formyl5-(hydroxymethyl)-1H-pyrrole-1-butanoic acid
–
Qi et al. (2019)
73
Butyl 2-formyl-5-butoxymethyl-1H-pyrrole-1-butanoate
–
Qi et al. (2019)
74
Hemerocallisamine II
–
Qi et al. (2019)
75
Butyl 2-pyrrolidone-5-carboxylate
–
Qi et al. (2019)
76
3-[6,7-furano-9-hydroxy-4-(2″,3″-dihydroxy-3″-methylbutyloxy)]-phenyl propionic acid.
C16H20O7
Matsuo et al. (2020)
77
3-[6,7-furano-9-(β-D-glucopyranosyloxy)-4-(2″,3″-dihydroxy-3″-methylbutyloxy)]-phenyl propionic acid
C22H30O12
Matsuo et al. (2020)
78
3-[6,7-furano-9-(β-D-glucopyranosyloxy)-4-(2″,3″-dihydroxy-3″-methylbutyloxy)]-phenyl propionic acid methyl ester
C23H32O12
Matsuo et al. (2020)
79
cnidioside A
C17H20O10
Matsuo et al. (2020)
80
methylcnidioside A
C18H22O10
Matsuo et al. (2020)
81
methylpicraquassioside
C19H24O11
Matsuo et al. (2020)
82
1-Methoxy-4-[(Z)-prop-1-enyl]benzene
C10H12O
Sun et al. (2017)
83
1-Caryophyllene
C15H24
Sun et al. (2017)
84
Cyclododecane
C12H24
Sun et al. (2017)
85
Oxacyclotetradecan-2-one
C13H24O2
Sun et al. (2017)
86
Tridecanoic acid
C13H26O2
Sun et al. (2017)
87
Hexadecanoic acid, ethyl ester
C18H36O2
Sun et al. (2017)
88
α-Pinene
C10H16
Sun et al. (2017)
89
Iso-bergaptene
C12H8O4
Sun et al. (2017)
90
E-1,9-tetradecadiene
C14H26
Sun et al. (2017)
91
Linoleic acid
C18H32O2
Sun et al. (2017)
92
Dodecyl alcohol
C12H26O
Sun et al. (2017)
93
1-Pentadecanol
C15H32O
Sun et al. (2017)
94
Linoleic acid ethyl ester
C20H36O2
Sun et al. (2017)
95
Ethyl oleate
C20H38O2
Sun et al. (2017)
96
1-Methylcyclooctene
C9H16
Sun et al. (2017)
97
Suberosin
C15H16O3
Sun et al. (2017)
98
Ethyl15-methylheptadecanoate
C20H40O2
Sun et al. (2017)
99
Imperatorin
C16H14O4
Sun et al. (2017)
100
Corydaldine
C11H13NO3
Sun et al. (2017)
101
Elemene
C15H24
Sun et al. (2017)
102
2-Ethylhexyl hydrogen phthalate
C16H22O4
Sun et al. (2017)
103
Cyclopropane carboxylic acid, 3-methyl phenyl ester
C11H12O2
Sun et al. (2017)
104
9Z,12Z-octadecadienoic acid
C18H32O2
Liu et al. (2011)
105
10Z,13Z-nonadecadienoic acid
C19H34O2
Liu et al. (2011)
106
12Z-octadecenoic acid
C18H34O2
Liu et al. (2011)
107
8Z,11Z-feptadecadienoic acid
C17H30O2
Liu et al. (2011)
108
14Z,17Z-tricosadienoic acid
C21H38O2
Liu et al. (2011)
109
17Z,20Z-hexacosadienoic acid
C27H50O2
Liu et al. (2011)
110
Dotriacontanoic acid
C32H64O2
Liu et al. (2011)
111
2,3-dihydroxy propyl decanoate
C13H26O2
Liu et al. (2011)
112
caffeic acid
C9H8O4
Liu et al. (2011)
113
9Z-1,9-heptadecadiene-4,6-diyne-3,8,11-trio
C17H24O3
Liu et al. (2011)
114
2-hydroxy-3-[(1-oxo-dodecyl)oxy]propyl-b-D-glucopyranoside
C16H40O9
Liu et al. (2011)
115
stigmasterol-3-O-b-glucosides
C35H57O7
Liu et al. (2011)
116
3-hydroxy-6-pyridine methanol
C6H7O2N
Liu et al. (2011)
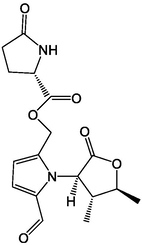
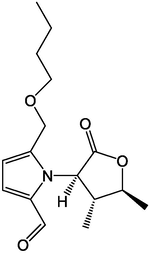
2.3 Alkaloids
Qi et al. (2019) isolated 12 alkaloids from AD, among which compounds 70 and 71 were isolated for the first time from plants. In addition, they detected the inhibitory effects of 12 compounds on acetylcholinesterase and found that compounds 65, 66, 67, 73 and 74 had an inhibitory effect. The alkaloids in AD had a certain inhibitory effect on the growth of cervical cancer (Hela) cells in a dose-dependent manner (Wang and Ma, 2014). AD alkaloids could effectively inhibit the reproduction of mouse U14 cervical cancer cells and reduce the content of Ki-67 (a protein similar to the change of DNA content during cell division) and mutant P53 protein (a protein expressed by mutant tumor suppressor genes) (Li et al., 2012). In summary, alkaloids, the active AD components, could be used as a prospective drug for cancer treatment. The main alkaloids isolated from AD are listed in Table 4. Serial number: 64–75.
2.4 Benzofurans
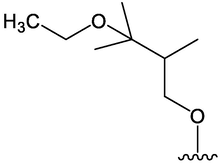
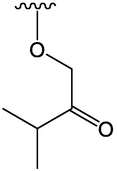
PPAR-γ was a receptor related to fat and glucose metabolism. Compounds 76, 80, and 81 showed obvious PPAR-γ ligand activity at 500 μmoL/L, and compound 76 had the strongest activity. Moreover, compound 76 could induce adipocyte differentiation, which was expected to become a leading compound for anti-obesity drugs (Matsuo et al. 2020). In Table 4, six benzofuran compounds had been identified from AD. Serial number: 76–81.
2.5 Volatile oil
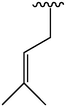
The volatile oil from AD was the most abundant chemical component in AD. Modern studies had shown that the volatile oil extracted from AD had clinical application effects, such as analgesia, treatment of cold, and rheumatism. Sun et al. (2017) isolated 22 kinds of volatile oil from the ethanol extract of AD and found that volatile oil had a significant therapeutic effect on migraine in rats. It could reduce the content of NO in the serum and brain of rats, reduce the level of CGRP in plasma, and increase the level of ET. Therefore, they concluded that AD volatile oil might treat migraine in rats by regulating the level of vasoactive substances. In addition, Zhang et al. (2019) reviewed the types and contents of volatile oils from AD in six regions of China. There were 12 quinones, 48 alkenes, 6 ketones, 6 aldehydes, 14 alkanes, and 25 others. A total of 111 kinds of volatile oils were found. Alcohols had the highest content, indicating that alcohols were the main components of volatile oils from AD. The main volatile oils components are shown in Table 4. Serial number: 82–103.
2.6 Fatty acids
Liu et al. (2011) isolated 13 compounds from the ethyl acetate extract of AD by biological induction technology, including 10 fatty acids and 3 derivatives. Srchomol-ogy-2domain-containing protein tyrosine phosphatase (Shp2) was considered to be a proto-oncogene that promotes tumorigenesis. It was found that compounds 107, 108, 112, and 114 showed significant selective inhibitory activity against Shp2. HepG2 cells (A human liver cancer cell) were treated with a fatty acid mixture. Compound 108 strongly induced poly (ADP-ribose) polymerase (PARP) cleavage in a dose and time-dependent manner and increased the activities of caspase-3, caspase-8, and caspase-9 at 100 mL Promoting apoptosis of HepG2 cells. In Table 4, 13 fatty acids isolated from AD were listed. Serial number: 104–116.
2.7 Amino acids
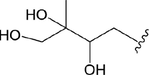
Niu et al. (2016) determined 17 common amino acids in AD from different areas by pre-column derivatization HPLC. AD from different regions contains eight essential amino acids, such as Lysine and Phenylalanine. Chen et al. (2015) detected the content of amino acids in AD in Hunan Province. They found that the highest content of amino acids in AD was Arg, accounting for 31.12% of the total amino acids. The total essential amino acids were 27.01%, of which the highest content was Leu, accounting for 24.14%. They found that the content of amino acids in AD in Hunan is in line with the recommended intake value of WHO, and thus, it can be used as a healthy food.
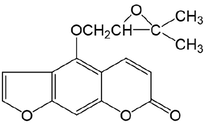
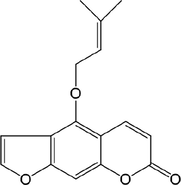
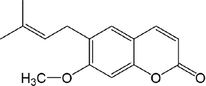
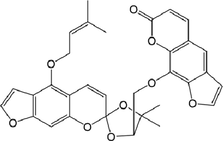
11
12
13
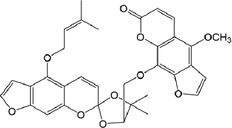
29
34
35
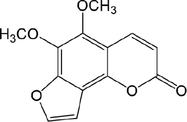
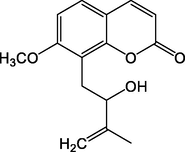
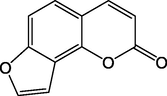
40
43
44
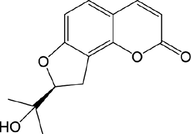
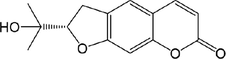
49
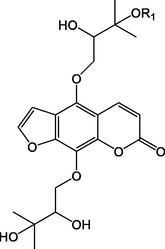
50
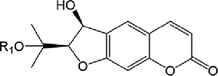
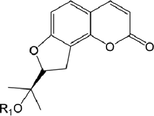
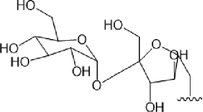
52.R1 = Glc
53.R1=
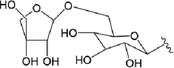
54.R1 = Glc
55.R1=
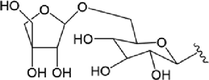
Compound
R1
R2
56
H
57
SO3H
H
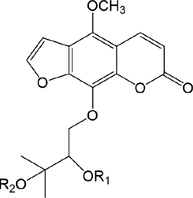
Compound
R1
R2
58
H
Glc
59
H
60
H
61
SO3H
H
62.R1 = H
63.R1 = Glc
Compound
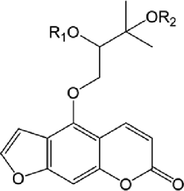
R1
R2
14
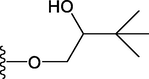
H
15
OCH3
OH
16
OCH3
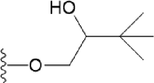
17
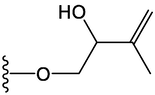
H
18
OH
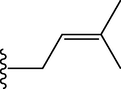
19
OCH3
20
OCH3
21
OCH3
22
H
OH
23
OCH3
OH
24
H
OCH3
25
OCH3
OCH3
26
OCH3
H
27
H
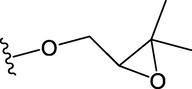
28
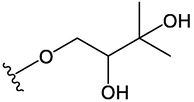
OCH3
30
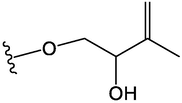
OCH3
31
H
32
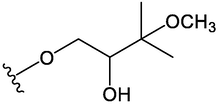
OCH3
33
OCH3

38
H
H
39
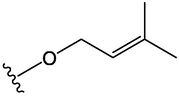
OCH3
42
OCH3
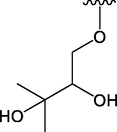
46
OCH3
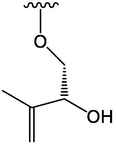
47
H
48
OCH3
Compound
R1
R2
R3
36
OCH3
OH
H
37
H
OCH3
41
H
OH
45
H
OH
H
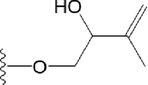
51
OH
H
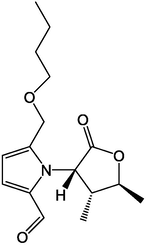
64
65
66
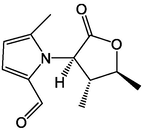
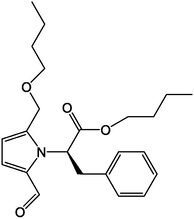
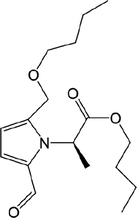
67
68
69
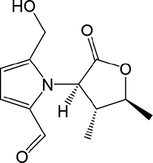
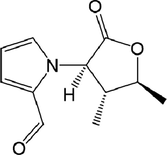
70
71
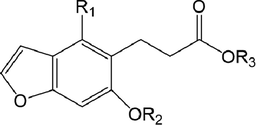 ,
,
C5
Compound
R1
R2
R3
76
C5
H
H
77
C5
Glc
H
78
C5
Glc
CH3
79
H
Glc
H
80
H
Glc
CH3
81
OCH3
Glc
CH3
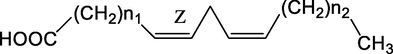
Compound
n1
n2
104
7
4
105
8
4
107
6
4
108
10
4
109
16
4
106
110
111
112
113
114
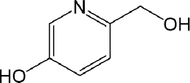
115
116
3 Pharmacological effects
3.1 Wound healing
Zhang et al. (2017) used streptozotocin to delay the healing rate of diabetic wounds in rats and then treated them with ADEE (AD ethanol extract). It was found that ADEE could promote angiogenesis and tissue formation to accelerate wound healing in rats. They also used HUVEC (human umbilical vein endothelial cells) for in vitro experiments to study the mechanism of ADEE and found that ADEE promoted angiogenesis with the increase of angiogenesis regulators, which was similar to that of VEGF (vascular endothelial growth factor). One analysis using network pharmacology and in vivo experiments proved that AD had 54 targets in diabetic wound healing, which could regulate the polarisation of macrophages M1 and M2 to produce anti-inflammatory effects to accelerate wound healing (Hu et al., 2021). In the healing experiment on diabetic trauma that AD treatment of trauma was accompanied by the expression of the HIF-1α gene and the increase of PDGF-β protein (Guo et al., 2020). Increased sebum secretion was one of the pathogenesis of acne. IMP was an effective component of AD, which could inhibit the promotion of insulin factor (IGF-1) on sebum formation and cytokine expression, and could be used to treat acne (Hwang et al., 2016). Yang et al. (2017) found that the extracts of AD and rhubarb (ARE) could increase the content of collagen fibers and myofibroblasts in the wounds of rats, thereby accelerating wound healing. They conducted experiments on rhubarb and AD extracts to treat Staphylococcus aureus infection, further confirming the function of AD in promoting wound healing (Yang et al., 2020). In patients with bedsores, it was found that AD could promote the increase of transforming growth factor (TGF-β), epidermal growth factor (EGF), and VEGF (vascular endothelial growth factor) after external application (Gong et al., 2016). AD polysaccharides played a significant noticeable effect in the coagulation process of wound healing, increasing the FIB (fibrinogen) content through endogenous or exogenous pathways to promote coagulation (Wang et al., 2021).
3.2 Antitumor activity
Tumor has always been a difficult problem in modern medical treatment. The traditional treatment is based on radiotherapy and chemotherapy, but the side effects are large, and low cure rate. Patients need to suffer great pain during the treatment. With the modern medical research of traditional Chinese medicine, it has been found that many components in traditional Chinese medicine have great help in the treatment of cancer. Modern medical experiments have proved that AD extracts have a good effect in treating cancer.
Some experiments found that the organic solvent extract of AD had a good therapeutic effect on cancer and stimulated the activation of the caspase pathway. Still, the specific components had not been shown (Zheng et al., 2016; Liu et al., 2011). Some later studies found that IMP was a chemical component of AD, which had been proved to be a very effective antitumor substance. Advanced liver cancer would resist cytotoxic drugs, resulting in little drug effect. IMP could act on the Mcl-1 target (An anti-apoptotic factor), inhibit its expression, make BAK express in mitochondria, and promote mitochondrial oligomerization to induce apoptosis of cancer cells (Li et al., 2014). In Choochuay's experiment, it was also shown that IMP could act on Mcl-1 targets and inhibit the metastasis and growth of lung cancer cells, promoting apoptosis of cancer cells (Choochuay et al., 2013). IMP could inhibit the synthesis of hypoxia-inducible factor protein (Mi et al., 2017); it showed an inhibitory effect on human colon cancer cells through caspase joint reaction (Zheng et al., 2016) and had a remarkable curative effect on skeletal muscle atrophy characterized by cancer (Chen et al., 2020). In addition, Oxypeucedanin (12) could inhibit liver cancer cells' G2/M division cycle, which was related to the regulation of p53 in cancer cells (Park et al., 2020). In summary, the mitochondrial pathway is an important way for the apoptosis of IMP.
In addition, it was found in previous studies that coumarin compounds extracted from AD could promote the apoptosis of various cancer cells (Liu et al., 2011; Luo et al., 2011; Kim et al., 2007). A polysaccharide extracted from AD could significantly increase the content of spleen lymphocytes and NK cells in tumor mice and improve the apoptosis rate of tumor cells at G1 (Dong et al., 2021). Bai et al. (2021) found that iso-imperatorin extracted from AD could significantly increase the apoptosis rate of female cervical cancer (Hela) cells. The apoptosis mechanism promotes the expression of apoptotic proteins in cervical cancer cells through mitochondrial and external apoptotic pathways. AD extract activated the mitochondrial apoptosis pathway of mouse melanoma cells, increased the intracellular reactive oxygen species content and Bax/Bcl-2 expression, induced apoptosis of cancer cells but also reduced the activities of metalloproteinases (MMP-2, 9, and 4) reduced the colony and migration rate of melanoma cells (Hwangbo et al., 2020). The molecular mechanism is concisely shown in Fig. 3.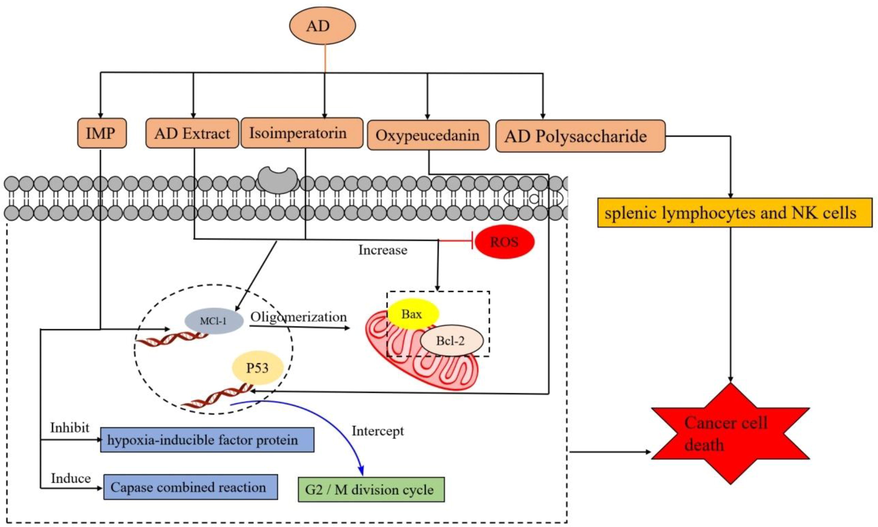
The component molecular mechanism of AD in anticancer.
Mcl-1: myeloid cell leukaemia 1, Bax: Bcl-2-Associated X, Bcl-2: B-cell lymphoma-2, BAK: Bcl-2-associated protein X, P53: tumor suppressor gene.
3.3 Neurological diseases
Modern research showed that AD extracts had the effects of alleviating pain, treating Alzheimer's disease, and depression (Nong, 2016). In traditional Chinese medicine, prescriptions composed of AD were widely used in analgesic treatment, such as migraine. In 2007, Dr. Yuan published an article on the analgesic effect of Yuan Hu Zhi Tong Formula, a prescription composed of AD and Corydalis Rhizoma, and found that it had an obvious analgesic effect and was dose-dependent. Still, the mechanism was not clear (Yuan et al., 2004). Later, in the study of Liao et al. (2010) it was pointed out that Cou (total coumarin) and VO (volatile oil) of AD could enhance the content of di-ThP (an effective component of tetrahydropalmatine alkaloids, with obvious analgesic effect) in mouse plasma. Mi et al. (2020) used network pharmacology to analyze the analgesic mechanism of YB (Yuanhu-Baizhi herbal pair). A total of 78 target genes were screened and then folded with pain-related genes. Finally, 34 related genes, 10 Hub genes and 23 important related compounds were obtained, indicating that YB was closely related to the central nervous system. AD and Ligusticum chuanxiong Hort. were common drug components for migraine treatment. DL (Duliang Soft Capsule) was a traditional Chinese medicine prescription composed of Chuanxiong and AD. In the experiment, it was found that DL extract could reduce the pain response of rats, reduce the content of CGRP, NO, and DA in rat plasma, and increase the content of ET and 5-HT (Hou et al., 2017). Tou feng yu pill (TFY) was a traditional Chinese medicine prescription composed of AD, Chuanxiong, and camellia. it was found that after oral administration of TFY extract, it could effectively reduce the pain reaction of mice caused by acetic acid and formalin, reduce the contents of CGRP, NO and dopamine in rats, improve the contents of endothelin, 5-HT and norepinephrine, effectively resist vasospasm, improve the cerebral blood flow of normal rabbits, and reduce the cerebral vascular resistance index (Li et al., 2011). Studies by Feng et al. (2018) had shown that the analgesic process of AD total coumarin extracts was also consistent with the above process. Auraptenol (43) was an effective analgesic substance in AD. Auraptenol (43) could significantly improve the foot withdrawal threshold of mice, and the receptor was mainly 5-HT mediated 1A (Wang et al., 2013). Chen and his colleagues found that IMP, the AD extract, could regulate pain by activating the TRPV1 channel but could not change the heat rejection threshold of animals (Chen et al., 2014).
AD polysaccharide (ADP) could increase the expression of CD86 and MHC-II in dendritic cells (DC), increase the contents of IL-12, IL-1β, and TNF-α, and significantly enhance the activation of allogeneic T cells in DC, Increase DC cell viability. Phosphorylation of ERK, JNK, and P38 MAPKs increased after ADP binds to TLR4. This suggests that ADP enhances the immunity of DC cells by activating the ERK/JNK pathway (Kim et al., 2013). In the study of AD in the treatment of Parkinson's disease (PD), it was found that the mixed extracts of peony bark, AD, and Bupleurum could significantly improve the decrease of OXPHOS complex 1 activity caused by tyrosinase inhibition and mitochondrial damage, and increase mitochondrial membrane potential and reactive oxygen species levels, promote apoptosis of damaged brain cells (Jeong et al., 2018). In the PD mouse model, oral AD extracts could increase dopamine content. In vitro and in vivo experiments, the extract could improve AKT phosphorylation and mitochondrial gene expression under the insulin signaling pathway, and could significantly improve the decrease of cell viability caused by 6-OHDA, stimulate the activity of antioxidant enzymes to inhibit cell apoptosis, inhibit the movement disorder caused by 6-OHDA, and reduce the nerve transfer between dopamine neurons. This indicated that AD exerted a therapeutic effect on PD by restoring neural cell vitality and increasing dopamine content (Eo et al., 2019). Bax and caspase were the key apoptosis factors of brain cell apoptosis induced by cerebral ischemia. A study showed that IMP could reduce the number of SH-SY5Y cells apoptosis induced by OGD-R (oxygen-glucose deprivation/reperfusion) and inhibit the expression of apoptosis factors (Wang et al., 2013). Cao et al. (2017) established a rat model of depression by prenatal stress and observed the role of IMP in treating depression. It was found that IMP could increase sucrose intake in offspring rats and restore the decrease of 5-HT caused by depression. In addition, Gu et al. (2014) found that auraptenol (43) in AD could shorten the resting time of tail suspension and swimming model mice, which proved that the antidepressant effect was related to the activation of 5-HT. In addition, IMP could improve the cognitive impairment and hippocampal neuronal damage caused by vascular dementia in rats, improve synaptic Structure, increase SAZ (synaptic active zone) length and PSD (postsynaptic density) thickness, and inhibit neuronal apoptosis by regulating Bax and caspase-3 (Huang et al., 2021). The molecular mechanisms are shown in Fig. 4.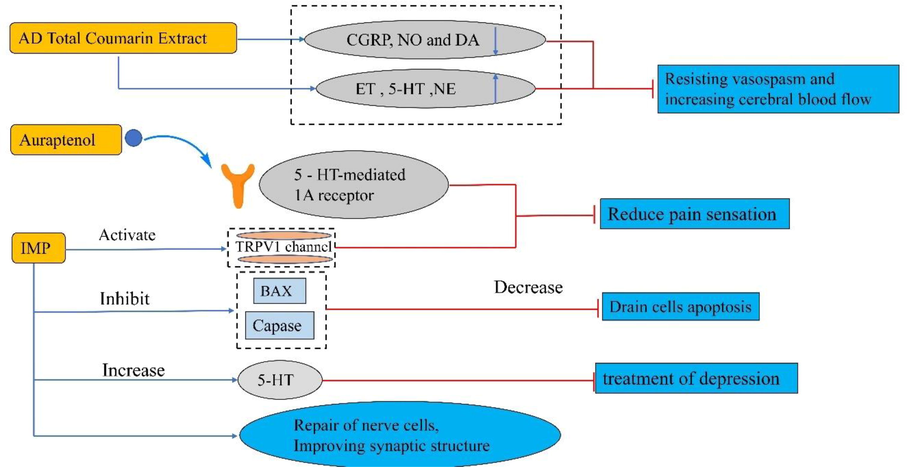
The component molecular mechanism of AD in Nerve.
CGRP: calcitonin gene-related to peptide, DA: dopamine, ET: endothelin, 5-HT: 5-hydroxytryptamine, NE: norepinephrine, TRPV 1: proteins form calcium channels that transmit pain to the central nervous system.
3.4 Anti-inflammatory
Inflammation is the body's immune response to the external invasion, usually manifested as swelling, fever, and pain. Cytokines secreted by immune cells (also known as inflammatory factors), such as TNF-α, TGF-β, IL-1β, IL-6, and COX-2, are important mediators for inflammatory response.
Modern studies had shown that inducible NO synthase (INOS) production can lead to an excessive increase of NO, a key factor in inducing inflammation. Methanol extract of AD inhibited NO synthesis in macrophages and reduced the expression of INOS by reducing the phosphorylation of ERK1/2 (Kang et al., 2008). Coumarins in AD showed inhibitory effects on NO synthesis (Yang et al., 2017; Wei et al., 2016). COX-2 gene was a key factor in subsequent inflammation and could be induced and expressed by various proinflammatory factors. The COX-2/PGE2 pathway was a classic proinflammatory pathway, and COX-2 was a major enzyme in the synthesis of PG. Iso-imperatorin isolated from AD could reduce the expression of COX-2 (Moon et al., 2008). Subsequent studies had found that furanocoumarins in AD could inhibit PGE production by inhibiting COX-2 and mPGES (Hong et al., 2008). Studies by Moon et al. (2012) showed that oral administration of AD extract (100 mg/kg) for 14 days decreased the levels of TNF-α, IL-1β, IL-6, INOS, COX-2, reactive oxygen species (ROS), etc. Lee et al. (2011) also found that AD ethanol extract could reduce the number of inflammatory factors in cells, reduce the mucus production and immunoglobulin (IGE) in airway inflammation model cells, in order to reduce excessive cellular immune response. In addition, they showed that AD ethanol extraction reduced airway inflammation by stimulating the expression of HO-1, TARC/CCL17, and MDC/CCL22. AD extract could inhibit the expression of TARC and MDC, preventing TH2 cell aggregation (Lee et al., 2012). AD volatile oil components could alleviate ear swelling and foot swelling in inflammatory model mice and reduce ankle synovial hyperplasia, excessive inflammatory cells, cartilage damage symptoms, and reduce plasma NO, TNF-α, PGE2, and NOS levels. NF-κB was responsible for the transcription of many pro-inflammatory factors and chemokines genes (Wang et al., 2016). The expression of NF-κB was related to TNF-α-induced IKKα/β phosphorylation, IκB phosphorylation, degradation, and NF-κB p65 nuclear translocation. One study showed that IMP inhibited NF-κB expression by inhibiting TNF-α, down-regulated the expression of inflammatory genes induced by lipopolysaccharide, and produced anti-inflammatory effects (Wang et al., 2017). Lee et al. (2017) also found the inhibitory effect of AD on NF-κB. In addition, AD could reduce the contents of IL-1β, IL-6, IL-8, and IFN-γ (interferon-gamma) in gingival tissue cells and inhibit the expression of the genes related to the above four inflammatory factors and the synthesis of COX-2 and INOS in mouse macrophages (RAW264.7). In the colitis mouse model experiment, IMP promoted the synthesis and expression of PXR, thereby inhibiting the activity of NF-κB (Liu et al., 2018). AD, rhubarb extract showed an inhibitory effect on mastitis in dairy cows, reduced mastitis indicators: LDH (lactate dehydrogenase), TNF-α, IL-6, IL-8 content, the body would not easily develop drug resistance (Yang et al., 2019). Another study showed that AD and rhubarb extracts had therapeutic effects on inflammation caused by Staphylococcus aureus (Yang et al., 2020). Byakangelicin (16) was an extract from AD roots. In vitro cell experiments showed that Byakangelicin (16) inhibited the production of INOS, COX-2, TNF-α, and IL-6 induced by IL-1β. In vivo experiments showed that Byakangelicin (16) had a protective effect on osteoarthritis in mice. Byakangelicin (16) had a good prospect in the treatment of osteoarthritis (Zhang et al., 2020). An experiment on airway inflammation showed that IMP could inhibit the activation of Mast cells and inhibit the phosphorylation of CAMK II and ERK. CAMK II was the upstream protein of ERK, which could guide the phosphorylation of ERK. MRGPRX2 was the inducer of airway inflammation. Experiments showed that CAMK II was involved in MRGPRX2-mediated LAD2 (Human Laboratory of Allergic Disease 2) cells activation and IMP was the antagonist of MRGPRX2. It had a therapeutic effect on airway inflammation through CAMK II/ERK pathway (Wang et al., 2021). The molecular mechanisms are shown in Fig. 5.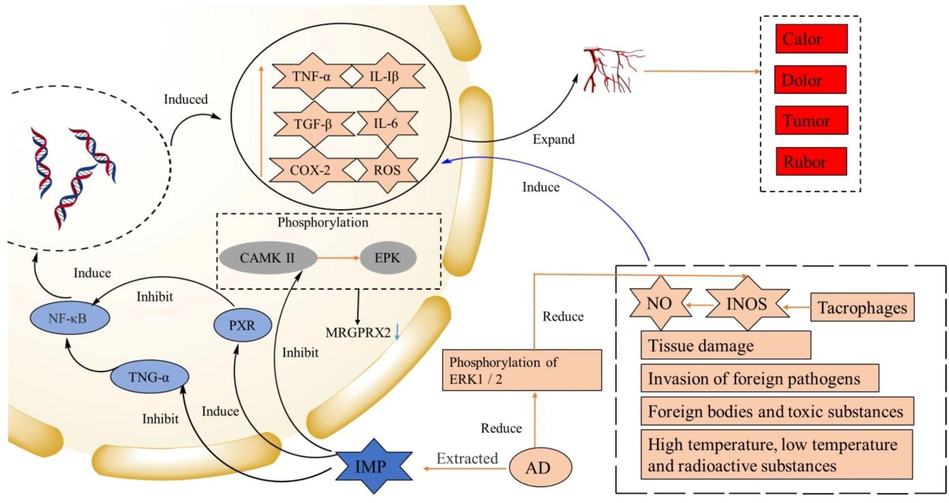
The anti-inflammatory molecular mechanism of AD.
INOS: Inducible nitric oxide synthase, TNF-α: Tumor necrosis factor, TGF-β: Transforming growth factor-β, IL: Interleukin, COX-2: cycloxygenase-2, ROS: reactive oxygen species, TNF-α: tumor necrosis factor-α, NF-κB: nuclear factor kappa-light-chain-enhancer of activated B cells, PXR: pregnane X receptor, CAMK Ⅱ: Ca2+/calmodulin-dependent kinase Ⅱ, ERK: extracellular regulated protein kinases, MRGPRX2: Mas-related G protein-coupled receptor X2.
3.5 Antioxidant activity
Antioxidation refers to the inhibition of free radicals. cancer and aging were related to many free radicals. DPPH, ABTS, and ROS were commonly used antioxidant indicators. Modern research showed that some components in AD had the free radical scavenging effect. One experiment showed that the antioxidant index of fermented AD on DPPH, ABTS, and FRAP models was significantly higher than that of unfermented AD, fermentation enhanced the antioxidant activity of AD (Zhou et al., 2019). Mehanz et al. (2013) found that the scavenging capacity of AD aqueous extract on DPPH and ABTS was 0.32 and 0.20 mg/mL, respectively, and that of ethanol extract was 0.24 and 0.13 mg/mL, respectively. The ethanol extract had a better antioxidant effect than the aqueous extract. Phenolic compounds in AD had significant antioxidant activity, and these phenolics were easily soluble in organic solvents. Phenolic compounds in AD could inhibit the peroxidation of linoleic acid, showing the same activity as superoxide dismutase and protecting DNA. A study of IMP on spontaneously hypertensive rats (SHR) showed that the urinary 8-iso-PGF-2α and urinary protein contents in rats were lower than those in the control group after IMP administration. It also inhibited the NADPH, AKT, and MAPK pathways. The experiment showed that IMP protected renal injury caused by hypertension through an antioxidant effect (Cao et al, 2014). Excessive reactive oxygen species (ROS) content could cause oxidative stress and damage human DNA and protein. One study found that the Coumarin mixture in AD could significantly reduce the ROS content and protect the cells from oxidative damage caused by H2O2 (Kang et al., 2019). AD was best preserved at 70 °C, at which the phenolics content is high. The antioxidant activity of the extract obtained at 70 °C was higher than that of the freeze-dried extract (Liang et al., 2018). These results showed that the active substances obtained from AD had obvious antioxidant activity.
3.6 Antibacterial and antiviral activities
AD extract could inhibit the reproduction of Staphylococcus aureus (minimum inhibitory concentration, 8–32 mg/ml) (Lechner et al., 2004). Another study showed that AD and rhubarb extracts could accelerate the healing of wounds caused by Staphylococcus aureus (Yang et al., 2020). Lee et al. (2020) isolated five known furanocoumarins from AD: iso-imperatorin (13), oxypeucedanin (12), oxypeucedanin hydrate (13), and imperatorin (11). Oxypeucedanin (12) had significant inhibitory effects on the cytopathogenic effect induced by H1N2 and H9N2. In vitro experiments had shown that within 1–2 h, the CPE of infected cells treated with oxypeucedanin (12) decreased by 80–90%, but after 3 h, the effect decreased sharply. The viral NA and NP contents in cells decreased to below 20% after 2 h. They concluded that oxypeucedanin (12) might have antiviral effects at the early stage of viral replication but not directly interact with the proteins.
3.7 Other activities
Through experimental studies, IMP could increase the content of cells in low diploidy during cell division, promote the activation of the Caspase-3 pathway, and induce apoptosis in HL-60 cells. This indicated that IMP could induce apoptosis of human promyelocytic leukemia (HL-60) cells, resulting in DNA fracture and changes in nuclear morphology (Pae et al. 2002). Eun et al. (2005) found that oxypeucedanin (12) could inhibit the Hkv1.5 channel, which caused a rapid decrease in the cardiac action potential duration (APD) in cardiac cells, and the effect was voltage-dependent. The experiment had shown that Oxypeucedanin (12) was a potential antiarrhythmic drug for atrial fibrillation. GPR119 was a receptor involved in insulin secretion and glucagon peptide-1 (GLP-1) release. It was found that AD extract had a therapeutic effect on diabetes. AD extract could activate GPR119 in cells, cAMP, GLP-1, and significantly increase insulin content in cells. In vivo experiments showed that AD extract could improve mice's glucose tolerance and insulin secretion (Park et al., 2016). The methanol extract of AD could inhibit vasocontraction of aortic rings induced by PE or KCl, and prevent extracellular Ca2+ from flowing into cells through ROCC and VDCC pathways. These results suggested that the ADE has a vasorelaxant effect and the vasorelaxant activity was mediated by an endothelium-independent pathway that includes the blockade of extracellular calcium influx through the receptor-operated Ca2+ channel and voltage-dependent calcium channel pathways. It indicated that AD could be used as a potential drug for hypertension (Lee et al., 2015).
4 Pharmacokinetics
Zhao et al. (2016) measured the concentration changes of 16 coumarins in the blood of rats using the LC-ESI-MS/MS method. All coumarins in the test extract were absorbed from the gastrointestinal tract. After oral administration, the absorption of (-)-marmesin (50) and columbianetin (49) peaked at 6 and 7 h. The Tmax values of oxypeucedanin hydrate (13), byakangelicin (16), psoralen (38), xanthotoxin (24), bergapten (26), imperatorin (11), and iso-imperatorin (13) were 2.2, 2.3, 1.9, 2.0, 2.4, 1.8 and 2.8 h. After oral administration of AD in rats, most of the effective components reached the maximum at about 2 h, providing a reference for the best efficacy of AD. They also found that the coexistence of multiple coumarins may lead to changes in metabolic interactions and pharmacokinetics, thus, the dosage of AD should be monitored in the clinic. Hwang et al. (2017) compared the concentrations of three furanocoumarins in colitis rats and normal rats. The peak time of IMP and iso-imperatorin in colitis rats was 133–144 min, significantly longer than in normal rats. The peak plasma concentrations (Cmax) of oxypeucedanin (12), IMP, and iso-imperatorin (13) decreased significantly to approximately 50% (p < 0.05). Conversely, the mean residence times (MRT) of oxypeucedanin, IMP, and iso-imperatorin (13) were prolonged by 40%-65% (p < 0.05), So they concluded that colitis delayed Tmax (time) and decreased Cmax (concentration) of three furanocoumarins.
5 Edibility
In Chinese history, the traditional application of AD was not limited to medicine but also could be used as food seasonings or cooking materials. Stewed fish head with Ligusticum chuanxiong Hort and AD was a dish with a long history. It was often used as a health care dish after an illness in winter. Boiling Ligusticum chuanxiong Hort, AD, and Aristichthys nobilis heads in a pot had the effect of dispersing cold, promoting blood circulation, and opening up the channels. This dish could be used to relieve dizziness, migraine, and cervical spondylosis (Jiang and Lei, 2020). When boiling sheep soup in Heze County, Shandong Province, China, it was customary to add AD, which could remove the fishy smell but did not destroy the unique odor of mutton and add fragrance to the sheep soup. It played the role of integrating various tastes. Adding AD to dishes such as beef soup had a good effect on removing the fishy smell. AD had a convergence effect, on the roast chicken, duck, and goose, which could prevent water loss, and roast dishes were fresh and tender, not dry. Bai Zhi Han Zheng Ya (白芷旱蒸鸭), this dish added AD to the traditional chopped white duck, which sublimated the fragrance of duck meat. Meanwhile, the cooking method of steamed duck was adopted to maintain the freshness and nutrition of raw materials. Bai zhi turbot (白芷多宝鱼) Decoction was cooked with AD and turbot. This diet had a delicious taste and could regulate qi (气) and blood, dilute pigments, promote blood circulation, and remove spots to have fair and beautiful skin, which was very suitable for women who yearn for fair skin (Yuan, 2018). Fig. 6 showed the cooking appearance of four dishes.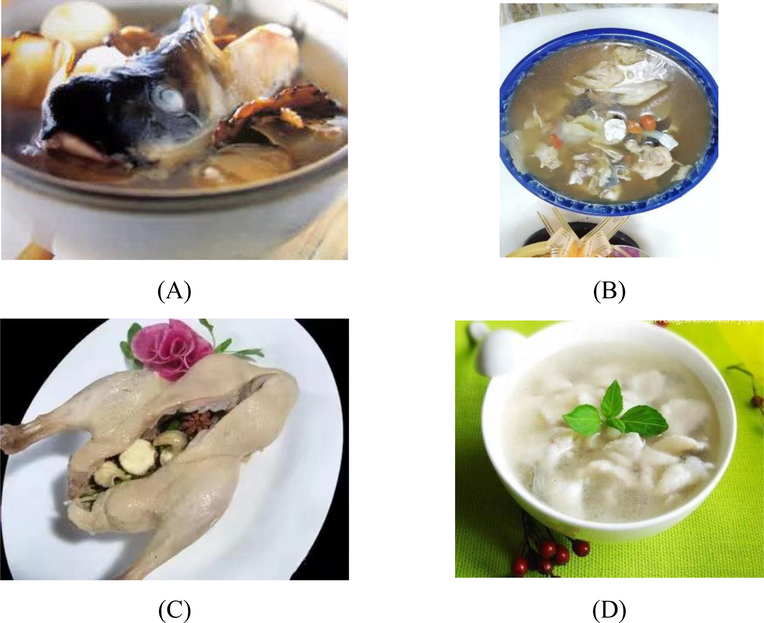
The pictures of the stewed fish head (A), sheep soup (B), Bai Zhi Han Zheng Ya (白芷旱蒸鸭) (C), and Bai Zhi Duo Bao Yu (白芷多宝鱼) decoction (D).
6 Conclusion
AD has a long history and is an ancient Chinese herbal medicine. AD was not used as a drug at first. According to the records, AD was initially used to praise a person's excellent quality and was the object of the poet's description. Later, the medicinal value of AD was gradually discovered by people, from the simple effect of deworming and deodorant to later becoming a commonly used traditional Chinese medicine. AD has received more attention from modern researchers. In this review, the chemical constituents in AD were introduced in detail. Among them, the volatile oils were the most abundant, with more than 100 species. Although the content of volatile oil is rich, there are few studies on its pharmacological effects, and its mechanism of action is still unclear, requiring further research by follow-up researchers. Coumarins are also one of the components with high content in AD. IMP is one of the most active compounds in AD, with multiple targets and pathways in the human body. Its medicinal effects mainly include accelerating wound healing, treating skin trauma, antitumor, analgesic, antidepressant, and anti-inflammatory. Based on the multiple curative effects of IMP, in future research, IMP can be used as the main component of drug preparations to develop a variety of drug formulations for different diseases to play its maximum medicinal use. In addition, polysaccharides in AD have an anticoagulant effect, alkaloids, and fatty acids have an antitumor effect, and benzofurans have an anti-obesity effect, which can be further studied to make its medical mechanism clearer and play a greater medical use.
AD has a wide range of pharmacological activities, and its mechanism of action in humans is also very complex. AD can accelerate wound healing, mainly acting on blood vessels and tissue cells and accelerating their formation to promote wound healing. The mitochondrial pathway is the main pathway for AD to exert an antitumor effect. AD can act on the Mcl-1 target and promote mitochondrial oligomerization to induce apoptosis of cancer cells. AD also possesses a very excellent neuroprotective effect. AD is often used in combination with other traditional Chinese medicine to treat migraine. In addition, AD has a certain effect on Parkinson's disease, depression, dementia, and other neurological diseases. The synthesis of inflammatory factors is the main pathogenesis of inflammation. AD can inhibit the synthesis of inflammatory factors from cells and play an anti-inflammatory role. Diabetes has always been a difficult problem for human beings. In recent years, the incidence of diabetes has tended to be among young people, so the treatment of diabetes has become very urgent. Modern studies have found that AD can increase insulin secretion and has a certain therapeutic effect on diabetes, but there are few studies on this aspect. Therefore, it is necessary to conduct further research on it, hoping to provide an additional and efficient drug for the treatment of diabetes. AD can be used not only as a drug but also as a nutrient-rich food supplement and a condiment for adjusting food taste. Studies have shown that AD contains many essential amino acids for the human body and has high nutritional value.
In conclusion, this paper provides a detailed comparative analysis of the existing literature on AD and identifies the origins, traditional applications, and therapeutic uses of AD to provide a reference for the safe clinical use of AD and the development of new drugs.
CRediT authorship contribution statement
Qingquan Wang: Conceptualization, Writing – original draft. Yanan Li: Conceptualization, Writing – original draft. Shengguang Wang: Methodology, Software, Resources, Data curation. Zedong Xiang: Methodology, Software, Resources, Data curation. Weichao Dong: Validation, Formal analysis, Investigation, Visualization. Xiaoyu Li: Validation, Formal analysis, Investigation, Visualization. Yumin Wei: Validation, Formal analysis, Investigation, Visualization. Peng Gao: Validation, Supervision. Long Dai: Conceptualization, Validation, Writing – review & editing, Project administration, Funding acquisition.
Acknowledgments
This research was funded by the key technology of preparation of Chinese herbal formula containing volatile oil and the support of an industrialization research project (YDZX2021050). Furthermore, all the authors of the manuscript also thank and acknowledge their respective Universities and Institutes.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Studies on the mechanism of Alloimperatorin on the proliferation and apoptosis of HeLa cells. J. Oncol.. 2021;2021:6617312.
- [Google Scholar]
- Antioxidant effect of imperatorin from Angelica dahurica in hypertension via inhibiting NADPH oxidase activation and MAPK pathway. J. Am. Soc. Hypertens.. 2014;8:527-536.
- [Google Scholar]
- Antidepressive-like effect of imperatorin from Angelica dahurica in prenatally stressed offspring rats through 5-hydroxytryptamine system. Neuroreport. 2017;28:426-433.
- [Google Scholar]
- Analysis of amino acid content and composition of Angelica dahurica in Hunan Daodi. Guihaia. 2015;35:569-573.
- [Google Scholar]
- Furanocoumarins are a novel class of modulators for the transient receptor potential vanilloid type 1 (TRPV1) channel. J. Biol. Chem.. 2014;289:9600-9610.
- [Google Scholar]
- Imperatorin alleviates cancer cachexia and prevents muscle wasting via directly inhibiting STAT3. Pharmacol. Res.. 2020;158:104871
- [Google Scholar]
- Imperatorin sensitizes anoikis and inhibits anchorage-independent growth of lung cancer cells. J. Nat. Med.. 2013;67:599-606.
- [Google Scholar]
- Structural characterization of a water-soluble polysaccharide from Angelica dahurica and its antitumor activity in H22 tumor-bearing mice. Int. J. Biol. Macromol.. 2021;193(Pt A):219-227.
- [Google Scholar]
- Protective effects of DA-9805 on dopaminergic neurons against 6-hydroxydopamine-induced neurotoxicity in the models of Parkinson's disease. Biomed, Pharmacother.. 2019;117:109184
- [Google Scholar]
- Effects of oxypeucedanin on hKv1.5 and action potential duration. Biol. Pharm. Bull.. 2005;28:657-660.
- [Google Scholar]
- A metabolism-based synergy for total Coumarin extract of radix Angelicae Dahuricae and Ligustrazine on migraine treatment in rats. Molecules. 2018;23:1004.
- [Google Scholar]
- Clinical effects of Angelica dahurica dressing on patients with I-II phase pressure sores. Die Pharmazie. 2016;71:665-669.
- [Google Scholar]
- Angelica dahurica promoted angiogenesis and accelerated wound healing in db/db mice via the HIF-1α/PDGF-β signaling pathway. Free. Radic. Biol. Med.. 2020;160:447-457.
- [Google Scholar]
- Lead compounds for anti-inflammatory drugs isolated from the plants of the traditional oriental medicine in Korea. Inflamm. Allergy Drug Targets. 2008;7:195-202.
- [Google Scholar]
- Pharmacodynamic action and mechanism of Du Liang soft capsule, a traditional Chinese medicine capsule, on treating nitroglycerin-induced migraine. J. Ethnopharmacol.. 2017;195:231-237.
- [Google Scholar]
- Angelica Dahurica regulated the polarization of macrophages and accelerated wound healing in diabetes: a network pharmacology study and in vivo experimental validation. Front. Pharmacol.. 2021;12:678713
- [Google Scholar]
- Effects of imperatorin on apoptosis and synaptic plasticity in vascular dementia rats. Sci. Rep.. 2021;11:8590.
- [Google Scholar]
- Inhibitory effect of imperatorin on insulin-like growth factor-1-induced sebum production in human sebocytes cultured in vitro. Life Sci.. 2016;144:49-53.
- [Google Scholar]
- Simultaneous determination of three Furanocoumarins by UPLC/MS/MS: application to pharmacokinetic study of Angelica dahurica radix after oral administration to normal and experimental colitis-induced rats. Molecules. 2017;22:416.
- [Google Scholar]
- Suppression of tumor growth and metastasis by ethanol extract of Angelica dahurica Radix in murine melanoma B16F10 cells. Biosci. Trends. 2020;14:23-34.
- [Google Scholar]
- Triple herbal extract DA-9805 exerts a neuroprotective effect via amelioration of mitochondrial damage in experimental models of Parkinson's disease. Sci. Rep.. 2018;8:15953.
- [Google Scholar]
- Two new coumarin biosidesfrom Angelica dahurica. Chem. Nat. Compd.. 2008;44:692-695.
- [Google Scholar]
- Know the traditional Chinese medicine - Angelica dahurica. TCM Healthy Life-Nurturing. 2020;6:28-29.
- [Google Scholar]
- Anti-nociceptive and anti-inflammatory effects of Angelicae dahuricae radix through inhibition of the expression of inducible nitric oxide synthase and NO production. Am. J. Chin. Med.. 2008;36:913-928.
- [Google Scholar]
- Furanocoumarins from the roots of Angelica dahurica with inhibitory activity against intracellular reactive oxygen species accumulation. J. Nat. Prod.. 2019;82:2601-2607.
- [Google Scholar]
- Antiproliferative effect of furanocoumarins from the root of Angelica dahurica on cultured human tumor cell lines. Phytother. Res.. 2007;21:288-290.
- [Google Scholar]
- Constituents of Corydalis heterocarpa and their anti-proliferative effects on human cancer cells. Food Chem. Toxicol.. 2010;48:722-728.
- [Google Scholar]
- Dendritic cell activation by polysaccharide isolated from Angelica dahurica. Food Chem. Toxicol.. 2013;55:241-247.
- [Google Scholar]
- Antimicrobial constituents of Angelica dahurica roots. Phytochemistry. 1997;44:887-889.
- [Google Scholar]
- Dian Nan Ben Cao (arganised by Yunnan Provincial Department of Health). People’s Publishing House: Kunming; 1959.
- The anti-staphylococcal activity of Angelica dahurica (Bai Zhi) Phytochemistry. 2004;65:331-335.
- [Google Scholar]
- Antiviral activity of furanocoumarins isolated from Angelica dahurica against influenza a viruses H1N1 and H9N2. J. Ethnopharmacol.. 2020;259:112945
- [Google Scholar]
- Angelicae Dahuricae Radix inhibits dust mite extract-induced atopic dermatitis-like skin lesions in NC/Nga mice. Evid. Based Complement. Alternat. Med.. 2012;2012:743075
- [Google Scholar]
- Angelica dahurica ameliorates the inflammation of gingival tissue via regulation of pro-inflammatory mediators in experimental model for periodontitis. J. Ethnopharmacol.. 2017;205:16-21.
- [Google Scholar]
- Anti-asthmatic effects of Angelica dahurica against ovalbumin-induced airway inflammation via upregulation of heme oxygenase-1. Food Chem. Toxicol.. 2011;49:829-837.
- [Google Scholar]
- Investigation of the mechanisms of Angelica dahurica root extract-induced vasorelaxation in isolated rat aortic rings. BMC Complement. Altern. Med.. 2015;15:395.
- [Google Scholar]
- Study on antitumor activity of Angelica alkaloids against U14 cervical cancer in mice. J. Yanshan Univ.. 2012;36:89-94.
- [Google Scholar]
- Analgesic effect and mechanism of the three TCM-herbal drug-combination Tou Feng Yu pill on treatment of migraine. Phytomedicine. 2011;18:788-794.
- [Google Scholar]
- Research progress on pharmacological effects and mechanisms of imperatorin. Chin. J. Exp. Tradit. Med. From.. 2020;26:196-201.
- [Google Scholar]
- Imperatorin induces Mcl-1 degradation to cooperatively trigger Bax translocation and Bak activation to suppress drug-resistant human hepatoma. Cancer Lett.. 2014;348:146-155.
- [Google Scholar]
- Research progress on chemical constituents and pharmacological activities of Angelica dahurica. Sci. Technol. Innov.. 2019;3:36-37.
- [Google Scholar]
- Effects of drying methods on contents of bioactive compounds and antioxidant activities of Angelica dahurica. Food Sci. Biotechnol.. 2018;27:1085-1092.
- [Google Scholar]
- Correlation between synergistic action of Radix Angelica dahurica extracts on analgesic effects of Corydalis alkaloid and plasma concentration of dl-THP. J. Ethnopharmacol.. 2010;129:115-120.
- [Google Scholar]
- Liu, D., Kong, G., Chen, Q.C., Wang, G., Li, J., Xu, Y., lin, T., Tian, Y., Zhang, X., Yao, X., Feng, G., Lu, Z., Chen, H., 2011. Fatty acids as natural specific inhibitors of the proto-oncogenic protein Shp2. Bioorg. Med. Chem. Lett. 21, 6833–6837
- Furocoumarin derivatives from radix Angelicae dahuricae and their effects on RXRα transcriptional regulation. Molecules. 2011;16:6339-6348.
- [Google Scholar]
- Activating the pregnane X receptor by imperatorin attenuates dextran sulphate sodium-induced colitis in mice. Br. J. Pharmacol.. 2018;175:3563-3580.
- [Google Scholar]
- Anticancer effects of imperatorin isolated from Angelica dahurica: induction of apoptosis in HepG2 cells through both death-receptor-and mitochondria-mediated pathways. Chemotherapy. 2011;57:449-459.
- [Google Scholar]
- Benzofuran and coumarin derivatives from the root of Angelica dahurica and their PPAR-γ ligand-binding activity. Phytochemistry. 2020;173:112301
- [Google Scholar]
- Antioxidant, anti-inflammatory and antiproliferative activity of Angelica dahurica root extracts. J. Food Biochem.. 2013;38:281-292.
- [Google Scholar]
- A network pharmacology study on analgesic mechanism of Yuanhu-Baizhi herb pair. BMC Complement. Med. Ther.. 2020;20:284.
- [Google Scholar]
- Imperatorin suppresses proliferation and angiogenesis of human colon cancer cell by targeting HIF-1α via the mTOR/p70S6K/4E-BP1 and MAPK pathways. J. Ethnopharmacol.. 2017;203:27-38.
- [Google Scholar]
- The effects of isoimperatorin isolated from Angelicae dahuricae on cyclooxygenase-2 and 5-lipoxygenase in mouse bone marrow-derived mast cells. Arch. Pharm. Res.. 2008;31:210-215.
- [Google Scholar]
- Inhibition of inflammation and oxidative stress by Angelica dahuricae radix extract decreases apoptotic cell death and improves functional recovery after spinal cord injury. J. Neurosci. Res.. 2012;90:243-256.
- [Google Scholar]
- Evolution and change of baizhidao real estate area and germplasm. Anhui Agric. Sci. Bull.. 2018;24(13):39-40.
- [Google Scholar]
- Constituents from the roots of Heracleum rapula Franch. J. Asian Nat. Prod. Res.. 2002;4:33-41.
- [Google Scholar]
- Determination of amino acid content in Angelica dahurica by pre-column derivatisation RP-HPLC. Yunnan J. Tradit. Chin. Med. Mater. Med.. 2016;37:68-70.
- [Google Scholar]
- Nong, X.X., 2016. Baizhi. Health Preserving. 37, 606-607
- Imperatorin, a furanocoumarin from Angelica dahurica (Umbelliferae), induces cytochrome c-dependent apoptosis in human promyelocytic leukaemia, HL-60 Cells. Pharmacol. Toxicol.. 2002;91:40-48.
- [Google Scholar]
- Chemical constituents of Angelica dahurica and its therapeutic effect on nervous system diseases. Chinese J. Pharmacol. Toxicol.. 2021;35:690-691.
- [Google Scholar]
- The Antiproliferative activity of Oxypeucedanin via induction of G2/M phase cell cycle arrest and p53-dependent MDM2/p21 expression in human hepatoma cells. Molecules. 2020;25:501.
- [Google Scholar]
- Angelica dahurica extracts improve glucose tolerance through the activation of GPR119. PloS One. 2016;11:e0158796.
- [Google Scholar]
- Determination of coumarins in the roots of Angelica dahurica by supercritical fluid chromatography. J. Pharm. Biomed. Anal.. 2016;129:246-251.
- [Google Scholar]
- Estrogenic activity of furanocoumarins isolated from Angelica dahurica. Arch. Pharm. Res.. 2006;29(9):741-745.
- [Google Scholar]
- Pyrrole 2-carbaldehyde derived alkaloids from the roots of Angelica dahurica. J. Nat. Med.. 2019;73:769-776.
- [Google Scholar]
- Song, S.S., 1988. Tu Jing Ben Cao (copy). Fujian Science & Technology Publishing House: Fuzhou
- Chemical composition and antimigraine activity of essential oil of Angelicae dahuricae Radix. J. Med. Food.. 2017;20:797-803.
- [Google Scholar]
- Tang, S.W., 1957. Revised He Zheng Material Medical for Emergency from Classics and History Documents. The peoples medical publishing house: Beijing
- Tao, H.J., 1994. Collective Notes to the Canon of Materia medica. The peoples medical publishing house: Beijing
- Furocoumarin glucosides of Angelica archangelica sub-Species litoralis. Phytochemistry. 1983;22:2035-2037.
- [Google Scholar]
- Native American food and medicinal plants, 8. Water-soluble constituents of Lomatium dissectum. J. Nat. Prod.. 1988;51:136-141.
- [Google Scholar]
- Wang, M.Y., Jia, M.R., 2004. Herbal Research of Angelica dahurica. Chin. J. Chin. Mater Med. 27 (2004) 382-385. 27, 382-385.
- Auraptenol attenuates vincristine-induced mechanical hyperalgesia through serotonin 5-HT1A receptors. Sci. Rep.. 2013;3:3377.
- [Google Scholar]
- Study on the original plants of Angelica dahurica IV. Discussion on the original plants, cultivation history and evolution of related wild plants of Angelica dahurica. Chin. J. Chin. Mater. Med.. 2001;2002:74-78.
- [Google Scholar]
- Purification, characterisation and procoagulant activity of polysaccharides from Angelica dahurice roots. Chem. Cent. J.. 2017;11:17.
- [Google Scholar]
- Imperatorin efficiently blocks TNF-α-mediated activation of ROS/PI3K/Akt/NF-κB pathway. Oncol. Rep.. 2017;37:3397-3404.
- [Google Scholar]
- Inhibition of Angelica alkaloids on HeLa cells. Chin. J. Gerontol.. 2014;34:1886-1888.
- [Google Scholar]
- In vivo anti-inflammatory activities of the essential oil from Radix Angelicae dahuricae. J. Nat. Med.. 2016;70:563-570.
- [Google Scholar]
- The protective activity of imperatorin in cultured neural cells exposed to hypoxia re-oxygenation injury via anti-apoptosis. Fitoterapia. 2013;90:38-43.
- [Google Scholar]
- Imperatorin ameliorates mast cell-mediated allergic airway inflammation by inhibiting MRGPRX2 and CamKII/ERK signaling pathway. Biochem. Pharmacol.. 2021;184:114401
- [Google Scholar]
- Herbal research of Angelica dahurica in famous classical prescriptions. Mod. Chin. Med.. Jun. 2020;22:1320-1330.
- [Google Scholar]
- Preparative isolation of imperatorin, oxypeucedanin and isoimperatorin from traditional Chinese herb “bai zhi”Angelica dahurica (Fisch. ex Hoffm) Benth. et Hook using multidimensional high-speed counter-current chromatography. J. Chromatogr. A. 2006;1115:112-117.
- [Google Scholar]
- Anti-inflammatory coumarins with short- and long-chain hydrophobic groups from roots of Angelica dahurica cv. Hangbaizhi. Phytochemistry. 2016;123:58-68.
- [Google Scholar]
- An efficient strategy based on MAE, HPLC-DAD-ESI-MS/MS and 2D-prep-HPLC-DAD for the rapid extraction, separation, identification and purification of five active coumarin components from Radix Angelicae Dahuricae. Phytochem Anal.. 2010;21:473-482.
- [Google Scholar]
- Chemical composition and antioxidant activities of different polysaccharides from the roots of Angelica dahurica. Chem. Biodivers.. 2011;8:1121-1131.
- [Google Scholar]
- Xu S., 2013. Shuo Wen Jie Zi. China Publishing House: Beijing
- Yang, G., Su, F., C., 2021. Origin and prospect of medicinal and edible homology. Modern Chinese medicine. 23, 1851 – 1856
- Effects of Angelica dahurica and Rheum officinale extracts on excisional wound healing in rats. Evid. Based Complement. Alternat Med.. 2017;2017:1583031.
- [Google Scholar]
- Effective treatment of Bovine Mastitis with intramammary infusion of Angelica dahurica and Rheum officinale extracts. Evid. Based Complement. Alternat Med.. 2019;2019:7242705.
- [Google Scholar]
- Antimicrobial and anti-inflammatory potential of Angelica dahurica and Rheum officinale extract accelerates wound healing in Staphylococcus aureus-infected wounds. Sci. Rep.. 2020;10:5596.
- [Google Scholar]
- Dimeric furanocoumarins from the roots of Angelica dahurica. Nat. Prod. Res.. 2017;31:870-877.
- [Google Scholar]
- Legends and beauty effects of Angelica dahurica. J. Beneficial Readines Drug Inform. Med. Advices. 2018;9:48-49.
- [Google Scholar]
- Effects of Corydalis yanhusuo and Angelicae dahuricae on cold pressor-induced pain in humans: a controlled trial. J. Clin. Pharmacol.. 2004;44:1323-1327.
- [Google Scholar]
- Chu Ci Yi Zhu. Jinan: Shandong Education Press; 1986.
- Tissue distribution study of Angelica dahurica cv. Yubaizhi in rat by ultra-performance liquid chromatography with tandem mass spectrometry. J. Pharm. Biomed. Anal.. 2019;174:43-49.
- [Google Scholar]
- Rapid separation and identification of furocoumarins in Angelica dahurica by high-performance liquid chromatography with diode-array detection, time-of-flight mass spectrometry and quadrupole ion trap mass spectrometry. Rapid Commun. Mass Spectrom.. 2009;23:2167-2175.
- [Google Scholar]
- Byakangelicin inhibits IL-1β-induced mouse chondrocyte inflammation in vitro and ameliorates murine osteoarthritis in vivo. Int. Immunopharmacol.. 2020;85:106605
- [Google Scholar]
- Angelica Dahurica ethanolic extract improves impaired wound healing by activating angiogenesis in diabetes. PloS One. 2017;12:e0177862.
- [Google Scholar]
- Comparative analysis of volatile components in Angelica dahurica from six different producing areas. Preserv. Process.. 2019;19:176-183.
- [Google Scholar]
- Simultaneous determination and pharmacokinetics of sixteen Angelicae dahurica coumarins in vivo by LC-ESI-MS/MS following oral delivery in rats. Phytomedicine. 2016;23:1029-1036.
- [Google Scholar]
- Anti-oxidant and anti-cancer activities of Angelica dahurica extract via induction of apoptosis in colon cancer cells. Phytomedicine. 2016;23:1267-1274.
- [Google Scholar]
- Imperatorin exhibits anticancer activities in human colon cancer cells via the caspase cascade. Oncol. Rep.. 2016;35:1995-2002.
- [Google Scholar]
- Simultaneous characterization and quantitation of 11 coumarins in Radix Angelicae Dahuricae by high performance liquid chromatography with electrospray tandem mass spectrometry. J. Pharm. Biomed. Anal.. 2010;51:599-605.
- [Google Scholar]
- Zhou, Y., Na, L.x., 2022. Research progress on medicinal and food homology of Angelica dahurica. Asian-Pacific traditional medicine. 18, 213-217
- Analysis and evaluation of coumarins and polysaccharides in Angelica dahurica from different habitats. Acta. Univ. Med. Nanjing. 2015;31(01):68-73.
- [Google Scholar]
- On-line screening and identification of free radical scavenging compounds in Angelica dahurica fermented with Eurotium cristatum using an HPLC-PDA-Triple-TOF-MS/MS-ABTS system. Food. Chem.. 2019;272:670-678.
- [Google Scholar]
- Zhu, C., Wang, M., Guo, J., Su, S.L., Yu, G., Yang ,Y., Zhou, Y., Tang, Z., 2022. Angelica dahurica Extracts Attenuate CFA-Induced Inflammatory Pain via TRPV1 in Mice. Evid. Based Complement Alternat Med.4684830







