Translate this page into:
A systematic strategy for rapid identification of chlorogenic acids derivatives in Duhaldea nervosa using UHPLC-Q-Exactive Orbitrap mass spectrometry
⁎Corresponding authors. yangjianbo@nifdc.org.cn (Jian-bo Yang), wwh815@hotmail.com (Wei-hua Wu)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Duhaldea nervosa (Wallich ex Candolle) A. Anderberg was widely used for food spice and folk medicine. However, it is still insufficient in the constituent’s characterization of D. nervosa. In this study, a systematic strategy for rapid detection and identification of constituents was proposed based on UHPLC-Q-Exactive Orbitrap mass spectrometry in parallel reaction monitoring mode combining anion exchange resin separation, expected compounds predicted and diagnosis fragmentation ions techniques. Finally, 149 chlorogenic acids derivatives were unanimously and tentatively characterized from D. nervosa, 102 of them were report for the first time. This results widely extended the chemical constituents of D. nervosa, which will facilitate understanding the effective substance and quality control. Meantime, it is possible for this strategy to exhibit a wide application for chemical’s characterization in different sample.
Keywords
Duhaldea nervosa (Wallich ex Candolle) A. Anderberg
UHPLC-Q-Exactive Orbitrap mass spectrometry
Chlorogenic acids derivatives
1 Introduction
Duhaldea nervosa (Wallich ex Candolle) A. Anderberg (D. nervosa), commonly called Maoxiucai or Xiaoheiyao, belongs to the Asteraceae family (Xiao, 2004; Editorial Board, 2010). It has been widely used as food flavor and folk medicine especially in Dong minority for treating traumatic injury and relieving rheumatism (Xiao, 1997; Long, 2004). Previous investigations had shown that D. nervosa contained steroid, terpenes, polysaccharide, and chlorogenic acids derivatives (CGAs) (Yan et al., 2011; Guan et al., 2017), especially CGAs, which has multiple biological activities, including promoting cell proliferation and differentiation, anti-inflammatory (Naveed et al., 2018; Zhang et al., 2014). However, it is still insufficient in the constituent's characterization of D. nervosa, which is very helpful for understanding the material basis and quality control. Therefore, it is necessary to develop a systematic strategy for rapid detection and identification of constituents in D. nervosa.
In the past few decades, Liquid Chromatography-Mass Spectrometry (LC-MS), especially Ultra-High performance liquid chromatography-high resolution mass spectrometry (UHPLC-HRMS) has become the most powerful and reliable analytical instruments in detection and characterization of constituents from traditional Chinese medicine, drug, or biological samples (Wang et al., 2019; Cai et al., 2017; Koley et al., 2020). However, numerous mass spectrometric data acquired by HRMS will be a new challenge for structure identification. Therefore, several algorithms including metabolic reaction network-based recursive (Shen et al., 2019) and mass spectral trees similarity filter (MTSF) were proposed to solve this problem. In our previous work, MTSF was established and applied in the detection and identification of CGAs in D. nervosa (Liu et al., 2018). Generally, the parent ion of constituents (MS1) and subsequent fragments (MSn) were used for structural elucidation and also for the construct of the mass spectral trees data. However, the parent ion of trace constituents especially when they co-eluted with higher content constituents could not be acquired, and the subsequent fragments could not be trigged due to the relatively lower content in the mass analyzer, which result in the insufficient of CGAs in D. nervosa. In order to obtain the fragments of relatively lower content, the parallel reaction monitoring (PRM) mode (Xiang et al., 2017) was adapted in this experiment. Hence, a systematic strategy was proposed for rapid detection and identification of CGAs in D. nervosa using UHPLC-Q-Exactive Orbitrap mass spectrometry in PRM mode based on expected compounds predicted and diagnosis fragmentation ions techniques.
In this study, anion exchange resin column was used to enrich the trace amount of CGAs at first. Then, the sample was performed on UHPLC-Q-Exactive Orbitrap MS in negative mode to gain the high resolution mass spectrum, which was processed by Compound Discover version 3.0 using high resolution extracted ion chromatography and expected compounds predicted. The MS2 data of expected compounds was obtained by PRM mode. Finally, the diagnosis fragmentation ions were established and used to rapidly identify 149 CGAs from D. nervosa, 102 of them were report for the first time.
2 Materials and methods
2.1 Materials and chemicals
Reference standards trans-3-caffeoylquinic acid (trans-3-CQA, neochlorogenic acid, X-014–170309), trans-4-caffeoylquinic acid (trans-4-CQA, cryptochlorogenic acid, Y-067-180320), trans-5-caffeoylquinic acid (trans-5-CQA, chlorogenic acid, L-007-171216), 3,5-dicaffeoylquinic acid (3,5-DiCQA, isochlorogenic acid A, Y-068-170903), 3,4-dicaffeoylquinic acid (3,4-DiCQA, isochlorogenic acid B, Y-069-180105), 4,5-dicaffeoylquinic acid (4,5-DiCQA, isochlorogenic acid C, Y-070-170515) were provided by Chengdu Herbpurify CO., LTD (Chengdu, China). Anion exchange resin column (WondaSep MAX, 500 mg/6mL) was purchased from Shimadzu Corporation. HPLC grade of water, methanol, acetonitrile, and formic acid were from Fisher scientific (New jersey, USA). Other reagents were of analytical grade.
D. nervosa was purchased from Yunyao company (Yunan, China). The voucher specimen was deposited at School of Pharmaceutical Sciences, Hunan university of medicine.
2.2 Standard and sample preparation
Each reference standard was accurately weighted and dissolved in methanol.
The dried powder of D. nervosa (10 g) was reflux-extracted in 50 mL 70% aqueous ethanol for 1 h, and then the extracted solution was filtrated and dried under reduce pressure to yield the brown residues, which was dissolved in water with 2% formic acid then subjected to anion exchange resign column (WondaSep MAX, 500 mg/6mL), eluting with water and methanol with 2% formic acid, successively. The eluted was evaporated under nitrogen at room temperature. The residue was re-dissolved in 1 mL methanol/water (1:1) and centrifuged at 13000 rpm for 30 min. A volume of 2 μL was injected into UHPLC-Q-Exactive Orbitrap MS for analysis.
2.3 Instrument and condition
All LC-MS analysis were performed on a Q-Exactive Focus Orbitrap MS (Thermo Electron, Bremen, Germany) connected to the Thermo Scientific Dionex Ultimate 3000 RS (Thermo Fisher Scientific, California, USA) via an ESI source. An HYPERSIL GOLD C18 column (100 × 2.1 mm, 1.9 μm) was used for chromatographic separation at 35 °C. The mobile phase consisted of 0.1% formic acid (A) and Acetonitrile (B) at a flow rate of 0.3 mL/min in the following gradient: 0 min, 5% B; 2 min, 8% B; 5 min, 10% B; 20 min, 40% B; 22 min, 95% B; 23 min, 95% B; 23.1 min, 5% B; 25 min, 5% B.
All Sample were analyzed in the negative mode as the following tune method. The nitrogen (purity ≥ 99.99%) served as sheath gas and auxiliary gas at the flow rate of 30 and 10 (arbitrary unit), respectively; the capillary temperature is 320 °C; the auxiliary gas heater temperature is 350 °C; spray voltage is 3.2 KV. High resolution mass spectrum was acquired at full scan in a mass range of m/z 100–1200 at a resolution of 70,000 detected by Orbitrap analyzer. The MS2 data at a resolution of 35,000 was obtained by parallel reaction monitoring mode triggered by inclusion ions list, which was built by molecule predicted. The nitrogen (purity ≥ 99.999%) served as collision gas to generate the fragment ions and the energy was set as normalized collision energy 30%.
2.4 Expected compounds prediction
It is well known that constituents in plant including traditional Chinese medicine could be classified into several families and the chemical constituents in the same family usually share the same carbon skeleton for the similar biosynthetic pathways. For example, CGAs analogues are a large family of esters formed between quinic acid or shikimic acid and one to four or three special residues, most commonly p-coumaric acid, caffeic acid, sinapic acid and ferulic acid. Therefore, the CGAs analogues can be predicted. In this method, shikimic acid (C7H10O5), and quinic acid (C7H12O6) were set as the carbon skeleton, and substituents was summarized according to published paper, including methyl (CH2), ethyl (C2H4), p-coumaroyl (C9H6O2), caffeoyl (C9H6O3), sinapoyl (C11H10O4), feruloyl (C10H8O3), and glucoside (C6H10O5), xyloside (C5H8O4), rhamnoside (C6H10O4). Expected compounds prediction and high resolution extracted ions chromatography (HREIC) were performed by Compound Discover version 3.0 and Xcalibur version 4.1 (Thermo Fisher Scientific, California, USA).
2.5 The establishment of diagnosis fragmentation ions
It is easily understood that CGAs analogues with the same carbon skeletons will generate the similar fragmentations, which can be define as diagnosis fragmentation ions for the screening and characterization of CGAs analogues. The fragmentation patterns of 6 reference standards were investigated by UHPLC-Q-Exactive Orbitrap MS in negative mode to establish the diagnosis fragmentation ions, such as 191.056 (C7H11O6), 173.045 (C7H10O5) generated from quinic acid moiety, 179.034 (C9H7O3), 135.045 (C8H7O2) yielded by caffeic acid moiety.
3 Result and discussion
3.1 Analytical strategy
In order to detect and identify CGAs analogues fully, a strategy based on UHPLC-Q-Exactive Orbitrap MS was proposed in this study. First, anion exchange resin column was used to enrich the trace amount of CGAs because CGAs as a weak acid is destined to enrich by anion exchange resin column. Second, the sample contained CGAs was injected into UHPLC-Q-Exactive Orbitrap MS to gain the high resolution mass data acquired by full MS scanning. Third, metabolism workflow of Compound Discover was modified to predict the molecule of CGAs by setting the parameter as followed: the drug was set as shikimic acid, and quinic acid. The transformations were set as the substituents list mentioned above. The molecule of CGAs was confirmed by data processing including compound discover and high resolution extracted ion chromatography (HREIC) to generated an inclusion ions list. Fourth, the fragmentation ions were acquired using UHPLC-Q-Exactive Orbitrap MS by parallel reaction monitoring mode triggered by inclusion ions list built above. Finally, The CGAs candidates were identified based on diagnosis fragmentation ions, retention time, and bibliography.
3.2 Optimization of UHPLC-Q-Exactive Orbitrap MS condition
In order to obtain satisfactory separation for all the CGAs analogues, the UHPLC parameter were optimized based on single factor experiment including the kind of mobile phase (acetonitrile/water, and methanol/water), the kind and content of acid (formic acid and acetic acid, 0.05, 0.1, and 0.2%), column (HYPERSIL GOLD C18 column, 100 × 2.1 mm, 1.9 μm and Waters ACQUITY BEH C18 column, 100 × 2.1 mm, 1.7 μm), flow rate of mobile phase (0.2, 0.3, and 0.4 mL/min), compartment temperature (25, 30, 35, 40 °C) and the mobile phase gradient. The MS parameters including the flow rate of sheath gas and auxiliary, the temperature of capillary and auxiliary, spray voltage, et al were examined. In the optimization condition of UHPLC-Q-Exactive Orbitrap MS, most of the CGAs analogues have shown good separation, quasi-molecular ions and fragmentation ions.
3.3 Structure elucidation of CGAs analogous
A total of 149 CGAs analogous was tentatively characterization in D. nervosa by UHPLC-Q-Exactive Orbitrap MS, 102 of them were report for the first time. The chromatographic and mass data of those detected constituents are summarized in Table 1 and table 1S, and the HREICs are shown in Fig. 1.
Peak
tR
Theoretical Mass m/z
Experimental Mass m/z
Error (ppm)
Formula [M−H]
Identification
Peak
tR
Theoretical Mass m/z
Experimental Mass m/z
Error (ppm)
Formula [M−H]
Identification
1
1.64
353.10894
353.10833
−1.71
C13H21O11
QA-hexoside
76
10.58
677.17232
677.16992
−3.55
C31H33O17
DiCQA-hexoside
2
1.64
677.19345
677.19128
−3.20
C28H37O19
CQA-Dihexoside
77
10.89
677.17232
677.16980
−3.71
C31H33O17
DiCQA-hexoside
3
1.81
353.10894
353.10812
−2.31
C13H21O11
QA-hexoside
78
11.05
677.17232
677.16913
−4.72
C31H33O17
DiCQA-hexoside
4
1.93
353.10894
353.10845
−1.37
C13H21O11
QA-hexoside
79
11.09
559.14571
559.14508
−1.13
C27H27O13
SCQA
5
2.06
677.19345
677.19312
−0.49
C28H37O19
CQA-Dihexoside
80
11.20
559.14571
559.14429
−2.55
C27H27O13
SCQA
6
2.10
353.10894
353.10825
−1.94
C13H21O11
QA-hexoside
81
11.22
677.17232
677.16962
−3.99
C31H33O17
DiCQA-hexoside
7
2.30
353.10894
353.10848
−1.29
C13H21O11
QA-hexoside
82
11.47
677.17232
677.16974
−3.81
C31H33O17
DiCQA-hexoside
8
2.31
677.19345
677.19269
−1.12
C28H37O19
CQA-Dihexoside
83
11.56
515.11950
515.11835
−2.23
C25H23O12
1,4-DiCQA
9
2.40
353.10894
353.10822
−2.02
C13H21O11
QA-hexoside
84
11.83
515.11950
515.11829
−2.35
C25H23O12
3,4-DiCQA
10
2.65
353.08781
353.08707
−2.08
C16H17O9
Cis-3-CQA
85
11.89
677.17232
677.17023
−3.09
C31H33O17
DiCQA-hexoside
11
2.99
677.19345
677.19263
−1.21
C28H37O19
CQA-Dihexoside
86
11.91
559.14571
559.14441
−2.33
C27H27O13
SCQA
12
3.04
529.15628
529.15509
−2.25
C23H29O14
3-FQA-hexoside
87
11.99
515.11950
515.11792
−3.07
C25H23O12
3,5-DiCQA
13
3.08
677.19345
677.19180
−2.44
C28H37O19
CQA-Dihexoside
88
12.09
677.17232
677.17346
1.68
C31H33O17
DiCQA-hexoside
14
3.21
515.14063
515.13934
−2.50
C22H27O14
CQA-4′-hexoside
89
12.21
559.14571
559.14463
−1.94
C27H27O13
SCQA
15
3.27
353.08781
353.08682
−2.79
C16H17O9
trans-3-CQA
90
12.25
677.15119
677.14978
−2.09
C34H29O15
Cis-TriCQA
16
3.35
499.14571
499.14459
−2.25
C22H27O13
4-pCoQA-hexoside
91
12.35
559.14571
559.14441
−2.33
C27H27O13
SCQA
17
3.49
341.08781
341.08701
−2.33
C15H17O9
CA-hexoside
92
12.47
515.11950
515.11853
−1.88
C25H23O12
1,5-DiCQA
18
3.64
341.08781
341.08691
−2.63
C15H17O9
CA-hexoside
93
12.65
559.14571
559.14392
−3.02
C27H27O13
SCQA
19
3.72
499.14571
499.14536
−0.71
C22H27O13
4-pCoQA-hexoside
94
12.84
677.15119
677.15101
−0.27
C34H29O15
Cis-TriCQA
20
3.80
515.14063
515.13959
−2.02
C22H27O14
CQA-3′-hexoside
95
12.94
515.11950
515.11804
−2.83
C25H23O12
4,5-DiCQA
21
4.14
529.15628
529.15521
−2.02
C23H29O14
4-FQA-hexoside
96
13.07
499.12458
499.12323
−2.71
C25H23O11
Cis-3-pCo, 5CQA
22
4.19
515.14063
515.13954
−2.11
C22H27O14
CQA-4′-hexoside
97
13.24
721.17741
721.17786
0.63
C36H33O16
DiCSQA
23
4.20
341.08781
341.08734
−1.36
C15H17O9
CA-hexoside
98
13.29
499.12458
499.12363
−1.91
C25H23O11
3-pCo, 5CQA
24
4.21
499.14571
499.14407
−3.29
C22H27O13
5-pCoQA-hexoside
99
13.35
529.13515
529.13409
−2.00
C26H25O12
3F,4CQA
25
4.48
353.08781
353.08701
−2.25
C16H17O9
Cis-4-CQA
100
13.43
499.12458
499.12363
−1.91
C25H23O11
3C, 5-pCoQA
26
4.50
337.09289
337.09232
−1.69
C16H17O8
Tran-3-pCoQA
101
13.45
721.17741
721.17828
1.97
C36H33O16
DiCSQA
27
4.50
397.11402
397.11374
−0.70
C18H21O10
3-SQA
102
13.61
529.13515
529.13428
−1.64
C26H25O12
3C,4FQA
28
4.51
515.14063
515.13947
−2.25
C22H27O14
CQA-3′-hexoside
103
13.66
499.12458
499.12341
−2.35
C25H23O11
4-pCo, 5CQA
29
4.68
341.08781
341.08688
−2.71
C15H17O9
CA-hexoside
104
13.78
529.13515
529.13391
−2.34
C26H25O12
3F,5CQA
30
4.69
337.09289
337.09225
−1.90
C16H17O8
Cis-3-pCoQA
105
13.78
677.15119
677.14990
−1.91
C34H29O15
1,3,5-TriCQA
31
4.69
397.11402
397.11395
−0.18
C18H21O10
4-SQA
106
13.81
497.10893
497.10910
0.33
C25H21O11
DiCQL
32
4.69
499.14571
499.14447
−2.49
C22H27O13
CQA-pentoside
107
13.92
529.13515
529.13416
−1.87
C26H25O12
3C,5FQA
33
4.74
397.11402
397.11322
−1.99
C18H21O10
5-SQA
108
13.93
497.10893
497.10834
−1.20
C25H21O11
DiCSA
34
4.75
529.15628
529.15594
−0.64
C23H29O14
4-FQA-hexoside
109
14.07
721.17741
721.17737
−0.05
C36H33O16
DiCSQA
35
4.80
515.14063
515.13916
−2.85
C22H27O14
CQA-3′-hexoside
110
14.15
497.10893
497.10867
−0.53
C25H21O11
DiCQL
36
4.83
499.14571
499.14526
−0.91
C22H27O13
CQA-pentoside
111
14.17
677.15119
677.14978
−2.09
C34H29O15
1,3,4-TriCQA
37
4.84
341.08781
341.08685
−2.80
C15H17O9
CA-hexoside
112
14.28
499.12458
499.12402
−1.13
C25H23O11
Cis-4-pCo, 5CQA
38
4.95
497.13006
497.12930
−1.54
C22H25O13
CSA-hexoside
113
14.29
721.17741
721.18115
5.95
C36H33O16
DiCSQA
39
5.10
353.08781
353.08682
−2.79
C16H17O9
Trans-5-CQA
114
14.41
515.11950
515.11847
−2.00
C25H23O12
Tran-4-Cis-5-DiCQA
40
5.11
515.14063
515.13910
−2.97
C22H27O14
CQA-4′-hexoside
115
14.53
529.13515
529.13422
−1.76
C26H25O12
4F,5CQA
41
5.25
341.08781
341.08710
−2.07
C15H17O9
CA-hexoside
116
14.55
499.12458
499.12378
−1.61
C25H23O11
4C, 5-pCoQA
42
5.39
839.22515
839.22614
1.18
C37H43O22
DiCQA-Dihexoside
117
14.58
497.10893
497.10822
−1.44
C25H21O11
DiCSA
43
5.42
367.10346
367.10273
−1.98
C17H19O9
Tran-3-FQA
118
14.68
721.17741
721.18005
4.42
C36H33O16
DiCSQA
44
5.43
353.08781
353.08682
−2.79
C16H17O9
Trans-4-CQA
119
14.72
677.15119
677.14984
−2.00
C34H29O15
1,4,5-TriCQA
45
5.51
515.14063
515.13995
−1.32
C22H27O14
CQA-4′-hexoside
120
14.73
529.13515
529.13446
−1.30
C26H25O12
4C,5FQA
46
5.61
499.14571
499.14488
−1.67
C22H27O13
5-pCoQA-hexoside
121
14.78
497.10893
497.10852
−0.83
C25H21O11
DiCQL
47
6.00
337.09289
337.09229
−1.78
C16H17O8
Tran-5-pCoQA
122
14.91
721.17741
721.18073
5.37
C36H33O16
DiCSQA
48
6.08
529.15628
529.15546
−1.55
C23H29O14
4-FQA-hexoside
123
15.10
661.15628
661.15533
−1.44
C34H29O14
pCoDiCQA
49
6.31
497.13006
497.12994
−0.25
C22H25O13
CSA-hexoside
124
15.12
691.16684
691.16681
−0.05
C35H31O15
DiCFQA
50
6.31
839.22515
839.22431
−1.00
C37H43O22
DiCQA-Dihexoside
125
15.12
721.17741
721.17316
−5.13
C36H33O16
DiCSQA
51
6.38
337.09289
337.09229
−1.78
C16H17O8
Cis-4-pCoQA
126
15.23
691.16684
691.16638
−0.67
C35H31O15
DiCFQA
52
6.51
497.13006
497.12903
−2.08
C22H25O13
CQL-hexoside
127
15.28
661.15628
661.15533
−1.44
C34H29O14
pCoDiCQA
53
6.55
367.10346
367.10239
−2.90
C17H19O9
Cis-3-FQA
128
15.40
661.15628
661.15485
−2.16
C34H29O14
pCoDiCQA
54
6.65
529.15628
529.15540
−1.66
C23H29O14
5-FQA-hexoside
129
15.54
497.10893
497.10815
−1.58
C25H21O11
DiCQL
55
6.76
839.22515
839.22369
−1.73
C37H43O22
DiCQA-Dihexoside
130
15.58
721.17741
721.17554
−2.59
C36H33O16
DiCSQA
56
6.80
497.13006
497.12918
−1.78
C22H25O13
CSA-glycoside
131
15.62
661.15628
661.15473
−2.34
C34H29O14
pCoDiCQA
57
6.85
529.15628
529.15594
−0.64
C23H29O14
4-FQA-hexoside
132
15.62
691.16684
691.16608
−1.10
C35H31O15
DiCFQA
58
6.90
353.08781
353.08694
−2.45
C16H17O9
Cis-5-CQA
133
15.92
677.15119
677.14996
−1.82
C34H29O15
3,4,5-TriCQA
59
7.18
335.07724
335.07678
−1.37
C16H15O8
5-CSA
134
16.29
721.17741
721.17627
−1.58
C36H33O16
DiCSQA
60
7.26
335.07724
335.07687
−1.11
C16H15O8
4-CSA
135
16.35
497.10893
497.10785
−2.18
C25H21O11
DiCSA
61
7.40
337.09289
337.09222
−1.99
C16H17O8
Tran-4-pCoQA
136
16.99
661.15628
661.15466
−2.45
C34H29O14
pCoDiCQA
62
7.69
337.09289
337.09210
−2.35
C16H17O8
Cis-5-pCoQA
137
17.01
721.17741
721.17603
−1.15
C36H33O16
DiCSQA
63
7.73
367.10346
367.10264
−2.20
C17H19O9
Cis-4-FQA
138
17.13
661.15628
661.15479
−2.25
C34H29O14
pCoDiCQA
64
8.08
335.07724
335.07654
−2.09
C16H15O8
3-CSA
139
17.22
691.16684
691.16644
−0.58
C35H31O15
DiCFQA
65
8.09
515.11950
515.11835
−2.23
C25H23O12
1,3-DiCQA
140
17.31
661.15628
661.15453
−2.65
C34H29O14
pCoDiCQA
66
8.23
335.07724
335.07663
−1.82
C16H15O8
3-CQL
141
17.36
691.16684
691.16632
−0.76
C35H31O15
DiCFQA
67
8.47
367.10346
367.10242
−2.82
C17H19O9
Tran-4-FQA
142
17.36
721.17741
721.17743
0.03
C36H33O16
DiCSQA
68
8.62
367.10346
367.10242
−2.82
C17H19O9
Tran-5-FQA
143
17.49
691.16684
691.16628
−0.81
C35H31O15
DiCFQA
69
8.69
335.07724
335.07645
−2.36
C16H15O8
1-CQL
144
17.87
721.17741
721.17682
−0.06
C36H33O16
DiCSQA
70
9.35
335.07724
335.07687
−1.11
C16H15O8
4-CQL
145
18.34
691.16684
691.16608
−1.10
C35H31O15
DiCFQA
71
9.80
367.10346
367.10257
−2.41
C17H19O9
Cis-5-FQA
146
18.49
721.17741
721.17639
−1.41
C36H33O16
DiCSQA
72
9.83
677.17232
677.17023
−3.09
C31H33O17
DiCQA-hexoside
147
18.82
721.17741
721.17578
−1.50
C36H33O16
DiCSQA
73
9.98
839.22515
839.22376
−1.65
C37H43O22
DiCQA-Dihexoside
148
19.07
721.17741
721.17584
−2.17
C36H33O16
DiCSQA
74
10.10
677.17232
677.17297
0.96
C31H33O17
DiCQA-hexoside
149
19.17
721.17741
721.17761
1.04
C36H33O16
DiCSQA
75
10.28
677.17232
677.16852
−5.62
C31H33O17
DiCQA-hexoside
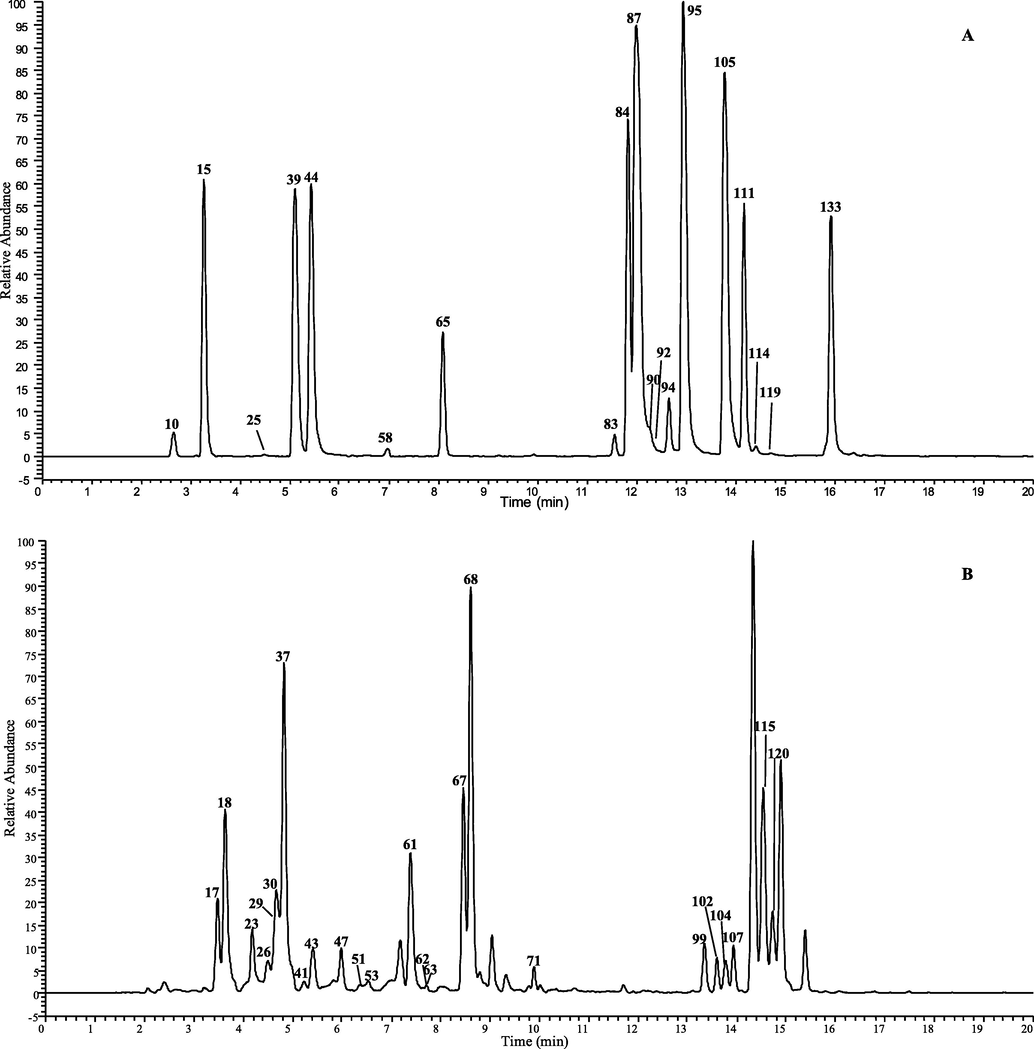
The high-resolution extracted ion chromatogram (HREIC) in 5 ppm for the multiple compounds in Duhaldea nervosa. (A) m/z 353.08781, 515.11950, 677.15119; (B) m/z 337.09289, 341.08781, 367.10346, 529.13515; (C) m/z 335.07724, 353.10894, 497.10893, 499.12458, 515.14063, 529.15628; (D) m/z 499.14571, 559.14571, 661.15628, 677.17232, 691.16684; (E) m/z 397.11402, 677.19345, 721.17741, 839.22515.
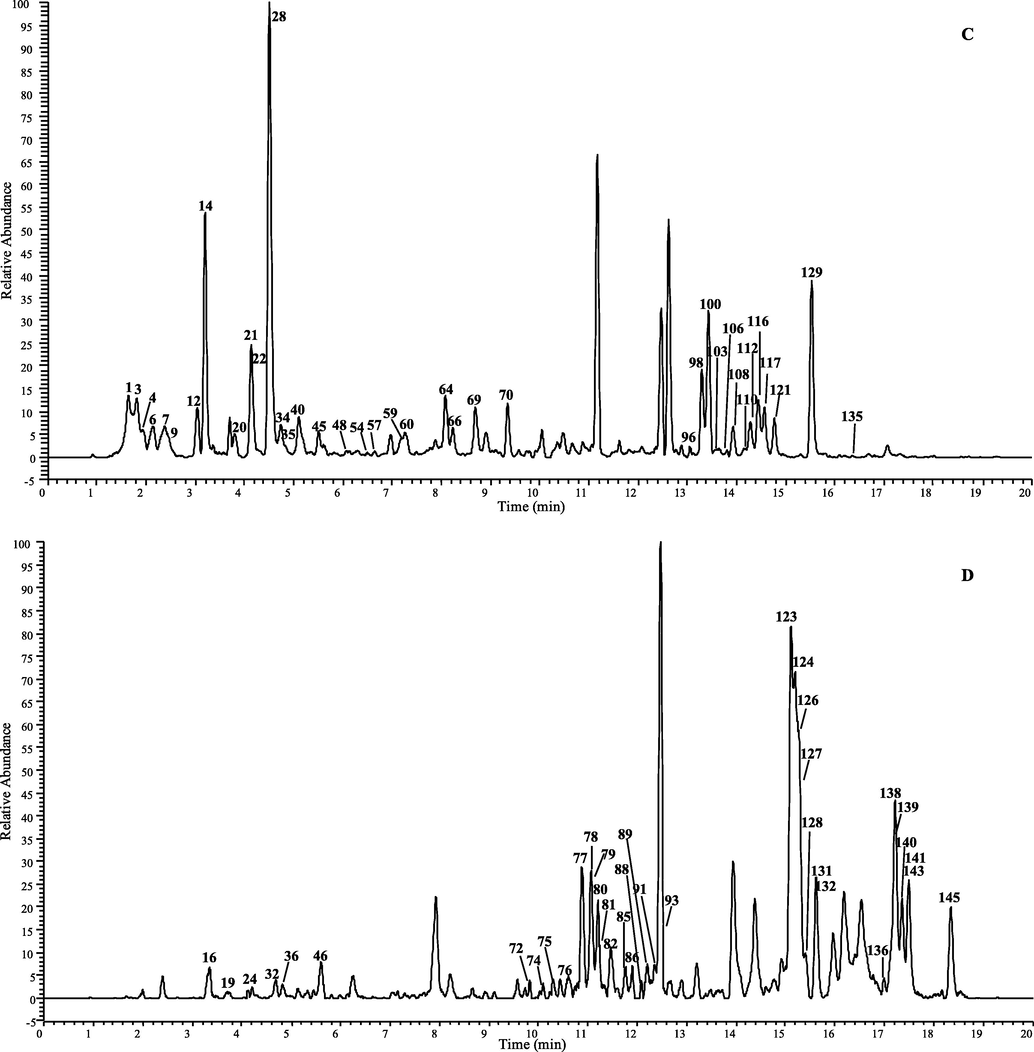
The high-resolution extracted ion chromatogram (HREIC) in 5 ppm for the multiple compounds in Duhaldea nervosa. (A) m/z 353.08781, 515.11950, 677.15119; (B) m/z 337.09289, 341.08781, 367.10346, 529.13515; (C) m/z 335.07724, 353.10894, 497.10893, 499.12458, 515.14063, 529.15628; (D) m/z 499.14571, 559.14571, 661.15628, 677.17232, 691.16684; (E) m/z 397.11402, 677.19345, 721.17741, 839.22515.
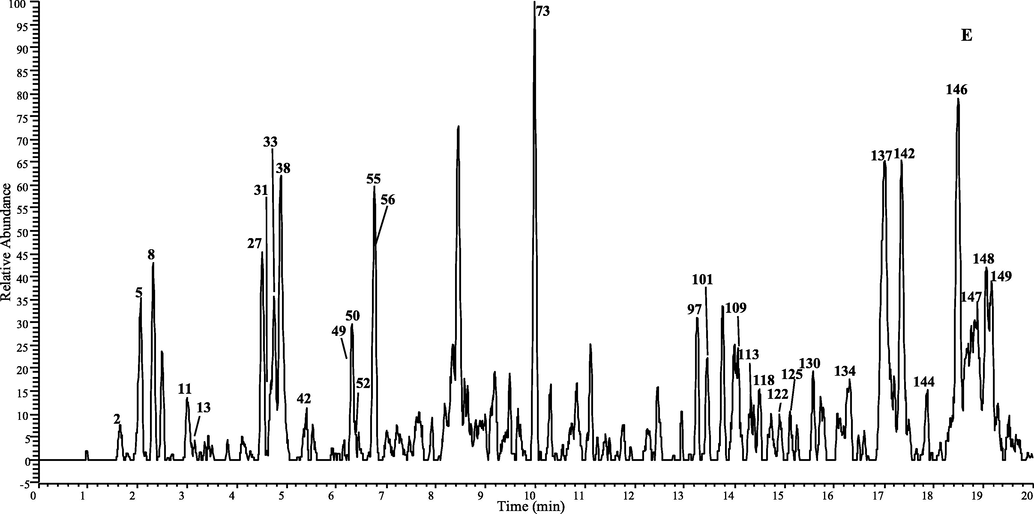
The high-resolution extracted ion chromatogram (HREIC) in 5 ppm for the multiple compounds in Duhaldea nervosa. (A) m/z 353.08781, 515.11950, 677.15119; (B) m/z 337.09289, 341.08781, 367.10346, 529.13515; (C) m/z 335.07724, 353.10894, 497.10893, 499.12458, 515.14063, 529.15628; (D) m/z 499.14571, 559.14571, 661.15628, 677.17232, 691.16684; (E) m/z 397.11402, 677.19345, 721.17741, 839.22515.
3.3.1 Identification of monoacyl-quinic acids or monoacyl-shikimic acids
Compounds 59, 60, 64, 66, 69, and 70 were eluted at 7.18, 7.26, 8.08, 8.23, 8.69, and 9.35 min, with the same quasi-molecular ions [M−H]− at m/z 335.076 (C16H15O8), which could be caffeoylquinic acid lactones (CQL) or caffeoylshikimic acids (CSA). Quinic acid lactones are prone to generate ion at m/z 161.023 by the loss of the lactone and H2O moiety, which can be used as the distinguished ions between CQLs and CSAs (Jaiswal et al., 2010, 2012, 2014a, 2014b, 2014c). Therefore, they were tentatively identified as 5-CSA, 4-CSA, 3-CSA, 3-CQL, 1-CQL, and 4-CQL, respectively. Compounds 38, 49, 52, and 56 generated the same deprotonated molecular ion m/z 497.129 (C22H25O13), 162 Da (C6H10O5) more than that of CSA or CQL, suggesting they were the hexoside of CQL or CSA, which were further confirmed by the present of m/z 335.076 (C16H15O8), 179.033 (C9H7O4), 135.043(C8H7O2) in MS2 data. The ion at m/z 161.023 (C9H5O3) in MS2 of compound 52 possessed a higher intensity than m/z 179.033 (C9H7O4), indicated that compound 52 was CQL-hexoside. The others (38, 49, and 56) were tentatively inferred as CSA-hexoside.
Compounds 26, 30, 47, 51, 61, and 62 eluted at 4.50, 4.69, 6.00, 6.38, 7.40, and 7.69 min and showed a deprotonated molecular ion [M−H]− at m/z 337.09232 (−1.69 ppm, C16H17O8), 337.09225 (−1.90 ppm, C16H17O8), 337.09229 (−1.78 ppm, C16H17O8), 337.09229 (−1.78 ppm, C16H17O8), 337.09222 (−1.99 ppm, C16H17O8), and 337.09210 (−2.35 ppm, C16H17O8), respectively. According to the published paper (Xiang et al., 2017; Clifford et al., 2003), they were tentatively assigned as Tran-3-O-p-coumaroylquinic acid (pCoQA), Cis-3-pCoQA, Tran-5-pCoQA, Cis-4-pCoQA, Tran-4-pCoQA, and Cis-5-pCoQA based on the different base peak ion in MS2 spectrum. Compounds 16, 19, 24, 32, 36, and 46 was eluted at 3.35, 3.72, 4.21, 4.69, 4.83, and 5.61 min, with the deprotonated ions [M−H]− at m/z 499.14459 (−2.25 ppm, C22H27O13), 499.14536 (−0.71 ppm, C22H27O13), 499.14407 (−3.29 ppm, C22H27O13), 499.14447 (−2.49 ppm, C22H27O13), 499.14526 (−0.91 ppm, C22H27O13), and 499.14488 (−1.67 ppm, C22H27O13), respectively, 162 Da (C6H10O5) and 146 Da (C6H10O4) more than pCoQA (C16H17O8) and CQA (C16H17O9), respectively. The fragment ion at m/z 337.09 (C16H17O8) by loss the C6H10O5 moiety was detected in MS2 spectrum of compounds 16, 19, 24 and 46, suggesting they were hexoside of pCoQA. The base peak at m/z 173.044 and 191.054 was shown in the MS2 spectrum of compounds 16, 19, and compounds 24, 46, respectively, indicated that compounds 16, 19, 24, and 46 were 4-pCoQA-hexoside, 4-pCoQA-hexoside, 5-pCoQA-hexoside, and 5-pCoQA-hexoside, respectively (Jaiswal et al., 2014a, 2014b, 2014c). The fragment ions at m/z 179.033 (C9H7O4) and 191.054(C7H11O6) of compounds 32, 36 were similar to the MS2 spectrum of CQA. Therefore, compounds 32, 36 were tentatively identified as CQA-pentoside.
Compounds 10, 15, 25, 39, 44, and 58 with the same deprotonated ions [M−H]− at m/z 353.08 (C16H17O9) were eluted at 2.65, 3.27, 4.48, 5.10, 5.43, and 6.90 min, of which compounds 15, 39, and 44 were accurately characterized as Tran-3-CQA, Tran-5-CQA, and Tran-4-CQA by comparing the retention time, MS data with those reference standards. Meantime, compounds 10, 25 and 58 were tentatively presumed as Cis-3-CQA, Cis-4-CQA and Cis-5-CQA, respectively (Clifford et al., 2008; Jaiswal et al., 2011). Compounds 14, 20, 22, 28, 35, 40, and 45 eluted at 3.21, 3.80, 4.19, 4.51, 4.80, 5.11, and 5.51 min, with the quasi-molecular ions [M−H]− at m/z 515.139 (C22H27O14), 162 Da (C6H10O5) more than CQA, suggesting they were the hexoside of CQA. The presence of fragment ion at m/z 323.076 (C15H15O8) was used to distinguish the position of hexoside (Clifford et al., 2007; Jaiswal et al., 2014a, 2014b, 2014c). Therefore, compounds 20, 28, and 35 were tentatively identified as CQA-3′-hexoside. The others (14, 22, 40, and 45) might be CQA-4′-hexoside. Compounds 2, 5, 8, 11, and 13 were detected 1.64, 2.06, 2.31, 2.99, 3.08 min and yielded a deprotonated ion [M−H]− at m/z 667.19128 (−3.20 ppm, C28H37O19), 667.19312 (−0.49 ppm, C28H37O19), 667.19269 (−1.12 ppm, C28H37O19), 667.19263 (−1.21 ppm, C28H37O19), and 667.19180 (−2.44 ppm, C28H37O19), 162 Da (C6H10O5) more than CQA-hexoside. Therefore, Compounds 2, 5, 8, 11, and 13 were tentatively characterized as CQA-Dihexoside.
Compounds 43, 53, 63, 67, 68, and 71, possessing a deprotonated ion [M−H]− at m/z 367.10273 (−1.98 ppm, C17H19O9), 367.10239 (−2.90 ppm, C17H19O9), 367.10264 (−2.20 ppm, C17H19O9), 367.10242 (−2.82 ppm, C17H19O9), 367.10242 (−2.82 ppm, C17H19O9), and 367.10257 (−2.41 ppm, C17H19O9), were detected at 5.42, 6.55, 7.73, 8.47, 8.62, and 9.80 min, suggesting that they might be feruloylquinic acid (FQA). The isomers were identified to be as follows: Cis-3-FQA and Tran-3-FQA produced the base peak ion at m/z 193.049 (C10H9O4); Cis-4-FQA and Tran-4-FQA yielded the base peak ion at m/z 173.044 (C7H9O5); Cis-5-FQA and Tran-5-FQA yielded the base peak ion at m/z 191.054 (C7H11O6); and the configuration of Cis or Tran was judged by the intensity of those peaks that cis-compound show lower intensity for its instability (Clifford et al., 2003, 2008). Therefore, they were tentatively characterized as Tran-3-FQA, Cis-3-FQA, Cis-4-FQA, Tran-4-FQA Tran-5-FQA, and Cis-5-FQA, respectively. Compounds 12, 21, 34, 48, 54, and 57, possessed the same quasi-molecular ion [M−H]− at m/z 529.155(C26H25O12), 162 Da (C6H10O5) more than FQA, suggesting they were the hexoside of FQA (Jaiswal et al., 2014a, 2014b, 2014c), which were further confirmed by the existence of fragment ions m/z 193.049, 173.044, and 367.102. The base peak (the second higher peak of compound 54) of m/z 529 can also be used to discriminate the submitted position as above. Therefore, compounds 12 and 54 were tentatively characterized as 3-FQA-hexoside, 5-FQA-hexoside, respectively. The others were 4-FQA-hexoside.
Compounds 27, 31, and 33 were detected at 4.50, 4.69, and 4.74 min, with a quasi-molecular ion [M−H]− at m/z 397.11374 (−0.70 ppm, C18H21O10), 397.11395 (−0.18 ppm, C18H21O10), and 397.11322 (−1.99 ppm, C18H21O10), respectively. The present of m/z 173.044 (C7H9O5), 191.054 (C7H11O6), and 205.049 (C11H9O4) in MS2 spectrum of those compounds indicated that they were Sinapoylquinic acids (SQA). The positional isomers can be distinguished by the base peak of MS2 data. The base peak at 191.0548 (−6.86 ppm, C7H11O6), 173.0442 (−7.78 ppm, C7H9O5), and 205.0496 (−5.03 ppm, C11H9O4) were detected in MS2 spectrum of those compound, respectively, indicated they were 3-SQA, 4-SQA, and 5-SQA by referring to the literature data (Zhang et al., 2016).
3.3.2 Identification of diacyl-quinic acids or diacyl-shikimic acids
Compounds 106, 108, 110, 117, 121, 129, and 135 were eluted at 13.81, 13.93, 14.15, 14.58, 14.78, 15.54, and 16.35 min and possessed a deprotonated ion [M−H]− m/z 497.10910 (0.33 ppm, C25H21O11), 497.10867 (−0.53 ppm, C25H21O11), 497.10834 (−1.20 ppm, C25H21O11), 497.10867 (−1.44 ppm, C25H21O11), 497.10852 (−0.83 ppm, C25H21O11), 497.10815 (−1.58 ppm, C25H21O11), and 497.10785 (−2.18 ppm, C25H21O11), which could be Dicaffeoylquinic acid lactones (DiCQL) or Dicaffeoylshikimic acids (DiCSA). Compounds 106, 110, 121, and 129 afforded a same base peak at m/z 161.0230, which were formed by losing the lactone and H2O moiety from quinic acids lactone (Jaiswal et al., 2014a, 2014b, 2014c), thus, they were tentatively characterized as DiCQL. The others (108, 117, and 43) were assigned as DiCSA (Jaiswal et al., 2010).
Compounds 96, 98, 100, 103, 112, and 116 showed the deprotonated ion [M−H]− m/z 499.12323 (−2.71 ppm, C25H23O11), 499.12363 (−1.91 ppm, C25H23O11), 499.12363 (−1.91 ppm, C25H23O11), 499.12341 (−2.35 ppm, C25H23O11), 499.12402 (−1.13 ppm, C25H23O11), and 499.12378 (−1.61 ppm, C25H23O11), respectively. the appearance of fragment ions m/z 173.044 (C7H9O5), 179.033 (C9H7O4), and 191.054 (C7H11O6) in MS2 spectrum of those compounds indicated they were p-coumaroylcaffeoylquinic acids (pCoCQA). The absence of base peak at m/z 173.044 (C7H9O5) of compounds 96, 98, and 100 are consistent with their being 3,5-pCoCQA. thus, compounds 96, 98, and 100 were tentatively identified as Cis-3-pCo,5CQA, 3-pCo,5CQA, and 3C, 5-pCoQA according the base peak and retention time (Jaiswal et al., 2010; Clifford et al., 2006). Likewise, compounds 103, 112, and 116 were tentatively characterized as 4-pCo,5CQA, Cis-4-pCo,5CQA, and 4C, 5-pCoQA, respectively.
Compounds 84, 87, and 95 possessed the same retention time, mass spectrum data with these reference standards 3,4-DiCQA, 3,5-DiCQA, and 4,5-DiCQA, respectively. Thus, they were unambiguously assigned as 3,4-DiCQA, 3,5-DiCQA, and 4,5-DiCQA. Compounds 65, 83, 92, and 114 generated the same deprotonated ion [M−H]− m/z 515.118 (C25H23O12) and fragment ions m/z 173.044 (C7H9O5), 179.033 (C9H7O4), and 191.054 (C7H11O6) with compounds above, suggesting they are isomers. The present of base peak m/z 173.044 (C7H9O5) in MS2 of 83 and 114 indicated they are n, 4-DiCQA. According the retention time (Liu et al., 2018; Clifford et al., 2005), compounds 65, 83, 92, and 114 were tentatively characterized as 1,3-DiCQA, 1,4-DiCQA, 1,5-DiCQA, and Tran-4-Cis-5-DiCQA, respectively. Compounds 74, 75, 76, 77, 78, 81, 82, 85, and 88 were eluted at 10.10, 10.28, 10.58, 10.89, 11.05, 11.22, 11.47, 11.89, and 12.09 min, with the same deprotonated ion [M−H]− m/z 677.169 (C31H33O17), 162 Da (C6H10O5) more than DiCQA, suggesting they were the hexoside of DiCQA, which were further confirmed by the presence of fragment ions m/z 515.117 (C25H23O12), 173.044 (C7H9O5), 179.033 (C9H7O4), and 191.054 (C7H11O6). Therefore, they were inferred as DiCQA-hexoside (Clifford et al., 2008; Jaiswal et al., 2014a, 2014b, 2014c). Compounds 42, 50, 55, and 73 generated a deprotonated ion [M−H]− m/z 839.22614 (1.18 ppm, C37H43O22), 839.22431 (−1.00 ppm, C37H43O22), 839.22369 (−1.73 ppm, C37H43O22), and 839.22376 (−1.65 ppm, C37H43O22), 162 Da (C6H10O5) more than DiCQA-hexoside, indicated they were the dihexoside of DiCQA. Therefore, they were tentatively identified as DiCQA-dihexoside.
Compounds 99, 102, 104, 107, 115, and 120 yielded a quasi-molecular ion [M−H]− at m/z 529.13409 (−2.00 ppm, C26H25O12), 529.13428 (−1.64 ppm, C26H25O12), 529.13391 (−2.34 ppm, C26H25O12), 529.13416 (−2.00 ppm, C26H25O12), 529.13422 (−1.76 ppm, C26H25O12), and 529.13444 (−1.30 ppm, C26H25O12) and were eluted at 13.35, 13.61, 13.78, 13.92, 14.53, and 14.73 min, respectively. All of those compounds showed the fragment ions at m/z 173.044 (C7H9O5), 193.049 (C10H9O4) or 353.086 (C16H17O9), 367 (C17H19O9), which were consistent with caffeoylferuloylquinic acids (CFQA). According the retention time and diagnosis ions in bibliography (Liu et al., 2018; Clifford et al., 2003), Compounds 99, 102, 104, 107, 115, and 120 were tentatively identified as 3F, 4CQA, 3C,4FQA, 3F,5CQA, 3C,5FQA, 4F,5CQA, 4C,5FQA, respectively.
Compounds 79, 80, 86, 89, 91, and 93 were detected at 11.09, 11.20, 11.91, 12.21, 12.35, 12.65, and 15.10, with the same deprotonated ion [M−H]− m/z 559.144 (C27H27O13). All of those compounds yield the fragment ions m/z 173.044 (C7H9O5), 191.054 (C7H11O6), 179.033 (C9H7O4), and 397.112 (C18H21O10), which were consistent with Caffeoylsinapoylquinic acids (CSQA) (Lin and Harnly, 2008).
3.3.3 Identification of triacyl-quinic acids or triacyl-shikimic acids
Compounds 123, 127, 128, 131, 136, 138, and 140 were eluted at 15.10, 15.28, 15.40, 15.62, 16.99, 17.13, and 17.31 min and yielded a deprotonated ion [M−H]− m/z 661.15533 (−1.44 ppm, C34H29O14), 661.15533 (−1.44 ppm, C34H29O14), 661.15485 (−2.16 ppm, C34H29O14), 661.15473 (−2.34 ppm, C34H29O14), 661.15466 (−2.45 ppm, C34H29O14), 661.15479 (−2.25 ppm, C34H29O14), and 661.15453 (−2.65 ppm, C34H29O14), respectively. the appearance of ions m/z 173.044 (C7H9O5), 179.033 (C9H7O4), 337.092 (C16H17O8), and 353.087 (C16H17O9) indicated they are p-coumaroyl-dicaffeoylquinic acids (pCoDiCQA).
Compounds 90, 94, 105, 111, 119, and 133 generated a quasi-molecular ion [M−H]− at m/z 677.14978 (−2.09 ppm, C34H29O15), 677.15101 (-0.27 ppm, C34H29O15), 677.14990 (−1.91 ppm, C34H29O15), 677.14978 (−2.09 ppm, C34H29O15), 677.14984 (−2.00 ppm, C34H29O15), and 677.14996 (−1.82 ppm, C34H29O15), respectively. The existence of fragment ions m/z 353.0869 (C16H17O9), m/z 515.1180 (C25H23O12) and the absence of ions m/z 497.1070 (C25H21O11) of compound 133 was consistent assignment as 3,4,5-TriCQA. Likewise, the others (92–96) were characterized as Cis-TriCQA, Cis-TriCQA, 1,3,5-TriCQA, 1,3,4-TriCQA, 1,4,5-TriCQA according the published paper (Liu et al., 2018).
Compounds 124, 126, 132, 139, 141, 143, and 145 eluted at 15.12, 15.23, 15.62, 17.22, 17.36, 17.49, and 18.34, with a quasi-molecular ion [M−H]− at m/z 691.166 (C35H31O15). The fragment ions m/z 529.133 (C26H25O12), 367.118(C21H19O6), 173.044(C7H9O5), 179.033 (C9H7O4), and 179.033 (C9H7O4) were detected in MS2 data of those compound, suggesting that they were dicaffeoylferuloylquinic acids (DiCFQA). Likewise, compounds 97, 101, 109, 113, 118, 122, 125, 130, 134, 137, 142, 144, 146, 147, 148 and 149 were tentatively characterized as Dicaffeoylsinapoylquinic acids (DiCSQA).
3.3.4 Others
Compounds1, 3, 4, 6, 7, and 37 were detected between 1.64 and 2.40 min, possessing the quasi-molecular ion [M−H]− at m/z 353.10833 (−1.71 ppm, C13H21O11), 353.10812 (−2.31 ppm, C13H21O11), 353.10845 (−1.37 ppm, C13H21O11), 353.10825 (−1.94 ppm, C13H21O11), 353.10848 (-1.29 ppm, C13H21O11), and 353.10822 (−2.02 ppm, C13H21O11), respectively. all of those compounds yielded fragment ions at m/z 191.054 (C7H11O6), 173.044 (C7H9O5), 129.054 (C6H9O3), and 101.059 (C5H9O2), which is consisted to the fragment of quinic acid moiety (Zhang et al., 2016). Thus, they might be considered as hexoside of quinic acid (QA-hexoside).
Compounds 17, 18, 23, 29, 37, and 41 yielded a deprotonated ion [M−H]− m/z 341.08701 (−2.33 ppm, C15H17O9), 341.08691 (−2.63 ppm, C15H17O9), 341.08734 (−1.36 ppm, C15H17O9), 341.08688 (−2.71 ppm, C15H17O9), 341.08685 (−2.80 ppm, C15H17O9), 341.08710 (−2.07 ppm, C15H17O9), which show a fragment ion at m/z 179.033 (C9H7O4) by losing the saccharide moiety 162 Da in the MS2 experiment. The base peak of m/z 135.043 (C8H7O2) and the fragment ion 179.033 (C9H7O4) were consisted with the caffeic acids (Gavrilova et al., 2011) [28, therefore, they were tentatively identified as caffeoyl hexoside (CA-hexoside).
4 Conclusion
In this study, a systematic strategy was proposed for rapid detection and identification of CGAs by UHPLC-Q-Exactive Orbitrap mass spectrometry combining anion exchange resin separation, expected compounds predicted and diagnosis fragmentation ions techniques. Using this strategy, 149 CGAs were unanimously and tentatively characterized from D. nervosa, 102 of them were report for the first time. This results widely extended the chemical constituents of D. nervosa, which will facilitate understanding the effective substance and quality control. Meantime, it is possible for this strategy to exhibit a wide application for characterization and profile of compounds in other kinds of sample, such as, fruits, vegetable, beverage and so on.
Acknowledgements
The authors would like to thank the financial support from the National Natural Science Foundation of China (no. 81603393), the Natural Science Foundation of Hunan Province (no. 2018JJ3376), the Scientific Research Fund of Hunan Provincial Education Department (no. 19A353).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Detection and characterization of the metabolites of rutaecarpine in rats based on ultra-high-performance liquid chromatography with linear ion trap orbitrap mass spectrometer. Pharm. Biol.. 2017;55:294-298.
- [Google Scholar]
- Hierarchical scheme for LC-MSn identification of chlorogenic acids. J. Agr. Food. Chem.. 2003;51:2900-2911.
- [Google Scholar]
- LC-MSn analysis of the cis isomers of chlorogenic acids. Food. Chem.. 2008;106:379-385.
- [Google Scholar]
- Discriminating between the six isomers of dicaffeoylquinic acid by LC-MSn. J. Agr. Food. Chem.. 2005;53:3821-3832.
- [Google Scholar]
- Characterization by LC-MSn of four new classes of p-coumaric acid-containing diacyl chlorogenic acids in green coffee beans. J. Agr. Food. Chem.. 2006;54:4095-4101.
- [Google Scholar]
- Profiling the chlorogenic acids and othercaffeic acid derivatives of herbal Chrysanthemum by LC–MSn. J. Agr. Food. Chem.. 2007;55:929-936.
- [Google Scholar]
- Flora of China. Beijing: Science Press; 2010. p. :20-21.
- Separation, characterization and quantification of phenolic compounds in blueberries and red and black currants by HPLC-DAD-ESI-MSn. J. Agr. Food. Chem.. 2011;59:4009-4018.
- [Google Scholar]
- Determination of isochlorogenic acid A and isochlorogenic acid C in Duhaldea nervosa by HPLC. Lishizhen Med. Mater. Med. Res.. 2017;28:1032-1034.
- [Google Scholar]
- Profiling the chlorogenic acids of Rudbeckia hirta, Helianthus tuberosus, Carlina acaulis and Symphyotrichum novae-angliae leaves by LC-MSn. Phytochem. Anal.. 2011;22:432-441.
- [Google Scholar]
- Identification and characterisation of the phenolics of Ilex glabra L. Gray (Aquifoliaceae) leaves by liquid chromatography tandem mass spectrometry. Phytochemistry. 2014;106:141-155.
- [Google Scholar]
- Understanding the fate of chlorogenic acids in coffee roasting using mass spectrometry based targeted and non-targeted analytical strategies. Food. Funct.. 2012;3:976-984.
- [Google Scholar]
- Does roasted coffee contain chlorogenic acid lactones or/and cinnamoylshikimate esters? Food. Res. Int.. 2014;61:214-227.
- [Google Scholar]
- Identification and characterization of chlorogenic acids, chlorogenic acid glycosides and flavonoids from Lonicera henryi L. (Caprifoliaceae) leaves by LC-MSn. Phytochemistry. 2014;108:252-263.
- [Google Scholar]
- Profiling and characterization by LC-MSn of the chlorogenic acids and hydroxycinnamoylshikimate esters in mate (Ilex paraguariensis) J. Agr. Food. Chem.. 2010;58:5471-5484.
- [Google Scholar]
- High resolution LC-MS characterization of phenolic compounds and the evaluation of antioxidant properties of a tropical purple radish genotype. Arab. J. Chem.. 2020.;13:1355-1366.
- [Google Scholar]
- Identification of hydroxycinnamoylquinic acids of arnica flowers and burdock roots using a standardized LC-DAD-ESI-MS profiling method. J. Agr. Food. Chem.. 2008;56:10105-10114.
- [Google Scholar]
- Liu, L.H., Zhang, J.Y., Zheng, B.J., Guan, Y., Wang, L.T., Chen, L., Cai., 2018. Rapid characterization of Chlorogenic Acids in Duhaldea nervosa based on UHPLC LTQ-Orbitrap-MS and mass spectral trees similarity filter technique. J. Sep. Sci. 41, 1764–1774
- Clinical experience of “maoshoucai” for treating traumatic injury. J. Med. Phar. Chin. Min.. 2004;12:231-232.
- [Google Scholar]
- Chlorogenic acid (CGA): a pharmacological review and call for further research. Biomed. Pharmacother.. 2018;97:67-74.
- [Google Scholar]
- Metabolic reaction network-based recursive metabolite annotation for untargeted metabolomics. Nat. Commun.. 2019;10:1516-1529.
- [Google Scholar]
- Characterization of stress degradation products of curcumin and its two derivatives by UPLC–DAD–MS/MS. Arab. J. Chem.. 2019;12:3998-4005.
- [Google Scholar]
- Comprehensive analysis of acylcarnitine species in db/db mouse using a novel method of high-resolution parallel reaction monitoring reveals widespread metabolic dysfunction induced by diabetes. Anal. Chem.. 2017;89:10368-10375.
- [Google Scholar]
- Textual research of dong medicine and a famous doctor for treating traumatic injury. J. Med. Phar. Chin. Min.. 1997;5:41-42.
- [Google Scholar]
- Explore Secret of Dong Medicine. Changsha.: Yuelu Press; 2004. p. :46.
- Phytane and neoclerodane diterpenes from the aerial parts of Inula nervosa Wall. Biochem. Syst. Ecol.. 2011;39:700-703.
- [Google Scholar]
- A strategy for comprehensive identification of sequential constituents using ultra-high-performance liquid chromatography coupled with linear ion trap-Orbitrap mass spectrometer, application study on chlorogenic acids in Flos Lonicerae Japonicae. Talantal. 2016;47:16-27.
- [Google Scholar]
- Chlorogenic acid promotes rat osteoblast proliferation via Shp2 activation Erk1/2. Chin. Tradit. Pat. Med.. 2014;36:693-697.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2020.01.007.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







