Translate this page into:
An efficient one-pot multi component synthesis of polyhydroquinoline derivatives through Hantzsch reaction catalysed by Gadolinium triflate
⁎Corresponding author. Tel.: +91 9944093020. smansoors2000@yahoo.co.in (S. Sheik Mansoor)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.

Abstract
Gadolinium(III) trifluoromethanesulfonate (Gadolinium triflate) Gd(OTf)3 catalysed efficient Hantzsch reaction via four-component coupling reactions of aldehydes, 5,5-dimethyl-1,3-cyclohexaedione (dimedone), ethyl acetoacetate and ammonium acetate at ambient temperature was described as the preparation of polyhydroquinoline derivatives. The process presented here is operationally simple, environmentally benign and has excellent yield. Furthermore, the catalyst can be recovered conveniently and reused efficiently.
Keywords
Gadolinium triflate
1,4-Dihydropyridine
Hantzsch reaction
One-pot synthesis
Multi component reaction
1 Introduction
Multi component reactions (Domling and Ugi, 2000; Ugi, 2001, 2003; Zhu, 2003; Domling, 2006) allow the creation of several bonds in a single operation and are attracting increasing attention as one of the most powerful emerging synthetic tools for the creation of molecular diversity and complexity (Burke and Schreiber, 2004). They also have considerable advantages in terms of user and environmental friendliness because of the step reduction and atom economy associated to their use.
4-Substituted 1,4-dihydropyridines (DHPs) comprise a large family of medicinally important compounds. In recent years, an increasing interest has been focused on the synthesis of 1,4-dihydropyridine compounds owing to their significant biological activity (Di Stilo et al., 1998; Kawase et al., 2002; Suarez et al., 2003). In particular, dihydropyridine drugs such as nifedipine, nicardipine, amlodipine (Fig. 1) and others are effective cardiovascular agents for the treatment of hypertension (Buhler and Kiowski, 1987; Reid et al.,1985). 4-Aryl-1,4-dihydropyridines are analogues of NADH coenzymes, which have been explored for their calcium channel activity and the heterocyclic rings are found in a variety of bioactive compounds such as vasodilator, bronchodilator, antiatherosclerotic, antitumour, antidiabetic, geroprotective and heptaprotective agents (Godfraid et al., 1986; Sausins and Duburs, 1988; Mager et al., 1992; Mannhold et al., 1992). Quinolines having 1,4-dihydropyridine nucleus are very important compounds because of their pharmacological properties. Members of this family are being used as antimalarial, anti-inflammatory, anti-asthmatic, antibacterial and tyrosine kinase inhibiting agents (Chen et al., 2001; Roma et al., 2000).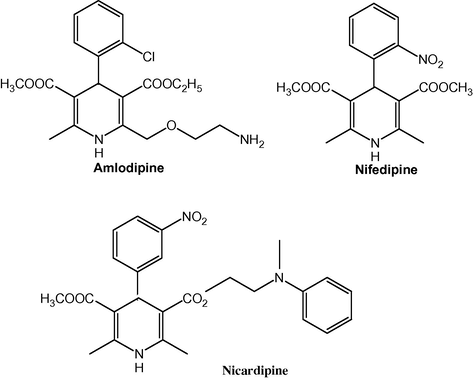
Dihydropyridine drugs.
Numerous methods have been reported for the synthesis of polyhydroquinoline derivatives. The classical methods involve the three-component condensation of an aldehyde with ethyl acetoacetate, and ammonia in acetic acid or in refluxing alcohol (Hantzsch and Liebigs, 1882; Loev and Snader, 1965).
In recent years, several new efficient methods have been developed including the use of MCM-41 (Nagarapu et al., 2007), microwave (Tu et al., 2001), TMS iodide (Sabitha et al., 2003), ionic liquid (Ji et al., 2004), autoclave (Watanabe et al., 1983), fluoroboric acid (Chen et al., 2007), K7[PW11CoO40] (Heravi et al., 2007), metal triflates (Wang et al., 2005; Donelson et al., 2006), molecular iodine (Ko et al., 2005), silica-supported acids (Maheswara et al., 2006; Gupta et al., 2007), ceric ammonium nitrate (Ko and Yao, 2006; Reddy and Raghu, 2008), PTSA-SDS (Kumar and Maurya, 2008), tris(pentafluorophenyl)borane (Chandrasekhar et al., 2008), boronic acids (Sridhar and Perumal, 2005; Debache et al., 2008), grinding (Kumar et al., 2008), organo-catalyst (Kumar and Maurya, 2007; Baghbanian et al., 2010) and Hafnium(IV)bis(perfluorooctanesulfonyl) imide complex (Hong et al., 2010). These methods, however, suffer from drawbacks such as unsatisfactory yields, acidic or basic catalysts, extended reaction times, elevated temperatures, tedious work-up, anhydrous organic solvents and the use of stoichiometric and/or relatively expensive reagents. Moreover, the main disadvantage of almost all existing methods is that the catalysts are destroyed in the work-up procedure and cannot be recovered or reused. Therefore, the search continues for a better catalyst for the synthesis of 1,4-DHPs and polyhydroquinolines in terms of operational simplicity, reusability, economic viability, and greater selectivity.
Lanthanide triflates are unique Lewis acids that are currently of great research interest. They are quite stable in water and reusable, as well as highly effective for the activation of nitrogen containing compounds. Therefore, lanthanide triflates are unique catalysts compared to conventional Lewis acids in several carbon–carbon bond forming reactions and have found a wide application in organic synthesis (Green and Wuts, 1999). In addition, these metal triflates can be used either in aqueous or in non-aqueous media and the reactions can be conveniently carried out under mild conditions and do not require anhydrous conditions or an inert atmosphere.
In 1994 Kobayashi and Hachiya (1994) used Godolinium triflate as a water-tolerant Lewis acid in the aldol reactions of silyl enol ethers with aldehydes in aqueous media. Gadolinium triflate has been used extensively as Lewis acid catalyst in acetylation of alcohols and amines (Alleti et al., 2005), alkylation of pyrroles (Unaleroglu and Yazici, 2007), Michael additions (Alleti et al., 2008) and acetylation of alcohols and phenols (Yoon et al., 2008).
Gadolinium triflate has several advantages over other Lewis acids, it is stable in water and therefore does not decompose under aqueous work-up conditions, unlike other conventional Lewis acids. Thus, recyclization of the Gadolinium triflate is often possible and renders the procedure relatively environmentally acceptable by utilizing these properties; this catalyst has been successfully applied to several synthetic reactions. However, there is no report on the use of Gadolinium triflate for the synthesis of polyhydroquinoline derivatives.
In continuation of our interest towards the development of new routes to the synthesis of heterocyclic compounds, such as 3,4-dihydropyrimidin-2(1H)-ones/-thiones/imines (Mansoor et al., 2016), β-amino ketone compounds (Mansoor et al., 2012a), amidoalkyl naphthols (Mansoor et al., 2012b) and 2-amino-4,6-diphenylpyridine-3-carbonitrile derivatives (Mansoor et al., 2012c) by multi-component reactions, we turned our attention towards the one-pot synthesis of polyhydroquinoline derivatives through Hantzsch a four component coupling reaction of aldehyde, dimedone, ethyl acetoacetate, and ammonium acetate in the presence of a reusable Gadolinium triflate catalyst at room temperature. In this paper, we wish to highlight our finding about the Gd(OTf)3 catalysed four-component Hantzsch reaction using ethanol as a solvent at ambient temperature.
2 Experimental
2.1 Methods and apparatus
All reactions were performed at room temperature. All chemicals were purchased from Aldrich Chemical Co. and solvents were used without further purification. Analytical thin-layer chromatography was performed with E. Merck silica gel 60F glass plates. Visualization of the developed chromatogram was performed by UV light. Melting points were determined with Shimadzu DS-50 thermal analyser. 1H NMR spectra were recorded at Bruker AM 300 (300 MHz) in CDCl3 using TMS as internal standard. FT-IR spectra were obtained as KBr discs on Shimadzu spectrometer. Mass spectra were determined on a Saturm 2000GC/MS instrument. Elemental analysis was measured by means of Perkin Elmer 2400 CHN elemental analyser flowchart.
2.2 General experimental procedure for the synthesis of polyhydroquinolines
Aldehyde (2 mmol), dimedone (2 mmol), ammonium acetate (2 mmol), ethyl acetoacetate (2 mmol) and Gd(OTf)3 (5 mol%) in ethanol (5 mL) were successively charged into a 50 mL round bottomed flask, equipped with a magnetic stirrer. Then the reaction mixture proceeded at room temperature for about 5–6 h and a solid product was gradually formed. After completion of reaction as indicated by thin layer chromatography (TLC), the resulting solid product was filtered and recrystallized to give the pure product. The filtrate was concentrated and then diluted with ethyl acetate, washed with water and the aqueous layer containing the catalyst could be evaporated under reduced pressure to give a white solid, which could be reused without losing catalytic activity.
2.3 Spectral data for the synthesized compounds
2.3.1 2,7,7-Trimethyl-5-oxo-4-phenyl-1,4,5,6,7,8-hexahydroquinoline-3-carboxylic acid ethyl ester (compound 4a)
IR (KBr): 3285, 3080, 2960, 1696, 1610, 1530 cm−1; 1H NMR (CDCl3, 300 MHz) δ: 0.93 (s, 3H), 1.07 (s, 3H), 1.19 (t, J = 7.1 Hz, 3H), 2.13–2.32 (m, 4H), 2.37 (s, 3H), 4.05 (q, J = 7.2 Hz, 2H), 5.05 (s, 1H), 6.21 (s, 1H), 7.06–7.31 (m, 5H). MS (EI): m/z 339 (M+). Anal. Calcd. for C21H25NO3: C, 74.34; H, 7.37; N, 4.13. Found: C, 74.45; H, 7.40; N, 4.10%.
2.3.2 2,7,7-Trimethyl-5-oxo-4-(4-chlorophenyl)-1,4,5,6,7,8-hexahydroquinoline-3-carboxylic acid ethyl ester (compound 4b)
IR (KBr): 3275, 3075, 2965, 1705, 1650, 1605 cm−1; 1H NMR (CDCl3, 300 MHz) δ: 0.93 (s, 3H), 1.07 (s, 3H), 1.19 (t, J = 7.2 Hz, 3H), 2.12–2.35 (m, 4H), 2.37 (m, 3H), 4.00 (q, J = 7.2 Hz, 2H), 5.02 (s, 1H), 6.13 (s, 1H), 7.15 (d, J = 8 Hz, 2H), 7.31 (d, J = 8 Hz, 2H). MS (EI): m/z 374 (M+). Anal. Calcd. for C21H24ClNO3: C, 67.48; H, 6.43; N, 3.75. Found: C, 67.45; H, 6.38; N, 3.80%.
2.3.3 2,7,7-Trimethyl-5-oxo-4-(4-hydroxyphenyl)-1,4,5,6,7,8-hexahydroquinoline-3-carboxylic acid ethyl ester (compound 4c)
IR (KBr): 3365, 2955, 1700, 1645, 1590, 1480, 1385, 1220,782 cm−1; 1H NMR (CDCl3, 300 MHz) δ: 0.93 (s, 3H), 1.07 (s, 3H), 1.19 (t, J = 7.2 Hz, 3H), 2.09–2.22 (m, 3H), 2.20–2.34 (m, 4H), 4.06 (q, J = 7.8 Hz, 2H), 4.98 (s, 1H), 5.61 (s, 1H), 6.10 (s, 1H), 6.65 (d, J = 8.0 Hz, 2H), 7.17 (d, J = 7.8 Hz, 2H). MS (EI): m/z 355 (M+). Anal. Calcd. for C21H25NO4: C, 70.99; H, 7.04; N, 3.94. Found: C, 70.96; H, 7.00; N, 3.90%.
2.3.4 2,7,7-Trimethyl-5-oxo-4-(4-methylphenyl)-1,4,5,6,7,8-hexahydroquinoline-3-carboxylic acid ethyl ester (compound 4d)
IR (KBr): 3275, 3080, 2960, 1700, 1650 cm−1; 1H NMR (CDCl3, 300 MHz) δ: 0.94 (s, 3H), 1.08 (s, 3H), 1.21 (t, J = 7.1 Hz, 3H), 2.10–2.24 (m, 4H), 2.26 (s, 3H), 2.37 (s, 3H), 4.06 (q, J = 7.1 Hz, 2H), 5.03 (s, 1H), 5.96 (s, 1H), 7.02 (d, J = 8 Hz, 2H), 7.19 (d, J = 8 Hz, 2H). MS (EI): m/z 353 (M+). Anal. Calcd for C22H27NO3: C, 74.79; H, 7.65; N, 3.97. Found: C, 74.84; H, 7.69; N, 3.95%.
2.3.5 2,7,7-Trimethyl-5-oxo-4-(4-methoxyphenyl)-1,4,5,6,7,8-hexahydroquinoline-3-carboxylic acid ethyl ester (compound 4e)
IR (KBr): 3275, 3085, 2960, 1705, 1605, 1498, 1382, 1217, 1032, 766 cm−1; 1H NMR (CDCl3, 300 MHz) δ: 0.94 (s, 3H), 1.07 (s, 3H), 1.21 (t, J = 7.2 Hz, 3H), 2.13–2.27 (m, 3H), 2.31–2.37 (m, 4H), 3.74 (s, 3H), 4.06 (q, J = 7.2 Hz, 2H), 5.00 (s, 1H), 6.01 (s, 1H), 6.72–6.75 (m, 2H), 7.20–7.26 (m, 2H). MS (EI): m/z 369 (M+). Anal. Calcd. for C22H27NO4: C, 71.54; H, 7.32; N, 3.79. Found: C, 71.50; H, 7.28; N, 3.83%.
2.3.6 2,7,7-Trimethyl-5-oxo-4-(4-bromophenyl)-1,4,5,6,7,8-hexahydroquinoline-3-carboxylic acid ethyl ester (compound 4f)
IR (KBr): 3285, 2960, 1702, 1605, 1510, 1380, 1230, 1020, 764 cm−1; 1H NMR (CDCl3, 300 MHz) δ: 0.93 (s, 3H), 1.07 (s, 3H), 1.19 (t, J = 7.2 Hz, 3H), 2.19–2.27 (m, 3H), 2.34–2.41 (m, 4H), 4.05 (q, J = 7.2 Hz, 2H), 5.03 (s, 1H), 5.78 (s, 1H), 7.19 (d, J = 8 Hz, 2H), 7.34 (d, J = 8 Hz, 2H). MS (EI): m/z 417 (M+). Anal. Calcd. for C21H24BrNO3: C, 60.30; H, 5.74; N, 3.35. Found: C, 60.35; H, 5.78; N, 3.33%.
2.3.7 2,7,7-Trimethyl-5-oxo-4-(4-nitrophenyl)-1,4,5,6,7,8-hexahydroquinoline-3-carboxylic acid ethyl ester (compound 4g)
IR (KBr): 3288, 3077, 2964, 1705, 1605, 1530 cm−1; 1H NMR (CDCl3, 300 MHz) δ: 0.93 (s, 3H), 1.07 (s, 3H), 1.22 (t, J = 7.1 Hz, 3H), 2.12–2.41 (m, 4H), 2.12–2.32 (m, 3H) 3.99 (q, J = 7.1 Hz, 2H), 5.15 (s, 1H), 6.86 (s, 1H), 7.35 (t, J = 7.9 Hz, 1H), 7.72 (d, J = 7.9 Hz, 1H). MS (EI): m/z 384 (M+). Anal. Calcd. for C21H24N2O5: C, 65.62; H, 6.25; N, 7.29. Found: C, 65.60; H, 6.22; N, 7.32%.
2.3.8 2,7,7-Trimethyl-5-oxo-4-(4-fluorophenyl)-1,4,5,6,7,8-hexahydroquinoline-3-carboxylic acid ethyl ester (compound 4h)
IR (KBr): 3290, 2960, 1695, 1610, 1490, 1380, 1220, 1025, 764 cm−1; 1H NMR (CDCl3, 300 MHz) δ: 0.93 (s, 3H), 1.07 (s, 3H), 1.19 (t, J = 7.2 Hz, 3H), 2.12–2.35 (m, 4H), 2.37 (m, 3H), 4.06 (q, J = 7.2 Hz, 2H), 5.02 (s, 1H), 6.13 (s, 1H), 7.15 (d, J = 8 Hz, 2H), 7.31 (d, J = 8 Hz, 2H). MS (EI): m/z 357 (M+). Anal. Calcd. for C21H24FNO3: C, 70.59; H, 6.72; N, 3.92. Found: C, 70.64; H, 6.70; N, 3.88%.
2.3.9 2,7,7-Trimethyl-5-oxo-4-(3-bromophenyl)-1,4,5,6,7,8-hexahydroquinoline-3-carboxylic acid ethyl ester (compound 4i)
IR (KBr): 3290, 2960, 1700, 1612, 1500, 1385, 1220, 1020, 764 cm−1; 1H NMR (CDCl3, 300 MHz) δ: 0.93 (s, 3H), 1.07 (s, 3H), 1.19 (t, J = 7.2 Hz, 3H), 2.12–2.35 (m, 4H), 2.37 (m, 3H), 4.06 (q, J = 7.2 Hz, 2H), 5.02 (s, 1H), 6.13 (s, 1H), 7.15 (d, J = 8 Hz, 2H), 7.31 (d, J = 8 Hz, 2H). MS (EI): m/z 417 (M+). Anal. Calcd. for C21H24BrNO3: C, 60.30; H, 5.74; N, 3.79. Found: C, 60.25; H, 5.70; N, 3.75%.
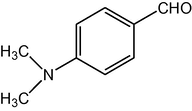
2.3.10 2,7,7-Trimethyl-5-oxo-4-(4-dimethylaminophenyl)-1,4,5,6,7,8-hexahydroquinoline-3-carboxylic acid ethyl ester (compound 4j)
IR (KBr): 3285, 3080, 2960, 1705, 1605, 1530 cm−1; 1H NMR (CDCl3, 300 MHz) δ: 0.93 (s, 3H), 1.07 (s, 3H), 1.19 (t, J = 7.2 Hz, 3H), 2.12–2.35 (m, 4H), 2.87 (s, 6H), 2.37 (m, 3H), 4.06 (q, J = 7.2 Hz, 2H), 5.02 (s, 1H), 6.13 (s, 1H), 7.15 (d, J = 8 Hz, 2H), 7.31 (d, J = 8 Hz, 2H). MS (EI): m/z 382 (M+). Anal. Calcd. for C23H30N2O3: C, 72.25; H, 7.85; N, 7.33. Found: C, 72.30; H, 7.88; N, 7.32%.
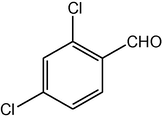
2.3.11 2,7,7-Trimethyl-5-oxo-4-(2,4-dichlorophenyl)-1,4,5,6,7,8-hexahydroquinoline-3-carboxylic acid ethyl ester (compound 4k)
IR (KBr): 3285, 3080, 2960, 1705, 1650, 1600, 1520 cm−1; 1H NMR (CDCl3, 300 MHz) δ: 0.93 (s, 3H), 1.07 (s, 3H), 1.19 (t, J = 7.2 Hz, 3H), 2.12–2.35 (m, 4H), 2.37 (m, 3H), 4.06 (q, J = 7.2 Hz, 2H), 5.02 (s, 1H), 6.13 (s, 1H), 7.22 (d, J = 8 Hz, 1H), 7.31 (d, J = 8 Hz, 2H). MS (EI): m/z 407 (M+). Anal. Calcd. for C21H23Cl2NO3: C, 61.78; H, 5.64; N, 3.43. Found: C, 61.80; H, 5.68; N, 3.40%.
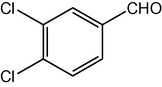
2.3.12 2,7,7-Trimethyl-5-oxo-4-(3,4-dichlorophenyl)-1,4,5,6,7,8-hexahydroquinoline-3-carboxylic acid ethyl ester (compound 4l)
IR (KBr): 3282, 3080, 2960, 1710, 1650, 1600, 1490 cm−1; 1H NMR (CDCl3, 300 MHz) δ: 0.93 (s, 3H), 1.07 (s, 3H), 1.19 (t, J = 7.2 Hz, 3H), 2.12–2.35 (m, 4H), 2.37 (m, 3H), 4.06 (q, J = 7.2 Hz, 2H), 5.02 (s, 1H), 6.13 (s, 1H), 7.25 (m, J = 8 Hz, 1H), 7.31 (d, J = 8 Hz, 2H). MS (EI): m/z 407 (M+). Anal. Calcd. for C21H23Cl2NO3: C, 61.78; H, 5.64; N, 3.43. Found: C, 61.75; H, 5.62; N, 3.45%.
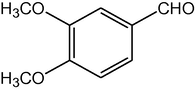
2.3.13 2,7,7-Trimethyl-5-oxo-4-(3,4-dimethoxyphenyl)-1,4,5,6,7,8-hexahydroquinoline-3-carboxylic acid ethyl ester (compound 4m)
IR (KBr): 3245, 2955, 1696, 1650, 1605, 1505, 1380, 1217, 1027, 753 cm−1; 1H NMR (CDCl3, 300 MHz) δ: 0.93 (s, 3H), 1.07 (s, 3H), 1.19 (t, J = 7.2 Hz, 3H), 2.12–2.35 (m, 4H), 2.37 (m, 3H), 4.06 (q, J = 7.2 Hz, 2H), 3.87 (s, 3H), 3.78 (s, 3H), 5.02 (s, 1H), 6.13 (s, 1H), 7.08 (m, J = 8 Hz, 1H), 7.31 (d, J = 8 Hz, 2H). MS (EI): m/z 399 (M+). Anal. Calcd. for C23H29NO5: C, 69.17; H, 7.27; N, 3.51. Found: C, 69.20; H, 7.30; N, 3.46%.
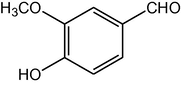
2.3.14 2,7,7-Trimethyl-5-oxo-4-(4-hydroxy-3-methoxyphenyl)-1,4,5,6,7,8-hexahydroquinoline-3-carboxylic acid ethyl ester (compound 4n)
IR (KBr): 3385, 2954, 1701, 1644, 1509, 1497, 1385, 1218, 1025, 782 cm−1; 1H NMR (CDCl3, 300 MHz) δ: 0.93 (s, 3H), 1.07 (s, 3H), 1.19 (t, J = 7.2 Hz, 3H), 2.12–2.35 (m, 4H), 2.37 (m, 3H), 5.61 (s, 1H), 3.80 (s, 3H),4.06 (q, J = 7.2 Hz, 2H), 5.02 (s, 1H), 6.13 (s, 1H), 7.10 (m, J = 8 Hz, 1H), 7.31 (d, J = 8 Hz, 2H). MS (EI): m/z 385 (M+). Anal. Calcd. for C22H27NO5: C, 68.57; H, 7.01; N, 3.64. Found: C, 68.56; H, 7.06; N, 3.60%.
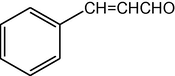
2.3.15 2,7,7-Trimethyl-5-oxo-4-cinnamyl-1,4,5,6,7,8-hexahydroquinoline-3-carboxylic acid ethyl ester (compound 4o)
IR (KBr): 3300, 2966, 1695, 1602, 1502 cm−1; 1H NMR (CDCl3, 300 MHz) δ: 0.93 (s, 3H), 1.07 (s, 3H), 1.19 (t, J = 7.2 Hz, 3H), 2.12–2.35 (m, 4H), 2.37 (m, 3H), 4.06 (q, J = 7.2 Hz, 2H), 5.02 (s, 1H), 6.13 (s, 1H), 7.10–7.38 (m, 5H) 7.18 (m, 1H), 7.38(d, 1H). MS (EI): m/z 365 (M+). Anal. Calcd. for C23H27NO3: C, 75.62; H, 7.40; N, 3.83. Found: C, 75.60; H, 7.45; N, 3.85%.
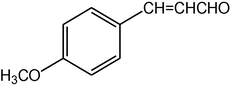
2.3.16 2,7,7-Trimethyl-5-oxo-4-(4-methoxycinnamyl)-1,4,5,6,7,8-hexahydroquinoline-3-carboxylic acid ethyl ester (compound 4p)
IR (KBr): 3285, 2966, 1704, 1605, 1530 cm−1; 1H NMR (CDCl3, 300 MHz) δ: 0.93 (s, 3H), 1.07 (s, 3H), 1.19 (t, J = 7.2 Hz, 3H), 2.12–2.35 (m, 4H), 2.37 (m, 3H), 3.68 (s, 3H), 4.06 (q, J = 7.2 Hz, 2H), 5.02 (s, 1H), 6.13 (s, 1H), 7.15 (d, J = 8 Hz, 2H), 7.31 (d, J = 8 Hz, 2H) 7.18 (m, 1H), 7.38(d, 1H). MS (EI): m/z 395 (M+). Anal. Calcd. for C24H29NO4: C, 72.91; H, 7.34; N, 3.54. Found: C, 72.90; H, 7.38; N, 3.50%.
3 Results and discussion
3.1 Effect of catalysis
In recent years, metal triflates have received considerable attention as a mild Lewis acid for an array of organic transformation (Wang et al., 2005; Donelson et al., 2006). Our initial work started with the screening of catalyst loading so as to identify optimal reaction conditions for the synthesis of polyhydroquinoline derivatives. The mixture of benzaldehyde, dimedone, ethyl acetoacetate and ammonium acetate was chosen as the model reaction (Scheme 1) to detect whether the use of Gadolinium triflate was efficient and investigate the optimized conditions. The results were summarized in Table 1.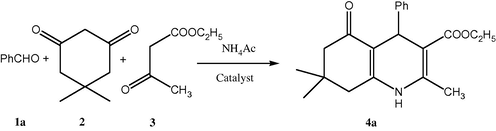
Optimizing the reaction conditions.
Entry
Catalyst
Amount of catalyst (mol%)
Time (h)
Yieldb
1
None
24
32
2
ZnCl2
100
24
42
3
AlCl3
100
24
48
4
FeCl3
100
24
40
5
NdCl3
25
12
70
6
La(OTf)3
10
12
76
7
Nd(OTf)3
20
24
60
8
Yb(OTf)3
10
6
80
9
Gd(OTf)3
10
5
82
10
Gd(OTf)3
5
5
89
11
Gd(OTf)3
1
5
72
12
Gd(OTf)3
5
5
89, 90, 91, 89c
First of all, a number of Lewis acid catalysts such as ZnCl2, AlCl3, FeCl3, NdCl3, La(OTf)3, Nd(OTf)3, Yb(OTf)3 and Gd(OTf)3 have been screened using the model reaction in ethanol (Table 1). Gd(OTf)3 was found to be the best catalyst under these conditions. The results show that Gd(OTf)3 (5 mol%) is effective for good yield (Table 1, entry 10). The Hantzsch condensation of dimedone, benzaldehyde, ethyl acetoacetate, and ammonium acetate in the presence of Gd(OTf)3 at room temperature results in the formation of 2,7,7-Trimethyl-5-oxo-4-phenyl-1,4,5,6,7,8-hexahydroquinoline-3-carboxylic acid ethyl ester in 89% yield (Scheme 2).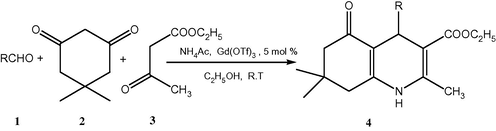
Ga(OTf)3 catalysed synthesis of polyhydroquinoline derivatives via the Hantzsch reaction.
After the reaction was completed, the product was filtered directly and the catalyst can be extracted by water from the residue. Lanthanide triflates are more soluble in water than in organic solvents. The catalyst could be recovered almost quantitatively from the aqueous layer, which could be subsequently reused several times. As indicated in Table 1, it showed almost no loss of activity after four successive runs. The yields obtained were from 91% to 88% (with yields of product 4a being 89%, 90%, 91%, 88% in the first, second, third and fourth run, respectively). In view of environmentally friendly methodologies, recovery and reuse of the catalyst is highly preferable.
3.2 Effect of solvent
To handle the procedure more easily, we then continued to optimize the model process mentioned above by detecting the efficiency of several classic solvents chosen as the medium for comparison (Table 2). In each case, the substrates were mixed together with 5 mol% Gd(OTf)3 agitated with 3–5 ml solvent. As indicated in Table 2, the polar solvents such as ethanol, methanol and acetonitrile (entry 1–3) were much better than non-polar solvents (entry 4–9). The results could be interpreted with much better solubility of the catalyst and the reagents in the polar solvents. When acetone was applied (entry 6), it was found that the reaction proceeded quickly but the obtained yellow solid contained many other by-products which were probably due to the fast self-assembling of reagents or some competitive reactions promoted by Gd(OTf)3 in acetone. Thus, we selected the optimized reaction condition to study the universality of the application of the catalyst.
Entry
Solvent
Time (h)
Yield%
1
Ethanol
5
89
2
Methanol
5
78
3
Acetonitrile
5
80
4
t-BuOH
8
58
5
1,4-Dioxane
8
53
6
Acetone
5
50
7
Toluene
24
24
8
DCM
24
34
9
Cyclohexane
24
20
3.3 Synthesis of various polyhydroquinoline derivatives
A variety of aromatic aldehydes were selected to undergo the Hantzsch reaction in the presence of catalytic amount of Gd(OTf)3 in ethanol at room temperature (Scheme 2). The results of this study are summarized in Table 3. As can be seen from the results in Table 3, aromatic aldehydes containing both electron withdrawing and electron donating groups reacted smoothly to produce moderate to high yields of products.
Entry
R
Product
Time (h)
Yield (%)
Melting point (°C)
1
4a
5
89
204–206
2
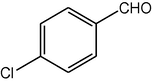
4b
5.5
83
250–252
3
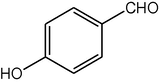
4c
6
85
228–230
4
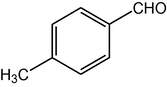
4d
5
89
260–262
5
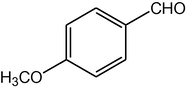
4e
5
88
258–260
6
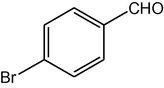
4f
5.5
85
252–254
7
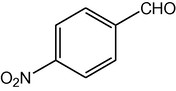
4g
5
82
244–246
8
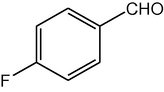
4h
6
88
184–186
9
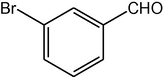
4i
5.5
85
234–236
10
4j
6
84
232–234
11
4k
5.5
85
242–244
12
4l
5.5
83
214–216
13
4m
6
89
197–199
14
4n
5.5
88
211–213
15
4o
6
86
204–206
16
4p
6
86
198–200
4 Conclusions
In conclusion, we successfully developed a facile and efficient method for preparing a variety of 4-substituted-1,4-dihydropyridines from the reactions of different aromatic aldehydes, dimedone, ethyl acetoacetate and ammonium acetate in the presence of a catalytic amount of Gd(OTf)3 at room temperature. The catalytic activity of Gd(OTf)3 is remarkable and the use of the environmentally benign, commercially available Gd(OTf)3 as catalyst in the synthesis of 4-substituted-1,4-dihydropyridines in good yield is also significant. The present method has many obvious advantages compared to those reported in the previous literature, including the avoidance of discharging harmful organic solvents, the generality, the simplicity of the methodology and recycling of the catalyst.
Acknowledgements
Author Mansoor gratefully acknowledges University Grants Commission, Government of India, New Delhi for the financial support (Major Research Project 40-44/2011(SR)). The authors wish to thank C. Abdul Hakeem College Management, Dr. W. Abdul Hameed, Principal, Dr. M.S. Dastageer, Head of the Research Department of Chemistry for the facilities and support.
References
- J. Mol. Cat. A: Chem.. 2005;226:57.
- Tetrahedron Lett.. 2008;49:3466.
- Chin. Chem. Lett.. 2010;21:563.
- Angew. Chem., Int. Ed.. 2004;43:46.
- Synthesis 2008:1737.
- Catal. Commun.. 2007;8:123.
- J. Med. Chem.. 2001;44:2374.
- Synletter 2008:509.
- J. Med. Chem.. 1998;41:5393.
- Chem. Rev.. 2006;106:17.
- Angew. Chem., Int. Ed.. 2000;39:3168.
- J. Mol. Catal. A: Chem.. 2006;256:309.
- Pharmacol. Rev.. 1986;38:321.
- Productive Groups in Organic Synthesis (third ed). New York: Wiley; 1999.
- Synthesis 2007:2835.
- J. Ann. Chem.. 1882;1:215.
- J. Mol. Catal. A: Chem.. 2007;264:50.
- J. Fluorine Chem.. 2010;131:111.
- Synletter 2004:831.
- Bioorg. Chem.. 2002;10:1051.
- J. Org. Chem.. 1994;59:3590.
- Tetrahedron Lett.. 2005;46:5771.
- Tetrahedron Lett.. 2006;62:7293.
- Synletter. 2008;6:883.
- Tetrahedron. 2008;64:536.
- Tetrahedron. 2007;63:1946.
- J. Org. Chem.. 1965;30:1914.
- Drug Des. Discovery. 1992;8:273.
- Mol. Catal. A: Chem.. 2006;17:179.
- Eur. J. Med. Chem.. 1992;27:229.
- Mansoor, S.S., Shafi, S.S., Ahmed, S.Z., 2016. An efficient one-pot multi component synthesis of 3,4-dihydropyrimidine-2-(1H)-ones/thiones/imines via a Lewis base catalyzed Biginelli-type reaction under solvent-free conditions. Arab. J. Chem. 9, S846–S851.
- Mansoor, S.S., Aswin, K., Logaiya, K., Sudhan, S.P.N., 2012a. An efficient synthesis of β-amino ketone compounds through one-pot three-component Mannich-type reactions using bismuth nitrate as catalyst. J. Saudi Chem. Soc., http://dx.doi.org/10.1016/j.jscs.2012.04.008.
- Mansoor, S.S., Aswin, K., Logaiya, K., Sudhan, S.P.N., 2012b. ZrOCl2·8H2O: an efficient and recyclable catalyst for the three-component synthesis of amidoalkyl naphthols under solvent-free conditions. J. Saudi Chem. Soc., http://dx.doi.org/10.1016/j.jscs.2012.06.003.
- Mansoor, S.S., Aswin, K., Logaiya, K., Sudhan, S.P.N., 2012c. [Bmim]BF4 ionic liquid: an efficient reaction medium for the one-pot multi-component synthesis of 2-amino-4,6-diphenyl pyridine-3-carbonitrile derivatives. J. Saudi Chem. Soc., http://dx.doi.org/10.1016/j.jscs.2012.07.011.
- Catal. Commun.. 2007;8:1871.
- Chin. Chem. Lett.. 2008;19:775.
- Cardiovasc. Pharmacol.. 1985;S18:7.
- Eur. J. Med. Chem.. 2000;35:1021.
- Tetrahedron Lett.. 2003;44:4129.
- Heterocycles. 1988;27:269.
- Tetrahedron. 2005;61:2465.
- Tetrahedron. 2003;59:9179.
- Chin. J. Org. Chem.. 2001;21:313.
- Pure Appl. Chem.. 2001;73:187.
- Molecules. 2003;8:53.
- Tetrahedron. 2007;63:5608.
- Tetrahedron. 2005;61:1539.
- Synthesis 1983:761.
- Tetrahedron Lett.. 2008;49:3165.
- Eur. J. Org. Chem. 2003:1133.







