Translate this page into:
An appraisal of Luffa aegyptiaca extract and its isolated triterpenoidal saponins in Trichinella spiralis murine models
⁎Corresponding author at: Medicinal Chemistry Department, Theodor Bilharz Research Institute, Kornaish El-Nile, Warrak El-Hadar, Imbaba (P.O. 30), Giza 12411, Egypt. m.ghareeb@tbri.gov.eg (Mosad A. Ghareeb),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract

Abstract
Trichinella spiralis is an intestinal and tissue parasitic nematode, emerging and re-emerging causative agent of a serious foodborne parasitic infection. This study aimed to evaluate the effect of Luffa aegyptiaca leaf extract and its triterpene glycosides on the intestinal and muscle stages of T. spiralis infection in vitro and in vivo. Phytochemical investigations of the extract led to the isolation of five compounds, namely (1) 3-O-β-d-glucopyranosyl-16-O-β-hydroxyolea12-en 23, 28-β-d-diglucopyranoside ester, (2) 3β-hydroxylolea12-en-28-oic acid (Oleanoic acid), (3) oleanolic acid 3-O-α-l-rhamnopyranosyl-(1 → 4)-β-d-glucopyranoside, (4) 3-O-β-d-glucopyranosyl-28-β-d-glucopyranosyl oleanolate, and (5) stigmast-5, 22-dien-3-O-β-d-glucopyrano-side. Moreover, the in vitro study showed marked degeneration and destruction of adult worms and larval teguments with tested drugs. Also, in the in vivo study, mice were divided into six groups; group I: infected and untreated, group II: received leaf extract as prophylaxis, group III: infected and treated with leaf extract, group IV: treated with compound (4), group V: treated with compound (1), and group VI: treated with albendazole. Furthermore, the treatment efficacy was assessed by the adult and total larval counts, histopathological study of the small intestinal and muscle tissues, and immunohistochemical staining of CD34 in muscles. The results revealed a significant reduction of total adult and larval counts in prophylactic and treated groups compared to the positive control group, with a reduction of total adult count by 63.48% and 74.4% in compound (1) and compound (4) treated groups, respectively. Also, a reduction was detected in larval counts by 36.5%, and 93.6% in compound (1) and compound (4) treated groups during both the muscular and intestinal phases, respectively.
Additionally, histopathological examination of the small intestine and muscles showed marked improvement with a reduction in the inflammatory infiltrates in treated groups. CD34 expressions were reduced in treated groups with more reduction in compound (4) treated group. In conclusion, this study implies that L. aegyptiaca leaf extract and its tested triterpene glycosides might be used for anti-trichinellosis treatments.
Keywords
Trichinella spiralis
Luffa aegyptiaca
Triterpenoidal saponins
Albendazole
Inflammation
CD34
- 1H NMR:
-
Proton Nuclear Magnetic Resonance
- 13C NMR:
-
Carbon-13 Nuclear Magnetic Resonance
- 2D NMR:
-
Two-dimension Nuclear Magnetic Resonance
- CC:
-
Column Chromatography
- DMSO:
-
Dimethylsulfoxide
- HMBC:
-
Heteronuclear Multiple Bond Connectivities
- HSQC:
-
The Heteronuclear Single Quantum Coherence
- NMR:
-
Nuclear Magnetic Resonance
- TLC:
-
Thin Layer Chromatography
- TMS:
-
Tetramethylsilane
- UV:
-
Ultra-Violet
Abbreviations
1 Introduction
It is not uncommon that natural compounds possess anti-parasitic activity, suggesting that plant products might supplement or eventually replace chemical medications in treating human and animal parasites. This has resulted in a never-ending search for safer and more effective medicines, particularly those derived from natural sources. Plants have long been a major target for innovative therapeutic substances, as evidenced by the huge research on medicinal plants documented in scientific databases (Rocha et al., 2005; Wright, 2010; Izumi et al., 2011; Ibrahim et al., 2014).
Trichinella spp. is unique among helminthes in that the parasite, infective larvae, and adult all develop in the same host. Trichinella spiralis is a microscopic human nematode parasite that unusually forms a small cystic structure within the afflicted host. Trichinellosis is a major health issue; it is a serious zoonotic disease that infects humans by consuming improperly cooked meat infected with the T. spiralis larvae. Then larvae are released from the nurse cell due to the acidic pH of the stomach and migrate into the small intestine, where they enter the intestinal mucosa, molt, and then become adults. Adult male and female worms mate and give birth to newborn larvae, which travel from the small intestine to the striated muscle via the circulatory system. They enter individual muscle cells, transforming them into specialized cysts known as nurse cells, infecting other hosts (Panigrahi et al., 2017). The first symptom is gastrointestinal irritation, followed by periorbital edema, muscle pain, fever, and eosinophilia. Stool examination is used to identify adult male, female, and rare larvae later confirmed by serologic tests, while muscle biopsy is used to diagnose the presence of encysted larvae. Human trichinellosis was treated with anthelmintic medications, mainly benzimidazole derivatives (such as albendazole and mebendazole). However, these medications are not strong enough to kill encysted larvae (Shalaby et al., 2010; Attia et al., 2015). Some had a high level of resistance and were banned for children under three and during pregnancy, while others are suspected of being carcinogenic (Yadav, 2006). These findings highlight the critical need for a new, safe, and effective treatment of Trichinella spp. infection.
The plant Luffa aegyptiaca is a member of the Cucurbitaceae family, commonly called sponge gourd and loofa (Partap et al., 2012). It is a vigorous climbing annual vine with numerous lobed cucumber-like fruits. The fruits were also cucumber-like in shape when mature, with a network of fibers surrounding many flat blackish seeds. It is widely distributed in the tropics and subtropics as a cultivated or naturalized plant (Stephen, 2003). In Egypt, L. aegyptiaca is grown for medicinal and nutritional purposes (Lawal et al., 2010).
Different classes of phytochemicals have been isolated from the plant and showed notable bioactivities, including antimicrobial (Mankilik and Mikailu, 2014), cytotoxic (Rahman et al., 2014), anthelmintic (Rahman et al., 2014; Maamoun et al., 2021) and anti-inflammatory activities (Kakate, 1997). Phytochemical screening of L. aegyptiaca revealed that the plant possesses many bioactive phytochemicals such as saponins, alkaloids, flavonoids, tannins, sterols, and glycosides (Mhya and Mankilik, 2014). L. aegyptiaca is a widely known edible plant with anthelmintic (Rahman et al., 2014) and anti-parasitic activities (Othman and Shoheib, 2016). Tannins and triterpenoid saponins, two of the most active ingredients in the L. aegyptiaca fruit, showed a broad spectrum of biological activities. The anthelmintic effect of tannins from L. aegyptiaca is well known. They can bind to free proteins in the host animal's gastrointestinal tract or glycoprotein on the cuticle, giving the parasite its shape, nutrition, and protection (Maestrini et al., 2020). They are also essential for osmoregulation (Thompson and Geary, 1995; Djurković-Djaković et al., 2013), so this damage may cause death. Moreover, pentacyclic triterpenoids are a class of plant-derived components that have beneficial and negative effects on various organisms; however, their effect on cell structure remains uncharacterized (Isah et al., 2016). Nevertheless, saponins offer enormous potential as therapeutic agents due to their detergent properties, ability to increase cell permeability, and their cytotoxic and cytostatic activities (Francis et al., 2002). Verifying new safe and efficient anti-trichinellosis drugs is an urgent need, and a drug of plant origin may be a promising opportunity. Therefore, this study aimed to assess the therapeutic potential of L. aegyptiaca leaf extract and its triterpenoid glycosides against experimental Trichinella spiralis infection in mice.
2 Materials and methods
2.1 Experimental procedures
NMR analysis was performed using Bruker AscendTM 850 spectrometer operating at 850, 500, and 400 MHz for 1H; 213, 125, and 100 MHz for 13C. All compounds have been prepared in deuterated dimethyl sulfoxide (DMSO‑d6). Tetramethylsilane (TMS) was utilized as the internal reference. Chemical shifts were expressed in δ ppm while coupling constant (J) was expressed in Hz. The ESI-MS were recorded on an Agilent instrument (Santa Clara, USA). Column chromatography was carried out on silica gel 60 (0.060–0.200 mm, 70–230 mesh). Sephadex LH-20 (Sigma Aldrich, Germany), precoated TLC silica gel 60G F₂₅₄ sheets (Sigma Aldrich, Germany) were used to check the purity of compounds, and detection was accomplished by visualizing with a UV lamp at 254 and 365 nm, followed by spraying with a solution of 10% ethanol/H2SO4 (v/v) on TLC and then heating at 120 °C.
2.2 Plant collection, extraction, and chromatographic isolation
Luffa aegyptiaca leaves were collected in May 2018 from Bilbies, Ash Sharqia Governorate, Egypt (31.11660 ◦N and 30.63333 ◦E). The plant material was collected in compliance with relevant institutional, national, and international guidelines and legislation. The planters approved the permit for the collection of leaf specimens. The collected sample was kindly authenticated by Dr. Therisa Labib, consultant of plant taxonomy at Orman Botanical Garden, Giza, Egypt. A voucher specimen (Reg. No. La 37) was deposited at the herbarium of the Pharmacognosy Department, Faculty of Pharmacy (for Girls), Al-Azhar University, Cairo, Egypt. Air-dried powdered L. aegyptiaca leaf (1 kg) was macerated with 70% methanol at room temperature (4 × 3 L). The combined methanol extract was evaporated under reduced pressure at 60 °C. The sticky brown residue from the total extract (120 g) was defatted by methylene chloride to obtain a 40 g methylene chloride fraction and 80 g residue (Mohammed et al., 2019). The residue was chromatographed on a silica column; elution was started with methylene chloride followed by methylene chloride/methanol mixture and increased polarities up to 100% methanol. Fractions were collected, subjected to TLC, and examined by VIS, UV, and spray reagents visualization; similar fractions were collected. Fractions were subjected to successive column chromatography on Sephadex LH-20, silica gel with different solvent systems, and subsequently purified to yield five pure compounds.
2.3 Animals, parasites, and materials
Swiss albino male mice aged 5–6 weeks and weighing 20–25 g (at the beginning of the experiment) were obtained from the Schistosome Biological Supply Centre (SBSC), Theodor Bilharz Research Institute (TBRI), Giza, Egypt. The mice were kept on a standard commercial pelleted diet with free accessible water and good sanitary conditions throughout the study. All procedures were conducted in adherence to the Guide for the Care and Use of Laboratory Animals (Eighth edition) of the National Institutes of Health and approved by the Research Ethics Committee of the Faculty of Pharmacy, Al-Azhar University, Egypt, for the conduct of animal experiments (IRB#339). The strain of T. spiralis was obtained from the Parasitology Department, TBRI. Swiss albino mice were orally infected with 200 T. spiralis larvae (Wassom et al., 1988; Abou Rayia et al., 2017). Albendazole was purchased as Bendax 200 mg tablets from Sigma Pharmaceutical Industries, Egypt. T. spiralis adult worms and muscle larvae were isolated from the infected mice according to reported procedures (Ozkoc et al., 2009). Animals fasted overnight with free access to water. Scarification was performed on day 7 post-infection for obtaining adult T. spiralis in the intestinal phase and on day 35 for obtaining larvae in the muscular phase. Muscles were separated and minced, and muscle larvae were digested by immersion in the acid pepsin solution (Dennis et al., 1970). The mixture was incubated at 37 °C for 2 h, continuously mixing with an electric stirrer (Dunn and Wright, 1985). The digest was filtered according to the reported study (Kapel et al., 2005). The collected larvae were washed 2–3 times with tap water and suspended in a conical flask for half an hour to allow sedimentation. T. spiralis adult worms were isolated from the small intestines of infected untreated mice six days post-infection (p.i.). The intestine was washed, opened longitudinally along its entire length, cut into small pieces 2 cm each, and placed in normal saline at 37 °C for 3–4 h to allow the worms to migrate out of the tissue (Wakelin and Margaret, 1980).
2.4 In vitro experimental study
T. spiralis adult worms (30 parasites per well) were cultured in a 24-well tissue culture plate prepared with an incubation medium consisting of RPMI-1640 Medium (containing 20% fetal bovine serum, 200 U/ml penicillin, and 200 μg/ml streptomycin). Five groups were established in this study. Group I contains adult worms cultured in the incubation medium only. In contrast, in group II, adult worms were cultured in the incubation medium containing leaf extract dissolved in dimethyl sulfoxide (DMSO) at a concentration of 100 μg/ml (Vila-Nova et al., 2013). In group III, adult worms were cultured in the incubation medium containing albendazole dissolved in DMSO at a concentration of 100 μg/ml (Tritten et al., 2012); in group IV, larvae of T. spiralis were cultured in the incubation medium containing leaf extract. For group V, larvae of T. spiralis were cultured in the incubation medium containing albendazole that was dissolved in DMSO at a concentration of 100 μg/ml. Three wells were used for each group, and the plate was placed in the incubator at 37 °C and 5% carbon dioxide for 24 h. The same in vitro study groups were conducted using compounds (1) and (4), then after 24 h, the adult worms and larvae were collected for scanning electron microscopic study.
2.5 Scanning electron microscopy (SEM)
After 24 h, adult worms and larvae were processed according to reported procedures (Bughdadi, 2010). Worms from each group were directly pipetted and immediately fixed in a fresh fixation solution of 2.5% glutaraldehyde solution buffered with 0.1 M sodium cacodylate at pH = 7.2 and left overnight at 4 °C. The fixed specimens were then washed in 0.1 M sodium cacodylate buffer at pH = 7.2 for 5 min, post-fixed in 2% osmium tetroxide for 1 h, and washed with distilled water. The specimens were dehydrated in ascending grades of ethyl alcohol, mounted on carbon-coated adhesive material, and examined using a Jeol scanning electron microscope (Jeol GSM- IT200, Japan), Electronic Microscope Unit, Faculty of Science, Alexandria University.
2.6 In vivo experimental study
In the in vivo study, mice (n = 108) were divided into six groups, with 18 in each. Group I; infected non-treated (control positive), group II; receiving leaf extract for seven days before infection (as prophylaxis), group III; infected and treated with leaf extract (given orally at a dose of 100 mg/kg) and dissolved in distilled water (Saxena et al., 2011), group IV; infected mice were treated with compound (4) according to reported studies (Ferreira et al., 2013), group V; infected mice were treated with compound (1), and group VI; infected and treated with albendazole (given orally at a 50 mg/kg dose and suspended in 10% tween 80 and 90% deionized water just before oral administration) (Attia et al., 2015). Each is divided into three subgroups (a, b, and c); each comprises six animals to assess the drug's effect during the intestinal phase only. Subgroup (a) was 3–5 days p.i. and muscular phase only, (b) was 30–32 days p.i. and intestinal then muscular phases, and (c) was 3–5 days p.i. and 30–32 days p.i. separately.
2.7 Histopathological studies
Parts of the intestine and skeletal muscles from the studied groups were fixed in 10% formalin for 24 h, washed in water for 12 h, dehydrated in ascending grades of alcohols, and cleared in xylene. Impregnation was performed in pure soft paraffin for 2 h at 55 °C. Then, hard paraffin sections of 5 μm thickness were cut by microtome. Sections were stained with hematoxylin and eosin (Drury and Wallington, 1980). Sections from the intestine were histopathologically examined for the presence or absence of Trichinella larva, any villous abnormality, and intensity of inflammatory cellular reaction. Sections from skeletal muscles were examined for the presence of Trichina capsule, counted, and comments on the integrity of the capsule, the content, and the inflammatory cellular reaction were reported in each group.
2.8 Immunohistochemical (IHC) technique
Paraffin sections from skeletal muscle specimens underwent de-paraffinization and rehydration. Antigen retrieval was performed by microwaving the sections in citrate buffer, pH = 6.0. Endogenous peroxidase was blocked with methanol containing 3% hydrogen peroxide. Sections were incubated overnight at 4 °C in a humid chamber with the primary antibodies: anti-CD34 antibody, Clone QBEnd-10, and Mouse anti-Human (Supplier: Dako; Catalog Number: M7165), in an optimal dilution of 1:50–1:100, followed by application of secondary antibody (Biotin-streptavidin link) (DAKO). Then, the antigen was localized by adding 3,3′diaminobenzidine tetrahydrochloride (DAB) substrate chromogen solution (Universal Detection Kit, Dako Envision, Denmark). Finally, slides were counterstained with hematoxylin (El-Wakil et al., 2022), dehydrated in alcohol, and mounted. For each setting, positive and negative control slides were included as a negative control, and skeletal muscle tissue was processed in the sequences mentioned above. However, the primary antibodies were not added; instead, non-immune immunoglobulin G (IgG; DAKO, Glostrup, Copenhagen, Denmark) was added.
2.9 Interpretation of immunostaining and scoring analysis
Immunohistochemical analysis of skeletal muscle tissue sections was blind quantified by two pathologists. The sections were examined using a light microscope (Scope A1, Axio, Zeiss, Germany). Photomicrographs were taken using a microscope camera (AxioCam, MRc5, Zeiss, Germany). CD34 positivity was considered when the cytoplasm of endothelial cells got brownish discoloration. The percentage of positive cells was calculated in 5 HPF, and the intensity of the color was graded from 1 + to 3 +.
2.10 Statistical analysis
The data were analyzed using Microsoft Excel 2016 and the statistical package for social science IBM SPSS Statistics for Windows, version 26 (IBM Corp., Armonk, New York, USA). Continuous normally distributed variables were represented as mean ± standard deviation, and a P-value < 0.05 was considered statistically significant. The student's t-test was performed to compare the means of normally distributed variables between groups.
3 Results
3.1 Chemical constituents of L. aegyptiaca leaf extract
Chromatographic isolation of the defatted 70% alcoholic extract of L. aegyptiaca leaves led to the isolation of five off-white compounds needle crystals. From their chromatographic properties, compounds from 1 to 5 gave violet spot response to 10% ethanol/H2SO4 on TLC at 120 °C as well as a positive Salkowski's test for compounds (1–4) to deduce them as triterpenoid saponins. In contrast, compound (5) gave a negative test to deduce it as a steroid. The isolated compounds were identified as (1) 3-O-β-d-glucopyranosyl-16-O-β-hydroxyolea12-en 23, 28-diglucopyranoside ester, (2) 3β-hydroxylolea12-en-28-oic acid (Oleanoic acid), (3) oleanolic acid 3-O-α-l-rhamnopyranosyl-(1 → 4)-O-β-d-glucopyranoside, (4) 3-O-β-d-glucopyranosyl-28-β-d-glucopyranosyl oleanolate, and (5) stigmast-5, 22-dien-3-O-β-d-glucopyranoside, which were elucidated by using spectroscopic techniques as 1D, 2D NMR and ESI-MS (Fig. 1, Table 1, and Supplementary materials: Fig. 1S − 13S. Compounds (1) and (4) were isolated for the first time from L. aegyptiaca.
Structures of phytoconstituents isolated from L. aegyptiaca leaf extract.
Compound 1
Compound 4
No.
13C NMR/
DEPT-135
(213 MHz)
1H NMR
(850 MHz)
APT
(100 MHz)
1H NMR
(400 MHz)
1
45.98 (CH2) (t)
41.21 (CH2) (t)
2
23/48 (CH2) (t)
26.06 (CH2) (t)
3
78.20 (CH) (d)
4.02 (dd, J = 6.8, 14.45 Hz)
78.20 (CH) (d)
4.42 (dd, J = 5.6, 11.2 Hz)
4
46.38 (Q) (s)
46.38 (Q) (s)
5
55.14 CH) (d)
55.21 CH) (d)
6
18.25 (CH2) (t)
22.41 (CH2) (t)
7
32.05 (CH2) (t)
33.33 (CH2) (t)
8
39.39 (Q) (s)
39.37 (Q) (s)
9
47.48 CH) (d)
47.63 CH) (d)
10
37.51 (Q) (s)
37.52(Q) (s)
11
22.95 (CH2) (t)
23.48 (CH2) (t)
12
122.02 (CH) (d)
5.18, t
122.03 (CH) (d)
5.18, t
13
143.97 (Q) (s)
143.98 (Q) (s)
14
40.40 (Q) (s)
40.62 (Q) (s)
15
32.61 (CH2) (t)
32.35 (CH2) (t)
16
65.93 (CH) (d)
4.35 brs
22.96 (CH2) (t)
17
41.77 (Q) (s)
41.77 (Q) (s)
18
41.20 (CH) (d)
2.75 (dd, J = 13.6, 3.0 Hz)
41.21 (CH) (d)
2.75 (dd, J = 13.6, 3.0 Hz)
19
46.98 (CH2) (t)
46.38 (CH2) (t)
20
30.78 (Q) (s)
30.78 (Q) (s)
21
33.68 (CH2) (t)
33.65 (CH2) (t)
22
27.62 (CH2) (t)
27.60 (CH2) (t)
23
170.83 (Q) (s)
28.34 (CH3) (q)
α 1.05 (s)
Compound 1
Compound 4
No.
13C NMR/
DEPT-135
(213 MHz)
1H NMR
(850 MHz)
APT
(100 MHz)
1H NMR
(400 MHz)
24
14.56 (CH3) (q)
β 1.04 (s)
16.76 (CH3) (q)
β 0.69 (s)
25
16.75 (CH3) (q)
β 0.91 (s)
17.14 (CH3) (q)
β 0.80 (s)
26
18.09 (CH3) (q)
β 0.87 (s)
18.09 (CH3) (q)
β 0.89 (s)
27
25.97 (CH3) (q)
α 1.11 (s)
25.97 (CH3) (q)
α 1.09 (s)
28
175.68 (Q) (q)
175.67 (Q) (s)
–
29
23.81 (CH3) (q)
α 0.88 (s)
23.82 (CH3) (q)
α 0.88 (s)
30
33.20 (CH3) (q)
β 0.79 (s)
33.20 (CH3) (q)
β 0.92 (s)
No.
Compound 1 Compound 4
3β-O-glucopyranosyl 3β-O-glucopyranosyl
1
105.34 (CH)
4.17
(d, J = 7.65 Hz)105.3 (CH)
4.18 (d, J = 8 Hz)
2
74.30 (CH)
3.00–3.90
71.7 (CH)
3.00–3.90
3
77.11 (CH)
77.1 (CH)
4
70.33 (CH)
70.02 (CH)
5
77.11 (CH) (d)
73.31(CH)
6
60.23 (CH2)
61.30 (CH2)
23β-COO glucopyranosyl
28 β-COO-glucopyranosyl
1
94.54 (CH) (d)
5.29 (d, J = 7.65 Hz)
94.5(CH)
5.25 (d, J = 8 Hz)
2
72.82 (CH) (d)
3.00–3.90
72.83(CH) (d)
3.00–3.90
3
77.16 (CH) (d)
77.17 (CH) (d)
4
69.99 (CH) (d)
70.35 (CH) (d)
5
77.16 (CH) (d)
74.3 (CH) (d)
6
61.12 (CH2) (t)
61.14 (CH2) (t)
28 β-COO-glucopyranosyl
1
95.02 (CH) (d)
5.245 (d, J = 7.65 Hz)
2
72.82(CH) (d)
3.00–3.90
3
77.16 (CH) (d)
4
69.99 (CH) (d)
5
77.16 (CH) (d)
6
61.24 (CH2) (t)
3.2 Scanning electron microscope (SEM) findings of the adult T. spiralis
In the infected control group, when cultured in the incubation medium only, the cuticle of the adult worm teguments retained the normal structure in the form of ridges, transverse creases, and annulations, with the appearance of openings of the hypodermal gland. In leaf extract groups, adult worms showed shrinking, sloughing, and loss of the normal annulations of the cuticle. The compound (4) group showed marked destruction of the adult worm cuticle and complete sloughing of some cuticle areas. In the compound (1) group, the adult worms showed loss of the normal annulations and multiple fissures in the cuticle; the albendazole group showed destruction of the adult worms and sloughing with loss of annulations. The destruction of the cuticle was more severe and obvious with the compound (4) group (Fig. 2).
Scanning electron microscope findings of the cultured T. spiralis adult worm: (A); infected control group showing adult worm with intact annulated cuticle, (B); infected control group showing normal adult worm cuticle with hypodermal glands openings, (C); infected control group showing normal adult worm cuticle with tapering end, (D); leaf extract group showing shrunk T. spiralis adult, sloughing of some areas of the cuticle (yellow arrow) and loss of the normal annulations in the cuticle, (E & F); compound (4) group showing marked destruction of the adult worm cuticle and sloughing of some areas of the cuticle (red arrows), (G); compound (1) group, adult worms showed loss of the normal annulations of the cuticle and multiple fissures in the cuticle (blue arrows) are seen, and (H); albendazole group showing the destruction of the adult worms and sloughing (yellow arrows) with loss of annulations.
3.3 SEM findings of T. spiralis larval stage
The infected control group showed a coiled posterior end with intact cuticular folds, transverse striations, and shallow longitudinal grooves. The leaf extract group showed shrunken T. spiralis larva, destructed and sloughed cuticle with loss of the normal annulations in the cuticle. In the compound (4) group, larval cuticles showed large blebs and multiple small vesicles. In the compound (1) group, larval cuticles showed multiple vesicles with a loss of normal cuticular folds and striations. The albendazole group showed large blebs with loss of the normal cuticular folds and striations (Fig. 3).
Scanning electron microscope findings of the cultured T. spiralis larvae: A; infected control group showing normal comma-shaped larva with intact cuticle and coiled posterior end, B; infected control group showing larva with intact cuticular folds, transverse striations, and shallow longitudinal grooves, C; leaf extract group showing shrunked T. spiralis larva, destructed, and sloughed cuticle (yellow arrow) with loss of the normal annulations in the cuticle, D & E; compound (4) group larval cuticle showing large blebs (blue arrow) and multiple small vesicles formation (red arrow), F; compound (1) group larval cuticle showed areas with multiple vesicles (red arrows) with loss of annulations, and G & H; albendazole group showing large blebs (blue arrows) with loss of the normal cuticular folds and striations.
3.4 In vivo studies
3.4.1 Adult worm count in the small intestine
Prophylactic treatment of the infected mice by the extract from leaf extract (G-II) significantly reduced the mean adult worm count (47 ± 5.65) with an efficacy of 47.19% compared to the control infected untreated group (G-I) (89 ± 8.49) (P < 0.05). A significant decrease in the mean number of adult worms was obtained in all treated groups compared to the control-infected untreated group (P < 0.05). Among the tested extract and compounds, the least mean adult count was found in G-IVa, which received compound (4) therapy (22.5 ± 6.36) and showed the most effective eradication of T. spiralis adult worms, with a drug efficacy of 74.72%, followed by G-IIIa, which received leaf extract (28.5 ± 12.02) with the efficacy of 67.98%. In comparison, the mean adult worm count was (32.5 ± 0.71) in G-Va, which received compound (1) with a satisfactory reduction of 63.48% (Table 2). P-value is significantly different compared with the control, depending on the Student t-test. *Initial P-value < 0.05 is significant. **Initial P-value < 0.01 is highly significant. Group-I: Positive control, Group-II: prophylactically treated with leaf extract for seven days before infection, Group-IIIa: infected and treated with leaf extract, Group-Iva: infected and treated with compound (4), Group-Va: infected and treated with compound (1), and Group VIa: infected and treated with albendazole. The drugs were given during the (a) intestinal phase only (3–5 days p.i.).
Intestinal phase groups
Mean ± SD
P-value
% Reduction
Group-I
89 ± 8.49
–
–
Group-II
47 ± 5.65
< 0.05*
47.19
Group-IIIa
28.5 ± 12.02
< 0.05*
67.98
Group-Iva
22.5 ± 6.36
< 0.05*
74.72
Group-Va
32.5 ± 0.71
< 0.05*
63.48
Group-Via
7 ± 1.41
< 0.01**
92.13
3.5 Encysted larvae count in muscles
As regards the effect of the drug on the muscular phase, the prophylactic treatment of the infected mice by leaf extract revealed a significant reduction with P < 0.01, and the mean larval count was (897 ± 104.9) per gram of muscle with an efficacy of 31.1% compared to the control infected untreated group (1302 ± 146.7). Also, a significant decrease in the mean larval count was detected in all treated groups with P < 0.01 compared to the control-infected untreated group. To compare the drug effects on the muscular phase, the drugs were given in two regimens, either giving the drugs for a single dose in the muscular phase only or giving the drugs for two doses, one dose in the intestinal phase and a second dose of the same drugs during the muscular phase. The larvae eradication was better in groups that received the drugs in both intestinal and muscular phases than in groups that only received the drug in the muscular phase. As regards the two doses regimen, the best reduction of the mean larval count in the tested extract and isolated compounds were found in G-IVc, which received compound (4) therapy (82.6 ± 15.7) with an efficacy of 93.6%, followed by the mice group (G-IIIc) that received leaf extract (399.3 ± 56.8) with the efficacy of 69.3%. In the mice group that received compound (1) (G-Vc), the mean larval count was 659 ± 84.3, with a 49.4% reduction. Concerning the single-dose regimen, the best reduction of the larval count was observed in G-IVb, which received compound (4) therapy (136.3 ± 8.7) with an efficacy of 89.5%. Followed by the mice group (G-IIIb) that received leaf extract, with mean larval counts of 413.3 ± 33.16 and percentages of reduction of 67.15%, followed by the mice group that received compound (1) (G-Vb), the mean larval count was 827 ± 40.6 with a 36.5% reduction (Table 3). P-value is significantly different compared with the control, depending on the Student t-test. *Initial P-value < 0.05 is significant. **Initial P-value < 0.01 is highly significant. Group-I: Positive control, Group-II: prophylactically treated with leaf extract for seven days before infection, Group-III: infected and treated with leaf extract, Group-IV: infected and treated with compound (4), Group-V: infected and treated with compound (1), and Group-VI: infected and treated with albendazole. The drugs were given during the (b) muscular phase only (30–32 days p.i.); and (c) intestinal then muscular phases (3–5 days p.i. and 30–32 days p.i.) separately.
Muscular phase groups
Mean ± SD
P-value
% Reduction
Group-I
1302 ± 146.7
–
–
Group-II
897 ± 104.9
0.01 **
31.1
Group-IIIb
413.3 ± 33.16
0.001***
67.15
Group-IIIc
399.3 ± 56.8
0.001***
69.3
Group-IVb
136.3 ± 8.7
0.001***
89.5
Group-IVc
82.6 ± 15.7
0.001***
93.6
Group-Vb
827 ± 40.6
0.01**
36.5
Group-Vc
659 ± 84.3
0.01**
49.4
Group-VIb
70.7 ± 13.7
0.001***
94.6
Group-Vic
61.3 ± 5.7
0.001***
95.3
3.6 Histopathological results
3.6.1 Small intestine changes
Histopathological examination of sections from the small intestine of the infected control group (G-I) showed dense intervillous inflammatory cellular infiltration consisting of mononuclear cellular infiltrate in the form of lymphocytes and plasma cells. There was broadening and atrophy of the intestinal villi with crypt hyperplasia. Moreover, fragments of the adult worms were detected within the intestinal lumen (Fig. 4a). Intestinal sections of Group II that received prophylactic treatment before infection showed occasional Trichina capsules and mostly preserved villous pattern (Fig. 4b). As regards results of the sections examined from the treated groups, an evident decrease in the intensity of the inflammatory cellular infiltration was observed, together with remarkable improvement of the other histopathological changes of the intestine, as in sections in the intestine of Group IIIa, which treated with leaf extract, showed absence of Trichina capsules with mostly preserved villous pattern and mild inflammation (Fig. 4c). Also, the normal villous pattern in G-IVa (Fig. 4d) was returned with a mostly preserved villous pattern. In compound (1) treated group (G-Va) showed a mostly preserved villous pattern (Fig. 4e) and absence of Trichina capsules and a mostly preserved villous pattern.
(a) Sections in the intestine of positive control mice Group I; show some Trichina capsules and a distorted villous pattern (H & E stain, upper X200; lower X400). (b) Sections in the intestine of Group II; prophylactic group, mice showing occasional Trichina capsules and a mostly preserved villous pattern (H & E stain, X200). (c) Sections in the intestine of Group IIIa; mice showing absence of Trichina capsules, a mostly preserved villous pattern, and mild inflammation (H & E stain, X200). (d) Sections in the intestine of group IVa; mice treated with compound (4) showing a mostly preserved villous pattern (H & E stain, X200). (e) Sections in the intestine of Group Va; mice treated with compound (1) showing a mostly preserved villous pattern (H & E stain, X200).
Skeletal muscle changes concerning the histopathological examination of muscular sections showed that the infected control group (G-I) revealed a massive number of encysted T. spiralis larvae present diffusely in the sarcoplasm of the muscles. It also revealed several chronic inflammatory cells in the form of lymphocytes, plasma cells, and histiocytes infiltrating muscle bundles and surrounding the encysted larvae (Fig. 5a). The muscles from the prophylaxis group (G-II) showed many Trichina capsules with focally degenerated capsules and pericapsular histio-lymphocytic inflammatory cellular infiltration (Fig. 5b).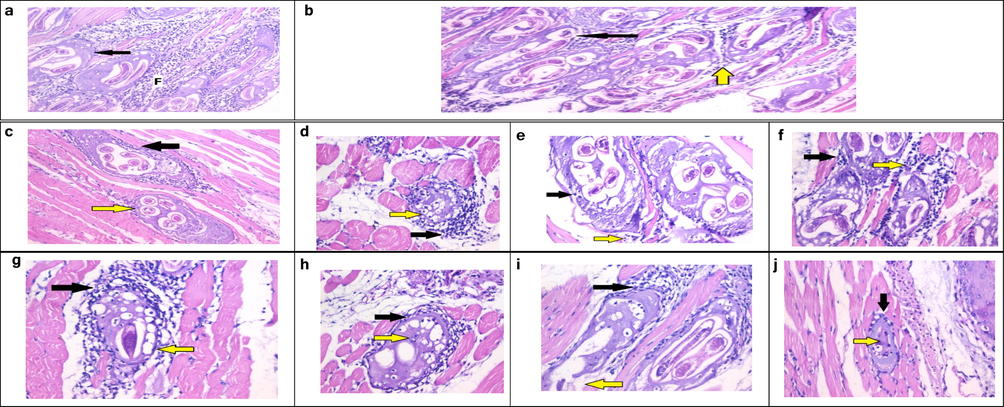
(a) Histopathological examination of sections from skeletal muscle of G-I, showing intact capsules (arrow) and pericapsular plasma-lymphocytic inflammatory cellular infiltration (F) (H & E stain, X200). (b) Histopathological examination of sections from skeletal muscle of G-II, showing many Trichina capsules with degenerated capsules (black arrow) invaded by many macrophages (yellow arrow) (H & E stain, X200). (c) Group IIIb, showing mild degeneration of the capsule (yellow arrow) surrounded and invaded by many macrophages (black arrow) (H & E stain, X200). (d) Group IVb, showing degenerated capsules (yellow arrow) and focal pericapsular plasma-lymphocytic inflammatory cellular infiltration (black arrow) (H & E stain, X400). (e) Group Vb, histopathological examination of sections from skeletal muscle of G-IIIc, showing degenerated capsules (yellow arrow) and focal pericapsular plasma lymphocytic inflammatory cellular infiltration (black arrow) (H&E stain, X400). (f) Group VIb, showing degenerated capsules (black arrow) and pericapsular plasma-lymphocytic inflammatory cellular infiltration (yellow arrow) (H & E stain, X 400). (g) Group IIIc, showing degenerated capsules (black arrow) and focal pericapsular plasma-lymphocytic inflammatory cellular infiltration (yellow arrow) (H & E stain, X400). (h) Group IVc, showing markedly degenerated capsules (black arrow) and larva with invasion by many macrophages (yellow arrow) H & E stain, X400). (i) Group Vc, showing focally degenerated capsules (yellow arrow) and dense pericapsular plasma-lymphocytic inflammatory cellular infiltration (black arrow) (H & E stain, X200). (j) Group VIc: Showing smaller Trichina capsule with degenerated content (yellow arrow) and mild pericapsular plasma-lymphocytic inflammatory cellular infiltration (black arrow) (H&E stain X200).
Concerning the histopathological examination of muscular sections from mice groups that received the drugs for a single dose in the muscular phase only (G- IIIb, G-IVb, and G-Vb), there were fewer Trichina capsules with focally degenerated capsules and dense pericapsular plasma-lymphocytic inflammatory cellular infiltration (Figs. 5c–f). Examination of muscular sections from mice groups that received the drugs for two doses (Fig. 5g-j) showed that one dose in the intestinal phase followed by a second dose of the same drugs during the muscular phase revealed marked improvement of the histopathological finding compared to the infected control.
3.7 Immunohistochemical findings
3.7.1 Immunostaining for CD34 (a marker for vascular endothelial cells)
The infected control group showed strong (+3) CD34 expression. The positive immunostaining was detected in the cytoplasm of the inflammatory cells (Fig. 6b). Regarding the prophylactic group treated with leaf extract before infection (Group II), sections in skeletal muscle showed dense expression of CD34 in skeletal muscles around the Trichina capsules (Fig. 6c). Concerning drug-receiving groups, CD34 expression was reduced to mild expression in compound (4) treated groups (Figs. 6f, 6g) and moderate in leaf extract-treated groups (Figs. 6d, 6e), with statistically significant differences. However, in compound (1) receiving groups, a significant reduction of the CD34 immunoreactivity was observed in Group Vc (Fig. 6i), which received the drug in both the intestinal and muscular phases. Nevertheless, the reduction in CD34 expression was non-significant in G-Vb (Fig. 6h), which received the compound (1) in the muscular phase only, and the prophylactic group (P > 0.05). CD34 expression was reduced to weak expression in the albendazole-treated group G-VIc (+1) (Fig. 6k) and moderate in the albendazole-treated group G-VIb (+2) (Fig. 6j); showing highly significant (P < 0.001) and significant (P < 0.05) differences, respectively (Table 4). P-value is significantly different compared with the control, depending on the Student t-test. *Initial P-value < 0.05 is significant. **Initial P-value < 0.01 is highly significant. Group I: positive control, Group II: prophylactically treated with leaf extract for seven days before infection, Group III: infected and treated with leaf extract, Group IV: infected and treated with compound (4), Group V: infected and treated with compound (1), and Group VI: infected and treated with albendazole. The drugs were given during the (b) muscular phase only (30–32 days p.i.); and (c) intestinal then muscular phases (3–5 days p.i. and 30–32 days p.i.) separately.
(a) A section in skeletal muscle of -ve control group showing negative expression of CD34, except in a few capillaries (arrows) (IHC for CD34, DAB, X400). (b) A section in skeletal muscle of + ve control group (G-I) showing dense expression of CD34 in skeletal muscles around Trichina capsules (arrows) (IHC for CD34, DAB, X200 (left), X400 (right)). (c) A section in skeletal muscle of Group II, showing dense expression of CD34 in skeletal muscles around Trichina capsules (arrows) (IHC for CD34, DAB, X400). (d) A section in skeletal muscle of G-IIIb, showing moderate expression of CD34 in skeletal muscles around Trichina capsules (arrows) (IHC for CD34, DAB, X400). (e) A section in skeletal muscle of G-IIIc, showing moderate expression of CD34 in skeletal muscles around Trichina capsules (arrow) (IHC for CD34, DAB, X400). (f) A section in skeletal muscle of G-IVb, showing mild expression of CD34 in skeletal muscle capillaries and around Trichina capsules (arrow) (IHC for CD34, DAB, X400). (g) A section in skeletal muscle of G-IVc showing mild expression of CD34 in skeletal muscles around degenerated Trichina capsules (arrow) (IHC for CD34, DAB, X400). (h) A section in skeletal muscle of G-Vb, showing dense expression of CD34 in skeletal muscle capillaries and around Trichina capsules (arrow) (IHC for CD34, DAB, X400). (i) A section in skeletal muscle of G-Vc, showing moderate expression of CD34 in skeletal muscles around Trichina capsules (arrow) (IHC for CD34, DAB, X400). (j) A section in skeletal muscle of G-VIb, showing very mild expression of CD34 in skeletal muscle capillaries and around degenerated Trichina capsules (arrow) (IHC for CD34, DAB, X400). (k) A section in skeletal muscle of G-Vic, showing scattered mild expression of CD34 in skeletal muscle capillaries around small degenerated Trichina capsules (arrow) (IHC for CD34, DAB, X400).
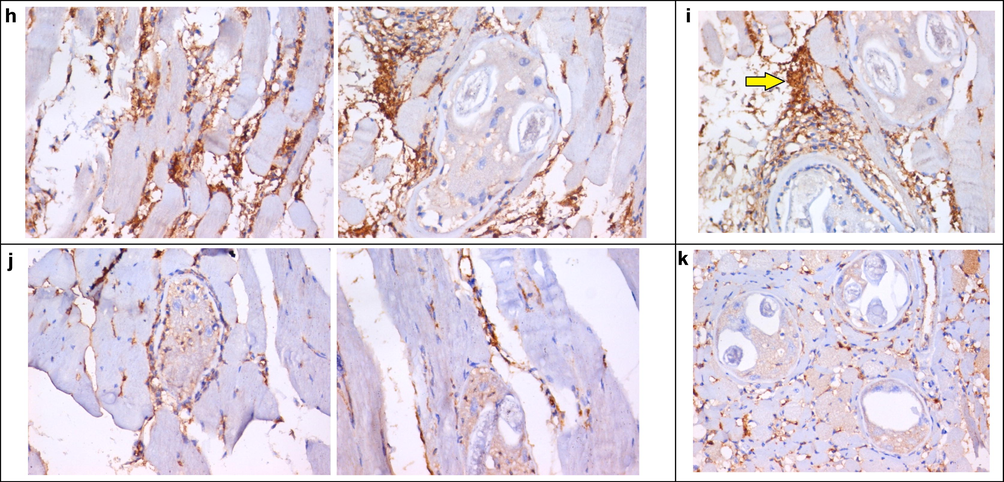
(a) A section in skeletal muscle of -ve control group showing negative expression of CD34, except in a few capillaries (arrows) (IHC for CD34, DAB, X400). (b) A section in skeletal muscle of + ve control group (G-I) showing dense expression of CD34 in skeletal muscles around Trichina capsules (arrows) (IHC for CD34, DAB, X200 (left), X400 (right)). (c) A section in skeletal muscle of Group II, showing dense expression of CD34 in skeletal muscles around Trichina capsules (arrows) (IHC for CD34, DAB, X400). (d) A section in skeletal muscle of G-IIIb, showing moderate expression of CD34 in skeletal muscles around Trichina capsules (arrows) (IHC for CD34, DAB, X400). (e) A section in skeletal muscle of G-IIIc, showing moderate expression of CD34 in skeletal muscles around Trichina capsules (arrow) (IHC for CD34, DAB, X400). (f) A section in skeletal muscle of G-IVb, showing mild expression of CD34 in skeletal muscle capillaries and around Trichina capsules (arrow) (IHC for CD34, DAB, X400). (g) A section in skeletal muscle of G-IVc showing mild expression of CD34 in skeletal muscles around degenerated Trichina capsules (arrow) (IHC for CD34, DAB, X400). (h) A section in skeletal muscle of G-Vb, showing dense expression of CD34 in skeletal muscle capillaries and around Trichina capsules (arrow) (IHC for CD34, DAB, X400). (i) A section in skeletal muscle of G-Vc, showing moderate expression of CD34 in skeletal muscles around Trichina capsules (arrow) (IHC for CD34, DAB, X400). (j) A section in skeletal muscle of G-VIb, showing very mild expression of CD34 in skeletal muscle capillaries and around degenerated Trichina capsules (arrow) (IHC for CD34, DAB, X400). (k) A section in skeletal muscle of G-Vic, showing scattered mild expression of CD34 in skeletal muscle capillaries around small degenerated Trichina capsules (arrow) (IHC for CD34, DAB, X400).
Group
CD34 expression
Mean ± SD (of positive cells)
Intensity of expression
P-value
Positive control (I)
70 ± 13.1
+++
–
Group II
61.7 ± 6.9
+++
>0.05
Group IIIb
51.7 ± 6.9
+++
<0.05*
Group IIIc
48.3 ± 9
+++
<0.01**
Group IVb
34.3 ± 4.9
++
<0.001**
Group IVc
30.8 ± 7.3
++
<0.001**
Group Vb
68.6 ± 9
+++
>0.05
Group Vc
58 ± 5
+++
<0.05*
Group VIb
40.7 ± 6.8
++
<0.001**
Group VIc
30.7 ± 6.2
+
<0.001**
4 Discussion
Based on extensive spectroscopic methods, including 1D (1H and 13C) and 2D NMR (HSQC, HMBC) experiments as well as negative ESI/MS analysis, the aglycone of compound (1) was predicted to be an oleanane type triterpene by 1H and 13C analysis (Table 1) (Altunkeyik et al., 2012). 1H NMR spectrum of the aglycone moiety of compound (1) displayed signals for six tertiary methyl groups at δH 1.04 (β, s), 0.91 (β, s), 0.87 (β, s), 1.11 (α, s), 0.88 (α, s), and 0.79 (β, s) assigned for Me-24, 25, 26, 27, 29, and 30, respectively. Also, two oxymethin protons at δH 4.02 (dd, J = 6.8 & 14.4 Hz) and δH at 4.35 brs were assigned for H-3 & 16, respectively. In addition, the olefinic proton at δH 5.18 (t) was assigned for H-12 to deduce the aglycone as 3,16-β-dihydroxyolea 12-en, 28,23-dioic acid. All previous data were confirmed by 13C NMR (Table 1), which showed that two olefinic carbon resonances at δc 122.02 and 143.97 assigned for C-12 & 13, respectively, indicated atypical Δ12 pentacyclic triterpene derivative (Altunkeyik et al., 2012). In the 13C NMR spectrum, carbon resonance δC at 78.2, 65.93, 170.83, and 175.68 ppm was assigned for the presence of two oxygenated methin and carboxylic groups. The chemical shift δc at 78.20 ppm, attributed to C-3, revealed that sugar was connected at this point, while δc at 65.93 ppm, attributed to C-16, revealed that no sugar was connected at this carbon. A carboxyl function was located at C-17 based on the HMBC correlation between the proton signal at δH 2.75 ppm (H-18) and δC 175.68 (C-28) (2JH-18, C-28). Moreover, HMBC correlations of δH 4.02 (H-3), 1.04 (Me-24), and δC 170.83 (C-23) revealed carboxylic carbon at C-23 (3J H-3,24&C-23). Also, the upfield of carbon resonance of C-24 at δC 14.56 deduces the occurrence of COOH at C-23 (Masullo et al., 2014). Based on the above data, the aglycone was identified as 16-β-hydroxygypsogenic acid [3,16-β-dihydroxyolea12-en, 23,28 dioic acid]. The 1H NMR spectrum in the aliphatic region (Table 1) showed three anomeric proton signals at δH 4.17 (d, J = 7.65 Hz), 5.24 (d, J = 7.65 Hz), and 5.24 (d, J = 7.65 Hz) whose carbon resonances were assigned unambiguously by HSQC experiments at δc 105.34, 94.54, and 95.02, respectively (Table 1). These data revealed an ether bond at (C-3) and ester bonds at (C-23 and C-28) due to shifts that were observed for C-3 (δc 78.20), C-23 (δc 170.83), and C-28 (δc 175.68), respectively. From the carbon resonances of sugars region (Table 1) assigned for 3-β-types of glucose moieties to expect the compound as 3-O-β-d-glycopyranosyl-16-β-hydroxyolea 12-en 23, 28-β-d-diglucopyranoside ester. The compound was confirmed by negative HR-ESI-MS, which by 1H & 13C NMR data expected the molecular weight at 988 for molecular formula C48H76O21; negative ESI-MS showed fragment ion peak at m/z 987.3795 which was assigned to [M−H]−. The MS/MS of this ion showed a peak at m/z 828.3224 assigned for [M−H−162]−, due to loss of hexose unit, and finally a fragment at m/z 482.7722 assigned for [M−H−3(162)–H2O]−. Therefore, compound (1) could be identified as 3-O-β-glucopyranosyl-16-O-β-hydroxyolea-12en 23, 28- β-d-diglucopyranoside ester, a new 16-hydroxygypsogenic acid derivative.
Concerning compound (4), the 1H and 13C NMR spectra (Table 1) revealed an ester triterpenoid glycosidic structure. The 1H NMR spectrum of compound (4) displayed signals arising from seven tertiary methyl groups at δH 1.05, 0.69, 0.80, 0.89, 1.09, 0.88, and 0.92 assigned for Me-23–27, 29, and 30, an oxymethine at δH 4.42 (dd, J = 5.6, 11.2 Hz) and an olefinic proton at δH 5.18 in the aglycon moiety. These findings, along with the 30 carbon resonances observed in the 13C NMR spectrum, indicated the aglycon as oleanolic acid (Tian et al., 1993). Additionally, the resonances of anomeric protons observed at δH 4.18 and 5.25 (each one d, J = 8 Hz) in the 1H NMR spectrum indicated the presence of two β-linked glucose units. The absence of any glycosidation shift for both sugar units suggested that compound (4) was bidesmosidic. The chemical shifts of the anomeric proton (δH 5.25) and carbon (δC 94.5) resonances of the β-d-glucopyranosyl unit indicated that this sugar unit was attached to the C-28 carboxyl group via an ester linkage (Kirmızıbekmez et al., 2006). The other chemical shift of anomeric proton (δH 4.18) and carbon (δC 105.3) indicated the location of the other β-d-glucopyranosyl unit was found at C-3 via ether linkage confirmed by carbon resonance of C-3 at 78.2 in comparison with its previous data in compound (1). In addition, the proton and carbon resonances were assigned to the aglycon and sugar portions. Based on these data and previously reported data (Tian et al., 1993; Kirmızıbekmez et al., 2006); therefore the structure of compound (4) was identified as 3-O-β-d-glucopyranosyl −28-β-d-glucopyranosyl oleanolate.
The destructive effects of leaf extract and its triterpene glycosides against some adult parasites were analyzed by other researchers (Athanasiadou et al., 2001). In the present study, we tried to emphasize the nematocidal activity of leaf extract and its isolated triterpene glycosides against T. spiralis infection in experimental mice. The use of leaf extract against nematodes is very recent, so an in vitro study was performed first to assess its ability to affect adult worms and larvae of T. spiralis. Cuticular injury and transcuticular passive diffusion of the medication into T. spiralis are proposed damaging mechanisms against the adult parasite and its larvae in our research. After scanning with electron microscopy, the adult worm cuticle was destructed under the effect of leaf extract, compounds (1) and (4), and albendazole, but it retained its normal morphology when incubated in the culture medium only. The destruction and sloughing of the cuticle were more obvious and significant in the group treated with compound (4).
In vivo experiments are more useful than in vitro experiments, as many active drugs in vitro are inactive in living organisms for distinct reasons, e.g., metabolism by liver enzymes or gut microflora, rapid absorption, detoxification, and excretion (Saeed et al., 2016). Consequently, we maintained our study in experimental animals to be sure while reporting the results. Regarding the present study's in vivo prospective therapeutic effects, our results revealed a significant reduction in total adult worm count compared to the infected control group. The reduction percentage was 47.19% in the leaf extract prophylactic group, 67.98% in the leaf extract-treated group, 74.72% with compound (4), and 63.48% with compound (1), which were less than that found in the albendazole-treated group (92.13%). Regarding the effects of the drug on the muscular phase, a significant reduction in the mean larval count per gram of muscle was detected in all treated groups (P < 0.01) compared to the control-infected untreated group. Their levels of efficacy varied according to the drug regimen. Better larvae reduction was found in the subgroups that received the two-dose drug regimen; treatment during intestinal then muscular phases. The best reduction of the mean larval count in muscle was found in the two-dose drug regimen of the albendazole-treated group (95.3%), and compound (4) treated group (93.6%). The prophylactic and therapeutic efficacy against T. spiralis has been evaluated in previous studies and reported a significant reduction in adult and larval counts on using Nigella sativa, consistent with our results (Nada et al., 2018). In the same context, few studies on the anti-parasitic effects of leaf extract reported in vivo and in vitro nematocidal activity (Rahman et al., 2014; Abd El-Aal et al., 2021). In addition to its well-known antiprotozoal properties, oleanolic acid (OA) exhibits appreciable anti-parasitic effects against Plasmodium falciparum, Toxoplasma gondii, Trypanosoma cruzi, and Leishmania spp. (Sibley and Price, 2012). The triterpenes gypsogenic acid also possesses trypanocidal activity (Cunha et al., 2003).
In this study, histopathological examination of the small intestine of the infected control group showed dense intravillous inflammatory cellular infiltration, also distorted villous pattern. Moreover, fragments of the adult worms were found within the intestinal lumen. Muscular sections from the infected control group revealed several encysted T. spiralis larvae diffusely present in muscle and many chronic inflammatory cells. This result agrees with previous findings (Dyab et al., 2019). The reduction of these destructive and inflammatory changes was obvious in the treated groups. The two-dose drug regimen therapy group showed the best improvement in restoring the normal architecture, the presence of the least number of Trichinella spiralis larvae with degenerated capsules, and focal pericapsular lymphocytic inflammatory cellular infiltration.
Host tissue damage in the muscle phase of trichinellosis is caused not only by the invading parasite itself but also due to the presence of inflammatory cells that produce superior levels of reactive oxygen species and several stress markers as transferases and cyclooxygenases (Bruschi et al., 2003). In the current study, CD34 marker expression in T. spiralis infected muscles was elevated during the encapsulation phase, owing to inflammatory myositis caused by infection. However, it was significantly reduced with leaf extract and its derivatives treatment, with a greater reduction in its expression in the compound-(4) treated group. This agreed with Hollemann et al. (2008), who found that CD34-expressing cells invade skeletal muscle under inflammatory settings, with some of these cells undergoing myoendothelial differentiation. They observed a significant increase in the number of CD34 + cells in polymyositis and inclusion body myositis patients as compared to controls. Abirami et al. (2011) also confirmed that L. aegyptiaca extracts had anti-inflammatory properties, proving the plant's medicinal promise for treating T. spiralis infection.
5 Conclusion
Based on the results of this investigation, L. aegyptiaca leaf extract and its oleanolic acid derivatives might be an effective, safe, natural preventive, and therapeutic alternative against T. spiralis in its intestinal and muscular stages, both in vitro and in vivo. L. aegyptiaca oleanolic acid derivatives were shown to be the most effective in terms of anthelmintic effectiveness. In the future, we recommend the urgent need to conduct a comprehensive study to identify the chemical components of the tested extract using the LC-ESI-MS/MS technique.
Author contributions
H.Sh.M. & M.A.G. & S.A.A.: Conceptualization, formal analysis, data curation, visualization, investigation, methodology, writing the original draft, review & editing. T.A.: Performing histopathological study, investigation, writing the original draft, review & editing. E.A.H: Performing parasitological study, investigation, methodology, writing the original draft, review & editing.
Data availability statement
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Funding
This research received no specific grant from the public, commercial, or not-for-profit funding agencies.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- In vivo and in vitro management of Meloidogyne incognita (Tylenchida: Heteroderidae) using rhizosphere bacteria, Pseudomonas spp. and Serratia spp. compared with oxamyl. Saudi J. Biol. Sci.. 2021;28:4876-4883.
- [CrossRef] [Google Scholar]
- Evaluation of the wound healing and anti-inflammatory activity of whole plant of Luffa cylindrica (Linn) in rats. Pharmacologyonline.. 2011;3:281-285.
- [Google Scholar]
- Implication of artemisinin nematocidal activity on experimental trichinellosis: in-vitro and in-vivo studies. Parasitol. Int.. 2017;66:56-63.
- [CrossRef] [Google Scholar]
- Triterpene saponins from Cyclamen hederifolium. Phytochemistry. 2012;73:127-133.
- [CrossRef] [Google Scholar]
- Direct anthelmintic effects of condensed tannins towards different gastrointestinal nematodes of sheep: in vitro and in vivo studies. Vet. Parasitol.. 2001;99:205-219.
- [CrossRef] [Google Scholar]
- Effect of myrrh and thyme on Trichinella spiralis enteral and parenteral phases with inducible nitric oxide expression in mice. Mem. Inst. Oswaldo. Cruz.. 2015;110:1035-1041.
- [CrossRef] [Google Scholar]
- Up-regulation of the 31 kDa dehydroascorbate reductase in the modified skeletal muscle cell (nurse cell) during Trichinella spp. infection. Int. J. Parasitol.. 2003;33:1035-1042.
- [CrossRef] [Google Scholar]
- Ultrastractural studies on the parasitic worm Trichinella spiralis. J. Tibah Univ. Sci.. 2010;3:33-38.
- [CrossRef] [Google Scholar]
- In vitro trypanocidal activity of triterpenes from Miconia species. Planta Med.. 2003;69:470-472.
- [CrossRef] [Google Scholar]
- Infectivity of the newborn larva of Trichinella spiralis in the rat. J. Parasitol.. 1970;56:974-977.
- [CrossRef] [Google Scholar]
- Pork as a source of human parasitic infection. Clin. Microbiol. Infect.. 2013;19:586-594.
- [CrossRef] [Google Scholar]
- Drury, R., Wallington, E., 1980. Carlton's histological technique. 5thedn., Oxford University Press, Oxford, New York.
- Cell injury caused by Trichinella spiralis in the mucosal epithelium of B10A mice. J. Parasitol.. 1985;71:757-766.
- [CrossRef] [Google Scholar]
- Prevalence and histopathology of Trichinella spiralis larvae of slaughtered pigs in Cairo governorate. Egypt. J. Egypt. Soc. Parasitol.. 2019;49:439-442.
- [CrossRef] [Google Scholar]
- Chemical profiling of Verbena officinalis and assessment of its anticryptosporidial activity in experimentally infected immunocompromised mice. Arab. J. Chem.. 2022;15:103945
- [CrossRef] [Google Scholar]
- Ferreira, D.D., Esperandim, V.R., Toldo, M.P.A., Kuehn, C.C., do Prado Júnior, J.C., Cunha, W. R. Silva, M.L.A., deAlbuquerque, S., 2013. In vivo activity of ursolic and oleanolic acids during the acute phase of Trypanosoma cruzi infection. Exp. Parasitol. 134, 455–459. https://doi.org/10.1016/j.exppara.2013.04.005.
- The biological action of saponins in animal systems: A review. Br. J. Nutr.. 2002;88:587-605.
- [CrossRef] [Google Scholar]
- Endothelial and myogenic differentiation of hematopoietic progenitor cells in inflammatory myopathies. J. Neuropathol. Exp. Neurol.. 2008;67:711-719.
- [CrossRef] [Google Scholar]
- Anti-trypanosomal activity of African medicinal plants: a review update. J. Ethnopharmacol.. 2014;154:26-54.
- [CrossRef] [Google Scholar]
- A systematic review of pentacyclic triterpenes and their derivatives as chemotherapeutic agents against tropical parasitic diseases. Parasitology. 2016;143:1219-1231.
- [CrossRef] [Google Scholar]
- Natural products and Chagas' disease: a review of plant compounds studied for activity against Trypanosoma cruzi. Nat. Prod. Rep.. 2011;28:809-823.
- [CrossRef] [Google Scholar]
- Kakate, K.C. 4th ed. Delhi: Vallabh Prakashan; 1997. Practical Pharmacognosy; p. 218.
- Muscle distribution of sylvatic and domestic Trichinella larvae in production animals and wildlife. Vet. Parasitol.. 2005;132:101-105.
- [CrossRef] [Google Scholar]
- Triterpene saponins from Calendula arvensis. Z. Naturforsch B.. 2006;61(9):1170-1173.
- [CrossRef] [Google Scholar]
- Ethnomedicinal information on collation and identification of some medicinal plants in research Institutes of South-west Nigeria. Afr. J. Pharm. Pharmacol.. 2010;4:001-007.
- [Google Scholar]
- Mapping metabolome changes in Luffa aegyptiaca Mill fruits at different maturation stages via MS-based metabolomics and chemometrics. J. Adv. Res.. 2021;29:179-189.
- [Google Scholar]
- In vitro Anthelmintic activity of saponins from Medicago spp. against sheep gastrointestinal nematodes. Molecules. 2020;25:242.
- [CrossRef] [Google Scholar]
- Phytochemical content and antimicrobial activities of Luffa aegyptiaca (sponge gourd) leaves extracts. Int. J. Res. Pharm. Biosci.. 2014;1:1-4.
- [Google Scholar]
- Saponins with highly hydroxylated oleanane-type aglycones from Silphium asteriscus L. Phytochemistry. 2014;97:70-80.
- [CrossRef] [Google Scholar]
- Phytochemical screening of aqueous extract of Luffa aegyptiaca (Sponge gourd) leave sample from northern Nigeria: A short communication. Int. J. Pharm. Sci. Res.. 2014;5:344-345.
- [Google Scholar]
- Antibacterial and potential antidiabetic activities of flavone C-glycosides isolated from Beta vulgaris subspecies cicla L. var. flavescens (Amaranthaceae) cultivated in Egypt. Curr. Pharm. Biotechnol.. 2019;20:595-604.
- [CrossRef] [Google Scholar]
- Therapeutic effect of Nigella sativa and ivermectin versus albendazole on experimental trichinellosis in mice. J. Egypt. Soc. Parasitol.. 2018;48:85-92.
- [CrossRef] [Google Scholar]
- Detrimental effects of geldanamycin on adults and larvae of Trichinella spiralis. Helminthologia. 2016;53:126-132.
- [CrossRef] [Google Scholar]
- Akisu C. In-vitro effects of resveratrol on Trichinella spiralis. Parasitol. Res.. 2009;105:1139-1143.
- [CrossRef] [Google Scholar]
- Luffa cylindrica: An important medicinal plant. J. Nat. Prod. Plant Resour.. 2012;2:127-134.
- [Google Scholar]
- In-vitro evaluation of cytotoxic and anthelmintic activity of Luffa acutangula, Luffa aegyptiaca and Momordica cochinchinensis. Br. J. Pharm. Res.. 2014;4:267-277.
- [Google Scholar]
- A review of natural products with antileishmanial activity. Phytomedicine. 2005;12:514-535.
- [CrossRef] [Google Scholar]
- Antischistosomal activity of artemisinin derivatives in vivo and in patients. Pharmacol. Res.. 2016;110:216-226.
- [CrossRef] [Google Scholar]
- Antidiabetic activity of Luffa aegyptiaca (Mill) in alloxan induced diabetic rats. J. Chem. Pharm. Res.. 2011;3:522-525.
- [Google Scholar]
- Effect of methanolic extract of Balanites aegyptiaca fruits on enteral and parenteral stages of Trichinella spiralis in rats. Parasitol. Res.. 2010;107:17-25.
- [CrossRef] [Google Scholar]
- Monitoring antimalarial drug resistance: applying lessons learned from the past in a fast-moving present. Int. J. Parasitol.: Drugs Drug Resist.. 2012;2:126-133.
- [CrossRef] [Google Scholar]
- Gourd Luffa-Luffa cylindrical, Luffa aegyptiaca and Luffa acutangula. Horticul Sci. Univ. Florida.. 2003;3:19-21.
- [Google Scholar]
- Thompson, D.P., Geary, T.G., 1995. The structure and function of helminth surfaces. In: Biochemistry and Molecular Biology of Parasites. Academic Press, pp. 203–232. https://doi.org/10.1016/B978-012473345-9/50013-1.
- Two triterpenoid saponins from Pterocephalus bretschneidri. Phytochemistry. 1993;32(6):1539-1542.
- [CrossRef] [Google Scholar]
- In-vitro and in-vivo efficacy of tribendimidine and its metabolites alone and in combination against the hookworms Heligmosomoides bakeri and Ancylostoma ceylanicum. Acta Trop.. 2012;122:101-107.
- [CrossRef] [Google Scholar]
- Different susceptibilities of Leishmania spp. Promastigotes to the Annona muricata acetogenins annonacinone and corossolone, and the Platymiscium floribundum coumarin scoparone. Exp. Parasitol.. 2013;133:334-338.
- [CrossRef] [Google Scholar]
- Immunity to Trichinella spiralis in irradiated mice. Int. J. Parasitol.. 1980;10:37-41.
- [CrossRef] [Google Scholar]
- Trichinella spiralis infections of inbred mice: immunologically specific responses induced by different Trichinella isolates. J. Parasitol.. 1988;74:283-287.
- [CrossRef] [Google Scholar]
- Recent developments in research on terrestrial plants used for the treatment of malaria. Nat. Prod. Rep.. 2010;27:961-968.
- [CrossRef] [Google Scholar]
- Anthelmintic activity of Gynura angulosa against Trichinella spiralis infections in mice. Pharmacologyonline. 2006;2:299-306.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2022.104258.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







