Translate this page into:
Amides from Zanthoxylum bungeanum Maxim. (Rutaceae) are promising natural agents with neuroprotective activities
⁎Corresponding authors. wuchunjie@cdutcm.edu.cn (Chunjie Wu), pengwei@cdutcm.edu.cn (Wei Peng)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Neurodegenerative diseases are neurological diseases that are characterized by neuronal degeneration and apoptosis; they threaten populations around the world and place a great burden on the social economy. Unfortunately, the drugs currently used to treat neurodegenerative diseases have obvious side effects, making them much less effective. Therefore, discovery of new safe and effective drugs is urgently needed. Zanthoxylum bungeanum Maxim. (ZBM) has a long medicinal history in China and can also be used as food. ZBM contains many types of chemical components, of which amides are the most abundant. In preparing this review, we searched and integrated the relevant literature in PubMed, Web of Science, Elesvier, Wliey and Springer using the keywords “ZBM”, “amide”, “physicochemical properties”, and “neurodegenerative diseases”. It was found that the amides that are present in ZBM (AZB), although structurally unstable, are the likely material basis for an irritating sensation in the mout,. In addition, AZB can play a therapeutic role in Alzheimer's disease, Parkinson's and depression by exerting antioxidant and anti-inflammatory effects. The main targets of AZB are the TRPV1, TRPA1 and PI3K/AKT signalling pathways. This review will discuss the physical and chemical properties of AZB and its use in the treatment of neurodegenerative diseases to provide a reference for the development of ZBM for use in the treatment of neurodegenerative diseases.
Keywords
Neurodegenerative diseases
Amides
Zanthoxylum bungeanum Maxim.
Natural agents
- AD
-
Alzheimer's disease
- PD
-
Parkinson's disease
- HD
-
Huntington's disease
- ZBM
-
Zanthoxylum bungeanum Maxim.
- AZB
-
Amides of ZBM
- HAS
-
Hydroxy-α-sanshool
- HBS
-
Hydroxy-β-sanshool
- TRPV1
-
Transient receptor potential vanilloid type 1
- TRPA1
-
Transient receptor potential A1
- Ach
-
Acetylcholine
- BDNF
-
Brain-derived neurotrophic factor
- CREB
-
c-AMP response element-binding protein
- MDA
-
Malondialdehyde
- MMP
-
Mitochondrial membrane potential
- SOD
-
Superoxide dismutase
- CAT
-
Catalase
- GSH-Px
-
Glutathione peroxidase
- D-gal
-
D-galactose
- HAS-LPs
-
HAS-liposomes
- Aβ
-
Amyloid-beta
- AβPP-Tg
-
Amyloid-β protein precursor transgenic
- GSK-3
-
Glycogen synthase kinase-3
- GF-β1
-
Transforming growth factor β1
- α7nAChR
-
α7 nicotinic acetylcholine receptor
- TOMM
-
Translocase of outer mitochondrial membrane
- NGF
-
Nerve growth factor
- α-syn
-
α-synuclein
- TRPV1
-
Transient receptor potential vanilloid 1
- TRPA1
-
Transient receptor potential anchoring protein 1
Abbreviations
1 Introduction
Neurodegenerative diseases are highly prevalent in the elderly population. With the increase in the elderly population, the number of patients with neurodegenerative diseases has increased dramatically, making the treatment of neurodegenerative diseases particularly important (Rehman et al., 2019). Neurodegenerative diseases are a major problem worldwide. The number of patients over 65 years of age with Alzheimer's disease (AD) in developed countries is expected to reach 7.1 million by 2025, and this number will increase to 13.8 million by 2050. The main causes of neurodegenerative diseases are the destruction of nerve cells in the brain and spinal cord and nervous system dysfunction that is closely related to ageing. Due to the inability of neurons to regenerate, the damage caused by this disease is irreversible and eventually fatal (Bhat et al., 2017).
Neurodegenerative diseases can be subdivided into acute neurodegenerative diseases and chronic neurodegenerative diseases. Acute neurodegenerative diseases are defined as those in which there is acute damage to neurons, including stroke caused by insufficient cerebral blood flow and retinal ischaemia. Chronic neurodegenerative diseases mainly include Alzheimer's disease (AD), Parkinson's disease (PD), depression, Huntington's disease (HD), epilepsy and multiple sclerosis (Perlikowska, 2021; Raafat et al., 2019). According to relevant data provided by the World Health Organization, the incidence of AD, PD and HD is increasing exponentially, and high medical expenses will bring a massive burden to patients and to the social economy (Li et al., 2022). As a heterogeneous group of diseases, neurodegenerative diseases have different biochemical sources, most of which are related to neurotoxin damage, metabolism, and attack by pathogens. At the same time, genetic and environmental factors and lifestyle can affect the development of the disease. After decades of research, common features of neurodegenerative diseases have gradually been identified; they include mitochondrial dysfunction, neuroinflammation, synaptic dysfunction and oxidative stress (Ahat et al., 2019; Peng et al., 2022). These conditions damage neurons and lead to memory loss, cognitive dysfunction, anxiety, and motor dysfunction. However, although AD and other diseases have been known for more than 100 years, there is still no effective way to prevent and treat these diseases (Bading, 2017). Nonsteroidal anti-inflammatory drugs, riluzole and caffeine A2A receptor antagonists minimize the risk of complications of neurodegenerative diseases, but long-term use of these agents leads to nonnegligible side effects (Gupta et al., 2021). Therefore, discovery of new safe and effective drugs is imperative.
Neuroprotection refers to the protection of neurons through various mechanisms and strategies designed to ensure normal brain function. To a certain extent, neuroprotection can delay or prevent nerve cell dysfunction, correct slow or abnormal developmental processes, promote regeneration of the nervous system, and thus effectively treat or prevent neurodegenerative diseases (Perlikowska, 2021; Campos et al., 2016). Some studies have reported that natural products exert neuroprotective effects through a wide range of pharmacological and biological activities. At the same time, the wide availability of natural products also makes them ideal sources of drugs.
Zanthoxylum bungeanum Maxim. (ZBM) is a species of the genus Zanthoxylum and is also a natural product that is widely distributed in Asian countries, including China, Japan, India, and Korea (Zhang et al., 2017). The pericarp of ZBM, commonly known as “Huajiao” in China, is not only a commonly used food additive but also has a long history of medicinal use. ZBM has been used to treat abdominal pain, dyspepsia and eczema due to its spleen- and stomach-warming medical functions (Wang et al., 2019). With the development of ZBM research, an increasing number of compounds have been identified. Phytochemical studies have shown that ZBM contains secondary metabolites such as alkaloids, flavonoids, terpenoids, and free fatty acids (Cho et al., 2003; Yang, 2008; Yang et al., 2013). Analysis of the aromatic components of ZBM using headspace solid-phase microextraction combined with gas chromatography-mass spectrometry showed that linalyl acetate, linalool and limonene are the main components of the leaves and pericarp of ZBM at different harvest stages. Amides, as special active ingredients in ZBM, contribute greatly to the unique flavour of ZBM (Zhu et al., 2019). Pharmacological research has shown that amides found in ZBM (AZB) have excellent analgesic, antibacterial, anti-inflammatory, and antioxidant activities and that they have certain effects on toothache, obesity, type Ⅰ diabetes, atherosclerosis, and other conditions (Wang et al., 2019, 2020; Dossou et al., 2013; Devkota et al., 2013). It was discovered in recent years that AZB is potentially useful in the treatment of neurodegenerative diseases, including AD, PD and depression; in this context, its anti-inflammatory and antioxidant effects are fully exploited to treat the disease (Fig. 1). In this review, we will focus on the efficacy and underlying mechanism of action of AZB in the treatment of neurodegenerative diseases, especially AD, PD and depression. This work provides a valuable reference for future research and for the development of clinical applications of AZB.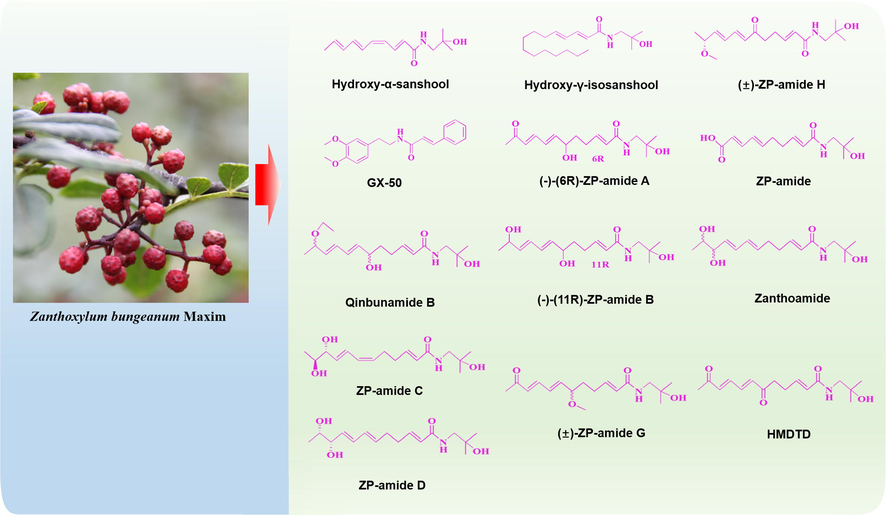
Amides from Zanthoxylum bungeanum Maxim. (Rutaceae) are promising natural agents with neuroprotective activities.
2 Physicochemical properties of AZB
As mentioned earlier, ZBM is often used as a condiment or in the treatment of toothache because it produces an irritating sensation in the mouth, which is also known as “Ma” in China. The amides isolated from ZBM, which include α-sanshool, β-sanshool, γ-sanshool, δ-sanshool and their derivatives, are thought to be the basis for “Ma” (Yang, 2008; Marcos et al., 1990). These amides form white crystals that are only slightly soluble in ethanol and water but easily soluble in hot ethanol, ethyl acetate and other organic solvents (Zhang et al., 2017; Greger, 1984). AZB are structurally similar and usually contain two or more conjugated double bonds (Koo et al., 2007). The structural characteristics of AZB make them extremely unstable at room temperature and highly susceptible to oxidation or polymerization, reactions that produce yellow, sticky, paste-like substances. Therefore, when preserving AZB, the material is often stored in sealed ampoules under nitrogen. In addition, because hydroxy-α-sanshool (HAS), one of the components of AZB, can be converted to its isomer hydroxy-β-sanshool (HBS) by UV light, HAS should be protected from light (Greger, 1984; Zhang et al., 2017). For these reasons, the “Ma” of ZBM decreases significantly after 1 year of storage at room temperature. In a sensory evaluation conducted by Sugai et al., “Ma” was subdivided into numbness, burning, and tingling. Notably, all-trans alkylamides, β-sanshool, and HBS were found to produce numbness, while HAS was the most important component of ZBM that produced irritation (Sugai et al., 2005).
There are two views regarding the stimulatory properties of HAS. One of these holds that HAS activates the transient receptor potential vanilloid type 1 (TRPV1) and transient receptor potential A1 (TRPA1) channels and thereby depolarizes sensory nerves and promotes Ca2+ influx (Koo et al., 2007; Sugai et al., 2005; Bryant and Mezine, 1999). The other view holds that HAS activates somatosensory nerves by blocking the two-pore potassium channels KCNK3, KCNK9 and KCNK18 (Bautista et al., 2008).
3 Pathway through which AZB exerts its protective effect against neurodegenerative diseases
AZB have shown protective effects against neurodegenerative diseases in recent studies, and their mechanisms of action can be divided into the following main categories. First, AZB represented by HAS scavenges active free radicals of 2,2-diphenyl-1-picrylhydrazyl (DPPH). When free radicals accumulate excessively, they can induce oxidative stress in cells and cause oxidative damage to nerve cells. Therefore, scavenging of free radicals is essential in the treatment of neurodegenerative diseases. Notably, HAS reduces the production and accumulation of intracellular ROS and inhibits the occurrence of oxidative stress at multiple levels (Li et al., 2020). Second, some pathological proteins such as amyloid precursor protein (APP), amyloid β protein (Aβ) and p-tau protein exacerbate the development of neurodegenerative disease. gx-50, a component of AZB, was shown to have superior ability to hydrolyse Aβ oligomers (Tang et al., 2013). Third, AZB acts as an inhibitor of cholinesterase (AChE), the enzyme that degrades ACh. Notably, ACh is an important mediator of signal transmission between neurons, and reduction in ACh levels often leads to the development of AD. Fortunately, HAS is able to inhibit AChE activity and thus increase ACh content, which has a mitigating effect on AD (Zhang et al., 2019). Finally, AZB, represented by gx-50, can alleviate neurotoxicity by reducing neuroinflammation and thereby exert a protective effect on nerve cells (Tang et al., 2013).
4 Neuroprotective activities of AZB and its possible mechanisms
4.1 Alzheimer’s disease
AD is a classic neurodegenerative disease. Various theories that suggest that cholinergic, Aβ cascade, oxidative stress or inflammatory pathways are involved have been proposed to explain the pathogenesis of AD, but there is no unified definitive conclusion regarding its pathogenesis. The hypothesis that AZB might serve as an effective treatment for AD was first derived from Satoh's experiment. It was found that a traditional prescription, Daikenchuto, which is also known as “Da-Jian-Zhong-Tang” in China and contains ZBM, improves gastrointestinal transit by affecting acetylcholine (ACh) release (Satoh et al., 2001). ACh, an important neurotransmitter that is part of the central cholinergic system, is considered to act as a key mediator of learning and memory by regulating the cholinergic system, and reduced ACh release often causes the development of AD. Therefore, Nakamura et al. investigated whether ZBM could improve learning and memory by affecting ACh release in the central nervous system. In those experiments, the Morris water maze test was used to evaluate learning and memory ability. Administration of ZBM extract significantly decreased the platform finding time in scopolamine-induced mice. In addition, the effect of HAS on learning and memory ability was stronger under the same dose conditions (Nakamura et al., 2006). Unfortunately, this experiment did not address in depth whether ZBM and HAS improve learning and memory ability through ACh and or determine the underlying mechanism of action. Since then, numerous researchers have begun experimental studies of AZB.
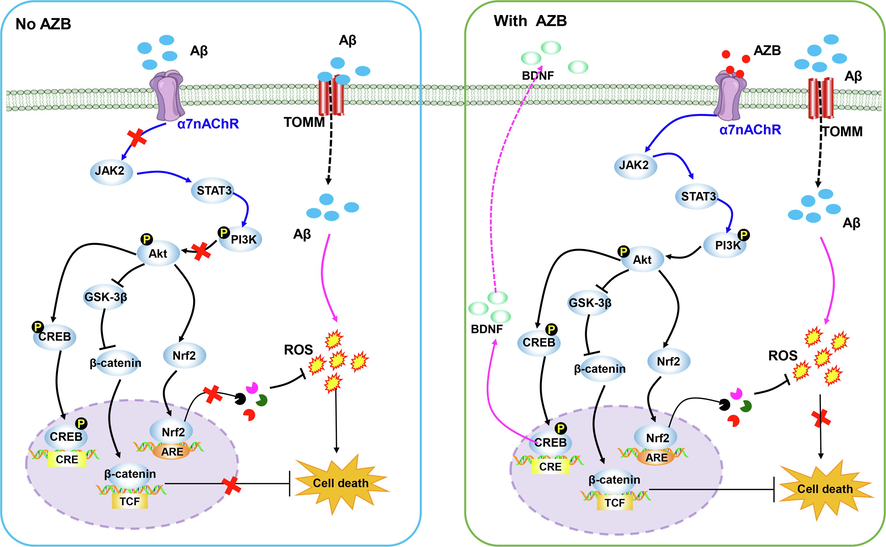
As previously mentioned, HAS is an important active component of ZBM, and it is very abundant in AZB. In the experiments of Zhang et al., scopolamine was used to construct AD models, followed by oral administration of HAS. Based on the results of their experiments, these investigators reached a conclusion similar to that of Nakamura et al., namely, that HAS significantly alleviated learning and memory impairment and inhibited apoptosis of hippocampal neurons. To further explore the mechanism of action of HAS, Zhang et al. measured ACh and acetylcholinesterase (AChE) content in the brains of mice. AChE is an enzyme that rapidly hydrolyses ACh and is considered an important component of the pathway that regulates ACh content. Donepezil, a drug that is widely used in the treatment of AD, is a typical AChE inhibitor. Zhang et al.’s results showed that HAS inhibited the activity of AChE, thereby increasing ACh content in the brains of the model mice (Zhang et al., 2019). Since brain-derived neurotrophic factor (BDNF) is a neurotrophic factor that is closely related to neuronal survival, its deletion in the hippocampus leads directly to the development of learning and memory impairment. In addition, cAMP response element-binding protein (CREB), an intranuclear factor that regulates gene transcription, is regulated by its own phosphorylation. Previous studies have shown that CREB is closely related to learning and memory function and that BDNF is an important target (Xue et al., 2015). Therefore, Zhang et al. continued to examine the expression of BDNF and CREB. HAS significantly upregulated the expression of BDNF and p-CREB in the hippocampi of model mice. This led to the conclusion that HAS exerts a therapeutic effect on AD by activating the cholinergic system and the CREB/BDNF signalling pathway (Zhang et al., 2019). Subsequently, Li and Zhang et al. continued to explore the mechanism of action of HAS using H2O2-induced PC12 cells as an in vitro model. They found that HAS significantly inhibited oxidative stress in the cells, as evidenced by the fact that it reduced intracellular ROS and malondialdehyde (MDA) content and increased mitochondrial membrane potential (MMP) and the levels of intracellular antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GSH-Px). Through these effects, HAS maintained redox homeostasis in PC12 cells, which in turn inhibited apoptosis induced by H2O2. Similar results were obtained when the investigators identified the PI3K/AKT signalling pathway as the signalling pathway through which HAS inhibits cellular oxidative stress. The PI3K/AKT signalling pathway produces extensive antioxidant activity in both central and peripheral neurons and is considered a potential target in the treatment of neurodegenerative diseases. PI3K phosphorylates and activates AKT, a serine/threonine kinase that is activated by recruitment to the plasma membrane. Activation of the PI3K/AKT signalling pathway further affects the expression of downstream apoptosis-related proteins. In the experiments of Li et al., HAS activated the PI3K/AKT signalling pathway, thereby reducing the expression of the proapoptotic proteins cleaved caspase-3 and BAX and increasing the expression of the antiapoptotic protein Bcl-2 (Li et al., 2020). Liu et al. used mice to which D-galactose (D-gal) and AlCl3 had been administered as in vivo models to explore the mechanism of action of HAS in experiments that continued to focus on apoptosis induced by oxidative stress. The results showed that orally administered HAS inhibited oxidative stress and apoptosis in mice via the Nrf2/HO-1 signalling pathway and that it exerted a protective effect on hippocampal neurons and improved the learning and memory ability of the mice (Liu et al., 2022). To avoid problems associated with the structural instability of HAS, HAS-liposomes (HAS-LPs) were prepared and used to treat AD mice by nasal administration. This not only improved the stability of HAS but also resulted in a certain degree of brain targeting, thereby improving the therapeutic effect of HAS (Tang et al., 2013). Additional details of this work are shown in Table 1 and Fig. 2. α7 nAChR, α7 nicotinic acetylcholine receptor; Ach, acetylcholine; AchE, acetylcholinesterase; AD, Alzheimer’s disease; Aβ, amyloid-beta; BDNF, brain-derived neurotrophic factor; cAMP response element-binding protein; CAT, catalase; CREB, cDNA, complementary DNA; CHO, Chinese hamster ovary; Con., concentration; CORT, corticosterone; D-gal, D-galactose; DRGN, dorsal root ganglia neurons; GSH-Px, glutathione peroxidase; GSK-3, glycogen synthase kinase; GX-50, N-[2-(3,4-Dimethoxyphenyl)ethyl]-3-phenylacrylamide; HAS, Hydroxy-α-sanshool; HAS-LPs, hydroxy-α-sanshool liposomes; HBS, Hydroxy-β-sanshool; HMDTD, (2E,7E,9E)-N-(2-hydroxy-2-methylpropyl)-6,11-dioxo-2,7,9-dodecatrienamide; MDA, malondialdehyde; MMOP, mitochondrial membrane potential; MWM, Morris water maze test; NGF, Nerve growth factor; PCN, Primary cortical neurons; SOD, superoxide dismutase; TDN, Tetrahydrobungeanool; TGN, trigeminal ganglion neurons.
Compounds
Compound’s structure
Doses/Con.
Experimental models
Detail effects and mechanisms
Related targets
References
Alzheimer’s disease
HAS
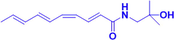
5 mg/kg, p.o., for 3 days
Scopolamine
Induced miceEnhancing the memory and learning of ddY mice
(Nakamura et al., 2006)
HAS

1.25, 2.5, 5 mg/kg, p.o.
Scopolamine
Induced miceInhibiting scopolamine-induced cognitive impairments in mice;
Inhibiting morphology changes and apoptosis in hippocampal neuron;
Decreasing AChE activity and increasing ACh content;
Up-regulating BDNF and CREBBDNF, CREB,AChE
(Zhang et al., 2019)
HAS
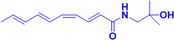
15,30,60 μM
H2O2 induced PC12 cells
Protecting PC12 cells from H2O2 induced injury;
Reducing H2O2-induced apoptosis in PC12 cells via reduction of intracellular ROS and increase of MMOP; Iincreasing SOD, CAT, GSH-Px, and decreasing MDA.
Upregulating p-PI3k, Akt, p-Akt, Bcl-2, and down-regulating caspase-3, Bax in H2O2 induced PC12 cells.
SOD, CAT, GSH-Px, PI3K, Akt, Bcl-2, MDA, Bax, Caspase-3
(Li et al., 2020)
HAS

1.25, 2.5, 5 mg/kg, p.o.
D-gal/AlCl3 induced mice
Ameliorating spontaneous locomotion deficit of D-gal/AlCl3 induced mice;
Improving the spatial learning and memory;
Alleviating morphological changes and increasing Nissl neurons in hippocampus;
Reducing MDA and increasing SOD, GSH-Px and CAT;
Increasing HO-1 and Nrf2 in the hippocampus;
Iinhibiting neuronal apoptosis by decreasing Cyt-c, Bax and Caspase-3, and increasing Bcl-2 in hippocampus.
SOD,GSH-Px, CAT,HO-1,Nrf2, Bcl-2,Caspase-3
(Liu et al., 2022)
HAS-LPs
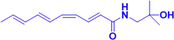
1.25,2.5,5mg/kg, Intranasal
D-gal/AlCl3 induced mice
Exhibiting slow drug release and low toxic to the nasal mucosa;
Alleviating D-galactose-induced learning memory deficits and protecting hippocampal neuronal cells.
Being enriched in plasma and brain tissue via intranasal aministration.
(Li et al., 2022)
Gx-50
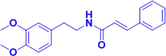
1 mg/kg, i.p.
Aβ transgenic mouse AβPP-Tg mice
Disassembling Aβ oligomers;
Improve cognitive performance;
Inhibiting Ca2+ inward flow.
(Tang et al., 2013)
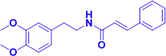
1 μM
Aβ42 induced primary cortical neurons of SD rats
Elevating the Akt phosphorylation and inhibiting GSK-3 to restoring the CREB’s transcriptional activity, and
finally upregulating BDNF.
Inhibiting Aβ-induced neuronal apoptosis and apoptotic gene expression, and Reducing neuronal calcium toxicity.Akt, CREB, BDNF, GSK-3
(Tang et al., 2014)
Gx-50
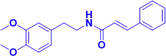
Aβ-induced primary microglia
Inhibiting the chemotactic migration of microglia and CCL5 induced by Aβ.
Upregulating TGF-β1 and enhancing phosphorylation of GSK-3β in Aβ-induced microglia.TGF-β1,GSK-3β, CCL5
(Guo et al., 2014)
Gx-50
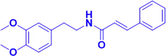
2.5,5, 10,20 μM
Aβ-induced microglia
Activating α7 nAChR;
upregulating JAK2/STAT3 and PI3K/Akt signaling pathways to inhibit IL-1β release.
α7nAChR, JAK2, STAT3, PI3K, Akt, IL-1β
(Shi et al., 2016)
Qinbunamide B
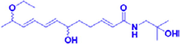
20 μM
PC12 cells
Potentiating activity of NGF to stimulate neurite outgrowth from PC12 cells.
NGF
(Tian et al., 2016)
ZP-amide C
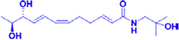
ZP-amide D
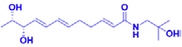
Hydroxy-γ-isosanshool
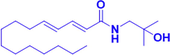
IC50 = 4.08 μM
Erastin induced HT-22 cells
Inhitibitng the erastin induced feroptosis of hippocampal neuron cell HT22 cell with the IC50 of 4.08 μM.
(Zhang et al., 2020)
Parkinson's disease
HAS
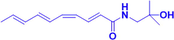
1 mM
HEK293T cells,
Primary TGN,
primary DRGNCausing Ca2+ influx in cells transfected with TRPV1 or TRPA1 and evoking robust inward currents in cells transfected with TRPV1 or TRPA1;
Inducing inward currents and Ca2+ influx which could be
diminished in TRPV1–/– mice.
TRPV1, TRPA1
(Riera et al., 2009)
Depression
(−)-(6R)-ZP-amide A
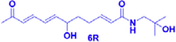
3.125–100 μM
CORT induced PC 12 cells
Protecting against CORT-induced PC12 cells damage
(Chen et al., 2018)
(−)-(11R)-ZP-amide B
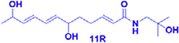
3.125–100 μM
(±)-ZP-amide G
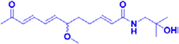
100 μM
(±)-ZP-amide H
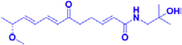
50–100 μM
ZP-amide I
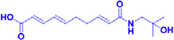
25–54 μM
Zanthoamide
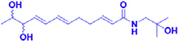
12.5–100 μM
HMDTD
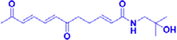
25–100 μM
Amides from Zanthoxylum bungeanum Maxim. (Rutaceae) are promising natural agents for the treatment of Alzheimer’s disease.
AZBs other than HAS that have therapeutic potential for AD are also present in ZBM. N-[2-(3,4-dimethoxyphenyl)ethyl]-3-phenyl-acrylamide, also known as gx-50, penetrates the blood–brain barrier and acts directly on the brain to exert therapeutic effects. gx-50 has shown great therapeutic potential in a number of recent studies. It was initially found to reduce the level of amyloid-beta (Aβ) oligomers in the brains of amyloid-β protein precursor transgenic (AβPP-Tg) mice and to improve cognitive performance in mice. In in vitro experiments, gx-50 showed superior ability to break down Aβ oligomers, and this resulted in reduced apoptosis of primary cortical neurons induced by Aβ and inhibition of inward Ca2+ flow (Li et al., 2022). Gene microarray technology was subsequently used to explore the mechanism of this effect. After pathway analysis of changes in gene expression, immunoblotting, immunohistochemistry and real-time fluorescence quantitative PCR, it was concluded that gx-50 inhibits glycogen synthase kinase-3 (GSK-3) activity by promoting p-Akt phosphorylation and that this, in turn, restores the transcriptional activity of CREB and ultimately promotes BDNF expression (Tang et al., 2014). In studies using Aβ-induced primary microglia, gx-50 was found to downregulate the candidate chemokine CCL5 by activating the transforming growth factor β1 (GF-β1)/Smad2 signalling pathway, thereby inhibiting microglial migration to Aβ. In addition, gx-50 was found to inactivate GSK-3β by enhancing the phosphorylation of GSK-3β, a change that synergistically helped gx-50 inhibit microglial migration (Guo et al., 2014). In Shi et al.'s subsequent study, the effects of gx-50 on inflammation were investigated using Aβ-induced microglia as a model. The researchers found that gx-50 acts as a specific ligand to activate the α7 nicotinic acetylcholine receptor (α7nAChR) (Shi et al., 2016). The α7nAChR is a nicotinic receptor that occurs widely in the nervous system. It impairs the inflammatory response in AD via the cholinergic anti-inflammatory pathway and has both neuroprotective and anti-inflammatory effects that may reduce cognitive impairment (Conejero-Goldberg et al., 2008). During the pathogenesis of AD, Aβ binds not only to the translocase of the outer mitochondrial membrane (TOMM), thereby inducing oxidative stress, but also to the α7nAChR, thereby blocking neuroprotection. Surprisingly, gx-50 binds competitively to α7nAChR. Upon activation of α7nAChR by gx-50, JAK2, a downstream signalling partner of the α7nAChR, phosphorylates the transcription factor STAT3 and activates the PI3K/AKT signalling pathway, and this in turn inhibits the release of proinflammatory factors (Shi et al., 2016).
Qinbunamides B, ZP-amide C and ZP-amide D are isobutyl hydroxylamines that have been isolated from ZBM and identified; qinbunamide B is the first ethyl-containing isobutyl hydroxylamine to be isolated (Tian et al., 2016). Nerve growth factor (NGF), as a neurotrophic factor, promotes the growth and development of neurons. It is also an essential part of the mechanism that repairs the nervous system after damage. However, because NGF is a high-molecular-weight peptide, it is difficult for exogenous NGF to cross the blood–brain barrier (Allen et al., 2013). Therefore, promotion of endogenous NGF activity has been a popular topic in the treatment of AD in recent years. In experiments conducted by Tian et al., all three compounds (qinbunamides B, ZP-amide C and ZP-amide D) enhanced NGF activity and thereby stimulated the neuronal differentiation of PC12 cells, with potential neuroprotective effects (Tian et al., 2016).
Hydroxy-γ-isosanshool is another isobutyl amide compound. In a series of experiments, erastin was used to induce iron-related death of mouse hippocampal neuronal HT22 cells. After treatment with hydroxy-γ-isosanshool, cell morphology and cell survival improved. The ability of hydroxy-γ-isosanshool to inhibit iron-related death of neurons suggests that it may potentially be useful in the treatment of AD (Zhang et al., 2020). Additional details of this work are presented in Table 1.
4.2 Parkinson's disease
PD is the second most common neurodegenerative disease, and its symptoms manifest as nonmotor symptoms and motor deficits. The pathology of PD is mainly characterized by delayed development of dopaminergic neurons in the dense region of the substantia nigra and aggregation of Lewy vesicles containing misfolded α-synuclein (α-syn) (Li et al., 2022). Transient receptor potential vanilloid 1 (TRPV1) and transient receptor potential anchoring protein 1 (TRPA1), both of which are members of the transient receptor potential channel protein family (Khan et al., 2019), are abundantly expressed in sensory nerves and jointly regulate Ca2+ influx. In previous studies, cannabinoids attenuated seizures, but their alleviating effects could be reversed by TRPV1 antagonists (Gaston and Szaflarski, 2018). Activation of TRPV1 promotes the inward flow of Ca2+ and increases glutamate release and neural activity and therefore has some therapeutic potential for the treatment of PD (Patricio et al., 2020). HAS induces an inward current and inward flow of Ca2+ in HEK cells transfected with TRPV1 or TRPA1, whereas HBS does not. A similar conclusion was reached when primary cultured sensory nerves were used to further explore the role of HAS. In an in vivo experiment, TRPV1-⁄- mice were used to explore whether the effects of HAS are mediated by TRPV1. The results were surprising in that inhibition of TRPV1 expression resulted in a significant reduction in both Ca2+ inward flow and current, even after HAS treatment (Koo et al., 2007; Riera et al., 2009).
4.3 Depression
Depression is an extremely common psychiatric disorder in which patients often suffer from cognitive impairment, fatigue, feelings of worthlessness, and even suicidal tendencies. The pathogenesis of depression is still being explored, and corticosterone-induced PC12 cells are often used as a model to screen drugs for the treatment of depression. In experiments conducted by Chen et al., a 95% ethanol extract of ZBM was prepared, and 21 isobutylamides were isolated from the extract, identified, and applied to corticosterone-induced PC12 cells as a way to screen for possible active compounds. In those experiments, seven compounds, (−)-(6R)-ZP-amide A, (−)-(11R)-ZP-amide B, (±)-ZP-amide G, (±)-ZP-amide H, ZP-amide I, zanthoamide and (2E,7E,9E)-N-(2-hydroxy-2-methylpropyl)-6,11-dioxo-2,7,9-dodecatrienamide, were found to effectively improve the survival rate of corticosterone-induced PC12 cells. In addition, the protective effects of these compounds were found to be closely related to their conformations. Isobutyl hydroxylamides with the R-configuration exhibited protective activity, while amides with the S-configuration showed no protective ability. The protective ability of isobutyl amides was also found to decrease with increasing unsaturated fatty chain length (Chen et al., 2018).
5 Toxic effects of AZM
The fruit of Z. bungeanum has been used for hundreds of years as an important traditional herbal medicine in China and has been found to have notable health benefits and little toxicity. However, it has been reported that these fruits may induce shortness of breath, toothache and blurred vision when taken in large doses (Wang, 2007; Tian, 1999). At present, data on the relative systemic toxicity and safety of AZM are lacking, and there are few data on its possible target-organ toxicity or side effects. However, it was reported that oral administration of HAS, an important amide isolated from the fruit of Z. bungeanum, at a dose of 72 mg/kg did not cause death in rats (Wang et al., 2019), and the LD50 of HAS in mice after oral administration has been reported to be 73 mg/kg.
6 Conclusion and perspective
From the above, it is clear that AZB has a wide range of therapeutic effects in neurodegenerative diseases. At the same time, due to its wide source of raw material, it is an excellent source of natural drugs. However, after reviewing the previous work on AZB, we found that there is still much work to be done before AZB can actually be applied in clinical treatment.
First, although extensive studies of the efficacy of the amide components of AZB in the treatment of AD and their possible mechanisms of action have been conducted, most of this work does not combine in vivo and in vitro experiments, resulting in different focuses on efficacy and mechanism of action. In addition, clinical trials and data on amide therapy have not yet progressed, and there is a lack of real and effective clinical data to support disease research. From previous reports, we found that AZB has unstable physicochemical properties. To develop AZB as a drug that can be directly used in clinical practice, the stability issue must be resolved. In previous experiments by Li et al. in which HAS-containing liposomes were prepared to improve the compound’s stability, the drug loading was low, and a reliable process for the production of such liposomes has not been developed. In recent research, silica gel, Sephadex LH-20, reversed-phase C18 and other methods were used to separate and purify AZB. Although new AZBs that possess anti-inflammatory activity have been isolated and identified, the low purity of the compounds and their unclear mechanisms of action also limit their further development. It is worth noting that there is still a lack of data on the ability of AZB to penetrate the blood–brain barrier and on its bioavailability in the brain, and this places great limitations on the development of AZB as a drug. In addition, although the possible targets TRPV1 and TRPA1 have been identified, the efficacy of HAS in alleviating the symptoms of PD has not been further studied. Finally, with respect to the treatment of depression, only a few compounds that may be effective have been screened, and studies of their efficacy and mechanisms of action are scarce. Therefore, in future studies, we need to deepen our research based on these issues with the goal of developing effective treatments for neurodegenerative diseases.
Author contribution
All authors made a significant contribution to the work reported, whether that is in the conception, execution, acquisition of data, analysis and interpretation. Wei Peng and Chunjie Wu conceived this paper; Ruolan Li, Hu-Xinyue Duan and Lingyu Wang took part in drafting, revising and critically reviewing the article; Qi Liang gave final approval of the version to be published; Wei Peng and Chunjie Wu have agreed on the journal to which the article has been submitted and agree to be accountable for all aspects of the work.
Acknowledgements
This work was supported by Natural Science Foundation of Sichuan Province (No. 2022NSFSC0720) and State Administration of Traditional Chinese Medicine of Sichuan Province of China (No. 2020HJZX001).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- New Insights Into the Golgi Stacking Proteins. Front. Cell Dev. Biol.. 2019;7:131.
- [CrossRef] [Google Scholar]
- GDNF, NGF and BDNF as therapeutic options for neurodegeneration. Pharmacol. Ther.. 2013;138:155-175.
- [CrossRef] [Google Scholar]
- Therapeutic targeting of the pathological triad of extrasynaptic NMDA receptor signaling in neurodegenerations. J. Exp. Med.. 2017;214:569-578.
- [CrossRef] [Google Scholar]
- Pungent agents from Szechuan peppers excite sensory neurons by inhibiting two-pore potassium channels. Nat. Neurosci.. 2008;11:772-779.
- [CrossRef] [Google Scholar]
- Synopsis on Managment Strategies for Neurodegenerative Disorders: Challenges from Bench to Bedside in Successful Drug Discovery and Development. Curr. Top. Med. Chem.. 2017;17:1371-1378.
- [CrossRef] [Google Scholar]
- Alkylamides that produce tingling paresthesia activate tactile and thermal trigeminal neurons. Brain Res.. 1999;842:452-460.
- [CrossRef] [Google Scholar]
- Cannabidiol, neuroprotection and neuropsychiatric disorders. Pharmacol. Res.. 2016;112:119-127.
- [CrossRef] [Google Scholar]
- Isobutylhydroxyamides from Sichuan Pepper and Their Protective Activity on PC12 Cells Damaged by Corticosterone. J. Agric. Food Chem.. 2018;66:3408-3416.
- [CrossRef] [Google Scholar]
- Study on the inhibitory effects of Korean medicinal plants and their main compounds on the 1,1-diphenyl-2-picrylhydrazyl radical. Phytomedicine. 2003;10:544-551.
- [CrossRef] [Google Scholar]
- Alpha7 nicotinic acetylcholine receptor: a link between inflammation and neurodegeneration. Neurosci. Biobehav. Rev.. 2008;32:693-706.
- [CrossRef] [Google Scholar]
- Isobutylhydroxyamides from the pericarp of Nepalese Zanthoxylum armatum inhibit NF1-defective tumor cell line growth. J. Nat. Prod.. 2013;76:59-63.
- [CrossRef] [Google Scholar]
- Identification of CB1/CB2 ligands from Zanthoxylum bungeanum. J. Nat. Prod.. 2013;76:2060-2064.
- [CrossRef] [Google Scholar]
- Cannabis for the Treatment of Epilepsy: an Update. Curr. Neurol. Neurosci. Rep.. 2018;18:73.
- [CrossRef] [Google Scholar]
- Alkamides: structural relationships, distribution and biological activity. Planta Med.. 1984;50:366-375.
- [CrossRef] [Google Scholar]
- The suppressive effects of gx-50 on Aβ-induced chemotactic migration of microglia. Int. Immunopharmacol.. 2014;19:283-289.
- [CrossRef] [Google Scholar]
- Neuroprotective Potential of Ellagic Acid: A Critical Review. Adv. Nutr.. 2021;12:1211-1238.
- [CrossRef] [Google Scholar]
- Insight into Pain Modulation: Nociceptors Sensitization and Therapeutic Targets. Curr. Drug Targets. 2019;20:775-788.
- [CrossRef] [Google Scholar]
- Hydroxy-alpha-sanshool activates TRPV1 and TRPA1 in sensory neurons. Eur. J. Neurosci.. 2007;26:1139-1147.
- [CrossRef] [Google Scholar]
- Development and in vivo Evaluation of Hydroxy-α-Sanshool Intranasal Liposomes as a Potential Remedial Treatment for Alzheimer's Disease. Int. J. Nanomed.. 2022;17:185-201.
- [CrossRef] [Google Scholar]
- Hydroxy-α-sanshool Possesses Protective Potentials on H2O2-Stimulated PC12 Cells by Suppression of Oxidative Stress-Induced Apoptosis through Regulation of PI3K/Akt Signal Pathway. Oxid. Med. Cell. Longev.. 2020;2020:3481758.
- [CrossRef] [Google Scholar]
- Regulation of mitochondrial dysfunction induced cell apoptosis is a potential therapeutic strategy for herbal medicine to treat neurodegenerative diseases. Front. Pharmacol.. 2022;13:937289
- [CrossRef] [Google Scholar]
- Protective effects of hydroxy-α-sanshool from the pericarp of Zanthoxylum bungeanum Maxim. On D-galactose/AlCl3-induced Alzheimer's disease-like mice via Nrf2/HO-1 signaling pathways. Eur. J. Pharmacol.. 2022;914:174691
- [CrossRef] [Google Scholar]
- Lignans and other constituents from South and central american zanthoxylum species. Planta Med.. 1990;56:89-91.
- [CrossRef] [Google Scholar]
- Memory and learning-enhancing effect of Daikenchuto, a traditional Japanese herbal medicine, in mice. J. Nat. Med.. 2006;60:64-67.
- [CrossRef] [Google Scholar]
- Cannabidiol as a Therapeutic Target: Evidence of its Neuroprotective and Neuromodulatory Function in Parkinson's Disease. Front. Pharmacol.. 2020;11:595635
- [CrossRef] [Google Scholar]
- Paeoniflorin is a promising natural monomer for neurodegenerative diseases via modulation of Ca2+ and ROS homeostasis. Curr. Opin. Pharmacol.. 2022;62:97-102.
- [CrossRef] [Google Scholar]
- Whether short peptides are good candidates for future neuroprotective therapeutics? Peptides. 2021;140:170528
- [CrossRef] [Google Scholar]
- Phytochemical and anti-neuropathic investigations of Crocus sativus via alleviating inflammation, oxidative stress and pancreatic beta-cells regeneration. Chin Herb Med.. 2019;12:47-55.
- [CrossRef] [Google Scholar]
- Neuroprotective Strategies for Neurological Disorders by Natural Products: An update. Curr. Neuropharmacol.. 2019;17:247-267.
- [CrossRef] [Google Scholar]
- Compounds from Sichuan and Melegueta peppers activate, covalently and non-covalently, TRPA1 and TRPV1 channels. Br. J. Pharmacol.. 2009;157:1398-1409.
- [CrossRef] [Google Scholar]
- Mechanism of atropine-resistant contraction induced by Dai-kenchu-to in guinea pig ileum. Jpn. J. Pharmacol.. 2001;86:32-37.
- [CrossRef] [Google Scholar]
- Gx-50 Inhibits Neuroinflammation via α7 nAChR Activation of the JAK2/STAT3 and PI3K/AKT Pathways. J. Alzheimers Dis.. 2016;50:859-871.
- [CrossRef] [Google Scholar]
- Pungent qualities of sanshool-related compounds evaluated by a sensory test and activation of rat TRPV1. Biosci. Biotech. Bioch.. 2005;69:1951-1957.
- [CrossRef] [Google Scholar]
- A novel drug candidate for Alzheimer's disease treatment: gx-50 derived from Zanthoxylum bungeanum. J. Alzheimers Dis.. 2013;34:203-213.
- [CrossRef] [Google Scholar]
- GSK-3/CREB pathway involved in the gx-50's effect on Alzheimer's disease. Neuropharmacology. 2014;81:256-266.
- [CrossRef] [Google Scholar]
- Ben Cao Xing Chang. In: Chinese Materia Medica. Beijing: China Publishing House; 1999.
- [Google Scholar]
- Characterization of isobutylhydroxyamides with NGF-potentiating activity from Zanthoxylum bungeanum. Bioorg. Med. Chem. Lett.. 2016;26:338-342.
- [CrossRef] [Google Scholar]
- Shao Xing Ben Cao Jiao Zhu. Beijing: Chinese Medicine Ancient Books Publishing House; 2007.
- Antiobesity, Regulation of Lipid Metabolism, and Attenuation of Liver Oxidative Stress Effects of Hydroxy-α-sanshool Isolated from Zanthoxylum bungeanum on High-Fat Diet-Induced Hyperlipidemic Rats. Oxid. Med. Cell. Longev.. 2019;2019:5852494.
- [CrossRef] [Google Scholar]
- Network Pharmacology-Based Strategy for the Investigation of the Anti-Obesity Effects of an Ethanolic Extract of Zanthoxylum bungeanum Maxim. Front. Pharmacol.. 2020;11:572387
- [CrossRef] [Google Scholar]
- CREB-regulated transcription coactivator 1: important roles in neurodegenerative disorders. Sheng Li Xue Bao. 2015;67:155-162.
- [Google Scholar]
- Aroma constituents and alkylamides of red and green huajiao (Zanthoxylum bungeanum and Zanthoxylum schinifolium) J. Agric. Food Chem.. 2008;56:1689-1696.
- [CrossRef] [Google Scholar]
- Polyphenolics composition of the leaves of Zanthoxylum bungeanum Maxim. grown in Hebei, China, and their radical scavenging activities. J. Agric. Food Chem.. 2013;61:1772-1778.
- [CrossRef] [Google Scholar]
- Study on isobutyl amide compounds from Zanthoxylum bungeanum and their activities against ferroptosis in HT22 hippocampal cells. Nat. Prod. Res. Develop.. 2020;32:6.
- [Google Scholar]
- Zanthoxylum bungeanum Maxim. (Rutaceae): A Systematic Review of Its Traditional Uses, Botany, Phytochemistry, Pharmacology, Pharmacokinetics, and Toxicology. Int. J. Mol. Sci.. 2017;18:2172.
- [CrossRef] [Google Scholar]
- Hydroxy-α-sanshool isolated from Zanthoxylum bungeanum attenuates learning and memory impairments in scopolamine-treated mice. Food Funct.. 2019;10:7315-7324.
- [CrossRef] [Google Scholar]
- Comparative studies on flavor substances of leaves and pericarps of Zanthoxylum bungeanum Maxim. at different harvest periods. Tropical J Pharmaceutical Res.. 2019;18:279-286.
- [CrossRef] [Google Scholar]







