Translate this page into:
A review on multi-component green synthesis of N-containing heterocycles using mixed oxides as heterogeneous catalysts
⁎Corresponding author. jonnalagaddas@ukzn.ac.za (Sreekantha B. Jonnalagadda)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
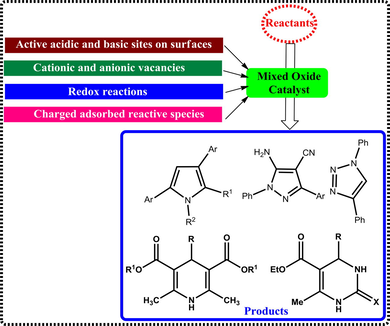
The use of mixed oxides is a well-appreciated approach in the fields of material science and synthesis, due to remarkable tunable surface properties such as acidic and basic characteristics, oxidation/reduction capabilities, and high agility of lattice oxygen, which makes them ideal choices as heterogeneous catalysts. The activity of the mixed oxides broadly relies on the nature of support and active material used and on the preparation method, calcination temperatures. Wide range of techniques for preparation of mixed oxide materials are adoptable, viz. sol-gel, co-precipitation, wet impregnation, microwave irradiation and hydrothermal methods. Use of mixed oxides as solid catalysts have gained popularity in many valued organic transformations, via alkylation, oxidation, condensation, dehydration, dehydrogenation, cycloaddition and isomerization. Application of mixed oxides in the area of green organic synthesis is a valuable strategy, which contributed significantly to the design of many novel heterocyclic scaffolds. The chemistry of N-heterocycle scaffolds, which generally possess five and six membered rings, is an interesting area for both synthetic and medicinal chemistry research constituting over 60% organics used in various arenas. The position and number of nitrogen atoms in the rings, distinguish them as pyrroles, pyrazoles, imidazoles, triazoles, pyridines and pyramidines classes. In this review, we focus on the scope, importance and versatile applications of mixed metal oxides and their synergetic effects as heterogeneous catalysts in the synthesis of variety of N-heterocyclic derivatives. The scientific aspects of the mixed oxides as catalytic active materials to design efficient synthetic protocols for the organic transformations is also discussed.
Keywords
Heterogeneous catalysis
Bimetallic catalysts
Mixed oxides
One-pot synthesis
Green chemistry
N-heterocycles
Multicomponent reactions
Nanocomposites
1 Introduction
The concept of green chemistry has made significant impact on many frontages including the use of green solvents, bio-renewable resources and sustainable catalyst materials (Cioc et al., 2014; Varma 2014). Environmentally benign protocols have been explored for heterocyclic synthesis to improve energy consumption, atom economy and reaction yields (Gawande et al., 2013; Anastas and Eghbali, 2010). Designing the high efficiency reactions that work at room temperature and use of alternative energy sources has become an attractive choice. A radical evolution in the field of synthetic organic chemistry is the practice of multicomponent reactions (MCRs), which involve more than three reactants in one-pot reaction (Ganem, 2009; Ryabukhin et al., 2012) In addition to avoidance of separation and purification of intermediates, MCRs are generally environment friendly, selective, atom-efficient and time saving (Graaff et al., 2012; Toure, 2009; Trost 2002). Thus, MCR approach has gained significant popularity in the fields of pharmaceutical chemistry and drug development, including control of stereo isomers (Cho and Morken (2014)). In the sequence of the reaction, inter-coordination between the reactants, solvent and catalyst is crucial for the success of MCRs (Gu, 2012; Climent et al., 2012; Kozhevnikov, 1998). Consequently, with choice for diverse molecular entities as reactants, MCRs have cherished in the designing of different organic blocks to prepare various fascinating heterocyclic frameworks (Kozhevnikov, 1998; Rotstein et al., 2014a; Martins et al., 2009).
While the nature of hetero atoms and the strain in the ring influence the properties of heterocycles, in broad sense, they are all-pervading, as one heterocyclic structure or other are integral part of almost every physiologically and biologically active organic compounds known (De Coen et al., 2016; Bhardwaj et al., 2015; Eftekhari-Sis et al., 2013a). Many of the heterocyclic entities are starting materials in the synthesis of varied drugs related to antimalarial (Karad et al., 2016), antiulcer (Peng-Hui et al., 2016), diuretic (Anderson et al., 2012), anthelmintic (Omar et al., 2015), antidepressants (Engers et al., 2015), anticancer (Kuroiwa et al., 2015), antineoplastic and antipsychotic (Castro et al., 2015; Chen et al., 2015). Depending on the reaction, heterocycles can exhibit both electrophilic and nucleophilic characteristics (Godoi et al., 2011). Among the heterocycles, nitrogen based compounds more dominant as sub-units in many of the pharmaceuticals and agrochemical products, due to their intriguing capabilities (Akhtar et al., 2016; Eftekhari-Sis et al., 2013b; Singh, 2012). The design and development of new synthetic strategies based on green principles and using renewable and recyclable materials will be the route for sustainable growth, which is anticipated from the R&D research.
Supported catalysts in general, and bimetallic or trimetallic mixed oxide catalysts in particular have attracted significant attention, in the recent past due their superb capabilities to steer the organic reactions with high selectivity and efficiency (Wachs, 2005; Guo et al., 2014; Dastan et al., 2012). Metal oxides are highly crystalline for their catalytic efficacy (Jabłonska and Palkovits, 2016; Shi, 2013). The combination of two or more metals and their curing processes allow the tuning of the surface properties of the materials, making them opt for specific purpose (Lin-Bing et al., 2015; Zhang et al., 2012). Thus, mixed oxide catalysts and their nanocomposites offer wide range of advantages including atom efficiency and green principles (Climent et al., 2012; Wachs and Routray, 2012). For instance, in perovskites like scheelites (ABO3) and spinels (ABO4), the metal cations, spatial orientations and type of bonding viz A-O-B-O, A-O-A-O or B-O-B-O in both can be predicted precisely, which is useful in identification of their structure-property relationship (Tessier and Marchand, 2003; Kudo and Miseki, 2009). The surface properties such as charged/adsorbed entities (Heiz and Bullock, 2004), acidic and basic characteristics (Rafiee and Eavani, 2016; Copeland et al., 2013), tendency of cations to undergo redox reactions (Trizio and Manna, 2016), and oxygen vacancies all contribute to their catalytic activity (Xiaoyang et al., 2013). Many catalytic procedures established using such mixed oxide catalysts are simple preparation (Johnson, 2003), prudent (Gawande et al., 2011), efficient and environmentally friendly (Dastan et al., 2012), selectively producing the anticipated products (Bartoli et al., 2010; Nakamura and Yamamoto, 2004). These are applied both for their acidic and basic possessions (Gawande et al., 2015), and their reduction-oxidation properties which make them vital class in heterogeneous catalysis (Shi, 2013). Mixed oxide catalysts address the sustainability concerns and provide alternative and efficient methods for various important organic transformation reactions (Brauch et al., 2013; Cho and Morken (2014); Eftekhari-Sis et al., 2013). Many novel mixed oxide catalysts with superb activity, excellent chemical and thermal stability, high surface area, low-cost and recyclability have been reported with precise applications in synthetic organic chemistry (Sambasivarao et al., 2015; Rotstein et al., 2014b; Singh and Chowdhury, 2012; Laurent et al., 2008; Corma and Garcia, 2008). Their heterogeneous nature facilitates easy separation from the reactants and products, making them attractive for reusability (Polshettiwar et al., 2011; Prasad and Singh, 2013). The bonding environment of metal cations in mixed oxides facilitate selective reaction of the approaching molecule at the active sites, enriching the reaction (Chandler et al., 1993; Manser et al., 2016; Fernandez-Garcia et al., 2004). One or more phases of mixed oxides could be active catalytic centers, which enable the chosen reaction. For example, in fluorapatites, the presence of both Lewis acidic and basic sites on its surface due to Ca2+ and PO4 phases boost the reaction, but one of its active catalytic phase characters renders the given reaction more efficiently (Yin et al., 2006). Mixed oxides are exceptionally better than the individual constituent oxides in terms of their catalytic activity in diverse reactions and sustainability, because of the subtle differences in surface area and nature of the active sites (Yin et al., 2006; Gangu et al., 2016a; Gangu et al., 2017a). Thus, in modern chemistry, the mixed oxides have undeniably contributed as valuable catalysts for different organic transformations, such as oxidation, dehydration, dehydrogenation, isomerization, condensation and may other catalysis science applications (Gangu et al., 2017b; Gangu et al., 2016b; Gawande et al., 2012).
For the catalytic activity of the mixed oxides, the interaction between the active phase and support material are crucial and such interactions occur through their surface and interfacial free energies (Dai et al., 2013; Chetty et al., 2012). Many transition metal oxides with known catalytic activity such as CeO2, MnO2, MoO3, V2O5, NiO etc. have comparatively low surface free energies relative to the oxide support materials (Al2O3, SiO2, TiO2, and ZrO2) normally used (Maddila et al., 2013, 2015, 2016a). The scale of these interfacial free energies are not exactly known, but the role of the interactions in overall activity of the material is acknowledged. Thermal treatment of the oxide mixtures at higher temperatures is known to facilitate the spreading and wetting phenomena, which induces mobility of the active oxides (Maddila et al., 2016b, 2016c).
The characteristics and capabilities of material as catalysts squarely depend on the support material used and the active metal used in addition to their relative proportions and the preparation method adopted (Maddila et al., 2015a, 2015b; Rana et al., 2015a). Main purpose of the support material is to afford surface area, thermal stability and mechanical strength, besides the active sites (Maddila et al., 2016d, 2016e, 2016f). Commonly used supports are active carbon, Al2O3, SiO2, TiO2, CeO2, ZrO2, hydroxyapatites, etc. The list of active materials is wide and many transition metals are commonly used, which also possess good thermal stability and moderate mechanical strength (Maddila et al., 2016g, 2016h; Pagadala et al., 2014a). Cost effectiveness also one of the crucial factors that decide the choice of active metal (Maddila et al., 2014). While broad choice of synthetic approaches such as sol-gel, co-precipitation, wet impregnation, microwave irradiation and hydrothermal method are attractive options, the preparation method and calcination temperature and protocols significantly influence the distribution of the active material on the support and the overall characteristics of the material (Maddila et al., 2016i; Oseghe et al., 2015; Rana et al., 2015b; Gangu et al., 2016c).
The present communication describes the scope of varied mixed oxides as catalysts for the synthesis of one and two nitrogen containing five and/or six membered heterocycles via one-pot multi-component reactions. Literature reports have been selected to illustrate the importance of mixed oxides as solid catalysts and interpret the chemistry between the catalyst and the reactant molecules. This review gives deep insight into the comprehensive utility of mixed oxides as catalysts and their structure functionality relationship.
2 Five membered heterocycles
Based on the number and position of nitrogen atoms in five-membered ring of heterocycles, they have classified into pyrroles, pyrazoles, imidazoles and triazoles. The following sections describe their facile synthetic routes by employing different mixed oxides as catalysts or catalyst supports and illustrate the role of mixed oxides in achieving remarkable yields of products in short reaction times.
2.1 One nitrogen containing heterocycles (Pyrroles)
Pyrroles are one of the preferred heterocyclic compounds, both in medicinal and synthetic chemistry, due to the possibility for a huge number of usual and unusual molecules with noteworthy characteristics. The pyrrole structural fragments are common in many natural products and show varied biological activities. The majority of these alkaloids are usually developed from a distinctive tetracyclic ring structure that contains a pyrrole fragment. Azgomi and Mokhtary (2015) have explored the advantages of Fe3O4@SiO2 nanoparticles and ionic liquid, namely 1-methyl-3-(3-trimethoxysilylpropyl)-1H-imidazol-3-ium chloride (IL) in organic synthesis for the preparation of 1,3-thiazolidin-4-ones (Scheme 1). Initially, Fe3O4@SiO2 nanoparticles were prepared and the resultant products were treated with the selected IL, under stirring for 16 h at 60 °C producing MNP@SiO2-IL (Fig. 1). The catalytic efficiency of prepared material has examined in the three-component reaction between varied aldehydes, and anilines with thioglycolic acid at 70 °C under no solvent condition. The yield (86–94%) and reaction times (55–70 min) with MNP@SiO2-IL have offered an economical protocol. The magnetic property of the catalyst makes its easy recoverability and reusability.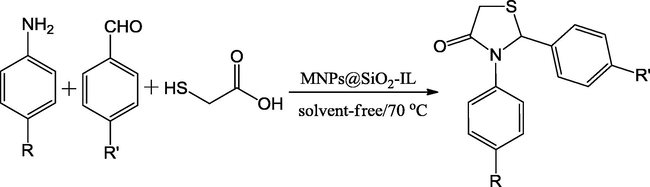
One-pot synthesis of 1,3-thiazolidin-4-ones catalyzed by MNPs@SiO2-IL.
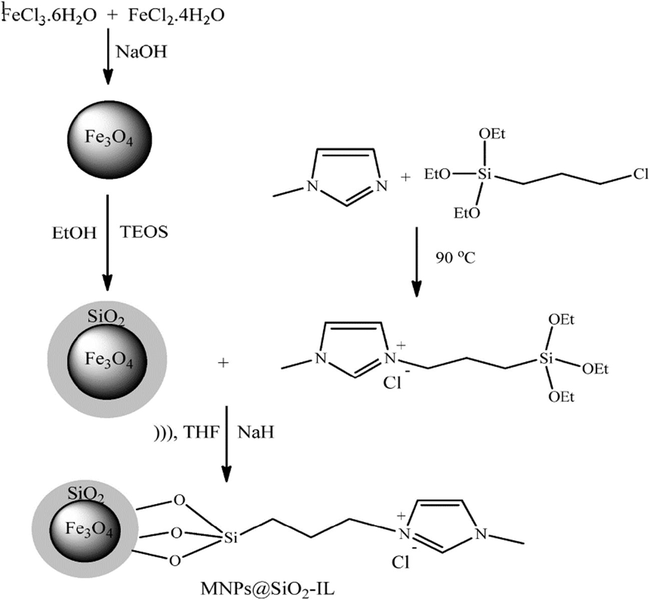
Preparation steps for synthesis of MNP@SiO2-IL nano catalyst.
Source: Reprinted from Azgomi and Mokhtary (2015) with permission from Elsevier.
Rajesh et al. (2016) have prepared novel heterogeneous catalyst, hydromagnetise (HM) supported copper (Cu/HM) by a simple impregnation procedure. The structure viability of HM with large cavities opens a new vista for its application in the catalysis as supporting substrate for many transition metals. The authors have stabilized the copper on HM and characterized the successful grafting of copper on HM. The XPS studies revealed that the copper existed in +2 oxidation state and it was confirmed by the Cu 2p1/2 and Cu 2p3/2 peaks in XPS spectrum. The catalytic performance was investigated in the preparation of pyrrolo[1,2-a]quinolone derivatives by employing the mixture of quinoline-2-carboxaldehye, secondary amine and phenyl acetylene at 110 °C in diethylene glycol as solvent. The varied pyrrolo[1,2-a]quinolone molecules with 87–95% have produced by varying the amine and alkyne precursors. The high catalytic activity showed by the Cu/HM has ascertained the presence of more active sites generated from the synergistic effect of Cu(II) and Mg(II) (Scheme 2).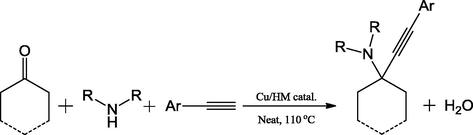
Cu/HM catalyzed synthesis of substituted propargylamines via KA2 coupling.
Bamoniri1 et al. (2015) have synthesized a nano-silica supported TiCl4 (Nano-TiCl4/SiO2) as heterogeneous catalyst by stirring the mixture of TiCl4 and nano-silica for 1h at room temperature in the presence of chloroform. The Nano-TiCl4/SiO2 has promoted in the acid-based catalyzed reaction to produce the dihydro-2-oxopyrroles derivatives. The 80 mg of catalyst was optimized for reaction between amine, dialkyl acetylenedicarboxylates and formaldehyde in ethanol at 70 °C. The reaction times and yields of dihydro-2-oxopyrroles were at the range of 30 min to 3 h and 65–95%, respectively (Scheme 3). The catalyst facilitated the Mannich reaction for intermolecular addition between the substrates. This protocol simplified the preparation of nitrogen-containing heterocycles like dihydro-2-oxopyrroles over other earlier reported catalysts.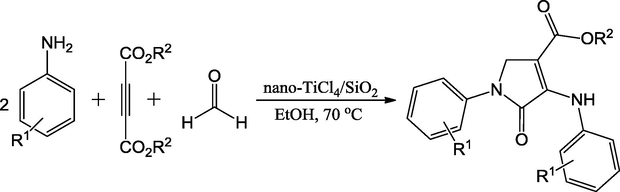
Nano-TiCl4/SiO2 catalyzed synthesis of substituted dihydro-2-oxopyrroles.
Li et al. (2014a) have reported an active catalyst, namely [CoFe2O4@SiO2-PrNH2-Mo(acac)2] by using cobalt ferrite (CoFe2O4), tetraethyl orthosilicate (TEOS), 3-aminopropyltriethoxysilane (APTES) and molybdenyl acetylacetonate precursors. The authors have used the TEOS for silica coating on CoFe2O4 and APTES used for amine functionalization of CoFe2O4@SiO2. Finally, the molybdenyl acetylacetonate complex, [Mo(acac)2] used to anchor the aminated-CoFe2O4@SiO2. The vibrating sample magnetometery (VSM) analysis evaluated that the material exhibits super paramagnetic nature with 21.0 emu/g magnetic saturation value (Msat) and noticed that it was high for CoFe2O4@SiO2 (25.0 emu/g). The decrease in the Msat value in the [CoFe2O4@SiO2-PrNH2-Mo(acac)2] is attributed that the non-magnetic character of [PrNH2-Mo(acac)2] has surrounded the surface of the CoFe2O4 nanoparticles (Fig. 2). The material was employed as catalyst in the four component one-pot synthesis of pyrroles and its derivatives in thermal conditions (90 °C) (Scheme 4). The catalyst was well worked towards the production of yields from good to excellent. The magnetic nature of catalyst is an added advantage, which made the separation for reusability simpler. In similar lines, the same team have proposed CoFe2O4 supported Sb nano-magnetic material ([CoFe2O4@SiO2–DABCO–Sb]) (Li et al., 2014b) as catalyst for the synthesis of multi-substituted pyrroles via three-component one-pot synthesis. The catalytic nature of antimony (III) chloride was hindered by its hygroscopic nature and it was overcome by immobilization of antimony trichloride on cobalt ferrite substrate. In the preparation of the catalyst, the CoFe2O4 nanoparticles were prepared by using the co-precipitation method by using the precursors FeCl3·6H2O and CoCl2·6H2O (Fig. 3). The tetraethyl orthosilicate (TEOS) and 1,4-diazabicyclo-[2.2.2]octane (DABCO) and antimony trichloride were used for the grafting of CoFe2O4 nanoparticles. The prepared catalyst showed cobalt ferrite diffraction pattern even after the functionalization and catalyst retained magnetic behavior. The reaction between amines, nitro-olefin and 1,3-dicarbonyl compounds under optimal conditions of 0.5 mol% of CoFe2O4@SiO2–DABCO–Sb as catalyst (Scheme 5) and ethanol as solvent progressed well at 80 °C, giving good to excellent yields. The catalyst usable up to five cycles stimulates the industrial sector in the preparation of novel pyrroles.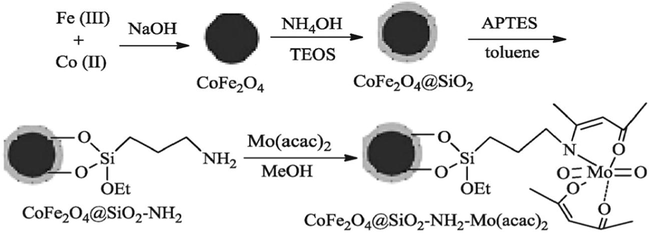
Synthesis of CoFe2O4@SiO2-NH2-Mo(acac)2.
Source: Reprinted from Li et al. (2014a) with permission from Royal Society of Chemistry.
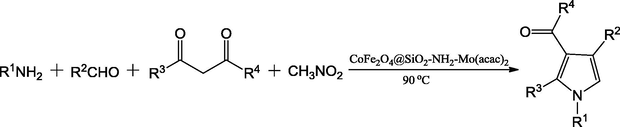
Synthesis of pyrroles in the presence of CoFe2O4@SiO2-NH2-Mo(acac)2.
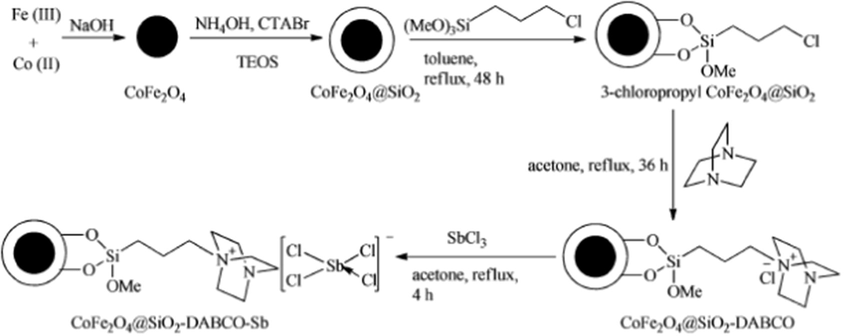
Synthesis of CoFe2O4@SiO2–DABCO–Sb.
Source: Reprinted from Li et al. (2014b) with permission from Royal Society of Chemistry.
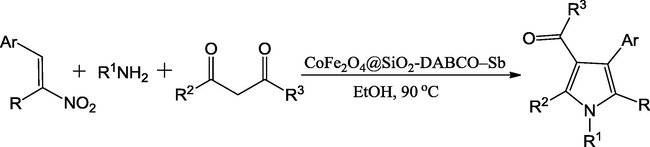
Synthesis of pyrroles in the presence of CoFe2O4@SiO2–DABCO–Sb.
Saha et al. (2016) have recently prepared the nanostructured copper ferrites (CuFe2O4) for the catalyzed organic synthesis. The presence of synergetic catalytic effect due to Fe3+ and Cu2+ in CuFe2O4 made it, the choice as catalyst. The catalyst was prepared by using Fe(NO3)3·9H2O (4 mmol), Cu(NO3)2·3H2O (2 mmol) and citric acid (9 mmol) as reactants and their solution in distilled water were heated with continuous stirring at 90 °C. The nano-sized CuFe2O4 particles were obtained after calcination of the resultant powder at 500 °C. The catalytic activity of CuFe2O4 was examined in the one-pot three-component synthesis of chromeno-[4,3-b]pyrrol-4(1H)-one derivatives from the building blocks of amine, 4-aminocoumarin and glyoxal monohydrate (Scheme 6). The optimal conditions such as temperature (70 °C), water, and 10 mol% of catalyst produced the impressive yields of chromeno-[4,3-b]pyrrol-4(1H)-one products in the range of 70–92%. The X-ray diffraction and IR studies disclosed that the structural integrity was not disturbed even after the sixth run of the catalyst, which induces the viability of catalyst as eco-friendly synthesis of organic molecules.![Synthesis of chromeno = [4,3-b]pyrrol-4(1H)-one derivatives.](/content/184/2020/13/1/img/10.1016_j.arabjc.2017.09.016-fig10.png)
Synthesis of chromeno = [4,3-b]pyrrol-4(1H)-one derivatives.
Dou et al. (2008) have reported the synthesis of pyrroles by using the TiCl4/Sm system as catalyst. The one-pot three-component reaction between 1,3-diketones, aldehydes, and amines yielding the poly-substituted pyrroles in desired manner. After investigation of different low-valent titanium systems, the three equivalents of TiCl4/Sm reagent were more superior for the preparation of new pyrrole derivatives. The reaction at room temperature in anhydrous tetrahydrofuran encouraged the reaction with good yields in short reaction time (15 min) (Scheme 7). The protocol is useful for the development of wide range of regio-isomeric products. The authors have established that the protocol is applied for both the aromatic and aliphatic aldehydes and amines for the preparation of many biologically imperative pyrroles.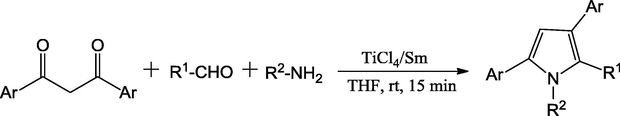
Synthesis of polysubstituted pyrroles derivatives.
Atar et al. (2015) have reported a new protocol for the expeditious synthesis of tetra substituted pyrroles using one-pot four-component technique in the presence of ceric ammonium nitrate supported on silica (CAN·SiO2) as catalyst (Scheme 8). The authors have chosen the CAN·SiO2 as catalyst due to its ease of preparation, inexpensive, and recycling of catalyst. The acidic nature of the catalyst has drawn enduring consideration in the building of C—C bond and C-hetero atom bonds in a range of organic transformations. The catalyst was prepared by mixing the 9.01 g of silica gel with a solution of CAN (1.02 g) in 2.0 mL of water. The yellowish powder obtained after evaporation of water and it could be in active up to six months provided stored in a tight sealed bottle. To push the reaction smoothly between the aldehyde, amine, dialkyl acetylenedicarboxylates and nitromethane under reflux conditions, the 10 mol% of CAN.SiO2 was optimized and more interestingly the reactant nitromethane was served as solvent. By alternating the aldehydes and amines, several substituted pyrroles have been reported with good yields in the range of 84–93%. This protocol has the scope in the synthesis of substituted pyrroles, which are widely used in the image diagnosis, for laser manufacture, and as chemosensors along others in pharmaceutical industry.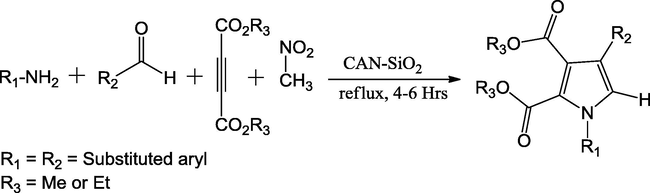
Synthesis of fully tetrasubstituted pyrrole functionalities catalyzed by CAN.SiO2.
In another study, Moghaddam et al. (2015) have outlined the limitations of previously reported extensive procedure for preparation of CoFe2O4@SiO2-PrNH2-Mo(acac)2 as catalyst for the synthesis of poly-substituted pyrroles. Authors have proposed the nickel ferrite (NiFe2O4) as a viable alternative catalyst. The co-precipitation method was employed to prepare the nano-sized NiFe2O4 particles from the solutions of Fe(NO3)3·9H2O and Ni(NO3)2·6H2O The synergistic effect of the two cations, chemical stability and magnetic nature of NiFe2O4 were exploited in the conversion of reaction mixture containing aldehyde, amine, nitromethane and 1,3-dicarbonyl into substituted pyrroles at 100 °C. The possessing of large surface area of nano-structured NiFe2O4 and low activation energy accelerated the reaction. High to excellent yields (70–96%) in relatively shorter times (3–4 h) and intact of catalytic efficiency up to nine cycles makes the NiFe2O4 an efficient catalyst for the facile production of pyrrole derivatives under neat conditions (Scheme 9).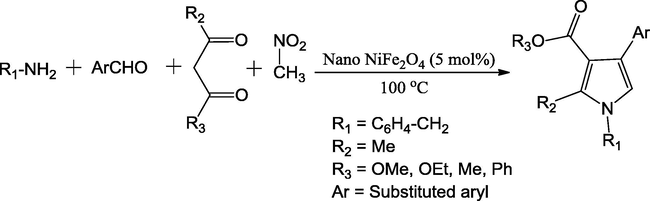
The preparation of different substituted pyrroles derivatives.
Atar and Jeong (2013) have reported silica supported tungstic acid (STA) as heterogeneous catalyst for the facile synthesis of tetra substituted pyrrole derivatives. The prepared STA could be mitigated the difficulties with earlier reported homogeneous catalysts. The STA is considered as acid catalyst, which fructify the making of new bonds in the organic transformations. While the catalyst is synonymous with ease of preparation, recyclability, low cost and isolation of organic products with high purity and yields add to the glitter. The introduction of 10 mol% of STA in refluxed condition accelerated the four-component reaction between aldehyde, amine, methylene ketones and nitro methane to afford impressive yields of substituted pyrrole compounds (Scheme 10). The mixture of silica chloride and sodium tungstic in hexane in 4 h reflux produced the STA. As far as yields are concerned, the STA showed a real effectiveness in the organic conversion over other silica supported ZnCl2, BF3 and TiO2 catalysts.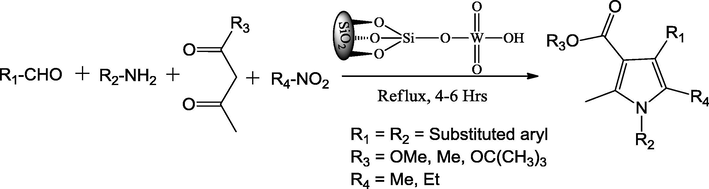
The preparation of different diverse tetra substituted pyrrole derivatives.
2.2 Two nitrogen containing heterocycles (Pyrazoles)
Pyrazole ring possess three C atoms and two N atoms in adjacent positions. It was firstly prepared by Knorr, which led to the discovery of antipyrine and its derivatives. Pyrazoles are very important group of heterocyclic compounds and different substituted pyrazoles are eminent as metal chelating and extracting reagents (Kramer, 2016; Ahmed and Mezei, 2015). Pyrazole represents as interesting template for combinatorial chemistry. Many condensed heterocyclic systems with pyrazoles as constituents have varied medicinal and agricultural applications (Kramer, 2016; Ahmed and Mezei, 2015; Liu et al., 2016). Due to excellent antibacterial (Panda et al., 2014), antifungal (Nasr et al., 2014), herbicidal (Ma et al., 2015; Janin, 2012), and antiviral (Lucas-Hourani and Munier-Lehmann, 2015; Kucukguzel and Senkardes, 2015) activities exhibited by pyrazoles, pharmacologically they are most important class among the heterocyclic compounds. Some of the pyrazole derivatives are known to possess antiarrhythmic (Liu et al., 2015), sedative (Meanwell, 2011a), hypoglycemic (Datar and Jadhav, 2015), and anti-inflammatory properties (Li et al., 2015; Viveka et al., 2015). Pyrazolines and annelated pyrazoles exhibit antimicrobial activity (Lucas-Hourani and Munier-Lehmann, 2015; Khan and Faidallah, 2015; Veenugopal and Vasavi, 2015). N-substituted pyrazole derivatives are capable to inhibit and deactivate of liver alcohol dehydrogenase (Bondock et al., 2012). Difenamizole and Metamizole exhibit higher analgesic activity than aspirin (Soltesz et al., 2008). Tactically functionalized fluorine substituents, especially the trifluoromethyl substituted group, in heterocyclic compounds show a significant role in pharmaceuticals and agrochemicals (Meanwell, 2011b; Giornal et al., 2013; Fustero et al., 2011). The fluorinated pyrazoles, in particular possess high biological activity as herbicides, fungicides, insecticides and analgesics agents (Yamamoto et al., 1992; Yang et al., 2015; Zhang et al., 2014; Faour et al., 2015; Gurib-Fakim, 2006; Majumdar et al., 2014).
Using CeO2/SiO2 as catalyst and a four-component condensation of phenylhydrazine, β-keto ester, 2-napthol and aromatic aldehydes, Akondi et al., have reported the synthesis of 1H-3-pyrazolones in excellent yields with water as solvent under reflux conditions (Scheme 1d) (Akondi et al., 2016). A new Ce/SiO2 complex synthesized via the sol–gel process was effectively applied as a heterogeneous Lewis acid (Scheme 11). The intrinsic possessions of Ce/SiO2 mixed oxide catalyst such as superior selectivity, eco-friendly compatibility, non-corrosiveness, recyclability, operational simplicity, low cost and ease of isolation makes this catalyst useful to carry out the reaction.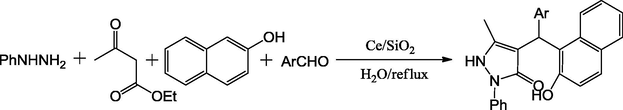
General synthesis of 1H-3-pyrazolones.
Twelve novel pyrazole-4-carbonitrile derivatives were synthesized using CuO/ZrO2 catalyst and three-component Mannich type reaction of phenyl hydrazine, malononitrile and substituted aromatic aldehydes in good to excellent yields within in 2 h (Scheme 12). The new mixed oxide catalyst, which was prepared by a simple wet-impregnation technique, revealed higher activity and long term stability. The heterogeneous catalyst is amply reusable for over five cycles with its high activity undiminished (Maddila et al., 2015c).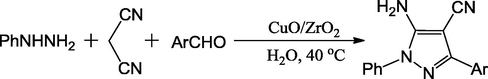
Synthesis of pyrazole-4-carbonitrile derivatives.
A one-pot, three-component reaction of 1,4-dihydropyrano[2,3-c]pyrazole derivatives using aldehydes, malononitrile and 3-methyl-1-phenyl-1H-pyrazol-5(4H)-one was developed using H14[NaP5W30O110] as a mixed oxide heteropolyacid catalyst (Scheme 13) (Heravi et al., 2010). This catalyst has remarkable properties like strong Bronsted acidity and flexible conversions, which are vital in catalytic reactions. Importance of heteropolyacids as environmentally benign catalysts is also due to their other advantages such as cost-effectiveness, long thermal stability, simple synthesis and reusability. Aldehydes with both electron-withdrawing and electron-donating substituents gave good to excellent yields (84–95%). The use of barbituric acid in place of 3-methyl-1-phenyl-1H-pyrazol-5(4H)-one again gave good yields. Catalyst was reusable with similar activity up to five cycles.![H14[NaP5W30O110] Catalyzed one-pot three-component synthesis.](/content/184/2020/13/1/img/10.1016_j.arabjc.2017.09.016-fig17.png)
H14[NaP5W30O110] Catalyzed one-pot three-component synthesis.
Highly efficient ultrasound irradiation technique using rare-earth based manganite, La0.7Sr0.3MnO3 (LSMO) as catalyst was reported for 4H-pyrano[2,3-c]pyrazoles. The three component/one-pot reaction involved aromatic aldehyde, malononitrile and 3-methyl-1-phenyl-2-pyrazolin-5-ones at room temperature (Scheme 14). EtOH as a solvent gave better yields in less time relative to H2O and CH3CN. LSMO, the mixed oxide nano catalyst was synthesized by hydrothermal process (Azarifar et al., 2013). The magnetic nano mixed oxide can act also as superb adsorbent for an extensive diversity of heterocyclic molecules to develop their activity in organic reactions. LSMO offers several benefits such as higher yields (97%), long-term stability, robust oxidizing power, non-toxicity, easy recyclability, and high catalytic activity owing to their more number of surface atoms.![Nano-LSMO catalyzed synthesis of 4H-pyrano[2,3-c]pyrazoles.](/content/184/2020/13/1/img/10.1016_j.arabjc.2017.09.016-fig18.png)
Nano-LSMO catalyzed synthesis of 4H-pyrano[2,3-c]pyrazoles.
NiFe2O4@SiO2–H3PW12O40, a magnetic nano-sized acid catalyst was successfully employed in synthesis of pyrazole moieties by Maleki et al. NiFe2O4@SiO2–H3PW12O40, was synthesized by combination of Keggin heteropolyacid with silica loaded NiFe2O4 magnetic nanoparticles. Pyrano[2,3-c]pyrazole derivatives were prepared through one-pot, three component catalyzed condensation of aldehyde, malonitrile and 1,3-dicarbonyl compounds. The catalyst is separable using external magnetic field. (Scheme 15) (Maleki et al., 2016).![One-pot synthesis of tetrahydrobenzo[b]pyrans and pyrano-[2,3-c]pyrazoles.](/content/184/2020/13/1/img/10.1016_j.arabjc.2017.09.016-fig19.png)
One-pot synthesis of tetrahydrobenzo[b]pyrans and pyrano-[2,3-c]pyrazoles.
Shaterian and Kangani (2014) have investigated the use of P2O5/SiO2, H3PO4/Al2O3, starch/H2SO4 and Cellulose/H2SO4 as catalysts for synthesis of 1,4-dihydropyrano[2,3-c]pyrazoles. Equi molar ratio of aryl aldehyde, malononitrile, ethyacetoacetate and hydrazine monohydrates in the presence of either 80 mg of P2O5/SiO2, 80 mg of H3PO4/Al2O3, 50 mg of Cellulose/H2SO4 or 50 mg of starch/H2SO4 under solvent-free conditions at 100 °C were reported as ideal reaction conditions for good yields (Scheme 16).![Preparation of 6-amino-4-aryl-3-methyl-1,4-dihydropyrano[2,3-c]pyrazole-5-carbonitriles using heterogeneous catalysts under solvent-free and thermal conditions.](/content/184/2020/13/1/img/10.1016_j.arabjc.2017.09.016-fig20.png)
Preparation of 6-amino-4-aryl-3-methyl-1,4-dihydropyrano[2,3-c]pyrazole-5-carbonitriles using heterogeneous catalysts under solvent-free and thermal conditions.
Nano magnetic crystalline CuFe2O4 as a Lewis acidic heterogeneous mixed oxide catalyst was employed by Pradhan et al., for the synthesis of functionalized dihydropyrano[2,3-c]pyrazoles by MCR in excellent yields using dialkyl acetylenedicarboxylates, malononitrile, substituted hydrazine derivatives and ethyl acetoacetate with water as solvent (Scheme 17). These catalyst particles were easily separated using with external magnet. As these magnetic materials reportedly contain highly active and precise centers, these features may assist to enhance the scope of this material for other applications. Catalyst stayed with similar activity for six cycles. All the reactions occurred with high efficiency under very simple and mild conditions (Pradhan et al., 2014).![Synthesis of dihydropyrano-[2,3-c]pyrazole derivatives.](/content/184/2020/13/1/img/10.1016_j.arabjc.2017.09.016-fig21.png)
Synthesis of dihydropyrano-[2,3-c]pyrazole derivatives.
An improved approach was reported by Suman et al., where they employed a strategy of sulfonic acid functionalized silica-coated CuFe2O4 magnetic (CuFe2O4@SiO2-SO3H) mixed oxide nanoparticles (Scheme 18). The evident benefit of the approach is the paramagnetic nature of magnetic nanoparticles assists the separation of catalyst through external magnet which makes the recovery of the catalyst easier and prevents loss of catalyst associated with conventional filtration. As a result, minor CuFe2O4 nanoparticles vastly distributed onto the silica could be homogeneously obtained. The catalyst also revealed high performance in the three-component Knoevenagel–Michael cyclization coupling reaction of 2-pyrazole-3-aminoimidazo-fused polyheterocyclic compounds with excellent yields in the presence of the ethanol as solvent (Suman et al., 2016).![Synthesis of 2-pyrazole-3-amino-imidazo-[1,2-a]pyridines derivatives.](/content/184/2020/13/1/img/10.1016_j.arabjc.2017.09.016-fig22.png)
Synthesis of 2-pyrazole-3-amino-imidazo-[1,2-a]pyridines derivatives.
Recently, dioxomolybdenum complex supported on functionalized Fe3O4 magnetite nanoparticles was reported by Jamshid and coworkers (Rakhtshah et al., 2016). They modified iron oxide nanoparticles with dioxomolybdenum complex through a Stober method followed by silica core–shell to generate Fe3O4@Si@MoO2 mixed oxide catalyst (Fig. 4). The catalytic method conserves active sites free for efficient catalysis. In the one-pot multicomponent reaction of malononitrile, aromatic aldehyde and phenylhydrazine to pyrazoles, the Knoevenagel–Michael cyclization reactions in solvent-free condition using Fe3O4@Si@MoO2 as catalyst afforded the target products with excellent yields (Scheme 19).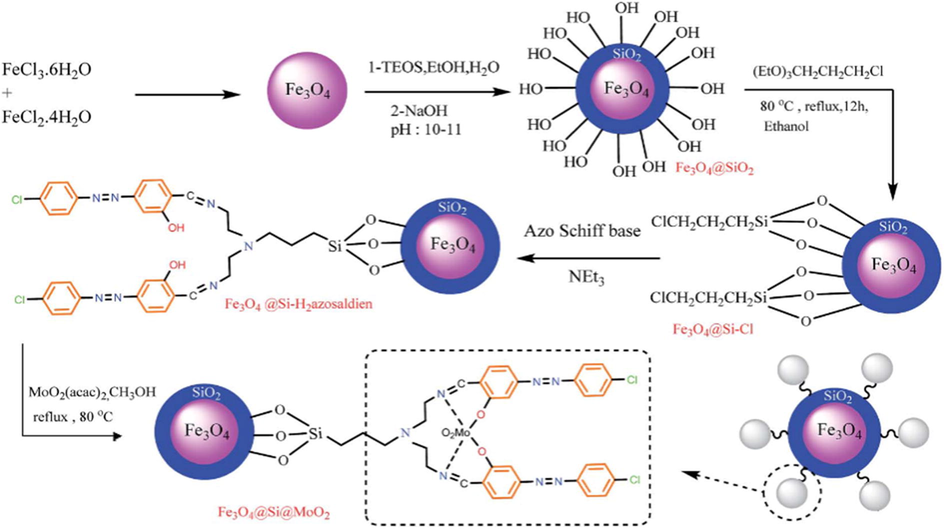
The sequence of events in the preparation of immobilized dioxomolybdenum complex supported on MNPs.
Source: Reprinted from Rakhtshah et al. (2016) with permission from Royal Society of Chemistry.
The synthesis of pyrazole derivatives using molybdenum complex supported on functionalized Fe3O4 magnetite nanoparticles containing Schiff base ligand.
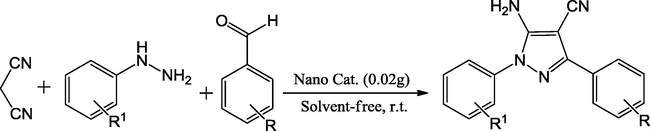
Zinc aluminate nanoparticles (ZnAl2O4) were used as a mixed oxide basic solid, recyclable for catalyzing the three-component cyclocondensation reaction of hydrazine hydrate, ethyl acetoacetate and substituted aromatic aldehydes to synthesize 4,4′-(arylmethylene)bis(3-methyl-1H-pyrazol-5-ol) derivatives (Ghomi et al., 2014). The products were obtained in good yields in few minutes. The benefits offered by this approach are easy method, very short reaction times, good yields, cost-effective catalyst, atom economy, use of no hazard solvent and no need of chromatographic purification (Scheme 20).
Synthesis of 4,4′-(arylmethylene)bis(3-methyl-1H-pyrazol-5-ol).
2.3 Two nitrogen containing heterocycles (Imidazoles)
Imidazole is an aromatic heterocyclic compound with the formula C3H4N2, which is an alkaloid. While imidazole refers to the parent compound, imidazoles are a class of heterocycles with similar ring structure, but with varying substituents. This ring system is present in many important biological building blocks such as histidine, related hormone histamine etc. Imidazole can serve as a base and as a weak acid. Many drugs such as antifungal drugs and nitroimidazole, contain an imidazole ring. Mohmoud et al., have used CuO in Fe3O4 nanoparticles as Lewis acids to motivate the carbonyl group in aldehydes for nucleophilic attack. CuFe3O4 magnetic nano mixed oxide catalysts can simply be alienated and recycled from the products by an external magnet. Its catalytic performance is enhanced by the accessible surface area of the nonporous magnetic nanoparticles. They established that the nanoparticles could catalyze the cyclic condensation reaction of aldehyde, benzyl, propargylamine and ammonium acetate (NH4OAc) in mixture of aqueous ethanol to form high yields of 1,2,4,5-tetrasubstitutedimidazole derivatives (Scheme 21) (El-Remaily and Abu-Dief, 2015). Easy workup, short reaction time, and high reusability of the catalyst are the attractive features.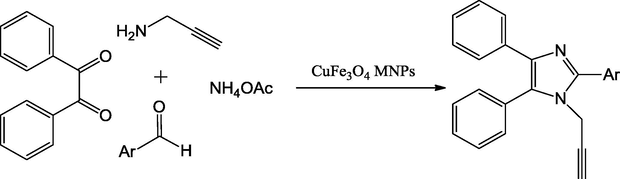
Synthesis of 1,2,4,5-tetrasubstituted imidazoles.
1,2,4,5-Tetrasubstituted-1H-imidazoles were produced from aldehydes, benzil, primary amines and NH4OAc catalyzed by a silica-supported SbCl3 (SbCl3/SiO2) as a heterogeneous mixed oxide catalyst under microwave irradiation (Scheme 22) (Safari et al., 2014). The catalyst was simply a mechanical mixture of SbCl3 and silica and the solvent-free process gave products in good to excellent yields in 15 min.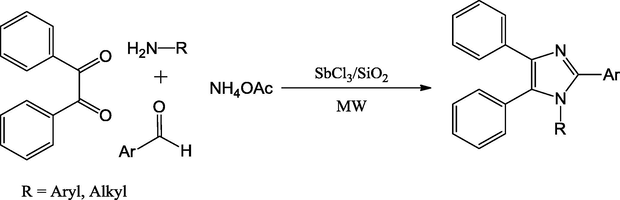
Synthesis of substituted imidazoles.
A novel urea-functionalized Fe3O4/SiO2 magnetic nanocatalyst was also synthesized by Ali and coworkers (Maleki et al., 2015). This mixed oxide catalyst contains highly distributed, nanosized iron oxide/silica nanoparticles that could be evenly imbedded in urea material. The catalyst also exhibited excellent performance in the coupling reactions in the presence of the green solvents (Scheme 23). The study displayed that the catalyst worked well in the preparation of substituted imidazoles. The catalyst is reusable and separable and by the use of an external magnet.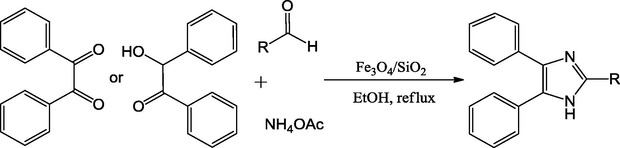
Synthesis of functionlized imidazoles.
Using iron oxide nanoparticle as support copper functionalized recoverable catalysts, CuFe2O4 nanoparticles initially were prepared and employed in the preparation of Cu/Co-Fe2O4 mixed oxide catalysts. A representative example of such nanoparticles is magnetically recoverable Cu/Co-Fe2O4 nanoparticles described by the Paul and co-workers (Scheme 24). Those were applied in condensation to synthesize 2,4,5-tri substituted imidazole derivatives with ethanol as solvent. These catalysts exhibited very efficient catalytic activity and excellent reusability. These mixed oxide nanocatalysts use common chemicals and thus have prospective applications in industries (Sanasi et al., 2014).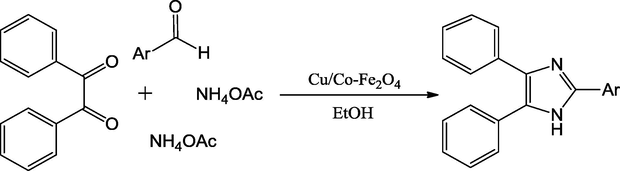
One-pot synthesis of 2,4,5,-trisubstituted imidazoles.
Safari et al., have proposed a protocol for the preparation of tetrasubstituted imidazoles using aldehyde, 1,2-diketone, NH4OAc and primary amine via cyclo-condensation employing SbCl3/SiO2 as catalyst. High catalytic activity of SbCl3/SiO2 was reported to be due to a superb dispersion of antimony trichloride over high surface area SiO2 support. Further, it reveals that distributed antimony trichloride harmonizes with the surface of OH groups leading to formation Cl—O—Sb as a stable Lewis acidic positions under the reaction conditions. In the solvent free reaction, optimum reaction conditions were reported as 120 °C, where the anticipated product was obtained in good to excellent yields with short reaction time (Scheme 25) (Safari et al., 2013).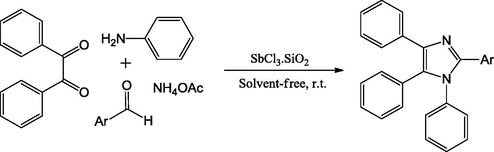
Synthesize 1,2,4,5-tetrasubstituted imidazoles in the presence of aromatic amine.
(PS@SiTPA30), tungstophosphoric acid supported on core-shell polystyrene-silica microspheres silica catalyst was also synthesized by Marina and coworkers. This method used polystyrene-silica as a coating and tungstophosphoric acid as reductant and stabilizer to form PS@SiTPA30 mixed oxide catalyst. Active material highly dispersed and evenly embedded in polystyrene-silica core-shells with Lewis acidic sites is expressed as cause for high activity. The cyclic condensation of aromatic aldehyde, benzyl and NH4OAc under absence of solvent and at 130 °C heating conditions gave good yield of the target products in 10–15 min (Scheme 26) (Gorsd et al., 2016).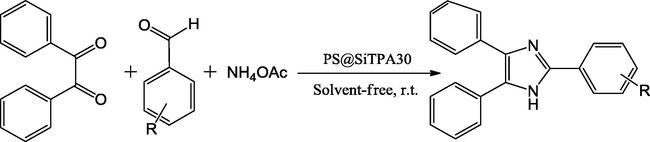
Trisubstituted imidazole synthesis.
Using potassium dodecatugstocobaltate trihydrate (K5CoW12O40H2O) mixed oxide catalyst. Lingaiah and co-workers have designed a facile preparation for 1,2,4,5-tetrasubstituted imidazole derivatives. K5CoW12O40H2O contains large reactive surface area, Brønsted acidic nature and good active sites proved good catalyst up to six cycles (Nagarapu et al., 2007). The reaction mixture of arylaldehyde, benzyl amines and NH4OAc gave the imidazoles in good to excellent yields under both conventional heating and microwave irradiation conditions in short reaction time. The catalyst offers reusability, atom economy, flexibility, fast and eco-friendly protocols (Scheme 27).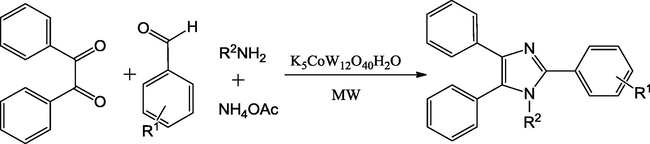
K5CoW12O40·3H2O catalysed synthesis of 1,2,4,5-tetrasubstituted imidazoles.
Recently, the synthesis of imidazo[1,2-a]pyridines was achieved by cyclocondensation of benzaldehyde, aminopyridine and cyclohexyl isocyanide in presence of Perovskite type LaMnO3 mixed oxide nano catalyst in a solvent-free reaction (Scheme 28) (Sanaeishoar et al., 2014). All products were obtained in excellent yields (92–99%). Further, the use of nano LaMnO3 as Lewis acidic catalysts gave the higher yield. High activity of the LaMnO3 along with Lewis acidic center depends on the high surface area and the perovskite structure, these structures playing a significant role to synthesis of anticipated targets. The main advantages of this procedure include excellent yields, short reaction time, mild condition, non-volatile, thermally stable, very low loading of catalyst and high performance of catalyst. Moreover the catalysts are separated by filtration, are reusable and lead to low leaching into solution.![Synthesis of imidazo-[1,2-a]pyridines compounds.](/content/184/2020/13/1/img/10.1016_j.arabjc.2017.09.016-fig33.png)
Synthesis of imidazo-[1,2-a]pyridines compounds.
Zhang and coworkers have reported interesting results, using magnetic carbon nanotube supported copper (CoFe2O4/CNT-Cu) mixed oxide catalyst, which was synthesized by a chemical co-precipitation method (Fig. 5). This catalyst have become one of the popular ones mainly due to its excellent chemical thermal stability, high surface area, high catalytic activity, noncorrosive, eco-friendly and inert nature. This catalyst could be rapidly separated from the reaction mixture by the use of an external magnet and reused eight times without obvious decrease in its catalytic activity. Consequently, the reactions were accomplished with various aldehydes, 2-aminopyridines and nitromethane, the corresponding 3-nitro-2-arylimidazo-[1,2-a]pyridines with yields between 80 and 95% for 2 h (Scheme 29) (Zhang et al., 2016).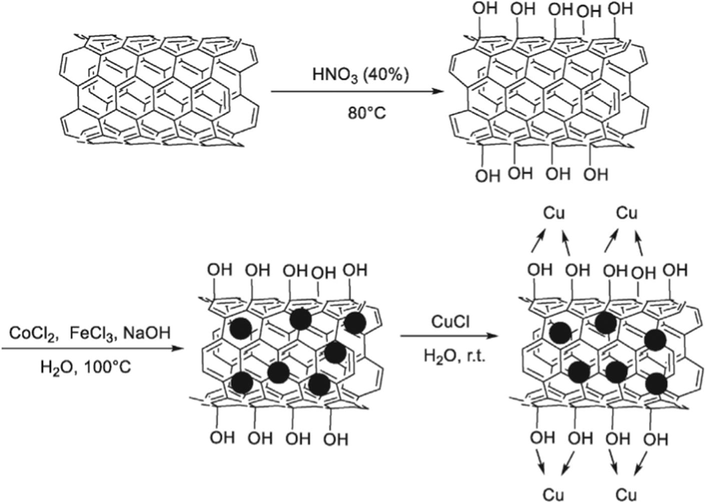
Synthesis of CoFe2O4/CNT–Cu.
Source: Reprinted from Zhang et al. (2016) with permission from Elsevier.
![Synthesis of 3-nitro-2-arylimidazo-[1,2-a]pyridines.](/content/184/2020/13/1/img/10.1016_j.arabjc.2017.09.016-fig35.png)
Synthesis of 3-nitro-2-arylimidazo-[1,2-a]pyridines.
Guntreddi and co-workers have established the catalytic use of magnetic nano-Fe3O4.KHSO4.SiO2 for a facile, efficient one-pot synthesis of imidazo-[1,2-a]pyridine derivatives with excellent yields (Scheme 30) (Guntreddi et al., 2012). Bharate et al., also improved another novel method for the preparation of imidazo-[1,2-a]pyridines using the similar method of coupling reaction (Scheme 31) (Bharate et al., 2013). The mixed Cu and Mn spinel oxide catalyzed domino multicomponent coupling of various aldehydes, 2-aminopyridines and alkyne examined by 5-exo-dig cyclo isomerization formed imidazo-[1,2-a]-pyridine derivatives in excellent yields.![One pot synthesis of imidazo-[1,2-a]pyridines.](/content/184/2020/13/1/img/10.1016_j.arabjc.2017.09.016-fig36.png)
One pot synthesis of imidazo-[1,2-a]pyridines.
![Synthesis of imidazo-[1,2-a]pyridines.](/content/184/2020/13/1/img/10.1016_j.arabjc.2017.09.016-fig37.png)
Synthesis of imidazo-[1,2-a]pyridines.
2.4 Three nitrogen containing heterocycles (Triazoles)
Triazoles consist of the three heteroatoms in adjacent position. The biological properties of triazole and its derivatives were broadly studied. Pharmacologically, its derivatives represent one of the most important classes of organic heterocyclic compounds, possessing antibacterial, antifungal, herbicidal, and antiviral activities. Some of its derivatives have been reported to exhibit significant antiarrhythmic, sedative, hypoglycemic, and anti inflammatory activities. In this reaction, a representative copper-(II)-1,4-dihydroxyanthraquinone supported on super paramagnetic Fe3O4@SiO2 was described by Saeed and co-workers. In their work, they immobilized copper-(II)-1,4-dihydroxyanthraquinone on silica layered iron oxide nanoparticles via an ion-pair strategy to form Cu(II)-DAQ-Fe3O4@SiO2 catalyst. This mixed oxide catalyst showed good selectivity in the reaction of alkyne, aryl boronic acid, sodium azide to form 1-aryl-1,2,3-triazole derivatives as product with aqueous acetonitrile as a solvent. The benefits of this reaction are that no need of surfactants, toxic reagents or solvents, no chromatography for purification. This magnetic renewable catalyst could be reused effectively at least six runs without obvious decrease in its catalytic performance in the further reaction (Scheme 32) (Zahmatkesh et al., 2016).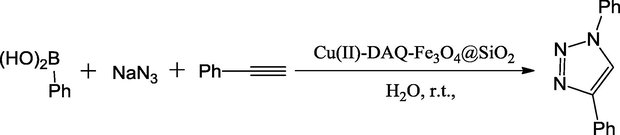
Fe3O4@SiO2–DAQ–Cu(II) catalyzed one-pot synthesis of 1-aryl-1,2,3-triazole.
In addition to the use of Fe3O4 nano catalyst particle as magnetic support for the synthesis of Cu-functionalized renewal catalysts, CuFe2O4 mixed oxide nanoparticles and CoFe2O4 magnetic nano catalyst could also be utilized in the preparation of Cu-functionalized renewal catalysts. A representative example of these nano catalyst is magnetically recoverable CuFe2O4 mixed oxide catalyst reported by the Kumar and co-workers (Kumar et al., 2012). Catalysts were used in alkyne, organic halide and azide cycloaddition to synthesize 1,4-disubstituted-1,2,3-triazoles (Scheme 33). This Cu-functionalized mixed oxide catalyst exhibited greater activity and good reusability. In the one-pot, multicomponent synthesis of 1,4-diaryl-1,2,3-triazoles, substituted benzyl-halides were reacted with NaN3 via in situ formation of benzyl azides, which could be easily reacted with alkynes using water as solvent in excellent yields. The CuFe2O4 nano catalysts could also be applied to synthesize 1,4-diaryl-1,2,3-triazole derivatives from acetylenes, sodium azide and boronic acid via the one-pot cycloaddition reaction as detailed by Kumar and coworkers (Scheme 34) (Kumar et al., 2013).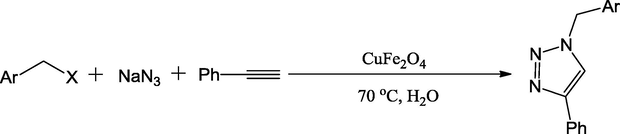
Synthesis of triazoles using CuFe2O4 nano particles in water.
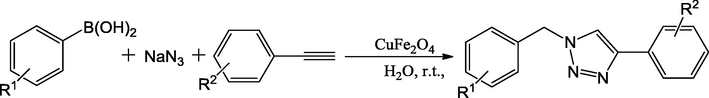
Magnetically catalyzed one-pot synthesis of 1,4-aryl-1,2,3-triazoles.
Notably, the Zhang et al., reported the synthesis of NiFe2O4 loaded Glutamate-functionalized Cu and its application in the organic transformations. Importantly, Cu nano catalyst particles were reproducibly strengthened on Glutamate coated NiFe2O4 through ultrasonication and simple wet-impregnation of divalent of copper ions on glutamate loaded NiFe2O4 followed by in situ reaction (Fig. 6). The catalytic activity of NiFe2O4-Gltamate-Cu has studied in the one-pot three-component click reactions of terminal alkynes, sodium azide and various benzyl chlorides to synthesize 1,2,3-triazole derivatives. At room temperature, with water as solvent and 0.005 mg of catalyst produced the impressive yields (85–96%) (Scheme 35) (Lu et al., 2015).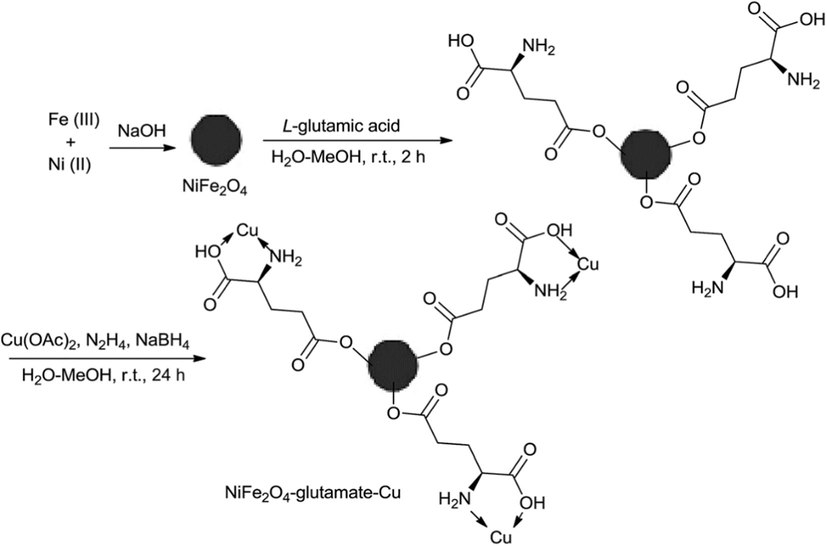
Synthesis of NiFe2O4–glutamate–Cu.
Source: Reprinted from Lu et al. (2015) with permission from Royal Society of Chemistry.
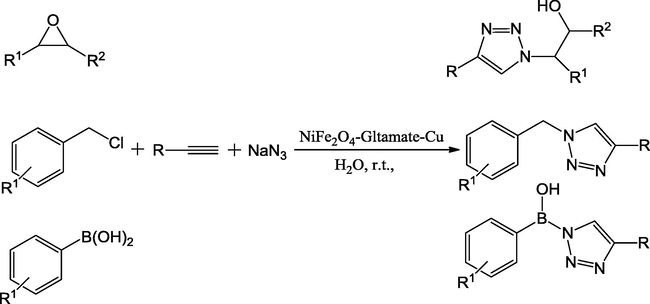
Synthesis of 1,2,3-triazoles catalyzed by NiFe2O4–glutamate–Cu in water.
Recently, two similar Cu-functionalized magnetic catalysts were designed by Xingquan and Lei. They altered ferrate nanoparticles either with [3-aminopropyl]-trimethoxysilane or [3-(2-aminoethylamino)propyl]-trimethoxysilane through a post grafting technique followed by complexation with copper bromide to produce novel magnetic supported Cu catalysts (Fig. 7). Those catalysts were employed in the one-pot reaction of phenylacetylene, sodium azide and benzyl chloride to synthesize 1,2,3-triazole derivatives via cycloaddition reactions in green solvent condition under microwave irradiation and obtained the target molecules in 45–65 min in good to excellent yields (85–96%). The microwave irradiation specifically reduced the reaction time and concurrently increased the reaction yields in contrast with conventional methods (Scheme 36) (Xiong and Cai, 2013).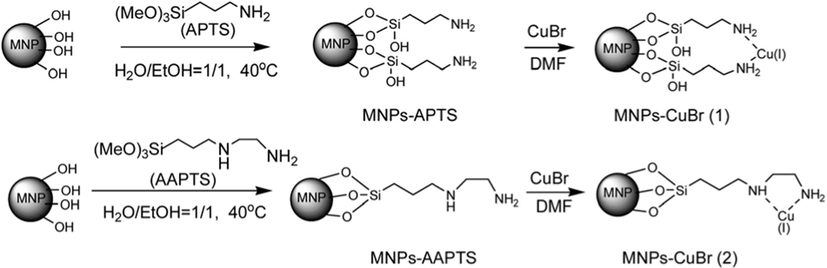
Synthetic route of MNPs–CuBr catalysts.
Source: Reprinted from Xiong and Cai (2013) with permission from Royal Society of Chemistry.
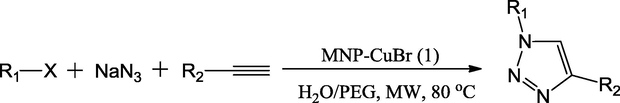
MNPs–CuBr catalyzed one-pot synthesis of 1,4-disubstituted 1,2,3-triazoles.
Mukherjee et al. (2013) have reported solvent-free, one-pot synthesis of 1,2,3-triazoles by using the Cu/Al2O3 mixed oxide as catalyst. The catalyst was prepared by using ball-milling method in the absence of any solvent and additive. A facile, multicomponent coupling reaction between 1,3-terminal alkynes, sodium azide and alkyl halides/aryl boronic acids yielded the 1,2,3-triazole derivatives. The azide reactants were generated in situ and thus this method dodges the handling of harmful azides. The authors have established that the protocol offers wide scope for access to various substituted 1,2,3-triazoles, the use of ball-milling method, use of no hazardous organic solvent, reusability of the catalyst and good yields in short reaction time (25 min) (Scheme 37) are attractive features of the study.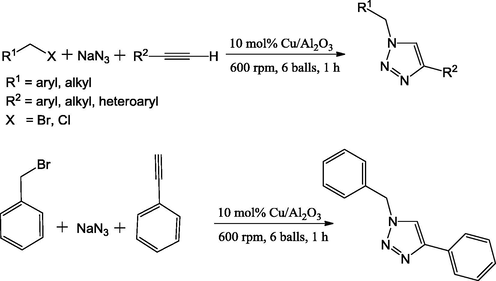
Solvent-free one-pot synthesis of 1,2,3-triazole derivatives.
Xiong et al. (2014) have reported a nanocomposite, GO/Fe3O4–CuBr for Cu(I)-catalyzed azide-alkyne 1,3-dipolar cycloaddition (CuAAC) reaction. The preparation of 1,2,3-triazole derivatives via CuAAC is recognized as a green synthetic approach but the homogeneous propensity of copper catalysts limited their applicability. The immobilization of copper salts on support is an attractive option for CuAAC reaction as well as the residual copper metal with the products. The authors have chosen the graphene oxide (GO) as viable support material and initially, prepared the GO/Fe3O4 nanocomposite by using the co-precipitation method. The GO/Fe3O4 obtained was treated with the CuBr (200 mg) to afford the GO/Fe3O4–CuBr catalyst (Fig. 8). The thermal studies disclosed that GO/Fe3O4-CuBr nanocomposite exhibits higher thermal stability than GO and GO/Fe3O4. The copper content in the GO/Fe3O4-CuBr before and after six cycles were identified to be 10.115 and 8.504 wt%, respectively by using atomic absorption spectroscopy studies, which disclosed the reusability potential of the catalyst. Using the catalyst, one-pot three-component reaction between primary halides, alkynes and sodium azide in the presence of 480 W of microwave irradiation at 80 °C in water was reported with good to excellent yields (92–98%) of the 1,2,3-triazoles. Large surface area and good accessibility of GO was mentioned as the cause of profound catalytic activity of GO/Fe3O4-CuBr (Scheme 38).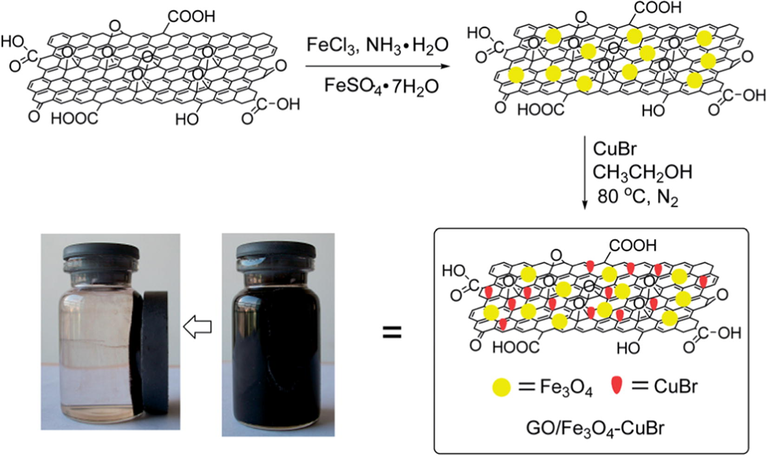
Schematic representation of the preparation and magnetic separation of GO/Fe3O4–CuBr catalyst from water by an external magnet after 30 s.)
Source: Reprinted from Xiong et al. (2014 with permission from Royal Society of Chemistry.
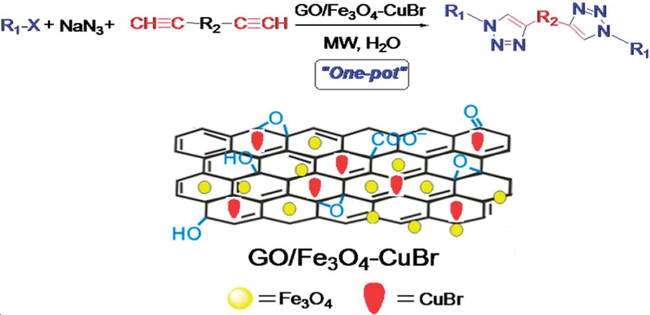
One-pot synthesis of 1,4-disubstituted mono/bis-1,2,3- triazoles catalyzed by GO/Fe3O4–CuBr in water under microwave irradiation condition.)
Source: Reprinted from Xiong et al. (2014 with permission from Royal Society of Chemistry.
Naeimi and Nejadshafiee have reported a Cu(I)@phosphorated SiO2 (CPSi) catalyst for the synthesis of β-hydroxy-1,2,3-triazoles (Scheme 39). The silica gel was modified with PCl3 in dry triethyl amine as base to afford the phophorated silica. The CPSi was employed as catalyst in the multi-component reaction between sodium azide, epoxide, and alkyne in water at 60 °C to produce β-hydroxy-1,2,3-triazoles. The catalyst in this protocol acted as bifunctional role since the catalyst promoted the epoxide ring opening as well as its activation for the cycloaddition. The copper loading of 0.64 mol% in CPSi facilitated the forward of the reaction smoothly. Distinguishing from the other copper contain catalysts, the CPSi showed a superiority in the yields and reaction times (Naeimi and Nejadshafiee, 2014).
Three-component synthesis of β-hydroxy-1,2,3-triazoles.
Jahanshahi and Akhlaghinia have reported a novel heterogeneous catalyst, namely CuII immobilized on guanidinated epibromohydrin functionalized γ-Fe2O3@TiO2 (γ-Fe2O3@TiO2-EG-CuII) for strengthening the Cu(I)-catalyzed azide–alkyne cycloaddition (CuAAC) reaction for the synthesis of 1,4-disubstituted 1,2,3-triazoles (Scheme 40). The magnetic nanoparticles on other hand have significant importance in catalysis and their usability is increased with incorporation of MNPs into some core shell structures. The authors have synthesized the catalyst in four steps starting from γ-Fe2O3@TiO2 nanoparticle preparation to immobilization of CuII (Fig. 9). The preparation of catalyst is followed the order γ-Fe2O3@TiO2 → γ-Fe2O3@TiO2-E → γ-Fe2O3@TiO2-EG → γ-Fe2O3@TiO2-EG-CuII (where, E = epibromohydrin, G = guanidine). The 4.0 mol% of catalyst was employed in the solution containing the three components viz. terminal alkynes, sodium azide and bromides at 50 °C in water. The TGA studies of catalyst disclosed that about 0.70 mmol/g of organic part supported on the surface of the γ-Fe2O3@TiO2. The catalyst showed the enhanced performance when compared with other catalysts in 1,3-dipolar cycloaddition step between the intermediates. The leaching of Cu metal from the catalyst is very low up to sixth run and it was evidenced from the ICP results which stated that 0.74 mmol of Cu per gram of freshly prepared γ-Fe2O3@TiO2-EG-CuII has reduced to 0.64 mmol only in the sixth cycle of γ-Fe2O3@TiO2-EG-CuII (Jahanshahi and Akhlaghinia, 2016).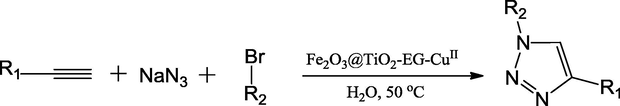
Synthesis of different structurally 1,4-disubstituted 1,2,3-triazoles.
Preparation of CuII immobilized on guanidinated epibromohydrin functionalized g-Fe2O3@TiO2 (g-Fe2O3@TiO2-EG-CuII).
Source: Reprinted from Jahanshahi and Akhlaghinia (2016) with permission from Royal Society of Chemistry.
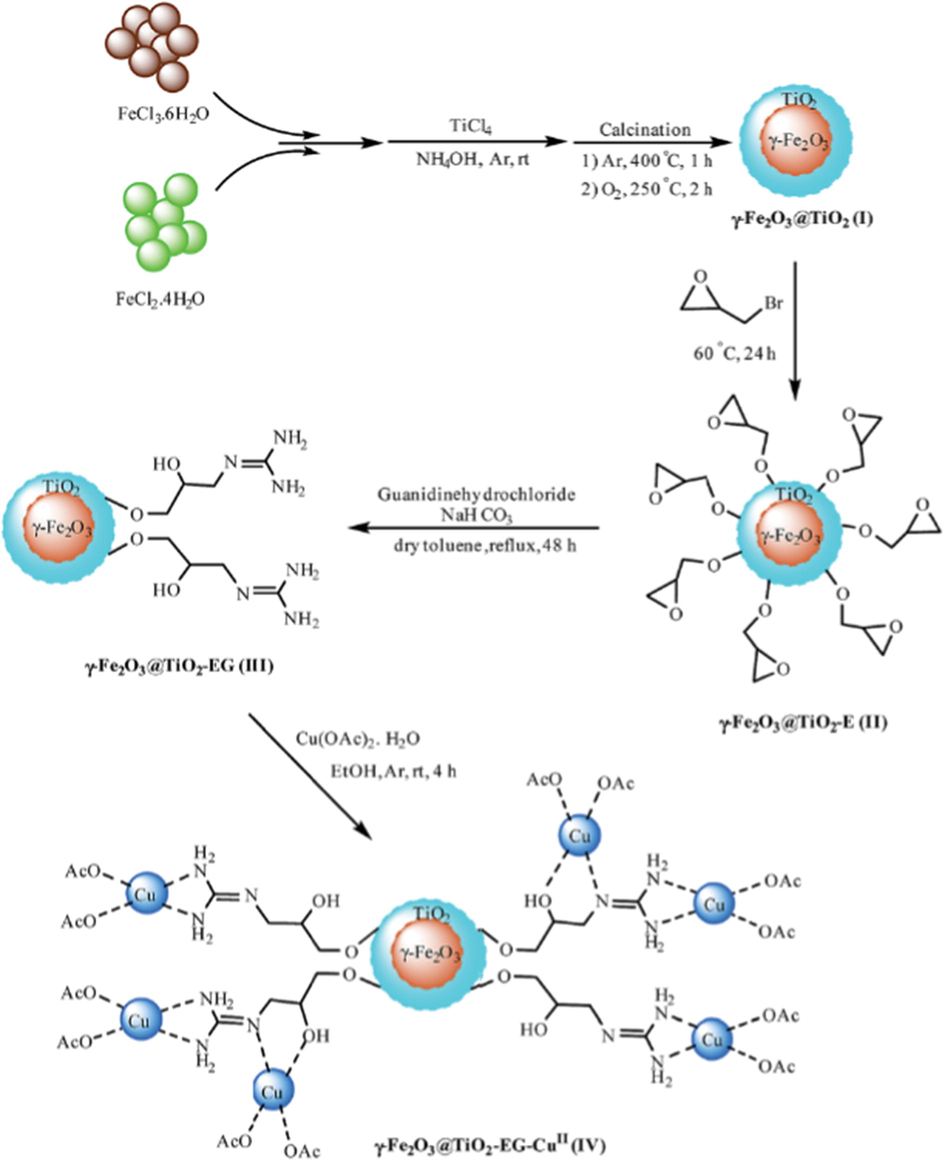
Tajbakhsh et al. (2015) have found the efficacy of 2,2′-biimidazole (H2Biim) complexes as homogeneous catalyst in the synthetic organic chemistry. The limitation accrues from the separation and recyclability of such catalyst lessens their prominence in the catalysis. To overcome the limitation, authors have functionalized the H2Biim complexes with solid supports for creating attractive heterogeneous catalysts. Primarily, the magnetic nanoparticles (MNPs) of ferrite (Fe3O4) were synthesized by using the FeCl3 and FeCl2 precursors via co-precipitation method (Fig. 10). The so prepared Fe3O4 nanoparticles were modified with silica and 3-(chloropropyl)-triethoxysilane (CPTES) to improve the chemical stability of MNPs. Finally, the Fe3O4 nanoparticles were treated with biimidazole followed by the metal salts affording MNP@BiimM M: Cu(I), Cu(II), Ni(II), Co(II). The magnetic studies revealed that the magnetic nature of MNPs still persist even after immobilization of non-magnetic materials. The catalytic performance of MNP@BiimCu(I) was investigated in the coupling of terminal alkyne, sodium azide and alkyl halide to produce 1,2,3-triazoles (Scheme 41). With 5 mg of catalyst at room temperature in water was reported optimal condition for reaction. Authors have commented on the other metal catalytic activities and asserted that Cu(I) nanoparticles exhibited better catalytic activity compared to Cu(II), Ni(II) and Co(II) nanoparticles. The Cu(I) species facilitated the azide-alkyne 1,3-dipolar cycloaddition reaction during the formation of 1,2,3-triazoles. No agglomeration, no metal leaching and several recycling ability of catalyst marked to be more advantageous to the industrial sector.
Stepwise preparation of MNP@BiimM nanoparticles as a magnetically separable system.
Source: Reprinted from Tajbakhsh et al. (2015) with permission from Royal Society of Chemistry.
Synthesis of 1,4-disubstituted 1,2,3-triazoles in water.
3 Six membered heterocycles
The six membered heterocycles are categorized into pyridines and pyrimidines based on number of nitrogen atoms in the ring. A number of synthetic procedures have been reported by using different heterogeneous catalysts including zeolites, mixed oxides etc. The low cost, ease of synthesis, and recyclable mixed oxides has produced variety of six membered heterocycles in good to excellent yields. The following sections interpret the efficient synthesis of pyridines and pyrimidines by using diverse mixed oxides as catalyst materials.
3.1 One nitrogen containing heterocycles (Pyridines)
Pyridine is a six membered, one-nitrogen containing heterocyclic ring compound. It was developed by Thomas Anderson in 1849 (Elwahy and Shaaban, 2015). Various pyridine derivatives have been explored as novel molecules with anti-inflammatory, antiviral, antimicrobial, analgesic, antineoplastic, antipsychotic, antidepressant, antifungal, antioxidant and insecticidal activities (Heller and Hapke, 2007; Hill, 2010) Consequently, varied pyridine derivatives have been prepared as target structures by many researchers and were evaluated for their biological activities.
Dam et al. reported the synthesis of 1,4-dihydropyridine (1,4-DHP) derivatives via an one-pot reaction of aldehydes, dimedone or 4-hydroxycoumarine and NH4OAc involving Knoevenagel-Michael reaction using Fe3O4@SiO2 mixed oxide as a heterogeneous catalyst and water as solvent medium. In this protocol, a wide range of substituted 1,4-dihydropyridines were reported with high yields (82–95%) under reflux condition in short reaction times (10–30 min) (Scheme 42). For the preparation of Fe3O4@SiO2 mixed oxide, at first ferrate nanoparticles were prepared from mixture of ferric nitrate and ferrous sulphate in ammonia solution by using co-precipitation method. Then, to develop the chemical thermal stability of ferrate particles, its external alteration was effectively done with appropriate deposition of SiO2 on to the ferrate mixed oxide particles by the NH3 catalyzed hydrolysis of precursor silicate. The TEM micrograph of Fe3O4@SiO2 catalyst showed that the samples constitute a well dark spherical metal particle core bounded by SiO2 core–shell structure (Dam et al., 2014).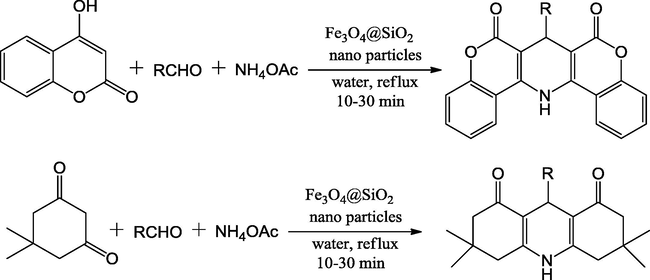
On-water synthesis of 1,4-DHPs.
Naik and Shivashankar have developed a MCR for synthesis of 1,4 dihydropyridines from ethyl acetoacetate, substituted aldehydes and NH4OAc by using the 10 mg of nano Zn-Fe2O4 metal oxide catalyst in aqueous medium (Scheme 43). TEM image revealed that the ZnO nanoparticles were entrapped successfully in the iron oxide. The effective features of this method are the mild reaction conditions, clean reaction profiles, high atom efficiency, non-toxicity and high yields (87–95%) (Naik and Shivashankar, 2016).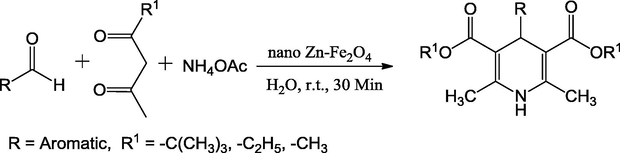
Synthesis of 1,4-dihydropyridine.
A facile Zn-VCO3 hydrotalcite was used as an environmental friendly catalyst for the synthesis of triphenylpyridine-3,5-dicarboxamide derivatives via Hantzsch reaction of aldehydes and acetoacetanilide with ammonium hydroxide in good to excellent yields (Scheme 44). Zn-VCO3 hydrotalcite was prepared through a modified co-precipitation process. The high specific surface area and total pore volume of the prepared catalyst confirmed from N2 adsorption–desorption isotherms were 13.4 m−2 g−1 and 0.044 cm−3 g−1, respectively. The reactions were carried out in water gave corresponding products in good to excellent yields (83–95%) in 2–3 h. The catalyst could also be successfully recycled up to five runs without significant loss of activity (Pagadala et al., 2014b).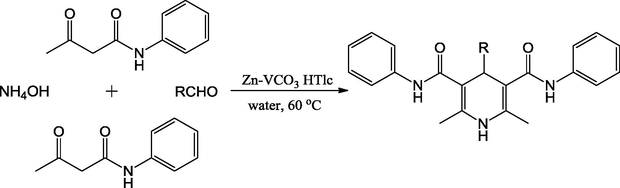
Multicomponent reaction catalyzed by hydrotalcite.
Zhang et al., have synthesized a series of spirooxindole dihydropyridines by reaction of malononitrile, isatins and anilinolactones in mixture of choline chloride/urea as a green solvent under Fe3O4/GO-Mo catalyst in the presence of microwave irradiation. The catalyst was synthesized via a consecutive four-step manner. Initially, graphene oxide (GO) was prepared with oxidation of graphite residue adapting to a marginally amended Hummer's method. Next the prepared GO was deferred into H2O and treated with FeCl3 and FeCl2 to afford ferrate Fe3O4/GO nanocomplex. Further, amine-functionalized Fe3O4 based on GO material were synthesized by sonicating Fe3O4/GO with dopamine in H2O. Moreover, the addition of bis-(acetylacetonato)-dioxomolybdenum into the Fe3O4/GO suspension in methanol yielded magnetically separable Fe3O4/GO-Mo mixed oxide nanocatalyst (Fig. 11). Low crystalline structure of the synthesized catalyst particles was identified by powder XRD instrument. The peaks in Fe3O4/GO-Mo at 2θ = 30.1°, 35.5°, 43.5°, 57.0° and 62.6° could be indexed as (2 2 0), (3 1 1), (4 0 0), (4 2 2), (5 1 1), (4 4 0) and (5 3 3), which are characteristics of single-phase cubic spinel structure (JCPDS card no. 19-0629). The SEM image of Fe3O4/GO and Fe3O4/GO-Mo approves that these particles are non-uniform sized and most of the particles have a spherically shaped. Using this molybdenum on magnetic Fe3O4/GO as a reusable catalyst, a wide range of substituted target molecules were reported with high yields (85–96%) (Scheme 45) (Zhang et al., 2017).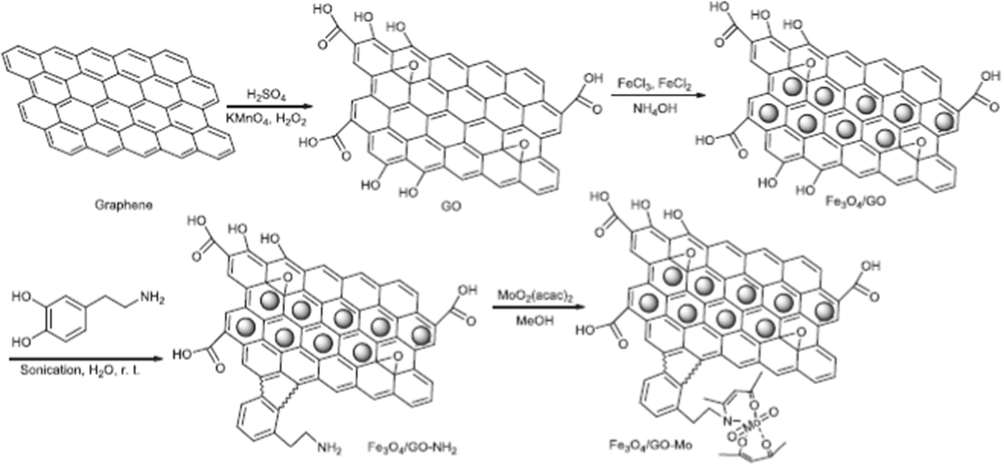
Preparation of Fe3O4/GO-Mo.
Source: Reprinted from Zhang et al. (2016) with permission from Royal Society of Chemistry.
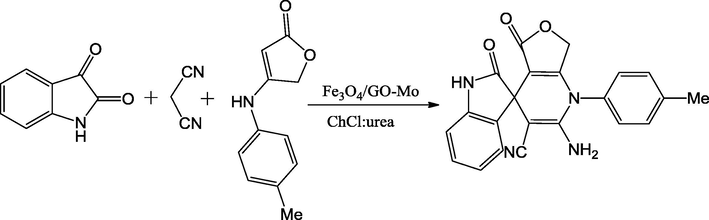
Synthesis of spirooxindole dihydropyridine derivatives.
Jain et al. (2008) explored a green mixed oxide catalyst alumina loaded with MoO3 to synthesize the 3,4-dihydropyridine-2(1H)-one derivatives (Scheme 46). The MoO3 doped alumina was prepared by the wet-impregnation method using ammonium heptamolybdate and calcined at 550 °C. Mo content of catalyst analysed by ICP-AES was 16%. Powder XRD pattern of the MoO3/Al2O3 revealed the amorphous nature. No specific peak of MoO3 was displayed as MoO3 is well dispersed over alumina surface. The molybdenum surface area 248.9 m2/g with pore size distribution 10 Å was investigated by the BET analysis. The prepared novel heterogeneous material was found to display high catalytic activity in one-pot, three component reaction of ethylacetoacetate, benzaldehyde and urea under solvent-free conditions. It provided an efficient method for the synthesis of 3,4-dihydropyridine-2(1H)-ones in high to excellent yields (up to 98%). The catalytic system can be successfully reused several times with a small decrease of its catalytic performance.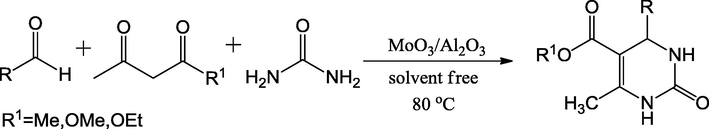
Synthesis of 3,4-dihydropyridine-2(1H)-ones under solvent free conditions.
Substituted novel indeno[1,2‐b]pyridines were synthesized with good to excellent yields from 1,3‐indandione, substituted aldehydes, acetophenone and NH4OAc in one pot using novel nanocrystalline Cu/ZnO catalyst (Scheme 47), at room temperature in the presence of mixture of aqueous ethanol solvent. The significant feature of this method comprises mild reaction conditions, short reaction time and high purity of product, good yield and recyclable catalyst without noticeable decrease in catalytic activity. Cu loaded ZnO nanocrystalline complex was synthesized using with commercially available salts (Zn(NO3)2·6H2O and CuSO4·5H2O) based on co-precipitation method under mild conditions (Alinezhad et al., 2014).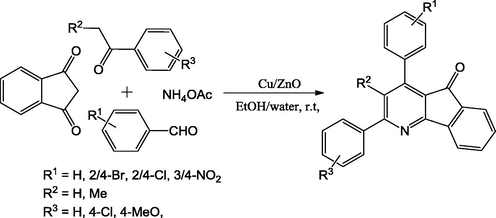
Synthesis of indenopyridines using Cu-doped ZnO nanocrystalline catalyst.
Bamoniri and Fouladgar (2015) reported the usage of Fe3O4@SiO2–SnCl4 mixed oxide catalyst for the preparation of 1,4-dihydropyridine derivatives shorter reaction time with excellent yield up to 98% (Scheme 48). The reaction was carried out with mild conditions and product yields were quite high. The one-pot multicomponent reaction between aldehyde, 1,3-dicarbonyl compound, and NH4OAc under ultrasonic irradiation by using Fe3O4@SiO2–SnCl4 as a heterogeneous catalyst, which can act as a Lewis acid catalyst. Fe3O4 nanoparticles were synthesized by co- precipitation method. Fe3O4@SiO2 was prepared by stirring the solution of tetraethylorthosilicate and Fe3O4, and then the preparation of Fe3O4@SiO2–SnCl4 was done by Fe3O4@SiO2 with SnCl4 using ultrasonication for 30 min (Fig. 12). The powder XRD pattern of Silica-coated Fe3O4nanoparticles displayed a broad peak due to non-crystalline nature. This method is hygienic, safe, cost effective and selective and hence very suitable for practical organic synthesis. The energy dispersive EDX examination of Fe3O4@SiO2–SnCl4 conformed of the estimated the presence of the elemental for Fe, O, Si, Sn and Cl individually.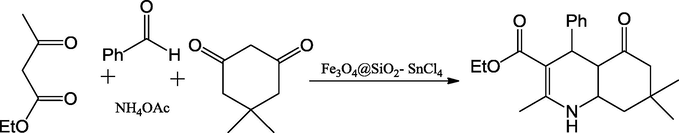
Synthesis of 1,4-dihydropyridine derivatives under Fe3O4@SiO2–SnCl4.
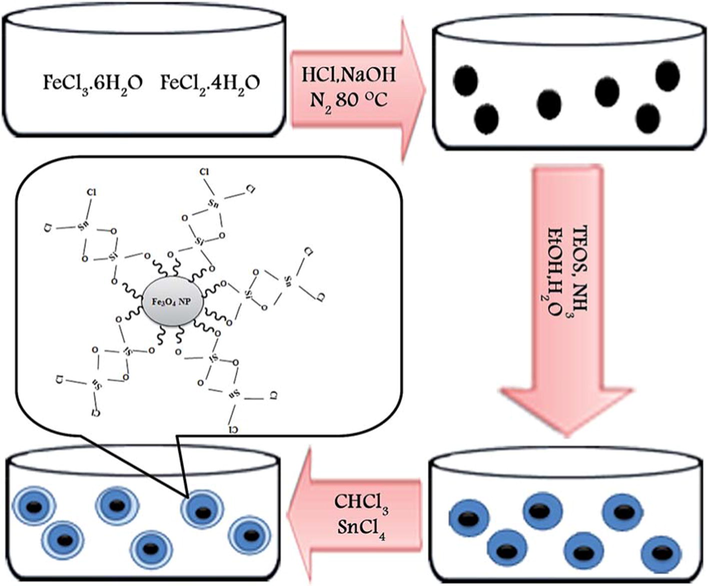
A schematic diagram for the synthesis and suggested structure of Fe3O4@SiO2–SnCl4.
Source: Reprinted from Bamoniri1 et al. (2015) with permission from Royal Society of Chemistry.
Demirci et al. (2016) reported a novel, efficient PdRuNi@GO catalyst for the synthesis of wide-range of substituted 1,4-dihydropyridine via multicomponent condensation of dimedone, aldehydes, ethyl acetoacetate and NH4OAc refluxed at 70 °C in DMF as reaction medium in a short time period with high yields (Scheme 49). The PdRuNi@GO nanocatalysts were synthesized using the sono-chemical binary solvent reduction approach. The morphology and particle size of PdRuNi@GO material were evaluated by TEM micrograph. The results show that these mixed oxide catalysts contain of spherical particles with the crystallite size between 5 and 10 nm, approving the results calculated from Scherrer’s formula based on the XRD pattern. Additionally, the high resolution image for monodisperse PdRuNi@GO was also used to analyze the atomic lattice fringes. This one pot novel catalytic procedure for Hantzsch synthesis is simple and efficient, as well as remarkably reusable.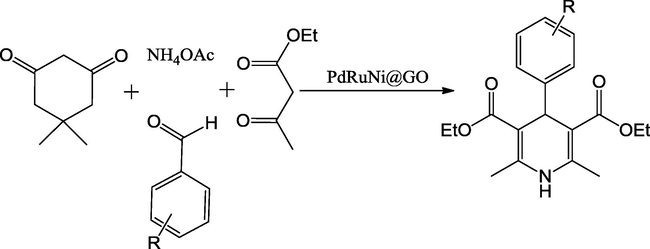
Synthesis of 1,4-dihydropyridine derivatives.
Azarifar et al., reported the usage of catalyst guanidinium chloride-functionalized γ-Fe2O3/HAp magnetic nanoparticles for the synthesis of fully substituted pyranopyridine derivatives. The most arguable magnetic nanoparticles as the core magnetic support, Fe2O3 is the most extensively premeditated, because of their high surface-area resulting in low catalyst loading capacity, conductivity, magnetic susceptibility, catalytic activity and striking stability (Fig. 13). In addition, guanidinium chloride is used to form the core-shell magnetic nanostructure, which is an important class of chaotropic agent. In this work, the γ-Fe2O3@HAP-GndCl mixed oxide nanocatalyst, as recyclable catalyst was very effective in the solvent-free condensation reaction of aromatic aldehydes, malononitrile and 3-cyano-6-hydroxy-4-methylpyridin-2(1H)-one accomplished with good yields of the corresponding pyranopyridine derivatives at 80 °C in short reaction times (10–40 min) (Azarifar et al., 2016). This new technique has remarkable gains such as excellent yields and simple work-up. Furthermore, thermal chemical stability, easy separation of the catalyst makes it a good heterogeneous system and a useful alternative to other heterogeneous catalysts.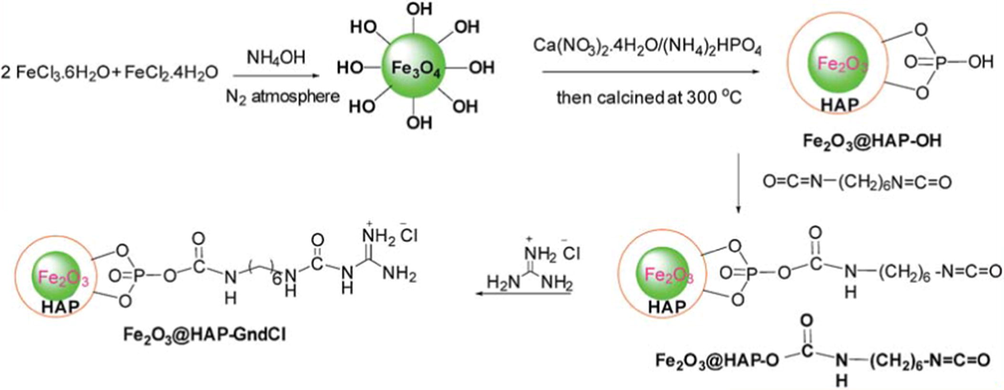
Steps for the synthesis of the catalyst g-Fe2O3@HAp-GndCl MNPs.
Source: Reprinted from Azarifar et al. (2016) with permission from Royal Society of Chemistry.
An effective protocol for the synthesis of synthesis of functionalized 1,4-dihydropyridine derivatives using a four-component, one-pot condensation reaction of substituted aldehydes, malononitrile, dimethylacetylenedicarboxylate and 4-fluoroaniline in the presence of Sm2O3/ZrO2 under ethanol solvent conditions is pronounced (Scheme 50) (Shabalala et al., 2016). The reaction using a catalyst was employed under mild conditions with high yields up to 96%. The methodology has number of advantages such as environmentally benign, shorter reaction times, higher yields, no chromatographic purification, milder reaction conditions, and catalyst reusability up to seven cycles.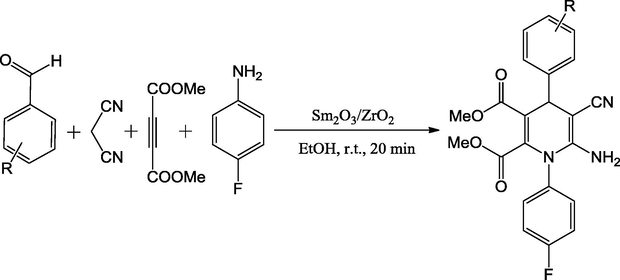
Synthesis of functionalized 1,4-dihydropyridine derivatives.
A simple and convenient approach has been achieved to prepare an AAPTMS composite on m-zirconia as support (Pagadala et al., 2015a). The surface of the mesoporous zirconia was improved by reaction with the diamine functionalized [N-(2 amino ethyl)-3-amino propyl trimethoxy silane (AAPTMS). The FTIR spectra of prepared catalyst contained representative peaks at 1386, 1621 and 3410 cm−1, most possibly attributable to symmetric and asymmetric stretching vibrations of —(O—H—O)—, —(H—O—H)— and —OH bonds. The peak at 730 cm−1 was ascribed to the presence of O—Zr bond. The information supports the successful grafting of organic amine group onto the surface of m-ZrO2. The modified catalyst was used as an effective and reusable catalyst for one-pot, four-component condensation of aldehydes, malononitrile, thiazolidine-2,4-dione and NH4OAc to obtain functionalized pyridine derivatives under water as solvent conditions at 70 °C with high yields (Scheme 51).
Four-component synthesis of heterocycle-fused pyridines.
A new, efficient and green Cu(II)/L-His@Fe3O4 catalyst has been established by Norouzi et al. (2016). The XRD pattern with quite narrow lines and strong intensities identifies good crystallinity of the calcined powder. The morphology and spherical particle size of Cu(II)/LHis@Fe3O4 powder prepared by molten-salt synthesis technique were examined by scanning electron microscopy (SEM). The SEM image showed that prepared catalyst were observed as spherical particles with an average diameter of 8–20 nm. The SEM-electron dispersive spectroscopy spectra of the Cu(II)/L-His@Fe3O4 was taken at random points on the surface. SEM-EDX spectrum Cu(II)/LHis@Fe3O4 presented the elemental compositions are (Si, C, O, N, Fe, and Cu) of core–shell nanoparticles (Fig. 14). The protocol for the synthesis of 2-amino-6-(arylthio)pyridine-3,5-dicarbonitrile derivatives using a four-component and one-pot condensation reaction of aromatic aldehydes, ethyl acetoacetate, dimedone and NH4OA in the presence of the above catalyst under ethanol solvent conditions is described. The reaction using a catalyst was carried out under mild condition with high yields up to 98% (Scheme 52). The reported method offers several advantages such as short reaction times, excellent yields, environmentally benign, easy recovery of catalyst with external magnet.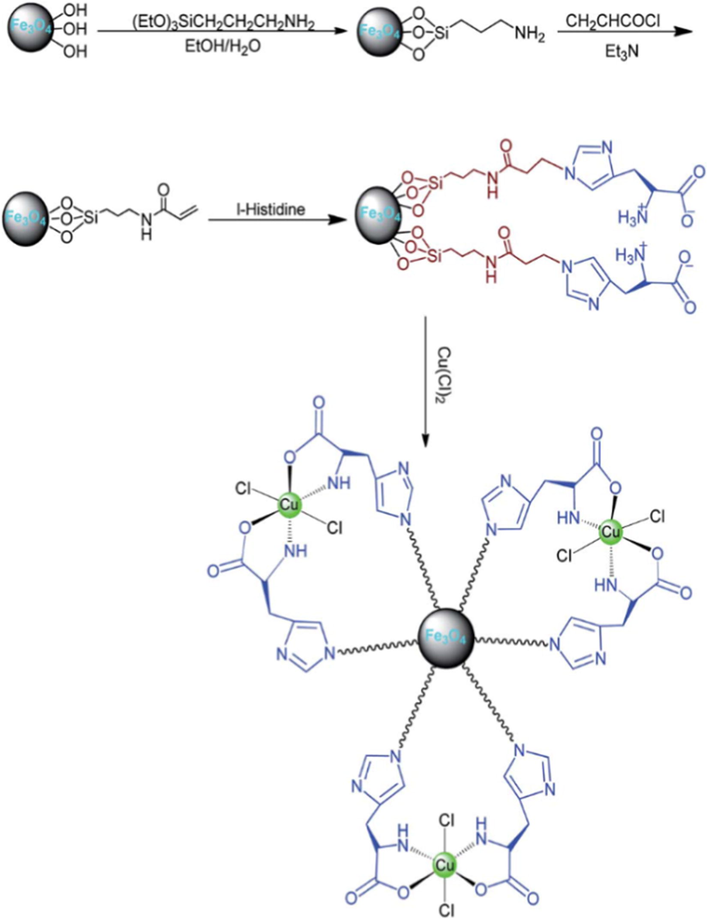
Synthesis of Cu(II)/L-His@Fe3O4.
Source: Reprinted from Norouzi et al. (2016) with permission from Royal Society of Chemistry.
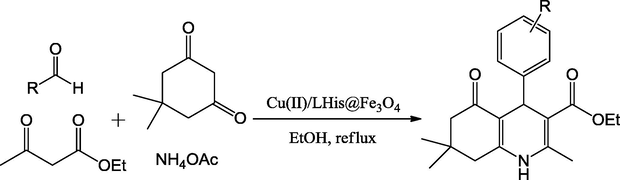
Synthesis of polyhydroquinoline derivatives.
Kantevari et al. (2007) have synthesized novel potassium dodecatangestocobaltate trihydrate (K5CoW12O40·3H2O) mixed oxide electrophilic catalyst. These Keggin-type heteropoly acid catalysts are a class of highly acidic solid acid catalysts having cobalt metal heteropolyanions synchronized by octahedral oxygen as the simple structural unit. It has been significant importance in the use of heteropolyacids as environmentally benign catalysts due to their unique properties such as high chemical thermal stability, cost-effective, ease of preparation and ease of recyclability. An efficient method for the one-pot, multicomponent reaction of aldehydes, β-dicarbonyl compounds and NH4OAc were carried out under solvent-free conditions to afford target active 2,3,6-trisubstituted pyridine derivatives (Scheme 53). The existing technique delivers a number of advantages like a simple workup, short reaction times, excellent yields (85–95%), easy catalyst separation, and slight catalyst loading.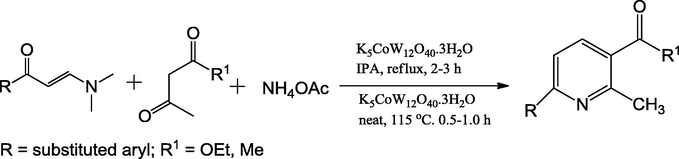
Synthesis of 2,3,6-trisubstituted pyridines using K5CoW12O40·3H2O catalyst.
Zolfigol et al. (2016) has reported {Fe3O4@SiO2@(CH2)3Im}C(CN)3 mixed oxide catalyst for the four-component synthesis of 2-amino-3-cyanopyridine derivatives through the reaction of aromatic aldehyde, malononitrile, acetophenone and NH4OAc. The reaction was completed solvent-free and refluxed at 100 °C with in short reaction times (30 min) (Scheme 54). The investigations showed that the reaction of a variety of aldehydes with acetophenone resulted in the formation of the corresponding products in moderate to good yields (80–91%). The prepared mixed oxide catalyst can be detached by using external magnet (Fig. 15).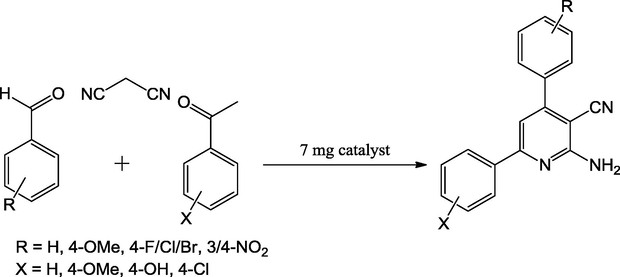
Catalytic applicability of the {Fe3O4@SiO2@(CH2)3Im} C(CN)3 in one-pot synthesis of 2-amino-3-cyanopyridines.
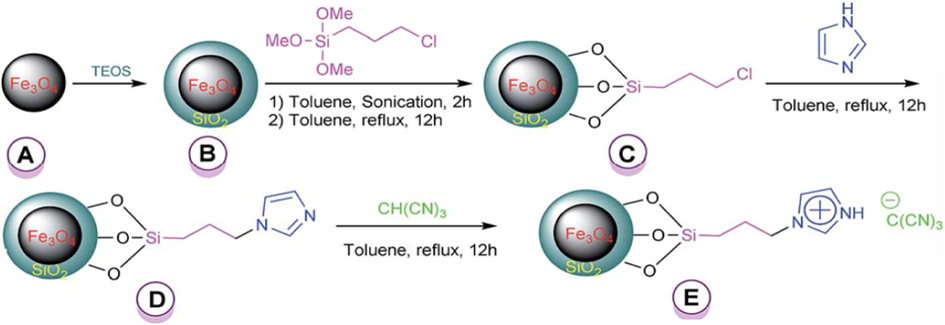
Preparation of silica-coated magnetic nanoparticles with a tag of ionic liquid catalyst.)
Source: Reprinted from Zolfigol et al. (2016 with permission from Royal Society of Chemistry.
3.2 Two nitrogen containing heterocycles (Pyrimidines)
Pyrimidine is a six membered heterocyclic aromatic compound with molecular formula C4H4N2 and it consists of two nitrogen atoms at positions 1 and 3. Many substituted pyrimidines are explored as important starting materials for medicinal, industrial and agricultural chemicals (De Coan et al., 2016; Ismail et al., 2016; Mohana et al., 2013).
Naeimi and co-workers have reported a new route for the synthesis of pyrido‑dipyrimidines through a one-pot, multicomponent reaction (Naeimi et al., 2016). The reaction was premeditated using 2-thiobarbituric acid with aromatic aldehydes and NH4OAc in the presence of CuFe2O4 mixed oxide nanoparticles as a Lewis acidic catalyst in water as a green solvent under microwave-assisted conditions, which resulting good to excellent yields (Scheme 55). The CuFe2O4 catalyst was prepared by co-precipitation method and possess an ample of magnetic property. This microwave orientated heterocyclic synthesis with recyclable the catalyst is ideal method for an enhance yields and reduced reaction times.![Reaction leading to the synthesis of novel pyrido[2,3-d]pyrimidine derivatives.](/content/184/2020/13/1/img/10.1016_j.arabjc.2017.09.016-fig71.png)
Reaction leading to the synthesis of novel pyrido[2,3-d]pyrimidine derivatives.
Novel mixed oxide magnetic nano catalysts were described by Shaterian and Aghakhanizadeh. In their work, they used 3-aminopropyltriethoxysilane (APTES) material as a support (Fig. 16), synthesized APTES supported on SBA-15 and APTES supported on magnetic Fe3O4 nano catalysts and were applied in the condensation of malononitrile, salicylaldehyde and secondary amines under solvent-free room temperature condition to afford chromeno[2,3-d]pyrimidines with good to excellent yields (87–93%) (Scheme 56). The catalyst is easily recoverable and reusable (Shaterian and Aghakhanizadeh, 2013).![The preparation of 3-aminopropyltriethoxysilane coated on magnetic Fe3O4 nanoparticles [APTES-MNPs].](/content/184/2020/13/1/img/10.1016_j.arabjc.2017.09.016-fig72.png)
The preparation of 3-aminopropyltriethoxysilane coated on magnetic Fe3O4 nanoparticles [APTES-MNPs].
Source: Reprinted from Shaterian and Kangani (2014) with permission from Royal Society of Chemistry.
![The preparation of chromeno-[2,3-d]pyrimidine derivatives.](/content/184/2020/13/1/img/10.1016_j.arabjc.2017.09.016-fig73.png)
The preparation of chromeno-[2,3-d]pyrimidine derivatives.
Ghomi et al. (2013) have proposed nanosilica-supported tin(II) chloride as mixed oxide catalyst for the green synthesis of 3,4-dihydropyrimidine-2(1H)-one/thiones from the reaction of urea/thiourea, substituted aldehydes, and ethyl acetoacetate. The catalyst was prepared by the addition of anhydrous SnCl2 to a suspension of nano particles of silica gel in dichloromethane solvent. The powder X-ray diffraction for SnCl2/nano SnO2 that designates the presence of highly crystalline nature and sharp intense peaks. Catalyst Lewis acidic sites were confirmed by temperature programmed desorption. The optimal conditions such as reflux at 78 °C, ethanol as a solvent and 0.45 mol% of catalyst produced the impressive yields of target molecules in the range of 88–97% (Scheme 57). The X-ray diffraction and SEM analysis disclosed that the structural integrity was not disturbed even after the fifth run of the catalyst, which induces the viability of catalyst as eco-friendly synthesis of heterocyclic molecules.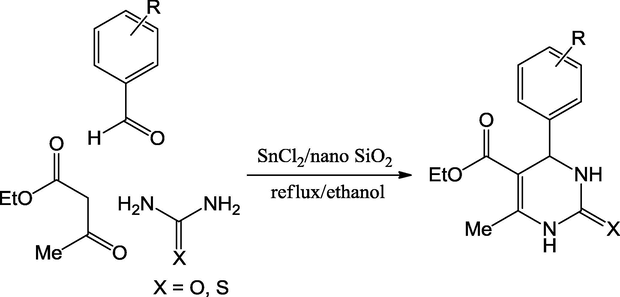
Synthesis of 3,4-dihydropyrimidine-2(1H)-one/thione derivatives.
Many heteropolyacids have been used in organic transformations and petrochemical industry as reusable catalysts (Palermo et al., 2015). Recently, Valeria and coworkers have reported the immobilization of H3PMo12O40 on the surface of silica-encapsulated vanadia mixed oxide particles. These catalysts are mostly sphere-shaped and have a typical size of nearly 50 nm. Fascinatingly, these catalysts have more active surface sites and uniform analogs. This contributed to their catalytic efficacy in one-pot multicomponent acid-condensation reaction of benzaldehyde, ethyltrifluoroacetoacetate and urea in solvent-free condition to form substituted hexahydropyrimidine derivatives with good yields in short reaction times (Scheme 58).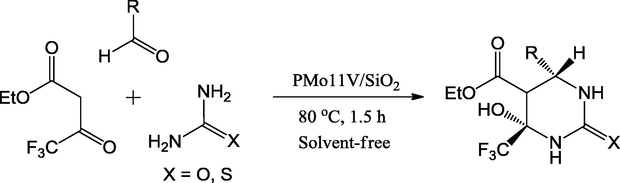
Synthesis of fluorinated hexahydropyrimidine.
Al-HMS-20 mixed oxide catalyst was utilized as a solid, reusable acid for catalyzing the multicomponent cyclo-condensation reaction of malononitrile with pyrimidine compounds and substituted aromatic aldehydes to prepare a serious pyrano[2,3-d]pyrimidine and pyrido[2,3-d]pyrimidine derivatives (Scheme 59) (Sabour et al., 2015). The target molecules were obtained at room temperature in good to excellent yields (87–95%) and also catalyst was recycled up to 6 runs without loss of its activity.![Synthesis of pyrano-[2,3-d]pyrimidines.](/content/184/2020/13/1/img/10.1016_j.arabjc.2017.09.016-fig76.png)
Synthesis of pyrano-[2,3-d]pyrimidines.
Recently, clay supported nanocomplexes with ample structures have found varied applications in the fields of medicine, food progression, agriculture, wastewater treatment and textile industries. Owing to their considerably high catalytic activity, the insertion of metal as ferrate into the clay complex increases the capacity of clay supported catalysts of interaction by complexation to form efficient catalytic reagents. Maleki and Aghaei reported a simple, efficient, environmentally benign, ultrasound assisted, one-pot multicomponent Michael-type addition reaction for the synthesis of polycyclic imidazo(thiazolo)pyrimidines by the reaction of dimedone, 2-aminobenzothiazole and aromatic aldehydes using catalytic amount of Fe3O4@clay as reusable catalyst in aqueous medium at room temperature in excellent yields (93–98%) (Scheme 60) (Maleki and Aghaei, 2016).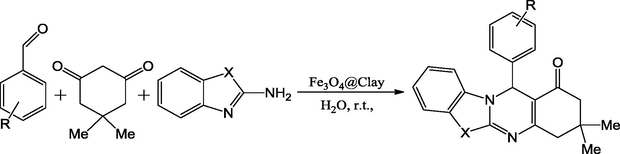
Ultrasonic-assisted synthesis of imidazo-(thiazolo)pyrimidines by using Fe3O4@clay nanocatalyst.
Firouzeh and Raheleh reported a silica-coated sulfonic acid-functionalized magnet renewal mixed oxide catalyst (Fe3O4@SiO2–SO3H) (Nemati and Saeedirad, 2013). It was used in the preparation of different functionalized pyrimido-[4,5-b]quinoline and indeno fused pyrido[2,3-d]pyrimidine derivatives through Knoevenagel condensation and Michael addition in green solvent. The investigations displayed that the reaction of a diversity of dimedone with arylaldehydes and 6-amino-1,3-dimethyl uracil resulted in the formation of the corresponding target molecules in good yields (Scheme 61). Moreover, Fe3O4@SiO2–SO3H could be readily recovered by using an external magnet and reused several runs without any loss of catalytic activity.![Synthesis of pyrimido-[4,5-b]quinolines and indeno fused pyrido[2,3-d]-pyrimidines.](/content/184/2020/13/1/img/10.1016_j.arabjc.2017.09.016-fig78.png)
Synthesis of pyrimido-[4,5-b]quinolines and indeno fused pyrido[2,3-d]-pyrimidines.
Mohsenimehr et al. (2015) have prepared pyrido-[2,3-d:6,5-dl]-dipyrimidine derivatives through one-pot reaction of aromatic aldehydes with butylthiopyrimidin-4(3H)-one, under DMF as solvent conditions at room temperature by using mixed oxide Brønsted acidic heterogeneous catalyst as γ-Fe2O3@HAp-SO3H with good to excellent yields in short reaction times (Scheme 62). The magnetic nanoparticles (γ-Fe2O3) were synthesized by a chemical co-precipitation method using ferric ions. Then, hydroxyapatite coated on the surface of the ironoxide particles, functionalization with a sulfonic acid group was accomplished by treatment with ClSO3H loading to afford efficient magnetic γ-Fe2O3@HAp-SO3H nanocatalyst. The SEM analysis of the catalyst noticeably revealed the size (30–45 nm) of the nano particles. The catalyst has consistent chemical thermal stability and flexibility in surface modification. Moreover, catalyst could be easily recovered by using a simple external magnet and reused several times without any significant loss of catalytic activity.![Synthesis of pyrimido-[4,5−b]quinolines derivatives.](/content/184/2020/13/1/img/10.1016_j.arabjc.2017.09.016-fig79.png)
Synthesis of pyrimido-[4,5−b]quinolines derivatives.
Recently, Dam et al. (2016) have developed a magnetic separable ferrate loaded silica sulfuric acid (Fe3O4@SiO2-HSO3) as mixed oxide catalyst and used for one-pot synthesis of fused dihydropyrimidines in solvent-free condition (Scheme 63). SiO2 is the best communal solid for coating the surfaces of ferrate materials. Such a layering is well-known for its facility to inhibit magnetic aggregation in solution and its ability to increase chemical thermal stability. Further, the surface of silica-coated Fe3O4 can react with HSO3 coupling agent for covalent attachment of functional groups to form Fe3O4@SiO2-HSO3 (Fig. 17). The ferrate particles offer a huge surface area to anchor the acidic sites. The synthesis was done by Knoevenagel- Michael condensing aldehydes, β-dicarbonyl compounds and 2-aminobenzimidazole in solvent-free condition at 60 °C. The main advantage of these catalyst is that they could be magnetically separated from the reaction mixture with minimal effort and be recycled in subsequent reactions.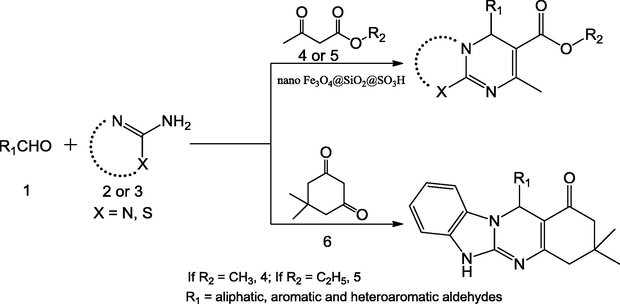
Synthesis of fused pyrimidine derivatives.
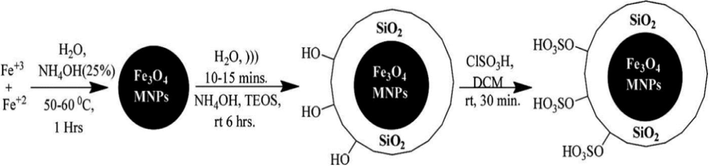
Schematic diagram for synthesis of nano-Fe3O4 encapsulated silica particles bearing sulfonic groups.)
Source: Reprinted from Dam et al. (2016 with permission from Royal Society of Chemistry.
Ramalingam (2009) have reported ideal reaction using an efficient, one-pot ultrasound irradiation procedure for the synthesis of 3,4-Dihydropyrimidin-2-(1H)-ones under Yttria-Zirconia loaded Lewis acidic catalyst (Scheme 64). The acidic nature of the catalyst has drawn persistent consideration in the building of C—C and C—N bonds in a range of organic transformations. The catalyst was prepared by mixing the appropriate amount of yttrium nitrate with zirconium nitrate in a solution of NH3 (pH 8.5). The white powder formed after evaporation of ammonia was modified with H2SO4 to obtain yttria-zirconia based Lewis acid catalyst. It efficiently catalyzed the MCR between the aldehyde, keto ester and urea or thiourea in the presence of aqueous CH3CN at reflux condition. The catalyst can be simply removed by filtration after the reaction and reused many times without significant loss of its activity.
Synthesis of dihydropyrimidones (DHPM) by using yttria-zirconia–based Lewis acid.
3.3 Synthsis of various heterocyclic compounds by mixed-oxide catalysts
The following table illustrates our research group’s contributions in the syntheses of wide range of heterocyclic compounds and valued added organic transformations, using multicomponent/one-pot protocols and employing varied mixed oxides as heterogeneous catalysts in impressive yields (see Table 1).
Mixed oxide
Products
Yield
Reactants
Reaction conditions
Fe-CaOx (Gangu et al., 2017b)
Synthesis of 8 substituted pyranopyrazoles
91–98%
Substituted Aromatic aldehyde, malononitrile, hydrazine hydrate and Dimethyl acetylenedicarboxylic acid
Heating at 50 °C
Sm2O3/ZrO2 (Sebenzile et al., 2016)
Synthesis of 11 functionalized 1,4-dihydropyridine derivatives
87–96%
Substituted aldehydes, malononitrile, dimethylacetylenedicarboxylate and 4-fluoroaniline
Ethanol as solvent at Room temperature
CuO/ZrO2 (Maddila et al., 2015c)
Synthesis of 9 different pyrazole-4-carbonitrile
88–92%
Substituted aldehyde phenylhydrazine and malononitrile
Water as a solvent at room temperature
Mn-ZrO2 (Maddila et al., 2015d)
Synthesis of 11 different pyrano[2,3-d]-pyrimidines
84–90%
1,3-dimethylbarbituric acid, substituted aldehyde and malononitrile
EtOH: H2O (1:1) at Room temperature
Diamine functionalized mesoporous ZrO2 (Pagadala et al., 2015b)
Synthesis of 8 different pyridines derivatives
84–95%
Substituted benzaldehyde, malononitrile, thiazolidine-2,4-dione and NH4OAc
Water as a solvent at Room temperature
Decorated MWCNT Sm-fluorapatites (Gangu et al., 2016d)
Synthesis of 5 different 1,2,4-triazole
90–96%
Substituted aromatic aldehyde and thiosemicarbazide
Ethanol as a solvent at Room temperature
Sm2O3/Fluoroapatite (Maddila et al., 2017a)
Synthesis of 14 different triazolidine-3-thione derivatives
92–97%
Aromatic aldehyde and thiosemicarbazide
Water as a solvent at Room temperature
Iron doped fluorapatite (Gangu et al., 2017c)
Synthesis of 6 different 1,2,4-triazolidine-3-thione derivatives
91–96%
Aromatic aldehyde and thiosemicarbazide
Ethanol as a solvent at Room temperature
RuCaHAp (Maddila et al., 2016f)
Synthesis of 11 different pyrano[2,3-c]pyrazole-3-carboxylates
88–97%
Dimethylacetylenedicarboxylate, hydrazine hydrate, malononitrile and aromatic aldehydes
Aqueous ethanol as a solvent at Room temperature
Chitosan/CaHAp (Maddila et al., 2017b)
Synthesis of 9 different 2,6-diamino-pyran-3,5-dicarbonitriles
86–96%
Malononitrile, substituted aldehydes, and cyanoacetamide
Ethanol as a solvent at Room temperature
Sm-fluorapatites (Gangu et al., 2016c)
Synthesis of 5 different 1,2,4-triazole moiety
92–98%
Different benzaldehydes and thiosemicarbazide
Ethanol as a solvent at Room temperature
Ru-hydroxyapatite (Maddila et al., 2016g)
Synthesis of 11 different pyranopyrazoles
89–98%
Aromatic aldehydes, malononitrile, hydrazine hydrate and ethyl acetoacetate
Ethanol-water mixture as a solvent at Room temperature
Ce-V/SiO2 (Maddila et al., 2016h)
Synthesis of 9 diverse 2-amino-3-cyano-4H-pyrans
87–95%
5,5-dimethylcyclohexane-1,3-dione, aromatic aldehyde, and malononitrile
Ethanol as solvent at Room temperature
Ag/SiO2 (Maddila et al., 2016i)
Synthesis of 13 diverse 3-methyl-4-(phenyl)-methylene-isoxazole-5(4H)-ones
88–93%
Substituted aromatic aldehydes, Hydroxylamine and ethylacetoacetate
Water as a solvent at Room temperature
Ag-SiO2 (Maddila et al., 2016j)
Synthesis of 10 different chloro-8-substituted-9H-purines
89–94%
Pyrimidine-4,5-diamine and substituted aromatic acid
Ethanol as a solvent at Room temperature
Mg-V/CO3 Hydrotalcite (Maddila et al., 2015e
Synthesis of 11 different multisubstituted pyridines
82–94%
Different aldehydes, acetanilide and malononitrile
Ethanol as a solvent at Room temperature
Ce-V/alumina (Maddila et al., 2016k)
synthesis of 11 different substituted pyridines
86–94%
Substituted Benzaldehyde, malononitrile and ethanol
Water as a solvent at Room temperature
4 Conclusions
The review searches the scope for the synthesis of a wide variety of nitrogen containing heterocycles and illustrates the significance of varied mixed oxides as catalyst carriers and catalysts. The mixed oxides employed as reusable catalysts represent an interesting class of materials for sustainable development of chemical industry, due to their intriguing and designable surface properties. The impressive acid-base characteristics of active sites, ability of metal cations to undergo redox reactions etc. positively contribute to the acceleration and selectivity of the chosen reactions. Ample of synthetic procedures like sol-gel, wet impregnation and hydrothermal, etc. practiced, and choice of characterizing tools such as XRD, FT-IR, microscopic analysis, TG analysis, acidic and basic measurements and XPS have enormously enhanced the scope of mixed oxides as potential catalyst materials with benefits to academia and industry. The mixed oxides play a vital role in organic reactions, green chemistry, petroleum industry and fine chemical synthesis and occupy over 30% share among industrially employed heterogeneous catalysts. The most commonly used oxides like SiO2, CeO2, ZrO2, TiO2, Al2O3 hydroxyapatites etc as catalyst supports and the deposition of active constituents’ viz. Fe, Co, V, Mn, Cu, Ni on their surfaces and their interchanging roles contribute the active sites for the selectivity of catalyzed reactions. The heterocycles with nitrogen in their ring have profound applications in many sectors including medicinal and agricultural fields, which necessitate their efficacious synthetic routes. Universally, diverse metal oxides have been explored and exploited to facilitate the C-C bond formation in the substrate molecules and to achieve the synthesis of many novel pyrrole, pyrazole and triazole derivatives through multicomponent reactions. The one-pot green approach using varied mixed oxides as catalysts provides a broad platform for design and development of innovative novel heterocyclic compounds with promising features and characteristics. Much to be understood in interpreting the on-going reaction mechanisms on the catalyst surfaces and huge latitude exists for modification and improvement of the potential of mixed oxides as catalysts and catalyst supports.
Acknowledgements
The authors sincerely acknowledge the receipt of financial assistance and research facilities from the National Research Foundation, Pretoria, South Africa, and the University of KwaZulu-Natal, Durban, South Africa respectively.
References
- Green protection of pyrazole, thermal isomerization and deprotection of tetrahydropyranylpyrazoles, and high-yield, one-pot synthesis of 3(5)-alkylpyrazoles. RSC Adv.. 2015;5:24081-24093.
- [Google Scholar]
- Recent advances in the synthesis and anticancer activity of molecules other than nitrogen containing heterocyclic moeities. Mini Rev. Med. Chem. 2016 (Epub ahead of print)
- [Google Scholar]
- Ce/SiO2 composite as an efficient catalyst for the multicomponent one-pot synthesis of substituted pyrazolones in aqueous media and their antimicrobial activities. J. Mol. Catal. A: Chem.. 2016;411:325-336.
- [Google Scholar]
- Cu-doped ZnO nanocrystalline powder catalyzed one-pot synthesis of fully substituted new indeno [1,2-β]pyridines at room temperature by a multi-component reaction. Chin. J. Catal.. 2014;35:560-564.
- [Google Scholar]
- Nanomolar potency and metabolically stable inhibitors of kidney urea transporter UT-B. J. Med. Chem.. 2012;55:5942-5950.
- [Google Scholar]
- Heterogenized tungsten complex: an efficient and high yielding catalyst for the synthesis of structurally diverse tetra substituted pyrrole derivatives via four-component assembly. Tetrahedron Lett.. 2013;54:5624-5628.
- [Google Scholar]
- Bridging homogeneous and heterogeneous catalysis with CAN. SiO2 as a solid catalyst for four-component reactions for the synthesis of tetra substituted pyrroles. New J. Chem.. 2015;39:396-402.
- [Google Scholar]
- Synthesis and biological evaluation of new pyranopyridine derivatives catalyzed by guanidinium chloride-functionalized γ-Fe2O3/Hap magnetic nanoparticles. RSC Adv.. 2016;6:92028-92039.
- [Google Scholar]
- Kobaisi, D. Azarifar. Magnetic La0.7Sr0.3MnO3 nanoparticles: Recyclable and efficient catalyst for ultrasound-accelarated synthesis of 4H-chromenes, and 4H-pyrano[2,3-c]pyrazoles. J. Iran. Chem. Soc.. 2013;10:439-446.
- [Google Scholar]
- Nano-Fe3O4@SiO2 supported ionic liquid as an efficient catalyst for the synthesis of 1,3-thiazolidin-4-ones under solvent-free conditions. J. Mol. Catal. A Chem.. 2015;398:58-64.
- [Google Scholar]
- SnCl4-functionalized nano-Fe3O4 encapsulated-silica particles as a novel heterogeneous solid acid for the synthesis of 1,4-dihydropyridine derivatives. RSC Adv.. 2015;5:78483-78490.
- [Google Scholar]
- Nano-TiCl4/SiO2: an efficient catalyst for the one-pot synthesis of highly substituted dihydro-2-oxopyrroles. Monatsh Chem.. 2015;146:2107-2115.
- [Google Scholar]
- Applications of CeCl3 as an environmental friendly promoter in organic chemistry. Chem. Rev.. 2010;110:6104-6143.
- [Google Scholar]
- Cu-Mn spinel oxide catalyzed synthesis of imidazo[1,2-a]pyridines through domino three-component coupling and 5-exo-dig cyclization in water. RSC Adv.. 2013;3:20869-20876.
- [Google Scholar]
- Pyrrole: A resourceful small molecule in key medicinal hetero-aromatics. RSC Adv.. 2015;5:15233-15266.
- [Google Scholar]
- Synthesis and antitumor evaluation of some new 1,3,4-oxadiazole-based heterocycles. Eur. J. Med. Chem.. 2012;48:192-199.
- [Google Scholar]
- Higher-order multicomponent reactions: beyond four reactants. Chem. Soc. Rev.. 2013;42:4948-4962.
- [Google Scholar]
- New 1,4-anthracenedione derivatives with fused heterocyclic rings: Synthesis and biological evaluation. RSC Adv.. 2015;5:1244-1261.
- [Google Scholar]
- Chemical aspects of solution routes to perovskite-phase mixed-metal oxides from metal-organic precursors. Chem. Rev.. 1993;93:1205-1241.
- [Google Scholar]
- Synthesis and evaluation of amide, sulfonamide and urea – Benzisoxazole derivatives as potential atypical antipsychotics. Med. Chem. Commun.. 2015;6:831-1838.
- [Google Scholar]
- Ozone initiated Ni/metal oxide catalyzed conversion of 1,2-dichlorobenzene to mucochloric acid in aqueous solutions. ACS-Indu. Eng. Chem. Res.. 2012;51:2864-2873.
- [Google Scholar]
- Catalytic bismetallative multicomponent coupling reactions: scope, applications, and mechanisms. Chem. Soc. Rev.. 2014;43:4368-4380.
- [Google Scholar]
- Green Chem.. 2014;16:2958-2975.
- Homogeneous and heterogeneous catalysts for multicomponent reactions. RSC Adv.. 2012;2:16-58.
- [Google Scholar]
- Surface interactions of glycerol with acidic and basic metal oxides. J. Phys. Chem. C. 2013;117:21413-21425.
- [Google Scholar]
- Supported gold nanoparticles as catalysts for organic reactions. Chem. Soc. Rev.. 2008;37:2096-2126.
- [Google Scholar]
- Catalytic combustion of chlorobenzene over Ru-doped ceria catalysts: Mechanism study. Appl. Catal. B: Environ.. 2013;129:580-588.
- [Google Scholar]
- B. Dam, S. Nandi, A.K. Pal. An efficient ‘on-water’ synthesis of 1,4-dihydropyridines using Fe3O4@SiO2 nanoparticles as a reusable catalyst. Tetrahedron Lett.. 2014;55(2014):5236-5240.
- [Google Scholar]
- Nano-Fe3O4@silica sulfuric acid as a reusable and magnetically separable potent solid acid catalyst in Biginelli-type reaction for the one-pot multicomponent synthesis of fused dihydropyrimidine derivatives: A greener NOSE and SFRC approach. Syn. Commun.. 2016;46:275-286.
- [Google Scholar]
- Environmentally benign synthesis of heterocyclic compounds by combined microwave-assisted heterogeneous catalytic approaches. Green Chem.. 2012;14:17-37.
- [Google Scholar]
- Design and synthesis of pyrazole-3-one derivatives as hypoglycaemic agents. Int. J. Med. Chem. 2015:670-679.
- [Google Scholar]
- Synthetic entries to and biological activity of pyrrolopyrimidines. Chem. Rev.. 2016;116(1):80-139.
- [Google Scholar]
- One-pot synthesis of Hantzsch dihydropyridines using a highly efficient and stable PdRuNi@GO catalyst. RSC Adv.. 2016;6:76948-76956.
- [Google Scholar]
- Highly regioselective synthesis of polysubstituted pyrroles through three-component reaction induced by low-valent titanium reagent. J. Comb. Chem.. 2008;10:810-813.
- [Google Scholar]
- Arylglyoxals in synthesis of heterocyclic compounds. Chem. Rev.. 2013;113:2958-3043.
- [Google Scholar]
- Arylglyoxals in synthesis of heterocyclic compounds. Chem. Rev.. 2013;113(5):2958-3043.
- [Google Scholar]
- CuFe2O4 nanoparticles: An efficient heterogeneous magnetically separable catalyst for synthesis of some novel propynyl-1Himidazoles derivatives. Tetrahedron. 2015;71:2579-2584.
- [Google Scholar]
- Synthesis of heterocycles and fused heterocycles catalyzed by nanomaterials. RSC Adv.. 2015;5:75659-75710.
- [Google Scholar]
- Discovery of a selective and CNS penetrant negative allosteric modulator of metabotropic glutamate receptor subtype 3 with antidepressant and anxiolytic activity in rodents. J. Med. Chem.. 2015;58:7485-7500.
- [Google Scholar]
- Synthesis of some new amide-linked bipyrazoles and their evaluation as anti-inflammatory and analgesic agents. J. Enzyme. Inhib. Med. Chem.. 2015;20:1-16.
- [Google Scholar]
- Nanostructured oxides in chemistry: characterization and properties. Chem. Rev.. 2004;104:4063-4104.
- [Google Scholar]
- Strategies for innovation in multicomponent reaction design. Acc. Chem. Res.. 2009;42(3):463-472.
- [Google Scholar]
- Decorated multi-walled carbon nanotubes with Sm doped fluorapatites: Synthesis, characterization and catalytic activity. RSC Adv.. 2016;6:58226-58235.
- [Google Scholar]
- Nanostructured Samarium doped fluorapatites and their catalytic activity towards synthesis of 1,2,4-triazole moiety. Molecules.. 2016;21:1281-1296.
- [Google Scholar]
- Decorated multi-walled carbon nanotubes with Sm doped fluorapatites: Synthesis, characterization and catalytic activity. RSC Adv.. 2016;6:58226-58235.
- [Google Scholar]
- A review on contemporary metal-organic framework materials. Inorg. Chim. Acta.. 2016;446:61-74.
- [Google Scholar]
- A study on behavioral influence of glutamic acid and histidine on morphology and fluorescence activity of Cadmium-doped fluorapatite. J. Alloys Comp.. 2017;690:817-824.
- [Google Scholar]
- Novel iron doped calcium oxalates as promising heterogeneous catalysts for one-pot multi-component synthesis of pyranopyrazoles. RSC Adv.. 2017;7:423-432.
- [Google Scholar]
- Efficient synthetic route for thio-triazole derivatives catalyzed by iron doped fluorapatite. Res. Chem. Intermed.. 2017;43:1793-1811.
- [Google Scholar]
- Benign by design: catalyst-free in-water, on-water green chemical methodologies in organic synthesis. Chem. Soc. Rev.. 2013;42:5522-5551.
- [Google Scholar]
- Synthesis and characterization of versatile MgO-ZrO2 mixed metal oxide nanoparticles and their applications. Catal. Sci. Technol.. 2011;1:1653-1664.
- [Google Scholar]
- Role of mixed metal oxides in catalysis science–versatile applications in organic synthesis. Catal. Sci. Technol.. 2012;2:1113-1125.
- [Google Scholar]
- Integrated Nanocatalysts: A unique class of heterogeneous catalysts. J. Mater. Chem. A.. 2015;3:8241-8245.
- [Google Scholar]
- A green synthesis of 3,4-dihydropyrimidine-2(1H)-one/thione derivatives using nanosilica-supported tin-(II) chloride as a heterogeneous nanocatalyst. Monatsh Chem.. 2013;144:1865-1870.
- [Google Scholar]
- Pseudo five-component process for the synthesis of 4,4′-(arylmethylene)bis(3-methyl-1H-pyrazol-5-ol) derivatives using ZnAl2O4 nanoparticles in aqueous media. RSC Adv.. 2014;4:46106-46113.
- [Google Scholar]
- Synthesis of diversely fluorinated pyrazoles as novel active agrochemical ingredients. J. Fluor. Chem.. 2013;152:2-11.
- [Google Scholar]
- Synthesis of heterocycles via electrophilic cyclization of alkynes containing heteroatom. Chem. Rev.. 2011;111(4):2937-2980.
- [Google Scholar]
- Tungstophosphoric acid supported on core-shell polystyrene-silica microspheres or hollow silica spheres catalyzed trisubstituted imidazole synthesis by multicomponent reaction. J. Mol. Catal. A: Chem.. 2016;420:294-302.
- [Google Scholar]
- Recent developments in asymmetric multicomponent reactions. Chem. Soc. Rev.. 2012;41:3969-4009.
- [Google Scholar]
- Multicomponent reactions in unconventional solvents: State of the art. Green Chem.. 2012;14:2091-2128.
- [Google Scholar]
- Simple and efficient one-pot synthesis of imidazo[1,2-a]pyridines catalyzed by magnetic nano-Fe3O4–KHSO4·SiO2. Synlett. 2012;23:2635-2638.
- [Google Scholar]
- Recent advances in heterogeneous selective oxidation catalysis for sustainable chemistry. Chem. Soc. Rev.. 2014;43:3480-3524.
- [Google Scholar]
- Medicinal plants: Traditions of yesterday and drugs of tomorrow. Mol. Aspect. Med.. 2006;27:1-93.
- [Google Scholar]
- Fundamental aspects of catalysis on supported metal clusters. J. Mater. Chem.. 2004;14:564-577.
- [Google Scholar]
- The fascinating construction of pyridine ring systems by transition metal-catalysed [2+2+2] cycloaddition reactions. Chem. Soc. Rev.. 2007;36:1085-1094.
- [Google Scholar]
- H14[NaP5W30O110] Catalyzed one-pot three-component synthesis of dihydropyrano[2,3-c]pyrazole and pyrano[2,3-d]pyrimidine derivatives. J. Iran. Chem. Soc.. 2010;7:615-620.
- [Google Scholar]
- Recent strategies for the synthesis of pyridine derivatives. Chem. A Euro. J.. 2010;16:2052-12062.
- [Google Scholar]
- Rabah A.T. Serya, Dalal A. Abou El Ella, Pyrazolo-[3,4-d]pyrimidine based scaffold derivatives targeting kinases as anticancer agents. Future J. Pharmaceu. Sci.. 2016;2:20-30.
- [Google Scholar]
- Nitrogen oxide removal over hydrotalcite-derived mixed metal oxides. Catal. Sci. Technol.. 2016;6:49-72.
- [Google Scholar]
- CuII immobilized on guanidinated epibromohydrin functionalized γ-Fe2O3@TiO2 (γ-Fe2O3@TiO2-EGCuII): A novel magnetically recyclable heterogeneous nanocatalyst for the green one-pot synthesis of 1,4-disubstituted 1,2,3-triazoles through alkyne–azide cycloaddition in water. RSC Adv.. 2016;6:29210-29219.
- [Google Scholar]
- Alumina supported MoO3: An efficient and reusable heterogeneous catalyst for synthesis of 3,4-dihydropyridine-2(1H)-ones under solvent free conditions. Catal. Commun.. 2008;9:499-503.
- [Google Scholar]
- Preparation and chemistry of 3/5-halogenopyrazoles. Chem. Rev.. 2012;112:3924-3958.
- [Google Scholar]
- Highly efficient regioselective one-pot synthesis of 2,3,6-trisubstituted pyridines and 2,7,7-trisubstituted tetrahydroquinolin-5-ones using K5CoW12O40.3H2O as a heterogeneous recyclable catalyst. Tetrahedron. 2007;63:13024-13031.
- [Google Scholar]
- Novel morpholinoquinoline nucleus clubbed with pyrazoline scaffolds: Synthesis, antibacterial, antitubercular and antimalarial activities. Eur. J. Med. Chem.. 2016;112:270-279.
- [Google Scholar]
- 1-Substituted carbamoyl and thiocarbamoyl-4,5-dihydro-1H-pyrazoles as possible cytotoxic and antimicrobial agents. J. Enzyme. Inhib. Med. Chem.. 2015;26:1-9.
- [Google Scholar]
- Catalysis by heteropoly acids and multicomponent polyoxometalates in liquid-phase reactions. Chem. Rev.. 1998;98(1):171-198.
- [Google Scholar]
- C.S. Kramer, Pyrazoles, Book Chapter Privileged Scaffolds in Medicinal Chemistry: Design, Synthesis, Evaluation, 2016, pp. 115–131.
- Heterogeneous photocatalyst materials for water splitting. Chem. Soc. Rev.. 2009;38:253-278.
- [Google Scholar]
- Magnetically separable CuFe2O4 nano particles catalyzed multicomponent synthesis of 1,4-disubstituted 1,2,3-triazoles in tap water using ‘click chemistry’. Tetrahedron Lett.. 2012;53:4595-4599.
- [Google Scholar]
- Magnetically recoverable CuFe2O4 nanoparticles: Catalyzed synthesis of aryl azides and 1,4-diaryl-1,2,3-triazoles from boronic acids in water. Eur. J. Org. Chem.. 2013;4674–4680
- [Google Scholar]
- Synthesis and structure–activity relationship study of 1-phenyl-1-(quinazolin-4-yl)ethanols as anticancer agents. ACS Med. Chem. Lett.. 2015;6:287-291.
- [Google Scholar]
- Elst, R.N. Muller. Magnetic iron oxide nanoparticles: synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem. Rev.. 2008;108:2064-2110.
- [Google Scholar]
- Nano-CoFe2O4 supported molybdenum as an efficient and magnetically recoverable catalyst for a one-pot, four-component synthesis of functionalized pyrroles. New J. Chem.. 2014;38:2435-2442.
- [Google Scholar]
- Nano CoFe2O4 supported antimony (III) as an efficient and recyclable catalyst for one-pot three component synthesis of multisubstituted pyrroles. RSC Adv.. 2014;4:12929-12943.
- [Google Scholar]
- Synthesis and biological evaluation of 1,3-diaryl pyrazole derivatives as potential antibacterial and anti-inflammatory agents. Bioorg. Med. Chem. Lett.. 2015;15:5052-5057.
- [Google Scholar]
- Design and fabrication of mesoporous heterogeneous basic catalysts. Chem. Soc. Rev.. 2015;44:5092-5147.
- [Google Scholar]
- Efficient approach to discover novel agrochemical candidates: intermediate derivatization method. J. Agric. Food Chem.. 2016;64:45-51.
- [Google Scholar]
- S-Adenosyl-L-methionine-competitive inhibitors of the histone methyltransferase EZH2 induce autophagy and enhance drug sensitivity in cancer cells. Anticancer Drugs.. 2015;26:139-147.
- [Google Scholar]
- One-pot three-component synthesis of 1,2,3-triazoles using magnetic NiFe2O4–glutamate–Cu as an efficient heterogeneous catalyst in water. RSC Adv.. 2015;5:59167-59185.
- [Google Scholar]
- F.EI. Mazouni, N.A. Malmquist, J. Harpon, E.P. Coutant, S. Guillou, O. Helynck, A. Noel, A. Scherf, M.A. Phillips, F. Tangy, P.O. Vidalain, Y.L. Janin. 2-(3-Alkoxy-1H-pyrazol-1-yl)azines inhibitors of human dihydroorotate dehydrogenase (DHODH) J. Med. Chem.. 2015;23:5579-5598.
- [Google Scholar]
- Design, synthesis and herbicidal evaluation of novel 4-(1H-pyrazol-1-yl)pyrimidine derivatives. Pest. Manag. Sci.. 2015;71:1189-1196.
- [Google Scholar]
- Ozone initiated dechlorination and degradation of trichlorophenol using Ce-Zr loaded metal oxides as catalysts. Appl. Catal. B: Environ.. 2013;142–143:129-141.
- [Google Scholar]
- Ce-V loaded metal oxides as catalysts for dechlorination of chloronitrophenol by ozone. Appl. Catal. B: Environ.. 2014;150–151:305-314.
- [Google Scholar]
- Synthesis of pyrazole-4-carbonitrile derivatives in aqueous media with CuO/ZrO2 as recyclable catalyst. Catal. Commun.. 2015;61:26-30.
- [Google Scholar]
- Mg-V/CO3 Hydrotalcite: An efficient and reusable catalyst for one-pot synthesis of multisubstituted pyridines. Res. Chem. Intermed.. 2015;41:8269-8278.
- [Google Scholar]
- Mn doped ZrO2 as a green, efficient and reusable heterogeneous catalyst for the multicomponent synthesis of pyrano[2,3-d]-pyrimidine derivatives. RSC Adv.. 2015;5:37360-37366.
- [Google Scholar]
- S.B. Jonnalagadda, Synthesis of pyrazole-4-carbonitrile derivatives in aqueous media with CuO/ZrO2 as recyclable catalyst. Catal. Commun.. 2015;61:26-30.
- [Google Scholar]
- Mn doped ZrO2 as a green, efficient and reusable heterogeneous catalyst for the multicomponent synthesis of pyrano[2,3-d]-pyrimidine derivatives. RSC Adv.. 2015;5:37360-37366.
- [Google Scholar]
- Swift and green protocol for one-pot synthesis of pyrano[2,3-c]pyrazole-3-carboxylates with RuCaHAp as catalyst. Curr. Org. Chem.. 2016;20:2125-2133.
- [Google Scholar]
- Ce-V/SiO2 catalyzed aascade for C-C and C-O bond activation: Green one-pot synthesis of 2-amino-3-cyano-4H-pyrans. ChemistryOpen.. 2016;5:38-42.
- [Google Scholar]
- Ag/SiO2 as a recyclable catalyst for the facile green synthesis of 3-methyl-4-(phenyl)methylene-isoxazole-5(4H)-ones. Res. Chem. Intermed.. 2016;42:2553-2566.
- [Google Scholar]
- Reusable Ce-V loaded alumina catalyst for multicomponent synthesis of substituted pyridines in green media. J. Heterocyc. Chem.. 2016;53:658-664.
- [Google Scholar]
- Ag loaded on SiO2 as an efficient and recyclable heterogeneous catalyst for the synthesis of chloro-8-substituted-9H-purines. J. Heterocyc. Chem.. 2016;53:319-324.
- [Google Scholar]
- Photocatalysed ozonation by Ce doped TiO2 catalyst degradation of pesticide, Dicamba in water. J. Chem. Tech. & Biotech.. 2016;91:385-393.
- [Google Scholar]
- Cesium loaded on silica as an efficient and recyclable catalyst for the novel synthesis of selenophenes. Arab. J. Chem.. 2016;9:891-897.
- [Google Scholar]
- One-pot synthesis of pyranopyrazoles using Ru-hydroxyapatite as reusable catalyst- a green approach. Curr. Org. Syn.. 2016;13:893-900.
- [Google Scholar]
- Ag/SiO2 as a recyclable catalyst for the facile green synthesis of 3-methyl-4-(phenyl)-methylene-isoxazole-5(4H)-ones. Res. Chem. Intermed.. 2016;42:2553-2566.
- [Google Scholar]
- Ag loaded on SiO2 as an efficient and recyclable heterogeneous catalyst for the synthesis of chloro-8-substituted-9H-purines. J. Heterocyclic Chem.. 2016;53:319-324.
- [Google Scholar]
- Reusable Ce-V loaded alumina catalyst for multicomponent synthesis of substituted pyridines in green media. J. Heterocyclic Chem.. 2016;53:658-664.
- [Google Scholar]
- Sm2O3/Fluoroapatite as a reusable catalyst for the facile, green, one-pot synthesis of triazolidine-3-thione derivatives under aqueous conditions. J. Fluorine Chem.. 2017;195:79-84.
- [Google Scholar]
- A facile, efficient and sustainable Chitosan/CaHAp catalyst and one-pot synthesis of novel 2,6-diamino-pyran-3,5-dicarbonitriles. Mol. Divers.. 2017;21:247-255.
- [Google Scholar]
- Acid hydrazides, potent reagents for synthesis of oxygen-, nitrogen-, and/or sulfur-containing heterocyclic rings. Chem. Rev.. 2014;114:2942-2977.
- [Google Scholar]
- Design. Preparation and characterization of urea-functionalized Fe3O4/SiO2 magnetic nanocatalyst and application for the one-pot Multi component synthesis of substituted imidazole derivatives. Catal. Commun.. 2015;69:29-33.
- [Google Scholar]
- A. Maleki, M. Aghaei, Ultrasonic assisted synergetic green synthesis of polycyclic imidazo (thiazolo)pyrimidine by using Fe3O4@clay core-shell, Ultrasonics Sonochem. (2016) (in press) 10.1016/j.ultsonch.2016.08.024.
- Silica-coated magnetic NiFe2O4 nanoparticles supported H3PW12O40; synthesis, preparation, and application as an efficient, magnetic, green catalyst for one-pot synthesis of tetrahydrobenzo[b]pyran and pyrano[2,3-c]pyrazole derivatives. Res. Chem. Intermed.. 2016;42:3071-3093.
- [Google Scholar]
- Intriguing optoelectronic properties of metal halide perovskites. Chem. Rev.. 2016;116:12956-13008.
- [Google Scholar]
- Improving drug candidates by Design: A focus on physicochemical properties as a means of improving compound disposition and safety. Chem. Res. Toxicol.. 2011;24:1420-1456.
- [Google Scholar]
- Synopsis of some recent tactical application of bioisosteres in drug design. J. Med. Chem.. 2011;54:2529-2591.
- [Google Scholar]
- Nickel ferrite nanoparticles: an efficient and reusable nano catalyst for a neat, one-pot and four component synthesis of pyrroles. RSC Adv.. 2015;5:18092-18096.
- [Google Scholar]
- Synthesis and biological activity of some pyrimidine derivatives. Drug Invent. Today. 2013;5(3):216-222.
- [Google Scholar]
- One-pot synthesis of novel pyrimido[4,5-b]quinolines and pyrido[2,3-d:6,5d|]dipyrimidines using encapsulated-γ -Fe2O3 nanoparticles. J. Chem. Sci.. 2015;127:1895-1904.
- [Google Scholar]
- Solvent-free one-pot synthesis of 1,2,3-triazole derivatives by the ‘Click’ reaction of alkyl halides or aryl boronic acids, sodium azide and terminal alkynes over a Cu/Al2O3 surface under ball-milling. Green Chem.. 2013;15:389-397.
- [Google Scholar]
- Microwave-assisted synthesis of pyrido-dipyrimidines using magnetically CuFe2O4 nanoparticles as an efficient, reusable, and powerful catalyst in water. J. Iran. Chem. Soc. 2016
- [CrossRef] [Google Scholar]
- Efficient one-pot click synthesis of b-hydroxy-1,2,3-triazoles catalyzed by copper(I) phosphorated SiO2 via multicomponent reaction in aqueous media. New J. Chem.. 2014;38:5429-5435.
- [Google Scholar]
- Potassium dodecatugstocobaltate trihydrate (K5CoW12O40·3H2O): A mild and efficient reusable catalyst for the one-pot synthesis of 1,2,4,5-tetrasubstituted imidazoles under conventional heating and microwave irradiation. J. Mol. Catal. A: Chem.. 2007;266:104-108.
- [Google Scholar]
- Heterogeneous bimetallic ZnFe2O4 nanopowder catalyzed synthesis of Hantzsch 1,4-dihydropyridines in water. Tetrahedron Lett.. 2016;57:4046-4049.
- [Google Scholar]
- Transition-metal-catalyzed reactions in heterocyclic synthesis. Chem. Rev.. 2004;104:2127-2198.
- [Google Scholar]
- Design, synthesis, studies of some new thiophene, pyrazole and pyridone derivatives bearing sulfisoxazole moiety. Eur. J. Med. Chem.. 2014;12:491-504.
- [Google Scholar]
- Nano-Fe3O4 encapsulated-silica particles bearing sulfonic acid groups as a magnetically separable catalyst for green and efficient synthesis of functionalized pyrimido[4,5-b]quinolines and indeno fused pyrido[2,3-d]pyrimidines in water. Chin. Chem. Lett.. 2013;24:370-372.
- [Google Scholar]
- Heterogeneous Cu-(II)/L-His@Fe3O4 nanocatalyst: a novel, efficient and magnetically-recoverable catalyst for organic transformations in green solvents. RSC Adv.. 2016;6:92387-92401.
- [Google Scholar]
- Design, facile synthesis and anthelmintic activity of new O-substituted 6-methoxybenzothiazole-2-carbamates. Med. Chem. Commun.. 2015;6:795-805.
- [Google Scholar]
- Photocatalytic degradation of 4-chloro-2-methylphenoxyacetic acid using W-doped TiO2. J. PhotoChem. & Photobiol. A-Chem.. 2015;312:96-106.
- [Google Scholar]
- Ce-Zr/SiO2: A versatile reusable heterogeneous catalyst for three-component synthesis and solvent free oxidation of benzyl alcohol. RSC Adv.. 2014;4:6602-6607.
- [Google Scholar]
- Zn-VCO3 hydrotalcite: A highly efficient and reusable heterogeneous catalyst for the Hantzsch dihydropyridine reaction. Catal. Commun.. 2014;45:148-152.
- [Google Scholar]
- Multicomponent synthesis of pyridines via diamine functionalized mesoporous ZrO2 domino intermolecular tandem Michael type addition. RSC Adv.. 2015;5:5627-5632.
- [Google Scholar]
- Multicomponent synthesis of pyridines via diamine functionalized mesoporous ZrO2 domino intramolecular tandem Michael type addition. RSC Adv.. 2015;5:5627-5632.
- [Google Scholar]
- New vanadium Keggin heteropolyacids encapsulated in a silica framework: Recyclable catalysts for the synthesis of highly substituted hexahydropyrimidines under suitable conditions. Catal. Lett.. 2015;145:1022-1032.
- [Google Scholar]
- Discovery of pyrazolopyridones as a novel class of noncovalent dpre1 inhibitor with potent anti-mycobacterial activity. J. Med. Chem.. 2014;57:4761-4771.
- [Google Scholar]
- Synthesis and mechanism studies of 1,3-benzoazolyl substituted pyrrolo[2,3-b]pyrazine derivatives as nonintercalative topoisomerase II catalytic inhibitors. J. Med. Chem.. 2016;59(1):238-252.
- [Google Scholar]
- Magnetically retrievable nano crystalline CuFe2O4 catalyzed multi-component reaction: a facile and efficient synthesis of functionalized dihydropyrano[2,3-c]pyrazole, pyrano[3,2-c]coumarin and 4H-chromene derivatives in aqueous media. Catal. Sci. Technol.. 2014;4:822-831.
- [Google Scholar]
- A novel route of single step reactive calcination of copper salts far below their decomposition temperatures for synthesis of highly active catalysts. Catal. Sci. Technol.. 2013;3:3326-3334.
- [Google Scholar]
- Heterogenization of heteropoly compounds: a review of their structure and synthesis. RSC Adv.. 2016;6:46433-46466.
- [Google Scholar]
- Cu-(II)-Hydromagnesite catalyzed synthesis of tetrasubstituted propargylamines and pyrrolo [1,2-a]quinolines via KA2, A3 couplings and their decarboxylative versions. ACS Sustainable Chem. Eng.. 2016;4:3409-3419.
- [Google Scholar]
- Synthesis of pyrazole derivatives in the presence of a dioxomolybdenum complex supported on silica-coated magnetite nanoparticles as an efficient and easily recyclable catalyst. RSC Adv.. 2016;6:104875-104885.
- [Google Scholar]
- Yttria-Zirconia based lewis acid catalysis of the biginelli reaction: an efficient one-pot synthesis of 3,4-dihydropyrimidin-2-(1H)-ones. Syn. Commun.. 2009;39:1299-1309.
- [Google Scholar]
- Covalent modification of organo functionalized graphene oxide and its scope as catalyst for one-pot pyrazolo-pyranopyrimidine derivatives. ChemistryOpen.. 2015;4:703-707.
- [Google Scholar]
- Synthesis and characterization of novel bifunctional mesoporous silica catalyst and its scope for one-pot deacetalization-Knoevenagel. J. Porous Mater.. 2015;22:353-360.
- [Google Scholar]
- Small heterocycles in multicomponent reactions. Chem. Rev.. 2014;114(16):8323-8359.
- [Google Scholar]
- Approach to the library of 3-hydroxy-1,5-dihydro-2h-pyrrol-2-ones through a three-component condensation. ACS Comb. Sci.. 2012;14:631-635.
- [Google Scholar]
- Al-HMS-20 catalyzed synthesis of pyrano[2,3-d]pyrimidines and pyrido[2,3-d]pyrimidines three-component reaction. Res. Chem. Intermed.. 2015;41:1343-1350.
- [Google Scholar]
- Efficient, green and solvent-free synthesis of tetrasubstituted imidazoles using SbCl3/SiO2 as heterogeneous catalyst. J. Chem. Sci.. 2013;125:827-833.
- [Google Scholar]
- Applications of microwave technology to rapid synthesis of substituted imidazoles on silica-supported SbCl3 as an efficient heterogeneous catalyst. J. Taibah Univer. Sci.. 2014;8:323-330.
- [Google Scholar]
- Facile and eco-friendly synthesis of chromeno[4,3-β] pyrrol-4(1H)-one derivatives applying magnetically recoverable nano crystalline CuFe2O4 involving a domino three-component reaction in aqueous media. RSC Adv.. 2016;6:55033-55038.
- [Google Scholar]
- Diversity-oriented approaches to polycyclics and bioinspired molecules via the Diels-Alder strategy: Green chemistry, synthetic economy, and beyond. ACS Comb. Sci.. 2015;17:253-302.
- [Google Scholar]
- A facile and eco-friendly synthesis of imidazo[1,2-a]pyridines using nano-sized LaMnO3 perovskite-type oxide as an efficient catalyst under solvent-free conditions. Appl. Catal. A: Gen.. 2014;470:56-62.
- [Google Scholar]
- Nano copper and cobalt ferrites as heterogeneous catalysts for the one-pot synthesis of 2,4,5-tri substituted imidazoles. J. Chem. Sci.. 2014;126:1715-1720.
- [Google Scholar]
- A facile, efficacious and reusable Sm2O3/ZrO2 catalyst for the novel synthesis of functionalized 1,4-dihydropyridine derivatives. Catal. Commun.. 2016;79:21-25.
- [Google Scholar]
- A facile, efficacious and reusable Sm2O3/ZrO2 catalyst for the novel synthesis of functionalized 1,4-dihydropyridine derivatives. Catal. Commun.. 2016;79:21-25.
- [Google Scholar]
- Aminopropyl coated on magnetic Fe3O4 and SBA-15 nanoparticles catalyzed mild preparation of chromeno[2,3-d]pyrimidines under ambient and solvent-free conditions. Catal. Sci. Technol.. 2013;3:425-428.
- [Google Scholar]
- Synthesis of 6-amino-4-aryl-3-methyl-1,4-dihydropyrano[2,3-c]pyrazole-5-carbonitriles by heterogeneous reusable catalysts. Res. Chem. Intermed.. 2014;40:1997-2005.
- [Google Scholar]
- On the synergetic catalytic effect in heterogeneous nanocomposite catalysts. Chem. Rev.. 2013;113:2139-2181.
- [Google Scholar]
- Synthesis and fungicidal activity of novel 3-(Substituted/unsubstituted phenylselenonyl)-1-ribosyl/deoxyribosyl-1H-1,2,4-triazole. J. Agric. Food Chem.. 2012;60(23):5813-5818.
- [Google Scholar]
- Recent developments in solvent-free multicomponent reactions: a perfect synergy for eco-compatible organic synthesis. RSC Adv.. 2012;2:4547-4592.
- [Google Scholar]
- Parecoxib versus dipyrone (metamizole) for postoperative pain relief after hysterectomy. Clin. Drug Invest.. 2008;28:421-428.
- [Google Scholar]
- Sulfonic acid functionalized silica-coated CuFe2O4 core-shell nanoparticles: an efficient and magnetically separable heterogeneous catalyst for syntheses of 2-pyrazole-3-aminoimidazo- fused poly heterocycles. New J. Chem.. 2016;40:9788-9794.
- [Google Scholar]
- Nano magnetite supported metal ions as robust, efficient and recyclable catalysts for greensynthesis of propargylamines and 1,4-disubstituted 1,2,3-triazoles in water. New J. Chem.. 2015;39:1827-1839.
- [Google Scholar]
- Ternary and higher order rare-earth nitride materials: synthesis and characterization of ionic-covalent oxynitride powders. J. Solid State Chem.. 2003;171:143-151.
- [Google Scholar]
- D. G. Hall. Natural product synthesis using multicomponent reaction strategies. Chem. Rev.. 2009;109:4439-4486.
- [Google Scholar]
- Forging colloidal nanostructures via cation exchange reactions. Chem. Rev.. 2016;116:10852-10887.
- [Google Scholar]
- Green Chem.. 2014;16:2027-2041.
- Pyrazolones based compounds are core units biological evaluation. Int. J. Inst. Pharm. Life Sci.. 2015;5:240245.
- [Google Scholar]
- Ballav and S. Kerkar. Design and synthesis of some new pyrazolyl-pyrazolines as potential anti-inflammatory, analgesic and antibacterial agents. Eur. J. Med. Chem.. 2015;28:442-451.
- [Google Scholar]
- Recent conceptual advances in the catalysis science of mixed metal oxide catalytic materials. Catal. Today.. 2005;100(1–2):79-94.
- [Google Scholar]
- Defective TiO2 with oxygen vacancies: synthesis, properties and photocatalytic applications. Nanoscale. 2013;5:3601-3614.
- [Google Scholar]
- Application of magnetic nanoparticle-supported CuBr: a highly efficient and reusable catalyst for the one-pot and scale-up synthesis of 1,2,3-triazoles under microwave-assisted conditions. Catal. Sci. Technol.. 2013;3:1301-1307.
- [Google Scholar]
- Supported CuBr on graphene oxide/Fe3O4: a highly efficient, magnetically separable catalyst for the multi-gram scale synthesis of 1,2,3-triazoles. RSC Adv.. 2014;4:9830-9837.
- [Google Scholar]
- S. Yamamoto, T. Sato, K. Morimoto, T. Nawamaki, New pyrazole sulfonylureas synthesis and herbicidal activity. Synthesis and Chemistry of Agrochemicals III, Chapter 5 (1992) 34–42 (ACS Symposium Series, Volume 504).
- Facile synthesis of spirooxindole-pyrazolines and spirobenzofuranone-pyrazolines and their fungicidal activity. Org. Biomol. Chem.. 2015;13:4869-4878.
- [Google Scholar]
- Synthesis of diphenylmethane derivatives in Lewis acidic ionic liquids. J. Mol. Catal. A: Chem.. 2006;245:260-265.
- [Google Scholar]
- 1,4-Dihydroxyanthraquinone–copper-(II) supported on superparamagnetic Fe3O4@SiO2: an efficient catalyst for N-arylation of nitrogen heterocycles and alkylamines with aryl halides and click synthesis of 1-aryl-1,2,3-triazole derivatives. RSC Adv.. 2016;6:90154-90164.
- [Google Scholar]
- Synthesis and insecticidal evaluation of novel anthranilic diamides containing N-substitued nitrophenylpyrazole. Bioorg. Med. Chem.. 2014;22:186-193.
- [Google Scholar]
- Magnetic carbon nanotube supported cu (CoFe2O4/CNT-Cu) catalyst: a sustainable catalyst for the synthesis of 3-nitro-2-arylimidazo[1,2-a]pyridines. Catal. Commun.. 2016;78:26-32.
- [Google Scholar]
- Supported molybdenum on graphene oxide/Fe3O4: An efficient, magnetically separable catalyst for one-pot construction of spiro-oxindole dihydropyridines in deep eutectic solvent under microwave irradiation. Catal. Commun.. 2017;88:39-44.
- [Google Scholar]
- Deep eutectic solvents: syntheses, properties and applications. Chem. Soc. Rev.. 2012;41:7108-7146.
- [Google Scholar]
- Experimental and theoretical studies of the nanostructured {Fe3O4@SiO2@(CH2)3Im}C(CN)3 catalyst for 2-amino-3-cyanopyridine preparation via an anomeric based oxidation. RSC Adv.. 2016;6:50100-50111.
- [Google Scholar]







