Translate this page into:
Antioxidant activity of crude extracts and essential oils from flower buds and leaves of Cistus creticus and Cistus salviifolius
⁎Corresponding author. mahmoud.qudah@yu.edu.jo (Mahmoud A. Al-Qudah)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Volatile oils from flowers and leaves of C. creticus L. and C. salviifolius L. were extracted by two extraction methods; namely, hydrodistillation and solid-phase micro-extraction (SPME). The chemical composition of essential oils was analyzed by GC and GC–MS. The volatile extracted from leaves and flowers of C. criticus using SPME was dominated by monoterpenes and sesquiterpenes hydrocarbon with α-pinene, camphene and α-cubebene as major components. In hydrodistillation, the oil extracted from leaves was dominated by oxygenated diterpenes and diterpenes hydrocarbon with manoyl oxide and sclarene as major components, whereas, the oil extracted from flowers was dominated by oxygenated diterpenes and diterpenes hydrocarbon with manoyl oxide and abietatriene as major components. The volatile from flowers and leaves of C. salviifolius obtained by SPME were dominated by monoterpenes and sesquiterpenes with δ-3-carene, α-pinene, β-pinene, and E-caryophyllene as major constituents. On the other hand, the oils from flowers and leaves of C. salviifolius obtained by hydrodistillation were dominated by oxygenated diterpenes, diterpenes hydrocarbon and esters with dehydro abietol, abietol, manoyl oxide and methyl octadecenoate as major components. In the leaves, the major components of the oil were manoyl oxide, E-ethyl cinnamate, and Z-ethyl cinnamate. These oils showed weak antioxidant activity when compared to the positive controls α-tocopherol, ascorbic acid, and EDTA, while the crude extracts aq. MeOH, butanol, and water showed good antioxidant activity. Discriminating between the studied plants based on the extraction method was also possible upon applying Principle component analysis (PCA) to the obtained GC–MS data.
Keywords
Antioxidant activity
Cistus creticus L.
Cistus salviifolius L.
PCA
Essential oils
1 Introduction
Rock-rose plants and shrubs consist of 175 species of eight herbaceous genera. Genera Cistus creticus L. and Cistus salviifolius L. of the species (Cistaceae) are grown in the Mediterranean region, Europe, North America temperate areas and a limited number of them is found in South America and the northern area of Jordan (Comandini et al., 2006; Al-Eisawi, 1982; Guimarães et al., 2009). Cistus species are commonly used in many Mediterranean countries in traditional folk medicine as antidiarrheic and for the treatment of various skin diseases (Madaus et al., 1938; Barrajón-Catalán et al., 2010). The use of the plant in this way has multiple functions as an antiinflammatory (Demetzos et al., 2001), antiulcerogenic, wound healing, antimicrobial (Demetzos et al., 1999), antifungal (Bayoub et al., 2010), antiviral, antitumor (Dimas et al., 2000), cytotoxic (Ben Jemia et al., 2013) and antinociceptive (Barrajón-Catalán et al., 2010). Phytochemical studies on different Cistus species revealed the presence of several flavonoid compounds (Pascual et al., 1977; Danne et al., 1994; Kreimyeret al., 1998; Vogt and Gulz, 1986; Petereit et al., 1991; Vogt et al., 1987; Santagati et al., 2008), labdane diterpenes (Demetzos et al., 1990; Anastasaki et al., 1999; Chinou et al., 1994; Demetzos et al., 1994) and polyphenolic glycosides (Demetzos et al., 1989). The studies on essential oil composition of Cistus species revealed the presence of oxygenated monoterpenes, sesquiterpenes, aromatics, oxygenated sesquiterpenes and traces of carbonyl compounds (Demetzos et al., 1997; Demetzos et al., 1995; Costa et al., 2009; Mastino et al., 2017; Maggi et al., 2016). Leaves of all Cistus species are covered with glands secreting resin and essential oil consisting mainly of terpenoids (Mastino et al., 2017).
In Jordanian traditional medicine Cistus species are used in the treatment of multiple ailments such as anti-inflammatory, gout, ulcers, gastrointestinal disorders, diabetes, as well as reduction of blood glucose (Farley and McNeilly, 2000; Al-Khalil, 1995; Yesilada et al., 1999). Previous studies on essential oil composition in Cistus salviifolius, and Cistus creticus showed that they are rich in oxygenated diterpenes such as manoyl oxide, labd-13-en-8-yl acetate, and 13-epi-manoyl oxide, as well as in oxygenated sesquiterpenes such as viridiflorol, caryophyllene oxide, vitispirane, and bulnesol and hydrocarbon sesquiterpenes such as α-cadinene and δ-cadinene (Demetzos et al., 1997; Demetzos et al., 1995; Maggi et al., 2016).
As part of our continuous effort in investigating the chemical composition of essential oil and antioxidant activity of medicinal plants (Al-Qudah, 2013, 2016; Al-Qudah et al., 2014, 2018). The objective of the present work is to investigate the chemical composition of essential oils extracted from fresh flower buds and leaves of C. creticus and C. salviifolius using hydro-distillation and solid-phase micro-extraction (SPME) followed by GC and GC–MS analysis. Also, we have examined the antioxidant activities of the oils and crude extract fractions prepared from these plants.
2 Materials and methods
2.1 Chemical reagents
Chemical reagents used in this study were: helium (high purity 99%), n-alkanes (C7-C30) GC grade AR., 5% diphenyl, 95% dimethyl polysiloxane (DP-5) grade AR, 2,2′-azino-bis-3-ethylbenzthiazoline-6-sulphonic acid (ABTS), 2,2-diphenyl-1-picrylhydrazyl (DPPH), ascorbic acid, a-tocopherol, methanol, potassium persulfate, ferrous chloride, ferrozine, Sodium sulfate, EDTA (Ethylenediaminetetraacetic acid) and internal reference compounds. All chemicals used in this investigation were purchased from Sigma-Aldrich (Buchs, Switzerland).
2.2 Sampling and materials
The present study was carried out in April 2016. Samples of fresh flower buds and leaves of Cistus creticus and C. salviifolius were collected at maturity stage from different sites in Ajloun and Jerash areas in the northern part of the Hashemite Kingdom of Jordan. A voucher specimen of each has been deposited in the herbarium of the Department of Biological Sciences-Yarmouk University, Irbid, Jordan, C. creticus L. (YU/1/CC/1001) and C. salviifolius L. (YU/1/CC/1002).
2.3 Preparation of essential oils
The essential oils from fresh leave and flower buds of C. creticus and C. salviifolius were isolated as described (Al-Qudah, 2013; Al-Qudah et al., 2014, 2018). Fresh leaves and flower buds of C. creticus and C. salviifolius (200 g), were chopped into small parts and hydrodistillation for 4 h using a Clevenger-type apparatus. Subsequently, oils were dried over anhydrous sodium sulfate and immediately stored in GC-grade n-hexane at 4 °C until the analysis by gas chromatography/mass spectrometry (GC/MS) is carried out.
2.4 Solid phase micro-extraction (SPME) of volatile oils
The SPME experiments were performed using the fiber assemblies (PDMS/DVB; df 65 μm, length 1 cm) for manual sampling (Supelco, USA). About 0.1 g of freshly leave and flower buds of C. creticus and C. salviifolius were put into 5.0 mL amber glass vials, tightly capped with PTFE-coated septa, and SPME extraction was performed for 2.0 min at RT. Desorption of the analytes was carried out at 240 °C for 60 s. Each sample was repeated twice.
2.5 GC and GC–MS analysis
For chemical identification, a small portion of 1 µL extracted oils were diluted to 10.0 μL with GC grade n-hexane, then analyzed by GC–MS (Model Varian Chrompack CP-3800 GC/MS, Saturn, Netherlands) system, equipped with a DB-5 GC capillary column (5% diphenyl, 95% dimethyl polysiloxane, 30 m × 0.25 mm i.d., 0.25 μm film thicknesses). For mass spectroscopy detection, an electron ionization mode of 70 eV energy was used with a specific mass range. The flame ionization detector (FID) and injector temperature in the MS source were set at 180 °C. The temperature column was also programmed from 60 °C for 1 min (isothermal) to 246 °C at a constant rate of 3 °C/min, with the lower and upper temperatures being held for 3 min. The carrier gas was helium and was set at a flow rate of 0.9 mL/min. Quantitative analysis was performed using the Hewlett-Packard HP-8590 gas chromatography (Hewlett-Packard Co., Palo Alto, CA, USA) equipped with a split-splitless injector (split ratio 1:50) and a flame ionization detector (FID) was used. The device was connected to a 5% diphenyl, 95% dimethyl polysiloxane (optima-5) fused silica capillary column (30 m × 0.25 mm, 0.25 μm film thickness) (Varian Capillary Column) and under the same conditions described for the GC/MS analysis part.
2.6 Identification of the chemical constituents
A hydrocarbon mixture of n-alkanes (C7–C30) was analyzed separately under similar chromatographic conditions using the same DP-5 column. The identification of separated volatile components was achieved by matching their recorded mass spectra with the built-in library spectra (NIST, Gaithersburg, MD, USA, and Wiley Co., Hoboken, NJ, USA) and by comparing their calculated Kovats retention index (KI) relative to (C7–C30) n-alkanes values measured with the column of identical polarity. Further identification of major components of the extracts was confirmed by injecting authentic standard reference compounds on the same chromatography column and comparing their retention times with those of their counterparts from the oil samples.
2.7 Preparation of crude fractions
The fresh plants material from the flowers and leaves of C. salviifolius and C. creticus were air-dried in the shade for 1 month as previously described (Al-Qudah et al., 2018). Afterward, they were ground to fine powders and defatted with petroleum ether in Soxhlet extractor. After this, the plant residue was extracted in the same apparatus in methanol. The obtained alcoholic gummy residue was then partitioned between CHCl3 and H2O (1:1). The dried chloroform residue was then subjected to partitioning between 10% aqueous methanol (aq.MeOH) and hexane. The polar organic compounds were extracted from water by n-butanol. The different fractions obtained were assayed for their total phenol contents (TPC), total flavonoid contents (TFC) and in vitro and antioxidant activities.
2.8 Determination of total flavonoid (TFC)and phenol contents (TPC)
The total flavonoids contents of the crudes (Aq. MeOH, Butanol and water extracts) from the flowers and leaves of C. salviifolius and C. creticus were determined by the Folin-Ciocalteu method (Al-Qudah et al., 2018). 1.0 mL aliquot from the stock solution (1 mg/mL) of each extract, diluted in 4.0 mL distilled water, were introduced into a 10.0 mL volumetric flask, to which 0.30 mL of sodium nitrite solution (5% NaNO2, w/v) were added. The resulting mixture was allowed to stand for 5 min and then, 0.30 mL of aluminum chloride solution (10% AlCl3, w/v) was added. The resulting solution was incubated for another 6 min after which, 2.0 mL of 1.0 M NaOH solution was added and the final volume was adjusted to 10.0 mL with distilled water. After 15 min, the absorbance was measured at the wavelength, λ, of 510 nm. Methanol was used as blank. The total flavonoids content is expressed, in mg/g, as mass of quercetin with respect to the mass of the dry extract.
The phenols' contents of the crudes from the flowers and leaves of C. salviifolius and C. creticus was determined by aluminum chloride assay (Al-Qudah et al., 2018) 0.5 mL aliquot from the stock solution (1 mg/mL) of each extract was treated with 2.5 mL of Folin–Ciocalteu reagent (2 N) (diluted ten folds) and 2 mL of Na2CO3 (75 g/L). The mixture was allowed to stand at room temperature for 15 min and the absorbance was then recorded at the wavelength, λ, of 765 nm. Methanol was used as a blank solution. The total phenol content in the different extracts of both plants was expressed as mg gallic/g dry extract.
2.9 Determination of antioxidant activity
Also, the antioxidant activity of the crude and essential oils was determined by DPPH (2,2-diphenyl-1-picrylhydrazyl), ABTS (2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt) radical scavenging, hydrogen peroxide scavenging and ferrous ion chelating activity assay (Al-Qudah, 2013, 2016; Al-Qudah et al., 2014, 2018).
2.9.1 DPPH free radical scavenging activity
Briefly, to 1.0 mL of 0.1 mM DPPH• solution (dissolved in MeOH), 2.0 mL of various concentrations (0.005–0.5) mg/mL of each methanolic extract solutions were added. The solutions were allowed to stand at room temperature in dark for 30 min and then the absorbance of the solutions was measured at the wavelength, λ, of 517 nm against blank samples using UV–VIS spectrophotometer.
2.9.2 ABTS radical scavenging assay
The ABTS•+ cation radical solution was prepared by reaction of similar quantities of 7 mM of ABTS and 2.4 mM of potassium persulfate (K2S2O8) solution and allowed to react for 16 h at R.T in the dark. Before use; this solution was diluted with methanol to get an absorbance of 0.75 ± 0.02 at 734 nm. The reaction mixture comprised 3.0 mL of ABTS•+ solution and 1 mL of the extracts at various concentration (0.005–0.50) mg/mL. The absorbance of the mixture was measured at the wavelength, λ, of 734 nm by using a UV–Vis spectrophotometer. The blank was run in each assay and all measurements were done after at least 5 min.
2.9.3 Hydroxyl radical assay
1 mL of different concentrations of the extract solution in methanol (0.005–0.50) mg/mL were added to a 0.5 mL FeSO4 solution (6 mM). Then, 0.5 mL of 6 mM H2O2 was added to the mixture. Subsequently, after shaking and incubation of the reaction mixture for 10 min at room temperature, a 1 mL of 6 mM salicylic acid was added and further incubated for 30 min at room temperature. The absorbance was at the wavelength, λ, of 510 nm.
2.9.4 Ferrous ion chelating (FIC) effect
3.0 mL of methanol solution containing the different concentrations of crudes and essential oils (0.005–0.50) mg/mL was added to a 0.25 mL of 2 mM ferrous chloride (FeCl2) reagent. Subsequently, a 0.2 mL of 5 mM ferrozine solution was added to the mixture and allowed to stand at r.t. for 10 min after vigorous shaking. Then, the reduction in the absorbance of the visible radiation of red color was measured spectrophotometrically at 562 nm. The scavenging activity of the tested extracts and essential oils was compared to those of the positive control's ascorbic acid, α- tocopherol, and EDTA under similar conditions.
The percentage of scavenging activity of the tested extracts and essential oils was calculated using the equation: Scavenging activity (%) = (Ac − As/Ac) × 100; where Ac is the absorbance of the control and As is the absorbance in the presence of either extracts or control substance.
Non-linear regression analysis of GraphPad Prism 6 (GraphPad Software, San Diego, California, USA) was applied for the determination of IC50 in all of the antioxidant assays from the sigmoidal curve which was obtained by plotting the percentages of scavenging relative to the control versus logarithmic concentration of test compound. Each concentration was tested three times in 3 independent experiments.
2.10 Data analysis
MAT LAB 7.0.4 (Math Works, MA, USA) with PLS Toolbox 4.0 (Eigenvector Research, Inc, WA, and the USA) software were used for data processing and PCA analysis.
3 Results and discussion
3.1 Essential oil composition of flowers and leaves from Cistus salviifolius
The essential oils yield of leaves and flowers from C. salviifolius was 0.05% w/w and 0.03% w/w, respectively. Analysis of extracted oils from leaves and flowers of genus salviifolius was achieved by GC and GC–MS. Also, the 48 and 17 constituents which represent 99.9% and 99.8% of the oils obtained by hydrodistillation were identified, respectively (Table 1).
No.
KI (reported)
KI (Exp.)
Compound
% Area
C. salviifolius L.% Area
C. creticus L.Method of identification
SPME
Hydrodistillation
SPME
Hydrodistillation
Flowers
Leaves
Flowers
Leaves
Flowers
Leaves
Flowers
Leaves
1
821
826a
2E-Octene
1.1
–
–
–
0.1
–
0.1
–
MSb, RI,
2
830
835
2E-Hexenal
3.4
0.4
–
0.9
–
–
2.8
1.3
MS, RI
3
856
863
E-Salvene
–
2.3
–
–
–
–
0.1
–
MS, RI
4
888
895
santene
0.7
3.9
–
–
–
–
0.1
–
MS, RI
5
901
903
Ethyl pentanoate
1.0
–
–
–
–
–
–
–
MS, RI
6
926
926
tricyclene
0.3
0.2
–
–
0.8
0.7
–
–
MS, RI
7
939
934
α-pinene
12.8
11.9
–
0.3
25.9
19.4
–
–
MS, RI, RCc
8
952
951
α-fenchene
3.1
1.4
–
–
0.8
–
–
MS, RI
9
954
952
camphene
–
–
–
12.0
9.3
–
–
MS, RI
10
975
973
sabinene
0.9
0.8
–
–
0.1
–
–
–
MS, RI
11
979
984
β-pinene
7.0
5.2
–
–
8.5
6.3
–
–
MS, RI, RC
12
990
990
myrcene
–
–
–
3.0
7.5
–
–
MS, RI
13
1004
1007
p-mentha-1(7),8-diene
–
–
–
0.8
0.8
–
–
MS, RI
14
1017
1023
α-terpinene
0.3
0.4
–
–
0.7
0.6
–
–
MS, RI
15
1024
1029
p-cymene
4.8
5.7
–
–
0.6
0.9
–
–
MS, RI
16
1031
1034
δ-3-carene
15.7
15.5
–
–
6.4
5.9
–
–
MS, RI, RC
17
1050
1046
E-β-ocimene
0.7
0.5
–
–
0.1
0.3
–
–
MS, RI
18
1059
1061
γ-terpinene
1.7
0.6
–
–
1.6
1.2
–
–
MS, RI
19
1070
1064
cis-sabinene hydrate
0.6
0.4
–
–
–
–
MS, RI
20
1088
1087
terpinolene
0.2
0.3
–
–
0.7
0.7
–
–
MS, RI
21
1091
1091
p-cymenene
0.7
–
–
–
–
–
–
–
MS, RI
22
1096
1101
linalool
0.7
0.8
–
–
–
–
–
–
MS, RI
23
1100
1106
n-Undecane
–
0.7
–
–
–
–
0.6
MS, RI,
24
1100
1107
n-Nonanal
0.3
0.5
0.4
–
–
–
–
–
MS, RI
25
1102
1108
cis-thujone
0.3
–
–
–
–
–
–
–
MS, RI
26
1146
1147
camphor
0.2
–
–
3.0
–
–
–
–
MS, RI
27
1177
1180
terpinen-4-ol
–
–
–
–
0.1
0.4
0.8
MS, RI
28
1188
1194
α-terpineol
–
–
–
–
–
0.1
2.2
MS, RI
29
1200
1200
n-Dodecane
0.5
0.4
–
–
–
–
–
–
MS, RI
30
1201
1206
n-Decanal
0.4
0.7
–
–
–
–
–
–
MS, RI
31
1225
1232
citronellol
–
2.0
–
0.4
–
0.1
–
–
MS, RI
32
1248
1249
Benzeneacetic acid, ethyl ester
–
–
–
0.4
–
–
–
–
MS, RI
33
1258
1258
carvenone
–
–
–
11.2
–
–
–
–
MS, RI, RC
34
1265
1264
cis-chrysanthenyl acetate
–
–
–
0.2
–
–
–
–
MS, RI
35
1285
1288
bornyl acetate
–
–
–
0.7
–
–
–
–
MS, RI
36
1299
1298
Z-Methyl cinnamate
–
–
–
0.3
–
–
–
–
MS, RI
37
1300
1305
n-tridecane
0.2
0.5
–
–
–
–
–
–
MS, RI
38
1346
1346
Benzyl butanoate
–
–
–
0.5
–
–
–
–
MS, RI
39
1351
1353
α-cubebene
1.4
1.0
–
–
10.4
12.0
0.8
4.2
MS, RI, RC
40
1369
1356
eugenol
–
–
–
–
–
–
10.2
–
MS, RI
41
1370
1360
silphiperfol-5,7(14)-diene
–
–
–
–
0.3
0.2
MS, RI
42
1371
1367
cyclosativene
0.4
1.3
–
0.3
–
–
–
–
MS, RI
43
1376
1374
α-copaene
1.1
3.3
–
–
–
–
–
–
MS, RI
44
1381
1375
daucene
–
–
–
5.6
4.0
0.3
–
MS, RI
45
1377
1383
Z-Ethyl cinnamate
–
–
–
11.3
0.7
0.5
–
–
MS, RI, RC
46
1378
1386
E-Methyl cinnamate
–
–
–
1.3
–
–
–
–
MS, RI
47
1388
1388
β-cubebene
0.3
0.3
–
–
1.2
1.6
0.6
2.8
MS, RI
48
1398
1398
methyl eugenol
–
–
–
–
–
–
0.5
–
MS, RI
49
1400
1400
n-Tetradecane
0.2
0.4
–
–
–
–
–
–
MS, RI
50
1409
1404
α-gurjunene
–
0.4
–
–
0.3
0.3
–
–
MS, RI
51
1419
1417
E-caryophyllene
2.9
10.4
0.58
1.6
4.4
8.4
0.6
2.7
MS, RI, RC
52
1420
1427
β-copaene
–
–
–
–
0.2
0.1
MS, RI
53
1450
1446
cis-muurola-3,5-diene
0.2
0.5
–
–
0.6
0.6
0.4
1.4
MS, RI
54
1454
1453
α-humulene
–
1.0
–
0.4
0.3
0.5
–
–
MS, RI
55
1460
1457
Allo-aromadendrene
0.6
1.4
–
0.4
0.1
–
–
–
MS, RI
56
1467
1462
E-Ethyl cinnamate
–
–
0.66
17.5
–
–
–
–
MS, RI
57
1473
1467
drima-7,9(11)-diene
–
–
–
–
–
0.3
0.8
3.1
MS, RI
58
1477
1469
trans-cadina-1(6),4-diene
0.4
0.6
–
–
1.7
1.5
0.8
2.5
MS, RI
59
1480
1472
γ-muurolene
0.3
0.8
–
–
0.3
0.3
–
–
MS, RI
60
1485
1478
germacrene D
0.4
–
–
0.1
0.1
0.1
–
–
MS, RI
61
1484
1488
γ-amorphene
–
0.4
–
–
0.1
–
0.5
1.5
MS, RI
62
1492
1489
selinene
0.4
–
–
0.5
1.7
2.2
–
–
MS, RI
63
1493
1489
trans-muurola-4(14),5 diene
–
–
–
–
0.5
0.6
0.8
2.8
MS, RI
64
1500
1495
n-Pentadecane
0.3
0.5
–
–
0.7
0.9
–
–
MS, RI
65
1500
1498
bicyclogermacrene
–
–
–
2.39
–
–
–
–
MS, RI
66
1500
1500
α-muurolene
0.4
1.5
–
–
0.4
0.3
–
–
MS, RI
67
1505
1503
(E,E)-α-farnesene
4.8
1.2
0.8
–
0.1
–
–
–
MS, RI,
68
1511
1508
δ-amorphene
–
–
–
–
0.2
0.1
–
–
MS, RI
69
1513
1510
γ-cadinene
0.4
1.8
–
–
0.1
–
3.8
10.8
MS, RI
70
1523
1515
δ-cadinene
1.8
4.5
–
–
4.2
3.8
0.9
2.1
MS, RI
71
1529
1520
zonarene
0.3
0.4
–
–
1.5
1.3
–
–
MS, RI
72
1534
1529
trans-cadina-1(2),4-diene
0.4
0.4
–
–
1.2
1.5
0.5
1.5
MS, RI
73
1532
1534
Z-nerolidol
–
–
–
–
0.1
0.2
0.4
–
MS, RI
74
1538
1536
α-cadinene
–
0.4
–
–
–
–
–
–
MS, RI
75
1566
1569
3Z-hexenyl benzoate
0.3
1.1
–
0.5
–
–
–
–
MS, RI
76
1571
1572
dendrolasin
0.8
0.5
–
–
–
–
–
–
MS, RI
77
1583
1582
caryophyllene oxide
–
0.3
–
0.9
0.2
0.5
0.4
–
MS, RI
78
1587
1593
davanone
–
–
–
4.77
–
–
–
–
MS, RI
79
1619
1605
1,10-di-epi-cubenol
0.4
0.7
–
–
0.2
–
3.9
5.3
MS, RI
80
1625
1613
citronellyl pentanoate
0.2
–
–
–
–
–
–
–
MS, RI
81
1640
1634
caryophylla-4(14),8(15)-diene-5,α-ol
–
–
–
–
–
0.2
–
0.4
MS, RI
82
1642
1640
α-muurolol (Torreyol)
–
–
–
–
0.2
–
1.9
3.1
MS, RI
83
1650
1652
7-epi-α-eudesmol
–
0.4
–
–
0.1
0.3
–
–
MS, RI
84
1668
1663
E-citronellyl tiglate
0.4
–
–
–
–
–
–
–
MS, RI
85
1672
1677
n-Tetradecanol
2.5
–
–
–
–
–
–
–
MS, RI
86
1845
1855
Z-ternine
0.3
–
2.0
0.4
–
–
–
–
MS, RI
87
1878
1879
cubitene
–
–
–
–
0.1
–
1.2
2.5
MS, RI
88
1894
1896
catalponone
–
–
–
–
–
–
2.7
–
MS, RI
89
1900
1900
n-Nonadecane
–
–
0.4
–
–
–
–
–
MS, RI,
90
1905
1907
isopimara-9(11),15-diene
–
–
–
0.3
–
–
–
–
MS, RI
91
1922
1916
totarene
–
–
–
0.3
–
–
0.2
–
MS, RI
92
1921
1922
methyl hexadecanoate
–
–
0.6
–
MS, RI
93
1934
1929
isohibaene
–
–
–
0.3
–
–
–
–
MS, RI
94
1938
1953
cembrene
–
–
–
1.5
–
–
–
–
MS, RI
95
1948
1957
3E-cembrene A
–
–
0.5
0.1
–
–
0.2
–
MS, RI
96
1960
1959
nootkatin
–
–
–
–
0.2
–
0.2
–
MS, RI
97
1974
1979
dolabradiene
–
–
–
–
–
–
0.5
–
MS, RI
98
1974
1987
sclarene
–
–
–
0.5
–
–
7.2
13.3
MS, RI, RC
99
1978
1991
bifloratriene
–
–
–
0.4
–
–
–
–
MS, RI
100
1988
1996
1-Eicosene
–
–
–
–
–
–
0.3
–
MS, RI
101
2003
2007
manoyl oxide
4.5
0.5
4.1
13.2
0.9
2.7
19.5
27.0
MS, RI, RC
102
2010
2017
epi-13-manoyl oxide
0.4
–
–
–
–
–
–
–
MS, RI
103
2056
2053
abietatriene
0.4
–
4.4
12.3
–
–
12.0
–
MS, RI
104
2057
2060
manool
–
–
–
–
–
0.1
1.1
2.1
MS, RI
105
2077
2082
octadecanol
–
–
–
–
–
–
1.2
4.0
MS, RI
106
2087
2088
abietadiene
–
1.0
–
0.9
–
–
3.3
–
MS, RI
107
2100
2098
n-heneicosane
–
–
–
–
–
–
1.2
–
MS, RI
108
2116
2110
laurenan-2-one
–
–
–
0.7
–
–
–
–
MS, RI
109
2125
2118
methyl octadecanoate
–
–
9.2
–
–
–
–
–
MS, RI
110
2133
2125
nezukol
–
–
0.3
0.9
–
–
–
–
MS, RI
111
2141
2136
osthole
1.1
–
–
0.6
–
MS, RI,
112
2184
2175
incensole acetate
–
–
2.6
–
–
–
0.3
–
MS, RI
113
2184
2180
sandaracopimarinal
–
–
0.5
0.5
–
–
–
–
MS, RI
114
2200
2198
n-docosane
–
–
–
–
–
0.1
–
MS, RI
115
2203
2204
α-santonine
–
–
–
–
–
1.2
–
MS, RI
116
2210
2212
phyllocladanol
–
–
–
0.6
–
–
–
–
MS, RI
117
2237
2235
7-α-hydroxy-manool
–
–
–
0.9
–
–
–
MS, RI
118
2241
2242
Z-isoeugenyl phenylacetate
–
–
–
–
–
1.2
–
MS, RI
119
2275
2270
dehydroAbietal
–
–
0.4
0.4
–
–
–
MS, RI
120
2310
2305
isopimarol
–
–
–
0.6
–
–
0.4
–
MS, RI
121
2313
2310
abietal
–
–
–
0.3
–
–
–
–
MS, RI
122
2314
2317
trans-totarol
–
–
–
0.7
–
–
–
–
MS, RI
123
2332
2328
trans-ferruginol
–
–
–
1.4
–
–
–
–
MS, RI
124
2360
2360
3-α-acetoxy-manool
–
–
–
1.0
–
–
5.9
5.6
MS, RI
125
2368
2371
dehydro abietol
–
–
59.0
0.9
–
–
–
–
MS, RI
126
2401
2408
abietol
–
–
12.7
0.5
–
–
–
–
MS, RI
127
2422
2420
labd-13E-8,15-diol
–
–
1.4
–
–
–
–
–
MS, RI
Total
85.1%
92.1%
99.9%
99.8%
98.9%
98.0%
96.7%
100%
Monoterpenes Hydrocarbon
131 (48.8)2
13 (48.9)
0
1 (0.3)
13 (61.2)
13 (53.5)
2 (0.2)
0
Oxygenated monoterpenes
4 (1.8)
3 (3.2)
0
5 (15.5)
1 (0.1)
3 (0.6)
2 (3.0)
0
Sesquiterpenes Hydrocarbon
17 (16.5)
19 (31.6)
2 (1.4)
7 (5.7)
23 (34.2)
20 (38.6)
12 (11.1)
11 (35.2)
Oxygenated Sesquiterpenes
4 (1.9)
4 (1.8)
0
2 (5.6)
6 (0.9)
4 (1.0)
6 (8.1)
3 (8.7)
Diterpenes Hydrocarbon
(1) 0.4
1 (1.0)
2 (4.9)
9 (16.6)
1 (0.1)
0
7 (24.7)
2 (16.0)
Oxygenated Diterpenes
4 (4.9)
1 (0.5)
7 (81.0)
15 (23.0)
1 (0.9)
2 (2.8)
6 (27.9)
3 (34.7)
Esters
2 (1.5)
1 (1.1)
2 (9.8)
7 (31.8)
1 (0.7)
1 (0.5)
2 (1.8)
Others
10 (9.3)
8 (4.0)
3 (2.8)
2 (1.3)
2 (0.8)
1 (0.9)
10 (19.9)
2 (5.3)
The total high percentage of principal oxygenated diterpenes oil in flowers (81.0%), associated with dehydro abietol (59.0%) and abietol (12.7%), was detected. Esters were found in a percentage of (9.9%) associated with methyl octadecanoate (9.2%) and E-ethyl cinnamate (0.7%). The analysis of the components of hydrodistilled oil from flowers contained two diterpene hydrocarbons (4.9%) and two sesquiterpenes (1.4%) as seen in Table 1. The essential oil of the leaves has E-ethyl cinnamate (17.5%), manoyl oxide (13.2%), abietatriene (12.3%), Z-ethyl cinnamate (11.3%) and carvenone (11.2%), which seem to be the major components of the essentail oil from leaves of C. salviifolius.
The volatiles from the flowers and leaves of C. salviifolius were extracted and collected by the SPME method for analysis using the GC–MS technique, as mentioned in the literature (Saleh et al., 2017). The analysis of volatiles from were identified 53 components from flowers and 50 components from leaves, with a percentage of 85.1% up to 92.1% of the total composition, respectively. The results of the analysis are shown in Table 1. Table 1 shows flower oil consisting of 13 monoterpene hydrocarbons (48.8%), associated with δ-3-carene (15.7%), α-pinene (12.8%) and β-pinene (7.0%) as major constituents. The identification reveals 17 sesquiterpene hydrocarbons (16.5%), 10 other compounds (9.3%), two oxygenate diterpenes (4.9%), four oxygenated monoterpenes (1.8%), four oxygenated sesquiterpenes (1.9%), two ester compounds (1.5%) and one diterpene hydrocarbon (0.4) from flower oil of C. salviifolius, which indicates that there is a high similarity in active essential oil components with C. creticus L. A different set of extracted oils was detected from leaves, which gave different components including 13 monoterpene hydrocarbons (48.9%), associated with δ-3-carene (15.5%) and α-pinene (11.9%) along with 19 sesquiterpene hydrocarbons (31.6%), associated with E-caryophyllene (10.4%), three oxygenated monoterpenes (3.2%), 8 other compounds (4.0%) and four oxygenated sesquiterpenes (1.8%) all of which may be considered as major compounds.
3.2 Essential oil composition of flowers and leaves from C. creticus
The distilled essential oils from leaves and flower buds are characterized to range from yellowish to yellow color and the yields were 0.02% w/w and 0.01% w/w, respectively. In terms of chemical structure, volatiles and essential oil components are classified into (8) classes associated with the calculated Kovats indices and mass spectra compared to those stored in the GC–MS built in libraries. Table 1 represents the GC and GC–MS analysis of the C. creticus oils from leaves and flower buds, where the results led to the identification of 21 and 47 constituents (100%) and (96.7%), respectively.
Experimentally, flower oil contents are manoyl oxide and 3-α-acetoxy-manool with a percentage of (19.5%) and (5.9%), respectively, whereas oxygenated diterpenes (27.9%) represent the major content of the total oil weight. Diterpenes (24.7%), abietatriene (12.0%) and sclarene (7.2%) were found to be the major components of the oils. The percentage composition of flower oil constituents obtained by the hydrodistillation method are summarizes in Table 1. It contains eight categories of volatiles with six oxygenated sesquiterpenes (8.1%), twelve sesquiterpene hydrocarbons (11.1%), ten aliphatic compounds (19.9%), two oxygenated monoterpenes (3.0%) and two monoterpene hydrocarbons (0.2%).
In comparison, the essential oil of the leaves has manoyl oxide (27.0%), sclarene (13.3%), γ-cadinene (10.8%), and 3-α-acetoxy-manool (5.6%) as major components. Organic active components of volatile oils extracted from flowers and leaves of C. creticus L. were obtained by Solid Phase Micro-Extraction (SPME) method and GC–MS technique using the procedure mentioned in the literature (Saleh et al., 2017). Table 1 shows the results of volatile principle active components of C. creticus L., which was analyzed by using GC–MS techniques. The results led to the identification of forty-eight flower and forty-four leave constituents, amounting up to 98.9% and 98.0% of the total composition, respectively. There have been 13 monoterpene hydrocarbons (61.2%) in flower oil, associated with α-pinene (25.9%) and camphene (12.0%) as major constituents. Furthermore, the experimental analysis indicated twenty-three sesquiterpene hydrocarbons (34.2%), associated with α-cubebene (10.4%). Sex oxygenated sesquiterpenes (0.9%), one oxygenated diterpene (0.9%), one diterpene (0.1%), and two aliphatic compound (0.8%) are the main active compounds. Oil extracted from leaves contains 13 monoterpene hydrocarbons (53.5%), associated with α-pinene (19.4%), camphene (9.3%), 20 sesquiterpene hydrocarbons (38.6%), α-cubebene (12.0%) E-caryophyllene (8.4%), in addition to two oxygenated diterpenes (2.8%), four oxygenated sesquiterpenes (1.0%) and three oxygenated monoterpenes (0.6%) as major contents as seen in Table 1.
In comparison to the previously published data on the oil composition of C. criticus and C. salviifolius (Mastino et al., 2017; Morales-Soto et al., 2015), our results show significant differences in the concentrations of the reported main components. The volatile components of the aerial parts of C. salviifolius from Spain were reported camphor (43.86%), E-caryophyllene (19.26%), eucalyptol (19.14%) and (β-bourbonene) (13.27%) as mainly compounds (Morales-Soto et al., 2015). But the oil from the aerial parts of C. salviifolius from Sardinia showed a high quantity of manoyl oxide (Mastino et al., 2017). The chemical composition from the aerial parts of C. creticus from Torre Beregna showed a high quantity of linolenic (39.2%), hexadecanoic (14.7%) and linoleic (12.9%) (Maggi et al., 2016). Whereas from the essential oils of the leaves of C. creticus subsp. from Greece were rich in sesquiterpenes such as δ-cadinene (5.6%), a-cadinene (6.5%), bulnesol (6.3%), and viridiflorol (5.4%) (Demetzos et al., 1997). These changes in the essential oil compositions might arise from several factors such as geological, geographical, seasonal, and climatic (Perry et al., 1999).
3.3 Screening of the total phenolic and flavonoid contents and evaluation of antioxidant activity
Table 2 summarizes the total phenols in mg/g gallic acid for aq. MeOH, butanol and aqueous extracts, which varied among the different extracts of plant ranging from 111 to 183.8 mg/g gallic acid in flowers of C. salviifolius and from 196 to 283 mg gallic/g dry extract in flowers of C. creticus, as well as from 126 to 393 mg gallic/g dry extract in leaves of C. salviifolius. The screening of aq. MeOH fraction from flowers and leaves of C. salviifolius showed the highest phenolic and flavonoid compound concentrations, compared with aqueous methanol and water extracts. Whereas, the butanol extract from flowers and leaves of C. creticus. contained the highest phenolic and flavonoid concentrations concerning the two different extracts (mg quercetin/g of a plant).
Crude
C. salviifolius L.
C. creticus L.
Flowers
Leaves
Flowers
Leaves
Total flavonoids
(mg quercetin/g of extract)Total phenol
mg gallic/g dry extractTotal flavonoids
(mg quercetin/g of extract)Total phenol
mg gallic/g dry extractTotal flavonoids
(mg quercetin/g of extract)Total phenol
mg gallic/g dry extractTotal flavonoids
(mg quercetin/g of extract)Total phenol
mg gallic/g dry extract
aq. MeOH
393 ± 8
183.8 ± 0.1
313 ± 4
199 ± 2
288 ± 9
221 ± 5
261 ± 8
172 ± 3
BuOH
173 ± 6
149.5 ± 0.3
284 ± 5
187.0 ± 0.2
369 ± 9
283 ± 4
273 ± 8
182 ± 5
Water
125 ± 1
110.9 ± 0.4
223 ± 5
161.0 ± 0.6
217 ± 9
196 ± 2
208 ± 1
112.4 ± 0.6
Table 3 represents the evaluation results of antioxidant activity of essential oils and crude extracts from flowers and leaves of C. salviifolius and C. creticus using different methods described in (Al-Qudah, 2013, 2016; Al-Qudah et al., 2014, 2018). Weak scavenging effects are shown in all the different antioxidant assays. Generally, the oils from leaves of C. salviifolius and C. creticus exhibited a higher scavenging effect than the ones obtained from the flower parts. The antioxidant activities for the different extract fractions from flowers and leaves of C. salviifolius and C. creticus showed strong scavenging effects in all the different antioxidant assays. The aq. MeOH crude from flowers and leaves of C. salviifolius exhibited a higher scavenging effect than the other crudes. Butanol extracted from flowers and leaves of C. creticus showed a higher scavenging effect than the other crudes.
C. salviifolius L.
Parts
Crude
DPPH
ABTS
Hydroxyl
FIC
Flowers
Essentail Oil
0.15 ± 0.06
0.09 ± 0.02
0.36 ± 0.02
0.37 ± 0.02
aq.MeOH
0.01 ± 0.00
0.01 ± 0.00
0.02 ± 0.00
0.04 ± 0.00
BuOH
(1.6 ± 0.06) * 10−2
(1.5 ± 0.06) * 10−2
0.12 ± 0.04
0.07 ± 0.00
Water
(1.5 ± 0.06) * 10−2
(1.9 ± 0.06) * 10−2
0.05 ± 0.00
0.04 ± 0.00
Leaves
Essentail Oil
0.16 ± 0.01
0.13 ± 0.01
0.28 ± 0.05
0.29 ± 0.03
aq.MeOH
(1.8 ± 0.06) * 10−2
0.01 ± 0.00
0.01 ± 0.00
0.11 ± 0.02
BuOH
(2.4 ± 0.06) * 10−2
0.01 ± 0.00
0.11 ± 0.00
0.12 ± 0.00
Water
(2.5 ± 0.06) * 10−2
0.01 ± 0.00
0.09 ± 0.01
0.14 ± 0.00
C. criticus L.
Flowers
Essentail Oil
0.36 ± 0.06
0.31 ± 0.01
0.61 ± 0.10
0.58 ± 0.07
aq.MeOH
(1.5 ± 0.06) * 10−2
(1.2 ± 0.06) * 10−2
0.14 ± 0.01
0.21 ± 0.01
BuOH
(2.3 ± 0.16) * 10−3
(6.0 ± 0.24) * 10−3
0.10 ± 0.00
0.10 ± 0.00
Water
(6.3 ± 0.01) * 10−3
0.01 ± 0.00
0.15 ± 0.00
0.19 ± 0.00
Leaves
Essentail Oil
0.33 ± 0.01
0.31 ± 0.11
0.57 ± 0.02
0.55 ± 0.07
aq.MeOH
(2.4 ± 0.06) * 10−2
0.02 ± 0.00
0.06 ± 0.00
0.16 ± 0.01
BuOH
(1.7 ± 0.06) * 10−2
(1.4 ± 0.06) * 10−2
0.03 ± 0.00
0.11 ± 0.00
Water
(1.7 ± 0.06) * 10−2
(1.6 ± 0.06) * 10−2
0.04 ± 0.00
0.19 ± 0.00
Ascorbic acid
(1.8 ± 0.06) * 10−3
(1.9 ± 0.06) * 10−3
(2.6 ± 0.03) * 10−3
(1.9 ± 0.02) * 10−3
α-tocopherol
(2.3 ± 0.04) * 10−3
(1.8 ± 0.01) * 10−3
(2.8 ± 0.05) * 10−3
(2.9 ± 0.02) * 10−3
EDTA
(1.3 ± 0.02) * 10−3
(2.2 ± 0.01) * 10−3
Principle component analysis (PCA), is a multivariate data analysis method that can be used for finding differences or similarities among a given dataset. In PCA, the score plot or model contains information about samples (objects), while the PCA loading plot involves variable information (Obeidat et al., 2014).
PCA has been applied to the data obtained from the mass spectrometry (Table 1) to investigate chemical composition differences between the two studied plants, or among the different organs within the plant using different extraction procedures. The first few PCs were studied; the best result was obtained upon having a two-dimensional PCA model that accounts for more than 45% of the total variation in the data set using the first and the fifth PCs. Fig. 1 represents the resulted score PCA model, as it appears in this figure the leaves and flowers from each plant were almost overlapped when extracted with the SPME method. In predicting the PCA score model, the greater the distance between the points in the model the more different they are (Al-Qudah et al., 2018). Therefore, it can be concluded that flowers and leaves of the same plant produced almost similar chemical compounds when extracted with the SPME method. On the other hand, the large distance in the PCA model between the points that represent SPME extracts of C. salviifolius. (leaves and flowers) and that of C. creticus. (leaves and flowers), reflects the variance in chemical composition between the two plants when extracted via the SPME method. Discriminating among plants based on chemical composition extracted via the hydrodistillation method was not possible using the score PCA model (Fig. 1) indicating that almost similar compounds were extracted from the leaves and flowers in the two studied plants when extracted with the hydro-distillation method.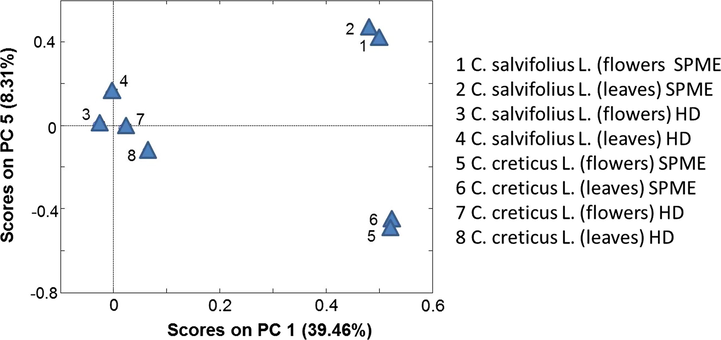
The PCA score plot for chemical composition of the extracts obtained by hydrodistillation HD and solid phase micro-extraction (SPME), from flowers and leaves of C. creticus and C. salviIfolius.
Further investigations of the PCA loading model (Fig. 2) showed that the responsible compound for distinguishing between C. saliifolius. and C. creticus. on the first PC was α-Pinene, while the compound δ-3-Carene had the greatest impact on the variation in the fifth PC, and for a less extent come to the compounds Camphene and α-Cubebene, respectively.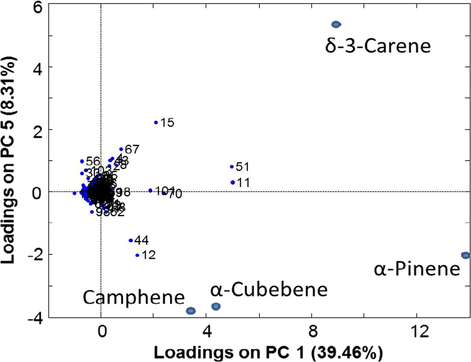
The PCA score plot for chemical composition of the extracts obtained by hydrodistillation HD and solid phase micro-extraction (SPME), from flowers and leaves of C. creticus and C. salviIfolius.
4 Conclusions
The extraction methods used, such as solid-phase micro-extraction method, showed that monoterpenes and sesquiterpenes are the most volatile constituents. However, hydrodistillation method indicated that diterpenes and oxygenated diterpenes are the principal classes represented in the volatile constituents of the essential oils. There are differences in chemical composition between the two organs, leaves, and flower buds in both plants. These oils showed weak antioxidant activity when compared to the positive controls, α-tocopherol, ascorbic acid, and EDTA, while crude extracts (aq. MeOH, butanol, water) showed good antioxidant activity, which could advocate their use as a source of natural antioxidants. PCA score plot showed that compounds extracted from the leaves and flowers of each plant were different upon extracted with SPME, while almost no significant difference between the two plants (C. salviifolius and C. creticus) was detected when extracted using hydrodistillation method.
Acknowledgments
This work was generously supported by the Deanship of Scientific Research and Graduate Studies at Yarmouk University, Irbid, Jordan.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Al-Eisawi, D.M. 1982. List of Jordan Vascular Plants. Mitteilungen der Botanischen Staatssammlung München, 18, pp. 79–182.
- survey of plants used in Jordanian traditional medicine. Pharmacognomics J.. 1995;33(4):317-323.
- [Google Scholar]
- Antioxidant acitvity and chemical composition of essential oils of fresh and air-dried Jordanian Nepeta curviflora Boiss. JBAPN. 2016;6(2):101-111.
- [Google Scholar]
- Chemical compositions of the essential oil from the Jordanian Plant lupinus varius. Arab. J. Chem.. 2013;6:225-227.
- [Google Scholar]
- Volatile components analysis, total phenolic, flavonoid contents, and antioxidant activity of Phlomis species collected from Jordan. JEOP. 2018;21(3):583-599.
- [Google Scholar]
- Antioxidant Activity and Chemical Composition of Essential Oils from Jordanian Ononis natrix L. and Ononis sicula Guss. JBAPN. 2014;4(1):52-61.
- [Google Scholar]
- Analysis of labdane-type diterpenes from Cistus creticus (subsp. creticus and subsp. eriocephalus) by GC and GC-MS. Planta Med.. 1999;65(8):735-739.
- [Google Scholar]
- Cistaceae aqueous extracts containing ellagitannins show antioxidant and antimicrobial capacity, and cytotoxic activity against human cancer cells. Food Chem. Toxicol.. 2010;48:2273-2282.
- [Google Scholar]
- Antibacterial activities of the crude ethanol extracts of medicinal plants against List eriamonocy to genes and some other pathogenic strains. Afr. J. Biotechnol.. 2010;9:4251-4258.
- [Google Scholar]
- Antipro life rative activity of hexane extracts from Tunisian Cistus libanotis, Cistus Monspeliensis and Cistus villosus. Chem. Cent. J.. 2013;47:1-7.
- [Google Scholar]
- Cytotoxic and antibacterial labdane-type diterpenes from the aerial parts of Cistus incanus subsp. creticus. Planta Med.. 1994;60(1):34-36.
- [Google Scholar]
- Application of a new GC-MS library, designed with a retention index filter tool, to the analysis of the essential oil of Cistus ladanifer. Acta Horticulturae. 2009;826:271-276.
- [Google Scholar]
- Flavon-3-ols, prodel- phinidins and further polyphenols from Cistus salvifolius. Phytochemistry. 1994;37:533-538.
- [Google Scholar]
- Cytotoxic and anti-inflammatory activity of labdane and cis-clerodane typediterpenes. Planta Med.. 2001;67:614-618.
- [Google Scholar]
- A new labdane-type diterpene and other compoundsfrom the leaves of Cistus incanus subsp. creticus. J. Nat. Prod.. 1990;53:1365-1368.
- [Google Scholar]
- Composition and antimicrobial activity of the essential oil of Cistus creticus subsp. Planta Med.. 1997;63(5):477-479.
- [Google Scholar]
- Composition and antimicrobial activity of the essential oil of Cistus creticus L. J. Essent. Oil Res.. 1995;7:407-410.
- [Google Scholar]
- Polyphenolic glycosides from Cistus creticus L. leaves. Ann. Pharm. Fr.. 1989;47:314-318.
- [Google Scholar]
- Diterpene esters of malonic acid from the resin ‘Ladano’ of Cistus creticus. Phytochemistry. 1994;35(4):979-981.
- [Google Scholar]
- Chemical analysis and antimicrobial activity of the resins Ladano, of its essential oil and of the isolated compounds. Planta Med.. 1999;65:76-78.
- [Google Scholar]
- Biological activity of myricetin and its derivatives against human leukemic cell lines in vitro. Pharmacol. Res.. 2000;42:475-478.
- [Google Scholar]
- Diversity and divergence in Cistus salvifolius L. populations from contrasting habitats. Hereditas. 2000;132:183-192.
- [Google Scholar]
- Aromatic plants as a source of important phytochemicals: Vitamins, sugars and fatty acids in Cistus ladanifer, Cupressus lusitanica and Eucalyptus gunnii leaves. Ind. Crops Prod.. 2009;30:427-430.
- [Google Scholar]
- Separations of favon-3-ols and dimeric proanthocyanidins by capillary electrophoresis. Planta Med.. 1998;64(1):63-67.
- [Google Scholar]
- Madaus, G., Lehrbuch der Biologischen Heilmittel Bd. II, 1938, p. 1003. Thieme, Leipzig.
- Phytochemical analysis of the labdanum-poor Cistus creticus subsp. eriocephalus (Viv.) Greuter et Burdet growing in central Italy. Biochem. Syst. Ecol.. 2016;66:50-57.
- [Google Scholar]
- Comparison of essential oils from Cistus species growing in Sardinia. Nat. Prod. Res.. 2017;31(3):299-307.
- [Google Scholar]
- Volatile profile of Spanish Cistus plants as sources of antimicrobials for industrial applications. Ind. Crops Prod.. 2015;74:425-433.
- [Google Scholar]
- Study of fuel assessment and adulteration using EEMF and multiway PCA. Energy Fuels. 2014;28(8):4889-4894.
- [Google Scholar]
- Flavonoids de Cistaceas. II Cistus populifolius L. Cistushirsutus. Lam. An. Quím.. 1977;73:1047.
- [Google Scholar]
- Essential oils from Dalmation Sage (Salvia officinalis L.): variations among individuals, plant parts, seasons and sites. J. Agric. Food Chem.. 1999;47:2048-2054.
- [Google Scholar]
- Flavan-3-ols and proanthocyanidins from Cistus incanus. Phytochemistry. 1991;30:981-985.
- [Google Scholar]
- Comprehensive analysis of the chemical composition and cytotoxicity mechanisms of Pallines spinosa flower and leaf essential oils against breast cancer cells. Cell Physiol. Biochem.. 2017;42:2043-2065.
- [Google Scholar]
- Simultaneous determination of catechins, rutin, and gallic acid in Cistus species extracts by HPLC with diode array detection. J. Chromatogr. Sci.. 2008;46:150-156.
- [Google Scholar]
- Methylated flavonoids in the leaf resin of Cistus albanicus and C. parviflorus. Planta Med.. 1986;52(6):527.
- [Google Scholar]
- Epicuticular flavonoids in the genus Cistus, Cistaceae. J. Plant Physiol.. 1987;131:25-36.
- [Google Scholar]
- Screening of Turkish anti-ulcerogenic folk remedies for anti-Helicobacter pylori activity. J. Ethnopharmacol.. 1999;66(3):289-293.
- [Google Scholar]







