Translate this page into:
Adsorption characteristics of Direct Red 23 azo dye onto powdered tourmaline
⁎Corresponding author at: Department of Civil and Ecological Engineering, I-Shou University, Da-Shu District, Kaohsiung 84001, Taiwan. Tel.: +886 929552662; fax: +886 76577461. chweng@isu.edu.tw (Chih-Huang Weng)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Conceptual scheme illustrates adsorption of diazo dye DR23 onto powdered tourmaline. The high electric fields on the surface of tourmaline significantly enhanced electrostatic interactions during adsorption.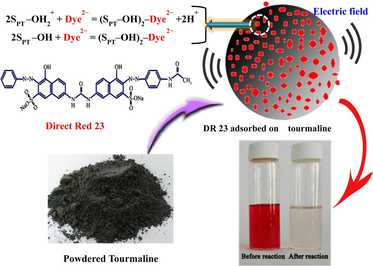
Abstract
The high electric field on the surface of tourmaline particles has a potential of enhancing electrostatic reactions during adsorption. However, information concerning the adsorption characteristics of dyes onto tourmaline is currently unavailable. In the present study, the behavior and efficiency of powdered tourmaline (PT) in removing the diazo Direct Red 23 (DR23) dye from aqueous solution were investigated. The observations from batch adsorption experiments indicated that the adsorption was more favorable under low adsorbate surface loading, low pH, high temperature, and low ionic strength conditions. A homogeneous particle diffusion model (HPDM) was used to characterize the process, and the rate of adsorption was found to be controlled by intra-particle diffusion. An activation energy of 4.54 kcal/mol was calculated, suggesting that the adsorption proceeded with a low energy barrier and that a physisorption was involved. The functional groups binding anionic DR23 on the PT particles were also identified. A maximum adsorption capacity of 153 mg/g was determined according to the Langmuir isotherm. The PT was subjected to a total of 5 regeneration runs without losing much of its dye-adsorption capacities. Due to its low price, abundant availability, and superb adsorption capacity, PT has a great potential for use as an effective adsorbent in removing DR23 from aqueous solutions.
Keywords
Dye
Tourmaline
Adsorption
Decolorization
1 Introduction
The wastewater of textile industries is usually heavily colored due to the contamination by dyeing chemicals. The color of dyes in water is highly visible which can be visually detected at low concentrations (Weng et al., 2013a). Azo dyes are the largest and most important dye class of synthetic organic dyes (Tunc et al., 2013). The molecules of azo dyes are characterized by the bearing of azo-groups (–) in association with aromatic rings (naphthalene and/or benzene). They may also carry sulfonic groups (Behnajady et al., 2007). Many azo dyes are thought to be carcinogenic to human when ingested. They can also be harmful to aquatic organisms. Without proper treatment, a dye-laden wastewater can pose significant threats to human health, as well as the integrity of aquatic ecosystems (Weng and Tsai, 2016). Due to complex molecular structures and their synthetic origin, azo dyes are usually non-biodegradable and don’t decompose naturally (Robinson et al., 2001). Conventional biological processes are usually inadequate for the treatment of dye under stringent effluent standards (Weng et al., 2013b).
The development of advanced oxidation processes (AOPs) for the treatment of trace organic contaminants has gained much attention. AOPs such as Fenton, persulfate, and photocatalyst, have been shown to be promising in removing color in dye-laden wastewaters (Avetta et al., 2014; Ribeiro et al., 2015; Su et al., 2011; Weng et al., 2014; Weng and Huang, 2015; Weng and Tsai, 2016; Yola et al., 2014a, 2014b; Zhang et al., 2014). However, concerns such as the release of residual radical oxidants and the possibilities of producing toxic degradation products remain for the application of these processes. Moreover, the effectiveness of these oxidants can be significantly undermined in the presence of radical scavengers in wastewater (Ribeiro et al., 2015).
Adsorption has been proven to be efficient in removing dye molecules from wastewater (Achmad et al., 2012; Ardejani et al., 2007; Atar et al., 2008, 2011; Atar and Olgun, 2009; Fathi et al., 2015; Holliman et al., 2008; Konicki et al., 2012; Liu et al., 2015; Olgun and Atar, 2009; Somasekhara Reddy et al., 2017; Wang et al., 1998; Weng and Pan, 2006; Weng et al., 2009; Weng et al., 2013a). Owing to its superior adsorption performance, activated carbon (AC) in the form of powder, granular, fiber and cloth has been extensively used as adsorbents for a wide variety of trace organic pollutants (Ayranci and Duman, 2009; Duman and Ayranci, 2006; Huang and Blankenship, 1984). However, due to high costs of the material and its regeneration, a general application of AC in wastewater treatment has been limited so far, particularly in the underdeveloped countries (Weng and Wu, 2012). Nonetheless, adsorption remains a simple and effective process for the removal of synthetic dyes, among other organic toxins, in wastewater when appropriate adsorbents are used. Attempts for the search of low cost and high capacity adsorbents continued for the past decades (Duman et al., 2015a, 2015b).
Tourmaline is a naturally-occurring borosilicate mineral with complex and variable compositions. It occurs in the forms of Fe tourmaline (schorl), Mg tourmaline (dravite), and alkali tourmaline depending on the relative abundance of these elements (Harraz and EL-Sharkawy, 2001). Tourmalines are widely available distributed in metamorphic formations, but are abundant in certain aplites and pegmatites. Tourmaline possesses some unique properties similar to ferroelectrics, including the emission of far infrared rays, the existence of spontaneous polarization and permanent poles, and therefore the formation of electric dipoles (Ruan et al., 2004; Yu et al., 2016). The occurrence of an electric field with 106–107 V/m intensity on the surface of micron-sized tourmaline is widely recognized (Yeredla and Xu, 2008). Owing to such unique properties, tourmaline has been used for the enhancement of some photocatalytic decontaminants. For example, Yu et al. (2016) used tourmaline to enhance the photocatalytic activities of TiO2 nano semiconductors through its obstruction on the recombination of electron–holes to prevent aggregation of the nanoparticles.
Tourmaline is a mixed Fe(II)–Fe(III) bearing mineral, and therefore can function as a catalyst in the breakdown of synthetic dyes. In particular, the presence of Fe(II) in Fe-bearing tourmaline significantly enhances the production of active radicals (Wang et al., 2013) from Fenton’s reaction. Furthermore, the electric field on the surface of tourmaline may induce Fe3+ ions to Fe2+ ions in aqueous solutions. This process enhances the oxidation of organic molecules in a conventional Fenton process (Yu et al., 2014). Recent studies have also found that tourmalines are capable of absorbing uranyl molecules (Guerra et al., 2012a) and divalent metals (Guerra et al., 2012b; Liu et al., 2013) in aqueous media. Other studies have also found that phosphates are sorbable to La-modified tourmaline (Li et al., 2015). The high electric fields on the surface of tourmaline particles promote enhancement of electrostatic interactions during adsorption. Therefore, tourmalines have great potentials for application in the adsorptive removal of ionic textile dyes from wastewater, and a closer examination of their adsorption behaviors is of great interest.
The purpose of this study was to investigate the potential application of tourmaline for the adsorptive removal of dyes, in particular diazo direct dye, from aqueous solutions. Experiments have been conducted using tourmaline for removal of diazo direct dye Direct Red 23 (DR23) from aqueous solutions. DR23 was chosen as a target chemical because it is the most frequently used anionic dye in the textile industry. Key parameters affecting adsorptive behaviors of tourmaline on DR23 were studied, including temperature, pH, and ionic strength of the solution, as well as surface loadings of the adsorbate. The fitting of experimental data to the kinetic and isotherm models has been tested. The DR23 adsorption capacity of tourmaline was compared with other adsorbents reported in literatures. Efficiency of regeneration of the tourmaline adsorbent was also evaluated. Findings of this study provide useful information on the applicability of this natural material as an adsorbent for the treatment of dye-laden wastewaters.
2 Materials and methods
2.1 Materials
A water-soluble anionic direct azo dye, viz, Direct Red 23 (DR23, Fig. 1) manufactured by Sigma-Aldrich (USA) was used as the adsorbate. All chemical reagents used were of analytical grade. Powdered Tourmaline (PT) with a particle size smaller than 0.074 mm (passing No. 200 ASTM sieve) was purchased from the Inner Mongolia Embellish Lung Chemical Co., China. The cost for the PT is 400 USD/ton. The PT as purchased was used without pretreatment. Major properties of the powder were determined using pertinent techniques viz. X-ray diffraction (XRD), scanning electron microscope (SEM), and specific surface area (SSA) measurements. BET-N2 (Brunauer, Emmett, Teller) SSA and average pore volume were determined using a surface area analyzer (SSA-4200C, Beijing oda Electronic Technology Co., China). Crystal structure of the tourmaline was determined using a XRD analyzer (DX2700, Dandong Fangyuan Instrument Co., China). A SEM (JSM-6700F, JEOL, Japan) was used to determine surface morphology of the tourmaline. A Fourier transform infrared spectroscopy (FTIR, spectrometer Nicolet Avatar 370DTGS, Thermo, USA) covering a wave number between 400 and 4000 cm−1 was used to identify the functional groups on the tourmaline particles before and after adsorption. A laser particle size analyzer (Bettersize 2000, Dandong Baxter Instrument Co., China) was used for the determination of particle sizes.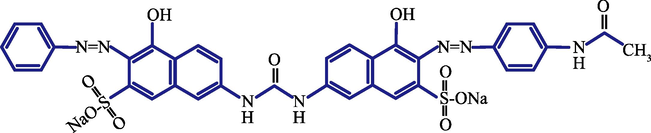
Molecular structure of DR23.
The point of zero charge (pHzpc) is a fundamental surface property of hydrated adsorbents. The pHzpc of PT was determined in accordance with Srivastava et al. (2011). Solutions of a constant ionic strength of 0.01 mol/L NaCl were prepared. The pH of the solutions was adjusted using either 0.1 mol/L HCl or NaOH to various values within a range between 2.0 and 12.0. At each pH, a volume of 50 mL of the solution was transferred to a test tube, followed by the addition of 1.0 g PT. The resultant solution was then shaken for 2 h before its pH was measured. A graph between pHfinal and pHinitial of the solution was plotted and pHzpc of the adsorbent was determined from the inflection point of the “pHfinal vs pHinitial” curves.
2.2 Analysis
The concentration of DR23 in solutions was determined from its absorbance at a wavelength of 507 nm using a UV–visible spectrophotometer (Thermo Scientific Evolution 201, USA). A calibration curve was established to correlate measured absorbance with the concentration of DR23.
2.3 Kinetic adsorption experiments
Batch adsorption experiments were conducted to characterize dynamic behaviors of the adsorption process and to quantify the adsorption rates. The effects of adsorbent dosage and solution temperature were also investigated. The procedures adopted are summarized as follows. A 1 L solution of fixed DR23 concentration (1.0 × 10−4 mol/L) and an ionic strength of 0.01 mol/L NaCl were prepared. The pH of the solution was adjusted to 3.0 using HCl or NaOH as necessary. A known amount of PT was added to the solution. The resultant solution was agitated at 800 rpm using a mechanical stirrer (Shin-Kwang, Taiwan) under room temperature (25 °C) for 3 h. Volumes of 5 mL each were taken from the agitated solution at a fixed interval and filtered immediately through 0.45 μm membranes (Advantec, Japan). The residual concentration of DR23 in the supernatant of each solution was then determined. Each run of the experiment was triplicated with average values used for data analysis. To compensate for the adsorption from glass beakers and membrane filters, blank tests without PT addition were conducted in parallel. Experiments using procedures as described were also conducted with various adsorbent concentrations (0.8–4.0 g/L) to evaluate the effect of adsorbent dosage on adsorption rate. The effect of temperature on adsorption kinetics was investigated using temperatures of 5, 15, 25, 35, and 45 ± 0.5 °C by immersing the mixtures in a water bath.
The amount of DR23 adsorbed by PT at a contact time t (qt) was calculated from the following:
2.4 Equilibrium adsorption experiments
Procedures for the test of adsorption equilibrium as affected by solution pH were as follows: a 1-L solution with an ionic strength of 0.01 mol/L NaCl and a DR23 concentration of 2.0 × 10−5 mol/L were prepared; volumes of 100 mL each of the solution were transferred to 125-mL polyethylene (PE) bottles; initial pH of the samples was adjusted to cover a range between 2.0 and 10.5 using either HCl or NaOH; a given amount of PT (1.0 g/L) was added to each of the samples; the sample bottles were then shaken on a reciprocal shaker for 3 h at 150 excursions/min under a temperature of 25 °C. The contact time was determined based on the results from kinetic experiments and was found to be adequate for reaching an equilibrium. The pH of the mixed liquors was recorded at the end of contact time; the mixed liquors were filtered through 0.45 μm membranes (Advantec, Japan) and residual DR23 in the filtrate was determined. Textile wastewater often contains high concentration of ions. The effect of such ions on DR23 adsorption was evaluated using solutions of various NaCl concentrations (0–0.05 mol/L).
2.5 Regeneration experiments
The cost of commercial powdered tourmaline is 400 USD/ton. The cost of PT can be significantly lower when used with regeneration. The efficiency of regeneration was evaluated using five consecutive cycles. Each cycle (adsorption–desorption) consisted of an adsorption equilibrium test followed by a desorption test. Procedures similar to the adsorption tests as described in Section 2.4 were adopted except that a DR23 concentration of 4.0 × 10−5 mol/L and a PT concentration of 4.0 g/L were used. After completion of the adsorption equilibrium test, the mixed liquor was centrifugally separated at 7500 rpm (6040g) for 10 min (Hsiangtai CN-10001, Taiwan). The concentration of residual dye in the supernatant was then determined. The DR23-saturated-PT was transferred immediately to a solution with an initial pH of 11.0 and proceeded with the desorption test. The mixture was agitated on a reciprocal shaker for 1 h at 150 excursions/min under a temperature of 25 °C. The PT was separated again from the mixture by centrifugation upon completion of desorption, and concentration of the desorbed dye in the supernatant was determined.
3 Results and discussion
3.1 Characterization of the powdered tourmaline
Crystal phase confirmation of the tourmaline powder was carried out using XRD (Fig. 2). The diffraction peaks were at 13.8°, 21.0°, 22.0°, 25.5°, 26.7°, 30.3°, 34.7°, 44.3°, 47.1°, 55.3°, 57.7°, 61.5°, 63.9°. The tourmaline was determined to have a chemical formula of Na(Fe2+, Fe3+)3Al6(Si6O18)(BO3)3(OH)4. It is classified as schorl, consistent with its relatively high iron content. Morphology of the PT is shown in Fig. 3. The image shows that PT particles formed aggregates of various sizes and shapes. Surface characteristics of the particles were examined using FTIR. Fig. 4 shows the FTIR spectra of bare and DR23 loaded PT particles. The absorption at 3563.6 cm−1 is attributed to O–H bond stretching. The peak at 1156.8 cm−1 was caused by the stretching vibration of C–C bonds. And the band at 1034 cm−1 was a result of stretching vibration of the Si–O–Si bonds (Li et al., 2015). The FTIR analysis reveals a definite chemical structure of the tourmaline. The spectra of OH band shifted prominently after adsorption when the tourmaline particles were loaded with DR23 molecules, suggesting that the OH functional group was likely to have participated in dye binding. Judging from the FTIR spectra, it is concluded that the chemical nature of tourmaline remained virtually unchanged after dye adsorption.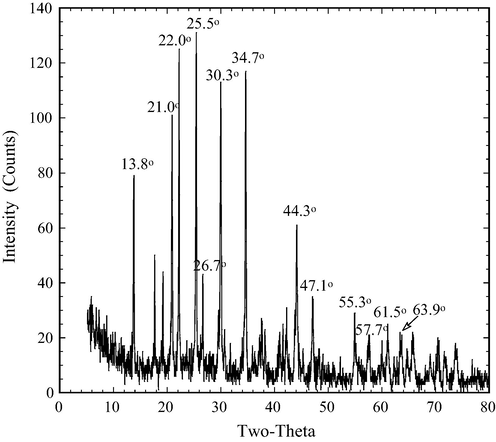
XRD pattern of powdered tourmaline.

Scanning electron microscope image (magnification × 7500) showing the surface morphology of powdered tourmaline.
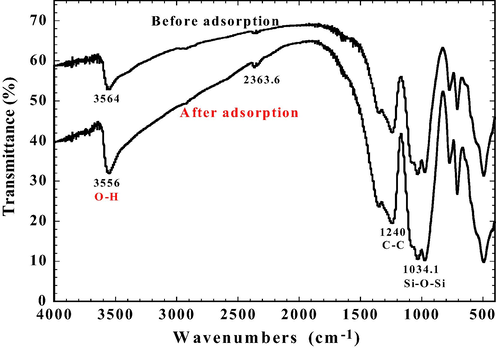
FTIR spectra of powdered tourmaline before and after DR23 adsorption; letters in red are the functional groups likely involved in DR23 binding.
The basic properties of PT are listed in Table 1. The value of pHzpc as determined was 6.6, which is close to those of iron rich minerals such as α-FeOOH (7.6), Fe2O3 (6.5), and α-Fe2O3 (7.0) (Stumm and Morgan, 1996; Zhang et al., 2016). The surface of PT was positively charged at a pH below 6.6 providing a favorable condition for adsorption of the anionic DR23 dye. A median diameter (D50) of 6.451 μm for the particles of PT was obtained and is used for the determination of intra-particle diffusion parameters in the subsequent kinetic studies.
Parameters
Value
Particle size distribution (μm)
D10
1.272
D50 (the median diameter)
6.451
D90
19.52
pH
5.87
pHzpc
6.6
BET specific surface area (m2/g)
7.34
Pore volume (cm3/g)
0.0152
Pore size (Å)
8.29
Density (kg/m3)
3.10 × 103
3.2 Kinetic studies
3.2.1 Effect of tourmaline dosage
To determine the required contact time for achieving adsorption equilibrium, experiments were carried out using various tourmaline dosages and a constant initial DR23 concentration. The results (Fig. 5) indicate that the uptake of DR23 by tourmaline particles occurred rapidly at an early stage. The adsorption then proceeded slowly with an equilibrium that was reached within 2.5 h under a surface adsorbate loading (ratio of initial dye concentration and tourmaline dosage) of 2.5 × 10−5 to 1.25 × 10−4 mol/g. The fast initial adsorption was mainly due to a rapid attachment of anionic DR23 molecules to favorable sites on the surface of PT particles through mass transfer. The subsequent adsorption was achieved through molecular diffusion of the dye into pores of the adsorbent (Weng and Pan, 2006).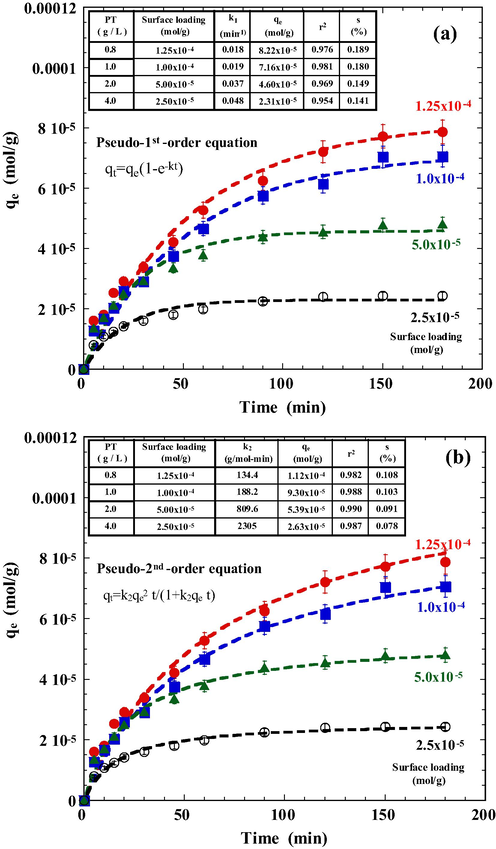
Effect of tourmaline dosage on the adsorption of DR23. Conditions: initial pH 3.0, NaCl 0.01 mol/L, DR23 1.0 × 10−4 mol/L, 25 °C. The dashed lines represent the best fit of (a) pseudo-first-order and (b) pseudo-second-order equation. Surface loading (mol/g) is defined as the ratio between DR23 concentration and tourmaline dosage.
To characterize adsorption process, the kinetic data were fitted with two empirical models namely the pseudo-first-order (PFO) and the pseudo-second-order (PSO) models. The Lagergren expression of a PFO model measures the rate of adsorption by assuming that one ion is adsorbed in one adsorption site, and has the form (Lagergren, 1998):
A graphic software, viz. KaleidaGraph™ (Version 3.6, Synergy Software, USA), was used to solve for the parameters of a nonlinear equation by calibrating the equation against experimental data. A normalized standard deviation (s %) was calculated to assess the validity of these models,
Dashed lines in Fig. 5 represent the best fits of both equations with their constants being summarized in the inset of the table. As shown in Fig. 5, both models fit the experimental data well with low standard deviations s. By comparing the values of r2 and s, it was determined that the adsorption kinetics was best described by a PSO model. The pseudo rate constant was found to be inversely proportional to surface loadings. As the surface loading increases from 2.5 × 10−5 to 1.25 × 10−4 mol/g, the PSO rate constant k2 decreases from 2305 to 134.4 g/(mol min). Higher surface loadings result in a lower diffusion efficiency where more ions are competition for a fixed number of activated surface sites; consequently, a lower k2 value was observed.
3.2.2 Intra-particle diffusion
In order to gain insights into the adsorption mechanisms involved, a homogeneous particle diffusion model (HPDM) as shown in Eq. (5), originally proposed by Boyd et al. (1947), was used to describe the process of diffusive adsorption. In this model, the rate-limiting step is usually described by either an intra-particle diffusion or a film diffusion mechanism:
A further formula deformation gives the following:
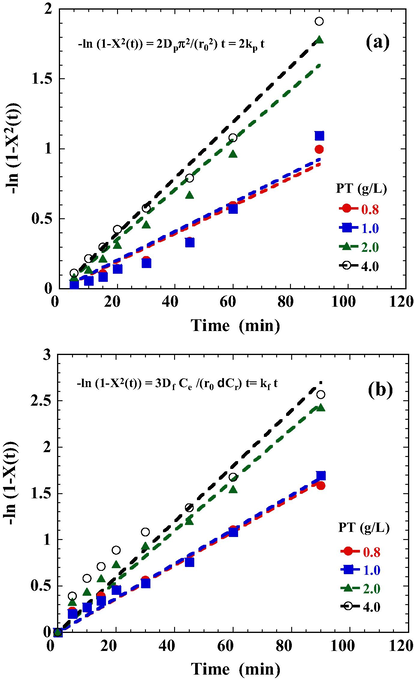
Homogeneous particle diffusion plots for DR23 adsorption onto PT (a) −ln(1 − X2(t)) vs t plots; (b) −ln(1 − X(t)) vs t plots.
Eq. (8) can be used when the rate of adsorption is controlled by liquid film diffusion (Valderrama et al., 2008):
A further formula deformation of Eq. (8) gives the following:
The results of linear regression analysis for Eqs. (7) and (9) are shown in Table 2. In general, both intra-particle and film diffusion kinetic data correlate well with HPDM as judged from the high r2 values. The intra-particle diffusion coefficient increased slightly with an increasing of PT dosage. A higher PT dosage provides more active sites for dye adsorption, and therefore, a higher rate of DR23 diffusion and adsorption. Additionally, Michelson et al. (1975) suggested that film diffusion is in control at Df values ranging from 10−10 to 10−12 (m2/s), while intra-particle diffusion is the rate-limiting step at Dp values in the range of 10−15 to 10−18 (m2/s). In this study, Df values in the order of 10−14 were found which were much lower than the lower limit of 10−12 (m2/s) for film diffusion. Conversely, the Dp values in the order of 10−16 were just in the range of 10−15 to 10−18 (m2/s) for intra-particle diffusion. The results indicate that the intra-particle diffusion controls the sorption of DR23 onto PT particles, which is in agreement with the PSO kinetic model.
Surface loading (mol/g)×10−4
PT (g/L)
kp × 10−4 (1/s)
r2
Dp × 10−16 (m2/s)
kf × 10−4 (1/s)
r2
Df × 10−14
1.25
0.8
0.825
0.951
0.870
3.03
0.972
0.544
1.00
1.0
0.860
0.921
0.905
3.10
0.983
0.801
0.50
2.0
1.485
0.969
1.560
4.67
0.964
12.70
0.25
4.0
1.160
0.984
1.755
5.00
0.924
18.70
3.2.3 Effect of temperature
The temperature dependence of DR23 adsorption is shown in Fig. 7a where dashed lines correspond to the best fit of PSO equations. Adsorption data ranging from 5 to 45 °C were well represented by the PSO equations as judged from the high correlation coefficient (i.e., r2 > 0.970) and low s values. The PSO rate constant, k2, increased from 122.3 to 339.5 g/mol min as the temperature rose from 5 to 45 °C, indicating that the adsorption of DR23 onto PT is more favorable and faster at higher temperatures. An increase in temperature elevates the driving force of diffusion across the boundary layer of an adsorbing particle and raises the rate of diffusive adsorption within its pores (Weng and Wu, 2012). The increasing DR23 adsorption with temperature suggests an endothermic reaction of the adsorption.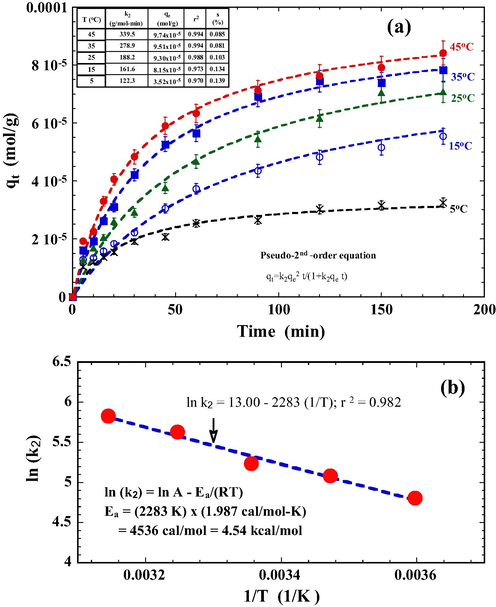
Effect of temperature on the adsorption of DR23. Conditions: tourmaline 1.0 g/L, initial pH 3.0, NaCl 0.01 mol/L, DR23 1.0 × 10−4 mol/L. The dashed lines represent the best fit of a pseudo-second-order equation.
The activation energy (Ea) of DR23/PT adsorption system was determined using the Arrhenius Equation (Eq. (10)):
3.3 Equilibrium study
3.3.1 Effect of pH
The pH dependence of DR23 adsorption was investigated using a series of experiments with initial pH of the solutions ranged from 2.5 to 10.5. An initial DR23 concentration of 1 × 10−4 mol/L and a PT dosage of 1 g/L were used for all experiments conducted at a temperature of 25 °C. As shown in Fig. 8, the adsorption of DR23 increased sharply as pH varied from 7.0 to 2.5. The adsorption is favored under acidic conditions owing to the formation of positively charged PT surfaces and fewer competitions from OH− ions.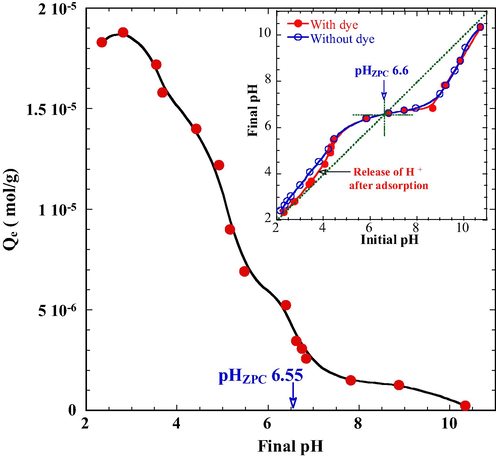
Effect of pH on the adsorption of DR23. Conditions: tourmaline 1.0 g/L, NaCl 0.01 mol/L, DR23 1.0 × 10−4 mol/L, contacting time 3 h, 25 °C.
Complexation and electrostatic interaction play important roles in determining the efficiency of an adsorption process (Weng et al., 2001; Budyanto et al., 2015). Upon hydration, hydroxyl groups form on the solid surface of an adsorbent (Huang and Stumm, 1973):
3.3.2 Effect of ionic strength on the isotherm
Dye wastewaters, particularly those of textile industries, often contain high level of soluble salts. Therefore, a knowledge on the interferences from these ions is necessary for a proper assessment of the efficiency of an adsorbent. In this study, a series of adsorption isotherm experiments were conducted under NaCl concentrations ranging from 0 to 0.05 mol/L. The effect of ionic strength on adsorption isotherm is shown in Fig. 9. In general, the amount of DR23 adsorbed decreases with increasing NaCl concentrations.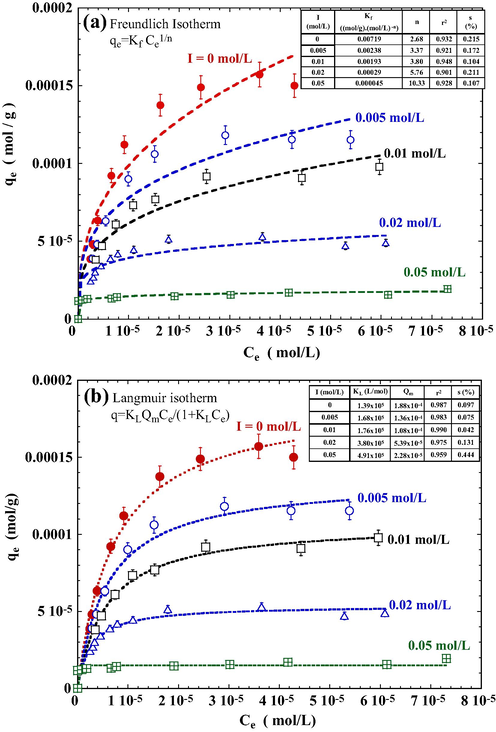
Effect of ionic strength on the adsorption isotherm of tourmaline for DR23. Conditions: NaCl 0.01 mol/L, DR23 1.0 × 10−4 mol/L, contacting time 3 h, initial pH 3.0, 25 °C. The dashed lines represent the best fit of (a) Freundlich isotherm and (b) Langmuir isotherm.
The two commonly used adsorption isotherms, viz. Freundlich and Langmuir equations, were adopted for the DR23/PT system. The adsorption on a heterogeneous surface is commonly characterized using an empirical Freundlich equation, which takes the form of Freundlich (1906)
Fig. 9a shows the data of adsorption isotherms as fitted using Eq. (15). The Freundlich isotherms suggested a decreased adsorption capacity of PT with an increasing ionic strength of the solution. As a rule of thumb, a value of n between 1 and 10 indicates a favorable sorption process (Goldberg, 2005). According to data shown in the inset table of Fig. 9a, values of n for I < 0.05 mol/L are smaller than 10, indicating that the sorption of DR23 onto tourmaline is a favorable one. The data also show that an increase in ionic strength led to a decrease in Kf and an increase in n, implying that ionic strength of the solution can reduce the favorability of adsorption.
The adsorption isotherm data were well represented using the Langmuir equation as judged from the relatively high coefficients of correlation (i.e., r2 > 0.959) (Fig. 9b). The calculated Langmuir constants are listed in the inset table of Fig. 9b. In general, the value of Qm decreases with increasing ionic strength.
Ionic strength is a key parameter defining the electrical double layer (EDL) of a hydrated particle (Weng and Pan, 2006). The thickness of an EDL (1/κ (1/m)) can be determined from the following:
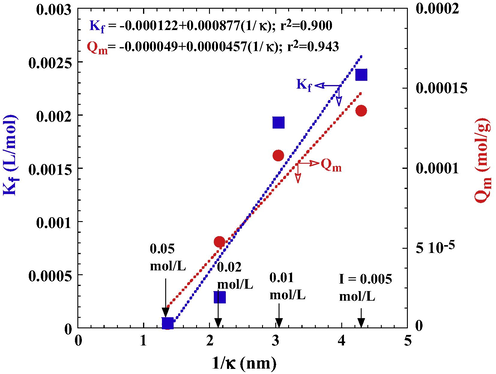
The relationships between EDL thickness and maximum adsorption capacity of tourmaline for DR23.
3.4 Regeneration test of PT
As shown in Fig. 11, the adsorption capacity of PT deteriorated gradually with regeneration. The removal efficiency of DR23 by PT is 100% at the 1st cycle. It declined to 49% at the 5th cycle. The efficiency of DR23 desorption from PT also deteriorated with regeneration. A complete decolorization was achieved on the first test cycle using fresh PT while only 89% of the adsorbed dye was desorbed. Some of the dye molecules may have trapped deep inside the aggregates of tourmaline particles and were not readily desorbable in alkaline solutions. The result shows that the PT losses only 51% of its adsorbing capacity after 5 consecutive cycles.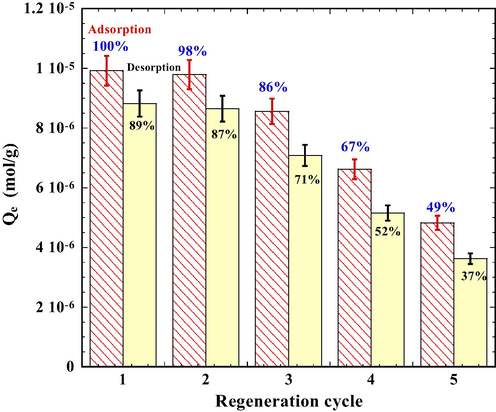
Regeneration test for DR23 adsorption onto tourmaline. Conditions for all cycles: tourmaline 4.0 g/L DR23 4.0 × 10−5 mol/L, NaCl 0.01 mol/L, DR23 1.0 × 10−4 mol/L, contacting time 3 h, 25 °C, initial pH 3.0 for adsorption, initial pH 11.0 for desorption (contacting time 1 h).
3.5 Comparison of various adsorbents for DR23 adsorption
The adsorption capacities (Qm) of various adsorbents for DR23 are compared in Table 3. The Qm of PT is significantly higher than those of TiO2 and the low-cost adsorbents. Although the magnetic multi-walled carbon nanotubes nanocomposite (MCNN) exhibits a higher adsorptive capacity, its application in the treatment of dyeing chemicals has been limited due to prohibitively high costs of the material. On the other hand, the low-cost adsorbents listed cannot be regenerated as PT or other regenerable adsorbents. Moreover, tourmaline is a relatively abundant mineral which can be obtained in large quantities at reasonable prices. Due to its low cost, reusability, and high adsorption capacity, PT has a high potential for application in the adsorptive removal of color from dye-laden wastewaters.
Adsorbent
Qm (mg/g)
pH
Temp. (°C)
Refs.
Powdered tourmaline
153.0
3.0
25
This study
Magnetic multi-walled carbon nanotubes–Fe3C nanocomposite
172.4
<7.0
30
Konicki et al. (2012)
Cationized sawdust
65.8
6.5
30
Hebeish et al. (2011)
Corn stalks
27.0–52.0
3.0
25
Fathi et al. (2015)
Uncaria gambir extract
26.7
2.0
30
Achmad et al. (2012)
Rhizophora apiculata bark
21.6
<7.0
30
Tan et al. (2010)
Orange peel
10.7
2.0
25
Ardejani et al. (2007)
Rice husk
2.4–4.4
–
25
Abdelwahab et al. (2005)
TiO2
2.2–35.2
<4.5
–
Holliman et al. (2008)
4 Conclusions
Powdered tourmaline is a highly efficient adsorbent in removing diazo direct dye DR23 with an adsorption capacity much higher than those of low-cost adsorbents reported in literatures. The efficiency of DR23 adsorption by PT is sensitive to the surface loading of adsorbent, and the temperature, pH, and ionic strength of dye-laden solutions. A proper control of these parameters is necessary in maximizing the efficiency of such an adsorptive system. The chemical structure of tourmaline is maintained after application, with OH being the major surface functional group for dye adsorption. A quick initial adsorption of DR23 by PT is achieved through physisorption, which is followed by a stage of slower molecular diffusion. Powdered tourmaline is effective for DR23 removal. It is also abundantly available and highly regenerable. It has great potentials for application in the treatment of colored textile effluents.
Acknowledgments
This research was partially supported by the Ministry of Science and Technology, Taiwan through Grant NSC102-2221-E214-002-MY3).
References
- Use of rice husk for adsorption of direct dyes from aqueous solution: a case study of Direct F. Scarlet. Egypt. J. Aquat. Res.. 2005;31:1-11.
- [Google Scholar]
- Equilibrium, kinetic and thermodynamic studies on the adsorption of direct dye onto a novel green adsorbent developed from Uncaria Gambir extract. J. Phys. Sci.. 2012;23:1-13.
- [Google Scholar]
- A study of the static and dynamic adsorption of Zn(II) ions on carbon materials from aqueous solutions. J. Colloid Interface Sci.. 2005;288:335-341.
- [Google Scholar]
- Numerical modelling and laboratory studies on the removal of Direct Red 23 and Direct Red 80 dyes from textile effluents using orange peel, a low-cost adsorbent. Dyes Pigm.. 2007;73:178-185.
- [Google Scholar]
- Thermodynamic, equilibrium and kinetic study of the biosorption of basic blue 41 using Bacillus maceran. Eng. Life Sci.. 2008;8:499-506.
- [Google Scholar]
- Removal of basic and acid dyes from aqueous solutions by a waste containing boron impurity. Desalination. 2009;249:109-115.
- [Google Scholar]
- Adsorption of anionic dyes on boron industry waste in single and binary solutions using batch and fixed-bed systems. J. Chem. Eng. Data. 2011;58:508-516.
- [Google Scholar]
- Activation of persulfate by irradiated magnetite: implications for the degradation of phenol under heterogeneous photo-Fenton-like conditions. Environ. Sci. Technol.. 2014;49:1043-1050.
- [Google Scholar]
- In-situ UV–visible spectroscopic study on the adsorption of some dyes onto activated carbon cloth. Sep. Sci. Technol.. 2009;44:3735-3752.
- [Google Scholar]
- A kinetic model for the decolorization of C.I. Acid Yellow 23 by Fenton process. J. Hazard. Mater.. 2007;148:98-102.
- [Google Scholar]
- The exchange adsorption of ions from aqueous solution by organic zeolites. II. Kinetics. J. Am. Chem. Soc.. 1947;69:2836-2848.
- [Google Scholar]
- Adsorption and precipitation of fluoride on calcite nanoparticles: a spectroscopic study. Sep. Purif. Technol.. 2015;150:325-331.
- [Google Scholar]
- Removal of strontium from aqueous solutions by adsorption onto activated carbon: kinetic and thermodynamic studies. Desalination. 2009;235:306-318.
- [Google Scholar]
- Adsorption of methylene blue onto hazelnut shell: kinetics, mechanism and activation parameters. J. Hazard. Mater.. 2009;164:172-181.
- [Google Scholar]
- Adsorption characteristics of benzaldehyde, sulphanilic acid and p-phenolsulfonate from water, acid or base solutions onto activated carbon cloth. Sep. Sci. Technol.. 2006;41:3673-3692.
- [Google Scholar]
- Adsorptive removal of triarylmethane dye (Basic Red 9) from aqueous solution by sepiolite as effective and low-cost adsorbent. Microporous Mesoporous Mater.. 2015;210:176-184.
- [Google Scholar]
- Determination of adsorptive properties of expanded vermiculite for the removal of C.I. Basic Red 9 from aqueous solution: kinetic, isotherm and thermodynamic studies. Appl. Clay Sci.. 2015;109–110:22-32.
- [Google Scholar]
- Removal of Direct Red 23 from aqueous solution using corn stalks: isotherms, kinetics and thermodynamic studies. Spectrochim. Acta, Part A. 2015;135:364-372.
- [Google Scholar]
- Über die adsorption in lösungen. Zeitschrift für Physikalische Chemie (Leipzig). 1906;57:385-470.
- [Google Scholar]
- Goldberg, S., 2005. Equations and models describing adsorption processes in soils. Soil Science Society of America, 677 S. Segoe Road, Madison, WI 53711, USA. Chemical Processes in Soils. SSSA Book Series, No. 8, pp. 489–517 (Chapter 10).
- Adsorption of uranyl on beryl and tourmaline; kinetics and thermodynamic investigation. Int. J. Miner. Process.. 2012;102–103:25-31.
- [Google Scholar]
- Characterization and application of tourmaline and beryl from Brazilian pegmatite in adsorption process with divalent metals. Int. J. Min. Sci. Technol.. 2012;22:711-718.
- [Google Scholar]
- Origin of tourmaline in the metamorphosed Sikait pelitic belt, south Eastern Desert, Egypt. J. Afr. Earth Sci.. 2001;33:391-416.
- [Google Scholar]
- An effective adsorbent based on sawdust for removal of direct dye from aqueous solutions. Clean Technol. Environ. Policy. 2011;13:713-718.
- [Google Scholar]
- Pseudo-second order model for sorption processes. Process Biochem.. 1999;34:451-465.
- [Google Scholar]
- Studies of dye sensitisation kinetics and sorption isotherms of direct red 23 on titania. Int. J. Photoenergy. 2008;2008:1-7.
- [Google Scholar]
- Adsorption of cations on hydrous Al2O3. J. Colloid Interface Sci.. 1973;43:409-414.
- [Google Scholar]
- The removal of mercury (II) from dilute aqueous solution by activated carbon. Water Res.. 1984;18:37-46.
- [Google Scholar]
- Adsorption of anionic dye Direct Red 23 onto magnetic multi-walled carbon nanotubes-Fe3C nanocomposite: kinetics, equilibrium and thermodynamics. Chem. Eng. J.. 2012;210:87-95.
- [Google Scholar]
- Zur theorie der sogenannten adsorption gelöster stoffe. Kungliga Svenska Vetenskapsakademiens. Handlingar. 1998;24:1-39.
- [Google Scholar]
- The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc.. 1918;40:1361-1403.
- [Google Scholar]
- Efficient adsorption behavior of phosphate on La-modified tourmaline. J. Environ. Chem. Eng.. 2015;3:515-522.
- [Google Scholar]
- Competitive adsorption of Cd(II), Zn(II) and Ni(II) from their binary and ternary acidic systems using tourmaline. J. Environ. Manage.. 2013;128:727-734.
- [Google Scholar]
- Characterization of biochars derived from agriculture wastes and their adsorptive removal of atrazine from aqueous solution: a comparative study. Bioresour. Technol.. 2015;198:55-62.
- [Google Scholar]
- Michelson, L.D., Gideon, P.G., Pace, E.G., Kutal, L.H., 1975. Removal of soluble mercury from wastewater by complexing technique. US Department of Industry, Office of Water Research and Technology, Bull No. 74.
- Equilibrium and kinetic adsorption study of Basic Yellow 28 and Basic Red 46 by a boron industry waste. J. Hazard. Mater.. 2009;161:148-156.
- [Google Scholar]
- An overview on the advanced oxidation processes applied for the treatment of water pollutants defined in the recently launched Directive 2013/39/EU. Environ. Int.. 2015;75:33-51.
- [Google Scholar]
- Remediation of dyes in textile effluent: a critical review on current treatment technologies with a proposed alternative. Bioresour. Technol.. 2001;77:247-255.
- [Google Scholar]
- Structure and properties of regenerated cellulose/tourmaline nanocrystal composite films. J. Polym. Sci. B: Polym. Phys.. 2004;42:367-373.
- [Google Scholar]
- Somasekhara Reddy, M.C., Nirmala, V., Ashwini, C., 2017. Bengal Gram Seed Husk as an adsorbent for the removal of dye from aqueous solutions – batch studies. Arab. J. Chem. 10 (Suppl. 2), S2554–S2566.
- Adsorption of nickel ions from aqueous solutions by nano alumina: kinetic, mass transfer, and equilibrium studies. J. Chem. Eng. Data. 2011;56:1414-1422.
- [Google Scholar]
- Aquatic Chemistry – Chemical Equilibria and Rates in Natural Waters (third ed.). New York: John Wiley & Sons; 1996.
- Effect of operating parameters on decolorization and COD removal of three reactive dyes by Fenton’s reagent using fluidized-bed reactor. Desalination. 2011;278:211-218.
- [Google Scholar]
- Adsorption of textile dye from aqueous solution on pretreated mangrove bark, an agricultural waste: equilibrium and kinetic studies. J. Appl. Sci. Environ. Sanitation. 2010;5:283-294.
- [Google Scholar]
- Monitoring the decolorization of Acid Orange 8 and Acid Red 44 from aqueous solution using Fenton’s reagents by on-line spectrophotometric method: effect of operation parameters and kinetic study. Ind. Eng. Chem. Res.. 2013;52:1414-1425.
- [Google Scholar]
- Sorption kinetics of polycyclic aromatic hydrocarbons removal using granular activated carbon: intraparticle diffusion coefficients. J. Hazard. Mater.. 2008;157:386-396.
- [Google Scholar]
- Adsorption characteristics of dye onto sludge particulates. J. Colloid Interface Sci.. 1998;208:518-528.
- [Google Scholar]
- Oxidative degradation of azo dyes using tourmaline. J. Hazard. Mater.. 2013;260:851-859.
- [Google Scholar]
- Cr(VI) adsorption onto hydrous concrete particles from groundwater. J. Environ. Eng.–ASCE. 2001;127:1124-1131.
- [Google Scholar]
- Removal of methylene blue from aqueous solution by adsorption onto pineapple leaf powder. J. Hazard. Mater.. 2009;170:417-424.
- [Google Scholar]
- Adsorption characteristics of methylene blue from aqueous solution by sludge ash. Colloids Surf. A. 2006;274:154-162.
- [Google Scholar]
- Adsorption of Cr(VI) from aqueous solution by spent activated clay. J. Hazard. Mater.. 2008;155:65-75.
- [Google Scholar]
- Potential low-cost biosorbent for copper removal: pineapple leaf powder. J. Environ. Eng.–ASCE. 2012;138:286-292.
- [Google Scholar]
- Spent green tea leaves for decolorization of raw textile industry wastewater. Color. Technol.. 2013;129:298-304.
- [Google Scholar]
- Rapid decoloration of Reactive Black 5 by an advanced Fenton process in conjunction with ultrasound. Sep. Purif. Technol.. 2013;117:75-82.
- [Google Scholar]
- Enhancement of the advanced Fenton process by ultrasound for decolorisation of real textile wastewater. Color. Technol.. 2014;130:133-139.
- [Google Scholar]
- Application of Fe0 aggregate in ultrasound enhanced advanced Fenton process for decolorization of methylene blue. J. Ind. Eng. Chem.. 2015;28:153-160.
- [Google Scholar]
- Ultrasound and heat enhanced persulfate oxidation activated with Fe0 aggregate for the decolourization of C.I. Direct Red 23. Ultrasonics Sonochem.. 2016;29:11-18.
- [Google Scholar]
- Incorporating strong polarity minerals of tourmaline with semiconductor titania to improve the photosplitting of water. J. Phys. Chem. C. 2008;112:532-539.
- [Google Scholar]
- A novel efficient photocatalyst based on TiO2 nanoparticles involved boron enrichment waste for photocatalytic degradation of atrazine. Chem. Eng. J.. 2014;250:288-294.
- [Google Scholar]
- Adsorptive and photocatalytic removal of reactive dyes by silver nanoparticle-colemanite ore waste. Chem. Eng. J.. 2014;242:333-340.
- [Google Scholar]
- The adsorption mechanism of anionic and cationic dyes by Jerusalem artichoke stalk-based mesoporous activated carbon. J. Environ. Chem. Eng.. 2014;2:220-229.
- [Google Scholar]
- Catalytic oxidative degradation of bisphenol A using an ultrasonic-assisted tourmaline-based system: influence factors and mechanism study. Chem. Eng. J.. 2014;252:346-354.
- [Google Scholar]
- Investigating the synergistic effects in tourmaline/TiO2-based heterogeneous photocatalysis: underlying mechanism insights. J. Mol. Catal. A: Chem.. 2016;411:1-8.
- [Google Scholar]
- Removal of COD from landfill leachate by an electro/Fe2+/peroxydisulfate process. Chem. Eng. J.. 2014;250:76-82.
- [Google Scholar]
- Phase transformation of crystalline iron oxides and their adsorption abilities for Pb and Cd. Chem. Eng. J.. 2016;284:247-259.
- [Google Scholar]







