Translate this page into:
A simple and efficient synthesis of substituted 2H-1-benzopyran-2-ones using natural acids and their bio evaluation
⁎Corresponding author. sgbhuna@hau.ac.in (Susheel Gulati)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
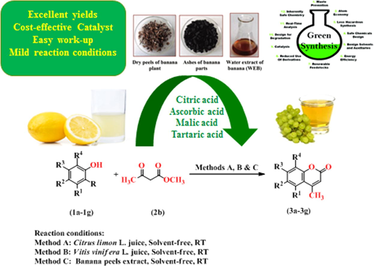
A simple and efficient route for the synthesis of coumarin derivatives (3a-3g) from reaction between substituted phenols (1a-1g) and methyl acetoacetate (2b) in presence of Citrus limon L. juice, Vitis vinifera L. juice and banana peels extract has been carried out. The homogeneity of the compounds were routinely checked by thin layer chromatography and melting points reported are uncorrected. The compounds (3a-3g) were characterized by using 1HNMR and FTIR spectral techniques and evaluated for in vitro herbicidal activity against Raphanus sativus L. (Radish seeds). The compounds (3a-3g) were also screened for their fungicidal activity against Rhizoctonia solani and Colletotrichum gloeosporioides by poisoned food techniques. Antibacterial activity was also determined against Erwinia cartovora and Xanthomonas citri by inhibition zone method. From activity data, it was found that compounds 3a and 3b were most active against Raphanus sativus L. (root) and Raphanus sativus L. (shoot) respectively. Compound 3b was found most active against R. solani fungus and Xanthomonas citri bacterium at highest concentration. Compound 3e has shown maximum percentage inhibition i.e. 83.17 against C. gloeosporioides at 2000 µg/mL concentration. Erwinia cartovora bacterium was most susceptible to compound 3 g giving 8.00 mm inhibition zone at 2000 µg/mL concentration. Less reaction time, excellent yields, simple work-up, cost effective and mild reaction conditions are some merits of present protocol.
Keywords
Coumarins
Citrus limon L. juice
Vitis vinifera L. juice
Banana peels extract
1 Introduction
Recently organic chemists are attracted toward the development of greener and more eco-friendly procedures for synthesis of biological active heterocyclic molecules (Fiorito et al., 2016). The application of fruits juice, vegetables juice and waste waters from agricultural and industrial processes (Pal et al., 2013; Pranamik and Pathan, 2014; Bakht, 2015) being environmentally benign, non-toxic, easily available and inexpensive makes a natural choice to chemist towards the synthesis of heterocyclic functionality. A wide range of biological activities such as antimicrobial, antithrombotic, anticoagulants, antipsoriasis, anticancer, anti-HIV, antioxidant, antiproliferative, inhibitory on viral proteases, estrogen like-effects and central nervous system modulating (Costova et al., 1993; Mitra et al., 1998; Bravic et al., 1968; Palmer et al., 2002; Palmer and Josephs, 1995; Taniguchi et al., 1999; Nettleton, 1996; Jacquot et al., 2003; Noeldner et al., 1996) shown by coumarins (2H-1-benzopyran-2-ones). Coumarins compounds are the important class in the domain of natural products and organic synthesis. They possess an oxygen heterocyclic benzopyrones system belonging to lactone family. Coumarins have been synthesized by various reactions including Pechmann, Perkin, Knoevenagel, Reformatsky and Witting reactions. Among these reactions, the Pechmann reaction most widely used method in which substituted phenols react to 1,3-dicarbonyl compounds in presence of acidic reagents such as H2SO4, P2O5, FeCl3, ZnCl2, POCl3, AlCl3, HCl, phosphoric acid and trifluoroacetic acid (Li et al., 1998). The Knoevenagel condensation is generally catalyzed by bases including pyridine, piperidine, sodium ethoxide and ammonia. Recently, metal oxides nanoparticles and many ionic liquids have been applied in Knoevenagel reaction to production of coumarins. However, most of these methods suffer from various limitations including the use of toxic catalysts, long reaction times, high cost, environmental toxicity, high purity requirements and not easy to work up. The use of these toxic acids is not environmentally friendly. With increasing environmental concerns, the development of waste minimized organic synthesis has become crucial and demanding area of organic chemist. Therefore, in this paper we reported waste minimized, simple and efficient method of synthesis of substituted coumarin derivatives via one-pot reaction between substituted phenols and 1,3-dicarbonyl compounds in presence of fruit juices viz. Citrus limon L. juice, Vitis vinifera L. juice and Banana peels extract.
2 Experimental
All chemicals and solvent used were of analytical grade. Melting points were determined on Ganson electric melting point apparatus and are uncorrected. The progress of the reaction was monitored by (TLC) thin layer chromatography. The 1HNMR spectra were recorded in CDCl3 or DMSO‑d6 using tetra methyl silane (TMS) as internal reference on “Brucker Ac 400F“(400 MHz) nuclear magnetic resonance spectrometer. The chemical shifts values were expressed in delta (parts per million). The following abbreviations correlate with the multiplicity of NMR signals: s = singlet, d = doublet, t = triplet, m = multiplet and brs = broad singlet. Infrared spectra (4000–350 cm−1) of the synthesized compounds were recorded in KBr pellets on Perkin Elmer FT-IR-R2X spectrophotometer and frequency was quoted in cm−1.
3 Bioevaluation
3.1 Herbicidal activity
Solutions of 50 µg/ mL, 100 µg/ mL, 150 µg/ mL and 200 µg/ mL of the test compounds in DMSO were prepared. Agar powder (5 g) was put into boiling distilled water (1L) until it dissolved, and then cooled down to 40–50 °C. The solution (2 mL) containing test compounds and melting agar (18 mL) was mixed and this mixture was added to a Petridish with 4.5 cm diameter. The agar plate without test compound was used as an untreated control. Then 15 seeds of Raphanus Sativus L. (Radish) were put on the surface of the agar plate. The Petridishes were covered with glass lids, and the cultivation conditions were kept at 25 ± 1 °C and 12 h in light and 12 h in dark alternating for seven days. Seven days later, the root lengths and shoot lengths of Raphanus sativus L. were measured. The growth inhibitory rate related to untreated control was determined by given formula (Roe et al., 1997).
% inhibition =
3.2 Antifungal activity
Amongst the several methods available, poisoned food technique (Groves and Moore, 1962) which is the most common was used for testing antifungal activity. The test fungus was grown on Potato dextrose agar medium. The required amount of synthesized compounds dissolved in 1 mL of DMSO was incorporated aseptically into 99 mL aliquots of sterilized potato dextrose agar cooled at 45o C after brief shaking. Each lot of medium was poured into Petri dishes and allowed to solidify. 1 mL DMSO in media was taken as control. Each dish was inoculated centrally with a 5 mm mycelial disc cut from the periphery of 2–3 days old fungal colonies. Inoculated Petri plates were incubated in the dark 25 ± 2 °C for 48–72 h and colony diameters were measured periodically till the control dishes were nearly completely covered with fungus growth. Three replicates were used for each concentration of a chemical together with three dishes containing only the solvent and no toxicant. The degree of inhibition of growth was calculated from the mean differences between treatments and the control as percentage of latter by using the formula.
% inhibition =
Control = mycelial growth in control dish
Treated = mycelial growth in treated dish
3.3 Antibacterial activity
The inhibition zone method (Thornberry, 1950) was followed for screening the synthesized compounds for their antibacterial activity. The bacterial suspension was prepared from 48 h old culture. The bacterial growth from five slants was taken and mixed in 100 mL sterilized distilled water aseptically. The medium was melted and cooled at 45 °C, needed medium was poured aseptically in sterilized Petri plates and rotated gently for even distribution of the medium and was allowed to solidify. 250, 500, 1000 and 2000 µg/mL concentrations of synthesized compounds were prepared from the stock solution by taking appropriate amount and diluting with DMSO. The circular paper discs of 10 mm diameter were prepared from Whatman’s Filter paper No. 1. The disc were kept in Petri plate and autoclaved at 15 lbs pressure 20 min. Two paper discs were used for each concentration of the synthesized compounds. The excess of solution absorbed by paper discs was removed by holding them vertically by sterile forecep. Such soaked discs were transferred aseptically to Petri plates containing media and bacterial suspension spread over the surface. Each concentration and chemical was replicated 3 times. Such Petri plates were inverted and kept at 5 °C for two hour for better diffusion of the chemicals in agar medium. Later, on the Petri plates were incubated at 25 ± 2 °C for 48 h. The zone of inhibition for each concentration of the chemicals was recorded in mm after 48 h of incubation.
3.4 Statistical analysis
The experiments were performed in triplicates for each treatment and the mean value were recorded and expressed as mean ± S.D. The descriptive statistics in form of box-and-whisker diagram were also presented in this paper. The spacing between the different parts of the box indicates the degree of dispersion and skewness in the data.
3.5 Composition and preparation of green catalyst
General procedure for extraction of Citrus limon L. juice: The main component of lemon juice are moisture (85%), carbohydrate (11.2%), citric acid (5–7%), protein (1%), ascorbic acid or vitamin-C (0.5%), fat (0.9%), minerals (0.3%), fibers (1.6%) and some other organic acids. Due to presence of citric and ascorbic acids (Vitamin C) in lemon juice, it acts as acid catalyst in organic synthesis. Fresh lemon was cut using knife and then pieces were pressed in a fruit juicer to get the juice extract. Then the juice was filtered through cotton and then through whatman filter paper no 1 to remove solid material, to get clear juice which was used as a catalyst (Pal, 2013).
Method for preparation of Vitis vinifera L. juice: Fresh grapes were purchased from the local market. Then washed thoroughly under running tap water followed by rinsing thrice with distilled water. Grapes were squeezed and juice were strained initially through a muslin cloth then passed through whatman filter paper No. 1 (Zia et al., 2016).
Method of preparation of Banana peels extract: The main ingredient of banana peels are starch (3%), crude protein (6–9%), crude fat (3.8–11%), dietary fibre (43.2–49.7%), lignin (6–12%), pectin (10–12%), cellulose (7.6–9.6%) and Hemicellulose (6.4–9.4%). The water extract of banana peels has been synthesized by drying the banana peels in sunlight at open atmosphere; burning the banana peels to ash at 300 °C for about 30 min until complete combustion has occurred. Then 5 g of banana peels ash was suspended in 100 mL distilled water in beaker and stirred well for 15 min at room temperature. The mixture was then filtered through whatman filter paper no 1 to remove solid material and to get clear extract which used as a catalyst (Bagul et al., 2017).
3.6 General method for the preparation of substituted coumarins derivatives (3a-3 g)
3.6.1 By Citrus limon L. Juice (Method A)
A mixture of substituted phenols (1a-1 g) (20 mmol), methyl acetoacetate (2b) (20 mmol) and Citrus limon L. juice (6 mL) was taken in round-bottom flask and stirred at room temperature. The completion of reaction was monitored by TLC. The separated solid was filtered and washed with cold water to get the product (3a-3 g), which was further recrystallized with ethylacetate and then characterized by 1H NMR and FTIR spectroscopy.
3.6.2 By Vitis vinifera L. Juice (Method B)
A mixture of substituted phenols (1a-1g) (20 mmol), methyl acetoacetate (2b) (20 mmol) was taken in round-bottom flask. To this mixture Vitis vinifera L. juice (10 mL) was added and resulting mixture was stirred at room temperature. The completion of reaction was monitored by TLC. The separated solid was filtered and washed with cold water to get the product (3a-3g), which was further recrystallized with ethylacetate and then characterized by 1H NMR and FTIR spectroscopy.
3.6.3 By Banana peels extract (Method C)
A mixture of substituted phenols (1a-1g) (20 mmol), methyl acetoacetate (2b) (20 mmol) and water extract of banana peels (8 mL) was taken in round-bottom flask and stirred at room temperature. The completion of reaction was monitored by TLC. The separated solid was filtered and washed with cold water to get the product (3a-3g), which was further recrystallized with ethylacetate and then characterized by 1H NMR and FTIR spectroscopy.
All the coumarin derivatives (3a-3g) were prepared according to Method A, B and C.
4 Results and discussion
The synthesis of coumarin derivatives (3a-3g) were carried out by reaction between 3,5-Dimethylphenol (1a), 4-Chloro-3,5-dimethylphenol (1b), 2,5-Dimethylphenol (1c), 2,3-Dimethylphenol (1d), 2,6-Dimethylphenol (1e), 1-Naphthol (1f), 4-Bromophenol (1g) and methyl acetoacetate (2b) in presence of Citrus limon L. juice, Vitis vinifera L. juice, Banana peels extract (Scheme 1). The progress of reaction was monitored by thin layer chromatography using Hexane: Ethyl acetate (80:20, v/v) as an eluent.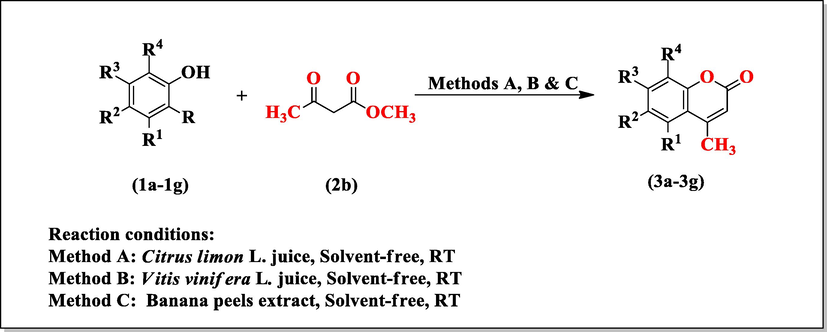
Synthesis of substituted coumarins derivatives (3a-3g).
To find the most favourable reaction conditions, we selected the 3, 4-Dimethylphenol (20 mmol) and methyl acetoacetate (20 mmol) as model substrate. Initially, the reaction was carried out using solvent-free conditions in presence of Citrus limon L. juice. It was observed that when amount of Citrus limon L. juice was taken 6 mL, the yield of product was 87% and reaction was completed in ten minutes (Table 1, Entry 3). This result encourage us to explored the same model reaction in a solvent-free medium at room temperature in presence of Vitis vinifera L. juice and Banana peels extract (Table 2, Entry 1–4) and maximum yield of product i.e. 86% and 82% was obtained when concentration of Vitis vinifera L. juice and Banana peels extract was 10 mL and 8 mL respectively in reaction mixture and reaction time was also reduced (Table 2, Entry 3–4). After completion of the reaction, the solid products were collected by simple filtration and then recrystallized with ethylacetate to afford pure coumarin derivatives (3a-3g) as shown in Fig. 1. The physical data of substituted coumarin derivatives (3a-3g) was shown in Table 3. The structure of synthesized compounds was confirmed by 1HNMR, FTIR spectral analysis as well as comparison of their melting points with those of reported compounds. The 1HNMR spectrum of compound 4,5,7-trimethyl-2H-1-benzopyran-2-one (3a) in CDCl3, displayed a singlet at 2.24 δ for methyl group attached at position 4 integrating three protons. The protons at C-5 position were appeared as singlet at 2.30 δ. The most distinguishing C3-H proton appeared as singlet at 6.63 δ while the protons at position C-6 observed downfield as doublet at 6.69 δ. The appearance of doublet at 7.04 δ integrating for proton was assigned to C8-H. The compound (3a) also showed IR absorptions at 3357.6, 1720.5, 1525.7 and 1121.0 cm−1 indicating the presence of C = CH, C = O, C = C aromatic and C-O respectively. The 1HNMR spectrum of compound 6-chloro-4,5,7-trimethyl-2H-1-benzopyran-2-one (3b) in DMSO‑d6, exhibited two singlets at 2.24 δ for methyl group at C-4 and C-5 position respectively integrating six protons. The most diagnostic C3-H proton appeared as singlet at 6.57 δ. The compound (3b) showed IR absorptions at 3322.6, 1700.5, 1588.2, 1024.3 and 858.7 cm−1 indicating the presence of C = CH, C = O, C = C aromatic, C-O and C-Cl respectively. The 1HNMR spectrum of compound 4,5,8-trimethyl-2H-1- benzopyran-2-one (3c) in CDCl3, showed a singlet at 2.14 δ for methyl group attached at position 4 integrating three protons. The protons at C-5 position were appeared as singlet at 2.23 δ. The most characteristic C3-H proton appeared as singlet at 6.66 δ while the protons at position C-6 observed downfield as doublet at 6.51 δ. The appearance of doublet at 6.93 δ integrating for proton was assigned to C7-H. The 1HNMR spectrum of compound 4,7,8-trimethyl-2H-1- benzopyran-2-one (3d) in CDCl3, exhibited a singlet at 2.23 δ of methyl group attached at position 4 integrating three protons. The protons at position C-5 and C-6 observed downfield as doublet at 6.68 δ and 7.03 δ respectively. The protons at C-7 position were appeared as singlet at 2.30 δ. The most indicative C3-H proton appeared as singlet at 6.63 δ. The comparison of activity of different catalysts with respect to time and yield of reaction as shown in Table 4. It was found that Citrus limon L. juice, Vitis vinifera L. juice, Banana peels extract gives the best catalytic activity in terms of product yield, reaction conditions and reaction time as compared to other catalyst in literature viz. KF/Al2O3, Triethylbenzylammonium chloride (TEBA), Cellulose sulfuric acid, 2,4,6-Trichloro-1,3,5-triazine, p-TSA, and ZrOCl2. The catalyst used in present study is nature derived, easily available and cost effective which makes this procedure eco-friendly. The possible mechanism for the formation of substituted coumarin derivatives (3a-3g) is shown in Scheme 2. According of this mechanism first of all there is ring formation which involves the trans esterification, followed by nucleophilic attack of the phenol ring at the carbonyl group and subsequent dehydration in presence of Citrus limon L. juice, Vitis vinifera L. juice and Banana peels extract give coumarin derivatives (3a-3g).
Entry
Catalyst Concentration (mL)
Method A
Time (min)
Yield (%)
1
2.0
80
58
2
4.0
40
74
3
6.0
10
87
Entry
Catalyst Concentration (mL)
Method B
Method C
Time (h)
Yield (%)
Time (h)
Yield (%)
1
4.0
14
70
8.5
70
2
6.0
11
54
8
86
3
8.0
9
74
7
82
4
10.0
7
86
6
66
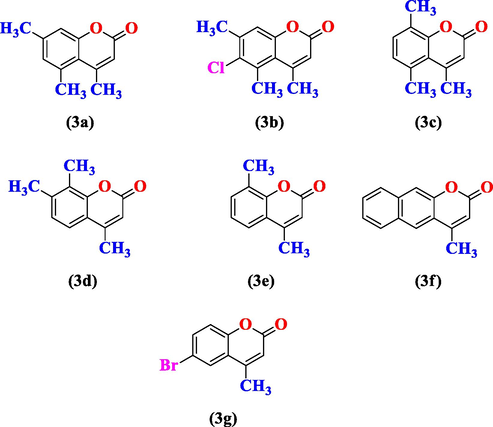
Substituted coumarins derivatives (3a-3g).
S. No
Product
R1
R2
R3
R4
Method A
Method B
Method C
m.p. (°C)
Time (min)
Yield (%)
Time (h)
Yield (%)
Time (h)
Yield (%)
1
3a
CH3
H
CH3
H
10
82
7
83
8
80
179–181 (Lit.179–180)(Bahekara and Shinde, 2004)
2
3b
CH3
Cl
CH3
H
10
86
6
81
8
78
165–167
3
3c
CH3
H
CH3
H
20
81
6
78
4
84
173–176
4
3d
H
H
CH3
CH3
10
82
8.5
80
8
82
173–175
5
3e
H
H
H
CH3
30
74
4
82
7
84
144–145
6
3f
Ph
Ph
H
H
20
85
7
86
6
75
184–185
7
3 g
H
Br
H
H
30
75
6
81
7.5
81
162–163
S.No.
Catalyst
Solvent
Temperature (°C)
Time (h)
Yield (%)
Reference
1
KF/Al2O3
Ethanol
80
–
83
Li et al., 2005
2
Triethylbenzylammonium chloride (TEBA)
Water
90
5
86
Wang et al., 2005
3
Cellulose sulfuric acid
Solvent free
RT, Grinding
0.5
96
Wang et al., 2005
4
2,4,6-Trichloro-1,3,5-triazine
Solvent free
120
2.5
93
Zhang et al., 2008
5
p-TSA
Water
90
0.5
83
Nagarapu et al., 2012
6
ZrOCl2
CH3CN
80
8
20
Mirjafary et al., 2009
7
ZrOCl2
DMSO
120
8
No reaction
Mirjafary et al., 2009
8
Citrus limon L. juice
Solvent free
RT
10 min
87
Present work
9
Vitis vinifera L. juice
Solvent free
RT
7
86
Present work
10
Banana peels extract
Solvent free
RT
7
82
Present work
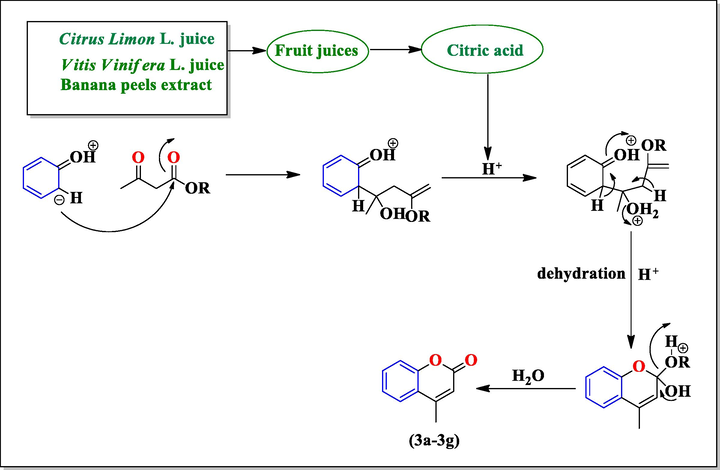
Possible mechanism for synthesis of substituted coumarins (3a-3g).
5 Characterization data of selected compounds
4,5,7-trimethyl-2H-1-benzopyran-2-one (3a): m.p. 179–181 °C [ Lit. 179–180 °C, Bahekara and Shinde (Bahekara and Shinde, 2004)]; 1H NMR (400 MHz, CDCl3): δ 2.24 (s, 3H, C4-CH3); 2.30 (s, 3H, C5-CH3); 6.63 (s, 1H, C3-H); 6.69 (d, 1H, C6-H); 7.04 (d, 1H, C8-H); IR (νmax cm−1) (neat): 3357.6 (C = CH), 1525.7 (C = C, aromatic), 1121.0 (C-O), 1720.5 (C = O)
6-chloro-4,5,7-trimethyl-2H-1-benzopyran-2-one (3b): m.p. 165–167 °C; 1H NMR (400 MHz, DMSO‑d6): δ 2.24 (s, 3H, C4-CH3); 2.24 (s, 3H, C5-CH3); 6.57 (s, 1H, C3-H); IR (νmax cm−1) (neat): 3322.6 (C = CH), 1700.5 (C = O); 1588.2 (C = C, aromatic), 1024.3 (C-O), 858.7 (C-Cl)
4,5,8-trimethyl-2H-1-benzopyran-2-one (3c): m.p. 173–176 °C; 1H NMR (400 MHz, CDCl3): δ 2.14 (s, 3H, C4-CH3); 2.23 (s, 3H, C5-CH3); 6.66 (s, 1H, C3-H); 6.51 (d, 1H, C6-H); 6.93 (d, 1H, C7-H)
4,7,8-trimethyl-2H-1-benzopyran-2-one (3d): m.p. 173–175 °C; 1H NMR (400 MHz, CDCl3): δ 2.23 (s, 3H, C4-CH3); 2.30 (s, 3H, C7-CH3); 6.63 (s, 1H, C3-H); 6.68 (d, 1H, C5-H); 7.03 (d, 1H, C6-H)
5.1 Herbicidal activity
All compounds (3a-3 g) were screened for herbicidal activity against Raphanus sativus L. at 200, 150, 100 and 50 µg/mL concentrations (Table 5). Results were shown in the form of primary screening. All compounds were diluted to 1000 µg/mL concentration as a stock solution. Herbicidal activities of compounds were evaluated against Raphanus sativus L. by inhibitory effect of compounds on the growth of weed roots and shoots. The percentage of inhibition growth was calculated from mean differences between treated and control. From the herbicidal activity results, we observed that compound 3a was exhibited maximum percentage growth inhibition i.e. 96.60 against Raphanus sativus L. (root) whereas compound 3b was exhibited maximum percentage growth inhibition i.e. 96.92 against Raphanus sativus L. (shoot) respectively at 200 µg/mL concentration. The growth inhibition may be attributed to substitution of methyl and chloro groups on phenyl ring. The box plot and graphical representation of herbicidal activity of all synthesized compounds (3a-3 g) against Raphanus sativus L. were shown in Figs. 2-5
All values are mean ± S.D.
Compounds
Growth Inhibition (%)
Root
Shoot
50 (µg/mL)
100 (µg/mL)
150 (µg/mL)
200 (µg/mL)
50 (µg/mL)
100 (µg/mL)
150 (µg/mL)
200 (µg/mL)
3a
73.33 ± 0.03
83.33 ± 1.50
90.00 ± 0.80
96.60 ± 0.60
75.38 ± 0.60
84.61 ± 2.05
87.69 ± 1.52
92.30 ± 2.71
3b
53.33 ± 0.03
70.00 ± 0.50
83.33 ± 0.08
90.00 ± 1.50
76.92 ± 0.92
87.69 ± 2.07
92.30 ± 2.00
96.92 ± 1.53
3c
42.12 ± 0.12
60.59 ± 0.70
75.36 ± 0.06
89.38 ± 0.50
40.12 ± 1.50
55.54 ± 1.99
70.58 ± 1.46
88.22 ± 1.38
3d
60.00 ± 0.02
73.33 ± 0.03
86.66 ± 0.10
93.33 ± 0.66
76.92 ± 1.14
83.07 ± 2.00
87.69 ± 1.55
90.76 ± 1.24
3e
63.33 ± 0.03
76.66 ± 0.39
90.00 ± 2.02
96.66 ± 1.20
78.46 ± 1.65
84.61 ± 3.48
92.30 ± 0.96
96.92 ± 1.18
3f
39.38 ± 0.03
51.33 ± 0.08
70.59 ± 0.59
84.32 ± 0.32
35.36 ± 1.51
49.98 ± 1.83
65.61 ± 1.51
82.22 ± 2.64
3 g
51.32 ± 0.02
62.35 ± 0.05
78.58 ± 0.58
91.33 ± 0.90
48.39 ± 1.94
63.63 ± 1.21
74.69 ± 3.33
89.36 ± 2.66
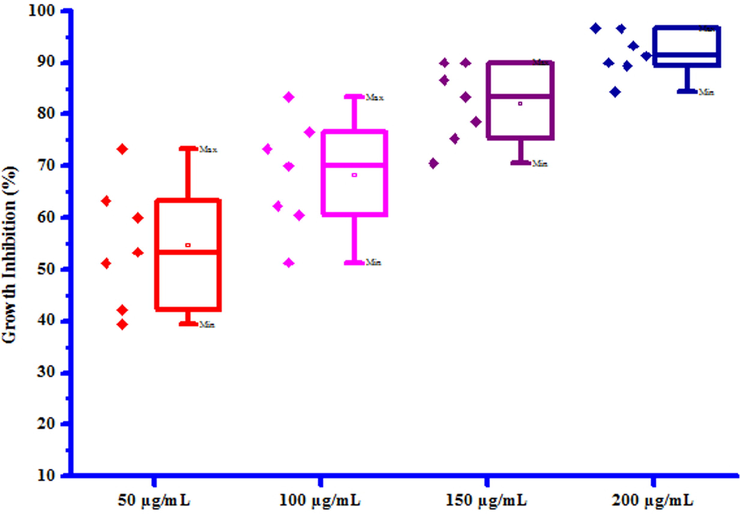
Box plot of substituted coumarins (3a-3g) against Raphanus sativus L. (root).
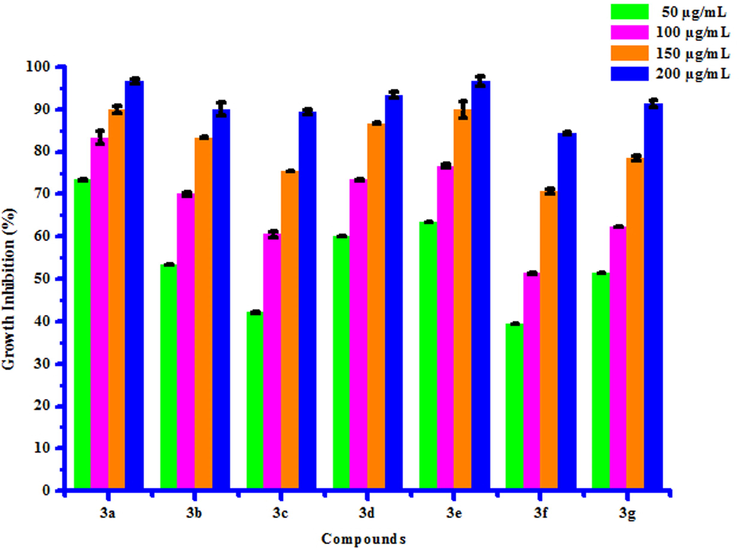
Herbicidal activity of substituted coumarins (3a-3g) against Raphanus sativus L. (root).
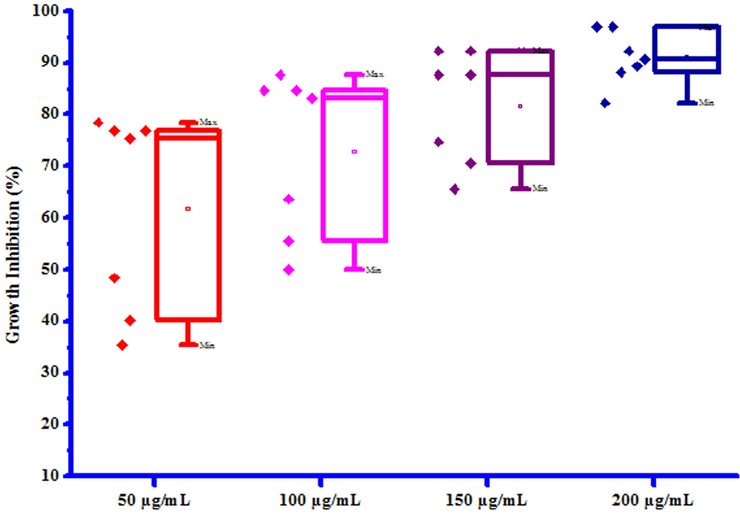
Box plot of substituted coumarins (3a-3g) against Raphanus sativus L. (shoot).
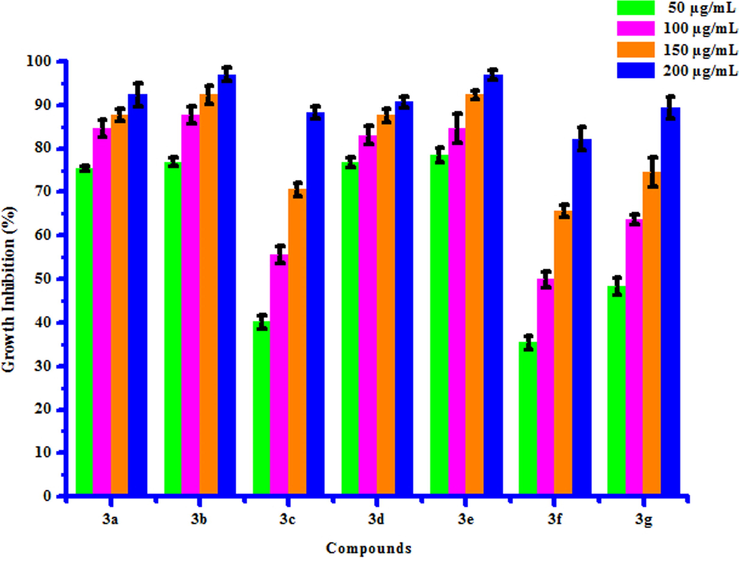
Herbicidal activity of substituted coumarins (3a-3g) against Raphanus sativus L. (shoot).
6 Antifungal activity
All compounds (3a-3 g) were tested for their in vitro antifungal activity against Rhizoctonia solani and Colletotrichum gloeosporioides. The percentage growth inhibition of compounds against R. Solani and C. Gloeosporioides is presented in Table 6. DMSO was used as control against both the test fungi. A culture of test fungi was grown on Potato Dextrose Agar (PDA) medium at ambient temperature (25 ± 2 °C). The stock solution (2000 µg/mL) of test compounds were prepared in DMSO and further dilutions were made to 1000, 500 and 250 µg/mL concentrations and stored at 4 °C for further use. Potato Dextrose Agar Media, containing specific concentration of test compounds was poured on Petri plates. After solidification, small disc (0.5 cm diameter) of the fungus culture was cut with a sterile cork borer and transferred aseptically upside down in centre of Petri plate. Petri plates were incubated in BOD incubator at 25 ± 2 °C. Growth of fungal colony was measured after every 24 h till the fungus in control plates (containing DMSO) completely occupied it. From the fungicidal activity results, we concluded that compounds 3b and 3e was found to be most likely against R. solani and C. gloeosporioides respectively. This result may be due to presence of chloro and methyl groups on phenyl ring. The box plot and graphical representation of antifungal activity of all synthesized compounds (3a-3 g) against Rhizoctonia solani and Colletotrichum gloeosporioides were shown in Fig. 6-9
All values are mean ± S.D. a: No Growth inhibition.
Compounds
Growth inhibition (%)
Fungi
Rhizoctonia solani (conc.) µg/mL
Colletotrichum gloeosporioides (conc.) µg/mL
250
500
1000
2000
250
500
1000
2000
3a
23.68 ± 1.50
35.87 ± 2.58
52.80 ± 0.98
77.85 ± 0.95
11.80 ± 0.92
21.09 ± 1.65
37.68 ± 1.24
58.96 ± 0.52
3b
30.58 ± 2.94
52.24 ± 2.76
72.57 ± 2.52
85.67 ± 2.16
a
a
a
a
3c
12.92 ± 1.63
25.98 ± 2.31
48.78 ± 1.27
69.37 ± 3.13
17.64 ± 1.63
33.98 ± 2.03
54.80 ± 1.20
78.18 ± 0.56
3d
a
a
a
a
a
a
13.36 ± 2.47
31.68 ± 0.93
3e
a
a
a
25.69 ± 2.00
21.89 ± 1.02
40.25 ± 2.00
68.98 ± 1.87
83.17 ± 1.28
3f
a
a
a
a
15.34 ± 1.57
31.21 ± 2.00
53.46 ± 1.84
79.51 ± 0.50
3 g
13.64 ± 1.68
31.68 ± 2.47
48.25 ± 2.37
68.19 ± 1.75
19.96 ± 1.42
37.65 ± 1.56
58.74 ± 0.57
82.13 ± 1.00
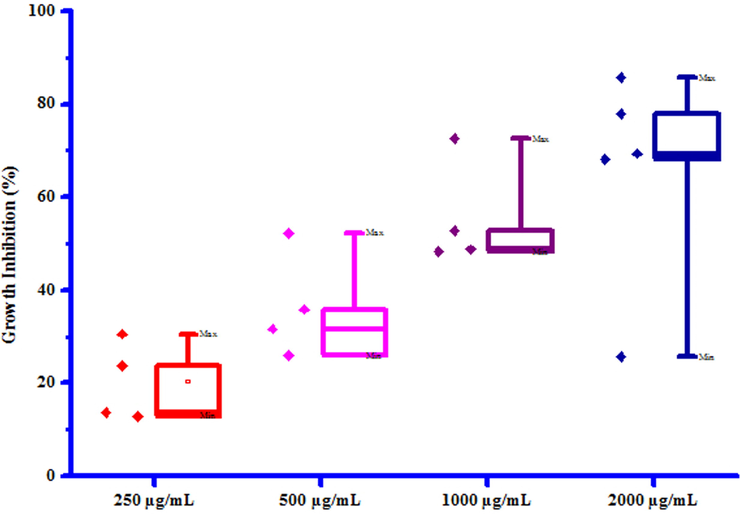
Box plot of substituted coumarins (3a-3g) against Rhizoctonia solani.
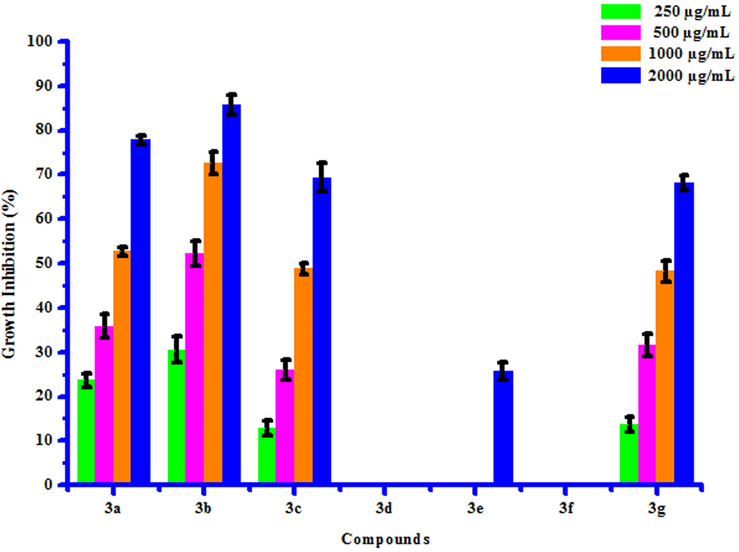
Antifungal activity of substituted coumarins (3a-3g) against Rhizoctonia solani.
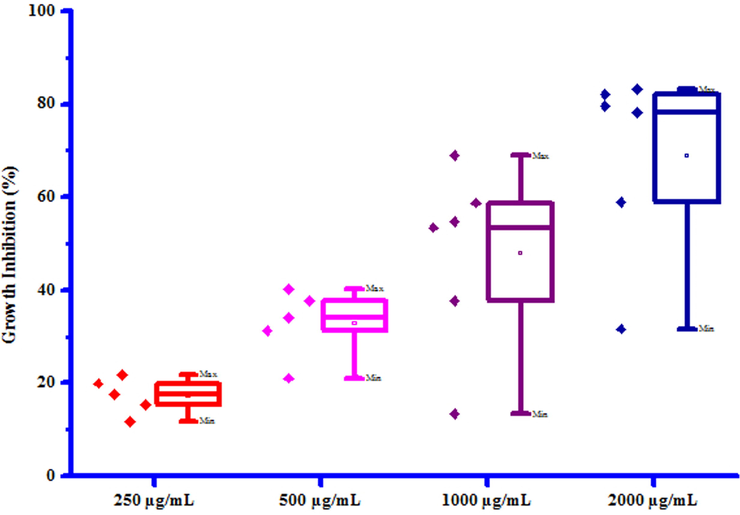
Box plot of substituted coumarins (3a-3g) against Colletotrichum gloeosporioides.
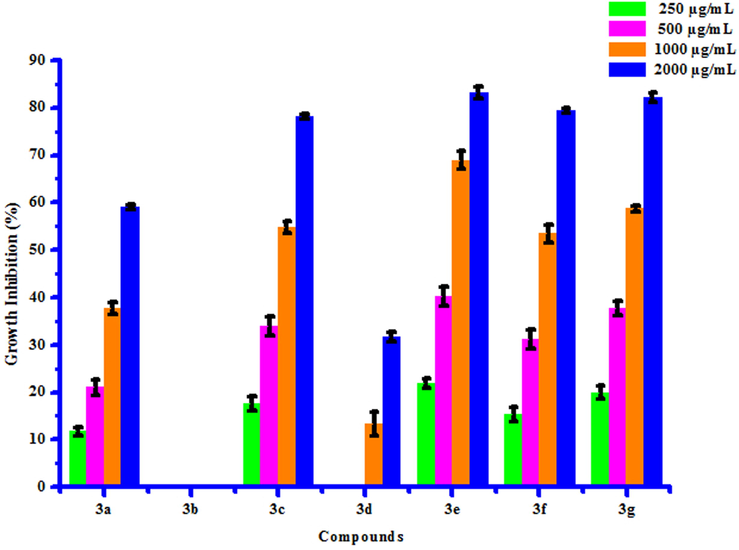
Antifungal activity of substituted coumarins (3a-3g) against Colletotrichum gloeosporioides.
7 Antibacterial activity
All compounds (3a-3 g) were screened for their inhibitory effect on the growth of two bacterial species viz. Erwinia cartovora and Xanthomonas citri at various concentrations i.e. 250, 500, 1000 and 2000 µg/mL. Luria- Bertani medium was sterilized by auto-claving at 15 psi pressure at 121 °C for 15 to 20 min. The plates were prepared by pouring 30–35 mL of the sterilized media into sterilized Petri plates. Media was then allowed to solidify and then suspension of 3–4 days old broth of test organism was then spread on specific medium plates. Sterile filter paper discs moistened with test compounds solution in DMSO were carefully placed on the medium inoculated with the specific bacterial suspension. Sterile filter paper discs dipped in DMSO served as control. These plates were incubated at 25 ± 2 °C and the diameter of growth inhibition zone was measured after 24 h. The results of antibacterial screening of synthesized compounds (3a-3g) were presented in Table 7. The results revealed that some of synthesized compounds showed no inhibition against Erwinia cartovora and Xanthomonas citri. Maximum Erwinia cartovora growth was inhibited by compound 3 g showing inhibition zone 3.00–8.00 mm. Maximum Xanthomonas citri growth was inhibited by compound 3b showing inhibition zone 14.00–27.00 mm. This inhibition may be caused by substitution of bromo and chloro groups on phenyl ring. The box plot and graphical representation of antibacterial activity of all synthesized compounds (3a-3g) against Erwinia cartovora and Xanthomonas citri were shown in Figs. 10-13. All values are mean ± S.D. a: No inhibition zone.
Compounds
Inhibition Zone (mm)
Bacteria
Erwinia cartovora (conc.) µg/mL
Xanthomonas citri (conc.) µg/mL
250
500
1000
2000
250
500
1000
2000
3a
a
a
a
a
a
a
a
a
3b
2.00 ± 0.01
5.00 ± 0.26
6.00 ± 0.15
7.00 ± 0.25
14.0 ± 0.25
18.0 ± 0.07
22.0 ± 0.36
27.0 ± 0.75
3c
0.80 ± 0.03
1.20 ± 0.15
1.80 ± 0.05
2.20 ± 0.10
6.00 ± 0.40
9.00 ± 0.07
14.2 ± 0.25
18.0 ± 0.45
3d
a
a
1.00 ± 0.07
3.00 ± 0.20
3.50 ± 0.25
5.00 ± 0.14
7.50 ± 0.25
10.0 ± 0.55
3e
a
a
a
a
a
a
a
a
3f
a
a
a
a
a
a
a
a
3 g
3.00 ± 0.13
4.50 ± 0.24
6.00 ± 0.36
8.00 ± 0.40
2.50 ± 0.15
4.50 ± 0.35
6.00 ± 0.50
8.50 ± 0.26
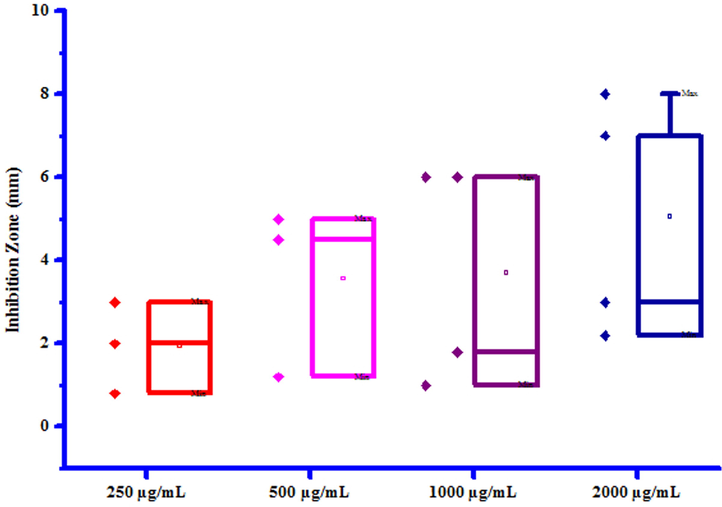
Box plot of substituted coumarins (3a-3g) against Erwina cartovora.
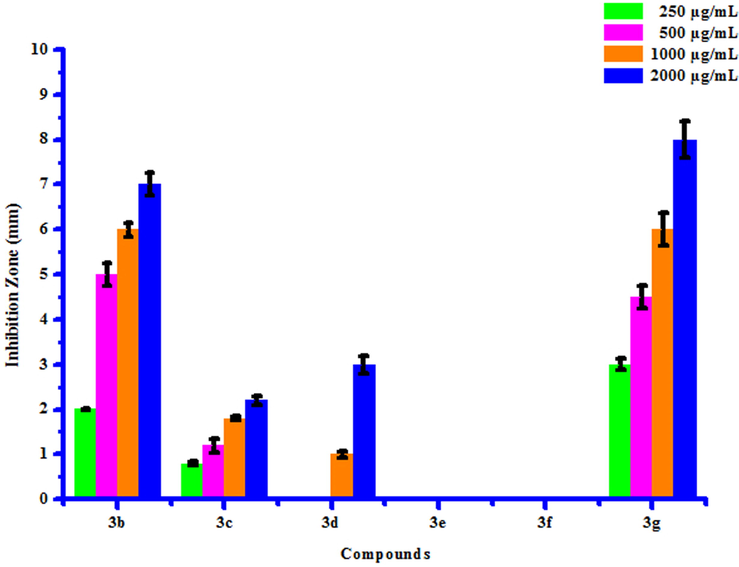
Antibacterial activity of substituted coumarins (3a-3g) against Erwina cartovora.
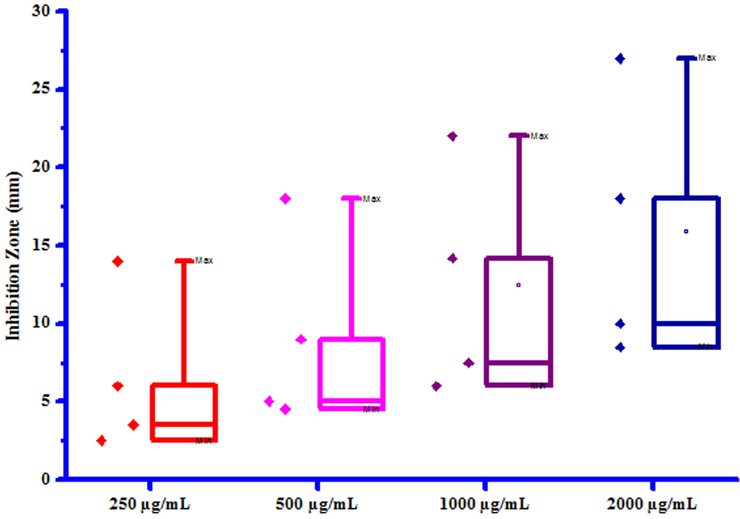
Box plot of substituted coumarins (3a-3g) against Xanthomonas citri.
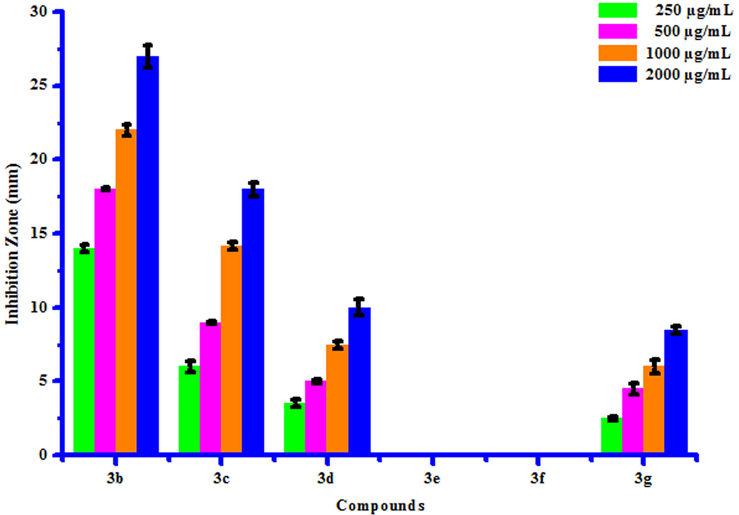
Antibacterial activity of substituted coumarins (3a-3g) against Xanthomonas citri.
8 Conclusions
A simple and efficient synthesis of substituted coumarins (3a-3g) using natural acids present in Citrus limon L. juice, Vitis vinifera L. juice and Banana peels extract was reported in this paper. Solvent-free medium, non-toxic side products, inexpensive reagents and mild reaction conditions etc. are some advantages of present methodology over conventional methods. All synthesized compounds (3a-3g) were also screened for their bio efficacy in terms of herbicidal activity against Raphanus sativus L. (Radish) seeds, fungicidal activity against R. solani, C. gloeosporioides and antibacterial activity against Erwinia cartovora and Xanthomonas citri. Based on activity data, it can be concluded that some of synthesized compounds possessed good activity due to presence of chloro, bromo and methyl groups on phenyl ring.
Acknowledgements
The authors are thankful to the Department of Chemistry, Chaudhary Charan Singh Haryana Agricultural University, Hisar for providing the necessary facilities. Financial assistance from Department of Science and Technology (DST), New Delhi, India is gratefully acknowledged. Authors are also thankful to SAIF, Punjab University Chandigarh, for providing analytical facilities for characterization of compounds.
Declaration of Competing Interest
Authors declare that there is no conflict of interest regarding the publication of this paper.
References
- Synthesis of 3-carboxycoumarins at room temperature in water extract of banana peels. Environ. Chem. Lett.. 2017;15:725-731.
- [Google Scholar]
- Samarium (III) catalyzed one-pot construction of coumarins. Tetrahedron Lett.. 2004;45:7999-8001.
- [Google Scholar]
- Lemon Juice Catalyzed Ultrasound Assisted Synthesis of Schiff’s Base: a Total Green Approach. Bull. Env. Pharmacol. Life Sci.. 2015;4:94-100.
- [Google Scholar]
- Structure crystalline et moleculaire du dicoumarol. Acad. Sci. Paris. 1968;267:1790.
- [Google Scholar]
- Antimicrobial properties of some hydroxycoumarins and Fraxinus ornus bark extracts. J. Ethnopharm. 1993;39:205.
- [Google Scholar]
- A green chemical synthesis of coumarin-3-carboxylic and cinnamic acids using crop-derived products and waste waters as solvents. Tetrahedron Lett.. 2016;57:4795-4798.
- [Google Scholar]
- Phytopathology. Toximetric studies of fungicides against brown rot organism Sclerotina fruticola. 1962;52:876-880.
- [Google Scholar]
- Recent advances in the development of phytoestrogens and derivatives: an update of the promising perspectives in the prevention of postmenopausal diseases. Mini- Rev Med Chem.. 2003;3:387.
- [Google Scholar]
- Reaction of substituted salicylaldehyde with dimedone catalyzed by KFAl2O3. Chin. J. Org. Chem.. 2005;25:846-849.
- [Google Scholar]
- An environmentally friendly procedure for the synthesis of coumarins via Pechmann condensation of phenols with ethyl acetoacetate. J. Chem. Res.. 1998;38
- [Google Scholar]
- Microwave-Assisted Synthesis of 3-Substituted Coumarins Using ZrOCl2. 8H2O as an Effective Catalyst. Scientia Iran.. 2009;16
- [Google Scholar]
- Synthesis of Coumarins in Search of Better Nonpeptidic HIV Protease Inhibitors. J. Indian Chem. Soc.. 1998;75:666.
- [Google Scholar]
- Efficient, high-yield protocol for the one-pot synthesis of benzopyran derivatives catalyzed by p-TSA in aqueous media. Synth. Commun.. 2012;42:967-974.
- [Google Scholar]
- Drugs Future. 1996;34:1257.
- Fruit juice: a natural, green and biocatalyst system in organic synthesis. Open J. Org. Chem.. 2013;1:47-56.
- [Google Scholar]
- First application of fruit juice of Citrus limon for facile and green synthesis of bis-and tris (indolyl) methanes in water. Chem. J.. 2013;3:7-12.
- [Google Scholar]
- Inhibition of cell cycle progression in human leukemia HL-60 cells by esculetin. Cancer Lett.. 2002;183:163.
- [Google Scholar]
- Synthesis of the Calophyllum coumarins. Part 2. J. Chem. Soc. Perkin Trans.. 1995;1:3135.
- [Google Scholar]
- Exploring the utility of fruit juices as green medium for Biginelli reaction. Res. J. Pharm. Biol. Chem. Sci.. 2014;5:444-449.
- [Google Scholar]
- Roe, R.M., Burton, J.D., Kuhr, R.J., Sandmann, G., Boger, P., 1997. Toxicology, Biochemistry and Molecular Biology of Herbicide Activity, Eds; IOS Press: Amsterdam, The Netherlands., 1997, pp. 111-141.
- Rivulobirin E and Rivulotririn C from Pleurospermum rivulorum. Chem. Pharm. Bull.. 1999;47:713.
- [Google Scholar]
- Thornberry, H.H., 1950. Phytopathology. A paper-disk plate method for the quantitative evaluation of fungicides and bactericides, 1950, 40.
- A clean synthesis of 1-oxo-hexahydroxanthene derivatives in aqueous media catalyzed by TEBA. Synth. Commun.. 2005;35:97-104.
- [Google Scholar]
- 2, 4, 6-Trichloro-1, 3, 5-triazine as an efficient catalyst for synthesis of benzopyran derivatives under solvent-free conditions. Synth. Commun.. 2008;38:4474-4479.
- [Google Scholar]
- Green synthesis of silver nanoparticles from grape and tomato juices and evaluation of biological activities. IET Nanobiotechnol.. 2016;11:193-199.
- [Google Scholar]







