Translate this page into:
A systematic review on the origin, anti-inflammatory effect, mechanism, pharmacokinetics, and toxicity of albiflorin
⁎Corresponding authors at: School of Pharmacy, Anhui University of Chinese Medicine, No. 350, Longzihu Road, Yaohai District, Hefei, Anhui 230012, China. jinchsh@ahtcm.edu.cn (Chuanshan Jin), hanr@ahtcm.edu.cn (Rongchun Han)
⁎⁎Corresponding author at: School of Life Sciences, Anhui University of Chinese Medicine, No. 350, Longzihu Road, Yaohai District, Hefei, Anhui 230012, China. twentytong@hotmail.com (Xiaohui Tong),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Albiflorin, a principal monoterpene glycoside isolated from Paeonia lactiflora Pall., is known for its extensive pharmacological properties and medicinal value in a number of traditional Chinese medicine recipes. Herein, we consolidate current research on the biological profile, anti-inflammatory capabilities, pharmacokinetics, tissue distribution, and toxicity of albiflorin with the, aim of delineating its therapeutic potential and safety profile. Albiflorin exhibits remarkable anti-inflammatory effects in various disease models, including respiratory, urinary, gastrointestinal, and rheumatic conditions, as well as metabolic and nutritional diseases, blood system disorders, mental illnesses, and pain. Pharmacokinetic studies have revealed its efficient absorption, distribution, and elimination, indicating favorable bioavailability with minimal toxicity, as supported by traditional toxicity assessments and next-generation metabolomics analyses. Despite its proven efficacy in a broad spectrum of health conditions, comprehensive toxicological studies are scarce, highlighting the need for further research to ascertain its safety in clinical applications. This review underscores the significant therapeutic promise of albiflorin, rooted in its efficacy as the key active ingredient in relevant traditional Chinese medicine formulas and reinforced by modern scientific inquiry. Albiflorin stands at the intersection of historical wisdom and contemporary pharmacological research, warranting further exploration to harness its full medical potential, especially for the treatment of inflammation-related diseases.
Keywords
Albiflorin
Anti-inflammation
Paeonia lactiflora
Toxicity
- 4CL
-
4-coumarate-CoA ligase
- albiflorin-NGSTH
-
albiflorin nanogel loaded self-assembled thermosensitive hydrogel system
- AKT
-
Protein Kinase B
- AS
-
Arteriosclerosis
- ARDS
-
acute respiratory distress syndrome
- AUC
-
area under concentration–time curve
- BALF
-
bronchoalveolar lavage fluid
- β-EP
-
β-endorphin
- BRPP
-
binding rate of plasma protein
- BYHWD
-
Buyang Huanwu Decoction
- cAMP
-
cyclic adenosine monophosphate
- CD-14
-
cluster of differentiation 14
- COX-2
-
cyclooxygenase-2
- CCI
-
chronic constriction injury
- Cmax
-
peak concentration
- CL/F
-
clearance
- CLP
-
cecal ligation and puncture
- CNKI
-
China National Knowledge Infrastructure
- DMAPP
-
dimethylallyl diphosphate
- DXP/MEP
-
1-deoxy-d-xylulose-5-phosphate/methyl-erythritol-4-phosphate
- DSS
-
dextran sulfate sodium
- DVT
-
deep vein thrombosis
- DXP
-
1-deoxy-d-xylulose-5-phosphate
- EGFR
-
epidermal growth factor receptor
- ERK
-
extracellular regulated protein kinase
- FAEW
-
Free and Easy Wanderer
- GPP
-
geranyl diphosphate
- GSH-Px
-
glutathione peroxidase
- GSK3β
-
glycogen synthase kinase
- HE
-
hematoxylin and eosin
- HDCG
-
high-dose control group
- HDMG
-
high-dose model group
- HIE
-
hypoxic brain injury
- HK-2
-
human kidney 2
- HFD
-
high fat diet group
- HFDA
-
high fat diet group with albiflorin
- HMCs
-
human mesangial cells
- HPLC
-
high-performance liquid chromatography
- HPA axis
-
hypothalamic pituitary adrenal axis
- HQJZT
-
Huangqi Jianzhong Tang
- HUVECs
-
human umbilical vein endothelial cells
- IκBα
-
recombinant inhibitory subunit of NF-κB Alpha
- IKK
-
inhibitor of kappa B kinase
- IL
-
interleukin
- IRAK1
-
interleukin 1 receptor-associated kinases
- IVC
-
Inferior vena cava
- IPP
-
isopentenyl diphosphate
- JGD
-
Jakyak-Gamcho decoction
- JNK
-
c-Jun N-terminal kinase
- LDCG
-
low-dose control group
- LDMG
-
low-dose model group
- LOX-1
-
Lectin-like oxidized low-density lipoprotein receptor-1
- LPS
-
lipopolysaccharides
- LC-MS/MS
-
liquid chromatography − mass spectrometry
- MAPK
-
mitogen-activated protein kinase
- MCAO
-
middle cerebral artery occlusion
- MD-2
-
myeloid differential protein-2
- MDA
-
malondialdehyde
- MEP
-
methyl-erythritol-4-phosphate
- MetS
-
Metabolic syndrome
- MG
-
model group
- MPGN
-
mesangial proliferative glomerulonephritis
- MVA
-
mevalonate
- NC
-
control group
- NLRP3
-
nucleotide-binding oligomerization domain, leucine-rich repeat and pyrin domain-containing 3
- NO
-
nitric oxide
- NF-κB
-
nuclear factor kappa B
- NG
-
normal group
- OARSI
-
Osteoarthritis Research Society International
- Ox-LDL
-
oxidized low-density lipoprotein
- PARP1
-
ADP-ribose polymerase-1
- PAN
-
puromycin aminonucleoside
- PI3K
-
phosphatidylinositol 3-kinase
- PDE
-
phosphodiesterase
- PGE2
-
prostaglandin E2
- PGF2α
-
prostaglandin F2α
- p-NF-κB
-
phosphorylated nuclear factor κB
- PTSD
-
posttraumatic stress disorder
- RIPA
-
radio immunoprecipitation assay
- RPA
-
Radix Paeoniae Alba
- RPR
-
Radix Paeoniae Rubra
- RANK
-
the receptor activator of the NF-кB ligand
- SD
-
Sprague Dawley
- SGT
-
Shaoyao-Gancao Tang
- SKT
-
Shakuyakukanzoto
- SOD
-
superoxide dismutase
- T2D
-
type 2 diabetes
- TAK1
-
transforming growth factor beta-activated kinase 1
- TCM
-
traditional Chinese medicine
- TLR
-
toll-like receptor
- TNF
-
tumor necrosis factor
- t1/2
-
biological half-time
- tmax
-
peak time
- TRAF6
-
tumor necrosis factor receptor-associated factor 6
- TRIF
-
TIR-domain-containing adaptor inducing interferon-β
Abbreviations
1 Introduction
As a traditional Chinese medicine (TCM), Paeonia lactiflora Pall. (Paeoniaceae) was first utilized to treat diseases and documented in the Prescriptions for Fifty-two Aliments that dates back to more than 2000 years ago (Tan et al., 2020). Radix Paeoniae Alba (RPA, also called baishao in Chinese), in the form of the dried root with its bark removed, has been used for centuries to treat various disorders including inflammation and pain (He and Dai, 2011, Jang et al., 2021, Lee et al., 2022). In TCM, RPA is used to tonify blood and smoothen the liver (Zhao et al., 2022). TCM practitioners often combined RPA with other herbs to create remedies that are believed to have synergistic effects and balance body systems. Modern pharmacological studies have shown that RPA has analgesic, anti-inflammatory, immuno-modulatory and antioxidant effects; therefore, it is used clinically for the treatment of various illnesses, including fever, muscle cramps, rheumatoid arthritis, systemic lupus erythematosus, hepatitis, dysmenorrhea, and spasms (Li et al., 2017, Chen et al., 2021). Various active components (e.g., paeoniflorin, oxypaeoniflorin, benzoylpaeoniflorin, lactiflorin and paeonol have been isolated and characterized from various parts of P. lactiflora (root, stem, leaf, flower and fruit), with albiflorin (C23H28O11) as the key compound.
Albiflorin (C23H28O11), a monoterpene glycoside with a molecular weight of 480.5 Da, contains a unique cage-like pinane skeleton (Zhou et al., 2019). It consists of a glucose molecule (glycone) linked to a cyclic monoterpene aglycone with 10 carbon atoms derived from isoprene units. A glycosidic bond connects the glycone to the hydroxyl group of monoterpene aglycone. Albiflorin can undergo various transformations including hydrolysis, oxidation or enzymatic reactions depending on specific conditions such as pH, temperature and the presence of catalysts. For example, the microbial conversion between albiflorin and paeoniflorin occurs in the presence of two fungi (Liu et al., 2010).
Recently, albiflorin has been demonstrated to exhibit strong pharmacological effects in combating inflammation, oxidation, apoptosis, cancer, bacterial infection, depression and cognitive dysfunction, indicating its great potential for treating related illnesses (Hu et al., 2021, Zhang et al., 2022, Han et al., 2023, Liu et al., 2023b). Albiflorin can be readily obtained from the root of P. lactiflora's, with seversl other medicinal plants, including P. emodi, P. obovata, P. suffruticosa, P. anomala and P. veitchii containing albiflorin. However, the natural production of albiflorin is not restricted to the Paeoniaceae family. Kunzea pomifera, a shrub from the family Myrtaceae native to Australia, has also been reported to produce albiflorin (Table 1).
Species
Distribution area
Source of albiflorin
Reference
Paeoniaceae
Paeonia lactiflora
China, Japan, South Korea, Mongolia, Russia
Root
(Zheng et al., 2021)
Paeonia veitchii
China, Japan
Root
(Zhu et al., 2015)
Paeonia rockii
China
Pollen
(Wang et al., 2019)
Paeonia suffruticosa
China
Seed
(Wang et al., 2023a)
Paeonia anomala
Russia, China, Mongolia
Root
(Oidovsambuu et al., 2013)
Paeonia emodi
Afghanistan, Tibet, India
Root
(Joshi et al., 2023)
Paeonia obovata
China, Korea, Russia, Japan
Root
(Bae et al., 2015)
Paeonia ostii
China
Leaf
(Bai et al., 2024)
Paeonia delavayi
China
Root
(Wu et al., 2007)
Myrtaceae
Kunzea pomifera
England, Australia
Fruit
(Ali et al., 2022)
In this review, a total of 282 articles were identified by searching the PubMed and Web of Science databases from February 6, 2024, using albiflorin and alibiflorin (a synonym for albiflorin) as key words. The same parameters were applied to the China National Knowledge Infrastructure (CNKI) database, which identified 368 items. This review captured 650 publications from these three public repositories. The focus was on understanding the origin, anti-inflammatory effects, mechanism, pharmacokinetics, and toxicity of albiflorin.
2 Biological profile of albiflorin and P. lactiflora
In the 1970s, albiflorin was extracted from the roots of Radix Paeoniae Alba (RPA) and its absolute structure was reported for the first time (Kaneda et al., 1972). In Latin, alba means white and albiflorin perfectly embodies the intimate relationship between this compound and the source plant P. lactiflora. As a well-known bulk medicinal material in China, P. lactiflora is grown in multiple provinces to produce large quantities of RPA, which is a stable source for the purification and application of albiflorin. P. lactiflora, native to China and commonly known as the perennial herbaceous peony, exhibits distinctive characteristics. The plant features thick roots and dark brown branches, with hairless stems reaching a height of 70 cm. The lower stem leaves contain two or three compound leaves, whereas the upper stem leaves are composed of three compound leaves. The leaflets adopt an elliptic, lanceolate and ovate shape with a pointed apex and base that is either wedge-shaped or oblique. The edges are white and finely toothed, and the leaves are smooth on both sides, with occasional sparse hair along the veins on the back. The flowers are positioned at the top of the stem and on the leaf axils with diameters ranging from 8 to 11.5 cm. Petals are obovate, 3.5–6 cm long, 1.5–4.5 cm broad, and mostly white with sporadic deep purple spots at the bottom. They range in size from 9 cm to 13 cm. The flowers of P. lactiflora blossom from May to June followed by fruiting in August (Editorial Board of Flora of China, 1979). RPA and Radix Paeoniae Rubra (RPR, also called chishao in China), both derived from the radix of P. lactiflora, are used as crude pharmaceuticals and listed as distinct Chinese medicines in the Chinese Pharmacopoeia. RPR is processed by directly drying fresh roots in the sun. In contrast, boiling the root in water is necessary for the processing of RPA to remove impurities and soften its tough texture, followed by peeling. The root is then dried under the sun or a gentle heat source. Albiflorin is present in both RPA and RPR (Fig. 1).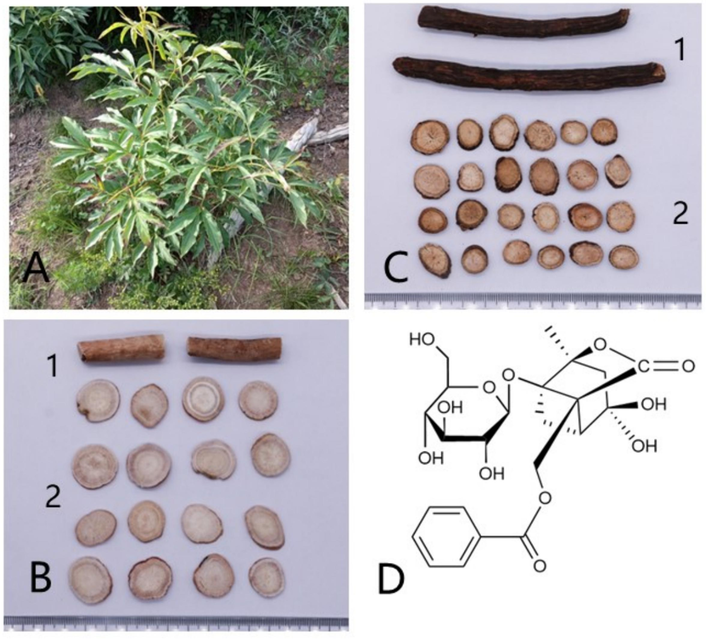
P. lactiflora and albiflorin. (A) vegetative body of P. lactiflora; (B1) Radix Paeoniae Alba; (B2) Decoction pieces made of RPA; (C1) Radix Paeoniae Rubra; (C2) Decoction pieces made of RPR; (D) Structure of albiflorin.
The biosynthesis of albiflorin begins with the condensation of two cinnamic acid derivatives, coniferyl alcohol and coumaryl alcohol, which are derived from the general phenylpropanoid pathway. The subsequent modifications, such as hydroxylation, methylation, and glycosylation, give rise to the final albiflorin molecule. 4-coumarate-CoA ligase (4CL), an enzyme that catalyzes the conversion of coniferyl alcohol and coumaryl alcohol into the CoA thioester, is one of the essential enzymes in the biosynthesis of albiflorin. To the best of our knowledge, no detailed information is available on the biosynthetic pathway of albiflorin. However, albiflorin and paeoniflorin are both isomers and therefore understanding the biosynthetic pathway of the latter can provide valuable insights into the potential pathway of albiflorin, given their structural similarities (Gao et al., 2015). The terpene routes, namely the 1-deoxy-d-xylulose-5-phosphate/methyl-erythritol-4-phosphate (DXP/MEP) and mevalonate (MVA) pathways, control the manufacture of the monoterpene glucoside paeoniflorin. The commonly occurring precursor, isopentenyl-PP (IPP), is synthesized via the DXP/MEP and MVA pathways. Geranyl diphosphate synthase catalyzes the condensation of one IPP and one DMAPP to generate geranyl diphosphate (GPP), the monoterpene precursor form, in the second stage. The final stage then involves the development and post-modification of the basic pinane skeleton. GPP is directly catalyzed by terpenoid synthase, which is situated near the isoprenoid pathway’s branch point, to create the monoterpenoid skeleton (Yuan et al., 2013).
3 Anti-inflammatory activity of albiflorin
Investigations on albiflorin, a prominent bioactive constituent of P. lactiflora have revealed its notable and multifaceted anti-inflammatory effects. In this review, we combine and organize relevant research on albiflorin trials conducted in China and abroad, emphasizing the anti-inflammatory properties of the compound. To sort distinct symptoms that are related to or caused by inflammation, the contents are organized according to diseases in different systems (Table 2), and an overview of the findings regarding the mechanisms of action are also provided. Effect on diseases of the respiratory system Effect on diseases of the urinary system Effect on gastrointestinal diseases Effect on metabolic and nutritional diseases Effect on diseases of the blood system Effect on rheumatic disease Effect on mental illness Effect on analgesia Effect on other diseases
Models
Mechanisms of action
Reference
Cecal ligation and puncture (CLP)-induced sepsis mice model and RAW 264.7 cells
Albiflorin ameliorates inflammation and oxidative stress induced by LPS through suppressing the TLR-4/NF-κB pathway.
(Wei et al., 2023)
Asthmatic mice model
Albiflorin suppresses the proliferation of inflammatory cells and inflammatory cytokines, such as TNF-α, IL-6, and IL-1β.
(Cai et al., 2019)
Epithelial cells
Albiflorin may target the MAPK1 signaling pathway in treating coronavirus-induced acute respiratory distress syndrome (ARDS).
(Tao and Jiming, 2022)
Nephrotic rats model induced with puromycin aminonucleoside (PAN)
Albiflorin intervenes in sodium retention by restricting the kidneys' uPA-mediated plasmin production and plasmin activity.
(Yang et al., 2022)
Human umbilical vein endothelial cells (HUVECs) and male SD rat model
Albiflorin alleviates mesangial proliferative glomerulonephritis (MPGN) via inhibiting the over-proliferation of mesangial cells, inflammation, and fibrosis through the activation of PI3K/AKT/NF-кB signaling.
(Yu et al., 2023)
Mice model of diabetic kidney disease and HK-2 cells
Albiflorin protects against diabetes-related vascular problems by interfering with EGFR and AKT phosphorylation.
(Liu et al., 2023b)
Ulcerative colitis rat model
Albiflorin hinders anti-inflammatory reaction in rats with ulcerative colitis, which is related to the NF-κB/COX-2 signaling pathway.
(Huang and Yan, 2022)
Enteritis rat model
Albiflorin reduces oxidative stress and inflammation in enteritis caused by methotrexate via controlling the NF-кB/NLRP3 (NOD-like receptor thermal protein domain linked protein 3) pathway.
(Zhang et al., 2022)
SD rats and human liver hepatocytes (L-O2)
Albiflorin protects against SAP-induced liver injury through suppression of inflammation and oxidative stress via the P38MAPK/NF-κB signaling pathway.
(Li et al., 2024)
Diabetic mice
Albiflorin in SKT is crucial for pain relief in diabetic neuropathy, enhancing adrenaline secretion and α2-adrenoceptor activation to adjust nociceptive transmission in the spinal cord.
(Lee et al., 2011)
HUVECs
Albiflorin encourages the growth and transfer of HUVECs while suppressing apoptosis and the production of IL-1β, TNF-α and IL-6.
(Yang and Yang, 2023)
Metabolic syndrome (MetS) mouse model
Albiflorin reverses elevated levels of liver TNF-α mRNA, plasma aspartate aminotransferase, and liver triglycerides.
(Zhou et al., 2019)
Middle cerebral artery occlusion (MCAO) model and HT22 cells
Albiflorin inhibits ferroptosis and activates autophagy through the PI3K/AKT signaling pathway.
(Zhao et al., 2023)
Rat arthritis model and mice capillary permeability model
Albiflorin mediates suppression of cAMP-phosphodiesterase (PDE) in neutrophils, which exerts an anti-inflammatory effect.
(Jiang et al., 2011)
ApoE-/-mouse arteriosclerosis model and human monocyte line THP-1 induced foam cell model
1. Albiflorin suppresses the LOX-1/NF-кB signaling pathway, which lowers the deposition of fat in macrophage cytoplasm, and prevents ox-LDL from inducing foaming cells.
2. Albiflorin can inhibit arteriosclerosis by modulating the LOX-1/NF-кB pathway and inflammatory molecule expression in vivo.
(Sun, 2017)
Rat cerebral ischemia model
Albiflorin reduces the inflammatory response of cerebral ischemia and apoptosis by binding with MAPK1, SRC, EGFR, MAPK14 and Caspase 7.
(Zhou et al., 2021)
HUVECs
Albiflorin slows the course of atherosclerosis via suppression of the IRAK1/TAK1 pathway.
(Liu et al., 2022)
Rat chondrocytes
By triggering the NF-кB signaling pathway, the receptor activator of the NF-кB ligand (RANKL) partially reverses the mitigating effect of albiflorin on IL-1β-induced chondrocyte damage.
(Zhou et al., 2023)
SD rat and ICR mouse model
Albiflorin alleviates depressive behavior and reduces proinflammatory cytokine levels.
(Xu et al., 2021)
T98G brain cells and HEK293 cells
Albiflorin primarily binds to DNA and ATP binding sites on p65-RelA in determinants of posttraumatic stress disorder.
(Hong et al., 2017)
Acetic acid induced writhing mice
The mechanism of albiflorin in analgesia may be related to the increase of β-endorphin (β-EP) level in serum and cerebral cortex and the reduction or release of PGE2 in the cerebral cortex.
(Wu et al., 2018a)
Primary dysmenorrhea mouse model
The mechanism of albiflorin relieving dysmenorrhea could be connected to the increased NO level and decreased PGF2α and Ca2+ concentration in uterine tissue.
(Wu et al., 2018b)
A rat model of chronic sciatic nerve compression injury
Albiflorin can effectively relieve neuropathic pain by controlling NLRP3 the spinal cord.
(Xu and Zhou, 2022)
RAW 264.7 cells
Albiflorin regulates the expression of inflammatory factors by activation of the NF-кB signaling pathway.
(Bi et al., 2017)
U937 cells
Albiflorin inhibits production of inflammatory cytokines TNF- α, IL- 1β and INF- γ from macrophages.
(Nöst et al., 2019)
RAW 264.7 cells
Albiflorin inhibits IL-6 and COX-2 mRNA expression.
(Wang et al., 2014)
RAW 264.7 cells
Albiflorin suppresses the production of IL-1β, TNF-α and PGE2 by downregulating IRAK1.
(Guo et al., 2007)
Hypoxic brain injury (HIE) mouse model
Albiflorin relieves oxidative stress and inflammation in mice with neonatal hypoxic-ischemic brain injury through activation of the PI3K/AKT/mTOR signaling pathway.
(Yang et al., 2022)
3.1 Diseases of the respiratory system
Respiratory diseases are a group of disorders that affect the lungs, airways and other organs involved in breathing. They are characterized by various signs, including sneezing, shortness of breath, tightness of the chest and coughing. Experimental results suggest that albiflorin exerts anti-inflammatory, antioxidant and bronchodilatory effects, which might be advantageous for the alleviation of respiratory conditions such as asthma and ARDS. Judging from hematoxylin and eosin (HE) staining of lung tissue sections from different groups of mice (sham, sham + 20 mg/kg, sham + 40 mg/kg, CLP, CLP + 20 mg/kg albiflorin, CLP + 40 mg/kg albiflorin and CLP + dexamethasone) showed, that the therapeutic effect of albiflorin on pulmonary damage was comparable to that of dexamethasone (Wei et al., 2023). The results from another experimental research on mice indicated that albiflorin hindered the expression of cytokines associated with inflammation including IL-1β, IL-6, and TNF-α. In addition, albiflorin reduced malondialdehyde (MDA) levels and increased superoxide dismutase (SOD) performance. Albiflorin also lowered airway hyperactivity and minimized inflammation of the lungs in mice with asthma by modifying the mitogen-activated protein kinase (MAPK) /nuclear factor κB (NF-κB) signaling process (Cai et al., 2019). By evaluating the therapeutic possibilities of Xuebijing injection in treating of coronavirus-induced ARDS, albiflorin was discovered among 30 active components that preferentially targeted the MAPK signaling pathway (Tao and Jiming, 2022).
3.2 Diseases of the urinary system
The urinary system is a vital component of the human body that is responsible for filtering waste products and excess substances from the blood. Albiflorin has recently gained attention as a potential cure for diseases affecting the urinary system. In vivo and in vitro investigations performed on human mesangial cells (HMCs) and male Sprague Dawley (SD) rats showed that albiflorin decreased the expression of ki67 (a marker for determining the growth fraction of a given cell population) in lipopolysaccharide (LPS)-treated HMCs and rats with mesangial proliferative glomerulonephritis (MPGN). Moreover, albiflorin reduced the mRNA expression of inflammation-associated cytokines and fibrosis in LPS-treated HMCs and rats with MPGN. Furthermore, albiflorin successfully decreased 24-hour urine protein levels, promoted kidney activity and alleviated dyslipidemia and pathologic damage in rats with MPGN by blocking the PI3K/AKT/NF-κB pathway (Yu et al., 2023). The Osteoarthritis Research Society International (OARSI) investigation of an in vitro model of diabetic renal proximal tubulopathy revealed that albiflorin inhibited high glucose or high lipid-induced epidermal growth factor receptor (EGFR) and phosphorylation of AKT, and imparted protective properties in a human kidney 2 (HK-2) cell model of diabetic kidney disease (Liu et al., 2023b).
3.3 Gastrointestinal diseases
Gastrointestinal diseases encompass a wide range of disorders that affect the digestive system including the stomach, liver, and intestines. Experimental studies have revealed the therapeutic effects of albiflorin for the management of these conditions. In vivo experiments have demonstrated that albiflorin could be used to treat dextran sulfate sodium (DSS) − induced murine colitis, an inflammatory bowel disease via adrenodoxin activation. Further investigations have shown that albiflorin may hinder the adrenodoxin isoform, a key component in the synthesis of adrenal steroid hormones. This inhibitory effect enabled the stimulation of phosphorylated NF-κB p65, which in turn repressed p38 MAPK, extracellular regulated protein kinase (ERK), and c-Jun N-terminal kinase (JNK) phosphorylation. Albiflorin also diminished the immuno-inflammatory response and increased Foxp3 expression in colon tissues (Wang et al., 2023b). In addition, albiflorin reduced the inflammatory response and exacerbated oxidative stress by reducing the infiltration of CD68+ cells, blocking myeloperoxidase activity, and reducing the expression of cyclooxygenase-2 (COX-2) and intercellular adhesion molecule-1 in a rat model of methotrexate-induced enteritis. Albiflorin also decreased the formation of reactive oxygen species, glutathione levels, superoxide dismutase activity, and malondialdehyde (Zhang et al., 2022). Ulcerative colitis (UC) is a severe inflammatory disease of the intestinal tract characterized by, recurrence and uncontrollability, and the effect of common therapeutic drugs available is not significant. Huang and Yan (2022) established a rat model of UC to investigate the effects of albiflorin. By assessing proteins of interest in rat colon tissue using western blotting, the levels of COX-2, p-NF-κB (phosphorylated nuclear factor κB) and NF-κB in the albiflorin group were found to be considerably lower than those in the model group. Therefore, albiflorin inhibited the occurrence of inflammatory and oxidation reactions of colon tissue through the NF-кB/COX -2 signaling pathway. In an acute liver injury mouse model established using carbon tetrachloride, Ren et al. (2020) measured the levels of glutathione peroxidase (GSH-Px) and SOD in liver tissue and observed pathological changes. In comparison with the model group, the levels of SOD (133.43 ± 7.47 vs 154.46 ± 17.89 U/mg·port) and GSH-Px (451.31 ± 65.81 µmol/L) increased in the medium-dose paeoniflorin plus albiflorin group. GSH-Px was reported to alleviate inflammation through the elimination of oxidative species (Li et al., 2018), thus we speculate that the improved pathological changes observed in hepatocytes using albiflorin may be associated with the suppression of inflammatory reactions.
3.4 Metabolic and nutritional diseases
Nutritional diseases arise when a person’s diet does not contain sufficient nutrients for a healthy body or when the person is unable to properly absorb nutrients from food. Metabolism is a term used to describe all chemical processes that occur continuously inside the body, allowing life and normal functioning. Albiflorin is a promising agent for the alleviation of obesity, glucose intolerance, and hepatic abnormalities in mouse models. Its potential therapeutic effects extend to type 2 diabetes (T2D), non-alcoholic steatohepatitis and metabolic syndrome, indicating its multifaceted impact on metabolic and nutritional health. The distinct mechanisms of albiflorin and its promotion of glucose uptake in muscles suggest its relevance in addressing T2D, insulin resistance and metabolic syndromes, such as obesity and glucose intolerance (Zhou et al., 2019). Albiflorin is a component of Shakuyakukanzoto (SKT), a formula that was shown to selectively activate the descending noradrenergic systems to modify nociception in streptozotocin-induced diabetic mice. The experimental results showed that orally administered albiflorin at doses of (1, 3 and10 mg/kg) exhibited a tendency to elevate the nociceptive threshold. Notably, the 10 mg/kg dose was effective within the first hour of administration (Lee et al., 2011). Another in vitro analysis showed that albiflorin, at doses ranging from 5 to 20 μM, influenced NF-κB signaling and targeted poly (ADP-ribose) polymerase-1 (PARP1) to mitigate the viability loss of high glucose-induced human umbilical vein endothelial cells (HUVECs). Moreover, albiflorin prevented HUVECs from undergoing apoptosis and from releasing TNF-α, IL-6, and IL-1β, indicating that it may be used to treat vascular problems associated with diabetes(Yang and Yang, 2023).
3.5 Diseases of the blood system
The circulatory system, or bloodstream, is composed of the cardiovascular system, blood vessels and heart. Diseases of the blood system occur in the blood or hematopoietic organs. These diseases include leukemia, primary thrombocytopenia and anemia. According to chemical profile analysis, albiflorin is one of the main constituents of the RPR dispensing granules, which are used in Chinese medicine to eliminate blood stasis. HE staining indicated that albiflorin improved the morphology of the cortex and hippocampus, and ameliorated middle cerebral artery occlusion (MACO)-induced neuronal injury in the trialed groups, which implied its underlying mechanism could be related to the regulation of ferroptosis and autophagy (Zhao et al., 2023). Jiang et al. (2011) studied baishao extract, in which albiflorin was one of the five main components, to investigate its effects on cAMP-phosphodiesterase activity and associated inflammation. Baishao inhibited PDE activity in vitro in a dose-dependent manner. In neutrophils, baishao was evaluated at low and high doses and the high dose dramatically suppressed PDE. Arteriosclerosis is the deposition of lipids, complex carbohydrates and calcium salts in the intima of arteries, accompanied by the proliferation of fibrous tissues, causing a combined action of lipid metabolic disorder and chronic inflammation. Using a mouse arteriosclerosis model, Sun (2017) randomly divided ApoE-/-mice into three groups which are high fat diet group (HFD), control (NC), and high fat diet with albiflorin (HFDA). Investigations conducted on proteins extracted from the thoracic aorta revealed that LOX-1 and NF-κB expressions in the HFD group were considerably higher than those in the NC group; however, these expressions were lower in the HFDA group than those in the HFD group.
3.6 Rheumatic disease
Rheumatism is a group of diseases that mainly involvs the joints, musculoskeletal blood vessels, soft tissues, and connective tissues. Most are autoimmune diseases, the onset of which is subtle and slow, with clinical manifestations of joint swelling, pain and activity limitations. Additional rheumatic illnesses are characterize by dominant aggravation (immune system arthritis) and tissue weakening (Adami et al., 2019). Albiflorin was examined in a rat model of osteoarthritis, and the effects of various concentrations (2.5, 5 or 10 mg/kg) of albiflorin on animals with osteoarthritis were examined. Albiflorin was found to reduce OARSI scores associated with osteoarthritis. Additionally, it increased the number of rat chondrocytes, decreased the number of cells that died, and inhibited the inflammatory response, oxidative stress, and extracellular matrix that were brought on by IL-1β. Moreover, it also prevented chondrocyte damage triggered by IL-1β by inhibiting the NF-κB signaling pathway (Zhou et al., 2023).
3.7 Mental illness
Mental illness or mental health disorders refer to a wide range of mental health conditions that affect behavior, thoughts, emotions, or a combination of all. These disorders can manifest in various forms, ranging from common issues such as anxiety and depression to more severe conditions such as post-traumatic stress disorder (PTSD).
Albiflorin improves depressive behavior and decreases pro-inflammatory cytokines levels. Alginate nanogels were constructed to load albiflorin and subsequently form an albiflorin nanogel loaded self-assembled thermosensitive hydrogel system (albiflorin-NGSTH). According to preliminary findings from behavioral despair tests in mice, albiflorin-NGSTH considerably shortened the time that the mice remained immobile in the tail suspension test but had no effect on anxiety or independent exploratory behavior. Furthermore, low-dose intranasal administration of albiflorin-NGSTH alleviated depressive behavior and reduced proinflammatory cytokine levels. Albiflorin-NGSTH was used to reverse corticosterone and PGE2, which are markers of the hypothalamic pituitary adrenal axis (HPA axis) and proinflammatory cytokines, respectively. Additionally, albiflorin restored neuronal damage in rats with chronic unpredictable mild stress, suggesting grea promise in treating depression (Xu et al., 2021). Using the phytochemical components of Free and Easy Wanderer (FAEW), a poly-herbal concoction that is frequently used in Chinese clinics to treat depression, as a basis for molecular docking and data mining, albiflorin was identified as one of the active ingredients. In alleviating PTSD, albiflorin has been shown to mainly bind to p65-RelA (transcription factor p65 encoded by the RelA gene) through ATP-binding sites and DNA. Importantly, albiflorin has been shown to enter the brain tissue and cross the blood–brain barrier which explains the effects of FAEW on the brain and spinal cord (Hong et al., 2017).
3.8 Analgesia
Three categories can be used to broadly classify pain: nociceptive, pathological and inflammatory. Pain is a common feature of inflammation and injury. Wu et al., (2018a) used the acetic acid writhing method to create a mouse pain model and administered albiflorin via intraperitoneal injection. Using radio immunoprecipitation assay (RIPA), compared with the model group, the contents of β-endorphin (β-EP) in serum and cerebral cortex in the low dose albiflorin group increased, but the content of PGE2 in the cerebral cortex decreased. In TCM, RPA is frequently prescribed for dysmenorrhea. Wu et al., (2018b) examined the effects of albiflorin, an active ingredient in RPA, in a mouse model of primary dysmenorrhea. After injecting oxytocin into the abdominal cavity of mice, the writhing times were recorded, and then the uterine tissues were isolated to determine the content of prostaglandin F2α (PGF2α), Ca2+ ions and nitric oxide (NO). Compared to the model group, PGF2α and Ca2+ contents decreased significantly, NO level in uterine tissue increased substantially, suggesting that the mechanism of action of albiflorin in relieving pain is related to the suppression of prostaglandins that often play a vital role in the initiation of inflammatory responses. Xu and Zhou (2022) created a rat model of chronic constriction injury (CCI) and conducted an immunofluorescence test (samples from the L4-L5 segment of the spinal cord). The results demonstrated that the expression level of NLRP3 in the spinal dorsal horn of the CCI group was higher than that in the sham group, whereas treatment with albiflorin significantly lowered NLRP3 levels.
3.9 Other diseases
Upon combining the retrieved articles, two cell lines were found to be used in examining the potential anti-inflammatory effects of albiflorin in vitro: RAW 264.7 and U937. RAW 264.7 is a murine macrophage cell line derived from a tumor in a male mouse induced with the Abelson murine leukemia virus, whereas U937 is a human histiocytic lymphoma cell line. Both cell lines have the ability to exhibit inflammatory responses when stimulated, making them ideal models for studying the anti-inflammatory effects of albiflorin.
The potential mechanism of action of albiflorin in suppressing inflammation involves the regulation of inflammatory factors through which LPS in RAW 264.7 cells markedly triggers the NF-κB signaling pathway (Bi et al., 2017). Huangqi Jianzhong Tang (HQJZT) has been used to treat several chronic gastrointestinal inflammatory disorders. The phytochemical composition of the extracts was analyzed and albiflorin was identified as one of the active compounds. By examining its inhibitory effects on the production of pro-inflammatory cytokines in U937 cells, the anti-inflammatory activity of HQJZT was determined. In vitro analyses showed that albiflorin inhibited the unveiling of inflammatory cytokines IL-1β, TNF-α and IFN-γ (Nöst et al., 2019). In LPS-induced RAW 264.7 cells, albiflorin decreased the mRNA expression of COX-2 and IL-6 (Wang et al., 2014). In another experimental research, the Jakyak-Gamcho Decoction (JGD), which contains Glycyrrhiza uralensis and P. lactiflora with albiflorin as the active compound, was used to treat pain and muscle spasms. In addition to the target indications, anti-inflammatory properties were also demonstrated following a reduction in IL-1β and TNF-α production, as well as PGE2 in LPS-stimulated RAW 264.7 cells (Guo et al., 2007). HIE leads to brain cell apoptosis. Yang et al. (2022) established a HIE model using neonatal mice and used fresh brain tissue for analysis. The model group exhibited significantly increased rates of brain cell apoptosis, as well as Cleaved Caspase-3 and Cleaved Caspase-9 production in comparison to the sham surgery group. However, there was a declining pattern in the previously mentioned markers in the albiflorin-treated group compared with that in the model group.
4 Metabolism and tissue distribution
Pharmacokinetic studies are essential for monitoring dynamic changes of drugs in the body and for rational drug use in clinical settings (Nakamura et al., 2023). By reviewing the literature, we focused on albiflorin pharmacokinetic parameters, including peak time (tmax), biological half-life (t1/2), peak concentration (Cmax), clearance (CL/F), area under the concentration–time curve (AUC), and the binding rate of plasma protein (BRPP). The pharmacokinetic behavior of albiflorin was compared between acute cholestatic hepatitis and normal rat models. In the low-dose control group (LDCG) and model group (LDMG), rats were orally administered powdered RPR at a dose of 7 g/kg (equivalent to 99 mg/kg albiflorin). In the high-dose control group (HDCG) and model group (HDMG), the same intervention was administered, except that the dose was doubled. By employing liquid chromatography-mass spectrometry (LC-MS/MS) at ten intervals for observation, LDMG plasma albiflorin showed a higher AUC0-∞ (3.16 ± 2.41 vs 1.18 ± 0.25), a longer tmax (0.17 ± 0.09 vs 0.08 ± 0.00), and a lower CL/F (31.37 ± 11.98 vs 87.06 ± 17.90) than those in the LDCG, with the same trend identified when the HDCG and HDMG were compared. Furthermore, the abliflorin concentrations in the model group were higher than those in the normal group at most monitoring points (Jiang et al., 2012). Liu (2009) used equilibrium dialysis to determine the BRPP of albiflorin in two mediators (human and mouse plasma) at different concentrations (1.0, 5.0, 10.0, 15.0, 20.0, 30.0 µg/ml); using high-performance liquid chromatography (HPLC) examination, the highest amount of albiflorin binding to plasma protein in vitro was 10 µg/ml and the BRPP of albiflorin in human plasma was 85.65%.
Using triple-quadrupole linear ion-trap tandem mass spectrometry in conjunction with ultra-high performance liquid chromatography to investigate Weikangling Capsules containing albiflorin as the active ingredient, Liu et al., (2023a) separated rats into two groups: the chronic gastritis model group (MG) and the normal group (NG), each of which was administered the capsules at a dose of 2.16 g/kg following a 12-hour fast. Subsequently, samples from eight organ tissues were collected four times. The results showed that albiflorin reached its Cmax in the liver, kidneys and stomach after 1 h in the NG. Albiflorin could be rapidly taken up by the small intestine and circulate through the bloodstream to reach the stomach. To reduce drug buildup in the body, the liver and kidneys quickly process and eliminate albiflorin. The amount of albiflorin in the stomach and small intestine of the MG were noticeably higher than that in the NG. Albiflorin was administered intragastrically to 10 rats at a dose of 5 mg/kg and urine was collected from five rats within 24 h. Five rats were first administered 10% chloral hydrate intraperitoneally, and bile was collected through a catheter within 24 h after oral administration. The main metabolic pathway of albiflorin is determined to be via the hydrolysis of ester bonds to produce M4, M5, and M6, loss of C7H4O fragment to produce M7 and subsequently followed by the hydrolysis of glycoside bonds (M1, M2) and metabolic binding with glucuronic acid (Fig. 2) (Cao et al., 2015).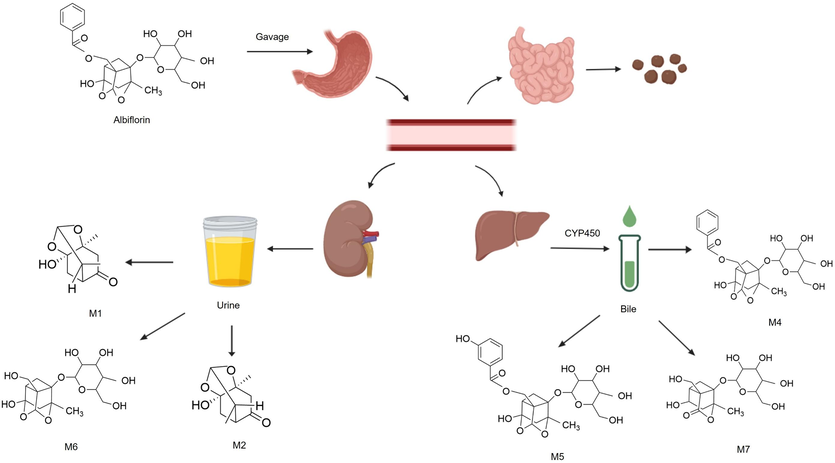
Graphic scheme of pharmacokinetics of albiflorin in vivo. Albiflorin reaches the stomach by gavage. It is then absorbed in the digestive system and distributed to various organs (liver, kidney and small intestine) through blood circulation. In the liver, albiforin is further metabolized into M4, M5 and M7 catalyzed by CYP450s. Most of the parent components and its metabolites are mainly excreted through urine.
5 Toxicity
Toxicity research is of great significance for clinically rational and safe drug use; however, toxicological studies on albiflorin are rare. Zhao collected the blood samples from the forelimb veins of Beagles at 12 time points after oral administration of albiflorin (1 mg/kg) for 7 days, and used LC-MS/MS to measure the amount of albiflorin present in the plasma. The results showed that albiflorin was absorbed and eliminated rapidly in Beagles dogs (t1/2 of was 2 h), and the bioavailability was 2.66%, with no significant drug accumulation, induction or inhibition after repeated administration (Zhao, 2015). Han et al. (2018) used a traditional approach (acute oral toxicity) to evaluate the potential toxicity of albiflorin. It was found that albiflorin administered orally at a dose of 5000 mg/kg (3000 times the clinical dose) did not cause significant changes in the food intake and body weight of Beagles. To further investigate the potential subtle toxicities, next-generation metabolomics was used for retesting. Urine metabolites were analyzed in model rats after oral administration of albiflorin (7 mg/kg) and the top 30 metabolites with the greatest differences before and after albiflorin treatment were determined by data mining using Metaboanalyst 3.0. Disease-related metabolite databases found no statistically significant differences, which again indicated that albiflorin did not have tangible toxic effects on rat metabolism (Han et al., 2018). In summary, low toxicity or non-toxicity of albiflorin is important for a wide range of rational clinical application.
6 Conclusion
This comprehensive review of albiflorin, a pivotal compound derived from P. lactiflora, highlights its therapeutic potential in traditional and modern medicine. Through an intricate examination of its biological profile, anti-inflammatory capabilities, metabolism, tissue distribution, and toxicity, albiflorin has emerged as a promising candidate for treating a diverse array of health conditions involving inflammation (Fig. 3).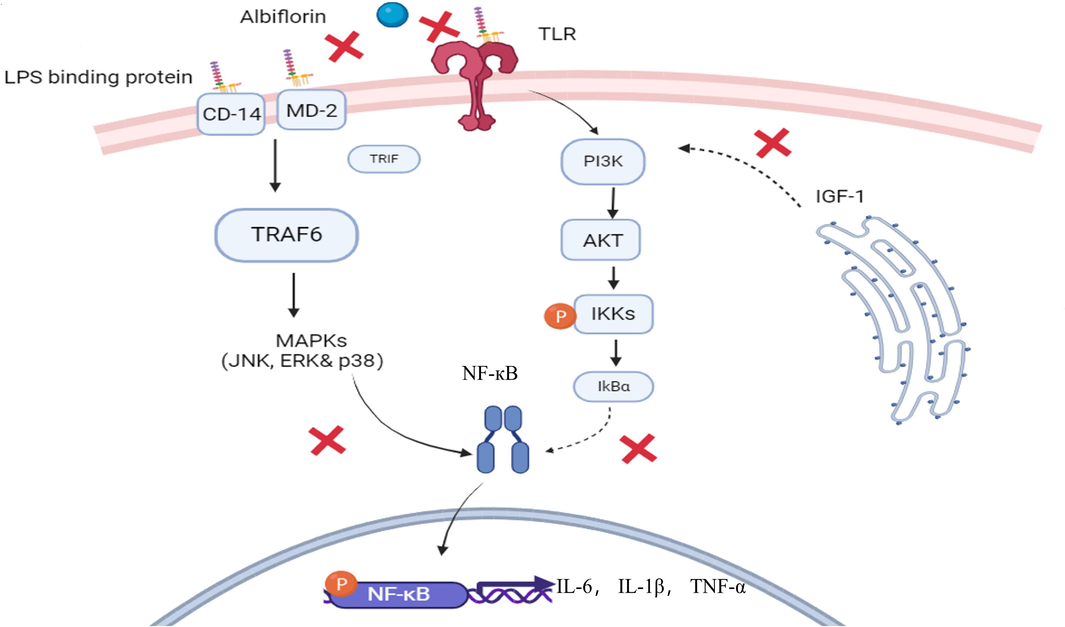
Graphic scheme of potential targets for albiflorin’s anti-inflammatory effects. Albiflorin influences LPS-induced inflammatory responses via inhibiting LPS binding to the lipopolysaccharide complex (CD14, MD-2, and TLR) on the cell membrane. As a result, it interferes with the MARKs and AKT/PI3K pathways. Subsequently, the activation of TRAF6 and the phosphorylation and degradation of IKK are inhibited. Eventually, downstream of p-NF-κB is inhibited by albiflorin, which reducing the production of relevant pro-inflammatory factors.
At present, paeoniflorin is normally regarded as the marker component for RPA and RPR. The European Pharmacopoeia stipulates that the content of paeoniflorin should not be less than 1.6% in RPA and 1.8% in RPR, which is also documented in the 2020 edition of the Chinese Pharmacopoeia (European pharmacopeia, 2023; Chinese pharmacopeia, 2020). On the other hand, according to the Japanese Pharmacopoeia, its content in peony root and powdered peony root should not be less than 2% (Japanese pharmacopeia, 2021). Xu et al. (2022) determined the contents of albiflorin and paeoniflorin in RPA decoction pieces and found that both compounds were abundant. For example, results from 15 different batches indicated that the concentrations of albiflorin were 3.000, 3.259, 3.490, 1.634, 2.690, 2.067, 2.265, 2.238, 2.044, 1.789, 2.079, 1.705, 1.807, 2.200, 2.225 mg/g, while those of paeoniflorin were 2.737, 2.500, 3.365, 3.586, 3.666, 3.966, 3.035, 4.161, 4.028, 3.659, 3.456, 3.266, 3.866, 4.042, 4.175 mg/g. Based on such findings, it is advisable to investigate the use of albiflorin as a characteristic compound for quality evaluation in the future.
Moreover, researchers have suggested that albiflorin could be extracted from other parts of Paeonia suffruticosa in addition to its roots, and found that it can also be obtained from Paeonia rockii pollen (Wang et al., 2018, Wang et al., 2019).
This study elucidates the multifaceted roles of albiflorin in treating diseases across various systems, including respiratory, urinary, gastrointestinal, metabolic, rheumatic, blood system disorders, mental illness, and pain management. Its pharmacokinetic properties and metabolic pathways underscore its compound’s efficient absorption and elimination, suggesting favorable bioavailability and minimal toxicity. This aligns with findings of toxicity studies, which indicate the safety of albiflorin for clinical use at the recommended therapeutic doses. The broad spectrum pharmacological effects of albiflorin, particularly its anti-inflammatory and analgesic properties, make it a valuable resource in the pharmacopeia of natural compounds. The absence of significant toxic effects, even at doses substantially higher than the clinical recommendations, further supports its safe therapeutic application. However, the limited scope of the toxicological studies requires further research to establish its safety profile.
In conclusion, albiflorin is a compound with potent therapeutic benefits, rooted in centuries of TCM use and has been validated by contemporary scientific research. Its efficacy in treating a wide range of diseases, coupled with its favorable pharmacokinetics and safety profile, underscores the need for continued exploration of its mechanisms of action, potential applications, and long-term effects on human health. Despite the positive pharmacological finding on albiflorin, one cold fact is noteworthy. A large proportion of the research deals with formulas containing albiflorin other than the compound itself. The interpretation of albiflorin efficacy might be distorted by the presence of other chemicals; in other words, studies on pure albiflorin deserve more attention. Future studies are warranted to fully harness the therapeutic potential of albiflorin, integrate it into modern medical practices, and strategically utilize its versatile resources, thereby bridging the gap between traditional knowledge and scientific innovations.
CRediT authorship contribution statement
Shasha Sun: Formal analysis, Methodology, Validation, Writing – original draft. Rutendo Betty Jimu: Investigation. Abdillah Khatib Lema: Investigation. Hanaa Elmamoune: Formal analysis, Investigation. Zhiwei Fan: Data curation, Investigation. Chuanshan Jin: Funding acquisition, Methodology. Xiaohui Tong: Conceptualization, Project administration, Writing – review & editing. Rongchun Han: Conceptualization, Supervision, Writing – original draft.
Acknowledgments
This work was supported by the Department of Science and Technology of Anhui Province (202303a07020010), Scientific Research Team Program of Anhui Colleges and Universities (2022AH010036) and Anhui University of Chinese Medicine (RH2300001171, 2021LCTH22).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Phytochemicals, antioxidant activities, and toxicological screening of native Australian fruits using zebrafish embryonic model. Foods. 2022;11:4038.
- [CrossRef] [Google Scholar]
- Differences in the chemical profiles and biological activities of Paeonia lactiflora and Paeonia obovata. J. Med. Food. 2015;18:224-232.
- [CrossRef] [Google Scholar]
- Integrated metabolomics approach reveals the dynamic variations of metabolites and bioactivities in Paeonia ostii ‘Feng Dan’ leaves during development. Int. J. Mol. Sci.. 2024;25(10):59.
- [CrossRef] [Google Scholar]
- Anti-inflammatory effects, SAR, and action mechanism of monoterpenoids from Radix Paeoniae Alba on LPS-stimulated RAW 264.7 cells. Molecules. 2017;22:715.
- [CrossRef] [Google Scholar]
- Albiflorin alleviates ovalbumin (OVA)-induced pulmonary inflammation in asthmatic mice. Am. J. Transl. Res.. 2019;11:7300-7309.
- [Google Scholar]
- Studies on metabolism of total glucosides of paeony from Paeoniae Radix Alba in rats by UPLC-Q-TOF-MS/MS. Biomed. Chromatogr.. 2015;29:1769-1779.
- [CrossRef] [Google Scholar]
- Exploration of the effect and mechanism of Fructus Lycii, Rehmanniae Radix Praeparata, and Paeonia lactiflora in the treatment of AMD based on network pharmacology and in vitro experimental verification. Drug Des. Devel. Ther.. 2021;15:2831-2842.
- [CrossRef] [Google Scholar]
- Editorial Board of Flora of China. 1979, Flora of China. Science Press, Beijing, China, 27, 51.
- Comparison of paeoniflorin and albiflorin on human CYP3A4 and CYP2D6. Evid. Based Complement. Alternat. Med.. 2015;2015:470219
- [CrossRef] [Google Scholar]
- Orthogonal array design for optimizing extraction efficiency of active constituents from Jakyak-Gamcho Decoction, the complex formula of herbal medicines, Paeoniae Radix and Glycyrrhizae Radix. J. Ethnopharmacol.. 2007;113:306-311.
- [CrossRef] [Google Scholar]
- Effect of Paeoniae Radix Rubra (Paeonia lactiflora Pall.) extract on mucin secretion, gene expression in human airway epithelial cells. J. Ethnopharmacol.. 2023;303:115959
- [CrossRef] [Google Scholar]
- Next-generation metabolomics in the development of new antidepressants:using albiflorin as an example. Curr. Pharm. Des.. 2018;24:2530-2540.
- [CrossRef] [Google Scholar]
- Anti-inflammatory and immunomodulatory effects of Paeonia lactiflora pall., a traditional chinese herbal medicine. Front. Pharmacol.. 2011;2:10.
- [CrossRef] [Google Scholar]
- Identification of NF-κB as determinant of posttraumatic stress disorder and its inhibition by the Chinese herbal remedy free and easy wanderer. Front. Pharmacol.. 2017;8:181.
- [CrossRef] [Google Scholar]
- An integrated strategy for the identification and screening of anti-allergy components from natural products based on calcium fluctuations and cell extraction coupled with HPLC-Q-TOF-MS. Anal. Bioanal. Chem.. 2021;413:6253-6266.
- [CrossRef] [Google Scholar]
- Effect of albiflorin on ulcerative colitis model rats based on NF-κB/COX-2 signaling pathway. Acta Chin. Med.. 2022;37:365-370.
- [CrossRef] [Google Scholar]
- Paeonia lactiflora extract suppresses cisplatin-induced muscle wasting via downregulation of muscle-specific ubiquitin E3 ligases, NF-κB signaling, and cytokine levels. J. Ethnopharmacol.. 2021;266:113403
- [CrossRef] [Google Scholar]
- Influence of Paeonia lactiflora roots extract on cAMP-phosphodiesterase activity and related anti-inflammatory action. J. Ethnopharmacol.. 2011;137:914-920.
- [CrossRef] [Google Scholar]
- Comparative pharmacokinetic study of paeoniflorin and albiflorin after oral administration of Radix Paeoniae Rubra in normal rats and the acute cholestasis hepatitis rats. Fitoterapia. 2012;83:415-421.
- [CrossRef] [Google Scholar]
- Age-dependent variations in bioactive compounds in the roots of Himalayan peony (Paeonia emodi Royle) J. Appl. Res. Med. Aromat. Plants. 2023;34:100479
- [CrossRef] [Google Scholar]
- The absolute structures of paeoniflorin, albiflorin, oxypaeoniflorin and benzoylpaeoniflorin isolated from chinese paeony root. Tetrahedron. 1972;28:4309-4317.
- [Google Scholar]
- Paeonia lactiflora Pallas extract alleviates antibiotics and DNCB-induced atopic dermatitis symptoms by suppressing inflammation and changing the gut microbiota composition in mice. Biomed. Pharmacother.. 2022;154:113574
- [CrossRef] [Google Scholar]
- Antinociceptive effect of paeoniflorin via spinal α(2)-adrenoceptor activation in diabetic mice. Eur. J. Pain. 2011;15:1035-1039.
- [CrossRef] [Google Scholar]
- Activation of glutathione peroxidase 4 as a novel anti-inflammatory strategy. Front. Pharmacol.. 2018;9:1120.
- [CrossRef] [Google Scholar]
- Li, H.T., Zeng, X.P., Sun, D.J., Qi, X.F., Li, D.Z., Wang, W., Lin, Y., 2024. Albiflorin alleviates severe acute pancreatitis-associated liver injury by inactivating P38MAPK/NF-κB signaling pathway. Biochem. Genet. Epub ahead of print. doi: 10.1007/s10528-024-10686-9.
- Inhibitory effect of an aqueous extract of Radix Paeoniae Alba on calcium oxalate nephrolithiasis in a rat model. Ren. Fail.. 2017;39:120-129.
- [CrossRef] [Google Scholar]
- Metabolism of Paeoniflorin and Albiflorin. Master’s diss.: Hebei Medical University; 2009.
- Microbiological transformation of paeoniflorin and albiflorin. Zhongguo Zhong Yao Za Zhi. 2010;35:872-875.
- [CrossRef] [Google Scholar]
- Pharmacokinetics and tissue distribution of 12 major active components in normal and chronic gastritis rats after oral administration of Weikangling capsules. J. Ethnopharmacol.. 2023;316:116722
- [CrossRef] [Google Scholar]
- Exploring the possible mechanism(s) underlying the nephroprotective effect of Zhenwu decoction in diabetic kidney disease: an integrated analysis. Phytomedicine. 2023;119:154988
- [CrossRef] [Google Scholar]
- Albiflorin alleviates Ox-LDL-induced human umbilical vein endothelial cell injury through IRAK1/TAK1 pathway. Biomed Res. Int.. 2022;2022:6584645.
- [CrossRef] [Google Scholar]
- Efficacy, safety, and pharmacokinetics of teduglutide in adult Japanese patients with short bowel syndrome and intestinal failure: two phase III studies with an extension. Surg. Today. 2023;53:347-359.
- [CrossRef] [Google Scholar]
- Identification of constituents affecting the secretion of pro-inflammatory cytokines in LPS-induced U937 cells by UHPLC-HRMS-based metabolic profiling of the traditional Chinese medicine formulation Huangqi Jianzhong Tang. Molecules. 2019;24:3116.
- [CrossRef] [Google Scholar]
- Protective effect of Paeonia anomala extracts and constituents against tert-butylhydroperoxide-induced oxidative stress in HepG2 cells. Planta Med.. 2013;79:116-122.
- [CrossRef] [Google Scholar]
- Pharmacopoeia, C.o.C., 2020. Chinese pharmacopeia 2020 Edition. The Medicine Science and Technology Press of China, Beijing, China.
- Protective effects of total glucosides of paeony and its main components paeoniflorin and albiflorin in tetrachlorid-induced acute liver injury. Chin. Arch. Tradit. Chin. Med.. 2020;38 244–247+283
- [CrossRef] [Google Scholar]
- Impact of Paeoniae Albiflorin on Formation of Foam Cells and Atherosclerosis. Doctor’s diss.: Jilin University; 2017.
- Efficacy, chemical constituents, and pharmacological actions of Radix Paeoniae Rubra and Radix Paeoniae Alba. Front. Pharmacol.. 2020;11:1054.
- [CrossRef] [Google Scholar]
- Molecular mechanisms revealed by network pharmacology of Xuebijing on the treatment of acute respiratory distress syndrome caused by novel coronavirus infection. Eur. Rev. Med. Pharmacol. Sci.. 2022;26:2651-2661.
- [CrossRef] [Google Scholar]
- The European Pharmacopoeia Commission, 2023. European Pharmacopoeia 11th ed. Druckerei C.H. Beck, Nördlingen, Germany.
- The Ministry of Health, Labour and Welfare, 2021. The Japanese pharmacopeia 18th ed. Hirokawa-Shoten Ltd., Gunma, Japan.
- Comparative studies of paeoniflorin and albiflorin from Paeonia lactiflora on anti-inflammatory activities. Pharm. Biol.. 2014;52:1189-1195.
- [CrossRef] [Google Scholar]
- Extraction, purification, and component identification of monoterpene glycosides from Paeonia suffruticosa seed meal. Molecules. 2023;28:3498.
- [CrossRef] [Google Scholar]
- Advances in chemical constituents of Paeonia suffruticosa. Chin. Tradit. Patent Med.. 2018;40(01):171-176.
- [Google Scholar]
- Chemical constituents from ethyl acetate extract of Paeonia rockii pollen. Nat. Prod. Res. Dev.. 2019;31:1912-1918.
- [Google Scholar]
- Albiflorin alleviates DSS-induced ulcerative colitis in mice by reducing inflammation and oxidative stress. Iran. J. Basic Med. Sci.. 2023;26:48-56.
- [CrossRef] [Google Scholar]
- Albiflorin attenuates sepsis-induced acute lung injury (ALI) via the TLR-4/ NF-κB pathway. J. Food Bioact.. 2023;107
- [CrossRef] [Google Scholar]
- Monoterpene glycosides from Paeonia delavayi. Fitoterapia. 2007;78:76-78.
- [CrossRef] [Google Scholar]
- Analgesic effect of paeoniflorin and albiflorin on acetic acid induced writhing mice and the expression of β-EP and PGE2. China J. Tradit. Chin. Med. Pharm.. 2018;33:915-918.
- [Google Scholar]
- Effect of paeoniflorin and albiflorin on the spasmolysis and analgesic in mice model of primary dysmenorrhea. Glob. Tradit. Chin. Med.. 2018;11:1670-1674.
- [Google Scholar]
- Quantitative analysis of the multicomponent and spectrum-effect correlation of the antispasmodic activity of Shaoyao-Gancao Decoction. J. Anal. Methods Chem.. 2022;2279404
- [CrossRef] [Google Scholar]
- Alginate nanogels-based thermosensitive hydrogel to improve antidepressant-like effects of albiflorin via intranasal delivery. Drug Deliv.. 2021;28:2137-2149.
- [CrossRef] [Google Scholar]
- Albiflorin relieve neuropathic pain by inhibiting the activation of the spinal cord NLRP3 inflammasome. J. Chengde Med. Coll.. 2022;39:361-365.
- [CrossRef] [Google Scholar]
- Experimental study on the neuronprotection of albiflorin in mice with neonatal hypoxic-ischemic brain injury. Hebei Med.. 2022;28:1414-1420.
- [Google Scholar]
- Screening bioactive compounds from Danggui-shaoyao-san for treating sodium retention in nephrotic syndrome using bio-affinity ultrafiltration. J. Ethnopharmacol.. 2022;292:115171
- [CrossRef] [Google Scholar]
- Albiflorin attenuates high glucose-induced endothelial apoptosis via suppressing PARP1/NF-κB signaling pathway. Inflamm. Res.. 2023;72:159-169.
- [CrossRef] [Google Scholar]
- Albiflorin ameliorates mesangial proliferative glomerulonephritis by PI3K/AKT/NF-κB pathway. Hum. Exp. Toxicol.. 2023;42:9603271221145386
- [CrossRef] [Google Scholar]
- Functional diversity of genes for the biosynthesis of paeoniflorin and its derivatives in Paeonia. Int. J. Mol. Sci.. 2013;14:18502-18519.
- [CrossRef] [Google Scholar]
- Albiflorin ameliorates inflammation and oxidative stress by regulating the NF-κB/NLRP3 pathway in methotrexate-induced enteritis. Int. Immunopharmacol.. 2022;109:108824
- [CrossRef] [Google Scholar]
- Preclinical Pharmacokinetic Study of Albiflorin. Master’s diss.: Jinan University; 2015.
- Paeoniae Radix Rubra extract attenuates cerebral ischemia injury by inhibiting ferroptosis and activating autophagy through the PI3K/AKT signalling pathway. J. Ethnopharmacol.. 2023;315:116567
- [CrossRef] [Google Scholar]
- Study of antidepressant-like effects of albiflorin and paeoniflorin through metabolomics from the perspective of cancer-related depression. Front. Neurol.. 2022;13:828612
- [CrossRef] [Google Scholar]
- Comparative elucidation of age, diameter, and “pockmarks” in roots of Paeonia lactiflora Pall. (Shaoyao) by qualitative and quantitative methods. Front. Plant Sci.. 2021;12:802196
- [CrossRef] [Google Scholar]
- Characterization of the therapeutic profile of albiflorin for the metabolic syndrome. Front. Pharmacol.. 2019;11:1151.
- [CrossRef] [Google Scholar]
- Albiflorin alleviation efficacy in osteoarthritis injury using in-vivo and in-vitro models. J. Pharm. Pharmacol.. 2023;75:1332-1343.
- [CrossRef] [Google Scholar]
- Mechanism of Chuanxiong Rhizoma-Paeoniae Radix Rubra drug pair on intervention of cerebral ischemia based on network pharmacology-molecular docking. Zhongguo Zhong Yao Za Zhi. 2021;46:3007-3015.
- [CrossRef] [Google Scholar]
- Genetic and chemical characterization of white and red peony root derived from Paeonia lactiflora. J. Nat. Med.. 2015;69:35-45.
- [CrossRef] [Google Scholar]







