Translate this page into:
Adsorption of organochlorine pesticides on modified porous Al30/bentonite: Kinetic and thermodynamic studies
⁎Corresponding author. rfarghaly@sci.cu.edu.eg (R.A. Farghali)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Adsorption of pesticides (heptachlor epoxide, dieldrin and endrin) onto modified bentonite by Keggin cation [Al30O8(OH)56(H2O)24]18+ denoted Al30 cation to form composite (Al30/B), has been investigated as a possible alternative method for their removal from aqueous solutions. The study was aimed to use a low-cost material as a step towards cleaner environment. Interestingly, these chemical modifications altered the physicochemical characteristics of bentonite in term of morphology, surface area and functionality which has been confirmed by using nitrogen adsorption–desorption isotherm, scanning electronic microscopy (SEM) and X-ray diffraction (XRD). Gas chromatography coupled to mass spectrometry (GC–MS) was used to identify and analyze the pesticides. Different physicochemical parameters were analyzed: contact time, adsorbent dose, pH, and temperature. The results showed that the removal percentage of pesticides on Al30/B was the highest at contact time of 5 h, adsorbent dosage of 25 mg, at pH 7.5, and at optimum temperature of 45 °C. Furthermore, the Kinetic study indicated that the adsorption of pesticides on Al30/B was well adapted to the pseudo-first order kinetic with a correlation coefficient near unity. The results of adsorption were fitted to the Langmuir and Freundlich isotherms. The Freundlich model represented the adsorption process better than Langmuir model, with correlation coefficients (R2) values range from 0.986 to 0.989. The Thermodynamic study suggested that the adsorption of pesticides was chemisorption, spontaneous and endothermic process. Therefore, Al30/B composite can be utilized effectively for removal of pesticides with efficiency up to 98%.
Keywords
Natural bentonite
Keggin Al30
Porous materials
Chlorinated pesticides
Adsorption
1 Introduction
Among the organic pollutants, the cyclodienes such as endosulfan, endrin, dieldrin and heptachlor are usually used as insecticides. They have been established to be highly toxic to aquatic life, as they exhibit carcinogenic activity, low biodegradability, and highly bio-concentrate in organisms at various trophic levels (Bandala et al., 2006; Kapustka et al., 1996). Indeed, serious environmental problems have been arisen from the release of organic pollutants into wastewaters (Kalnay & Cai, 2003; Ouyang et al., 2006). Therefore, the removal of organic pollutants from the aqueous phase is of a great interest. Among several chemical and physical methods, adsorption is one of the most useful techniques that has been successfully employed for organic pollutants removal from wastewater because of simplicity of design, ease of operation and convenience (Bhatnagar et al., 2015; De Gisi et al., 2016). Activated carbon, as commercial adsorbent, is very effective but expensive due to their high costs of manufacturing and regeneration (Amin et al., 2015). Accordingly, research interest is focused on natural materials that remove dyes from wastewater as they are affordable eco-friendly materials (F. Allouche & Yassaa, 2018).
Bentonite is a clay mineral of phyllosilicate groups, occurs as a silicate layers in a three-phase crystalline structure that promotes intrinsic characteristics. The clay mineral surface poses a negative charge owning to the edge hydroxyls groups, giving raises to intermolecular hydrogen bonds with hydrophilic medium. Furthermore, the presence of inorganic cations can stabilize its silicate layers by Van der Waals forces formation (Motawie et al., 2014). Additionally, the surface Brønsted, Lewis acid sites and ion exchange sites are the main reasons for bentonite clay excellent absorptivity. It is well known, bentonite as a natural mineral, is eco-friendly, inexpensive, and accessible (Issaabadi et al., 2017).
Actually, introducing intercalating long chains and/or grafting with different functional groups, interlayer spacing of clay will have influenced results in enhancing its mechanical and physical properties especially adsorption ability (Mockovčiaková & Orolínová, 2009). Nowadays, the modification of clay by several chemical and physical methods shows extensive attention. Magnetic modification in which, e.g., bentonite coated with iron can be obtained (Oliveira et al., 2003), the use of catalyst support as surfactant or nanomaterials (Bhorodwaj & Dutta, 2010), and the addition of ceramic materials with their refractory nature, good chemical stability, high-temperature strength, low thermal conductivity and expansion coefficient will influence clay properties. Lately, inorganic polycationic species has been widely studied, as these materials converted into a firm metal oxide pillars after calcination. The pillars got inserted between the sheets of the clay mineral and kept them separated from each other, which prevents the collapse of the structure on high temperature application use (Gil et al., 2000). The most commonly used polycation in the pillaring process is the Keggin polycation, [Al13O4(OH)24(H2O)12]7+ denoted Al13, due to its big size (0.9 nm), high charge (+7), so after using as a pillaring agent it yields large surface area and thermally stable composite (Johansson, 1960).
The aim of this work is improving the specific surface area and pore size and in turn adsorption property of bentonite by combining with aluminum Keggin polycation. The recently discovered Al30 Keggin cation was used in pillaring bentonite because this cation is promising due to its bigger size (1.0 nm) and even higher charge (+18) than Al13 (Zhu et al., 2017). The preparation of Al30/B composite and possible mechanisms of pesticides adsorption were presented. In the present work, the structure and morphology of the modified bentonite were confirmed by different techniques. The effect of the adsorption conditions on adsorption capacity of pesticides onto the modified bentonite was extensively investigated. Finally, the experimental data were described using kinetic and adsorption isotherm models. Simultaneously, the heats of adsorption and the Gibbs free adsorption energy were predicted from the thermodynamic study at equilibrium adsorption isotherms.
2 Experimental
2.1 Method and material
Commercial bentonite as starting material was purchased from (CMB Co., Egypt) and its cation exchange capacity CEC value was measured by [Co(NH3)6]3+ adsorption method and found to be 0.56 meq/g (Aran et al., 2008). Pesticides standard set including heptachlor epoxide, dieldrin and endrin were purchased from (AccuStandard, Inc., USA). The other materials that used in this study including anhydrous Na2SO4, AlCl3·6H2O, Ba(NO3)2, o-phenanthroline, Ferron, hydroxylamine, AgNO3 were obtained from (SIGMA-ALDRICH Co., Germany) and all are of analytical grade.
2.2 Synthesis of Al30-modified bentonite
Al30-modified bentonite (Al30/B) was prepared similar to the methods described in previous studies (Aouad et al., 2006; Chen et al., 2007). A 0.154 g of Al30 nitrate was grinded with 1.0 g bentonite till homogeneity and stirred in a water bath shaker for one hour at 30 °C. It was then kept soaked in the solution for 24 h. Then the Al30/B filtered from the mixture, washed with distilled water and, air dried for 24 h, then calcined at 300 °C for three hours.
2.3 Surface characterizations
Ferron assay has been performed as an important method for aluminum speciation analysis (Jardine & Zelazny, 1986). Absorbance of Al-Ferron complexes were monitored by using advanced (HACH, DR3900 UV–visible spectrophotometer-USA). Scanning electron microscopy (SEM) images were examined with (QUANTA FEG250 instrument-Netherlands). The specific surface area and pore size of the adsorbents were carried out by nitrogen-adsorption/desorption method (Gregg et al., 1967) at 77 K (boiling point of nitrogen) with the aid of gas sorption analyzer (Nova touch LX2, surface area and pore size analyzer–Quanta Chrome instruments). X-ray diffraction technique was conducted to Al30/B sample using Cu Kα beam of wavelength λ = 1.54 Å emitted from D8 Discover, Bruker diffractometer–Germany, working at potential 40 kV and current 40 mA and a scanning rate of 1 scan/0.01° and 2θ° range between 3° and 80°.
2.4 Pesticides adsorption, separation and quantification
Batch adsorption experiments were performed by adding known amounts of Al30/B to 25 mL solutions of 0.2 mg/L heptachlor epoxide, dieldrin and endrin each. The pH of the tested solutions were adjusted using 0.1 M NaOH and 0.1 M HCl solutions to study the effect pH in the range from (3–10). The mixture was placed in a water bath shaker (120 rpm) for various temperature values ranging from 25 to 55 °C and duration time ranging from (0.5–20 h). After adsorption and filtration the residual heptachlor epoxide, dieldrin and endrin in filtrate was extracted according to EPA 508 (Graves et al., 1988). The concentration of each pesticide was measured using gas chromatography device (Agilent Technologies, 7890A GC System) coupled with mass spectrometer (Agilent Technologies, 5975C inert MSD with triple-axis Detector). The amount of pesticide adsorbed on the material surface at time of contact (t) can be calculated from the following equation:
2.5 Adsorption, kinetics and thermodynamic studies
The pseudo-first order module describes the quantity of adsorbate on the heterogeneous adsorbent surface over time (t) (Lagergren, 1898), according to Eq. (3):
The pseudo-second order was established to simulate the real reaction as possible and considers a chemisorption reaction as the rate determining step (Ho, 2006). It is expressed by Eq. (4):
Many isotherms are established to prove the adsorption processes such as Langmuir and Freundlich isotherms which are valid for most of the adsorption reactions. Langmuir isotherm suggests that maximum adsorption capacity is a monolayer of adsorbate covers the adsorbent surface where the homogeneous surface possess equal chances for adsorbate molecules adsorption, and no interaction between adsorbate molecules themselves (Langmuir, 1918). The Langmuir equation is represented by:
On the other side, Freundlich isotherm module suggests an interaction between adsorbate molecules which represent surface heterogeneity (Freundlich, 1907), according to Freundlich equation:
The thermodynamic parameters, enthalpy (ΔH°, J/mole), Gibbs free energy (ΔG°, J/mole) and entropy (ΔS°, J/mole/K) were determined to define the adsorption reaction (Yang & Al-Duri, 2005).
3 Results and discussion
3.1 Structure and characterization of modified Al30/bentonite
3.1.1 Studies of time-developed Al-Ferron complex colorimetry
Ferron kinetic method was used to evaluate Al30 content in Al30/B. The absorbance/time curve of hydrolytic polymeric aluminum (HPA) solutions measured by Al-Ferron method is shown in Fig. 1a. The change in absorbance was monitored for 60 min and the Al reacted at 30 min was assumed to represent [Ala + Alb] (Parker & Bertsch, 1992). The quantity [Alc] was computed as the difference between [Al]T and [Ala + Alb]. The quantitative calculation results of three species, Al monomer (Ala), oligomer (Alb), and polymer (Alc), as well as rate constant (k) concluded that the real content of [Al30] is 78.2% which means success formation of keggin Al30 (Chen et al., 2007). With increasing total Al concentration, Ala and Alb contents decrease, while Alc content increases indicating Al30 polymeric formation. This could be explained based on the fact that during aluminum hydrolysis both Al13 and Al30 species are kinetically intermediate products and the highly charged Al30 is more stable than Al13 as it is consists of two δ-Al13 Keggin units connected by a crown made of four hexa-coordinated aluminum (AlO6) (L. Allouche et al., 2000; L. Allouche & Taulelle, 2003).![Structure and characterization of modified Al30/bentonite: (a) Timed Ferron colorimetric analysis of HPA solution ([Al]T = 2.4 × 10−5 M) showing the increment in absorbance with progress of time. (b) SEM images for natural bentonite (i and iii) and Al30/B (ii and iv) with different magnifications. (c) XRD pattern of raw bentonite and Al30/B composite.](/content/184/2020/13/8/img/10.1016_j.arabjc.2020.06.027-fig1.png)
Structure and characterization of modified Al30/bentonite: (a) Timed Ferron colorimetric analysis of HPA solution ([Al]T = 2.4 × 10−5 M) showing the increment in absorbance with progress of time. (b) SEM images for natural bentonite (i and iii) and Al30/B (ii and iv) with different magnifications. (c) XRD pattern of raw bentonite and Al30/B composite.
3.1.2 Morphological studies
SEM analyses have been used to compare the surface morphologies and micro-structures changes before and after modification of bentonite. Fig. 1b(i, iii) shows that raw bentonite displays rock-like macro particles with rough and heterogeneous surfaces. After Al30 modification, meso particles were noticed giving compact, large surface area and lamellae like structure were also observed on the surface of the Al30/B (Fig. 1b(ii, iv)), indicating the partial exfoliation of the bentonite layer during Al30 modification process.
The XRD patterns of the bentonite was studied to confirm the intercalation of the Al30 into the bentonite layers and presented in Fig. 1c. Sharp peak of quartz was observed in raw bentonite, 2θ, 27.0°, and attributed to impurity of the sample. During modification processes, the basal spacing reflection of bentonite shifts gradually from 2θ, 4.75° to 4.19°, implying the formation of micro and meso porous structure resulting from the Al polycation addition. Also, a broad peak was noticed at 22° after modification confirming that the Al30/B was successfully synthesized.
The raw and modified-bentonite samples showed a similar N2-adsorption/desorption isotherm behavior of H2-type hysteresis loop for aggregates of plate-like particles according to IUPAC classification giving rise to meso and micro pores (Fig. 2a) with higher performance for Al30/B if compared with raw bentonite. The microporosity of the Al30/B allows their use in the adsorption and separation of small ions, while the mesoporosity favors the adsorption of bigger molecules, such as aromatic compounds even biomolecules.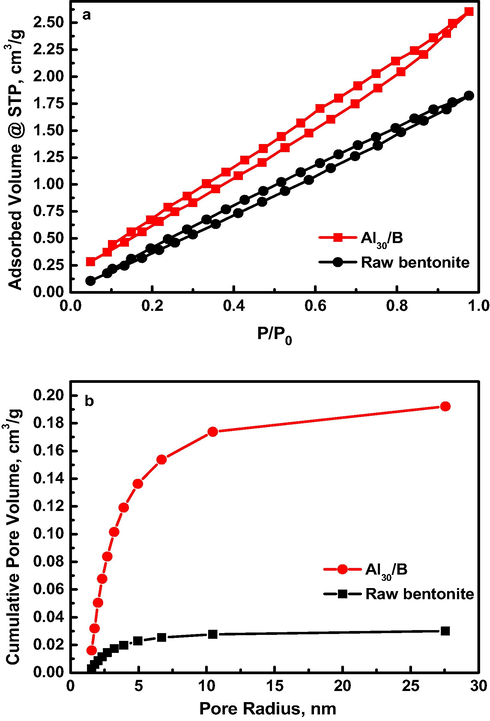
(a) N2 adsorption/desorption isotherms of raw bentonite and modified Al30/B composite and (b) their corresponding pore size distribution curves.
The specific surface areas were estimated to be 164.2 and 29.6 m2/g for modified and raw bentonite, respectively. The higher surface area of modified bentonite is attributed to generation of micro and meso pores after success pillaring of bentonite surface under the hydrothermal conditions. The raw and modified bentonite exhibited uniform pore size distribution of 5 and 10 nm, respectively (Fig. 2b). It was concluded that, Al30/B has more attractive receptor sites compared to raw-bentonite confirming the effect exerted by inserting Al30 onto bentonite layers, as proved by XRD characterization. Therefore, the higher pore size distribution for modified bentonite could play a significant role in pesticides adsorption.
3.2 Adsorption study of chlorinated pesticides
3.2.1 Effect of contact time and adsorbent dosage
The effect of contact time on adsorption of pesticides was explored in presence of 0.2 mg/L pesticides onto 25 mg of Al30/B in a solution of pH 7.5 at 25 °C (Fig. 3a). The results revealed that ∼95% of the three pesticides were adsorbed onto Al30/B surface in the first 5 h followed by equilibrium for the three chosen pesticides. The rapid increase in the adsorbed amount of pesticides was attributed to the available pores and vacant adsorption sites in Al30/B, which fill up gradually and the adsorption becomes slower with time till achieving complete saturation. It was observed that the adsorption capacities, qe, were 0.188, 0.196 and 0.191 mg/g after 5 h for heptachlor epoxide, dieldrin and endrin, respectively. The adsorbent dosage effect was also studied by varying the mass of Al30/B from 10 to 50 mg in a solution of pH 7.5 containing pesticides of 0.2 mg/L at 25 °C and for contacting time of 5 h (Fig. 3b). The removal efficiency of modified bentonite for pesticides was increased from 40% to 95%, which might be attributed to increase in the total surface area of modified bentonite sample (Alzahrani & Ahmed, 2016; Alzahrani et al., 2020; Mudzielwana et al., 2019; Nameni et al., 2008). It was observed that, as the adsorbent dosage increases, the removal efficiency increases till 25 mg adsorbent and then no significant effect was noticed. Consequently, the subsequent experiments were conducted during a contact time of 5 h and Al30/B dose of 25 mg as optimum conditions.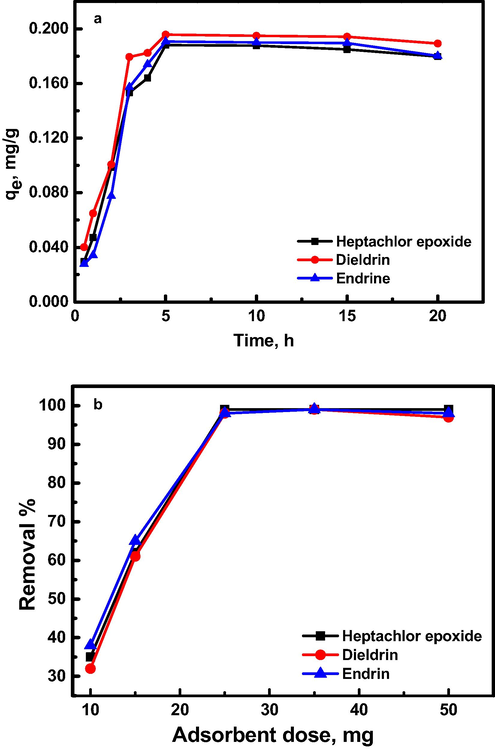
(a) Effect of contact time on the adsorption capacity of 0.2 mg/L organochlorine pesticides at pH 7.5 in presence of 25 mg of Al30/B dosage at 25 °C. (b) The removal percent of organochlorine pesticides (0.2 mg/L) as a function of Al30/B dose at natural pH 7.5 and ambient temperature 25 °C and with contacting time of 5 h.
3.2.2 Effect of solution pH
It is well known that, the adsorption is affected greatly by the pH of solution. All the tested pesticides have epoxide group, i.e. oxacyclopropane or oxirane which is the main active site in their structures. This group is strained and easily affected by adjacent substituents acids and bases (Grabowsky et al., 2011). In aqueous solution, oxacyclopropane group maintains a positive charge ion, while the bentonite surface is negatively charged. Thus, the solution pH affected both the surface charge of the modified bentonite and the degree of polarization of pesticides molecules. Thus, as the solution pH increases the adsorption of pesticides on modified bentonite increases (Fig. 4a). The adsorption is an electrophilic attack of pesticide carbon [C+] on bentonite surface. In presence of acid, there are two contrary actions; the first is the catalyzed ring breakage and a true positive charge on carbon atom according to the following mechanism.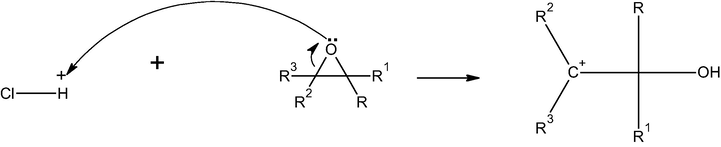
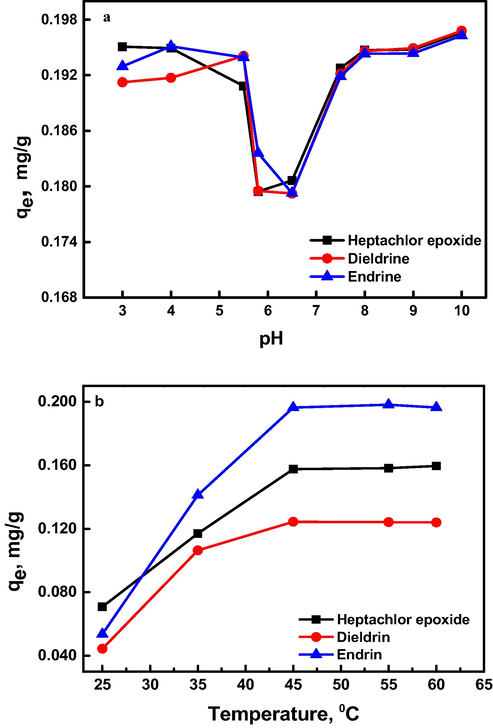
(a) Effect of solution pH on the adsorption capacity of 2.00 mg/L pesticides on 25 mg Al30/B dosage after 5 h at 25 °C. (b) Effect of solution temperature on the amount of pesticides (initially 2.00 mg/L) adsorbed on 25 mg Al30/B dosage at pH 7.5 after 5 h.
The true cationic pesticide moiety is enforced to adsorb on Al30/B and the amount of pesticides adsorbed increased as shown in Fig. 4a. The second is a competition between the H+ proton in the acid and the slightly positive carbon in pesticide molecule to adsorb on the Al30/B surface (Hameed et al., 2008). So the amount of pesticide adsorbed was decreased and this can be noticed at pH values 5.5 and 6.5 (i.e. in slightly acidic solutions). In case of basic solution, the OH group of the base attacks the slightly positive carbon and ring opening takes place to give a negatively charged ionized oxygen R-O− Na+ as shown in the mechanism.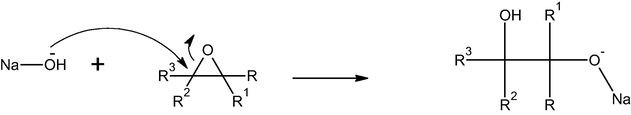
In addition, the Lewis acidity of bentonite was enhanced by pillaring with Al30 (TOMUL & Balci, 2008), and therefore the pillars of Al2O3 attract pesticides molecules of negatively oxygen at high pH so the amount of pesticides adsorbed increases with rise of pH as illustrated in Fig. 4a.
3.2.3 Effect of temperature
The effect of temperature on the adsorption of pesticides was also investigated in presence of 25 mg of modified bentonite sample and 0.2 mg/L initial pesticides concentration at pH 7.5 for 5 h contact time. The results showed that as the temperature increases the amount of pesticides adsorbed increases from 25 to 45 °C then no noticeable increase was observed up to about 60 °C (Fig. 4b). Hence, increasing temperature can affect solubility of pesticides and their orientation on bentonite surfaces (El-Sayed, 2011). Moreover, high temperature activates Al30/B active sites which enhance adsorption of sparingly soluble pesticides, so the optimum temperature was chosen to be 45 °C. In Table 1, the Adsorption capacities and other parameters for the removal of pesticides in different adsorbent materials and modifications of bentonite of the proposed method are compared with those obtained by some reported methods. In comparison with some other adsorbent materials, Al30/B showed advantages in several aspects. The modified bentonite is prepared in simple way. This gives the Al30/B more advantages over other adsorbent used in the literature. Al30/B bentonite showed relatively higher percent adsorption in neutral medium.
Adsorbents
Adsorbate
Surface Area/Particle Size of Adsorbent (m2/g)
Adsorption Capacity (mg/g)
Concentration Range (mg/L)
Contact Time (h)
Temp. (°C)
pH
Percent Adsorption
Reference
Triolein embedded activated carbon composite
dieldrin
721
0.002
0.001–0.05
48
25
4–8
—
Ru et al. (2007)
Pine bark sawdust
Heptachlor
—
—
2–1000
24
—
—
93.6
Ratola et al. (2003)
Organo-bentonite complexes
bromoxynil residues
—
—
1.2–31
48
5
3–7
88
El-Nahhal and Safi (2008)
Montmorillonite Kaolinite clays
endrin
14.1
17.7
500–5000
—
5.4–7.2
Peng et al. (2009)
Fe3O4 magnetic nanospheres coated with polystyrene
heptachlor epoxide
dieldrin
endrin129
—
0.01
1
25
93.3
Lan et al. (2014)
Al30/bentonite
heptachlor epoxide
dieldrin
endrin164.2
0.188
0.196
0.1910.2
5
45
7.5
95
Our work
3.3 Adsorption kinetics and isotherms
3.3.1 Adsorption kinetics
The kinetic of organochlorine pesticides removal by Al30/B have been evaluated in this study using the pseudo-first order and pseudo-second order kinetics. The various model parameters are illustrated in Table 2 and the fitting plots are illustrated in Fig. 5(a–b). A good fit for either kinetic model would require correlation coefficient (R2) values close to unity as well as calculated adsorption capacity (qe) values that come in a good agreement with the experimental values (Toor & Jin, 2012). In both regards, it was observed that the pseudo-first order kinetics gave the best fits and was more appropriate in describing the adsorption of pesticides on Al30/B than the second order kinetics. Although the correlation coefficient values for the latter are around 0.98, the experimental qe values do not agree with the calculated ones, obtained from the second order linear plots (Table 2).
Organochlorine pesticides
Pseudo-first order
Pseudo-second order
k1 (min−1)
qe (mg/g)
R2
k2 (g mg−1 min−1)
qe (mg/g)
R2
Heptachlor epoxide
0.0114
0.190
0.995
0.041
0.234
0.987
Dieldrin
0.0115
0.185
0.997
0.050
0.236
0.983
Endrin
0.0115
0.192
0.998
0.049
0.237
0.986
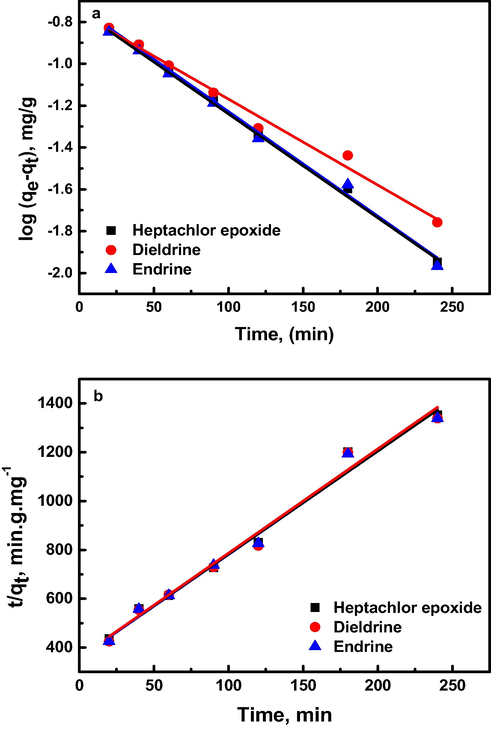
(a) Pseudo-first order and (b) Pseudo-second order kinetics for adsorption of organochlorine pesticides on Al30/B at 45 °C.
3.3.2 Adsorption isotherms
The equilibrium adsorption isotherm is fundamental in describing the interactive behavior between solute and adsorbent, and is important for the design of adsorption system Fig. 6a. The analysis of the isotherm data by fitting them to different isotherm models is an important step to find the suitable model that can be used for design purpose. Adsorption isotherm study is carried out on two well-known isotherms, Langmuir and Freundlich. Langmuir isotherm assumes monolayer adsorption onto a surface containing a finite number of adsorption sites of uniform strategies of adsorption with no transmigration of adsorbate in the plane of surface. While, Freundlich isotherm model assumes heterogeneous surface energies, in which the energy term in Langmuir equation varies as a function of the surface coverage. The applicability of the isotherm equation is compared by judging the correlation coefficients, R2. Table 3 shows the values of the parameters of the two isotherms and the related correlation coefficients. A comparison is also made between two isotherms plotted in Fig. 6(b-c), which shows the experimental data points and the fitted lines for the three organochlorine pesticides examined for different initial concentrations at pH 7.5 and 45 °C. As seen from Table 3, the Freundlich model yields better fit (R2 ∼ 0.99) than the Freundlich model (R2 ∼ 0.97). In addition, the maximum monolayer adsorption capacity, qm, calculated using Langmuir isotherm, as predicted by Eq. (5), do not agree with the experimental values (derived from the effect of contact time study at 5 h which are 0.188, 0.196 and 0.191 mg/g for heptachlor epoxide, dieldrin and endrin, respectively). As also illustrated in Table 3, the values of 1/n (the slope of Freundlich linear Eq. (7)) are 2.09, 2.18 and 2.15 for heptachlor epoxide, dieldrin and endrin, respectively. The three 1/n values are greater than one which indicate favorable and cooperative adsorption.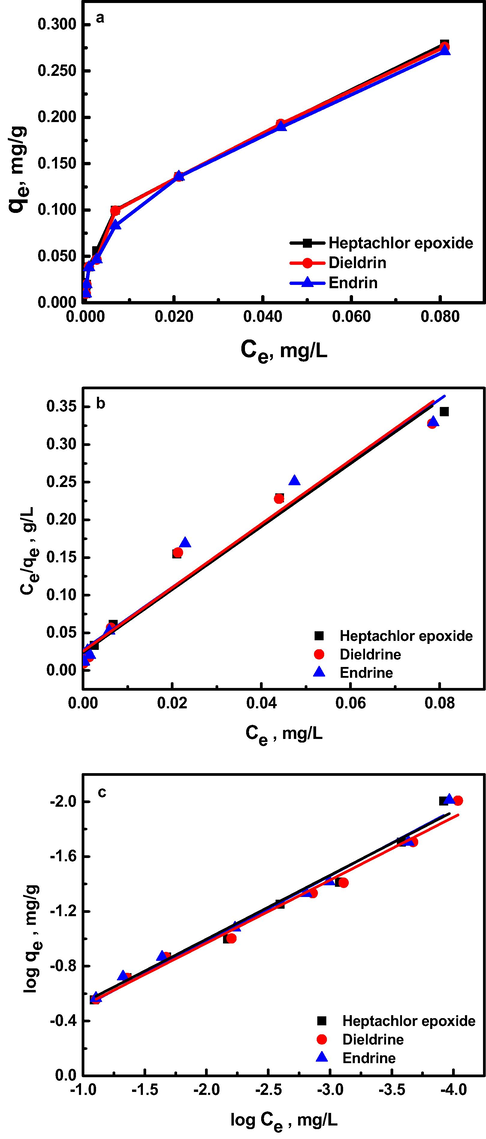
(a) The equilibrium adsorption (b) Langmuir adsorption and (c) Freundlich adsorption isotherms of organochlorine pesticides on Al30/B at 45 °C.
Organochlorine pesticides
Langmuir isotherm
Freundlich isotherm
qm (mg/g)
kL (L/mg)
R2
RL
KF
1/n
R2
Heptachlor epoxide
0.241
152.3
0.972
0.032
0.940
2.09
0.989
Dieldrin
0.239
175.7
0.966
0.028
0.889
2.18
0.986
Endrin
0.237
164.4
0.962
0.030
0.858
2.15
0.989
Langmuir isotherm assumes monolayer coverage on a homogeneous surface with identical adsorption sites. But these assumptions are valid for gas adsorption on solid surface. In solution-solid systems, with the hydration forces, mass transport effects etc. the system is much more dynamic and complicated, and Langmuir isotherm does not necessarily reflect the validity of the aforementioned assumptions. In such systems, the isotherm adequacy can be seriously affected by the experimental conditions, in particular, the range of concentration of the solute/adsorbate (Jeppu & Clement, 2012; Potgieter, 1991). In the current system, the adsorbate pesticide concentration range is significantly small and the Al30/B adsorbent possesses more available sites for extra adsorbates. Furthermore, the Al-pillared adsorbent has more pores and sites to catch pesticides molecules after the monolayer accomplished by a different way. In conclusion, Freundlich isotherm model shows the best fit compared to Langmuir isotherm for adsorption of organochlorine pesticides on Al30/B at the experimental conditions applied in this study.
3.3.3 Thermodynamic study
Fig. 7 shows a relation between ln KL versus 1/T represented as a straight line with the gradient (−ΔH°/R) from which ΔH° may be calculated using the least-squares method (Table 4) which confirms the endothermic nature of the process. This conclusion coincides with the results obtained from the effect of temperature experiment. In addition, the higher magnitude of ΔH° (>20 kJ. mol−1) suggests that the organochlorine pesticides are chemisorbed on Al30/B.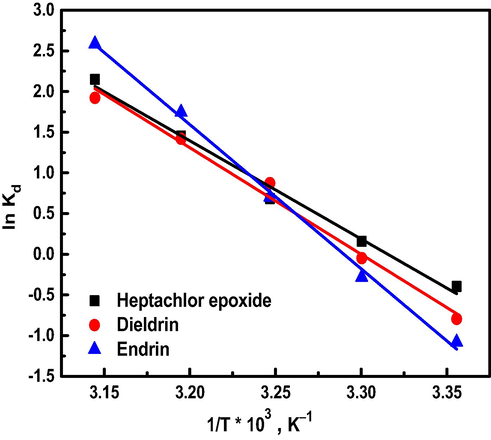
Plot of ln Kd against 1/T for adsorption organochlorine pesticides on Al30/B.
Organochlorine pesticides
ΔH°
kJ. Mol−1
ΔS°
kJ. Mol−1. K−1
ΔG° at 45 °C
kJ. Mol−1
Heptachlor epoxide
100
0.33
−4.57
Dieldrin
109
0.36
−5.38
Endrin
149
0.48
−6.81
The positive values of ΔS° (Table 4) suggest the increased randomness at the solid-solution interface with the increasing of temperature (Lian, Guo, & Guo, 2009). Taken into consideration the values of both ΔH° and ΔS°, the Gibbs free energy, ΔG°, at 45 °C was calculated as depicted in Table 4. The negative values of ΔG° for the three investigated pesticides indicates that the adsorption of these pesticides on Al30/B are spontaneous and feasible (Dawood & Sen, 2012).
4 Conclusion
A Successful Al30/B was prepared from raw bentonite and Al30 and was used in adsorption of three important pesticides from aqueous solutions. The Al30/B has more porosity and larger surface area compared to raw-bentonite confirming the effect exerted by Al30 cations on the surface of bentonite, as proved by SEM and XRD characterization. The results of N2 adsorption-desorption isotherms indicated that the new mesoporous and microporous structure was significantly increased after the insertion of Al30 allowing adsorption of large organic molecules. The parameters affecting the adsorption of pesticides were studied. The results obtained showed that the optimal adsorbent dose and the contact time to reach equilibrium were 25 mg and 5 h, respectively. Also, the adsorption was strongly influenced by temperature which had a positive effect on pesticides adsorption by Al30/B. In addition, the adsorption of pesticides was better at neutral pH. The overall kinetic study showed pseudo first-order kinetic model with correlation coefficient close to unity. Adsorption behavior was best described by a Freundlich type isotherm. The thermodynamic study indicated that the adsorption was chemisorption, spontaneous and endothermic in nature. Al30/B has a promising potential in environment fields as an effective, economical, and ecological adsorbent for the removal of pesticides from aqueous medium.
Acknowledgment
The authors express appreciation to the Department of Chemistry, Cairo University and would like to thank Central Laboratories for Environmental Quality Monitoring (CLEQM), National Water Research Center (NWRC), Egypt for technical and consulting support.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Potential adsorption of methylene blue from aqueous solution using green macroalgaePosidonia oceanica. In: Paper presented at the IOP Conference Series: Materials Science and Engineering. 2018.
- [Google Scholar]
- Conversion of Al13 Keggin into Al30: a reaction controlled by aluminum monomers. Inorg. Chem. Commun.. 2003;6(9):1167-1170.
- [Google Scholar]
- Synthesis of copper nanoparticles with various sizes and shapes: Application as a superior non-enzymatic sensor and antibacterial agent. Int. J. Electrochem. Sci.. 2016;11:4712-4723.
- [CrossRef] [Google Scholar]
- TiO2NPs embedded in chitosan membrane for efficient photodegradation of various dyes. Orient. J. Chem.. 2020;36:144-160.
- [CrossRef] [Google Scholar]
- Adsorptive removal of reactive black 5 from wastewater using bentonite clay: isotherms, kinetics and thermodynamics. Sustainability. 2015;7(11):15302-15318.
- [Google Scholar]
- Al-pillared montmorillonite obtained in concentrated media. Effect of the anions (nitrate, sulfate and chloride) associated with the Al species. Clays Clay Miner.. 2006;54(5):626-637.
- [Google Scholar]
- A spectrophotometric measurement of soil cation exchange capacity based on cobaltihexamine chloride absorbance. C.R. Geosci.. 2008;340(12):865-871.
- [Google Scholar]
- Removal of aldrin, dieldrin, heptachlor, and heptachlor epoxide using activated carbon and/or Pseudomonas fluorescens free cell cultures. J. Environ. Sci. Health Part B. 2006;41(5):553-569.
- [Google Scholar]
- Agricultural waste peels as versatile biomass for water purification – a review. Chem. Eng. J.. 2015;270:244-271.
- [Google Scholar]
- Heteropoly acid supported modified Montmorillonite clay: an effective catalyst for the esterification of acetic acid with sec-butanol. Appl. Catal. A. 2010;378(2):221-226.
- [Google Scholar]
- Effect of thermal treatment on the formation and transformation of Keggin Al13 and Al30 species in hydrolytic polymeric aluminum solutions. Colloids Surf. A: Physicochem. Eng. Aspects. 2007;292(2–3):110-118.
- [Google Scholar]
- Removal of anionic dye Congo red from aqueous solution by raw pine and acid-treated pine cone powder as adsorbent: equilibrium, thermodynamic, kinetics, mechanism and process design. Water Res.. 2012;46(6):1933-1946.
- [Google Scholar]
- Characteristics and adsorption capacities of low-cost sorbents for wastewater treatment: a review. Sustain. Mater. Technol.. 2016;9:10-40.
- [Google Scholar]
- El-Nahhal, Y., Safi, J.M., 2008. Removal of pesticide residues from water by organo-bentonites. IWTC12, Alexandria, Egypt 1711.
- Removal of methylene blue and crystal violet from aqueous solutions by palm kernel fiber. Desalination. 2011;272(1–3):225-232.
- [Google Scholar]
- Recent advances in the synthesis and catalytic applications of pillared clays. Catal. Rev.. 2000;42(1–2):145-212.
- [Google Scholar]
- Effect of electron-withdrawing substituents on the epoxide ring: an experimental and theoretical electron density analysis of a series of epoxide derivatives. J. Org. Chem.. 2011;76(5):1305-1318.
- [Google Scholar]
- Graves, R., Schirmann, D.M., Ciampone, A.M., 1988. Method 508; determination of chlorinated pesticides in water by gas chromatography with an electron capture detector Methods for the determination of organic compounds in drinking water. EPA. pp. 171–198.
- Equilibrium modeling and kinetic studies on the adsorption of basic dye by a low-cost adsorbent: coconut (Cocos nucifera) bunch waste. J. Hazard. Mater.. 2008;158(1):65-72.
- [Google Scholar]
- Review of second-order models for adsorption systems. J. Hazard. Mater.. 2006;136(3):681-689.
- [Google Scholar]
- Green synthesis of the copper nanoparticles supported on bentonite and investigation of its catalytic activity. J. Cleaner Prod.. 2017;142:3584-3591.
- [Google Scholar]
- Mononuclear and polynuclear aluminum speciation through differential kinetic reactions with ferron 1. Soil Sci. Soc. Am. J.. 1986;50(4):895-900.
- [Google Scholar]
- A modified Langmuir-Freundlich isotherm model for simulating pH-dependent adsorption effects. J. Contaminant Hydrol.. 2012;129:46-53.
- [Google Scholar]
- On the crystal structures of some basic aluminum salts. Acta Chem. Scand.. 1960;14(3):771-773.
- [Google Scholar]
- Evaluating risk predictions at population and community levels in pesticide registration-hypotheses to be tested. Environ. Toxicol. Chem.: Int. J.. 1996;15(4):427-431.
- [Google Scholar]
- About the theory of so-called adsorption of soluble substances. Sven. Vetenskapsakad. Handingarl. 1898;24:1-39.
- [Google Scholar]
- Effective organochlorine pesticides removal from aqueous systems by magnetic nanospheres coated with polystyrene. J. Wuhan Univ. Technol.-Mater. Sci. Ed.. 2014;29(1):168-173.
- [Google Scholar]
- The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc.. 1918;40(9):1361-1403.
- [Google Scholar]
- Adsorption of Congo red from aqueous solutions onto Ca-bentonite. J. Hazard. Mater.. 2009;161(1):126-131.
- [Google Scholar]
- Adsorption properties of modified bentonite clay. Cheminé Technologija. 2009;1(50):47-50.
- [Google Scholar]
- Physico-chemical characteristics of nano-organo bentonite prepared using different organo-modifiers. Egypt. J. Pet.. 2014;23(3):331-338.
- [Google Scholar]
- Enhanced As(III) and As(V) adsorption from aqueous solution by a clay based hybrid sorbent. Front. Chem.. 2019;7(913):1-10.
- [Google Scholar]
- Adsorption of hexavalent chromium from aqueous solutions by wheat bran. Int. J. Environ. Sci. Technol.. 2008;5(2):161-168.
- [Google Scholar]
- Clay–iron oxide magnetic composites for the adsorption of contaminants in water. Appl. Clay Sci.. 2003;22(4):169-177.
- [Google Scholar]
- Assessing impact of urbanization on river water quality in the Pearl River Delta Economic Zone, China. Environ. Monit. Assess.. 2006;120(1–3):313-325.
- [Google Scholar]
- Identification and quantification of the“ Al13” tridecameric aluminum polycation using ferron. Environ. Sci. Technol.. 1992;26(5):908-914.
- [Google Scholar]
- Sorption of endrin to montmorillonite and kaolinite clays. J. Hazard. Mater.. 2009;168(1):210-214.
- [Google Scholar]
- Adsorption of methylene blue on activated carbon: an experiment illustrating both the Langmuir and Freundlich isotherms. J. Chem. Educ.. 1991;68(4):349.
- [Google Scholar]
- The use of pine bark as a natural adsorbent for persistent organic pollutants–study of lindane and heptachlor adsorption. J. Chem. Technol. Biotechnol.. 2003;78(2–3):347-351.
- [Google Scholar]
- Removal of dieldrin from aqueous solution by a novel triolein-embedded composite adsorbent. J. Hazard. Mater.. 2007;141(1):61-69.
- [Google Scholar]
- Synthesis and characterization of Al-pillared interlayered bentonites. Gazi Univ. J. Sci.. 2008;21(1):21-31.
- [Google Scholar]
- Adsorption characteristics, isotherm, kinetics, and diffusion of modified natural bentonite for removing diazo dye. Chem. Eng. J.. 2012;187:79-88.
- [Google Scholar]
- Kinetic modeling of liquid-phase adsorption of reactive dyes on activated carbon. J. Colloid Interface Sci.. 2005;287(1):25-34.
- [Google Scholar]
- Keggin-Al30 pillared montmorillonite. Microporous Mesoporous Mater.. 2017;242:256-263.
- [Google Scholar]







