Translate this page into:
Adsorption characteristics of used granular activated carbon regenerated by ultrasonic backwashing
⁎Corresponding author. wangzheng@njfu.edu.cn (Zheng Wang)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Activated carbon is used in water-treatment processes owing to its strong adsorption capacity. Ultrasonic-backwash regeneration of particulate activated carbon in an ozone activated carbon filter was investigated with sodium benzoate as the model pollutant, and the effects of regeneration parameters (ultrasonic frequency, power, regeneration time, and backwashing intensity) on the regeneration efficiency of the activated carbon were studied. The regenerated carbon was characterized by BET, SEM, XPS, and strength, and compared with used activated carbon. The ideal regeneration parameters were determined to be 120 kHz ultrasonic frequency, 360 W ultrasonic power, 1 L/min backwashing intensity, and 10 min regeneration time. The results showed that ultrasonic treatment changes part of the pore structure of the activated carbon; that the high-speed microjets generated by cavitation as well as the high-pressure microbubbles play important roles in the regeneration of the activated carbon; and that backwashing facilitates a range of ultrasonic action on the activated carbon, positively affecting the regeneration process.
Keywords
Ultrasonic
Backwashing
Regeneration
Used activated carbon
1 Introduction
Compared with other methods of water treatment, the adsorption method is favored because of its simplicity, convenience, high efficiency, and low cost (Lin et al., 2017). To address deteriorating raw water quality, increasing water quality requirements, and the treatment of wastewater, the ozone granular activated carbon process is one of the most widely used technologies for removing pollutants in water purification plants (Korotta-Gamage and Sathasivan, 2017), including that of inorganic and organic pollutants (Ferrández-Gómez et al., 2021). The bioactivated carbon process has a good treatment effect. Microorganisms can attach to the surface of the activated carbon, distributing on its surface or in the pores to form biofilms and utilize the organic matter on the surface as nutrients (Simpson, 2008). However, with increasing process operation time, the adsorption sites of the activated carbon gradually tend to saturation, adsorption performance decreases, and the treatment effect of the process is gradually weakened (Klimenko et al., 2002). When the adsorption performance of the activated carbon decreases to a certain extent, the activated carbon in the filter is prone to failure, making the effluent water quality difficult to ensure (Liu et al., 2020a), so it needs to be replaced or regenerated (Nahm et al., 2012) in order to ensure the effectiveness of the process. Saturated activated carbon, if not treated accordingly, becomes waste activated carbon, causing environmental pollution. Accordingly, several waste reduction policies are being implemented to increase waste carbon usage through recycling and circular economy strategies (Ferrández-Gómez et al., 2023).
Regeneration of activated carbon takes place through desorption and decomposition of adsorbates (Ma et al., 2022). There are various ways of regenerating activated carbon, such as thermal regeneration (Chen et al., 2022), electrochemical regeneration (Santos et al., 2022), Fenton-like oxidative regeneration(Zhang et al., 2023a), microwave regeneration (Gagliano et al., 2021), and ultrasonic regeneration (Liu et al., 2020b). Currently, thermal regeneration is commonly used in industry for the reuse of activated carbon. However, the loss of activated carbon during thermal regeneration is very large, with losses generally ranging from 10 % ∼ 30 % (Heidari et al., 2014), and there are problems with by-products, such as sulfur dioxide, produced during thermal regeneration (Zhang et al., 2023b). Moreover, electrochemical regeneration and other regeneration methods are difficult to be applied in large scale in actual projects.
Ultrasonic wave propagation in a liquid medium causes cavitation. Ultrasonic cavitation utilizes sound waves with frequencies higher than the human audible range (>18 kHz) to irradiate a liquid medium and form microbubbles (Mason and Peters, 2002). When a bubble compresses and expands several times, it reaches critical state and collapses, generating a large amount of energy called cavitation (Mason and Peters, 2002). When the bubbles burst, a large amount of energy is emitted, producing microjets (Breitbach and Bathen, 2001) with velocities of up to kilometers per hour, in addition to the formation of high-pressure shock waves and localized high temperatures (Wang et al., 2007). Ultrasound not only promotes desorption, it also enhances mass transfer in adsorption processes (Li et al., 2006).
In recent years, the technology of ultrasonic regeneration of activated carbon has been compounded with many other technologies, such as combining with low-temperature thermal regeneration or microwave (Fu et al., 2020, Zheng et al., 2021), and the method of regenerating activated carbon by coupling ultrasonic waves with the backwashing process has not been investigated yet. Therefore, in this study, the ability of ultrasonic waves to regenerate activated carbon was explored, and the desorption potential of ultrasonic backwashing on activated carbon was reflected by using the adsorption capacity of regenerated carbon with sodium benzoate as the target pollutant. Sodium benzoate is a preservative widely used in food and cosmetics in life (Atkins et al., 2018). The relative molecular mass is 144.12, which is a small molecule, and both the micropores and mesopores of the activated carbon can adsorb sodium benzoate. This study comprehensively evaluated the regeneration potential of the combined ultrasonic-backwashing technique on activated carbon was comprehensively evaluated, focusing on the effect of ultrasonic frequency. BET, SEM, XPS, strength, and other indexes before and after regeneration as well as the adsorption performance of the activated carbon were also characterized. The results of the study lay a certain foundation for subsequent research on activated carbon and have practical significance for the further development of combined ultrasonic regeneration processes.
2 Materials and methods
2.1 Materials
Sodium benzoate, sodium thiosulfate, methylene blue, and sodium hydroxide (all analytically pure) were purchased from Nanjing Chemical Reagent Co. Iodine, potassium iodide (all analytical grade), and starch indicator (concentration 5 g/L) were obtained from Tianjin Komeo Chemical Reagent Co.
The activated carbon used in the experiment was granulated activated carbon (GAC) from coal, used for four years in an ozone activated carbon filter of a water plant in Nanjing City, Jiangsu Province, China, and the manufacturer was Inner Mongolia Taixi Coal Chemical Co. The characteristics of the activated carbon are shown in Table 1. The used activated carbon was rinsed with pure water three times before the experiment to remove soluble impurities. The cleaned activated carbon was dried in an oven at 60 °C to a constant weight and stored. The representative pollutant selected for the experiment was sodium benzoate with a relative molecular mass of 144.12.
Sample
Useful life (years)
Iodine value (mg/g)
Methylene blue value (mg/g)
Specific surface area (m2/g)
Total pore volume (cm3/g)
Ash (%)
Used activated carbon
4
339.86
57.00
79.740
0.0510
25.80
2.2 Instrumentation
The ultrasonic device was designed independently (working power: 40 kHz, 80 kHz, 120 kHz; power range: 0–400 W; ultrasonic time: 0 ∼ 60 min; gas flushing intensity: 0–5 L/min, which can be adjusted according to the specific experimental needs). An external metal column ultrasonic generation system was used, which can simulate the real environment in which mechanical waves are scattered to the activated carbon. The metal column also has the of filtration and gas–water backwashing functions, which can realize ultrasonic in-situ regeneration. A diagram of the device is shown in Fig. 1, each device provides only one frequency, and each ultrasonic generation device operates independently, water in at the top and out at the bottom, flowmeter provides air impact strength.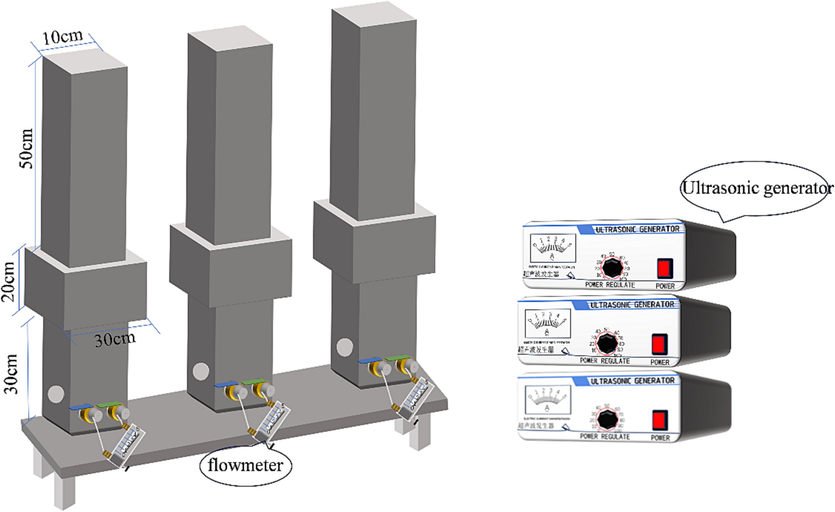
Self-made ultrasonic back-washing regeneration device.
2.3 Experimental method
The regeneration method was as follows: 100 g of used activated carbon was placed in an oven at 60 °C and subjected to ventilation drying for 2 h and then placed in the ultrasonic-backwash regeneration device for regeneration, controlling the four independent variables (ultrasonic frequency, power, regeneration time, and intensity of gas flushing). At the end of regeneration, the product was taken out and dried in an oven at 60 °C for 8 h, sealed, dried, and bagged.
The amount of sodium benzoate adsorbed on the activated carbon before and after regeneration was determined by static adsorption experiments. The experimental steps were as follows: 5 g of activated carbon and 1000 mL of 100 mg/L sodium benzoate solution was poured into a 1000 mL beaker. The beaker was placed in a multifunctional mixer (HJ-4) and oscillated at a rotational speed of 160 r/min at 28 °C ± 2 °C for 2 h in order to ensure that the adsorption equilibrium was reached. The absorbance value of sodium benzoate solution was determined by UV spectrophotometer. The residual concentration of the sodium benzoate solution was then determined. The equilibrium adsorption of sodium benzoate by used activated carbon was 0.690 ± 0.04 mg/g.
All experimental data were examined using parallel experiments for activated carbon and sodium benzoate solution related indicators, and each experiment was repeated three times. The data in the Fig. 2 and Fig. 8 are the average values of the three experiments, and the error bars indicate the standard deviation of the results of the three parallel experiments.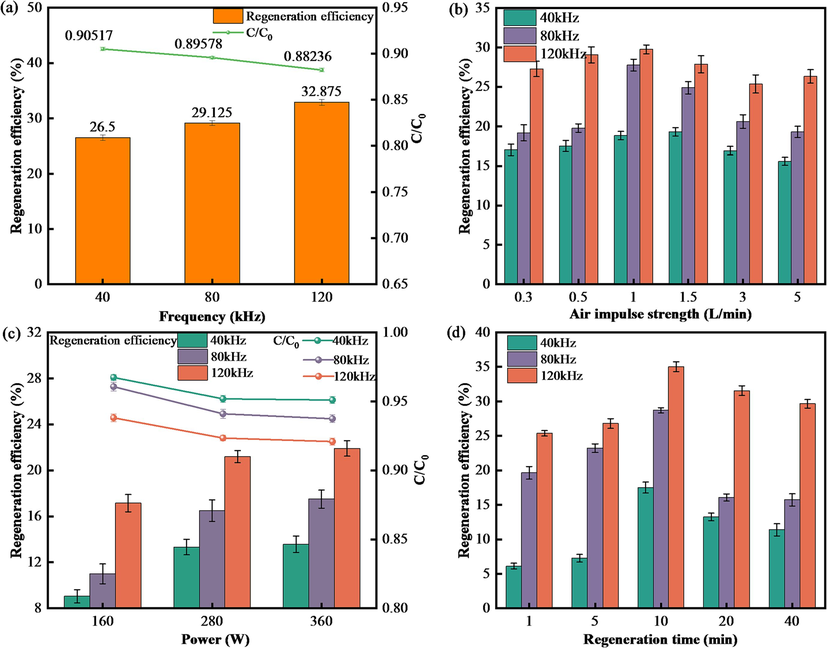
Factors affecting ultrasonic regeneration efficiency. (a) Frequency (gas impulse intensity 1.0 L/min, time 10 min, power 360 W). (b) Gas impulse intensity (power 360 W, time 10 min). (c) Power (gas impulse intensity 1.0 L/min, time 10 min). (d) Time (gas impulse intensity 1.0 L/min, power 360 W).
2.4 Analytical methods
2.4.1 Characterization of activated carbon
Iodine value was determined by Chinese standard method GB/T 7702.7–2008. After fully oscillating the adsorption of the iodine standard solution with the quantitative test material, the residual iodine content of the solution was determined by titration method, the number of iodine adsorption in each test material was determined, and the adsorption isotherms were drawn. The adsorption value of activated carbon on iodine was indicated by the amount of iodine adsorbed per gram of test material with residual iodine concentration of 0.02 mol/L.
Methylene blue value by Chinese standard method GB/T 7702.6–2008. The remaining concentration of methylene blue solution was mixed with methylene blue solution, and the value of methylene blue adsorption was calculated.
Ash content was analyzed according to national standard GB/T 7702.15–2008.
Activated carbon particle size was analyzed by a laser particle size analyzer (Mastersizer 2000, Malvern Panalytical, UK).
The surface morphology of the activated carbon was observed by field emission scanning electron microscopy (SEM, Prisma E, Thermo Fisher Scientific, USA).
The Brunauer-Emmett-Teller specific surface area and porosity of the activated carbon were analyzed using a fully automated specific surface area and porosity analyzer (BET, Autosorb IQ MP, Quantachrome, USA), and the carbon specific surface area was determined from N2 adsorption isotherms obtained at 77.137 K. The N2 adsorption–desorption isotherm of the sample was obtained at −196 °C after degassing at 120 °C for 8 h. Specific surface area was calculated by the BET method, and the void volume and pore sizes of the micropores and mesopores were evaluated using BJH (Barrett-Joyner-Halenda) and H–K theory (Horvath-Kawazoe) (Barrett et al., 1951).
The strength of activated carbon before and after regeneration was theoretically calculated according to the strength determination method in GB/T 7702.3–2008. Under the prescribed conditions, the test material is placed in a drum with a steel ball, rotated mechanically by the drum, the test material is worn. To determine the change of the particle size of the damaged test material, the test material quality station's mass fraction retained on the test sieve as the test material strength.
The elemental composition of the activated carbon before and after regeneration were analyzed by X-ray photoelectron spectroscopy (XPS, K-Alpha, Thermo Fisher Scientific, USA).
2.4.2 Removal effect
To further evaluate the effect of ultrasonic regeneration, indicators such as regeneration efficiency were used. At the end of each regeneration, the adsorption experiments were performed again under the same conditions as the initial experiment, and the adsorption capacity Qe (mg/g) obtained was calculated by Equation (1), i.e., the saturated adsorption amount was used to obtain the regeneration efficiency E compared to the initial adsorption amount, which was calculated by Equation (2):
where C0 and Ce are the initial and equilibrium concentrations of sodium benzoate (mg/L), respectively; V is the solution volume (L); m is the mass of activated carbon (g); Qp is the adsorption capacity of regenerated carbon adsorption of sodium benzoate; and Q0 is the adsorption capacity of activated carbon adsorption of sodium benzoate before regeneration.
Orthogonal tests were designed to determine the main factors affecting the regeneration of activated carbon. The regeneration efficiency of the activated carbon was evaluated using four factors and three levels in an orthogonal test. Four main factors were considered: activated carbon regeneration time, ultrasonic frequency, power, and backwashing intensity at room temperature (28 °C ± 2 °C).
3 Results and discussion
3.1 Ultrasonic regeneration effect
Fig. 2 shows the effect of frequency, air punch strength, power and regeneration time on activated carbon regeneration. According to Fig. 2a, the used activated carbon regeneration efficiency increases with ultrasonic frequency. When the ultrasonic frequency increases from 40 to 120 kHz, the used activated carbon regeneration efficiency increases from 26.50 % to 32.88 %, indicating that high-frequency ultrasound is more effective than low-frequency ultrasound for regeneration. In the regeneration process, with the increase of ultrasonic frequency, the bubbles generated by cavitation are collapsed in one cycle, generating energy and localized high temperature and high pressure inside the fluid, thus generating high-speed microjets and high-pressure shock waves, which promote the breakage of chemical bonds between adsorbent and adsorbate(Hamdaoui et al., 2003) to achieve desorption of sodium benzoate, regenerating the activated carbon. In addition, backwashing conditions were also considered, and high-frequency ultrasonic backwashing was found to be more effective for regeneration. However, the higher the ultrasound frequency, the faster the energy decreases (Breitbach et al., 2003), the cavitation intensity is weakened, and the number of small bubbles produced is reduced (Hamdaoui et al., 2005b), so it is difficult to gather a large amount of energy.
Fig. 2b and 2d show that with the increase of gas impulse strength and regeneration time, the sodium benzoate adsorption capacity of the regenerated carbon at all three frequencies shows a trend of increasing and then decreasing. The regeneration efficiency of activated carbon at a frequency of 40 kHz reaches the highest at a gas impulse strength of 1.5 L/min, and the regenerated carbon at 120 kHz has the best regeneration effect at a gas impulse strength of 1.0 L/min. The regeneration efficiency of the regenerated activated carbon at the three frequencies is highest when the regeneration time is 10 min. In the case of low gas impulse intensity, the intensity is very small, failing to fully wash up the activated carbon at the bottom, so the activated carbon is still in the agglomerated state, and the ultrasonic waves do not completely penetrate the activated carbon layer, which makes the regeneration effect poor. Moreover, the regeneration efficiency for the three frequencies decreases after the maximum efficiency is reached, which is because in the gas impulse conditions, the activated carbon layer is in the fluidized state, and the particles are uniformly distributed in the device. The ultrasonic wave can effectively propagate inside the activated carbon, and as the air flushing intensity increases, the size of the bubbles generated increases, the cavitation intensity generated by the cavitation bubbles is weakened, and the activated carbon is backwashed by the air and water at too high an intensity, which makes the activated carbon float on the water surface. Furthermore, the water flow is in a state of turbulence, which has an attenuation effect on the sound waves generated by ultrasonication, so under the conditions of high air flushing intensity, the regeneration effect decreases.
In the early regeneration time, the regeneration efficiency increases more quickly, probably because of the energy scattered by ultrasonic waves and the cavitation effect. An overly long regeneration time (40 min) leads to a decrease in the regeneration efficiency of activated carbon, and more energy is required. In addition, a long period of ultrasonic treatment may lead to a decline in the performance of the activated carbon because it is damaged, breaking up into small particles (de Carvalho Costa et al., 2022). Thus, the activated carbon yield rate is reduced. In this experiment, it was found that when the regeneration time was 40 min, the turbidity of the solution in the device was higher and the number of fine particles increased. Therefore, an appropriate extension of the regeneration time is conducive to improving the regeneration of activated carbon. After a certain time of ultrasonic treatment, the concentration of cavitation bubbles in the solution reaches saturation (Zheng et al., 2021), and after the ultrasonic regeneration time is extended for a certain period of time, the regeneration efficiency of the activated carbon remains unchanged, or even decreases to a certain extent.
According to the Fig. 2c, with the increase of ultrasonic power, the regeneration effect increases. When the power increases from 160 to 360 W, the regeneration efficiency at an ultrasonic frequency of 40 kHz increases from 9.05 % to 13.57 %. However, the regeneration efficiency at an ultrasonic frequency of 120 kHz grows from 17.14 % to 21.90 %, which is more substantial. The greater increase observed when the power was increased from 160 W to 280 W may be due to the fact that for ultrasound to produce cavitation, the power input to the reaction solution must be greater than the cavitation threshold. As the ultrasound power increases, so does the intensity of the ultrasonic field, so more cavitation chances can be generated, and the stronger the high-pressure shock wave and high-speed microjets that act on the activated carbon will be (Hamdaoui et al., 2005a), thus desorbing more substances from its surface. However, from 280 to 360 W, the increase was very small, when the power increases to a certain value, the cavitation effect tends to saturation, so more power input will produce a large number of useless bubbles, increasing the scattering attenuation (Wang et al., 2021). Thus, the ultrasonic energy utilization is reduced and the cavitation effect is weakened, so that the activated carbon particles are broken.
Orthogonal design (L9(34)) were conducted with a four-factor, three-level experimental design (i.e., regeneration times of 1 min, 10 min, and 40 min; ultrasonic frequencies of 40 kHz, 80 kHz, and 120 kHz; ultrasonic powers of 160 W, 280 W, and 360 W; and backwashing intensities of 0.3 L/min, 1.0 L/min, and 5.0 L/min) (Table 2). A: Ultrasonic frequency. B: Backwashing strength. C: Regeneration time. D: Ultrasonic power.
Number
Factor
Regeneration efficiency%
A
B
C
D
1
1 (40 kHz)
1 (0.3 L/min)
1 (1 min)
1 (160 W)
6.00
2
1
2 (1.0 L/min)
2 (10 min)
2 (280 W)
16.50
3
1
3 (5.0 L/min)
3 (40 min)
3 (360 W)
10.38
4
2 (80 kHz)
1
2
3
17.02
5
2
2
3
1
8.81
6
2
3
1
2
10.69
7
3
1
3
2
19.19
8
3
2
1
3
25.36
9
3
3
2
1
15.31
K1
32.88
42.21
42.05
30.12
K2
36.52
50.67
48.83
46.38
K3
59.86
36.38
38.38
52.76
R
6.98
8.46
6.78
22.64
Order of priority
A > D > B > C
Superior level
120 kHz, 360 W, 1.0 L/min, 1 min
Where Kij is the sum of test results corresponding to level i in column j; Rj = max {Kij} – min {Kij}. For the main effect relative index Kij, the larger its value indicates that the level of the greater impact on the test results, the size of Rj indicates the size of the impact of the factor on the test results, the larger its value indicates that the greater the impact of the factor on the results of the test, and finally select the level corresponding to the maximum value of Kij among the various factors can be constituted as a better combination of levels, to form a preferred experimental program.
From the influence index R in Table 2, it can be seen that the primary and secondary order of the factors influencing the regeneration efficiency of activated carbon is ultrasonic frequency > ultrasonic power > backwashing intensity > regeneration time, and ultrasonic frequency is the primary influencing factor. By analyzing the K value, it can be seen that the optimal regeneration conditions are: ultrasonic frequency 120 kHz, ultrasonic power 360 W, backwashing intensity 1.0 L/min, and regeneration time 1 min. In this experiment, the regeneration time is the smallest factor of the influence index, so the regeneration time was reevaluated with different times under the condition that the first three factors remain unchanged, and the experimental results show that the regeneration time is appropriately extended to help improve the regeneration efficiency of activated carbon, and that the regeneration time of 10 min is optimal.
3.2 Changes in adsorption properties of activated carbon after ultrasound treatment
Adsorption performance, especially for iodine and methylene blue, is a commonly used parameter in activated carbon research to evaluate the quality of adsorbents. The effect of ultrasound (regeneration time 10 min, air impulse strength 1.0 L/min, power 360 W) at different frequencies (40 kHz, 80 kHz, 120 kHz) on the adsorption indexes of the used activated carbon was investigated (Fig. 3). Ultrasonic treatment can improve the adsorption performance index (methylene blue value) of activated carbon by 8.57 %–22.86 %. The methylene blue value mainly characterizes the mesopore content of activated carbon (Benadjemia et al., 2011). The methylene blue value shows a rapid initial increase and then slows when the ultrasonic frequency is further increased, which may be due to the high-speed microjets generated during the collapse of cavitation bubbles and the role of high-pressure shock waves. Under this effect, the surface pore wall became thinner, and the adjacent microporous structure collapsed, and thus connected with each other to form mesopores or macropores. The iodine value decreases under the action of ultrasonic waves compared with that of the used activated carbon, but the iodine value increases gradually with the increase of ultrasonic wave frequency, and slowly recovers from 304.02 to 331.90 mg/g, which is probably due to fragmentation of the particles of the wasted activated carbon after ultrasonic wave treatment (Breitbach and Bathen, 2001). Although the iodine adsorption value of the ultrasonically regenerated activated carbon was slightly lower than that of the used activated carbon, the regenerated activated carbon significantly increased the removal of sodium benzoate from water, indicating that its adsorption capacity was partially restored.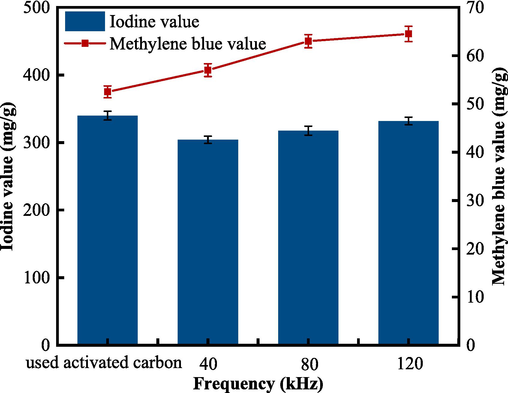
The effect of ultrasound frequency on the adsorption properties of GAC after ultrasound treatment (different frequencies, regeneration time 10 min, gas impulse strength 1.0 L/min, power 360 W).
3.3 Characterization of activated carbon
3.3.1 SEM
The difference in morphology of the adsorbent before and after regeneration can be seen in the SEM images. The surface of the used activated carbon is nearly smooth, and the pores are covered with impurities, which might have formed a biofilm on the surface (Fig. 4a). Fig. 4b, 4c, and 4d show the microscopic morphology of the regenerated carbon, the surface of which shows obvious cracks. With increasing ultrasonic frequency, the surface of the regenerated carbon shows more pores. Sharper edges of regenerated activated carbon particles in Fig. 4d. Probably due to the effect of the ultrasonic cavitation, a large amount of surface-attached compounds are desorbed, and the pore structure is more obvious. The samples after ultrasonic treatment show more pore structures at increasing frequency levels, and more mesopores and macropores are generated on the surface of the activated carbon.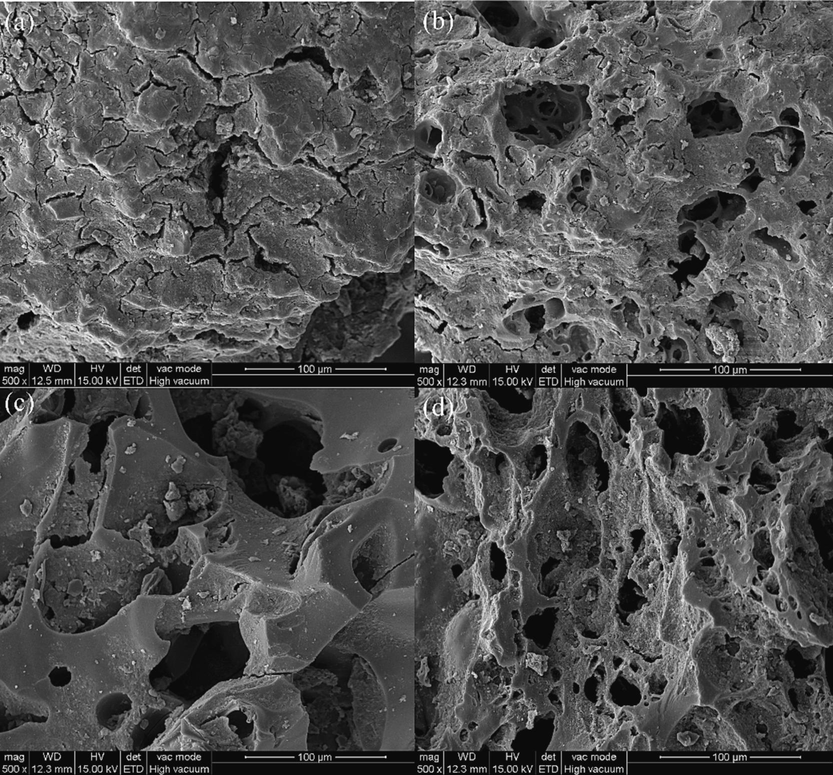
SEM images of activated carbon before and after regeneration. (a) Initial carbon; (b) 40 kHz, 1.0 L/min, 10 min, 360 W regenerated carbon; (c) 80 kHz, 1.0 L/min, 10 min, 360 W regenerated carbon; (d) 120 kHz, 1.0 L/min, 10 min, 360 W regenerated carbon.
3.3.2 BET and pore-size distribution analysis
Due to the size limitation of cavitation bubbles and their corresponding microjets and microfluidics, ultrasound can only affect activated carbon particles locally. Changes in pore space and specific surface area after regeneration of activated carbon by ultrasound-backwashing were determined by N2 adsorption desorption. The isotherms for used activated carbon and carbon regenerated at frequencies of 40, 80, and 120 kHz are shown in Fig. 5, and the pore structure parameters are shown in Table 3.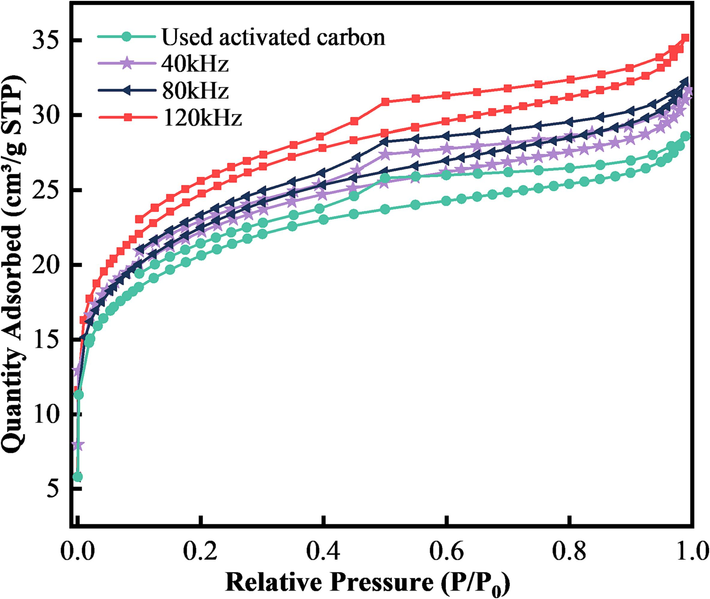
N2 adsorption–desorption isotherms of activated carbon before and after regeneration.
Carbon sample
Specific surface area (m2/g)
Pore volume (cm3/g)
Pore diameter (nm)
BET
t-Plot external surface area
t-Plot micropore surface area
Total volume
t-Plot volume of micropore
Volume of mesopore and macropore
Mean diameter
Used activated carbon
79.740
56.154
23.586
0.0680
0.0136
0.0545
2.73
40 kHz
71.788
51.963
19.825
0.0632
0.0116
0.0515
2.87
80 kHz
72.581
51.556
21.025
0.0620
0.0121
0.0499
2.75
120 kHz
67.001
44.465
21.536
0.0566
0.0123
0.0443
2.68
The adsorption of the four activated carbons is mainly observed at lower pressure (P/P0 < 0.2). The filling of micropores, a rapid increase of N2 adsorption (Sun et al., 2016), and hysteresis loops appear between P/P0 = 0.1 and 1.0. According to IUPAC classification, both the used activated carbon and the regenerated activated carbon present type-II isotherms (Schneider, 1995). The adsorption and desorption curves did not completely coincide, a trailing tail begins to appear at P0 = 1.0, and the area of the hysteresis loop is larger, which indicates that the carbon has a wider pore-size distribution and contains a larger proportion of medium and large pores. This usually indicates saturation of monolayer adsorption, and the hysteresis loop belongs to the H4 type, which appears for solids with slits, such as activated carbon. Fig. 5 shows that the pore structure of this activated carbon(Zou et al., 2008) is dominated by mesopores.
According to results in Table 3, the specific surface area of the regenerated carbon after ultrasonic treatment decreases to a certain extent, which may be due to the fact that ultrasonic energy causes the regenerated carbon to break into small particles. For the regenerated carbon after ultrasonic backwashing, with the increase of ultrasonic frequency, although the specific surface decreases, the microporous area of the regenerated carbon increases; the micropore volume increases from 0.0116 cm3/g to 0.0123 cm3/g. The microporous content increases after ultrasonic regeneration, and the high-speed microjet and high-pressure excitation waves generated by the rupture of cavitation bubbles mainly act on the activated carbon surface and mesopores (Zheng et al., 2021), which cause the mesopore walls to be eroded into micropores, and the mesopore pore volume decreased from 0.0515 cm3/g to 0.0443 cm3/g, which indicates that high-frequency ultrasonics have a stronger effect on the activated carbon mesopores. The microporous area and micropore volume of the regenerated activated carbon (120 kHz, 1 L/min, 10 min, 360 W) increase by 8.63 % and 5.97 %, respectively, compared to those of regenerated activated carbon (40 kHz, 1 L/min, 10 min, 360 W). The micropores are mainly concentrated in the interior of the activated carbon (Liu et al., 2017), which is connected to the outside world through mesopores and macropores. Therefore, the frequency of ultrasonic wave increases, and the degree of action on activated carbon also increases, and it can pass through the activated carbon to enter the interior, indicating that the high-speed microjets and the high-pressure shockwaves generated by cavitation pass through the surface of the activated carbon to affect the micropores inside, regenerating the activated carbon to provide more usable binding sites. The average pore size of the regenerated carbon decreases compared with that of the used activated carbon. The average pore size of the ultrasonically treated activated carbon is decreased from 2.73 nm to 2.68 nm (120 kHz, 1.0 L/min, 10 min, 360 W). It is further shown that even though the higher ultrasonic frequency may damage the pore structure of the activated carbon to a certain extent, the cavitation effect during the ultrasonic process removes the impurities blocking the pores in the used activated carbon, and micropores and mesopores are formed, which can adsorb more impurities in water.
As can be seen from the micropore map in Fig. 6a, the volume of the micropores after ultrasonic treatment is higher, and the maximum amount of nitrogen adsorbed at the ultrasonic frequency of 120 kHz indicates that the micropores are well developed. In Fig. 6b, with dV/dlog(D) as the vertical coordinate and the distribution of pore sizes as the horizontal coordinate, the higher the value of dV/dlog(D) corresponding to a certain horizontal coordinate, the pores will correspond to that pore size. It can be seen that the pore size of the regenerated activated carbon treated with low-frequency ultrasound is not very different from that of the used activated carbon, the pore size range is distributed between 0 and 200 nm, and the highest value between 0 and 2.5 nm indicates that the number of pores under the corresponding pore size is higher. The mesopore or macroporous structure in the activated carbon treated with ultrasound contributes more to the pore diameter. The particle size distribution of activated carbon before and after ultrasound was basically the same. According to Fig. 6c, there is a peak value around 0.7–0.8 nm before and after regeneration, which indicates that the micropores before and after regeneration are mainly distributed in this range. Fig. 6d of the cumulative volume-pore size distribution indicates that the volume of mesopores between 0.6 and 2.4 nm grows significantly with increasing pore size, and the trend of the cumulative pore volume growth is higher than that of the old saturated charcoal, which indicates that the content of the mesopore increases after ultrasonic regeneration.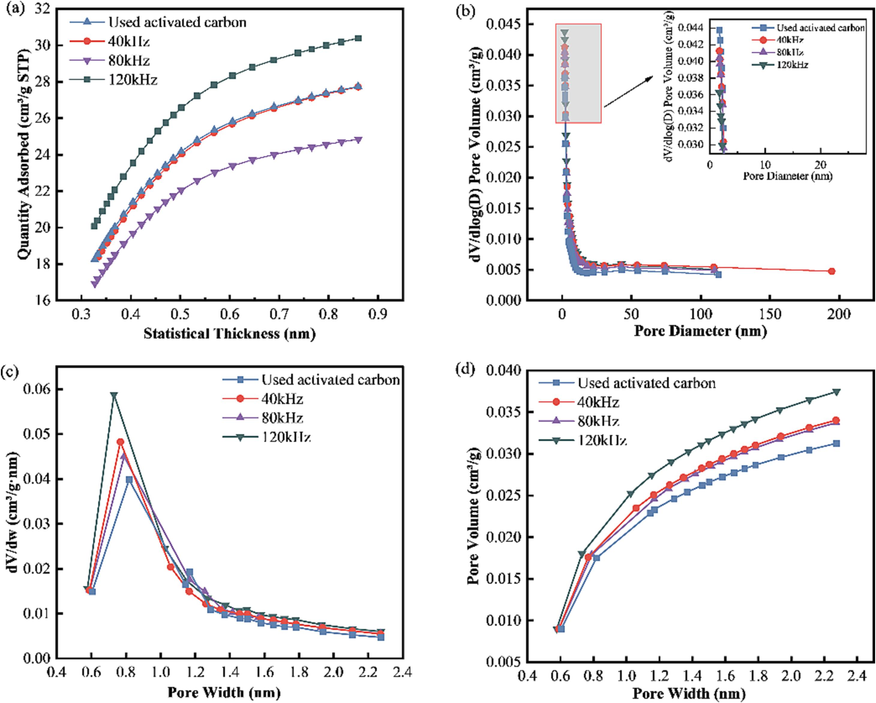
Pore-size variation. (a) t-plot microporosity variation; (b) BJH dV/dlog(D)-porosity variation; (c) H–K pore size variation; (d) H–K cumulative pore volume.
3.3.3 Granularity
Laser particle-size analysis of activated carbon before and after regeneration showed that, as can be seen in Fig. 7, the curves before and after ultrasonic regeneration are largely the same, but the curve distribution of the regenerated activated carbon is shifted to the left compared with that of used activated carbon as a whole. It shows that the particle size of activated carbon particles regenerated by ultrasonic backwashing decreases. The activated carbon has the highest concentration of particle size between 300.0 and 500.0 μm, and the used activated carbon has the largest distribution of particle size at 447.7 μm. The particle size of carbon regenerated at 40 kHz is mainly concentrated at 399.0 μm, and the maximum concentration is at 355.6 μm when the frequency is 120 kHz. Thus, the ultrasonic frequency has an effect on the particle size of the activated carbon, with higher frequency is, the smaller is the particle size corresponding to the maximum content. Pore structure is the main determinant of adsorption performance(Lee et al., 2023).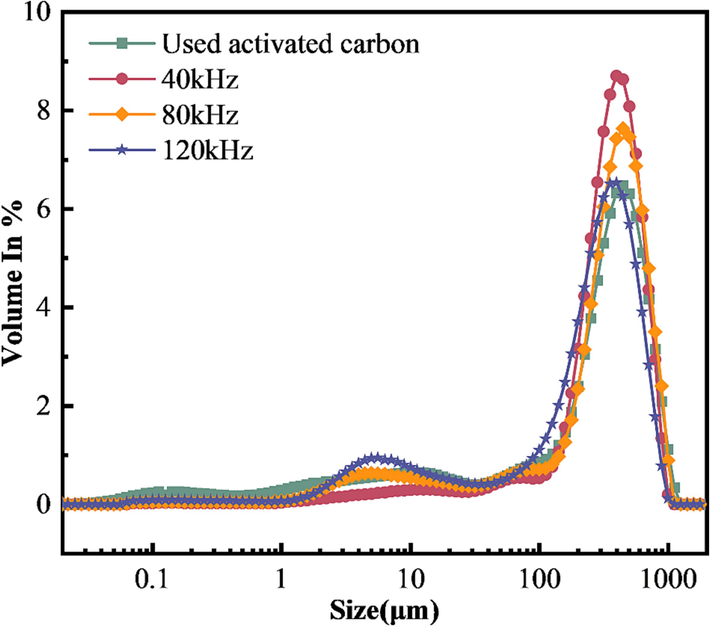
Particle-size distribution curves.
According to Table 4, for the regenerated carbon, the average particle surface area and the average particle volume decrease with increasing frequency, which indicates that the porosity of activated carbon under ultrasonic regeneration increases non-linearly with the ultrasonic frequency. As the frequency increases from 40 to 120 kHz, the average particle surface area decreases by 70.37 %, and the average particle volume decreases by 22.93 %.
Carbon sample
Average surface area particle size (μm)
Volume average particle size (μm)
Used activated carbon
2.447
346.434
40 kHz
25.347
396.481
80 kHz
11.688
385.154
120 kHz
7.511
305.563
Although the particle size decreases, there is still adsorption capacity, and as the adsorption capacity gradually increases, the particle size decreasesy, which further indicates that with increasing ultrasonic frequency, the cavitation effect and the shockwaves caused by cavitation cause damage to the activated carbon particle morphology, breaking it into smaller particles and enlarging the activated carbon surface, which increases the number of active contact points on the surface of the activated carbon particles.
3.3.4 Strength
Activated carbon strength refers to its wear-resistant crushing performance, which is an important index regarding the physical properties of activated carbon. Wear resistance has an important impact on the performance and operating cost of activated carbon. The criteria of failure and replacement for coal-based bioactivated carbon in urban water-supply plants in Jiangsu Province (DB 32/T 4245–2022) include an activated carbon strength lower than 80 % and particle homogeneity coefficient K80 > 3.0.
In this study, the method of determining the strength in the Test Method for Coal Granular Activated Carbon (GB/T 7702.3–2008) was used to investigate the effect of different parameters of ultrasonic-backwash regeneration, and therefore the regenerated activated carbon with different ultrasonic frequencies as well as the used activated carbon of the water plant were tested. However, the strength of this specimen was difficult to detect according to the ball-and-disk (grinding) method of the national standard. The reasons for this are, firstly, from the appearance, both the used activated carbon from the water plant and the activated carbon regenerated by ultrasonic wave-backwashing have small particles; in addition, the regenerated activated carbon appears to be pulverized in different degrees. Therefore, it was decided to use the crushing force to show the crushing resistance of the activated carbon, and the related experimental results are shown in Fig. 8.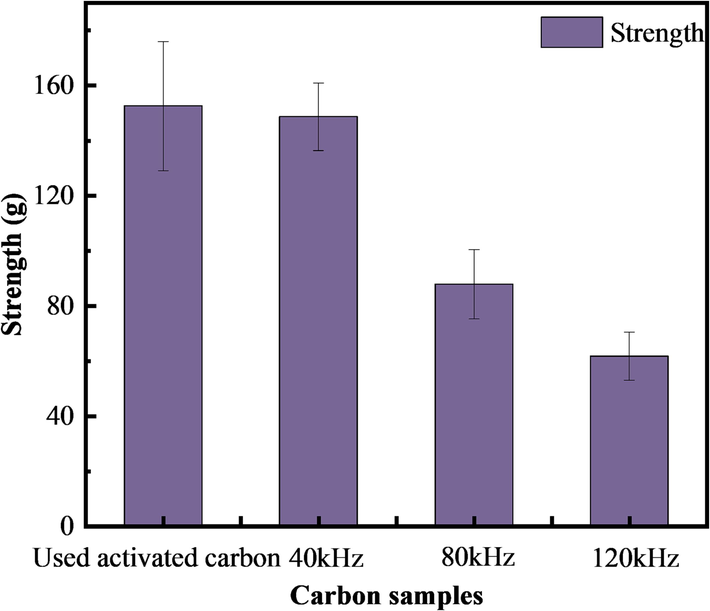
Variation of carbon strength activated under different ultrasonic frequencies (regeneration time 10 min, gas impulse strength 1.0 L/min, power 360 W).
According to Fig. 8, the crushing force of the used activated carbon in the water plant is 152.08 g. The crushing force of the carbon regenerated at ultrasonic frequencies of 40, 80, and 120 kHz is 148.60, 87.81, and 67.71 g, respectively. Thus, at a low ultrasonic frequency, the strength of the activated carbon (the crushing force) is almost unchanged, and it only decreases slightly. The higher the frequency, the lower the strength of the carbon. For the high ultrasonic frequency treatment (120 kHz), the strength of the activated carbon is less than half of the strength of the used activated carbon from the water plant. Specifically, its strength decreases by 55.44 %, which may be due to the fact that the higher the frequency of the ultrasonic waves, the higher the corresponding ultrasonic energy, resulting in the activated carbon internal pore space being more developed. Therefore, the activated carbon strength is bound to decrease (Zhao et al., 2023), allowing pulverization and crushing to occur. And according to Fig. 7, the particle sizes of both the used activated carbon and the regenerated carbon are mainly distributed at < 0.45 mm, accounting for about 10 % of the total, indicating that this activated carbon belongs to the small particle size category, and according to the “Coal Granular Activated Carbon for Water Purification” (GB/T 7701.2–2008), the proportion of activated carbon with particle size of < 0.45 mm and > 1.60 mm should be less than 5 %. Activated carbon with a smaller particle size may exhibit less strength and is prone to pulverization.
In the process of activated carbon use, it is subjected to wear and tear, and the strength of activated carbon is related to its service life. Some studies have shown that after two years of operation in a biological activated carbon filter in a water plant, the strength of activated carbon decreases from 90 % to 86 % (Li, 2016), while other studies have shown that after six or seven years of use, its strength is still more than 90 % (Gao, 2018). Thus, the attenuation of the strength of activated carbon is related to a variety of factors.
3.3.5 XPS
XPS provides information on the elements inside a material or covering its surface (Marandi et al., 2021) and qualitatively analyze the elements by comparing the different binding energies of the elements and the intensity of their corresponding peaks. XPS scans of activated carbon before and after regeneration were performed to assess the changes in their elemental composition. The XPS spectra of activated carbon before and after regeneration are presented in Fig. 9. There is no change in the elemental composition of activated carbon before and after ultrasonic regeneration. The peaks observed are C 1 s, O 1 s, N 1 s, Al 2p, and Si 2p, indicating that the main elements are C and O, with traces of N, Al, and Si. The presence of Al 2p and Si 2p peaks may be due to coagulation and precipitation processes in the drinking water treatment process. In addition, the O atom content of the regenerated activated carbon is higher compared with that of the used activated carbon, indicating that the increase in the surface O content of the ultrasonically treated activated carbon may be a factor affecting its adsorption performance.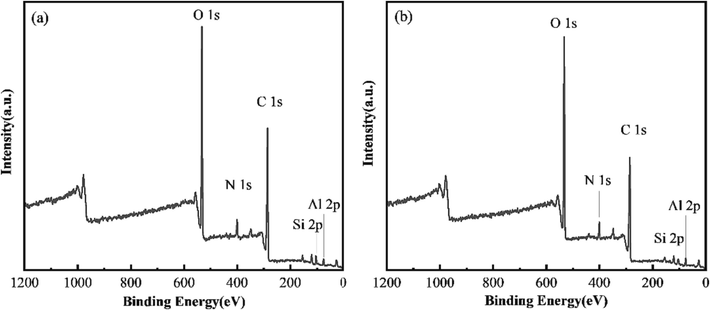
XPS full spectra of activated carbon. (a) used activated carbon; (b) regenerated carbon (40 kHz, 1 L/min, 10 min, 360 W).
The C 1 s and O 1 s peak profiles are shown in Fig. 10. The used activated carbon presents two peaks at 285.43 and 532.25 eV related to C 1 s and O 1 s, respectively, and similar peaks are observed at 285.56 and 532.33 eV for the carbon regenerated by ultrasonic backwashing. The XPS corresponding to the carbon is given in Fig. 10a, b spectra, the high-resolution XPS spectra show a complex envelope showing a variety of carbon states on the surface of the carbon. As can be seen from the figure, the C 1 s spectrum can be deconvoluted into three peaks. C 1 s (1), C 1 s (2), and C 1 s (3) are attributed to the C atoms (Yang et al., 2020), the carbons in the carbonyl groups (Puziy et al., 2008), and the carboxyl or ether groups (Xu et al., 2022), respectively. The contents of several functional groups in the C 1 s spectrum are listed in Table 5. According to Table 5, the content of C 1 s (3) band decreases by 16.85 % after ultrasonic treatment, the possible reason for which is the reduction of carboxyl group to alcohol group (Marandi et al., 2022). In addition, the content of C 1 s (2) and C 1 s (3) decreases and the content of C 1 s (1) increases, which may be the result of the cavitation generated by ultrasonic waves.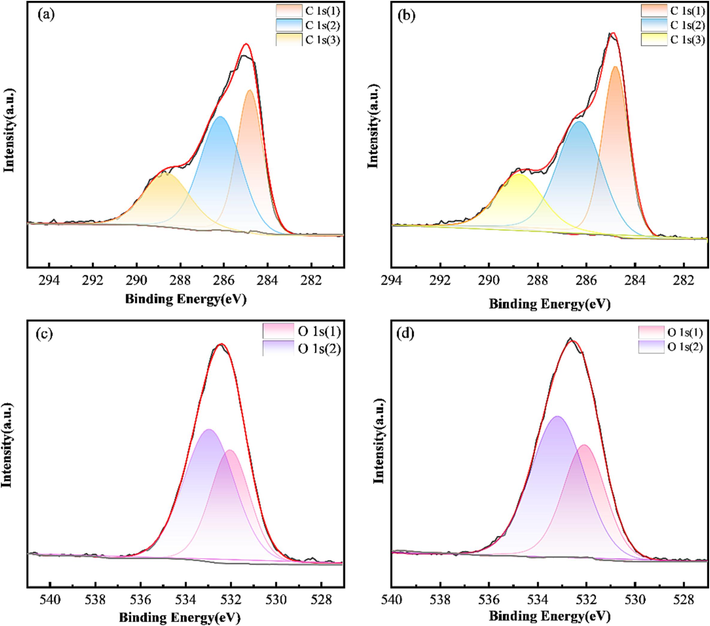
C 1 s XPS spectra. (a) Used activated carbon; (b) regenerated activated carbon (40 kHz, 1 L/min, 10 min, 360 W). O 1 s XPS spectra. (c) Used activated carbon; (d) regenerated activated carbon (40 kHz, 1.0 L/min, 10 min, 360 W).
Sample
Distribution of carbon functional groups (%)
C (%)
C 1 s (1)
C 1 s (2)
C 1 s (3)
Used activated carbon
20.63
54.31
25.05
99.99
Regenerated carbon
33.13
46.03
20.83
99.99
Fig. 10c and 10d show O 1 s scans of the activated carbon before and after regeneration. The O 1 s peak deconvolution provides relevant information about the surface oxygen groups. In this sense, the back-convolution of the O 1 s spectrum results in two peaks: the O 1 s (1) peak centered around 532 eV, which corresponds to carbonyl and hydroxyl groups (C = O, R-OH) in lactones, esters, as well as phenols (Jiang et al., 2020), and the O 1 s (2) peak centered at around 533 eV, which corresponds to lactone and acid anhydride groups (Sánchez-Díaz et al., 2022). The functional group binding energies and peak intensities of activated carbon are enhanced after ultrasonic treatment, and after regeneration, there is no significant change in the O 1 s region for activated carbon.
3.4 Regeneration mechanism
Based on the above characterizations of the activated carbon regenerated by ultrasonic backwashing, the regeneration mechanism is analyzed, the mechanism diagram is shown in Fig. 11. The characteristic XPS peak composition of the activated carbon before and after regeneration (Fig. 10) is basically unchanged, with some changes in the binding energies and peak intensities of the C and O elements, which are probably related to the cavitation effect of ultrasonic waves, and C = O bond broken, oxygen content increased, further indicating the impurity shedding on the used activated carbon. The irregularity and sharp edges of the regenerated carbon surface (Fig. 4) also indicate that the energy generated acts on the pores of the activated carbon surface, thus partially altering its pore structure and generating localized high temperatures that contribute to the removal of impurities.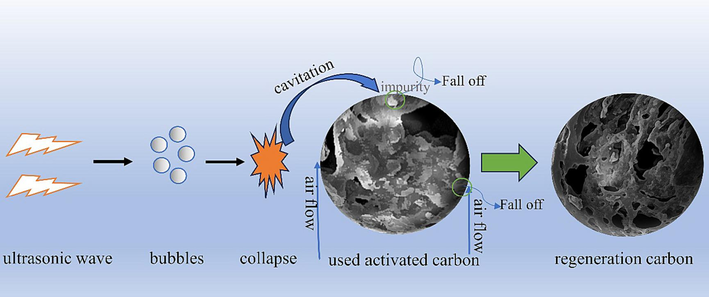
Mechanism of the regeneration of activated carbon by ultrasound-backwashing.
4 Conclusion
In this study, ultrasonic-backwashing was used to reactivate used activated carbon from a water plant. The use of backwash on the fluidization of activated carbon, so that ultrasound can have a more comprehensive ultrasonic effect on the activated carbon. The effects of different ultrasonic frequencies on the regeneration of activated carbon were compared, and it was found that an increase in ultrasonic frequency significantly improves regeneration efficiency, when the ultrasonic frequency is 120 kHz, the regeneration efficiency of the activated carbon can reach 32.88 %. The higher the ultrasonic power, the more significant the foulant-desorption effect. The regeneration efficiency of activated carbon increases and then decreases with the increase of backwashing frequency and regeneration time. SEM revealed that increasing ultrasonic frequency more effectively dislodges adherents on the surface of the activated carbon. The specific surface areas of the activated carbon before and after regeneration were analyzed, revealing that the micropore content of the activated carbon is directly proportional to the frequency of the ultrasonic waves, it was further shown that the adsorption performance of regenerated charcoal increased with the increase of ultrasonic frequency.
The strength of the activated carbon decreases after ultrasonic treatment, which is related to many factors, including service life and original performance of activated carbon. XPS results showed that ultrasonic regeneration does not affect the elemental composition of the activated carbon, but the contents of functional groups changes, indicating that the ultrasonic backwashing regeneration method has no effect on the structural properties of the activated carbon itself. Therefore, the ultrasonic-backwashing process for the regeneration of used activated carbon has considerable economic and environmental benefits and helps to advance the transition of water purification plants to a low-carbon model and reduce the generation of waste carbon.
CRediT authorship contribution statement
Binbin Wu: Conceptualization, Formal analysis, Data curation, Visualization, Writing – original draft. Bingjie Zhou: Writing – review & editing. Zhendong Liu: Writing – review & editing. Lu Li: Data curation. Kemei Zhou: Supervision, Writing – review & editing. Zhiwei Wang: Data curation. Yuanxiang Shan: Data curation. Wanting Feng: Data curation. Ziwen Shao: Writing – review & editing. Hongqin Xue: Writing – review & editing. Zheng Wang: Supervision, Project administration, Methodology, Writing – review & editing.
Acknowledgments
The authors express their sincere gratitude to the Nanjing Water Group Co., Ltd. Technology Innovation Project (YF2022-003).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Stability of extemporaneously prepared sodium benzoate Oral suspension. Int. J. Pharm. Compd.. 2018;22:326-328.
- [Google Scholar]
- The determination of pore volume and area distributions in porous substances. I. Computations from nitrogen isotherms. J. Am. Chem. Soc.. 1951;73:373-380.
- [CrossRef] [Google Scholar]
- Preparation, characterization and methylene blue adsorption of phosphoric acid activated carbons from globe artichoke leaves. Fuel Process. Technol.. 2011;92:1203-1212.
- [CrossRef] [Google Scholar]
- Influence of ultrasound on adsorption processes. Ultrason. Sonochem.. 2001;8:277-283.
- [CrossRef] [Google Scholar]
- Effect of ultrasound on adsorption and desorption processes. Ind. Eng. Chem. Res.. 2003;42:5635-5646.
- [CrossRef] [Google Scholar]
- Coke powder improving the performance of desulfurized activated carbon from the cyclic thermal regeneration. Chem. Eng. J.. 2022;448:137459
- [CrossRef] [Google Scholar]
- Evaluation of efficiency and capacity of thermal, chemical and ultrasonic regeneration of tetracycline exhausted activated carbon. Environ. Technol.. 2022;43:907-917.
- [CrossRef] [Google Scholar]
- Feasibility of electrochemical regeneration of activated carbon used in drinking water treatment plant. reactor configuration design at a pilot scale. Process Saf. Environ. Prot.. 2021;148:846-857.
- [CrossRef] [Google Scholar]
- Effect of concentration and flow rate of electrolyte on electrochemical regeneration of activated carbon at pilot-plant scale. J. Electroanal. Chem.. 2023;946:117727
- [CrossRef] [Google Scholar]
- Study on the effect of oxidation-ultrasound treatment on the electrochemical properties of activated carbon materials. Ultrason. Sonochem.. 2020;69:104921
- [CrossRef] [Google Scholar]
- Microwave regeneration of granular activated carbon saturated with PFAS. Water Res.. 2021;198:117121
- [CrossRef] [Google Scholar]
- Selection, failure determination and operation management of activated carbon in water supply deep treatment process. Water Purif. Technol.. 2018;37:1-5.
- [CrossRef] [Google Scholar]
- Effects of ultrasound on adsorption–desorption of p-chlorophenol on granular activated carbon. Ultrason. Sonochem.. 2003;10:109-114.
- [CrossRef] [Google Scholar]
- Desorption of metal ions from activated carbon in the presence of ultrasound. Ind. Eng. Chem. Res.. 2005;44:4737-4744.
- [CrossRef] [Google Scholar]
- Ultrasonic desorption of p-chlorophenol from granular activated carbon. Chem. Eng. J.. 2005;106:153-161.
- [CrossRef] [Google Scholar]
- Adsorptive removal of CO2 on highly microporous activated carbons prepared from Eucalyptus camaldulensis wood: effect of chemical activation. J. Taiwan Inst. Chem. Eng.. 2014;45:579-588.
- [CrossRef] [Google Scholar]
- Activated carbons prepared by indirect and direct CO2 activation of lignocellulosic biomass for supercapacitor electrodes. Renew. Energy.. 2020;155:38-52.
- [CrossRef] [Google Scholar]
- Role of the physico-chemical factors in the purification process of water from surface-active matter by biosorption. Water Res.. 2002;36:5132-5140.
- [CrossRef] [Google Scholar]
- A review: potential and challenges of biologically activated carbon to remove natural organic matter in drinking water purification process. Chemosphere.. 2017;167:120-138.
- [CrossRef] [Google Scholar]
- Activation temperature and particle size of palm kernel shell vs. the surface properties of activated carbon. BioResources.. 2023;18:1714-1730.
- [CrossRef] [Google Scholar]
- Experimental study on regeneration of saturated bioactivated carbon in water plants. Shandong University of Architecture 2016
- [CrossRef] [Google Scholar]
- Effect of ultrasound on desorption kinetics of phenol from polymeric resin. Ultrason. Sonochem.. 2006;13:225-231.
- [CrossRef] [Google Scholar]
- Quantitative effects of amination degree on the magnetic iron oxide nanoparticles (MIONPs) using as adsorbents to remove aqueous heavy metal ions. J. Hazard. Mater.. 2017;335:47-55.
- [CrossRef] [Google Scholar]
- Performance and mechanism of low-frequency ultrasound to regenerate the biological activated carbon. Ultrason. Sonochem.. 2017;34:142-153.
- [CrossRef] [Google Scholar]
- Comparison of two typical regeneration methods to the spent biological activated carbon in drinking water. Environ. Sci. Pollut. Res.. 2020;27:16404-16414.
- [CrossRef] [Google Scholar]
- Variation in the biological characteristics of BAC during ultrasonic regeneration. Ultrason. Sonochem.. 2020;61:104689
- [CrossRef] [Google Scholar]
- Experimental study on Fenton oxidation regeneration of adsorbed toluene saturated activated carbon. Environ. Technol.. 2022;43:524-533.
- [CrossRef] [Google Scholar]
- Fabrication of activated carbon sulfuric acid as an excellent and novel solid acid catalyst, evaluating its catalytic activity in synthesizing 1,8-dioxo-octahydroxanthenes and 14-aryl-14H-dibenzo[a, j]xanthenes. Res. Chem. Intermed.. 2021;47:3145-3163.
- [CrossRef] [Google Scholar]
- Immobilization of –OSO3H on activated carbon powder and its use as a heterogeneous catalyst in the synthesis of phthalazine and quinoline derivatives. Diamond Relat. Mater.. 2022;124:108908
- [CrossRef] [Google Scholar]
- 1 - an introduction to the uses of power ultrasound in chemistry. In: Practical Sonochemistry ((Second Edition).). Woodhead Publ; 2002. p. :1-48.
- [CrossRef] [Google Scholar]
- Thermal and chemical regeneration of spent activated carbon and its adsorption property for toluene. Chem. Eng. J.. 2012;210:500-509.
- [CrossRef] [Google Scholar]
- XPS and NMR studies of phosphoric acid activated carbons. Carbon.. 2008;46:2113-2123.
- [CrossRef] [Google Scholar]
- Elucidating the low-frequency ultrasound effect on mass transfer resistances during phenol adsorption over microporous materials. J. Water Process Eng.. 2022;47:102790
- [CrossRef] [Google Scholar]
- Regeneration of activated carbon adsorbent by anodic and cathodic electrochemical process. Process Saf. Environ. Prot.. 2022;159:1150-1163.
- [CrossRef] [Google Scholar]
- Adsorption isotherms of microporous-mesoporous solids revisited. appl. catal. A.. 1995;129:157-165.
- [CrossRef] [Google Scholar]
- Biofilm processes in biologically active carbon water purification. Water Res.. 2008;42:2839-2848.
- [CrossRef] [Google Scholar]
- Characterization and ciprofloxacin adsorption properties of activated carbons prepared from biomass wastes by H3PO4 activation. Bioresour. Technol.. 2016;217:239-244.
- [CrossRef] [Google Scholar]
- Investigation on the sonocatalytic degradation of acid red B in the presence of nanometer TiO2 catalysts and comparison of catalytic activities of anatase and rutile TiO2 powders. Ultrason. Sonochem.. 2007;14:545-551.
- [CrossRef] [Google Scholar]
- Enhanced regeneration of spent FCC catalyst by using oxalic acid-sulfuric acid mixture under ultrasonic irradiation. J. Mater. Res. Technol.. 2021;15:7085-7099.
- [CrossRef] [Google Scholar]
- Magnetically separable fe-base deposited on different carbon sources for ultrasound/persulfate-like heterogeneous activation: optimized synthesis and field driving process. Chemosphere.. 2022;298:134270
- [CrossRef] [Google Scholar]
- Mechanistic insights into adsorptive and oxidative removal of monochlorobenzene in biochar-supported nanoscale zero-valent iron/persulfate system. Chem. Eng. J.. 2020;400:125811
- [CrossRef] [Google Scholar]
- Ultrasonic-thermal regeneration of spent powdered activated carbon. Sustainability.. 2023;15
- [CrossRef] [Google Scholar]
- FeOCl-confined activated carbon for improving intraparticle Fenton-like oxidation regeneration. J. Hazard. Mater.. 2023;442:130026
- [CrossRef] [Google Scholar]
- Study on the mechanical strength and iodine adsorption behavior of coal-based activated carbon based on orthogonal experiments. Energy.. 2023;282:128450
- [CrossRef] [Google Scholar]
- Characteristics of ultrasonically enhanced low-temperature thermal regeneration of powdered activated carbon: a case study of acetone and aniline. Water.. 2021;13
- [CrossRef] [Google Scholar]
- Using activated carbon electrode in electrosorptive deionisation of brackish water. Desalination.. 2008;225:329-340.
- [CrossRef] [Google Scholar]







