Adsorption of thallium from wastewater using disparate nano-based materials: A systematic review
⁎Corresponding author. sumihutapea@gmail.com (Sumihar Hutapea)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Development of promising technologies to remove thallium as a highly poisonous contaminant is of great attention to guarantee the sustainable supplement of safe potable water and human well-being all around the world. Recently, adsorption has been introduced as a noteworthy technique to remove trace amount of thallium. In the past, the rate of thallium removal using the adsorption technique was relatively low due to the fact that this method was significantly influenced by the co-existing cations. To overcome this problem, more promising adsorbents such as nano-based materials have been developed. These adsorbents have shown great potential in the process of thallium removal due to their large surface area and superior selectivity. The main objective of this paper is to present a state-of-the-art review about the potential of nano-based form of disparate materials (i.e., titanium compounds, MnO2, ZnO, Al2O3 and multiwall carbon nanotubes) to separate thallium from water/waste water sources. Then, a systematic overview about acute/chronic toxicities of thallium for humans is aimed to be provided. Throughout the review, the authors aim to compare the negative and positive aspects of each treatment technique and offer promising technologies for thallium removal. At the end, an outlook on the recent advancements in the adsorption process of thallium using nanomaterials is provided.
Keywords
Adsorption
Nano-based materials
Thallium
Wastewater treatment
1 Introduction
Separation and removal of impurities from water and wastewater streams is of vital importance from environmental point of view. These impurities could be in different forms such as microbial, ions, organic molecules, etc. (He, 2021; Ni et al., 2021; Yang et al., 2020; Moondra et al., 2021; Chen et al., 2018; Zhao, 2020; Yang et al., 2021; Handschuh Wang et al., 2021; Liu et al., 2020). Among various elements which exist in wastewater, thallium (Tl) is a scarce element, which its high dispersion in the environment has caused it to be prioritized as an important contaminant by the U.S. Environmental Protection Agency (USEPA) (EPAToxic, U., Toxic and Priority Pollutants Under the Clean Water Act. US EPAAvailable online: https://www. epa. gov/eg/toxic-and-priority-pollutants-under-clean-water-act (accessed on 24 January 2021; Xu, 2019; Aslam et al., 2021). This rare metal, which was discovered in 1861, is of paramount importance in different industries such as chemical, pharmaceutical, aerospace and superconducting materials (Belzile and Chen, 2017; Liu et al., 2019; Babanezhad et al., 2020).
Apart from various applications of thallium in industrial-based activities, this metal has shown extreme toxicity on humans' body even more than mercury, lead and cadmium (Babanezhad et al., 2020; ZITKO, 1975; Li, 2020). The fatal dose of thallium for adults is just 8–10 mg.kg−1 but the uptake of even much lower dose by humans' body may cause adverse acute/chronic poisoning with various symptoms such as vomiting, diarrhea, hair loss and kidney/liver failure (Birungi and Chirwa, 2015; Puccini et al., 2018; Osorio-Rico et al., 2017; Li, 2019; Marjani, 2021). Adsorption has shown to be a promising technology for removal of various pollutants such as thallium from water and wastewater effluents (Chen, 2021; He, 2021; Moondra, 2021; Shi, 2021; Wang, 2021; Wong, 2017; Yang, 2020, 2021, Zhang, 2020, 2021; Yang et al., 2020). Adsorption is based on separation and mass transfer between a solution and solid phase. Various adsorbents have been synthesized by chemical and physical techniques for efficient removal of ions and organic molecules from water streams (Cao et al., 2021; Chen, 2018; Feng, 2020; Guan, 2020; Han, 2021; Handschuh Wang, 2021; He, 2021; Lin, 2019; Liu, 2020). Beside adsorption separation process, membrane separation technology has shown to be efficient in removal of ions and organic matters from water by which the separation can be driven mainly by pressure, electrical, temperature, or concentration gradient (Böning et al., 2018; Wick et al., 2018).
Thallium has very low solubility in alkali and liquid ammonia, but it possesses significant solubility in nitric acid. The reaction rate of thallium with dilute sulfuric acid is much lower than dilute nitric acid. This rare element with the atomic number 81 belongs to the thirteenth group of the Periodic Table of the Elements can be present in two oxidation states including Tl(I) and Tl(III), which demonstrates different chemistry in aqueous media. Monovalent thallium possesses more thermodynamic stability, which makes it the governing thallium species in environment (Belzile and Chen, 2017; Liu et al., 2014; Li et al., 2019; Antonia López Antón et al., 2013; Li, 2020). Fig. 1 shows the exact location of thallium in the Periodic Table of the Elements.
- The exact location of thallium in the Periodic Table of the Elements (ETF).
Due to the existence of numerous dispersion sources to nature along with great solubility in aqueous environment, thallium compounds has been recently considered to have potential harms to humans' potable water or food chain (Babanezhad et al., 2020; Li et al., 2018; Li et al., 2018; Li et al., 2017; Hosseini and Hassan-Abadi, 2008). Fig. 2 renders a simplified cycle of thallium in the environment. Therefore, thallium removal from wastewater seems to be vital to mitigate its deleterious impacts on human well-being. In last decades, several techniques such as precipitation, flotation, electrochemical deposition, ion exchange and solvent extraction have been emerged to separate thallium from water/waste water sources (Ussipbekova, 2015. 2015.; Escudero et al., 2013; Nabipour et al., 2020; xxxx; Twidwell and Williams-Beam, 2002). Table 1 gives detailed information about the performance of disparate employed techniques to remove thallium from water/wastewater sources.
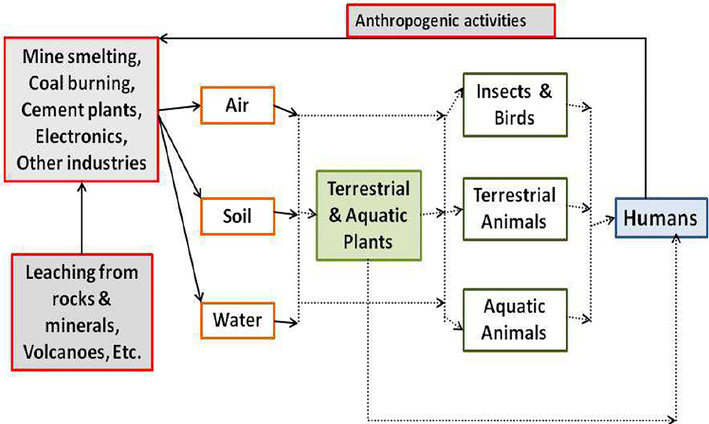
- Simplified illustration of thallium cycle in the environment. Reprinted from (Belzile and Chen, 2017) with permission from Elsevier.
| Removal technique | Advantages | Disadvantages | Ref. |
|---|---|---|---|
| Chemical precipitation |
|
|
(Liu et al., 2019; Chen et al., 2013; Li et al., 2017; Yang et al., 2017) |
| Ion exchange |
|
|
(Vincent et al., 2014) |
| Solvent extraction |
|
|
(Wan et al., 2014) |
| Adsorption |
|
|
(Ussipbekova, 2015. 2015.; Li et al., 2017; Huangfu et al., 2015; Tian et al., 2017; Wang et al., 2018) |
| Biotechnology |
|
|
(Wang, 2020; Marjani et al., 2020) |
| Electrocoagulation |
|
|
(Agency, 2002) |
Despite the variety of existed techniques, very few of them have shown their potential of application in industries due to the fact that the abovementioned procedures often have some drawbacks such as low performance, production of large scale of waste and high energy consumption (Ghadiri et al., 2020; Ecer and Şahan, 2018; Zhao, 2020). Recently, adsorption has shown appropriate potential for use in industrial-based wastewater processing for thallium removal due to having important privileges such as great purification performance, low energy consumption, and eco-friendly characteristics (Yang, 2019). Some efficient and promising adsorbents such as Prussian blue analogues, biosorbents and metal oxides have been synthetically investigated to evaluate their efficiency to separate trace amount Tl (I) from wastewater (Kumar et al., 2015).
In recent decades, nano-based substances have been identified as an appropriate approach to separate heavy/poisonous metals from various types of surface/ underground wastewater sources. Over the past decades, nano-based adsorbents have achieved numerous attentions (Liang et al., 2005; Ni et al., 2021; Yang, 2021; Yap et al., 2017; Zhang, 2020; Zhao, 2020). In various scientific fields such as wastewater treatment, health care, membrane-based separation process and energy due to illustrating numerous noteworthy properties including high surface area and small size/quantum effect (Pishnamazi, 2020; Pishnamazi et al., 2020; Shirazian, 2020; Cao, 2021; Pumera, 2011; Marjani, 2020; Yinghua et al., 2006; Nguyen et al., 2020; Vunain et al., 2016). These positive characteristics are due to their brilliant adsorption capacity and reactivity, which are desirable for heavy/poisonous metals removal (Yaqoob, 2020; Karthigadevi, 2021; Pishnamazi, 2020; Babanezhad et al., 2021; Nnaji, 2018). Despite the abovementioned advantages, high cost and time-consuming processes are considered as the main disadvantages of nano-based materials, which have confined their application for heavy/poisonous metals removal (Babanezhad et al., 2020; Williams-Beam and Twidwell, 2003; Zhang et al., 2018).
This paper presents a state-of-the-art review about the feasibility of various sorts of nano-based materials to separate thallium from disparate water/waste water sources. Then, a systematic overview about the acute/chronic toxicities of thallium for humans is aimed to be provided. Investigation of several feasible nano-based materials towards thallium separation is another aim of this paper. Throughout the review, the authors aim to compare the advantages and disadvantages of each treatment technique and offer promising technologies for thallium removal. Ultimately, an outlook on the recent advancements in the adsorption process of thallium using nanomaterials is provided.
2 Standard amount of thallium in water/wastewater sources
Global environmental standards are regulated to evaluate the effects of various human-based pollutants on the environment and consequently the ecosystem's protection from their abnormal dispersion/emission. Standards for water pollutant discharge (SWPD) specify the highest amounts of contaminants discharged by human-based water pollution sources to gain appropriate water quality standards (Xu, 2019; National Primary Drinking Water Regulations: Thallium (Technical Version)., 1995). Detailed summarization of the environmental standards for toxic element of thallium in water and wastewater is presented in Table 2. The USEPA notifies the fact that the most efficient current technologies (TMECTs) must decrease the thallium concentration to lower than 140 mg.L-1. However, in China where the pollution of water sources with thallium is more serious, stricter standards for thallium discharge are stipulated. According to their standards, the thallium concentration should be less than 5 mg mg.L-1 and decreased to less than 2 mg.L-1 after 2020 (Xu, 2019).
| Classification | Source/Agency | Limited value (µg.L-1) | Ref. |
|---|---|---|---|
| Water quality standard | Water + organism/USEPA | 0.24 | (Deng et al., 2016) |
| Organism only/USEPA | 0.47 | (Deng et al., 2016) | |
| Drinking water, maximum contaminant level goal (MCLG)/USEPA | 0.5 | (CCME, 2002) | |
| Drinking water, maximum contaminant level (MCL)/USEPA | 2 | (CCME, 2002) | |
| Sea water/USEPA | 4 | (China, GB3838-2002, 2002.) | |
| Fresh water/Canada | 0.8 | (Jin, et al., 2006) | |
| Water quality/China | 0.1 | (MH , S., Standards for drinking water quality (GB 5749-, 2006) | |
| Drinking water/China | 0.1 | (Rosengrant and Craig, 1990; Flegal and Patterson, 1985) | |
| Discharge standard of contaminants | Best demonstrated available technology (BDAT) for thallium wastewater/U.S.A | 140 | (Cheam, 2001) |
| Inorganic chemical industry/China | 5 | (Xu, 2019) | |
| Industrial wastewater in Hunan province/China | 5 | (Xu, 2019; Liu et al., 2019) |
3 Existence of thallium in natural waters
To the base of the author’s knowledge, little is known about the fate of thallium in the aquatic environments. In fact, before the current years, the detection of thallium in water sources was extremely difficult (Cheam et al., 1995). Accurate evaluations of the recent years have illustrated that the amount of dissolved thallium concentrations in seawater, unpolluted and polluted freshwater is 10–15, 5–10 and 20–50 ng.L-1, respectively (Peter and Viraraghavan, 2005; Shand et al., 1998; Wallwork-Barber et al., 1985). Flegal et al. concluded that the thallium concentrations in phytoplankton, zooplankton and ichthyoplankton from the central pacific ocean were 0.02–0.8, 0.03–0.5 and 0.1 attogram.liter-1 (ag.L-1), respectively (Cheam et al., 1995). The data obtained showed that the thallium concentration in seawater is approximately constant. Thallium was investigated in surface waters gathered from low-order upland streams from an extensive variety of sedimentary/igneous/metamorphic rock sorts. It was perceived the value of thallium in the majority of surface waters was lower than the detection limit but high value of thallium (up to 490 ng.L-1) was found in waters near to auriferous ore bodies (Gramlich et al., 2001). Thallium concentrations through river-/ground water have been reported 0.04 ag.L-1 and 800 ag.L-1, respectively. Certain transport of thallium took place through water, fish and plants but there isn't any substantial thallium transport between sand and other ecosystem components (Šídlo et al., 2021).
Tl (III) ion- 4-(2-pyridylazo) re-sorcinol (PAR) azodye ligand builds complexes of 1:1 and 1:2 stoichiometry. The concentration of this complex in solution relies on a number of important parameters including pH and ligand–metal ion ratio. At pH values greater than 3, the formation of 1:2 complex takes place quantitatively whereas the formation of 1:1 complex can be seen at pH values around 1 (Musso, 1993; Chen et al., 2000). Due to the intervention of prevalent buffers with high concentration (more than 0.05 M) of Tl(III) complex formation, the acetate buffer (with the concentration 0.01 M) was applied to regulate the pH value around 4 to hinder the feasible reduction of Tl3+ to Tl+ in presence of halide X− (Cvjetko et al., 2010; Rickwood et al., 2015).
4 Acute and chronic toxicity of thallium for humans
Vomiting, diarrhea, hair loss and serious failure in the functions of kidneys/hear/lungs can be considered as the serious toxicity of the large-amounts ingestion of thallium over a short period of time from (Osorio-Rico et al., 2017). The major characteristics of acute thallium toxicity on humans are gastro-intestinal disorders, polyneuropathy, alopecia, acne and anhydrosis (Wallwork-Barber et al., 1985; Manikandan, 2020; Elveny, 2021; Merian and Clarkson, 1991; Nakhjiri and Roudsari, 2016). It is worth noting that the central/peripheral nervous system is the major human-related organ damaged by abnormal exposure of thallium. Of course, the severity of symptoms significantly depends on age, dosage and administration procedure (Nakhjiri and Heydarinasab, 2020; Riyaz et al., 2013; Ramsden, 2002).
In the case of chronic symptoms, the 350 years history of thallium mining in several countries has led to the appearance of disproportionate symptoms of chronic thallium poisoning such as anorexia, abdominal pain, blindness and even death in the extreme condition (Becking and Chen, 1998). White streaks might be appeared on finger-/toe nails if the long-term absorption of thallium takes place (Manikandan, 2020; Hoffman and Hoffman, 2000). Despite the limited information available on the impacts of thallium on human reproduction, some literatures have reported adverse effects such as menstrual cycle imbalance and sperm inadequacy (Nuvolone, 2021; Singh et al., 2020; Zhang, 2009). Some major acute/chronic adverse effects of thallium on the humans' health are illustrated in Fig. 3.
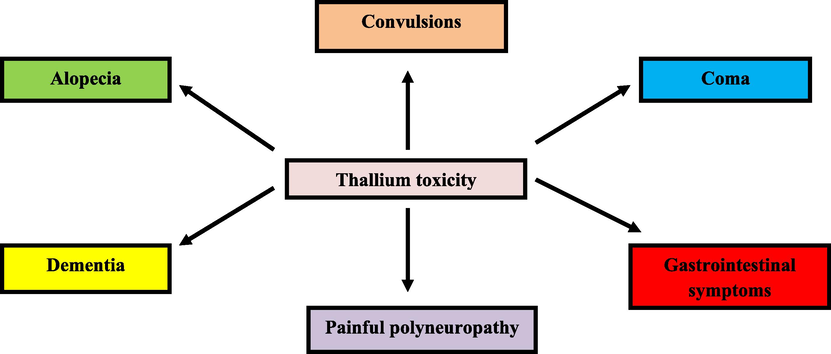
- Important acute/chronic adverse effects of thallium on the humans' health. Reprinted from (Singh et al., 2020) with permission from Elsevier.
5 Common nano-based materials for thallium adsorption
5.1 Titanium compounds
Titanium compounds including titanium dioxide nanoparticles (TiO2NPs), titanium dioxide (TiO2), titanate nanotubes (TNTs) and titanium peroxide (TiPO) are the first classification of adsorption materials, which is comprehensively scrutinized in this paper. It has been analytically reported that TiO2 nanoparticles possess great capability to remove thallium from water/wastewater sources with the maximum adsorption capacity of 258 mg.g−1 (Li et al., 2017). In recent years, TNTs and TiPO have received the most attention due to their brilliant capabilities, such as greater adsorption capacity and strong selectivity to remove thallium from water/wastewater sources (Antonia López Antón et al., 2013; Li et al., 2017). Despite significant positive characteristics for thallium removal, the regeneration by titanium peroxide is described as very difficult (Li et al., 2017). Zhang et al. concluded that the regeneration process of TiPO through HNO3 is entirely impossible due to the existence of strong affinity between Tl (I) and the surface of adsorbent (Li et al., 2017). The adsorption of Tl (III) applying TiO2NPs and TNTs have recently been under consideration. TNTs have demonstrated a superior adsorption capacity for Tl (III) compared to TiO2NPs (388.3 mg.g−1 vs 24.09 mg.g−1) (Antonia López Antón et al., 2013; Zhang, 2008). Fig. 4 schematically illustrates the adsorption mechanisms of Tl (I) and Tl (III) using TNTs.
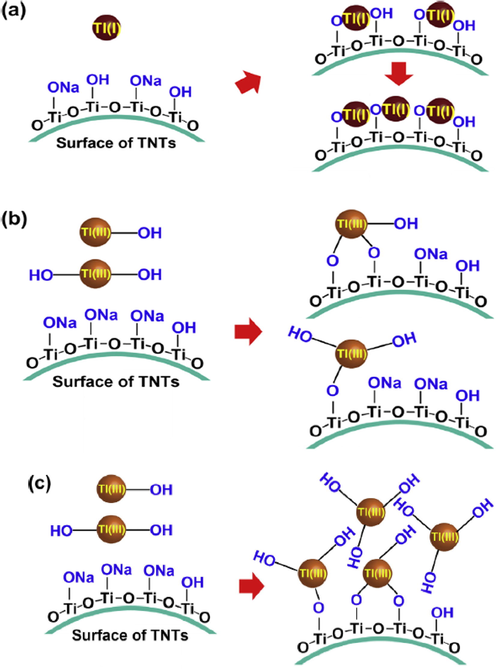
- Schematic illustration of (a) Tl(I) and (b), (c) Tl(III) adsorption mechanisms applying TNTs. Reprinted from (Liu et al., 2014) with permission from Elsevier.
5.2 Al2O3 nanoparticles
Currently, Al2O3 nanoparticles (Al2O3NPs) have been introduced as a promising absorbent to remove thallium from aqueous solution. At present, nanoparticles are promising functional materials of great interest owing to their noteworthy properties. Due to the unsaturated nature of nanoparticles on the surface of the most atoms, they are capable of binding to other atoms easily. Nanoparticles possess significant adsorption capacity, easy operation and fast adsorption process and therefore, it could be operationally applied as adsorbents (Mahdavi et al., 2013; Martel et al., 1998). It has been shown experimentally that Al2O3NPs are significantly effective for the adsorption of Tl (III) from aqueous solutions such as water/wastewater sources. To put the issue into the perspective view, Al2O3NPs can remove almost all of the existed Tl (III) from solution at pH 4.5 (Martel et al., 1998; Hrapovic et al., 2004). Fig. 6 schematically illustrates the molecular structure of Al2O3NPs.
5.3 Multiwall carbon nanotubes (MWCNTs)
Carbon nanotubes (CNTs) have been extensively used in disparate applications such as electronic transistors, biosensors, gas separation and optical component because of their brilliant chemical, mechanical and electronic characteristics (Ramsden, 2002; Liu et al., 2012; Pishnamazi et al., 2020; Edgington et al., 2010; Bai et al., 2010). However, rapid advances in the production and industrial uses of CNTs can render incidental exposure to human/environment receptors (Klaine, 2008; Wang et al., 2010; Pu et al., 2013). Due to their large surface area, CNTs has the ability of altering the destiny, transmission and bioaccessibility of pollutants by powerful adsorptive interactions (Zhang et al., 2011; Sheng, 2010). By elapsing the time, Tl (I) and disparate types of CNTs are likely to emerge in the aquatic environment. Despite an extensive study of the adsorption process of numerous pollutants (i.e., heavy metals) in CNTs, very little research have been implemented to evaluate the interaction mechanisms of Tl (I) -CNT. Owing to the fact that the modification of surface may considerably increase colloidal consistency of CNTs and consequently affects adsorption performance, the dynamism of Tl(I) related to the modified surface of CNTs might be changed in environment (Zhang et al., 2011; Yu et al., 2011). Fig. 5 illustrates the TEM images of pristine and three-surface modified MWCNTs. The figure demonstrates that an isolated MWCNT consists of a concentrically nested array of single-walled CNTs following an outer diameter in the range of 5–25 nm and the hollow interior tube diameter of approximately 2 nm. The purification and oxidation treatment on the surface of the modified MWCNTs considerably enhanced their specific surface areas compared to the pristine-MWCNTs, while the surface modification have reduced the volume of micropores and the mean diameter of the pores. This can be attributed to the removal of impurities from MWCNTs and the increment of functional groups on the surface of MWCNTs (Babanezhad et al., 2020; Dahal et al., 1998; Chen et al., 2017).
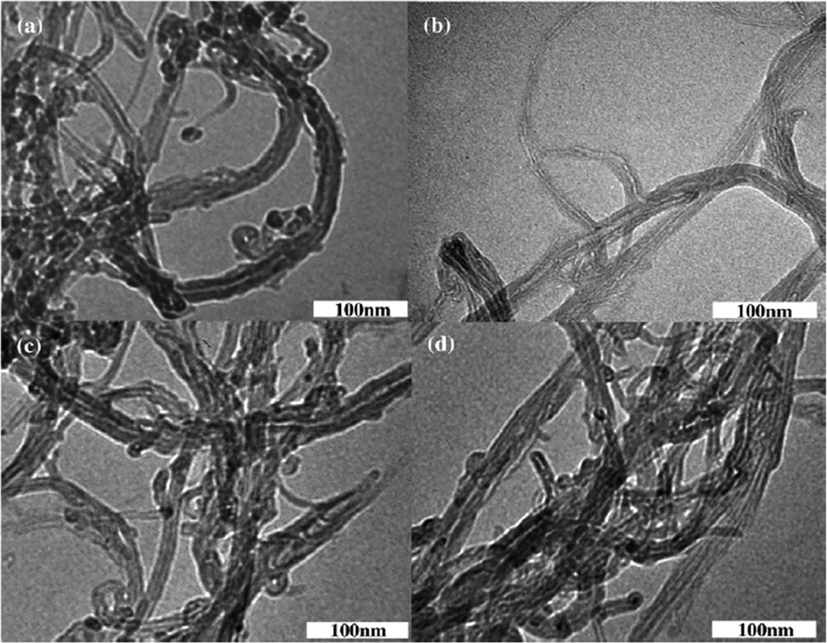
- TEM illustrations of (a) pristine-MWCNTs, (b) purified-MWCNTs (c) H2SO4-MWCNTs and d) Na2S2O8-MWCNTs. Reprinted from (Pu et al., 2013) with permission from Elsevier.

- Representation of ZnO nanoparticles for thallium removal. Reprinted from (Azonano).
It is worth noting that the adsorption of Tl (I) on MWCNTs profoundly relies on pH and ionic strength. Those MWCNTs that possess more hydrophilic groups and negative surface charges may adsorb the highest amount of Tl(I).
5.4 Manganese dioxide (MnO2) nanoparticles
MnO2 is a promising adsorbing agent to remove heavy metals (Pan et al., 2014). In recent years, nano-sized MnO2 has attracted attention compared to conventional forms because it has various advantages such as a large surface area, a large porous structure, and strong interaction between pollutants and absorbents. Therefore, the use of nano-sized MnO2 can significantly improve absorption performance compared to conventional forms. For instance, the maximum absorption capacity of nano-sized MnO2 for thallium removal is 672 mg Tl (I) g−1, which is much bigger than the highest absorption capacity of conventional MnO2 (203 mg Tl (I) g−1) (Wang et al., 2018; DASHTI, K.H., H. Aghaie, and M. Shishehbore, Adsorption of thallium (III) ion from aqueous solution using modified ZnO nanopowder., 2011). Amorphous hydrous manganese dioxide (AHMO) and polymer-based nano-sized MnO2 are regarded as two novel sorts of modified MnO2, which have been currently under investigation by Wan et al (Tian et al., 2017). They perceived that despite the lower adsorption capacity of AHMO compared to nano-sized MnO2, AHMO can be an appropriate alternative to decrease the amount of thallium in potable water to meet the water standards regulated by China (Tian et al., 2017; Dhiman and Kondal, 2021).
5.5 Zinc oxide (ZnO) nanoparticles
Adsorption is considered as an efficient procedure among various techniques to remove heavy/toxic contaminant (such as thallium) owing to its noteworthy features including low cost, simplicity of availability and low operation time. After modification with sodium phosphate solution (SPS), ZnO nanopowder has great potential as an absorbent for removing Tl (I) ions from aqueous solutions (National Primary Drinking Water Regulations: Thallium (Technical Version)., 1995; Deng et al., 2016; CCME, 2002; China, GB3838-2002, 2002.). Dashti et al. experimentally investigated the effect of adding sodium phosphate solution on improving the efficiency of ZnO nanopowder in removing thallium. They resulted that 5% w/v of SPS eventuated superior adsorption efficiency. Experimental data has shown that the adsorption efficiency rely on some momentous operational/functional factors including initial solution's pH, contact time, amount of employed adsorbent, temperature and the thallium concentration at the beginning of the process. They also perceived that under the optimum situations (solution pH = 6; contact time = 1 hr; adsorbent amount = 0.1 g; thallium concentration = 50 mg.L-1 and temperature = 25 °C), the highest adsorption percentage of Tl (I) ion applying modified ZnO nanopowder was achieved 92.8% (xxxx). Compared to different types of nano-based adsorbents, modified ZnO nanopowder shows better performance due to its abundance, affordability and high adsorption capability (Wu, 2019). Fig. 6 presents an illustration of ZnO nanoparticles.
5.6 Nano-magnetite-based biochar
In recent years, much attention has been paid to the development of promising/regenerated nano-based materials in conjunction with advanced oxidation to remove thallium (Tl) from a variety of surface/groundwater resources. Nano-magnetite-based biochar (Fe3O4/C) is known as a new type of nano-adsorbent due to its luminous properties such as high porous structure and abundant active binding sites (Babanezhad et al., 2021). Li et al. experimentally investigated the efficiency of Fe3O4/C for the removal of Tl (I) from wastewater sources. They corroborated that the capacity of Tl(I) adsorption from wastewater applying Fe3O4/C nano-adsorbent is significantly increased at 1123 mg.g−1 (Li, 2020).
6 Conclusions and future outlook
This review article aims to provide both expert and non-expert readers with a comprehensive and systematic overview of the latest promising nano-based materials for the removal of thallium as one of the most toxic chemical elements present in water/wastewater sources. Despite the existence of promising and efficacious techniques to control and manage thallium-containing wastewater, these technologies are still at the infancy stage in comparison with other metal contaminants. With the increasing amount of thallium in the world, cost-intensiveness of conventional methods (i.e., precipitation, ion exchange and solvent extraction) and its high toxicity to various microorganisms and human-health, the development of affordable technologies to mitigate its distribution in various aqueous/ non-aqueous sources are of great importance. Adsorption technology applying nano-based materials have recently become a viable option to overcome the operational/functional drawbacks of traditional techniques that are emerging as an economical, reliable and eco-friendly method to control the distribution of thallium in water/wastewater sources. These novel techniques need to be further developed to prepare theoretical foundations for industrial-based utilizations at the commercial level.
Acknowledgment
A.K. is thankful to the Russian Government and Institute of Engineering and Technology, Department of Hydraulics and Hydraulic and Pneumatic Systems, South Ural State University, Lenin prospect 76, Chelyabinsk, 454080, Russian Federation for their support to this work through Act 211 Government of the Russian Federation, contract No. 02. A03.21.0011.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- National recommended water quality criteria. DC: Office of Water Washington; 2002.
- Recent development in the green synthesis of titanium dioxide nanoparticles using plant-based biomolecules for environmental and antimicrobial applications. J. Ind. Eng. Chem. 2021
- [Google Scholar]
- Prediction of turbulence eddy dissipation of water flow in a heated metal foam tube. Sci. Rep.. 2020;10(1)
- [CrossRef] [Google Scholar]
- Babanezhad, M., et al., Performance and application analysis of ANFIS artificial intelligence for pressure prediction of nanofluid convective flow in a heated pipe. Scientific Reports, 2021. 11(1): p. 1-18.
- Pattern recognition of the fluid flow in a 3D domain by combination of Lattice Boltzmann and ANFIS methods. Sci. Rep.. 2020;10(1)
- [CrossRef] [Google Scholar]
- Prediction of gas velocity in two-phase flow using developed fuzzy logic system with differential evolution algorithm. Sci. Rep.. 2021;11(1)
- [CrossRef] [Google Scholar]
- Repeated administrations of carbon nanotubes in male mice cause reversible testis damage without affecting fertility. Nat. Nanotechnol.. 2010;5(9):683-689.
- [Google Scholar]
- International Programme on Chemical Safety (IPCS) environmental health criteria on boron human health risk assessment. Biol. Trace Elem. Res.. 1998;66(1-3):439-452.
- [Google Scholar]
- Thallium in the environment: a critical review focused on natural waters, soils, sediments and airborne particles. Appl. Geochem.. 2017;84:218-243.
- [Google Scholar]
- The adsorption potential and recovery of thallium using green micro-algae from eutrophic water sources. J. Hazard. Mater.. 2015;299:67-77.
- [Google Scholar]
- Thallium dynamics in the southern North Sea. Geochim. Cosmochim. Acta. 2018;227:143-155.
- [Google Scholar]
- Recent advancements in molecular separation of gases using microporous membrane systems: A comprehensive review on the applied liquid absorbents. J. Mol. Liq. 2021116439
- [Google Scholar]
- Mathematical modeling and numerical simulation of CO2 capture using MDEA-based nanofluids in nanostructure membranes. Process Saf. Environ. Prot.. 2021;148:1377-1385.
- [Google Scholar]
- CCME, Canadian water quality guidelines for the protection of aquatic life: total particulate matter. 2002, CCME Winnipeg, Manitoba.
- Thallium contamination of water in Canada. Water Quality Res. J.. 2001;36(4):851-877.
- [Google Scholar]
- Dissolved and total thallium in Great Lakes waters. J. Great Lakes Res.. 1995;21(3):384-394.
- [Google Scholar]
- Multi-criteria design of shale-gas-water supply chains and production systems towards optimal life cycle economics and greenhouse gas emissions under uncertainty. Comput. Chem. Eng.. 2018;109:216-235.
- [CrossRef] [Google Scholar]
- Fabrication of cellulosic paper containing zeolitic imidazolate framework and Its application in removal of anionic dye from aqueous solution. BioResources. 2021;16(2):2644-2654.
- [CrossRef] [Google Scholar]
- Multi-criteria design of shale-gas-water supply chains and production systems towards optimal life cycle economics and greenhouse gas emissions under uncertainty. Comput. Chem. Eng.. 2018;109:216-235.
- [Google Scholar]
- Equilibria with the thallium (III) triethylenetetraminehexaacetate anion [Tl (ttha)] 3− in aqueous solution. Anal. Chim. Acta. 2000;406(2):317-323.
- [Google Scholar]
- Environmental exposure and flux of thallium by industrial activities utilizing thallium-bearing pyrite. Sci. China Earth Sci.. 2013;56(9):1502-1509.
- [Google Scholar]
- FeOOH-loaded MnO2 nano-composite: An efficient emergency material for thallium pollution incident. J. Environ. Manage.. 2017;192:31-38.
- [Google Scholar]
- China, E., Environmental quality standards for surface water. MEP, China, GB3838-2002, 2002.
- Thallium toxicity in humans. Arhiv za higijenu rada i toksikologiju. 2010;61(1):111-118.
- [Google Scholar]
- Kinetics of heavy metal ion adsorption on to, and proton release from, electrolytic manganese dioxide. Adsorpt. Sci. Technol.. 1998;16(1):39-50.
- [Google Scholar]
- DASHTI, K.H., H. Aghaie, and M. Shishehbore, Adsorption of thallium (III) ion from aqueous solution using modified ZnO nanopowder. 2011.
- Adsorption of Tl (I) on Na–montmorillonite and kaolinite from aqueous solutions. Environ. Earth Sci.. 2016;75(9)
- [CrossRef] [Google Scholar]
- ZnO Nanoadsorbents: A potent material for removal of heavy metal ions from wastewater. Colloid Interface Sci. Commun.. 2021;41:100380
- [Google Scholar]
- A response surface approach for optimization of Pb (II) biosorption conditions from aqueous environment with Polyporus squamosus fungi as a new biosorbent and kinetic, equilibrium and thermodynamic studies. Desalin. Water Treat.. 2018;102:229-240.
- [Google Scholar]
- The influence of natural organic matter on the toxicity of multiwalled carbon nanotubes. Environ. Toxicol. Chem.. 2010;29(11):2511-2518.
- [Google Scholar]
- A state-of-the-art review on the application of various pharmaceutical nanoparticles as a promising technology in cancer treatment. Arabian J. Chem. 2021103352
- [Google Scholar]
- EPAToxic, U., Toxic and Priority Pollutants Under the Clean Water Act. US EPAAvailable online: https://www. epa. gov/eg/toxic-and-priority-pollutants-under-clean-water-act (accessed on 24 January 2021).
- Sensitive determination of thallium species in drinking and natural water by ionic liquid-assisted ion-pairing liquid–liquid microextraction and inductively coupled plasma mass spectrometry. J. Hazard. Mater.. 2013;244-245:380-386.
- [Google Scholar]
- The significance of dispersion of nano-SiO2 on early age hydration of cement pastes. Mater. Des.. 2020;186:108320
- [CrossRef] [Google Scholar]
- Modelling tyramine extraction from wastewater using a non-dispersive solvent extraction process. Environ. Sci. Pollut. Res.. 2020;27(31):39068-39076.
- [Google Scholar]
- The Stability of Metal N, N, N′, N′-Tetrakis (2-aminoethyl) ethane-1, 2-diamine (= penten) Complexes and the X-Ray Crystal Structure of [Tl (NO3)(penten)](NO3) 2. Helv. Chim. Acta. 2001;84(3):623-631.
- [Google Scholar]
- Chemical environment and magnetic moment effects on point defect formations in CoCrNi-based concentrated solid-solution alloys. Acta Mater.. 2020;187:122-134. In this issue
- [CrossRef] [Google Scholar]
- Crop evapotranspiration prediction by considering dynamic change of crop coefficient and the precipitation effect in back-propagation neural network model. J. Hydrol. (Amsterdam). 2021;596:126104
- [Google Scholar]
- Ultrathin diamond nanofilms—development, challenges, and applications. Small 20212007529
- [CrossRef] [Google Scholar]
- Enhanced adsorption of Cu(II) and Zn(II) from aqueous solution by polyethyleneimine modified straw hydrochar. Sci. Total Environ.. 2021;778
- [Google Scholar]
- Novel coagulation waste-based Fe-containing carbonaceous catalyst as peroxymonosulfate activator for pollutants degradation: Role of ROS and electron transfer pathway. J. Hazard. Mater.. 2021;417:126113
- [CrossRef] [Google Scholar]
- Enhanced adsorption of Cu(II) and Zn(II) from aqueous solution by polyethyleneimine modified straw hydrochar. Sci. Total Environ.. 2021;778:146116
- [CrossRef] [Google Scholar]
- Thallium poisoning during pregnancy: a case report and comprehensive literature review. J. Toxicol. Clin. Toxicol.. 2000;38(7):767-775.
- [Google Scholar]
- Flotation separation and electrothermal atomic absorption spectrometric determination of lead in water samples. Int. J. Environ. Anal. Chem.. 2008;88(3):199-207.
- [Google Scholar]
- Hrapovic, S., et al., Electrochemical biosensing platforms using platinum nanoparticles and carbon nanotubes. Analytical chemistry, 2004. 76(4): p. 1083-1088.
- Adsorption and oxidation of thallium (I) by a nanosized manganese dioxide. Water Air Soil Pollut.. 2015;226(1)
- [CrossRef] [Google Scholar]
- Jin, Y., et al., Standards for drinking water quality (GB-5749-2006). Beijing Ministry of Health of the People’s Republic of China, 2006.
- Chemico-nanotreatment methods for the removal of persistent organic pollutants and xenobiotics in water–A review. Bioresour. Technol. 2021124678
- [Google Scholar]
- Nanomaterials in the environment: behavior, fate, bioavailability, and effects. Environ. Toxicol. Chem.: Int. J.. 2008;27(9):1825-1851.
- [Google Scholar]
- Graphene, carbon nanotubes, zinc oxide and gold as elite nanomaterials for fabrication of biosensors for healthcare. Biosens. Bioelectron.. 2015;70:498-503.
- [Google Scholar]
- Synthesis of manganese dioxide with different morphologies for thallium removal from wastewater. J. Environ. Manage.. 2019;251:109563
- [Google Scholar]
- Enhanced thallium (I) removal from wastewater using hypochlorite oxidation coupled with magnetite-based biochar adsorption. Sci. Total Environ.. 2020;698:134166
- [Google Scholar]
- Highly efficient removal of thallium (I) from wastewater via hypochlorite catalytic oxidation coupled with adsorption by hydrochar coated nickel ferrite composite. J. Hazard. Mater.. 2020;388:122016
- [Google Scholar]
- Simultaneous removal of thallium and chloride from a highly saline industrial wastewater using modified anion exchange resins. J. Hazard. Mater.. 2017;333:179-185.
- [Google Scholar]
- Removal of thallium from aqueous solutions using Fe-Mn binary oxides. J. Hazard. Mater.. 2017;338:296-305.
- [Google Scholar]
- Removal and recovery of thallium from aqueous solutions via a magnetite-mediated reversible adsorption-desorption process. J. Cleaner Prod.. 2018;199:705-715.
- [Google Scholar]
- Efficient removal of thallium (I) from wastewater using flower-like manganese dioxide coated magnetic pyrite cinder. Chem. Eng. J.. 2018;353:867-877.
- [Google Scholar]
- Biochar derived from watermelon rinds as regenerable adsorbent for efficient removal of thallium (I) from wastewater. Process Saf. Environ. Prot.. 2019;127:257-266.
- [Google Scholar]
- Determination of trace rare earth elements by inductively coupled plasma atomic emission spectrometry after preconcentration with multiwalled carbon nanotubes. Spectrochim. Acta, Part B. 2005;60(1):125-129.
- [Google Scholar]
- Accelerated microbial reductive dechlorination of 2,4,6-trichlorophenol by weak electrical stimulation. Water Res.. 2019;162:236-245.
- [CrossRef] [Google Scholar]
- Wear and heat shock resistance of Ni-WC coating on mould copper plate fabricated by laser. J. Mater. Res. Tech.. 2020;9(4):8283-8288.
- [CrossRef] [Google Scholar]
- Thallium pollution in China and removal technologies for waters: a review. Environ. Int.. 2019;126:771-790.
- [Google Scholar]
- 2 μm passive Q-switched mode-locked Tm3+: YAP laser with single-walled carbon nanotube absorber. Opt. Laser Technol.. 2012;44(4):960-962.
- [Google Scholar]
- Wear and heat shock resistance of Ni-WC coating on mould copper plate fabricated by laser. J. Mater. Res. Technol.-Jmr&T. 2020;9(4):8283-8288.
- [Google Scholar]
- Adsorption mechanisms of thallium (I) and thallium (III) by titanate nanotubes: ion-exchange and co-precipitation. J. Colloid Interface Sci.. 2014;423:67-75.
- [Google Scholar]
- Mahdavi, S., M. Jalali, and A. Afkhami, Heavy metals removal from aqueous solutions using TiO2, MgO, and Al2O3 nanoparticles. Chemical Engineering Communications, 2013. 200(3): p. 448-470.
- Emerging nano-structured innovative materials as adsorbents in wastewater treatment. Bioresour. Technol. 2020124394
- [Google Scholar]
- Modification of polyethersulfone membrane using MWCNT-NH2 nanoparticles and its application in the separation of azeotropic solutions by means of pervaporation. PLoS ONE. 2020;15(7):e0236529
- [Google Scholar]
- Evaluation of potassium glycinate, potassium lysinate, potassium sarcosinate and potassium threonate solutions in CO2 capture using membranes. Arabian J. Chem.. 2021;14(3):102979
- [Google Scholar]
- Effect of graphene oxide on modifying polyethersulfone membrane performance and its application in wastewater treatment. Sci. Rep.. 2020;10(1)
- [CrossRef] [Google Scholar]
- Martel, R., et al., Single-and multi-wall carbon nanotube field-effect transistors. Applied physics letters, 1998. 73(17): p. 2447-2449.
- Merian, E. and T.W. Clarkson, Metals and their compounds in the environment 1991:Vch.
- MH , S., Standards for drinking water quality (GB 5749-2006). 2006, Standards Press of China Beijing.
- Microalgal-bacterial consortia: An alluring and novel approach for domestic wastewater treatment. Water Conser. Manage.. 2021;4(1):51-56.
- [CrossRef] [Google Scholar]
- Musso, S., Untersuchung von Gleichgewichten in wässriger Lösung und Kristallstrukturbestimmung von Komplexen des Thalliums (III) mit Chelatliganden. 1993, ETH Zurich.
- Prediction of Nanofluid Temperature Inside the Cavity by Integration of Grid Partition Clustering Categorization of a Learning Structure with the Fuzzy System. ACS Omega. 2020;5(7):3571-3578.
- [Google Scholar]
- Efficiency evaluation of novel liquid potassium lysinate chemical solution for CO2 molecular removal inside the hollow fiber membrane contactor: Comprehensive modeling and CFD simulation. J. Mol. Liq.. 2020;297:111561
- [Google Scholar]
- Modeling and simulation of natural convection heat transfer process in porous and non-porous media. Appl. Res. J. 2016;2:199-204.
- [Google Scholar]
- National Primary Drinking Water Regulations: Thallium (Technical Version). 1995; Available from: https://nepis.epa.gov/Exe/ZyNET.exe/9100PO2M.TXT?ZyActionD=ZyDocument&Client=EPA&Index=1995+Thru+1999&Docs=&Query=&Time=&EndTime=&SearchMethod=1&TocRestrict=n&Toc=&TocEntry=&QField=&QFieldYear=&QFieldMonth=&QFieldDay=&IntQFieldOp=0&ExtQFieldOp=0&XmlQuery=&File=D%3A%5Czyfiles%5CIndex%20Data%5C95thru99%5CTxt%5C00000029%5C9100PO2M.txt&User=ANONYMOUS&Password=anonymous&SortMethod=h%7C-&MaximumDocuments=1&FuzzyDegree=0&ImageQuality=r75g8/r75g8/x150y150g16/i425&Display=hpfr&DefSeekPage=x&SearchBack=ZyActionL&Back=ZyActionS&BackDesc=Results%20page&MaximumPages=1&ZyEntry=1&SeekPage=x&ZyPURL.
- Thermal and flow visualization of a square heat source in a nanofluid material with a cubic-interpolated pseudo-particle. ACS Omega. 2020;5(28):17658-17663.
- [Google Scholar]
- Facile synthesis of copper(I) oxide nanochains and the photo-thermal conversion performance of its nanofluids. Coatings. 2021;11(7)
- [CrossRef] [Google Scholar]
- Engineered nanomaterials for wastewater treatment: current and future trends. In: Fundamentals of Nanoparticles. Elsevier; 2018. p. :129-168.
- [Google Scholar]
- Thallium Contamination of Drinking Water: Health Implications in a Residential Cohort Study in Tuscany (Italy) Int. J. Environ. Res. Public Health. 2021;18(8):4058.
- [Google Scholar]
- Thallium toxicity: general issues, neurological symptoms, and neurotoxic mechanisms. Neurotoxicity of Metals 2017:345-353.
- [Google Scholar]
- Recyclable polymer-based nano-hydrous manganese dioxide for highly efficient Tl (I) removal from water. Sci. China Chem.. 2014;57(5):763-771.
- [Google Scholar]
- Thallium: a review of public health and environmental concerns. Environ. Int.. 2005;31(4):493-501.
- [Google Scholar]
- Computational fluid dynamics simulation of NO2 molecular sequestration from a gaseous stream using NaOH liquid absorbent through porous membrane contactors. J. Mol. Liq. 2020113584
- [Google Scholar]
- Mechanistic modeling and numerical simulation of axial flow catalytic reactor for naphtha reforming unit. PLoS ONE. 2020;15(11):e0242343
- [Google Scholar]
- ANFIS grid partition framework with difference between two sigmoidal membership functions structure for validation of nanofluid flow. Sci. Rep.. 2020;10(1)
- [CrossRef] [Google Scholar]
- Molecular investigation into the effect of carbon nanotubes interaction with CO 2 in molecular separation using microporous polymeric membranes. Sci. Rep.. 2020;10(1)
- [CrossRef] [Google Scholar]
- Adsorption and desorption of thallium (I) on multiwalled carbon nanotubes. Chem. Eng. J.. 2013;219:403-410.
- [Google Scholar]
- Development of a chemosensor for the in situ monitoring of thallium in the water network. Water Air Soil Pollut.. 2018;229(7)
- [CrossRef] [Google Scholar]
- Graphene-based nanomaterials for energy storage. Energy Environ. Sci.. 2011;4(3):668-674.
- [Google Scholar]
- Assessing the fate and toxicity of thallium I and thallium III to three aquatic organisms. Ecotoxicol. Environ. Saf.. 2015;115:300-308.
- [Google Scholar]
- Riyaz, R., et al., A fatal case of thallium toxicity: challenges in management. J. Med. Toxicol., 2013. 9, 1, p. 75–78.
- Final best demonstrated available technology (BDAT) background document for P and U Thallium wastes. National Technical Information Services PB90-234188l; 1990.
- Shand, P., W.M. Edmunds, and J. Ellis. The hydrogeochemistry of thallium in natural waters. in Water–Rock Interaction” Proceedings of the 9th International Symposium on Water–Rock Interaction—WRI-9. Taupo, New Zealand. 1998.
- Adsorption of copper (II) on multiwalled carbon nanotubes in the absence and presence of humic or fulvic acids. J. Hazard. Mater.. 2010;178(1–3):333-340.
- [Google Scholar]
- Ultrasonic desulfurization of amphiphilic magnetic-Janus nanosheets in oil-water mixture system. Ultrason. Sonochem.. 2021;76:105662
- [CrossRef] [Google Scholar]
- Theoretical investigations on the effect of absorbent type on carbon dioxide capture in hollow-fiber membrane contactors. PLoS ONE. 2020;15(7):e0236367
- [Google Scholar]
- Colorimetric Chemosensor Array for Determination of Halides. Chemosensors. 2021;9(2):39.
- [Google Scholar]
- Low-cost bio-adsorbent for emerging inorganic pollutants. In: Inorganic Pollutants in Water. Elsevier; 2020. p. :205-220.
- [Google Scholar]
- Electrochemical oxidation of thallium (I) in groundwater by employing single-chamber microbial fuel cells as renewable power sources. Int. J. Hydrogen Energy. 2017;42(49):29454-29462.
- [Google Scholar]
- Potential technologies for removing thallium from mine and process wastewater: an annotation of the literature. Eur. J. Miner. Process Environ. Prot.. 2002;2(1):1-10.
- [Google Scholar]
- Electrochemical deposition and dissolution of thallium from sulfate solutions. Int. J. Analyt. Chem. 2015. 2015.
- [Google Scholar]
- Thallium (I) sorption using Prussian blue immobilized in alginate capsules. Carbohydr. Polym.. 2014;99:517-526.
- [Google Scholar]
- Dendrimers, mesoporous silicas and chitosan-based nanosorbents for the removal of heavy-metal ions: a review. Int. J. Biol. Macromol.. 2016;86:570-586.
- [Google Scholar]
- Thallium movement in a simple aquatic ecosystem. J. Environ. Sci. Health Part A. 1985;20(6):689-700.
- [Google Scholar]
- Selective capture of thallium (I) ion from aqueous solutions by amorphous hydrous manganese dioxide. Chem. Eng. J.. 2014;239:200-206.
- [Google Scholar]
- One-step removal of thallium (I) from groundwater by electrocoagulation using an aluminum anode. Int. J. Electrochem. Sci. 2020;15:1329-1337.
- [Google Scholar]
- Exceptional high and reversible ammonia uptake by two dimension few-layer BiI3 nanosheets. ACS Appl. Mater.. 2021;13(22):25918-25925.
- [CrossRef] [Google Scholar]
- Adsorption of dialkyl phthalate esters on carbon nanotubes. Environ. Sci. Technol.. 2010;44(18):6985-6991.
- [Google Scholar]
- Spontaneous thallium (I) oxidation with electricity generation in single-chamber microbial fuel cells. Appl. Energy. 2018;209:33-42.
- [Google Scholar]
- Removal of thallium from wastewater. Electrometall. Environ. Hydrometall.. 2003;2:1717-1727.
- [Google Scholar]
- Natural treatment technology for cleaning wastewater. Water Conser. Manage.. 2017;1(1):7-10.
- [CrossRef] [Google Scholar]
- A scientometric review of biochar research in the past 20 years (1998–2018) Biochar. 2019;1(1):23-43.
- [Google Scholar]
- May 1, 2021]; Available from: https://www.etf.com/sections/features-and-news/1944-thallium-a-poison-and-more-much-more.
- Nanomaterials for the removal of heavy metals from wastewater. Nanomaterials. 2019;9(3):424.
- [Google Scholar]
- Flexible carbon-fiber/semimetal Bi nanosheet arrays as separable and recyclable plasmonic photocatalysts and photoelectrocatalysts. ACS Appl. Mater. Interfaces. 2020;12(22):24845-24854.
- [CrossRef] [Google Scholar]
- Simulating a combined lysis-cryptic and biological nitrogen removal system treating domestic wastewater at low C/N ratios using artificial neural network. Water Res.. 2021;189:116576
- [CrossRef] [Google Scholar]
- Removing and recycling mercury from scrubbing solution produced in wet nonferrous metal smelting flue gas purification process. J. Environ. Sci. (China). 2021;103:59-68.
- [CrossRef] [Google Scholar]
- Removing and recycling mercury from scrubbing solution produced in wet nonferrous metal smelting flue gas purification process. J. Environ. Sci.. 2021;103:59-68.
- [Google Scholar]
- The efficient removal of thallium from sintering flue gas desulfurization wastewater in ferrous metallurgy using emulsion liquid membrane. Environ. Sci. Pollut. Res.. 2017;24(31):24214-24222.
- [Google Scholar]
- Microwave induced synthesis of magnetic biochar from agricultural biomass for removal of lead and cadmium from wastewater. J. Ind. Eng. Chem.. 2017;45:287-295.
- [Google Scholar]
- Role of nanomaterials in the treatment of wastewater: A review. Water. 2020;12(2):495.
- [Google Scholar]
- The application and prospect of nanotechnology in animal husbandry. J. Northwest Sci.-Tech. Univ. Agric. Forestry 2006
- [Google Scholar]
- Adsorption of lead (II) on O2-plasma-oxidized multiwalled carbon nanotubes: thermodynamics, kinetics, and desorption. ACS Appl. Mater. Interfaces. 2011;3(7):2585-2593.
- [Google Scholar]
- Studies on the capability and behavior of adsorption of thallium on nano-Al2O3. J. Hazard. Mater.. 2008;157(2–3):352-357.
- [Google Scholar]
- Sorption of thallium (III) ions from aqueous solutions using titanium dioxide nanoparticles. Microchim. Acta. 2009;165(1–2):73-78.
- [Google Scholar]
- Preparation of PI porous fiber membrane for recovering oil-paper insulation structure. J. Mater. Sci.: Mater. Electron.. 2020;31(16):13344-13351.
- [CrossRef] [Google Scholar]
- Effects of Al3+ on the microstructure and bioflocculation of anoxic sludge. J. Environ. Sci. (China). 2020;91:212-221.
- [CrossRef] [Google Scholar]
- Effect of Fe3+ on the sludge properties and microbial community structure in a lab-scale A2O process. Sci. Total Environ.. 2021;780:146505
- [CrossRef] [Google Scholar]
- Superior adsorption of thallium (I) on titanium peroxide: performance and mechanism. Chem. Eng. J.. 2018;331:471-479.
- [Google Scholar]
- Adsorption behavior of multi-walled carbon nanotubes for the removal of olaquindox from aqueous solutions. J. Hazard. Mater.. 2011;197:389-396.
- [Google Scholar]
- One-step fabrication of biomass-derived hierarchically porous carbon/MnO nanosheets composites for symmetric hybrid supercapacitor. Appl. Surf. Sci.. 2020;526
- [Google Scholar]
- Adsorptive removal of trace thallium (I) from wastewater: A review and new perspectives. J. Hazard. Mater.. 2020;393:122378
- [Google Scholar]
- Zinc Oxide (ZnO) Nanoparticles – Properties & Applications. Available:https://www.azonano.com/article.aspx?ArticleID=3348. May 13, 2021]; Available from: https://www.azonano.com/article.aspx?ArticleID=3348.
- One-step fabrication of biomass-derived hierarchically porous carbon/MnO nanosheets composites for symmetric hybrid supercapacitor. Appl. Surf. Sci.. 2020;526:146696
- [CrossRef] [Google Scholar]
- Toxicity and pollution potential of thallium. Sci. Total Environ.. 1975;4(2):185-192.
- [Google Scholar]







