Translate this page into:
Antitumor effect of guava leaves on lung cancer: A network pharmacology study
⁎Corresponding author at: Hechi University, No. 42 Longjiang Road, Hechi 546300, China. qinyuehechi@outlook.com (Yue Qin)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Guava is known for its hypoglycemic, antivirus, antibacterial, anti-inflammatory, antioxidant, and antitumor properties. In this study, triterpenoids, sesquiterpenes, and flavonoids were examined as potential targets of constituents of guava leaves. Our study was aimed to reveal the antitumor mechanism and construct the network pharmacology network of guava leaf constituents and lung cancer. The potential targets of guava leaf constituents were searched in target databases, while the disease genes were searched in the GeneCards database. The common targets of drugs and diseases were screened out. A network map was constructed by the Cytoscape software, and the GO and KEGG pathways were analyzed. The existing cases were studied by SystemsDock molecular docking and cBioPortal tumor database study. Among the 66 chemical constituents of guava leaves, 153 of their targets were the lung cancer genes involved in many signaling pathways, such as the PI3K-Akt signaling pathway, in small cell lung cancer and non-small cell lung cancer. There was a binding activity between ligand compounds and receptor proteins. Guava leaves inhibited tumor through a gene regulatory network, and may play an important role in gene-targeting therapy. Through network pharmacology, we found that guava leaves had potential targets that interacted with various tumors, regulating the signaling pathways of cancers. This study preliminarily verified the pharmacological basis and the mechanism of the antitumor effect of guava leaves, providing a foundation for further research.
Keywords
Guava leaf
Antitumor
Network pharmacology
SystemsDock molecular docking
1 Introduction
Guava (Psidium guajava), a member of the Myrtaceae family, is an evergreen shrub or small arbor with a wide range of habitats. Guava is found in countries in tropical or subtropical areas such as South America, Africa, and Southern Asia (Gutierrez et al., 2008; Feng et al., 2015). Guava leaves, also known as Folium Psidii Guajavae, are the dry leaves and leafy shoots of guava. The substances in guava leaves are triterpenoids (Shao et al., 2012a), flavonoids, tannins (Seo et al., 2014), sesquiterpenes, miscellaneous quinones, volatile oils, and benzophenone glycosides. Guava is known for its hypoglycemic, antivirus, antibacterial, anti-inflammatory, antioxidant, and antitumor properties (Seo et al., 2014). These substances in guava leaves are of great research value. In a previous study, researchers identified novel types of aldehyde terpenes with their spectral characteristics and summarized the chemical structures of 17 heterodialdehyde compounds (Ouyang et al., 2015). Moreover, Psiguadial C and Psiguadial D showed significant biological activities, including the inhibitions of protein tyrosine phosphatase 1B (PTP1B) and human hepatoma cells (HepG2) (Shao et al., 2012b). Triterpenoids are known to exert antitumor effects (Song and Zhu, 2011; Lu et al., 2016). Flavonoids are one of the main functional components in guava leaves and have various pharmacological effects (Alnaqeeb et al., 2019; Luo et al., 2019). Researchers extracted flavonoids from guava leaves and obtained approximately 9.89 mg/g of total flavonoids (Wang et al., 2016b). Flavonoids in plants are also known for their antitumor activity, which mainly involves regulation of immune function, repression of tumor cell adhesion and signal transmission, and inhibition of cellular proliferation and tumor angiogenesis (Kandaswami et al., 2005). In this study, triterpenoids, sesquiterpenes, and flavonoids were examined as potential targets of guava leaves.
Previous studies have investigated antitumor substances in guava leaves. It was found that guava leaf extracts exhibit potent antitumor activity (Ashraf et al., 2016)and play an inhibitory role in HeLa and Ec109 cells (Lee and Park, 2010).
Lung cancer has been the leading cause of cancer deaths among men since the early 1950s. A total of 1,824,701 lung cancer cases were estimated worldwide in 2012, accounting for nearly 32% for women and 68% for men (Rafiemanesh et al., 2016). In contrast, a total of 1,589,925 lung cancer deaths were estimated in 2012, of which 31% were women and 69% were men (Rafiemanesh et al., 2016). The number of new lung cancer cases has risen to 7,328,000, and 5,807,000 deaths occurred in China in 2013 (Chen et al., 2017). Lung cancer is the most common malignant tumor in China with high morbidity and mortality rates (Xing et al., 2019). Therefore, finding ways to treat lung cancer is of vital importance.
The concept of network pharmacology is based on multidisciplinary theories such as systems biology and multi-directional pharmacology (Boezio et al., 2017). Utilizing various techniques, such as omics, high-throughput screening, network visualization, and network analysis can help us better understand the molecular mechanism of diseases and the pharmacological mechanism of drugs from a multi-dimensional perspective (Wu and Wu, 2015; Danhof, 2016; Boezio et al., 2017). The method of network pharmacology and the databases available for research also tend to be diverse (Hu et al., 2014; Lee, 2015; Wang et al., 2019). It is straightforward in visual analysis of the results through target prediction, pharmacological mechanism research, active component research, and construction of network graphs (Boezio et al., 2017).
Herein, we aimed to construct the network pharmacology of constituents from guava leaves and investigate the potential of these constituents on lung cancer.
2 Materials and methods
2.1 Materials
The databases used in this study included TCMSP, PubChem, PharmMapper, STRING, UniProt, GeneCards, Venny 2.1.0, KOBAS 3.0, SystemsDock, DisGeNET, and CbioPortal. The software used included ChemDraw Office 2010 (PerkinElmer), Cytoscape 3.7.1 (Cytoscape), and FunRich 3.1. 3 (http://www.funrich.org/download).
2.2 Methods
2.2.1 Collection of active constituents and chemical structures
Ouyang et al. (2015) systematically sorted out the compounds found in guava leaves through literature research; thus, these two documents were used as standards. The 2D or 3D structure of the target compound was searched in the PubChem (Kim et al., 2016) database, but for some of the compounds that were not included in the database, the chemical structure was drawn using the ChemBioDraw Ultra 12.0 software (PerkinElmer).
2.2.2 Screening of potential targets and acquisition of disease genes
Screening of potential targets: the TCMSP (Ru et al., 2014) database was used for the screening of potential targets, using “Chemical name” as the key word and the English name of the target compound as the potential target. PharmMapper (Liu et al., 2010) was used to screen potential targets. The 2D or 3D structures of the compound were used as input to screen potential targets. All potential targets of guava leave constituents were converted to gene names using the STRING database and the UniProt database, and the species selected was Homo Sapiens.
2.2.3 Acquisition of disease genes
Through the human gene database GeneCards, using “Lung cancer” as a key word, disease target genes with greater correlation with lung cancer were extracted, and the first 500 genes (based on the descending order of the correlation score) were selected as disease genes.
The searched potential target genes were compared with the disease genes in the Venny database, and the common genes were selected to obtain potential targets for the treatment of lung cancer with guava leaf constituents.
2.2.4 Topological analysis of target protein networks and gene assignment
The STRING database was used for gene regulatory network construction based on potential targets of guava leaf constituents and lung cancer genes. The species was set to “Homo sapiens” and the minimum interaction threshold was set to 0.97. PPI network interaction maps of potential targets of guava leaf constituents derived from the database was downloaded. Network topology analysis was performed using the Cytoscape (Kohl et al., 2011) database. The gene type was assigned to the gene through the DisGeNET (Bauer-Mehren et al., 2010) database, and the protein/gene was sequentially input for retrieval of the related gene and the target type (protein class) information. Interactions between compounds and target proteins were analyzed by constructing a network map of “Guava leaf - constituent category - active constituent - gene”.
2.2.5 GO and KEGG enrichment analysis
GO analysis was performed using the ClueGO plug-in in the Cytoscape software, and the gene symbols of targets of guava leaf constituent for lung cancer were input into ClueGO for gene ontology (GO) enrichment analysis (Biology Process, Molecular Function and Cellular Component). The gene symbols of targets of guava leaf constituent for lung cancer were converted into Entrez ID by the Funrich software, and KEGG pathway enrichment analysis was performed using KOBAS 3.0.
2.2.6 Molecular docking
The 10 genes that were relatively strong in the network diagram of “Guava leaf - constituent category - active constituent - gene” were molecularly docked with five compounds by systemsDock (Hsin et al., 2013; Hsin et al., 2016).
2.2.7 cBioportal analysis
The gene expression of existing case samples in the database was analyzed by cBioportal. cBioportal was financially funded by the Memorial Sloan-Kettering Cancer Center. It mainly addresses a large number of data problems obtained from large sample tumor genomic studies so that the results can be easily explored and directly applied to oncology (Wu et al., 2019).
2.2.8 Gene expression analysis and pathway activity analysis
The mRNA expression and the pathway activity of genes of interest were analyzed in the GSCALite database (http://bioinfo.life.hust.edu.cn/web/GSCALite/) following the instructions provided in this platform.
3 Results
3.1 Collection of active ingredients and their chemical structures
The 2D or 3D structures of all compounds were searched in the PubChem database, and the files in the sdf format were saved. Compounds not included in the database were identified by chemical structure, which were then drawn using the ChemDraw Ultra 12.0 software, and the sdf file was saved (Fig. 1). The structures of the compounds were searched by PubChem, and their molecular formulas and accession numbers in the database (PubChem CID) were retrieved. The relevant information of the active constituents of Guava leaves is listed in Table 1. In total, 66 active components of guava leaves, including 17 triterpenoids such as ursane and oleanane pentacyclic triterpenes, 19 sesquiterpenoids, and 30 flavonoids.
The structural formula of six compounds, which were found but not included in the PubChem database. T2: 3β-O-trans-p-coumaroylmaslinicacid; T15: psidiumoic acid; F12: guavinoside C; F24: quercetin-3-O-(6″-feruloyl); S1: Diguajadial; S2: Guadial A.
Compound Name
Molecular type
Number
Molecular Formula
Molecular structure
PubChem CID
Pharmmapper JOB ID
2α-hydroxyoleanolic acid
Triterpenoid
T1
C30H48O4
15,560,128
190,417,034,302
3β-O-trans-p-coumaroylmaslinicacid
Triterpenoid
T2
C39H54O5
/
190,417,034,505
asiatic acid
Triterpenoid
T3
C30H48O5
119,034
190,417,034,630
corosolic acid
Triterpenoid
T4
C30H48O4
6,918,774
190,417,034,747
goreishic acid I
Triterpenoid
T5
C30H46O4
3,081,756
190,318,032,131
guajanoic acid
Triterpenoid
T6
C32H50O6
101,211,343
190,417,035,053
guavacoumaric acid
Triterpenoid
T7
C39H54O7
101,211,344
190,417,035,342
guavanoic acid
Triterpenoid
T8
C32H50O6
101,211,343
190,417,035,431
ilelatifol D
Triterpenoid
T9
C30H46O4
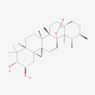
102,572,108
190,318,031,159
isoneriucoumaric acid
Triterpenoid
T10
C39H54O6
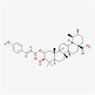
10,100,394
190,417,035,723
jacoumaric acid
Triterpenoid
T11
C39H54O6
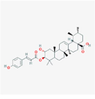
11,700,083
190,417,035,813
obtusinin
Triterpenoid
T12
C15H18O6
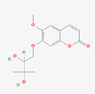
3,604,942
190,318,030,756
obtusol (3β, 27-dihydroxy-urs-12-ene)
Triterpenoid
T13
C30H50O2
15,895,316
190,417,040,026
oleanolic acid
Triterpenoid
T14
C30H48O3
10,494
190,417,040,126
psidiumoic acid
Triterpenoid
T15
C32H50O5
/
190,417,040,228
ursolic acid
Triterpenoid
T16
C30H48O3
64,945
190,417,040,330
uvoal
Triterpenoid
T17
C30H50O2
92,802
190,417,040,432
Apigenin
Flavonoid
F1
C15H10O5
5,280,443
190,414,093,403
Avicularin
Flavonoid
F2
C20H18O11
5,490,064
190,414,093,603
Biochanin
Flavonoid
F3
C16H12O5
5,280,373
190,403,023,437
Daidzein
Flavonoid
F4
C15H10O4
5,281,708
190,414,094,000
demthoxymatteucinol
Flavonoid
F5
C17H16O4
180,550
190,414,094,140
formononetin
Flavonoid
F6
C16H12O4
5,280,378
190,414,094,323
Genistein
Flavonoid
F7
C15H10O5
5,280,961
190,414,094,458
Genistin
Flavonoid
F8
C21H20O10

5,281,377
190,414,094,600
Glycitin/daidzin
Flavonoid
F9
C22H22O10
187,808
190,414,094,811
guaijaverin
Flavonoid
F10
C20H18O11
5,481,224
190,414,095,056
guavaric A
Flavonoid
F11
C32H50O6
101,211,343
190,414,095,209
guavinoside C
Flavonoid
F12
C27H22O15
/
190,414,095,324
hyperin
Flavonoid
F13
C21H20O12
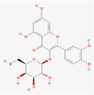
133,568,467
190,417,041,614
isoquercetin(Isoquercitrin)
Flavonoid
F14
C21H20O12
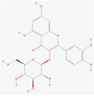
5,280,804
190,414,095,613
kaempferol
Flavonoid
F15
C15H10O6
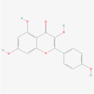
5,280,863
190,414,095,757
kaempferol-3-glucoside
Flavonoid
F16
C21H20O11
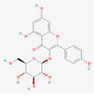
5,282,102
190,414,095,928
Leucocyanidin
Flavonoid
F17
C15H14O7
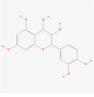
71,629
190,414,100,124
morin-3-O-α-L-lyxopyranoside
Flavonoid
F18
C20H18O11
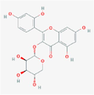
10,455,578
190,414,100,422
myricetin
Flavonoid
F19
C15H10O8
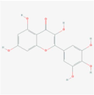
5,281,672
190,414,100,546
Ononin
Flavonoid
F20
C22H22O9
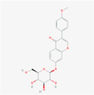
442,813
190,414,100,718
prunetin
Flavonoid
F21
C16H12O5
5,281,804
190,414,100,847
quercetin
Flavonoid
F22
C15H10O7
5,280,343
190,414,101,024
quercetin3-O-β-D-xylopyranoside
Flavonoid
F23
C20H18O11
5,320,861
190,414,101,158
quercetin-3-O-(6″-feruloyl) -β-D-galactopyranoside
Flavonoid
F24
C31H28O15
/
190,414,101,321
quercetin-3-O-gentiobioside
Flavonoid
F25
C27H30O17
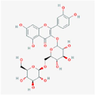
13,915,963
190,414,101,504
quercetin-3-O-β-D-glucuronide
Flavonoid
F26
C21H18O13
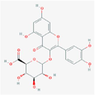
13,258,914
190,414,101,626
quercitrin
Flavonoid
F27
C21H20O11
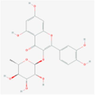
5,280,459
190,414,101,724
reynoutrin
Flavonoid
F28
C20H18O11
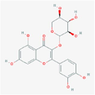
5,320,863
190,414,101,824
rutin
Flavonoid
F29
C27H30O16
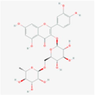
5,280,805
190,414,101,941
xanthone
Flavonoid
F30
C13H8O2
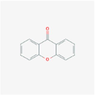
7020
190,414,102,035
Diguajadial
Sesquiterpenoids
S1
C60H66O9
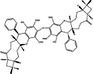
/
190,321,031,034
Guadial A
Sesquiterpenoids
S2
C25H26O5
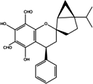
/
190,321,032,624
Guadial B
Sesquiterpenoids
S3
C25H26O5
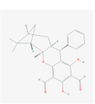
122,377,745
190,319,051,501
Guadial C
Sesquiterpenoids
S4
C25H26O5

122,377,746
190,319,051,610
Guajadial
Sesquiterpenoids
S5
C30H34O5
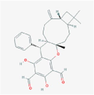
101,447,677
190,318,025,632
Guajadial B
Sesquiterpenoids
S6
C30H34O5
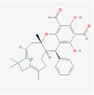
137,346,032
190,319,050,244
Guajadial C
Sesquiterpenoids
S7
C30H34O5
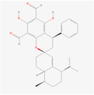
134,714,902
190,319,050,403
Guajadial D
Sesquiterpenoids
S8
C30H34O5
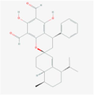
134,714,901
190,319,050,732
Guajadial E
Sesquiterpenoids
S9
C30H34O5
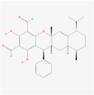
134,714,904
190,319,050,851
Guajadial F
Sesquiterpenoids
S10
C30H34O5
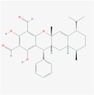
134,714,903
190,319,050,938
Guapsidial A
Sesquiterpenoids
S11
C29H32O5
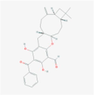
122,377,744
190,319,051,859
Guajadial
Sesquiterpenoids
S12
C30H34O5
46,197,930
190,320,031,551
Psidial A
Sesquiterpenoids
S13
C30H36O6
45,104,960
190,318,025,129
Psidial B
Sesquiterpenoids
S14
C30H36O6
45,104,961
190,318,025,249
Psidial C
Sesquiterpenoids
S15
C30H34O5
49,844,493
190,318,025,408
Psiguadial A
Sesquiterpenoids
S16
C30H34O5
49,844,493
190,318,025,837
Psiguadial B
Sesquiterpenoids
S17
C30H34O5
102,052,649
190,318,025,954
Psiguadial C
Sesquiterpenoids
S18
C30H34O6
122,224,646
190,319,051,306
Psiguadial D
Sesquiterpenoids
S19
C30H34O5
77,984,632
190,321,030,934
3.2 Screening of potential targets and acquisition of disease genes
A total of 115 potential targets for four triterpenoids and 303 potential targets for the 19 flavonoids were obtained from the TCMSP database (Table 2). Molecular information on guava leaves, including Lipinski's “five-law” parameters, namely relative molecular mass (MV), octanol–water partition coefficient (AlogP), possible hydrogen bond donor number (Hdon), possible hydrogen, number of bond receptors (Hacc), number of bonds allowed to rotate freely (RBN), oral bioavailability (OB), and drug-like degree (DL) were obtained. The targets of the active ingredients of guava leaves were obtained from the PharmMapper database, and targets with z'-score ≥ 1 were screened as potential targets of guava leaf active ingredients. From the definition in the database website, “Fit Score” and “z'-score” are scores generated by the metric's Fit score, which is a pre-calculated library score matrix, and a large positive z'-score represents the target-to-query combination. A total of 198 target genes for triterpenoids, 215 target genes for sesquiterpenoids, and 302 target genes for flavonoids were retrieved from the PharmMapper database. A total of 246 potential targets for triterpenoids, 215 potential targets for sesquiterpenoids, and 535 potential targets for flavonoids were obtained from TCMSP and Pharmmapper. The names of all potential targets were imported into the STRING database and Uniprot database, and converted into Gene Symbols.
Compound Name
Mol ID
MW
AlogP
Hdon
Hacc
RBN
OB (%)
DL
Apigenin
MOL000008
270.25
2.33
3
5
1
23.06
0.21
Avicularin
MOL007979
434.38
−0.08
7
11
4
2.06
0.7
Daidzein
MOL000390
254.25
2.33
2
4
1
19.44
0.19
formononetin
MOL000392
268.28
2.58
1
4
2
69.67
0.21
Genistein
MOL000481
270.25
2.07
3
5
1
17.93
0.21
Genistin
MOL000480
432.41
0.16
6
10
4
13.35
0.75
daidzin
MOL009720
416.41
0.43
5
9
4
14.32
0.73
guaijaverin
MOL000702
434.38
−0.08
7
11
3
29.65
0.7
hyperin
MOL004368
464.41
−0.59
8
12
4
6.94
0.77
isoquercetin
MOL000437
302.25
0.34
5
7
1
5.92
0.28
kaempferol
MOL000422
286.25
1.77
4
6
1
41.88
0.24
kaempferol-3-glucoside
MOL001415
448.41
−0.32
7
11
4
2.77
0.74
Leucocyanidin
MOL007214
306.29
1.09
6
7
1
37.61
0.27
myricetin
MOL002008
318.25
1.24
6
8
1
13.75
0.31
Ononin
MOL000391
430.44
0.68
4
9
5
11.52
0.78
prunetin
MOL000486
284.28
2.32
2
5
2
5.41
0.24
quercetin
MOL000098
302.25
1.5
5
7
1
46.43
0.28
quercetin-3-O-β-D-glucuronide
MOL001001
450.38
−0.42
8
12
3
30.66
0.74
quercitrin
MOL000701
448.41
0.3
7
11
3
4.04
0.74
rutin
MOL000415
610.57
−1.45
10
16
6
3.2
0.68
2α-hydroxyoleanolic acid
MOL012969
471.77
4.78
2
4
1
17.38
0.74
asiatic acid
MOL006861
488.78
4.41
4
5
2
16.69
0.72
oleanolic acid
MOL000263
456.78
6.42
2
3
1
29.02
0.76
ursolic acid
MOL000511
456.78
6.47
2
3
1
16.77
0.75
olmelin
MOL000510
284.28
2.32
2
5
2
25.21
0.24
In the Genecards database, with “Lung Cancer” as the key word, a total of 20,649 results were associated with lung cancer, and the top 500 targets with “Score” values ranged in descending order.
The genes associated with the three types of compounds were compared with the lung cancer genes in the Venny 2.1.0 database, and a chart of gene interaction was obtained (Fig. 2A). A total of 153 genes were obtained from lung cancer, and the target genes of guava leaf constituents against lung cancer were obtained. The total number of genes was 16.4%. Among them, there were 4 (0.4%) cross-reactive genes in three sputum and lung cancer; 68 (7.3%) cross-genes between flavonoids and lung cancer; 3 (0.3%) genes between triterpenoids, sesquiterpenes, and lung cancer; 9 (1%) cross-linking genes for scorpion, flavonoids, and lung cancer; 27 (2.9%) cross-linking genes for triterpenoids, flavonoids, and lung cancer; and 42 (4.5%) cross-linking genes for triterpenoids, sesquiterpenes, flavonoids, and lung cancer.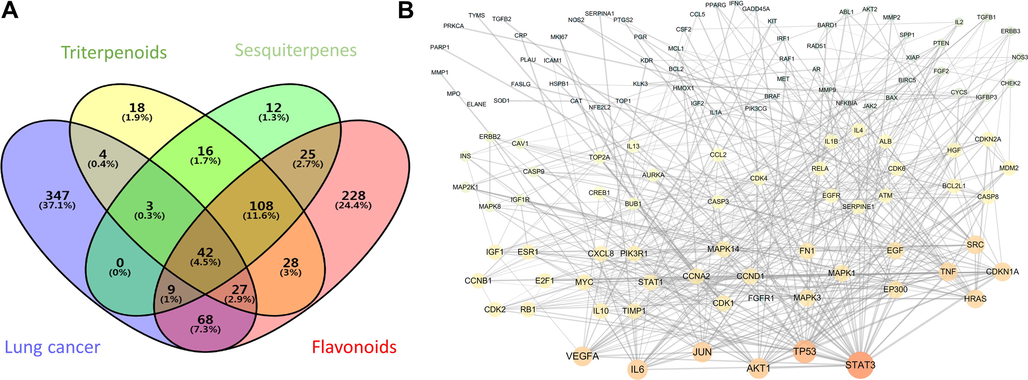
Drug-disease interaction network analysis (A) Screening of guava leaf-lung cancer common gene. (B) Topological analysis of drug-disease interactive gene network.
3.3 Topological analysis of target protein networks and gene assignment
The 153 targets related to lung cancer and identified as targets for guava leaf constituents were introduced into the STRING database to obtain a protein interaction network. Topological analysis of the target protein network was performed in Cytoscape. The topological analysis results are shown in Fig. 2B. The color depth and size of the nodes represent the strength of interaction between the gene and other genes. The interaction network had a total of 153 number of nodes. The number of edges associated with the target protein was 443, and the average node degree was 5.79, with p value (PPI enrichment p-value) < 1.0e−16. The target genes with a degree greater than or equal to 10 in the guava leaf constituent and lung cancer cross-linking network topology analysis were sequentially introduced into the DisGeNET database to obtain the protein type corresponding to the target. The results showed that the types of these proteins were nucleic acid binding, transcription factor, calcium-binding protein, kinase, transferase, signaling molecule, enzyme modulator, receptor, hydrolase, protease, and transfer/carrier protein (Table 3). The network topological analysis of genes associated with lung cancer in guava leaves genes with a score of 10 or higher and their genes are also shown in Table 3.
Gene
Full Name
Degree
Uniprot ID
Protein class
STAT3
signal transducer and activator of transcription 3
35
P40763
nucleic acid binding; transcription factor
TP53
tumor protein p53
27
P04637
transcription factor
AKT1
AKT serine/threonine kinase 1
22
P31749
calcium-binding protein; kinase; transferase; transfer/carrierprotein
IL6
interleukin 6
21
P05231
None
JUN
Transcription factor AP-1
21
P05412
nucleic acid binding; transcription factor
VEGFA
vascular endothelial growth factor A
20
P15692
signaling molecule
SRC
non-receptor tyrosine kinase
18
P12931
None
TNF
tumor necrosis factor
18
P01375
signaling molecule
HRAS
HRas proto-oncogene, GTPase
18
P01112
enzyme modulator
CDKN1A
cyclin dependent kinase inhibitor 1A
18
P38936
None
EGF
epidermal growth factor
15
P01133
None
EP300
E1A binding protein p300
15
Q09472
nucleic acid binding; transcription factor; transferase
FN1
fibronectin 1
14
P02751
signaling molecule
MAPK3
mitogen-activated protein kinase 3
14
P27361
kinase; transferase
MAPK1
mitogen-activated protein kinase 1
14
P28482
kinase; transferase
MAPK14
mitogen-activated protein kinase 14
13
Q16539
kinase; transferase
CCNA2
cyclin A2
13
P20248
enzyme modulator
CDK1
cyclin dependent kinase 1
13
P06493
kinase; transferase
CCND1
cyclin D1
13
P24385
enzyme modulator
PIK3R1
phosphoinositide-3-kinase regulatory subunit 1
12
P27986
enzyme modulator
CXCL8
C-X-C motif chemokine ligand 8
12
P10145
signaling molecule
MYC
MYC proto-oncogene
12
P01106
nucleic acid binding; transcription factor
IL10
interleukin 10
12
P22301
None
TIMP1
TIMP metallopeptidase inhibitor 1
12
P01033
enzyme modulator
STAT1
signal transducer and activator of transcription 1
12
P42224
nucleic acid binding; transcription factor
ESR1
estrogen receptor 1
11
P03372
nucleic acid binding; receptor; transcription factor
IGF1
insulin like growth factor 1
11
P05019
None
CCNB1
cyclin B1
11
P14635
enzyme modulator
CDK2
cyclin dependent kinase 2
11
P24941
kinase; transferase
RB1
RB transcriptional corepressor 1
11
P06400
nucleic acid binding; transcription factor
E2F1
E2F transcription factor 1
11
Q01094
nucleic acid binding; transcription factor
CDKN2A
cyclin dependent kinase inhibitor 2A
10
P42771
None
HGF
hepatocyte growth factor
10
P14210
hydrolase; protease
BCL2L1
BCL2 like 1
10
Q07817
signaling molecule
CASP8
caspase 8
10
Q14790
enzyme modulator; hydrolase; protease
MDM2
MDM2 proto-oncogene
10
Q00987
nucleic acid binding
3.4 Cytoscape network interaction analysis
There is a genetic interaction between drugs and diseases. This is a characteristic of multi-component and multi-target network pharmacology analysis. Through Cytoscape network visualization analysis, a “Guava leaf-compound class-active ingredient-gene” interaction network was obtained. The compound and target information on nodes ≥20 are listed in Table 4, where the Average Shortest Path Length is the average shortest path, Closeness Centrality is the center proximity, and Radiality is the radial degree.
Node Name
Degree
Average Shortest
Path LengthCloseness
CentralityRadiality
quercetin
85
1.96396396
0.50917431
0.80720721
genistein
62
2.15315315
0.46443515
0.76936937
apigenin
53
2.22522523
0.44939271
0.75495495
ursolic acid
43
2.31531532
0.43190661
0.73693694
daidzein
38
2.37837838
0.42045455
0.72432432
kaempferol
33
2.45045045
0.40808824
0.70990991
myricetin
30
2.45045045
0.40808824
0.70990991
rutin
22
2.6036036
0.38408304
0.67927928
formononetin
21
2.53153153
0.39501779
0.69369369
demthoxymatteucinol
21
2.51351351
0.39784946
0.6972973
Guadial B
20
2.56756757
0.38947368
0.68648649
VDR
36
2.44594595
0.40883978
0.71081081
CDK2
29
2.31981982
0.43106796
0.73603604
MAP2K1
27
2.6981982
0.3706177
0.66036036
CDK6
26
2.18468468
0.45773196
0.76306306
MET
25
2.90540541
0.34418605
0.61891892
ABL1
24
2.42792793
0.41187384
0.71441441
PPARG
22
2.28378378
0.43786982
0.74324324
IL2
22
2.37387387
0.42125237
0.72522523
EGFR
22
2.3018018
0.43444227
0.73963964
PGR
21
2.40990991
0.41495327
0.71801802
MAPK14
21
2.58108108
0.38743455
0.68378378
KIT
21
2.77927928
0.35980551
0.64414414
RARB
20
2.77927928
0.35980551
0.64414414
NOS2
20
2.53603604
0.39431616
0.69279279
As shown in Fig. 3 and Table 4, the three compounds in guava leaves that interacted strongly with lung cancer were quercetin, genistein, and apigenin. Moreover, the top ten targets were vitamin D3 receptor (VDR), cyclin-dependent kinase 2 (CDK2), dual specificity mitogen-activated protein kinase 1 (MAP2K1), cyclin-dependent kinase 6 (CDK6), hepatocyte growth factor receptor (MET), tyrosine-protein kinase ABL1 (ABL1), peroxisome proliferator-activated receptor gamma (PPARG), interleukin-2 (IL2), epidermal growth factor receptor (EGFR), and progesterone receptor (PGR).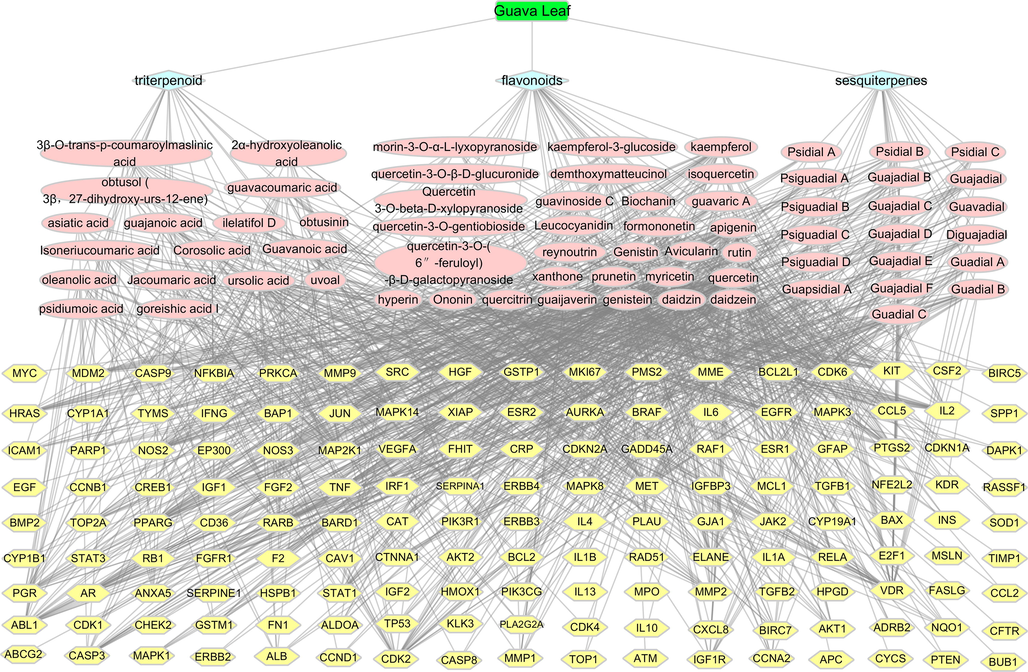
Interactive network of ‘guava leaf - compound class – active compound – gene’.
The network diagram of “Guava leaf-compound class-active constituents-gene” generated by Cytoscape is shown in Fig. 3, in which the green rectangle shape represents the traditional Chinese medicine guava leaf, and the three pale blue diamond shapes represent sesquiterpene, flavonoids, and triterpenoids; pink ovals represent compounds and yellow hexagons represent genes for the treatment of lung cancer with guava leaves. In the interaction network, the target information corresponding to the compound having a degree value of >20 and the corresponding gene are listed in Table 5.
Compound
Targets
Genes
apigenin
RAC-alpha serine/threonine-protein kinase
AKT1
apigenin
Adenomatous polyposis coli protein
APC
apigenin
Androgen receptor
AR
apigenin
Apoptosis regulator BAX
BAX
apigenin
Apoptosis regulator Bcl-2
BCL2
apigenin
Bcl-2-like protein 1
BCL2L1
apigenin
Caspase-3
CASP3
apigenin
Caspase-9
CASP9
apigenin
G2/mitotic-specific cyclin-B1
CCNB1
apigenin
G1/S-specific cyclin-D1
CCND1
apigenin
Cell division control protein 2 homolog
CDK1
apigenin
Cell division protein kinase 2
CDK2
apigenin
Cell division protein kinase 4
CDK4
apigenin
Cell division protein kinase 6
CDK6
apigenin
Cyclin-dependent kinase inhibitor 1
CDKN1A
apigenin
Cyclin-dependent kinase inhibitor 2
CDKN2A
apigenin
Cytochrome c
CYCS
apigenin
Cytochrome P450 19A1
CYP19A1
apigenin
Estrogen receptor
ESR1
apigenin
Estrogen receptor beta
ESR2
apigenin
Prothrombin
F2
apigenin
Basic fibroblast growth factor receptor 1
FGFR1
apigenin
Heme oxygenase 1
HMOX1
apigenin
Intercellular adhesion molecule 1
ICAM1
apigenin
Interferon gamma
IFNG
apigenin
Insulin-like growth factor 1 receptor
IGF1R
apigenin
Interleukin-13
IL13
apigenin
Interleukin-2
IL2
apigenin
Interleukin-4
IL4
apigenin
Insulin
INS
apigenin
Transcription factor AP-1
JUN
apigenin
Vascular endothelial growth factor receptor 2
KDR
apigenin
Induced myeloid leukemia cell differentiation protein Mcl-1
MCL1
apigenin
E3 ubiquitin-protein ligase Mdm2
MDM2
apigenin
Interstitial collagenase
MMP1
apigenin
Matrix metalloproteinase-9
MMP9
apigenin
NF-kappa-B inhibitor alpha
NFKBIA
apigenin
Nitric oxide synthase, endothelial
NOS3
apigenin
Phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit, gamma isoform
PIK3CG
apigenin
Phospholipase A2, membrane associated
PLA2G2A
apigenin
Urokinase-type plasminogen activator
PLAU
apigenin
Prostaglandin G/H synthase 2
PTGS2
apigenin
Retinoblastoma-associated protein
RB1
apigenin
Transcription factor p65
RELA
apigenin
Alpha-1-antitrypsin
SERPINE1
apigenin
Proto-oncogene tyrosine-protein kinase Src
SRC
apigenin
Tumor necrosis factor
TNF
apigenin
DNA topoisomerase II
TOP2A
apigenin
Cellular tumor antigen p53
TP53
apigenin
Vitamin D3 receptor
VDR
apigenin
Vascular endothelial growth factor A
VEGFA
apigenin
Baculoviral IAP repeat-containing protein 4
XIAP
daidzein
Beta-2 adrenergic receptor
ADRB2
daidzein
Ubiquitin carboxyl-terminal hydrolase BAP1
BAP1
daidzein
BRCA1-associated RING domain protein 1
BARD1
daidzein
Apoptosis regulator BAX
BAX
daidzein
Caspase-3
CASP3
daidzein
Catalase
CAT
daidzein
Caveolin-1
CAV1
daidzein
Cell division protein kinase 2
CDK2
daidzein
Cell division protein kinase 6
CDK6
daidzein
Cyclin-dependent kinase inhibitor 1
CDKN1A
daidzein
Histone acetyltransferase p300
EP300
daidzein
Estrogen receptor
ESR1
daidzein
Estrogen receptor beta
ESR2
daidzein
Prothrombin
F2
daidzein
Growth arrest and DNA damage-inducible protein GADD45 alpha
GADD45A
daidzein
Intercellular adhesion molecule 1
ICAM1
daidzein
Insulin-like growth factor 1 receptor
IGF1R
daidzein
Interleukin-4
IL4
daidzein
Interleukin-6
IL6
daidzein
Transcription factor AP-1
JUN
daidzein
Mitogen-activated protein kinase 14
MAPK14
daidzein
Mitogen-activated protein kinase 8
MAPK8
daidzein
Antigen KI-67
MKI67
daidzein
Neprilysin
MME
daidzein
Nitric oxide synthase, inducible
NOS2
daidzein
Nitric oxide synthase, endothelial
NOS3
daidzein
Progesterone receptor
PGR
daidzein
Phosphatidylinositol 3-kinase regulatory subunit alpha
PIK3R1
daidzein
Peroxisome proliferator activated receptor gamma
PPARG
daidzein
Peroxisome proliferator activated receptor gamma
PPARG
daidzein
Prostaglandin G/H synthase 2
PTGS2
daidzein
DNA repair protein RAD51 homolog 1
RAD51
daidzein
Transcription factor p65
RELA
daidzein
Signal transducer and activator of transcription 1-alpha/beta
STAT1
daidzein
Tumor necrosis factor
TNF
daidzein
Cellular tumor antigen p53
TP53
daidzein
Vascular endothelial growth factor A
VEGFA
demthoxymatteucinol
Proto-oncogene tyrosine-protein kinase ABL1
ABL1
demthoxymatteucinol
Serine/threonine-protein kinase 6
AURKA
demthoxymatteucinol
Bone morphogenetic protein 2
BMP2
demthoxymatteucinol
Cell division protein kinase 6
CDK6
demthoxymatteucinol
Catenin alpha-1
CTNNA1
demthoxymatteucinol
Leukocyte elastase
ELANE
demthoxymatteucinol
Basic fibroblast growth factor receptor 1
FGFR1
demthoxymatteucinol
Insulin-like growth factor 1 receptor
IGF1R
demthoxymatteucinol
Tyrosine-protein kinase JAK2
JAK2
demthoxymatteucinol
Vascular endothelial growth factor receptor 2
KDR
demthoxymatteucinol
Mast/stem cell growth factor receptor
KIT
demthoxymatteucinol
Dual specificity mitogen-activated protein kinase kinase 1
MAP2K1
demthoxymatteucinol
Nitric oxide synthase, endothelial
NOS3
demthoxymatteucinol
NAD(P)H dehydrogenase [quinone] 1
NQO1
demthoxymatteucinol
Progesterone receptor
PGR
demthoxymatteucinol
Phospholipase A2, membrane associated
PLA2G2A
demthoxymatteucinol
Urokinase-type plasminogen activator
PLAU
demthoxymatteucinol
Peroxisome proliferator activated receptor gamma
PPARG
demthoxymatteucinol
Thymidylate synthase
TYMS
demthoxymatteucinol
Vitamin D3 receptor
VDR
formononetin
Beta-2 adrenergic receptor
ADRB2
formononetin
Androgen receptor
AR
formononetin
C-C motif chemokine 5
CCL5
formononetin
Cyclin-A2
CCNA2
formononetin
Cell division protein kinase 2
CDK2
formononetin
Cell division protein kinase 6
CDK6
formononetin
Estrogen receptor
ESR1
formononetin
Estrogen receptor beta
ESR2
formononetin
Prothrombin
F2
formononetin
Interleukin-4
IL4
formononetin
Transcription factor AP-1
JUN
formononetin
Mitogen-activated protein kinase 14
MAPK14
formononetin
Mitogen-activated protein kinase 8
MAPK8
formononetin
Neprilysin
MME
formononetin
Nitric oxide synthase, inducible
NOS2
formononetin
Nitric oxide synthase, endothelial
NOS3
formononetin
Progesterone receptor
PGR
formononetin
Peroxisome proliferator activated receptor gamma
PPARG
formononetin
Peroxisome proliferator activated receptor gamma
PPARG
formononetin
Prostaglandin G/H synthase 2
PTGS2
genistein
RAC-alpha serine/threonine-protein kinase
AKT1
genistein
Androgen receptor
AR
genistein
Serine-protein kinase ATM
ATM
genistein
Apoptosis regulator BAX
BAX
genistein
Apoptosis regulator Bcl-2
BCL2
genistein
Baculoviral IAP repeat-containing protein 5
BIRC5
genistein
Baculoviral IAP repeat-containing protein 7
BIRC7
genistein
Mitotic checkpoint serine/threonine-protein kinase BUB1
BUB1
genistein
Caspase-3
CASP3
genistein
Caspase-9
CASP9
genistein
C-C motif chemokine 2
CCL2
genistein
Cyclin-A2
CCNA2
genistein
G2/mitotic-specific cyclin-B1
CCNB1
genistein
Cell division control protein 2 homolog
CDK1
genistein
Cell division protein kinase 2
CDK2
genistein
Cyclin-dependent kinase inhibitor 1
CDKN1A
genistein
Cyclin-dependent kinase inhibitor 2
CDKN2A
genistein
Cystic fibrosis transmembrane conductance regulator
CFTR
genistein
Serine/threonine-protein kinase Chk2
CHEK2
genistein
Interleukin-8
CXCL8
genistein
Epidermal growth factor receptor
EGFR
genistein
Leukocyte elastase
ELANE
genistein
Receptor tyrosine-protein kinase erbB-2
ERBB2
genistein
Estrogen receptor
ESR1
genistein
Estrogen receptor beta
ESR2
genistein
Prothrombin
F2
genistein
Basic fibroblast growth factor receptor 1
FGFR1
genistein
Fibronectin
FN1
genistein
Glial fibrillary acidic protein
GFAP
genistein
15-hydroxyprostaglandin dehydrogenase [NAD + ]
HPGD
genistein
Intercellular adhesion molecule 1
ICAM1
genistein
Insulin-like growth factor 1 receptor
IGF1R
genistein
Interleukin-1 beta
IL1B
genistein
Insulin
INS
genistein
Transcription factor AP-1
JUN
genistein
Prostate-specific antigen
KLK3
genistein
Mitogen-activated protein kinase 1
MAPK1
genistein
Mitogen-activated protein kinase 14
MAPK14
genistein
Mitogen-activated protein kinase 3
MAPK3
genistein
E3 ubiquitin-protein ligase Mdm2
MDM2
genistein
Neprilysin
MME
genistein
Matrix metalloproteinase-9
MMP9
genistein
Mesothelin
MSLN
genistein
Nitric oxide synthase, inducible
NOS2
genistein
Nitric oxide synthase, endothelial
NOS3
genistein
Progesterone receptor
PGR
genistein
Phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit, gamma isoform
PIK3CG
genistein
Urokinase-type plasminogen activator
PLAU
genistein
Peroxisome proliferator activated receptor gamma
PPARG
genistein
Peroxisome proliferator activated receptor gamma
PPARG
genistein
Phosphatidylinositol-3,4,5-trisphosphate 3-phosphatase and dual-specificity protein phosphatase PTEN
PTEN
genistein
Prostaglandin G/H synthase 2
PTGS2
genistein
Transcription factor p65
RELA
genistein
Signal transducer and activator of transcription 1-alpha/beta
STAT1
genistein
Signal transducer and activator of transcription 3
STAT3
genistein
Transforming growth factor beta-1
TGFB1
genistein
Transforming growth factor beta-2
TGFB2
genistein
Metalloproteinase inhibitor 1
TIMP1
genistein
Tumor necrosis factor
TNF
genistein
Cellular tumor antigen p53
TP53
genistein
Vascular endothelial growth factor A
VEGFA
3.5 GO and KEGG enrichment analysis
Through ClueGO analysis, a total of 1430 GO biological processes were obtained, with 7536 interactions between the biological processes; 35 molecular functions, with 28 interactions between the molecular functions; and 203 GO cell components, with 470 interactions between the cell components. KEGG analysis was performed by KOBAS 3.0, and 217 pathway enrichment results were obtained. GO analysis results of the interacting genes between guava leaves and lung cancer are shown in Table 6.
GO ID
GO Term
Percentage of genes
P-Value
Corr P-Value
GO:0006915
apoptotic process(BP)
112/2458 (4.56%)
9.13E−64
1.18E−60
GO:0012501
programmed cell death (BP)
114/2605(4.38%)
1.59E−63
2.05E−60
GO:0043067
regulation of programmed cell death (BP)
103/1991(5.17%)
4.85E−62
6.24E−59
GO:0010941
regulation of cell death(BP)
105/2153(4.88%)
4.24E−61
5.45E−58
GO:0042981
regulation of apoptotic process(BP)
101/1971(5.12%)
5.21E−60
6.69E−57
GO:0042127
regulation of cell population proliferation(BP)
100/2092(4.78%)
2.59E−56
3.32E−53
GO:1901700
response to oxygen-containing compound(BP)
98/2088(4.69%)
4.63E−54
5.94E−51
GO:0051247
positive regulation of protein metabolic process(BP)
97/2115(4.59%)
2.12E−52
2.72E−49
GO:0043069
negative regulation of programmed cell death(BP)
78/1182(6.60%)
2.21E−51
2.83E−48
GO:0060548
negative regulation of cell death(BP)
78/1299(6.16%)
1.23E−50
1.58E−47
GO:0031093
platelet alpha granule lumen(CC)
15/88(17.05%)
3.46E−16
1.14E−14
GO:0031091
platelet alpha granule(CC)
16/118(13.56%)
1.51E−15
4.83E−14
GO:0031983
vesicle lumen(CC)
24/470(5.11%)
4.76E−13
1.48E−11
GO:0060205
cytoplasmic vesicle lumen(CC)
23/469(4.90%)
3.51E−12
1.05E−10
GO:0034774
secretory granule lumen(CC)
22/448(4.91%)
1.05E−11
3.06E−10
GO:0000307
cyclin-dependent protein kinase holoenzyme complex(CC)
10/57(17.54%)
2.54E−11
7.12E−10
GO:0045121
membrane raft(CC)
21/441(4.76%)
5.74E−11
1.55E−09
GO:0098857
membrane microdomain(CC)
21/442(4.75%)
5.98E−11
1.56E−09
GO:0098589
membrane region(CC)
21/457(4.60%)
1.11E−10
2.77E−09
GO:1902911
protein kinase complex(CC)
11/137(8.03%)
1.36E−08
3.26E−07
GO:0004672
protein kinase activity(MF)
72/1534(4.69%)
1.14E−36
2.51E−34
GO:0043085
positive regulation of catalytic activity(MF)
76/1809(4.20%)
9.45E−36
2.07E−33
GO:0016773
phosphotransferase activity, alcohol group as acceptor(MF)
73/1661(4.39%)
2.32E−35
5.06E−33
GO:0016301
kinase activity(MF)
75/1798(4.17%)
5.68E−35
1.23E−32
GO:0004674
protein serine/threonine kinase activity(MF)
60/1094(5.48%)
1.79E−33
3.87E−31
GO:0051338
regulation of transferase activity(MF)
64/1298(4.93%)
3.03E−33
6.51E−31
GO:0043549
regulation of kinase activity(MF)
61/1153(5.29%)
3.28E−33
7.01E−31
GO:0071900
regulation of protein serine/threonine kinase activity(MF)
50/708(7.06%)
1.36E−32
2.89E−30
GO:0045859
regulation of protein kinase activity(MF)
58/1067(5.44%)
4.95E−32
1.05E−29
GO:0033674
positive regulation of kinase activity(MF)
50/7755(6.45%)
9.88E−31
2.08E−28
The top 10 enriched biological processes are shown in Fig. 4A. The enrichment results included apoptosis, programmed cell death, regulation of programmed cell death, regulation of cell death, regulation of apoptotic process, regulation of cell population proliferation, response to oxygen-containing compound, negative regulation of programmed cell death, and negative regulation of cell death. The top 10 enriched cellular components are shown in Fig. 4B. The enrichment results included platelet alpha granule lumen, platelet alpha granules, vesicle lumen, cytoplasmic vesicle lumen, secretory granule lumen, cyclin-dependent protein kinase holoenzyme complex, membrane raft, membrane microdomain, membrane region, and protein kinase complex. The top 10 enriched molecular function terms are shown in Fig. 4C. The enrichment results included protein kinase activity, positive regulation of catalytic activity, phosphotransferase activity, alcohol group as acceptor, phosphokinase activity, kinase activity, protein serine/threonine kinase activity, regulation of transferase activity, regulation of kinase activity, regulation of protein serine/threonine kinase activity, regulation of protein kinase activity, and positive regulation of kinase activity.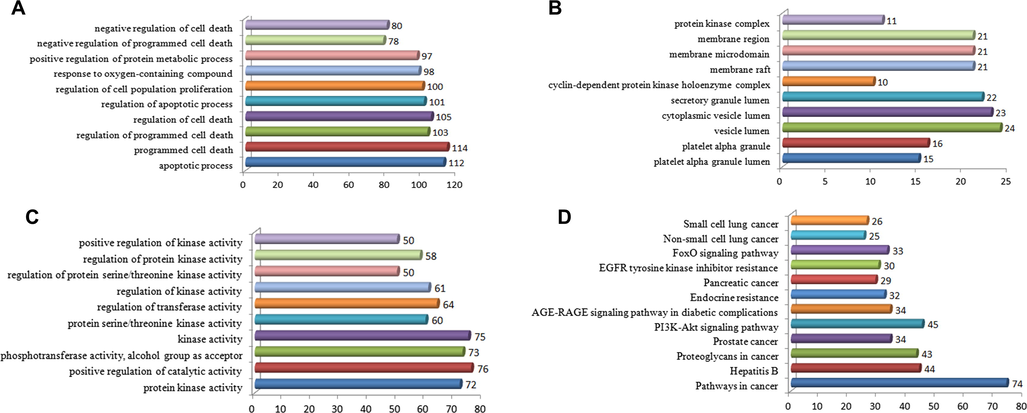
GO and KEGG analysis of ‘guava leaf – lung cancer’ interactive gene with P-values from small to large. (A) GO biological process analysis of ‘guava leaf – lung cancer’ interactive gene with P-values from small to large. (B) GO cell components analysis of ‘guava leaf – lung cancer’ interactive gene with P-values from small to large. (C) GO molecular function analysis of ‘guava leaf – lung cancer’ interactive gene with P-values from small to large. (D) KEGG analysis of ‘guava leaf – lung cancer’ cross-gene.
KEGG enrichment analysis results of common genes between guava leaf constituent target and lung cancer related genes are shown in Fig. 4D, Table 7 and Table 8. The top 10 common enriched pathways are listed below: cancer pathway, hepatitis B, proteoglycans in cancer, prostate cancer, PI3K-Akt signaling pathway, AGE-RAGE signaling pathway in diabetic complications, endocrine resistance, pancreatic cancer, EGFR tyrosine kinase inhibitor resistance, and FoxO signaling pathway. The non-small cell lung cancer and small cell lung cancer pathways were also recorded.
KEGG ID
GO Term
Percentage of genes
P-Value
Corr P-Value
hsa05200
Pathways in cancer
74/397(18.64%)
1.63E−101
3.53E−99
hsa05161
Hepatitis B
44/146(30.14%)
1.06E−66
1.15E−64
hsa05205
Proteoglycans in cancer
43/205(20.98%)
3.33E−59
2.41E−57
hsa05215
Prostate cancer
34/89(38.20%)
2.56E−54
1.39E−52
hsa04151
PI3K-Akt signaling pathway
45/342(13.16%)
9.08E−54
3.94E−52
hsa04933
AGE-RAGE signaling pathway in diabetic complications
34/101(33.66%)
9.60E−53
3.47E−51
hsa01522
Endocrine resistance
32/97 (32.99%)
2.15E−49
6.68E−48
hsa05212
Pancreatic cancer
29/66(43.94%)
8.75E−48
2.37E−46
hsa01521
EGFR tyrosine kinase inhibitor resistance
30/81(37.04%)
1.39E−47
3.35E−46
hsa04068
FoxO signaling pathway
33/134(24.63%)
2.60E−47
5.64E−46
hsa05223
Non-small cell lung cancer
25/56(44.64%)
2.17E−41
3.63E−40
hsa05222
Small cell lung cancer
26/86(30.23%)
2.45E−39
2.95E−38
KEGG ID
Enriched genes in the KEGG pathway
hsa05200
CCND1,BCL2,BCL2L1,PIK3CG,TGFB2,XIAP,KIT,BIRC5,MAP2K1,PTGS2,NOS2,JUN,RAD51,ABL1,PTEN,CASP3,TP53,GSTP1,CXCL8,DAPK1,IGF1,RB1,EP300,CDK4,BMP2,AKT2,CDK6,FGFR1,FGF2,TGFB1,HGF,MMP1,MMP2,RASSF1,APC,AKT1,BAX,MMP9,IGF1R,CYCS,BIRC7,BRAF,MDM2,EGFR,RAF1,EGF,MYC,E2F1,PPARG,MET,STAT3,NFKBIA,FN1,CDKN1A,MAPK3,MAPK1,CASP8,PRKCA,MAPK8,ERBB2,IL6,CDKN2A,STAT1,FASLG,KLK3,CDK2,VEGFA,AR,RARB,HRAS,RELA,PIK3R1,CASP9,CTNNA1
hsa05161
CCND1,BCL2,PIK3CG,TGFB2,BIRC5,MAP2K1,JUN,TNF,PTEN,CASP3,TP53,CXCL8,RB1,EP300,CDK4,CDK2,CCNA2,PIK3R1,AKT1,BAX,MMP9,AKT2,CYCS,RAF1,TGFB1,MYC,E2F1,CDK6,SRC,STAT3,NFKBIA,CASP9,CDKN1A,MAPK3,MAPK1,CASP8,PRKCA,MAPK8,IL6,STAT1,FASLG,HRAS,RELA,CREB1
hsa05205
CCND1,PIK3CG,MYC,MAP2K1,ESR1,TNF,CAV1,CASP3,TP53,KDR,IGF1,MMP9,AKT2,FGFR1,FGF2,HGF,MMP2,AKT1,IGF2,IGF2,IGF1R,MDM2,BRAF,EGFR,RAF1,TGFB1,TGFB2,MAPK14,SRC,MET,PLAU,FN1,CDKN1A,MAPK3,MAPK1,PRKCA,STAT3,ERBB2,FASLG,VEGFA,HRAS,PIK3R1,ERBB3,ERBB4
hsa05215
CDK2,CDKN1A,CREB1,E2F1,EGF,EGFR,EP300,ERBB2,AKT1,AKT2,FGFR1,GSTP1,HRAS,IGF1,IGF1R,KLK3,INS,AR,MDM2,NFKBIA,PIK3CG,PIK3R1,MAPK1,MAPK3,MAP2K1,PTEN,RAF1,RB1,CCND1,BCL2,RELA,BRAF,TP53,CASP9
hsa04151
CDK2,CDK4,CDK6,CDKN1A,CREB1,EGF,EGFR,AKT1,AKT2,FGF2,FGFR1,FN1,HGF,HRAS,IGF1,IGF1R,IL2,FASLG,IL4,IL6,INS,JAK2,KDR,KIT,MCL1,MDM2,MET,MYC,NOS3,PIK3CG,PIK3R1,PRKCA,MAPK1,MAPK3,MAP2K1,PTEN,RAF1,CCND1,BCL2,RELA,BCL2L1,SPP1,TP53,VEGFA,CASP9
hsa04933
CDK4,MAPK14,AKT1,AKT2,FN1,HRAS,ICAM1,IL1A,IL1B,IL6,CXCL8,JAK2,JUN,MMP2,NOS3,SERPINE1,PIK3CG,PIK3R1,PRKCA,MAPK1,MAPK3,MAPK8,BAX,CCND1,BCL2,RELA,CCL2,STAT1,STAT3,TGFB1,TGFB2,TNF,VEGFA,CASP3
hsa01522
CDK4,CDKN1A,CDKN2A,MAPK14,E2F1,EGFR,ERBB2,AKT1,AKT2,ESR1,ESR2,HRAS,IGF1,IGF1R,JUN,MDM2,MMP2,MMP9,PIK3CG,PIK3R1,MAPK1,MAPK3,MAPK8,MAP2K1,BAX,RAF1,RB1,CCND1,BCL2,SRC,BRAF,TP53
hsa05212
CDK4,CDK6,CDKN2A,E2F1,EGF,EGFR,ERBB2,AKT1,AKT2,PIK3CG,PIK3R1,MAPK1,MAPK3,MAPK8,MAP2K1,RAD51,RAF1,RB1,CCND1,RELA,BCL2L1,BRAF,STAT1,STAT3,TGFB1,TGFB2,TP53,VEGFA,CASP9
hsa01521
EGF,EGFR,ERBB2,ERBB3,AKT1,AKT2,FGF2,HGF,HRAS,IGF1,IGF1R,IL6,JAK2,KDR,MET,PIK3CG,PIK3R1,PRKCA,MAPK1,MAPK3,MAP2K1,PTEN,BAX,RAF1,BCL2,BCL2L1,SRC,BRAF,STAT3,VEGFA
hsa04068
CDK2,CDKN1A,MAPK14,GADD45A,EGF,EGFR,EP300,AKT1,AKT2,HRAS,IGF1,IGF1R,FASLG,IL6,IL10,INS,MDM2,ATM,PIK3CG,PIK3R1,MAPK1,MAPK3,MAPK8,MAP2K1,PTEN,RAF1,CCND1,BRAF,STAT3,TGFB1,TGFB2,CAT,CCNB1
hsa05223
CDK4,CDK6,CDKN2A,RASSF1,E2F1,EGF,EGFR,ERBB2,AKT1,AKT2,FHIT,HRAS,PIK3CG,PIK3R1,PRKCA,MAPK1,MAPK3,MAP2K1,RAF1,RARB,RB1,CCND1,BRAF,TP53,CASP9
hsa05222
CDK2,CDK4,CDK6,E2F1,AKT1,AKT2,FHIT,FN1,XIAP,MYC,NFKBIA,NOS2,PIK3CG,PIK3R1,CYCS,PTEN,PTGS2,RARB,RB1,CCND1,BCL2,RELA,BCL2L1,TP53,BIRC7,CASP9
One of these pathways is the PI3K-Akt signaling pathway, which has been shown to play an important regulatory role in tumor therapy. The PI3K-Akt signaling pathway is an important pathway for cell survival, metabolism, angiogenesis, apoptosis, proliferation and differentiation. The substrate is used to control key cellular processes, and many targets are involved in this pathway and AKT also plays an important role in the regulation of this pathway (Ebrahimi et al., 2017). In order to investigate the effectiveness of guava leaf constituents on lung cancer via the PI3K-Akt signaling pathway, we analyzed the expression (Fig. 5A) and the pathway activities (Fig. 5) of these genes in lung squamous cell carcinoma (LUSC) and lung adenocarcinoma (LUAD) samples from TCGA database in the GSCAlite online platform. The results indicated that the PI3K-Akt signaling pathway genes that could be potentially affected by the guava leaf constituents showed differential expression with some of them being downregulated (Fig. 5A, blue color dot) or upregulated (Fig. 5A, red color dot) in both LUSC and LUAD. In addition, we found that some of the target genes of the guava leaf constituents did not show differential expression in lung cancer (Fig. 5A). Furthermore, in pathway activity analysis, we found that these target genes were involved in the inhibition or activation of important pathways such as PI3K-Akt, apoptosis, cell cycle, EMT, DNA damage response, hormone AR, TSC/mTOR, RAS/MAPK, RTK and hormone ER pathways.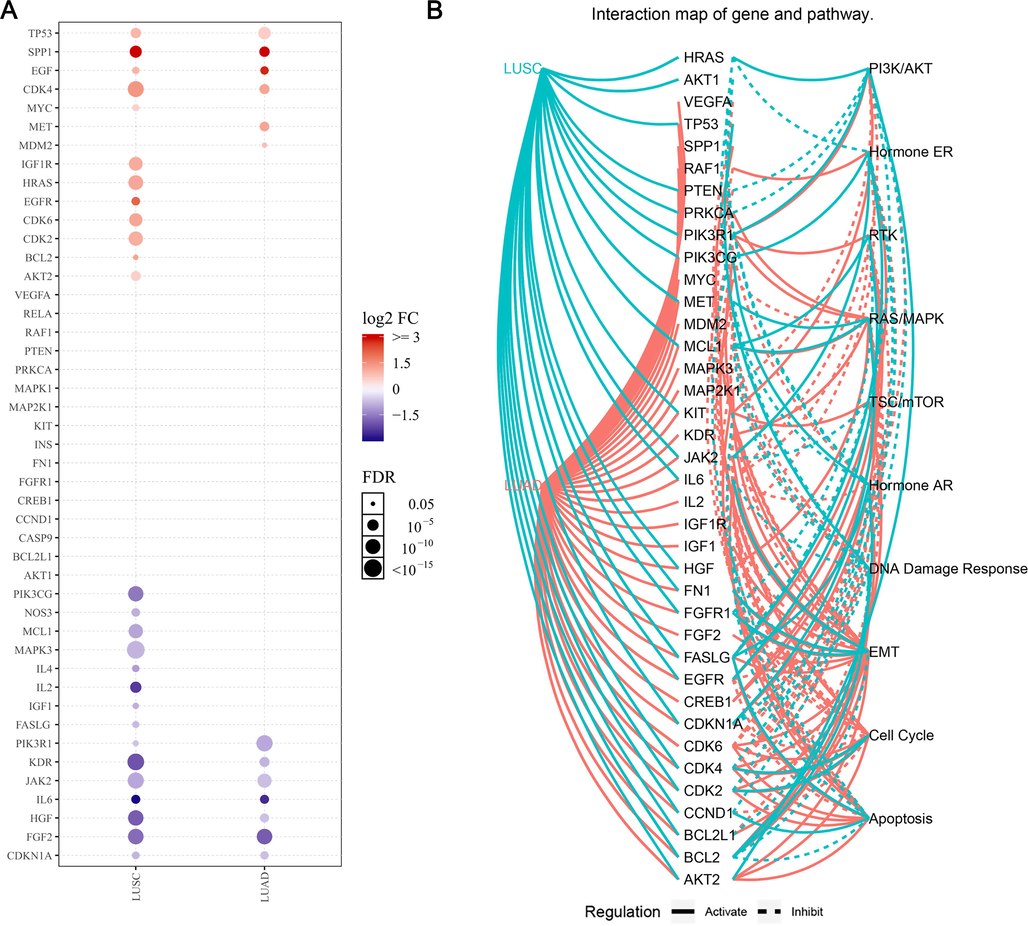
The expression and pathways activity analysis of guava leaf constituent target genes in the PI3K-Akt signaling pathway. (A) The mRNA expression profile of genes. The dots represent the fold change; the blue color indicates downregulation while the red color indicates upregulation. The size of the dot is proportional to the expression foldchange (FC). (B) Pathway activity analysis.
3.6 Molecular docking
The previous drug-disease interaction gene network topological analysis showed that the 10 genes with the strongest interaction were STAT3, TP53, AKT1, JUN, IL6, VEGFA, SRC, TNF, HRAS, and CDKN1A, which are effective in the “Guava leaf-constituent category” network. The Cytoscape interaction network of component-genes showed that the five compounds with strong interaction between guava leaf constituents and lung cancer genes were quercetin, genistein, apigenin, ursolic acid, and daidzein. The five compounds and 10 target proteins were molecularly docked by online molecular docking on the systemsDock website. The score of the database system docking was between 0 and 10. The larger the docking score, the better the docking effect, and the greater the binding activity between the docking molecule and the target. A docking score greater than 4.25 indicates that a ligand has a certain binding activity with a receptor; a score greater than 5.0 indicates a better binding activity; a score greater than 7.0 indicates a strong binding activity (Hsin et al., 2016). The PDB ID of the 10 genes was molecularly docked with the five compounds. The docking scores of all compounds and proteins were greater than 4.25, indicating the presence of binding activity. The molecular docking results are shown in Table 9.
Ligand ID
compounds
Receptor proteins
PDB ID
gene
Docking results
5,280,343
quercetin
Signal transducer and activator of transcription 3
1BG1
STAT3
7.475
5,280,863
kaempferol
Signal transducer and activator of transcription 3
1BG1
STAT3
7.437
5,280,961
Genistein
Signal transducer and activator of transcription 3
1BG1
STAT3
7.809
5,281,708
Daidzein
Signal transducer and activator of transcription 3
1BG1
STAT3
7.811
64,945
ursolic acid
Signal transducer and activator of transcription 3
1BG1
STAT3
4.88
5,280,343
quercetin
Cellular tumor antigen p53
5MGT
TP53
6.658
5,280,863
kaempferol
Cellular tumor antigen p53
5MGT
TP53
6.689
5,280,961
Genistein
Cellular tumor antigen p53
5MGT
TP53
6.669
5,281,708
Daidzein
Cellular tumor antigen p53
5MGT
TP53
6.662
64,945
ursolic acid
Cellular tumor antigen p53
5MGT
TP53
5.464
5,280,343
quercetin
RAC-alpha serine/threonine-protein kinase
3CQW
AKT1
6.847
5,280,863
kaempferol
RAC-alpha serine/threonine-protein kinase
3CQW
AKT1
6.858
5,280,961
Genistein
RAC-alpha serine/threonine-protein kinase
3CQW
AKT1
6.824
5,281,708
Daidzein
RAC-alpha serine/threonine-protein kinase
3CQW
AKT1
6.85
64,945
ursolic acid
RAC-alpha serine/threonine-protein kinase
3CQW
AKT1
8.331
5,280,343
quercetin
Interleukin-6
1ALU
IL6
6.684
5,280,863
kaempferol
Interleukin-6
1ALU
IL6
6.656
5,280,961
Genistein
Interleukin-6
1ALU
IL6
6.632
5,281,708
Daidzein
Interleukin-6
1ALU
IL6
6.663
64,945
ursolic acid
Interleukin-6
1ALU
IL6
4.936
5,280,343
quercetin
Transcription factor AP-1
5TO1
JUN
6.569
5,280,863
kaempferol
Transcription factor AP-1
5TO1
JUN
6.548
5,280,961
Genistein
Transcription factor AP-1
5TO1
JUN
6.59
5,281,708
Daidzein
Transcription factor AP-1
5TO1
JUN
6.637
64,945
ursolic acid
Transcription factor AP-1
5TO1
JUN
4.892
5,280,343
quercetin
Vascular endothelial growth factor A
6BFT
VEGFA
5.849
5,280,863
kaempferol
Vascular endothelial growth factor A
6BFT
VEGFA
5.937
5,280,961
Genistein
Vascular endothelial growth factor A
6BFT
VEGFA
6.601
5,281,708
Daidzein
Vascular endothelial growth factor A
6BFT
VEGFA
6.65
64,945
ursolic acid
Vascular endothelial growth factor A
6BFT
VEGFA
5.799
5,280,343
quercetin
Proto-oncogene tyrosine-protein kinase Src
2BDJ
SRC
6.46
5,280,863
kaempferol
Proto-oncogene tyrosine-protein kinase Src
2BDJ
SRC
6.785
5,280,961
Genistein
Proto-oncogene tyrosine-protein kinase Src
2BDJ
SRC
6.246
5,281,708
Daidzein
Proto-oncogene tyrosine-protein kinase Src
2BDJ
SRC
6.789
64,945
ursolic acid
Proto-oncogene tyrosine-protein kinase Src
2BDJ
SRC
7.927
5,280,343
quercetin
Tumor necrosis factor
3ALQ
TNF
4.904
5,280,863
kaempferol
Tumor necrosis factor
3ALQ
TNF
5.01
5,280,961
Genistein
Tumor necrosis factor
3ALQ
TNF
5.109
5,281,708
Daidzein
Tumor necrosis factor
3ALQ
TNF
5.063
64,945
ursolic acid
Tumor necrosis factor
3ALQ
TNF
5.945
5,280,343
quercetin
GTPase HRas
6D5W
HRAS
6.434
5,280,863
kaempferol
GTPase HRas
6D5W
HRAS
6.582
5,280,961
Genistein
GTPase HRas
6D5W
HRAS
6.595
5,281,708
Daidzein
GTPase HRas
6D5W
HRAS
6.605
64,945
ursolic acid
GTPase HRas
6D5W
HRAS
7.867
5,280,343
quercetin
Cyclin-dependent kinase inhibitor 1
3TS8
CDKN1A
5.02
5,280,863
kaempferol
Cyclin-dependent kinase inhibitor 1
3TS8
CDKN1A
5.023
5,280,961
Genistein
Cyclin-dependent kinase inhibitor 1
3TS8
CDKN1A
4.663
5,281,708
Daidzein
Cyclin-dependent kinase inhibitor 1
3TS8
CDKN1A
4.94
64,945
ursolic acid
Cyclin-dependent kinase inhibitor 1
3TS8
CDKN1A
8.148
A heat map of the molecular docking is shown in Fig. 6A. The deeper the color, the better the docking effect. The effect of molecular docking is also shown by the heat map. Table 9 and Fig. 6A showed that the compound with the highest binding activity to the protein receptor STAT3 was genistein; that with the highest binding activity to the protein receptor TP53 was kaempferol; that with the highest binding activity to the protein receptor AKT1 was kaempferol; that with the highest binding activity to the protein receptor AKT1 was quercetin; that with the highest binding activity to the protein receptor JUN was daidzein; that with the highest binding activity to the protein receptor VEGFA was genistein; that with the highest binding activity to the protein receptor SRC was ursolic acid; that with the highest binding activity to the protein receptor TNF was ursolic acid; that with the highest binding activity to the protein receptor HRAS was ursolic acid; and that with the highest binding activity to the protein receptor CDKN1A was ursolic Acid.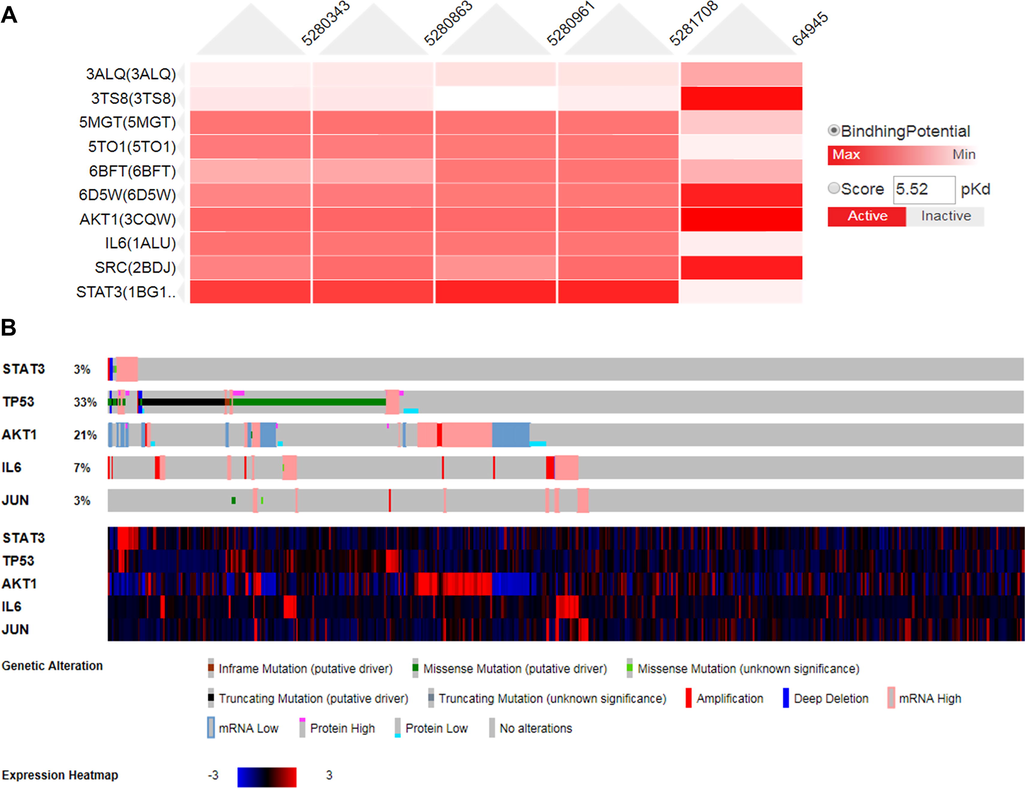
Molecular docking. (A) A heat map showing the docking of receptor protein and ligand. (B) A diagram showing the genetic variation in lung squamous cell carcinoma.
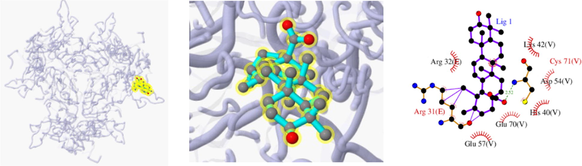
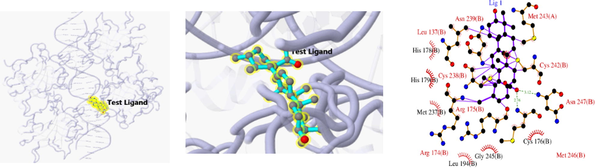
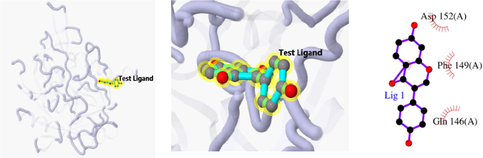
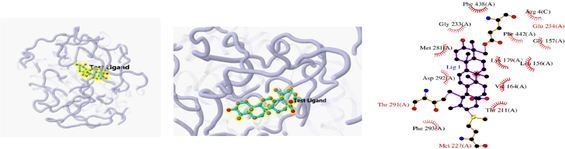
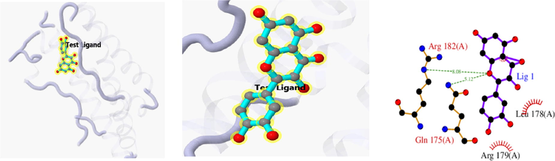
The 3D and 2D structures of their docking are shown in Table 10. In the 2D docking diagram of the third column, there is a specific case where the ligand binds to the receptor, and the surrounding red bar represents a bond-free protein residue within the ligand; the structure linked by the bat is a ligand compound and is subjected to the body protein. Purple represents a ligand compound; yellow represents a receptor protein. Red font represents the binding mode between the ligand and the receptor; the green dotted line represents a hydrogen bond. In these docking results, the docking activity of the receptor protein AKT1 and the ligand ursolic acid was 8.331, which was bound by Met 227 (A), Glu234 (A), and Thr291 (A); the receptor protein CDKN1A and the ligand compound ursolic acid, with docking activity of 8.148, and bound by a hydrogen bond with Asn247 (B) and Met246 (B), with bond lengths of 3.12 and 2.76, respectively, as well as with Cys242 (B), Met243 (A), Asn239 (B), Leu137 (B), Cys238 (B), Arg175 (B), and Arg174 (B), which directly bound.
Docking result of 3ALQ and ursolic acid
Docking result of 3TS8 and ursolic acid
Docking result of 5GMT and kaempferol

Docking result of 5TO1 and Daidzein
Docking result of 6BFT and Genistein
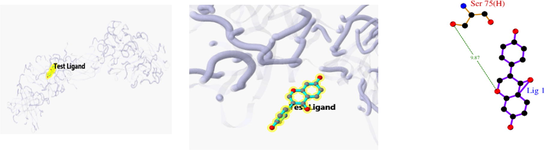
Docking result of 6D5W and ursolic acid
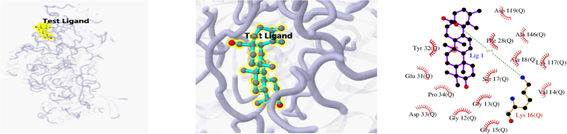
Docking result of 3CQW and kaempferol
Docking result of 1ALU and quercetin
Docking result of 2BDJ and ursolic acid
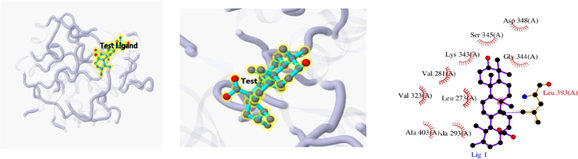
Docking result of 1BG1 and Daidzein
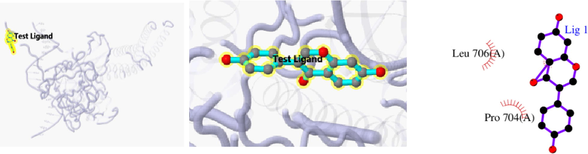
3.7 cBioPortal analysis
cBioPortal is a database used for tumor research. The expression of the genes STAT3, TP53, AKT1, IL6, and JUN in 501 patients with lung squamous cell carcinoma was studied. The expression of these genes in the selected lung cancer samples is shown in Fig. 6B. The first part is the expression of genes, including inframe mutation, missense mutation, truncating mutation, amplification, deep deletion, mRNA level upregulation (mRNA High), mRNA level downregulation (mRNA Low), and no alterations. The second part is a heat map of the gene mRNA levels.
According to the data in Fig. 6B, TP53 and AKT1 were highly expressed in these 501 cancer patients. TP53 was mainly expressed in patients with mutations (129 cases, 25.75%), increased copy number (1 case, 0.2%), mRNA level upregulation (6 cases, 1.2%), high protein level (5 cases, 1%), low protein level (8 cases, 1.8%), and multiple alterations (17 cases, 3.39%). AKT1 was expressed mainly in patients with mutations (1 case, 0.2%), increased copy number (5 cases, 1%), mRNA level upregulation (34 cases, 6.79%), mRNA level downregulation (34 cases, 6.79%), high protein level (2 cases, 0.4%), low protein level (15 cases, 2.99%), and multiple alterations (16 cases, 3.19%).
4 Discussion
Guava leaves are medicinal herbs with various pharmacological effects and a wide range of research and development significance. Guava leaves also have certain antitumor effects. Lung cancer is a common tumor. Currently, there is no particularly effective method for the diagnosis and treatment of lung cancer. Image detection is a commonly used diagnostic method (Hong et al., 2019), followed by genetic diagnosis (Munne and Wells, 2002). Lung cancer has a very low cure rate, and recent approaches to treat lung cancer include immunotherapy, radiotherapy, and targeted therapy (Alasti et al., 2006; Petrosyan et al., 2012; Tsang et al., 2014; Zhang et al., 2019). In this study, the role of guava leaves in lung cancer was studied by network pharmacology to elucidate their correlation and provide a relevant basis for experimental research. In recent years, the pharmacology of traditional Chinese medicine has been continuously developed and has occupied a place in medical research.
From the research point of view, examination of the potential intersection of guava leaf potential target and lung cancer genes revealed 153 common genes, and the interaction between STAT3, TP53, AKT1, IL6, JUN, and VEGFA was the strongest. Using the 66 compounds in guava leaves and the 153 genes, we constructed a “guava leaf-constituent category-active constituent-gene” network“, and our results showed that the compounds quercetin, genistein, apigenin, ursolic acid, daidzein, and lung cancer showed the strongest effect. The proteins with the strongest interaction were VDR, CDK2, MAP2K1, CDK6, MET, ABL1, PPARG, IL2, EGFR, and PGR, indicating that these genes interacted with most constituents of guava leaves, showing that these constituents have high antitumor activities.
GO annotation is an important means to examine the function of gene products (Leale et al., 2018). Through GO analysis, in biological processes, enrichment information includes apoptosis, programmed cell death, cell proliferation, etc.; in terms of cellular components, enrichment Information includes platelets, cell membranes, protein kinases, etc.; in terms of molecular function, it is mainly reflected in enzyme activity and regulation of enzyme activity.
KEGG enrichment analysis provides information on integrated metabolic pathways including metabolism, membrane trafficking, signal processing, and cell cycle. The PI3K-Akt signaling pathway is a typical tumor signaling pathway. In many primary and metastatic human cancers, PTEN activity is lost owing to mutations, deletions, or high-frequency silencing of promoter methylation. It is important for prediction in targeted therapy (Fresno Vara et al., 2004; Carnero et al., 2008; Ma and Hu, 2013). EGFR tyrosine kinases are important for the treatment of non-small cell lung cancer, including monoclonal antibodies. The current clinical representatives are cetuximab and panitumumab as well as EGFR-tyrosine kinase inhibitor. The combination inhibits the binding site of ATP and tyrosine kinase, thereby cutting the downstream signaling pathway and exerting antitumor effect (Toulabi and Ryan, 2018). EGFR is of great significance in the study of non-small cell lung cancer and has potential implications for drug therapy (Mead et al., 1980; Marchetti et al., 2005). In the small cell lung cancer pathway, the tumor suppressor genes are p53, PTEN, RB, and FHIT. ECM activates membrane receptors, and through ITGA and ITGB conduction, activates FAK conduction to activate PI3K activity and express the PKB/AKT signaling pathway. Through continuous phosphorylation and activation of IκBα, the free NF-κB signal is activated into the nucleus for gene network regulation. STAT3 and VEGF may play a reverse regulatory role on the metastasis of lung cancer (Wang et al., 2011). The p53 gene also has an antitumor effect, and Ad-p53 combined with chemotherapy can reverse the chemoresistance of tumor cells and produce a synergistic antitumor effect (Matsubara et al., 2001; Meng and El-Deiry, 2002). In addition, there are other genes expressed in lung cancer that were targeted by guava leaves, representing a potential research direction as clinical anticancer targets.
Analysis of lung squamous cell carcinoma by the cBioPortal tumor database revealed that the genes targeted by Chinese medicine and disease genes are highly expressed in cancer patients, and can undergo mutations, such as addition and deletion, and expression regulation. TP53 and AKT1 were the two most strongly expressed genes in the cancer patients surveyed. Hence, different compounds in guava leaves regulated the same target, and one compound regulated multiple targets and participated in multiple pathways and biological processes, reflecting the multi-target and multi-channel characteristics of guava leaves.
5 Conclusions
Through network pharmacology research, we found that guava leaves had potential targets that interacted with various tumors, regulating the signaling pathways of cancers. The PI3K-Akt signaling pathway, an important signaling pathway in the study of tumor processes, regulates the proliferation of tumor cells and plays a role in tumor cell migration, tumor adhesion, tumor angiogenesis, and extracellular matrix degradation (Chatterjee et al., 2013; Liu et al., 2014; Wang et al., 2016a). The TP53 gene is an important tumor suppressor gene involved in many gene network regulation processes, and has a reference significance in the research and development of new drugs and targeted therapy.
Many of the pharmacological effects of guava leaves are yet to be developed. There are many compounds in guava leaves. At present, antitumor studies on guava leaves are still lacking, although some studies have shown that guava leaves exert antitumor effects. This study preliminarily verified the pharmacological basis and the related mechanism of the antitumor effect of guava leaves, providing a foundation for further research. It is also hoped that specific experiments will be carried out to verify the pharmacological effects of guava leaves against tumors. If successful, the lack of cancer research on guava leaves will be overcame. In addition, network pharmacology is a hot topic of research; we hope to conduct more research projects in the future to provide a basis for future research on new drugs.
6 Data availability
The data used to support the findings of this study are included within the article.
Author contribution
LJ has made substantial contributions to conception and design, interpretation of data, and manuscript writing. WJ has made substantial contributions to acquisition and analysis of data. YQ and JL received the funding supporting for the study. YW has made substantial contributions to acquisition of data.
Funding
This work was supported by the Guangxi Natural Science Foundation Program [Grant number 2018GXNSFAA138140]; the Basic Competence Improvement Project for Middle and Young Teachers in Guangxi universities [Grant number KY2016LX281]; the Hechi University School Research Project [Grant number 2014QN-N005]; and the Hechi University High-level Talent Research Startup Project [Grant number XJ2018GKQ014].
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- A novel four-dimensional radiotherapy method for lung cancer: imaging, treatment planning and delivery. Phys. Med. Biol.. 2006;51:3251-3267.
- [CrossRef] [Google Scholar]
- Critical pharmacokinetic and pharmacodynamic drug-herb interactions in rats between warfarin and pomegranate peel or guava leaves extracts. BMC Complement. Altern. Med.. 2019;19:29.
- [CrossRef] [Google Scholar]
- Chemical composition, antioxidant, antitumor, anticancer and cytotoxic effects of Psidium guajava leaf extracts. Pharm. Biol.. 2016;54:1971-1981.
- [CrossRef] [Google Scholar]
- DisGeNET: a Cytoscape plugin to visualize, integrate, search and analyze gene-disease networks. Bioinformatics. 2010;26:2924-2926.
- [CrossRef] [Google Scholar]
- The PTEN/PI3K/AKT signalling pathway in cancer, therapeutic implications. Curr. Cancer Drug Targets. 2008;8:187-198.
- [CrossRef] [Google Scholar]
- The PI3K/Akt signaling pathway regulates the expression of Hsp70, which critically contributes to Hsp90-chaperone function and tumor cell survival in multiple myeloma. Haematologica. 2013;98:1132-1141.
- [CrossRef] [Google Scholar]
- Lung cancer incidence and mortality in China in 2013. Zhonghua Zhong Liu Za Zhi. 2017;39:795-800.
- [CrossRef] [Google Scholar]
- Systems pharmacology – towards the modeling of network interactions. Eur. J. Pharm. Sci.. 2016;94:4-14.
- [CrossRef] [Google Scholar]
- Targeting the Akt/PI3K signaling pathway as a potential therapeutic strategy for the treatment of pancreatic cancer. Curr. Med. Chem.. 2017;24:1321-1331.
- [CrossRef] [Google Scholar]
- Cytotoxic and antioxidant constituents from the leaves of Psidium guajava. Bioorg. Med. Chem. Lett.. 2015;25:2193-2198.
- [CrossRef] [Google Scholar]
- PI3K/Akt signalling pathway and cancer. Cancer Treat. Rev.. 2004;30:193-204.
- [CrossRef] [Google Scholar]
- Psidium guajava: a review of its traditional uses, phytochemistry and pharmacology. J. Ethnopharmacol.. 2008;117:1-27.
- [CrossRef] [Google Scholar]
- Added value of bone suppression image in the detection of subtle lung lesions on chest radiographs with regard to reader's expertise. J. Korean Med. Sci.. 2019;34:e250
- [CrossRef] [Google Scholar]
- Combining machine learning systems and multiple docking simulation packages to improve docking prediction reliability for network pharmacology. PLoS ONE. 2013;8:e83922
- [CrossRef] [Google Scholar]
- systemsDock: a web server for network pharmacology-based prediction and analysis. Nucleic Acids Res.. 2016;44:W507-513.
- [CrossRef] [Google Scholar]
- VNP: interactive visual network pharmacology of diseases, targets, and drugs. CPT Pharmacometrics Syst. Pharmacol.. 2014;3:e105
- [CrossRef] [Google Scholar]
- PubChem substance and compound databases. Nucleic Acids Res.. 2016;44:D1202-D1213.
- [CrossRef] [Google Scholar]
- Cytoscape: software for visualization and analysis of biological networks. Methods Mol. Biol.. 2011;696:291-303.
- [CrossRef] [Google Scholar]
- Inferring unknown biological function by integration of GO annotations and gene expression data. IEEE/ACM Trans. Comput. Biol. Bioinform.. 2018;15:168-180.
- [CrossRef] [Google Scholar]
- Anticancer activity of guava (Psidium guajava L.) branch extracts against HT-29 human colon cancer cells. J. Med. Plants Res.. 2010;4:891-896.
- [Google Scholar]
- Systems biology – a pivotal research methodology for understanding the mechanisms of traditional medicine. J. Pharmacopuncture. 2015;18:11-18.
- [CrossRef] [Google Scholar]
- Glutaminase 2 negatively regulates the PI3K/AKT signaling and shows tumor suppression activity in human hepatocellular carcinoma. Oncotarget. 2014;5:2635-2647.
- [CrossRef] [Google Scholar]
- PharmMapper server: a web server for potential drug target identification using pharmacophore mapping approach. Nucleic Acids Res.. 2010;38:W609-614.
- [CrossRef] [Google Scholar]
- Terpenoids progress in the anti-tum or research. Heilongjiang Sci. Technol. Inform.. 2016;30:149-151.
- [Google Scholar]
- Antioxidant and anti-diabetic activities of polysaccharides from guava leaves. Molecules. 2019;24
- [CrossRef] [Google Scholar]
- Targeting PI3K/Akt/mTOR cascade: the medicinal potential, updated research highlights and challenges ahead. Curr. Med. Chem.. 2013;20(24):2991-3010.
- [CrossRef] [Google Scholar]
- EGFR mutations in non-small-cell lung cancer: analysis of a large series of cases and development of a rapid and sensitive method for diagnostic screening with potential implications on pharmacologic treatment. J. Clin. Oncol.. 2005;23:857-865.
- [CrossRef] [Google Scholar]
- Combinatory anti-tumor effects of electroporation-mediated chemotherapy and wild-type p53 gene transfer to human esophageal cancer cells. Int. J. Oncol.. 2001;18:825-829.
- [CrossRef] [Google Scholar]
- Meng, R.D., El-Deiry, W.S., 2002. “CHAPTER 18 - Cancer Gene Therapy with the p53 Tumor Suppressor Gene,” in Gene Therapy of Cancer (Second Edition), eds. E.C. Lattime & S.L. Gerson. (San Diego: Academic Press), 299-313.
- Preimplantation genetic diagnosis. Curr. Opin. Obstet. Gynecol.. 2002;14:239-244.
- [CrossRef] [Google Scholar]
- Spectroscopic characteristics of novel Psidium meroterpenoids isolated from guava leaves. Zhongguo Zhong Yao Za Zhi. 2015;40:2898-2902.
- [Google Scholar]
- Epidemiology, incidence and mortality of lung cancer and their relationship with the development index in the world. J. Thorac. Dis.. 2016;8:1094-1102.
- [CrossRef] [Google Scholar]
- TCMSP: a database of systems pharmacology for drug discovery from herbal medicines. J. Cheminform.. 2014;6:13.
- [CrossRef] [Google Scholar]
- Study to find the best extraction solvent for use with guava leaves (Psidium guajava L.) for high antioxidant efficacy. Food Sci. Nutr.. 2014;2:174-180.
- [CrossRef] [Google Scholar]
- Four new triterpenoids from the leaves of Psidium guajava. J. Asian Nat. Prod. Res.. 2012;14:348-354.
- [CrossRef] [Google Scholar]
- Guadial A and psiguadials C and D, three unusual meroterpenoids from Psidium guajava. Org. Lett.. 2012;14:5262-5265.
- [CrossRef] [Google Scholar]
- Research advancement of the antitumor effect and mechanisms of triterpenoid comprised by traditional Chinese medicine. J. Modern Oncol.. 2011;19:1880-1883.
- [CrossRef] [Google Scholar]
- Stressing the need to overcome EGFR tyrosine kinase inhibitor resistance. Transl. Lung Cancer Res.. 2018;7:S123-s126.
- [CrossRef] [Google Scholar]
- Non-surgical treatment of lung cancer: personalised stereotactic ablative radiotherapy. Hong Kong Med. J.. 2014;20:529-536.
- [CrossRef] [Google Scholar]
- PTP4A3 is a target for inhibition of cell proliferatin, migration and invasion through Akt/mTOR signaling pathway in glioblastoma under the regulation of miR-137. Brain Res.. 2016;1646:441-450.
- [CrossRef] [Google Scholar]
- Fingerprint analysis and quality consistency evaluation of flavonoid compounds for fermented Guava leaf by combining high-performance liquid chromatography time-of-flight electrospray ionization mass spectrometry and chemometric methods. J. Sep. Sci.. 2016;39:3906-3916.
- [CrossRef] [Google Scholar]
- Significance of CXCR4, phosphorylated STAT3 and VEGF-A expression in resected non-small cell lung cancer. Exp. Ther Med.. 2011;2:517-522.
- [CrossRef] [Google Scholar]
- Prediction of quality markers of traditional Chinese medicines based on network pharmacology. Chinese Herbal Med. 2019
- [CrossRef] [Google Scholar]
- Integration and analysis of CPTAC proteomics data in the context of cancer genomics in the cBioPortal. Mol. Cell. Proteomics. 2019;18:1893-1898.
- [CrossRef] [Google Scholar]
- Network pharmacology: a new approach to unveiling Traditional Chinese Medicine. Chin. J. Nat. Med.. 2015;13:1-2.
- [CrossRef] [Google Scholar]
- Spatial association between outdoor air pollution and lung cancer incidence in China. BMC Public Health. 2019;19:1377.
- [CrossRef] [Google Scholar]
- Immune checkpoint blockade mediated by a small-molecule nanoinhibitor targeting the PD-1/PD-L1 pathway synergizes with photodynamic therapy to elicit antitumor immunity and antimetastatic effects on breast cancer. Small. 2019;e1903881
- [CrossRef] [Google Scholar]







