Translate this page into:
An efficient synthesis of styryl 1,3,4-thiadiazoles using Lawesson’s reagent and Propylphosphonic anhydride-precursors for bis heterocycles
2nd Heterocyclic Update
*Corresponding author. Tel.: +91 877 2289303; fax: +91 877 2249532 adivireddyp@yahoo.co.in (Adivireddy Padmaja)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Available online 29 August 2014

Peer review under responsibility of King Saud University.
Abstract
Abstract
The compounds styryl 1,3,4-thiadiazoles were prepared adopting one and two step methodologies to optimize the yield of the products. The two-step methodology via benzohydrazide followed by treatment with Lawesson’s reagent in the presence of Propylphosphonic anhydride and triethylamine produced styryl 1,3,4-thiadiazoles in excellent yields. The olefin moiety in these compounds is utilized to develop pyrazole and isoxazole rings by 1,3-dipolar cycloaddition methodology followed by oxidation.
Keywords
Nitrile imine
Nitrile oxide
Manganese dioxide
Pyrazolyl thiadiazole
Isoxazolyl thiadiazole
1 Introduction
The five membered heterocycles, particularly pyrazoles, isoxazoles and 1,3,4-thiadiazoles are the primary skeletons of a large number of compounds produced by nature and play a vital role in pharmacological chemistry (Bhat et al., 2011; Kritsanida et al., 2002). Pyrazole framework plays an essential role in biologically active compounds and therefore represents an interesting template for combinatorial as well as medicinal chemists (Foks et al., 2005; Gilbert et al., 2006; Liu et al., 2008; Shamroukh et al., 2007). One of the most important methods used for the synthesis of pyrazole derivatives is 1,3-dipolar cycloaddition utilizing diazomethane, nitrile imine to activated olefins or [2+3] cyclocondensation of α,β-unsaturated ketones with hydrazine hydrate (Wen et al., 2011). Using the dipolar cycloadditions for the synthesis of heterocycles the regiochemistry can be also controlled (Bonini et al., 2009; Chandanshive et al., 2010). The pyrazoles are also reported by the nucleophilic attack of hydrazines to chromones, flavones or isoxazoles (Levai et al., 2006; Sviridov et al., 2007). The isoxazoles besides being potential pharmaceutical agents are also precursors to useful intermediates such as γ-amino alcohols and β-hydroxy ketones (Kozikowski, 1984). The 1,3-dipolar cycloaddition of nitrile oxides to alkynes is the most direct and frequently used approach for the synthesis of isoxazoles (Dadiboyena et al., 2007; Jawalekar et al., 2011; Sanders et al., 2011). Apart from these, 1,3,4-thiadiazole constitutes the active part of several biologically active compounds including antibacterial (Foroumadi et al., 2003; Karaku and Rollas, 2002; Thomasco et al., 2003), antimycotic (Dogan et al., 2002; Mamolo et al., 1996) and anti-inflammatory activities (Palaska et al., 2002; Santagati et al., 1994; Schenone et al., 2006). The common method for the preparation of 1,3,4-thiadiazoles involves the reaction of aldehydes, hydrazine hydrate and elemental sulfur under conventional and microwave conditions (Mazzone et al., 1983; Mounim et al., 2005). Thionation of dibenzoylhydrazines using Lawesson’s reagent or Phosphorus pentasulfide followed by cyclization and dehydrosulfurization is also one of the methods to produce 1,3,4-thiadiazoles (Gierczyk and Zalas, 2005; Kiryanov et al., 2001). In addition, the cyclization of 1,2-diacylhydrazine or its thia analogs in the presence of a coupling agent such as SOCl2 or POCl3 and a strong mineral acid also resulted in 1,3,4-thiadiazoles (Borg et al., 1995; Mavrova et al., 2009; Sun et al., 2001; Xu et al., 1998). The other important route is via exchange of oxygen atom in 1,3,4-oxadiazole to sulfur using thiourea and tetraphosphorus decasulfide (Padmaja et al., 2012; Linganna and Rai, 1998). In fact, we have been continuously focusing on the synthesis of a variety of bis heterocycles held by different pharmacophoric units (Padmaja et al., 2011a,b; Padmavathi et al., 2008, 2009, 2011; Reddy et al., 2013). Our successful efforts in this direction made us to design molecules having pyrazole and isoxazole moieties in combination with 1,3,4-thiadaizoles.
2 Experimental
2.1 Chemistry
Melting points were determined in open capillaries on a Mel-Temp apparatus and are uncorrected. The purity of the compounds was checked by TLC (silica gel H, BDH, EtOAc/hexane, 1:3). The IR spectra were recorded on a Thermo Nicolet IR 200 FT-IR spectrometer as KBr pellets and the wave numbers are given in cm−1. The 1H NMR spectra were recorded in DMSO-d6 on a Bruker-400 spectrometer (400 MHz). The 13C NMR spectra were recorded in DMSO-d6 on a Bruker spectrometer operating at 100 MHz. All chemical shifts are reported in δ (ppm) using TMS as an internal standard. The mass spectra were recorded on Jeol JMS-D 300 and Finnigan Mat 1210 B at 70 eV with an emission current of 100 μA. The microanalyses were performed on a Perkin–Elmer 240C elemental analyzer. The 2-((arylsulfonyl)aminosulfonyl)acetohydrazide (1) and Z-styrylsulfonylacetic acid (2) were prepared by the literature procedure (Reddy et al., 2013).
2.2 Typical one-pot procedure for the synthesis of 2-(((arylsulfonyl)amino-sulfonyl)methyl)-5-[Z-(styrylsulfonyl)methyl]-1,3,4-thiadiazole 3a–c
To a mixture of 2-((arylsulfonyl)aminosulfonyl)acetohydrazide (1) (1.0 mmol), Z-styrylsulfonylacetic acid (2) (1.0 mmol) and Phosphorus pentasulfide (P2S5) (0.66 g, 1.5 mmol) in ethyl acetate (EtOAc) (5 mL), triethylamine (TEA) (0.34 mL, 2.5 mmol) followed by Propylphosphonic anhydride (T3P) (0.36 mL, 1.2 mmol) in EtOAc (3 mL) was added dropwise under nitrogen atmosphere and heated to 60 °C for 5–7 h. Then, the reaction mixture was cooled, poured into ice-water and extracted with EtOAc. The combined organic phase was washed successively with saturated sodium hydrogen carbonate solution and brine. The organic phase was dried (an. MgSO4) and the solvent was removed under reduced pressure. The resultant residue was purified by column chromatography (silica gel, 60–120 mesh) using hexane/EtOAc (6:1) as eluent.
Method A: The compound 5 (1.0 mmol), Lawesson’s reagent (LR) (0.60 g, 1.5 mmol) and EtOAc (5 mL) were refluxed for 8–12 h at 65 °C. After completion of the reaction, the solvent was evaporated under vacuum and the resultant semi-solid was chromatographed on silica gel (60–120 mesh) using EtOAc/hexane (7:3) as eluent to afford pure product.
Method B: To a solution of compound 5 (1.0 mmol) in EtOAc (10 mL), LR (0.60 g, 1.5 mmol), TEA (0.34 mL, 2.5 mmol), T3P (0.36 mL, 1.2 mmol) in EtOAc (7 mL) was added dropwise. The reaction mixture was heated to 55 °C for 4–6 h, cooled and poured into ice-water. The separated solid was extracted with dichloromethane. The solvent was removed in vacuo. The resultant residue was purified by column chromatography (silica gel, 60–120 mesh) using hexane/EtOAc (4:1) as eluent.
2.2.1 2-(((Phenylsulfonyl)aminosulfonyl)methyl)-5-[Z-(styrylsulfonyl)methyl]-1,3,4-thiadiazole (3a)
Yield 39% (0.19 g, One-step method), 55% (0.26 g, Method A), 85% (0.41 g, Method B) as a white solid. M.p. 157–159 °C. IR (KBr): ν = 3225 (NH), 1632 (C⚌C), 1570 (C⚌N), 1319, 1151 (SO2) cm−1. 1H NMR (DMSO-d6): δ = 4.74 (s, 2H, CH2-(C-5)), 5.02 (s, 2H, CH2-(C-2)), 6.56 (d, 1H, HB, J = 9.4 Hz), 7.14–7.72 (m, 11H, HA, ArH), 10.26 (bs, 1H, NH) ppm. 13C NMR (DMSO-d6): δ = 43.2 (CH2-(C-5)), 48.2 (CH2-(C-2)), 121.2 (C-HB), 123.1, 123.7, 124.2, 125.6, 127.8, 128.4, 129.2, 130.4 (ArC), 140.1 (C-HA), 155.3 (C-5), 157.4 (C-2) ppm. MS (m/z): 499.61 (M+·). Anal. Calcd. for C18H17N3O6S4: C 43.27, H 3.43, N 8.41; found C 43.51, H 3.58, N 8.76.
2.2.2 2-(((4-Methylphenylsulfonyl)aminosulfonyl)methyl)-5-[Z-(4-methylstyryl-sulfonyl)methyl]-1,3,4-thiadiazole, (3b)
Yield 36% (0.18 g, One-step method), 52% (0.27 g, Method A), 90% (0.46 g, Method B) as a white solid. M.p. 135–137 °C. IR (KBr): ν = 3220 (NH), 1627 (C⚌C), 1562 (C⚌N), 1305, 1145 (SO2) cm−1. 1H NMR (DMSO-d6): δ = 2.16 and 2.24 (s, 6 H, Ar–CH3), 4.62 (s, 2H, CH2-(C-5)), 4.94 (s, 2H, CH2-(C-2)), 6.52 (d, 1H, HB, J = 9.2 Hz), 7.10–7.56 (m, 9 H, HA, ArH), 10.14 (bs, 1H, NH) ppm. 13C NMR (DMSO-d6): δ = 21.5 and 21.7 (Ar–CH3), 42.4 (CH2-(C-5)), 47.3 (CH2-(C-2)), 120.2 (C-HB), 122.1, 123.4, 124.2, 124.9, 125.5, 126.9, 127.5, 128.6 (ArC), 136.4 (C-HA), 152.2 (C-5), 154.6 (C-2) ppm. MS (m/z): 527.67 (M+·). Anal. Calcd. for C20H21N3O6S4: C 45.52, H 4.01, N 7.96; found C 45.67, H 4.29, N 7.91.
2.2.3 2-(((4-Chlorophenylsulfonyl)aminosulfonyl)methyl)-5-[Z-(4-chlorostyryl-sulfonyl)methyl]-1,3,4-thiadiazole, (3c)
Yield 38% (0.21 g, One-step method), 49% (0.27 g, Method A), 88% (0.49 g, Method B) as a white solid. M.p. 171–173 °C. IR (KBr): ν = 3228 (NH), 1638 (C⚌C), 1575 (C⚌N), 1324, 1158 (SO2) cm−1. 1H NMR (DMSO-d6): δ = 4.90 (s, 2H, CH2-(C-5)), 5.12 (s, 2H, CH2-(C-2)), 6.61 (d, 1H, HB, J = 9.6 Hz), 7.19–7.84 (m, 9 H, HA, ArH), 10.35 (bs, 1H, NH) ppm. 13C NMR (DMSO-d6): δ = 46.6 (CH2-(C-5)), 49.8 (CH2-(C-2)), 122.4 (C-HB), 126.2, 127.6, 128.4, 129.7, 130.2, 132.6, 133.8, 135.4 (ArC), 143.6 (C-HA), 157.4 (C-5), 159.2 (C-2) ppm. MS (m/z): 568.51 (M+·). Anal. Calcd. for C18H15Cl2N3O6S4: C 38.02, H 2.65, N 7.39; found C 38.41, H 2.84, N 7.70.
2.3 Typical procedure for the synthesis of N′-(2-((arylsulfonyl)aminosulfonyl)acetyl)-2-(styrylsulfonyl)acetohydrazide 5a–c
The Z-styrylsulfonylacetic acid (2) (1.0 mmol) was dissolved in dry N,N-Dimethylformamide (DMF) (10 mL). To this, O-(7-Azabenzotriazole-1-yl)-N,N,N′,N′-tetramethyluroniumhexafluorophosphate (HATU), (0.38 g, 1.0 mmol) was added at room temperature and stirred for 10 min. Then 2-((arylsulfonyl)aminosulfonyl)acetohydrazide (1) (2.0 mmol) was added and stirred for 20 min followed by N,N-Diisopropylethylamine (DIPEA) (0.52 mL, 3.0 mmol) via syringe. The reaction mixture was further stirred for 22–25 h and then saturated solution of sodium chloride was added. The mixture was cooled to 4 °C and the separated precipitate was isolated by vacuum filtration over sintered glass, washed with deionized water and recrystallized from 2-propanol.
2.3.1 N′-(2-((Phenylsulfonyl)aminosulfonyl)acetyl)-2-(styrylsulfonyl)acetohydrazi-de (5a)
Yield 94% as a white solid (0.47 g). M.p. 149–151 °C. IR (KBr): ν = 3264 (NH), 1682 (C⚌O), 1618 (C⚌C), 1316, 1154 (SO2) cm−1. 1H NMR (DMSO-d6): δ = 5.30 (s, 2H, CH2–CONH), 5.46 (s, 2H, CH2–SO2NH), 6.84 (d, 1H, HB, J = 9.7 Hz), 7.41–7.94 (m, 11H, HA, ArH), 8.24 (bs, 1H, CH2CO–NH), 8.82 (bs, 1H, NH–COCH2), 10.44 (bs, 1H, NH–SO2Ar) ppm. 13C NMR (DMSO-d6): δ = 51.8 (CH2–SO2NH), 53.4 (CH2–CONH), 125.2 (C-HB), 125.7, 126.6, 127.4, 128.8, 130.2, 131.5, 132.7, 133.8 (ArC), 137.2 (C-HA), 168.4 (NH–CO), 170.2 (CO–NH) ppm. MS (m/z): 501.56 (M+·). Anal. Calcd. for C18H19N3O8S3: C 43.11, H 3.82, N 8.38; found C 43.59, H 3.99, N 8.64.
2.3.2 N′-(2-((4-Methylphenylphenylsulfonyl)aminosulfonyl)acetyl)-2-(4-methyl-styrylsulfonyl)acetohydrazide (5b)
Yield 90% as a white solid (0.46 g). M.p. 121–123 °C. IR (KBr): ν = 3256 (NH), 1676 (C⚌O), 1610 (C⚌C), 1312, 1148 (SO2) cm−1. 1H NMR (DMSO-d6): δ = 2.41 and 2.54 (s, 6 H, Ar–CH3), 5.27 (s, 2H, CH2–CONH), 5.40 (s, 2H, CH2–SO2NH), 6.79 (d, 1H, HB, J = 9.6 Hz), 7.32–7.92 (m, 9 H, HA, ArH), 7.98 (bs, 1H, CH2CO–NH), 8.75 (bs, 1H, NH–COCH2), 10.46 (bs, 1H, NH–SO2Ar) ppm. 13C NMR (DMSO-d6): δ = 22.6 and 23.8 (Ar–CH3), 50.2 (CH2–SO2NH), 52.6 (CH2–CONH), 124.3 (C-HB), 124.6, 125.5, 126.2, 127.5, 128.4, 130.1, 131.3, 132.6 (ArC), 136.8 (C-HA), 165.9 (NH–CO), 169.4 (CO–NH) ppm. MS (m/z): 529.61 (M+·). Anal. Calcd. for C20H23N3O8S3: C 45.36, H 4.38, N 7.93; found C 45.41, H 4.49, N 7.98.
2.3.3 N′-(2-((4-Chlorophenylphenylsulfonyl)aminosulfonyl)acetyl)-2-(4-chloro-styrylsulfonyl)acetohydrazide (5c)
Yield 92% as a white solid (0.52 g). M.p. 162–164 °C. IR (KBr): ν = 3276 (NH), 1694 (C⚌O), 1621 (C⚌C), 1321, 1159 (SO2) cm−1. 1H NMR (DMSO-d6): δ = 5.34 (s, 2H, CH2–CONH), 5.52 (s, 2H, CH2–SO2NH), 6.92 (d, 1H, HB, J = 9.7 Hz), 7.46–7.98 (m, 9 H, HA, ArH), 8.38 (bs, 1H, CH2CO–NH), 8.85 (bs, 1H, NH–COCH2), 10.49 (bs, 1H, NH–SO2Ar) ppm. 13C NMR (DMSO-d6): δ = 52.4 (CH2–SO2NH), 54.8 (CH2–CONH), 125.8 (C-HB), 126.4, 127.2, 128.7, 129.6, 130.4, 131.2, 133.4, 134.1 (ArC), 138.1 (C-HA), 168.9 (NH–CO), 170.8 (CO–NH) ppm. MS (m/z): 570.45 (M+·). Anal. Calcd. for C18H17Cl2N3O8S3: C 37.90, H 3.00, N 7.37; found C 38.21, H 3.51, N 7.78.
2.4 Typical procedure for the synthesis 2-(((arylsulfonyl)aminosulfonyl)methyl)-5-((4′,5′-dihydro-1′,3′-diphenyl-5′-aryl-1′H-pyrazol-4′-ylsulfonyl)methyl)-1,3,4-thiadiazole 6a–c
The compound 3 (1.0 mmol), benzaldehyde phenylhydrazone (1.2 mmol), chloramine-T (0.33 g, 1.2 mmol) and methanol (20 mL) were refluxed for 23–25 h. The precipitated inorganic salts were filtered off. The filtrate was concentrated and the residue was extracted with dichloromethane. The organic layer was washed with water, brine and dried (an. Na2SO4). Evaporation of the solvent under reduced pressure yielded a solid which was purified by column chromatography (silica gel, 60–120 mesh) using hexane/EtOAc (4:1) as eluent.
2.4.1 2-(((Phenylsulfonyl)aminosulfonyl)methyl)-5-((4′,5′-dihydro-1′,3′-diphenyl-5′-phenyl-1′H-pyrazol-4′-ylsulfonyl)methyl)-1,3,4-thiadiazole (6a)
Yield 73% as a pale yellow solid (0.50 g). M.p. 189–191 °C. IR (KBr): ν = 3230 (NH), 1563 (C⚌N), 1318, 1147 (SO2) cm−1. 1H NMR (DMSO-d6): δ = 4.68 (s, 2H, CH2-(C-5)), 5.08 (s, 2H, CH2-(C-2)), 5.15 (d, 1H, C4′-H, J = 7.1 Hz), 5.30 (d, 1H, C5′-H, J = 7.2 Hz), 7.20–7.72 (m, 20 H, ArH), 10.42 (bs, 1H, NH) ppm. 13C NMR (DMSO-d6): δ = 46.2 (CH2-(C-5)), 51.1 (CH2-(C-2)), 64.1 (C-4′), 82.5 (C-5′), 123.6, 124.3, 125.5, 126.1, 127.3, 128.2, 130.1, 131.3, 132.4, 132.8, 133.2, 133.9, 134.2, 134.8, 136.4, 138.2 (ArC), 153.4 (C-3′), 155.8 (C-5), 156.8 (C-2) ppm. MS (m/z): 693.84 (M+·). Anal. Calcd. for C31H27N5O6S4: C 53.66, H 3.92, N 10.09; found C 53.82, H 4.21, N 10.20.
2.4.2 2-(((4-Methylphenylsulfonyl)aminosulfonyl)methyl)-5-((4′,5′-dihydro-1′,3′-diphenyl-5′-(4-methylphenyl)-1′H-pyrazol-4′-ylsulfonyl)methyl)-1,3,4-thiadiazole (6b)
Yield 67% as a pale yellow solid (0.48 g). M.p. 204–206 °C. IR (KBr): ν = 3226 (NH), 1555 (C⚌N), 1311, 1135 (SO2) cm−1. 1H NMR (DMSO-d6): δ = 2.19 & 2.36 (s, 6 H, Ar–CH3), 4.62 (s, 2H, CH2-(C-5)), 4.98 (s, 2H, CH2-(C-2)), 5.09 (d, 1H, C4′-H, J = 6.8 Hz), 5.24 (d, 1H, C5′-H, J = 6.8 Hz), 7.12–7.58 (m, 18 H, ArH), 10.38 (bs, 1H, NH) ppm. 13C NMR (DMSO-d6): δ = 21.9 & 22.3 (Ar–CH3), 45.6 (CH2-(C-5)), 51.8 (CH2-(C-2)), 62.8 (C-4′), 81.1 (C-5′), 122.3, 123.2, 124.5, 125.5, 126.1, 126.6, 127.5, 128.4, 130.2, 131.6, 132.9, 133.2, 133.8, 134.6, 135.3, 137.4 (ArC), 151.8 (C-3′), 154.1 (C-5), 155.6 (C-2) ppm. MS (m/z): 721.89 (M+·). Anal. Calcd. for C33H31N5O6S4: C 54.90, H 4.32, N 9.70; found C 55.24, H 4.69, N 9.91.
2.4.3 2-(((4-Chlorophenylsulfonyl)aminosulfonyl)methyl)-5-((4′,5′-dihydro-1′,3′-diphenyl-5′-(4-chlorophenyl)-1′H-pyrazol-4′-ylsulfonyl)methyl)-1,3,4-thiadiazole (6c)
Yield 79% as a pale yellow solid (0.60 g). M.p. 218–220 °C. IR (KBr): ν = 3235 (NH), 1567 (C⚌N), 1327, 1151 (SO2) cm−1. 1H NMR (DMSO-d6): δ = 4.79 (s, 2H, CH2-(C-5)), 5.13 (s, 2H, CH2-(C-2)), 5.22 (d, 1H, C4′-H, J = 7.4 Hz), 5.33 (d, 1H, C5′-H, J = 7.3 Hz), 7.25–7.72 (m, 18 H, ArH), 10.45 (bs, 1H, NH) ppm. 13C NMR (DMSO-d6): δ = 47.8 (CH2-(C-5)), 52.2 (CH2-(C-2)), 66.6 (C-4′), 84.2 (C-5′), 124.4, 125.6, 126.1, 127.2, 128.8, 130.2, 131.7, 132.3, 132.9, 134.2, 135.1, 136.5, 137.2, 138.6, 139.2, 139.9 (ArC), 154.6 (C-3′), 156.3 (C-5), 158.2 (C-2) ppm. MS (m/z): 762.74 (M+·). Anal. Calcd. for C31H25Cl2N5O6S4: C 48.81, H 3.30, N 9.18; found C 49.01, H 3.54, N 9.26.
2.5 Typical procedure for the synthesis of 2-(((arylsulfonyl)aminosulfonyl)methyl)-5-((4′,5′-dihydro-3′-phenyl-5′-arylisoxazol-4′-ylsulfonyl)methyl)-1,3,4-thiadiazole 7a–c
A mixture of compound 3 (1.0 mmol), benzaldehyde oxime (1.2 mmol), chloramine-T (0.33 g, 1.2 mmol) and methanol (20 mL) was refluxed for 17–20 h. The precipitated inorganic salts were filtered off. The filtrate was concentrated and the residue was extracted with dichloromethane. The organic layer was washed with water, brine and dried (an. Na2SO4). The solvent was removed under vacuum. The resultant residue was purified by column chromatography (silica gel, 60–120 mesh) using hexane/EtOAc (4:1) as eluent.
2.5.1 2-(((Phenylsulfonyl)aminosulfonyl)methyl)-5-((4′,5′-dihydro-3′-phenyl-5′-phenylisoxazol-4′-ylsulfonyl)methyl)-1,3,4-thiadiazole (7a)
Yield 74% as a white solid (0.45 g). M.p. 174–176 °C. IR (KBr): ν = 3240 (NH), 1565 (C⚌N), 1326, 1132 (SO2) cm−1. 1H NMR (DMSO-d6): δ = 4.88 (s, 2H, CH2-(C-5)), 5.08 (d, 1H, C4′-H, J = 7.5 Hz), 5.14 (s, 2H, CH2-(C-2)), 5.41 (d, 1H, C5′-H, J = 7.5 Hz), 7.21–7.80 (m, 15 H, ArH), 10.50 (bs, 1H, NH) ppm. 13C NMR (DMSO-d6): δ = 47.4 (CH2-(C-5)), 52.8 (CH2-(C-2)), 63.4 (C-4′), 84.4 (C-5′), 125.6, 126.4, 127.3, 128.6, 129.2, 130.4, 131.1, 132.1, 132.8, 133.6, 134.3, 136.2 (ArC), 154.2 (C-3′), 156.2 (C-5), 157.4 (C-2) ppm. MS (m/z): 618.73 (M+·). Anal. Calcd. for C25H22N4O7S4: C 48.53, H 3.58, N 9.05; found C 48.61, H 3.72, N 9.09.
2.5.2 2-(((4-Methylphenylsulfonyl)aminosulfonyl)methyl)-5-((4′,5′-dihydro-3′-phenyl-5′-(4-methylphenyl)isoxazol-4′-ylsulfonyl)methyl)-1,3,4-thiadiazole (7b)
Yield 68% as a white solid (0.43 g). M.p. 196–198 °C. IR (KBr): ν = 3236 (NH), 1560 (C⚌N), 1321, 1124 (SO2) cm−1. 1H NMR (DMSO-d6): δ = 2.26 & 2.38 (s, 6 H, Ar–CH3), 4.80 (s, 2H, CH2-(C-5)), 5.01 (d, 1H, C4′-H, J = 7.1 Hz), 5.06 (s, 2H, CH2-(C-2)), 5.36 (d, 1H, C5′-H, J = 7.1 Hz), 7.18–7.73 (m, 13 H, ArH), 10.47 (bs, 1H, NH) ppm. 13C NMR (DMSO-d6): δ = 22.4 & 23.9 (Ar–CH3), 46.2 (CH2-(C-5)), 51.4 (CH2-(C-2)), 61.8 (C-4′), 83.6 (C-5′), 123.8, 124.2, 126.4, 127.2, 128.5, 129.3, 130.5, 131.1, 132.4, 133.6, 134.2, 135.4 (ArC), 152.2 (C-3′), 155.6 (C-5), 156.3 (C-2) ppm. MS (m/z): 646.79 (M+·). Anal. Calcd. for C27H26N4O7S4: C 50.14, H 4.05, N 8.66; found C 50.08, H 3.95, N 8.62.
2.5.3 2-(((4-Chlorophenylsulfonyl)aminosulfonyl)methyl)-5-((4′,5′-dihydro-3′-phenyl-5′-(4-chlorophenyl)isoxazol-4′-ylsulfonyl)methyl)-1,3,4-thiadiazole (7c)
Yield 78% as a white solid (0.53 g). M.p. 210–212 °C. IR (KBr): ν = 3250 (NH), 1573 (C⚌N), 1332, 1139 (SO2) cm−1. 1H NMR (DMSO-d6): δ = 4.92 (s, 2H, CH2-(C-5)), 5.14 (d, 1H, C4′-H, J = 7.7 Hz), 5.18 (s, 2H, CH2-(C-2)), 5.48 (d, 1H, C5′-H, J = 7.6 Hz), 7.26–7.89 (m, 13 H, ArH), 10.52 (bs, 1H, NH) ppm. 13C NMR (DMSO-d6): δ = 48.2 (CH2-(C-5)), 53.6 (CH2-(C-2)), 64.5 (C-4′), 84.7 (C-5′), 125.9, 126.4, 127.6, 128.5, 129.8, 130.4, 131.8, 133.6, 134.2, 135.3, 136.6, 137.4 (ArC), 154.9 (C-3′), 157.9 (C-5), 158.8 (C-2) ppm. MS (m/z): 687.62 (M+·). Anal. Calcd. for C25H20Cl2N4O7S4: C 43.67, H 2.93, N 8.14; found C 44.01, H 3.41, N 8.37.
2.6 Typical procedure for the synthesis of 2-(((arylsulfonyl)aminosulfonyl)methyl)-5-((1′,3′-diphenyl-5′-aryl-1′H-pyrazol-4′-ylsulfonyl)methyl)-1,3,4-thiadiazole 8a–c/2-(((arylsulfonyl)aminosulfonyl)methyl)-5-((3′-phenyl-5′-arylisoxazol-4′-ylsulfonyl)-methyl)-1,3,4-thiadiazole 9a–c
To a solution of compound 6/7 (1.3 mmol) in benzene (50 mL), Manganese dioxide (MnO2) (0.43 g, 5.0 mmol) was added and stirred for 6–8 h at room temperature. After completion of the reaction, the reaction mixture was filtered. Removal of the solvent under reduced pressure resulted in a residue which was purified by column chromatography (chloroform/EtOAc, 2:1).
2.6.1 2-(((Phenylsulfonyl)aminosulfonyl)methyl)-5-((1′,3′-diphenyl-5′-phenyl-1′H-pyrazol-4′-ylsulfonyl)methyl)-1,3,4-thiadiazole (8a)
Yield 86% as a white solid (0.59 g). M.p. 180–182 °C. IR (KBr): ν = 3246 (NH), 1622 (C⚌C), 1584 (C⚌N), 1330, 1145 (SO2) cm−1. 1H NMR (DMSO-d6): δ = 5.05 (s, 2H, CH2-(C-5)), 5.28 (s, 2H, CH2-(C-2)), 7.12–7.76 (m, 20 H, ArH), 10.58 (bs, 1H, NH) ppm. 13C NMR (DMSO-d6): δ = 45.4 (CH2-(C-5)), 48.9 (CH2-(C-2)), 122.7, 123.8, 124.2, 125.9, 126.4, 127.3, 128.6, 129.2, 130.4, 131.7, 132.4, 133.5, 134.1, 135.8, 136.4, 137.2 (ArC), 137.8 (C-4′), 144.2 (C-3′), 147.2 (C-5′), 153.1 (C-5), 154.6 (C-2) ppm. MS (m/z): 691.83 (M+·). Anal. Calcd. for C31H25N5O6S4: C 53.81, H 3.64, N 10.12; found C 53.86, H 3.71, N 10.47.
2.6.2 2-(((4-Methylphenylsulfonyl)aminosulfonyl)methyl)-5-((1′,3′-diphenyl-5′-(4-methylphenyl)-1′H-pyrazol-4′-ylsulfonyl)methyl)-1,3,4-thiadiazole (8b)
Yield 83% as a white solid (0.58 g). M.p. 193–195 °C. IR (KBr): ν = 3238 (NH), 1615 (C⚌C), 1572 (C⚌N), 1324, 1138 (SO2) cm−1. 1H NMR (DMSO-d6): δ = 2.20 & 2.34 (s, 6 H, Ar–CH3), 5.01 (s, 2H, CH2-(C-5)), 5.20 (s, 2H, CH2-(C-2)), 7.06–7.54 (m, 18 H, ArH), 10.54 (bs, 1H, NH) ppm. 13C NMR (DMSO-d6): δ = 21.4 & 22.6 (Ar–CH3), 44.8 (CH2-(C-5)), 49.6 (CH2-(C-2)), 122.1, 122.9, 123.4, 124.3, 125.5, 125.9, 126.4, 127.2, 128.6, 129.3, 130.6, 132.7, 133.2, 134.6, 135.4, 136.2 (ArC), 136.4 (C-4′), 142.6 (C-3′), 145.4 (C-5′), 152.8 (C-5), 155.2 (C-2) ppm. MS (m/z): 719.88 (M+·). Anal. Calcd. for C33H29N5O6S4: C 55.05, H 4.06, N 9.72; found C 55.34, H 4.09, N 10.01.
2.6.3 2-(((4-Chlorophenylsulfonyl)aminosulfonyl)methyl)-5-((1′,3′-diphenyl-5′-(4-chlorophenyl)-1′H-pyrazol-4′-ylsulfonyl)methyl)-1,3,4-thiadiazole (8c)
Yield 88% as a white solid (0.66 g). M.p. 225–227 °C. IR (KBr): ν = 3254 (NH), 1626 (C⚌C), 1590 (C⚌N), 1335, 1149 (SO2) cm−1. 1H NMR (DMSO-d6): δ = 5.11 (s, 2H, CH2-(C-5)), 5.32 (s, 2H, CH2-(C-2)), 7.22–7.94 (m, 18 H, ArH), 10.60 (bs, 1H, NH) ppm. 13C NMR (DMSO-d6): δ = 46.2 (CH2-(C-5)), 50.4 (CH2-(C-2)), 124.3, 124.6, 125.9, 126.3 127.6 128.5, 129.8, 130.5, 131.6 132.3, 133.6, 134.2, 135.4, 136.6, 137.2, 138.1 (ArC), 138.6 (C-4′), 146.3 (C-3′), 149.6 (C-5′), 153.8 (C-5), 157.8 (C-2) ppm. MS (m/z): 760.72 (M+·). Anal. Calcd. for C31H23 Cl2N5O6S4: C 48.94, H 3.04, N 9.21; found C 49.15, H 3.37, N 9.08.
2.6.4 2-(((Phenylsulfonyl)aminosulfonyl)methyl)-5-((3′-phenyl-5′-phenylisoxazol-4′-ylsulfonyl)methyl)-1,3,4-thiadiazole (9a)
Yield 85% as a white solid (0.52 g). M.p. 186–188 °C. IR (KBr): ν = 3256 (NH), 1642 (C⚌C), 1580 (C⚌N), 1342, 1160 (SO2) cm−1. 1H NMR (DMSO-d6): δ = 5.28 (s, 2H, CH2-(C-5)), 5.39 (s, 2H, CH2-(C-2)), 7.30–7.82 (m, 15 H, ArH), 10.62 (bs, 1H, NH) ppm. 13C NMR (DMSO-d6): δ = 46.8 (CH2-(C-5)), 50.6 (CH2-(C-2)), 125.7, 126.2, 127.4, 128.8, 129.2, 131.4, 132.8, 133.5, 134.2, 135.6, 136.1, 136.8 (ArC), 138.2 (C-4′), 145.6 (C-3′), 150.2 (C-5′), 155.2 (C-5), 156.1 (C-2) ppm. MS (m/z): 616.72 (M+·). Anal. Calcd. for C25H20N4O7S4: C 48.68, H 3.26, N 9.08; found C 48.83, H 3.84, N 9.22.
2.6.5 2-(((4-Methylphenylsulfonyl)aminosulfonyl)methyl)-5-((3′-phenyl-5′-(4-methylphenyl)isoxazol-4′-ylsulfonyl)methyl)-1,3,4-thiadiazole (9b)
Yield 82% as a white solid (0.52 g). M.p. 197–199 °C. IR (KBr): ν = 3252 (NH), 1638 (C⚌C), 1582 (C⚌N), 1335, 1152 (SO2) cm−1. 1H NMR (DMSO-d6): δ = 2.24 & 2.41 (s, 6 H, Ar–CH3), 5.16 (s, 2H, CH2-(C-5)), 5.35 (s, 2H, CH2-(C-2)), 7.22–7.71 (m, 13 H, ArH), 10.55 (bs, 1H, NH) ppm. 13C NMR (DMSO-d6): δ = 22.1 & 23.4 (Ar–CH3), 44.6 (CH2-(C-5)), 50.2 (CH2-(C-2)), 124.5, 125.2, 126.4, 127.8, 128.7, 129.4, 130.6, 132.9, 133.4, 134.2, 135.6, 136.2 (ArC), 137.6 (C-4′), 143.5 (C-3′), 149.4 (C-5′), 154.4 (C-5), 155.8 (C-2) ppm. MS (m/z): 644.77 (M+·). Anal. Calcd. for C27H24N4O7S4: C 50.29, H 3.75, N 8.68; found C 50.38, H 3.61, N 8.74.
2.6.6 2-(((4-Chlorophenylsulfonyl)aminosulfonyl)methyl)-5-((3′-phenyl-5′-(4-chlorophenyl)isoxazol-4′-ylsulfonyl)methyl)-1,3,4-thiadiazole (9c)
Yield 90% as a white solid (0.61 g). M.p. 206–208 °C. IR (KBr): ν = 3260 (NH), 1651 (C⚌C), 1585 (C⚌N), 1350, 1165 (SO2) cm−1. 1H NMR (DMSO-d6): δ = 5.37 (s, 2H, CH2-(C-5)), 5.45 (s, 2H, CH2-(C-2)), 7.28–7.89 (m, 13 H, ArH), 10.64 (bs, 1H, NH) ppm. 13C NMR (DMSO-d6): δ = 47.2 (CH2-(C-5)), 51.6 (CH2-(C-2)), 126.7, 127.4, 128.2, 129.6, 130.3, 131.9, 133.2, 134.8, 135.4, 136.2, 137.6, 138.1 (ArC), 138.8 (C-4′), 147.2 (C-3′), 152.6 (C-5′), 155.9 (C-5), 156.8 (C-2) ppm. MS (m/z): 685.61 (M+·). Anal. Calcd. for C25H18Cl2N4O7S4: C 43.80, H 2.64, N 8.17; found C 44.31, H 2.92, N 8.39.
3 Results and discussion
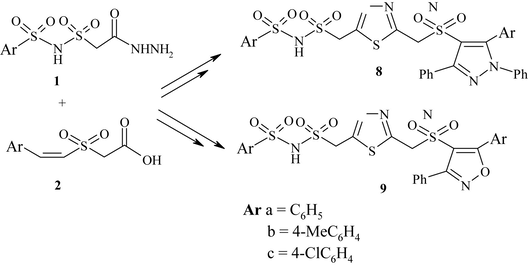
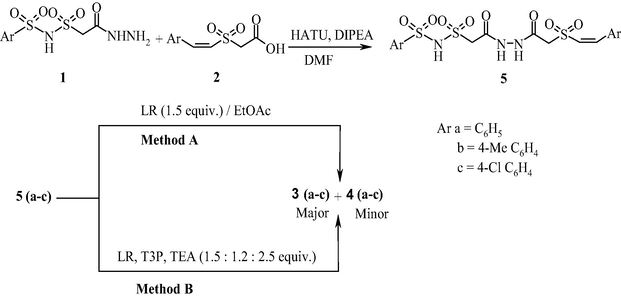
The bis heterocycles, pyrazolyl and isoxazolyl 1,3,4-thiadiazoles were synthesized from the synthetic intermediate 2-(((arylsulfonyl)aminosulfonyl)methyl)-5-[Z-(styrylsulfonyl) methyl]-1,3,4-thiadiazole (3). The compound 3 was prepared in different routes in order to optimize the yield. Earlier 1,3,4-thiadiazole moiety was built by the exchange of oxygen atom in oxadiazole with sulfur (Padmaja et al., 2012). However this transformation failed when 2-(((arylsulfonyl)aminosulfonyl)methyl)-5-((styrylsulfonyl)methyl)-1,3,4-oxadiazole was treated with thiourea. In order to prepare compound 3, initially one-pot reaction of 2-((phenylsulfonyl)aminosulfonyl)acetohydrazide (1a) and Z-styrylsulfonylacetic acid (2a) with P2S5 in the presence of T3P and TEA in equimolar ratio was carried out (Scheme 1). After heating for 5 h, the product 2-(((phenylsulfonyl)aminosulfonyl)methyl)-5-[Z-(styrylsulfonyl)methyl]-1,3,4-thiadiazole (3a) was isolated in 35% yield along with the by product 2-(((phenylsulfonyl)aminosulfonyl)methyl)-5-[Z-(styrylsulfonyl)methyl]-1,3,4-oxadiazole (4a) in 20% yield. In the literature, it was reported that the reaction of 1 equivalent of 4-toluic acid and 3-fluorophenylhydrazide with 1.5 equivalents of P2S5 in the presence of 1.2 equivalents of T3P and 2.5 equivalents of TEA gave the desired 1,3,4-thiadiazole in 92% yield (Augustine et al., 2009). Employing similar reaction conditions, we repeated the reaction of 1a and 2a with 1.5 equivalents of P2S5, 1.2 equivalents of T3P and 2.5 equivalents of TEA but there was no appreciable increase in the yield of compound 3 (Table 1). Later a two-step route via benzohydrazide followed by cyclization was adopted. The hydrazide, N′-(2-((arylsulfonyl)aminosulfonyl)acetyl)-2-(styrylsulfonyl)acetohydrazide (5) was prepared by the reaction of 1 with 2 in the presence of HATU and DIPEA in DMF. The compound 5 was obtained in almost quantitative yields. The compound 5a was heated with 1.5 equivalents of LR in EtOAc for 8 h. The product 3a was formed in 55% along with 1,3,4-oxadiazole (4a) in 19% yield (Method A). However, when the same reaction was performed with 1.5 equivalents of LR, 1.2 equivalents of T3P and 2.5 equivalents of TEA compound 3a was obtained in 85% yield along with a minor amount of 4a in 2% yield (Method B). Adopting similar methodology, the compounds 3b and 3c were prepared (Scheme 2 and Table 2).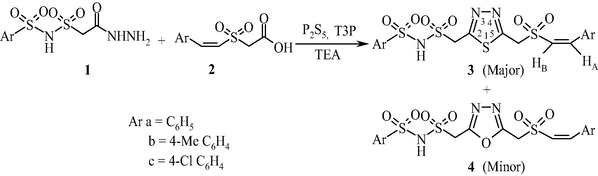
Single-step synthesis of 1,3,4-thiadiazoles.
Entry
Reagent (equivalents)
Ar
Product 3
Product 4
P2S5:T3P:TEA
Yield (%)
mp (°C)
Yield (%)
mp (°C)
1
1:1:1
C6H5
35
157–159
20
128–130
2
1.5:1.2:2.5
C6H5
39
157–159
18
128–130
3
1.5:1.2:2.5
4-Me C6H4
36
135–137
23
115–117
4
1.5:1.2:2.5
4-Cl C6H4
38
171–173
21
139–141
Two-step synthesis of 1,3,4-thiadiazoles.
Entry
Ar
Method A, Yield (%)
Method B, Yield (%)
Product 3
Product 4
Product 3
Product 4
1
C6H5
55
19
85
2
2
4-Me C6H4
52
14
90
2
3
4-Cl C6H4
49
16
88
3
The (3+2) π 1,3-dipolar cycloaddition reaction of dipolar reagents to Michael acceptors is a simple and a facile technique to prepare five-membered heterocycles. The olefin moiety present in compound 3 was exploited to synthesize pyrazoles and isoxazoles by cycloaddition of dipolar reagents—nitrile imines and nitrile oxides. Thus, 2-(((arylsulfonyl)amino-sulfonyl)methyl)-5-((4′,5′-dihydro-1′,3′-diphenyl-5′-aryl-1′H-pyrazol-4′-ylsulfonyl)methyl)-1,3,4-thiadiazole (6) and 2-(((arylsulfonyl)amino-sulfonyl)methyl)-5-((4′,5′-dihydro-3′-phenyl-5′-arylisoxazol-4′-ylsulfonyl)methyl)-1,3,4-thiadiazole (7) were prepared by cycloaddition of nitrile imine and nitrile oxide generated from benzaldehyde phenylhydrazone and benzaldoxime in the presence of chloramine-T to 3 (Scheme 3). In 1,2-disubstituted ethylenes bearing two vicinal electron withdrawing substituents a regioisomeric mixture of cycloadducts is expected. However, the reaction of 1-aryl-2-aroylethylenes with diazomethane and its derivatives produced exclusively 3-aroyl-4-aryl-2-pyrazolines (Bhaskar Reddy et al., 1986). Similarly, the cycloaddition of nitrile imines and nitrile oxides to 1,4-bis-((E)-2(aroylsulfonyl)vinyl)benzene gave pyrazolines and isoxazolines. In the present study, the addition of nitrile imines and nitrile oxides to 3 resulted in only one pure regioisomer 6 and 7 respectively. A small amount of the other isomer if any, formed could not be isolated by this process. There are several methods for oxidation of pyrazolines and isoxazolines to the corresponding aromatized products (Padmaja et al., 2011a,b; Srivastava et al., 2003). We focused our attention toward the transformation of pyrazolines and isoxazolines to pyrazoles and isoxazoles adopting the oxidation protocol using activated MnO2 to achieve better yields. In fact, the oxidation of compounds 6 and 7 with MnO2 in benzene led to the formation of 2-(((arylsulfonyl)aminosulfonyl)methyl)-5-((1′,3′-diphenyl-5′-aryl-1′H-pyrazol-4′-ylsulfonyl)methyl)-1,3,4-thiadiazole (8)/2-(((arylsulfonyl)aminosulfonyl)meth-yl)-5-((3′-phenyl-5′-arylisoxazol-4′-ylsulfonyl)methyl)-1,3,4-thiadiazole (9) in 80–90% yield (Table 3). All the new compounds (3, 5, 6–9) were characterized by IR, 1H NMR, 13C NMR, mass and elemental analyses.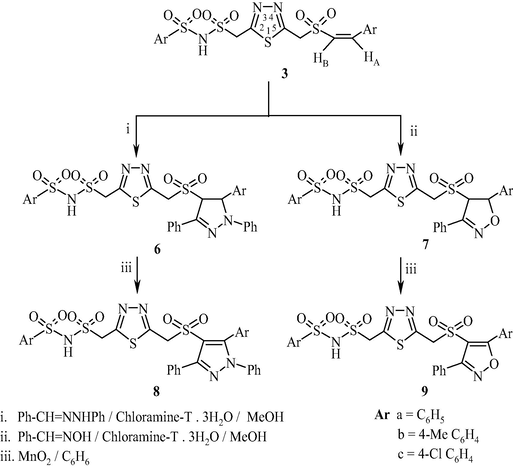
Synthesis of bis heterocycles.
Entry
Compound
Yield
Entry
Compound
Yield
1
6a
73
7
8a
86
2
6b
67
8
8b
83
3
6c
79
9
8c
88
4
7a
74
10
9a
85
5
7b
68
11
9b
82
6
7c
78
12
9c
90
4 Conclusion
The compounds styryl 1,3,4-thiadiazoles were prepared from different routes to optimize the yield of the products. The two-step methodology via benzohydrazide followed by treatment with Lawesson’s reagent in the presence of Propylphosphonic anhydride and triethylamine produced styryl 1,3,4-thiadiazoles in excellent yields. The olefin moiety in these compounds is utilized to develop pyrazole and isoxazole rings by 1,3-dipolar cycloaddition methodology followed by oxidation.
Acknowledgments
One of the authors (A. Padmaja) is grateful to COUNCIL OF SCIENTIFIC AND INDUSTRIAL RESEARCH (CSIR), New Delhi, for financial assistance under major research project (Sanction Lr. No. 01(2439)/10/EMR-II dated 28.12.2010).
References
- Tetrahedron. 2009;65:9989-9996.
- Acta Chim. Hungarica. 1986;122:19-24.
- Eur. J. Med. Chem.. 2011;46:3158-3166.
- Synlett. 2009;14:2328-2332.
- J. Org. Chem.. 1995;60:3112-3120.
- Eur. J. Org. Chem. 2010:6440-6447.
- Tetrahedron Lett.. 2007;48:1295-1298.
- Bioorg. Med. Chem.. 2002;10:2893-2898.
- Il Farmaco. 2005;60:513-517.
- Eur. J. Med. Chem.. 2003;38:851-854.
- Org. Prep. Proced. Int.. 2005;37:213-222.
- J. Med. Chem.. 2006;49:6027-6036.
- Chem. Commun.. 2011;47:3198-3200.
- Il Farmaco. 2002;57:577-581.
- J. Org. Chem.. 2001;66:7925-7929.
- Acc. Chem. Res.. 1984;17:410-416.
- Il Farmaco. 2002;57:253-257.
- Eur. J. Org. Chem. 2006:2825-2832.
- Synth. Commun.. 1998;28:4611-4617.
- Bioorg. Med. Chem.. 2008;16:4075-4082.
- Il Farmaco. 1996;51:71-74.
- Eur. J. Med. Chem.. 2009;44:63-69.
- J. Heterocycl. Chem.. 1983;20:1399-1401.
- J. Heterocycl. Chem.. 2005;42:991-994.
- Eur. J. Med. Chem.. 2011;46:5034-5038.
- Chem. Pharm. Bull.. 2011;59:1509-1517.
- J. Chem. Pharm. Res.. 2012;4:294-302.
- Chem. Pharm. Bull.. 2008;56:815-820.
- Chem. Pharm. Bull.. 2009;57:1200-1205.
- Eur. J. Med. Chem.. 2011;46:5317-5326.
- Il Farmaco. 2002;57:101-107.
- Arch. Pharm. Chem. Life Sci.. 2013;346:154-162.
- J. Am. Chem. Soc.. 2011;133:949-957.
- Pharmazie. 1994;49:880-884.
- Bioorg. Med. Chem.. 2006;14:1698-1705.
- Arch. Pharm. Chem. Life Sci.. 2007;340:345-351.
- Bioorg. Med. Chem.. 2003;11:1821-1827.
- Indian J. Chem. Sect. B. 2001;40:15-19.
- Tetrahedron. 2007;63:12195-12201.
- Bioorg. Med. Chem. Lett.. 2003;13:4193-4196.
- Tetrahedron. 2011;67:9618-9621.
- Indian J. Chem. Sect. B. 1998;37:127-131.







