Translate this page into:
Accumulation of selected metals in the fruits of medicinal plants grown in urban environment of Islamabad, Pakistan
⁎Corresponding author. Fax: +92 51 90642241. mhshahg@qau.edu.pk (Munir H. Shah) munir_qau@yahoo.com (Munir H. Shah)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The present study is based on the measurement of selected metals (Ca, Cd, Co, Cr, Cu, Fe, Mg, Mn, Pb, Sr and Zn) in the fruits of eight medicinal plants (Carrisa opeca, Phyllanthus emblica, Solanum nigrum, Zizyphus nummularia, Zizyphus mauritiana, Physalis minima, Opuntia dillenii and Phoenix dactylifera) and relevant soil samples by atomic absorption spectrometry. Highest average concentrations of Cu (14.4 mg/kg), Cr (19.0 mg/kg), and Zn (125 mg/kg) were found in the fruits of P. minima, C. opeca and Z. nummularia, respectively, while O. dillenii showed the elevated mean levels of Cd (3.49 mg/kg), Sr (61.4 mg/kg), Mg (0.21%), Ca (6.62%) and Mn (44.6 mg/kg). However, highest average levels of Pb (41.7 mg/kg) and Co (38.4 mg/kg) were found in Z. mauritiana. Overall, most of the fruit samples showed higher contributions of Ca and Mg, followed by Fe, Zn, Co and Pb. In the case of soil samples, highest concentration was observed for Ca, followed by Fe, Mg, Mn and Sr, while lowest concentration was shown by Cd. Bioaccumulation factors exhibited significantly higher accumulation of Co (0.813–1.829) and Pb (0.060–2.350) from the soil to the fruits. Principal component analysis revealed significant anthropogenic contributions of Pb, Fe and Co in the fruit samples. Contamination factors and enrichment factors of Cd and Pb in the soil indicated very high contamination and extreme enrichment of these metals.
Keywords
Bioaccumulation
Fruit
Medicinal plant
Metal
Enrichment
Health risk
1 Introduction
The use of medicinal plants for therapeutic applications has been on the rise throughout the world, particularly in Asia (Abbasi et al., 2012; Ahmed et al., 2010; Chandrasekaran and Bahkali, 2013; Jaijoy et al., 2010). The popularity of medicinal plants is mainly due to their ease of access, therapeutic effectiveness, relatively low cost as compared to allopathic medicines and assumption that they are free from adverse effects (Bohm, 2008; Huang et al., 2010; Nathiya and Dorrcus, 2012). Most of the population in developing countries rely on the unconventional medicine in their primary health care (Chan, 2003; Pandey et al., 2010). However, it has been noted that not all the natural therapies/products related to complementary or alternative medicines are free from adverse effects (Meena et al., 2010). The efficiency of medicinal plants in treating various ailments/therapeutic actions is partially dependant on trace metal concentrations and their complexation with some chemotherapeutic agents (Desideri et al., 2010). In medicinal plants, trace metals play very significant roles in the reactions which lead to the formation of the active chemical constituents and are, therefore, responsible for their curative as well as toxic properties (Tokalioglu, 2012). The analysis of essential and toxic metals can be used to decide the dosage of the herbal drugs prepared from these plants. It is thus of great interest to establish the levels of trace metals in medicinal plants because at elevated levels, they might exert toxic effects (Ajasa et al., 2004; Abugassa et al., 2008).
Contamination of medicinal plants is mainly caused by the pollution of soil with toxic metals which may originate from polluted irrigation water, automobile/industrial emissions, atmospheric dusts, pesticides and fertilizers (Baye and Hymete, 2010). The toxic metals interact with soil matrix and may persist for longer time creating long-term hazards. Their availability in soil is used as a key indicator of potential risks to the environment and human health (Barthwal et al., 2008). Moreover, plants can also accumulate the metals for which no direct benefit and no significant physiological functions have been recognized. These metals may not be so harmful for the plants, but are hazardous for human health as medicinal plants are part of the food chain (Razic et al., 2006). Thus, it is of particular importance to evaluate the concentrations of essential and toxic metals in the plants and relevant soil. This study was taken up to evaluate the bioaccumulation of selected metals (Ca, Cd, Co, Cr, Cu, Fe, Mg, Mn, Pb, Sr and Zn) in therapeutically important medicinal plants grown in urban environment in relation to the metal contents in the soil. Plausible health risks associated with the metal contents in the plants were also estimated. Besides, contamination and enrichment of the metals in soil were assessed in this study. It is anticipated that the study would provide a baseline data related to the metal pollution in the soil and their uptake by the medicinal plants in urban environment.
2 Materials and methods
2.1 Study area
The wild edible fruits and relevant soil samples were collected from urban areas of Islamabad, the capital city of Pakistan. It is located at 33.43°N & 73.04°E in the northwest of the country against the backdrop of Margalla Hills. The city extends over 906 km2 and it has an estimated population of more than one million. The climate is tropical with two diverse seasons, viz. winter (October-February) and summer (March-September); average temperature ranges from 13 °C in January to 38 °C in June (Shah and Shaheen, 2007).
2.2 Sample collection
Composite samples of the wild edible fruits of Carrisa opeca, Phyllanthus emblica, Solanum nigrum, Zizyphus nummularia, Zizyphus mauritiana, Physalis minima, Opuntia dillenii and Phoenix dactylifera were collected from various urban areas of Islamabad during October 2010-April 2011 (Table 1). The plant identification and verification were carried in the Herbarium of Quaid-i-Azam University, Islamabad, Pakistan, where the voucher specimen for each plant was submitted. The plant species were collected from different urban localities along with the soil sample. Most of the samples were collected in the vicinity of medium to high traffic density areas. The fresh fruit samples were carefully collected in clean polyethene bags and transported to the laboratory for further analysis. About 18–20 composite fruit samples of each medicinal plant were collected from the study area. Composite soil samples (0–30 cm, top layer) were also collected from the place of the collection of fruit samples. Three composite soil samples were collected from the locality of each plant, and each composite sample consisted of 3–5 sub-samples that were mixed in equal proportions. About 500 g of the soil samples was collected in polyethene bags and labelled and transported to the laboratory for further analysis (Radojevic and Bashkin, 1999).
S. No.
Botanical name
Family
Vernacular name
Therapeutic effects
1.
Solanum nigrum
Solanaceae
Kach mach
Antipyretic, diuretic, hepatoprotective effects and anticancer (Huang et al., 2010)
2.
Physalis minima
Solanaceae
Rasbhari
Diuretic, purgative, analgesic, cure spleen disorder, anti-cancer (Nathiya and Dorrcus, 2012)
3.
Zizyphus nummularia
Rhamnaceae
Jharberi
Astringent, appetizer, stomachic, cure mucous, increase biliousness effect (Pandey et al., 2010)
4.
Zizyphus mauritiana
Rhamnaceae
Ber
Anodyne and tonic, stop vomiting, antidote to aconite poisoning and abdominal pain (Meena et al., 2010)
5.
Phyllanthus emblica
Euphorbiaceae
Amla
Diarrhoea, jaundice, inflammatory disorders, antitumor, antibacterial (Jaijoy et al., 2010)
6.
Carrisa opeca
Apocynaceae
Karounda
Cure fever and eye disorders (Ahmed et al., 2010)
7.
Opuntia dillenii
Cactaceae
Nagphana
Antioxidant activities, antidiabetic drug, antispermatogenic effect (Bohm, 2008)
8.
Phoenixdactelifera
Arecaceae
Kharjura
Antioxidant, antimutagenic, antimicrobial, anti-inflammatory, gastro protective, hepatoprotective, neoprotective, nephroprotective, anticancer and immunostimulant (Chandrasekaran and Bahkali, 2013)
2.3 Sample preparation
The fresh weights (FW) of the fruit samples were recorded just after sample collection. The samples were then washed with distilled water and dried at 70–80 °C for 48 h in an electric oven. The dried samples were ground into fine powder by porcelain mortar and pestle. The powdered samples were placed in tightly closed clean/labelled sample bottles and stored at room temperature in desiccators until further analysis. Afterwards, about 1.0 g of the powdered sample was digested using 20 mL HNO3 and 10 mL HClO4. The mixture was heated to obtain clear solution and the final volume (50 mL) was adjusted with 0.1 M HNO3. Along with each batch of five samples, a blank (containing all the reagents except the fruit sample) was also processed through the same procedure in order to maintain the accuracy of results (Radojevic and Bashkin, 1999).
In the case of soil samples, large items such as stones, grass, and pieces of wood were manually removed and samples were dried in an electric oven at 70–80 °C for 48 h to achieve the constant weight. Dried samples were ground to smallest possible size and stored in desiccators until further processing (Guvenc et al., 2003). Every precaution was taken to avoid contamination during the sampling, drying, grinding and storage. For digestion, the exactly weighed quantity of the soil sample (∼1.000 g) was taken in the digestion vessel, to which 15 mL of freshly prepared aqua-regia was added and left for 16 h. Then the vessel was placed on heating block and continued heating at 50 °C for 30 min. Afterwards, the temperature was raised to 120 °C and continued the heating for 2 h. Then the vessel was cooled and 10 mL of 0.25 M HNO3 was added. The solution was filtered and the filtrate transferred to a 50 mL flask and made up to the mark with 0.25 M HNO3. Blank samples were also processed in the similar manner along with each batch of five samples (Radojevic and Bashkin, 1999).
2.4 Instrumental analysis
The quantitative measurement of selected metals (Ca, Cd, Co, Cr, Cu, Fe, Mg, Mn, Pb, Sr and Zn) in the digested fruits and soil samples was performed on flame atomic absorption spectrophotometer (Model: AA-670 Shimadzu, Japan) using optimum analytical condition (Table 2). Standard stock solutions (1000 mg/L) were used to prepare the working standard for each metal. The working standards were prepared afresh on the day of analysis by successive dilutions. Accuracy of the quantified results was also checked by analysing the standard reference materials of plant (NIST SRM-1515, USA) and soil (NIST SRM-2711a, USA) origin and the recoveries were 98 to 101% as shown in Table 2. Some of the samples were also analysed at an independent laboratory for cross comparison and the results were found to be within ±2.5%. All the reagents used were of ultrahigh purity (certified >99.99%) procured from E-Merck and BDH.
Metal
Limit of detection (mg/L)
Limit of quantification (mg/L)
Calibration ‘R’
SRM-1515 (apple leaves)
SRM-2711a (soil)
Certified level
Measured level
Recovery (%)
Certified level
Measured level
Recovery (%)
Ca
0.003
0.010
0.998
15,260 ± 150
15,310 ± 160
100
24,200 ± 600
23,980 ± 610
99
Mg
0.001
0.004
0.997
2710 ± 80
2730 ± 65
101
10,700 ± 600
10,510 ± 560
98
Zn
0.002
0.006
0.998
12.5 ± 0.3
12.3 ± 0.4
98
414 ± 11
418 ± 13
101
Fe
0.006
0.018
0.997
83.0 ± 5.0
82.2 ± 5.3
99
28,200 ± 400
28,010 ± 345
99
Co
0.004
0.013
0.996
(0.09)a
0.09 ± 0.01
98
9.9 ± 0.2
9.7 ± 0.2
98
Sr
0.005
0.016
0.998
25.0 ± 2.0
25.2 ± 1.9
101
242 ± 10
238 ± 11
98
Cr
0.005
0.016
0.997
(0.30)a
0.30 ± 0.02
100
52.3 ± 2.9
52.8 ± 2.8
101
Pb
0.009
0.028
0.998
0.470 ± 0.024
0.465 ± 0.022
99
1400 ± 10
1375 ± 10
98
Mn
0.003
0.009
0.999
54.0 ± 3.0
53.6 ± 3.1
99
675 ± 18
680 ± 16
101
Cu
0.003
0.010
0.999
5.64 ± 0.24
5.53 ± 0.28
98
140 ± 2
141 ± 3
101
Cd
0.004
0.013
0.997
0.013 ± 0.002
0.013 ± 0.003
100
54.1 ± 0.5
52.9 ± 0.7
98
Plant ability to accumulate the metals from soil can be estimated using the bioaccumulation factor (BAF), which is defined as the ratio of metal concentrations in the fruits samples to that in soil (Kovacik et al., 2012; Gebrekidan et al., 2013):
2.5 Statistical analysis
Statistical analysis of the analytical data was carried out using STATISTICA software version 6.0 (StatSoft, 1999). Basic statistical parameters and correlation coefficients were calculated for the metal data of fruits and soil. Multivariate analysis may be particularly valuable when there is a huge volume of experimental results and sometime they provide insight into the multidimensional patterns in the data that might be overlooked with univariate analysis (Shtangeeva et al., 2009). Multivariate principal component analysis was performed using varimax normalized rotation on the data set. It is generally used for the pattern recognition and apportionment of the variables.
2.6 Ecological risk assessment
Potential ecological risks associated with the concentrations of selected metals in the soil were assessed using contamination factor (CF) and degree of contamination (Cdeg). The CF is calculated using the following equation as suggested by Hakanson (1980):
In this study, mean concentrations of the metals in background soil of Islamabad (Iqbal et al., 2015) were used as reference values.
Enrichment factor (EF) can be used to assess the extent of metal pollution in soil and represents the contamination level in the soil and is a good tool to differentiate among the anthropogenic and natural sources of the metals. Enrichment factor is usually taken as double ratio of the target element and a reference element in soils and earth crust:
2.7 Health risk assessment
A detailed door-to-door survey with open response and multiple-choice questions was administered to the adults in local population. Information acquired included the fruiting season, the consumption duration, the intake rate, the number of family members and the source of the fruits. A total of 351 respondents participated in the survey. The average daily intake of each fruit by an adult in a year was then calculated (Cherfi et al., 2014). Risk to human health was evaluated by computing the health risk index (HRI) which depends on daily intake of the metals through consumption of the fruits (Cn × Dn) and then compares them to the prescribed reference oral dose (Abbasi et al., 2013; Li et al., 2012). This index was calculated using following relationship:
Target hazard quotient (THQ) is used to assess the non-carcinogenic risks to humans from food consumption (Abbasi et al., 2013; Cherfi et al., 2014). The method to estimate THQ was provided in USEPA Region III Risk-Based Concentration Table (USEPA, 2006):
The hazard index (HI) can be expressed as the sum of the hazard quotients for all metals (USEPA, 2006):
3 Results and discussion
Table 1 shows the description of the medicinal plants included in this study and the therapeutic effects of their fruits as reported in the literature. These wild plants are commonly found in the study area and have high cultural importance because they are frequently used by the local population in a number of recipes. These data showed that the fruits have several therapeutic properties; hence, evaluation of metal levels is desirable.
3.1 Metal concentrations in the fruits
Distribution of selected metal levels (mg/kg, dry weight) in the fruits of medicinal plants is shown in Table 3. It is clear from the results that Ca and Mg were the most abundant elements in the fruit samples. Highest average concentration of Ca was noted for O. dillenii (6.62%), followed by Z. nummularia (0.48%), Z. mauritiana (0.28%), P. emblica (0.19%), C. opeca (0.18%), P. dactilifera (0.14%), S. nigrum (0.05%), and P. minima (0.05%). Exceptionally higher concentration of Ca found in fruits of O. dillenii indicated the uptake and enrichment of the metal in this plant, which may be used as a food supplement to overcome the Ca-deficiency. High concentration of Ca is considered important in medicinal plants because of its role in maintaining healthy bones and teeth (Rihawy et al., 2010). Like the previous case, Mg contents were the highest (0.21%) in O. dillenii, while lowest concentrations (0.05%) were noted in P. emblica. Mg along with K is essential electrolyte for maintaining normal fluid balance in cells. Moreover, normal cardiac rhythm is maintained and increase in blood pressure is prevented by a delicate balance of these two elements (Desideri et al., 2010).
Ca
Mg
Zn
Fe
Co
Sr
Cr
Pb
Mn
Cu
Cd
S. nigrum
Range
466–491
901–918
17.8–18.9
45.3–47.4
30.4–33.0
1.88–2.16
14.2–17.0
35.7–39.3
10.5–10.9
2.75–3.24
0.008–0.12
Mean ± SD
480 ± 15
910 ± 32
18.5 ± 1.8
46.5 ± 2.5
31.6 ± 1.4
2.03 ± 0.14
15.7 ± 1.8
37.8 ± 2.2
10.7 ± 0.1
3.00 ± 0.27
0.10 ± 0.02
P. minima
Range
458–482
1105–1191
9.05–9.37
61.2–65.8
29.4–31.6
4.16–5.10
15.1–18.1
21.4–24.0
9.05–9.88
13.5–15.5
3.19–3.70
Mean ± SD
470 ± 18
1150 ± 36
9.25 ± 1.05
63.2 ± 3.3
30.9 ± 1.1
4.33 ± 0.26
16.3 ± 1.3
22.8 ± 1.3
9.50 ± 0.52
14.4 ± 1.2
3.48 ± 0.20
Z. nummularia
Range
4680–4790
645–710
116–133
23.5–25.6
33.9–36.8
9.9–11.3
2.15–2.74
14.5–16.9
10.7–11.4
5.03–5.89
2.84–3.23
Mean ± SD
4750 ± 85
680 ± 30
125 ± 6
24.9 ± 1.8
35.4 ± 1.4
10.4 ± 0.5
2.33 ± 0.82
15.6 ± 1.5
11.1 ± 0.1
5.47 ± 0.52
3.01 ± 0.20
Z. mauritiana
Range
2740–2850
532–568
3.52–3.69
62.4–67.9
37.0–39.6
20.1–22.9
16.4–18.5
39.6–43.2
6.00–6.63
8.64–9.55
1.11–1.58
Mean ± SD
2800 ± 67
550 ± 27
3.61 ± 0.64
65.5 ± 3.1
38.4 ± 1.4
21.3 ± 1.9
17.3 ± 1.2
41.7 ± 2.0
6.31 ± 0.31
9.01 ± 0.44
1.30 ± 0.22
P. emblica
Range
1845–1910
471–523
12.9–13.8
53.5–56.8
36.5–39.1
14.0–16.1
13.9–15.2
26.1–28.8
10.7–11.0
0.41–0.67
0.19–0.29
Mean ± SD
1890 ± 46
500 ± 20
13.5 ± 1.3
55.0 ± 2.7
37.9 ± 1.5
14.7 ± 1.0
14.6 ± 1.5
27.8 ± 1.8
10.9 ± 0.2
0.50 ± 0.18
0.25 ± 0.04
C. opeca
Range
1814–1852
781–818
16.4–17.3
47.5–50.2
28.9–31.6
2.20–2.87
17.6–20.7
34.5–38.1
10.9–11.4
6.31–7.20
2.38–2.66
Mean ± SD
1840 ± 44
800 ± 34
16.9 ± 1.5
48.9 ± 2.5
30.2 ± 1.4
2.50 ± 0.26
19.0 ± 1.7
36.1 ± 2.0
11.2 ± 0.3
6.97 ± 0.68
2.52 ± 0.10
O. dillenii
Range
65,980–66,315
2105–2147
44.2–46.1
26.9–29.8
32.3–35.0
59.6–63.7
14.7–17.0
3.06–3.51
43.1–46.2
5.91–6.79
3.20–3.69
Mean ± SD
66,200 ± 300
2130 ± 53
45.7 ± 2.2
28.4 ± 2.0
33.8 ± 1.4
61.4 ± 2.8
15.7 ± 1.2
3.24 ± 0.25
44.6 ± 2.3
6.48 ± 0.64
3.49 ± 0.21
P. dactelifera
Range
1382–1448
654–722
79.6–84.7
55.1–59.7
26.5–28.7
14.6–16.9
17.5–19.9
28.7–31.3
3.71–3.99
11.3–13.6
1.51–1.95
Mean ± SD
1420 ± 37
690 ± 32
81.5 ± 3.9
57.5 ± 3.0
27.8 ± 1.1
15.4 ± 1.2
18.7 ± 1.4
29.9 ± 1.6
3.90 ± 0.31
12.5 ± 1.2
1.73 ± 0.24
Zinc is known to be involved in immunomodulatory functions and its deficiency in diet could be extremely detrimental to human health (Alexander et al., 2006). In the present study, its concentration varied from 3.61 mg/kg in Z. mauritiana to 125 mg/kg in Z. nummularia. Significant concentrations of Zn were found in P. dactylifera (81.5 mg/kg) and O. dillenii (45.7 mg/kg). FAO/WHO recommended 27.4 mg/kg of Zn as the permissible limit in edible plants (FAO/WHO, 1984). Average concentrations of Zn found in S. nigrum, P. emblica, C. opeca, P. minima and Z. mauritiana during the present study were within the FAO/WHO limit, while Z. nummularia, O. dillenii and P. dactylifera revealed elevated levels than the recommended limit. However, the daily intake of Zn associated with the consumption of these fruits was well below the maximum limits of 21 mg/day (USEPA, 2002; Bagdatlioglu et al., 2010). Somewhat consistent levels were noted for Fe in the fruit samples, ranging from 24.9 mg/kg in Z. nummularia to 65.5 mg/kg in Z. mauritiana. Appreciably higher Fe levels were noted in the fruits of P. minima (63.2 mg/kg), P. dactylifera (57.5 mg/kg) and P. emblica (55.0 mg/kg). The average daily intake of Fe through the fruit consumption was found to be within the WHO provisional maximum tolerable daily intake limits of 56 mg/day (Goldhaber, 2003; Bagdatlioglu et al., 2010). Similarly, cobalt was measured in the fruits samples at almost comparable levels ranging from 27.8 mg/kg in P. dactilifera to 38.4 mg/kg in Z. mauritiana. However, relatively large variations in the Sr levels were observed in the fruit samples ranging from 2.03 mg/kg in S. nigrum to 61.4 mg/kg in O. dillenii. Like Ca and Mg, O. dillenii depicted highest concentrations of Sr. Although there are no known physiological effects of Sr at low concentration, its accumulation in the body could be detrimental to health (Desideri et al., 2010).
Among the fruits, C. opeca depicted highest concentrations of Cr (19.0 mg/kg), followed by P. dactilifera (18.7 mg/kg), Z. mauritiana (17.3 mg/kg), P. minima (16.3 mg/kg), O. dillenii (15.7 mg/kg), S. nigrum (15.7 mg/kg) and P. emblica (14.6 mg/kg). Most of the fruits samples showed significant uptake of Cr, except Z. nummularia which showed lowest average levels at 2.33 mg/kg. The permissible limit for Cr in edible plants was set by FAO/WHO (1984) at 0.02 mg/kg. In the present study, all fruit samples contained Cr far above than this limit. The elevated Cr levels in the fruits samples may be attributed to the anthropogenic sources of Cr in the urban areas from where the samples were collected (Shah and Shaheen, 2007; Shah et al., 2012). The environmental risks of Cr are not considered very high since most of the Cr in soil exists in form of Cr3+ which is not toxic and also relatively immobile due to strong sorption by soil (Moodley et al., 2012). Significantly high concentrations of Pb were noted in all fruit samples except O. dillenii (3.24 mg/kg); however, highest concentration of Pb was observed in the fruits of Z. mauritiana (41.7 mg/kg), followed by S. nigrum (37.8 mg/kg) and C. opeca (36.1 mg/kg). According to WHO, the permissible limit of Pb for medicinal plants based on acceptable daily intake (ADI) is 10 ppm (WHO, 1992). Thus, the medicinal plants under investigation accumulated the metal at a significantly higher level compared to the permissible level, except O. dilleni. The weekly intake of Pb through the consumption of these fruits was also found to be considerably higher than the FAO/WHO provisional tolerable weekly intake limit of 1.750 mg/week (FAO/WHO, 1999; Bagdatlioglu et al., 2010). Higher accumulation of Pb in the fruits samples may be associated with various anthropogenic sources of the metals in the local urban environment (Shah and Shaheen, 2007; Shah et al., 2012). It is recognized as the most toxic environmental contaminant which accumulates in the skeleton, predominantly in bone marrow. It is a neurotoxin, retard intelligence and mental development and causes behavioural abnormalities (Oymak et al., 2009).
The measured levels of Mn in the fruits samples ranged from 3.90 mg/kg in P. dactelifera to 44.6 mg/kg in O. dillenii. Permissible limit set by FAO/WHO (1984) for Mn in edible plant is 2 mg/kg. Comparison of the present results with the proposed limit revealed that all the medicinal plants accumulated Mn in their fruits higher than the limit. Copper has critical biological functions in plant as a micronutrient; nonetheless, higher concentration in plant tissues could be toxic (Kubova et al., 2008). In the present study, highest concentration of Cu was observed in fruits of P. minima (14.4 mg/kg), while the fruits of P. emblica exhibited lowest contents (0.50 mg/kg). The permissible limit for Cu in edible plants was set by FAO/WHO (1984) at 3 mg/kg; hence, most of the samples in present study exceeded the limit except S. nigrum and P. emblica. Nonetheless, the average daily intake of Cu through the fruit consumption in the present study was found within the WHO maximum limit of daily intake (35 mg/day) (Goldhaber, 2003; Bagdatlioglu et al., 2010).
Highest average concentrations of Cd were noted in the fruits of O. dillenii (3.49 mg/kg) and P. minima (3.48 mg/kg), followed by Z. nummularia (3.01 mg/kg), C. opeca (2.52 mg/kg), P. dactilifera (1.73 mg/kg), and Z. mauritiana (1.30 mg/kg). All these fruits showed Cd concentrations exceeding the limit of 0.3 mg/kg recommended for medicinal plants (WHO, 2005). Nevertheless, fruits of P. emblica (0.250 mg/kg) and S. nigrum (0.010 mg/kg) exhibited Cd concentrations within the permissible limit. The weekly intake of Cd through the consumption of these fruits was noticeably higher than the FAO/WHO provisional tolerable weekly intake limit of 490 µg/week (FAO/WHO, 1999; Bagdatlioglu et al., 2010). Excessive accumulation of Cd in the fruits samples can be linked with various anthropogenic activities in the urban areas (Shah and Shaheen, 2007; Shah et al., 2012). Environmental risks of Cd are of major concern because it showed adverse effects on brain metabolism and other severe effects such as prostate cancer, liver, kidney, lungs and bone damage (Baldwin and Marshall 1999).
3.2 Metal concentrations in the soil
The distribution of selected metal levels (mg/kg, dry weight) in the soil samples is shown in Fig. 1. An examination of the metals data in soil revealed relatively broad distribution for Sr, Pb, Zn, Cu and Cd while relatively narrow ranges were observed for Fe, Cr, Co, Mn, Mg and Ca. Among the metal dominant mean levels were shown by Ca (2.01%), Fe (0.83%) and Mg (0.14%), followed by Mn (520 mg/kg), Sr (510 mg/kg), Zn (73 mg/kg), Cr (43 mg/kg), Cu (43 mg/kg), Pb (35 mg/kg), and Co (29 mg/kg), while Cd showed the lowest average concentration (5.12 mg/kg). Consequently, the soil in the study area was mostly calcite in nature (showing comparatively higher concentrations of the alkaline earth metals) and almost similar results were reported in an earlier study (Iqbal and Shah, 2011). Soil samples collected from the sites of S. nigrum showed lowest concentrations of Ca and Sr while the highest concentrations were found in the samples collected from the vicinity of P. minima. Highest concentration of Mg (0.34%) was recorded in the soil samples related to P. datylifera, while its lowest concentration (0.09%) was present in soil samples related to S. nigrum.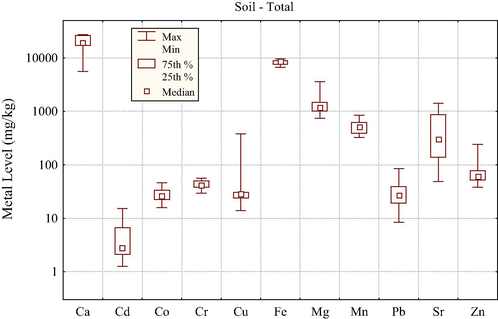
Distribution of selected metal levels (mg/kg, dry weight) in the soil samples.
Uptake of Fe by plant at very high concentration could be toxic and may inhibit their growth. In the present study, Fe showed highest levels (0.91%) in the soil samples related to S. nigrum while it showed minimum levels (0.69%) in the soil sample related to P. minima. Although Fe concentration in the soil samples was relatively high only a small fraction was taken up by the plants. Cobalt concentrations in soil samples were restricted to narrow range, lowest of 21.1 mg/kg to the highest of 30.8 mg/kg in the soil samples taken from the vicinity of Z. mauritian and P. dactylifera, respectively. Soil samples related to Z. nummularia exhibited highest concentration of Zn (133 mg/kg), while soil sample collected from the vicinity of S. nigrum showed very high levels of Cu (148 mg/kg). The permissible levels for Zn and Cu in agricultural soil are 150–300 mg/kg and 50–140 mg/kg (Kabata-Pendias and Pendias, 2001). Zn contents in all soil samples were within the permissible limit; however in case of Cu, the metal levels were below the permissible levels except the soil samples related to S. nigrum.
Highest concentration of Cr (52.9 mg/kg) was found in the soil samples related to Z. mauritiana, while its lowest concentration (35.6 mg/kg) was noted in the soil sample related to Z. nummularia. Under moderately oxidizing/reducing conditions and neutral pH values, Cr shows low mobility and Cr6+ adsorption decreases with increasing pH while that of Cr3+ increases with increasing pH (Gowd et al., 2010). Highest concentration of Cd (8.77 mg/kg) was noted in the soil sample taken from vicinity of O. dillenii. The permissible level for Cd in agricultural soil is 1–3 mg/kg (Kabata-Pendias and Pendias, 2001). Present results revealed that most of the soil samples contained Cd contents above this limit; it may be attributed to the anthropogenic activities in the urban environment. Highest concentration of Pb (54.0 mg/kg) was found in the soil samples related to O. dillenii, followed by those related to C. oppeca (47.6 mg/kg), S. nigrum (44.1 mg/kg), Z. nummularia (42.6 mg/kg), P. emblica (27.2 mg/kg), P. dactylifera (26.0 mg/kg), P. minima (18.0 mg/kg) and Z. mauritiana (17.7 mg/kg). In the present study, most of the soil samples were found to be slightly basic in nature showing mean pH value of 8.01.
Concentrations of the selected metals in the atmosphere from the study area have been reported by Shah et al. (2012); mean levels of Cd, Co, Cr, Cu, Fe, Mn, Pb and Zn in the air of the study area were reported at 3.43, 12.2, 7.18, 38.1, 1343, 38.8, 63.5 and 3325 ng/m3, respectively. Overall, Pb, Cd, Zn, Co, Cr and Cu were mainly associated with the anthropogenic enrichment in the atmospheric particulates of Islamabad, Pakistan (Shah and Shaheen, 2007; Shah et al., 2012).
3.3 Correlation study
Inter-relationships among selected metals in the fruits and soil samples were computed in terms of correlation coefficients (r), which revealed very strong relationships (p < 0.001) between following metal pairs in the fruits: Ca-Mn (r = 0.98), Ca-Sr (r = 0.95), Pb-Cd (r = 0.93), Mg-Mn (r = 0.92), Ca-Mg (r = 0.90), Mg-Sr (r = 0.79), Sr-Mn (r = 0.76), Fe-Pb (r = 0.70), Cu-Cr (r = 0.56) and Pb-Cu (r = 0.55). Some significant inverse associations were also observed as shown by negative correlations among various metal pairs: Pb with Ca (r = −0.76) & Mg (r = −0.74), Fe-Mn (r = −0.63), Co-Cr (r = −0.58), Fe-Ca (r = −0.57), Co-Cu (r = −0.52) and Fe-Sr (r = −0.46). These results indicated mutual relationships among Ca, Mg, Sr and Mn in the fruits samples while inverse associations of these metals were noted with Pb and Fe which showed common associations in the fruit samples. This aspect would be further explored by multivariate statistical analysis. Very strong positive correlations (p < 0.001) in the soil samples were noted between Mn-Sr (r = 0.89) and Ca-Sr (r = 0.80), followed by significant positive relationships between Mg-Cd (r = 0.70), Fe-Mn (r = 0.63), Ca-Mg (r = 0.59), Mg-Sr (r = 0.52) and Mn-Cd (r = 0.49). The correlation study thus indicated common variations of these metals in the soil. Some significantly negative correlations among the metals were also noted: Sr with Fe (r = −0.74) & Pb (r = −0.64), Fe with Ca (r = −0.70) & Zn (r = −0.65), Mn with Ca (r = −0.54) & Pb (r = −0.77), and Pb with Cd (r = −0.62). The inverse relationships among these metals revealed their divergent variations in the soil samples. Although very complex in nature, the correlation study suggested that Ca, Mg and Sr find their way through common sources in soil while Fe and Mn constitute another common group based on mutual distribution.
3.4 Bioaccumulation factor
Accumulation of the metals in the plants was assessed by bioaccumulation factor (BAF) which helps in comparing the ability of different plants in taking up metal from soil (Kovacik et al., 2012; Gebrekidan et al., 2013). In the present study most of the fruits species revealed relatively low BAF values (on the average) which showed limited ability of the plants to accumulate the metals (Table 4). Highest accumulation of Ca, Mg and Mn was observed in O. dillenii while S. nigrum and P. dactylifera revealed maximum BAF for Cr and Zn, respectively. Maximum accumulation of Co, Sr and Pb was observed in Z. mauritiana, whereas P. minima showed comparatively higher accumulation of Cd, Cu and Fe from the soil. Most of the metals exhibited relatively lower BAF values (below 1.0) in the fruits except Co and Pb, which showed somewhat higher BAF values (above 1.0 in most of the cases). Consequently, the bioaccumulation of Co and Pb indicated uptake and storage of the metals in the fruits samples of the plants grown in the urban environment. Overall, based on the average BAF of all fruit samples, plants were most efficient in taking up Co (1.204), followed by Pb (0.978), Mg (0.753), Zn (0.522), Ca (0.489), Cd (0.432), Cr (0.337), Cu (0.282), Sr (0.431), Mn (0.0291) and Fe (0.005).
Plant
Ca
Mg
Zn
Fe
Co
Sr
Cr
Pb
Mn
Cu
Cd
S. nigrum
0.039
1.008
0.193
0.005
1.317
0.003
0.439
0.857
0.017
0.020
0.100
P. minima
0.017
0.802
0.189
0.009
0.813
0.004
0.410
1.266
0.028
0.720
1.243
Z. nummularia
0.234
0.545
0.930
0.003
1.143
0.014
0.300
0.367
0.025
0.185
0.425
Z. mauritiana
0.132
0.428
0.051
0.008
1.829
0.133
0.326
2.350
0.008
0.326
0.563
P. emblica
0.111
0.504
0.225
0.006
1.307
0.076
0.343
1.021
0.018
0.019
0.102
C. opeca
0.114
0.822
0.270
0.006
1.077
0.009
0.428
0.759
0.020
0.229
0.345
O. dillenii
3.213
1.715
0.717
0.004
1.252
0.093
0.340
0.060
0.106
0.167
0.398
P. dactelifera
0.055
0.204
1.603
0.007
0.897
0.017
0.408
1.148
0.010
0.588
0.384
3.5 Ecological risk assessment
The ecological risks associated with the metal levels in the soil were evaluated in terms of the contamination factor (CF) and degree of contamination (Cdeg) and their results were interpreted as proposed by Hakanson (1980): CF < 1 = low contamination, 1 ≤ CF < 3 = moderate contamination, 3 ≤ CF < 6 = considerable contamination, 6 ≤ CF = very high contamination, Cdeg < 8 = low degree of contamination, 8 ≤ Cdeg < 16 = moderate degree of contamination, 16 ≤ Cdeg < 32 = considerable degree of contamination, 32 ≤ Cdeg = very high degree of contamination. The CFs of the metals in the soil are shown in Fig. 2 which revealed that Mg and Fe showed low contamination while Ca, Cr, Mn, Co, Zn and Sr exhibited moderate to considerable contamination. However, Cu and Pb showed considerable to very high contamination and Cd demonstrated very high contamination in the soil samples. The Cdeg was also calculated and it showed a range of 15–155 with a mean value of 53; thus, it fluctuated from moderate degree of contamination to very high degree of contamination but on the average basis it indicated very high degree of contamination in the soil.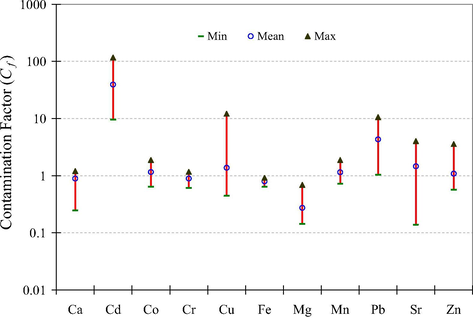
Contamination factors of selected metals in the soil samples.
Enrichment factors (EFs) of selected metals were considered to assess the anthropogenic intrusions of the metals in the soils. If EF approaches unity or less, the element is considered crustal in origin; however, the element with EF higher than 20 is considered to originate primarily from anthropogenic sources (Sutherland, 2000). Fig. 3 shows the minimum, mean and maximum values of EFs for the metals in soil samples. Average EFs of the metals were as follows: Cd (557) > Pb (40) > Sr (23) > Co (19) > Zn (17) > Cu (12) > Mn (8.9) > Ca (7.9) > Cr (6.9) > Fe (2.4). These values were interpreted as suggested by Sutherland (2000) and Loska et al. (2004); EF < 2 showed deficiency to minimal enrichment; EF = 2–5 indicated moderate enrichment; EF = 5–20 exhibited significant enrichment; EF = 20–40 demonstrated very high enrichment; and EF > 40 revealed extremely high enrichment. In the present study, mean values of EF for Cd and Pb were greater than 40 indicating extreme enrichment of these metals in the soil samples which may be linked with excessive anthropogenic uses and uncontrolled emissions of the metals in urban environment. The metals were mainly contributed by traffic emissions and other anthropogenic activities, such as industrial emissions, fertilizers, waste incineration and fossil fuel burning. Both metals are relatively volatile, and thus can also undergo long-range transport (Dragovic and Mihailovic, 2009). Among rest of the metals, soil samples were found to be significantly enriched by Ca, Cu, Co, Cr, Mn, and Zn, whereas highly enriched by Sr. Therefore, EFs revealed significant anthropogenic enrichment of most of the metals in the soil samples from local urban areas.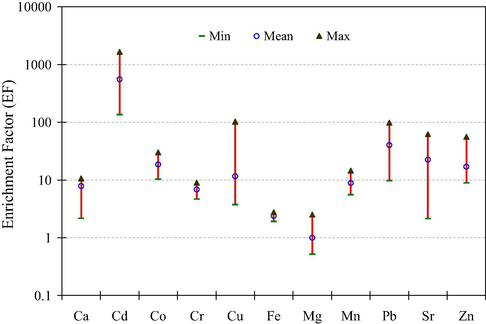
Enrichment factors of selected metals in the soil samples.
3.6 Principal component analysis
Multivariate principal component analysis (PCA) was applied to the metal data pertaining to the fruit samples using varimax normalized rotation of the data set and the results are presented in Table 5. Three significant principal components (PCs) with eigenvalues greater than 1.0 were obtained explaining more than 90% variance of the data. First PC explaining highest variance of the data (43.89%) showed significantly elevated loadings for Ca, Mg, Mn and Sr while PC 2 exhibited maximum loadings for Cd, Cr, Cu and Zn. Third PC revealed significant loadings in favour of Pb, Fe and Co. First PC was mostly contributed by the crustal materials and hence predominantly natural in origin. These findings are well supported by the CF and EF results discussed above. Second PC indicated mixed contributions of natural and anthropogenic sources such as fertilizers, pesticides and domestic wastes. The third PC was mostly derived from anthropogenic activities such as automobile/industrial emissions and domestic wastes. Although Fe is one of the major constituents of the soil most of its lithogenic form is not bioavailable while the anthropogenic contributions are generally bioavailable and plants can assimilate them readily. A mutual PC of Fe with Pb and Co indicated the anthropogenic contributions of Fe in the soil as also supported by the EFs in the previous section. Overall, multivariate PCA revealed significant anthropogenic contributions of some metals in the fruit samples which may be of concern to the consumers.
PC 1
PC 2
PC 3
Eigen value
4.928
2.368
1.651
Total variance (%)
43.89
31.08
15.46
Cumulative variance (%)
43.89
74.97
90.43
Ca
0.985
–
–
Cd
–
0.839
–
Co
–
–
0.738
Cr
–
0.676
–
Cu
–
0.847
–
Fe
–
–
0.917
Mg
0.932
–
–
Mn
0.986
–
–
Pb
–
–
0.924
Sr
0.908
–
–
Zn
0.265
0.901
–
3.7 Health risk assessment
The calculated values of health risk index (HRI), target hazard quotient (THQ) and hazard index (HI) for the metal levels in the fruits of medicinal plants are shown in Table 6. The HRI values of Mg, Sr, Fe, Zn, Cu, Cr and Cd in each fruit species were less than unity (1.0), which is considered to be safe for human consumption. Nonetheless, the HRI values for Pb, Co and Mn were greater than unity for majority of the plant species; therefore, the consumers are at risk with respect to these metal contents in the fruits, which may be associated with adverse health effects (Abbasi et al., 2013). The HRI value of Ca was also below unity for most of the plant species except O. dillenii which exhibited significantly higher HRI value. The health protection standard of lifetime risks for THQ and HI is 1.0 (USEPA, 2006). As shown in Table 6, the THQ values for all of the metal levels in the medicinal plants were substantially lower than the permissible limit (1.0); therefore, the present study revealed that the consumption of these fruits would not result in any significant non-carcinogenic risks related to these metals to the consumers. A significantly lower value of HI (0.0112) demonstrated that the consumption of these fruits was overall safe and would not result in any long-term non-carcinogenic risks to the local consumers.
Health risk index (HRI)
THQ
S. nigrum
P. minima
Z. nummularia
Z. mauritiana
P. emblica
C. opeca
O. dillenii
P. dactelifera
Total
Ca
0.026
0.025
0.255
0.150
0.101
0.099
3.549
0.076
4.280
5.13E−04
Mg
0.111
0.141
0.083
0.067
0.062
0.098
0.261
0.084
0.907
1.09E−04
Sr
0.044
0.022
0.294
0.009
0.032
0.040
0.109
0.194
0.744
8.91E−05
Fe
0.047
0.064
0.025
0.067
0.056
0.050
0.029
0.059
0.398
4.77E−05
Zn
0.376
0.368
0.422
0.457
0.451
0.359
0.402
0.331
3.166
3.80E−04
Cu
0.002
0.005
0.012
0.025
0.017
0.003
0.073
0.018
0.157
1.88E−05
Mn
3.738
3.886
0.555
4.107
3.476
4.521
3.738
4.452
28.474
3.41E−03
Co
7.500
4.532
3.099
8.264
5.516
7.171
0.643
5.933
42.657
5.11E−03
Cr
0.055
0.048
0.057
0.032
0.056
0.057
0.227
0.020
0.552
6.62E−05
Cd
0.054
0.258
0.098
0.161
0.009
0.124
0.116
0.223
1.042
1.25E−04
Pb
0.071
2.486
2.136
0.929
0.179
1.779
2.493
1.179
11.250
1.35E−03
Hazard index (HI)
1.12E−02
4 Conclusions
In this study, concentration of selected metals (Ca, Cd, Co, Cr, Cu, Fe, Mg, Mn, Pb, Sr, and Zn) in the fruits of eight medicinal plants and related soil samples was evaluated in relation to the metal transfer to food chain. In the fruit samples highest concentration was noted for Ca, followed by Mg, Fe, Zn, Co, and Pb. O. dillenii showed the elevated levels of Ca, Mg, Mn, Sr and Cd in the fruit samples while maximum concentrations of Cu, Cr and Zn were found in the fruits of P. minima, C. opeca, and Z. nummularia, respectively. Highest levels of Pb and Co were observed in the fruits of Z. mauritiana. In the case of soil samples, elevated concentrations were noted for Ca, Fe, Mg, Mn and Sr while lowest concentration was shown by Cd. Bioconcentration factor of the metals from soil to fruits exhibited highest values for Co, followed by Pb. Very high contamination and extremely high enrichment of Cd and Pb were noted in the soil samples, which exhibited very high degree of contamination for the metals on the whole. Multivariate PCA revealed significant anthropogenic intrusions of Pb, Co and Fe, followed by Cr, Cd, Cu and Zn in the fruit samples. Although HRI values for Pb, Co and Mn were higher than the safe limit the THQ and HI showed insignificant non-carcinogenic risks to the consumers.
Acknowledgments
The research funding awarded by Quaid-i-Azam University, Islamabad, to carry out this project is thankfully acknowledged.
References
- Medicinal Plant Biodiversity of Lesser Himalayas-Pakistan. New York: Springer; 2012.
- Health risk assessment and multivariate apportionment of trace metals in wild leafy vegetables from Lesser Himalayas, Pakistan. Ecotoxicol. Environ. Saf.. 2013;92:237-244.
- [Google Scholar]
- Characterization of trace elements in medicinal herbs by instrumental neutron activation analysis. J. Radioanal. Nucl. Chem.. 2008;278:559-563.
- [Google Scholar]
- Nutritional and antimicrobial studies on leaves and fruit of Carrisa opeca Stapf Ex haines. Elect. J. Environ. Agric. Food Chem.. 2010;9(10):1631-1640.
- [Google Scholar]
- Heavy trace metals and macronutrients status in herbal plants of Nigeria. Food Chem.. 2004;85:67-71.
- [Google Scholar]
- Genotypic variations in the accumulation of Cd, Cu, Pb and Zn exhibited by six commonly grown vegetables. Environ. Pollut.. 2006;144(3):736-745.
- [Google Scholar]
- Heavy metal levels in leafy vegetables and some selected fruits. J. Verbr. Lebensm.. 2010;5:421-428.
- [Google Scholar]
- Heavy metal poisoning and its laboratory investigation. Ann. Clin. Biochem.. 1999;36:267-300.
- [Google Scholar]
- Heavy metal accumulation in medicinal plants collected from environmentally different sites. Biomed. Environ. Sci.. 2008;21:319-324.
- [Google Scholar]
- Lead and cadmium accumulation in medicinal plants collected from environmentally different sites. Bull. Environ. Contam. Toxicol.. 2010;84:197-201.
- [Google Scholar]
- ‘Opuntia dillenii’ – an interesting and promising Cactaceae Taxon. J. Prof. Assoc. Cactus Dev.. 2008;10:148-170.
- [Google Scholar]
- Some aspects of toxic contaminants in herbal medicines. Chemosphere. 2003;52:1361-1371.
- [Google Scholar]
- Valorization of date palm (Phoenix dactylifera) fruit processing by-products and wastes using bioprocess technology – review. Saudi J. Biol. Sci.. 2013;20:105-120.
- [Google Scholar]
- Food survey: levels and potential health risks of chromium, lead, zinc and copper content in fruits and vegetables consumed in Algeria. Food Chem. Toxicol.. 2014;70:48-53.
- [Google Scholar]
- Determination of essential and non-essential elements in some medicinal plants by polarised X-ray fluorescence spectrometer (EDPXRF) Microchem. J.. 2010;95:174-180.
- [Google Scholar]
- Analysis of mosses and topsoils for detecting sources of heavy metal pollution: multivariate and enrichment factor analysis. Environ. Monit. Assess.. 2009;157:383-390.
- [Google Scholar]
- EC, 2006. Commission Regulation No 1881/2006: Setting Maximum Levels for Certain Contaminants in Foodstuffs. European Commission (EC). <http://www.eurlex.europa.eu/LexUriServ/site/en/oj/2006/l_364/l_36420061220en00050024.pdf>. Accessed 10 May, 2011.
- FNB, 2004. Dietary Reference Intakes (DRIs): Recommended Intakes for Individuals. Food and Nutrition Board, Institute of Medicine, National Academies. <http://www.iom.edu/Global/NewsAnnouncements/∼/media/Files/ActivityFiles/Nutrition/DRIs/DRISummaryListing2.ashx>. Accessed 13 March 2012.
- FAO/WHO, 1984. Contaminants. In: Codex Alimentarius, vol. XVII, Edition 1. FAO/WHO, Codex Alimentarius Commission, Rome.
- FAO/WHO, 1999. Joint FAO/WHO Expert Committee on Food Additives. Summary and conclusions. In: 53rd meeting, Rome, 1–10 June.
- Toxicological assessment of heavy metals accumulated in vegetables and fruits grown in Ginfel river near Sheba Tannery, Tigray, Northern Ethiopia. Ecotoxicol. Environ. Saf.. 2013;95:171-178.
- [Google Scholar]
- Trace element risk assessment: essentially vs. toxicity. Regul. Toxicol. Pharmacol.. 2003;38:232-242.
- [Google Scholar]
- Assessment of heavy metal contamination in soils at Jajmau (Kanpur) and Unnao industrial areas of the Ganga Plain, Uttar Pradesh, India. J. Hazard. Mater.. 2010;174:113-121.
- [Google Scholar]
- Investigation of soil multi-element composition in Antalya, Turkey. Environ. Int.. 2003;29:631-640.
- [Google Scholar]
- An ecological risk index for aquatic pollution control. A sedimentological approach. Water Res.. 1980;14:975-1001.
- [Google Scholar]
- Chemical composition of Solanum nigrum linn extract and induction of autophagy by leaf water extract and its major flavonoids in AU565 breast cancer cells. J. Agric. Food Chem.. 2010;58(15):8699-8708.
- [Google Scholar]
- Distribution, correlation and risk assessment of selected metals in urban soils from Islamabad, Pakistan. J. Hazard. Mater.. 2011;192:887-898.
- [Google Scholar]
- Distribution, source identification and risk assessment of selected metals in sediments from freshwater lake. Int. J. Sed. Res.. 2015;30:241-249.
- [Google Scholar]
- Anti-inflammatory and analgesic activities of the water extract from the fruit of Phyllanthus emblica Linn. Int. J. Appl. Res. Nat. Prod.. 2010;3(2):28-35.
- [Google Scholar]
- Trace Elements in Soils and Plants (third ed.). Boca Raton, FL, USA: CRC Press; 2001.
- Accumulation of metals and selected nutritional parameters in the field-grown chamomile anthodia. Food Chem.. 2012;131:55-62.
- [Google Scholar]
- Utilization of optimized BCR three step sequential extraction and dilute HCl single extraction procedures for soil-plant metal transfer prediction in contaminated lands. Talanta. 2008;75:1110-1122.
- [Google Scholar]
- Health risk of heavy metals in food crops grown on reclaimed tidal flat soil in the Pearl River Estuary, China. J. Hazar. Mater.. 2012;227:148-154.
- [Google Scholar]
- CRC Handbook of Chemistry and Physics, Geophysics, Astronomy, and Acoustics, Abundance of Elements in the Earth's Crust and in the Sea, Section 14 (85th ed.). Boca Raton, USA: CRC Press; 2005.
- Metal contamination of farming soils affected by industry. Environ. Int.. 2004;30:159-165.
- [Google Scholar]
- Estimation of heavy metals in commonly used medicinal plants: a market basket survey. Environ. Monit. Assess.. 2010;170:657-660.
- [Google Scholar]
- Elemental composition and fatty acid profile of the edible fruits of Amatungula (Carissa macrocarpa) and impact of soil quality on chemical characteristics. Anal. Chim. Acta. 2012;730:33-41.
- [Google Scholar]
- Preliminary phytochemical and anti-bacterial studies on Physalis minima Linn. Int. J. Curr. Sci. 2012:24-30.
- [Google Scholar]
- Determination of lead and cadmium in food samples by the coprecipitation method. Food Chem.. 2009;113:1314-1317.
- [Google Scholar]
- Exploring the potential of Ziziphus nummularia (Burm. f.) Wight et Arn. from drier regions of India. Genet. Resour. Crop Evol.. 2010;57:929-936.
- [Google Scholar]
- Practical Environmental Analysis. Cambridge, UK: Royal Society of Chemistry; 1999.
- Inorganic analysis of herbal drugs. Part II. Plant and soil analysis-diverse bioavailability and uptake of essential and toxic elements. J. Serb. Chem. Soc.. 2006;71(10):1095-1105.
- [Google Scholar]
- Elemental investigation of Syrian medicinal plants using PIXE analysis. Nucl. Instrum. Methods Phys. Res. B. 2010;268:2790-2793.
- [Google Scholar]
- Statistical analysis of atmospheric trace metals and particulate fractions in Islamabad, Pakistan. J. Hazard. Mater.. 2007;147:759-767.
- [Google Scholar]
- Assessment of the trace elements level in urban atmospheric particulate matter and source apportionment in Islamabad, Pakistan. Atmos. Pollut. Res.. 2012;3:39-45.
- [Google Scholar]
- Multivariate statistical analysis of nutrients and trace elements in plants and soil from northwestern Russia. Plant Soil. 2009;322:219-228.
- [Google Scholar]
- STATISTICA for Windows. Tulsa, OK: Computer Program Manual; 1999.
- Bed sediment-associated trace metals in an urban stream, Oahu, Hawaii. Environ. Geol.. 2000;39:611-627.
- [Google Scholar]
- Determination of trace elements in commonly consumed medicinal herbs by ICP-MS and multivariate analysis. Food Chem.. 2012;134:2504-2508.
- [Google Scholar]
- Application of a three-stage sequential extraction procedure for the determination of extractable metal contents in highway soils. Turk. J. Chem.. 2003;27:333-346.
- [Google Scholar]
- Integrated Risk Information System (IRIS) for Zinc. Washington: National Center for Environmental Assessment, Office of Research and Development, US Environmental Protection Agency; 2002.
- USEPA Region III Risk-based Concentration Table: Technical Background Information. Washington: US Environmental Protection Agency; 2006.
- Quality Control Methods for Medicinal Plant Materials. Geneva: World Health Organization; 2005. Revised
- WHO, 1992. Expert Committee on Specification for Pharmaceuticals Preparation. Report Geneva WHO 32 (pp. 44–52, 75–76). WHO technical report series 823.







