Translate this page into:
An in vitro study on probable inhibition of cerebrovascular disease by salidroside as a potent small molecule against induction of protein amyloid fibrils and cytotoxicity
⁎Corresponding author at: NO.301, Yanchang Middle Road, Jing'an District, Shanghai 200072, China. cjr.limaoquan@vip.163.com (Maoquan Li)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Protein aggregation and associated amyloid formation is linked with several harmful human pathophysiologies including Alzheimer's, Parkinson's, and cerebrovascular diseases. A potential approach for modulating and exploring amyloid fibrillization is the control of protein self-assembly. Herein, anti-aggregation effects of salidroside, its influence on the kinetics of amyloid fibrillization of Aβ1-42 peptide and its cytotoxicity against cerebrovascular endothelial cells (bEnd.3) were assessed by using a wide range of spectroscopic and cellular techniques. The present outcome of Thioflavin T (ThT) and 8-anilino-1-naphthalenesulfonic acid (ANS) fluorescence, Congo red (CR), and circular dichroism (CD) analyses indicated that salidroside potentially inhibits protein fibril formation. The cellular studies inferred that salidroside protects bEnd.3 cells against Aβ1-42 oligomers -triggered cytotoxicity through modulation of oxidative stress [reactive oxygen species (ROS), superoxide dismutase (SOD) and catalase (CAT) activities] and apoptosis (caspase-3 activity). Therefore, the data signifies the role of salidroside as a promising small molecule in inhibiting Aβ1-42 aggregation and associated cerebrovascular endothelial cell toxicity. Hence, salidroside can serve as a potential inhibitor in the therapeutic advancement to combat cerebrovascular diseases.
Keywords
Amyloid β
Salidroside
Cerebrovascular endothelial cell
1 Introduction
Cerebrovascular dysfunction is an important contributor in the protein aggregation-stimulated neurodegenerative disorders (Kamat et al., 2015; Bos et al., 2017). During this process, the blood vessels function in the cerebral cells is downregulated (Smith and Greenberg, 2009). Indeed, several reports have indicated that amyloid plaque formation results in hypoxia-triggered inappropriate blood supply causing improper function in vascular activity (Peers et al., 2009; Zlokovic, 2011). The vascular damage influences the endothelial cells in the vascular system, and amyloid β (Aβ) peptide leads to cerebrovascular endothelial cytotoxicity (Thomas et al., 1997). Aβ-triggered adverse effects cause downregulation of the relaxing mediators in the endothelium (Xi et al., 2012). Therefore, Aβ peptide-stimulated endothelial dysfunction is heavily associated with the vasoconstriction (Smith et al., 2004). In addition, several reports have indicated that different vascular factors are associated with neurodegeneration in neurodegenerative diseases (Iadecola, 2010; Sweeney et al., 2018; Cheng et al., 2020). Structural and molecular alterations in endothelium like the formation of Aβ are known as the key parameters influencing cerebrovascular dysfunction in neurodegenerative diseases (Han et al., 2008).
Structural changes of Aβ with a molecular weight of around 4 kDa can cause its aggregation into amyloid fibrils which become neurotoxic (Maltsev et al., 2011). Aβ peptides are one of the natural products of metabolism, which is composed of 36–43 amino acids, where Aβ1-40 is more common than others. (Maltsev et al., 2011) Aβ peptides are formed by proteolysis of the amyloid precursor protein (APP) by the activity of the secretase family (Maltsev et al., 2011). Most of the processes related to the neurotoxic influences of Aβ are related to type Aβ1-42 (Marshall et al., 2016), which causes damage to synaptic activity. APP is a membrane protein located at the end of presynaptic axons (Portelius et al., 2011). This protein has a large extracellular amine portion and a small intracellular carboxylic portion (Portelius et al., 2011; Vaillant-Beuchot et al., 2021). This part of the precursor protein is a stimulus factor for neuronal survival (Portelius et al., 2011; Vaillant-Beuchot et al., 2021).
Plant extracts and their bioactive compounds regulate the production and accumulation of Aβ peptides associated with neurodegenerative diseases in the laboratory (Kwon et al., 2011). It has been shown that bioactive compounds of plant extracts can prevent the formation of amyloid structures in Aβ peptides (Kumar et al., 2012; Doig and Derreumaux, 2015). The bioactive natural products have long been proven to be powerful anti-amyloid agents. Indeed, many researchers are exploring small molecules as potential inhibitors against protein aggregation in vitro as a starting point and the next step is to formulate these molecules in order to investigate their ability to interfere with the formation of amyloid plaque in vivo (Velander et al., 2017). For this reason, the bioactive compounds should cross the blood brain barrier and prevent the aggregation of Aβ peptides in the brain, especially to interfere with cerebrovascular/neurovegetative diseases-related plaques (Brahmachari et al., 2017). Together, the researchers should assess the new molecule's ability to inhibit the potential toxicity of amyloid plaques containing proteins.
Salidroside as one of the major phenolic glycosides is known as the major bioactive compound extracted from Rhodiola crenulata, has been demonstrated to provide promising influences on inhibiting oxidative stress and cell mortality (Cao et al., 2005; Zuo et al., 2007; Zhang et al., 2009; Ye et al., 2011; Zhu et al., 2011) and has benefit for several disorders including ischemic cardiomyocytes (Zhong et al., 2010), lung damage (Guan et al., 2012) and diabetes (Li et al., 2011). It has been also indicated that salidroside can prevent H2O2 or Aβ-triggered oxidative damage and subsequent apoptosis in neuron-like cells (Cai et al., 2008; Jang et al., 2003) and neuroblastoma cells (Zhang et al., 2010) and hippocampal neurons (Chen et al., 2009). In vivo data have also shown the ability of salidroside to mitigate cerebral ischemia–reperfusion damage by its potential reactive oxygen species (ROS)-scavenging characteristics (Zou et al., 2009; Shi et al., 2012).
Although, it has been shown that salidroside can prevent the Aβ-stimulated neurotoxicity, its anti-amyloid properties against Aβ aggregation and associated cytotoxicity against cerebrovascular endothelial cell line (bEnd.3) have not been well explored in detail. Therefore, in this study as the main novelty of this work we aimed to evaluate the inhibitory characteristics of salidroside against Aβ aggregation and relevant cytotoxicity.
2 Experiments
2.1 Sample preparation
The Aβ1-42 was prepared as explained in literature (Wang et al., 2011; Du et al., 2015 Jan 23) For formation of Aβ1-42 aggregation, 70 μM of protein sample was incubated at 37 °C for 50 h under constant stirring (100 rpm) with and without 35 and 70 μM of salidroside prepared in DMSO based on the previous study (Wang et al., 2011; Du et al., 2015; Alam et al., 2016).
2.2 ThT fluorescence spectroscopy assay
ThT fluorescence analysis was done using Shimadzu fluorescence spectrophotometer (RF-6000). ThT powder was dissolved in double distilled water and filtered. Then, the Aβ1-42 samples co-incubated with or without salidroside, from each sample were taken out at different time intervals and well-mixed with ThT to reach final Aβ1-42 and ThT concentration of 20 μM. The samples were then incubated in dark for 15 min at room temperature followed by excitation at 440 nm and reading the emission spectra at 480 nm with both excitation and emission slit widths of 10 nm. The protein samples were diluted with sodium phosphate buffer (20 mM, pH 7.4) and resulting data were subtracted from associated blanks and were fitted as explained previously (Chaturvedi et al., 2015).
2.3 ANS fluorescence measurements
Aβ1-42 samples with a final concentration of 15 μM were mixed with ANS (15 μM) and incubated in dark for 30 min at room temperature. ANS fluorescence intensity was then read with excitation at 380 nm and emission between 440 and 600 nm. Both excitation and emission slit width were set at 10 nm.
2.4 CR binding analysis
CR was prepared in a phosphate buffer (20 mM, pH 7.4) supplemented with NaCl (50 mM) and filtered and the final concentration was calculated employing εM 45,000 M−1 cm−1 at 495 nm. CR (15 μM) and Aβ1-42 (15 μM) samples were mixed and incubated in the dark for 30 min at room temperature. The CR absorbance spectra (440–650 nm) were read using a UV–Visible spectrophotometer (Perkin Elmer Lambda 25).
2.5 Far-UV CD analysis
The CD study was performed on a J-815-JASCO spectropolarimeter, where the analysis was performed with Aβ1-42 . Signals were read in the range of 260–190 nm using a cuvette with 0.1 cm path length at scanning rate of 100 nm/min.
2.6 Cell culture
bEnd.3 endothelial cells (ATCC, VA, USA) were cultured in Dulbecco's Modified Eagle's Medium (DMEM, #30–2002 from ATCC, VA, USA) supplemented with 10 % FBS (Gibco, UK) and incubated at 37 °C with 5 % CO2 in accordance with the supplier's instruction.
2.7 Cell viability assay
MTT assay was performed to assess the cell viability of bEnd.3. Sample solutions of Aβ1-42 with and without salidroside were added to the bEnd.3 cells for 24 h and MTT assay was done based on the literature (Telli et al., 1999) using a microplate absorbance reader (Bio-Rad). Cell viability was reported statistically significant as compared to control cells. The cells exposed to Aβ1-42 monomer (7 µM) and salidroside (7 µM) were also used to determine the cytotoxicity of native protein and salidroside at the highest studied concentration.
2.8 ROS assay
The level of ROS was quantified by using 2′, 7′-dichlorodihydrofluorescein as a fluorescence probe as determined in the literature (Chen et al., 2010). The fluorescence intensity (λex = 485 nm; λem = 535 nm) was read using the Perkin Elmer Multimode reader (Waltham, USA).
2.9 Superoxide dismutase (SOD), catalase (CAT), and caspase-3 activities assays
After preparation of protein extract and determination of protein concentration in the supernatant by Bradford assay (Jones et al., 1989), 25 µg of protein was used to determine the SOD and CAT activity using the relative kits (Abcam, UK) based on the manufacturer's protocol.
2.10 Statistical analysis
Data were reported as mean ± SD of three independent assays and analyzed by Student t-test to explore the significant differences between samples. P < 0.05 was reported as significant.
3 Results and discussion
3.1 Spectroscopic studies
The kinetic of Aβ1-42 aggregation was explored via ThT fluorescence analysis. The sigmoidal ThT fluorescence plot was detected for Aβ1-42 aggregation with a detectable lag phase followed by fibrillization step and finally a plateau step, indicating a probable nucleation dependent aggregation rate. To analyze the preventive influence of salidroside, Aβ1-42 aggregation was performed in the presence of different concentrations of salidroside. As depicted in Fig. 1a, in addition to an obvious influence on the lag phase, salidroside led to an apparent reduction in the steady-state fluorescence (Fmax) in a concentration-dependent fashion. It can be indicated that Fmax is directly proportional to the level of aggregation and the reduction in the amount of Fmax can be used to determine the inhibitory effect of salidroside (Sabaté et al., 2003). The obvious reduction in the Fmax can propose that salidroside can inhibit the Aβ1-42 aggregation. As demonstrated in Fig. 1a, salidroside with various concentrations of 35 and 70 µM potentially prevented Aβ1-42 aggregation as calculated by about 44 % and 63 % reduction in Fmax, respectively. Moreover, the apparent rate growth constant (kapp) can be regarded as an important indicator of protein aggregation. It was found that the kapp values were 0.331 ± 0.029 h−1, 0.213 ± 0.014 h−1, 0.133 ± 0.011 h−1 upon interaction of Aβ1-42 with various concentrations of salidroside; 0, 35 and 70 µM, respectively.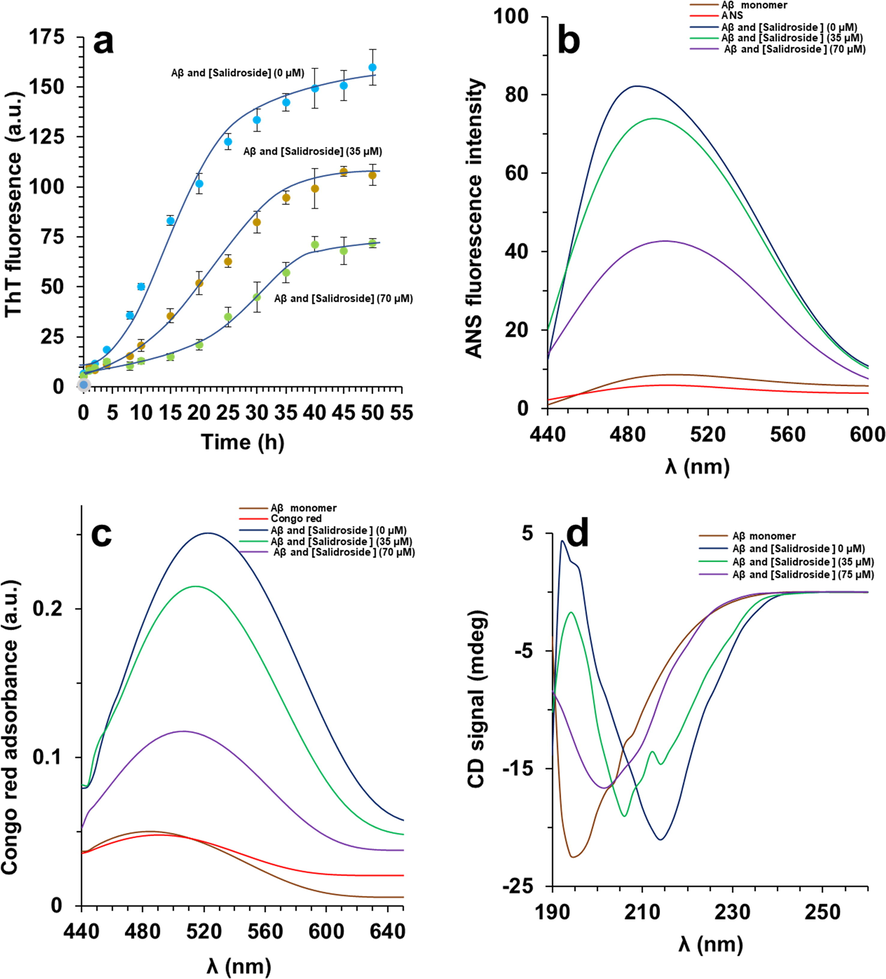
(a) ThT fluorescence assay of Aβ1-42 aggregation with or without salidroside. (b) ANS fluorescence assay of Aβ1-42 aggregated samples with or without salidroside. (c) Congo red absorbance assay of Aβ1-42 aggregated samples with or without salidroside. (d) CD analysis of Aβ1-42 aggregated samples with or without salidroside.
It has been detected that structural alterations of biomolecules occuring upon protein aggregation can be determined by formation of hydrophobic moieties on the protein surface (Sirangelo et al., 1998). The increase in ANS fluorescence examination as a hydrophobic probe proved the appearance of obvious hydrophobic patches on the surface of the Aβ1-42 aggregated species (Fig. 1b). However, the presence of salidroside mitigated the ANS fluorescence intensity accompanied by an apparent red shift, suggesting that salidroside prevented the induction of hydrophobic patches in Aβ1-42 structure. This data in agreement with ThT fluorescence analysis suggested that salidroside potentially inhibited Aβ1-42 aggregation through inhibiting the formation of hydrophobic moieties on the protein.
To further study the inhibitory influence of salidroside against Aβ1-42 aggregation, CR adsorption analysis was carried out. Actually, the influence of salidroside on the Aβ1-42 aggregation was assessed through detecting maximal CR optical density. It was found that salidroside was outstandingly potent in preventing Aβ1-42 aggregation and this protective effect was directly related with the concentration of salidroside, as shown by the obvious decrease and blue shift detected in CR absorbance of Aβ1-42 samples incubated with salidroside (Fig. 1c).
To explore whether secondary structural changes of Aβ1-42 could be prevented by salidroside, far-UV CD technique was employed. As depicted in Fig. 1d, the ellipticity changes of Aβ1-42 after aggregation show an apparent transition from 195 nm to 218 nm, indicating the predominant formation of β-sheet structures (Böhm et al., 1992). Nevertheless, salidroside demonstrated a promising inhibition on secondary structural changes of Aβ1-42 and this aggregation inhibition potency was more pronounced in the case of higher concentration of this small molecule than lower concentrations. Also, it was realized that salidroside does not trigger any conformational changes on the random coil structure of Aβ1-42 monomers (data not shown).
This data also proved that salidroside protects Aβ1-42 against secondary structural changes induced by an aggregation buffer.
Since, Aβ1-42 oligomeric species are cytotoxic and the inhibition of protein aggregation by small molecules like salidroside can mitigate this associated cytotoxicity (Xu et al., 2019), cellular assays were performed to further support the spectroscopic data.
For the cellular assay, Aβ1-42 samples diluted to a final concentration of 7 µM with corresponding salidroside concentrations of 3.5 µM and 7 µM.
3.2 MTT assay
The cytotoxicity of different Aβ1-42 samples against bEnd.3 cells for 24 h was explored via MTT assay (Fig. 2). It was seen that not only salidroside does not induce a significant cytotoxicity, but also the toxicity of Aβ1-42 oligomeric species aged for 25 h with salidroside against bEnd.3 cells was remarkably lower than that of Aβ1-42 aggregated species alone, and this protective effect was more pronounced in the case of higher concentration of this small molecule than lower concentration. This outcome indicated that the salidroside as a biocompatible compound can prevent the Aβ1-42 aggregation and associated cytotoxicity.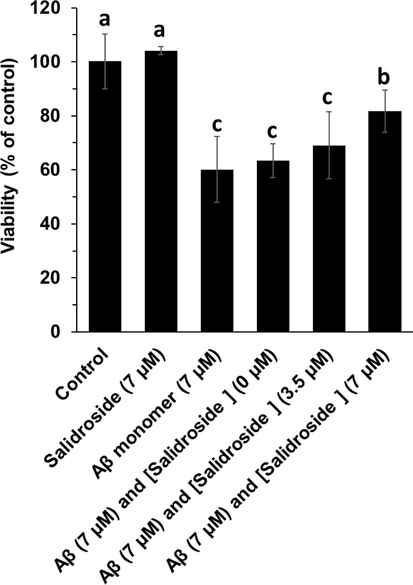
MTT assay of bEnd. 3 cells after incubation with different Aβ1-42 samples (aged for 25 h) for 24 h. a, b, and c letters show the significance (P < 0.05).
3.3 Oxidative stress assay
The effect of different Aβ1-42 samples alone or with salidroside on generation of intracellular ROS and SOD and CAT activity was assessed to examine the protective effect of salidroside against Aβ1-42 oligomeric samples-induced oxidative stress in bEnd.3 cells after 24 h (Fig. 3). It was determined that Aβ1-42 oligomers stimulated a significant enhancement in the generation of intracellular ROS (Fig. 3a), and reduction in the SOD activity (Fig. 3b) and CAT activity (Fig. 3c) in bEnd. 3 cells. Nevertheless, treatment of cells with Aβ1-42 oligomers co-incubated with salidroside reduced the generation of ROS and recovered the SOD and CAT activity. Therefore, it was deduced that the level of oxidative stress induced by Aβ1-42 oligomers can be mitigated upon treatment of cells with salidroside.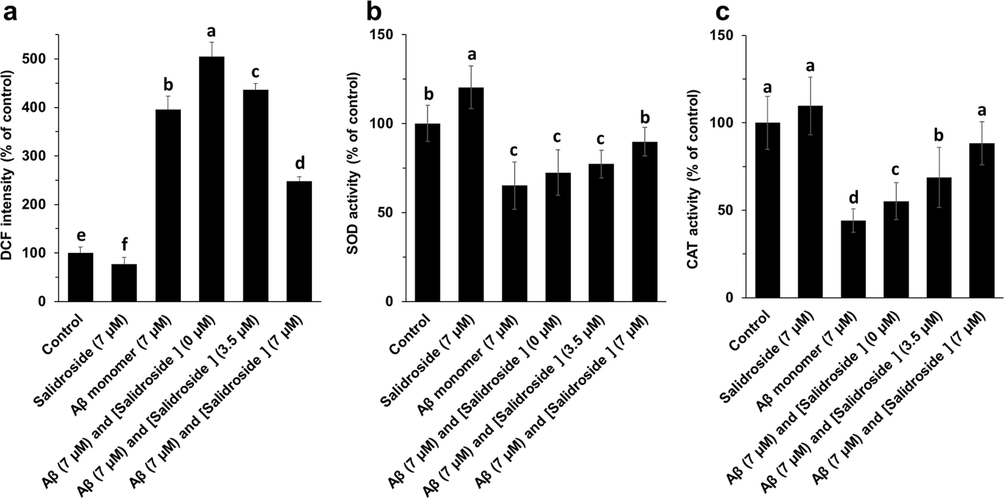
ROS (a), SOD activity (b), CAT activity (c) assays of bEnd. 3 cells after incubation with different Aβ1-42 samples (aged for 25 h) for 24 h. a, b, c, and d letters show the significance (P < 0.05).
3.4 Caspase-3 activity
The effect of different Aβ1-42 oligomers alone or with salidroside on caspase-3 activity was explored to assess the probable inhibitory effect of salidroside against Aβ1-42 samples-triggered apoptosis in bEnd.3 cells after 24 h (Fig. 4). It was observed that Aβ1-42 oligomers induced a significant increase in caspase-3 activity in bEnd. 3 cells, whereas treatment of cells with Aβ1-42 samples co-incubated with salidroside mitigated the caspase-3 activity. Therefore, it was determined that the probable apoptosis stimulated by Aβ1-42 oligomers can be reduced after treatment of cells with salidroside.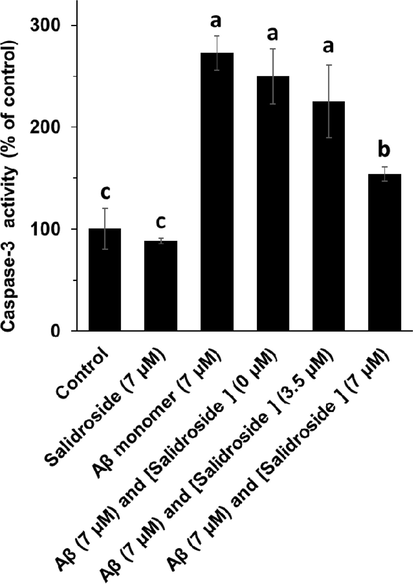
Caspase-3 assay of bEnd. 3 cells after incubation with different Aβ1-42 samples (aged for 25 h) for 24 h. a, b, and c letters show the significance (P < 0.05).
4 Discussion
In recent years, several emerging diseases have been identified in humans directly related to abnormal and expanded aggregation of protein (Chiti and Dobson, 2017). The ability to form amyloid structures is one of the general characteristics of proteins. Therefore, the development of amyloid structures in model proteins and the investigation of the protective effect of small molecules on the formation of these structures can be useful in designing potentially effective drug compounds for disease-related amyloid (Alam et al., 2017). The present study was designed to investigate the effect of salidroside as a flavonoid small molecule on the formation of amyloid structure in Aβ1-42 as a model protein and its associated cytotoxicity in bEnd.3 endothelial cells as a marker of cerebrovascular diseases.
There is ample evidence that any defect in the control and clearance mechanisms of misfolded proteins may lead to protein amyloid formation and the development of neurodegenerative diseases (Kylkilahti et al., 2021). On the other hand, the aggregation of proteins stimulates the cellular stress and appearance of neurodegenerative diseases (Zaman et al., 2019). Several human proteins that cause amyloid disease have been identified as capable of forming structures called amyloid fibrils in various tissues, including neurodegenerative diseases such as Huntington's, Alzheimer's and cerebrovascular (Tabner et al., 2001). Studies have shown that proteins with very different structures can advance to these particular clusters by changing their natural structure (Gadad et al., 2011).
Different proteins that are prone to aggregation do not have identical amino acid sequence, or three-dimensional structures, but despite their different origins, the amyloids formed have similar structures with a fold structure and a similar cytotoxicity mechanism (Gadad et al., 2011). Due to the presence of unprotected hydrophobic groups, the misfolded protein has the potential to form larger clusters, leading to toxicity, cell damage, and eventually programmed cell death (apoptosis) (Li et al., 1996).
Despite extensive research on the process of amyloid formation and related diseases, few treatments are currently available for these diseases. Studies in various animal cells and models indicate that the prevention of amyloid formation is useful in reducing the complications of amyloidosis (Fändrich et al., 2018). In addition, anti-amyloid activity of various compounds such as antibodies, synthetic peptides, proteins known as chaperones, and chemical compounds have been identified (Härd and Lendel, 2012). Also, a large number of small molecules with different concentrations ranging from nanomolar to millimolar have been found to provide high potential for inhibiting the formation of amyloid aggregates, especially Aβ. Medicinal plants and their bioactive compounds as natural molecules have been shown to potentially inhibit the formation and accumulation of amyloid and related diseases (Doig and Derreumaux, 2015). Based on the outcomes from in vitro and in vivo assays, it was found that Aβ could induce oxidative stress and apoptosis in cerebrovascular endothelial cells, which is in agreement with previous reports (Park et al., 2011; Song et al., 2017; Chen et al., 2018).
It has been shown that phenolic compounds can mitigate the bEnd.3 cytotoxicity from Aβ peptide-stimulated oxidative stress (Xi et al., 2012; Liu et al., 2017). Salidroside as a phenolic compound has been shown to protect neuron-like cells against Aβ-triggered apoptosis by modulation of AKT signaling pathway (Liao et al., 2019).
It can be noted that cerebrovascular-targeted small molecules relieving oxidative stress may provide potential results for delayed progression of neurodegenerative diseases.
5 Conclusion
In conclusion, with the use of several biophysical and cellular techniques, we demonstrated that salidroside prevented Aβ1-42 peptide aggregation and mitigated against amyloid triggered cerebrovascular endothelial cytotoxicity. The fluorescence analysis indicated that salidroside prevents the Aβ1-42 peptide aggregation and this potential inhibitory effect was more pronounced in the presence of higher concentration of salidroside. The aggregated species that appeared in the presence of salidroside showed fewer β-sheet structures, as evidenced by the CD and CR spectra. Interestingly salidroside was shown to be safe against bEnd.3 cells and also mitigated the cells against oligomers-triggered cytotoxicity. Though much more studies in vivo investigation is demanded to support it as a potential and clinically viable drug. This study will further expand the pool of promising drugs for cerebrovascular disease from bioactive compounds.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Vitamin k3 inhibits protein aggregation: implication in the treatment of amyloid diseases. Sci. Rep.. 2016;6(1):1.
- [Google Scholar]
- Protein aggregation: from background to inhibition strategies. Int. J. Biol. Macromol.. 2017 Oct;1(103):208-219.
- [Google Scholar]
- Quantitative analysis of protein far UV circular dichroism spectra by neural networks. Protein Eng. Des. Sel.. 1992 Apr 1;5(3):191-195.
- [Google Scholar]
- Cerebrovascular and amyloid pathology in predementia stages: the relationship with neurodegeneration and cognitive decline. Alzheimers Res. Ther.. 2017 Dec;9(1):1.
- [Google Scholar]
- Inhibition of amyloid oligomerization into different supramolecular architectures by small molecules: mechanistic insights and design rules. Future Med. Chem.. 2017 May;9(8):797-810.
- [Google Scholar]
- Salidroside inhibits H2O2-induced apoptosis in PC12 cells by preventing cytochrome c release and inactivating of caspase cascade. Acta Biochim. Biophys. Sin. (Shanghai). 2008;40:796-802.
- [Google Scholar]
- Influence of salidroside on mitochondria injury induced by sodium azide. Yao Xue Xue Bao. 2005;40:700-704.
- [Google Scholar]
- Biophysical insight into the anti-amyloidogenic behavior of taurine. Int. J. Biol. Macromol.. 2015 Sep;1(80):375-384.
- [Google Scholar]
- Aβ1-42 induces cell damage via RAGE-dependent endoplasmic reticulum stress in bEnd. 3 cells. Exp. Cell Res.. 2018;362(1):83-89.
- [Google Scholar]
- Protective influence of salidroside against H2O2-induced cell apoptosis in primary culture of rat hippocampal neurons. Mol. Cell. Biochem.. 2009;332:85-93.
- [Google Scholar]
- 2′, 7′-Dichlorodihydrofluorescein as a fluorescent probe for reactive oxygen species measurement: forty years of application and controversy. Free Radic. Res.. 2010 Jan 1;44(6):587-604.
- [Google Scholar]
- The contribution of vascular risk factors in neurodegenerative disorders: from mild cognitive impairment to Alzheimer’s disease. Alzheimers Res. Ther.. 2020 Dec;12(1):1.
- [Google Scholar]
- Protein misfolding, amyloid formation, and human disease: a summary of progress over the last decade. Annu. Rev. Biochem. 2017 Jun;20(86):27-68.
- [Google Scholar]
- Inhibition of protein aggregation and amyloid formation by small molecules. Curr. Opin. Struct. Biol.. 2015 Feb;1(30):50-56.
- [Google Scholar]
- Brazilin inhibits amyloid β-protein fibrillogenesis, remodels amyloid fibrils and reduces amyloid cytotoxicity. Sci. Rep.. 2015 Jan 23;5(1):1.
- [Google Scholar]
- Amyloid fibril polymorphism: a challenge for molecular imaging and therapy. J. Intern. Med.. 2018 Mar;283(3):218-237.
- [Google Scholar]
- Targeting oligomers in neurodegenerative disorders: lessons from α-synuclein, tau, and amyloid-β peptide. J. Alzheimers Dis.. 2011 Jan 1;24(s2):223-232.
- [Google Scholar]
- Protective influences of salidroside from Rhodiola rosea on LPS-induced acute lung injury in mice. Immunopharmacol. Immunotoxicol.. 2012;34:667-672.
- [Google Scholar]
- Cerebrovascular dysfunction in amyloid precursor protein transgenic mice: contribution of soluble and insoluble amyloid-β peptide, partial restoration via γ-secretase inhibition. J. Neurosci.. 2008 Dec 10;28(50):13542-13550.
- [Google Scholar]
- The overlap between neurodegenerative and vascular factors in the pathogenesis of dementia. Acta Neuropathol.. 2010 Sep 1;120(3):287-296.
- [Google Scholar]
- Salidroside from Rhodiola sachalinensis protects neuronal PC12 cells against cytotoxicity induced by amyloid-beta. Immunopharmacol. Immunotoxicol.. 2003;25:295-304.
- [Google Scholar]
- Measuring plant protein with the Bradford assay. J. Chem. Ecol.. 1989 Mar;15(3):979-992.
- [Google Scholar]
- Homocysteine induced cerebrovascular dysfunction: a link to Alzheimer’s disease etiology. The open neurology journal.. 2015;9:9.
- [Google Scholar]
- Experimental inhibition of fibrillogenesis and neurotoxicity by amyloid-beta (Aβ) and other disease-related peptides/proteins by plant extracts and herbal compounds. Protein Aggregation and Fibrillogenesis in Cerebral and Systemic Amyloid Disease. 2012:295-326.
- [Google Scholar]
- Rapid identification of bioactive compounds reducing the production of amyloid β-peptide (Aβ) from South African plants using an automated HPLC/SPE/HPLC coupling system. Biomol. Ther.. 2011;19(1):90-96.
- [Google Scholar]
- Achieving brain clearance and preventing neurodegenerative diseases—A glymphatic perspective. J. Cereb. Blood Flow Metab. 2021 Jan 18:0271678X20982388
- [Google Scholar]
- β-Amyloid induces apoptosis in human-derived neurotypic SH-SY5Y cells. Brain Res.. 1996 Nov 4;738(2):196-204.
- [Google Scholar]
- Protective influence of salidroside from Rhodiolae Radix on diabetes-induced oxidative stress in mice. Molecules. 2011;16:9912-9924.
- [Google Scholar]
- Salidroside protects PC-12 cells against amyloid β-induced apoptosis by activation of the ERK1/2 and AKT signaling pathways. Int. J. Mol. Med.. 2019 Apr 1;43(4):1769-1777.
- [Google Scholar]
- Lutein protects against β-amyloid peptide-induced oxidative stress in cerebrovascular endothelial cells through modulation of Nrf-2 and NF-κb. Cell Biol. Toxicol.. 2017 Feb;33(1):57-67.
- [Google Scholar]
- The role of β-amyloid peptide in neurodegenerative diseases. Ageing Res. Rev.. 2011 Sep 1;10(4):440-452.
- [Google Scholar]
- A critical role for the self-assembly of Amyloid-β1-42 in neurodegeneration. Sci. Rep.. 2016 Jul 22;6(1):1-3.
- [Google Scholar]
- Scavenger receptor CD36 is essential for the cerebrovascular oxidative stress and neurovascular dysfunction induced by amyloid-β. Proc. Natl. Acad. Sci.. 2011 Mar 22;108(12):5063-5068.
- [Google Scholar]
- A novel pathway for amyloid precursor protein processing. Neurobiol. Aging. 2011 Jun 1;32(6):1090-1098.
- [Google Scholar]
- An autocatalytic reaction as a model for the kinetics of the aggregation of β-amyloid. Pept. Sci.. 2003;71(2):190-195.
- [Google Scholar]
- Neuroprotective influences of Salidroside and its analogue tyrosol galactoside against focal cerebral ischemia in vivo and H2O2-induced neurotoxicity in vitro. Neurotox. Res.. 2012;21:358-367.
- [Google Scholar]
- Apomyoglobin folding intermediates characterized by the hydrophobic fluorescent probe 8-anilino-1-naphthalene sulfonate (ANS) Biochimica et Biophysica Acta (BBA)-Protein Structure and Molecular Enzymology.. 1998;1385(1):69-77.
- [Google Scholar]
- Soluble β-amyloid (Aβ) 40 causes attenuation or potentiation of noradrenaline-induced vasoconstriction in rats depending upon the concentration employed. Neurosci. Lett.. 2004 Aug 26;367(1):129-132.
- [Google Scholar]
- Adiponectin controls the apoptosis and the expression of tight junction proteins in brain endothelial cells through AdipoR1 under beta amyloid toxicity. Cell Death Dis.. 2017;8(10):e3102.
- [Google Scholar]
- The role of brain vasculature in neurodegenerative disorders. Nat. Neurosci.. 2018 Oct;21(10):1318-1331.
- [Google Scholar]
- Production of reactive oxygen species from aggregating proteins implicated in Alzheimer's disease, Parkinson's disease and other neurodegenerative diseases. Curr. Top. Med. Chem.. 2001 Dec 1;1(6):507-517.
- [Google Scholar]
- Evaluation of the cytotoxicity of calcium phosphate root canal sealers by MTT assay. J. Endod.. 1999 Dec 1;25(12):811-813.
- [Google Scholar]
- Cerebrovascular endothelial dysfunction mediated by β-amyloid. Neuroreport. 1997 Apr 14;8(6):1387-1391.
- [Google Scholar]
- Accumulation of amyloid precursor protein C-terminal fragments triggers mitochondrial structure, function, and mitophagy defects in Alzheimer’s disease models and human brains. Acta Neuropathol.. 2021 Jan;141(1):39-65.
- [Google Scholar]
- Natural product-based amyloid inhibitors. Biochem. Pharmacol.. 2017 Sep;1(139):40-55.
- [Google Scholar]
- Structural, morphological, and kinetic studies of β-amyloid peptide aggregation on self-assembled monolayers. PCCP. 2011;13(33):15200-15210.
- [Google Scholar]
- Flavonoids protect cerebrovascular endothelial cells through Nrf2 and PI3K from β-amyloid peptide-induced oxidative damage. Curr. Neurovasc. Res.. 2012 Feb 1;9(1):32-41.
- [Google Scholar]
- Regulation of artemisinin and its derivatives on the assembly behavior and cytotoxicity of amyloid polypeptides hIAPP and Aβ. ACS Chem. Nerosci.. 2019 Oct 2;10(11):4522-4534.
- [Google Scholar]
- Influences of salidroside on proliferation, apoptosis, phagocytosis, ROS and NO production of murine peritoneal macrophages in vitro. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2011;27:237-241.
- [Google Scholar]
- Protein misfolding, aggregation and mechanism of amyloid cytotoxicity: an overview and therapeutic strategies to inhibit aggregation. Int. J. Biol. Macromol.. 2019 Aug;1(134):1022-1037.
- [Google Scholar]
- Salidroside protects cardiomyocyte against hypoxia-induced death: a HIF-1alpha-activated and VEGF-mediated pathway. Eur. J. Pharmacol.. 2009;607:6-14.
- [Google Scholar]
- Neuroprotective influences of salidroside against beta-amyloid-induced oxidative stress in SH-SY5Y human neuroblastoma cells. Neurochem. Int.. 2010;57:547-555.
- [Google Scholar]
- Salidroside attenuates apoptosis in ischemic cardiomyocytes: a mechanism through a mitochondria-dependent pathway. J. Pharmacol. Sci.. 2010;114:399-408.
- [Google Scholar]
- Salidroside protects against hydrogen peroxide-induced injury in cardiac H9c2 cells via PI3K-Akt dependent pathway. DNA Cell Biol.. 2011;30:809-819.
- [Google Scholar]
- Neurovascular pathways to neurodegeneration in Alzheimer's disease and other disorders. Nat. Rev. Neurosci.. 2011 Dec;12(12):723-738.
- [Google Scholar]
- Influences of salidroside -pretreatment on neuroethology of rats after global cerebral ischemia-reperfusion. Zhong Xi Yi Jie He Xue Bao. 2009;7:130-134.
- [Google Scholar]
- Activity of compounds from Chinese herbal medicine Rhodiola kirilowii (Regel) Maxim against HCV NS3 serine protease. Antiviral Res.. 2007;76:86-92.
- [Google Scholar]







