Translate this page into:
Antioxidant potential of non-oil seed legumes of Indonesian’s ethnobotanical extracts
⁎Corresponding author at: Department of Food Science and Biotechnology, Graduate School, Kyungpook National University, Daegu 41566, Republic of Korea. sang@knu.ac.kr (Sang-Han Lee)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
This study investigated the in vitro antioxidant properties (DPPH, ABTS, CUPRAC and FRAP), total phenolic content and flavonoid content of extracts from three non-oil seed legumes (Phaseolus lunatus red and white, and Canavalia ensiformis), local edible seeds from Indonesia, obtained using different solvent system (distilled water, 70% ethanol, and 100% ethanol). The variety of legume was a major source of variation in the phenolic contents, flavonoid content and antioxidant activity. HPLC analysis of the non-oil seed legume extracts identified gallic acid, epicatechin and coumaric acid. Among the varieties of non-oil seed legume extracts, the phenolic content varied from 15.21–38.60 mg gallic acid equivalents/g dry weight and the flavonoid content was 11.73–24.61 mg catechin equivalents/g dry weight. The antioxidant activity of the extracts suppressed the reactive oxygen species (ROS) generation and cellular damage induced by UV-B in HaCaT cells. These results showed that antioxidant activity (1.83–19.42% of inhibition DPPH; 2.99–37.29% of inhibition ABTS; 0.20–2.47 µM CUPRAC value; and 0.96–1.10 µM of FRAP value) of extracts possessed strong radical scavenging activity as well as inhibited ROS generation in a dose-dependent manner without showing any cytotoxicity. Collectively, the data presented that antioxidant of the extracts have potent antioxidant activity and decreasing ROS generation in HaCaT cells. It can be intimately used as alternative criterion for antioxidant and antiradical activities that can be utilized as a functional food and nutraceutical ingredients.
Keywords
Antioxidant
Canavalia ensiformis
HPLC
Phaseolus lunatus
Pholyphenol
1 Introduction
Underutilized plants are genetically very diverse groups grown in temperate, subtropical and tropical regions and have been recognized for their human health benefits. Most of the underutilized plants have high content of non-nutritive, nutritive, and bioactive compounds such as flavonoids, phenolics, anthocyanins, phenolic acids, and as well as nutritive compounds such as sugars, essential oils, carotenoids, vitamins, and minerals (Dogan et al., 2014; Mollica et al., 2017; Tsukamoto et al., 2018; Fazenda et al., 2019; Sadeer et al., 2019).
Non-oil seed legumes are commonly planted (bean) in marginal land with minus of concern, considered to have originated in Southeast Asia, including Indonesia. The young pods are boiled separately as a vegetable, or together with another vegetables as a soup. The dry seeds are cooked together with rice after soaking water. The seeds are utilized as raw materials of tempeh, a traditional Indonesian fermented food. They are ingested by humans throughout the world, including Indonesia, and are an essential commodity in the optimal human diet because their seed structure and composition confer physiological benefits to the total diet. In addition, the consumption of a legume-rich diet has been associated with a decreased prevalence of many chronic diseases (Hayat et al., 2014), including tumor, diabetes, and cancer (Yeap et al., 2014). Some non-oil grain legumes, including lima bean (Phaseolus lunatus) and jack bean (Canavalia ensiformis [CES]), have been used for thousands of years as a food and therapeutic plant in Indonesia, yet they have not been well-studied and are underutilised.
Besides their biological benefits, such as anti-glycative, anti-angiogenic, and hepatoprotective effects (Sun et al., 2012; Kim O et al., 2013; Kumar and Reddy, 2014), non-oil seed legumes have high nutritional potential. Edible beans contain appreciable amounts of various nutrients, such as protein, carbohydrate, minerals and vitamins (Ekanayake et al., 2000). In recent years, the human health benefits associated with the polyphenols, fibre and various other components in beans have attracted increasing interest (Hayat et al., 2014; Ombra et al., 2016). Natural antioxidants, mainly the polyphenols, are found abundantly in the seed coats than cotyledons and show potent antioxidant and antiradical activities (Diaz-Batalla et al., 2006). Phenolic compounds exert antioxidant action because they participate in redox reactions by donating electrons and hydrogen atoms, and acting as reducing agents, singlet oxygen suppressers and metal chelators (Tsao and Deng, 2004). There are many varieties of non-oil seed legumes, and their chemical composition and nutritive value vary considerably.
The process of extraction is an essential step in analysing the composition of a plant because it allows obtaining a crude extract of the target compounds for further separation and identification. Each plant has unique characteristics, and so the choice of extraction conditions can greatly influence the recovery of the compounds (Chirinos et al., 2007). The traditional solid–liquid extraction with acetone, ethanol, methanol, water, either alone or as mixtures, is commonly utilized for phenolic constituents. The choice of solvent and its concentration will depend on the nature of the target phenolic constituents and the characteristics of the sample (e.g., seeds, leaves, peel). Other factors known to affect the rate of extraction, yield and purity of extracted polyphenolics, include the particle size of the sample, temperature, pH and duration of the extraction (Chew et al., 2011).
Starting from such hypotheses, this research explored the in vitro antioxidant properties and polyphenols (including total phenolic content [TPC] and flavonoid contents) of the extracts from three non-oil seed legume (P. lunatus red [PLR] and white [PLW], and CES) from Indonesia, obtained using different solvent systems.
2 Experimental
2.1 Materials
2,2-Diphenyl-1-picrylhydrazyl (DPPH), 2,2′-azino-bis (3-ethyl benzothiazoline-6-sulphonic acid) (ABTS), α,α'-azodiisobutyramidine dihydrochloride, gallic acid, catechin and Folin–Ciocalteu reagent were procured from Sigma–Aldrich (USA). As the extraction solvents, 70% and 100% ethanol, acetonitrile, formic acid (high-performance liquid chromatography [HPLC] grade), were from Merck (Germany), and distilled water was acquired from a water distillation plant in our laboratory. All other chemicals were of analytical grade. UV–visible spectra were acquired on a Multiskan GO spectrophotometer (Thermo Fisher Scientific, Japan).
2.2 Sample collection
The non-oil seed legumes (PLR, PLW and CES) (Fig. 1A-C i) were collected from Bondowoso district, East Java, Indonesia. The seeds were collected in polyethylene bags and stored at 4 °C until needed. Seed samples (500 g) were washed, soaked in water for 1 day, peeled, cut, sun-dried, dried in a hot air oven, ground to a fine powder (60-mesh) and stored at 4 °C before extraction. Voucher specimens of the non-oil seed legume samples were deposited in our laboratory (Food Enzyme Biotechnology, Kyungpook National University, Korea) for future reference (2019-Plr, 2019-Plw and 2019-Ce).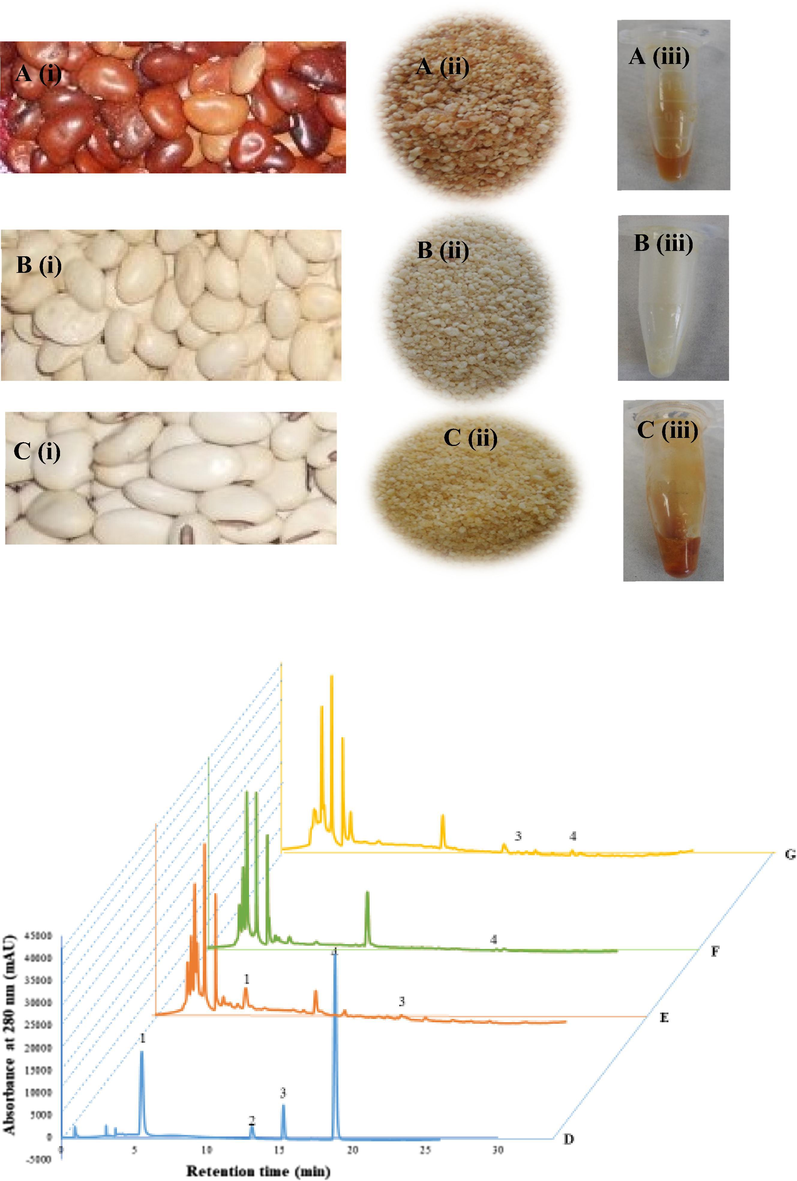
Photo of non-oil seed legumes varieties, Phaseolus lunatus red (PLR) (A), Phaseolus lunatus white (PLW) (B), Canavalia ensiformis (CES) (C) bean (i), powder (ii), and extract (iii). HPLC (absorbance at 280 nm) – profile of phenolic standards (D); Phaseolus lunatus red (PLR) (E); Phaseolus lunatus white (PLW) (F); Canavalia ensiformis (CES) (G). Peaks: gallic acid (1); catechin (2); epicatechin (3); and coumaric acid (4).
2.3 Extraction
The powder of non-oil seeds (30 g) (Fig. 1A-C ii) was extracted three times with i) distilled water, ii) 70% ethanol and iii) 100% ethanol (30 × 300 mL) using an ultrasonic water bath (Powersonic 420, 50/60 Hz) at 40 kHz and 50 °C for 120 min. The supernatants were collected and filtered through filter paper, and the solvent was evaporated using a vacuum rotary evaporator (Eyela N-1000, Tokyo Rikakikai Co. Ltd., Tokyo, Japan). The extracts were lyophilised and dissolved in distilled water (Fig. 1A-C iii) to determine their antioxidant activity.
2.4 HPLC analysis
HPLC was conducted to identify the phytochemical characteristics of the non-oil seed legume extracts. The Shimadzu Prominence HPLC system (Shimadzu, Kyoto, Japan) was equipped with an autosampler (SIL-201), an SPD-M20A diode array detector, LC solution 1.22 SPI software and a reverse-phase Phenomenex C18 column (4.6 mm × 250 mm, 5 μm) (Merck, Germany). A stepwise gradient of solvent A (acetonitrile) and solvent B (1% formic acid solution) was utilized with the ration changing each minutes at λ = 280 nm, as described by Brito et al. (2015).
2.5 TPC determination
The crude extracts of PLR, PLW and CES were analysed for TPC by the Folin–Ciocalteu assay as described by Gul et al (2011), using gallic acid as a standard. Specifically, the sample (2 μL) and 100 μL of sodium carbonate (7%) were added to 10 μL of Folin–Ciocalteau regent (10% v/v). The absorbance was monitored at 595 nm after 10 min of reaction at room temperature. Result were expressed as gallic acid equivalent (GAE) per gram of extract.
2.6 Determination of total flavonoids
Total flavonoid content of PLR, PLW and CES were determined by aluminium chloride colourimetric method, as described by Zengin et al. (2015), 2 μL of sample and 5% sodium nitrite (5 μL), 10% aluminium chloride (10 μL), 1 M sodium hydroxide (40 μL) and distilled water (43 μL) were mixed. The absorbance was measured at 405 nm after 10 min at room temperature. Catechin was used to construct the calibration curve. Flavonoid content was expressed as milligrams of catechin equivalents per gram of extract (mg CE/g).
2.7 DPPH• free radical-scavenging activity
To evaluate the free radical scavenging activity of extracts we used DPPH method as described by Alam et al (2017). The non-oil seed legume of extracts of different concentrations (3, 10, 30 and 100 μg/mL, respectively) and ascorbic acid (as the standard) were dispensed into a 96-well plate. The volume of each well was adjusted to 200 μL by adding 4% methanolic solution of DPPH. The plate was left at 37 °C for 10 min in the dark before the absorbance was measured at 520 nm. An appropriate reagent blank was run simultaneously in each assay.
2.8 ABTS•+ free radical-scavenging activity
The evaluation of ABTS•+ scavenging by the extracts was assayed, as described previously (Mocan et al, 2016) with modification. ABTS•+ chromogenic radical reagent solution was prepared by reacting aqueous ABTS (7 mM) with K2S2O8 to produce a final concentration of 2.5 mM persulphate, then kept for 12–24 h in the dark at room temperature before use. Ethanol (50% v/v) was added to the blue–green solution at a 1:10 ratio, and the absorbance was 1.28 ± 0.04 at 595 nm. Various concentrations of the sample extract (2 μL) were added to 198 μL of final ABTS•+ solution, and the change in absorbance at 595 nm was recorded for 20 min.
2.9 Cupric ion-reducing antioxidant capacity (CUPRAC)
CUPRAC was evaluated by following the method of Uysal et al. (2017) with some modification, utilising copper (II)– neocuproine reagent as the chromogenic oxidising agent, and ascorbic acid as the standard. In this assay, 2 μL of extract was carefully mixed with 198 μL of working reagent of neocuproine (75 mM) and CuCl2 (10 mM), and the absorbance read at 450 nm after 20 min.
2.10 Ferric-reducing antioxidant power (FRAP)
The FRAP assay of Dezsi et al (2015) with modification was carried out on the sample extracts diluted appropriately with distilled water to provide an absorbance in the linear range. The FRAP reagent was freshly-prepared by mixing 300 mM acetate buffer (pH 3.6), 10 mM 2,4,6-tris(2-pyridyl)-s-triazine (TPTZ) in 40 mM HCl and 20 mM FeCl3·6H2O (10:1:1 v/v/v). The sample extracts (2 μL) at various concentrations were added to 198 μL of working FRAP reagent and incubated at 37 °C for 10 min. The absorbance was read at 520 nm, and the FRAP value was expressed as micromoles of Fe2+ equivalents per gram of extract or gram of seeds.
2.11 Cell culture and 3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyl tetrazolium bromide (MTT) assay
The human keratinocyte cell line (HaCaT) was prepared at 37 °C in an incubator with 5% CO2 humidified atmosphere. Cells were cultured in high-glucose-containing Dulbecco’s modified Eagle’s medium supplemented with penicillin–streptomycin mixture (100 U/mL) and 10% foetal bovine serum. HaCaT cells were seeded in 96-well plates (1 × 105 cell/mL), incubated for 24 h, and treated with extracts of the non-oil seed legumes at various concentrations, and gallic acid as a standard, respectively. MTT solution was added to each well after 1 h incubation, followed by dimethyl sulphoxide, and the absorbance read at 595 nm. The MTT colourimetric assay is described elsewhere (de Oliveira et al., 2014).
2.12 Detection of intracellular reactive oxygen species (ROS)
The fluorogenic substrate 2′7′-dichlorodihydrofluorescein diacetate (H2DCFDA) was used to detect intracellular ROS generated by UV-B (Bender et al., 2014) with modification. HaCaT cells were seeded in a 96-well plate at a density of 1 × 105 cell/mL and treated for 24 h with gallic acid and the extracts of non-oil seed legumes, respectively. Cells were exposed to UV-B (60 mJ/cm2) after incubation for 1 h at 37 °C in a 5% CO2 incubator. Afterwards, the cells were washed with PBS, then H2DCFDA solution was added. 2′7′-Dichlorofluorescein fluorescence was detected 10 min later, using a spectrofluorometer (PerkinElmer, Waltham, USA) at 485 nm excitation and 535 nm emission.
2.13 Statistical analysis
All data are represented as mean and standard deviation of three parallel measurements and were analysed by SPSS 10.07 (SPSS, Chicago, IL, USA). Differences between means were calculated by one-way analysis of variance and considered significant if p < 0.05. Antioxidant activity assays and HPLC separations were conducted at least in three replications. Pearson’s correlation coefficient (r) was determined to establish the relationship between the phenolic and flavonoids contents and the antioxidant activity.
3 Results
3.1 HPLC analysis of 70% ethanolic extracts
In this study, the HPLC phenolic profiles of the non-oil seed legume extracts (PLR, PLW and CES) were analysed by comparison of the retention times with those of known standard antioxidants. A representative chromatogram of these samples and standards is shown in Fig. 1D. Notably, the retention times of 5.536, 13.109, 15.276 and 18.822 min corresponded to the presence of gallic acid, catechin, epicatechin and coumaric acid, respectively (Fig. 1E-G). By employing the peak areas of notable concentrations of standards, the amounts of these polyphenols in each of the non-oil seed legume extracts were determined. As shown in Fig. 1E-G, peaks for gallic acid and epicatechin were identified in PLR extract, and coumaric acid in PLW extract, while CES extract contained epicatechin and coumaric acid.
3.2 Measurements of TPC and total flavonoid content
The TPC of the extracts (Fig. 2A) was based on the Folin–Ciocalteu method, using gallic acid as the standard. Among the aqueous and ethanolic (70% and 100%) extracts, the 70% ethanolic extract of CES at 100 μg/mL presented the highest TPC (38.60 mg GAE/g dry weight), and the lowest TPC was represented by the 70% ethanolic extract of PLW (15.21 mg GAE/g dry weight). The quantitative differences in TPC for CES (16.27–38.60 mg GAE/g dry weight), PLW (15.21–31.68 mg GAE/g dry weight) and PLR extract (15.33–23.16 mg GAE/g dry weight) were both dose- and solvent-dependent. The TPC values differed significantly according to the solvent and variety of non-oil seed legume (p < 0.05).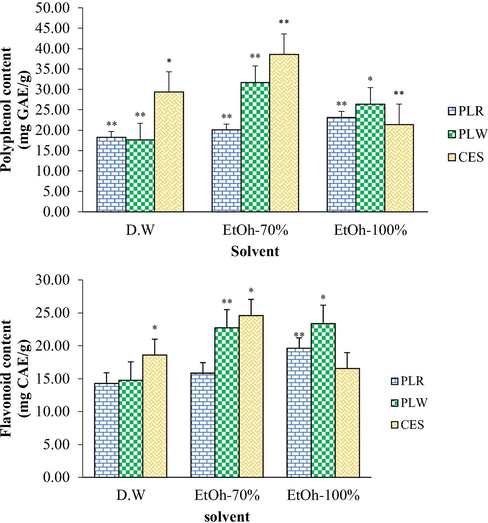
Total phenolic content (A) and flavonoids content (B) in extracts of Phaseolus lunatus red (PLR), Phaseolus lunatus white (PLW) and Canavalia ensiformis (CES) in different solvents (distilled water, D.W; ethanol 70%, EtOH-70%; ethanol 100%, EtOH-100%).
The flavonoid contents of the extracts were determined by the aluminium chloride colourimetric method and are described in Fig. 2B. The total flavonoid contents in the extracts of the non-oil seed legumes, PLR, PLW and CES, varied in the ranges 12.64–19.63, 11.73–23.39 and 12.72–24.61 mg CE/g dry weight, respectively. The lowest (11.73 mg CE/g dry weight) and highest flavonoid contents (24.61 mg CE/g dry weight) were detected in the extracts of PLW and CES, respectively. Similarly to the TPC, the flavonoid contents were significantly influenced by the solvent and variety of non-oil seed legume (p < 0.05). The TPC and flavonoid contents data promoted the analysis of the free radical-scavenging and reducing properties of the extracts.
3.3 Antioxidant assays
In the present study, the DPPH, ABTS, CUPRAC and FRAP antioxidant abilities of the non-oil seed extracts were explored. All four assays were simple, reproducible and inexpensive, accordingly employed together to estimate the antioxidant capacity in vitro.
The DPPH and ABTS antioxidant capacities of the extracts were fundamentally based on their ability to quench free radicals by donating a hydrogen atom (DPPH assay) or an electron (ABTS assay) (Re et al., 1999). For these assays, the antioxidant capacity is related to the degree of the decolourisation of the free radical (Prior et al., 2005), while the CUPRAC method is based on the principle of utilizing copper (II)–neocuproine reagent as the chromogenic oxidising agent (Apak et al., 2008). The non-oil seed extracts (PLR, PLW and CES) displayed electron-donating potential and reducing power capacity, and these effects were concentration- and solvent-dependent (Fig. 3A–D). Statistical assessments highlighted significant differences in the DPPH• and ABTS•+ antiradical activities among the non-oil seed varieties and solvent systems (p < 0.05). The 70% ethanolic extract of CES exhibited both the highest DPPH and ABTS antioxidant capacity, respectively (19.42% DPPH inhibition and 37.28% ABTS inhibition; Fig. 3A, B). Another mode of action of an antioxidant is its capability to engage in redox reactions. Based on the CUPRAC and FRAP value, PLR, PLW and CES all possessed a notable ability to act as reducing agents (Fig. 3C, D). The FRAP assay defines an antioxidant as any substance in the reaction medium that has reducing power (Benzie and Strain, 1996). Considering this procedure, the 70% ethanolic extracts of CES (100 μg/mL) had the highest reducing power, and the aqueous extract of PLW had the lowest FRAP (Fig. 3D).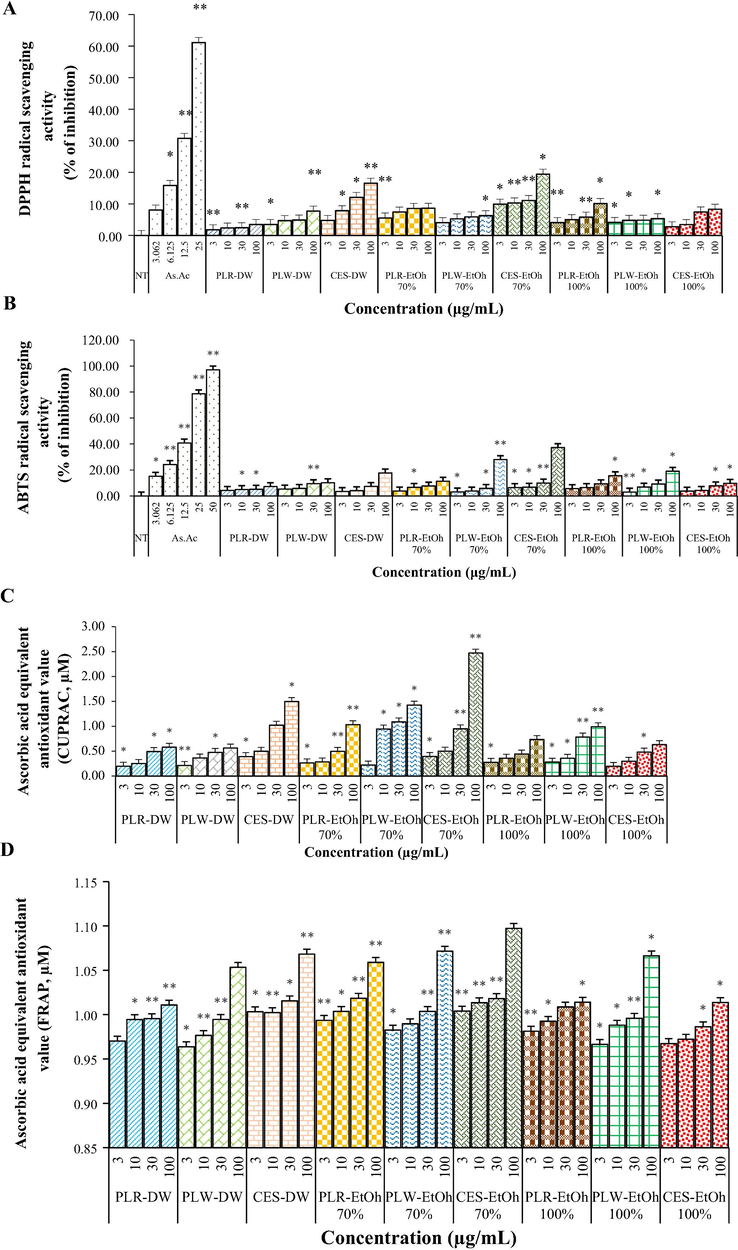
DPPH (A), ABTS (B), CUPRAC (C) and FRAP (D) antioxidant activity of Phaseolus lunatus red (PLR), Phaseolus lunatus white (PLW) and Canavalia ensiformis (CES) extracts in different solvents (distilled water, D.W; ethanol 70%, EtOH-70%; and ethanol 100%, EtOH-100%).
3.4 Pearson’s correlation analysis
Pearson’s correlation analysis was performed to assess the relationship between the antioxidant activities of the extracts and their TPC and flavonoid content (Lesaffre et al., 2009). Pearson’s correlation coefficients between these variables are provided in Table 1. Significant positive correlations were observed between the TPC and antioxidant activity of the aqueous, 70% ethanolic and 100% ethanolic extracts measured by the ABTS (r = 0.9013, 0.9327 and 0.9182), CUPRAC (r = 0.9424, 0.9485, and 0.8369) and FRAP assay (r = 0.7.861, 0.8531 and 0.8767), respectively. Moderate correlations were established between the DPPH antioxidant activity of the 70% ethanolic (r = 0.6608) and 100% ethanolic extracts (r = 0.6834) and their TPC when compared with the aqueous extract (r = 0.8740). In addition, inferior correlations were noticed between the DPPH• scavenging activity and the flavonoid contents of the 70% and 100% ethanolic extracts (r = 0.5110 and 0.4766, respectively). On the contrary, however, there were strong positive correlation between the flavonoid content of the aqueous, 70% ethanolic and 100% ethanolic extracts and the ABTS (r = 0.8554, 0.9413 and 0.9424), CUPRAC (r = 0.9731, 0.9144 and 0.9448), FRAP activities (r = 0.7568, 0.8685 and 0.8834), respectively.
In vitro assay
Flavonoid (R value)
Polyphenol (R value)
D.W
EtOH-70%
EtOH-100%
D.W
EtOH-70%
EtOH-100%
DPPH
0.9465*
0.5110
0.4766
0.8740*
0.6608*
0.6834*
ABTS
0.8554*
0.9413*
0.9424*
0.9013*
0.9327*
0.9182*
CUPRAC
0.9731*
0.9144*
0.9448*
0.9424*
0.9485*
0.8369*
FRAP
0.7568*
0.8685*
0.8834*
0.7861*
0.8531*
0.8767*
3.5 Cytotoxicity and decrease of ROS by the extracts
The MTT assay was carried out to explore the cytotoxic effects of extracts on UV-B-irradiated. Exposure to UV-B caused cell damage. HaCaT cells are well-established cell line used to explore the protective effect of extract compounds against oxidative stress-induced conditions. Here, the viability of HaCaT cells exposed to UV-B following pre-treatment with PLR, PLW and CES extracts, respectively, were analysed using the MTT assay. Pre-treatment with PLR, PLW and CES significantly protected HaCaT cells against cell death from oxidative stress induced by UV-B (60 mJ/cm2), in a dose-dependent manner (Fig. 4A). Antioxidants may inhibit cellular damage induced by oxidative stress immediately or progressively. As described in Fig. 4B, UV-B irradiation significantly enhanced ROS generation compared non-irradiated cells. Pre-treatment of PLR, PLW and CES extracts significantly lessened ROS generation compared with the UV-irradiated control.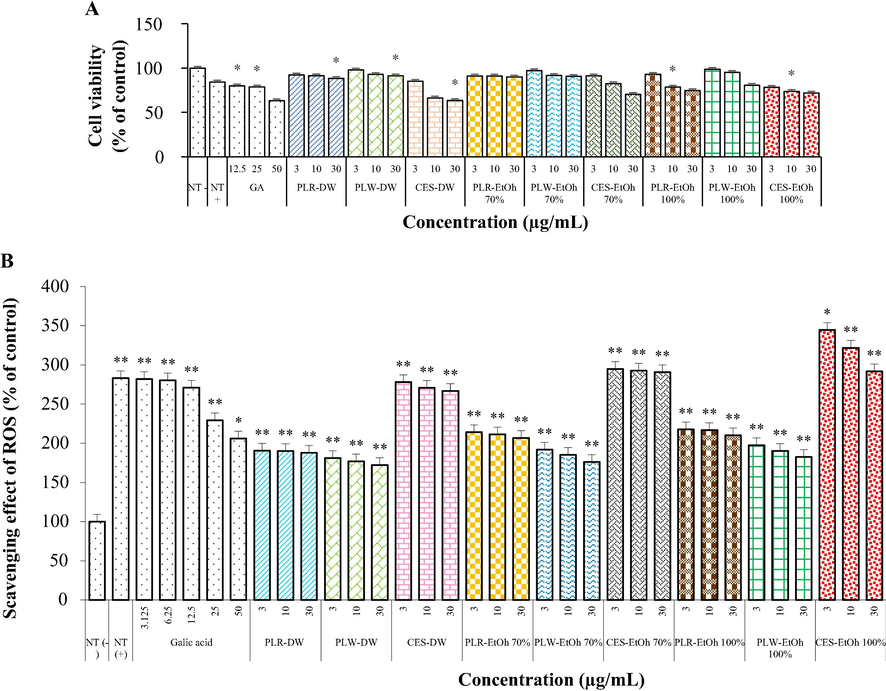
HaCat cell viability. HaCaT cells were seeded at a density of 1 × 105 cell/mL, and the MTT assay was performed (A). Phaseolus lunatus red (PLR), Phaseolus lunatus white (PLW) and Canavalia ensiformis (CES) extracts in different solvents (distilled water, D.W; ethanol 70%, EtOH-70%; 100% ethanol, EtOH-100%) were evaluated for their ability to scavenge intracellular ROS generated by UV-B 60 mJ/cm2, by the H₂DCF-DA assay (B).
4 Discussion
There are many varieties of non-oil seed legumes in Indonesia that can grow in high productivity even in a marginal area. Nonetheless, the non-oil seed legumes are currently underutilised. Here, three varieties of non-oil seed legumes, namely PLR, PLW and CES, were collected from a farm in Indonesia and studied for their polyphenolic contents and antioxidant properties. All three varieties of non-oil seed legumes are commonly consumed as food sources in the local region. The profile of the bean (i), powder (ii) and extracts (iii) of these varieties were shown in Fig. 1A–C.
The HPLC phenolic profiles of the 70% ethanolic extract varieties (Fig. 1E-G) were notable for the polyphenolics, gallic acid and epicatechin (PLR), coumaric acid (PLW) and epicatechin and coumaric acid (CES). Correspondingly, Luthria and Pastor-Corrales (2006) identified p-coumaric acid, ferulic acid and sinapic acid in the extracts of 15 varieties of P. vulgaris, mainly from the base-hydrolysed fraction, and the TPC was 19.1–48.3 mg/100 g of bean. These same three polyphenolics were also established in seven different P. vulgaris cultivars after alkaline hydrolysis of the extracts (Chen et al., 2015). Several researcher isolated and identified gallic acid as a major compound in the legume. Gallic acid and its derivatives have potent antioxidant capacity and are therefore thought to be largely responsible for the high antioxidant capacity of the red and black sword bean coats (Badhani et al., 2015; Gan et al., 2017).
Phenolic compounds are known as free radical scavengers and account for the majority of the antioxidant activity of a plant. The antioxidant mechanism of polyphenolic compounds is mostly derived from their metal ion-chelating and hydrogen-donating abilities (Jacobo-Velazquez and Cisneros-Zevallos, 2009). The TPC and flavonoid contents differed significantly (p < 0.05) among the extracts of the non-oil seed legume varieties (Fig. 2A, B). In the present study, the 70% ethanolic PLR, PLW and CES extracts presented the highest amount of phenolic compounds. According to the results, 70% ethanol was the most powerful solvent for phenolic extraction, and distilled water was the least potent. In a previous study, Abdille et al., (2005) reported the antioxidant activity of Dillenia indica fruit extracts decreased in the order of methanol extract > ethyl acetate extract > water extract, as analysed through various in vitro assays. Additionally, Wijekoon et al., (2011) notified that for phenolic extraction from Etlingera elatior Jack inflorescence, water was the least powerful solvent in comparison to methanol and acetone (50%, 90% and 100% v/v). Although there is no common appropriate solvent, 70% ethanol is usually preferred for extracting phenolics from plants (Prior et al., 2005).
In general, some preliminary comparative research of numerous legumes and bean varieties showed that the TPC of part bean have different value (Gan et al., 2017). It was further suggested that the solvent polarity and bean variety affected the extractability of polyphenols and flavonoids (Agostini-Costa et al., 2015; Orak et al., 2016). Some studies demonstrated that the bean cultivar was the main source of variance in the yield of tannins, and TPC and in vitro antioxidant activity (Gan et al., 2017).
The determination of free radical-scavenging activity of natural antioxidants by DPPH and ABTS analyses provides simplicity and high sensitivity. In this analysis, the DPPH radicals receive a hydrogen atom or an electron from an antioxidant donor (Moon and Shibamoto, 2009). The colour changes from purple to yellow upon the reduction of the DPPH• to the stable diamagnetic molecule. Our results showed that the PLR, PLW and CES extracts exhibited a dose-dependent scavenging activity (Fig. 3A). It means that the antioxidants of non-oil seed legumes extracts were competent to decolourise DPPH• and had free radical-scavenging potential. The phenolic compounds are indicated as the antioxidants of non-oil seed legumes with scavenging potential, due to their hydrogen-donating abilities. The free radical-scavenging potential was further verified by the ABTS method (Fig. 3B). In this present study, the CES 70% ethanolic extract had the highest antioxidant activity. Regarding the hydroalcoholic extracts, the most effective scavenger against ABTS•+ was obtained when 70% ethanol was the solvent. Prior et al. (2005) suggested that the sample’s antioxidant capacity correlated with the degree of cation radical decolourisation. Based on of these results, it should be noted that hydrophilic compounds are effective free radical scavengers. Moreover, like the literature studies discussed above, the antioxidant properties depended on the bean variety, the solvent and the concentration of the extract.
In the present study, it has been suggested that the type of antioxidant compound, antioxidant activity and capacity being dissolved in the solvent also varies with the change of solvent polarity, different concentration of solvent and solvent system. A solvent with low viscosity, low density and high diffusivity can easily diffuse into the pores of the plant materials to extract the plant components (Alothman et al., 2009).
The reducing ability of antioxidants is evaluated in the FRAP assay. In the presence of an antioxidant, the yellow Fe3+–TPTZ complex is reduced to the intensely blue Fe2+–TPTZ complex, in an acidic medium (Moon and Shibamoto, 2009). In this study, all non-oil seed legumes extract presented Fe3+-reducing power and Fe2+-chelating activity. These properties were higher for the 70% ethanolic than aqueous extracts (Fig. 3D). Although the FRAP antioxidant method is well-established, the CUPRAC method is a relatively new method expanded by Apak et al. (2008). This assay is based on the cupric ion-reducing capability in the presence of copper (II)–neocuproine. From the CUPRAC antioxidant activity results of the evaluated non-oil seed legume extracts, the cupric ion-reducing capability increased in a concentration-dependent manner and was in the order of 70% ethanol > distilled water > 100% ethanol (Fig. 3C), consistent with the trend in the free radical-scavenging activity (DPPH• and ABTS•+)
UV-B was used treat HaCaT cells to analyse oxidative damage. Several studies have verified the protective influences of phytochemicals against oxidative stress-induced cytotoxicity. Likewise, in our study, PLR, PLW and CES pre-treatment attenuated UV-B induced cell death and ROS formation in HaCaT cells (Fig. 4A, B). Flavonoids have the ability to inhibit ROS-producing enzymes, as well as the potential to immediately scavenge these ROS through the up-regulation of antioxidant enzymes (Alam et al., 2017). A healthy cell condition was maintained in the control samples, and cell viability decreased after UV-B exposure. The antioxidant pre-treatments significantly reduced cell death.
Pearson’s correlation coefficients were evaluated to indicate the extent to which the antioxidant activities displayed by the extracts was particularly induced by the phenolic compounds and associated with the great number of flavonoids present in the extracts. A remarkable correlation between the antioxidant activities and the phenolic compounds and flavonoid content in plants has been confirmed in another research (Chaudhari and Mahajan, 2015; Teixeira et al., 2017). Several studies have identified a direct and positive correlation between these variables (Marathe et al., 2011; Quiroga et al., 2013). Phenolic compounds other than flavonoids are likely to be the greatest contributors to the antioxidant capacity of the non-oil seed legume extracts studied in this research because the flavonoids content was inferiorly correlated with the antioxidant activity in comparison to the TPC. Generally, the extracts with the highest TPC showed the highest antioxidant activities.
In this study, we investigated the TPC, flavonoid content and antioxidant capacity of the extracts (aqueous, 70% ethanol, 100% ethanol) obtained from three non-oil seed legumes (PLR, PLW and CES). Generally, the ethanolic 70% extracts had the greater level of in vitro antioxidant capacity, TPC and flavonoid content. Collectively, the data indicated that the non-oil seed legumes could provide health advantages against oxidative stress-related chronic diseases and could be used as a functional food, nutraceutical ingredient and as cosmetic agents. Many chronic diseases derive from molecular inflammation in the tissues in the body. Identifying natural sources of antioxidants represents an important step in confronting various oxidative stress conditions. Further studies are required for the isolation and identification of the individual phenolic compounds in the studied legumes, and in vivo studies are required for supporting their mechanism of action as an antioxidant.
Author contributions
N.D.D. performed the experiments. N.D.D., M.B.A., and S.-H.L. designed the research and analyzed the data. N.D.D. and S.-H.L. wrote the paper. N.D.D., M.B.A. and S.-H.L. revised the paper.
Acknowledgements
This work was funded by Islamic Development Bank, Project Management Unit 4in1 Project D1.1/PR/4in1/X/2018, University of Jember, Indonesia, through a postgraduate fellowship award.
Declaration of Competing Interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
References
- Antioxidant activity of the extracts from Dillenia indica fruits. Food Chem.. 2005;90(40):891-896.
- [Google Scholar]
- Total phenolics, flavonoids, tannins and antioxidant activity of Lima beans conserved in a Brazilian Genebank. Ciencia Rural. 2015;45(2):335-341.
- [Google Scholar]
- DNA protecting activities of Nymphea nouchali (burm. F) flower extract attenuate t-bhp-induced oxidative stress cell death through nrf2-mediated induction of heme oxygenase-1 expression by activating map-kinases. Int. J. Mol. Sci.. 2017;18(2069):1-17.
- [Google Scholar]
- Antioxidant capacity and phenolic content of selected tropical fruits from Malaysia, extracted with different solvents. Food Chem.. 2009;115:785-788.
- [Google Scholar]
- Mechanism of antioxidant capacity assays and the CUPRAC (cupric ion reducing antioxidant capacity) assay. Microchim. Acta. 2008;160(4):413-419.
- [Google Scholar]
- Gallic acid: a versatile antioxidant with promising therapeutic and industrial applications. RSC Adv.. 2015;5:27540-27557.
- [Google Scholar]
- Antioxidant potential of aqueous plant extracts assessed by the cellular antioxidant activity assay. Am. J. Biol. Life Sci.. 2014;2(3):72-79.
- [Google Scholar]
- The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal. Biochem.. 1996;239:70-76.
- [Google Scholar]
- Analysis of bioactivities and chemical composition of Ziziphus joazeiro mart. Using HPLC-DAD. Food Chem.. 2015;186:185-191.
- [Google Scholar]
- Comparative antioxidant activity of twenty traditional Indian medical plants and its correlation with total flavonoid and phenolic content. Int. J. Pharmaceut. Sci. Rev. Res.. 2015;30(1):105-111.
- [Google Scholar]
- Characterization of free, conjugated and bound phenolics and lipophilic antioxidants in regular and non-darkening cranberry beans (Phaseolus vulgaris L.) Food Chem.. 2015;185:298-308.
- [Google Scholar]
- Effect of ethanol concentration, extraction time and extraction temperature on the recovery of phenolic compounds and antioxidant capacity of Centella asiatica extracts. Int. Food Res. J.. 2011;18:571-578.
- [Google Scholar]
- Optimization of extraction conditions of antioxidant phenolic compounds from mashua (Tropaeolum tuberosum Ruiz and Pavon) tubers. Sep. Purif. Technol.. 2007;55(2):217-225.
- [Google Scholar]
- Sulforaphane induces DNA damage and mitotic abnormalities in human osteosarcoma MG-63 cells: correlation with cell cycle arrest and apoptosis. Nutrition Cancer. 2014;66(2):325-334.
- [Google Scholar]
- Antimicrobial and antioxidant activities and phenolic profile of Eucalyptus globulus Labill. And Corymbia ficifolia (F. Muell.) K.D Hill & LAS Johnson leaves. Molecules. 2015;20:4720-4734.
- [Google Scholar]
- Chemical components with health implications in wild and cultivated Mexican common bean seeds (Phaseolus vulgaris L.) J. Agric. Food Chem.. 2006;54(6):2045-2052.
- [Google Scholar]
- Diversity of chemical content and biological activity in flower buds of a wide number of wild grown caper (Capparis ovate Desf.) genotypes from Turkey. C R Acad. Bulg. Sci.. 2014;67:1593-1600.
- [Google Scholar]
- Literature review of an underutilized legume: Canavalia gladiate L. Plant Foods Hum. Nutr.. 2000;55:305-321.
- [Google Scholar]
- Identification and validation of microsatellite markers in strawberry tree (Arbutus unedo L.) Turk. J. Agric. For.. 2019;43:430-436.
- [Google Scholar]
- Bioactive compounds and bioactives of germinated edible seeds and sprouts: an updated review. Trends Food Sci. Technol.. 2017;59:1-14.
- [Google Scholar]
- Evaluation of Abelmoschus moschatus extracts for antioxidant, free radical scavenging, antimicrobial and antiproliferative activities using in vitro assays. BMC Complement. Alternat. Med.. 2011;11(64):1-12.
- [Google Scholar]
- Nutritional and health perspectives of beans (Phaseolus vulgaris L.): an overview. Crit. Rev. Food Sci. Nutrit.. 2014;54(5):580-592.
- [Google Scholar]
- Correlations of antioxidant activity against phenolic content revisited: a new approach in data analysis for food and medicinal plants. J. Food Sci.. 2009;74(9):107-113.
- [Google Scholar]
- Protective effect of Canavalia gladiate on gastric inflammation induced by alcohol treatment in rats. J. Korean Soc. Food Sci. Nutrit.. 2013;42:690-696.
- [Google Scholar]
- Protective effect of Canavalia gladiate (sword bean) fruit extracts and its flavonoidal contents, against azathioprine-induced toxicity in hepatocytes of albino rats. Toxicol. Environ. Chem.. 2014;96:474-481.
- [Google Scholar]
- Statistical and Methodological Aspects of Oral Health Research. Cornwall: John Wiley & Sons Ltd; 2009.
- Phenolic acids content of fifteen dry edible bean (Phaseolus vulgaris L.) varieties. J. Food Composit. Anal.. 2006;19(2006):205-211.
- [Google Scholar]
- Comparative study on antioxidant activity of different varieties of commonly consumed legumes in India. Food Chem. Toxicol.. 2011;49:2005-2012.
- [Google Scholar]
- Determination of lignans and phenolic components of Schisandra chinensis (Turcz.) Baill. using HPLC-ELS-ToF-MS and HPLC-online TEAC: contribution of individual components to overall antioxidant activity and comparasion with traditional antioxidant assays. J. Funct. Foods. 2016;24:579-594.
- [Google Scholar]
- An assessment of the neutraceutical potential of Juglans regia L. leaf powder in diabetic rats. Food Chem. Toxicol.. 2017;107:554-564.
- [Google Scholar]
- Antioxidant assays for plants and food components. J. Agric. Food. Chem.. 2009;57:1655-1666.
- [Google Scholar]
- Phenolic composition and antioxidant and antiproliferative activities of the extracts of twelve common bean (Phaseolus vulgaris L.) endemic ecotypes of Southern Italy before and after cooking. Oxid. Med. Cell. Longevity. 2016;2016:1-12.
- [Google Scholar]
- Antioxidant potential and phenolic compounds of some widely consumed Turkish white bean (Phaseolus vulgaris L.) varieties. Polish J. Food Nutrit. Sci.. 2016;66(4):253-260.
- [Google Scholar]
- Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food. Chem.. 2005;53(10):4290-4302.
- [Google Scholar]
- Chemical composition, antioxidant activity and anti-lipase activity of Origanum vulgare and Lippia turbinate essential oils. Int. J. Food Sci. Technol.. 2013;48(3):642-649.
- [Google Scholar]
- Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biol. Med.. 1999;26:1231-1237.
- [Google Scholar]
- Chemical profiling, antioxidant, enzyme inhibitory and molecular modelling studies on the leaves and stem bark extracts of three African medicinal plants. J. Pharm. Biomed. Anal.. 2019;174:19-33.
- [Google Scholar]
- Cynarin-rich sunflower (Helianthus annus) sprouts possess both antiglycative and antioxidant activities. J. Agric. Food. Chem.. 2012;60:3260-3265.
- [Google Scholar]
- Antioxidant potential and its correlation with the contents of phenolic compounds and flavonoids of methanolic extracts from different medicinal plants. Revista Virtual de Quimica. 2017;9(4):1546-1559.
- [Google Scholar]
- Separation procedures for naturally occurring antioxidant phytochemicals. J. Chromatogr. B. 2004;812(1–2):85-99.
- [Google Scholar]
- Isoflavone profile diversity in Korean wild soybeans (Glycine soja Sieb. & Zucc.) Turk. J. Agric. For.. 2018;42:248-261.
- [Google Scholar]
- Cytotoxic and enzyme inhibitory potential of two Potentilla species (P. speciose L. and P. reptans Willd.) and their chemical composition. Front. Pharmacol.. 2017;8:290.
- [Google Scholar]
- Effect of extraction solvents on the phenolic compounds and antioxidant activities of bunga kantan (Etlingera elatior Jack.) inflorescence. J. Food Chomposit. Anal.. 2011;24:615-619.
- [Google Scholar]
- Optimizing soaking and germination conditions to improve gamma-aminobutyric acid content in japonica and indica germinated brown rice. J. Funct. Foods. 2014;10:283-291.
- [Google Scholar]
- Enzyme inhibitory properties, antioxidant activities, and phytochemical profile of three medical plants from Turkey. Adv. Pharmacol. Sci.. 2015;2015:1-8.
- [Google Scholar]







