Translate this page into:
Advances in coatings on Mg alloys and their anti-microbial activity for implant applications
⁎Corresponding author. nayem.hossain@iubat.edu (Nayem Hossain),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Magnesium matrix composites reinforced by calcium phosphate could not show the desired effect on the magnesium breakdown rate. Rapid disintegration rate limited the magnesium alloys used as biodegradable implant material. The rate of degradation can be minimized and biological activity can be improved in the magnesium alloy by Hydroxyapatite (HA) coating with the improvement of bone induction and conduction abilities. Various alkali post-treatment and conversion coating methods are applied to deposit HA coatings and biocompatible dicalcium phosphate dihydrate (DCPD) on magnesium alloy so that corrosion resistance and surface biocompatibility can be improved to be used in bone tissue engineering applications. Magnesium's corrosion resistance will weaken its antibacterial properties, which are linked to and proportional to the alkaline pH at the time of breakdown. The goal of this study is to bring together and compare contemporary research on different coatings on magnesium and related alloys in relation to antibacterial functionalized activities. A though review has been performed on in vivo and in vitro cytocompatibility, material property, corrosion resistance, and antibacterial properties of the coatings. Increased degradation behavior, biocompatibility, and bioactivity have been achieved following multiple procedures such as alkali treatment with HA electrochemical deposition on magnesium alloy. Multifunctional coatings can make safe and bioactive magnesium alloy surfaces for biodegradable implant applications.
Keywords
Mg
Implant
Antimicrobial activity
Biocompatibility
Corrosion resistance
1 Introduction
Magnesium, as a new implant material, has a number of advantages over typical orthopedic equipment. Biodegradability is the most appealing property, as it allows magnesium-based materials to avoid secondary procedures so that the implant materials can be removed. The weight of magnesium is less with less density, in addition to being biodegradable. Human bone and magnesium have a similar elastic modulus which is significant to eliminate the stress-shielding effect that typical orthopedic material provides. Mg and its related alloys have gotten a lot of press recently as biodegradable implant materials, and they're regarded as a cutting-edge research topic in biomedical engineering (Witte, 2015; Witte et al., 2008; Bellucci et al., 2010; Mouriño and Boccaccini, 2009; Eliezer et al., 1998; Lin and Kuo, 2009; Staiger et al., 2006; Zeng et al., 2008). Cobalt-based alloys, titanium-based alloys, and stainless steel can also be used as an implant material for temporary metal implants for bone tissue restoration as these materials are not biodegradable but a second procedure is necessary after a certain period of bone implantation. Because of these, intact biodegradable implant material allows sick tissue in the human body to recuperate before being gradually dissolved, absorbed, expelled, or devoured, avoiding the need for a second surgery, is an appealing concept (Shaw et al., 2008). Numerous concerns have been raised as excellent mechanical and biocompatible properties made metallic implants are employed for bone replacement or regeneration (Witte, 2015; Chen and Thouas, 2015; Niinomi et al., 2012; Crubzy et al., 1998; Ratner et al., 2006). Table 1 listed the mechanical characteristics and density of metallic implant materials and natural bone.(See Table 2.).
Materials
Density (gm/cm3)
Modulus of Elasticity (GPa)
Tensile strength (MPa)
Fracture Toughness (MPa m1/2)
Ref.
Natural Bone
1.8–2.1
3–20
130–180
3–6
(Hornberger et al., 2012)
AZ91
1.81
45
160
N/A
(Shadanbaz and Dias, 2012)
WE43
1.84
44
170
N/A
(Dorozhkin, 2014)
PLLA
1.25
2.86
–
55
(Burg et al., 2000)
ZK60
–
320
–
12
(Su et al., 2012)
Synthetic Hydroxyapatite
3.1
73–117
600
0.7
(Su et al., 2013)
Molecular Type
Structural Formula
Atomic Ratio of Ca/P
Calcium Phosphate(anyhydrous)
CaHPO4
1.00 (Posner and Betts, 1975)
Monocalcium Phosphate(MCP)
Ca(H2PO4)2
0.50 (Posner and Betts, 1975)
Di-calcium Phosphate dehydrate (brushite,DCPD)
CaHPO4·2H2O
1.00 (Heughebaert and Montel, 1982)
Calcium Phosphate(Amorphous)
Cag(PO4)6·nH2O
1.50(Heughebaert and Montel, 1982)
Octacalcium Phosphate(OCP)
CagH2(PO4)
1.33 (Koutsopoulos, 2002)
Fluorapatite(FA)
Ca10(PO4)6F2
1.67 (Tudela et al., 2014)
Hydroxyapatite(HA)
Ca5OH(PO4)3/Ca10(PO4)6(OH)2
1.67 (Tudela et al., 2014)
It has been demonstrated that covering Mg and its alloys with protective bioactive ceramic coatings improves their biocompatibility and slow their rate of corrosion in physiological patterns. DCPD, HA, and tricalcium phosphate (TCP) have intrinsic biocompatible and bioactive properties as Ca and P are the major constituents of bone minerals (Wang et al., 2012; Wu et al., 2013). Because of having structural and chemical similarity and bone concrescence speed ability, HA has become a popular coating material (Paital and Dahotre, 2009). A simple convenient technique was applied in previous work to prepare coating on MgAl by DCPD and HA which was uniform and adhered and useful for orthopedic implants having complex-shaped components (Su et al., 2012). A similar technique can be applied to magnesium composites to improve surface biocompatibility and corrosion resistance. The second phase plays a crucial role while the coating conversion takes place as the magnesium phase and magnesium alloys phase have different chemical potentials. Established galvanic coupling due to more anodic sites during the corrosion process makes electrochemical heterogeneity occurs in Mg composites (Su et al., 2013). However, when generating the conversion coating on Mg composites, this might potentially be a favorable component in the electrochemical reactions. The investigation was done on the coating deposition process by applying DCPD conversion coating on the surface of magnesium alloys. Later, an alkali post-treatment was employed to change the DCPD coatings into various HA coatings with the variation of the deposition times. The best HA and DCPD coatings were identified by investigating the electrochemical polarization behavior and the conversion coating duration effect. Studies were also done on simulated bodily fluid (SBF) best coatings immersion corrosion and electrochemical behaviors.
Highly reactive surfaces can be formed by the sol–gel method with the help of bioactive glass. A hydroxy carbonated apatite layer is formed when the bioactive glass is immersed in biological fluid. This integrates the surrounding bone by enhancing protein absorption to the implant surface (Xie et al., 2022; Qin et al., 2022). Ca:P ratio, microstructure, and composition determine the rate of ion release from the surface of bioglass. Some bioactive glasses increase the local pH value of some bioactive glasses with biological fluids which is beneficial for HA production and cell activity (Shaikh et al., 2022). Currently, bioactive glasses are available in particulate form, sol-gels, and sintered porous bulk (Wu et al., 2023).
One of the most serious surgical consequences is infection. Preoperative infection management has gotten a lot of attention in recent years, thanks to an increase in traffic accidents and a rise in the number of difficult orthopedic procedures. The pathogenic factor of peri-implantitis is bacterial biofilm formation, which is induced by bacterial adhesion and colonization and leads to implant loss. However, once a bacterial biofilm has formed, the immune system will have a difficult time removing it. As a result, supplementing magnesium with adequate antibacterial properties to prevent biofilm formation is a good strategy to avoid peri-implantitis. One of the most effective ways to get excellent antibacterial qualities is to coat with antibacterial substances. Zou et al. (Onuma and Serruys, 2011) have found that zinc-loaded montmorillonite coating has good antibacterial ability and corrosion resistance on magnesium alloy. In their investigation, they examined the gentamicin-loaded polymeric multilayers HA coating antibacterial properties and corrosion resistance on magnesium alloy and came to clear results (Zou et al., 2019). To improve the antibacterial capabilities of the coatings on magnesium and its alloys, many antibacterial substances were used. Antibiotics (Ji et al., 2019; Ji et al., 2019; Zhuk et al., 2014) or antibacterial metallic elements including copper, silver and zinc (Onuma and Serruys, 2011; Rezk et al., 2019; Zeng et al., 2013) were added to polymer coatings (Shao et al., 2020; Zeng et al., 2016; El-Kamel et al., 2019; Huang et al., 2015) or calcium phosphates (Cap) coatings (Perkins et al., 2015; Zhao et al., 2016; Tian et al., 2015).To improve the antibacterial coatings' combination qualities, a variety of physical and chemical surface modification procedures were used, including plasma electrolytic oxidation (PEO), Sol-Gel, dipping, and Lay-by-Lay (LbL) assembly, and plasma electrolytic oxidation (PEO). This study aims to gather antibacterial functionalized coating research on magnesium and related alloys and make a comparison. This review paper will be used as a reliable source for the researchers to explore the new insight to understand the properties of magnesium alloy in depth. The elaborate discussion of material characterization, biological, corrosion, and mechanical properties in relation to different coating processes can contribute to the development of more effective and biocompatible coatings for the repair of the human bone without any health concerns.
2 HA coating on Mg alloys for biomedical applications
The mineral compound of HA, teeth, and bone has the same density which is 3.16 g/cm3, and is spontaneously formed from a calcium phosphates (CaP) crystal phase and calcium apatite. CaP has gotten a lot of interest in the fields of chemistry, medicine, biology, and geology, as well as other interdisciplinary fields. Berzelius (Berzelius, 1845) was the first to attempt to determine the chemical composition of CaP compounds in the mid-eighteenth century. The identical phases of CaP crystal and CaP crystal combinations by Hausenet al. (Hausen, 1929) were known popularly as apatite later.
HA compounds have recently received a lot of attention in catalysis, medicinal products, water treatment, protein chromatography applications, and the development of biocompatible materials. Due to its inorganic nature and low water solubility, HA crystals have been discovered to easily calcify in skull bones and hard tissues, etc. (Koutsopoulos, 2001; Uchida et al., 1992; Kawasaki, 1991; Walsh and Guzelsu, 1994; Xie et al., 2014). Furthermore, HA coating is a time-saving and cost-effective processing technology, and compared to CaP, its chemical composition has more similarity to natural bone (Killian et al., 2010; Poinern et al., 2009). HA has osteointegration property which is useful for bone repair. Moreover, it is an inorganic compound of human bone which is biocompatible and biodegradable (Yang et al., 2016; Liu et al., 2009). Moreover, it has advantageous osteoconductive and osteoinductive properties for orthopedic applications (Walker and Walker, 1973). However, HA is brittle in nature and that is why it cannot be used in load-bearing applications (Ulaeto et al., 2017). Because of this, it is difficult to achieve nano HA, HA composite coating, conformal HA, homogeneous coating thickness, or crack-free coatings on magnesium alloy.
3 Surface coatings techniques for Mg alloys
3.1 Sol − gel process
In the preparation of coating, this approach uses two processes: hydrolysis and condensation. Sol-gel is a broad category of operations that involve the formation of a solid phase from a colloidal solution known as “sol.” After drying the gel, heat treatment could be applied to remove any leftover (unreacted) organic dregs, improve its density, acquaint it with crystallinity, and stabilize the gel (Ganguli, 1993). Environmentally acceptable coatings are usually used to make it. This one is a low-temperature method that makes the coated metal more resistant to corrosion, oxidation, and wear. Polymerization of organic functionalized metal alkoxide or inorganic metal alkoxide is performed by a systematic polymer. The possible chemical reactions are as follows:
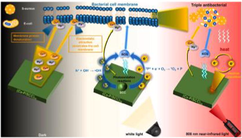
The first step in the operation is represented by equation (1), while the second and third steps are represented by equations (2) and (3). Monomeric metal or metalloid alkoxide precursors are denoted by M, organic group by R and monomeric metal or metalloid alkoxide precursor by M(RO)n. The hydroxylated metal centers are denoted by MOH, and oxo-polymers are denoted by MOM. The sol then gels and transforms into a coated layer. Inorganic sol–gel coatings and hybrid-based inorganic organic sol–gel coatings can both be made using this method (Wang and Bierwagen, 2009). Fig. 1 depicts a schematic representation of the sol–gel coating process.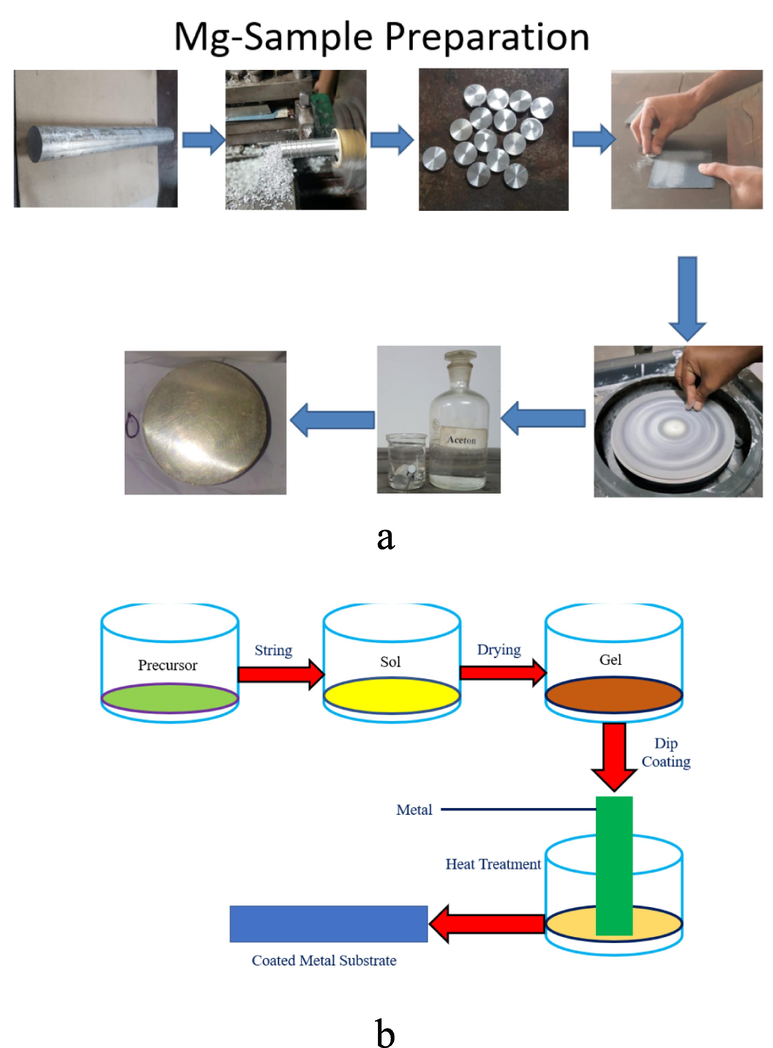
(a) Mg Sample Preparation and (b) Sol gel coating technique.
The sol–gel coating technique is depicted in Fig. 1. The following are some of the benefits of the sol–gel coating technique: (Barranco et al., 2010; Zheludkevich et al., 2012).
• Good quality adhesion of the coating material can be obtained by building diverse shapes and thin film coating by using liquid precursor. Furthermore, no machining or melting is required.
• It can produce high-purity goods, allowing for precise composition control.
• It can serve as an excellent matrix for encasing a wide range of chemical and inorganic compounds, as well as biologically important molecules.
• Keeping the processing temperature low, reduction of the thermal degradation or volatilization is possible in this process. The sintering process can also be performed at 200–600 °C.
• Sol-gel films are created using a “green” method. As starting materials, the used compounds do not introduce contamination into the end product. Preparation produces no trash and eliminates the need for washing.
• The sol–gel coating technique is a simple, cost-effective, and efficient way to make high-quality coatings. • A wide range of product compositions is conceivable, and their chemical and thermal durability is excellent. Sol-gel silica-based films could produce a SiO metal layer, resulting in a stable metal oxide/sol–gel film interface and, as a result, lowering metal corrosion. These films are good pretreatment solutions because they have good adhesive characteristics and mechanical strength (Castro et al., 2002). Achieving less thickness by mechanical deposition such as dip coating, spin coating or spraying is the prime limitation of the sol–gel method (Fernández-Hernán et al., 2021).
Surface tension, density, viscosity, withdrawal speed, and function of gravity are the parameters that have an effect on the structural properties of sol–gel-derived coatings. The effect can be described with the help of the Landau-Levich Equation (Takagi et al., 1998):
(η.ʋ)2/3.
Here, η is the viscosity of the solution.
ʋ is the withdrawal speed from the solution.
ρ is the density of the solution.
g is the acceleration due to gravity.
γ is the liquid vapor surface tension.
3.2 Cap based antibacterial functionalized coatings
Utilization of calcium phosphates (CaP) has been done as protective coatings for a long time and has a high level of biocompatibility (Ramselaar et al., 1991; Coelho et al., 2009). Cap is a biological compound that belongs to the orthophosphate family and has been found in a variety of biological structures, including teeth and bone. Furthermore, synthetic hydroxyapatite has been found to have characteristics that are similar to the Cap found naturally in the inorganic component of bone. CaP coatings have been shown to improve the biocompatibility of metallic implants while also promoting osteogenesis at the implantation site (Sridhar et al., 2003; Tan et al., 2017). As a result, Extensive investigation has been performed on CaP films as a modified membrane layer to improve the corrosion resistance of magnesium alloy. Recent research on calcium phosphate/tetracycline (CaP/TC) composite coating fabrication on magnesium observed that CaP coating becomes more uniform and compact by TC additives along with providing the CaP coating optical antibacterial ability (Hussein et al., 2013).
PEO-based polymer coating and plasma electrolyte oxidation coating corrosion process is shown in Fig. 2. Hydroxyapatite (HA, Ca10(PO4)6(OH)2) is the primary mineral component of teeth and bone which is the naturally occurring mineral form of calcium apatite. Because of its excellent osteoconductive and osteo inductivity, HA has been utilized to induce and stimulate the production of new bone (Ozeki, K.; Goto, T.; Aoki, H.; Masuzawa, T. Influence of the crystallinity of a sputtered hydroxyapatite film on its osteocompatibility. Bio-Med. Mater. Eng., 2015; Predoi et al., 2020; Pang et al., 2015; Tan et al., 2011; Wang et al., 2022). Efforts are made to combine antibacterial elements with HA. Table 3 shows some typical formation processes of HA for antibacterial preparation.(See Table 4.).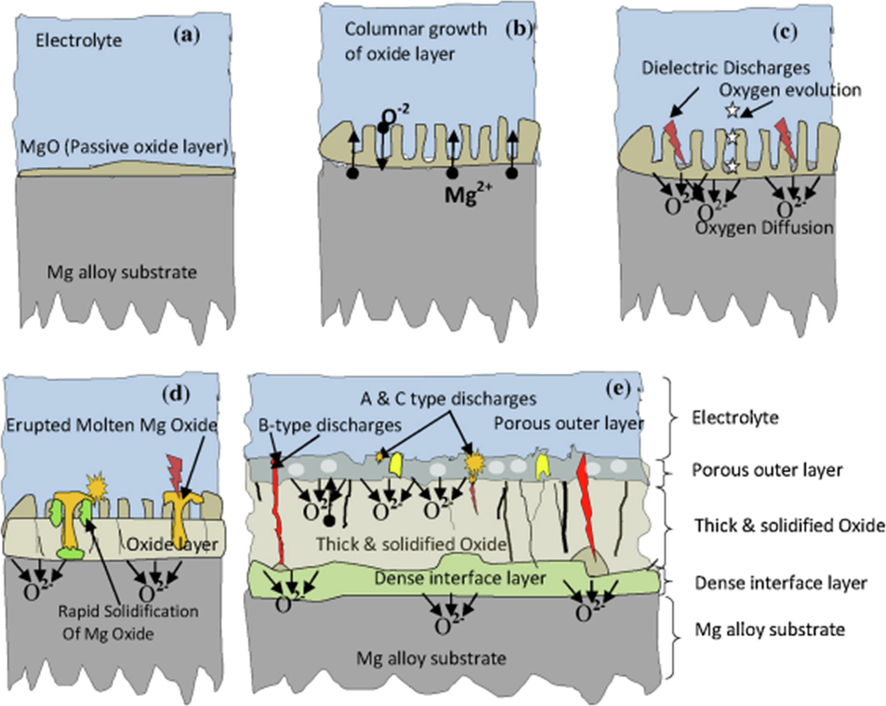
A schematic of the plasma electrolytic oxidation (PEO) coating(Tesavibul et al., 2015).
year
Coatings
Substrate
Solution
Immersion Time
Temperature (°C)
Ref.
2022
HANFsCoating
Methacrylic anhydride-modified gelatin
0.5 g NaOH solution, 6 g ethanol, 6 g oleic acid, 10 mL CaCl2, (0.11 g) and 5 mL NaH2PO4··2H2O (0.2 g)
23 h
180
(HadiSamadian and MahmoudAzami, 2020)
2020
HA crystals coating
carbonized nanofbers
Acrylonitrile, N,N-dimethylformamide, MG-63 cell line, nutrient mixture F-12, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, fetal bovine serum, Lactate dehydrogenase
4 h
290
(PeizhenDuan, 2018)
2018
NRHA coatings
Graphene oxide
NaCl, NaHCO3, KCl, K2HPO4$3H2O, MgCl2$6H2O, Na2SO4, Tris-HCl, and HCl
2 h
Room temperature
(Le et al., 2020)
2020
Col − HAcoating
synthetic analogues of NCPs
NaCl, >99 %, K2HPO4··3H2O, >99 %, TPP, 85 %, NaHCO3, >99 %, FeCl2··4H2O, ≥99 %, MnCl2··4H2O, ≥99 %, CaCl2, >99 %, MgCl2··6H2O, >99 %, NaOH, 1 N, and 4-(2-hydroxyethyl)-1- piperazineethanesulfonic acid (HEPES, >99 %)
23 h
37
(Bai et al., 2017)
Type of coatings
Antibacterial property and related observation
Implant and relatedapplications
characteristics of coating
Ref.
Fluoride Coating
(Sun et al., 2016; Li et al., 2017; Zhang et al., 2022)
Mg-Mn LDH coatings

(Pan et al., 2022)
Aliphatic polycarbonate (APC) coatings


(Cui et al., 2021)
Chitosan /deoxyribonucleic acid (CHI/DNA)5 coating
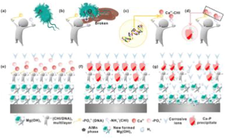
(Zhang et al., 2022)
Cu-bearing chlorophyllin-induced Ca–P coating
(Zhang et al., 2018)
Catechol /polyethyleneimine conversion coating

(Yuan et al., 2022)
Magnesium hydroxide/graphene oxide/hydroxyapatite composite coating
(William, 2020)
PFLX (Pefloxacin), Ag-FHA (Silver fluoridated hydroxyapatite), Zn (Zinc), and Mg (Magnesium).
HA is often used on magnesium alloy as drug-loading coatings because it is a substantial mineral ingredient of the bone matrix. Bai et al. created the hydroxyapatite (HA)/pefloxacin (PFLX) drug-eluting layer on AZ91. Biomimetic mineralization was used to create the HA coating, which was then dipped in 1-mg/mL, 10-mg/mL, and 100-mg/mL PFLX aqueous solutions (Wang et al., 2017). The results demonstrated that an appropriate dose of PFLX (10 mg/mL) will improve the HA coating's corrosion resistance. However, if PFLX levels rise, the acidity of PFLX will erode the HA coating's integrity. E. coli was used in their investigation to evaluate the antibacterial performance of the HA/PFLX coating. Antibacterial metal ions can also be combined with HA. A mussel-inspired nano-multilayered coating was proposed by Wang et al. (Zasloff, 2002) which will be on AZ31 magnesium alloy containing a CaP periodic unit that will combine the benefits of antibacterial activity of silver nanoparticles, excellent bioactivity of biomimetic CaP nanoparticles, and osteoconductive, PDA (polydopamine strong)'s adhesion, and chitosan's biocompatibility (Zasloff, 2002). All living things produce antimicrobial peptides (AMPs) which are popularly known as innate immune components (De Smet and Contreras, 2005). On a broad spectrum, these peptides can resist both gram-positive and gram-negative bacterial (Kazemzadeh-Narbat et al., 2012). Unlike conventional antibiotics, AMPs become insusceptible when the antibacterial process happens and that is why it offers reliable antibacterial activity, especially against antibiotic-resistant bacteria. AMPs have cationic properties which make them interact with bacterial cells avoiding mammalian cells. This makes it highly antibacterial without much damage to the host cell (Jamesh et al., 2012). There are currently just a few types of research on the use of AMPs to modify the surface of magnesium alloys. In vivo and in vitro experiments help to demonstrate the osteogenesis impacts, antibacterial ability, and biocompatibility of the HA-AMP coating. Besides, HA coating has controllable slow-release rates of the antimicrobial peptides with high drug-loading efficiency.
3.3 Electrochemical methods
James et al. (Li et al., 2019) generated a HA coating using an electro-deposition approach on a pure Mg sample in a mixture of electrolytes made of 0.06 M (NH4)3PO4, 0.1 M Ca (NO3)2, and 10 mL/L of 30 vol% H2O2 at 27 °C and a pH of 4. The electro-deposition of HA coating on an Mg plate sample is depicted in Fig. 3. The coating was made up of DCPD crystals after electro-deposition. HA coating was obtained by submerging as-deposited pure Mg samples for 120 min in 1 M NaOH solution at 80 °C. On the pure Mg surface, a homogeneous flake-like crystal structure was discovered, which facilitated osseointegration. The HA coating procedure through the electro-deposition technique is shown in Fig. 3.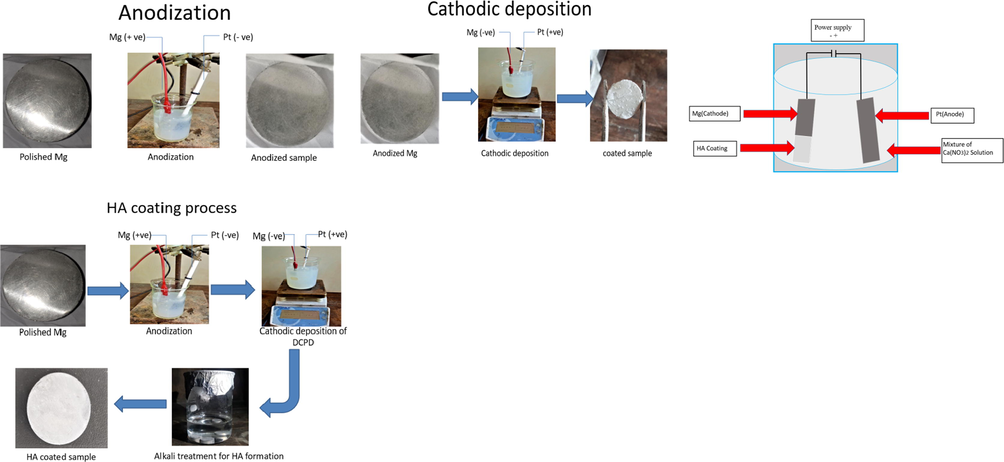
HA coating procedure through electro-deposition technique.
Micron particles are removed effectively from the cathode surface with weak bonding by the ultrasonic vibration and resulting jets (Poinern et al., 2009). With the solution flow, the particles are then disseminated throughout the electrolyte (Fig. 4 (1)). Bubbles are created around the cathode during the electrodeposition process by the electrolysis of water. Without ultrasonic treatment, buoyancy reaction repelled the bubbles in an upward direction along the braids’ surface. An ultrasonic field, on the other hand, causes the bubbles to vibrate in a radial, uniform linear pattern. Bubbles are subsequently transferred and distributed across the surface of the micron HA particle in the electrolyte, and over the surface of the particles, a mesoscopic eddy was initiated by the spherical wave. It achieves mesoscopic uniform mixing, removes unequal local electrolyte concentrations, and manipulates crystal nucleus formation. The use of ultrasonic therapy on the same sedimentary substrate in the same location helps to produce more nucleation sites minimizing the distance between nucleation sites and resulting in a denser needle-like structure. Ultrasonic effect microscopic view of the HA crystal formation process reveals that crystal growth rate and nucleation site numbers are related to the crystal size. Crystal nuclei and a large number of nucleation sites should be available during the nucleation stage so that nucleation growth can be determined during the crystallization process (Fig. 4). A sufficient amount of nucleation energy is required during the development of a critical nucleus at the stage of nucleation within the system via energy fluctuations. The nucleation energy is highly increased by partial high energy by strengthening per unit volume energy fluctuations in an ultrasonic environment (Fernández-Hernán et al., 2021). As a result, the system's sub-nucleus can readily reach the requisite nucleation energy, enhancing nucleation probability and forming a large crystal nucleus almost rapidly. Furthermore, the ultrasonic cavitation effect can effectively manipulate crystal nucleation development during the crystal growth period, as seen by the denser needle- Fig. 4. Model of ultrasonic effects on HA electrodeposition.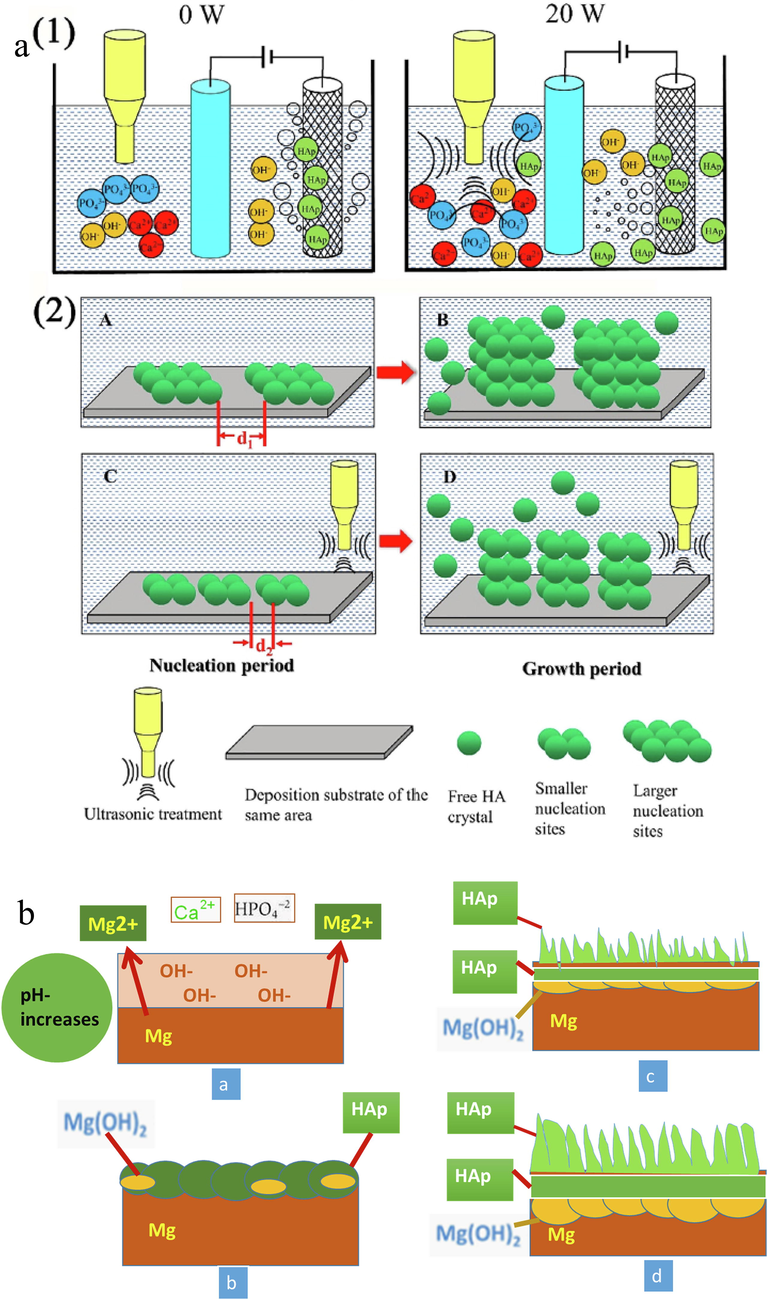
(a) Model of ultrasonic effects on electrodeposition of HA (Tomozawa and Hiromoto, 2011) and (b) Schematic illustration of the formation mechanism of HA crystal structures on Mg substrate.
Tomozawa et al. (Li et al., 2019) various crystal-shaped HA covering on a Magnesium substrate employing a hydrothermal treatment technique. Immerging magnesium substrate the HA coatings were created in a solution made by Ca-EDTA and KH2PO4 for 0.6 to 28.8 ks at 60 °C. The solution pH was 8.9 and the duration of processing was 0.6 to 28.8 ks. Fig. 4 shows the HA crystal formation procedure on magnesium substrate. Certain chemical reactions start the corrosion in the HA-coated magnesium sample after immersing in the treatment solution and thus the pH value increases (Fig. 4a). Mg(OH)2 coated layer is created with the rapid nucleation of HA crystals due to the increase of pH value on the magnesium sample. Ca2+ ions are supplied continuously to encourage the crystal growth of HA nucleation over the Mg(OH)2layer creation. Because of the magnesium surface's thick coating with dome-shaped HA crystals and frequent nucleation, the rate of corrosion is less compared to the previous one. As a result, the pH value is raised and Mg2+ discharge is controlled. pH value controllable rod-like structured HA crystals are created during the hydrothermal process and formed on the magnesium substrate surface. Li et al. (Wen et al., 2021) made glucose-induced CaP coatings on pure magnesium with an alkaline solution that had Ca deficient hydroxyapatite, HA, and dicalcium phosphate anhydrous. They found significantly increased corrosion resistance of pure magnesium by CaP coating. The electrodeposition technique can be performed at room temperature. H2 is formed in the cathode due to the substrate to anode's slow rate of ion transfer which is a negative side of the standard electrodeposition process. Furthermore, this process is less sticky and more porous.
3.4 Hydrothermal synthesis
Hydrothermal synthesis is the compound's chemical synthesis in the liquid solution at the pressure of 1 MPa to 1 GPa and a temperature of 100 to 1000 °C. The rate of dissolution and crystallinity of HA coating are both high made by hydrothermal technique.
Wen et al. (Li et al., 2019) created HA coating on AZ31B Mg alloy surface by hydrothermal bonding. Good structure, coating morphology, and corrosion resistance were obtained at the 1.67 calcium phosphate ratio and 0.1 mol/L concentrated solutions. Delaying the early corrosion of the magnesium alloy substrate, produced coatings corrosion potential increased 1.18 V from 1.51 V and its impedance climbed to 1.0 105 Wcm2 whereas a reduced Ca/P ratio was found to 1.58. A high-pressure torsion was used by Li et al. (Zhang et al., 2019) for the magnesium alloy pre-deform a ZEK100 followed by creating HA coating with different percentages of Mg(OH)2nano-powders using a hydrothermal synthesis method on the surface. The adhesive tape test results show that 0.3 mg/mL Mg(OH)2 containing HA coating had interfacial bonding strength of 4B which contained less than 5 % coating peeling area. Whereas, the untreated magnesium alloy had the interfacial bonding strength of 2B that contained 15–35 % of peeling area. More nucleation sites were offered for HA coating compared to the magnesium alloy by the HPT-treated magnesium alloy microstructure that contained many grains, grain boundaries, and twins. Significant improvement took place in the interfacial bonding strength of the HA coatings by the promoted HA deposition made by 0.3 mg/mL Mg(OH)2nano-powder. A hydrothermally synthesized HA coating was created by Zhang et al. [8 9 1] on the fluorinated Az31 magnesium alloy surface. Good corrosion resistance with an average thickness of 10 µm was obtained by the HA/MgF2 coating. The bonding strength between the magnesium alloy matrix and HA coating was improved by the MgF2 interlayer. Delaying the magnesium alloy deterioration, segregation was performed in the interface between the magnesium alloy matrix and SBF by the HA covering and the dense MgF2 interlayer bonding. The MG63 cell growth was tested on alloy surfaces by live/dead staining and CCK8 assay. Early cell attachment was aided by the HA/MgF2-coated nanocrystal structure. The biocompatibility of the HA/MgF2-coated sample was good after 7 days when it was fused with MG63 cells. Promoting bone formation and regeneration by limiting bone resorption and preventing osteoporosis in some trials has been demonstrated by strontium (Sr) (Ni et al., 2006; Reginster et al., 2009; Yang and Wang, 2020). Yang et al. (Zhou et al., 2020) used a hydrothermal synthesis process to produce a strontium substitute HA coating (Sr-HAC) on the alloy of AZ91D. The findings show that the release of Mg2+ ions concentration was lowered by Sr-HAC and increased the viability of the MG63 cells successfully. The viability of osteoblasts was greatly boosted with the improvement of magnesium alloy biocompatibility by Sr-HAC and nano-structured lamellar surface in an experiment performed in vitro cell culture. Installing a HA coating becomes difficult on magnesium alloy in hydrothermal synthesis because of the absence of adsorption. A PDA interlayer has been used by Zhou et al. (Li et al., 2019) on an AZ31 magnesium alloy to promote HA coating production. The pure HA coating is thinner and less dense than the dopamine-induced HA coating according to Fig. 5. The dopamine-induced HA coating also reduces the corrosion rate significantly. Moreover, using cells and extract of co-cultured alloy investigation was done on cell proliferation and it was discovered that after 5 days, cells coated with dopamine HA had a survival rate of 120 percent. On the coated samples, there were a lot of polygonal cells, and there were filopodia between the cells and the matrix.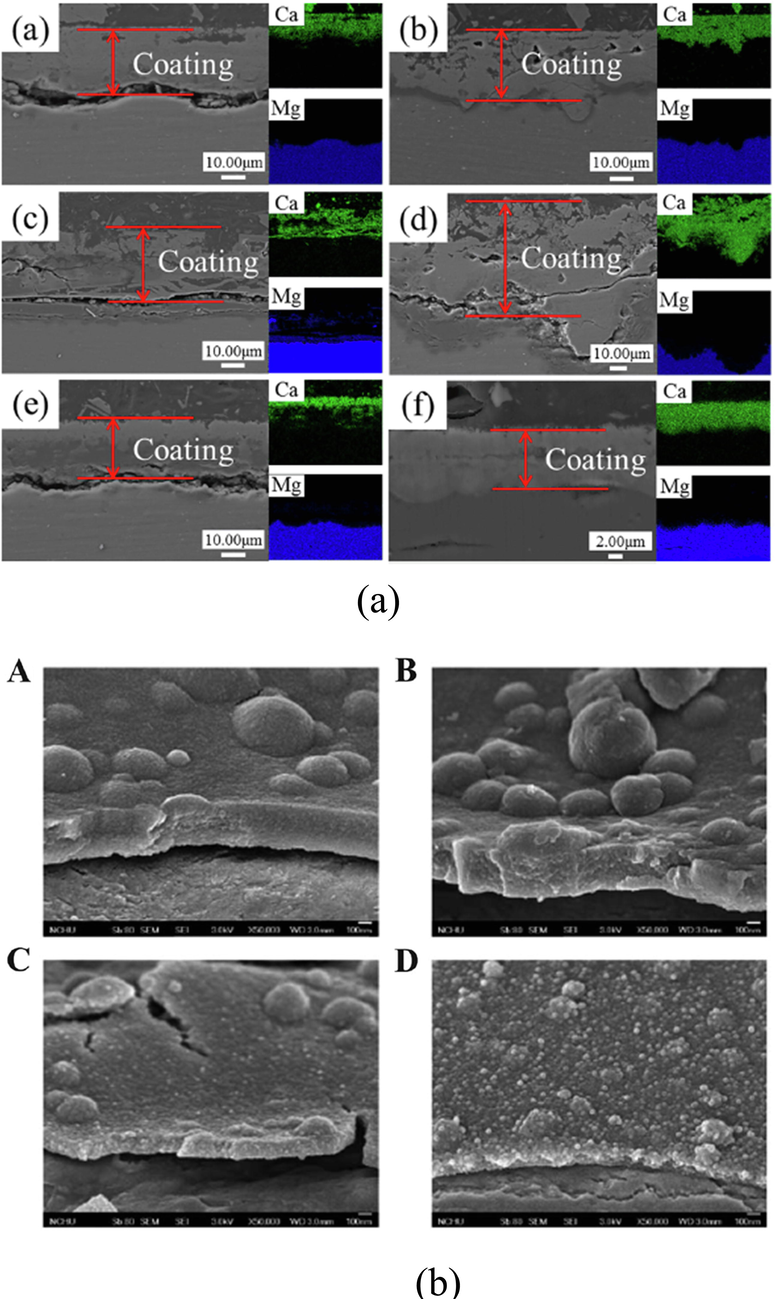
(a) Cross-sectional images of HA coating(Ho and Ding, 2015) and (b) PDA-HA coating(Zheng et al., 2021).
3.5 Chemical conversion method
Chemical conversion is another method used to from coating on Mg and its alloys. In this process, chemical transformation happens and the product differs from the starting material chemically. It has sequenced steps in which changes in chemical makeup, energy level, phase state, or combination of these. The step is called unit operation if the changes are only physical in nature. The step is called unit process if the changes are chemical in nature. Some steps involve both of these. The process is said to be a chemical conversion for both of the processes either the overall process involving chemical transformation or certain of the unit process (Liu et al., 2005).
4 Surface morphology of HA-coated Mg alloys
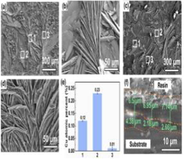
Coatings SEM micrographs of the surface of AZ91 magnesium alloy are shown in Fig. 6. The SEM micrographs of the magnesium alloy surface show that the HA coating was microporous having plate-like morphology with petal-shaped crystals. Tinierpetal shape crystals appeared in the AMP-loaded HA-coated AZ91 compared to without AMP-loaded HA-coated AZ91. A comparatively higher number of corroded pores appeared on the AMP-loaded naked AZ91 surface than the bare AZ91 sample.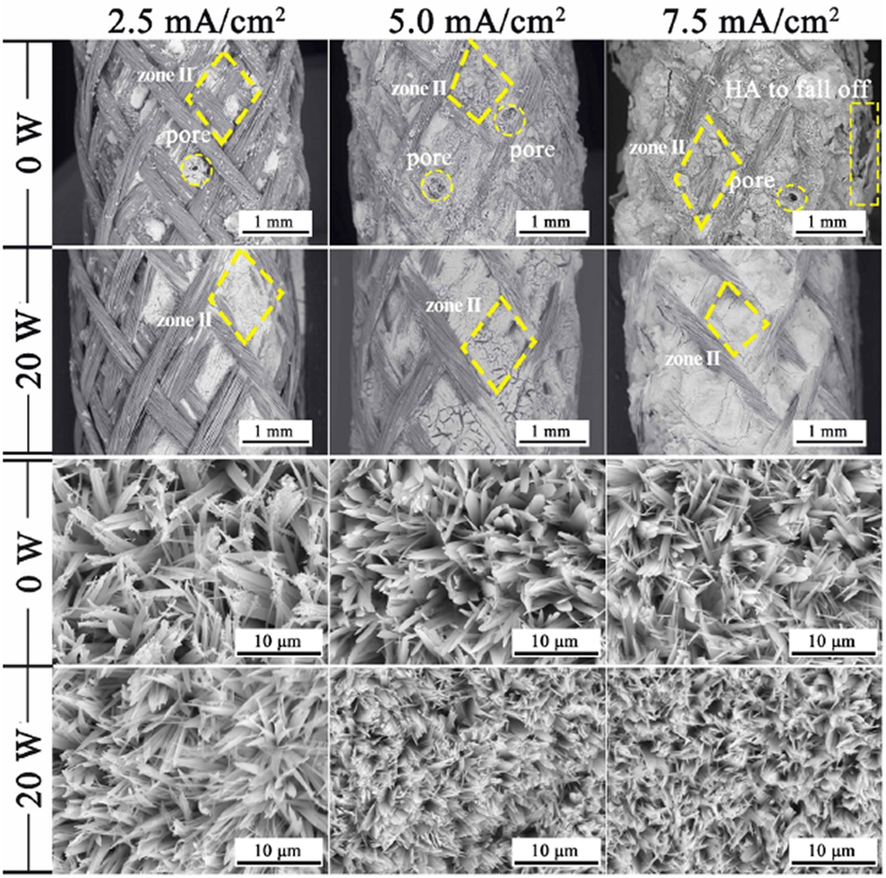
SEM images of the electro-deposited HA coating over the braid surface (Lee et al., 2009).
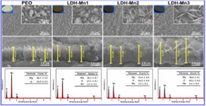
Calcium phosphate layer development on AZ91 magnesium alloy was evaluated with the help of the FTIR spectrum shown in Fig. 7 (b,d,f). Distinctive peaks of phosphate groups of the mineralized particles are shown by the FTIR spectra. Symmetric stretching vibration peak O—H was attributed at 3420 cm−1. 1035 cm−1 and 962 cm−1 were attributed to the stretching mode of PO43 and 601 cm−1 and 562 cm−1 were attributed to the bending mode of PO43. Carbonate group CO32– present at 1420 cm-1and 876 cm−1 indicated the production of carbonated hydroxyapatite Ca10(PO4)3(CO3)3(OH)2 (Wang et al., 2009). Fig. 7h shows the XRD patterns of the HA coating deposited on magnesium alloy. Large characteristic diffraction peaks were observed at 2 h of 26 and at 32.5 in the HA coating XRD analysis.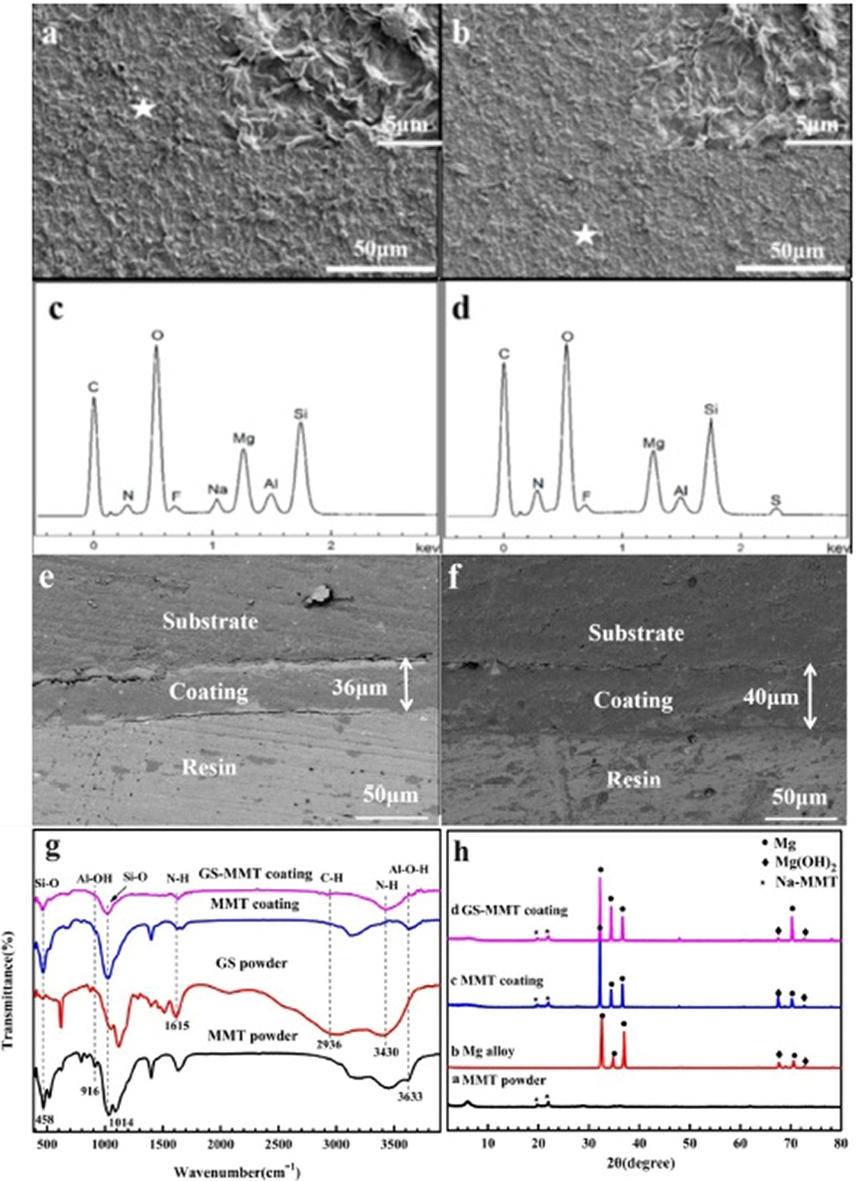
SEM morphologies, corresponding EDS spectra, cross-sectional micro-graphs of the MMT (a, c, e) and GS/MMT (b, d, f) coating and FT-IR spectra (g) and XRD patterns (h) (Dvorsky et al., 2020).
Peaks of 32.5, 34.5, 36.0, and 48.0 diffractions were weakened after 2 h. The maxima for HA and Mg (Zhang et al., 2022; Ji et al., 2019) were found to be overlapping in this study.
5 Magnesium alloy degradation in vitro
Table 5 shows each sample's degradation profile. The naked magnesium alloy lost mass more quickly than the HA-coated magnesium alloy. The bare magnesium mass loss percentage was 36.13 for the first 15 days whereas the HA-coated magnesium alloy mass loss percentage was only 7.62 in the same days. A biomimetic solution mineralized HA-coated magnesium alloy. The corrosion of magnesium alloy could be reduced by covering the sample's surface (Surmeneva et al., 2015).(See Table 6.).
Type of coating
Corrosion results
Ref.
Fluoride Coating

(Fan et al., 2022; Li et al., 2008; Jorgimara de et al., 2020)
Aliphatic polycarbonate (APC) coatings

(Kazemzadeh-Narbat et al., 2010)
chitosan/deoxyribonucleic acid (CHI/DNA)5 coating
(Diosa et al., 2020)
Hydroxyapatite coating induced by polyacrylic acid and gentamicin sulfate
(Leonor et al., 2009)
Catechol /polyethyleneimine conversion coating
(Liu et al., 2003)
Magnesium hydroxide/graphene oxide/hydroxyapatite composite coating
(Wang et al., 2021)
Gentamicin-montmorillonite coating
(Kumar et al., 2016)
Cu-bearing chlorophyllin-induced Ca–P coating
(Tian et al., 2019)
Coatings
Mechanical Properties
Ref.
Fluoride Coatings
(Rojaee et al., 2013)
Chitosan/graphene oxide- magnesium oxide (CS/GO-MgO) nanocomposite coatings
(Jo et al., 2011)
Electrophoretic (EPD) coatings
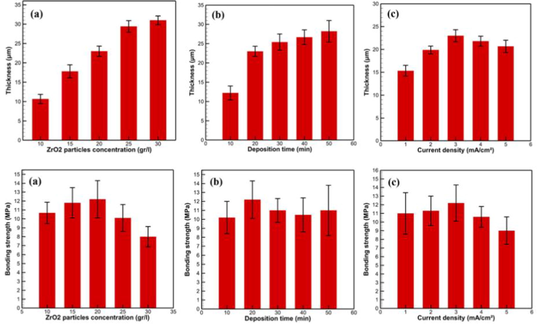
(Xu et al., 2009)
Cast and annealed AZ91 Mg alloy
(Meng et al., 2011)
Nano-to-submicron hydroxyapatite coatings
(Liu et al., 2019)
Plasma electrolytic oxidation (PEO) coatings
(Hiromoto et al., 2015)
AZ91/HA bio-nanoMgcomposite
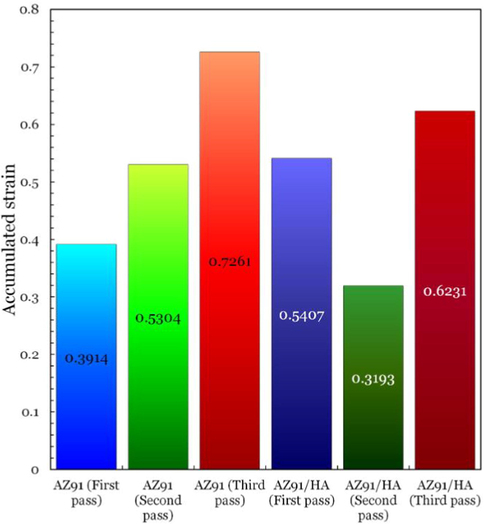
(Mousa et al., 2015)
Layer-by-Layer Engineered Hybrid Coating
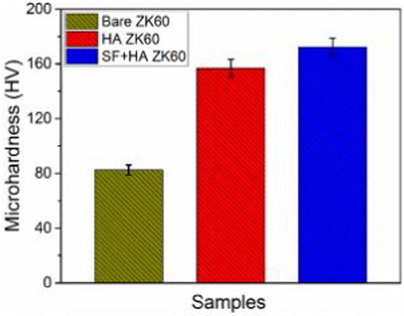
(Dunne et al., 2016)
The biocompatibility of Mg increases in the physiological medium due to its reaction with the increase of time in which Mg2+ ions are released and form the Mg(OH)2 layer. Another layer of either amino acids or organic matter forms when corrosion slows down. These layers enable cells to grow and can shield the physiological environment around the material (Dubey et al., 2019). The reaction happens as follows:
6 Antimicrobial peptide loading and release
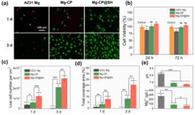
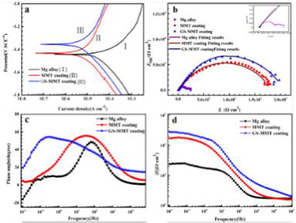
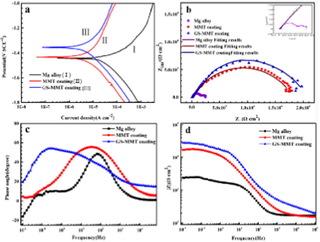
AMP PSI 10 put quantity onto the surface of AZ91 magnesium alloys is shown in Fig. 7a. The concentration of magnesium alloy with AMP loaded onto HA coating is higher than the bare magnesium alloy with AMP. The critical function was performed by the HA coating in adsorption (Bakhsheshi-Rad et al., 2016). Fig. 7b shows the HA-coated magnesium alloy as well as bare magnesium alloy with AMP PSI 10 release profile. Fast and burst discharge of AMP PSI 10 was observed in the initial hours from the bare magnesium alloy. Around 54 % release of the loaded AMP PSI 10 was done in the first 6 h. The gradual release was observed by the other parts over a five-day period. The slow and constant release of the AMP was found during the experiment from the HA-coated magnesium alloy. 57 % release of the loaded AMP was performed in two days. Fig. 8 shows the HEA curves of AZ31, MMT, and GS/MMT coatings. The loaded amount and release profiles of AMP onto the magnesium alloy surface can be seen in Fig. 9.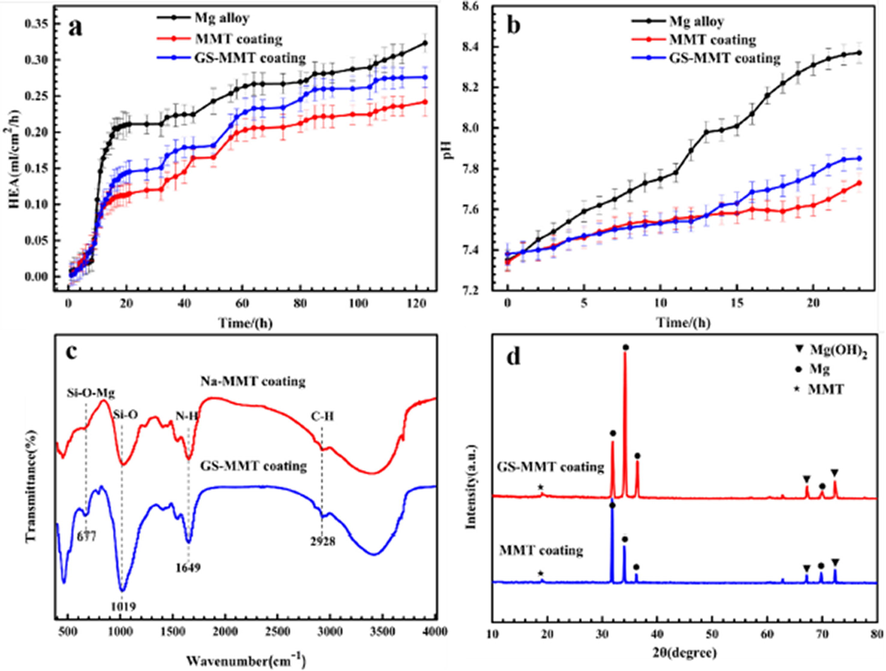
HEA curves of AZ31, MMT and GS/MMT coatings immersed for 120 h (a), variation in pH values for AZ31, MMT and GS/MMT coatings in DMEM for 24 h (b), FT-IR spectra (c) and XRD patterns (d) of the MMT coating and GS/MMT coating immersed in DMEM for 5 days (Baghbaderani et al., 2022).
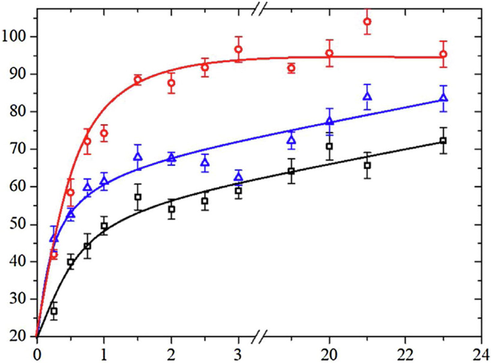
The loaded amount and release profiles of AMP onto themagnesium alloy surface: the loaded amount of AMP onto themagnesium alloy surface(Saji, 2021).
7 Antimicrobial test against S. Aureus
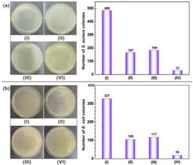
The test bacterial strain was killed by AMP placed in either the naked magnesium alloy or the HA Coated alloy. For both specimens, a distinct inhibition zone was found (Fig. 10). Larger inhibition zone was observed from the HA-coated AZ91 PSI 10 than AZ91 PSI 10. The antibacterial efficacy of the HA-coated AZ91 PSI 10 and AZ91 PSI 10 was observed against S. aureus at different times. In the first 6 h, the HA-coated AZ91 PSI 10 showed 50 % more antibacterial inhibition efficiency than the AZ91 PSI 10. In the first 4 days, the HA-coated AZ91 PSI 10 bacterial inhibition rate exceeded 50 % and the AMP activity of HA-coated AZ91 PSI 10 was maintained due to the HA coatings supporting the delivery of the AMP. The activity of AMP was retained when it was integrated into the HA crystals (Najm et al., 2022; Tian et al., 2019).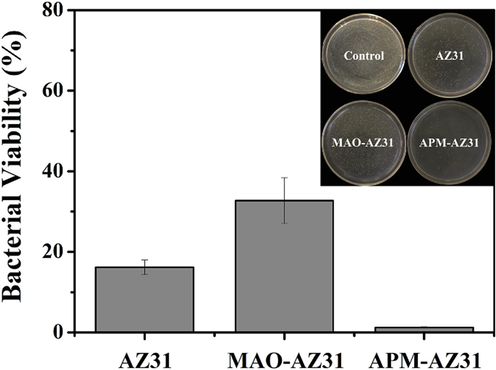
Histogram of the viability of S. aureus treated by AZ31, MAO-AZ31 and APM-AZ31. Insets are the corresponding photographs of bacterial colonies (control is S. aureus without treatment) (Pezzato et al., 2019).
8 Mechanical properties of HA-coated Mg alloys
Common uses of metallic biomaterials are seen in orthopedic implants because of their load-bearing capacity and mechanical qualities. Fracture toughness, hardness, and elastic modulus of coated and uncoated biomaterials can be determined using a variety of test methods, including micro- and nano-indentation testing. Kumar et al. (Yousefpour and RoohollahJamaati, and HamedJamshidiAval. , 2022) used an electrodeposition technique to create a HA coating on an Mg-3Zn substrate for orthopedic purposes. They used instrumented micro-indentation to evaluate the HA-coated alloys’ mechanical properties including hardness, modulus of elasticity, and fracture toughness. Furthermore, it was stated that when fracture toughness decreased, hardness increased. Li et al. (Rahman et al., 2022) used a hydrothermal approach to synthesize HA coating on ZEK100 magnesium alloy to compare with an uncoated sample and found that HA-coated alloy had superior mechanical properties. Tian et al. (McConnell, 2012) synthesized nano-structured HA coating on magnesium substrate to improve mechanical properties and corrosion resistance. The mechanical properties improved dramatically of the HA-coated magnesium compared to uncoated magnesium. Surmeneva et al. (Song et al., 2008) improved the of the substrate coated with HA both on micro and nanoscale and increased wear resistance significantly when compared to the uncoated substrate by creating nanostructured HA coating on AZ31 magnesium alloy by using radio frequency (RF) magnetron sputtering technique. Magnesium alloys’ mechanical properties are improved using ceramic materials recently as a reinforcement material. HA reinforced magnesium alloy composites were manufactured by Dubey et al. (Mena-Morcillo and Veleva, 2020) and they found improved mechanical properties compared to Mg-3Zn alloy. It was also claimed that after 3 days of immersion, the composite materials maintained mechanical integrity with a 66 percent ultimate tensile strength. Furthermore, on Mg alloy, a combination of organic and inorganic nanocomposite coatings can improve mechanical properties. nFHA/polycaprolactone (PCL) nanocomposite has been synthesized by Bakhsheshi-Rad et al (Kang et al., 2013) on magnesium alloy to increase corrosion resistance and mechanical properties. Mg-2Zn-3Ce magnesium alloy coated with nFHA/PCL composite degrades slowly compared to nanolayered PCL coated substrate because of the magnesium substrate/coating interface adhesive strength, which delays the passage of bodily fluid, resulting in reduced corrosion attack. As a result, the compressive strength of the implant material was reduced significantly, resulting in improved mechanical integrity and corrosion resistance, as well as enough support for post-fracture bone tissue repair. Mechanical properties can be improved using this method to make anticorrosion coating which can then be followed by an appropriate surface treatment to prevent the Mg surface from being exposed to bodily fluid. When magnesium comes into touch with water, it reacts quickly and begins to corrode, resulting in a loss of mechanical integrity.
9 Corrosion behavior of HA-coated Mg alloys
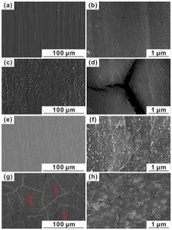
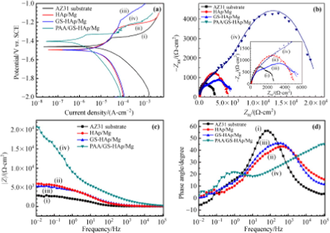
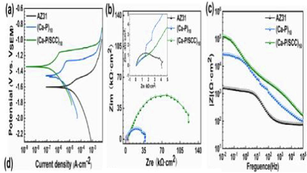
The corrosion performance, as well as bioactivity of HA-coated magnesium alloy, are heavily influenced by the surface shape, microstructure, and composition. Because of their exceptional thermal stability, high corrosion resistance is possessed by the HA-coated magnesium alloy substrate in the physiological environment (Ji et al., 2019). Traditional Mg alloys' poor corrosion resistance, as well as their lack of biocompatibility and bioactivity, limit their use in orthopedics and cardiovascular devices. The electrochemical deposition technique was used to produce an HA coating on the AZ91D alloy surface to improve biocompatibility, bioactivity, and corrosion resistance (Zhang et al., 2016). A mixture of electrolytes of 0.025 mol/L NH4H2PO4; 0.1 mol/L NaNO3, and 0.042 mol/L Ca(NO3)2 at 85 °C having a pH value of 5 at an immersion time of 60 min prior to the immersion of the coated magnesium alloy for 4 h in NaOH solution at 80 °C and dried for 4 h at 60 °C. Radiating plate-like structure was observed in the as deposited state and a needle-like structure was observed after the post-treatment on the magnesium alloy deposited by HA in morphological analysis. Fig. 11 shows the SEM images of the HA-coated AZ31 and uncoated AZ31 after immersing in SBF solution. The uncoated sample had more cracks and pits compared to other samples. Post-treated samples were more corrosion resistive, and bioactive, and had a lower degradation rate.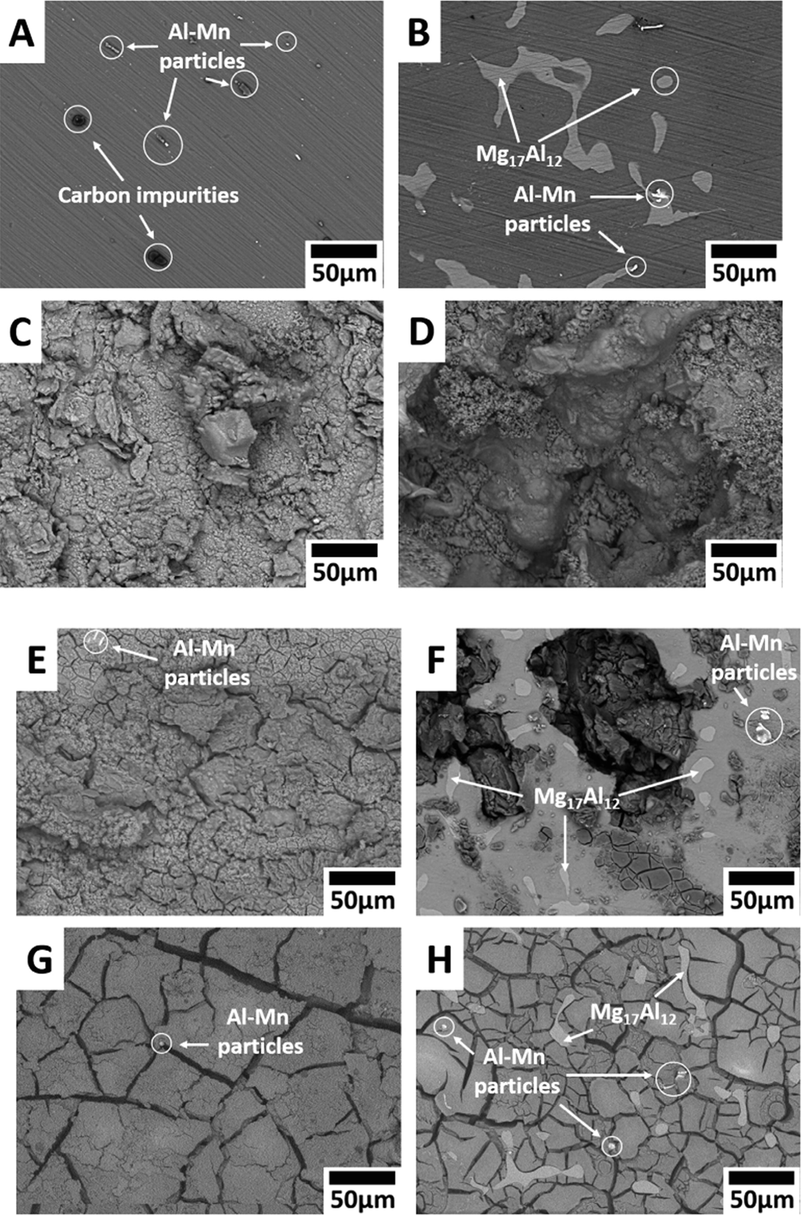
SEM images of Mg-alloys surface before exposure: (A) AZ31, (B) AZ91; and after their exposure to Ringer’s: (C) AZ31, (D) AZ91; Hanks: (E) AZ31, (F) AZ91; and SBF: (G) AZ31, (H) AZ91 solutions at 37 °C (Bakhsheshi-Rad et al., 2016).
HA coating has been synthesized by Kang et al. (Chen et al., 2012) on biodegradable magnesium having a needle-shaped crystal structure that showed good corrosion resistance in SBF solution indicating that it might be used in implant applications. In the physiological environment, an alkali treatment can help improve the corrosion resistance of magnesium alloys. Rojaee et al. (Zhang et al., 2012) used an electrophoretic deposition technique to create a nanoHA (nHA) coating on an anodized AZ91 Magnesium alloy and found that corrosion resistance was greatly improved compared to uncoated Magnesium alloy, tested by potentiodynamic polarization tests. A double-layer coating considerably improved the corrosion resistance and biomedical properties of Mg alloys (Guan et al., 2012).HA coating was created on an Mg-Mn-Zn alloy treated with phosphate to improve corrosion resistance and tested by EIS test. The results showed that the corrosion resistance increased with the increase of coating thickness compared to naked Mg-Mn-Zn alloy (Makkar et al., 2020). Hydroxyapatite (nFHA) coating doped with nano fluorine was synthesized in another study on Mg-Zn-Ca alloy and tested by immersion and potentiodynamic polarization tests. The obtained result indicated the implication of the nFHA coating on magnesium alloy because of its excellent corrosion resistance and can be applied in clinical applications as implant material due to its excellent biodegradability (Kim et al., 2014). Magnesium and its alloys can’t be used in clinical applications as these materials are low corrosion resistive. The antibacterial properties and corrosion resistance of magnesium alloys can be improved by inducing antibiotic, protein, or polymeric components. DNA doped bioinspired Ca-P coatings have been synthesized by Liu et al. (Wang et al., 2010) on AZ31 magnesium alloy to improve corrosion resistance and bonding strength. The results indicated that improved corrosion resistance and bonding strength were obtained by DNA addition to the electrolyte. The adhesion between the coating and the substrate has been improved by the gentamicin sulfate (GS)/polyacrylic acid (PAA) containing HA coating with the improvement of antibacterial performance and corrosion resistance. The corrosion resistance of OCP and HA coating on magnesium alloy has been compared by Hiromoto et al. (Zhou et al., 2020) in NaCl solution. The obtained result showed greater corrosion resistance by the HA coating due to its denser inner layer. Magnesium-based alloy does not show long-term corrosion resistance for biological applications. The polarization test and the cyclic wet and dry test shows that the HA-coated pure magnesium having a high crystal structure has higher corrosion resistance than bare magnesium. Better corrosion resistance was obtained by the HA-coated AZ31B magnesium alloy compared to the bare AZ31B magnesium alloy at 20 V shown in Fig. 12(a) (Iqbal et al., 2020). CoBlastTM process has been used by Dunne et al. (Peng et al., 2020) to synthesize HA coating on EW10X40, WE43, and EW62 magnesium alloy and found that the HA coating improved the corrosion resistance significantly compared to the bare magnesium alloy shown in Fig. 12(b). Both organic and inorganic multilayer coating shows better corrosion resistance. Ji et al. (Stuart et al., 2022) synthesized multilayered polymeric and HA coating on AZ31 magnesium alloy and found that improved corrosion resistance due to the film’s barrier performance on the outer surface of the multilayer coating. The dense structure and large thickness of this multilayer HA coating on AZ31 substrate provided the optimum corrosion protection. The application of a compact and dense coating to a magnesium substrate can help increase bonding strength and corrosion resistance between the coating and substrate (Shahin et al., 2022). A bi-layer covering that combined silica (SiO2)/silver-doped fluorohydroxyapatite (AgFHAp) was synthesized by Bakhsheshi-Red et al. (Albalwi et al., 2022) on Ma-1.2Ca-4.5Zn substrate by an electrodeposition method followed by physical vapor deposition and found better corrosion resistance because of the appropriate coating thickness and compact coating structure creation.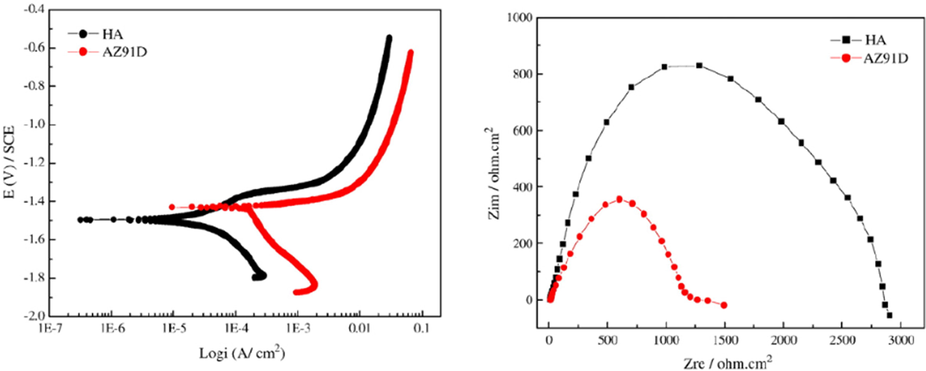
Potentiodynamic curves of HA-coated and uncoated Mg alloys: (a) AZ31B (Shahin et al., 2022) and (b) WE43, EW10X40, and EW62 (Albalwi et al., 2022).
Bio-absorbed magnesium alloy is degraded by the produced H2 and increased alkalinity during deterioration. The demand for magnesium alloy is created by this having precise breakdown rates with regularity. Besides, the rate of corrosion can be delayed by the alkaline treated HA coating or biocompatible composite coating that allows the magnesium-based implant material to be used till the advanced recovery phases preserve mechanical integrities. Oxide, HA, and OCP composite coating has been created on Mg-Zn-Ca alloy by Chen et al. (Ren et al., 2022) to improve corrosion resistance and bone responsiveness. The result showed that the degradation of the implant slowed by decreasing magnesium ion escape at the interface from the substrate. Furthermore, induction of the new bone tissue production and the rapid bone response was increased with the improvement of corrosion resistance by the composite coating on magnesium alloy. Satiric acid and HA composite coating were created on AZ91D magnesium alloy by the electrodeposition method in another study and found porous composite coating having good osseointegration capabilities with excellent corrosion resistance I SBF solution (Russo et al., 2017).
10 In vitro and in vivo assessment of HA-coated Mg alloys
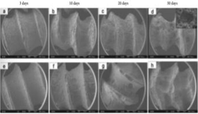
The in vivo and in vitro characteristics with the related mechanism of the biodegradable magnesium-based materials have been studied in recent years. The in vivo and in vitro tests are vital for gathering data on Mg alloys that will be utilized in clinical applications, but in the future, a larger emphasis will need to be made on a better understanding of the degradation mechanism and corrosion behavior of magnesium-based alloys in a biological environment. The types of corrosion, corrosion products, and bodily fluid composition that influence material degradation of magnesium-based alloy are evaluated by in vitro evaluations in a physiological environment. Biological responses of the host environment including new bone formation, bone tissue engagement, degradation performance of implants every time, and cell adhesion are evaluated by in vivo investigations. Mg-Zn-Ca-Zr alloy was coated with HA by Guan et al. (Godoy-Gallardo et al., 2021) to compare degradation rate, hemolysis, corrosion performance, and cytocompatibility with bare magnesium alloy. Compared to bared magnesium alloy, delayed degradation rate and improved corrosion resistance were observed in SBF solution demonstrated by in vivo and in vitro experiments. The corrosion potentials of pure magnesium, Mg-Ca, uncoated Mg-Zn-Ca-Zr, and Mg-Zn-Ca-Zr coated with HA were − 1.95, −1.97, −1.72, and − 1.51 respectively indicated higher corrosion resistance by Mg-Zn-Ca-Zr coated with HA. 4.35 and 4.12 were the hemolysis rates of the magnesium alloy coated with HA and uncoated magnesium alloy. The result indicates that significant hemolysis did not occur because of less than a 5 % rate. Magnesium alloy can be used as implant material in biomedical applications as the hemolysis rate meets the required criteria. The biocompatibility and corrosion resistance of the magnesium alloy coated with CaP can be improved by adding Ag or Sr to the electrolyte. Mg-Zn-Ca alloy was coated by CaP coating doped with Sr by Makkar et al. (Zhang et al., 2022) and found that the biocompatibility, in vivo cytotoxicity, bioactivity, bone regeneration, and corrosion resistance has increased significantly. Besides, being more corrosion resistive and having superior biological characteristics magnesium alloy with a rod-shaped, needle-shaped, plate-shaped, and sphere-shaped magnesium-based alloy with HA coating can be synthesized. In vivo and in vitro studies were performed to increase bone response, biocompatibility, and bio-corrosion of pure magnesium having HA coating (Fan et al., 2022). The bio-corrosion resistance of the pure magnesium with needle-shaped crystal structure HA coating was increased due to increased contact surface area in SBF. HA coating significantly improved the cell engagement, proliferation, differentiation, biological response, and mechanical stability of the magnesium sample as an implant decreasing the degradation and corrosion rate in vivo. The performance of corrosion and bone tissue response of HA and OCP coating on AZ31 was investigated in vivo and in vitro (Wang et al., 2022). The uncoated AZ31 implant was covered by the corrosion product with some cracks after immersing in the solution and implementing in a mouse shown in Fig. 13 (a) and 13 (b). The bioactivity of the plate-like crystals of OCP coating on the alloy was increased shown in Fig. 13 (c) and Fig. 13 (d). Fig. 13 (f) shows the rod-like crystals of HA after the immersion of 14 weeks and 52 weeks in a biological environment. Fig. 13 (e) shows the plate-like crystals of OCP coated implant and found that the crystals were becoming thinner and smaller in vivo evaluation. However, rod-like crystals of HA coated implant did not show much change even after implementation in mouse shown in Fig. 13 (f).
SEM images of AZ31 sample surfaces: (a, b) without coating; (c–e) with OCP coating; and (f) with HA coating. (Wang et al., 2022) (not open access).
The majority of investigations have been performed to reduce the degradation rate and corrosion of the HA-coated Mg-based alloys but little focus has been paid to improving mechanical integrity. Wang et al. (Zhang et al., 2022) used the pulse electro-deposition approach to reduce the degradation rate with the improvement of mechanical properties by creating a Ca-deficient HA coating on an Mg-Zn-Ca alloy. The tensile testing method with a slow strain rate and in vitro evaluation testing method demonstrated a significantly slower rate of deterioration and better mechanical integrity. Excellent adherence was obtained by the Ca-deficient HA coating on the Mg–Zn–Ca implant material, according to the researchers. In the physiological milieu, biocompatible coatings like HA coating are always useful for stimulating cellular response. Inducing polydopamine interlayer, Zhou et al. [1 6 8] synthesized HA coating on AZ31 magnesium alloy to increase cellular responsiveness. The synergic effect of polydopamine and alkali treatment increased the corrosion resistance of the HA-coated substrate revealed by the potentiodynamic test and immersion test. The better cellular response was obtained from magnesium alloy substrate coated by composite compared to the single and uncoated polydopamine layer substrate evaluated by cytotoxicity test in terms of proliferation and adhesion. Using PCL and zinc-doped hydroxyapatite zeolite (ZnHAZeo), a composite coating was synthesized on magnesium substrate by Iqbal et al. [1 6 9] to increase corrosion resistance and antibacterial properties. The presence of zinc increases the antibacterial performance of the zinc-coated composite coated substrate more than with zinc-coated composite and bare magnesium substrate. Combining graphene oxide (GO) and HA, a composite coating was created on AZ31 magnesium alloy by Peng et al. [1 7 0] to improve biocompatibility where they used graphene oxide on the outer layer and HA coating on the inner layer. The bi-layer coating increased the biocompatibility with the best growth and adherence performance of the MC3T3-E1 cells evaluated by the cytotoxicity test. The result indicates that the synthesized HA/GO coated substrate can be used in clinical applications.
11 Future prospect and challenges of bone implant materials
Despite being a ground-breaking medical implant, Mg-based alloys still have many shortcomings that need to be addressed before they can be used on a large scale in early clinical trials. Using 3D printed biodegradable porous magnesium implants; researchers [171, 172] have successfully eliminated implant-related infections in both in vitro and in vivo studies. The porous scaffolds made of Zn-Mg alloy in additive manufacturing can improve osseointegration. The 3D porous bioactive materials have a promising aspect in bone implant applications. The 3D manufactured bone implant process is illustrated in Fig. 14.![The 3D manufactured bone implant process [171–172].](/content/184/2022/15/11/img/10.1016_j.arabjc.2022.104214-fig14.png)
The 3D manufactured bone implant process [171–172].
Magnesium alloy can be doped with bioactive nanoparticles and other nanobiological substances to enhance the bone implant's characteristics. Numerous studies have previously been conducted to gain a comprehensive understanding of the incorporation of doping nanoparticles, but there are still many prospects for their use to lessen post-operative problems. In a critical-sized bone defect in the rat femur shaft, bioresorbable magnesium-based alloys with strontium-doped nanohydroxyapatite improve bone regeneration [1 7 3]. Nanohydroxyapatite and strontium-substituted hydroxyapatite were successfully used to reinforce the pure magnesium, enhancing its grain refinement, corrosion resistance, biocompatibility, and mechanical properties at low concentrations. Citrate-based bioadhesives inspired by mussels were gelled almost instantly through hydrogen bonding using the natural polyphenolic compound tannic acid [1 7 4]. These bioadhesives also had anti-oxidant, anti-inflammatory, and antibacterial properties. The resultant materials had high mechanical strength, elasticity, and adhesion together with low swelling ratios and self-healing properties. For use in the next generation of implant coatings, active agents of Ga and Ag were introduced into PBG thin-film matrices to control the release of osteogenic (Ca, P, Mg) and antibacterial (Ga, Ag) components [1 7 5]. Graphene, Zr, ytterbium oxide, Cu, CeO2, and Au nanoparticles have superior prospects in future implant applications for resolving the challenges that have occurred now [176–180]. The inclusion of bioactive nanoparticles and other nanobiological substances for the reduction of postoperative complications is shown in Fig. 15.![The inclusion of bioactive nanoparticles and other nanobiological substances [174–176].](/content/184/2022/15/11/img/10.1016_j.arabjc.2022.104214-fig15.png)
The inclusion of bioactive nanoparticles and other nanobiological substances [174–176].
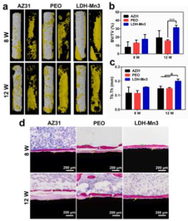
The best absorbable metals for bone fracture fixation implants are thought to be magnesium alloys. The fundamental issue with absorbable magnesium alloys is the necessity to regulate the high rate of corrosion and degradation. Magnesium alloys have received a variety of treatments to slow down their corrosion rates so that they are comparable to the regeneration rate of bone fracture. Still now, the high rate of corrosion degradation is considered as challenging issue. The enhanced corrosion resistance and synergistic bio functions of Mn and Mg ions, which facilitated cell adhesion, spreading and proliferation, and osteogenic differentiation in vitro, and accelerated bone regeneration in vivo, are found in a black Mn-containing layered double hydroxide (LDH) nanosheet-modified Mg-based implants [1 8 1]. Layer-by-layer assembly was used to create the Ca-P coating, which was applied to the AZ31 magnesium alloy and showed good biocompatibility, antibacterial activity, and corrosion resistance [1 8 2].Mg-0.8Ca-5Zn-1.5Ag surface is coated with an (Mg(OH)2/GO/HA) nanocomposite, which exhibits strong bonding power, hydrophilicity, and corrosion resistance [1 8 3]. Studies conducted in vitro demonstrate that Mg(OH)2 does really enhance the substrate's antibacterial activity. The subsequent GO and GO/HA coating techniques both support osteogenic differentiation of MC3T3-E1 cells and exhibit no negative effects on the antibacterial activity of Mg(OH)2 coating, while the latter displays the strongest supporting impact. Studies conducted in vivo show that the Mg alloy with the composite coating not only reduces osteolysis brought on by bacterial invasion but also encourages bone regeneration in both healthy and diseased situations. Some other research [184–186] has already been done by incorporating the different coating materials but the proper selection of coating on bone-implant still be a tough issue. The coating process for enhancement of bone implant property is depicted in Fig. 16.![Coating process for enhancement of bone implant property [181–183].](/content/184/2022/15/11/img/10.1016_j.arabjc.2022.104214-fig16.png)
Coating process for enhancement of bone implant property [181–183].
12 Conclusion
Because of the increase in pH value during its disintegration in the bacterial solution, Mg alloys have antibacterial characteristics. It has clinical significance in terms of preventing infections linked with surgical implants. Rapid depreciation indicates the biomedical Mg facility will fail, which is contrary to our original goal. Coatings having antibacterial properties are a viable option. Many obstacles are still there to improving magnesium alloy coating for clinical applications. Self-degradable, biocompatible, and corrosion-resistive magnesium alloy coatings are good for biomedical applications. However, fabricating coatings with all of the qualities stated thus far is nearly impossible. It is difficult to regulate the degradation rate by corrosion of magnesium alloy as implant material. Sol-gel processing, CaP-based antibacterial coating, electrochemical deposition, and hydrothermal synthesis on magnesium alloy are overviewed here. Mg-based implants may benefit from anodic gradient coatings. Though biomedical Mg alloys and their coatings have numerous flaws, their benefits cannot be overlooked. Shortcomings will be reduced, if not eradicated, as a result of the ongoing efforts of researchers.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Medical applications of ternary nanocomposites based on hydroxyapatite/ytterbium oxide/graphene oxide: potential bone tissue engineering and antibacterial properties. J. Mater. Res. Technol.. 2022;18:4834-4845.
- [Google Scholar]
- Baghbaderani, Mohammad Zolfaghari, SomayehAbazari, Hamid Reza Bakhsheshi-Rad, Ahmad Fauzi Ismail, Safian Sharif, AliakbarNajafinezhad, Seeram Ramakrishna, MohammadrezaDaroonparvar, and FilippoBerto. “Dual Synergistic Effects of MgO-GO Fillers on Degradation Behavior, Biocompatibility and Antibacterial Activities of Chitosan Coated Mg Alloy.” Coatings 12, no. 1 (2022): 63.
- Study on the corrosion resistance and anti-infection of modified magnesium alloy. Biomed. Mater. Eng.. 2017;28:339-345.
- [Google Scholar]
- Deposition of nanostructured fluorine-doped hydroxyapatite–polycaprolactone duplex coating to enhance the mechanical properties and corrosion resistance of Mg alloy for biomedical applications. Mater. Sci. Eng., C. 2016;60:526-537.
- [Google Scholar]
- Novel bi-layered nanostructured SiO2/Ag-FHAp coating on biodegradable magnesium alloy for biomedical applications. Ceram. Int.. 2016;42:11941-11950.
- [Google Scholar]
- Electrochemical study of tailored sol−gel thin films as pre-treatment prior to organic coating for AZ91 magnesium alloy. Prog. Org. Coat.. 2010;68:347-355.
- [Google Scholar]
- A new generation of scaffolds for bone tissue engineering. Adv. Sci. Technol., Trans. Tech. Publcation. 2010;31:48-53.
- [Google Scholar]
- Biomaterial developments for bone tissue engineering. Biomaterials. 2000;21:2347-2359.
- [Google Scholar]
- Thick sol−gel coatings produced by electrophoretic deposition. Adv. Mater.. 2002;14:505-508.
- [Google Scholar]
- In vivo degradation and bone response of a composite coating on Mg–Zn–Ca alloy prepared by microarc oxidation and electrochemical deposition. J. Biomed. Mater. Res. Part B: Appl. Biomater.. 2012;100:533-543.
- [Google Scholar]
- Corrosion resistance evaluation of a Ca-and P-based bioceramic thin coating in Ti-6Al-4V. J. Mater. Sci. Mater. Med.. 2009;20:215-222.
- [Google Scholar]
- Cui, Lan-Yue, Ling Gao, Jing-Chao Zhang, Zhe Tang, Xiao-Li Fan, Jia-Cheng Liu, Dong-Chu Chen, Rong-Chang Zeng, Shuo-Qi Li, and Ke-QianZhi. “In vitro corrosion resistance, antibacterial activity and cytocompatibility of a layer-by-layer assembled DNA coating on magnesium alloy.” Journal of Magnesium and Alloys 9, no. 1 (2021): 266-280.
- Human antimicrobial peptides: defensins, cathelicidins and histatins. Biotechnol. Lett.. 2005;27:1337-1347.
- [Google Scholar]
- Formation mechanisms of chitosan-silica hybrid materials and its performance as solid support for KR-12 peptide adsorption: impact on KR-12 antimicrobial activity and proteolytic stability. J. Mater. Res. Technol.. 2020;9(1):890-901.
- [Google Scholar]
- Calcium orthophosphate coatings on magnesium and its biodegradable alloys. Acta Biomater.. 2014;10:2919-2934.
- [Google Scholar]
- Mechanical integrity of biodegradable Mg–HA composite during in vitro exposure. J. Mater. Eng. Perform.. 2019;28:800-809.
- [Google Scholar]
- Corrosion behaviour of biodegradable magnesium alloys with hydroxyapatite coatings. Surf. Coat. Technol.. 2016;289:37-44.
- [Google Scholar]
- Dvorsky, Drahomir, Jiri Kubasek, Eva Jablonska, JirinaKaufmanova, and DaliborVojtech. “Mechanical, corrosion and biological properties of advanced biodegradable Mg–MgF2 and WE43-MgF2 composite materials prepared by spark plasma sintering.” Journal of Alloys and Compounds 825 (2020): 154016
- Magnesium science, technology and applications. Adv. Performance Mater.. 1998;5:201-212.
- [Google Scholar]
- Electrochemical, biodegradation and cytotoxicity of graphene oxide nanoparticles/polythreonine as a novel nano-coating on AZ91E Mg alloy staple in gastrectomy surgery. Mater. Sci. Eng. C. 2019;103:109780
- [Google Scholar]
- Fan, Lijun, Wenxin Sun, YuhongZou, Qian-qianXu, Rong-Chang Zeng, and JingruiTian. “Enhanced corrosion resistance, antibacterial activity and biocompatibility of gentamicin-montmorillonite coating on Mg alloy-in vitro and in vivo studies.” Journal of Materials Science & Technology 111 (2022): 167-180
- Enhanced corrosion resistance, antibacterial activity and biocompatibility of gentamicin-montmorillonite coating on Mg alloy-in vitro and in vivo studies. J. Mater. Sci. Technol.. 2022;111:167-180.
- [Google Scholar]
- Influence of roughness and grinding direction on the thickness and adhesion of sol-gel coatings deposited by dip-coating on AZ31 magnesium substrates. a Landau-Levich equation revision. Surf. Coat. Technol.. 2021;408:126798
- [Google Scholar]
- Sol-gel processing: a versatile concept for special glasses and ceramics. Bull. Mater. Sci.. 1993;16:523-531.
- [Google Scholar]
- Antibacterial approaches in tissue engineering using metal ions and nanoparticles: from mechanisms to applications. Bioact. Mater.. 2021;6(12):4470-4490.
- [Google Scholar]
- Electrodeposition of hydroxyapatite coating on Mg-4.0 Zn-1.0 Ca-0.6 Zr alloy and in vitro evaluation of degradation, hemolysis, and cytotoxicity. J. Biomed. Mater. Res. Part A. 2012;100:999-1015.
- [Google Scholar]
- Osteoconductive and electroactive carbon nanofbers/hydroxyapatite nanocomposite tailored for bone tissue engineering: in vitro and in vivo studies. Sci. Rep.. 2020;10:14853.
- [Google Scholar]
- Die Apatite, derenchemischeZusammensetzung und ihrverhältniszu den physikalischen und morphologischenEigenschaften. Acta Acad. Abo. Ser. B Mat. Phys. Mat. Natur.Teknik.. 1929;5:62-65.
- [Google Scholar]
- Conversion of amorphous tricalcium phosphate into apatitictricalcium phosphate. Calcif. Tissue Int.. 1982;34:S103-S108.
- [Google Scholar]
- In vitro and in vivo biocompatibility and corrosion behaviour of a bioabsorbable magnesium alloy coated with octacalcium phosphate and hydroxyapatite. Acta Biomater.. 2015;11:520-532.
- [Google Scholar]
- Novel SiO 2/PDA hybrid coatings to promote osteoblast-like cell expression on titanium implants. J. Mater. Chem. B. 2015;3(13):2698-2707.
- [Google Scholar]
- Biomedical coatings on magnesium alloys − a review. Acta Biomater.. 2012;8:2442-2455.
- [Google Scholar]
- Evaluation of the novel three-dimensional porous poly (L-lactic acid)/nano-hydroxyapatite composite sca old. Bio-Med. Mater. Eng.. 2015;26:S197-S205.
- [Google Scholar]
- The application of plasma electrolytic oxidation (PEO) to the production of corrosion resistant coatings on magnesium alloys: a review. Corros. Mater.. 2013;38(1):55-65.
- [Google Scholar]
- N. Iqbal, S. Iqbal, T. Iqbal, H. Bakhsheshi-Rad, A. Alsakkaf, A. Kamil, M.R.A. KADIR, M.H. Idris, H.B. Raghav, Zinc-doped hydroxyapatite—zeolite/polycaprolactone composites coating on magnesium substrate for enhancing in-vitro corrosion and antibacterial performance, Transactions of Nonferrous Metals Society of China, 30 (2020) 123-133
- Electrodeposition of hydroxyapatite coating on magnesium for biomedical applications. J. Coat. Technol. Res.. 2012;9:495-502.
- [Google Scholar]
- Corrosion resistance and antibacterial effects of hydroxyapatite coating induced by polyacrylic acid and gentamicin sulfate on magnesium alloy. Front. Mater. Sci.. 2019;13:87-98.
- [Google Scholar]
- Corrosion resistance and antibacterial effects of hydroxyapatite coating induced by polyacrylic acid and gentamicin sulfate on magnesium alloy. Front. Mater. Sci.. 2019;13(1):87-98.
- [Google Scholar]
- Corrosion resistance and antibacterial effects of hydroxyapatite coating induced by polyacrylic acid and gentamicin sulfate on magnesium alloy. Front. Mater. Sci.. 2019;13:87-98.
- [Google Scholar]
- Corrosion resistance and antibacterial properties of hydroxyapatite coating induced by gentamicin-loaded polymeric multilayers on magnesium alloys. Colloids Surf. B Biointerfaces. 2019;179:429-436.
- [Google Scholar]
- Hydroxyapatite coating on magnesium with MgF2 interlayer for enhanced corrosion resistance and biocompatibility. J. Mater. Sci. Mater. Med.. 2011;22:2437-2447.
- [Google Scholar]
- Jorgimara de O. Braga, Sandhra M. de Carvalho, Lucas M.C. Silva, Renata B. Soares, Vanessa F.C. Lins, Eric M. Mazzer, Manuel Houmard, Roberto B. Figueiredo, Eduardo H.M. Nunes, Fabrication and characterization of dicalcium phosphate coatings deposited on magnesium substrates by a chemical conversion route, Surface and Coatings Technology, 386, 2020, 125505.
- Production and bio-corrosion resistance of porous magnesium with hydroxyapatite coating for biomedical applications. Mater. Lett.. 2013;108:122-124.
- [Google Scholar]
- Hydroxyapatite as a liquid chromatographic packing. J. Chromatogr. A. 1991;544:147-184.
- [Google Scholar]
- Antimicrobial peptides on calcium phosphate-coated titanium for the prevention of implant-associated infections. Biomaterials. 2010;31(36):9519-9526.
- [Google Scholar]
- Drug release and bone growth studies of antimicrobial peptide-loaded calcium phosphate coating on titanium. J. Biomed. Mater. Res. Part. B. 2012;100:1344-1352.
- [Google Scholar]
- Functionalization of metallic magnesium with protein layers via linker molecules. Langmuir. 2010;26:12044-12048.
- [Google Scholar]
- Hydroxyapatite-coated magnesium implants with improved in vitro and in vivo biocorrosion, biocompatibility, and bone response. J. Biomed. Mater. Res. Part A. 2014;102:429.
- [Google Scholar]
- Kinetic study on the crystal growth of hydroxyapatite. Langmuir. 2001;17:8092-8097.
- [Google Scholar]
- Synthesis and characterization of hydroxyapatite crystals: a review study on the analytical methods. J. Biomed. Mater. Res. Part A. 2002;62:600-612.
- [Google Scholar]
- Electrophoretic deposition of hydroxyapatite coating on Mg–3Zn alloy for orthopaedic application. Surf. Coat. Technol.. 2016;287:82-92.
- [Google Scholar]
- Le Yu, David W. Rowe, Inosh P. Perera, Jiyao Zhang, Steven L. Suib, Xiaonan Xin, Mei Wei, Intrafibrillar Mineralized Collagen−Hydroxyapatite-Based Scaffolds for Bone Regeneration, (2020).
- Biomimetic apatite-coated alginate/chitosan microparticles as osteogenic protein carriers. Biomaterials. 2009;30(30):6094-6101.
- [Google Scholar]
- Effects of protein incorporation on calcium phosphate coating. Mater. Sci. Eng.. 2009;29(3):913-918.
- [Google Scholar]
- Corrosion resistance of glucose-induced hydrothermal calcium phosphate coating on pure magnesium. Appl. Surf. Sci.. 2019;465:1066-1077.
- [Google Scholar]
- The development of binary Mg–Ca alloys for use as biodegradable materials within bone. Biomaterials. 2008;29(10):1329-1344.
- [Google Scholar]
- Effects of ultrasonic treatment and current density on the properties of hydroxyapatite coating via electrodeposition and its in vitro biomineralization behavior. Mater. Sci. Eng., C. 2019;105:110062
- [Google Scholar]
- In vitro and in vivo corrosion, mechanical properties and biocompatibility evaluation of MgF2-coated Mg-Zn-Zr alloy as cancellous screws. Mater. Sci. Eng., C. 2017;75:1268-1280.
- [Google Scholar]
- Improving the corrosion resistance of ZEK100 magnesium alloy by combining high-pressure torsion technology with hydroxyapatite coating. Mater. Design. 2019;181:107933
- [Google Scholar]
- Li, Qite, Wenbo Ye, Hong Gao, and LilanGao. “Improving the corrosion resistance of ZEK100 magnesium alloy by combining high-pressure torsion technology with hydroxyapatite coating.” Materials & Design 181 (2019): 107933
- Application of an integrated RE/RP/CAD/CAE/CAM system for magnesium alloy shell of mobile phone. J. Mater. Process. Technol.. 2009;209:2818-2830.
- [Google Scholar]
- On the study of BSA-loaded calcium-deficient hydroxyapatite nano-carriers for controlled drug delivery. J. Control. Release.. 2005;107(1):112-121.
- [Google Scholar]
- Proteins incorporated into biomimetically prepared calcium phosphate coatings modulate their mechanical strength and dissolution rate. Biomaterials. 2003;24(1):65-70.
- [Google Scholar]
- In vitro effects of nanophase hydroxyapatite particles on proliferation and osteogenic differentiation of bone marrow-derived mesenchymal stem cells. J. Biomed. Mater. Res. Part A. 2009;90:1083-1091.
- [Google Scholar]
- Corrosion resistance of bioinspired DNA-induced Ca–P coating on biodegradable magnesium alloy. J. Magnesium Alloys. 2019;7:144-154.
- [Google Scholar]
- In-vitro and in-vivo evaluation of strontium doped calcium phosphate coatings on biodegradable magnesium alloy for bone applications. Appl. Surf. Sci.. 2020;510:145333
- [Google Scholar]
- Apatite: its crystal chemistry, mineralogy, utilization, and geologic and biologic occurrences. Berlin: Springer Science & Business Media; 2012.
- Degradation of AZ31 and AZ91 magnesium alloys in different physiological media: effect of surface layer stability on electrochemical behaviour. J. Magn. Alloys. 2020;8(3):667-675.
- [Google Scholar]
- Effect of electrodeposition modes on surface characteristics and corrosion properties of fluorine-doped hydroxyapatite coatings on Mg–Zn–Ca alloy. Appl. Surf. Sci.. 2011;257:4811-4816.
- [Google Scholar]
- Bone tissue engineering therapeutics: controlled drug delivery in three-dimensional scaffolds. J. R. Soc. Interface. 2009;7:20090379.
- [Google Scholar]
- One-step anodization deposition of anticorrosive bioceramic compounds on AZ31B magnesium alloy for biomedical application. Ceram. Int.. 2015;41:10861-10870.
- [Google Scholar]
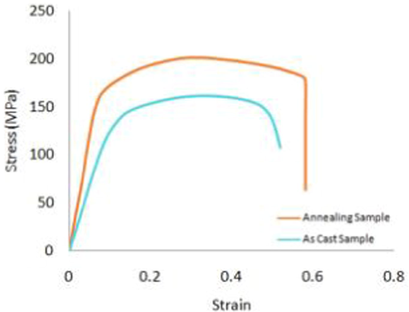
- Annealing and coating influence on the mechanical properties, microstructure, and corrosion properties of biodegradable Mg Alloy (AZ91) J. Bio-Tribo-Corros.. 2022;8(2):1-8.
- [Google Scholar]
- Interfacial behaviour of strontium-containing hydroxyapatite cement with cancellous and cortical bone. Biomaterials. 2006;27:5127-5133.
- [Google Scholar]
- Development of new metallic alloys for biomedical applications. Acta Biomater.. 2012;8:3888-3903.
- [Google Scholar]
- Bioresorbablesca old: the advent of a new era in percutaneous coronary and peripheral revascularization? Circulation. 2011;123:779-797.
- [Google Scholar]
- Ozeki, K.; Goto, T.; Aoki, H.; Masuzawa, T. Influence of the crystallinity of a sputtered hydroxyapatite film on its osteocompatibility. Bio-Med. Mater. Eng. 2015, 26, 139–147. Coatings 2020, 10, 828 22 of 22.
- Calcium phosphate coatings for bioimplant applications: materials, performance factors, and methodologies. Mater. Sci. Eng., R. 2009;66:1-70.
- [Google Scholar]
- Pan, Kai, Xiaojie Li, Hui Shi, Miao Dai, Zhenyu Yang, Maohua Chen, Wei Wei, Xiaoya Liu, and YufengZheng. “Preparation of photo-crosslinked aliphatic polycarbonate coatings with predictable degradation behavior on magnesium-alloy stents by electrophoretic deposition.” Chemical Engineering Journal 427 (2022): 131596.
- Biologic properties of nano-hydroxyapatite: An in vivo study of calvarial defects, ectopic bone formation and bone implantation. Bio-Med. Mater. Eng.. 2015;25:25-38.
- [Google Scholar]
- Guohong Zou, Xu Xia, Bo Jin, Biomimeticmineralization and cytocompatibility of nanorod hydroxyapatite/graphene oxidecomposites. Front. Chem. Sci. Eng.. 2018;12:798-805.
- [Google Scholar]
- Enhanced corrosion resistance and biocompatibility of magnesium alloy by hydroxyapatite/graphene oxide bilayer coating. Mater. Lett.. 2020;264:127322
- [Google Scholar]
- Direct writing of polymeric coatings on magnesium alloy for tracheal stent applications. Ann. Biomed. Eng.. 2015;43:1158-1165.
- [Google Scholar]
- Pezzato, Luca, Leonardo Bertolucci Coelho, RacheleBertolini, Alessio Giorgio Settimi, Katya Brunelli, Marjorie Olivier, and ManueleDabalà. “Corrosion and mechanical properties of plasma electrolytic oxidation‐coated AZ80 magnesium alloy.” Materials and Corrosion 70, no. 11 (2019): 2103-2112.
- Synthesis and characterisation of nanohydroxyapatite using an ultrasound assisted method. UltrasonicsSonochemistry. 2009;16:469-474.
- [Google Scholar]
- Synthetic amorphous calcium phosphate and its relation to bone mineral structure. Acc. Chem. Res.. 1975;8:273-281.
- [Google Scholar]
- Obtaining and characterizing thin layers of magnesium doped hydroxyapatite by dip coating procedure. Coatings. 2020;10:510.
- [Google Scholar]
- Additive manufacturing of Zn-Mg alloy porous scaffolds with enhanced osseointegration: in vitro and in vivo studies. Acta Biomater.. 2022;145:403-415.
- [Google Scholar]
- Rahman, Mostafizur, RajkamalBalu, Naba Kumar Dutta, and Namita Roy Choudhury. “In Vitro Corrosion Resistance of a Layer-by-Layer Engineered Hybrid Coating on ZK60 Magnesium Alloy.” Sustainability 14, no. 4 (2022): 2459
- Biodegradation of four calcium phosphate ceramics: in vivo rates and tissue interactions. J. Mater. Sci. Mater. Med.. 1991;2:63-70.
- [Google Scholar]
- Biomaterials science: An introduction to materials in medicine. MRS Bull.. 2006;31:59.
- [Google Scholar]
- Long-term treatment of postmenopausal osteoporosis with strontium ranelate: Results at 8 years. Bone. 2009;45:1059-1064.
- [Google Scholar]
- Cerium oxide nanoparticles loaded nanofibrous membranes promote bone regeneration for periodontal tissue engineering. Bioact. Mater.. 2022;7:242-253.
- [Google Scholar]
- Strategic design of a Musselinspired in situ reduced Ag/Au nanoparticle coated magnesium alloy for enhanced viability, antibacterial property and decelerated corrosion rates for degradable implant applications. Sci. Rep.. 2019;9:117.
- [Google Scholar]
- Electrophoretic deposition of nanostructured hydroxyapatite coating on AZ91 magnesium alloy implants with different surface treatments. Appl. Surf. Sci.. 2013;285:664-673.
- [Google Scholar]
- Preliminary focus on the mechanical and antibacterial activity of a PMMA-based bone cement loaded with gold nanoparticles. Bioact. Mater.. 2017;2(3):156-161.
- [Google Scholar]
- Electrophoretic (EPD) coatings for magnesium alloys. J. Ind. Eng. Chem.. 2021;103:358-372.
- [Google Scholar]
- Calcium phosphate coatings on magnesium alloys for biomedical applications: a review. Acta Biomater.. 2012;8:20-30.
- [Google Scholar]
- Mechanical and corrosion properties of graphene nanoplatelet–reinforced Mg–Zr and Mg–Zr–Zn matrix nanocomposites for biomedical applications. J. Magn. Alloys. 2022;10(2):458-477.
- [Google Scholar]
- Shaikh, Shazia, Irfan Qayoom, R. Sarvesha, and Ashok Kumar. “Bioresorbable magnesium-based alloys containing strontium doped nanohydroxyapatite promotes bone healing in critical sized bone defect in rat femur shaft.” Journal of Magnesium and Alloys (2022).
- Shao, Y.; Zeng, R.C.; Li, S.Q.; Cui, L.Y.; Zou, Y.H.; Guan, S.K.; Zheng, Y.F. Advance in Antibacterial Magnesium Alloys and Surface Coatings on Magnesium Alloys: A Review. Acta Metall. Sin. Engl. Lett. 2020, 33, 615–629.[.
- Fix, heal, and disappear: a new approach to using metals in the human body. Electrochem. Soc. Interface. 2008;17:1745.
- [Google Scholar]
- Electrodeposition of hydroxyapatite coating on AZ91D magnesium alloy for biomaterial application. Mater. Lett.. 2008;62:3276-3279.
- [Google Scholar]
- Preparation and characterisation of electrophoretically deposited hydroxyapatite coatings on type 316 L stainless steel. Corros. Sci.. 2003;45:237-252.
- [Google Scholar]
- Magnesium and its alloys as orthopedic biomaterials: a review. Biomaterials. 2006;27:1728-1734.
- [Google Scholar]
- New solutions for combatting implant bacterial infection based on silver nano-dispersed and gallium incorporated phosphate bioactive glass sputtered films: a preliminary study. Bioact. Mater.. 2022;8:325-340.
- [Google Scholar]
- A chemical conversion hydroxyapatite coating on AZ60 magnesium alloy and its electrochemical corrosion behaviour. Int. J. Electrochem. Sci.. 2012;7:11497-11511.
- [Google Scholar]
- Preparation and corrosion behavior of calcium phosphate and hydroxyapatite conversion coatings on AM60 magnesium alloy. J. Electrochem. Soc.. 2013;160:C536-C541.
- [Google Scholar]
- 2e fluoride coated AZ31B magnesium alloy improves corrosion resistance and stimulates bone formation in rabbit model. Mater. Sci. Eng., C. 2016;63:506-511.
- [Google Scholar]
- Enhancement of the mechanical properties of AZ31 magnesium alloy via nanostructured hydroxyapatite thin films fabricated via radio-frequency magnetron sputtering. J. Mech. Behav. Biomed. Mater.. 2015;46:127-136.
- [Google Scholar]
- Takagi, S.; Chow, L.C.; Ishikawa, K. Formation of hydroxyapatite in new calcium phosphate cements.vBiomaterials 1998, 19, 1593–1599
- Osteoconductivity and growth factor production by MG63 osteoblastic cells on bioglass-coated orthopedic implants. Biotechnol. Bioeng.. 2011;108:454-464.
- [Google Scholar]
- Fabrication of multifunctional CaP-TC composite coatings and the corrosion protection they provide for magnesium alloys. Biomed. Eng.. 2017;62:375-381.
- [Google Scholar]
- Biocompatibility of hydroxyapatite scaolds processed by lithography-based additive manufacturing. Bio-Med. Mater. Eng.. 2015;26:31-38.
- [Google Scholar]
- Tian, Qiaomu, Jiajia Lin, Laura Rivera-Castaneda, AmitTsanhani, Zachary S. Dunn, Alexis Rodriguez, ArashAslani, and Huinan Liu. “Nano-to-submicron hydroxyapatite coatings for magnesium-based bioresorbable implants–deposition, characterization, degradation, mechanical properties, and cytocompatibility.” Scientific reports 9, no. 1 (2019): 1-27.
- Nano-to-submicron hydroxyapatite coatings for magnesium-based bioresorbable implants-deposition, characterization, degradation, mechanical properties, and cytocompatibility. Scient. Rep.. 2019;9:810.
- [Google Scholar]
- Investigation of the antimicrobial activity and biocompatibility of magnesium alloy coated with HA and antimicrobial peptide. J. Mater. Sci. Mater. Med.. 2015;26:66.
- [Google Scholar]
- Growth mechanism of hydroxyapatite-coatings formed on pure magnesium and corrosion behavior of the coated magnesium. Appl. Surf. Sci.. 2011;257:8253-8257.
- [Google Scholar]
- Ultrasound-assisted electrodepositionof composite coatings with particles. Surf. Coat. Technol.. 2014;259:363-373.
- [Google Scholar]
- Slow release of anticancer drugs from porous calcium hydroxyapatite ceramic. J. Orthop. Res.. 1992;10:440-445.
- [Google Scholar]
- Developments in smart anticorrosive coatings with multifunctional characteristics. Prog. Org. Coat.. 2017;111:294-314.
- [Google Scholar]
- Effect of ultrasonic agitation on some properties of electrodeposits. Electrodeposition Surf. Treatment. 1973;1(6):457-469.
- [Google Scholar]
- Compressive properties of cortical bone: mineral-organic interfacial bonding. Biomaterials. 1994;15:137-145.
- [Google Scholar]
- Sol−gel coatings on metals for corrosion protection. Prog. Org. Coat.. 2009;64:327-338.
- [Google Scholar]
- Wang, Chang, Bo Zhang, Sen Yu, Hao Zhang, Wenhao Zhou, Rifang Luo, Yunbing Wang, WeiguoBian, and Genwen Mao. “Incorporation of Mg-phenolic networks as a protective coating for magnesium alloy to enhance corrosion resistance and osteogenesis in vivo.” Journal of Magnesium and Alloys (2022).
- Effects of structure and composition of the CaP composite coatings on apatite formation and bioactivity in simulated body fluid. Appl. Surf. Sci.. 2009;255(7):4074-4081.
- [Google Scholar]
- In vitro degradation and mechanical integrity of Mg–Zn–Ca alloy coated with Ca-deficient hydroxyapatite by the pulse electrodeposition process. Acta Biomater.. 2010;6:1743-1748.
- [Google Scholar]
- Biomimetic mineralized hydroxyapatite nanofiber-incorporated methacrylated gelatin hydrogel with improved mechanical and osteoinductive performances for bone regeneration. Int. J. Nanomed.. 2022;17:1511-1529.
- [Google Scholar]
- Surface modification of magnesium alloys developed for bioabsorbable orthopedic implants: a general review. J. Biomed. Mater. Res., Part B. 2012;100B:1691-1701.
- [Google Scholar]
- Enhanced anticorrosive and antibacterial performances of silver nanoparticles/polyethyleneimine/MAO composite coating on magnesium alloys. J. Mater. Res. Technol.. 2021;11:2354-2364.
- [Google Scholar]
- Mussel-inspired nano-multilayered coating on magnesium alloys for enhanced corrosion resistance and antibacterial property. Colloids Surf. B Biointerfaces. 2017;157:432-439.
- [Google Scholar]
- Hydrothermal synthesis of hydroxyapatite coating on the surface of medical magnesium alloy and its corrosion resistance. Prog. Nat. Sci. Mater. Int.. 2021;31:324-333.
- [Google Scholar]
- AccessScience, s.v. “Chemical conversion,” by William F. Furter, last reviewed January 2020.
- Reprint of: The history of biodegradable magnesium implants: a review. Acta Biomater.. 2015;23:S28-S40.
- [Google Scholar]
- Degradable biomaterials based on magnesium corrosion. Curr. Opin. Solid State Mater. Sci.. 2008;12:63-72.
- [Google Scholar]
- Surface design of biodegradable magnesium alloys - a review. Surf. Coat. Technol.. 2013;233:2-12.
- [Google Scholar]
- Anti-oxidant anti-inflammatory and antibacterial tannin-crosslinked citrate-based mussel-inspired bioadhesives facilitate scarless wound healing. Bioact. Mater.. 2023;20:93-110.
- [Google Scholar]
- Xie, Kai, Nanqing Wang, Yu Guo, Shuang Zhao, Jia Tan, Lei Wang, Guoyuan Li et al. “Additively manufactured biodegradable porous magnesium implants for elimination of implant-related infections: An in vitro and in vivo study.” Bioactive Materials 8 (2022): 140-152.
- Silver nanoparticles and growth factors incorporated hydroxyapatite coatings on metallic implant surfaces for enhancement of osteoinductivity and antibacterial properties. ACS Appl. Mater. Interfaces. 2014;6:8580-8589.
- [Google Scholar]
- Phosphating treatment and corrosion properties of Mg–Mn–Zn alloy for biomedical application. J. Mater. Sci. Mater. Med.. 2009;20:859.
- [Google Scholar]
- Effect of hydrothermal (Sr)-hydroxyapatite coatings on the corrosion resistance and Mg(2+) ion release to enhance osteoblastic cell responses of AZ91D alloy. Materials. 2020;13:591.
- [Google Scholar]
- Growth, in vitro biodegradation and cytocompatibility properties of nano-hydroxyapatite coatings on biodegradable magnesium alloys. J. Alloy. Compd.. 2016;672:366-373.
- [Google Scholar]
- Yousefpour, Foroozan, RoohollahJamaati, and HamedJamshidiAval. “Investigation of microstructure, crystallographic texture, and mechanical behavior of magnesium-based nanocomposite fabricated via multi-pass FSP for biomedical applications.” Journal of the Mechanical Behavior of Biomedical Materials 125 (2022): 104894
- Yuan, Bo, Hewei Chen, Rui Zhao, Xuangeng Deng, Guo Chen, Xiao Yang, Zhanwen Xiao et al. “Construction of a magnesium hydroxide/graphene oxide/hydroxyapatite composite coating on Mg–Ca–Zn–Ag alloy to inhibit bacterial infection and promote bone regeneration.” Bioactive Materials 18 (2022): 354-367
- Progress and challenge for magnesium alloys as biomaterials. Adv. Eng. Mater.. 2008;10:1-14.
- [Google Scholar]
- Self-assembled silane film and silver nanoparticles coating on magnesium alloys for corrosion resistance and antibacterial applications. Acta Metall. Sin.. 2013;26:681-686.
- [Google Scholar]
- In vitro corrosion and cytocompatibility of a microarc oxidation coating and poly (L-lactic acid) composite coating on Mg-1Li-1Ca alloy for orthopaedic implants. Acs Appl. Mater. Interfaces. 2016;8:10014-10028.
- [Google Scholar]
- In vitro degradation, photo-dynamic and thermal antibacterial activities of Cu-bearing chlorophyllin-induced Ca–P coating on magnesium alloy AZ31. Bioact. Mater.. 2022;18:284-299.
- [Google Scholar]
- Zhang, Hao, LingxiaXie, XiaolongShen, Tengda Shang, RifangLuo, Xin Li, Tianxue You, Jin Wang, Nan Huang, and Yunbing Wang. “Catechol/polyethyleneimine conversion coating with enhanced corrosion protection of magnesium alloys: potential applications for vascular implants.” Journal of Materials Chemistry B 6, no. 43 (2018): 6936-6949.
- Zhang, Dongdong, Shi Cheng, Ji Tan, JuningXie, Yu Zhang, Shuhan Chen, Huihui Du et al. “Black Mn-containing layered double hydroxide coated magnesium alloy for osteosarcoma therapy, bacteria killing, and bone regeneration.” Bioactive Materials (2022).
- Zhang, Zhen-Yu, Yan-Lin An, Xiao-Shi Wang, Lan-Yue Cui, Shuo-Qi Li, Cheng-Bao Liu, Yu-Hong Zou, Fen Zhang, and Rong-Chang Zeng. “In vitro degradation, photo-dynamic and thermal antibacterial activities of Cu-bearing chlorophyllin-induced Ca–P coating on magnesium alloy AZ31.” Bioactive materials (2022).
- Zhang, Dongdong, Shi Cheng, Ji Tan, JuningXie, Yu Zhang, Shuhan Chen, Huihui Du et al. “Black Mn-containing layered double hydroxide coated magnesium alloy for osteosarcoma therapy, bacteria killing, and bone regeneration.” Bioactive materials 17 (2022): 394-405.
- Zhang, Dongdong, Feng Peng, Ji Tan, Yu Zhang, Fang Wang, JuningXie, Ru Xu et al. “Self-assembled ferric oxyhydroxide nanosheet on PEO-coated magnesium alloy with photocatalytic/photothermal antibacterial and enhanced osteogenesis activities.” Chemical Engineering Journal 437 (2022): 135257.
- Fabrication of hydroxyapatite/stearic acid composite coating and corrosion behavior of coated magnesium alloy. Mater. Lett.. 2012;88:76-77.
- [Google Scholar]
- The formation of hydroxyapatite layer onto hopeite coating on stainless steel substrate. Corros. Sci.. 2016;111:216-229.
- [Google Scholar]
- Hydroxyapatite coating on fluorine-treated magnesium alloy by hydrothermal method and its electrochemical corrosion behaviour in hank’s solution. Prot. Met. Phys. Chem. Surf.. 2019;55:127-135.
- [Google Scholar]
- Ag-incorporated FHA coating on pure mg: degradation and in vitro antibacterial properties. ACS Appl. Mater. Interfaces. 2016;8:5093-5103.
- [Google Scholar]
- Smart coatings for active corrosion protection based on multi-functional micro and nanocontainers. Electrochim. Acta. 2012;82:314-323.
- [Google Scholar]
- L. Zheng, K. Li, C. Ning, and J. Sun, “Study on antibacterial and fluoride-releasing properties of a novel composite resin with fluorine-doped nano-zirconia fillers,” Journal of Dentistry, vol. 113, Article ID 103772, 2021.
- Zhou, Zhiwei, Bo Zheng, YuanpingGu, Chong Shen, Jian Wen, ZhengbingMeng, Shuoping Chen, Jun Ou, and Aimiao Qin. “New approach for improving anticorrosion and biocompatibility of magnesium alloys via polydopamine intermediate layer-induced hydroxyapatite coating.” Surfaces and Interfaces 19 (2020): 100501.
- New approach for improving anticorrosion and biocompatibility of magnesium alloys via polydopamine intermediate layer-induced hydroxyapatite coating. Surf. Interfaces. 2020;19:100501
- [Google Scholar]
- Self-defensive layer-by-layer films with bacteria-triggered antibiotic release. ACS Nano. 2014;8:7733-7745.
- [Google Scholar]
- Corrosion resistance and antibacterial activity of zinc-loaded montmorillonite coatings on biodegradable magnesium alloy AZ31. Acta Biomater.. 2019;98:196-214.
- [Google Scholar]







