Translate this page into:
Antimicrobial air filter made of chitosan-ZnO nanoparticles immobilized on white silica gel beads
⁎Corresponding author at: Graduate School of Mathematics and Applied Science, Universitas Syiah Kuala, Darussalam Banda Aceh 23111, Indonesia. adlim@unsyiah.ac.id (Muhammad Adlim) adlim@usk.ac.id (Muhammad Adlim)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University. Production and hosting by Elsevier.
Abstract
Airborne disease transmitted by biological air pollution threatens public space, therefore developing antimicrobial air filters is a crucial study. This study investigated the environmentally friendly preparation of synthetic zinc oxide nanoparticles (ZnOnano-synth) at ambient temperature and the ZnO immobilization technique on silica gel beads coated with chitosan. The immobilized ZnO was used as an air filter in the simulated air chamber. Methanol swelled the 4.59 ± 0.5 wt% of the chitosan layer causing ZnO particles to stick to it, being stabilized, and dispersed. The dispersed ZnO particles of 5.18 ± 1.70 nm, thereby the efficacy preparation procedure is confirmed. The ZnOnano-synth exhibited high antibacterial properties against Gram-positive (Staphylococcus aureus) and Gram-negative (Escherichia coli) bacteria in agar media with inhibition diameters up to (33.50 ± 0.10 mm). Air filters containing the ZnOnano-synth-chi-SiG exhibited high and prolonged antibacterial activity against Bacillus subtilis in the air with 92% efficacy. These synthetic ZnO nanoparticles show better characteristics than commercial ZnO nanoparticles in terms of the particle size and the antibacterial properties of Staphylococcus aureus and Escherichia coli.
Keywords
Zinc oxide nanoparticles
Immobilization
Chitosan
Silica gel beads
Antibacterial properties
Air filter
1 Introduction
Air quality has been the interest of study by researchers worldwide, due to the uncontrolled rise in population and human activities that have led to the emission of significant amounts of pollutants and biohazards into the environment. People spend about 16 h a day indoors and in working environments, which are mostly closed spaces (Vu et al., 2022). Hence the concern for environmental safety becomes a crucial issue. Among various tools and technologies such as mechanical collectors, electrostatic precipitators, and fabric filters; air purifiers are used to improve air quality indoors. Air purifiers are equipped with a High-Efficiency Particulate Air (HEPA) filter which is known to remove 99.97% of particles with a size of ≥ 0.3 µm diameter (Dubey et al., 2021; Zhang et al., 2022). Air purifiers with HEPA filters allow air to pass while capturing particles physically through four mechanisms (interception, inertial impaction, diffusion, and sieving). The smallest particles are removed by diffusion while the other three mechanisms work more effectively on large particles (Lowther et al., 2020). However, studies of air filter technologies for the elimination of bacteria and other microorganisms in the air are still rising (Li et al., 2018; Li et al., 2022; Watson et al., 2022).
The bacteria entrainment in indoor air can cause high risks of respiratory disease especially when the enclosed space has inadequate ventilation (Forthomme et al., 2014). In long periods with high relative humidity (>80% room humidity), a proliferation of bacteria in the air that are subsequently stuck at the air filter can be hazardous even at low concentrations, especially for children (Harbizadeh et al., 2019). Thereby, antimicrobial air filter media is a crucial choice to reduce airborne risk (Nunes et al., 2020).
The previous new design for antibacterial air filters loaded with silver nanoparticles (Ag NPs) (Hidayat et al., 2022; Ciorîță et al., 2023; Ali et al., 2018), is still not cost-effective. This study focused on zinc oxide (ZnO) nanoparticles to substitute the silver nanoparticles. ZnO nanoparticles have several advantages including antibacterial and fungal properties (Gudkov et al., 2021; Ahmad et al., 2021; Decelis et al., 2017). The preparation and immobilization of ZnO nanoparticles on the solid support as a photocatalyst and potential air filter media have also been reported by our group (Muktaridha et al., 2021). Zinc oxide/graphene immobilized on glass microfiber inactivated bacteria after ZnO was photochemically irradiated with a 60 Watt LED (Zhai et al., 2022). ZnO nanorods immobilized polytetrafluoroethylene (ePTFE) matrix were antibacterial air filters but reduced the filter's adequate porous size (Zhong et al., 2015). Nanofiber (ZnO@NF) hybrid membranes as air filters were fabricated by immobilized ZnO on PVA-co-PE nanofiber yarns (Yao et al., 2022). The antibacterial filter was prepared from polypropylene melt-blown nonwoven materials, in which ZnO was the electret material to absorb contaminants including bacteria (Han et al., 2022). When the filter fibers/cloth or membrane was coated permanently with ZnO then the air filter porosity becomes lessened, is easily fouled, and requires energy to pump in the air (Jatoi et al., 2019). Therefore, this current study altered the common ZnO preparation by immobilizing the ZnO nanoparticles on white silica gel beads to avoid airflow stuck, as part of newly designed effective and potentially reusable air filters. Low-cost and green preparation, immobilized and stable ZnO nanoparticles on the support are part of this study agenda. ZnO is usually synthesized involving sodium hydroxide and heating at a high temperature, while the preparation at room temperature as reported in this paper is still rare. The study continued with the antibacterial properties of the new environmentally friendly air filter design, providing possibilities for controlling pollutants indoors which are crucial in vulnerable places (De Almeida et al., 2022).
Green synthesized ZnO nanoparticles showed a strong bactericidal effect on a wide variety of pathogenic bacteria due to their biocompatibility, low toxicity, and high specific surface area (Huang et al., 2020; Amininezhad et al., 2014). Chitosan was used as a template for nanoparticle attachment, acted as a stabilizing agent, and prevent the agglomeration of ZnO (Adlim et al., 2019), so the utilization of ZnO nanoparticles would be effective to apply as antibacterial air filters. Chitosan functioned as the stabilizer for metal nanoparticles is verified in the literature (Zarharan et al, 2023).
In this current study, the survival rates of Staphylococcus aureus and Escherichia coli bacteria which are typically Gram-positive and Gram-negative bacteria after contact with synthetic ZnO nanoparticles were investigated. Staphylococcus aureus and Escherichia coli themselves are pathogens, then their strains are pathogens. Staphylococcus aureus is notorious for causing skin and soft-tissue infections and it has the ability to infect nearly every organ system in the human body with often fatal consequences (Bose & Bayles, 2013). Escherichia coli is a foodborne pathogen that causes intestinal disease in humans (Govindarajan et al., 2020). The antibacterial filter experiment is only tested on gram-positive bacteria in the air since they were the dominant species producing flying spores in the air (Harbizadeh et al., 2019). Then Bacillus subtilis survival rates before and after penetration of the air filter chamber, are compared. These antibacterial performances provide possibilities for the future design of antibacterial air filters in consideration of their environmental friendliness, controlling pollutants indoors, when used correctly (with periodic maintenance) can provide better air quality in the environment (De Almeida et al., 2022).
2 Materials and method
2.1 Material
Chitosan of medium molecular weight (∼400,000) purchased from Sigma-Aldrich (CAS: 9012–76-4, USA; DD > 75 %), symbolized as ‘chi-‘ and white silica gel beads (sorbed India production: spherical with specification refer to https://www.silicagel-desiccant.com/silica-gel-white (Desiccants, 2023) were purchased from the local market (Indonesia) and indicated as ‘SiG’. Acetic acid glacial solution (CH3COOH 98%), zinc nitrate hexahydrate crystal (Zn(NO3)2·6H2O), commercially available zinc oxide nanopowder (ZnO) denoted as ZnOnano-com, and methanol (CH3OH), and sodium hydroxide (NaOH) were of analytical grade and purchased from Merck KGaA (Indonesia). Distilled water (H2O) was purchased from the Chemistry Laboratory of Syiah Kuala University (Indonesia). Bacteria of Escherichia coli (E. coli, ATCC 25922), Staphylococcus aureus (S. aureus, ATCC 25923), Bacillus subtilis (B. subtilis, ATCC 6633), Mueller-Hinton agar (MHA), and nutrient agar (NA) were all obtained from Dipa Puspa Labsains (Indonesia). All chemicals were used without further purification.
2.2 Equipment
A binocular microscope stereo (Olympus SZ61, Indonesia) equipped with an optical camera (OptiLab, Indonesia) was used to observe all samples including chi-SiG (original, before, and after immersing with several solvents), ZnO immobilized on chi-SiG, spore, and Gram staining. A scanning electron microscope (SEM, JEOL JSM 6360 LA, Indonesia) was used to observe the sample surface area. A transmission electron microscope (TEM, JEOL JEM-1400, Malaysia) was used to determine the particle size of ZnO. The zinc content on samples was analyzed with atomic absorption spectroscopy (AAS, ICE 3000 Series, Indonesia). Crystallinity analysis was carried out using X-ray diffraction (XRD, Shimadzu 7000, Indonesia). Spore and Gram staining were observed using a microscope (Olympus CX-21, Indonesia). Fourier-transform infrared spectroscopy (FTIR, Shimadzu IR Prestige-21, Indonesia) was used to analyze the bonding of synthesized nanoparticles with functional groups of chitosan.
2.3 Procedure
2.3.1 Coating and characterization of chitosan on white silica gel beads
The coating procedure was prepared according to a previously described procedure (Adlim et al., 2021) with minor modifications. White silica gel beads (20.866 g) were soaked within 50 mL of chitosan solution for 60 min at room temperature, then it was dried in the oven for 60 min at 40 °C (called the first coating) and denoted as chi-SiG. Then, it was repeated to get the third coating. The drying process was carried out until a constant weight was obtained. The sample was characterized by using a binocular microscope stereo and SEM. The third coating chi-SiG was then used in the following experiments.
The coating stability of chi-SiG was verified by suspending the chi-SiG in several solvents. Several granules of chi-SiG were soaked into a separated small beaker with 5 mL of methanol, 5 mL of 0.1 M sodium hydroxide, and 5 mL of 1:1 (v/v) methanol-0.1 M sodium hydroxide for 60 min at room temperature. All chi-SiG was then dried in the oven for 60 min at 100 °C. The chi-SiG was observed using a binocular microscope stereo. The experiments were repeated for swelling studies but drying was excluded from the procedure. The different weights of dried and wet chi-SiG were recorded with an analytical balance (dried sample; and wet sample; . The wet chi-SiG piece was air-dried and dried in the oven until the constant weight and denoted as . The swelling studies were referred to in the previous study (Wu et al., 2018) with minor modifications.
The swelling property (
was calculated by using the equation:
Chitosan stability (
was calculated by using the equation:
= swelling property
= chitosan solubility
= weight of the dried sample before immersing it into the solvent
= weight of the wet sample after immersing into the solvent
= weight of the dried sample after immersing into the solvent
2.3.2 Immobilization and characterization of ZnO nanoparticles on chi-SiG
The synthesis of ZnO nanoparticles was prepared according to a previously described procedure (Donia et al., 2021; Haase et al., 1988) with some modifications and immobilized on chi-SiG. The chitosan layer on chi-SiG was used as the adhesive and stabilizer for ZnO in the immobilization process. Crystal Zn(NO3)2·6H2O (5.130 g) was dissolved in 10 mL methanol and transferred into a 100 mL flask. After all the crystals had been dissolved, 10 mL of 0.1 M NaOH was added, a cloudy white suspension was formed and then, the chi-SiG (30.140 g ∼ 942 granules) was added into the flask. The flask was set in a rotary evaporator and rotated at 225 rpm, equipped with a water bath without heating. Furthermore, the Tungsten lamp (40 W at a 7 cm distance from the round flask) irradiated the round flask for 150 min and the temperature was maintained at ∼ 40 °C. The sample was dried in the oven for 72 h at 60 °C and the color all changed to white. The sample is denoted as ZnOnano-synth-[chi-SiG]. Two other samples were also prepared as a control. The first immobilization was ZnO nanopowder commercially available which was denoted as ZnOnano-com-[chi-SiG] and the second was Zn(NO3)2 symbolized as Znsalt-[chi-SiG] without the addition of NaOH. Immobilization of ZnOnano-com on chi-SiG was prepared by mixing wet chi-SiG (1.125 g) with 1.000 g of ZnOnano-com in a round flask and rotated with similar conditions as mentioned above before drying in the oven for 60 min at 60 °C. The samples were characterized with the light microscope, SEM, TEM, XRD, AAS, and FTIR methods.
The amount of zinc in all samples was calculated by using the AAS method. Each of ZnOnano-synth-[chi-SiG] (3.1628 g), ZnOnano-com-[chi-SiG] (3.0779 g), or Znsalt-[chi-SiG] (3.1272 g) was put into each 250 mL beaker and 5 mL of concentrated HNO3 was added. The solution of each beaker was then mixed for 20 min by using a glass spatula. The distilled water (100 mL) was added and mixed thoroughly. The mixture was boiled until the volume was ∼ 30 mL to expel nitric acid digestion gases and dissolve all soluble salts. The solution of each sample was filtered with a Whatman filter paper 2 into a 50 mL volumetric flask. The distilled water was added to each volumetric flask to rinse the filter paper up to 50 mL before the AAS analysis. Preparations of all zinc samples without SiG immobilization were repeated to prepare for XRD and antibacterial analysis.
2.3.3 The antibacterial property of ZnOnano-synth-[chi-SiG] against Gram-positive and Gram-negative bacteria
The antibacterial activity was performed by a previous study (Hidayat et al., 2021). All samples were tested against S. aureus and E. coli by determining the inhibition zone diameters after exposure to the sample. As a comparison, the use of SiG and chi-SiG as the control. The bacterial suspension was set to a 0.5 McFarland standard equal to 1.5 × 108 CFU/mL bacteria and inoculated on an agar plate of Mueller-Hinton agar (MHA). Three granules of each sample were put into a petri dish that contained MHA and bacterial suspensions. The agar was then incubated at 37 °C for 24 h before measuring the inhibition zone diameters. Screened the antibacterial activity of all samples, and then, the highest antibacterial activities were chosen to be applied in the simulated air filter. The research methodology flowchart is shown in Fig. 1.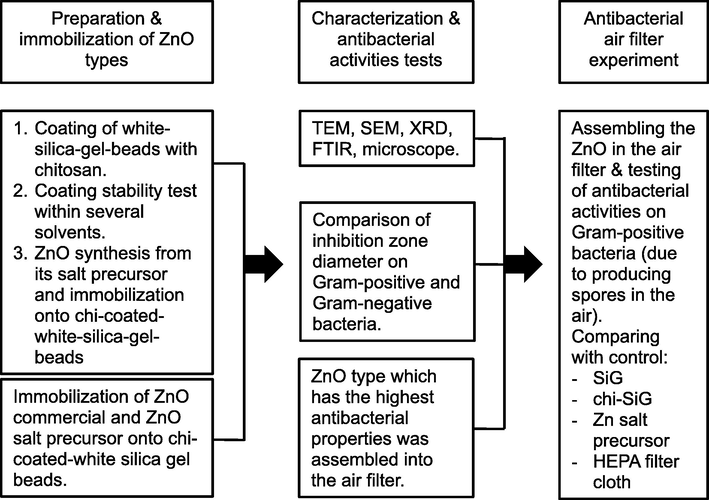
The research methodology flowchart.
2.3.4 Preparation of air filter chamber and air filter for the simulation
The air filter chamber was made of acrylic glass (34 cm × 12 cm × 11 cm) that was equipped with a small fan 5 V (8.8 cm × 9 cm × 3.7 cm) on the left side and another fan 12 V (10 cm × 10 cm × 2 cm) on the right side. Chamber was in the horizontal position. Two agar media were prepared; the first agar was contaminated with bacterial suspension of B. subtilis (on the left side) and another was a sterile agar media (on the right side) as presented in Fig. 2. The left fan was turned on to dry and flow media containing bacteria or spore, while the right fan was also turned on to blow the air out from the chamber. The air-containing bacteria was allowed to pass through the air filter. The experiment was performed for two days and both fans were run for 60 min per day at a velocity of 3.1 m/s. Bacteria colony before and after exposure was compared. Bacteria stuck on air filters was also observed. The detailed procedure for the antibacterial air filter test is described in Section 2.3.5. Furthermore, the air filter was made from pieces of nylon wire (10 cm × 10 cm) as presented in Fig. 3. This air filter has a pore size ± 1 mm to cover all granules in the filter. The prepared samples of ZnOnano-synth-[chi-SiG] were filled into the air filter to test. Air filters containing SiG, chi-SiG, Znsalt-[chi-SiG], and HEPA filter cloth (Sharp FZ-F30, Indonesia) were also prepared as the control for antibacterial testing. Each air filter was a single layer containing ∼ 629 granules. Furthermore, both the air filter chamber and filter were sterilized using UV light irradiation (ESCO airstream) at 250 nm for 30 min before the test run.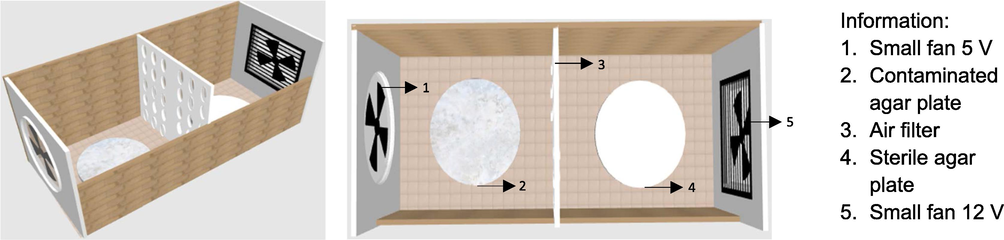
Air filter chamber.
![Air filter containing (a) SiG, (b) ZnOnano-synth-[chi-SiG], and (c) HEPA filter.](/content/184/2023/16/8/img/10.1016_j.arabjc.2023.104967-fig3.png)
Air filter containing (a) SiG, (b) ZnOnano-synth-[chi-SiG], and (c) HEPA filter.
2.3.5 Antibacterial air filter containing ZnOnano-synth-[chi-SiG]
A bacterial suspension of 1 McFarland standard that is equal to 3 × 108 CFU/mL bacteria was inoculated on an agar plate of nutrient agar and put into the air filter chamber. A single air filter containing ZnOnano-synth-[chi-SiG] was placed between B. subtilis agar and sterile agar media. The air filter chamber was incubated at 37 °C for 48 h and the air was flown in from B. subtilis agar media to penetrate the air filter containing ZnOnano-synth-[chi-SiG], and then the air was flown out from the chamber by a small fan. In the air filtration process, a small fan blew air slowly into the agar plate surface containing B. subtilis bacteria, and another fan drew air from the inside to get out from the air filter chamber. The agar plate was slowly dried and B. subtilis produced the spores (Setlow, 2006) which are rod-shaped and non-pathogenic bacteria present in low-temperature environments (Wightman et al., 2011; Headd & Bradford, 2016). Air passed containing bacteria spores was flown into a sterile agar plate. The number of bacteria colonies before and after air filter penetration was investigated. The experiment was observed after 48 h incubation of the air filter chamber. Colonies were counted by using the colony counters (BISQUIT Industrial Co, Indonesia). A similar procedure was carried out for the air filter containing SiG, chi-SiG, Znsalt-[chi-SiG], HEPA filter cloth, and no filter (control) tested against B. subtilis. A comparison between total survival bacteria colonies detected passed through the filter with and without zinc content (control) was stated as the filter capacity for inactivating the bacteria or inactivation rate (%)
Furthermore, the bacteria staining procedure was conducted according to the Gram staining protocol (Gerhardt, 1980), and the spore staining procedure followed the Schaeffer-Fulton method using malachite green and safranin (Hussey & Zayaitz, 2007) toward air filter containing SiG and ZnOnano-synth-[chi-SiG] granules. Both Gram and spore staining were observed using a microscope.
3 Results and discussion
3.1 Coating and characterization of chitosan-ZnOnano-synth on SiG
Swelling properties and chitosan film covering the SiG in several solvents are analyzed from microscope images displayed in Table 1. Microscope images show that the chitosan layer was stable in methanol but dissolved or destroyed in a diluted sodium hydroxide solution. The image is consistent with Table 2 data showing that swelling in methanol caused less absorbed the solvent with Wsp of 4.59 ± 0.5 % and without chitosan weight loss. In aqueous NaOH, however, most of the chitosan film disappears and then the SiG looks bald. Table 2 shows the swelling property was 24.45 ± 1.0 % and 19.12 ± 1.9 % of chitosan loss. The film breakage is due to deprotonating chitosan-acetic acid film that causes chitosan film loss from SiG. Chitosan dissolves in aqueous acetic acid by protonating the amino groups (Adlim et al., 2008). Others also found that chitosan-acetic acid solution precipitated in NaOH solution (Soon et al., 2018). The swelling moderately occurred in a mixture of methanol-diluted NaOH. The swelling was 23.35 ± 3.5 % but only 4.49 ± 0.3 % chitosan loss. Less chitosan loss in methanol-diluted NaOH might be due to the low dissociation of hydroxide ions in methanol, causing a weaker deprotonating chitosan-acetic acid film. Unlike in water, NaOH dissolved slowly in methanol according to the previous report (PubChem, 2005). Moderate swelling is required to activate the adhesive property of chitosan film to attach ZnO without losing chitosan from the SiG support. Therefore, the last solvent formula is chosen for the following experiments. Wo = dried sample weight, W1 = wet sample weight after immersion, W2 = dried sample weight after immersion, Wsp = swelling property, Wcs = weight chitosan loss.
Solvent
Before immersion
After immersion
Methanol

Sodium hydroxide 0.1 M


Methanol-sodium hydroxide 0.1 M


Solvent
No.
(g)
(g)
(g)
Wsp (%)
Wcs (%)
Methanol
1
0.046
0.048
0.046
4.16
0
2
0.044
0.047
0.044
4.48
0
3
0.039
0.041
0.039
5.13
0
mean ± SD
4.59 ± 0.5
0
Sodium hydroxide 0.1 M
1
0.059
0.077
0.049
23.37
16.95
2
0.050
0.067
0.040
25.37
20.00
3
0.049
0.065
0.039
24.61
20.41
mean ± SD
24.45 ± 1.0
19.12 ± 1.9
Methanol-sodium hydroxide 0.1 M
1
0.073
0.091
0.070
19.78
4.11
2
0.065
0.085
0.062
23.53
4.61
3
0.063
0.086
0.060
26.74
4.76
mean ± SD
23.35 ± 3.5
4.49 ± 0.3
Furthermore, chitosan film solubility was investigated from the swelling properties and presented in Table 2. Table 2 showed that all solvents caused the chitosan film to swell, but methanol performed the lowest swelling properties and sodium hydroxide was the highest. The result showed 4.59 ± 0.5 %, 24.45 ± 1.0 %, and 23.35 ± 3.5 % for methanol, sodium hydroxide, and methanol-sodium hydroxide respectively. No chitosan dissolution occurred in methanol. Therefore, the mixture of methanol and sodium hydroxide is needed to minimize the swelling property that can lead to the dissolution of chitosan film on SiG. Our work showed that methanol-sodium hydroxide has lower chitosan solubility rather than sodium hydroxide only. Based on Table 2, chitosan film soluble in sodium hydroxide was 19.12 ± 1.9 % while in methanol-sodium hydroxide was 4.49 ± 0.3 %, thus it proved to limit the excessive dissolution (weight loss) of chitosan film. Thereby, methanol-sodium hydroxide was chosen as the solvent for the immobilization of ZnOnano-synth onto chi-SiG.
ZnOnano-com which is commercially available was directly immobilized on chi-SiG and the synthetics ZnO was prepared from the nitrate salt and methanol-NaOH. The control and sample microscope images are presented in Fig. 4. ZnOnano-com was stuck directly on the chi-SiG so that ZnOnano-com covered almost all SiG surfaces as shown in Fig. 4(a) which is a different appearance from synthetics ZnO (Fig. 4b) and chi-SiG as control (Fig. 4c).![Cross-section image of (a) ZnOnano-com-[chi-SiG], (b) ZnOnano-synth-[chi-SiG], and (c) chi-SiG through binocular microscope stereo with 15 × magnification.](/content/184/2023/16/8/img/10.1016_j.arabjc.2023.104967-fig4.png)
Cross-section image of (a) ZnOnano-com-[chi-SiG], (b) ZnOnano-synth-[chi-SiG], and (c) chi-SiG through binocular microscope stereo with 15 × magnification.
Based on the Sigma Aldrich database, the Zn precursor price and the preparation cost of ZnO nanoparticles have a lower price than gold and silver nanoparticles and are comparable with the commercial ZnO nanoparticles, which all have antibacterial properties (Sigma Aldrich, 2023a,b,c; Bankar et al., 2020; Ahmad et al., 2021). Immobilization of synthetic ZnO on chi-SiG involves several steps. It initiates Zn(OH)2 formation and conversion of Zn(OH)2 into ZnO after the heating process. Heating was at a moderate temperature (60 °C) to avoid chitosan film burning since chitosan starts decomposing significantly at 230 °C (Muktaridha et al., 2022). Formation of ZnO at low temperatures has been recently known but the zinc precursors were mostly not zinc nitrate (Tadji et al., 2022). Also, previous literature confirmed that ZnO formation had been proven in an alcoholic solution containing a small amount of water, and the process involved a complex mechanism (Haase et al., 1988).
The surface and shape studies of the synthesized nanoparticles were carried out by optical microscope and SEM. Microscope images revealed that chitosan film covered the entire chi-SiG (Fig. 4a) as proven by the yellowish transparent color and allowed the attachment of the ZnOnano-synth (Fig. 4b) and ZnOnano-com (Fig. 4c) as confirmed by the white color on the chi-SiG surface. A closer appearance of chitosan film and ZnOnano-synth observed in SEM image showed the chitosan film has smooth layers and its microfibril covered the entire surface of SiG (indicated by the yellow line in Fig. 5a). At higher magnification (5000 x), some spherical and sheet-like shaped existed (Fig. 5b). The presence of chitosan film on SiG provides sites for ZnO and it prevents ZnO from agglomeration. The different appearance observed on ZnOnano-com images confirmed agglomerated nanopowder, with larger particle size and chitosan film was not obvious (Fig. 5c). According to AAS data, the zinc weight in each granule sample of ZnOnano-synth-[chi-SiG] and ZnOnano-com-[chi-SiG] was 0.304 mg and 0.498 mg, respectively.![SEM images of (a) chi-SiG, (b) ZnOnano-synth-[chi-SiG], and (c) ZnOnano-com-[chi-SiG] with 5000 × magnification.](/content/184/2023/16/8/img/10.1016_j.arabjc.2023.104967-fig5.png)
SEM images of (a) chi-SiG, (b) ZnOnano-synth-[chi-SiG], and (c) ZnOnano-com-[chi-SiG] with 5000 × magnification.
The synthesis of ZnOnano-synth-[chi-SiG] involves a two-stage process, i.e., hydroxylation and drying. In the present paper, the reaction temperature was carried out at ∼ 40 °C and the drying temperature at a low degree (60 °C). In this work, the drying temperature plays a role in the formation of ZnO, as it allows the dehydration of residual Zn(OH)2 to form ZnO. According to Biron et al (2020), the syntheses follow the reaction step:
Another possible reaction of the interaction between NaOH, methanol, water, and zinc salt is the involvement of methoxide ion (–OCH3) as the reaction product NaOH and CH3OH. Although the reaction is in equilibrium with small K, during slow heating, the water evaporated and the equilibrium goes to the right side, thereby the methoxide ion might exist. The proposed reaction has been previously published (Xiong et al., 2014). NaOCH3 then dissociates into sodium and methoxide ions.
According to Crystal Field Theory, zinc ions in the water will be surrounded by four H2O molecules, which is a common coordination number for Zn complexes. Then, the H2O molecules are replaced by hydroxyl and methoxide (–OCH3) ligands as the proposed reaction:
The comparative FTIR spectra of unmodified chitosan and chitosan-ZnOnano-synth were depicted and studied (shown in Fig. 6). The specific absorption peaks of the original chitosan were observed at 3420 cm−1 (NH2 and OH stretching vibrations), 2944 cm−1 and 2881 cm−1 (asymmetric and symmetric stretching vibrations of –CH2 groups), 1640 cm−1 and 1579 cm−1 (C-O stretching vibration of amide I and –NH2 bending vibration of amide II) which are consistent with previously reported work (Sarojini et al., 2019; Kumar et al., 2019; Sun et al., 2020). In the chitosan-ZnOnano-synth spectra, compared with chitosan, the broader and stronger peak moved noticeably to wavenumber at 3486 cm−1 which indicated the interaction between ZnO molecules and chitosan's NH2 and OH groups (Balogun et al., 2020). In addition, the absorption peaks formed at 675 cm−1 and 449 cm−1 are due to the attachment of the amide group and stretching mode of ZnO, respectively (Balogun et al., 2020; Vijayalakshmi et al., 2015). Thus, a broader and stronger peak in chitosan-ZnO hypothesizes the weak chemical interaction between ZnO molecules and the binding sites of chitosan.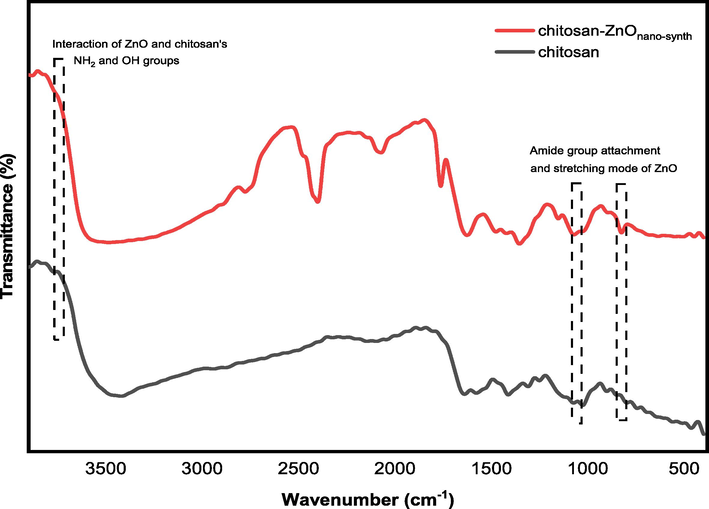
FTIR spectra of chitosan and chitosan-ZnOnano-synth.
When chitosan and zinc oxide dissolved; thereby; regulating the acidity of the solution, Zn2+ ions were formed. At pH = 6–9 the unstable compound Zn(OH)2 was formed, according to the following equation:
Chitosan's NH2 and OH groups can form coordination bonds with metal ions (Ardean et al., 2021), by increasing the pH of the solution to pH = 10 dropwise addition of NaOH, the stable complex of chitosan-ZnOnano-synth is formed. In this work, chitosan may have played a major role in stabilizing zinc oxide nanoparticles.
The size and distribution of ZnO were analyzed using TEM images of ZnOnano-synth-[chi-SiG] and ZnOnano-com-[chi-SiG]. The solid samples were immersed in water and the slurry was analyzed with TEM methods. The particle size distribution was calculated from around 300 particles with diameter calculated using software of image-J, where the data were presented as mean ± standard deviation (SD) (Aljabali et al., 2018) as tabulated in Fig. 7 and Fig. 8. The TEM images of ZnOnano-synth exhibited well-dispersed spherical shapes with an average particle size of 5.18 ± 1.70 nm. The presence of chitosan as a stabilizer prevents particles from agglomeration (Ranjani et al., 2019). However, ZnOnano-com was aggregated with a sheet-like shape and an average particle size of 30.83 ± 0.75 nm. The particles were not dispersed well and had smaller size deviations. The different distribution of ZnO onto the chi-SiG surface has a relation to the immobilization technique; ZnOnano-synth-[chi-SiG] was synthesized from the salt, while ZnOnano-com-[chi-SiG] was directly used from the nanopowder. The appropriate immobilization technique causes the ZnO surface to be assessable for bacteria to contact. This finding was verified in the previous study of the immobilization of chi-Pd/Au on titania (Adlim & Bakar, 2013) and the immobilization of Ag NPs on chi-pumice (Adlim et al., 2021). Therefore, this promising-immobilization technique was confirmed as an appropriate method with several advantages including being environmentally friendly, low energy, not toxic, and low cost.![TEM analysis of (a) ZnOnano-synth from ZnOnano-synth-[chi-SiG] (scale bar 100 nm), and (b) particle size distribution.](/content/184/2023/16/8/img/10.1016_j.arabjc.2023.104967-fig7.png)
TEM analysis of (a) ZnOnano-synth from ZnOnano-synth-[chi-SiG] (scale bar 100 nm), and (b) particle size distribution.
![TEM analysis of (a) ZnOnano-com from ZnOnano-com-[chi-SiG] (scale bar 200 nm), and (b) particle size distribution.](/content/184/2023/16/8/img/10.1016_j.arabjc.2023.104967-fig8.png)
TEM analysis of (a) ZnOnano-com from ZnOnano-com-[chi-SiG] (scale bar 200 nm), and (b) particle size distribution.
Analysis was conducted to compare the crystallinity and impurity of the ZnOnano-synth-[chi-SiG] with ZnOnano-com-[chi-SiG]. The XRD measurement was performed at room temperature with an X-ray, continuous scan (2θ) in the range of 10 to 70° with a 0.02-degree step. The diffraction patterns are matched with the ZnO standard diffractogram (marked by the •; JCPDS: 036–1451) as a wurtzite structure and no characteristic peaks were observed other than ZnO. Miller indices marked by the •; (1 0 0), (0 0 2), (1 0 1), (1 0 2), (1 1 0), (1 0 3), and (2 0 0) from left to right (Bindu & Thomas, 2014). The diffractogram shows that ZnOnano-synth-[chi-SiG] (ZnO synthesized at 60 °C) has low crystallinity as shown in Fig. 9. In contrast, the synthesized ZnOnano-synth at 150 °C exhibits seven diffraction peaks located at 31.7, 34.4, 36.2, 47.5, 56,5, 62.8, and 67.9°. These peaks are indexed as the spherical to the hexagonal phase of ZnO with high crystallinity of the particles (Khoshhesab et al., 2011; Faisal et al., 2021). It was revealed that all of the characteristic peaks were of ZnOnano-synth and no such impurities exist in the ZnOnano-synth.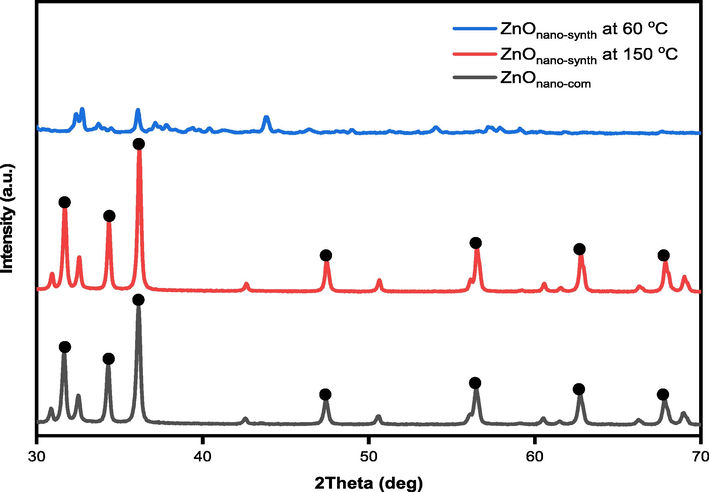
Diffractogram of ZnOnano-synth (at 60 °C and 150 °C) and ZnOnano-com.
The diameter of ZnO crystallites was calculated by the Debye-Scherrer formula, on the bases of θ (Bragg's diffraction angle) and β (full width at half maximum (FWHM)) of the most intense peaks corresponding to the (1 0 1) planes located at approximately 36.2°. The crystallite size is about 31.9 nm for ZnOnano-synth (synthesized at 150 °C) while the crystallite size is about 23.1 nm for ZnOnano-com as presented in detail in Table 3. The indexation confirms the standard hexagonal wurtzite structure (JCPDS: 036–1451) of ZnOnano-synth as previously reported in other studies (Arakha et al., 2015). ZnO crystallizes in the wurtzite structure in which the oxygen atoms are arranged in a hexagonal close-packed type with zinc atoms occupying half the tetrahedral sites (Bindu & Thomas, 2014). Zn and O atoms are tetrahedrally coordinated with each other and have, therefore, an equivalent position.
Plane
Diffraction angle ZnOnano-synth
Diffraction angle ZnOnano-com
Crystallite size ‘D’ (nm)
ZnOnano-synth
ZnOnano-com
(1 0 0)
31.7
31.7
30.3
27.5
(0 0 2)
34.4
34.3
33.4
30.9
(1 0 1)
36.2
36.1
31.9
23.1
(1 0 2)
47.5
47.4
31.9
27.9
(1 1 0)
56.5
56.5
31.2
26.6
(1 0 3)
62.8
62.7
30.2
25.3
(2 0 0)
67.9
67.8
28.2
24.1
The comparison of lattice parameters between ZnOnano-synth (synthesized at 150 °C) and ZnOnano-com are listed in Table 4. The a and b are equivalent to wurtzite crystallite dimension and c is much larger. The (1 0 0) plane was used to determine a and b, while the (0 0 2) plane determine c (Bindu & Thomas, 2014). The ratio of c/a, bond length, the volume of the unit cell, and atomic packing factor (APF) are all matched with the ZnO standard (JCPDS: 036–1451). The APF of ideal hexagonal ZnO is 74% (Kanchana et al., 2016) and it was found about 75.5%, which means that the APF of nanocrystals is slightly more than that of bulk materials. It may be due to the size effect in nanocrystalline powders. Furthermore, all the lattice parameters are well-matched with the ZnO standard of the JCPDS.
Sample
Lattice constant
(c/a)
Bond length ‘L’ (Å)
The volume of the unit cell ‘V’
Atomic packing factor ‘APF’ (%)
1 0 0
0 0 2
a (Å)
c (Å)
ZnOnano-synth
2.8
2.6
3.3
5.2
1.6
1.9
47.9
75.5
ZnOnano-com
2.8
2.6
3.3
5.2
1.6
1.9
48.1
75.5
ZnO-JCPDS
2.8
2.6
3.3
5.2
1.6
1.9
47.6
75.5
3.2 Antibacterial study for ZnO without immobilization sample
Antibacterial activities were tested for several samples that were ZnO without immobilization, the ZnOnano-synth–[chi-SiG], and the air filter containing ZnOnano-synth-[chi-SiG]. The antimicrobial properties are compared among ZnOnano-synth synthesis at 60 °C and 150 °C, and ZnOnano-com. The antibacterial data for ZnO without immobilization are presented in Table 5. All samples exhibit an inhibition zone against S. aureus and E. coli as shown in Fig. 10. ZnOnano-synth synthesized at 60 °C has less crystalline characteristic according to XRD data but it shows the highest antibacterial activity against both types of bacteria with an inhibition zone diameter of 33.50 ± 0.10 mm (S. aureus) and 30.08 ± 0.03 mm (E. coli). The ZnOnano-synth synthesized at 150 °C also exhibit a moderate inhibition zone against S. aureus and E. coli with a diameter zone of 27.62 ± 0.08 mm and 22.49 ± 0.11 mm, respectively. ZnOnano-com shows the lowest antibacterial activity against S. aureus with 6.26 ± 0.15 mm inhibition zone diameter, although according to AAS data, ZnOnano-com has higher zinc content compared to synthetic ZnOnano-synth. All experiments were performed in triplicates and reported as mean ± standard deviation (SD).
Bacteria
Sample codes of ZnO without immobilization on chi-SiG
Inhibition zone diameters (mm) (mean ± SD)
Staphylococcus aureus
ZnOnano-com
ZnOnano-synth synthesized at 60 °C
ZnOnano-synth synthesized at 150 °C6.26 ± 0.15
33.50 ± 0.10
27.62 ± 0.08
Escherichia coli
ZnOnano-com
ZnOnano-synth synthesized at 60 °C
ZnOnano-synth synthesized at 150 °C0
30.08 ± 0.03
22.49 ± 0.11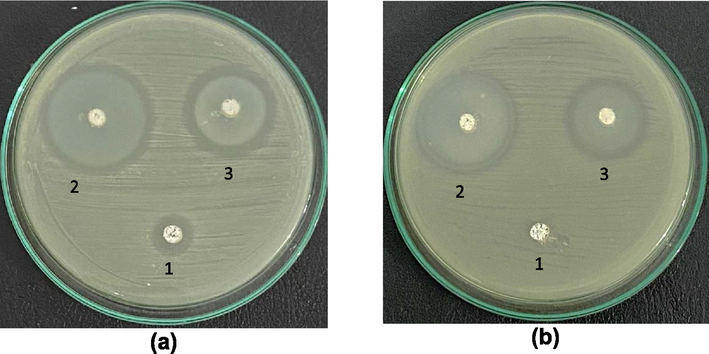
Antibacterial activity against (a) S. aureus and (b) E. coli; (1) ZnOnano-com, (2) ZnOnano-synth synthesized at 60 °C, and (3) ZnOnano-synth synthesized at 150 °C.
Furthermore, the finding suggested that ZnOnano-synth had a stronger antibacterial effect on Gram-positive (S. aureus) than on Gram-negative (E. coli) bacteria due to their different cell wall components. The Gram-negative (E. coli) bacteria have multilayer structures of cell walls containing peptidoglycan layer, lipoprotein layer, phospholipids, or lipopolysaccharide layer (Siripatrawan & Vitchayakitti, 2016). These complex layer structures caused weaker interaction between ZnO and cells if it is compared to the Gram-positive cell wall properties. Since the ZnOnano-synth synthesized at 60 °C has stronger antibacterial then this sample was involved in further study of antibacterial tests for the ZnOnano-synth immobilization and the antibacterial air filter samples.
3.3 Antibacterial study for ZnO immobilized on chi-SiG sample
After samples of ZnO without immobilization on chi-SiG showed high antibacterial characteristics, then study the immobilized ZnO samples because they were prepared differently. The ZnOnano-synth-[chi-SiG] granules were evaluated for their antibacterial properties before being assembled as the air filter. The characteristics of synthetics ZnOnano-synth-[chi-SiG] (prepared at 60 °C) were compared with some of the control sample properties including SiG, chi-SiG, Znsalt-[chi-SiG], and ZnOnano-com-[chi-SiG], based on the inhibition zone diameter. As presented in Table 6 that all samples exhibited the inhibition zone against S. aureus and E. coli except SiG. The chi-SiG displayed antibacterial activity with an inhibition zone diameter of 13.06 ± 0.11 mm (S. aureus) and 15.29 ± 0.38 mm (E. coli). Similarly, other samples such us ZnOnano-com-[chi-SiG], Znsalt-[chi-SiG], and ZnOnano-com-[chi-SiG] exhibited the antibacterial properties against both types of bacteria (shown in Fig. 11), this indicated that zinc ions and zinc oxide nanoparticles act as antibacterial agents, but the ZnOnano-synth-[chi-SiG] performed the strongest. The inhibition zone diameter of ZnOnano-synth-[chi-SiG] against S. aureus and E. coli was 33.33 ± 0.07 mm and 29.54 ± 0.33 mm, respectively. These indicated that ZnO particles along with chitosan inhibit bacterial growth by diffusion of the soluble species into the agar medium.
Bacteria
Sample codes
Inhibition zone diameters (mm)
(mean ± SD)
Staphylococcus aureus
SiG
chi-SiG
Znsalt-[chi-SiG]
ZnOnano-synth-[chi-SiG]
ZnOnano-com-[chi-SiG]0
13.06 ± 0.11
32.05 ± 0.03
33.33 ± 0.07
20.28 ± 0.98
Escherichia coli
SiG
chi-SiG
Znsalt-[chi-SiG]
ZnOnano-synth-[chi-SiG]
ZnOnano-com-[chi-SiG]0
15.29 ± 0.38
28.41 ± 0.26
29.54 ± 0.33
18.43 ± 0.84![Antibacterial activity against (a) S. aureus and (b) E. coli; (1) SiG, (2) chi-SiG, (3) Znsalt-[chi-SiG], (4) ZnOnano-synth-[chi-SiG], and (5) ZnOnano-com-[chi-SiG].](/content/184/2023/16/8/img/10.1016_j.arabjc.2023.104967-fig11.png)
Antibacterial activity against (a) S. aureus and (b) E. coli; (1) SiG, (2) chi-SiG, (3) Znsalt-[chi-SiG], (4) ZnOnano-synth-[chi-SiG], and (5) ZnOnano-com-[chi-SiG].
Chitosan is effective against a wide range of target organisms and is available in a variety of formulations including films (Costa-Pinto et al., 2021) as shown in chi-SiG. However, chitosan only has weak antibacterial without combining with ZnO. The suggested mechanisms of action are based on the electrostatic interaction between chitosan positively charged amino groups and the predominantly anionic lipopolysaccharide on Gram-negative bacteria or teichoic acid on Gram-positive bacteria (Kulawik et al., 2020). In Gram-positive bacteria, chitosan interacted with lipopolysaccharide molecules and displaced divalent cations within bacteria cells (e.g., Ca2+ and Mg2+) inducing depolarization of the cell membrane, leading to increased permeability, osmotic damage, intercellular leakage and ultimately cell death. In Gram-negative bacteria, chitosan diffused inside the cell bonded to the cellular membrane and inhibited their growth (Duan et al., 2019).
Zinc ions are involved in the regulation of cell growth and differentiation of the membrane structure of bacterial cells (Jafarirad et al., 2016), for example in Znsalt-[chi-SiG] granules, an excess of Zn2+ may compete with other metals and provoke a metal mismatch in various metal-binding proteins resulting in protein malnutrition, enzymatic inactivation, or protein denaturation, thus leading to the bacterial cell off balance (Godoy-Gallardo et al., 2021). However immobilized zinc salt (representing Zn2+ sample) without converting into ZnO, will easily leach from the chi-SiG support in a humid environment thereby it might be not long-lasting and not easily regenerated for reusable.
The toxicity mechanism mediated by zinc oxide nanoparticles, in ZnOnano-synth-[chi-SiG], is mainly linked to the release of Zn2+ ions (Deng et al., 2009) and attributed to the nanoparticles themselves (Yang et al., 2009). The proposed mechanisms are listed as follows: (1) loss of cellular integrity by direct contact of ZnOnano-synth with the cell wall and/or membrane and (2) release of Zn2+ ions upon ZnOnano-synth dissolution and followed by intercellular reactive oxygen species (ROS) production subsequent and damage of biomolecules (Kadiyala et al., 2018). Although the solubility of ZnO in water is small (Ksp 3.7 × 10-17), a trace amount of Zn2+ is enough to retard bacteria cell growth. Therefore, the antibacterial activity of ZnOnano-synth-[chi-SiG] is higher than Znsalt-[chi-SiG] against Gram-positive and Gram-negative bacteria, although Zn2+ from Znsalt-[chi-SiG] is more mobile than from ZnO. This finding confirmed the antimicrobial ZnO either alone or with other metal oxides as previously reported in the literature although with different preparation methods (Alabyadh et al, 2022; Yazdi et al, 2021; Mousavi-Kouhi, 2021).
All experiments were performed in triplicates and reported as mean ± standard deviation (SD).
3.4 Antibacterial studies of air filter assembled immobilized ZnO
Each granule of ZnOnano-synth-[chi-SiG], Znsalt-[chi-SiG], chi-SiG, and SiG was assembled as an air filter panel (Fig. 3) and inserted into the air filter chamber (Fig. 2). The HEPA filter bacterial activity was also compared to ZnOnano-synth-[chi-SiG] filter efficacy. Since the dominant bacteria were Bacillus sp. in the indoor air (Harbizadeh et al., 2019), therefore the experiment was limited to Bacillus subtilis which can produce spores in relatively dry conditions. In the air filter chamber, an agar petri dish containing B. subtilis was blown by a small fan, and then when the humidity slowly decreased, thus B. subtilis became stressed and produced endospores (Higgins & Dworkin, 2012) that would be flown to penetrate the filter.
The number of bacteria colonies before and after contacting the filter was compared as presented in Table 7 and some representative colony images are shown in Table 8. The counted colonies in 24 and 48 h observations were displayed in Table 7. The number of bacterial colonies in the air before passing through the filter was 330–342 colonies. The number of colonies was extremely smaller in the area shared by the filter because not all bacteria were flown to penetrate the filter. The flown bacteria in control (without filter) was only 24–26 colonies observed in 24 and 48 h. The colonies were considered as the maximum survival colonies (control) flown toward the filter in 24 h (24 colonies) and 48 h incubation (26 colonies) that were used for the calculation of the inactivation rate of filters. The ZnOnano-synth-[chi-SiG] filter performed the highest antibacterial property compared to other filters except for chi-SiG. Although chi-SiG seems to show the highest antibacterial activity it was in only a short time (24 h), then the bacteria grew after 48 h. The ZnOnano-synth-[chi-SiG] filter retard colony growth into 2 colonies (92% efficacy) in 24 and 48 h. If it is compared to the initial colonies which were 342, then the survival was only 2 colonies in 24 and 48 h, then the inactivation rate would be 99% efficacy for the ZnOnano-synth-[chi-SiG] filter. This finding confirmed might relate to ZnOnano-synth particle size that has a high surface-to-volume ratio leading to improve antibacterial activity against bacteria and spores. The data are consistent with the above ZnO-type characteristics. These results confirmed that both chitosan and zinc ions showed moderate antibacterial activity, but the chitosan-only filter showed that the colonies continue growth after 24 h. It is proven that a filter containing ZnOnano-synth-[chi-SiG] prevents air contamination in prolonged activity.
Filter
Initial colonies before filter penetration
Incubation time (h)
The survival colonies after penetration
Inactivation rate (%)
No filter
330
24
4824
26Control
SiG
336
24
489
2663
0
HEPA filter cloth
342
24
4819
2421
8
chi-SiG
339
24
480
6100
69
Znsalt-[chi-SiG]
340
24
484
583
81
ZnOnano-synth-[chi-SiG]
342
24
482
292
92
Filter
Incubation time (24 h)
Incubation time (48 h)
SiG

HEPA filter cloth
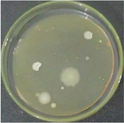
ZnOnano-synth-[chi-SiG]


To compare the existence of bacteria after penetration of air SiG filter (control) and after contact or penetration with ZnOnano-synth-[chi-SiG] filter was also studied. After penetration SiG, many bacteria were still alive and had coccus or basil shapes (Fig. 12a). The spores staining found that spores and vegetative cells still exist, indicating the alive cells and metabolically active (Fig. 12b). It was very different after bacteria penetrate ZnOnano-synth-[chi-SiG] filter, the spore, and Gram staining show the cells were shrunk and had an irregular shape and unusual color (Fig. 12c). The spores were not found inside the vegetative cells (Fig. 12d). Therefore, the existence of ZnOnano-synth with air filter is confirmed to stop the sporulation process and significantly modify metabolism in B. subtilis (Rosi & Mirkin, 2005; Eymard-Vernain et al., 2018). The findings are also consistent with the previous studies on B. subtilis that showed ZnO nanoparticles initiate the sporulation and inhibit the cell growth of B. subtilis bacteria (Godoy-Gallardo et al., 2021; Hsueh et al., 2015; Matyszczuk & Krzepilko, 2022).![Microscopic images of B. subtilis (a) Gram staining after through SiG filter, (b) spore staining after through SiG filter, (c) Gram staining after through ZnOnano-synth-[chi-SiG] filter, and (d) spore staining after through ZnOnano-synth-[chi-SiG] filter with 1000 × magnification.](/content/184/2023/16/8/img/10.1016_j.arabjc.2023.104967-fig12.png)
Microscopic images of B. subtilis (a) Gram staining after through SiG filter, (b) spore staining after through SiG filter, (c) Gram staining after through ZnOnano-synth-[chi-SiG] filter, and (d) spore staining after through ZnOnano-synth-[chi-SiG] filter with 1000 × magnification.
However, ZnO nanoparticles selectively harm bacteria while having little effect on human and animal tissues. Zinc is recognized as an essential trace element for the physiological and biochemical processes in humans and animals (Baek & An, 2011). Therefore, the Food and Drug Administration (FDA) considered ZnO as a generally recognized safe substance (GRAS). The GRAS designation mostly encompasses materials ranging from microns to larger sizes so, conversion of these macro substances to nanoscale will attain distinctive properties including toxicity.
ZnO bactericidal performance in this current study is aligned with the previous studies proving ZnO to retard cancer cell growth in vitro of human liver hepatocellular carcinoma (HepG2) (Tanino et al., 2020). ZnO nanoparticles can activate ROS fabrication in cancer cells and ultimately destroy the cancer cell which consequently leads to cancer cell death (Yazdi et al., 2021). Cancer cells are different from normal cells, especially in terms of metabolic requirements; this leads to different cytotoxicity results (Rajendran et al., 2017). The cytotoxicity effect of ZnO nanoparticles against a normal cell revealed less toxicity than a cancerous cell (Yadav et al., 2022; Mousavi-Kouhi et al., 2021; Yazdi et al., 2021; Alabyadh et al., 2022).
4 Conclusion
Synthesis and immobilization of ZnOnano-synth on white silica gel beads coated with chitosan were proved to be a low-cost and environmentally friendly procedure with high antibacterial properties. After testing several solvents, methanol-sodium hydroxide was confirmed as the proper reagent for the preparation and immobilization of ZnOnano-synth onto chi-SiG. Subsequently, the drying process at 60 °C for 72 h affected the dispersed and nanoparticle size of ZnO. The chitosan coat was proved to act as the stabilizer and the adhesive for ZnOnano-synth on the white silica gel beads surface. The ZnOnano-synth-[chi-SiG] which has dispersed small nanoparticle size, exhibited the strongest antibacterial activity against Gram-positive and Gram-negative bacteria in agar media, if it is compared to the ZnO commercial nanopowder, and the Zn precursor bacterial activities. The air filter containing ZnOnano-synth-[chi-SiG] showed high antibacterial performance against B. subtilis in the air.
Acknowledgment
This work was supported by the PMDSU Scholarship for Muhammad Iqbal Hidayat and a Research fund from The Ministry of Education, Culture, Research, and Technology, Republic of Indonesia (grant number 060/E5/PG.02.00.PT/2022); and PKPI PMDSU (grant number 085.36/E4.4/KU/2022). Part of this work was also supported by the bridging grant (304/PKIMIA/6316598) from Universiti Sain Malaysia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- The properties of Pd/Au bimetallic colloidal catalysts stabilized by chitosan and prepared by simultaneous and stepwise chemical reduction of the precursor ions. Kinet. Catal.. 2013;54:586-596.
- [CrossRef] [Google Scholar]
- Adlim, M., Hidayat, M.I., Azmi, N., Ramayani, R.F.I., 2021. Preparation of chitosan-silver nanoparticles immobilized onto pumice for antibacterial testing against Escherichia coli. Proceedings of the 2nd ICECME, Banda Aceh, Indonesia. p. 63-72. 10.1007/978-981-16-0736-3_7.
- Nutrient release properties of a urea-magnesium-natural rubber composite coated with chitosan. Environ. Technol. Innov.. 2019;16:100442
- [CrossRef] [Google Scholar]
- Adlim., Bakar, M.A., 2008. Preparation of chitosan-gold nanoparticles: part 2 (finish). The role of chitosan. Indo. J. Chem. 8(3), 320-326. 10.22146/ijc.21585.
- Synthesis of yttrium and cerium doped ZnO nanoparticles as highly inexpensive and stable photocatalysts for hydrogen evolution. J. Rare Earths. 2021;39(4):440-445.
- [CrossRef] [Google Scholar]
- ZnO/CeO2 nanocomposites: metal-organic framework-mediated synthesis, characterization, and estimation of cellular toxicity toward liver cancer cells. J. Funct. Biomater.. 2022;13:139.
- [CrossRef] [Google Scholar]
- Performance of silver, zinc, and iron nanoparticles-doped cotton fibers against airborne E. coli to minimize bioaerosol exposure. Air Qual. Atmos. Health. 2018;11:1233-1242.
- [CrossRef] [Google Scholar]
- Synthesis of gold nanoparticles using leaf extract of Ziziphus zizyphus and their antimicrobial activity. Nanomaterials (Basel). 2018;8(3):1-5.
- [CrossRef] [Google Scholar]
- Amininezhad, S.M., Amininejad, S.M., Eslamian, S., 2014. Disinfection of Water and Nanotechnology, in Handbook of Engineering Hydrology, Ch. 3, Vol. 3: Environmental Hydrology and Water Management, Ed. By Eslamian, S., Taylor and Francis, CRC Group, USA, 51-64.
- The effects of interfacial potential on antimicrobial propensity of ZnO nanoparticle. Sci. Rep.. 2015;5:9578.
- [CrossRef] [Google Scholar]
- Factors influencing the antibacterial activity of chitosan and chitosan modify by functionalization. Int. J. Mol. Sci.. 2021;22(14):7449.
- [CrossRef] [Google Scholar]
- Microbial toxicity of metal oxide nanoparticles (CuO, NiO, ZnO, and Sb2O3) to Escherichia coli, Bacillus subtilis, and Streptococcus aureus. Sci. Total Environ.. 2011;409(8):1603-1608.
- [CrossRef] [Google Scholar]
- Green synthesis and characterization of zinc oxide nanoparticles using bashful (Mimosa pudica), leaf extract: a precursor for organic electronics applications. SN Appl. Sci.. 2020;2:504.
- [CrossRef] [Google Scholar]
- Facile synthesis of nanostructured Ni-Co/ZnO material: an efficient and inexpensive catalyst for Heck reactions under ligand-free conditions. Arab. J. Chem.. 2020;13:9005-9018.
- [CrossRef] [Google Scholar]
- Estimation of lattice strain in ZnO nanoparticles: X-ray peak profile analysis. J. Theor. Appl. Phys.. 2014;8:123-134.
- [CrossRef] [Google Scholar]
- Synthesis and characterization of the zinc oxide obtained by combining zinc nitrate with sodium hydroxide in polyol medium. Mater. Res.. 2020;23(2):e20200080.
- [Google Scholar]
- Staphylococcus aures. Omaha: University of Nebraska Medical Center; 2013.
- Antibacterial enhancement of high-effieciency particulate air filters modified with graphene-silver hybrid material. Microorganisms. 2023;11:74.
- [CrossRef] [Google Scholar]
- Chitosan and hydroxyapatite based biomaterials to circumvent periprosthetic joint infections. Materials. 2021;14(4):804.
- [CrossRef] [Google Scholar]
- Air pollution control for indoor environments using nanofiber filters: A brief review and post-pandemic perspectives. Chem. Eng. J. Adv.. 2022;11:100330
- [CrossRef] [Google Scholar]
- Assessing the anti-fungal efficiency of filters coated with zinc oxide nanoparticles. R. Soc. Open Sci.. 2017;4:161032
- [CrossRef] [Google Scholar]
- Nanosized zinc oxide particles induce neural stem cell apoptosis. Nanotechnology. 2009;20(11):115101
- [CrossRef] [Google Scholar]
- Room temperature syntheses of ZnO and their structures. Symmetry. 2021;13(4):733.
- [CrossRef] [Google Scholar]
- Chitosan as a preservative for fruits and vegetables: a review on chemistry and antimicrobial properties. J. Bioresour. Bioprod.. 2019;4(1):11-21.
- [CrossRef] [Google Scholar]
- Assessing effectiveness of air purifiers (HEPA) for controlling indoor particulate pollution. Heliyon. 2021;7(9):e07976.
- [Google Scholar]
- The polygamma-glutamate of Bacillus subtilis interacts specifically with silver nanoparticles. PLoS ONE. 2018;13(5):1-19.
- [CrossRef] [Google Scholar]
- Green synthesis of zinc oxide (ZnO) nanoparticles using aqueous fruit extracts of myristica fragrans: their characterizations and biological and environmental applications. ACS Omega. 2021;6(14):9709-19022.
- [CrossRef] [Google Scholar]
- Microbial aerosol filtration: growth and release of a bacteria–fungi consortium collected by fibrous filters in different operating conditions. J. Aerosol Sci.. 2014;72:32-46.
- [CrossRef] [Google Scholar]
- Antibacterial approaches in tissue engineering using metal ions and nanoparticles: from mechanisms to applications. Bioact. Mater.. 2021;6(12):4470-4790.
- [CrossRef] [Google Scholar]
- Adherence patterns of Escherichia coli in the intestine and its role in pathogenesis. Medicine in Microecology. 2020;5:100025
- [CrossRef] [Google Scholar]
- A mini review of antibacterial properties of ZnO nanoparticles. Front. Phys.. 2021;9:641481
- [CrossRef] [Google Scholar]
- Photochemistry and radiation chemistry of colloidal semiconductors 23 electron storage on zinc oxide particles and size quantization. J. Phys. Chem.. 1988;92(2):482-487.
- [CrossRef] [Google Scholar]
- High-performance electret and antibacterial polypropylene meltblown nonwoven materials doped with boehmite and ZnO nanoparticles for air filtration. Fibers Polym.. 2022;23:1947-1955.
- [CrossRef] [Google Scholar]
- Indoor and outdoor airborne bacterial air quality in day-care centers (DCCs) in greater Ahvaz. Iran. Atmos. Environ.. 2019;216:116927
- [CrossRef] [Google Scholar]
- Use of aerobic spores as a surrogate for cryptosporidium oocysts in drinking water supplies. Water Res.. 2016;90:185-202.
- [CrossRef] [Google Scholar]
- Immobilization of silver nanoparticles on chitosan-coated silica-gel-beads and the antibacterial activity. Key Eng. Mater.. 2021;892:36-42.
- [CrossRef] [Google Scholar]
- Green synthesis of chitosan-stabilized silver-colloidal nanoparticles immobilized on white-silica-gel beads and the antibacterial activities in a simulated-air-filter. Arab. J. Chem.. 2022;15(2):103596
- [CrossRef] [Google Scholar]
- Recent progress in Bacillus subtilis sporulation. FEMS Microbiol. Rev.. 2012;36(1):131-148.
- [CrossRef] [Google Scholar]
- ZnO nanoparticles affect Bacillus subtilis cell growth and biofilm formation. PLos ONE. 2015;10(6):e0128457.
- [Google Scholar]
- Antibacterial nanomaterials for environmental and consumer product applications. Nanoimpact. 2020;20:100268
- [CrossRef] [Google Scholar]
- Endospore stain protocol. Am. Soc. Microbiol.. 2007;8
- Biofabrication of zinc oxide nanoparticles using fruit extract of Rosa canina and their toxic potential against bacteria: a mechanistic approach. Mater. Sci. Eng. C. 2016;59:296-302.
- [CrossRef] [Google Scholar]
- Characterization and application of CA/ZnO/AgNP composite nanofibers for sustained antibacterial properties. Mater. Sci. Eng. C. 2019;105:110077
- [CrossRef] [Google Scholar]
- Unexpected insights into antibacterial activity of zinc oxide nanoparticles against methicillin resistant: Staphylococcus aureus (MRSA) Nanoscale. 2018;10(10):4927-4939.
- [CrossRef] [Google Scholar]
- Violet emission from Fe doped ZnO nanoparticles synthesized by precipitation method. J. Lumin.. 2016;176:6-14.
- [CrossRef] [Google Scholar]
- Precipitation of ZnO nanostructures by chemical precipitation method. Synth. React. Inorg. Met. Org. Nano-Met. Chem.. 2011;41(7):814-819.
- [CrossRef] [Google Scholar]
- Chitosan role for shelf-life extension of seafood. Environ. Chem. Lett.. 2020;18:61-74.
- [CrossRef] [Google Scholar]
- Bio-based (chitosan/PVA/ZnO) nanocomposites film: thermally stable and photoluminescence material for removal of organic dye. Carbohydr. Polym.. 2019;205:559-564.
- [CrossRef] [Google Scholar]
- Antibacterial capability of air filter fiber materials treated with triclosan against indoor environmental microbes. Atmosphere. 2022;13:1104.
- [CrossRef] [Google Scholar]
- An overview about varieties and detection methods of pathogenic microorganisms in indoor air. Chin. Sci. Bull.. 2018;63(21):2116-2127.
- [CrossRef] [Google Scholar]
- How efficiently can HEPA purifiers remove priority fine and ultrafine particles from indoor air? Environ. Int.. 2020;144:106001
- [CrossRef] [Google Scholar]
- Model study for interaction of sublethal doses of zinc oxide nanoparticles with environmentally beneficial bacteria Bacillus thuringiensis and Bacillus megaterium. Int. J. Mol. Sci.. 2022;23(19):11820.
- [CrossRef] [Google Scholar]
- Silver-zinc oxide nanocomposite: from synthesis to antimicrobial and anticancer properties. Ceram. Int.. 2021;47:21490-21497.
- [CrossRef] [Google Scholar]
- Progress of 3d metal-doped zinc oxide nanoparticles and the photocatalytic properties. Arab. J. Chem.. 2021;14(6):103175
- [CrossRef] [Google Scholar]
- Highly reusable chitosan-stabilized Fe-ZnO immobilized onto fiberglass cloth and the photocatalytic degradation properties in batch and loop reactors. J. Saudi Chem. Soc.. 2022;26(3):101452
- [CrossRef] [Google Scholar]
- Thinking the future of membranes: perspectives for advanced and new membrane materials and manufacturing processes. J. Membr. Sci.. 2020;598:117761
- [CrossRef] [Google Scholar]
- PubChem, 2005. Compound summary of sodium hydroxide. https://pubchem.ncbi.nlm.nih.gov/compound/Sodium-hydroxide (accessed 26 January 2023).
- Evaluation of cytotoxicity of hematite nanoparticles in bacteria and human cell lines. Colloids Surf. B Biointerfaces. 2017;157:101-109.
- [CrossRef] [Google Scholar]
- D-glucosamine chitosan base molecule-assisted synthesis of different shape and sized silver nanoparticles by a single pot method: A greener approach for sensor and microbial applications. Int. J. Biol. Macromol.. 2019;133:1280-1287.
- [CrossRef] [Google Scholar]
- Mahua oil-based polyurethane/chitosan/nano ZnO composite films for biodegradable food packaging applications. Int. J. Biol. Macromol.. 2019;124:163-174.
- [CrossRef] [Google Scholar]
- Spores of Bacillus subtilis: their resistance to and killing by radiation, heat and chemicals. J. App. Microbiol.. 2006;101(3):514-525.
- [CrossRef] [Google Scholar]
- Sigma Aldrich, 2023a. Gold(III) chloride solution 99.99% trace metals basis. https://www.sigmaaldrich.com/ID/en/product/aldrich/484385 (accessed 15 April 2023).
- Sigma Aldrich, 2023b. Silver nitrate 99.99% trace metals basis. https://www.sigmaaldrich.com/ID/en/product/aldrich/204390 (accessed 15 April 2023).
- Sigma Aldrich, 2023c. Zinc nitrate hydrate 99.99% trace metals basis. https://www.sigmaaldrich.com/ID/en/product/aldrich/230006 (accessed 15 April 2023).
- Silica-Gel Desiccants, 2023. Silica gel white beads & crystals. https://www.silicagel-desiccant.com/silica-gel-white (accessed 5 January 2023).
- Improving functional properties of chitosan films as active food packaging by incorporating with propolis. Food Hydrocoll.. 2016;61:695-702.
- [CrossRef] [Google Scholar]
- Extraction and physicochemical characterization of chitin and chitosan from Zophobas morio larvae in varying sodium hydroxide concentration. Int. J. Biol. Macromol.. 2018;108:135-142.
- [CrossRef] [Google Scholar]
- Multifunctional bionanocomposite films based on konjac glucomannan/chitosan with nano-ZnO and mulberry anthocyanin for active food packaging. Food Hydrocoll.. 2020;107:105942
- [CrossRef] [Google Scholar]
- Facile preparation of nanostructured ZnO via low-temperature hydrothermal method upon changing the precursor anion: the study of structural, morphological, and optical properties. Mater. Today Chem.. 2022;31:103789
- [CrossRef] [Google Scholar]
- Anticancer activity of ZnO nanoparticles against human small-cell lung cancer in an orthopaedic mouse model. Mol. Cancer Ther.. 2020;19(2):502-512.
- [CrossRef] [Google Scholar]
- Influence of coating parameter and sintering atmosphere on the corrosion resistance behavior of electrophoretically deposited composite coatings. Mater. Manuf. Process. 2015;31(6):95-106.
- [CrossRef] [Google Scholar]
- Assessing the contributions of outdoor and indoor source to air quality in London homes of the SCAMP cohort. Build. Environ.. 2022;222:109359
- [CrossRef] [Google Scholar]
- Efficacy of antimicrobial and anti-viral coated air filters to prevent the spread of airborne pathogens. Sci. Rep.. 2022;12:2803.
- [CrossRef] [Google Scholar]
- Measurement of bacterial surface protonation constants for two species at elevated temperatures. Geochim Cosmochim Acta. 2011;65(21):3657-3669.
- [CrossRef] [Google Scholar]
- Novel chitosan films with laponite immobilized Ag nanoparticles for active food packaging. Carbohydr. Polym.. 2018;199:210-218.
- [CrossRef] [Google Scholar]
- Preparing sodium methoxide from sodium hydroxide by reaction coupling with separation processes. Adv. Mater. Res.. 2014;986–987:101-105.
- [CrossRef] [Google Scholar]
- Cytotoxicity and DNA fragmentation-mediated apoptosis response of hexagonal ZnO nanorods against human prostate cancer cells. Appl. Surf. Sci. Adv.. 2022;9:100237
- [CrossRef] [Google Scholar]
- Comparative study of cytotoxicity, oxidative stress and genotoxicity induced by four typical nanomaterials: the role of particle size, shape, and composition. J. Appl. Toxicol.. 2009;29(1):69-78.
- [CrossRef] [Google Scholar]
- A hierarchical structure of flower-like zinc oxide and poly(vinyl alcohol-co-ethylene) nanofiber hybrid membranes for high-performance air filters. ACS Omega. 2022;7(3):3030-3036.
- [CrossRef] [Google Scholar]
- Ultrasound-based synthesis of ZnO.Ag2O3 nanocomposite: characterization and evaluation of its antimicrobial and anticancer properties. Res. Chem. Intermed.. 2021;47:1285-1296.
- [CrossRef] [Google Scholar]
- The anti-angiogenesis and antioxidant activity of chitosan-mediated synthesized selenium-gold nanostructure. Arab. J. Chem.. 2023;16:104806
- [CrossRef] [Google Scholar]
- Viscosity simulation of glass microfiber and an unusual air filter with high-efficiency antibacterial functionality enabled by ZnO/graphene-modified glass microfiber. ACS Omega. 2022;7(16):14211-14221.
- [CrossRef] [Google Scholar]
- Energy consumption performance optimization of PTFE HEPA filter media during dust loading through compositing them with the efficient filter medium. Sustain. Cities Soc.. 2022;78:103657
- [CrossRef] [Google Scholar]
- Unusual air filters with ultrahigh efficiency and antibacterial functionality enabled by ZnO nanorods. ACS Appl. Mater. Interfaces. 2015;7(38):21538-21544.
- [CrossRef] [Google Scholar]







