Translate this page into:
Antibacterial, antifungal and in vivo anticancer activities of chitin-binding lectins from Tomato (Solanum lycopersicum) fruits
⁎Corresponding author at: Department of Biochemistry and Molecular Biology, University of Rajshahi, Matihar, Rajshahi 6205, Bangladesh. hasanimtiaj@yahoo.co.uk (Imtiaj Hasan)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
A mixture of chitin-binding lectins from Tomato (Solanum lycopersicum) fruits, designated as Tomato chitin-binding lectins (TCLs), was isolated through affinity chromatography using an acetylated chitin column. Molecular weights of TCLs were determined to be 30 to 115 KDa which possessed mild toxicity with an LC50 value of 521 µg/ml examined by the brine shrimp nauplii toxicity assay. Strong antibacterial activity of TCLs was found against Escherichia coli, Staphylococcus aureus and Shigella boydii at a concentration of 500 µg/ml by using disc diffusion method. Minimum inhibitory concentrations (MIC) of TCLs against Staphylococcus aureus and Escherichia coli were found to be 200 µg/ml and 140 µg/ml, respectively whereas minimum bactericidal concentrations (MBC) against the same bacterial species were 840 and 600 µg/ml, respectively. TCLs also exerted antibiofilm activity (53.32% at 250 μg/ml) against Escherichia coli. Strong antifungal activity of TCLs against Aspergillus niger was found at 600 µg/ml whereas the lectin mixture agglutinated A. niger spores at 200 µg/ml. TCLs exhibited 19.63% and 59.91% anti-proliferative activity against Ehrlich ascites carcinoma (EAC) cells in vivo in Swiss albino mice when intraperitonealy injected at doses 1.0 mg/kg/day and 2.0 mg/kg/day, respectively for five consecutive days. Morphological changes of apoptosis in EAC cells under fluorescence microscope and alteration of the expression of apoptosis-related genes (Fas, Caspase 8 and Caspase 3) had also been observed. MTT assay showed 27.61%, 38.74% and 49.23% of in vitro anticancer activity of the tomato lectins at concentrations of 37.5, 75 and 150 µg/ml, respectively.
Keywords
Acetylated chitin
Antibiofilm
Antifungal
Anticancer
Apoptosis-related genes
1 Introduction
Lectins are a group of multivalent proteins or glycoproteins that bind cell-surface glycoconjugates to get involved in direct defense against pathogens and immune regulation in various biological systems (Barre et al., 2019; Coelho et al., 2017; Hasan et al., 2019; Souza et al., 2013). Beside their physiological roles, functions of lectins in determining the structures of carbohydrates and elucidating mechanisms of biological phenomena are now well-studied. A number of lectins are being used for performing diagnostic and therapeutic perspectives as well.
Chitin is the biopolymer of N-acetylglucosamine units linked by glycosidic bonds, distributed abundantly within arthropods, fungi, crustaceans and marine organisms. A number of plants and animals possess chitin-binding lectins contributing in their immune defense system against pathogens containing chitin through the stimulation of antimicrobial, anticancer and insecticidal activities. Chitin-binding lectins belong to the hevein family possessing one or more hevein domains. These lectins showed varying binding specificity to LacNAc and chito-oligosaccharides (Itakura et al., 2017).These lectins interact mainly with chito-oligosaccharides moieties in different degrees to bring on antimicrobial effects by various mechanisms. They hamper the growth of bacteria through antibiofilm and antiquorum sensing properties whereas pore formation, changes in cell permeability and disintegration of cell wall are the mechanisms involved in their bactericidal and fungicidal activities, apoptosis and autophagy (Gomes et al., 2012; Hasan et al., 2014a,b, Yao et al., 2010).Chitin-binding lectins or CBLs are widely known for their role in plant defense (Chen et al., 2018). Tomato lectins had been used in histochemical analysis, analyzing structural changes during oncogenesis as well as a tool for drug delivery (Oguri et al., 2008).
N-acetylglucosamine specificity of 74-kDa lectin from Tomato (Solanum lycopersicum) fruits was exploited to purify it by affinity adsorption in 1980 (Kilpatrick, 1980). Nachbar et al also reported the purification of a Tomato lectin (71 kDa) by affinity chromatography at the same year (Nachbar et al., 1980). Two isolectins with a molecular weight of 68 kDa were also purified by chromatofocusing (Kilpatrick et al., 1984). Until then, all the purified lectins were similar in size. Later, bigger lectins (130 and 100 KDa) from tomato fruits were isolated using erythroglycan-sepharose column and affinity chromatography on a chitin column, respectively (Zhu and Laine, 1989; Saito et al., 1996).In recent years, molecular structure and properties of Tomato lectins have been elucidated and it became evident that Tomato lectins are typical chitin-binding lectins, chimeric in nature with at least two chitin-binding domains (Peumans et al., 2003; Jain et al., 2021). They possess a range of molecular weights (45–138 kDa) due to the presence of highly glycosylated extension-like domains (Merkle and Cummings, 1987; Naito et al., 2001; Qin et al., 2003; Oguri et al., 2008).
This study focused on biological activities of Tomato lectins rather than the purification as there is insufficient information about their physiological roles, despite of their long history. Previously, antifungal and antimicrobial activities of two lectins from Lycopersicon esculentum and Solanum integrifolium were evaluated (Roh et al., 2010; Chen et al., 2018). This work reports partial purification of a mixture of chitin-binding Tomato lectins (TCLs) through a modified affinity chromatographic procedure, their toxicity as well as antibacterial and antifungal effects in detail. In addition, in vivo and in vitro antiproliferative activities of TCLs against Ehrlich’s ascites carcinoma (EAC) cells were reported for the first time.
2 Experimental
2.1 Materials
We purchased DMEM medium, fetal calf serum and Hoechst-33342 dye from Sigma Chemicals Co., USA. Streptomycin and penicillin were bought from CarlRoth Co., Germany. All other reagents were bought from Merck, Germany, Wako Pure Chemical Co., Japan and Sigma Chemicals Co., USA and were of the highest purity grades. Tomato (Solanum lycopersicum) fruits were purchased from a local market at Rajshahi city, Bangladesh.
2.2 Acetylation of chitin and preparation of gel
Acetylation of chitin powder was obtained by following the procedure from Nishi et al., 1979, with few modifications (Nishi et al., 1979). Perchloric acid (ice-cold 70%) was added by drops to glacial acetic acid with agitating at 0 °C and the mixture was kept at 4 °C for overnight. Acetic anhydride and chitin powder were mixed to the above solution by shaking for 5 h at 0 °C and kept at 0 °C overnight. For precipitating the above mixture, ice-water was added and precipitation was procured by filtration. Then washed with water and again re-suspended in distilled water to neutralize the mixture at pH 7.0 with ammonium hydroxide and boiled for an hour. The product was filtered, washed with water and treated with boiling ethanol for 90 min. The final product, acetylated chitin was picked up by filtration, again washed with ethanol, filtered and then air-dried.
2.3 Purification of TCLs and checking the hemagglutination activity
The acetylated chitin powder was dissolved in 10 mM Tris-HCl buffer (pH 8.0) and packed in a column of desired length. The column was equilibrated with the same buffer, pH 8.0. After washing, Tomato pulps were grated and homogenized with 50 mM Tris-HCl buffer (pH 8.0) containing 1% NaCl and 0.02% sodium metabisulfite, to prevent browning. The centrifuged homogenate or crude protein was subjected to affinity chromatography on that acetylated chitin column which was washed well by the same buffer. Finally, the proteins were eluted by 0.5 M acetic acid and absorbance of each fraction was measured by an UV Spectrophotometer at 280 nm. The protein was dialyzed in 10 mM Tris-HCl buffer and hemagglutination activity of each fraction was confirmed with human and mice red blood cells according to a standard protocol (Hasan et al., 2021). Purity of the protein fractions (TCLs) showing hemagglutination activity was estimated with 16% (w/v) polyacrylamide gel by sodium dodecyl sulfate polyacrylamide gel electrophoresis (Laemmli, 1970).
2.4 Brine shrimp nauplii lethality assay
Artificial sea water was prepared through dissolving 38 g of NaCl in 1000 ml of distilled water and pH 7.0 was adjusted by adding sodium tetraborate salt. 1 gm cysts of brine shrimp (Artemia salina L.) were added to the water and after hatching, incubated at 30 °C under constant light and aeration for 24 h. After 24 h, aeration was halted and to separate hatched cysts from unhatched cysts, light source was moved to the other side. Because of their phototropic nature, the nauplii were migrated toward the light source. Five vials, each containing ten brine shrimp nauplii to which TCLs were added at a concentration of 0.0, 25.0, 50.0, 100.0 and 200.0 µg/ml and to adjust the volume of each vial, artificial sea water was added. Each experiment was performed in triplicates. Number of dead nauplii was counted after 24 h for each experiment and the LC50 value of TCLs were calculated by Probit analysis (Finney, 1971).
2.5 Antimicrobial activity of TCLs
2.5.1 Antibacterial activity against gram-positive and gram-negative bacteria by TCLs checked by disc diffusion assay
Three bacteria, Shigella boydii (ATCC 231903), Shigella dysenteriae (ATCC 238135), Staphylococcus aureus (ATCC 25923) and Escherichia coli (ATCC 27853) were grown in nutrient broth and incubated them at 37 °C overnight. Absorbance was adjusted by liquid nutrient medium to 1.0 at 630 nm. Sterilized petri dishes containing solidified nutrient medium where pathogenic bacteria were spread out separately on them and paper discs (5 mm) were placed on the top of the surface. TCLs (500 µg/disc) and standard antibiotic (15 µg/disc of Ampicillin) were soaked by the discs and bacterial cells were allowed to grow at 37 °C overnight. After 24 h, zone of inhibition formed against discs containing TCLs and antibiotic as well as control discs were measured and compared.
2.5.2 Determination of bacterial growth inhibition activity of TCLs by turbidity method
Escherichia coli, Staphylococcus aureus and Shigella boydii were taken in test tubes for this study. Various concentrations of TCLs were added to the test tubes and shaken in a temperature-controlled shaker at 37 °C overnight. Test tubes with turbid solutions indicated bacterial growth whereas clear solutions showed no growth. Based on optical density (at 630 nm), minimum inhibitory concentration (MIC) and minimum bactericidal concentrations (MBC) of TCLs were determined for these bacteria.
2.5.3 Antibiofilm activity of TCLs
Escherichia coli were grown in a liquid nutrient media for overnight at 37 °C. Antibiofilm activity assay of TCLs against E. coli was performed following a method published earliar (Hasan et al., 2021). In another experiment, two test tubes (‘Control’ and ‘Test’) were filled up with the bacterial suspension. TCLs (500 µg/ml) were added to the ‘Test’ whereas the ‘Control’ was not treated with those lectins. Both test tubes were allowed to keep in an incubator until the formation of biofilm.
2.5.4 Antifungal activity of TCLs by disc diffusion method
Mycelia of fungal strain- Aspergillus niger was dissolved in distilled water and spread out on petri dishes with potato dextrose agar media. Sterile paper discs were placed on top of the surface. TCLs (600 µg/disc) and standard antifungal agent (1% Clioquinol + 0.02% Flumetasone Pivalate, 200 µg/disc) were soaked by the discs. The petri dishes were then incubated at 30 °C as long as the mycelia were grown. Antifungal activity of the lectins was observed by the formation of transparent rings around the discs.
2.5.5 Agglutination of Aspergillus niger spores
A number of spores of Aspergillus niger were dissolved in Phosphate buffer saline (PBS) and taken into two petri dishes. TCLs were added to one petri dish at a concentration of 200 µg/ml whereas the same amount of PBS was added to another. After 10 min, both petri dishes were checked under a microscope to find any agglutination activity of TCLs to fungal spores.
2.6 In vivo antiproliferative activity of TCLs against EAC cells in Swiss albino mice
2.6.1 Growth inhibition assay of Ehrlich ascites carcinoma (EAC) cells in vivo
Female Swiss albino mice aged 6–8 weeks weighing 25–35 g were bought from the ICDDR’B (International Center for Diarrheal Diseases Research, Bangladesh) and had ad libitum access to laboratory pellet diet and water. Viable tumor cells (0.1 ml) were intraperitonealy injected into eighteen Swiss albino mice to develop ascite tumors. Concentration of EAC cells were adjusted to 4 × 106 cells/ml. There were six mice in three groups (‘Control’, ‘low-dose’ and ‘high-dose’). After an interval of 24 h, TCLs were injected to the mice from ‘low-dose’ group at a dose of 1.0 mg/kg/day and to the mice from ‘high-dose’ group at a dose of 2.0 mg/kg/day. After continuing this treatment for five days, mice from all three groups were sacrificed on the sixth day. Using 0.9% saline solution, EAC cells from each mouse were collected and later were counted by a haemocytometer. Percentage of growth inhibition of EAC cells treated by TCLs was determined by following formula: Percentage of inhibition = 100 – {(cells from TCLs-treated mice/cells from control mice) × 100}.
2.6.2 Morphological changes of EAC cells observed by fluorescence microscopy
After treatment of Swiss albino mice with TCLs (at 1 mg/kg/day and 2 mg/kg/day) and without TCLs, EAC cells were collected from the mice and washed thrice with PBS. The cells were stained with 0.1 µg/ml of Hoechst 33342, kept for 20 min in the dark at 37 °C and washed again with PBS to remove free dye. Changes in size and shape of EAC cells were observed under a fluorescent microscope.
2.6.3 Isolation of RNA from EAC cells and expression of apoptosis-related genes
Total RNA was extracted from the EAC cells of TCLs-treated and control Swiss albino mice with a commercial kit (TIANGEN Biotech reagent kit, Beijing, China). Purity and concentration of the RNA was confirmed by a spectrophotometer and with the help of 1.4% agarose gel electrophoresis. Ten μg/ml of ethidium bromide were used to stain the bands to visualize those in a gel documentation system (Cleaver Scientific Ltd, DI-HD, UK). cDNA samples were isolated from the RNAs following the manufacturer’s protocol (Applied Biosystems, USA). Expression of p53, Fas, FADD, Bak, Caspase-3, Caspase-8, MAPK, NF-kB, TNF-α, mTOR, EGFR, Notch1 and STAT-1 genes was evaluated and 18S gene was used as the housekeeping gene.
Following a standard protocol, temperature settings in the amplification reactions were at 95 °C for 3 min, 94 °C for 30 sec, 55 °C 30 sec, 72 °C for 50 sec and 72 °C for 10 min (Gene, Atlas 482, Japan). At the end of the reaction, the amplification products were analyzed using 1.4% agarose gel electrophoresis. A 100 bp DNA ladder (Sigma-Aldrich, USA) was used in the experiment.
2.7 In vitro antiproliferative activity of TCLs against EAC cells determined by MTT assay
EAC cells from a tumor-bearing Swiss albino mouse were collected following a standard procedure (Hasan et al., 2021). Cells (2 × 104 in 100 µl) were cultured in a 96-well flat-bottomed cell culture plate containing DMEM media and incubated at 37 °C for 24 h. TCLs were serially diluted and added to the wells at concentrations of 8–256 µg/ml. There were three ‘Control’ wells with untreated EAC cells. Then the media was removed and 10 mM of PBS and 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyl Tetrazolium Bromide or MTT reagent (20 µl, 5 mg/ml MTT in PBS) were further added. The plate was again incubated for 6 h at 37 °C. After draining out the aliquot, 200 µl of acidic isopropanol was added. After 30 min, the plate was taken out from the incubator and absorbance values were recorded at 570 nm. Percentage of EAC cell growth inhibition in the presence if TCLs was calculated following the formula given below: Proliferation inhibition ratio (%) = {(A- B) × 100}/A.
A is the OD570 nm of the cellular homogenate from control wells and B is the OD570 nm of the cellular homogenate treated with TCLs.
2.8 Statistical analysis
Statistical analyses were performed and compared by One-way ANOVA with Dunnett’s t test using SPSS software version 21 (SPSS Inc., Chicago, IL, USA). Data were considered significant when p < 0.05.
3 Results
3.1 Purification and determination of molecular mass of TCLs
Chitin-binding proteins with hemagglutinating activity were isolated through a single-step affinity chromatography using acetylated chitin powder (Fig. 1A). Four major bands with molecular masses ranging from 30 to 115 kDa were found in 16% SDS-PAGE (Fig. 1B, Lane 2). This protein was designated as Tomato Chitin-binding lectins or TCLs.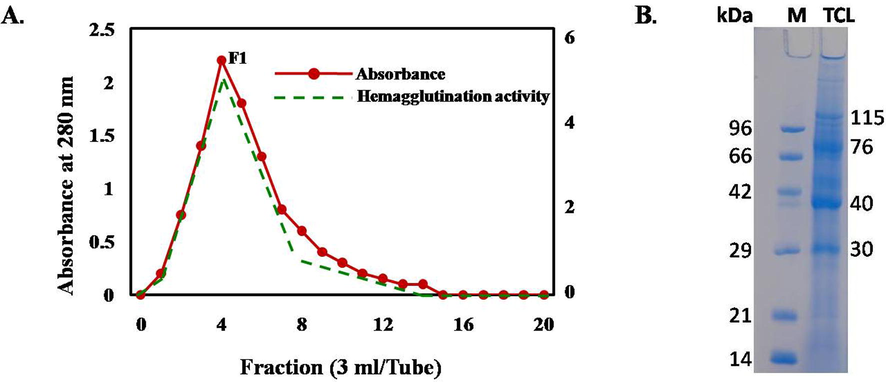
(A) The acetylated chitin powder was packed in a chromatographic column and the column was equilibrated with 10 mM Tris-HCl buffer, pH 8.0.The crude Tomato protein was applied and the column was washed by the same buffer. The proteins were eluted by 0.5 M acetic acid (3 ml/tube) and hemagglutination activity of each tube was determined after dialysis. B. The isolated protein fraction showing hemagglutination activity showed four major bands (115, 76, 40 and 30 kDa) in SDS-PAGE (sodium dodecyl sulfate polyacrylamide gel electrophoresis).
3.2 Toxicity of TCLs against Artemia nauplii
TCLs dose-dependently killed the shrimp nauplii whereas 40% mortality rate was found at a concentration of 200 µg/ml. Mild toxicity of the protein mixture was indicated by an LC50 value of 261.0 μg/ml (Fig. 2).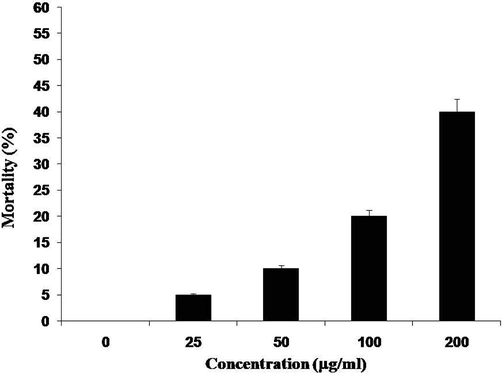
Toxicity of TCLs. Percentages of mortality brine shrimp nauplii treated with different concentrations of TCLs after a 24-hour exposure. Data are expressed in mean ± S. D.; p < 0.05.
3.3 Antibacterial activity of TCLs against pathogenic bacteria
After the incubation of Shigella boydii for 8, 16 and 24 h with TCLs, percentages of growth inhibition were calculated to be 2.58%, 6.57% and 4.03%, respectively, comparing to the growth of control bacteria (Fig. 3A). After treating with TCLs for 8, 16 and 24 h, time-dependent growth inhibition was found for Shigella dysenteriae as 5.17%, 3.67% and 2.85%, respectively (Fig. 3B). This trend continued for Staphylpcoccus aureus as well. TCLs inhibited their growth at the rate of 6.84% and 0.51% when treated for 8 and 16 h, respectively, but lost the activity during a 24 h treatment (Fig. 3C). In case of Escherichia coli, TCLs could not inhibit their growth and mitogenic growth (7–9%) was observed instead (not shown in the figure).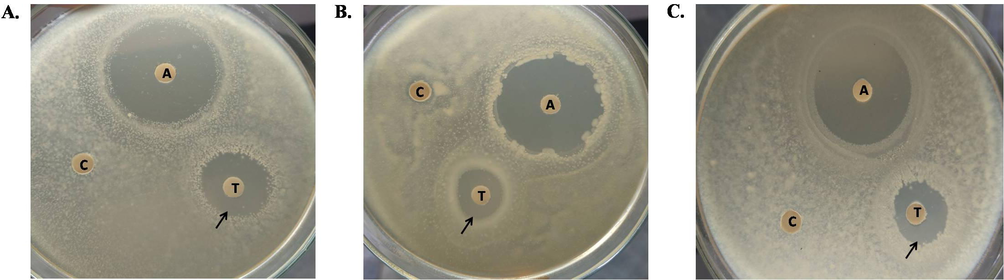
Growth inhibition of various bacterial species treated with TCLs comparing to their corresponding controls. (A) Shigella boydii (B) Shigella dysenteriae (C) Staphylococcus aureus. ‘A’, ‘T’ and ‘C’ represent antibiotic (15 µg/disc of Ampicillin), TCLs-treated (500 µg/disc) and Control discs, respectively.
3.4 Determination of MIC and MBC values of TCLs against different bacteria
By following the Turbidity method, minimum inhibitory concentration (MIC) values of TCLs against Escherichia coli, Staphylococcus aureus and Shigella boydii were determined which showed higher activity of these lectins against Escherichia coli than the other two (Table 1). MBC/MIC ratios were similar for Escherichia coli and Staphylococcus aureus comparing to Shigella boydii. ‘−’ denotes Gram-negative and ‘+’ denotes Gram-positive bacteria. MIC and MBC values are expressed in µg/ml of protein. MIC, minimal inhibitory concentration corresponds to the lowest lectin concentration at which there was ≥ 50% reduction in OD630 in comparison with the control. MBC, minimal bactericidal concentration corresponds to the minimum lectin concentration at which no bacterial growth was observed in OD630 in comparison with the control.
Bacteria
MIC
MBC
MBC/MIC Ratio
Escherichia coli (−)
140
600
4.29
Staphylococcus aureus (+)
200
840
4.20
Shigella boydii (−)
280
1480
5.29
3.5 Antibiofilm activity of TCLs
Though TCLs could not inhibit the growth of Escherichia coli, it showed antibiofilm activity against the bacteria. The lectin could inhibit the formation of biofilm by 53.32%, 62.62% and 77.29% at concentrations of 250, 500 and 1000 μg/ml (Fig. 4A). In another experiment, formation of biofilm was observed in the test tube containing E. coli bacterial suspension whereas no biofilm was found in the TCLs-treated bacterial suspension (Fig. 4B).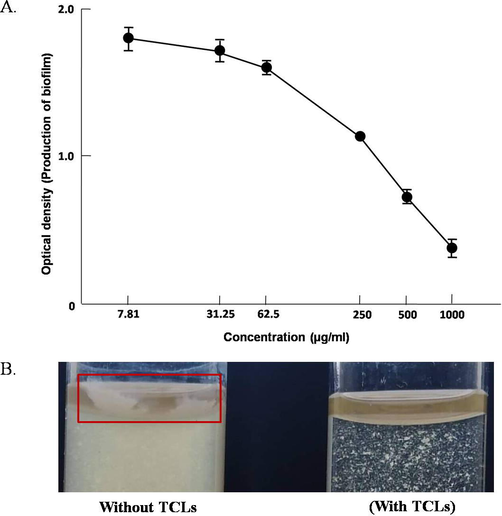
(A) Antibiofilm activity of TCLs against Escherichia coli. Data are expressed in mean ± S.D; p < 0.05. (B) Inhibition of biofilm formation in TCLs-treated E. coli suspension.
3.6 Antifungal activity of TCLs by disc diffusion method and agglutination of fungal spores
TCLs exerted moderate antifungal activity against the fungi Aspergillus niger by inhibiting fungal growth at a concentration of 600 µg/ml (Fig. 5A). Fungal spores from Aspergillus niger became agglutinated at a concentration of 200 µg/ml of TCLs whereas no agglutination was observed without TCLs (Fig. 5B).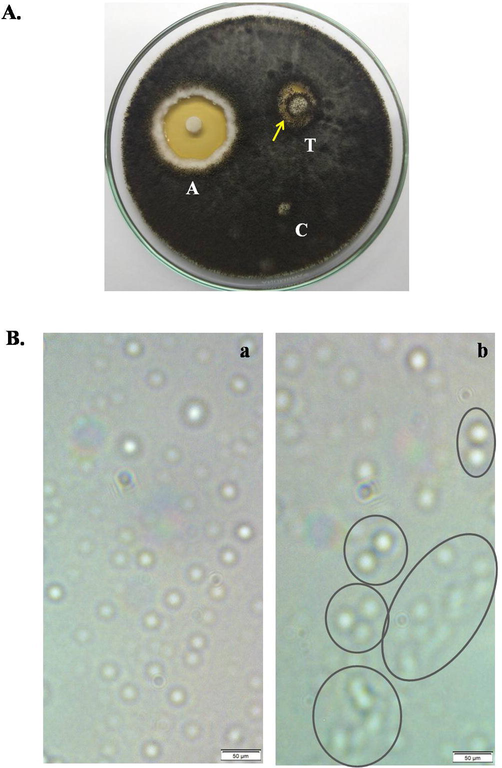
Antifungal activity of TCLs against Aspergillus niger. (A) A disc (‘T’) soaked with 600 µg/ml of TCLs inhibited the fungal growth, ‘A’ represents standard antifungal agent (1% Clioquinol + 0.02% Flumetasone Pivalate and ‘C’ is the control disc. (B) Agglutination of fungal spores in the absence (a) and presence (b) of 200 µg/ml of TCLs. Scale bar indicates 50 µm.
3.7 Growth inhibition assay of Ehrlich ascites carcinoma (EAC) cells in vivo and in vitro
TCLs could inhibit the growth of EAC cells when treated for five days. Comparing to the untreated or control cells, 19.63% and 59.91% of in vivo cell growth inhibition was observed at low (2 mg/kg/day) and high (4 mg/kg/day) doses of TCLs (Fig. 6A). Proliferation of EAC cells was measured in vitro by MTT assay after treating those with various doses of TCLs. At concentrations 37.5, 75 and 150 μg/ml, percentage of growth inhibition slowly increased from 27.61% to 38.74% and 49.23%, respectively (Fig. 6B).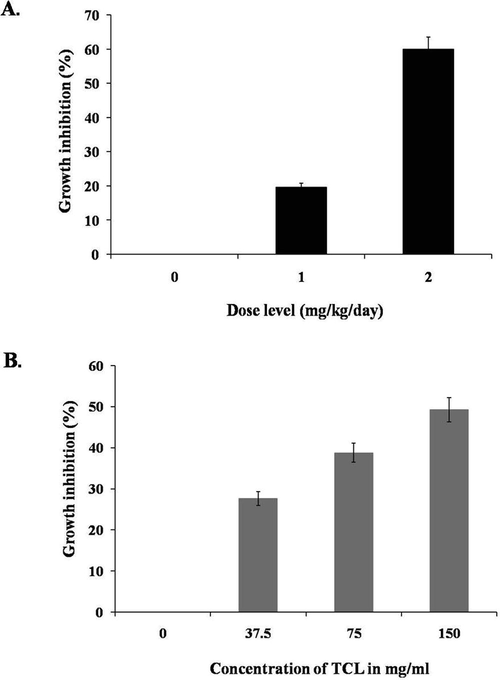
Growth inhibition of EAC cells from mice treated with TCLs. Data are expressed in mean ± S.D (n = 6); p < 0.05. Dose-dependent growth inhibition of TCLs-treated EAC cells cultured in DMEM media for 48 h. Data are expressed in mean ± S.D. (n = 3); p < 0.05.
3.8 Observation of morphological changes and nuclear damages of EAC cells through fluorescence microscope
Comparing to control mice, number of EAC cells got reduced in the lectin-treated mice (Fig. 7A). EAC cells from Swiss albino mice treated with TCLs were stained with Hoechst-33342 to observe the morphological alterations. EAC nuclei in cells from the control group were found to be in regular round shape in fluorescence microscope (Fig. 7B) and homogeneously stained with Hoechst-33342. On the other hand, cells from TCLs-treated mice were found to be irregular in shape and exhibited apoptotic morphological changes (e.g., membrane blebbing, cell shrinkage, chromatin condensation, and nuclear fragmentation) when observed in fluorescence microscope (Fig. 7C & D).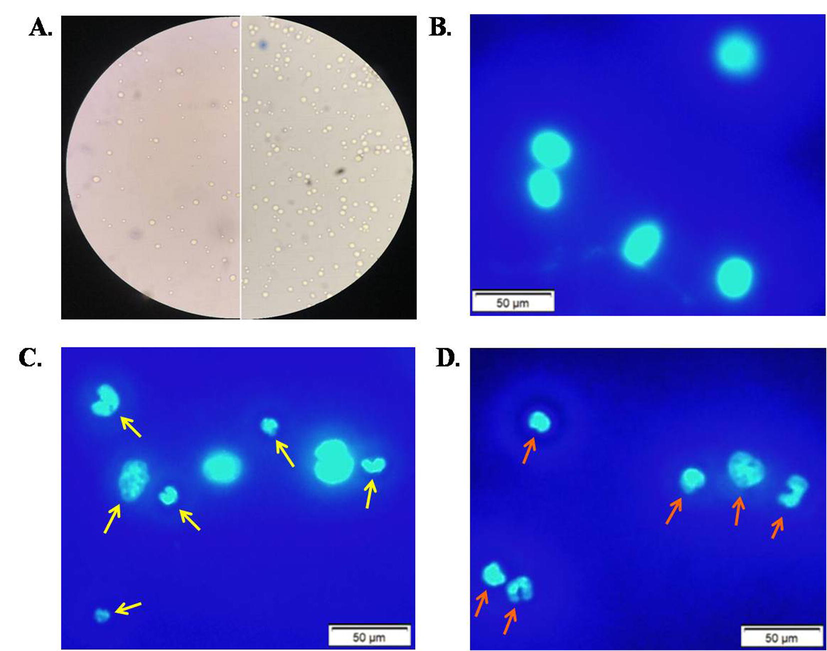
Observation of morphological features of EAC cells (A) Reduced number of EAC cells in lectin-treated mice (left) comparing to untreated mice (right) (B) EAC cells (stained) from untreated or control group of mice, EAC cells (stained) from mice treated with (B) 1 mg/kg/day and (C) 2 mg/kg/day of TCLs. Scale bar indicates 50 µm.
3.9 Isolation of RNA from EAC cells and expression of apoptosis-related genes
Considerable appearance of apoptosis-related genes like Fas, Caspase 8 and Caspase 3 was found. Expression of these genes from EAC cells of TCLs-treated mice were upregulated than those cells from the control mice. Expression of caspase 3 was much stronger than that of caspase 8. Quality of mRNA from lectin-treated and control EAC cells was confirmed through the ample expression of 18S gene (Fig. 8).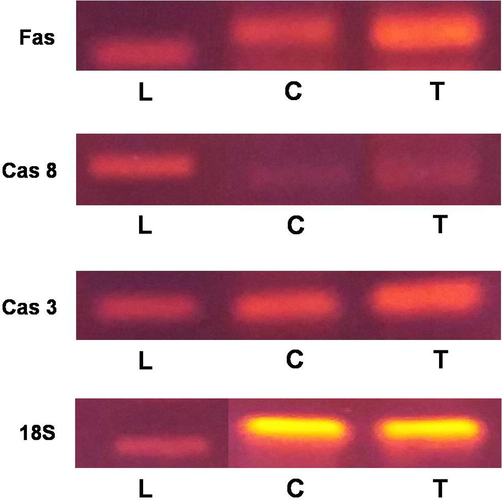
Expression of apoptosis-related genes. PCR reactions were carried out with primers specific for Fas, Caspase 8, Caspase 3 and 18S whereas products were separated on 1.5% agarose gel and stained with ethidium bromide. L represents DNA ladder; T and C represents RNA from TCLs-treated EAC cells and untreated control EAC cells, respectively.
4 Discussion
Tomato lectins were actually isolated long ago following various isolation procedures. Physicochemical properties of Tomato lectins were revealed to be parallel to other solanaceae lectins from Solanum tuberosum (Potato) and Datura stramonium (Datura) (Marinkovich, 1964; Allen et al., 1978; Kilpatrick and Yeoman, 1978). These three lectins were similar in structure though both potato and tomato lectins were fusion proteins consisting of Cys-rich and Hyp-rich domains (Kieliszewski et al., 1994; Allen et al., 1996). TCLs with apparent molecular weights of 30, 40, 76 and 115 KDa migrated in the SDS-PAGE as four major bands and were eluted as a mixture of proteins. Previously, mixtures of Potato lectins had been reported to possess antimicrobial and anticancer activities (Hasan et al., 2014a,b). Antifungal property of another mixture of lectins from cherry tomato was also observed (Roh et al., 2010). All these results encouraged us to investigate the antibacterial, antifungal and in vivo anticancer activities of this mixture of Tomato lectins, instead of a purified lectin.
TCLs were found to be mildly toxic with an LC50 value of 261.0 µg/ml against brine shrimp nauplii. Two other solanaceae lectins, STL-S and STL-D, showed more toxic nature against the nauplii with LC50 values 75 and 90 µg/ml, respectively (Hasan et al., 2014a,b). Plant lectins from Momordica charantia, Nymphaea nouchali, Moringa oleifera and Sebastiania jacobinensis possessed varying toxicity (49.7, 120 ± 29, 131 and 715.89 ± 1 µg/ml) (Kabir and Reza, 2014); Kabir et al., 2011; Asaduzzaman et al., 2018; Vaz et al., 2010) whereas a lectin from snake gourd shared very similar LC50 value (261 ± 29 μg/ml) with TCLs (Kabir et al., 2012).
It is already recognized that gram-positive bacteria are surrounded by much thicker layers of peptidoglycan comparing to gram-negative bacteria (Silhavy et al., 2010). Glycan strands in bacterial peptidoglycan are composed of repeating units of N-acetylglucosamine and N-acetylmuramic acids with different degrees of polymerization. As a result, chitin (polymer of N-acetylglucosamine units)-binding lectins can bind and interact with cell surface glycans present in bacterial peptidoglycan and this is probably the reason behind the susceptibility of gram-positive bacteria (S. aureus) towards TCLs comparing to gram-negative bacteria (E. coli and S. boydii). StL-20, a potato lectin, also showed strong antibacterial activity against gram-positive bacteria than gram-negative bacteria (Hasan et al., 2014a,b). Moreover, antibacterial activity of Eugenia uniflora lectin (EuniSL) against gram-positive and gram-negative bacteria was very much in line with the finding of this study (Oliveira et al., 2008). Minimum inhibitory concentration (MIC) values of TCLs against S. aureus and E. coli were 140 and 200 µg/ml, respectively which indicated their potential as antibiotic. Two seed lectins from Archidendron jiringa and Eugenia uniflora showed bacteriostatic activity against S. aureus (with MIC values 56.7 and 1.5 µg/ml). In case of gram negative E. coli bacteria, strong growth inhibition (MIC 16.5 µg/ml) was observed by E. uniflora seed lectin (EuniSL) though A. jiringa seed lectin could not affect their growth (Charungchitrak et al., 2011; Oliveira et al., 2008). Minimum bactericidal concentrations (MBC) of EuniSL against the same bacterial species were 16.5 µg/ml and 180 µg/ml, respectively (Oliveira et al., 2008) whereas for TCLs, MBC values were 600 and 840 µg/ml, respectively. TCLs moderately (53.32% at 250 μg/ml) suppressed the formation of biofilm by E. coli. StL-20 exerted stronger anti-biofilm activity (20% at 80 μg/ml) against P. aeruginosa whereas that activity of AGL (Amaranthus gangeticus lectin) was weaker (37.14% at 250 μg/ml) than TCLs (Hasan et al., 2014a,b; Hasan et al., 2021).
TCLs showed strong antifungal activity against the fungi, Aspergillus niger, at a concentration of 600 µg/ml and agglutinated fungal spores at a concentration of 200 µg/ml. Potent fungal growth inhibition by the lectin from cherry tomato against Cladosporium cucumerinum and Monosporascus cannonballus was also reported (Roh, 2010). Other antifungal lectins from solanaceae family (Solanum tuberosum, Capsicum annuum and Solanum integrifolium) had also been reported (Hasan et al., 2014a,b; Gozia et al., 1993; Kuku et al., 2009; Chen et al., 2018). Another 48 kDa chitin-binding lectin from Setcreasea purpurea rhizome exerted fungicidal activity against a number of fungi but was inactive against Aspergillus niger even at much higher concentration (1.51 mg/ml) (Yao et al., 2010). Strong fungal growth inhibition was also documented by lectins from Archidendron jiringa (Charungchitrak et al., 2011), Phaseoulus vulgaries and Glycine max (Mohsen et al., 2018).
Cancer is a group of diseases identified according to their preliminary affected cell types. Various plant lectins have been shown to have antiproliferative properties against various cancer cell types (Kabir et al., 2016; Kabir and Reza, 2014). Given the lack of H2 histocompatibility antigen, EAC cells are very suitable to check the potentiality of newly synthesized or purified chemopreventive agents because of their proliferative capacity (Chen and Watkins, 1970). In this work, TCLs dose dependently inhibited the growth of EAC cells and its antiproliferative activity (49.23% inhibition of cell growth at a concentration of 150 µg/ml) was higher than potato lectins (36% at 160 µg/ml) (Kabir et al., 2016). Other plant lectins from Keampferia rotunda and Geodorum densiflorum repressed 43.7% and 60% of EAC cell growth in similar (160 µg/ml) concentrations (Ahmed et al., 2017; Kabir et al., 2019). At lower concentrations of 120 µg/ml and 128 µg/ml, anti-proliferative activity of lectins from Pisum sativum and Amaranthus gangeticus varied a lot, giving values like 84% and 33.7%, respectively (Kabir et al., 2013; Hasan et al., 2021). In the in vivo experiment, growth inhibition of EAC cells by TCLs was 59.91% at a dose of 2 mg/kg/day. KRL-2 and Pea lectins were weaker in action 41% and 63% at concentrations 3 and 2.8 mg/kg/day whereas Potato lectins showed stronger effects (83% at 1.38 mg/kg/day) (Ahmed et al., 2017; Kabir et al., 2013; Hasan et al., 2014a,b). So, it can be recommended that TCLs showed moderate anticancer activity comparing to the lectins mentioned here.
Apoptosis is characterized by shrinking of cells, blebbing of plasma membrane, fragmented DNA and nuclear condensation (Procopio et al., 2017). Signs of apoptosis in TCLs-treated EAC cells became evident due to the presence of apoptotic symptoms when compared to control EAC cells . Similar signs of apoptosis in EAC cells have also been induced by other lectins from plants and invertebrates (Kabir et al., 2013; Ahmed et al., 2017; Asaduzzaman et al., 2018; Kabir et al., 2019; Swarna et al., 2019). Fas-mediated pathway is well-reported among several extrinsic pathways of apoptosis. Fas ligands bind with cell-surface death receptors (Fas receptors) to send the ‘First Apoptotic Signal’. Fas associated death domains (FADD) become recruited causing the formation of death-inducing signaling complex or DISC including caspase 8, the initiator caspase. Caspase 8 directly or indirectly cleaves downstream caspases and cell death finally occurs due to the action of effector caspases like caspase 3. Expression of Fas, caspase 8 and caspase 3 genes in the TCLs-treated EAC cells suggested a possible Fas-mediated pathway of their apoptotic death. Involvement of caspase 8 and 3 in apoptosis was previously observed in CNE-1 cancer cells treated by another chitin-binding lectin from Setcreasea purpurea (Yao et al., 2010).
5 Conclusion
From this present study, it became obvious that TCLs were mildly toxic with low chitinase activity. It is demonstrated that TCLs has strong antibacterial and antifungal activities. To dispel biofilm from medical instruments, TCLs may contribute effective roles in clinical microbiology because of their antibiofilm activity. In vitro and in vivo studies indicated that TCLs has also moderate anti-proliferative activities against Ehrlich ascites carcinoma cells. These lectins have the ability to change the morphology of EAC cells by inducing apoptosis. These observations are commencing that TCLs has potent biomedical properties and further investigations are required to explore their chemopreventive properties in more details.
Ethics approval and consent to participate
Ethical clearance of the experiments using Swiss albino mice was provided by the Institutional Animal, Medical Ethics, Bio-safety and Bio-security Committee (IAMEBBC) for Experimentations on Animals, Human, Microbes and Living Natural Sources (Memo No. 102(6)/320/IAMEBBC/IBSc), Institute of Biological Sciences (IBSc), University of Rajshahi, Bangladesh.
Authorship contribution statement
Nawshin Arfin: Methodology, Writing – original draft. Munna Kumar Podder: Methodology, Validation. Syed Rashel Kabir: Methodology, Validation, Writing – review & editing. A.K.M. Asaduzzaman: Methodology, Data curation, Validation. Imtiaj Hasan: Conceptualization, Supervision, Validation, Writing – original draft, review & editing.
Consent for publication
Authors declare consent for publication.
Funding
This study was partly supported by the research grant from Ministry of Science and Technology (Grant No. 39.00.0000.009.14.011.20-BS-231/1567), Government of Bangladesh.
References
- Antitumor properties of a methyl-β-d-galactopyranoside specific lectin from Kaempferia rotunda against Ehrlich ascites carcinoma cells. Int. J. Biol. Macromol.. 2017;102:952-959.
- [CrossRef] [Google Scholar]
- Potato lectin: a three-domain glycoprotein with novel hydroxyproline-containing sequences and sequence similarities to wheat-germ agglutinin. Int. J. Biochem. Cell Biol.. 1996;28(11):1285-1291.
- [CrossRef] [Google Scholar]
- Properties of potato lectin and the nature of its glycoprotein linkages. Biochem.. 1978;171(3):665-674.
- [CrossRef] [Google Scholar]
- Moringa oleifera seed lectin inhibits Ehrlich ascites carcinoma cell growth by inducing apoptosis through the regulation of Bak and NF-κB gene expression. Int. J. Biol. Macromol.. 2018;107(Pt B):1936-1944.
- [CrossRef] [Google Scholar]
- Overview of the Structure-Function Relationships of Mannose-Specific Lectins from Plants, Algae and Fungi. Int. J. Mol. Sci.. 2019;20(2):254.
- [CrossRef] [Google Scholar]
- Antifungal and antibacterial activities of lectin from the seeds of Archidendron jiringa Nielsen. Food Chem.. 2011;126(3):1025-1032.
- [CrossRef] [Google Scholar]
- Functional characterization of chitin-binding lectin from Solanum integrifolium containing anti-fungal and insecticidal activities. BMC Plant Biol.. 2018;18(1):3.
- [CrossRef] [Google Scholar]
- Evidence against the presence of H2 histocompatibility antigens in Ehrlich ascites tumour cells. Nature. 1970;225(5234):734-735.
- [CrossRef] [Google Scholar]
- Lectins, Interconnecting Proteins with Biotechnological/Pharmacological and Therapeutic Applications. Evid.-based Complement Altern. Med.. 2017;2017(1594074)
- [CrossRef] [Google Scholar]
- Finney, D.J., 1971.Probit Analysis, 3rd ed., Cambridge University Press. J Pharm Sci. 60 (9), 1432–1432.
- Antimicrobial lectin from Schinus terebinthifolius leaf. J. Appl. Microbiol.. 2012;114(3):672-679.
- [CrossRef] [Google Scholar]
- Antifungal properties of lectin and new chitinases from potato tuber. C R Acad. Sci. III.. 1993;316(8):788-792.
- [Google Scholar]
- Antiproliferative activity of cytotoxic tuber lectins from Solanum tuberosum against experimentally induced Ehrlich ascites carcinoma in mice. Afr. J. Biotechnol.. 2014;13(15):1679-1685.
- [CrossRef] [Google Scholar]
- Histochemical localization of N-acetylhexosamine-binding lectin HOL-18 in Halichondria okadai (Japanese black sponge), and its antimicrobial and cytotoxic anticancer effects. Int. J. Biol. Macromol.. 2019;124:819-827.
- [CrossRef] [Google Scholar]
- Purification of a novel chitin-binding lectin with antimicrobial and antibiofilm activities from a Bangladeshi cultivar of potato (Solanum tuberosum) Int. J. Biochem. Biophys.. 2014;51(2):142-148.
- [Google Scholar]
- A N-acetyl-D-galactosamine-binding lectin from Amaranthus gangeticus seeds inhibits biofilm formation and Ehrlich ascites carcinoma cell growth in vivo in mice. Int. J. Biol. Macromol.. 2021;181:928-936.
- [CrossRef] [Google Scholar]
- Sugar-Binding Profiles of Chitin-Binding Lectins from the Hevein Family: A Comprehensive Study. Int. J. Mol. Sci.. 2017;18(6):1160.
- [CrossRef] [Google Scholar]
- Comparative structural and functional analysis of STL and SLL, chitin-binding lectins from Solanum spp. J. Biomol. Struct. Dyn.. 2021;39(13):4907-4922.
- [CrossRef] [Google Scholar]
- Geodorum densiflorum rhizome lectin inhibits Ehrlich ascites carcinoma cell growth by inducing apoptosis through the regulation of BAX, p53 and NF-κB genes expression. Int. J. Biol. Macromol.. 2019;125:92-98.
- [CrossRef] [Google Scholar]
- A new lectin from the tuberous rhizome of Kaempferia rotunda: isolation, characterization, antibacterial and antiproliferative activities. Protein Pept. Lett.. 2011;18(11):1140-1149.
- [CrossRef] [Google Scholar]
- Purification, characterizations of a snake guard seeds lectin with antitumor activity against Ehrlich ascites carcinoma cells in vivo in mice. Protein Pept. Lett.. 2012;19(3):360-368.
- [CrossRef] [Google Scholar]
- Pea lectin inhibits growth of Ehrlich ascites carcinoma cells by inducing apoptosis and G2/M cell cycle arrest in vivo in mice. Phytomedicine. 2013;20(14):1288-1296.
- [CrossRef] [Google Scholar]
- Solanum tuberosum lectin inhibits Ehrlich ascites carcinoma cells growth by inducing apoptosis and G 2/M cell cycle arrest. Tumor Biol.. 2016;37(6):8437-8444.
- [CrossRef] [Google Scholar]
- Antibacterial activity of Kaempferia rotunda rhizome lectin and its induction of apoptosis in Ehrlich ascites carcinoma cells. Appl. Biochem. Biotechnol.. 2014;172(6):2866-2876.
- [CrossRef] [Google Scholar]
- Purification and characterization of a Ca2+-dependent novel lectin from Nymphaea nouchali tuber with antiproliferative activities. Biosci. Rep.. 2011;31(6):465-475.
- [CrossRef] [Google Scholar]
- Potato lectin: a modular protein sharing sequence similarities with the extensin family, the hevein lectin family, and snake venom disintegrins (platelet aggregation inhibitors) Plant J.. 1994;5(6):849-861.
- [CrossRef] [Google Scholar]
- A comparison of tomato (Lycopersicon esculentum) lectin with its deglycosylated derivative. Biochem.. 1984;220(3):843-847.
- [CrossRef] [Google Scholar]
- Purification of the lectin from Datura stramonium. Biochem.. 1978;175(3):1151-1153.
- [CrossRef] [Google Scholar]
- Purification and some properties of a lectin from the fruit juice of the tomato (Lycopersicon esculentum) Biochem.. 1980;185(1):269-272.
- [CrossRef] [Google Scholar]
- Purification of a mannose/glucose-specific lectin with antifungal activity from pepper seeds (Capsicum annuum) Afr. J. Biochem. Res.. 2009;3(6):272-278.
- [Google Scholar]
- Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227(5259):680-685.
- [Google Scholar]
- Purification and characterization of the hemagglutinin present in potatoes. J. Immunol.. 1964;93(5):732-741.
- [Google Scholar]
- Tomato lectin is located predominantly in the locular fluid of ripe tomatoes. Plant Sci.. 1987;48(2):71-78.
- [Google Scholar]
- Isolation and Antifungal Activity of Plant Lectins against some Plant Pathogenic Fungi. Alex Sci. Exch.. 2018;39:161-167.
- [Google Scholar]
- Lectins in the US Diet. Isolation and characterization of a lectin from the tomato (Lycopersicon esculentum) J. Biol. Chem.. 1980;255(5):2056-2061.
- [Google Scholar]
- Domain construction of cherry-tomato lectin: relation to newly found 42-kDa protein. Biosci. Biotechnol. Biochem.. 2001;65(1):86-93.
- [CrossRef] [Google Scholar]
- Molecular structure and properties of lectin from tomato fruit. Biosci. Biotechnol. Biochem.. 2008;72(10):2640-2650.
- [CrossRef] [Google Scholar]
- Purification of a lectin from Eugenia uniflora L. seeds and its potential antibacterial activity. Lett. Appl. Microbiol.. 2008;46(3):371-376.
- [CrossRef] [Google Scholar]
- The tomato lectin consists of two homologous chitin-binding modules separated by an extensin-like linke. Biochem J.. 2003;376(Pt 3):717-724.
- [CrossRef] [Google Scholar]
- Procopio, T.F., de Siqueira Patriota, L.L., de Moura, M.C., da Silva, P.M., de Oliveira, A.P., do Nascimento Carvalho, L.V., de Albuquerque Lima, T., Soares, T., da Silva, T.D., Coelho, L.C., da Rocha Pitta, M.G., 2017. CasuL: A new lectin isolated from Calliandra surinamensis leaf pinnulae with cytotoxicity to cancer cells, antimicrobial activity and antibiofilm effect. Int. J. Biol. Macromol. 98, 419–429. https://doi.org/10.1016/j.ijbiomac.2017.02.019.
- Characterization of a tomato protein that inhibits a xyloglucan-specific endoglucanase. Plant J.. 2003;34(3):327-338.
- [CrossRef] [Google Scholar]
- Antifungal activity and biochemical characterization of lectin isolated from locular fluid of cherry tomato fruit. Kor. Soc. Biotechnol. Bioeng. J.. 2010;25(3):289-296.
- [Google Scholar]
- Purification, properties and carbohydrate binding specificity of cherry tomato (Lycopersicon esculentum var. Cherry) lectin. Oyo Toshitsu Kagaku (J. Appl. Glycosci.).. 1996;43:331-345.
- [Google Scholar]
- The bacterial cell envelope. Cold Spring Harb. Perspect. Biol.. 2010;2(5):a000414.
- [CrossRef] [Google Scholar]
- The immunomodulatory effect of plant lectins: A review with emphasis on ArtinM properties. Glycoconj. J.. 2013;30(7):641-657.
- [CrossRef] [Google Scholar]
- Antiproliferative and Antimicrobial Potentials of a Lectin from Aplysia kurodai (Sea Hare) Eggs. Mar Drugs. 2021;19(7):394.
- [CrossRef] [Google Scholar]
- Biocontrol of Fusarium species by a novel lectin with low ecotoxicity isolated from Sebastiania jacobinensis. Food Chem.. 2010;119(4):1507-1513.
- [CrossRef] [Google Scholar]
- A new chitin-binding lectin from rhizome of Setcreasea purpurea with antifungal, antiviral and apoptosis-inducing activities. Process Biochem.. 2010;45(9):1477-1485.
- [CrossRef] [Google Scholar]
- Purification of acetyllactosamine-specific tomato lectin by erythroglycan-sepharose affinity chromatography. Prep Biochem. 1989;19(4):341-350.
- [CrossRef] [Google Scholar]







