Translate this page into:
Antihypertensive activity of indole and indazole analogues: A review
⁎Corresponding authors. lijia33233@jlu.edu.cn (Jia Li), ylq666666@jlu.edu.cn (Li-Quan Yin)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Heterocyclic compounds occupy an important position in chemistry because of their wide range of uses in drug design, photochemistry, agrochemicals, and other fields. Indole and indazole scaffolds are available from natural and synthetic sources, and molecules containing these scaffolds have been shown to have various biological effects, including anti-inflammatory, antibacterial, antiviral, antifungal, analgesic, anticancer, antioxidant, anticonvulsant, antidepressant, and antihypertensive activities. Indole and indazole molecules bind to receptors with high affinity, and thus are useful for the study of bioactive compounds involved in multiple pathways. In this review, we highlight the antihypertensive activity and the mechanisms of action of indole and indazole derivatives. In addition, structure–activity relationship studies of the antihypertensive effect are presented.
Keywords
Indole
Indazole
Synthetic approach
Antihypertensive activity
Structure–activity relationships
- ACE
-
angiotensin converting enzyme
- α1-AR
-
α1-adrenergic receptors
- MR
-
mineralocorticoid receptor
- HR
-
heart rate
- SHR
-
spontaneously hypertensive rats
- RHR
-
renal hypertensive rats
- SBP
-
systolic blood pressure
- MBP
-
mean blood pressure
- SAR
-
structure–activity relationships
- IOP
-
intraocular pressure
- α-ARs
-
α-adrenoceptors
- WHO
-
world health organization
- ED50
-
median effective dose
- LD50
-
median lethal dose
- MAP
-
mean arterial pressure
- ECG
-
electrocardiogram
- 5-HIAA
-
5-hydroxyindoleaceticacid
- ODQ
-
1H-[1,2,4]oxadiazolo [4,3-a]quinoxalin-1-one
- CDI
-
N,N-carbonyldiimidazole
- DIC
-
disseminated intravascular coagulation
- RAS
-
renin angiotensin system
- DPTS
-
post-traumatic stress disorder
- Ang-II
-
angiotensin II
- p.o
-
orally administered
- i.v
-
intravenous
- DMF
-
N,N-dimethylformamide
- AT1
-
angiotensin II type 1
- THF
-
tetrahydrofuran
- L-NAME
-
NG-Nitro-L-arginine Methyl Ester
Abbreviations
1 Introduction
Hypertension is often referred to as the “silent killer” because most people are unaware that they are affected by hypertension. Hypertension is regarded as one of the most important risk factors for cardiovascular illnesses, affects approximately one billion people globally, and is closely related to congestive heart exhaustion, stroke, and coronary heart disease. As per WHO hypertension report 2019, about 1.13 billion people globally have hypertension (Britannica encyclopedia, 2021, Bai et al., 2018, Gilyarevskiy, 2017, Höcht et al., 2019, Kishi and Fujii, 2019, Kucan et al., 2018, Perez et al., 2015). Reducing hypertension can decrease cardiovascular illness complications and mortality. Recently, the adjustment of vasomotor tension and the theory of central antihypertensive medicines have been widely investigated (Burnier et al., 2019, Bialy et al., 2018, Garkani-Nejad and Poshteh-Shirani, 2013, Tamargo et al., 2015). The vasodilating effects of the third generation β-adrenolytics are produced via diverse mechanisms, for instance, α1-blockade, α2-adrenoceptor agonistic action, β2-agonistic action, NO release, antioxidant action, and calcium (Ca2+) blockade, Krasavin, 2015, Zeng et al., 2016, Liu et al., 2021). Despite the availability of many antihypertensive drugs, it is often necessary to combine several of these drugs for the effective treatment of hypertension.
Nitrogen-containing heterocycles are important pharmacological structures that are present in many commercial medicines. Heterocyclic chemistry is a branch of organic chemistry that plays a pivotal role in the construction of synthetic molecules. Indole and indazole are considered to be important heterocyclic moieties because of the diverse biological activities of compounds containing these structures, and are useful molecules in drug discovery. Indole and indazole are aromatic heterocyclic organic compounds, with the chemical formulae C8H7N and C7H6N2, respectively, which have a bicyclic structure consisting of a benzene ring fused with a pyrrole or pyrazole ring, respectively. Indole and indazole derivatives can have biological activity, for example, anti-malarial, anti-HIV, antifungal, antibacterial, anti-convulsion, anticancer, anti-inflammatory, antihypertensive, and anti-diabetic activity (Aingh et al., 2017, Ates-Alagoz et al., 2005, Ali et al., 2013, Cerecetto et al., 2005, Chu et al., 2017, Dong et al., 2018, Dousson et al., 2016, El-Ayoubi et al., 2002, Garg et al., 2019, Stec et al., 2016, Zhang et al., 2017, Pérez-Villanueva et al., 2018, Sun et al., 2017, Kim et al., 2013, Zhao et al., 2013, Praveen et al., 2011). Thus, the discovery of new indolyl and indazolyl derivatives is of interest.
To date, few reviews have reported the synthesis, medicinal use, and SAR of indole and indazole derivatives in terms of antihypertensive activity. Therefore, in this review, we summarize the various synthetic routes for indole- and indazole-based analogues with a focus on analogues with antihypertensive activity and present information from SAR studies. We believe that this review will provide substantial guidance for further research on drugs to alleviate hypertension. Information was compiled on the synthesis, and antihypertensive activity from in vitro and in vivo studies, of various indole and indazole derivatives from various research publications obtained from searching scientific databases (e.g., PubMed and ScienceDirect) focused on the period 1980–2021.
2 Synthetic strategies and SAR studies of the antihypertensive activity
Indole analogues are known as “privileged structures” that can bind to a variety of high affinity receptors. Indole and indazole derivatives have been demonstrated to have antihypertensive effects (Kornicka et al., 2017, Klioze and Ehrgott, 1979, Lavrado et al., 2010, Ozaki, 1989, Shi et al., 1992, Song et al., 2000, Bunch et al., 2004, Horton et al., 2013, Rajan et al., 2018, Singh, T.P. and Singh, O.M, 2018). This review describes the synthesis and in vivo, and in vitro studies of various indole and indazole derivatives.
2.1 Analogues containing an indole skeleton
Kreighbaum et al. (Kreighbaum et al., 1980) a adopted a three-step process to synthesize 25 phenoxypropanolamine-bearing indole analogues. First, epichlorohydrin (2) reacted with a substituted phenol in piperidine (1) to obtain the intermediate (3), which was transformed to an epoxide (4) under conditions of NaOH and tetrahydrofuran. Then, 2-nitropropane and a substituted gramine (5) were refluxed with KOH to provide 3-(2-methyl-2-nitropropyl)-1H-indole (6) and the amine derivatives (indolyl tert-butylamines) (7) were obtained using Raney nickel and hydrazine hydrate. Ultimately, the indolyl tert-butylamine was refluxed with the epoxide (4) to form the final target compound (8), as shown in Scheme 1. The generated analogues were assessed for antihypertensive activity in vivo from the vasodilating effect at a dosage of 100 mL·kg−1 orally administered (p.o.) and 2 mL·kg−1 intravenous injection (i.v.) in rats with ganglion block and SHR, respectively. Compound 8a displayed a remarkable antihypertensive activity in SHR and compounds 8b and 8c substituted with 2-methyl and 2-cyano groups, respectively, on the benzene ring of the phenylpropanolamine moiety, and indoles with 5-methoxy substitution exhibited better vasodilation properties than the other synthesized compounds (Fig. 1).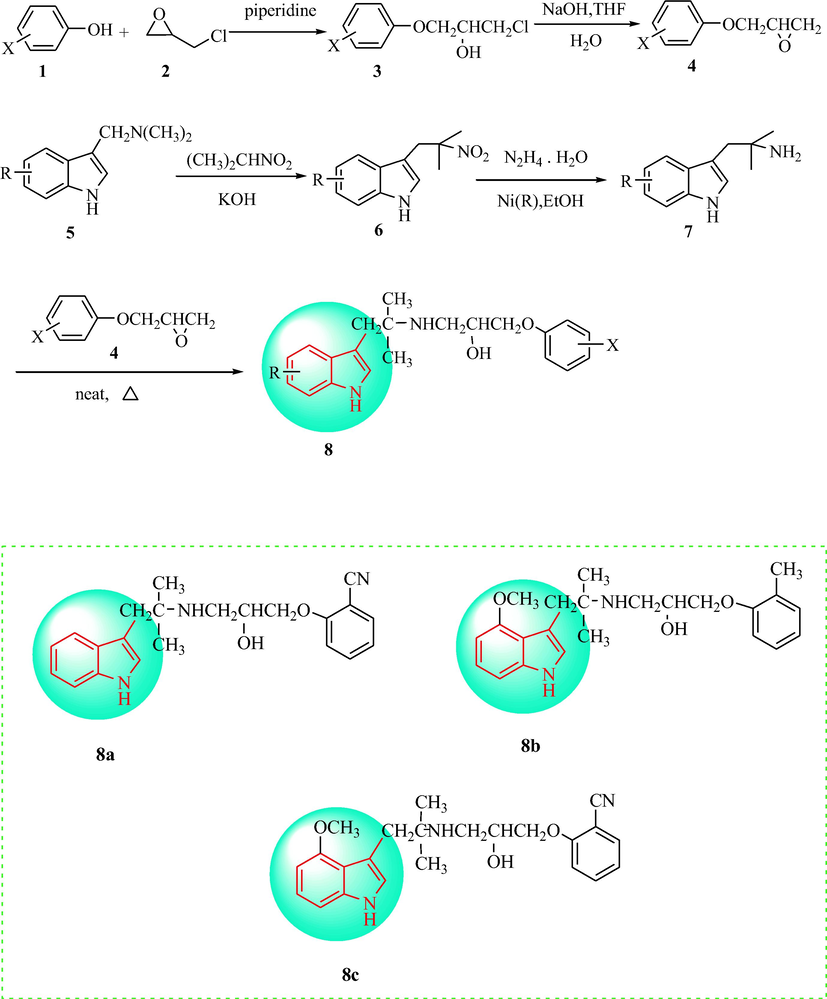
Synthesis of 25-indole derivatives of phenoxypropanolamines.
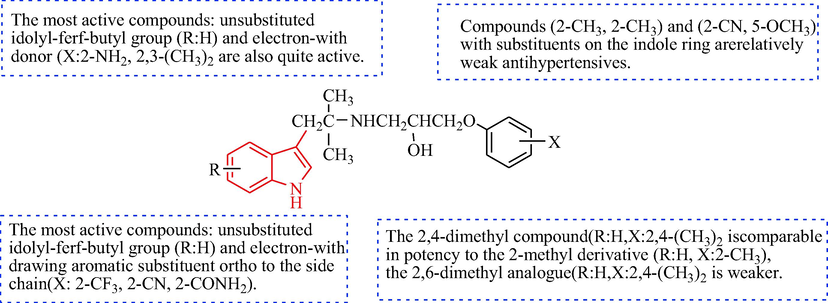
The SAR of the antihypertensive activity of phenoxypropanolamine-bearing indole analogues.
Monge Vega et al. (Monge Vega et al., 1982) prepared a series of compounds, compounds 12a-12d and 13a-13d in Fig, 1, and assessed the antihypertensive properties. Compounds 9 were reacted with hydrazine hydrate to undergo cyclization to form compounds 10. Then, under basic conditions, P2S5 was used to transform the keto group into a thioketone (11). And then, the derivatives (12) were obtained with hydrazine hydrate. Finally, the derivatives 12 underwent cyclization with substituted formaldehyde form the target derivatives 13 as shown in Scheme 2. The changes in the arterial pressure in SHR were measured after intraperitoneal injection (i.p) of 1, 2, and 5 mL·kg−1 and oral administration of 2.5 mL·kg−1 of the synthesized compounds. Administration of compounds 12a and 12b resulted in a marked decrease in the arterial pressure. The most active compounds 12c and 12d were assessed in desoxycorticosterone-saline rats (2 mL·kg−1 and 5 mL·kg−1, i.p), the results showed that12c and 12d remarkably decreased the arterial blood pressure. Compounds 13 exhibiting antihypertensive effects do not necessarily have a hydrazine group, indicating that the hydrazine is not necessary for antihypertensive activity (Fig. 1).![Synthetic route of pyridazino[4,5-b]indole derivatives.](/content/184/2022/15/5/img/10.1016_j.arabjc.2022.103756-fig3.png)
Synthetic route of pyridazino[4,5-b]indole derivatives.
Kim et al. produced compounds 17 and assessed their antihypertensive activity (Kim et al., 1983). Compounds 14 were acylated with methacryloyl chloride to obtain substituted ethyl 1-acryloyl-indoline-2-carboxylated derivatives 15, which were hydrolyzed to give 1-acryloyl-indoline-2-carboxylic acids (16) under basic conditions. The subsequent treatment of the 1-acryloyl indoline-2-carboxylic acids (16) with thiobenzoic acid provided the substituted 1-(3-mercaptopropanoyl)indoline-2-carboxylic acid derivatives (17) (predominant product 70%), as shown in Scheme 3. Among the compounds 17, compound 17b (S, S) displayed the greatest activity with IC50 = 3.7 × l0-9 M, which is three times the activity of captopril. Compared with captopril, the hydrophobicity of 17b (S, S) was increased, which indicated that there is a hydrophobic group in the active site of ACE. Compound 17b (S, S) showed oral antihypertensive activity in spontaneously hypertensive rats that was 27 times greater than captopril. The benzoyl 17a (S, S) was 24 times more potent than captopril. The considerable antihypertensive activity shown by 17a and 17b indicated that as well as peripheral ACE inhibitory activity, other antihypertensive mechanisms may also be involved (Fig. 2).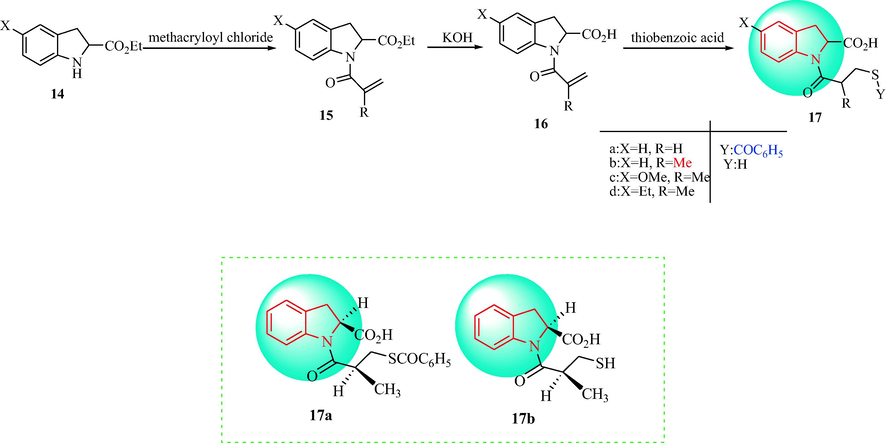
Synthesis of 1-(3-mercaptopropanoyl)indoline-2-carboxylic acids.
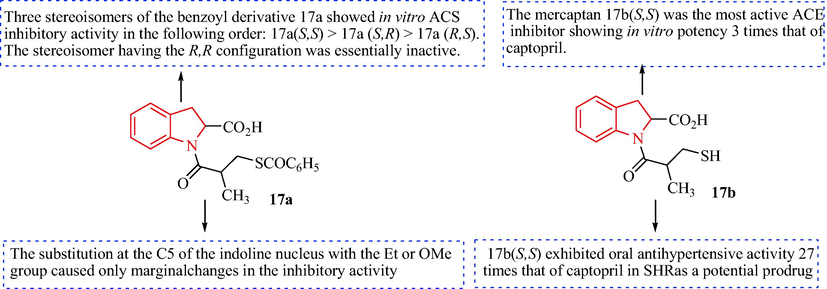
The SAR of the antihypertensive activity of indoline-2-carboxylic acid derivatives.
In 1986, Asselin et al. (Asselin et al., 1986) prepared and appraised the antihypertensive activity of compounds 25, as shown in Scheme 4. First, the condensation of l-acetylindoline (18) with chloroacetyl chloride provided a 93% yield of the 5-chloroacetyl compound 19 in 1,2-dichloroethane. Then, the 5-chloroacetyl compound (19) was reacted with dibenzylamine to obtain a dibenzylamino ketone compound 20, which was reduced with NaBH4 to obtain an amino ethanol compound 21. Further reaction with cuprous potassium cyanide and cyanide gave the nitrile compound 22. The nitrile compound 22 was reacted with NaOH to provide the 7-cyanoindoline compound 23. Hydrolysis of the cyano group to prepare the carboxamide group (23) was effected by mixing NaOH in hydrogen peroxide. The indole-7-carboxamide (24) was obtained using manganese (IV) oxide. The compounds 24 were reacted under conditions of sodium cyanoborohydride to give the target compounds 25a and 25b. Compounds 25a and 25b could reduce blood pressure in SHR with an ED50 value of 5 mL·kg−1 (p.o) (Table 1). Isolated tissue studies of 25a and 25b indicated that these compounds were non-selective β-adrenergic receptor antagonists. Additionally, 25a and 25b showed significant β-adrenoceptor intrinsic sympathomimetic effects. The β-adrenergic receptor antagonist and agonist actions of 25a and 25b were generated though the formation of a hydrogen bond with the receptor.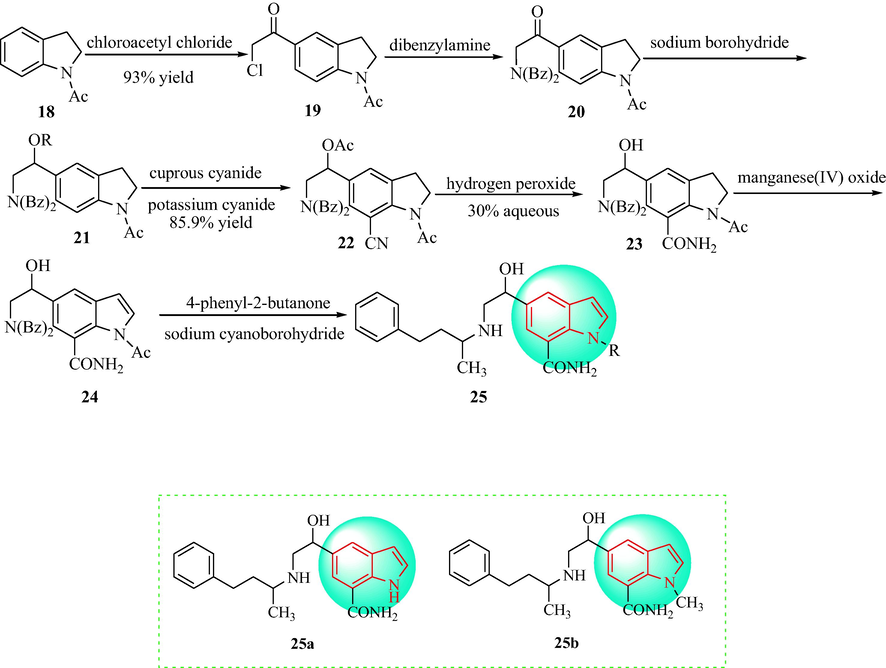
Synthetic route of 1H-indole-7-carboxamide 25a and 25b.
Compound
Receptor pA2 valuesa
β1
β2
α1
α2
25a
7.4 ± 0.1b
7.3 ± 0.2
6.6 ± 0.1
5.4 ± 0.2
25b
<6.0
<5.0
6.2 ± 0.04
<5.0
β1-ISAc
β2-ISAd
% changee
concn M
% relaxation
concn M
25a
17.0 ± 2.4f
1 × 10-6
89.0 ± 6.1 g
3 × 10-7
25b
2.7 ± 1.4
1 × 10-6
34.6 ± 5.3
1 × 10-5
In 1987, Monge et al. (Monge et al., 1987) designed and prepared 5H-pyridazino[4,6-b]indole derivatives 31, as illustrated in Scheme 5. The target products were synthesized starting from derivatives 26 that were reacted under conditions of POCl3 and DMF to give derivatives 27, which is the Vilsmeier-Haack reaction. Derivatives 28 were obtained from derivatives 27 treated with 90 % hydrazine hydrate, followed by reaction with phosphorus pentasulfide and pyridine to provide derivatives 29. The derivatives 29 were mixed and reacted with hydrazine hydrate to afford derivatives 30. Finally, derivatives 31 were obtained by reaction of derivatives 28 with boiling acetic acid or formic acid, and nitrosation (NaNO2/H+). Table 2 shows the effect of i.v. administration at a dosage of 1 mg·kg−1 i.p of compounds 30a-30c on the systolic blood pressure in rats over time. The compounds decreased the blood pressure in the order 30c > hydralazine = 30a > 30b. The increasing order of stimulation was observed: hydralazine = 30a = 30b ≪ 30c and at a higher dose 30b = 30a ≪ 30c in a dose of 1 mg·kg−1 i.p. But derivatives 31 displayed weakly effects. And then, the LD50 values for 30a, 30b, 30c are about 2.2–2.5 times less toxic than for hydralazine. The acute toxicity in mice for 30a is 225 ± 12 mg·kg−1. All the above-reported results suggest that 30a is a potentially promising antihypertensive agent. (aIv (vehicle normal saline) on the jugular vein. b(*) no significant values (p ≦ 5%).![Synthesis of 5H-pyridazino[4,6-b]indole derivatives.](/content/184/2022/15/5/img/10.1016_j.arabjc.2022.103756-fig7.png)
Synthesis of 5H-pyridazino[4,6-b]indole derivatives.
Compound
Percentage falls in systolic blood pressure, directly measured on carotida
Dose mg/kg
Control
Minutes
5
10
15
30
45
60
75
90
120
180
240
30a
1
250
30
-
36
40
-
60
54
-
40
36
30
30b
1
220
16
18
20
32
36
41
32
25
*b
*
*
30c
1
225
44
60
56
56
67
56
56
*
*
*
Hydralazine
1
250
40
48
48
52
60
20
*
*
250
40
48
In 1990, Squadrito et al. (Squadrito et al., 1990) explored the effect of compound 32 (Fig. 3) on the systolic blood pressure in rats with spontaneous hypertension and Grollman hypertension. Administration of 32 markedly reduced the systolic blood pressure in model of hypertension at a dosage of 100 mL·kg−1 by p.o. gavage for 10 days. In addition, 32 partially inhibited the development of hypertension, when administered daily to 5-week-old SHR for 7 weeks. Administration of 32 also enhanced the levels of tryptophan and 5-HIAA in the diencephalon and cortex. The brain stem serotonin content in SHR was also increased by treatment with 32. These results indicated that 32 can reduce the blood pressure in dissimilar rat models of hypertension.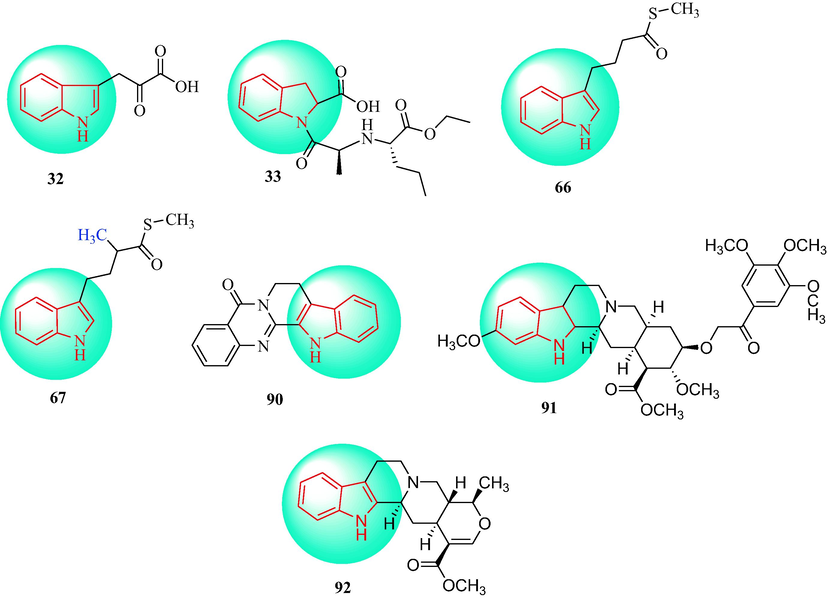
Chemical structure of indole derivatives 32, 33, 66, 67, 90–92.
In 1991, Todd and Fitton (Todd, Fitton, 1991) reported that perindopril (33), a prodrug, was a long-acting ACE inhibitor (Fig. 3). Compound 33 is a lipophilic small molecule with an indole ring. 33 is used to control blood pressure in patients with mild to moderate the basic hypertension, at dose of 4–8 mg once daily. In addition, the study showed that 33 may be a useful alternative to other ACE inhibitors for the treatment of all types of hypertension (Alfakih and Hall, 2006, Ghiadoni, 2011, De leeuw, 2011).
In 2001, EI-Gendy et al. (EI-Gendy et al., 2001) synthesized two series of Mannich bases derivatives 34 and 38 and tested the antihypertensive effects (Scheme 6). 2-Ethoxycarbonylindoles 26 were reacted with formaldehyde and the appropriate secondary amines to give derivatives 34 by heating under reflux in ethanol containing drops of acetic acid. The formylation of derivatives 26 with POCl3 and DMF gave 2-ethoxycarbonyl-3-formylindoles (35). The alkylation of derivatives 35 with ethyl iodide was carried out using benzyltriethylammonium chloride as catalyst to give 2-ethoxycarbonyl-l-ethyl-3-formylindoles (36) in 50% NaOH/benzene. Heating of derivatives 36 with hydrazine hydrate provided 5H-pyridazino[4,5-b]indoles (37). Refluxing of 37 with formalin and the appropriate secondary amine supplied the N-Mannich bases (38) in ethanol. The synthesized compounds were administered i.p. at a dosage of 40 mg·kg−1. Blood pressure measurements were taken at 10, 30, 60, 120, 180, and 240 min after injection (Table 3). 34a, 34b, 34c and 34d significantly reduced the blood pressure. Nevertheless, derivatives 38 did not display diminished the blood pressure. Three anaesthetized dogs weighing 12–16 kg were injected with 35 mg·kg−1 pentobarbital sodium and administered compound 34b. Compound 34b at a dose of 40 mg·kg−1, significantly decreased the systolic blood pressure from 70 ± 10 to 120 ± 10 mmHg with a LD50 value of 180 mg·kg−1, and displayed antihypertensive activity. *Indicates statistically significant changes with respect to the vehicle treated control group, P < 0.05.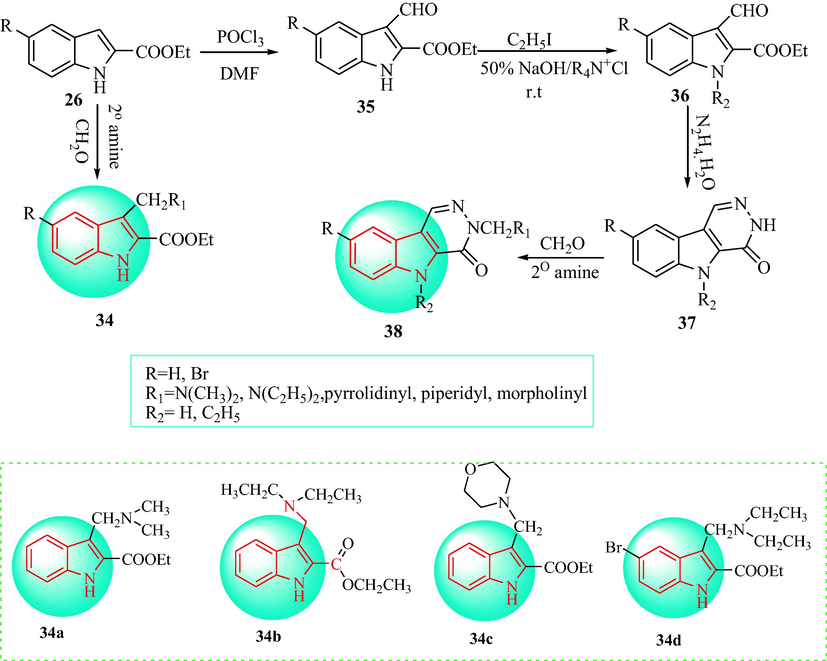
Synthesis of indole analogues 34 and 38.
Compound
Control mean ± SEM
Systolic arterial blood pressure (mmHg)
10 min
30 min
60 min
120 min
180 min
240 min
34a
119 ± 8
80 ± 11*
72 ± 11*
75 ± 12*
75 ± 12*
84 ± 10*
85 ± 11*
34b
138 ± 4
63 ± 8*
69 ± 9*
61 ± 8*
69 ± 8*
66 ± 9*
73 ± 10*
34c
137 ± 4
90 ± 11*
99 ± 10*
86 ± 11*
84 ± 10*
92 ± 12*
88 ± 9*
34d
116 ± 8
87 ± 9*
80 ± 8*
83 ± 9*
83 ± 10*
91 ± 10*
92 ± 8*
vehicle
117 ± 1
115 ± 3
117 ± 2
115 ± 2
116 ± 2
114 ± 2
117 ± 2
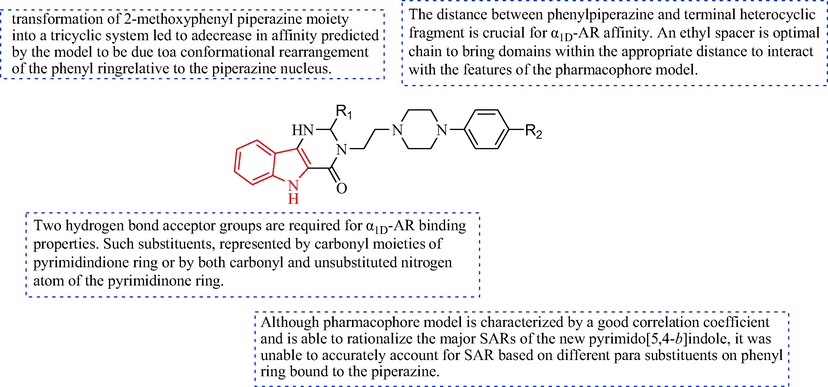
In 2003, the derivatives 44 were synthesized, as shown in Scheme 7. The starting aminoester (39) was reacted with triethylorthoesters to afford the intermediate iminoethers (40). The intermediate tricyclic alcohols (41) were obtained from the iminoethers (40) with 3-amino-1-propanol or 2-ethanolamine. By reaction with SOCl2, the tricyclic alcohols (41) were transformed into alkyl chloride hydrochlorides (42). The final products (44) were obtained at 140 °C with 1-(2-ethoxy-phenyl)piperazine or 1-(2-methoxyphenyl)piperazine (43). The target compounds 44 and 46 were examined in binding experiments with three cloned human α1-AR subtypes and the antihypertensive effects were evaluated (Romeo et al., 2003). Most of the compounds displayed selective affinity. Compounds 46a and 46b displayed moderate affinities for α1D-AR [(pK i) 7.45 and 7.70, respectively] with no measurable affinity for α1A-AR, α1B-AR subtypes, when tested for binding experiments on the 3 human cloned. In addition, among compounds 44a-44 g, 44a and 44b showed the highest affinity (pKi = 9.14 and 9.39), a slight selectivity for the α1D-AR (Table 4). The SAR of based on fitting a pharmacophore model for α1D-AR of the antihypertensive is discussed in Fig. 4 (Romeo et al., 2014).![Synthesis of pyrimido[5,4-b]indol-4(5H)-one with piperazin.](/content/184/2022/15/5/img/10.1016_j.arabjc.2022.103756-fig10.png)
Synthesis of pyrimido[5,4-b]indol-4(5H)-one with piperazin.
Compound
pKi (M) or [% of inhibition at 1 µM]a
α1A-AR
α1B-AR
α1D-ARb
44ac
8.85 ± 0.08
7.86 ± 0.11
9.14 ± 0.08 (9.46)
44bd
8.52 ± 0.03
7.68 ± 0.05
9.39 ± 0.19 (9.38)
44 cc
7.31 ± 0.13
6.61 ± 0.05
8.16 ± 0.10 (8.43)
44dc
7.78 ± 0.17
6.79 ± 0.04
7.79 ± 0.12 (7.74)
44e
8.31 ± 0.14
7.57 ± 0.08
8.59 ± 0.16
44fc
7.97 ± 0.11
7.56 ± 0.12
7.90 ± 0.11 (7.82)
44gd
8.01 ± 0.03
7.21 ± 0.07
8.22 ± 0.12 (7.70)
46ad
4.89 ± 0.18
5.02 ± 0.13
7.45 ± 0.14 (5.36)
46bc
[8 ± 3]
[2 ± 4]
7.70 ± 0.08 (6.77)
46 cc
[26 ± 4]
[4 ± 3]
7.22 ± 0.014 (7.43)
46dc
[19 ± 6]
[6 ± 5]
6.61 ± 0.07 (6.96)
46ec
[7 ± 2]
[18 ± 5]
[3 ± 2] (6.74)
The SAR of based on fitting a pharmacophore model for α1D-AR of the antihypertensive.
In 2006, a series of new pyrimido[5,4-b]indole and [1]benzothieno[3,2-d]pyrimidine derivatives was prepared, as shown in Scheme 8. 3-Amino-2-ethoxycarbonylbenzo[b]thiophene (47) was reacted with 1,1,1-triethoxyethane to provide the ethyl-3-(1-ethoxyethylideneamino)benzo[b]thiophene-2-carboxylate (48). Then, derivatives 49 was obtained with 2-ethanolamine, in which was converted to derivatives 50 in thionyl chloride and toluene. Finally, the target compounds (51) were obtained with 1-(2-methoxyphenyl)piperazine (50) by heating at 140 °C. The synthesized compounds were assessed in binding experiments using human α1-AR, α1B-AR, and α1D-AR subtypes expressed in HEK293 cells with [125I] BE2254 as a radioligand (Romeo et al., 2006). Among them, 51b with 3,4-disubstituted chloro groups showed modest affinity. Compound 51a with a 2-methoxy and a 5-chloro group showed high affinity for the α1D-AR subtype (pKi = 9.78). The 2-methoxy derivative 51c exhibited high affinity for the α1D-AR subtype (pKi = 9.40). The effects of compounds 51a-51d were investigated in α1-AR subtypes from isolated rat spleen (α1B-AR), prostate vas deferens (α1A-AR), and thoracic aorta (α1D-AR) (Table 5). In addition, compounds 51a and 51c displayed high affinity and a slight selectivity for the α1D-AR in binding experiments in human cloned receptors. The SAR of indole and pyrimidine derivatives for α1D-AR of the antihypertensive is discussed in Fig. 5.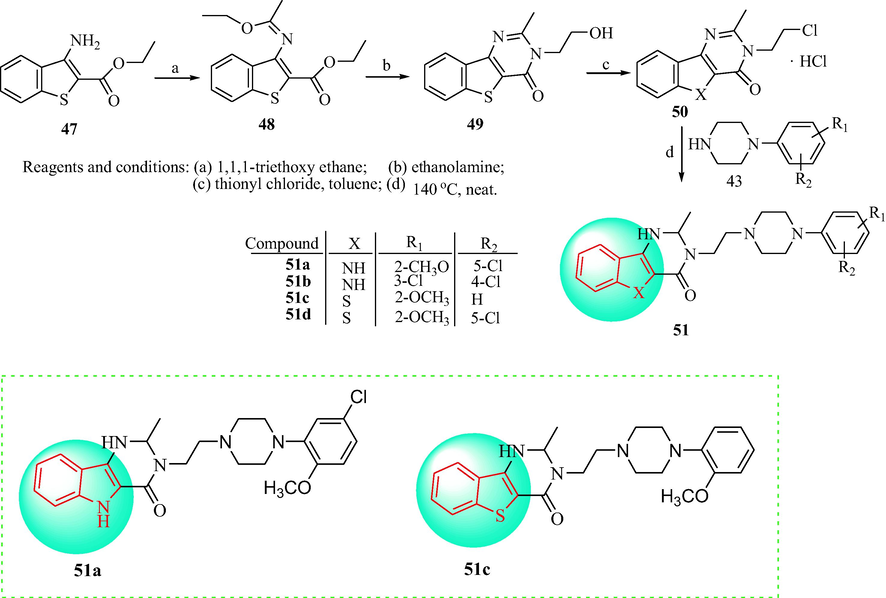
Synthetic route of indole derivatives 51.
Compounds
pKia (M)
α1A-AR
α1B-AR
α1D-AR
51a
8.88 ± 0.04
8.35 ± 0.04
9.78 ± 0.03
51b
7.52 ± 0.13
7.08 ± 0.07
8.13 ± 0.05
51c
8.83 ± 0.03
7.76 ± 0.03
9.40 ± 0.05
51d
8.46 ± 0.05
7.76 ± 0.03
9.08 ± 0.05
pKbb(M)
51a
7.60 ± 0.19
8.68 ± 0.07
8.39 ± 0.07
51b
8.65 ± 0.09
7.63 ± 0.14
8.98 ± 0.01
51c
7.52 ± 0.14
8.91 ± 0.09
7.57 ± 0.06
![The SAR of pyrimido[5,4-b]indole and [1]benzothieno[3,2-d]pyrimidine for α1D-AR.](/content/184/2022/15/5/img/10.1016_j.arabjc.2022.103756-fig13.png)
The SAR of pyrimido[5,4-b]indole and [1]benzothieno[3,2-d]pyrimidine for α1D-AR.
Bell et al. (Bell et al., 2007) synthesized a new compound 57. Compound 57 was made in a five-step synthesis, as shown in Scheme 9. Benzyl-1H-indol-7-yl-carbamate (53) was made by hydrogenation of the commercially available 52 followed by reaction with BnCO2Cl to give the protected amino indole. Then, under acidic conditions, 55 was condensed with 53 to obtain 54. Finally, after deprotection, chiral resolution of the obtained aniline 56, mesylation, and recrystallization, the product 57 was obtained. Compound 57 was shown to be an effective, selective, and orally active MR antagonist. Compound 32 was a high affinity (hMR Ki) 0.494 (0.23 nM), and functional antagonist (hMR Kb) 19 (12.8 nM), which had moderate efficacy in vivo. In salt-containing, mononephrectomized in Sprague–Dawley rats, after 14 days (p.o., qd) administration of 57 at a dose of 3 mpk, resulted in a decrease in blood pressure, which was further decreased to 1 at 30 mpk. Interestingly, addition of the ortho-F in 57 resulted in a remarkable effect (Bell et al., 2004).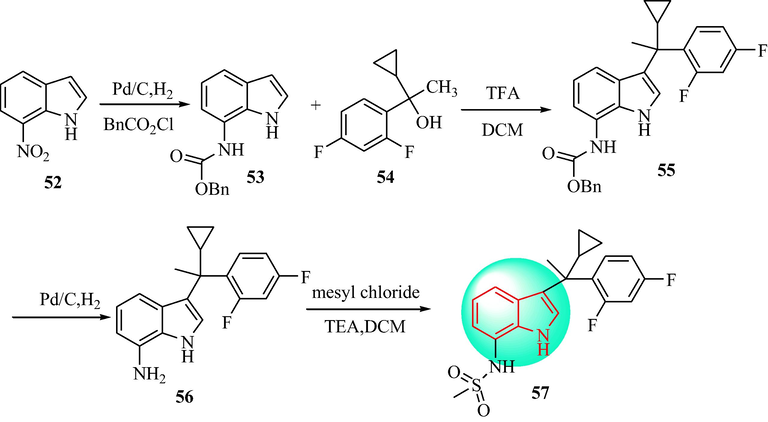
Synthetic route of compound 57.
Zhou et al. (Zhou et al., 2010) reported the compounds rhynchophylline (58) and isorhynchophylline (59) from the Chinese herb Uncaria (Fig. 6). Compound 58 displayed antihypertensive effects, including a reduction in blood pressure with eventually, a return to normal blood pressure (Zhang et al., 1978, Sun et al., 1996, Song et al., 2000). Compound 59 at a dosage of 10 or 20 mg·kg−1 by the duodenum resulted in hypotensive activity at 10 and 20 min after administration, and the effectiveness continued for at least 3 h. Compound 59 at doses from 10 to 20 mg·kg−1 (i.v.) decreased the blood pressure of hypertensive rats and the cardiac rate of anesthetized dogs (Zhou, J.Y. and Zhou, S.W., 2012).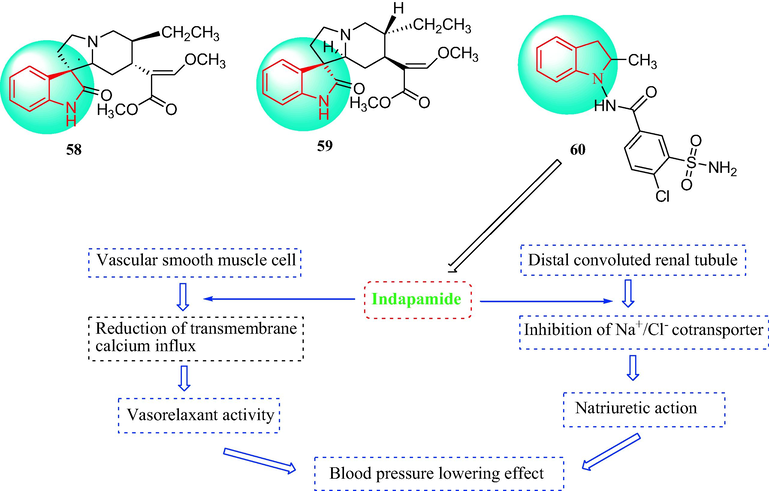
The chemical structures of indole derivatives 58–60 and the mechanisms of 60.
Indapamide (compound 60) contains an indole ring and is a sulfonamide diuretic (Fig. 6). Experimental data showed that 60 had a vasodilating action. Clinical investigations have shown that straightforward vascular actions contributed to the antihypertensive effects of 60 (Neglia et al., 2011, Ambrosioni et al., 1998). Compound 60 is a strongly lipophilic molecule that can reduce trans-membrane calcium influx as well as lead to vascular dilation. Therefore, several of the hypotensive actions of 60 may be concerned with calcium antagonist-like activity (Waeber et al., 2012).
In 2014, Zhu et al. (Zhu et al., 2014) synthesized a sequence of indole derivatives 65 (Scheme 10). The derivatives 63 were obtained after reaction of derivatives 61 with compound 62 in the presence of K2CO3, and hydrolysis with aqueous NaOH. The derivatives 63 were reacted with 2-fluorobenzonitrile and K2CO3 to give derivatives 64. The final target compounds (65) were acquired after hydrolysis of derivatives 64 in NaOH. The in vitro effects on angiotensin II, as well as in vivo antihypertensive effects, were assessed for indole derivatives 65 (Table 6). Radioligand binding assays indicated that 65c showed affinity for the angiotensin II type 1 receptor (IC50 = 1.03 ± 0.26 nM). In SHR and renal hypertensive rats, administration of 65c led to a prominent reduction in the mean blood pressure in a dose-dependent manner. The best responses were reductions in the mean blood pressure of 30 and 41 mmHg in the 5 and 10 mg·kg−1 (p.o.) groups, respectively, and the remarkable antihypertensive activity continued for>24 h. In addition, 65c was more effective antihypertensive agent compared with losartan. Accordingly, 65c may be a potentially useful antihypertensive drug. The SAR of 5-nitro benzimidazole with 1,4-disubsituted or 1,5-disubsituted indole derivatives of the antihypertensive is discussed in Fig. 7.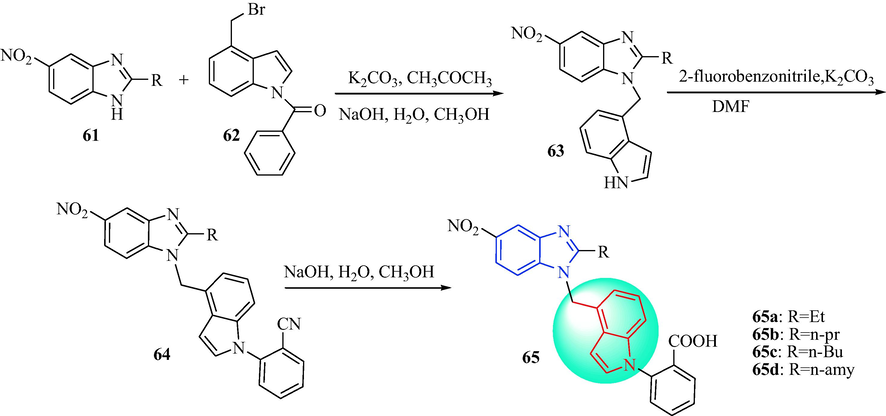
Synthetic route of derivatives 65.
Compounds
IC50 ± SEM (nM)
Ki(nM)
65a
4.12 ± 1.15
3.04 ± 1.17
65b
5.43 ± 0.27
4.23 ± 0.47
65c
1.03 ± 0.26
0.97 ± 0.43
65d
15.74 ± 0.32
13.42 ± 2.78
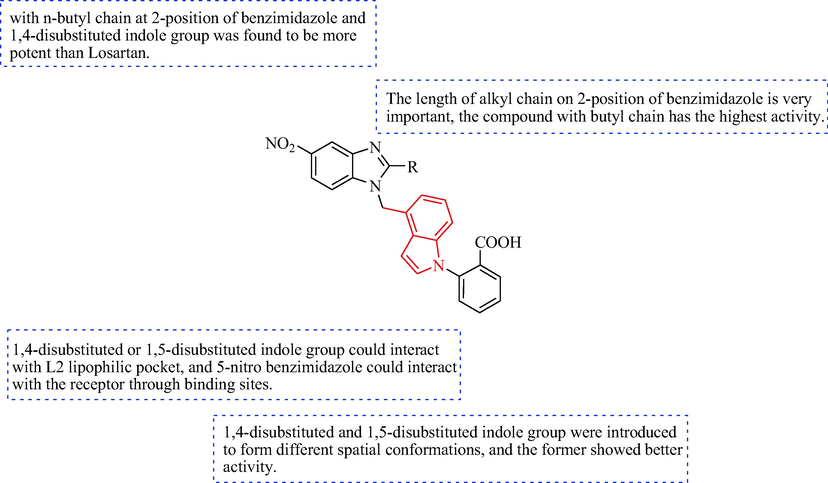
The SAR of 5-nitro benzimidazole with indole derivatives of the antihypertensive.
In 2014, the indole sulfur-containing alkaloids, N-demethylglypetelotine (66) and glypetelotine (67) (Fig. 3), were extracted from the foliage of Glycosmis petelotii. The ability of the two compounds to relax aortic rings, and the effect on the L-type Ba2+ current [IBa(L)] in a single muscle cell isolated from the tail artery were evaluated. Compounds 66 and 67 reduced phenylephrine-triggered shrinkage of the aortic ring (IC50 = 50 and 20 μM, respectively). In addition, 66 and 67 did not affect calcium ion delivery from the sarcoplasmic reticulum induced by phenylephrine. The antispasmodic effect of 67 decreased with membrane depolarization. At K+ concentrations of 60 mM, 66 and 67 reduced the shrinkage caused by the accumulation of Ca2+ in a concentration-dependent manner; the inhibition was inversely proportional to the Ca2+ concentration. Compound 67 reduced the IBa(L) value, and impacted the IBa(L) kinetics. Therefore, 67 was determined to be new type of vasodilator that can antagonize L-type Ca2+ channels (Cuong et al., 2014).
In 2015, Zhu and colleagues (Zhu et al., 2015) designed an analogue of indoloquinolizidine that had antihypertensive activity. The synthetic route is shown in Scheme 11. Condensation between compound 68 in the presence of DIC and DPTS gave compound 69. Removal of the Boc group of compound 69 under acidic conditions resulted in the target 70. Compound 70 had a significant influence on the contractile response of thoracic aorta rings in male SD rats (IC50 = 1.129 × 10-9) and reduced the SBP and HR of SHR. Mechanism studies indicated that 70 induced vasodilation in endothelium-dependent and independent manners. L-NAME (1 × 10-6 mol∙L-1) and ODQ (1 × 10-6 mol∙L-1) inhibited the relaxation of the endothelium of intact aortic rings induced by 70. In addition, compound 70 inhibited intracellular Ca2+ release and blocked Ca2+ influx through L-type Ca2+ channels, while not influencing K+ channels (Fig. 3).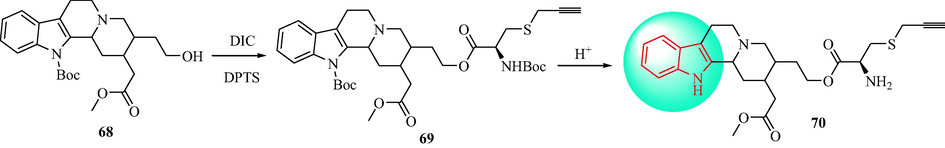
Synthesis of indoloquinolizidine 70.
Sączewski et al. (Sączewski et al., 2012, Sączewski et al., 2016) developed the series of the derivatives 73. The derivatives 73 were prepared from the corresponding indoles (71) and compound 72 in sodium hydride and anhydrous THF, as outlined in Scheme 12. Then, the target indole analogues were evaluated in α1-adrenoceptor and α2-adrenoceptor binding assays and via radioligand binding in vitro. The nature and position of the substituents affected the affinity of the compounds for the α1-AR and α2-AR, that is, 7-Cl > 7-Br > 7-CH3 > 7-F for the α1-AR and 7-Cl > 7-F > 7-CH3 > 7-Br for the α2-AR. Clonidine-based antihypertensive drugs have been shown to bind to the imidazoline I1 and I2 receptors. Compounds 3a, 73c and 73e displayed good antihypertensive effects.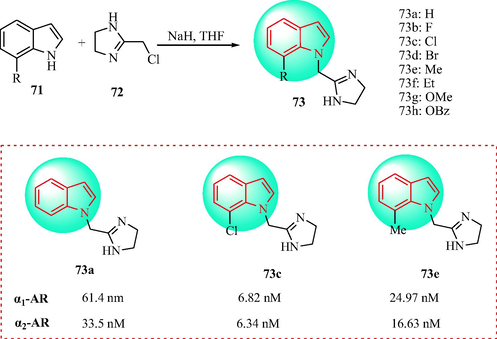
Synthesis of imidazol containing indole derivatives 73.
Bednarski et al. (Bednarski et al., 2016) synthesized the series of indole derivatives 76 (Scheme 13). Compound 74 was obtained through a nucleophilic substitution using 4-hydroxyindole and epichlorohydrin in NaOH. Next, through Gabriel synthesis, the Manske modification was used with the appropriate alkyl halide to obtain substituted 2-phenoxyethylamine derivatives (75). Finally, 74 and the substituted 2-phenoxyethanamine derivatives (75) were reacted in salicylic aldehyde to obtain the target compounds 76. The hypotensive effect of the compounds was measured after intravenous administration to anesthetized rats with normal blood pressure. In addition, the hypotensive effect of indole derivatives under study was measured after intravenous administration to anesthetized rats with normal blood pressure. Compounds 76a, 76b, 76d, and 76e exhibited a high correlation of hypotensive activity of spatial configuration. As hypertensive agent, the most active is enantiomer S, while the R-enantiomer is 2–32 times weaker. The hypotensive activity probably results from adrenoceptor blockade (α1 in arteries and β1 in heart), and found the difference only in affinity of enantiomers to β1-not α1-adrenoceptor in radioligand binding studies. Compound 76a in the form of a racemic mixture decreased systolic and diastolic blood pressure at a dose of 0.25–1.0 mg·kg−1; hypotensive activity occurred 30 min after intravenous administration of 0.25 mg·kg−1. The compounds containing methyl and dimethoxy groups and their enantiomers showed α1-adrenolytic and β1-adrenolytic activity, and had anti-hypertensive pharmacological effects. The most active hypertensive agents were the S-enantiomers. The docking analysis is shown in Fig. 8.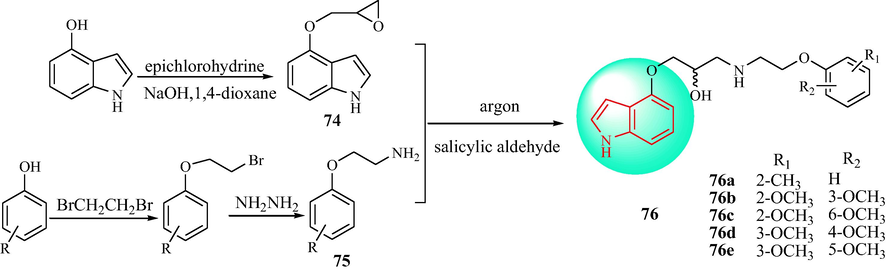
Synthesis of indole including phenoxy and ethylaminopropan-2-ol derivatives.
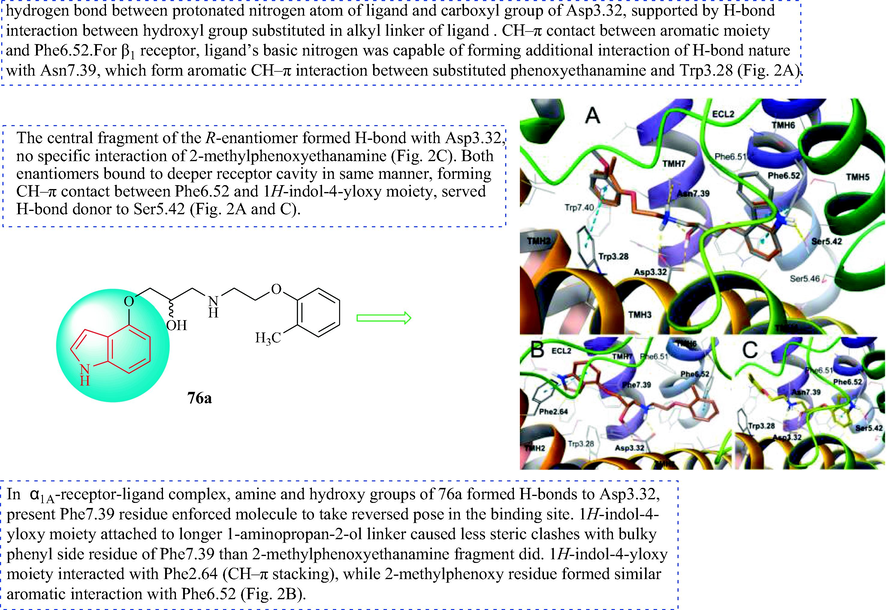
The predicted binding mode of S-enantiomer (eutomer) of 76a (orange), displayed together with reference carvedilol molecule (gray). (A) R-enantiomer (distomer, yellow); (B) in site of adrenergic β1-receptor. S-enantiomer of 76a docked in site of α1A-receptor;(C) Amino acid residues engaged in ligand binding are displayed as sticks, whereas crucial residues, e.g., forming H-bonds (dotted yellow lines) or π-π/CH-π stacking interactions (dotted cyan lines) are represented as thick sticks.
In 2016, Zhu et al. (Zhu et al., 2016a) synthesized a series of 5-oxo-1,2,4-oxadiazole analogues with 1,4-disubsituted or 1,5-disubsituted indoles, and studied the pharmacological actions as AT1 antagonists. The preparation of compounds is shown in Scheme 14. The disubstituted indole analogues 77 were obtained from the reaction of 2-(4 or 5-chloromethyl-1H-indol-1-yl)benzonitrile with compound 78. The obtained products were oxidized to obtain the carboxylic acid compounds 79, which were treated with methyl iodide to generate the methyl benzoate compounds 80. Then, 80 were reacted with hydroxylamine hydrochloride to obtain the N-hydroxyformamidine compounds 81. CDI was used to cyclize the formamidines (82) through a condensation reaction to obtain the target compounds 83. Table 7 shows the evaluation of the in vitro AII antagonistic activity of 83a-83d and the in vivo antihypertensive effects. Compounds 83a-83d exhibited obvious antagonism of the AT1 receptor with similar activity to losartan. Compound 82a with a 1,4-disubsituted indole group (IC50 = 5.01 ± 1.67 nM) displayed a strong anti-hypertensive effect in SHR. The maximum response of compound 82a (10 mg·kg-1p.o.) was to lower the average blood pressure by 30 mmHg. The antihypertensive effect of 82a lasted for>24 h.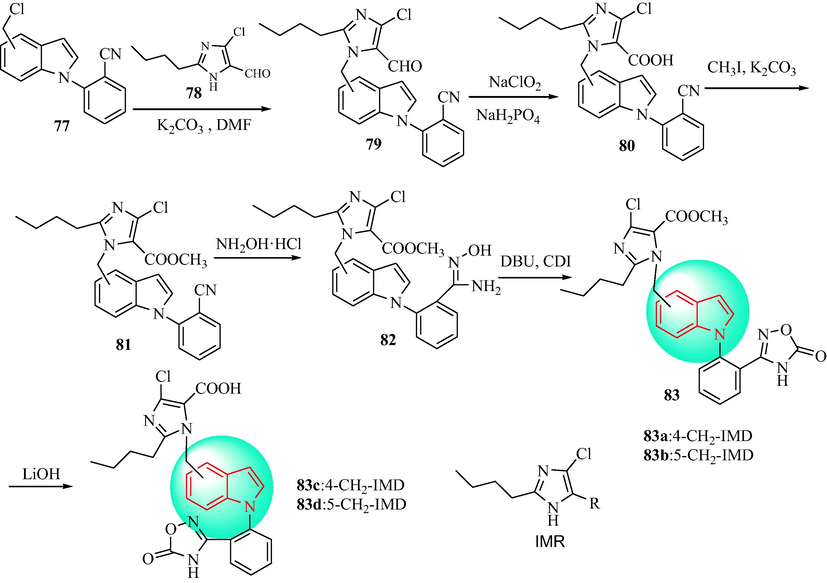
Synthetic route of indole derivatives 83.
Compounds
R
IC50 (nM)
Ki (nM)
83a
4-CH2-
5.01 ± 1.67
3.63 ± 1.21
83b
4-CH2-
9.40 ± 2.12
6.81 ± 1.54
83c
5-CH2-
8.95 ± 1.39
6.48 ± 1.00
83d
5-CH2-
6.67 ± 2.12
4.83 ± 1.54
losartan
/
10.51 ± 2.19
7.61 ± 1.59
lrbesartan
/
1.30 ± 0.06
0.94 ± 0.04
Kornicka et al. (Kornicka et al., 2017) designed a sequence of 1-((imidazolidine-2-yl)imino)-1H-indole analogues (Scheme 15). 1-Amino-1H-indoles (84) were reacted with N-Boc-2-methylthio-4,5-dihydro-1H-imidazole (85) in acetic acid to obtain, after deprotection of the formed intermediates, and then, under alkaline conditions to give the target free indoles analogues 87. In the presence of HgCl2, compounds 84 and 86 bind to N, N'-bis-boc-imidazolidine-2-thione (88), then reacted to give the Boc-protected intermediate and the protecting groups were cleaved with TFA and CH2Cl2 to give compound 89 (Mundla et al., 2000, Sączewski et al., 2011, Dardonville et al., 2000). The indole analogs 87 and 89 were evaluated for selective α1 and α2 adrenoceptor affinity though radioligand binding assays in vitro (Table 8). The indole analogs 87 and 89 displayed high receptor binding affinity for the α2-AR with varying selectivity towards the α2-AR vs I1 binding positions (I1/α2 selectivity ratios: 592–9636). The biggest difference in affinity between the α2-AR and I1-imidazoline receptor was shown for the unsubstituted compound 87a, compared with the 5-CH3-, 7-F, and 7-CH3 substituted compounds 87b, 87c and 89, respectively (I1/α2 selectivity ratios of 1300, 9636, 1269, and 591.6, respectively). The 7-fluoro analogue 87c (10 μg/kg i.v.) displayed the most obvious anti-blood pressure effect and bradycardia activity (Fig. 9).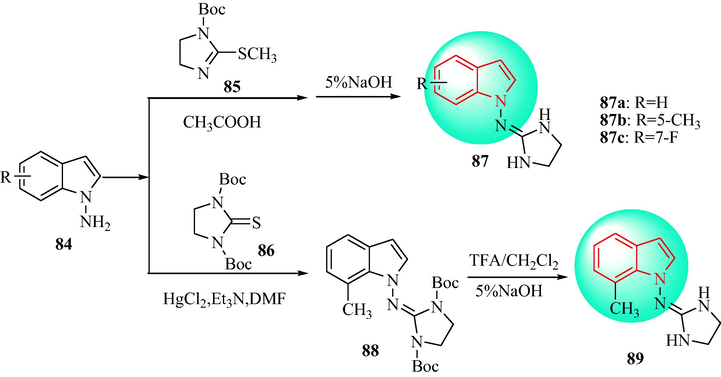
Synthetic route of indole derivatives 87 and 89.
Compounds
Binding affinities a Selectivity ratio
α1Ki (nM)b
α2 Ki (nM)b
I1 IC50(nM)c
I2Ki (nM)b
α 1/α 2
I1 /α2
87a
70.2
5.33
6930
1840
13
1300
87b
118
16.5
159,000
354
7
9636
87c
167
12
15,230
1087
14
1269
89
166
12.1
7152
2536
13.7
591.6
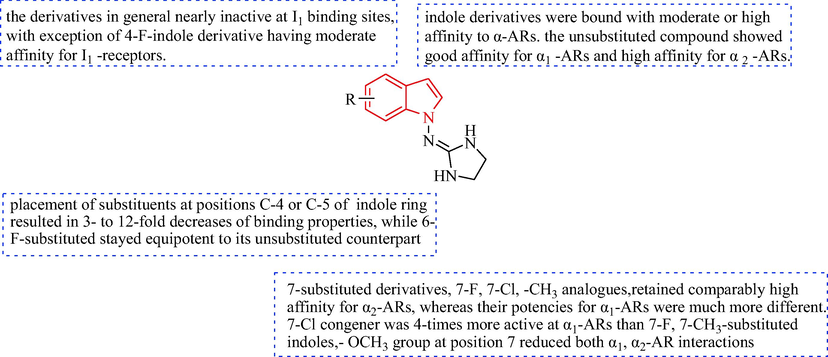
The SAR of indole analogues is discussed.
In 2019, Tian et al. (Tian et al., 2019) isolated rutaecarpine (90) from Evodiarutaecarpa (Wu Zhu Yu, Family: Rutaceae) (Fig. 3), which is a potential clinical candidate for the treatment of hypertension. Compound 55 reduced the right ventricular systolic and mean pulmonary artery pressure induced in PHR by monocrotaline. In addition, compound 90 also reduced the systolic blood pressure of the right ventricle. In a hypertensive rat model, 90 showed anti-hypertensive effects after 2-kidney and 1-clip surgical operation. Rutaecarpine, as an inhibitor of Ang-II type I receptors, significantly reduced systolic blood pressure and Ang-II secretion in a dose-dependent way (Deng et al., 2004, Li et al., 2014, Li et al., 2016, Ding et al., 2008, Jia and Hu, 2010, Qin et al., 2007).
In 2021, Irshad and Khatoon (Irshad and Khatoon, 2021) reported two antihypertensive monoterpenoid indole alkaloids in Rauvolfia species from northern India (91 and 92) (Fig. 3). The monoterpenoid indole alkaloids 91 and 92 are the major phytochemicals reported from plants and are well known for their potential antihypertensive activity (Leão et al., 2017, Shamon and Perez, 2016, Douglas, 2015, Dey, A. and Dey, J.N., 2011).
2.2 Analogues containing an indazole skeleton
Benzene and pyrazole rings are fused to obtain an aromatic heterocyclic molecule called an indazole, which has three tautomeric constructions (Fig. 10). A 1H-Indazole and analogues are the most thermodynamically stable of these tautomers (Kuhn et al., 2014, Ali et al., 2013).
The chemical structure of tautomers of indazole.
In 2010, Meyers et al. discovered a new type of non-steroidal pyrazoline antagonists of the MR receptor for the potential treatment of nephropathy and hypertension (Meyers et al., 2010). A sequence of conformationally confined pyrazolines was prepared (Scheme 16). The cyclic ketones derivatives 93 were reacted with an alkyl or aryl aldehyde to obtain the flavone analogues 94. Then, condensation with aryl hydrazine analogues gave the pyrazoline derivatives 93 predominantly as the cis diastereomers. The final pyrazolines derivatives 96 were produced via ethyl ester hydrolysis. The target compounds were resolved by chiral supercritical fluid chromatography. Compounds 96c-96d were potent MR antagonists with selectivity over other nuclear receptors (Table 9). The SAR of pyrazoline analogues of the antihypertensive is discussed in Scheme 16. Compound 96b had four times the functional productivity (MR binding IC50 = 2.7 nM). In the same way, the binding affinity of 96b for the PR was two times the functional PR strength. The binding affinity of 96b for the MR was 115 times that for the PR, which was compared with spironolactone (MR binding IC50 = 8.1 nM vs. PR binding IC50 = 2440 nM; 301-fold) and eplerenone (MR binding IC50 = 138 nM vs. PR binding IC50 > 10000 nM; > 72-fold). To identify the potential binding mode of the pyrazoline of 96b, the 1.95 Å MR/corticosterone X-ray crystal structure 2A3I (Bledsoe et al., 2005, Li et al., 2005) was used to prepare induced fit models with 96b. The N1 cyanophenyl group was in the A-ring pocket and formed hydrogen bonds with R817 and Q776, which mimicked the A-ring 3-carbonyl group of corticosterone in this model (Sun et al., 2006; Bohl et al., 2008). The dangling cyclopentyl group forces the L960 side chain into a higher energy conformation and displaces the N770 side chain from its normal position, disrupting the hydrogen bond network stabilized by the 11β -hydroxyl group of corticosterone. This feature might be an important component of these pyrazoline antagonistic MR, namely the formation of hydrogen bonds between ligands and N770 in helix 3 and T945 in helix 10. In addition, the ligand carboxylate replaces the side chains of L848, especially F941, opening the pathway to the solvent under helix 11. Compound 96b was selected as a clinical candidate for the treatment of diabetic nephropathy because of its promising in vitro potency and selectivity, in vivo potency, pharmacokinetic properties, and preclinical safety profile, and is currently undergoing clinical research (Fig. 11).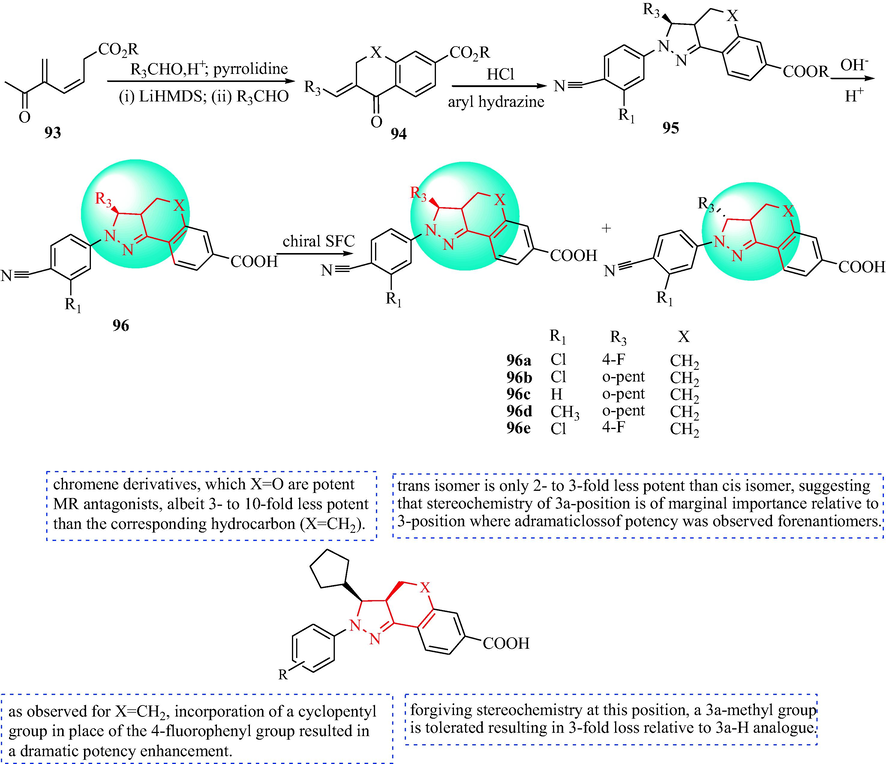
Synthesis and SAR of pyrazoline derivatives 96.
Compounds
MR IC50 nM
AR IC50 nM
GR IC50 nM
PR IC50 nM
ER IC50
96b
38
>10000
>10000
3180
n.d.
96c
9
>8910
>10000
416
>10000
96d
26
>10000
>10000
1880
n.d.
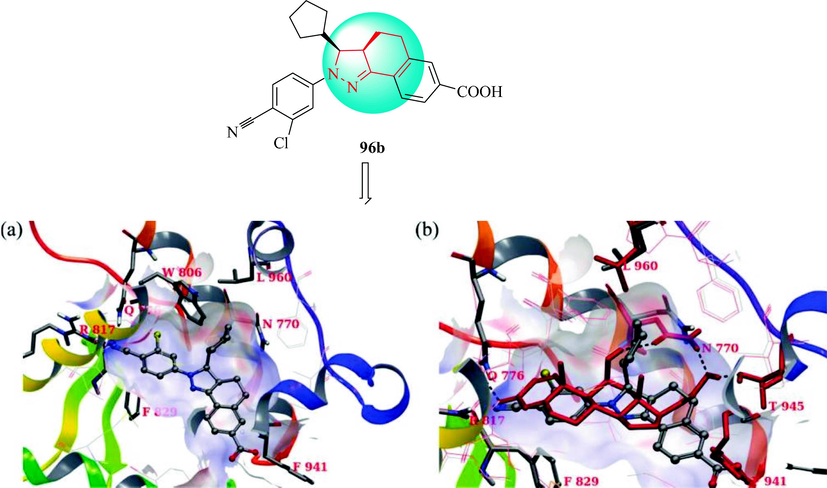
Induced-fit model of 96b din MR: (a) ball and stick, showing residues L960 (helix12), F941 (helix11), N770 (helix 3), Q776 and R817 (ketosteroid recognition), and F829, tube; (b) overlay of native crystal MR/corticosterone crystal structure 2A3I(red)with the 56-MR induced-fit model.
In 2013, Wróblewska et al. (Wróblewska et al., 2013) described the synthesis of two imidazoline compounds: marsanidine (97) and 7-methylmarsanidine (98). Compound 97, with a selectivity ratio α2/I1 of 3879, was a highly selective α2-adrenergic receptor ligand. Compound 98, with a selectivity ratio α2/I1 of 7.2, was a mixed α2-adrenergic receptor/imidazoline I1 receptor agonist. Intravenous injection of 97 and 98 induced a reduction in the heart rate and blood pressure and resulted in antihypertensive effects in Wistar rats. The hypotensive activity of the imidazolines was mediated not only by a decrease in renal sympathetic nerve activity and direct actions on peripheral receptors but also by activating central α2 and/or I1 receptors (Fig. 12). Compound 98 was significantly more effective as an antihypertensive agent than 97, which may be because of its moderate affinity for the I1-imidazoline receptor.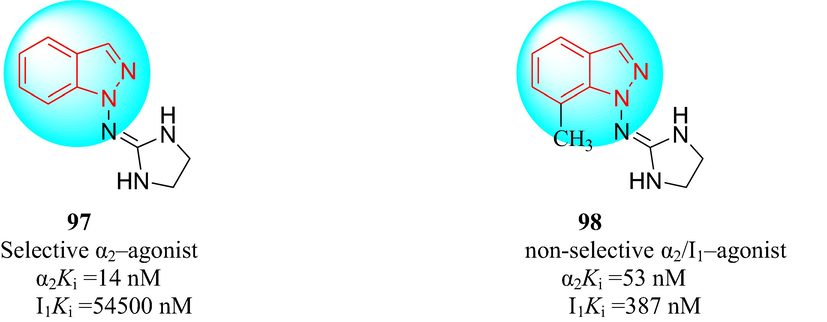
The structure of the new hypotensive imidazolines.
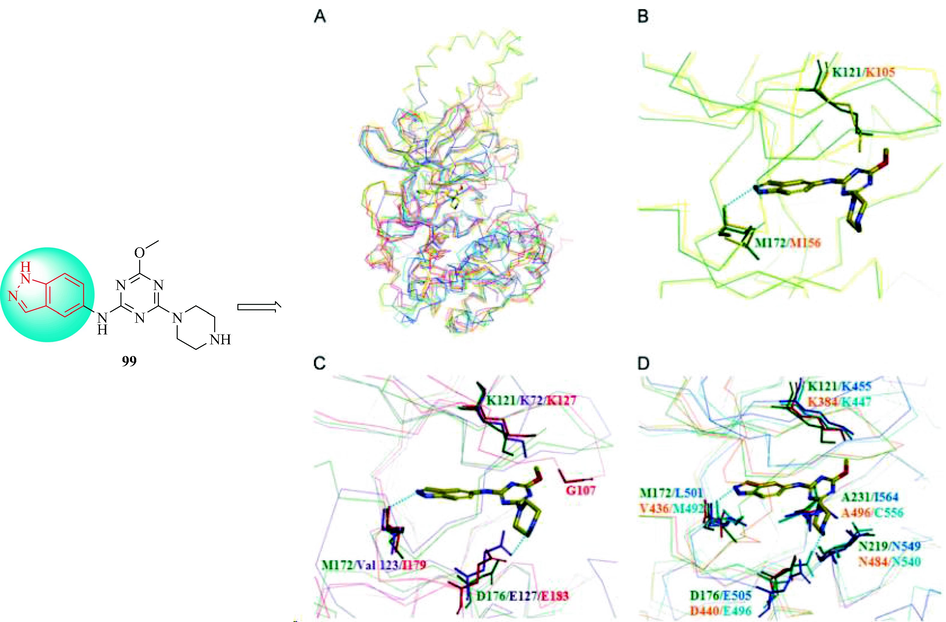
In 2013, the Rho kinase inhibitor 99 was discovered and investigated in biochemical, tissue, animal, and cellular experiments. Compound 99 demonstrated inhibition of the kinase activity of Rho kinase 1 and Rho kinase 2 in vitro. In analysis of a panel of 13 kinases, compound 99 showed high selectivity for Rho kinase (Table 10) and as a selective Rho kinase inhibitor, 99 was 10 times more effective than fasudil in inhibiting the activity of Rho kinase. In a study of isolated vascular tissues, 99 exerted a vasodilator effect on phenylephrine or 5-hydroxytriphenylamine-induced contractions in a concentration-dependent manner. The administration of 99 resulted in a significant dose-dependent decrease in the blood pressure of spontaneously hypertensive rats. In addition, 99 blocked the formation of stress fibers and cell hypertrophy induced by angiotensin II in rat heart-derived H9C2 cells. These results showed that 99 can reduce the pathophysiological effects of Rho kinase, such as cell hypertrophy, stress fiber formation, and hypertension, and that 99 was a highly selective and effective Rho kinase inhibitor (Oh et al., 2013). The crystal structures of seven AGC family kinases, mitogen, Rho kinase 2, Rho kinase 1, stress-activated protein kinase 1, protein kinase Cη, p90 ribosomal S6 kinase 1, and protein kinase A and glucocorticoid- and serum-induced protein kinases were combined (Fig. 13A) to investigate the selectivity of 99 in molecular modeling studies, and identify the residues involved in the binding in the active site. The piperazine portion of 99 tended to interact with the Asp176 of Rho kinase 2, thereby enhancing the activity of the compound (Fig. 13C). The Met172 residue in the hinge region of Rho kinase 2 clearly interacted with 99 as shown in Fig. 13B. Additionally, the long fatty chain containing the Gly107 residue of the serum- and glucocorticoid-induced protein kinases, and the long fatty chain of the protein kinase A Glu183 collided with the methoxy group of 99. In p90 ribosomal S6 kinase 1 and protein kinase Cη, there was steric hindrance between the extended Lys447 and Lys384 residues, respectively, and the 99 methoxy group, which may have caused the low binding to these receptors (Fig. 13D).
Enzymes
IC50 value (nM)
Rho kinase 2 (ROCK2)
0.02
Rho kinase 1 (ROCK1)
0.1
AKT1
>10
Cyclin-dependent kinase 1 (CDK1)
>10
IκB kinase (IKKβ)
>10
c-Jun N-terminal kinase 1 (JNK1)
>10
LIM kinase 1 (LIMK1)
>10
Mitogen- and stress-activated protein kinase 1 (MSK1)
>10
Protein kinase A (PKA)
>10
Protein kinase Cη(PKCη)
>10
Polo-like kinase 1 (PLK1)
>10
p38a Mitogen-activated protein kinase (p38a)
>10
p70S6 Kinase (S6K)
9.8
p90 Ribosomal S6 kinase 1(RSK1)
9.2
Serum- and glucocorticoid-induced protein kinase (SGK)
>10
Modeling of 99 with Rho kinase 2 (pdb entry; 2H9V) and Rho kinase 1 (pdb entry: 3NCZ), mitogen- and stress-activated protein kinase 1 (pdb entry: 3KN5), protein kinase A (pdb entry: 3POO), protein kinase Cη (pdb entry: 3TXO), p90 ribosomal S6 kinase 1 (pdb entry: 2WNT), and serum- and glucocorticoid-induced protein kinase (pdb entry: 3HDM).
In 2016 (Zhu et al., 2016b), the synthesis of derivatives 107 was outlined in Schemes 17. Substituted benzimidazoles derivatives 100 were obtained with methyl 4-amino-3-methylbenzoate (1 0 0) in DCM with alkyl acid chloride, nitrated with concentrated nitric acid, hydrogenated with hydrogen, and cyclized in acetic acid. The bisbenzimidazole (1 0 2) were obtained to react with benzimidazoles (1 0 1) in NaOH, cyclization with N-methylbenzene-1,2-diamine in the presence of polyphosphorous acid (PPA). The derivatives 104 were obtained with 5-bromomethyl-1H-indol-1-phenylmethanone (1 0 5) in NaH, then derivatives 104 in NaOH to derivatives 105. The compounds 105 were alkylated with 2-fluorobenzonitrile to obtain benzonitrile (1 0 6) after deprotection of the benzoyl group, finally, hydrolyzed to derivatives 107 with NaOH in methanol. Compounds 107 were evaluated as angiotensin II receptor antagonists in vivo and in vitro (Table 11). According to the results of radioligand binding analysis, several benzimidazole derivatives displayed high-affinity binding to the angiotensin II type 1 receptor, in the same order of magnitude as telmisartan. Compound 107c reduced the MBP in a dose-dependent manner in spontaneously hypertensive rats. The maximum response was a reduction of 53 mmHg in MBP when 107c was administered orally at a dose of 5 mg∙kg−1, and the MBP was reduced by 64 mmHg when the dose was 10 mg∙kg−1. The antihypertensive effect lasted>24 h, which was comparable to telmisartan and losartan. These results indicated that 107c was worthy of further research for application as an active and long-lasting antihypertensive agent.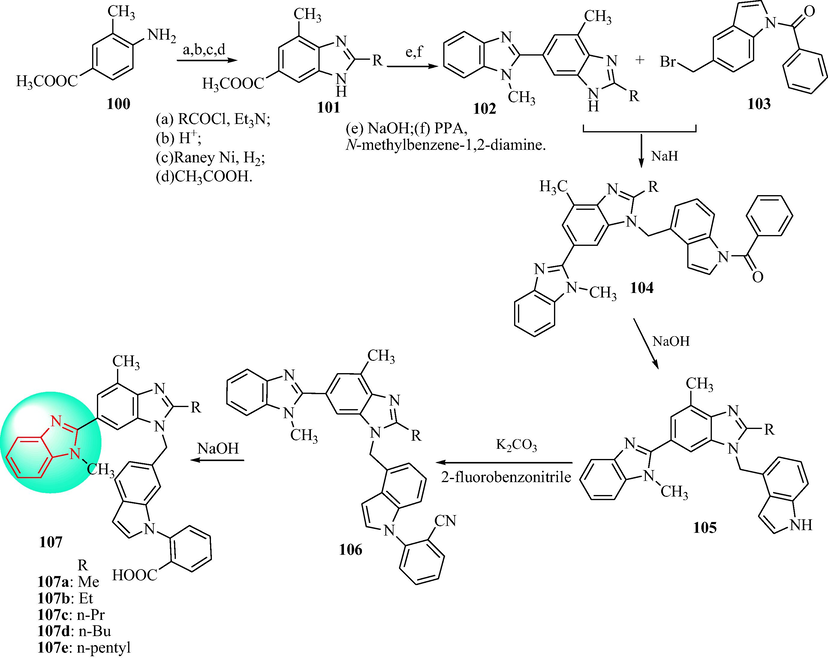
Synthesis of N-phenyl indole derivatives 107.
Compounds
R
IC50 ± SEM (nM)
Ki (nM)
107a
Me
7.46 ± 0.16
4.66 ± 0.78
107b
Et
1.83 ± 0.19
1.14 ± 0.23
107c
n-Pr
0.36 ± 0.18
0.23 ± 0.17
107d
n-Bu
2.61 ± 0.26
1.63 ± 0.19
107e
n-pentyl
11.30 ± 5.89
7.06 ± 4.14
losartan
–
20.09 ± 0.11
13.06 ± 0.07
telmisartan
–
3.80 ± 0.22
2.75 ± 0.17
In 2014, Boblewski et al. (Boblewski et al., 2014) synthesized indazole derivatives that exerted promising actions on the heart rate and blood pressure in Wistar rats. In 2017, Boblewski et al. (Boblewski et al., 2017) evaluated 108–111 that had different affinities to the α2-adrenorecptor and to the I1 imidazoline receptor (Table 12). Among them, 108 showed the lowest affinity for the α2-adrenoreceptor and the highest affinity for the I1 receptor. The effects of 108–111 on the blood pressure, heart rate, and diuresis were compared. Male Wistar rats received the tested compounds at two doses: 10 and 100 µg∙kg−1 i.v. The MAP, ECG, and HR were recorded continuously. Compounds 108–111 caused a profound decrease in the MAP. Compound 111 at a dose of 100 µg∙kg−1. caused the strongest drop in the MAP. The weakest and the shortest duration of effect were observed after administration. The HR was reduced after administration of each compound and the greatest effect was observed after 110 was administered at a dose of 100 µg∙kg−1. The weakest and the shortest duration of effect was observed after 108 administration. These data suggested that a methyl substituent at the 7-position of the indazole ring was the most effective substitution for improving the hypotensive effects of the synthesized imidazolidine derivatives.
Compounds
R
α2Ki (nM)
Ii (nM)
α2/ Ii
108
–
14.05
54,500
3879
109
7-CH3
53.5
387
7.23
110
7-Cl
30.3
46,800
1544
111
7-F
30.9
7740
250
In 2018, a series of 3,4-dihydropyrazino[1,2-b]indazol-1(2H)-one derivatives were synthesized by Furlotti et al. (Furlotti et al., 2018), as outlined in Scheme 18. The preparation of the piperidine 114 was obtained: (a) the N-protection and the subsequent basic hydrolysis (NaOH) of the aminoester 112 to the acid intermediate, then the direct reduction of intermediate to primary alcohol 113; (c) the conversion of the hydroxyl moiety to a better leaving group. The tricyclic initial scaffold 117 was obtained through the deprotection and the ring closure of the intermediate 116, which, in turn, was prepared starting from the simple indazole-3-carboxylic acid 115 upon esterification and subsequent N-alkylation. The derivatives 118 were obtained with 117 under NaOH, then derivatives 18 reacted with 1-(2-bromoethyl)-2-fluorobenzene under Cs2CO3 to give the target compound 119. Then, 3,4-dihydropyrazino[1,2-b]indazol-1(2H)-one derivatives be studied as a potential ocular hypotensive compound. Compound 119 acted on two receptors (serotonin 2A, 5-HT2A, and adrenergic α1) related to the regulation of aqueous humor fluid dynamics as a potential ocular hypotensive agent (Table 13). Compound 119, which was a potent 5-HT2A antagonist (IC50 = 18 nM), was>100 times more selective for 5-HT2A than other serotonin subtype receptors, and had a positive effect on the α1 receptors (IC50 values ranging from 220 nM to 1.5 µM). In addition, compared with the clinically used reference compound timolol, compound 119 showed a greater ability to lower the intraocular pressure in vivo when administered topically (Fig. 14).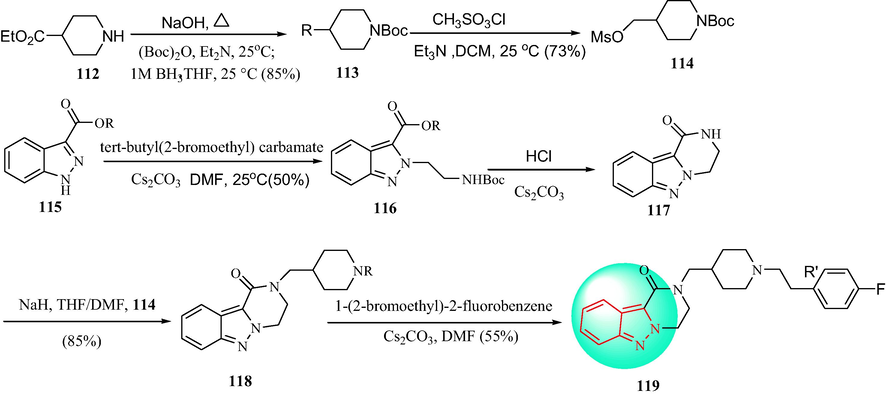
Synthetic route of indazol derivatives 119.
Receptor
IC50 nM
Kb nM
Functional activity
5-HT2A
18
4
antagonist
α1A
1500
190
antagonist
α1B
220
24
antagonist
α1D
910
260
antagonist
α2B
67,000
8800
antagonist
α2C
3600
1700
antagonist
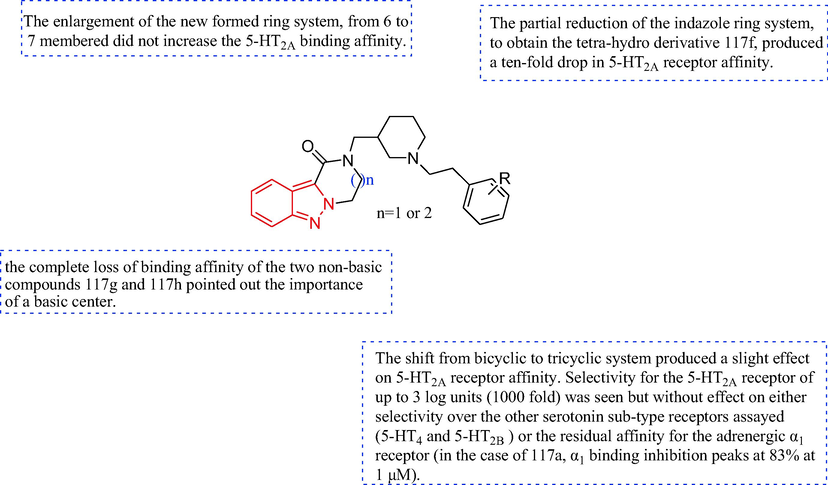
The SAR of indazol-1(2H)-one derivatives of the antihypertensive.
To find new hypotensive drug candidates, imidazo[1,2-a]benzimidazole and pyrimido[1,2-a] benzimidazole derivatives were screened (Marcus et al., 2018). The compound were studied in rats with normal blood pressure, and a rebound tonometer was employed to estimate the IOP. Compounds 120–123 were administered by a single drop, at 0.1%, 0.2%, and 0.4% concentrations (Table 14). Compounds120-123 reduced the IOP in ocular normotensive rats. A relationship between the ability to lower intraocular pressure and the ability to lower blood pressure has been shown. According to pharmacophore analysis, pyrimido[1,2-a]benzimidazole is a better scaffold than imidazo[1,2-a]benzimidazole to provide compounds with a high intraocular pressure reduction effect. The key features for high intraocular pressure reducing activity were phenylalkyl and methoxyphenyl fragments, as well as non-conjugated six-membered heterocycles.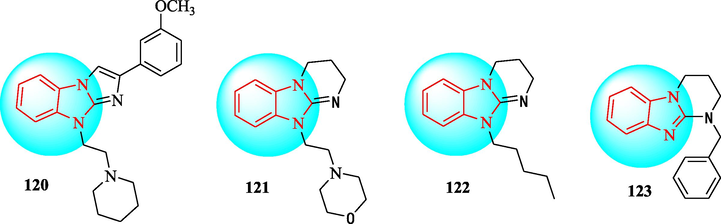
Compounds
%
Max IOP decrease
from baseline (%)Max IOP decrease from control
Duration of action
AUC
Timolol
0.5
20.00
12.50
5 ± 0.0
7.25 ± 0.66
120
0.1
0.2
0.427.91
31.83
30.2226.21
31.83
30.224 ± 1.00
5 ± 2.89
4 ± 0.5816.3 ± 4.04a
20.3 ± 2.10 a
13.0 ± 6.91 a
121
0.1
0.2
0.420.00
27.70
31.1321.73
5 ± 0.00
13.1 ± 2.90 a
27.70
5 ± 0.58
16.5 ± 2.90 a
27.91
5 ± 0.58
17.9 ± 3.70 a
122
0.1
29.19
29.19
5 ± 0.58
18.8 ± 2.20 a
0.2
30.00
27.06
4 ± 0.58
17.2 ± 1.70 a
0.4
31.91
28.87
5 ± 0.58
18.5 ± 2.00 a
123
0.1
19.53
19.53
5 ± 0.58
14.1 ± 1.80 a
0.2
23.24
21.43
5 ± 0.58
16.0 ± 3.20 a
0.4
25.54
17.92
5 ± 0.58
10.4 ± 4.40
3 Conclusions
Hypertension is an important risk factor for cardiovascular complications. The treatment of hypertension is fundamental for the prevention of cardiovascular events. Effective methods for designing drug-like molecules include fragment-based drug discovery. The indole and indazole scaffolds are widely present in many biomolecules and medical products. This review describes the synthesis and antihypertensive bioactivity of indole and indazole analogues. It has been reported that indole- and indazole-substituted derivatives have antihypertensive activity. Interestingly, most of the indole and indazole derivatives were identified by scaffold hopping strategies or screening approaches. The review focused on the antihypertensive effects of recently developed indole and indazole derivatives effected via several targets. Analysis of the structural characteristics revealed that (I) the N1 indole nitrogen atom has biological significance because of its hydrogen bond donor and acceptor properties; (II) the pyrrole ring of the indole skeleton can form hydrogen bonds with amino acid residues in the hinge region of enzymes, acting as a hydrogen bond donor or acceptor, which can result in inhibitory actions. The phenyl moiety of indole contributes to good hydrophobic gated interactions; and (III) the indazole supplies a coordination site for metals. The π-π and dipolar interactions between the indazole fragment and nitrogenous bases can heighten the antihypertensive activity. The information provided in this review regarding the activities of indole and indazole derivatives will assist in the design of new compounds with improved biological properties and lead to the development of novel synthetic strategies. These advancements may lead to the development of indole and indazole derivatives for clinical use in the eradication and control of various diseases.
Acknowledgement
This work was supported by the Jilin Province Department of Education change This work was supported by the Jilin Province Department of Education, China (No. JJKH20200461KJ, JJKH20211144KJ), and Jilin Science and Technology Development Project, China(20190201148JC).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Novel indole-2-carboxylic acid linked 3-phenyl-2-alkoxy propanoicacids: synthesis, molecular docking and in vivo antidiabetic studies. Med. Chem. Res.. 2017;26:745-759.
- [Google Scholar]
- Chemistry and biology of indoles and indazoles: a mini-review. Mini Rev. Med. Chem.. 2013;13:1792-1800.
- [Google Scholar]
- Low-dose antihypertensive therapywith 1.5 mg sustained-releaseindapamide: results of randomized double-blind controlled studies. J. Hypertens. 1998;16:1677-1684.
- [Google Scholar]
- Indole-phenol bioisosterism. Synthesis and antihypertensive activity of a pyrrolo analogue of labetalol. J. Med. Chem.. 1986;29:1009-1015.
- [Google Scholar]
- A Comparative study: evaluation of antioxidant activity of melatonin and some indole derivatives. Med Chem Res. 2005;14:169-179.
- [Google Scholar]
- (S)-N-{3-[1-Cyclopropyl-1-(2,4-difluoro-phenyl)-ethyl]-1H-indol-7-yl}-methanesulfonamide: A potent, nonsteroidal, functional antagonist of the mineralocorticoid receptor. J. Med. Chem.. 2007;50:6443-6445.
- [Google Scholar]
- Preparation of indole derived modulators of steroid hormone nuclear receptors. PCT Int. Appl. 2004:243. WO 2004067529, A1 20040812
- [Google Scholar]
- Synthesis and pharmacological activity of a new series of 1-(1H- indol-4-yloxy)-3-(2-(2-methoxyphenoxy)ethylamino)propan-2-ol analogs. Arch Pharm (Weinheim). 2016;349:211-223.
- [Google Scholar]
- Mucosal delivery systems of antihypertensive drugs: A practical approach in general practice. Biomed Pap Med FacUnivPalacky Olomouc Czech Repub. 2018;162:71-78.
- [Google Scholar]
- A ligand-mediatedhydrogen bond network required for the activation of the miner-alocorticoid receptor. J. Biol. Chem.. 2005;280:31283-31293.
- [Google Scholar]
- Vagotomy reveals the importance of the imidazoline receptors in the cardiovascular effects of marsanidine and 7-ME-marsanidine in rats. Pharmacol. Rep.. 2014;66:874-879.
- [Google Scholar]
- Comparison of the effects of marsanidine derivatives on rat cardiovascular system. Acta Pol. Pharm.. 2017;74:579-586.
- [Google Scholar]
- Effect of B-ring substitution pattern on binding mode of propionamide selective androgen receptor modulators. Bioorg. Med. Chem. Lett.. 2008;18:5567-5570.
- [Google Scholar]
- Britannica encyclopedia, http://www.britannica.com /plant /Rauvolfia. Last accessed,17.6.2021.
- BVT.28949 as a potential ocular hypotensive agent in monkeys. Invest. Ophth. Vis. Sci.. 2004;45:5040.
- [Google Scholar]
- New data on antihypertensive drugs and risk of cancer: should we worry? Blood Press.. 2019;28:1-3.
- [Google Scholar]
- Pharmacological properties of indazole derivatives: recent developments. Mini Rev. Med. Chem.. 2005;25:869-878.
- [Google Scholar]
- Rational drug design of indazole-based diarylurea derivatives as anticancer agents. Chem. Biol. Drug Des.. 2017;90:609-617.
- [Google Scholar]
- Vascular L-type Ca2+ channel blocking activity of sulfur-containing indole alkaloids from Glycosmispe- telotii. J. Nat. Prod.. 2014;77:1586-1593.
- [Google Scholar]
- New aromatic iminoimidazolidine derivatives as α 1 -adrenoceptor antagonists: a novel synthetic approach and pharmacological activity. Bioorg. Med. Chem.. 2000;8:1567-1577.
- [Google Scholar]
- Combination perindopril/indapamide for the treatment of hypertension: A review. Expert Opin. Pharmacother.. 2011;12:1827-1833.
- [Google Scholar]
- Stimulation of calcitonin gene-related peptide synthesis and release: mechanisms for a novel antihypertensive drug. Rutaecarpine. J Hypertens. 2004;22:1819-1829.
- [Google Scholar]
- Ethnobotanical aspects of Rauvolfia serpentina (L.) Benth ex Kurz in India Nepal and Bangladesh. J Med Plant Res. 2011;5:144-150.
- [Google Scholar]
- Solid dispersion of rutaecarpine improved its antihypertensive effect in spontaneously hypertensive rats. Biopharm. Drug Dispos.. 2008;29:495-500.
- [Google Scholar]
- Recent advances in the development of indazole-based anticancer agents. ChemMedChem. 2018;13:1490-1507.
- [Google Scholar]
- Discovery of the aryl-phospho-indole IDX899, a highly potent anti-HIV non-nucleoside reverse transcriptase inhibitor. J. Med. Chem.. 2016;59:1891-1898.
- [Google Scholar]
- Imidazoline receptors in the heart: characterization, distribution and regulation. J. Cardiovasc. Pharmacol.. 2002;39:875-883.
- [Google Scholar]
- Synthesis and antihypertensive activity of certain mannich bases of 2-ethoxycarbonylindoles and 5H-pyridazino[4,5-b]indoles. Arch. Pharm. Res.. 2001;24:21-26.
- [Google Scholar]
- Targeting serotonin 2A and adrenergic α1 receptors for ocular antihypertensive agents: discovery of 3,4-dihydropyrazino[1,2-b] indazol-1(2H)-one derivatives. ChemMedChem. 2018;13:1597-1607.
- [Google Scholar]
- Prediction of antihypertensive activity of pyridazinone derivatives through multivariate image analysis applied to QSAR. Med. Chem. Res.. 2013;22:3389-3397.
- [Google Scholar]
- An insight into the medicinal perspective of synthetic analogs of indole: A review. Eur. J. Med. Chem.. 2019;180:562-612.
- [Google Scholar]
- The role of the three-drug combination antihypertensive in improving the treatment of arterial hypertension. Kardiologiia. 2017;57:62-67.
- [Google Scholar]
- Perindopril for the treatment of hypertension. Expert Opin. Pharmacother.. 2011;12:1633-1642.
- [Google Scholar]
- Factors influencing hepatic metabolism of antihypertensive drugs: impact on clinical response. Expert Opin. Drug Metab. Toxicol.. 2019;15:1-13.
- [Google Scholar]
- The combinatorial synthesis of bicyclic privileged structures or privileged substructures. Chem. Rev.. 2013;103:893-930.
- [Google Scholar]
- A validated HPTLC method for the simultaneous determination of seasonal alterations of two antihypertensive monoterpenoid indole alkaloids in Rauvolfia species from northern India. South African J Botany. 2021;142:193-200.
- [Google Scholar]
- Pharmacological effects of rutaecarpine as a cardiovascular protective agent. Molecules. 2010;15:1873-1881.
- [Google Scholar]
- Discovery of a new HIV-1 inhibitor scaffold and synthesis of potential prodrugs of indazoles. Bioorg. Med. Chem. Lett.. 2013;23:2888-2892.
- [Google Scholar]
- (Mercaptopropanoyl)indoline-2-carboxylic acids and related compounds as potent angiotensin converting enzyme inhibitors and antihypertensive agents. J. Med. Chem.. 1983;26:394-403.
- [Google Scholar]
- Carvedilol and bisoprolol as initial therapy for adult hypertension without compelling indications. Hypertens. Res.. 2019;42:496-503.
- [Google Scholar]
- Synthesis and antihypertensive activity of 2-benzamido-1,2,3,4,6,7,12,12b -octahydroindolo[2,3-a]quinolizine. J. Med. Chem.. 1979;22:1497-1504.
- [Google Scholar]
- 1-[(Imidazolidin-2-yl)imino]-1H-indoles as new hypotensive agents: synthesis and in vitro and in vivo biological studies. Chem. Biol. Drug Des.. 2017;89:400-410.
- [Google Scholar]
- Biologically active compounds based on the privileged 2-imidazoline scaffold: The world beyond adrenergic/imidazoline receptor modulators. Eur. J. Med. Chem.. 2015;97:525-537.
- [Google Scholar]
- Antihypertensive indole derivatives of phenoxypropanolamines with beta-adrenergic receptor antagonist and vasodilating activity. J. Med. Chem.. 1980;23:285-289.
- [Google Scholar]
- Antihypertensive drugs in Croatia: What changes the drug usage patterns? Clin. Ther.. 2018;40:1159-1169.
- [Google Scholar]
- Osmium(III) analogues of KP1019: electrochemical and chemical synthesis, spectroscopic characterization, X-ray crystallography, hydrolytic stability, and antiproliferative activity. Inorg. Chem.. 2014;53:11130-11139.
- [Google Scholar]
- Indoloquinolines as scaffolds for drug discovery. Curr. Med. Chem.. 2010;17:2348-2370.
- [Google Scholar]
- Spontaneously hypertensive rats (SHR) are resistant to a reserpine-induced progressive model of parkinson's disease: differences in motor behavior, tyrosine hydroxylase and a -synuclein expression. Front. Aging Neurosci.. 2017;9:78.
- [Google Scholar]
- Effects of rutaecarpine on right ventricular remodeling in rats with monocrotaline-induced pulmonary hypertension. Zhongguo Ying Yong Sheng Li XueZaZhi. 2014;30:405-410.
- [Google Scholar]
- Rutaecarpine attenuates hypoxia-induced right ventricular remodeling in rats. Naunyn Schmiedebergs Arch. Pharmacol.. 2016;389:757-767.
- [Google Scholar]
- Structural and biochemical mechanisms for the specificity of hormone binding andcoactivator assembly by mineralocorticoid receptor. Mol. Cell. 2005;19:367-380.
- [Google Scholar]
- Gut microbiota-derived tryptophan metabolism mediates renal fibrosis by aryl hydrocarbon receptor signaling activation. Cell. Mol. Life Sci.. 2021;78:909-922.
- [Google Scholar]
- Intraocular pressure lowering effect and structure-activity relationship of imidazo[1,2-a]benzimidazole and pyrimido[1,2-a]benzimidazolecompounds in ocular normotensive rats: Insight on possible link withhypotensive activity. Eur. J. Pharmaceut. Sci.. 2018;114:245-254.
- [Google Scholar]
- Discovery of (3S,3aR)-2-(3-chloro-4-cyanophenyl)-3-cyclopentyl-3,3a,4,5-tetrahydro-2H-benzo[g]indazole-7-carboxylic acid (PF-3882845), an orally efficacious mineralocorticoid receptor (MR) antagonist for hypertension and nephropathy. J. Med. Chem.. 2010;53:5979-6002.
- [Google Scholar]
- Antihypertensive agents: pyridazino(4,5-b)indole derivatives. J. Pharm. Sci.. 1982;71:1406-1408.
- [Google Scholar]
- Selective thromboxane synthetase inhibitors and antihypertensive agents. new derivatives of 4-hydrazino-5H-pyridazino[4,5-b]indole, 4-hydrazino- pyridazino[4,5-a]indole, and related compounds. J. Med. Chem.. 1987;30:1029-1035.
- [Google Scholar]
- A novel method for the efficient synthesis of 2-arylamino-2-imidazolines. Tetrahedron Lett.. 2000;41:6563-6566.
- [Google Scholar]
- Perindopril and indapamide reversecoronary microvascular remodelling and improve flow in arterial hypertension. J. Hypertens.. 2011;29:364-372.
- [Google Scholar]
- Cardiovascular effects of a novel selective Rho kinase inhibitor, 2-(1H-indazole-5-yl)amino-4-methoxy-6-piperazino triazine (DW1865) Eur. J. Pharmacol.. 2013;702:218-226.
- [Google Scholar]
- Pharmacological studies of indole alkaloids obtained from domestic plants, uncariarhyn- chophylla MIQ and amsoniaelliptica roem. Et Schult. Nippon YakurigakuZassh.. 1989;94:17-26.
- [Google Scholar]
- Indolo[2,3-a]quinolizidines and derivatives: bioactivity and asymmetric synthesis. Curr. Pharm. Des.. 2015;21:5518-5546.
- [Google Scholar]
- Novel indole and triazole based hybrid molecules exhibit potent anti-adipogenic and antidyslipidemic activity by activating Wnt3α/β-catenin pathway. Eur. J. Med. Chem.. 2018;143:1345-1360.
- [Google Scholar]
- Practical synthesis, anticonvulsant, and antimicrobial activity of N-allyl and N-propargyl di(indolyl)indolin-2-ones. Bioorg. Med. Chem. Lett.. 2011;21:4072-4077.
- [Google Scholar]
- Calcitonin gene-related peptide-mediated depressor effect and inhibiting vascular hypertrophy of rutaecarpine in renovascular hypertensive rats. J. Cardiovasc. Pharmacol.. 2007;50:654-659.
- [Google Scholar]
- Interrater and intrarater agreement on the 2018 consensus statement on classification of tremors. Mov. Disord.. 2018;33:1966-1967.
- [Google Scholar]
- High affinity ligands and potent antagonists for the α1D-adrenergic receptor. Novel 3,8-disubstituted [1]benzothieno[3,2-d]pyrimidine derivatives. Eur. J. Med. Chem.. 2014;83:419-432.
- [Google Scholar]
- New pyrimido[5,4-b]indoles as ligands for α1-adrenoceptor subtypes. J. Med. Chem.. 2003;46:2877-2894.
- [Google Scholar]
- New pyrimido[5,4-b]indoles and [1]benzothieno[3,2-d]pyrimidines: High affinity ligands for the α1-adrenoceptor subtypes. Bioorg. Med. Chem. Lett.. 2006;16:6200-6203.
- [Google Scholar]
- 3-[(Imidazolidin-2-yl)imino]indazole with selectivity for the α2-adrenoceptor compared to the imidazoline I 1 receptor. Bioorg. Med. Chem.. 2011;19:321-329.
- [Google Scholar]
- Synthesis and biological activities of 2[(heteroaryl) methyl]-imidazolines. Bioorg. Med. Chem.. 2012;20:108-116.
- [Google Scholar]
- Transfer of SAR information from hypoten- sive indazole to indole derivatives acting at α-adrenergic receptors: in vitro and in vivo studies. Eur. J. Med. Chem.. 2016;115:406-415.
- [Google Scholar]
- Effects of rhynchophylline and isorhynchophylline on blood pressure and blood flow of organs in anesthetized dogs. Zhongguoyao li xuebao. 1992;13:35-38.
- [Google Scholar]
- Recent progress in biological activities of indole and indole alkaloids. Mini Rev. Med. Chem.. 2018;18:9-25.
- [Google Scholar]
- Blood pressure-lowering efficacy of reserpine for primary hypertension. Cochrane Database Syst. Rev.. 2016;21:CD007655.
- [Google Scholar]
- Different hypotentive effects of various active constituents isolated from Uncariarhynchophylla. Chin. Tradit. Herb Drugs. 2000;31:762-764.
- [Google Scholar]
- Antihypertensive activity of indolepyruvic acid: A keto analogue of tryptophan. J. Cardiovasc. Pharmacol.. 1990;15:102-108.
- [Google Scholar]
- Indole-2-carboxamide-based MmpL3 inhibitors show exceptional antitubercular activity in an animal model of tuberculosis infection. J. Med. Chem.. 2016;59:6232-6247.
- [Google Scholar]
- Discovery of novel anti-angiogenesis agents. Part 8: Diarylthiourea bearing 1H-indazole-3-amine as multi-target RTKs inhibitors. Eur. J. Med. Chem.. 2017;141:373-385.
- [Google Scholar]
- Compared antihypertensive effect of dihydralazinewith rhynchophylline or total alkaloids of Uncaria macrophylla in conscious animals. Chin. Pharmacolo. Bull.. 1996;12:513-515.
- [Google Scholar]
- Discovery of potent, orally-active, and muscle-selective androgen receptor modulators based on an N-aryl-hydroxy bicyclohydantoin scaffold. J. Med. Chem.. 2006;49:7596-7599.
- [Google Scholar]
- Rutaecarpine: A promising cardiovascular protective alkaloid from Evodiarutaecarpa (Wu Zhu Yu) Pharmacol. Res.. 2019;141:541-550.
- [Google Scholar]
- A review of its pharmacological properties and therapeutic use in cardiovascular disorders. Drugs. 1991;42:90-114.
- [Google Scholar]
- Position of indapamide, a diureticwith vasorelaxant activities in antihype- rtensive therapy. Expert Opin. Pharmacother.. 2012;13:1515-1526.
- [Google Scholar]
- Marsanidine and 7-Me-marsanidine, the new hypotensive imidazolines augment sodium and urine excretion in rats. Pharmacol. Rep.. 2013;65:1025-1032.
- [Google Scholar]
- Use of FDSS/μCell imaging platform for preclinical cardiac electrophysiology safety screening of compounds in human induced pluripotent stem cell-derived cardiomyocytes. J. Pharmacol. Toxicol.. 2016;81:217-222.
- [Google Scholar]
- Synthesis and antifungal activity of novel indole-replaced streptochlorin analogues. Eur. J. Med. Chem.. 2017;126:669-674.
- [Google Scholar]
- Hypotensive effect of Uncaria total alkaloid and rhynchophylline. Chin. Med. J.. 1978;58:408-409.
- [Google Scholar]
- Synthesis of indazole based diarylurea derivatives and their antiproliferative activity against tumor cell lines. Bioorg. Med. Chem. Lett.. 2013;23:1989-1992.
- [Google Scholar]
- Antihypertensive and neuroprotective activities of rhynchophylline: The role of rhynchophylline in neurotransmission and ion channel activity. J. Ethnopharmacol.. 2010;132:15-27.
- [Google Scholar]
- Isorhynchophylline: A plant alkaloid with therapeutic potential for cardiovascular and central nervous system diseases. Fitoterapia. 2012;83:617-626.
- [Google Scholar]
- Design synthesis and biological evaluation of new 5-nitro benzimidazole derivatives as AT1 antagonists with anti-hypertension activities. Bioorg. Med. Chem.. 2014;22:2294-2302.
- [Google Scholar]
- The novel analogue of hirsutine as an anti- hypertension and vasodilatary agent both in vitro and in vivo. PLoS ONE. 2015;10:e0119477
- [Google Scholar]
- Design synthesis and pharmacological evaluation of 5-oxo-1,2,4-oxadiazole derivatives as AT1 antagonists with antihypertension activities. Clin. Exp. Hypertens.. 2016;38:435-442.
- [Google Scholar]
- N-Phenyl indole derivatives as AT1 antagonists with anti-hypertension activities: design, synthesis and biological evaluation. Eur. J. Med. Chem.. 2016;115:161-178.
- [Google Scholar]







