Translate this page into:
Antibiotics-loaded nanofibers fabricated by electrospinning for the treatment of bone infections
⁎Corresponding author at: Laboratório de Laboratório de Desenvolvimento Galênico, Departamento de Fármacos e Medicamentos, Faculdade de Farmácia, Universidade Federal do Rio de Janeiro (FF/UFRJ), Avenida Carlos Chagas Filho s/n CCS, Ilha do Fundão, Rio de Janeiro, 21941-590, RJ, Brazil. ricci@pharma.ufrj.br (Eduardo Ricci-Junior)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Electrospinning is one of the most promising fabrication techniques of nanostructured membranes for guided bone regeneration. In association with antibiotics, membranes consisting of fibers with sub-micrometric and nanometric dimensions have been tested to prevent and treat bone infections. Electrospinning was recently applied to produce metallic implant coatings for biofilm inhibition. Despite the numerous in vitro and in vivo studies conducted with nanofibers from several polymeric matrices and fabrication methods, there is no consensus on the best conditions to optimize their antimicrobial activity. This study analyzed recent advances in nanofiber fabrication by the electrospinning technique for clinical applications in the treatment of bone infections. An integrative review from MEDLINE/PubMed, Web of Science, and Scielo databases selected 16 works focused on nanofibers' in vitro and in vivo evaluations. It was found that manufacturing methods significantly influence fiber composition, structure, morphology, pore size, and biodegradability. Thus, standardizing these parameters in the production of nanofibers at an industrial scale is one of the challenges in improving drug loading control on the fiber network and its release profiles. Further in vivo studies need to be conducted to optimize the dose effect of antibiotic-loaded membranes in inhibiting the proliferation of pathogens and inflammatory processes without promoting toxicity and reducing bone regenerating capacity.
Keywords
Antibiotics-loaded nanofibers
Bone infection treatment
Antimicrobial activity
Metallic implants recovering
Biocompatibility
1 Introduction
The bone tissue is susceptible to infections by many pathogenic microorganisms, such as Methicillin-resistant Staphylococcus aureus (MRSA) (Brunotte et al., 2019), Escherichia coli, Fusobacterium nucleatum, Streptococcus sanguinis, Porphyromonas gingivalis and Actinomyces naeslundii (Bottino et al., 2019; Ho et al., 2020; Xue et al., 2015, 2014b). In general, the prevention and treatment of bone infections use the administration of antibiotics through oral and injectable routes and also as grafts implanted both into and onto bone tissues affected by diseases and trauma. Biocompatible antibiotic carriers are an alternative for preventing and treating bone infections because of their ability to insert the drug locally into the infected area and promote its controlled release in the diseased bone tissue (Dhandayuthapani et al., 2011). Polymeric, ceramics, and composite grafts loaded with antibiotics and antimicrobials such as chlorhexidine and metronidazole (Bottino et al., 2019; Ho et al., 2020, 2017, Xue et al., 2015, 2014a, 2014b), gentamicin, doxycycline, minocycline, and vancomycin have been tested in clinics to treat dental and orthopedic bone infections (Gao et al., 2016; Jang et al., 2015; Yu et al., 2020; Zhang et al., 2014).
With the emergence of nanostructured grafts, membranes consisting of nanometric fibers started to be produced using the electrospinning technique. Polymeric nanofibers with diameters varying from 1 to 1000 nm have a porous structure, high specific surface area, permeability of biological media and cellular adhesion, and proliferation (Costa et al., 2012a). The porous networks allow the association with drugs (Bhattarai et al., 2018; Costa et al., 2012a; Dahlin et al., 2011; Zupančič, 2019) for applications in drug delivery, medical devices, tissue engineering, and healing patches (Costa et al., 2012b). A large variety of synthetic and natural polymers are used to produce nanofibers, such as polycaprolactone (PCL), poly-lactic acid (PLA), poly-lactic acid-co-glycolic acid (PLGA), and sodium alginate (Xue et al., 2014b; Zhang et al., 2014). The main methods to fabricate nanofibers are phase separation, self-assembly, and electrospinning. Phase separation is based on the principle of a thermodynamically unstable environment with two phases: a polymeric phase and a poor polymeric phase; self-assembly utilizes non-covalent molecular interactions to synthesize fiber segments (Dahlin et al., 2011; Eatemadi et al., 2016; Zhao et al., 2011).
Electrospinning is the most used method to produce polymeric nanofibers due to its easiness to work and low-cost equipment (Fig. 1A). The principles of the technique may be found in many papers (Bhardwaj and Kundu, 2010; Costa et al., 2012a; Dhandayuthapani et al., 2011). The polymeric solution is added to a plastic or glass syringe and pressed only enough to form a single drop at the tip of the needle. A high electrical voltage is applied in the system, which makes the drop change into a conical shape called the Taylor cone, which is considered the critical step in forming nanofibers. When the repulsive electric forces can overcome the superficial tension of the drop, it distends, and the solvent evaporates, making it possible to place the scaffold on the collector surface, often made of aluminum (Bhardwaj and Kundu, 2010; Costa et al., 2012a; Dhandayuthapani et al., 2011).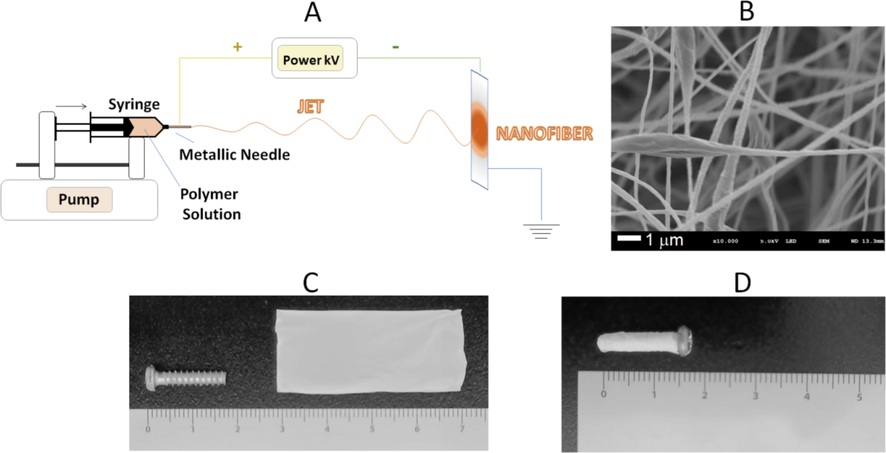
Nanofibers produced by electrospinning (A), Membrane of nanofibers observed by scanning electron microscopy (SEM) (B), Macroscopic image of the membrane formed by nanofibers (C) and Metallic implant coated with membrane of nanofibers. Font: Authors.
It is possible to modify the nanofiber structure (Fig. 1B) according to the experiment's goal, for example, using co-electrospinning or coaxial electrospinning. The principle of both is the same as monolithic nanofiber electrospinning. However, in the case of co-electrospinning, two pumping bombs are functioning simultaneously with different polymer solutions and one rotating collector. Coaxial or core–shell electrospinning uses one single bomb and a needle with two compartments: one for the outside polymer (shell) and the other for the inside polymer (core). Both techniques may help in the combination of immiscible polymers, encapsulating molecules that are sensitive to organic solvents, preventing burst effect on drug release, and improving biocompatibility and biomechanical properties (Sutrisno et al., 2018; Zupančič, 2019).
The main challenge of nanofiber fabrication is to combine biocompatibility, biodegradation, and high clinical efficiency for guided bone regeneration. Metallic implants can be coated with a nanofiber membrane (Fig. 1C and 1D) to prevent and treat infections. This involves complex issues, such as polymer selection, nanofibers fabrication method, and deposition parameters, the physicochemical characterization at an industrial scale, antibiotic association to the fibers network, the control of its release in a biological medium, and finally the in vitro and in vivo evaluation of the membrane efficacy.
This article reviews in vitro and in vivo studies published in scientific journals about electrospinning nanostructured membranes doped with antibiotics to prevent and treat bone infections. Our group previously published a literature review on the subject (Silva et al., 2021), but an integrative review has been done for the current work. Here, it is analyzed several aspects of the production of the nanostructured membranes and their biological and microbiological evaluations: materials and fabrication methods; physicochemical techniques used to characterize fibers at a micro and nanoscale; membrane and fibers composition, morphology, and porosity; antibiotics association to fibers structure, and their release profiles; cell culture procedures and animal models; antimicrobial activity, toxicity, and regenerative properties; potential for clinical applications.
Since the current treatment of bone diseases involves hospitalization and continuous use of antibiotics, which increases the risk of adverse effects and bacterial resistance, it is shown in this study the importance of an alternative therapy using nanotechnology. By comparing the issues addressed and the obtained results by each selected work, this revision evaluates advances and challenges to improving antibiotic-loaded nanofiber‘s efficacy in treating bone infections and severe inflammation by promoting guided bone regeneration (Fig. 2).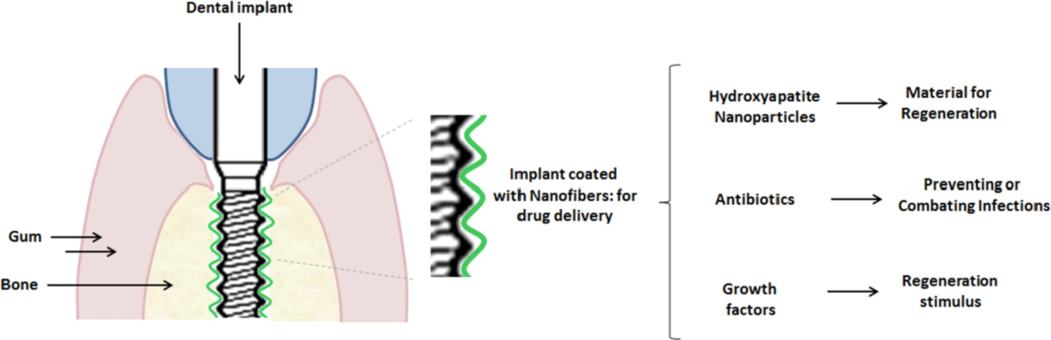
Implantation of the nanofiber scaffold in the bone.
2 Methodology
This integrative review involved a search in the scientific databases MEDLINE/PubMed, Web of Science, and Scielo between January 2010 and April 2021 using keywords “nanofibers”, “nanofibers and antibiotics”, “nanofibers and bone infections”, and “nanofibers and bone infections and antibiotics”. Three individuals conducted the integrative review to ensure the quality of study selection. Inclusion factors consisted of i) biomaterials, ii) techniques to produce nanofibers, iii) use of antibiotics, iv) prevention or healing, v) in vivo models and in vitro assays, vi) physicochemical characterization, vii) antibiotic release profile, viii) cell culture. Exclusion factors were duplicated articles, patents, review articles, book chapters, congress resumes, and articles out of the proposed context. After the article selection, the following information was considered: publishing year, name of the journal, impact factor, cell culture assays, in vivo experiments and type of animals, ethics committee, route of administration and placement of the implant, results, and conclusions of the work.
The research in Pubmed, Web of Science, and Scielo found 77,248 studies containing the keyword “nanofibers”, 1471 containing the keywords “nanofibers and antibiotics”, 177 containing “nanofibers and bone infections”, and 70 containing “nanofibers and bone infections and antibiotics” (Table 1). Scielo and Web of Science databases had the lowest and highest number of studies containing the keywords “nanofibers and bone infection”.
Keywords
PubMed
Web of Science
Scielo
Total
Nanofibers
15,761
61,374
113
77,248
Nanofibers and antibiotics
862
609
0
1471
Nanofibers and bone infections
53
124
0
177
Nanofibers and bone infections and antibiotics
32
38
0
70
From the 70 selected articles using the keywords “nanofibers and bone infection and antibiotics”, only 16 papers fulfilled all inclusion criteria (Fig. 3): 29 studies did not contemplate in vivo studies, 24 were excluded due to duplication (16), patents (2), review articles (1), book chapter (1) and a study contemplating 3D-printed scaffolds (1). The remaining articles were evaluated according to the topics addressed, such as Title, Abstract, Introduction, Contextualization, Objective, Methods, Ethics statement, Study design, Full physicochemical characterization, and nanometric size. The topic with discussion added one point, whereas incomplete or not discussed added no point (Table 2). The Arrive Guide list of verification was used to evaluate the quality of the articles in which a = in vivo study without preexistent infection; b = in vivo study with induced infection.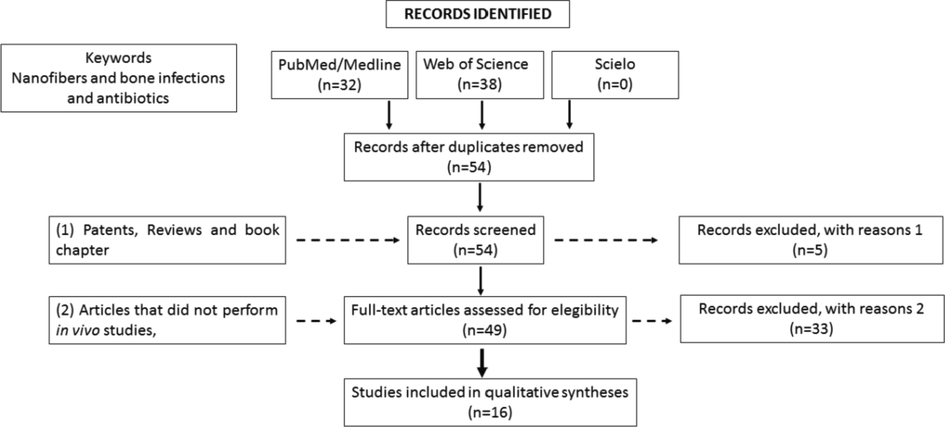
Flow diagram of literature selection process.
Reference
(Gilchrist et al., 2013)
(Zhang et al., 2014)
(Yu et al., 2020)
(Gao et al., 2016)
(Xue et al., 2014b)
(Ho et al., 2020)
(Ho et al., 2017)
(Bottino et al., 2019)
(Ashbaugh et al., 2016)
(Eren Boncu et al., 2020)
(Miller et al., 2019)
(Xue et al., 2015)
(Xue et al., 2014a)
(Jang et al., 2015)
(Song et al., 2017)
(Sutrisno et al., 2018)
Title
1
1
1
1
1
1
1
1
0
1
1
1
1
1
0
1
Abstract
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
Introduction
Contextualization
1
1
1
1
1
1
1
1
1
1
0
1
1
0
1
1
Objective
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
Methods
Ethics statement
0
0
1
0
1
1
1
1
1
0
0
0
0
1
0
1
Study design
1
1
1
1
1
1
1
1
0
1
1
1
1
1
1
1
Full physicochemical characterization
1
1
1
1
1
1
1
0
0
1
0
1
1
1
0
1
Nanometric size
1
1
1
1
1
1
1
1
0
0
0
1
1
1
0
1
In vivo experimental procedures
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
In vivo biocompatibility
0
1
1
1
1
1
1
1
1
1
0
1
1
0
1
0
Animals
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
Housing
1
0
0
1
0
0
0
1
0
0
1
0
0
1
0
1
Sample size
1
1
1
1
1
1
1
1
1
1
1
0
1
1
1
0
Anesthesia
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
Analgesia
0
0
0
0
0
0
0
1
0
0
1
0
0
0
0
0
Preventive effect a
0
0
1
0
1
1
1
0
0
1
0
1
1
0
1
1
Healing effect b
1
1
0
1
0
0
0
1
1
1
1
0
0
1
1
0
Statistics
1
1
0
1
1
1
1
0
1
1
1
1
1
0
1
1
Results and discussion
Confidence interval
1
1
1
1
1
1
1
0
1
1
1
1
1
1
1
1
Interpretation
1
1
1
1
1
1
1
1
1
1
1
0
1
1
1
1
Limitations
0
1
1
0
0
1
0
0
0
0
1
0
0
1
1
0
Funding
1
1
1
1
0
1
1
0
1
1
0
1
1
0
1
1
Points
17
18
18
18
17
19
18
16
14
17
15
15
17
16
16
17
Clinical trials were also searched on the ClinicalTrials.gov website using the keyword “nanofiber”. The research was done by evaluating complete, withdrawn, and recruiting trials.
According to Table 2, most articles had similar points because they contemplated both in vitro and in vivo studies. Also, the majority made at least two important physicochemical characterization assays like electronic microscopy and nanofiber diameter. Ho et al. (2020) scored the highest because they accomplished almost all requirements. In contrast, the article by Ashbaugh et al. (2016) had the lowest score as electronic microscopy to determine fiber diameter was missing. The selected articles were evaluated according to the materials used to fabricate the fibers, sample preparation, physicochemical characterization methods, in vitro antimicrobial activity and cell studies, and animal models in vivo studies. These topics will be discussed throughout this work. Though the theme proposed in this study referred to the use of nanostructured fiber implants, four articles did not inform the fiber diameter or whether or not the diameter was above 1000 nm, which characterizes a microfiber (Ashbaugh et al., 2016; Eren Boncu et al., 2020; Miller et al., 2019; Song et al., 2017).
3 Results
Most of the works used the electrospinning technique while two of them used co-electrospinning (Ashbaugh et al., 2016; Miller et al., 2019), and one used coaxial electrospinning (Song et al., 2017). Thus, electrospinning was the primary technique used to produce nanofiber-based membranes (Table 3). PLGA = poly lactic-co-glycolic acid; PCL = polycaprolactone; PDLLA = poly D-L lactic acid); PEO = poly (ethylene oxide); PVA = poly(vinyl alcohol); CHIT = chitosan; SEM = scanning electron microscopy; TEM = transmission electron microscopy; XRD = X-ray diffraction; DSC = differential scanning calorimetry; FTIR = Fourier-transform infrared spectroscopy; ATR-FTIR = attenuated total reflectance with Fourier-transform infrared spectroscopy; AFM = atomic force microscopy. a = Sample with highest amount of Rifampicin; b = Sample with highest amount of Metronidazole.
Reference
Composition
Method of preparation
Parameters: Flow rate (mL/h) Electric tension (kV) Distance (cm)
Fiber diameter (nm)
Porosity or pore mean size
Physicochemical essays
(Gilchrist et al., 2013)
PLGA/ Fusidic acid/ Rifampicin
Electrospinning
1 mL/h; 15 kV;10 cm
993.9 ± 178.7 nm (control) 618.7 ± 28.8 (w/ Rifampicin) a
–
SEM, XRD, DSC, encapsulation efficiency, in vitro drug release profile
(Zhang et al., 2014)
PLGA/ Vancomycin
Electrospinning
2.5 mL/h;12 kV;15 cm
983 ± 86 nm (control) 728 ± 72 nm (w/ Vancomycin)
–
SEM, encapsulation efficiency, in vitro drug release profile
(Yu et al., 2020)
PLGA/ Vancomycin/ ceftazidime
Electrospinning
1.2 mL/h;15 kV; 15 cm
1040 ± 0.38 nm (control) 111.68 ± 45.11 nm (w/ Vancomycin and ceftazidime)
54.23 %
SEM, TEM, contact angle, in vitro drug release profile
(Gao et al., 2016)
PLGA/ Vancomycin
Electrospinning
3 mL/h; kV; 10 cm
70 – 1200 nm
1 – 5 µm
SEM, in vitro drug release profile
(Xue et al., 2014b)
PCL/ Metronidazole
Electrospinning
–
480 ± 0.04 nm (control) 350 ± 0.10 (w/ Metronidazole) b
60 – 80 %
SEM, FTIR, DSC, XRD, contact angle, encapsulation efficiency, in vitro drug release profile, degradation study
(Ho et al., 2020)
PDLLA/ Metronidazole
Electrospinning
0.15 mL/h; 18 kV; 10 cm
799.47 ± 80.47 nm (c/ Metronidazole)
–
SEM, encapsulation efficiency, in vitro drug release profile
(Ho et al., 2017)
PDLLA/ Amoxicillin
Electrospinning
0.15 mL/h; 18 kV; 6 cm
737 ± 128 nm (control) 775 ± 174 nm (w/ Amoxicillin)
–
SEM, encapsulation efficiency, in vitro drug release profile
(Bottino et al., 2019)
PDO/ Metronidazole/ Ciprofloxacin/ Minocycline
Electrospinning
2 mL/h;;15–19 kV; 18 cm
1026.1 nm (control) 898.5 nm (w/ antibiotics)
–
SEM, ATR-FTIR, mechanical properties
(Ashbaugh et al., 2016)
PCL/PLGA/ Vancomycin/ Rifampicin/ Linezolid/ Daptomycin
Co-electrospinning
0.5 mL/h
6–7 kV
10 cm–
–
In vitro drug release profile
(Eren Boncu et al., 2020)
PCL/PLGA/ Linezolid
Electrospinning
2 mL/h
14 kV
14 cm1412.2 ± 44.4 (w/ Linezolid in vivo)
10.78 %
Viscosity, DSC, SEM, mechanical properties, contact angle, porosity, encapsulation efficiency, in vitro drug release profile, sterilization, stability
(Miller et al., 2019)
PCL/PLGA/ Linezolid/ Rifampicin
Co-electrospinning
25 mL/h (PLGA)
1.5 mL/h (PCL)
6–7 kV
10 cm–
–
SEM, in vitro drug release profile
(Xue et al., 2015)
PCL/ Gelatin/ Metronidazole
Electrospinning
1 mL/h
8–12 kV
20 cm540 ± 0.33 (w/ Metronidazole in vivo) 640 ± 0.32 (w/ Metronidazole in vivo)
60 – 80 %
SEM, DSC, XRD, FTIR, contact angle, in vitro drug release profile
(Xue et al., 2014a)
PCL/ Gelatin/ Metronidazole
Electrospinning
1 mL/h
8–12 kV
20 cm970 ± 0.20 (w/ Metronidazole)
60 – 80 %
SEM, FTIR, DSC, XRD, mechanical properties, contact angle, encapsulation efficiency, in vitro drug release profile
(Jang et al., 2015)
PCL/ PEO/ Vancomycin
Electrospinning
0.25 mL/h (PCL)
1 mL/h (PEO)
10 kV (PCL)
13 kV (PEO)
10 cm1720 ± 0.18 (PCL) 299 ± 48 (PEO/Vancomycin)
–
Optical microscopy, SEM, in vitro drug release profile
(Song et al., 2017)
PCL/ PVA/ Doxycycline
Coaxial electrospinning
0.5 mL/h
18 kV
10 cm–
–
Raman spectroscopy, adhesion to titanium pin, SEM
(Sutrisno et al., 2018)
PCL/ CHIT/ Tannic acid/ Gentamicin
Electrospinning
1 mL/h
15 kV
10 cm88.82 ± 28.57 (control) 95.29 ± 33.30 (w/ Tannic acid + Gentamicin)
–
SEM, AFM, contact angle, substrate adsorption, in vitro release profile, degradation study
The seventeen articles analyzed in this review developed nanostructured membranes consisting of fibers from one or more synthetic polymers. The most used polymers were PLGA (Ashbaugh et al., 2016; Eren Boncu et al., 2020; Gao et al., 2016; Gilchrist et al., 2013; Miller et al., 2019; Yu et al., 2020; Zhang et al., 2014) and PCL (Ashbaugh et al., 2016; Eren Boncu et al., 2020; Jang et al., 2015; Miller et al., 2019; Song et al., 2017; Sutrisno et al., 2018; Xue et al., 2015, 2014a, 2014b). Also, some studies combined PLGA and PCL with poly-(D-l-lactic acid) (PDLLA), polydioxanone (PDO), poly-(ethylene oxide) (PEO), poly-(vinyl alcohol) (PVA), and natural polymers, such as chitosan (CHIT), and gelatin (Table 3).
The main parameters of the electrospinning equipment are flow rate (mL/h), electric tension (kV), and the distance between the needle and collector (cm). These parameters differed widely among the 16 analyzed studies (Table 3): the flow rate varied from 0.3 to 3 mL/h, the electric tension from 6 kV to 30 kV, and the distance between the needle and collector from 10 cm to 18 cm.
The macroscopic features of the scaffolds were reported in 8 articles, whereas just one article discussed mechanical characteristics (Table 3) (Eren Boncu et al., 2020; Gao et al., 2016; Gilchrist et al., 2013; Sutrisno et al., 2018; Xue et al., 2015, 2014a, 2014b).
Fiber structure, morphology, and mean diameters were characterized by scanning electronic microscopy (SEM), Raman spectroscopy, Fourier-transform infrared spectroscopy (FTIR), and X-ray diffraction (XRD). Twelve papers determined fiber’s mean diameters by SEM, varying from 480 nm to 1040 nm. According to six papers (Bottino et al., 2019; Gilchrist et al., 2013; Sutrisno et al., 2018; Xue et al., 2014b; Yu et al., 2020; Zhang et al., 2014), fiber’s mean diameters increased after introducing antibiotics to the polymeric matrix, whereas one work found the opposite behavior (Ho et al., 2017). 6 works out of 16 used XRD, FTIR, and Raman spectroscopy to obtain information from sample composition and crystallinity (Bottino et al., 2019; Gilchrist et al., 2013; Song et al., 2017; Xue et al., 2015, 2014a, 2014b) (Table 3).
Many antibiotics, such as rifampicin, vancomycin, ceftazidime, gentamicin, metronidazole, amoxicillin, ciprofloxacin, minocycline, rifampicin, linezolid, and doxycycline were associated with the nanofibers. Not all articles evaluated and determined the percentage of the incorporated drug in the polymer matrix. Drug release tests were executed in 14 articles (Ashbaugh et al., 2016; Eren Boncu et al., 2020; Gilchrist et al., 2013; Ho et al., 2017; Jang et al., 2015; Miller et al., 2019; Sutrisno et al., 2018; Xue et al., 2015, 2014a,2014b; Yu et al., 2020; Zhang et al., 2014) (Table 4). Legend: PLGA = Poly(lactic)–co-glycolic acid; PCL = polycaprolactone; PDLLA = poly D-L(lactic acid); PDO = polydioxanone; PEO = polyethylene oxide; PVA = poly (vinyl alcohol); CHIT = chitosan; MIC = minimum inhibitory concentration.
Reference
Biomaterial
Antibiotic
Adsorption capacity
Release profile
Mean conclusion
(Gilchrist et al., 2013)
PLGA
Rifampicin
92 % − 100 %
Burst release over 1 – 2 days followed by slow and controlled release for 35 days
Only 40 – 60 % of the drug was released possibly due to degradation. Burst release may have been caused by interaction with hydrophilic structures
(Zhang et al., 2014)
PLGA
Vancomycin
–
Burst release of 50.3 % on day 1; controlled release of 82.7 % after 28 days
Biphasic pattern of drug release
(Yu et al., 2020)
PLGA
Vancomycin
Ceftazidime–
Peaks at day 1 and 6 followed by sustained release for Vancomycin and Ceftazidime until day 30 (86.4 % and 83.9 % respectively)
Effective sustained release to prevent or control infections
(Gao et al., 2016)
PLGA
Vancomycin
–
Burst effect on days 1 – 2 with slow release cumulative of 96 % for 30 days
Concentration higher than 90 % of MIC during all the experiment
(Xue et al., 2014b)
PCL
Metronidazole
81.7 – 92.3 %
90 % released on the first 7 days followed by linear release until day 14
Drug releases through diffusion. Higher drug concentrations led to higher burst effects
(Ho et al., 2020)
PDLLA
Amoxicillin
81.16 % ± 10.51 %
60 % released on the first 7 days followed by sustained release for 28 days (∼95 %)
Biphasic pattern caused by polymer interaction with burst effect caused by drug diffusion
(Ho et al., 2017)
PDLLA
Metronidazole
82.19 % ± 15.13 %
Sustained release for 28 days with little to no burst effect on day 1 and more than 80 % released
Controlled drug release that promotes antibacterial activity and chemotaxis
(Bottino et al., 2019)
PDO
Metronidazole
Ciprofloxacin
Minocycline–
–
–
(Ashbaugh et al., 2016)
PCL
PLGAVancomycin
Rifampicin
Linezolid
Daptomycin–
Vancomycin released above MIC for 3 – 5 days; Linezolid released above MIC for 14 days; Daptomycin released above MIC for 5 – 10 days; Rifampicin released below MIC when combined with all three antibiotics
Rifampicin was released faster than all three antibiotics no matter the combination, possibly due to interactions or to PCL ability in releasing the drug faster
(Eren Boncu et al., 2020)
PCL
PLGALinezolid
56 % − 92 %
Burst effect followed by sustained release for 20 days
Biphasic pattern and drug release decreased by concentrating polymer solution. 10 % PLGA allowed the most controllable drug release
(Miller et al., 2019)
PCL
PLGALinezolid
Rifampicin–
Linezolid presented burst effect followed by sustained release, whereas Rifampicin showed lesser burst effect with sustained release for 18 days
Drug release above MIC for 8 – 10 days. Presence of PLGA promotes slower release rates
(Xue et al., 2015)
PCL
GelatinMetronidazole
84.6 % − 89.6 %
Burst effect of 60 – 70 % of drug release up to day 2 followed by a sustained linear rate up to day 7
Burst effect important to eliminate local bacteria. Local concentration above MIC and low systemic distribution
(Xue et al., 2014a)
PCL
GelatinMetronidazole
84 % − 93 %
Burst effect of 60 % of drug release up to day 3 with sustained linear rate until 3 weeks
Drug release through diffusion, Gelatin biodegradation and is maintained slower due to PCL crystallinity
(Jang et al., 2015)
PCL
PEOVancomycin
–
First peak on day 1 followed by sustained release with second peak on day 4 and sustained release up to day 7
Triphasic pattern with controlled profile due to interaction of the sandwiched scaffolds
(Song et al., 2017)
PCL
PVADoxycycline
–
–
–
(Sutrisno et al., 2018)
PCL
CHITGentamicin
–
Burst effect on the first 3 h followed by sustained release for 6 days
Higher drug delivery affected by lower pH (5.8). Nanofiber scaffolds presented more sustained release
Fifteen papers evaluated the antibacterial activity, and most of them tested Metronidazole (Bottino et al., 2019; Ho et al., 2020, 2017, Xue et al., 2015, 2014a, 2014b) and Vancomycin (Gao et al., 2016; Jang et al., 2015; Yu et al., 2020; Zhang et al., 2014) associated with the nanofiber membranes. Staphylococcus strains, especially S. aureus, were the preferred pathogen in microbiological evaluations (Ashbaugh et al., 2016; Eren Boncu et al., 2020; Gao et al., 2016; Gilchrist et al., 2013; Jang et al., 2015; Miller et al., 2019; Song et al., 2017; Sutrisno et al., 2018; Zhang et al., 2014) because this bacteria is the leading cause of bone infections and it is the most antibiotic-resistant strain (Brunotte et al., 2019). Other studies used Escherichia coli, Fusobacterium nucleatum, Streptococcus sanguinis, Porphyromonas gingivalis, and Actinomyces naeslundii (Bottino et al., 2019; Ho et al., 2020; Xue et al., 2015, 2014b).
Eight papers performed in vitro cell viability assays to evaluate the cytotoxicity of the antibiotic-loaded nanofibers (Gilchrist et al., 2013; Ho et al., 2020, 2017; Sutrisno et al., 2018; Xue et al., 2015, 2014a,2014b; Zhang et al., 2014). These works used cell lines such as L929 fibroblasts, human periodontal fibroblasts, rat osteogenesis cells, immortalized periodontal ligament cells, human gingival squamous carcinoma cells, mesenchymal stem cells, MC3T3-E1 osteoblasts and human umbilical vein endothelial cells (Table 5). Legend: PLGA = Poly(lactic)–co-glycolic acid; PCL = polycaprolactone; PDLLA = poly D-L(lactic acid); PDO = polydioxanone; PEO = polyethylene oxide; PVA = poly (vinyl alcohol); CHIT = chitosan; HUVEC = human umbilical vein endothelial cells; MRSA = methicillin-resistant Staphylococcus aureus; hPDLFs = human periodontal ligament fibroblasts; ROS = rat osteogenesis sample; iPDLs = immortalized periodontal ligament cells; CA922 = cells derived from human gingival squamous cell carcinoma; MSCs = mesenchymal stem cells.
Reference
Biomaterial
Antibiotic
Cell culture
Bacteria culture
Mean conclusion
(Gilchrist et al., 2013)
PLGA
Rifampicin
HUVEC
Staphylococcus aureus
Staphylococcus epidermidis
MRSA
MRSA Newman strainCell viability of 83 ± 10 %
Antibacterial activity after 48 h implanted, but with bacteriostatic effect only
(Zhang et al., 2014)
PLGA
Vancomycin
MC3T3-E1 osteoblasts
Staphylococcus aureus
PLGA with and without Vancomycin promoted more cell growth than the bare group on days 3 and 5
(Yu et al., 2020)
PLGA
Vancomycin
Ceftazidime–
Staphylococcus aureus
Escherichia coli
Data not shown
(Gao et al., 2016)
PLGA
Vancomycin
–
MRSA
Inhibition activity for 28 days with a peak on day 1 and slowly decreasing throughout the experiment
(Xue et al., 2014b)
PCL
Metronidazole
L929 fibroblasts
hPDLFs
ROSFusobacterium nucleatum
No cytotoxicity to the fibroblasts up to 40 % Metronidazole (w/w). Over 100 % viability of hPDLFs and ROS
Antibacterial activity occurs with greater than 5 % Metronidazole (w/w)
(Ho et al., 2020)
PDLLA
Amoxicillin
iPDLs
CA922
Streptococcus sanguinis
Porphyromonas gingivalis
Higher viability for iPDLs cells than CA922 with adhesion and proliferation onto the scaffolds
Greater antibacterial activity
(Ho et al., 2017)
PDLLA
Metronidazole
MSCs
–
PDLLA alone showed higher viability than PDLLA/Metronidazole. Homogeneous distribution of cells
(Bottino et al., 2019)
PDO
Metronidazole, ciprofloxacin and minocycline
–
Actinomyces naeslundii
Bacterial reduction of 99.1 – 99,94 %
(Ashbaugh et al., 2016)
PCL and PLGA
Vancomycin
Rifampicin
Linezolid
Daptomycin–
Staphylococcus aureus Xen36
Combination of antibiotics promotes greater activity also against biofilm formation
(Eren Boncu et al., 2020)
PCL and PLGA
Linezolid
–
MRSA
Longer activity as drug content and polymer concentration increases
(Miller et al., 2019)
PCL and PLGA
Linezolid and Rifampicin
–
Staphylococcus aureus SAP321
Bacterial inhibition for 10 days
(Xue et al., 2015)
PCL and Gelatin
Metronidazole
L929 fibroblasts
hPDLFs
ROSFusobacterium nucleatum
Increasing Gelatin concentration promotes higher viability, better cell adhesion and proliferation
Bacterial inhibition for 7 days
(Xue et al., 2014a)
PCL and Gelatin
Metronidazole
L929 fibroblasts
hPDLFs
ROSFusobacterium nucleatum
No cytotoxicity for fibroblasts until 40 % Metronidazole. 30 % (w/w) is the ideal for cell proliferation, viability and bactericidal activity
(Jang et al., 2015)
PCL and PEO
Vancomycin
–
MRSA
Antibacterial activity for 14 days and no biofilm formation
(Song et al., 2017)
PCL and PVA
Doxycycline
–
Staphylococcus aureus
Little to no bacterial activity on the Doxycycline-nanofiber sample
(Sutrisno et al., 2018)
PCL and CHIT
Gentamicin
Osteoblasts
Staphylococcus aureus
Escherichia coli
greater than 90 % antibacterial activity for 72 h. Polymer + antibiotic layer by layer technique promotes better viability
In vivo studies using different animal models were performed to evaluate the preventive antimicrobial activity and biocompatibility of nanofiber scaffolds implanted in the bone defect or subcutaneously (Ho et al., 2020, 2017, Xue et al., 2014a,2014b; Yu et al., 2020) to analyze tissue biocompatibility, biodegradability, osteogenesis, inflammatory processes, and prevention of infections after surgical procedures (Table 6). Also, in vivo studies were performed to combat and treat bone infections (Ashbaugh et al., 2016; Eren Boncu et al., 2020; Gao et al., 2016; Jang et al., 2015; Miller et al., 2019; Zhang et al., 2014). In the studies, metallic implants were recovered with the nanofiber, and these nanofibers were inserted in the fractured bone tissue, followed by an injection of microorganisms in the damaged area (Table 6). Legend: PLGA = Poly(lactic)–co-glycolic acid; MRSA = Methicillin-resistant Staphylococcus aureus; PCL = polycaprolactone; BMP2 = bone morphogenetic protein 2; PDLLA = poly D-L(lactic acid); PDGF = platelet-derived growth factor; PDO = polydioxanone; PEO = polyethylene oxide; PVA = poly (vinyl alcohol); CHIT = chitosan.
Reference
Nanofiber sample
Animal model
Experimental period
Mean conclusions
(Gilchrist et al., 2013)
PLGA + 10 % (w/w) Fusidic acid/Fusidic acid sodium salt + 5 % (w/w) Rifampicin
13 Sprague-Dawley rats
7 days
99.9 % less MRSA attached to the nanofiber implant, promotion of neovascularization and less inflammatory response
(Zhang et al., 2014)
PLGA + Vancomycin 10 % (w/w)
36 New Zealand rabbits
28 days
New bone formation in the Vancomycin group with osteolysis, necrosis and tissue swelling in bare PLGA group
(Yu et al., 2020)
PCL + PLGA + Vancomycin/ Ceftazidime
PCL + PLGA + Vancomycin/ Ceftazidime + BMP215 New Zealand rabbits
42 days
Presence of small blood vessels with higher bone callus formation and bone strength for the polymers/antibiotics/BMP2 group
(Gao et al., 2016)
PLGA + Vancomycin + deproteinized bone
36 New Zealand rabbits
8 weeks
Vancomycin + deproteinized bone promoted more effective osteogenesis with no significant inflammation and no infection
(Xue et al., 2014b)
PCL + Metronidazole 30 % (w/w)
40 New Zealand rabbits
8 months
No signs of inflammation, necrosis or adverse effects. Fibroblasts adhesion, neovascularization and tissue healing was seen
(Ho et al., 2020)
PDLLA + Amoxicillin 20 % (w/w)
27 Sprague-Dawley rats
7 days
Reduced inflammation and swelling on Amoxicillin group with presence of small blood vessels and collagen matrix
(Ho et al., 2017)
PDLLA + Metronidazole 3 %
12 C57BL/6 mice
15 Sprague-Dawley rats14 days
No visible swelling, no abscess formation. Production of extracellular matrix. Better bone healing when combined with PDGF
(Bottino et al., 2019)
PDO + Metronidazole + ciprofloxacin + Minocycline
1 Beagle dog
3 months
Reduction of bacterial burden, less inflammatory response and presence of osteoblast and odontoblast-like cells
(Ashbaugh et al., 2016)
PCL + PLGA + Vancomycin + Rifampicin + Linezolid + Daptomycin
10 C57BL/6 mice
14 days
No systemic distribution of antibiotics, no biofilm formation. Combination of antibiotics promote better bone healing
(Eren Boncu et al., 2020)
PCL + PLGA + Linezolid 20 % (w/w)
64 Wistar albine rats
28 days
More effective than intraperitoneally Linezolid and had the lowest MRSA count (or not observed at all). The nanofiber may have induced faster bone healing
(Miller et al., 2019)
PCL + PLGA + Linezolid + Rifampicin
6 Dutch Belted rabbits per group
7 days
Knee-joint with signs of infection in the non-antibiotic group. No presence of MRSA on antibiotic nanofiber
(Xue et al., 2015)
PCL + Gelatin + Metronidazole 30 % (w/w)
New Zealand rabbits
8 months
No inflammation or adverse effects. From 12 weeks on, presence of fibroblasts and blood vessels
(Xue et al., 2014a)
PCL + Gelatin + Metronidazole 1 – 40 % (w/w)
40 New Zealand rabbits
8 months
Reduction of inflammatory response with fibroblast adhesion and good wound healing. 30 % (w/w) is considered the ideal concentration
(Jang et al., 2015)
PCL + Vancomycin + PEO
10 guinea pigs
7 days
Mild inflammation and recovery of hearing with no infection
(Song et al., 2017)
PCL + PVA + Doxycycline
Sprague-Dawley rats
16 weeks
Induction of osteoblasts adhesion and proliferation with new bone formation
(Sutrisno et al., 2018)
PCL + CHIT + Gentamicin
Sprague-Dawley rats
8 weeks
Greater structural stability for long enough to promote cell adhesion and bone repair
The selected papers in this revision conducted in vivo studies focusing on the effect of antibiotics-doped nanofibers in i) the prevention of bone infections (Ho et al., 2020, 2017; Sutrisno et al., 2018; Xue et al., 2015, 2014a,2014b; Yu et al., 2020), ii) the treatment of infections (Ashbaugh et al., 2016; Bottino et al., 2019; Gao et al., 2016; Gilchrist et al., 2013; Jang et al., 2015; Miller et al., 2019; Zhang et al., 2014), iii) both prevention and treatment assays (Eren Boncu et al., 2020; Song et al., 2017) and iv) bone biocompatibility. Samples were implanted in rats, mice, rabbits, dogs, guinea pigs, and different animal models were tested.
Eight clinical trials were found on the ClinicalTrials.gov website, with two completed, one terminated, one withdrawn, three unknowns, and one recruiting. Solely the 3 unknown clinical trials used nanofibers to perform endodontics procedures in the years 2017 and 2018 (NCT03264105, NCT03690960, and NCT03242291). In the study NCT03690960, 30 randomized participants with immature necrotic teeth will either receive a nanofiber implant associated with a triple antibiotic mixture (TAP) made of ciprofloxacin, metronidazole, and minocycline or the conventional treatment using TAP paste. The outcome of this study has not been provided.
The studies NCT03264105 and NCT03242291 used hydroxyapatite-reinforced nanofibers to treat demineralized pits and fissures. Twenty-six participants will randomly receive the conventional resin-based flowable composite or the hydroxyapatite nanofiber flowable composite implant. However, the outcomes have been provided in neither of the studies.
Only eight articles mentioned the identification code of the Ethics Committee (Ashbaugh et al., 2016; Bottino et al., 2019; Ho et al., 2020, 2017; Jang et al., 2015; Sutrisno et al., 2018; Xue et al., 2014a; Yu et al., 2020) and 6 mentioned housing conditions (Bottino et al., 2019; Gao et al., 2016; Gilchrist et al., 2013; Jang et al., 2015; Miller et al., 2019; Sutrisno et al., 2018). Three articles did not mention the number of animals used in the experiments (Gilchrist et al., 2013; Sutrisno et al., 2018; Xue et al., 2015). In preventive studies of bone infections, the antibiotic-doped nanostructured membranes implants were inserted subcutaneously into the animals (Ho et al., 2020, 2017; Sutrisno et al., 2018; Xue et al., 2015, 2014a,2014b; Yu et al., 2020). Nanofibers decreased the inflammatory response after their implantation (Ho et al., 2020, 2017; Jang et al., 2015; Xue et al., 2015, 2014a, 2014b), preventing biofilm formation and minimizing bacterial proliferation (Ashbaugh et al., 2016; Bottino et al., 2019; Eren Boncu et al., 2020; Gilchrist et al., 2013; Miller et al., 2019; Zhang et al., 2014). In addition, blood vessel formation and infiltration of osteoblasts and fibroblasts have been observed, indicating the nanofiber’s ability to induce myogenesis and osteogenesis in most cases (Ashbaugh et al., 2016; Bottino et al., 2019; Eren Boncu et al., 2020; Gao et al., 2016; Ho et al., 2017; Song et al., 2017; Xue et al., 2015, 2014a,2014b; Yu et al., 2020).
Although previously most studies implanted the nanofiber via a subcutaneous route, in the combative studies, only the study of Gilchrist et al. (2013) chose that methodology. Other studies preferred to implant the nanofiber scaffold directly into the bone or around it (Ashbaugh et al., 2016; Bottino et al., 2019; Eren Boncu et al., 2020; Gao et al., 2016; Jang et al., 2015; Miller et al., 2019; Song et al., 2017; Zhang et al., 2014). To cause the infection, hence a combative study, all the researchers injected MRSA at the local site. It was observed, however, that some of the studies (Gilchrist et al., 2013; Jang et al., 2015; Miller et al., 2019) lack a critical step which is tissue biocompatibility, to evaluate the adhesion and penetration of cells and consequently their proliferation to promote complete healing of the damaged tissue.
4 Discussion
4.1 Nanofibers preparation and structural characterization
Two articles use co-electrospinning (Ashbaugh et al., 2016; Miller et al., 2019) and one uses coaxial electrospinning (Song et al., 2017), but the others use regular electrospinning to produce monolithic nanofibers. Most works used the electrospinning technique to produce nanofiber-based membranes because of their low production cost and versatility for applications in different areas (Costa et al., 2012b).
Electrospinning is commonly used in tissue engineering since it allows the construction of a scaffold mimics the extracellular matrix and facilitates cell adhesion and proliferation. Further, the high porosity and surface area of nanofibers allow the incorporation of drugs, proteins, and growth factors and control the release of these substances (Costa et al., 2012b; Keirouz et al., 2020; Ye et al., 2019). Electrospinning parameters involve high voltage applied, polymer ejection flow rate, and distance between the needle tip and the collector, and it can be controllable to produce adequate nanofiber (Li and Xia, 2004).
The most used polymers for the produced nanofiber-based membrane were PLGA and PCL due to their biocompatible and biodegradable characteristics, the capability of keeping the biomolecule stable for a longer time and allowing interactions with hydrophilic and hydrophobic molecules (Mir et al., 2017). Also, the association of polymers with different characteristics was used since it improves drug stability in the nanofibers network (Costa et al., 2012b; Dahlin et al., 2011; Xue et al., 2014a).
In a study by Eren Boncu et al. (2020), the addition of PCL to PLGA fibers initially increased fiber diameter, but polymer concentrations above 25 % decreased in the diameter. The combination of polymers aims to enhance the thermal and mechanical stability of the scaffold (Bhardwaj and Kundu, 2010) and the increase in fiber diameter was due to the increase in PLGA concentration, which leads to a more viscous polymer solution and high viscosity rates increase the diameter.
Humidity and temperature are ambient parameters that also directly affect the production of fibers since increasing humidity leads to the formation of circular pores, and continuously increasing it can create coalescing pores (Bhardwaj and Kundu, 2010). Higher temperatures can form smaller fibers by increasing solvent evaporation and decreasing polymer viscosity (Haider et al., 2018).
Most articles analyzed the influence of the preparation parameters on the nanofiber‘s structural and morphological characteristics. The precise knowledge of structural and morphological parameters such as fibers morphology and size, thermal degradation and heat sensitivity, time-related stability, polymorphisms, and degradation of active compounds will directly influence the control of drug-loading to the polymeric matrix and its release profile to the biological medium.
Despite fifteen articles having used SEM to determine fiber morphology, only thirteen of them conducted a complete characterization of the fiber morphology and diameter (Bottino et al., 2019; Eren Boncu et al., 2020; Gao et al., 2016; Gilchrist et al., 2013; Ho et al., 2020, 2017; Jang et al., 2015; Sutrisno et al., 2018; Xue et al., 2014b, 2015,2014a; Yu et al., 2020; Zhang et al., 2014). None of the works evaluated the fiber diameter distribution, and the articles with the complete characterization studies were Eren Boncu et al. (2020), Gilchrist et al. (2013), Sutrisno et al. (2018), and Xue et al. (2015, 2014a, 2014b).
As mentioned, the complete characterization is necessary to explain, for example, drug behavior within the polymeric matrix, drug release profile, drug degradation, and if there are risks to the patient, and identify the presence of possible contaminants (De Oliveira et al., 2011).
4.2 Antibiotics association and release profile
A total of 7 articles determined the amount of antibiotic encapsulated and executed in vitro drug release (Eren Boncu et al., 2020; Gilchrist et al., 2013; Ho et al., 2020, 2017; Miller et al., 2019; Xue et al., 2015, 2014a,2014b; Zhang et al., 2014).
The association of rifampicin and the sodium salt of the antibiotic fusidic acid in PLGA nanofibers led to rifampicin’s burst release (∼35 %) on the first two days with a sustained and slow release for the following 35 days. It may have happened due to interactions between the two molecules since they are both hydrophilic (Gilchrist et al., 2013).
Zhang et al. (2014) used PLGA loaded with vancomycin, and it was seen that on the first day, the drug was already released by 50.3 %. For the next 28 days, it presents a sustained release of 82.7 %, demonstrating a biphasic drug release pattern.
Yu et al. (2020) co-loaded vancomycin and ceftazidime in PLGA nanofibers recovered by a 3D-printed PCL mesh. Vancomycin had two peaks throughout the 30-day experiment, the first on day 1 (25–30 % released) and the second on day 6. Ceftazidime showed a similar profile but with higher variation in the amount released. Both antibiotics were able to release more than 80 %.
Gao et al. (2016) produced PLGA nanofibers loaded with vancomycin by electrospinning onto the bovine deproteinized cancellous bone and showed that the drug release kept above the minimum inhibitory concentration (MIC) for 30 days. It also presented a burst effect since ∼ 35 % of the drug was released on the first day. By the end of the experiment, 96 % of vancomycin had been released.
Xue et al. (2014b) loaded different concentrations of metronidazole in PCL nanofibers. Around 90 % is released in the first week, showing an intense burst effect followed by a linear and sustained release for the next seven days through a diffusion mechanism. It was also observed that the burst effect is more intense when drug concentration is increased because the drug was dispersed on the surface of the scaffold.
Ho et al. (2020) used PDLLA nanofibers loaded with amoxicillin. The drug showed a biphasic pattern initially with burst release due to the diffusional mechanism followed by a sustained and controlled release over 28 days. It is also mentioned that 60 % of the drug is released during the first week.
Ashbaugh et al. (2016) combined four antibiotics (vancomycin, linezolid, rifampicin, and daptomycin) in PLGA/PCL nanofibers. It was seen that rifampicin was released faster when combined with linezolid and daptomycin, but slower when combined with vancomycin. However, the result was not explained by the authors.
Boncu et al. (2020) concluded that the addition of PCL to PLGA fibers led to an increase in fiber diameter in polymer concentrations up to 25 % and a decrease in fiber diameter for higher concentrations of PCL. These non-linear profile changes when increasing the PCL: PLGA ratio also impacted the antibiotic release profile. Adding PCL in different concentrations accelerated drug release, with higher burst effects due to increased PCL concentration. The nanofiber with 10 % PCL released 85 % of linezolid in the first eight days, and 50 % PCL led to 85 % of release in only two days, but then it kept sustained for 28 days.
Miller et al. (2019) used the combination of PCL and PLGA to release linezolid and rifampicin. It was seen that the system released antibiotics for 18 days, concluding that PLGA can keep antibiotic release slower, making it possible to alter polymeric concentrations. This study also observed that both antibiotics were above the minimum inhibitory concentration for ten days, with a decrease after.
Xue et al. (2015) incorporated metronidazole at a concentration of 30 % (w/w) in PCL/Gelatin nanofibers. Within two days, 60–70 % of the drug is released, showing a prominent burst effect followed by a linear release over the seven days of the experiment. Another article from the same group (Xue et al., 2014a) also incorporated metronidazole in PCL/Gelatin nanofibers, varying drug concentration from 1 to 40 %. It was seen that the drug released 60 % of its content in 3 days, which characterized a burst effect as well and followed by a linear release until three weeks. The burst effect may have been caused because of the presence of the drug on the surface of the nanofibers, diffusing faster into the medium.
Jang et al. (2015) combined two outside layers of PCL and one inside layer of PEO and vancomycin, making a “sandwich” nanofiber. The sandwiched effect aims to enhance the release characteristics of the drug, making it possible to control better and prolong it. Polymers mixture with different polarities had vancomycin presenting a prolonged triphasic release profile as it showed a burst effect on the first day. It sustained until day 4, and on day 5 it reached its peak, maintaining another sustained release up to day 7.
Song et al. (2017) used PCL and PVA to make core–shell nanofibers, with PVA as the fiber core containing the antibiotic doxycycline and PCL as the external fiber coating. The slow degradation rate of PCL protected the fiber core containing the antibiotic encapsulated by PVA. The polymer mixture eliminated the burst effects providing a slow and controlled drug release.
Sutrisno et al. (2018) used PCL and CH mixture to produce nanofibers on a titanium foil soaked in dopamine solution. This entire structure was soaked in several layers of a solution containing tannic acid and the antibiotic gentamicin. The release profile showed that gentamicin releases in a higher amount when the pH is lower than 7.4 and was seen as a burst effect in the first 3 h of the experiment. It was also shown that the nanofibers with dopamine solution highly favor drug delivery.
From these studies, it is possible to conclude that the nanofiber‘s porous structure allows the incorporation of different drugs and molecules. Drug release must occur in a slow and controlled manner for periods adjusted to the clinical application, enhancing efficacy and reducing the risk of toxicity and adverse effects (Zupančič, 2019). Such a statement was fulfilled since most of the scaffolds were able to release in a controlled manner for days and even weeks. The combination of polymers demonstrated the ability to overcome issues such as the burst effect and to provide controllable drug delivery. The choice of polymers appears to have dictated how the drug leaves the matrix.
4.3 Antibacterial and cytotoxicity in vitro studies
Among the 16 studies, the most chosen antibiotics encapsulated in polymeric nanofibers were metronidazole and vancomycin (Table 5). Metronidazole is a nitroimidazole whose mechanism of action involves oxidative damage to the DNA of pathogenic microorganisms like protozoans and bacteria. Metronidazole has antibacterial activity against anaerobic gram-negative bacilli, sporulated gram-positive bacilli, and all anaerobic cocci that cause bone infections. This active compound is widely used to treat teeth and gingival infections caused by anaerobic bacteria (Ho et al., 2017; Hsu et al., 2014).
Vancomycin is the antibiotic of choice for the treatment of bone infections, being efficient against the main causes of this pathology, such as Staphylococcus aureus, Staphylococci sp., and Pseudomonas. The mechanism of action of vancomycin is related to the inhibition of cell wall synthesis during bacterial multiplication, resulting in osmotic lysis (Rubinstein and Keynan, 2014).
In the study of Xue et al. (2014b), PCL membranes loaded with metronidazole were used for an antibacterial in vitro study. F. nucleatum inhibition is dose-dependent using drug concentrations up to 5 % (w/w). It was observed that inhibition of bacterial growth at an area size higher (∼20 mm) than the size of the nanofiber sample (10 mm2). The same group developed hybrid PCL/Gelatin nanofibers to encapsulate metronidazole to obtain a guided bone regeneration scaffold with antimicrobial activity (Xue et al., 2015). The in vivo biodegradation from the PCL/Gelatin nanofiber was improved, promoting cell adhesion, and the nanofibers were able to reduce inflammation and produce new blood vessels. However, the in vitro antimicrobial study showed that the activity against F. nucleatum lasted seven days.
Another study from the same group also encapsulated metronidazole in PCL/Gelatin nanofibers in different concentrations. It was seen that F. nucleatum inhibition occurs from 5 % of antibiotics to 40 % (w/w) (Xue et al., 2014a).
The association of Metronidazole, Ciprofloxacin, and Minocycline already exists in the clinics as a paste for treating dental pulp infections. However, it is followed by cytotoxicity and risk of reinfection. Bottino et al. (2019) study showed that the inhibition of A. naeslundii biofilm reached 99.94 % when using the nanofiber loaded with the three antibiotics, with the same efficacy as conventional pastes and lesser toxic effects on the cells during the seven days of the experiment.
Zhang et al. (2014) encapsulated vancomycin in PLGA nanofibers aiming to develop an antimicrobial membrane to recover metallic implants used in bone repairs. Antimicrobial in vitro evaluation against S. aureus showed that light inhibition zones were formed in all bacterial plates from the antibiotic group. Besides, the inhibition zone in the vancomycin group was at its maximum on the first day (average diameters of 12.7 ± 0.4 mm). The diameters of the inhibition zone remained around 8 mm during the 28 days of the experiment.
Yu et al. (2020) developed a PCL mesh by 3D printing to recover two layers of nanofiber: the first was made of PLGA monolithic nanofibers containing encapsulated vancomycin and ceftazidime for antimicrobial activity, and the second was made of co-axial PLGA (shell) fibers with BMP2 (core) for bone lesion repair. The association of vancomycin and ceftazidime increased the spectrum of action against both Gram-positive and negative, respectively (Calero Castro et al., 2019; Yu et al., 2020). Antimicrobial activity against S. aureus and E. coli lasted 30 and 14 days for vancomycin and ceftazidime, and the activity rates varied from 25 % to 100 % (Calero Castro et al., 2019; Yu et al., 2020), respectively.
Xue et al. (2015, 2014a) combined PCL and Gelatin and pointed out the disadvantages of PCL, such as its slow biodegradation and low cell adhesion and proliferation. Both of them can be minimized by using Gelatin, which has, in turn, bond domains that facilitate cell adhesion and proliferation. PCL/Gelatin combination to produce nanofibers was positive because it promoted the cell interactions desired for biocompatible implants.
Sutrisno et al. (2018) produced nanofibers using the combination of CHIT and PCL because the molecular characteristics of CHIT allow more interaction with hydrophilic substances and cell substrates, as PCL enhances the mechanical properties of the scaffold. The combination of polymers originated a robust film with a hydrophilic surface capable of providing cell interactions (Sutrisno et al., 2018).
Gao et al. (2016) encapsulated vancomycin in electrospinning PLGA nanofibers over a deproteinized cancellous bone to develop an implantable scaffold to treat MRSA infection and for bone regeneration. In vitro, results showed that the biodegradable scaffolds released vancomycin for 30 days in concentrations superior to the minimum inhibitory concentration (MIC) for MRSA. In vitro antibacterial studies confirmed that antibacterial efficacy lasted approximately-four weeks, and during the entire experiment, antibiotic concentration remained above MIC but below toxic concentrations.
Jang et al. (2015) produced a sandwich-like PCL/PEO/PCL nanofiber with vancomycin encapsulated. The nanofiber had two external layers and one internal layer containing the antibiotic. This type of membrane was developed for both prevention and combat of the formation of MRSA biofilm. It showed in vitro antimicrobial activity and prevented MRSA biofilm for 14 days.
Eight of these studies performed cytotoxicity assays in human cells (Gilchrist et al., 2013; Ho et al., 2020, 2017; Sutrisno et al., 2018; Xue et al., 2015, 2014a,2014b; Zhang et al., 2014). Gilchrist et al. (2013) encapsulated rifampicin and fusidic acid in PLGA nanofibers to prevent and combat infections after orthopedical invasive surgeries. They evaluated in vitro cytotoxicity by using human umbilical vein endothelial cells. Cell viability was at 83 %, and the authors concluded that the nanofiber-based membrane was safe for the cells, making possible the performance of in vivo studies of antimicrobial activity and biocompatibility.
Zhang et al. (2014) produced vancomycin encapsulated in PLGA nanofibers to recover metallic titanium implants aiming for preventive action against post-surgery infections and bone reconstruction. The authors concluded that the recovery of the implant was not cytotoxic for the MC3T3-E1 osteoblastic cell line, which also enabled the execution of in vivo studies with an excellent antimicrobial activity using an antibiotic dose of approximately 528.2 μg.
In the study of Xue et al. (2014b), the authors encapsulated metronidazole in PCL nanofibers to develop an implantable membrane to prevent and combat bone infections. The nanofibers concentrated with 0 %, 5 %, 10 %, 20 %, 30 %, and 40 % (w/w) metronidazole were analyzed to see cytotoxicity in different cell lines, such as L929 fibroblasts, human periodontal fibroblasts (hPDLFs) and rat osteogenesis cells (ROS). The study concluded that the nanofibers did not significantly alter cell viability in any of the lineages studied.
Metronidazole was encapsulated in PCL/Gelatin nanofibers by Xue et al. (2015) aiming for guided bone tissue regeneration. The authors evaluated the 40 % (w/w) metronidazole nanofiber cytotoxicity by using L929 fibroblasts, hPDLFs, and ROS. It was concluded that the nanofibers were not only not cytotoxic but able to promote cell expansion exponentially in seven days.
In another study by Xue et al. (2014a), metronidazole was also encapsulated in PCL/Gelatin nanofibers in concentrations of 1 %, 5 %, 10 %, 20 %, 30 %, and 40 % (w/w), and a cytotoxicity assay was performed on days 3 and 7 using the same cell lines as mentioned above. For the hPDLFs, and ROS cells, there was no sign of cytotoxicity, but for L929 fibroblasts, there was no cytotoxicity except for the concentration of 40 % Metronidazole.
Ho et al. (2020) encapsulated amoxicillin in PDLLA nanofibers to prevent bone infections by stimulating guided tissue regeneration. In vitro cytotoxicity studies in immortalized periodontal ligament cells (iPDLs) showed that the nanofiber membranes with 20 % (w/w) of antibiotics are not cytotoxic and allow in vivo studies in animals.
Ho et al. (2017) developed a multifunctional implantable membrane loaded with metronidazole for antimicrobial activity and platelet-derived growth factor (PDGF) to promote bone tissue regeneration. The membrane was built from PDLLA nanofibers with 3 % metronidazole (w/w), and cytotoxicity was evaluated through metabolic activity and cultivated mesenchymal stem cells viability. Metabolic activity and the number of cells increased between the first and fourth day of the study, showing that the nanofiber is not cytotoxic.
The drug-loaded nanofibers displayed great antibacterial activity since the drug entrapped inside the polymeric matrix was able to be delivered slowly, which made it possible to inhibit bacterial growth for weeks. Despite differences regarding the composition of nanofibers and cell lines, the results from the cytotoxicity essays demonstrated that nanofibers combined with antibiotics did not alter cell viability and were able to promote cell adhesion and proliferation on the membrane surface, favoring guided tissue regeneration. The success of in vitro cell cytotoxicity shows that the nanofibers do not change viability compared to the control groups. That allows the execution of in vivo biocompatibility and antimicrobial activity studies to prevent bacterial growth and the formation of an infectious biofilm.
4.4 In vivo studies: preventive antimicrobial activity and biocompatibility
In the in vivo studies of preventive antimicrobial activity, the nanofiber scaffolds were implanted in the bone defect or subcutaneously (Ho et al., 2020, 2017, Xue et al., 2014a,2014b; Yu et al., 2020) to analyze tissue biocompatibility, biodegradability, osteogenesis, inflammatory processes and prevention of infections after surgical procedures.
Yu et al. (2020) implanted the nanofiber containing vancomycin, ceftazidime, and BMP2 in femur injuries in rabbits. The groups of animals were divided into three: 1) 3D PCL mesh; 2) 3D PCL mesh + PLGA monolithic nanofiber with vancomycin / ceftazidime; 3) 3D PCL mesh + PLGA monolithic nanofiber with vancomycin / ceftazidime + PLGA/BMP2 co-axial nanofiber. After eight weeks, the implant had not suffered biodegradation, along with the presence of regenerated bone tissue. The best osteogenesis results came from the PCL-PLGA/antibiotics/BMP2 group. The groups that received both PLGA/antibiotics and PLGA/antibiotics/BMP2 had a better torsional strength than a healthy femur.
Xue et al. (2014b) developed PCL nanofibers with metronidazole to prevent infections and promote guided bone regeneration. The researchers implanted the nanofiber subcutaneously in rabbits. After a week, the antibiotic implant promoted a softer inflammatory response due to inhibition of bacterial growth compared to the implant without antibiotics. After 5 and 7 weeks, it was possible to observe a thin fibrous capsule with fibroblast adhesion in the nanofibers with antibiotics, indicating that the implant with the drug was biocompatible.
PDLLA nanofibers loaded with amoxicillin were developed to prevent periodontal infections that impair guided bone regeneration (Ho et al., 2020). During the prevention and in vivo biocompatibility studies in rats, the implants were inserted in the periodontal pouch. After four days, inflammation was milder in the antibiotic group in comparison with the non-antibiotic group. After seven days, the group with an antibiotic showed a better integration between the nanofiber and the gingival tissue, a more organized collagen matrix, and the presence of small blood vessels.
Ho et al. (2017) also developed a PDLLA nanofiber to encapsulate metronidazole or platelet-derived growth factor (PDGF). The nanofiber was implanted in mice subcutaneous tissue and exhibited excellent in vivo biocompatibility. Bone cells adhered to the membranes and produced an extracellular matrix. The nanofiber loaded with the combination of metronidazole and PDGF was the most biocompatible implant, capable of promoting cell proliferation. Pre-clinical studies showed wound dehiscence was lower when metronidazole and PDGF were combined in the nanofiber, and bone volume fraction was higher in the PDGF, Metronidazole -PDGF, and metronidazole groups, respectively (Ho et al., 2017).
Xue et al. (2014a) implanted PCL/Gelatin nanofibers loaded with metronidazole subcutaneously on rabbits' backs to evaluate biocompatibility and prevention of infections. Within one week, there were no neutrophils surrounding the nanofibers with metronidazole in concentrations of 5 %, 10 %, and 30 %. On the 16th week, nanofibers with metronidazole 5 % and 10 % showed blood vessels and a thin fibrous tissue capsule. After six months, all membranes had been absorbed, and no animal showed complications, inflammatory responses, or necrosis, demonstrating that the nanofibers were biocompatible (Xue et al., 2014a).
Sutrisno et al. (2018) developed CHIT/PCL nanofibers and polyelectrolyte multilayers made of tannic acid and gentamicin sulfate to deliver an antioxidant and antibiotic locally onto the surface of a metallic implant. In vitro cytotoxicity was evaluated using rat osteoblasts for 4 and 7 days. The highest viability cells and osteoblastic differentiation rates were in the nanofibers with multiple layers loaded with antibiotics, tannic acid, and polydopamine.
Even though different animal models were evaluated, these in vivo studies demonstrated that the drug-loaded nanofibers could induce tissue regeneration due to their resemblance to the extracellular matrix. Biocompatible polymers were chosen, and along with antibiotics, there was no sign of intense inflammation response or bone compromise.
4.5 In vivo studies: combative action in the treatment of bone infections
In vivo studies using different animal models were performed to combat and treat bone infections (Ashbaugh et al., 2016; Eren Boncu et al., 2020; Gao et al., 2016; Jang et al., 2015; Miller et al., 2019; Zhang et al., 2014). In the studies, metallic implants were recovered with the nanofiber, and these nanofibers were inserted in the fractured bone tissue, followed by an injection of microorganisms in the damaged area. Also, four articles performed combative in vivo studies in parallel with biocompatibility and biodegradation studies (Ashbaugh et al., 2016; Eren Boncu et al., 2020; Gao et al., 2016; Zhang et al., 2014).
Zhang et al. (2014) studied the effect of Titanium metallic implants coated with vancomycin encapsulated in PLGA nanofibers in rabbits’ tibia defects with local injection of S. aureus. There was no mortality in the group of animals that received the nanofiber loaded with vancomycin. The leukocyte counting of this group, after 28 days, was at a similar level to the pre-operation counting. The group with the antibiotic inhibited pus formation, bacterial activity, and tissue necrosis. It observed bone cell migration and new bone tissue.
Vancomycin was also encapsulated in PLGA nanofibers to form a membrane over a deproteinized bone (Gao et al., 2016). The scaffold aimed to prevent and combat bone infection and promote guided bone regeneration. The authors implanted the nanofiber inside the rabbit’s radius along with MRSA injection. After eight weeks, the implant suffered biodegradation, and the group of animals that received the deproteinized bone covered with the antibiotic nanofiber showed cell migration and osteogenesis.
Ashbaugh et al. (2016) covered a metallic implant with a nanofiber. The PLGA nanofibers were incorporated into a PCL film with combinations of different antibiotics (vancomycin, linezolid, daptomycin, and rifampicin) to combat infections and regenerate the bone. The authors implanted the nanofibers in mice femurs and injected the S. aureus Xen36 bioluminescent strain. In the animals with the implants covered with antibiotic nanofibers, biofilm formation was not seen after fourteen days. Also, in the antibiotic-nanofiber group, femur density and osteointegration capacity were higher.
Linezolid was encapsulated in PLGA and PLGA/PCL nanofibers for the prophylaxis and treatment of MRSA-induced prosthetic infections (Eren Boncu et al., 2020). The linezolid nanofiber was inserted in the rat’s tibia after its fracture, followed by an MRSA injection. The membranes showed antimicrobial and regenerative activity for 28 days. On the 28th day, MRSA was detected in the group that did not receive the antibiotic nanofiber. Apart from that, the study concluded that the dose of antibiotic administered was 37 times lower in the treatment with antibiotic nanofiber compared to the conventional injectable treatment, which makes it possible to avoid antibiotic resistance (Eren Boncu et al., 2020).
Miller et al. (2019) used co-electrospinning to produce PLGA/PCL nanofibers to recover metallic implants and used rifampicin and linezolid to prevent and treat orthopedic bone infections. The recovered implant was inserted in the rabbits’ femurs with a late S. aureus injection. After seven days, the group which did not receive antibiotic nanofiber exhibited swollen knees and the presence of pus in the tissues, both signs of inflammation and infection. However, the group that received the antibiotic nanofiber had normal knees with no indication of infection or bacteria in the surrounding tissues.
Jang et al. (2015) encapsulated vancomycin in PCL/PEO nanofibers to prevent and treat periprosthetic infection and biofilm formation. The researchers implanted the nanofiber in guinea pigs' tympanic bullae with MRSA injection at the site and analyzed it for seven days. The control group with non-antibiotic nanofibers exhibited an intense inflammatory response with the presence of pus, edema, and biofilm. The group with antibiotic nanofibers retrieved hearing and did not show an inflammatory response or biofilm formation, concluding that the antibiotic-loaded nanofibers can prevent MRSA infection and biofilm.
Nanofibers can adhere to the infected area, promoting a healthy environment for the stem cells to differentiate into osteoblasts. It can be seen that these implants provide stronger bone tissue with little to no inflammation and eliminate bacteria and biofilm formation at a much faster rate than a conventional treatment would. Since the action is local, the dose administered can be reduced, which leads to fewer adverse effects and bacterial resistance.
4.6 Clinical trials
The three clinical trials mentioned used nanofiber implants to treat bone defects in the endodontics area. The first one used TAP paste and TAP nanofibers groups. However, the TAP paste presents disadvantages, such as tooth discoloration and meaningful dental stem cell death if used at concentrations above 1 mg/mL (Albuquerque et al., 2017). It is thought that the nanofiber would diminish these outcomes since the nanofibers can promote antibacterial activity along with cell proliferation. The last two trials use hydroxyapatite to reinforce and functionalize the nanofiber. Hydroxyapatite is composed of the bone's mineral phase, which is responsible for its mechanical strength. Inside the scaffolds, this mineral acts as a biocompatible osteoinduction agent, providing the proper means for the local cells to differentiate into osteoblasts, hence leading to the regeneration of the damaged bone (Silva et al., 2021).
From these clinical trials, it is possible to observe that, even though the number of studies increases yearly, it is still far from enough to provide treatment for the patients. One can infer that it is easier to develop nanofibers for endodontic procedures because the implant size is small, so it can be simpler to develop inside a laboratory. Nevertheless, it has been developed to fabricate nanofibers industrially so they can reach many patients.
5 Conclusion
This review article approaches the uses of nanofiber-based membranes loaded with antibiotics to both prevent and treat bone infections, and it emphasizes that polymers like PCL and PLGA are the most widely used materials to produce such nanofibers. The main antibiotics encapsulated within nanofibers are metronidazole, a large spectrum bactericidal antibiotic, and vancomycin, which combats and prevents MRSA infections, the main pathogenic microorganisms that cause bone infections. Electrospinning is the most used method due to its easiness to work with and reproducibility. However, the main challenge is the production on a large scale to obtain the necessary amount to begin human clinical studies. The drug release is usually sustained and prolonged, enhancing the antibacterial activity and decreasing possible harm to human tissue cells. It is possible to conduct both preventive and combative in vivo studies, but the lack of homogeneity and reproducibility makes it challenging to advance into further steps. The establishment of robust and reproducible pre-clinical protocols is crucial, to begin with, for the initial clinical studies in humans. Finally, it is also essential to develop a method in which nanofibers can be produced on a larger scale so the patients can benefit from it.
Funding
This work was supported by the Carlos Chagas Filho Foundation for Research Support of Rio de Janeiro State (FAPERJ) - Cientista do Nosso Estado (CNE) [grant number: E-26/201.077/2021] and Rede Nano Saude [grant number: E-26/010.000981/2019), Rio de Janeiro, Brazil. This work was supported by Coordination for the Improvement of Higher Education Personnel (CAPES) for scholarship.
Ethics approval
Not applicable as no human or animals were involved in this study
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Antimicrobial efficacy of triple antibiotic–eluting polymer nanofibers against multispecies biofilm. J. Endod.. 2017;43:S51-S56.
- [CrossRef] [Google Scholar]
- Polymeric nanofiber coating with tunable combinatorial antibiotic delivery prevents biofilm-associated infection in vivo. Proc. Natl. Acad. Sci. U. S. A.. 2016;113:E6919-E6928.
- [CrossRef] [Google Scholar]
- Electrospinning: a fascinating fiber fabrication technique. Biotechnol. Adv.. 2010;28:325-347.
- [CrossRef] [Google Scholar]
- A review on properties of natural and synthetic based electrospun fibrous materials for bone tissue engineering. Membranes (Basel). 2018;8
- [CrossRef] [Google Scholar]
- A novel patient-specific three-dimensional drug delivery construct for regenerative endodontics. J. Biomed. Mater. Res. - Part B Appl. Biomater.. 2019;107:1576-1586.
- [CrossRef] [Google Scholar]
- A new small animal model for simulating a two-stage-revision procedure in implant-related methicillin-resistant Staphylococcus aureus bone infection. Injury. 2019;50:1921-1928.
- [CrossRef] [Google Scholar]
- Proof of concept, design, and manufacture via 3-D printing of a mesh with bactericidal capacity: behaviour in vitro and in vivo. J. Tissue Eng. Regen. Med.. 2019;13:1955-1964.
- [CrossRef] [Google Scholar]
- Eletrofiação de polímeros em solução. parte I: fundamentação teórica. Polímeros Ciência e Tecnol.. 2012;22:170-177.
- [CrossRef] [Google Scholar]
- Eletrofiação de polímeros em solução. parte II: aplicações e perspectivas. Polímeros Ciência e Tecnol.. 2012;22:178-185.
- [CrossRef] [Google Scholar]
- Polymeric nanofibers in tissue engineering. Tissue Eng. - Part B Rev.. 2011;17:349-364.
- [CrossRef] [Google Scholar]
- Análise térmica aplicada a fármacos e formulações farmacêuticas na indústria farmacêutica. Quim. Nova. 2011;34:1224-1230.
- [CrossRef] [Google Scholar]
- Polymeric scaffolds in tissue engineering application: a review. Int. J. Polym. Sci.. 2011;2011
- [CrossRef] [Google Scholar]
- Nanofiber: synthesis and biomedical applications. Artif. Cells, Nanomed. Biotechnol.. 2016;44:111-121.
- [CrossRef] [Google Scholar]
- In vitro and in vivo evaluation of linezolid loaded electrospun PLGA and PLGA/PCL fiber mats for prophylaxis and treatment of MRSA induced prosthetic infections. Int. J. Pharm.. 2020;573:118758
- [CrossRef] [Google Scholar]
- A biodegradable antibiotic-eluting PLGA nanofiber-loaded deproteinized bone for treatment of infected rabbit bone defects. J. Biomater. Appl.. 2016;31:241-249.
- [CrossRef] [Google Scholar]
- Fusidic acid and rifampicin co-loaded PLGA nanofibers for the prevention of orthopedic implant associated infections. J. Control. Release. 2013;170:64-73.
- [CrossRef] [Google Scholar]
- A comprehensive review summarizing the effect of electrospinning parameters and potential applications of nanofibers in biomedical and biotechnology. Arab. J. Chem. 2018
- [CrossRef] [Google Scholar]
- PDGF-metronidazole-encapsulated nanofibrous functional layers on collagen membrane promote alveolar ridge regeneration. Int. J. Nanomed.. 2017;12:5525-5535.
- [CrossRef] [Google Scholar]
- The treatment response of barrier membrane with amoxicillin-loaded nanofibers in experimental periodontitis. J. Periodontol.. 2020;1–25
- [CrossRef] [Google Scholar]
- Hsu, Y.H., Chen, D.W. e. C., Tai, C. Der, Chou, Y.C., Liu, S.J., Ueng, S.W. e. N., Chan, E.C., 2014. Biodegradable drug-eluting nanofiber-enveloped implants for sustained release of high bactericidal concentrations of vancomycin and ceftazidime: in vitro and in vivo studies. Int. J. Nanomedicine 9, 4347–4355. https://doi.org/10.2147/IJN.S66526
- Antibacterial effect of electrospun polycaprolactone/polyethylene oxide/vancomycin nanofiber mat for prevention of periprosthetic infection and biofilm formation. Int. J. Pediatr. Otorhinolaryngol.. 2015;79:1299-1305.
- [CrossRef] [Google Scholar]
- 2D and 3D electrospinning technologies for the fabrication of nanofibrous scaffolds for skin tissue engineering: a review. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol.. 2020;12:1-32.
- [CrossRef] [Google Scholar]
- Electrospinning of nanofibers: reinventing the wheel? Adv. Mater.. 2004;16:1151-1170.
- [CrossRef] [Google Scholar]
- In vivo bioluminescence imaging in a rabbit model of orthopaedic implant-associated infection to monitor efficacy of an antibiotic-releasing coating. J. Bone Jt. Surg. - Am.. 2019;101:e12.
- [Google Scholar]
- Mir, M., Ahmed, N., Rehman, A. ur, 2017. Recent applications of PLGA based nanostructures in drug delivery. Colloids Surfaces B Biointerfaces 159, 217–231. https://doi.org/10.1016/j.colsurfb.2017.07.038
- Silva, G.L.G. e, Monteiro, M.S. de S. de B., Matos, A.P. dos S., Santos-Oliveira, R., Kenechukwu, F.C., Ricci-Júnior, E., 2021. Nanofibers in the treatment of osteomyelitis and bone regeneration. J. Drug Deliv. Sci. Technol. 67, 102999. https://doi.org/10.1016/j.jddst.2021.102999
- Song, W., Seta, J., Chen, L., Bergum, C., Zhou, Z., Kanneganti, P., Kast, R.E., W., G., 2017. Doxycycline-loaded coaxial nanofiber coating of titanium implants enhances osseointegration and inhibits Staphylococcus aureus infection. Biomed. Mater. 12
- Construction of three-dimensional net-like polyelectrolyte multilayered nanostructures onto titanium substrates for combined antibacterial and antioxidant applications. J. Mater. Chem. B. 2018;6:5290-5302.
- [CrossRef] [Google Scholar]
- Drug loaded homogeneous electrospun PCL/gelatin hybrid nanofiber structures for anti-infective tissue regeneration membranes. Biomaterials. 2014;35:9395-9405.
- [CrossRef] [Google Scholar]
- Preparation and in vivo efficient anti-infection property of GTR/GBR implant made by metronidazole loaded electrospun polycaprolactone nanofiber membrane. Int. J. Pharm.. 2014;475:566-577.
- [CrossRef] [Google Scholar]
- Fabrication of drug-loaded anti-infective guided tissue regeneration membrane with adjustable biodegradation property. Colloids Surf. B Biointerfaces. 2015;135:846-854.
- [CrossRef] [Google Scholar]
- Electrospun nanofibers for tissue engineering with drug loading and release. Pharmaceutics. 2019;11:1-17.
- [CrossRef] [Google Scholar]
- A three-dimensional printed polycaprolactone scaffold combined with co-axially electrospun vancomycin/ceftazidime/bone morphological protein-2 sheath-core nanofibers for the repair of segmental bone defects during the masquelet procedure. Int. J. Nanomed.. 2020;15:913-925.
- [CrossRef] [Google Scholar]
- Electrospun vancomycin-loaded coating on titanium implants for the prevention of implant-associated infections. Int. J. Nanomed.. 2014;9:3027-3036.
- [CrossRef] [Google Scholar]
- Preparation, structure and crystallinity of chitosan nano-fibers by a solid-liquid phase separation technique. Carbohydr. Polym.. 2011;83:1541-1546.
- [CrossRef] [Google Scholar]
- Core-shell nanofibers as drug delivery systems. Acta Pharm.. 2019;69:131-153.
- [CrossRef] [Google Scholar]







