Translate this page into:
Antihistaminic and other biological activities of 2-methylpropanamide and benzamide derivatives of carboxyterfenadine
⁎Corresponding author. msarayne@gmail.com (M. Saeed Arayne),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
In the present study the 2-methylpropanamide and benzamide derivatives of carboxyterfenadine, have been synthesized by nucleophilic substitution at carboxylic acid moiety (C-33) of tertiary carbon C-19 substituted with benzene ring. The proficient and high yielding series was carried out in two steps by continuous monitoring with TLC. The carboxylic group was utilized in the formation of 2-methylpropanamide and benzamide derivatives of carboxyterfenadine. The derivatives were characterized by spectroscopic techniques including mass. The starting material (2-[4-[1-hydroxy-4-[4-(hydroxydiphenylmethyl)piperidino]butyl]phenyl]-2-methylpropanoic acid) itself is H1 receptor antagonist. Hence, all these compounds A–E were biologically evaluated for antihistaminic and anticholinergic activity, using isolated guinea pig ileum tissues.
Keywords
Fexofenadine
Anticholinergic activity
Carboxyterfenadine
1 Introduction
H1 antagonists are the drugs that competitively inhibit the action of histamine on tissue containing H1. Usually H1 receptors have been evaluated in vitro in terms of their ability to inhibit histamine induced spasms in an isolated strip of guinea pig ileum (Meeves and Appajosyula, 2003). Antihistamines may be assessed in vivo in terms of their ability to protect animals against the toxic effects of histamine administered intravenously or by aerosol (Suhagia et al., 2006; Saxena et al., 2006). Fexofenadine (2-[4-[1-hydroxy-4-[4-(hydroxydiphenylmethyl)piperidino]butyl] phenyl]-2-methylpropanoic acid) (Fig. 1) is a selective and peripherally acting H1-receptor antagonist and is a nonsedative active metabolite of terfenadine (see Figs. 2 and 3).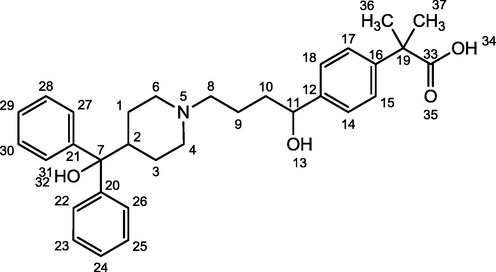
Fexofenadine.
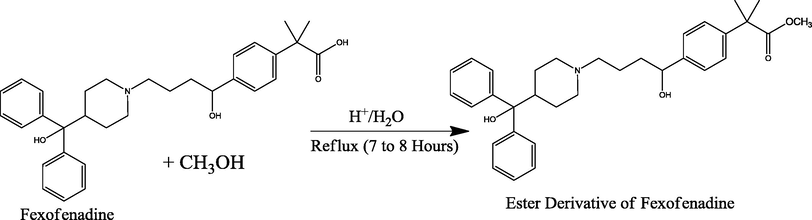
Esterification of fexofenadine.
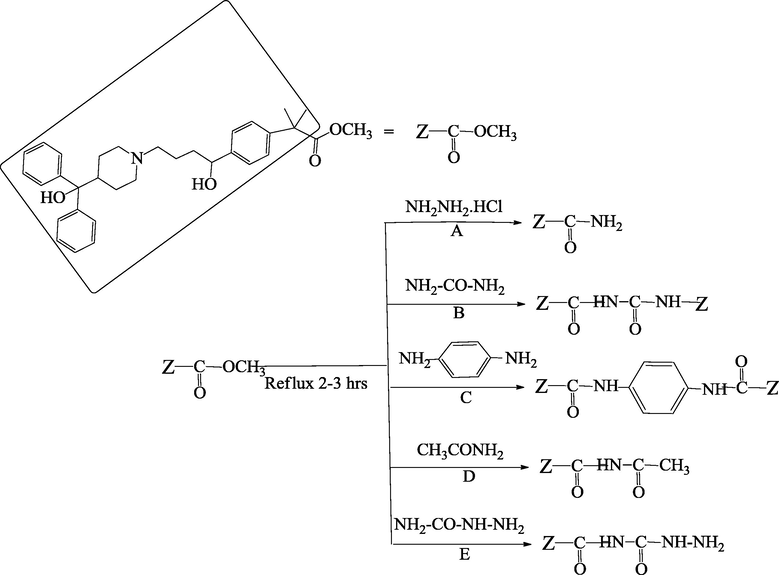
Synthetic pathways of compound A–E.
It has a chiral carbon in its chemical structure and is orally administered as a racemic mixture of R- and S-enantiomers (Robbins et al., 1998), it has the capacity to exist as Zwitter-ionic form and does not cross blood–brain barrier (BBB) that is why it does not cause drowsiness or sedation (Lappin et al., 2010).
Fexofenadine is used clinically for treating seasonal allergic rhinitis (Segall et al., 2008) and daily 180 mg of the drug, given for 2 weeks caused statistical and clinical improvement in symptoms and less drowsiness in patients with modest to stern SAR (Hampel et al., 2003). The most common adverse events were headache, throat irritation, viral infection, nausea, dysmenorrhea, drowsiness, dyspepsia and fatigue (Markham and Wagstaff, 1998). Complex of fexofenadine with α-cyclodextrin in aqueous medium was studied which gives the evidence of the attachment of phenyl ring into α-cyclodextrin cavity (Ali et al., 2012). Thus, it is important to understand the mechanism of action of fexofenadine in the presence of chemically active compounds. Fexofenadine has poor bioavailability in humans and its microdose is used to predict the pharmacokinetics of the therapeutic drug because it has low intestinal membrane permeability due to hydrophilicity (Ohura et al., 2012).
In vivo and in vitro antihistaminic studies of fexofenadine have been established and oral administration on guinea pig indicates that fexofenadine antagonised histamine-induced skin wheals in a dose-dependent manner (Neuhaus et al., 2012; Malick and Grant, 1997). The isolated guinea pig ileum studies of fexofenadine and terfenadine reveal that both these compounds have antihistaminic property. Both drugs antagonised the contractile effects of histamine, although, fexofenadine is more selective anti-histamine than terfenadine (Zhang et al., 1994).
The present study uses fexofenadine as model compound and derivatives with hydrazine hydrochloride, urea, p-phenelediamine, acetamide and semicarbazide have been prepared. In addition, the antihistaminic and anticholinergic activities of fexofenadine derivatives assessed and compared with fexofenadine and pyrilamine maleate taken as standard for the examination of biological activities.
The carboxylic group at tertiary carbon-19 through condensation reaction was esterified and by nucleophilic substitution reaction at the acyl (carbonyl) carbon form tetrahedral intermediate after the elimination of the leaving group it returns to a carbonyl carbon (Arayne et al., 2009). Various reactions have been recognized on the basis of the above reaction mechanism by reacting various amines to yield amides (Sultana et al., 2013).
2 Experimental
2.1 Material and equipments
Fexofenadine was a gift from Getz Pharmaceutical Laboratories Ltd., Karachi, Pakistan. Hydrazine hydrochloride, urea, p-phenelediamine, acetamide and semicarbazide were of Merck, Darmstadt, Germany. Acetylcholine chloride (ACh), pyrilamine maleate, histamine diphosphate and potassium chloride were purchased from Sigma Chemicals Co, St Louis, MO, USA, magnesium chloride, magnesium sulfate, potassium dihydrogen phosphate, glucose, calcium chloride, sodium bicarbonate, sodium dihydrogen phosphate were purchased from E. Merck, Darmstadt, Germany and sodium chloride was purchased from BDH Laboratory Supplies, Poole, England. Analytical grade reagents were used and all the glassware was washed with chromic acid followed by a thorough washing with freshly prepared de-ionized water, freshly prepared salt solutions were used on the day of the experiment.
Melting points were obtained manually by capillary method. The IR spectra were obtained on Shimadzu prestige-21 200 VCE coupled to a PIV-PC and loaded with IR solution version 1.2 software (KBr disks). NMR spectra were recorded on Bruker FT-NMR 500 MHz with the compounds dissolved in deuterated methanol. Chemical shifts are reported in (δ ppm) relative to tetramethylsilane as an internal standard. Significant 1H NMR data are tabulated in the order of multiplicity (s, singlet; d, doublet; t, triplet; q, quartet; and m, multiplet) and number of protons. Mass spectra were recorded on Finnign-MAT212 under electron impact (EI) ionization condition. Thin layer chromatography (TLC) was performed on HSF-254 TLC plate and compounds visualized under UV lamp. Tissue bath connected with thermocirculator which has an inlet and an outlet for salt solutions, tube for carbogen, finally connected with transducer, amplifier, oscillograph which monitors the tissue response on chart paper.
2.2 General procedure for preparation of derivatives
Synthesis of amide derivatives of fexofenadine with hydrazine hydrochloride, urea, p-phenelediamine, acetamide and semicarbazide was carried out by adding 0.1 Mole fexofenadine in 60 mL of methanol with 1:2 drops of concentrated sulfuric acid and the whole content was refluxed for about 7–8 h. By continuous monitoring the flask contents, 0.2 M amine solutions were added individually into reaction flask and again refluxed for about 2–3 h till the completion of the reaction which was monitored by TLC. Excess solvent was removed by rotary evaporator and slowly crystallized out of the solution. The materials were recrystallized in suitable solvents till constant melting point.
2.3 Animals
Guinea pig (500–550 g) of local breed and either sex were used for this study. Animals were housed at the animal house of Aga Khan University Hospital, Karachi, maintained at 23–25 °C and were given a standard diet and tap water. Animals had free access to water but food was withdrawn 24 h prior to experiment. Guinea pigs were killed through cervical dislocation. The experiment performed complied with the rulings of the Institute of Laboratory Animal Resources, Commission of life Sciences, National Research Council (Office of Laboratory Animal Welfare, 2002).
Male and female guinea pigs were used in the experiment. Handling and use of animals was according to the Office of Laboratory Animal Welfare policy (Office of Laboratory Animal Welfare, 2002). The animals were housed and fed in a laboratory kept at constant temperature of 23–25 °C under standard conditions i-e 12:12 h light–dark cycle, standard pellet diet and tap water.
2.4 Isolated guinea pig ileum
Guinea pigs were sacrificed by cervical dislocation; ileum was cut into approximately 2.5-cm segments. Segments were placed in a carbogen-aerated organ bath (37 °C) containing the Tyrode’s solution in mM was: KCl 2.68; NaCl 136.9; MgCl2 1.05; NaHCO3 11.90; NaH2PO4 0.42; CaCl2 1.8 and glucose 5.55, with pH 7.4. One end of the ileal segment was attached to the transducer (FDT10-A), and the resting tension was adjusted to a load of 0.5 g. Samples were equilibrated for at least 30 min before the contraction measurement. The isotonic contractions were recorded with a Bioscience transducer coupled to a Harvard oscillograph. After equilibration period of 30 min, each tissue preparation was repeatedly treated with sub-maximal concentration (0.3 μM) of ACh with 3 min interval until constant responses were recorded.
2.5 Experimental protocol
After stabilization of the tissues, control peak responses of acetylcholine chloride (1 μM) and histamine (1 μM) were obtained and then these spasmogens were repeated in the presence of increasing concentrations of the compounds A–E (Willard et al., 1999; Gilani, 1992). Data were analyzed with Graphpad Prism version 5, GraphPad InStat 3 and expressed as means ± SEM (Standard error of mean). One way ANOVA was used to compare the means between groups followed by Tukey’s test for multiple comparisons.
2.6 Spectral data
1-Fexofenadine: 2-(4-(1-hydroxy-4-(4-(hydroxydiphenylmethyl)piperidin-1-yl)butyl)phenyl)-2-methylpropanoic acid, Color: white, m.p. 190 °C, UV nm (ε): 1.775(218 nm), IR (KBr) νmax: 3400–3350 db, s, 3058.04 s, 1700 s, 1600 and 1475 sm 1447.60 m, 1403.55 m, 1279 m, 1166.41 m,1099 s, 1068 m, 983, 965 db, sm, 834 ms, 747, 702, 638, 577, 525.53 dp, s. 1H NMR (MeOD, 500 MHz) δ: 7.06, 7.19 (m, J = 1.16 Hz), 7.11 (m, J = 0.5 Hz) aromatic, 4.5 (db, J = 1.0 Hz) —CH2 open chain, 4.95 (s) alcoholic, 2.0 N—H, 2.29, 2.19 CH2—N, 2.34 (t, J = 12.2 Hz) —CH2—CH2—N, 2.36 (t, J = 14.0 Hz), 1.39, 1.77 and 1.52 CH3. MS (m/z, %): (500.29, 35.2%) M+. Calculated for C32H39NO4: C, 49.14; H, 5.96; N, 3.18. Found: C, 49.01; H, 5.74; N, 4.33.
Compounds A to E: 2-(A): 2-(4-(1-hydroxy-4-(4-(hydroxydiphenylmethyl)piperidin-1-yl)butyl)phenyl)-2-methylpropanamide, m.p. 115 °C, UV nm (ε): 171(223 and 259 nm), IR (KBr) νmax: 3350, 3100 cm−1 (N—H), 1650 cm−1 (C⚌O), 1390 cm−1 (C—N). 1H NMR (MeOD, 500 MHz) δ: 7.19 (m, J = 7.02 Hz), 7.11 (m, J = 3.5 Hz) and 7.06 aromatic, 6.92 (s, pri amide), 4.41 methine, 2.51, 2.41 CH2—N, 2.2(s) alcoholic, 1.77 CH3, 1.59, 1.3 (t, J = 6.2 Hz), CH2—CH2—N. MS (m/z, %): (500, 9.2) M+. Calculated for C32H40N2O3: C, 76.77; H, 8.05; N, 5.6. Found: C, 76.9; H, 7.99; N, 5.4.
3-(B) N,N′-carbonylbis(2-(4-(1-hydroxy-4-(4-(hydroxydiphenylmethyl)piperidin-1-yl)butyl)phenyl)-2-methylpropanamide), m.p. 120 °C, UV nm (ε): 2415 (223 and 259 nm), IR (KBr) νmax: 3380, 3180 cm−1 (N—H), 1650 cm−1 (C⚌O) 1622 cm−1 (N—H bending), 1420 cm−1 (C—N). 1H NMR (MeOD, 500 MHz) δ: 9.6 (s, imide), 7.54 (m, J = 3.5 Hz), 7.38 (m, J = 7.02 Hz) and 7.28 aromatic, 3.9 methine, 3.6(s) alcoholic, 1.8 CH3, 2.5, 1.7 CH2—N, 1.4, 1.3 (t, J = 8 Hz) CH2—CH2—N. MS (m/z, %): (1026.59, 7.9) M+. Calculated for C65H78N4O7: C, 75.99; H, 7.65; N, 5.45. Found: C, 76.01; H, 7.69; N, 5.4.
4-(C) N,N′-(1,4-phenylene)bis(2-(4-(1-hydroxy-4-(4-(hydroxydiphenylmethyl)piperidin-1-yl)butyl)phenyl)-2-methylpropanamide), m.p. 229 °C, UV nm (ε): 5400 (223 and 259 nm), IR (KBr) νmax: 3390, 3130 cm−1 (N—H), 1680 cm−1 (C⚌O) 1640 cm−1 (N—H bending), 1370 cm−1 (C—N). 1H NMR (MeOD, 500 MHz) δ: 8 (s, sec amide), 7.64 (m, J = 5.3 Hz), and 7.22 and 7.11 (m, J = 6 Hz) aromatic, 4.6 methine, 3.65(s) alcoholic, 2.75, 2.41 CH2—N, 1.6, 1.27 (t, J = 4.2 Hz) CH2—CH2—N, 1.24 CH3. MS (m/z, %): (1074.62, 6.5) M+. Calculated for C70H82N4O6: C, 78.18; H, 7.69; N, 5.21. Found: C, 78.01; H, 7.61; N, 5.29.
5-(D) N-carbamoyl-2-(4-(1-hydroxy-4-(4-(hydroxydiphenylmethyl)piperidin-1-yl)butyl)phenyl)-2-methylpropanamide, m.p. 125 °C, UV nm (ε): 4090 (216 and 246 nm), IR (KBr) νmax: 3380, 3178 cm−1 (N—H), 1600–1630 cm−1 (C⚌O) 1625 cm−1 (N—H bending), 1570 cm−1 (C—N). 1H NMR (MeOD, 500 MHz) δ 8.8 (s, imide), 7.7 (m, J = 3.5 Hz), 7.46 (m, J = 7.02 Hz), 7.22 aromatic, 5.7 (s, urea) 3.7 methine, 3.2(s) alcoholic, 3.1, 2.6 CH2—N, 1.8 CH3, 1.6, 1.5 (t, J = 4.1 Hz) CH2—CH2—N. MS (m/z, %): (586.32, 8.5) M+. Calculated for C34H42N4O5: C, 69.60; H, 7.22; N, 9.55. Found: C, 69.4; H, 7.31; N, 9.71.
6-(E) N-(2-(4-(1-hydroxy-4-(4-(hydroxydiphenylmethyl)piperidin-1-yl)butyl)phenyl)-2-methylpropanoyl)benzamide, m.p. 79 °C, UV nm (ε): 5740 (202 and 234 nm), IR (KBr) νmax: 3200, 3190 cm−1 (N—H), 1620 cm−1 (C⚌O), 1670 cm−1 (N—H bending), 1545 cm−1 (C—N). 1H NMR (MeOD, 500 MHz) δ: 9.5 (s, imide), 8.08 aromatic, 7.28 (s, J = 3.5 Hz), 7.23 (s, J = 7.02 Hz), 2.1, 3.5 CH2—N, 2.32 methine, 3.65(s) alcoholic, 1.63, 1.58 CH3, 1.35 (t, J = 4.1 Hz) CH2—CH2—N. MS (m/z, %): (604.33, 5.8) M+. Calculated for C32H44N2O4: C, 77.45; H, 7.33; N, 4.63. Found: C, 77.20; H, 7.31; N, 4.38.
2.7 Spectral analysis
In the IR spectra of fexofenadine, the OH stretching vibration of carboxylic acid appeared as a broadband ranging from 3400 to 3350 cm–1 and C—H stretching at 3058 cm–1, fexofenadine showed a strong band at 1700 cm–1 due to C⚌O stretching and at 1279 cm–1 for C—O stretching. C⚌C aromatic strong bands were observed at 1475 and 1600 cm–1, while in all compounds from A to E the hydroxyl peak of acid was modified and changed into broad to sharp band. The spectra of all the compounds showed the absence of acidic OH peak and amide has been formed. 1H NMR data supported the evidence of amide formation. The methyl groups of tertiary carbon and acidic proton have been shifted while other signals of fexofenadine were present. All compounds A to E showed a singlet in the region 6.9–9.5 ppm which provide evidence for the absorption of amide. In addition, the spectral data also mentioned other signals consequent to the particular chemical structure of the compounds. All the compounds showed very low percentage of (M+) peak because they were not as sturdy as fexofenadine. The electron impact mass spectrum (EIMS) of fexofenadine showed molecular ion (M+) peak at (m/z, %): (320, 35).
2.8 Effect on guinea-pig ileum
Fexofenadine a non-anticholinergic, non-sedative antihistamine (Caballero et al., 1996) is prescribed for oral treatment of allergic rhinitis and chronic urticaria (Amichai et al., 2001). The nonsedating fexofenadine is actually an active metabolite of terfenadine (Turncliff et al., 2006; Amon et al., 2000), a drug taken off the market because of its cardio toxicity. Recent data subjected to indicate whether the compounds A–E are antihistaminic and anticholinergic in nature or not. The Guinea pig ileum is commonly used to define whether a potential drug has an antihistaminic effect (Larsson et al., 2007).
It is envisaged that all compounds are antihistaminic in nature but they did not block acetylcholine chloride (Fig. 4). The effect of different doses of fexofenadine was same to pyrilamine a standard histamine receptor (H1) antagonist for blocking the contractile effect of the guinea pig ileum while the acetylcholine chloride stimulant effects were not blocked (Corcóstegui et al., 2006).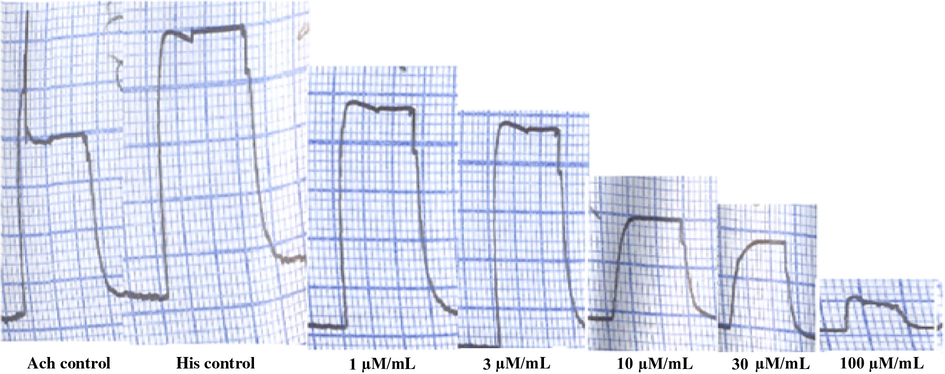
A tracing showing the inhibitory effect of 2-(4-(1-hydroxy-4-(4-(hydroxydiphenylmethyl)piperidin-1-yl)butyl)phenyl)-2-methylpropanamide.
In guinea-pig ileum, compounds A–E inhibit the histamine (1 μM) responses at dose 3–100 μM/mL (Fig. 4). Compound A: A dose of 3 μM/ml 81.67 ± 3.827%, 10 μM/ml 69.50 ± 5.920% and 30 μM/ml 47.00 ± 4.382% and complete inhibition obtained at a dose of 100 μM/ml 11.33 ± 1.783. Compound B: A dose of 3 μM/ml 65.83 ± 7.935%, 10 μM/ml 53.00 ± 3.759% and 30 μM/ml 41.67 ± 4.580% and complete inhibition obtained at a dose of 100 μM/ml 12.67 ± 2.728 (see Fig. 5).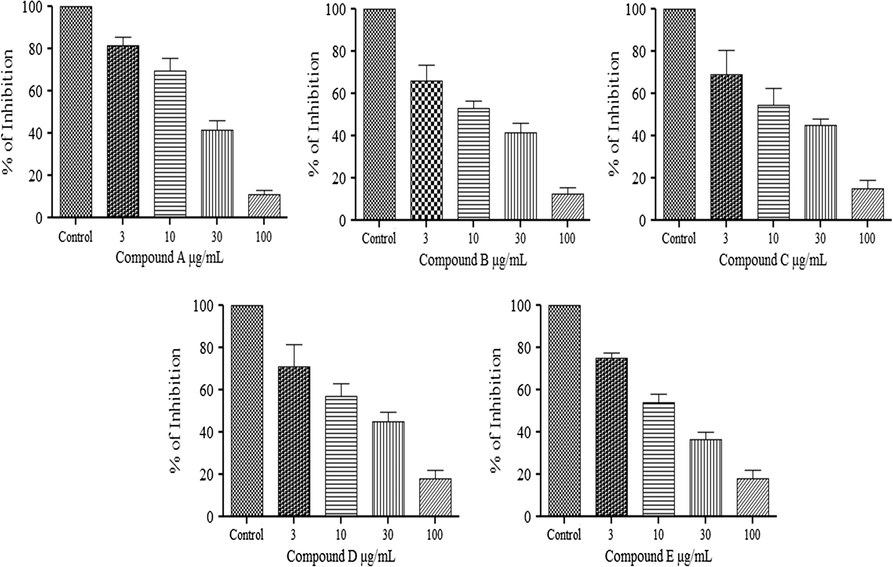
Inhibitory effect of histamine (1 μM) responses in the presence of compound A–E on the guinea-pig ileum. Value shown are mean ± S.E.M, n = 6. (∗P < 0.05; ∗∗P < 0.0001; compared with control group; One way ANOVA, Tukey’s multiple comparison).
Compound C: A dose of 3 μM/ml 69.00 ± 11.60%, 10 μM/ml 54.50 ± 8.094% and 30 μM/ml 45.00 ± 2.933% and complete inhibition obtained at a dose of 100 μM/ml 15.17 ± 3.978. Compound D: A dose of 3 μM/ml 70.83 ± 10.85%, 10 μM/ml 57.17 ± 5.700% and 30 μM/ml 45.00 ± 4.494% and complete inhibition obtained at a dose of 100 μM/ml 18.00 ± 4.258. Compound E: A dose of 3 μM/ml 74.83 ± 2.833%, 10 μM/ml 54.17 ± 3.978% and 30 μM/ml 36.83 ± 3.016% and complete inhibition obtained at a dose of 100 μM/ml 18.00 ± 4.258. And its parent compound fexofenadine cause inhibition of histamine (1 μM) responses at dose 0.000003 μM/ml 23.14 + 4.48, 0.00001 μM/ml 40.87 + 8.98, 0.00003 μM/ml 51.87 + 11.74, 0.0001 μM/ml 89.05 + 1.28 and 0.0003 μM/ml cause complete inhibition. Similar patterns of inhibition were seen with pyrilamine at dose 0.03 μM/ml 13.52 + 7.83%, 0.1 μM/ml 70.96 + 7.05%, 0.3 μM/ml 88.69 + 1.84% and the 1.0 show the complete inhibition of histamine (1 μM) responses.
3 Conclusion
The current study was the first attempt in which five derivatives (2-methylpropanamide and benzamide) of fexofenadine were synthesized, characterized by TLC, IR, UV, NMR and mass spectroscopy. The antihistaminic and anticholinergic mechanisms were tested on guinea pig ileum. The compounds A–E have antihistaminic action but not anticholinergic as shown by the tissue response.
References
- Structure determination of fexofenadine-α-cyclodextrin complex by quantitative 2D ROESY analysis and molecular mechanics studies. Magn. Reson. Chem.. 2012;50(4):299-304. Epub 2012 Mar 14. <http://www.ncbi.nlm.nih.gov/pubmed/22416034>
- [CrossRef] [Google Scholar]
- Fexofenadine hydrochloride – a new anti-histaminic drug. Isr. Med. Assoc. J.. 2001;3(3):207-209. <http://www.ima.org.il/IMAJ/ViewJournal.aspx?JournalId=142>
- [Google Scholar]
- In vitro studies with fexofenadine, a new non sedating histamine H1 receptor antagonist, on isolated human basophils. Inflamm. Res.. 2000;49(Suppl 1):S13-S14. <http://www.ncbi.nlm.nih.gov/pubmed/10864400>
- [Google Scholar]
- Synthesis and biological evaluations of enoxacin carboxamide derivatives. Arch. Pharm. Res.. 2009;32(7):967-974. <http://www.ncbi.nlm.nih.gov/pubmed/19641876>
- [CrossRef] [Google Scholar]
- Fexofenadine: a antihistaminic. Review of its practical characteristics. Rev. Med. Univ. Navarra.. 1996;43(2):93-97. <http://www.ncbi.nlm.nih.gov/pubmed/11256010>
- [Google Scholar]
- In vivo pharmacological characterisation of bilastine, a potent and selective histamine H1 receptor antagonist. Drugs R and D. 2006;7(4):219-231. <http://link.springer.com/article/10.2165/00126839-200607040-00002>
- [Google Scholar]
- Fexofenadine hydrochloride, 180 mg, exhibits equivalent efficacy to cetirizine, 10 mg, with less drowsiness in patients with moderate-to-severe seasonal allergic rhinitis. Ann. Allergy Asthma Immunol.. 2003;91(4):354-361. <http://www.ncbi.nlm.nih.gov/pubmed/14582814>
- [Google Scholar]
- Pharmacokinetics of fexofenadine: evaluation of a microdose and assessment of absolute oral bioavailability. Eur. J. Pharm. Sci.. 2010;40:125-131. <http://www.ncbi.nlm.nih.gov/pubmed/20307657>
- [Google Scholar]
- A new class of nitric oxide-releasing derivatives of cetirizine; pharmacological profile in vascular and airway smooth muscle preparations. Br. J. Pharmacol.. 2007;151:35-44. <http://onlinelibrary.wiley.com/>
- [CrossRef] [Google Scholar]
- Antihistamines in the treatment of asthma. Allergy. 1997;52(34 Suppl):55-66. <http://onlinelibrary.wiley.com/>
- [CrossRef] [Google Scholar]
- Fexofenadine Drugs. 1998;55(2):269-274. discussion 275–6. <http://www.ncbi.nlm.nih.gov/pubmed/9506246>
- Efficacy and safety profile of fexofenadine HCl: a unique therapeutic option in H1-receptor antagonist treatment. J. Allergy Clin. Immunol.. 2003;112:S69-S77. <http://download.journals.elsevierhealth.com/pdfs/journals/0091-6749/PIIS0091674903022188.pdf>
- [Google Scholar]
- Blood-brain barrier in vitro models as tools in drug discovery: assessment of the transport ranking of antihistaminic drugs. Pharmazie. 2012;67(5):432-439. <http://pharmazie.govi.de/contents_0512.htm>
- [CrossRef] [Google Scholar]
- Office of Laboratory Animal Welfare, 2002. In Public Health Service Policy on Humane Care and Use of Laboratory Animals. Washington D.C: The National Academies Press. <http://grants.nih.gov/grants/olaw/Guide-for-the-care-and-use-of-laboratory-animals.pdf>.
- Effect of intestinal first-pass hydrolysis on the oral bioavailability of an ester prodrug of fexofenadine. J. Pharm. Sci.. 2012;101(9):3264-3274. <http://www.ncbi.nlm.nih.gov/pubmed/22628163>
- [CrossRef] [Google Scholar]
- Dose proportionality and comparison of single and multiple dose pharmacokinetics of fexofenadine (MDL 16455) and its enantiomers in healthy male volunteers. Biopharm. Drug Dispos.. 1998;19(7):455-463. <http://www.ncbi.nlm.nih.gov/pubmed/9818712>
- [Google Scholar]
- Synthesis of some substituted pyrazinopyridoindoles and 3D QSAR studies along with related compounds: piperazines, piperidines, pyrazinoisoquinolines, and diphenhydramine, and its semi-rigid analogs as antihistamines H1. Bioorg. Med. Chem.. 2006;14(24):8249-8258. <http://www.sciencedirect.com/science/article/pii/S0968089606007498>
- [Google Scholar]
- Pharmacokinetics, safety and tolerability of an oral suspension of fexofenadine for children withallergic rhinitis. Allergy Asthma Proc.. 2008;29(4):380-385. <http://www.ncbi.nlm.nih.gov/pubmed/18702885>
- [CrossRef] [Google Scholar]
- Design, synthesis and pharmacological screening of a series of N1-(substituted) aryl-5,7-dimethyl-2-(substituted)pyrido(2,3-d)-pyrimidin-4(3H)-ones as potential histamine H1 receptor antagonists. J. Enzyme. Inhib. Med. Chem.. 2006;21(6):681-691. <http://informahealthcare.com/doi/pdfplus/10.1080/14756360600851104>
- [Google Scholar]
- Identification of anti-inflammatory and other biological activities of 3-carboxamide 3-carbohydrazide and ester derivatives of gatifloxacin. Chem. Cent. J.. 2013;14(1):6. 7. <http://journal.chemistrycentral.com/content/7/1/6>
- [CrossRef] [Google Scholar]
- Hepatobiliary disposition of a drug/metabolite pair: comprehensive pharmacokinetic modeling in sandwich-cultured rat hepatocytes. J. Pharmacol. Exp. Ther.. 2006;318(2):881-889. <https://www.pubmed.com/pubmed/?term=%20Hepatobiliary%20disposition%20of%20a%20drug/metabolite%20pair:%20Comprehensive%20pharmacokinetic%20modeling%20in%20sandwich-cultured%20rat%20hepatocytes>
- [Google Scholar]
- Willard, H.H., Mervitt, L.L., Jr., Dean J.A., Settle, F.A., 1999. Instrumental Methods of Analysis, Wadsworth Publishing Company Belmont California., seventh ed., 287. <http://www.gobookee.org/instrumental-methods-of-analysis-by-willard/>.
- Zhang, M.Q., Caldirola, P., Timmerman, H., 1994. Chiral manipulation of Drug Selectivity: Studies on a Series of Terfenadine-Derived Dual Antagonists on H1-Receptors and Calcium Channels. Agents Actions. 41, Spec No., C140-2. <http://www.ncbi.nlm.nih.gov/pubmed/7976802>.







