Translate this page into:
A robust computational investigation on C60 fullerene nanostructure as a novel sensor to detect SCN−
⁎Corresponding authors. andrew.ng@xmu.edu.my (Andrew Ng Kay Lup), lotfor@ums.edu.my (Md. Lutfor Rahman)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
This study explored on the adsorption properties and electronic structure of SCN− via density functional theory analysis on the exterior surfaces of C60 and CNTs using B3LYP functional and 6-31G** standard basis set. Then adsorption of SCN− through nitrogen atom on the C60 fullerene is electrostatic (−48.02 kJ mol−1) in comparison with the C59Al fullerene that shows covalently attached to fullerene surface (−389.10 kJ mol−1). Our calculations demonstrate that the SCN− adsorption on the pristine and Al-doped single-walled CNTs are −173.13 and −334.43 kJ mol−1, indicating that the SCN− can be chemically bonded on the surface of Al-doped CNTs. Moreover, the adsorption of SCN− on the C60 surface is weaker in comparison with C59B, C59Al, and C59Ga systems but its electronic sensitivity improved in comparison with those of C59B, C59Al, and C59Ga fullerenes. The evaluation of adsorption energy, energy gap, and dipole moment demonstrates that the pure fullerene can be exploited in the design practice as an SCN− sensor and C59Al can be used for SCN− removal applications.
Keywords
C60 fullerene
Adsorption
Density functional theory
SCN
1 Introduction
Over these past decades, various carbon based structures have been found such as C60 fullerene by Kroto et al. (1985) in 1985 and SWCNTs through Iijima (1991) in 1991, encouraging scientists to have drastic attention on their chemical, physical, mechanical, and electronical phenomena of these novel materials. Due to technological availability, the most ample C60 molecule has the most widely adaptation of fullerenes. Thus, it has been of the most focused material to be under research by various chemists of their research areas, including gas storage, pharmaceutical studies, batteries, transistors, sensors and micro-electromechanical systems and sensor applications, due to its exclusive properties of unusual stability, superconductivity, ferroelectricity, non-linear optical properties, ultra-stiffness to ferromagnetism (Ren et al., 2006; Krainara et al., 2012; Sabirov et al., 2008; Liu, 2009; Rondeau-Gagne et al., 2010; Funasaka et al., 1995; Wilson, 1999; Bakry et al., 2007).
Fullerene, particularly C60, has been used as a good electron acceptor without changes to its electronic properties during its chemical bonding with other organic molecules (Liu et al., 2008; Shinohara, 2000). Hetero fullerenes have been the source of attention both experimentally and theoretically (Shi et al., 2016; Bezi Javan et al., 2010; Neyts et al., 2011). Doping inside of the fullerene molecule and substitute of heteroatoms (hetero fullerene) with one or more carbon atoms of the molecules of fullerene are two approaches of doping on C60. Abundant experimental investigations for endohedrally doped or exohedrally doped fullerenes via metal atoms were performed. Among them, the point of interest has been given to cage structures endohedrally doped with many atoms including some gases, transition metallic, and rare earth atoms (Ren et al., 2008; Murata et al., 2006; Mauser et al., 1997; Larssona and Greer, 2002). The interaction properties between gas molecules and fullerene-doped structures are known among scientists as one of the most important aspects when considering intermolecular interactions (Hernández et al., 2021). For example, the interactions of some polar molecules with endohedral metallo C80 fullerene was investigated by Jalbout et al. (Jalbout, 2009) who considered interaction energy of H2O, CH3OH, HF and NH3 molecules with endohedral metallo fullerene. Recently, Bucher et al. (Bucher, 2012) have reported the behavior of water trapped C60 fullerene. They indicated that the interaction of water with the carbon cage is weak. Also, Neyts et al. (Baei et al., 2011) have studied the interaction of dopant atom trapped inside and bound outside to the carbon cage (endo- and exohedral) or the metal atom via substituting one carbon atom. The adsorption energy of SCN− on the exterior surfaces of C, BN, BP, AlN, and AlP nanostructures have been reported (Soltani et al., 2012; Soltani et al., 2012; Soltani et al., 2014; Kanani et al., 2014; Frisch et al., 2009). Herein this work, the adsorption behavior and electronic structures of interacting SCN− towards the exterior surfaces of pristine and doped C60 nanostructures were examined to provide further insights on designing novel gas sensor materials.
2 Computational details
The equilibrium geometry, natural bond orbital (NBO), and density of states (DOS) calculations were performed (Alireza, 2012) through Gaussian 09 suite of programs (Cao et al., 2021). B3LYP level (Soltani et al., 2017; Soltani et al., 2018; Baei et al., 2014; Kia et al., 2013) was used for geometry development through the 6-31G** basis set. B3LYP/6-31G** level of theory were illustrated as a dependable and usually utilized level to evaluate of carbon fullerenes (Baei et al., 2017; Baei et al., 2017; Hassani and Tavakol, 2014). Three additional nanostructures were examined by substituting one carbon atom in C60 with one Al, Ga or B atom to form doped C60 heterofullerenes. The adsorption energies (
) of SCN− on the nanostructures of C60, C59Al, C59Ga, C59B were determined through the following equation:
3 Result and discussion
3.1 Adsorption state of SCN− on the carbon nanocages
Based on present computational analysis, the bond lengths of C1-C2 and C1-C3 in the pure C60 are about 1.395 and 1.453 Å with sp2 hybridization (Fig. 1). The outputs are similar to the results which is reported by Gallo and co-worker (Chen et al., 2021). After adsorption of SCN through N-down on carbon atom of C60 (Fig. 1b), the bond lengths of C1-C2 and C1-C3 in this complex changed to 1.511 and 1.548 Å. These results imply that the adsorption of SCN− on the wall of the pristine C60 has noticeable influence on bond lengths of carbon atoms (Peyghan et al., 2013). The most stable configuration for the SCN− adsorption (N-down) on the pristine C60 has adsorption energy of −48.06 kJ mol−1 and the interaction distance is 1.479 Å. Similarly, the most stable configuration for the SCN− adsorption (parallel position) over the pristine C60 has adsorption energy of −52.41 kJ mol−1 and the interaction distance is 2.53 Å. After the use of BSSE calculation, the values of Eads for the SCN− adsorption through N-down and parallel position on the pristine C60 have adsorption energies of −43.21 and −47.08 kJ mol−1, suggesting the nature of this the interaction in the terms of Ead has mainly electrostatic character. Upon the adsorption of SCN− with C60 fullerene (Fig. 1c), the average distances of C1-C2 and C1-C3 change to sp3 hybridization of lengths 1.51 and 1.54 Å while the bond lengths of C—N and C—S upon the adsorption of SCN− on the wall of fullerene is 1.186 and 1.607 Å, respectively. For this system, the results of NBO analysis exhibits that there is large charge transfer (0.70 e) from SCN− to the surface of C60 fullerene. Moreover, the computed adsorption energy of SCN− toward the wall of fullerene is obviously higher than those on the surfaces of CNT and BNNT (Soltani et al., 2012; Soltani et al., 2012). The previous report represents that the value of
for the SCN− on the perfect (6, 0) BNNT was −148 kJ mol−1 (Soltani et al., 2012) and it increased on the outer surfaces of the AlPNT (−318.163 kJ mol−1), AlNNT (−262.145 kJ mol−1), and BPNT (−255.845 kJ mol−1) (Soltani et al., 2014). It is observed from the outputs that the adsorption behavior of SCN− on the fullerene surface is weak electrostatic interaction. While the
value for the SCN− on BNNT, BPNT, AlPNT, and AlNNT surfaces are chemisorption and a covalent bound is expected to produce through interaction of each configuration with SCN− (Soltani et al., 2014; Frisch et al., 2009). Our last investigation on the effect of doping atoms on chemical interaction encouraged us to scrutinizing it on fullerene (Figs. 2 and 3).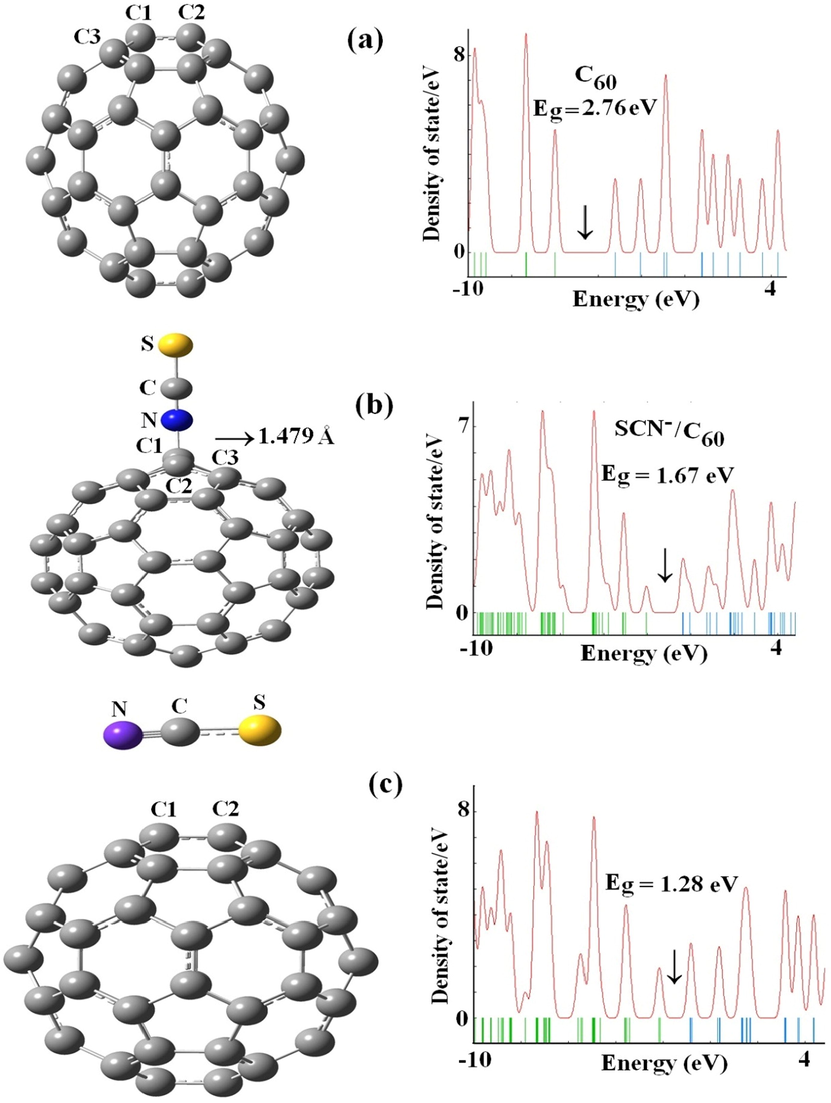
The relaxed structures and density of states for the pure C60 and SCN− interacting with C60 systems in different positions.
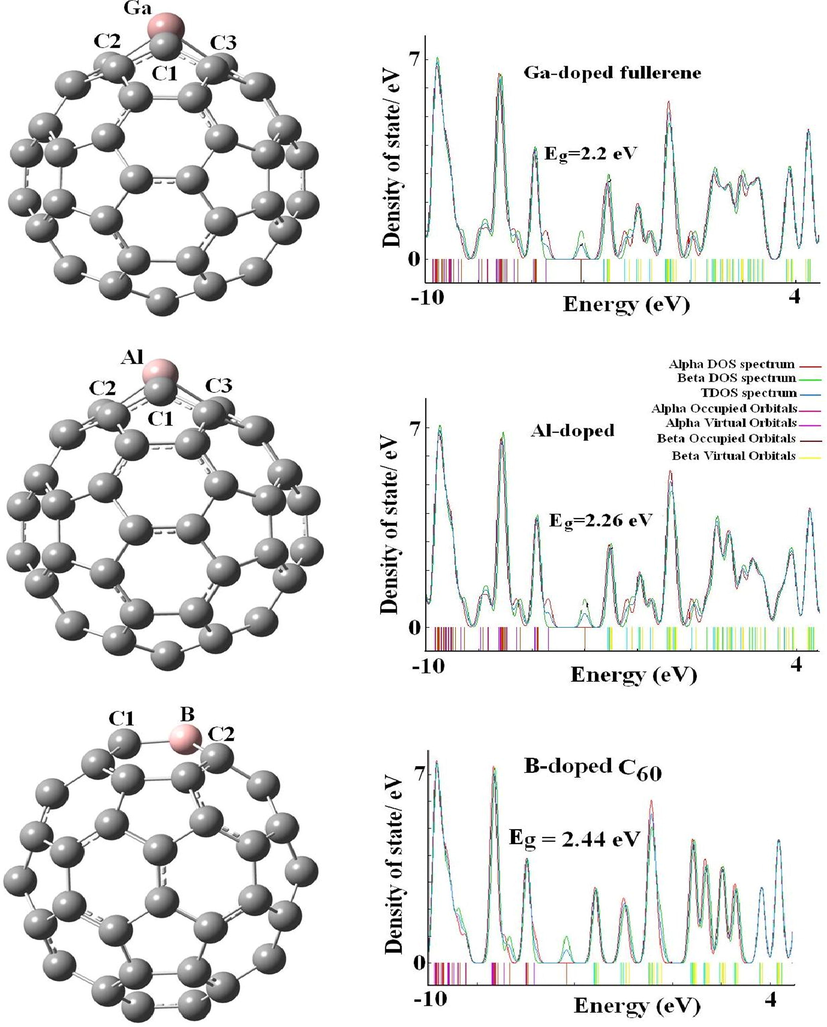
The relaxed structures and density of states for the pure Ga-, Al-, and B-C59 systems.
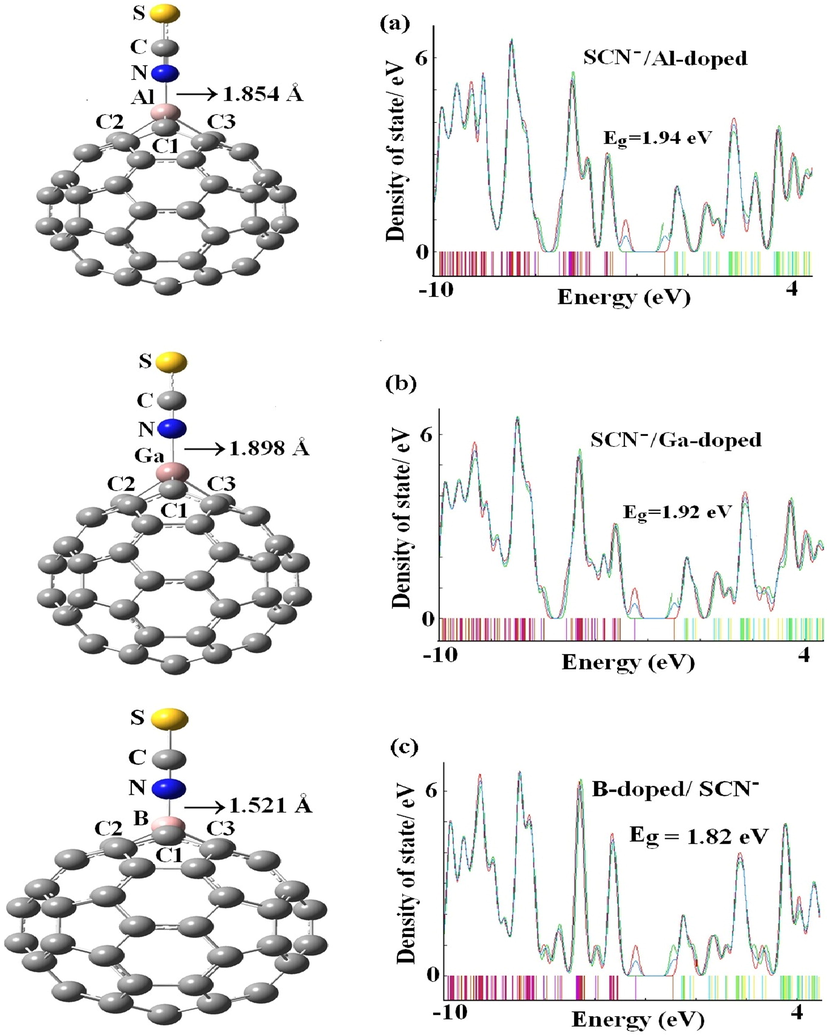
The relaxed structures and density of states for the most stable state (N-down) of (a) SCN−/C59Al, (b) SCN−/C59Ga, and (c) SCN−/C59B systems.
The computations of SCN− corresponding to C59B, C59Al, and C59Ga fullerenes confirmed our expectation with adsorption amount of −258.28, −389.10, and −347.71 kJ mol−1 respectively, with the interaction distances of 1.52, 1.85, and 1.90 Å (Figs. 2 and 3). Outcomes verifying a chemisorption process as SCN− close to C59B, C59Al, and C59Ga fullerenes. The adsorption and interaction distance of SCN− through S-down attached to C59Al fullerene in order are −308.11 kJ mol−1 and 2.31 Å. In contrast, the increment in the interaction of SCN− with C59B, C59Ga, and C59Al fullerenes can be ascribed to the strong hybridization between dopant p orbitals of fullerene and the nitrogen p orbitals of anion. Moreover, the doping of Al can further enhance the adsorption of SCN− onto the fullerene in comparison with the B- and Ga dopant atoms (Einert et al., 2021). These days, different significant approaches like functionalization, doping and size-dependent were applied in order to enhance or control the attributes of substances (Kurban et al., 2021; Muz et al., 2022; Muz et al., 2020; Olea Ullo et al., 2018; Soltani et al., 2016, 1105). To better understand the adsorption results, we performed the charge analysis which shows a strong charge transfer of approximately 0.50, 0.32, and 0.29
from the SCN− to the C59B, C59Ga, and C59Al fullerenes. The analysis of NBO reveals that more ionic-bond-type process of electron transfer ameliorates the adsorption of SCN− upon the C59B, C59Ga, and C59Al fullerenes. The strong chemical adsorption between the SCN− and the C59B, C59Ga and C59Al fullerenes leads to elongated bond lengths of B-C, Ga-C, and Al-C from 1.548, 1.887, and 1.884 Å to 1.616, 1.942, and 1.950 Å after the adsorption processes, respectively. The calculation thus exhibits that adsorbed N atom of SCN− on C59B, C59Ga, and C59Al fullerenes had strong covalent bonds. The evaluation of our previous study (adsorption of SCN− on the pure and Al-doped SWCNTs) exhibited that the single point energy (SPE) cannot purvey significant validity because of the lack of geometry relaxation of complexes provided (Soltani et al., 2012). According to our calculations (Fig. 4), the adsorption of SCN− through N-down on the pure and Al-doped SWCNTs are −173.13 and −334.43 kJ mol−1, suggesting that the SCN− can be chemically bonded on the surface of Al-doped CNTs. Based on SPE calculations, the adsorption of SCN− on the pure and Al-doped SWCNTs are found to be −26.08 and −286.38 kJ mol−1, respectively (Soltani et al., 2012). On the SCN− adsorption with SWCNT (Fig. 4a), the average distances of C1-C2 and C1-C3 shift to sp3 hybridization of lengths 1.42 and 1.44 Å while the bond lengths of C—N and C—S upon the adsorption of SCN− on the wall of nanotube in order is 1.185 and 1.604 Å (Ramezanitaghartapeh et al., 2022).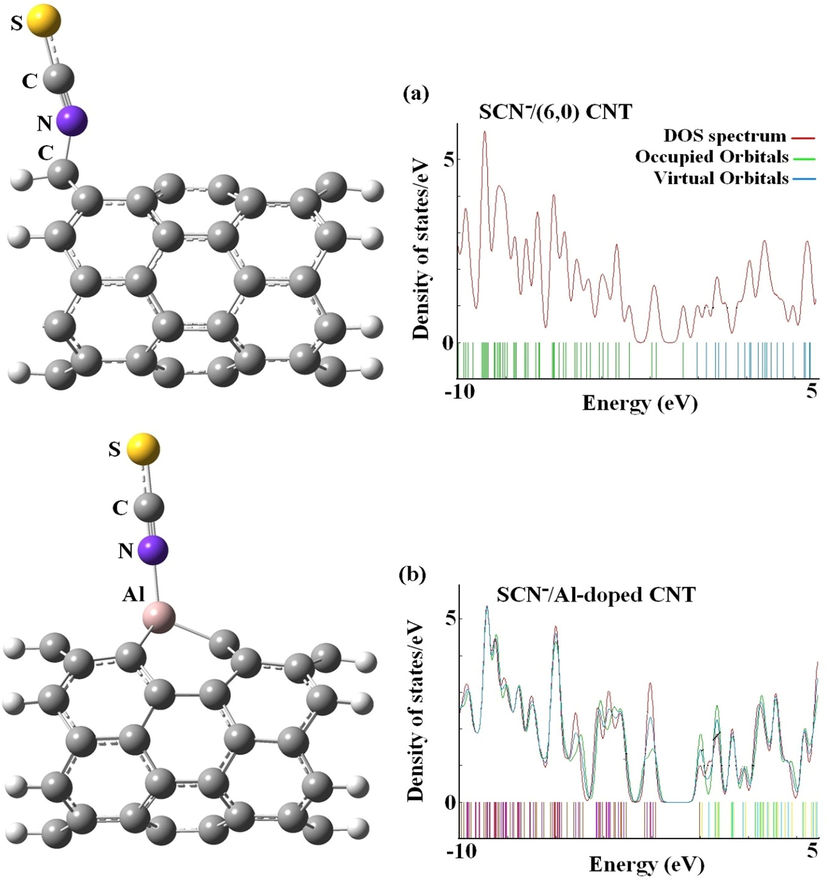
The relaxed structures and density of states for the most stable state (N-down) of (a) SCN−/(6,0) SWCNT and (b) SCN−/Al-SWCNT systems.
3.2 Electronic features
Considering the HOMO (high occupied molecular orbital) and the LUMO (low unoccupied molecular orbital), the most effective HOMO and LUMO (Rimadani, 2020) distribution plots for every form are proven in Fig. 5. The electro-positive and electro-negative densities, illustrated in order by the green and red colors, also are determined as almost equivalent in every shape.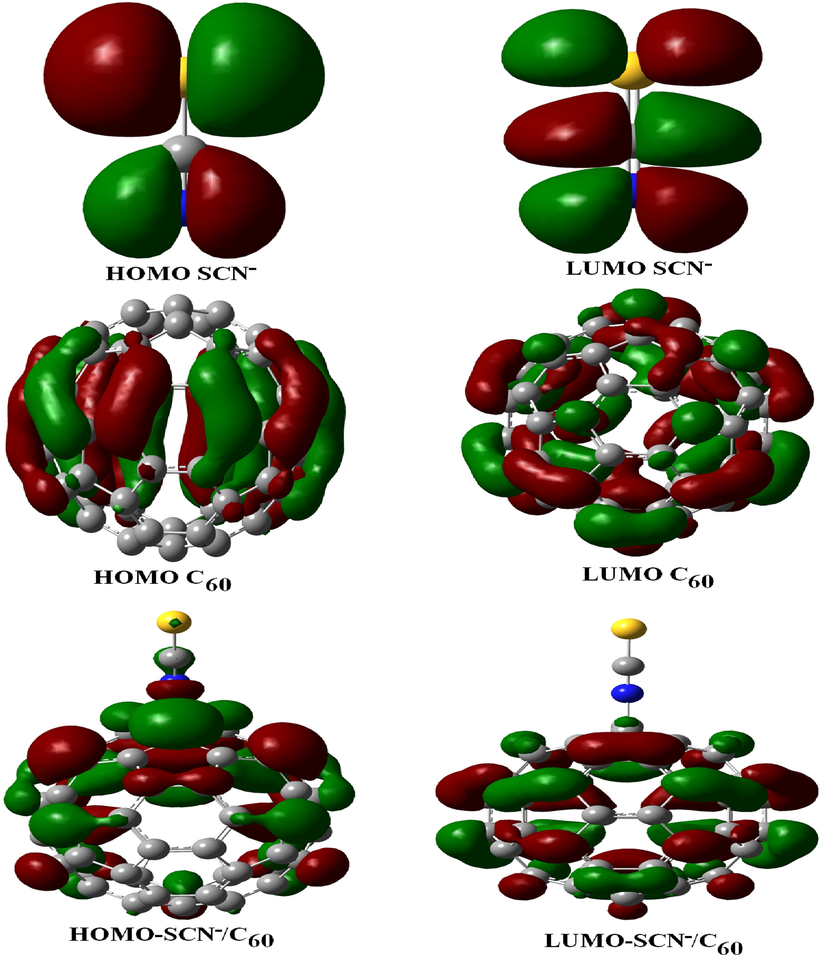
Charge distribution of HOMO and LUMO orbitals upon the pure SCN−, C60, and, SCN− adsorbed on C60.
Fig. 5 exhibits that the HOMO for SCN−/C60 is slightly located upon the S—C—N orbital and is uniformly distributed upon the carbon atoms of C60 in energy level of −2.05 eV. The LUMO is more located on the carbon–carbon orbitals in the center of the C60 in energy level of −0.38 eV (Mohammad, 2014). In contrast, the HOMO of SCN− is more resided on the carbon atoms in the center and near of the C59Al, C59Ga, and C59B, and is slightly located on electronegative nitrogen atoms of SCN− anion (Fig. 6) in energy levels of −2.45, −2.51, and −2.39 eV, while the LUMO orbitals for whole configurations are more dispensed upon the carbon–carbon bonds of fullerene cage in order in energy level of −0.51, −0.59, and −0.57 eV (Table 2). Fig. 7 presents the molecular electrostatic potential (MEP) map with an isodensity surface of 0.02 a.u. for pure C60 fullerene (Gassoumi, 2022). Analysis of MEP represents that the blue color of the pristine C60 fullerene acts as electron-rich regions and the red color acts as the electron-poor regions (Avramopoulos et al., 2016).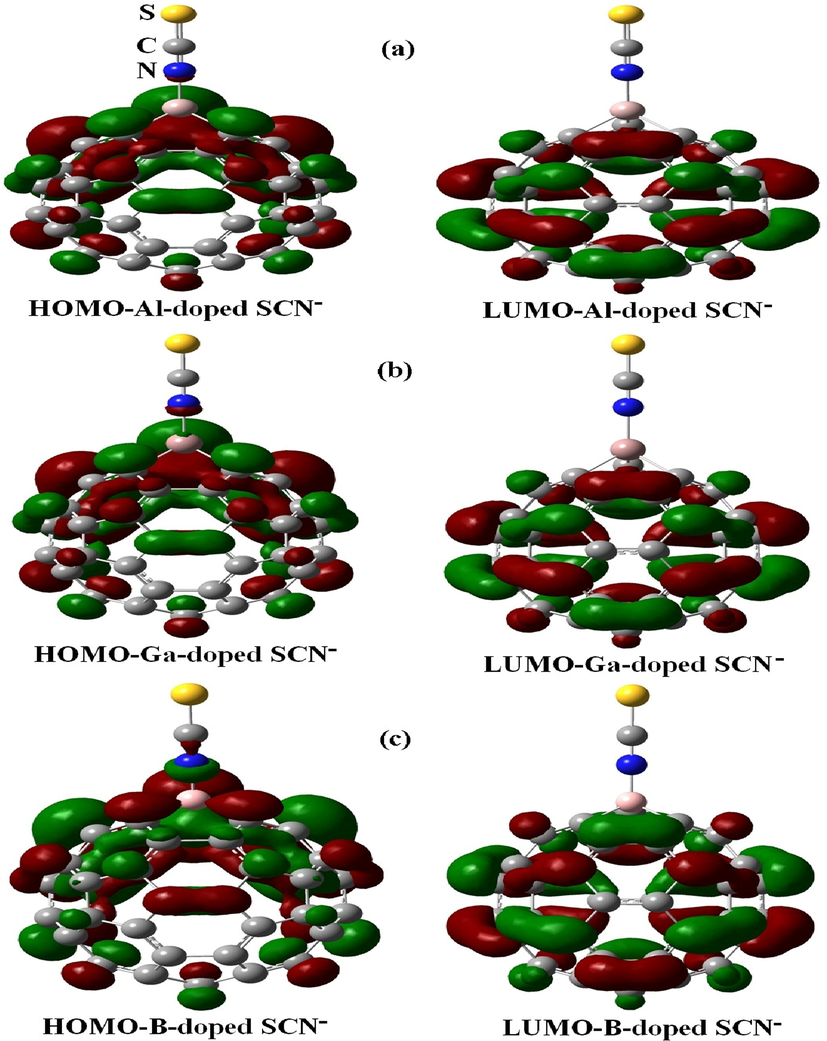
Charge distribution of HOMO and LUMO orbitals upon the pure Ga-, Al-, and B-C59 loaded with one SCN−.
Property
SCN−
C60
SCN−/C60(N-down)
SCN−/C60(Parallel)
EHOMO/eV
−5.38
−5.99
−2.05
−2.14
ELUMO/eV
−3.12
−3.23
−0.38
−0.86
[ELUMO − EHOMO]/eV
2.26
2.76
1.67
1.28
μD/Debye
2.64
0.00
8.27
34.40
EF/eV
−4.25
−4.61
−1.21
−1.50
ΔEg/eV
–
–
1.09
1.48
Property
AlC59
SCN−/C59Al
C59Ga
SCN−/C59Ga
C59B
SCN−/C59B
EHOMO/eV
−5.38
−2.45
−5.44
−2.51
−5.66
−2.39
ELUMO/eV
−3.12
−0.51
−3.24
−0.59
−3.22
−0.57
[ELUMO − EHOMO]/eV
2.26
1.94
2.20
1.92
2.44
1.82
μD/Debye
2.64
18.35
1.61
30.01
0.573
19.75
EF/eV
−4.25
−1.48
−4.34
−1.55
−4.44
−1.48
ΔEg/eV
–
0.32
–
0.28
–
0.62
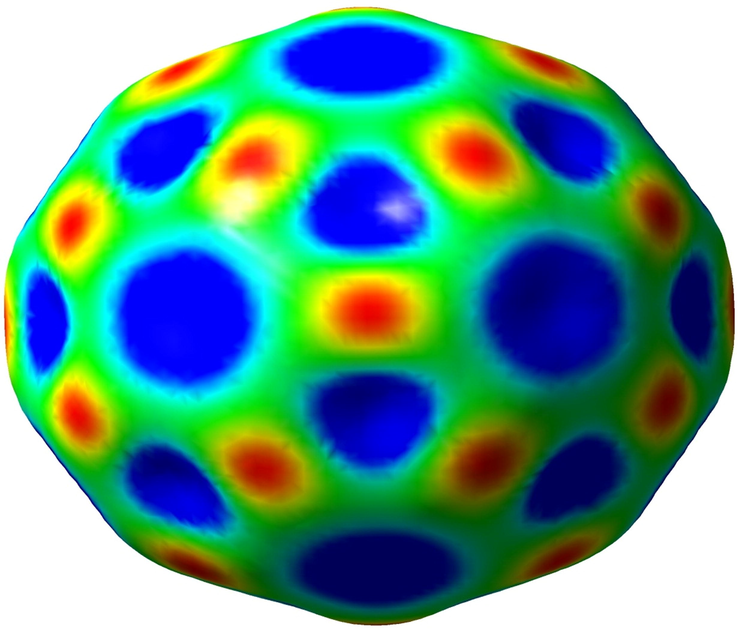
MEP map of C60 fullerene.
As it is clear from the Table 1 and 2, there is a significant diminish in energy gaps from C60 to C59Al, C59B, and C59Ga fullerenes from 2.76 eV for C60 to 2.26, 2.20, and 2.44 eV for C59Al, C59B, and C59Ga fullerenes (Avramopoulos et al., 2016; Bahrami et al., 2014). The diminish is due to approaching of SCN− to the exterior walls of C59Al, C59B, and C59Ga fullerenes with high reduced placing at SCN−/C59B complex from 2.44 eV for basic stage to 1.82 eV for mixture plot, as gap energy for C59Al and C59Ga fullerenes in order are 1.94 and 1.92 eV. Also, the value of Eg is 2.67 eV for the mixture of SCN− and C60. Therefore, the reactivity of C59Al, C59B, and C59Ga fullerenes would be increased as SCN− approaches to the aforementioned configurations. Therefore, unlike the C59Al and C59Ga fullerenes, the B-doping improves the sensitivity of the fullerene interacting with the SCN− anion. The reduction of Eg improves the complexes electrical conductivity, which can then be converted to an electrical signal by C59B for SCN−detection (Bahrami et al., 2014) (Javarsineh, 2018).
C60 has icosahedral symmetry (Ih point group) and composed of sixty carbon atoms (Soltani et al., 2012). The symmetry of the C59Al, C59B, and C59Ga fullerenes is Cs, so their symmetry is not similar than for the C60 case, as SCN− have C∞V symmetry. Besides, the point groups of SCN− on C60, C59Al, C59B, and C59Ga fullerenes are C1. NBO analysis demonstrates that the electronic configurations of C59Al and C59Ga models are [Ne] 3 s0.333p0.39 and [Ar] 4 s0.384p0.37, while SCN−/C59Al and SCN−/C59Ga models are of [Ne] 3 s0.253p0.42 and [Ar] 4 s0.634p0.99, respectively. Hence, the more reactivity has taken place the more charge transfer has occurred for SCN− adsorbed on C60, C59Al, C59B, and C59Ga fullerenes. A substantial characteristic that demonstrates the charge distribution when gases are adsorbed to the system is the dipole moment (μD) vector of species (Alireza, 2012). As a gas approaches or remotes from a fullerene, alteration of the size and direction of the μD is predictable. The results of the μD for whole configurations are scrutinized and speculated that total μD upraised drastically as SCN− approaches to varied configuration of fullerene. Our calculations suggest that the dipole moment (μD) value for pure C60 and SCN− are 0.00 and 2.64 Debye, respectively that with the adsorption of SCN− in the positions of N-down and parallel both complexes in order increased to 8.27 and 34.40 Debye (Table 1). The μD values are found to be 1.61, 2.64, and 0.57 for C59Al, C59Ga, C59B fullerenes, respectively. While it significantly increased as a result of SCN− closeness to the outer surface of considered configurations, with 18.35, 30.01, and 19.75 Debye for C59Al, C59Ga, C59B fullerenes (Table 2). To observe the adsorption behavior in these complexes, the DOS of SCN−/C60 complex in comparison with other complexes including SCN−/C59Al, SCN−/C59Ga, and SCN−/C59B was analyzed. As depicted in Figs. 1 and 3, the DOS of practical complexes are remarkably altered when the SCN− adsorbed upon the surface of fullerene while it is more noticeable in pure model (parallel position). These data illustrate that DOS close the Fermi level (EF) are influenced by the SCN− and C60, C59Al, C59Ga, and C59B. Moreover, the great charge transfer is probable as extracted from EF outcomes revealing as basic amount of −4.61 eV for the pure C60 to −1.21 eV and −1.50 eV for SCN− through N-down and parallel positions on the surface of C60 fullerene, respectively. The values of EF for the pure C59Al, C59Ga, and C59B are changed from −4.25, −4.34, and −4.44 eV to −1.48, −1.55, and −1.48 eV for SCN− interacting with C59Al, C59Ga, and C59B systems, respectively. Our findings introduce fullerene and its doped models as high sensitivity materials to SCN− that resemble a gas sensor.
3.3 Scrutinizing of quantum molecular descriptor for SCN−
Results of Quantum Molecular Descriptor (QMD) represents a decrement on energy gap (Eg: [ELUMO − EHOMO]) that results in ionization potential increase from 2.05 eV for SCN− approaching to pure C60 to 2.45 and 2.51 eV for C59Al and C59Ga respectively, and consequently raise in global hardness (η) from 0.83 eV for SCN− on the pristine complex to 0.97 and 0.96 eV for C59Al and C59Ga when SCN− is added to them. Similar plot is predictable for electron affinity (A) with 0.51 and 0.59 eV for SCN− on the surfaces of the C59Al and C59Ga fullerenes from basic amount of 0.38 eV for SCN− approaching to perfect C60. Chemical potential (μ) increased from 1.21 eV for pure matrix of SCN/C60 to 1.48 and 1.55 eV for SCN/C59Al and SCN/C59Ga complexes. An increase in electrophilicity index (ω) for SCN−/Ga-doped C59 complex is noticeable than that of SCN/C59Al complex with certain amount of 1.25 eV. Considering softness (S), it has decreased from 0.60 eV at SCN−/C60 complex to 0.51 eV and 0.52 eV for SCN/C59Al and SCN/C59Ga complexes, respectively (see Table 3).
Property
SCN−
C60
SCN−/C60
C59Al
SCN−/C59Al
C59Ga
SCN−/C59Ga
I/eV
0.41
5.99
2.05
5.38
2.45
5.44
2.51
A/eV
−7.41
3.23
0.38
3.12
0.51
3.24
0.59
η/eV
3.91
1.38
0.83
1.13
0.97
1.10
0.96
µ/eV
−3.50
−4.61
−1.21
−4.25
−1.48
−4.34
−1.55
S/eV−1
0.13
0.36
0.60
0.44
0.51
0.45
0.52
ω/eV
1.57
7.69
0.89
7.99
1.13
8.56
1.25
ΔNmax/a.u.
1.65
−3.34
−1.46
3.76
−1.52
3.86
−1.61
4 Conclusion
SCN− adsorption capacity on outer surface of perfect C60 in comparison with C59B, C59Al, and C59Ga fullerenes have been investigated through density functional theory calculations. Then interaction of the SCN− with C59Al is covalent in nature with the strong adsorption energy about −389.10 kJ mol−1. These results indicate that the chemisorption of SCN− on surface of C59Al with a strong charge transfer about 0.29 e. Our computation study shows that the adsorption property of SCN− on the C59Al surfaces is most notable as compared to C59Ga and C59B for SCN− storages. Moreover, the eligible C59Al nano-cage has significant energetic endurances and slightly larger energy gaps than the dopant Ga and B atoms. Therefore, the presence of C59B, C59Al, and C59Ga fullerenes can lower the electrophilicity, ionization potential, energy gaps and then upraise the softness or global hardness with the adsorption properties of SCN−.
Acknowledgements
–Rami M. Alzhrani would like to acknowledge Taif University Researchers Supporting Project Number (TURSP-2020/209), Taif University, Taif, Saudi Arabia.
–The authors would like to thank the Deanship of Scientific Research at Umm Al-Qura University for supporting this work by Grant Code :(22UQU4290565DSR98).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Ab initio investigation of Al- and Ga-doped single-walled boron nitride nanotubes as ammonia sensor. Applied surface science 2012
- [CrossRef] [Google Scholar]
- Ab initio investigation of Al- and Ga-doped single-walled boron nitride nanotubes as ammonia sensor. applied surface science 2012
- [CrossRef] [Google Scholar]
- A Computational Study of the Interaction and Polarization Effects of Complexes Involving Molecular Graphene and C60 or a Nucleobases. J. Phys. Chem. A. 2016;120(2):284-298.
- [Google Scholar]
- Adsorption properties of SCN- on (6,0), (7,0), (8,0), and Al-doped (6,0) zigzag single-walled carbon nanotubes: A density functional study. Monatshefte. 2011;142:979-984.
- [Google Scholar]
- Al12N12 nanocage as a potential sensor for phosgene detection. J. Chem.. 2014;92:605.
- [Google Scholar]
- Structural and Electronic Properties of XY-Doped (AlN, AlP, GaN, GaP) C58 Fullerenes: a DFT Study. Russ. J. Inorg. Chem.. 2017;62:1067-1076.
- [Google Scholar]
- Interaction of Pure and Metal Atom Substituted Carbon Nanocages with CNCl: a DFT Study, Russian. J. Phys. Chem. B. 2017;11:354-360.
- [Google Scholar]
- A DFT study on doping assisted changing of B80 electronic structure: Promising candidates for NH3 sensor. Sens. Actuators, B. 2014;191:457-463.
- [Google Scholar]
- Influence of 3d transitionmetals (Fe, Co) on the structural, electrical and magnetic properties of C60 nano-cage. Phys. B. 2010;405:4937-4942.
- [Google Scholar]
- Orientational relaxation of water trapped inside C60 fullerenes. Chem. Phys. Lett.. 2012;534:38-42.
- [Google Scholar]
- Investigations of adsorption behavior and anti-cancer activity of curcumin on pure and platinum-functionalized B12N12 nanocages. J. Mol. Liquids. 2021;334
- [Google Scholar]
- Penicillamine functionalized B12N12 and B12CaN12 nanocages act as potential inhibitors of proinflammatory cytokines: A combined DFT analysis, ADMET and molecular docking study. Arab. J. Chem.. 2021;14(7):103200.
- [Google Scholar]
- Aromaticity Survival in Hydrofullerenes: The Case of C66H4 with Its π-Aromatic Circuits. Chem. Eur. J.. 2021;27(2):802-808.
- [Google Scholar]
- Quasi-homogenous photocatalysis of quantum-sized Fe-doped TiO2 in optically transparent aqueous dispersions. Sci. Rep.. 2021;11:17687.
- [Google Scholar]
- Gaussian 09, Revision A01. Wallingford, CT: Gaussian, Inc.; 2009.
- Magnetic properties of Gd@C82 metallofullerene. Chem. Phys. Lett.. 1995;232:273-277.
- [Google Scholar]
- Carbon-carbon bonds functioning as electron shuttles: The generation of electron-rich manganese(II) Schiff base complexes and their redox chemistry. J. Am. Chem. Soc.. 1997;119:5144-5154.
- [Google Scholar]
- Spectroscopic characterization, host-guest charge transfer, Hirshfeld surfaces, AIM-RDG and ELF study of adsorption and chemical sensing of heavy metals with new derivative of Calix [4]quinone: A DFT-D3 computation. Materials Chemistry and physics 2022
- [Google Scholar]
- A DFT, AIM and NBO study of adsorption and chemical sensing of iodine by S-doped fullerenes. Sens. Actuators, B. 2014;196:624-630.
- [Google Scholar]
- Physical-chemical properties of (Znm)@C60 and (Znm)@C70 endohedral metallofullerenes (m = 1–5): A DFT approach. Surf. Interfaces. 2021;23:101012.
- [Google Scholar]
- Endohedral metallo [80] fullerene interactions with small polar molecules. Comput. Mater. Sci.. 2009;44:1065-1070.
- [Google Scholar]
- A Computational Study on the Purinethol Drug Adsorption on the AlN Nanocone and Nanocluster. Scientific reports 2018
- [Google Scholar]
- Adsorption mechanism of single OCN- and SCN− upon single-walled BP nanotubes. Phys. E. 2014;59:66-74.
- [Google Scholar]
- A first-principles study of functionalized clusters and carbon nanotubes or fullerenes with 5-Aminolevulinic acid as vehicles for drug delivery. Superlatt. Microstruct.. 2013;62:251-259.
- [Google Scholar]
- Interaction of adenine Cu (II) complexes with BN-doped fullerene differentiates electronically equivalent tautomers. Chem. Phys. Lett.. 2012;537:88-93.
- [Google Scholar]
- Effect of Mg content on electronic structure, optical and structural properties of amorphous ZnO nanoparticles: A DFTB study. J. Non-Crystall. Solids. 2021;560 120726(1–6)
- [Google Scholar]
- Phosphorous trapped within buckminsterfullerene. J. Chem. Phys.. 2002;116:7849-7854.
- [Google Scholar]
- Heterofullerene molecules C58X (X = S, Se, Te): A DFT study. Chem. Phys. Lett.. 2009;471:116-121.
- [Google Scholar]
- DFT study of a heterofullerene molecule containing fifty-eight carbon atoms and one sulphur atom. Chem. Phys.. 2008;353:19-24.
- [Google Scholar]
- Stabilization of atomic nitrogen inside C60. Angew. Chem. Int. Ed. Engl.. 1997;36:2835-2838.
- [Google Scholar]
- Computational study of OCN−chemisorption over AlN nanostructures. Superlattices and Microstructures 2014
- [Google Scholar]
- Synthesis and properties of endohedral C60 encapsulating molecular hydrogen. J. Am. Chem. Soc.. 2006;128:8024-8033.
- [Google Scholar]
- 3d-transition metals (Cu, Fe, Mn, Ni, V and Zn)-doped pentacene π-conjugated organic molecule for photovoltaic applications: DFT and TD-DFT calculations. Theor. Chem. Acc.. 2020;139:23.
- [Google Scholar]
- A density functional theory study on favipiravir drug interaction with BN-doped C60 heterofullerene. Physica E. 2022;135:114950.
- [Google Scholar]
- A density-functional theory simulation of the formation of Ni-doped fullerenes by ion implantation. Carbon. 2011;49:1013-1017.
- [Google Scholar]
- Formation of Coinage-Metal···Fullerene Adducts. Evaluation of the Interaction Nature between Triangular Coinage Metal Complexes (M3 = Cu, Ag, and Au) and C60 through Relativistic Density Functional Theory Calculations. J. Phys. Chem. C. 2018;122(43):25110-25117.
- [Google Scholar]
- Adsorption of CO molecule on AlN nanotubes by parallel electric field. J. Mol. Model.. 2013;19(2):859-870.
- [Google Scholar]
- Sustainable cyanide-C60 fullerene cathode to suppress the lithium polysulfides in a lithium-sulfur battery. Sustain. Mater. Technol.. 2022;32:e00403.
- [Google Scholar]
- Endohedral complex of fullerene C60 with tetrahedrane, C4H4@C60. J. Mol. Graph. Model.. 2008;27:558-562.
- [Google Scholar]
- State of hydrogen molecules confined in C60 fullerene and carbon nanocapsule structures. Carbon. 2006;44:397-406.
- [Google Scholar]
- Reactivity and Stability of Metalloporphyrin Complex Formation: DFT and Experimental Study. MDPI 2020
- [Google Scholar]
- Synthesis, characterization and DFT calculations of new ethynyl-bridged C60 derivatives. Tetrahedron. 2010;66:4230-4242.
- [Google Scholar]
- Ozone addition to C60 and C70 fullerenes: A DFT study. J. Mol. Graph. Model.. 2008;27:124-130.
- [Google Scholar]
- Structural, Electronic and Adsorption Characteristics of Transition Metal doped TM@C70 Endohedral Fullerenes. Communications biology 2020
- [Google Scholar]
- A tumor-specific cleavable nanosystem of PEG-modified C60@Au hybrid aggregates for radio frequency-controlled release, hyperthermia, photodynamic therapy and X-ray imaging. Acta Biomater.. 2016;29:282-297.
- [Google Scholar]
- A first-principles study of the SCN- chemisorption on the surface of AlN, AlP, and BP nanotubes. Appl. Surf. Sci.. 2012;259:637-642.
- [Google Scholar]
- Ab initio investigation of the SCN- chemisorption of single-walled boron nitride nanotubes. Appl. Surf. Sci.. 2012;258:9536-9543.
- [Google Scholar]
- Pahlevani: The study of SCN- adsorption on B12N12 and B16N16 nano-cages. Superlatt. Microstruct.. 2014;75:716-724.
- [Google Scholar]
- The interaction of 2,6-dichlorobenzylidene-2,4-dichloroaniline (2,6-DBDA) and 2,4-dichlorobenzylidene-2,4-dichloroaniline (2,4-DBDA) with single-walled carbon nanotube: A DFT study. J. Mol. Struct.. 2016;1105:128-134.
- [Google Scholar]
- Interaction of hydrogen with Pd- and co-decorated C24 fullerenes: Density functional theory study. Synth. Met.. 2017;234:1-8.
- [Google Scholar]
- Serine adsorption through different functionalities on the B12N12 and Pt-B12N12 nanocages. Mater. Sci. Eng., C. 2018;92:216-227.
- [Google Scholar]
- Improvement of anti-inflammatory and anticancer activities of poly(lactic-co-glycolic acid)-sulfasalazine microparticle via density functional theory, molecular docking and ADMET analysis. Arab. J. Chem.. 2022;15:103464.
- [Google Scholar]
- Improved anti-inflammatory and anticancer properties of celecoxib loaded zinc oxide and magnesium oxide nanoclusters: A molecular docking and density functional theory simulation. Arab. J. Chem.. 2022;15
- [Google Scholar]
- How to face COVID-19: proposed treatments based on remdesivir and hydroxychloroquine in the presence of zinc sulfate. Docking/DFT/POM structural analysis. Joiurnal of Biomolecular Structure and dynamics 2021
- [CrossRef] [Google Scholar]
- Electrostatic interaction assisted Ca-decorated C20 fullerene loaded to anti-inflammatory drugs to manage cardiovascular disease risk in rheumatoid arthritis patients. J. Mole. Liquids. 2022;350
- [Google Scholar]
- Medical applications of fullerenes and metallofullerenes. Electrochem. Soc. Interface. 1999;8:24-28.
- [Google Scholar]







