Translate this page into:
Antifungal activity and identification of bioactive peptide from Etawa crossbreed goat (Capra hircus) milk protein hydrolyzed using trypsin enzyme
⁎Corresponding author. trijr_mipa@ugm.ac.id (Tri Joko Raharjo)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
There had been a growing interest in biopreservation in recent years due to the increasing demand for food products that were free of synthetic preservatives and therefore safer to consume. This made it necessary to explore natural bioactive with antifungal properties that can inhibit microbial spoilage and extend shelf life. Etawa crossbreed goat milk (Capra hircus) peptide fraction has antifungal activity. Therefore, this study aimed to determine the peptide with antifungal activity resulting from the casein and whey proteins of goat milk hydrolysis by trypsin. The hydrolyzate was fractionated using a Strong Cationic Exchange (SCX) catridge and the antifungal activity test was performed against Aspergillus sp. using the disc diffusion method. The most active peptide fraction with the highest antifungal activity was identified using Liquid Chromatography-High Resolution Mass Spectrometry (LC-HRMS). Based on the results, the ratio of the enzyme substrates used to hydrolyze casein and whey proteins is 1:10; 1:20; 1:30 and 1:40. The greatest degree of hydrolysis of casein and whey proteins was obtained at a 1:40 enzyme-substrate ratio of 66.8% and 75.4%, respectively. The analysis of Sodium Dodecylsulfate - Polyacrylamide Gel Electrophoresis (SDS PAGE) showed that goat milk casein contained αS1-casein and β-casein, while the whey proteins contained beta-lactoglobulin and α-lactalbumins. The casein and whey protein hydrolysates were fractionated using an SCX with pH variations of 3–9. The Goat Milk Casein (GMC) 5, 6, 7 dan 8 peptide fractions inhibited Aspergillus sp, and the most active was a fraction from GMC6 with an inhibition zone of 26.95 ± 0.63 mm and Minimum Inhibitory Concentration (MIC) value of 31.25 μg/mL. Furthermore, the morphology of Aspergillus sp. mycelia after treatment with peptide fraction showed that the mycelia were wrinkled and withered. The fractions with the highest antifungal activity contained 4 peptide sequences, namely YNVPQLEIVPK, KENNINELSK, GLSPEVPNENLLR, and YLGYLEQLLK. These results showed that peptide from goat milk had potential applications as a natural antifungal compound.
Keywords
Antifungal
Goat milk
Peptides
Trypsin
MIC
Aspergillus sp
1 Introduction
Food security is a global issue encountered by all countries, with 70% of the approximately 1.5 billion diseases being foodborne, according to WHO. This is because some fungal genera can cause food spoilage and poisoning, including Penicillium, Aspergillus, Cladosporium, and Neurospora. Many foods are highly susceptible to spoilage by fungal growth, such as processed foods, fruits, vegetables, dairy products, meat, and bakery product (Adeyeye, 2016). To overcome this issue, the use of synthetic preservatives has been adopted to prolong the shelf-life of the products.
Some synthetic preservatives that inhibit fungal growth, such as benzoate, sorbate, and sulfite, have the potential to harm the environment and human health, while their excessive application can also lead to microbial resistance (Arulrajah et al., 2020). However, there is a growing interest in the use of natural preservatives to prolong the shelf life of products due to their safety and sustainability (Luz et al., 2018; Leyva Salas et al., 2017; Jabeen and Khanum, 2017). Antimicrobial peptide (AMP) has been used as a natural preservative in bakery, meat, and processed food products because it can prevent fungal contamination and preserve food without changing quality (Wang et al., 2016; Upendra et al., 2016; Perez Espitia et al., 2012).
Several studies reported that peptides had antifungal activity, with Kenaf seed peptide (KSPM) delaying fungal growth on tomato pulp by 14 and 23 days at 25 and 4 °C, significantly reducing the number of A. niger and Fusarium sp. (Arulrajah et al., 2021). Palm kernel cake (PKC) peptide successfully inhibited the growth of A. flavus (69.15%), A. niger (88.08%), Fusarium sp. (87.14%), and Penicillium sp. (71.84%), as well as prolonged the shelf life of the product up to 10 days when added at a concentration of 2000 mg/kg (Mohamad Asri et al., 2020). According to (Bondaryk et al., 2017), natural antimicrobial peptides usually show no or little toxicity against human cells.
Goat milk is a potential source of peptides because it is easily digested and absorbed, and low-allergenic (Verma et al., 2020; Kostić et al., 2021; Verruck et al., 2019; Zhu et al., 2022). A recent study showed that whey hydrolyzate from goat milk cheese could inhibit Penicillium sp. and extend the shelf life of bakery products (Luz et al., 2020). Although goat milk protein with hydrolyzed bromelain enzymes could inhibit Escherichia coli, Salmonella thypimurium, and Listeria monocytogenes (Kusumaningtyas et al., 2015), the bioactive peptides that act as antifungals inhibiting Aspergillus sp. are not yet known. There is limited information on the identification of antifungal peptide sequences, particularly on peptides derived from animal products. Therefore, this study aimed to obtain active peptide fractions from goat milk, identify amino acid peptides using LC-HRMS, and determine their antifungal activities against Aspergillus sp.
2 Material and methods
2.1 Materials
The main ingredients used in the preparation of peptides include goat milk from the experimental farm, Jenderal Soedirman University, Purwokerto, BCA (Bicinchoninic Acid Solution) protein kit (Sangon Biotech; Shanghai China), USP Trypsin enzyme (Bioworld USA), Supelco DSC-SCX (Strong Cationic Exchange) Cartridge (Sigma), Hypersep Retain PEP (Polar Enhanced Polymer) (Thermo Scientific). Subsequently, an antifungal activity test was performed using Aspergillus sp. (IPBCC 151255), Potato Dextrose Agar (PDA) (Merck), blank disk (Oxoid), and Nystatin NS disk (Oxoid, UK). Acetonitrile (hyper grade for LC-MS LiChrosolv, Merck, Germany), water (MS grade, Merck, Germany), and trifluoroacetic acid (TFA; Merck, Germany) were used during peptide identification.
2.2 Protein isolation of goat milk
A total of 1 L of goat milk was centrifuged at 7000 rpm for 30 min at 4 °C and the fat layer was separated. Milk was pasteurized at 72 °C for 15 min and added with 2 M HCl at 40 °C to a pH of 4.6 to isolate casein by isoelectric precipitation. Subsequently, the milk was centrifuged at 7000 rpm for 30 min to separate casein and whey. The casein precipitate was rinsed with distilled water 3 times at 7000 rpm for 5 min. Goat milk casein (GMC) and goat milk whey (GMW) were freeze-dried.
2.3 Hydrolysis of goat milk
Approximately 60 mL of casein and whey were dissolved in 5 mL of ammonium bicarbonate and homogenized for 1 min. Subsequently, the mixture was heated at 90 °C for 15 min and cooled for 15 min. This was followed by the addition of trypsin to each sample at a ratio of 1:10, 1:20, 1:30, and 1:40 (w/w) and homogenized for 1 min. The mixture was incubated at 37 °C for 20 h, followed by heating at 80 °C for 15 min, and the samples were centrifuged at 4500 rpm. The supernatant was put in an Amicon® Ultra – 15 Centrifugal Filter Devices with a molecular weight cut-off (MWCO) of 3,000 Da and centrifuged at 4500 rpm. The filtrate that passed through the filter was used for further experiments and subjected to a microplate reader with a BCA (Bicinchoninic Acid Solution) protein assay kit at a wavelength of 562 nm. To confirm the hydrolysis, the samples were analyzed with SDA PGE (Biorad Mini Protein II, California) using a method developed by (Sharma and Singh, 2022).
2.4 Fractionation of hydrolyzed protein
Casein and whey protein hydrolized were fractionated using SPE SCX with buffer pH 3–9., which complied with the previous study (Raharjo et al., 2021). The results of fractionation are peptides fraction was neutralized pH 7 and purified using a solid phase extraction polar enhance polymer (SPE-PEP) catridge with methanol as an eluent. Than, GMC or GMW pH X will be called GMCX or GMWX (X = pH value 3–9). The peptides fraction from the purification was dried by flowing nitrogen gas into the surface of the solution. Subsequently, the dried peptide fraction was dissolved in 1 mL of distilled water. The concentration and mass were determined using a BCA protein assay kit, and the absorbance was measured at the wavelength of 562 nm.
2.5 Antifungal activity test
A 200 µL of Aspergillus sp. suspension containing 1x106 spore /mL was dispersed equally to Potato Dextrose Agar (PDA) media. A paper disc with a 6 mm diameter was impregnated with 20 µL of the peptide fraction sample, dried, and placed on the PDA media previously inoculated with Aspergillus sp. culture. The media was incubated at 30 °C for 48 h and the inhibition activity was measured from the average diameter of the inhibition zone.
MIC determination was based on a previous study (Singh et al., 2021) using 12 wells of microplate from Biologik, Indonesia. The RPMI 1640 media with Aspergillus sp. culture and media were used as control positive and negative, respectively. The samples, RPMI 1640 media with Aspergillus sp. culture, and media were put on wells number 1–10, 11, and 12, respectively. The concentrations of the well ranged from 1.953 to 1000 µg/mL, in which the concentration of the next well was half of the previous one. The wells were further incubated at 30 °C for 48 h and the MIC value was determined with a clear medium, indicating no growth of Aspergillus sp.
2.6 Morphology analysis using scanning electron microscope (SEM)
A scanning Electron Microscope (SEM) was used to observe the morphology of the mycelia of the fungus Aspergillus sp. as previously described by Khani et al. (2020) with modifications. A total of 100 µL of spore solution containing (106 spores/mL) was inoculated on PDA media treated with GMC5, GMC6, GMC7 and GMC8. The PDA media without the addition of peptide fraction was used as a control. Samples and controls were incubated at 30 °C for 4 days. Mycelia were placed on stubs using carbon adhesive, coated with a thin layer of gold, and observed under SEM (Jeol JSM-6510LA, Japan).
2.7 Identification of peptide using LC-HRMS
A total of 5 µL sample fraction was injected into the LC-HRMS using a C18, 75 μm × 150 cm, and 2 μm particles catridge (Acclaim® PepMap RSLC, Germany). Mobile phase A contained water and 0.05% TFA while mobile phase B contained water: acetonitrile at 20:80 and 0.1% TFA. Subsequently, mobile phases A and B were used with a gradient of 10% B for 3 min, increased to 95% B at 90th minutes with a flow rate of 0.3 µL/min. The MS detection was performed using the full-MS/dd-MS2 positive ion scanning mode, while the peptide was measured in the m/z range of 350–1800. The full-MS parameter was set with a resolving power of 140,000, while the dd-MS2 parameter was used at 17,500. The Chromatogram was obtained from the Xcalibur software and the peptide was identified using Proteome Discoverer with raw data from Xcalibur and Capra hircus downloaded from the UniProt.org database.
3 Results and discussion
3.1 Goat milk protein hydrolysate
Goat milk contained 20% whey and 80% caseins, which were the major proteins in goat milk. Casein was divided into αS1-casein, αS2-casein β-casein, and κ-casein, while whey was categorized into α-lactalbumin, β-lactoglobulin, serum albumin, immunoglobulin, and glycomacropeptide (Mohanty et al., 2016). Fresh goat milk was prepared before separating fat from milk protein by centrifugation. Casein from milk protein was isolated by adding 2 M HCl at 40 °C until it reached an isoelectric point of pH 4.6, which was indicated by the formation of lumps. The centrifuged pellet was casein and the supernatant was whey. The preparation of 1 L of goat milk produced 1.35% (g/mL) casein and 6.121% (g/mL) whey. Casein and whey hydrolized with variation enzyme (trypsin): substrat ratios. Fig. 1 showed the degree of hydrolysis (DH) of casein and whey, which was performed using enzyme: substrate ratios of 1:10, 1:20, 1:30, and 1:40. The degree of hydrolysis of casein respectively were 47, 65, 56, and 66.8%. On other hand, the degree hydrolysis of whey were 49.2, 69.4, 67.8 and 75.5% respectively. Moreover, the degree of hydrolysis of protein was measured by calculating the total protein mass and supernatant before and after hydrolysis.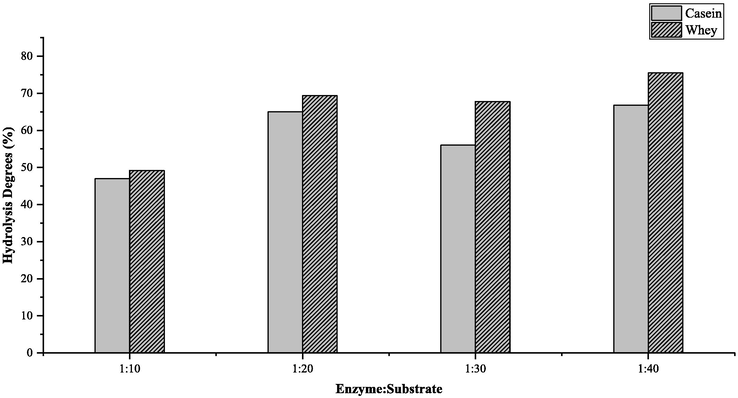
Degree of hydrolysis of casein and whey.
Fig. 1 showed that the degree of hydrolysis value increased with the increase of enzyme-substrate ratio but in ratio 1:30, the degree of hydrolysis obtained slightly decreased. This is influenced by parameters such as time, quantity and quality of enzymes used. The enzyme-substrate ratio of 1: 40 produced the greatest degree of hydrolysis percentage. According to (Charoenphun et al., 2013), the higher degree of hydrolysis (DH) indicated an effective hydrolysis process in breaking peptide bonds, which can represent the greater number of short-chain peptides in the hydrolyzate (Yang et al., 2019). This value can also indicate that the process of protein hydrolysis was getting better. From the results of the degree of hydrolysis obtained, more than half of the amount of protein was hydrolyzed by enzymes. These values were quite large, where the trypsin enzyme had specificity for cutting certain amino acids, causing the hydrolysis process to not reach 100%.
The result showed that the ratio of 1:40 caused the maximum interaction of the enzyme with goat milk protein to hydrolyze the substrate optimally and obtain the most significant concentration. (Charoenphun et al., 2013) also reported that the greater the concentration, the more peptides were produced. Some studies used an enzyme: protein ratio of 1:40 up to 1:100 (Heissel et al., 2018; Doellinger et al., 2020). However, the excessive application of the trypsin enzyme can increase the occurrence of peptide cutting at non-specific amino acids. This was due to the run out of the cutting points at the amino acids lysine (K) and arginine (R) and the results of autodigestion/autolysis.
Casein and Whey protein hydrolyzate were analyzed using SDS PAGE and the results showed that small proteins migrated faster than large proteins. Furthermore, SDS PAGE analysis also showed the purity level of the resulting proteins to examine their molecular sizes of the proteins. The protein bands from the hydrolysis of samples 1, 2, 3, and 4 for both casein and whey were thinner compared to casein and whey without hydrolysis (6 and 7) and goat milk as a control (8), indicating that they were hydrolyzed by the trypsin enzyme. However, the hydrolysis of casein and whey with an enzyme: substrate ratio of 1:10, 1:20, 1:30, and 1:40 did not yield different protein bands. The hydrolyzate analysis also showed that the casein protein had been hydrolyzed into α-s1 and beta-casein proteins with molecular weights of 33 and 25 kDa, respectively. The whey protein hydrolyzate revealed that casein was hydrolyzed into beta-lactoglobulin and α-lactalbumin with molecular weights of 15 kDa and 13 kDa, respectively. The molecular weights of α-S1 casein, α-S2 casein, β-casein, β-lactoglobulin, and α-lactalbumin were 33.37 kDa, 29.49 kDa, beta 25.59 kDa, 15.97 kDa, and 13.70 kDa, respectively (Widodo et al., 2021). Peptide bands did not appear because the hydrolyzed peptide fraction had a molecular weight below 10 kDa. The results of the SDS PAGE analysis of casein and whey protein hydrolyzate were presented in Fig. 2.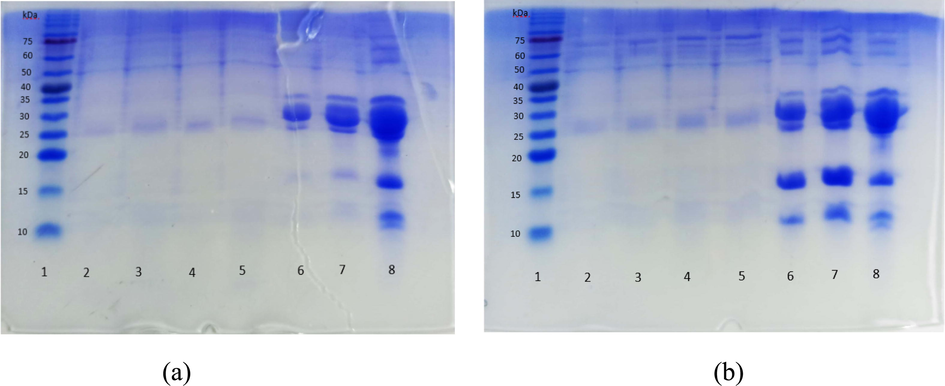
Results of SDS PAGE protein hydrolyzate (a) casein and (b) whey of goat milk. (a) 1 (Marker), 2 (Casein 1:10), 3 (Casein 1:20), 4 (Casein 1:30), 5 (Casein 1:40), 6 (Casein 50 µg), 7 (Casein 100 µg), 8 (milk). (b) 1 (Marker), 2 (Whey 1:10), 3 (Whey 1:20), 4 (Whey 1:30), 5 (Whey 1:40), 6 (Whey 50 µg), 7 (Whey 100 µg), 8 (milk).
3.2 Antifungal activity of goat milk casein peptide fraction against Aspergillus sp
Casein and whey protein hydrolized were fractionated using SPE SCX with buffer pH 3–9. The results of fractionation are peptides fraction were further neutralized to pH 7. Peptida fraction purified using an SPE HyperSep Retain PEP catridge with methanol. The peptides fractions gave a colorless solution. The result of fractionation were obtained GMC3-9 and GMW3-9 peptide fractions.
The data obtained in Table 1, the peptide in the GMC4 and GMW3 had the highest number, namely 2442 and 1108 µg and yield 45,67% and 32.59%, respectively. The peptides in the trypsin hydrolyzate of goat milk protein were dominated by those with an isoelectric point value at pH 4. The fractionated process showed that not all of the eluted peptides were performed by the buffer. The number of different peptides in each fraction was also influenced by the protein hydrolysis process using the trypsin enzyme and the fractionation process with the SCX catridge. This led to variations in the number of amino acids that made up peptides, which resulted in differences in peptide concentrations. Therefore, the amount of peptide in one fraction was different from others. The GMC3-9 and GMW3-9 than tested its activity against the fungus Aspergillus sp.
Fraction
Massa (µg)
Yield (%)
GMC3
2288
42.79
GMC4
2442
45.67
GMC5
1760
32.92
GMC6
1388
25.96
GMC7
952
17.80
GMC8
1432
26.78
GMC9
956
17.88
GMW3
1108
32.59
GMW4
866
25.47
GMW5
808
23.77
GMW6
1036
30.48
GMW 7
832
24.47
GMW8
766
22.53
GMW9
936
27.53
The antifungal activity of GMC3-9 and GMW3-9 was examined using the disc diffusion method at a concentration of 1000 µg/ml. The antifungal activity were presented in Table 2 and Fig. 3. Based on the results of one-way ANOVA analysis on the antifungal activity test, it showed a P value (P value) of 0.000 (<0.05) so it can be concluded that there was a significantly different effect of the peptide fraction used to inhibit fungal growth activity. Furthermore, the Duncan Multiple Range Test (DMRT) was carried out to determine the most significantly different peptide fractions. The results of the antifungal DMRT test showed that the diameter of the inhibition zone for GMC6 was 26.95 ± 0.63d mm, GMC8 was 22.40 ± 0.84c mm, GMC7 was 20.44 ± 0.09b mm and GMC5 was 8.73 ± 0.74a mm. GMC6 produced the largest and most significant (significantly different) inhibition zone with other treatments. GMC6, 7, and 8 had very strong antifungal activity, while that GMC5 was moderate. GMC6 presented the highest antifungal activity. The difference in antifungal activity for each fraction could be related to the synergetic and antagonistic effect in the fraction. Hilal A. Syahrir et al. (2016) explained that the synergistic effect occurred due to two or more active compounds in a particular fraction or sample reinforcing each other in their activity. The antagonistic effect occurred because the compounds contained in a sample gave opposite responses, thereby weakening their activity. Within a column, values with differing superscript alphabets are significantly different (p < 0.05).
Peptide fraction
Inhibition zone diameter (mm)
MIC (µg/ml)
GMC5
8.73 ± 0.74a
500.00
GMC6
26.95 ± 0.63d
31.25
GMC7
20.44 ± 0.09b
500.00
GMC8
22.40 ± 0.84c
250.00
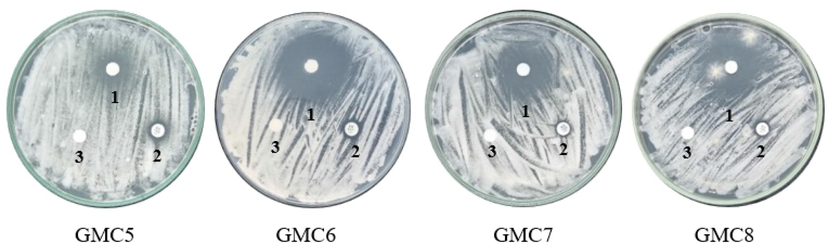
The antifungal activity fraction of GMC peptide against Aspergillus sp., sample (1), positive control (2), solvent control (3).
The result showed that GMC6 exhibited the lowest MIC of 31.25 μg/mL against Aspergillus sp, followed by GMC8, GMC7, and GMC5 fractions of 250, 500, and 500 μg/mL, respectively. These values were also obtained through the antifungal activity test using the diffusion disc method. The MIC obtained in this study was lower than that previously reported by (Schmidt et al., 2019) on peptide from cowped a seed against A. niger (>500 μg/mL). The MIC of the kenaf seed peptides mixture (KSPM) against A. niger and A. flavus was 43 µg/mL (Arulrajah et al., 2022). However, the lower MIC obtained in this study could be attributed to the different enzymes and microbes used. The MIC fraction of GMC5-8 peptide against Aspergillus sp. were presented in Table 2.
Based on the morphology of Aspergillus sp. mycelia observed using the SEM method, it was discovered that peptide fraction with no treatment looked normal, uniform, smooth surface, flat, and arranged in order. After treatment with GMC5, GMC6, GMC7, and GMC8 at their MIC, the mycelia were wrinkled and withered, and their surfaces were concave, as shown in Fig. 4 . Furthermore, some mycelia were broken, indicating that goat milk peptides destroyed the integrality and physiology function of mycelium. These results were in line with a previous study on the antifungal activity of kenaf seed peptide against Fusarium sp. reported by Arulrajah et al. (2023). The treatment with kenaf seeds peptide caused significant deformation, shrinkage, and folded appearance compared to its control. (Han et al., 2019) and (Devi and Sashidhar, 2019) also observed that Fusarium oxysporum and Aspergillus flavus mycelia showed deformation and wrinkling membrane cells after treatment with antifungal peptide. According to Khani et al. (2020), Skh-AMP1 antifungal peptide caused conidia and mycelia shrinkage as well as deformation in A. fumigatus. The changes in the mycelium in this study might be due to the variation in membrane permeability as previously reported by Arulrajah et al. (2023), where severe damage found on the membrane cells was consistent with the membrane permeability test.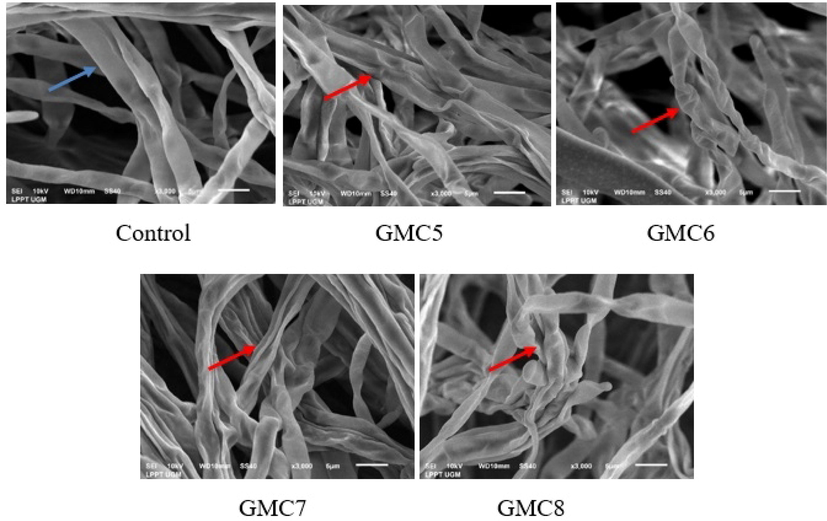
SEM images of Aspergillus sp. indicating the morphological change after treatment with GMC5-8 Fractions. Blue arrow normal mycelia dan red arrow wrinkled mycelia.
3.3 Identification of bioactive peptide
The most active antifungal fraction, GMC6 was identified for its amino acid sequence by LC-HRMS, which was performed using a Proteome Discoverer 2.5 software with Capra hircus goat milk database. The peptide was categorized based on mass-to-charge ratio, net charge, hydrophobic ratio, peptide origin, molecular weight, and gravy. LC-HRMS MS2 spectra of GMC6 fraction and peptide sequence were presented in Table 3 and Fig. 5, respectively.
Sequence
M/Z ratio (Da)
Net charge
Hydrophobic Ratio
pI
Mol. Wt (Da)
Protein Name
YNVPQLEIVPK
650.36890
0
36
6.00
1299.73071
αS1-casein
KENINELSK
537.79382
0
22
6.14
1074.57896
αS1-casein
GLSPEVPNENLLR
719.38934
−1
31
4.53
1437.76962
αS1-casein
YLGYLEQLLK
620.35303
0
40
6.00
1239.69835
αS1-casein
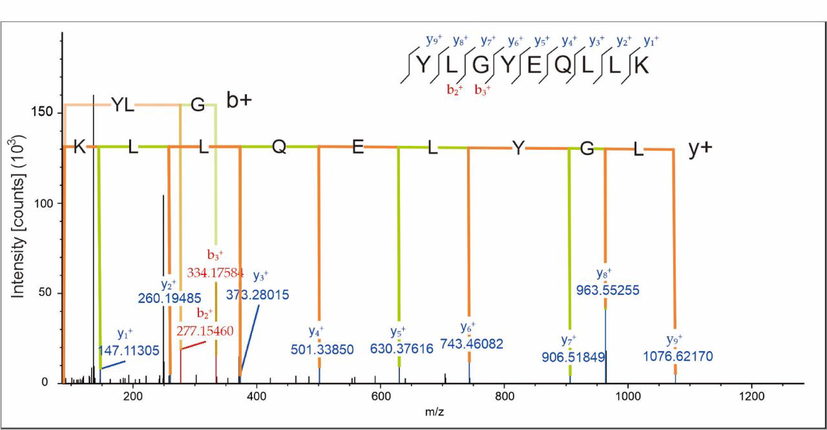
LC-HRMS MS2 Spectra of YLGYLEQLLK Peptide.
The GMC6 fraction contained 4 peptide sequences with antifungal activity. Based on the LC-HRMS identification results, a total of 4 peptides were obtained, namely YNVPQLEIVPK, KENINELSK, GLSPEVPNENLLR, YLGYLEQLLK, with a molecular weight ranging from 1074.57896 to 1299.73071 Da. Peptides with a z ion charge of + 2 had m/z values of 650.36969, 537.79382, 719.3893, and 620.35303 Da, respectively. The net charge on the peptide sequence showed a significant impact of interaction with the fungal surface, which ranged from −1 to 0. Furthermore, 3 out of 4 antifungal peptides had a neutral net charge of 0, and one peptide was anionic (GLSPEVPNENLLR) with a net charge of −1. The 4 antifungal peptides were derived from casein proteins, mainly αS1-casein, as presented in Table 3. The hydrophobicity of the antifungal peptides ranged from 22 to 40%, with hydrophobic peptide YLGYLEQLLK exhibiting the highest hydrophobicity of 40%, as presented in Table 3. Short peptides were reported to be potent antimicrobials that can penetrate the cell wall of microorganisms, causing growth retardation and lethality (Sari et al., 2018). Most resulting peptide residues were positively charged Lys (K) and Arg (R) amino acid residues. Similarly, (Heissel et al., 2018) stated that trypsin specifically cut the carboxylic group of peptide bonds on the lysine (K) and arginine (R) amino acids and produced peptides with 9–13 amino acid residue lengths. Besides arginine and lysine, leucine, glutamic acid, valine, proline, and threonine amino acids were also responsible for antimicrobial activity (Sah et al., 2016). Peptides with hydrophobic positively charged amino acids lysine and arginine act as cationic peptides and interact with the fungal cell membrane, thereby penetrating and disrupting the integrity of the cell membrane. Antifungal Peptides (AFP) had been classified into 2 groups based on their effect on the cell membrane. These included (1) peptides that cross the cell membrane and cause pore formation or act on specific targets such as β-glucan, chitin, and enzyme synthesis, and (2) peptides that cooperate with the cell membrane and cause cell lysis (Neelabh et al., 2016).
In this study, it was suspected that peptide inhibition against the fungus Aspergillus sp was due to an interaction with the membrane. According to Manju Devi et al. (2021), short synthetic peptides such as PPD1-FRLHF, 66–10-FRLKFH, and 77–3-FRLKFHF inhibited fungal growth and showed membranolytic activity. Docking studies on the interaction of the peptides with a trans-membrane protein calcium ATPase of A. flavus also showed that all the peptides bound to the protein with a high z-rank score. The activity of the calcium ATPase was significantly decreased in peptides-treated fungal samples, thereby validating the docking results. Among all the tested peptides, 77–3 peptides exhibited the maximal membrane damage property.
4 Conclusion
Goat milk peptides obtained in this study demonstrated potential as antifungal compounds. The highest antifungal activity was obtained on GMC6 fraction, with an inhibition zone of 26.95 ± 0.63 mm and MIC of 31.25 µg/ml. The goat milk peptides with the highest antifungal activity were identified as YNVPQLEIVPK, KENINELSK, GLSPEVPNENLLR, and YLGYLEQLLK. The SEM results suggested that peptides changed the morphology of mycelium. However, further investigation was recommended to fully understand the interaction of goat milk peptide with the membrane.
Acknowledgment
Tri Wahyudi was acknowledged for performing LC-HRMS analysis for peptide while Yusuf Umardani was acknowledged for performing SEM of Aspergillus sp. mycelia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Lacto-fermented Kenaf (Hibiscus cannabinus L.) seed protein as a source of bioactive peptides and their applications as natural preservatives. Food Control. 2020;110:106969
- [CrossRef] [Google Scholar]
- Production of cationic antifungal peptides from kenaf seed protein as natural bio preservatives to prolong the shelf-life of tomato puree. Int. J. Food Microbiol.. 2021;359:109418
- [CrossRef] [Google Scholar]
- Antibacterial and antifungal activity of kenaf seed peptides and their effect on microbiological safety and physicochemical properties of some food models. Food Control. 2022;140:109119
- [CrossRef] [Google Scholar]
- Antifungal efficacy of kenaf seed peptides mixture in cheese, safety assessment and unravelling its action mechanism against food spoilage fungi. Food Biosci.. 2023;52:102395
- [CrossRef] [Google Scholar]
- Natural antimicrobial peptides as inspiration for design of a new generation antifungal compounds. J. Fungi 2017
- [CrossRef] [Google Scholar]
- Calcium-binding peptides derived from tilapia (Oreochromis niloticus) protein hydrolysate. Eur. Food Res. Technol.. 2013;236:57-63.
- [CrossRef] [Google Scholar]
- Antiaflatoxigenic effects of selected antifungal peptides. Peptides. 2019;115:15-26.
- [CrossRef] [Google Scholar]
- Sample preparation by easy extraction and digestion (SPEED) - A universal, rapid, and detergent-free protocol for proteomics based on acid extraction. Mol. Cell. Proteomics. 2020;19:209-222.
- [CrossRef] [Google Scholar]
- Effect of a novel antifungal peptide P852 on cell morphology and membrane permeability of Fusarium oxysporum. Biochim. Biophys. Acta - Biomembr.. 2019;1861:532-539.
- [CrossRef] [Google Scholar]
- Enhanced trypsin on a budget: Stabilization, purification and high-temperature application of inexpensive commercial trypsin for proteomics applications. PLoS One. 2018;14:1-16.
- [CrossRef] [Google Scholar]
- Isolation and characterization of potential food preservative peptide from Momordica charantia L. Arab. J. Chem.. 2017;10:S3982-S3989.
- [CrossRef] [Google Scholar]
- Effects of the antifungal peptide Skh-AMP1 derived from Satureja khuzistanica on cell membrane permeability, ROS production, and cell morphology of conidia and hyphae of Aspergillus fumigatus. Peptides. 2020;123:170195
- [CrossRef] [Google Scholar]
- Kostić, A., Milinčić, D.D., Stanisavljević, N.S., Gašić, U.M., Lević, S., Kojić, M.O., Lj. Tešić, Ž., Nedović, V., Barać, M.B., Pešić, M.B., 2021. Polyphenol bioaccessibility and antioxidant properties of in vitro digested spray-dried thermally-treated skimmed goat milk enriched with pollen. Food Chem. 351. https://doi.org/10.1016/j.foodchem.2021.129310.
- Kusumaningtyas, Widiastuti, R., Dewantari Kusumaningrum, H., Thenawidjaja Suhartono, M., 2015. Aktivitas Antibakteri Dan Antioksidan Hidrolisat Hasil Hidrolisis Protein Susu Kambing Dengan Ekstrak Kasar Bromelin. J. Teknol. dan Ind. Pangan 26, 179–188. https://doi.org/10.6066/jtip.2015.26.2.179.
- Antifungal microbial agents for food biopreservation—a review. Microorganisms. 2017;5:1-35.
- [CrossRef] [Google Scholar]
- Evaluation of biological and antimicrobial properties of freeze-dried whey fermented by different strains of Lactobacillus plantarum. Food Funct.. 2018;9:3688-3697.
- [CrossRef] [Google Scholar]
- Antifungal and antimycotoxigenic activity of hydrolyzed goat whey on Penicillium spp: An application as biopreservation agent in pita bread. LWT Food Sci. Technol.. 2020;118:108717
- [CrossRef] [Google Scholar]
- Efficacy of short-synthetic antifungal peptides on pathogenic Aspergillus flavus. Pestic. Biochem. Physiol.. 2021;174:104810
- [CrossRef] [Google Scholar]
- Low molecular weight peptides generated from palm kernel cake via solid state lacto-fermentation extend the shelf life of bread. LWT Food Sci. Technol.. 2020;134:110206
- [CrossRef] [Google Scholar]
- Milk derived antimicrobial bioactive peptides: A review. Int. J. Food Prop.. 2016;19:837-846.
- [CrossRef] [Google Scholar]
- Neelabh, Singh, K., Rani, J., 2016. Sequential and Structural Aspects of Antifungal Peptides from Animals, Bacteria and Fungi Based on Bioinformatics Tools. Probiotics Antimicrob. Proteins 8, 85–101. https://doi.org/10.1007/s12602-016-9212-3.
- Perez Espitia, P.J., De Fátima, N., Soares, F., Sélia, J., Coimbra, R., José De Andrade, N., Cruz, R.S., Antonio, E., Medeiros, A., 2012. Bioactive Peptides: Synthesis, Properties, and Applications in the Packaging and Preservation of Food 11, 18. https://doi.org/10.1111/j.1541-4337.2011.00179.x.
- Antibacterial peptides from tryptic hydrolysate of Ricinus communis seed protein fractionated using cation exchange chromatography. Indones. J. Pharm.. 2021;32:74-85.
- [CrossRef] [Google Scholar]
- Antibacterial and antiproliferative peptides in synbiotic yogurt-Release and stability during refrigerated storage. J. Dairy Sci.. 2016;99:4233-4242.
- [CrossRef] [Google Scholar]
- Sari, M., Suryanto, D., Yurnaliza, 2018. Antimicrobial activity of lactic acid bacteria isolated from bekasam against staphylococcus aureus ATCC 25923, escherichia coli ATCC 25922, and salmonella sp. IOP Conf. Ser. Earth Environ. Sci. 130. https://doi.org/10.1088/1755-1315/130/1/012011.
- Isolation and characterisation of the antifungal activity of the cowpea defensin Cp-thionin II. Food Microbiol.. 2019;82:504-514.
- [CrossRef] [Google Scholar]
- Effect of atmospheric pressure cold plasma treatment time and composition of feed gas on properties of skim milk. Lwt. 2022;154:112747
- [CrossRef] [Google Scholar]
- Singh, U., Singh, P., Singh, A.K., Laxmi, Kumar, D., Tilak, R., Shrivastava, S.K., Asthana, R.K., 2021. Identification of antifungal and antibacterial biomolecules from a cyanobacterium, Arthrospira platensis. Algal Res. 54, 102215. https://doi.org/10.1016/j.algal.2021.102215.
- Hilal A. Syahrir, N., Mochamad Afendi, F., Susetyo, B., 2016. Efek Sinergis Bahan Aktif Tanaman Obat Berbasiskan Jejaring dengan Protein Target. J. Jamu Indones. 1, 35–46. https://doi.org/10.29244/jjidn.v1i1.30594.
- Bacteriocin production from indigenous strains of lactic acid bacteria isolated from selected fermented food sources. Int. J. Pharma Res. Heal. Sci.. 2016;4:982-990.
- [Google Scholar]
- Functional milk proteome analysis of genetically diverse goats from different agro climatic regions. J. Proteomics. 2020;227:103916
- [CrossRef] [Google Scholar]
- Functionality of the components from goat’s milk, recent advances for functional dairy products development and its implications on human health. J. Funct. Foods. 2019;52:243-257.
- [CrossRef] [Google Scholar]
- Antimicrobial peptides as potential alternatives to antibiotics in food animal industry. Int. J. Mol. Sci.. 2016;17
- [CrossRef] [Google Scholar]
- Identification of goats’ and cows’ milk protein profile in Banyumas Regency by sodium dedocyl sulphate gel electrophoresis (Sds-Page) Anim. Prod.. 2021;23:27-33.
- [CrossRef] [Google Scholar]
- Hydrolysis process optimization and functional characterization of yak skin gelatin hydrolysates. J. Chem.. 2019;2019
- [CrossRef] [Google Scholar]
- Changes of extracellular vesicles in goat milk treated with different methods. Lwt. 2022;170:114038
- [CrossRef] [Google Scholar]







