Translate this page into:
Alleviation of chromium toxicity by trehalose supplementation in Zea mays through regulating plant biochemistry and metal uptake
⁎Corresponding authors. drnudrataisha@gcuf.edu.pk (Nudrat Aisha Akram), parvaizbot@yahoo.com (Parvaiz Ahmad)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Exogenously applied trehalose is effective for improving plant tolerance to some abiotic stresses, but whether it has function in alleviating maize (Zea mays) tolerance to chromium (Cr) stress is unknown. This study investigated the effects of exogenous application of trehalose (0, 25 and 50 mM) on the growth, physiological parameters and oxidative defense systems of maize seedlings exposed to chromium (Cr) stress at the rate of 100 and 500 µM. Our results depicted that Cr stress reduced plant growth, biomass, leaf chlorophyll contents, transpiration rate, photosynthetic properties, stomatal conductance and antioxidant enzymes. The Cr-induced increase was observed in oxidative defense system, malondialdehyde, hydrogen peroxide contents and membrane leakage. Although, activities of POD, SOD and CAT enzymes, total phenolics and proline concentrations increased at 100 µM Cr but were significantly diminished at 500 µM compared to non-Cr plants. However, the negative impacts of Cr on plant growth and physiological traits were reduced by the foliar application of trehalose. Trehalose significantly enhanced plant biomass, improved photosynthetic apparatus and antioxidants in addition to ascorbic acid, free proline, GB and total phenolic compounds. The application of trehalose diminished the exudation of organic acids and indicator of oxidative stress in roots and shoots by decreasing Cr retention in plant parts. The findings suggest that 50 mM trehalose spray efficiently mitigated the Cr toxicity in maize plants as compared to 25 m trehalose spray. However, the exogenous application of trehalose alleviated Cr toxicity by regulating Cr uptake in plant tissues by enhancing the antioxidative defense system.
Keywords
Heavy metals stress
Exogenous application
Maize
Peroxidase (POD)
Superoxide dismutase (SOD)
Catalase (CAT)
1 Introduction
Metals are elements that have moderately high density as compared to water (Wang et al., 2017). During the last few decades, metal accumulation in soil has been increasing dramatically as well as alarming for human health due to the environmental contamination with these metals (Rizwan et al., 2017). Human exposed to these metals have mounted intensely in response to the increase consumption in numerous domestic, technological, industrial, and agricultural uses. Cadmium, chromium, copper and lead are the major toxic heavy metals, particularly in areas which face a high anthropogenic pressure. Heavy metals depositions in soil constitute a significant problem for the agricultural productivity owing to their negative influence on safety of food and food products, marketability and health of soil organisms (Ali et al., 2019). Plants growing in metal contaminated soils exhibit changes in metabolism (physiological and biochemical) which reduced plant growth, yield and metal accumulation (Yang et al., 2014; Adrees et al., 2015b;Yang et al., 2021).
Chromium (Cr) as a metal and pollutant found in water, soil and residues due to its wide-ranging industrial usage resulting in various environmental problems. Cr exists in two stable oxidation states in nature, e.g. trivalent (Cr3+) and hexavalent (Cr6+). Both Cr states are unlike in availability as well toxicity. Cr hexavalent is found to be more poisonous due to its higher toxicity than Cr trivalent (Shanker et al., 2009). There is no uptake system for chromium in plants. Its uptake is done together with necessary elements such as sulfate and sulfate transporter. Hexavalent forms of Cr, chromate and dichromate are readily dissolved in water. Chromium trivalent, on the other hand, has less solubility in water. Plants growing under Cr stress exhibit substantial toxicity concerning reduced growth, yield and structural changes. It also constrains photosynthetic and respirational routes, and water and mineral uptake systems. Cr toxicity inhibited enzymatic activities either by direct interaction with enzymes or by increasing the amount of reactive oxygen radicals (ROS). It also triggers oxidative injury via influencing lipid membrane and DNA (Singh et al., 2013). The hexavalent (Cr6+) is vigorously absorbed by plants and can easily get down to the plasma membrane and affects the cellular functions of plants (Sharma et al., 2020; Wakeel et al., 2020). Once Cr enters into plant system, it generates a variety of cytotoxic effects which may lead to plant death (Rizvi and Khan, 2019). Previous reports indicate that Cr toxicity critically inhibits plant growth and production, chlorophyll pigments, photosynthetic attributes as well as enzymatic antioxidant defense system, but induces the intensification in malondialdehyde, electrolyte leakage (EL) and hydrogen peroxide (H2O2) in plants e.g., rice (Basit et al., 2022; AbdElgawad et al., 2022), sunflower (Mallhi et al., 2020), cauliflower (Ahmad et al., 2020) and soybean (Basit et al., 2023).
Maize is an economically important, extensively cultivated in erratic soils as well as under environmental disorders worldwide. It has a great value for human and animal nutrition (Rosas-Castor et al., 2014). It is also known as a crop of industry due to its usage in ethanol preparation. Both China and United State are chief aspirants for maize intake as compared to other countries (Gale et al., 2014). It is also a proficient plant for accumulation of heavy metals from soil with better phytoextraction prospective and high discharge rate (Wuana and Okieimen, 2010). The present yield of maize is almost 0.250 billion tons/year. Its estimated yield reached to 0.29 billion tons/year in the East and South Asia in 2020 (Rosegrant et al., 2001). Being a C4 plant, maize has the ability to survive under water scarcity and oxidative stress. As per reports, the production of maize is reduced due to the negative impacts of several abiotic factors such as increased concentration of salts, changing temperature regime, water scarcity and metal toxicity (Boyer, 1970). Moreover, maize is considered as a hyper accumulator plant for heavy metals (Shanker et al., 2005). About thirty-five metals are present in nature. Out of which twenty-five are known to be heavy metals (Zubair et al., 2016). These metals cause hazardous effects on plants and human health if present in food chains. Cr stress also inhibits seed germination, transpiration rate and mineral nutrition (Zou et al., 2009). The deleterious impact of Cr on maize growth and biomass were also reported by Shanker et al. (2005).
Trehalose is a type of sugars, found in plants. It protects plant structures from aridity and other damages (Jain and Roy, 2010). Trehalose induces osmoprotection in response to stress conditions in plants. It supports the maintenance of membrane and stabilization of the biological macromolecules such as proteins. Similar to glycine betaine and proline, trehalose protects plants against harsh environmental stresses (Fernandez et al., 2010). Its concentration is low in plants but it is necessary for various metabolic stresses (Ponnu et al., 2011). The exogenous spray of trehalose enhances the defense role of proteins in plants (Redillas et al., 2012). Previous study indicates that application of trehalose is readily taken from soil and its translocation from plant roots to aerial plant parts (Luo et al., 2010). Thus, the main intentions of this study were to observe the ameliorative effects of trehalose on maize plants biomass, yield, oxidative stress and Cr concentration under Cr toxicity.
2 Material and methods
2.1 Soil analysis
Soil was collected from Faisalabad, Pakistan and sieved through a 2 mm sieve. Initial characterization was measured by hydrometer such as particle size. The electrical conductivity (EC) of soil was 2.92 dS m−1 measured by EC meter. The pH of the soil was 7.65 (1:25 in water). The type of soil used in the current study was sandy clay loam. The soil was composed of 46, 32 and 22 % of sand, clay and silt particles, respectively. The soil was concentrated with 6.59 mmol/L, 3.8 mmol/L, 0.07 mmol/L, 0.30 mg kg−1, 0.18 mg kg−1 of SO42-, Na+, K+, Cu2+ and Cr2+, respectively.
2.2 Pot experiment
An experimentation was planned to alleviate the toxic impact of Cr stress in maize plants by foliar application of trehalose. Seeds of the maize cultivar ‘Malka 2016′ were used in this experiment conducted during February to June 2019. Five seeds were sown in pots filled with 8 kg of the sandy clay loam soil. After sprouting, plant thinning was performed. Three plants were kept in each pot. After two weeks of germination, the NPK (nitrogen-phosphorous-potassium) fertilizers were applied with water. The NPK was used at a rate of 120:50:20 kg ha−1, correspondingly.
After four weeks of germination, two levels of Cr stress (100 µM and 500 µM) were added in the soil medium along with control after sprouting of seeds. The 500 mL of prepared solution applied in each pot. Each pot flooded with 500 mL of Cr solution. Every time when irrigation needed after first application of Cr solution, plants irrigated with Cr solution about three times with the regular interval of 3 weeks. After three weeks of chromium stress, two different levels of trehalose 25 and 50 mM along with control were applied foliarly to plant leaves. Trehalose was applied four times during the whole trial. The first foliar application was completed after 7 weeks of sprouting, 2nd, 3rd and 4th sprays were done after 9, 11 and 13 weeks of sowing respectively. Plants were accessed 16 weeks after sowing.
2.3 Morphological attributes
Two maize plants were uprooted after 16 weeks from each potted medium and soil particles were removed using a double deionized water. The plant shoot and root heights, total leaf area and total number of leaves/plants, were determined. The plant samples oven dried for three days at 65 °C to obtain the dry weight (DW).
2.4 Estimation of chlorophyll contents
Fresh leaf (500 mg) was homogenized into 80 % acetone solution at 4 °C overnight. The filtrate was centrifuged for 5 min and the absorbance was spectrophotometrically recorded at 480, 645 and 663 nm (Arnon, 1949).
2.5 Determination of proline and glycinebetaine (GB) contents
Proline contents in the leaves were determined by a method proposed by Bates et al. (1973). The fresh leaf was extracted in sulfosalicylic acid (10 mL; 3 %) and filtered. Then, 2 mL acid ninhydrin, 2 mL filtrate and 2 mL glacial acetic acid were mixed in the test tubes and incubated at 95 °C for 60 min. The filtrate was cooled in ice bath and 4 mL toluene was added and shaken. The absorbance of the reaction mixture was taken at 520 nm.
The GB contents in fresh leaf were calculated following the methodology of Grieve and Grattan (1983). The absorbance of the lower layer was noted spectrophotometrically at 365 nm.
2.6 Estimation of electrolyte leakage, H2O2 and malondialdehyde (MDA) content
The leaf EL was calculated following Dionisio-Sese and Tobita (1998). The method of Velikova et al. (2005) was employed for estimation of hydrogen peroxide (H2O2). The OD was spectrophotometrically noted at 390 nm.
Lipid peroxidation (MDA) was calculated following Cakmak and Horst (1991). Fresh plant material (500 mg) was ground in solution containing trichloroacetic acid (TCA). The 4 mL filtrate was mixed with 4 mL of thiobarbituric acid (TBA) solution and OD was noted read at 532 as well as 600 nm.
2.7 Phenolics estimation
Shoot and root phenolics were analyzed following the procedure of Julkunen-Tiitto (1985). In which 0.1 g fresh leaf and root material was ground in 80 % (5 mL) acetone. The obtained mixture was centrifuged and 0.1 mL of the supernatant was mixed into deionized water (2 mL) as well as Folin Ciocalteu’s phenol reagent (1 mL). The absorbance value of the supernatant was taken at 750 nm.
2.8 Determination of antioxidant enzymes activities
Fresh 0.5 g leaf or root samples were extracted in phosphate buffer (5 mL). Following Giannopolitis and Ries (1977), the superoxide dismutase (SOD) enzyme activity was noted at absorbance value of 560 nm. The method of Chance and Maehly (1955) was used for the determination of enzyme activities of catalase (CAT) at 240 nm absorbance while peroxidase (POD) was taken at 470 nm absorbance.
2.9 Determination of ascorbic acid (AsA)
Following Mukherjee and Choudhuri (1983), plant samples of fresh weight 0.25 g were ground in TCA solution to this extract (4 mL) were added into 2 mL dinitrophenyl hydrazine solution. Then 0.5 mL of thiourea was then mixed and the solution was then heated up for 15 min. After cooling the reaction mixture, 80 % H2SO4 were mixed and OD was noted at 530 nm.
2.10 Gas exchange attributes
The physiological parameters rate of photosynthesis (A), stomatal conductance (gs) and rate of transpiration (E) were determined following Shakoor et al. (2014) by the use of Infra-Red Gas Analyzer (IRGA). The data were recorded at a maximum light intensity between 10.00 and 12.00 a.m.
2.11 Measurement of Cr content in plant tissues
Plant sample (0.5 g) were digested by adding 15 mL of nitric acid (concentrated) in a 100 mL flask. After this, mixture was placed on hot plate until yellow fumes appeared. When fumes quantity becomes very low then H2O2 added until fumes were disappeared. Then flasks were removed from hot plate and the volume of solution made up to 25 mL by adding DW. Then, reaction mixture used for measuring the determination of Cr contents in both root and shoot tissues by the method of Rehman et al. (2015). The Cr contents were determined using atomic absorption spectrophotometer.
2.12 Statistical analysis
The data were analyzed to determine the significance of variance at 0.05 level with the help of SPSS software.
3 Results
3.1 Plant growth
The observed impact of trehalose on the plant growth of maize cultivar Malka 2016 raised under chromium stress were recorded. Results showed that root dry weights, height of plants, and total leaf area/plant enhanced upon the treatment with trehalose. Under chromium stress (100 and 500 µM), the foliar application of trehalose (25 and 50 mM) enhanced the plant height, root dry weight and total leaf area than control ones (Fig. 1).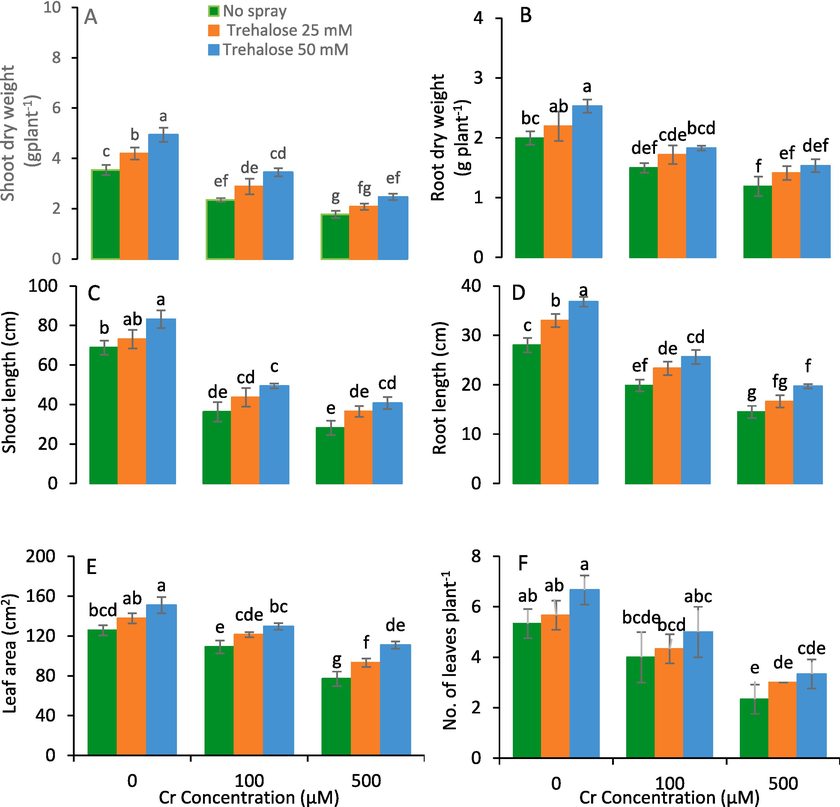
Shoot dry weight (A), root dry weight (B), shoot length (C), root length (D), leaf area (E) and number of leaves (F) of maize (Zea mays L.) plant subjected to foliarly applied varying levels of trehalose under different concentrations of chromium (Cr) stress (Mean ± S.D.).
3.2 Impacts of trehalose on chlorophyll contents and gas exchange parameters
Chlorophyll (a, b) contents of plant leaves were improved by foliar spray of trehalose under chromium toxicity. The higher contents were observed at the maximum concentration of trehalose application and lowest were found in control plants (Fig. 2).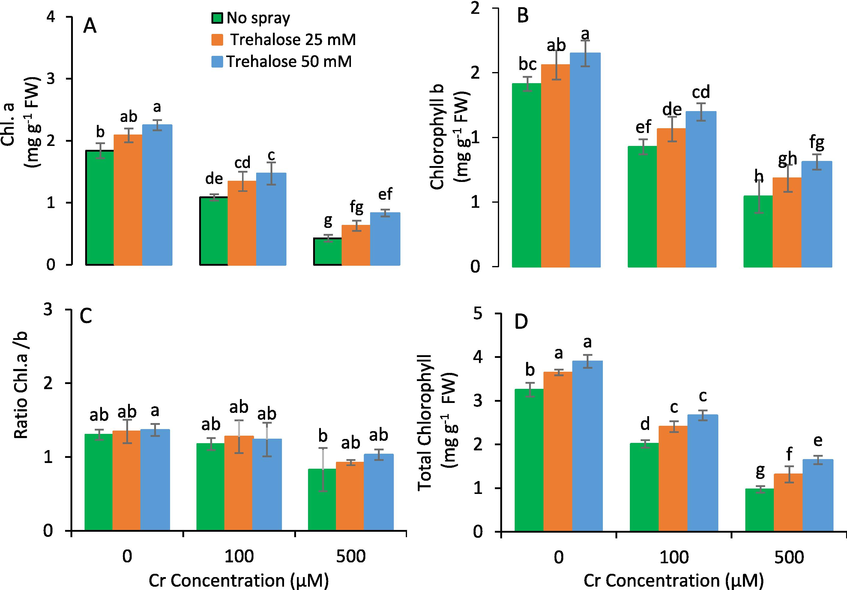
Chlorophyll a (A), chlorophyll b (B), total chlorophyll (C) and chlorophyll a/b ratio (D) of maize (Zea mays L.) plant subjected to foliarly applied varying levels of trehalose under different concentrations of chromium (Cr) stress (Mean ± S.D.).
The results also indicated that the gas exchange attributes were expressively improved by the foliar applied trehalose under Cr stress than control plants. The best results for photosynthetic attributes were noticed at 50 mM trehalose treatment, while, the minimum values were recorded in untreated plants. At 50 mM trehalose, the (gs), rate of photosynthetic (A) and transpiration (E) were considerably enhanced as compared to control plants (Fig. 3).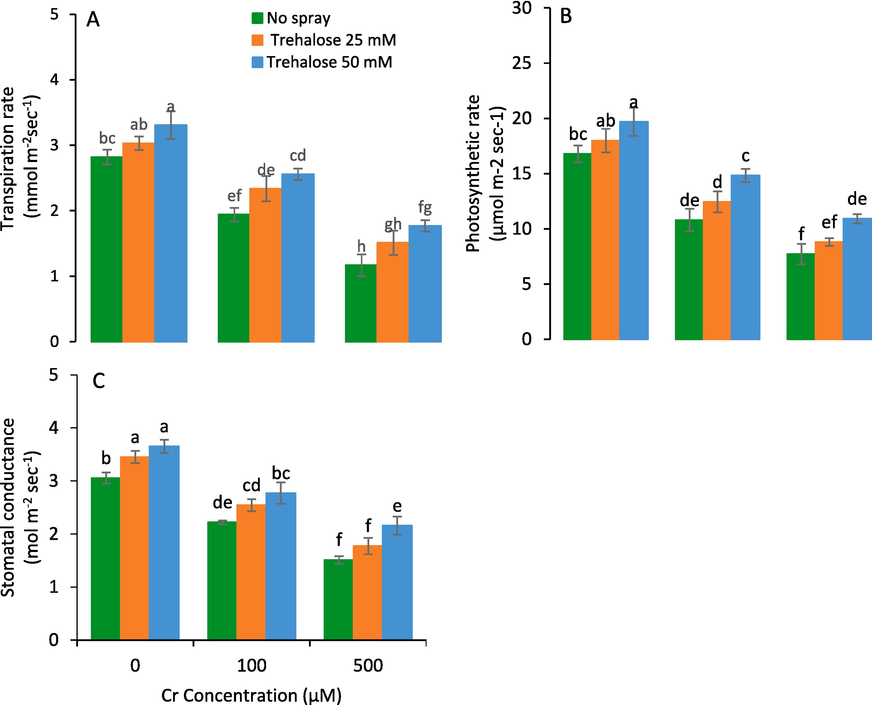
Photosynthetic rate (A), transpiration rate (B) and stomatal conductance (C) of maize (Zea mays L.) plant subjected to foliarly applied varying levels of trehalose under different concentrations of chromium (Cr) stress (Mean ± S.D.).
3.3 Impact of trehalose on EL, MDA and H2O2
Chromium stress increased levels of EL, H2O2 and MDA contents in shoots and roots of plants under study (Fig. 4). While as the foliar spray of trehalose suppressed the levels of these oxidative markers in maize plants grown under Cr stress. The maximum reductions in the levels of EL, H2O2 and MDA were recorded at 50 mM trehalose (Fig. 4).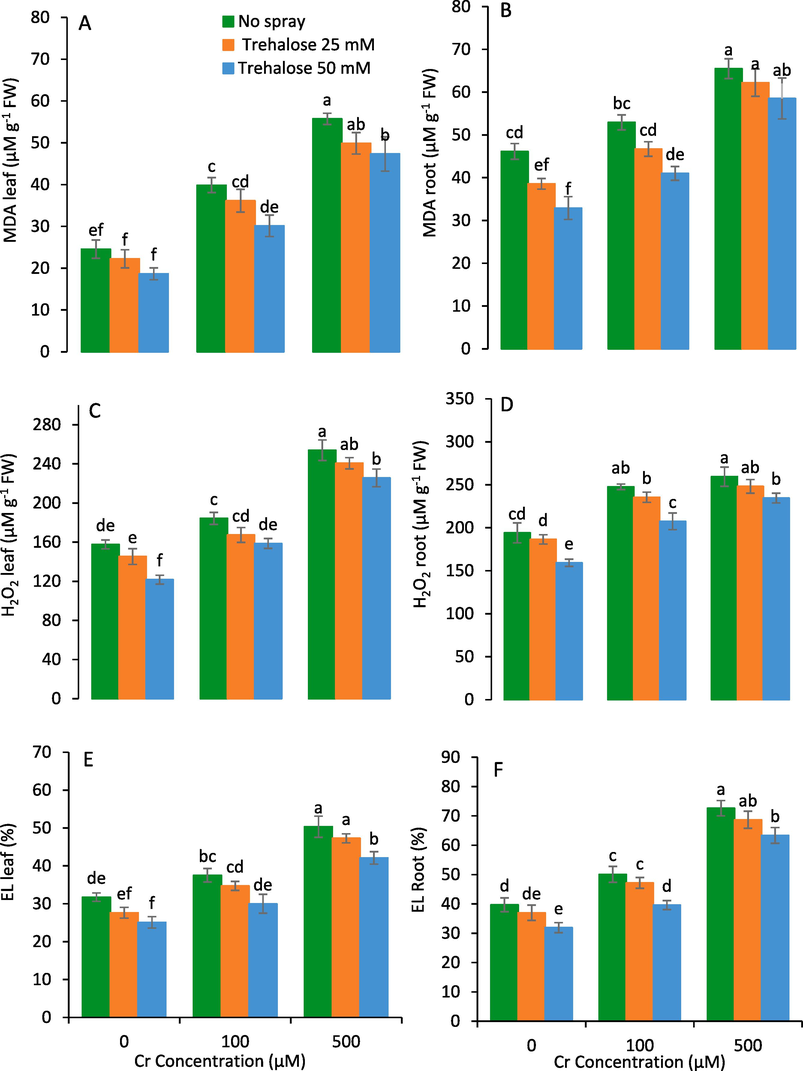
Leaf malondialdehyde (MDA) (A), root MDA (B), leaf hydrogen peroxide (H2O2) (C), root H2O2 (D), leaf electrolyte leakage (EL) (E) and root EL (F) of maize (Zea mays L.) plant subjected to foliarly applied varying levels of trehalose under different concentrations of chromium (Cr) stress (Mean ± S.D.).
3.4 Impact of trehalose on activities of antioxidant enzymes
The foliar spray of trehalose improved the activities SOD, POD and CAT enzymes in the leaf and root of Cr-treated maize plants in comparison to the control plants (Fig. 5). The maximum increase in leaf and root activities of these enzymes were recorded at 50 mM trehalose (Fig. 5).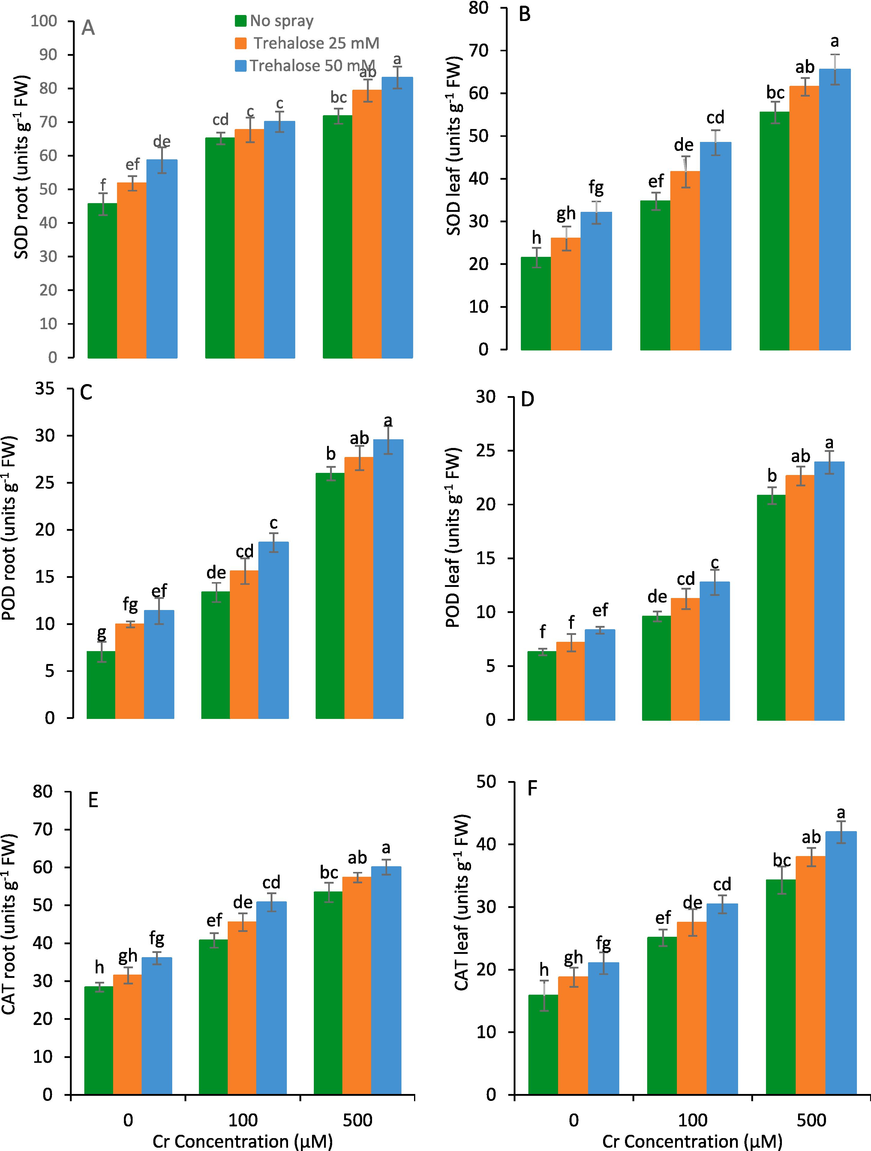
Root superoxide dismutase (SOD) (A), leaf SOD (B), root peroxidase (POD) (C), leaf POD (D), root catalase (CAT) (E) and leaf CAT (F) of maize (Zea mays L.) plant subjected to foliarly applied varying levels of trehalose under different concentrations of chromium (Cr) stress (Mean ± S.D.).
3.5 Effect of trehalose in proline, GB, phenolics and ascorbic acid contents
The foliar spray of trehalose induced the contents of GB, proline, phenolics, and AsA in maize leaves and roots raised under Cr stress than control plants (Figs. 6 & 7). Moreover, 50 mM trehalose treatment exhibited the highest contents of GB, proline, phenolics and AsA in maize leaves and roots (Figs. 6 & 7).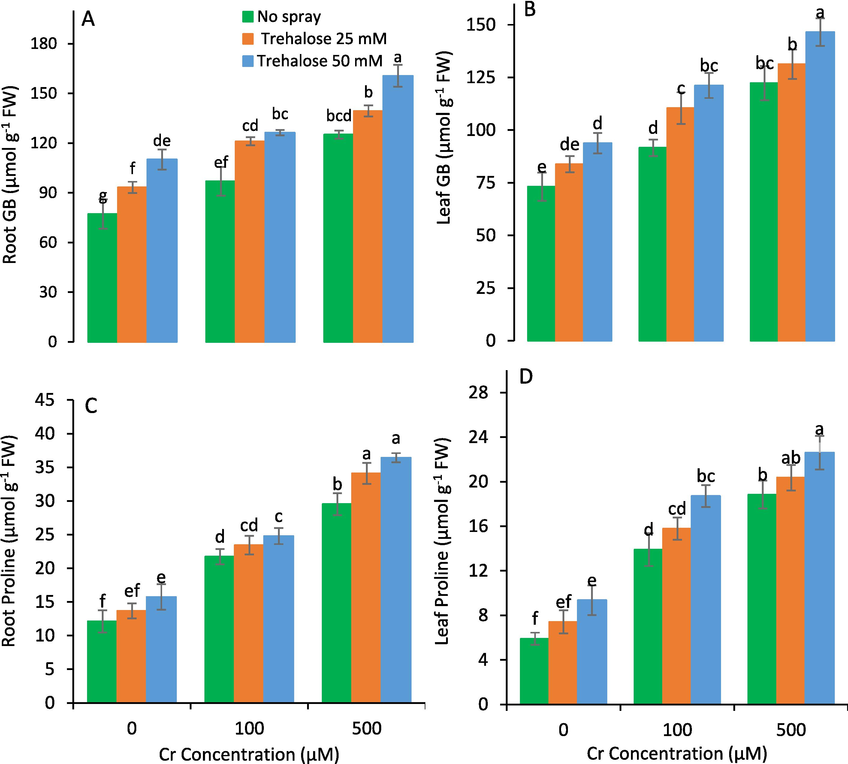
Glycinebetaine (GB) and proline of maize (Zea mays L.) plant subjected to foliarly applied varying levels of trehalose under different concentrations of chromium (Cr) stress (Mean ± S.D.).

Root total phenolics (A), leaf total phenolics (B), root ascorbic acid (AsA) (C) and leaf AsA (D) of maize (Zea mays L.) plant subjected to foliarly applied varying levels of trehalose under different concentrations of chromium (Cr) stress (Mean ± S.D.).
3.6 Chromium accumulation in roots and leaves
Chromium concentration in maize tissues were increased with the increasing concentration of dipotassium chromate used as a source of Cr. However, the root tissues maintained higher Cr concentration that the shoot parts. On the other hand, the foliar spray of trehalose significantly reduced concentration of Cr in maize leaves and roots under all levels of chromium stress (Fig. 8). Moreover, 50 mM trehalose treatment exhibited the lowest concentration of Cr in maize leaves and roots.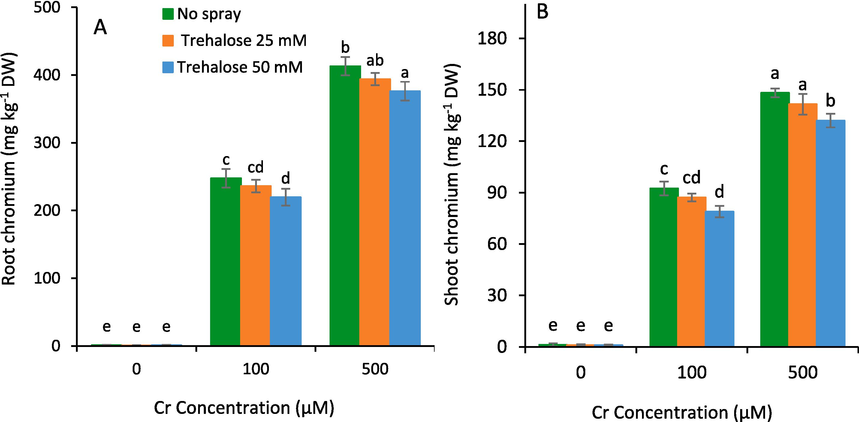
Concentration of chromium metal in root (A) and shoot (B) in maize (Zea mays L.) plant subjected to foliarly applied varying levels of trehalose under different concentrations of chromium (Cr) stress (Mean ± S.D.).
4 Discussion
Chromium (Cr) toxicity affects the plant growth, biomass and yield severely. Toxicity symptoms vary based on the concentration and duration of application of Cr. Higher Cr concentration expressively declines the nutrient uptake, resulting in stunted plant growth (Tauqeer et al., 2016). In the current work, the growth inhibition under Cr stress in comparison to control. Similar findings are previously recorded and indicated that Cr causes the inhibition of transpiration rate, stomatal conductance and carbon dioxide assimilation via the limitation of photosynthetic rate and chlorophyll contents (Atta et al., 2013). Sunflower biomass was also decreased under Cr (50 ppm) toxicity (Saleem et al., 2015). This inhibition might be due to the resulting changes in cell size, shape and cell wall permeability (Antoniadis et al., 2017). To stabilize such challenges, plants have a tendency to adopt a variety of defensive mechanisms. Conversely, cellular osmoregulation in transgenic tobacco plants exhibits a significant role to cope this effect (Holmström et al., 2000). Previous reports revealed that the foliar spray of osmoregulators is a promising method for minimizing the damages of stress conditions on plants (Mahmood et al., 2009; Farooq et al., 2010). Trehalose as an osmoregulator could alleviate the destructive properties of Cr on proteins and lipid membranes and protects plant structures from such damaging effects (Garg et al., 2002). In the present work, the exogenous introduction of trehalose increases the growth attributes of maize plants grown under chromium stress. Current observations are in accordance to previous findings which indicated that externally applied trehalose could augment the growth of maize (Zeid, 2009), Triticum (Ibrahim and Abdellatif, 2016), and Raphanus sativus (Akram et al., 2016) plants grown under stress conditions. Similar findings of previous studies indicated the enhanced growth of Sinorhizobium saheli L. under salt stress due to the foliar spray of trehalose (Gouffi et al., 1999).
Cr toxicity reduced the photosynthetic parameters in maize plants. These results coincide the previous findings (Atta et al., 2013; Anjum et al., 2016) which showed that Cr considerably reduced photosynthetic pigments (chl. a and b) in sunflower and maize plants. Reduction in photosynthetic contents were also noticed in wheat, Brassica and mung bean plants subjected to metal toxicity (Yang et al., 2014; Afshan et al., 2015; Jabeen et al., 2016). In contrast, carotenoids and photosynthetic pigments were enhanced in Pisum sativum in relation to Cr stress (Rodriguez et al., 2012). Like chromium toxicity, metals like Cu, Cd and Ni also show declines in gas exchange parameters (Farooq et al., 2016). The decreasing concentration of photosynthetic pigments might be owing to the ultrastructure changes in chloroplast (Najeeb et al., 2011). In the current study, the decline in pigments might be due to the increasing oxidative stress due to Cr toxicity. The increased concentration observed at the maximum level of growth regulator might be owing to the ameliorative role of trehalose under Cr stress in contrast to control. The present results were in accordance with that reported in maize plants treated with trehalose as a foliar spray under salinity stress (Zeid, 2009). Similarly, improvements in chlorophyll contents were recorded in Brassica napus plants grown under varied metals stress (Afshan et al., 2015). Moreover, it has been found that the exogenous applied trehalose showed positive effects on various plants such as drumstick tree (Anwar et al., 2006), sunflower (Akram et al., 2011) and cotton (Ahmad et al., 2008) subjected to stresses.
In this study, maize plants produced ROS which induces the electrolyte leakage due to the resulting oxidative damage under chromium stress e.g., likewise reported previously (Ahmad et al., 2017). The oxidative injury and induced electrolyte leakage levels were reported in barley and wheat seedlings when exposed to Cr toxicity (Adrees et al., 2015a). Metal stress also induces oxidative damage in plants (Noman et al., 2015; Arshad et al., 2016; Pereira et al., 2018). In this study, trehalose application expressively declined the levels of ROS and EL in-maize plants exposed to Cr stress via increasing antioxidants levels. Antioxidants play a considerable role in decreasing ROS (Islam et al., 2016). The neutralizing/scavenging influence of growth regulators recorded in different plants (Aravind et al., 2016; Habiba et al., 2019) stress was studied. The uptake of water from plant root to stem as well as the stomatal conductance due to stomatal closure are affected by Cr which further causes declines in rate of photosynthesis and transpiration (Wahid et al., 2007). The destruction of gas exchange reduced plant growth as well as development (Saleem et al., 2015). Likewise, such findings were already recorded in wheat and Brassica grown under chromium stress (Afshan et al., 2015). Disturbing water balance also leads to disturbing the functions of gas exchange system (Demirevska et al., 2010; Rapacz et al., 2010). However, the foliar spray of trehalose plays an important ameliorative role under stress conditions as previously reported (Zeid, 2009). In this study, foliar-applied trehalose suppressed the effects of Cr stress on maize plants. Similar findings were previously reported (Habiba et al., 2019). The current study also indicated that the sprayed application of trehalose improved the activities of different antioxidants in maize plants subjected to Cr stress. Similarly, earlier reports indicated that trehalose foliar spray remarkably enhanced the activities of different antioxidant enzymes in Brassica(Alam et al., 2014), maize (Ali and Ashraf, 2011b) and wheat (Aldesuquy and Ghanem, 2015) under water deficit stress. Furthermore, the results indicated that the foliar spray of trehalose upregulates the antioxidant defense system and ultimately leads to scavenging ROS besides enhanced growth and progress of stressed maize seedlings.
Stress conditions induces ROS formation and oxidative damage in maize plants (El-Esawi et al., 2018; El-Esawi et al., 2019; El-Esawi and Alayafi, 2019; Abdelaal et al., 2020; Ali et al., 2020; El-Esawi et al., 2020; Imran et al., 2020; Naveed et al., 2020; Zafar-ul-Hye et al., 2020). Metabolites and genetic diversity technology are important in cells exposed to stress conditions (El-Esawi et al., 2015; El-Esawi et al., 2016). Phenolics and ascorbic acid have antioxidative roles in decreasing ROS in plant tissues (Posmyk et al., 2009). In this trial, the phenolics and ascorbic acid contents were induced under chromium toxicity. The current results are in association with previous findings observed in wheat (Sečenji et al., 2010). Moreover, increases in AsA contents were previously reported under drought stress (Reddy et al., 2004). The foliar spray of trehalose increased the secondary metabolites under Cr stress conditions. Similarly, increases in phenolics and AsA contents due to the foliar spray of trehalose have been reported earlier in radish (Akram et al., 2016) and maize (Ali and Ashraf, 2011a) grown under stress conditions. It is prospective that phenolic composites enhanced the plant growth through upregulating the antioxidant defense systems. The osmoprotectants such as GB and proline plays a pivotal protective role in plants subjected to stress conditions (Anjum et al., 2011). In current work, the proline and GB enhanced in maize plants grown under stressful Cr conditions, which were in agreement with the previously reported findings in rapeseed (Razaji et al., 2014). However, trehalose spray enhanced the concentration of proline in maize plants treated under toxic Cr conditions. Present results were in concordance with the previously observed conditions which showed significant increases in proline concentration in trehalose-treated Brassica plants grown under water deficient environmental settings (Wahid et al., 2007). Trehalose acts as a regulatory and signaling molecule for plant adaptation under chromium toxicity (Zeid, 2009). In this work, concentration of higher Cr in medium of growth induced Cr uptake and deposition of Cr in underground and aerial parts of maize plants roots. The present observations are in association with those previously reported findings in maize plants (Anjum et al., 2016; Bukhari et al., 2016). However, maize roots maintained higher Cr concentrations than leaf tissues. On the other hand, the foliar-applied trehalose considerably condensed the higher Cr translocation and storage from below to upper ground plant parts. This decrease in Cr translocation might be due to the ameliorative role of the exogenously applied trehalose against adverse environmental conditions. Moreover, citric acid application enhanced the metal accretion and translocation in maize plants (Farid et al., 2017).
5 Conclusion
Chromium stress remarkably suppressed the plant morphological, physiological and biochemical characteristics of maize seedlings. Metal uptake and accumulation were increased with an increase in soil Cr content. Foliar-applied trehalose showed great potential for enhancing maize tolerance to Cr stress via increasing gas exchange attributes, chlorophyll pigments, secondary metabolites, osmoprotectants and antioxidant activities of enzymes. Exogenous application of trehalose also reduced the concentration of Cr in shoot and root tissues. Taken together, maize growth was greatly improved in response to trehalose spray by suppressing the levels of MDA, H2O2, and EL in Cr-stressed plants of maize. These findings suggested that the use of trehalose has the potential to be advantageous for enhancing overall plant growth, irrespective of stress conditions. So, the capacity of trehalose to boost stress tolerance in crop plants holds promising benefits for farmers, particularly those grappling with Cr stress conditions.
Ethics approval
Not applicable.
Consent to participate
All authors consent to participate in the manuscript publication.
Consent for publication
All authors approved the manuscript to be published.
Acknowledgement
The authors would like to extend their sincere appreciation to the Researchers Supporting Project Number (RSPD2023R1106) King Saud University, Riyadh, Saudi Arabia.
Author Contributions:
MR, NAK and PA drafted the experimental design and MR performed the experiments. YC and MSS analyzed the data and corrected the language of this manuscript. All authors read this manuscript before submission.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Abdelaal, K.A., EL-Maghraby, L.M., Elansary, H., Hafez, Y.M., Ibrahim, E.I., El-Banna, M., El-Esawi, M., Elkelish, A., 2020. Treatment of sweet pepper with stress tolerance-inducing compounds alleviates salinity stress oxidative damage by mediating the physio-biochemical activities and antioxidant systems. Agronomy 10, 26.
- Elevated CO2 differentially mitigates chromium (VI) toxicity in two rice cultivars by modulating mineral homeostasis and improving redox status. Chemosphere. 2022;307:135880
- [Google Scholar]
- The effect of excess copper on growth and physiology of important food crops: a review. Environ. Sci. Pollut. Res.. 2015;22:8148-8162.
- [Google Scholar]
- Mechanisms of silicon-mediated alleviation of heavy metal toxicity in plants: a review. Ecotoxicol. Environ. Saf.. 2015;119:186-197.
- [Google Scholar]
- Citric acid enhances the phytoextraction of chromium, plant growth, and photosynthesis by alleviating the oxidative damages in Brassica napus L. Environ. Sci. Pollut. Res.. 2015;22:11679-11689.
- [Google Scholar]
- Promotive role of 5-aminolevulinic acid on chromium-induced morphological, photosynthetic, and oxidative changes in cauliflower (Brassica oleracea botrytis L.) Environ. Sci. Pollut. Res.. 2017;24:8814-8824.
- [Google Scholar]
- Hydrogen sulfide alleviates chromium stress on cauliflower by restricting its uptake and enhancing antioxidative system. Physiol. Plant.. 2020;168:289-300.
- [Google Scholar]
- Reactive oxygen species, antioxidants and signaling in plants. Journal of Plant Biology. 2008;51:167-173.
- [Google Scholar]
- Aminolevulinic acid-induced changes in yield and seed-oil characteristics of sunflower (Helianthus annuus L.) plants under salt stress. Pak J Bot. 2011;43:2845-2852.
- [Google Scholar]
- Akram, N., Irfan, I., Ashraf, M., 2016. Trehalose-induced modulation of antioxidative defence system in radish (Raphanus sativus L.) plants subjected to water-deficit conditions. Trehalose-induced modulation of antioxidative defence system in radish (Raphanus sativus L.) plants subjected to water-deficit conditions, 186-198.
- Trehalose-induced drought stress tolerance: A comparative study among different Brassica species. Plant Omics. 2014;7:271-283.
- [Google Scholar]
- Exogenous salicylic acid and trehalose ameliorate short term drought stress in wheat cultivars by up-regulating membrane characteristics and antioxidant defense system. Journal of 2015 Horticulture
- [Google Scholar]
- Foliar spray of Fe-Asp confers better drought tolerance in sunflower as compared with FeSO4: Yield traits, osmotic adjustment, and antioxidative defense mechanisms. Biomolecules. 2020;10:1217.
- [Google Scholar]
- Exogenously applied glycinebetaine enhances seed and seed oil quality of maize (Zea mays L.) under water deficit conditions. Environ. Exp. Bot.. 2011;71:249-259.
- [Google Scholar]
- Induction of drought tolerance in maize (Zea mays L.) due to exogenous application of trehalose: growth, photosynthesis, water relations and oxidative defence mechanism. J. Agron. Crop Sci.. 2011;197:258-271.
- [Google Scholar]
- Ali, H., Khan, E., Ilahi, I., 2019. Environmental chemistry and ecotoxicology of hazardous heavy metals: environmental persistence, toxicity, and bioaccumulation. Journal of chemistry 2019.
- Morphological, physiological and biochemical responses of plants to drought stress. Afr. J. Agric. Res.. 2011;6:2026-2032.
- [Google Scholar]
- Chromium toxicity induced alterations in growth, photosynthesis, gas exchange attributes and yield formation in maize. Pak. J. Agric. Sci.. 2016;53
- [Google Scholar]
- Bioavailability and risk assessment of potentially toxic elements in garden edible vegetables and soils around a highly contaminated former mining area in Germany. J. Environ. Manage.. 2017;186:192-200.
- [Google Scholar]
- Characterization of Moringa oleifera seed oil from drought and irrigated regions of Punjab, Pakistan. Grasas Aceites. 2006;57:160-168.
- [Google Scholar]
- Efficacy of chelating agents in phytoremediation of cadmium using Lemna minor (Linnaeus, 1753) Nat. Environ. Pollut. Technol.. 2016;15:509-514.
- [Google Scholar]
- Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta Vulgaris. Plant Physiology. 1949;24:1.
- [Google Scholar]
- Phosphorus amendment decreased cadmium (Cd) uptake and ameliorates chlorophyll contents, gas exchange attributes, antioxidants, and mineral nutrients in wheat (Triticum aestivum L.) under Cd stress. Arch. Agron. Soil Sci.. 2016;62:533-546.
- [Google Scholar]
- Assessing some emerging effects of hexavalent chromium on leaf physiological performance in sunflower (Helianthus annuus L.) Int. J. Sci. Eng. Res. 2013;4:945-949.
- [Google Scholar]
- Seed priming with nitric oxide and/or spermine mitigate the chromium toxicity in rice (Oryza sativa) seedlings by improving the carbon-assimilation and minimising the oxidative damages. Funct. Plant Biol.. 2022;50:121-135.
- [Google Scholar]
- Nitric oxide and brassinosteroids enhance chromium stress tolerance in Glycine max L. (Merr.) by modulating antioxidative defense and glyoxalase systems. Environ. Sci. Pollut. Res.. 2023;30:51638-51653.
- [Google Scholar]
- Rapid determination of free proline for water-stress studies. Plant and Soil. 1973;39:205-207.
- [Google Scholar]
- Differing sensitivity of photosynthesis to low leaf water potentials in corn and soybean. Plant Physiol.. 1970;46:236-239.
- [Google Scholar]
- Genotype-dependent effect of exogenous 24-epibrassinolide on chromium-induced changes in ultrastructure and physicochemical traits in tobacco seedlings. Environ. Sci. Pollut. Res.. 2016;23:18229-18238.
- [Google Scholar]
- Effect of aluminium on lipid peroxidation, superoxide dismutase, catalase, and peroxidase activities in root tips of soybean (Glycine max) Physiol. Plant.. 1991;83:463-468.
- [Google Scholar]
- Chance, B., Maehly, A., 1955. [136] Assay of catalases and peroxidases.
- Response of oryzacystatin I transformed tobacco plants to drought, heat and light stress. J. Agron. Crop Sci.. 2010;196:90-99.
- [Google Scholar]
- Antioxidant responses of rice seedlings to salinity stress. Plant Sci.. 1998;135:1-9.
- [Google Scholar]
- Overexpression of rice Rab7 gene improves drought and heat tolerance and increases grain yield in rice (Oryza sativa L.) Genes. 2019;10:56.
- [Google Scholar]
- AFLP analysis of genetic diversity and phylogenetic relationships of Brassica oleracea in Ireland. C. R. Biol.. 2016;339:163-170.
- [Google Scholar]
- Serratia liquefaciens KM4 improves salt stress tolerance in maize by regulating redox potential, ion homeostasis, leaf gas exchange and stress-related gene expression. Int. J. Mol. Sci.. 2018;19:3310.
- [Google Scholar]
- Overexpression of AtWRKY30 transcription factor enhances heat and drought stress tolerance in wheat (Triticum aestivum L.) Genes. 2019;10:163.
- [Google Scholar]
- Serratia marcescens BM1 enhances cadmium stress tolerance and phytoremediation potential of soybean through modulation of osmolytes, leaf gas exchange, antioxidant machinery, and stress-responsive genes expression. Antioxidants. 2020;9:43.
- [Google Scholar]
- Cellular metabolites modulate in vivo signaling of Arabidopsis cryptochrome-1. Plant Signal. Behav.. 2015;10:e1063758.
- [Google Scholar]
- Phyto-management of Cr-contaminated soils by sunflower hybrids: physiological and biochemical response and metal extractability under Cr stress. Environ. Sci. Pollut. Res.. 2017;24:16845-16859.
- [Google Scholar]
- Silicon-induced reversibility of cadmium toxicity in rice. J. Exp. Bot.. 2016;67:3573-3585.
- [Google Scholar]
- Drought stress: comparative time course action of the foliar applied glycinebetaine, salicylic acid, nitrous oxide, brassinosteroids and spermine in improving drought resistance of rice. J. Agron. Crop Sci.. 2010;196:336-345.
- [Google Scholar]
- Trehalose and plant stress responses: friend or foe? Trends Plant Sci.. 2010;15:409-417.
- [Google Scholar]
- Prospects for China’s corn yield growth and imports. Department of Agriculture Economic Research Service, Washington DC: United States; 2014.
- Garg, A.K., Kim, J.-K., Owens, T.G., Ranwala, A.P., Do Choi, Y., Kochian, L.V., Wu, R.J., 2002. Trehalose accumulation in rice plants confers high tolerance levels to different abiotic stresses. Proceedings of the National Academy of Sciences 99, 15898-15903.
- Superoxide dismutases: I. Occurrence in Higher Plants. Plant Physiology. 1977;59:309-314.
- [Google Scholar]
- Disaccharides as a new class of nonaccumulated osmoprotectants for Sinorhizobium meliloti. Appl. Environ. Microbiol.. 1999;65:1491-1500.
- [Google Scholar]
- Rapid assay for determination of water soluble quaternary ammonium compounds. Plant and Soil. 1983;70:303-307.
- [Google Scholar]
- Alleviative role of exogenously applied mannitol in maize cultivars differing in chromium stress tolerance. Environ. Sci. Pollut. Res.. 2019;26:5111-5121.
- [Google Scholar]
- Improved tolerance to salinity and low temperature in transgenic tobacco producing glycine betaine. J. Exp. Bot.. 2000;51:177-185.
- [Google Scholar]
- Effect of maltose and trehalose on growth, yield and some biochemical components of wheat plant under water stress. Annals of Agricultural Sciences. 2016;61:267-274.
- [Google Scholar]
- Molybdenum supply alleviates the cadmium toxicity in fragrant rice by modulating oxidative stress and antioxidant gene expression. Biomolecules. 2020;10:1582.
- [Google Scholar]
- Combined ability of chromium (Cr) tolerant plant growth promoting bacteria (PGPB) and salicylic acid (SA) in attenuation of chromium stress in maize plants. Plant Physiol. Biochem.. 2016;108:456-467.
- [Google Scholar]
- Glycinebetaine mediates chromium tolerance in mung bean through lowering of Cr uptake and improved antioxidant system. Arch. Agron. Soil Sci.. 2016;62:648-662.
- [Google Scholar]
- Jain, N.K., Roy, I., 2010. Trehalose and protein stability. Current protocols in protein science 59, 4.9. 1-4.9. 12.
- Phenolic constituents in the leaves of northern willows: methods for the analysis of certain phenolics. J. Agric. Food Chem.. 1985;33:213-217.
- [Google Scholar]
- Exogenously-supplied trehalose protects thylakoid membranes of winter wheat from heat-induced damage. Biol. Plant.. 2010;54:495-501.
- [Google Scholar]
- Does exogenous application of glycinebetaine as a pre-sowing seed treatment improve growth and regulate some key physiological attributes in wheat plants grown under water deficit conditions. Pak J Bot. 2009;41:1291-1302.
- [Google Scholar]
- Mallhi, A.I., Chatha, S.A.S., Hussain, A.I., Rizwan, M., Bukhar, S.A.H., Hussain, A., Mallhi, Z.I., Ali, S., Hashem, A., Abd_Allah, E.F., 2020. Citric acid assisted phytoremediation of chromium through sunflower plants irrigated with tannery wastewater. Plants 9, 380.
- Implications of water stress-induced changes in the levels of endogenous ascorbic acid and hydrogen peroxide in Vigna seedlings. Physiol. Plant.. 1983;58:166-170.
- [Google Scholar]
- Insights into cadmium induced physiological and ultra-structural disorders in Juncus effusus L. and its remediation through exogenous citric acid. J. Hazard. Mater.. 2011;186:565-574.
- [Google Scholar]
- Mitigation of nickel toxicity and growth promotion in sesame through the application of a bacterial endophyte and zeolite in nickel contaminated soil. Int. J. Environ. Res. Public Health. 2020;17:8859.
- [Google Scholar]
- Foliar application of ascorbate enhances the physiological and biochemical attributes of maize (Zea mays L.) cultivars under drought stress. Arch. Agron. Soil Sci.. 2015;61:1659-1672.
- [Google Scholar]
- An interdisciplinary approach to evaluate the mobility and toxicity of cadmium in a soil–plant system. Clean (Weinh). 2018;46:1-8.
- [Google Scholar]
- Trehalose-6-phosphate: connecting plant metabolism and development. Front. Plant Sci.. 2011;2:70.
- [Google Scholar]
- Antioxidant enzymes activity and phenolic compounds content in red cabbage seedlings exposed to copper stress. Ecotoxicol. Environ. Saf.. 2009;72:596-602.
- [Google Scholar]
- Different patterns of physiological and molecular response to drought in seedlings of malt-and feed-type Barleys (Hordeum vulgare) J. Agron. Crop Sci.. 2010;196:9-19.
- [Google Scholar]
- The effects of seed priming by ascorbic acid on some morphological and biochemical aspects of rapeseed (Brassica napus L.) under drought stress condition. Int. J. Biosci. 2014;4:432-442.
- [Google Scholar]
- Differential antioxidative responses to water stress among five mulberry (Morus alba L.) cultivars. Environ. Exp. Bot.. 2004;52:33-42.
- [Google Scholar]
- Accumulation of trehalose increases soluble sugar contents in rice plants conferring tolerance to drought and salt stress. Plant Biotechnology Reports. 2012;6:89-96.
- [Google Scholar]
- Rehman, M.Z.-u., Rizwan, M., Ghafoor, A., Naeem, A., Ali, S., Sabir, M., Qayyum, M.F., 2015. Effect of inorganic amendments for in situ stabilization of cadmium in contaminated soils and its phyto-availability to wheat and rice under rotation. Environmental Science and Pollution Research 22, 16897-16906.
- Heavy metal-mediated toxicity to maize: oxidative damage, antioxidant defence response and metal distribution in plant organs. Int. J. Environ. Sci. Technol.. 2019;16:4873-4886.
- [Google Scholar]
- Role of organic and inorganic amendments in alleviating heavy metal stress in oil seed crops. Oil Seed Crops: Yield and Adaptations under Environmental Stress. 2017;12:224-235.
- [Google Scholar]
- Chromium (VI) induces toxicity at different photosynthetic levels in pea. Plant Physiol. Biochem.. 2012;53:94-100.
- [Google Scholar]
- Arsenic accumulation in maize crop (Zea mays): a review. Sci. Total Environ.. 2014;488:176-187.
- [Google Scholar]
- Rosegrant, M.W., Paisner, M.S., Meijer, S., Witcover, J., 2001. 2020 Global food outlook: Trends, alternatives, and choices, Intl Food Policy Res Inst.
- Gibberellic acid in combination with pressmud enhances the growth of sunflower and stabilizes chromium (VI)-contaminated soil. Environ. Sci. Pollut. Res.. 2015;22:10610-10617.
- [Google Scholar]
- Differences in root functions during long-term drought adaptation: comparison of active gene sets of two wheat genotypes. Plant Biol.. 2010;12:871-882.
- [Google Scholar]
- Citric acid improves lead (Pb) phytoextraction in Brassica napus L. by mitigating Pb-induced morphological and biochemical damages. Ecotoxicol. Environ. Saf.. 2014;109:38-47.
- [Google Scholar]
- Chromium interactions in plants: current status and future strategies. Metallomics. 2009;1:375-383.
- [Google Scholar]
- Chromium bioaccumulation and its impacts on plants: an overview. Plants. 2020;9:100.
- [Google Scholar]
- Phytoremediation of heavy metals by Alternanthera bettzickiana: growth and physiological response. Ecotoxicol. Environ. Saf.. 2016;126:138-146.
- [Google Scholar]
- Localized ozone fumigation system for studying ozone effects on photosynthesis, respiration, electron transport rate and isoprene emission in field-grown Mediterranean oak species. Tree Physiol.. 2005;25:1523-1532.
- [Google Scholar]
- Effects of cadmium on carbon and nitrogen assimilation in shoots of mungbean [Vigna radiata (L.) Wilczek] seedlings. J. Agron. Crop Sci.. 2007;193:357-365.
- [Google Scholar]
- Chromium-induced reactive oxygen species accumulation by altering the enzymatic antioxidant system and associated cytotoxic, genotoxic, ultrastructural, and photosynthetic changes in plants. Int. J. Mol. Sci.. 2020;21:728.
- [Google Scholar]
- Remediation of Cr (VI)-contaminated acid soil using a nanocomposite. ACS Sustain. Chem. Eng.. 2017;5:2246-2254.
- [Google Scholar]
- Phytoremediation potential of maize (Zea mays L.). A review. African Journal of General Agriculture. 2010;6:275-287.
- [Google Scholar]
- Bioaccumulation and translocation of cadmium in wheat (Triticum aestivum L.) and maize (Zea mays L.) from the polluted oasis soil of Northwestern China. Chem. Spec. Bioavailab.. 2014;26:43-51.
- [Google Scholar]
- Salicylic acid underpins silicon in ameliorating chromium toxicity in rice by modulating antioxidant defense, ion homeostasis and cellular ultrastructure. Plant Physiol. Biochem.. 2021;166:1001-1013.
- [Google Scholar]
- Effect of cadmium-tolerant rhizobacteria on growth attributes and chlorophyll contents of bitter gourd under cadmium toxicity. Plants. 2020;9:1386.
- [Google Scholar]
- Trehalose as osmoprotectant for maize under salinity-induced stress. Res. J. Agric. Biol. Sci.. 2009;5:613622
- [Google Scholar]
- Antioxidant response system and chlorophyll fluorescence in chromium (VI)-treated Zea mays L. seedlings. Acta Biol. Cracov. Bot.. 2009;51:23-33.
- [Google Scholar]
- Rhizobacteria and phytoremediation of heavy metals. Environ. Technol. Rev.. 2016;5:112-119.
- [Google Scholar]







