Translate this page into:
Antimicrobial activity of biosynthesized Cuo/Se nanocomposite against Helicobacter pylori
⁎Corresponding author. dohaboubaker@gmail.com (Doha H. Abou Baker)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Recently, nanotechnology has been considered one of most frontiers in scientific sector, which increasingly attracts researchers’ interest due to its variable and valuable applications in all areas. The synthesis of nanoparticles represents a promising era in therapeutic research that may lead to the development of new approaches in pharmaceutical studies. Selenium nanoparticles (SeNPs) share a status of high repute owing to their remarkable therapeutic potential. Biological synthesis of environment-friendly SeNPs using plant extracts has emerged as a beneficial alternative approach to chemical synthesis. In this regard, we have synthesized biogenic PG-SeNPs using pomegranate peels aqueous extract (PPAE) as a stabilizing and reducing agent. The PG-SeNPs were evaluated for their anti-Helicobacter pylori and anti-stomach cancer potential. The PG-SeNPs were efficacious against H. pylori. The PG-SeNPs showed dose-dependent restriction of the growth of H. pylori. The anti-stomach cancer ability of the PG-SeNPs was evaluated against SNU-16 stomach cancer. As evident from the MTT results, PG-SeNPs reduced cell viability in a dose-dependent manner. Briefly, the PG-SeNPs evolved with synergistically emerging attributes that were effective against H. pylori; Moreover, the PG-SeNPs and SeNPs@CuO embody intriguing anticancer potential against stomach cancer cells.
Keywords
Green nanotechnology
Pomegranate peels
PG-SeNPs
H. pylori
Stomach cancer
1 Introduction
In 2005, the Nobel Prize in physiology or medicine was granted to Barry J. Marshall and Robin Warren, two Australian researchers who figured out H. pylori and their relationship with gastritis and peptic ulcer disease (Ahmed, 2005). Billions of people in the world, primarily in developing countries, are considered to have H. pylori infections. Millions of people are suffering from peptic ulcers, which may progress to gastric cancer. The possible ways of the pathogen transmission are through contaminated food and water sources or infected person to healthy person in one family (Salih, 2009). The prevalence of H. pylori infections arises chiefly in developing countries, and many of these countries suffer extremely from a test and treatment approach. Nowadays, there is a crucial need to take action for the treatment of such infections (Salih, 2009; Kusters et al., 2006).
Usually, the patient infected with H. pylori will feel a burning pain in the abdomen as a result for stomach ulcer, and non-invasive tests like antigen in stool, and invasive tests such as polymerase chain reaction, and histology, requiring the use of endoscopically surgery and biopsy specimens can be used in H. pylori diagnosis (Best et al., 2018; Rimbara et al., 2013; Perry et al., 2009). The virulence factors of H. pylori can be classified into 3 main pathogenic progressions, including invasion, immune escape, and induction of diseases. The colonization factors for virulence include urease, flagella, chemotaxis, and adhesions (Kao et al., 2016; Baj et al., 2020). The absence of urease, flagella, or chemotaxis causes a failure to develop an infection (Baj et al., 2020). However, Immune escape virulence factors help the bacteria avoid the host immune system, allowing for their survival in the human bowel, and other virulence factors are related to gastric adenocarcinoma development.
Furthermore, the lack of appropriate treatment for H. pylori infection causes serious long-term complications due to H. pylori colonization. The major reason for the failure of its eradication is antibiotic resistance (Zali, 2011). In 2017, clarithromycin resistant H. pylori was listed as a highly significant bacterium in antibiotic research and development by the World Health Organization (WHO) (Hu et al., 2017; Savoldi, et al., 2018). In particular, in countries like Egypt, quadruple-based therapy is superior to clarithromycin-containing triple therapy. Recently, growing thoughts for resistance to levofloxacin and reduction of efficacy as a second-line therapy have led us to think of levofloxacin as an alternative regimen of treatment for improved bacteria eradication in Egypt, including doxycycline and nitazoxanide (Afifi et al., 2020; Arj et al., 2020).
Significance of nanotechnology in developing novel approaches to the treatment of H. pylori infection can be seen by the strong antibacterial effects on metallic nanoparticles, and advantages of green nanotechnology by using plant extracts in biosynthesis of metallic nanoparticles such as rapid and low-cost technique (Amin et al., 2014; Chuh et al., 2022). Pomegranate, punica granatum (punicaceae) as herbal plant is a very large source of a wide range of phytochemicals, including phenolic acids, flavonoids and tannins etc., which belong to different chemical groups (Sreekumar et al., 2014). This is the first study use PP and membranes for the biosynthesis of PG-SeNPs and SeNPs@CuO depending on the main components present in the plant extract. Furthermore, evaluating the cytotoxicity of SeNPs and SeNPs@CuO against stomach cancer and normal cell lines and studying their antimicrobial efficiency against multidrug resistant H. pylori.
2 Material and methods
2.1 Plant material
Ripe pomegranate fruits were purchased from the market. The peels were separated manually from the fruit, dried in shade until reaching a moisture content of 10%, and then kept at room temperature for further study.
2.2 Extraction of phenolic compounds
Five hundred grams of the powdered pomegranate peels (PP) were extracted with hot distilled water in room temperature. The PP aqueous extract (PPAE) was evaporated by rotary evaporator.
2.3 Isolation preparations
For further fractionation, the extract was resuspended in H2O, and then extracted successively with n-hexane, chloroform, and ethyl acetate. The ethyl acetate-soluble portion was applied to a Diaion™ HP-20 column, and then eluted by a gradient with increasing methanol (MeOH) in H2O. The active fraction eluted by the H2O and MeOH (50/50, v/v) was further purified on a Sephadex™ LH-20 column in which the column was eluted with MeOH–H2O (7:3), and 5 ml/vial was collected. The pure compound from fraction was obtained by HPTLC using a 3:1:2 butanol: acetic: water as solvent system.
2.4 Structure elucidation
MS and NMR spectra (1H NMR in deuterated methanol) were used for the identification of the purified compounds. The NMR spectra (500 MHz) (Bruker BioSpin GmbH, Rheinstetten, Germany). The EI-MS spectra were recorded on a JMS-SX102A spectrometer.
2.5 Biosynthesis of SeNPs and SeNPs@CuO
For selenium NPs biosynthesis, 50 ml of pomegranate aqueous extract is mixed with 50 ml of deionized water containing 0.2 g of sodium selenite at room temperature. In another set of experiments, a copper sulphate solution (0.2 g/100 ml) was added to biogenic selenium nanosuspension in a volume ratio of 1:1 at ambient temperature for the synthesis of SeNPs@CuO.
2.6 Evaluation the role of caffeic and cinnamic acid in synthesis of selenium NPs
Caffeic and cinnamic acids were added to sodium selenite in a volume ratio of 1:1 at ambient temperature for evaluation of selenium NPs synthesis. The nanoparticulates were examined by UV–VIS (Specord Plus 210, Analytic Jena, Germany).
2.7 Characterization of nanomaterials
The reduction of selenium ions by pomegranate extract into SeNPs and SeNPs@CuO nanocomposite was checked by using UVVIS Spectra Analysis (UV–VIS (Specord Plus 210, analytic Jena, Germany) Plant chemistry Lab, Egyptian Drug Authority), high-resolution transmission electron microscope (HRTEM, JEOL-JEM-2010), Energy Disperse X-Ray, and a Fourier transform infrared spectroscope (FTIR) (Bruker).
2.8 Collection of H. pylori isolates
Ten H. pylori strains were received from Dr. Tarek AM Ismaeil from laboratories in some Egyptian hospitals (Cairo governorate, Egypt).
Bacteria samples were cultivated on Colombia blood agar plate (Oxoid, Basingstoke, UK) containing antibiotic supplements (vancomycin 6 mg/L, polymyxin-B 2500 IU/L, and amphotericin-B 2 mg/L) with 7% sheep blood. The plates were incubated at 37 °C in microaerophilic conditions (10% CO2, 85% N2, 5% O2) and observed after 72 h. Organisms were identified as H. pylori based on colony morphology, modified Gram staining, and positive oxidase, catalase, and rapid urease tests (Van der Hulst et al. 1996; Mishra et al. 2002 a and b).
2.9 Antibiotics susceptibility test for H. pylori
The antimicrobial sensitivity of Twenty-five H. pylori isolates was detected by the agar disk-diffusion method. Bacterial suspensions (McFarland tube no. 3) of H. pylori were inoculated on Mueller-Hinton Agar (Oxoid, Basingstoke, UK) supplemented with 10% horse blood. The disks (6-mm diameter) of different antibiotics (Metronidazole (5 µg), Clarithromycin (15 µg), Levofloxacin (5 µg), Amoxicillin/clavulanic acid (20/10 µg), Tetracycline (30 µg) and Amoxicillin (10 µg) placed on the plates, and incubated at 37 °C under microaerophilic conditions for 72 h and checked for the diameter of the inhibition zone, which was measured in millimetres. Based on Clinical and Laboratory Standards Institute (CLSI) guidelines for the fastidious organism H. influenza. Zone size ≤ 18 mm was resistant to amoxicillin (an analog of ampicillin); ≤19 mm was resistant to amoxicillin/clavulanic acid; ≤10 mm was resistant to clarithromycin; ≤25 mm for tetracycline; and ≤17 mm for levofloxacin (CLSI, 2018). For metronidazole ≤ 16 mm was resistant (Mishra et al. 2006).
2.10 Molecular identification of H. pylori using Hp1, Hp2 and Hp3
In this work, a nested PCR approach has been used to detect H. pylori DNA. It was performed in two sequential amplification rounds using three oligonucleotide primers, Hp1, Hp2, and Hp3. A positive control of DNA H. pylori SS1 and a negative control of double-distilled water were used per amplification in a thermal cycler (Gene Amp PCR System 9600, Perkin-Elmer, USA).
Primer sequences used in this study are as follows:
HP1(5′- CTGGAGAGACTAAGCCCTCC-3′).
HP2 (5′- ATTACTGACGCTGATTGTGC-3′).
HP3 (5′- AGGATGAAGGTTTAAGGATT-3′).
The first amplification round was performed with a 5 μl template DNA, 3 μl Hp1 and Hp3 primers, 25 μl Taq PCR master mix (2x), 17 μl nuclease free water. The second amplification round was performed with a 1 μl first amplification product, 3 μl Hp1 and Hp2 primers, 15 μl Taq PCR master mix (2x), 11 μl nuclease free water. The first and second amplifications were performed as follows: 92 °C, 2 min; 50 °C, 2 min; 72 °C, 2 min; a final step at 72 °C, 4 min (35 cycles). The products of the second PCR, with an expected size of 109 bp, were visualized by gel electrophoresis (Cirak et al. 2003).
2.11 Antimicrobial studies using agar disc diffusion method
The antimicrobial activity of SeNPs, and SeNPs@CuO was investigated against multidrug resistant H. pylori by using the agar disc diffusion method. A 0.5 McFarland turbidity H. pylori strains were inoculated on Muller–Hinton agar (MHA) plates. A sterile cork-borer was used to make wells in the plates.Then 100 μl of SeNPs, and SeNPs@CuO in 20% DMSO solutions were distributed into each well. The 20% DMSO solutions were used as negative control, and the petri-plates were incubated at 37 °C for 24 h. The diameter of the inhibition zone was measured in mm.
2.12 Determination of the minimum inhibitory concentration (MIC) based on broth micro dilution method
Determination of the MIC based on a modified broth microdilution method according to DIN 58940 Part 8 and Weseler et al., 2005. 96-well-microtitre plates were prepared with two-fold dilutions ranging from 1024 to 8 µg/ml of SeNPs@CuO, respectively. The tested nanomaterials were added to H. pylori inoculated brucella broth, which is supplemented with 5% horse serum in a 1:1 (v/v) ratio. At least 100 µl of the inoculated brucella broth supplemented with 5% horse serum was used as a negative control on a test tray. Each test was performed in duplicate and repeated three times. Plates were incubated at 37 C in microaerophilic condition. In order to ascertain the MIC values, 40 ml of a p-iodonitrophenyl tetrazolium violet (INT) solution (0.6 mg/ml) was added to each well. After 2 h of further incubation at 37C the MIC was determined visually as the lowest concentration of antimicrobial test substance at which no color change occurred.
2.13 Cell viability assay
A 10 × 103 concentration of SNU-16 cancer stomach cell lines were seeded in fresh complete growth medium in 96-well microtiter plates and incubated for 24 h at 37 °C in a water jacketed CO2 incubator. A fresh medium was added, and cells were incubated for 48 h alone or with, selenium, PE, SeNPs, Se@CuO nanocomposite, and staurosporine as a standard drug to give a final concentration of 0.39, 1.56, 6.25, 25, and 100 µg (El-Menshawi et al. (2010)).
3 Result and discussion
3.1 Identification of the isolated compounds
The phytochemical investigation of PP led to the isolation of two compounds (Fig. 1). They were identified, on the basis of their MS and 1H data, as caffeic acid (compound 1), cinnamic acid (compound 2).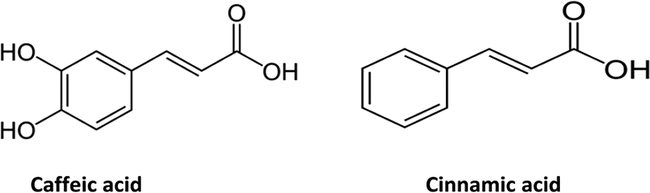
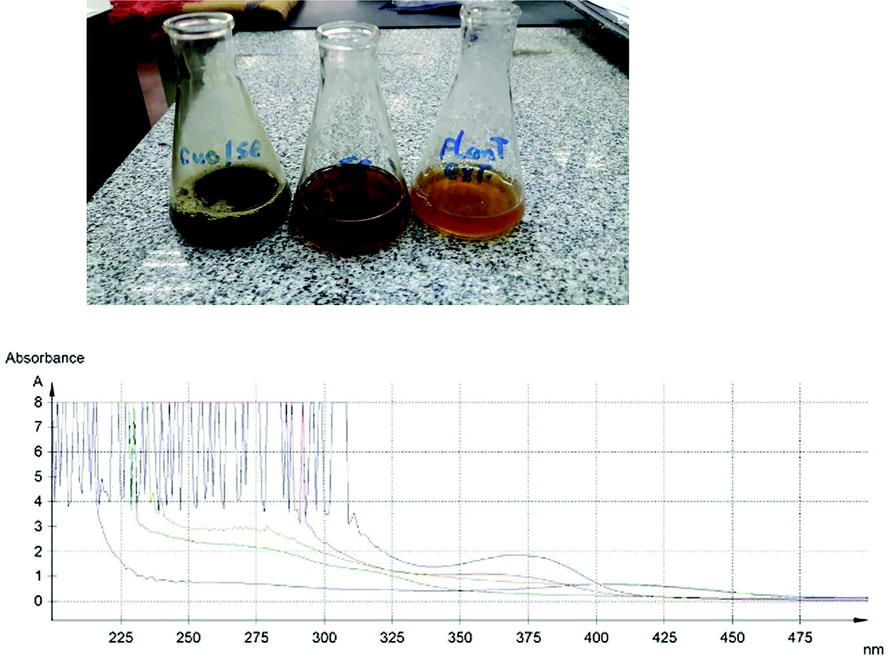
UV spectroscopy of PE, SeNPE, SENPCo, SeNPCI, and Se@CuO, PPAE: Pomegranate peel Aqueous Extract, SeNPE: Selenium NPs synthesized by PPAE, SeNPCo: Selenium NPs synthesized by isolated caffeic acid, SeNPCI: Selenium NPs synthesized by isolated cinnamic acid.
Compound 1 was obtained as an amorphous powder, and exhibited an [M] + ion peak at m/z 180 as a base peak, [M−COOH]+ and [M−OH] + ions at m/z 135 and 163, respectively. In the 1H NMR spectrum there were aromatic methine protons at 7.03, 6.93, and 6.77. Based on the above spectroscopic data, the active compound was identified as caffeic acid.
Compound 2: 1H NMR: 6.47 (C8—H), 7.42 (C3—H, C4—H and C5—H), 7.57(C2—H, C6—H), 7.81 (C7—H), 11.01(—COOH), According to 1H NMR, compound 2 was identified as cinnamic acid.
3.2 Biosynthesis of SeNPs and SeNPs@CuO nanocomposite
In this investigation, SeNPs were synthesized using pomegranate extract (PE). After 24 h a visible color change from a slight yellowish to a faint brick red color confirmed the reduction of sodium selenite into SeNPs. After adding copper sulfate solution, the color changed to black, as shown in photograph (1). Abbas and Abou bakr (Abbas and Abou Baker (2020)) synthesized SeNPs using Fusarium semitectum culture filtrate, and a color change from yellow to red orange was observed, which confirmed the presence of α – Se. UV- spectroscopy was performed for the synthesized SeNPs by PE, caffeic and cinnamic, CuO coated SeNPs, and PE as a negative control. Different absorption peaks indicated the enrichment of plant extracts with polyphenols (200–300 nm). The formation of SeNPs due to combinations of several polyphenol substances in PE prompted the reduction process. The maximum absorption peaks for SeNPs were 357, and 369 nm in the case of using PE and caffeic, respectively. This indicates the role of caffeic acid in PE constituents in the biosynthesis of SeNPs. However, no characteristic absorption peak was noticed in the case of the use of cinnamic acid in the biosynthesis of SeNPs (Fig. 1). A major factor in the biosynthesis of SeNPs is the presence of reducing and stabilizing agents. There are several biomolecules present in plant extracts, including polysaccharides, phenolic compounds, flavonoids, tannins, saponins, amino acids, enzymes, proteins, and sugars, that act as potential reducers. In agreement of our study, phytochemicals found in plant extracts were reported to be stabilizers by some authors (Guleria et al., 2020; Alagesan and Venugopal 2019).
The coating of SeNPs biosynthesized by PE extract was determined by UV spectroscopy. Subsequent color changes take place to black color, where the orange color is characteristic for CuONPs. The redshift for the wavelength from 357 nm to 408 nm occurs after coating SeNPs by CuONPs.
The mode of Se@CuO nanocomposite synthesis depends on the polyphenols, proteins, and carbohydrates of the PE. IR spectra of dried PP revealed various functional groups of these components as follows: 3,291.25 cm–1 peak corresponds to O—H stretching vibration of phenols, 2920 cm−1 represents the stretching vibration of C—H alkane,1627 cm−1 peak corresponds C⚌C— stretching vibration of alkene. A 1409 cm–1 band indicated the presence of O—H bending of carboxylic acid (Fig. 2). To elucidate the functional group responsible for the biosynthesis of CuO-coated Se NPs, their IR was compared with the dried PP IR spectrum (Fig. 2b). However, in the case of CuO-coated Se NPs, characteristic blue shifts for the absorption bands 1627 cm−1 into 1607 cm−1, which is assigned to —C⚌C— stretching vibration of alkene, 3,291.25 cm–1 into 3261 cm−1, and in 1409 into 1359 cm−1 (Fig. 4). further, a slightly red shift for the absorption band 1028 into 1071 cm−1, which is corresponding to C—O stretching. Our findings estimated that phenols, phenolic acids, sugars, and proteins aided the reduction of sodium selenite and copper salts and the formation of nanocomposite.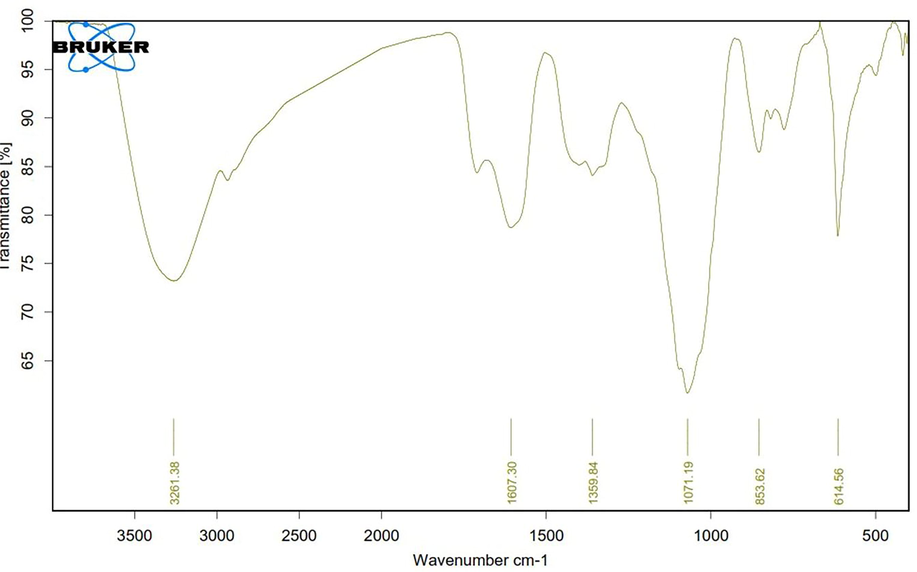
FTIR of SeNPs@CuO nanocomposite.
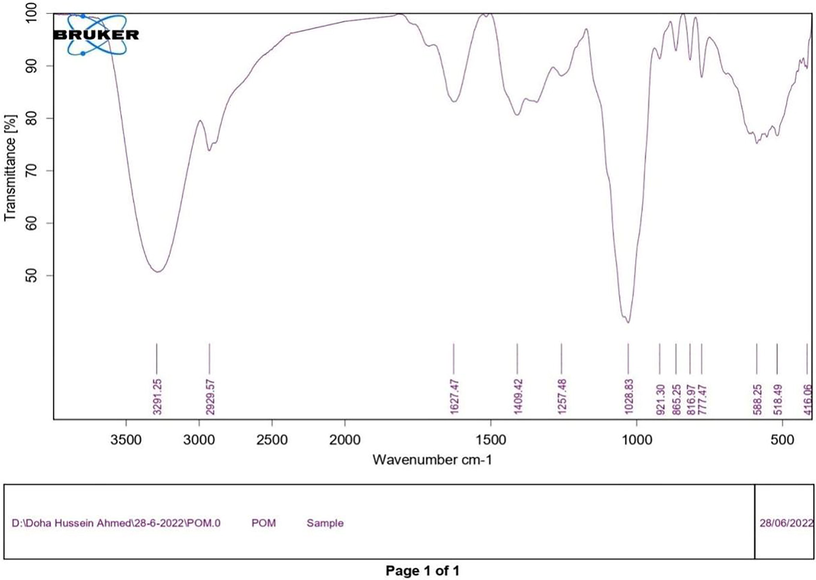
FTIR of pp extract.
HRTEM micrographs showed spherical shaped uncoated SeNPs with an average size of 5.89 nm. The size of uncoated SeNPs synthesized by PE was ranged from 1.97 nm to 10.56 nm (Fig. 3). This et al. (2017), where their sizes ranged from 24 to 134 nm depending on the various sodium selenite salt concentrations utilized. In that regard, Abbas et al. (2021) synthesized a spherical shaped SeNPs from cyanobacteria extract with an average size of 79.40 ± 44.26 nm.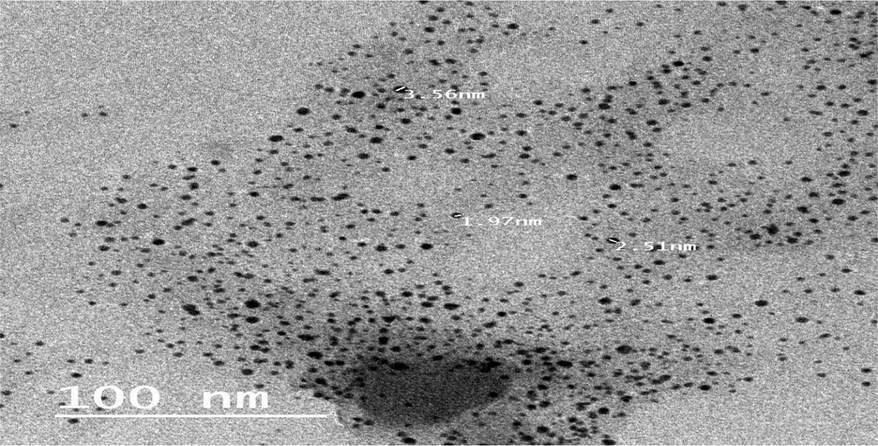
High Resolution Transmission Electron Microscope of SeNPs synthesized by PE.
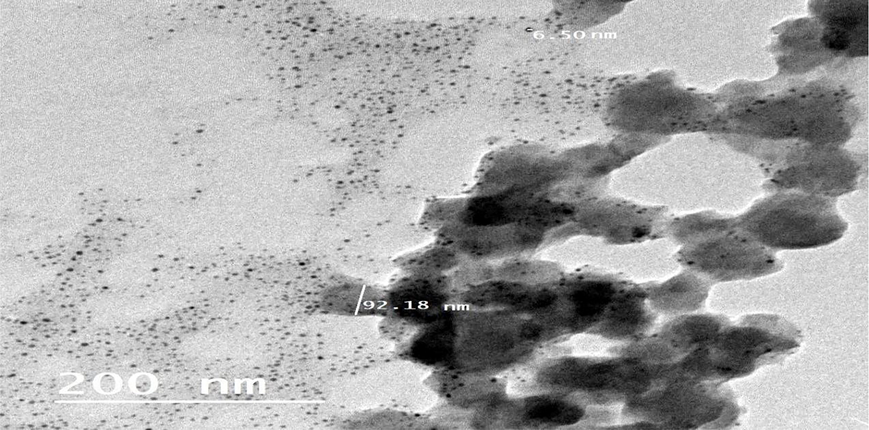
High Resolution Transmission Electron Microscope of SeNPs@CuO nanocomposite.
However, HRTEM images in Fig. 4 represent a spherical shape of CuO@SeNPs with an approximately average size of 92.18 nm. From this observation, the size of uncoated SeNPs was smaller than coated CuO@SeNPs. The outer SeNPs coat will integrate with the inner CuONPs. The size of CuO@SeNPs nanocomposite has been increased 18 times. Similar findings were reported by Abbas et al. (2022) in the case of biosynthesized αFe2 O3 NPs@ZnO nanocomposite. Also, Malathi et al. (2018), synthesized a rod-shaped α-FeOOH/BiOI nanocomposite with an average length of 180 nm. No more research literature concerns the synthesis of CuO@SeNPs nanocomposite by using biological methods.
3.2.1 Anti H. pylori
To evaluate the antimicrobial effect of the prepared SeNPs, and SeNPs@CuO nanocomposite against Helicobacter pylori strains, ten strains were identified as Helicobacter pylori according to the 16 s rRNA PCR assay (Fig. 5) and the PCR result, which was 109 bp, was as anticipated. Our findings were similar to those of Kargar and Doosti's (2012) study, which used a 16S rRNA PCR technique to check 150 stomach biopsy samples for the presence of H. pylori. Antibiotic susceptibility was evaluated for ten isolates. The ten isolates showed resistance against Metronidazole (5 µg), Clarithromycin (15 µg), Levofloxacin (5 µg), Amoxicillin/clavulanic acid (20/10 µg), Tetracycline (30 µg), Amoxicillin (10 µg) antibiotics based on Clinical and Laboratory Standards Institute (CLSI, 2015) guidelines for the fastidious organism H. influenza (Table 1). The antimicrobial efficacy of SeNPs, and coated CuO@SeNPs was assessed against MDR H. pylori by Agar well diffusion method. The diameter of inhibition zones in SeNPs and coated CuO@SeNPs showed very week inhibition zones. The inhibition zones for SeNPs ranged from zero to 9 mm. However, for the coated SeNPs, the inhibition zones increased to 11–15 mm (Fig. 6). By using the broth dilution method, the MIC for CuO @ SeNPs was 8 µg/ml for inhibition of the growth of 100% MDR H. pylori (Fig. 7). However, no effect on H. pylori appears by increasing the concentration of CuO @ SeNPs. An electromagnetic interaction between the positively charged nanoparticles and the negatively charged bacterium is one potential explanation for the antibacterial activity of metal nanoparticles. Once this attraction happens, the bacteria oxidize and perish right away (Haq et al., 2022). The majority of ions produced by nanoparticles interact with the -SH groups of the proteins on the surface of existing bacterial cells, leading to cell death (Rezaei-Zarchi et al., 2010; Zhang and Chen, 2009). It was proposed that highly concentrated metal oxide NPs would electrostatically repel negative bacteria, resulting in minimal antibacterial efficacy. Due to oxidative stress caused by reactive oxygen species, however, the positive potential metal oxide NPs with low concentrations had a robust contact with the surface of the negative bacterium (ROS) (Arakha et al., 2015), which totally agrees with our study. Recently, environmentally friendly metal oxide nanoparticle antibacterial investigations are gaining popularity in the fields of health and pharmaceutical development.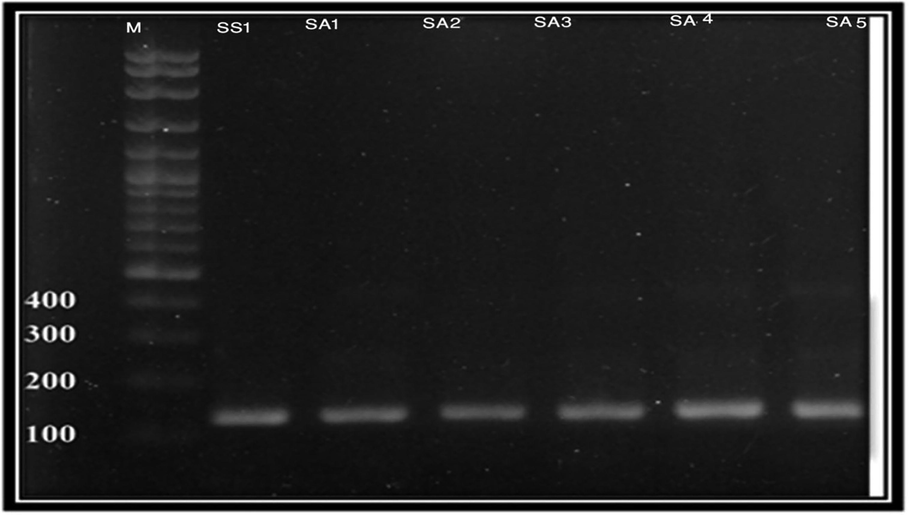
Molecular Identification of H. pylori using Hp1, Hp2 and Hp3.
Antibiotics
Isolates
Zone of Inhibition (cm)
CLR(15mcg)
AX(25mcg)
TE(30mcg)
LEV(5 µg)
MET(5mcg)
OFX(5 µg)
DO(30 µg)
CIP(5mcg)
SAM(20 µg)
AP(10 µg)
1
1.2
0
0
0
0
0
0
0
0
0
2
1
0
1.9
2.3
0
2.1
0.8
3.1
0
1
3
1.5
1.5
1.2
1.5
0
2.4
1.7
2.3
0.7
0.8
4
0.8
1.6
0
2.7
0
2.5
0.7
1.8
0
0
5
1.2
0
0
0
0
0
0
0
0
0
6
1
0.7
1.1
2
0
1.8
1
2.2
0
1
7
1.7
0
1
2.2
0
0
0
0
0
0
8
1.4
0
0
2
0
0
0
0.8
0
0
9
1.1
1
1.3
2.7
0
2.7
1.2
3
0.7
0.9
10
1
0
1.4
0
0
1.7
1
0.9
0
1

Screening for antibacterial activity for coated SeNPs@CuO, and SeNPs against H pylori.

Determination MIC of SeNPs@CuO using Broth dilution Method. (The concentrations were ranged from 1024 to 8 µg /ml from left to right, last vertical row is for negative control).
3.3 Cytotoxicity results
Among the various biological causes of gastric cancer, H. pylori infection is a well-known bacterial infection that can lead to the development of gastric cancer. H. pylori infection can have a direct epigenetic effect on gastric epithelial cells and indirectly induce an inflammatory response in the gastric mucosa (Yang et al., 2020; Machlowska et al., 2020; Thrift and El-Serag, 2020; Wagner et al., 2017; Zitvogel et al., 2008; Lodesani and Costa, 2005). H. pylori is a cross-resistance phenomenon not only to chemotherapeutic agents, but also to many anticancer agents that have different functional mechanisms and structures (Gottesman and Ling, 2006). In this context, the antiproliferative role of PG-SeNPs and SeNPs@CuO nanocomposites against gastric cancer cells (SNU-16) was evaluated using the MTT assay. As shown in Table 2, treatment of SNU-16 cells with SeNPs@CuO and PG-SeNPs nanocomposites resulted in inhibition of cell viability, indicating a decrease in the rate of proliferation in a concentration-dependent manner. The PG-SeNPs and SeNPs@CuO nanocomposites showed a half-maximum value (IC50) of 7–12 µg/ml. Results were in agreement with Mi et al., (2022) who found that SeNPs mediated by silymarin significantly inhibited gastric cancer cells without affecting normal cells. By increasing the expression of caspase proteins, cytochrome c, and Bax/Bcl-2. Moreover, inhibiting PI3K/AKT/mTOR pathways in gastric cancer cells. Percent cell viability was scored following treatment with 0–100 µg/ml of PG-SeNPs and SeNPs@CuO nanocomposite concentrations. Data are presented as the mean ± SE of three independent experiments.
Sample
IC50 µg/ml
SNU-16
PPAE
14.02 ± 0.84
SeNPs@CuO nanocomposite
7.11 ± 0.43
PG-SeNPs
12.91 ± 0.78
Selenium
22.63 ± 1.36
Staurosporine
8.88 ± 0.53
4 Conclusions and recommendations
Chronic infection with H. pylori is a direct cause of serious gastric diseases such as gastric cancer (Shah et al., 2021). All patients infected with H. pylori should receive eradication treatment. However, due to antibiotic resistance, the eradication rate of H. pylori is much lower than before. The development of new and effective strategies to enhance H. pylori eradication is urgent. Crossing the gastric mucus barrier, killing intracellular H. pylori, relieving oxidative stress, and destroying H. pylori biofilms are major anti-H. pylori difficulties in treatment. Nanostructures have great potential to increase antibacterial activity (Wong et al., 2020). Here, we synthesized biogenic SeNPs useful for destroying H. pylori and stomach cancer cells. This type of antibiotic-free strategy offers a new direction for the multidimensional treatment of H. pylori infections. As part of our future endeavours in nanoparticle toxicity analysis, our focus must be on anticancer and antimicrobial in vitro studies.
Funding
This research funded by National Research Center, Dokki, Egypt.
Ethics approval and consent to participate
This manuscript does not contain studies involving human participants, human data or human tissue or animals.
Consent for publication
This manuscript does not contain any individual person’s data in any form (including individual details, images or videos).
Acknowledgements
We acknowledge National Research Centre for funding this study (project No. 12020209).
Author information and contribution
Doha Abou baker Prof of Medicinal and Aromatic Plants Department. Pharmaceutical and Drug Industries Division, National Research Centre.
The PI of the project (12020209) and responsible for chemistry, cytotoxicity part, writing and publishing the manuscript.
Heba Abbas, Microbiology Department, National Organization for Drug Control and Research (NODCAR), responsible for microbiology and nanotechnology part.
And also participate in writing and reviewing the manuscript.
Availability of data and materials
Data will be available.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Biological evaluation of selenium NP biosynthesized by Fusarium semitectum as antimicrobial and anticancer agents. Egypt. J. Chem.. 2020;63(4):1119-1133.
- [Google Scholar]
- Cytotoxicity and antimicrobial efficiency of selenium nanoparticles biosynthesized by Spirulina platensis. Arch. Microbiol.. 2021;203:523-532.
- [Google Scholar]
- Helicobacter Pylori Treatment Eradication in Egypt: Standard Clarithromycin-based Triple versus Quadruple Regimen Therapy. Afro-Egypt. J. Infect. Endemic Dise. 2020:199-206.
- [Google Scholar]
- 23 years of the discovery of Helicobacter pylori: is the debate over? Ann. Clin. Microbiol. Antimicrob.. 2005;4:17. Published 2005 Oct 31
- [Google Scholar]
- Green synthesis of selenium nanoparticles using leaves extract of Withania somnifera and its biological applications and photocatalytic activities. BioNanoScience. 2019;9:105-116.
- [Google Scholar]
- Green synthesis of silver nanoparticles: structural features and in vivo and in vitro therapeutic effects against helicobacter pylori induced gastritis. Bioinorg. Chem. Appl.. 2014;2014:135824
- [CrossRef] [Google Scholar]
- Antimicrobial activity of iron oxide nanoparticle upon modulation of nanoparticle-bacteria interface. Sci. Rep.. 2015;5:1-12.
- [Google Scholar]
- The comparison of levofloxacin- and clarithromycin-based bismuth quadruple therapy regimens in helicobacter pylori eradication. J. Res. Pharm. Pract.. 2020;9(2):101-105. Published 2020 Jun 26
- [CrossRef] [Google Scholar]
- Helicobacter pylori virulence factors-mechanisms of bacterial pathogenicity in the gastric microenvironment. Cells.. 2020;10(1):27.
- [CrossRef] [Google Scholar]
- Non-invasive diagnostic tests for Helicobacter pylori infection. Cochrane Database Syst. Rev.. 2018;3(3):CD012080.
- [CrossRef] [Google Scholar]
- Chuh, L.F., Klemeš, J.J., Bokhari, A., Asif, S., Cheng, Y.W., Chong, C.C., Show, P.L., 2022. Chapter 3 - A review of intensification technologies for biodiesel production, Editor(s): Claudia Gutiérrez-Antoni, Fernando Israel Gómez Castro, Biofuels and Biorefining, Elsevier, 2022, pp. 87–116.
- Detection of helicobacter pylori and Its CagA gene in tonsil and adenoid tissues by PCR. Arch Otolaryngol. Head Neck Surg.. 2003;129:1225-1229.
- [Google Scholar]
- CLSI, 2015. Methods for Antimicrobial Dilution and Disk Susceptibility Testing of Infrequently Isolated or Fastidious Bacteria. 3rd ed. CLSI guideline M45. Wayne, PA: Clinical and Laboratory Standards Institute.
- CLSI. 2018. Performance Standards for Antimicrobial Disk Susceptibility Tests. 13th ed. CLSI standard M02; Wayne, PA: Clinical and Laboratory Standards Institutes.
- Screening of natural products for therapeutic activity against solid tumors. IJEB. 2010;48:258-264.
- [Google Scholar]
- Screening of natural products for therapeutic activity against solid tumors. Indian J. Exp. Biol. 2010:258-264.
- [Google Scholar]
- The molecular basis of multidrug resistance in cancer: the early years of P-glycoprotein research. FEBS Lett.. 2006;580(4):998-1009.
- [Google Scholar]
- Room temperature ionic liquid assisted rapid synthesis of amorphous Se nanoparticles: their prolonged stabilization and antioxidant studies. Mater. Chem. Phys.. 2020;253:123369
- [Google Scholar]
- A study on the uptake of methylene blue by biodegradable and eco-friendly carboxylated starch grafted polyvinyl pyrrolidone. Environ. Res.. 2022;215, Part 1
- [Google Scholar]
- Novel and effective therapeutic regimens for helicobacter pylori in an era of increasing antibiotic resistance. Front. Cell. Infect. Microbiol.. 2017;7
- [CrossRef] [Google Scholar]
- Helicobacter pylori infection: An overview of bacterial virulence factors and pathogenesis. Biomed J.. 2016;39(1):14-23.
- [CrossRef] [Google Scholar]
- Pathogenesis of Helicobacter pylori infection. Clin. Microbiol. Rev.. 2006;19(3):449-490.
- [CrossRef] [Google Scholar]
- Limits of chemotherapy in beekeeping: development of resistance and the problem of residues. Bee World. 2005;86(4):102-109.
- [Google Scholar]
- Gastric cancer: epidemiology, risk factors, classification, genomic characteristics and treatment strategies. Int. J. Mol. Sci.. 2020;21(11):4012.
- [Google Scholar]
- Rod-on-flake α-FeOOH/BiOI nanocomposite: facile synthesis, characterization and enhanced photocatalytic performance. Colloids Surf. A: Physicochem. Eng.. 2018;537:435-445.
- [Google Scholar]
- Biosynthesis and cytotoxic effect of silymarin-functionalized selenium nanoparticles induced autophagy mediated cellular apoptosis via downregulation of PI3K/Akt/mTOR pathway in gastric cancer. Phytomedicine. 2022;99:154014
- [Google Scholar]
- Genotype of Helicobacter pylori isolated from various acid peptic diseases in and around Lucknow. Curr. Sci.. 2002;83:749-755.
- [Google Scholar]
- Antibiotic susceptibility of helicobacter pylori clinical isolates: comparative evaluation of disk-diffusion and E-test methods. Curr. Microbiol.. 2006;53:329-334.
- [Google Scholar]
- Helicobacter pylori. In: Brachman P., Abrutyn E., eds. Bacterial Infections of Humans. Boston, MA: Springer; 2009.
- [CrossRef] [Google Scholar]
- Comparative study of antimicrobial activities of TiO2 and CdO nanoparticles against the pathogenic strain of Escherichia coli. Iran J. Pathol.. 2010;5:83-89.
- [Google Scholar]
- PCR detection of Helicobacter pylori in clinical samples. Methods Mol. Biol.. 2013;943:279-287.
- [CrossRef] [Google Scholar]
- Helicobacter pylori infection in developing countries: the burden for how long? Saudi J. Gastroenterol.. 2009;15(3):201-207.
- [CrossRef] [Google Scholar]
- Prevalence of antibiotic resistance in helicobacter pylori: A systematic review and meta-analysis in world health organization regions. Gastroenterology. 2018;155(5):1372-1382.e17.
- [CrossRef] [Google Scholar]
- AGA clinical practice update on the diagnosis and management of atrophic gastritis: expert review. Gastroenterology. 2021;161(4):1325-1332.
- [Google Scholar]
- 2014Pomegranate fruit as a rich source of biologically active compounds. BioMed Res. Int. 2014
- [Google Scholar]
- Effect of specimen collection techniques, transport media, and incubation of cultures on the detection rate of helicobacter pylori. Eur. J. Clin. Microbiol. Infect. Dis.. 1996;15:211-215.
- [Google Scholar]
- Wagner, A.D., Syn, N.L., Moehler, M., Grothe, W., Yong, W.P., Tai, B.C., Ho, J., Unverzagt, S., 2017. Chemotherapy for advanced gastric cancer. Cochrane database of systematic reviews, (8).
- Nanomaterials for nanotheranostics: tuning their properties according to disease needs. ACS Nano. 2020;14(3):2585-2627.
- [Google Scholar]
- Gastric cancer: Epidemiology, risk factors and prevention strategies. Chin. J. Cancer Res.. 2020;32(6):695.
- [Google Scholar]
- Facing resistance of H.pylori infection. Gastroenterol. Hepatol. Bed. Bench.. 2011;4(1):3-11.
- [Google Scholar]
- Potent antibacterial activities of Ag/TiO2 nanocomposite powders synthesized by a one-pot sol-gel method. Environ. Sci. Technol.. 2009;43:2905-2910.
- [Google Scholar]
- Potent antibacterial activities of Ag/TiO2 nanocomposite powders synthesized by a one-pot sol–gel method. Environ. Sci. Technol.. 2009;43:2905-2910.
- [Google Scholar]
- Weseler, A.H.K.R., Geiss, H.K., Saller, R. and Reichling, J.J.D.P., 2005. A novel colorimetric broth microdilution method to determine the minimum inhibitory concentration (MIC) of antibiotics and essential oils against Helicobacter pylori. Die Pharmazie-An International Journal of Pharmaceutical Sciences, 60(7), pp.498-502.
- Abbas, H.S., Mahmoud, A.M., Wahed, R.A., Elsantawy, M.A.A., Hamdy, N.M., Ismail, S.E. and Nabil, M.A., 2022. Prospects of using bioactive compounds in nanomaterials surface decoration and their biomedical purposes. International Nano Letters, 12 (2), pp.125-138.







