Translate this page into:
A systematic review on triterpenoids from genus Schisandra: Botany, traditional use, pharmacology and modern application
⁎Corresponding author at: School of Pharmacy, Shaanxi University of Chinese Medicine, No.1, Middle Section of Century Avenue, Qindu District, Xianyang, Shaanxi Province 712046, PR China. zhangnatprod@163.com (Dongdong Zhang)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University. Production and hosting by Elsevier.
Abstract
The genus Schisandra belongs to the family Schisandraceae and grows mainly in south-central and southwestern China. Most of the plants in this genus are used for medicine by their fruits, usually for the treatment of inflammatory diseases. In traditional medicine, Schisandra is also used to stop bleeding, relieve pain, and clear heat. Based on this, many domestic and foreign scholars have conducted systematic studies on its chemical composition, and experimental data show that triterpenoid components are the important material basis for the pharmacological effects of this genus. This paper summarizes the relevant literature on triterpenes of the genus Schisandra from 1983 to 2023. All information and research about this paper was obtained from libraries and digital databases (SciFinder, Medline PubMed, Google Scholar, and CNKI, etc.). At present, there are 335 different kinds of triterpenes isolated from the genus Schisandra, including lanostanes, cycloartanes, nortriterpenoids and pentacyclic triterpenoids, which are mostly found in fruits and vine stems. They have been found to possess various activities such as antitumor, antioxidant, anti-inflammatory, immunomodulatory, neuroprotective, nephroprotective and hepatoprotective.
This review systematically summarizes the literature on triterpenoid composition, traditional applications, pharmacology, and exploitation of the genus Schisandra. We hope this paper will provide a valuable reference for further research and development of the resources of this genus.
Keywords
Schisandra
Traditional uses
Triterpenoids
Pharmacological
Development utilization
Review
1 Introduction
The Schisandraceae contains Schisandra and Kadsura with distributions in southeastern Asia and southeastern North America, with 22 species of the genus Schisandra in China (See Table 1 for specific names and main distribution). At present, research on the genus Schisandra has focused on S. chinensis, S. sphenanthera, S. arisanensis, S. rubriflora, S. propinqua and S. arisanensis. Research on this genus has been increasing this year, especially on its fruits and vine parts. Among them, the fruit of S. chinensis (habitually known as Bei Wu Wei Zi) and the fruit of S. sphenanthera (habitually known as Nan Wu Wei Zi) are included in the Chinese Pharmacopoeia (Gao and Liu, 2010). The genus Schisandra has the properties of astringent, benefiting the qi, nourishing the kidneys and nourishing the heart (Xu et al., 2008). Its efficacy is documented in Shennon’s Herbal Classic and the Compendium of Materia Medica. It is a traditional medicinal plant with a long history of use in China and other parts of Asia. This herb can be applied to treat a wide range of conditions, including respiratory, digestive, and inflammation, as well as fatigue and stress. In traditional medicine, it is often used as a soup and as a powder to treat illnesses (Zhao, 2008). In comparison with the summary by Zhang et al. (Zhang et al., 2022) this paper focuses on the collation of triterpenoids from the genus Schisandra, with a total of 335 compounds, in addition to the traditional uses of the genus and the current status of related exploitation.
No.
Species
Main distribution
Ref.
a
Schisandra sphenanthera
(S. sphenanthera)China (Shanxi, Shannxi, Gansu, Shandong, Jiangsu, Guizhou, Yunnan)
(Liu et al., 2023)
b
Schisandra chinecnsis
(S. chinensis)China (Heilongjiang, Jilin, Liaoning, Inner Mongolia, Hebei), Korea, Japan, Russia
(Liu et al., 2023)
c
Schisandra arisanensis
(S. arisanensis, Subspecies: S. Viridis)China (Taiwan)
(Liu et al., 2023)
d
Schisandra glaucescens
(S. glaucescens)China (Hubei, Sichuan)
(Liu et al., 2023)
e
Schisandra rubriflora
(S. rubriflora)China (Gansu, Hubei, Sichuan, Yunnan, Tibet)
(Liu et al., 2023)
f
Schisandra longipes
(S.longipes)China Southeast
(Liu et al., 2023)
g
Schisandra bicolor
(S. bicolor, Synonym: S. wilsoniana)China (Zhejiang, Jiangxi, Hunan)
(Liu et al., 2023)
h
Schisandra grandiflora
(S. grandiflora)China (Tibet, Yunnan), Nepal, Bhutan, Sikkim,
(Liu et al., 2023)
i
Schisandra henryi
(S. henryi)China (Zhejiang, Jiangxi, Fujian, Henan), Vietnam
(Liu et al., 2023)
j
Schisandra incarnata
(S. incarnata)China (Hubei), East Himalaya
(Liu et al., 2023)
k
Schisandra lancifolia
(S. lancifolia)China (Sichuan, Yunnan)
(Liu et al., 2023)
l
Schisandra micrantha
(S. micrantha)China (Guangxi, Guizhou, Yunnan), Assam, Myanmar
(Liu et al., 2023)
m
Schisandra neglecta
(S. neglecta)China (Sichuan, Yunnan, Tibet), Assam, Bangladesh, China South-Central, East Himalaya, Myanmar, Nepal
(Liu et al., 2023)
n
Schisandra plena
(S. plena)China (Yunnan), India, East Himalaya
(Liu et al., 2023)
o
Schisandra propinqua
(S. propinqua)China (Yunnan, Tibet), Bhutan, Assam, Himalaya, Jawa, Lesser Sunda Is, Myanmar, Nepal, Thailand
(Liu et al., 2023)
p
Schisandra pubescens
(S. pubescens)China (Sichuan)
(Liu et al., 2023)
q
Schisandra sphaerandra
(S. sphaerandra)China (Sichuan, Tibet)
(Liu et al., 2023)
r
Schisandra tomentella
(S. tomentella)China (Sichuan)
(Liu et al., 2023)
s
Schisandra macrocarpa
(S. macrocarpa)China (Yunnan)
(Liu et al., 2023)
t
Schisandra parapropinqua
(S. parapropinqua)China South-Central
(Liu et al., 2023)
u
Schisandra pubinervis
(S. pubinervis)China South-Central
(Liu et al., 2023)
v
Schisandra repanda
(S. repanda)China Southeast
(Liu et al., 2023)
In recent years, scientific research has focused on the potential health benefits of this genus, particularly its antioxidant, anti-inflammatory and neuroprotective properties. Pharmacological studies have shown that the triterpenoid component of the genus is one of the important material bases for this pharmacological action. As research continues, some structurally novel triterpenes have also been discovered, and their pharmacological activities and mechanisms of action are gradually becoming a hot topic of interest for researchers. The triterpenoids micranoic acid B (319) isolated from S. pubescens have been used in the preparation of anti-tumor drugs with significant pharmacological activity against human lung cancer (A549) and nasopharyngeal carcinoma (KB) (Lu et al., 2016). Notably, the triterpenoids from this genus can also be used to develop food and beverages as well as health products, which have good market prospects (Li and Chen, 2013; Cao and Zhang, 2012).
Accordingly, this paper aims to systematically classify the triterpenes in the genus Schisandra and to present the progress made in their traditional applications, pharmacology and development, to provide an important scientific basis for the subsequent comprehensive development of the triterpenoids in this genus. All species queries in Table 1 are from Catalogue of Life China: 2023 (Liu et al., 2023).
2 Search strategy
This paper presents a comprehensive study and analysis of the previously published literature to investigate the traditional uses, phytochemistry and pharmacological activities of plants of the genus Schisandra. Articles from 1987 to 2023 were searched using databases such as Medline PubMed, Science Direct, Sci Finder, Baidu Scholar, Google Scholar and CNKI by using the keywords such as genus Schisandra, S. chinensis, Triterpenoids and uses of Schisandra. Part of the analyzed studies was got by a manual search of articles in the reference lists of the included studies. The chemical structures were drawn using Chem Draw Professional 20.0 software.
3 Physiology, description and distribution
Woody vines, glabrous throughout (branches, leaf backs, and petioles of S. chinensis, S. pubescens and S. sphaerandra pubescent), branchlets with petioles decurrent on both sides of the base into longitudinal stripes or sometimes narrowly winged; with long branches and spur-like short branches growing from axillary buds on long branches. Bud scales 6–8, imbricate, bud's axillary alone or two together or many clustered in leaf axils or at the tips of short branches. Leaves papery, margins membranous decurrent to petiole into narrow wings, flesh with hyaline dots; leaf scars rounded, slightly elevated. Flowers are unisexual, dioecious, rarely monoecious, borne singly in leaf axils or bract axils, often on short branches, in clusters of several due to dense internodes. Tepals 5–12(20), usually the largest in the middle whorl, smaller in the outer and inner whorls; male flowers: stamens 5–60, filaments slender or short, or adnate to the receptacle without filaments. Female flowers: pistils 12–120, free, spirally and densely arranged on the receptacle, which gradually elongates and becomes sparse after pollination. Ovules 2 (3) per locule, superimposed on the ventral suture. The mature carpels are small berries, arranged on the receptacle, forming sparse or dense long spikes of aggregated fruit. The seeds are 2 (3) or sometimes only 1 developed, with a conspicuous hilum, usually U-shaped, Testa smooth or rugose or tuberculate-like projection (Yang et al., 2002; Guo et al., 2015; Sun, 2006).
The entire genus has about 30 species, mainly in eastern and south-eastern Asia, with only one species (Schisandra glabra) in the American continent (Jose and Patricia, 1998). 22 species in China, distributed throughout the country (except Xin Jiang), mostly growing in valleys and among jungles (Schisandra in Flora of China @ efloras.org, 2020). Fig. 1 illustrates the distribution of the genus Schisandra.
Distribution of the genus Schisandra (the distribution of this genus in China is marked in red).
4 Traditional use of Schisandra
The genus Schisandra is widely distributed throughout China and has a long history of use in China for its rich biological and pharmacological activity, which is why it is often used to treat a variety of diseases. In the genus Schisandra, the fruit is the main part of the medicine. In different regions, the fruits of many species are used as a substitute for “S chinensis” (the fruit of S. chinensis). In Yunnan Province, the fruits of S. propinqua, S. rubriflora, S. neglecta, S. lancifolia, and S. micrantha are used as substitutes. In Sichuan Province, the fruits of S. henryi and S. rubriflora are used as substitutes; in Zhejiang Province, the fruits of S. viridis are used as substitutes. In the Ming Dynasty, Li Shizhen recorded the difference between the efficacy of the northern and southern species of Schisandra, describing that there are northern and southern varieties of Schisandra, with the southern variety being red and the northern variety black, with the northern variety being more tonic. In the Tang Materia, it is recorded that Schisandra has the effect of stopping loss, sweating and diarrhea. It is worth mentioning that its wide distribution has also given rise to ethnic uses with regional characteristics. In Tibetan areas of China, the fruit of Schisandra is often used to treat indigestion and diarrhea from enteritis. In addition, similarly distinctive ethnomedicines include Mongolian medicine and Miao medicine. Its development to date has also been helpful to modern medicine, being used to treat rheumatism, stomach pain, gastritis, dysentery and urticaria. Table 2 describes the applications of different plants of the genus Schisandra in traditional medicine.
Species
Local name
Dosage form
parts
Traditional clinical uses
Ref
S. sphenanthera
Man shan xiang,
Yan pi pa,
Hong ling ziDecoction, Liquor, Powder(orally)
fruits
insomnia, palpitations, calming and tranquilizing, cough.
(Jiang, 2005; Zhang et al., 2014)
Powder (external application)
stem, root
dysmenorrhea.
S. chinensis
Xuan ji, Hui ji,
Shan hua jiao,
Wu wei, Wu mei ziDecoction, Liquor, Powder(orally),
Liniment (external application)fruits
palpitations, night sweating, rheumatism, cough.
(Jiang, 2005; Zhang et al., 2014)
stem, root
chronic gastritis, acute gastroenteritis, stomachache.
S. arisanensis
Jin bei teng,
Jin la baLiquor (orally)
fruits
insomnia, palpitations, calming and tranquilizing.
(Jiang, 2005; Zhang et al., 2014)
S. glaucescens
Xi wu wei,
Cheng gan maFruits, Decoction(orally)
fruits
cough, rheumatism, gall tumors, goiter.
(Liu et al., 2012b)
S. rubriflora
Dian wu wei, Hong xue teng, Guo shan long, Xiang xue teng
Decoction(orally), Fruits
fruits
rheumatism, neurological disorders.
(Jiang, 2005; Zhang et al., 2014)
stem
rheumatism, stomachache, indigestion.
S. bicolor
Xiang su zi,
Er se nei feng xiaoDecoction, Liquor (orally)
fruits
chest tightness, gastrointestinal distress.
(Jiang, 2005; Zhang et al., 2014)
Wine(orally)
stem, root
traumatic injuries, excessive fatigue.
S. grandiflora
Decoction(orally)
fruits
analgesic, cough, tonics for the kidneys.
(Liu et al., 2012b; Zhang et al., 2014)
stem, root
tonics for the kidneys.
S. henryi
Da feng teng,
Yao wu wei,
Huang zuanDecoction, Wine(orally)
fruits
back pain, lumbar strain.
(Liu et al., 2012b; Wei et al., 2009)
stem, root
soreness in the limbs, irregular menstruation, stomachache.
S. incarnata
Xi wu wei
Fruits
fruits
lumbar strain, gastrointestinal
discomfort, cough, night sweating.(Liu et al., 2012b)
S. lancifolia
Diao diao xiang
Decoction(orally)
stem, root
bruises and injuries, fractures, hemostasis.
(Liu et al., 2012b)
Fruits
fruits
Neurasthenia.
S. micrantha
Xiang shi teng,
Da shen jin,
Jie jin tengDecoction, Wine(orally)
fruits
nephrosis, menstrual irregularities.
(Liu et al., 2012b)
stem, root
rheumatism, abdominal pain.
S. neglecta
Xiao xue teng
Decoction, Wine(orally), External
application, Fruitsfruits
cough, clears heat.
(Liu et al., 2012b)
stem
diarrhea, relieving pain.
S. plena
Fu ban huang long teng
Decoction
whole plant
clears heat, relieving pain.
(Liu et al., 2012b)
S. propinqua
Zhong jian wu wei zi
Decoction(orally), External
applicationfruits
stops bleeding, clears heat.
(Liu et al., 2012b)
stem, root
stomach pain, gastritis, menstrual disorders, fractures, blood clots.
S. pubescens
Mao mai wu wei zi
Fruits
fruits
cough, night sweating.
(Liu et al., 2012b)
Decoction(orally)
stem
exertional injuries, diarrhea.
S. sphaerandra
Shan bao gu,
Shan hua jiao,
Xiao xue pianFruits, Liquor (orally)
stem, root
rheumatic arthritis, joint pain, abdominal distension, dysentery.
(Jiang, 2005😉
S. tomentella
Mao bei wu wei zi
vinum, wine(orally)
fruits
diarrhea, palpitation, insomnia.
(Jiang, 2005)
S. viridis
Guo shan feng,
Nei feng xiao,
Bai zuanDecoction (orally or external
application)fruits
urticaria, zoster, rheumatism, stomachache.
(Jiang, 2005)
Therefore, in this article, we have collected information on the characteristic folk uses of the Schisandra genus, including empirical prescriptions and hospitalized preparations for folk use (Liu et al., 2012b; Jiang, 2005; Zhang et al., 2014; Wei et al., 2009).
5 Triterpenoids
Structurally diverse triterpenoids have been isolated from the genus Schisandra. There are 335 triterpenoids in total, and these compounds are divided into four major groups: 72 lanostanes, 42 cycloartanes, 207 nortriterpenoids and 14 pentacyclic triterpenoids. Compound names and sources are shown in Table 3, and their chemical structures are shown in Figs. 2-15.
NO.
Compound
Source
Parts
Ref
5.1.1. Intact lanostanes
1
schiglausin R
g
stems
(Liu, 2018)
2
schisandronic acid
b
stems
(Suh, 2014)
3
3β-hydroxy-lanosta-8,24Z-dien-26-oic acid
o
stems
(Liu and Tao, 1992)
4
schipropinqua acid E
o
stems and leaves
(Ding, 2018)
5
schipropinqua acid F
o
stems and leaves
(Ding, 2018)
6
schipropinqua acid G
o
stems and leaves
(Ding, 2018)
7
schipropinqua acid H
o
stems and leaves
(Ding, 2018)
8
schiglausin R
d
furits
(Yu et al., 2016)
9
(24E)-3α,12α-dihydroxy-lanost-24-en-26-oic acid
d
stems
(Wu, 2016)
10
coccinic acid
d
stems
(Wu, 2016)
11
anwuweizonic acid
d
stems
(Wu, 2016)
12
iso-anwuweizic acid
a
furits
(Zhang, 2008)
13
schisactone D
a
furits
(Zhang, 2008)
14
schisanlactone
a
furits
(Zhang, 2008)
15
3β-hydroxyl-5α,8α-epi-dioxyergosta-6,22-diene
a
furits
(Li and Sun, 2004)
16
12β-hydroxycoccinic acid
a
furits
(Du, 2007)
17
iso-schizandrlic acid
a
furits
(Du, 2007)
18
schizandronic acid
a
furits
(Du, 2007)
19
schisanol
c
leaves
(Hou et al., 2016)
5.1.2. 3,4-secolanostane
20
manwuweizic acid
g
stems
(Liu, 2018)
21
schiglausin G
g
stems
(Liu, 2018)
22
kadsuric acid
b
stems
(Guo et al., 2019)
23
schipropinqua acid N
o
stems and leaves
(Ding, 2018)
24
kadcoccinic acid O
j
stems
(Zhou, 2018)
25
29-hydroxyl schiglausin D
c
stems
(Wang et al., 2021)
26
6-hydroxyl schiglausin G
c
stems
(Wang et al., 2021)
27
schisanlactone A
b
furits
(Liu et al., 1983)
28
schinalactone D
b
stems and leaves
(Qiu et al., 2018)
29
schisanchinlactone B
b
stems and leaves
(Zhao et al., 2020)
30
schisanlactone C
b
stems and leaves
(Qiu et al., 2018)
31
schisphenthin C
a
furits
(Liu et al., 2017a)
32
schisanlactone I
b
stems and leaves
(Qiu et al., 2018)
33
schiglausin P
d
furits
(Yu et al., 2016)
34
schiglausin Q
d
furits
(Yu et al., 2016)
35
schisanlactone H
a
furits
(Du, 2007)
36
schinchinenlactone D
b
roots
(Song et al., 2015)
37
schisphendilactone B
b
stems and leaves
(Qiu et al., 2018)
38
schinchinenlactone B
b
stems and leaves
(Song et al., 2013)
39
schinchinenlactone C
b
stems and leaves
(Song et al., 2013)
40
schisanchinlactone A
b
stems and leaves
(Zhao et al., 2020)
41
schisanchinlactone C
b
stems and leaves
(Zhao et al., 2020)
42
schinchinenin B
b
stems and leaves
(Song et al., 2013)
43
schinchinenin C
b
stems and leaves
(Song et al., 2013)
44
schinchinenin D
b
stems and leaves
(Song et al., 2013)
45
schinchinenin E
b
stems and leaves
(Song et al., 2013)
46
schinchinenin F
b
stems and leaves
(Song et al., 2013)
47
henrischinin A
b
stems and leaves
(Song et al., 2013)
48
henrischinin B
b
stems and leaves
(Song et al., 2013)
49
schinchinenin G
o
stems
(Liu and Tao, 1992)
50
schipropinqua acid k
o
stems and leaves
(Ding, 2018)
51
schipropinqua acid L
o
stems and leaves
(Ding, 2018)
52
schipropinqua acid M
o
stems and leaves
(Ding, 2018)
53
schipropinqua acid M 3-methyl ester
o
stems and leaves
(Ding, 2018)
54
kadsuric acid 3-methyl ester
d
stems
(Wu, 2016)
55
kadsuricacid
a
furits
(Du, 2007)
56
kadpolysperin J
d
stems
(Wu, 2016)
57
schinalactone E
j
stems
(Zhou, 2018)
58
12-hydroxyschiglausin B
d
stems
(Liu et al., 2018)
59
sphendilactone
a
stems
(Huang and Qin, 2016)
60
sphenantherain A
a
furits
(Zhang, 2008)
61
sphenantherain B
a
furits
(Zhang, 2008)
62
sphenantherain C
a
furits
(Zhang, 2008)
63
schiglausin A
a
furits
(Liu et al., 2017a)
64
6-hydroxyl schiglausin A
c
stems
(Wang et al., 2021)
65
schisanlactone E
a
furits
(Du, 2007)
66
schisphenthin A
a
furits
(Liu et al., 2017a)
67
schisphenthin B
a
furits
(Liu et al., 2017a)
68
schiglausin C
a
furits
(Liu et al., 2017a)
69
schisanlactone G
a
furits
(Ren, 2012)
70
schisanlactone B
a
furits
(Liu et al., 2012a)
71
henrischinin A
i
stems and leaves
(Xue et al., 2011)
72
henrischinin B
i
stems and leaves
(Xue et al., 2011)
5.2.1. Intact cycloartanes
73
ganwuweizic acid
b
stems
(Guo et al., 2019)
74
schipropinqua acid I
o
stems and leaves
(Ding, 2018)
75
schipropinqua acid J
o
stems and leaves
(Ding, 2018)
76
schiglausin S
d
furits
(Yu et al., 2016)
77
cycloart-23-ene-3,25-diol
a
furits
(Li and Sun, 2004)
78
schinensin D
a
roots
(Tanaka et al., 2021)
79
lancifodilactone H
k
stems and leaves
(Xiao et al., 2006b)
5.2.2. 3,4 secocycloartane
80
schisanbilactone A
g
stems
(Ma et al., 2009)
81
schisanbilactone B
g
stems
(Ma et al., 2009)
82
kadsulactone A
g
stems
(Ma et al., 2009)
83
kadsudilactone C
b
roots
(Song et al., 2015)
84
schisphendilactone A
a
stems
(Liang et al., 2013a)
85
schinensin C
a
roots
(Tanaka et al., 2021)
86
kadsudilactone B
j
stems
(Zhou, 2018)
87
kadsuphilactone B
b
stems and leaves
(Qiu et al., 2018)
88
renchanglactone A
a
furits
(Liu et al., 2017a)
89
wuweizilactone acid
b
stems and leaves
(Huang et al., 2008)
90
schisanlactone J
b
stems and leaves
(Qiu et al., 2018)
91
propiniclactone A
b
roots
(Song et al., 2015)
92
schinchinenin G
b
stems and leaves
(Song et al., 2013)
93
schinchinenin H
b
stems and leaves
(Song et al., 2013)
94
henrischinin C
b
stems and leaves
(Song et al., 2013)
95
schipropinqua acid A
o
stems and leaves
(Ding, 2018)
96
schipropinqua acid B
o
stems and leaves
(Ding, 2018)
97
schipropinqua acid B 3-methy1 ester
o
stems and leaves
(Ding, 2018)
98
schipropinqua acid C
o
stems and leaves
(Ding, 2018)
99
schiglausin O
d
stems
(Wu, 2016)
100
6β-hydroxyl nigranoic acid
d
stems
(Wu, 2016)
101
3,4-seco(24Z)-cycloart-4(28),24-diene-3,26-dioic-3-methyl ester
d
stems
(Wu, 2016)
102
abiesatrine O
j
stems
(Zhou, 2018)
103
nigranoic acid
p
stems
(Sun et al., 1996)
104
schipropinqua acid D
o
stems and leaves
(Ding, 2018)
105
schiglausin T
d
furits
(Yu et al., 2016)
106
lancifoic acid A
d
stems
(Wu, 2016)
107
propindilactone Z
o
stems and leaves
(Ding, 2018)
108
12-hydroxykadsuphilactone B
d
stems
(Liu et al., 2018)
109
kadsudilactone A
j
stems
(Zhou, 2018)
110
20R-hydroxyschinalactone C
d
stems
(Liu et al., 2018)
111
propinic lactone A
a
furits
(Liu et al., 2017a)
112
3-O-methylchangnanic acid
a
roots
(Tanaka et al., 2021)
113
henrischinin C
i
stems and leaves
(Xue et al., 2011)
114
propinic lactone C
j
stems
(Zhou, 2018)
5.3.1. Schiartane
115
wuweizidilactone C
b
aerial parts
(Huang et al., 2007b)
116
wuweizidilactone D
b
aerial parts
(Huang et al., 2007b)
117
wuweizidilactone E
b
aerial parts
(Huang et al., 2007b)
118
wuweizidilactone F
b
aerial parts
(Huang et al., 2007b)
119
2β-hydroxy-micrandilactone C
b
leaves and stems
(Wang et al., 2011)
120
schinchinenlactone A
b
stems and leaves
(Qiu et al., 2018)
121
schinchinenin A
b
stems and leaves
(Song et al., 2013)
122
rubriflordilactone B
e
stems and leaves
(Xiao et al., 2006c)
123
schinensin B
a
roots
(Tanaka et al., 2021)
124
henridilactone J
i
stems and leaves
(He et al., 2020)
125
henridilactone k
i
stems and leaves
(He et al., 2020)
126
lancifodilactone F
k
stems and leaves
(Xiao et al., 2005a)
127
micrandilactone B
l
stems and leaves
(Li, 2005)
128
micrandilactone C
l
stems and leaves
(Li, 2005)
129
propindilactones k
o
stems
(Lei, 2009)
130
propindilactone L
o
stems
(Lei, 2009)
131
propindilactone M
o
stems
(Lei, 2009)
132
propindilactone O
o
stems
(Lei, 2009)
133
propindilactone P
o
stems
(Lei, 2009)
134
hydroxymicrandilactone C
b
stems
(Wang et al., 2011)
135
propincilactone E
o
stems
(Lei et al., 2008)
136
propincilactone F
o
stems
(Lei et al., 2008)
137
propincilactone G
o
stems
(Lei et al., 2008)
138
propincilactone H
o
stems
(Lei et al., 2008)
139
propincilactone I
o
stems
(Lei et al., 2008)
140
propincilactone J
o
stems
(Lei et al., 2008)
5.3.2. 18-Norschiartane
141
wuweizidilactone A
b
aerial parts
(Huang et al., 2007b)
142
wuweizidilactone B
b
aerial parts
(Huang et al., 2007b)
143
wuweizidilactone G
b
stems and leaves
(Huang et al., 2008)
144
wuweizidilactone H
b
stems and leaves
(Huang et al., 2008)
145
wuweizidilactone I
b
furits
(Xue et al., 2010)
146
19(R)-hydroxylwuwei-zidilactone H
b
furits
(Li et al., 2017)
147
propindilactone W
o
stems and leaves
(Ding, 2018)
148
propindilactone X
o
stems and leaves
(Ding, 2018)
149
propindilactone Y
o
stems and leaves
(Ding, 2018)
150
schirbidilactone F
e
stems and leaves
(Xiao et al., 2010a)
151
rubriflordilactone A
e
stems and leaves
(Xiao et al., 2006c)
152
schinensin A
a
roots
(Tanaka et al., 2021)
153
wilsonianadilactone F
g
stems and leaves
(Yang et al., 2011)
154
henridilactone F
i
stems and leaves
(He et al., 2020)
155
henridilactone G
i
stems and leaves
(He et al., 2020)
156
henridilactone H
i
stems and leaves
(He et al., 2020)
157
henridilactone I
i
stems and leaves
(He et al., 2020)
158
wuwezidilactone Q
k
stems
(Liu et al., 2015)
159
wuwezidilactone R
k
stems
(Liu et al., 2015)
160
lancifodilactone A
k
stems and leaves
(Li et al., 2002)
161
wuweizidilactone Q
c
stems and leaves
(Liu et al., 2017b)
5.3.3. Wuweiziartane and Lancischiartane
162
schintrilactone A
b
furits
(Huang et al., 2007c)
163
schintrilactone B
b
furits
(Huang et al., 2007c)
164
schintrilactone C
a
furits
(Jiang, 2011)
165
schintrilactone D
a
furits
(Jiang, 2011)
166
propintrilactone A
o
stems
(Lei et al., 2010)
167
propintrilactone B
o
stems
(Lei et al., 2010)
168
propintrilactone C
a
stems
(Jiang et al., 2011)
169
arisandilactone A
a
furits
(Cheng et al., 2010a)
170
schicagenin C
a
stems
(Liang, 2013)
171
arisanlactone A
c
furits
(Cheng et al., 2010b)
172
propinqtrilactone A
o
stems and leaves
(Ding, 2018)
173
propinqtrilactone B
o
stems and leaves
(Ding, 2018)
174
schilancitrilactone A
a
stems
(Liang, 2013)
175
schilancidilactone A
k
stems and leaves
(Luo et al., 2009)
176
schilancidilactone B
k
stems and leaves
(Luo et al., 2009)
177
schilancitrilactone B
k
stems
(Luo et al., 2012)
178
schilancitrilactone C
k
stems
(Luo et al., 2012)
5.3.4. Preschisanartane
179
2β-hydroxyarisanlactone C
c
furits
(Cheng et al., 2010b)
180
schindilactone D
c
furits
(Cheng et al., 2010b)
181
schindilactone E
c
furits
(Cheng et al., 2010b)
182
preschisanartanin A
c
furits
(Cheng et al., 2010b)
183
preschisanartanin B
c
furits
(Cheng et al., 2010b)
184
pre-schisanartanin A
b
aerial part
(Huang et al., 2007a)
185
pre-schisanartanin B
b
stems and leaves
(Huang et al., 2008)
186
preschisanartanin Q
e
stems and leaves
(Hu et al., 2019)
187
preschisanartanin R
e
stems and leaves
(Hu et al., 2019)
188
preschisanartanin S
e
stems and leaves
(Hu et al., 2019)
189
preschisanartanin T
e
stems and leaves
(Hu et al., 2019)
190
preschisanartanin U
e
stems and leaves
(Hu et al., 2019)
191
preschisanartanin X
e
stems and leaves
(Hu et al., 2019)
192
preschisanartanin V
e
stems and leaves
(Hu et al., 2019)
193
preschisanartanin W
e
stems and leaves
(Hu et al., 2019)
194
preschisanartanin Y
e
stems and leaves
(Hu et al., 2019)
195
preschisanartanin Z
e
stems and leaves
(Hu et al., 2019)
196
preschidilactone A
e
stems and leaves
(Hu et al., 2019)
197
pre-schisanartanin C
a
stems
(He, 2009)
198
pre-schisanartanin D
a
stems
(He, 2009)
199
pre-schisanartanin E
a
stems
(He, 2009)
200
pre-schisanartanin H
a
stems
(He, 2009)
201
pre-schisanartanin F
a
stems
(He, 2009)
202
pre-schisanartanin G
a
stems
(He, 2009)
203
pre-schisanartanin I
a
stems
(He, 2009)
204
pre-schisanartanin J
a
stems
(He, 2009)
205
schilancidilactone V
g
furits
(Gao et al., 2013)
206
schilancidilactone W
g
furits
(Gao et al., 2013)
207
henridilactone E
i
stems and leaves
(He et al., 2020)
208
schisanartanin A
j
stems
(Zhou, 2018)
209
schisanartanin B
j
stems
(Zhou, 2018)
210
pre-schisanartanin P
c
stems and leaves
(Liu et al., 2017b)
5.3.5. 16,17-secopreschisanartane and 14,15-secopreschisanartan
211
schisdilactone H
b
stems
(Li et al., 2013a)
212
schisdilactone I
b
stems
(Li et al., 2013a)
213
schicagenin A
a
rattans
(Li et al., 2013b)
214
schicagenin F
a
stems
(Liang, 2013)
215
schicagenin G
a
stems
(Liang, 2013)
216
schicagenin H
a
stems
(Liang, 2013)
217
isoschicagenin C
a
stems
(Liang, 2013)
218
schicagenin B
a
stems
(Liang, 2013)
219
lancifonin A
k
stems
(Shi et al., 2014)
220
lancifonin B
k
stems
(Shi et al., 2014)
221
lancifonin C
k
stems
(Shi et al., 2014)
222
lancifonin D
k
stems
(Shi et al., 2014)
223
schicagenin D
m
stems
(Liang et al., 2013b)
224
schicagenin E
m
stems
(Liang et al., 2013b)
225
schisdilactone J
a
stems
(Wang et al., 2012)
226
spiroschincarin A
j
furits
(Song et al., 2017)
227
spiroschincarin B
j
furits
(Song et al., 2017)
228
spiroschincarin C
j
furits
(Song et al., 2017)
229
spiroschincarin D
j
furits
(Song et al., 2017)
230
spiroschincarin E
j
furits
(Song et al., 2017)
5.3.6. Lancifoartane and 12,22-Cyclopreschisanartane
231
lancifonin E
k
stems
(Shi et al., 2014)
232
lancifonin F
k
stems
(Shi et al., 2014)
233
lancolide A
k
stems
(Shi et al., 2013)
234
lancolide C
k
stems
(Shi et al., 2013)
235
lancolide B
k
stems
(Shi et al., 2013)
236
lancolide D
k
stems
(Shi et al., 2013)
5.3.7. 14-Nor-16,17-secopreschis anartane and 15,17-Dicyclolancifoartan
237
schinesdilactone A
a
stems
(Wang et al., 2012)
238
schinesdilactone B
a
stems
(Wang et al., 2012)
239
schisphenin A
a
stems
(Liang, 2013)
240
schisphenin B
a
stems
(Liang, 2013)
241
schisphenin C
a
stems
(Liang, 2013)
242
schisphenin D
a
stems
(Liang, 2013)
5.3.8. Pchisanartane
243
schinarisanlactone A
c
furits
(Lin et al., 2011)
244
arisanlactone B
c
furits
(Cheng et al., 2010b)
245
arisanlactone C
c
furits
(Cheng et al., 2010b)
246
arisanlactone D
c
furits
(Cheng et al., 2010b)
247
schindilactone A
b
aerial part
(Huang et al., 2007a)
248
schindilactone B
b
aerial part
(Huang et al., 2007a)
249
schindilactone C
b
aerial part
(Huang et al., 2007a)
250
schindilactone F
b
stems and leaves
(Huang et al., 2008)
251
sphenadilactone F
a
stems
(He, 2009)
252
lancifodilactone O
k
stems and leaves
(Xiao et al., 2010b)
253
lancifodilactone P
k
stems and leaves
(Xiao et al., 2010b)
254
lancifodilactone Q
k
stems and leaves
(Xiao et al., 2010b)
255
20-hydroxymicrandilactone D
k
stems and leaves
(Xiao et al., 2010c)
256
schindilactone G
b
stems and leaves
(Huang et al., 2008)
257
schindilactone H
b
furits
(Xue et al., 2010)
258
propindilactone V
o
stems and leaves
(Ding, 2018)
259
schirbidilactone A
e
stems and leaves
(Xiao et al., 2010a)
260
wilsonianadilactone D
g
stems and leaves
(Yang et al., 2011)
261
wilsonianadilactone E
g
stems and leaves
(Yang et al., 2011)
262
wilsonianadilactone B
g
stems and leaves
(Yang et al., 2008)
263
wilsonianadilactone C
g
stems and leaves
(Yang et al., 2008)
264
lancifodilactone L
k
stems and leaves
(Xiao et al., 2006a)
265
lancifodilactone M
k
stems and leaves
(Xiao et al., 2006a)
266
rubriflorin A
e
stems and leaves
(Xiao et al., 2007c)
267
rubriflorin B
e
stems and leaves
(Xiao et al., 2007c)
268
rubriflorin C
e
stems and leaves
(Xiao et al., 2007c)
269
rubriflorin D
e
stems and leaves
(Xiao et al., 2007a)
270
rubriflorin E
e
stems and leaves
(Xiao et al., 2007a)
271
rubriflorin F
e
stems and leaves
(Xiao et al., 2007a)
272
rubriflorin G
e
stems and leaves
(Xiao et al., 2007a)
273
rubriflorin H
e
stems and leaves
(Xiao et al., 2007a)
274
rubriflorin i
e
stems and leaves
(Xiao et al., 2007a)
275
rubriflorin J
e
stems and leaves
(Xiao et al., 2007a)
276
schirbidilactone B
e
stems and leaves
(Xiao et al., 2010a)
277
schirbidilactone C
e
stems and leaves
(Xiao et al., 2010a)
278
schirbidilactone D
e
stems and leaves
(Xiao et al., 2010a)
279
schirbidilactone E
e
stems and leaves
(Xiao et al., 2010a)
280
sphenadilactone A
a
stems and leaves
(Xiao et al., 2006d)
281
sphenadilactone B
a
stems and leaves
(Xiao et al., 2006d)
282
sphenalactone A
a
stems and leaves
(Xiao et al., 2007b)
283
sphenalactone B
a
stems and leaves
(Xiao et al., 2007b)
284
sphenalactone C
a
stems and leaves
(Xiao et al., 2007b)
285
sphenalactone D
a
stems and leaves
(Xiao et al., 2007b)
286
sphenadilactone C
a
stems and leaves
(Xiao et al., 2008)
287
sphenadilactone D
a
stems and leaves
(He et al., 2012)
288
sphenadilactone E
a
stems and leaves
(He et al., 2012)
289
wilsonianadilactone A
g
stems and leaves
(Yang et al., 2008)
290
schigrandilactone A
h
stems
(Xiao et al., 2009)
291
schigrandilactone B
h
stems
(Xiao et al., 2009)
292
schigrandilactone C
h
stems
(Xiao et al., 2009)
293
henridilactone A
i
stems and leaves
(Li et al., 2004a)
294
henridilactone B
i
stems and leaves
(Li et al., 2004a)
295
henridilactone C
i
stems and leaves
(Li et al., 2004a)
296
henridilactone D
i
stems and leaves
(Li et al., 2004a)
297
henridilactone L
i
stems and leaves
(He et al., 2020)
298
henridilactone M
i
stems and leaves
(He et al., 2020)
299
henridilactone N
i
stems and leaves
(He et al., 2020)
300
henridilactone O
i
stems and leaves
(He et al., 2020)
301
lancifodilactone R
k
stems and leaves
(Xiao et al., 2010b)
302
lancifodilactone I
k
stems and leaves
(Xiao et al., 2006a)
303
lancifodilactone J
k
stems and leaves
(Xiao et al., 2006a)
304
lancifodilactone L
k
stems and leaves
(Xiao et al., 2006a)
305
lancifodilactone N
k
stems and leaves
(Xiao et al., 2006a)
306
lancifodilactone G
k
stems and leaves
(Xiao et al., 2005b)
307
lancifodilactone E
k
stems and leaves
(Li et al., 2004b)
308
micrandilactone A
l
stems and leaves
(Li et al., 2003a)
309
negleschidilactone A
m
stems
(Liang et al., 2013b)
310
negleschidilactone B
m
stems
(Liang et al., 2013b)
311
lancifodilactone D
k
stems and leaves
(Li et al., 2004b)
312
lancifodilactone C
k
stems and leaves
(Li et al., 2004b)
313
lancifodilactone B
k
stems and leaves
(Li et al., 2004b)
5.3.9. 8,9-seco-12,22-cyclopreschisanartane and 9,23:12,22-Dicyclolancischiartane
314
schincalactone A
j
stems
(Song et al., 2018)
315
schincalactone B
j
stems
(Song et al., 2018)
316
schincalide A
j
stems
(Zhou, 2018)
317
schincalide B
j
stems
(Zhou et al., 2016)
5.3.10. Octanortriterpenoids and others
318
micranoic acid A
l
stems and leaves
(Li et al., 2003b)
319
micranoic acid B
l
stems and leaves
(Li et al., 2003b)
320
11β-hydroxylkadsuphilactone A
p
stems
(Wang et al., 2016)
321
chinorlactone A
b
stems
(Yang et al., 2022)
5.4. Pentacyclic triterpenoids
322
oleanolic acid
a
furits
(Du, 2007)
323
β-Amyrin
h
stems
(Zong et al., 2013)
324
taraxerol
h
stems
(Zong et al., 2013)
325
maslinic acid
h
stems
(Zong et al., 2013)
326
ursolic acid
h
stems
(Shi et al., 2012; Zong et al., 2013)
327
acetylursolic acid
h
stems
(Zong et al., 2013)
328
2α, 3α-dihydroxyurs-12-en-28-oic acid
h
stems
(Zong et al., 2013)
329
corosolic acid
h
stems
(Zong et al., 2013)
330
asiatic acid
h
stems
(Zong et al., 2013)
331
2α, 3α, 23-trihydroxyurs-12-en-28-oic acid
h
stems
(Zong et al., 2013)
332
23-hydroxyursolic acid
h
stems
(Zong et al., 2013)
333
granditriol
h
stems
(Shi et al., 2016)
334
lupeol
h
stems
(Zong et al., 2013)
335
betulinic acid
h
stems
(Zong et al., 2013)
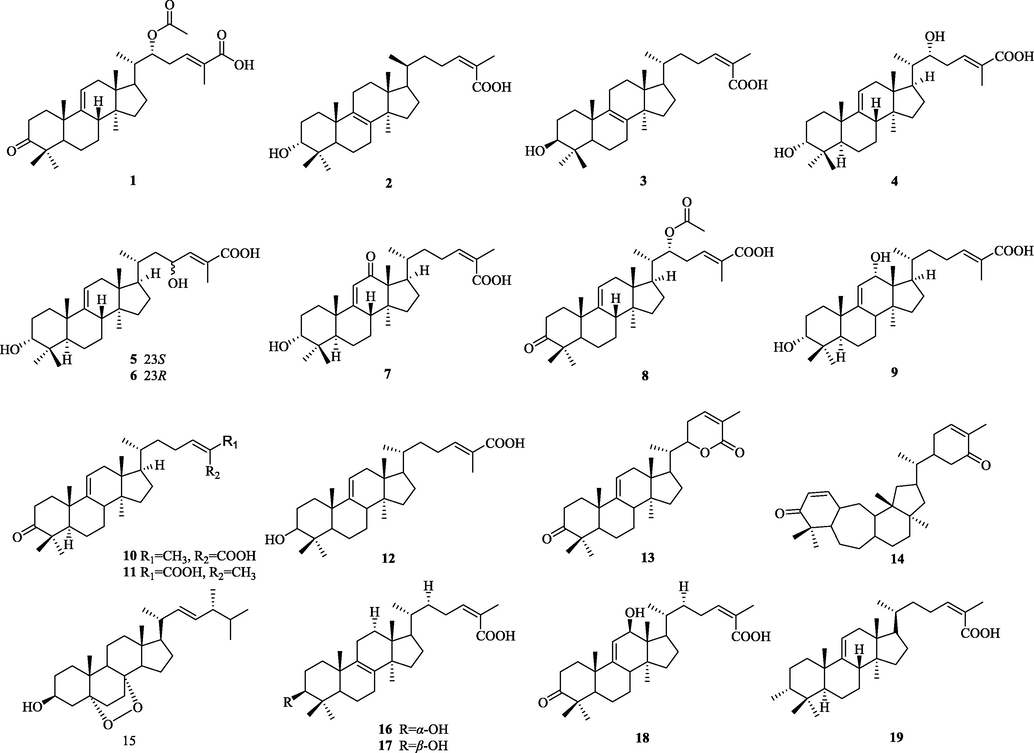
The chemical structure of 1–19.
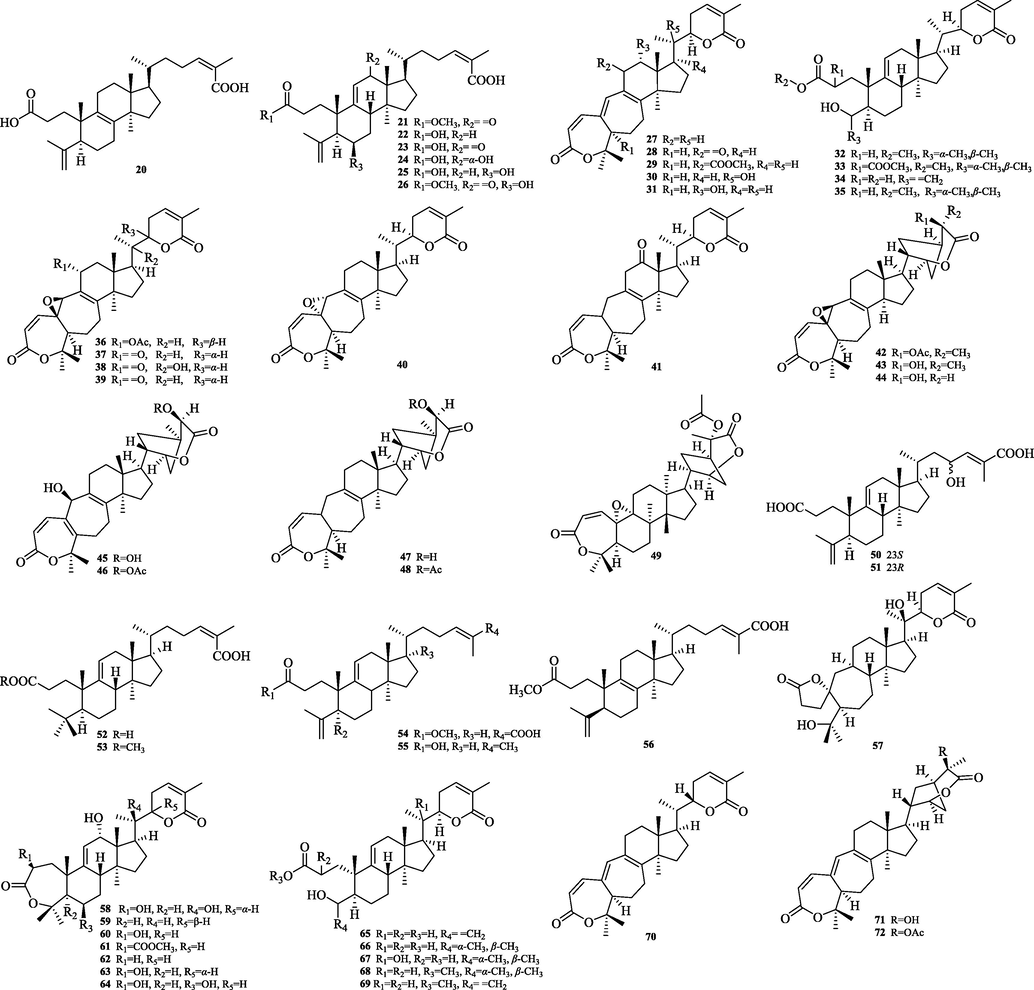
The chemical structure of 20–72.
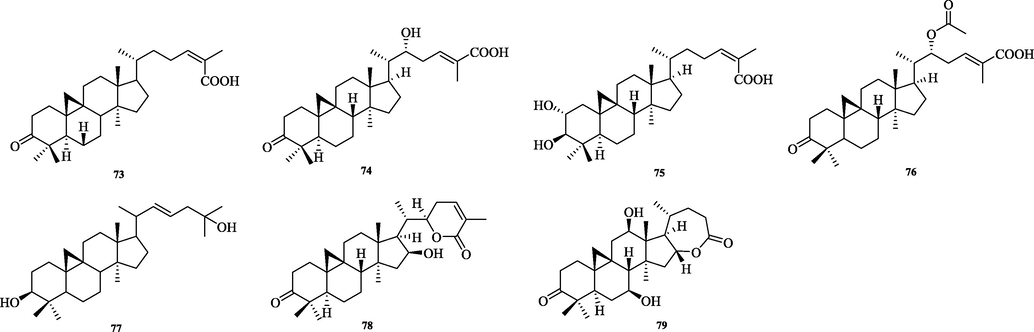
The chemical structure of 73–79.
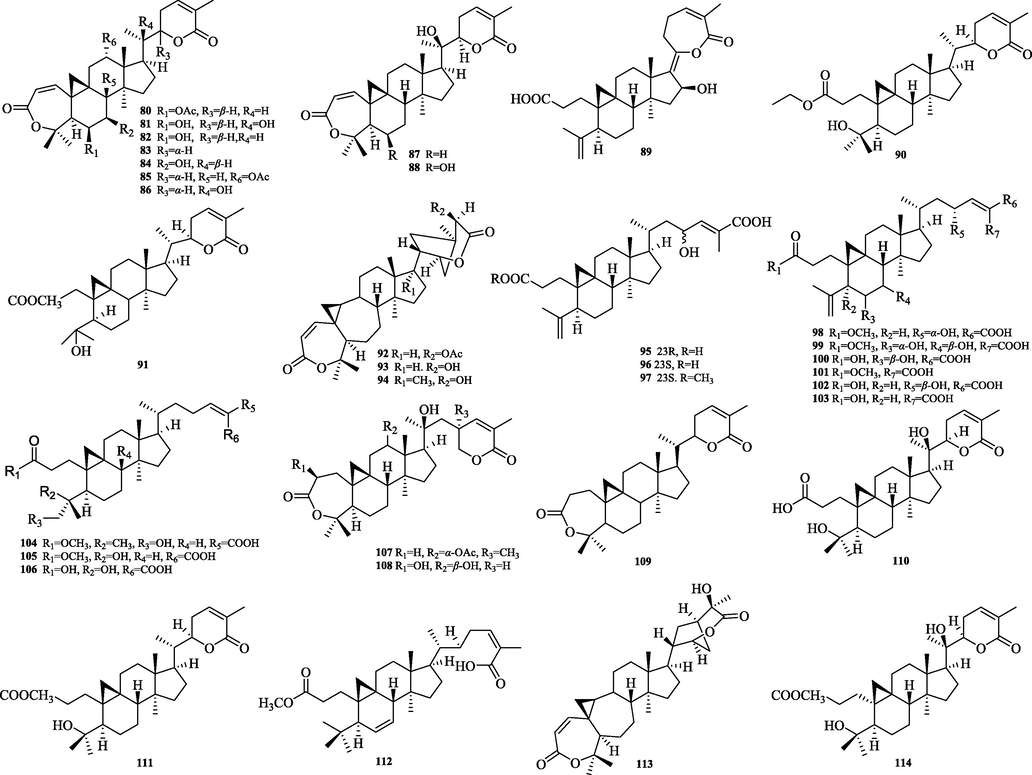
The chemical structure of 80–114.
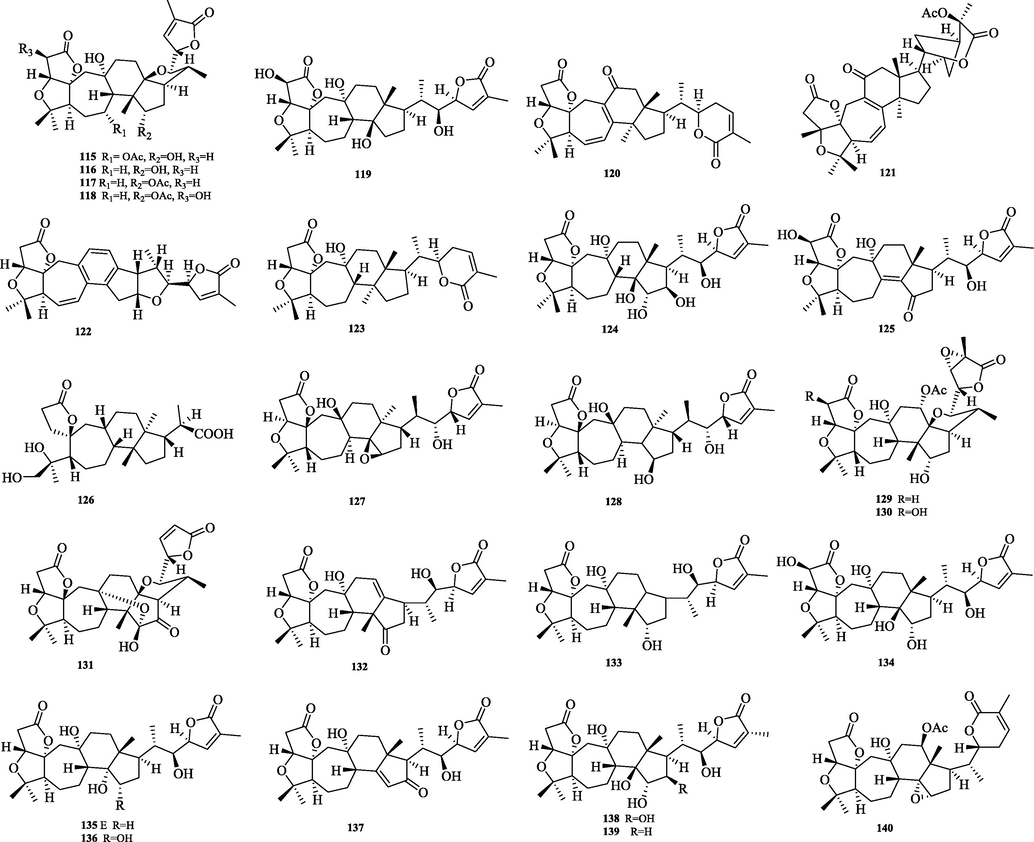
The chemical structure of 115–140.
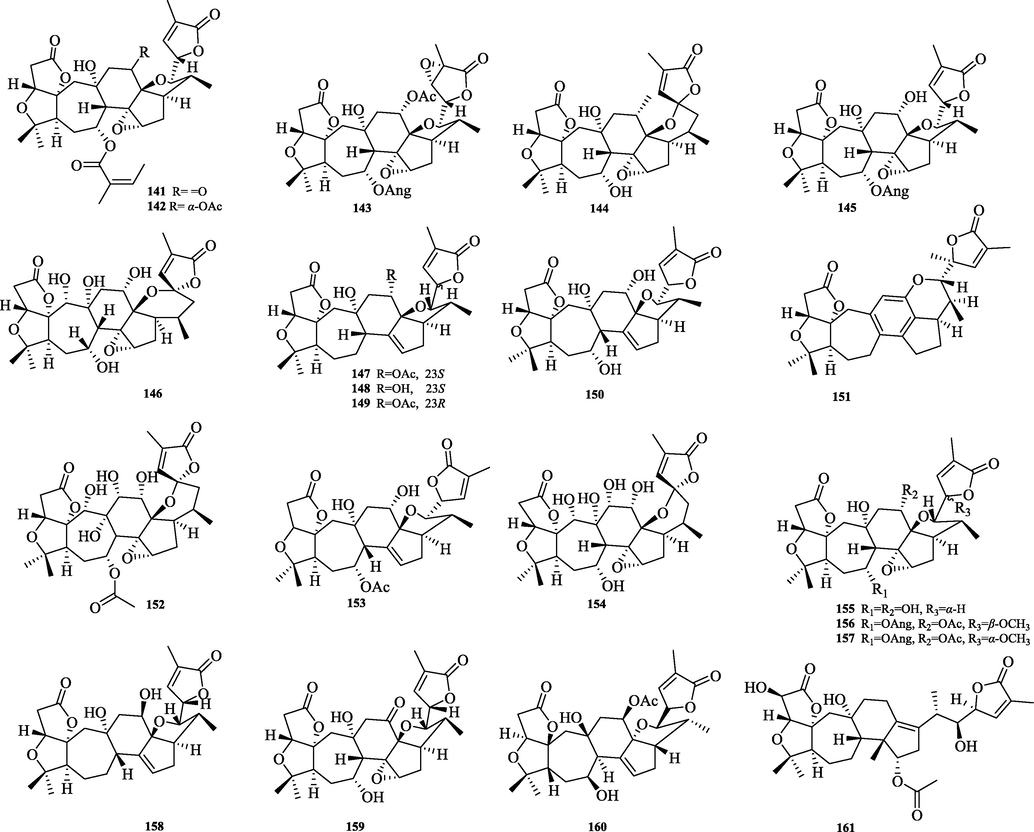
The chemical structure of 141–161.
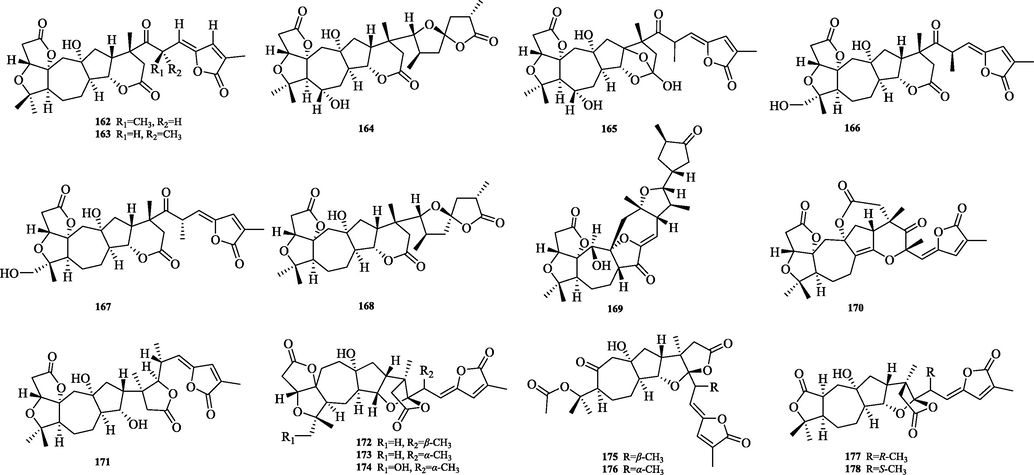
The chemical structure of 162–178.
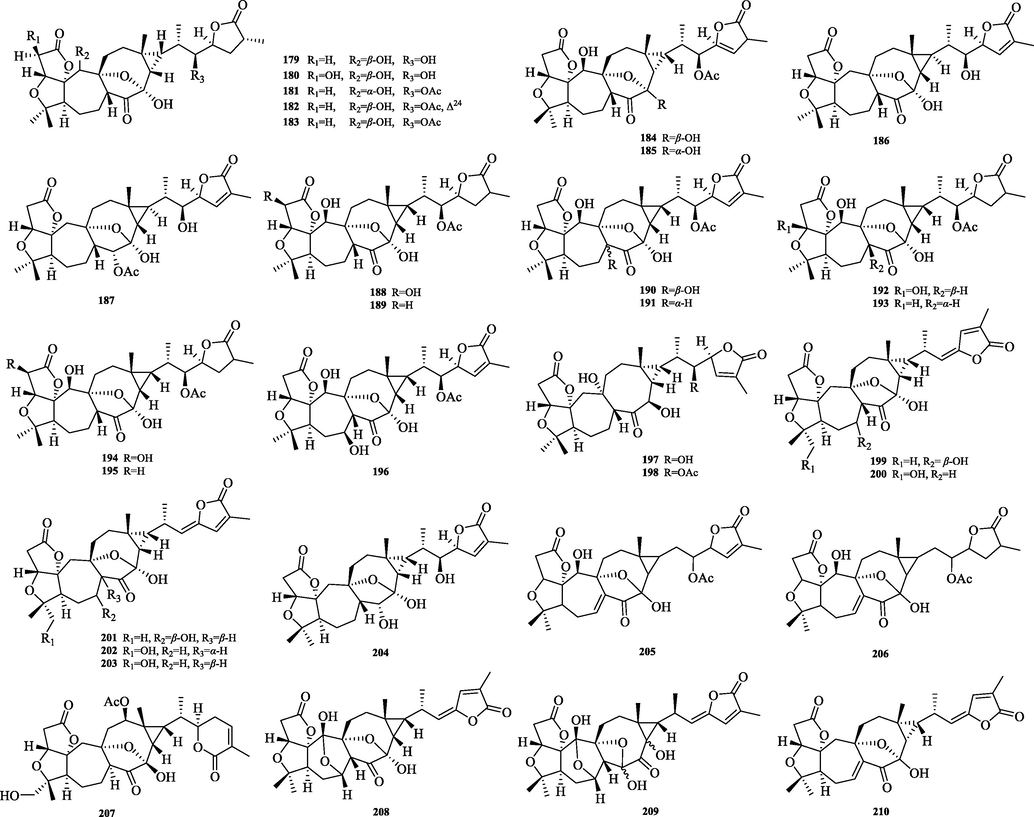
The chemical structure of 179–210.
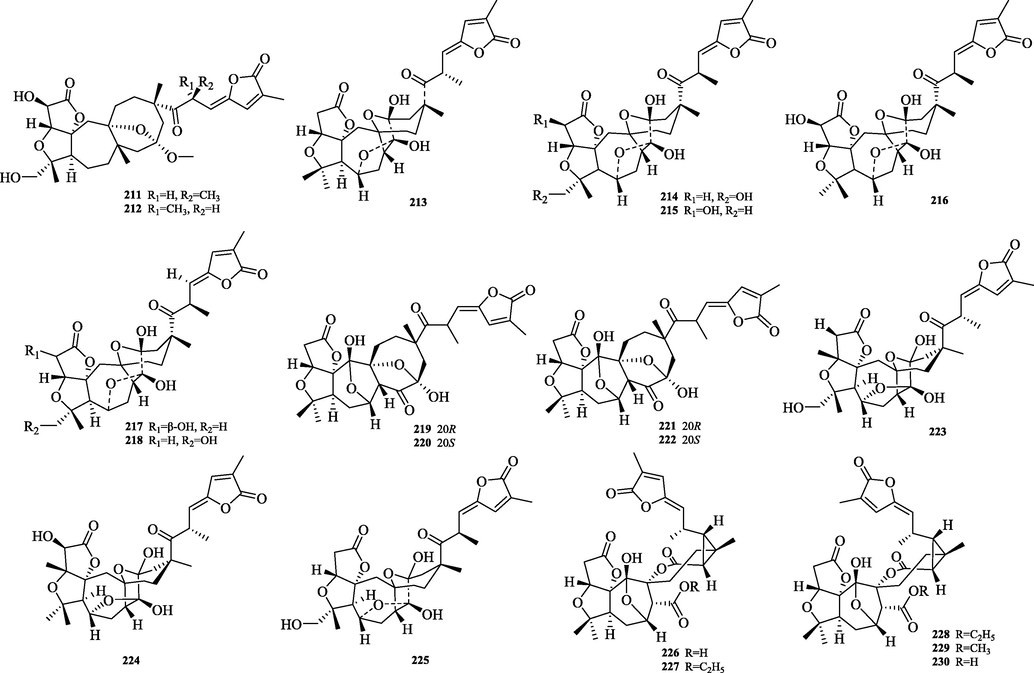
The chemical structure of 211–230.
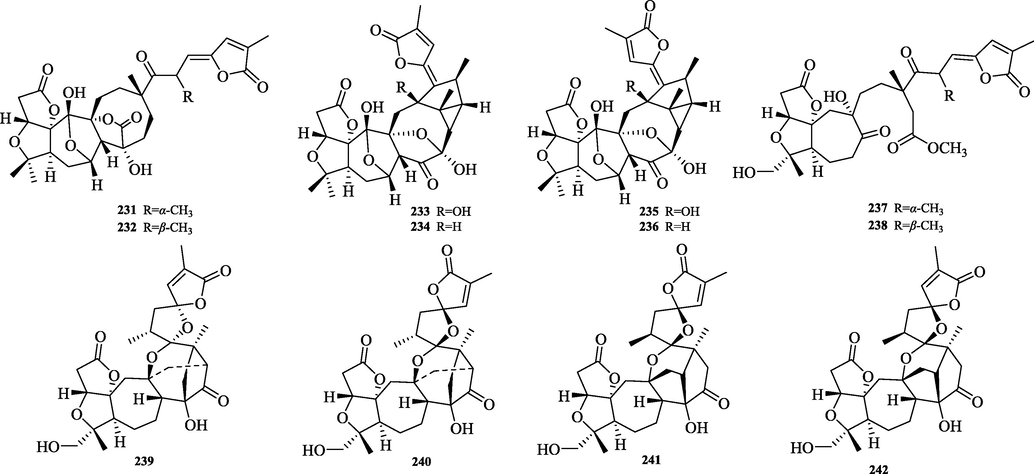
The chemical structure of 231–242.
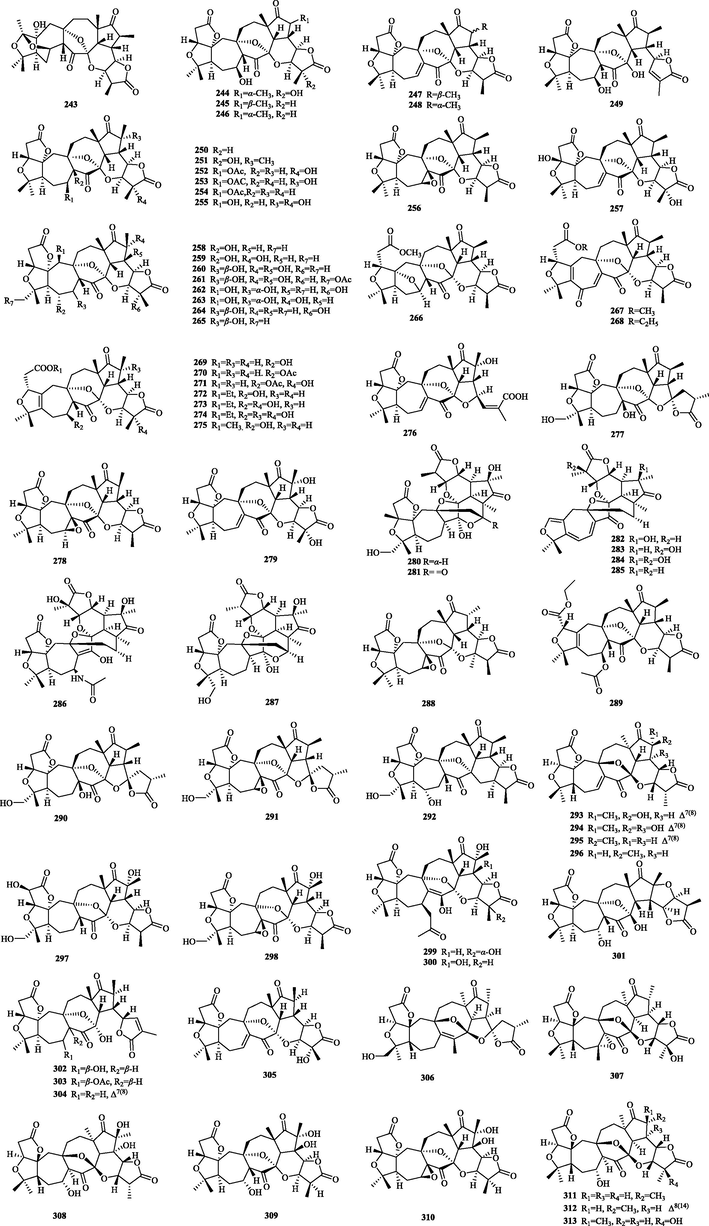
The chemical structure of 243–313.
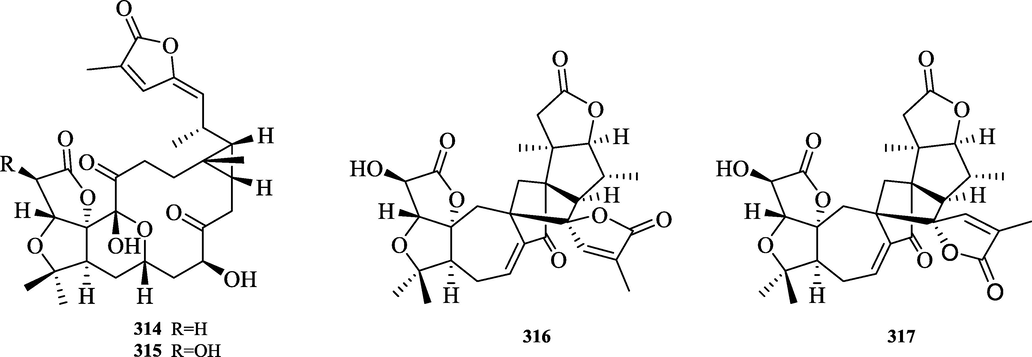
The chemical structure of 314–317.
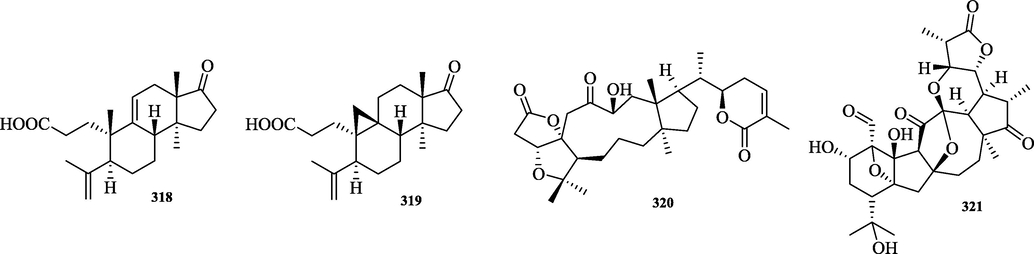
The chemical structure of 318–321.
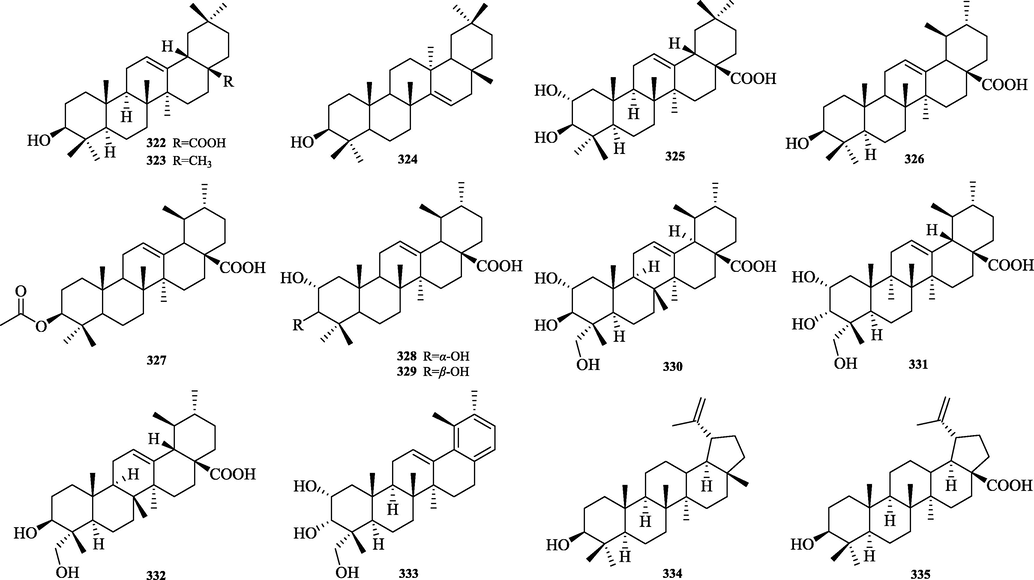
The chemical structure of 322–335.
5.1 Lanostane
5.1.1 Intact lanostanes
The structural features of this type (1–19) of triterpenoids: the C-3 position is mainly substituted by carbonyl or hydroxyl group, and the double bonds in the ring are mostly in C-8 (9), C-9, and C-12 positions are mostly substituted by –OH. And most of the compounds are substituted by carbonyl group at C-3 position (Fig. 2).
5.1.2 3,4-Secolanostanes
The structure of these compounds is characterized by the breakage of the carbon bond at the C-3 and C-4 positions to open the ring, the formation of carboxylic acid at the C-3 position, in a few cases, the formation of carboxylic acid derivatives (20–72). The side chains are mainly 24(Z)-ene-26-acids or 22, 26-lactone rings. Most of the lanostane compounds isolated so far belong to this type (Fig. 3).
5.2 Cycloartane
The cycloartane triterpenes (73–79) are typically characterized by the ternary ring formed by C9 and C19 in their structural skeleton, and are distinguished from other types of triterpene structures by this. In this paper, we classify them into two categories, intact cycloartanes (Fig. 4) and 3,4-seco-cycloartanes (Fig. 5). The structural difference between these two types of cycloartanes is that the former has a carbonyl or hydroxyl group at the C-3 position, while the latter has a broken carbon bond at the C-3 and 4 positions to form a carboxylic acid or a carboxylic acid derivative at the C-3 position (80–114).
5.3 Nortriterpeonids
5.3.1 Schiartane
Schiartane (115–140) is the simplest class of nortriterpenes with a 7/6/5 ring system skeleton and possessing 29 carbon atoms. 26 compounds were obtained and their structures are shown in Fig. 6. The differences in the structure of the skeleton are: C-2 or C-16 are easily substituted with hydroxyl groups. Side chain ring formation: The hydroxyl group of C-22/C-23 and the carboxyl group of C-26 are dehydrated to form a six or five-membered lactone ring.
5.3.2 18-Norschiartane
The 18-Norschiartane type triterpenes (141–161), with a 7/6/5 carbon shelf parent nucleus, are presumably derived from the oxidative decarboxylation of the schiartane type Me-18 (Fig. 7). So far, 23 compounds of this type have been isolated. The difference between its structural features and those of schiartane: the hydroxyl group at C-13 can form a five-membered oxygen ring by intramolecular dehydration with the hydroxyl group at C-22, in addition to a six-membered oxygen ring by intramolecular dehydration with the hydroxyl group at C-23. The C-23 position has methoxy substitution of different orientations.
5.3.3 Wuweiziartane and lancischiartane
The wuweiziartane type (162–178) has a 7/5 carbon frame parent nucleus and the differences in skeletal structure are: Me-21 has a different conformation. 24,25 Carbon-carbon double bonds are partially present. C-30 has hydroxyl substitution. lancischiartane and wuweiziartane have the same planar structure, except for the absolute C-13 configuration, which is β- for wuweiziartane and α- for lancischiartane (Fig. 8).
5.3.4 Preschisanartane
Preschisanartane type (179–210), with a 7/8/3 carbon frame parent nucleus, are presumably derived from schiartane type triterpenes undergoing oxidative rearrangement of C-13, C-14, and cyclization of C-13, C-16 (Fig. 9). The structure is characterized by the easy introduction of hydroxyl groups at C-2, C-19, C-30 and the hydroxyl group introduced at the C-19 position has two conformations. Compound 94 has no oxygen bridge fragment.
5.3.5 16,17-Secopreschisanartane and 14,15-secopreschisanartan
The 16,17-secopreschisanartane type (211–225), presumably derived from preschisanartane type triterpenes by C-16, C-17 oxidative rearrangement (Fig. 10), is the only backbone type with a 7/8-carbon parent core and a 9-carbon side chain at present. C-2, C-8, C-19, C-30 are generally hydroxyl substituted. hydroxyl and methoxy are easily introduced at the C-15 position. The 14,15-Secopreschisanartan type (226–230) has a 1-oxospiro [6.6] tridecane system.C-22,23-position double bond conformation has both Z and E conformations.
5.3.6 Lancifoartane and 12,22-Cyclopreschisanartane
Lancifoartane (231–232), which possesses a 7/7-carbon nucleus and has a lactone bridge between C-9 and C-14, may be derived from 16,17-secopreschisanartane-type triterpenes by keto-alcohol condensation rearrangement. 12,22-Cyclopreschisanartane type (233–236), possessing a 7/8/3/5 carbon frame parent nucleus, presumably derived from preschisanartane type by oxidation and keto-alcohol condensation rearrangement reaction (Fig. 11).
5.3.7 14-Nor-16,17-secopreschis anartane and 15,17-Dicyclolancifoartan
The 14-Nor-16,17-secopreschisanartane type (237–238), which possesses a 7-carbon backbone parent nucleus, is derived from the 16,17-secopreschisanartane type by the removal of the C-14 atom after the 14,15 oxidative rearrangement reaction. 15,17-Dicyclolancifoartan type triterpenes (239–242), with [9.2.1.02.8] tetradecane system. So far, four of these compounds have been isolated. The specific structures are shown in Fig. 11.
5.3.8 Schisanartane
Schisanartane type (243–313), possessing a 7/8/5 carbon frame parent nucleus, presumably derived from the cyclic reaction at the C-16, C-22 positions of 16,17-secopreschisanartanes type triterpenes. The hydroxyl group is generally substituted on C-2, C-8, C-19, C-30. Double bond position: generally between C-1 and C-10, C-5 and C-6/C-10, C-8 and C-7/C-14, C-24 and C-25 positions (Fig. 12).
5.3.9 8,9-Seco-12,22-cyclopreschisanartane and 9,23:12,22-Dicyclolancischiartane
The 8,9-Seco-12,22-cyclopreschisanartane type (314–315) has a unique 5/5/6/11/3 ring system and is the first type with a characteristic thirteen-membered carbon ring structure found in Schisandraceae. The schincalactone A and B isolated from S. incarnata belong to this conformation. 9,23:12,22-Dicyclolancischiartane type, with a [5.2.1.01,6] decane system. Two compounds (316–317) of this type, schincalide A-B, were isolated so far and their chemical structures are shown in Fig. 13.
5.3.10 Octanortriterpenoids and others
The compounds micranoic acid A (318) and B (319) are highly degraded triterpenoids in which the fission of the C-17/C-20 bond is achieved by oxidation. 319 differed from 318 in which the Me-19 was replaced by a cyclopropyl methylene group. In addition, two other classes of triterpenes, 320 and 321, were isolated. Their chemical structures are shown in Fig. 14.
5.4 Pentacyclic triterpenoids
Pentacyclic triterpenes (322–335) are widely found in Chinese herbal medicine, which are triterpenoids consisting of five closed rings linked by six isoprene units as the parent, and the three categories of Oleanane, Ursane and Lupane are summarized in this paper. The structures are shown in Fig. 15.
6 Pharmacology of Schisandra spp
In recent years, the medicinal value of the Schisandra genus has gradually received attention from scholars, and the research on its chemical components and pharmacological effects has intensified. As one of the components with great potential for development, triterpenes have also received much attention in pharmacological effects, especially in hepatoprotective, neuroprotective and antitumor effects.
6.1 Hepatoprotective and anti-inflammatory effects
Wang et al. explored the effect of triterpenoid derivatives of S. chinensis on HDAC and NLRP3 inflammatory vesicle activity and found that the derivatives showed significant inhibition of HDAC with IC50 of 1.139–10.558 μM and low cytotoxicity (CC50 greater than 20 μM) against J774A.1 compared to HDAC inhibitor class of antitumor drugs, showing potential for development as potential for development as an anti-inflammatory immune drug. In addition, this class of derivatives modulates LPS and Nig levels and reduces IL-1β and caspase-1 expression (Wang, 2021a). A new triterpene, 11β-hydroxylkadsuphilactone A (320), was isolated from the stem of S. pubescens and the structure of the compound was determined through wave spectroscopy, while the hepatoprotective activity of the new triterpene against D-GalN-induced cell death of QSG7701 cells was later assessed. The test results showed that 11β-hydroxyl kadsuphilactone A (320) showed hepatoprotective activity at 10 μM with a survival rate of 60.5% (Silybin served as a positive control with a survival rate of 66.2 ± 4.0%) (Wang et al., 2016). Kang et al. investigated the effect of ethanolic extract of S. chinensis fruit (dose of 500 μg/mL) on the expression and production of pro-inflammatory mediators and inflammatory cytokines in RAW264.7 cells and the possibility of SF having anti-inflammatory properties was investigated by determining the effect on the levels of NO, etc. The results showed that it significantly blocked the production of nitric oxide (NO), tumor necrosis factor-α (TNF-α) and IL-1β induced by lipopolysaccharide (LPS) stimulation and that this effect did not trigger a cytotoxic response (Kang, 2014). Similarly, Jeong et al. investigated the anti-inflammatory effect of the ethanolic extract of S. chinensis fruit and found that the ethanolic extract of S. chinensis fruit inhibited the expression and activity of IL-1β-stimulated matrix proteases in SW1353 cells, and this effect may be related to the expression of nuclear factor-κB and c-Jun N-terminal kinase/p38MAPK (Jeong, 2015).
A new triterpene propindilactone L (130) isolated from S. propinqua was found to significantly inhibit hepatitis B surface antigen (HBsAg) and hepatitis B e antigen (HBeAg), which is the first report on the anti-hepatitis B virus effect of nortriterpenoids, and achieved good therapeutic effect with SI values of 2.68 and 1.11 (Lei, 2009). The compounds arisanlactone B (244) and D (246) were found to have a slight anti-inflammatory effect, with 22.24% and 18.47% inhibition of elastase at 10 μg/mL, correspondingly (Cheng et al., 2010b).
6.2 Anti-tumor effects
Gao et al. found that schilancidilactone V (205) isolated from S. wilsoniana had significant cytotoxic activity against oral epithelial carcinoma cells and breast cancer cells MDA-MB-231 cells and was effective in inhibiting the proliferation of cancer cells with IC50 values of 3.18 and 5.22 μmol/L in that order (with camptothecin as the positive control, IC50 values of 1.62 and 2.18 μmol/L) (Gao et al., 2013). Tanaka et al. tested the anti-proliferative activity of triterpenoids from the roots of S. chinensis against human cancer cell lines A549 (lung cancer), RPMI8226 (leukemia), HeLa (uterine cancer) and MCF-7 (breast cancer) cells and showed that propiniclactone A (91), schinensin C (85), schinensin D (78) and schisanbilactone A (80), which are triterpenes with α,β-unsaturated δ-lactone moiety, showed more significant anti-proliferative activity against MCF-7 cells with IC50 values of 9.3–12.8 μM, and their activity was close to that of the positive drug Cisplatin (IC50 of 8.5 μM) (Tanaka et al., 2021). Liu et al. performed human tumor cytotoxicity tests on 12-hydroxyschiglausin B (58) and 12-hydroxykadsuphilactone B (108) using the MTT method. The data showed that 12-hydroxykadsuphilactone B (108) had higher cytotoxic activity, with the strongest toxicity against HepG2 cells with an IC50 of 11.3 μM (Liu et al., 2018). Five new triterpenes, Schiglausins P-T (33–34, 8, 76, 105), were isolated from S. glaucescens fruit and tested for cytotoxicity by YU et al. Pharmacological experiments confirmed that all five compounds had toxic effects on B16 mouse melanoma cell lines with IC50 values ranging from 3.64 to 27.00 μM, exhibiting moderate inhibition of tumor cell proliferation (Doxorubicin hydrochloride as the positive control, IC50 values of 2.61 μM) (Yu et al., 2016).
Pharmacological studies have shown that manwuweizic acid (20) inhibited lung cancer, brain tumour-22 and solid liver cancer in mice. Additionally, anwuweizonic acid (11) and nigranoic acid (103) also have been reported to have antitumor activity, showing high inhibition of human decidual cells and rat luteal cells (20 ug/ml for rat luteal cells and 40 ug/ml for human decidual cells). That is the first that a plant from the family Schisandraceae can inhibit these two tumor cells (Chen et al., 2001). S. chinensis extract can affect the function of the immune organs of the spleen and thymus and influence the activity of immune cells, thus enhancing the ability of the body to clear cancer cells. Hou et al. studied S. viridis as an anti-tumor active ingredient. Using the MTT assay, Fluorouracil (5-FU) was used as a positive control, and Schisanol (19) was found to have an inhibitory effect on human tongue cancer CAL27 cells with an IC50 of 11.7 mg/mL. In further studies, Schisanol (19) was also shown to have toxic activity against human breast cancer MCF7 cells, with significant inhibitory effects on cancer cell proliferation (Hou et al., 2016).
6.3 Anti-HIV effects
At present, the use of anti-AIDS drugs remains the main response to AIDS and therefore anti-AIDS natural products have become the focus of scholarly attention in various countries. In recent years, researchers have conducted numerous studies on the biological activities, pharmacological effects and mechanisms of action of the fruits, roots and leaves of the genus Schisandra, leading to the isolation of a series of novel highly oxidized triterpenoids with anti-HIV effects. Xiao et al. isolated three new highly oxidized hypo triterpenoids rubriflorins A-C (266–268) from S. rubriflora, which have a unique A-ring opening feature. Pharmacological experiments showed that rubriflorins A-C (266–268) exhibited anti-HIV effects with EC50 of 10.0–81.3 μg/mL (AZT as a positive control with EC50 of 0.0043 µg/mL) (Xiao et al., 2007c). The compounds schisphendilactone A (84) and B (37) were isolated from S. sphenanthera by Liang et al. In vitro experiments demonstrated their significant anti-HIV effects. Their EC50 values were 8.79 and 1.09 μg/mL respectively, while the positive control AZT EC50 was 0.0053 μg/mL (Liang et al., 2013a).
Xiao et al. isolated two new triterpenes lancifodilactone F (126) and G (306) from S. lancifolia, and pharmacological experiments showed that both compounds had an anti-HIV activity with EC50 values of 20.69 and 95.47 μg/mL, respectively (Xiao et al., 2005a; Xiao et al., 2005b). In 2015, the anti-HIV effect of S. chinensis was discovered and identified a lead compound, diphenylamine ester, with potent anti-HIV activity. Diphenylamine esters have low toxicity and a good broad spectrum and are effective against both experimental and clinical strains. It is currently undergoing preclinical studies and is expected to become a new class of anti-HIV drugs.
6.4 Neuroprotective effects
Researchers isolated 11 compounds from S. henryi and pharmacological assays revealed that only henridilactone O (300) exhibited moderate neuroprotective activity with a cell differentiation rate of 11.1% (He et al., 2020). It has been reported in the literature that S. chinensis extract prevents hydrogen peroxide-induced neuronal cell death and improves cognitive dysfunction in rats. The experimental data suggest that this is associated with an increase in brain-derived neurotrophic factor, downstream molecules pERK, pATK and pCREB (Park et al., 2019). Jin et al. investigated the protective effect of an aqueous extract of S. chinensis on neuronal cells in the cerebral cortex of mice. The results illustrated that S. chinensis aqueous extract could improve the learning ability of APP/PS1 double transgenic mice, enhance the number of nisin bodies, improve the cell quality of the cerebral cortex, and promote the survival of neural cells. In addition, mice treated with S. chinensis aqueous extract showed a decrease in MDA content and a significant increase in SOD content in the cerebral cortex. Its neuroprotective effect may be related to the reduction of damage caused by oxidative stress, and its mechanism of action should be further explored (Jin, 2017).
6.5 Immunomodulation effects
The triterpenoids schisanlatone C (30) showed selective inhibition of B-lymphocyte proliferation with IC50 with 20.51 μM; the compounds schisanlactone B (70) and 3,4-seco-(24Z)-cyclo-4(28),24-diene-3,26-dioic-3-methyl ester (101) had a selective inhibitory effect on T-lymphocyte proliferation with an IC50 range of 14.28–19.33 μM (positive control: cisplatin, IC50 = 3.16 µg/mL); Propinic lactone A (111) had an inhibitory effect on both T- and B-lymphocyte proliferation with an IC50 of 6.97–16.93 μM (positive control: 5-Fluorouracil, IC50 = 8.48 μM). Among them, schisanlactone B (70) and 3,4-seco-(24Z)-cycloart-4(28),24-diene-3,26-dioic-3-methyl ester (101) specifically inhibited the proliferation of B lymphocytes by more than 90% (Qiu et al., 2018; Zhao et al., 2020; Liu et al., 2017a).
kadsudilactone A (109) showed moderate inhibition of ConA-induced T-lymphocyte proliferation with an IC50 of 6.32 μM. Significant inhibition of LPS-induced B lymphocyte proliferation was observed with the same intensity as the positive drug mycophenolate mofetil (MMF) with an IC50 of 11.49 μM (IC50 of MMF was 17.8 μM). Moreover, there was no significant toxicity to mouse spleen lymphocytes at a concentration of 100 μM compared to the positive drugs CsA and MMF (Zhou, 2018). Studies have found that S. chinensis extract has a bidirectional immunomodulatory effect, on the one hand enhancing the secretion of immune cells and on the other hand, increasing the activity of related cells and accelerating the clearance of antigens (Chen, 2021).
6.6 Anti-oxidation activity
The intrinsically low levels of catalase (CAT), glutathione peroxidase (POD) and superoxide dismutase (SOD) make immune cells more susceptible to damage from oxidative stress induced by high levels of glucose and free fatty acids. Thus, the high antioxidant activity and free radical scavenging capacity of S. chinensis extracts play an invaluable role in protecting cells from dysfunction or death (Jo et al., 2011). The total triterpenoids of S. chinensis had a strong antioxidant capacity overall and there was an obvious quantitative-effect relationship. The total triterpene scavenging ability of S. chinensis stems and leaves were flowering > spreading > budding > deciduous, and only the total triterpene scavenging ability of vine stems was spreading > flowering > budding > deciduous. The reducing ability of total triterpenes of S. chinensis stems and leaves were stronger than VE and weaker than VC, and the antioxidant ability of stems was the strongest compared with other parts (Wang, 2021b). The scavenging ability of S. chinensis vine stem triterpenes on DPPH radicals was correlated with the concentration of added triterpenes, the higher the added amount, the stronger the scavenging capacity, with an IC50 of 0.077 mg/mL, and the IC50 of the positive control VC was 0.692 mg/mL. Besides, there was an inhibitory effect on lipid oxidation (Li, 2012).
6.7 Antimicrobial activity
The ethanolic extract of S. chinensis has been reported to have an antibacterial effect. In recent years, it has been found to have a significant inhibitory effect on Staphylococcus aureus, Escherichia coli, Salmonella typhi and Escherichia coli, and the extract has shown good stability to heat treatment (Zou et al., 2011; Chen, 2018). In vitro inhibition experiments showed that the total triterpenes of S. chinensis leaves had some inhibitory effect on bacteria, but not on Candida albicans. The inhibitory ability against Bacillus subtilis and Pseudomonas aeruginosa was more pronounced and weaker against Staphylococcus aureus and Escherichia coli. The MIC of the total triterpenes of the stem and leaves of S. chinensis at different periods ranged from 0.63 to 5.00 mg/mL and the MBC from 2.50 to 20.00 mg/mL, and the growth rate and cycle of the bacteria were effectively limited (Zhou, 2018).
6.8 Insecticidal activity
Jiang et al. screened the insecticidal activity of triterpenes from S. chinensis and found that manwuweizic acid (20) showed toxic to Aphis gossypii and Plutella xylostella with a pest mortality rate of 66% (Jiang, 2011). The compounds acetyl ursolic acid (327), 2α, 3α-dihydroxy urs-12-en-28-oic acid (328) and corosolic acid (329) exhibited significant cytotoxicity against marine shrimp larvae with IC50 of 17.0–25.2 μM. Preliminary structure–activity relationship studies indicated that the 3-position hydroxylation of the arcane-type triterpene acids and acetylation of the urethane-type triterpene acids could greatly enhance their insecticidal activity (Zong et al., 2013).
6.9 Others
Ding et al. isolated a series of triterpenoids with good activity from S. chinensis, among which propindilactone Z (107) and schipropinqua acid J (75) showed moderate inhibitory activity against COLL-induced platelet aggregation in rabbits with 12.6 ± 4.5 and 10.4 ± 9.5, respectively, as inhibition percentage (%) (Ding, 2018). The research reports that the compound manwuweizic acid (20) obtained from S. propinqua exhibited cholesterol biosynthesis inhibitory activity with 19.2% inhibition (Liu et al., 1989). Scholars conducted in vitro cytotoxicity experiments with anwuweizonic acid (11) and manwuweizic acid (20). The data showed that the mortality rate of the human metaphase cell line and murine corpus luteum cell line exceeded 98.5% after the addition of 20 and 40 mg/mL of both triterpenes. This indicated that the triterpenes had strong cytotoxic activity against the human metaphase cell line and murine luteal cell line and had anti-reproductive effects (Chen et al., 2002; Chen et al., 2001). All the pharmacological effects of this genus are summarized in Table 4 (active: IC50 < 10 μM; moderately active: 10 μM < IC50 < 20 μM; not active: IC50 greater than 20 μM).
Pharmacologic
Action
Effective Fraction/
Compounds
Model
Response and Critical Assessment
Target or
Possible
Mechanism
Ref
Anti-inflammation effects
niqranoic acid derivatives
Macrophage J774A.1
IC50 = 1.139–10.558 μM (Inhibition of IL-1β and caspase-1)
LPS and Nig↑, IL-1β and caspase-1↓
(Wang, 2021a)
11β-hydroxylkadsuphilactone A
QSG7701 cells
survival rates of 60.5%
p38 MAPK↓, Increases intracellular glucocorticoid levels, p38 MAPK-C/EBPβ pathway
(Wang et al., 2016)
propindilactone L
HepG 2.2.15cells
SI value of 2.68 and 1.11 (Inhibition of HBsAg, HBeAg)
Not mentioned
(Lei, 2009)
propindilactone N
HepG 2.2.15cells
SI value of 1.62 (HBsAg)
arisanlactone B
Human neutrophils
Inhibition rate of 22.24%
Inhibition of superoxide anion production and elastase release by neutrophils
(Cheng et al., 2010b)
arisanlactone D
Inhibition rate of 18.47% (Inhibition of elastase)
schisanbilactone A
LPS-induced NO production
IC50 = 10.6 μM
Inhibiting NO
(Song et al., 2015)
Anti-tumor effects
schilancidilactone V
KB
IC50 = 3.18 μmol/L
Not mentioned
(Gao et al., 2013)
MDA-MB-231 cells
IC50 = 5.22 μmol/L
HL-60
IC50 = 11.5 μmol/L (Inhibits tumor cell proliferation)
schilancidilactone W
KB
IC50 = 8.21 μmol/L
MDA-MB-231 cells
IC50 = 12.44 μmol/L
HL-60
IC50 = 4.15 μmol/L
propinic lactone A
MCF-7 cells
IC50 = 9.3–12.8 μM (Inhibits tumor cell proliferation)
Key groups: α, β-unsaturalted δ-lactone moiety
(Tanaka et al., 2021)
schinensin C
schinensin D
schisanbilactone A
12-hydroxy
schiglausin BHepG2 cells
IC50 = 14.0 μM
Not mentioned
(Liu et al., 2018)
12-hydroxy
kadsuphilactone BIC50 = 11.3 μM
(positive control: Doxorubicin 0.02 μM)
anwuweizonic acid
human decidual cells and rat luteal cells
Effective inactivation rate: 98.5–100 %
Not mentioned
(Chen et al., 2001)
nigranoic acid
schisanol
human tongue cancer CAL27 cells
IC50 of 11.7 mg/mL
C-2 and C-3 positions and removal of hydroxyl at C-7 greatly enhancedthe activity
(Hou et al., 2016)
human breast cancer MCF7 cells
IC50 of 10.6 mg/mL (Medium activity)
schisphenthin A
HepG2 cells
IC50 = 18.12–49.52 μM (Medium activity)
Not mentioned
(Liu et al., 2017a)
schisphenthin C
schisanlactone H
propinic lactone A
3β-hydroxy-lanosta-8,24Z-dien-26-oic acid
Cdc25A
phosphataseinhibitory rates of 85.5% (significant inhibition)
Not mentioned
(Zhao et al., 2020)
3,4-seco-(24Z)-cycloart-4(28),24-diene- 3,26-dioic-3-methyl ester
Lung cancer A-549 cells
IC50 = 28.11 ± 1.49 μM
Not mentioned
(Ding, 2018)
Liver cancer SMMC-7721 cells
IC50 = 25.33 ± 1.39 μM
schigrandilactone A
K562 cells
IC50 = 0.13 μg/mL
Not mentioned
(Xiao et al., 2009)
HepG2 cells
IC50 = 0.19 μg/mL
schigrandilactone B
K562 cells
IC50 = 3.19 μg/mL
HepG2 cells
IC50 = 0.20 μg/mL (significant cytotoxicity)
6-hydroxyl schiglausin A
BGC-823 cells
IC50 = 7.3 μM
Not mentioned
(Wang et al., 2021)
W480 cells
IC50 = 7.9 μM (significant cytotoxicity)
Anti-HIV effects
rubriflorin A
C8166 cells
EC50 of 10.0–81.3 microg/mL
Not mentioned
(Xiao et al., 2007c)
rubriflorin B
rubriflorin C
schisphendilactone A
C8166 cells
EC50 = 8.79 μg/mL
Not mentioned
(Liang et al., 2013a)
schisphendilactone B
EC50 = 1.09 μg/mL
lancifodilactone F
C8166 cells
EC50 = 20.69 μg/mL
Not mentioned
(Xiao et al., 2005a; Xiao et al., 2005b)
lancifodilactone G
EC50 = 95.47 μg/mL
lancifodilactone G
C8166 cells
EC50 = 95.47 ± 14.19 μg/mL
(Low activity)
rubriflorin D
C8166 cells
EC50 = 19.1 μg/mL
AZT as apositive control (EC50 = 0.0043 ug/mL)Not mentioned
(Xiao et al., 2007a)
Neuro-
protective effectshenridilactone E
PC12 cells
survival rate is 67.39%
(He et al., 2020)
Not mentioned
henridilactone O
PC12 cells
survival rate is 64.52%
positive control: desipramine (DIM)
S. chinensis extract
PC12 cells
75, 150, or 300 mg/kg/day
Increase in downstream molecules pERK, pATK and pCREB
(Park et al., 2019)
S. chinensis extract
C57BL/6 mice
10、30、50 mg/kg
MDA content decreased and SOD content increased
(Jin, 2017)
Immunomodulation effects
schisanlatone C
B lymphocytes
IC50 = 20.51 μM
Inhibits proliferation of T and B lymphocytes
(Chen, 2021)
schisanlactone B
T lymphocytes
IC50 = 14.28–19.33 μM
(moderate inhibitory)
3,4-seco-(24Z)-cycloart-4(28),24-diene- 3,26-dioic-3-methyl ester
kadsudilactone A
ConA-induced T-lymphocyte
IC50 of 6.32 μM
Inhibits proliferation of T and B lymphocytes
(Zhou, 2018)
LPS-induced B lymphocytes
IC50 of 11.49 μM
Anti-bacterial activity
ethanolic extract of S. chinensis
Staphylococcus aureus
The diameter of the inhibition circle is 11.3–19.3 mm
Not mentioned
(Zou et al., 2011; Chen, 2018)
Trichoderma
Salmonella typhi
Escherichia coli
total triterpenes of S. chinensis leaves
Staphylococcus aureus
MIC was 0.63–5.00 mg/mL and MBC was 2.50–20.00 mg/mL
Not mentioned
(Zhou, 2018)
Escherichia coli
Insecticidal activity
manwuweizic acid
Aphis gossypii
Mortality rate of 66% (moderate inhibitory)
Not mentioned
(Jiang, 2011)
Plutella xylostella
acetylursolic acid
marine shrimp larvae
IC50 of 17.0–25.2 μM
Not mentioned
(Zong et al., 2013)
2α, 3α-dihydroxyurs-12-en-28-oic acid
corosolic acid
micranoic B
Plutella xylostella
mortality rate of 66% (Medium activity)
Not mentioned
(Jiang, 2011)
Cotton worms
Anti-platelet aggregation
propindilactone Z
rabbits
Inhibition rate = 12.6 ± 4.5%
Not mentioned
(Ding, 2018)
schipropinqua acid J
Inhibition rate = 14.2 ± 11.0%
23-hydroxyursolic acid
Inhibition rate = 10.4 ± 9.5% (moderate inhibitory)
lancolide A
PAF-induced aggregation
IC50 = 79.95 μg/mL
Best activity at 100 μg/ml
(Shi et al., 2013)
lancolide D
IC50 = 52.56 μg/mL
Anti-oxidation activity
lancifonin E
Caco-2 cells
EC50 = 0.26 mM
Blocking phosphorylation of JNK 1/2/3 MAPK in CaCO-2 cells
(Shi et al., 2014)
cytotoxicity
henrischinin A
HSV-2
SI = 23.31
The acetyl and hydroxyl groups of C-25 may be crucial for the role of triterpenoids in the inhibition of HSV-2 and adenovirus.
(Song et al., 2013)
henrischinin B
SI = 29.55
henrischinin C
SI = 19.49
schinchinenin A
Adenovirus
SI = 13.75
schinchinenin G
SI = 11.43
henrischinin A
SI = 13.67
henrischinin B
SI = 11.45 (most active inhibitor of HSV-2)
kadsuphilactone A
SMMC-7721 cell lines
IC50 = 29.9 μM
Not mentioned
(He et al., 2012)
A549 cell lines
IC50 = 32.5 μM
MCF-7 cell lines (modest cytotoxicity)
IC50 = 24.4 μM
7 Treatment potential
7.1 Liver injury
Non-alcoholic fatty liver disease (NAFLD) includes simple fatty liver and the evolution of steatohepatitis, cirrhosis and liver cancer from it. Up to now, its pathogenesis is still unclear, and treatment is mainly through lipid-lowering, alleviation of insulin resistance and anti-oxidative stress to alleviate NAFLD. The hepatoprotective active component of S. chinensis, triterpenes 19R-hydroxylwuwei-zidilactone H (146), had significant effects on hepatic lesions, and such components showed significant alleviation of high-fat diet-induced NASH in rats and also modulated related enzyme activities, thus providing relief from NAFLD (Li et al., 2017). Researchers found that total triterpenes of S. chinensis had significant intervention effects on both acute and chronic liver injury induced by alcohol intake. It is speculated that the mechanism of action may be to alleviate the lipid peroxidation triggered by ethanol metabolism, scavenge oxygen free radicals and protect cellular organelles. Secondly, it regulates the expression of CYP2E1, a key enzyme in ethanol metabolism, and reduces its activity to improve the liver injury caused by alcohol intake (Zhu, 2014). Leng et al (Leng, 2015). used S. chinensis to treat patients with drug-induced liver injury, with Silybin Meglumine as the control group, both treated for two courses of treatment (one course of treatment is 30 days). Clinical results showed that the total effective rate of the treatment group (86.67%) was better than that of the control group (73.33%), and there was a significant difference between the two groups, which was statistically significant (P < 0.05). In addition, it can effectively reduce alanine transferase, aspartate transferase and alkaline phosphatase, and significantly improve liver function, which is worth applying and popularizing in the clinic.
7.2 Diabetes
The production of reactive oxygen species (ROS) and the accumulation of glycosylation end-products in the body cause apoptosis in kidney cells, leading to hyperglycemia in diabetes. It was found that ursolic acid (326) could scavenge early ROS production and prevent apoptosis due to hyperglycemia. In addition, ursolic acid (326) has mimetic and insulin-sensitizing activities and promotes insulin receptor tyrosine phosphorylation. It has a positive effect on insulin-mediated signaling and the translocation of glucose transporter 4 in 3 T3-L1 adipocytes for the treatment of type 1 and type 2 diabetes (Oh et al., 2007; Jung et al., 2007).
7.3 Cardiovascular disease
The literature reports that oleanolic acid (322) and corosolic acid (329) are the main pentacyclic triterpenes against cardiovascular diseases. Scholars conducted experiments using ex vivo perfusion of rat atria. The results showed that oleanolic acid (322) treated with 10–30 mg/kg-d could increase the secretion and synthesis of ANP induced by atrial myocardial traction and achieve the purpose of regulating cardiovascular homeostasis. It also reversed the ox-LDL-induced cell proliferation inhibition and exerted anti-atherosclerotic effects (Kim et al., 2013; Pan et al., 2018). Sun et al. obtained derivatives by hydroxyl esterification of corosolic acid (329), which have different degrees of inhibition on glycogen phosphorylase and play a role in the prevention and treatment of atherosclerosis and other diseases (Sun et al., 2009). Xu et al. explored the clinical effect of S. chinensis in the treatment of cardiac arrhythmia, and the results showed that the number of premature beats of patients treated with S. chinensis was significantly less than that of the control group (Amiodarone Hydrochloride Tablets), the total effective rate of treatment in the treatment group was 97.06%, which was higher than that of the control group (82.35%), and the difference was statistically significant. Demonstrates its potential for the treatment of arrhythmia (Xu et al., 2018). The main pharmacological activities and mechanisms of the triterpenes of S. chinensis and the related therapeutic potential are shown in Fig. 16.
Major pharmacological activities and therapeutic potential of triterpenes from genus Schisandra.
8 Exploitation
The genus Schisandra is named after the fruit's sweet, sour, pungent, bitter, and salty flavors. It is native to China and other parts of East Asia. The fruits of most species in this genus are edible and have cough-relieving properties. Its leaves and seeds can extract aromatic oil, which can be used as industrial raw materials and lubricants. The stem bark is pliable and can be used for rope. Due to their attractive leaves and flowers, these plants are also popular as ornamental plants. In addition, Schisandra plants are valued for their medicinal properties. They have been used for centuries in traditional Chinese medicine to treat a variety of ailments, including coughs, asthma and liver disease.
8.1 New drug development
A variety of monomeric compounds in the genus Schisandra are rich in the structural skeleton, and their pharmacological activities are also rich and diverse, which are important discoveries beyond the traditional functions. It is of great significance to the clinical practice of TCM and the development of new drug varieties in Chinese medicine. Modern studies have shown that micranoic acid B (319), a cyclic pineapple alkane type hypo triterpene isolated from the stem of S. pubescens, has good antitumor activity, with significant cytotoxic effects against several types of human cancer cells, including lung cancer (A549), prostate cancer (PC-3), and nasopharyngeal carcinoma (KB and KBvin), with GI50 of 4.07 ∼ 4.40 μg/mL. It is shown to have potential as a chemotherapeutic agent for the treatment of cancer and has positive implications for the preparation of antitumor drugs. This finding highlights the potential of S. pubescens as a source of natural compounds with significant antitumor properties (Lu et al., 2016). Li et al. isolated a new 3,4-secolanostane from S. sphenanthera, which has a novel structure, easy extraction and isolation method, and relatively high content of the obtained compound, which is conducive to its further pharmacological study and creates conditions for the development of new antitumor and anti-AIDS drugs with good efficacy and low side effects (Li et al., 2008).
8.2 Economic importance
Schisandra genus is a dual-use food material with nutritional value and healthcare effects and has been widely used in the food industry. Yang et al. investigated the application of extracts of S. wilsoniana in cigarettes and found that various components of S. wilsoniana, such as triterpenes, their lactones, and flavonoids, are commonly used as aroma components in tobacco. In this regard, a preliminary analysis of the extraction process optimization and the effect of the additive on harmful substances in a cigarette was carried out, and the results showed that the additive can improve cigarette quality, scavenge free radicals and reduce the content of harmful components in cigarette smoke (Yang, 2008; Li et al., 2016). According to a study by Russian scholar Vikorov, the fruit of Schisandra contains 1.1 mg of anti-aging substances in 100 ml of juice, indicating its high nutritional value (Wang and Zhang, 2003). Therefore, Schisandra fruit is becoming an important raw material for food and beverage production abroad, and there are precedents for its application in Eastern Europe. In addition, it is also used in the dye and cosmetic industries (Wang and Chen, 2007; Zheng et al., 2020).
With the continuous research on the Schisandra genus, its functions and values are increasing with each passing day, and with its wide distribution and abundant resources, it shows enormous exploitation value for exploitation. On the whole, Schisandra spp. has economic and cultural importance in China and other parts of Asia.
9 Discussion, development status and prospects
Schisandra genus is widely distributed in East Asia that has a long history of use in traditional medicine and has given rise to many related medical prescriptions in Chinese folklore. The best-known and most widely studied species of this genus are S. sphenanthera and S. chinensis, renowned for their adaptogenic and hepatoprotective properties. Among them, triterpenoids are dominant in S. sphenanthera and S. chinensis and also represent an essential component of compounds in the genus Schisandra, which have been investigated broadly for their potential health benefits. After years of research, more than 335 triterpenoids have been identified from the genus Schisandra with diverse chemical structures, especially in recent years, a large quantity of highly oxygenated descending triterpenes have been extracted and isolated from this genus, greatly enriching the composition of triterpenoids. It can be seen from Fig. 17 that nortriterpeonids have the largest percentage of triterpenoids in the genus Schisandra. This class of compounds has good anti-tumor and anti-HIV activities. It has been developed and explored by several scholars for new drugs and has received patents. Moreover, its hypolipidemic, enzyme activity-regulating and kidney cell-protecting effects have positive effects on the prevention and treatment of liver damage and diabetes. Triterpenoids have proved to be a promising source of valuable natural compounds that deserve further research and development.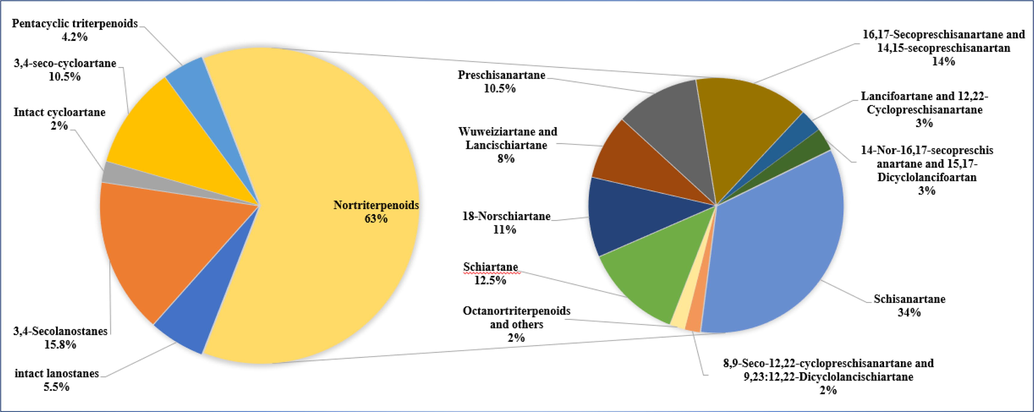
Distribution of triterpenoids in the Schisandra genus.
The pharmacological activities of the triterpenoid components in the Schisandra genus are mainly antitumor, anti-HIV, antioxidant and hepatoprotective (Fig. 18). Some studies suggest that it may also help improve cognitive function, enhance immunity, and reduce symptoms of depression and anxiety disorders. One area of focus is the anti-inflammatory and antioxidant properties of the Schisandra genus, which have been shown to exert anti-inflammatory effects by modulating inflammatory signaling pathways and reducing the production and release of inflammatory factors such as TNF-α, IL-6 and IL-1β, in combination with protecting cells from oxidative stress. These properties make it effective in the treatment of liver disease, acute inflammation and Alzheimer's disease. In clinical practice, it is often found in the form of traditional formulations. Its roots, stems, leaves and fruits are used as raw materials and are commonly available in the dosage forms of wine, pills and tablets.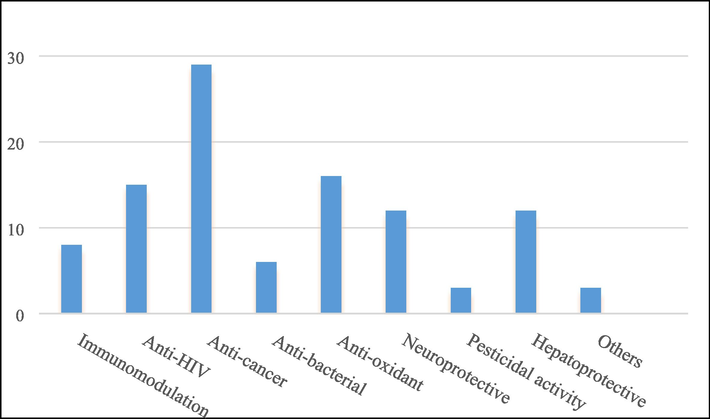
Major biological properties of the triterpenoids of the genus Schisandra.
Although research on the genus Schisandra is promising, however, there are several areas still in need of further study. First, the genus is rich in triterpenoid species and has been extensively reported in the literature, but there are few kinds of literature reports on S. plena and S. tomentella in this genus (Fig. 19 and Fig. 20). Besides, the vine stems, roots and fruits from this genus have been well studied, while the study of other parts is relatively blank. Therefore, a comprehensive research program should have built to systematically study this genus to enrich the chemical composition of triterpenoids of this genus. Secondly, to strengthen the research on monomeric compounds, schisanlactone H from S. sphenanthera has significant inhibitory activity against either HIV or tumor cells, which is expected to be the next generation of anti-tumor drugs. Therefore, in-depth exploration of monomeric compounds with good physiological activity is an essential source of avenues for new drug development and drug preparation, which plays an essential role in the development of innovative drugs. Third, although the activity of triterpenoids of this genus has been widely studied, it is necessary to note that most of its activity tests have been performed in animal models or in vitro, while the mechanism of its action is still unknown (As shown in Table 4, there are relative gaps in mechanistic studies for most compounds). In this regard, more research data are necessary to confirm the confidence of its activity and to continue exploring its mechanism of action and potential side effects. Fourth, its clinical application has some limitations, and its therapeutic potential can be improved by using modern technological tools and developing new formulations and delivery systems. Its potential interactions with other drugs should also be investigated to ensure the safety of Schisandra's use. Fifthly, the genus Schisandra is rich in resources, most of the plants are for medicinal or edible purposes and have received scholarly attention for their potential health effects. Appropriate commercial products can be developed, such as health products, beverages, skin care products, etc.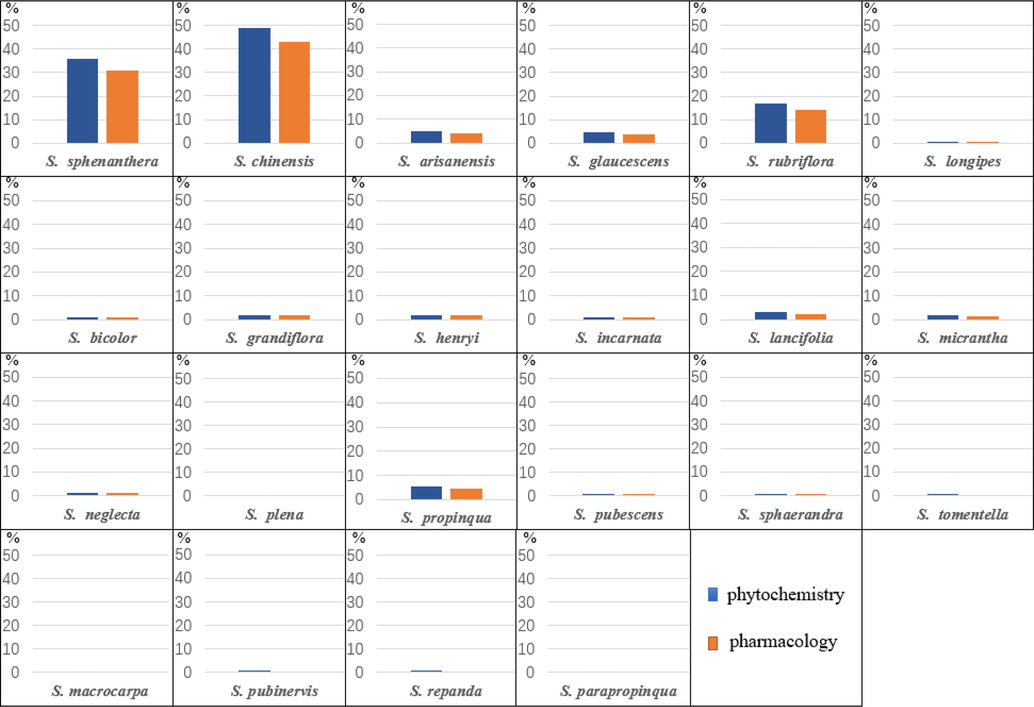
Relative percentages of all published chemical and biological reports for each species.
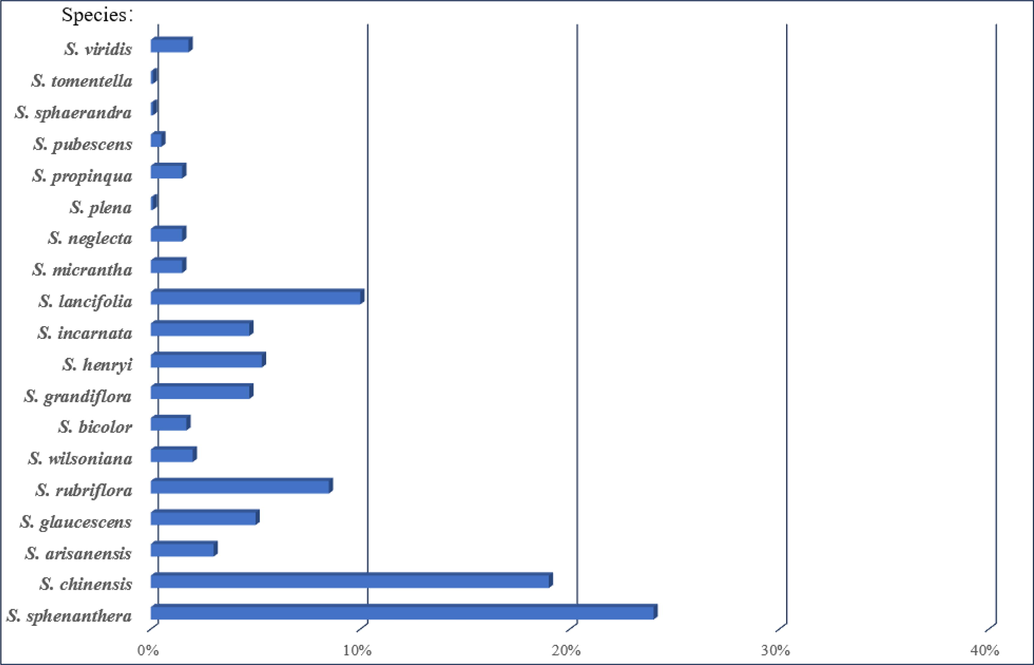
Percentage distribution of triterpenoids in each species.
All of the above shows that triterpenoids have an indispensable position in the genus Schisandra, with a variable chemical structure and rich biological activity, which has long been the focus of researchers' attention. It has been used in clinical treatment, drug development and the food industry, contributing well to human health and economic development. In this paper, we systematically introduce the recent research on triterpenoids and present our views, hoping to provide scientific data to further explore their potential biological activities and develop first-in-class drugs and novel commercial products in the future.
Author contributions
All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
Funding
This work was supported by the National Natural Science Foundation of China (82174111, 82104368), Shaanxi Provincial Science and Technology Department Project (2021JQ-744), Shaanxi University of Chinese Medicine Postgraduate Innovation Program (2021CX06) and Shaanxi University of Chinese Medicine Science and Technology Innovation Team Project (2019-YL12), and Sci-Tech Innovation Talent System Construction Program of Shaanxi University of Chinese Medicine(2023-CXTD-05).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Development of compound healthy tea with Schisandra chinensis. Heilongjiang Agri Sci.. 2012;10:118-121.
- [Google Scholar]
- Studies on the chemical constituents and bioactivities of stems of Schisandra incarnata from Muyu town in Hubei. Huazhong Univ Sci Technol. 2021
- [CrossRef] [Google Scholar]
- Triterpenoid acids from Schisandra propinqua with cytotoxic effect on rat luteal cells and human decidual cells in vitro. Fitoterapia.. 2001;72:435-437.
- [CrossRef] [Google Scholar]
- Triterpenoid acids from Schisandra propinqua with cytotoxic effect on rat luteal cells and human decidual cells in vitro. Fitoterapia.. 2001;72:435-437.
- [CrossRef] [Google Scholar]
- Antifertility triterpenoid acids from Kadsura angustifolia. Chin J Mod Appl Pharm.. 2002;19:103-105.
- [Google Scholar]
- Chen, W.Y., 2018. Research on antibacterial activity of Schisandra chinensis extracts [J]. Pesticide Science and Administration. 39, 53-56+61.
- Arisandilactone A, a new triterpenoid from the fruits of Schisandra arisanensis. Org Lett.. 2010;12:1016-1019.
- [CrossRef] [Google Scholar]
- Nortriterpene lactones from the fruits of Schisandra arisanensis. J Nat Prod.. 2010;73:1228-1233.
- [CrossRef] [Google Scholar]
- Studies on the secondary metabolites and bioactivities of Schisandra propingua var. propinqua. Yunnan Univ; 2018. [Dissertation]
- Studies on bioactive constituents of three species of medicinal plants [Dissertation]. Naval: Medical Univ; 2007.
- New Triterpenoids from the fruits of Schisandra wilsoniana and their biological activities. Bull Korean Chem Soc.. 2013;34:827-830.
- [CrossRef] [Google Scholar]
- Overview of research on the medicinal resources of conman Magnolcavine fruit. Guide China Med.. 2010;8:66-69.
- [CrossRef] [Google Scholar]
- Chemical constituents isolated from stems of Schisandra chinensis and their antifeedant activity against Tribolium castaneum. Nat Prod Res.. 2019;3:1-7.
- [CrossRef] [Google Scholar]
- Progress of traditional application of Schisandraceae plants and the systematic research on them. World Chin Med.. 2015;10:1133-1138.
- [Google Scholar]
- Nortriterpene constituents from Schisandra sphenanthera. Tetrahedron.. 2012;68:440-446.
- [CrossRef] [Google Scholar]
- Neuroprotective schinortriterpenoids with diverse scaffolds from Schisandra henryi. Bioorg Chem.. 2020;105:104353
- [CrossRef] [Google Scholar]
- He, F., 2009. Chemical constituents of three medicinal plants with their biological activities [Dissertation]. Kunming Institute of Botany.
- Cytotoxic ethnic Yao medicine Baizuan, leaves of Schisandra viridis A. C. Smith. J Ethnopharmacol.. 2016;194:146-152.
- [CrossRef] [Google Scholar]
- Structural determination of eleven new preschisanartane-type schinortriterpenoids from two Schisandra species and structural revision of preschisanartanin J using NMR computation method. Chin J Nat Medicine.. 2019;17:970-981.
- [CrossRef] [Google Scholar]
- Isolation and characterization of biogenetically related highly oxygenated nortriterpenoids from Schisandra chinensis. Org Lett.. 2007;9:2079-2082.
- [CrossRef] [Google Scholar]
- Chemical constituents from canes of Schisandra sphenanthera. Chin Trad Herb Drugs.. 2016;47:3374-3378.
- [Google Scholar]
- Wuweizidilactones A-F: Novel highly oxygenated nortriterpenoids with unusual skeletons isolated from Schisandra chinensis. Chemistry.. 2007;13:4816-4822.
- [CrossRef] [Google Scholar]
- Structural characterization of schintrilactone, a new class of nortriterpenoids from Schisandra chinensis. Org Lett.. 2007;9:4175-4178.
- [CrossRef] [Google Scholar]
- Isolation and characterization of miscellaneous terpenoids of Schisandra chinensis. Tetrahedron.. 2008;64:4260-4267.
- [CrossRef] [Google Scholar]
- Schisandrae fructus inhibits IL-1β-induced matrix metalloproteinases and inflammatory mediators production in SW1353 human chondrocytes by suppressing NF-κB and MAPK activation. Drug Dev Res.. 2015;76:474-483.
- [CrossRef] [Google Scholar]
- Dictionary of Medicinal Plants. Tianjin, China: Tianjin Science and Technology Press; 2005.
- Studies on the chemical constituents and biological activities of Schisandra sphenanthera [Dissertation]. South-Central Minzu Univ; 2011.
- Terpenes from Schisandra sphenanthera. Helv Chim Acta.. 2011;94:491-496.
- [CrossRef] [Google Scholar]
- Protective effect of Schisandrae water extract on neuronal cells from cortex in APP/PS1 mice. J Liaoning Univ Trad Chin Med.. 2017;19:30-33.
- [CrossRef] [Google Scholar]
- In vitro and in vivo anti-hyperglycemic effects of Omija (Schizandra chinensis) fruit. Int J Mol Sci.. 2011;12:1359-1370.
- [CrossRef] [Google Scholar]
- The family Schisandraceae: a new record for the flora of Mexico. Brittonia.. 1998;50:87-90.
- [CrossRef] [Google Scholar]
- Insulin-mimetic and insulin-sensitizing activities of a pentacyclic triterpenoid insulin receptor activator. Biochem J.. 2007;403:243-250.
- [CrossRef] [Google Scholar]
- Anti-inflammatory effects of Schisandra chinensis (Turcz.) Baill fruit through the inactivation of nuclear factor-κB and mitogen-activated protein kinases signaling pathways in lipopolysaccharide-stimulated murine macrophages. J Cancer Prev.. 2014;19:279-287.
- [CrossRef] [Google Scholar]
- Oleanolic acid increases plasma ANP levels via an accentuation of cardiac ANP synthesis and secretion in rats. Eur J Pharmacol.. 2013;710:73-79.
- [CrossRef] [Google Scholar]
- Propindilactones E-J, schiartanenortriterpenoids from Schisandra propinqua var.propinqua. J Nat Prod.. 2008;7:1228-1232.
- [CrossRef] [Google Scholar]
- A class of 18(13→14)-abeo-schiartane skeleton nortriterpenoids from Schisandra propinqua var. propinqua. Tetrahedron.. 2009;65:164-170.
- [CrossRef] [Google Scholar]
- Pre-schisanartanins C-D, propintrilactones A-B, two classes of new nortriterpenoids from Schisandra propinqua var. propinqua. Tetrahedron.. 2010;66:2306-2310.
- [CrossRef] [Google Scholar]
- A study on the correlation between the decoction of the Chinese herb Schisandra chinensis and the treatment of drug-induced liver injury. Information on Traditional Chinese Medicine.. 2015;32:53-55.
- [Google Scholar]
- Li, R. T., Li, H.M., Zhou, S.Y., Wang, W.G., Zhang, R.B., Luo, Y.M., Li, H.Z., 2008. New triterpene compound schisanlactone H and its extraction and isolation method. CN101376652A. 2008, 10, 10.
- Comparative study on the processing of health green tea made by schisandra chinensis leaves. Forest By-Prod Speciality in China.. 2013;3:37-39.
- [Google Scholar]
- Li, B., Li, Y.S., Meng, X.J., Xue, X., Zhu, L.J., 2012. Study on antioxidant activity of triterpenes from caculis of Schisandra Chinensis (Turcz.) baill. Sci Technol Food Ind. 33, 121-123+128. https://doi.org/10.13386/j.issn1002-0306.2012.03.007.
- Micranoic Acids A and B: two new octanortriterpenoids from Schisandra micrantha. Chem Pharm Bull.. 2003;51:1174-1176.
- [CrossRef] [Google Scholar]
- Review on the extraction of main effective active ingredients and function of Schisandra chinensis (Turcz.) Baill. Sci Technol Food Ind.. 2016;37:386-390.
- [CrossRef] [Google Scholar]
- Schicagenin A, a rare nortriterpenoid from Schisandra sphenanthera and its molecular interaction studies with human serum albumin. J Cent South Univ.. 2013;47:805-812.
- [CrossRef] [Google Scholar]
- Lancifodilactone A, a novel bisnortriterpenoid from Schisandra lancifolia. Tetrahedron Lett.. 2002;44:3531-3534.
- [CrossRef] [Google Scholar]
- Structure and anti-HIV activity of micrandilactones B and C, new nortriterpenoids possessing a unique skeleton from Schisandra micrantha. Chem Commun (Camb).. 2005;21:2936-2938.
- [CrossRef] [Google Scholar]
- Two new schisdilactone-type compounds from Schisandra chinensis. J Asian Nat Prod Res.. 2013;15:1168-1172.
- [CrossRef] [Google Scholar]
- Four novel nortriterpenoids isolated from Schisandra henryi var. yunnanensis. Eur J Org Chem.. 2004;4:807-811.
- [CrossRef] [Google Scholar]
- Studies on the chemical constituents and bioactivities of five Schisandra medicinal species and Elsholtzia bodinieri. J Univ Chin Acad Sci.. 2004;25:569-575.
- [Google Scholar]
- A New nortriterpenoid, a sesquiterpene and hepatoprotective lignans isolated from the fruit of Schisandra chinensis. Molecules.. 2017;22:1931.
- [CrossRef] [Google Scholar]
- Micrandilactone A: a novel triterpene from Schisandra micrantha. Org Lett.. 2003;5:1023-1026.
- [CrossRef] [Google Scholar]
- Lancifodilactones B-E, new nortriterpenes from Schisandra lancifolia. J Nat Prod.. 2004;67:94-97.
- [CrossRef] [Google Scholar]
- Studies on chemical constituents and bioactivities of three Schisandraceae plants. Yunnan Univ; 2013. [Dissertation]
- Structure and bioactivity of triterpenoids from the stems of Schisandra sphenanthera. Arch Pharm Res.. 2013;37:168-174.
- [CrossRef] [Google Scholar]
- Five new nortriterpenoids from the stems of Schisandra neglecta. Helv Chim Acta.. 2013;96:1376-1385.
- [CrossRef] [Google Scholar]
- Schinarisanlactone A, a new bisnortriterpenoid from Schisandra arisanensis. Org Lett.. 2011;13:446-449.
- [CrossRef] [Google Scholar]
- Studies on the chemical constituents and bioactivities of Schisandra bicolor var. In: Tuberculata and Pausinystalia yohimbe. Huazhong Univ Sci Technol; 2018. [Dissertation]
- [Google Scholar]
- Cytotoxic lanostane triterpenoids from the stems of Schisandra glaucescens. J Asian Nat Prod Res. 2018;20:727-733.
- [CrossRef] [Google Scholar]
- Synthesis of manwuweizic acid, an anticancer triterpenoid. Tetrahedron.. 1992;48:6793-6798.
- [CrossRef] [Google Scholar]
- Schisanlactone B, a new triterpenoid from a Schisandra sp. Tetrahedron Lett.. 1983;24:2355-2358.
- [CrossRef] [Google Scholar]
- Anwuweizonic acid and manwuweizic acid, the putative anticancer active principle of Schisandra propinqua. Can J Chem.. 1989;66:414-415.
- [CrossRef] [Google Scholar]
- Chemical constituents of petroleum ether extract of fruits of Schisandra sphenanthera. China J Chin Materia Med.. 2012;37:1597-1601.
- [Google Scholar]
- Two new 18-norschiartane-type schinortriterpenoids from Schisandra lancifolia. Nat Prod Commun.. 2015;10:2045-2047.
- [Google Scholar]
- Ethno-pharmacological investigation of Schisandraceae plants in China. China J Chin Materia Med.. 2012;37:1353-1359.
- [Google Scholar]
- Triterpenoids and lignans from the fruit of Schisandra sphenanthera. Fitoterapia.. 2017;116:10-16.
- [Google Scholar]
- Nortriterpenoids from the stems and leaves of Schisandra viridis. Fitoterapia.. 2017;118:38-41.
- [CrossRef] [Google Scholar]
- Lu, Y., Chen, D.F., Li, Y.Q., Li, G.W., 2016. Use of cycloartane nortriterpenoids in the preparation of antitumor drugs. CN103800308B. 2016. 03. 30.
- Schilancidilactones A and B: two novel tetranortriterpenoids with an unprecedented skeleton from Schisandra lancifolia. Tetrahedron Lett.. 2009;50:5962-5964.
- [CrossRef] [Google Scholar]
- Schilancitrilactones A-C: three unique nortriterpenoids from Schisandra lancifolia. Org Lett.. 2012;14:1286-1289.
- [CrossRef] [Google Scholar]
- Two new triterpenoids from the stems of Schisandra bicolor. Helv Chim Acta. 2009;92:2086-2091.
- [CrossRef] [Google Scholar]
- Ursolic acid regulates high glucose-induced apoptosis. Free Radical Res.. 2007;41:638-644.
- [CrossRef] [Google Scholar]
- Oleanolic acid protects against pathogenesis of atherosclerosis, possibly via FXR-mediated angiotensin (Ang)-(1–7) upregulation. Biomed Pharmacother.. 2018;97:1694-1700.
- [CrossRef] [Google Scholar]
- Synergistic neuroprotective effect of Schisandra chinensis and ribes fasciculatum on neuronal cell death and scopolamine-induced cognitive impairment in rats. Int J Mol Sci.. 2019;20:4517.
- [CrossRef] [Google Scholar]
- Isolation, structural elucidation of three new triterpenoids from the stems and leaves of Schisandra chinensis (Turcz) Baill. Molecules.. 2018;23:1624.
- [CrossRef] [Google Scholar]
- Study on the chemical composition and anti-inflammatory activity of Schisandra sphenanthera. Kunming Univ Sci Technol; 2012. [Dissertation]
- Schisandraceae plant in Flora of China @ efloras.org., 2020. Schisandraceae plant in Flora of Chloranthus 2020.
- Triterpenoids from the stems of Schisandra grandiflora and their biological activity. J Asian Nat Prod Res.. 2016;18:711-718.
- [CrossRef] [Google Scholar]
- Lancolides, antiplatelet aggregation nortriterpenoids with tricyclo [6.3.0.0(2,11)] undecane-bridged system from Schisandra lancifolia. Org Lett.. 2013;15:5068-5071.
- [CrossRef] [Google Scholar]
- Structural characterization and antioxidative activity of lancifonins: unique nortriterpenoids from Schisandra lancifolia. Org Lett.. 2014;16:1370-1373.
- [CrossRef] [Google Scholar]
- Eleven new highly oxygenated triterpenoids from the leaves and stems of Schisandra chinensis. Org Biomol Chem.. 2013;11:1251-1258.
- [CrossRef] [Google Scholar]
- Highly oxygenated triterpenoids from the roots of Schisandra chinensis and their anti-inflammatory activities. J Asian Nat Prod Res.. 2015;18:189-194.
- [CrossRef] [Google Scholar]
- Spiroschincarins A-E: five spirocyclic nortriterpenoids from the fruit of Schisandra incarnata. Org Lett.. 2017;19:1196-1199.
- [CrossRef] [Google Scholar]
- Schincalactones A and B, two 5/5/6/11/3 fused schinortriterpenoids with a 13-membered carbon ring system from Schisandra incarnata. Org Lett.. 2018;20:2499-2502.
- [CrossRef] [Google Scholar]
- 21014. The Antiproliferative effects of compounds isolated from Schisandra chinensis. Korean. J Food Sci Technol. 2014;46:665-670.
- [CrossRef] [Google Scholar]
- Sun, H.B., Zhang, L.Y., Liu, J., 2009. Ursane-type pentacyclic triterpene amino acid derivatives, their preparation methods and their pharmaceutical uses. CN101337984. 2009, 01, 07.
- Morphological diversity of Schisandra (Schisandraceae) seeds and its taxonomic implication. Plant Divers.. 2006;4:383-393.
- [Google Scholar]
- Nigranoic acid, a triterpenoid from Schisandra sphaerandra that inhibits HIV-1 reverse transcriptase. J Nat Prod.. 1996;59:525-527.
- [CrossRef] [Google Scholar]
- Studies on non-medicinal parts of plant materials: Triterpenes from the roots of Schisandra chinensis. Fitoterapia.. 2021;152:104939
- [CrossRef] [Google Scholar]
- Evaluation of the effect of Callicarpa arborea diterpen and Schisandra chinensis triterpene drivatives on inhibiting the activation of inflammasome. Yunnan Univ 2021 [Dissertation]
- [CrossRef] [Google Scholar]
- Extraction of total triterpenes from vine stems and leaves of Schisandra sphenanthera at different periods and antioxidant and antibacterial studies. Shaanxi Normal Univ 2021 [Dissertation]
- [CrossRef] [Google Scholar]
- Pharmacology function of Schisandra and its exploitation. Journal of Beihua Univ.. 2007;8:128-133.
- [Google Scholar]
- Hepatoprotective triterpenoids and lignans from the stems of Schisandra pubescens. Nat Prod Res.. 2016;31:1855-1860.
- [CrossRef] [Google Scholar]
- Structure and Absolute Stereochemistry of Nortriterpenoids from Schisandra chinensis (Turcz.) Baill. Eur J Org Chem.. 2012;28:5471-5482.
- [CrossRef] [Google Scholar]
- Cytotoxic lanostane triterpenoids from the ethanol extract of Schisandra viridis. J Asian Nat Prod Res.. 2021;24:321-327.
- [CrossRef] [Google Scholar]
- Current research status and prospect of Schisandra chinensis. Non-wood Forest Res.. 2003;4:126-127.
- [Google Scholar]
- A new highly oxygenated nortriterpenoid from Schisandra chinensis. J Asian Nat Prod Res.. 2011;13:551-555.
- [CrossRef] [Google Scholar]
- A new highly oxygenated nortniterpenoid from Schisandra chinensis. J Asian Nat Prod Res.. 2011;13:551-555.
- [CrossRef] [Google Scholar]
- Medicinal value of the fruits of wild fruit trees in Fujian Province. China Trop Agri.. 2009;4:37-40.
- [Google Scholar]
- China Checklist of Higher Plants. In: the Biodiversity Committee of Chinese Academy of Sciences ed., Catalogue of Life China. Beijing, China.: 2023 Annual Checklist; 2023.
- [Google Scholar]
- Wu, W.M., 2016. Studies on the Chemical constituents and bioactivities of Lycoris sprengeri, Abies fargesii & Schisandra glaucescens. [Dissertation]. Huazhong Univ Sci Technol.
- Lancifodilactone F: A novel nortriterpenoid possessing a unique skeleton from Schisandra lancifolia and its anti-HIV activity. Org Lett.. 2005;7:1263-1266.
- [CrossRef] [Google Scholar]
- Lancifodilactone G: a unique nortriterpenoid isolated from Schisandra lancifolia and its anti-HIV activity. Org Lett.. 2005;7:2145-2148.
- [CrossRef] [Google Scholar]
- Nortriterpenoids from Schisandra lancifolia. J Nat Prod.. 2006;69:650-653.
- [CrossRef] [Google Scholar]
- Triterpenoids from Schisandra lancifolia with anti-HIV-1 activity. J Nat Prod.. 2006;69:277-279.
- [CrossRef] [Google Scholar]
- Rubriflordilactones A and B, two novel bisnortriterpenoids from Schisandra rubriflora and their biological activities. Org Lett.. 2006;8:991-994.
- [CrossRef] [Google Scholar]
- Sphenadilactones A and B, two novel nortriterpenoids from Schisandra sphenanthera. Org Lett.. 2006;8:1475-1478.
- [CrossRef] [Google Scholar]
- Isolation and structure elucidation of nortriterpenoids from Schisandra rubriflora. Helv Chim Acta.. 2007;90:1505-1513.
- [CrossRef] [Google Scholar]
- Sphenalactones A-D, a new class of highly oxygenated nortriterpenoids from Schisandra sphenanthera. Tetrahedron Lett.. 2007;48:5543-5546.
- [CrossRef] [Google Scholar]
- Triterpenoids from Schisandra rubriflora. J Nat Prod.. 2007;70:1056-1059.
- [CrossRef] [Google Scholar]
- Nortriterpenoids and lignans from Schisandra sphenanthera. Phytochemistry.. 2008;69:2862-11866.
- [CrossRef] [Google Scholar]
- Bioactive nortriterpenoids from Schisandra grandiflora. J Nat Prod.. 2009;72:1678-1681.
- [CrossRef] [Google Scholar]
- Chemical constituents from the leaves and stems of Schisandra rubriflora. J Nat Prod.. 2010;73:221-225.
- [CrossRef] [Google Scholar]
- Four new nortriterpenoids from Schisandra lancifolia. Helv Chim Acta.. 2010;93:94-97.
- [CrossRef] [Google Scholar]
- Chemical constituents from the leaves and stems of Schisandra lancifolia. Chem Pharm Bull.. 2010;58:852-855.
- [CrossRef] [Google Scholar]
- A preliminary pharmacophylogenetic investigation in Schisandraceae. Clin J Chin Med.. 2008;5:692-723.
- [Google Scholar]
- Effective evaluation on treating arrhythmia of the Xinqi Buzu type with the Wuweizi decoction. J Syst Evol.. 2018;10:47-48.
- [Google Scholar]
- Nortriterpenoids and lignans from the fruit of Schisandra chinensis. Chem Pharm Bull.. 2010;58:1606-1611.
- [CrossRef] [Google Scholar]
- Henrischinins A-C: three new triterpenoids from Schisandra henryi. Org Lett.. 2011;13:1564-1567.
- [CrossRef] [Google Scholar]
- Study on the chemical composition of Schisandra wilsoniana and its application in cigarette. Kunming Institute of Botany. 2008 [Dissertation]
- [Google Scholar]
- Chinorlactone A: a schinortriterpenoid with a 6/5/8/5-fused carbocyclic core from the stems and leaves of Schisandra chinensis. Org Chem Front.. 2022;9:1917-1923.
- [CrossRef] [Google Scholar]
- Seed morphology of the genus Schisandra and its taxonomic significance. Plant Divers.. 2002;5:627-637.
- [Google Scholar]
- Nortriterpenoids from Schisandra wilsoniana. Helv Chim Acta.. 2008;91:1871-1878.
- [CrossRef] [Google Scholar]
- Three new nortriterpenoids from Schisandra wilsoniana and their anti-HIV-1 activities. Nat Prod Bioprospect.. 2011;1:33-36.
- [CrossRef] [Google Scholar]
- Triterpenoids from the fruit of Schisandra glaucescens. Fitoterapia.. 2016;113:64-68.
- [CrossRef] [Google Scholar]
- Study on the chemical composition of Schisandra sphenanthera and the structural modification of its lignans. Yunnan Univ; 2008.
- Schisandraceae triterpenoids: A review of phytochemistry, bioactivities and synthesis. Fitoterapia.. 2022;161:105230
- [Google Scholar]
- Investigation of wild plant resources of Schisandra in Qinling mountains. J Plant Genet Resourc.. 2014;15:236-241.
- [Google Scholar]
- Advanced research on terpenoids in schisandra and their biological activity. Lishizhen Med Mater Medica Res.. 2008;1:228-230.
- [Google Scholar]
- Triterpenoids and lignans from Schisandra chinensis and their inhibition activities of Cdc25A/B phosphatases. Nat Prod Res.. 2020;36:754-759.
- [CrossRef] [Google Scholar]
- Study on the processing technology of dried Schisandra chinensis vinegar powder. Food R&D.. 2020;41:114-121.
- [Google Scholar]
- Schincalide A, a schinortriterpenoid with a tricyclo [5.2.1.0 (1,6)] decane-bridged system from the stems and leaves of Schisandra incarnate. Org Lett.. 2016;18:4558-4561.
- [CrossRef] [Google Scholar]
- Zhou, M., 2018. Studies on the chemical constituents and bioactivities of the stems of Schisandra incarnata and the herbs of Parasenecio subglaber [Dissertation]. Huazhong Univ Sci Technol.
- Zhu, L.J., 2014. Protective effect of total triterpenoid and lignan from Schisandra chinensis (Turcz.) Baill on alcohol-induced liver injury and its mechanism of action [Dissertation]. Shenyang Agr Univ.
- Chemical Constituents from Schisandra grandiflora. Acta Agr Boreali-occidentalis Sinica.. 2013;22:156-161.
- [Google Scholar]
- Study on the antibacterial activity of Schisandra chinensis extract. Jiangsu Agr Sci.. 2011;39:400-402.
- [Google Scholar]







