Translate this page into:
A review on the therapeutic applications of aptamers and aptamer-conjugated nanoparticles in cancer, inflammatory and viral diseases
⁎Corresponding authors at: Department of Tissue Engineering, School of Medicine, Shahroud University of Medical Sciences, Shahroud, Iran (M. Sharifi). hualinlin2009@126.com (Linlin Hua), h-derakhshankhah@alumnus.tums.ac.ir (Hossein Derakhshankhah), Sharifi@shmu.ac.ir (Majid Sharifi)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The advancement in early diagnosis and precise treatments options result in more predictable and powerful health care modalities. Aptamers are known as nucleic acid structures with three-dimensional conformation to selectively bind a target site. Physicochemical properties of aptamers, their conjugation with nanoparticles (NPs) in theranostics applications and their internalization have been found to be of interest in development of aptamer-based drug delivery systems. Therefore, we aimed to present an overview on the structure and generation of aptamers followed by advantages of aptamers-conjugated NPs and their theranostics applications in various diseases such as oncology, inflammatory diseases and viral diseases. Afterward, we discussed several reports on the internalization approaches of aptamers, efficiency of aptamers vs. their analogous, and implications of aptamers in clinical trials. Finally, we discussed the current challenges and future perspectives of actively targeted aptamers for clinical application. In conclusion, this review may hold a great promise for development of aptamer-based therapeutic platforms in clinical trials.
Keywords
Aptamer
Cancer disease
Inflammatory disease
Nanoparticles
Viral disease
1 Introduction
The development of accurate diagnosis with successful therapy is important for favorable clinical application. So, the effort to develop small molecules capable of modulating the target's activities was increased. In the recent year, aptamer was introduced to fulfill desired goals related to clinical applications. Aptamers are the unique synthetic fold up structure of single-stranded DNA or RNA molecules with the capability to form secondary and tertiary structures. These unique characteristics make them potential candidates to bind several target molecules such as peptides, proteins, metal ions, bacteria, viruses, and other cellular targets. Aptamers research field is extending because of their remarkable potential for instance as potent anti-tumor activity, excellent circulation stability, biocompatibility, multimodal diagnostic functionalities, and high loading efficiency (Ni et al. 2011, Wan et al. 2019). Most conventional aptamer therapies stop cell transfer machines by dysregulation of transcription activator. In this regard, aptamers modulate cellular function through interfere with the DNA binding of the transcription activator (Zhao et al. 2006, Shi et al. 2007). Recently, the use of oligonucleotide-based drug has grown, providing new ways for the treatment of cancer, autoimmunity and inflammatory diseases. The treatment of wet form of age-related macular degeneration (wet AMD) was possible by Pegaptanib sodium, i.e. Macugen® (first FDA approved commercialized oligonucleotide-based drug) (Röthlisberger and Hollenstein 2018). Also, aptamers used in clinical phases include, Edifoligide (E2F Decoy), Metastatic Renal Cell Carcinoma (AS1411) and von Willebrand (ARC1779) (Nimjee and Sullenger 2020). Aptamers have also gained wide attention in the treatment of neurodegenerative, autoimmune, and bacterial or viral infections. Although aptamers are going to find their own niche of theranostic applications, their limitations still remained as challenges such as aptamer degradation, metabolic clearance, renal filtration, control of the duration of action, cross-reactivity, irreversible tissue uptake and generation by automated synthetic methods (Röthlisberger and Hollenstein 2018).
This review presents the applications and progress of aptamers in various diseases. First, we consider aptamer discovery, generation, possible modification to tackle aptamer degradation and aptamer internalization. Subsequently, recent progress in the use of aptamer-conjugated nanoparticles (NPs) and their theragnostic applications in various diseases such as oncology, inflammatory, and viral diseases were discussed. Afterwards, internalization approaches of aptamers, efficiency of aptamers vs. their analogous, and implications of aptamers in clinical trials were surveyed (Ni et al. 2011).
2 Aptamers
Aptamers are oligonucleotide compounds in the range of 15–100 nucleotides that exhibit a complex tertiary or quaternary structure. Aptamer detects targets in the micro- to picomolar range, which is comparable to antibodies (Morita et al. 2018). The first step in the aptamer coin, accompanied with identifying a chemical composition of the cell which is called nucleic acids (deoxyribonucleic acid or DNA) in 1869 by Friedrich Miesche (Dahm 2008). Likewise, studies continued in order to determine DNA structures, DNA functions, its effect on regulation of cellular pathways and ultimately its chemical synthesis and preparation in vitro (Alshaer et al. 2018). In 1990, aptamer was introduced by Tuerk and Gold in order to bind the T4 DNA polymerase (Tuerk and Gold 1990). High-affinity aptamer ligands were isolated by a procedure known as Systematic Evolution of Ligands by Exponential Enrichment (SELEX): process relies on ligand selection with alternate cycles from pools of different sequences and amplification of the bound species. In fact, they selected and enriched two variant types of RNA ligands from one eight-base random region library (65,536 species) which can interact with the T4 DNA polymerase. In the same year, the word of aptamer was coined by Ellington and Szostak to name RNA molecules with the ability to bind organic dyes. They isolated the aptamer that binds to organic dyes from 1013 different sequences of pools (Ellington and Szostak 1990).
Compared to antibodies (their proteinaceous counterparts), commercial success of aptamers still proceeds at a weak pace. However their unique features such as higher sensitivity and selectivity, small size, rapid penetration into target tissues, ease of chemical production on large scale, facile chemical modification, low cost, low immunogenicity, limited batch to batch variation, high thermal stability, simple storage, and resistance to denaturation reflect this fact that they can find their own niche of bioanalytical and pharmaceutical applications or even are expected to become an alternative platform for antibody applications (Dehghani et al. 2018, Röthlisberger and Hollenstein 2018, Ahmadyousefi et al. 2019). However, despite these favorable functions, aptamers suffer from two major shortcomings that often thwart their applications as therapeutic and diagnostic agents: limited stability and insufficient binding affinity and specificity (Röthlisberger and Hollenstein 2018, Nimjee and Sullenger 2020).
2.1 Structure
Aptamers are usually willing to form complementary base pairs based on their tendency to have certain structures. They can fold into different secondary structures such as internal loops, stems, pseudoknots, bugles, kissing complexes, tetra loops, hairpins, and G-quadruplexes. Followed by the specific and unique complex, three-dimensional structures can be formed from these secondary structures that are capable of specific molecular recognition of their cognate targets (Reinemann and Strehlitz 2014). Mixtures of interaction containing base stacking of aromatic rings, hydrogen bonding, van der Waals forces, complementarity in the geometrical shape, and electrostatic interactions, result in binding affinity and specificity of the aptamer (Zhou and Rossi 2017). In addition, enabling them to distinguish between conformational isomers, recognizing a distinct epitope of a target molecule and amino acid mutation, differentiate various functional groups or even closely similar targets such as theophylline and caffeine (Reinemann and Strehlitz 2014). Many of the selected aptamers show potential affinities comparable to those observed for monoclonal antibodies.
3 The generation of aptamers (SELEX Process)
Systematic evolution of ligands by exponential enrichment (SELEX) is a conventional aptamer engineering method that is used for in vitro selecting target-specific aptamers. Although there are numerous types of the SELEX process in new selection protocols, the main principles remain the same. In fact, the operation mimics a Darwinian type process, driving the selection towards relatively few (but optimized) structural motifs, which show the highest specificities and affinities to the selected target. Generally, the basic process can be divided into two repetitive stages of selection and enzymatic amplification. At the first level, the pool of original oligonucleotides with optimum concentration (around 1013-1015 sequences) is chemically synthesized. Before starting the RNA SELEX process, the DNA library must be turned into the RNA library (Fig. 1A) (Röthlisberger and Hollenstein 2018). Initial library qualities play a crucial role in successful SELEX experiments. After incubating the oligonucleotide pools with the target and washing steps, the small amounts of the target bound oligonucleotides are amplified via a reverse transcription PCR (RT-PCR) for RNA and polymerase chain reaction (PCR) for DNA. Subsequently, this new enriched pool of selected oligonucleotides is re-exposed to the target in the next SELEX round. Iterative rounds up to the saturation concentration of target-interacting sequences dominate the population (Fig. 1A) (Röthlisberger and Hollenstein 2018). The selected aptamer pool is cloned to obtain individual aptamers and corresponding sequencing, which further analyzed to select representative aptamers in binding assays to characterize their binding characterization, including the affinities and specificities (Proske et al. 2005, Reinemann and Strehlitz 2014, Zhou and Rossi 2017, Röthlisberger and Hollenstein 2018).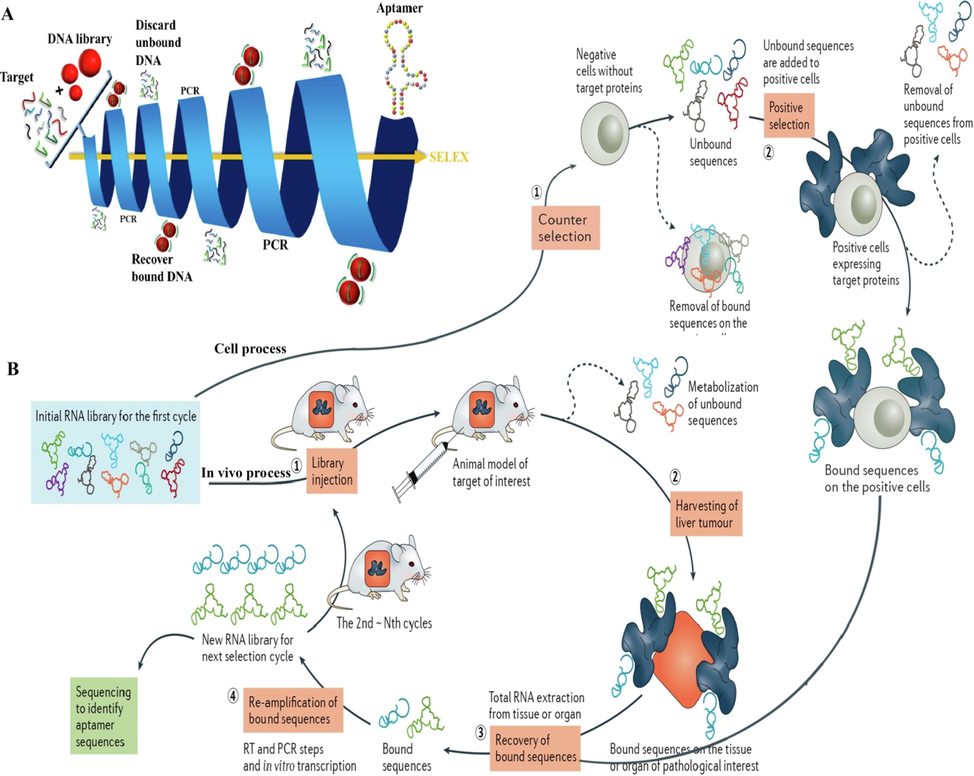
A) Schematic representation of the SELEXmethod. An initial DNA library (of typically 1014 molecules) is incubated with the solid support-bound target. Unbound DNA molecules are discardedwhile the active species are recovered, amplified by PCR, and injected into subsequent rounds of selection. The stringency of the selection protocol can bemodulated by altering physicochemical parameters such as concentration, pH, temperature, or buffer composition. At the end of the protocol, the enriched population is sequenced and the individual aptameric sequences evaluated for their capacity at binding to the target (Röthlisberger and Hollenstein 2018). B) Cell-process: Step 1 involves counter selection by incubating the RNA library with negative cells that do not express the target protein. Step 2 involves positive selection by incubating recovered unbound sequences with positive cells expressing the target protein. Step 3 involves recovery of target-bound sequences. Step 4 involves re-amplification of recovered species and generation of a new RNA pool for the next selection round. In vivo process: After intravenous administration and circulation of an RNA library in the animal model (step 1), the tissue or organ of pathological interest is harvested (step 2) and the bound sequences are extracted (step 3). Subsequently, the recovered RNA sequences are re–amplified to make a new RNA library for the next selection cycle (step 4) (Zhou and Rossi 2017). Copyright 2018, reprinted with permission from Elsevier, (Röthlisberger and Hollenstein 2018) and Copyright 2017, Macmillan Publishers Limited, part of Springer Nature (Zhou and Rossi 2017).
Over the last few years, considerable efforts have focused on automating in vitro selection procedures. Since the advent of SELEX, the original method has evolved and improved in terms of time–cost optimization and efficiency. Despite considerable success of aptamers, they contain some complications that prevent their widespread utilization in various applications particularly in biomedical fields. Aptamer degradation in biological media by nucleases is the first drawback. To tackle this issue, modified nucleotides before or after SELEX round, mirror image aptamers, and aptamer displacement screening are generally used. For instance, modification of 2′ sugar position (2′-amino pyrimidine nucleosides [20, 21], 2′-fluoropyrimidine nucleosides [22, 23], 2′-O-methyl purine, and 2′-O-methyl pyrimidine nucleosides [24, 25]) or 3′- and 5′-nucleotides, located L-ribose or L-deoxyribose in oligonucleotide backbone and displace aptamer with low-molecular-weight compound from the binding site of a target molecule, improve pharmacokinetics of the aptamer in blood. In the terms of the second problem, renal filtration of aptamer, conjugation with polyethylene glycol (PEG) and thus increasing aptamer size is a good way to increase the bloodstream circulation time. Third problem related to control action duration of aptamer, the use of polycationic biopolymers like porphyrin and conversion of an inactive aptamer to an active form are the most common solutions to this problem. Furthermore, Cell-SELEX and in vivo SELEX (Fig. 1B, (Zhou and Rossi 2017)), SELEX negative selection, automated SELEX and CE –SELEX were used to avoid aptamer generation with purified target molecules, cross-reactivity of aptamer, and automation of aptamer generation limitations, respectively (Lakhin et al. 2013).
4 Advantages of aptamer-conjugated nanoparticles (NPs) and their theranostic applications in various diseases
In recent years, combinations of aptamer and NPs are extensively used in the development of theranostic platforms because of their unique potential in targeted drug delivery systems, diagnosis and monitoring response to treatment (Khan et al. 2021b). In fact, Warner coined the new term ‘theranostics’ in order to implement simultaneous diagnosis and treatment into a single system. This is a useful concept when designing nanotechnology-based imaging contrasting agents and imaging-guided therapeutics (Ahmed et al. 2012, Morshed et al. 2020).
4.1 Oncology
Although significant advances in cancer treatment such as molecular biology, surgical procedures, radiotherapy, and chemotherapy have been achieved recently, cancer remains the most common cause of death worldwide. Various factors comprising microenvironment, genetics, and epigenetics affect tumor cells that ultimately, can lead to enhance the risk of therapeutic failure and thereby tumor relapse. In principle, the ultimate goal of cancer therapy is the development of targeted drug delivery systems (Liu et al. 2014). Rapid development of nanotechnology with the essential needs of selective inhibition of cancer cell proliferation at the initial phases of growth, making hybrid nanostructures as potential and powerful active targeting materials (Khan et al. 2021a, Khan et al. 2021c). Interaction of aptamers with nanomaterial has made this aim possible by increasing the efficacy of antitumor drugs on their target (Sharifi et al. 2019). Actually, the ability of aptamers to identify specific epitopes on cell surfaces can result in improved drug accumulation inside cancer cells (Reinemann and Strehlitz 2014, Alshaer et al. 2018). Furthermore, we summarized recent progress in the development of aptamer-NPs structures (Fig. 2A) that can deliver anticancer drugs to the specific tumor site in Table 1 (Grabowska-Jadach et al. 2019).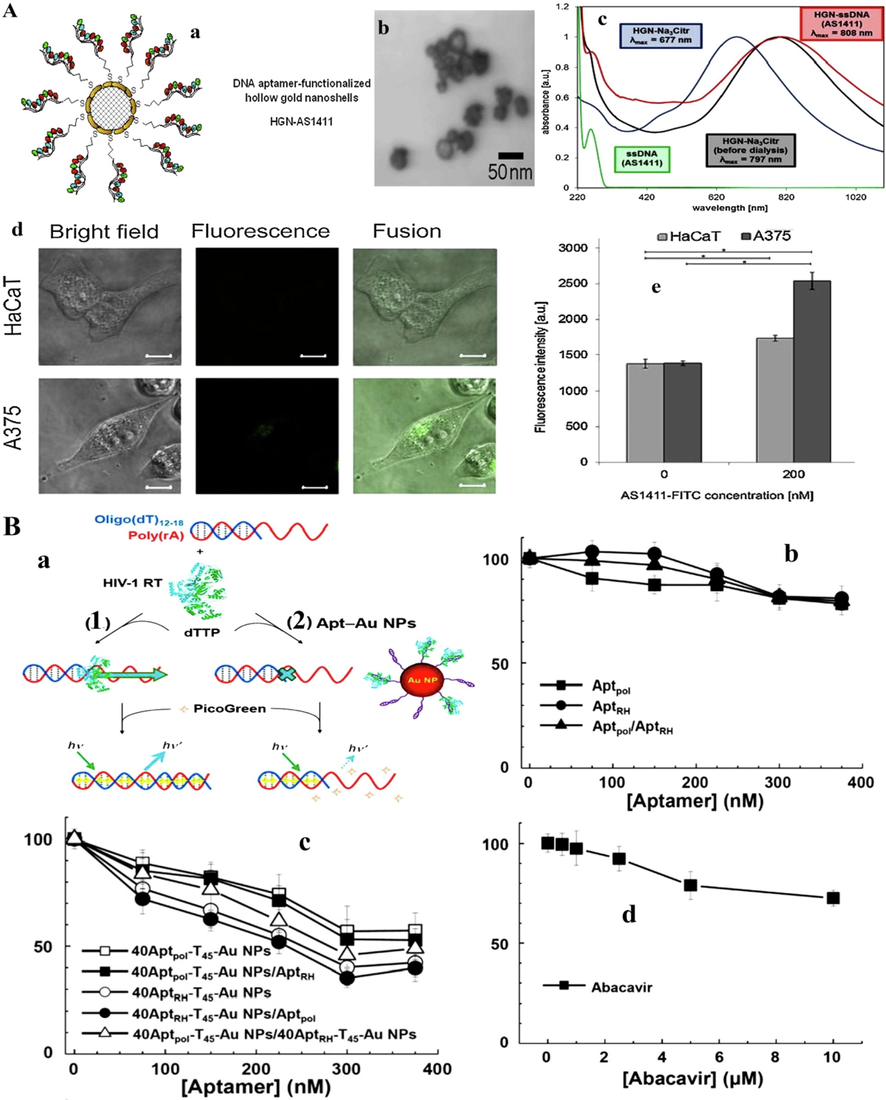
A) a; Schematic illustration of HGN surface modification with thiolated ssDNA aptamer. b; TEM image of HGNs before ssDNA attachment. c; Absorption spectra of HGN samples at various stages of modification with ssDNA (AS1411 aptamer). Normalized spectra represent NPs: citrate estabilized before dialysis (black line), citrate-stabilized after dialysis (blue line) and DNA aptamer (red line). Green line represents spectrum of free ssDNA (AS1411) in solution. AS1411-FITC uptake by HaCaT and A375 cells after 24 h of incubation with cell cultures: d; confocal microscope images (scale bar 10 μm), e; measurement of fluorescence intensity (Grabowska-Jadach et al. 2019). B) a; Schematic representation of the binding and enzymatic activity of HIV-1 RT toward poly(rA): oligo (dT)12–18 in the absence (A) and presence (B) of Apt–Au NPs. Inhibition of viral infectivity to HepG2 cells by (b) aptamers, (c) Apt_Au NPs or (d) nucleoside RT inhibitor (Abacavir). The green fluorescent protein signal was measured after infection for 4 days. Results are expressed as a percentage of infectivity (Shiang et al. 2013). Copyright 2019, reprinted with permission from Elsevier, Under the terms of the Creative Commons CC BY license (Grabowska-Jadach et al. 2019) and Copyright 2013, Royal Society of Chemistry publishing group (Shiang et al. 2013).
Nanomaterials
drug
Aptamer
Target cell lines
Cancer type
Therapy method
Imaging
ref
Organic
Ru(bpy)32+- SiO2 NPs
miRNA-21
AS1411
MCF-7
Breast cancer
Chemotherapy
Fluorescence
(Li et al. 2014)
Poly (ethylene glycol)- poly (caprolactone) NPs
Docetaxel
GMT8
U87 cells
Drain Glioblastoma
Chemotherapy
Fluorescence
(Gao et al. 2012b)
Poly (ethylene glycol)- poly (caprolactone) NPs
Docetaxel
AS1411
C6 cells
Brain glioma
Chemotherapy
Fluorescence
(Gao et al. 2012a)
CA-PLGA-b-TPGS NPs
Docetaxel
AS1411
MCF-7
Breast
CancerChemotherapy
Fluorescence
(Tao et al. 2016)
Exosome
Doxorubicin
sgc8
Ramos cells and CEM cells
Lymphoblast
Chemotherapy
Fluorescence
(Zou et al. 2019a)
Inorganic
Gold (Au)
–
AS1411
A375
Skin cancer
Photothermal therapy (PTT)
Fluorescence
(Grabowska-Jadach et al. 2019)
Silica (SiO2) NPs
YQ26
HEK293 BNL-CL2 H22
B16
Fluorescence
(Tan et al. 2017)
Mesoporous SiO2 NPs
Doxorubicin
MUC1
MDA-MB-231 cells
Breast cancer
Chemotherapy
SPECT
(Pascual et al. 2017)
Au nanocluster
Doxorubicin
AS1411
U87MG
cellsBrain glioblastoma
Chemotherapy
Fluorescence
(Chen et al. 2016)
Silver (Ag) nanocluster
miR-34a
MUC1
MCF-7
breast cancer
Fluorescence
(Chen et al. 2017)
Fe3O4
Epirubicin
5TR1
C26 cells
Colon
carcinomaChemotherapy
MRI
(Jalalian et al. 2013)
Fe3O4
Doxorubicin
PSMA
LNCaP
cellsProstate-cancer
Chemotherapy
MRI
(Yu et al. 2011)
CdTe/CdS quantum dots
Doxorubicin
MUC1
MCF-7
Breast cancer
Chemotherapy
Fluorescence
(Du et al. 2015)
Au@ γ-Fe2O3 NPs
–
MUC-1aptamer
L929
CHO
HT-29Colon cancer
PTT
MRI
(Azhdarzadeh et al. 2016)
Au@Ag/Au
–
S6 aptamer
A549
Lung cancer
PTT
Fluorescence
(Shi et al. 2014)
Fe3O4@carbon
Doxorubicin
sgc8c aptamers
A549
Lung cancer
Chemo– PTT
MRI imaging
(Zhao et al. 2019)
Fe3O4–Au
nanocompositeEpirubicin
MUC-1 aptamer
MCF-7 and HT-29
Breast and colorectal cancer
Chemotherapy
Fluorescent imaging
(Binaymotlagh et al. 2019)
Au nanocage/ SiO2
–
AS1411
MCF-7
Breast cancer
PTT
SERS
Imaging(Wen et al. 2019)
Fe3O4 co-loaded
(PEG-PLGA) NPsDoxorubicin
AS1411
C26 cells
Colon carcinoma cancer
Chemotherapy
MRI
(Mosafer et al. 2017)
Although the use of aptamers can significantly reduce tumor activity according to Table 1, Kang et al. (2015) exposed that the use of aptamer E-selectin reduces the activity of breast cancer metastasis by inhibiting the adhesion of CD44 + breast cancer cells to blood vessels. They showed that intravenous injection of aptamer E-selectin reduced metastasis in syngeneic or xenogeneic breast cancer models without relocating the metastasis site (Kang et al. 2015). Moreover, Heo et al. (2016) in an animal model using an aptamer-antibody complex (oligobody) were able to prohibit the angiogenesis of xenograft A549 tumor (human adenocarcinoma cells) similar to the growth of tumor compared to control group with cotinine-specific antibody. Also, the oligobody increased the half-life in serum to 8.2 h, which can be very promising in drug stability. Meanwhile, in an animal model, it was determined that aptamer PD-L1 could induce lymphocyte proliferation and tumor growth by mimicking antibody functions, with a chemical nature without immunogenicity or inducing hepatotoxicity (Lai et al. 2016). The results of this study not only reduce angiogenesis compared to the previous study, but also significantly increase T cells with the markers CD4 + and CD8+, Interlukin-2, TNF-α, interferon-γ and the chemokines CXCL9 and CXCL10. These chemokines can further adsorb T cells to tumor tissues (Lai et al. 2016). In the following, Jain et al. (2018) revealed that the function of doxorubicin in the treatment of diffuse B-cell lymphoma (DLBCL) and other blood cancers depends on nucleolin silencing and non-binding to topoisomerase-II-α. Amplification of nucleolin quenching by aptamer AS1411 or Nocant N6L increases the activity of doxorubicin in DLBCL cells, which ultimately induces apoptosis significantly by DNA fragmentation. This finding is of potential clinical importance due to the high accuracy of aptamer in regulating the expression of nucleolin with low concentration.
4.2 Inflammatory diseases
Inflammation, which is primarily caused by immune molecules, acts an important role in promotion of a state of low-grade diseases due to the increased immune response to infections or injuries. Therefore, there is evidence that early detection of inflammatory molecules as well as their reduction by aptamers can accelerate therapeutic activity earlier than the clinical onset of the disease (Table 2). For example, cerebral inflammations, which generally have few clinical manifestations, can be detected and controlled by aptamers (Shahdadi Sardou et al. 2020). In this regard, Giorgi-Coll et al. (2020) in an animal model using an optical nanobiosensors based on the aggregation of AuNPs coated with two anti-murine interleukin-6 aptamers (ATW0082 and ATW0077) were able to detect interleukin-6 with a detection limit of 1.95 μg/mL (linear range of 3.3 to 125 μg/mL) as an indicator of acute inflammation. Interleukin-6 concentration, which is considered as an indicator of rheumatoid arthritis, has been reported in plasma samples from mice with different health conditions between concentrations of 1 and 1500 pg/mL (Nukina et al. 2001). In this line, Hekmatimoghaddam et al. (2019) to reduce brain inflammation induced by proteolipid protein and parathion showed that the use of gelatin hydrogel containing cerium oxide NPs coated with interleukin-17 aptamers could decrease the level of brain inflammation significantly by reducing the expression of interleukin-17, −10 and −6 genes as well as their serum concentrations.
Nr.
Aptamer
Target
Action
1
DEK-binding
Nuclear chromatin protein DEK
Juvenile idiopathic arthritis
2
Aptamer M.G (RNA)
Acetylcholine receptors
Control of myasthenia gravis
3
ADR58 (RNA)
gp130 receptor
Control of rheumatoid arthritis
4
CD4-specific aptamer 14 (RNA)
Antigen-presenting cells
Immunosuppressant
5
DD7, ED1 (RNA)
hNE-specific ligand
Anti-inflammatory
6
IGEL1.2 and D17.4 (DNA)
Human IgE
Antiallergic response
7
LD201t1 (DNA)
L-selectin
Anti-inflammatory action
8
2′–NH2 −30-ligand (RNA)
IFN-a
Immunoregulatory
9
Spiegelmers NOX 2149
ORL-1R
Decrease in pain and stress
10
SE RNA
Hepatitis C virus NS3
Viral proliferation in chronic hepatitis
11
LIGAND 1.1
HIV-1 RT
Anti-HIV
4.3 Viral diseases
Aptamers are oligonucleotides that can be easily used as targeted agents in drug delivery and even in the development of biosensors to detect infectious agents (Eilers et al. 2020). Also, aptamers can target viral proteins involved in various stages of viral infection (Table 2) (Zou et al. 2019b). In this regard, Shiang et al. (2013) by designing an AuNPs (13 nm in diameter) containing two aptamers including RT1t49 and ODN 93 as a very effective suppressors for human immuno-deficiency virus type 1, showed that in the early stages of the HIV-lentiviral replication, the inhibitory effect of the loaded aptamers on AuNPs in the presense of the virus increases by 40.2% (Fig. 2B). Likewise, it was found that magnetic NPs containing E1E2 glycoprotein-aptamers reduce the amount of hepatitis C virus in human plasma samples with more than 91% capturing efficiency (Delaviz et al. 2015).
5 Internalization approaches of aptamers
The cellular internalization of aptamer is an important factor for development of in vivo targeted drug delivery systems to reach the targeted site without harmful side effects to off-targeted cells (Wan et al. 2019). The cellular internalization depends on some factor such as charge, size, and even the stability of aptamers. The negatively charged aptamer and cell surface lead to electrostatic repulsion. In addition, cellular internalization is usually reduced in the case of aptamers with oligonucleotides longer than 25 bases due to self-hybridized conformations (Patil et al. 2005)
Since the advent of aptamers, exploring different signaling pathways mediating their cellular internalization have received a great interest in the development of aptamer-based diagnostic and therapeutic platforms. Endocytosis is a main pathway that is used by cells to internalize different types of aptamers. Generally, four pathways comprise of phagocytosis, micropinocytosis, clathrin-mediated endocytosis (CME) and caveolae mediated-endocytosis are introduced for endocytic mechanisms to internalized aptamer from the cell surface membranes. Besides, the mechanism of cellular internalization depends on aptamer targeting the specific receptor. The analysis of the localization of fluorescently labeled transferrin is used for determining CME of aptamers.
6 Efficiency of aptamers vs. Their analogous
Although, a variety of biological agents such as peptides and antibodies are used in biochemical assays, aptamers are able to detect very specific molecules by creating a variety of secondary structures like a loop, bugle, pseudoknot, and G-quadruplex, and even three-dimensional structures. This structural diversity and other advantages listed below make the use of aptamers much more desirable than others. One of the most important advantages of using aptamers compared to other cases is the shorter production time of aptamers (1–3 months) compared to antibodies (4–6 months). Also, unlike antibodies and other proteins that require immunogenicity tests to be used in diagnostic and therapeutic processes, the use of aptamers show significantly lower immunogenicity (Dhar et al. 2020, Ni et al. 2020b). Also, unlike antibodies and other proteins that require immunogenicity tests to be used in diagnostic and therapeutic processes, the use of aptamers could have significantly lower immunogenicity (Jayasena 1999, Avci-Adali et al. 2013). On the other hand, the small size of aptamers along with flexible structures not only increases the concentration of aptamers compared to other compounds (Chen and Yang 2015), which can be more effective (5–10 times) in medical activities such as treatment (Ni et al. 2020b), but also their small size leads to more accurate tracking of biomaterials with sizes less than 60 Daltons (Zhang et al. 2014), which ultimately increases the accuracy of detection. Thus, their small size can reduce the restrictions on the penetration of compounds into tumors and the blood–brain barrier, although their small size accelerates the filtration rate of aptamers from the kidney (Chen et al. 2020). Furthermore, modification of aptamers to increase the detection accuracy in vitro through chemical synthesis is much simpler than other compounds, especially antibodies obtained through in vivo methods. Meanwhile, the production cost along with levels of impurities or contaminants of aptamers are much lower than those of other compounds. Finally, the results of the reports show that the shelf life of aptamers is much higher (∼up to 1 month) compared to other compounds and is very resistant to adverse environmental conditions such as changes of pH and temperature (Sun et al. 2014). In contrast, their level of stability in the body or tissues due to kidney filtration and nuclease activity are much lower than antibodies and other biological compounds that can be very effective in reducing aptamers toxicity. However, various results indicate that pharmacological modifications can improve the stability of aptamers for imaging and drug delivery activities (Han et al. 2019, Odeh et al. 2020).
7 Implications of aptamers in clinical trials
Despite extensive efforts to use aptamers in medical practice, their use faces numerous challenges and drawbacks. However, the first commercial and therapeutic example of the use of aptamers is Pegaptanib, which has been used to treat age-related macular degeneration (Ng et al. 2006). This aptamer acts as a vascular endothelial growth factor antagonist. However, clinical models have shown that this aptamer has no drastic effect on oncology applications. Nevertheless, successful aptamers have been developed in the treatment of cancer, such as AS1411 (a nucleolin-targeting DNA aptamer) and NOX-A12, which have shown good clinical activity (Ireson and Kelland 2006). The first aptamer has a desirable half-life due to its unique structure and using PEG (Ireson and Kelland 2006). The NOX-A12 aptamer with L-form also has a good half-life due to its high nuclear resistance (Ludwig et al. 2017). Aptamer AS1411 has shown approximately 47% success in the treatment of kidney cancer and myeloid leukemia after 4 to 6 months treatment (Soundararajan et al. 2009). However, phase II trials of this aptamer show that it needs other auxiliary biomarkers that can more effectively detect kidney tumors in all patients. Clinical activity in the field of hematologic malignancies of aptamer NOX-A12 shows that it not only reduced bone marrow niche microenvironment receptivity to multiple myeloma cells, but also effectively forbade chemotaxis of tumor cells towards CXCL12 along with reducing drug resistance by mediating adhesion in cancer cells (Ludwig et al. 2017, Waldschmidt et al. 2017). After treatment with NOX-A12 aptamer, approximately 86% of patients recovered within 16 months. However, the activity of this aptamer in the treatment of metastatic colorectal and pancreatic cancer with pembrolizumab is still under study. Summary of approved aptamers in clinical activities are presented in Table 3.
Aptamers
Nucleotide
target
Disease
Ref.
REG1
RNA
Coagulation Factor IX
Coronary Artery
(Vavalle and Cohen 2012)
E10030
DNA
PDGF
Age-Related Macular
(Jaffe et al. 2016)
NU172
DNA
Thromin
Heart
(Troisi et al. 2018)
ARC1779
DNA
vWF
Thrombotic thrombocytopenic purpura
(Mayr et al. 2010)
NOX-E36
RNA
CCL2
Type 2 Diabetes mellitus
(Oberthür et al. 2015)
BX499
RNA
Tissue Factor
Pathway InhibitorHemophilia
(Chang et al. 2012)
gp120
RNA
gp120
HIV therapy (pre-clinical)
(Shrivastava et al. 2020)
A10-3.2
RNA
PSMA
Prostate cancer (pre-clinical)
(Singh 2019)
Zimura
RNA
Anti-c5
Age-Related Macular (pre-clinical)
(Petrukhin 2020)
Rn-DsDsDs-44
DNA
Von Willebrand
Factor(pre-clinical)
(Matsunaga et al. 2017)
Kall1-T4
RNA
Kallikrein
(pre-clinical)
(Steen Burrell et al. 2017)
8 Challenges and future perspective
Despite the widespread attention in the field of using aptamers, they show several drawbacks in pre-clinical and clinical applications. In order to optimally use aptamers and reduce their side effects, it is necessary to pay special attention to these limitations as follows:
-
The rapid clearance of aptamers in the blood and other biological environments due to the activity of endonucleases has challenged the diagnostic activity or drug delivery based on aptamers. Reports indicate that changes in the conformational structure of aptamers can be very effective in increasing their shelf life (Roxo et al. 2019, Odeh et al. 2020). However, time is still considered as a limiting factor, and this reduction in retention time in medical practice is not desirable. The use of SELEX methods (Kovacevic et al. 2018) with oligonucleotides containing modified nucleotides and NPs (Mignani et al. 2020, Thevendran et al. 2020) can have a significant effect on the stability of aptamers. However, it should be noted that the use of the above methods should not adversely affect their binding to target tissues or cells.
-
One of the challenges of using aptamers to load drug compounds within the nucleus is the interaction of aptamers with cell surface receptors, which can reduce access to intracellular target molecules. To reduce this problem, it is recommended to use compounds as inducers of cell surface receptor-dependent endocytosis on the drug carrier (Engelberg et al. 2018). After intracellular penetration of drug compounds, targeted access to intracellular organs can be provided by using protected aptamers via harpins in regions the 3′- and 5′- termini (Futami et al. 2019).
-
One of the most important pathways for the removal of aptamer in the body is filtration through the kidneys due to a molecular weight of less than 50 Da (Wang et al. 2019). Therefore, therapeutic applications of aptamers without preservatives are problematic. In this regard, the use of polymers, proteins and fats to increase their molecular weight can increase the shelf life of aptamers in the blood (Jeevanandam et al. 2020).
-
Another major challenge in using aptamers for medical activities is the complex and lengthy process of their production. Also, purification of produced aptamers is a difficult process and requires complicated processes. In addition, sometimes aptamers produced by microbial methods have no interaction with human cells due to detectable epitope changes. In this regard, it is possible to produce and purify aptamers using methods with negative selection approaches through normal cells and positive selection with modified cells (Sefah et al. 2010, Catuogno and Esposito 2017). Also, it is possible to use an organism to isolate and amplify aptamers by injecting them into the bloodstream and isolating the target tissue to extract aptamers.
-
Aptamers simply interact with the target molecule, so they can easily off-interact with structures similar to the target molecule (Liu et al. 2020), which can seriously challenge aptamer-based therapeutic activities. Although negative selection with molecules similar to the target molecule can drastically reduce these drawbacks, the results of some reports suggest that cross-activity based on the type of treatment is possible.
-
The use of unnatural nucleotides can increase chemical toxicity or immune responses. For example, an experiment has shown that nucleic acids as an aptamer increase the toxicity against liver cells (Burdick et al. 2014). Therefore, it is necessary to investigate the toxicity of the aptamers used and, for example, reduce their toxicity by their modification. On the other hand, the response of immune system against aptamer and additives such as polymers have raised concerns about the use of these compounds in therapeutic activities (Lincoff et al. 2016).
Funding
This research was made possible by China Postdoctoral Science Foundation grant number “2020 M672291" and Henan Province Science and Technology attack plan project (212102310044).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Ahmed, N., Fessi, H., Elaissari, A.J.D.d.t., 2012. Theranostic applications of nanoparticles in cancer. 17, 928–934.
- Alshaer, W., Hillaireau, H., Fattal, E.J.A.d.d.r., 2018. Aptamer-guided nanomedicines for anticancer drug delivery. Advanced drug delivery reviews. 134,122–137.
- Potential capacity of aptamers to trigger immune activation in human blood. PLoS ONE. 2013;8:e68810
- [Google Scholar]
- Azhdarzadeh, M., Atyabi, F., Saei, A.A., Varnamkhasti, B.S., Omidi, Y., Fateh, M., Ghavami, M., Shanehsazzadeh, S., Dinarvand, R.J.C., Biointerfaces, S.B., 2016. Theranostic MUC-1 aptamer targeted gold coated superparamagnetic iron oxide nanoparticles for magnetic resonance imaging and photothermal therapy of colon cancer. 143, 224–232.
- Binaymotlagh, R., Haghighi, F.H., Aboutalebi, F., Mirahmadi-Zare, S.Z., Hadadzadeh, H., Nasr-Esfahani, M.-H.J.N.J.o.C., 2019. Selective chemotherapy and imaging of colorectal and breast cancer cells by a modified MUC-1 aptamer conjugated to a poly (ethylene glycol)-dimethacrylate coated Fe 3 O 4–AuNCs nanocomposite. 43, 238–248.
- Aptamers against pro-and anti-inflammatory cytokines: a review. Inflammation.. 2017;40:340-349.
- [Google Scholar]
- Sequence motifs associated with hepatotoxicity of locked nucleic acid—modified antisense oligonucleotides. Nucleic Acids Res.. 2014;42:4882-4891.
- [Google Scholar]
- Studies on the mechanism of action of the aptamer BAX499, an inhibitor of tissue factor pathway inhibitor. Thromb. Res.. 2012;130:e151-e157.
- [Google Scholar]
- Replacing antibodies with aptamers in lateral flow immunoassay. Biosens. Bioelectron.. 2015;71:230-242.
- [Google Scholar]
- Chen, D., Li, B., Cai, S., Wang, P., Peng, S., Sheng, Y., He, Y., Gu, Y., Chen, H.J.B., 2016. Dual targeting luminescent gold nanoclusters for tumor imaging and deep tissue therapy. 100, 1–16.
- Chen, H.-Y., Albert, K., Wen, C.-C., Hsieh, P.-Y., Chen, S.-Y., Huang, N.-C., Lo, S.-C., Chen, J.-K., Hsu, H.-Y.J.C., Biointerfaces, S.B., 2017. Multifunctional silver nanocluster-hybrid oligonucleotide vehicle for cell imaging and microRNA-targeted gene silencing. 152, 423–431.
- Chen, J., Wang, J., Luo, Z., Fang, X., He, L., Zhu, J., Qurat ul ain, Z., He, J., Ma, H., Zhang, H., Liu, M., He, L., 2020. Productive screening of single aptamers with ddPCR. Analyst. 145, 4130-4137.
- Dahm, R.J.H.g., 2008. Discovering DNA: Friedrich Miescher and the early years of nucleic acid research. 122, 565–581.
- Dehghani, S., Nosrati, R., Yousefi, M., Nezami, A., Soltani, F., Taghdisi, S.M., Abnous, K., Alibolandi, M., Ramezani, M.J.B., Bioelectronics. 2018. Aptamer-based biosensors and nanosensors for the detection of vascular endothelial growth factor (VEGF): A review. Biosensors and Bioelectronics. 110, 23–37.
- Aptamer-conjugated magnetic nanoparticles for the efficient removal of HCV particles from human plasma samples. RSC Adv.. 2015;5:79433-79439.
- [Google Scholar]
- Antibodies, Nanobodies, or Aptamers—Which Is Best for Deciphering the Proteomes of Non-Model Species? Int. J. Mol. Sci.. 2020;21:2485-2491.
- [Google Scholar]
- Du, W., Yuan, Y., Wang, L., Cui, Y., Wang, H., Xu, H., Liang, G.J.B.c., 2015. Multifunctional bioconjugate for cancer cell-targeted theranostics. 26, 2571–2578.
- Eilers, A., Witt, S., Walter, J., 2020. Aptamer-Modified Nanoparticles in Medical Applications.
- Ellington, A.D., Szostak, J.W.J.n., 1990. In vitro selection of RNA molecules that bind specific ligands. 346, 818–28.
- Cancer cell-selective, clathrin-mediated endocytosis of aptamer decorated nanoparticles. Oncotarget.. 2018;9:20993-20999.
- [Google Scholar]
- Genetic alphabet expansion provides versatile specificities and activities of Unnatural-Base DNA aptamers targeting cancer cells. Molecular Therapy-Nucleic Acids.. 2019;14:158-170.
- [Google Scholar]
- Gao, H., Qian, J., Cao, S., Yang, Z., Pang, Z., Pan, S., Fan, L., Xi, Z., Jiang, X., Zhang, Q.J.B., 2012a. Precise glioma targeting of and penetration by aptamer and peptide dual-functioned nanoparticles. 33, 5115–5123.
- Gao, H., Qian, J., Yang, Z., Pang, Z., Xi, Z., Cao, S., Wang, Y., Pan, S., Zhang, S., Wang, W.J.B., 2012b. Whole-cell SELEX aptamer-functionalised poly (ethyleneglycol)-poly (ε-caprolactone) nanoparticles for enhanced targeted glioblastoma therapy. 33, 6264–6272.
- Aptamer-modified gold nanoparticles for rapid aggregation-based detection of inflammation: an optical assay for interleukin-6. Microchim. Acta. 2020;187:13.
- [Google Scholar]
- Grabowska-Jadach, I., Kalinowska, D., Drozd, M., Pietrzak, M.J.B., Pharmacotherapy. 2019. Synthesis, characterization and application of plasmonic hollow gold nanoshells in a photothermal therapy—New particles for theranostics. 111, 1147–1155.
- Multivalent aptamer-modified tetrahedral DNA nanocage demonstrates high selectivity and safety for anti-tumor therapy. Nanoscale.. 2019;11:339-347.
- [Google Scholar]
- Gelatin hydrogel containing cerium oxide nanoparticles covered by interleukin-17 aptamar as an anti- inflammatory agent for brain inflammation. J. Neuroimmunol.. 2019;326:79-83.
- [Google Scholar]
- An aptamer-antibody complex (oligobody) as a novel delivery platform for targeted cancer therapies. J. Control. Release. 2016;229:1-9.
- [Google Scholar]
- Discovery and development of anticancer aptamers. Mol. Cancer Ther.. 2006;5:2957-2962.
- [Google Scholar]
- A phase 1 study of intravitreous E10030 in combination with ranibizumab in neovascular age-related macular degeneration. Ophthalmology. 2016;123:78-85.
- [Google Scholar]
- Targeting nucleolin for better survival in diffuse large B-cell lymphoma. Leukemia. 2018;32:663-674.
- [Google Scholar]
- Jalalian, S.H., Taghdisi, S.M., Hamedani, N.S., Kalat, S.A.M., Lavaee, P., ZandKarimi, M., Ghows, N., Jaafari, M.R., Naghibi, S., Danesh, N.M.J.E.J.o.P.S., 2013. Epirubicin loaded super paramagnetic iron oxide nanoparticle-aptamer bioconjugate for combined colon cancer therapy and imaging in vivo. 50, 191–197.
- Aptamers: an emerging class of molecules that rival antibodies in diagnostics. Clin. Chem.. 1999;45(9):1628-1650.
- [Google Scholar]
- Advancing Aptamers as Molecular Probes for Cancer Theranostic Applications—The Role of Molecular Dynamics Simulation. Biotechnol. J.. 2020;15:1900368-1900375.
- [Google Scholar]
- Blocking the adhesion cascade at the premetastatic niche for prevention of breast cancer metastasis. Mol. Ther.. 2015;23:1044-1054.
- [Google Scholar]
- Applications of aptamers in nanodelivery systems in cancer, eye and inflammatory diseases. Nanomedicine.. 2010;5:1435-1445.
- [Google Scholar]
- Enzyme–polymeric/inorganic metal oxide/hybrid nanoparticle bio-conjugates in the development of therapeutic and biosensing platforms. J. Adv. Res.. 2021;33:227-239.
- [Google Scholar]
- In vivo guiding inorganic nanozymes for biosensing and therapeutic potential in cancer, inflammation and microbial infections. Talanta. 2021;224:121805-121812.
- [Google Scholar]
- Polymeric micelles functionalized with cell penetrating peptides as potential pH-sensitive platforms in drug delivery for cancer therapy: A review. Arabian J. Chem. 2021 15.103264-70
- [Google Scholar]
- Pharmacokinetics, pharmacodynamics and safety of aptamers. Adv. Drug Deliv. Rev.. 2018;134:36-50.
- [Google Scholar]
- A novel PD-L1-targeting antagonistic DNA aptamer with antitumor effects. Molecular Therapy-Nucleic Acids.. 2016;5:e397
- [Google Scholar]
- Lakhin, A., Tarantul, V., Gening, L.J.A.N., 2013. Aptamers: problems, solutions and prospects. 5.1–10.
- Li, H., Mu, Y., Lu, J., Wei, W., Wan, Y., Liu, S.J.A.c., 2014. Target-cell-specific fluorescence silica nanoprobes for imaging and theranostics of cancer cells. 86, 3602–3609.
- Lincoff, A.M., Mehran, R., Povsic, T.J., Zelenkofske, S.L., Huang, Z., Armstrong, P.W., Steg, P.G., Bode, C., Cohen, M.G., Buller, C., 2016. Effect of the REG1 anticoagulation system versus bivalirudin on outcomes after percutaneous coronary intervention (REGULATE-PCI): a randomised clinical trial. The Lancet. 387, 349–356.
- Liu, Q., Jin, C., Wang, Y., Fang, X., Zhang, X., Chen, Z., Tan, W.J.N.A.m., 2014. Aptamer-conjugated nanomaterials for specific cancer cell recognition and targeted cancer therapy. 6, e95.
- Tuning Biosensor Cross-Reactivity Using Aptamer Mixtures. Anal. Chem.. 2020;92:5041-5047.
- [Google Scholar]
- Olaptesed pegol, an anti-CXCL12/SDF-1 Spiegelmer, alone and with bortezomib–dexamethasone in relapsed/refractory multiple myeloma: A Phase IIa Study. Leukemia. 2017;31:997-1000.
- [Google Scholar]
- High-affinity DNA aptamer generation targeting von Willebrand factor A1-domain by genetic alphabet expansion for systematic evolution of ligands by exponential enrichment using two types of libraries composed of five different bases. J. Am. Chem. Soc.. 2017;139:324-334.
- [Google Scholar]
- The aptamer ARC1779 blocks von Willebrand factor–dependent platelet function in patients with thrombotic thrombocytopenic purpura ex vivo. Transfusion.. 2010;50:1079-1087.
- [Google Scholar]
- Dendrimer–and polymeric nanoparticle–aptamer bioconjugates as nonviral delivery systems: a new approach in medicine. Drug Discovery Today. 2020;25(6):1065-1073.
- [Google Scholar]
- DEK-targeting DNA aptamers as therapeutics for inflammatory arthritis. Nat. Commun.. 2017;8:14252-14258.
- [Google Scholar]
- Non-viral delivery systems of DNA into stem cells: Promising and multifarious actions for regenerative medicine. J. Drug Delivery Sci. Technol.. 2020;60:101861-101871.
- [Google Scholar]
- Mosafer, J., Abnous, K., Tafaghodi, M., Mokhtarzadeh, A., Ramezani, M.J.E.J.o.P., Biopharmaceutics. 2017. In vitro and in vivo evaluation of anti-nucleolin-targeted magnetic PLGA nanoparticles loaded with doxorubicin as a theranostic agent for enhanced targeted cancer imaging and therapy. 113, 60–74.
- Pegaptanib, a targeted anti-VEGF aptamer for ocular vascular disease. Nat. Rev. Drug Discovery. 2006;5:123-132.
- [Google Scholar]
- Recent Progress in Aptamer Discoveries and Modifications for Therapeutic Applications. ACS Appl. Mater. Interfaces. 2020;13(8):9500-9519.
- [Google Scholar]
- Ni, X., Castanares, M., Mukherjee, A., Lupold, S.E.J.C.m.c., 2011. Nucleic acid aptamers: clinical applications and promising new horizons. 18, 4206-4214.
- Therapeutic Aptamers: Evolving to Find their Clinical Niche. Curr. Med. Chem.. 2020;27:4181-4193.
- [Google Scholar]
- Restraint stress elevates the plasma interleukin-6 levels in germ-free mice. J. Neuroimmunol.. 2001;115:46-52.
- [Google Scholar]
- Crystal structure of a mirror-image L-RNA aptamer (Spiegelmer) in complex with the natural L-protein target CCL2. Nat. Commun.. 2015;6:1-11.
- [Google Scholar]
- Aptamers chemistry: Chemical modifications and conjugation strategies. Molecules. 2020;25:3-9.
- [Google Scholar]
- Pascual, L., Cerqueira-Coutinho, C., García-Fernández, A., de Luis, B., Bernardes, E.S., Albernaz, M.S., Missailidis, S., Martínez-Máñez, R., Santos-Oliveira, R., Orzaez, M.J.N.N., Biology, Medicine. 2017. MUC1 aptamer-capped mesoporous silica nanoparticles for controlled drug delivery and radio-imaging applications. 13, 2495–2505.
- Patil, S.D., Rhodes, D.G., Burgess, D.J.J.T.A.j., 2005. DNA-based therapeutics and DNA delivery systems: a comprehensive review. 7, E61–E77.
- Recent Developments in Agents for the Treatment of Age-Related Macular Degeneration and Stargardt Disease. Drug Delivery Challenges and Novel Therapeutic Approaches for Retinal Diseases.. 2020;2020:125-160.
- [Google Scholar]
- Proske, D., Blank, M., Buhmann, R., Resch, A.J.A.m., biotechnology. 2005. Aptamers—basic research, drug development, and clinical applications. 69, 367–374.
- Reinemann, C., Strehlitz, B.J.S.m.w., 2014. Aptamer-modified nanoparticles and their use in cancer diagnostics and treatment. 1, 144–149.
- Röthlisberger, P., Hollenstein, M.J.A.d.d.r., 2018. Aptamer chemistry. 134, 3-21.
- G-quadruplex-forming aptamers—characteristics, applications, and perspectives. Molecules. 2019;24:3781-3788.
- [Google Scholar]
- Sefah, K., Shangguan, D., Xiong, X., O'donoghue, M.B., Tan, W., 2010. Development of DNA aptamers using Cell-SELEX. Nature protocols. 5, 1169–1175.
- Dual function of interleukin-23 Aptamer to suppress brain inflammation via attachment to macrophage stimulating 1 kinase and interleukin-23. Colloids Surf., B. 2020;185:110619-110628.
- [Google Scholar]
- Involvement of planned cell death of necroptosis in cancer treatment by nanomaterials: Recent advances and future perspectives. J. Control. Release. 2019;299:121-137.
- [Google Scholar]
- RNA aptamers directed to discrete functional sites on a single protein structural domain. Proc. Natl. Acad. Sci.. 2007;104:3742-3746.
- [Google Scholar]
- Shi, H., Ye, X., He, X., Wang, K., Cui, W., He, D., Li, D., Jia, X.J.N., 2014. Au@ Ag/Au nanoparticles assembled with activatable aptamer probes as smart “nano-doctors” for image-guided cancer thermotherapy. 6, 8754–8761.
- Highly efficient inhibition of human immunodeficiency virus type 1 reverse transcriptase by aptamers functionalized gold nanoparticles. Nanoscale.. 2013;5:2756-2764.
- [Google Scholar]
- Nucleic Acid Aptamers as a Potential Nucleus Targeted Drug Delivery System. Curr. Drug Deliv.. 2020;17:101-111.
- [Google Scholar]
- Singh, R. 2019. Repurposing Thymoquinone as Therapy for Metastatic Castration-Resistant Prostate Cancer. Morehouse School of Medicine Inc. Atlanta United States.
- Plasma membrane nucleolin is a receptor for the anticancer aptamer AS1411 in MV4-11 leukemia cells. Mol. Pharmacol.. 2009;76:984-991.
- [Google Scholar]
- A kallikrein-targeting RNA aptamer inhibits the intrinsic pathway of coagulation and reduces bradykinin release. J. Thromb. Haemost.. 2017;15:1807-1817.
- [Google Scholar]
- Oligonucleotide aptamers: new tools for targeted cancer therapy. Mol. Therapy-Nucleic Acids.. 2014;3:e182
- [Google Scholar]
- Tan, J., Yang, N., Zhong, L., Tan, J., Hu, Z., Zhao, Q., Gong, W., Zhang, Z., Zheng, R., Lai, Z.J.T., (2017). A new theranostic system based on endoglin aptamer conjugated fluorescent silica nanoparticles. 7, 4862–4869.
- Tao, W., Zeng, X., Wu, J., Zhu, X., Yu, X., Zhang, X., Zhang, J., Liu, G., Mei, L.J.T., (2016). Polydopamine-based surface modification of novel nanoparticle-aptamer bioconjugates for in vivo breast cancer targeting and enhanced therapeutic effects. 6, 470–477.
- Strategies to bioengineer aptamer-driven nanovehicles as exceptional molecular tools for targeted therapeutics: A review. J. Control. Release. 2020;323:530-548.
- [Google Scholar]
- Several structural motifs cooperate in determining the highly effective anti-thrombin activity of NU172 aptamer. Nucleic Acids Res.. 2018;46:12177-12185.
- [Google Scholar]
- Tuerk, C., Gold, L.J.S., 1990. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. 249, 505–510.
- The REG1 anticoagulation system: a novel actively controlled factor IX inhibitor using RNA aptamer technology for treatment of acute coronary syndrome. Future Cardiol.. 2012;8:371-382.
- [Google Scholar]
- CXCL 12 and CXCR 7 are relevant targets to reverse cell adhesion-mediated drug resistance in multiple myeloma. Br. J. Haematol.. 2017;179:36-49.
- [Google Scholar]
- Wan, L.-Y., Yuan, W.-F., Ai, W.-B., Ai, Y.-W., Wang, J.-J., Chu, L.-Y., Zhang, Y.-Q., Wu, J.-F.J.E.o.o.d.d., (2019). An Exploration of aptamer internalization mechanisms and their applications in drug delivery. Expert opinion on drug delivery. 16(3), 207–218.
- Three decades of nucleic acid aptamer technologies: Lessons learned, progress and opportunities on aptamer development. Biotechnol. Adv.. 2019;37:28-50.
- [Google Scholar]
- Wen, S., Miao, X., Fan, G.-C., Xu, T., Jiang, L.-P., Wu, P., Cai, C., Zhu, J.-J.J.A.s., 2019. Aptamer-Conjugated Au Nanocage/SiO2 Core-Shell Bifunctional Nanoprobes with High Stability and Biocompatibility for Cellular SERS Imaging and Near-Infrared Photothermal Therapy. ACS sensors. 4(2), 301–308.
- Yu, M.K., Kim, D., Lee, I.H., So, J.S., Jeong, Y.Y., Jon, S.J.S., 2011. Image‐guided prostate cancer therapy using aptamer‐functionalized thermally cross‐linked superparamagnetic iron oxide nanoparticles. 7, 2241–2249.
- An ensemble of aptamers and antibodies for multivalent capture of cancer cells. Chem. Commun.. 2014;50:6722-6725.
- [Google Scholar]
- Image-guided cancer therapy using aptamer-functionalized cross-linked magnetic-responsive Fe3O4@ carbon nanoparticles. Anal. Chim. Acta. 2019;1056:108-116.
- [Google Scholar]
- An RNA aptamer that interferes with the DNA binding of the HSF transcription activator. Nucleic Acids Res.. 2006;34:3755-3761.
- [Google Scholar]
- Zhou, J., Rossi, J.J.N.r.D.d., 2017. Aptamers as targeted therapeutics: current potential and challenges. 16, 181–190.
- Zou, J., Shi, M., Liu, X., Jin, C., Xing, X.-J., Qiu, L., Tan, W.J.A.c., 2019a. Aptamer-Functionalized Exosomes: Elucidating the Cellular Uptake Mechanism and the Potential for Cancer-Targeted Chemotherapy. Anal. Chem. 91(3), 2425–2430.
- Zou, X., Wu, J., Gu, J., Shen, L., Mao, L., 2019b. Application of Aptamers in Virus Detection and Antiviral Therapy. Front. Microbiol. 10, 1462–1462.







