Translate this page into:
Alkaloid-rich plant Tylophora indica; current trends in isolation strategies, chemical profiling and medicinal applications
⁎Corresponding author. majaz172@yahoo.com (M.A. Hussain)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Tylophora indica, a threatened medicinal plant of family Asclepiadaceae, grows profoundly in Asia, Africa, Australia, Oceanic Islands, Ceylon, Malay island, and Borneo. The plant is a twinning herb that is excessively used in folk medicine as a substitute of ipecac, an expectorant. Different parts of the plant are accredited for the anti-asthmatic, antibacterial, anti–psoriasis, antimicrobial, antiulcer, antiallergic, antidiarrhoeal, hypolipidemic, and anxiolytic properties. Antidiabetic, hepatoprotective, antiangiogenic, anti-tumor, antioxidant, anticonvulsant, anti-rheumatic, and diuretic activities are also attributed to the magnificent medicinal plant owing to the occurrence of various active phytochemicals such as alkaloids, saponins, phytosterols, tannins, and primary metabolites. The plant has been exploited by the ethnic people due to the immense medicinal importance, leading to the endangerment of the plant species. The review brings to light the important pharmacological attributes, folk medicinal uses, and biotechnological approaches that must be followed to save the plant from extinction.
Keywords
Tylophora indica
Endangered species
Phytochemistry
Toxicity
Folk medicinal uses
Pharmacological attributes
1 Introduction
Nature has endowed great diversity to the flora of the biological world (Tagliapietra and Sigovini, 2010), which can be reckoned by almost 250,000 (Ayensu and DeFilipps, 1978) species of the flowering plants. More than 50,000 plants have already been applied for therapeutic objectives (Schippmann et al., 2002). However, a small fraction of the plants has been screened for bioactivity (about 6%) and phytochemical investigations (15%) (Verpoorte, 2000). During the previous years, there has been a revival of heed in utilizing plants as traditional medicine/complementary and alternative medicines (Hussain et al., 2012; Muhammad et al., 2015, 2016; Ashraf et al., 2016). Owing to fewer side effects and more significant pharmacological potential, plant-based medicines have been used in all conventional systems of therapies including Greco–Arab (Unani–Tibb), Ayurveda, and Chinese (Gilani and Atta–ur Rahman, 2005; Nazar et al., 2020; Anwar et al., 2016; Saleem et al., 2018).
T. indica (Burm f.) Merill. (Syn: Tylophora asthmatica; Asclepias asthmatica), a twining perennial woody plant of family Asclepidaceae, is widely distributed in Africa, Asia, Australia, and Oceanic islands (Nema et al., 2007). The plant has been extensively used in Ayurveda (Anonymous 1978; Chopra et al., 1986) and Siddha (Ram et al., 2009) system of medicines since long and is known in local communities with a variety of vernacular names (Ignacimuthu and Ayyanar, 2006; Jayaweera, 2006; Reddy et al., 2006; Prajapati et al., 2003; Ram et al., 2009; Sankaranarayanan et al., 2010; Gurav et al., 2011; Kumar et al., 2012; Ved et al., 2016) displayed in Table 1.
Language
Name
Sanskrit
Lataksiri
Kannda
Nipaladaberu
Hindi
Antmool, Jamgli pikvam
Gujrati
Damvel, Dumvel
Malayalam
Verripalla
English
Emetic swallow–wort, Country ipeacacuanha, Vomiting Swallow–wort
Tamil
Nangilai, Nacharuppan
Siddha
Kurinjan
Telugu
Mekameyani aku
Marathi
Khadari, Pitthakaadi, Pitthamaari, Pitvel
Traditionally, the plant is being implied for curing asthma, cough (Reddy et al., 2006), inflammation, jaundice (Chopra et al., 1986; Kirtikar and Basu, 1991), snakebite (Ignacimuthu and Ayyanar, 2006), syphilitic rheumatism and gout (Nadkarni, 1954; Kirtikar and Basu, 1981), diarrhea, and dysentery (Walter, 2005). The plant has been traditionally used as emetic, diaphoretic, expectorant, purgative, and stimulant (Wealth of India, 1969; Varrier et al., 1994; Mohiuddin, 2019).
The plant is admired for the anti–asthmatic (Umamaheswari et al., 2017; Mohiuddin, 2019), antibacterial (Balasubramanian et al., 2010), anti–psoriasis (Sarma and Misra, 1995), antimicrobial (Parekh and Chanda, 2007; Reddy et al., 2009), antiulcer (Ghodekar et al., 2010), antiallergic (Gaitonde, 2001), antidiarrhoeal (Patel et al., 2006), antifeedant (Reddy et al., 2009), hypolipidemic (Swathi et al., 2012), anxiolytic (Manikkoth et al., 2013), and antidiabetic (Swathi et al., 2012) properties. Hepatoprotective (Gujrati et al., 2007; Kumar et al., 2012; Manikkoth and Rao, 2013), antiangiogenic, anti–tumor (Saraswati et al., 2013), antioxidant (Bhatia et al., 2013), anticonvulsant (Hafis et al., 2017), anti–rheumatic (Reddy et al., 2009), anti-neuroinflammatory (Gupta et al., 2020), and diuretic activities (Meera et al., 2009) are also ascribed to different organs of the multipurpose plant. The major phytochemical fraction consists of alkaloids which rendered the pharmacological activities to the plant. Out of the alkaloids, tylophorine, tylophorinine, and tylophorinidine are worth mentioning (Shahzad et al., 2016). Apart from the pivotal importance in pharmacology, many side effects such as giddiness (Gupta et al., 1979), loss of taste for salt, mouth pain, upset stomach, nausea, vomiting, chest pain, skin rashes, and hives itchy or swollen skin are also associated with a high dosage of the plant (Acharya et al., 2010). The use of any plant part for nutritional purposes is not yet reported.
Owing to the broad medicinal uses, the plant has been incorporated as an official drug in the Bengal pharmacopeia of 1884 (Gopalakrishnan et al., 1979, 1980). Medicinal properties, phytochemical composition, and pharmaceutical potential of the endangered multiuse plant have inspired us to write an encyclopedic review on bioactives, pharmacological attributes, and folk medicinal uses. The emphasis is also to discuss the clinical trials, toxicity, and pharmacological characteristics of the miraculous plant critically. The review will be fruitful for pharmacists, phytochemists, and pharmacologists working on phytochemistry and medical applications of the useful plant.
1.1 Methods
We searched PubMed, ISI Web of Science, Science Direct, acs.org and SciFinder-CAS for studies on phytochemical potential and bioactives of Tylophora indica plant published between January 2012-April 23, 2020. Studies in English language were included. From the eligible publications according to relevance, the information was extracted regarding: (i) isolation of secondary metabolites/phytochemicals, (ii) Bioactives, (iii) folk medicinal uses, (iv) pharmacological activities, (v) biotechnological application and (vi) clinical trials, etc. As well as, some historical references, taxonomy and miscellaneous applications/aspects have also been gathered from literature resources and discussed.
2 Taxonomy and distribution
T. indica is a threatened medicinal plant of family Asclepiadaceae (Hindi, Antamul). The plant is a perennial, twining or climbing laticiferous, and suffruticose herb (Prajapati et al., 2003; Pandey et al., 1993). Based on the morphology of roots (Saroya, 2011), the plant is locally known as Indian Ipecac or Antmool (Bhavan, 1992). The plant is distributed in planes, hilly slopes, moist, humid forests, and narrow valleys (Nadkarni, 1996) of Asia, Africa, Australia, Oceanic islands, Ceylon, Malay island, and Borneo (Nema et al., 2007). The plant can flourish in areas where annual rainfall is about 1000–1500 mm (Nadkarni, 1996) at an altitude range of 900 m at sea level (Schmelzer, 2012) up to 1260 m in the sub-Himalayan track (Gupta et al., 2010a) and optimum temperature, 5–25 °C (Nadkarni, 1996). The morphological characteristics of different parts of the plant are summarized in Table 2.
Plant part
Morphological characteristics
References
Stem
Long hairy stem that attains a height of 1.5 m and climbs with the help of tendrils.
Jayaweera, 2006; Gurav et al., 2011; Muthumperumal and Parthasarathy, 2009
Leaves
Leaves are oblong, ovate to elliptical with length and diameter of 6.0–10.5 and 3.8–6.0 cm, respectively. Leaves are arranged in opposite decussate fashion with 12 mm long petioles.
Shah and Kapoor, 1976; Kirtikar and Basu, 2001; Ved et al., 2016
Flowers
Minute flowers at the end of hairy peduncles of leaf axils are arranged into umbellate cymes (1–1.5 cm across) with haired calyx and greenish-yellow to the greenish-purple corolla. Flowers blossom from September to January.
Gupta, 2003; Jayaweera, 2006
Roots
Roots are long and fleshy bearing knots and light brown corky bark.
Gupta, 2003; Schmelzer, 2012
Fruits
Fruit is a fusiform follicle, 5–8.5 cm long, divaricate, ovoid lanceolate, tapering at the apex, and glabrous.
Gupta, 2003
Seeds
Seeds are oval-shaped (0.6–0.8 × 0.3–0.4 cm) with 1.8 cm hairy coma.
Jayaweera, 2006; Gupta, 2003
3 Phytochemistry
A rich phytochemical profile such as alkaloids, saponins, tannins, terpenoids, etc., is associated with T. indica. Major phytochemicals are investigated in the aerial parts of the plant mainly leaves (Rao et al., 1971; Gupta et al., 2010b; Kumar, 2011; Joshi et al., 2012; Rani et al., 2012). Recent studies (Khanna et al., 2018) have also reported some important phytochemicals in the roots of T. indica.
3.1 Alkaloids
The plant is admired for the valuable bioactive phytoconstituents, particularly alkaloids. The alkaloids are known to lend the analgesic, antispasmodic, and bactericidal potential to the plants (Okwu, 2004). The existence of alkaloids in the methanolic extract of leaves was authenticated by applying dragendorff’s reagent in thin layer chromatography (Kumar, 2011). Most of the alkaloids extracted from the plant have the basic framework of phenanthroindalizidine and furoquinoline (Karnick, 1975; Etherington et al., 1977). The presence of a crystalline alkaloid, tylophorine in roots, was first reported by Hooper (1891) using characteristic colour reactions (Hooper, 1891). Tylophorine and tylophorinine were isolated from T. indica, and the chemical structures were confirmed using X-ray studies (Govindachari et al., 1957, 1960, 1965, Govindachari, 2002). Three new alkaloidal compounds were extracted from the leaves of the plant with 0.5% methanolic acetic acid, along with already reported tylophorine and tylophorinine. New alkaloids were characterized but could not be assigned the structural formulae (Rao et al., 1971). The structural formulas of some pivotal alkaloids are shown in Fig. 1.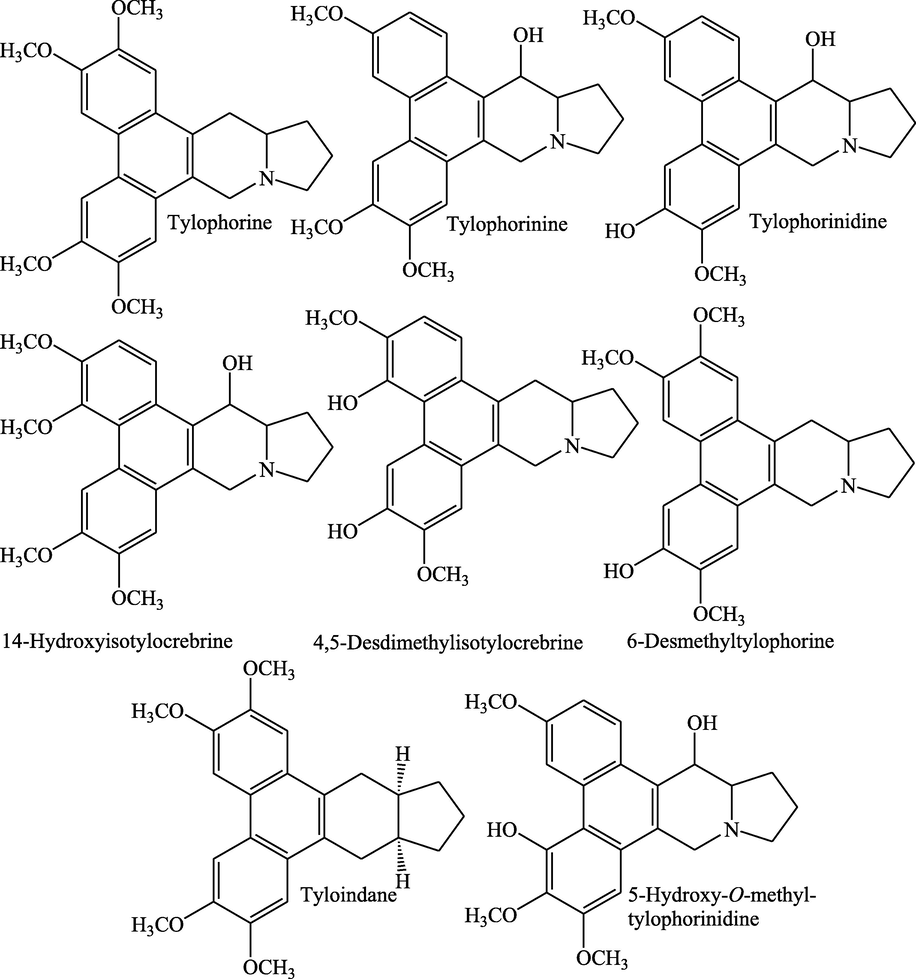
Structures of the alkaloids isolated from different parts of T. indica.
Seven new alkaloids such as tyloindicine A, tyloindicine–B, 14–hydroxyisotylocrebrine, 4,6–desdimethylisotylocrebrine, tyloindicine C, tyloindicine D, and tyloindicine E were isolated from methanolic leaf extract along with four previously reported alkaloids (tylophorine, 6–desmethyltylophorine, tylophorinidine, and 5–hydroxy–O–methyltylophorinidine). All aforesaid alkaloids possessed dibenzo–[f, h] pyrrolo [1 2b] isoquinoline skeleton except tyloindicine–B which contained substituted phenanthrene nucleus and an angular methyl group on the indolizidine portion. However, nature, number, and distribution of the oxygen-bearing substituents are different in aforesaid alkaloids. The alkaloids also differ in the presence or absence of C–13a or benzylic hydroxyl groups and an angular methyl function (Ali and Bhutani, 1989). Rao et al. (1971) have also reported the existence of desmethyl–tylophorine and desmethyltylophorinine in aqueous methanolic extract (1:1) of stem and leaves. Tyloindicine phenanthroindolizidine alkaloids (Fig. 2) such as tyloindicine F, tyloindicine G, tyloindicine H, tyloindicine I, and tyloindicine J were extracted from the aerial parts of the plant (Rao et al., 1971). A substituted phenanthrene hydrocarbon tyloindane was also isolated with tylophorine from the leaves and stem of the plant (Ali et al., 1991). Apart from the alkaloids, some minor alkaloids like (+)–septicine and d–isotylocrebrine have been isolated from the plant (Govindachari et al., 1975). The alkaloids have been reported from the phytochemical analysis of methanolic and aqueous extracts of T. indica roots but the category of alkaloids has not been classified yet (Khanna et al., 2018).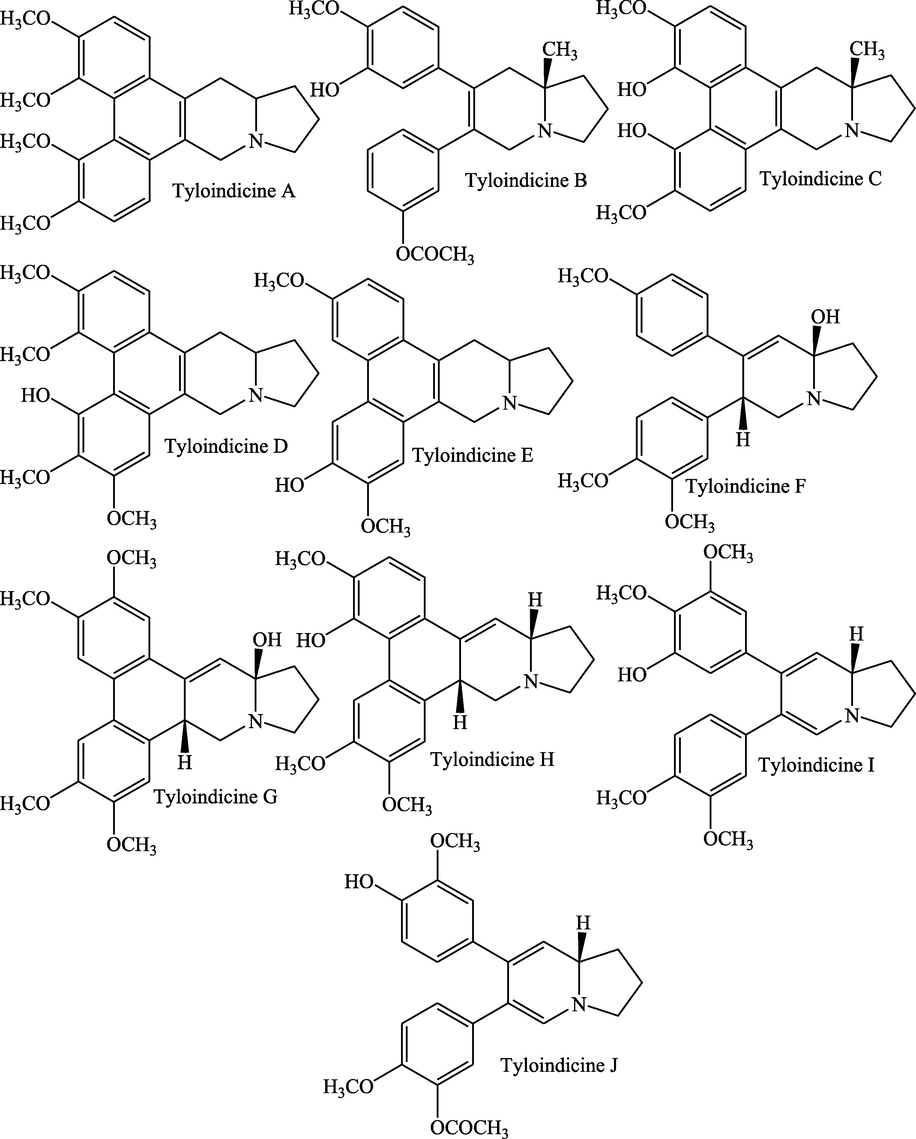
Structures of important tyloindicine phenanthroindolizidine alkaloids.
3.2 Saponins and terpenoids
Saponins are admired for the ability to fight against the microbes and boosting the immune system of the body (Surendra, 2009; Tiwari et al., 2018). Anticancerous, antioxidant, antimicrobial, and anti-inflammatory properties of different plant parts could also be attributed to the saponins (Aiyelaagbe and Osamudiamen, 2009). The tannins also possess the ability to check the growth and reproduction of bacteria and viruses, especially HIV (Aiyelaagbe and Osamudiamen, 2009). Tannins and terpenoids are reported from aqueous extract, while saponins have been investigated from the methanolic as well as aqueous extracts (Kumar, 2011). Saponins, terpenoids, and tannins have also been reported from the aqueous and alcoholic extracts of roots (Khanna et al., 2018).
3.3 Other metabolites
Apart from alkaloids, saponins, and terpenoids, other major phytoconstituents isolated from different organs of the plant are stigmasterol, tetratriacontanol, β–sitosetrol, and octaosanyl octacosanoate. Quercetin, α– and β–amyrins, cetyl–alcohol, phytosterol, wax, resin, coutchone, pigments, glucose, calcium salts, and potassium chloride have also been reported from various plant parts (Gupta et al., 2010b; Joshi et al., 2012; Rani et al., 2012). Some minerals such as Zn, Cu, Mn, and V, were also reported from the plant (Pattar et al., 2018). Alcoholic root extract was explored to contain small quantities of p–methoxy salicylaldehyde and oily matter when subjected to steam distillation (Ratanagiriswaran and Venkatachalam, 1935; Gopalakrishnan et al., 1979; Ali, 2008; Vivean et al., 2014). Phytochemical analyses of methanolic and aqueous extracts of roots have confirmed the existence of carbohydrates, flavonoids, steroids, glycosides, and amino acids. The elemental analysis reported the presence of Ca, K, Si, Mg, Al, Na, Fe, P, Cl, S, and Ti (Khanna et al., 2018). The phytochemical composition of plant leaves (Kaushik et al., 2010) is enlisted in Table 3.
Phyto–constituents
Average estimated value (mg/g fresh weight)
Alkaloids
1.46 ± 0.7
Flavonoids
4.5 ± 1.1
Saponins
2.0 ± 0.6
Protein
0.80 ± 0.036
Lipid
0.212 ± 0.00
Carbohydrates
0.620 ± 0.0026
Starch
0.737 ± 0.003
4 Isolation of phytochemicals
4.1 Isolation of alkaloids
Leaves of the plant were ground to a fine powder after washing with running tap water and shade dried. Before soaking in ethyl acetate, lipophilic substances were removed with n-hexane. The pH of the filtrate was adjusted to 3–4 by adding HCl dropwise. After concentrating the extract with flash evaporator at 55–60 °C, it was washed thrice with dichloromethane, and the pH was raised to the range of 11–13 by NaOH. The suspension of concentrated extract in chloroform was applied to pre-coated silica gel plates (10 × 10) for isolation of alkaloids using high-performance thin-layer chromatography (TLC). Nitrogen was used as a spray agent. The plates were run in the solvent system (toluene:chloroform:ethanol:ammonia) and then sprayed with nitrogen. The chromatograph was scanned in scanner III at 258 nm using a lamp in absorption mode with a spectrum scan speed of 100 nm/s. Quantification of tylophorine was accomplished using the following formula (Kaur et al., 2011);
In another method of alkaloid isolation, leaves were extracted with a mixture of 95% ethanol and 2% citric acid. After concentrating the extract with a rotary vacuum evaporator, it was purified using an acid-base purification technique, as discussed in the above study. Tylophorine was obtained with a high yield using pilot batch extraction (Gupta et al., 2012). In another study, leaves of the plant were extracted with methanol at low temperatures. The solvent-free extract was then re-extracted with aqueous acetic acid and dichloromethane to remove the neutral, acidic, and water-soluble materials. Acetic acid present in the aqueous layer was neutralized, and the organic layer of dichloromethane containing alkaloids was separated (Hadi and John, 2001). The extract obtained from finely ground plant material (5.0 g) after soaking for 4 h in 200 mL of 10% acetic acid in ethanol was concentrated on a water bath. Alkaloids were precipitated from the extract using concentrated ammonium hydroxide solution (Mohan et al., 2014).
4.2 Isolation of flavonoids
Aluminium chloride colorimetric method (Chang et al. 2002) was used with slight alterations for the determination of flavonoids (Mohan et al., 2014). The absorbance of the solution containing 1.0 mL aqueous root extract, 1.0 mL methanol, 0.5 mL potassium acetate (0.1176%), and 0.5 mL aluminium chloride (1.2%) was measured at the wavelength of 415 nm after keeping solution for 30 min at room temperature. Flavonoid content is expressed in terms of quercetin equivalent (mg/g) of the extracted compound).
4.3 Isolation of saponins
The ground plant material (20.0 g) was extracted with 20% aqueous ethanol for 4 h at 55 °C. The mixture was then filtered, and the residue was subjected to re–extraction with 20% aqueous ethanol. The resultant was combined with the first filtrate and concentrated to 40 mL by heating on a water bath at 90 °C. This concentrated solution was shaken with diethyl ether to separate the aqueous layer. Furthermore, n–butanol was added to the aqueous layer and washed with 5% saline. The final butanol layer was evaporated and then dried in the oven until the weight became constant (Obadoni and Ochuko, 2002).
4.4 Isolation of phytosterols
Ground plant leaves were heated with petroleum ether at (60–80 °C) to remove lipophilic substances. The residual plant material was extracted with ethanol before subjecting to TLC analysis using stigmasterol as a reference compound, hexane–acetone (8:2) as solvent and silica gel as a stationary phase. The appearance of grey colour (Rf 0.91) with 5% concentrated H2SO4 on TLC plate is the indication of stigmasterol in the leaves of the plant. Moreover, HPTLC analysis was applied to quantify stigmasterol (Chaturvedi and Chowdhary, 2014).
4.5 Isolation of kaempferol
Methanolic extract of six-week old leaf calli was analyzed qualitatively by TLC and quantitatively by HPTLC. After placing in toluene–ethyl acetate (14:2) mixture, silica gel HPTLC plates were sprayed with inert gas. The analysis was done at a wavelength of 430 nm (Chaturvedi and Chowdhary, 2013).
5 Folk medicinal uses
Plants have been used as a remedy for different ailments since time immemorial (Marwat et al., 2011; Shah et al., 2013). Primary healthcare mainly depends on traditional herbal medicines all over the world (WHO, 1998). The indigenous medicinal systems enlisted various medicinal plants as Ayurveda (7 0 0), Unani (7 0 0), Siddha (6 0 0), Amchi (6 0 0), and Allopathy (30) plant species (Rabe and Staden, 1997). In Pakistan, about 1500 species of medicinal plants have been implied traditionally for the cure of a number of ailments (Chaudhary, 1961). In the arena of potential therapeutic plants, the plant has proved the worth as a new medicinal plant. Ethnomedicinal literature is evident in the plant used for curing a wide range of ailments since prehistoric times (Yuan et al., 2016). The traditional medicinal uses of the plant are briefly discussed in Table 4.
Plant parts
Folk medicinal applications
References
Whole plant
Treatment of respiratory disorders particularly bronchial asthma
Faisal and Anis, 2005; Kumar et al., 2012; Shivpuri et al., 1969
Curing allergy, bronchitis, and dermatitis
Shivpuri et al., 1968
Antidote to poisons
Sankaranarayanan et al., 2010
Acts as cathartic, diaphoretic, emetic, laxative, purgative and stimulant
Shah and Kapoor, 1976
Muscle relaxant
Rani et al., 2012
Treatment of cold, dysentery, hay fever, arthritis diarrhea, and syphilitic rheumatism
Gore et al., 1980; Walter, 2005
Jaundice
Rani et al., 2012
Blood purifier
Nadkarni, 1996
As an alternative for Ipecacuanha
Vivean et al., 2014
used as an anti–psoriasis agent
Sarma and Misra, 1995
Leaves
Treatment of asthma
Saroya, 2011; Mohiuddin, 2019
Curing tuberculosis, cough
Dahanukar and Thatte, 1989
Treatment of asthma using an extract of leaves with black pepper and garlic
Reddy et al., 2006
Decoction of leaves is an antidote to snake poisons
Ignacimuthu et al., 2008; Sankaranarayanan et al., 2010
Used to kill intestinal worms
Rani et al., 2012
Food preservative
Nadkarni, 1954; Kirtikar and Basu, 1981; Reddy et al., 2009
Roots
Decoction used as an expectorant
Sankaranarayanan et al., 2010
Roots paste is applied on the forehead to get rid of headache and neuralgia
Jayaweera, 2006
Food preservative
Nadkarni, 1954; Kirtikar and Basu, 1981; Reddy et al., 2009
Leaves and roots
Paste of leaves and roots mixed with paste of Rauvolfia serpentia roots is applied on snake bite.
Ignacimuthu and Ayyanar, 2006; Sankaranarayanan et al., 2010
Treatment of hydrophobia
Rani et al., 2012
6 Pharmacological activities
The plant has been used for the treatment of various diseases such as hepatitis, asthma, arthritis, diarrhea, dysentery, and inflammatory disorders in the traditional system of medicines (Reddy et al., 2006; Chopra et al., 1986; Kirtikar and Basu, 1991). Inspired from the folk medicinal uses of the plant, many preclinical and clinical trials using various animal models have been piloted. Among these, antibacterial, antiulcer, antidiarrhoeal, hypolipidemic, anxiolytic, anti-diabetic, hepatoprotective, and antioxidant are significant (Meera et al., 2009; Balasubramanian et al., 2010; Gaitonde, 2001; Reddy et al., 2009; Swathi et al., 2012; Manikkoth et al., 2013; Saraswati et al., 2013; Hafis et al., 2017). The biological attributes are ascribed to the bioactives, particularly alkaloids (tylophorine, tylophorinine, and tylophorinidine) present in various extracts (Shahzad et al., 2016).
6.1 Anxiolytic activity
In the ever-changing and challenging world of today, pathological anxiety is one of the most common mental disorders. It is a kind of emotional reaction associated with decreased brain inhibitory and elevated excitatory neurotransmissions. The stress was induced in one group of Wistar albino rats by administering ethanol (20%) twice a day for 30 days at a dose of 1.5 mL/200 g body weight. The elevated plus maze and light-dark arena tests were applied to confirm the induction of anxiety. The rats were then treated with the extract of plant leaves (100 mg/kg body weight). The brains of rats were then dissected and subjected to dopamine assay. The significant anxiolytic effect was observed in the group treated with ethanolic extract of leaves. The protective action of the ethanolic extract against anxiety can be attributed to the modulating role of different neurotransmitters or due to the antioxidant potential (Manikkoth et al., 2013, 2016).
6.2 Anti-asthmatic activity
Asthma is spreading worldwide and affecting the children primarily. The adults of both sexes are equally attacked by the disease after the age of 40 (Dodge et al., 1986). The global death toll of asthma raised to 383,000 in 2015, along with 235 million asthmatic patients surviving through to 2016, according to the reports of WHO published in December 2017 (WHO, 2017). The global burden of disease was estimated to be 339.4 million in 2016, according to the report published by The Global Asthma Network (Marks et al., 2018). T. indica is considered as one of the best folk remedies for asthma (Butani et al., 2007). The leaves of the plant (150 mg of the leaf by weight) relieved the patients from asthma in a clinical trial when chewed and swallowed with an empty stomach in the morning (Shivpuri et al., 1969). Different cases of the controlled clinical trials favoured the use of the plant as an anti-asthmatic agent (Shivpuri et al., 1969; Shivpuri et al., 1972; Mathew and Shivpuri, 1974; Thiruvengadam et al., 1978; Gupta et al., 1979). The plant has been proved useful in causing significant relief from the symptoms of bronchial asthma and allergenic rhinitis even in the dose of 3–6 leaves only. The leaves of the plants are also beneficial for diminution of nasal impediment and sneezing with the effect lasting for approximately 10 days (Gore et al., 1980). Another causal agent of asthma is the increased concentration of inflammatory mediators released in response to the used allergens (Church et al., 1997; Barnes et al., 1998). Histamine, an inflammatory mediator, caused the deficiency of oxygen in the system, leading to the convulsion in Guinea pigs. The convulsions are characterized by contraction of smooth muscles, hypotension, and dilated capillaries. Histamine also induced the grave bronchoconstriction leading to asphyxia and eventually death. The plant lengthened the latent periods of spasms induced by the use of histamine aerosol at the dose of 100 mg/kg. The plant extract exhibited 43.15% protection against the histamine-induced bronchoconstriction in Guinea pigs supporting the anti-asthmatic properties of the plant (Paliwal et al., 2011). The alcoholic extract has the potential to thwart anaphylaxis in guinea pigs induced by egg albumin and horse serum-induced broncho–contraction in sensitized rat lungs (Gupta, 1975; Nayampalli and Sheth, 1979).
6.3 Anti–cancerous activity
Cancer has become one of the leading causes of mortality around the globe over the last few years (Madhusudan and Middleton, 2005). The skin cancer or melanoma is a multifactorial and multi–mechanistic type of cancer that is immensely affecting the human population (Stratigos et al., 2007). The leaf extract of the plant has been used as a folk remedy to treat tumors, but little is known about the mechanism of action (Chitnis et al., 1972). Most of the anti-tumor agents work by virtue of the induction of apoptosis in the target cancerous cells (Yin et al., 2012). The condensation of the nucleus, the overturning of membrane phosphatidylserine, activation of caspase–3, formation of apoptotic bodies, and release of mitochondrial cytochrome c confirmed the induction of apoptosis in K562 cells by the plant (Elmore, 2007). The ethanolic extract of the plant significantly retarded the escalation of the SKMEL–28 cells. The activity of the plant extract increased with concentration. Antiproliferative property of the plant extract was further established by comet assay. The extract induced nuclear damage in the head region of the comet resulting in apoptosis (Smitha et al., 2016). The anti–tumor activity may be attributed to the presence of an array of alkaloids in the crude fraction of the plant extract (Hammerová et al., 2011). Ethanolic extract of the plant reduced the viability of HCT–15 cells (A cell line of human colon, colorectal adenocarcinoma). The anti–proliferative potential of plant extract was confirmed by MTT (3–(4,5–dimethylthiazol–2–yl)–2,5–diphenyltetrazolium bromide) cell viability assay, Neutral Red uptake assay, and Lactate dehydrogenase assay. The protocols exhibited anti-cancerous potential of the plant by inducing apoptosis and membrane damage. The elevated concentration of crude extract aggravates the deterioration of cells (Pratheesh et al., 2014). The sprouting of new blood vessels from already existing ones is termed as angiogenesis (Carmeliet and Jain, 2011), one of the critical features of tumor build-out (Eikesdal et al., 2008). Blocking the sprouting arrested the growth and development of cancer (Ferrara, 2004). Tylophorine, an alkaloid from the plant (Gellert, 1987), is known to halt the cell cycle of HepG2, HONE–1, and NUGC–3 carcinoma cells in G1 phase and downregulates the articulation of cyclin A2 (Wu et al., 2009). Tylophorine retarded the process of angiogenesis in human umbilical vascular endothelial cells by virtue of VEGFR2 (Vascular Endothelial Growth Factor Receptor) signaling pathway, making it an expedient therapy for anti–angiogenetic cancer treatment (Saraswati et al., 2013). Tylophorine can retard the growth of tumor cells by exerting the CycA2 inhibitory function and countermand the HCV replication. It is further demonstrated that two tylophorine intermediates, 5–oxo–1–[(2,3,6,7–tetramethoxy–9–phenanthrenyl) methyl]–L–proline and 2,3,6,7–tetramethoxy–9–phenanthrenecarboxylic acid displayed anti–HCV activity with an EC50 value of 38.25 μM and 29.11 ∼ 35.3 μM, respectively in various HCV genotypes (Nguyen et al., 2019).
6.4 Anti–convulsant activity
Plant-based antiepileptic drugs have a vital role in the treatment of epilepsy because of the curative potential and cost-effectiveness (Joy et al., 2012). The ethanolic extract of the plant leaves were evaluated for the anti–convulsant potential against Maximal electroshock (MES), and Pentylene tetrazole (PTZ) induced seizure models in rats. The results of the said experiments indicated the anticonvulsant potential of ethanolic extract in MES and PTZ induced seizure models. The hind limb extension was completely prevented by administration of ethanol extract (100 mg/kg body weight) in MES model however in case of PTZ model, oral administration of the ethanolic extract at a dose of 100 mg/kg body weight completely eradicated the convulsions (Hafis et al., 2017). The curing potential of the extract owes to blockade of sodium channels (MES model) and T type calcium channels in the thalamus (PTZ model) (Manikkoth et al., 2011). The extract may mimic the activity of GABA and elevate the monoaminergic activity (Hafis et al., 2017). The activity of monoamino oxidase enzyme in the brain can be restrained by the isoquinoline alkaloid isolated from the ethanolic extract of the plant leaves. The tylophorinidine increased the concentration of monoamines by prohibiting the action of monoamino oxidase enzyme resulting in the interception of the MES and PTZ induced seizures (Manikkoth et al., 2016).
6.5 Anti-diabetic activity
Diabetes mellitus refers to the cellular impotency to uptakeglucose from the bloodstream. The inability may be congenital (type I insulin-dependent diabetes mellitus) or acquired (type II non–insulin-dependent diabetes mellitus) (Abesundara et al., 2004). It is marked by elevated blood glucose levels in the postprandial and /or fasting state and its severe forms are characterized by ketosis and protein wasting (Bell, 1991). The hypoglycemic activity of the plant was investigated by the application of methanolic plant extract on male diabetic Balb/c mice. The oral administration of the extract was carried out at a dose of 50 mg/kg body weight. For the analysis of blood glucose level, blood was collected from the tail vein at the time of administration of extract and 24 h after application. The blood samples were analyzed by Accu–chek blood glucose analyzer from Roche (Shukla et al., 2004). The results confirmed the hypoglycemic activity, which could be attributed to the presence of gymnemic acid in the stem and leaf extracts of the plant. Thus, the plant can be a substitution of the drugs as a natural remedy for diabetes in folk medication as well as the pharmaceutical industry (Najafi et al., 2010).
6.6 Antidiarrheal activity
One of the principal causes of infant mortality in developing countries is diarrhea (Jousilahti et al., 1997). The conventional treatment is the use of indigenous medicinal plants such as Andrographis paniculata, Asparagus racemosus, Butea monosperma, and Cassia auriculata (Chopra and Chopra, 1956). Little scientific evidence is available for the safe use of such plants as a remedy for diarrhea and dysentery. To check the anti–diarrheal potential of the plant, aqueous and alcoholic extracts of roots were prepared and administrated orally to the rats. The rats were divided into groups, and diarrhea was induced by castor oil (1.0 mL/100 g body weight) and prostaglandin–E2 (100 μg/kg in 2% v/v tween 80 orally). Another group was treated with 1.0 mL of the charcoal meal (3% deactivated charcoal in 2% aqueous tween 80). The parameters observed were first defecation time, frequency of defecation and cumulative wet faecal mass, contents of the intestine from the pylorus to cecum, and distance moved by the charcoal meal from the pylorus to the caecum. The alcoholic and aqueous extracts of roots caused significant inhibition of gastrointestinal propulsion as well as fluid secretion. The activity of the plant is exploited for its use as a folk anti-diarrheal agent (Patel et al., 2006). The bioactive compounds could be accredited for the anti-diarrheal activity of the plant included alkaloids, saponins, reducing sugars, tannins, and flavonoids (Okudo et al., 1989; Evans, 1997). Flavonoids reduced the motility of intestinal masses and also caused a decrease in hydro–electrolytic secretion (Carlo et al., 1993; Rao et al., 1997). Reduction in prostaglandin E2 induced intestinal secretory response is also attributed to the flavonoid content of the plant extracts (de Medina et al., 1997).
6.7 Antimicrobial activity
It is the call of the day to explore plant-based novel antimicrobials as microbes are getting more resistant to the available germicides because of the excessive use of the drugs (Chan et al., 2007; Gull et al., 2015). Attention must be paid to the folk remedies for the development of new drugs (Colombo and Bosisio, 1996). Different phytoconstituents such as homoisolavonoids, isoflavones, and gamma thionin are considered responsible for the antimicrobial potential of the plant extracts (Franco et al., 2006; Mhaeswara and Rao, 2006). Sulphur is also found as an active antibacterial component of plants such as Satureja pilosa, S. icarica, and Mentha piperita in some studies (Azaz et al., 2002; Iscan et al., 2002; Kalemba and Kunicka 2003). T. indica was studied for the antifungal potential against fungal species such as Aspergillus niger, A. fumigatus, and Trichoderma viride. The significant antifungal activity was manifested by the crude methanolic plant extracts as well as the isolated pure compounds except for stem crude extract against A. niger and root crude extract against A. fumigatus. Tylophorine and O–methyl tylophorinidine restrained the growth of A. niger and T. viride more efficiently with the inhibition zone of 10–15 mm as compared to septicine and simple aliphatic acid (5–10 mm). A. fumigatus was found to be most resistant, least inhibited even by pure compounds (Reddy et al., 2009). The crude extracts of the plant showed potent activity for checking the growth of fungal strains, A. niger, and Fusarium species (Reddy, 2009). The alcoholic leaf extract showed reasonable fungicidal potential against A. niger, A. flavus, A. fumigatus, and Penicillium species. The aqueous extract of leaf callus exhibited conspicuous activity against Candida parapsilosis, while parent plant extract was investigated inactive. The minimal inhibitory concentrations (MICs) of alcoholic leaf extract against the tested A. spergillus species ranged from 6.25 × 10–3 to 18.7 × 10–3 μg/mL, and MICs against Penicillium species was found to be 9.38 × 10–3 μg/mL (Shahid et al., 2012). In another study, the antifungal potential of in vitro raised plant extract was investigated as compared to parent plant extract. The alcoholic leaf callus extract excelled the parent plant in activity against C. albicans and C. krusei, in addition to activity against A. flavus, A. niger, A. fumigatus, and Penicillium species. The heightened activity of in vitro raised the plant could be attributed to the enhanced nutritional and hormonal concentrations in the culture medium. The MICs of the alcoholic leaf extracts of the parent plant, in vitro raised plant extract, and callus extract ranged from 12.0 to 98.0, 1.53–49.0, and 3.05–24.0 μg/mL, respectively (Khatoon et al., 2013). The methanolic extracts of wild and tissue cultured T. indica plants showed moderate control with the concentration of 60 μL against two fungal species viz., A. fumigatus and Verticillum lecanii with the zone of inhibition of 7.0 and 8.0 mm for wild and 8.0 and 9.0 mm, respectively for tissue-cultured plants (Vanitha et al. 2019).
The plant extracts are esteemed for the bactericidal activity against different bacterial strains (Garg and Jain, 1998; Ogbulie et al., 2007). The bacterial cultures of B. subtilis, S. aureus, M. luteus, E. coli, and P. aerginosa were incubated in petri dishes with test compounds for 24 h to detect the antibacterial capacity of the plant. The methanolic plant extract and isolated pure compounds were studied. Tylophorine and O–methy tylophorinidine manifested the highest antibacterial activity with an inhibition zone of 10–20 mm. The plant restrained the growth of all bacterial cultures except E. coli. The pure compounds displayed better bactericidal potential than crude extract (Reddy et al., 2009). Similar results were obtained against bacterial strains such as S. aureus, P. vulgaris, P. aergenosa, and E. coli (Reddy, 2009).
Another study was conducted to investigate the activity of alkaloid extract of the plant against E. coli, P. aeruginosa (gram-negative), and S. aureus (gram-positive). The alkaloid extract (1.0 mg of crude extract in 1.0 mL of 7.0% methanol) was applied at the concentrations of 5, 10, 15, and 20 μL/mL. The phytochemical extract of the plant was found to be effective in all applied concentrations; the activity is increased by increasing concentration. S. aureus and E. coli were found to be more susceptible to the plant extracts as compared to P. aeruginosa (Sathyabama and Kingsley, 2013).
Another study reported the use of a different solvent (benzene, isopropyl alcohol, and ethyl acetate) extracts as bactericidal agents against S. typhi, E.coli, and S. aureus. The maximum inhibition was exhibited by the ethyl acetate extract with the inhibition zone of 25–28 mm against S. aureus (Ponnanikajamideen et al., 2013). Another study reported the activity of different solvent extracts of root and leaves against P. aeruginosa, K. pneumoniae, E. coli, S. aureus, and S. typhi. Ethyl acetate extract of the root successfully inhibited the growth of all the bacterial strains. The methanolic leaf extract proved to have significant antifungal activity against all the stains except S. typhi. In contrast, methanolic root extract showed concentration-dependent activity against all the tested bacterial types. The growth of P. aerginosa, K. pneumonia, and S. typhi was restrained by the methanolic extract of leaves (Balasubramanian et al., 2010).
The methanolic and ethanolic extracts of wild and tissue cultured plants in different concentrations (20, 40, and 60 μL) were subjected to test for the antibacterial activity against E. coli, B. subtilis, S. paratyphi, and Klebsiella pneumonia. The best results were obtained by the application of ethanolic extracts with a concentration of 60 μL against B. subtilis and E. coli with a zone of inhibition of 13 mm and 12 mm, respectively (Vanitha et al., 2019). The leaves of the native and in vitro plant were extracted using different solvents for the evaluation of antimicrobial potential against bacterial (S. aureus and E. coli) and fungal strains (A. niger and P. chrysogenum). The results of the agar well diffusion method revealed that methanolic extract inhibited the growth of strains mentioned above significantly at all concentrations (25, 50, and 100 μg/mL). Among all the extracts, the acetonic extract showed the least antimicrobial activity (Anand et al., 2020).
6.8 Antioxidant activity
The antioxidants possessed the ability to detain the oxidation of living substrates by reactive oxygen species and free radicals (Kasote et al., 2015). The plants have been assumed to hold antioxidant potential for long. Two-third of the world flora has been bestowed with medicinal properties, including excellent antioxidant capacity (Krishnaiah et al., 2011). Gupta et al., 2011 investigated the antioxidant potential of the methanolic extract by quantification of the phenolic content of leaves. The phenolic content was estimated by the Folin–Ciocalteu assay method. The antioxidant capacity of a compound is measured in terms of DPPH (2, 2–diphenyl–1–picrylhydrazyl) radical scavenging activity (Pisoschi and Negulescu, 2011). The methanolic extract of the plant exhibited maximum (30.74%) activity at the concentration of 100 μL as compared to standard ascorbic acid (45.43%). The increase in the concentration of extract enhanced % DPPH scavenging activity. The IC50 value required for 50% inhibition was found higher for the plant (199.58 μg/mL) than ascorbic acid (194.58 μg/mL). The intense DPPH radical scavenging activity recommended its use as an antioxidant (Gupta et al., 2011). The generation of lipid peroxides is a despotic indication of build-out of oxidative stress (Sreepriya et al., 1998). In another study, the antioxidant potential of native as well as micropropagated plants was investigated using lipid peroxidation, superoxide dismutase, and catalase activity methods. IC50 was found to be 10 μg/mL by lipid peroxidation inhibition and 6 μg/mL by superoxide dismutase and catalase methods (Bhatia et al., 2013). The antioxidative capability of the plant was also ascertained by similar experiments carried out by Malathi et al. (2012).
The plant was also found useful against paracetamol-induced lipid peroxidation in the liver of male Wistar rats. The paracetamol (acetaminophen) was inoculated intraperitoneally at a dose of 1.0 g/kg body weight. The resultant liver damage was cured by treatment with methanolic extract of dried leaves. The lowered levels of lipid peroxides in the treated animal group led to the confirmation of the antioxidative potential of the plant (Malathi et al., 2011). The methanolic root extract of in vitro plants showed significant antioxidant potential and captured more than 75% free radicals at a concentration of 1000 μg/mL (Manju et al., 2020).
6.9 Anti-ulcer activity
One of the significant calamities associated with the consumption of non–steroidal anti-inflammatory drugs and alcohol is the formation of ulcerative lesions in the mucosal membranes of the body, particularly in the gastrointestinal tract. Among medicinal plants, T. indica has also been employed for the treatment of different types of ulcers, including peptic ulcer (Kannappan et al., 2008). The plant has been found beneficial in the treatment of histamine and naproxen induced ulcers in rats. The ulcer was caused by separately using 300 mg/kg histamine hydrochloride and 80 mg/kg naproxen intraperitoneally. The methanolic extract of the plant leaves was investigated useful for the inhibition of ulcers in different doses. In histamine induced ulcers, methanolic extract of the leaves showed 6.57, 8.95, and 18.68% inhibition with the injection of 50, 100, and 200 mg/kg of the plant extract, respectively. The percentage inhibition of the plant extract was noted to be 9.66, 16.09, and 47.53% for similar doses of naproxen (Ghodekar et al., 2010). The mechanism of action of the plant in both treatments is different depending upon the ulcer-inducing drug. The plant protects naproxen induced ulceration by aggravating the action of enzymatic antioxidants such as glutathione peroxidase and catalase (Parks, 1989). The histamine stimulated H2–receptor, an increase in gastric acid secretion, and vasodilation led to ulcer development. The possible route of action is the blockage of H2–receptors leading to the inhibition of ulcer development (Amagase and Okabe, 2003).
6.10 Diuretic activity
The osmotic balance of the body is regulated by diuretic drugs. The drugs are responsible for checking the rate of urine flow and excretion of sodium from the body. The drawback associated with synthetic diuretics is the rapid loss of potassium from the body. Hence, a natural diuretic is required that allows the inhibition of Na+ reabsorption and retains the K+ concentration of the body (Meera et al., 2009). The alcoholic and aqueous extracts of leaves were investigated for the diuretic activity of the plant. The ethanolic extract was the most effective diuretic at active dose of 100 mg/kg body weight. Ethanol enhanced the activity of leaf extract by increasing the urinary electrolyte concentration (Kumar et al., 2010). Thus the plant shows good diuretic activity, increasing the volume of urine, cation, and anion excretion, particularly that of sodium, potassium, and chloride. The active dose of the extract was examined to be 100 mg/kg body weight. Ethanol extract proved to be the most effective, followed by chloroform and aqueous extracts (Meera et al., 2009). The diuretic activity of the plant can be attributed to the bioactive components found in the plant, such as flavonoids, saponins, and terpenoids (Rizvi et al., 1980; Sood et al., 1985; Chodera et al., 1991). The control of acid-base balance in the kidney is closely related to the maintenance of the sodium to potassium balance. The excessive loss of potassium ion due to the use of diuretics can cause hypokalemia as a side effect. Therefore, such diuretics are preferred, which spare the loss of potassium (Sturat, 2002). The level of potassium in urine was considerably high, leading to a risk of hypokalemia. The aspect of plant diuretic is still to be investigated (Meera et al., 2009).
6.11 Hepatoprotective activity
The liver is considered as the warehouse of metabolism of animal bodies, playing a vital role in physiological processes. The autoimmune diseases, excessive consumption of alcohol, and toxic chemicals may lead to hepatotoxicity. The hepatotoxicity is mainly caused by the induction of lipid peroxidation in liver cells. A number of plants have been implemented for the protection of the liver (Kumar et al., 2011). The hepatoprotective potential of the plant was subjected to a test against the liver damage caused by carbon tetrachloride in Wistar albino rats. The hepatoprotective capability of methanolic extract of leaves was estimated in terms of biochemical parameters such as serum glutamate oxaloacetate transaminase, serum glutamate pyruvate transaminase, total protein and level of serum bilirubin. The methanolic extract of leaves showed a remarkable diminution in the level of serum hepatic enzymes at a dose of 200 mg/kg and 300 mg/kg body weight (Koratala and Kazory, 2017). The plant has also proven useful against artesunate induced liver injury. The hepatic injury was caused by the oral administration of artesunate at the rate of 110 mg/kg body weight of Wistar albino rats. The damage was ameliorated by treatment with 90% aqueous ethanolic extracts of leaves. The efficiency of plant extract was assessed on the basis of various biochemical parameters like alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, γ‐glutamyltransferase, bilirubin, and albumin in the serum, and the levels of antioxidant enzymes like superoxide dismutase and catalase in liver tissues (Jahas et al., 2014). The methanolic extract of the leaves was found useful against CCl4 induced hepatotoxicity by studying serum glutamate oxaloacetate transaminase, serum glutamate pyruvate transaminase, serum alkaline phosphatase and bilirubin content (Mujeeb et al., 2009). The hepatoprotective activity of the plant is accredited to an active phytoconstituent, tylophorine, which provides the membrane-stabilizing and antioxidant property to the therapeutic gem (Gujrati et al., 2007; Manikkoth and Rao, 2013).
6.12 Nitric oxide scavenging activity
Nitric oxide (NO) is one of the important bioregulatory molecules essentially required for many physiological processes such as immune response, the transmission of nerve impulse, vasodilation, and control of blood pressure (Pacher et al., 2007; Hosking, 2009). However, prolonged exposure of the body to NO may lead to various ailments, including cancer (Chen et al., 2014). The aqueous ethanolic extract (50%) of the leaves was found useful in scavenging NO. The highest NO scavenging activity (79.28%) was manifested by the extract concentration of 1000 µg/mL. Even the lowest concentration of the tested extract (32.25 µg/mL) was an efficient NO scavenger (Jagetia and Baliga, 2004). Another study reported potent NO radicals scavenging activity of the plant at different concentrations (125, 250, and 500 μg) (Pratheesh et al., 2014).
7 Biotechnological applications
7.1 Antifeedant activity
The pests and microorganisms caused massive damage to the productivity of crops. The pesticides used against the pests made the situation even worse by deteriorating the environment. Hence there is a dire need for drugs that possessed antifeedant and insecticidal properties (Reddy et al., 2009). The crude methanolic extract of different parts of the plant and isolated pure compounds were investigated for the antifeedant activity against Spodoptera litura, a polyphagous pest damaging crops. The extract showed antifeedant activity in dose-dependent fashion. The crude leaf extract manifested maximum activity (71.85%) as compared to crude extracts of the stem (62.90%) and root (56.04%) at the concentration of 500 ppm with an interval of 24 h. With the elevation of concentration from 500 ppm to 1000 ppm, the activity of crude extracts of leaf, stem, and root increased to 95.85, 90.82, and 81.75%, respectively. Tested pure compounds included tylophorine, O–methyl tylophorinidine, septicine, and simple aliphatic extracts. Among the compounds, tylophorine and O–methyl tylophorinidine exhibited maximum (100%) activity at the concentration of 500 ppm, followed by the activity of septicine (90.35%) and simple aliphatic acids (72.68%) within 24 h (Reddy et al., 2009). Tylophorine has also been useful as an antifeedant against Spilosoma oblique walker as reported by Verma et al. (1986) and Thripathi et al. (1990).
7.2 Larvicidal and insect-repellent activities
Vector-borne diseases are becoming epidemic nowadays (Wei et al., 2007; Lemon et al., 2008). Recent research has revealed the fact that different parts of the plants can be used as mosquito repellents or pesticides without bearing any side–effect to the non–target organisms and ecosystem because of the presence of certain insecticidal phytochemicals. The phytoconstituents are reported from the fruit pulp, kernel, root, bark, and leaves of different plants (Ansari et al., 2005; Ezeonu et al., 2001; Sen-Sung et al., 2003; Chapagain et al., 2007; Rawani et al., 2013). Methanol, n-hexane, and ethyl acetate extracts of the plant leaves were tested against Culex quinquefasciatus and Aedes aegypti for the larvicidal and insect-repellent activities. Among the extracts, n-hexane extract was found to be most effective for the larval mortality with the lethal concentration (LC50) of 324 and 619 ppm against C. quinquefasciatus and A. Aegypti, respectively. Appreciable screening time of 187 min against C. quinquefasciatus and 122 min against A. aegypti was exhibited by the n-hexane extract of the leaves at the concentration of 5.0 mg/cm2. The larvicidal and insect-repellent activities of methanol and ethyl acetate extracts were negligible. This property of T. indica can be exploited for the commercial production of insect repellents and insecticides (Gandhi et al., 2014). The summary of all the biological and biotechnology attributes is given in Table 5.
Activity
Nature of plant extract
In vitro/in vivo study
References
Anxiolytic
Ethanolic plant extract
Wistar albino rats
Manikkoth et al., 2013, 2016
Anti-asthmatic
Alcoholic plant extract
Guinea pigs
Paliwal et al., 2011
Anticancerous
Ethanolic leaf extract
SKMEL–28 cells, HCT–15 cells
Pratheesh et al., 2014; Smitha et al., 2016
Anti-convulsant
Ethanolic leaf extract
Rats
Hafis et al., 2017
Anti-diabetic
Methanolic plant extract
Mice
Shukla et al., 2004
Antidiarrhoeal
Aqueous and alcoholic root extracts
Rats
Patel et al., 2006
Antifeedant
Crude methanolic leaf extract
Spodoptera litura and Spilosoma oblique walker pests
Verma et al., 1986; Thripathi et al., 1990; Reddy et al., 2009
Antimicrobial
Methanolic plant extract; aqueous leaf callus extract; alcoholic leaf callus extract; ethyl acetate root extract; methanolic leaf extract, ethanolic plant extract
In vitro
Reddy et al., 2009; Reddy, 2009; Balasubramanian et al., 2010;Shahid et al., 2012; Khatoon et al., 2013; Sathyabama and Kingsley, 2013; Ponnanikajamideen et al., 2013; Vanitha et al., 2019
Antioxidant
Methanolic leaf extract
In vitro
Gupta et al., 2011; Bhatia et al., 2013
Plant
Wistar rats
Malathi et al., 2011; Malathi et al., 2012;
Anti-ulcer
Methanolic leaf extract
Rats
Ghodekar et al., 2010
Diuretic
Alcoholic leaf extract
Wistar rats
Meera et al., 2009
Hepatoprotective
Methanolic leaf extract
Wistar rats
Koratala and Kazory 2017; Jahas et al., 2014;
Larvicidal and insect repellent
Hexane leaf extract
Culex quinquefasciatus and Aedes aegypti larvae
Gandhi et al., 2014
Nitric oxide scavenging
Ethanolic plant extract
In vitro
Jagetia and Baliga, 2004; Pratheesh et al., 2014
8 Propagation of the plant
Traditionally T. indica has been propagated through seeds. The optimum conditions for the propagation included organic matter–rich loamy soil, shady environment with an ambient supply of heat, and light. Added organic manure also promoted the growth of the plant. The germination of seeds starts within 10 days and could be transplanted into the fields after 90 days. The transplantation brings about the best results when done in the rainy season (Rani et al., 2012).
The magnificent activity of the plant against asthma has brought it to the limelight in the folk medicinal systems. The plant has been used extensively, leading to overexploitation (Thomas, 2009). Owing to the unsystematic collection and mismanagement of the cultivation, the plant is facing the threat of extinction in the Southeast Asian subcontinent. It is the call of the day to devise such methods that can overcome the present critical situation of the plant and meeting the increasing demand (Faisal and Anis, 2003). One of the ways is micropropagation, which includes the development of a large number of plants from the explant. Micropropagation takes a very short period as compared to the time take conventional cultivation practices (Rani et al., 2012). Many studies authenticated the uses of modern biotechnological approaches for the mass cultivation of the plant. The propagation through somatic embryogenesis has been carried out from mature leaves (Jayanthi and Mandal, 2001; Choudhury et al., 2004; Chandrasekhar et al., 2006; Archana, 2018). In another study, plants were rapidly multiplied by means of enhanced axillary bud proliferation from the nodal explants collected from young shoots (Faisal et al., 2007). Faisal and Anis (2005) used petiole cells for the development of callus and regeneration of plants. The plant has been regenerated through mesophyll protoplast (Thomas, 2009) and callus regeneration (Faisal and Anis, 2005). The transformations in plant were brought using the bacterial strain of Agrobacterium rhizogenes on excised leaf explants (Gupta et al., 2010b) and Agrobacterium tumefaciens on stem apices (Alagumanian et al., 2014).
9 Toxicity
Besides its enormous pharmacological benefits, T. indica is found to be very toxic at higher concentrations. The allergic reactions of the plant have been previously reported in some cases. Tylophorine, phenanthroindolizidine alkaloid is considered as an allergen, but further confirmation is required. Among the aqueous, methanolic, and ethanolic extracts of the leaves and stem of the plant, the ethanolic extract was found to be the most toxic against the baby hamster kidney fibroblast cells (BHK–21) (Kannan et al., 2013). The plant extract is found to be toxic in concentrations as low as 20 μg/mL (Yang et al., 2005). A study claimed that the alkaloids, tylophorine, and tylophorinine from the plant might cause dermatitis as a side effect, but no skin testing report is evident from the literature. A single dose (12–100 mg/kg) of pure alkaloids suspended in peanut oil causes indolence, salivation, respiratory obstruction, and diarrhea in male rats. The LD50 value of the alkaloid was investigated to be 35.32 mg/kg (Dikshith et al., 1990). Another study claims plant juice to cause vomiting, unconsciousness, and eventually death (Basher and Islam, 2015; Mohiuddin, 2019).
10 Conclusion and prospects
Owing to the diverse range of phytoconstituents occurring in the plant, T. indica has found extensive uses in the traditional as well as the modern system of medicines. A wide arena of pharmacological attributes is accredited on the part of the plant such as antiasthmatic, bronchodilator, antioxidant, anti-cancerous, hepatoprotective, anti–diabetic, diuretic, and many others. The plant is rich in alkaloids, major of them being tylophorine and tylophorinine. Other phytochemicals include flavonoids, saponins, tannins, terpenoids, phytosterols, and a fair concentration of primary metabolites.
In vitro raised plants showed improved qualities as compared the conventionally raised plants. The methanolic extract of callus checks the growth of C. albicans and C. krusei, which was unable to be done with the parent plant extract. The activity could be exploited to reduce the risk of diseases caused by infectious organisms. The plant extract could be used as a raw material for the development of fungicides. The chemical formulations of the plant are already available in the market. The extracts could be used on the commercial industrial stage for the development of new novel drugs. The variety of bioactivities shown by the plant invites the intrigued explorers of nature to further uncover the veil of the plant. Further studies are required to check the toxicity of the plant and the methods that could minimize the side effect of the plant. As every boon carries a bane with it, the pharmacologically active nature of the plant has brought it to the advent of extinction due to the indiscriminate barbarian collection of the plant from the natural habitat. The present situation necessitates such steps that restore the population of the plant as well as meet the increasing demand of the plant. The biotechnological tools should be used for the rapid mass cultivation of the plant, particularly in the areas where the plant is unable to grow due to a lack of favorable environmental conditions.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- α–Glucosidase nhibitory activity of some Sri Lanka plant extracts, one of which, Cassia auriculata, exerts a strong antihyperglycemic effect in rats comparable to the therapeutic drug acarbose. J. Agric. Food Chem.. 2004;52:1541-1545.
- [Google Scholar]
- Acharya, D., Shrivastava, A., Pawar, S., Sancheti, G., 2010. Rare Herb of Patalkot: Tylophora indica–Tylophora asthmatica. Retrieved from https://www.disabled–world.com/medical/alternative/herbal/tylophora–indica.php.
- Phytochemical screening for active compounds in Mangifera indica. Leaves from Ibadan. Oyo State. 2009;2(1):11-13.
- [Google Scholar]
- In vitro studies and Agrobacterium mediated transformation in Tylophora indica L. (Burm. F.). Merr. Int. J. Curr. Res. Biosci. Plant Biol.. 2014;1(3):110-116.
- [Google Scholar]
- Pharmacognosy (pharmacognosy & phytochemistry). New Delhi: CBS Publisher & Distributors; 2008. p. :653.
- Rare phenanthroindolizidine alkaloids and a substituted phenanthrene, tyloindane, from Tylophira indica. J. Nat. Prod.. 1991;54(5):1271-1278.
- [Google Scholar]
- On the mechanisms underlying histamine induction of gastric mucosal lesions in rat with partial gastric vascular occlusion. J. Pharmacol. Sci.. 2003;92:124-136.
- [Google Scholar]
- Antimicrobial and antioxidant potential of Tylophora indica (Burm. f.) Merrill and development of an efficient micropropagation system for its therapeautic use. Med. Plants Int. J. Phytomed. Related Indus.. 2020;12(1):82-89.
- [Google Scholar]
- Anonymous, 1978. The wealth of India. Raw materials. Vol. X. CSIR, New Delhi, India. 398.
- Larvicidal and mosquito repellent activities of pine (Pinus longifolia, Family: Pinaceae) oil. J. Vec. Bor. Dis.. 2005;42:95-99.
- [Google Scholar]
- Capparis spinosa L.: a plant with high potential for development of functional foods and nutraceuticals/pharmaceuticals. Int. J. Pharmacol.. 2016;12:201-219.
- [Google Scholar]
- In vitro propagation and rapid multiplication of Tylophora indica (Burm. f.) Merrill. Int. J. Bot. Studies. 2018;3(2):127-128.
- [Google Scholar]
- Cydonia oblonga M., a medicinal plant rich in phytonutrients for pharmaceuticals. Front. Pharmacol.. 2016;7:163.
- [Google Scholar]
- Ayensu, E.S., DeFilipps, R.A., 1978. Endangered and Threatened Plants of the United States. Smithsonian Institution, Washington, DC.
- Antimicrobial activity of some Satureja essential oils. Zeitschrift für Naturforschung C.. 2002;57:817-821.
- [Google Scholar]
- Balasubramanian, B., Dhanabal, M., Perumal1, A., George, S., 2010. Studies on the antibacterial activity and phytochemical screening of Tylophora indicalinn on opportunistic bacterial pathogens co–infected with HIV. Drug Invention Today. 2 (9), 402–404.
- Plants and herbal poisoning in Bangladesh. Clinical Toxinol. Asia Pacific Afr. 2015:609-631.
- [Google Scholar]
- Lilly lecture, Molecular defects in diabetes mellitus. Diabetes.. 1991;40(4):413-422.
- [Google Scholar]
- Antioxidant activity of native and micropropagated Tylophora Indica leaves extract: A comparative study. J. Nat. Prod. Plant Resour.. 2013;3(1):1-7.
- [Google Scholar]
- Bhavan, B.V., 1992. Selected medicinal plants of India. Bombay, India: Tata Press, 33.
- Tylophora indica–An ancient anti–asthmatic medicinal plant: A Review. Int. J. Green Pharma.. 2007;1(2):2-6.
- [Google Scholar]
- Inhibition of intestinal motility and secretion by flavonoids in mice and rats: structure-activity relationships. J. Pharma. Pharmacol.. 1993;45(12):1054-1059.
- [Google Scholar]
- Molecular mechanisms and clinical applications of angiogenesis. Nature.. 2011;473:298-307.
- [Google Scholar]
- Antioxidant and antibacterial activity of leaves of Etingera species (Zingiberaceae) in Peninsular Malaysia. Food Chem.. 2007;104:1586-1593.
- [Google Scholar]
- Somatic embryogenesis of Tylophora indica (Burm. f.) Merril, an important medicinal plant. Int. J. App. Sci. Eng.. 2006;4(1):33-40.
- [Google Scholar]
- Estimation of total flavonoid content in Propolis by two complementary colorimetric methods. J. Food Drug Anal.. 2002;10:178-182.
- [Google Scholar]
- Larvicidal activity of saponins from Balanites aegyptiaca callus against Aedes aegypti mosquito. Biores. Technol.. 2007;99(5):1165-1168.
- [Google Scholar]
- Chaturvedi, P., Chowdhary, A., 2014. Tylophora indica: Phytochemical, biotechnological and pharmacological approach, Munich, GRIN Verlag. https://www.grin.com/document/270256.
- Enhancement of antioxidant compound in Tylophora indica (Asclepeadaceae) callus. Adv. App. Sci. Res.. 2013;4(2):325-330.
- [Google Scholar]
- Distribution of some important medicinal plants of West Pakistan. Pak. J. Sci. Ind. Res.. 1961;4(4):207-211.
- [Google Scholar]
- Traffic–related air pollution and lung cancer: A meta–analysis. Thoracic Cancer.. 2014;6:307-318.
- [Google Scholar]
- Anti–cancer activity of the extracts of stem and leaf of Tylophora indica. Indian J Med Res.. 1972;60(3):359-362.
- [Google Scholar]
- Effect of flavonoid fractions of Solidago virgaurea L. on diuresis and levels of electrolytes. Acta Pol. Pharm.. 1991;48:35-37.
- [Google Scholar]
- Chopra, R.N., Chopra, S.L., 1956. Glossary of Indian medicinal plants New Delhi: Council of Scientific and Industrial Research.
- Chopra, I.C., Chopra, R.N., Nayar, S.L., 1986. Glossary of Indian medicinal plants, CSIR, New Delhi, 5.
- The root: a potential new source of competent cells for high–frequency regeneration in Tylophoraindica. Plant cell report. 2004;22(10):731-740.
- [Google Scholar]
- Church, M.K., Bradding, P., Walls, A.F., 1997. Allergy and allergic diseases, Human mast cells and basophils. Oxford. 149–170.
- Pharmacological activities of Chelidonium majus L (Papavaraceae) Pharmacol. Res.. 1996;33:127-134.
- [Google Scholar]
- Dahanukar, S.A., Thatte, U.M., 1989. Therapeutic approaches in Ayurveda Revisited, Popular Prakashan, Mumbai. 74–130.
- Effects of quercetin on epithelial chloride secretion. Life Sci.. 1997;61(20):2049-2055.
- [Google Scholar]
- Toxicity of pure alkaloid of Tylphora asthmatica in male rat. Indian J. Exp. Bio.. 1990;28:208-212.
- [Google Scholar]
- Comparisons of asthma, emphysema, and chronic bronchitis diagnoses in a general population sample. Am. Rev. Respir. Dis.. 1986;133:981-986.
- [Google Scholar]
- Identification of amino acids essential for the antiangiogenic activity of tumstatin and its use in combination antitumor activity. Proc. Natl. Acad. Sci. USA. 2008;105:15040-15045.
- [Google Scholar]
- Apoptosis: a review of programmed cell death. Toxicol. Pathol.. 2007;35(4):495-516.
- [Google Scholar]
- Furoquinoline alkaloids from Tylophora asthmatica. Phytochemistry. 1977;16(7):1125-1126.
- [Google Scholar]
- Evans, W.C., 1997. Pharmacognosy, Singapore: Har court brace and Co. Pvt. Ltd.
- Insecticidal properties of volatile extracts of orange peels. Biores. Technol.. 2001;76:273-274.
- [Google Scholar]
- Rapid mass propagation of Tylophora indica Merrill via leaf callus culture. Plant Cell, Tissue Organ Culture. 2003;75:125-129.
- [Google Scholar]
- An efficient micropropagation system for Tylophora indica: an endangered, medicinally important plant. Plant Biotechnol Rep.. 2007;1:155-161.
- [Google Scholar]
- Faisal, M., Anis, M., 2005. In vitro regeneration and plant establishment of Tylophora indica: Petiole callus culture. In vitro Cell. Dev. Biol. Plant. 41, 511–515.
- Vascular endothelial growth factor as a target for anticancer therapy. Oncologist. 2004;9:2-10.
- [Google Scholar]
- Identification of a cowpea γ–thionin with bactericidal activity. FEBS.. 2006;273:3489-3497.
- [Google Scholar]
- Gaitonde, B.B., 2001. Research, drug development and manufacture of herbal drugs In: Traditional medicines in Asia, WHO. 247–270.
- Larvicidal and repellent activities of Tylophora indica (Burm. F.) Merr. (Asclepiadaceae) against Culex quinquefasciatus Say and Aedes aegypti L. (Diptera: Culicidae) Int. J. Pure Appl. Zoo.. 2014;2(2):113-117.
- [Google Scholar]
- Antimicrobial efficacy of essential oil from Curcuma caesia. Ind. J. Microbial.. 1998;38:169-170.
- [Google Scholar]
- Gellert, E., 1987. In Alkaloids: Chemical and biological perspectives. Edited by Pelletier SW. New York: Academic Press. 55–132.
- Antiulcer activity of methanolic extract of leaf of Tylophora indica on histamine and naproxen induced gastric lesions in rats. Pharmacologyonline.. 2010;1:141-147.
- [Google Scholar]
- Gilani, A. H., Atta–ur Rahman., 2005. Trends in ethnopharmacology. J. Ethnopharmacol. 100, 43–9.
- Pharmacological investigation of tylophorine. Indian J. Med. Res.. 1979;69:513-520.
- [Google Scholar]
- Effect of tylophorine, a major alkaloid of Tylophora indica, onimmunopathological and inflammatory reactions. Indian J. Med. Res.. 1980;71:940-948.
- [Google Scholar]
- Physiological studies with Tylophora asthmatica in bronchial asthma. Ind. J. Med. Res.. 1980;71:144-148.
- [Google Scholar]
- Five decades in the study of natural products. Proc. Indian Acad. Sci.. 2002;3:114.
- [Google Scholar]
- Govindachari, T.R., Lakshmikantham, M.V., Nagarajan, B.K., Pai, B.R., 1957. Chem. Ind. 1484.
- Chemical examination of Tylophora asthmatica—III: The complete structure of tylophorine. Tetrahedron.. 1960;9(1–2):53-57.
- [Google Scholar]
- Hepatoprotective activity of alcoholic and aqueous extracts of leaves of Tylophora indica (Linn.) in rats. Indian J. Pharmacol.. 2007;39:43-47.
- [Google Scholar]
- Antibacterial potential of Capparis spinosa and Capparis decidua extracts. Int. J. Agric. Biol.. 2015;17:727-733.
- [Google Scholar]
- Pharmacological basis for the use of Tylaophora indica in bronchial asthma. Aspect Aller. Appl. lmmunol.. 1975;8:95-100.
- [Google Scholar]
- Extraction process optimization of tylophorine from Tylophora asthmatica Wight and Arn. Phcog. J.. 2012;4(28):19-23.
- [Google Scholar]
- Tylophora indica in Bronchial Asthma: A double–blind study. Indian J. Med. Res.. 1979;69:981-989.
- [Google Scholar]
- Anti-neuroinflammatory potential of Tylophora indica (Burm. f) Merrill and development of an efficient in vitro propagation system for its clinical use. Plos one. 2020;15(3):e0230142
- [Google Scholar]
- Phyto–pharmacological and plant tissue culture overview of Tylophora indica (Burm. f) Merill. J. Pharma. Sci. Res.. 2010;2(7):401-411.
- [Google Scholar]
- HPTLC Fingerprinting of different leaf extracts of Tylophora indica (Burm f.) Merill. Pharma. J.. 2010;2(11):381-385.
- [Google Scholar]
- Int. J. Pharma. Sci. Res.. 2011;2(1):121-126.
- Gupta, A.K., 2003. Quality standards of Indian medicinal plants. 1, 221.
- Gurav, S., Devprakash, Senthilkumar, G.P., Tembare, R., Mani, T., 2011.Tylophora indica: A review on its ethnobotany, phytochemical and pharmacological profile. Asian J. Biochem.Pharma. Res. 1 (3), 405–414.
- Initial Studies on alkaloids from Lombok medicinal plants. Molecules.. 2001;6:117-129.
- [Google Scholar]
- Pharmacological evidence for the anticonvulsant activity of Tylophora indica in experimental animal models. Int. J. Basic Clin. Pharmacol.. 2017;6:750-753.
- [Google Scholar]
- Benzo[c]phenanthridine alkaloidsexhibit strong anti–proliferative activity in malignant melanoma cells regardless of their p53 status. J. Dermatol. Sci.. 2011;62(1):22-35.
- [Google Scholar]
- Hooper., 1891. Pharm. J. 21, 617
- Nitric oxide and the immune system: a literature review. The Plymouth Student Scientist.. 2009;2(2):270-278.
- [Google Scholar]
- Hussain, S., Malik, F., Khalid, N., Qayyum, M. A., Riaz, H., 2012. Alternative and traditional medicines systems in Pakistan: history, regulation, trends, usefulness, challenges, prospects and limitations. A compendium of essays on alternative therapy, Dr. Arup Bhattacharya (Ed.), ISBN: 978–953–307–863–2, InTech, Available from: http://www.intechopen.com/books/acompendium–of–essays–on–alternative therapy/alternative–and–traditional–medicines–systems–in–pakistanhistory–regulation–trends–usefulness–chall
- Ethnobotanical investigations among tribes in Madurai District of Tamil Nadu (India) J. Ethnobio. Ethnomed.. 2006;2(1):25.
- [Google Scholar]
- Ethnobotanical study of medicinal plants used by Paliyar tribals in Theni district of Tamil Nadu, India. Fitoterapia. 2008;79(7–8):562-568.
- [Google Scholar]
- Antimicrobial screening of Mentha piperita essential oils. J. Agric. Food Chem.. 2002;50:3943-3946.
- [Google Scholar]
- The evaluation of nitric oxide scavenging activity of certain Indian medicinal plants In Vitro: A preliminary study. J. Med. Food.. 2004;7(3):343-348.
- [Google Scholar]
- Protective role of Tylophora indica ethanolic extract on artesunate induced liver toxicity. Int. J. App. Bio. Pharma. Tech.. 2014;5(4):206-210.
- [Google Scholar]
- Jayanthi, M., Mandal, P.K., 2001. Plant regeneration through somatic embryogenesis and RAPD analysis of regenerated plants in Tylophora indica (Burm. f. Merrill.). In Vitro Cell. Dev. Biol–Plant. 37 (5), 576–580.
- Jayaweera, D.M.A., 2006. Medicinal plants (indigenous and exotic) used in ceylon. The National Science Foundation, Colombo, Sri Lanka.
- Diarrhoeal disease morbidity and home treatment practical in Egypt. Public Health.. 1997;111(1):5-10.
- [Google Scholar]
- Anticonvulsant activity of ethanolic extract of Moringa concanensis leaves in swiss albino mice. Arch. Med. Health Sci.. 2012;1(1):6-9.
- [Google Scholar]
- Antibacterial and antifungal properties of essential oils. Curr. Med. Chem.. 2003;10:813-829.
- [Google Scholar]
- Evaluation of phytochemical constituents, antibacterial activities, cytopathic and cytotoxic effects of extracts of Tylophora indica, Curcuma amada and Urtica dioica. J. Recent Adv. Appl. Sci.. 2013;28:1-11.
- [Google Scholar]
- Antiulcer activity of methanolic extract of Jatropha curcas (linn.) on aspirin induced gastric lesionsin Wistar Rats. Pharmacol. Online.. 2008;1:125-126.
- [Google Scholar]
- Phytochemical investigations of some Tylophora species found in India. Planta Medica.. 1975;27:333-336.
- [Google Scholar]
- Significance of antioxidant potential of plants and its relevance to therapeutic applications. Int. J. Bio. Sci.. 2015;11(8):982.
- [Google Scholar]
- Extraction of tylophorine from in vitro raised plants of Tylophora indica. J. Med. Plants Res.. 2011;5(5):729-734.
- [Google Scholar]
- Biochemical assessment of in vitro and in vivo culture of Tylophora indica (Burm F.) Merr. Kathmandu Uni. J. Sci. Engr. Tech.. 2010;6(2):1-5.
- [Google Scholar]
- Physicochemical, qualitative, and high profile thin–layer chromatography study of Tylophora indica (Burm. f) Merr. leaves and roots. Int. J. Green Pharma. 2018;12(02):136-141.
- [Google Scholar]
- Comparison of antifungal activity of medicinal plant Tylophora indica Merr. with its in vitro raised plant and callus. J. App. Pharma. Sci.. 2013;3(8):41-45.
- [Google Scholar]
- Kirtikar, K.R., Basu, B.D., 1981. Indian medicinal plants. 3, 1636–1638.
- Kirtikar, K.R., Basu, B.D., 1991. Indian medicinal plants. 2nd Ed. Periodic expert book agency.1.
- Kirtikar K.R., Basu, B.D., 2001. Indian medicinal plants. Vol. 1. 2nd ed. Dehradun publisher Ltd, India. 1, 830–832.
- Extracorporeal ultrafiltration for acute heart failure: lost battle or lasting opportunity? Blood Purification. 2017;43(1–3):1-10.
- [Google Scholar]
- A review of the antioxidant potential of medicinal plant species. Food Bioprod. Process.. 2011;89:217-233.
- [Google Scholar]
- Qualitative studies of bioactive compounds in leaf of Tylophora indica (Burm. F.) Merr. Int. J. Res. Pharma. Biomed. Sci.. 2011;2(3):1188-1192.
- [Google Scholar]
- A review on hepatoprotective activity of medicinal plants. Int. J. Pharma. Sci. Res.. 2011;2(3):501-515.
- [Google Scholar]
- Folk medicinal plants used in the treatment of asthma in Polavaram forest area, West Godavari District. A. P., India. Int. J. Ayur. Herbal Med.. 2012;2(6):947-953.
- [Google Scholar]
- Lemon, S.M., Sparling, P.F., Hamburg, M.A., Relman, D.A., Choffnes, E.R., Mack, A., 2008. Vector–borne diseases: understanding the environmental, human health, and ecological connections. Washington, DC: The National Academies Press. 1.
- The emerging role of DNA repair proteins as predictive, prognostic and therapeutic targets in cancer. Cancer Treat. Rev.. 2005;31(8):603-617.
- [Google Scholar]
- Tylophora asthmatica L. prevents lipid peroxidation in acetaminophen induced hepatotoxicity in rats. Asian J. Res. Pharm. Sci.. 2011;1(3):71-73.
- [Google Scholar]
- Antioxidant activity of extract from the leaves of Tylophora asthmatica. Asian J. Res. Pharm. Sci.. 2012;2(2):86-88.
- [Google Scholar]
- Anti convulsant activity of Phyllanthus amarus in experimental animal models. Int. J. Appl. Biol. Pharm.. 2011;2(4):144-149.
- [Google Scholar]
- Antianxiety effect of ethanolic extract of leaves of Tylophora indica in wistar albino rats. Int. J. Res. Ayur. Pharm.. 2013;4(1):127-129.
- [Google Scholar]
- Effect of ethanolic extract of Phyllanthus amarus and Tylophora indica on isoniazid induced hepatic injury in wistar albino rats. Int. J. App. Biol. Pharma. Tech.. 2013;4(2):141-149.
- [Google Scholar]
- Assessment of brain dopamine levels to evaluate the role of Tylophora indica ethanolic extract on alcohol induced anxiety in Wistar albino rats. J. Young Pharm.. 2016;8(2):1-10.
- [Google Scholar]
- Marks, G., Pearce, N., Strachan, D., Asher, I., Ellwood, P., 2018. Global burden of disease due to asthma in the global asthma report, Auckland, New Zealand. 20.
- Medicinal folk recipes used as traditional phytotherapies in District Dera Ismail Khan, KPK. Pakistan. Pak. J. Bot.. 2011;43(3):1453-1462.
- [Google Scholar]
- Treatment of asthma with alkaloids of Tylophora indica: A double–blind study. Aspects Allergy Appl. Immunol.. 1974;7:166-179.
- [Google Scholar]
- Evaluation of diuretic activity from Tylophora indica leaves extracts. J. Pharma. Sci. and Res.. 2009;1(3):112-116.
- [Google Scholar]
- Two new homo–iso–flavonoids from Caesalpinia pulcherrima. Chem. Pharm. Bull.. 2006;54:1193-1195.
- [Google Scholar]
- Phytochemical investigation and micropropogation of Tylophora indica (Burm F.)Merill from nodal explants. J. Indian Bot. Soc.. 2014;93(1):42-49.
- [Google Scholar]
- Indigenous plants as sources of pharmacological interests. J. Global Biosci.. 2019;8(1):5900-5915.
- [Google Scholar]
- Alhagi: a plant genus rich in bioactives for pharmaceuticals. Phytother. Res.. 2015;29(1):1-13.
- [Google Scholar]
- Mimosa pudica L., a high-value medicinal plant as a source of bioactives for pharmaceuticals. Comp. Rev. Food Sci. Food Saf.. 2016;15(2):303-315.
- [Google Scholar]
- Hepatoprotective activity of the methanolic extract of Tylophora indica (Burm. f.) Merill. leaves. Int. J. Green Pharma.. 2009;3(2)
- [Google Scholar]
- Angiosperms, climbing plants in tropical forests of Southern Eastern Ghats, Tamil Nadu. India. Check List.. 2009;5(1):92-111.
- [Google Scholar]
- Nadkarni, K.M., editor, 1996. [Indian materia medica]; Dr. K.M. Nadkarni's Indian materia medica: with Ayurvedic, Unani-Tibbi, Siddha, allopathic, homeopathic, naturopathic & home remedies, appendices & indexes. 1. Popular Prakashan.
- Assessing postural control and postural control strategy in diabetes patients using innovative and wearable technology. J. Diabetes Sci. Technol.. 2010;4(4):780-791.
- [Google Scholar]
- Evaluation of anti–allergic activity of Tylophora indica using rat lung perfusion. Indian J. Pharmacol.. 1979;11:229-232.
- [Google Scholar]
- Capparis decidua Edgew (Forssk.): A comprehensive review of its traditional uses, phytochemistry, pharmacology and nutrapharmaceutical potential. Arabian J. Chem.. 2020;13(1):1901-1916.
- [CrossRef] [Google Scholar]
- 5–Oxo–1–[(2, 3, 6, 7–tetramethoxy–9–phenanthrenyl) methyl]–L–proline inhibits hepatitis C virus entry. Sci.Reports. 2019;9(1):7288-7298.
- [Google Scholar]
- Phytochemical studies and comparative efficacy of the crude extracts of some homeostatic plants in Edo and Delta States of Nigeria. Global J. Pure Appl. Sci.. 2002;8:203-208.
- [Google Scholar]
- Antibacterial activities and toxicological potentials of crude ethanolic extracts of Euphorbia hirta. Afr. J. Biotechnol.. 2007;6(13):1544-1548.
- [Google Scholar]
- Phytochemical and vitamin content of indigenous spices of South-Eastern Nigeria. J. Sustain. Agric. Environ.. 2004;6:30-34.
- [Google Scholar]
- Nitric oxide and peroxynitrite in health and disease. Physiol. Rev.. 2007;87(1):315-424.
- [Google Scholar]
- Comparative evaluation of some plant extracts on bronchoconstriction in experimental animals. Asian J. Pharma. Life Sci.. 2011;1(1):52-57.
- [Google Scholar]
- Medicinal plants of Kumaon Himalaya: strategies for conservation. Himalayan Biodivers. Conserv. Strategies. 1993;3:293-302.
- [Google Scholar]
- Antibacterial and phytochemical studies on twelve species of Indian medicinal plants. Afr. J. Biol. Res.. 2007;10:175-181.
- [Google Scholar]
- Oxygen radicals: mediators of gastrointestinal pathophysiology. Gut.. 1989;30:293-298.
- [Google Scholar]
- Antidiarrhoeal activity of alcoholic and aqueous extracts of roots of Tylophora indica (wight & arn.) in rodents. Pharmacologyonline. 2006;1:19-29.
- [Google Scholar]
- Determination of some minerals and trace elements in medicinal plants–Acalypha indica (L.), Datura metel (L.) and Tylophora indica used in the treatment of asthma. Eur. J. Med. Plants. 2018;22(2):1-11.
- [Google Scholar]
- Methods for total antioxidant activity determination: A Review. Biochem Anal. Biochem.. 2011;1:106.
- [Google Scholar]
- Antibacterial activity of different solvent extracts of Tylophora asthmatica (leaves) against different bacterial strains. Int. J. Res. Bot.. 2013;3(1):13-18.
- [Google Scholar]
- Prajapati, N.D., Purohit, S.S., Sharma, A.K., Kumar, T., 2003. A Handbook of Medicinal Plants’ A complete source book. First Edition. Agrobios (India).
- Study on the anti–cancer activity of Tylophora indica leaf extracts on human colorectal cancer cells. Int. J. Pharma. Phytochem. Res.. 2014;6(2):355-361.
- [Google Scholar]
- Antibacterial activity of South African plants used for medicinal purposes. J. Ethnopharmacol.. 1997;56:81-87.
- [Google Scholar]
- Medicinal plants from Siddha system of medicine useful for treating respiratory diseases. Int. J. Pharma. Anal.. 2009;1(2):20-30.
- [Google Scholar]
- Review of Tylophora indica–An antiasthmatic plant. FS. J. Res. Basic Appl. Sci.. 2012;1(3):20-21.
- [Google Scholar]
- Investigations on the gastroprotective and antidiarrhoeal properties of ternatin, a tetramethoxyflavone from Egletes viscosa. Planta medica.. 1997;63(02):146-149.
- [Google Scholar]
- Alkaloids of tylophora III: New alkaloids of Tylophora indica (burm) merrill and Tylophora dalzellii hook. F. J. Pharma. Sci.. 1971;60(11):1725-1726.
- [Google Scholar]
- The chemical examination of T. asthmatica and isolation of the alkaloids, tylophorine and tylophorinine. Indian. J. Med. Res.. 1935;22(3):433-441.
- [Google Scholar]
- Mosquito larvicidal activity of Solanum nigrumberry extracts. Ind. J. Med. Res.. 2013;137:972-976.
- [Google Scholar]
- Antimicrobial activity of Datura stramonium (L) and Tylophora indica (Buru L) New Pharmacol. online.. 2009;1:1293-1300.
- [Google Scholar]
- Antifeedant and antimicrobial activity of Tylophora indica. Afr. J. Bioch. Res.. 2009;3(12):393-397.
- [Google Scholar]
- Ethnobotanical survey on respiratory disorders in Eastern Ghats of Andhra Pradesh, India. Ethnobotanical Leaflets.. 2006;10:139-148.
- [Google Scholar]
- Two diuretic triterpenoids from Antiderma menasu. Phytochemistry.. 1980;19(11):2409-2410.
- [Google Scholar]
- A comprehensive review of phytochemical profile, bioactives for pharmaceuticals, and pharmacological attributes of Azadirachta indica. Phytother. Res.. 2018;32(7):1241-1272.
- [Google Scholar]
- Ethnobotanical study of medicinal plants used by traditional users in Villupuram district of Tamil Nadu. India. J. Med. Plant Res.. 2010;4(12):1089-1101.
- [Google Scholar]
- Tylophorine, a phenanthraindolizidine alkaloid isolated from Tylophora indica exerts antiangiogenic and antitumor activity by targeting vascular endothelial growth factor receptor 2–mediated angiogenesis. Mol. Cancer.. 2013;12(1):82.
- [Google Scholar]
- Carakokta sarvasrestha vatasamakdravya “rasna“ ka adhunik sarveksan. Sachitra Ayurved.. 1995;48(4):443-446.
- [Google Scholar]
- Herbalism, phytochemistry and ethnopharmacology. USA: Enfield: Science Publishers; 2011. p. :384-388.
- Antibacterial activity and the mode of action of alkaloid compound isolated from the leaves of Tylophora indica. Int. J. Ethno. Pharma. Res.. 2013;1(1):59-70.
- [Google Scholar]
- Schippmann, U., Leaman, D.J., Cunninghan, A.B., 2002. Impact of cultivation and gathering of medicinal plants on Biodiversity. FAO biodiversity and the ecosystem approach in agriculture, forestry and fisheries. Satellite event on the occasion of the ninth regular session of the commission on genetic resources for food and agriculture. Rome, Inter–Departmental Working Group on Biological Diversity for Food and Agric. Rome. 12–13.
- Schmelzer, G.H., 2012. Tylophora indica (Burm.f.) Merr. In Schmelzer, G.H., Gurib–Fakim, A., (Editors). PROTA (Plant resources of tropical Africa / Ressources végétales de l’Afrique tropicale), Wageningen, Netherlands.
- Sen–Sung, C., Hui–Ting, C., Shang–Tzen, C., Kum–Hsien, T., Wei–June, C., 2003. Bioactivity of selected plant essential oils against the yellow fever mosquito Aedesaegypti larvae. Biores. Technol. 89, 99–102.
- Botany of Tylophora indica (Burm. f.) Merr. Quarterly J. Crude. Drug Res.. 1976;14(1):27-34.
- [Google Scholar]
- Ethnobotanical Study of Medicinal Plants of Semi-Tribal Area of Makerwal and Gulla Khel (Lying between Khyber Pakhtunkhwa and Punjab Provinces) Pakistan. Am. J. Plant Sci.. 2013;4:98-116.
- [Google Scholar]
- Antimicrobial potential of Balanites aegyptiaca (L.) Del, Stevia rebaudiana (Bert.), Bertoni, Tylophora indica (Burm.f.) Merrill and Cassia sophera (Linn.) Open Conference Proc J.. 2012;3:63-69.
- [Google Scholar]
- Shahzad, A., Upadhyay, A., Sharma, S., Saeed, T., 2016. Tylophora indica (Burm. f.) Merrill: Medicinal uses, propagation, and replenishment. In: Biotechnological strategies for the conservation of medicinal and ornamental climbers. Springer Cham. 239–258.
- Preliminary studies in Tylophora indica in the treatment of asthma and allergic rhinitis. J. Assoc. Physicians India.. 1968;16(1):9-15.
- [Google Scholar]
- A crossover double–blind study on Tylophora indica in the treatment of asthma and allergic rhinitis. J. Allergy.. 1969;43:145-150.
- [Google Scholar]
- Treatment of asthma with an alcoholic extract of Tylophora indica: a cross–over, double–blind study. Ann. Allergy.. 1972;30:407-412.
- [Google Scholar]
- Synthesis structural properties and insulin–enhancing potential of bis (quercetinato) oxovanadium (IV) conjugate. Bioorganic Med. Chem. Lett.. 2004;14:4961-4965.
- [Google Scholar]
- Tylophora Indica Extracts Down Regulates Bcl2 and Induces Apoptosis in Melanoma Cells. Am. J. Pharma. Health Res.. 2016;4(2):25-35.
- [Google Scholar]
- Pharmacological and biological studies on saponins. Indian. J. Pharmacol.. 1985;17(3):178-179.
- [Google Scholar]
- Anti–oxidant effects of L–arginine against isoproterenol–induced myocardial stress in rats. Med. Sci. Res.. 1998;26(9):587-593.
- [Google Scholar]
- Melanoma/skin cancer screening in a Mediterranean country: results of the Euromelanoma Screening Day Campaign in Greece. J. Eur. Acad. Dermatol. Venereol.. 2007;21(1):56-62.
- [Google Scholar]
- Sturat, I.F., 2002. Human Physiology, Wm. C. Brown publishers, Dubuque, lowa. 2, 500–503.
- Surendra, L.A., 2009. Assessment of some species of Adiantum with reference to secondary metabolites, amylase inhibitors and antioxidants (Doctoral dissertation).
- Evaluation of anti hyperglycemic and anti hyperlipidemic activity of ethanolic extract of Tylophora indica in alloxan induced diabetic rats. Int. J. Curr. Pharm. Res.. 2012;4(1):25-31.
- [Google Scholar]
- Biological diversity and habitat diversity: a matter of Science and Perception. NEAR Curriculum in Natural Environmental Science. Terre Environ.. 2010;88:147-155.
- [Google Scholar]
- Tylophora indica in Bronchial Asthma. A Controlled Comparison with a Standard Anti-Asthmatic Drug. J. Indian Med. Assoc.. 1978;71:172-176.
- [Google Scholar]
- Isolation, callus formation and plantlet regeneration from mesophyll protoplasts of Tylophora indica (Burm. f.) Merrill: an important medicinal plant. Vitro Cell. Dev. Biol. Plant.. 2009;45:591-598.
- [Google Scholar]
- Persistency of Tylophorine as an insect antifeedant against Spilosoma oblique walker. Phytotherapy Res.. 1990;4(4):144-147.
- [Google Scholar]
- Tiwari, R., Latheef, S.K., Ahmed, I., Iqbal, H., Bule, M. H., Dhama, K., Samad, H.A., Karthik, K., Alagawany, M., El–Hack, M.E., Yatoo, M.I., 2018. Herbal Immunomodulators–A Remedial Panacea for Designing and Developing Effective Drugs and Medicines: Current Scenario and Future Prospects. Curr. Drug Met. 19 (3), 264–301.
- Role of Tylophora indica in treatment of bronchial asthama. Int. J. Life Sci. Pharma. Res.. 2017;7(1):17-21.
- [Google Scholar]
- Phytochemical screening and Antimicrobial activity of wild and tissue cultured plant extracts of Tylophora indica. Asian J. Pharma. Pharmacol.. 2019;5(1):21-32.
- [Google Scholar]
- Tylophora indica Indian medicinal plants–a compendium of 500 species. New Delhi. Orient Longman. 1994;5:66-68.
- [Google Scholar]
- Ved, D.K., Sureshchandra, S.T., Barve, V., Srinivas, V., Sangeetha, S., Ravikumar, K., Kartikeyan R., Kulkarni, V., Kumar, A.S., Venugopal, S.N., Somashekhar, B.S., Sumanth, M.V., Begum, N., Rani, S., Surekha, K.V., Desale, N., 2016. (envis.frlht.org / frlhtenvis.nic.in). FRLHT's ENVIS Centre on Medicinal Plants, Bengaluru.
- Insect feeding deterrants from the medicinal plant Tylophora asthmatica. Entomol. Exp. Appl.. 1986;40:99-105.
- [Google Scholar]
- Pharmacognosy in the new millennium: leadfinding and biotechnology. J. Pharm, Pharmacol.. 2000;52:253-262.
- [Google Scholar]
- Walter, T.M., 2005. Catalogue of Siddha medicinal plants. Available from Internet: http://openmed.nic.in/2055/01/Siddha_herbs.pdf.
- Wealth of India, 1969. NISCOM, CSIR publications, Vol–(VI). 398–402.
- An epidemic model of a vector–borne disease with direct transmission and time delay. J. Math. Anal. App.. 2007;342:895-908.
- [Google Scholar]
- World Health Organization (WHO), 1998. Quality control methods for medicinal plant materials. Published by WHO, Geneva.
- World Health Organization (WHO), 2017. WHO strategy for prevention and control of chronic respiratory diseases. Published by WHO, Geneva.
- Tylophorine arrests carcinoma cells at G1 phase by downregulating cyclin A2 expression. Biochem. Biophys. Res. Commun.. 2009;386:140-145.
- [Google Scholar]
- Anti-Inflammatory mechanisms of phenanthroindolizidine alkaloids. Mol. Pharmacol.. 2005;749–758
- [CrossRef] [Google Scholar]
- Anti–tumor activity and apoptosis–regulation mechanisms of bufalin in various cancers: new hope for cancer patients. Asian Pac. J. Cancer Prev.. 2012;13(11):5339-5343.
- [Google Scholar]
- The traditional medicine and modern medicine from natural products. Molecules.. 2016;21(5):559.
- [Google Scholar]







