Translate this page into:
Antibacterial activity of flavonoids and triterpenoids isolated from the stem bark and sap of Staudtia kamerunensis Warb. (Myristicaceae)
⁎Corresponding authors. edwinm@uj.ac.za (Edwin M. Mmutlane), dndinteh@uj.ac.za (Derek T. Ndinteh)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
The chemical investigation of the ethyl acetate extract of the stem bark of Staudtia kamerunensis and sap led to the isolation of six compounds which included three isoflavonoids: biochanin A (1), formononetin (2) and 3-(1,3-benzodioxol-5-yl)-5,6,7-trimethoxy-4H-1-benzopyran-4-one (3), one flavonoid: (-) epicatechin (4) and two pentacyclic triterpenoids (oleanan-12-ene-2α,3β -diol (5) and 2α,3β-dihydroxylup-20-ene (6). They were characterized by HREIMS (High Resolution Electron Ionisation Mass Spectrometry), NMR spectroscopy (1D and 2D) and comparison with existing data in literature. The crude extract and isolates were tested against twelve bacterial strains namely; Bacillus subtilis, Staphylococcus epidermidis, Enterococcus faecalis, Mycobacterium smegmatis, Staphylococcus aureus, Enterobacter cloacae, Klebsiella oxytoca, Pseudomonas aeruginosa, Proteus vulgaris, Escherichia coli, Proteus mirabilis and Klebsiella pneumonia. Streptomycin, nalidixic acid and ampicillin were used as standard antibacterial drugs. The results revealed significant antibacterial activity for both the ethyl acetate partition and for the tested compounds, with the lowest MIC value being 15.625 μg/mL. A synergistic activity of the isolated triterpenoids was evaluated with interesting results. On a general note, the antibacterial activity of compound 5 was doubled specifically against Gram-negative bacterial strains. This could be a therapeutic antimicrobial pathway in face of the rising bacterial resistance. To the best of our knowledge, it is the first time that flavonoids and triterpenoids are isolated from this genus and species. It is also the first report of antibacterial studies on this species.
Keywords
Myristicaceae
Staudtia kamerunensis
Flavonoids
Triterpenoids
Antibacterial activity
- 13C
-
NMR
- Carbon-13
-
nuclear magnetic resonance
- 1H NMR
-
Proton nuclear magnetic resonance
- 1H–1H COSY
-
Correlation spectroscopy
- d
-
Doublet
- dd
-
Doublet of doublets
- DEPT-135
-
Distortionless Enhancement by Polarization Transfer-135
- EtOAc
-
Ethyl acetate
- UV
-
Ultra-violet
- HMBC
-
Heteronuclear Multiple Bond Correlation
- HSQC
-
Heteronuclear Single Quantum Coherence
- J
-
Coupling constant
- CD3OD
-
Deuterated Methanol
- NMR
-
Nuclear Magnetic Resonance
- s
-
Singlet
- t
-
Triplet
- m
-
multiplet
- TLC
-
Thin Layer Chromatography
- TMS
-
Tetramethyl silane
Abbreviations
1 Introduction
The use of therapeutic plants is as old as human civilization. Man’s knowledge of his environment has greatly improved over time and hence, his living conditions as well. This folkloric knowledge is the foundation of modern ethnopharmacology that is transmitted from generation to generation. By the middle of the 19th century, three-quarter of all existing drugs were from plant origin (Raphael, 2011) and many pharmaceutical companies were already preparing crude therapeutic formulations (Estes, 1988). These therapeutic effects have been attributed to the many secondary metabolites synthetized by plants for multiple purposes including: protection, interaction with their environment and attraction of pollinators (Pagare et al., 2015). Secondary metabolites have increasingly attracted the interest of researchers, resulting in the invention of ultra-modern methods for isolating pure compounds from plants that can solve the health issues of humans (Salihu et al., 2020). To date, two-thirds of the world’s population rely on herbal medicine taken in diverse forms for therapeutic purposes. This is justified by their efficacy not only in providing lead molecules against multiple drug resistant (MDR) conditions, but also because they are less toxic and much more available compared to synthetic drugs (Nikaido, 2009). As part of our on-going search for new bioactive compounds that can counteract bacterial resistance, this study was aimed at investigating the antibacterial effect of the extract and isolates from the stem bark and sap of Staudtia kamerunensis Warb (Myristicaceae), a plant that grows in the Central region of Africa.
Staudtia kamerunensis Warb. (Myristicaceae), is an evergreen tree, medium sized to large, and can reach up to 40 m high. The stem has a diameter of 90–110 cm; it is branchless up to 25 m and cylindrical. The fruit is an ellipsoid drupe of 2–5 cm × 1.5–4.5 cm that usually occurs in clusters of up to 20. They are fleshy when ripe and possess a single ovoid seed which is dark brown with a red or pink aril as envelop and is lobed at the apex (Oyen & Louppe, 2012). These species are identified by many synonyms namely; Staudtia congensis, Staudtia niohue Pierre, Staudtia spititata and Staudtia kamerunensis that has one variant, Staudtia gabonensis, whose fruits are smaller than the later. It has been identified as the only species of the genus Staudtia (Oyen & Louppe, 2012). Different parts of the tree are believed to possess multiple curative properties; bark decoction is used to treat menstrual issues, diarrhoea, lung ailments and cough in Central African traditional medicine. In the Democratic Republic of Congo (DRC), bark decoction is used to treat coughs, skin problems, oedema, wounds and oral infections while bark sap is used to treat snakebites, irritated eyes, bleeding, wounds and diarrhoea. In Gabon, the sap is rubbed against the mouths of new-borns to help them with teething and the pulverized bark mixed with padouk wood powder (Pterocarpus soyauxii Taub.) is used to treat ulcers, especially yaws. Boiled wood chips are used in treating gonorrhoea and rheumatism while fresh twigs are crushed, salted, and eaten as an aphrodisiac in Congo (Oyen & Louppe, 2012). Previous chemical investigations carried out on this species and other species of the Myristicaceae family reported the isolation of lignans as one of the major components of the species. Noumbissie et al., 1992 isolated a diterpene known as staudtienic acid (Noumbissie et al., 1992) from the stem bark. The work of Yankep et al., 1999 on the seeds and the stem bark of the species yielded seven lignans and one glycerol trimyristate.
Microbial resistance remains a major concern to public health and search for new potent antimicrobial agent is crucial (Baker et al., 2022). The scarcity of biological investigations on this plant species, coupled to its usage for bacteria-related diseases in ethno-pharmacology prompted us to the chemical investigations of its stem bark and sap, to isolate the biologically active compounds that can serve as lead for new antimicrobial agents, and to justify its use in traditional pharmacopeia.
2 Material and methods
2.1 Material
The reagents used for extraction and isolation were obtained from Sigma Aldrich. Pre-coated silica gel 60 F254 sheets were used for thin layer chromatography with a mixture of hexane and ethyl acetate, hexane and chloroform and chloroform and methanol, as eluents. UV lamps (254 nm and 365 nm), iodine and sulphuric acid were used to visualize the spots. Column chromatography was packed with Merck silica gel 60 (0.040–0.060 mm).
3 Instrumental
NMR analysis was carried out in CDCl3 and DMSO‑d6 solvents, on a magnet of 500 MHz coupled to an Avance III HD 500 MHz console. All spectra were referenced using the chemical shifts of the different solvents, both for 1H and 13C. Tetramethylsilane (TMS) was used as the internal standard. A last generation of Quadrupole Time-of-Flight (Q-TOF) Mass Spectrometry system (Bruker Compact), was used for the analysis of the pure isolates, using HR-ESIMS positive mode as ionisation method.
3.1 General experimental procedures
3.1.1 Plant material
The stem bark of Staudtia kamerunensis warb. was collected from Minkam Mengale Menkommegan forest (South Region, Republic of Cameroon) in October 2017. Dr Nono, from the department of Plant Biology (Faculty of Science, University of Yaoundé I) identified it. The sap of the tree was collected after the stem was wounded. A voucher specimen documenting the collection is deposited at the National Herbarium of Cameroon (49184 HNC).
3.1.2 Extraction and isolation
3.1.2.1 The stem bark
After collection, the plant material was cut into pieces, dried for two weeks at ambient temperature and crushed to powder to yield a mass of 1,057 g. The powdered residue was soaked in 5 L of methanol and macerated for 48 h. The extract was then filtered and the filtrate was evaporated to dryness under reduced pressure using a rotavapor to give a viscous dark red paste. The paste was further fractionated using ethyl acetate to yield 83.12 g of a brown residue. This ethyl acetate residue was mixed with the same amount of silica gel (0.040–0.060 mm) in ethyl acetate and the solvent was allowed to evaporate completely. A slurry of silica gel was prepared using 100% n-hexane and mounted over silica gel in a glass column chromatography of 4.2 cm of diameter, and 35.5 cm length. It was eluted using a binary system of solvents with increasing polarity starting from hexane, then a mixture of hexane and ethyl acetate, and then 100% ethyl acetate. 281 fractions of 200 mL each were collected and pooled based on TLC. Compound 1 (3.12 mg) precipitated as a white powder from 15% EtOAc in hexane, from fractions 73–86, and was recrystallized into chloroform. Compound 2 (2.01 mg) was obtained from 20% EtOAc in hexane as a brown precipitate, from fractions 130–141. Compound 3 (2.02 mg) was obtained from 30% EtOAc in hexane as a red crystal, from fraction 161–180) and compound 4 (4.01 mg) was obtained from 50% EtOAc in hexane from fraction 235–251 as a reddish-brown crystal.
3.1.2.2 The sap
Some species of the Myristicaceae family are characterized by their red exudate whenever the stem is wounded. Compsoneura excelsa from Costa Rica (Janovec and Amer-, 2004) and S. Kamerunensis are examples of trees which exudate a red sap when the stem is wounded. In order to give an account of the plausible metabolites that can be responsible for this red colour and also to characterize and evaluate their biological activity, the sap of this tree was collected. After collection of 500 mL of sap, the liquid was dissolved in a mixture of chloroform/methanol and mixed with silica gel (0.040–0.060 mm). The mixture was subjected to successive steps of column chromatography for purification. A slurry of silica gel was prepared with 100% n-hexane. It was eluted with a binary system of solvents with gradient polarity starting from hexane, then a mixture of hexane and chloroform, then chloroform 100%, a mixture of chloroform and methanol, and finally with methanol. The isolation procedure led to the collection of 350 fractions that were monitored by TLC. A mixture of compound (5) and (6) were obtained as a white powder (2.8 g) from fraction 99–107 at 40% chloroform in hexane. This fraction was further chromatographed over silica gel. A preliminary evaluation was made to identify the best separating system of solvents. A mixture of Hexane and dichloromethane and of hexane and ethyl acetate were used, with a gradient of polarity. The hexane and ethyl acetate was found to be the best to separate our mixture at a polarity of 20% EtOAc in Hexane. Thus, 1.5 g of the mixture was mixed with 76.6 g of silica gel and loaded in a glass column (length: 64.5 cm, diameter: 25 mm). A slurry of silica gel was prepared using hexane and elution was done starting with pure hexane, then a mixture of hexane and ethyl acetate with increasing polarity, then pure ethyl acetate. Two hundred and six (2 0 6) fractions were collected overall. Fifty-three fractions (53) fractions of 13 mL each were collected from fraction 1 to 53, from pure hexane to 7% ethyl acetate in hexane. Then, from fraction 54 to 200, 50 mL of elute was collected from the column, from 7% ethyl acetate in hexane to 20% ethyl acetate in hexane. Fraction 200 to fraction 206 were collected from pure ethyl acetate. Precipitates started forming from fraction 95, but only fractions 129 and 130 were found pure and to be compound (5)-(17.03 mg and 10.22 mg respectively).
3.1.3 Antibacterial assay
The ethyl acetate fractions of the crude extract, compound 4, compound 5 and a mixture of compound 5 and 6 were evaluated for their antibacterial activity against twelve bacterial strains that included both Gram-positive and Gram-negative strains. The Gram-positive strains were Bacillus subtilis (BS) (ATCC 19659), Enterococcus faecalis (EF) (ATCC13047), Staphylococcus epidermidis (SE) (ATCC14990), Mycobacterium smegmatis (MS) (MC 2155) and Staphylococcus aureus (SA) (ATCC25923). The Gram-negative strains were Enterobacter cloacae (ECL) (ATCC13047), Proteus vulgaris (PV) (ATCC33420), Klebsiella oxytoca (KO) (ATCC13882), Proteus mirabilis (PM) (ATCC 7002), Escherischia coli (EC) (ATCC 25922) and Klebsiella Pneumonia (KP) (ATCC13882). Broth microdilution technique was employed and the minimum inhibitory concentration (MIC) of the compounds and crude extract were evaluated following the procedure described by Fonkui et al., 2018, with minor modifications. Briefly, 4 mg of each compound and crude extracts were weighed and dissolved in 4 mL of DMSO. These solutions were then serially diluted (6 times) to the appropriate concentrations (500, 250, 125, 62.5, 31.25, and 15.625 μg/mL) in 100 μL of nutrient broth in 96 well plates. Following that, 100 μL of each of these solutions was duplicated and seeded with 100 μL of an overnight bacterial culture grown to 0.5 Mc Farland in nutrient broth. The result was incubated overnight in a 5% CO2 incubator at 37 0C. Positive controls were ampicillin, nalidixic acid and streptomycin while the negative control was made up of 50% nutrient broth in DMSO. The presence of living cells was assessed using resazurin dye after 4 h of incubation and MIC values were recorded.
3.1.4 Physical and spectral data of the compounds
Compound 1, Biochanin A: white crystal (3.12 mg); 1H NMR (DMSO‑d6): 8.35 (1H,s, H-2); 12.92 (1H,s, –OH at position 5), 6.23 (1H, d, J = 2.0 Hz, H-8), 10.89 (1H, br s, –OH, at position 7); 6.39 (1H, d, J = 2.0 Hz, H-6); 7.49 (2H, d, J = 8 Hz, H-2′ and H-6′); 6.99 (2H, d, J = 8 Hz, H-3′ and H-5′) 3.78 (3H, s, –OCH3 at position 4′). 13C NMR (DMSO‑d6): 154.7 (C-2), 122.4 (C-3), 180.5 (C-4), 162.5 (C-5), 99.5 (C-6), 164.8 (C-7), 94.2 (C-8), 159.6 (C-9), 104.9 (C-10); 123.4 (C-1′), 130.6 (C-2′&C-6′), 114.2 (C-3′&C-5′), 158.1 (C-4′), 55.6 (–OCH3). This data corroborates with those reported by Zhao et al. (2009).
Compound 2, Formononetin - brown powder (2.01 mg); 1H NMR (DMSO‑d6): 8.33 (s, H-2); 6.99 (d, J = 8.6 Hz, H-3′ and H-5′); 7.51 (d, J = 8.7 Hz, H-2′ and H-6′); 7.96 (d, J = 8.7 Hz, H-5); 6.86 (d, J = 2.2 Hz, H-8); 6.93 (dd, J = 8.8, 2.2 Hz, H-6); 3.79 (s, –OCH3); 13C NMR (DMSO‑-d6): 153.0 (C-2); 123.1 (C-3); 174.5C = O (C-4); 127.2 (C-5); 115.2 (C-6); 162.8 (C-7); 102.0 (C-8), 158.9 (C-9); 116.4 (C-10); 124.2 (C-1′); 130.0 (C-2′&C-6′); 113.5 (C-3′&C-5′); 158.9 (C-4′); 115.2 (C-6′); 55.1 (–OCH3). These spectral data were further confirmed by comparison with the findings of (Ha et al., 2010).
Compound 3, 3-(1,3-Benzodioxol-5-yl)-5,6,7-trimethoxy-4H-1-benzopyran-4-one: light red crystal; 1H NMR (CDCl3): 7.75 (s, H-2), 7.04 (t, J = 1.8 Hz, H-6′), 6.87 (d, J = 5 Hz, H-2′), 6.78 (d, J = 7.9 Hz, H-5′), 6.61 (s, H-8), 5.89 (s, O-CH2-O), 3.88 (s, –OCH3, position 5), 3.87 (3H,s, –OCH3, position 6), 3.83 (3H,s, –OCH3, position 7). 13C NMR (CDCl3): 175.0 (C-4, C = O), 157.7 (C-7), 154.5 (C-5), 153.0 (C-9), 150.6 (C-2), 147.5 (C-3′), 147.5 (C-4′), 140.6 (C-6), 125.6 (C-3), 125.5 (C-1′), 110.0 (C-6′), 113.5 (C-10), 122.5 (C-2′), 108.2 (C-5′), 101.1 (O-CH2-O), 96.0 (C-8), 62.1 (–OCH3, position 5), 61.5 (–OCH3, position 7), 56.2 (–OCH3, position 6). The data were confirmed by those published by Yankep et al. (1998).
Compound 4, (-)-Epicatechin: red crystal: (4.01 mg); 1H NMR (DMSO‑d6): 4.21 (m, H-2); 4.20 (m, H-3); 2.88 (dd, J = 16.7, 4.6 Hz, H-4a); 2.76 (dd, J = 16.8, 3.1 Hz, H-4b); 5.94 (1H, d, J = 7.9 Hz, H-6); 5.96 (1H, d, J = 8.8 Hz, H-8); 7.00 (1H, s, H-6′); 6.77 (m, H-5′); 6.82 (m, H-2′); 13C NMR (DMSO-d6): 79.9 (C-2); 67.5 (C-3); 29.2 (C-4); 157.7 (C-5); 96.5 (C-6); 158.0 (C-7); 95.9 (C-8); 157.3 (C-9); 100.1 (C-10); 132.3 (C-1′); 115.9 (C-2′); 145.9 (C-3′); 145.8 (C-4′); 115.3 (C-5′); 119.4 (C-6′). These data corroborates what was published by Téné and collaborators (Téné et al., 2021).
Compound 5, (2α, 3β) Oleanan-12-ene-2,3-diol (27.25 mg): white powder; 1H NMR (CDCl3): 0.80 (3H, s, Me-24); 0.83 (3Hs, Me-28); 0.83 (3H, s, Me-29); 0.88 (3H, s,); 0.80 (6H, s, Me-26 & Me-25); 0.99 (3H, s,); 1.13 (3H, s, Me-27); 1.91 (1H, m, H-18); 2.98 (1H, td, J = 3.32 and 11.36 Hz, H-3); 3.67 (1H, d, J = 3.25 Hz, H-2); 5.22 (1H, t, J = 5.65 Hz, H-12). 13C NMR (CDCl3): 46.6 (C-1), 69.0 (C-2); 83.8 (C-3); 39.2 (C-4); 55.3 (C-5); 18.4 (C-6); 32.5 (C-7); 39.9 (C-8); 47.6 (C-9); 38.2 (C-10); 23.6 (C-11); 121.5 (C-12); 145.2 (C-13); 41.8 (C-14); 26.1 (C-15); 26.9 (C-16); 31.1 (C-17); 47.2 (C-18); 46.8 (C-19); 31.1 (C-20); 34.8 (C-21); 37.1 (C-22); 28.6 (C-23); 16.8 (C-24); 16.8 (C-25); 16.9 (C-26); 26.00 (C-27); 28.4 (C-28); 33.3 (C-29), 23.7 (C-30). These data are in agreement with those reported by Alessandra and colleagues (Braca et al., 2001).
Compound 6, 2α,3β-dihydroxylup-20-ene: white powder; 1H NMR (CDCl3): 0.77 (3H,s, Me-28), 0.79 (3H,s, Me-24), 0.91 (3H, s, H-27), 0.94 (3H, s, H-25), 1.00 (3H,s, Me-26), 1.02 (3H,s, Me-23), 1.65 (3H, s, H-30), 1.98 (2H, m, H-1), 2.97 (d, J = 9.4 Hz, 1H), 3.68 (td, J = 10.7, 4.5 Hz, H-2); 4.55 (1H, br s, H-29a); 4.66 (1H, d, J = 2.4 Hz). 13C NMR: 43.0 (C-1); 69.3 (C-2); 83.9 (C-3); 38.0 (C-4); 55.4 (C-5); 18.0 (C-6); 29.9 (C-7); 40.9 (C-8); 50.4 (C-9); 38.0 (C-10); 22.7 (C-11); 25.1 (C-12); 34.2 (C-13); 42.9 (C-14); 27.4 (C-15); 31.9 (C-16); 43.0 (C-17); 48.3 (C-18); 48.0 (C-19); 150.8 (C-20); 29.6 (C-21); 38.6 (C-22); 28.5 (C-23); 16.5 (C-24); 17.4 (C-25); 16.1 (C-26); 14.5 (C-27); 18.3 (C-28); 19.3 (C-29); 109.2 (C-30). These data were in agreement with what was published by Hisham et al. (1996).
4 Results and discussion
The phytochemical study of the ethyl acetate extract of the stem bark and sap of S. Kamerunensis, resulted in the isolation of six compounds namely; biochanin A (1), formononetin (2), 3-(1,3-Benzodioxol-5-yl)-5,6,7-trimethoxy-4H-1-benzopyran-4-one (3), and epicatechin (4) from the stem bark, (2α, 3β) Oleanan-12-ene-2,3-diol (5) and 2α,3β-dihydroxylup-20-ene (6) from the sap.
Compound 1, 2, 3, 4 have the characteristic C6-C3-C6 backbone and hence belong to the large class of flavonoids. Compounds belonging to the flavan-3-ol and other polyphenolic compounds are generally omnipresent in plant species, and are responsible for the colour observed in fruits, flowers, (Amalesh et al., 2011). They play an important role in the protection of plants against microbial aggression (Amalesh et al., 2011). To the best of our knowledge, this is the first report of flavonoids, from this plant species. Yankep and collaborators (1999) reported the isolation of lignans from the seed and stem bark of this species. From biosynthetic viewpoint, lignans and flavonoids both originates from the phenylpropanoid pathway, which helps us to understand the existence of both in the stem bark of this specie. In his review, Valderrama reports the isolation of many flavonoids among which (-) epicatechin from Myristica fragans seeds, biochanin A from the seed of Virola surinamensis (Martínez Valderrama, 2000). In this review, several isoflavonoids are reported with the methylene dioxy moiety attached to ring Zeng et al. (1994) isolated formononetin from the stem bark of Knema glomerata Merr. Compound 5 and 6 are two isomeric pentacyclic triterpenoids. The difference between the two compounds is on the fifth ring. In compound 6, the fifth ring is a five membered ring with an isopropenyl moiety attached at carbon C-19, while compound 5 has the normal six-membered ring of pentacyclic triterpenoids. These compounds were obtained from the same fraction as a mixture. A further purification of the mixture was carried out and compound 5 was obtained as pure entity. Compound 6 rather, was difficult to obtain pure. To the best of our knowledge, this is the first phytochemical investigation of the sap of species belonging to this genus and species of the family and the first report of this class of compounds from the genus. Fig. 1 depicts the chemical structures of the isolated compounds from the extract of the stem bark and sap of the tree.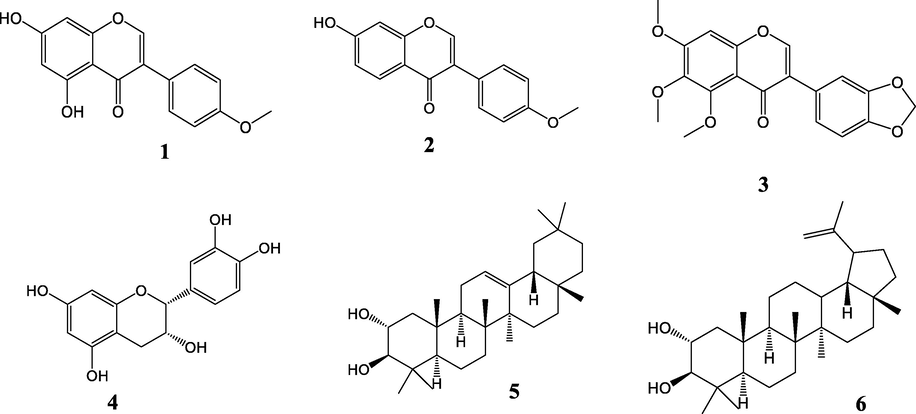
Structures of compounds (1–6) isolated from the stem bark and sap of S. kamerunensis.
Table 1 presents the MIC values of the crude extract and of the ethyl acetate partition (EASK), (-) epicatechin (4), oleanan-12-ene-2α,3β –diol, (5) and the mixture of oleanan-12-ene-2α,3β -diol and 2α,3β-dihydroxylup-20-ene (6), to study their synergistic effects. The crude extracts showed significant antibacterial activity against both Gram-positive and Gram-negative bacteria strains. The lowest MIC value of the crude extract was 15.625 µg/mL and was against Bacillus subtilis, Enterococcus faecalis among the Gram-positive, against Enterobacter cloacae, Proteus vulgaris, Klebsiella oxytoca, and Proteus mirabilis, among Gram-negative strains. This makes the crude extract 1.7 times more potent than Ampicillin against Bacillus subtilis, Enterococcus faecalis, Enterobacter cloacae, Klebsiella oxytoca, and Proteus mirabilis, 26.6 times more potent against Proteus vulgaris, and 4.1 times more potent against Pseudomonas aeruginosa. These results justify the uses of the stem bark of this plant species against many bacteria-related diseases (Cough, lung complaints, wounds etc.). AMP, Ampicillin; NLD, nalidixic acid; STM, Streptomycin; BS, Bacillus subtilis; EF, Enterococcus faecalis; SE, Staphylococcus epidermidis; SA, Staphylococcus aureus; MS, Mycobacterium smegmatis; ECL, Enterobacter cloacae; PV, Proteus vulgaris; KO, Klebsiella oxytoca; PA, Pseudomonas aeruginosa; PM, Proteus mirabilis; EC, Escherichia coli; KP, Klebsiella pneumonia; EASK, Ethyl Acetate Extract of Staudtia kamerunensis.
Minimum inhibitory concentration (MIC, ug/mL)
Test compounds
Gram-positive
Gram-negative
BS
EF
SE
SA
MS
ECL
PV
KO
PA
PM
EC
KP
EASK
15.625
15.625
250
125
62.5
15.625
15.625
15.625
15.625
15.625
250
250
4
15.625
15.625
500
15.625
125
15.625
62.5
15.625
15.625
15.625
250
250
5&6
32.5
15.625
125
62.5
125
15.625
125
15.625
15.625
125
125
125
5
15.625
15.625
250
62.5
250
15.625
250
15.62
250
250
250
250
AMP
26
26
26
26
26
26
416
26
64
26
26
26
STM
16
128
8
256
4
512
128
16
128
128
64
512
NLD
16
>512
64
64
512
16
128
8
128
32
512
256
A comparative study was carried out between the structures of oleanan-12-ene-2α, 3β -diol and maslinic acid isolated by Kamdem et al., (2022). The only difference between the two structures is the presence of a carboxylic acid at position 17 of the maslinic acid. Maslinic acid displayed excellent and consistent antibacterial characteristic against the same strains that were used in this study. A structure activity study can lead us to conclude that the carboxylic acid enhanced the activity of maslinic acid. The activity of maslinic acid is 16 times enhanced against P. aeruginosa, P. mirabilis, E. coli, K. pneumonia among Gram-negative strains, against S. epidermidis, M. smegmatis among Gram-positive strains, in comparison with oleanan-12-ene-2α, 3β-diol. These findings gives us an insight in the mechanism of action of maslinic acid in killing bacteria: the reactive site being the carboxylic acid. Deeper studies are required to have a clear understanding of its way of action. Oleanan-12-ene-2α, 3β-diol showed better but almost similar activity to maslinic acid against Bacillus subtilis, Enterococcus faecalis and Klebsiella oxytoca. The difference may be attributed to the presence of the carboxylic acid, but further studies are needed for clarification.
The biological activity of compounds can improve when combined with others. This effect is known as synergistic activity. The synergistic effect of pentacyclic triterpenoids has been studied as a pathway to counteract the emergence of antibacterial resistance. Chung and collaborators studied the synergistic effect of combining three pentacyclic triterpenoids with existing antibacterial medicines. The study of the combination of α-amyrin and betulinic acid revealed the synergistic effect of these compounds against Staphyloccocus aureus with an FIC (fractional inhibitory concentration of individual compounds) of 0.5 or less. A combination of α-amyrin, betulinic acid and betulinaldehyde did not inhibit the growth of the bacterial cells at the tested concentrations thus suggesting lack of synergy (Chung et al., 2011). Oleanan-12-ene-2α, 3β -diol and 2α, 3β-dihydroxylup-20-ene, are very similar in their structure, except for their fifth ring. A synergistic activity was observed; the activity of Oleanan-12-ene-2α, 3β -diol was doubled especially against Gram-negative strains (P. vulgaris, P. mirabilis, E. coli, K. pneumonia) and against Mycobacterium smegmatis among Gram-positive. Its activity was increased 16 times against P. aerogenes. The activity is instead decreased against Bacillus subtilis. Further studies can be developed in the pathway of utilizing the combination of these tritepenoids from the sap of this species against microbial attacks, more specifically Gram-negative aggressions.
Epicatechin also portrayed excellent antimicrobial activity compared to the standard drugs (see Table 1). Masika et al. (2004) reported the antimicrobial activity of epicatechin against four strains namely (B. subtilis, S. aureus, E. coli, P. aeruginosa). The MIC reported for E. coli (62.5 µg/mL) was 4 times better than what is reported here. The compound presented weak activity compared to ours, against the other strains (B.S −250 µg/mL, S.A. – 250 µg/mL, P.A. 62.5 µg/mL). Esquezani and co-workers attributed the antimicrobial effect of fractions from coconut husks to the relatively high abundance of catechin and epicatechin against S. aureus bacteria strains and acyclovir- resistant Herpes simplex virus type 1 (HSV-1-ACVr) (Esquenazi et al., 2002). Cetin-Karaca and Newman tested epicathecin against three different strains of E. coli (FTJ, ATCC43895, ATCC 35150) at pH = 5.6, and found it active with MIC being more or less equal to 20.0, 20.0 and 15.0 (mg/L) respectively (Cetin-Karaca and Newman, 2015). Escandón and collaborators reported epicatechin active against Helicobacter pylori (Escandón et al., 2016). Epicatechin also demonstrated synergistic effects with theaflavin from black tea when tested against eight clinical isolates of Stenotrophomonas maltophilia and Acinetobacter baumannii (Betts et al., 2011). Theaflavin tested alone was very active (p ), at concentration . When coupled with epicatechin at a ratio of 2:1 (Theaflavin: epicatechin), and a concentration level above 1 mg/mL, the effectiveness was significantly increased (p ).
5 Conclusion
The chemical investigation of the stem bark and sap of S. kamerunensis led to the isolation of six compounds, three isoflavonoids and one flavonoid from the stem bark, and a mixture of two pentacyclic triterpenoids from the sap. This is the first report of the isolation of flavonoids and triterpernoids from the species as well as of the antimicrobial studies. The species is known to be a rich source of lignans as the other members of the Myristicaceae family. The ethyl acetate extract, epicatechin, Oleanan-12-ene-2α, 3β -diol and the mixture of triterpenoids have shown significant antibacterial activity against strains tested compared to standard drugs. This justifies the local use of this plant against bacteria-related infections, and can serve as a source for the development of potent antibacterial treatment.
Funding.
The Centre for Natural Product Research (CNPR), and Drug Discovery and Smart Molecules Research Laboratory are acknowledged for providing funding for this research project, as well as the National Research Foundation, South Africa (Grant numbers 116740).
CRediT authorship contribution statement
Jordan L. Tonga: Investigation, Conceptualization, Writing – original draft. Michael H.K. Kamdem: Investigation, Formal analysis, Writing – review & editing. Julio I. M. Pagna: Supervision, Formal analysis. Thierry Y. Fonkui: . Charlotte M. Tata: Supervision, Writing – review & editing. Marthe C.D. Fotsing: Formal analysis, Writing – review & editing. Ephrem A. Nkengfack: Project administration, Supervision, Writing – review & editing. Edwin M. Mmutlane: Supervision, Writing – review & editing. Derek T. Ndinteh: Supervision, Writing – review & editing.
Acknowledgment
This work is partially supported by The Centre for Natural Product Research (CNPR), and Drug Discovery and Smart Molecules Research Laboratory. The National Research Foundation, South Africa (Grant numbers 116740) is also acknowledged for providing funding.
Declaration of competing Interest
No competing interest
References
- Baker, J.R., Cossar, P.J., Blaskovich, M.A.T., Elliott, A.G., Zuegg, J., Cooper, M.A., Lewis, P.J., Mccluskey, A., 2022. Amino Alcohols as Potential Antibiotic and Antifungal Leads.
- Antibacterial effects of theaflavin and synergy with epicatechin against clinical isolates of Acinetobacter baumannii and Stenotrophomonas maltophilia. Int. J. Antimicrob. Agents. 2011;38:421-425.
- [CrossRef] [Google Scholar]
- Antimicrobial efficacy of plant phenolic compounds against Salmonella and Escherichia Coli. Food Biosci.. 2015;11:8-16.
- [CrossRef] [Google Scholar]
- Synergistic antimicrobial activity between pentacyclic triterpenoids and antibiotics against staphylococcus aureus strains. Ann. Clin. Microbiol. Antimicrob.. 2011;10:1-6.
- [CrossRef] [Google Scholar]
- Antibacterial effect of kaempferol and (−)-epicatechin on Helicobacter pylori. Eur. Food Res. Technol.. 2016;242:1495-1502.
- [CrossRef] [Google Scholar]
- Antimicrobial and antiviral activities of polyphenolics from Cocos nucifera Linn. (Palmae) husk fiber extract. Res. Microbiol.. 2002;153:647-652.
- [CrossRef] [Google Scholar]
- The Pharmacology of Nineteenth-Century Patent Medicines. Am. Inst. Hist. Pharm.. 1988;30:3-18.
- [CrossRef] [Google Scholar]
- Synthesis, Characterization and Biological Applications of Novel Schiff Bases of 2-(Trifluoromethoxy)aniline. J. Chin. Pharm. Sci.. 2018;27(5):307-323.
- [Google Scholar]
- Formononetin prevents ovariectomy-induced bone loss in rats. Arch. Pharm. Res.. 2010;33:625-632.
- [CrossRef] [Google Scholar]
- 20,29-epoxysalacianone and 6β-hydroxysalacianone, two lupane triterepenes from Salacia beddomei. Phytochemistry. 1996;42:789-794.
- [CrossRef] [Google Scholar]
- Janovec, J.P., Amer-, S., 2004. Myristicaceae TROPICAL FORESTS | Myristicaceae.
- Pentacyclic Triterpenoids, Phytosteroids and Fatty Acid Isolated from the Stem-bark of Cola lateritia K. Schum. (Sterculiaceae) of Cameroon origin; Evaluation of Their Antibacterial Activity. Arab. J. Chem.. 2022;15:103506
- [CrossRef] [Google Scholar]
- Distribution of flavonoids in the Myristicaceae. Phytochemistry. 2000;55:505-511.
- [CrossRef] [Google Scholar]
- Staudtienic acid, a diterpene acid from staudtia kamerunensis. J. Nat. Prod.. 1992;55:137-139.
- [CrossRef] [Google Scholar]
- Oyen, L. P. A., & Louppe, D. 2012. Staudtia kamerunensis Warb.
- Secondary metabolites of plants and their role: Overview. Curr. Trends Biotechnol. Pharm.. 2015;9:293-304.
- [Google Scholar]
- Traditional medicine in Nigeria: Current status and the future. Res. J. Pharmacol.. 2011;5:90-94.
- [CrossRef] [Google Scholar]
- Antibacterial and antioxidant activities of compounds isolated from the leaves of Symphonia globulifera (Clusiaceae) and their chemophenetic significance. Biochem. Syst. Ecol.. 2021;99
- [CrossRef] [Google Scholar]
- O-geranylated isoflavones and a 3-phenylcoumarin from Millettia griffoniana. Phytochemistry. 1998;49:2521-2523.
- [CrossRef] [Google Scholar]
- Note Chemical Investigation of the Stem Bark and Seeds of Staudtia kamerunensis. Pharm. Biol.. 1999;37:155-157.
- [CrossRef] [Google Scholar]
- Isolation and characterisation of the isoflavones from sprouted chickpea seeds. Food Chem.. 2009;114:869-873.
- [CrossRef] [Google Scholar]
- Kneglomeratanol, Kneglomeratanones A and B, and Related Bioactive Compounds from Knema glomerata. J. Nat. Prod.. 1994;57(3):376-381.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2022.104150.
Appendix A
Supplementary data
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







